- 1Department of Biology, Faculty of Science, Marmara University, Istanbul, Türkiye
- 2Department of Medical Services and Techniques, Akyazi Vocational School of Health Services, Sakarya University of Applied Science, Sakarya, Türkiye
- 3Biology Program, Institute of Pure and Applied Sciences, Tekirdag Namık Kemal University, Tekirdag, Türkiye
- 4Department of Medical Laboratory Techniques, Vocational School of Health Services, Bahcesehir University, Istanbul, Türkiye
- 5Application and Research Centre for Testing and Analysis, EGE MATAL, Chromatography and Spectroscopy Laboratory, Ege University, Izmir, Türkiye
- 6Department of Civil Engineering, Faculty of Engineering and Natural Sciences, Bahcesehir University, Istanbul, Türkiye
- 7Department of Biology, Faculty of Arts and Sciences, Tekirdag Namık Kemal University, Tekirdag, Türkiye
- 8Department of Biology, Faculty of Science, Kyrgyz-Turkish Manas University, Bishkek, Kyrgyzstan
- 9Department of Agricultural Biotechnology, Faculty of Agriculture, Ondokuz Mayis University, Samsun, Türkiye
Plants are the sources of many bioactive secondary metabolites which are present in plant organs including leaves, stems, roots, and flowers. Although they provide advantages to the plants in many cases, they are not necessary for metabolisms related to growth, development, and reproduction. They are specific to plant species and are precursor substances, which can be modified for generations of various compounds in different plant species. Secondary metabolites are used in many industries, including dye, food processing and cosmetic industries, and in agricultural control as well as being used as pharmaceutical raw materials by humans. For this reason, the demand is high; therefore, they are needed to be obtained in large volumes and the large productions can be achieved using biotechnological methods in addition to production, being done with classical methods. For this, plant biotechnology can be put in action through using different methods. The most important of these methods include tissue culture and gene transfer. The genetically modified plants are agriculturally more productive and are commercially more effective and are valuable tools for industrial and medical purposes as well as being the sources of many secondary metabolites of therapeutic importance. With plant tissue culture applications, which are also the first step in obtaining transgenic plants with having desirable characteristics, it is possible to produce specific secondary metabolites in large-scale through using whole plants or using specific tissues of these plants in laboratory conditions. Currently, many studies are going on this subject, and some of them receiving attention are found to be taken place in plant biotechnology and having promising applications. In this work, particularly benefits of secondary metabolites, and their productions through tissue culture-based biotechnological applications are discussed using literature with presence of current studies.
1 Introduction
Plants produce a large number of secondary metabolites that do not appear to be involved in primary biological activities. Although secondary metabolites are not essential for the continuity of the plant’s vital functions, they provide great benefits in optimizing plant growth, adapting to changing environmental conditions and protecting the plant from environmental damage (Li et al., 2021; Seker and Erdogan, 2023). This shows the great structural diversity of secondary metabolites in different plants as well as in their different parts. While some of these secondary compounds can be very selectively produced in floral tissues to attract pollinators, some can be synthesized in roots or in leaf tissues for defense purposes (Ritmejeryte et al., 2020; Jasuja, 2022). Some derivatives of these secondary metabolites are vital for plant growth as they form hormones such as auxins, brassinosteroids, gibberellins, and strigolactones (Mihalache et al., 2015; Chopra and Samuel, 2020; Sathyanathan and Varadarajan, 2021; Waratadar et al., 2021; Ozbilen et al., 2022).
Previous researches have focused on the effects of growth conditions and environmental stressors (such as drought, salinity, extreme temperatures, and high light intensity) on secondary metabolite synthesis. Jasmonic acid and methyl jasmonate are critical regulators of secondary metabolite synthesis. For example, in presence of pathogens or other stress conditions, the concentrations of jasmonic acid in plant tissues increase (Gutierrez-Gamboa et al., 2021; Nabi et al., 2021; Kuru et al., 2023). Besides jasmonic acid, cytokinins and ethylene are effective on the regulation of secondary metabolites. Cytokinin affects cell division and plant growth at almost all stages especially tuber formation and lateral bud elongation in rice (Zhang et al., 2010; Kaur et al., 2021). On the other hand, ethylene induces the synthesis of some important secondary metabolites such as anthocyanins in most fruits (Ni et al., 2021). Ethylene also induces the release of cell wall–modifying enzymes such as pectate lyase, pectin methyl esterase and polygalacturonase, and affects the regulation of stress-responsive genes (Uluisik et al., 2016; Iqbal et al., 2017; Wang et al., 2019).
Secondary metabolites are often categorized into three major classes based on their biosynthesis pathways: phenolics, terpenes, and alkaloids (Ozyigit, 2008; Eguchi et al., 2019; Jain et al., 2019; Movahedi et al., 2021; Diyabalanage, 2022). These phytochemicals exhibit enormous chemical and biological diversity, are species- and organ-specific, and are produced in response to a variety of biotic and abiotic stimuli (Sharma et al., 2022). As being one of the major classes of plant secondary metabolites, phenolics are common in all higher plants and are involved in lignin biosynthesis (Ozyigit et al., 2007a; Ozyigit, 2008; Khalofah et al., 2021; Bahrami-Rad et al., 2022; Magray et al., 2023), and pigmentation (Pospisil et al., 2021). Among secondary metabolites, polyphenols are present in all plant species and acts in chemical defense systems against the deleterious effects of UV radiations, pathogens, and oxidative stress (Erb and Kliebenstein, 2020; Pradhan et al., 2020). Phenolics are an essential group of active compounds in phytonutrients and complementary medical systems. Modified polyphenols are also known for having various biological roles including antibacterial, and antioxidant activities (Cano-Avendaño et al., 2021). It has also been reported that plant phenolics have potential to be used as antiviral agents against influenza viruses (Wani et al., 2021) and human coronavirus (Aati et al., 2022). A recent study implied that virus-host cell interactions can be disrupted by phenolic secondary metabolites through interfering enzymatic reactions; thus, the severity of viral diseases is reduced (Arimboor, 2021).
Today, there are more than 25,000 terpenoids proven to exist in plants. Terpenes, also known as isoprenoids or terpeneoids, are organic compounds that can be classified as monoterpenes (10 Cs), sesquiterpenes (15 Cs), diterpenes (20 Cs), triterpenes (30 Cs) and other terpenes according to the number of isoprene units (hydrocarbons containing 5 carbon). The large number of different terpene synthases, found in plants, are the primary cause of the molecular diversity of terpenoids in plants; hence, some terpene synthase enzymes can produce different products from the same substrate. Moreover, it has been reported that terpenes have antimicrobial properties against antibiotic-resistant bacteria through inhibiting protein or DNA synthesis or through disrupting the structure of the cell membrane that cause cell breakdown (Masyita et al., 2022). On the other hand, other compounds including alkaloids are rarely existed and more specific to some plant genera and species (Yang and Stöckigt, 2010; Choi et al., 2022; Zhao et al., 2022). Alkaloids are the important part of the defense mechanisms in various plant species and are getting attention worldwide due to their potential to be used in cancer treatment and other therapeutic purposes (Srivastava and Tiwari, 2022). These low molecular weight organic compounds contain alkyl substituted peptide rings; therefore, they can form chemical interactions with many other molecules. Alkaloids have antibacterial, anti-inflammatory and antimicrobial properties (Yan et al., 2021). Alkaloids suppress oncogenesis by modulating some signaling pathways, related to cell division and proliferation as well as metastasis, and due to having these properties, alkaloids have become the focus of many clinical anti-cancer studies (Bello-Martínez et al., 2022). Paclitaxel, vinblastine, vincristine and vitexin are the primary alkaloid-based molecules that are frequently used as anti-cancer molecules in clinical studies (Desam and Al-Rajab, 2022).
The identities of secondary metabolites are used as the basis for chemotaxonomical and chemical ecology studies (Singh, 2016). Compounds with known biological functions in the secondary metabolite class include dyes (shikonin, indigo, etc.), fragrances (lavender, rose, and other essential oils, etc.), spices (mustard oil, capsaicin, vanillin, etc.), stimulants (caffeine, nicotine, ephedrine, etc.), insecticides (nicotine, rotenone, pyrethrin, piperine, etc.), hallucinogens (morphine, scopolamine, cocaine, tetrahydrocannabinol, etc.), some poisons (aconite, coniine, colchicine, cardiac glycosides, strychnine, etc.) and therapeutic drugs (atropine, cardenolide, codeine, quinine, etc.) have been recognized by humans for thousands of years, but today they are the subject of many new studies (Anulika et al., 2016; Alamgir, 2018; Twaij and Hasan, 2022).
Secondary metabolites can be used in industry as raw materials for the production of pharmaceuticals and cosmetics, and as food additives in food industry as well as protecting crops in agriculture. Because of having great importance, there is a wide range of studies on production of secondary metabolites, including gene modification and biosynthesis-related prospects for meeting high value agroecosystem demands. These unique bioactive plant-derived molecules are used as insecticides (laurine, chlorobutanol etc.) (Zhang et al., 2017), hallucinogens (morphine, scopolamine, tetrahydrocannabinol etc.) (Batool et al., 2020), therapeutic agents (codeine, atropine, cardenolide etc.) (Rashid et al., 2021), antioxidants (carsonic acid, rosemary oil etc.) (Sahoo et al., 2022) as well as flavors (capsaicin, vanillin, mustard oils etc.) (Zachariah and Leela, 2018), oils and fragrances (rose and lavender oils, pulegone etc.). Because secondary metabolites coexist in various plant parts such as leaves, flowers, and stems, extraction, and isolation approaches for obtaining a specific targeted molecule from secondary metabolites play an important role. Regarding this, several techniques have been developed for the extraction of secondary metabolites from plant tissues that are also used in the production of high-volume commercial products. A number of techniques developed as an alternative to traditional extraction techniques (maceration, Soxhlet, and steam distillation) where some limiting conditions exist during applications are as follows: Microwave Assisted Extraction (MAE); Ultrasound-Assisted Extraction (UAE); Supercritical Fluid Extraction (SFE); Pulsed-Electric Field extraction (PEFE); and Enzyme-Assisted Extraction (EAE) (Zhang et al., 2018; Getachew et al., 2020). These latter methods, developed for the extraction of secondary metabolites from plants by preserving their biological activities, both increase the commercial value of these bioactive molecules and increase the performance of their use in the medical field.
Today, modern biotechnology shows rapid growth and unlimited potential, and become as a central branch of science regarding with applications in the fields of agriculture, forestry, environment, medicine and pharmacy, military and different industries. Biotechnology covers the studies related with various physiological and biochemical properties of microorganisms, cell and tissue culture operations, the production of some secondary chemicals, proteins, hormones, antibodies, vitamins, antibiotics and vaccines. Based on genetic recombination, modern biotechnology deals with improving plant or animal properties, and developing microorganisms for specific usages, in agricultural and remediation fields through employing genetic engineering methods (Aslan et al., 2021; Ozyigit, 2020; Vitolo, 2021). Plant biotechnology can be defined as “the use of tissue culture and genetic engineering techniques to produce genetically modified plants that exhibit new or improved desirable characteristics” (Agrios, 2005; Bhatia, 2017).
New approaches based on plant tissue culture practices provide provisions as promoting the modification of source easily and of the extraction of secondary compounds in higher qualities and quantities. Tissue culture methods for plants including callus and in vitro propagation ensure the production rate of secondary compounds in higher quantities without inhibiting the effects of the atmosphere (Beigmohamadi et al., 2019; Kong et al., 2019; Erdem and Uysal, 2021). Through using recombinant DNA technology, certain genes including GUS (a reporter gene), NPT II (as a marker gene), dehE and dehD (herbicide tolerance) can be transferred between species (Mohamed et al., 2016; Kaya et al., 2020; Nandy et al., 2020; Kapusi and Stoger, 2022; Ozyigit et al., 2022). Related with this, recombinant DNA techniques have also been utilized for promoting increases in the yields of some secondary chemicals through modification of the secondary metabolite pathways (Zolfaghari et al., 2020; Sreenikethanam et al., 2022).
As mentioned above, the systems adapted from using tissue culture techniques should be used to produce different compounds as biotechnological products. In order to produce secondary metabolites, the most successful tissue culture techniques for biotechnological applications include using callus culture, hairy root culture, protoplast culture, and micropropagation approaches.
There are very comprehensive and valuable studies pinpointing the importance of plant secondary metabolites in terms of their functions, biological properties, and activities that are changed under the influence of environmental factors, as well as their potential in the medical and economic fields (Pant et al., 2021; Sudheer and Praveen, 2021; Zheng et al., 2021; Mattosinhos et al., 2022).
Especially plant tissue culture methods have a substantial ground in the productions of secondary metabolites from plants that are used in broad range of different industries including agricultural, dye, food processing and cosmetic industries as well as being used as pharmaceutical raw materials by humans in general. In this study, rather than other studies, the emphasis was not on gene transfer systems but on tissue culture systems, and information about secondary metabolites obtained using classical tissue culture was given. It is our hope that this article emphasizing of the importance of plant secondary metabolites in biotechnological studies will be a useful resource, especially for researchers working in the field of tissue culture.
2 Secondary metabolites
2.1 Phenolic compounds
Phenolic compounds are one of the largest and most complex classes of secondary metabolites produced by plants and they arise via the pentose phosphate, shikimate, and phenylpropanoid pathways in plants. They have been the subject of numerous chemical, biological, agricultural, and medical studies (Laganà et al., 2019; Chiocchio et al., 2021). As chemically defined, phenolic compounds have hydroxylated aromatic rings at the centers where the hydroxy group is being attached directly to the phenyl, substituted phenyl, or other aryl groups (Alara et al., 2021). Phenolic compounds as exhibiting anti-inflammatory, anticancer, and antioxidant activities have been studied for their roles in the treatment of diabetes, neurodegenerative diseases, hypertension, and cancer (Chen and Zhang, 2021; Rahman et al., 2021a; Singh et al., 2021a). These roles may also be associated with their protective properties against oxidative stress and some diseases (Rahman et al., 2021b; Seker and Erdogan, 2023). Besides, simple phenolics have bacteriocidal, antiseptic, and anti-helminthic activities (Bekkar et al., 2021; Patil et al., 2021). It is a large group of compounds containing phenolic acids, phlorotannins, bromophenols, and flavonoids (Murray et al., 2018).
Till now, more than 8,000 structures of phenolic materials from plants including simple ones (i.e., phenolic acids) to highly polymerized ones (i.e., tannins) are identified. Their roles in growth, reproduction, and providing a contribution to plants’ colours as well as involvement in the facilitating of resistance against ultraviolet radiation, pathogens, parasites, and predators are reported. Due to being ever-present in all plant organs, flavonoids are ubiquitously found in the human diet. Phenolics are the compounds found to be widespread in plant foods including fruits, vegetables, cereals, olives, legumes, chocolate, and in beverages including tea, coffee, beer, wine (Saha et al., 2019; Dable-Tupas et al., 2023). Furthermore, organoleptic activity is at least partially attributed to plant food properties (Dai and Mumper, 2010; Yasien et al., 2022). Simple phenolic acids and flavonoids are found in plants with insoluble free, soluble esterified, and insoluble-bound configurations (Gulsunoglu et al., 2019).
Regarding the oxidation state of the central C ring, there are six subgroups of flavonoids, which are flavones, flavonols, flavanones, isoflavones, anthocyanins, and polyphenols, and they are most abundantly found in our diets (Reddy et al., 2020; Kumar et al., 2021). They show a very wide distribution of plants. In higher plants, flavonoids are involved in UV (ultraviolet) filtration, symbiotic nitrogen fixation, and floral pigmentation as well as playing roles in many processes as chemical messengers, physiological regulators, and cell cycle inhibitors (Baskar et al., 2018; Gupta et al., 2021; Liu et al., 2022).
Due to having a range of biological activities (antioxidant, anti-mutagenic, anti-inflammatory, and anti-viral properties), these compounds are considered to be a fundamental source of therapeutic applications (Ginwala et al., 2019). As putative inducers, certain flavonoids including naringenin, luteolin, and quercetin exert effects on PPAR-γ activation and escalate insulin sensitivity (Saini, 2010). Among the flavonoids, quercetin has the effects on relieving symptoms of diseases including high fever, eczema, asthma, and sinusitis (Sreeram et al., 2021; Sangeetha et al., 2022). Epidemiological studies have shown that heart diseases are inversely related to flavonoid intake. In addition, it is known that flavonoids have a preventive effect on the occurrence of the oxidation of low-density lipopolysaccharides and reduce the risk of the formation of atherosclerosis (Ginwala et al., 2019; Li et al., 2020).
Flavonoids provide health benefits with their wide spectrum of effects and are essential being as constituents in a variety of nutraceutical, pharmaceutical, medical, and cosmetic applications. This is due to their free radical scavenging properties being as strong anti-oxidants, along with their capacity to modulate basic cellular enzyme functions (Karak, 2019; Carsono et al., 2022). As a result of in vivo and/or in vitro research conducted on flavonoids has shown that flavonoids have anti-oxidant, anti-inflammatory, antipyretic, anti-allergic, anti-ulcer, anti-bacterial, anti-cancer, anti-viral, anti-protozoal, anti-platelets, anti-atherogenic activities (Lesnik and Bren, 2021; Mumtaz et al., 2021; Demir et al., 2022; Puangpraphant et al., 2022; Vo et al., 2022).
Tannin derived from the French “Tanin” is used for defining a range of naturally occurring water-soluble polyphenolic compounds (Khanbabaee and Van Ree, 2001). Tannins, which have two subgroups as hydrolyzable and condensed, form a large group among polyphenols. Hydrolyzable ones have a central core of glucose, or another type of polyol esterified with gallic acid (gallotannins), or with hexahydroxydiphenic acid (ellagitannins) (Dai and Mumper, 2010; Mal and Pal, 2022). As polyphenolic secondary metabolites of higher plants, structurally occurrences of tannins are either as galloyl esters and their derivatives, in which galloyl moieties or their derivatives are attached to a variety of polyol-, catechin- and triterpenoid cores, or as oligomeric and polymeric proanthocyanidins that have possessed of different interflavanyl coupling and substitution patterns (condensed tannins) (Fraga-Corral et al., 2021; Rajasekar et al., 2021).
It is known that tannins, as flavonoids, have antioxidant properties with their free radical scavenging effect and are involved in the complex antioxidant defence system by chelation of transition metals and inhibition of prooxidative enzymes (Koleckar et al., 2008; Pizzi, 2021). Tannins are actively used in the preparation of herbal-based medicines. According to studies, herbal tannins are used as astringents (stopping bleeding, constricting vessels) against diarrhea and as an auretic and anti-inflammatory against stomach and duodenal tumors (Brito-Arias, 2007; Fujiki et al., 2012). In addition, anti-tumor, cardioprotective, anti-inflammatory, and antimicrobial activities are defined for tannins (Hossain et al., 2021; Jing et al., 2022; Maugeri et al., 2022).
2.2 Terpenes
The synthesis of terpenoids proceeds via using of isoprenoid units (two five-carbon building blocks). Because of having a large number of building blocks, terpenoids are classified as: monoterpenes such as carvone, geraniol, D-limonene, and peril alcohol; diterpenes such as retinol and retinoic acid; triterpenes such as betulinic acid, lupeol, oleanolic acid, and ursolic acid; and tetraterpenes such as α-carotene, β-carotene, lutein, and lycopene (Thoppil and Bishayee, 2011; Ninkuu et al., 2021; Thomas and Pronin, 2021). The terpene synthases are involved in the biosynthesis of terpenes and related to this; they can easily be modified including new catalytic properties through minor changes in their structures. In the synthesis of monoterpenes, the first step is the formation of geranyl carbocation through dephosphorylation, and ionization of geranyl diphosphate (Bergman and Phillips, 2021). The first step of the sesquiterpene synthesis begins with the ionization of farnesyl diphosphate to farnesyl cation. Also, the formation of nerolidyl cation via isomerization can occur from farnesyl cation (Liang et al., 2021; Kirschning et al., 2022). Two routes are known for the synthesis of diterpenes and the main enzymes for synthesis are diterpene synthases. One route includes a class I type enzyme, which catalyzes the reaction via the ionization of diphosphate and the other route includes a class II type enzyme, which catalyzes the reaction via the substrate protonation at the 14,15-double bond of geranyl diphosphate (Veneziani et al., 2017; Liu et al., 2022). The generation of nonsteroidal triterpenoids is facilitated through the conversion of squalene into oxidosqualene and cyclization following the formation of dammarenyl cation. The enzymes, that catalyze the reaction are oxidosqualene cyclases (Singh and Sharma, 2015; Goyal et al., 2022). Many terpenoid compounds display a wide range of pharmaceutical properties and due to having these properties; nowadays, they are now gaining increased interest for their use in clinical practices. As well-known examples, taxol (diterpene) isolated from Taxus baccata and artemisinin (sesquiterpene lactone) isolated from Artemisia annua can be given for their antineoplastic and antimalarial potential (Croteau et al., 2006; Pollier et al., 2011). The terpenes have activities related to plant interactions, plant defences, and other environmental stresses (Abbas et al., 2017; Ninkuu et al., 2021).
Monoterpenes are a terpene type that consists of two isoprene units and has the molecular formula of C10H16 (Sundriyal, 2022). Studies by various researchers have also reported that monoterpenes have antiseptic, anti-cancer, antibacterial, and antifungal properties (Scariot et al., 2021; Silva et al., 2021). Various monoterpene types are used in foods as a flavoring and fragrant additive (Li et al., 2021; Wackett, 2021; Ignea et al., 2022), and in agriculture and animal husbandry due to their insecticidal and pesticide effects (Alam et al., 2022; Almadiy et al., 2022; Song et al., 2022).
Sesquiterpenes, the largest class of terpenes, consist of three isoprene units and are represented by the molecular formula of C15H24 (dos Santos Franciscato et al., 2022). Some of the important known sesquiterpenes are: bisabolol found in Matricaria recutita (Herrera et al., 2022); chamazulene found in Artemisia absinthium (Mohammed, 2022); farnesol and cumin found in Vachellia farnesiana; guaiazulene found in Cuminum cyminum (Gilbertson and Koenig, 1981); and dicarabrol found in Carpesium abrotanoides L. (Asteraceae) and Lactarius indigo (Jie-Wei et al., 2021).
Diterpenes having four isoprene units are shown by the formula of C20H32 (Somantri et al., 2022). They are widely found in nature and are defined as compounds having various pharmacological activities (Kemboi et al., 2021). For example, it has been reported by various researchers that diterpenes obtained from the plants belonging to the genus Taxus are used in the treatment of prostate, ovarian, lung, and breast cancer (Chen et al., 2021; Acquaviva et al., 2022; Tomiotto-Pellissier et al., 2022).
Triterpenes are terpenes having six isoprene units and being shown by the formula C30H48 (Luo et al., 2021). Triterpenes are produced by all animals, plants, and fungi (Chaudhary, 2022). Examples of this group are squalene (SKU) found in shark liver oil and stigmasterol, oleanan, and ursan found in soybeans, legumes, and nuts. Triterpenes are used in food, cosmetic, and pharmaceutical industry due to their antioxidant, anti-viral, anti-inflammatory, and anti-tumor activities (Sureda et al., 2021; Darshani et al., 2022; Miranda et al., 2022).
Tetraterpenes (Carotenoids) are defined by the formula C40H56 (da Silveira Vasconcelos et al., 2020; Zia-Ul-Haq, 2021). Examples of tetraterpenes consisting of eight isoprene units are the carotenoids found in peaches, carrots, apricots, spinach, and peppers. Carotenes are tetraterpenes with important biological functions including light capture, antioxidant activity and protection against free radicals, synthesis of plant hormones, and structural components of membranes (Săvescu, 2021; Adil et al., 2022; Seker and Erdogan, 2023). In addition, carotenoids are high-value compounds for the food and pharmaceutical industries and can be synthesized via photosynthetic and non-photosynthetic organisms. Xanthophylls are another group of tetraterpene pigments commonly found in nature (Siziya et al., 2022). Carotenoids function as antioxidants, anti-inflammatory, anti-cancer, anti-diabetic, anti-microbial, and autoinflammatory compounds (Sathasivam and Ki, 2018; Karpiński et al., 2021; Zia-Ul-Haq et al., 2021).
2.3 Alkaloids
Some alkaloids containing basic nitrogen atoms are well recognized as biologically active natural compounds in chemistry and medicine. Target-oriented achievements for the synthesis of alkaloids in laboratory conditions can make possible the study and optimization of their biological properties; however, proceeding in their preparations cannot be that much simpler because of the basicity and nucleophilicity of nitrogen, its susceptibility to oxidation, and its ability to alter reaction outcomes in unexpected ways (Parr et al., 2015; Thawabteh et al., 2021). The main key in alkaloid classification is related to the structure of the molecule containing a basic nitrogen atom at any position that does not bear nitrogen in an amide or peptide bond (Bribi, 2018). Some groups of alkaloids also contain bonding properties related to neutral or weak acidity. In addition to carbon, hydrogen, and nitrogen groups, they also contain groups including oxygen, sulphur, and albeit very little, bromine, chlorine, and phosphorus. Compounds such as amino acids, proteins, peptides, nucleic acids, and amines are generally not called alkaloids (Nicolaou and Chen, 2011; Chen et al., 2021). Alkaloids with complex and diverse structures can be classified most commonly and correctly based on their C-N skeleton profiles. Pyrrolidine, pyridine, quinoline, isoquinoline, indole, quinazoline, steroidal, diterpenoid, and other alkaloids are the groups that alkaloids fall into based on the last signature (Archana and Nagadesi, 2022). They can also be produced by a wide variety of organisms such as bacteria, fungi, animals, and plants (especially). Many are toxic to other organisms and have a wide variety of pharmacological activities (Al-Snafi, 2021; Yan et al., 2021; Cano Ortiz et al., 2022). Alkaloids exhibit various activities including toxicity at organismal and cellular levels in herbivores and vertebrates, and in certain bacteria, fungi, and viruses because of having antibacterial, antifungal, and antiviral properties as well as having effects on molecular targets related to mutagenicity or carcinogenicity. Several alkaloids including nicotine and anabasine are useful in controlling insects as insecticides. Many alkaloids with activity on the nervous system are known in animals (Bribi, 2018; Badri et al., 2019; Hussein and El-Anssary, 2019). The most typical example is morphine, a benzylisoquinoline alkaloid formed as a result of the phenol coupling reaction. Alkaloids such as caulerpin, abisindole alkaloid obtained from algae are isolated due to having anti-inflammatory, anti-tumor, and growth regulatory activities (Bai et al., 2021; Haghighi and Ali, 2021; Zhou et al., 2021).
3 Secondary metabolite extraction from plant material
Secondary metabolites are distinguished by their ability to accumulate in high concentrations in specific tissues or organs of the plants from which they are synthesized. Secondary metabolites can account for up to 1-3% of a plant’s dry weight (Morris et al., 2021). Having different molecular structures and chemical activities, the unique functions of plant secondary metabolites are more apparent, especially in their pure forms. Plant secondary metabolites are an important starting material for many industrial products and are also valuable for a variety of medicinal products and applications. Therefore, the extraction of these plant secondary metabolites with special biological activity with high efficiency is important both commercially and medically.
