- 1State Key Laboratory of Grassland and Agro-ecosystems, College of Ecology, Lanzhou University, Lanzhou, Gansu, China
- 2Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou, Gansu, China
- 3College of Life Science and Engineering, Hexi University, Zhangye, Gansu, China
- 4Aix Marseille Univ, Centre national de la recherche scientifique, Avignon Université, Institut de Recherche pour le Développement, Institut Méditerranéen de Biodiversité et d’Écologie marine et continentale, Technopôle Arbois-Méditerranée, Aix-en-Provence, France
Biodiversity is essential for the provision of multiple ecosystem functions simultaneously (ecosystem multifunctionality EMF). Yet, it remains unclear whether and how dominant plant species impact EMF. Here, we aimed at disentangling the direct from indirect above- and belowground pathways by which dominant plant species influence EMF. We evaluated the effects of two dominant plant species (Dasiphora fruticosa, and the toxic perennial plant Ligularia virgaurea) with expected positive and negative impacts on the abiotic environment (soil water content and pH), surrounding biological communities (plant and nematode richness, biomass, and abundance in the vicinity), and on the EMF of alpine meadows, respectively. We found that the two dominant plants enhanced EMF, with a positive effect of L. virgaurea on EMF greater than that of D. fruticosa. We also observed that dominant plants impacted on EMF through changes in soil water content and pH (indirect abiotic effects), but not through changes in biodiversity of surrounding plants and nematodes (indirect biotic pathway). Our study suggests that dominant plants may play an important role in promoting EMF, thus expanding the pervasive mass-ratio hypothesis originally framed for individual functions, and could mitigate the negative impacts of vegetation changes on EMF in the alpine meadows.
1 Introduction
Biodiversity is essential for the provision of multiple ecosystem functions simultaneously (ecosystem multifunctionality; EMF, Hector and Bagchi, 2007), such as litter decomposition, ecosystem production, food web stability, and climate regulation (Van Der Heijden et al., 2008; Bodelier, 2011; Bardgett and Van Der Putten, 2014; Handa et al., 2014). While multiple facets of biodiversity can impact on EMF (e.g. Flynn et al., 2011; Soliveres et al., 2016; Le Bagousse-Pinguet et al., 2019; Le Bagousse-Pinguet et al., 2021; Yuan et al., 2021), focusing solely on diversity stricto sensu ignores the overwhelming influence of species dominance on ecosystem functions (Grime, 1998; Garnier et al., 2004). According to the mass-ratio hypothesis (Grime, 1998), the effects of plant species on ecosystem functioning are proportional to plant biomass (Grime, 1998). However, this hypothesis was originally proposed for single functions (Grime, 1998; Smith and Knapp, 2003; Garnier et al., 2004), but yet remains less clear when focusing on EMF (Le Bagousse-Pinguet et al., 2019). Determining the key role of dominant species on EMF not only expands our fundamental understanding of the Biodiversity-EMF relationships, but could also help to prioritize relevant biodiversity attributes in conservation programs (Balvanera et al., 2014; Brum et al., 2017).
Dominant species can impact on ecosystem functioning through multiple pathways. On the one hand, dominant species and their traits can affect directly individual ecosystem functions, such as biomass production or nutrient cycling (e.g. Garnier et al., 2004). For instance, species assemblages dominated by recalcitrant species (i.e. the dominant species that exhibit high leaf lignin concentration) have been shown to decrease EMF, particularly the functions related to decomposition processes (Austin and Ballare, 2010; Le Bagousse-Pinguet et al., 2021). On the other hand, dominant species could also have indirect effects on ecosystem functioning, i.e. through the changes in local above- and belowground diversities. Dominant plant species can have negative effects on plant growth and establishment (e.g. due to asymmetric light competition, Grime, 1973), decreasing local plant biodiversity (Hejda et al., 2021), and ultimately ecosystem functioning (Livingstone et al., 2020). However, these species could also have positive effects (i.e. facilitation, Bertness and Callaway, 1994), and play as ecosystem engineers that enhance abiotic conditions (e.g. soil water content and pH) in their vicinity (Cavieres et al., 2014; Ellison, 2019). In these cases, dominant plants could promote plant (Michalet et al., 2006; Le Bagousse-Pinguet et al., 2014) and soil biodiversity and activity, including soil bacterial (Hortal et al., 2015), and fungal diversity and abundance (Delgado-Baquerizo et al., 2016b; Le Bagousse-Pinguet et al., 2021). Disentangling the direct (positive and negative) from indirect above- and belowground pathways by which dominant plant species influence EMF remains poorly explored, although such an integrative framework could contribute to the global understanding of the impact of biodiversity on ecosystem functioning.
Finally, the mediating role of soil nematodes in the influence of dominant plants on EMF has rarely been explored. Nematodes include a wide variety of trophic groups, such as herbivores, omnivores, predators, and microbial feeders, and are known to play important roles in soil food webs (Ferris, 2010). Furthermore, dominant plants can play a key role in regulating soil nematode abundances (De Deyn et al., 2004) or their richness through changes in understory plant and microbial diversity (Wang et al., 2019b). Altogether, soil nematodes represent key organisms influencing ecosystem functioning, e.g. by grazing on plant roots (Yeates et al., 2009) or by regulating microbial communities, litter decomposition, and nutrient cycling (Yeates et al., 2009; García-Palacios et al., 2016; Yeates and Coleman, 2021). Yet, exploring the impacts of soil nematodes on EMF and their role in the indirect pathways by which dominant plants influence EMF is essential for maintaining high levels of ecosystem function in the context of grassland vegetation change.
Previous studies have shown that soil pH and water content are important drivers of soil biological communities (Fierer and Jackson, 2006), and ecosystem functions or processes (e.g. soil organic carbon accumulation, nitrogen mineralization, and plant productivity) (Chen et al., 2013; Jing et al., 2015; Li et al., 2017). Therefore, here we investigated the role of dominant plant species on the abiotic environment (soil pH and water content), on biological communities (surrounding plants, nematodes, and microbial richness, abundances, and biomasses), and on the EMF of grassland ecosystems using nine soil functions related with biological productivity, nutrient cycling, and build-up of nutrient pools. We focused on alpine grasslands because they cover 86% of the Qinghai-Tibet Plateau (Wang and Cheng, 2001), are biodiversity hot-spots (Bengtsson et al., 2019), and support the provision of essential ecosystem services such as animal husbandry, forage production or carbon sequestration (Clough et al., 2014; Newbold et al., 2016). We used two common plant species with potentially negative and positive effects on biodiversity and EMF: Ligularia virgaurea Mattf. ex Rehder. & Kobuski. (Ligularia Cass, Asteraceae) and Dasiphora fruticosa (L.) Rydb. (Dasiphora, Rosaceae). L. virgaurea is a perennial herb widely distributed in the alpine meadows of the Qinghai-Tibet Plateau, and it is a poisonous weed plant (Wang et al., 2008) that can be fatal for animals (Ma et al., 2006; Shi et al., 2011). D. fruticosa is a common shrub of the alpine meadows within an elevation range from 2700 to 4500 m.a.s.l (Wang et al., 2017). D. fructicosa has been shown to promote the survival of surrounding graminoids (Xu et al., 2010; Michalet et al., 2014) or through indirect effects that promote nematode abundance by increasing grass biomass (Wang et al., 2018). However, these positive effects can be hidden by complex indirect interactions among plant functional groups (Xu et al., 2010; Michalet et al., 2014). We hypothesized that: (1) D. fruticosa would increase and L. virgaurea would reduce the EMF; (2) dominant plants would influence EMF through modifying the abiotic drivers, such as soil water content and pH; (3) dominant species would also indirectly affect EMF by influencing biodiversity, especially soil nematodes.
2 Materials and methods
2.1 Study site
The experiment was conducted in the alpine grasslands of the eastern Qinghai-Tibet Plateau, i.e. at the Gansu Gannan Grassland Ecosystem National Observation and Research Station in Maqu (33°40′N, 101°51′E) at 3550 m.a.s.l, Gansu, China. The mean annual temperature is 1.2°C, with the lowest temperatures occurring in January (-10°C) and the highest in July (11.7°C). The mean annual precipitation reaches 564 mm, mostly concentrated from May to September. Yaks have been grazing the alpine grassland in the study area since 1999, with a density of 1.6 head ha-1 (Hu et al., 2015). Vegetation did not experience significant degradation over the least 20 years. The vegetation cover of the study site is dominated by the shrub Dasiphora fruticosa (Rosaceae) and Ligularia virgaurea (Asteraceae), but also includes other perennial plant species such as Carex atrofusca (Cyperaceae), Agrostis hugoniana (Poaceae), Euphorbia altotibetica (Euphorbiaceae) and Halenia elliptica (Gentianaceae) (See Supplementary Table 1 for more information about each plant species).
