- 1Key Laboratory of Crop Physiology Ecology and Production Management of Ministry of Agriculture, Nanjing Agricultural University, Nanjing, Jiangsu, China
- 2National Agricultural Exhibition Center (China Agricultural Museum), Chaoyang District, Beijing, China
Shoot branching is inhibited by a low red/far-red ratio (R/FR). Prior studies have shown that the R/FR suppressed Arabidopsis thaliana branching by promotes bud abscisic acid (ABA) accumulation directly. Given that wheat tiller buds are wrapped in leaf sheaths and may not respond rapidly to a R/FR, systemic cytokinin (CTK) may be more critical. Here, systemic hormonal signals including indole-3-acetic acid (IAA), gibberellins (GA) and CTK and bud ABA signals in wheat were tested under a low R/FR. The results showed that a low R/FR reduced the percentage of tiller occurrence of tiller IV and the tiller number per plant. The low R/FR did not rapidly induced ABA accumulation in the tiller IV because of the protection of the leaf sheath and had little effect on IAA content and signaling in the tiller nodes. The significant change in the CTK levels was observed earlier than those of other hormone (ABA, IAA and GA) and exogenous cytokinin restored the CTK levels and tiller number per plant under low R/FR conditions. Further analysis revealed that the decrease in cytokinin levels was mainly associated with upregulation of cytokinin degradation genes (TaCKX5, TaCKX11) in tiller nodes. In addition, exposure to a decreased R/FR upregulated the expression of GA biosynthesis genes (TaGA20ox1, TaGA3ox2), resulting in elevated GA levels, which might further promote CTK degradation in tiller nodes and inhibit tillering. Therefore, our results provide evidence that the enhancement of cytokinin degradation is a novel mechanism underlying the wheat tillering response to a low R/FR.
Introduction
Tillering of wheat (Triticum aestivum L.) is an important determinant of spike density and grain production. The appropriate tiller number and spike density in wheat can be obtained by changing the cultivation measures such as planting density. A higher planting density results in earlier tillering cessation and fewer tillers per plant, which is related to the red/far-red ratio (R/FR) to a certain extent (Evers et al., 2006; Sparkes et al., 2006). In plant populations, red light is absorbed by the surrounding canopy, whereas most far-red light is transmitted and reflected, causing the decrease in the R/FR. Increased planting density results in a faster decline in the R/FR (Sparkes et al., 2006). A high R/FR increases, but a low R/FR decreases the total tiller number (Ugarte et al., 2010). Cessation of tiller bud outgrowth starts at a relatively conservative R/FR of 0.2-0.4 (Evers et al., 2006; Sparkes et al., 2006; Dreccer et al., 2013). However, few studies have reported the physiological mechanisms of a low R/FR on tillering in wheat, which limits cultivation and genetic improvement for regulating the tillering response to a low R/FR and planting density in wheat.
A low R/FR is detected by the phytochromes family, including the phytochrome B (phyB) which is the key receptor (Sawers et al., 2005; Casal, 2013). A low R/FR inactivated phyB, and elongation-promoting PHYTOCHROME INTERACTING FACTORs (PIFs) are released to regulate downstream genes expression (Leivar and Monte, 2014). In Arabidopsis (Arabidopsis thaliana), phyB deficiency and a low R/FR have been shown to inhibit shoot branching by regulating hormone signals (Finlayson et al., 2010; Reddy et al., 2013; Yao and Finlayson, 2015). Several studies have stated that abscisic acid (ABA) is the key signal regulates Arabidopsis axillary bud outgrowth responses to the R/FR (Reddy et al., 2013; Yao and Finlayson, 2015; Holalu and Finlayson, 2017). ABA abundance in buds rapidly decreased in response to a high R/FR before Arabidopsis axillary bud growth changes (Holalu and Finlayson, 2017). Under a decreased R/FR, elevated ABA was related to upregulated biosynthesis gene expression (Reddy et al., 2013) and was found to downregulate the expression of cell cycle-related genes to inhibit Arabidopsis axillary bud outgrowth (Yao and Finlayson, 2015). Importantly, Arabidopsis axillary buds exposed to air can directly perceive a low R/FR; however, wheat tiller buds before they grew out, may not directly perceive a low R/FR due to the protection of leaf sheaths. In other words, wheat tiller buds could not produce ABA signals as quickly, so ABA probably is not the key hormone mediates the inhibiting effect of a low R/FR on wheat tillering.
Systemic hormonal signals from the main stem such as indole-3-acetic acid (IAA), gibberellins (GA) and cytokinin (CTK) are involved in the regulation of shoot branching (Wang et al., 2018) and may be more critical for the regulation of wheat tillering by the R/FR. IAA synthesized at the shoot apex is well known for its role in apical dominance, but it does not enter the bud directly from the main stem (Cline and Choonseok, 2006). One model proposes that IAA can reduce CTK levels in nodes by downregulating the expression of biosynthesis genes and therefore inhibit branching (Tanaka et al., 2006). Active GA, such as GA1 and GA4, inhibit tiller bud outgrowth. GA levels in nodes are determined by biosynthesis, including the actions of GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox), and degradation including the action of GA 2-oxidase (GA2ox) (Binenbaum et al., 2018). GA-deficient mutants exhibit higher shoot branching than wild types and reduced GA levels produce increased branching phenotypes in rice (Lo et al., 2008). GA-inhibition of tillering is mainly due to decreased CTK levels by upregulating CTK degradation genes by GA (Zhuang et al., 2019), indicating that CTK could act as the key messenger. CTK from roots and basal nodes can directly move into the bud to promote outgrowth (Barbier et al., 2019). The trans-zeatin (tZ) and trans-zeatin riboside (tZR; transport form of tZ) are natural cytokinin that are mainly synthesized by the actions of phosphate-isopentenyltransferase (IPT) and cytochrome P450 monooxygenases (CYP735As), degraded by cytokinin oxidase/dehydrogenase (CKX), conjugated by cytokinin glucosyltransferase (CGT), or reverted by glucosidase (GLU) (Chen et al., 2021). An intuitively clear mechanism for CTK-mediated bud outgrowth is promoting the cell cycle by inducing the expression of cell-cycle-related genes (Müller and Leyser, 2011).
Previous studies have shown that a low R/FR promotes the IAA content in young Arabidopsis seedlings (Tao et al., 2008; Hornitschek et al., 2012), and systemic auxin signaling is necessary for maintained Arabidopsis bud suppression by a decreased R/FR (Holalu et al., 2021). Two studies have reported that the GA biosynthetic genes GA20ox and GA3ox in Rumex palustris (Pierik et al., 2011) and KAO in Pinus tabuliformis (Li et al., 2020) are upregulated by a low R/FR. A study on sorghum (Sorghum bicolor) suggested that the dormant state of phyB-1 buds is correlated with the regulation of cytokinin degradation genes (Kebrom and Mullet, 2016). Furthermore, PIFs was found to directly induce AtCKX5 expression based on ChIP-seq results (Leivar and Monte, 2014). Since both IAA and GA inhibit shoot branching by reducing CTK content in nodes, we addressed the question whether CTK abundance and degradation were the key factors mediate the inhibiting effect of a low R/FR on wheat tillering. It was hypothesized that a low R/FR would alter CTK homeostasis by inducing degradation genes expression, thus inhibiting wheat tillering. To prove our hypotheses, we monitored the temporal patterns of tiller number per plant, tiller bud outgrowth and hormonal state under a low R/FR. And we used two techniques to characterize hormonal state, involving the measurement of hormone levels and gene expression.
Materials and methods
Materials and experimental design
A pot experiment was conducted at the Pailou Experimental Station of NJAU, Nanjing, China (32°04′N, 118°76′E) during the 2020–2021 growing season. The concentration of organic matter, total nitrogen, available phosphorus and available potassium in the sieved soil were 19.84 g kg-1, 0.76 g kg-1, 28.24 mg kg-1, and 86.42 mg kg-1, respectively. Each pot (length 49.5 cm, width 21 cm, height 20 cm) was filled with 16 kg of sieved soil and placed outdoors. Each pot was supplied with 10.5 g of compound fertilizer (15% nitrogen, 15% phosphorus, and 15% potassium) before sowing, and the other 1.0 g nitrogen was top-dressed at the jointing stage. Yangmai 25 (YM25, Triticum aestivum L.), one of the popular wheat varieties in Jiangsu province, was selected to be sown in the drill and thinned to 14 seedlings with a seedling spacing of 3.2 cm per pot at the three-leaf stage, and coleoptile tillers were removed simultaneously. Two most marginal seedlings in each pot were not sampled to reduce the marginal effect.
Two treatments were designed: natural radiation supplemented with far-red light (Low R/FR) and a control (Control). The experimental setting and measurement of the R/FR were performed as described by Ugarte et al. (2010) with minor modifications, as shown in Supplementary Figure S1. A 40 W LED plant light provided far-red light (15 μmol m-2 s-1, 730 ± 10 nm). Each lamp was east-west oriented, 50 cm away from the pots, placed at plant height, and the plants were horizontally irradiated from the south side from 8 am to 5 pm daily from the five-leaf stage to the booting stage. The whole plant was exposed to FR light, and the red/far-red ratio at the base of the plant was characterized by an SKR 116 dual-channel radiometer (Skye Instruments, Llandrindod Wells, UK). The red/far-red ratio was calculated as the average of the ratio measured facing towards the light sources and the ratio measured facing against the light sources. Low R/FR treatment reduced the red/far-red ratio received by the plants from 0.78 (Control) to 0.38 at noon on a sunny day.
