- 1Institute of Plant Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China
- 2College of Agriculture and Forestry Technology, Hebei North University, Zhangjiakou, China
- 3College of Plant Protection, Shanxi Agricultural University, Taigu, China
Acylsugars are secondary metabolites that are produced in the trichomes of some solanaceous species and can help control several herbivorous insect pests. Previously, knockout mutations (asat2 mutants) were shown to significantly reduce the acylsugar content of Nicotiana benthamiana, and significantly improve the fitness of six generalist insect herbivores. The current study compared the significant mortality and fitness costs in Spodoptera litura conferred by acylsugar protection of N. benthamiana (wild-type plants) compared to S. litura strains reared in acylsugar-deficient plants with depleted acylsugar biosynthesis. Acylsugar protection prolonged the developmental duration and decreased viability in the larval stages. Further, the fecundity of females and the hatching rate of eggs significantly decreased under acylsugar protection. For F1 offspring, acylsugar protection still exerted significant negative effects on larval survival rate and fecundity per female. The net reproductive rate and relative fitness of the S. litura strain were strongly affected by acylsugar. Altogether, these results indicate that acylsugar could contribute to plant protection due to toxicity to pests, diffused availability, and low environmental persistence. This could represent a complementary and alternative strategy to control populations of insect pests.
Introduction
Plants generate molecules with low-molecular mass that are considered secondary metabolites, and they show various mechanisms of defense against different herbivores (Schuman and Baldwin, 2016). In addition to physical characteristics such as low digestibility, spines, and leaf toughness, it has been reported that, in plants, many published metabolites could be used to control insect pests (Bérdy, 2005). However, many insect pests display the ability to resist the defensive traits from metabolites in their preferred species of plants which could be against by more sporadically distributed chemical defenses. For example, the extensively investigated Brassicaceae provides outstanding instances of plants that generate extra chemical defenses beyond the canonical glucosinolates characteristic of this plant family (Fahey et al., 2001). Two lineages of Barbarea vulgaris, glabrous (G-type) and pubescent (P-type), display different content of triterpenoid saponins, and show distinct levels of resistance against Plutella xylostella (Agerbirk et al., 2003). Erysimum contains cardiac glycosides which negatively affect feeding behavior and oviposition of Pieris rapae (Sachdev-Gupta et al., 1990, 1993). Other cases of chemical defenses, such as cucurbitacins in Iberis umbellate, alliarinoside in Alliaria petiolate, and tropane alkaloids in Cochlearia officinalis have been demonstrated previously (Nielsen et al., 1977; Haribal et al., 2001; Brock et al., 2006). These kinds of deterrent or toxic metabolites from various plants can be utilized to enhance resistance to insect pests in crops if reasonable and rational strategies are established with current biotechnologies (Zhou and Jander, 2021).
Acylsugars are insect-deterrent metabolites generated by the family Solanaceae, and are produced and exuded from glandular trichomes of the plants (Goffreda et al., 1988, 1989; Wagner, 1991), resulting in significant negative effects like antibiosis or insect-repellent on various tomato herbivores (Hawthorne et al., 1992; Rodriguez et al., 1993; Juvik et al., 1994; Leckie et al., 2012; Ben-Mahmoud et al., 2018). Similarly, although Nicotiana benthamiana has been extensively utilized in the study of plant–microbe interactions (Goodin et al., 2008; Bally et al., 2018), it may not be the most appropriate host plant for studying herbivore–plant interactions (Hagimori et al., 1993; Simón et al., 2003) and the undesirable performance of herbivores on N. benthamiana could be partially ascribed to acylsugars (Feng et al., 2021). Specifically, the Nicotiana species showing resistance to aphids contained acylsugars, yet acylsugars cannot be measured in the more susceptible species of the genus (Hagimori et al., 1993). Similarly, compared with Solanum lycopersicum, the cultivated tomatoes, acylsugars could be detected in the wild tomato species S. pennellii, which displayed higher resistance to the pest species Bemisia tabaci and Myzus persicae (Rodriguez et al., 1993; Marchant et al., 2020). Recently, Feng et al. (2021) reported that changed profiles of acylsugar could reduce levels of resistance to six insect pests such as B. tabaci, M. persicae, Macrosiphum euphorbiae, Trichoplusia ni, Heliothis virescens, and Helicoverpa zea. This type of plant resistance to herbivore pests could be strengthened via bioengineering to enhance amounts of defensive metabolites, alter available biochemical pathways, or transfer the biosynthesis of novel types of defensive metabolites into target plants. Nevertheless, present strategies of bioengineering are limited owing to several factors, such as inadequate references for the biosynthetic pathways of plant metabolites, unexpected byproducts originating from plant metabolites, and demands for the spatial specificity of metabolite production to increase resistance to insect pests.
Spodoptera litura (Fabricius), the tobacco cutworm, is one notorious polyphagous and destructive herbivore pest that feeds on various economic and horticultural crops, including cotton, soybeans, tobacco, tomatoes, and peanuts. The extensive range of host plants suggests that S. litura could neutralize the traits of resistance of different plants (Shi et al., 2022), and some specific secondary metabolites of the plants significantly inhibit the growth of S. litura in the larval stages (Kundu et al., 2018). Because the application of chemical agents has been the primary step against S. litura for the most recent few decades, an increasing number of studies has indicated that several field-collected S. litura populations have evolved significant levels of resistance to a variety of chemical agents such as carbamate, organophosphate, chlorantraniliprole, pyrethroids, abamectin, indoxacarb, and emamectin benzoate, and the wide application of these chemical agents is no longer a suitable strategy for environment-friendly plant protection (Tong et al., 2013; Saleem et al., 2016; Wang et al., 2018; Xu et al., 2020). Considering that N. benthamiana acylsugars showed defensive effects of metabolites against lepidopteran pests, it may be possible to enhance resistance of plant by transgenic methods of transferring biosynthetic pathways (Feng et al., 2021). Typically, establishment of the life-table has been shown as one important method for evaluating and understanding the effects of exogenous elements on the individual and the entire population of insect pests. The analysis of the life-table could be used for precisely estimating the growth rate of the population and the fitness costs, and on this basis, strategies of pest management could be formulated more reasonably (Kliot and Ghanim, 2012). In the present work, mortality and fitness costs in a lab-reared population of S. litura with acylsugar protection of N. benthamiana were systematically examined, and the results indicated the plant chemical defenses conferred by acylsugar, and these results can supply important data for using acylsugar for controlling pests via chemical plant defenses in the field.
