- College of Biological and Environmental Sciences, Zhejiang Wanli University, Ningbo, China
Hydrogen-rich water (HRW) treatment has been reported to delay the softening and senescence of postharvest okras, but its regulatory mechanism remains unclear. In this paper, we investigated the effects of HRW treatment on the metabolism of several phytohormones in postharvest okras, which act as regulatory molecules in fruit ripening and senescence processes. The results showed that HRW treatment delayed okra senescence and maintained fruit quality during storage. The treatment upregulated all of the melatonin biosynthetic genes such as AeTDC, AeSNAT, AeCOMT and AeT5H, contributing to the higher melatonin content in the treated okras. Meanwhile, increased transcripts of anabolic genes but lower expression of catabolic genes involved in indoleacetic acid (IAA) and gibberellin (GA) metabolism were observed in okras when treated with HRW, which was related to the enhanced levels of IAA and GA. However, the treated okras experienced lower abscisic acid (ABA) content as compared to the non-treated fruit due to the down-regulation of its biosynthetic genes and up-regulation of the degradative gene AeCYP707A. Additionally, there was no difference in γ-aminobutyric acid between the non-treated and HRW-treated okras. Collectively, our results indicated that HRW treatment increased levels of melatonin, GA and IAA, but decreased ABA content, which ultimately delayed fruit senescence and prolonged shelf life in postharvest okras.
Introduction
Horticulture is a dynamic sector where a wide variety of crops and their products are continuously innovating, and new market opportunities are considered and explored. It includes the cultivation of fruits, vegetables, nuts, seeds, herbs, sprouts, mushrooms, algae, flowers, seaweeds, and non-food crops such as grass and ornamental trees and plants. However, this sector faces many challenges, from retaining economic competitiveness, achieving full economic and environment sustainability and responding to climate change (Fawad et al., 2021; Taskesenlioglu et al., 2022). In addition, growing population and rise in income level will lead to increase in demand of high-value agriculture (HVA) produce (Ghosh, 2012).
Okra (Abelmoschus esculentus L.) as a vegetable, is now becoming more and more popular in China because of its rich nutritive value (Petropoulos et al., 2018). Generally, okra is extremely vulnerable to mechanical damage and therefore quite difficult for storage after harvest. According to statistics, the loss rate of postharvest okra ranges from 50% to72% (Gajanan et al., 2018). Therefore, it is of great importance to develop its preservation methods and prolong its shelf life.
In recent years, the role of hydrogen against abiotic stress in plants has been widely studied (Wu et al., 2019; Yan et al., 2022; Yu et al., 2023). In addition, hydrogen also plays an important role in maintaining fruit quality and delaying senescence process in several postharvest vegetables and fruit. For instance, 25% hydrogen-rich water (HRW) treatment improved the activities of antioxidant enzymes and inhibited the decay incidence of mushrooms (Chen et al., 2017). HRW treatment also increased fruit quality through regulating antioxidant capacity and energy metabolism in Rosa sterile fruit (Dong et al., 2022).
Plant hormones are chemical compounds present in very low concentration in plant, appearing to be multi-regulatory molecules during fruit development, ripening and senescence (Poveda, 2020). Indolacetic acid (IAA), which exists in almost all plant organs, is the main form of plant auxin (Yue et al., 2019). It was reported that IAA content accumulated during fruit ripening but decreased rapidly during fruit senescence (Trainotti et al., 2007; Chen et al., 2014). Exogenous treatment with IAA could delay the ripening process in harvested kiwifruit (Gan et al., 2021). The protective effects of melatonin as an antioxidant has been frequently investigated (Reiter et al., 2001; Iriti et al., 2006). Its effect on prolonging shelf life and improving fruit quality has been demonstrated in postharvest strawberries and table grapes (Liu et al., 2018; Sun et al., 2020). Abscisic acid (ABA) has a key role in regulating fruit senescence (McAtee et al., 2013) and conferring tolerance to environmental stresses. Previous studies have showed that ABA induced fruit senescence in postharvest sweet cherries (Luo et al., 2014) and enhanced the resistance against chilling in harvested zucchini (Carvajal et al., 2017). A tetracyclic diterpenoid hormone known as gibberellic acid (GA) has been reported to maintain fruit quality and prolong shelf life in postharvest okras (Xiao et al., 2022), Chinese flowering cabbage (Fan et al., 2021), persimmons (Maurer et al., 2019).
In a previous study, postharvest okra softening was delayed and storage life was prolonged by treatment with 0.22 mM HRW (Dong et al., 2023). However, there is no information available on the effect of hydrogen (H2), as an important signal molecule, on the regulation of metabolism of plant hormones in postharvest fruit or vegetables. Therefore, in order to further understand the mechanism of H2 in the preservation of okras after harvest, here we determined the levels of several hormones such as melatonin, IAA, ABA and GA3 together with their metabolizing genes in okras treated with HRW to reveal the relationship between H2 and other signal molecules.
Materials and methods
Hydrogen-rich water preparation, okra treatment and storage
HRW was prepared as the protocol of Dong et al. (2023). Fresh okras were purchased from a farm in Fenghua, Ningbo, China. The sample of the same size and maturity, free from disease and mechanical damage were selected. Okras were randomly divided into two experimental groups with two hundred okras in each. The HRW treatment was same as our previous study (Dong et al., 2023). After treatment, all the okras were surface-dried and packed in 0.03 mm polyethylene bags (5 mm × 5 mm diameter holes per bag, 10 fruit per bag). Then The fruit were stored in a constant temperature and humidity incubator at 25 ± 1°C and 80% ~ 90% relative humidity for 12 d. Thirty okras were taken from the control and treatment groups every 3 d for analysis.
Determination of senescence index and ΔE value
Senescence index manifested as browning discoloration and appearance on the surface of ten okras from each replicate were evaluated visually. For each okra, senescence was scored based on a five-grade system, where 0 means no shrinkage, no browning, mildew and bright color; 1 means slight shrinkage and browning but no mildew; 2 means obvious shrinkage, browning, mildew and yellowing; 3 means severe shrinkage, browning and mildew, yellow and black color with pungent odor; 4 means rotten into mud with strong pungent odor. The results were expressed as a senescence index calculated according to the formula below: Senescence index (between 0 and 4) = [(senescence level) * (number of okras at the senescence level)]/(total number of fruit *4) *100%. After measuring the L*, a*, b* values of okras with a colorimeter (KONICA MINOLTA, Japan), the ΔE value was calculated using the following formula: ΔE*ab=∑, where L1*/a1*/b1*= measured value of sample before treatment; L2*/a2*/b2*= measured value of sample at the sampling point.
