
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 18 April 2023
Sec. Plant Nutrition
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1121073
This article is part of the Research TopicOmics Techniques to Optimize Plant-Microbe Interactions under Climate ChangeView all 7 articles
 Prabhu Govindasamy1*†
Prabhu Govindasamy1*† Senthilkumar K. Muthusamy2†
Senthilkumar K. Muthusamy2† Muthukumar Bagavathiannan3*
Muthukumar Bagavathiannan3* Jake Mowrer3
Jake Mowrer3 Prasanth Tej Kumar Jagannadham4
Prasanth Tej Kumar Jagannadham4 Aniruddha Maity5
Aniruddha Maity5 Hanamant M. Halli6
Hanamant M. Halli6 Sujayananad G. K.7
Sujayananad G. K.7 Rajagopal Vadivel6
Rajagopal Vadivel6 Das T. K.1
Das T. K.1 Rishi Raj1
Rishi Raj1 Vijay Pooniya1
Vijay Pooniya1 Subhash Babu1
Subhash Babu1 Sanjay Singh Rathore1
Sanjay Singh Rathore1 Muralikrishnan L.8
Muralikrishnan L.8 Gopal Tiwari1
Gopal Tiwari1Nitrogen (N) is an essential element required for the growth and development of all plants. On a global scale, N is agriculture’s most widely used fertilizer nutrient. Studies have shown that crops use only 50% of the applied N effectively, while the rest is lost through various pathways to the surrounding environment. Furthermore, lost N negatively impacts the farmer’s return on investment and pollutes the water, soil, and air. Therefore, enhancing nitrogen use efficiency (NUE) is critical in crop improvement programs and agronomic management systems. The major processes responsible for low N use are the volatilization, surface runoff, leaching, and denitrification of N. Improving NUE through agronomic management practices and high-throughput technologies would reduce the need for intensive N application and minimize the negative impact of N on the environment. The harmonization of agronomic, genetic, and biotechnological tools will improve the efficiency of N assimilation in crops and align agricultural systems with global needs to protect environmental functions and resources. Therefore, this review summarizes the literature on nitrogen loss, factors affecting NUE, and agronomic and genetic approaches for improving NUE in various crops and proposes a pathway to bring together agronomic and environmental needs.
A rapidly growing global population places considerable pressure on agricultural lands to produce more food and energy per unit area. For sustainable production, agricultural practices must both intensify productivity and simultaneously protect the environment and human and animal health. Improving nitrogen use efficiency (NUE) is an element of this framework (Zhang et al., 2015; Xiong et al., 2018). Nitrogen (N) is a key constituent of all living cells and is essential for the growth and development of plants. Fertilizer N is the second largest requirement after water in crop production, and N is the most common yield-limiting nutrient deficiency (Marschner, 1995). The ratio of N taken up versus the unit applied to a crop is referred to as NUE (Fageria and Baligar, 2005). The low N use of the crop indicates that uptake is inefficient or higher than the plant’s requirement (Anas et al., 2020). Cereal crops like rice, wheat, and maize require large amounts of N for healthy growth and higher yields (Linquist et al., 2012). Hence, varieties with higher NUE should be a priority for breeders developing new varieties (Balyan et al., 2016; Mălinaş et al., 2022).
The global estimates of N stored in soil are 65 Pg to 30 cm depth and 92–140 Pg to 100 cm depth (Zinke et al., 1986; Batjes, 2014). The largest portion of stores is in the form of organic N, which is not directly plant available. Chemical fertilizers and manures add 200 Tg of N each year (Potter et al., 2010). Biological N fixation provides an additional input of 258 Tg of N (Fowler et al., 2013). Ammonium (NH4+) and nitrate (NO3−) are the two forms of plant-available N. Globally, only 50% of applied N is converted and the rest is wasted (Mălinaş et al., 2022).
Crop NUE is influenced by environmental factors, plants’ physiological activity, and their interactions. Biochemical transformations of N in soil are complex and are best considered as being in a state of continual flux (Table 1). The fluxes of biochemical transformation in the soil system are primarily responsible for constraints to NUE. However, physical losses of N from the plant or soil system also decrease NUE. The major forms of N loss are the volatilization of ammonia (NH3) gas, leaching of dissolved NO3−, and overland runoff of all soluble forms. Changes in temperature and precipitation patterns affect biological and enzyme activity rates, which are important for most transformations listed in Table 1.
The simplest approach to quantifying NUE is to divide the crop yield (Y) by the nitrogen inputs (N) (Eq. 1).
However, several authors have suggested that yield may be defined in several ways, including the mass of the harvested portion of the crop, total (aboveground) biomass of the crop, N content contained in the harvestable portion, and N content of the total biomass. Fageria and Baligar (2005) proposed a number of general “groups” of approaches to calculate NUE that may be considered (Eqs. 2-7).
Where Gf and Gu are the grain yields (kg) of the fertilized and unfertilized plots, respectively, and Na is the rate of N applied (kg).
Where Yf and Yu are the total aboveground biomass (kg) of the crop in fertilized and unfertilized plots, respectively, and Nf and Nu are the N contents (kg) of the aboveground biomass in the fertilized and unfertilized plots, respectively.
Where Gf and Gu are the grain yield in fertilized and unfertilized plots, respectively.
All of the above equations rely on the assumption that varied nitrogen rate as fertilizer input is the independent variable. Naturally, as the mass of N inputs decreases, the calculated efficiency increases in equations using N rate or difference in N accumulation in the denominator. It would therefore be quite easy to interpret these as suggesting that the lowest rates of N fertilizer inputs result in the best NUE. This outcome ignores the importance of crop productivity.
Berendse and Aerts (1987) proposed a “biologically meaningful” definition of NUE as the product of nitrogen productivity (An/Ln) and the mean residence time (1/Ln) of nitrogen in the plant (Eq. 7).
This approach avoids the same pitfalls in Eqs. 1-6 but somehow fails to provide an interpretation of NUE necessary to evaluate the direct effects of climate change or advancements in crop management to adapt to climate change. It is indeed likely that future studies will not employ varied rates of N inputs to study NUE but will instead evaluate changes in other practices, varieties, genetic enhancements, and emerging biotechnologies. In this case, new approaches to the calculation of NUE will be needed. Preferably, these will also include mass balances of native soil plant-available N (PAN) and potential PAN in addition to fertilizer or manure inputs.
When considering the pressures of climate change, increased atmospheric carbon dioxide (CO2) will impact the ultimate equilibrium states of many of these processes. Higher temperatures will reduce soil N inventories by 5%–10% due to increased mineralization (Fowler et al., 2015). With the twin pressures of population expansion and climate change, management and breeding will need to focus on fundamental problems to make progress in NUE. Consider, for example, that leaf expansion and photosynthetic rates are affected by low N and that root traits are chiefly responsible for N uptake and NUE in maize (Wijewardana et al., 2015). Inbred maize lines exhibiting higher NUE were those with larger root diameters (Wijewardana et al., 2015). Root-ABA1, a major quantitative trait locus (QTL) for root development in maize, plays a vital role in NUE along with four other QTLs, viz., Qaer3.10, Qaer5.05–6, aer9.07–8, and Qaer10.04, responsible for aerenchyma cell development. In rice, the transcriptomic approach has helped to identify 62 candidate NUE genes. SHORT ROOT and SCARECROW are root-patterning genes responsible for root development and architecture. AUX1 and PIN proteins regulate the auxin movement and lead to lateral root development. NUE is a complex trait governed by the crop’s agronomic, physiological, environmental, and genetic traits. The integration of association mapping and genomics approach accompanied by the phenomic approach will be a major contributor to improve the NUE of global crops (Wani et al., 2021). Therefore, it is increasingly important to improve our understanding of factors affecting NUE and possible management measures for improving the NUE of crops.
This review focuses on describing different forms of N loss in the environment, analyzing the factors influencing NUE, discussing the consequences of poor NUE, and suggesting possible management practices for enhancing the NUE in various crops. Overall, better agronomic management of crops, genetic resources, breeding programs, and biotechnological tools to improve NUE are presented as potential solutions to low NUE of crops.
The negative effect of N loss on water, the environment, and human and animal health has been well reported (Singh et al., 2010). Soil N is transient and moves rapidly away from the point of application through various mechanisms. The processes responsible for N loss include volatilization, nitrification, denitrification, leaching, surface runoff, ammonium fixation, and immobilization (Baggs et al., 2000). Overall, the amount of mineral N in the soil at any given time can be described by the following N balance equation (Eq. 19) (Di and Cameron, 2002).
where Np is the precipitation and dry deposition, Nb is the biological fixation (Eq. 10, Table 1), Nf is the fertilizer, Nu is the urine and dung return to the soil, Nm is the mineralization, Npl is the plant uptake (Eqs. 11 and 12, Table 1), Ng is the gaseous losses, Ni is the immobilization, Nl is the leaching loss, and Ne is the erosion and surface runoff.
The gaseous loss of NH3 is known as volatilization. Volatilization is a complex process that is controlled by the physical, chemical, and biological properties of soil and the environment (Fan et al., 2011). Agriculture activities account for 50% of the total annual global NH3 loss (32 Tg year−1) to the atmosphere through volatilization (Liu et al., 2019a). Fertilizer and manure application and livestock activity are the primary sources of NH3 emissions in agriculture. Chemical N fertilizer alone is responsible for 34% of the loss (He et al., 2014). In particular, urea-based fertilizers are more susceptible than other N fertilizers because of the temporary increase in soil pH through the consumption of H+ ions during hydrolysis (Eq. 13). There is an equilibrium between NH4 and NH3 in soil solution (Eq. 16). The pKa for equilibrium in Eq. 16 is 9.3. Therefore, alkaline conditions favor greater proportions of NH3 (Havlin et al., 2014). When soil pH exceeds 7.5, temperatures increase up to 45°C, sufficient air movement is present to remove NH3 gas at the soil–atmosphere interface, and losses of N as NH3 are maximized (Bock and Kissel, 1988; Havlin et al., 2014). Application to acidic soils raises a little risk of volatilization. Application to sandy soils with low native cation exchange capacity (CEC) raises the risk. The common management approaches to improve the NUE of NH4/NH3 fertilizers include incorporation into the soil through injection or tillage to protect NH4/NH3 through the association of NH4 with clay colloid cation exchange sites. When animal wastes are used as nutrient sources for crops, volatilization has been markedly diminished by incorporation or pretreatment with acidifying agents (Marshall et al., 1998; Choi and Moore, 2008; Doydora et al., 2011). Splitting applications between pre-plant and one or more subsequent applications later in the growing season is also commonly recommended to reduce the time that NH4/NH3 fertilizers are exposed to environmental conditions that promote loss.
Urea hydrolysis (Eq. 13) may be considered the final step in the mineralization of organic N. The urease enzymes (urea amidohydrolases, EC 3.5.1.5) are produced by a large number of organisms filling a variety of ecological niches including plants, bacteria, algae, fungi, and invertebrates (Sigurdarson et al., 2018). In most soils, the enzyme is more than sufficiently present and free in solution to rapidly hydrolyze urea to NH3 (Klose and Tabatabai, 1999). Therefore, management to avoid losses of NH3 through volatilization following urea application has commonly involved the inhibition of ureases to prevent the reaction from occurring until the urea itself may be safely incorporated into the subsurface soil.
Conventional urease inhibitors include N-(n-butyl) thiophosphoric triamide (NBPT), perhaps the most widely employed, with demonstrated effectiveness in rice, cotton, wheat, maize, and pasture grasses (Zaman et al., 2009; Kawakami et al., 2012; Marshall et al, 1998; Martins et al., 2017; Wallace et al., 2020). Urease inhibition with NBPT and cyclohexylphopshoric triamide (CHPT) may also be effective in preventing N losses from manure sources (Svane et al., 2020). Plant-based materials such as those isolated from Canavalia ensiformis (jack bean), Eucalyptus camaldulensis (eucalyptus), allicin from Allium sativum (garlic), and certain Acacia spp. have been shown to inhibit ureases in soil (Mathialagan et al., 2017; Rana et al., 2021). This raises the possibility of the increased entrance of plant biotechnologies into this area. Finally, as with any N source, urea may also be split applied and/or subsurface applied to prevent exposure to environmental conditions that lead to losses.
Higher rates of animal manure or commercial N fertilizer application increase NO3− leaching as a result of increased available N concentration in soil solution. Nitrate N is highly susceptible to leaching due to the negative charge associated with NO3− which prevents its association with negatively charged soil colloids, whereas NH4+ is electrostatically attracted to colloids and therefore protected from leaching (Lodhi et al., 2009). Therefore, rain and irrigation would take the NO3− out of the system. Nitrate leaching takes place mainly after the heavy rainy season and the period of slow crop growth. Pande et al. (1985) reported that the N leaching process accounted for 2%–60% of the applied N loss. It has been estimated that the irrigated wheat fields account for 5 to 12.5 kg N ha−1 N leaching loss, where farmers have applied 250 kg N ha−1 with two splits in northern Mexico (Riley et al., 2001). Clay soil typically has lower NO3− leaching than sandy soil due to limited hydraulic conductivity. In clay soils, NO3− measured in soil samples to 60 cm can be subtracted from maize N fertilizer recommendations due to the reduced leaching potential (Fromme et al., 2017).
Nitrification is a microbial process (Eq. 14, Table 1), in which the ammonium is converted into nitrate by the oxidation process (Ward et al., 2011). It is a two-stage process (Eqs. 8 and 9) and is mediated by autotrophic bacteria.
The first stage is initiated by the ammonia-oxidizing bacteria like Nitrosospira and Nitrosomonas, which perform the oxidation of NH4+ to nitrite (NO2−) by means of the membrane-bound ammonia monooxygenase enzyme associated with hydroxylamine oxygenase (Jiang et al, 2018). The second step involves the conversion of NO2− to NO3− mediated by Nitrobacter. The last stage is much faster and more effective than the first stage; hence, nitrite rarely accumulates in the soil (Linn and Doran, 1984). Nitrification takes place in an aerobic soil environment with optimal soil moisture (60% water-filled pore space) (Linn and Doran, 1984). However, it is a very slow process in anaerobic soil environments (rice ecosystem) (Linn and Doran, 1984). The process is also regulated by soil temperature, pH, NH4+/NH3 concentration, and microbial population (Sharma and Ahlert, 1977). Nitrate produced by this process can be leached, absorbed by plants, and immobilized by soil microorganisms.
Denitrification is also a microbe-mediated, though strictly anaerobic, process (Eq. 15, Table 1) wherein NO2− is reduced to N2 gas using intermediate products such as nitrogen dioxide [NO2, nitric oxide (NO), and nitrous oxide (N2O) (Figure 1)]. The production of N2O is a major concern because of its greenhouse gas (GHG) function, with approximately 300 times the GHG potential of CO2. Soil N loss through denitrification as a percentage of applied N varies widely and is a function of soil water content, soluble carbon (C), the presence of NO3−, temperature, and time. Global loss of N from denitrification is estimated to be 96 Tg year−1 in 2000 and would probably increase to 142 Tg year−1 by 2050 (Bouwman et al., 2013). The process is carried out by a group of facultative anaerobic bacteria and catalyzed by nitrate reductase and nitrite reductase enzymes (Garbeva et al., 2007; Ranatunga et al., 2018). Two different electron acceptors are used during the denitrification process in aerobic conditions; oxygen acts as an electron acceptor, while NO3− is used as an electron acceptor in anaerobic conditions (Bock et al., 1995). Chemo-denitrification is another process responsible for nitrous oxide emission, but the quantity is smaller than biological production (Kool et al., 2011). Likewise, the nitrification process also releases N2O through the spontaneous oxidation of hydroxylamine, which is an intermediate in the nitrification process (Kool et al., 2011).