Materials and methods
Insects and plants
The reference strain of S. litura, Lab-S strain, was used in this study and was reared on an artificial diet in one insect-rearing room without exposure to chemical agents for over 5 years (Zhang et al., 2022). The wild-type (WT) and the acylsugar-deficient asat2-1 line (ASAT2) plants of N. benthamiana were obtained from the Boyce Thompson Institute, Ithaca, New York, USA, and the ASAT2 plants showed an almost complete absence of acylsugar compared to the WT plants (Feng et al., 2021). All plants of WT and the ASAT2 mutant of N. benthamiana were reared at 23°C and a 16:8 h light:dark photoperiod in a well-controlled chamber. All bioassays and fitness cost evaluation work were performed at 26°C under a 16:8 h light:dark photoperiod in a well-controlled growth chamber.
Bioassays
The lethal activity of acylsugar toward various stages of larvae was examined by bioassays. S. litura eggs were maintained on an artificial diet, and five larval stages (the 2nd, 3rd, 4th, 5th, and 6th stages), were measured. For each tested instar of larvae, one hundred 12-h-old larvae were selected and fed with the leaves of WT or ASAT2 plants. Ten larvae were placed on one WT or ASAT2 plant as one tested group, and 10 of the tested groups were set as replicas for each bioassay. The immobile larvae in each stage were considered as dead, and the number of larvae that survived was recorded after 48 h. Comparisons were made between the WT and ASAT2 using the Student's t-test.
Defensive effects of acylsugar on S. litura of F0
This study evaluated the defensive effects of acylsugar on second-instar larvae of S. litura. Six hundred one-day-old second-instar larvae were randomly collected, and 300 of them were fed with N. benthamiana leaves of WT plants, while the other 300 were fed with N. benthamiana leaves of ASAT2 plants. The total number of deformed pupae was counted, and, within 24 h, all healthy pupae were weighed, and the rate of pupation was recorded. After the adults emerged, 15 pairs of female and male adults were coupled in the first 12-h after emergence, and each couple was placed into one plastic cup (3-cm diameter and 5-cm height). Each of the tested couples was introduced into new plastic cups daily, and the fecundity of each female, oviposition, and egg hatching rate was recorded every day. Comparisons were made between the plants of WT and ASAT2 using the Student's t-test.
Transgenerational defensive effects of acylsugar on F1 offspring
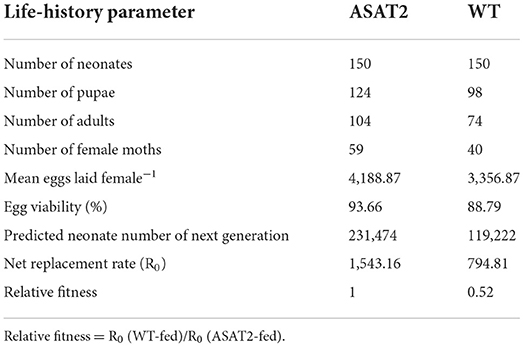
To determine whether acylsugar exerts transgenerational defensive effects on the F1 population, the egg hatching rate was assessed by sampling 20 egg masses (more than 250 eggs per mass) on the fourth day of the oviposition duration for F0 females, which were fed on acylsugar (the WT plants) or acylsugar-depleted (the ASAT2 plants) from the second larval instar. Further, 100 collections from four masses of eggs (20–30 eggs from each mass) were utilized to establish the life table for each tested population of S. litura. Neonates of the F1 generation were transferred individually into one plastic tube and fed with artificial diet in the tube. The developmental time of larval-instar stages and survival rates were checked daily, and pupation rate, duration of pupae, the longevity of adults, and emergence rate were recorded every day. Newly emerged males and females of the F1 generation were coupled and put into one plastic cup for oviposition. The fecundity of females, oviposition duration of females, and hatchability of the eggs were checked daily. Comparisons were made between the WT and ASAT2 using the Student's t-test. Net reproductive rate (R0) and the relative fitness were evaluated according to a previously published method (Wang and Wu, 2014).
Results
Toxicity of acylsugar on different instar larvae in S. litura
To confirm if the depletion of acylsugar in the ASAT2 mutants enhances the adaptability of S. litura on Nicotiana benthamiana, we performed bioassays with the 2nd, 3rd, 4th, 5th, and 6th instars of S. litura. When each of the specific instar larvae was put onto the leaves of the ASAT2 mutant or wildtype (WT), survival rates of S. litura on WT plants were significantly lower compared to their counterparts reared on the ASAT2 plants (Figure 1). The 2nd instar larvae of S. litura on WT plants had the lowest survival rate, ~53%, while the survival rate of 2nd instar larvae on ASAT2 plants was ~96% (Figure 1). For other stages of larvae in the bioassays, survival rates of S. litura on WT plants decreased more significantly than on ASAT2 plants (Figure 1).
