- 1Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 2Biology Department, College of Science, Jouf University, Sakaka, Saudi Arabia
- 3Biological Sciences Department, Faculty of Science, University of Jeddah, Jeddah, Saudi Arabia
- 4Department of Biology, College of Science, Imam Abdulrahman Bin Fasial University, Dammam, Saudi Arabia
- 5Department of Biology, Faculty of Science, Tabuk University, Tabuk, Saudi Arabia
- 6Department of Biology, College of Science and Humanities in Al-Kharj, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 7Department of Botany and Microbiology, Faculty of Science, Cairo University, Giza, Egypt
- 8Department of Biology, Faculty of Science, Taibah University, Yanbu, Saudi Arabia
- 9Department of Biology, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
- 10Institute of Life Sciences, Faculty of Food Science and Technology, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania
- 11Department of Food Engineering, Faculty of Food Science and Technology, University of Agricultural Science and Veterinary Medicine Cluj-Napoca, Cluj-Napoca, Romania
The present study was conducted to examine the potential role of Plantago ovata Forsk leaf extract (POLE) which was applied at various concentration levels (control, hydropriming, 10, 20, 30, and 40% POLE) to the wheat (Triticum aestivum L.) seedlings. Drought stressed was applied at 60% osmotic potential (OM) to the T. aestivum seedlings to study various parameters such as growth and biomass, photosynthetic pigments and gas exchange characteristics, oxidative stress and response of various antioxidants and nutritional status of the plants. Various growth parameters such as gaseous exchange attributes, antioxidants and nutritional status of T. aestivum were investigated in this study. It was evident that drought-stressed condition had induced a negative impact on plant growth, photosynthetic pigment, gaseous exchange attributes, stomatal properties, and ion uptake by different organs (roots and shoots) of T. aestivum. The decrease in plant growth resulted from oxidative stress and overcome by the antioxidant (enzymatic and non-enzymatic) compounds, since their concentration increased in response to dehydration. Seed priming with POLE positively increased plant growth and photosynthesis, by decreasing oxidative stress indicators and increasing activities of antioxidant (enzymatic and non-enzymatic) compounds, compared to the plants which were grown without the application of POLE. Our results also depicted that optimum concentration of POLE for T. aestivum seedlings under drought condition was 20%, while further increase in POLE (30 and 40%) induced a non-significant (P < 0.05) effect on growth (shoot and root length) and biomass (fresh and dry weight) of T. aestivum seedling. Here we concluded that the understanding of the role of seed priming with POLE in the increment of growth profile, photosynthetic measurements and nutritional status introduces new possibilities for their effective use in drought-stressed condition and provides a promising strategy for T. aestivum tolerance against drought-stressed condition.
Introduction
Clean sufficient water supplies are vital for all communities, industries, and ecosystems for drinking, farming, sanitation, and energy production. Yet, the world’s water systems face formidable threats. UNESCO (World Water Assessment Programme, 2012) estimates that around 700 million people in 43 countries suffer from water scarcity and predict that “by 2025, 1.8 billion people will be living in countries or regions with absolute water scarcity, and two-thirds of the world’s population could be living under water stressed conditions.” Pakistan is already experiencing a shortage in freshwater resources. In the wake of growing population, per capita water availability declined from 5,600 m3 in 1951 to 1,200 m3 in 2003, and is reaching close to the water scarcity threshold level of 1,000 m3 (Waraich et al., 2011; Aslam et al., 2013). A proper amount of soil moisture is compulsory for crop growth, transpiration, and also for transportation of food prepared in leaves to sink and in drought condition, crop growth, and yield decreases (Hasanuzzaman et al., 2018; Rana et al., 2020; Saleem et al., 2020b,d). Drought conditions can lead to a number of changes in plants, including reduced growth, lower fresh and dry biomass, lower rates of photosynthesis, and reduced absorption of essential nutrients from the soil (Ahmad et al., 2017; Akram et al., 2018; Khan et al., 2019a; Dola et al., 2022; Farooq et al., 2022; Faryal et al., 2022). Reductions in chlorophyll content and oxidation of membrane lipids and proteins are the crucial reasons for plant damage caused by the production of drought-induced highly reactive oxygen species (ROS), leading to the modification of the cellular redox status (Alam et al., 2020; Saleem et al., 2020c, 2022; Yaseen et al., 2020; Afridi et al., 2022; Wahab et al., 2022). However, the negative impacts of drought depend on the total length of the plant growth cycle, the degree of drought stress and frequency of drought conditions, growth conditions, and plant species (Wu et al., 2018; Goharrizi et al., 2020; Sakya and Prahasto, 2020). Under environmental stress, imbalance in ROS accumulation and generation can occur, which results in the formation of hydrogen peroxide (H2O2), superoxide radicles (O–2), and hydroxide ions (OH), all of which are known as stress indicators at cellular levels (Imran et al., 2020a,b; Ma et al., 2022a, b). These ROS are toxic to plants and scavenged by various types of antioxidants like SOD (superoxide dismutase), POD (peroxidase), catalase (CAT), and ascorbate peroxidase (APX), etc. to maintain cellular homeostasis (Imran et al., 2019; Rehman et al., 2019; Saleem et al., 2020a,e,g). Previously, many researchers concluded that varieties of antioxidants have increased their activities under drought environments in Brassica napus L. (Khan et al., 2019b), Triticum aestivum (Nikolaeva et al., 2010), and Zea mays L. (Anjum et al., 2017). Additionally, drought conditions reduce the photosynthetic rates, expansion of leaves, increase stomatal closure, the levels of ROS and early leaf catabiotic, and decrease the translocation within the plant, resulting in overall decreased crop yield (Ahmad et al., 2017; Anjum et al., 2017). Adaptive agricultural strategies are urgently needed in these changing environments (Singh et al., 2022). Because different organs show various levels of sensitivity to water stress, a whole-plant approach is required in research rather than focusing only on single components (Liang et al., 2018).
Wheat (Triticum aestivum L.) is grown in nearly all parts of the world (Ahmad et al., 2022; Khan et al., 2023). Pakistan stands fourth in Asia and eleventh in the world as far as wheat production are concerned (Mahmood et al., 2020; Saeed et al., 2022) and in Pakistan, the worst threat for the production of wheat is drought (Ahmad et al., 2017). In addition, wheat is a main grain cereal widely consumed worldwide and is a staple food for more than 50% of world population (Azooz et al., 2012; Amna Ali et al., 2021; Adnan et al., 2022). Physiological responses in wheat include closure of stomata, decrease in the activity of photosynthesis, development of oxidative stress, alteration in the integrity of cell wall, production of metabolites which are toxic and cause plants’ death, signal recognition of roots, turgor loss and adjustment of osmosis, reduction in water potential of leaf, decrease in stomata conductance to CO2, reduction of internal CO2 concentration, and reduction of growth rates (Nezhadahmadi et al., 2013; Mehmood et al., 2021; Zainab et al., 2021; Saleem et al., 2022). According to researchers, there is a relationship between different physiological responses of wheat and their resistance functions under drought such as high amount of relative water and potential water (Dimkpa et al., 2020; Yaseen et al., 2020). Cysteine is expressed in wheat leaf organs and its contribution in proteolysis activity rises under drought (Dehghani and Mostajeran, 2020). According to the study of Denčić et al. (2000), wheat is paid special attention due to its morphological traits during drought stress including leaf (shape, expansion, area, size, senescence, pubescence, waxiness, and cuticle tolerance) and root (dry weight, density, and length). Although due to the shortage of large genetic tolerant assets and the intricacy in physiological, biochemical and genetic attributes, the progress toward water resistance cultivars is considerably vulnerable. Therefore, identification of numerous genetic ways and also to recognize the strategies of water stress tolerance that might lead to high levels of drought resistance is more important.
Different approaches are being explored to resolve this issue and to improve the crop yields (Zaheer et al., 2020a,b). These approaches include the development of stress-tolerant genotypes (Singh et al., 2022), use of plant extract, or enhance stress tolerance by the exogenous applications of various organic compounds (Haider et al., 2022). The response of plants to water stress depends on the growth stage, duration, and species. The most sensitive stage of the plant life cycle is the reproductive stage that significantly influences the final productivity (Fahad et al., 2017). Accordingly the increase in the use of plant extracts depends or seed priming with various chemicals depends up on the concentration of the substance used and growth stage of plant. Seed priming is known as pre-sowing treatment that involves the exposure of seeds to lower external water potential (Khan et al., 2019b). Recent evidences showed that seed priming improves stress-tolerance throughout the subsequent plant growth and development stages. Although the germination process is not obvious during the process of seed priming, it is advised that priming initiates metabolic accomplishments required for embryonic growth and radical projection (Farooq et al., 2013; Hassini et al., 2017). As a consequence of priming, germination rates increase than emergence of uniform seedling compared with non-primed seeds (Jiang et al., 2016). Recently, Plantago ovata (also known as desert Indian wheat or ispaghula) leaf extract becomes interestingly popular in ameliorating abiotic stress in many crops (Asgharipour and Rafiei, 2010; Rahimi et al., 2010). Plantago ovata is an important medicinal plant which has different compounds such as phenolic compounds (caffeic acid derivatives), flavonoids, alkaloids, terpenoids, vitamin C, antioxidants, and anti-inflammatory agents (Saghir et al., 2008). A hypothesis made that POLE extract as an agent against abiotic stress may be studied in wheat as a drought-tolerant agent. Hence, we conducted the current study to determine (i) the impact of drought stress on seed germination, plant growth and biomass, photosynthetic pigment, gaseous exchange attributes, stomatal properties and nutritional status in wheat, when primed with P. ovata extract and (ii) various oxidative stress indicators and the response of antioxidant (enzymatic and non-enzymatic) compounds in the roots and leaves of wheat seedlings. According to best of our knowledge, this study is among the few studies which focus on the drought tolerance and resistance using POLE in wheat in order to investigate their suitability for water-deficient conditions. Findings from the present study will add to our understanding the mechanism of drought tolerance and resistance using POLE in T. aestivum seedlings.
Materials and methods
Experimental design and growth conditions
‘V12304’ wheat line was used for this experiment and this line is more tolerant to drought stress than other wheat lines and has been used in a number of previous studies conducted under drought stress conditions (Zulkiffal et al., 2018). Seeds were placed in Petri dishes, which were placed in growth chamber (100 W, Guangdong PHILIPS Co., Guangdong, China) with a day/night temperature of 25 ± 2°C and day/night humidity of 80%. Before starting a Petri dish experiment, the seeds were surface-sterilized with 0.1% HgCl2 for the prevention of surface fungal/bacterial contamination, followed by de-ionized water and for priming seeds that were soaked in the POLE solutions of varying concentrations (control, hydropriming, 10, 20, 30, and 40% POLE). Plantago ovata leaf was collected from the garden and the leaves of P. ovata were washed carefully with distilled water and then dried and crushed with the help of grinding machine. Then, the filtrate (as it is water soluble) was used for seed priming at various concentrations. After 12 h of seeds soaked in the various levels of POLE, the seeds were dried to attain their original moisture contents with the help of blotting papers. The effect of control and drought stressed environment in wheat grown under the seed priming with POLE is shown in Supplementary Figure 1. The Petri dishes used in this study for wheat lines have up and down (two) filter papers. Three replicates with 15 seedlings were used for each treatment and were arranged in completely randomized design (CRD). All seedlings were also divided into two subgroups: (I) control [100% OM (osmotic potential)] and (II) drought [60% OM [using polyethylene glycol (PEG-8000)]. The plant seedlings were subjected to PEG-8000 concentration along with control treatments for 24 h. About 10 mL of Hoagland’s (compositions are given in Supplementary Table 1) and PEG solution were given to each Petri dish, and water levels and other intercultural operations were monitored on daily basis. All seedlings were harvested after 21 days, from the beginning of the experiment for measuring different traits. All seedlings were harvested for measuring various morphological, physiological, and nutritional status from wheat seedlings.
Morphological traits and data collection
All seedlings were rooted-up in July 2019 to study different growth, germination, and other morphological and physiological parameters. Analysis of different biological parameters was performed in College of Agriculture and Food Sciences, King Faisal University, Al-Ahsa 31982, Saudi Arabia. The leaf in each treatment was picked at a rapid growth stage during 09:00–10:30 a.m. The sampled leaves were washed with distilled water, immediately placed in liquid nitrogen, and stored in a freezer at low temperature (−80°C) for further analysis. Germination index, time to 50% germination, co-efficient of uniformity of emergence, mean germination time, and germination energy (E) were measured by following the method presented by Coolbear et al. (1984), Wiesner (1990), Ruan et al. (2002), and Bewley and Black (2013). Germination percentage was calculated by the following formula:
Stomata were counted at random in 30 visual sections on the abaxial epidermis, and final tallies were used to calculate stomatal density. We used Image J software (Schneider et al., 2012) for measuring stomatal lengths, widths, and apertures.
Plants in each treatment were harvested and separated into roots and shoots for growth and morphology traits. Shoot length was defined as the length of the plant from the surface growth medium line of the Petri dish to the tip of the uppermost shoot and root length was also measured. Shoot fresh weight was measured by measuring the weight of shoots with the help of a digital weighing balance and root fresh weight was also measured. After that plant samples were oven-dried for 1 h at 105°C, then 65°C for 72 h until the weight become uniform, and dry biomass was recorded. Roots were washed with distilled water and dipped in 20 mM Na2EDTA for 15–20 min, washed thrice with distilled water and finally with de-ionized water, and then oven-dried for further analysis.
Determination of photosynthetic pigments and gas exchange parameters
For chlorophyll determination, 0.1 g of fresh leaf sample was extracted with 8 mL of 95% acetone for 24 h at 4°C in the dark. The absorbance was measured by a spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan) at 646.6, 663.6, and 450 nm. Chlorophyll content was calculated by the standard method of Arnon (1949) and Ali et al. (2022a,c).
Gas exchange parameters were also measured during the same day. Net photosynthesis (Pn), leaf stomatal conductance (Gs), transpiration rate (Ts), and intercellular carbon dioxide concentration (Ci) were measured from three different plants in each treatment group. Measurements were conducted between 11:30 and 13:30 on days with clear sky. Rates of leaf Pn, Gs, Ts, and Ci were measured with a LI-COR gas-exchange system (LI-6400; LI-COR Biosciences, Lincoln, NE, United States) with a red-blue LED light source on the leaf chamber. In the LI-COR cuvette, CO2 concentration was set as 380 mmol mol–1 and LED light intensity was set at 1,000 mmol m–2 s–1, which is the average saturation intensity for photosynthesis in T. aestivum (Austin, 1990).