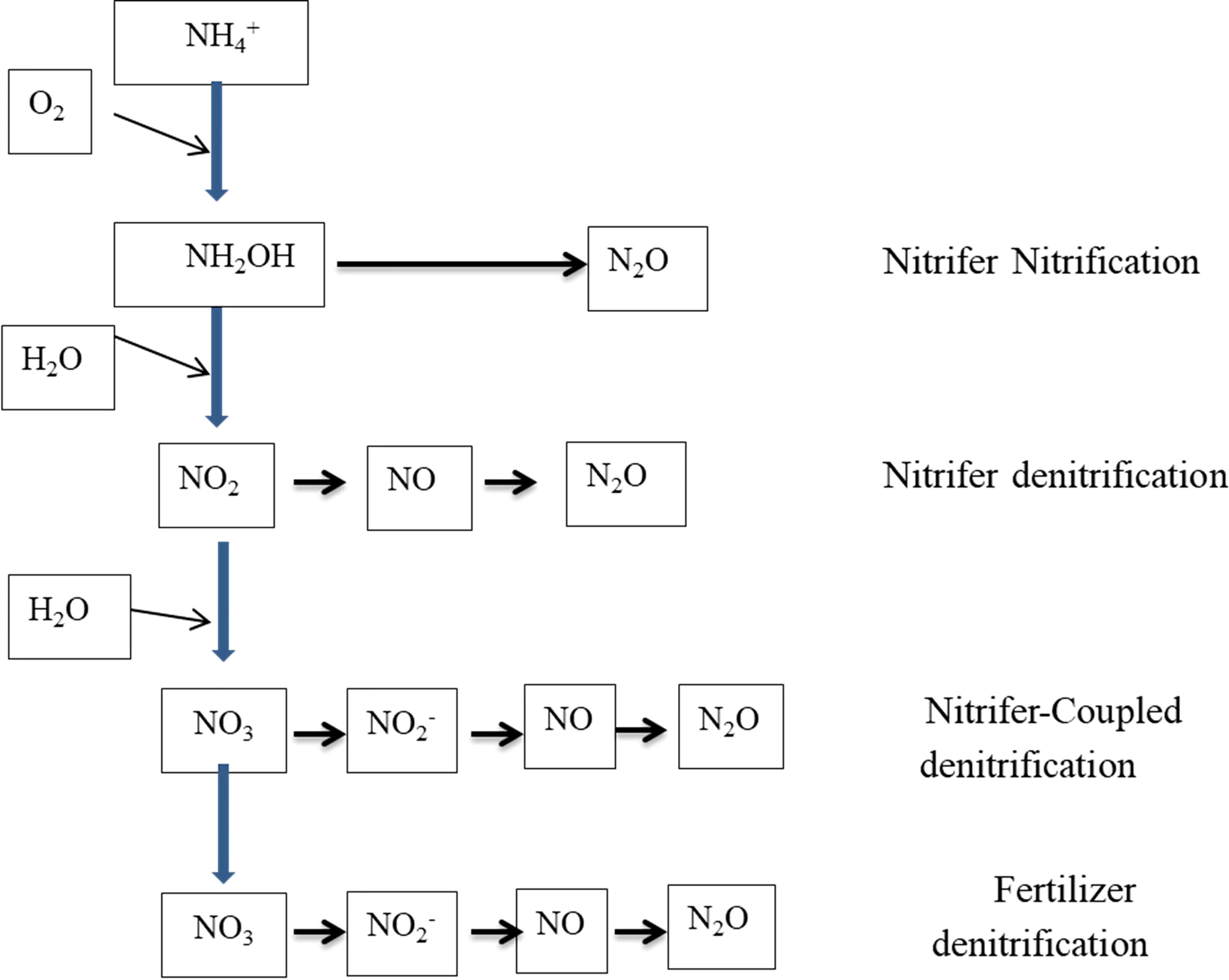
Figure 1 Different pathways of N2O production in soil (Kool et al., 2011).
Conventional management for the prevention of denitrification losses has conventionally been through inhibition of nitrification in soil. Nitrification inhibition prevents the formation of NO3−, the substrate for denitrification, from NH4+ (Eq. 14). There are a number of chemistries known and used in agriculture for nitrification inhibition. These include nitrapyrin (and various other pyridines), thiourea, thiophosphoryl triamide (also a urease inhibitor), 3,4-dimethylpyrazole phosphate (DMPP), and dicyandiamide (DCD) (McGinn et al, 2016; Ruser and Schulz, 2015; Alonso-Ayuso et al., 2016). Each of these chemistries is known to increase the production and release of nitrous oxide (N2O) from soils, a fact that should be considered in all efforts to increase NUE.
Research into biological nitrification inhibition (BNI) is advancing rapidly (Coskun et al., 2017). The current state of BNI research suggests that both plant-derived compounds (direct inhibition) and indirect mechanisms may be simultaneously responsible. For a thorough review of isolated plant exudates and metabolomics responsible for BNI, please see Nardi et al. (2020). Harnessing BNI for agricultural scale use will continue to be a fecund area of research in the near future for plant biotechnology and breeding disciplines.
Slope, rainfall intensity, soil type, and vegetation are key determinants of soil and nutrient loss and transport (Kang et al., 2001). Soil nearest to the surface often contains the greatest concentrations of N and organic matter which can be readily transported through runoff and erosion. It is possible that up to 70% of surface-applied N fertilizer may be lost to a runoff if rain occurs on the same day (Mandal et al., 2012).
Management of cropping systems to reduce such physical losses of N will improve NUE. Conventional approaches to minimizing erosion and runoff include reduced tillage or no-tillage, cover cropping, surface residue retention (conservation tillage), contour tillage, terracing, and grassed waterways (Boincean and Dent, 2019; Farzadfar et al., 2021; Young et al., 2021). While no-till and reduced till systems tend to protect or increase soil organic matter, which includes organic nitrogen, Canisares et al. (2021) reported that no-till increased mineralization rates without affecting the optimal corn fertilization response. In this case, yields were greater under no-till (~1,000 kg ha−1), though the response to N fertilizer was unchanged. Depending on how it is defined (Eqs. 1-7), NUE may or may not have been improved in this case. However, efforts to control erosion and loss of N through reduced tillage should improve soil stocks of N through both conservation and enhanced mineralization and continue to be the best recommended practices.
When cover crops are included in cropping systems, there are multiple mechanisms that can lead to increased NUE. Reduction of erosion caused by overland flow is more effective when covers with finer roots such as cereal rye or oats are used as opposed to covers with thick roots such as mustards or radishes (De Baets et al., 2011). Leguminous cover crops fix N2 gas from the atmosphere into plant-available NH3 (Eq. 10) and incorporated into the plant biomass. Upon senescence of the cover crop, the biomass N may then be remineralized (Eq. 18). Any measure of NUE which simply considers the reduction of fertilizer requirement will naturally be improved by increasing soil stores in this way. Cereal covers have the potential to reduce leaching by scavenging N from soils into biomass and releasing to the following cash crop through mineralization as well. Ranells and Wagger (1997) reported that the legumes hairy vetch and crimson clover could release 132 and 60 kg N ha−1, respectively, while the non-legume cereal rye released 24 kg ha−1.
Ammonium fixation occurs with 2:1 type of clay minerals such as illite, vermiculite, and smectite because they have negative charges and have the ability to expand interlayer spacing when soil water enters the basal oxygen plane (Nieder et al., 2011). The NH4+ ion is comparable to that of K+ with respect to ionic radii and low energy of hydration (Nieder et al., 2011). Therefore, the NH4+ ion is fitted exactly in the ditrigonal holes, or interlayers, in the basal oxygen plane of 2:1 clay mineral when soil water is present (Kunze and Jeffries, 1953). The clay mineral interlayers collapse approximately 1 nm upon drying, and NH4+ ions are then trapped between silicate sheets and largely removed from further exchange reactions (Juang et al., 2001).
Manure and residues are applied to the soil as a source of nutrients (Figure 2). The first step after applying organic matter to the soil is mineralization (Eq. 17, Table 1), which converts the unavailable nutrient form into the available form NH4+ (Chen et al., 2014). The C:N ratio of organic matter influences the N mineralization process because microbial biomass production requires both N and C (Chen et al., 2014). The wider the C:N ratio (e.g., >30:1) could hinder the mineralization process due to insufficient N content, and this condition leads to the immobilization of N (Eq. 18) (Quemada and Cabrera, 1995; Yassen et al, 2010). Immobilization is a process by which applied N can be incorporated into microbial biomass to provide for protein synthesis and reproduction. When mineral N + mineralizable organic N are insufficiently present to meet these needs, immobilization will remove plant-available N from the system (Sakala et al., 2000; Bird et al., 2001). Immobilization is considered negligible when the C:N ratio is <20:1.
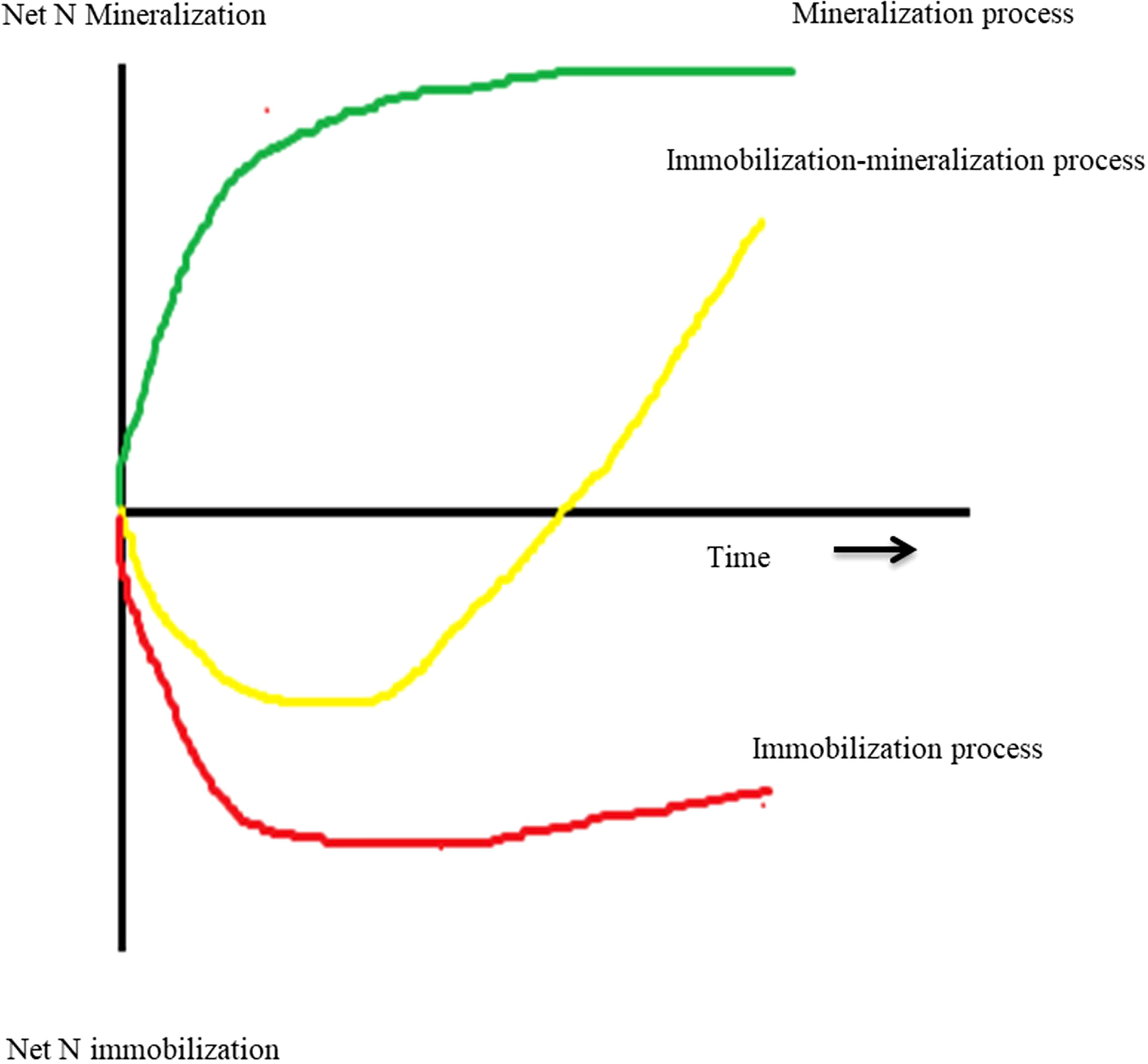
Figure 2 Three different process types regarding the effects of returning plant residues on soil inorganic N over the limited experimental period (Chen et al., 2014).
In addition, when the N concentration is insufficient at the early stage of residue decomposition, the N content of the microbe’s own tissues may be increased through the remineralization process (Zelenev et al., 2006). Remineralization is a natural process by which the microbes requiring N can meet by mineralization of dead microorganisms using the enzymolysis process. Shindo and Nishio (2005) reported that the remineralization rates of wheat straw were 0.71, 0.55, and 0.29 mg N kg−1 day−1 after 7, 28, and 54 days, respectively. The high rate of remineralization is usually happening due to high consumption and low assimilation of N by microbes (Braun et al., 2018).
The agronomic N use efficiency of crops is greatly influenced by crop characteristics, environmental variability, and management practices.
Crops and crop varieties differ considerably in their ability to uptake N per unit of biomass production. The agronomic NUE of major crops is given in Table 2. Crops grown in well-irrigated conditions have a greater agronomic NUE than in unirrigated/rainfed conditions. A study conducted on various irrigation regimes on wheat in China concluded that the nitrogen partial factor productivity was higher for 40 mm per irrigation (41.57 to 43.69 kg grain per kg N applied) compared with 20 mm per irrigation (32.24 to 32.47 kg grain per kg N applied) (Si et al., 2020). High crop growth rate, yield, and N uptake in crops can be achieved by maintaining optimal soil moisture conditions (Giller et al., 2004; Ding et al., 2021). Annual crops have a higher agronomic NUE than perennial crops due to the higher N uptake efficiency and N concentration (Weih et al., 2011). However, yield-specific N efficiency was more for perennial crops than wheat (Weih et al., 2011). Compared with food crops, fodder crops have a higher agronomic NUE because of the higher biomass production per unit area and time.
Important environmental factors that affect the agronomic NUE are photosynthetic active radiation (PAR), temperature, and rainfall. Environmental factors that affect the agronomic NUE of crops in decreasing order are temperature > rainfall > irradiance (Balasubramanian et al., 2004). The temperature requirement of crops may vary greatly (Table 3). For crops like rice and wheat, NUE increased significantly with increasing growing season temperature, but it decreased for corn, which may be due to the variation in plant N demand and uptake responses to temperature (Yu et al., 2022). An et al. (2005) reported that when the crop suffers because of lower than optimal temperature, an increase in seasonal air temperature suddenly increases crop growth and nitrogen demand, which could increase NUE. At low temperatures, the ability to absorb N by the roots is greatly reduced due to the high affinity of the temperature and nitrate influx systems in the roots (Glass, 2003). However, the increase in temperature may lead to a high loss of N, thus reducing the NUE (Bai et al., 2013). The N loss and crop N uptake are highly influenced by the intensity, duration, and frequency of rainfall in a crop season. The occurrence of rainfall within a day of N fertilizer application had a positive impact on the NUE. A strong correlation between the total rainfall and NUE was observed for the dryland summer sorghum in Australia (Rowlings et al., 2022). The highest NUE was reported for 125% simulated rainfall for wheat and corn in a silt loam soil of Kentucky, USA (Shahadha et al., 2021). Photosynthetic active radiation is a major driving force affecting crop growth and N uptake (Shahadha et al., 2021). However, it is only important for tropical and subtropical regions but not for temperate regions (Balasubramanian et al., 2004). Studies have observed that crop growth and nitrogen uptake vary significantly during the dry and wet seasons, mainly due to variations in PAR in the tropics (Balasubramanian et al., 2004).
Globally, 50% of the nitrogen applied to crops is lost to the environment, resulting in resource wastage and increased GHG emissions (Grizzetti et al., 2013). The 50-year data from 124 countries suggest that increased N fertilization involved low agronomical benefits and higher environmental risks. Different management practices have resulted in reduced NUE. Basically, the selection of crops or varieties with poor N uptake and assimilation followed by inefficient utilization through reduced N remobilization resulted in a lower N use efficiency (Dong and Lin, 2020). Furthermore, it is responsible for the loss of N from the soil and plant residue after harvesting the economic part (Kant et al., 2011). Galloway et al. (2003) reported that extensive crop cultivation over grasslands exposes the protected and stored soil organic carbon pool. Thus, it increases nitrate leaching and NH3 or NO2 and N2O emission, leading to environmental pollution. In South America, Africa, and Asia, reduced NUE was reported in areas devoid of cropping systems with biological N fixation such as soybean, beans, and groundnut (Herridge and Peoples, 1990; Liu et al., 2010). Similarly, intensive cropping without integration of livestock systems also reduced the N use efficiency at the local and global levels (Lassaletta et al., 2014). The promotion of synthetic N fertilizers rather than symbiotic N fixation resulted in poor N use efficiency (Lassaletta et al., 2014). Likewise, uncontrolled flood irrigation resulted in NO3− leaching due to a negative charge and high solubility; furthermore, it creates anoxic conditions which lead to the development of denitrifying microorganisms (Chattha et al., 2022; Shabbir et al., 2022).