Techniques applied for the extraction of secondary metabolites are basically divided into two groups: traditional and untraditional. In traditional extraction techniques such as maceration, Soxhlet, and steam distillation, water or organic solvents are used in terms of employing the extraction power of the solvents as well as heat or mechanical mixing for the extraction. To ovecome the most important restrictions of the traditional methods for example reducing the bioactivity and bioavailability of the target biomolecule, modern techniques such as MAE, UAE, SFE, PEFE and EAE were developed to be used in the extraction of plant secondary metabolites.
3.1 Maceration
Being as simple and common, this technique is applied through using of the grounded plant material with a suitable organic solvent (hexane, acetone, methanol, ethanol etc.) together in a reaction vessel (Ashibuogwu et al., 2022). In this method, where the extraction rate can be increased by factors such as heat and mechanical mixing, the extraction process is stopped when the secondary metabolite quantities remaining in the extract and plant material reach equilibrium. The maceration technique, of which works with relatively small volumes and is quite suitable for laboratory-scale extractions, has its own disadvantages and they are: the need for separating of the extract and plant material from each other by a second process (filtration, centrifugation etc.) at the end of the extraction; the need of the time for processing, in which vary from a few hours to several days; the need of a large amount of solvents in the step-applied maceration; and significant losses of secondary metabolites during isolation process after maceration, in which is resulted in low extraction yields (Silva et al., 2021; Pataro et al., 2022; Wela et al., 2022).
3.2 Soxhlet extraction
Soxhlet extraction is a technique preferred for the secondary metabolite extraction because of its ease of use. The grounded plant material is placed into a cellulose filled thimble and after following is put into the extraction tube. A suitable solvent is introduced into the extraction flask and reflux is initiated by heat application. The solvent applied gone and condensed into the extraction tube reaches to the plant material in the thimble and descends from there to the following collection chamber. The solvent that is reheated in the chamber passes into the gas phase and then returns to the plant material for a new wash (Khongthaw et al., 2022). The process shows a continuous character with requiring shorter time and taking less solvent consumption compared to maceration (Tzanova et al., 2020). However, it is a significant disadvantage that the extract is kept around the boiling point of the solvent throughout the process (Adewale et al., 2022).
3.3 Steam-distillation
This technique is mainly applied for the extraction of volatile plant components such as essential oils, dry or wet plant materials that are dispersed in water (Kshyap et al., 2021). This mixture, being in a container connected to the condenser, is heated, and the resulting steam formed after condenses in the condenser, which is having a two-phase system consisting of extracted essential oil and water in the condensation vessel. Phases are separated using a simple separatory funnel. This technique is suitable for processing large quantities of plant materials, especially for industrial extractions. However, it is not recommended as a suitable technique for the extraction of thermolabile metabolites (Lo et al., 2021).
3.4 Microwave-assisted extraction
Microwave-assisted extraction (MAE) is a widely used technique supporting for extracting valuable bioactive molecules (secondary metabolites etc.) from plant materials. MAE is based on the principle that the electromagnetic radiation sent into a polar solvent is absorbed by the substance, and the absorbed energy by the substance increases the intermolecular and intramolecular mobility, resulting in releasing of heat under the “friction force” of the molecules (Delazar et al., 2012). The determinative factors for the efficiency of MAE include the molecular structures of the solvents used (polar or non-polar etc.), the sample/solvent ratio used, the microwave power applied, the time and temperature applied. Although the applied microwave power increases the extraction efficiency, the application of high power may cause overheating of the extraction solution and the subsequent degradation of thermolabile secondary metabolites under the influence of heat. Also, the kind of solvent used in MAE is another important parameter that affects the extraction efficiency. Although the use of water is quite successful due to its ability to absorb microwave energy and provide a homogeneous distribution of the resulting heat, the majority of secondary metabolites derived from plants have very low solubility in aquatic environments. For this reason, various solvent mixtures have been tested by previous studies in order to find a polar and effective solvation environment and their results have been presented (He et al., 2013; Abdelhamid et al., 2019). Besides, numerous studies have also been carried out for the examination of the effectiveness of various reaction parameters in terms of having increased extraction yield using statistical methods (Dang et al., 2018: Poole et al., 2019).
One of the leading advantages of MAE is that the solution system responds very quickly to application, allowing for much greater extraction of total phenolic compounds compared to traditional methods. Microwave energy, being in the frequency range of 300 MHz-1000 GHz, penetrates deeply into the sample, permitting the material to heat up quickly as a whole. Compared to the conditions with long-term and high temperature (110°C) applied in traditional methods, MAE paves the way for shorter-term and higher-efficiency extraction procedures (Magnusson et al., 2017).
3.5 Ultrasound-assisted extraction
This technique works based on the fact that ultrasound waves interact with the material, creating changes in the material at the molecular level as well as in physical properties. The ultrasound wave sent on the sample is dispersed in the solvent medium promoting solvent penetration into the cellular matrices, as well as having a disruptive effect on the cell membrane; thereby, significantly increases the secondary metabolite extraction efficiency (Awad et al., 2012). UAE, using microwave frequencies in the range of 20 kHz-200 kHz, stands out as a technique that operates with short processing times and provides to have high extraction efficiencies with very high qualities (Ho et al., 2016). UAE is also a clean technique, not requiring large quantities of solvents in extraction processes. Compared to traditional techniques such as maceration, steam distillation or Soxhlet, MAE provides benefits including simplification of the working procedure, higher efficiency in a shorter time, high purity level in the final product obtained, and low energy consumption (Mushtaq et al., 2020).
3.6 Supercritical fluid extraction
Supercritical fluid refers to any substance, found at certain temperatures over the critical level as well as, being found under certain pressure conditions over the critical level (Maliki et al., 2022). The properties of a substance such as density, viscosity, surface tension and diffusibility under supercritical conditions are distinctly different compared to the properties of the same substance stored under standard atmospheric conditions (Radivojac et al., 2021).
Today, the increasing sensitivity for the environmental pollution pushes both industrial production centers and researchers to take different precautions to eliminate pollution sources and to reduce pollution levels. In SFE technique, the most commonly used supercritical fluid is CO2 (31.4°C-73.8 bar for CO2), but water, ammonia, nitrous oxide and low molecular weight organic compounds (ethane, propane, butane etc.) kept under supercritical conditions can also be used as supercritical fluids (Gumerov et al., 2021; Kessler et al., 2022). The main advantages of SFE technique are that the usage of predominantly non-toxic supercritical fluids, being safer in terms of preventing contamination, allowing selective extraction depending on the supercritical fluid used, and minimizing the oxidative and thermal degradation of the targeted secondary metabolites (Thota et al., 2022). There are also some limitations/disadvantages associated with SFE technique. Among these, additional costs incurred during the generation of supercritical pressure conditions and the necessity of taking additional precautions due to working under high pressure conditions are of importance (Geow et al., 2021).
3.7 Pulsed-electric field extraction
PEFE is an unthermal technique for secondary metabolite extraction with minimal processing using plant material. It is an application of repetitive short high voltage pulse (μs-ms) that the applied electric field strengths is in the range of 0.1-3 kV.cm-1 and the energy applied is in the level of 1 - 20 kJ.kg-1. The logical basis of this technique relies on the creation of pores on the cell membranes and thereby increasing the cell membrane permeability that is gained via leaving the viable cells of the sample under the influence of an electric field. Furthermore, the use of this technique, which causes irreversible damage to the sample’s cells, is sometimes referred to as a “pre-extraction process” (Baiano and Del Nobile, 2016).
As a “high capital cost” technology that provides a medium extraction efficiency, PEFE has very short application time and is suitable for working with thermolabile molecules (Sun et al., 2020). However, in order to increase the yield for the targeted secondary metabolites, the process should be applied carefully. For example, prolonging of the application time or increasing of the number of applied electric field pulses may cause an increase in temperature in the sample (Brahim et al., 2021).
Enzyme-Assisted Extraction (EAE) is a simpler and safer extraction technique than traditional extraction techniques, with advantages such as the use of non-toxic solvents and the ability to work in low temperature conditions. The underlying fact of EAE is being involving of the enzymatic degradation of cell membranes and the release of secondary metabolites from the cells in an enzyme-containing extraction medium (Cheng et al., 2015). Another important advantage of this technique is that it allows the selective extraction of secondary metabolites (Marathe et al., 2017). Extractions performed in moderate solvent environments yield significant yield percentages for the targeted secondary metabolite(s) (Wijesinghe and Jeon, 2012). Although EAE is a good alternative to traditional extraction techniques, particularly for highly valuable products, it does have some limitations in terms of applicability, particularly for large volume extractions. As final remarks, the high cost and easily degradable nature of enzymes, difficulty in the isolation of the target product from the final reaction mixture at the end of the process, and the low reproducibility make the technique as choice of a high-cost alternative (Wang et al., 2015; Wang et al., 2019).
4 Tissue culture-based biotechnological approaches for obtaining secondary metabolites
4.1 Plant tissue cultures
The plant tissue culture applications introduced by Haberlandt (1902) have been used for over 100 years. A media composition widely used in tissue culture protocols with modifications was designed by Murashige and Skoog in, 1962 (Sehgal and Khan, 2020). The model systems established regarding to plant tissue culture applications are often used in research to bring solutions to the various problems related to physiological, biochemical, genetic, and structural conditions of plants (Torres, 2012). Organizations around the world put in use plant tissue culture techniques as well as micropropagation to improve economically important crops widely via transforming different explants and following regenerating them under optimized culture conditions (Chandana et al., 2018; Sehgal and Khan, 2020; Erdem and Uysal, 2021).
Tissue culture approaches mainly include the methods used to obtain and grow plant cells or organs in aseptic conditions using direct and indirect ways, and their usage areas are involved in (1) obtaining molecules having high economic worth such as plant-derived secondary metabolites and recombinant proteins used as biopharmaceuticals, (2) plant reproduction by micropropagation method, (3) conservation of rare or endangered plant species, (4) screening of cells rather than plants for advantageous characters (e.g. herbicide resistance/tolerance), (5) obtaining plants free from pathogens, (6) obtaining hybrid species by interspecies hybridization, (7) obtaining plants having new features when somaclonal variation occurs, (8) production of identical sterile hybrid species, (9) generating haploid plants by anther and microspore culture, (10) cross-pollinating of distantly related species and then application of tissue culturing for creating embryos (embryo rescue), (11) creating chromosome duplication and induction of polyploidy, usually achieved by applications of antimitotic agents such as colchicine or oryzalin (e.g. doubled haploids, tetraploids, and other forms of polyploids), (12) generating new species between cross distantly related species by protoplast fusion and regeneration of the novel hybrids, (13) providing a quick studying way of the molecular basis for physiological, biochemical and reproductive mechanisms in plants (e.g. in vitro selection for stress tolerant plants), (14) storage of gene resources, (15) utilization of cell cultures in in vitro selection studies, (16) large-scale production of artificial seeds by somatic embryogenesis, (17) carrying out germplasm collections and seed conservation, (18) putting into practices for making automated control of cell growth and rational regulation of metabolite processes in order to have contribution to reduce labor costs, and improvements in productivity and (19) using tissue culture serving as a basic tool for transgenic plant production (Ozyigit, 2012; Mukta et al., 2017; Dagustu, 2018; Huyop et al., 2019; Phillips and Garda, 2019; Secgin and Okumus, 2022).
Totipotency special to plants that are highly useful in biotechnological research provides the ability to regenerate a whole plant from a plant part (Finer and Dhillon, 2016). A basic concept in totipotency follows the regeneration of the whole plant from explants, which are the small parts of the plant prepared by dissection of the plant (body, organ, any tissue, etc.) (Bhatia and Dahiya, 2015). Young plants, seedlings, calli, and somatic embryos are preferred to be used in tissue culture systems (Isah et al., 2017; Secgin and Okumus, 2022). Propagating plants in vitro using the tissue culture techniques involve two consecutive steps: the first one is the formation of in vitro cell/callus/cell suspension and protoplast culture and the latter one is direct or indirect organogenesis or somatic embryogenesis via in vitro regeneration (Aasim et al., 2019).
Organogenesis is called the process that involves in vitro formation of plant structures including roots, shoots, and leaves through being derived directly from the meristem or indirectly from the callus (Figure 1). The plant regeneration process via organogenesis is to be enabled through plant growth regulators that are modified by altering their concentrations in nutrient medium to act on is the formation of callus and differentiation of adventitious meristems into organs (Hussain et al., 2012; Pradhan et al., 2022).
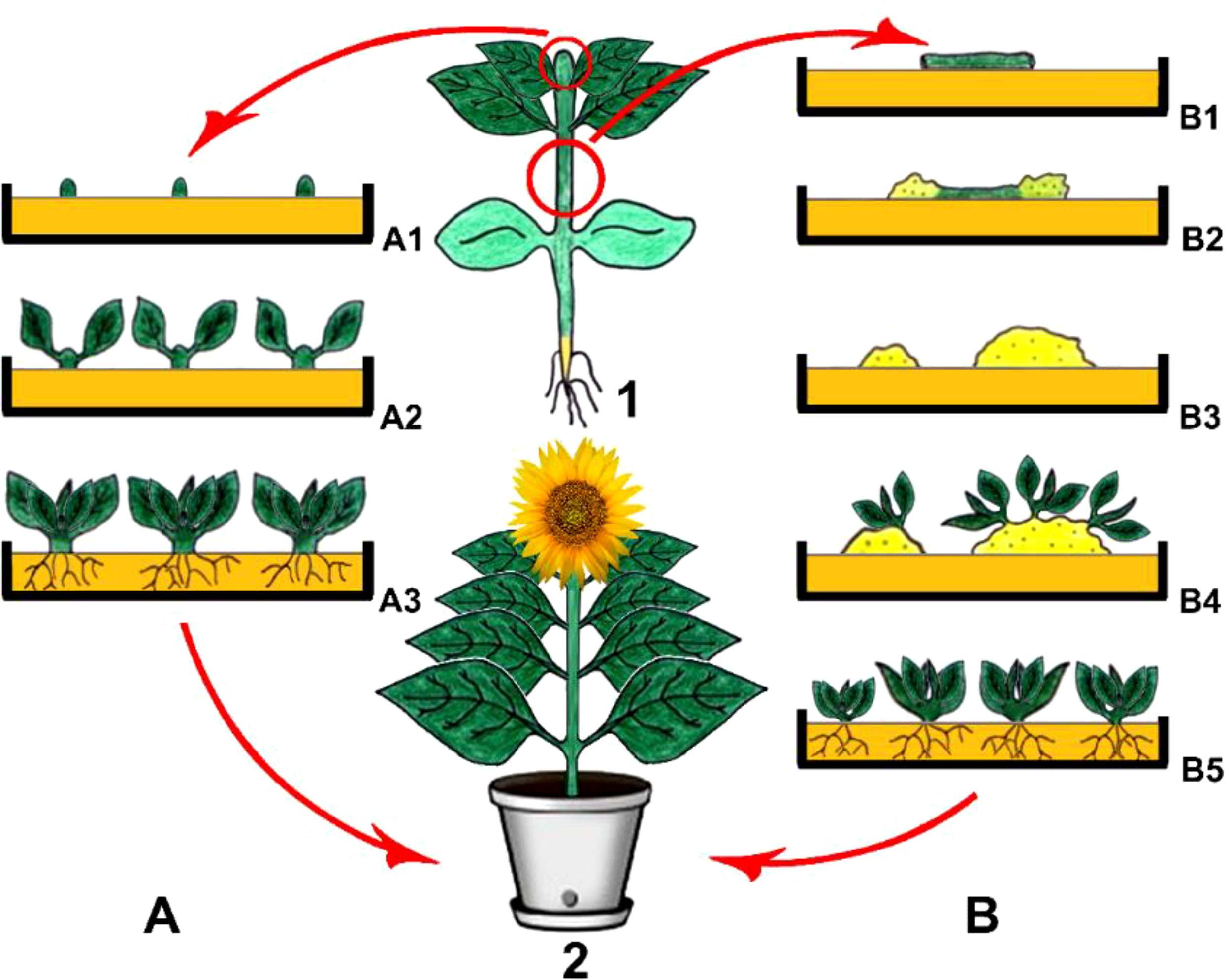
Figure 1 Direct (A) and indirect (B) organogenesis. 1. Main plant and explant sources, (A1) Isolation of meristematic shoot tips as explants and their culturing, (A2) Direct organogenesis and shoot formation, (A3) Rooting and obtaining young plantlets, (B1) Isolation of hypocotyls as explants and their culturing, (B2) Callus induction, (B3) Young calli, (B4) Indirect organogenesis on callus tissues and shoot formation, (B5) Rooting and obtaining young plantlets, 2 Obtained plant via direct and/or indirect organogenesis (Copyrighted illustration from Prof. Ozyigit).
Being a non-zygotic embryonic production process, a new plant can be generated through somatic embryogenesis (Secgin and Okumus, 2022). The somatic embryo formation can be carried out either directly from the explant or after the culturing process of the callus (Figure 2), involving the first formation of the embryogenic clumps in an auxin-rich medium and the subsequent transfer of the embryogenic clumps into a medium without auxins. Complete embryonic development requirements include adequate delivery of auxin and nitrogen, which are found in the medium, and at the end, whole plant formation is a result of a process involving asexual reproduction (Hussain et al., 2012; Shahzad et al., 2017; Erdem and Uysal, 2021).
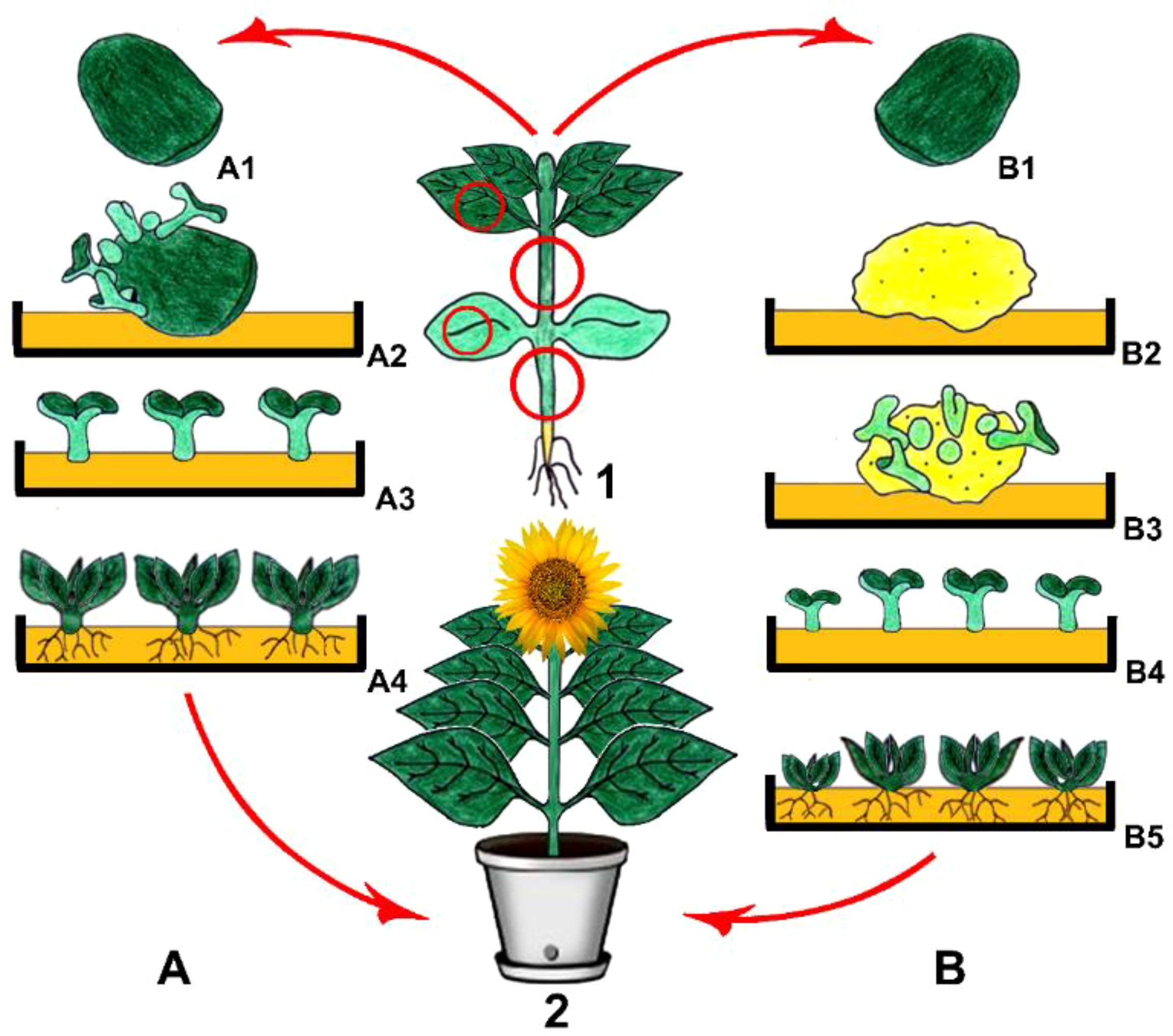
Figure 2 Direct (A) and indirect (B) somatic embryogenesis. 1. Main plant and explant sources, (A1) Isolation of cotyledones as explants, (A2) Direct somatic embryo development on the explant, (A3) Isolation of somatic embryos and their culturing, (A4) Shoot and root formation and then obtaining young plantlets (B1) Isolation of cotyledonary nodes as explants, (B2) Callus induction, (B3) Indirect somatic embryo development on a young callus, (B4) Isolation of somatic embryos and their culturing, (B5) Shoot and root formation and then obtaining young plantlets, 2. Obtained plant via direct and/or indirect somatic embryogenesis (Copyrighted illustration from Prof. Ozyigit).
The formation of new plants through in vitro tissue culture can be defined as a model having mainly six fundamental stages, including: (1) setting up a suitable laboratory environment, (2) choosing a donor plant (identification of plant species and plant parts to be used), (3) determining, preparing and sterilizing of suitable nutrient media for the selected plant species, (4) arranging of callus and cell suspensions, promoting plant regeneration from callus or cell suspensions or regenerating of plants directly from somatic or gamete cells via meristem propagation, organogenesis or somatic embryogenesis, (5) proliferating and extending of the formed shoots, and maturating of somatic embryos, and finally (6) rooting of extended shoots and acclimating of rooting plants (Cui et al., 2019; Joo et al., 2019; Cavallaro et al., 2022).
Productions of important compounds including secondary metabolites via plant tissue culture have also been successfully accomplished. These metabolites are released by plants to provide protection against pathogenic attacks or wounding. Secondary metabolites play important roles in adaptation to stressful conditions (Chandana et al., 2018; Erb and Kliebenstein, 2020). Induction of the biosynthesis of secondary metabolites in plants starts via treatment with any elicitor, which is any stress factor causing triggering of defense responses in plants. (Naik and Al-Khayri, 2016; Khanam et al., 2022). Based on their natures, elicitors are broadly classified into two major categories, being as abiotic (AgNO3, CaCl2, CdCl2, ethanol, and methyl jasmonate, etc.) and biotic (chitosan pectin, chitin, elicitin, yeast extract, and fungal homogenate, etc.) (Kaur and Pati, 2018; Piatczak et al., 2018).
Several elicitation and biotransformation techniques have been utilized to provide a high yield in secondary metabolite production in various plant species (Naik and Al-Khayri, 2016; Halder et al., 2019; Bhaskar et al., 2021; Khanam et al., 2022). Besides, as an alternative tool to increase the growth of cultures by bringing down the cost of the requirements of energy, labor, and space, bioreactors have been started to be used (Shahzad et al., 2017; Abahmane, 2020).
Cell line selection was previously proven to be used in the production of cell lines that can provide great increases in secondary metabolite production (Wawrosch & Zotchev, 2021). In vitro production of the berberine in selected cell lines of Coptis japonica was reported by Sato and Yamada (1984) with a production of up to 13.2% (DW). Catharanthus roseus was utilized by Hall and Yeoman (1987) in the production of high amount of anthocyanin using cell line. Camptothecin formation was realized in suspension cultures of Ophiorrhiza mungos through cell line selection, nutrient medium optimization, and jasmonic acid elicitation of 1.12 mg g-1 DW compared to 0.06 mg g-1 DW in the original cell line (Deepthi and Satheeshkumar, 2017). Recently, Heydari et al. (2020) improved the production of some phenolic acids (rosmarinic, salvianolic-B, ferulic, and cinnamic) in the cell suspension cultures of Woodland Sage through attaining high-yielding cell lines and carboxyl functionalized multi-walled carbon nanotubes elicitation. To provide the needs required by the pharmaceutical industry, attempts have been done in terms of realization of increase in the secondary metabolite production (Hussain et al., 2012).
As mentioned above, it is possible to produce only the relevant plant part for the production of secondary compounds (Mishra, 2015). These strategies include gene cloning and repeated selection of high-yielding strains from heterogeneous cell populations using plant tissue culture techniques such as clonal micropropagation, callus, hairy root, and protoplast cultures (Solle et al., 2016). Several secondary compounds, being produced by using tissue culture techniques from various explant sources, are identified as follows: phenolics including caffeic acid, rosmarinic acid and rosmarinic acid hexoside, salvianolic acid, salvianolic acid K, salvianolic acid F isomer I, salvianolic acid F isomer II, caffeic acid derivative I, caffeic acid derivative II, and methyl rosmarinate from the leaves and shoots of Salvia bulleyana (Wojciechowska et al., 2020); iridoid glycosides (aucubin, harpagide, harpagoside) and phenylethanoid glycosides (verbascoside and isoverbascoside) from the seeds, leaves and shoots of Rehmannia elata (Piatczak et al., 2019); podophyllotoxin-related compounds (6-methoxy-podophyllotoxin, podophyllotoxin and deoxypodophyllotoxin) from the hypocotyls of Linum flavum (Renouard et al., 2018); psoralen, daidzein and genistein bioactive compounds from the cotyledon callus cultures of Cullen corylifolium (Singh et al., 2020); triterpenoids (madecassoside, asiaticoside, madecassic acid, and asiatic acid) from the petioles and leaves of Centella asiatica (Baek et al., 2020); crocin, pircorcrocin, safronal from the corms of Crocus sativus (Ahamed, 2019); phenolic acids (Caffeic acid, Syringic acid, p-Coumaric acid, ferulic acid, Salicylic acid) and flavonoids (rutin, Myricetin and Kaempferol) from the nodes, internodes and leaves of Sphagneticola calendulacea (Kundu et al., 2018); meroterpene bakuchiol from the cotyledone-derived callus, seeds, leaves, internodes and roots of Psoralea drupacea (Lystvan et al., 2010); tryptophan-derived quinoline alkaloid camptothecin from the shoots and leaves of Ophiorrhiza alata (Ya-ut et al., 2011); monoterpene-derived indole alkaloid camptothecin from the radicle-derived roots of Pyrenacantha volubilis (Hima et al., 2019); and phenolic acids (Rosmarinic acid, Caffeic acid, Lithospermic acid, Chlorogenic acid, Cinnamic acid) from the leaves and shoots of Mentha spicata (Yousefian et al., 2020); flavonoids, phenylpropanoids, alkaloids, fatty acids and aromatic glycosides from callus and suspension cultures of Carthamus tinctorius (Liu et al., 2021); epigallocatechin and chlorogenic acid from callus and suspension cultures of Oryza sativa (El-Beltagi et al., 2022) and phenolics, flavonoids, tannins and essential oils from nodal segments of Artemisia arborescens (Riahi et al., 2022).