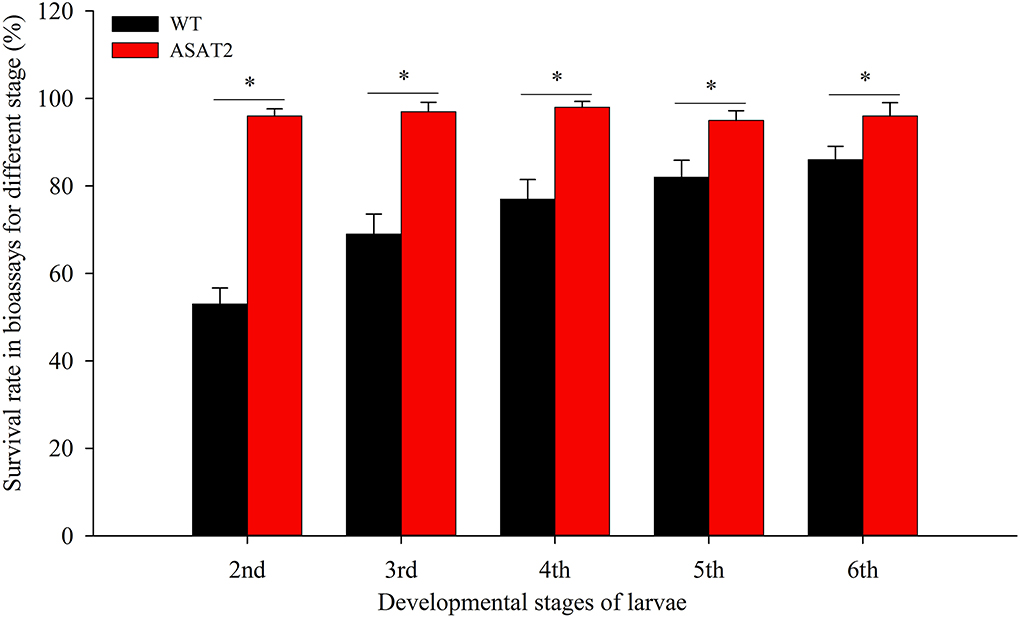
Figure 1. Survival rate of bioassays using specific stages of larval instars on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05).
Effect of acylsugar on larvae and adults of S. litura F0 generation
Biological components including survival rate and developmental time, larval, and pupal weight, the fecundity of females, duration of oviposition, and egg hatching rate for the F0 generation grown from 2nd instar larvae fed with or without acylsugar were studied. Compared to those fed on ASAT2 plants, the survival rate of the second- to sixth instar larvae from the F0 group fed with WT significantly decreased in each stage (Figure 2A), and their weight significantly decreased in each stage from second instar larvae to pupae (Figure 2B). In comparison with the ASAT2 group, the development time of second- to sixth instar larvae of F0 fed with WT was significantly prolonged by 2.2 days (Figure 3A). However, pupal duration and female and male longevity were not significantly different between those reared on WT and ASAT2 plants (Figures 3A,B). Further, compared to the mean fecundity of ASAT2-fed females (3,815.53 eggs per female), WT-fed females displayed significantly reduced fecundity, with 2,565.93 eggs per female (Figure 4A). Similarly, a significant decrease in the egg hatch rate of WT-fed females (79.58%) was observed compared with ASAT2-fed females (94.20%; Figure 4C). However, there was no detectable difference in the duration of oviposition between the two populations (Figure 4B).
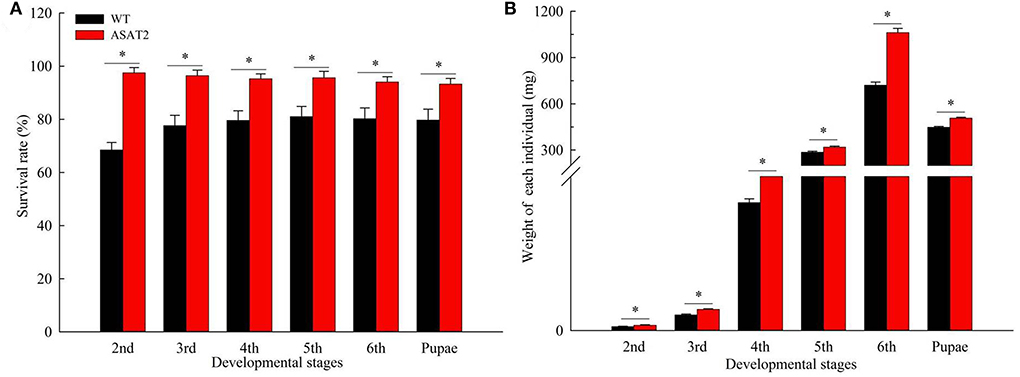
Figure 2. Survival rate (A) and weight of individual (B) in each larval stage of the F0 generation on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05).
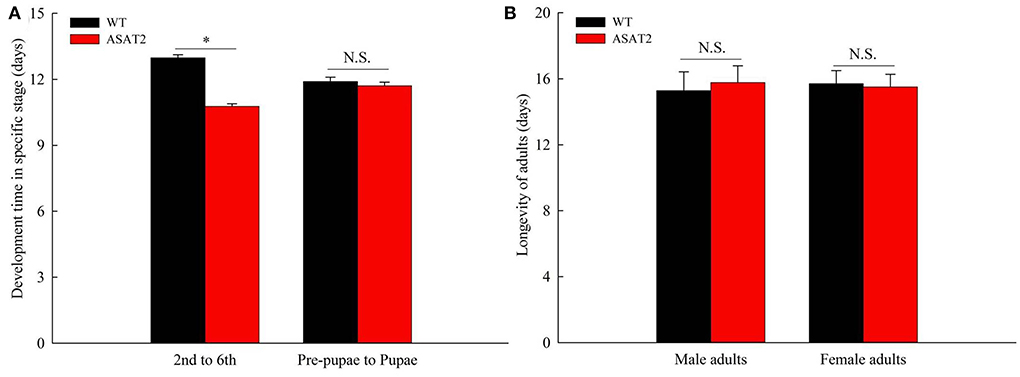
Figure 3. Development time (A) and longevity of adults (B) of the F0 generation on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05), and n.s. indicates not significant (P > 0.05).