Environmental factors, mainly higher temperature and wind speed, increase the risk of NH3 volatilization (Chattha et al., 2022). It was found that an increase in soil temperature due to climate change increases the nitrification rate resulting in N loss and poor NUE (Engel et al., 2011). Higher soil compactness and wet conditions promote the denitrification process, whereas no-till and coarse soils showed higher leaching or volatilization/loss of nitrogen. In coarse soils, NH4NO3 fertilizer is subject to severe leaching and denitrification losses (Chattha et al., 2022).
Globally, the majority of countries are facing a decreasing trend of NUE (from 68% to 47%) over a period of five to six decades (1960–1970) (Lassaletta et al., 2014). Greater NUE in the initial years was probably due to higher native soil fertility, less use of additional nutrients, and favorable soil conditions (physical, chemical, and biological) (Figure 3). During the last decade, intensive management practices, monoculture, and increased use of off-farm input resources have resulted in low NUE (Lassaletta et al., 2014).
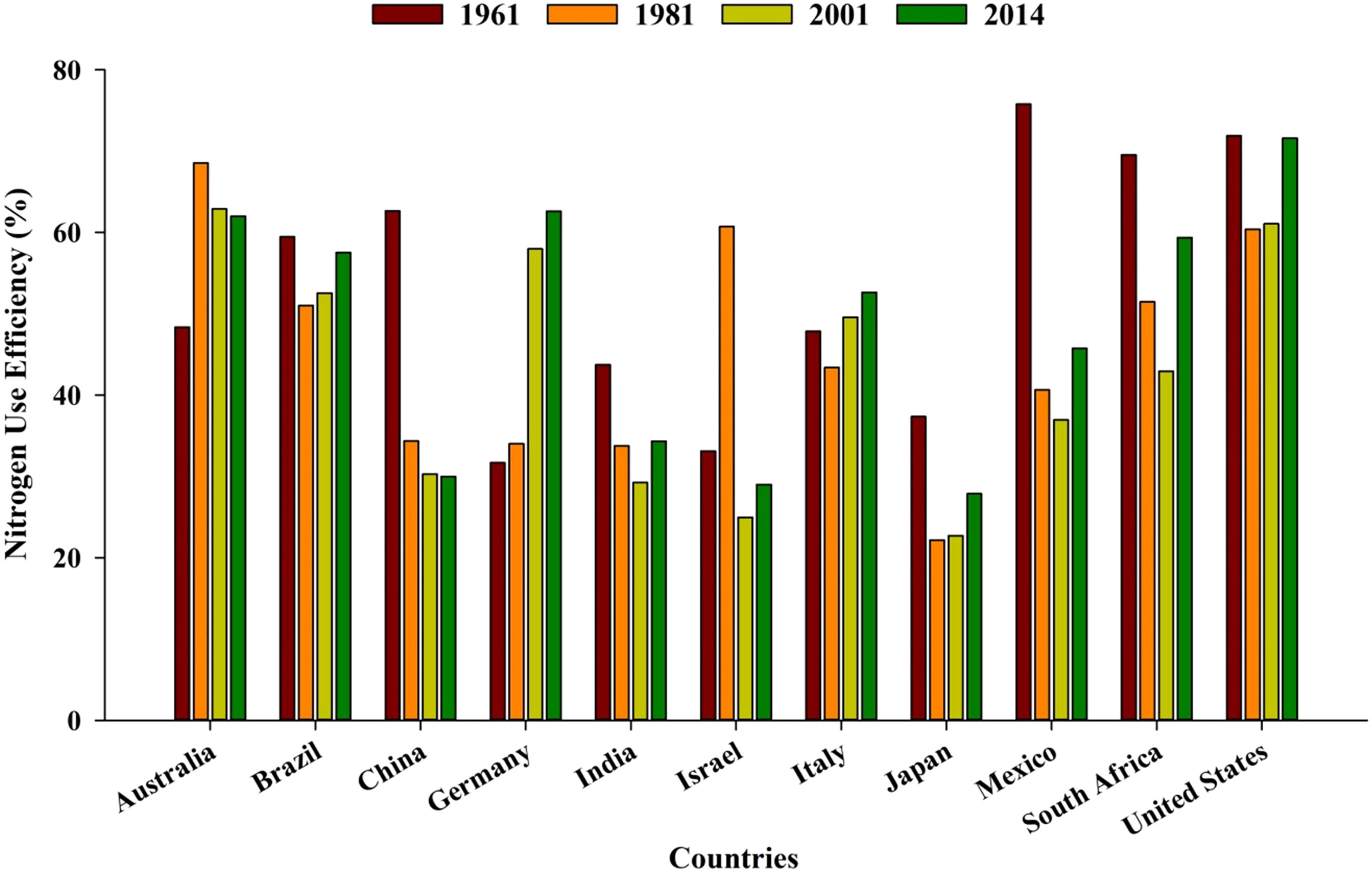
Figure 3 Average nitrogen use efficiency in different countries over the years (source: Lassaletta et al., 2014). https://ourworldindata.org/fertilizers http://creativecommons.org/licenses/by/4.0/deed.en_US.
Modern agriculture is entirely dependent on excessive N fertilizer application leading to ecosystem degradation and environmental pollution (Brender et al., 2013). According to estimates, 70% of applied nitrogen fertilizer is lost in the biosphere and affects the local and global atmospheric chemistry (Suthar et al., 2009). Nitrate pollution of groundwater in particular has led to numerous socioeconomic and environmental issues (Suthar et al., 2009). Nitrate contamination of drinking water is a major concern, particularly for children (Suthar et al., 2009; Brender et al., 2013). Continued consumption of NO3-contaminated drinking water (recommended limit of 10 mg NO3-N L−1) results in methemoglobinemia in children and gastric cancer among adults (Taneja et al., 2017). Moreover, NO3 or NH4+ contamination of water bodies promotes the growth of algae and other aquatic plants, which lowers the water’s oxygen level (Wild et al., 2001).
The oxide forms of N are highly reactive and harmful to the environment in many ways (Liu et al., 2019a). Excessive emissions of nitrous oxide and nitric oxide contribute to the formation of nitric acid, which is the key component of acid rain (Liu et al., 2019a). It significantly affects soil microbial communities and damages infrastructure (Liu et al., 2019a). Moreover, the atmospheric pollutant ozone is created when nitrous oxide combines with volatile organic pollutants (Karlsson et al., 2017). In this way, the loss of N leads to serious health and environmental problems. To avoid these consequences, the NUE of crops needs to be improved on a global basis.
The level of soil disturbance induced by different tillage practices affects soil N dynamics and plant N availability (Power and Peterson, 1998). For example, Francis and Knight (1993) reported that compared with conventional tillage systems, conservation tillage techniques reduced nitrogen availability. The absence of soil disturbance under the conservation tillage system can reduce the N mineralization rate, thereby decreasing the N availability to crops as well as the loss of N. In the conventional tillage system, however, increased oxidation of soil organic matter due to disturbance and exposure, as well as increased soil erosion, hastens the loss of soil organic matter (Schillinger et al., 1999). Soil organic matter loss caused by conventional tillage systems results in poor soil quality and low N availability. Therefore, the role of the tillage system will be vital for improving NUE.
The relationship between the conservation tillage system and NUE varies between studies, but overall NUE is often improved by the conservation tillage system (McConkey et al., 2002; Giacomini et al., 2010). Long-term conservation tillage systems (10–15 years) enhance the quantity of soil organic matter and increase the concentration of mineralizable organic nutrients at the soil surface layer (Sirivedhin and Gray, 2006), thereby improving the nutrient-supplying capacity of the soil (Van Den Bossche et al., 2009). As a result, conservation tillage systems that retain crop residues often result in higher crop yields and NUE compared with conventional tillage systems with a similar N application level (Stahl et al., 2019).
A long-term (10-year) study conducted in the southern United States of America showed that with the optimum application of N, cotton yields were higher in conservation tillage than in conventional tillage plots (Boquet et al., 2004). However, without N fertilizer application, the yields were lower in the conservation tillage system as a result of slow mineralization and immobilization of soil N (Boquet et al., 2004). For instance, in a study conducted in Kentucky, Phillips et al. (1980) found that fertilizer N applied on well-drained soil in a no-tillage system produced a greater (13.2 kg greater) corn yield per kilogram of applied N than under conventional tillage, but without N fertilizer, the corn yield was lower under a no-tillage system. On the contrary, crop residue retention, wetter soil surface, and anaerobic environments in no-till systems promote N immobilization, NH4 volatilization, and denitrification, negatively affecting N availability and NUE. In a wheat–fallow cropping system under the conventional tillage system, the N uptake was greater than that of stubble mulch systems. This is probably due to increased N immobilization in the stubble mulch system (Rasmussen and Rohde, 1991). Therefore, changes in N management, rate of application, and type of N fertilizer can improve NUE under conservation tillage systems. Overall, the role of conservation tillage and NUE requires more research to find practical compatibility.
It has been demonstrated that NUE could be improved through management practices such as timing, rate, source, and placement of fertilizer application. These practices are considered fundamentals to N management and may be refined or supplemented by emerging and future technologies, but not replaced.
The chemical composition of N fertilizers influences the NUE of crops. Urea-based N sources can be lost through volatilization when hydrolyzed to ammonia (Eq. 16) and the effect is intensified when urea is surface applied (Chien et al., 2009). Slow-release N fertilizers have the potential to minimize N leaching and denitrification losses and to improve the synchronization of N release and uptake in accordance with crop demand (Shapiro et al., 2016). Similarly, coated N sources such as neem-coated urea, sulfur-coated urea, and slow-release synthetic urea-based fertilizers such as isobutylidene diurea (IBDU) and crotobylidene diurea (CDU) have also improved the NUE. Polymer-coated urea was also found to reduce N volatilization loss (23%–62%) and ammonia emissions (51.3%–91.3%) and improve NUE (3%–34%). The combined application of 150 kg N through urea + 2,000 kg manure and 90 kg N + 2,000 kg manure under normal and dry years, respectively, has recorded maximum grain yield and NUE by improving the nitrogen nutritive index and nitrogen productivity of wheat in dry land area (Liu et al., 2023). Furthermore, the combined application of N fertilizer (276 kg ha−1) with biochar (15 t ha−1) produced the maximum yield of maize and the NUE (46.3%). Zhang et al. (2023) demonstrated that integrated application of 180 kg N ha−1 + 900 g Se ha−1 utilized the maximum resources and recorded maximum apparent recovery efficiency of N, agronomic N use efficiency, partial factor productivity, NUE, and grain yield of wheat.
Before determining the amount of fertilizer to apply, consider the soil’s nutrient-supplying capacity through a regionally appropriate soil testing program. Excessive fertilizer application leads to losses from the system, environmental problems, and economic losses to farmers. On the other hand, an insufficient nutrient application can exhaust soil fertility and lead to nutrient mining (degradation) and poor long-term soil productivity. Optimizing the nutrients’ rate based on soil status and crop requirement is the right way to improve NUE.
Typically, N fertilizers are applied either in single or two split applications. Split application of N at various crop stages is effective at increasing NUE. The application of 120 kg N ha−1 proved optimal to produce a higher grain yield and NUE in direct-seeded rice than 60 and 180 kg N ha−1 (Mahajan et al., 2012). A higher fodder maize seed yield (3.80 t ha−1) and N utilization were recorded for 120 kg N ha−1 in a semiarid region (Halli et al., 2019). Application of 180 kg ha−1 has been recommended to achieve higher grain yield, NUE, and protein yield of buckwheat (Wan et al., 2023). Hu et al. (2023) reported that among the rates of N studied (0, 150, 200, 250, and 300 kg ha−1), fertilization at the rate of 250 kg N ha−1 recorded a maximum grain yield, maximum grain N accumulation, improved aboveground dry biomass and N metabolism enzymes, and increased NUE in corn.
Site-specific N scheduling could be an alternative option to the blanket application of N. Results from a study conducted in 107 farmers’ fields indicated that the leaf color chart (LCC)-guided N management in hybrid rice had decreased N requirement by 25% without compromising the crop yield (Bhatia et al., 2012). Therefore, LCC can be further explored as a diagnostic tool to help farmers make appropriate decisions about N fertilizer applications throughout the crop cycle. However, the use of sensor-based N application techniques is still at a nascent stage in many parts of the world.
Fertilizer placement nearer to the root zone of crop plants, as opposed to even distribution in the field, has the potential to minimize N losses. The incorporation of fertilizers in the soil (via tillage or injection) is recommended over broadcasting (Ladha et al., 2005). The placement of N fertilizer under the seeds at the time of planting, band application, and fertilizer injection increased the NUE and reduced the NH3 volatilization compared with broadcasting in winter wheat (Dao, 1998; Ladha et al., 2005).
Deep placement of the USG fertilizer resulted in better N recovery efficiency (49%) compared with the broadcasting method (37%) in Australia (Schmidt et al., 2002). Granular ammonium nitrate fertilization at the depth of 20 cm below the soil surface recorded the highest NUE (134%), N recovery efficiency (18.1%), and grain yield (11%) in spring wheat and barley (Rychel et al, 2020). Qiang et al. (2022) noted that placement of controlled release urea at the depth of 16 cm achieved maximum grain yield, water productivity, partial factor productivity, and NUE in rainfed spring maize in Northern China.
Fertigation, or co-application of N with irrigation, is a viable option for the improvement of NUE. N fertilization at 15 cm depth increased grain yield (13.9%–98.9%), NUE (7.1%–44.3%), and N absorption (6.5%–38.0%) in summer maize (Chen et al., 2023). This approach gives the farmer with the proper equipment the flexibility to engage in multiple applications of low rates to minimize exposure to losses and optimize the opportunity of the crop to take up the right amount at the right time.
The timing of fertilizer application should coincide as close as possible with crop nutrient demand to avoid nutrient loss. For instance, in single applications, part of the applied nutrient is absorbed by plants, while a substantial portion is vulnerable to loss. Improved N partial factor productivity, agronomic N efficiency, N recovery efficiency, physiological efficiency, grain yield, and N uptake may be optimized when N is applied in four splits at the sowing, 6th leaf stage, 12th leaf stage, and silking stage in maize (Zhou et al., 2019). However, commercial-scale agriculture will likely avoid multiple trips across the field and traffic when the crop canopy has closed to reduce fuel, compaction, and crop damage. Likewise, the application of N fertilizer in three splits has increased the wheat grain yield and N recovery use efficiency (Liu et al., 2019b).
Ranatunga et al, 2018 indicated that more than 6 t ha−1 grain yields can be achieved in dry direct-seeded rice production systems when urea application is delayed by 10 days after sowing or split application compared with the blanket application. Although optimized in this way, the commercial-scale application on flooded rice will be impossible without aerial application. Split application of N at the time of sowing and later stages (V12, R1, and R2) increased the plant uptake, photosynthetic efficiency, and grain yield and improved NUE in summer maize (Deng et al., 2023). Late and split application of N during jointing, booting, anthesis, and grain filling stages through microsprinkler irrigation increased grain yield, protein concentration, and NUE of wheat by 5.8%, 8.6%, and 15.8%, respectively, as compared with the conventional method of fertilization and irrigation (Yao et al., 2023). N application with basal to top dressing ratio of 2:8 between the sowing and jointing stages recorded maximum dry matter yield, crude protein, N recovery, water, and N use efficiency of forage maize in a semiarid region of China (Ma et al., 2023). Hence, the split application of N would be superior to the blanket application, though the number and timing of these applications will be limited due to the practical considerations mentioned above.
