
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 11 August 2022
Sec. Crop and Product Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.956391
This article is part of the Research TopicBiostimulants in Agriculture II: Towards a Sustainable FutureView all 78 articles
Co-inoculation of arbuscular mycorrhizal fungi (AMF) and bacteria can synergically and potentially increase nitrogen use efficiency (NUE) in plants, thus, reducing nitrogen (N) fertilizers use and their environmental impact. However, limited research is available on AMF-bacteria interaction, and the definition of synergisms or antagonistic effects is unexplored. In this study, we adopted a response surface methodology (RSM) to assess the optimal combination of AMF (Rhizoglomus irregulare and Funneliformis mosseae) and Bacillus megaterium (a PGPR—plant growth promoting rhizobacteria) formulations to maximize agronomical and chemical parameters linked to N utilization in maize (Zea mays L.). The fitted mathematical models, and also 3D response surface and contour plots, allowed us to determine the optimal AMF and bacterial doses, which are approximately accorded to 2.1 kg ha–1 of both formulations. These levels provided the maximum values of SPAD, aspartate, and glutamate. On the contrary, agronomic parameters were not affected, except for the nitrogen harvest index (NHI), which was slightly affected (p-value of < 0.10) and indicated a higher N accumulation in grain following inoculation with 4.1 and 0.1 kg ha–1 of AMF and B. megaterium, respectively. Nonetheless, the identification of the saddle points for asparagine and the tendency to differently allocate N when AMF or PGPR were used alone, pointed out the complexity of microorganism interaction and suggests the need for further investigations aimed at unraveling the mechanisms underlying this symbiosis.
Nitrogen (N) represents a major nutrient for plants, being an essential component of proteins, nucleotides, chlorophyll, and a broad range of secondary metabolites. Cereals grains, providing 60% of the food necessary to feed the world’s population, require significant inputs of N to achieve optimum yields. Nevertheless, N availability can represent a limiting condition since nitrate (NO3–) and ammonium (NH4+), representing the readily available N pool, account for only 2% of total soil N content (Moreau et al., 2019).
At the same time, the increasing use of synthetic N fertilizers over the last decades has posed concerns about the contamination of surface and groundwater bodies by nitrate, the impairment of biodiversity, and the emission of greenhouse gases (Ahmed et al., 2017). In line with the EU “Farm to fork” strategy, which aims at increasing the agricultural land managed under organic farming by 25%, scientific efforts are being done to maintain (or even increase) crop productivity by efficiently using organic fertilizers, which have been recognized to increase N losses very often, especially if applied inappropriately (Maris et al., 2021). Such an increase in N use efficiency can significantly minimize N losses and the consequent adverse impacts on ecosystems while decreasing costs for organic fertilizers (Galloway et al., 2014).
All the pathways of N cycling in soils mainly depend on the edaphic conditions, agronomic management, climate, crop genetics, and finally determines N availability, transfer, transformation, and losses (Congreves et al., 2021). Many scientific contributions have reported best agronomic practices and breeding solutions to enhance NUE, commonly defined as the plant biomass accumulation per unit of soil N available (Peng et al., 2006; van Bueren and Struik, 2017; Anas et al., 2020). N assimilation by plants involves the GS–GOGAT pathway, for which glutamine, asparagine, glutamate, and aspartate are upstream key intermediates; once incorporated into organic compounds, N is then distributed to a broad range of different N-containing compounds. Over the last two decades, biotechnological engineering of the amino acid metabolism has led to promising results for the improvement of NUE, especially the content of glutamine, asparagine, glutamate, and aspartate has been adopted as an interesting target to carry out these studies, being involved in plant N utilization and storage after assimilation (Dellero, 2020; The et al., 2021). Recently, especially in a framework of sustainable crop production, the inoculation with beneficial microorganisms has gained importance in such plant NUE increase (Di Benedetto et al., 2017; Verzeaux et al., 2017; Dalla Costa et al., 2021).
Arbuscular mycorrhizal fungi (AMF) have been reported to increase NUE by developing the symbiotic association with most terrestrial plants, favoring access to N uptake in a larger soil volume (Verzeaux et al., 2017). Interestingly, the specific up-regulation of NO3– and NH4+ transporters has been observed in AMF colonized roots compared with the non-colonized plants (Courty et al., 2015; Garcia et al., 2016). In cereals such as sorghum, maize, and rice, AMT3.1 plant NH4+ transporter transcripts were specifically up-regulated following the mycorrhizal colonization (Koegel et al., 2017). Similarly, higher NUE has been observed following beneficial associations with N2-fixing bacteria such as Rhizobia, Frankia sp., and Cyanobacteria, and some other diazotrophs like Azospirillum spp., Herbaspirillum spp., and Paenibacillus spp. Interestingly, a community of mycorrhizosphere bacteria living strictly associated with AMF has been suggested to encompass improved crop performances, acting synergistically with AMF (Oldroyd et al., 2011; Santi et al., 2013; Udvardi and Poole, 2013; Agnolucci et al., 2015; Kollah et al., 2016; Mus et al., 2016; Rosenblueth et al., 2018). In this regard, a high degree of specificity between bacteria and AMF has been proposed, and this tripartite association can potentially increase NUE in plants (Tajini et al., 2012; Giovannini et al., 2020; Paul et al., 2020).