4.1.1 Clonal micropropagation
Micropropagation is the process involving the generation of plants from vegetative parts or seeds of plants through growth and multiplication by applying various plant tissue culture techniques and is executed in aseptic and favorable conditions on growth media (Figure 3) (Zhou and Wu, 2006; Sidhu, 2011). Micropropagation also presents an advantage as being an effective way of regenerating tissues of genetically transformed material (Bravo-Ruiz et al., 2022; Ozyigit et al., 2022). The regeneration of a plant through the process involves isolation of a plant part (leaf, bud, meristem, etc.) under aseptic conditions and following usage of the plant part isolated via application of a different source of hormone and media regime (Ozyigit and Gozukirmizi, 2008; Sehgal and Khan, 2020). Micropropagation has been shown to be faster and less expensive than traditional cell and tissue culture methods, and it can be used to produce true-type plant material if meristem culture is used (Ozyigit, 2009; Ozyigit and Gozukirmizi, 2009; Musacchi and Neri, 2019; Sehgal and Khan, 2020; Eisa et al., 2022).
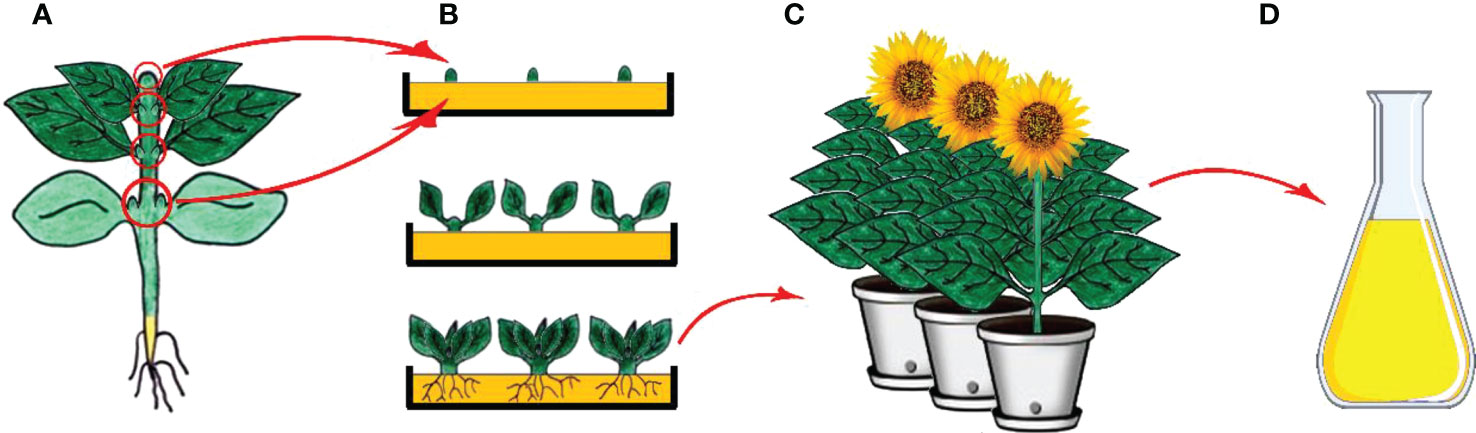
Figure 3 Clonal micropropagation. (A) Main plant and meristem derived (shoot tips, leaf nodes and cotyledonary nodes) explants (B) Isolation of explants, and in vitro direct organogenesis and obtaining clone plants, (C) Obtained clone plants, (D) Isolation of secondary metabolites from clone plants (Copyrighted illustration from Prof. Ozyigit).
The traditional system for agricultural practices allows for clonal propagation of plants, but reproduction rates remain relatively low, which explains why it may take many years for plant tissue culture methods to enter agricultural applications (García-Gonzáles et al., 2010). Through the application of a tissue culture method developed for living plant species, rapid propagations can be effectively achieved, even for the plant species having low multiplication rates (Aslam et al., 2020). As well, the land requirement for the growth of plant species via micropropagation is significantly smaller (Huyop et al., 2019). In conventional cultivation, due to unsuitable climatic conditions or taking a long time, the desired growth and reproduction or germination or flower and seed production by many plant species are insufficient in the field. Micropropagation provides applications to be realized allowing a regular supply of plants using minimum space and time (Prakash and Van Staden, 2007). The main advantages of in vitro micropropagation of plants can be listed as (1) Multiplication of plants at high rates in a short time and a small space, (2) Providing plant production all year round without being affected by regional or seasonal variations, (3) Obtaining of clones having desired characteristics, (4) Productions of genetically engineered plants that are newly created or improved, (5) Enabling of cryopreservation of genetic materials, (6) Using of it for growing of virus-free plants such as potato, banana, apple, and papaya, (7) Rapid and large-scale propagations of plants which are endangered or medicinal or economically important and enhancing of production of plant derivatives in high rates (Sidhu, 2011; Sehgal and Khan, 2020). The important point here is to have new plants in large numbers that are able to produce secondary metabolites naturally using micropropagation.
Plants, especially medicinal plants, have also been used in traditional medicine for years; therefore, they are subjects of ongoing research for their potential biotechnological uses due to having chemical properties in medicine and pharmacy (Veraplakorn, 2016; Debnath and Goyali, 2020; Karahan et al., 2020). The aim of using this approach being apart from other biotechnological approaches is not to obtain large-scale secondary compounds but rather to obtain large numbers of clonal plants in a short time from a selected mother plant and isolation of these chemicals from these clonal plants (Figure 3).
4.1.2 Callus cultures
As known, a plant cell in culture retains totipotency function; hence, has the ability to produce the substances found in the parent plant (Feher, 2019). In tissue culture practices, callus is formed as a result of unorganized growth seen following the wound healing process along with initiation of cell division, which is started on the surface of freshly dissected explant after transferring it into growth-promoting conditions (Neumann et al., 2009; Filová, 2014). Callus produced via dedifferentiation is plant tissue that predominantly contains an unorganized mass of parenchymatic cells (Figure 4) (Ozyigit et al., 2007b).
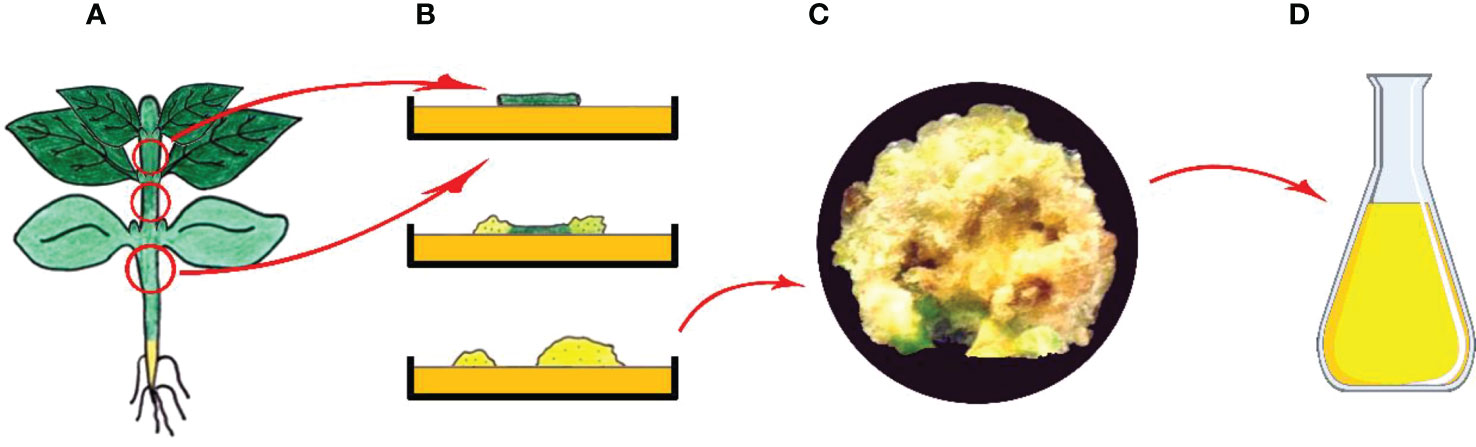
Figure 4 Callus induction and secondary metabolite production from callus cultures. (A) Main plant and explant sources, (B) Isolation of explant, and in vitro callus induction, (C) Obtained callus and (D) Isolation of secondary metabolites from callus (Copyrighted illustration from Prof. Ozyigit).
Callus culture formations have been successfully initiated in a wide variety of dicot and monocot species. Callus formation can be induced using explants from stems, anthers, fruits, apical regions, leaves, roots, flowers, or seeds (Patra et al., 2020). Vascular cambia, storage organs, pericycle of roots, endosperm, cotyledons, leaf mesophyll, and pro vascular tissue are the tissues used for callus production under in vitro culture conditions via induction (Torres, 2012; Patra et al., 2020; Ozbilen et al., 2022). Although sometimes seen that liquid medium is used, the solid medium is generally used for callus growth. There are several disadvantages to using solid cultures that make liquid cultures preferable, at least in some cases (Bridgen et al., 2018; Efferth, 2019). For instance, only part of a callus proliferates on the surface of the solid medium; hence, various factors including gradients in nutrients, the exchange of gases, and toxic waste products existing between the callus and the solid medium cause disparity in raising callus. Besides, gravity and variations in light intensity are the parameters that affect the proliferation of the callus by causing polarization. Also, a problem raising with callus growth in the solid medium is associated with limitations in certain directions by the medium or walls of the glassware. Finally, transferring callus grown in a solid medium to a liquid medium cannot be realized without some distortions occurring in the tissue. Solid cultures are still preferred as the method chosen for routine maintenance of callus formation, even though having drawbacks (Torres, 2012; Patra et al., 2020). The media supplemented with relatively high auxin concentrations or a combination of auxin and cytokinin provide in vitro conditions required for callus formation (Ozyigit et al., 2002; Shekhawat et al., 2021). Callus formed from explants at the initiative is called a primary callus or callus induction. And then, secondary callus formation starts from the primary callus (Phillips and Garda, 2019; Patra et al., 2020). Callus cultures prepared can be found as embryogenic callus or non-embryogenic callus. Differentiated embryonic competent cells found in embryogenic callus are capable of regenerating whole plants through the somatic embryo development process. Non-embryogenic callus cultures that retain homogenous clumps of dedifferentiated cells are utilized for the production of secondary metabolites (Filová, 2014).
The effective long-term maintenance of callus culture on the same medium cannot be provided due to the occurrence of cell losses causing a reduction in cell division and secondary metabolite production. Therefore, subculturing practice for callus should be regularly repeated over a period of 4-5 weeks (Patra et al., 2020; Wahyuni et al., 2020). Storage products accumulated within resting cells appear to be gradually lost during dedifferentiation. After the formation of new meristematic cells in the tissue, undifferentiated parenchymatous cells without any structural order are developed (Filová, 2014). The natural photosynthetic capacity in most plant cultures is lost as a result of the dedifferentiation process occurring. Consequences of this situation have probably arisen as variations occurring in the culture of callus tissue and the donor plant that have different metabolic profiles (Bhatia and Dahiya, 2015).
After undergoing growth resulting in the formation of a typical unorganized callus, the re-appearance of some kinds of specialized cells can be again seen in the following time of development. Such differentiation can arise randomly but may occur as being taking part of centers of morphogenesis that direct the formation of organs including roots, shoots, and embryos. Unorganized cultures are used for the de novo production of plants, often being known as plant regeneration (Filová, 2014). The first step in considering callus cultures to be used in the production of plant secondary metabolites by cell culture is to ensure that they are stable and optimized. For instance, preparing the liquid suspension cultures to be used as inocula. Many previous studies addressed the use of cell suspension cultures for the production of secondary metabolites and considered this technology as a way to overcome problems related to product quantity and quality of whole plants due to the effects of different environmental factors (Rao and Ravishankar, 2002; Yamamoto et al., 2002; Zhang et al., 2002; Filová, 2014).
In addition, some active ingredients and secondary compounds have successfully been produced by application of tissue culture approach using intact plants (Figure 4). The examples are as listed: phenolic molecules, including apigenin, p-coumaric acid, genistein, luteolin, rutin hydrate, trans-ferulic acid, salicylic acid and naringenin from Coryphantha macromeris (Karakas and Bozat, 2020); medicinally vital phenolic and flavonoid compounds, including apigenin, caffeic acid, catechin, gallic acid, hederagenin, myricetin, kaempherol, isorhamnetin, nahagenin, ursolic acid, betulinic acid from Fagonia indica (Khan et al., 2019); p-coumaric acid, hesperidin, cafeic acid, rosmarinic acid from Rosmarinus officinalis (Coskun et al., 2019); phenolics, including gallic acid, chlorogenic acid, caffeic acid, rutin, myricetin, quercetin, vanillic acid, luteolin and iso-rhamnetin, from Lycium barbarum (Karakas, 2020); gingerol, shogaol, and zingerone from Zingiber officinale (Arijanti and Suryaningsih, 2019); indole alkaloids, including echitamine, acetylechitamine, tubotaiwine and picrinine from Alstonia scholaris (Jeet et al., 2020); crocin from Crocus sativus (Moradi et al., 2018); anticancer alkaloids (vincristine and vinblastine) from Catharanthus roseus (Mekky et al., 2018); phenylethanoid (salidroside, tyrosol), phenylpropanoid (rosavin and rosarin) and phenolic acids (p-coumaric acid, gallic acid, and cinnamic acid) from Rhodiola imbricata (Rattan et al., 2020); eugenol and ursolic acid from Ocimum tenuiflorum (Sharan et al., 2018); bioactive compounds, including 1,2-benzenedicarboxylic acid (phthalic acid), 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 2-hexadecen-1-ol-3,7,11,15-tetrametil, hexadecanoic acid methyl ester (methyl palmitate), n-hexadecanoic acid (palmitic acid), 9,12-octadecadienoic acid methyl ester, 9,12,15-octadecatrienoic acid methyl ester, phytol, octadecanoic acid methyl ester (methyl stearate), 9,12,15-octadecatrienoic acid (linolenic acid) and squalene from Mucuna pruriens (Sweetlin and Daniel, 2020); several different metabolics, including acetamide, propanoic acid, α-thujene, linalool, 5-hydroxymethylfurfural, β-maaliene, epidolichodial, calarene, seychellene, α-curcumene, eremophilene, α-vatirenene, valencene, α-cadinol, ledol, meso-erythritol, α-gurjunene, viridiflorol, (-)-globulol, spirojatamol, dodecanoic acid, patchouli alcohol, jatamansone, xylitol, aristolone, protocatechuic acid, mannose, hexadecanoic acid, p-coumaric acid, talose, α-D-mannopyranose, α-D-galactopyranoside, D-mannitol, myo-inositol, -D-glucopyranoside, D-(+)-trehalose, D-(+)-cellobiose, melibiose, vitamin E, β-sitosterol from Nardostachys jatamansi (Bose et al., 2019); identified 11 organic acids, 16 phenolic acids, 8 flavonoids, and 17 metabolites of different classes from Coryphantha macromeris (Cabanas-Garcia et al., 2021); phenolic compounds (ferulic acid, isoquercitrin, rutin, quercetin, quercetin-7-O-glucoside and luteolin) from Hyssopus officinalis (Babich et al., 2021) and phenylethanoids and steroidal glycosides of the furostanol typefrom Digitalis lanata (Tomilova et al., 2022).
4.1.3 Protoplast and suspension cultures
Defined as protoplast, the cell has a spherical shape surrounded by a plasma membrane, but without having a cell wall and sensitive to osmotic pressure. The protoplast can regenerate a cell wall and can give rise to callus and shoot, root, or embryo as well as subsequent entire plant formations through the redifferentiation process (El-Sherif, 2018). The usage of protoplasts has wide potential for applications in genetic engineering and crop improvement programs in terms of being capable of making fusion and being able to take up genes. In protoplast creation, mesophyll tissues from leaves are generally used as the preferable source for isolation (Figure 5) (Patra et al., 2020).
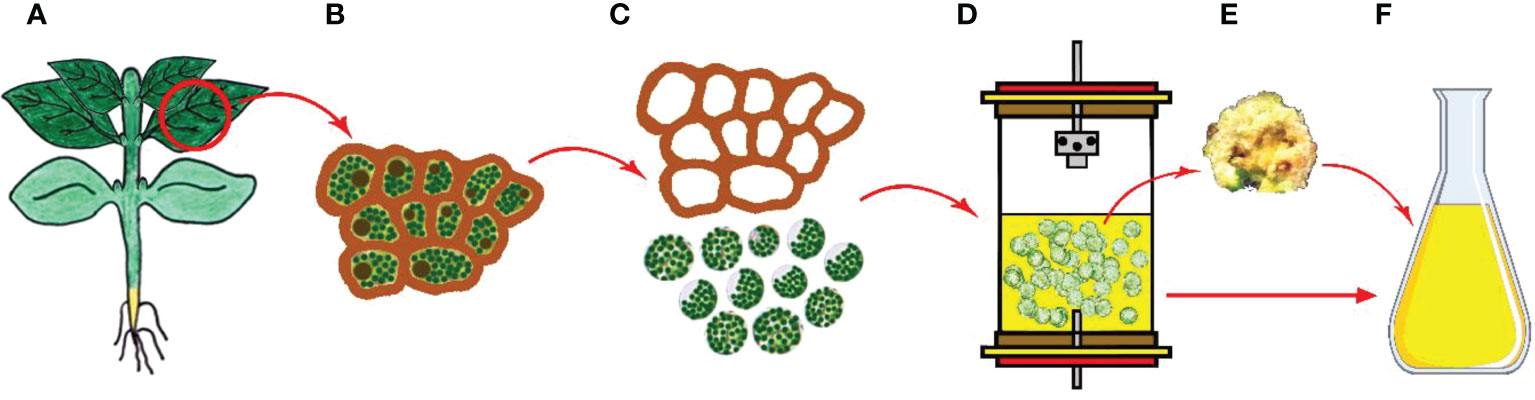
Figure 5 Protoplast cultures and secondary metabolite production. (A) Main plant and explant sources, (B) Isolation of plant cells from leaf tissue, (C) Cell wall removal and obtaining protoplasts, (D) Culturing the protoplasts in liquid media, (E) Obtaining callus, (F) Isolation of secondary metabolites from protoplast derived calli (Copyrighted illustration from Prof. Ozyigit).
Suspension culture conditions can be used for protoplasts, as being used for calli. Following the accomplishment of hydrolytic removal of cell wall building materials by using suitable enzymes, the remaining so-called naked cell, known as a protoplast, can be obtained (Kang et al., 2019). Mechanical and/or enzymatic method(s) can be employed for the isolation of protoplasts from cells. In mechanical application, protoplast obtaining is provided by the plasmolysis process resulting in the separation of the cell wall from the cell during shrinkage. The cell walls from the plasmolyzed cells are cut to release the protoplasts without damaging them using a sharp blade (Patra et al., 2020). The process of enzymatic hydrolysis includes the utilization of enzymes in a sequential or mixed fashion. Macerozyme and pectolyase are pectin hydrolyzing enzymes, one of which is used for the separation of the cells. After separation, the cells are washed using cell and protoplast enzyme-free washing (CPW) solution containing only plasmolyticum by gentle centrifugation. After centrifugation, the pellet is kept in the centrifuge tube containing second enzymes (i.e., cellulases, hemicellulases), which are used for hydrolysis of the remaining cell wall materials. CPW application is done for the removal of debris after the release of protoplasts (Silva et al., 2012; Patra et al., 2020). Following protoplast isolation, their viability test is performed and then, the viable protoplasts are cultivated in artificial media at a known concentration (Chadipiralla et al., 2020). An isotonic medium gives better survival ability to these protoplasts, and they stay healthy in such an environment. A wide range of physiological problems related to cell walls is actively studied using protoplasts (i.e., nutrient uptake at the cell wall, mechanisms involved in the cell wall synthesis) (Neumann et al., 2009).
As a suitable source, the best material for protoplast isolation in large quantities is young callus. The procedure for isolation from callus is basically equal to the procedure used for isolation from leaves. One difference is related to the optimal enzyme concentrations (i.e., cellulase) that are used less in the isolation of protoplasts from callus compared to leaf tissues. Being young and showing active growth, cell suspension culture is also proven to be a good source for isolating protoplasts (Torres, 2012). Secondary metabolites can be obtained directly and indirectly from protoplast cultures; however, studies are fewer than those in which secondary compounds are obtained with other methods.
The followings can be given as examples of secondary compounds obtained by protoplast cultures and the plants studied: benzoxazinoids from Zea mays (Gao et al., 2019); indole alkaloids from Catharanthus roseus (Aoyagi et al., 1998); chitinase, ajmalicine, and 5’-phosphodiesterase from Wasabia japonica and Catharanthus roseus (Akimoto et al., 1999); scopolamine from Hyoscyamus muticus (Oksman-Caldentey and Strauss, 1986); saponins from Maesa lanceolate (Lambert and Geelen, 2010); phenolics and flavonoids from Satureja sahendica (Tarigholizadeh et al., 2021); and 3-O-p-coumaroylquinic acid and 3-O-feruloylquinic acid from Bambusa multiplex (Nomura et al., 2021);
As rapidly dividing homogeneous cell aggregations in suspensions (King, 1984), the suspension cultures can be used in studies related to biochemistry and physiology including growth and metabolism at cellular levels as well as molecular biology and genetic engineering researches. Applications in the industrial level productions of secondary compounds, cell suspension cultures can also be utilized (Figure 5) (Loyola-Vargas et al., 2008; Sharifi-Rad et al., 2020).
Isolation of cells from in vitro plant materials for the preparation of suspension cultures can be obtained by either mechanical treatment or enzymatic digestion. Other than these, cells for suspension culture preparation can be made from callus induced from any explants. Actually, cell suspension culture can be practically derived from any part of the plant, as in callus cultures. A predictable pattern of growth curve depending upon multiple factors including light, temperature, and aeration can be drawn when a suspension culture is maintained under controlled conditions (Daffalla and Elsheikh, 2018; Patra et al., 2020). A peak with reaching a maximum cell biomass increase for a period of time is seen during incubation. By application of dilution at this point as subculturing, the occurrence of the repeating of the process for the growth and yield is realized (Santos et al., 2019; Wang et al., 2019). Following of entry into the stationary phase occurring as a result of exhaustion of some factors or the accumulation of toxic substances in the medium, a decrease in the viability of cells in the suspension as well as the growth rate for the whole culture is observed. By the addition of an aliquot of the cell suspension into the freshly prepared medium, which has the same composition as the original, new cell suspension can be prepared via the following step (Bhatia and Dahiya, 2015).
There are many options for the method used in the production of appropriate suspension culture. However, using an agitated (50-200 rpm) liquid medium with friable callus added provides the dispersion of the cells in most cases during incubation, after several passages. Mechanical agitation is the cause for most cell suspension cultures to arise from callus cultures. The initiation of suspension cultures can be done using sterile seedlings or embryos (Loyola-Vargas et al., 2008).
After breaking up soft callus using a hand-operated glass homogenizer, the transfer of homogenate to the liquid culture medium is executed (Torres, 2012; Efferth, 2019). Generally, a suspension can be prepared from stationary cultures grown on agar with the aid of a sterile glass rod or by squeezing with a scalpel. In particular, loosely attached cells generated on the opposite side of the agar medium with 2-4 D can be easily scraped off by using a sterile scalpel. An improvement driven by ammonia, being used as a nitrogen source can probably be attributed to the excretion of protons in exchange for its uptake by the cells (Neumann et al., 2009). In ideal conditions, suspension cultures consist of single cells, but rather this is the case rarely seen and appearances as small aggregates formed generally by 20-100 cells are usually observed (Loyola-Vargas et al., 2008). The ideal cell suspension culture is well defined by homogeneity that depends upon both morphologic and biochemical criteria (Torres, 2012). Suspension cultures consisting of a population of cells are nearly homogenous allowing them to be exposed to nutrients easily. Being as useful biological material, cell suspensions offer an opportunity for studying biosynthetic pathways (Shahzad et al., 2017). For the production of beneficial secondary compounds in suspension cultures of different plant species, applications using rolC genes have also been carried out for determining their possible stimulatory and inhibitory effects (Bulgakov et al., 2005). Involvement of rolC in Panax ginseng cells in the plant defence through the induction of related genes has been shown (Kiselev et al., 2006).
All cell culture methods applied for in vitro propagation of different plants follow general steps as directed: isolations of the plant cells from the cultured tissue by means of mechanical or enzymatic process; growth and subculturing of batch or continuous propagated suspension cultures; determining of selection and optimization of culture medium conditions for cell suspension culture; making synchronization of suspension cultures; performing physical selection via volume and temperature shock and chemical methods via application of starvation, inhibition, mitotic arrest; growth estimations in suspension cultures through measurements; determining of growth parameters via measurements of cell counting, packed cell volume, cell fresh, and dry weight; assessing cultured cell viability via using phase-contrast microscopy and performing tetrazolium salt reduction, fluorescein diacetate, Evans blue staining assays; and culturing of isolated single cells including involvements of using plating technique, filter paper raft nurse technique, microchamber technique, and scale-up technique (Bhatia and Dahiya, 2015).
Examples of secondary metabolites obtained using suspension cultures can be given as stigmasterol from Abutilon indicum (Rao et al., 2021); gymnemic acids from Gymnema sylvestre (Mahendran et al., 2021); catechin from Camellia sinensis (Ardianto et al., 2020); alkaloids (vincristine, vinblastine, ajmalicine and serpentine) from Catharanthus roseus (Mishra et al., 2019); triterpenoids from Ocimum basilicum (Pandey et al., 2019); artemisinin from Artemisia annua (Mir et al., 2017); plumbagin from Plumbago europaea (Beigmohamadi et al., 2019) and P. zeylanica (Roy and Bharadvaja, 2019); bacoside from Bacopa monnieri (Kharde et al., 2018); hydrolyzable tannin from Phyllanthus debilis (Malayaman et al., 2017); triterpenoids from Euphorbia hirta (Samkumar et al., 2019); atropine from Hyoscyamus muticus (Abdelazeez et al., 2022); triterpenic acids (betulinic acid, oleanolic acid, and ursolic acid) from Thymus persicus (Lamiaceae) (Bakhtiar and Mirjalili, 2022); and withanolides (withaferin A and withanolide A) from Withania coagulans (Mirjalili and Esmaeili, 2022).
4.1.4 Hairy root cultures
Undifferentiated plant cell cultures are generally found to grow faster compared to roots of higher plants and are considered an alternative to roots of higher plants because of easy handling in the harvesting process. As an alternative method for the production of compounds, the use of plant hairy root cultures is the most promising one (Pence, 2011; Hussain et al., 2012; Filová, 2014).
