
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 07 September 2022
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.960948
 Iftikhar Ali1,2,3*†
Iftikhar Ali1,2,3*† Ajab Khan2†
Ajab Khan2† Ahmad Ali2†
Ahmad Ali2† Zahid Ullah2
Zahid Ullah2 Dong-Qin Dai1*
Dong-Qin Dai1* Naveed Khan2
Naveed Khan2 Asif Khan4
Asif Khan4 Abdel Rahman Al-Tawaha5
Abdel Rahman Al-Tawaha5 Hassan Sher2
Hassan Sher2Malnutrition is mainly caused by iron and zinc micronutrient deficiencies affecting about half of the world's population across the globe. Biofortification of staple crops is the right approach to overcome malnutrition and enhance nutrient contents in the daily food of humans. This study aimed to evaluate the role of foliar application of iron and zinc in Trichoderma harzianum treated soil on various growth characteristics, quality, and yield of wheat varieties. Plants were examined in the absence/presence of T. harzianum, and iron and zinc micronutrients in both optimal and high-stress conditions. Although the symbiotic association of T. harzianum and common wheat is utilized as an effective approach for wheat improvement because of the dynamic growth promoting the ability of the fungus, this association was found tremendously effective in the presence of foliar feeding of micronutrients for the enhancement of various growth parameters and quality of wheat. The utilization of this approach positively increased various growth parameters including spike length, grain mass, biomass, harvest index, and photosynthetic pigments. The beneficial role of T. harzianum in combination with zinc and iron in stimulating plant growth and its positive impact on the intensities of high molecular weight glutenin subunits (HMW-GS) alleles make it an interesting approach for application in eco-friendly agricultural systems. Further, this study suggests a possible alternative way that does not merely enhances the wheat yield but also its quality through proper biofortification of iron and zinc to fulfill the daily needs of micronutrients in staple food.
Wheat (Triticum aestivum L.) belongs to the family Poaceae (grasses). It is an essential staple food and the most widely grown cereal crop in the world with a global annual production of more than 620 million tons (Mujeeb-Kazi et al., 2008). Approximately 36% of the world's population depends on wheat as a primary source of food due to the presence of 20% of the food calories, 55% carbohydrates (70–75% starch), 10–12% proteins, 14% water and lipids (2%) (Goel et al., 2021). Being staple food, wheat is mainly used as a source of food for humans while also utilized as a source of fodder for animals and as a substrate for mushroom cultivation (Shewry, 2009). The production of wheat is however severely affected by various biotic and abiotic factors (Waller et al., 2005). To encounter the need of a rapidly growing population, alternative approaches are required to enhance the wheat quality and yield (Cunha et al., 2022).
Micronutrients are required in lower amounts but are extremely vital for plant growth as compared to macronutrients (Tripathi et al., 2015). Deficiencies of micronutrients especially zinc (Zn) and iron (Fe) result in various diseases both in humans and plants (Pallavi and Sudha, 2017). In humans, micronutrient deficiencies are mainly due to their less intake in daily diet, while the growth and yield of plants are also affected when these micronutrients are less available in soil (Kihara et al., 2020). In cereal crops, Zn deficiency is a major problem because it reduces crop yields and human nutritional quality, particularly in those regions of the world which have a deficiency of Zn in soil (Cakmak and Kutman, 2018). Therefore, any increase in Zn and Fe concentrations in the cereal crops provides for healthy plants, enhancing crop yield and improving human health worldwide.
Trichoderma harzianum belongs to the genus Trichoderma and is characterized by rapid growth establishing a strong symbiotic association with many plant species (Visconti et al., 2020). T. harzianum can promote plant growth by enhancing the availability of nutrients to plants (Chun et al., 2018). It is present in almost every ecosystem and is involved in inhibiting plant pathogens in the soil to reduce plant diseases due to their mycoparasitic capacities (Hermosa et al., 2012; Guo Y. et al., 2019). Trichoderma has been used to protect different types of cereal crops from soil borne as well as air-borne diseases (Woo et al., 2014). Various species of Trichoderma improve plant growth by protecting them from bacteria and other pathogenic fungi (Błaszczyk et al., 2014).
Wheat has unique protein characteristics that serve as a vital resource of food and energy (Iftikhar and Ali, 2017). Among all cereals, wheat is the most extensively used crop accounting for about 20% of proteins and dietary calories required for human beings (Gupta et al., 2021). Glutenin, a type of storage protein in the wheat endosperm is considered to play a key role in controlling bread quality (Ali et al., 2013; Khalid et al., 2013; Ullah et al., 2016). Glutenin proteins contribute to 80–85% of the total wheat flour protein and are responsible for the viscoelastic properties of wheat flour and dough (Shewry et al., 1995). Further, the seeds of wheat contain proteins that determine the quality of bread making (Ali A. et al., 2015; Ali I. et al., 2015). Based on the mobility in SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), glutenin proteins are divided into two subunits, high molecular weight glutenin subunits (HMW-GS) and low molecular weight glutenin subunits (LMW-GS) (Payne et al., 1979). Both LMW-GS and HMW-GS are a long chain of amino acids linked together by disulfide bonds forming macropolymer glutenin. Among them, HMW-GS plays an important role in controlling bread-making quality (Ali et al., 2013). Glutenin proteins can also be utilized as a biochemical marker for the identification and validation of wheat cultivars to check the purity of seed (Ullah et al., 2016), and to determine the quality of wheat products and human nutrition (Xu et al., 2012).
Wheat growth and development are affected by various biotic and abiotic factors. Further, high increase in population growth, industrialization, stagnant wheat yield per unit area, lack of essential micro-macronutrients, the use of uncertified seeds and pathogens, and pest attacks significantly increase the wheat production requirement globally (Ali et al., 2014; Seleiman et al., 2021; Erenstein et al., 2022; Reynolds and Braun, 2022).
Nutrient foliar application is considered an important approach to enhancing crop yields and grain micronutrient content. Several studies have demonstrated that zinc and iron foliar application resulted in enhanced growth and yield characteristics of wheat crops (Cakmak et al., 2010; Li et al., 2016). However, little is known about the combined effect of Zn and Fe on the quality traits of wheat kernels. The current study was therefore conducted to investigate the combined effect of zinc, iron, and T. harzianum on wheat growth, yield attributes, and grain quality with a focus on the intensity of HMW-GS alleles.