The dynamic assembly of the rhizosphere microbial community depends on a large set of factors and is driven by an intricate set of belowground chemical communications (van Dam and Bouwmeester, 2016; Qu et al., 2020). However, very little information is available in the literature regarding optimizing AMF-bacteria co-inoculation to manipulate rhizomicrobiome and improve plant performance.
The present study aimed at optimizing the co-inoculation between mycorrhiza and Bacillus megaterium using the response surface methodology (RSM), with reference to enhanced N utilization in maize (Zea mays L.). The RSM approach has been chosen to account for the interaction(s) between the fungal and bacterial inoculum in the framework of the complex rhizosphere community. Similarly, different plant-based indices of NUE (i.e., NHI and NUtE; López-Bellido and López-Bellido, 2001) and yield of maize have been considered to account for the different assimilation, metabolization, mobilization processes, as well as the translocation to reproductive portions, to overcome the temporal and spatial edges of NUE indices (Congreves et al., 2021).
The field experiment was conducted over one maize cropping season—between May 2021 and September 2021—at the CERZOO experimental research station in Piacenza (45°00′21.6′′N, 9°42′27.1′′E; altitude 68 m a.s.l.), Northern Italy. At the experimental site, the climate is temperate (Cfa following Köppen classification), with an average annual temperature of 13.2°C and cumulative annual precipitation of 837 mm (20-yr data). The soil is a fine, mixed, mesic Udertic Haplustalf based on the Keys to Soil Taxonomy (Soil Survey Staff 2014). The physicochemical properties of soil (0–30 cm soil layer) measured before starting the experiment were: organic matter content 27 g kg–1; pH H2O 7.8; bulk density 1.36 g cm–3; sand 127 g kg–1; silt 445 g kg–1; clay 428 g kg–1; soil total N 1.3 g kg–1; available P (Olsen) 32 mg kg–1; exchangeable K (NH4+ Ac) 294 mg kg–1, and cation exchange capacity 30 cmol+ kg–1. Biostimulant treatments consisted of a seed dressing with the powder AMF-based product Aegis Sym irriga® (Rhizoglomus irregulare BEG72 and Funneliformis mosseae BEG234, 700 sp g–1 each species) formulation, or the PGPR Bactrium® (Bacillus megaterium BM77 e BM06, 5 × 109 CFU/g each species) liquid formulation, all from Athens, Agrotecnologia Naturales SL (Tarragona, Spain). The seed dressing was homogenized with maize seeds using an automated mixer.
To assess the effect of different doses of AMF and bacteria on yield and NUE in maize, nine different types of seed dressing were prepared as all the possible combinations of the three doses of AMF (low: 0.1 kg ha–1; medium: 2.1 kg ha–1; and high: 4.1 kg ha–1) and three doses of B. megaterium (low: 0.1 kg ha–1; medium: 2.1 kg ha–1; and high: 4.1 kg ha–1). As a result, the present field experiment was set up as a randomized complete block (RCB) design with 9 treatments and 4 replicates (blocks). Each plot was 28 m2: 10 m long and 2.8 m wide (4 maize rows at a 0.7 m inter-row distance). Before planting maize, the seed dressing was carried out in the Lab separately for each treatment. In brief, each level of the 3 × 3 AMF-B. megaterium doses were manually mixed with maize seeds (at around 82,000 seeds ha–1). Then, maize was planted with a common two-row plot seeder on 7 May 2021. Maize cropping management followed principles reported by the regulation (EU) 2018/848 on the organic farming production methods. To represent local practice under organic farming, a 170 kg N ha–1 slurry distribution was applied to all the plots before soil tillage (autumn 2020), and no fertilizer was applied during the cropping cycle of maize. To prevent water stress, maize was sprinkler-irrigated three times at doses of 30, 40, and 45 mm. Harvesting took place on 20 September 2021 with a plot-scale combination.
On 13 August, relative chlorophyll concentrations, defined as SPAD value, were estimated between 02:00 p.m. and 03:00 p.m. using a SPAD-502 chlorophyll meter (Minolta, Tokyo, Japan). The average value of five measurements of three leaves was taken as the maize SPAD value per plot.
On 13 August, the ninth leaf of five plants per plot was sampled and stored at −20°C. The frozen samples were used to determine the content of four amino acids, namely, asparagine, glutamine, aspartate, and glutamate.
Initially, the five leaves per plot were ground together with liquid nitrogen using a pestle and mortar to obtain a homogeneous and representative final sample. Two aliquots (2 × 1 g) per plot were extracted in 10 ml of 50% methanol, 50% deionized water, and 0.01% formic acid using an Ultra-Turrax (Ika T-25, Staufen, Germany) and successively centrifuged (12,000 × g).