Nitrogen use efficiency is also dependent on the ability of the cropping system (Ortiz-Monasterio et al., 1997; Reddy, 2011). Crop diversification can improve soil structure, soil health, vertical nutrient stratification, and mycorrhizal fungal interactions, as well as offer diversity in crop residues. A potential cropping system could help improve N availability and plant uptake (Tisdall and Oades, 1979; Lehman et al., 2012). Cereal- and legume-based cropping is the best system for leaving more residual N accumulation (Lehman et al., 2012). In a study with fallow followed by rice and legume followed by rice systems in Japan, the fertilizer NUE was higher for the legume (broad bean) followed by rice with 40 kg N application compared with fallow followed by rice in a clay loam soil (Rahman et al., 2009). Similarly, in a 20-year study on clay loam soil in Ontario, Canada, Gaudin et al. (2015) reported an increase in maize fertilizer NUE when winter wheat is inserted into a maize–soybean (especially when wheat is under-seeded with red clover) cropping system. In another study conducted in China, Li et al, 2022 found a higher N uptake and N harvest index in faba bean when intercropped with wheat compared with sole faba bean. The benefits associated with crop rotation and intercropping are mainly due to the facilitation through interaction between legumes and cereals and shallow-rooted and deep-rooted crops. Therefore, the rotation of crops with different depths of roots can improve soil structure and stability (Obalum and Obi, 2010) and enhance resource use efficiency (Halli et al, 2019). Tap-rooted crops can more easily penetrate compacted soil layers than shallow or fibrous-rooted crops, which serve to enhance the water and N use efficiency of the overall system (Chen and Ray, 2010). In Denmark, van Oosterom et al, 2010 observed a maximum mineralized N (81 kg N ha−1) within the rooting zone of pea–cabbage compared with the onion–cauliflower cropping sequence, where the mineralized N was only 52 kg ha−1 within the root zone. The selection of varieties/crops with different root systems, varied capacity to fix atmospheric N, and higher biomass production is an effective strategy to enhance NUE that deserves future research attention.
Nitrogen use efficiency of plants depends on the rate of soil N used by roots and accumulation in different plant parts such as the stem, leaf, and harvestable portions. Therefore, NUE is influenced by the inclusion of cover crops in a cropping system. The inclusion of high biomass-producing crops such as cover crops and dual-use forage crops can enhance the overall NUE of any system (Reicosky and Forcella, 1998). Cover crops are the crops planted in the off-season when the land is otherwise left uncultivated. Leaving land fallow increases the likelihood of soil erosion and nutrient leaching. Cover crops can help to protect the soil from loss, keep living roots in the soil as much of the year as possible, and recycle nutrients.
Cover crops with low C:N ratio residues (legume) can hasten the mineralization of organic N which may be responsible for the high NUE of the main crops (Franzluebbers et al., 2014). However, the cover crops with high biomass and high C:N ratio residues can lead to the immobilization of N, decreasing NUE for the following cash crop. A simulation model study using NLEAP (N Leaching and Economic Analysis Package) predicted that the inclusion of winter cover crops increased the NUE of lettuce by 3.1 kg per 4.5 kg of available N (Delgado, 1998). The cover crops in this study included winter wheat and rye, which were modeled to recover and retain soil NO3-N in tissue, preventing leaching loss and fertilizing the next crops. Similarly, planting cereal rye crops after no-till corn has reduced N leaching by 100% (McCracken, 1989). The CERES-N model modified by Quemada and Cabrera (1995) includes important considerations outside of simple C:N ratios to predict the mineralization or immobilization potential of cover crop residues. The model requires inputs for water-soluble carbohydrates, cellulose/hemicellulose, lignin, total C, total N, and C:N ratio.
Forage crops (often perennials) also contribute to the reduction in N loss and improved NUE. For example, a study from the USA reported that a perennial, such as alfalfa, reduced NO3-N leaching by 10-fold over a corn–soybean rotation or continuous corn systems (Randall and David, 2001). Moreover, persistent roots of forage grasses are important to bind the soil particles together to develop a stable soil structure and potentially capture N from 1.5 m deep in the soil. Thus, surface available N can be utilized by subsequent crops to improve NUE.
During the green revolution and post-green revolution, high fertilizer-responsive cultivars have been favored owing to low N-fertilizer costs. Though there are contradictory reports that under low N, more N-responsive modern varieties still perform better than historical varieties (Ding et al., 2005; Echarte et al., 2008), breeding efforts to develop high fertilizer-responsive cultivars under high fertilizer conditions have resulted in high-yielding cultivars with poor NUE (Garnett et al., 2015). As a consequence, yielding increases are fast approaching a theoretical limit with given physiological and genetic potential of crop cultivars under high N availability (Ali et al., 2018). To narrow down the demand–supply gap of food amid decreasing farmland and depleting soils around the globe without further magnifying environmental impacts, breeding strategies to improve the NUE of crop cultivars are becoming the prime focus of agricultural researchers (Fiaz et al., 2021; Ciampitti et al., 2022). Breeding for high input-responsive cultivars, occurring during the last five to six decades, is different from breeding for NUE. For NUE, the inherent capacity of the plant has to be improved and selected to facilitate efficient uptake and to use N and produce higher yield under moderate or marginal N availability (Anbessa and Juskiw, 2012). Therefore, breeding for high NUE is mainly aimed at realizing maximum benefit by reducing the N application rate while maintaining the high yield level.
Although there has been a consensus on the need to increase the NUE of crop plants through breeding, practically, no breeding program is primarily dedicated worldwide for this purpose, to the best of our knowledge. Theoretically, there may be different ways to improve NUE through breeding, such as overall consideration of grain yield or biomass growth under limited N conditions, selection and improvement of specific traits that contribute to high NUE, or introduction of the foreign gene. However, indirect selection for yield has been the common method for achieving higher NUE (Cormier et al., 2016).
NUE is considered a complex trait. Modifications in traits such as plant height, tiller number, dry weight of shoots and roots, grain yield, spikelet number, number of filled grains per panicle, 1,000-grain weight, and chloroplasts were reported to improve NUE (Lawlor, 2002; Zhao et al., 2011a; Hamaoka et al., 2013). Breeding targets for genetic improvement of the plant may be grouped into two major categories: first, improving N uptake efficiency by increasing uptake capacity (Le Gouis et al., 2000) and breeding for ideal root morphology (Liao et al., 2006) and, second, improving N utilization efficiency by modifying the leaf area index, specific leaf N, and biomass yield (Gastal and Lemaire, 2002) and by delaying the senescence (Foulkes et al., 2009).
Before initiating the new breeding efforts to create genetic variability for high NUE in modern crop cultivars, the rich genetic resources conserved in different gene/seed banks of the world should be explored for screening high NUE lines. There is proven genetic diversity for root N uptake in plants (Pereira et al, 2010; Le Gouis et al., 2000), and exploiting this property requires researchers to understand the underlying mechanism of higher root uptake.
Root morphology plays a critical role in modulating N uptake by plants (Garnett and Rebetzke, 2013). Plants with rapid root growth can minimize N losses that occur through various field processes (Gastal and Lemaire, 2002). Anbessa and Juskiw (2012) observed that barley plants with higher root dry weight and volume assessed at the five-leaf stage showed higher NUE than normal plants. Improvements in root traits such as length of root, root-length density, the radius of the root, root surface area, and number, length, and density of root hairs (Wang et al., 2006) are associated with greater N uptake in plants. Breeding efforts for enhancing root-related traits are essential for improving NUE. However, the limited scope of large-scale and high-throughput root phenotyping creates obstacles in breeding programs for selecting and screening specifically for such beneficial root architecture (Fiorani and Schurr, 2013).
The uptake of additional N must match with the metabolism of the plants to avoid systemic feedback control of metabolites representative of the whole-plant N status (Nacry et al., 2013). The uptake and utilization of N for the entire plant growth period can be separated into two phases: pre-anthesis and post-anthesis (Cormier et al., 2016). At the pre-anthesis stage, plants take up N, and the whole-plant system utilizes it upon receiving fractional interception of light at the start of the stem elongation phase. However, at post-anthesis, once grains appear, plants begin partitioning available N for higher grain yield, jeopardizing the simultaneous improvement in grain yield and protein content (Oury and Godin, 2007). Higher N utilization is possible under low N supply through an increased specific leaf N area (SLN), which is reported to be associated with the embryo size of the plant (López-Castañeda et al., 1996) and earlier canopy closure (Rebetzke and Richards, 1999). Physiological conditions wherein N is more efficiently utilized are associated with the abundance of prostrate leaves during vegetative growth and semi-erect to erect leaves during later vegetative and reproductive stages. This can be difficult for plant architecture to manipulate (Cormier et al., 2016). Normally, at the post-anthesis stage, the grains draw N from the stem and rachis in cereals and then from leaves if necessary. However, the stay-green plant types are prone to supply N to growing grains slowly and thus impact the balance in the N demand–supply framework (van Oosterom et al., 2010). Researchers are in consensus that physiologically important traits that directly or indirectly improve N utilization are taken into consideration in breeding programs, in addition to the common target traits. However, assessing those traits on the bulk scale is a question of technological advancement, resources available to the breeders, and practical limitations (Cormier et al., 2016).
The integration of molecular tools, such as genomics and marker-assisted breeding, into traditional breeding programs has revolutionized genetic enhancements for various intricate traits in crops (Jagannadham et al., 2019). The incorporation of these tools has significantly increased the efficiency of the selection process, resulting in a reduction in the time and resources required to develop improved varieties or hybrids. Recent advances in genomics have further accelerated the generation of genomic resources for many crops, providing breeders with more data and insights into the genetic makeup of crops, ultimately leading to more effective breeding strategies (Kumar et al., 2018c; Jagannadham et al., 2019). Ultimately, these resources can be exploited for identifying, characterizing, and developing molecular markers linked to N-responsive genes in crop plants (Yang et al., 2017; Lenka et al., 2018). Two molecular approaches can be explored for improving NUE in crops; one is through a traditional breeding strategy combined with genomic selection, and the other is a transgenic approach, which would target specific NUE-associated genes for the genetic engineering of the plant (Good and Beatty, 2011; McAllister et al., 2012; Kumar et al., 2018c).
It is of utmost importance to identify genes or QTLs that govern NUE to enable the breeding of crops with high NUE using approaches such as marker-assisted selection (MAS) and genomic selection. Nutrient use efficiency is a complex trait, and as a result, several research groups have undertaken efforts to map the genetic loci in correlation with specific traits (Balyan et al., 2016; Kumar et al., 2018b; Mălinaş et al., 2022). In rice, 20 single QTLs (S-QTLs) and 58 pairs of epistatic loci (E-QTLs) were identified for the grain N, straw N, shoot N, harvest index, grain yield, straw yield, and PE in low N and ordinary N conditions. Harvest index and grain yield were positively correlated with PE in both conditions (Cho et al., 2007). In another study carried out with rice, four QTL clusters harboring QTLs for both NDT and NUE traits were identified (Wei et al., 2012). In European winter wheat, a genome-wide association study using 214 varieties identified 333 genomic regions associated with 28 traits related to NUE (Cormier et al., 2014). For the second approach, specific NUE-associated genes should be identified. Some of the efforts successfully mapped genes and identified QTLs. In maize, a meta-analysis of published NUE QTLs revealed 37 “consensus” QTLs, of which 18 were detected under low N conditions. Comparing expressed sequence tags (ESTs) associated with low N stress response, N uptake and transport, and assimilation with the QTL map has resulted in identifying candidate NUE-associated genes. Among those genes, nine candidates introgressed into Ye478 have significantly altered grain yield/yield components (Liu et al., 2012). Five significant QTL clusters associated with large-rooted architecture and high N uptake efficiency (NupE) were identified in maize. The root system architecture (RSA), such as that found in maize, has an essential role in N acquisition. NupE had significant phenotypic correlations with RSA (Li et al., 2015). Three QTLs, NUE1a, NUE1b, and NUE2, were identified in maize for NUE (Mandolino et al., 2018). Under N starvation, the expression of TaNLP7 displayed enhanced expression in root and shoot tissues of the high NUE genotype (Kumar et al., 2018a). Forty-seven genes are known to involve N uptake, metabolism, and distribution in maize (Wani et al., 2021). In barley, 10 independent mapping studies were screened and a number of NUE-associated genes that control complex physiological traits were mapped (Han et al., 2016). Even though a large number of reports claim to be identifying QTLs for NUE, some of them are yet to be validated. Since NUE involves a myriad of factors, the traditional breeding strategy combined with MAS will be cumbersome. Therefore, exploiting genomic selection for improving NUE will speed up the development of superior genotypes by combining high-throughput phenotyping and genotyping (Han et al., 2016; Kumar et al., 2018c; Stahl et al., 2019). In wheat, four QTLs, viz., QNue.151-1D, QNue.151-4A, QNue.151-6A, and QNue.151-7D, were associated with NUE; one QTL, QNupe.151-4A, was associated with N uptake efficiency; and one QTL, QNute.151-4A, was associated with N utilization efficiency (Brasier et al., 2020). The details of the QTLs identified in the crop plants are given in Table 4.
The role of small RNAs in regulating the nutrition assimilation/starvation response is well documented in many crops (Balyan et al., 2016). A total of 126 long non-coding RNAs (lncRNAs) were altered during N starvation, and these RNAs regulate various protein-coding genes involved in diverse cellular functions (Chen et al., 2016). Forty-four miRNAs are differentially regulated under high and low N conditions (Li et al., 2016). Most of these targets were found to be the genes encoding for the transcription factors. The important miRNAs and transcription factors involved in the N starvation response in Arabidopsis are shown in Figure 4. In Arabidopsis and maize, the expression of miR167 was enhanced under N starvation conditions (Xu et al., 2011; Balyan et al., 2016). miR167 regulates the lateral root growth response to N starvation in Arabidopsis (Gifford et al., 2008). Conversely, downregulation of the transcription factors ARF10, ARF16, and ARF17 by N-responsive miR160 regulates the process of seed germination and development of the seedling after post-germination under N-deficient conditions (Liu et al., 2007; Hao et al., 2022). Downregulation of miR169 enhances the expression of the NFYA transcription factors; these genes regulate the function of the nitrate transporter genes, viz., AtNRT1.1 and AtNRT2.1 (Zhao et al., 2011b). These studies showed the involvement of small RNAs and their functional importance in inducing/repressing multiple genes in response to N assimilation/deprivation and regulation of root development in plants. In wheat, simple sequence repeat markers developed from miR171a effectively group the panel of wheat genotypes into N-efficient and non-efficient markers. These markers can be employed to characterize the wheat germplasm/breeding lines in crop breeding programs (Sagwal et al., 2022).
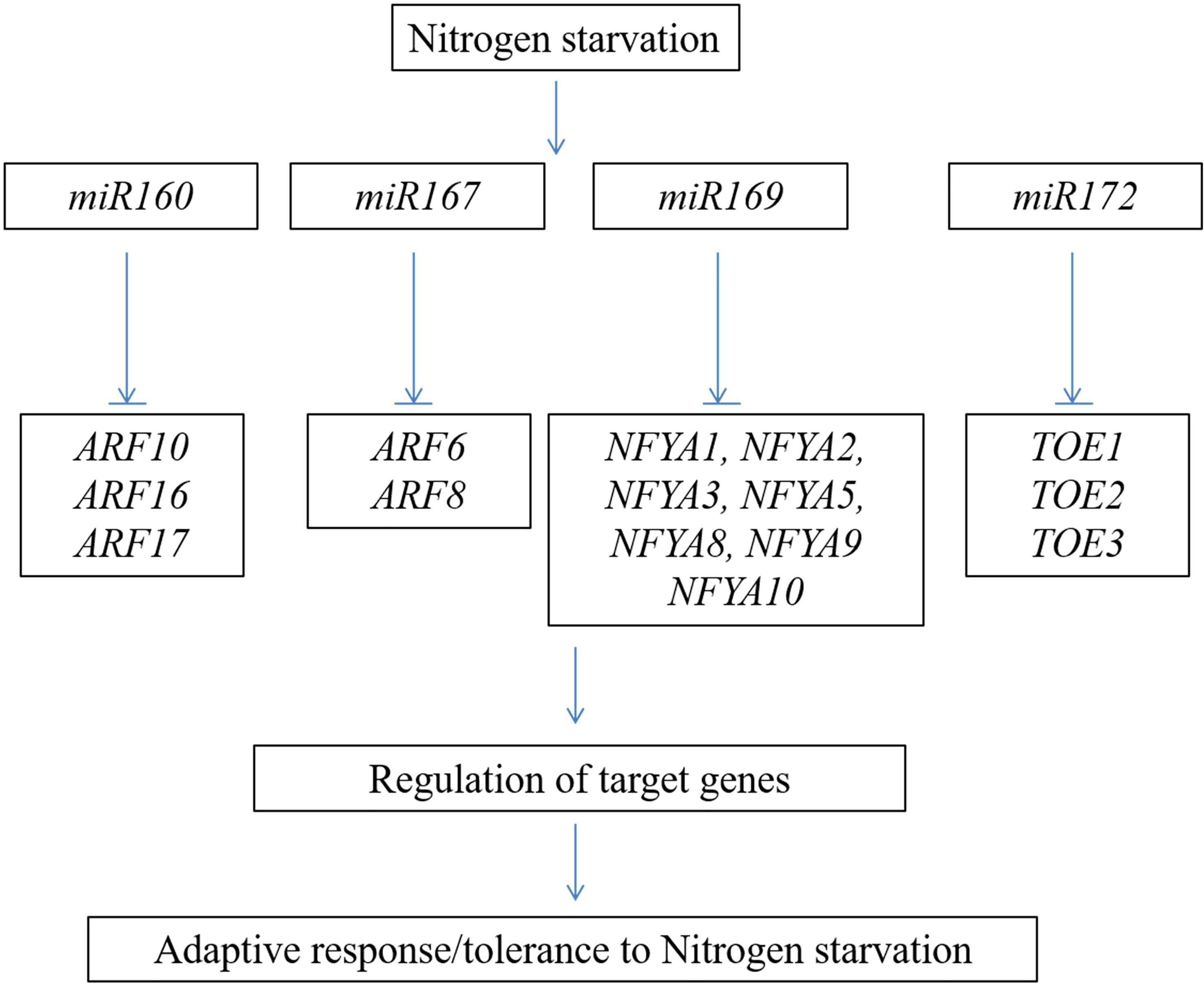
Figure 4 Schematic representation of important nitrogen-responsive miRNAs and their transcription factor targets involved in nitrogen starvation response.