The research was conducted at the Center for Plant Sciences and Biodiversity, University of Swat, Khyber Pakhtunkhwa, Pakistan. The experimental site is located at 34°3'N and 71°50'E, 940 m asl with an annual rainfall of 737.3 to 1,200 mm and an average temperature from 11.3 to 25.7°C. Detailed characterization of the experimental soil revealed its texture as silt loam with 3.2% sand, 26.7% clay, and 70.1% silt; having 1.2% organic matter; pH 6.4; EC 0.17 m.mohs cm−1; noncalcareous and non-saline in nature. The recommended fertilizer dose of 20–60–60 kg ha−1 (NPK) was applied before sowing. Healthy and clean seeds of Pirsabak 2005 were sown in 3 m long rows with 30 cm inter-row spacing which formed an experimental unit/plot. Compost was administered to all plots at the rate of 20 tons ha−1. The conidia suspension of T. harzianum strain G8 was prepared according to the method of Zhang et al. (2014). G8 strain obtained from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China was cultured at 25°C for 1 week in Petri dishes containing potato dextrose agar (PDA) solid medium. The conidia suspension of T. harzianum was then made by adding 5 ml of sterilized distilled water to the surface of the agar and the collected suspension was transferred into 500 ml beakers. The suspension was then adjusted to 100 ml, the conidia were separated and dispersed by a hand mixer and the conidia density was determined by a hemocytometer and adjusted to 1.5 × 107 conidia per ml. Then 1 ml of the conidia suspension of T. harzianum was inoculated into 60 ml of the sterilized liquid culture medium of potato dextrose broth (PDB) in a 150 ml flask. Then the flask was kept at 30°C for 9 days in a shaking incubator running at 120 rpm. The fungi were purified, and the spore suspension produced was inoculated in fresh PDB containing a drop of Tween-80. The spores were collected in sterile water and were filtered through a Whatman Paper and then through 0.22 mm Millipore membranes. The concentration was then adjusted to 1.5 × 107 spores per ml and was used for treatment. At a pre-anthesis stage, following Sesan et al. (2020) with minor modification as per the requirement of the experiment, micronutrients including zinc as ZnSO4 (zinc sulfate) and iron as FeSO4 (iron sulfate) were applied as foliar spray alone and in combination with T. harzianum, each in two concentrations at the rate of 9 and 18 mm, respectively. A total of 12 treatments were applied which comprised of control; T.harzianum; Zn-9mM; Fe-9mM; Zn-18mM; Fe-18mM; Fe-9mM/T.harzianum; Fe-18mM/ T.harzianum; Zn-9mM/T.harzianum; Zn-18mM/T.harzianum; Fe-9mM)/T.harzianum/Zn-9mM; and, Fe-18mM/T.harzianum/ Zn-18mM. The layout of the experiment followed a randomized complete block design having 3 replications (Li et al., 2016).
After 10 days of applying these treatments, fresh leaves were collected from every plot to measure chlorophyll quantity following the method of Hiscox and Israelstam (1979). Fresh leaves of 0.5 g were collected, wrapped in aluminum foil, and kept in the freezer for 72 h. Weighed leaf tissue in fractions was taken in a tube containing 6 ml dimethyl sulfoxide (DMSO) preheated to 65°C in a water bath for 30–40 min. Then 4 ml DMSO was added to all samples to make the final volume 10 ml. Finally, the solution extracted from leaves was observed in 645 and 663 nm with a spectrophotometer (BMS-UV 1602). Chlorophyll a, b, and total chlorophyll contents were calculated by using the equations of Arnon's (1949).
Phenological traits including days to heading (DH), days to physiological maturity (PM), spike length (SPL), seeds per spike (SPS), and grain mass (GM) were recorded as discussed in Afzal et al. (2018). Biomass and harvest index of the experimental plant material was also determined as described in Dreisigacker et al. (2021).
Identification of HMW-GS of the studied wheat was accomplished by crushing a single kernel from each treatment, followed by protein extraction and SDS-PAGE analysis according to Ali et al. (2013). The SDS-PAGE gel was stained in a solution containing 44% methanol, 6% acetic acid, and 0.22% Coomassie brilliant blue R350, while, de-stained in a solution of 20% methanol and 5% acetic acid. The allelic diversity at different locus Glu-A1, Glu-B1, and Glu-D1 was determined using 12 different quality standards obtained from CIMMYT, Mexico (Ullah et al., 2016). The intensity of HMW-GS under different treatments was also measured by a computer software image J. For this purpose, the de-stained gel was scanned with a high-resolution scanner, converted to a grayscale image, and processed as previously described in Ali et al. (2020) and Bonilla et al. (2020).
The data were recorded in triplicates for each treatment and analyzed by one-way analysis of variance. Multiple Duncan range test (DMRT) using SPSS version 21.0 depicted the differences among treatments at p < 0.05 level.
The foliar application of Zn and Fe, either alone or in combination with T. harzianum, enhanced all the studied attributes in the experimental wheat. Analysis of variance depicted differences among treatments with respect to the studied traits including days to heading (DH), physiological maturity (PM), plant height (PH), spike length (SPL), grain mass (GM), seeds per spike (SPS), chlorophyll-a (Chla), total chlorophyll (TChl) and biomass (Table 1; Supplementary Table S1).
The studied wheat also exhibited differential behavior in response to foliar application of micronutrients and T. harzianum (Figure 1; Table 2; Supplementary Table S2). It was observed that treatment either alone or in combination significantly stimulated days to heading, and a maximum enhanced effect of 3.6% was found in wheat plants treated with T. harzianum, while the least effect was noted in plants treated with Fe-9mM/T.harzianum/Zn-9mM when compared with control treatment. Regarding days to physiological maturity, the maximum effect (3.2%) was recorded for treatment Fe-18mM/T.harzianum/Zn-18mM. However, PM was negatively affected by treatment Fe-18mM/T.harzianum with an observed decrease of 18.9% when compared to plants grown in control conditions. Similarly, in comparison with control conditions, all the treatments resulted in enhanced plant height, however, the most effective amongst all the treatments was Zn-18mM/T.harzianum which led to the maximum recorded enhanced PH of 6.3%. The effect of other treatments including Fe-18mM/T.harzianum/Zn-18mM and Zn-9mM was negative which decreased PH by 22.7 and 13.8%, respectively. Concerning spike length, the treatment Fe-9mM/T.harzianum was much more promising as it enhanced SPL by 12.2%, while other treatments negatively affected the same studied trait which included Zn-18mM (21.8%), Fe-9mM/T.harzianum/Zn-9mM (15.1%) and Zn-9mM/T.harzianum 3.9%). The maximum harvest index was exhibited in the plants treated with Zn-9 mM/T.harzianum. While, as compared to control the least harvest index was exhibited in the plants treated with Zn-9mM. Similarly, the current research showed that maximum seeds per spike were exhibited by plants treated with Fe-9mM/T.harzianum.