Wheat tiller dynamics and plant height (two pots per replicate, three biological replicates) were observed every 7 days after treatment (DAT), and phenotypes were recorded at the booting stage. The tiller buds exposed from the leaf sheath and became new tillers were counted, excluding dying tillers whose newest leaves started yellowing. The percentage of tiller occurrence (%) for the tiller position was equal to the tiller number of the tiller position/total number of plants (%). Considered that the percentage of tillers I, II and III from the main stem was 100% at five-leaf stage, the tiller IV (from the axil of the fourth leaf on the main stem) was studied. For morphology, hormone levels, and gene expression analysis, the tiller node and tiller IV were harvested with three biological replicates at 7, 14, and 21 DAT. The seedlings were dug out and washed, and then the fourth leaf (from bottom) and its leaf sheath were peeled off. The tiller IV and its corresponding tiller node were cut respectively. All fresh samples were immediately placed in liquid N2 and then stored at -80°C. Samples were dried at 105 °C for 30 min and then at 75 °C for 72 h.
To verify that a low R/FR inhibited tillering by reducing CTK levels in wheat, a pharmacology experiment was conducted to study the alleviating effect of 6-benzyl aminopurine (synthetic cytokinin; 6-BA) on the suppressed tillering of wheat under low R/FR conditions in October 2021. Given that the tZ-type cytokinin were synthesized from the roots and basal nodes, 6-BA was applied to the roots using a hydroponic experiment. YM25 was planted in the Hoagland’s nutrient solution, containing 0.75 mM Ca(NO3)2, 1 mM KNO3, 1 mM KH2PO4, 0.5 mM CaCl2, 0.5 mM CaSO4, 1 mM MgSO4, 10 mM Fe-EDTA, 0.5 mM NaCl, 2.35 mM H3BO3, 0.0385 mM ZnSO47H2O, 0.55 mM MnSO4H2O, 0.0165 mM CuSO45H2O and 0.0065 mM H2MoO4, following the detailed hydroponic methods from Gao et al. (2018). The Control and Low R/FR treatments were remained the same as in the pot experiment, and 6-BA (25 μM) was added to the solutions at 7 DAT under low R/FR conditions (Low R/FR + 6-BA). The red/far-red ratios in the Control, Low R/FR and Low R/FR + 6-BA treatments were 0.75, 0.35, and 0.34, respectively, at noon on 14 DAT. Tiller number and tiller IV growth of wheat treated for 7 days and 14 days and the control, as well as the CTK levels in tiller nodes at 7 and 14 DAT and the control were measured.
Hormone levels assays
Plant hormone (ABA, IAA, GA1, GA4, tZ, and tZR) levels were measured according to Kojima et al. (2009) with minor modifications. Fresh powder samples (0.2 g) was homogenized in 5 mL pre-chilled acetonitrile for 12 h. The supernatant was collected by centrifugation at 12,000 x g for 15 min at 4°C. The pellet was resuspended in 5 mL pre-chilled acetonitrile for 2 h and then collected and combined with the supernatant after centrifugation as before. Next, 20 mg C18 powder and 0.5 g anhydrous magnesium sulfate powder was added to the combined supernatant, and the supernatant was collected after centrifugation as before. The supernatant was concentrated to near dryness using a centrifuge concentrator for 6–8 h and added with 0.2 mL methanol. The sample was filtered through a 0.22 μm organic nylon membrane and filled with a 2 mL sample bottle. The levels of plant hormones were measured using liquid chromatography-mass spectrometry (LC-MS/MS; QTRAP® 6500, AB Sciex, USA) fitted with a chromatographic column (ACQUITY UPLC HSS T3, 1.8 μm, 2.1 × 75 mm, Waters). Hormones were separated at a flow rate of 0.3 mL min-1 with the gradients of solvent A (0.1% formic acid) and solvent B (acetonitrile) set as follows: 0 min, 95% A + 5% B; 1 min, 95% A + 5% B; 8 min, 5% A + 95% B; 8.1 min, 95% A + 5% B; and 10 min, 95% A + 5% B.
RNA extraction and cDNA synthesis
Total RNA was extracted from tiller node and bud tissues using TRIzol reagent (Vazyme Bio, China) according to the manufacturer’s protocols. cDNA synthesis was performed using HiScript III Q RT SuperMix (Vazyme Bio, China), following the manufacturer’s instructions. The cDNA samples were diluted 5× before qPCR analysis.
Primers
To ensure the reliability of the results, two or three typical genes with high expression in each metabolic hormone process were tested in tiller nodes. The primer sequences for a part of target genes including TaNCED, TaABA3, TaCYP707A1, TaCYP707A2, TaIAA12, TaIAA13, TaARF13, TaARF15, TaGA20ox1, TaGA20ox2, TaGA3ox2, TaGA2ox1, TaGA2ox4, TaGA2ox6, TaGLU6, TaGLU7, TaGLU13, and TaADP-RF (reference gene) have been reported previously (Paolacci et al., 2009; Pearce et al., 2015; Son et al., 2016; Izydorczyk et al., 2018; Qiao et al., 2018; Shoaib et al., 2019; Lang et al., 2021). The primer sequences for other target genes were designed using the Primer3 software (https://bioinfo.ut.ee/primer3-0.4.0/) and are listed in Supplementary Table S1. The amplification efficiency of the total genes ranged from 90 to 105%. The sequences of some of these genes, including TaCYP735A1, and TaCYP735A2, have not yet been reported in wheat. Thus, the CDS sequences of Arabidopsis AtCYP735A1 (GenBank ID: AT5G38450), and AtCYP735A2 (GenBank ID: CYP735A2) published in NCBI were used for a BLAST search against the Chinese Spring gene (RefSeq v1.0 or v2.1) in WheatOmics 1.0 (Ma et al., 2021). Gene sequences with the highest similarity were selected, and the corresponding primers were previously described and are listed in Supplementary Table S1.
Real-time qPCR assay
qPCR was performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad, USA) with ChamQ SYBR qPCR Master Mix (Vazyme Bio, China). The PCR conditions involved a preliminary incubation for 30 s at 95 °C, followed by 40 cycles of 10 s at 95°C, and 30 s at 58 or 60°C. Relative transcript levels were calculated using the method described by Livak and Schmittgen (2001) with TaADP-RF as the reference gene. Three biological replicates and two technical replicates were performed for each gene.
Statistical analysis
Statistically significant differences between the control and treated samples were tested using Student’s t-test (P < 0.05) using SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA).
Results
Morphology trait
Exposure to a low R/FR resulted in a 16% decrease in tiller number per plant at 14 DAT and the effect lasted until the end of the treatment compared to the control (Figures 1A, B). Due to a decreased R/FR, tillering stopped at 14 DAT and declined at 28 DAT, which was earlier than that of the control at 21 DAT and 35 DAT, respectively (Figure 1B). The percentage of tiller occurrence of the tiller IV was zero at 7 DAT and significantly reduced by 67% at 14 and 21 DAT under low R/FR conditions (Figure 1C), indicating that tiller IV buds were wrapped in leaf sheaths and did not become new tillers at 7 DAT and tiller IV growth was inhibited by a low R/FR at 14 and 21 DAT. The tiller IV buds were not significantly different in size at 7 DAT and the length of tiller IV was reduced at 14 and 21 DAT under low R/FR conditions (Figure 1D). These results indicated that wheat tillering was inhibited significantly in response to a low R/FR.
