
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 03 April 2023
Sec. Sustainable and Intelligent Phytoprotection
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1154266
This article is part of the Research TopicPlant-Derived Natural Compounds in Drug Discovery: The Prism Perspective between Plant Phylogeny, Chemical Composition, and Medicinal Efficacy, Volume IIView all 9 articles
Lipid metabolism disorders may considerably contribute to the formation and development of atherosclerosis (AS). Traditional Chinese medicine has received considerable attention in recent years owing to its ability to treat lipid metabolism disorders using multiple components and targets. Verbena officinalis (VO), a Chinese herbal medicine, exhibits anti-inflammatory, analgesic, immunomodulatory, and neuroprotective effects. Evidence suggests that VO regulates lipid metabolism; however, its role in AS remains unclear. In the present study, an integrated network pharmacology approach, molecular docking, and molecular dynamics simulation (MDS) were applied to examine the mechanism of VO against AS. Analysis revealed 209 potential targets for the 11 main ingredients in VO. Further, 2698 mechanistic targets for AS were identified, including 147 intersection targets between VO and AS. Quercetin, luteolin, and kaempferol were considered key ingredients for the treatment of AS based on a potential ingredient target–AS target network. GO analysis revealed that biological processes were primarily associated with responses to xenobiotic stimuli, cellular responses to lipids, and responses to hormones. Cell components were predominantly focused on the membrane microdomain, membrane raft, and caveola nucleus. Molecular functions were mainly focused on DNA-binding transcription factor binding, RNA polymerase II-specific DNA-binding transcription factor binding, and transcription factor binding. KEGG pathway enrichment analysis identified pathways in cancer, fluid shear stress, and atherosclerosis, with lipid and atherosclerosis being the most significantly enriched pathways. Molecular docking revealed that three key ingredients in VO (i.e., quercetin, luteolin, and kaempferol) strongly interacted with three potential targets (i.e., AKT1, IL-6, and TNF-α). Further, MDS revealed that quercetin had a stronger binding affinity for AKT1. These findings suggest that VO has beneficial effects on AS via these potential targets that are closely related to the lipid and atherosclerosis pathways. Our study utilized a new computer-aided drug design to identify key ingredients, potential targets, various biological processes, and multiple pathways associated with the clinical roles of VO in AS, which provides a comprehensive and systemic pharmacological explanation for the anti-atherosclerotic activity of VO.
Atherosclerosis (AS) is a chronic inflammatory disease characterized by lipid deposition on vessel walls (Libby et al., 2011). Several risk factors are associated with its development, including hyperlipidemia, hypertension, chronic stress, glucose metabolism disorders, smoking, genetics, aging, and sex-related factors (Yao et al., 2019; Landowska et al., 2022). In modern society, the mortality and morbidity of AS are increasing; at present, it is a leading cause of death among adults (Dahlöf, 2010). The World Health Organization states that approximately 17.9 million people die from cardiovascular diseases every year, accounting for 32% of all deaths in both developed and developing countries (Fan and Watanabe, 2022). AS leads to endothelial dysfunction, the formation of a new endothelial layer, and the development of lipid deposits, foam cells, and plaques, which eventually rupture, resulting in the formation of thrombi and the narrowing of blood vessels. Further, it also causes ischemia and hypoxia in the tissues and organs involved, leading to symptoms such as chest tightness and pain and even sudden death sometimes (Grootaert et al., 2018). To date, the mechanism underlying AS remains unelucidated; however, studies suggest that it is associated with lipid metabolism disorders, inflammation, oxidative stress, autophagy disorder, and mitochondrial dysfunction (Zhang et al., 2021; Chen et al., 2022a). At present, three main types of statins are used in clinical settings: atorvastatin, resulvastatin, and lipocalin. Further, some fibrates, such as benzofibrate and fenofibrate, and aspirin are used as antithrombotic medications. However, statins are prone to muscle toxicity, including myositis, mild hypercreatine kinaseemia, and acute liver failure owing to rhabdomyolysis (Hilton-Jones, 2018). In addition, statins and fibrates can affect liver function, resulting in elevated alanine transferase and aspartate transferase levels, usually in the early stages, mostly because of the drug dose. This may lead to changes in hepatocyte membrane structure, mitochondrial dysfunction, apoptosis, and drug interactions. As a result, safe and effective medications are urgently warranted.
In traditional Chinese medicine, Verbena officinalis (VO) is a commonly used herb with abundant sources, a low price, and low toxicity. It was originally published in Mingyi Bielu (Records of Famous Physicians). VO is a perennial herb that grows worldwide in Europe, America, North and Central Africa, Asia, and Australia (Austin, 2004). It stimulates blood circulation, disperses blood stasis, clears heat, detoxifies the body, and suppresses coughing. High-performance liquid chromatographic analysis revealed that VO mainly comprises flavonoids, iridoid glycosides, triterpenoids, phenylpropanoids, and other components (El-Hela et al., 2010; Gibitz-Eisath et al., 2018). With bitter and cold properties, VO exerts its influence via the liver and spleen meridians. According to modern pharmacological research, the active ingredients in VO are lipid regulators and possess anti-inflammatory, analgesic, immunomodulatory, and neuroprotective properties. Owing to its ability to inhibit lipid metabolism (Steinberg, 2004; Konstantinov et al., 2007), some of its ingredients may be useful for treating AS (Tu et al., 2007; Xue et al., 2017); however, whether VO has an anti-atherosclerotic effect via multiple components and targets remains unclear.
A computer-aided drug design method combines network biology and multipharmacology, including molecular docking and molecular dynamics simulation (MDS), to develop an updated drug discovery method. In the present study, network pharmacology was used to examine the key ingredients in VO and its possible targets against AS. Further, molecular docking and MDS were used to explore the interaction between targets and active ingredients in VO. In addition to these techniques, we used a computerized target prediction system based on interactions among components in medicinal materials and targets and among genes and biological pathways to determine the relationship comprehensively and systematically between active ingredients in VO and their anti-atherosclerotic effects for the first time (Figure 1).
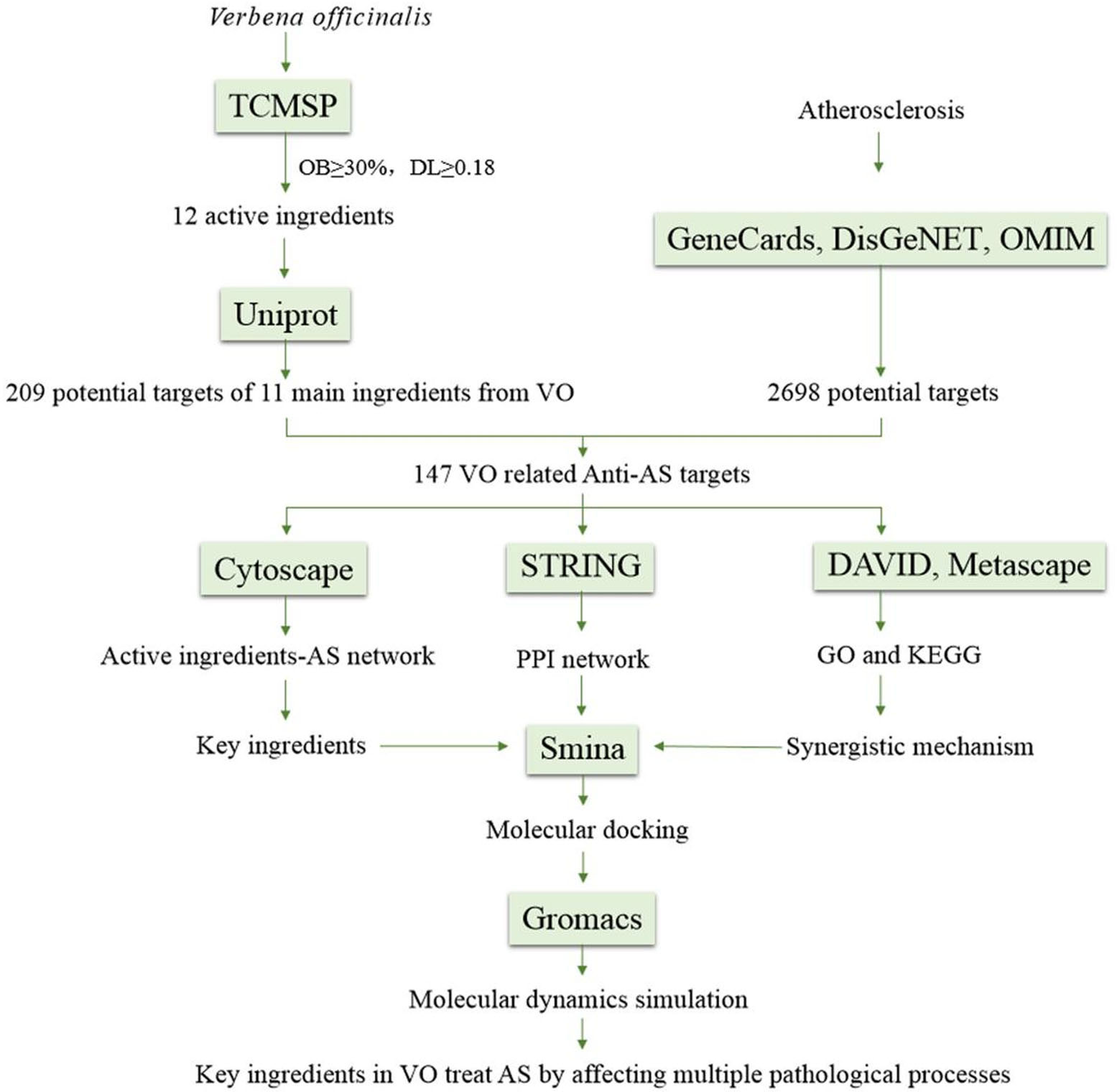
Figure 1 Flow chart showing the steps involved in investigating the pharmacological mechanism of VO against atherosclerosis.
The databases and software used were as follows: Traditional Chinese Medicine Systems Pharmacology (TCMSP; http://tcmspw.com/tcmsp.php), ClassyFire (https://cfb.fiehnlab.ucdavis.edu/), GeneCards (http://www.genecards.org), DisGeNET (https://www.disgenet.org/), OMIM (https://www.omim.org/), PANTHER Classification System (http://pantherdb.org/), UniProt (https://www.uniprot.org), Venny2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny), STRING (http://string-db.org/cgi/input.pl), DAVID (http://David.ncifcrf.gov), Bioinformatics (https://www.bioinformatics.com.cn/), Metascape (https://metascape.org/gp/index.html#/main/step1), PubChem (https://pubchem.ncbi.nlm.nih.gov/), Cytoscape 3.9.0 software, Smina software, Gromacs 2019.6, and Gmx_MMPBSA software.
Using the TCMSP database (Ru et al., 2014), the active ingredients in VO were identified according to the following basic criteria: oral bioavailability ≥30% and druglikeness ≥0.18 (Tao et al., 2012; Xu et al., 2012). PubChem was used to obtain the InChIKeys for each ingredient in VO. These InChIKeys were then entered into the ClassyFire database for classification (Djoumbou Feunang et al., 2016).