Agrobacterium-based hairy root culture formation for different plant species has been realized using different strains of Agrobacterium rhizogenes (a Gram-negative soil bacterium) through causing infection, which comprises of integration of its T-DNA of root inducing plasmid into the host genome (Ozyigit et al., 2013). The roles of rol genes (rolA, rolB, rolC, and rolD, which correspond to orf10, orf11, orf12, and orf15) involved in the induction of hairy root formation were first identified in plants infected by the A. rhizogenes A4 T-DNA mutants (White et al., 1985). The neoplastic phenotype of root growth arising from A. rhizogenes infection is described as showing a high growth rate, high degree of lateral branching, a profusion of root hairs, and lack of geotropism (Hu and Du, 2006; Hakkinen and Oksman-Caldentey, 2018).
Cell and tissue culture systems, which are considered biotechnological tools, have been applied to increase the production of secondary compounds, but their use has brought limited success mainly due to their undifferentiation. (Kaur and Pati, 2018). Genetically engineered root cultures with high stability and high productivity have become a viable choice as a useful biotechnological tool as they allow the use of hairy roots for the production of plant secondary compounds compared to intact plants (Figure 6) (Giri and Narasu, 2000; Srivastava and Srivastava, 2007; Pistelli et al., 2010). The intense progress in the development of hairy root technology was gained after having comprehensive knowledge of molecular mechanisms. The critical point in obtaining high yields to produce secondary compounds is the optimization of the nutrient composition (Hu and Du, 2006; Hussain et al., 2012). The main applications using hairy root cultures include biotransformation, production of high-value plant metabolites, phytoremediation, and production of artificial seeds (Georgiev et al., 2012; Guillon et al., 2006; Ozyigit et al., 2021). In addition, the hairy root cultures show distinguishing features as having high genetic stability and being more stable for metabolic production compared to undifferentiated cell cultures (Peebles et al., 2009; Häkkinen et al., 2016). This is mainly due to the high degree of chromosomic stability that hairy roots exhibit (Weber et al., 2008; Weber et al., 2010; Dehghan et al., 2012). Hairy roots have chromosome numbers and karyotypes, which are characteristically the same as that of found in the parent plant. Also, the stability of the growth capacity of hairy roots can be increased without exogenous auxin application. Exposure to growth regulators causes changes in even organized tissues, manifested as modification of chromosome numbers and somaclonal variation (Baíza et al., 1999; Hakkinen and Oksman-Caldentey, 2018).
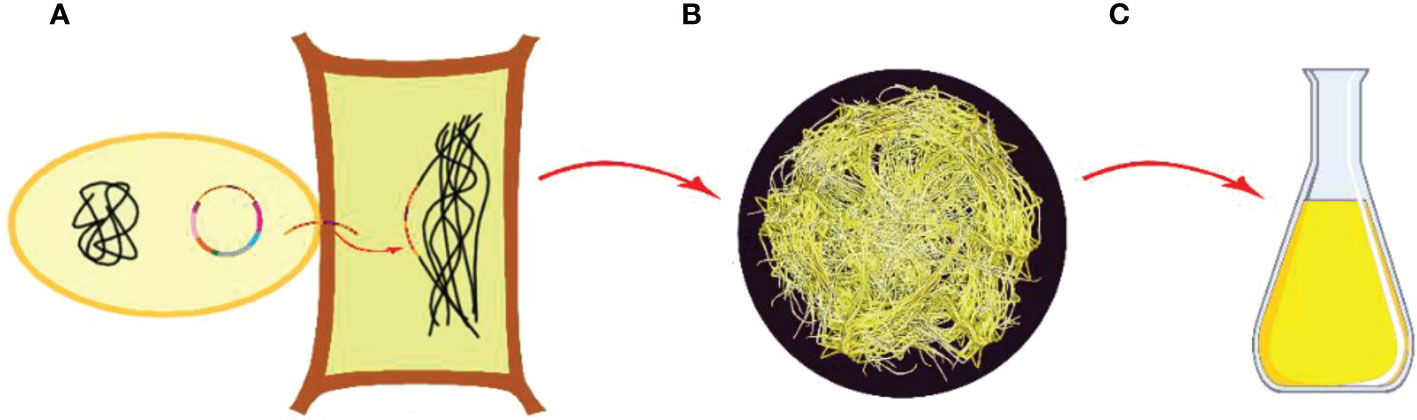
Figure 6 Obtaining hairy roots after gene transfer using Agrobacterium rhizogenes and secondary metabolite production from hairy root cultures. (A) Gene transfer to plant cell via A. rhizogenes, (B) In vitro induced hairy roots, (C) Isolation of secondary metabolites from hairy roots (Copyrighted illustration from Prof. Ozyigit).
Concerning the production of plant secondary compounds from hairy root cultures, a number of root nodule research were realized. Many studies have shown that the transformed roots produce high yields in many plant species for in vitro secondary compound and artificial seed production (Giri and Narasu, 2000; Erdem and Uysal, 2021). In conjunction with this issue, biosynthesis characteristics of plant secondary compounds in transformed root cultures were analyzed from this perspective (Kuzovkina and Schneider, 2006). The production of plant-based chemicals on large-scales was gained momentum using organized cell cultures after the observation of a strong correlation between secondary compound production and morphological differentiation. Root-shoot co-culturing was an effective way of improving generating tissue-specific secondary compounds using intergeneric co-culture of genetically transformed hairy roots and shooty teratomas (Filová, 2014).
For large-scale production of bioactive substances by employing hairy root-based biotechnology, the key point is the cultivation of bioreactors designed for optimal conditions for having high yields (See section 4.2.) (Georgiev and Weber, 2014). Hairy root cultures are susceptible to shear stress; thereby, bioreactor systems designed for the cultivation of hairy roots show differences from those used for suspension plant cell cultures (Mishra and Ranjan, 2008; Piatczak et al., 2018). The regenerations of the transformed plants by the application of genetic engineering approach with the use of A. rhizogenes from hairy roots often show the higher capability of accumulating secondary compounds compared to wild type counterparts and as an alternative and efficient method for obtaining plants for high production of a range of biologically active substances can be used (Korde et al., 2016; Piatczak et al., 2018). On the other hand, for the use of hairy root cultures in the production of bioactive compounds, the construction of a viable bioreactor system with optimum configuration for various physical and chemical parameters including controlled temperature, optimum pH, adequate substrate, salts for nutrition are required. For this purpose, oxygen, product and by-product removal, inoculation size and density, and product recovery should be considered. Also, the Agrobacterial concentration is found to be an important factor in the production of transformed roots (Mishra and Ranjan, 2008).
Although genetically controlled, the nutritional and environmental factors as drawbacks in the biosynthesis of secondary compounds in bioreactors using hairy root cultures can be influential. As an example, the results of a previous study showed that low hairy root growth, biomass, and ginsenoside content were obtained after the experiments using a bioreactor containing normal MS nutrient medium, indicating optimal mineral-element ratio adjustments were required for increasing hairy root growth and biomass. In addition, by exploiting numerous strategies, further enhancements in the accumulation of secondary compounds at both small- and large-scale levels can be gained (Mishra and Ranjan, 2008; Shahzad et al., 2017).
The techniques using hairy root cultures for the production of secondary compounds detected in non-transformed roots were determined (Jabran et al., 2015). The qualifications of hairy root culture techniques for the production of secondary compounds are known as allowing strong secondary compound production through consecutive generations; having genetic stability required for stable productivity; showing hairy root plagiotropism; having exponential growth as biomass increases that occurs as an effect of lateral root formation and results in exponential enlargement in the number of elongation formations, and offering genetic manipulation possibility via transformation to increase biosynthetic capacity. These specifications of the culture techniques were built considerable interest both as a basic study tool and as a source of secondary metabolites (Sathyanarayana and Mathews, 2007).
Hairy root cultures show greater genetic stability than plant suspension cultures. Hairy root plant cultures can be successfully obtained by using different strategies. One of which depends upon the infection occurring between suitable plant explants and A. rhizogenes that recognizes a special chemical (acetosyringone) exuded by susceptible wounded plant cells and attaches to them (Gul et al., 2018). The infection of explant with the bacteria causes to arise the development of hairy roots at the site of infection. This approach relies on A. rhizogenes mediated continuous hairy root culture together with random gain-of-function mutagenesis (Kayser and Wim, 2006). Later, genetically modified and/or transgenic hairy root cultures are developed, being a great achievement in the system (Mehrotra et al., 2010). A number of research using genetic engineering technology have been realized to improve the production of different kinds of pharmaceutically valuable plant-based bioactive metabolites (Grzegorczyk-Karolak et al., 2018; Mehrotra et al., 2018; Sahai and Sinha, 2022).
Biotechnological applications for the production of secondary metabolites using hairy root cultures of plants have been reported. Followings can be given as examples of secondary compounds produced from hairy roots: podophyllotoxin and related aryltretralin-lignans in Linum flavum, (Mikac et al., 2021); curcumin and curcumin monoglucoside in Atropa belladonna (Singh et al., 2021b); feruloyl-glucoside in Turbinicarpus lophophoroides (Solis-Castañeda et al., 2020); sapogenins (stigmasterol and hecogenin) in Chlorophytum borivilianum (Bathoju et al., 2017); alkaloids including eburenine, quebrachamine, fluorocarpamine, pleiocarpamine, tubotaiwine, tetrahydroalstonine and ajmalicine in Rhazya stricta (Akhgari et al., 2019); cryptotanshinone and tanshinone in Perovskia abrotanoides (Ebrahimi et al., 2017); tropane alkaloids, atropine (hyosciamine) and scopolamine (hyoscine) in Atropa komarovii (Banihashemi et al., 2017); flavonoids (rutin, quercetin, isorhamnetin, and isoliquiritigenin) in Isatis tinctoria (Jiao et al., 2018); caffeic acid, prolithospermic acid, salvianolic acid J, rosmarinic acid hexoside (I) and (II), salvianolic acid E, methyl rosmarinate, salvianolic acid F (or isomer I and II) in Salvia viridis (Grzegorczyk-Karolak et al., 2018); triterpenoids in Centella asiatica (Baek et al., 2022) and taxol in Taxus baccata sub sp. wallichiana (Sahai and Sinha, 2022).
4.2 Bioreactors
As a source of secondary compounds, plants are utilized for the production of pharmaceuticals, flavors, fragrances, coloring agents, food additives, and agrochemicals (Figure 7) (Wang et al., 2017). The productions using plants occur regardless of growth parameters and the productions are not in large quantities as well as their occurrences in different tissues of the same plant are at different rates (Atanasov et al., 2015). The roles of most of these compounds in plants are related to defense and as being the leading alternative, the plant cell/organ culture developed has been given the paving of the way for resulting in advancing of bioreactors for plant cell/organ culture (Srikantan and Srivastava, 2018). However, a number of problems observed as cell productivity below desirable level, slow-growing rate, having genetic instability in high-producing cell lines, poor control in cellular differentiation, and failure in maintaining photoautotrophic growth interfere with performance regarding the practices of plant cell cultures (Sajc et al., 2000; Dörnenburg, 2008).
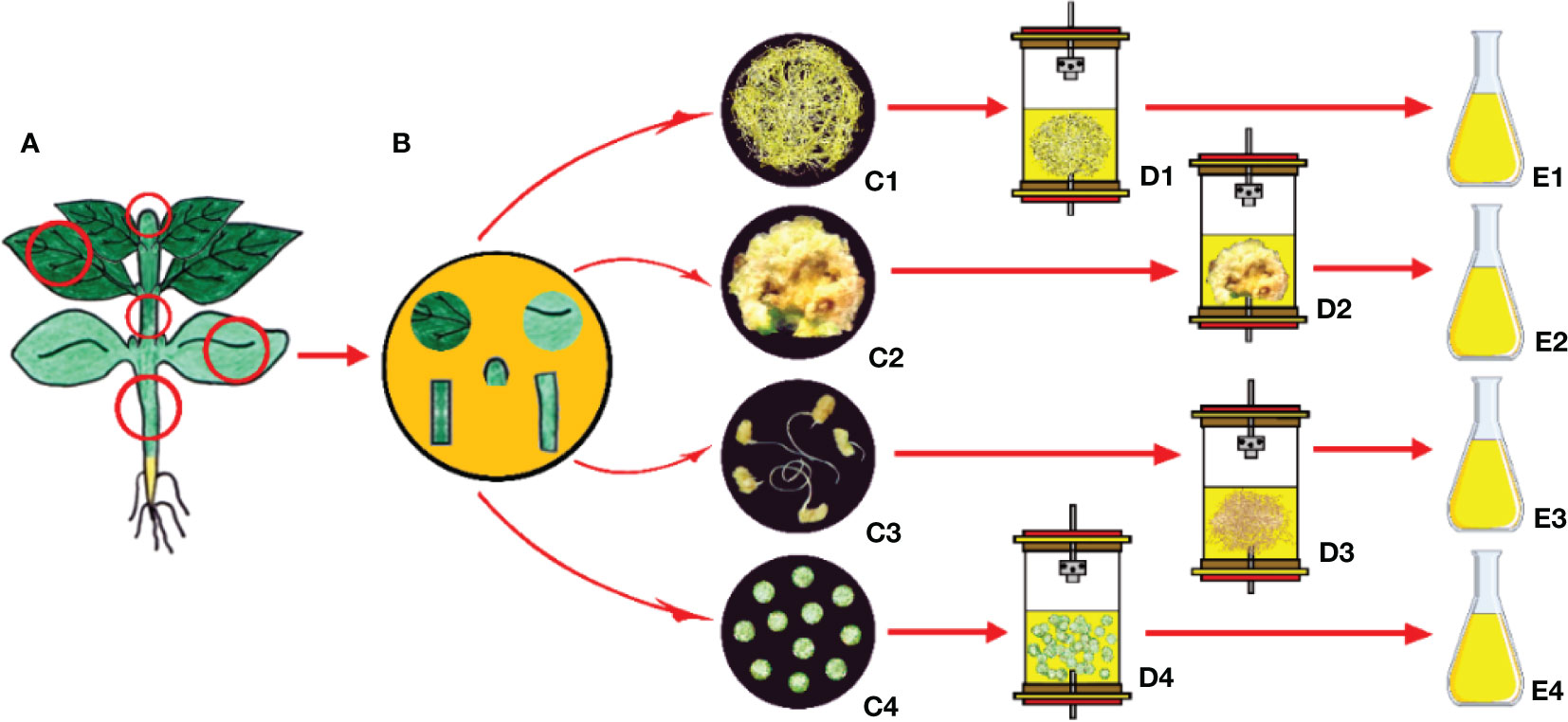
Figure 7 Using bioreactors for secondary metabolite production. (A) Main plant and explant sources, (B) Isolation of explants from different plant parts, obtaining (C1) Hairy roots, (C2) Callus, (C3) Adventitious roots and (C4) Protoplasts, obtaining secondary metabolites in bioreactors using (D1) Hairy root, (D2) Calllus, (D3) Adventitious roots and (D4) Protoplast cultures (E1-4) Isolation of secondary metabolites from different sources (Copyrighted illustration from Prof. Ozyigit).
As an efficient way to produce active biomolecules in vitro, the process used in plant culture depends on factors such as optimization and engineering that affect plant cells what is needed to scale up large bioreactor volumes (Srikantan and Srivastava, 2018). The cultivation strategy involving using bioreactors for plant cell and hairy root cultures is adopted for providing low shear stress, proper mixing, a suitable support system for organ cultures, and ease in scale-up (Honda et al., 2001). To increase biomass and achieve maximum productivity in plant cell suspensions, bioreactors are designed with sterile conditions and approved batch consistency. Regulatory approval systems compatible with bioreactors allow for more successful and faster operations. (Fischer et al., 2012). The bioreactor system offers some key advantages such as allowing cultures to grow rapidly, promoting the bulk transfer of nutrients and gases, promising to scale up the process and save labor costs. Moreover, constant micro-environmental conditions and a high degree of automation of the cultivation process for a liquid medium can be provided by using the bioreactor systems (Yancheva et al., 2019; Sidal, 2022). Similar to microbial and mammalian cell cultures grown in bioreactors that have fulfilled the requirements set by the FDA and EMEA over the past 20 years, plant cell cultures grown in bioreactors show advantages for the production of therapeutics (Huang and McDonald, 2012). Bioreactor configuration for plant cell cultures shows dependence on aerobic conditions with low shear and good mixing. Due to having bigger cell sizes and forming aggregates or organs in comparison with microbial cultures, the operation of bioreactor for cell suspension cultures or hairy root cultures at constant intervals is difficult in terms of the sampling of biomass (Vaghari et al., 2017; Srikantan and Srivastava, 2018).
Different bioreactor configurations have been developed to provide integrative ability with the biological systems to have efficient growth and better product yield (Sharma and Shahzad, 2013). Bioreactors show modifications, including the addition of mist spray, temporary immersion, having mesh or basket according to the cultivation process being performed for the organized plant structures such as hairy roots, somatic embryos, and micropropagation of plantlets (Paek et al., 2005; Srivastava and Srivastava, 2012). Plant cell bioreactors are designed depending on operative capacity which could be mechanically, hydraulically, pneumatically driven, immobilized, or perfusion types (Eibl and Eibl, 2009; Srikantan and Srivastava, 2018). Physical damage (wounding) and shear stress are the factors causing negative effects on the cultivations of hairy roots and callus in bioreactors, of which hairy roots are more sensitive compared to callus; therefore, the usages of low-shear impellers and external support (stainless steel plate or styrofoam mesh) are essential. Due to excessive branching of hairy roots causing self-immobilization by forming an interlocked matrix in the bioreactor, the biochemical mass transfer of nutrients and oxygen becomes limited (Eibl et al., 2018; Srikantan and Srivastava, 2018, Angulo-Bejarano et al., 2019).
Large-scale cultivations of plant cell-based products such as taxol, shikonin, and taliglucerase alfa in bioreactors have been carried out using plant cell suspension cultures (Hidalgo et al., 2018). A modified impeller bioreactor to reduce cell damage from the hydrodynamic stress on cell suspension that cells are found in a homogeneous state is used for cultivation. Bioreactor types with various configurations, including stirred tank, airlift, and bubble column with minor modifications have been commonly employed to grow plant cell suspension cultures. Cell aggregation, foaming, and cell aggregation are issues that cause problems in bioreactors used for plant cell suspension cultures. These difficulties can be overcome by using a suitable low shear impeller and implementing efficient aeration (Huang and McDonald, 2012; Srikantan and Srivastava, 2018).
As discussed above, there are several ways of improving the production yields of secondary compounds via using plant cell cultures or suspensions. They could be by application of biotic or abiotic elicitors; addition of a precursor leading to improvement in the desired compound; modifying the metabolic carbon flux that leads to promoting the expression pathway of the target compound; generating new genotypes by genetic manipulation using genetic engineering or protoplast fusion; treatment with mutagens causing rise the variability already existing in living cells; and using root cultures (Neumann et al., 2009; Bhaskar et al., 2021). For obtaining high productivity, yield, and concentration, a plant cell/tissue-based bioprocess can be designed by taking into consideration of following important parameters. These are the selection of cell lines giving high yields, media optimization, and strategy for optimal bioreactor operation (Srivastava and Srivastava, 2007; Srikantan and Srivastava, 2018).
As examples of recent studies using bioreactors, the followings can be given: phenolic acids, flavonoids and dibenzocyclooctadiene lignans in Schisandra chinensis (Szopa et al., 2018; Szopa et al., 2019); verbascoside, baicalin, wogonoside, luteolin, luteolin-7-glucoside in Scutellaria alpine (Grzegorczyk-Karolak et al., 2017); flavonoids in Gynura procumbens (Pramita et al., 2018); isoflavonoids in Pueraria tuberosa (Kanthaliya et al., 2019); essential oils, p-cymene, geranyl acetate, δ-cadinene, shyobuone, methyl everninate, alloaromadendrene, ledene oxide (II) in Ledum palustre (Jesionek et al., 2018); phenylethanoid glycosides (verbascoside,isoverbascoside) and aucubin in Castilleja tenuiflora (Cortes-Morales et al., 2018); thapsigargin in Thapsia garganica (Lopez et al., 2018); rosmarinic acid and phenolics in Salvia nemorosa (Heydari et al., 2020); flavonoids (Quercetin, Kaempferide, Epicatechin gallate, quercetin-3-o-glucose, Kaempferol-3-rutinoside) in Orostachys cartilaginous (Hao et al., 2020); antifungal saponins SC-2 and SC-3 in Solanum chrysotrichum (Salazar-Magallón and Huerta de la Peña, 2020); six phenolic acids [rosmarinic acid, methyl rosmarinate cafeic acid hexoside, cafeic acid, salvianolic acid F (I) and salvianolic acid F (II)] and four phenylethanoids (verbascoside, leucosceptoside, isoverbascoside and martynoside) in Salvia viridis (Grzegorczyk-Karolak et al., 2022); and some phenolic acids, flavonoids (diosmin, catechin, rutin, and myricetin), a stilbenoid (resver- atrol) and phenylethanoid glycosides (acteoside and echinacoside) in Scrophularia striata (Ahmadi-Sakha et al., 2022).
5 Other in vitro applications for obtaining secondary metabolytes
Photoautotrophic micro-propagation refers to sucrose-free propagation in culture media, in which carbohydrate accumulation in tissues grown under in vitro conditions and subsequent growth occur entirely depending upon photosynthesis and the presence of inorganic nutrients (Kozai, 2010; Gago et al., 2022). The use of photoautotrophic cultures is proven to be useful way of investigating various aspects of photosynthesis, source-sink regulation, nitrogen metabolism, production of secondary metabolites, and defense response subjects (Segečová et al., 2019). In vitro photo-autotrophy can be promoted by removing carbohydrates from the culture medium along with increasing gas exchange in the culture vessel (Xiao et al., 2011; Nguyen et al., 2020; Clapa et al., 2022). As shown by previous works, photoautotrophic micropropagation was applicable approach for a number of plant species to be produced by, as in case of photoautotrophic micro-propagation of Nicotiana tabacum, Lycopersicon esculentum, Solanum tuberosum, and Glycine max. However, now there is intense demand to establish photoautotrophic micropropagation of economically important crop plants (Roitsch and Sinha, 2002). Iarema et al. (2012) described the influence of the photoautotrophic system on increasing the production of secondary metabolites. Photoautotrophic system presents new prospects in terms of increasing commercial production in 20E levels for Pfaffia glomerata as well as providing to conduct basic studies aiming at elucidate the biosynthetic pathway of phytoecdisteroids in plants. The presence of an increasing number of photoautotrophic cultures of different economically important species brings into being the basis for secondary metabolite applications (Fazili et al., 2022).
Finding alternative sources of α-tocopherol by using sunflower species (Helianthus sp.) is having a priority for researchers working in this field. It was targeted to increase the level of α-tocopherol by various experiments performed under in vitro conditions, such as the addition of homogentisic acid as a biosynthetic precursor, in tissues (e.g. hypocotyls, stems, leaves) of different sunflower species and in vitro cultures (Caretto et al., 2004). Gala et al. (2005) showed that the volume of α-tocopherol production can be enlarged by adding jasmonic acid to the culture medium. In a following study performed by Fachechi et al. (2007), it was shown that an increase was achieved in α-tocopherol production by the reduction of the sucrose content via following of adding photomixotrophic to the culture medium.
Photomixotrophic is a suitable micropropagation approach which includes living organisms that are capable of utilizing sugar-containing medium in terms of having energy source. The degree of dependence is related with the sugar concentration found the medium, the presence of feeding with CO2, as well as with a higher photosynthetic photon flux (Emara et al., 2018), The cells grown under photomixotrophic conditions had plastids having photosynthetic activity in increased number in comparison with the cells grown under heterotrophic conditions. However, increased sugar as carbon and energy source utilization by photomixotrophic or photoheterotrophic metabolism is occurred in contrast to photoautotrophic metabolism (Madigan et al., 2003).
As well, sunflower cells cultured with photomixotrophically in comparison with sunflower cells cultured with heterotrophically gave result having more chloroplast in a certain extent as well as an increase in the gene expression of the tocopherol biosynthetic enzyme geranylgeranylpyrophosphate synthase (Fachechi et al., 2007). Plastids in higher plants are known to be the sole sites for the biosynthesis of α-tocopherol (Lichtenthaler et al., 1981) and particularly as in chloroplasts of photosynthetic tissues (Munné-Bosch and Alegre, 2002). Therefore, the α-tocopherol production occurred is related with light exposure given (Geipel et al., 2014).
Hence, using immobilized plant cells as practical way of establishing opportunity for realizing of enhancements in industrial production of secondary metabolites will be widely utilized in future. Research and development are important in the field of the immobilization of plant cells, of which has an important place among the potential benefits being gained by using plant cell cultures (Vitta et al., 2021). Among them, the followings can be mentioned: being prolonged viability of cells in the stationary (and producing) phase; supporting sustaining of biomass over an extended time period; making downstream processing easier (if products are secreted); induction of differentiation shown to be related with enhanced secondary metabolism; alleviating of contamination risk; lessening of shear sensitivity; being increased secondary metabolite secretion, under some circumstances; and lowering of fluid viscosity magnitude in terms of clearing up mixing and aeration problems in cell suspension (Vitta et al., 2021; Krasteva et al., 2021).
Calcium alginate having excellent physical characteristics for use as a cell immobilization agent which is the most common one was exploited in the productions of paclitaxel (Bentebibel et al., 2005; Ochoa-Villarreal et al., 2016), vanillin, ajmalicine and capsaicin (Rao and Ravishankar, 2002; Bentebibel et al., 2005). The application of immobilized plant cells may constitute a significant opportunity to enhance the future industrial production of secondary products. Considering of designing of the culture conditions in optimum level for the production of secondary metabolites and using of several approaches for manipulating the synthesis of these phyto-constituents can be brought into reality by exploiting of approaches such as cell line selection, elicitation, and precursor feeding (Gaosheng and Jingming, 2012).
6 Conclusion and future perspectives
Tissue culture techniques are one of the application areas for plant biotechnology, providing extraction of valuable plant metabolites under restrained conditions. Production of secondary metabolites using plant cell and tissue cultures have distinct advantages compared to classical methods. These advantages can be summarized as: the ability of producing the relevant metabolites under controlled conditions without being affected by environmental factors; the culture conditions being optimized in order to increase the production of secondary metabolites; by taking into account the supply-demand balances, sufficient production being provided when necessary, and thus, regularly controlling the market; a quick production without any political pressure; acquisition of disease-free and harmless plant material; and in vitro culturability of any plant, whether of having tropical or subtropical originality.
Secondary compounds being found in the groups of alkaloids, flavonoids, and terpenoids can exhibit a variety of biological activities related with therapeutic use, and some can also be effective in the prevention of various types of diseases. In addition to pharmaceutical industry, their applications find place in paint manufacturing, food processing, cosmetic field, and agricultural management. Therefore, the production of them in large volumes is an important issue. Large-scale production of secondary metabolites can be accomplished using post-harvest isolations from plants grown for specific purposes or using production technology (phytofermentations) in in vitro cultures. The combination of these strategies with synthetic biology approaches not only helps in showing the interaction natures between drugs and pathologies but also enhances the efficiencies in the development of new products within related industries.