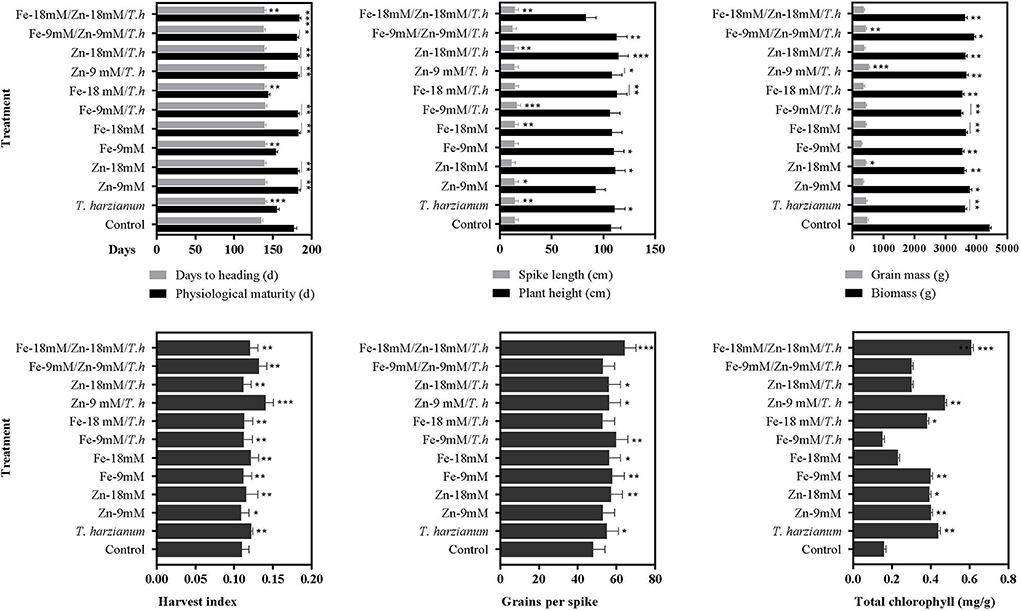
Figure 1. Effects of Fe, Zn, and Trichoderma harzianum on the studied traits. Where: *, ** and *** are depicting statistical significances at 0.05, 0.01, and 0.001 probability level, respectively. While the bars without * are showing no statistically significant differences (NS).
As compared to the control, the chlorophyll contents increased with the application of Zn, Fe, and T. harzianum (Figures 1, 2; Table 2). Likewise, chlorophyll contents were significantly increased in wheat plants treated with the Fe-18mM/T.harzianum and Zn-18mM/T.harzianum. It is pertinent to mention that chlorophyll content remained almost unaffected in response to both foliar applications of Zn and Fe. On the contrary, the treatment Fe-9mM/T.harzianum resulted in a slight decrease in total chlorophyll content. However, its combination with T. harzianum significantly enhanced chlorophyll content, but only at a high concentration (18mM).
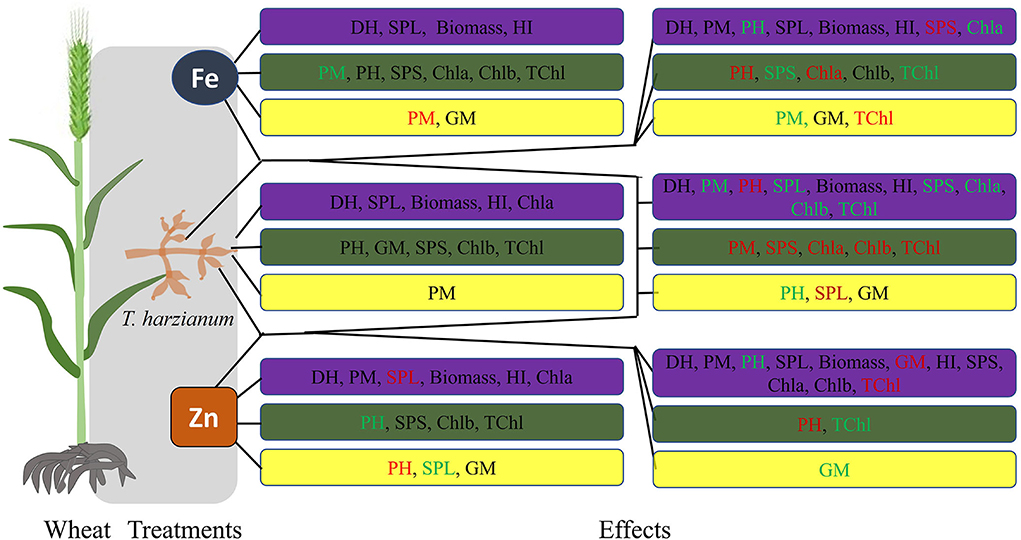
Figure 2. Overview of wheat responses for the studied traits to different treatments. Blue boxes represent enhanced effect; green boxes represent no significant differences; while, yellow boxes represent negative/decreased effect of the respective treatment, in comparison to control. Further, the black font color of the studied trait represents a response to both low and high concentration; the red color represents a response to low concentration; while, the green color represents a response to the high concentration of the respective treatment.