Determination of melatonin, abscisic acid, indolacetic acid, gibberellin and γ-aminobutyric acid levels
Melatonin, ABA, IAA and GA levels were determined using corresponding commercially ELISA kits under instruction (Jiangsu Meimian industrial Co., Ltd, Nanjing, China). GABA was extracted and determined using Wang’s methods (2019).
Gene expression analysis
Extraction and reverse transcription of total RNA were performed according to the method of Dong et al. (2023). Gene expression analysis was performed on a StepOnePlus™ real-time PCR instrument (BIO-RAD, Hercules, California, USA) using SYBR Green I Master Mix (Vazyme, Nanjing, Jiangsu, China) and specific primers (Supplementary Table 1). Gene expression was normalized to AeACT and calculated using the 2-ΔCt method.
Statistical analysis
GraphPad Prism 9 was used to analyze the experimental data. Differences between control and treatment were tested using multiple t tests (* P< 0.05, ** P< 0.01, and *** P< 0.001).
Results
Effects of hydrogen-rich water treatment on shelf life of postharvest okra
The controlled okras slowly became shrivelled and tarnished during storage, together with the gradual increase in senescence index and ΔE values. However, HRW treatment delayed okra senescence and maintained fruit quality during storage (Figure 1).
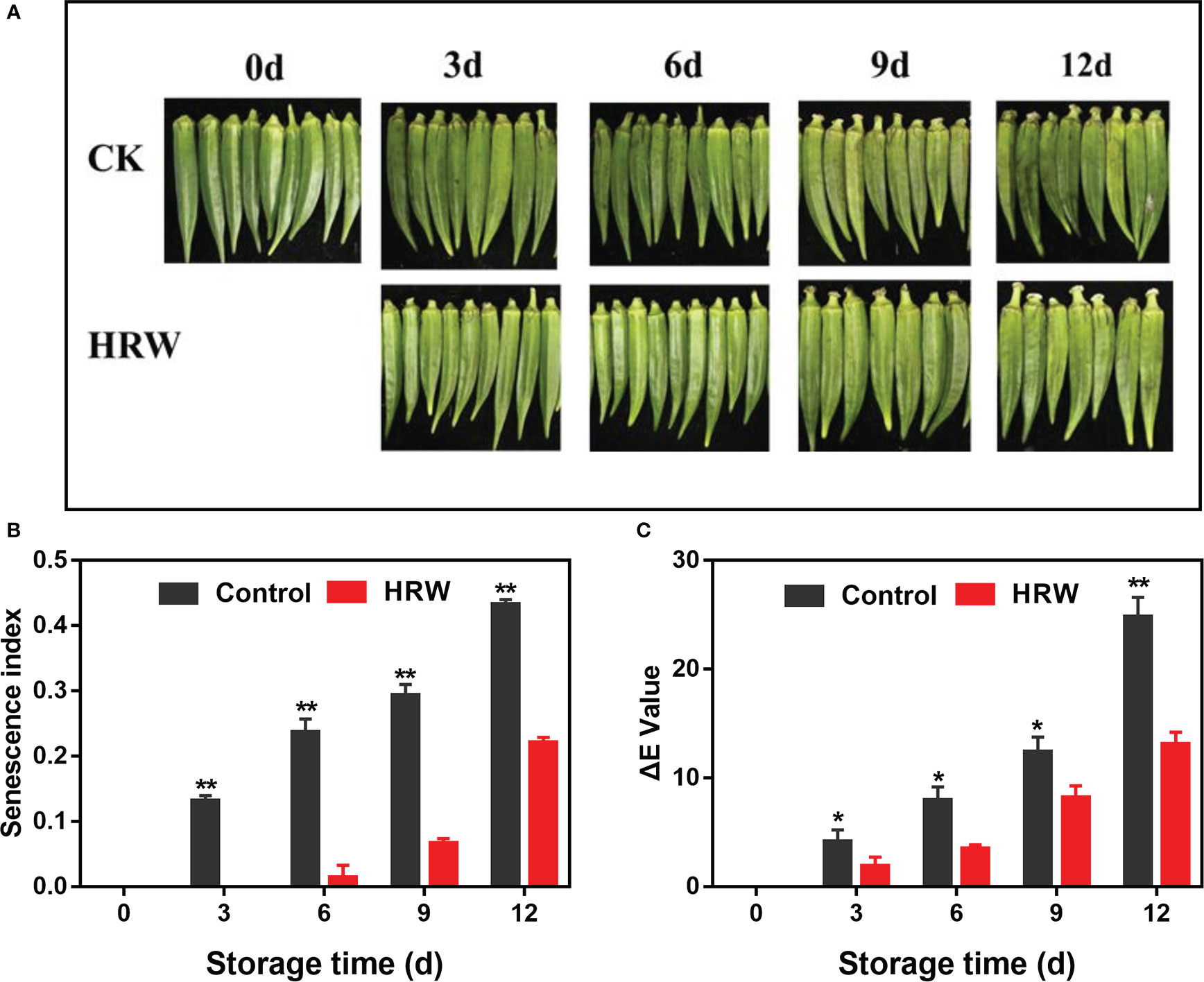
Figure 1 Hydrogen-rich water treatment affected appearance (A), senescence index (B) and ΔE value (C) of okras stored at 25°C. Asterisks indicate significant differences between Control and HRW treatment (*p < 0.05, **p < 0.01, and ***p < 0.001).
Effects of HRW treatment on melatonin content and expression of biosynthesis-related genes in okra during storage
The melatonin contents in both HRW- and non-treated okras increased firstly followed by a decline thereafter. HRW treatment increased its content in okra throughout the storage (Figure 2A). At the end of storage, the content was 14.2% higher than the untreated okras. Correspondingly, all of the melatonin biosynthetic genes investigated here exhibited an increasing trend firstly and then declined during the remaining storage time. HRW treatment increased the expressions of AeTDC and AeSNAT during the whole storage (Figures 2B, C). Higher transcripts of AeCOMT1/2 were observed in treated okras on day 3, 6 and 9 (Figures 2D, E). Meanwhile, the treatment upregulated the expression of AeT5H1/2 after 6 d and AeT5H3 on day 3, 6 and 9 of storage (Figures 2F–H).