2.2 Experimental design
Our observational design was set up in early June 2016 (See Supplementary Figure 1). Fifteen blocks were set up within a homogenous and flat alpine grassland landscape, which is one of the representative landscape types of the Tibetan Plateau and the main typical habitat of the distribution of D. fruticosa and L. virgaurea (Chu et al., 2014; Qian et al., 2022). Two hundred individuals of D. fruticosa could be observed (25% of the total cover), and thousands of L. virgaurea (15% of the cover) in this landscape. Each block included 3 plots of 30 cm × 30 cm: one grassland plot including the dominant shrub D. fruticosa, one including the dominant poisonous weed L. virgaurea and one grassland control without D. fruticosa or L. virgaurea. The canopy size of each shrub was around 50 cm × 70 cm in our study, so to ensure that the sample plot was completely under the canopy, we set up a 30 cm × 30 cm plot. The dominant plant represented the center of the plot. Altogether, our study included a total of 45 plots.
2.3 Sampling and measurement
In each plot, we collected a composite soil sample, resulting from three sub-samples (4 cm diameter) randomly taken by a soil auger at 15 cm depth. We mixed each composite soil sample and removed the gravel. We divided the composite soil samples into two replicates, and stored them at 4°C: one replicate was used to extract nematodes, and the other to measure soil physicochemical properties.
We measured 9 soil variables that were uncorrelated with each other (See Supplementary Figure 2), and together constitute good proxies for biological productivity, nutrient cycling, and nutrient pools establishment (Bowker et al., 2013; Soliveres et al., 2016; Delgado-Baquerizo et al., 2016a; Wang et al., 2019a): soil nitrate (NO3-), soil ammonium (NH4+), soil organic carbon (SOC), soil total phosphorus (TP), soil total nitrogen (TN), urease (URE), phosphatase (PHO), invertase (INV), protease (PRO).
We measured soil water content (SWC) by taking 5 g fresh soil and placing them in an oven at 90°C for 48 h until constant weight. After removing plant residues and gravel, the remaining soil was air-dried (avoiding direct sunlight), and then sieved (aperture of 0.25 mm). Soil pH was quantified in a 1:2.5 soil: deionized water slurry using a pH meter (PHSJ-3F, Shanghai INESA Scientific Instrument Co., Ltd, China). TP and TN were digested by concentrated H2SO4, followed by Mo-Sb antispetrophotography and semi micro-Kjeldahl (Bao, 2000) with an auto chemistry analyzer (SmartChem 200, AMS Alliance, Italy). SOC was determined following the wet oxidation method. Soil NH4+ and NO3- were extracted using 2 M KCl (1:10 soil: solution ratio) and analyzed with an auto chemistry analyzer.
The activity of URE was measured by the reaction of urease enzyme ammonia with phenol-sodium hypochlorite in an alkaline medium (Huang et al., 2015). The activity of INV was measured by using the dinitrosalicylic acid method (Asare-Brown and Bullock, 1988). PHO activity was determined by using disodium diphenyl phosphate colorimetry (Tabatabai and Bremner, 1969). Ninhydrin colorimetry was used to determine PRO activity (Watanabe and Hayano, 1995).
2.4 Biodiversity indices
We focused on plant species richness, biomass, and abundance as potential aboveground indirect biotic drivers, as they are known to have an impact on EMF (e.g. Maestre et al., 2012; Soliveres et al., 2016). In each plot, all herbaceous plants were thus identified at the species level to calculate the plant richness. We also counted the number of each individual plant species to evaluate the abundance of aboveground plant communities and then harvested per species to assess the aboveground biomasses. The plant material was oven-dried for 48 h at 80 ° before weighing. Note that the Shannon index of plant diversity was also calculated. However, this index was further removed from subsequent analyses due to high correlation with plant richness (r = 0.8, Supplementary Figure 3).
We also considered the richness, biomass, and abundance of nematodes as potential indirect soil biotic drivers of dominant plant species on EMF. We used the modified Baermann wet funnel technique to extract nematodes from 50 g. of fresh soil (Liu et al., 2008). We identified all nematodes in each sample to genus/species level and converted nematode abundances to the number of individuals per kg. of dry soil (ind. kg-1 dry soil). We also calculated the Shannon index of nematode diversity. However, this index was further removed from subsequent analyses due to high correlation with nematode richness (r = 0.9, Supplementary Figure 3). We finally measured the maximum width and length of all nematodes observed. We used Andrassy’s formula (Andrássy, 1967) to estimate nematode biomass:
where W is the maximal width of nematodes and the L is their length.
Finally, we considered microbial C and N biomasses as proxies of the C and N sources in the systems (Sanaullah et al., 2011). They were measured based on the Chloroform fumigation extraction method (Brookes et al., 1985). Then a microbial C:N biomass ratio was calculated and used as a surrogate of ecosystem productivity and soil fertility (Sanaullah et al., 2011; Cheng et al., 2020).
2.5 Multifunctionality indices
We evaluated EMF using both the averaging (Mouillot et al., 2011) and the multiple threshold approaches (Byrnes et al., 2014), which allowed us to assess whether multiple functions are simultaneously performing at high levels, and to consider potential trade-offs between the functions assessed (Le Bagousse-Pinguet et al., 2019; Le Bagousse-Pinguet et al., 2021). The averaged EMF index (EMFA) was calculated using each of the 9 soil variables (SOC, TN, TP, NH4+, NO3-, URE, PRO, PHO, INV). We scaled the 9 variables to range from 0 to 1 with the formula:
where x is the value of the function with its maximum (xmax) and minimum (xmin) values measured, then we averaged the standardized variables to obtain the EMFA for each plot. We also computed EMF-threshold values of 25% (MFT25), 50% (MFT50), 75% (MFT75), according to Byrnes et al. (2014).
2.6 Statistical analyses
Generalized linear mixed modeling (GLMM) was used to evaluate the effects of D. fruticosa, L. virgaurea, and the control on EMF, and on plant and nematode diversity and biomass and soil properties, with the treatments as fixed effects, and block as a random effect. The uniformity and dispersion of these models were checked and adjusted accordingly. The beta and poisson generalized linear models were used for proportional and counting data, respectively (Douma and Weedon, 2019). We also used “compois” or “genpois” distribution due to the presence of underdispersion (i.e. variance < mean) in the data (Zuur et al., 2007). Tukey’s HSD test was used for post-hoc analyses to determine significant differences between treatments.
Linear mixed effect models (LMM) were used to evaluate the impacts of abiotic and biotic drivers on EMF. We used the EMF indices as response variables, and soil (pH, SWC, and their quadratic term) and biotic attributes (dominant plants; plant richness, abundance, and biomass; nematode richness, abundance, and biomass; microbial biomass C:N) as predictors, and included the block as a random effect. Note that since some diversity index do not necessarily change linearly along environmentally strong gradients (e.g. soil pH and water content), we considered the quadratic term of soil pH and water content. Before regression analysis, the predictors highly correlated (r > 0.7) and the variance inflation factor (VIF) value of more than 10, such as plant and nematode Shannon diversity, were removed from all analyses (See Supplementary Figure 3 and Supplementary Table 2).
All response variables and predictors were Z-scored (standardized deviated) prior to analyses to account for parameter estimates within a comparable scale. To assess the relative effect of each predictor on EMF, we used a method similar to the variance decomposition. In short, the method can be simply calculated the ratio between the standardized regression coefficients of predictors and the sum of all standardized regression coefficients in the models (Gross et al., 2017; Yuan et al., 2021). We also repeated these analyses without random effect to ensure the robustness of our results (See Supplementary Table 3).
For each EMF index, a model selection procedure was used to select the most parsimonious set of predictors (Le Bagousse-Pinguet et al., 2017). We first generated all possible combinations of predictors, and then selected the set of best-supported models within a ΔAICc of 2 (See Supplementary Table 4). Before analysis, we scaled all predictors using the Z-scored (standardized deviated) method (Le Bagousse-Pinguet et al., 2017).
Finally, piecewise structural equation modeling (pSEM) was used to test for the direct and indirect effects of L. virgaurea and D. fruticosa on EMF through changes in abiotic and biotic attributes. We set up an a-priori models, while only considering dominant plants, and the significant biotic and abiotic indirect drivers found with the linear modeling procedure (Supplementary Figure 5). We thus considered the dominant plant type D. fruticosa or L. virgaurea) as predictors, SWC and pH as indirect abiotic drivers, and plant and nematode richness as indirect biotic drivers of EMF. The model fits of pSEM were assessed using Shipley’s test of d-separation through Fisher’s C statistic (Lefcheck, 2016).
