
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Plant Sci., 31 January 2023
Sec. Plant Pathogen Interactions
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1148261
This article is part of the Research TopicSustainable Strategies for the Management of Plant Parasitic NematodesView all 5 articles
Editorial on the Research Topic
Sustainable strategies for the management of plant parasitic nematodes
Plant-parasitic nematodes (PPN), “the unseen enemies” of plants, are a threat to economically important crops, affecting production, quality, and yields, with losses estimated at 173 billion US dollars/year (Elling, 2013). The top 10 PPN, based on their scientific and economic importance, include root-knot (RKN, Meloidogyne spp.), cyst (Heterodera/Globodera spp.), and root-lesion nematodes (RLN, Pratylenchus spp.), followed by other species.
For the last 50 years, PPN control has relied on the use of synthetic nematicides and soil fumigants that are rapid-acting, and specifically reliable. Nevertheless, due to environmental concerns and human health issues, most traditional nematicides have been exposed to an increasing regulatory pressure, with many products banned or withdrawn from the market. Nowadays, the adoption of control strategies based on the use of safer and selective bionematicides, biocontrol agents, cultural methods, and plant resistance is highly advisable, in order to achieve sustainable PPN control. However, some of these strategies, and their integration, are not always available or appear less consistent, compared to synthetic nematicides. In particular, microbial antagonists are selected to improve efficacy of bioformulations vs PPN, through direct application or active metabolites. However, the rhizosphere is a complex system including thousands of microbial species, whose effect is known only in part. A schematic representation of such tri-trophic (plant-nematode-antagonists) food web is given in Figure 1.
The main goal of this topic was indeed to bring together recent advances in sustainable management strategies for important PPN. The contributions include potential bionematicides, evaluation of their efficacy and mode of action (Maleita et al.); exploitation of biocontrol bacteria (Singh and Wesemael; Diaz-Manzano et al.); and a review on Nacobbus spp. management (Lax et al).
The effects of two natural compounds (juglone and 1,4-naphthoquinone), which can be extracted from walnut husk residues, were evaluated on the Meloidogyne luci life cycle (Maleita et al.). This RKN has been detected in several countries in Europe, associated to economically important crops. Although there are no data available on M. luci impacts, host suitability assays suggested that significant losses can be induced by this species (Maleita et al., 2018; Aydinli et al., 2019; Sen and Aydinli, 2021; Maleita et al., 2022). For this reason, M. luci has been included since 2017 in the Alert List of Pests of the European and Mediterranean Plant Protection Organization (EPPO, 2017; EPPO, 2021). Results showed that both compounds increased the in vitro mortality of the second-stage juveniles (J2) and reduced hatching by ≈50%. In pot assays with tomato plants, J2 infection was also reduced by ≈80%. Both compounds are thus promising in the development of novel, natural and effective bionematicides, contributing to limiting crop damage caused by RKN. Finally, the mode of action of both natural compounds was also tentatively inferred through the assessment of reactive oxygen species (ROS) generation, in vitro inhibitory activity of acetylcholinesterase, and gene expression analysis. Since only 1,4-naphthoquinone induced the formation of ROS, the authors conclude that the action modes of the compounds are probably different. Studies on the M. luci transcriptome will be needed to provide further insights on the nematode metabolism and the nematicidal mechanisms reported.