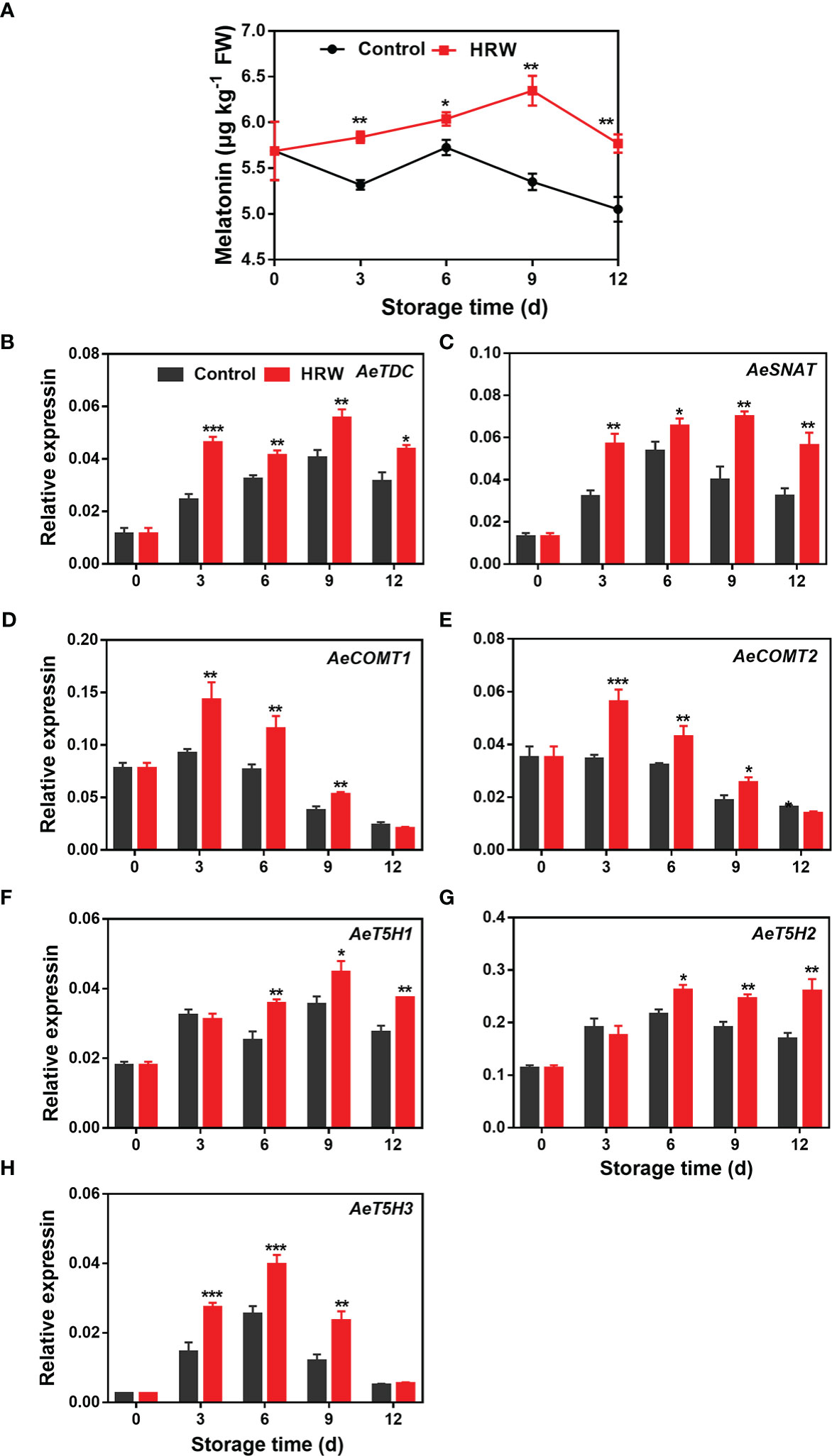
Figure 2 Hydrogen-rich water treatment affected melatonin content (A) and gene expression of AeTDC (B), AeSNAT (C), AeCOMT1 (D), AeCOMT2 (E), AeT5H1 (F), AeT5H2 (G), AeT5H3 (H) of okras stored at 25°C. Asterisks indicate significant differences between Control and HRW treatment (*p < 0.05, **p < 0.01, and ***p < 0.001).
Effects of HRW treatment on gibberellin content and expression of gibberellin metabolizing genes in okra during storage
As okras were stored for the first six days, the GA contents increased, followed by a decline thereafter. Higher content of GA was observed in okras after HRW treatment throughout the whole storage (Figure 3A). As compared to the non-treated okras, higher transcripts of AeKAO and AeKO were observed on day 3, 9 and 12 of storage and AeGA20OX on day 6 and 12 in fruit with HRW treatment (Figures 3B–D). The expression levels of AeGA20X1 and AeGA20X2 declined firstly in both control and treated okras. Their expression was downregulated throughout the storage by the treatment (Figures 3E, F). Meanwhile, AeDELLA expression was also down-regulated by HRW on day 3, 6, and 9 (Figure 3G).

Figure 3 Hydrogen-rich water treatment affected gibberellin content (A) and gene expression of AeKAO (B), AeGA20OX (C), AeKO (D), AeGA20X2 (E), AeGA20X1 (F), AeDELLA (G) of okras stored at 25°C. Asterisks indicate significant differences between Control and HRW treatment (*p < 0.05, **p < 0.01, and ***p < 0.001).
Effects of HRW treatment on indolacetic acid content and expression of indolacetic acid metabolizing genes in okra during storage
After HRW treatment, IAA content in okras increased rapidly during the first 6 d of storage. However, the level of IAA in control fruit declined firstly then increased slightly afterwards. IAA content in the treated-okras was higher than that in the controls (Figure 4A). In the first 3 d of storage, AeYUC6 transcript abundance decreased drastically and then continued to increase. HRW treatment increased its expression during storage (Figure 4B). In control okras, AeYUC10 and AeTAR expression initially increased, then declined. Compared to the untreated- okras, fruit treated with HRW had higher levels of AeYUC10 on day 3, 6 and 9 (Figure 4C). The treatment also upregulated AeTAR expression on day 3, 9 and 12 (Figure 4D). No significant difference was found in the abundance of AeMES transcripts between the control and treated okras during first 6 d of storage, but it was increased by the treatment after that (Figure 4E). For the degradative gene AeDAO, HRW down-regulated its expression within the storage (Figure 4F). AeSAUR71’s expression in both control and treated okras initially increased, then declined afterwards. Higher expression of AeSAUR71 was observed in HRW-treated okras during storage when compared to the control (Figure 4G).