The different extracts were diluted 1,000-fold with deionized water and then filtered in the HPAEC vials using 0.20 μm syringe filters until instrumental analysis. The amino acid content of the hydroalcoholic leaf extracts was then investigated through High-Performance Anion Exchange Chromatography with Pulsed Amperometric Detection (HPAEC–PAD). The analyses were carried out on a Dionex ICS-5000 + instrument (Thermo Fisher Scientific, Waltham, the United States) provided with an electrochemical cell consisting of a gold-working electrode and a pH–Ag/AgCl reference electrode. The separation was performed using a Thermo Scientific™ Dionex™ AMINOPAC™ PA10 Analytical column (2 × 250 mm). The chromatographic runs were executed in a total time of 80 min, with multi-gradient elution consisting of a mobile phase of deionized water (eluent A), 250 mM aqueous sodium hydroxide (eluent B), and 1 M sodium acetate solution (eluent C). The flow rate was 0.25 ml/min, and the injection volume was 25 μl, with a column and detector compartment temperature at 30°C. Gradient elution consisted of 0–12 min with 80% of eluent A and 20% of eluent B; 12–16 min with 68% of eluent A and 32% of eluent B; 16–40 min of 36% of eluent A, 24% of eluent B, and 40% of eluent C. Also, a 40 min of equilibration phase was considered, sing 80% of eluent A and 20% of eluent B. The amino acids were detected by an ICS-5000 + electrochemical detector in integrated pulsed amperometric detection mode applying the following waveform potentials and durations: E1 = 0.13 V (t1 = 0.40 s), E2 = 0.33 V (t2 = 0.210 s), E3 = 0.55 V (t3 = 0.460 s), E4 = 0.33 V (t4 = 0.560 s), E5 = −1.67 V (t5 = 0.580 s), E6 = 0.93 V (t6 = 0.590 s), and E7 = 0.13 V (t7 = 0.600 s). For the quantification step, a calibration curve (R2 > 0.98) of the four amino acids was prepared by appropriately diluting an amino acid standard mix (2.5 μmol/ml in 0.1M HCl solution, provided by Merck, Darmstadt, Germany), considering the following concentration range: 25-10-5-2.5-1 μM (Supplementary Figure 2). The chromatographic system was controlled through the Thermo Scientific™ Dionex™ Chromeleon™ software version 7.0 for the instrumentation command, chromatograms acquisition, and processing.
At the BBCH 89, maize yield was measured by manually harvesting 8 m2 per single plot. Plants were weighed and separated into grain and biomass (stalks). A 100-g sub-sample of each grain and biomass sample was oven-dried at 65°C until constant weight to measure dry matter content. Grain and biomass N-uptake were calculated by multiplying grain and biomass yield by their N-concentrations, determined by the Dumas combustion method with an elemental analyzer varioMax C:N (VarioMax C:NS, Elementar, Germany).
The two following N-efficiency parameters were calculated for each treatment according to López-Bellido and López-Bellido (2001): (i) N harvest index (NHI;%) as the ratio of N in grain to N in total plant biomass; and (ii) N-utilization efficiency (NUtE; kg kg–1) as the ratio of grain yield to total plant N-uptake.
Data analysis was performed with Rstudio 3.6.1 software (R Core Team, 2013). On each NUE parameter, two different two-way ANOVA (p < 0.05) were carried out. The first ANOVA was focused on the differences between treatments, thus, the combination of AMF and bacterial doses, as well as the block, were considered as factors, respectively, represented by 9 and 3 levels. The response profiles not impacted by the microbial inoculations were plotted as box plots using the ggplot package to represent the data distribution according to treatments (Wickham and Chang, 2007). Successively, the second ANOVA was performed to study the effects and the interactions of the AMF and bacteria factors.
After that, response-surface analysis was performed with the rsm package (Lenth, 2009). According to Kumar et al. (2020), the face-centered composite design (FCDD) was used and two independent variables with three levels were selected: AMF (x1: 0.1 kg ha–1; 2.1 kg ha–1; 4.1 kg ha–1) and B. megaterium (x2: 0.1 kg ha–1; 2.1 kg ha–1; 4.1 kg ha–1). A full factorial design of 27 experimental runs with 12 cube points, 12 axial points, and 3 center points was chosen (Table 1), while SPAD values, amino acid concentrations, and other NUE parameters were considered response profiles for modeling. The experimental runs were randomized to minimize the effect of unexpected variability on the observed responses. The following second-order quadratic model was used to develop the optimization and predictive model:
where Y is the predicted response profile, β0, βi, βj, and βij are the interactive regression coefficients, and x1 and x2 are the AMF and B. megaterium doses, respectively.