Determination of oxidative stress indicators
The degree of lipid peroxidation was evaluated as malondialdehyde (MDA) contents. Briefly, 0.1 g of frozen leaves were ground at 4°C in a mortar with 25 mL of 50 mM phosphate buffer solution (pH 7.8) containing 1% polyethene pyrrole. The homogenate was centrifuged at 10,000 × g at 4°C for 15 min. The mixtures were heated at 100°C for 15–30 min and then quickly cooled in an ice bath. The absorbance of the supernatant was recorded using a spectrophotometer (xMark™ Microplate Absorbance Spectrophotometer; Bio-Rad, United States) at wavelengths of 532, 600, and 450 nm. Lipid peroxidation was expressed as l mol g–1 using the formula: 6.45 (A532-A600)-0.56 A450. Lipid peroxidation was measured using a method previously published by Heath and Packer (1968) and Nawaz et al. (2022).
To estimate H2O2 content of plant tissues (root and leaf), 3 mL of sample extract was mixed with 1 mL of 0.1% titanium sulfate in 20% (v/v) H2SO4 and centrifuged at 6,000 × g for 15 min. The yellow color intensity was evaluated at 410 nm. The H2O2 level was computed by extinction coefficient of 0.28 mmol–1 cm–1. The contents of H2O2 were measured by the method presented by Jana and Choudhuri (1981).
Stress-induced electrolyte leakage (EL) of uppermost stretched leaves was determined using methodology of Dionisio-Sese and Tobita (1998) and Ali et al. (2022d). The leaves were cut into minor slices (5 mm length) and placed in test tubes having 8-mL distilled water. These tubes were incubated and transferred into water bath for 2 h prior to measuring the initial electrical conductivity (EC1). The samples were autoclaved at 121°C for 20 min, and then cooled down to 25°C before measuring the final electrical conductivity (EC2). Electrolyte leakage was calculated using the following formula:
Determination of antioxidant enzyme activities
To evaluate enzyme activities, fresh leaves (0.5 g) were homogenized in liquid nitrogen and 5 mL of 50 mmol sodium phosphate buffer (pH 7.0) including 0.5 mmol EDTA and 0.15 mol NaCl. The homogenate was centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant was used for the measurement of SOD and POD activities. Superoxidase dismutase activity was assayed in 3-mL reaction mixture containing 50 mM sodium phosphate buffer (pH 7), 56 mM nitro blue tetrazolium, 1.17 mM riboflavin, 10 mM methionine, and 100 μL enzyme extract. Finally, the sample was measured using a spectrophotometer (xMark™ Microplate Absorbance Spectrophotometer; Bio-Rad). Enzyme activity was measured using a method by Chen and Pan (1996) and expressed as U g–1 FW.
Peroxidase activity in the leaves was estimated using the method of Sakharov and Ardila (1999) and Ali et al. (2022b) using guaiacol as the substrate. A reaction mixture (3 mL) containing 0.05 mL of enzyme extract, 2.75 mL of 50 mM phosphate buffer (pH 7.0), 0.1 mL of 1% H2O2, and 0.1 mL of 4% guaiacol solution was prepared. Increases in the absorbance at 470 nm because of guaiacol oxidation were recorded for 2 min. One unit of enzyme activity was defined as the amount of the enzyme.
Catalase activity was analyzed according to Aebi (1984). The assay mixture (3.0 mL) was comprised of 100 μL enzyme extract, 100 μL H2O2 (300 mM), and 2.8 mL 50 mM phosphate buffer with 2 mM ETDA (pH 7.0). The CAT activity was measured from the decline in absorbance at 240 nm as a result of H2O2 loss (ε = 39.4 mM–1 cm–1).
Ascorbate peroxidase activity was measured according to Nakano and Asada (1981). The mixture containing 100 μL enzyme extract, 100 μL ascorbate (7.5 mM), 100 μL H2O2 (300 mM), and 2.7 mL 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0) was used for measuring APX activity. The oxidation pattern of ascorbate was estimated from the variations in wavelength at 290 nm (ε = 2.8 mM–1 cm–1).
Determination of non-enzymatic antioxidants, sugars, and proline contents
Plant ethanol extracts were prepared for the determination of non-enzymatic antioxidants and some key osmolytes. For this purpose, 50 mg of plant dry material was homogenized with 10 mL ethanol (80%) and filtered through Whatman No. 41 filter paper. The residue was re-extracted with ethanol and the two extracts were pooled together to a final volume of 20 mL. The determination of flavonoids (Pêkal and Pyrzynska, 2014), phenolics (Bray and Thorpe, 1954), ascorbic acid (Azuma et al., 1999), anthocyanin (Lewis et al., 1998), and total sugars (Dubois et al., 1956) was performed from the extracts.
Fresh leaf material (0.1 g) was mixed thoroughly in 5-mL aqueous sulpho salicylic acid (3%). The mixture was centrifuged at 10,000 × g for 15 min and aliquot (1 mL) was poured into a test tube having 1 mL acidic ninhydrin and 1 mL glacial acetic acid. The reaction mixture was first heated at 100°C for 10 min and then cooled in an ice bath. The reaction mixture was extracted with 4-mL toluene and test tubes are vortexed for 20 s and cooled. Thereafter, the light absorbance at 520 nm was measured by using UV–VIS spectrophotometer (Hitachi U-2910, Tokyo, Japan). The free proline content was determined on the basis of standard curve at 520 nm absorbance and expressed as μmol (g FW) –1 (Bates et al., 1973).
Determination of nutrient contents
For nutrient analysis, plant roots and shoots were washed twice in redistilled water, dipped in 20 mM EDTA for 3 s and then, again washed with deionized water twice for the removal of adsorbed metal on plant surface. The washed samples were then oven-dried for 24 h at 105°C. The dried roots and shoots were digested using wet digestion method in HNO3: HClO4 (7:3 V/V) until clear samples were obtained. Each sample was filtered and diluted with redistilled water up to 50 mL. The root and shoot contents of Fe2+, Mg2+, Ca2+, and P and were analyzed using Atomic Absorption Spectrophotometer (AAS) model Agilent 240FS-AA.
Statistical analysis
Statistical analysis of data was performed with analysis of variance (ANOVA) using a statistical program Co-Stat version 6.2, Cohorts Software, 2003, Monterey, CA, United States. All the data obtained were tested by one-way ANOVA. Thus, the differences between treatments were determined using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means. Logarithmic or inverse transformations were performed for data normalization, where necessary, prior to analysis. Pearson’s correlation analysis was performed to quantify relationships between various analyzed variables. The graphical presentation was carried out using Origin-Pro 2017. The RStudio (R Core Team, 2018) was used to calculate Pearson’s correlation through heat-map analysis. Furthermore, the plots of principal component analysis and heat map on T. aestivum parameters were carried out using the RStudio.
Results
Effect of different concentrations of Plantago ovata Forsk leaf extract on seed germination and growth of wheat grown under well-watered and drought-stressed condition
In the present study, the effects of different concentrations of POLE (control, hydropriming, 10, 20, 30, and 40% POLE) on seed germination, plant growth, and biomass were also determined in wheat seedlings when grown in well-watered and drought-stressed environment. The data regarding various germination parameters are presented in Figure 1, and data regarding various growth and morphological parameters of wheat seedlings are presented in Figure 2. The results showed that drought-stressed condition significantly (P < 0.05) increased germination index, time to 50% germination, co-efficient of uniformity of emergence, mean germination time, germination energy (E), germination percentage, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, shoot length, and root length in wheat seedlings, compared to those plants which were grown in well-watered environment. In addition, it was depicted that seed priming with POLE significantly (P < 0.05) increased germination index, time to 50% germination, co-efficient of uniformity of emergence, mean germination time, germination energy (E), germination percentage, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, shoot length, and root length in well-watered and drought-stressed plants, when compared with plant grown without POLE (0% POLE or hydropriming). Seed priming with POLE showing more significant results up to a level of 20% POLE, while further increase in the POLE levels (30 and 40% POLE) induces a negative impact of all seed germination parameters and growth in wheat seedlings in well-watered and drought-stressed environment.
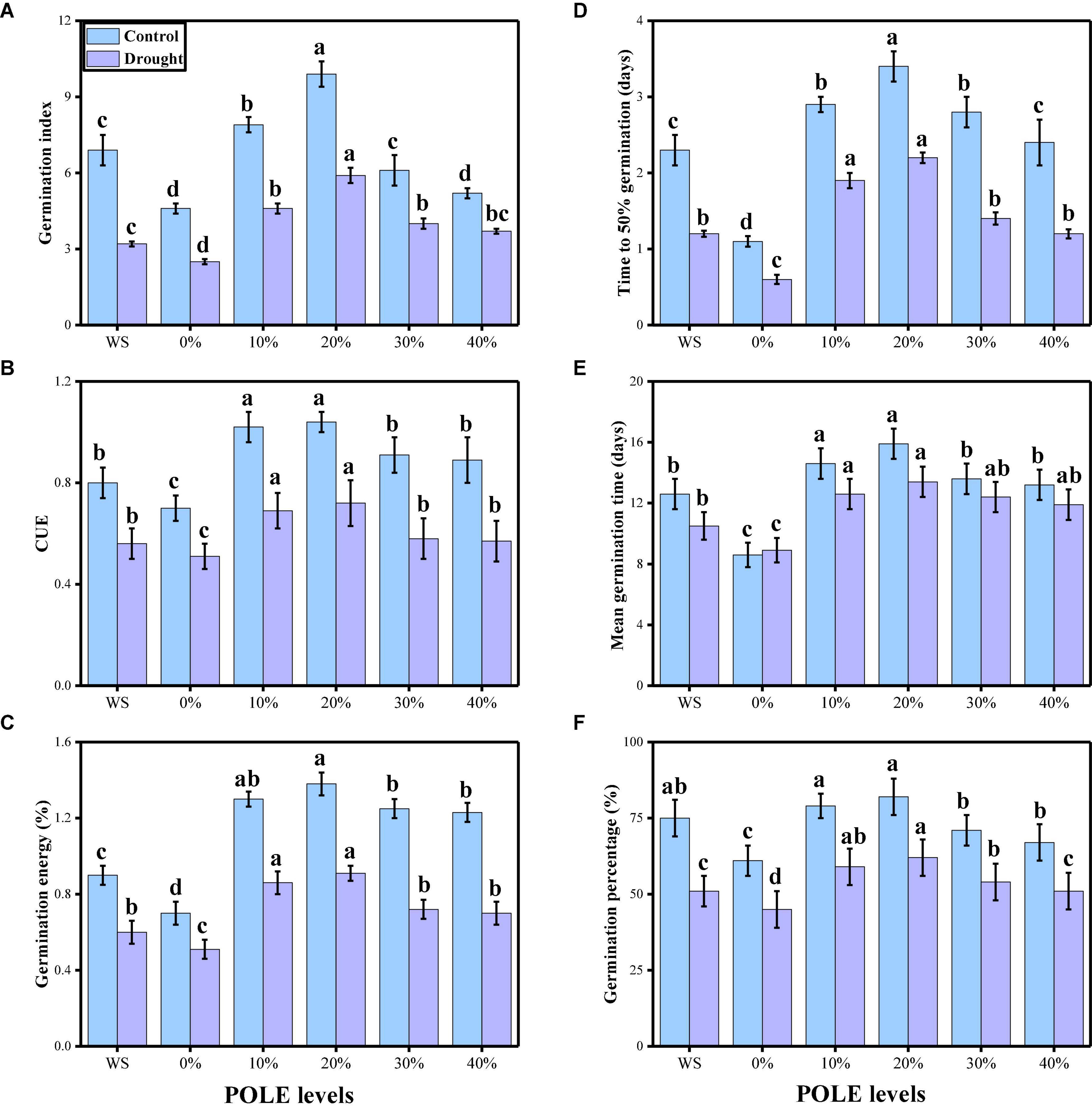
Figure 1. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on germination index (A), time to total 50% germination (B), co-efficient of uniformity of emergence (C), mean germination time (D), germination energy (E), and germination percentage (F) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
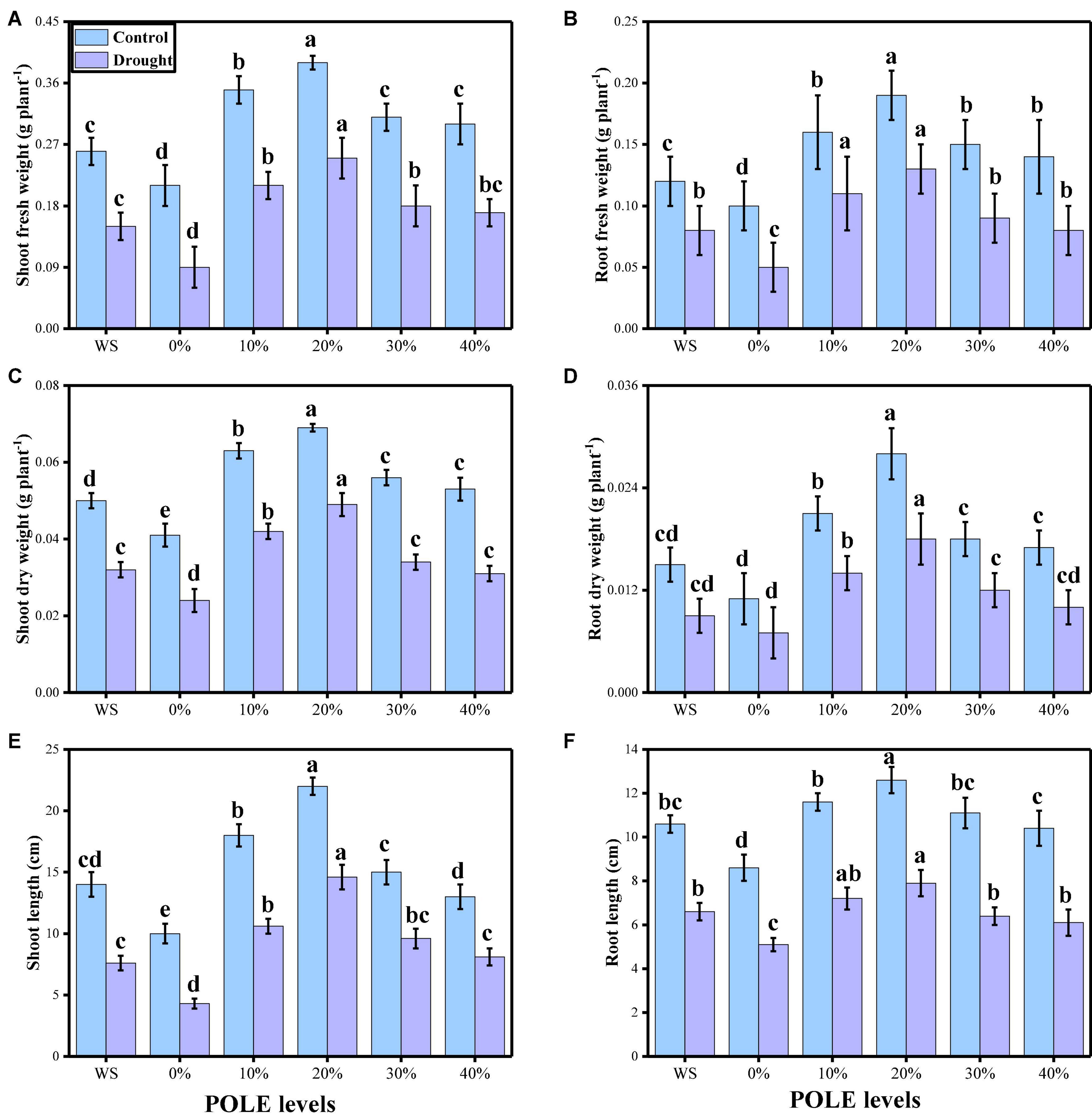
Figure 2. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on shoot fresh weight (A), root fresh weight (B), shoot dry weight (C), root dry weight (D), shoot length (E), and root length (F) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
Effect of different concentrations of Plantago ovata Forsk leaf extract on gaseous exchange parameters and stomatal properties of wheat grown under well-watered and drought-stressed condition
Various photosynthetic pigments (chlorophyll and carotenoid contents), gas exchange parameters [net photosynthesis (Pn), leaf stomatal conductance (Gs), transpiration rate (Ts), and intercellular carbon dioxide concentration (Ci)], and stomatal properties (stomatal density, stomatal width, stomatal length, stomatal aperture, efficiency of PSII, and quantum yield of PSII) were also measured in this study. The data regarding photosynthetic pigments and gaseous exchange parameters are presented in Figure 3, and the data regarding the stomatal properties in wheat seedlings are presented in Figure 4 under the application of POLE in well-watered and drought-stressed environment. The results showing that the chlorophyll contents, carotenoid contents, net photosynthesis, leaf stomatal conductance, transpiration rate, stomatal width, stomatal length, stomatal aperture, efficiency of PSII, and quantum yield of PSII were decreased in the plant which were grown in drought stress condition (60% OM), when compared to the plants which were grown in the well-watered condition (100% OM). Although stomatal density was increased in the plants which were grown in drought stress environment and intercellular carbondioxide concentration showed non-significant increase or decrease in all treatments of drought stress and POLE. Results also showing that photosynthetic pigments, gas exchange parameters, and stomatal properties in drought-stressed condition can be increased with the seed priming with POLE which significantly (P < 0.05) increased chlorophyll contents, carotenoid contents, net photosynthesis, leaf stomatal conductance, transpiration rate, stomatal width, stomatal length, stomatal aperture, efficiency of PSII, and quantum yield of PSII, compared to those plants which were grown in 0% POLE or hydropriming. In addition, the maximum contents of photosynthetic pigments, gas exchange attributes and stomatal properties showed maximum results up to 20% POLE, while further increase in the concentration of POLE (30 and 40%) induced a significant (P < 0.05) decrease in all these parameters, compared to those plants which were grown in 0% POLE or hydropriming.