To obtain targets related to the main ingredients in VO, data were retrieved from the TCMSP database, the Uniprot database was searched for all verified human genes and their corresponding gene names, and the target genes of VO were annotated according to their database names to determine potential action targets for the active ingredients in VO (UniProt Consortium, T, 2018). Then, genes with duplicate values were removed (such as NOS2, PTGS1, PTGS2, and DPP4), followed by the construction and visualization of an interconnected network graph of the effective ingredients and targets using Cytoscape 3.9.0 software (Chai et al., 2022).
Data from the GeneCards (Safran et al., 2010), DisGeNET (Zhao et al., 2021), and OMIM (Amberger et al., 2015) disease databases were retrieved using the keyword “AS.” We selected disease targets with a corresponding median of ≥1.12 based on GeneCards data. Simultaneously, disease targets with a score of ≥0.02 in the DisGeNET database were screened. Finally, AS targets were obtained by combining the disease targets from the three databases and removing duplicates. The targets for VO and AS were imported into Venny 2.1.0 to prepare Venn diagrams, and VO targets correlated with AS pathology were obtained (An et al., 2022).
VO targets were functionally annotated and classified using the Panther classification system (Mi et al., 2019), in which the VO targets were classified using Homo sapiens as the only organism. Results were plotted using Origin 2017 software.
The PPI network of the target proteins was constructed using the STRING11.5 database (Szklarczyk et al., 2016) and visualized using the Cytoscape 3.9.0 software (Shannon et al., 2003). A significant interaction score was determined as >0.4 only for the organism H. sapiens. Fifteen core targets were chosen based on their degree values, which were determined using NetworkAnalysis (a Cytoscape plugin).
A GO analysis of biological functions was performed using the DAVID database (Huang et al., 2009; Chen et al., 2017), which comprises biological processes (BP), cell components (CC), and molecular functions (MF). Terms were considered statistically significant when the p-value was<0.05. The top 10 terms were visually represented using an online drawing program called Bioinformatics (Xia et al., 2022). Enrichment bar graphs were created for KEGG pathways for the species H. sapiens using the Metascape database (Zhou et al., 2019b).
To validate the binding of the active ingredients in VO to the core targets, the PubChem database (Kim et al., 2020) was used to determine the three-dimensional (3D) structures of the active ingredients, and AlphaFold2 was used to obtain the protein structure files of the targets (Jumper et al., 2021). Molecular docking calculations were performed using the molecular docking software Smina (Masters et al., 2020). The size of the docking box was 126, and the docking approximation was 20, with 20 conformations generated each time. When a ligand binds to its receptor, hydrogen bonding and hydrophobic interactions are formed; hydrogen bonding plays an essential role in maintaining ligand–receptor binding stability. When a ligand binds with a receptor, it changes the structure and chemical properties of the receptor to obtain the ligand–receptor complex system with good stability.
The software Gromacs 2019.6 (Van Der Spoel et al., 2005), Amber14sb for proteins, Generation Amber Force Field for macromolecules, and TIP3P water model for water were used in addition to a sodium ion balance system to provide the complex system with a balanced electrolyte solution. The Verlet and Cg algorithms were used for elastic simulation. The particle mesh Ewald method was used to analyze electrostatic interactions, and the steepest descent method (5,000 steps) was chosen to minimize energy. Van der Waals (VDWAALS) radius and Coulomb force cutoff distances were both set at 1.4 nm. After balancing the regular and isothermal isobaric systems, MDS was performed for 100 ns at room temperature and pressure. An integral step of 2 fs was used for constraining hydrogen bonds in the MDS using the LINCS algorithm. Gmx_MMPBSA (Valdés-Tresanco et al., 2021) was used to implement the free energy of binding between a ligand and protein.
The formula above shows that a component known as inner energy contains Ebond, Eangle, and Etorsion. EVDW denotes VDWAALS action, and Eelec denotes electrostatic interaction. The free energy of solvation was defined by GGB and GGA together. The solvation energy in polar solutions is GGB while that in nonpolar solutions is GSA. Calculations were performed using the GB model (GB = 8) developed by Ukai et al. (2020). The solvent accessibility surface area (SASA) was multiplied by the surface tension of the nonpolar solvent to calculate its free energy of solvation (GSA = 0.0072 × SASA) (Weiser et al., 1999). Because computational resources were limited and precision was low, entropy changes were neglected in this study.
Using TCMSP, all the active ingredients in VO were identified. By referring to the pharmacokinetic parameters, 12 components, including quercetin, luteolin, kaempferol, and beta-sitosterol, were screened (Table 1). Using the ClassyFire online program, five component categories were identified: six flavonoids (50%), two steroids and steroid derivatives (17%), two prenol lipids (17%), one fatty acyl (8%), and one macrolide and its analogs (8%). Among the identified active ingredients, 1((4aS,6aR,6aS,6bR,8aR,10R,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid) was not involved in the construction of the network diagram, indicating that the efficacy of the active ingredients in VO was not explored.
Apart from confirming the targets of the VO ingredients, we identified 209 potential targets using the UniProt database. Figure 2 demonstrates that quercetin, luteolin, and kaempferol had the largest number of corresponding targets on the list containing the 11 active ingredients. Evidence suggests that these three ingredients in VO are vital for treating ailments.
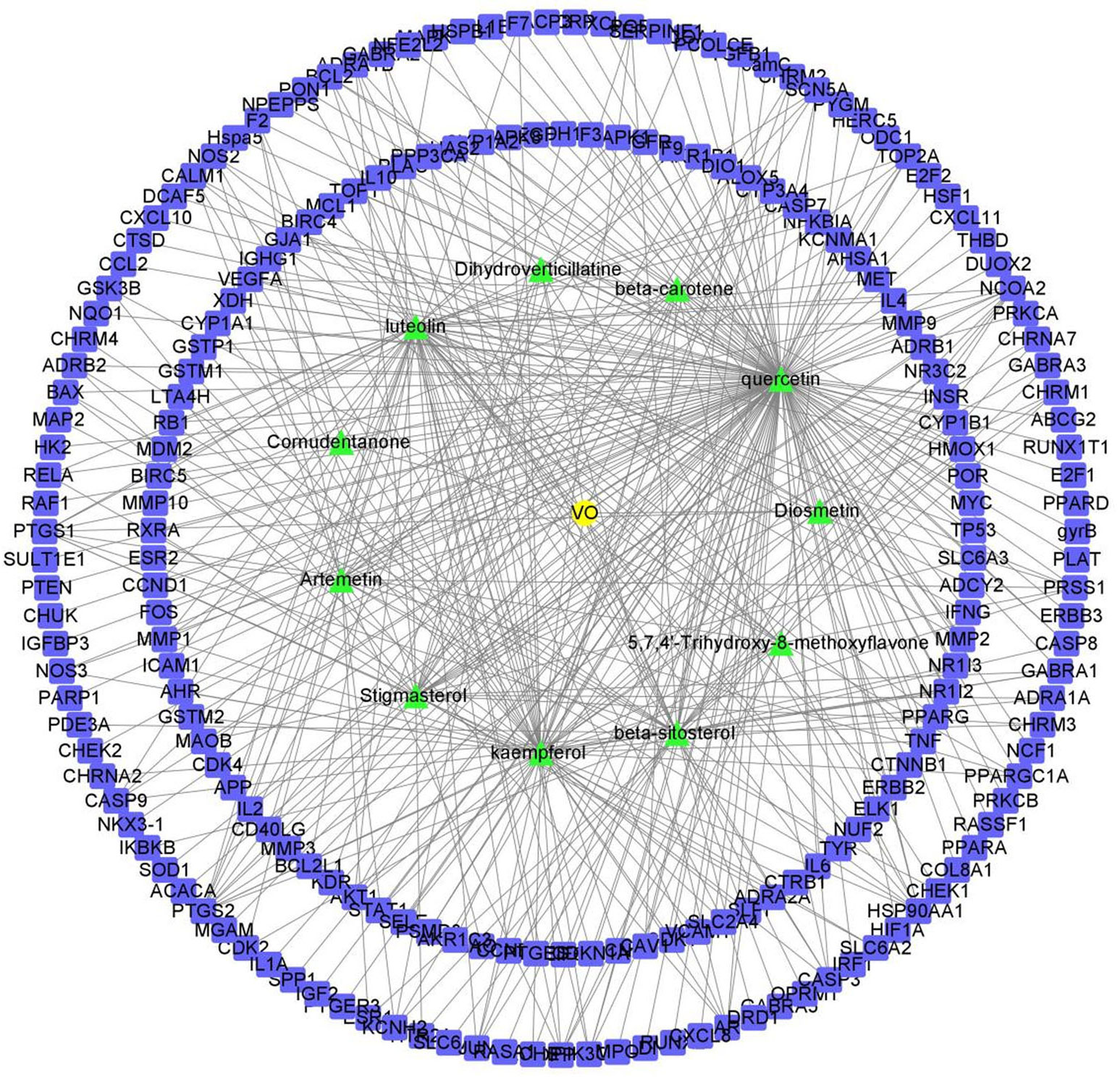
Figure 2 Network of the potential effective ingredients in VO and targets. The green nodes represent the 11 effective ingredients in VO, and the blue nodes represent the 209 corresponding target points of the effective ingredients. There are nodes in the network diagram that represent the potential targets of the genes, and the links between nodes represent the interactions between proteins.
To identify the targets of AS, keywords were entered into the GeneCards, DisGeNET, and OMIM databases; subsequently, 4,848, 2,044, and three targets, respectively, were screened. After combining the data from the three databases and removing duplicates, 2,698 AS targets were identified. To further determine the effectiveness of both VO and AS, a Venn diagram was constructed to summarize 147 VO-related anti-atherosclerotic targets (Figure 3A). Detailed information about these targets is provided in Table 2.