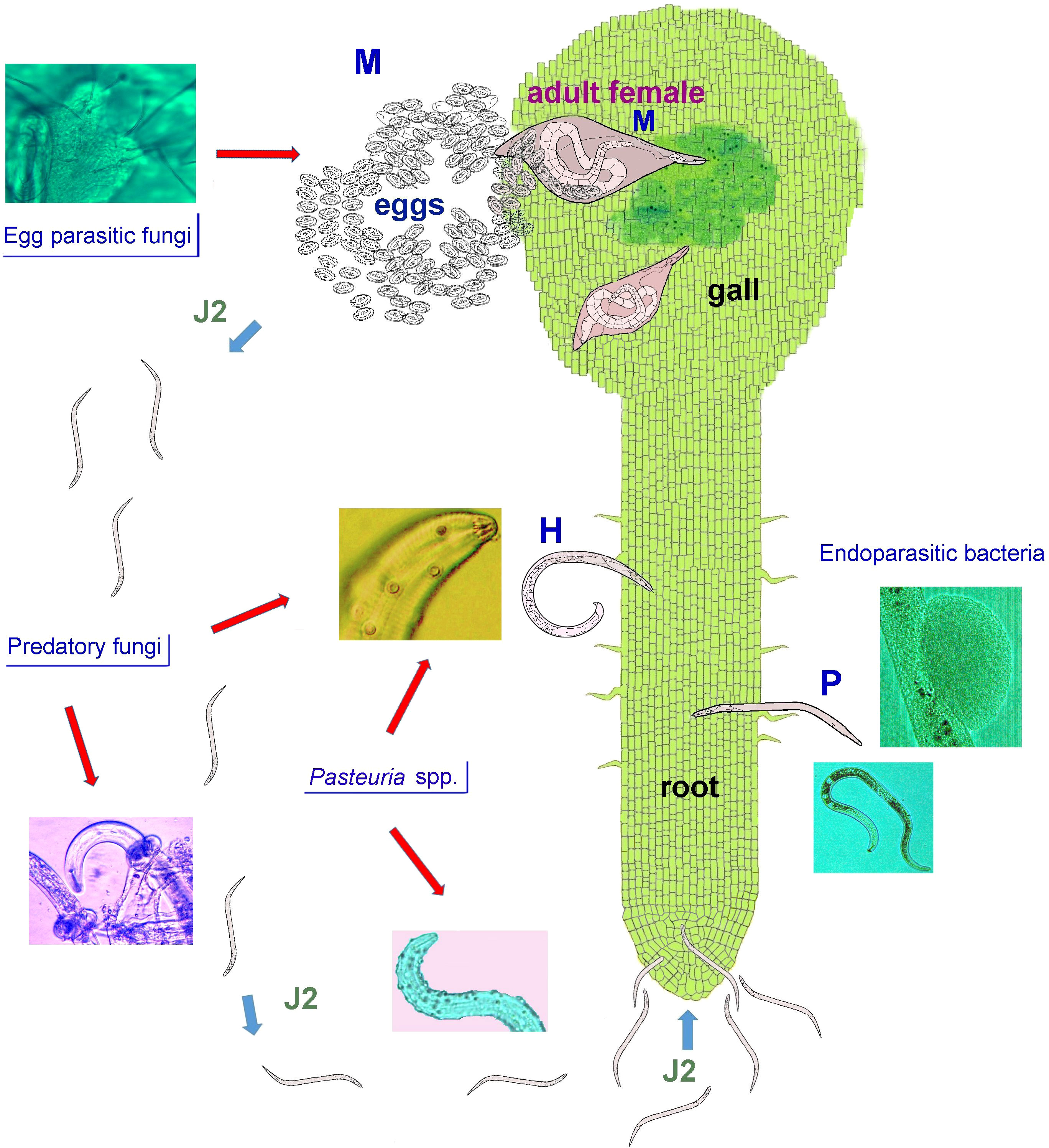
Figure 1 A schematic representation of a generic tri-trophic food web showing most common types of plant-parasitic nematode (PPN) antagonists, occurring in the rhizosphere environment. Three PPN with different lifestyles and trophic behaviour are shown: a sedentary root-knot nematode (RKN, M), Meloidogyne sp.; a migratory ectoparasite, such as the spiral nematode Helicotylenchus sp. (H), and a migratory endoparasite, Pratylenchus sp. (P). A number of fungi parasitize RKN eggs in soil or in colonized egg masses. The RKN second stage juveniles (J2), emerging from the remaining healthy eggs, migrate in soil toward the root apex. During this limited time period the J2 may encounter traps of other predatory fungi and/or infective propagules of bacterial endoparasites, i.e. Pasteuria spp. Once inside the roots, the J2 induce histological alterations yielding a root swelling (gall) with a feeding site, allowing the immature females development and adults reproduction. At this stage Pasteuria spp. complete their life-cycle in synchronism with the host and digest its body content, before being released in the rhizosphere environment (not shown). Also ecto- and endoparasitic nematodes are exposed in soil to fungal predators and/or to infection by bacteria, including other host-specific Pasteuria spp. and several unclassified species.