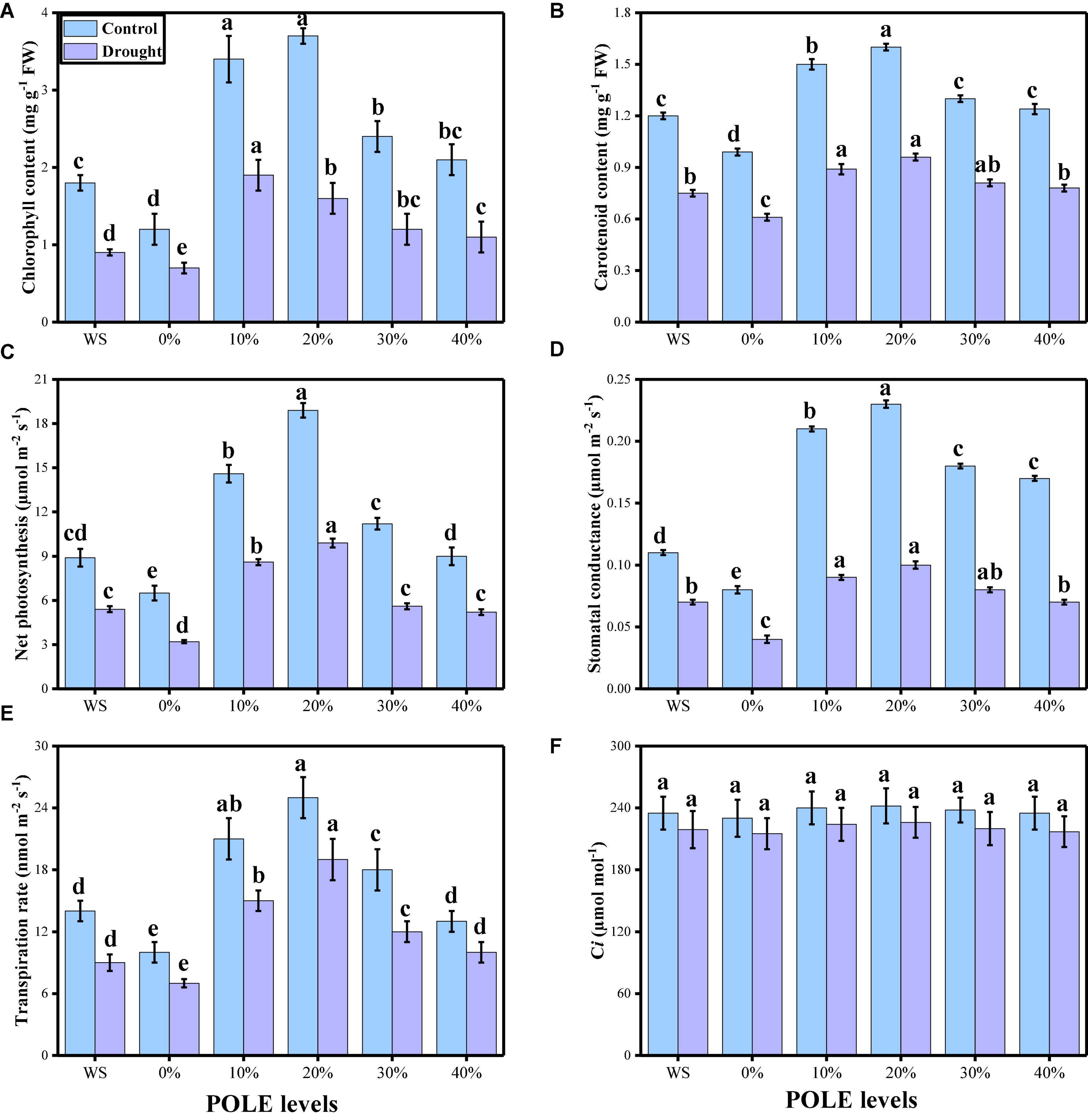
Figure 3. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on total chlorophyll contents (A), carotenoid contents (B), net photosynthesis (C) stomatal conductance (D), transpiration rate (E), and intercellular CO2 (F) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
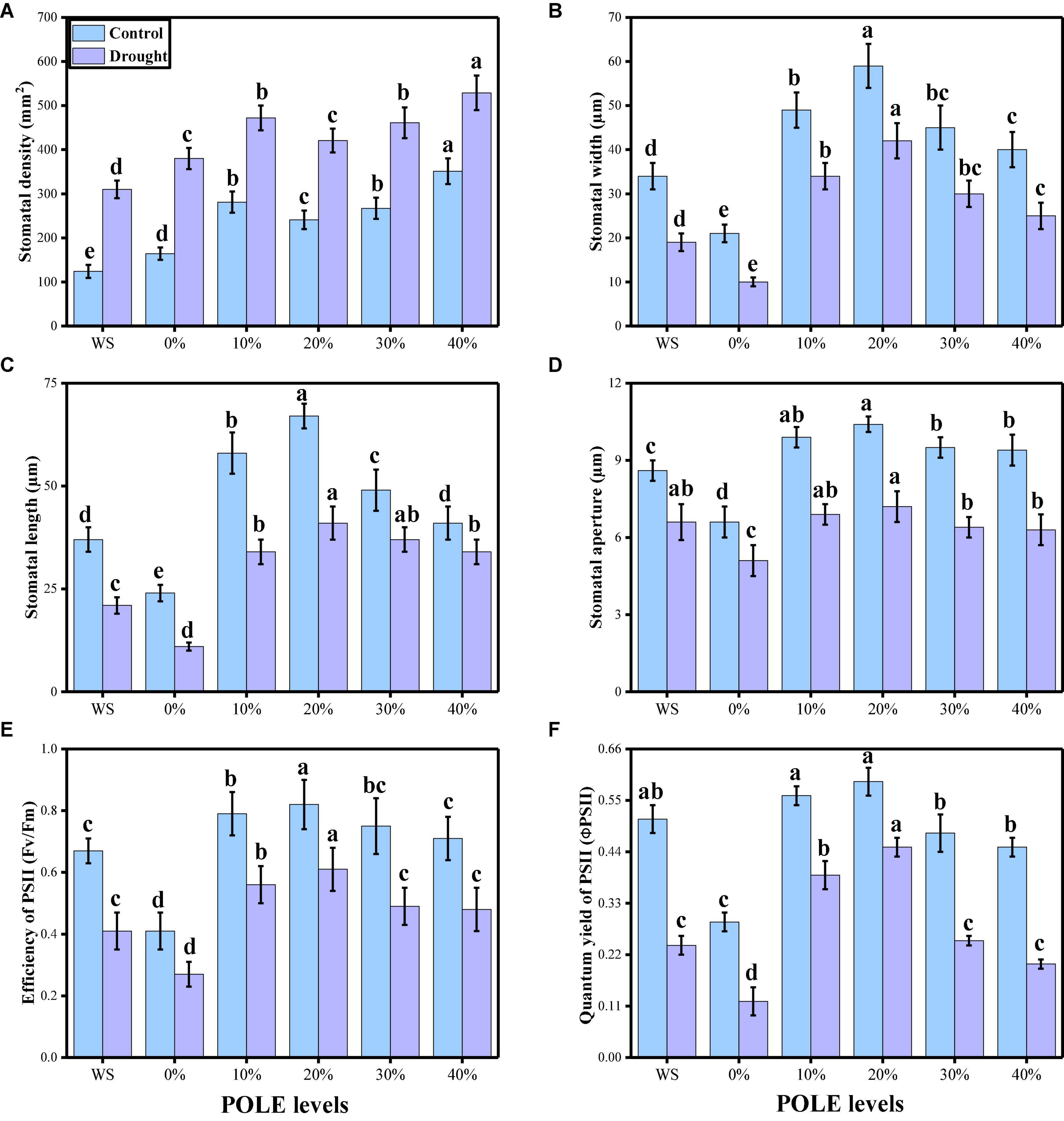
Figure 4. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on stomatal density (A), stomatal width (B), stomatal length (C), stomatal aperture (D), efficiency of PSII (E), and quantum yield of PSII (F) under well-watered and drought stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
Effect of different concentrations of Plantago ovata Forsk leaf extract on oxidative stress indicators and antioxidants response of wheat grown under well-watered and drought-stressed condition
In the present study, we also elucidated different oxidative stress parameters (MDA, H2O2 initiation, and EL) in the roots and shoots of wheat seedlings. The data regarding different oxidative stress parameters are presented in Figure 5, under the application of POLE in well-watered and drought-stressed environment. According to the given results, the contents of MDA, H2O2 initiation and EL (%) were increased in the roots and shoots of the plant grown in drought stress (60% OM), when compared to the plants which were grown in the well-watered condition (100% OM). Seed priming with POLE decreased the contents MDA, H2O2 initiation, and EL (%) in the roots and shoots of the plant, when grown in well-watered and drought-stressed condition. Although the maximum decrease in the content MDA, H2O2 initiation and EL (%) was observed in 20% POLE, further increase in POLE (30 and 40%) increases contents MDA, H2O2 initiation, and EL (%) in the roots and shoots of the wheat seedlings in well-watered and drought stress condition, compared to the plants grown in 0% POLE.