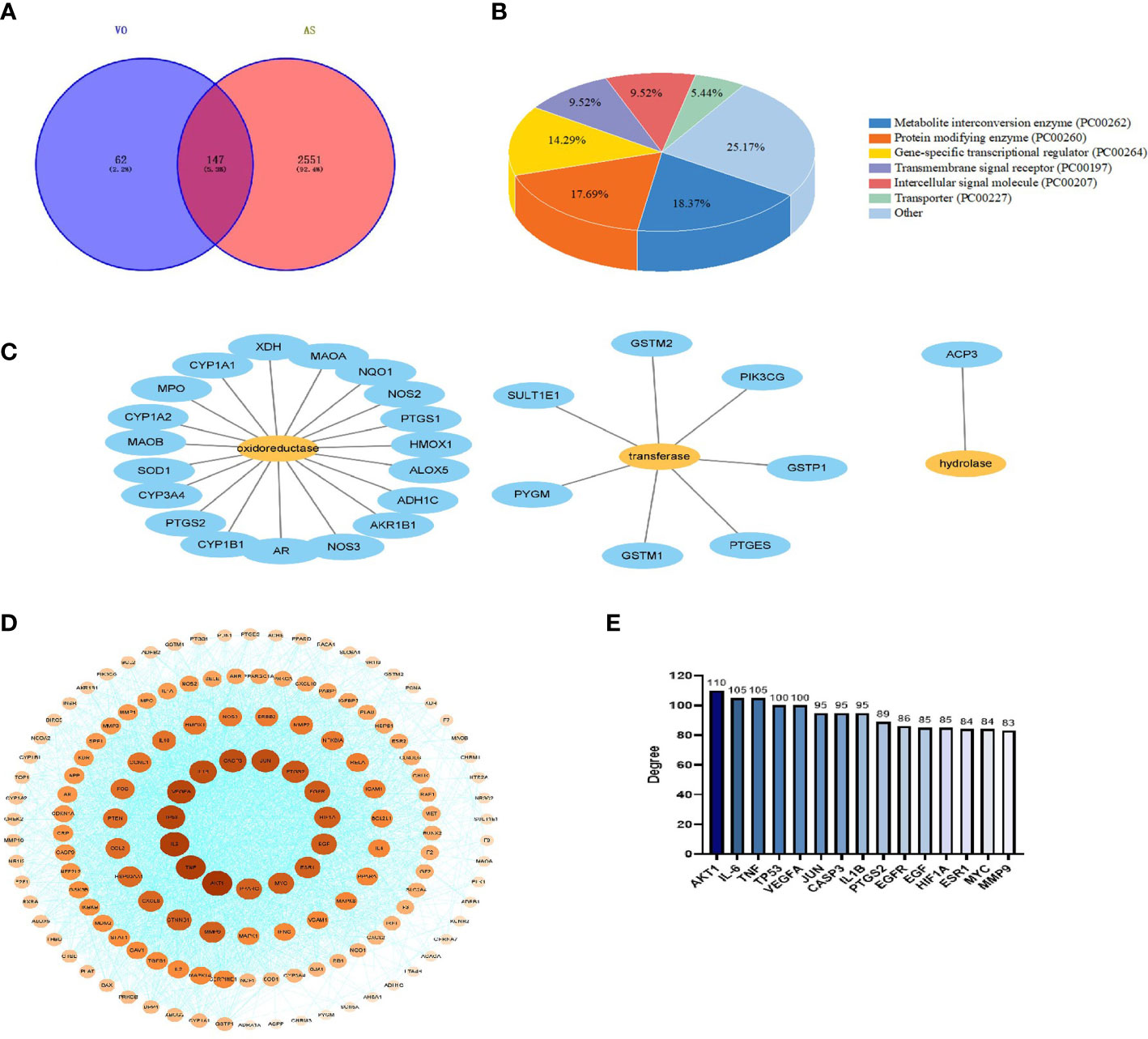
Figure 3 Construction of a PPI network for VO target proteins against AS. (A) The TCMSP database was screened, and 209 VO targets were identified. Further, using the DisGeNET, GeneCards, and OMIM databases, 2,698 genes related to AS were identified. VO and AS intersect at 147 points on the Venn diagram. (B) Panther classification was used to categorize the target proteins targeted by VO against AS. (C) As per Panther classification, the target proteins that modify proteins are classified as enzymes (PC00262). (D) PPI network for VO targets against AS. Proteins are represented by nodes, and interactions between targets are represented by edges. The color and degree of a node are correlated, with a darker color indicating a larger node. (E) The top 15 core targets were identified based on their degree of importance. Degrees are represented by the number on the vertical axis. Nodes are ranked based on the number of nodes that are directly connected to them. A node with a higher degree is considered an important node.
To confirm the function of the potential VO targets, the Panther classification system was used to classify the 147 VO targets against AS. These targets were classified into seven classes according to their cellular functions: metabolite interconversion enzyme (PC00262, 18.37%), protein-modifying enzyme (PC00260), gene-specific transcriptional regulator (PC00264), transmembrane signal receptor (PC00197), intercellular signal molecule (PC00207), transporter (PC00227), and others; of these, metabolite interconversion enzymes (18.37%) were the most abundant class (Figure 3B), followed by oxidoreductases (PC00176, 70.37%), transferases (PC00220, 25.93%), hydrolases (PC00121, 3.70%) (Figure 3C).
Using the STRING database, 147 AS and VO intersection targets were determined, followed by mapping in Cytoscape. The degree of a node indicates its importance based on the number of edges connecting it to other nodes in the PPI network diagram. A PPI network comprising 145 nodes, 2,988 edges, and a degree of average quality of 40.9 was constructed (Figure 3D). The potential targets were identified as AKT1, IL-6, TNF, TP53, VEGFA, JUN, CASP3, IL1B, PTGS2, EGFR, EGF, HIF1A, ESR1, MYC, and MMP9, depending on the degree of the node. Among them, AKT1, IL-6, and TNF were the first three targets with the highest correlation, with degree values of 110, 105, and 105, respectively (Figure 3E). These results indicate that the ingredients in VO are effective against AS via multiple targets and many biological mechanisms.
To determine the effective VO ingredients for treating AS, 147 possible targets and 11 effective VO ingredients were selected for constructing an effective ingredient network for VO and AS (Figure 4). Degree refers to how many edges are connected to a node; therefore, a higher degree value suggests the greater importance of the node. Notably, all these ingredients were associated with multiple targets, resulting in 327 ingredient–target connections for 11 effective VO ingredients and 147 targets. In total, 29.7 targets were identified per ingredient, and the average number of components per target was 2.2. These findings indicate that VO possesses the multicomponent and multitarget characteristics of traditional Chinese medicines. Among these bioactive ingredients, quercetin (degree = 120) had the most targets, followed by luteolin (degree = 47), kaempferol (degree = 46), beta-sitosterol (degree = 23), stigmasterol (degree = 21), beta-carotene (degree = 19), artemetin (degree = 17), 5,7,4’-trihydroxy-8-methoxyflavone (degree = 14), dihydroverticillatine (degree = 9), diosmetin (degree = 7), and cornudentanone (degree = 4). Taken together, these findings suggest that quercetin, luteolin, and kaempferol are probably the most important VO ingredients needed for treating AS.
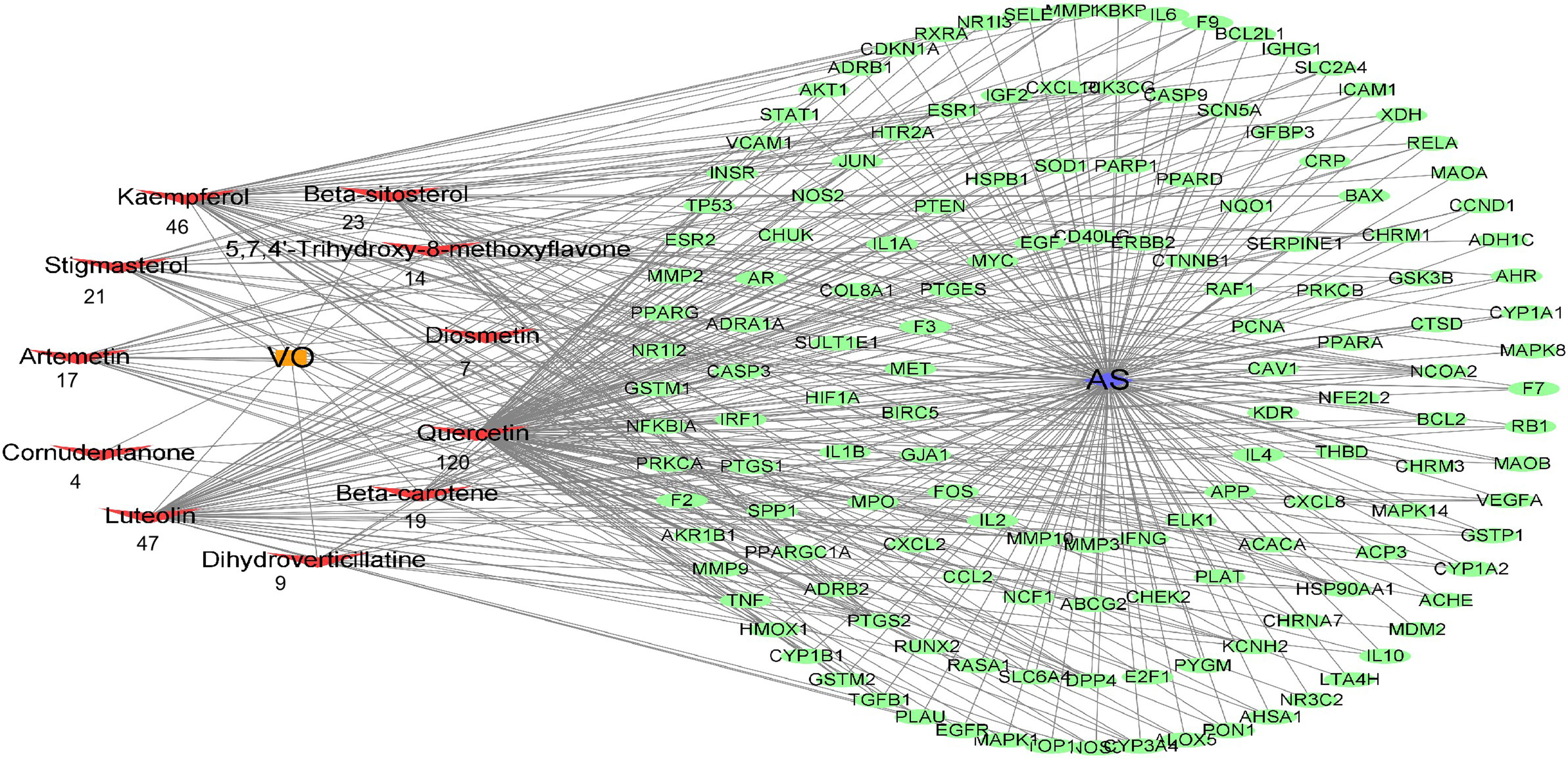
Figure 4 Construction of the drug–target–disease network. Each red node represents an effective ingredient, and the number beneath each node indicates how effective that ingredient is against AS. Cytoscape represents the green nodes as potential targets for VO and AS.
To investigate the synergistic mechanisms of the main VO ingredients against AS, the DAVID database was used to assess the 147 VO targets involved in AS pathology via GO enrichment analysis. Figure 5A presents the top 10 enriched GO terms based on their adjusted p-values. The major focus of BP was response to xenobiotic stimulus (GO:0009410), cellular response to lipid (GO:0071396), response to hormone (GO:0009725), and so on. Most CC research focused on the membrane microdomain (GO:0098857), membrane raft (GO:0045121), and caveola (GO:0005901) in Figure 5B. In Figure 5C, MF was mainly focused on DNA-binding transcription factor binding (GO:0140297), RNA polymerase II-specific DNA-binding transcription factor binding (GO:0061629), and transcription factor binding (GO:0008134). These findings suggest that VO plays an essential biological role in AS development.