Plants have evolved mechanisms to alter the molecular machinery in response to N availability (Gaudinier et al., 2018). Yang et al. (2017) identified 1,158 and 492 genes that were differentially expressed in leaf sheaths and roots, respectively, after 12 h of N starvation in rice. Conversely, in Dunaliella salina, 3,127 were differentially expressed (2,380 genes were upregulated and 747 were downregulated) under N starvation (Lv et al., 2019). In maize, ZmGLK5, bZIP108, CLC-a, and miRNA399b genes play a significant role in regulating genes in response to N (Jiang et al., 2018). The NIGT1/HRS1s transcriptional repressors are essential in regulating N starvation response during high N availability (Kiba et al., 2018). The CLE peptides and the CBL7 and TAR2 proteins regulate root architecture in response to N starvation (Kiba et al., 2018); the DUR3 and AMT family proteins play an important role in the uptake of urea and ammonium, respectively, during N starvation (Krapp et al., 2014). The nitrate transporters, NRT1/NPF and NRT2, regulate nitrate uptake (Krapp et al., 2014).
The availability of high-throughput genomics tools and efficient transformation systems in model crops further eases the functional validation of NUE (Muthusamy et al., 2016; Lenka et al., 2019). Several attempts have been made to develop transgenics with high NUE. Overexpression of AtDof1, AtGS1, and AtGS2 enhances the N assimilation in transgenic tobacco lines grown under N-starved conditions compared with wild-type plants (Wang et al., 2013). Transgenic overexpression of OsDof25 modulates C and N metabolism in transgenic Arabidopsis lines during an increased supply of N (Santos et al., 2012). Plant species comprising the C4 photosynthetic pathway have evolved highly efficient molecular mechanisms of carbon fixation. C4 plants exhibit high radiational, N, and water use efficiencies compared with species with the C3 photosynthetic mechanism (Ghannoum et al., 2011; Muthusamy et al., 2019). Engineering the genes involved in the C4 photosynthetic pathway in C3 plants remains an essential strategy for enhancing the NUE in C3 crops (Lin et al., 2019; Muthusamy et al., 2019). Moreover, the availability of N regulates the ethylene and jasmonic acid hormone signaling, thereby regulating the plant response to pathogen infection (Vega et al., 2015; Farjad et al., 2018). miRNAs are known to play an important role in regulating the function of N-responsive genes during N-limiting conditions (Nguyen et al., 2015; Zuluaga et al., 2017). Thus, the identification of gene regulatory networks, including small RNAs involved in regulating the stress response, will further help to understand the development of stress-responsive crops with high NUE (Muthusamy et al., 2017; Zuluaga et al., 2017; Farjad et al., 2018). The details of the QTLs identified in the crop plants are given in Table 5.
In global agriculture, the low-efficiency uptake by crops of applied N fertilizer is a major concern because of its negative impact on production costs and the environment. To improve NUE in crops, modern agronomic, breeding, and biotechnological strategies should be incorporated to supplement fundamental nutrient management. Agronomic practices such as precise timing and placement of N fertilizer, site-specific nutrient management, conservation tillage, crop residue retention, and cultivation of high biomass crops can enhance NUE under various soil and climatic conditions. NUE is a multifaceted trait that involves physiological, biochemical, and molecular regulations. Therefore, the engineering of N-responsive genes through genome editing has great potential for improving NUE in crops. To breed superior genotypes with high NUE, the use of genomic selection combined with speed breeding techniques in breeding programs is expected to be a valuable approach in the future.
PG, SM, MB, RV, PJ, AM, HH, RR, SB, VP, GT and ML: manuscript writing and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
N, nitrogen; NUE, nitrogen use efficiency; PAN, plant-available N; NH4+, ammonium; NO3−, nitrate; NH3, ammonia; PE, physiological nitrogen use efficiency; NDT, nitrogen deficiency tolerance; NE, nitrogen utilization efficiency; NupE, nitrogen uptake efficiency; QTL, quantitative trait loci; RSA, root system architecture.
Ali, J., Jewel, Z. A., Mahender, A., Anandan, A., Hernandez, J., Li, Z. (2018). Molecular genetics and breeding for nutrient use efficiency in rice. Int. J. Mol. Sci. 19, 1762. doi: 10.3390/ijms19061762
Alonso-Ayuso, M., Gabriel, J. L., Quemada, M. (2016). Nitrogen use efficiency and residual effect of fertilizers with nitrification inhibitors. Eur. J. Agron. 80, 1–8. doi: 10.1016/j.eja.2016.06.008
An, Y., Wan, S., Zhou, X., Subedar, A. A., Wallace, L. L., Luo, Y. (2005). Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Global Change Biol. 11 (10), 1733–1744. doi: 10.1111/j.1365-2486.2005.01030.x
Anas, M., Liao, F., Verma, K. K., Sarwar, M. A., Mahmood, A., Chen, Z. L., et al. (2020). Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 53 (1), 1–20. doi: 10.1186/s40659-020-00312-4
Anbessa, Y., Juskiw, P. (2012). Review: Strategies to increase nitrogen use efficiency of spring barley. Can. J. Plant Sci. 92, 617–625. doi: 10.4141/cjps2011-207
Baggs, E. M., Rees, R. M., Smith, K. A., Vinten, A. J. A. (2000). Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manage. 16 (2), 82–87. doi: 10.1111/j.1475-2743.2000.tb00179.x
Bai, E., Li, S., Xu, W., Li, W., Dai, W., Jiang, P. (2013). A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 199 (2), 441–451. doi: 10.1111/nph.12252
Balasubramanian, V., Alves, B., Aulakh, M. S., Bekunda, M., Cai, Z., Drinkwater, L., et al. (2004). “Crop, environmental and management factors affecting nitrogen use efficiency,” in Agriculture and the nitrogen cycle: Assessing the impacts of fertilizer use on food production and the environment. Eds. Mosier, A. R., Syers, J. K., Freney, J. R. (Washington, USA: Island Press), 19–33.
Balyan, H. S., Gahlaut, V., Kumar, A., Jaiswal, V., Dhariwal, R., Tyagi, S., et al. (2016). “Nitrogen and phosphorus use efficiencies in wheat: Physiology, phenotyping, genetics, and breeding,” in Plant breeding reviews, vol. 40. (Hoboken, New Jersey: John Wiley & Sons, Ltd), 167–234. doi: 10.1002/9781119279723.ch4
Batjes, N. H. (2014). Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 47, 151–163. doi: 10.1111/j.1365-2389.1996.tb01386.x
Bhatia, A., Pathak, H., Jain, N., Singh, P. K., Tomer, R. (2012). Greenhouse gas mitigation in rice–wheat system with leaf color chart-based urea application. Environ. Monit. Assess. 184 (5), 3095–3107. doi: 10.1007/s10661-011-2174-8
Bird, J. A., Horwath, W. R., Eagle, A. J., van Kessel, C. (2001). Immobilization of fertilizer nitrogen in rice: effects of straw management practices. Soil Sci. Soc Am. J. 65 (4), 1143–1152. doi: 10.2136/sssaj2001.6541143x
Bock, B. R., Kissel, D. E. (1988). Ammonia volatilization from urea fertilizers. (National Fertilizer Development Center, Tennessee Valley Authority 3, 189.
Bock, E., Schmidt, I., Stüven, R., Zart, D. (1995). Nitrogen loss caused by denitrifying nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch. Microbiol. 163 (1), 16–20. doi: 10.1007/BF00262198
Boincean, B., Dent, D. (2019). Farming the black earth. sustainable and climate-smart management of chernozem soil (Cham: Spring Nature Switherland AG), 125–149.
Boquet, D. J., Hutchinson, R. L., Breitenbeck, G. A. (2004). Long-term tillage, cover crop, and nitrogen rate effects on cotton: Yield and fiber properties. Agron. J. 96 (5), 1436–1442. doi: 10.2134/agronj2004.1436
Bouwman, A. F., Beusen, A. H. W., Griffioen, J., Van Groenigen, J. W., Hefting, M. M., Oenema, O., et al. (2013). Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philos. T. R. Soc Biol. Sci. 368 (1621), 20130112. doi: 10.1098/rstb.2013.0112
Brasier, K., Ward, B., Smith, J., Seago, J., Oakes, J., Balota, M., et al. (2020). Identification of quantitative trait loci associated with nitrogen use efficiency in winter wheat. PloS One 15, e0228775. doi: 10.1371/journal.pone.0228775
Braun, J., Mooshammer, M., Wanek, W., Prommer, J., Walker, T. W., Rütting, T., et al. (2018). Full 15N tracer accounting to revisit major assumptions of 15N isotope pool dilution approaches for gross nitrogen mineralization. Soil Biol. Biochem. 117, 16–26. doi: 10.1016/j.soilbio.2017.11.005
Brender, J. D., Weyer, P. J., Romitti, P. A., Mohanty, B. P., Shinde, M. U., Vuong, A. M., et al. (2013). Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Persp. 121 (9), 1083–1089. doi: 10.1289/ehp.1206249
Canisares, L. P., Grove, J., Miguez, F., Poffenbarger, H. (2021). Long-term no-till increases soil nitrogen mineralization but does not affect optimal corn nitrogen fertilization practices relative to inversion tillage. Soil Tillage Res. 213, 105080. doi: 10.1016/j.still.2021.105080
Chattha, M. S., Ali, Q., Haroon, M., Afzal, M. J., Javed, T., Hussain, S., et al. (2022). Enhancement of nitrogen use efficiency through agronomic and molecular based approaches in cotton. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.994306
Chen, B., Liu, E., Tian, Q., Yan, C., Zhang, Y. (2014). Soil nitrogen dynamics and crop residues. a review. Agron. Sus. Dev. 34 (2), 429–442. doi: 10.1007/s13593-014-0207-8
Chen, G., Ray, R. W. (2010). Penetration of cover crop roots through compacted soils. Plant Soil. 331 (1), 31–43. doi: 10.1007/s11104-009-0223-7
Chen, M., Wang, C., Bao, H., Chen, H., Wang, Y. (2016). Genome-wide identification and characterization of novel lncRNAs in populus under nitrogen deficiency. Mol. Genet. Genomics 291, 1663–1680. doi: 10.1007/s00438-016-1210-3
Chen, G., Wu, P., Wang, J., Zhou, Y., Ren, L., Cai, T., et al. (2023). How do different fertilization depths affect the growth, yield, and nitrogen use efficiency in rain-fed summer maize? Field Crops Res. 290, 108759. doi: 10.1016/j.fcr.2022.108759
Chien, S. H., Prochnow, L. I., Cantarella, A. H. (2009). Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 102, 267–322. doi: 10.1016/S0065-2113(09)01008-6
Cho, Y.-I., Jiang, W., Chin, J.-H., Piao, Z., Cho, Y.-G., McCouch, S., et al. (2007). Identification of QTLs associated with physiological nitrogen use efficiency in rice. Mol. Cells 23, 72–79.
Choi, I. H., Moore, J. P.A. (2008). Effect of various litter amendments on ammonia volatilization and nitrogen content of poultry litter. J. Appl. Poultry Res. 17 (4), 454–462. doi: 10.3382/japr.2008-00012
Ciampitti, I. A., Briat, J.-F., Gastal, F., Lemaire, G. (2022). Redefining crop breeding strategy for effective use of nitrogen in cropping systems. Commun. Biol. 5, 1–4. doi: 10.1038/s42003-022-03782-2
Cormier, F., Foulkes, J., Hirel, B., Gouache, D., Moënne-Loccoz, Y., Le Gouis, J. (2016). Breeding for increased nitrogen-use efficiency: a review for wheat (T. aestivum l.). Plant Breed. 135, 255–278. doi: 10.1111/pbr.12371
Cormier, F., Le Gouis, J., Dubreuil, P., Lafarge, S., Praud, S. (2014). A genome-wide identification of chromosomal regions determining nitrogen use efficiency components in wheat (Triticum aestivum l.). Theor. Appl. Genet. Theor. Angew. Genet. 127, 2679–2693. doi: 10.1007/s00122-014-2407-7
Coskun, D., Britto, D. T., Shi, W., Kronzucker, H. J. (2017). Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 3 (6), 1–10. doi: 10.1038/nplants.2017.74
Dao, T. H. (1998). Tillage and crop residue effects on carbon dioxide evolution and carbon storage in a paleustoll. Soil Sci. Soc Am. J. 62 (1), 250–256. doi: 10.2136/sssaj1998.03615995006200010032x
Davies, B., Coulter, J. A., Pagliari, P. H. (2020). Timing and rate of nitrogen fertilization influence maize yield and nitrogen use efficiency. PloS One 15 (5), e0233674. doi: 10.1371/journal.pone.0233674
De Baets, S., Poesen, J., Meersmans, J., Serlet, L. (2011). Cover crops and their erosion-reducing effects during concentrated flow erosion. Catena 85 (3), 237–244.
Delgado, J. A. (1998). Sequential NLEAP simulations to examine effect of early and late planted winter cover crops on nitrogen dynamics. J. Soil Water Conserv. 53 (3), 241–244.
Deng, T., Wang, J. H., Gao, Z., Shen, S., Liang, X. G., Zhao, X., et al. (2023). Late split-application with reduced nitrogen fertilizer increases yield by mediating source–sink relations during the grain filling stage in summer maize. Plants 12 (3), 625. doi: 10.3390/plants12030625
Devasirvatham, V., Tan, D. K. Y., Gaur, P. M., Raju, T. N., Trethowan, R. M. (2012). High temperature tolerance in chickpea and its implications for plant improvement. Crop Pasture Sci. 63 (5), 419–428. doi: 10.1071/CP11218
Di, H. J., Cameron, K. C. (2002). Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr. Cycling Agroecosyst. 64 (3), 237–256. doi: 10.1023/A:1021471531188
Ding, J., Li, F., Le, T., Xu, D., Zhu, M., Li, C., et al. (2021). Tillage and seeding strategies for wheat optimizing production in harvested rice fields with high soil moisture. Sci. Rep. 11 (1), 1–12. doi: 10.1038/s41598-020-80256-7
Ding, L., Wang, K. J., Jiang, G. M., Biswas, D. K., Xu, H., Li, L. F., et al. (2005). Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Ann. Bot. 96, 925–930. doi: 10.1093/aob/mci244
Dong, N. Q., Lin, H. X. (2020). Higher yield with less nitrogen fertilizer. Nat. Plants 6 (9), 1078–1079. doi: 10.1038/s41477-020-00763-3
Doydora, S. A., Cabrera, M. L., Das, K. C., Gaskin, J. W., Sonon, L. S., Miller, W. P. (2011). Release of nitrogen and phosphorus from poultry litter amended with acidified biochar. Int. J. Environ. Res. Pub. Health 8 (5), 1491–1502. doi: 10.3390/ijerph8051491
Ebrahim, M. K., Zingsheim, O., El-Shourbagy, M. N., Moore, P. H., Komor, E. (1998). Growth and sugar storage in sugarcane grown at temperatures below and above optimum. J. Plant Physiol. 153 (5-6), 593–602. doi: 10.1016/S0176-1617(98)80209-5
Echarte, L., Rothstein, S., Tollenaar, M. (2008). The response of leaf photosynthesis and dry matter accumulation to nitrogen supply in an older and a newer maize hybrid. Crop Sci. 48, 656–665. doi: 10.2135/cropsci2007.06.0366
Engel, R., Jones, C., Wallander, R. (2011). Ammonia volatilization from urea and mitigation by NBPT following surface application to cold soils. Soil Sci. Soc Am. J. 75, 2348–2357. doi: 10.2136/sssaj2011.0229
Fageria, N. K., Baligar, V. C. (2005). Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 88, 97–185. doi: 10.1016/S0065-2113(05)88004-6
Fageria, N. K., Moreira, A., Moraes, L. A. C., Moraes, M. F. (2014). Nitrogen uptake and use efficiency in upland rice under two nitrogen sources. Commun. Soil Sci. Plant Anal. 45 (4), 461–469. doi: 10.1080/00103624.2013.861907
Fan, X. H., Li, Y. C., Alva, A. K. (2011). Effects of temperature and soil type on ammonia volatilization from slow-release nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 42 (10), 1111–1122. doi: 10.1080/00103624.2011.566957
Farjad, M., Rigault, M., Pateyron, S., Martin-Magniette, M.-L., Krapp, A., Meyer, C., et al. (2018). Nitrogen limitation alters the response of specific genes to biotic stress. Int. J. Mol. Sci. 19, 3364. doi: 10.3390/ijms19113364
Farzadfar, S., Knight, J. D., Congreves, K. A. (2021). Soil organic nitrogen: an overlooked but potentially significant contribution to crop nutrition. Plant and Soil 462, 7–23.