Analysis of variance revealed a significant effect of the treatments on maize in terms of SPAD value and amino acid concentrations (Table 2). Interestingly, the highest value of SPAD (62.95 ± 1.41) was found with the intermediate dose of AMF and B. megaterium, corresponding to 2.1 kg ha–1 of each inoculum. Similarly, the same result was obtained for aspartate, whose highest concentration (0.81 ± 0.38 μM) was registered at the same treatment level, showing a content 4.7- and 3.5-folds higher than those, respectively, registered with the lowest (0.1 kg ha–1) and the highest (4.1 kg ha–1) doses of both inocula. Surprisingly, asparagine and glutamine concentrations reached the maximum concentration (respectively, 2.95 ± 0.52 and 0.86 ± 0.13 μM) at 2.1 + 2.1 kg ha–1 AMF-B. Megaterium combination, then decreased with the increasing dosage of AMF and raised again at the highest levels of mycorrhizal and bacterial inocula. At final, the inoculum with 2.1 + 4.1 kg ha–1 of AMF and B. megaterium combined reflected a higher glutamate content (0.82 ± 0.14 μM).
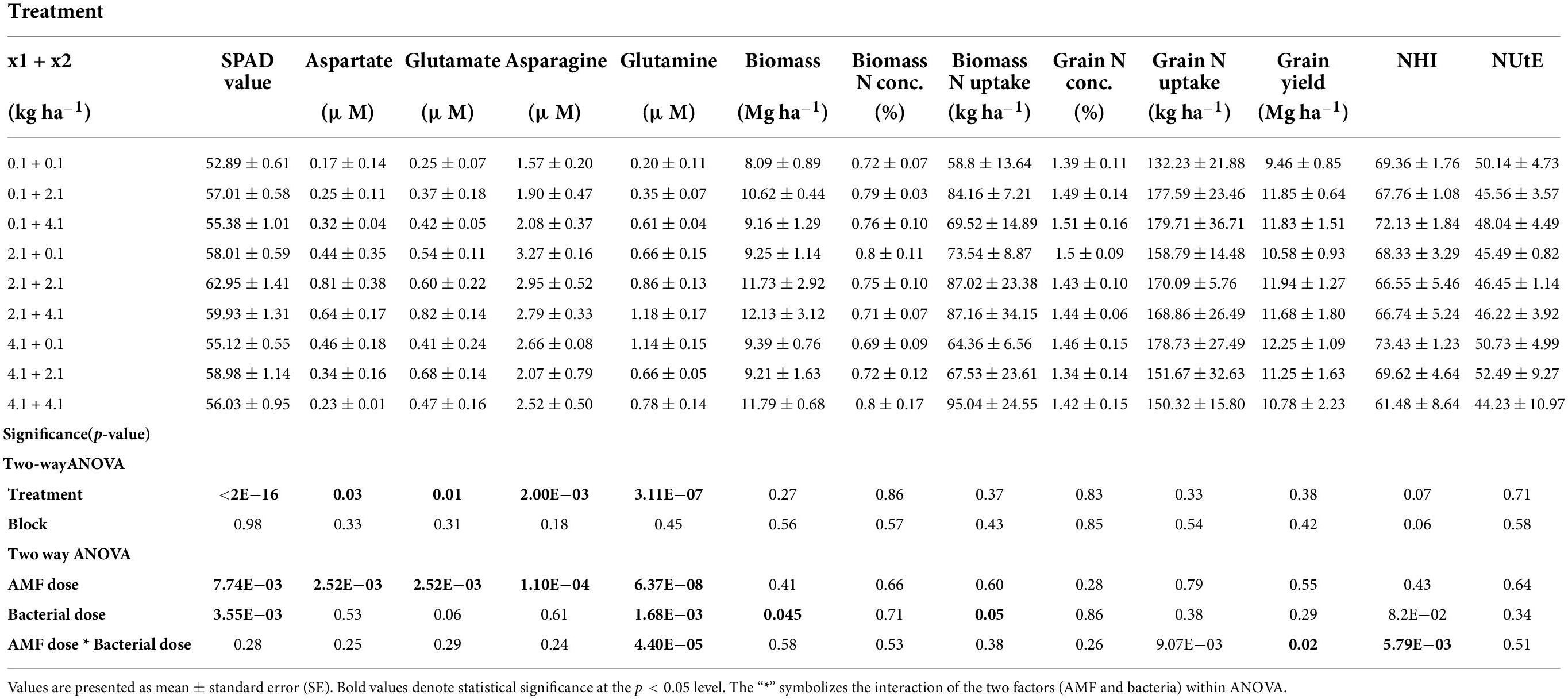
Table 2. Two−way ANOVA on SPAD value, amino acid concentrations, and agronomic parameters observed in the maize crop following AMF and B. megaterium treatment.