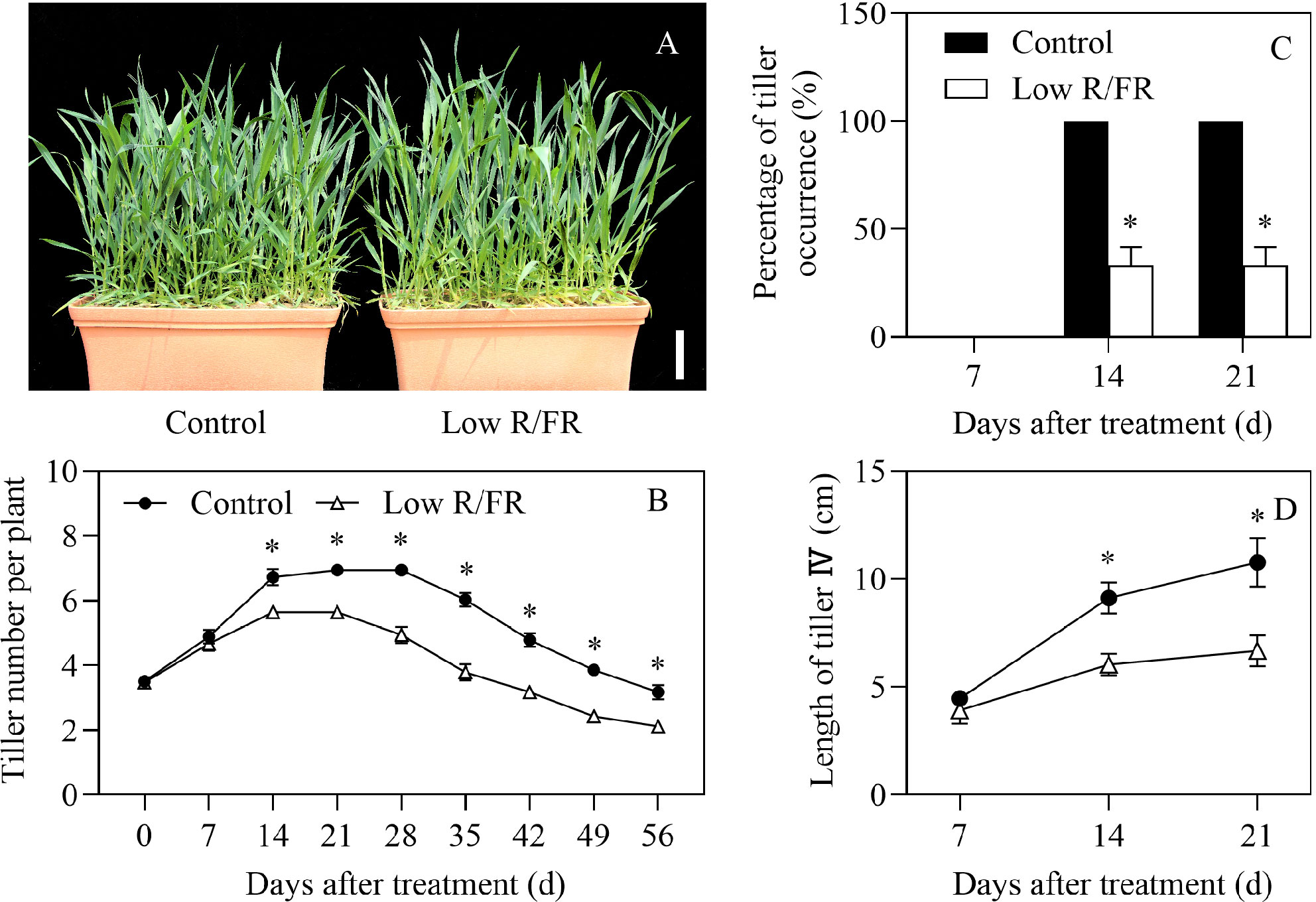
Figure 1 Plants and tillers phenotype under control and low R/FR conditions. (A) Comparison of phenotype at booting stage (56 DAT), and the white legend represents 10 cm. (B) Tiller number per plant was tested at 0, 7, 14, 21, 28, 35, 42, 49 and 56 DAT. (C) The percentage of tiller occurrence of tiller IV was counted at 7, 14 and 21 DAT. Tiller IV means the tiller bud from the axil of the 4th leaf from bottom on the main stem. (D) Length of tiller IV was determined at 7, 14 and 21 DAT. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples. DAT, days after treatment.
Hormone levels
There was no difference in ABA levels in the tiller IV at 7 DAT, and ABA levels were significantly higher in the tiller IV at 14 and 21 DAT (66–72%) by a low R/FR compared to the control (Figure 2A). These results indicated that a decreased R/FR did not rapidly induced ABA accumulation in the tiller IV wrapped in leaf sheaths until the tiller IV grew out. Tiller nodes IAA levels were not affected by a low R/FR at any DAT (Figure 2B), but GA levels were significantly increased at 14 and 21 DAT (23–25%) under low R/FR conditions relative to the control (Figure 2C). Tiller nodes CTK levels were decreased by 34% at 7 DAT and this effect was pronounced as the treatment continued for 14 d and 21 d (Figure 2D). Overall, CTK abundance in tiller nodes declined in response to a decreased R/FR prior to measured effects on tiller growth and other hormone levels.
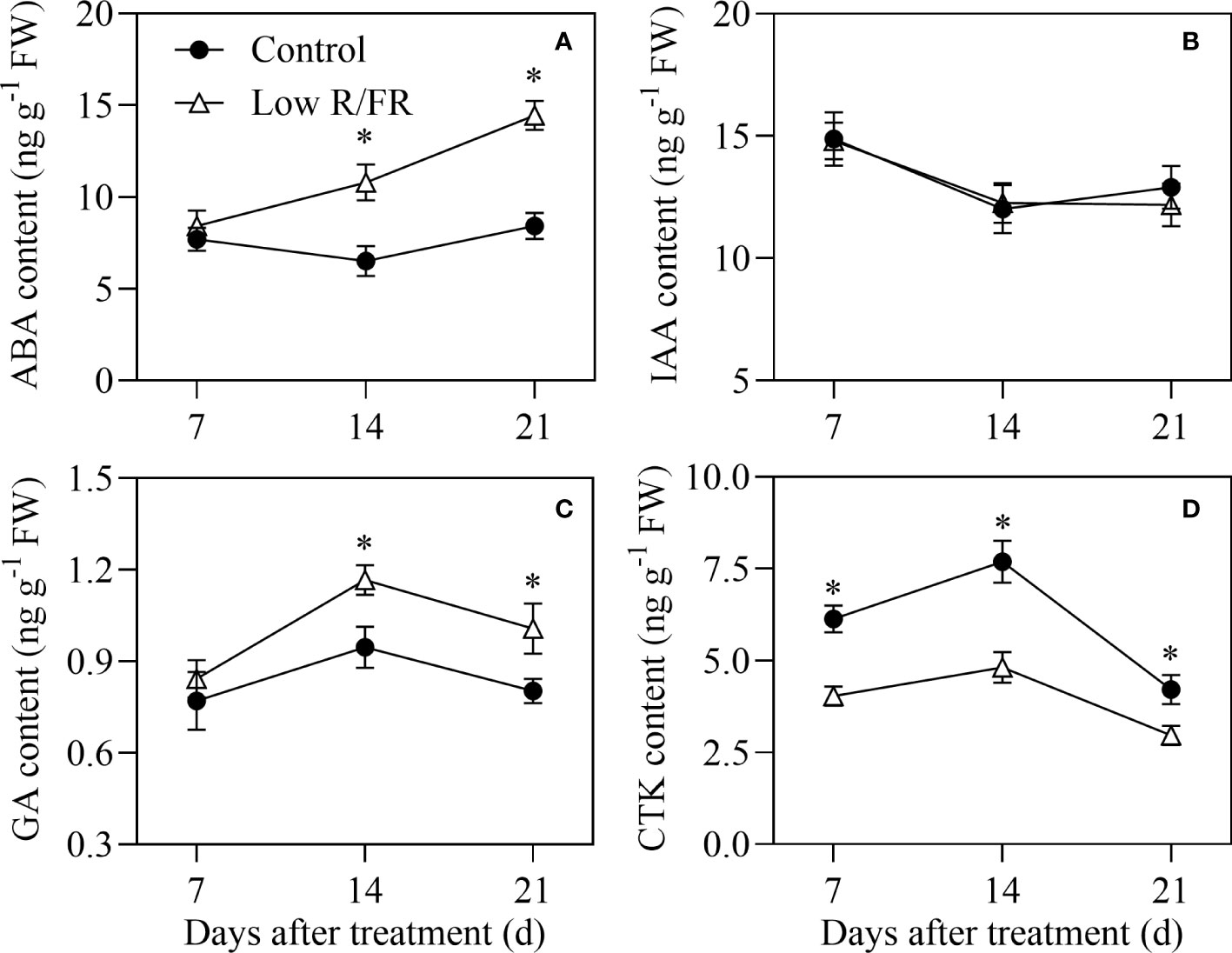
Figure 2 Abscisic acid (ABA) abundance in the tiller IV and auxin, gibberellin (GA) and cytokinin (CTK) levels in tiller nodes and under control and low R/FR conditions. ABA (A) levels in the tiller IV and indole-3-acetic acid (IAA;) (B), GA (C) and CTK (D) levels in tiller nodes treated for 7, 14 and 21 d. GA levels were the sum of GA1 and GA4. CTK levels were the sum of tZ and tZR. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Transcriptional regulation of ABA metabolism in the tiller IV
The relative expression levels of ABA biosynthesis (TaNCED, TaABA3, and TaAAO) and deconjugation (TaCYP707A1 and TaCYP707A1) genes in tiller nodes at all DATs were showed in Figure 3. No significant effect of a low R/FR on the transcripts of above genes was observed at 7 DAT (Figures 3A–E), further suggesting that a decreased R/FR did not rapidly induced ABA accumulation in the tiller IV. Compared to the control, the relative expression levels of TaNCED, TaABA3, and TaCYP707A1 were upregulated at 14 and 21 DAT by a low R/FR (Figures 3A, B, D). In addition, the relative expression levels of TaAAO were improved at 21 DAT and TaCYP707A1 was remained unchanged at all DATs under low R/FR conditions (Figures 3C, E). Thus, a low R/FR improved ABA levels by induced ABA biosynthesis genes (TaNCED and TaABA3) in the tiller IV.