Fazily, T., Thakral, S. K., Dhaka, A. K., Sharma, M. K. (2020). Effect of integrated nutrient management on fertilizer use efficiency in wheat (Triticum aestivum l.) under irrigated condition. Int. J. Adv. Agric. Sci. Technol. 7, 1–9. doi: 10.47856/ijaast.2021.v08i3.002
Fiaz, S., Wang, X., Khan, S. A., Ahmar, S., Noor, M. A., Riaz, A., et al. (2021). Novel plant breeding techniques to advance nitrogen use efficiency in rice: A review. GM Crops Food 12, 627–646. doi: 10.1080/21645698.2021.1921545
Fiorani, F., Schurr, U. (2013). Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 64, 267–291. doi: 10.1146/annurev-arplant-050312-120137
Foulkes, M. J., Hawkesford, M. J., Barraclough, P. B., Holdsworth, M. J., Kerr, S., Kightley, S., et al. (2009). Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crops Res. 114, 329–342. doi: 10.1016/j.fcr.2009.09.005
Fowler, D. M., Coyle, U., Skiba, M. A., Sutton, J. N., Cape, S., Reis-Sheppard, L. J., et al. (2013). The global nitrogen cycle in the twenty-first century. Philos. T. R. Soc B. 368 (1621), 20130164. doi: 10.1098/rstb.2013.0164
Fowler, D., Steadman, C. E., Stevenson, D., Coyle, M., Rees, R. M., Skiba, U. M., et al. (2015). Effects of global change during the 21st century on the nitrogen cycle. Atmos. Chemist. Phys. 15 (24), 13849–13893. doi: 10.5194/acp-15-13849-2015
Francis, G. S., Knight, T. L. (1993). Long-term effects of conventional and no-tillage on selected soil properties and crop yields in Canterbury, new Zealand. Soil Tillage Res. 26 (3), 193–210. doi: 10.1016/0167-1987(93)90044-P
Franzluebbers, A. J., Jorge, S., Taboada, M. A. (2014). Agronomic and environmental impacts of pasture–crop rotations in temperate north and south America. Agric. Ecosyst. Environ. 190, 18–26. doi: 10.1016/j.agee.2013.09.017
Fromme, D. D., Coker, D. L., McFarland, M. L., Mowrer, J. E., Provin, T. L., Schnell, R. W., et al (2017). Residual soil nitrogen credits for corn production along the upper Texas Gulf Coast region. J. Plant Nutr. 40, 23–32. doi: 10.1080/01904167.2016.1184279
Galloway, J. N., Aber, J. D., Erisman, J. W., Seitzinger, S. P., Howarth, R. W., Cowling, E. B., et al. (2003). The nitrogen cascade. Biosci. 53 (4), 341–356. doi: 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Garbeva, P., Baggs, E. M., Prosser, J. I. (2007). Phylogeny of nitrite reductase (nirK) and nitric oxide reductase (norB) genes from Nitrosospira species isolated from soil.". FEMS. Microbiol. Lett. 266 (1), 83–89. doi: 10.1111/j.1574-6968.2006.00517.x
Garnett, T., Plett, D., Heuer, S., Okamoto, M., Garnett, T., Plett, D., et al. (2015). Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals: challenges and future directions. Funct. Plant Biol. 42, 921–941. doi: 10.1071/FP15025
Garnett, T. P., Rebetzke, G. J. (2013). “Improving crop nitrogen use in dryland farming,” in Improving water and nutrient-use efficiency in food production systems (Hoboken, New Jersey: John Wiley & Sons, Ltd), 123–144. doi: 10.1002/9781118517994.ch8
Gastal, F., Lemaire, G. (2002). N uptake and distribution in crops: an agronomical and ecophysiological perspective. J. Exp. Bot. 53, 789–799. doi: 10.1093/jexbot/53.370.789
Gaudin, A. C., Janovicek, K., Deen, B., Hooker, D. C. (2015). Wheat improves nitrogen use efficiency of maize and soybean-based cropping systems. Agric. Ecosyst. Environ. 210, 1–10. doi: 10.1016/j.agee.2015.04.034
Gaudinier, A., Rodriguez-Medina, J., Zhang, L., Olson, A., Liseron-Monfils, C., Bågman, A.-M., et al. (2018). Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 563, 259–264. doi: 10.1038/s41586-018-0656-3
Getahun, B. B., Visser, R. G. F., van der Linden, C. G. (2020). Identification of QTLs associated with nitrogen use efficiency and related traits in a diploid potato population. Am. J. Potato Res. 97, 185–201. doi: 10.1007/s12230-020-09766-4
Ghaffar, A., Anjum, S. A., Cheema, M. (2012). Effect of nitrogen on growth and yield of sugarcane. J. Am. Soc Sugar Cane. Technol. 32, 75. doi: 10.1007/s10705-020-10074-w
Ghannoum, O., Evans, J. R., von Caemmerer, S. (2011). “Chapter 8 nitrogen and water use efficiency of C4 plants,” in C4 photosynthesis and related CO2 concentrating mechanisms advances in photosynthesis and respiration. Eds. Raghavendra, A. S., Sage, R. F. (Dordrecht: Springer Netherlands), 129–146. doi: 10.1007/978-90-481-9407-0_8
Giacomini, S. J., Machet, J. M., Boizard, H., Recous, S. (2010). (2010). dynamics and recovery of fertilizer 15N in soil and winter wheat crop under minimum versus conventional tillage. Soil Tillage Res. 108, 51–58. doi: 10.1016/j.still.2010.03.005
Gifford, M. L., Dean, A., Gutierrez, R. A., Coruzzi, G. M., Birnbaum, K. D. (2008). Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. U. S. A. 105, 803–808. doi: 10.1073/pnas.0709559105
Giller, K. E., Chalk, P., Dobermann, A., Hammond, L., Heffer, P., Ladha, J. K., et al. (2004). Emerging technologies to increase the efficiency of use of fertilizer nitrogen. Agric. nitrogen cycle: assessing impacts fertilizer Use Food production Environ. 65, 35–51.
Glass, A. D. (2003). Nitrogen use efficiency of crop plants: physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 22 (5), 453–470. doi: 10.1080/07352680390243512
Good, A. G., Beatty, P. H. (2011). Fertilizing nature: a tragedy of excess in the commons. PloS Biol. 9, e1001124. doi: 10.1371/journal.pbio.1001124
Grizzetti, B., Pretato, U., Lassaletta, L., Billen, G., Garnier, J. (2013). The contribution of food waste to global and European nitrogen pollution. Environ. Sci. Policy 33, 186–195. doi: 10.1016/j.envsci.2013.05.013
Guo, S., Klinkesorn, U., Lorjaroenphon, Y., Ge, Y., Na Jom, K. (2021). Effects of germinating temperature and time on metabolite profiles of sunflower (Helianthus annuus l.) seed. Food Sci. Nutt. 9 (6), 2810–2822. doi: 10.1002/fsn3.1983
Halli, H. M., Wasnik, V. K., Swami, S. R., Yadav, V. K. (2019). “Seed production of African tall fodder maize as influenced by plant population and nutrient application,” in Abstracts of the international symposium on innopreneurship: a need of sustainable agriculture (Hisar, India: CCSHAU), 79.
Hamaoka, N., Uchida, Y., Tomita, M., Kumagai, E., Araki, T., Ueno, O. (2013). Genetic variations in dry matter production, nitrogen uptake, and nitrogen use efficiency in the AA genome oryza species grown under different nitrogen conditions. Plant Prod. Sci. 16, 107–116. doi: 10.1626/pps.16.107
Han, M., Wong, J., Su, T., Beatty, P. H., Good, A. G. (2016). Identification of nitrogen use efficiency genes in barley: Searching for QTLs controlling complex physiological traits. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01587
Hao, K., Wang, Y., Zhu, Z., Wu, Y., Chen, R., Zhang, L. (2022). miR160: An indispensable regulator in plant. front. Plant Sci. 13, 833322. doi: 10.3389/fpls.2022.833322 [Accessed February 19].
Havlin, J. L., Beaton, J. D., Tisdale, S. L., Nelson, W. L. (2014). Soil fertility & fertilizers: 8th Ed. An introduction to nutrient management. Upper Saddle River, New Jersey. Indian reprint. pp, 516.
He, Y., Yang, S., Xu, J., Wang, Y., Peng, S. (2014). Soil fertility & fertilizers: 8th Ed. An introduction to nutrient management. Upper Saddle River. New Jersey, Indian reprint. pp:516.
Herridge, D. F., Peoples, M. B. (1990). Ureide assay for measuring nitrogen-fixation by nodulated soybean calibrated by n-15 methods. Plant Physiol. 93, 495–503. doi: 10.1104/pp.93.2.495
Hu, S., Qiao, B., Yang, Y., Rees, R. M., Huang, W., Zou, J., et al (2023). Optimizing nitrogen rates for synergistically achieving high yield and high nitrogen use efficiency with low environmental risks in wheat production–Evidences from a long-term experiment in the North China Plain. Eur. J. Agron. 142, 126681.
Huang, Y., Wang, H., Zhu, Y., Huang, X., Li, S., Wu, X., et al. (2022). THP9 enhances seed protein content and nitrogen-use efficiency in maize. Nature 612, 292–300. doi: 10.1038/s41586-022-05441-2
Jagannadham, P. T. K., Muthusamy, S. K., Chidambaranathan, P. (2019). “Micromics: A novel approach to understand the molecular mechanisms in plant stress tolerance,” in Recent approaches in omics for plant resilience to climate change. Ed. Wani, S. H. (Cham: Springer International Publishing), 93–108. doi: 10.1007/978-3-030-21687-0_5
Jewel, Z. A., Ali, J., Mahender, A., Hernandez, J., Pang, Y., Li, Z. (2019). Identification of quantitative trait loci associated with nutrient use efficiency traits, using SNP markers in an early backcross population of rice (Oryza sativa l.). Int. J. Mol. Sci. 20, 900. doi: 10.3390/ijms20040900
Jiang, L., Ball, G., Hodgman, C., Coules, A., Zhao, H., Lu, C. (2018). Analysis of gene regulatory networks of maize in response to nitrogen. Genes 9, 151. doi: 10.3390/genes9030151
Juang, T. C., Wang, M. K., Chen, H. J., Tan, C. C. (2001). Ammonium fixation by surface soils and clays. Soil Sci. 166 (5), 345–352. doi: 10.1097/00010694-200105000-00005
Kang, S., Zhang, L., Song, X., Zhang, S., Liu, X., Liang, Y., et al. (2001). Runoff and sediment loss responses to rainfall and land use in two agricultural catchments on the loess plateau of China. Hydrol. Process 15 (6), 977–988. doi: 10.1002/hyp.191
Kant, S., Bi, Y. M., Rothstein, S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62 (4), 1499–1509. doi: 10.1093/jxb/erq297
Karlsson, P. E., Klingberg, J., Engardt, M., Andersson, C., Langner, J., Karlsson, G. P., et al. (2017). Past, present and future concentrations of ground-level ozone and potential impacts on ecosystems and human health in northern Europe. Sci. Total Environ. 576, 22–35. doi: 10.1016/j.scitotenv.2016.10.061
Kawakami, E. M., Oosterhuis, D. M., Snider, J. L., Mozaffari, M. (2012). Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. Eur. J. Agron. 43, 147–154. doi: 10.1016/j.eja.2012.06.005
Keerthi, P., Pannu, R. J., Dhaka, A. K., Chaudhary, K. (2017). Effect of sowing time and nitrogen on growth, yield and nutrient uptake by Indian mustard (Brassica juncea l.) under Western haryana. Chem. Sci. Rev. let 6 (24), 2526–2532.
Khan, A., Ahmad, M., Ahmed, M., Iftikhar Hussain, M. (2020). Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 10 (1), p.43. doi: 10.3390/plants10010043
Kiba, T., Inaba, J., Kudo, T., Ueda, N., Konishi, M., Mitsuda, N., et al. (2018). Repression of nitrogen starvation responses by members of the arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell 30, 925–945. doi: 10.1105/tpc.17.00810
Klose, S., Tabatabai, M. A. (1999). Urease activity of microbial biomass in soils. Soil Biol. Biochem. 31, 205–211. doi: 10.1016/S0038-0717(98)00090-X
Kool, D. M., Dolfing, J., Wrage, N., Van Groenigen, J. W. (2011). Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 43 (1), 174–178. doi: 10.1016/j.soilbio.2010.09.030
Krapp, A., David, L. C., Chardin, C., Girin, T., Marmagne, A., Leprince, A.-S., et al. (2014). Nitrate transport and signalling in arabidopsis. J. Exp. Bot. 65, 789–798. doi: 10.1093/jxb/eru001
Kumar, A., Batra, R., Gahlaut, V., Gautam, T., Kumar, S., Sharma, M., et al. (2018a). Genome-wide identification and characterization of gene family for RWP-RK transcription factors in wheat (Triticum aestivum l.). PloS One 13, e0208409. doi: 10.1371/journal.pone.0208409
Kumar, S., Muthusamy, S. K., Mishra, C. N., Gupta, V., Venkatesh, K. (2018c). “Importance of genomic selection in crop improvement and future prospects,” in Advanced molecular plant breeding: Meeting the challenge of food security (Boca Raton, FL, USA: CRC Press), 275–296.