Singh and Wesemael and Díaz-Manzano et al. focused on bacterial biocontrol agents (Paenibacillus polymyxa, Bacillus paralicheniformi and B. subtilis) against RKN. The gram-positive, rod-shaped bacterium Paenibacillus polymyxa showed biological activity against different organisms, including bacteria, fungi, oomycetes, and PPN (Khan et al., 2008; Son et al., 2009; Cheng et al., 2017; Chávez-Ramírez et al., 2020; Chávez-Ramírez et al., 2021). Singh and Wesemael evaluated the dose-response effect of P. polymyxa on tomato susceptibility to M. incognita and plant growth. Lower numbers of second/third/fourth juveniles, galls, and females were observed in tomato roots treated with increasing concentrations of the bacterium. Tomato plants treated with different concentrations of P. polymyxa, with/without nematodes, showed a dose-dependent effect on growth. A negative effect was observed on root/shoot parameters, but it decreased in presence of M. incognita, suggesting a strong activation of plant defenses against the nematode attack. Paenibacillus polymyxa also showed a nematicidal activity inhibiting hatching, increasing the nematode mortality at the highest concentration tested. The authors hence suggest that the application of P. polymyxa as soil drenching, at seeding/planting, and at the end of the nematode life cycle (egg masses formation), may protect the host from nematodes penetration, and prevent hatching.
Díaz-Manzano et al. identified a potential nematode biological control strategy based on the combination of B. paralicheniformi and B. subtilis. The dual strain combination affected RKN hatching, survival, penetration and reproduction and RLN parasitism in soybean.
A further paper reviewed current insights into the biology, economic importance, and management of the false root-knot nematodes Nacobbus spp. (Lax et al.). Nematodes of this genus, native to the American continent, are important sedentary endoparasites responsible for significant economic losses on main food crops. However, the lack of knowledge on their life cycle, real impact on crops, and reliable methods of identification/detection made their management difficult. Lax et al. presented the latest outcomes on eco-friendly and sustainable management strategies of Nacobbus spp. They include the use of bacteria, entomopathogenic nematodes and their symbiotic bacteria, mycorrhizal and nematophagous fungi, essential oils, plant extracts, phytohormones, amendments, and plant resistance. The combined use and synergistic effects of the different strategies mentioned above were also discussed, together with strategies, such as crop rotation, nematicides, amendments, and biological control, biofumigation combined with arbuscular mycorrhizal fungi (AMF), as well as resistance, grafting and AMF symbiosis.
In many countries, the application of synthetic nematicides is still the most frequent strategy adopted by farmers for PPN control. Nevertheless, the concern for environmental protection and related issues arose not only within the scientific community but also among industry, farmers, and the society. This progressive concern is promoting the search for sustainable management strategies for important PPN. Several approaches have been developed, but their application is not ubiquitous, as many variables affect the soil food webs, related to climate, soil microbiome and texture, plant genotypes, and technical or agronomic specificities. Further progress is needed on many issues, including trainings of farmers and professionals, for the correct application of management practices and bioformulations. Main goal is also preventing the emergence of virulent populations and/or the insurgence of other PPN species that often coexist in the rhizosphere environment, at low density levels.