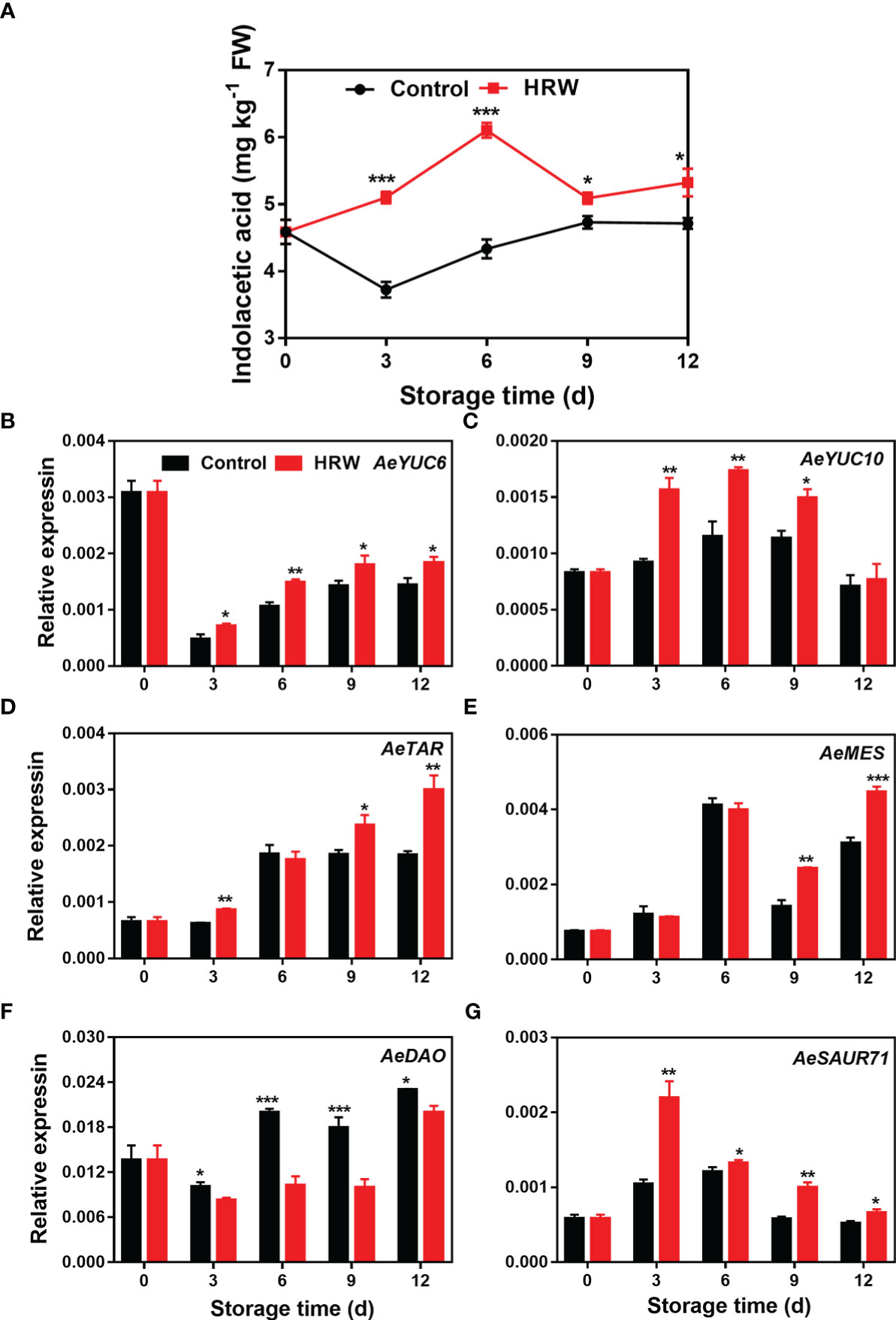
Figure 4 Hydrogen-rich water treatment affected indolacetic acid content (A) and gene expression of AeYUC6 (B), AeYUC10 (C), AeTAR (D), AeMES (E), AeDAO (F), AeSAUR71 (G) of okras stored at 25°C. Asterisks indicate significant differences between Control and HRW treatment (*p < 0.05, **p < 0.01, and ***p < 0.001).
Effects of HRW treatment on abscisic acid content and expression of abscisic acid metabolizing genes in okra during storage
ABA content in both HRW- and non-treated okras increased during the first 9 d of storage and then declined towards the end. Lower ABA content was found in HRW-treated okras during the storage (Figure 5A). The expression of AeNCED and AeAAO declined dramatically during the first 3 d of storage, then the level was maintained at a low level. HRW treatment down-regulated these two genes within the storage (Figures 5B, C). As compared to the okras treated with HRW, higher transcripts of AeZEP and lower ABA degradative gene AeCYP707A could be observed in non-treated fruit (Figures 5D, E). In addition, HRW treatment also down-regulated the expression of AePLY3/9 on day 3, 6 and 9 (Figures 5F, G) but decreased the transcripts of AeABF during the whole storage (Figure 5H).