Kumar, A., Sharma, M., Kumar, S., Tyagi, P., Wani, S. H., Gajula, M. N. V. P., et al. (2018b). Functional and structural insights into candidate genes associated with nitrogen and phosphorus nutrition in wheat (Triticum aestivum l.). Int. J. Biol. Macromol. 118, 76–91. doi: 10.1016/j.ijbiomac.2018.06.009
Kunze, G. W., Jeffries, C. D. (1953). X-Ray characteristics of clay minerals as related to potassium fixation. Soil Sci. Soc Am. J. 17 (3), 242–244. doi: 10.2136/sssaj1953.03615995001700030014x
Ladha, J. K., Pathak, H., Krupnik, T. J., Six, J., Van Kessel, C. (2005). Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Adv. Agron. 87, 85–156. doi: 10.1016/S0065-2113(05)87003-8
Lassaletta, L., Billen, G., Grizzetti, B., Anglade, J., Garnier, J. (2014). 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ.Res. Lett. 9 (10), 105011. doi: 10.1088/1748-9326/9/10/105011
Lawlor, D. W. (2002). Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J. Exp. Bot. 53, 773–787. doi: 10.1093/jexbot/53.370.773
Le Gouis, J., Béghin, D., Heumez, E., Pluchard, P. (2000). Genetic differences for nitrogen uptake and nitrogen utilisation efficiencies in winter wheat. Eur. J. Agron. 12, 163–173. doi: 10.1016/S1161-0301(00)00045-9
Lehman, R. M., Taheri, W. I., Osborne, S. L., Buyer, J. S., Douds, J. D.D. (2012). Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 61, 300–304. doi: 10.1016/j.apsoil.2011.11.008
Lenka, S. K., Muthusamy, S. K., Chinnusamy, V., Bansal, K. C. (2018). Ectopic expression of rice PYL3 enhances cold and drought tolerance in arabidopsis thaliana. Mol. Biotechnol. 60, 350–361. doi: 10.1007/s12033-018-0076-5
Lenka, S. K., Singh, A. K., Muthusamy, S. K., Smita, S., Chinnusamy, V., Bansal, K. C. (2019). Heterologous expression of rice RNA-binding glycine-rich (RBG) gene OsRBGD3 in transgenic arabidopsis thaliana confers cold stress tolerance. Funct. Plant Biol. FPB 46, 482–491. doi: 10.1071/FP18241
Li, P., Chen, F., Cai, H., Liu, J., Pan, Q., Liu, Z., et al. (2015). A genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 66, 3175–3188. doi: 10.1093/jxb/erv127
Li, M., Wang, T., Zhang, H., Liu, S., Li, W., Abou Elwafa, S. F., et al. (2022). TaNRT2.1-6B is a dual-affinity nitrate transporter contributing to nitrogen uptake in bread wheat under both nitrogen deficiency and sufficiency. Crop J. 10, 993–1005. doi: 10.1016/j.cj.2021.11.012
Liao, M., Palta, J. A., Fillery, I. R. P., Liao, M., Palta, J. A., Fillery, I. R. P. (2006). Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 57, 1097–1107. doi: 10.1071/AR05439
Limon-Ortega, A. (2021). Nitrogen use and agronomic efficiency of rainfed wheat in permanent beds as affected by n fertilizer, precipitation and soil nitrate. J. Agric. Sci. 159 (3-4), 199–205. doi: 10.1017/S0021859621000411
Lin, H. C., Coe, R. A., Quick, W. P., Bandyopadhyay, A. (2019). “Climate-resilient future crop: Development of C4 rice,” in Sustainable solutions for food Security:Combating climate change by adaptation. Eds. Sarkar, A., Sensarma, S. R., vanLoon, G. W. (Cham: Springer International Publishing), 111–124. doi: 10.1007/978-3-319-77878-5_6
Linn, D. M., Doran, J. W. (1984). Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc Am. J. 48 (6), 1267–1272. doi: 10.2136/sssaj1984.03615995004800060013x
Linquist, B., Van Groenigen, K. J., Adviento-Borbe, M. A., Pittelkow, C., Van Kessel, C. (2012). An agronomic assessment of greenhouse gas emissions from major cereal crops. Global Change Biol. 18 (1), 194–209. doi: 10.1111/j.1365-2486.2011.02502.x
Liu, Z., Fang, G., Yan, L., Jianqun, Y., Xiaoyv, Z., Xinxin, L., et al. (2019b). Timing and splitting of nitrogen fertilizer supply to increase crop yield and efficiency of nitrogen utilization in a wheat–peanut relay intercropping system in China. Crop J. 7 (1), 101–112. doi: 10.1016/j.cj.2018.08.006
Liu, P., Guo, X., Zhou, D., Zhang, Q., Ren, X., Wang, R., et al. (2023). Quantify the effect of manure fertilizer addition and optimal nitrogen input on rainfed wheat yield and nitrogen requirement using nitrogen nutrition index. Agric. Ecosyst. Environ. 345, 108319. doi: 10.1016/j.agee.2022.108319
Liu, P.-P., Montgomery, T. A., Fahlgren, N., Kasschau, K. D., Nonogaki, H. (2007). And carrington, J Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. C.Plant J. 52, 133–146. doi: 10.1111/j.1365-313X.2007.03218.x
Liu, S., Wang, X., Yin, X., Savoy, H. J., McClure, A. (2019a). Ammonia volatilization loss and corn nitrogen nutrition and productivity with efficiency enhanced UAN and urea under no-tillage. Sci. Rep. 9 (1), 1–12. doi: 10.1038/s41598-019-42912-5
Liu, J., You, L., Amini, M., Obersteiner, M., Herrero, M., Zehnder, A. J., et al. (2010). A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. 107 (17), 8035–8040. doi: 10.1073/pnas.0913658107
Liu, R., Zhang, H., Zhao, P., Zhang, Z., Liang, W., Tian, Z., et al. (2012). Mining of candidate maize genes for nitrogen use efficiency by integrating gene expression and QTL data. Plant Mol. Biol. Rep. 30, 297–308. doi: 10.1007/s11105-011-0346-x
Lodhi, A., Arshad, M., Azam, F., Sajjad, M. H. (2009). Changes in mineral and mineralizable n of soil incubated at varying salinity, moisture and temperature regimes. Pak. J. Bot. 41 (2), 967–980.
López-Bellido, L., López-Bellido, R. J., Redondo, R. (2005). Nitrogen efficiency in wheat under rainfed Mediterranean conditions as affected by split nitrogen application. Field Crops Res. 94 (1), 86–97. doi: 10.1016/j.fcr.2004.11.004
López-Castañeda, C., Richards, R. A., Farquhar, G. D., Williamson, R. E. (1996). Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Sci. 36, cropsci1996.0011183X003600050031x. doi: 10.2135/cropsci1996.0011183X003600050031x
Lv, H., Wang, Q., Qi, B., He, J., Jia, S. (2019). RNA-Seq and transcriptome analysis of nitrogen-deprivation responsive genes in dunaliella salina TG strain. Theor. Exp. Plant Physiol. 31, 139–155. doi: 10.1007/s40626-019-00138-w
Ma, R., Jiang, C., Shou, N., Gao, W., Yang, X. (2023). An optimized nitrogen application rate and basal topdressing ratio improves yield, quality, and water-and n-use efficiencies for forage maize (Zea mays l.). Agron. 13 (1), 181. doi: 10.3390/agronomy13010181
Mahajan, G., Chauhan, B. S., Timsina, J., Singh, P. P., Kuldeep, S. (2012). Crop performance and water-and nitrogen-use efficiencies in dry-seeded rice in response to irrigation and fertilizer amounts in northwest India. Field Crops Res. 134, 59–70. doi: 10.1016/j.fcr.2012.04.011
Mălinaş, A., Vidican, R., Rotar, I., Mălinaş, C., Moldovan, C. M., Proorocu, M. (2022). Current status and future prospective for nitrogen use efficiency in wheat (Triticum aestivum l.). Plants Basel Switz. 11, 217. doi: 10.3390/plants11020217
Mandal, U., Kumar., Sharma, K. L., Prasad, J. V. N. S., Reddy, B. S., Narsimlu, B., et al. (2012). Nutrient losses by runoff and sediment from an agricultural field in semi-arid tropical India. Indian J. Dryland Agricu. Res. Dev. 27 (1), 01–09.
Mandolino, C. I., D’Andrea, K. E., Olmos, S., Otegui, M. E., Eyhérabide, G. (2018) Maize nitrogen use efficiency: QTL mapping in a U.S (Maydica: Dent x Argentine-Caribbean Flint RILs population). Available at: https://www.semanticscholar.org/paper/Maize-Nitrogen-Use-Efficiency%3A-QTL-Mapping-in-a-x-Mandolino-D%E2%80%99Andrea/80dc02bd07978cb153e00a39e11703845693ffc4 (Accessed February 16, 2023).
Marschner, H. (1995). “Functions of mineral nutrients: Micronutrients,” in Mineral nutrition of higher plants, 2nd Edition (London: Academic Press), 313–404.
Marshall, S. B., Wood, C. W., Braun, L. C., Cabrera, M. L., Mullen, M. D., Guertal, E. A. (1998). Ammonia volatilization from tall fescue pastures fertilized with broiler litter Vol. 27 (Madison, WI: American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America), 1125–1129.
Martins, M. R., Sant’Anna, S. A. C., Zaman, M., Santos, R. C., Monteiro, R. C., Alves, B. J. R., et al. (2017). Strategies for the use of ure-ase and nitrification inhibitors with urea: Impact on N2O and NH3 emissions, fertilizer-15 n recovery and maize yield in a tropical soil. Agric. Ecosys. Environ. 247, 54–62. doi: 10.1016/j.agee.2017.06.021
Mathialagan, R., Mansor, N., Al-Khateeb, B., Mohamad, M. H., Shamsuddin, M. R. (2017). Evaluation of allicin as soil urease inhibitor. Proc. Eng. 184, 449–459. doi: 10.1016/j.proeng.2017.04.116
Mauceri, A., Abenavoli, M. R., Toppino, L., Panda, S., Mercati, F., Aci, M. M., et al. (2021). Transcriptomics reveal new insights into molecular regulation of nitrogen use efficiency in solanum melongena. J. Exp. Bot. 72, 4237–4253. doi: 10.1093/jxb/erab121
McAllister, C. H., Beatty, P. H., Good, A. G. (2012). Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol. J. 10, 1011–1025. doi: 10.1111/j.1467-7652.2012.00700.x
McConkey, B. G., Curtin, D., Campbell, C. A., Brandt, S. A., Selles, F. (2002). Crop and soil nitrogen status of tilled and no-tillage systems in semiarid regions of Saskatchewan. Can. J. Soil Sci. 82 (4), 489–498. doi: 10.4141/S01-036
McCracken, D. (1989) Control of nitrate leaching with winter annual cover crops. Available at: https://uknowledge.uky.edu, (Accessed 13 July 2019).
McGinn, S. M., Janzen, H. H., Coates, T. W., Beauchemin, K. A., Flesch, T. K. (2016). Ammonia emission from a beef cattle feedlot and its local dry deposition and re-emission. J. Environ. Qual. 45 (4), 1178–1185. doi: 10.2134/jeq2016.01.0009
Muthusamy, S. K., Dalal, M., Chinnusamy, V., Bansal, K. C. (2016). Differential regulation of genes coding for organelle and cytosolic ClpATPases under biotic and abiotic stresses in wheat. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00929
Muthusamy, S. K., Dalal, M., Chinnusamy, V., Bansal, K. C. (2017). Genome-wide identification and analysis of biotic and abiotic stress regulation of small heat shock protein (HSP20) family genes in bread wheat. J. Plant Physiol. 211, 100–113. doi: 10.1016/j.jplph.2017.01.004
Muthusamy, S. K., Lenka, S. K., Katiyar, A., Chinnusamy, V., Singh, A. K., Bansal, K. C. (2019). Genome-wide identification and analysis of biotic and abiotic stress regulation of C4 photosynthetic pathway genes in rice. appl. biochem. Biotechnol. 187, 221–238. doi: 10.1007/s12010-018-2809-0
Nacry, P., Bouguyon, E., Gojon, A. (2013). Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370, 1–29. doi: 10.1007/s11104-013-1645-9
Nardi, P., Laanbroek, H. J., Nicol, G. W., Renella, G., Cardinale, M., Pietramellara, G., et al. (2020). Biological nitrification inhibition in the rhizosphere: determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol. Rev. 44 (6), 874–908. doi: 10.1093/femsre/fuaa037
Naz, M., Luo, B., Guo, X., Li, B., Chen, J., Fan, X. (2019). Overexpression of nitrate transporter OsNRT2.1 enhances nitrate-dependent root elongation. Genes 10, 290. doi: 10.3390/genes10040290
Nguyen, G. N., Rothstein, S. J., Spangenberg, G., Kant, S. (2015). Role of microRNAs involved in plant response to nitrogen and phosphorous limiting conditions. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00629
Nieder, R., Benbi, D. K., Scherer, H. W. (2011). Fixation and defixation of ammonium in soils: a review. Biol. Fertil. Soils 47, 1–14. doi: 10.1007/s00374-010-0506-4
Nishad, A., Mishra, A. N., Chaudhari, R., Aryan, R. K., Katiyar, P. (2018). Effect of temperature on growth and yield of rice (Oryza sativa l.) cultivars. Int. J. Chem. Stud. 6 (5), 1381–1383.
Obalum, S. E., Obi, M. E. (2010). Physical properties of a sandy loam ultisol as affected by tillage-mulch management practices and cropping systems. Soil Tillage Res. 108 (1–2), 30–36. doi: 10.1016/j.still.2010.03.009
Ortiz-Monasterio, R., Sayre, K. D., Rajaram, S., McMahon, M. (1997). Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 37 (3), 898–904. doi: 10.2135/cropsci1997.0011183X003700030033x
Oury, F.-X., Godin, C. (2007). Yield and grain protein concentration in bread wheat: how to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 157, 45–57. doi: 10.1007/s10681-007-9395-5
Pande, N. C., Samantaray, R. N., Mohanty, S. K. (1985). Nutrient changes in direct-seeded submerged rice soils with varying nutrio-environments. Plant Soil 88 (2), 299–306. doi: 10.1007/BF02182459
Pereira, J., Misselbrook, T. H., Chadwick, D. R., Coutinho, J., Trindade, H. (2010). Ammonia emissions from naturally ventilated dairy cattle buildings and outdoor concrete yards in Portugal. Atm. Environ. 44 (28), 3413–3421. doi: 10.1016/j.atmosenv.2010.06.008
Phillips, R. E., Thomas, G. W., Blevins, R. L., Frye, W. W., Phillips, S. H. (1980). No-tillage agriculture. Sci. 208 (4448), 1108–1113.
Potter, P., Ramankutty, N., Bennett, E. M., Donner, S. D. (2010). Characterizing the spatial patterns of global fertilizer application and manure production. Earth Interac 14 (2), 1–22. doi: 10.1175/2009EI288.1
Power, J. F., Peterson, G. A. (1998). Nitrogen transformations, utilization, and conservation as affected by fallow tillage method. Soil Tillage Res. 49 (1-2), 37–47. doi: 10.1016/S0167-1987(98)00153-6
Qiang, S., Zhang, Y., Zhao, H., Fan, J., Zhang, F., Sun, M., et al. (2022). Combined effects of urea type and placement depth on grain yield, water productivity and nitrogen use efficiency of rain-fed spring maize in northern China. Agric. Water Manage. 262, 107442. doi: 10.1016/j.agwat.2021.107442
Quemada, M., Cabrera, M. L. (1995). CERES-n model predictions of nitrogen mineralized from cover crop residues. Soil Sci. Soc Am. J. 59 (4), 1059–1065. doi: 10.2136/sssaj1995.03615995005900040015x
Rahman, M. M., Amano, T., Shiraiwa, T. (2009). Nitrogen use efficiency and recovery from n fertilizer under rice-based cropping systems. Aus. J. Crop Sci. 3 (6), 336–351.
Rana, M. A., Mahmood, R., Ali, S. (2021). Soil urease inhibition by various plant extracts. PloS One 16 (10), e0258568. doi: 10.1371/journal.pone.0258568
Ranatunga, T., Hiramatsu, K., Onishi, T., Ishiguro, Y. (2018). Process of denitrification in flooded rice soils. Rev. Agric. Sci. 6, 21–33. doi: 10.7831/ras.6.21
Randall, G. W., David, J. M. (2001). Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. J. Environ. Qual 30 (2), 337–344. doi: 10.2134/jeq2001.302337x
Ranells, N. N., Wagger, M. G. (1997). Nitrogen-15 recovery and release by rye and crimson clover cover crops. Soil Sci. Soc. Am. J. 61 (2), 943–948.
Rasmussen, P. E., Rohde, C. R. (1991). Tillage, soil depth, and precipitation effects on wheat response to nitrogen. Soil Sci. Soc Am. J. 55 (1), 121–124. doi: 10.2136/sssaj1991.03615995005500010021x
Rawal, N., Pande, K. R., Shrestha, R., Vista, S. P. (2022). Nutrient use efficiency (NUE) of wheat (Triticum aestivum l.) as affected by NPK fertilization. PloS One 17 (1), e0262771. doi: 10.1371/journal.pone.0262771
Rebetzke, G. J., Richards, R. A. (1999). Genetic improvement of early vigour in wheat. Aust. J. Agric. Res. 50, 291–302. doi: 10.1071/a98125
Reicosky, D. C., Forcella, F. (1998). Cover crop and soil quality interactions in agroecosystems. J. Soil Water Conserv. 53 (3), 224–229.