By discovery of new secondary metabolites and the applications of new analytical methods of the chemical industry for large-scale biotechnological production will provide gains in the area. Therefore, this study aims to demonstrate the importance of secondary compounds produced by plants and to emphasize their evolution in research studies/technologies. Also, information about tissue culture based biotechnological applications is provided to cover in this study. Here, our intent is to give a leading prospect for the related industries in terms of providing sufficient quantity and quality production of these compounds using tissue culture based biotechnological applications.
Author contributions
IO: Validation, writing - review & editing, supervision. ID: Conceptualization, investigation. AH-O, BY, AE, IY: Original draft, writing, editing. EC: Investigation. YK: Editing and writing. All authors contributed to the article and approved the submitted version.
Funding
IO and YK acknowledged Kyrgyz Turkish Manas University Grant Number KTMU-BAP 2020.FB.01 for partly sponsoring this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aasim, M., Sameeullah, M., Karataş, M., Bakirci, S., Bakhsh, A., Akhtar, M. S. (2019). “An insight into biotechnological approaches used for the improvement of secondary metabolites from the medicinal aquatic plant, water hyssop (Bacopa monnieri l.),” in Natural bio-active compounds. Eds. Akhtar, M., Swamy, M. (Singapore: Springer), 123–152.
Aati, H. Y., Ismail, A., Rateb, M. E., AboulMagd, A. M., Hassan, H. M., Hetta, M. H. (2022). Garcinia cambogia phenolics as potent anti-COVID-19 agents: phytochemical profiling, biological activities, and molecular docking. Plants 11 (19), 2521. doi: 10.3390/plants11192521
Abahmane, L. (2020). A comparative study between temporary immersion system and semi-solid cultures on shoot multiplication and plantlets production of two Moroccan date palm (Phoenix dactylifera l.) varieties in vitro. Notulae Sci. Biol. 12 (2), 277–288. doi: 10.15835/nsb12210610
Abbas, F., Ke, Y., Yu, R., Yue, Y., Amanullah, S., Jahangir, M. M., et al. (2017). Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 246 (5), 803–816. doi: 10.1007/s00425-017-2749-x
Abdelazeez, W. M. A., Anatolievna, K. Y., Zavdetovna, K. L., Damirovna, A. G., El-Dis, A., Rayan, G., et al. (2022). Enhanced productivity of atropine in cell suspension culture of Hyoscyamus muticus l. Vitro Cell. Dev. Biol.-Plant 58, 593–605.
Abdelhamid, A., Lajili, S., Elkaibi, M. A., Ben Salem, Y., Abdelhamid, A., Muller, C. D., et al. (2019). Optimized extraction, preliminary characterization, and evaluation of the in vitro anticancer activity of phlorotannin-rich fraction from the brown seaweed, Cystoseira sedoides. J. Aquat. Food Prod. Technol. 28, 892–909. doi: 10.1080/10498850.2019.1662865
Acquaviva, R., Malfa, G. A., Loizzo, M. R., Xiao, J., Bianchi, S., Tundis, R. (2022). Advances on natural abietane, labdane and clerodane diterpenes as anti-cancer agents: sources and mechanisms of action. Molecules 27 (15), 4791. doi: 10.3390/molecules27154791
Adewale, A. O., Grace, O. A., Omotoso, A. O., Ayoola, B. K., Abraham, O. A., Paul, O. I. (2022). Kinetics of the process of oil extraction from Gmelina arborea seeds. World Sci. News 173, 27–42.
Adil, M., Nasir, A., Khan, N. M., Sikandar, A. (2022). “Phytotoxic effects of nanoparticles and defense mechanisms in plants,” in Plant and nanoparticles. Ed. Chen, J. T. (Singapore: Springer), 217–238.
Agrios, G. (2005). Plant pathology. 5th Edition (Amsterdam: Elsevier Academic Press), 922. Available at: https://doi.org/10.1016/C2009-0-02037-6">https://doi.org/10.1016/C2009-0-02037-6.
Ahamed, T. E. S. (2019). Bioprospecting elicitation with gamma irradiation combine with chitosan to enhance, yield production, bioactive secondary metabolites and antioxidant activity for saffron. J. Plant Sci. 7 (6), 137.
Ahmadi-Sakha, S., Sharifi, M., Niknam, V., Zali, H. (2022). Production of phenylethanoid glycosides under PEG-induced osmotic stress in Scrophularia striata boiss. cell culture in bioreactor. Ind. Crops Prod. 181, 114843. doi: 10.1016/j.indcrop.2022.114843
Akhgari, A., Laakso, I., Maaheimo, H., Choi, Y. H., Seppänen-Laakso, T., Oksman-Caldentey, K. M., et al. (2019). Methyljasmonate elicitation increases terpenoid indole alkaloid accumulation in Rhazya stricta hairy root cultures. Plants 8 (12), 534. doi: 10.3390/plants8120534
Akimoto, C., Aoyagi, H., Tanaka, H. (1999). Endogenous elicitor-like effects of alginate on physiological activities of plant cells. Appl. Microbiol. Biotechnol. 52 (3), 429–436. doi: 10.1007/s002530051542
Alam, T., Jilani, G., Chaudhry, A. N., Ahmad, M. S., Aziz, R., Ahmad, R. (2022). Terpenes and phenolics in alcoholic extracts of pine needles exhibit biocontrol of weeds (Melilotus albus and Asphodelus tenuifolius) and insect-pest (Plutella xylostella). J. King Saud University-Sci. 34 (4), 101913. doi: 10.1016/j.jksus.2022.101913
Alamgir, A. N. M. (2018). “Secondary metabolites: secondary metabolic products consisting of c and h; c, h, and o; n, s, and p elements; and O/N heterocycles,” in Therapeutic use of medicinal plants and their extracts, vol. 2. (Cham: Springer), 165–309.
Alara, O. R., Abdurahman, N. H., Ukaegbu, C. I. (2021). Extraction of phenolic compounds: a review. Curr. Res. Food Sci. 4, 200–214. doi: 10.1016/j.crfs.2021.03.011
Almadiy, A. A., Nenaah, G. E., Albogami, B. Z. (2022). Bioactivity of deverra tortuosa essential oil, its nanoemulsion, and phenylpropanoids against the cowpea weevil, a stored grain pest with eco-toxicological evaluations. Environ. Sci. Pollut. Res. 29 (43), 65112–65127. doi: 10.1007/s11356-022-20404-w
Al-Snafi, A. E. (2021). Medicinal plants alkaloids, as promising therapeutics-a review (part 1). IOSR J. Pharm. 11, 51–67.
Angulo-Bejarano, P. I., Gómez-García, M. D. R., Valverde, M. E., Paredes-López, O. (2019). Nopal (Opuntia spp.) and its effects on metabolic syndrome: New insights for the use of a millenary plant. Curr. Pharm. Design 25 (32), 3457–3477. doi: 10.2174/1381612825666191010171819
Anulika, N. P., Ignatius, E. O., Raymond, E. S., Osasere, O. I., Abiola, A. H. (2016). The chemistry of natural product: plant secondary metabolites. Int. J. Technol. Enhanc. Emerg. Eng. Res. 4 (8), 1–9.
Aoyagi, H., Sakamoto, Y., Asada, M., Tanaka, H. (1998). Indole alkaloids production by Catharanthus roseus protoplasts with artificial cell walls containing of guluronic acid rich alginate gel. J. Fermentation Bioengineering 85 (3), 306–311. doi: 10.1016/S0922-338X(97)85680-2
Archana, O., Nagadesi, P. K. (2022). Endophytic, non-endophytic fungal alkaloids and its applications. Saudi J. Pathol. Microbiol. 7 (1), 4–19. doi: 10.36348/sjpm.2022.v07i01.002
Arijanti, S., Suryaningsih, D. R. (2019). Biosynthesis of secondary metabolites (gingerol, shogaol, and zingerone) from callus of three ginger varieties. Drug Invent. Today 11 (2), 1–6.
Ardianto, C., Khotib, J., Purwanto, D. A., Muslihatin, W. (2020). Production of the secondary metabolite catechin by in vitro cultures of Camellia sinensis l. J. Basic Clin. Physiol. Pharmacol. 31 (5):1–7. doi: 10.1515/jbcpp-2019-0357
Arimboor, R. (2021). Plant phenolics with antiviral activities against human corona virus and structure-activity relationships–a review. Pharmacognosy Rev. 15 (30), 96–106. doi: 10.5530/phrev.2021.15.12
Ashibuogwu, A. I., Afieroho, O. E., Suleiman, M., Abo, K. A. (2022). Anti-urease and antioxidant activities of the leaf extracts from Murraya paniculata (L.) jack (Rutaceae). GSC Adv. Res. Rev. 11 (1), 156–164. doi: 10.30574/gscarr.2022.11.1.0099
Aslan, A., Engin, T. A., Kurmanbekova, G., Kayalar, F., Kayalar, F., Karagoz, Y., et al. (2021). Standing of biotechnology subjects found in biology courses of higher education and profiling of prospective teachers for their interests on biotechnology. Front. Life Sci. RT 1 (2), 51–57.
Aslam, R., Alam, M. S., Saeed, P. A. (2020). Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Eng. Rev. 12, 48–67. doi: 10.1007/s12393-019-09204-0
Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E. M., Linder, T., Wawrosch, C., Uhrin, P., et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 33 (8), 1582–1614. doi: 10.1016/j.biotechadv.2015.08.001
Awad, T. S., Moharram, H. A., Shaltout, O. E., Asker, D., Youssef, M. M. (2012). Applications of ultrasound in analysis, processing and quality control of food: a review. Food Res. Int. 48 (2), 410–427. doi: 10.1016/j.foodres.2012.05.004
Babich, O., Sukhikh, S., Pungin, A., Astahova, L., Chupakhin, E., Belova, D., et al. (2021). Evaluation of the conditions for the cultivation of callus cultures of Hyssopus officinalis regarding the yield of polyphenolic compounds. Plants 10 (5), 915. doi: 10.3390/plants10050915
Badri, S., Basu, V. R., Chandra, K., Anasuya, D. (2019). A review on pharmacological activities of alkaloids. World J. Curr. Med. Pharm. Res. 1 (6), 230–234. doi: 10.37022/WJCMPR.2019.01068
Baek, S., Han, J. E., Ho, T. T., Park, S. Y. (2022). Development of hairy root cultures for biomass and triterpenoid production in Centella asiatica. Plants 11 (2), 148. doi: 10.3390/plants11020148
Baek, S., Ho, T. T., Lee, H., Jung, G., Kim, Y. E., Jeong, C. S., et al. (2020). Enhanced biosynthesis of triterpenoids in Centella asiatica hairy root culture by precursor feeding and elicitation. Plant Biotechnol. Rep. 14 (1), 45–53. doi: 10.1007/s11816-019-00573-w
Bahrami-Rad, S., Haji Boland, R., Khatamian, M. (2022). Foliar application of nano tetraammine copper (II) sulfate complex influences Cu and fe homeostasis, phenolics and lignin biosynthesis in tobacco (Nicotiana rustica) plants. J. Sci. Islamic Republic Iran 33 (2), 105–116. doi: 10.22059/jsciences.2022.337223.1007696
Bai, R., Yao, C., Zhong, Z., Ge, J., Bai, Z., Ye, X., et al. (2021). Discovery of natural anti-inflammatory alkaloids: potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 213, 113165. doi: 10.1016/j.ejmech.2021.113165
Baiano, A., Del Nobile, M. A. (2016). Antioxidant compounds from vegetable matrices: biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 56 (12), 2053–2068. doi: 10.1080/10408398.2013.812059
Baíza, A. M., Quiroz-Moreno, A., Ruíz, J. A., Loyola-Vargas, V. M. (1999). Genetic stability of hairy root cultures of Datura stramonium. Plant Cell Tissue Organ Culture 59 (1), 9–17. doi: 10.1023/A:1006398727508
Bakhtiar, Z., Mirjalili, M. H. (2022). Long-term cell suspension culture of Thymus persicus (Lamiaceae): a novel approach for the production of anti-cancer triterpenic acids. Ind. Crops Prod. 181, 114818. doi: 10.1016/j.indcrop.2022.114818
Banihashemi, O., Khavari-Nejad, R. A., Yassa, N., Najafi, F. (2017). In vitro production of secondary metabolite using Atropa komarovii Bline&Shal (Solanaceae) hairy root culture via agrobacterium rhizogenes ATCC15834. J. Herbal Drugs (An Int. J. Med. Herbs) 8 (1), 51–58. doi: 10.18869/JHD.2017.51
Baskar, V., Venkatesh, R., Ramalingam, S. (2018). “Flavonoids (antioxidants systems) in higher plants and their response to stresses,” in Antioxidants and antioxidant enzymes in higher plants (Cham: Springer), 253–268.
Bathoju, G., Rao, K., Giri, A. (2017). Production of sapogenins (stigmasterol and hecogenin) from genetically transformed hairy root cultures of chlorophytum borivilianum (Safed musli). Plant Cell Tissue Organ Culture (PCTOC) 131 (3), 369–376. doi: 10.1007/s11240-017-1290-8
Batool, A., Batool, Z., Qureshi, R., Raja, N. I. (2020). Phytochemicals, pharmacological properties and biotechnological aspects of a highly medicinal plant: datura stramonium. J. Plant Sci. 8 (2), 29–40. doi: 10.11648/j.jps.20200802.12
Beigmohamadi, M., Movafeghi, A., Sharafi, A., Jafari, S., Danafar, H. (2019). Cell suspension culture of Plumbago europaea l. towards production of plumbagin. Iranian J. Biotechnol. 17 (2), e2169. doi: 10.21859/ijb.2169
Bekkar, N. E. H., Meddah, B., Cakmak, Y. S., Keskin, B. (2021). Phenolic composition, antioxidant and antimicrobial activities of Zizyphus lotus l. and Ruta chalepensis l. growing in mascara (Western Algeria). J. Microbiol. Biotechnol. Food Sci. 10 (5), e3004–e3004. doi: 10.15414/jmbfs.3004
Bello-Martínez, G., García-Ramírez, G., Olea-Flores, M., Navarro-Tito, N., Hernández-Moreno, A., Avila-Caballero, L. P., et al. (2022). Biological activity of Haematoxylum brasiletto in MCF7 and MDA-MB-231 breast cancer cell lines. South Afr. J. Bot. 146, 528–537. doi: 10.1016/j.sajb.2021.11.017
Bentebibel, S., Moyano, E., Palazón, J., Cusidó, R. M., Bonfill, M., Eibl, R., et al. (2005). Effects of immobilization by entrapment in alginate and scale-up on paclitaxel and baccatin III production in cell suspension cultures of taxus baccata. Biotechnol. Bioengineering 89 (6), 647–655. doi: 10.1002/bit.20321
Bergman, M. E., Phillips, M. A. (2021). Structural diversity and biosynthesis of plant derived p-menthane monoterpenes. Phytochem. Rev. 20 (2), 433–459. doi: 10.1007/s11101-020-09726-0
Bhaskar, R., Xavier, L. S. E., Udayakumaran, G., Kumar, D. S., Venkatesh, R., Nagella, P. (2021). Biotic elicitors: a boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Culture 149 (1-2), 7–24. doi: 10.1007/s11240-021-02131-1
Bhatia, S., Dahiya, R. (2015). Concepts and techniques of plant tissue culture science. Modern Appl. Plant Biotechnol. Pharm. Sci., 121–156. doi: 10.1016/B978-0-12-802221-4.00004-2
Bose, B., Tripathy, D., Chatterjee, A., Tandon, P., Kumaria, S. (2019). Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine 55, 58–69. doi: 10.1016/j.phymed.2018.08.010
Brahim, R. M. S., Lemrabout, A., Taleb, R. (2021). Effect of treatment with electric field pulse on the extraction of polyphenols. iKSP J. Emerging Trends Basic Appl. Sci. 1 (1), 22–29.
Bravo-Ruiz, I. N., González-Arnao, M. T., Castañeda-Castro, O., Pastelín-Solano, M. C., Cruz-Cruz, C. A. (2022). “Use of thin cell layer (TCL) to obtain somatic embryogenesis,” in Somatic embryogenesis: methods and protocols (New York: NY: Springer US), 183–201.
Bribi, N. (2018). Pharmacological activity of alkaloids: a review. Asian J. Bot. 1 (1), 1–6. doi: 10.63019/ajb.v1i2.467
Bridgen, M. P., Van Houtven, W., Eeckhaut, T. (2018). “Plant tissue culture techniques for breeding,” in Ornamental crops (Cham: Springer), 127–144.
Bulgakov, V. P., Veselova, M. V., Tchernoded, G. K., Kiselev, K. V., Fedoreyev, S. A., Zhuravlev, Y. N. (2005). Inhibitory effect of the agrobacterium rhizogenes rolC gene on rabdosiin and rosmarinic acid production in Eritrichium sericeum and Lithospermum erythrorhizon transformed cell cultures. Planta 221 (4), 471–478. doi: 10.1007/s00425-004-1457-5
Cabanas-Garcia, E., Areche, C., Gómez-Aguirre, Y. A., Borquez, J., Munoz, R., Cruz-Sosa, et al. (2021). Biomass production and secondary metabolite identification in callus cultures of Coryphantha macromeris (Engelm.) britton & rose (Cactaceae), a traditional medicinal plant. South Afr. J. Bot. 137, 1–9. doi: 10.1016/j.sajb.2020.10.002
Cano-Avendaño, B. A., Carmona-Hernandez, J. C., Rodriguez, R. E., Taborda-Ocampo, G., González-Correa, C. H. (2021). Chemical properties of polyphenols: a reviewfocusedonanti-inflammatory and anti-viral medical application. Biomedicine 41 (1), 3–8. doi: 10.51248/.v41i1.524
Cano Ortiz, A., JC, P. F., Cano, E. (2022). Some medicinal plants of interest for their content in alkaloids. I. Biomed. J. Sci. Tech. Res. 42 (3), 33702–33705. doi: 10.20431/2349-0381.0905005
Caretto, S., Bray Speth, E., Fachechi, C., Gala, R., Zacheo, G., Giovinazzo, G. (2004). Enhancement of vitamin e production in sunflower cell cultures. Plant Cell Rep. 23, 174–179. doi: 10.1007/s00299-004-0799-6
Carsono, N., Tumilaar, S. G., Kurnia, D., Latipudin, D., Satari, M. H. (2022). A review of bioactive compounds and antioxidant activity properties of piper species. Molecules 27 (19), 6774. doi: 10.3390/molecules27196774
Cavallaro, V., Pellegrino, A., Muleo, R., Forgione, I. (2022). Light and plant growth regulators on in vitro proliferation. Plants 11, 844. doi: 10.3390/plants11070844
Chadipiralla, K., Gayathri, P., Rajani, V., Reddy, P. V. B. (2020). “Plant tissue culture and crop improvement,” in Sustainable agriculture in the era of climate change (Cham: Springer), 391–412.
Chandana, B. C., Kumari Nagaveni, H. C., Heena, M. S., Shashikala, S. K., Lakshmana, D. (2018). Role of plant tissue culture in micropropagation, secondary metabolites production and conservation of some endangered medicinal crops. J. Pharmacogn. Phytochem. 7 (3S), 246–251.
Chaudhary, M. (2022). “Role of plant secondary metabolites as modulators of multidrug resistance in cancer therapy,” in Plant secondary metabolites (Singapore: Springer), 415–435.
Chen, E. W. C., Wong, S. K., Chan, H. T. (2021). An overview of the chemistry and anticancer properties of rosemary extract and its diterpenes. J. Herbmed Pharmacol. 11 (1), 10–19. doi: 10.34172/jhp.2022.02
Chen, H., Zhang, T. (2021). Isolation and structure elucidation of hypoglycemic compounds. Structure Health Effects Natural Prod. Diabetes Mellitus, 103–128. doi: 10.1007/978-981-15-8791-7_6
Cheng, X., Bi, L., Zhao, Z., Chen, Y. (2015). “Advances in enzyme assisted extraction of natural products,” in 3rd International conference on material, mechanical and manufacturing engineering (IC3ME 2015). 371–375 (Guangzhou, China: Atlantis Press).
Chiocchio, I., Mandrone, M., Tomasi, P., Marincich, L., Poli, F. (2021). Plant secondary metabolites: an opportunity for circular economy. Molecules 26 (2), 495. doi: 10.3390/molecules26020495
Choi, Y. J., Alishir, A., Jang, T., Kang, K. S., Lee, S., Kim, K. H. (2022). Antiskin aging effects of indole alkaloid n-glycoside from ginkgo fruit (Ginkgo biloba fruit) on TNF-α-Exposed human dermal fibroblasts. J. Agric. Food Chem. 70 (42), 13651–13660. doi: 10.1021/acs.jafc.2c05769
Chopra, S., Samuel, J. (2020). Co-Evolutionary relationship between plants and phytopathogens. Int. J. Res. Analytical Rev. 7 (2), 1133–1150.
Clapa, D., Nemes, S. A., Ranga, F., Hârta, M., Vodnar, D. C., Călinoiu, L. F. (2022). Micropropagation of Vaccinium corymbosum l.: an alternative procedure for the production of secondary metabolites. Horticulturae 8 (6), 480. doi: 10.3390/horticulturae8060480
Cortes-Morales, J. A., López-Laredo, A. R., Zamilpa, A., Bermúdez-Torres, K., Trejo-Espino, J. L., Trejo-Tapia, G. (2018). Morphogenesis and secondary metabolites production in the medicinal plant Castilleja tenuiflora benth. under nitrogen deficiency and starvation stress in a temporary immersion system. Rev. Mexicana Ingeniería Química 17 (1), 229–242.
Coskun, D., Deshmukh, R., Sonah, H., Menzies, J. G., Reynolds, O., Ma, J. F., et al. (2019). And The controversies of silicon's role in plant biology. New Phytol. 221 (1), 67–85. doi: 10.1111/nph.15343
Croteau, R., Ketchum, R. E., Long, R. M., Kaspera, R., Wildung, M. R. (2006). Taxol biosynthesis and molecular genetics. Phytochem. Rev. 5, 75–97. doi: 10.1007/s11101-005-3748-2
Cui, Y., Deng, Y., Zheng, K., Hu, X., Zhu, M., Deng, X., et al. (2019). An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae noot. & chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 9, 9634. doi: 10.1038/s41598-019-46050-w
Dable-Tupas, G., Tulika, V., Jain, V., Maheshwari, K., Brakad, D. D., Naresh, P. N., et al. (2023). “Bioactive compounds of nutrigenomic importance,” in Role of nutrigenomics in modern-day healthcare and drug discovery (Elsevier), 301–342.
Daffalla, H. M., Elsheikh, A. M. (2018). Secondary metabolites accumulation and production through in vitro cultures. Phytochem. Mar. Sources Ind. Applications Recent Adv. 131.
Dagustu, N. (2018). Use of plant tissue culture practices in breeding studies. Türktob Dergisi 25, 23–26.
Dai, J., Mumper, R. J. (2010). Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15 (10), 7313–7352. doi: 10.3390/molecules15107313
Dang, T. T., Bowyer, M. C., Van Altena, I. A., Scarlett, C. J. (2018). Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 53, 1711–1723. doi: 10.1080/01496395.2017.1414845
Darshani, P., Sen Sarma, S., Srivastava, A. K., Baishya, R., Kumar, D. (2022). Anti-viral triterpenes: a review. Phytochem. Rev. 21 (6), 1761–1842. doi: 10.1007/s11101-022-09808-1
da Silveira Vasconcelos, M., de Oliveira, L. M. N., Nunes-Pinheiro, D. C. S., da Silva Mendes, F. R., de Sousa, F. D., de Siqueira Oliveira, L., et al. (2020). “Analysis of tetraterpenes and tetraterpenoids (carotenoids),” in Recent advances in natural products analysis. Ed. Eryilmaz, K. (Amsterdam: Elsevier), 427–456.
Debnath, S. C., Goyali, J. C. (2020). In vitro propagation and variation of antioxidant properties in micropropagated vaccinium berry plants–a review. Molecules 25 (4), 788. doi: 10.3390/molecules25040788
Deepthi, S., Satheeshkumar, K. (2017). Cell line selection combined with jasmonic acid elicitation enhance camptothecin production in cell suspension cultures of Ophiorrhiza mungos l. Appl. Microbiol. Biotechnol. 101 (2), 545–558. doi: 10.1007/s00253-016-7808-x
Dehghan, E., Häkkinen, S. T., Oksman-Caldentey, K. M., Shahriari Ahmadi, F. (2012). Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus l.). Plant Cell Tissue Organ Culture (PCTOC) 110 (1), 35–44. doi: 10.1007/s11240-012-0127-8
Delazar, A., Nahar, L., Hamedeyazdan, S., Sarker, S. D. (2012). Microwave-assisted extraction in natural products isolation. Natural Prod. Isolation, 89–115. doi: 10.1007/978-1-61779-624-1_5
Demir, T., Akpınar, Ö., Kara, H., Güngör, H. (2022). Phenolic profile and investigation of biological activities of Allium scorodoprasum l. subsp. rotundum. Food Biosci. 46, 101548. doi: 10.1016/j.fbio.2022.101548
Desam, N. R., Al-Rajab, A. J. (2022). “Herbal biomolecules: anticancer agents,” in Herbal biomolecules in healthcare applications (Academic Press), 435–474.
Diyabalanage, T. (2022). Plant secondary metabolites as prospective pharmaceuticals and cosmeceuticals. Chem. Natural Prod.: Phytochem. Pharmacognosy Med. Plants 19, 19–39. doi: 10.1515/9783110595949-002
Dörnenburg, H. (2008). Plant cell culture technology–harnessing a biological approach for competitive cyclotides production. Biotechnol. Lett. 30, 1311–1321. doi: 10.1007/s10529-008-9704-7
dos Santos Franciscato, L. M. S., Ariati, A. M., Picolloto, A. M., Raia, R. Z., Barbosa, V. A., Bittencourt, P. R. S., et al. (2022). Thermal properties of cinnamon (Cinnamomum verum) essential oil and its antibacterial activity. Res. Soc. Dev. 11 (13), e567111335942–e567111335942. doi: 10.33448/rsd-v11i13.35942
Ebrahimi, S., Zaker, A., Abrishamchi, P., Bahrami, A. R., Ganjeali, A., Sodagar, N. (2017). Hairy root induction and secondary metabolite production in Perovskia abrotanoides karel. J. Plant Process Funct. 6 (20), 17–26.