In the currently studied wheat genotype (Pirsabak 2005), the observed compositions of HMW-GS were 2* (encoded by Glu-A1 b), 17+18 (encoded by Glu-B1i), and 2+12 (encoded by GLUD1a) (Figure 3; Supplementary Figure S1; Supplementary Table S2). This is denoted that in wheat, HMW-GS are coded by co-dominant genes GluA1, Glu-B1, and Glu-D1 which are present at Glu-1 loci on the long arms of the homologous group 1 chromosome. For correct scoring of HMW-GS 12 different standards were selected and were used in SDS-PAGE along with our samples. Variable densities of HMW-GS alleles were found in response to different foliar treatments (Figure 3). For the accuracy and validity of the observed data, the average area integrated densities were determined from three different replicates. The observed densities of Glu-A1 were enhanced by all the treatments except Zn-9mM, Fe-9mM, and Zn-18mM/T.harzianum. In comparison to control treatment, maximum increased densities were depicted by Fe-9mM/T.harzianum/Zn-9mM (31.1%), Fe-18mM/T.harzianum (16.7%), Zn-9mM/T.harzianum (10.8%) and Fe-18mM (3.3%). Regarding Glu-B1 alleles, increased densities were observed for the treatments Fe-18mM (41.6%), Fe-9mM (22.8%), Fe-9mM/T.harzianum/Zn-9mM (20.1%), Zn-9mM/T.harzianum (11.4%) and T.harzianum (6.3%). While the treatments Zn-18mM and Fe-9mM/T.harzianum negatively affected its relative densities. Similarly, with respect to Glu-D1, the treatments Fe-18mM (20.6%), Fe-9mM/T.harzianum/Zn-9mM (17.3%), T.harzianum (7.9%) and Fe-18mM/T.harzianum (2.2%) led to increased densities in comparison to control.
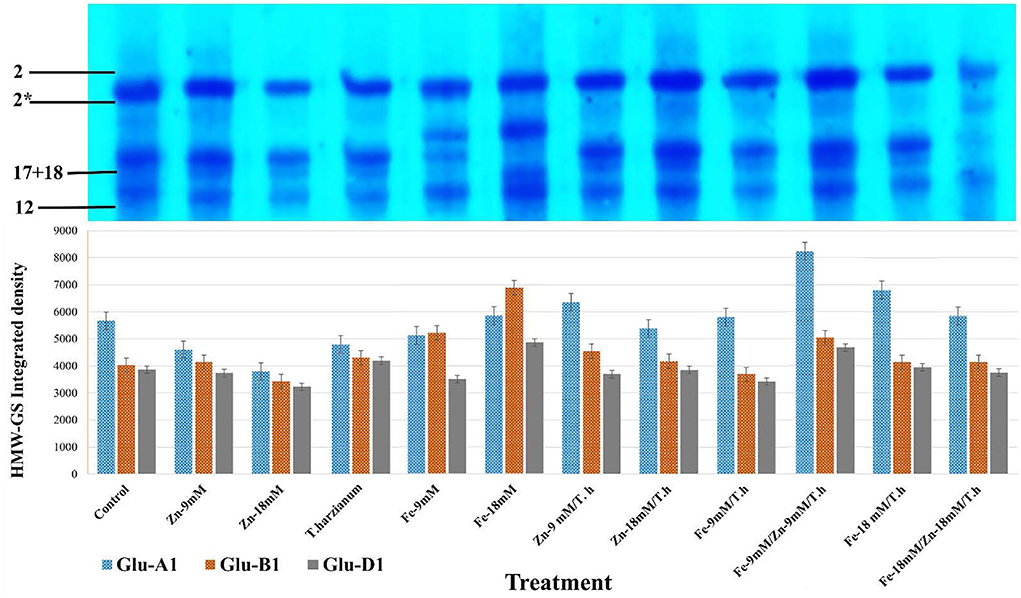
Figure 3. SDS-PAGE profile of HMW-GS alleles of the studied wheat cultivar and its area integrated densities under different treatments. For accuracy and validity of data, the average area integrated densities were determined for comparison. HMW-GS 2* asterisk.
Biofortification is a suitable approach to improving crop yield and the quality of human diet (White and Broadley, 2009). Particularly in areas having malnutrition, this current research identified new approaches that will result in the improvement of the nutritional quality of crops without reducing yields (Aziz et al., 2019). Minerals nutrition plays an important role in the plant's physio-molecular traits including photosynthesis, gene expression, carbohydrate accumulation, enzymatic activities, signal transduction pathways, respiration, and recovery from stress conditions (Li et al., 2016). However, the quantity of nutrients (zinc and iron) requirement considerably depends on its quantity as well as its available form in the soil, cultivar characteristics, and yield (Rashid and Ryan, 2004). The unavailability of micronutrients in the soil might be due to its complex form (chelation), access to CaCO3, organic matter, and pH of the soil (Salim and Raza, 2020). Foliar application of micronutrients is one of the best and most suitable approaches which can provide a practical way for the improvement of cereal crop yield with balanced nutrients (Aziz et al., 2019). Further, another approach to increase mineral availability to plants includes the use of plant growth promoting microbes (PGPM), which significantly improve the yield attributes of the wheat crop, like plant height, dry and fresh weight, net photosynthesis, grain yield, spike length and other physiochemical parameters of wheat plants (El-Katatny and Idres, 2014).
Foliar application of iron and zinc alone and in combination with T. harzianum significantly affected various growth traits of the studied wheat cultivar including increased leaf chlorophyll contents, spike length, plant height, biomass, and grain mass. This may be attributed to the potential promising role of Zn and Fe in the grain filling and reproductive stages of wheat as previously discussed by Zain et al. (2015) and Yaseen et al. (2018). Our findings are in general agreement with those reported by El-Katatny and Idres (2014), Colla et al. (2015), and Sani et al. (2020), which further support the potential effects of these micronutrients in combination with T. harzianum to enhance plant overall performances. Our results are also in general agreement with those reported by Jalal et al. (2020) who found that foliar application of Zn in combination with Fe significantly improved grain yield, grain nutrients, and biomass. Higher yield in the studied wheat under foliar application of micronutrients may be due to better availability of these micronutrients, as these nutrients may be less available when applied to soil due to their immobility in alkaline soils. Physio-biochemical roles of Fe and Zn in plant growth have been previously reported by Hanjagi and Singh (2017). Significant roles of Zn and Fe in the improvement of wheat physiology have also been reported by Babaeian et al. (2011) and Impa et al. (2019). Another reason for higher yield could be due to the presence of environmentally friendly microbe i.e., Trichoderma which can enhance crop quality and quantity in an eco-friendly manner (Ogut and Er, 2006). In contrast, in the access quantity, these minerals could also form chelates not easily available to plants though Trichoderma helps in the conversion of them into the simplest form due to which the soil becomes more fertile resulting in better plant growth and development (Visconti et al., 2020).