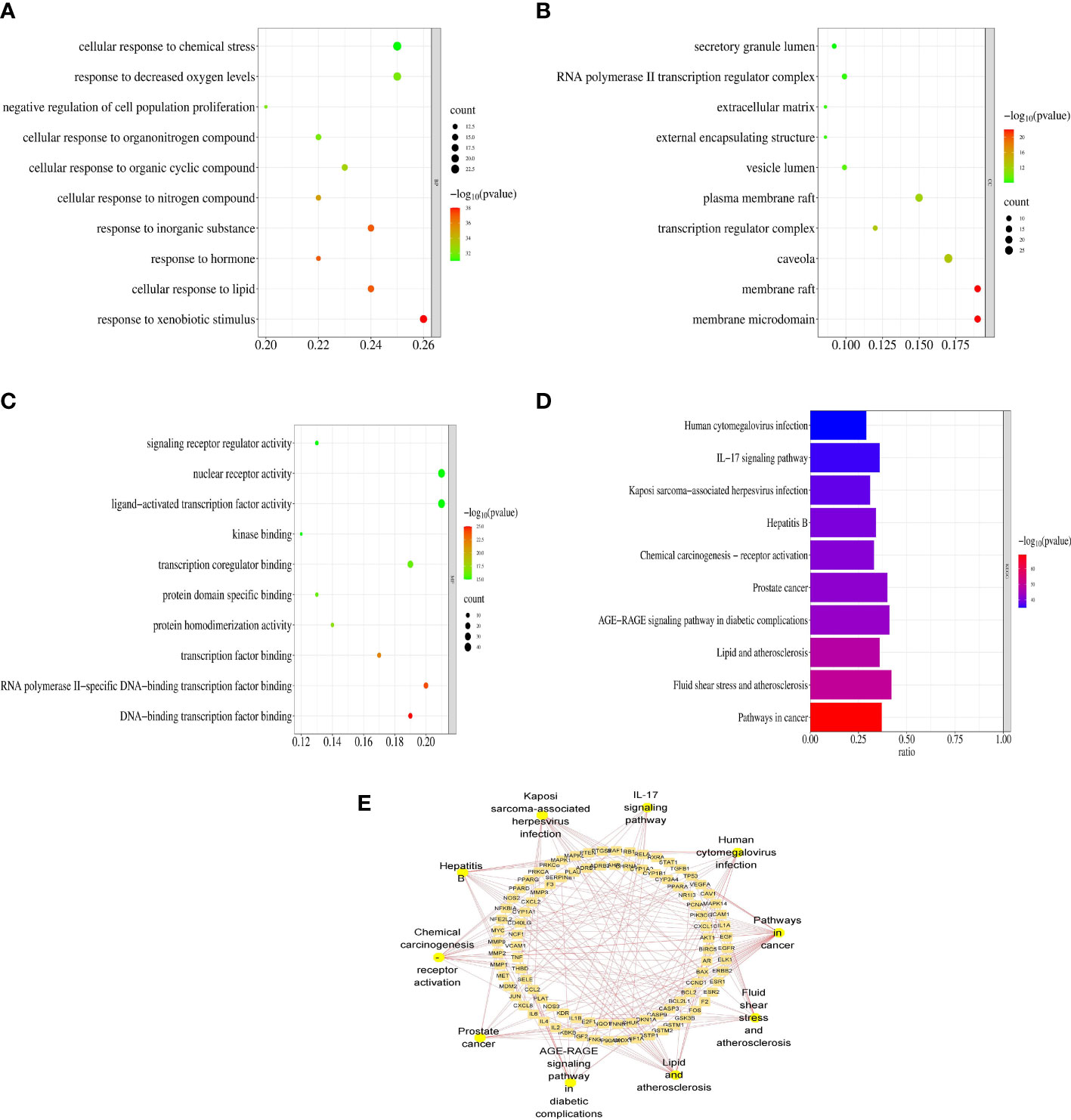
Figure 5 Functional enrichment analyses of the target proteins of VO against AS. (A) GO enrichment analysis reveals the top 10 biological processes. (B) A bubble chart illustrating the top 10 biological processes that are involved in cell components. (C) A bubble chart of the top 10 molecular functions derived from GO enrichment analysis. Rich factor is indicated on the X-axis, bubble size indicates target counts enriched in terms, and color indicates the significance as p-value. According to the order of the p-values from small to large, the top 10 vital GO terms were selected from the VO targets against AS. (D) VO anti-AS targets were analyzed for KEGG pathway enrichment. According to the order of the p-value from small to large, the 10 most significant KEGG pathways were chosen. (E) The top 10 pathways, along with the targets associated with them, as shown in the Cytoscape software.
To identify KEGG pathways related to effective VO ingredients against AS targets, the Metascape database, which contains 147 targets, was enriched for KEGG pathway enrichment analysis. The top 10 pathways with the highest significance are shown in Figure 5D. The main pathways involved in cancer (hsa05200), fluid shear stress and atherosclerosis (hsa05418), lipids and atherosclerosis (hsa05417), the AGE-RAGE signaling pathway in diabetic complications (hsa04933), and prostate cancer (hsa05215), along with the top 10 pathways along with the targets associated with them (Figure 5E). Table 3 provides a detailed report of the KEGG pathway enrichment analysis. Based on these findings, VO exerts its anti-atherosclerotic effects via multiple pathways.
To validate the binding of VO ingredients to the molecules involved in AS pathology, molecular docking was performed using Smina software. Table 4 presents the docking scores between quercetin, luteolin, and kaempferol and the main targets (AKT1, IL-6, and TNF). Lower scores were determined by better docking effects. In terms of affinity, quercetin exhibited the strongest affinity with AKT1, IL-6, and TNF-α, with scores of −9.6, −8.9, and −8.8 kcal/mol, respectively. The binding scores of luteolin to AKT1, IL-6, and TNF-α were −9.5, −8.4, and −7.3 kcal/mol and those of kaempferol were −9.0, −8.4, and −7.9 kcal/mol.
As shown in Figure 6, the binding pockets of AKT1, IL-6, and TNF-α were tightly bound by kaempferol, luteolin, and quercetin, which were stabilized by hydrogen bonds. Four amino acid residues, namely, GLU191, GLU198, LYS179, and ASP292 form hydrogen bonds with quercetin in AKT1, whereas ASP274 plays a hydrophobic role (Figure 6A). Furthermore, five amino acid residues, namely, GLU191, GLU198, LYS179, LYS276 and THR195, formed hydrogen bonds with luteolin in AKT1 (Figure 6B), and four amino acid residues, namely, GLU191, GLU198, LYS179, and THR195, in AKT1 formed hydrogen bonds with kaempferol (Figure 6C). IL-6 contains the amino acid residues LEU90 and ARG196 that form multiple binding sites for quercetin; these are surrounded by hydrophobic forces such as LEU193, LEU92, and MET95 (Figure 6D). Luteolin forms potential interactions with IL-6 via the amino acid residues ARG196 and SER204 and two hydrogen bonds (Figure 6E). The amino acid residues GLU200 and LEU92 in IL-6 form several hydrogen bonds with kaempferol (Figure 6F). The amino acid residue ALA109 in TNF-α forms a hydrogen bond with quercetin, whereas GLN225 plays a hydrophobic role in TNF-α (Figure 6G). Moreover, luteolin formed potential interactions with the amino acid residues PHE220 and PRO215 of TNF-α via two hydrogen bonds, with the amino acid residue GLY100 playing a hydrophobic role (Figure 6H). The amino acid residue ALA109 in TNF-α formed multiple binding locations with quercetin, whereas the amino acid residue ARG108 played a hydrophobic role (Figure 6I). All binding positions are shown in Supplementary Figure 1. Taken together, these findings suggest that the key ingredients in VO strongly bind to targets related to the lipid and AS pathways.
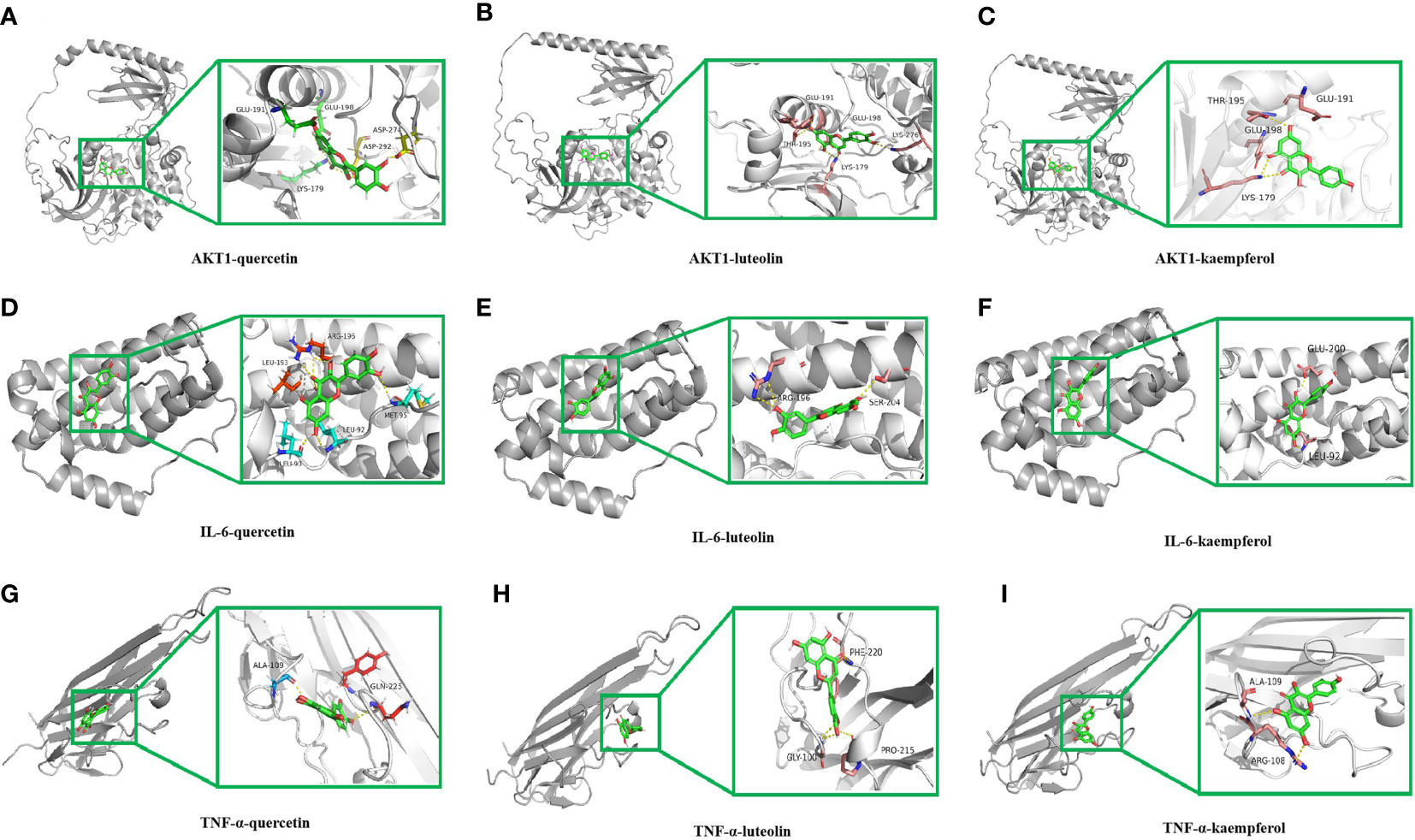
Figure 6 Molecular docking of the main ingredients in VO with AKT1, IL-6, and TNF-α. (A) 3D binding posture schematic diagram of AKT1 and quercetin. (B) 3D binding posture schematic diagram of AKT1 and luteolin. (C) 3D binding posture schematic diagram of AKT1 and kaempferol. (D) 3D binding posture schematic diagram of IL-6 and quercetin. (E) 3D binding posture schematic diagram of IL-6 and luteolin. (F) 3D binding posture schematic diagram of IL-6 and kaempferol. (G) 3D binding posture schematic diagram of TNF-α and quercetin. (H) 3D binding posture schematic diagram of TNF-α and luteolin. (I) 3D binding posture schematic diagram of TNF-α and kaempferol. (TNF-α was selected for docking with TNF.).