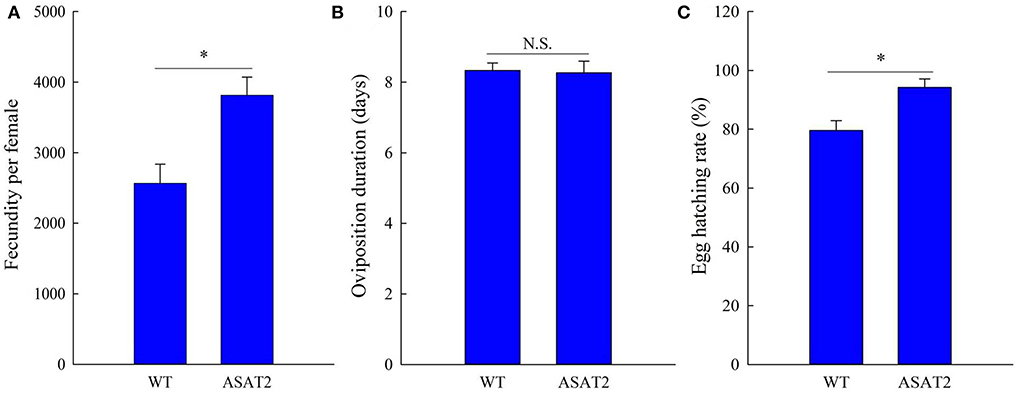
Figure 4. Fecundity (A), oviposition duration (B), and egg hatching rate (C) of the F0 generation of S. litura on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05), and n.s. indicates not significant (P > 0.05).
Transgenerational defensive effects of acylsugar on the F1 generation
No significant defensive effects of acylsugar on the period of various stages of life were detected between ASAT2-fed and the WT-fed group (Figure 5A). In addition, the pupation and emergence rate did not significantly differ between the two groups (Figure 5B). However, in comparison with the ASAT2-fed group, the larval survival of the WT-fed plant group significantly decreased (Figure 5B). Further, a significant difference in eggs laid per female of F1 was observed between the ASAT2-fed (4,188.87 ± 267.29) and WT-fed groups (3,356.87 ± 207.54; Figure 6A). On the contrary, no significant difference was observed in other reproduction parameters, such as oviposition duration (Figure 6B) and hatchability of the eggs (Figure 6C). All fitness parameters of F1 offspring are displayed in Table 1. Relative to the net replacement rate (R0) of the ASAT2-fed group, the fitness of the WT-fed group was 0.51 (Table 1).
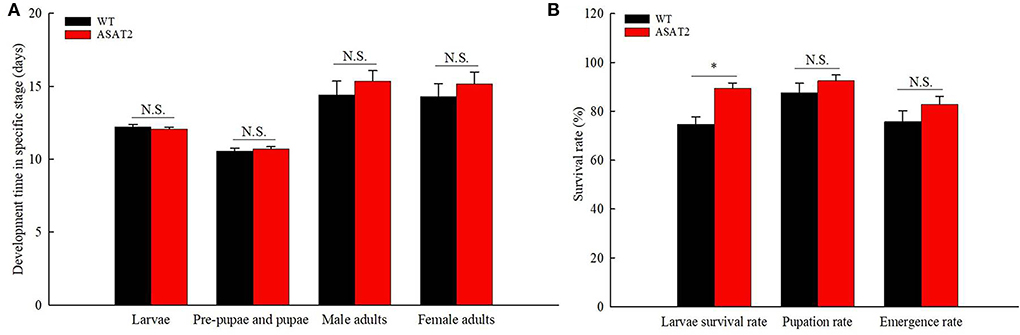
Figure 5. Development time (A) and survival rate (B) of the F1 generation on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05), and n.s. indicates not significant (P > 0.05).
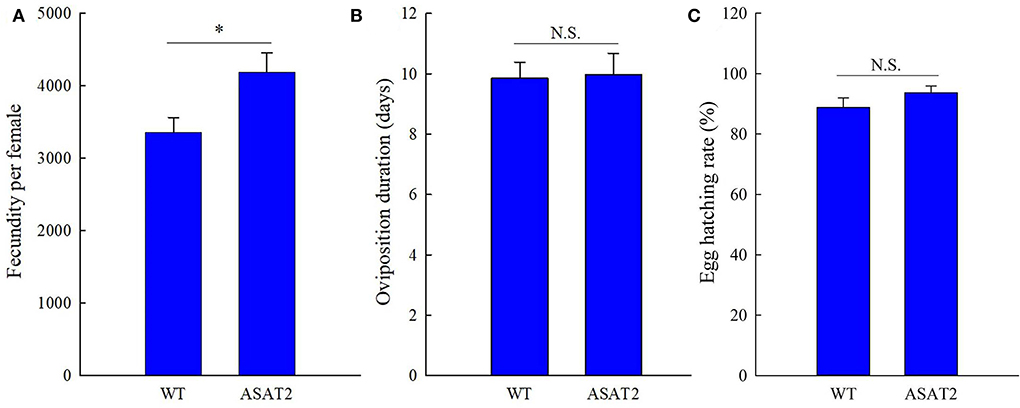
Figure 6. Fecundity (A), oviposition duration (B), and egg hatching rate (C) of the F1 generation of S. litura on WT and ASAT2 plants of N. benthamiana. Values are presented as means ± SE. Asterisks above error bars indicate significant differences (P < 0.05), and n.s. indicates not significant (P > 0.05).