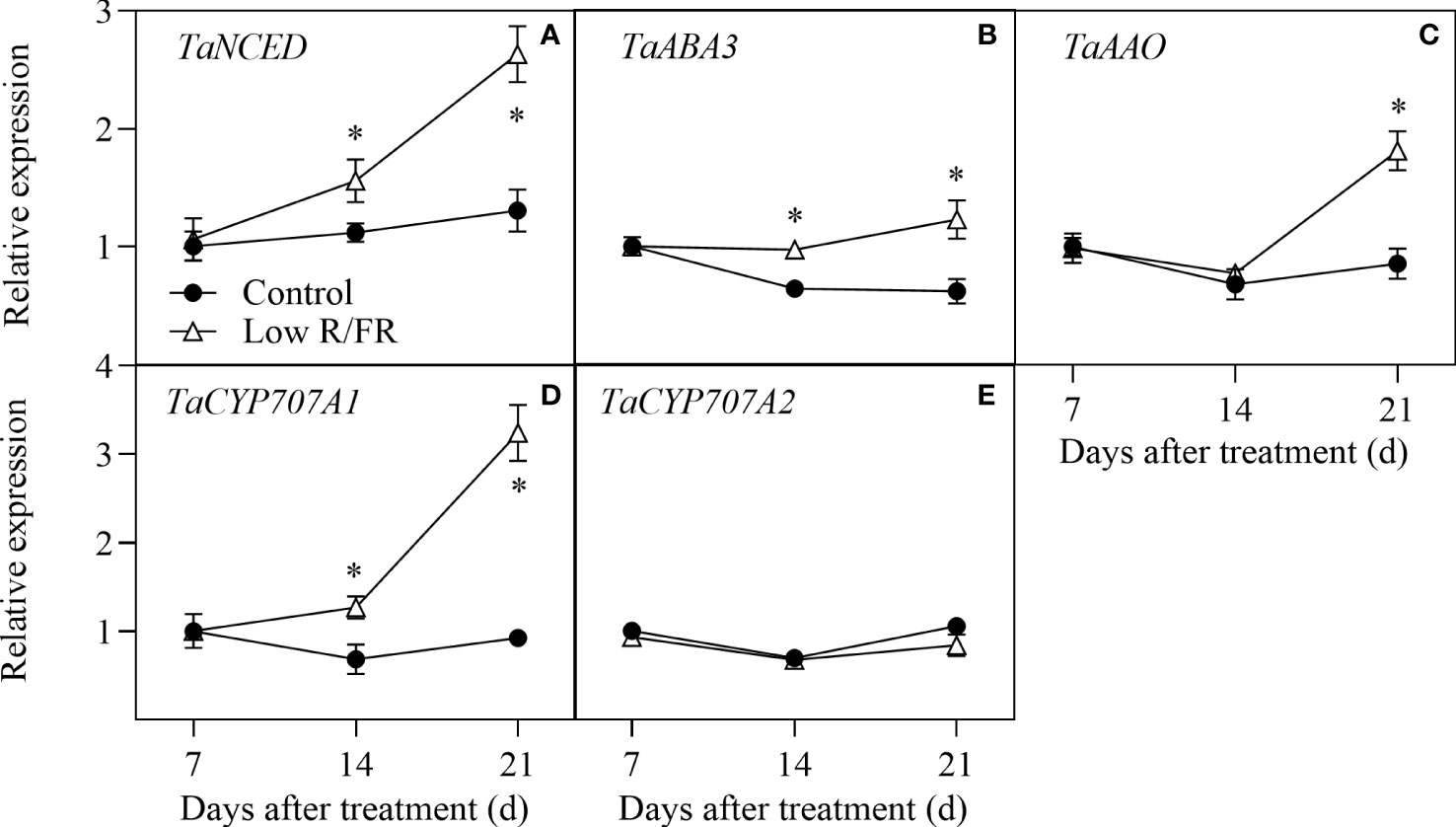
Figure 3 Expression of ABA metabolism genes in the tiller IV under control and low R/FR conditions. Relative transcript levels of TaNCED (A), TaABA3 (B), TaAAO (C), TaCYP707A1 (D), and TaCYP707A2 (E) in the tiller IV treated for 7, 14 and 21 d. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Transcriptional regulation of auxin signaling in tiller nodes
Auxin signaling status was assessed using a panel of IAA-responsive genes, including TaIAA12, TaIAA13, TaARF13, TaARF15, and TaGH3.2 in tiller nodes at all DATs. No significant effect of a low R/FR on the transcripts of TaIAA12 and TaIAA13 was observed at all DATs (Figures 4A, B). Relative to the control, the expression of TaARF13 and TaARF15 was upregulated at 14 d after treatment, but there were no differences at any other days (Figures 4C, D). In addition, the relative expression level of TaGH3.2 was decreased at 21DAT to a lesser extent by a low R/FR (Figure 4E). The average relative expression of the auxin signaling genes was not altered by the R/FR (Figure 4F). In summary, a low R/FR had little effect on auxin signaling in tiller nodes.
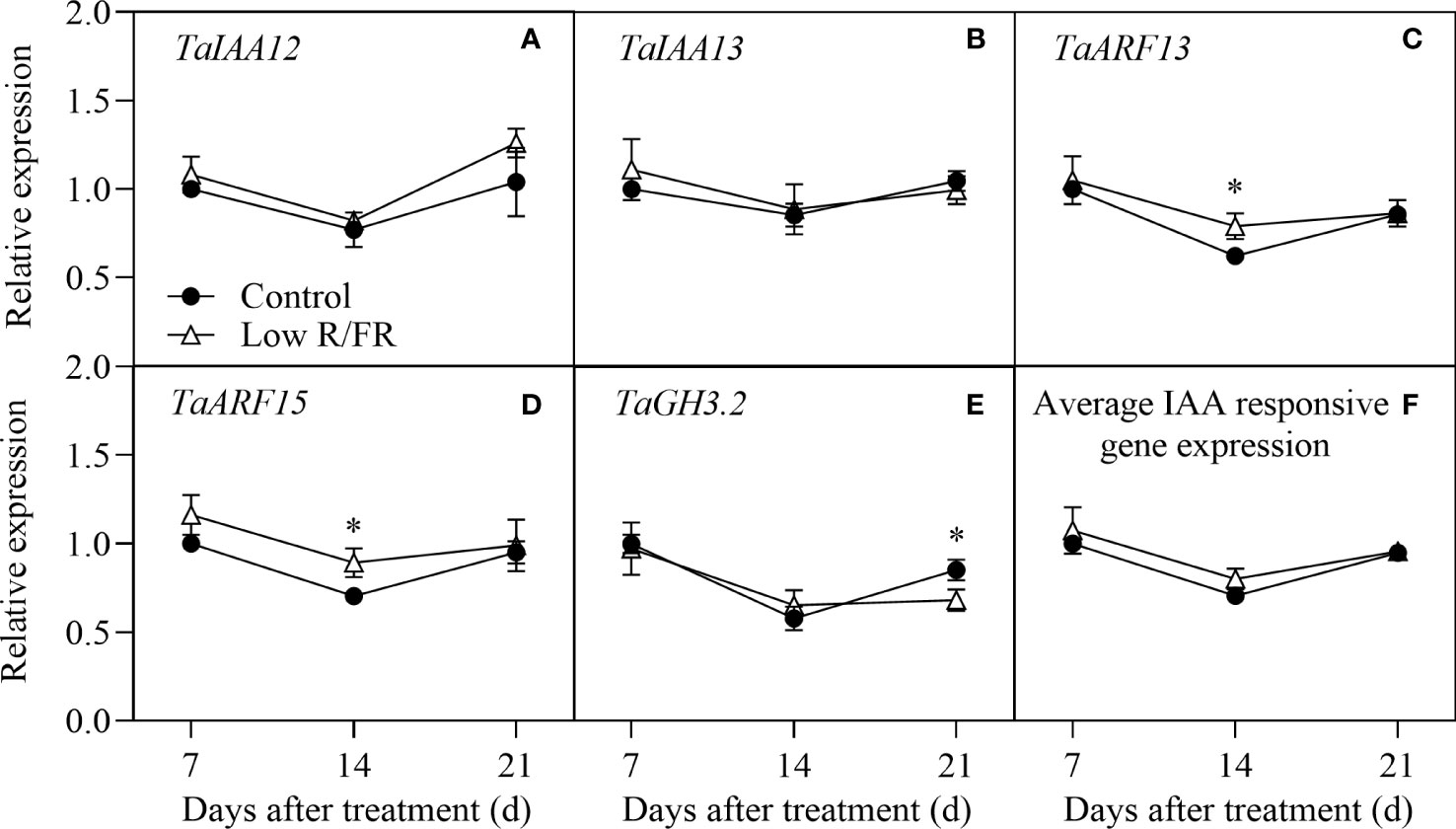
Figure 4 Expression of IAA-responsive genes in tiller nodes under control and low R/FR conditions. Relative transcript levels of TaIAA12 (A), TaIAA13 (B), TaARF13 (C), TaARF15 (D), TaGH3.2 (E), and average positive normalized expression (F) in tiller nodes treated for 7, 14 and 21 d. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Transcriptional regulation of GA metabolism in tiller nodes
The relative expression levels of GA biosynthesis (TaGA20ox1, TaGA20ox2, and TaGA3ox2) and degradation (TaGA2ox1, TaGA2ox4, and TaGA2ox6) genes at all DATs were showed in Figure 5. The relative expression levels of the GA biosynthetic genes TaGA20ox1 and TaGA3ox2 significantly increased at 7 DAT and that of TaGA20ox2 significantly increased at 14 DAT in response to a low R/FR (Figures 5A–C). Among the three degradative genes, the relative expression levels of TaGA2ox1 and TaGA2ox4 were significantly upregulated at 14 DAT, whereas TaGA2ox6 was not affected by a low R/FR (Figures 5D–F). These results suggested that the increase in GA levels was associated with upregulated biosynthesis genes (TaGA20ox1 and TaGA3ox2) in wheat under low R/FR conditions.