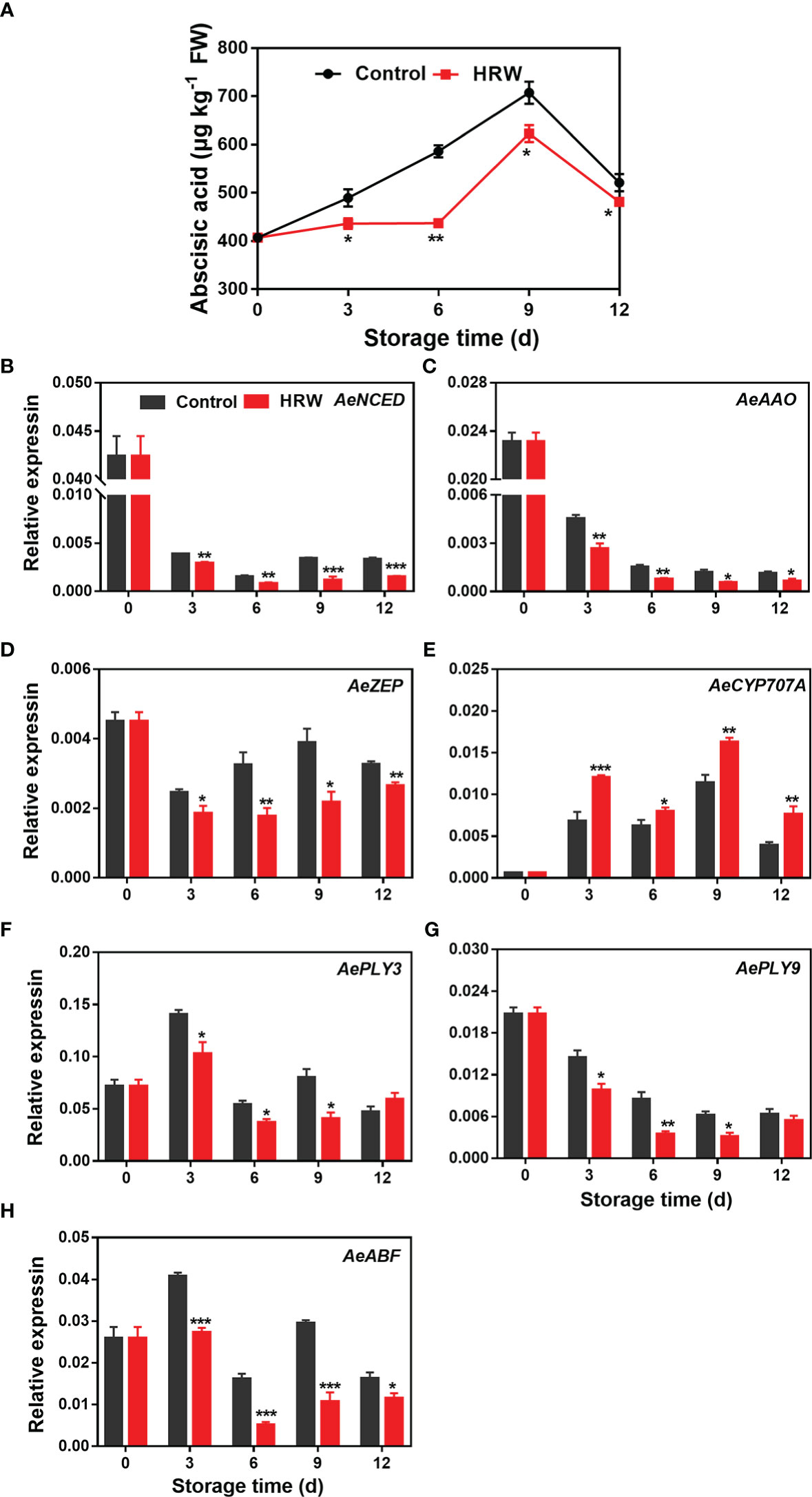
Figure 5 Hydrogen-rich water treatment affected abscisic acid content (A) and gene expression of AeNCED (B), AeAAO (C), AeZEP (D), AeCYP707A (E), AePLY3 (F), AePLY9 (G), AeABF (H) of okras stored at 25°C. Asterisks indicate significant differences between Control and HRW treatment (*p < 0.05, **p < 0.01, and ***p < 0.001).
Effects of HRW treatment on γ-aminobutyric acid content and expression of γ-aminobutyric acid biosynthesis-related genes in okra during storage
A slight increase of GABA content in both control and the treated okras was observed during storage, there was no significant difference in its content between the non- and treated okras (Figure 6A). The expression of AeALDH1/2 and AePAO1/2 was observed a significant decline during the first three days of storage, then decreased and remained stable till the end of storage. The expression level of AeGAD1 and AeGAD2 in the two groups experienced a similar change during storage. No significant difference in the transcripts of all these six GABA biosynthetic genes was observed between the non- and treated okras (Figures 6B–G).

Figure 6 Hydrogen-rich water treatment affected γ-aminobutyric acid content (A) and gene expression of AeALDH1 (B), AeALDH2 (C), AePAO1 (D), AePAO2 (E), AeGAD1 (F), AeGAD2 (G), of okras stored at 25°C.
Discussion
As an important bio-regulator in plants, hydrogen has critical roles in diverse processes in plant growth, development and stress responses (Ren et al., 2017a; Yu et al., 2023). Recently, attention to the role of hydrogen has shifted from plant to postharvest vegetables and fruit. For example, HRW treatment was found to improve antioxidant capacity and extend the shelf life of pakchoi (An et al., 2021). Additionally, kiwifruit and mushrooms treated with HRW showed higher free radical scavenging activity and lower rot rate as compared to the non-treated groups (Chen et al., 2017; Hu et al., 2018). In our previous study, we observed that hydrogen-rich water treatment delayed the softening process and prolonged the storage time by modifying the cell wall metabolism and cell wall composition in postharvest okras (Dong et al., 2023). Similarly, here we demonstrated again that HRW treatment could prolong the shelf life of postharvest okras.
In the last few years, a growing number of studies have revealed how hydrogen interacts with other plant hormone signalling pathways, indicating the key role it plays in protecting plants from stress (Zeng, 2013). H2 enhanced the drought resistance of tomato seedlings by regulating the expression of genes in ABA biosynthesis and signal transduction (Yan et al., 2022); H2 was also reported to be involved in auxin-induced lateral root formation (Cao et al., 2017). Melatonin, a well-known animal hormone that the brain produces in response to darkness, was first discovered in plants in 1995 (Dubbels et al., 1995), and since then its role in plants has been discovered and studied widely. Accumulating documents over the past decades demonstrated that melatonin could fortify plants against senescence and biotic stresses such as salt, chilling, drought and heavy metals (Cai et al., 2017; Wang et al., 2017; Yan et al., 2019). In addition to inducing stress resistance in plants, much progress has been made recently in understanding the roles of melatonin in regulating storage time in postharvest fruit. Melatonin application enhanced antioxidant defense and increased tolerance to chilling in cold-stored peaches (Cao et al., 2018). The postharvest treatment with melatonin inhibited decay incidence and delayed in strawberries (Liu et al., 2018). Through our experiments, we found that the melatonin content and its biosynthetic genes such as AeTDC, AeT5H, AeSNAT and AeCOMT were upregulated in okras after HRW treatment, indicating the increased melatonin biosynthesis in postharvest okras treated with HRW contribution to the delay of senescence and prolongation of shelf life. Furthermore, Su et al. (2021) reported that melatonin acted downstream of molecular hydrogen and its combination with molecular hydrogen induced salt tolerance in Arabidopsis. Therefore, it is reasonable to speculate that the crosstalk between molecular hydrogen and melatonin also plays a role in delaying senescence in postharvest fruit and vegetable.