Our data analyses were conducted in R software, ver. 4.0.3 (R core Team, 2020). The calculation of EMF indices was conducted using the getStdAndMeanFunctions function in the ‘multifunc’ package (Byrnes et al., 2014). Shannon diversity was calculated using the diversity function in the ‘vegan’ package (Oksanen et al., 2019). The GLMMs were performed using the glmmTMB function with a genpois link (i.e. count data for the underdispersion), or with a beta link (i.e. proportional data) in the ‘glmmTMB’ package (Brooks et al., 2017) and the lmer (i.e. normal data) and glmer function with a poisson link (i.e. count data for no dispersion) in the ‘lme4’ package (Bates et al., 2015). The model diagnosis of linear mixed models was conducted using the testUniformity function in the ‘DHARMa’ package (Hartig, 2020), and the testDispersion function in the ‘DHARMa’ package (Hartig, 2020) for the dispersion test. The dredge function in the ‘MuMIn’ package (Bartoń, 2020) for the model selection procedure. The marginal means (EMMS) of GLMMs and LMMs were estimated using the emmeans function in package ‘emmeans’ (Lenth et al., 2020). The pSEMs were conducted using the psem function in the R package ‘piecewiseSEM’ (Lefcheck, 2016). Package ‘ggplot2’ (Wickham, 2016) was used to plot figures.
3 Results
EMF indices strongly varied in response to dominant plants (Figure 1). The allopathic L. virgaurea significantly increased all EMF indices compared to the control plots (Figures 1A–D). This was particularly true for individual functions such as SOC, TN, NH4+, and for most of the enzymes considered (See Supplementary Figures 4A, C, E–I). The facilitative species D. fruticosa mostly led to intermediate values of EMF indices (Figures 1A, C, D), although a significant increase in EMF was observed when the ecosystem was performing low (MFT25: Figure 1B). This pattern mostly occurred because the effect of D. fruticosa on individual ecosystem functions were not consistent, and could be either positive (SOC, TN, NH4+, INV) (See Supplementary Figures 4A, C, E, F) or neutral (TP, NO3-, URE, PHO) (See Supplementary Figures 4B, D, H, I).
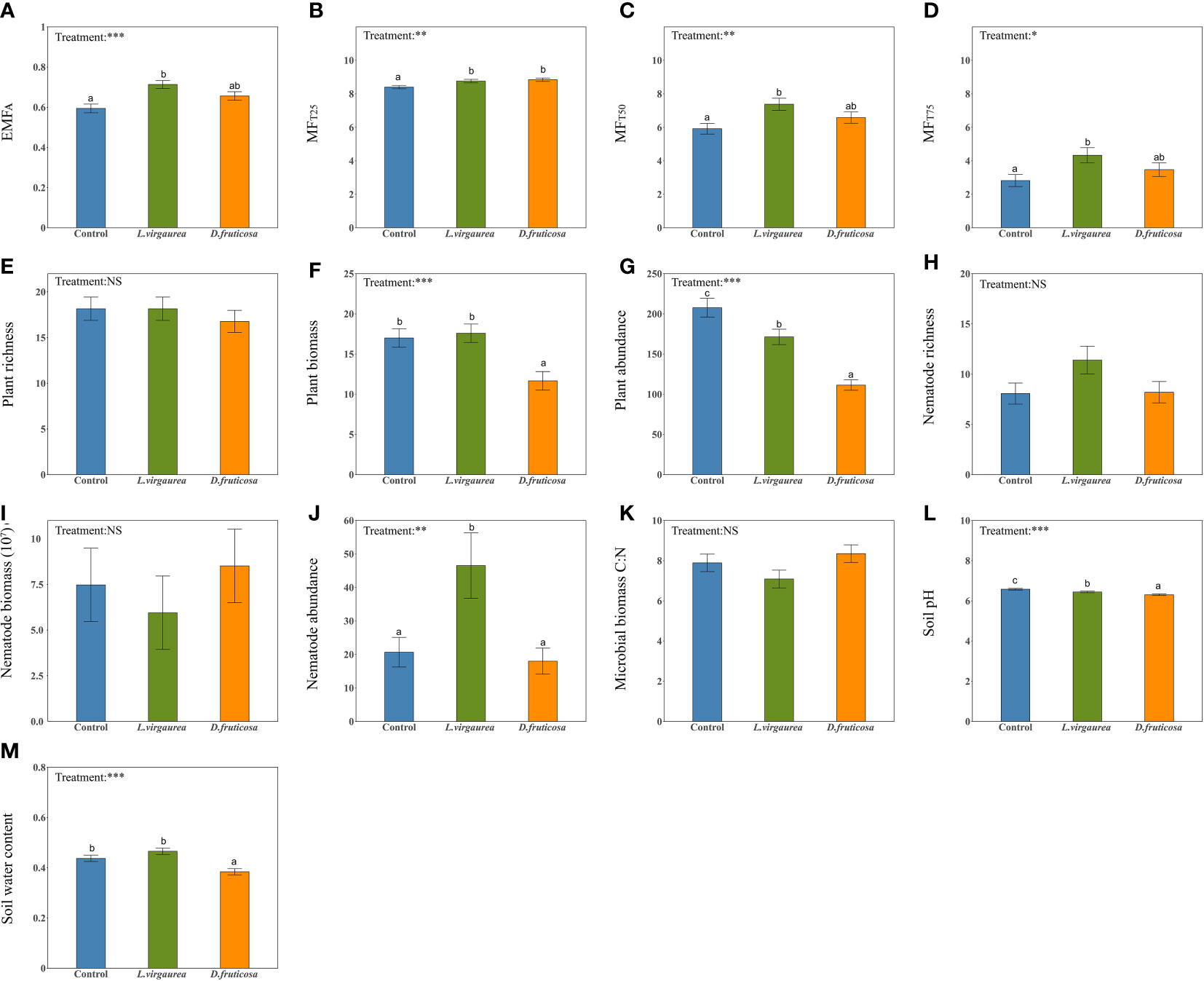
Figure 1 The effects of dominant plants on EMFA (A), MFT25 (B), MFT50 (C), MFT75 (D), plant richness (E), plant biomass (F), plant abundance (G), nematode richness (H), nematode biomass (I), nematode abundance (J), Microbial biomass C:N ratio (K), soil pH (L), soil water content (M). Different lowercase letters within panels indicate significant (p-value < 0.1) differences between treatment means, after using Tukey’s method to correct for multiple comparisons. Error bars represent means ± SE (NS, p> 0.05; *p <0.05; **p <0.01; ***p <0.001).
We found that dominant plants had contrasted effects on the indirect biotic and abiotic drivers considered (Figures 1E–M). D. fruticosa significantly decreased plant biomass and abundance (Figures 1F, G). L.virgaurea had a negative effect on plant abundance (Figure 1G), a positive effect on nematode abundance (Figure 1J), and a neutral effect on plant biomass (Figure 1F) compared to the control plots. In contrast, dominant plants had no impact on the richness of understory plant and nematode (Figures 1E, H), nematode biomass (Figure 1I), and microbial biomass C:N ratio (Figure 1K). Finally, dominant plants had significant effects on the abiotic attributes considered, particularly by significantly decreasing the soil pH (Figure 1L). D. fruticosa furthermore had a significant negative effect on SWC, while SWC under L. virgaurea remained as high as in the control plots (Figure 1M).
The multiple linear regression models explained a fair amount of variation in EMF, i.e. ~68%, 35%, 37%, and 43% of variations in EMFA, MFT25, MFT50, and MFT75 respectively (Figure 2). The dominant plants D. fruticosa and L. virgaurea together explained on average ~11% of the variation in EMF (5%~20%). In comparison, the abiotic attributes considered explained ~20% of the variations in EMF (2~38%), and the cumulative above- and belowground attributes accounted for ~9% (3%~16%) and 7% (3%~11%) of the variations in EMF respectively.