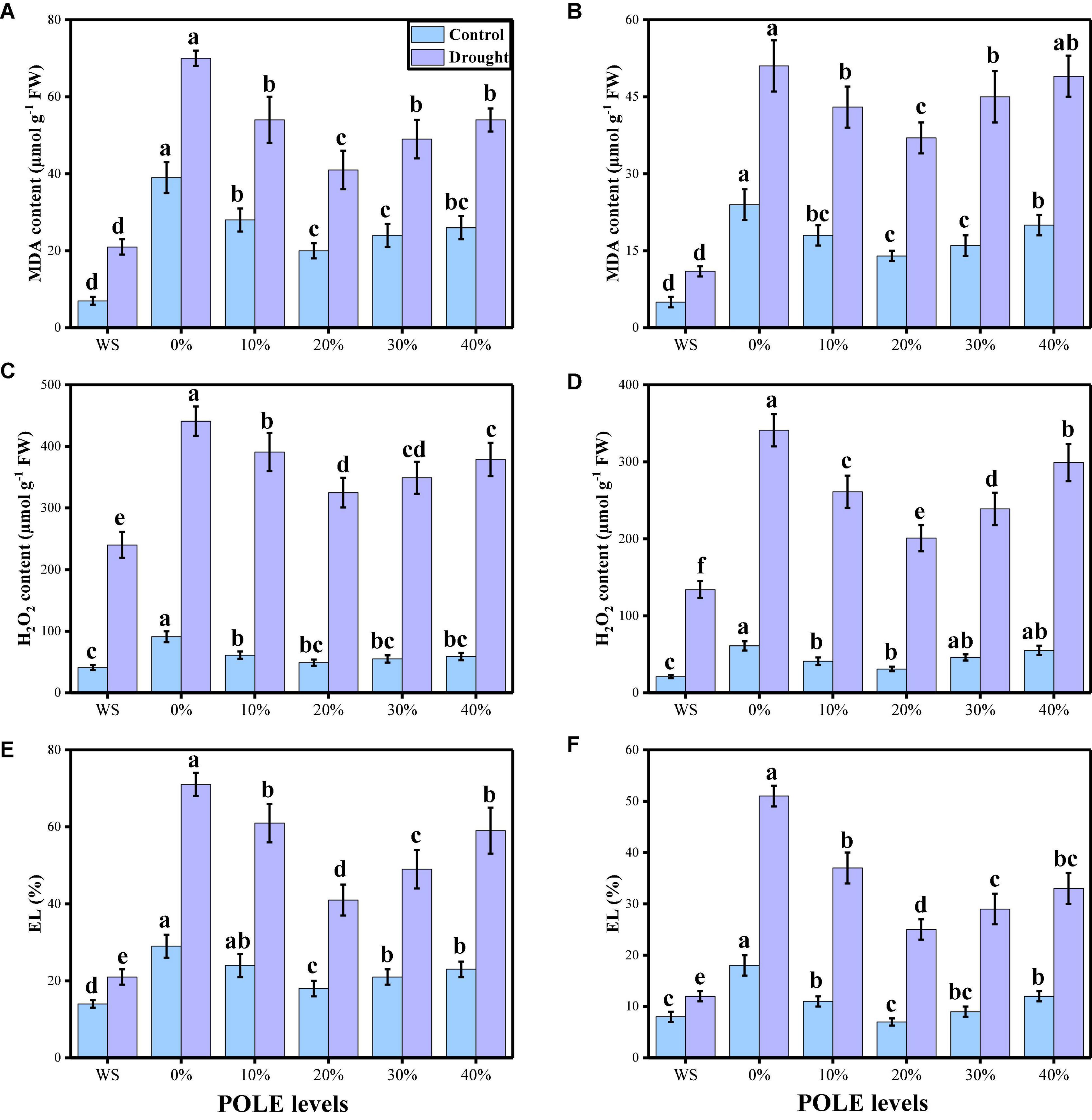
Figure 5. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on MDA contents in the roots (A), MDA contents in the leaves (B), H2O2 contents in the roots (C), H2O2 contents in the leaves (D), EL percentage in the roots (E), and EL percentage in the leaves (F) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
The activities of various enzymatic (SOD, POD, CAT, and APX) and non-enzymatic (ascorbic acid, anthocyanin, phenolics, and flavonoids) antioxidants were also increased due to low water contents in the mixture. The data regarding enzymatic antioxidants are presented in Figure 6, and the data regarding non-enzymatic antioxidants in wheat seedlings are presented in Figure 7. Compared to the plants grown in the well-watered conditions, the activities of enzymatic antioxidants (SOD, POD, CAT, and APX) in the roots and leaves and compounds of non-enzymatic antioxidants (ascorbic acid, anthocyanin, phenolics, and flavonoids) of T. aestivum seedlings were increased significantly (P < 0.05) in the plants grown in the drought-stressed condition. The results also showing that the activities of enzymatic and non-enzymatic antioxidants were further increased with the increasing levels of POLE in well-watered and drought-stressed plants, compared with those plants of 0% POLE. Moreover, the maximum increased in the activities of enzymatic and non-enzymatic antioxidants were found in the plants which were grown in 20% POLE, while further increase such as 30 and 40% POLE induces a significant (P < 0.05) decrease in the activities of enzymatic and non-enzymatic antioxidants, compared with those plants which were not primed with POLE.
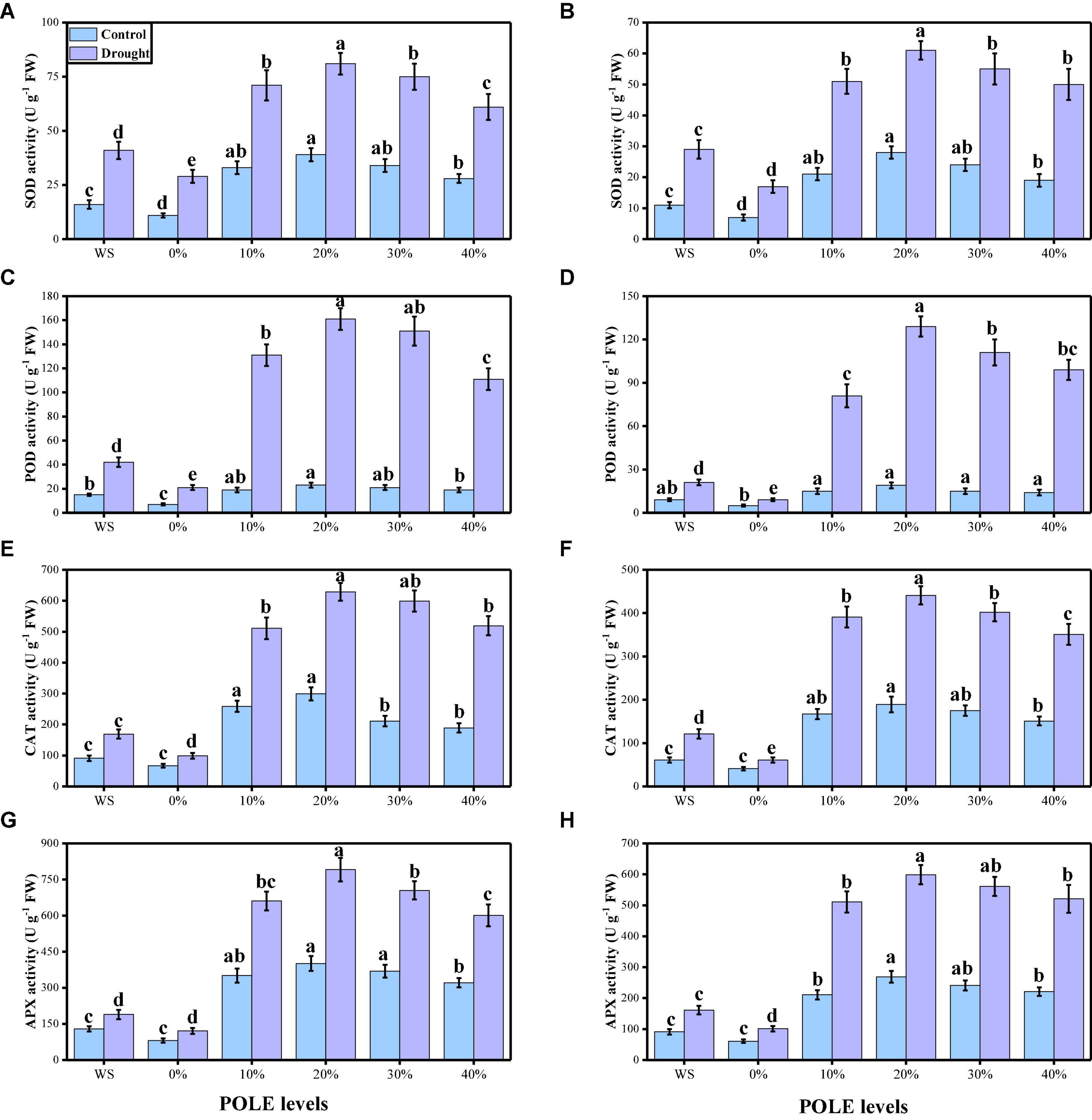
Figure 6. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on SOD activity in the roots (A), SOD activity in the leaves (B), POD activity in the roots (C), POD activity in the leaves (D), CAT activity in the roots (E), CAT activity in the leaves (F), APX activity in the roots (G), and APX activity in the leaves (H) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
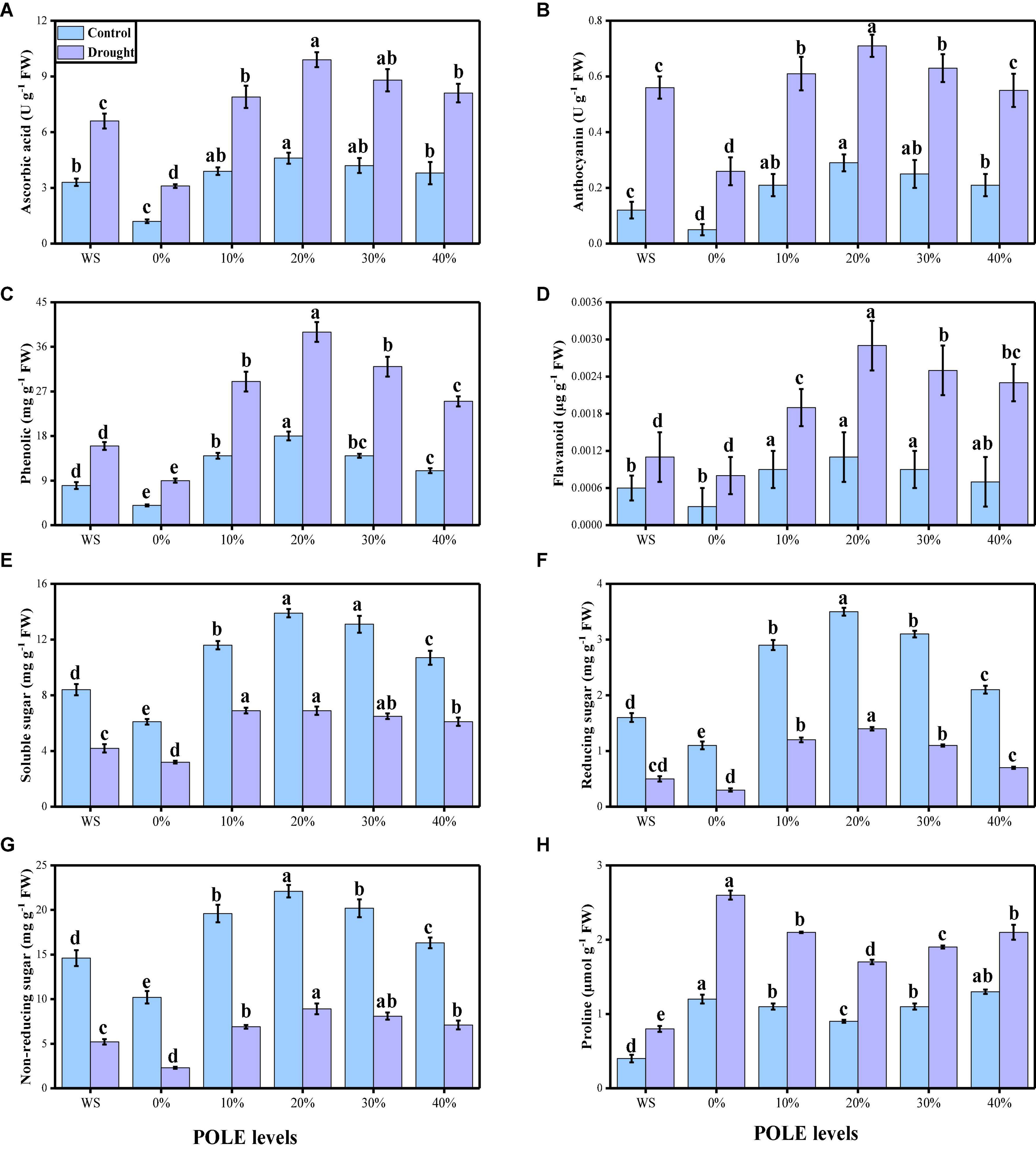
Figure 7. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on ascorbic acid contents (A), anthocyanin contents (B), phenolic contents (C), flavonoid contents (D), soluble sugar contents (E), reducing sugar contents (F), non-reducing sugar contents (G), and proline contents (H) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
The contents of sugar (soluble, reducing, and non-reducing) and proline from the leaves of T. aestivum seedlings were also determined and presented in Figure 7. Compared to the well-watered, drought-stressed condition significantly (P < 0.05) decrease the contents of soluble, reducing and non-reducing sugar while increase the contents of proline in the leaves of wheat. Results show that the contents of soluble, reducing and non-reducing sugar were increased with the seed priming with POLE while they induce a significant decrease in the contents of proline, compared with those plants which were not primed with 0% POLE.
Effect of different concentrations of Plantago ovata Forsk leaf extract on nutritional status of wheat grown under well-watered and drought-stressed condition
The contents of essential minerals such as magnesium (Mg2+), phosphorus (P), iron (Fe2+), and calcium (Ca2+) from the roots and shoots of T. aestivum seedlings were also measured in this study. The data regarding the contents of various minerals (Mg2+, P, Fe2+, and Ca2+) in the roots and shoots of wheat seedlings are presented in Figure 8, grown in well-watered and drought-stressed condition under the seed priming with various concentrations of POLE. Compared to the plants grown in the control treatment, the contents of various minerals (Mg2+, P, Fe2+, and Ca2+) in the roots and shoots of wheat seedlings were decreased significantly (P < 0.05) in the plants grown in the water-deficient condition. The results show that the contents of various minerals (Mg2+, P, Fe2+, and Ca2+) in the roots and shoots of wheat seedlings were increased with the increasing levels of POLE in control and drought-stressed plants, compared with those plants which were not primed (0% POLE). Moreover, the maximum increased in the contents of various minerals (Mg2+, P, Fe2+, and Ca2+) in the roots and shoots of wheat seedlings were found in the plants which were grown in 20% POLE, while further increase in POLE such as 30 and 40% POLE induce a significant (P < 0.05) decrease in the in the contents of various minerals, compared with those plants which were not primed with POLE.