Discussion
Acylsugars exuded by glandular trichomes are considered powerful natural pesticides (Puterka et al., 2003), and can directly kill some species of insect pests (Feng et al., 2021). In this study, we found that although larvae of S. litura grow well on the ASAT2 mutant line of N. benthamiana, significant insecticidal effects of acylsugar against larvae of S. litura were observed in the WT line of N. benthamiana. In particular, a 50% lethality effect was detected for the 2nd instar larvae. Similarly, it has been reported that knockout of acylsugar biosynthesis conferred a significantly higher survival rate for M. persicae and B. tabaci on the ASAT2 mutant line compared with their high mortality in wildtype N. benthamiana (Feng et al., 2021). Considering that acylsugars are defensive metabolites generated by various Solanaceae species, in which they provide deterrence against a large range of herbivores, acylsugar-associated herbivore resistance has huge promise against insect pests of tomato such as whiteflies, thrips, and aphids (Goffreda et al., 1988, 1989; Hawthorne et al., 1992; Rodriguez et al., 1993; Juvik et al., 1994; Liedl et al., 1995; Leckie et al., 2012).
Further, acylsugars can negatively affect the fitness of various insect pests by interfering with behaviors such as feeding and oviposition and have detrimental effects on their growth (Simmons et al., 2003; Resende et al., 2006). To investigate the underlying ecological effects of acylsugar on insect pests, we conducted systematic work on the defensive effects on S. litura. We observed that acylsugar shows insecticidal effects against S. litura larvae from the 2nd to the 6th stage, and it was previously observed that there was a high death rate of sucking insect pests such as B. tabaci and M. persicae on wild-type plants of N. benthamiana (Feng et al., 2021). In the acylsugar-fed group, S. litura larvae showed decreased body weight in each larval and pupal stage. They also displayed significant prolongation of the larval period, suggesting that acylsugar not only acts against larvae directly but also suppresses their development. More importantly, fecundity of females and egg hatching rate of the S. litura F1 generation were significantly affected by acylsugar. Similarly, these effects were also observed in Tetranychus urticae and Frankliniella occidentalis (Lucini et al., 2015; Ben-Mahmoud et al., 2019). It has also been reported that acylsugar could interfere with the oviposition and feeding of M. persicae and Tuta absoluta, and have detrimental effects on their growth (Simmons et al., 2003; Resende et al., 2006).
In addition to reducing the fitness of S. litura during the F0 generation, the transgenerational effects of acylsugar were detected. Here, we found that in the F1 generation of the WT-fed group, the larval survival rate and female fecundity were still significantly suppressed, even though the F1 generation of S. litura was reared on an artificial diet from hatching. A variety of studies have suggested that various chemical agents can affect insect pests by damaging their behavioral or physiological characteristics including longevity, duration of growth, host locating, feeding ability, and fecundity (Desneux et al., 2006; Biondi et al., 2013; Wang et al., 2016, 2017; Qu et al., 2017; Fang et al., 2018; Jam and Saber, 2018; Zhou et al., 2021). Most of these effects could also be transgenerational, indirectly affecting their offspring (Cui et al., 2018), and they could cause alterations in communities and ecosystems (Lu et al., 2012; Mohammed et al., 2019). Thus, the transgenerational effects induced by acylsugar might be contributed to delaying the outbreak of acylsugar in a short term. Recently, biopesticides (natural products) have emerged as a better alternative for pest control (Mostafiz et al., 2020), and acylsugars, one of the products of glandular trichomes that secrete secondary metabolites, could be repellent, toxic, and disturb oviposition and feeding of insect pests. They are involved in tritrophic interactions in plant defenses by tagging herbivores for predation through breaking down volatile acylsugar products (Weinhold and Baldwin, 2011) and efficiently protecting plants from attacks from microbes (Luu et al., 2017). In tomato plants, breeding measures have attempted to control the composition and content of acylsugar for increasing resistance to herbivores, and more enhanced breeding lines have been generated (Leckie et al., 2012, 2013, 2014; Smeda et al., 2016). Accordingly, acylsugars can provide an alternative to synthetic insecticides for the future environmentally-friendly control of insect pests.
In recent years, novel advances in ecotoxicology have been impacting the assessment of xenobiotic effects (Godfray, 1993; Sedaratian et al., 2013). Demography has been considered as one approach for evaluating the overall effects of xenobiotics because it can illustrate all the impacts of a xenobiotic on a population of insect pests (Hamedi et al., 2010). In addition, combining demography with biological parameters could better predict the impacts of xenobiotics at the population level. Fitness cost is considered as one essential biological component that must be assessed when formulating xenobiotics pest management strategies. The fitness cost can be observed when organisms face niche alteration and must adapt to novel surroundings (Kliot and Ghanim, 2012). In the present study, compared with the ASAT2-fed group, significant the fitness costs resulting from acylsugar displayed a fitness value of 0.52 in the WT-fed group. It has been shown that the more significant fitness cost, the longer it takes for insect pests to develop their populations, which is one vital element of the Integrated Pest Management (IPM) program (Kliot and Ghanim, 2012). Therefore, an overall understanding of fitness costs associated with defensive metabolites of plants could contribute to the design of more effective strategies for pest management against herbivore pests.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RW, BG, and CL conceived and designed the study. RW, BG, QZ, and ZZ performed the experiments and analyzed the data with the help of YL, QY, MZ, and WL. RW wrote the first draft of the manuscript. RW, BG, QZ, and CL participated in manuscript drafting and modification. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the China Agriculture Research System of MOF and MARA and the Scientific and Technological Innovation Capacity Construction Special Funds of the Beijing Academy of Agriculture and Forestry Sciences, Beijing, China (KJCX20210437).