Lately, Wu et al. (2020) revealed that HRW treatment enhanced the growth of mung bean seedlings by stimulating elongation of hypocotyl and root cells via increasing GA and IAA content, suggesting that hydrogen acted upstream of IAA and GA biosynthesis during plant growth. Meanwhile, GA and IAA have been demonstrated their roles in inhibiting the senescence process in postharvest okras and strawberries, respectively (Chen et al., 2014; Xiao et al., 2022). Therefore, to further reveal the crosstalk between hydrogen and GA or IAA, the biosynthetic and signalling pathways of these two phytohormones were investigated in okras after HRW treatment. In our present study, HRW upregulated the anabolic genes such as AeGA20OX, AeKO and AeKAO, but downregulated the catabolic gene AeGA20Xs and AeDELLA the negative regulator of gibberellin signalling, thereby increasing GA content and signalling in postharvest okras. In respect of IAA biosynthesis, the treatment also increased the IAA content along with the enhanced transcripts of biosynthetic genes including AeTAR, AeYUC and AeMES and the early auxin-responsive gene AeSAUR, however, the lower expression of the degradative gene AeDAO was observed in the treated okras. Moro et al. (2017) found that postharvest exogenous IAA treatment postponed fruit ripening by affecting anthocyanin biosynthesis in red raspberries. It has also been reported that the application of exogenous GA inhibited fruit softening and delayed the loss of ascorbic acid and soluble reducing sugar, thereby prolonging the shelf life of banana fruit (Huang et al., 2014). Recently, in carambola fruit, exogenous 2,4-epibrassinolide treatment maintained fruit quality and inhibited fruit ripening due to the increase in levels of IAA and GA in carambola fruit (Zhu et al., 2021). Therefore, taken together, we could conclude that HRW treatment delayed senescence and extended the shelf life of postharvest okras also due to the increase of levels of GA and IAA.
ABA, another plant hormone, regulates numerous aspects of fruit ripening, senescence and stress responses. It not only enhanced the ripening process of climacteric fruit like kiwifruit and mango (Zaharah et al., 2013; Gan et al., 2020), but also promoted the senescence of non-climacteric fruit such as sweet cherries (Luo et al., 2014). Previous studies have showed that the application of alginate oligosaccharide inhibited the accumulation of ABA and reduced the degradation of cell wall components, thereby maintaining fruit quality in strawberries (Bose et al., 2019). Gan et al. (2020) found silencing AeNCED1 the key gene in ABA biosynthesis in kiwifruit could decline ABA content and postpone fruit softening. The inhibition of fruit ripening in carambola fruit treated with 2,4-epibrassinolide was also related to the decrease in ABA content (Zhu et al., 2021). Our experimental results showed that HRW treatment reduced the expression of genes related to ABA synthesis such as AeNCED, AeZEP, and AeAAO but increased the transcripts of degradative gene AeCYP707A, thus finally reducing ABA content, which was associated with the inhibition of senescence in okras with HRW treatment. It has been reported that both PLYs and ABFs play important roles in regulating Arabidopsis leaf senescence triggered by ABA (Gao et al., 2016; Zhao et al., 2016). In our present study, corresponding to the ABA level, AePLY3/9 and AeABF were also downregulate by HRW treatment, suggesting that hydrogen could regulate ABA-associated signals to slow down the senescence process in postharvest okras.
The role of GABA in fruit ripening, senescence and tolerance against stresses was well reported in different kinds of postharvest fruit and vegetables (Sheng et al., 2017; Palma et al., 2019; Asgarian et al., 2022). However, in our present study, there was no difference in GABA content together with the expression of its metabolizing genes between the control and HRW treated okras, suggesting that no interaction between H2 and GABA existed in postharvest okras.
Conclusion
In conclusion, our data demonstrated that HRW treatment could regulate the contents of several phytohormones and consequently prolong shelf life in postharvest okras. Our results suggested that melatonin, IAA and GA were negatively while ABA was positively associated with fruit senescence in postharvest okras. The increased shelf life and delaying okra senescence by HRW treatment was due to its capability to increase melatonin, IAA and GA contents and inhibit ABA level resulted from its regulation of their metabolizing genes, indicating H2, as a signaling molecule, could interrelate to these hormones and influenced their biosynthesis and signaling and finally co-adjusted okra senescence after harvest. However, more evidence is needed to determine the underline cross-talk regulation and transduction relationship between H2 and other phytohormones for further exploration on the role of H2 in the shelf life prolongation of postharvest products.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WD: Perform the experiment, write the original manuscript. SC and QZ: Conceived and designed the experiment. SJ, CZ and QL: Formal analysis, Investigation. XL and WC: Conducted the experiments, analyzed the data. LS and ZY: Funding acquisition, supervision and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Key Research and Development Project of Zhejiang Province of China (2019C02079), the National Natural Science Foundation of China (32172646) and the Key Projects of Natural Science Foundation of Zhejiang Province (LZ21C200002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1108515/full#supplementary-material
References