Efferth, T. (2019). Biotechnology applications of plant callus cultures. Engineering 5 (1), 50–59. doi: 10.1016/j.eng.2018.11.006
Eguchi, R., Ono, N., Hirai Morita, A., Katsuragi, T., Nakamura, S., Huang, M., et al. (2019). Classification of alkaloids according to the starting substances of their biosynthetic pathways using graph convolutional neural networks. BMC Bioinf. 20, 380. doi: 10.1186/s12859-019-2963-6
Eibl, R., Meier, P., Stutz, I., Schildberger, D., Hühn, T., Eibl, D. (2018). Plant cell culture technology in the cosmetics and food industries: current state and future trends. Appl. Microbiol. Biotechnol. 102, 8661–8675. doi: 10.1007/s00253-018-9279-8
Eibl, R., Eibl, D. (2009). “Plant cell-based bioprocessing,” in Cell and tissue reaction engineering: principles and practice. Eds. Eibl, R., Eibl, D., Portner, R., Catapano, G., Czermak, P. (Berlin/Heidelberg: Springer-Verlag), 315–355.
Eisa, E. A., Tilly-Mándy, A., Honfi, P., Shala, A. Y., Gururani, M. A. (2022). Chrysanthemum: a comprehensive review on recent developments on In vitro regeneration. Biology 11 (12), 1774. doi: 10.3390/biology11121774
El-Beltagi, H. S., Mohamed, H. I., Aldaej, M. I., Al-Khayri, J. M., Rezk, A. A., Al-Mssallem, M. Q., et al. (2022). Production and antioxidant activity of secondary metabolites in hassawi rice (Oryza sativa l.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitro Cell. Dev. Biol.-Plant 58 (4), 615–629. doi: 10.1007/s11627-022-10264-x
El-Sherif, N. A. (2018). “Impact of plant tissue culture on agricultural sustainability,” in Sustainability of agricultural environment in Egypt: part II (Cham: Springer), 93–107.
Emara, H. A., Nower, A. A., Hamza, E. M., El Shaib, F. (2018). Evaluation of photomixotrophic technique and several carbohydrate sources as affecting banana micropropagation. Int. J. Curr. Microbiol. Appl. Sci. 7, 788–804. doi: 10.20546/ijcmas.2018.710.088
Erb, M., Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 184 (1), 39–52. doi: 10.1104/pp.20.00433
Erdem, M., Uysal, H. (2021). Sentetik tohum. Front. Life Sci. RT 2 (2), 68–74. doi: 10.51753/flsrt.943981
Fachechi, C., Nisi, R., Gala, R., Leone, A., Caretto, S. (2007). Tocopherol biosynthesis is enhanced in photomixotrophic sunflower cell cultures. Plant Cell Rep. 26, 525–530. doi: 10.1007/s00299-006-0268-5
Fazili, M. A., Bashir, I., Ahmad, M., Yaqoob, U., Geelani, S. N. (2022). In vitro strategies for the enhancement of secondary metabolite production in plants: a review. Bull. Natl. Res. Centre 46 (1), 1–12. doi: 10.1186/s42269-022-00717-z
Feher, A. (2019). Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front. Plant Sci. 10, 536. doi: 10.3389/fpls.2019.00536
Filová, A. (2014). Production of secondary metabolities in plant tissue cultures. Res. J. Agric. Sci. 46 (1), 236–245.
Finer, J., Dhillon, T. (2016). Transgenic plant production. Plant Biotechnol. Genetics: Principles Techniques Appl., 245–274.
Fischer, R., Schillberg, S., Hellwig, S., Twyman, R. M., Drossard, J. (2012). GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnol. Adv. 30 (2), 434–439. doi: 10.1016/j.biotechadv.2011.08.007
Fraga-Corral, M., Otero, P., Echave, J., Garcia-Oliveira, P., Carpena, M., Jarboui, A., et al. (2021). By-products of agri-food industry as tannin-rich sources: a review of tannins’ biological activities and their potential for valorization. Foods 10 (1), 137. doi: 10.3390/foods10010137
Fujiki, H., Imai, K., Nakachi, K., Shimizu, M., Moriwaki, H., Suganuma, M. (2012). Challenging the effectiveness of green tea in primary and tertiary cancer prevention. J. Cancer Res. Clin. Oncol. 138, 1259–1270. doi: 10.1007/s00432-012-1250-y
Gago, D., Sánchez, C., Aldrey, A., Christie, C. B., Bernal, M.Á., Vidal, N. (2022). Micropropagation of plum (Prunus domestica l.) in bioreactors using photomixotrophic and photoautotrophic conditions. Horticulturae 8 (4), 286. doi: 10.3390/horticulturae8040286
Gala, R., Mita, G., Caretto, S. (2005). Improving α-tocopherol production in plant cell cultures. J. Plant Physiol. 162 (7), 782–784. doi: 10.1016/j.jplph.2005.04.010
Gao, L., Shen, G., Zhang, L., Qi, J., Zhang, C., Ma, C., et al. (2019). An efficient system composed of maize protoplast transfection and HPLC–MS for studying the biosynthesis and regulation of maize benzoxazinoids. Plant Methods 15 (1), 1–13. doi: 10.1186/s13007-019-0529-2
Gaosheng, H., Jingming, J. (2012). Production of useful secondary metabolites through regulation of biosynthetic pathway in cell and tissue suspension culture of medicinal plants. Recent Adv. Plant Vitro Culture, 197–210. doi: 10.5772/53038
García-Gonzáles, R., Quiroz, K., Carrasco, B., Caligari, P. (2010). Plant tissue culture: current status, opportunities and challenges. Int. J. Agric. Natural Resour. 37 (3), 5–30. doi: 10.4067/S0718-16202010000300001
Geipel, J., Link, J., Claupein, W. (2014). Combined spectral and spatial modeling of corn yield based on aerial images and crop surface models acquired with an unmanned aircraft system. Remote Sens. 6 (11), 10335–10355. doi: 10.3390/rs61110335
Georgiev, M. I., Agostini, E., Ludwig-Müller, J., Xu, J. (2012). Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol. 30 (10), 528–537. doi: 10.1016/j.tibtech.2012.07.001
Georgiev, M. I., Weber, J. (2014). Bioreactors for plant cells: hardware configuration and internal environment optimization as tools for wider commercialization. Biotechnol. Lett. 36 (7), 1359–1367. doi: 10.1007/s10529-014-1498-1
Geow, C. H., Tan, M. C., Yeap, S. P., Chin, N. L. (2021). A review on extraction techniques and its future applications in industry. Eur. J. Lipid Sci. Technol. 123 (4), 2000302. doi: 10.1002/ejlt.202000302
Getachew, A. T., Jacobsen, C., Holdt, S. L. (2020). Emerging technologies for the extraction of marine phenolics: opportunities and challenges. Mar. Drugs 18 (8), 389. doi: 10.3390/md18080389
Gilbertson, G., Koenig, R. T. (1981). Essential oils and related products. Analytical Chem. 53 (5), 61–77. doi: 10.1021/ac00228a006
Ginwala, R., Bhavsar, R., Chigbu, D. G. I., Jain, P., Khan, Z. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 8 (2), 35. doi: 10.3390/antiox8020035
Giri, A., Narasu, M. L. (2000). Transgenic hairy roots: recent trends and applications. Biotechnol. Adv. 18 (1), 1–22. doi: 10.1016/S0734-9750(99)00016-6
Goyal, P., Manzoor, M. M., Gupta, A. P., Pandotra, P., Gupta, S. (2022). Molecular dissection of genes and promoters involved in glycyrrhizin biosynthesis revealed phytohormone induced modulation in Glycyrrhiza glabra l. Gene 836, 146682. doi: 10.1016/j.gene.2022.146682
Grzegorczyk-Karolak, I., Kuźma, Ł., Skała, E., Kiss, A. K. (2018). Hairy root cultures of salvia viridis l. for production of polyphenolic compounds. Ind. Crops Prod. 117, 235–244. doi: 10.1016/j.indcrop.2018.03.014
Grzegorczyk-Karolak, I., Rytczak, P., Bielecki, S., Wysokińska, H. (2017). The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tissue Organ Culture 128 (2), 479–486. doi: 10.1007/s11240-016-1126-y
Grzegorczyk-Karolak, I., Staniewska, P., Lebelt, L., Piotrowska, D. G. (2022). Optimization of cultivation conditions of Salvia viridis l. shoots in the plantform bioreactor to increase polyphenol production. Plant Cell Tissue Organ Culture 149 (1), 269–280. doi: 10.1007/s11240-021-02168-2
Guillon, S., Tremouillaux-Guiller, J., Pati, P. K., Rideau, M., Gantet, P. (2006). Hairy root research: recent scenario and exciting prospects. Curr. Opin. Plant Biol. 9 (3), 341–346. doi: 10.1016/j.pbi.2006.03.008
Gul, M. Z., Yasin Bhat, Y., Kumar, A., Rao, B. S. (2018). “Molecular pharming (pharmaceuticals): primary and secondary metabolites in plants,” in Advanced molecular plant breeding, meeting the challenges of food security (Palm Bay, FL: Apple Academic Press, Inc.(CRC Press), a Taylor & Francis Group), 397–432.
Gulsunoglu, Z., Karbancioglu-Guler, F., Raes, K., Kilic-Akyilmaz, M. (2019). Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. Int. J. Food Properties 22 (1), 1501–1510. doi: 10.1080/10942912.2019.1656233
Gumerov, F. M., Khairutdinov, V. F., Zaripov, Z. I. (2021). An additional condition of efficiency of the supercritical fluid extraction process. Theor. Foundations Chem. Eng. 55 (3), 348–358. doi: 10.1134/S0040579521030076
Gupta, S., Acharya, R., Gamit, R. V., Shukla, V. J. (2021). Quantitative analysis of tannins, alkaloids, phenols, and flavonoids in ficus semicordata leaf, stem, stem bark, root, and fruit powder. J. Indian System Med. 9 (3), 171. doi: 10.4103/JISM.JISM_16_21
Gutierrez-Gamboa, G., Mateluna-Cuadra, R., Díaz-Galvez, I., Mejia, N., Verdugo-Vasquez, N. (2021). Methyl jasmonate applications in viticulture: a tool to increase the content of flavonoids and stilbenes in grapes and wines. Horticulturae 7 (6), 133. doi: 10.3390/horticulturae7060133
Haberlandt, G. (1902). Uber die statolithefunktion der starkekoner. Ber. Dtsch. Bot. Ges. 20, 189–195.
Haghighi, K., Ali, M. (2021). Evaluation of the hormonal treatments effect on biosynthesis of endol alkaloids in tissue culture, suspension culture and field culture. Dev. Biol. 13 (1), 1–16. doi: 10.30495/JDB.2021.680719
Häkkinen, S. T., Moyano, E., Cusidó, R. M., Oksman-Caldentey, K. M. (2016). Exploring the metabolic stability of engineered hairy roots after 16 years maintenance. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01486
Hakkinen, S. T., Oksman-Caldentey, K. M. (2018). “Progress and prospects of hairy root research,” in Hairy roots (Singapore: Springer), 3–19.
Halder, M., Sarkar, S., Jha, S. (2019). Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 19 (12), 880–895. doi: 10.1002/elsc.201900058
Hall, R. D., Yeoman, M. M. (1987). Intercellular and intercultural heterogeneity in secondary metabolite accumulation in cultures of catharanthus roseus following cell line selection. J. Exp. Bot. 38 (8), 1391–1398. doi: 10.1093/jxb/38.8.1391
Hao, Y. J., Cui, X. H., Li, J. R., An, X. L., Sun, H. D., Piao, X. C., et al. (2020). Cell bioreactor culture of Orostachys cartilaginous a. bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis. Acta Physiol. Plantarum 42 (1), 1–10. doi: 10.1007/s11738-019-3008-5
He, Z., Chen, Y., Chen, Y., Liu, H., Yuan, G., Fan, Y., et al. (2013). Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin. J. Oceanol. Limnol. 31, 1045–1054. doi: 10.1007/s00343-013-2321-x
Herrera, E., Pacheco, C., Olivera, L. (2022). “Extraction and characterization of chamomile (Matricaria recutita l.) essential oil using the green technology of solvent-free microwave extraction,” in Biol. life sci. forum, vol. 2. (Basel, Switzerland: MDPI) .
Heydari, H. R., Chamani, E., Esmaeilpour, B. (2020). Effect of total nitrogen content and NH4+/NO3-ratio on biomass accumulation and secondary metabolite production in cell culture of salvia nemorosa. Iranian J. Genet. Plant Breed. 9 (1), 17–27. doi: 10.30479/IJGPB.2020.12321.1258
Hidalgo, D., Sanchez, R., Lalaleo, L., Bonfill, M., Corchete, P., Palazon, J. (2018). Biotechnological production of pharmaceuticals and biopharmaceuticals in plant cell and organ cultures. Curr. Med. Chem. 25 (30), 3577–3596. doi: 10.2174/0929867325666180309124317
Hima, S., Midhu, C. K., Krishnakumar, G., Satheeshkumar, K. (2019). In vitro seedlings as dynamic explants for establishment of root cultures of Pyrenacantha volubilis hook. for camptothecin production. Proc. Natl. Acad. Sci. India Section B.: Biol. Sci. 90, 405–413. doi: 10.1007/s40011-019-01113-w
Ho, W. W. S., Ng, H. K., Gan, S. (2016). Advances in ultrasound-assisted transesterification for biodiesel production. Appl. Thermal. Eng. 100, 553–563. doi: 10.1016/j.applthermaleng.2016.02.058
Honda, H., Liu, C., Kobayashi, T. (2001). “Large-Scale plant micropropagation,” in Advances in biochemical engineering/biotechnology. Ed. Zhong, J. J. (Berlin/Heidelberg: Springer), 157–182.
Hossain, M. T., Furhatun-Noor, M., Asadujjaman, M. A. M., Tabassum, F., Rashid, M. H. A. A. (2021). Review study on the pharmacological effects and mechanism of action of tannins. ejpmr 8 (8), 05–10.
Hu, Z. B., Du, M. (2006). Hairy root and its application in plant genetic engineering. J. Integr. Plant Biol. 48 (2), 121–127. doi: 10.1111/j.1744-7909.2006.00121.x
Huang, T. K., McDonald, K. A. (2012). Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol. Adv. 30 (2), 398–409. doi: 10.1016/j.biotechadv.2011.07.016
Hussain, M. S., Fareed, S., Ansari, S., Rahman, M. A., Ahmad, I. Z., Saeed, M. (2012). Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 6 (10), 1–28. doi: 10.4103/0975-7406.92725
Hussein, R. A., El-Anssary, A. A. (2019). Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal Med. 1, 13. doi: 10.5772/intechopen.76139
Huyop, F. Z., Yilmaz, K., Faraj, M. (2019). Tissue culture and transformation of eggplant with synthetic gene (Riga Latvia: Scholars' Press), 978–613-8-83340-6.
Iarema, L., da Cruz, A. C. F., Saldanha, C. W., Dias, L. L. C., Vieira, R. F., de Oliveira, E. J., et al. (2012). Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) pedersen]. Plant Cell Tissue Organ Culture (PCTOC) 110, 227–238. doi: 10.1007/s11240-012-0145-6
Ignea, C., Raadam, M. H., Koutsaviti, A., Zhao, Y., Duan, Y. T., Harizani, M., et al. (2022). Expanding the terpene biosynthetic code with non-canonical 16 carbon atom building blocks. Nat. Commun. 13 (1), 1–16. doi: 10.1038/s41467-022-32921-w
Iqbal, N., Khan, N. A., Ferrante, A., Trivellini, A., Francini, A., Khan, M. I. R. (2017). Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front. Plant Sci. 8, 475. doi: 10.3389/fpls.2017.00475
Isah, T., Umar, S., Mujib, A., Sharma, M. P., Rajasekharan, P. E., Zafar, N., et al. (2017). Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Culture (PCTOC) 132 (2), 239–265. doi: 10.1007/s11240-017-1332-2
Jabran, K., Mahajan, G., Sardana, V., Chauhan, B. S. (2015). Allelopathy for weed control in agricultural systems. Crop Prot. 72, 57–65. doi: 10.1016/j.cropro.2015.03.004
Jain, C., Khatana, S., Vijayvergia, R. (2019). Bioactivity of secondary metabolites of various plants: a review. Int. J. Pharm. Sci. Res. 10 (2), 494–504. doi: 10.13040/IJPSR.0975-8232.10(2).494-04
Jasuja, N. D. (2022). The role of salicylic acid in elicitation for production of secondary metabolites in Cayratia trifolia cell suspension culture. Med. Plants-International J. Phytomed. Related Industries 14 (1), 113–118. doi: 10.5958/0975-6892.2022.00012.0
Jeet, A., Singh, Y., Singh, P., Nimoriya, R., Bilung, C. J., Kanojiya, S., et al. (2020). Strategies for indole alkaloids enrichment through callus culture from alstonia scholaris (L.) r. br. Plant Growth Regul. 90, 383–392. doi: 10.1007/s10725-019-00570-7
Jesionek, A., Kokotkiewicz, A., Krolicka, A., Zabiegala, B., Luczkiewicz, M. (2018). Elicitation strategies for the improvement of essential oil content in rhododendron tomentosum (Ledum palustre) bioreactor-grown microshoots. Ind. Crops Prod. 123, 461–469. doi: 10.1016/j.indcrop.2018.07.013
Jiao, J., Gai, Q. Y., Wang, X., Qin, Q. P., Wang, Z. Y., Liu, J., et al. (2018). Chitosan elicitation of Isatis tinctoria l. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crops Prod. 124, 28–35. doi: 10.1016/j.indcrop.2018.07.056
Jie-Wei, W. U., Chun-Ping, T. A. N. G., Sheng, Y. A. O., Chang-Qiang, K. E., Yang, Y. E. (2021). Three new carabrane sesquiterpenoid derivatives from the whole plant of Carpesium abrotanoides l. Chin. J. Natural Medicines 19 (11), 868–873. doi: 10.1016/S1875-5364(21)60091-2
Jing, W., Xiaolan, C., Yu, C., Feng, Q., Haifeng, Y. (2022). Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 154, 113561. doi: 10.1016/j.biopha.2022.113561
Joo, S. J., Yoon, A. R., Kim, Y. G., Moon, B. C., Komakech, R., Kang, Y. (2019). In vitro propagation of trichosanthes kirilowii maxim. through nodal segment shoot proliferation. Vitro Cell Dev. Biol. Plant 55, 702–709. doi: 10.1007/s11627-019-10010-w
Kang, H. H., Naing, A. H., Kim, C. K. (2019). Optimization of an afficient protocol for protoplast isolation and callus induction from petunia hybrida cv. mirage rose. Optimization 252, 3.
Kanthaliya, B., Joshi, A., Arora, J. (2019). Evaluation of isoflavonoid content in context to tuber size and seed biology study of Pueraria tuberosa (Roxb. ex. willd.) DC: a vulnerable medicinal plant. Vegetos 32 (3), 247–253. doi: 10.1007/s42535-019-00042-3
Kapusi, E., Stoger, E. (2022). “Molecular farming in seed crops: gene transfer into barley (Hordeum vulgare) and wheat (Triticum aestivum),” in Recombinant proteins in plants (New York, NY: Humana), 49–60.
Karahan, F., Ozyigit, I. I., Saracoglu, I. A., Yalcin, I. E., Ozyigit, A. H., Ilcim, A. (2020). Heavy metal levels and mineral nutrient status in different parts of various medicinal plants collected from eastern Mediterranean region of Turkey. Biol. Trace Element Res. 197 (1), 316–329. doi: 10.1007/s12011-019-01974-2
Karak, P. (2019). Biological activities of flavonoids: an overview. Int. J. Pharm. Sci. Res. 10 (4), 1567–1574. doi: 10.13040/IJPSR.0975-8232.10(4).1567-74
Karakas, F. P. (2020). Efficient plant regeneration and callus induction from nodal and hypocotyl explants of goji berry (Lycium barbarum l.) and comparison of phenolic profiles in calli formed under different combinations of plant growth regulators. Plant Physiol. Biochem. 146, 384–391. doi: 10.1016/j.plaphy.2019.11.009
Karakas, F. P., Bozat, B. G. (2020). Fluctuation in secondary metabolite production and antioxidant defense enzymes in in vitro callus cultures of goat’s rue (Galega officinalis) under different abiotic stress treatments. Plant Cell Tissue Organ Culture (PCTOC) 142, 401–414. doi: 10.1007/s11240-020-01870-x
Karpiński, T. M., Ożarowski, M., Alam, R., Łochyńska, M., Stasiewicz, M. (2021). What do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 20 (1), 36. doi: 10.3390/md20010036
Kaur, P., Pandey, D. K., Gupta, R. C., Kumar, V., Dwivedi, P., Sanyal, R., et al. (2021). Biotechnological interventions and genetic diversity assessment in swertia sp.: a myriad source of valuable secondary metabolites. Appl. Microbiol. Biotechnol. 105 (11), 4427–4451. doi: 10.1007/s00253-021-11345-4
Kaur, K., Pati, P. K. (2018). “Stress-induced metabolite production utilizing plant hairy roots,” in Hairy roots. Eds. Srivastava, V., Mehrotra, S., Mishra, S. (Singapore: Springer), 123–145.
Kaya, Y., Aksoy, H. M., Edbeib, M. F., Wahab, R. A., Ozyigit, I. I., Hamid, A. A. A., et al. (2020). Agrobacterium-mediated transformation of Turkish upland rice (Oryza sativa l.) for dalapon herbicide tolerance. Indian J. Biotechnol. 19, 237–243.
Kayser, O., Wim, J. Q. (2006). Medicinal plant biotechnology: from basic research to industrial applications (WILEY-VCH Verlag GmbH & Co. KGaA).
Kemboi, D., Siwe-Noundou, X., Krause, R. W., Langat, M. K., Tembu, V. J. (2021). Euphorbia diterpenes: an update of isolation, structure, pharmacological activities and structure–activity relationship. Molecules 26 (16), 5055. doi: 10.3390/molecules26165055
Kessler, J. C., Vieira, V. A., Martins, I. M., Manrique, Y. A., Afonso, A., Ferreira, P., et al. (2022). Obtaining aromatic extracts from portuguese Thymus mastichina l. by hydrodistillation and supercritical fluid extraction with CO2 as potential flavouring additives for food applications. Molecules 27 (3), 694. doi: 10.3390/molecules27030694
Khalofah, A., Kilany, M., Migdadi, H. (2021). Assessment of morpho-physiological and biochemical responses of mercury-stressed Trigonella foenum-gracum l. @ to silver nanoparticles and sphingobacterium ginsenosidiumtans applications. Plants 10 (7), 1349. doi: 10.3390/plants10071349
Khan, T., Khan, T., Hano, C., Abbasi, B. H. (2019). Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of fagonia indica. Ind. Crops Prod. 129, 525–535. doi: 10.1016/j.indcrop.2018.12.048
Khanam, M. N., Anis, M., Javed, S. B., Mottaghipisheh, J., Csupor, D. (2022). Adventitious root culture-an alternative strategy for secondary metabolite production: a review. Agronomy 12 (5), 1178. doi: 10.3390/agronomy12051178
Khanbabaee, K., Van Ree, T. (2001). Tannins: classification and definition. Natural Prod. Rep. 18 (6), 641–649.
Kharde, A. V., Kore, S. V., Khetmalas, M. B. (2018). Elicitation of bacoside content using plant growth regulators in cell suspension culture of Bacopa monnieri (L.) wettst. Plant Tissue Culture Biotechnol. 28 (2), 191–199. doi: 10.3329/ptcb.v28i2.39678
Khongthaw, B., Chauhan, P. K., Dulta, K., Kumar, V., Ighalo, J. O. (2022). A comparison of conventional and novel phytonutrient extraction techniques from various sources and their potential applications. J. Food Measurement Characterization 17 (2), 1317–1342. doi: 10.1007/s11694-022-01697-4
King, P. J. (1984). “Induction and maintenance of cell suspension cultures,” in Cell culture and somatic cell genetics of plants, vol. 1 . Ed. Vasil, I. K. (Orlando: Academic Press), 130–138.
Kirschning, A., Dräger, G., Tran, C. D., Struwe, H., Siedenberg, L., Grunenberg, J., et al. (2022). Cyclopropylmethyldiphosphates are substrates for the sesquiterpene synthases: experimental and theoretical results. Organic Biomol. Chem. 20, 7833–7839. doi: 10.1039/D2OB01279K
Kiselev, K. V., Kusaykin, M. I., Dubrovina, A. S., Bezverbny, D. A., Zvyagintseva, T. N., Bulgakov, V. P. (2006). The rolC gene induces expression of a pathogenesis-related β-1, 3-glucanase in transformed ginseng cells. Phytochemistry 67 (20), 2225–2231. doi: 10.1016/j.phytochem.2006.07.019
Koleckar, V., Kubikova, K., Rehakova, Z., Kuca, K., Jun, D., Jahodar, L., et al. (2008). Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Medicinal Chem. 8 (5), 436–447. doi: 10.2174/138955708784223486
Kong, C. H., Xuan, T. D., Khanh, T. D., Tran, H. D., Trung, N. T. (2019). Allelochemicals and signaling chemicals in plants. Molecules 24 (15), 2737. doi: 10.3390/molecules24152737
Korde, V. V., Dhas, S. S., Gurave, N. A., Plant, A. J., Res, S. (2016). Hairy root culture: a promising approach in biotransformation. Asian J. Plant Sci. 6, 6–11.
Kozai, T. (2010). Photoautotrophic micropropagation-environmental control for promoting photosynthesis. Propagation Ornamental Plants 10 (4), 188–204.