Acknowledgments
The authors would like to thank Prof. Georg Jander from Boyce Thompson Institute for providing the seeds of the wild-type (WT) and the acylsugar-deficient (ASAT2) plants of N. benthamiana in the whole work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agerbirk, N., Olsen, C. E., Bibby, B. M., Frandsen, H. O., Brown, L. D., Nielsen, J. K., et al. (2003). A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J. Chem. Ecol. 29, 1417–1433. doi: 10.1023/A:1024217504445
Bally, J., Jung, H., Mortimer, C., Naim, F., Philips, J. G., Hellens, R., et al. (2018). The rise and rise of Nicotiana benthamiana: a plant for all reasons. Ann. Rev. Phytopathol. 56, 405–426. doi: 10.1146/annurev-phyto-080417-050141
Ben-Mahmoud, S., Anderson, T., Chappell, T. M., Smeda, J. R., Mutschler, M. A., Kennedy, G. G., et al. (2019). A thrips vector of tomato spotted wilt virus responds to tomato acylsugar chemical diversity with reduced oviposition and virus inoculation. Sci. Rep. 9, 17157. doi: 10.1038/s41598-019-53473-y
Ben-Mahmoud, S., Smeda, J. R., Chappell, T. M., Stafford-Banks, C., Kaplinsky, C. H., Anderson, T., et al. (2018). Acylsugar amount and fatty acid profile differentially suppress oviposition by western flower thrips, Frankliniella occidentalis, on tomato and interspecific hybrid flowers. PLoS ONE 13, e0201583. doi: 10.1371/journal.pone.0201583
Biondi, A., Zappal,à, L., Stark, J. D., and Desneux, N. (2013). Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 8, e76548. doi: 10.1371/journal.pone.0076548
Brock, A., Herzfeld, T., Paschke, R., Koch, M., and Dräger, B. (2006). Brassicaceae contain nortropane alkaloids. Phytochemistry 67, 2050–2057. doi: 10.1016/j.phytochem.2006.06.024
Cui, L., Yuan, H., Wang, Q., Wang, Q., and Rui, C. (2018). Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii glover (Hemiptera: Aphididae). Sci. Rep. 8, 8915. doi: 10.1038/s41598-018-27035-7
Desneux, N., Ramirez-Romero, R., and Kaiser, L. (2006). Multi-step bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 25, 2675–2682. doi: 10.1897/05-562R.1
Fahey, J. W., Zalcmann, A. T., and Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56, 5–51. doi: 10.1016/S0031-9422(00)00316-2
Fang, Y., Wang, J., Luo, C., and Wang, R. (2018). Lethal and sublethal effects of clothianidin on the development and reproduction of Bemisia tabaci (Hemiptera: Aleyrodidae) MED and MEAM1. J. Insect Sci. 18, 37. doi: 10.1093/jisesa/iey025
Feng, H., Acosta-Gamboa, L., Kruse, L. H., Tracy, J. D., Chung, S. H., Fereira, A. R. N., et al. (2021). Acylsugars protect Nicotiana benthamiana against insect herbivory and desiccation. Plant Mol. Biol. 109, 505–522. doi: 10.1007/s11103-021-01191-3
Godfray, H. C. J. (1993). Applied Demography for Biologists, with Special Emphasis on Insects, ed J. R. Carey (New York, NY: Oxford University Press), 4. doi: 10.1016/0169-5347(94)90043-4
Goffreda, J. C., Mutschler, M. A., Av,é, D. A., Tingey, W. M., and Steffens, J. C. (1989). Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomato, Lycopersicon pennellii. J. Chem. Ecol. 15, 2135–2147, doi: 10.1007/BF01207444
Goffreda, J. C., Mutschler, M. A., and Tingey, W. M. (1988). Feeding behavior of potato aphid affected by glandular trichomes of wild tomato. Entomol. Exp. Appl. 48, 101–107. doi: 10.1111/j.1570-7458.1988.tb01152.x
Goodin, M. M., Zaitlin, D., Naidu, R. A., and Lommel, S. A. (2008). Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol. Plant Microbe Interact. 21, 1015–1026. doi: 10.1094/MPMI-21-8-1015
Hagimori, M., Matsui, M., Matsuzaki, T., Shinozaki, Y., Shinoda, T., and Harada, H. (1993). Production of somatic hybrids between Nicotiana benthamiana and Nicotiana tabacum and their resistance to aphids. Plant Sci. 91, 213–222. doi: 10.1016/0168-9452(93)90144-O
Hamedi, N., Fathipour, Y., and Saber, M. (2010). Sublethal effects of fenpyroximate on life table parameters of the predatory mite Phytoseius plumifer. BioControl 55, 271–278. doi: 10.1007/s10526-009-9239-4
Haribal, M., Yang, Z., Attygalle, A. B., Renwick, J. A., and Meinwald, J. (2001). A cyanoallyl glucoside from Alliaria petiolata, as a feeding deterrent for larvae of Pieris napi oleracea. J. Nat. Prod. 64, 440–443. doi: 10.1021/np000534d
Hawthorne, D. J., Shapiro, J. A., Tingey, W. M., and Mutschler, M. A. (1992). Trichome-borne and artificially applied acylsugars of wild tomato deter feeding and oviposition of the leafminer Liriomyza trifolii. Entomol. Exp. Appl. 65, 65–73. doi: 10.1111/j.1570-7458.1992.tb01628.x