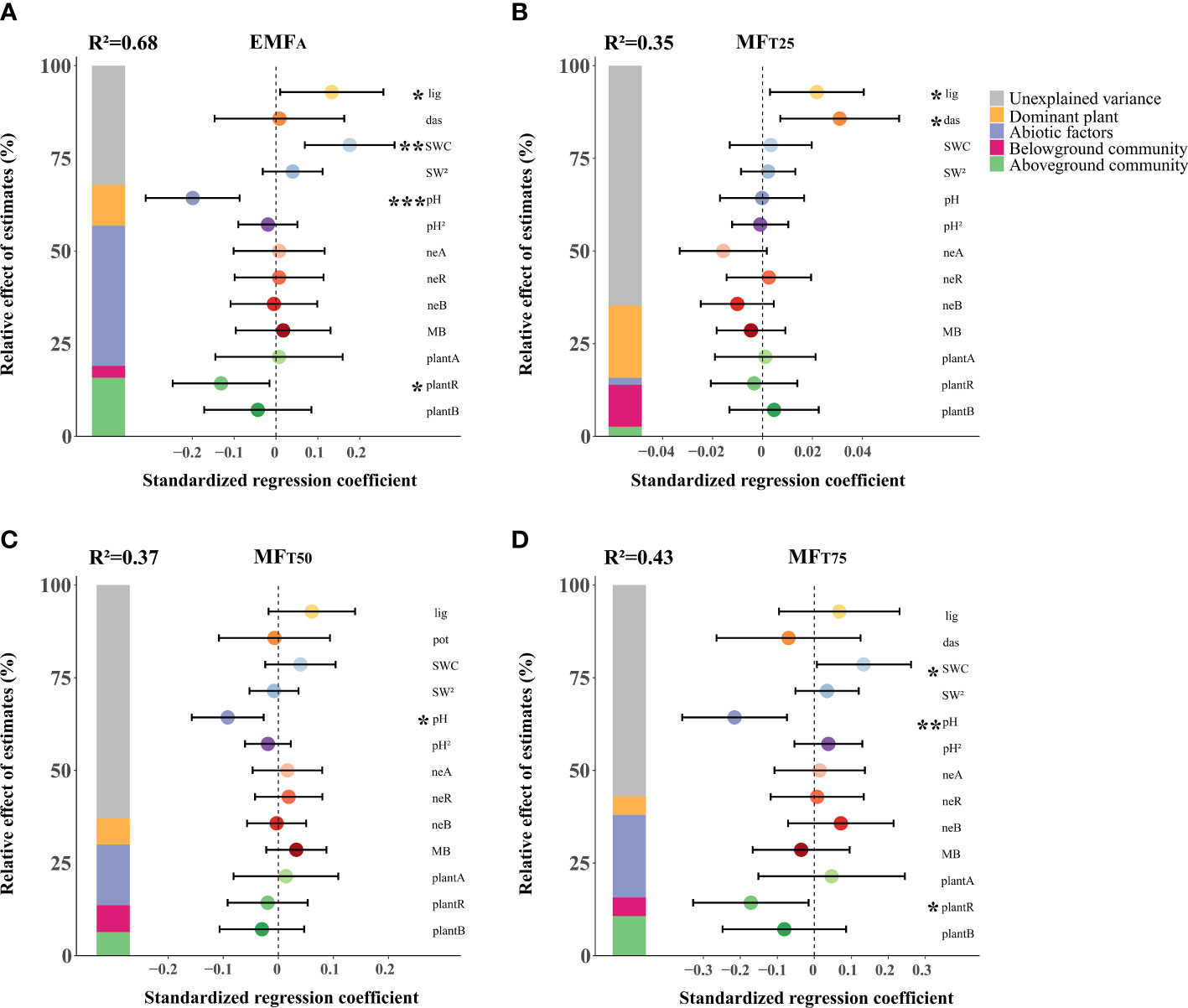
Figure 2 The effects of biotic and abiotic factors on EMFA (A), MFT25 (B), MFT50 (C), MFT75 (D). Standardized regression coefficients of model predictors, associated 95% confidence intervals and relative importance of each factor, expressed as the percentage of explained variance. The R2 of the averaged model and the p-value of each predictor are given as: *p < 0.05; **p < 0.01; ***p < 0.001. lig, L. virgaurea; das, D. fruticosa; SWC, soil water content; pH, soil pH; neA, nematode abundance; neR, nematode richness; neB, nematode biomass; MB, microbial biomass; C, N ratio; plantA, plant abundance; plantR, plant richness; plantB, plant biomass.
L. virgaurea, SWC, soil pH, and plant richness were the main predictors of EMF (Figure 2A). However, these effects were highly dependent on the level of performance of the system. D. fruticosa and L. virgaurea positively affected EMF at the low level of ecosystem performance (MFT25). Plant richness had a negative effect on EMF, specifically at the higher level of EMF (MFT75). Increasing SWC enhanced EMF, specifically at the higher level of performance (MFT75), and soil pH also reduced EMF in the case of higher levels of ecosystem performance (MFT50 and MFT75).
The pSEMs explained 52%, 39%, 49%, and 35% of the variations in EMFA, MFT25, MTT50, and MFT75 respectively (Figure 3). From the pSEMs results, we can find that the impacts of D. fruticosa and L. virgaurea on EMF were both a promotion effect, irrespective of the EMF threshold considered, and the promotion effect of L. virgaurea was greater than that of D. fruticosa (except MFT25). The pSEMs showed that L. virgaurea can directly enhance EMFA and MFT25, and D. fruticosa significantly improved MFT25 directly. Meanwhile, both D. fruticosa and L. virgaurea indirectly affected the EMF through the abiotic pathway. The pSEMs also showed indirect effects of L. virgaurea on EMFA and MFT50via soil pH (Figures 3A, E), while D. fruticosa influenced EMFA indirectly through soil pH and SWC (Figure 3A). We also considered other biological pathways, such as nematode and plant abundance (See Supplementary Figure 6), nematode, plant, and microbial biomass (See Supplementary Figure 7). The results also showed that the dominant plants affected EMF mainly through the direct path and abiotic indirect path, and the promotion effect of L. virgaurea on EMF was higher than that of D. fruticosa.
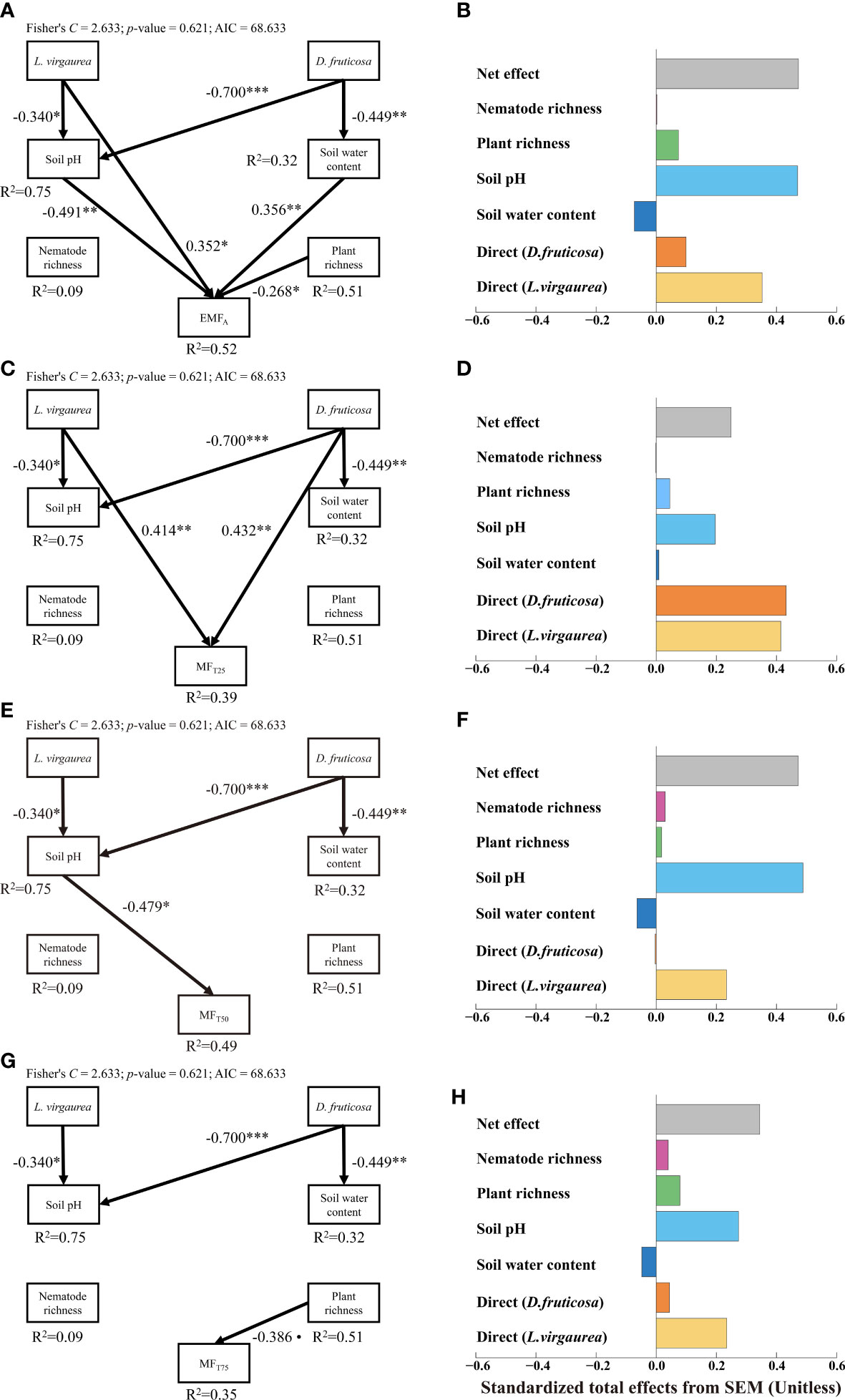
Figure 3 Structural equation model assessing the direct and indirect effects of dominant plants on EMFA (A), MFT25 (C), MFT50 (E) and MFT75 (G). Numbers adjacent to arrows are indicative of the effect size of the relationship. Only significant and marginally significant pathways were shown. Significance levels are as follows: •, p < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001. The right panel of the figure showed that total, direct and indirect standardized effects of the different drivers of EMFA (B), MFT25 (D), MFT50 (F) and MFT75 (H).