However, the ANOVA did not reveal a significant effect of the treatments for the considered agronomic parameters, except for NHI, which seemed to be slightly affected (p-value of < 0.10) and indicated a higher N accumulation in grain following 4.1 and 0.1 kg ha–1 of AMF and B. megaterium inoculation (Table 2). Box plots (Figure 1) provided high-level information regarding data symmetry, variance, and outliers, allowing comparisons between different treatments.
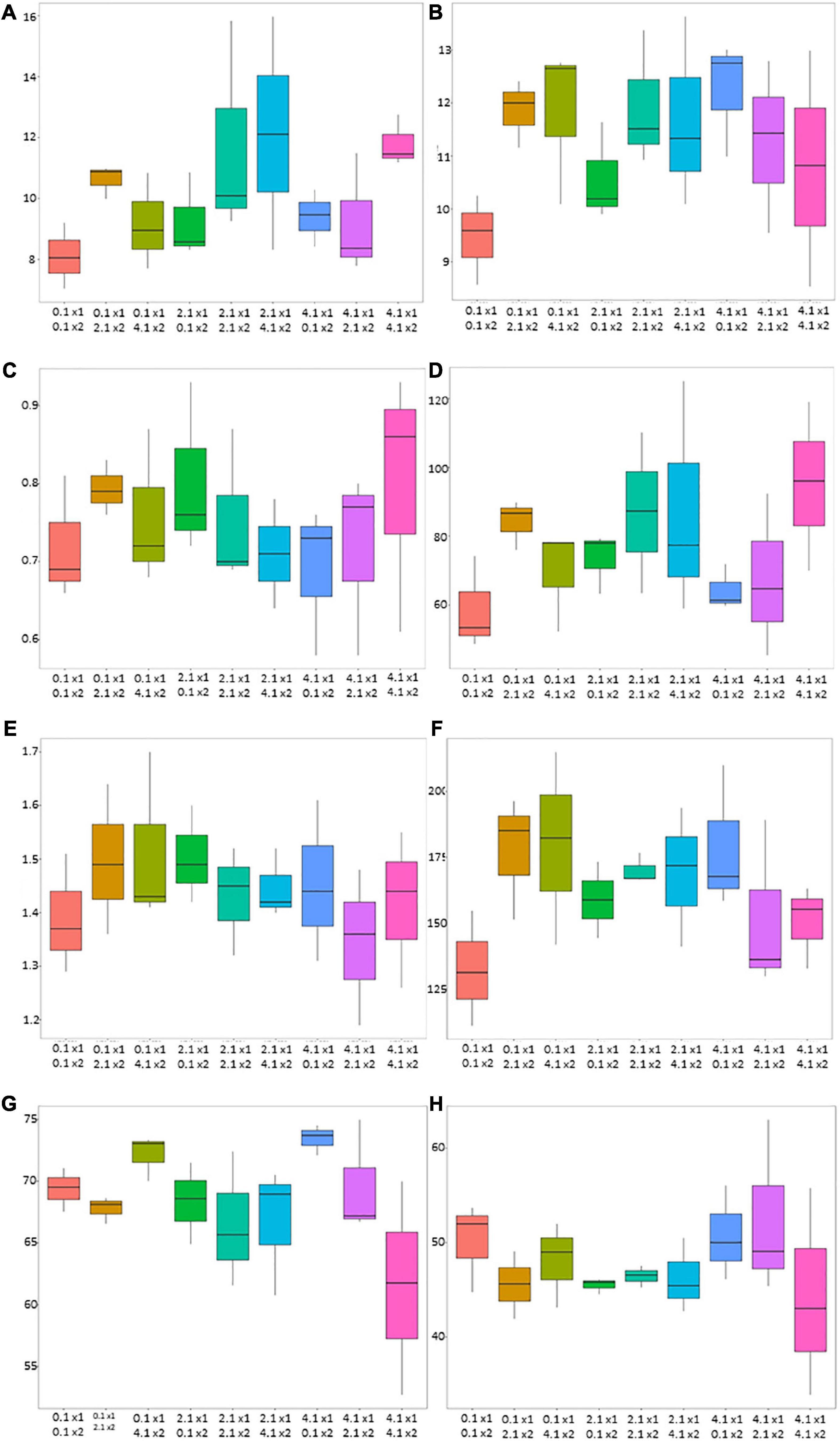
Figure 1. Box plot diagrams of agronomic parameters for AMF and Bacillus megaterium treatments on maize (Zea mays L.). The X-axis describes the combination of AMF and B. megaterium doses (0.1 kg ha–1, 2.1 kg ha–1, and 4.1 kg ha–1) while the Y-axis represents the values for maize biomass (mg ha–1) (A), grain yield (mg ha–1) (B), biomass nitrogen concentration (%) (C), biomass nitrogen-uptake (kg ha–1) (D), grain nitrogen concentration (%) (E), grain nitrogen-uptake (kg ha–1) (F), NHI (G), and NutE (H). Each box line corresponds to the median of the data while the ends of the box show the upper (Q3) and lower quartiles.