Jam, N. A., and Saber, M. (2018). Sublethal effects of imidacloprid and pymetrozine on the functional response of the aphid parasitoid, Lysiphlebus fabarum. Entomol. Gen. 38, 173–190. doi: 10.1127/entomologia/2018/0734
Juvik, J. A., Shapiro, J. A., Young, T. E., and Mutschler, M. A. (1994). Acylglucoses from wild tomatoes alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 87, 482-492. doi: 10.1093/jee/87.2.482
Kliot, A., and Ghanim, M. (2012). Fitness costs associated with insecticide resistance. Pest Manag. Sci. 68, 1431–1437. doi: 10.1002/ps.3395
Kundu, A., Mishra, S., and Vadassery, J. (2018). Spodoptera litura mediated chemical defense is differentially modulated in older and younger systemic leaves of Solanum lycopersicum. Planta 248, 981–997. doi: 10.1007/s00425-018-2953-3
Leckie, B., Halitschke, R., De Jong, D., Smeda, J., Kessler, A., and Mutschler, M. (2014). Quantitative trait loci regulating the fatty acid profile of acylsugars in tomato. Mol. Breed. 2014, 34, 1201–1213. doi: 10.1007/s11032-014-0110-7
Leckie, B. M., De Jong, D. M., and Mutschler, M. A. (2012). Quantitative trait loci increasing acylsugars in tomato breeding lines and their impacts on silverleaf whiteflies. Mol. Breed. 30, 1621–1634. doi: 10.1007/s11032-012-9746-3
Leckie, B. M., De Jong, D. M., and Mutschler, M. A. (2013). Quantitative trait loci regulating sugar moiety of acylsugars in tomato. Mol. Breed. 31, 957–970. doi: 10.1007/s11032-013-9849-5
Liedl, B. E., Lawson, D. M., White, K. K., Shapiro, J. A., Cohen, D. E., Carson, W. G., et al. (1995). Acylsugars of wild tomato Lycopersicon pennellii alters settling and reduces oviposition of Bemisia argentifolii (Homoptera: Aleyrodidae). J. Econ. Entomol. 88, 742–748. doi: 10.1093/jee/88.3.742
Lu, Y., Wu, K., Jiang, Y., Guo, Y., and Desneux, N. (2012). Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362. doi: 10.1038/nature11153
Lucini, T., Faria, M. V., Rohde, C., Resende, J., and de Oliveira, J. R. F. (2015). Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod. Plant Interact. 9, 45–53. doi: 10.1007/s11829-014-9347-7
Luu, V. T., Weinhold, A., Ullah, C., Dressel, S., Schoettner, M., and Gase, K. (2017). O-acyl sugars protect a wild tobacco from both native fungal pathogens and a specialist herbivore. Plant Physiol. 174:370–386. doi: 10.1104/pp.16.01904
Marchant, W. G., Legarrea, S., Smeda, J. R., Mutschler, M. A., and Srinivasan, R. (2020). Evaluating acylsugars-mediated resistance in tomato against Bemisia tabaci and transmission of tomato yyellow leaf curl virus. Insects 11, 842. doi: 10.3390/insects11120842
Mohammed, A. A. A. H., Desneux, N., Monticelli, L. S., Fan, Y., Shi, X., Guedes, R. N. C., et al. (2019). Potential for insecticide-mediated shift in ecological dominance between two competing aphid species. Chemosphere 226, 651–658. doi: 10.1016/j.chemosphere.2019.03.114
Mostafiz, M., Alam, M., Chi, H., Hassan, E., Shim, J. K., and Lee, K. Y. (2020). Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 10, 1313. doi: 10.3390/agronomy10091313
Nielsen, J. K., Larsen, L. M., and Søorensen, H. (1977). Cucurbitacin E and I in Iberis amara: feeding inhibitors for Phyllotreta nemorum. Phytochemistry 10, 1519–1522. doi: 10.1016/0031-9422(77)84014-4
Puterka, G. J., Farone, W., Palmer, T., and Barrington, A. (2003). Structure-function relationships affecting the insecticidal and miticidal activity of sugar esters. J. Econ. Entomol. 96, 636–644. doi: 10.1093/jee/96.3.636
Qu, C., Zhang, W., Li, F. Q., Tetreau, G., Luo, C., and Wang, R. (2017). Lethal and sublethal effects of dinotefuran on two invasive whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae). J. Asia Pac. Entomol. 20, 325–330. doi: 10.1016/j.aspen.2017.02.006
Resende, J. T. V., Maluf, W. R., Faria, M. V., Pfann, A. Z., and Nascimento, I. R. (2006). Acylsugars in tomato leaflets confer resistance to the South American tomato pinworm, Tuta absoluta Meyr. Sci. Agric. 63, 20–25. doi: 10.1590/S0103-90162006000100004
Rodriguez, A. E., Tingey, W. M., and Mutschler, M. A. (1993). Acylsugars of Lycopersicon pennellii deter settling and feeding of the green peach aphid (Homoptera, Aphididae). J. Econ. Entomol. 86, 34–39. doi: 10.1093/jee/86.1.34
Sachdev-Gupta, K., Radke, C., Renwick, J. A., and Dimock, M. B. (1993). Cardenolides from Erysimum cheiranthoides: feeding deterrents to Pieris rapae larvae. J. Chem. Ecol. 19, 1355-1369. doi: 10.1007/BF00984881
Sachdev-Gupta, K., Renwick, J. A., and Radke, C. D. (1990). Isolation and identification of oviposition deterrents to cabbage butterfly, Pieris rapae, from Erysimum cheiranthoides. J. Chem. Ecol. 16, 1059–1067. doi: 10.1007/BF01021010