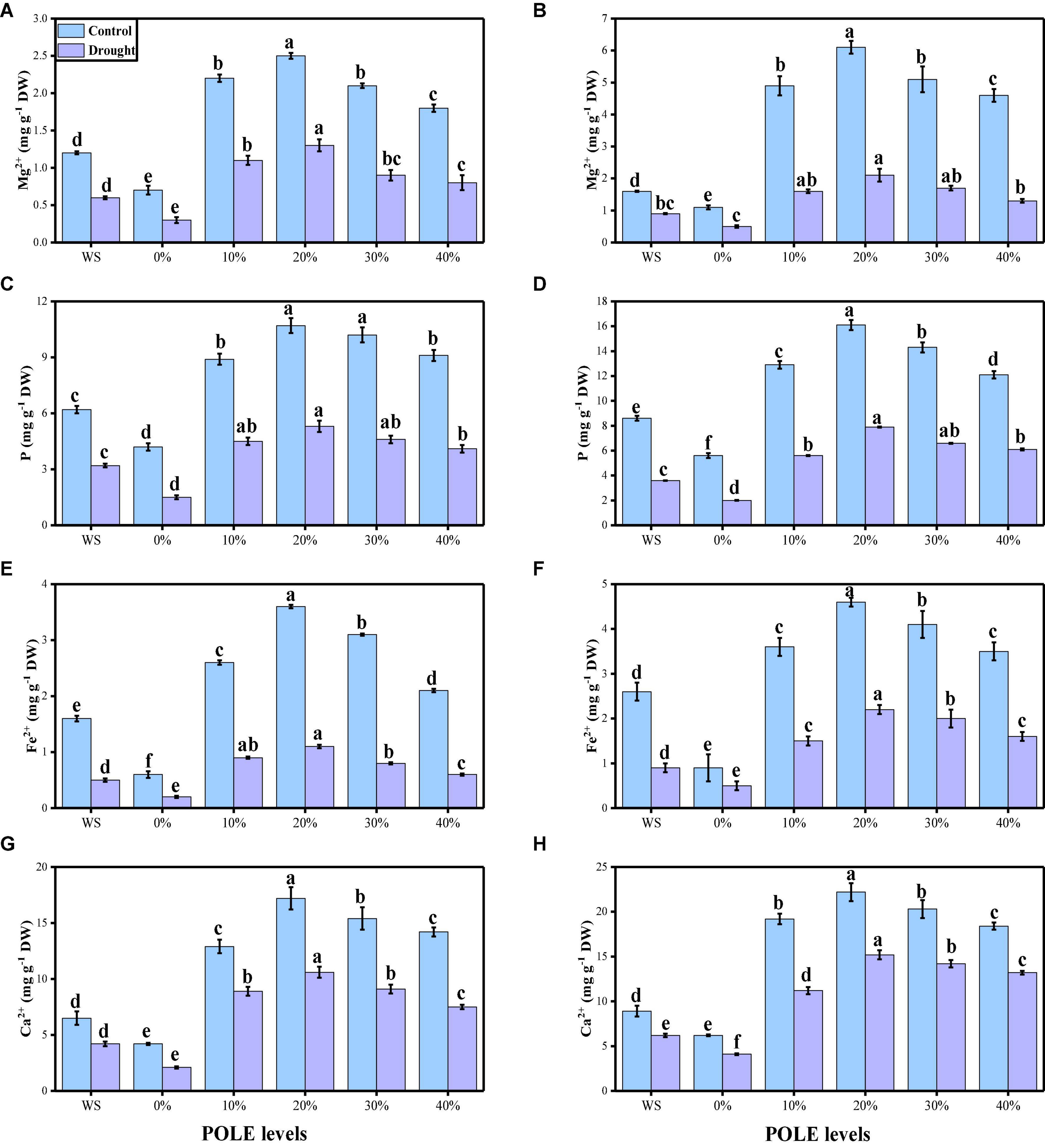
Figure 8. Effect of seed priming with Plantago ovata Forsk leaf extract (POLE) on magnesium contents in the roots (A), magnesium contents in the shoots (B), phosphorus contents in the roots (C), phosphorus contents in the shoots (D), iron contents in the roots (E), iron contents in the shoots (F), calcium contents in the roots (G), and calcium contents in the leaves (H) under well-watered and drought-stressed environment in wheat. Means sharing similar letter(s) within a column for each parameter do not differ significantly at P < 0.05. Data in the figures are means of four repeats (n = 4) of just one harvest of wheat plants ± standard deviation (SD). Different lowercase letters on the error bars indicate significant difference between the treatments. Different treatments of POLE used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE).
Relationship between growth, physiology, and nutritional status in various organs of Triticum aestivum
A heatmap-histogram analysis was also constructed to explore the relationship between the different growth and ions uptake in various parts of the plants (Figure 9). Heatmap-histogram analysis shows that 0% POLE showed a significant result with various oxidative stress indicators, proline contents while rest of heatmap-histogram is showing non-significant results in all other parameters studied in this study. The color blue in Figure 9 indicates the non-significant results of our study, and the aquamarine color is showing significant results in this figure. This histogram depicted a clear difference between the ion uptake abilities and growth attributes of wheat.
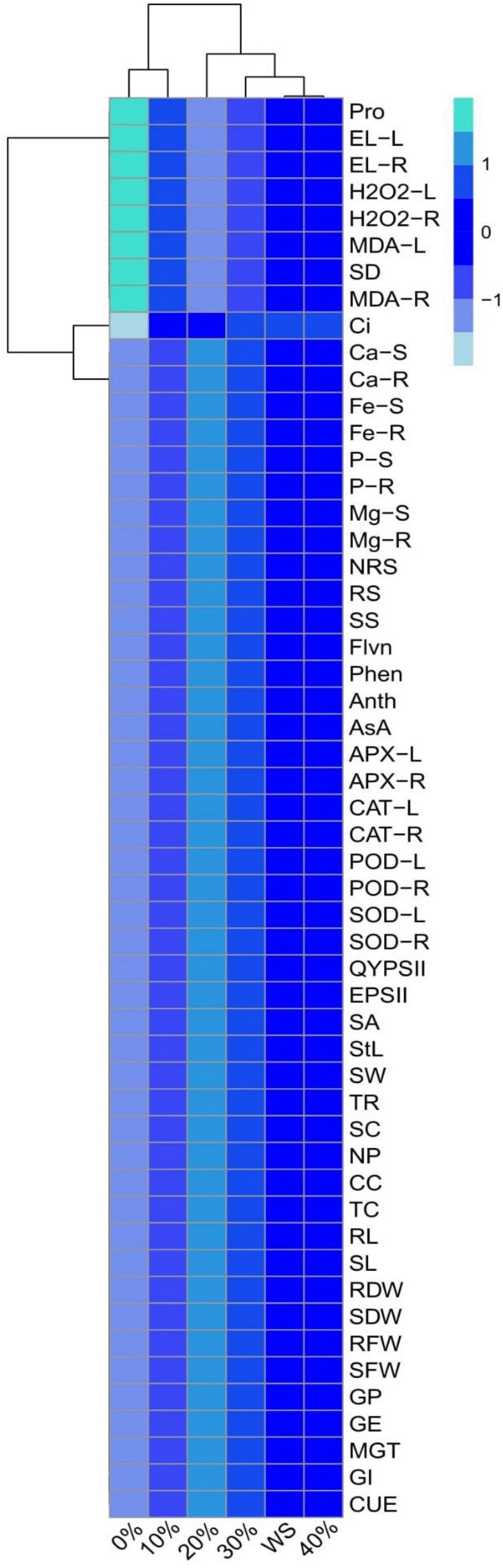
Figure 9. A heatmap between different studied attributes of wheat grown under well-watered and drought-stressed environment. Different treatments of Plantago ovata Forsk leaf extract (POLE) used in this study are as follows: WS (Control, without POLE + without water), 0% (0% POLE), 10% (10% POLE), 20% (20% POLE), 30% (30% POLE), and 40% (40% POLE). Different abbreviations used are as follows: Pro, proline contents; EL-L, electrolyte leakage in the leaves; EL-R, electrolyte leakage in the roots; H2O2-L, hydrogen peroxide initiation in the leaves; H2O2-R, hydrogen peroxide initiation in the roots; MDA-L, malondialdehyde contents in the leaves; SD, stomatal density; MDA-R, malondialdehyde contents in the roots; Ci, intercellular CO2; Ca-S, calcium contents in the shoots; Ca-R, calcium contents in the roots; Fe-S, iron contents in the shoots; Fe-R, iron contents in the roots; P-S, phosphorus contents in the shoots; P-R, phosphorus contents in the roots; Mg-S, magnesium contents in the shoots; Mg-R, magnesium contents in the roots; NRS, non-reducing sugars; RS, reducing sugars; SS, soluble sugars; Flvn, flavonoid contents; Phen, phenolic contents; Anth, anthocyanin contents; AsA, ascorbic acid contents; APX-L, ascorbate peroxidase activity in the leaves; APX-R, ascorbate peroxidase activity in the roots; CAT-L, catalase activity in the leaves; CAT-R, catalase activity in the roots; POD-L, peroxidase activity in the leaves; POD-R, peroxidase activity in the roots; SOD-L, superoxidase dismutase activity in the leaves; SOD-R, superoxidase dismutase activity in the roots; QYPSII, quantum yield of PSII; EPSII, efficiency of PSII; SA, stomatal aperture; StL, stomatal length; SW, stomatal width; TR, transpiration rate; SC, stomatal conductance; NP, net photosynthesis; CC, carotenoid contents; TC, total chlorophyll; RL, root length; SL, shoot length; RDW, root dry weight; SDW, shoot dry weight; RFW, root fresh weight; SFW, shoot fresh weight; GP, germination percentage; GE, germination energy; MGT, mean germination time; GI, germination index; and CUE, co-efficient of uniformity of emergence.
The loading plots of principal component analysis (PCA) to check the effect of drought stress with the seed priming with POLE on some selected attributes of wheat seedlings are presented in Figure 10. The loading plots of PCA show that the contents of Ca2+, Mg2+, P, and Fe2+ in the roots and shoots and soluble sugar, ascorbic acid contents, SOD activity in the roots, net photosynthesis, total chlorophyll contents, shoot length, germination index, and shoot fresh weight show a positive correlation with each other while proline contents, stomatal density, and MDA contents in the roots show a negative correlation.
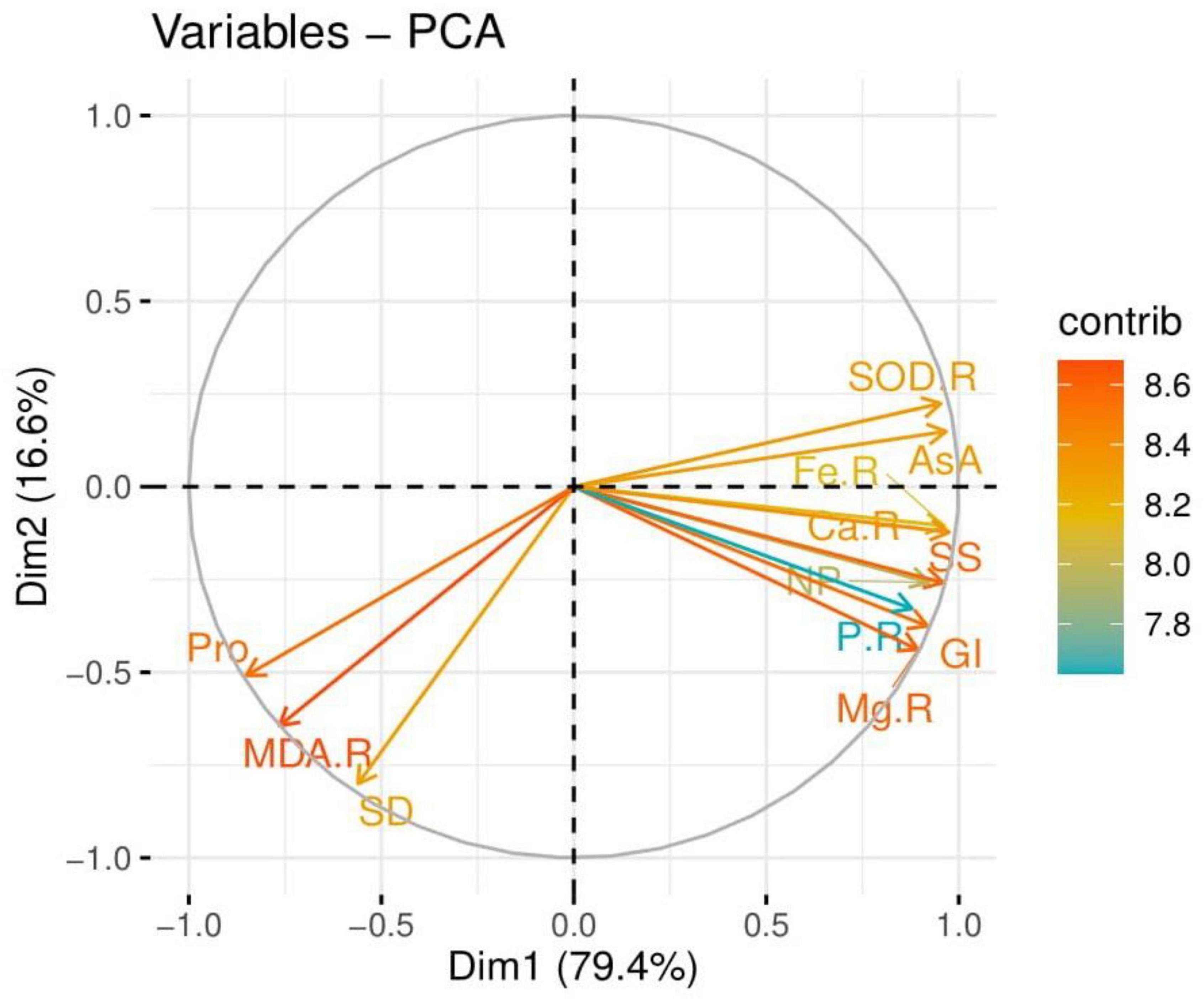
Figure 10. Loading plots of principal component analysis (PCA) on different studied attributes of wheat grown under well-watered and drought-stressed environment with various application levels of Plantago ovata Forsk leaf extract (POLE). Different abbreviations used are as follows: Ca-R, calcium contents in the roots; Fe-R, iron contents in the roots; P-R, phosphorus contents in the roots; Mg-R, magnesium contents in the roots; SS, soluble sugar; AsA, ascorbic acid contents; SOD-R, superoxidase dismutase activity in the roots; NP, net photosynthesis; GI, germination index; Pro, proline contents; SD, stomatal density; and MDA-R, malondialdehyde contents in the roots.
Discussion
Drought being as a stern environmental setback is associated with the deviation in surface hydrological processes and climate (Ahmad et al., 2017; Hasanuzzaman et al., 2018; Sakya and Prahasto, 2020). Water is one of the most limiting factors that influence many morphological, physiological, biochemical, metabolic, transcriptomic, and proteomic processes, thereby affecting plant survival, development, and crop yields (Fahad et al., 2017; Schmidthoffer et al., 2018; Khan et al., 2019a). In addition, drought induces various changes in morphological and physiological behavior of plants including altered plant water relation, lower growth rate, reduced stem elongation, expansion of leaf, stomatal movement, ion and nutrient imbalance and photosynthesis (Mahajan and Tuteja, 2005; Aslam et al., 2013). Soil drought is a predominant environmental factor that restricts plant growth worldwide and is known to slow plant growth rates and reduce crop yields by inhibiting plants’ carbon assimilation capacity (Bashir et al., 2020; Yaseen et al., 2020; Hussain et al., 2022). In the present study, we have noticed that low water contents in the medium (60% OM) significantly (P < 0.05) decrease seed germination, plant growth, and biomass, compared to those plants which were grown in the well-watered condition (Figures 1, 2). Drought indicates the unavailability of water for the plant uptake. Thus, the primary effect of drought stress on the plant is appeared as reduced water content. This reduction of water content resulted in growth and physiological disorder (Khan et al., 2019a). It was also specified that low soil moisture in the soil would suppress root growth, which eventually results in restricting water absorption from the subsoil and changing the plants’ ability to tolerate drought stress (Ahmad et al., 2017; Hasanuzzaman et al., 2018; Taha et al., 2020). Previous studies have documented that water stress hampered growth at seedling stage because of inhibited cell expansion and decrease in carbon acclimatization and partitioning (Nikolaeva et al., 2010; Aslam et al., 2013; Ahmad et al., 2017; Akram et al., 2018; Noman et al., 2018; Khan et al., 2019a).