Riley, W. J., Ortiz-Monasterio, I., Matson, P. A. (2001). Nitrogen leaching and soil nitrate, nitrite, and ammonium levels under irrigated wheat in northern Mexico. Nutr. Cycl. Agroecosyst. 61 (3), 223–236. doi: 10.1023/A:1013758116346
Rowlings, D. W., Lester, D. W., Grace, P. R., Scheer, C., De Rosa, D., Migliorati, M. D. A., et al. (2022). Seasonal rainfall distribution drives nitrogen use efficiency and losses in dryland summer sorghum. Field Crops Res. 283, 108527. doi: 10.1016/j.fcr.2022.108527
Ruser, R., Schulz, R. (2015). The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils–a review. J. Plant Nutt. Soil Sci. 178 (2), 171–188. doi: 10.1002/jpln.201400251
Sagwal, V., Sihag, P., Singh, Y., Mehla, S., Kapoor, P., Balyan, P., et al. (2022). Development and characterization of nitrogen and phosphorus use efficiency responsive genic and miRNA derived SSR markers in wheat. Heredity 128, 391–401. doi: 10.1038/s41437-022-00506-4
Sakala, W. D., Cadisch, G., Giller, K. E. (2000). Interactions between residues of maize and pigeonpea and mineral n fertilizers during decomposition and n mineralization. Soil Biol. Biochem. 32 (5), 679–688. doi: 10.1016/S0038-0717(99)00204-7
Santos, L. A., de Souza, S. R., Fernandes, M. S. (2012). OsDof25 expression alters carbon and nitrogen metabolism in arabidopsis under high n-supply. Plant biotechnol. Rep. 6, 327–337. doi: 10.1007/s11816-012-0227-2
Sawan, Z. M. (2017). Cotton production and climatic factors: Studying the nature of its relationship by different statistical methods. Cogent Biol. 3 (1), 1292882. doi: 10.1080/23312025.2017.1292882
Schillinger, W. F., Cook, R. J., Papendick, R. I. (1999). Increased dryland cropping intensity with no-till barley. Agron. J. 91 (5), 744–752. doi: 10.2134/agronj1999.915744x
Schmidt, J. P., De, J. A., Ferguson, R. B., Taylor, R. K., Young, R. K., Havlin, J. L. (2002). Corn yield response to nitrogen at multiple in-field locations. Agron. J. 94 (4), 798–806. doi: 10.2134/agronj2002.7980
Shabbir, R., Javed, T., Hussain, S., Ahmar, S., Naz, M., Zafar, H., et al. (2022). Calcium homeostasis and potential roles to combat environmental stresses in plants. South Afr. J. Bot. 148, 683–693. doi: 10.1016/j.sajb.2022.05.038
Shahadha, S. S., Wendroth, O., Ding, D. (2021). Nitrogen and rainfall effects on crop growth–experimental results and scenario analyses. Water 13 (16), 2219. doi: 10.3390/w13162219
Shapiro, C., Attia, A., Ulloa, S., Mainz, M. (2016). Use of five nitrogen source and placement systems for improved nitrogen management of irrigated corn. Soil Sci. Soc Am. J. 80 (6), 1663–1674. doi: 10.2136/sssaj2015.10.0363
Sharma, B., Ahlert, R. C. (1977). Nitrification and nitrogen removal. Water Res. 11 (10), 897–925. doi: 10.1016/0043-1354(77)90078-1
Shekara, B. G., Mahadevu, P., Chikkarugi, N. M., Manasa, N. (2020). Response of multi-cut fodder pearl millet (Pennisetum glaucum l.) genotypes to varied nitrogen levels in southern dry zone of karnataka. J. pharmacogn. Phytochem. 9 (5), 2665–2668.
Shindo, H., Nishio, T. (2005). Immobilization and remineralization of n following addition of wheat straw into soil: determination of gross n transformation rates by 15N-ammonium isotope dilution technique. Soil Biol. Biochem. 37 (3), 425–432. doi: 10.1016/j.soilbio.2004.07.027
Si, Z., Zain, M., Mehmood, F., Wang, G., Gao, Y., Duan, A. (2020). Effects of nitrogen application rate and irrigation regime on growth, yield, and water-nitrogen use efficiency of drip-irrigated winter wheat in the north China plain. Agric. Water Manage. 231, 106002. doi: 10.1016/j.agwat.2020.106002
Sigurdarson, J., Svane, S., Karrin, H. (2018). The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018), 17. doi: 10.1007/s11157-018-9466-1
Singh, R., Gautam, S., Kumar, A., Gautam, T., Singh, S., Gahlaut, V., et al. (2022). QTL analysis for nitrogen use efficiency in wheat (Triticum aestivum l.). Euphytica 219, 9. doi: 10.21203/rs.3.rs-1866820/v1
Singh, B. P., Hatton, B. J., Singh, B., Cowie, A. L., Kathuria, A. (2010). Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 39 (4), 1224–1235. doi: 10.2134/jeq2009.0138
Sirivedhin, T., Gray, K. A. (2006). Factors affecting denitrification rates in experimental wetlands: field and laboratory studies. Ecol. Eng. 26 (2), 167–181. doi: 10.1016/j.ecoleng.2005.09.001
Snider, J., Harris, G., Roberts, P., Meeks, C., Chastain, D., Bange, M., et al. (2021). Cotton physiological and agronomic response to nitrogen application rate. Field Crops Res. 270, 108194. doi: 10.1016/j.fcr.2021.108194
Sravanthi, D., Pratibha, G., Padmaja, B., Reddy, T. P. (2017). Enzyme activity, agronomic nitrogen use efficiency and yield of rainfed maize (Zea mays l.) as influenced by natural nitrification inhibitors. Int. J. Curr. Microbiol. App. Sci. 6 (10), 1485–1490.
Stahl, A., Vollrath, P., Samans, B., Frisch, M., Wittkop, B., Snowdon, R. J. (2019). Effect of breeding on nitrogen use efficiency-associated traits in oilseed rape. J. Exp. Bot. 70, 1969–1986. doi: 10.1093/jxb/erz044
Suthar, S., Bishnoi, P., Singh, S., Mutiyar, P. K., Nema, A. K., Patil, N. S. (2009). Nitrate contamination in groundwater of some rural areas of rajasthan, India. J. Hazard Mater. 171 (1–3), 189–199. doi: 10.1016/j.jhazmat.2009.05.111
Svane, S., Sigurdarson, J. J., Finkenwirth, F., Eitinger, T., Karring, H. (2020). Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 10 (1), 8503.
Taneja, P., Labhasetwar, P., Nagarnaik, P., Ensink, J. H. (2017). The risk of cancer as a result of elevated levels of nitrate in drinking water and vegetables in central India. J. Water Health 15 (4), 602–614. doi: 10.2166/wh.2017.283
Tang, W., Ye, J., Yao, X., Zhao, P., Xuan, W., Tian, Y., et al. (2019). Genome-wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 10, 5279. doi: 10.1038/s41467-019-13187-1
Tisdall, J. M., Oades, J. M. (1979). Stabilization of soil aggregates by the root systems of ryegrass. Soil Res. 17 (3), 429–441. doi: 10.1071/SR9790429
Ueda, Y., Kiba, T., Yanagisawa, S. (2020). Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J. 102, 448–466. doi: 10.1111/tpj.14637
Van Den Bossche, A., De Bolle, S., De Neve, S., Hofman, G. (2009). Effect of tillage intensity on n mineralization of different crop residues in a temperate climate. Soil Tillage Res. 103 (2), 316–324. doi: 10.1016/j.still.2008.10.019
van Oosterom, E. J., Chapman, S. C., Borrell, A. K., Broad, I. J., Hammer, G. L. (2010). Functional dynamics of the nitrogen balance of sorghum. II. grain filling period. Field Crops Res. 115, 29–38. doi: 10.1016/j.fcr.2009.09.019
Vega, A., Canessa, P., Hoppe, G., Retamal, I., Moyano, T. C., Canales, J., et al. (2015). Transcriptome analysis reveals regulatory networks underlying differential susceptibility to botrytis cinerea in response to nitrogen availability in solanum lycopersicum. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00911
Wallace, A. J., Armstrong, R. D., Grace, P. R., Scheer, C., Partington, D. L. (2020). Nitrogen use efficiency of 15 n urea applied to wheat based on fertiliser timing and use of inhibitors. Nutrient Cycling Agroecos. 116, 41–56. doi: 10.1007/s10705-019-10028-x
Wan, C., Gao, L., Wang, J., Lei, X., Tao, J., Feng, B., et al. (2023). Effects of nitrogen fertilizer on protein synthesis, accumulation, and physicochemical properties in common buckwheat. Crop J. doi: 10.1016/j.cj.2023.01.002
Wang, Y., Fu, B., Pan, L., Chen, L., Fu, X., Li, K. (2013). Overexpression of ArabidopsisDof1, GS1 and GS2 enhanced nitrogen assimilation in transgenic tobacco grown under low-nitrogen conditions. Plant Mol. Biol. Rep. 31, 886–900. doi: 10.1007/s11105-013-0561-8
Wang, Z., Gao, J., Ma, B. L. (2014). Concurrent improvement in maize yield and nitrogen use efficiency with integrated agronomic management strategies. Agron. J. 106 (4), 1243–1250. doi: 10.2134/agronj13.0487
Wang, H., Inukai, Y., Yamauchi, A. (2006). Root development and nutrient uptake. Crit. Rev. Plant Sci. 25, 279–301. doi: 10.1080/07352680600709917
Wani, S. H., Vijayan, R., Choudhary, M., Kumar, A., Zaid, A., Singh, V., et al. (2021). Nitrogen use efficiency (NUE): elucidated mechanisms, mapped genes and gene networks in maize (Zea mays l.). Physiol. Mol. Biol. Plants 27, 2875–2891. doi: 10.1007/s12298-021-01113-z
Ward, B. B., Arp, D. J., Klotz, M. G. (2011). Nitrification (Washington, DC: American Society for Microbiology Press), 454.
Wei, D., Cui, K., Ye, G., Pan, J., Xiang, J., Huang, J., et al. (2012). QTL mapping for nitrogen-use efficiency and nitrogen-deficiency tolerance traits in rice. Plant Soil 359, 281–295. doi: 10.1007/s11104-012-1142-6
Weih, M., Asplund, L., Bergkvist, G. (2011). Assessment of nutrient use in annual and perennial crops: A functional concept for analyzing nitrogen use efficiency. Plant Soil 339, 513–520. doi: 10.1007/s11104-010-0599-4
Wijewardana, C., Hock, M., Henry, B., Reddy, K. R. (2015). Screening corn hybrids for cold tolerance using morphological traits for early-season seeding. Crop Sci. 55 (2), 851–867. doi: 10.2135/cropsci2014.07.0487
Wild, M., Ohmura, A., Gilgen, H., Morcrette, J., Slingo, A. (2001). Evaluation of downward longwave radiation in general circulation models. J. Climate 14 (15), 3227–3239. doi: 10.1175/1520-0442(2001)014<3227:EODLRI>2.0.CO;2
Xiong, Q., Tang, G., Zhong, L., He, H., Chen, X. (2018). Response to nitrogen deficiency and compensation on physiological characteristics, yield formation, and nitrogen utilization of rice. Front. Plant Sci. 9, 1075. doi: 10.3389/fpls.2018.01075
Xu, Z., Zhong, S., Li, X., Li, W., Rothstein, S. J., Zhang, S., et al. (2011). Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PloS One 6, e28009. doi: 10.1371/journal.pone.0028009
Yang, H.-C., Kan, C.-C., Hung, T.-H., Hsieh, P.-H., Wang, S.-Y., Hsieh, W.-Y., et al. (2017). Identification of early ammonium nitrate-responsive genes in rice roots. Sci. Rep. 7, 16885. doi: 10.1038/s41598-017-17173-9
Yao, C., Ren, J., Li, H., Zhang, Z., Wang, Z., Sun, Z., et al. (2023). Can wheat yield, n use efficiency and processing quality be improved simultaneously? Agric. Water Manage. 275, 108006. doi: 10.1016/j.agwat.2022.108006
Yassen, A., Abou, E. N., Shedeed, S. (2010). Response of wheat to foliar spray with urea and micronutrients. J. Am. Sci. 6 (9), 14–22.
Young, M. D., Ros, G. H., de Vries, W. (2021). Impacts of agronomic measures on crop, soil, and environmental indicators: A review and synthesis of meta-analysis. Agric. Ecosyst. Environ. 319, 107551. doi: 10.1016/j.agee.2021.107551
Yu, X., Keitel, C., Zhang, Y., Wangeci, A. N., Dijkstra, F. A. (2022). Global meta-analysis of nitrogen fertilizer use efficiency in rice, wheat and maize. Agric. Ecosyst. Environ. 338, 108089. doi: 10.1016/j.agee.2022.108089
Zaman, M., Saggar, S., Blennerhassett, J. D., Singh, J. (2009). Effect of urease and nitrification inhibitors on n transfor-mation, gaseous emissions of ammonia and nitrous oxide, pasture yield and n uptake in grazed pasture system. Soil Biol. Biochem. 41, 1270–1280. doi: 10.1016/j.soilbio.2009.03.011
Zelenev, V. V., Van Bruggen, A., Leffelaar, P., Bloem, J., Semenov, A. (2006). Oscillating dynamics of bacterial populations and their predators in response to fresh organic matter added to soil: the simulation model ‘BACWAVE-WEB’. Soil Biol. Biochem. 38 (7), 1690–1711. doi: 10.1016/j.soilbio.2005.11.024
Zhang, X., Davidson, E. A., Mauzerall, D. L., Searchinger, T. D., Dumas, P., Shen, Y. (2015). Managing nitrogen for sustainable development. Nature. 528 (7580), 51–59. doi: 10.1038/nature15743
Zhang, H., Du, B., Jiang, S., Zhu, J., Wu, Q. (2023). Potential assessment of selenium for improving nitrogen metabolism, yield and nitrogen use efficiency in wheat. Agron. 13 (1), 110. doi: 10.3390/agronomy13010110
Zhang, M., Gao, M., Zheng, H., Yuan, Y., Zhou, X., Guo, Y., et al. (2019). QTL mapping for nitrogen use efficiency and agronomic traits at the seedling and maturity stages in wheat. Mol. Breed. 39, 71. doi: 10.1007/s11032-019-0965-8
Zhang, M., Wang, Y., Chen, X., Xu, F., Ding, M., Ye, W., et al. (2021). Plasma membrane h+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat. Commun. 12, 735. doi: 10.1038/s41467-021-20964-4
Zhao, M., Ding, H., Zhu, J.-K., Zhang, F., Li, W.-X. (2011b). Involvement of miR169 in the nitrogen-starvation responses in arabidopsis. New Phytol. 190, 906–915. doi: 10.1111/j.1469-8137.2011.03647.x
Zhao, Y., Liu, Z., Duan, F., An, X., Liu, X., Hao, D., et al. (2018). Overexpression of the maize ZmAMT1;1a gene enhances root ammonium uptake efficiency under low ammonium nutrition. Plant Biotechnol. Rep. 12, 47–56. doi: 10.1007/s11816-018-0471-1
Zhao, K., Tung, C.-W., Eizenga, G. C., Wright, M. H., Ali, M. L., Price, A. H., et al. (2011a). Genome-wide association mapping reveals a rich genetic architecture of complex traits in oryza sativa. Nat. Commun. 2, 467. doi: 10.1038/ncomms1467
Zhou, B., Ming, Z., Xuefang, S., Dan, W., Zaisong, D., Congfeng, L., et al. (2019). Integrated agronomic practice increases maize grain yield and nitrogen use efficiency under various soil fertility conditions. Crop J. 7 (4), 527–538.
Zinke, P. J., Stangenberger, A. G., Post, W. M., Emanuel, W. R., Olson, J. S., Millemann, R. E., et al. (1986). “Worldwide organic soil carbon and nitrogen dat). environmental system science data infrastructure for a virtual ecosystem (ESS-DIVE)(United states),” in Carbon dioxide information analysis center (CDIAC) (Oak Ridge, TN (United States: Oak Ridge National Laboratory (ORNL).
Keywords: conservation tillage system, NUE, nitrogen assimilation, nitrogen loss, QTLs
Citation: Govindasamy P, Muthusamy SK, Bagavathiannan M, Mowrer J, Jagannadham PTK, Maity A, Halli HM, G. K. S, Vadivel R, T. K. D, Raj R, Pooniya V, Babu S, Rathore SS, L. M and Tiwari G (2023) Nitrogen use efficiency—a key to enhance crop productivity under a changing climate. Front. Plant Sci. 14:1121073. doi: 10.3389/fpls.2023.1121073
Received: 11 December 2022; Accepted: 20 March 2023;
Published: 18 April 2023.
Edited by:
Victoria Fernandez, Polytechnic University of Madrid, SpainReviewed by:
Sharif Ahmed, International Rice Research Institute (IRRI), PhilippinesCopyright © 2023 Govindasamy, Muthusamy, Bagavathiannan, Mowrer, Jagannadham, Maity, Halli, G. K., Vadivel, T. K., Raj, Pooniya, Babu, Rathore, L. and Tiwari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muthukumar Bagavathiannan, bXV0aHVAdGFtdS5lZHU=; Prabhu Govindasamy, cHJhYm1hbmlrYW5kYW5AZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.