Krasteva, G., Georgiev, V., Pavlov, A. (2021). Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 21 (3-4), 68–76. doi: 10.1002/elsc.202000078
Kshyap, N., Kumari, A., Raina, N., Zakir, F., Gupta, M. (2021). Prospects of essential oil loaded nanosystems for skincare. Phytomed. Plus 100198. doi: 10.1016/j.phyplu.2021.100198
Kumar, P., Dixit, J., Saini, R., Verma, P., Mishra, A. K., NathTiwari, K. (2021). Potential of flavonoids as anticancer drugs. Phytopharmaceut.: Potential Ther. Appl., 135–159. doi: 10.1002/9781119682059.ch7
Kundu, S., Salma, U., Ali, M. N., Hazra, A. K., Mandal, N. (2018). Development of transgenic hairy roots and augmentation of secondary metabolites by precursor feeding in Sphagneticola calendulacea (L.) pruski. Ind. Crops Prod. 121, 206–215. doi: 10.1016/j.indcrop.2018.05.009
Kuru, I. S. (2023). The morpho-physiological responses of a tolerant and sensitive wheat (Triticum aestivum l.) cultivar to drought stress and exogenous methyl jasmonate. Front. Life Sci. RT 4 (1), 7–12. doi: 10.51753/flsrt.1162821
Kuzovkina, I. N., Schneider, B. (2006). Genetically transformed root cultures–generation, properties and application in plant sciences. Prog. Bot., 275–314. doi: 10.1007/3-540-27998-9_13
Laganà, P., Anastasi, G., Marano, F., Piccione, S., Singla, R. K., Dubey, A. K., et al. (2019). Phenolic substances in foods: health effects as anti-inflammatory and antimicrobial agents. J. AOAC Int. 102 (5), 1378–1387. doi: 10.5740/jaoacint.19-0131
Lambert, E., Geelen, D. (2010). High efficiency protoplast isolation from in vitro cultures and hairy roots of Maesa lanceolata. Afr. J. Biotechnol. 9 (42), 7071–7078. doi: 10.5897/AJB09.2011
Lesnik, S., Bren, U. (2021). Mechanistic insights into biological activities of polyphenolic compounds from rosemary obtained by inverse molecular docking. Foods 11 (1), 67. doi: 10.3390/foods11010067
Li, C., Cai, C., Zheng, X., Sun, J., Ye, L. (2020). Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Biosci. Biotechnol. Biochem. 84 (4), 774–779. doi: 10.1080/09168451.2019.1702871
Li, R., Li, Z., Leng, P., Hu, Z., Wu, J., Dou, D. (2021). Transcriptome sequencing reveals terpene biosynthesis pathway genes accounting for volatile terpene of tree peony. Planta 254 (4), 1–13. doi: 10.1007/s00425-021-03715-z
Liang, D., Li, W., Yan, X., Caiyin, Q., Zhao, G., Qiao, J. (2021). Molecular and functional evolution of the spermatophyte sesquiterpene synthases. Int. J. Mol. Sci. 22 (12), 6348. doi: 10.3390/ijms22126348
Lichtenthaler, H. K., Buschmann, C., Döll, M., Fietz, H. J., Bach, T., Kozel, U., et al. (1981). Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynthesis Res. 2, 115–141. doi: 10.1007/BF00028752
Liu, B., Liu, Q., Zhou, Z., Yin, H., Xie, Y. (2022). Overexpression of geranyl diphosphate synthase (PmGPPS1) boosts monoterpene and diterpene production involved in the response to pine wood nematode invasion. Tree Physiol. 42 (2), 411–424. doi: 10.1093/treephys/tpab103
Liu, N., Wu, X. Y., Song, Y. D., Gao, W., Huang, L. Q. (2021). Tissue culture of safflower and analysis of secondary metabolites in suspension cells. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater Med. 46 (17), 4380–4388. doi: 10.19540/j.cnki.cjcmm.20210523.105
Lo, M. M., Benfodda, Z., Bénimélis, D., Fontaine, J. X., Molinié, R., Meffre, P. (2021). Development of a HS-SPME/GC-MS method for the extraction and identification of the volatile compounds emitted by flowers of tillandsia xiphioides. ACS Omega 6 (19), 12691–12698. doi: 10.1021/acsomega.1c00917
Lopez, C. Q., Corral, P., Lorrain-Lorrette, B., Martinez-Swatson, K., Michoux, F., Simonsen, H. T. (2018). Use of a temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot cultures of thapsia garganica. Plant Methods 14 (1), 1–17. doi: 10.1186/s13007-018-0346-z
Loyola-Vargas, V. M., De-la-Peña, C., Galaz-Avalos, R. M., Quiroz-Figueroa, F. R. (2008). “Plant tissue culture,” in Molecular biomethods handbook (Humana Totowa, NJ: Humana Press), 875–904.
Luo, Y., Garmash, O., Li, H., Graeffe, F., Praplan, A. P., Liikanen, A., et al. (2021). Oxidation product characterization from ozonolysis of the diterpene ent-kaurene. Atmospheric Chem. Phys. Discussions. 22 (8), 5619–5637. doi: 10.5194/acp-2021-881
Lystvan, K., Belokurova, V., Sheludko, Y., Ingham, J. L., Prykhodko, V., Kishchenko, O., et al. (2010). Production of bakuchiol by in vitro systems of psoralea drupacea bge. Plant Cell Tissue Organ Culture (PCTOC) 101 (1), 99–103. doi: 10.1007/s11240-009-9657-0
Madigan, M. T., Martinko, J. M., Parker, J. (2003). Brock Biology of microorganisms (New Jersey: Pearson Education International, Upper Saddle River).
Magnusson, M., Yuen, A. K. L., Zhang, R., Wright, J. T., Taylor, R. B., Maschmeyer, T., et al. (2017). A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 23, 28–36. doi: 10.1016/j.algal.2017.01.002
Magray, J. A., Sharma, D. P., Deva, M. A., Thoker, S. A. (2023). Phenolics: accumulation and role in plants grown under heavy metal stress. Plant Phenolics Abiotic Stress Manage., 321–351. doi: 10.1007/978-981-19-6426-8_15
Mahendran, G., Iqbal, Z., Kumar, D., Verma, S. K., Rout, P. K., ur Rahman, L. (2021). Enhanced gymnemic acids production in cell suspension cultures of Gymnema sylvestre (Retz.) r. br. ex sm. through elicitation. Ind. Crops Prod. 162, 113234. doi: 10.1016/j.indcrop.2020.113234
Mal, S., Pal, D. (2021). Tannins and polyphenols extracted from natural plants and their versatile application. Bioactive Natural Products Pharm. Appl., 715–757.
Malayaman, V., Sisubalan, N., Senthilkumar, R. P., Ranjithkumar, R. (2017). Chitosan mediated enhancement of hydrolysable tannin in phyllanthus debilis Klein ex willd via plant cell suspension culture. Int. J. Biol. Macromol 104, 1656–1663. doi: 10.1016/j.ijbiomac.2017.03.138
Maliki, I. M., Misson, M., Teoh, P. L., Rodrigues, K. F., Yong, W. T. L. (2022). Production of lectins from marine algae: current status, challenges, and opportunities for non-destructive extraction. Mar. Drugs 20 (2), 102. doi: 10.3390/md20020102
Marathe, S. J., Jadhav, S. B., Bankar, S. B., Singhal, R. S. (2017). “Enzyme-assisted extraction of bioactives,” in Food bioactives (Cham: Springer), 171–201.
Masyita, A., Sari, R. M., Astuti, A. D., Yasir, B., Rumata, N. R., Emran, T. B., et al. (2022). Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X (13), 100217. doi: 10.1016/j.fochx.2022.100217
Mattosinhos, P. D. S., Sarandy, M. M., Novaes, R. D., Esposito, D., Gonçalves, R. V. (2022). Anti-inflammatory, antioxidant, and skin regenerative potential of secondary metabolites from plants of the brassicaceae family: a systematic review of In vitro and In vivo preclinical evidence (Biological activities brassicaceae skin diseases). Antioxidants 11 (7), 1346. doi: 10.3390/antiox11071346
Maugeri, A., Lombardo, G. E., Cirmi, S., Süntar, I., Barreca, D., Laganà, G., et al. (2022). Pharmacology and toxicology of tannins. Arch. Toxicol. 96, 1257–1277. doi: 10.1007/s00204-022-03250-0
Mehrotra, S., Mishra, S., Srivastava, V. (2018). “Hairy root cultures for monoterpene indole alkaloid pathway: investigation and biotechnological production,” in Hairy roots (Singapore: Springer), 95–121.
Mehrotra, S., Rahman, L. U., Kukreja, A. K. (2010). An extensive case study of hairy-root cultures for enhanced secondary-metabolite production through metabolic-pathway engineering. Biotechnol. Appl. Biochem. 56 (4), 161–172. doi: 10.1042/BA20100171
Mekky, H., Al-Sabahi, J., Abdel-Kreem, M. F. M. (2018). Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of catharanthus roseus. South Afr. J. Bot. 114, 29–31. doi: 10.1016/j.sajb.2017.10.008
Mihalache, G., Zamfirache, M. M., Stefan, M. (2015). Root associated bacteria–friends or enemies? a review. Mem. Sci. Sect. Rom. Acad. 38, 27–54.
Mikac, S., Markulin, L., Drouet, S., Corbin, C., Tungmunnithum, D., Kiani, R., et al. (2021). Bioproduction of anticancer podophyllotoxin and related aryltretralin-lignans in hairy root cultures of Linum flavum l. Plant Cell Tissue Differentiation Secondary Metabolites: Fundamentals Appl., 503–540. doi: 10.1007/978-3-030-30185-9_20
Mir, M. Y., Kamili, A. N., Hassan, Q. P., Tyub, S. (2017). Effect of light and dark conditions on biomass accumulation and secondary metabolite production in suspension cultures of Artemisia amygdalina decne. J. Himal. Ecol. Sustain. Dev. 12, 1–6.
Miranda, R. D. S., de Jesus, B. D. S. M., da Silva Luiz, S. R., Viana, C. B., Adão Malafaia, C. R., Figueiredo, F. D. S., et al. (2022). Antiinflammatory activity of natural triterpenes–an overview from 2006 to 2021. Phytother. Res. 36 (4), 1459–1506. doi: 10.1002/ptr.7359
Mirjalili, M. H., Esmaeili, H. (2022). Callus induction and withanolides production through cell suspension culture of Withania coagulans (Stocks) dunal. J. Med. Plants 21 (81), 79–91. doi: 10.52547/jmp.21.81.79
Mishra, A. (2015). Allelopathic properties of Lantana camara. Int. Res. J. Basic Clin. Study 3, 13–28. doi: 10.14303/irjbcs.2014.048
Mishra, B. N., Ranjan, R. (2008). Growth of hairy-root cultures in various bioreactors for the production of secondary metabolites. Biotechnol. Appl. Biochem. 49 (1), 1–10. doi: 10.1042/BA20070103
Mishra, M. R. M., Srivastava, R. K., Akhtar, N. (2019). Effect of nitrogen, phosphorus and medium pH to enhance alkaloid production from Catharanthus roseus cell suspension culture. Int. J. Secondary Metabolite 6 (2), 137–153. doi: 10.21448/ijsm.559679
Mohamed, E., Rahiman, F. A., Wahab, R. A., Zain, C. R. C. M., Javed, M. A., Huyop, F. (2016). A plant transformation vector containing the gene dehd for the development of cultivars resistant to monochloroacetic acid. J. Anim. Plant Sci. 26 (4), 1133–1139.
Mohammed, H. A. (2022). Phytochemical analysis, antioxidant potential, and cytotoxicity evaluation of traditionally used Artemisia absinthium L.(Wormwood) growing in the central region of Saudi Arabia. Plants 11 (8), 1028. doi: 10.3390/plants11081028
Moradi, A., Zarinkamar, F., Caretto, S., Azadi, P. (2018). Influence of thidiazuron on callus induction and crocin production in corm and style explants of crocus sativus l. . Acta Physiologiae Plantarum 40, 1–8. doi: 10.1007/s11738-018-2760-2
Morris, P., Carter, E. B., Hauck, B., Hughes, J. W., Allison, G., Theodorou, M. K. (2021). Responses of Lotus corniculatus to environmental change. 4: root carbohydrate levels at defoliation and regrowth climatic conditions are major drivers of phenolic content and forage quality. Planta 253 (2), 1–11. doi: 10.1007/s00425-020-03523-x
Movahedi, A., Almasi Zadeh Yaghuti, A., Wei, H., Rutland, P., Sun, W., Mousavi, M., et al. (2021). Plant secondary metabolites with an overview of populus. Int. J. Mol. Sci. 22 (13), 6890. doi: 10.3390/ijms22136890
Mukta, S., Ahmed, S. R., Afrin, D. (2017). Plant tissue culture–the alternative and efficient way to extract plant secondary metabolites. J. Sylhet Agril. Univ. 4 (1), 1–13.
Mumtaz, M. Z., Kausar, F., Hassan, M., Javaid, S., Malik, A. (2021). Anticancer activities of phenolic compounds from Moringa oleifera leaves: in vitro and in silico mechanistic study. Beni-Suef Univ. J. Basic Appl. Sci. 10 (1), 1–11. doi: 10.1186/s43088-021-00101-2
Munné-Bosch, S., Alegre, L. (2002). The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21 (1), 31–57. doi: 10.1080/0735-260291044179
Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 15 (3), 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Murray, M., Dordevic, A. L., Ryan, L., Bonham, M. P. (2018). The impact of a single dose of a polyphenol-rich seaweed extract on postprandial glycaemic control in healthy adults: A randomised cross-over trial. Nutrients 10 (3), 270. doi: 10.3390/nu10030270
Musacchi, S., Neri, D. (2019). Optimizing production of quality nursery plants for fruit tree cultivation. in achieving sustainable cultivation of temperate zone tree fruits and berries (London: Burleigh Dodds Science Publishing), 183–242.
Mushtaq, A., Roobab, U., Denoya, G. I., Inam-Ur-Raheem, M., Gullón, B., Lorenzo, J. M., et al. (2020). Advances in green processing of seed oils using ultrasound-assisted extraction: a review. J. Food Process. Preservation 44 (10), e14740. doi: 10.1111/jfpp.14740
Nabi, N., Singh, S., Saffeullah, P. (2021). Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. Vitro Cell. Dev. Biol.-Plant 57 (3), 341–355. doi: 10.1007/s11627-020-10140-6
Naik, P. M., Al-Khayri, J. M. (2016). Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J. Adv. Res. Biotech. 1 (2), 1–7.
Nandy, D., Maity, A., Mitra, A. K. (2020). Target-specific gene delivery in plant systems and their expression: insights into recent developments. J. Biosci. 45 (1), 1–12. doi: 10.1007/s12038-020-0008-y
Neumann, K. H., Kumar, A., Imani, J. (2009). Plant cell and tissue culture-a tool in biotechnology: basics and application. Springer Sci. Business Media. 2. edition, 1–459.
Nguyen, Q. T., Xiao, Y., Kozai, T. (2020). Photoautotrophic micropropagation. Plant Factory, 333–346. doi: 10.1016/b978-0-12-816691-8.00023-6
Nicolaou, K. C., Chen, J. S. (2011). “Classics in total synthesis. further targets, strategies, methods III III,” (Weinheim: Wiley-VCH).
Ni, J., Premathilake, A. T., Gao, Y., Yu, W., Tao, R., Teng, Y., et al. (2021). Ethylene-activated PpERF105 induces the expression of the repressor-type R2R3-MYB gene PpMYB140 to inhibit anthocyanin biosynthesis in red pear fruit. Plant J. 105 (1), 167–181. doi: 10.1111/tpj.15049
Ninkuu, V., Zhang, L., Yan, J., Fu, Z., Yang, T., Zeng, H. (2021). Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 22 (11), 5710. doi: 10.3390/ijms22115710
Nomura, T., Yoneda, A., Ogita, S., Kato, Y. (2021). Activation of cryptic secondary metabolite biosynthesis in bamboo suspension cells by a histone deacetylase inhibitor. Appl. Biochem. Biotechnol. 193 (11), 3496–3511. doi: 10.1007/s12010-021-03629-2
Ochoa-Villarreal, M., Howat, S., Hong, S., Jang, M. O., Jin, Y. W., Lee, E. K., et al. (2016). Plant cell culture strategies for the production of natural products. BMB Rep. 49 (3), 149. doi: 10.5483/BMBRep.2016.49.3.264
Oksman-Caldentey, K. M., Strauss, A. (1986). Somaclonal variation of scopolamine content in protoplast-derived cell culture clones of Hyoscyamus muticus. Planta Med. 52 (01), 6–12. doi: 10.1055/s-2007-969053
Ozbilen, A., Sezer, F., Taskin, K. M. (2022). Improving the adventitious rooting ability of hard-to-root olive (Olea europaea l.) cultivar cuttings through inhibiting strigolactone biosynthesis. Front. Life Sci. RT 3 (3), 134–137. doi: 10.51753/flsrt.1186955
Ozyigit, I. I. (2008). Phenolic changes during in vitro organogenesis of cotton (Gossypium hirsutum l.) shoot tips. Afr. J. Biotechnol. 7 (8), 1145–1150.
Ozyigit, I. I. (2009). In vitro shoot development from three different nodes of cotton (Gossypium hirsutum l.). Not Bot. Hort. Agrobo 37 (1), 74–78. doi: 10.15835/nbha3713144
Ozyigit, I. I. (2012). “Agrobacterium tumefaciens and its use in plant biotechnology,” in Crop production for agricultural improvement (Dordrecht: Springer), 317–361.
Ozyigit, I. I. (2020). About life sciences and related technologies. Front. Life Sci. Related Technol. 1 (1), 1–11.
Ozyigit, I. I., Bajrovic, K., Gozukirmizi, N., Semiz, B. D. (2002). Direct plant regeneration from hypocotyl and cotyledon explants of five different sunflower genotypes (Helianthus annuus l.) from Turkey. Biotechnol. Biotechnol. Equip. 16 (1), 8–11. doi: 10.1080/13102818.2002.10819148
Ozyigit, I. I., Can, H., Dogan, I. (2021). Phytoremediation using genetically engineered plants to remove metals: a review. Environ. Chem. Lett. 19 (1), 669–698. doi: 10.1007/s10311-020-01095-6
Ozyigit, I. I., Dogan, I., Artam-Tarhan, E. (2013). “Agrobacterium rhizogenes-mediated transformation and its biotechnological applications in crops,” In: Hakeem, K., Ahmad, P., Ozturk, M. (eds) Crop Improvement (Boston, MA: Springer), 1–48. doi: 10.1007/978-1-4614-7028-1_1
Ozyigit, I. I., Dogan, I., Kaya, Y., Bajrovic, K., Gozukirmizi, N. (2022). Cotton biotechnology: an efficient gene transfer protocol via agrobacterium tumefaciens for a greater transgenic recovery. J. Natural Fibers 19 (15), 11582–11596. doi: 10.1080/15440478.2022.2029662
Ozyigit, I. I., Gozukirmizi, N. (2008). High efficiency shoot and root formation from cotyledonary nodes of cotton (Gossypium hirsutum l.). Pak J. Bot. 40 (3), 1665–1672.
Ozyigit, I. I., Gozukirmizi, N. (2009). Efficient shoot and root formation from shoot apices of cotton (Gossypium hirsutum l.). Russ J. Plant Phys. 56 (4), 527–531. doi: 10.1134/S1021443709040128
Ozyigit, I. I., Gozukirmizi, N., Semiz, B. D. (2007b). Genotype dependent callus induction and shoot regeneration in sunflower (Helianthus annuus l.). Afr J. Biotechnol. 6 (13), 1498–1502.
Ozyigit, I. I., Kahraman, M. V., Ercan, O. (2007a). Relation between explant age, total phenols and regeneration response in tissue cultured cotton (Gossypium hirsutum l.). Afr. J. Biotechnol. 6 (1), 3–8.
Paek, K. Y., Chakrabarty, D., Hahn, E. J. (2005). Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult 81, 287–300. doi: 10.1007/s11240-004-6648-z
Pandey, P., Singh, S., Banerjee, S. (2019). Ocimum basilicum suspension culture as resource for bioactive triterpenoids: yield enrichment by elicitation and bioreactor cultivation. Plant Cell Tissue Organ Culture 137 (1), 65–75. doi: 10.1007/s11240-018-01552-9
Pant, P., Pandey, S., Dall'Acqua, S. (2021). The influence of environmental conditions on secondary metabolites in medicinal plants: a literature review. Chem. Biodiversity 18 (11), e2100345. doi: 10.1002/cbdv.202100345
Parr, B. T., Economou, C., Herzon, S. B. (2015). A concise synthesis of (+)-batzelladine b from simple pyrrole-based starting materials. Nature 525 (7570), 507–510. doi: 10.1038/nature14902
Pataro, G., Carullo, D., Ferrari, G. (2022). “Innovative processes for the extraction of bioactive compounds from winery wastes and by-products,” in Improving sustainable viticulture and winemaking practices (Academic Press), 281–303.
Patil, S. M., Ramu, R., Shirahatti, P. S., Shivamallu, C., Amachawadi, R. G. (2021). A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 7 (5), e07054. doi: 10.1016/j.heliyon.2021.e07054
Patra, J. K., Das, G., Das, S. K., Thatoi, H. (2020). “Plant tissue culture techniques and nutrient analysis,” in A practical guide to environmental biotechnology (Singapore: Springer), 135–164.
Peebles, C. A., Sander, G. W., Li, M., Shanks, J. V., San, K. Y. (2009). Five-year maintenance of the inducible expression of anthranilate synthase in catharanthus roseus hairy roots. Biotechnol. Bioengineering 102 (5), 1521–1525. doi: 10.1002/bit.22173
Pence, V. C. (2011). Evaluating costs for the in vitro propagation and preservation of endangered plants. Vitro Cell. Dev. Biol.—Plant. 47 (1), 176–187. doi: 10.1007/s11627-010-9323-6
Phillips, G. C., Garda, M. (2019). Plant tissue culture media and practices: an overview. Vitro Cell. Dev. Biol.-Plant 55 (3), 242–257. doi: 10.1007/s11627-019-09983-5
Piatczak, E., Grąbkowska, R., Skała, E. (2018). “pRi-transformed plants as a source of secondary metabolites,” in Hairy roots (Singapore: Springer), 45–70.
Piatczak, E., Jeleń, A., Makowczyńska, J., Zielińska, S., Kuźma, Ł., Balcerczak, E. (2019). Establishment of hairy root cultures of Rehmannia elata NE brown ex prain and production of iridoid and phenylethanoid glycosides. Ind. Crops Prod. 137, 308–314. doi: 10.1016/j.indcrop.2019.05.022
Pistelli, L., Giovannini, A., Ruffoni, B., Bertoli, A., Pistelli, L. (2010). Hairy root cultures for secondary metabolites production. Bio-farms Nutraceuticals, 167–184. doi: 10.1007/978-1-4419-7347-4_13
Pizzi, A. (2021). Tannins medical/pharmacological and related applications: a critical review. Sustain. Chem. Pharm. 22, 100481. doi: 10.1016/j.scp.2021.100481
Pollier, J., Moses, T., Goossens, A. (2011). Combinatorial biosynthesis in plants: a (p) review on its potential and future exploitation. Nat. Prod Rep. 28, 1897–1916. doi: 10.1039/c1np00049g
Poole, J., Diop, A., Rainville, L. C., Barnabé, S. (2019). Bioextracting polyphenols from the brown seaweed Ascophyllum nodosum from québec’s north shore coastline. Ind. Biotechnol. 15, 212–218. doi: 10.1089/ind.2019.0008
Pospisil, J., Konrádová, D., Strnad, M. (2021). Antileishmanial activity of lignans, neolignans, and other plant phenols. Prog. Chem. Organic Natural Prod. 115, 115–176. doi: 10.1007/978-3-030-64853-4_3
Pradhan, S. P., Adhikari, K., Nepal, S., Pandey, B. P. (2020). Determination of sun protective factor of selected medicinal plants from Western Nepal. J. Nepal Chem. Soc. 41 (1), 51–55. doi: 10.3126/jncs.v41i1.30487
Pradhan, S., Tarai, R. K., Panigrahi, I. (2022). “Plant growth regulators in passion fruit,” in Plant growth regulators in tropical and Sub-tropical fruit crops (London: CRC Press), 505–519.
Prakash, S., Van Staden, J. (2007). Micropropagation of Hoslundia opposita vahl a valuable medicinal plant. South Afr. J. Bot. 73, 60–63. doi: 10.1016/j.sajb.2006.07.001
Pramita, A. D., Kristanti, A. N., Utami, E. S. W., Manuhara, Y. S. W. (2018). Production of biomass and flavonoid of Gynura procumbens (Lour.) merr shoots culture in temporary immersion system. J. Genet. Eng. Biotechnol. 16 (2), 639–643. doi: 10.1016/j.jgeb.2018.05.007
Puangpraphant, S., Cuevas-Rodríguez, E. O., Oseguera-Toledo, M. (2022). “Anti-inflammatory and antioxidant phenolic compounds,” in Current advances for development of functional foods modulating inflammation and oxidative stress (Academic Press), 165–180.
Radivojac, A., Bera, O., Zeković, Z., Teslić, N., Mrkonjić, Ž., Bursać Kovačević, D., et al. (2021). Extraction of peppermint essential oils and lipophilic compounds: assessment of process kinetics and environmental impacts with multiple techniques. Molecules 26 (10), 2879. doi: 10.3390/molecules26102879
Rahman, M. M., Rahaman, M. S., Islam, M. R., Rahman, F., Mithi, F. M., Alqahtani, T., et al. (2021a). Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules 27 (1), 233. doi: 10.3390/molecules27010233
Rahman, M. M., Rahaman, M. S., Islam, M. R., Rahman, F., Mithi, F. M., Alqahtani, T., et al. (2021b). Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules 27 (1), 233. doi: 10.3390/molecules27010233
Rajasekar, N., Sivanantham, A., Ravikumar, V., Rajasekaran, S. (2021). An overview on the role of plant-derived tannins for the treatment of lung cancer. Phytochemistry 188, 112799. doi: 10.1016/j.phytochem.2021.112799
Rao, K., Chodisetti, B., Gandi, S., Giri, A., Kishor, P. K. (2021). Cadmium chloride elicitation of abutilon indicum cell suspension cultures for enhanced stigmasterol production. Plant Biosystems-An Int. J. Dealing All Aspects Plant Biol. 156 (3), 613–618. doi: 10.1080/11263504.2021.1891151
Rao, S. M., Ravishankar, G. A. (2002). Plant cell cultures: chemical factories of secondary metabolities. Biotechnol. Adv. 20, 101–153. doi: 10.1016/S0734-9750(02)00007-1
Rashid, S., Majeed, L. R., Nisar, B., Nisar, H., Bhat, A. A., Ganai, B. A. (2021). “Phytomedicines: diversity, extraction, and conservation strategies,” in Phytomedicine (Academic Press), 1–33.