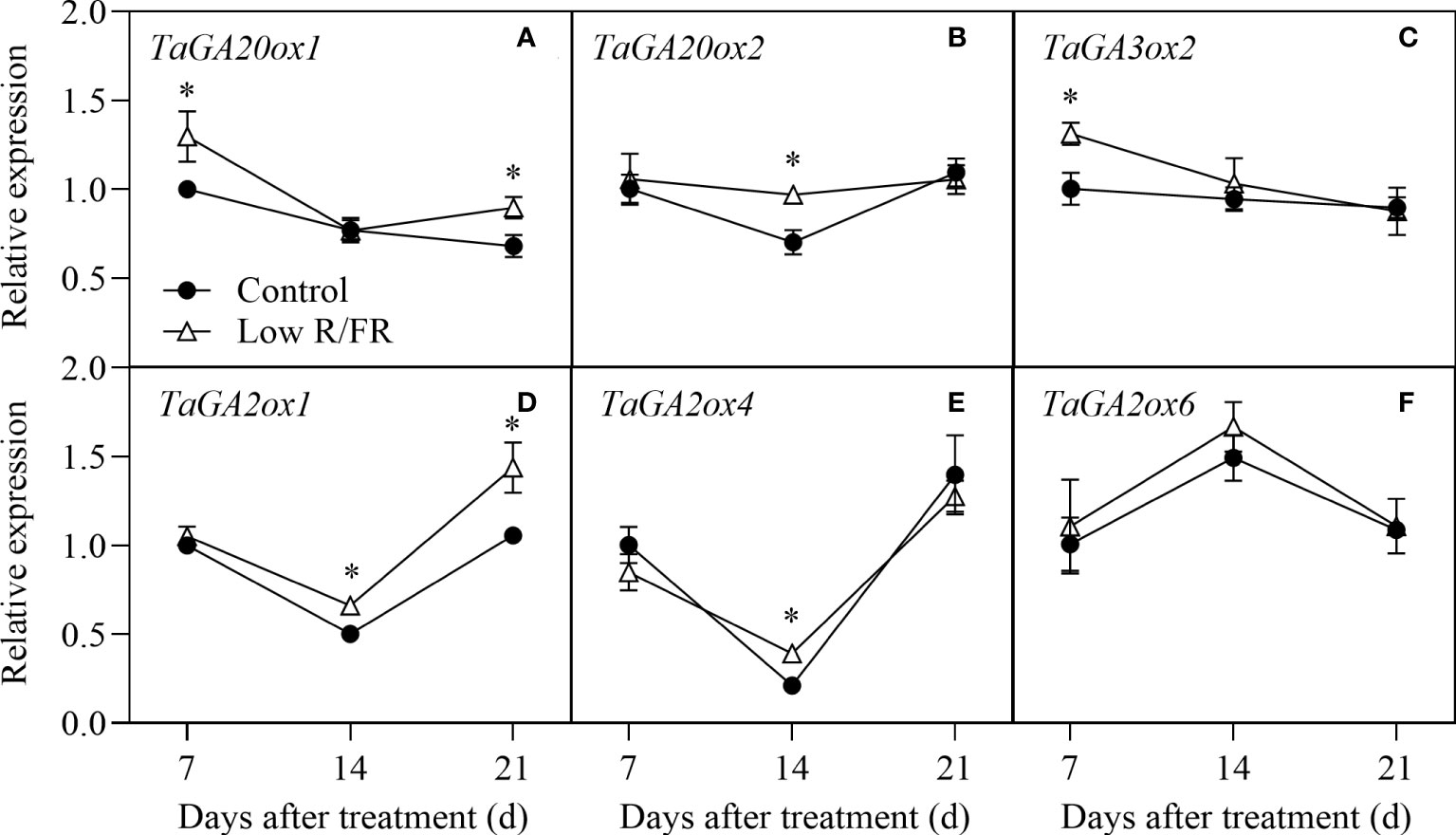
Figure 5 Expression of GA metabolism genes in tiller nodes under control and low R/FR conditions. Relative transcript levels of TaGA20ox1 (A), TaGA20ox2 (B), TaGA3ox2 (C), TaGA0ox1 (D), TaGA2ox4 (E), and TaGA2ox6 (F) in tiller nodes treated for 7, 14 and 21 d. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Transcriptional regulation of CTK metabolism in tiller nodes
The relative expression patterns of CTK biosynthesis (TaIPT3, TaIPT6, TaCYP735A1, and TaCYP735A2), degradation (TaCKX5, TaCKX9, and TaCKX11), deconjugation (TaGLU6, TaGLU7, and TaGLU13) and conjugation (TaCOGT and TaCNGT) genes in tiller nodes at all DATs were showed in Figure 6. Treatment with a low R/FR downregulated the relative expression levels of TaIPT3 and TaCYP735A2 at 14 and 21 DAT and the expression of TaCYP735A1 at 21 DAT; however, it did not affect the expression of TaIPT6 in tiller nodes (Figures 6A–D). The relative expression levels of TaCKX5 and TaCKX11 were higher in low R/FR conditions than that in the control conditions at 7 and 14 DAT, and the expression of TaCKX9 was unchanged under low R/FR conditions (Figures 6E–G). Low R/FR treatment did not alter the relative expression levels of TaGLU6, TaGLU7 and TaGLU13 in tiller nodes (Figures 6H–J). In addition, Low R/FR treatment resulted in a marked increase in the relative expression levels of TaCOGT and TaCNGT in tiller nodes at 14 and 21 DAT compared with the Control (Figures 6K, L). Overall, the decrease in CTK levels was associated with inhibited biosynthesis genes (TaIPT3, TaCYP735A2) and enhanced degradation (TaCKX5, TaCKX11) and conjugation (TaCOGT, TaCNGT) genes in wheat under low R/FR conditions.
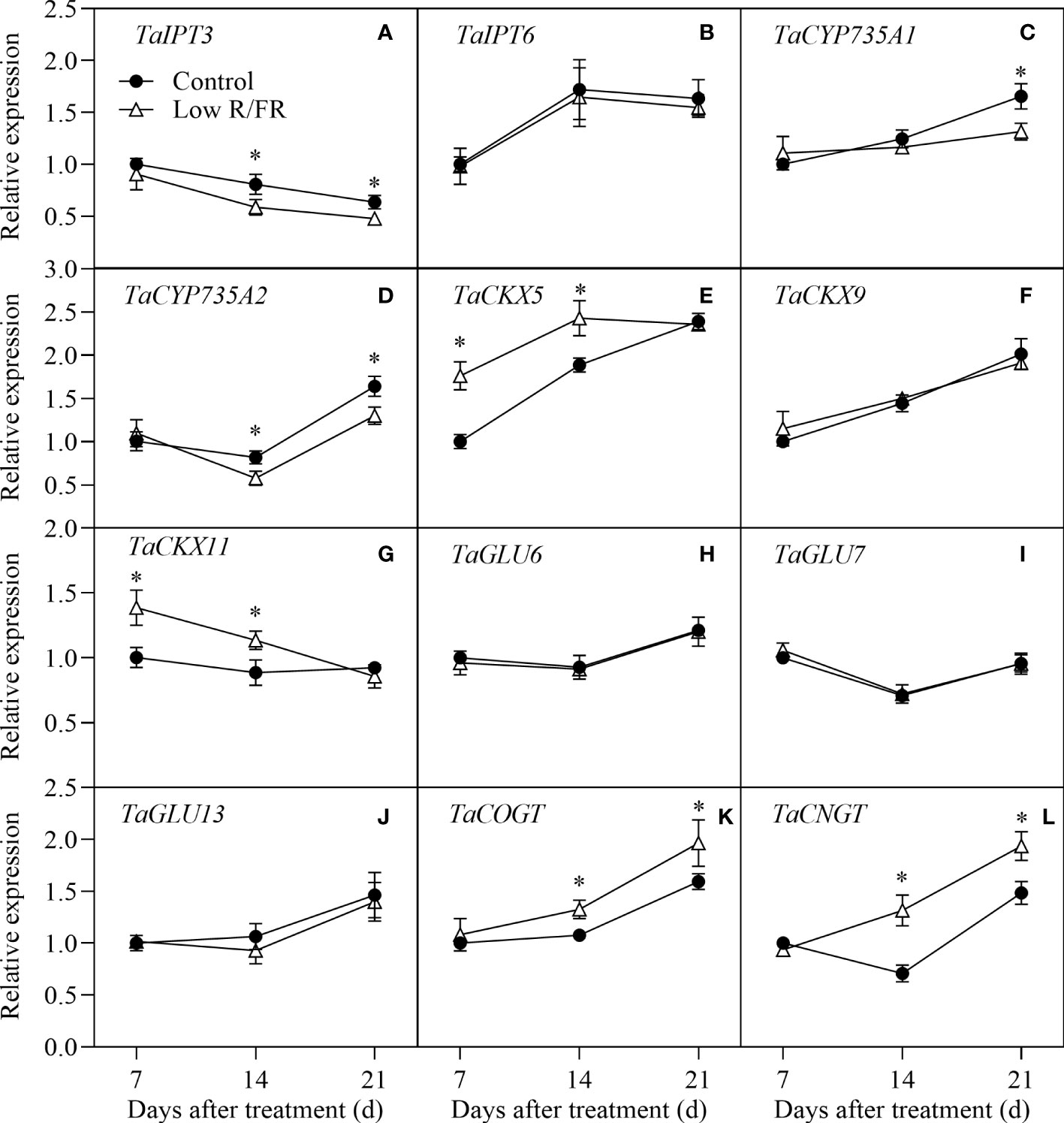
Figure 6 Expression of CTK metabolism genes in tiller nodes under control and low R/FR conditions. Relative transcript levels of TaIPT3 (A), TaIPT6 (B), TaCYP735A1 (C), TaCYP735A2 (D), TaCKX5 (E), TaCKX9 (F), TaCKX11 (G), TaGLU6 (H), TaGLU7 (I), TaGLU13 (J), TaCOGT (K) and TaCNGT (L) in tiller nodes treated for 7, 14 and 21 D. Data are the means of three biological replicates ± SE, and the asterisk denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Effect of 6-BA on tiller number and length under low R/FR conditions
Similarly, exposure to a low R/FR resulted in a marked decrease in tiller number per plant, length of tiller IV, and levels of tZ and tZR at 14 DAT in the hydroponic experiment (Figure 7). Importantly, root application of 6-BA (exogenous cytokinin) treatment significantly improved tiller number per plant, length of the tiller IV, and levels of tZ and tZR under low R/FR conditions (Figure 7). These results suggested that root-supplied 6-BA increased the levels of tZ-type CTK in tiller nodes and improved tillering under low R/FR conditions.