Although many stress factors suppress plant growth by targeting more than one physiological process, photosynthesis is the most severely affected, as reflected by changes in biomass production that may be due to a lower demand for assimilates (Nikolaeva et al., 2010; Khan et al., 2019b). Under such conditions, photosynthesis is influenced by several mechanisms, including stomatal limitations, the primary drought-stress response that leads to reduced stomatal conductance. When plants close their stomata to minimize water losses, that action also inhibits atmospheric CO2 diffusion into the leaf and chloroplasts, thereby decreasing the rate of photosynthesis and limiting carbon assimilation, which then reduces plant growth (Kosar et al., 2015; Saud et al., 2017). We have also noticed in the present study that drought-stressed condition significantly affected photosynthetic pigments, gas exchange parameters, and stomatal properties in wheat seedlings, compared to the plants grown in the well-watered condition (Figures 3, 4). Net photosynthesis is decreased by regulation mechanisms which include decreases in stomatal behavior and photosynthetic pigments when plants are exposed to drought (Liang et al., 2018). Previously, it was observed that normal or controlled water environment showed significantly higher photosynthetic apparatus and stomatal aperture, compared to the plants grown in the water-deficient condition (Khan et al., 2019a; Bashir et al., 2020; Sharma et al., 2020). Similar trend was also observed in T wheat seedlings, when exposed to drought stress condition (Mathur et al., 2019; Adrees et al., 2020) due to stomatal closure found due to water shortage as shown by Rivas et al. (2016) in Vigna unguiculata species. Moreover, the reduction in transpiration rate might be due to the plants that does not maintain water field capacity which was induced by rate of transpiration (Rivas et al., 2016). When plants undergoes water limited conditions, stomata progressively close, reducing stomatal conductance, resulting in decreased photosynthesis and transpiration as showed by Lotter et al. (2014) in fynbos legume under water-deficient environment.
Oxidation of photosynthetic pigments, membrane lipids, proteins, and nucleic acids are the crucial reasons of plant damage, which occur due to the drought-induced high ROS production leading to the modification of cellular redox status (Saleem et al., 2019a,b, 2020f; Kamal et al., 2022). Low water levels in the soil cause ultra-structural alterations, oxidative stress in plants and increased EL, MDA concentrations, whereas induced alterations in antioxidant enzyme activities are such as SOD, POD, CAT, and APX (Imran et al., 2019; Kamran et al., 2019; Alatawi et al., 2022; Azeem et al., 2022; Naveed et al., 2022). As reported by Taha et al. (2020) antioxidants, such as ascorbic acids and glutathione contents, additional ROS may be scavenged and therefore increased plant tolerance against harsh conditions may be achieved. Furthermore, osmo-protectants also constitute redox active molecules which overcome the production of ROS as well as participate in the ascorbate-glutathione cycle (Rana et al., 2020; Rehman et al., 2020; Imran et al., 2021; Akhtar et al., 2022; Mfarrej et al., 2022). In the present study, the contents of MDA, H2O2, and EL (%) were increased in the plants which were grown in the drought-stressed environment (Figure 5), compared to those plants which were grown in the well-watered conditions. Plant has strong defense system and increased enzymatic (SOD, POD, CAT, and APX) and non-enzymatic antioxidants (ascorbic acid, anthocyanin, phenolics, and flavonoids) to scavenge ROS production. The increase in the concentration of soluble sugars in drought-stressed plants (Figure 7) shows that improved cell osmotic adjustment would help to maintain higher water content in plant cells and lower electrolyte leakage (Anjum et al., 2017; Hasanuzzaman et al., 2018; Khan et al., 2019b; Afzal et al., 2021; Saleem et al., 2021).
Drought stress reduces plant productivity by inhibiting the uptake of common minerals. Although water uptake and nutrient absorption are independent processes in the roots, the need for water to support nutrient transport and plant growth makes those processes closely related (Waraich et al., 2011; Heile et al., 2021; Mumtaz et al., 2021; Sufyan et al., 2021). Most nutrients are absorbed by plant roots as ions, and water acts as a medium for their movement. Water deficits inhibit this flow of nutrients within the soil, the absorption of those elements, and their uptake by roots (Liang et al., 2018). Therefore, drought-stressed plants will have lower nutrient absorption because less water is available and the diminished power of the roots hinders the uptake process (Waraich et al., 2011). Adequate absorption of minerals is important for the maintenance of plant structural integrity and key physiological processes, and any changes in mineral uptake may negatively affect plant metabolism (Vurukonda et al., 2016; Bashir et al., 2020; Hassan et al., 2021; Javed et al., 2021; Ali et al., 2022a). Compared to the plants grown in the control treatment, the contents of various minerals (Mg2+, P, Fe2+, and Ca2+) in the roots and shoots of wheat seedlings were decreased significantly (P < 0.05) in the plants grown in the drought-stressed condition (Figure 8). The plant would take up sufficient quantities of essential nutrients to control plant structure and composition, and many other biological processes of a plant’s life cycle and any decreased in nutrient uptake not only impaired plant metabolism but also decreased plant growth and yield-related attributes (Delshadi et al., 2017; Liang et al., 2018; Ali et al., 2020; Kamran et al., 2020; Yaseen et al., 2020; Rehman et al., 2021).
Recently, priming seeds with different derivatives have become increasingly popular due to the effects of this treatment on plants, which ultimately increase plant growth and biomass in cereal crops (Dawood and El-Awadi, 2015; Abid et al., 2018). Plantago ovata leaves extract (POLE) (Psyllium) Forsk is an annual, medicinal (family: Plantaginaceae) and cultivated in the most countries of Asia (most cultivation is found in India) (Patel et al., 2016). Husks of P. ovata (also known as isabgol) are rich bioactive compounds and also essential for various primary and secondary metabolites (Talukder et al., 2015; Patel et al., 2016). Under abiotic stress condition such as drought, plants decrease their growth and biomass, due to increasing concentration of ROS which induce oxidative stress in the plants (Khan et al., 2019b; Mohamed et al., 2020). The use of medicinal plant leaf extracts such as P. ovata increased enzymatic and non-enzymatic activities in various plant species, which scavenged ROS production and thus ultimately increased growth and biomass due to active defense mechanism (Patel et al., 2016). There is a limited literature available on POLE to ameliorate abiotic stress in the plants, but the results from this study are depicting that this is a promising technique of seed priming and able to increase seed germination (Figure 1), plant growth (Figure 2), photosynthetic measurements (Figure 3), stomatal properties (Figure 4), and ions uptake (Figure 8) and decreasing oxidative stress (Figure 5) by optimizing the activities of various antioxidant compounds (Figures 6, 7) in the different organs of T. aestivum. Therefore (Tlili et al., 2019) studied the positive impacts of POLE along with many other medicinal plant extracts and noticed that POLE significantly increased plant growth, antibacterial activities and shrimp toxicity. However, decrease in growth and photosynthesis in severe high contents of 30 and 40% POLE might be due to the excessive amount or toxic amount of POLE to T. aestivum seedlings. Although this idea is new, but in another study by Nawaz et al. (2021), they studied the POLE extract to Zea mays seedlings under the drought stress and noticed that the drought stress induced a negative impact on plant growth, stomatal properties, gas enhance characteristics, and ion uptake in different organs of Z. mays but induced oxidative stress in the plants which were manifested by the increasing the activities of enzymatic and non-enzymatic antioxidant compounds. The use of POLE significantly ameliorates the negative impact of drought by increasing plant growth and biomass, stomatal properties, gas enhanced characteristics, and ion uptake in different parts of plants by decreasing oxidative damage to membrane-bounded organelles and increasing the activities of various antioxidant compounds. The use of POLE decreased the oxidative stress and increased antioxidant capacity, that is, one of the possible factors to enhance the plant growth and photosynthetic pigments in drought-stressed T. aestivum. Some previous studies also showed that the use of POLE is able to protect a plant species under low water content in the soil as showed by Rahimi et al. (2010) under different water regimes. These results indicated that POLE addition might alleviate the negative effects of drought stress manipulation on whole-plant growth of the plant. It is demonstrated that POLE addition might play a key role in maintaining plant productivity under different soil water conditions in the arid and semi-arid land. The schematic presentation of T. aestivum seedlings grown under the drought stressed with the application of POLE is presented in Figure 11.
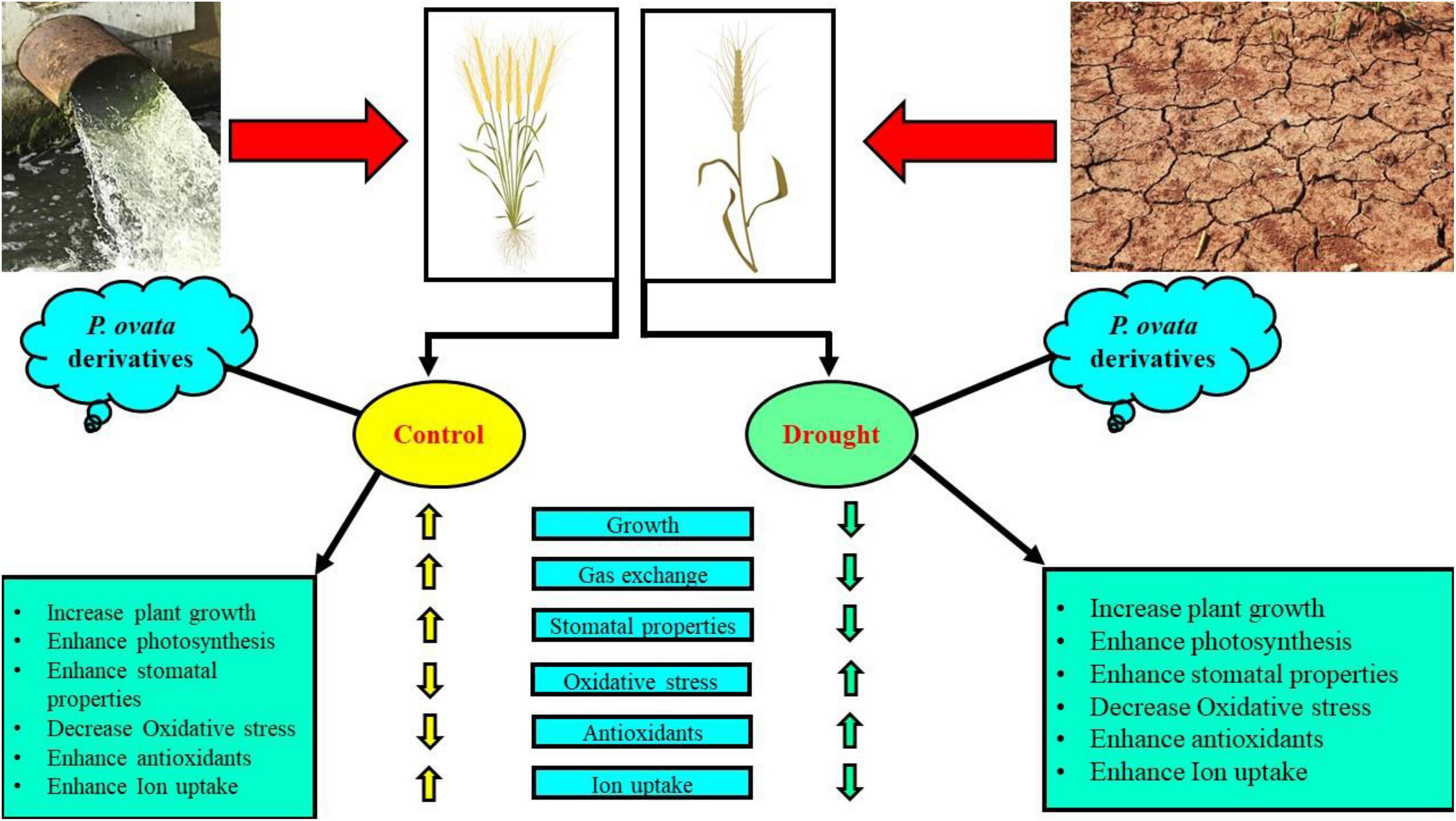
Figure 11. Schematic presentation interpreting the mechanistic role of Plantago ovata Forsk leaf extract (POLE) in alleviating the drought stress in wheat. The drought stress inhibited plant growth characteristics and higher ROS concentration was accumulated in the roots and shoots of wheat. In contrast, the application of POLE significantly alleviated the negative impact of drought stress and improved root and shoot growth and alleviated ROS accumulation. POLE addition regulated the antioxidant defense system while reduced the oxidative stress and relative membrane permeability. The current study demonstrated that POLE could relieve drought stress in wheat by reducing oxidative stress at the root surface, and regulating proficient antioxidant coordination in the roots and shoots of wheat.
Conclusion
The findings showed that the drought stress significantly (P < 0.05) decreased plant growth attributes, photosynthetic efficiency, and nutritional status of the plants, while increased ROS concentration in the plant tissues which induced oxidative damaged in the membranous-bounded organelles. Therefore, the findings also showed that the seed priming with POLE non-significantly (P < 0.05) enhanced plant growth and related attributes and ultimately improved plant yield and decreased the oxidative stress indicators by enhancing the activities of various antioxidant compounds. Therefore, long-term field studies should be executed to draw parallels amongst plants/crops nutrients mobility patterns and plant growth in order to gain insights into underlying mechanisms. Hence, based on the present findings, it is recommended that seed priming of field cultivated wheat with 20% POLE should be done to produce crop that is able to tolerate water scarcity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KA, HASA, and AAMA: conceptualization. WAA, SMA, and AAMA: data curation. MHS, DCV, MSA, and RAM: formal analysis. DCV and RAM: funding acquisition. KA, HASA, and AAMA: investigation. WAA and SMA: methodology. MHS, DCV, MSA, and RAM: validation. KA and HASA: writing original draft. KA, HASA, AAMA, WAA, SMA, AAMA, MHS, MSA, DCV and RAM: writing, review, and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R188), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This work was also supported by the National Research Development Projects to finance excellence (PFE)-14/2022-2024 granted by the Romanian Ministry of Research and Innovation and CASEE Fund for Incentives, project no: CASEE fund 2021–2022. We acknowledged to Dr. Muhammad Nawaz who help in performing some analysis and methodology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.999170/full#supplementary-material
References
Abid, M., Hakeem, A., Shao, Y., Liu, Y., Zahoor, R., Fan, Y., et al. (2018). Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 145, 12–20. doi: 10.1016/j.envexpbot.2017.10.002
Adnan, M., Fahad, S., Saleem, M. H., Ali, B., Mussart, M., Ullah, R., et al. (2022). Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 12:11997. doi: 10.1038/s41598-022-16035-3
Adrees, M., Khan, Z. S., Ali, S., Hafeez, M., Khalid, S., and Ur Rehman, Z. (2020). Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 238:124681. doi: 10.1016/j.chemosphere.2019.124681
Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/S0076-6879(84)05016-3
Afridi, M. S., Javed, M. A., Ali, S., De Medeiros, F. H. V., Ali, B., Salam, A., et al. (2022). New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 13:899464. doi: 10.3389/fpls.2022.899464
Afzal, J., Wang, X., Saleem, M. H., Sun, X., Hussain, S., and Khan, I. (2021). Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa L.). Biocell 45, 1631–1649. doi: 10.32604/biocell.2021.014934
Ahmad, M., Ishaq, M., Shah, W. A., Adnan, M., Fahad, S., and Saleem, M. H. (2022). Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 14:7669. doi: 10.3390/su14137669
Ahmad, Z., Waraich, E. A., Ahmad, R., and Shahbaz, M. (2017). Modulation in water relations, chlorophyll contents and antioxidants activity of maize by foliar phosphorus application under drought stress. Pak. J. Bot. 49, 11–19.