Saleem, M., Hussain, D., Ghouse, G., Abbas, M., and Fisher, S. W. (2016). Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 79, 177–184. doi: 10.1016/j.cropro.2015.08.024
Schuman, M. C., and Baldwin, I. T. (2016). The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 61, 373–394. doi: 10.1146/annurev-ento-010715-023851
Sedaratian, A., Fathipour, Y., Talaei-Hassanloui, R., and Jurat-Fuentes, J. L. (2013). Fitness costs of sublethal exposure to Bacillus thuringiensis in Helicoverpa armigera: a carryover study on offspring. J. Appl. Entomol. 137, 540–549. doi: 10.1111/jen.12030
Shi, Y., Li, W., Zhou, Y., Liao, X., and Shi, L. (2022). Contribution of multiple overexpressed carboxylesterase genes to indoxacarb resistance in Spodoptera litura. Pest Manag. Sci. 78, 1903–1914. doi: 10.1002/ps.6808
Simmons, A. T., Gurr, G. M., McGrath, D., Nicol, H. I., and Martin, P. M. (2003). Trichomes of Lycopersicon spp. and their effect on Myzus persicae (Sulzer) (Hemiptera: Aphididae). Aust. J. Entomol. 42, 373–378. doi: 10.1046/j.1440-6055.2003.00376.x
Simón, B., Cenis, J. L., Demichelis, S., Rapisarda, C., Caciagli, P., and Bosco, D. (2003). Survey of Bemisia tabaci (Hemiptera: Aleyrodidae) biotypes in Italy with the description of a new biotype (T) from Euphorbia characias. Bull. Entomol. Res. 93, 259–264. doi: 10.1079/BER2003233
Smeda, J. R., Schilmiller, A. L., Last, R. L., and Mutschler, M. A. (2016). Introgression of acylsugar chemistry QTL modifies the composition and structure of acylsugars produced by high-accumulating tomato lines. Mol. Breed. 36, 160. doi: 10.1007/s11032-016-0584-6
Tong, H., Su, Q., Zhou, X., and Bai, L. (2013). Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J. Pest Sci. 86, 599–609. doi: 10.1007/s10340-013-0505-y
Wagner, G. J. (1991). Secreting glandular trichomes: more than just hairs. Plant Physiol. 96, 675–679. doi: 10.1104/pp.96.3.675
Wang, R., and Wu, Y. (2014). Dominant fitness costs of abamectin resistance in Plutella xylostella. Pest Manag. Sci. 70, 1872–1876. doi: 10.1002/ps.3741
Wang, R., Zhang, W., Che, W., Qu, C., Li, F., Desneux, N., et al. (2017). Lethal and sublethal effects of cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci (Hemiptera: Aleyrodidae) MED. Crop Prot. 91, 108–113. doi: 10.1016/j.cropro.2016.10.001
Wang, R., Zheng, H., Qu, C., Wang, Z., Kong, Z., and Luo, C. (2016). Lethal and sublethal effects of a novel cis-nitromethylene neonicotinoid insecticide, cycloxaprid, on Bemisia tabaci. Crop Prot. 83, 15–19. doi: 10.1016/j.cropro.2016.01.015
Wang, X., Huang, Q., Hao, Q., Ran, S., Wu, Y., Cui, P., et al. (2018). Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 106, 110–116. doi: 10.1016/j.cropro.2017.12.020
Weinhold, A., and Baldwin, I. T. (2011). Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. U. S. A. 108, 7855–7859. doi: 10.1073/pnas.1101306108
Xu, L., Mei, Y., Liu, R., Chen, X., Li, D., and Wang, C. (2020). Transcriptome analysis of Spodoptera litura reveals the molecular mechanism to pyrethroids resistance. Pestic. Biochem. Physiol. 169, 104649. doi: 10.1016/j.pestbp.2020.104649
Zhang, Z., Gao, B., Qu, C., Gong, J., Li, W., Luo, C., et al. (2022). Resistance monitoring for six insecticides in vegetable field-collected populations of Spodoptera litura from China. Horticulturae 8, 255. doi: 10.3390/horticulturae8030255
Zhou, S., and Jander, G. (2021). Engineering insect resistance using plant specialized metabolites. Curr. Opin. Biotechnol. 70, 115–121. doi: 10.1016/j.copbio.2021.03.005
Keywords: acylsugar, Nicotiana benthamiana, chemical defenses, Spodoptera litura, toxicity, fitness cost, transgenerational effects
Citation: Wang R, Gao B, Zhang Q, Zhang Z, Li Y, Yang Q, Zhang M, Li W and Luo C (2022) Acylsugar protection of Nicotiana benthamiana confers mortality and transgenerational fitness costs in Spodoptera litura. Front. Plant Sci. 13:993279. doi: 10.3389/fpls.2022.993279
Received: 13 July 2022; Accepted: 05 August 2022;
Published: 02 September 2022.
Edited by:
Minmin Li, Institute of Food Science and Technology (CAAS), ChinaReviewed by:
Talha Javed, Fujian Agriculture and Forestry University, ChinaKangxu Wang, Michigan State University, United States
Huipeng Pan, South China Agricultural University, China
Copyright © 2022 Wang, Gao, Zhang, Zhang, Li, Yang, Zhang, Li and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Luo, bHVvY2hlbkBpcGVwYmFhZnMuY24=; Ran Wang, cndhbmcxMTA1QDEyNi5jb20=
†These authors have contributed equally to this work
 Ran Wang
Ran Wang Bingli Gao1†
Bingli Gao1†