4 Discussion
Here we aimed at disentangling the direct from indirect abiotic and biotic (above- and belowground diversities) pathways by which dominant plant species influence EMF. Our results indicated that the two dominant plants overall promoted the EMF of alpine grasslands. Our result brings new evidence on the importance of dominant plants for EMF and thus expands to multiple ecosystem functions simultaneously the mass-ratio hypothesis (Grime, 1998), originally framed for individual functions (Smith and Knapp, 2003; Garnier et al., 2004).
The positive effect of dominant plants on EMF occurred irrespective of their expected (positive and negative) effects. Two main reasons may explain this pattern. First, dominant plants can alter the spatial allocation of resources (e.g. fertilizer island effects) (Maestre and Puche, 2009; Eldridge et al., 2011; Ochoa-Hueso et al., 2018). For instance, Soliveres and Eldridge (2014) found that increasing shrub encroachment did not impede ecosystem functions, but instead had positive effects on plant and soil properties. Second, grazing may have played an important role in this process (Daryanto et al., 2013; Soliveres and Eldridge, 2014). Upon herbivore trampling effects on soil compaction and water redistribution that could reduce nematode diversity and ecosystem functioning (Castellano and Valone, 2007; Allington and Valone, 2010; Andriuzzi and Wall, 2017), the unexpected facilitative effect of L. virgaurea on EMF may arise from mechanisms of associational avoidance (see Milchunas and Noy-Meir, 2002 for a review). On the other hand, and according to the attractant decoy hypothesis (Milchunas and Noy-Meir, 2002), herbivores preferentially consume palatable plants, such as grasses, thereby altering the aboveground plant community composition of grasslands (control) and affecting nutrient input and decomposition (Abule et al., 2005; see Hempson et al., 2015 for a review). We acknowledge that our approach does not allow us to fully conclude on the mechanisms explaining the observed patterns, but this overall positive effect clearly stimulates the need for further research on the impact of dominants on EMF.
Contrary to the first hypothesis, we did not observe any positive effects of D. fruticosa on EMF indices (except MFT25), although it is often regarded as a nurse species (Michalet et al., 2016; Wang et al., 2017). Our result may relate to the environmental conditions under which our experimental design was performed. Species interactions can shift from competitive to facilitative interactions from low to moderate stress environmental conditions (Bertness and Callaway, 1994). Facilitation then diminishes from moderate to highly stressed conditions (Michalet et al., 2006; Michalet et al., 2014). In this context, and depending on the environmental conditions, the dominant plant D. fruticosa could have various effects on local species diversity (Le Bagousse-Pinguet et al., 2012; Liancourt et al., 2017).
The positive effects of L. virgaurea on EMF were greater than that of D. fruticosa, to significantly increase each component of EMF (e.g. soil organic carbon, total nitrogen, ammonium, and for most of the enzymes considered). L. virgaurea has been found to increase total soil organic carbon concentrations, soil organic C:N ratio, and enzymatic activity (Shi et al., 2011), or root secretions to promote multiple bacterial groups (Wang et al., 2022). Also, leaching from allelopathic plants such as L. virgaurea can also improve soil conditions in their vicinity (Hierro and Callaway, 2003). However, our results do not call for their expansion as a potential standardization of management. Previous studies have also found that the expansion of toxic weeds could reduce grassland areas (Van Auken, 2009), thus limiting the access to palatable food sources for herbivores (Ma et al., 2006). Therefore, and while beneficial to soil functions, this species may have negative effects on key functions on which human society depends. These antagonistic effects warn for the need of comprehensive efforts when formulating management policies to deal with the expansion of toxic weeds rather than a ‘one size fits all’ management approach.
Our results also showed that dominant plants mostly impacted on EMF through changes in soil pH and water content. Soil pH is known to be an important driver impacting on microbial communities (Lauber et al., 2009) and EMF (Delgado-Baquerizo et al., 2016b; Luo et al., 2018). Furthermore, soil pH was highly related to soil organic carbon, and soil acidification could contribute to soil organic carbon accumulation, thereby improving the ability of grassland ecosystems to maintain multiple functions (Jing et al., 2015). Soil water content had an indirect positive effect on ecosystem functions, which may relate to the increase of water holding capacity, further promoting nutrient cycling and grassland productivity (Guo et al., 2012).
Contrary to expectation, we found negligible indirect biotic effects, indicating that dominant plants weakly affected the EMF of alpine grasslands through changes in the richness of plants and key soil organisms such as nematodes. Furthermore, the effects of plant richness were only observed at high levels of ecosystem performance. Our results align with the view of considering other facets of biodiversity such as functional and phylogenetic diversity facets, which have been found to contribute more to EMF than taxonomic richness only (Flynn et al., 2011; Gross et al., 2017; Le Bagousse-Pinguet et al., 2019; Wen et al., 2019; Le Bagousse-Pinguet et al., 2021). Also, the absence of effect of belowground biodiversity may arise from the consideration of nematodes only as an indicator of belowground biodiversity, while multiple trophic levels are needed to promote EMF (Jing et al., 2015; Schuldt et al., 2018). The reason may be that the soil functions (e.g. soil enzymes) we selected may be more related to abiotic factors (e.g. soil pH and water content) than to nematode diversity. Studies on the relationship between soil species richness and ecosystem function also show that for nutrient cycling, it depends to some extent on species traits rather than species richness (Heemsbergen et al., 2004; Nielsen et al., 2011). In addition, there may be trade-offs in the relationship between nematodes of different feeding types and EMF. For example, Du et al. (2022) found that bacterial feeders were positively correlated with EMF, while fungal feeders and omnivorous feeders were negatively correlated with EMF.
5 Conclusions
Our findings provided evidence that contrasting dominant plants such as D. fruticosa and L. virgaurea can increase ecosystem multifunctionality in the alpine meadows, although our “one-shot” study should be complemented by longer-term and dynamical approaches. Finally, our results also showed that these effects not only arise from direct, but also indirect abiotic pathways through changes in soil conditions. Altogether, our study suggested that dominant plants may play a key role in promoting multiple ecosystem functions simultaneously, and could mitigate the negative impacts of vegetation changes on EMF in the alpine meadows.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JC, SC, HG, KL, LA, and SX contributed to conception and design of the study. ZL, HC, HS, JW, ZY, YW, XW, XY, and LM conducted the field experiment and organized the database. JC performed all the statistical analyses. JC and YB-P wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the project of the National Natural Science Foundation of China (41830321, 31870412, 32071532), the “111 Project” (BP0719040), the Natural Science Foundation of Gansu Province (22JR5RG564), and the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0302).
Acknowledgments
We thank the Gansu Gannan Grassland Ecosystem National Observation and Research Station (Maqu Sub-station) for allowing us to use their site.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1117903/full#supplementary-material
Supplementary Figure 1 | The schematic diagram of experimental design.
Supplementary Figure 2 | Pearson’s correlation coefficients between each individual function and their relationships with EMF indices. Red to black color indicates negative to positive correlations. The number represent correlation coefficient. TP: soil total phosphatase; TN: soil total nitrogen; SOC: soil organic carbon; NO3-: soil nitrate; NH4+: soil ammonium; INV: invertase; PRO: protease; URE: urease; PHO: phosphatase; EMFA: averaged multifunctionality index; MFT25: 25% threshold-based multifunctionality index; MFT50: 50% threshold-based multifunctionality index; MFT75: 75% threshold-based multifunctionality index.
Supplementary Figure 3 | Pearson’s correlation coefficients between pairs of abiotic and biotic factors. Red to black color indicates negative to positive correlations. The number represent correlation coefficient. lig: L. virgaurea; das: D. fruticosa; SWC: soil water content; pH: soil pH; plantA: plant abundance; plantR: plant richness; plantB: plant biomass; plantS: plant Shannon diversity. neA: nematode abundance; neR: nematode richness; neB: nematode biomass; neS: nematode Shannon diversity; MB:microbial biomass C:N ratio.