MDS was performed to further verify the binding between quercetin and AKT1, IL-6, and TNF-α. Root-mean-square deviation (RMSD) was used as a measure of the system’s stability. During the six dynamic simulation sets, it fluctuated slightly but remained stable at 0.20–0.25 nm/0.5–0.6 nm after simulation for 25 ns (Figure 7A). The radius of gyration (Rg) was used as a metric for determining the tightness of the architecture. It is an estimation of the distance between the center of mass of a protein atom and its terminal atoms. The fluctuation amplitude (degree of fluctuation) and RMSD of the six simulation groups were consistent, and the value of the AKT1 system was higher for both RMSD and Rg (Figure 7B). When a solvent molecule seeks the VDWAALS surface of a protein, it monitors a portion of the protein’s surface known as SASA. SASA steadily decreased from 0 to 100 ns, indicating that the protein was constricted/closed and that its hydrophilic surface area had decreased (Figure 7C). The root-mean-square function (RMSF) is used to observe the allotropy of the local site of the system during simulations (Figure 7D). Because hydrogen bonds contributed to the major interactions for complex formation and stability, they exerted a significant effect in determining the stability of the protein–ligand complex. Figures 7E, F illustrate the number of hydrogen bonds for small molecules and proteins and proteins and systems, respectively. AKT1 had four to six contacts with small molecules, IL-6 had two to five contacts, and TNF-α had one to three contacts. First, the binding free energies of the protein–ligand complex were calculated, followed by comparisons between bound and unbound solvated molecules. To achieve this, different conformations of the same molecule were compared in terms of their free energies (Figure 8). Solvation free energy can be determined by studying the interactions between polar and nonpolar residues of a protein. Using MDS, we found that the total free energy was negative after determining the change in free energy. This could have been because of interactions between the protein and small molecules. The affinity of the protein–ligand of the AKT1 system was −28.093 kcal/mol (Figure 8A), that of the IL-6 system was −26.52 kcal/mol (Figure 8C), and that of the TNF-α system was −17.77 kcal/mol (Figure 8E). The decomposition diagram of free energy residues can be used to understand which amino acid residues contribute to the binding during ligand simulation. In the AKT1 system, HIS194, GLY198, GLY294, and THR312 positively contributed to free energy, which was conducive to binding, whereas GLU191 was not conducive to binding (Figure 8B). Further, LEU90, PRO93, ARG196, and GLU200 positively contributed to free energy in the IL-6 system, which was conducive to binding (Figure 8D). Lastly, in the AKT1 system, ALA109, SER223, GLY224, and ALA226 positively contributed to free energy, which was conducive to binding (Figure 8F). Taken together, the results suggest that quercetin stably binds with AKT1, IL-6, and TNF-α, making it a promising agent for treating AS.
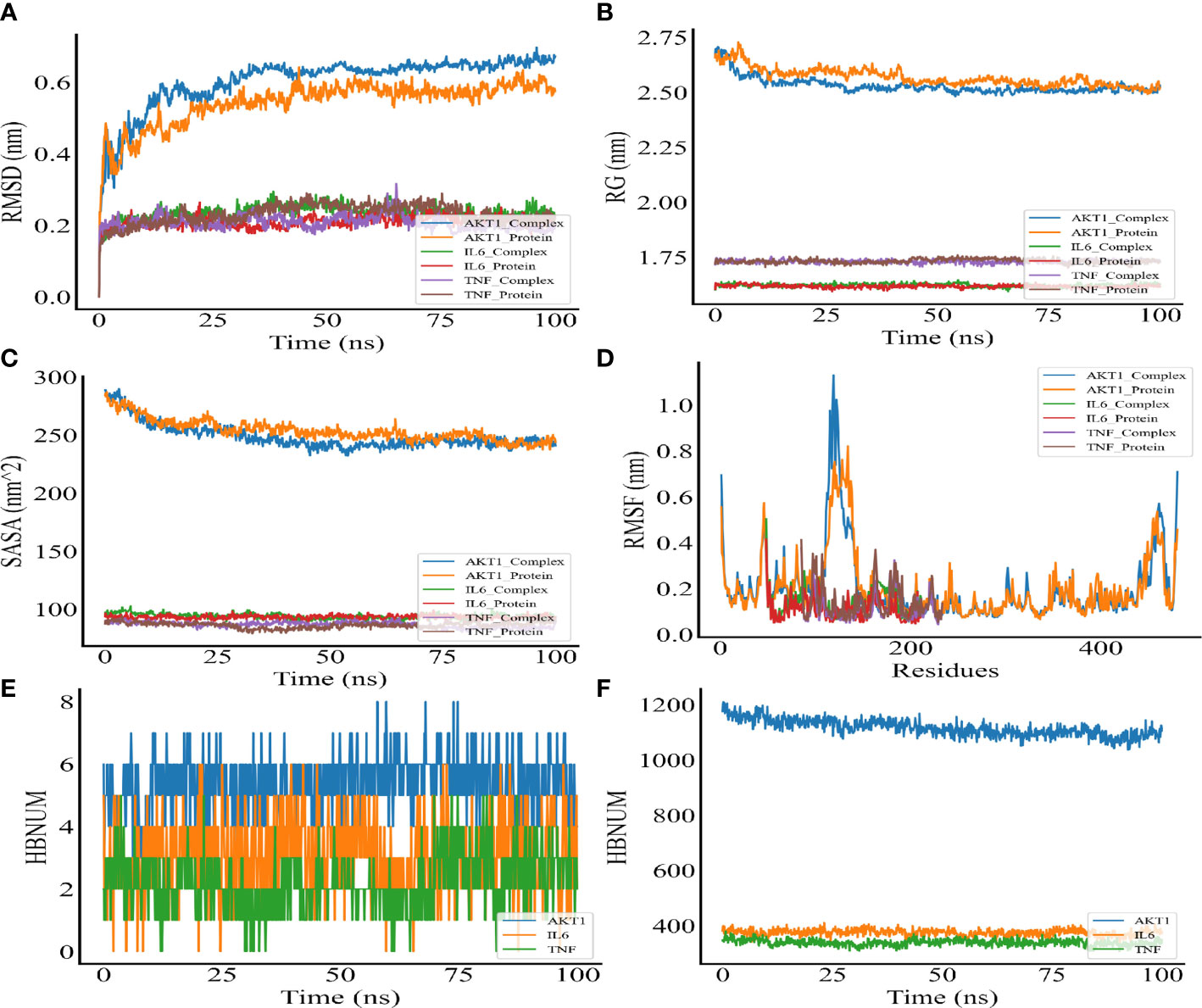
Figure 7 Molecular dynamic simulation. (A) RMSD of the complex MDS. (B) Rg variation diagram of the complex MDS. (C) SASA of the complex MDS. (D) RMSF of the complex MDS is displayed in the high fluctuation range of the surface. (E) HBNUM in the complex MDS. (F) Number of hydrogen bonds between proteins and water (HBNUM) (protein represents a blank protein, and complex represents core target proteins and core ligands).
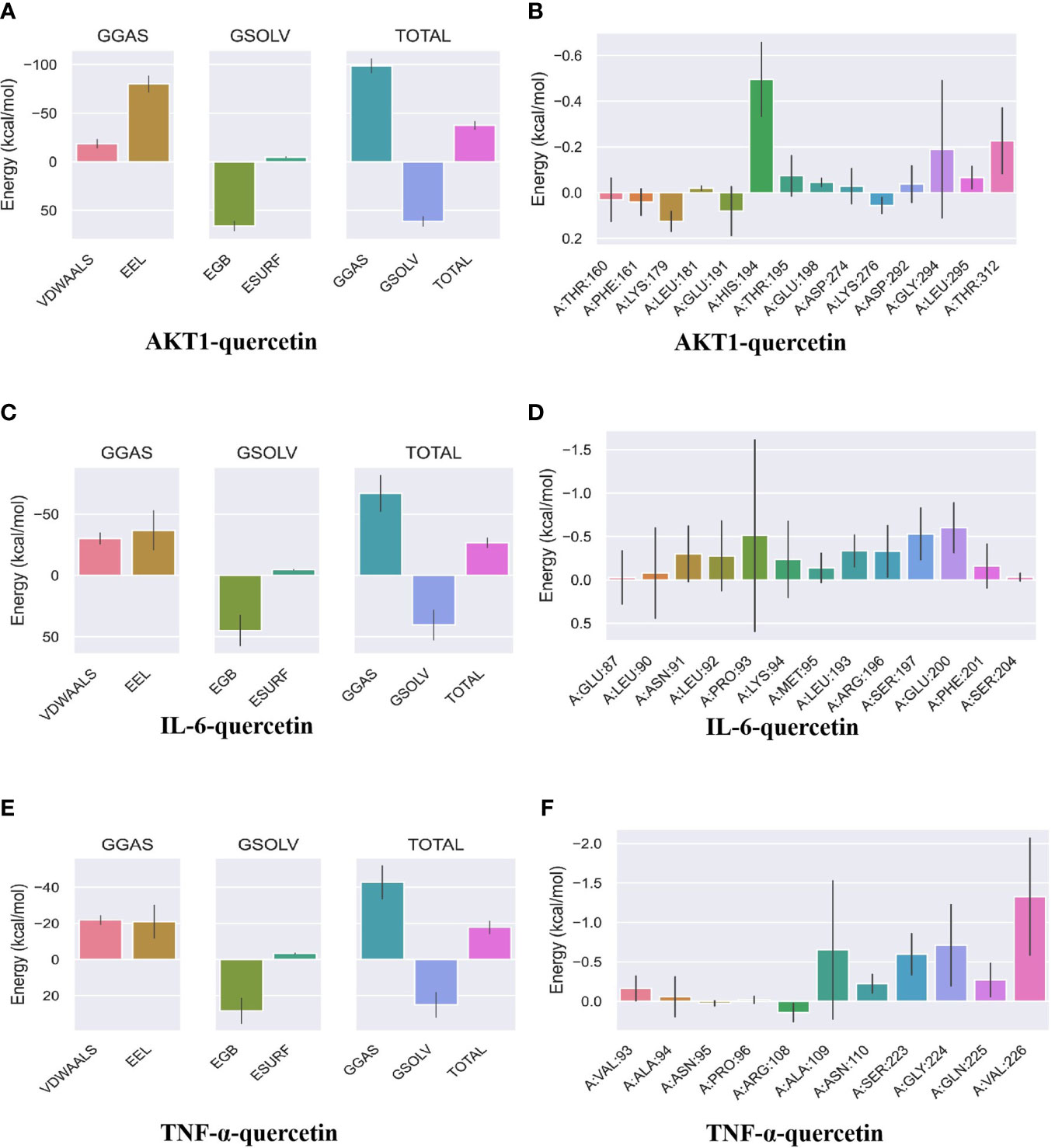
Figure 8 Binding free energy analysis. (A) Energy attribute breakdown diagram for AKT1–quercetin. (B) Energy amino acid decomposition diagram for AKT1–quercetin. (C) Energy attribute breakdown diagram for IL-6-quercetin. (D) Energy amino acid decomposition diagram for IL-6–quercetin. (E) Energy attribute breakdown diagram for TNF-α–quercetin. (F) Energy amino acid decomposition diagram for TNF-α–quercetin. A method based on MM-GBSA was used to calculate free energy and decompose residues. VDWAALS, van der Waals energy; EEL, polar solvation energy; EGB, polar solvation energy; ESURF, nonpolar solvation energy; GGAS, total gas phase free energy; GSOLV, Total solvation free energy; TOTAL, GSOLV + GGAS.