Overall, biomass and biomass N-uptake values showed a similar trend, with 2.1 + 2.1 kg ha–1, 2.1 + 4.1 kg ha–1, and 4.1 + 4.1 kg ha–1 doses of mycorrhizal and bacterial inocula that tended to increase maize performances. Biomass N-concentration tended to be the highest under 0.1 + 2.1 kg ha–1, 2.1 + 0.1 kg ha–1, and 4.1 + 4.1 kg ha–1 doses of mycorrhizal and bacterial inocula.
Grain yield values showed a tendency to be increased under 0.1 + 2.1/4.1 kg ha–1, 2.1 + 2.1 kg ha–1, and 4.1 + 0.1 kg ha–1 doses. Moreover, grain N-concentration tended to be higher (1.51 ± 0.16%) in the absence of mycorrhizal inoculation (0.1 + 4.1 kg ha–1 of AMF and B. megaterium). At last, the highest NUtE value was obtained with 4.1 kg ha–1 and 2.1 kg ha–1, respectively, of AMF and B. megaterium dose.
A prediction model was developed for those indices being significantly affected (p-value of < 0.05) by AMF and B. megaterium treatment. The quadratic equations of the final models are given below Eqs. 1–5:
Regarding the SPAD value, the model showed a high coefficient of determination (R2 = 0.9) and a high F-value (224.9), while the p-value was < 0.05 and no significant lack of fit values (> 0.05) were found. Similarly, a high-predictive model was found for glutamine concentration, exhibiting R2 = 0.8, F-value = 8.75, and a p-value of < 0.05. Glutamate, asparagine, and aspartate models showed lower R2, being 0.5, 0.6, and 0.4, respectively, corresponding to 4.3, 6.4, and 3.9 F-values. However, in all the three cases, the p-value was < 0.05, and a significant lack of fit was not provided (> 0.05).
The final regression analysis equations were used for plotting 3D response surface and contour plots which represented the interaction of the input factors—AMF and B. megaterium—to trace the stationary point of each variable for the desired response (Figure 2 and Supplementary Figure 1). This stationary point was a maximum for SPAD value, aspartate, and glutamate, indicating a maximum response of these profiles obtained with mycorrhizal and bacterial-specific doses (Figure 2). The RSM optimized values for maximum SPAD value (62.66) were 2.27 ka ha–1 for AMF and 2.35 ka ha–1 for B. megaterium. Moreover, 2.22 and 2.24 ka ha–1 indicated the optimized doses of mycorrhizal and bacterial inocula to reach the highest aspartate content (0.69), while 2.41 ka ha–1 and 3.31 ka ha–1 obtained the maximum glutamate concentration. Concerning asparagine and glutamine, the stationary point was represented by a saddle point (2.91 and 0.85), which consisted of an inflection point of the response surface and was, respectively, achieved with 2.40 ka ha–1 and 2.36 ka ha–1, and with 2.99 ka ha–1 and 2.03 ka ha–1 of AMF and B. megaterium inocula. The contour plots of the fitted response surfaces allowed us to visualize the behavior of the fitted surface around the stationary point (Supplementary Figure 2). Each plot displayed a color image overlaid by the contour lines of constant responses in a two-dimensional space.
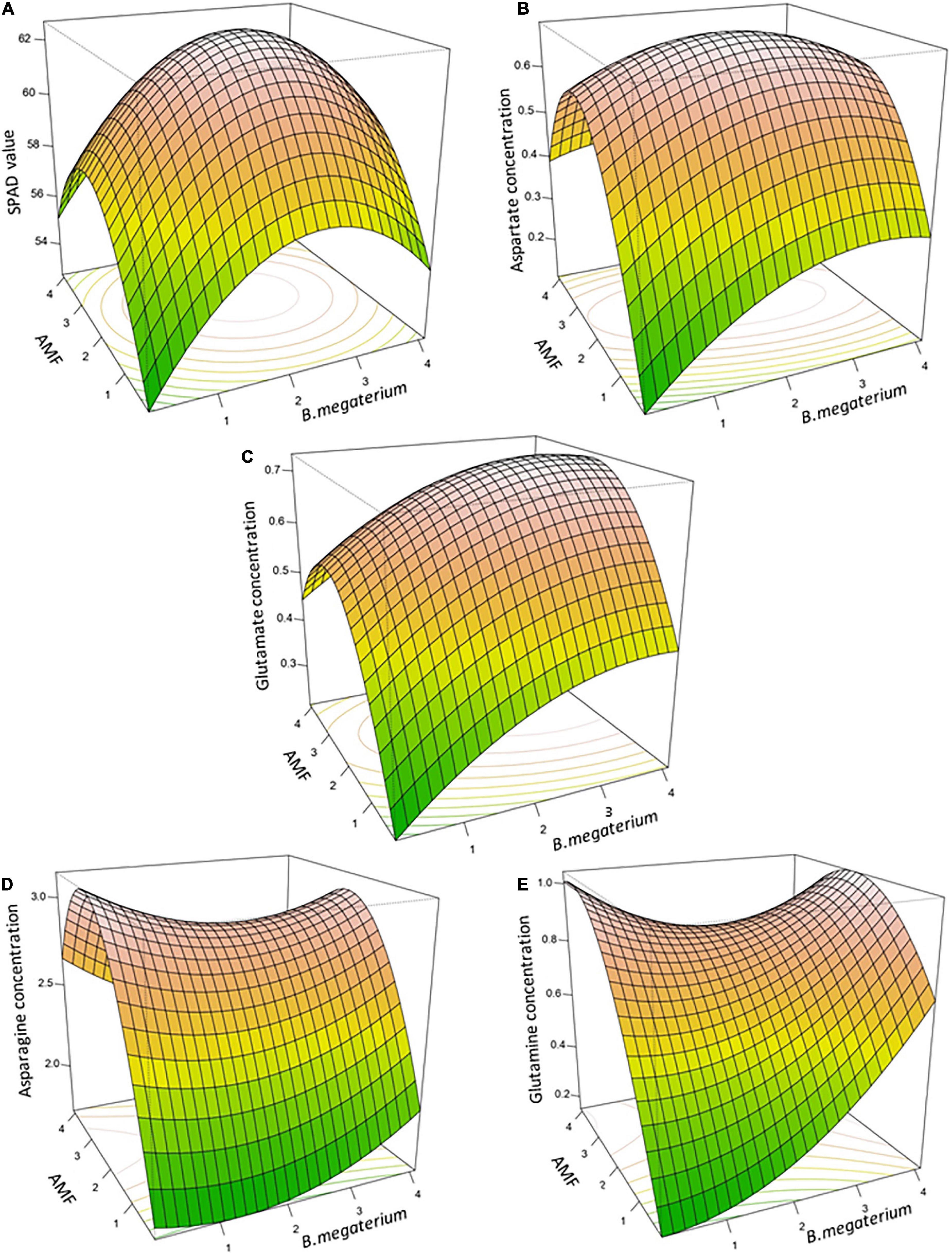
Figure 2. The three-dimensional response surface plots for interactive effects of B. megaterium and AMF co-inoculation on SPAD value (A), aspartate concentration (B), glutamate concentration (C), asparagine concentration (D), and glutamine concentration (E) in maize.