Micronutrients, specifically Zn and Fe have a significant role in plant physiology and biochemical metabolism (Moshfeghi et al., 2019). For instance, Hermosa et al. (2012) reported that the supply of zinc in proper amounts increased plant physiological activities. Previously it was also reported by Ma et al. (2017) that under adverse conditions, such as salt and drought stress, application of Zn and Fe significantly increased photosynthetic pigments, photosynthesis rate, and overall growth of plants. Soil or foliar application of Fe and Zn separately or in combination increased plant height, tiller number, spike length, seeds per spike, 1,000 grain weight, biomass, and harvesting index in wheat plants (Piri, 2012; Ramzan et al., 2020).
However, the availability of these micronutrients in alkaline and calcareous soils become insufficient and often leads to a reduction in plant growth (Gentili et al., 2018). As Fe and Zn have limited mobility inside the plant body, adding them directly over parts of the plants is a good strategy. Foliar applications can serve this purpose very efficiently. An increase in the uptake of micronutrients by foliar application in the current study indicated the effectiveness of the foliar application. Yaseen et al. (2018) suggested that this approach is particularly true for saline and alkaline soils. Moreover, an increase in the uptake of micronutrients can enhance the uptake of macronutrients in plants (Abbas et al., 2009).
When compared to control, the growth traits like spike length and plant and grain biomass were non-significantly affected. A similar finding was reported by Sahin and Işler (2021) where Zn foliar spray did not significantly increase the soybean quantity and quality. In contrast, Hosseinzadeh and Ahmadpour (2018) reported that Zn foliar spray somehow resulted in enhanced growth and development, especially at the flowering stage. Our results about spike length are in general agreement with those described by Abbas et al. (2009) who found that application of Zn significantly increased spike length. Similarly, Zain et al. (2015) also found that spike length was highly increased when micronutrients (ZnSO4 and FeSO4) were applied as a foliar application. Likewise, Afshar et al. (2020) also reported that foliar application of Zn meaningfully increased spike length than control. However, the reason could be due to the long-term exposure of plants to any sort of stress that adversely affect the growth and development of plants. It is one of the key factors causes that affect soil properties, making it more acidic, impacting cation exchanges and soil structure, and influencing microorganism's beneficial capabilities and useful oxidation-reduction reactions.
Photosynthesis in plants is regulated by several factors like nutrient availability, pH of the soil, light, water, heat, and carbon dioxide (Guo J. et al., 2019). These factors interfere directly and indirectly by altering the enzymatic and chemical processes in the photosynthesis of plants. In the current study, the chlorophyll contents were significantly improved with the application of Fe, Zn, and T. harzianum combined treatment. It has been hypothesized that Zn and Fe improved the synthesis of chlorophyll because they act as a structural component as well as a catalytic component of enzymes and proteins, and are also essential for the normal synthesis of photosynthetic pigment (Wani et al., 2022). Trichoderma sp. was previously reported to have significant impacts on drought stress, particularly T. harizianum have been reported to have beneficial applications for rice drought tolerance (Shukla et al., 2012; Khadka and Uphoff, 2019; Hewedy et al., 2020).
Our results are in agreement with the previous findings of Colla et al. (2015), who found that arbuscular mycorrhizal fungi have the capabilities of root colonization that ultimately increase the chlorophyll biosynthesis, functions, and efficacy, which could be associated with higher net CO2 assimilation rate in the plant's growth and development. Another possible reason could be the availability of maximum chlorophyll contents and micronutrients which are the basic structural and functional components, which they colonize during the photosynthetic organelle's biosynthesis (Moshfeghi et al., 2019; Niyigaba et al., 2019; Szerement et al., 2021).
The observed composition of HMW-GS for the studied wheat cultivar was 2*, 17 + 18, and 2 + 12. Further, variable integrated densities of HMW-GS alleles were found in response to different foliar treatments. For instance, in Glu-A1, maximum increased densities were depicted by Fe-18mM/T.harzianum, Fe-9mM/T.harzianum/Zn-9mM, Zn-9mM/T.harzianum and Fe-18mM, in comparison to control. Similarly, in Glu-D1, treatments including Fe-18mM, T.harzianum, Fe-9mM/T.harzianum/Zn-9mM, and Fe-18mM/T.harzianum led to increased densities in comparison to control. These results predict a potentially significant role of the provided treatments in cultivating wheat for different quality traits. For example, it has been reported that glutenin strength increases with the HMW-GS allelic combination of 5 + 10, 17 + 18, and 2* (Song et al., 2020). Similarly, Maryami et al. (2020) observed that Glu-A1 alleles including 2* were associated with higher gluten quality than the null allele and that Glu-B1 encoded subunit 17 + 18 was highly correlated with bread texture, volume, and cell traits. The beneficial role of T. harzianum in combination with zinc and iron in stimulating plant growth, efficient use of micronutrients, and its positive impact on the intensities of HMW-GS alleles make it interesting as an alternate approach for application in eco-friendly agricultural systems (Vukelic et al., 2021; Ali et al., 2022).
Application of T. harzianum in combination with foliar applied zinc and iron significantly enhanced plant height, spike length, grain yield, number of seeds per spike, and harvest index specifically in treatments Zn-9mM, Zn-18mM, Fe-9mM/T.harzianum/Zn-9mM, and Fe-18mM. Photosynthetic pigments such as chlorophyll a, chlorophyll b, and total chlorophyll were also improved with the application of micronutrients in combination with T. harzianum. The current study could be used successfully for bread wheat development programs and may serve to meet the need for bread wheat with enhanced yield attributes and appropriate gluten strength.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
AA and ZU designed the research. IA, AjK, and NK conducted the research. IA, AA, AjK, and AsK analyzed the data. HS, D-QD, and AA-T checked and revised the final content of the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (No. NSFC 31760013) and High-Level Talent Recruitment Plan of Yunnan Provinces Young Talents Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.960948/full#supplementary-material
Abbas, G., Khan, M. Q., Khan, M. J., Hussain, F., and Hussain, I. (2009). Effect of iron on the growth and yield contributing parameters of wheat (Triticum aestivum L.). J. Anim. Plant Sci. 19, 135–139.