CM, IE, AC and YO contributed to conception of the Editorial. CM wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was financed by Fundação para Ciência e a Tecnologia (FCT)/MCTES through national funds (PIDDAC) and at the European Regional Development Fund (FEDER) through the Programa Operacional Factores de Competitividade 2020 (COMPETE 2020) under contracts UIDB/00102/2020, UIDP/00102/2020 (CIEPQPF), UID/BIA/04004/2020 (CFE), POCI-01-0145-FEDER-029392, Centro-01-0145-FEDER-000007, and CEECIND/02082/2017 (IE).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aydinli, G., Kurtar, E. S., Mennan, S. (2019). Screening of Cucurbita maxima and Cucurbita moschata genotypes for resistance against Meloidogyne arenaria, M. incognita, M. javanica, and M. luci. J. Nematol. 51, e2019–e2057. doi: 10.21307/jofnem-2019-057
Chávez-Ramírez, B., Kerber-Diaz, J. C., Acoltzi-Conde, M. C., Ibarra, J. A., Vasquez-Murrieta, M. S., Estrada-De Los Santos, P. (2020). Inhibition of Rhizoctonia solani RhCh-14 and Pythium ultimum PyFr-14 by Paenibacillus polymyxa NMA1017 and Burkholderia cenocepacia CACua-24: A proposal for biocontrol of phytopathogenic fungi. Microbiol. Res. 230, 126347. doi: 10.1016/j.micres.2019.126347
Chávez-Ramírez, B., Rodríguez-Velázquez, N. D., Mondragón-Talonia, C. M., Avendaño-Arrazate, C. H., Martínez-Bolaños, M., Vásquez-Murrieta, M. S., et al. (2021). Paenibacillus polymyxa NMA1017 as a potential biocontrol agent of Phytophthora tropicalis, causal agent of cacao black pod rot in chiapas. Mexico. Antonie van Leeuwenhoek 114, 55–68. doi: 10.1007/s10482-020-01498-z
Cheng, W., Yang, J., Nie, Q., Huang, D., Yu, C., Zheng, L., et al. (2017). Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-16631-8
Elling, A. A. (2013). Major emerging problems with minor Meloidogyne species. Phytopathology 103, 1092–1102. doi: 10.1094/PHYTO-01-13-0019-RVW
EPPO (2017). EPPO alert list: Addition of meloidogyne luci together with m. ethiopica. In: EPPO reporting servic/2018. Available at: https://gd.eppo.int/reporting/article-6186 (Accessed January 12, 2023).
EPPO (2021). Meloidogyne luci. In: Distribution. Available at: https://gd.eppo.int/taxon/MELGLC/ (Accessed January 12, 2023).
Khan, Z., Kim, S., Jeon, Y., Khan, H., Son, S., Kim, Y. (2008). A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresour. Technol. 99, 3016–3023. doi: 10.1016/j.biortech.2007.06.031
Maleita, C., Esteves, I., Cardoso, J. M. S., Cunha, M. J., Carneiro, R. M. D. G., Abrantes, I. (2018). Meloidogyne luci, a new root-knot nematode parasitizing potato in Portugal. Plant Pathol. 67, 366–376. doi: 10.1111/ppa.12755
Maleita, C., Correia, A., Abrantes, I., Esteves, I. (2022). Susceptibility of crop plants to the root-knot nematode Meloidogyne luci, a threat to agricultural productivity. Phytopathol. Mediterr. 61, 169–179. doi: 10.36253/phyto-13369
Sen, F., Aydinli, G. (2021). Host status of cultivated crops to Meloidogyne luci. Eur. J. Plant Pathol. 161, 607–618. doi: 10.1007/s10658-021-02346-0
Keywords: biocontrol agents, bionematicides, cultural methods, Meloidogyne spp., Nacobbus spp., nematode control, plant-parasitic nematodes, resistance
Citation: Maleita C, Esteves I, Ciancio A and Oka Y (2023) Editorial: Sustainable strategies for the management of phytoparasitic nematodes. Front. Plant Sci. 14:1148261. doi: 10.3389/fpls.2023.1148261
Received: 19 January 2023; Accepted: 23 January 2023;
Published: 31 January 2023.
Edited and Reviewed by:
Yanfeng Hu, Center for Agricultural Technology (CAS), ChinaCopyright © 2023 Maleita, Esteves, Ciancio and Oka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Maleita, Y2FybGEubWFsZWl0YUB1Yy5wdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.