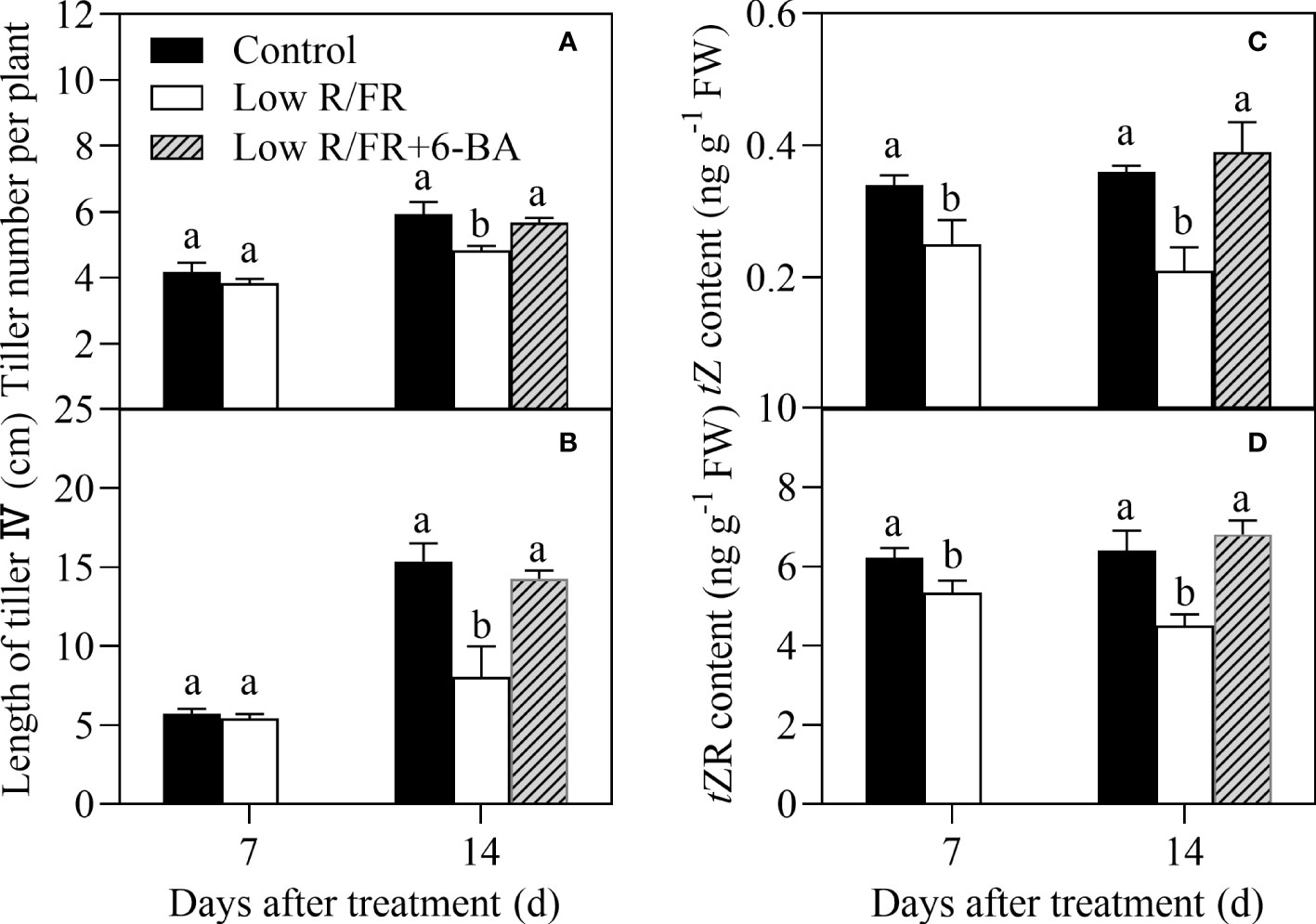
Figure 7 Effect of root application 6-BA on tiller number and tiller IV growth under low R/FR conditions (hydroponic experiment). Tiller numbers per plant (A), length of tiller IV (B) and levels of tZ (C) and tZR (D) in tiller nodes were tested at 7, 14 DAT. Data are the means of three biological replicates ± SE, and the lowercase letters denotes a statistically remarkable difference (P < 0.05) between treated and control samples.
Discussion
A low R/FR triggers shade avoidance syndrome, characterized by a decrease in branch number (Fernández-Milmanda and Ballaré, 2021). In this study, a low R/FR reduced tiller number and caused earlier tillering cessation and decline (Figures 1A, B). Similar effects of a decreased R/FR have also been reported in wheat (Ugarte et al., 2010; Xie et al., 2016). Further analysis revealed that the reduction in tiller number was associated with a lower percentage of tiller occurrence of tiller IV under low R/FR conditions (Figure 1C). The regulation of branching by a low R/FR is a complex process. Endogenous hormones, including IAA, GA, ABA, and CTK, are involved in the regulation of bud outgrowth (Wang et al., 2018). The present results showed CTK levels in tiller nodes declined in response to a low R/FR prior to measured effects on tiller growth and other hormone levels (Figure 1, 2). And the application of exogenous cytokinin (6-BA) in roots recovered endogenous CTK levels and tiller number (Figure 7), suggesting that cytokinin could be the key hormone mediating the effect of the R/FR on tiller bud outgrowth in wheat. Increase in endogenous CTK levels in the tiller nodes in response to the 6-BA application is in accordance with the reports available (Xu et al., 2015; Yang et al., 2020), and probably due to the promotion of cytokinin biosynthesis by the 6-BA application (Panda et al., 2018; He et al., 2020).
Initial work indicated that rapid responses to an increased R/FR might be mediated by changes in bud ABA in Arabidopsis thaliana (Reddy et al., 2013; Yao and Finlayson, 2015; Holalu and Finlayson, 2017). However, a low R/FR did not rapidly induce ABA accumulation in the tiller IV (Figure 2A), because the wheat tiller IV bud was wrapped by the leaf sheath within 7 DAT in our study (Figure 1C). Moreover, no significant effect of a decreased R/FR on the relative expression of ABA mechanism genes was observed at 7 DAT (Figures 3). These results confirmed our assumption that wheat tiller buds, unlike Arabidopsis axillary buds, respond weakly to a low R/FR before they were exposed from the leaf sheath. Thus, ABA was not the key hormone that inhibited wheat tillering by a low R/FR. Nonetheless, the effect of ABA could not be completely ignored, as a large accumulation of ABA was observed in the tiller IV at 14 and 21 DAT under low R/FR conditions (Figure 2A). Accumulation of ABA was attributed to upregulation of biosynthesis genes (TaNCED and TaABA3) by a decreased R/FR in our study, and these results are consistent with previous findings (Reddy et al., 2013). ABA promotes plant senescence (Zhang and Gan, 2012) and a low R/FR may initiate tiller death in wheat (Sparkes et al., 2006). In our study, elevated ABA levels may exacerbate tiller death, explaining that a low R/FR causes earlier decline in tiller number.