Afshar, R. K., Chen, C., Zhou, S., Etemadi, F., He, H., and Li, Z. (2020). Agronomic and economic response of bread wheat to foliar zinc application. Agron. J. 112, 4045–4056. doi: 10.1002/agj2.20247
Afzal, F., Ali, A., Ullah, Z., Sher, H., Gul, A., Mujeeb-Kazi, A., et al. (2018). Terminal drought stress adaptability in synthetic derived bread wheat is explained by alleles of major adaptability genes and superior phenology. Int. J. Agric. Biol. 20, 1623–1631. doi: 10.17957/IJAB/15.0680
Ali, A., Ali, Z., Quraishi, U. M., Kazi, A. G., Malik, R. N., Sher, H., et al. (2014). “Integrating physiological and genetic approaches for improving drought tolerance in crops,” in Emerging Technologies and Management of Crop Stress Tolerance eds P. Ahmad and S. Rasool (Massachusetts, MA: Academic Press), 74, 315–345.
Ali, A., Arshad, M., Mastrangelo, A. M., De Vita, P., Gul-Kazi, A., and Mujeeb-Kazi, A. (2013). Comparative assessment of glutenin composition and its relationship with grain quality traits in bread wheat and synthetic derivatives. Pak. J. Bot. 45, 289–296.
Ali, A., Arshad, M., Saqlan Naqvi, S. M., Rasheed, A., Sher, H., Kazi, A. G., et al. (2015a). Comparative assessment of synthetic-derived and conventional bread wheat advanced lines under osmotic stress and implications for molecular analysis. Plant Mol. Biol. Report. 33, 1907–1917. doi: 10.1007/s11105-015-0884-8
Ali, A., Ullah, Z., Alam, N., Naqvi, S. M., Jamil, M., Bux, H., et al. (2020). Genetic analysis of wheat grains using digital imaging and their relationship to enhance grain weight. Sci. Agric. 77, 1–10. doi: 10.1590/1678-992x-2019-0069
Ali, I., Sardar, Z., Rasheed, A., and Mahmood, T. (2015b). Molecular characterization of the puroindoline-a and b alleles in synthetic hexaploid wheats and in silico functional and structural insights into Pina-D1. J. Theor. Biol. 376, 1–7. doi: 10.1016/j.jtbi.2015.04.001
Ali, S., Khan, M. J., Anjum, M. M., Khan, G. R., and Ali, N. (2022). Trichoderma harzianum modulates phosphate and micronutrient solubilization in the rhizosphere. Gesunde Pflanzen 74, 1–10. doi: 10.1007/s10343-022-00643-0
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1. doi: 10.1104/pp.24.1.1
Aziz, M. Z., Yaseen, M., Abbas, T., Naveed, M., Mustafa, A., Hamid, Y., et al. (2019). Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 18, 1369–1378. doi: 10.1016/S2095-3119(18)62095-7
Babaeian, M., Tavassoli, A., Ghanbari, A., Esmaeilian, Y., and Fahimifard, M. (2011). Effects of foliar micronutrient application on osmotic adjustments, grain yield and yield components in sunflower (Alstar cultivar) under water stress at three stages. Afr. J. Agric. Res. 6, 1204–1208.
Błaszczyk, L. M. S. K. S., Siwulski, M., Sobieralski, K., Lisiecka, J., and Jedryczka, M. (2014). Trichoderma spp.–application and prospects for use in organic farming and industry. J. Plant Protect. Res. 54, 309–317. doi: 10.2478/jppr-2014-0047
Bonilla, J. C., Erturk, M. Y., Schaber, J. A., and Kokini, J. L. (2020). Distribution and function of LMW glutenins, HMW glutenins, and gliadins in wheat doughs analyzed with ‘in situ'detection and quantitative imaging techniques. J. Cereal Sci. 93, 102931. doi: 10.1016/j.jcs.2020.102931
Cakmak, I., Kalayci, M., Kaya, Y., Torun, A. A., Aydin, N., Wang, Y., et al. (2010). Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 58, 9092–9102. doi: 10.1021/jf101197h
Cakmak, I., and Kutman, U. B. (2018). Agronomic biofortification of cereals with zinc: a review. Eur. J. Soil Sci. 69, 172–180. doi: 10.1111/ejss.12437
Chun, J., Yang, H. E., and Kim, D. H. (2018). Identification of a novel partitivirus of Trichoderma harzianum NFCF319 and evidence for the related antifungal activity. Front. Plant Sci. 9, 1699. doi: 10.3389/fpls.2018.01699
Colla, G., Rouphael, Y., Bonini, P., and Cardarelli, M. (2015). Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int. J. Plant Prod. 9, 171–190. doi: 10.22069/IJPP.2015.2042
Cunha, M. C., Serpa, D., Marques, J., Keizer, J. J., and Abrantes, N. (2022). On sustainable improvements of agricultural practices in the Bairrada region (Portugal). Environ. Dev. Sustain. 24, 1–23. doi: 10.1007/s10668-022-02155-3
Dreisigacker, S., Burgue?o, J., Pacheco, A., Molero, G., Sukumaran, S., Rivera-Amado, C., et al. (2021). Effect of flowering time-related genes on biomass, harvest index, and grain yield in CIMMYT elite spring bread wheat. Biology. 10, 855. doi: 10.3390/biology10090855
El-Katatny, M. H., and Idres, M. M. (2014). Effects of single and combined inoculations with Azospirillum brasilense and Trichoderma harzianum on seedling growth or yield parameters of wheat (Triticum vulgaris L., Giza 168) and corn (Zea mays L., hybrid 310). J. Plant Nutr. 37, 1913–1936. doi: 10.1080/01904167.2014.911322
Erenstein, O., Jaleta, M., Mottaleb, K. A., Sonder, K., Donovan, J., and Braun, H. J. (2022). “Global trends in wheat production, consumption and trade,” in Wheat Improvement (Cham: Springer), 47–66.