Supplementary Figure 4 | The effects of dominant plants on each of ecosystem functions. Different lowercase letters within panels indicate significant (NS: p> 0.05; *: p <0.05; **: p <0.01; ***: p <0.001) differences between treatment means, after using Tukey’s method to correct for multiple comparisons. Error bars represent means ± SE. For abbreviations, see Supplementary Figure 2.
Supplementary Figure 5 | An a-priori conceptual model of piecewise structural equation modeling.
Supplementary Figure 6 | Structural equation model assessing the direct and indirect effects of dominant plants on averaged ecosystem multifunctionality (A), 25% threshold-based (C), 50% threshold-based (E) and 75% threshold-based (G) ecosystem multifunctionality via abiotic factors (soil water content and pH) and biotic factors (nematode and plant abundance). Numbers adjacent to arrows are indicative of the effect size of the relationship. Only significant and marginally significant pathways were shown. Significance levels are as follows: •, p < 0.10; *, p < 0.05; **, p < 0.01; ***, p < 0.001. The right panel of the figure showed that total, direct and indirect standardized effects of the different drivers of EMF.
Supplementary Figure 7 | Structural equation model assessing the direct and indirect effects of dominant plants on averaged ecosystem multifunctionality (A), 25% threshold-based (C), 50% threshold-based (E) and 75% threshold-based (G) ecosystem multifunctionality via abiotic factors (soil water content and pH) and biotic factors (nematode, plant and microbial biomass). Numbers adjacent to arrows are indicative of the effect size of the relationship. Only significant and marginally significant pathways were shown. Significance levels are as follows: •, p < 0.10; *, p < 0.05; **, p < 0.01; ***, p < 0.001. The right panel of the figure showed that total, direct and indirect standardized effects of the different drivers of EMF.
References
Abule, E., Smit, G. N., Snyman, H. A. (2005). The influence of woody plants and livestock grazing on grass species composition, yield and soil nutrients in the middle awash valley of Ethiopia. J. Arid Environ. 60, 343–358. doi: 10.1016/j.jaridenv.2004.04.006
Allington, G. R. H., Valone, T. J. (2010). Reversal of desertification: The role of physical and chemical soil properties. J. Arid Environ. 74, 973–977. doi: 10.1016/j.jaridenv.2009.12.005
Andrássy, L. (1967). The determination of volume and weight of nematodes. Eds. Zuckerman, B. M., Brzeski, M. W., Deubert, K. H. (East Wareham, Massachusetts: University of Massachusetts) 73–84. doi: 10.4238/gmr16039705
Andriuzzi, W. S., Wall, D. H. (2017). Responses of belowground communities to large aboveground herbivores: Meta-analysis reveals biome-dependent patterns and critical research gaps. Glob. Change Biol. 23, 3857–3868. doi: 10.1111/gcb.13675
Asare-Brown, E., Bullock, C. (1988). Simple practical investigations using invertase. Biochem. Educ. 16, 98–100. doi: 10.1016/0307-4412(88)90072-6
Austin, A. T., Ballare, C. L. (2010). Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. 107, 4618–4622. doi: 10.1073/pnas.0909396107
Balvanera, P., Siddique, I., Dee, L., Paquette, A., Isbell, F., Gonzalez, A., et al. (2014). Linking biodiversity and ecosystem services: Current uncertainties and the necessary next steps. Bioscience 64, 49–57. doi: 10.1093/biosci/bit003
Bardgett, R. D., van der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Bartoń, K. (2020). MuMIn: Multi-model inference. R Packag. version 1.43.17. Available at: https://cran.r-project.org/package=MuMIn.
Bates, D., Mächler, M., Bolker, B. M., Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Bengtsson, J., Bullock, J. M., Egoh, B., Everson, C., Everson, T., O’Connor, T., et al. (2019). Grasslands–more important for ecosystem services than you might think. Ecosphere 10, e02582. doi: 10.1002/ecs2.2582
Bertness, M. D., Callaway, R. (1994). Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. doi: 10.1016/0169-5347(94)90088-4
Bodelier, P. L. E. (2011). Toward understanding , managing , and protecting microbial ecosystems. Front. Microbiol. 2. doi: 10.3389/fmicb.2011.00080
Bowker, M. A., Maestre, F. T., Mau, R. L. (2013). Diversity and patch-size distributions of biological soil crusts regulate dryland ecosystem multifunctionality. Ecosystems 16, 923–933. doi: 10.1007/s10021-013-9644-5
Brookes, P. C., Landman, A., Pruden, G., Jenkinson, D. S. (1985). Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842. doi: 10.1016/0038-0717(85)90144-0
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/rj-2017-066
Brum, F. T., Graham, C. H., Costa, G. C., Hedges, S. B., Penone, C., Radeloff, V. C., et al. (2017). Global priorities for conservation across multiple dimensions of mammalian diversity. Proc. Natl. Acad. Sci. 114, 7641–7646. doi: 10.1073/pnas.1706461114
Byrnes, J. E. K., Gamfeldt, L., Isbell, F., Lefcheck, J. S., Griffin, J. N., Hector, A., et al. (2014). Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 5, 111–124. doi: 10.1111/2041-210X.12143
Castellano, M. J., Valone, T. J. (2007). Livestock, soil compaction and water infiltration rate: Evaluating a potential desertification recovery mechanism. J. Arid Environ. 71, 97–108. doi: 10.1016/j.jaridenv.2007.03.009
Cavieres, L. A., Brooker, R. W., Butterfield, B. J., Cook, B. J., Kikvidze, Z., Lortie, C. J., et al. (2014). Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett. 17, 193–202. doi: 10.1111/ele.12217
Chen, D., Lan, Z., Bai, X., Grace, J. B., Bai, Y. (2013). Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 101, 1322–1334. doi: 10.1111/1365-2745.12119
Cheng, Q., Xue, R., Wu, H., Hartley, W., Zhang, Y., Zhou, L., et al. (2020). Ecological stoichiometry of microbial biomass carbon, nitrogen and phosphorus on bauxite residue disposal areas. Geomicrobiol. J. 37, 467–474. doi: 10.1080/01490451.2020.1722768
Chu, H., Wang, S., Yue, H., Lin, Q., Hu, Y., Li, X., et al. (2014). Contrasting soil microbial community functional structures in two major landscapes of the Tibetan alpine meadow. Microbiologyopen 3, 585–594. doi: 10.1002/mbo3.190
Clough, Y., Ekroos, J., Báldi, A., Batáry, P., Bommarco, R., Gross, N., et al. (2014). Density of insect-pollinated grassland plants decreases with increasing surrounding land-use intensity. Ecol. Lett. 17, 1168–1177. doi: 10.1111/ele.12325
Daryanto, S., Eldridge, D. J., Throop, H. L. (2013). Managing semi-arid woodlands for carbon storage: Grazing and shrub effects on above- and belowground carbon. Agric. Ecosyst. Environ. 169, 1–11. doi: 10.1016/j.agee.2013.02.001
De Deyn, G. B., Raaijmakers, C. E., Van Ruijven, J., Berendse, F., van der Putten, W. H. (2004). Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106, 576–586. doi: 10.1111/j.0030-1299.2004.13265.x
Delgado-Baquerizo, M., Maestre, F. T., Eldridge, D. J., Bowker, M. A., Ochoa, V., Gozalo, B., et al. (2016a). Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytol. 209, 1540–1552. doi: 10.1111/nph.13688
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., et al. (2016b). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 7, 10541. doi: 10.1038/ncomms10541
Douma, J. C., Weedon, J. T. (2019). Analysing continuous proportions in ecology and evolution: A practical introduction to beta and dirichlet regression. Methods Ecol. Evol. 10, 1412–1430. doi: 10.1111/2041-210X.13234
Du, X., Liu, H., Li, Y., Li, B., Han, X., Li, Y., et al. (2022). Soil community richness and composition jointly influence the multifunctionality of soil along the forest-steppe ecotone. Ecol. Indic. 139, 108900. doi: 10.1016/j.ecolind.2022.108900
Eldridge, D. J., Bowker, M. A., Maestre, F. T., Roger, E., Reynolds, J. F., Whitford, W. G. (2011). Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 14, 709–722. doi: 10.1111/j.1461-0248.2011.01630.x
Ellison, A. M. (2019). Foundation species, non-trophic interactions, and the value of being common. iScience 13, 254–268. doi: 10.1016/j.isci.2019.02.020
Ferris, H. (2010). Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 42, 63–67. doi: 10.1111/j.1600-0684.2009.00388.x
Fierer, N., Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U. S. A. 103, 626–631. doi: 10.1073/pnas.0507535103
Flynn, D. F. B., Mirotchnick, N., Jain, M., Palmer, M. I., Naeem, S. (2011). Functional and phylogenetic diversity as predictors of biodiversity- ecosystem-function relationships. Ecology 92, 1573–1581. doi: 10.1890/10-1245.1
García-Palacios, P., Shaw, E. A., Wall, D. H., Hättenschwiler, S. (2016). Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol. Lett. 19, 554–563. doi: 10.1111/ele.12590
Garnier, E. R. I. C., Ortez, J. A. C., Ille, G. E. B. (2004). Plant functional markers capture ecosystem properties. Ecology 85, 2630–2637. doi: 10.1890/03-0799
Grime, J. P. (1973). Control of species density in herbaceous vegetation. J. Environ. Manage. 1, 151–167.