Rattan, S., Sood, A., Kumar, P., Kumar, A., Kumar, D., Warghat, A. R. (2020). Phenylethanoids, phenylpropanoids, and phenolic acids quantification vis-à-vis gene expression profiling in leaf and root derived callus lines of rhodiola imbricata (Edgew.). Ind. Crops Products 154, 112708. doi: 10.1016/j.indcrop.2020.112708
Reddy, A. V. B., Moniruzzaman, M., Madhavi, V., Jaafar, J. (2020). Recent improvements in the extraction, cleanup and quantification of bioactive flavonoids. Stud. Natural Prod. Chem. 66, 197–223. doi: 10.1016/B978-0-12-817907-9.00008-8
Renouard, S., Corbin, C., Drouet, S., Medvedec, B., Doussot, J., Colas, C., et al. (2018). Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 19 (4), 990. doi: 10.3390/ijms19040990
Riahi, L., Chograni, H., Ben Rejeb, F., Ben Romdhane, M., Masmoudi, A. S., Cherif, A. (2022). Efficient in vitro regeneration of the endangered species Artemisia arborescens l. through direct organogenesis and impact on secondary metabolites production. Horticult. Environment Biotechnol. 63 (3), 439–450. doi: 10.1007/s13580-021-00400-8
Ritmejeryte, E., Boughton, B. A., Bayly, M. J., Miller, R. E. (2020). Unique and highly specific cyanogenic glycoside localization in stigmatic cells and pollen in the genus lomatia (Proteaceae). Ann. Bot. 126 (3), 387–400. doi: 10.1093/aob/mcaa038
Roitsch, T., Sinha, A. K. (2002). Application of photoautotrophic suspension cultures in plant science. Photosynthetica 40, 481–492. doi: 10.1023/A:1024332430494
Roy, A., Bharadvaja, N. (2019). Establishment of root suspension culture of Plumbago zeylanica and enhanced production of plumbagin. Ind. Crops Prod. 137, 419–427. doi: 10.1016/j.indcrop.2019.05.007
Saha, P., Talukdar, A. D., Nath, R., Sarker, S. D., Nahar, L., Sahu, J., et al. (2019). Role of natural phenolics in hepatoprotection: a mechanistic review and analysis of regulatory network of associated genes. Front. Pharmacol. 10, 509. doi: 10.3389/fphar.2019.00509
Sahai, P., Sinha, V. B. (2022). Development of hairy root culture in Taxus baccata sub sp wallichiana as an alternative for increased taxol production. Mater. Today: Proc. 49, 3443–3448. doi: 10.1016/j.matpr.2021.03.407
Sahoo, M. M., Perach, O., Shachter, A., Gonda, I., Porwal, A., Dudai, N., et al. (2022). Spectral estimation of carnosic acid content in in vivo rosemary plants. Ind. Crops Prod. 187, 115292. doi: 10.1016/j.indcrop.2022.115292
Saini, V. (2010). Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J. Diabetes 1, 68–75. doi: 10.4239/wjd.v1.i3.68
Sajc, L., Grubisic, D., Vunjak-Novakovic, G. (2000). Bioreactors for plant engineering: an outlook for further research. Biochem. Eng. J. 4 (2), 89–99. doi: 10.1016/S1369-703X(99)00035-2
Salazar-Magallón, J. A., Huerta de la Peña, A. (2020). Production of antifungal saponins in an airlift bioreactor with a cell line transformed from Solanum chrysotrichum and its activity against strawberry phytopathogens. Preparative Biochem. Biotechnol. 50 (2), 204–214. doi: 10.1080/10826068.2019.1676781
Samkumar, R. A., Premnath, D., Raj, R. D. P. (2019). Strategy for early callus induction and identification of anti-snake venom triterpenoids from plant extracts and suspension culture of Euphorbia hirta l. Biotech. 9 (7), 1–11. doi: 10.1007/s13205-019-1790-9
Santos, L. C., Coelho, R. D., Barbosa, F. S., Leal, D. P., Júnior, E. F. F., Barros, T. H., et al. (2019). Influence of deficit irrigation on accumulation and partitioning of sugarcane biomass under drip irrigation in commercial varieties. Agric. Water Manage. 221, 322–333. doi: 10.1016/j.agwat.2019.05.013
Sangeetha, M., Anandaraj, B., Rajan, S. (2022). Antibacterial efficiency and phytochemical assessment of stereospermum chelonoides (LF) DC flower and leaf mixed powder extracts. Int. J. Life Sci. Pharma Res. 12 (3), L159–L172. doi: 10.22376/ijpbs/lpr.2022.12.3.L159-172
Sathasivam, R., Ki, J. S. (2018). A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 16 (1), 26. doi: 10.3390/md16010026
Sathyanarayana, B. N., Mathews, D. (2007). Plant tissue culture (New Delhi, Indıa: I K International Publishing House).
Sathyanathan, R., Varadarajan, G. S. (2021). “Cytotoxic efficiency of pulsed electric field treated Plectranthus amboinicus leaf extract on lung (A549) cancer cell lines,” in In 2021 IEEE 5th International Conference on Condition Assessment Techniques in Electrical Systems (CATCON). 163–169 (Kozhikode, India: IEEE).
Sato, F., Yamada, Y. (1984). High berberine-producing cultures of Coptis japonica cells. Phytochemistry 23 (2), 281–285. doi: 10.1016/S0031-9422(00)80318-0
Săvescu, P. (2021). Natural compounds with antioxidant activity-used in the design of functional foods. Funct. Foods: Phytochem. Health Promoting Potential, 169–193.
Scariot, F. J., Pansera, M. S., Longaray Delamare, A. P., Echeverrigaray, S. (2021). Antifungal activity of monoterpenes against the model yeast Saccharomyces cerevisiae. J. Food Process. Preservation 45 (5), e15433. doi: 10.1111/jfpp.15433
Secgin, Z., Okumus, A. (2022). Domates (Lycopsersicum esculentum l.)’te sentetik tohum üretiminde aljinat oranlarının depolama zamanına etkisi. Front. Life Sci. RT 3 (1), 30–35. doi: 10.51753/flsrt.1041120
Segečová, A., Pérez-Bueno, M. L., Barón, M., Červený, J., Roitsch, T. G. (2019). Noninvasive determination of toxic stress biomarkers by high-throughput screening of photoautotrophic cell suspension cultures with multicolor fluorescence imaging. Plant Methods 15 (1), 1–15. doi: 10.1186/s13007-019-0484-y
Sehgal, D., Khan, T. (2020). “Plant tissue culture: beyond being a tool for genetic engineering,” in Environmental microbiology and biotechnology. Eds. Singh, A., Srivastava, S., Rathore, D., Pant, D. (Singapore: Springer), 175–200.
Seker, M. E., Erdogan, A. (2023). Phenolic and carotenoid composition of rhododendron luteum sweet and ferula communis l. subsp. communis flowers. Front. Life Sci. RT 4 (1), 37–42. doi: 10.51753/flsrt.1214172
Shahzad, A., Parveen, S., Sharma, S., Shaheen, A., Saeed, T., Yadav, V., et al. (2017). “Plant tissue culture: applications in plant improvement and conservation,” in Plant biotechnology: principles and applications. Eds. Abdin, M., Kiran, U., Kamaluddin, A. (Singapore: Springer), 37–72.
Sharan, S., Sarin, N. B., Mukhopadhyay, K. (2019). Elicitor-mediated enhanced accumulation of ursolic acid and eugenol in hairy root cultures of ocimum tenuiflorum l. @ is age, dose, and duration dependent. South Afr. J. Bot. 124, 199–210. doi: 10.1016/j.sajb.2019.05.009
Sharifi-Rad, R., Bahabadi, S. E., Samzadeh-Kermani, A., Gholami, M. (2020). The effect of non-biological elicitors on physiological and biochemical properties of medicinal plant Momordica charantia l. Iranian J. Sci. Technol. Trans. A: Sci. 44 (5), 1315–1326. doi: 10.1007/s40995-020-00939-8
Sharma, S., Shahzad, A. (2013). “Bioreactors: a rapid approach for secondary metabolite production,” in Recent trends in biotechnology and therapeutic applications of medicinal plants. Eds. Shahid, M., Shahzad, A., Malik, A., Sahai, A. (Dordrecht: Springer Netherlands), 25–49.
Sharma, A., Sharma, S., Kumar, A., Kumar, V., Sharma, A. K. (2022). “Plant secondary metabolites: an introduction of their chemistry and biological significance with physicochemical aspect,” in Plant secondary metabolites (Singapore: Springer), 1–45.
Shekhawat, J. K., Rai, M. K., Shekhawat, N. S., Kataria, V. (2021). Synergism of m-topolin with auxin and cytokinin enhanced micropropagation of Maytenus emarginata. Vitro Cell. Dev. Biol.-Plant 57 (3), 418–426. doi: 10.1007/s11627-020-10132-6
Sidal, U. (2022). Citric acid production using rotating biodisc reactor (RBR). Front. Life Sci. RT 3 (1), 25–29. doi: 10.51753/flsrt.1035228
Sidhu, Y. (2011). In vitro micropropagation of medicinal plants by tissue culture. Plymouth Student Scientist 4 (1), 432–449.
Silva, B. I., Nascimento, E. A., Silva, C. J., Silva, T. G., Aguiar, J. S. (2021). Anticancer activity of monoterpenes: a systematic review. Mol. Biol. Rep. 48 (7), 5775–5785. doi: 10.1007/s11033-021-06578-5
Silva, J. M. D., Jr., Paiva, R., Campos, A. C. A. L., Rodrigues, M., Carvalho, M. A. D. F., Otoni, W. C. (2012). Protoplast production and isolation from Etlingera elatior. Acta Scientiarum. Agron. 34 (1), 45–50. doi: 10.1590/S1807-86212012000100007
Singh, R. (2016). Chemotaxonomy: a tool for plant classification. J. Med. Plants Stud. 4 (2), 90–93.
Singh, S., Pandey, P., Akhtar, M. Q., Negi, A. S., Banerjee, S. (2021b). A new synthetic biology approach for the production of curcumin and its glucoside in atropa belladonna hairy roots. J. Biotechnol. 328, 23–33. doi: 10.1016/j.jbiotec.2020.12.022
Singh, S., Raza, W., Parveen, S., Meena, A., Luqman, S. (2021a). Flavonoid display ability to target microRNAs in cancer pathogenesis. Biochem. Pharmacol. 189, 114409. doi: 10.1016/j.bcp.2021.114409
Singh, B., Sharma, R. A. (2015). Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. Biotech. 5 (2), 129–151. doi: 10.1007/s13205-014-0220-2
Singh, T., Yadav, R., Agrawal, V. (2020). Effective protocol for isolation and marked enhancement of psoralen, daidzein and genistein in the cotyledon callus cultures of Cullen corylifolium (L.) medik. Ind. Crops Prod. 143, 111905. doi: 10.1016/j.indcrop.2019.111905
Siziya, I. N., Hwang, C. Y., Seo, M. J. (2022). Antioxidant potential and capacity of microorganism-sourced C30 carotenoids–a review. Antioxidants 11 (10), 1963. doi: 10.3390/antiox11101963
Solis-Castañeda, G. J., Zamilpa, A., Cabañas-García, E., Bahena, S. M., Pérez-Molphe-Balch, E., Gómez-Aguirre, Y. A. (2020). Identification and quantitative determination of feruloyl-glucoside from hairy root cultures of Turbinicarpus lophophoroides (Werderm.) buxb. & Backeb.(Cactaceae). Vitro Cell. Dev. Biol.-Plant 56 (1), 8–17. doi: 10.1007/s11627-019-10029-z
Solle, H., Realista, L., Endang, S. (2016). Micropropagation of sandalwood (Santalum album l.) endemic plant from East nusa tenggara, Indonesia. AIP Conf. Proc. 1744, 020026. doi: 10.1063/1.495350
Somantri, R. U., Iriani, E. S., Sunarti, T. C. (2022). In vitro study on the antimicrobial activity of eleven essential oils against oral cavity microbiota. IOP Conf. Series: Earth Environ. Sci. 1063 (1), 012025. doi: 10.1088/1755-1315/1063/1/012025
Song, H. J., Yong, S. H., Kim, H. G., Kim, D. H., Park, K. B., Shin, K. C., et al. (2022). Insecticidal activity against Myzus persicae of terpinyl acetate and bornyl acetate in Thuja occidentalis essential oil. Horticulturae 8 (10), 969. doi: 10.3390/horticulturae8100969
Sreeram, S., Sathishkumar, R., Amritha, P. S. (2021). Targeting the ENV spike protein of HIV with naturally occurring compounds: an in-silico study for drug designing. Adv. Traditional Med. 23, 503–511. doi: 10.1007/s13596-021-00617-z
Srikantan, C., Srivastava, S. (2018). “Bioreactor design and analysis for Large-scale plant cell and hairy root cultivation,” in Hairy roots (Singapore: Springer), 147–182.
Srivastava, S., Srivastava, A. K. (2007). Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 27 (1), 29–43. doi: 10.1080/07388550601173918
Srivastava, R., Tiwari, P. (2022). Medicinal plant used against cancer: a review. Asian J. Pharm. Res. Dev. 10 (4), 76–85. doi: 10.22270/ajprd.v10i4.1150
Sudheer, W. N., Praveen, N. (2021). Phytochemical, pharmacological and tissue culture studies of some important species of the genus barleria L.(Acanthaceae)-a review. Plant Sci. Today 8 (3), 491–500. doi: 10.14719/pst.2021.8.3.1117
Sun, Y., Zhang, M., Fang, Z. (2020). Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: a review. Trends Food Sci. Technol. 105, 468–482. doi: 10.1016/j.tifs.2019.02.026
Sundriyal, A. (2022). “Essential oils and their biological applications: extraction methods, types, biological activities, antimicrobial fumes,” in Handbook of research on advanced phytochemicals and plant-based drug discovery (IGI Global), 395–412.
Sureda, A., Martorell, M., Capó, X., Monserrat-Mesquida, M., Quetglas-Llabrés, M. M., Rasekhian, M., et al. (2021). Antitumor effects of triterpenes in hepatocellular carcinoma. Curr. Med. Chem. 28 (13), 2465–2484. doi: 10.2174/0929867327666200602132000
Srivastava, S., Srivastava, A. K. (2012). And In vitro azadirachtin production by hairy root cultivation of azadirachta indica in nutrient mist bioreactor. Appl. Biochem. Biotechnol. 166, 365–378. doi: 10.1007/s12010-011-9430-9
Sweetlin, P., Daniel, R. R. (2020). Determination of bioactive copmounds in ethanolic extract of callus derived from mucuna pruriens using gas chromatography and mass spectroscopic technique. J. Natural Remedies 21(7 (7 (S2), 11–16.
Szopa, A., Kokotkiewicz, A., Bednarz, M., Jafernik, K., Luczkiewicz, M., Ekiert, H. (2019). Bioreactor type affects the accumulation of phenolic acids and flavonoids in microshoot cultures of Schisandra chinensis (Turcz.) baill. Plant Cell Tissue Organ Culture 139 (1), 199–206. doi: 10.1007/s11240-019-01676-6
Szopa, A., Kokotkiewicz, A., Król, A., Luczkiewicz, M., Ekiert, H. (2018). Improved production of dibenzocyclooctadiene lignans in the elicited microshoot cultures of Schisandra chinensis (Chinese magnolia vine). Appl. Microbiol. Biotechnol. 102 (2), 945–959. doi: 10.1007/s00253-017-8640-7
Tarigholizadeh, S., Motafakkerazad, R., Kosari-nasab, M., Movafeghi, A., Mohammadi, S., Sabzi, M., et al. (2021). Influence of plant growth regulators and salicylic acid on the production of some secondary metabolites in callus and cell suspension culture of satureja sahendica bornm. Acta Agricult. Slovenica 117 (4), 1–12. doi: 10.14720/aas.2021.117.4.773
Thawabteh, A. M., Thawabteh, A., Lelario, F., Bufo, S. A., Scrano, L. (2021). Classification, toxicity and bioactivity of natural diterpenoid alkaloids. Molecules 26 (13), 4103. doi: 10.3390/molecules26134103
Thomas, W. P., Pronin, S. V. (2021). New methods and strategies in the synthesis of terpenoid natural products. Accounts Chem. Res. 54 (6), 1347–1359. doi: 10.1021/acs.accounts.0c00809
Thoppil, R. J., Bishayee, A. (2011). Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 3, 228–249. doi: 10.4254/wjh.v3.i9.228
Thota, J. R., Maddula, R. K., Pandeti, S., Yerra, N. V. (2022). 11 advances in extraction and analysis of natural products. Chem. Natural Prod.: Phytochem. Pharmacognosy Med. Plants 203.
Tomilova, S. V., Kochkin, D. V., Tyurina, T. M., Glagoleva, E. S., Labunskaya, E. A., Galishev, B. A., et al. (2022). Specificity of growth and synthesis of secondary metabolites in cultures in vitro digitalis lanata ehrh. Russian J. Plant Physiol. 69 (2), 1–11. doi: 10.1134/S1021443722020200
Tomiotto-Pellissier, F., Gonçalves, M. D., Silva, T. F., Concato, V. M., da Silva Bortoleti, B. T., Arakawa, N. S., et al. (2022). Plant-derived diterpenes for breast cancer treatment: new perspectives and recent advances. Stud. Natural Prod. Chem. 74, 41–80. doi: 10.1016/B978-0-323-91099-6.00011-6
Torres, K. C. (2012). Tissue culture techniques for horticultural crops (New York: Springer Science & Business Media).
Twaij, B. M., Hasan, M. N. (2022). Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 13 (1), 4–14. doi: 10.3390/ijpb13010003
Tzanova, M., Atanasov, V., Yaneva, Z., Ivanova, D., Dinev, T. (2020). Selectivity of current extraction techniques for flavonoids from plant materials. Processes 8 (10), 1222. doi: 10.3390/pr8101222
Uluisik, S., Chapman, N. H., Smith, R., Poole, M., Adams, G., Gillis, R. B., et al. (2016). Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 34 (9), 950–952. doi: 10.1038/nbt.3602
Vaghari, H., Jafarizadeh-Malmiri, H., Anarjan, N., Berenjian, A. (2017). “Hairy root culture: a biotechnological approach to produce valuable metabolites,” in Agriculturally important microbes for sustainable agriculture (Singapore: Springer), 131–160.
Veneziani, R. C. S., Ambrósio, S. R., Martins, C. H. G., Lemes, D. C., Oliveira, L. C. (2017). Antibacterial potential of diterpenoids. Stud. Natural Prod. Chem. 54, 109–139. doi: 10.1016/B978-0-444-63929-5.00004-8
Veraplakorn, V. (2016). Micropropagation and callus induction of Lantana camara l.–a medicinal plant. Agric. Natural Resour. 50 (5), 338–344. doi: 10.1016/j.anres.2016.12.002
Veraplakorn, V. (2017). In vitro micropropagation and allelopathic effect of lantana (Lantana camara l.). Agric. Natural Resour. 51 (6), 478–484. doi: 10.1016/j.anres.2018.03.006
Vitolo, M. (2021). “Fundamentals of biotechnology,” in Pharmaceutical biotechnology: a focus on industrial application. Eds. Riaz, S., Vitolo, M., Long, P. F. (London: CRC Press), 1–28.
Vitta, F. A., Costa, S. M., Amaral, M. D. C. E., Shepherd, G. J., Thomas, W. W. (2021). New combinations and typifications in cryptangieae (Cyperaceae). Phytotaxa 502 (1), 86–92. doi: 10.11646/phytotaxa.502.1.6
Vo, G. T., Liu, Z., Chou, O., Zhong, B., Barrow, C. J., Dunshea, F. R., et al. (2022). Screening of phenolic compounds in australian grown grapes and their potential antioxidant activities. Food Biosci. 101644. doi: 10.1016/j.fbio.2022.101644
Wackett, L. P. (2021). Microbially produced flavors and fragrances: an annotated selection of world wide web sites relevant to the topics in microbial biotechnology. Microbial Biotechnol. 14 (6), 2711. doi: 10.1111/1751-7915.13961
Wahyuni, D. K., Huda, A., Faizah, S., Purnobasuki, H., Wardoyo, B. P. E. (2020). Effects of light, sucrose concentration and repetitive subculture on callus growth and medically important production in justicia gendarussa burm. f. Biotechnol. Rep. 27, e00473. doi: 10.1016/j.btre.2020.e00473
Wang, D., Li, Y., Hu, X., Su, W., Zhong, M. (2015). Combined enzymatic and mechanical cell disruption and lipid extraction of green alga Neochloris oleoabundans. Int. J. Mol. Sci. 16 (4), 7707–7722. doi: 10.3390/ijms16047707
Wang, J., Li, J. L., Li, J., Li, J. X., Liu, S. J., Huang, L. Q., et al. (2017). Production of active compounds in medicinal plants: from plant tissue culture to biosynthesis. Chin. Herbal Medicines 9 (2), 115–125. doi: 10.1016/S1674-6384(17)60085-6
Wang, D., Samsulrizal, N. H., Yan, C., Allcock, N. S., Craigon, J., Blanco-Ulate, B., et al. (2019). Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 179 (2), 544–557.
Wani, A. R., Yadav, K., Khursheed, A., Rather, M. A. (2021). An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microbial Pathogenesis 152, 104620. doi: 10.1016/j.micpath.2020.104620
Waratadar, A., Nirmalnath, P. J., Matiwade, P. S., Navi, V. (2021). Effectiveness of mycorrhizal biofertilizer in the management of orobanche in tomato (Lycopersicon esculentum l.). Biol. Forum–An Int. J. 13 (3), 295–302.
Wawrosch, C., Zotchev, S. B. (2021). Production of bioactive plant secondary metabolites through in vitro technologies–status and outlook. Appl. Microbiol. Biotechnol. 105 (18), 6649–6668. doi: 10.1007/s00253-021-11539-w
Weber, J., Georgiev, V., Haas, C., Bley, T., Pavlov, A. (2010). Ploidy levels in Beta vulgaris (red beet) plant organs and in vitro systems. Eng. Life Sci. 10 (2), 139–147. doi: 10.1002/elsc.200900021
Weber, J., Georgiev, V., Pavlov, A., Bley, T. (2008). Flow cytometric investigations of diploid and tetraploid plants and in vitro cultures of Datura stramonium and Hyoscyamus niger. Cytometry Part A: J. Int. Soc. Analytical Cytol. 73 (10), 931–939. doi: 10.1002/cyto.a.20628
Wela, N. D., Dali, S., Chairunnas, A., Amalia, H. A. M., Puspitasari, S. A. A. (2022). Extraction of the chemical components of dengen leaves (Dillenia serrata thunb) by MAE method and activity test as antioxidant and toxicity. Indonesian J. Chem. Res. 10 (2), 74–82. doi: 10.30598//ijcr.2022.10-wel
White, F. F., Taylor, B. H., Huffman, G. A. (1985). Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of agrobacterium rhizogenes. J. Bacteriol. 164), 33–44. doi: 10.1128/jb.164.1.33-44.1985
Wijesinghe, W. A. J. P., Jeon, Y. J. (2012). Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia 83 (1), 6–12. doi: 10.1016/j.fitote.2011.10.016
Wojciechowska, M., Owczarek, A., Kiss, A. K., Grąbkowska, R., Olszewska, M. A., Grzegorczyk-Karolak, I. (2020). Establishment of hairy root cultures of Salvia bulleyana diels for production of polyphenolic compounds. J. Biotechnol. 318, 10–19. doi: 10.1016/j.jbiotec.2020.05.002
Xiao, Y., Niu, G., Kozai, T. (2011). Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Culture (PCTOC) 105, 149–158. doi: 10.1007/s11240-010-9863-9
Yamamoto, H., Zhao, P., Inoue, K. (2002). Origin of two isoprenoid units in a lavandulyl moiety of sophoraflavanone G from Sophora flavescens cultured cells. Phytochemistry 60, 263–267. doi: 10.1016/S0031-9422(02)00111-5
Yan, Y., Li, X., Zhang, C., Lv, L., Gao, B., Li, M. (2021). Research progress on antibacterial activities and mechanisms of natural alkaloids: a review. Antibiotics 10 (3), 318. doi: 10.3390/antibiotics10030318
Yancheva, S., Georgieva, L., Badjakov, I., Dincheva, I., Georgieva, M., Georgiev, V., et al. (2019). Application of bioreactor technology in plant propagation and secondary metabolite production. J. Cent. Eur. Agric. 20 (1), 321–340. doi: 10.5513/JCEA01/20.1.2224
Yang, L., Stöckigt, J. (2010). Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod Rep. 27, 1469–1479. doi: 10.1039/c005378c
Yasien, S., Iqbal, M. M., Javed, M., Alnuwaiser, M. A., Iqbal, S., Mahmood, Q., et al. (2022). Comparative evaluation of various extraction techniques for secondary metabolites from Bombax ceiba l. flowering plants along with In vitro anti-diabetic performance. Bioengineering 9 (10), 486. doi: 10.3390/bioengineering9100486
Ya-ut, P., Chareonsap, P., Sukrong, S. (2011). Micropropagation and hairy root culture of ophiorrhiza alata craib for camptothecin production. Biotechnol. Lett. 33 (12), 2519–2526. doi: 10.1007/s10529-011-0717-2
Yousefian, S., Lohrasebi, T., Farhadpour, M., Haghbeen, K. (2020). Production of phenolic acids in hairy root cultures of medicinal plant Mentha spicata l. @ in response to elicitors. Mol. Biol. Res. Commun. 9 (1), 23. doi: 10.22099%2Fmbrc.2020.36031.1475
Zachariah, T. J., Leela, N. K. (2018). “Spices: secondary metabolites and medicinal properties,” in Indian Spices (Cham: Springer), 277–316.
Zhang, S., Li, G., Fang, J., Chen, W., Jiang, H., Zou, J., et al. (2010). The interactions among DWARF10, auxin and cytokinin underlie lateral bud outgrowth in rice. J. Integr. Plant Biol. 52 (7), 626–638. doi: 10.1111/j.1744-7909.2010.00960.x
Zhang, Q. W., Lin, L. G., Ye, W. C. (2018). Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 13 (1), 1–26. doi: 10.1186/s13020-018-0177-x
Zhang, W., Seki, M., Furusaki, S. (2002). Effect of temperature and its shift on growth and anthocyani anthocyanin production in suspension cultures of strawberry cells. Plant Sci. 127, 207–214. doi: 10.1016/S0168-9452(97)00124-6
Zhang, H. J., Zheng, L. H., Zhao, K., Chen, Y., Yi, Z. (2017). Insecticidal activities of constituents of Litsea cubeba fruit extracts effective against the maize weevil (Coleoptera: curculionidae). J. Insect Sci. 17 (5), 103. doi: 10.1093/jisesa/iex079
Zhao, G., Hong, Y., Li, L., Zhang, H., Xu, R., Hao, Y. (2022). Selection and characterization of plant-derived alkaloids with strong antialgal inhibition: growth inhibition selectivity and inhibitory mechanism. Harmful Algae 117, 102272. doi: 10.1016/j.hal.2022.102272
Zheng, R., Li, S., Zhang, X., Zhao, C. (2021). Biological activities of some new secondary metabolites isolated from endophytic fungi: a review study. Int. J. Mol. Sci. 22 (2), 959. doi: 10.3390/ijms22020959
Zhou, S., Huang, G., Chen, G. (2021). Synthesis and anti-tumor activity of marine alkaloids. Bioorg. Med. Chem. Lett. 41, 128009. doi: 10.1016/j.bmcl.2021.128009
Zhou, L. G., Wu, J. Y. (2006). Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Natural Prod. Rep. 23, 789–810. doi: 10.1039/b610767b
Zia-Ul-Haq, M. (2021). “Past, present and future of carotenoids research,” in Carotenoids: structure and function in the human body. Eds. Zia-Ul-Haq, M., Dewanjee, S., Riaz, M. (Cham: Springer), 827–854.
Keywords: Tissue culture, gene transfer, bioreactor, hairy root, bioengineering
Citation: Ozyigit II, Dogan I, Hocaoglu-Ozyigit A, Yalcin B, Erdogan A, Yalcin IE, Cabi E and Kaya Y (2023) Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 14:1132555. doi: 10.3389/fpls.2023.1132555
Received: 27 December 2022; Accepted: 22 May 2023;
Published: 29 June 2023.
Edited by:
Laigeng Li, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Mercedes Bonfill, University of Barcelona, SpainAriel D. Arencibia, Catholic University of Maule, Chile
Copyright © 2023 Ozyigit, Dogan, Hocaoglu-Ozyigit, Yalcin, Erdogan, Yalcin, Cabi and Kaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim Ilker Ozyigit, aWxrb3p5aWdpdEBtYXJtYXJhLmVkdS50cg==
 Ibrahim Ilker Ozyigit
Ibrahim Ilker Ozyigit Ilhan Dogan2
Ilhan Dogan2 Aysegul Erdogan
Aysegul Erdogan Yilmaz Kaya
Yilmaz Kaya