Traditional Chinese medicines can be used to treat diseases involving multiple targets and components. They exhibit the ability to inhibit endothelial dysfunction, platelet activation, lipid peroxidation, ROS production, and macrophage-induced AS (Kirichenko et al., 2020). However, the study of traditional Chinese medicines is extremely challenging owing to their complex ingredients (Han et al., 2020). A computer-aided drug design approach can be used to gain an in-depth and systematic understanding of the effectiveness and functions of the ingredients in traditional Chinese medicines (Man and Sai, 2022). Previous studies have reported the various pharmacological effects of VO, including regulating lipid metabolism, scavenging free radicals, inhibiting inflammation, and preventing tumors (Li et al., 2022). Therefore, in the present study, we comprehensively and systematically predicted the herb–ingredient–target and gene–pathway interactions for VO against AS using a computer-aided drug design approach involving network pharmacology, molecular docking, and MDS.
Twelve active ingredients were screened according to pharmacokinetic parameters, including flavonoids, steroids and steroid derivatives, prenol lipids, fatty acyls, and macrolides and analogs; this is in agreement with the findings of previous studies (El-Hela et al., 2010; Gibitz-Eisath et al., 2018). Although VO has 12 active ingredients, their action targets remain unknown, indicating that their efficacy has not yet been investigated. In our study, we identified 11 active ingredients in VO with 147 potential targets. Among the main ingredients screened, flavonoids, including quercetin, luteolin, and kaempferol, and steroids and steroid derivatives, including beta-sitosterol and stigmasterol, had high degrees of nodes, and quercetin had the most anti-atherosclerotic targets, with 120 core targets. Evidence suggests that quercetin suppresses the PI3K/AKT pathway via the lipid and atherosclerotic pathways, inhibiting downstream NF-KB and reducing inflammatory factors (Xu et al., 2018). Further, quercetin inhibits endothelial dysfunction in AS by reducing HOCl production by MPO/NADPH oxidase (Li et al., 2023). Several studies have demonstrated that luteolin prevents AS by regulating oxidative stress, reducing triglyceride and low-density lipoprotein (LDL) cholesterol levels, and inhibiting plaque development (Makino et al., 2016; Li et al., 2018; Ding et al., 2019). In atherosclerotic ApoE−/− mice, kaempferol targeted the plaque areas and inhibited macrophage-mediated inflammation, accompanied by a decrease in TNF-α proinflammatory cytokines and repolarization of M1 to M2 macrophages (Jianing et al., 2022). A study reported the antioxidant, anti-inflammatory, and cardioprotective effects of kaempferol and suggested its potential as a drug candidate for treating and preventing AS (Chen et al., 2022b). Beta-sitosterol can effectively reduce the production of the intestinal microbial metabolite trimethylamine to ameliorate atherosclerotic plaques in AS mice (Wu et al., 2022). Stigmasterol activates the nuclear receptor LXR to promote cholesterol secretion via the intestinal tract and inhibits the expression of proinflammatory mediators such as IL-6, IL-1β, COX-2, and TNF-α to ameliorate AS (Lifsey et al., 2019). Taken together, these findings suggest the anti-atherosclerotic effect of VO, with quercetin, luteolin, and kaempferol as the main ingredients.
In the PPI network, AKT1, IL-6, and TNF-α were chosen as potential targets owing to their high degree of nodality. The degree of a node is determined by the number of nodes directly linked to it. In general, nodes with a higher degree are considered to be more important than those with a lower degree (Zeng et al., 2021). AKT1 is an AKT kinase that regulates cell proliferation and growth and has an antiapoptotic function; its mechanism involves the PI3K/AKT signaling pathway (Meng et al., 2021). The PI3K/AKT pathway is implicated in AS pathogenesis because it participates in lipid metabolism, smooth muscle and fibrocyte proliferation, and collagen production in the arterial wall (Chen et al., 2009). Study has shown that the administration of Astragalus mongholicus extract helped improve the lipid profile in high-fat diet-induced mice by lowering adipogenesis, increasing lipolysis and lipid β-oxidation, downregulating AKT1 and CCND1 in the liver, and upregulating VEGFA and ESR1 in the adipose tissue and liver; these findings suggest that AKT1 is an important target for treating AS (Wang et al., 2022). NF-KB, as a vital effector downstream of the PI3K/AKT signaling pathway, promotes the phosphorylation of NF-KB inhibitory protein A (inhibitory subunit alpha of NF-KB); as a result, it dissociates from NF-KB and induces several inflammatory cytokines, including IL-6 and TNF-α, resulting in an inflammatory response (Song et al., 2018). IL-6 and TNF-α induce lipolysis and inhibit lipogenesis, whereas chemokines mediate the recruitment of macrophages and monocytes from the adipose tissue, thereby increasing the production of inflammatory factors to impair fat homeostasis (Chen et al., 2009; Hashizume and Mihara, 2011). A study has shown that TNF-α can participate in lipid metabolism by mediating the role of LXRα in regulating cholesterol efflux (Ikhlef et al., 2016). Overall, these targets are involved in AS progression, consistent with the findings of existing studies (Meng et al., 2021), and can be used as biomarkers for AS as well as targets for drug action. TNF-α is responsible for AS owing to its role as an inflammatory mediator secreted by macrophages (Businaro et al., 2012). A study found a correlation between atherogenic indexes and both IL-1 and IL-6 as a result of spondyloarthritis, and that serum IL-6 and IL-1 levels contribute to the development of AS (Ben Ali et al., 2021). The above studies suggest that VO may treat AS through these targets.
In our study, GO pathway analysis predicted that BP mainly focused on responses to xenobiotic stimuli, cellular responses to lipids, and responses to hormones. A study has shown that the response to xenobiotic stimuli is involved in AS development. The presence of chronic infectious stimuli may directly activate metabolic pathways (e.g., cholesterol esterification) in susceptible individuals, leading to the formation of foam cells (Conti et al., 2010). AS depends on the cellular response to lipids. In the arterial wall, macrophages play an important role in lipid accumulation and generating foam cells filled with atherosclerotic plaques (Sukhorukov et al., 2020). For the response to hormones, previous studies have reported that glucocorticoids can affect vascular responses by regulating vasoconstriction or vasodilation and are essential for regulating blood pressure and blood flow (Macleod et al., 2021). In terms of hormones, estrogen has positive effects on blood lipids, including increasing high-density lipoprotein levels, reducing LDL and LDL oxidation, and controlling triglyceride levels, thereby improving AS (Dobrescu et al., 2020). KEGG pathway enrichment analysis revealed that the intersection targets were mainly related to pathways in cancer, fluid shear stress and atherosclerosis, and lipid and atherosclerosis. A research study reported that AS, similar to cancer, develops via clonal proliferation of altered cells during local tissue damage, inflammation, and genomic instability (Ross et al., 2002). Lipid metabolism is important for the effects of AS. Zhou reported that Danhong injection (DHI) reduced the AS index and plaque areas in high-fat diet-induced atherosclerotic mice and that stimulation of the PI3K/AKT signaling pathway led to DHI inhibition of lipid accumulation by macrophages (Zhou et al., 2019a). Li explored the use of the lipid pathway to treat AS and found that the Qing-Xue-Xiao-Zhi formula inhibited lipid accumulation and inflammation in macrophages, regulated and promoted lipid efflux via the TLR4/MyD88/NF-κB pathway, inhibited macrophage-mediated inflammation, and exhibited therapeutic effects on AS (Li et al., 2021). These findings suggest the significant biological role of VO in AS development and that it exerts multiple protective effects on AS.
We found that three key ingredients in VO (quercetin, luteolin, and kaempferol) were linked to three AS target genes (AKT1, IL-6, and TNF-α). We used molecular docking to dock the three active ingredients with the three related potential target proteins (Malik et al., 2023). The negative value of the binding energy of the complex indicates that the energy released by the binding is easier to bind and that the complex is more stable (Alshammari, 2023). Molecular docking revealed that quercetin, luteolin, and kaempferol strongly bind with AKT1, IL-6, and TNF-α, respectively. Compared with other binding energy values, the lowest binding energy of quercetin indicated the highest binding energy for these targets. Based on these findings, the key ingredients in VO that bind to the targets related to lipids and AS may be AKT1, IL-6, and TNF-α.MDS can be used to place ligands and receptors in a system that mimics their natural binding. MDS can illustrate the microscopic evolution of a system at an atomic level and intuitively demonstrate the mechanism and law of experimental phenomena, which are critical for understanding the mechanism of ligand–receptor binding. In our study, MDS revealed that the binding between quercetin and AKT1, IL-6, and TNF-α was stable; however, the binding affinity of quercetin to AKT1 was higher. RMSD can be utilized to evaluate system stability, and Rg is an important index used to evaluate the tightness of the architecture. SASA refers to the solvent-accessible surface area of the protein. RMSF allows observation of the allotropy of the local site as the simulation proceeds (Miraz et al., 2023). The stability of protein ligands is greatly influenced by hydrogen bonding. In our study, the RMSD and Rg of the AKT1 system were higher. AKT1 had four to six contacts with small molecules, which was higher than that of IL-6 and TNF-α, further indicating that quercetin is strongly bound to AKT1. Analysis of free energy revealed that AKT1, IL-6, and TNF-α have the same positive and negative sign distribution. However, the affinity of AKT1 was −28.093 kcal/mol, which was stronger than that of IL-6 and TNF-α. This finding once again demonstrates that quercetin was strongly bound to AKT1. VDWAALS energy is used to calculate the total gas phase free energy, which was negative in our study (Mao et al., 2023). VDWAALS<0 indicates that the hydrophobic action is conducive to binding, and EEL indicates that the polar solvation energy is negative; these results indicate that hydrogen bonding and other actions are conducive to binding. Total solvation-free energy (GSOLV) represents a positive value of total solvation-free energy, indicating that it is not conducive to binding. GSOLV represents the interaction between nonpolar solvation energy and polar solvation energy (EGB). EGB is a positive value, indicating that it does not combine well and that the nonpolar effect is beneficial to binding. MDS revealed that the binding between AKT1, IL-6, and TNF-α and quercetin is stable, further suggesting that quercetin plays an anti-atherosclerotic role via multiple targets.