Auxin and GA were not the key hormones that mediated the regulation of a low R/FR in our study. Prior studies have shown that the indole-3-acetic acid (IAA) accumulation or signaling increases rapidly in young Arabidopsis seedlings under a low R/FR (Tao et al., 2008; Hornitschek et al., 2012; Reddy and Finlayson, 2014). However, IAA levels and expression levels of IAA signaling genes in tiller nodes were not altered by a low R/FR in this study (Figures 2B, 4). This could be because IAA mainly mediates the increase in plant height rather than the decrease in bud outgrowth response to a low R/FR (Tao et al., 2008). These results are in agreement with those of Holalu and Finlayson (2017), showing that the auxin content and signaling in the main stem presented a weak and transient response to low R/FR. However, GA may still be involved in inhibiting wheat tillering. These findings showed that the increase in GA levels was associated with improved biosynthesis in wheat (Figures 2C, 5), as suggested by Pierik et al. (2011) for Rumex palustris. The upregulation of gibberellin degradation might maintain gibberellin homeostasis under a low R/FR. Given that GA-inhibition of tillering was related to decreased CTK levels by enhancing CTK degradation in nodes (Zhuang et al., 2019), GA was most likely involved in promoting CTK degradation and inhibiting wheat tillering in our study.
Cytokinin levels are related to biosynthesis, degradation, conjugation, and deconjugation (Chen et al., 2021). The relative expression levels of CTK biosynthesis genes (TaIPT3 and TaCYP735A2) were downregulated by a low R/FR following 14 DAT (Figures 6A, D). This could be explained by the fact that these two genes are specifically induced by nitrate (Sakakibara et al., 2006) which was reduced in our study (data not shown). The relative expression levels of conjugation genes (TaCOGT, TaCNGT) were improved by a low R/FR at 14 and 21 DAT (Figures 6K, L), suggesting that enhanced conjugation by a low R/FR might contribute to lower levels of CTK. Cytokinin glucosyltransferase is thought to function in the overabundance or overproduction of CTK (Hoyerová and Hošek, 2020). Our results provide insights into the role of CGT in cytokinin homeostasis. The relative expression levels of degradation genes (TaCKX5, TaCKX11) were notably upregulated by a low R/FR at 7 DAT prior to other genes (Figures 6E,G), indicating the reduction in CTK levels was mainly related to enhanced degradation. A previous study reported that CTK levels in phyB-1 buds could be lower than those in wild-type buds due to increased expression of the cytokinin degradation gene (SbCKX1) (Kebrom and Mullet, 2016), suggesting that our results are credible. In other words, a low R/FR can induce cytokinin degradation resulting in the inhibition of wheat tillering. What needs to be considered is that the tiller node is not an organ that directly senses the R/FR, so there should be the other substance to transmit the signal. Prior work have shown that PIFs may directly improve AtCKX5 expression (Leivar and Monte, 2014). We speculated that PIFs would be shoot-to-root mobile signals which induce TaCKXs expression in the tiller nodes. Linking environmental control of branching with specific genes may provide a method to alter crop architecture (Doust, 2007). It is feasible to use gene editing technology such as CRISPR for inhibiting the expression of TaCKXs to regulate the sensitivity of wheat tillering to a low R/FR. For this, the relationship between a low R/FR and TaCKXs in wheat should be further verified using molecular genetics. It is important to note that increasing the tiller number in high-density populations would increase the risk of lodging. Therefore, this improvement method is suitable for varieties sensitive to a low R/FR and planting density.
Sucrose has been shown to play a signaling role in bud outgrowth (Mason et al., 2014; Wang et al., 2018). It is interesting to explore that whether sucrose is involved in a low R/FR regulation of wheat tillering and the interaction between sucrose and cytokinin in the control of tillering by the R/FR. The other limitation of the present study is a lack of research on strigolactone (SL), which is involved in bud outgrowth regulation by a low R/FR or shade (Kebrom et al., 2010; Yao and Finlayson, 2015). Because the difficulty in determining the SL level and the limited understanding of its metabolic process in biosynthesis, this study did not conduct in-depth research on the role of SL in regulating wheat tillering response to a low R/FR. Given that SL is mainly synthesized from roots, the inhibiting effect may need more time to manifest. Although SL signaling genes in axillary buds of Dendranthema grandiflorum were rapidly induced by a low R/FR (Yuan et al., 2018), this induction would be probably prevented by the presence of leaf sheaths in wheat. Therefore, physiological mechanisms in response to a low R/FR are different for plants with different branching structures, so that species characteristics need to be considered when modifying the sensitivity to a low R/FR.
Conclusion
This study provides evidence that a low R/FR can decrease CTK levels in tiller nodes by inducing degradation genes expression, thus inhibiting wheat tillering. The low R/FR did not rapidly induce ABA accumulation in the tiller bud because the bud was wrapped by the leaf sheath. Although the node IAA content and signaling were not altered under low R/FR conditions, the elevated GA levels might further promote CTK degradation in tiller nodes and inhibit tillering. Further studies will explore the relationship between a low R/FR and cytokinin degradation at the molecular level.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
KL, ZT and TD conceived and designed the research. KL, LX and LZ conducted experiments. SY and JH contributed to data collection. QT, LG and PH contributed to data analysis. KL wrote the manuscript. DJ and WC supported the resources, QT, YS, TD and ZT revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We want to thank Professor Zhigang Xu for his help in designing and manufacturing the far-red lights and thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 32272215), the National Key R&D Program of Jiangsu (BE2021361-1), and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.971003/full#supplementary-material
References
Barbier, F., Dun, E., Kerr, S., Chabikwa, T., Beveridge, C. (2019). Feature review: an update on the signals controlling shoot branching. Trends Plant Sci. 24 (3), 220–236. doi: 10.1016/j.tplants.2018.12.001
Binenbaum, J., Weinstain, R., Shani, E. (2018). Gibberellin localization and transport in plants. Trends Plant Sci. 23 (5), 410–421. doi: 10.1016/j.tplants.2018.02.005
Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64 (1), 403–427. doi: 10.1146/annurev-arplant-050312-120221
Chen, L., Zhao, J., Song, J. C., Jameson, P. E. (2021). Cytokinin glucosyl transferases, key regulators of cytokinin homeostasis, have potential value for wheat improvement. Plant Biotechnol. J. 19 (5), 878–896. doi: 10.1111/pbi.13595
Cline, M. G., Choonseok, O. H. (2006). A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann. Bot. 98 (4), 891–897. doi: 10.1093/aob/mcl173
Doust, A. N. (2007). Grass architecture: genetic and environmental control of branching. Curr. Opin. Plant Biol. 10 (1), 21–25. doi: 10.1016/j.pbi.2006.11.015
Dreccer, M. F., Chapman, S. C., Rattey, A. R., Neal, J., Song, Y. H., Christopher, J. T., et al. (2013). Developmental and growth controls of tillering and water-soluble carbohydrate accumulation in contrasting wheat (Triticum aestivum l.) genotypes: can we dissect them? J. Exp. Bot. 64 (1), 143–160. doi: 10.1093/jxb/ers317
Evers, J. B., Vos, J., Andrieu, B., Struik, P. C. (2006). Cessation of tillering in spring wheat in relation to light interception and red: far-red ratio. Ann. Bot. 97 (4), 649–658. doi: 10.1093/aob/mcl020
Fernández-Milmanda, G. L., Ballaré, C. L. (2021). Shade avoidance: expanding the color and hormone palette. Trends Plant Sci. 26 (5), 509–523. doi: 10.1016/j.tplants.2020.12.006
Finlayson, S. A., Krishnareddy, S. R., Kebrom, T. H., Casal, J. J. (2010). Phytochrome regulation of branching in arabidopsis. Plant Physiol. 152 (4), 1914–1927. doi: 10.1104/pp.109.148833
Gao, J. W., Wang, F., Hu, H., Jiang, S. Y., Muhammad, A., Shao, Y. H., et al. (2018). Improved leaf nitrogen reutilisation and rubisco activation under short-term nitrogen-deficient conditions promotes photosynthesis in winter wheat (Triticum aestivum l.) at the seedling stage. Funct. Plant Biol. 45 (8), 840–853. doi: 10.1071/FP17232
He, G., Yang, P., Tang, Y., Cao, Y., Qi, X., Xu, L., et al. (2020). Mechanism of exogenous cytokinins inducing bulbil formation in Lilium lancifolium in vitro. Plant Cell Rep. 39 (7), 861–872. doi: 10.1007/s00299-020-02535-x