The choice of the best AMF-bacteria combination to improve NUE and thus ensure efficient and sustainable food production has become a burning question in the perspective of developing new strategies for the ecological transition of agriculture (Agnolucci et al., 2019). B. megaterium and AMF are both supposed to be potential bio-fertilizer agents able to play a key role in plant growth promotion. Their single and dual inoculation has revealed beneficial emerging properties in plant symbiosis, mostly translated into enhanced plant growth, yield, and abiotic stress tolerance (Ortiz et al., 2015; Khalid et al., 2017). RSM allows testing of multiple factors with complex interactions, modeling the system mathematically and using a limited number of experimental trials. However, despite the presence of studies based on the application of RSM models to improve plant growth, yield, and NUE, the statistical optimization of the best performing inoculum which can boost plant N acquisition is still limited in literature (Peng et al., 2014; Gundi et al., 2018; Naili et al., 2018; Mazumdar et al., 2021).
Several studies have shed light on the ability of AMF or PGPR to increase plant NUE, reporting higher grain and biomass values, as well as higher levels of NUtE, in different crops. In maize, clear evidence of improved NUE have been observed following inoculation with bacterial strains of P. fluorescens S3X and C. necator 1C2 (Pereira et al., 2020), Bacillus megaterium (Ganugi et al., 2022), and other. Concerning AMF, positive results were reported by single inoculation with R. irregulare BEG72 and F. mosseae BEG234, and coupled application of Rhizophagus irregularis and Bacillus spp. (Adesemoye et al., 2008).
Nevertheless, understanding the specific mycorrhizal and bacterial synergic action requires considerable study since PGPR–AMF affinity and colonization efficiency seem to be strongly related to the fungus species and origin. Marulanda-Aguirre et al. (2008) found different results for B. megaterium when inoculated on lettuce (Lactuca sativa L.) coupled with Glomus constrictum autochthonous, G.constrictum from a collection or commercial G.intraradices. In addition, this study highlights how an optimized mycorrhizal-to-bacterial inoculation rate on maize seeds at planting might be a success factor for the establishment and effectiveness of an AMF–PGPR symbiosis. Interestingly, our optimization aimed at enhancing maize NUE revealed similar RSM values for maximum SPAD, aspartate, and glutamate concentrations, which correspond to the intermediate doses of AMF and B. megaterium. Together with indicating a treatment effect on these NUE indices, this point confirmed the tripartite synergy between plants, mycorrhizae, and microbial communities for plant nutrition. Such synergy can be translated into a significant increase in N acquisition compared with the single inoculation of AMF or B. megaterium (Hestrin et al., 2019). However, the identification of saddle points for asparagine and glutamine pointed to the complexity of microorganisms’ interactions. The presence of such intricate microbial interactions indicates the need for further deep analyses, focused on experimental runs at points along this path, using the observed response values for guidance on where to locate the next factorial experiment (Lenth, 2009; Jan et al., 2021).