In the present study, the main VO ingredients, potential pharmacological targets, and signal pathways were identified for treating AS using a computer-aided drug design approach. Our study results indicate that VO ameliorates multiple pathological features of AS via a direct synergistic effect on multiple targets and pathways. Further experiments are warranted to verify the biological mechanism by which VO regulates AS signaling pathways because there is still no evidence that VO antagonizes AS in animals or clinical experiments.
Using network pharmacology, molecular docking, and MDS, this study comprehensively and systematically revealed that the characteristics of the key ingredients in VO (including quercetin, luteolin, and kaempferol), potential targets (AKT1, IL-6, and TNF-α), various biological processes (response to xenobiotic stimulus, cellular response to lipid, and response to hormone), and multiple pathways (cancer, fluid shear stress and atherosclerosis, and lipid and atherosclerosis) prevent the occurrence and development of AS, providing an important theoretical basis for clinical treatment of AS and related research.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Data were processed by TYC, YYG, XJY, and XY. The manuscript was drafted by TYC and YYG. Data acquisition, analysis, and interpretation were conducted by WY. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the Hubei Province Research Innovation Team Project (T2021022) and the Xianning Natural Science Foundation (2021ZRKX026).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1154266/full#supplementary-material
Supplementary Figure 1 | Molecular docking of VO main ingredients with AKT1, TNF-α and IL-6 in 2D binding posture. (A): Two-dimensional (2D) binding posture schematic diagram of AKT1 and quercetin. (B): 3D binding posture schematic diagram of AKT1 and luteolin. (C): 2D binding posture schematic diagram of AKT1 and kaempferol. (D): 2D binding posture schematic diagram of IL-6 and quercetin. (E): 2D binding posture schematic diagram of IL-6 and luteolin. (F): 2D binding posture schematic diagram of IL-6 and kaempferol. (G): 2D binding posture schematic diagram of TNF-α and quercetin. (H): 2D binding posture schematic diagram of TNF-α and luteolin. (I): 2D binding posture schematic diagram of TNF-α and kaempferol. (TNF-α was selected for docking with TNF.)
Alshammari, A. (2023). Identification of novel inhibitors against hantaviruses through 2D fingerprinting and molecular modeling approaches. Front. Immunol 14, 1113321. doi: 10.3389/fimmu.2023.1113321
Amberger, J. S., Bocchini, C. A., Schiettecatte, F., Scott, A. F., Hamosh, A. (2015). OMIM.org: Online mendelian inheritance in man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res 43 (D1), D789–D798. doi: 10.1093/nar/gku1205
An, X., Fan, D., Yin, Z., Zhang, J., Zhou, Y., Tian, R., et al. (2022). Prediction of the potential mechanism of triptolide in improving diabetic nephropathy by utilizing a network pharmacology and molecular docking approach. Front. Bioscience-Landmark 27 (3), 94. doi: 10.31083/j.fbl2703094
Austin, D. F. (2004). Medicinal plants of the world. an illustrated scientific guide to important medicinal plants and their uses. Economic Botany 58 (3), 505. doi: 10.1663/0013-0001(2004)058[0505:dfabre]2.0.co;2
Ben Ali, K., Slouma, M., Kharrat, L., Tezeghdenti, A., Dhahri, R., Ghazouani, E., et al. (2021). Ab0071 relationship between pro-inflammatory cytokine and atherogenic indexes in axial radiographic spondyloarthritis. Ann. Rheumatic Diseases 80, 1066. doi: 10.1136/annrheumdis-2021-eular.1281
Businaro, R., Tagliani, A., Buttari, B., Profumo, E., Ippoliti, F., Di Cristofano, C., et al. (2012). Cellular and molecular players in the atherosclerotic plaque progression. Ann. New York Acad. Sci 1262, 134–114. doi: 10.1111/j.1749-6632.2012.06600.x
Chai, Y., Chen, F., Li, Z., Yang, P., Zhou, Q., Liu, W., et al. (2022). Mechanism of salidroside in the treatment of chronic myeloid leukemia based on the network pharmacology and molecular docking. Clin. Trans. Oncol 25 (2), 384–395. doi: 10.1007/s12094-022-02990-w
Chen, C.-H., Hsia, C.-C., Hu, P.-A., Yeh, C.-H., Chen, C.-T., Peng, C.-L., et al. (2022a). Bromelain ameliorates atherosclerosis by activating the TFEB-mediated autophagy and antioxidant pathways. Antioxidants 12 (1), 72. doi: 10.3390/antiox12010072
Chen, M., Xiao, J., El-Seedi, H. R., Woźniak, K. S., Daglia, M., Little, P. J., et al. (2022b). Kaempferol and atherosclerosis: From mechanism to medicine. Crit. Rev. Food Sci. Nutr, 1–19. doi: 10.1080/10408398.2022.2121261
Chen, X., Xun, K., Chen, L., Wang, Y. (2009). TNF-α, a potent lipid metabolism regulator. Cell Biochem. And Funct 27, 407–416. doi: 10.1002/cbf.1596
Chen, L., Zhang, Y.-H., Wang, S., Zhang, Y., Huang, T., Cai, Y.-D. (2017). Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS One 12 (9), e0184129. doi: 10.1371/journal.pone.0184129
Conti, M., Sanna, F., Farci, G. A., Uda, S., Porcu, G., Collu, M., et al. (2010). Abnormal macrophage response to microbial stimulus in a 43-year-old man with a severe form of atherosclerosis: a case report. J. Med. Case Rep 4, 183. doi: 10.1186/1752-1947-4-183
Dahlöf, B. (2010). Cardiovascular disease risk factors: epidemiology and risk assessment. Am. J. Cardiol 105 (1 Suppl), 3A–9A. doi: 10.1016/j.amjcard.2009.10.007
Ding, X., Zheng, L., Yang, B., Wang, X., Ying, Y. (2019). Luteolin attenuates atherosclerosis Via modulating signal transducer and activator of transcription 3-mediated inflammatory response. Drug Design Dev. Ther 13, 3899–3911. doi: 10.2147/DDDT.S207185
Djoumbou Feunang, Y., Eisner, R., Knox, C., Chepelev, L., Hastings, J., Owen, G., et al. (2016). ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J. Cheminformatics 8, 61. doi: 10.1186/s13321-016-0174-y
Dobrescu, M., Păun, D., Grigorie, D., Poiană, C. (2020). Hormonal mechanisms in atherosclerosis. Internal Med 17 (3), 19–35. doi: 10.2478/inmed-2020-0114
El-Hela, A. A., Al-Amier, H. A., Ibrahim, T. A. (2010). Comparative study of the flavonoids of some verbena species cultivated in Egypt by using high-performance liquid chromatography coupled with ultraviolet spectroscopy and atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A. 1217 (41), 6388–6393. doi: 10.1016/j.chroma.2010.08.025
Fan, J., Watanabe, T. (2022). Atherosclerosis: Known and unknown. Pathol. Int 72 (3), 151–160. doi: 10.1111/pin.13202
Gibitz-Eisath, N., Eichberger, M., Gruber, R., Sturm, S., Stuppner, H. (2018). Development and validation of a rapid ultra-high performance liquid chromatography diode array detector method for verbena officinalis l. J. Pharm. Biomed. Analysis 160, 160–167. doi: 10.1016/j.jpba.2018.07.047
Grootaert, M. O. J., Moulis, M., Roth, L., Martinet, W., Vindis, C., Bennett, M. R., et al. (2018). Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res 114 (4), 622–634. doi: 10.1093/cvr/cvy007
Han, Y., Sun, H., Zhang, A., Yan, G., Wang, X.-J. (2020). Chinmedomics, a new strategy for evaluating the therapeutic efficacy of herbal medicines. Pharmacol. Ther. 216, 107680. doi: 10.1016/j.pharmthera.2020.107680
Hashizume, M., Mihara, M. (2011). IL-6 and lipid metabolism. Inflammation Regeneration 31 (3), 325–333. doi: 10.2492/inflammregen.31.325
Hilton-Jones, D. (2018). Statin-related myopathies. Pract. Neurology 18 (2), 97–105. doi: 10.1136/practneurol-2017-001738
Huang, D. W., Sherman, B. T., Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4 (1), 44–57. doi: 10.1038/nprot.2008.211
Ikhlef, S., Berrougui, H., Kamtchueng Simo, O., Khalil, A. (2016). Paraoxonase 1-treated oxLDL promotes cholesterol efflux from macrophages by stimulating the PPARγ-LXRα-ABCA1 pathway. FEBS Lett. 590 (11), 1614–1629. doi: 10.1002/1873-3468.12198
Jianing, Z., Longbing, L., Wei, Z., Tianhao, Y., Tao, Y., Mengmeng, S., et al. (2022). M1/M2 re-polarization of kaempferol biomimetic NPs in anti-inflammatory therapy of atherosclerosis. J. Controlled Release 353, 1068–1083. doi: 10.1016/j.jconrel.2022.12.041
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596 (7873), 583–589. doi: 10.1038/s41586-021-03819-2
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., et al. (2020). PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49 (D1), D1388–D1395. doi: 10.1093/nar/gkaa971
Kirichenko, T. V., Sukhorukov, V. N., Markin, A. M., Nikiforov, N. G., Liu, P.-Y., Sobenin, I. A., et al. (2020). Medicinal plants as a potential and successful treatment option in the context of atherosclerosis. Front. Pharmacol 11, 403. doi: 10.3389/fphar.2020.00403
Konstantinov, I. E., Mejevoi, N., Anichkov, N. M. (2007). Nikolai N. anichkov and his theory of atherosclerosis. Texas Heart Institute J. 33 (4), 417–423.