Akhtar, M. N., Ul-Haq, T., Ahmad, F., Imran, M., Ahmed, W., and Ghaffar, A. (2022). Application of Potassium along with Nitrogen under Varied Moisture Regimes Improves Performance and Nitrogen-Use Efficiency of High- and Low-Potassium Efficiency Cotton Cultivars. Agronomy 12:502. doi: 10.3390/agronomy12020502
Akram, N. A., Iqbal, M., Muhammad, A., Ashraf, M., Al-Qurainy, F., and Shafiq, S. (2018). Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255, 163–174. doi: 10.1007/s00709-017-1140-x
Alam, H., Khattak, J. Z. K., Ksiksi, T. S., Saleem, M. H., Fahad, S., and Sohail, H. (2020). Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 172, 1336–1351. doi: 10.1111/ppl.13273
Alatawi, A., Wang, X., Maqbool, A., Saleem, M. H., Usman, K., and Rizwan, M. (2022). S-Fertilizer (Elemental Sulfur) Improves the Phytoextraction of Cadmium through Solanum nigrum L. Int. J. Environ. Res. Public Health 19:1655. doi: 10.3390/ijerph19031655
Ali, B., Wang, X., Saleem, M. H., Azeem, M. A., Afridi, M. S., and Nadeem, M. (2022a). Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 12:219.
Ali, B., Hafeez, A., Javed, M. A., Ahmad, S., Afridi, M. S., Sumaira Nadeem, M., et al. (2022c). Bacterial-mediated salt tolerance in maize: Insights into plant growth promotion, antioxidant defense system, oxidative stress, and surfactant production. Front. Plant Sci. 13:978291. doi: 10.3389/fpls.2022.978291
Ali, B., Hafeez, A., Ahmad, S., Javed, M. A., Sumaira Afridi, M. S., Dawoud, T. M., et al. (2022d). Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 13:921668. doi: 10.3389/fpls.2022.921668
Ali, B., Wang, X., Saleem, M. H., Sumaira, H. A., Afridi, M. S., and Khan, S. (2022b). PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 11:345. doi: 10.3390/plants11030345
Ali, M., Kamran, M., Abbasi, G. H., Saleem, M. H., Ahmad, S., Parveen, A., et al. (2020). Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 40, 2236–2248. doi: 10.3390/life12020219
Amna Ali, B., Azeem, M. A., Qayyum, A., Mustafa, G., Ahmad, M. A., Javed, M. T., et al. (2021). “Bio-fabricated silver nanoparticles: A sustainable approach for augmentation of plant growth and pathogen control,” in Sustainable agriculture reviews, Vol. 3, eds A. Goyal and E. Lichtfouse (Cham: Springer), 345–371.
Anjum, S. A., Ashraf, U., Tanveer, M., Khan, I., Hussain, S., Zohaib, A., et al. (2017). Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front. Plant Sci. 8:69. doi: 10.1007/s00344-020-10273-3
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.3389/fpls.2017.00069
Asgharipour, M., and Rafiei, M. (2010). Intercropping of Isabgol (Plantago Ovata L.) and lentil as influenced by drought stress. Am. Euras. J. Sustain. Agric. 4, 341–348. doi: 10.1104/pp.24.1.1
Aslam, M., Ibni Zamir, M. S., Afzal, I., and Yaseen, M. (2013). Morphological and physiological response of maize hybrids to potassium application under drought stress. J. Agric. Res. 51, 443–454.
Austin, R. B. (1990). Prospects for genetically increasing the photosynthetic capacity of crops. Plant Biol. 10, 395–409.
Azeem, M., Haider, M. Z., Javed, S., Saleem, M. H., and Alatawi, A. (2022). Drought Stress Amelioration in Maize (Zea mays L.) by Inoculation of Bacillus spp. Strains under Sterile Soil Conditions. Agriculture 12:50.
Azooz, M. M., Abou-Elhamd, M. F., and Al-Fredan, M. A. (2012). Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (’Triticum aestivum’cv. Hasaawi) at early growing stage. Aust. J. Crop Sci. 6:688. doi: 10.3390/agriculture12010050
Azuma, K., Nakayama, M., Koshioka, M., Ippoushi, K., Yamaguchi, Y., Kohata, K., et al. (1999). Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem. 47, 3963–3966.
Bashir, T., Naz, S., and Bano, A. (2020). Plant growth promoting rhizobacteria in combination with plant growth regulators attenuate the effect of drought stress. Pak. J. Bot. 52, 783–792. doi: 10.1021/jf990347p
Bates, L. S., Waldren, R. P., and Teare, I. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.30848/PJB2020-3(17)
Bewley, J. D., and Black, M. (2013). Seeds: Physiology of development and germination. Berlin: Springer Science & Business Media. doi: 10.1007/BF00018060
Bray, H., and Thorpe, W. (1954). Analysis of phenolic compounds of interest in metabolism. Methods Biochem. Anal. 1, 27–52. doi: 10.1007/978-1-4614-4693-4
Chen, C. N., and Pan, S. M. (1996). Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 37, 107–111. doi: 10.1002/9780470110171.ch2
Coolbear, P., Francis, A., and Grierson, D. (1984). The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot. 35, 1609–1617.
Core Team, R. (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. doi: 10.1093/jxb/35.11.1609
Dawood, M. G., and El-Awadi, M. E. (2015). Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biol. Colomb. 20, 223–235.
Dehghani, I., and Mostajeran, A. (2020). Does compatibility of wheat cultivars with Azospirillum brasilense strains affect drought tolerance? Cereal Res. Commun. 48, 121–129. doi: 10.15446/abc.v20n2.43291
Delshadi, S., Ebrahimi, M., and Shirmohammadi, E. (2017). Influence of plant-growth-promoting bacteria on germination, growth and nutrients’ uptake of Onobrychis sativa L. under drought stress. J. Plant Interact. 12, 200–208. doi: 10.1007/s42976-019-00001-3
Denčić, S., Kastori, R., Kobiljski, B., and Duggan, B. (2000). Evaluation of grain yield and its components in wheat cultivars and landraces under near optimal and drought conditions. Euphytica 113, 43–52. doi: 10.1080/17429145.2017.1316527
Dimkpa, C. O., Andrews, J., Sanabria, J., Bindraban, P. S., Singh, U., Elmer, W. H., et al. (2020). Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 722:137808. doi: 10.1023/A:1003997700865
Dionisio-Sese, M. L., and Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135, 1–9. doi: 10.1016/j.scitotenv.2020.137808
Dola, D. B., Mannan, M. A., Sarker, U., Mamun, M. A. A., Islam, T., Ercisli, S., et al. (2022). Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 13:992535. doi: 10.3389/fpls.2022.992535
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1016/S0168-9452(98)00025-9
Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 8:1147. doi: 10.1021/ac60111a017
Farooq, M., Irfan, M., Aziz, T., Ahmad, I., and Cheema, S. (2013). Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 199, 12–22. doi: 10.3389/fpls.2017.01147
Farooq, T. H., Rafay, M., Basit, H., Shakoor, A., Shabbir, R., Riaz, M. U., et al. (2022). Morpho-physiological growth performance and phytoremediation capabilities of selected xerophyte grass species toward Cr and Pb stress. Front. Plant Sci. 13:997120. doi: 10.3389/fpls.2022.997120
Faryal, S., Ullah, R., Khan, M. N., Ali, B., Hafeez, A., Jaremko, M., et al. (2022). Thiourea-capped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules 27:5744. doi: 10.3390/molecules27185744
Goharrizi, K. J., Baghizadeh, A., Kalantar, M., and Fatehi, F. (2020). Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci. Hortic. 261:108970. doi: 10.1111/j.1439-037X.2012.00521.x
Haider, M. W., Nafees, M., Ahmad, I., Ali, B., Maryam Iqbal, R., et al. (2022). Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 13:945256. doi: 10.3389/fpls.2022.945256
Hasanuzzaman, M., Nahar, K., Anee, T., Khan, M., and Fujita, M. (2018). Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 115, 50–57. doi: 10.1016/j.scienta.2019.108970
Hassan, A., Amjad, S. F., Saleem, M. H., Yasmin, H., Imran, M., Riaz, M., et al. (2021). Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 28, 4276–4290. doi: 10.1016/j.sajb.2017.12.006
Hassini, I., Martinez-Ballesta, M. C., Boughanmi, N., Moreno, D. A., and Carvajal, M. (2017). Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 226, 141–151. doi: 10.1016/j.sjbs.2021.03.045
Heath, R. L., and Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. doi: 10.1016/j.scienta.2017.08.030
Heile, A. O., Zaman, Q. U., Aslam, Z., Hussain, A., Aslam, M., Saleem, M. H., et al. (2021). Alleviation of Cadmium Phytotoxicity Using Silicon Fertilization in Wheat by Altering Antioxidant Metabolism and Osmotic Adjustment. Sustainability 13:11317. doi: 10.1016/0003-9861(68)90654-1
Hussain, S. Q., Rasheed, M., Saleem, M. H., Ahmed, Z. I., Hafeez, A., Jilani, G., et al. (2022). Salt tolerance in maize with melatonin priming to achieve sustainability in yield on salt affected soils. Pak. J. Bot. 55. doi: 10.30848/PJB2023-1(27)
Imran, M., Hussain, S., El-Esawi, M. A., Rana, M. S., Saleem, M. H., Riaz, M., et al. (2020a). Molybdenum Supply Alleviates the Cadmium Toxicity in Fragrant Rice by Modulating Oxidative Stress and Antioxidant Gene Expression. Biomolecules 10:1582. doi: 10.3390/su132011317
Imran, M., Sun, X., Hussain, S., Rana, M. S., Saleem, M. H., Riaz, M., et al. (2020b). Molybdenum supply increases root system growth of winter wheat by enhancing nitric oxide accumulation and expression of NRT genes. Plant Soil 459, 235–248. doi: 10.3390/biom10111582
Imran, M., Hussain, S., Rana, M. S., Saleem, M. H., Rasul, F., Ali, K. H., et al. (2021). Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol. Environ. Saf. 211:111911. doi: 10.1007/s11104-020-04765-0
Imran, M., Sun, X., Hussain, S., Ali, U., Rana, M. S., Rasul, F., et al. (2019). Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 20:3009. doi: 10.1016/j.ecoenv.2021.111911
Jana, S., and Choudhuri, M. A. (1981). Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 11, 67–77. doi: 10.3390/ijms20123009
Javed, M. T., Tanwir, K., Abbas, S., Saleem, M. H., Iqbal, R., and Chaudhary, H. J. (2021). Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ. Sci. Pollut. Res. 28, 27542–27554. doi: 10.1016/0304-3770(81)90047-4
Jiang, X., Li, H., and Song, X. (2016). Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 48, 1345–1352. doi: 10.1007/s11356-020-12269-8
Kamal, A., Saleem, M. H., Alshaya, H., Okla, M. K., Chaudhary, H. J., and Munis, M. F. H. (2022). Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalyticability against different organic and inorganic pollutants. J. Saudi Chem. Soc. 26:101445.
Kamran, M., Danish, M., Saleem, M. H., Malik, Z., Parveen, A., Abbasi, G. H., et al. (2020). Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 263:128169. doi: 10.1016/j.jscs.2022.101445
Kamran, M., Parveen, A., Ahmar, S., Malik, Z., Hussain, S., Chattha, M. S., et al. (2019). An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 21:148. doi: 10.1016/j.chemosphere.2020.128169
Khan, M. A., Adnan, M., Basir, A., Fahad, S., Hafeez, A., Saleem, M. H., et al. (2023). Impact of tillage and potassium levels and sources on growth, yield and yield attributes of wheat. Pak. J. Bot. 55. doi: 10.30848/PJB2023-1(30)
Khan, M. N., Zhang, J., Luo, T., Liu, J., Ni, F., Rizwan, M., et al. (2019a). Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 41:25. doi: 10.3390/ijms21010148
Khan, M. N., Zhang, J., Luo, T., Liu, J., Rizwan, M., Fahad, S., et al. (2019b). Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 140:111597. doi: 10.1007/s11738-019-2812-2
Kosar, F., Akram, N., and Ashraf, M. (2015). Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 96, 71–77. doi: 10.1016/j.indcrop.2019.111597
Lewis, C. E., Walker, J. R., Lancaster, J. E., and Sutton, K. H. (1998). Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J. Sci. Food Agric. 77, 45–57. doi: 10.1016/j.sajb.2014.10.015
Liang, B., Ma, C., Zhang, Z., Wei, Z., Gao, T., Zhao, Q., et al. (2018). Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 155, 650–661. doi: 10.1002/(SICI)1097-0010(199805)77:1<45::AID-JSFA1>3.0.CO;2-S
Lotter, D., Valentine, A. J., Van Garderen, E. A., and Tadross, M. (2014). Physiological responses of a fynbos legume, Aspalathus linearis to drought stress. S. Afr. J. Bot. 94, 218–223. doi: 10.1016/j.envexpbot.2018.08.016
Mahajan, S., and Tuteja, N. (2005). Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 444, 139–158. doi: 10.1016/j.sajb.2014.07.005
Mahmood, N., Arshad, M., Kaechele, H., Shahzad, M. F., Ullah, A., and Mueller, K. (2020). Fatalism, Climate Resiliency Training and Farmers’ Adaptation Responses: Implications for Sustainable Rainfed-Wheat Production in Pakistan. Sustainability 12:1650. doi: 10.1016/j.abb.2005.10.018
Mehmood, S., Khatoon, Z., Amna Ahmad, I., Muneer, M. A., Kamran, M. A., Ali, J., et al. (2021). Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021:1971654.