Gentili, R., Ambrosini, R., Montagnani, C., Caronni, S., and Citterio, S. (2018). Effect of soil pH on the growth, reproductive investment and pollen allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 9, 1335. doi: 10.3389/fpls.2018.01335
Goel, S., Singh, M., Grewal, S., Razzaq, A., and Wani, S. H. (2021). Wheat proteins: a valuable resources to improve nutritional value of bread. Front. Sustain. Food Syst. doi: 10.3389/fsufs.2021.769681
Guo, J., Jia, Y., Chen, H., Zhang, L., Yang, J., Zhang, J., et al. (2019a). Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-018-37838-3
Guo, Y., Ghirardo, A., Weber, B., Schnitzler, J. P., Benz, J. P., and Rosenkranz, M. (2019b). Trichoderma species differ in their volatile profiles and in antagonism toward ectomycorrhiza Laccaria bicolor. Front. Microbiol. 10, 891. doi: 10.3389/fmicb.2019.00891
Gupta, R., Meghwal, M., and Prabhakar, P. K. (2021). Bioactive compounds of pigmented wheat (Triticum aestivum): potential benefits in human health. Trends Food Sci. Technol. 110, 240–252. doi: 10.1016/j.tifs.2021.02.003
Hanjagi, P. S., and Singh, B. (2017). Interactive regulation of iron and zinc nutrition in wheat (Triticum aestivum L.). Indian J. Plant Physiol. 22, 70–78. doi: 10.1007/s40502-016-0272-x
Hermosa, R., Viterbo, A., Chet, I., and Monte, E (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158, 17–25. doi: 10.1099/mic.0.052274-0
Hewedy, O. A., Lateif, K. S., Seleiman, M. F., Shami, A., Albarakaty, F. M., and M El-Meihy, R. (2020). Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology. 9, 189. doi: 10.3390/biology9080189
Hiscox, J. D., and Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canad. J. Bot. 57, 1332–1334.
Hosseinzadeh, S. R., and Ahmadpour, R. (2018). Evaluation of vermicompost fertilizer application on growth, nutrient uptake and photosynthetic pigments of lentil (Lens culinaris Medik.) under moisture deficiency conditions. J. Plant Nutr. 41, 1276–1284. doi: 10.1080/01904167.2018.1450419
Iftikhar, A., and Ali, I. (2017). Kernel softness in wheat is determined by starch granule bound Puroindoline proteins. J. Plant Biochem. Biotechnol. 26, 247–262. doi: 10.1007/s13562-016-0387-1
Impa, S. M., Perumal, R., Bean, S. R., Sunoj, V. J., and Jagadish, S. K. (2019). Water deficit and heat stress induced alterations in grain physico-chemical characteristics and micronutrient composition in field grown grain sorghum. J. Cereal Sci. 86, 124–131. doi: 10.1016/j.jcs.2019.01.013
Jalal, A., Shah, S., Carvalho Minhoto Teixeira Filho, M., Khan, A., Shah, T., Ilyas, M., et al. (2020). Agro-biofortification of zinc and iron in wheat grains. Gesunde Pflanzen 72, 227–236. doi: 10.1007/s10343-020-00505-7
Khadka, R. B., and Uphoff, N. (2019). Effects of Trichoderma seedling treatment with System of Rice Intensification management and with conventional management of transplanted rice. Peer J. 7, e5877. doi: 10.7717/peerj.5877
Khalid, M., Mahmood, T., Rasheed, A., Kazi, A. G., Ali, A., and Kazi, A. M. (2013). Glu−DT1 allelic variation in synthetic hexaploid wheats derived from durum cultivar 'Decoy' x Aegilops tauschii accessional crosses. Pak. J. Bot. 45, 409–414.
Kihara, J., Bolo, P., Kinyua, M., Rurinda, J., and Piikki, K. (2020). Micronutrient deficiencies in African soils and the human nutritional nexus: opportunities with staple crops. Environ. Geochem. Health 42, 3015–3033. doi: 10.1007/s10653-019-00499-w
Li, M., Wang, S., Tian, X., Li, S., Chen, Y., Jia, Z., et al. (2016). Zinc and iron concentrations in grain milling fractions through combined foliar applications of Zn and macronutrients. Field Crops Res. 187, 135–141. doi: 10.1016/j.fcr.2015.12.018
Ma, D., Sun, D., Wang, C., Ding, H., Qin, H., Hou, J., et al. (2017). Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 8, 860. doi: 10.3389/fpls.2017.00860
Maryami, Z., Huertas-García, A. B., Azimi, M. R., Hernández-Espinosa, N., Payne, T., Cervantes, F., et al. (2020). Variability for glutenins, gluten quality, iron, zinc and phytic acid in a set of one hundred and fifty-eight common wheat landraces from Iran. Agronomy 10, 1797. doi: 10.3390/agronomy10111797
Moshfeghi, N., Heidari, M., Asghari, H. R., Abadi, M. B. F., Abbott, L. K., and Chen, Y. (2019). Effect of zinc foliar application and mycorrhizal inoculation on morpho-physiological traits and yield parameters of two barley cultivars. Ital. J.Agron. 14, 67–77. doi: 10.4081/ija.2019.1354
Mujeeb-Kazi, A., Gul, A., Farooq, M., Rizwan, S., and Ahmad, I. (2008). Rebirth of synthetic hexaploids with global implications for wheat improvement. Aust. J. Agric. Res. 59, 391–398. doi: 10.1071/AR07226
Niyigaba, E., Twizerimana, A., Mugenzi, I., Ngnadong, W. A., Ye, Y. P., Wu, B. M., et al. (2019). Winter wheat grain quality, zinc and iron concentration affected by a combined foliar spray of zinc and iron fertilizers. Agronomy, 9, 250. doi: 10.3390/agronomy9050250
Ogut, M., and Er, F. (2006). Micronutrient composition of field-grown dry bean and wheat inoculated with Azospirillum and Trichoderma. J. Plant Nutr. Soil Sci. 169, 699–703. doi: 10.1002/jpln.200520597
Pallavi, V., and Sudha, T. (2017). Effect of soil and foliar application of zinc and iron on productivity and quality of wheat. J Farm Sci, 30, 49–51.
Payne, P. I., Corfield, K. G., and Blackman, J. A. (1979). Identification of a high-molecular-weight subunit of glutenin whose presence correlates with bread-making quality in wheats of related pedigree. Theoret. Appl. Genet. 55, 153–159. doi: 10.1007/BF00295442
Piri, I. (2012). Effect of phosphorus fertilizer and micronutrients foliar application on sorghum yield. Ann. Biol. Res. 3, 3998–4001.