An, R. H., Luo, S. F., Zhou, H. S., Zhang, Y. T., Zhang, L. G., Hu, H. L., et al. (2021). Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. chinensis). Scientia Hortic. 289, 110469. doi: 10.1016/j.scienta.2021.110469
Asgarian, Z. S., Karimi, R., Ghabooli, M., Maleki, M. (2022). Biochemical changes and quality characterization of cold-stored ‘Sahebi’ grape in response to postharvest application of GABA. Food Chem. 373 (A), 131401. doi: 10.1016/j.foodchem.2021.131401
Bose, S. K., Howlader, P., Jia, X. C., Wang, W. X., Heng, Y. (2019). Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via abscisic acid signaling in strawberry. Food Chem. 283, 665–674. doi: 10.1016/j.foodchem.2019.01.060
Cai, S. Y., Zhang, Y., Xu, Y. P., Qi, Z. Y., Li, M. Q., Ahammed, G. J. (2017). Hsfa1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 62 (2), e12387. doi: 10.1111/jpi.12387
Cao, Z. Y., Duan, X. L., Yao, P., Cui, W. T., Cheng, D., Zhang, J., et al. (2017). Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int. J. Mol. Sci. 18 (10), 2084. doi: 10.3390/ijms18102084
Cao, S. F., Shao, J. R., Shi, L. Y., Xu, L. W., Shen, Z. M., Chen, W., et al. (2018). Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 8 (1), 806. doi: 10.1038/s41598-018-19363-5
Carvajal, F., Palma, F., Jimenez-Munoz, R., Jamilena, M., Pulido, A., Garrido, D. (2017). Unravelling the role of abscisic acid in chilling tolerance of zucchini during postharvest cold storage. Postharvest Biol. Technol. 133, 26–35. doi: 10.1016/j.postharvbio.2017.07.004
Chen, J. X., Mao, L. C., Mi, H. B., Zhao, Y. Y., Ying, T. J., Luo, Z. S. (2014). Detachment-accelerated ripening and senescence of strawberry (Fragaria x ananassa duch. cv. akihime) fruit and the regulation role of multiple phytohormones. Acta Physiologiae Plantarum 36 (9), 2441–2451. doi: 10.1007/s11738-014-1617-6
Chen, H., Zhang, J. J., Hao, H. B., Feng, Z. Y., Chen, M. J., Wang, H., et al. (2017). Hydrogen-rich water increases postharvest quality by enhancing antioxidant capacity in hypsizygus marmoreus. Amb Express 7, 221. doi: 10.1186/s13568-017-0496-9
Dong, W., Shi, L., Li, S., Xu, F., Yang, Z., Cao, S. (2023). Hydrogen-rich water delays fruit softening and prolongs shelf life of postharvest okras. Food Chem. 399, 133997. doi: 10.1016/j.foodchem.2022.133997
Dong, B. Y., Zhu, D. Q., Yao, Q. P., Tang, H. M., Ding, X. C. (2022). Hydrogen-rich water treatment maintains the quality of Rosa sterilis fruit by regulating antioxidant capacity and energy metabolism. Lwt-Food Sci. Technol. 161, 113361. doi: 10.1016/j.lwt.2022.113361
Dubbels, R., Reiter, R. J., Klenke, E., Goebel, A., Schnakenberg, E., Ehlers, C., et al. (1995). Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 18 (1), 28–31. doi: 10.1111/j.1600-079X.1995.tb00136.x
Fan, Z. Q., Wei, W., Tan, X. L., Shan, W., Kuang, J. F., Lu, W. J., et al. (2021). A NAC transcription factor BrNAC087 is involved in gibberellin-delayed leaf senescence in Chinese flowering cabbage. Postharvest Biol. Technol. 181, 111673. doi: 10.1016/j.postharvbio.2021.111673
Fawad, A., Nadeem, M. A., Habyarimana, E., Altaf, M. T., Barut, M., Kurt, C., et al. (2021). Identification of genetic basis associated with agronomic traits in a global safflower panel using genome-wide association study. Turk. J. Agric. For 45 (6), 834–849. doi: 10.3906/tar-2105-55
Gajanan, G., Gaur, R. S., Jyoti, S. D., Dinesh, S. (2018). Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packaging Shelf Life 18, 1–12. doi: 10.1016/j.fpsl.2018.08.002
Gan, Z. Y., Shan, N., Fei, L. Y., Wan, C. P., Chen, J. Y. (2020). Isolation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene from kiwifruit and its effects on postharvest softening and ripening. Scientia Hortic. 261, 109020. doi: 10.1016/j.scienta.2019.109020
Gan, Z. Y., Yuan, X., Shan, N., Wan, C. P., Chen, C. Y., Zhu, L. Q., et al. (2021). AcERF1B and AcERF073 positively regulate indole-3-acetic acid degradation by activating AcGH3.1 transcription during postharvest kiwifruit ripening. J. Agric. Food Chem. 69 (46), 13859–13870. doi: 10.1021/acs.jafc.1c03954
Gao, S., Gao, J., Zhu, X. Y., Song, Y., Li, Z. P., Ren, G. D., et al. (2016). ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in arabidopsis. Mol. Plant 9 (9), 1272–1285. doi: 10.1016/j.molp.2016.06.006
Ghosh, S. P. (2012). Carrying capacity of Indian horticultureTI carrying capacity of Indian horticulture. Curr. Sci. 102 (6), 889–893.
Hu, H. L., Zhao, S. P., Li, P. X., Shen, W. B. (2018). Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 135, 123–130. doi: 10.1016/j.postharvbio.2017.09.008
Huang, H., Jing, G., Wang, H., Duan, X., Qu, H., Jiang, Y. (2014). The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Scientia Hortic. 167, 36–42. doi: 10.1016/j.scienta.2013.12.028
Iriti, M., Rossoni, M., Faoro, F. (2006). Melatonin content in grape: myth or panacea? J. Sci. Food Agric. 86, 1432–1438. doi: 10.1002/jsfa.2537
Liu, C. H., Zheng, H. H., Sheng, K. L., Liu, W., Zheng, L. (2018). Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 139, 47–55. doi: 10.1016/j.postharvbio.2018.01.016
Luo, H., Dai, S. J., Ren, J., Zhang, C. X., Ding, Y., Li, Z., et al. (2014). The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant Growth Regul. 33 (2), 373–383. doi: 10.1007/s00344-013-9388-7
Maurer, D., Feygenberg, O., Tzoor, A., Atzmon, G., Glidai, S., Prusky, D. (2019). Postharvest dips of persimmon fruit in gibberellic acid: An efficient treatment to improve storability and reduce alternaria black spot. Horticulturae 5 (1), 23. doi: 10.3390/horticulturae5010023
McAtee, P., Karim, S., Schaffer, R., David, K. (2013). A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 4 (79). doi: 10.3389/fpls.2013.00079