Holalu, S. V., Finlayson, S. A. (2017). The ratio of red light to far red light alters arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J. Exp. Bot. 68 (5), 943–952. doi: 10.1093/jxb/erw479
Holalu, S. V., Reddy, S. K., Finlayson, S. A. (2021). Low red light : Far red light inhibits branching by promoting auxin signaling. J. Plant Growth Regul. 40 (5), 2028–2036. doi: 10.1007/s00344-020-10253-7
Hornitschek, P., Kohnen, M. V., Lorrain, S., Rougemont, J., Ljung, K., López-Vidriero, I., et al. (2012). Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71 (5), 699–711. doi: 10.1111/j.1365-313X.2012.05033.x
Hoyerová, K., Hošek, P. (2020). New insights into the metabolism and role of cytokinin n-glucosides in plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00741
Izydorczyk, C., Nguyen, T. N., Jo, S., Son, S., Tuan, P. A., Ayele, B. T. (2018). Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signalling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum l.). Plant Cell Environ. 41 (5), 1022–1037. doi: 10.1111/pce.12949
Kebrom, T. H., Brutnell, T. P., Finlayson, S. A. (2010). Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ. 33 (1), 48–58. doi: 10.1111/j.1365-3040.2009.02050.x
Kebrom, T., Mullet, J. (2016). Transcriptome profiling of tiller buds provides new insights into PhyB regulation of tillering and indeterminate growth in sorghum. Plant Physiol. 170 (4), 2232–2250. doi: 10.1104/pp.16.00014
Kojima, M., Kamada-Nobusada, T., Komatsu, H., Takei, K., Kuroha, T., Mizutani, M., et al. (2009). Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol. 50 (7), 1201–1214. doi: 10.1093/pcp/pcp057
Lang, J., Fu, Y., Zhou, Y., Cheng, M., Deng, M., Li, M., et al. (2021). Myb10-d confers PHS-3D resistance to pre-harvest sprouting by regulating NCED in ABA biosynthesis pathway of wheat. New Phytol. 230 (5), 1940–1952. doi: 10.1111/nph.17312
Leivar, P., Monte, E. (2014). PIFs: Systems integrators in plant development. Plant Cell. 26 (1), 56–78. doi: 10.1105/tpc.113.120857
Li, W., Liu, S. W., Ma, J. J., Liu, H. M., Han, F. X., Li, Y., et al. (2020). Gibberellin signaling is required for far-red light-induced shoot elongation in pinus tabuliformis seedlings. Plant Physiol. 182 (1), 658–668. doi: 10.1104/pp.19.00954
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Lo, S. F., Yang, S. Y., Chen, K. T., Hsing, Y. I., Zeevaart, J. A., Chen, L. J., et al. (2008). A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell. 20 (10), 2603–2618. doi: 10.1105/tpc.108.060913
Mason, M. G., Ross, J. J., Babst, B. A., Wienclaw, B. N., Beveridge, C. A. (2014). Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. U.S.A 111 (16), 6092–6097. doi: 10.1073/pnas.1322045111
Ma, S. W., Wang, M., Wu, J. H., Guo, W. L., Chen, Y. M., Li, G. W., et al. (2021). WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 14 (12), 1965–1968. doi: 10.1016/j.molp.2021.10.006
Müller, D., Leyser, O. (2011). Auxin, cytokinin and the control of shoot branching. Ann. Bot. 107 (7), 1203–1212. doi: 10.1093/aob/mcr069
Panda, B. B., Sekhar, S., Dash, S. K., Behera, L., Shaw, B. P. (2018). Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice. BMC Plant Biol. 18 (1), 89. doi: 10.1186/s12870-018-1279-4
Paolacci, A. R., Tanzarella, O. A., Porceddu, E., Ciaffi, M. (2009). Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 10, 11–37. doi: 10.1186/1471-2199-10-11
Pearce, S., Huttly, A. K., Prosser, I. M., Li, Y. D., Vaughan, S. P., Gallova, B., et al. (2015). Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biol. 15, 130–147. doi: 10.1186/s12870-015-0520-7
Pierik, R., De Wit, M., Voesenek, L. (2011). A Growth-mediated stress escape: convergence of signal transduction pathways activated upon exposure to two different environmental stresses. C. J. New Phytol. 189 (1), 122–134. doi: 10.1111/j.1469-8137.2010.03458.x
Qiao, L., Zhang, W., Li, X., Zhang, L., Zhang, X., Li, X., et al. (2018). Characterization and expression patterns of auxin response factors in wheat. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01395
Reddy, K. S., Finlayson, S. A. (2014). Phytochrome b promotes branching in arabidopsis by suppressing auxin signaling. Plant Physiol. 164 (3), 1542–1550. doi: 10.1104/pp.113.234021
Reddy, S. K., Holalu, S. V., Casal, J. J., Finlayson, S. A. (2013). Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 163 (2), 1047–1058. doi: 10.1104/pp.113.221895
Sakakibara, H., Takei, K., Hirose, N. (2006). Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci. 11 (9), 440–448. doi: 10.1016/j.tplants.2006.07.004
Sawers, R. J., Sheehan, M. J., Brutnell, T. P. (2005). Cereal phytochromes: targets of selection, targets for manipulation? Trends Plant Sci. 10 (3), 138–143. doi: 10.1016/j.tplants.2005.01.004
Shoaib, M., Yang, W. L., Shan, Q. Q., Sajjad, M., Zhang, A. M. (2019). Genome-wide identification and expression analysis of new cytokinin metabolic genes in bread wheat (Triticum aestivum l.). PeerJ 7, e6300. doi: 10.7717/peerj.6300
Son, S., Chitnis, V. R., Liu, A., Gao, F., Nguyen, T. N., Ayele, B. T. (2016). Abscisic acid metabolic genes of wheat (Triticum aestivum l.): identification and insights into their functionality in seed dormancy and dehydration tolerance. Planta 244 (2), 429–447. doi: 10.1007/s00425-016-2518-2
Sparkes, D. L., Holme, S. J., Gaju, O. (2006). Does light quality initiate tiller death in wheat? Eur. J. Agron. 24 (3), 212–217. doi: 10.1016/j.eja.2005.08.003
Tanaka, M., Takei, K., Kojima, M., Sakakibara, H., Mori, H. (2006). Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45 (6), 1028–1036. doi: 10.1111/j.1365-313X.2006.02656.x
Tao, Y., Ferrer, J. L., Ljung, K., Pojer, F., Hong, F., Long, J. A., et al. (2008). Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133 (1), 164–176. doi: 10.1016/j.cell.2008.01.049
Ugarte, C. C., Trupkin, S. A., Ghiglione, H., Slafer, G., Casal, J. J. (2010). Low red/far-red ratios delay spike and stem growth in wheat. J. Exp. Bot. 61 (11), 3151–3162. doi: 10.1093/jxb/erq140
Wang, B., Smith, S. M., Li, J. Y. (2018). Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 69 (1), 437–468. doi: 10.1146/annurev-arplant-042817040422
Xie, Q., Mayes, S., Sparkes, D. L. (2016). Optimizing tiller production and survival for grain yield improvement in a bread wheat x spelt mapping population. Ann. Bot. 117 (1), 51–66. doi: 10.1093/aob/mcv147
Xu, J., Zha, M., Li, Y., Ding, Y., Chen, L., Ding, C., et al. (2015). The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa l.). Plant Cell Rep. 34 (9), 1647–1662. doi: 10.1007/s00299-015-1815-8
Yang, D., Luo, Y., Kong, X., Huang, C., Wang, Z. (2020). Interactions between exogenous cytokinin and nitrogen application regulate tiller bud growth via sucrose and nitrogen allocation in winter wheat. J. Plant Growth Regul. 40, 329–341. doi: 10.1007/s00344-020-10106-3
Yao, C., Finlayson, S. A. (2015). Abscisic acid is a general negative regulator of arabidopsis axillary bud growth. Plant Physiol. 169 (1), 611–626. doi: 10.1104/pp.15.00682
Yuan, C. Q., Ahmad, S., Cheng, T. R., Wang, J., Pan, H. T., Zhao, L. J., et al. (2018). Red to far-red light ratio modulates hormonal and genetic control of axillary bud outgrowth in chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Int. J. Mol. Sci. 19 (6), 1590. doi: 10.3390/ijms19061590
Zhang, K., Gan, S. S. (2012). An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing arabidopsis leaves. Plant Physiol. 158 (2), 961–969. doi: 10.1104/pp.111.190876
Zhuang, L. L., Ge, Y., Wang, J., Yu, J. G., Yang, Z. M., Huang, B. R. (2019). Gibberellic acid inhibition of tillering in tall fescue involving crosstalks with cytokinins and transcriptional regulation of genes controlling axillary bud outgrowth. Plant Sci. 287, 110168. doi: 10.1016/j.plantsci.2019.110168
Keywords: wheat, tillering, low R/FR, hormone, cytokinin, degradation
Citation: Lei K, Tan Q, Zhu L, Xu L, Yang S, Hu J, Gao L, Hou P, Shao Y, Jiang D, Cao W, Dai T and Tian Z (2022) Low red/far-red ratio can induce cytokinin degradation resulting in the inhibition of tillering in wheat (Triticum aestivum L.). Front. Plant Sci. 13:971003. doi: 10.3389/fpls.2022.971003
Received: 16 June 2022; Accepted: 14 November 2022;
Published: 08 December 2022.
Edited by:
Ep Heuvelink, Wageningen University and Research, NetherlandsReviewed by:
Jochem B. Evers, Wageningen University and Research, NetherlandsJasmina Kurepa, University of Kentucky, United States
Copyright © 2022 Lei, Tan, Zhu, Xu, Yang, Hu, Gao, Hou, Shao, Jiang, Cao, Dai and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingbo Dai, dGluZ2JvZEBuamF1LmVkdS5jbg==; Zhongwei Tian, emh3dGlhbkBuamF1LmVkdS5jbg==
 Kangqi Lei1
Kangqi Lei1 Libing Xu
Libing Xu Jinling Hu
Jinling Hu Lijun Gao
Lijun Gao Pan Hou
Pan Hou Dong Jiang
Dong Jiang Weixing Cao
Weixing Cao Tingbo Dai
Tingbo Dai Zhongwei Tian
Zhongwei Tian