World Water Assessment Programme (2012). The United Nations world water development report 4, managing water under uncertainty and risk. Paris: World Water Assessment Programme. doi: 10.3390/su12041650
Ma, J., Saleem, M. H., Ali, B., Rasheed, R., Ashraf, M. A., Aziz, H., et al. (2022a). Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 13:950120. doi: 10.3389/fpls.2022.950120
Ma, J., Saleem, M. H., Yasin, G., Mumtaz, S., Qureshi, F. F., Ali, B., et al. (2022b). Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 13:973740. doi: 10.3389/fpls.2022.973740
Mathur, S., Tomar, R. S., and Jajoo, A. (2019). Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 139, 227–238. doi: 10.4324/9781849773355
Mfarrej, M. F. B., Wang, X., Fahid, M., Saleem, M. H., Alatawi, A., Ali, S., et al. (2022). Floating treatment wetlands (FTWs) is an innovative approach for the remediation of petroleum hydrocarbons-contaminated water. J. Plant Growth Regul. 1–19. doi: 10.1007/s00344-022-10674-6
Mohamed, I. A., Shalby, N., Ma El-Badri, A., Saleem, M. H., Khan, M. N., Nawaz, M. A., et al. (2020). Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants. Agron. 10:1186. doi: 10.1007/s00344-022-10674-6
Mumtaz, S., Saleem, M. H., Hameed, M., Batool, F., Parveen, A., Amjad, S. F., et al. (2021). Anatomical adaptations and ionic homeostasis in aquatic halophyte Cyperus laevigatus L. under high salinities. Saudi J. Biol. Sci. 28, 2655–2666. doi: 10.3390/agronomy10081186
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. doi: 10.1016/j.sjbs.2021.03.002
Naveed, M., Haleem, K. S., Ghazanfar, S., Tauseef, I., Bano, N., Adetunji, C. O., et al. (2022). Quantitative Estimation of Aflatoxin Level in Poultry Feed in Selected Poultry Farms. BioMed. Res. Int. 2022:5397561.
Nawaz, H., Ali, A., Saleem, M. H., Ameer, A., Hafeez, A., Alharbi, K., et al. (2022). Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 82:e261785.
Nawaz, M., Wang, X., Saleem, M. H., Khan, M. H. U., Afzal, J., Fiaz, S., et al. (2021). Deciphering Plantago ovata Forsk Leaf Extract Mediated Distinct Germination, Growth and Physio-Biochemical Improvements under Water Stress in Maize (Zea mays L.) at Early Growth Stage. Agronomy 11:1404. doi: 10.1155/2022/5397561
Nezhadahmadi, A., Prodhan, Z. H., and Faruq, G. (2013). Drought tolerance in wheat. Sci. World J. 2013:610721. doi: 10.3390/agronomy11071404
Nikolaeva, M., Maevskaya, S., Shugaev, A., and Bukhov, N. (2010). Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 57, 87–95. doi: 10.1155/2013/610721
Noman, A., Ali, Q., Maqsood, J., Iqbal, N., Javed, M. T., Rasool, N., et al. (2018). Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 195, 175–189. doi: 10.1134/S1021443710010127
Patel, M. K., Mishra, A., and Jha, B. (2016). Non-targeted metabolite profiling and scavenging activity unveil the nutraceutical potential of psyllium (Plantago ovata Forsk). Front. Plant Sci. 7:431. doi: 10.1016/j.chemosphere.2017.12.059
Pêkal, A., and Pyrzynska, K. (2014). Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 7, 1776–1782. doi: 10.3389/fpls.2016.00431
Rahimi, A., Hosseini, S. M., Pooryoosef, M., and Fateh, I. (2010). Variation of leaf water potential, relative water content and SPAD under gradual drought stress and stress recovery in two medicinal species of Plantago ovata and P. psyllium. Plant Ecophysiol. 2, 53–60. doi: 10.1007/s12161-014-9814-x
Rana, M., Bhantana, P., Sun, X. C., Imran, M., Shaaban, M., Moussa, M., et al. (2020). Molybdenum as an Essential Element for Crops: An Overview. Int. J. Sci. Res. Growth 24:18535.
Rehman, M., Liu, L., Wang, Q., Saleem, M. H., Bashir, S., Ullah, S., et al. (2019). Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 26:180016.
Rehman, M., Saleem, M. H., Fahad, S., Bashir, S., Peng, D., Deng, G., et al. (2021). Effects of rice straw biochar and nitrogen fertilizer on ramie (Boehmeria nivea L.) morpho-physiological traits, copper uptake and post-harvest soil characteristics, grown in an aged-copper contaminated soil. J. Plant Nutr. 45, 11–24. doi: 10.1007/s11356-019-05073-6
Rehman, M., Yang, M., Fahad, S., Saleem, M. H., Liu, L., Liu, F., et al. (2020). Morpho-physiological traits, antioxidant capacity and nitrogen metabolism in Boehmeria nivea L. under nitrogen fertilizer. Agron. J. 112, 2988–2997. doi: 10.1080/01904167.2021.1943675
Rivas, R., Falcão, H., Ribeiro, R., Machado, E., Pimentel, C., and Santos, M. (2016). Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 103, 101–107. doi: 10.1002/agj2.20212
Ruan, S., Xue, Q., and Tylkowska, K. (2002). The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci. Technol. 30, 61–67. doi: 10.1016/j.sajb.2015.08.008
Saeed, S., Ullah, A., Ullah, S., Noor, J., Ali, B., Khan, M. N., et al. (2022). Validating the impact of water potential and temperature on seed germination of wheat (Triticum aestivum L.) via hydrothermal time model. Life 12:983.
Saghir, S., Iqbal, M. S., Hussain, M. A., Koschella, A., and Heinze, T. (2008). Structure characterization and carboxymethylation of arabinoxylan isolated from Ispaghula (Plantago ovata) seed husk. Carbohydr. Polym. 74, 309–317.
Sakharov, I. Y., and Ardila, G. B. (1999). Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 65, 51–54. doi: 10.1016/j.carbpol.2008.02.019
Sakya, A., and Prahasto, D. (2020). “The application of phosphorus and potassium to increase drought tolerance in Pereskia bleo (Kunt) DC with proline and antioxidant indicators”, in: IOP Conference Series. Earth Environ. Sci. 423:012055. doi: 10.1016/S0308-8146(98)00160-5
Saleem, K., Asghar, M. A., Saleem, M. H., Raza, A., Kocsy, G., Iqbal, N., et al. (2022). Chrysotile-asbestos-induced damage in Panicum virgatum and Phleum pretense species and its alleviation by organic-soil amendment. Sustainability 14:10824. doi: 10.3390/su141710824
Saleem, K., Asghar, M. A., Saleem, M. H., Raza, A., Kocsy, G., Iqbal, N., et al. (2022c). Chrysotile-asbestos-induced damage in Panicum virgatum and Phleum pretense species and its alleviation by organic-soil amendment. Sustainability 14:10824. doi: 10.3390/su141710824
Saleem, M. H., Ali, S., Kamran, M., Iqbal, N., Azeem, M., Tariq Javed, M., et al. (2020b). Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 9:756. doi: 10.1088/1755-1315/423/1/012055
Saleem, M. H., Ali, S., Rehman, M., Rizwan, M., Kamran, M., Mohamed, I. A., et al. (2020d). Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innovat. 19:100895. doi: 10.3390/plants9060756
Saleem, M. H., Ali, S., Rehman, M., Hasanuzzaman, M., Rizwan, M., Irshad, S., et al. (2020c). Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 9:258. doi: 10.1016/j.eti.2020.100895
Saleem, M., Ali, S., Rehman, M., Rana, M., Rizwan, M., Kamran, M., et al. (2020a). Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 248:126032.
Saleem, M. H., Fahad, S., Khan, S. U., Ahmar, S., Khan, M. H. U., Rehman, M., et al. (2020e). Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 189:109915. doi: 10.3390/plants9020258
Saleem, M. H., Kamran, M., Zhou, Y., Parveen, A., Rehman, M., Ahmar, S., et al. (2020g). Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 257:109994. doi: 10.1016/j.chemosphere.2020.126032
Saleem, M. H., Fahad, S., Rehman, M., Saud, S., Jamal, Y., Khan, S., et al. (2020f). Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJ 8:e8321. doi: 10.1016/j.ecoenv.2019.109915
Saleem, M. H., Ali, S., Seleiman, M. F., Rizwan, M., Rehman, M., Akram, N. A., et al. (2019a). Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 8:545. doi: 10.1016/j.jenvman.2019.109994
Saleem, M. H., Rehman, M., Zahid, M., Imran, M., Xiang, W., and Liu, L. (2019b). Morphological changes and antioxidative capacity of jute (Corchorus Capsularis, Malvaceae) under different color light-emitting diodes. Braz. J. Bot. 42:590. doi: 10.7717/peerj.8321
Saleem, M. H., Wang, X., Ali, S., Zafar, S., Nawaz, M., Adnan, M., et al. (2021). Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiol. Biochem. 167, 884–900. doi: 10.1007/s00344-022-10681-7
Saud, S., Fahad, S., Yajun, C., Ihsan, M. Z., Hammad, H. M., Nasim, W., et al. (2017). Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky bluegrass plants. Front. Plant Sci. 8:983. doi: 10.3390/su14031462
Schmidthoffer, I., Szilák, L., Molnár, P., Csontos, P., and Skribanek, A. (2018). Drought tolerance of European barley (Hordeum vulgare L.) varieties. Agriculture 64, 137–142. doi: 10.1016/j.plaphy.2021.09.015
Schneider, C. A., Rasband, W. S., and Eliceiri, K. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.3389/fpls.2017.00983
Sharma, A., Wang, J., Xu, D., Tao, S., Chong, S., Yan, D., et al. (2020). Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 713:136675. doi: 10.2478/agri-2018-0014
Singh, D., Dhillon, T. S., Javed, T., Singh, R., Dobaria, J., Dhankhar, S. K., et al. (2022). Exploring the genetic diversity of carrot genotypes through phenotypically and genetically detailed germplasm collection. Agronomy 12:1921. doi: 10.3390/agronomy12081921
Sufyan, M., Ashfaq, U. A., Ahmad, S., Noor, F., Saleem, M. H., Aslam, M. F., et al. (2021). Identifying key genes and screening therapeutic agents associated with diabetes mellitus and HCV-related hepatocellular carcinoma by bioinformatics analysis. Saudi J. Biol. Sci. 28, 5518–5525. doi: 10.1038/nmeth.2089
Taha, R., Alharby, H., Bamagoos, A., Medani, R., and Rady, M. (2020). Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 128, 42–53. doi: 10.1016/j.scitotenv.2020.136675
Talukder, P., Talapatra, S., Ghoshal, N., and Raychaudhuri, S. (2015). Antioxidant activity and HPLC analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk and effect of exogenous additives on accumulation of phenolic compounds. J. Sci. Food Agric. 96, 232–244. doi: 10.1016/j.sjbs.2021.07.068
Tlili, H., Marino, A., Ginestra, G., Cacciola, F., Mondello, L., Miceli, N., et al. (2019). Polyphenolic profile, antibacterial activity and brine shrimp toxicity of leaf extracts from six Tunisian spontaneous species. Nat. Prod. Res. 35, 1057–1063. doi: 10.1016/j.sajb.2019.09.014
Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M., and Skz, A. (2016). Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184, 13–24. doi: 10.1002/jsfa.7086
Wahab, A., Abdi, G., Saleem, M. H., Ali, B., Ullah, S., Shah, W., et al. (2022). Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 11:1620. doi: 10.1080/14786419.2019.1616725
Waraich, E. A., Ahmad, R., and Ashraf, M. (2011). Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 5:764. doi: 10.1016/j.micres.2015.12.003
Wiesner, L. (1990). Rules for testing seeds. Washington, DC: Association of Official Seed Analysts. doi: 10.3390/plants11131620
Wu, W., Ma, B. L., and Whalen, J. K. (2018). “Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: Perspectives for stress adaptation from root system architecture,” in Advances in Agronomy, ed. D. Sparks (Amsterdam: Elsevier), 87–157.
Yaseen, R., Aziz, O., Saleem, M. H., Riaz, M., Zafar-Ul-Hye, M., Rehman, M., et al. (2020). Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability 12:6022.
Zaheer, I. E., Ali, S., Saleem, M. H., Ali, M., Riaz, M., Javed, S., et al. (2020a). Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol. Mol. Biol. Plants 26, 2435–2452. doi: 10.1016/bs.agron.2018.05.002
Zaheer, I. E., Ali, S., Saleem, M. H., Imran, M., Alnusairi, G. S. H., Alharbi, B. M., et al. (2020b). Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 155, 70–84. doi: 10.3390/su12156022
Zainab, N., Amna Khan, A. A., Azeem, M. A., Ali, B., Wang, T., et al. (2021). PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 11:1820. doi: 10.3390/agronomy11091820
Zulkiffal, M., Ahsan, A., Ahmed, J., Ur Rehman, A., Abdulah, M., and Gulnaz, S. (2018). Appraisal of bread wheat (Triticum aestivum l.). Genotypes under normal, drought and heat prone environments for morpho-physiological multiplicity and constancy. Int. J. Agric. Environ. Res. 4, 1298–1306. doi: 10.1007/s12298-020-00912-0
Keywords: agronomic crop, antioxidant compounds, isabgol extract, nutrient uptake, oxidative stress, water deficit stress
Citation: Alharbi K, Alhaithloul HAS, Alayafi AAM, Al-Taisan WA, Alghanem SM, Al-Mushhin AAM, Soliman MH, Alsubeie MS, Vodnar DC and Marc RA (2022) Impact of Plantago ovata Forsk leaf extract on morpho-physio-biochemical attributes, ions uptake and drought resistance of wheat (Triticum aestivum L.) seedlings. Front. Plant Sci. 13:999170. doi: 10.3389/fpls.2022.999170
Received: 20 July 2022; Accepted: 23 August 2022;
Published: 20 September 2022.
Edited by:
Iftikhar Ali, Institute of Genetics and Developmental Biology (CAS), ChinaReviewed by:
Noshin Ilyas, Pir Mehr Ali Shah Arid Agriculture University, PakistanManar Bani Mfarrej, Zayed University, United Arab Emirates
Javaria Afzal, Huazhong Agricultural University, China
Copyright © 2022 Alharbi, Alhaithloul, Alayafi, Al-Taisan, Alghanem, Al-Mushhin, Soliman, Alsubeie, Vodnar and Marc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mona H. Soliman, aG1vbmFAc2NpLmN1LmVkdS5lZw==; Dan C. Vodnar, ZGFuLnZvZG5hckB1c2FtdmNsdWoucm8=; Romina Alina Marc, cm9taW5hLnZsYWljQHVzYW12Y2x1ai5ybw==
†ORCID: Haifa Abdulaziz Sakit Alhaithloul, orcid.org/0000-0003-4153-0262; Mona H. Soliman, orcid.org/0000-0002-9102-4790
 Khadiga Alharbi1
Khadiga Alharbi1 Romina Alina Marc
Romina Alina Marc