Grime, J. P. (1998). Benefits of plant diversity to Ecosystems : Immediate , filter and founder effects. J. Ecol. 86, 902–910. doi: 10.1046/j.1365-2745.1998.00306.x
Gross, N., Le Bagousse-Pinguet, Y., Liancourt, P., Berdugo, M., Gotelli, N. J., Maestre, F. T. (2017). Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 1, 1–9. doi: 10.1038/s41559-017-0132
Guo, Q., Hu, Z., Li, S., Li, X., Sun, X., Yu, G. (2012). Spatial variations in aboveground net primary productivity along a climate gradient in Eurasian temperate grassland: Effects of mean annual precipitation and its seasonal distribution. Glob. Change Biol. 18, 3624–3631. doi: 10.1111/gcb.12010
Handa, I. T., Aerts, R., Berendse, F., Berg, M. P., Bruder, A., Butenschoen, O., et al. (2014). Consequences of biodiversity loss for litter decomposition across biomes. Nature 509, 218–221. doi: 10.1038/nature13247
Hartig, F. (2020). DHARMa: Residual diagnostics for hierarchical (Multi-level / mixed) regression models. R Packag. version 0.3.3.0. Available at: https://cran.r-project.org/package=DHARMa.
Hector, A., Bagchi, R. (2007). Biodiversity and ecosystem multifunctionality. Nature 448, 188–190. doi: 10.1038/nature05947
Heemsbergen, D. A., Berg, M. P., Loreau, M., Van Hal, J. R., Faber, J. H., Verhoef, H. A. (2004). Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Sci. (80-. ). 306, 1019–1020. doi: 10.1126/science.1101865
Hejda, M., Sádlo, J., Kutlvašr, J., Petřík, P., Vítková, M., Vojík, M., et al. (2021). Impact of invasive and native dominants on species richness and diversity of plant communities. Preslia 93, 181. doi: 10.23855/PRESLIA.2021.181
Hempson, G. P., Archibald, S., Bond, W. J., Ellis, R. P., Grant, C. C., Kruger, F. J., et al. (2015). Ecology of grazing lawns in Africa. Biol. Rev. 90, 979–994. doi: 10.1111/brv.12145
Hierro, J. L., Callaway, R. M. (2003). Allelopathy and exotic plant invasion. Plant Soil 256, 29–39. doi: 10.1023/A:1026208327014
Hortal, S., Bastida, F., Moreno, J. L., Armas, C., García, C., Pugnaire, F. I. (2015). Benefactor and allelopathic shrub species have different effects on the soil microbial community along an environmental severity gradient. Soil Biol. Biochem. 88, 48–57. doi: 10.1016/j.soilbio.2015.05.009
Hu, J., Wu, J., Ma, M., Nielsen, U. N., Wang, J., Du, G. (2015). Nematode communities response to long-term grazing disturbance on tibetan plateau. Eur. J. Soil Biol. 69, 24–32. doi: 10.1016/j.ejsobi.2015.04.003
Huang, J., Li, Z., Zhang, J. (2015). Improvement of indophenol blue colorimetric method on activity of urease in soil. J. Civil Archit. & Environ. Eng. 2012, 102–107. doi: 10.11835/j.issn.1674-4764.2012.01.020
Jing, X., Sanders, N. J., Shi, Y., Chu, H., Classen, A. T., Zhao, K., et al. (2015). The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 6, 8159. doi: 10.1038/ncomms9159
Lauber, C. L., Hamady, M., Knight, R., Fierer, N. (2009). Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120. doi: 10.1128/AEM.00335-09
Le Bagousse-Pinguet, Y., Gross, N., Maestre, F. T., Maire, V., de Bello, F., Fonseca, C. R., et al. (2017). Testing the environmental filtering concept in global drylands. J. Ecol. 105, 1058–1069. doi: 10.1111/1365-2745.12735
Le Bagousse-Pinguet, Y., Gross, N., Saiz, H., Maestre, F. T., Ruiz, S., Dacal, M., et al. (2021). Functional rarity and evenness are key facets of biodiversity to boost multifunctionality. Proc. Natl. Acad. Sci. U. S. A. 118, 1–8. doi: 10.1073/pnas.2019355118
Le Bagousse-Pinguet, Y., Liancourt, P., Gross, N., Straile, D. (2012). Indirect facilitation promotes macrophyte survival and growth in freshwater ecosystems threatened by eutrophication. J. Ecol. 100, 530–538. doi: 10.1111/j.1365-2745.2011.01931.x
Le Bagousse-Pinguet, Y., Soliveres, S., Gross, N., Torices, R., Berdugo, M., Maestre, F. T. (2019). Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. U. S. A. 116, 8419–8424. doi: 10.1073/pnas.1815727116
Le Bagousse-Pinguet, Y., Xiao, S., Brooker, R. W., Gross, N., Liancourt, P., Straile, D., et al. (2014). Facilitation displaces hotspots of diversity and allows communities to persist in heavily stressed and disturbed environments. J. Veg. Sci. 25, 66–76. doi: 10.1111/jvs.12064
Lefcheck, J. S. (2016). piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. doi: 10.1111/2041-210X.12512
Lenth, R., Singmann, H., Love, J., Buerkner, P., Herve, M. (2020) Emmeans: Estimated marginal means, aka least-squares means. Available at: https://CRAN.R-project.org/package=emmeans.
Li, J., Gilhooly, W. P., Okin, G. S., Blackwell, J. (2017). Abiotic processes are insufficient for fertile island development: A 10-year artificial shrub experiment in a desert grassland. Geophys. Res. Lett. 44, 2245–2253. doi: 10.1002/2016GL072068
Liancourt, P., Le Bagousse-Pinguet, Y., Rixen, C., Dolezal, J. (2017). SGH: Stress or strain gradient hypothesis? insights from an elevation gradient on the roof of the world. Ann. Bot. 120, 29–38. doi: 10.1093/aob/mcx037
Liu, M., Chen, X., Qin, J., Wang, D., Griffiths, B., Hu, F. (2008). A sequential extraction procedure reveals that water management affects soil nematode communities in paddy fields. Appl. Soil Ecol. 40, 250–259. doi: 10.1016/j.apsoil.2008.05.001
Livingstone, S. W., Isaac, M. E., Cadotte, M. W. (2020). Invasive dominance and resident diversity: Unpacking the impact of plant invasion on biodiversity and ecosystem function. Ecol. Monogr. 90, 1–19. doi: 10.1002/ecm.1425
Luo, G., Rensing, C., Chen, H., Liu, M., Wang, M., Guo, S., et al. (2018). Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 32, 1103–1116. doi: 10.1111/1365-2435.13039
Ma, R. J., Du, G. Z., Lu, B. R., Chen, J. K., Sun, K., Hara, T., et al. (2006). Reproductive modes of three ligularia weeds (Asteraceae) in grasslands in qinghai-Tibet plateau and their implications for grassland management. Ecol. Res. 21, 246–254. doi: 10.1007/s11284-005-0114-1
Maestre, F. T., Puche, M. D. (2009). Indices based on surface indicators predict soil functioning in Mediterranean semi-arid steppes. Appl. Soil Ecol. 41, 342–350. doi: 10.1016/j.apsoil.2008.12.007
Maestre, F. T., Quero, J. L., Gotelli, N. J., Escudero, A., Ochoa, V., Delgado-Baquerizo, M., et al. (2012). Plant species richness and ecosystem multifunctionality in global drylands. Sci. (80-. ). 335, 214–218. doi: 10.1126/science.1215442
Michalet, R., Brooker, R. W., Cavieres, L. A., Kikvidze, Z., Lortie, C. J., Pugnaire, F. I., et al. (2006). Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773. doi: 10.1111/j.1461-0248.2006.00935.x
Michalet, R., Le Bagousse-Pinguet, Y., Maalouf, J. P., Lortie, C. J. (2014). Two alternatives to the stress-gradient hypothesis at the edge of life: The collapse of facilitation and the switch from facilitation to competition. J. Veg. Sci. 25, 609–613. doi: 10.1111/jvs.12123
Michalet, R., Schöb, C., Xiao, S., Zhao, L., Chen, T., An, L., et al. (2016). Beneficiary feedback effects on alpine cushion benefactors become more negative with increasing cover of graminoids and in dry conditions. Funct. Ecol. 30, 79–87. doi: 10.1111/1365-2435.12507
Milchunas, D. G., Noy-Meir, I. (2002). Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99, 113–130. doi: 10.1034/j.1600-0706.2002.990112.x
Mouillot, D., Villéger, S., Scherer-Lorenzen, M., Mason, N. W. H. (2011). Functional structure of biological communities predicts ecosystem multifunctionality. PloS One 6, e17476. doi: 10.1371/journal.pone.0017476
Newbold, T., Hudson, L. N., Arnell, A. P., Contu, S., De Palma, A., Ferrier, S., et al. (2016). Has land use pushed terrestrial biodiversity beyond the planetary boundary? a global assessment. Sci. (80-. ). 353, 288–291. doi: 10.1126/science.aaf2201
Nielsen, U. N., Ayres, E., Wall, D. H., Bardgett, R. D. (2011). Soil biodiversity and carbon cycling: A review and synthesis of studies examining diversity-function relationships. Eur. J. Soil Sci. 62, 105–116. doi: 10.1111/j.1365-2389.2010.01314.x
Ochoa-Hueso, R., Eldridge, D. J., Delgado-Baquerizo, M., Soliveres, S., Bowker, M. A., Gross, N., et al. (2018). Soil fungal abundance and plant functional traits drive fertile island formation in global drylands. J. Ecol. 106, 242–253. doi: 10.1111/1365-2745.12871
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., et al. (2019). Vegan: Community ecology package. R Packag. version 2.5-6. Available at: https://cran.r-project.org/package=vegan.