Ramzan, Y., Hafeez, M. B., Khan, S., Nadeem, M., Batool, S., and Ahmad, J. (2020). Biofortification with zinc and iron improves the grain quality and yield of wheat crop. Int. J. Plant Prod. 14, 501–510. doi: 10.1007/s42106-020-00100-w
Rashid, A., and Ryan, J. (2004). Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: a review. J. Plant Nutr. 27, 959–975. doi: 10.1081/PLN-120037530
Reynolds, M. P., and Braun, H. J. (2022). “Wheat improvement,” in Wheat Improvement, eds M. P. Reynolds, and H. J. Braun (Cham: Springer).
Sahin, C. B., and Isler, N. (2021). Foliar applied zinc and iron effects on yield and yield components of soybean: Determination by PCA analysis. Commun Soil Sci. Plant Anal. 52, 212–221. doi: 10.1080/00103624.2020.1854297
Salim, N., and Raza, A. (2020). Nutrient use efficiency (NUE) for sustainable wheat production: a review. J. Plant Nutr. 43, 297–315. doi: 10.1080/01904167.2019.1676907
Sani, M. N. H., Hasan, M., Uddain, J., and Subramaniam, S. (2020). Impact of application of Trichoderma and biochar on growth, productivity and nutritional quality of tomato under reduced NPK fertilization. Ann. Agric. Sci. 65, 107–115. doi: 10.1016/j.aoas.2020.06.003
Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259. doi: 10.3390/plants10020259
Sesan, T. E., Oancea, A. O., Stefan, L. M., Manoiu, V. S., Ghiurea, M., Raut, I., et al. (2020). Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 8, 123. doi: 10.3390/microorganisms8010123
Shewry, P. R., Tatham, A. S., Barro, F., Barcelo, P., and Lazzeri, P. (1995). Biotechnology of breadmaking: unraveling and manipulating the multi-protein gluten complex. Biotechnology 13, 1185–1190. doi: 10.1038/nbt1195-1185
Shukla, N., Awasthi, R. P., Rawat, L., and Kumar, J. (2012). Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 54, 78–88. doi: 10.1016/j.plaphy.2012.02.001
Song, G., Genlou, S., Weihua, L., Daokun, S., Yanchun, P., and Xifeng, R. (2020). High-molecular-weight glutenin subunit compositions in current Chinese commercial wheat cultivars and the implication on Chinese wheat breeding for quality. Cereal Chem. 97, 762–771. doi: 10.1002/cche.10290
Szerement, J., Szatanik-Kloc, A., Mokrzycki, J., and Mierzwa-Hersztek, M. (2021). Agronomic Biofortification with Se, Zn, and Fe: an effective strategy to enhance crop nutritional quality and stress defense-A Review. J. Soil Sci. Plant Nutr. 22, 1–31. doi: 10.1007/s42729-021-00719-2
Tripathi, D. K., Singh, S., Singh, S., Mishra, S., Chauhan, D. K., and Dubey, N. K. (2015). Micronutrients and their diverse role in agricultural crops: advances and future prospective. Acta Physiol. Plant. 37, 1–14. doi: 10.1007/s11738-015-1870-3
Ullah, H., Ahmad, H., Shahzad, A., Ali, A., and Ali, G. M. (2016). Evaluation of allelic variation for HMW glutenin subunits through SDS-PAGE in diverse bread wheats. Int. J. Biosci. 9, 203–214. doi: 10.12692/ijb/9.1.203-214
Visconti, D., Fiorentino, N., Cozzolino, E., Woo, S. L., Fagnano, M., and Rouphael, Y. (2020). Can Trichoderma-based biostimulants optimize N Use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 10, 121. doi: 10.3390/agronomy10010121
Vukelic, I. D., Prokic, L. T., Racic, G. M., Pesic, M. B., Bojovic, M. M., Sierka, E. M., et al. (2021). Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. Int. J. Mol. Sci. 22, 6961. doi: 10.3390/ijms22136961
Waller, F., Achatz, B., Baltruschat, H., Fodor, J., Becker, K., Fischer, M., et al. (2005). The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Nat. Acad. Sci. U. S. A. 102, 13386–13391. doi: 10.1073/pnas.0504423102
Wani, S. H., Khan, H., Riaz, A., Joshi, D. C., Hussain, W., Rana, M., et al. (2022). Genetic diversity for developing climate-resilient wheats to achieve food security goals. Adv. Agron. 49, 255–303. doi: 10.1016/bs.agron.2021.08.006
White, P. J., and Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84. doi: 10.1111/j.1469-8137.2008.02738.x
Woo, S. L., Ruocco, M., Vinale, F., Nigro, M., Marra, R., Lombardi, N., et al. (2014). Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 8, 71–126. doi: 10.2174/1874437001408010071
Xu, Y., An, D., Liu, D., Zhang, A., Xu, H., and Li, B. (2012). Molecular mapping of QTLs for grain zinc, iron and protein concentration of wheat across two environments. Field Crops Res. 138, 57–62. doi: 10.1016/j.fcr.2012.09.017
Yaseen, M., Abbas, T., Aziz, M. Z., Wakeel, A., Yasmeen, H., Ahmed, W., et al. (2018). Microbial assisted foliar feeding of micronutrients enhance growth, yield and biofortification of wheat. Int. J. Agric. Biol. 20, 353–360. doi: 10.17957/IJAB/15.0498
Zain, M., Khan, I., Qadri, R. W. K., Ashraf, U., Hussain, S., Minhas, S., et al. (2015). Foliar application of micronutrients enhances wheat growth, yield and related attributes. Am. J. Plant Sci. 6, 864. doi: 10.4236/ajps.2015.67094
Keywords: wheat, Trichoderma harzianum, zinc, iron, HMW-GS
Citation: Ali I, Khan A, Ali A, Ullah Z, Dai D-Q, Khan N, Khan A, Al-Tawaha AR and Sher H (2022) Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 13:960948. doi: 10.3389/fpls.2022.960948
Received: 03 June 2022; Accepted: 05 July 2022;
Published: 07 September 2022.
Edited by:
Zamin Shaheed Siddiqui, University of Karachi, PakistanReviewed by:
Muhammad Imran, South China Agricultural University, ChinaCopyright © 2022 Ali, Khan, Ali, Ullah, Dai, Khan, Khan, Al-Tawaha and Sher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iftikhar Ali, aWZ0aWtoYXIuaXBmcC4yOEBuYWhlLmVkdS5waw==; Dong-Qin Dai, Y2ljaWRhaWRvbmdxaW5AZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.