Landowska, M., Żebrowska, A., Fajer, K., Adamek, P., Kruk, A., Kałuża, B., et al. (2022). Atherosclerosis risk factors in patients with reactive hypoglycemia. Diabetes Metab. Syndrome Obesity: Targets Ther. 15, 3133–3142. doi: 10.2147/dmso.s371706
Li, J., Dong, J.-Z., Ren, Y.-L., Zhu, J.-J., Cao, J.-N., Zhang, J., et al. (2018). Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages. Exp. Ther. Med. 16 (3), 2593–2599. doi: 10.3892/etm.2018.6499
Li, J.-X., Tian, R., Lu, N. (2023). Quercetin attenuates vascular endothelial dysfunction in atherosclerotic mice by inhibiting myeloperoxidase and NADPH oxidase function. Chem. Res. Toxicology 36 (3), 260–269. doi: 10.1021/acs.chemrestox.2c00334
Li, Y., Zhang, L., Ren, P., Yang, Y., Li, S., Qin, X., et al. (2021). Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine 93, 153812. doi: 10.1016/j.phymed.2021.153812
Li, R., Zhang, Z., Su, X., Yu, J., Lu, L., Liu, T. (2022). Nontargeted metabolomics study and pharmacodynamic evaluation of bidirectional fermentation for ganoderma lucidum with marsdenia tenacissima. Front. Pharmacol 13, 1012063. doi: 10.3389/fphar.2022.1012063
Libby, P., Ridker, P. M., Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature 473 (7347), 317–325. doi: 10.1038/nature10146
Lifsey, H. C., Kaur, R., Thompson, B. H., Bennett, L., Temel, R. E., Graf, G. A. (2019). Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J. Nutr. Biochem. 76, 108263. doi: 10.1016/j.jnutbio.2019.108263
Macleod, C., Hadoke, P. W. F., Nixon, M. (2021). Glucocorticoids: Fuelling the fire of atherosclerosis or therapeutic extinguishers? Int. J. Mol. Sci 22 (14), 7622. doi: 10.3390/ijms22147622
Makino, J., Asai, R., Hashimoto, M., Kamiya, T., Hara, H., Ninomiya, M., et al. (2016). Suppression of EC-SOD by oxLDL during vascular smooth muscle cell proliferation. J. Cell. Biochem. 117 (11), 2496–2505. doi: 10.1002/jcb.25542
Malik, M., Khan, S., Ullah, A., Hassan, M., Haq, M. U., Ahmad, S., et al. (2023). Proteome-wide screening of potential vaccine targets against brucella melitensis. Vaccines 11 (2), 263. doi: 10.3390/vaccines11020263
Man, X., Sai, Z. (2022). Study on the prognosis effect of traditional Chinese medicine treatment in DR patients based on the perspective of network pharmacology. Contrast Media Mol. Imaging. doi: 10.1155/2022/3528732
Mao, R., Zhang, H., Bie, L., Liu, L.-N., Gao, J. (2023). Million-atom molecular dynamics simulations reveal the interfacial interactions and assembly of plant PSII-LHCII supercomplex. RSC Advances 13 (10), 6699–6712. doi: 10.1039/D2RA08240C
Masters, L., Eagon, S., Heying, M. (2020). Evaluation of consensus scoring methods for AutoDock vina, smina and idock. J. Mol. Graphics Modelling 96, 107532. doi: 10.1016/j.jmgm.2020.107532
Meng, Q., Pu, L., Lu, Q., Wang, B., Li, S., Liu, B., et al. (2021). Morin hydrate inhibits atherosclerosis and LPS-induced endothelial cells inflammatory responses by modulating the NFκB signaling-mediated autophagy. Int. Immunopharmacology 100, 108096. doi: 10.1016/j.intimp.2021.108096
Mi, H., Muruganujan, A., Huang, X., Ebert, D., Mills, C., Guo, X., et al. (2019). Protocol update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 14 (3), 703–721. doi: 10.1038/s41596-019-0128-8
Miraz, M. M. H., Ullah, M. A., Nayem, A. A., Chakrobortty, B., Deb, S., Laskar, A., et al. (2023). Nigelladine a among selected compounds from nigella sativa exhibits propitious interaction with omicron variant of SARS-CoV-2: An in silico study. Int. J. Clin. Practice 2023, 9917306. doi: 10.1155/2023/9917306
Ross, J. S., Stagliano, N. E., Donovan, M. J., Breitbart, R. E., Ginsburg, G. S. (2002). Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann. New York Acad. Sci. 947, 271–292.
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 6, 13. doi: 10.1186/1758-2946-6-13
Safran, M., Dalah, I., Alexander, J., Rosen, N., Iny Stein, T., Shmoish, M., et al. (2010). GeneCards version 3: the human gene integrator. Database: J. Biol. Database Curation 2010, baq020. doi: 10.1093/database/baq020
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 (11), 2498–2504. doi: 10.1101/gr.1239303
Song, D., Fang, G., Mao, S.-Z., Ye, X., Liu, G., Miller, E. J., et al. (2018). Selective inhibition of endothelial NF-κB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice. Atherosclerosis 270, 68–75. doi: 10.1016/j.atherosclerosis.2018.01.027
Steinberg, D. (2004). Thematic review series: the pathogenesis of atherosclerosis. an interpretive history of the cholesterol controversy: part I. J. Lipid Res. 45 (9), 1583–1593. doi: 10.1194/jlr.r400003-jlr200
Sukhorukov, V. N., Khotina, V. A., Chegodaev, Y. S., Ivanova, E., Sobenin, I. A., Orekhov, A. N. (2020). Lipid metabolism in macrophages: Focus on atherosclerosis. Biomedicines 8 (8), 262. doi: 10.3390/biomedicines8080262
Szklarczyk, D., Morris, J. H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., et al. (2016). The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45 (D1), D362–D368. doi: 10.1093/nar/gkw937
Tao, W., Xu, X., Wang, X., Li, B., Wang, Y., Li, Y., et al. (2012). Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal radix curcumae formula for application to cardiovascular disease. J. Ethnopharmacology 145 (1), 1–10. doi:10.1016/j.jep.2012.09.051
Tu, Y.-C., Lian, T.-W., Yen, J.-H., Chen, Z.-T., Wu, M.-J. (2007). Antiatherogenic effects of kaempferol and rhamnocitrin. J. Agric. Food Chem. 55 (24), 9969–9976. doi: 10.1021/jf0717788
Ukai, S., Kato, S., Furukawa, T., Ohtsuka, S. (2020). High-temperature creep deformation in FeCrAl-oxide dispersion strengthened alloy cladding. Materials Sci. Engineering: A. 794, 139863. doi: 10.1016/j.msea.2020.139863
UniProt Consortium, T (2018). UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46 (5), 2699. doi: 10.1093/nar/gkw1099
Valdés-Tresanco, M. S., Valdés-Tresanco, M. E., Valiente, P. A., Moreno, E. (2021). gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Computation 17 (10), 6281–6291. doi: 10.1021/acs.jctc.1c00645
Van Der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E., Berendsen, H. J. C. (2005). GROMACS: Fast, flexible, and free. J. Comput. Chem. 26 (16), 1701–1718. doi: 10.1002/jcc.20291
Wang, L., Zheng, W., Yang, J., Ali, A., Qin, H. (2022). Mechanism of astragalus membranaceus alleviating acquired hyperlipidemia induced by high-fat diet through regulating lipid metabolism. Nutrients 14 (5), 954. doi: 10.3390/nu14050954
Weiser, J., Shenkin, P. S., Still, W. C. (1999). Approximate solvent-accessible surface areas from tetrahedrally directed neighbor densities. Biopolymers 50 (4), 373–380. doi: 10.1002/(SICI)1097-0282(19991005)50:4<373::AID-BIP3>3.0.CO;2-U
Wu, W., Liu, W., Wang, H., Wang, W., Chu, W., Jin, J. (2022). β-sitosterol inhibits trimethylamine production by regulating the gut microbiota and attenuates atherosclerosis in ApoE–/– mice. Front. Cardiovasc. Med. 9, 986905. doi: 10.3389/fcvm.2022.986905
Xia, Z., Gao, P., Chen, Y., Shu, L., Ye, L., Cheng, H., et al. (2022). Analysis of the key prognostic genes and potential traditional Chinese medicine therapeutic targets in glioblastoma based on bioinformatics and network pharmacology methods. Trans. Cancer Res. 11 (5), 1386–1405. doi: 10.21037/tcr-22-1122
Xu, W., Yang, Z., Xie, C., Zhu, Y., Shu, X., Zhang, Z., et al. (2018). PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J. Exp. Clin. Cancer Res. 37 (1), 198. doi: 10.1186/s13046-018-0795-2
Xu, X., Zhang, W., Huang, C., Li, Y., Yu, H., Wang, Y., et al. (2012). A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 13 (6), 6964–6982. doi: 10.3390/ijms13066964
Xue, F., Nie, X., Shi, J., Liu, Q., Wang, Z., Li, X., et al. (2017). Quercetin inhibits LPS-induced inflammation and ox-LDL-Induced lipid deposition. Front. Pharmacol 8, 40. doi: 10.3389/fphar.2017.00040
Yao, B.-C., Meng, L.-B., Hao, M.-L., Zhang, Y.-M., Gong, T., Guo, Z.-G. (2019). Chronic stress: a critical risk factor for atherosclerosis. J. Int. Med. Res. 47 (4), 1429–1440. doi: 10.1177/0300060519826820
Zeng, P., Su, H.-F., Ye, C.-Y., Qiu, S.-W., Tian, Q. (2021). Therapeutic mechanism and key alkaloids of uncaria rhynchophylla in alzheimer’s disease from the perspective of pathophysiological processes. Front. Pharmacol. 12, 806984. doi: 10.3389/fphar.2021.806984
Zhang, S., Hong, F., Ma, C., Yang, S. (2021). Hepatic lipid metabolism disorder and atherosclerosis. Endocrine Metab. Immune Disord Drug Targets 22 (6), 590–600. doi: 10.2174/1871530322666211220110810
Zhao, X., Liu, J., Yang, L., Niu, Y., Ren, R., Su, C., et al. (2021). Beneficial effects of mijianchangpu decoction on ischemic stroke through components accessing to the brain based on network pharmacology. J. Ethnopharmacology 285, 114882. doi: 10.1016/j.jep.2021.114882
Zhou, M., Ren, P., Li, S., Kang, Q., Zhang, Y., Liu, W., et al. (2019a). Danhong injection attenuates high-Fat-Induced atherosclerosis and macrophage lipid accumulation by regulating the PI3K/AKT insulin pathway. J. Cardiovasc. Pharmacol. 74 (2), 152–161. doi: 10.1097/FJC.0000000000000691
Keywords: Verbena officinalis (VO), atherosclerosis (AS), network pharmacology approach, molecular docking, molecular dynamics simulation
Citation: Chen Y, Gan Y, Yu J, Ye X and Yu W (2023) Key ingredients in Verbena officinalis and determination of their anti-atherosclerotic effect using a computer-aided drug design approach. Front. Plant Sci. 14:1154266. doi: 10.3389/fpls.2023.1154266
Received: 30 January 2023; Accepted: 16 March 2023;
Published: 03 April 2023.
Edited by:
Yu Xue, Nanjing University of Information Science and Technology, ChinaReviewed by:
Kun-Yi Hsin, National Chung Hsing University, TaiwanCopyright © 2023 Chen, Gan, Yu, Ye and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Yu, eXV3ZWkwODA1QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.