Qian, D., Du, Y., Li, Q., Guo, X., Fan, B., Cao, G. (2022). Impacts of alpine shrub-meadow degradation on its ecosystem services and spatial patterns in qinghai-Tibetan plateau. Ecol. Indic. 135, 108541. doi: 10.1016/j.ecolind.2022.108541
R core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Found. Stat. Comput).
Sanaullah, M., Blagodatskaya, E., Chabbi, A., Rumpel, C., Kuzyakov, Y. (2011). Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl. Soil Ecol. 48, 38–44. doi: 10.1016/j.apsoil.2011.02.004
Schuldt, A., Assmann, T., Brezzi, M., Buscot, F., Eichenberg, D., Gutknecht, J., et al. (2018). Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 9 2989. doi: 10.1038/s41467-018-05421-z
Shi, X. M., Li, X. G., Wu, R. M., Yang, Y. H., Long, R. J. (2011). Changes in soil biochemical properties associated with ligularia virgaurea spreading in grazed alpine meadows. Plant Soil 384, 289–301. doi: 10.1007/s11104-011-0818-7
Smith, M. D., Knapp, A. K. (2003). Dominant species maintain ecosystem function. Ecol. Lett. 6, 509–517. doi: 10.1046/j.1461-0248.2003.00454.x
Soliveres, S., Eldridge, D. J. (2014). Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Funct. Ecol. 28, 530–537. doi: 10.1111/1365-2435.12196
Soliveres, S., van der Plas, F., Manning, P., Prati, D., Gossner, M. M., Renner, S. C., et al. (2016). Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459. doi: 10.1038/nature19092
Tabatabai, M. A., Bremner, J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1, 301–307. doi: 10.1016/0038-0717(69)90012-1
Van Auken, O. W. (2009). Causes and consequences of woody plant encroachment into western north American grasslands. J. Environ. Manage. 90, 2931–2942. doi: 10.1016/j.jenvman.2009.04.023
Van Der Heijden, M. G. A., Bardgett, R. D., Van Straalen, N. M. (2008). The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310. doi: 10.1111/j.1461-0248.2007.01139.x
Wang, G., Cheng, G. (2001). Characteristics of grassland and ecological changes of vegetations in the source regions of Yangtze and yellow rivers. J. Desert Res. 2, 101–107. doi: 10.3321/j.issn:1000-694X.2001.02.001
Wang, L., Delgado-Baquerizo, M., Wang, D., Isbell, F., Liu, J., Feng, C., et al. (2019a). Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. U. S. A. 116, 6187–6192. doi: 10.1073/pnas.1807354116
Wang, W., Jia, T., Qi, T., Li, S., Degen, A. A., Han, J., et al. (2021). Interaction between root exudates and rhizosphere microorganisms facilitates the expansion of poisonous plant species in degraded grassland. iScience 25, 1052423. doi: 10.1016/j.isci.2022.105243
Wang, X., Michalet, R., Chen, S., Zhao, L., An, L., Du, G., et al. (2017). Contrasting understorey species responses to the canopy and root effects of a dominant shrub drive community composition. J. Veg. Sci. 28, 1118–1127. doi: 10.1111/jvs.12565
Wang, X., Nielsen, U. N., Yang, X., Zhang, L., Zhou, X., Du, G., et al. (2018). Grazing induces direct and indirect shrub effects on soil nematode communities. Soil Biol. Biochem. 121, 193–201. doi: 10.1016/j.soilbio.2018.03.007
Wang, X., Xiao, S., Yang, X., Liu, Z., Zhou, X., Du, G., et al. (2019b). Dominant plant species influence nematode richness by moderating understory diversity and microbial assemblages. Soil Biol. Biochem. 137, 107566. doi: 10.1016/j.soilbio.2019.107566
Wang, M. T., Zhao, Z. G., Du, G. Z., He, Y. L. (2008). Effects of light on the growth and clonal reproduction of ligularia virgaurea. J. Integr. Plant Biol. 50, 1015–1023. doi: 10.1111/j.1744-7909.2008.00645.x
Watanabe, K., Hayano, K. (1995). Seasonal variation of soil protease activities and their relation to proteolytic bacteria and bacillus spp in paddy field soil. Soil Biol. Biochem. 27, 197–203. doi: 10.1016/0038-0717(94)00153-R
Wen, Z., Zheng, H., Smith, J. R., Zhao, H., Liu, L., Ouyang, Z. (2019). Functional diversity overrides community-weighted mean traits in liking land-use intensity to hydrological ecosystem services. Sci. Total Environ. 682, 583–590. doi: 10.1016/j.scitotenv.2019.05.160
Xu, J., Michalet, R., Zhang, J. L., Wang, G., Chu, C. J., Xiao, S. (2010). Assessing facilitative responses to a nurse shrub at the community level: The example of potentilla fruticosa in a sub-alpine grassland of northwest China. Plant Biol. 12, 780–787. doi: 10.1111/j.1438-8677.2009.00271.x
Yeates, G. W., Coleman, D. C. (2021). “Role of nematodes in decomposition,” in Nematodes in soil ecosystems ed. Freckman, D. W. (New York: University of Texas Press), 55–80. doi: 10.7560/755260-007
Yeates, G. W., Ferris, H., Moens, T., van der Putten, W. H. (2009). “The role of nematodes in ecosystems,” in Nematodes as environmental indicators eds. Wilson, M. J., Kakouli-Duarte, T. (Wallingford, CABI International), 1–44. doi: 10.1079/9781845933852.0001
Yuan, Z., Ali, A., Loreau, M., Ding, F., Liu, S., Sanaei, A., et al. (2021). Divergent above- and below-ground biodiversity pathways mediate disturbance impacts on temperate forest multifunctionality. Glob. Change Biol. 27, 2883–2894. doi: 10.1111/gcb.15606
Keywords: ecosystem multifunctionality, plant biodiversity, soil biodiversity, dominant plants, alpine meadow
Citation: Chen J, Liu Z, Cui H, Song H, Wang J, Gao H, Chen S, Liu K, Yang Z, Wang Y, Wang X, Yang X, Meng L, An L, Xiao S and Le Bagousse-Pinguet Y (2023) Direct and indirect effects of dominant plants on ecosystem multifunctionality. Front. Plant Sci. 14:1117903. doi: 10.3389/fpls.2023.1117903
Received: 07 December 2022; Accepted: 17 February 2023;
Published: 02 March 2023.
Edited by:
Huakun Zhou, Northwest Institute of Plateau Biology (CAS), ChinaReviewed by:
Guofang Liu, Institute of Botany (CAS), ChinaJinlong Zhang, Kadoorie Farm and Botanic Garden, Hong Kong SAR, China
Copyright © 2023 Chen, Liu, Cui, Song, Wang, Gao, Chen, Liu, Yang, Wang, Wang, Yang, Meng, An, Xiao and Le Bagousse-Pinguet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyan Chen, Y2hlbnNoeUBsenUuZWR1LmNu
 Jingwei Chen1
Jingwei Chen1 Hanwen Cui
Hanwen Cui Shuyan Chen
Shuyan Chen Kun Liu
Kun Liu Xiaoli Yang
Xiaoli Yang Sa Xiao
Sa Xiao Yoann Le Bagousse-Pinguet
Yoann Le Bagousse-Pinguet