Moro, L., Hassimotto, N. M. A., Purgatto, E. (2017). Postharvest auxin and methyl jasmonate effect on anthocyanin biosynthesis in red raspberry (Rubus idaeus l.). J. Plant Growth Regul. 36 (3), 773–782. doi: 10.1007/s00344-017-9682-x
Palma, F., Carvajal, F., Jimenez-Munoz, R., Pulido, A., Jamilena, M., Garrido, D. (2019). Exogenous gamma-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 136, 188–195. doi: 10.1016/j.plaphy.2019.01.023
Petropoulos, S., Fernandes, A., Barros, L., Ferreira, I. (2018). Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 242 (MAR.1), 466–474. doi: 10.1016/j.foodchem.2017.09.082
Poveda, J. (2020). Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiol. Mol. Plant Pathol. 111, 101521. doi: 10.1016/j.pmpp.2020.101521
Reiter, R. J., Tan, D. X., Burkhardt, S., Manchester, L. C. (2001). Melatonin in plants. Nutr. Rev. 59 (9), 286–290. doi: 10.1111/j.1753-4887.2001.tb07018.x
Ren, A., Liu, R., Miao, Z. G., Zhang, X., Cao, P. F., Chen, T. X., et al. (2017a). Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in ganoderma lucidum. Environ. Microbiol. 19 (2), 566–583. doi: 10.1111/1462-2920.13498
Sheng, L., Shen, D. D., Luo, Y., Sun, X. H., Wang, J. Q., Luo, T., et al. (2017). Exogenous gamma-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 216, 138–145. doi: 10.1016/j.foodchem.2016.08.024
Su, J. C., Yang, X. H., Shao, Y. D., Chen, Z. P., Shen, W. B. (2021). Molecular hydrogen-induced salinity tolerance requires melatonin signalling in arabidopsis thaliana. Plant Cell Environ. 44, 476–490. doi: 10.1111/pce.13926
Sun, Y. D., Guo, D. L., Yang, S. D., Zhang, H. C., Wang, L. L., Min, L., et al. (2020). Melatonin treatment improves the shelf-life and postharvest quality of table grape (Vitis labrusca l. cv. 'Fengzao'). J. Berry Res. 10 (4), 665–676. doi: 10.3233/JBR-200569
Taskesenlioglu, M. Y., Ercisli, S., Kupe, M. (2022). History of grape in Anatolia and historical sustainable grape production in erzincan agroecological conditions in Turkey. Sustainability 14 (3), 1496. doi: 10.3390/su14031496
Trainotti, L., Tadiello, A., Casadoro, G. (2007). The involvement of auxin in the ripening of climacteric fruit comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 58 (12), 3299–3308. doi: 10.1093/jxb/erm178
Wang, L., Feng, C., Zheng, X. D., Guo, Y., Zhou, F. F., Shan, D. Q., et al. (2017). Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63 (3), e12429. doi: 10.1111/jpi.12429
Wu, Q., Su, N., Huang, X., Ling, X., Shabala, S. (2020). Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct. Plant Biol. 47 (9), 771–778. doi: 10.1071/FP19107
Wu, X., Zhu, Z. B., Chen, J. H., Huang, Y. F., Liu, Z. L., Zou, J. W., et al. (2019). Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis l.) by exogenous hydrogen-rich water. Chemosphere 216, 684–697. doi: 10.1016/j.chemosphere.2018.10.152
Xiao, X., Yang, M. J., Dong, W. Q., Zhou, C. J., Shi, L. Y., Chen, W., et al. (2022). Gibberellic acid inhibited chlorophyll degradation in post-harvest okras. Postharvest Biol. Technol. 190, 111951. doi: 10.1016/j.postharvbio.2022.111951
Yan, Y., Jing, X., Tang, H., Li, X., Gong, B., Shi, Q. (2019). Using transcriptome to discover a novel melatonin-induced sodic alkaline stress resistant pathway in Solanum lycopersicum l. Plant Cell Physiol. 60 (9), 2051–2064. doi: 10.1093/pcp/pcz126
Yan, M., Yao, Y. D., Mou, K. P., Dan, Y. Y., Li, W. T., Wang, C. L., et al. (2022). The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Scientia Hortic. 292, 110631. doi: 10.1016/j.scienta.2021.110631
Yu, Y., Zhang, H. N., Xing, H. Y., Cui, N., Liu, X. Y., Meng, X. N., et al. (2023). Regulation of growth and salt resistance in cucumber seedlings by hydrogen-rich Water. J Plant Growth Regul. 42, 134–153. doi: 10.1007/s00344-021-10536-7
Yue, P. T., Wang, Y. N., Bu, H. D., Li, X. Y., Yuan, H., Wang, A. D. (2019). Ethylene promotes IAA reduction through PuERFs-activated PuGH3.1 during fruit ripening in pear (Pyrus ussuriensis). Postharvest Biol. Technol. 157, 110955. doi: 10.1016/j.postharvbio.2019.110955
Zaharah, S. S., Singh, Z., Symons, G. M., Reid, J. B. (2013). Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol. Technol. 75, 37–44. doi: 10.1016/j.postharvbio.2012.07.009
Zeng, J. (2013). Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8 (8), e71038. doi: 10.1371/journal.pone.0071038
Zhao, Y., Chan, Z. L., Gao, J. H., Xing, L., Cao, M. J., Yu, C. M., et al. (2016). ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. United States America 113 (7), 1949–1954. doi: 10.1073/pnas.1522840113
Zhu, X. Y., Chen, Y. X., Li, J. Y., Ding, X. C., Xiao, S. L., Fan, S. L., et al. (2021). Exogenous 2, 4-epibrassinolide treatment maintains the quality of carambola fruit associated with enhanced antioxidant capacity and alternative respiratory metabolism. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.678295
Keywords: okra, hydrogen-rich water, melatonin, indoleacetic acid, gibberellin, abscisic acid
Citation: Dong W, Cao S, Zhou Q, Jin S, Zhou C, Liu Q, Li X, Chen W, Yang Z and Shi L (2023) Hydrogen-rich water treatment increased several phytohormones and prolonged the shelf life in postharvest okras. Front. Plant Sci. 14:1108515. doi: 10.3389/fpls.2023.1108515
Received: 26 November 2022; Accepted: 30 January 2023;
Published: 14 February 2023.
Edited by:
Viktorija Vastakaite-Kairiene, Vytautas Magnus University Academy of Agriculture, LithuaniaCopyright © 2023 Dong, Cao, Zhou, Jin, Zhou, Liu, Li, Chen, Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyu Shi, c2hpbGl5dUB6d3UuZWR1LmNu
†These authors have contributed equally to this work
 Wanqi Dong
Wanqi Dong Shifeng Cao
Shifeng Cao Qihang Zhou
Qihang Zhou Chujiang Zhou
Chujiang Zhou Xu Li
Xu Li Zhenfeng Yang
Zhenfeng Yang Liyu Shi
Liyu Shi