Despite the lack of significant treatment effect on most of the agronomic parameters, our data revealed interesting trends which is worth exploring in the future. On the one hand, it seems that maize plant biomass and biomass N-uptake could be increased by the concomitant action of AMF and B. megaterium at > 2.1. On the other hand, grain yield, grain N-uptake, and NHI showed a tendency to be increased preferentially by the single action of either AMF or B. megaterium, while lower values were registered under co-inoculation conditions. This latter statement was further corroborated by the fact that NHI (which was close to being significantly affected by the treatments) had the highest values with 4.1 kg ha–1 of AMF or B. megaterium, alternatively. These results suggest that (i) combining AMF and B. megaterium at a significant dose (> 2 kg ha–1) may have the potential to enhance plant tissues growth toward vegetative biomass, and (ii) if AMF and B. megaterium are inoculated alone, it is likely that grain-related agronomic parameters could benefit more than biomass-related ones. In fact, distinct effects provided by AMF, PGPR, and their co-inoculation in terms of drought resistance and essential oil yield have been previously reported in myrtle (Azizi et al., 2021). Similarly, it was reported that symbiotic efficiency may be hindered by interaction(s) with other biofertilizers together with being impacted by agro-practices (Kuila and Ghosh, 2022). In another work, Pacheco et al., evaluated the effect of AMF and Pseudomonas putida (PSB) single and co-inoculation on P uptake, productivity, and P concentration in maize (Pacheco et al., 2021). Interestingly, these authors reported that the microbial inoculants enhanced plant P uptake, that the presence of PSB increased biomass per unit of P taken up, and that the microbial inoculants altered P allocation within the plant, reducing grain P concentration (Pacheco et al., 2021). This distinct effect of the microbial inoculants on biomass production and nutrients allocation agrees with the results from Lozano Olivério Salvador and co-workers, who observed specific effects in terms of dry weight, symbiotic efficiency, chlorophyll content, and nitrogen accumulation when AMF or rhizobacteria were applied with compost to soybean (Salvador et al., 2022).
Despite the fact that mechanisms underlying the effect of single and combined inoculum are still unknown, amino acid metabolism represents an interesting target for crop NUE improvement, being actively involved in plant NUtE (Dellero, 2020). In particular, the glutamine synthetase/glutamate:2-oxoglutarate aminotransferase (GS/GOGAT) cycle represents the major route for plant ammonia assimilation, followed by asparagine synthetase (ASN) and glutamate dehydrogenase (GDH) (Harrison et al., 2000). Accordingly, experimental studies on maize have pointed out the increased concentration of aspartate and glutamate following R. irregularis and Gigaspora margarita inoculation (Matsumura et al., 2013; Hu and Chen, 2020), while the enhanced activity of ammonium assimilating enzymes (GS and GDH) have been detected with Azospirillum bacterial treatment (Ribaudo et al., 2001).
Here, for the first time, we reported how different rates of mycorrhiza-to-Bacillus co-inoculation may be considered—to some extent—as a driver of differential N partitioning between grain and vegetative biomass in the maize plant. This should be considered to address future studies to shed light on how such biotic interactions may steer plant physiology toward grain rather than vegetative tissues N accumulation.
Nevertheless, it should be stated some important aspects of the experiment. At first, it must be pointed out that the Aegis Sym irriga® product is predominantly but not totally made by AMF, being associated—as the most commercial inocula—to other bacteria. For this reason, the effect of this inoculum on plant-based indices of NUE, as well as that obtained following its interaction with the PGPR product, is not necessarily ascribable to the exclusive presence of mycorrhiza. Second, to further complicate matters, plant responses should be studied under a wide range of soil-climate conditions, including different soil types and soil fertility, and temperate to dry climates. Particularly, physico-chemical soil properties, such as nutrient availability, pH, structure, organic matter content, and texture, can affect the release of plant root exudates and, consequently, the availability and the interactions with soil microbial communities (Neumann et al., 2014). It is likely that plant-microbe symbiosis is profoundly influenced by external environmental conditions, namely, temperature and moisture (Cheng et al., 2019). As consequence, it should be clarified that our results are strictly related to a specific pedoclimatic experimental context and, for this reason, maximum and saddle points for each NUE index cannot be certainly considered as absolute values exactly reproducible under different plant growth conditions.
Equally, the lack of significant effects in some agronomic indices may be reasonable because of the peculiar temperate and fertile conditions underlying the study, which is why it would be unreasonable to draw a conclusion about a lack of general effect of microbial inocula on the plant NUE. Notwithstanding, the application of prediction models may considerably contribute to advancements in the agricultural sector, particularly, under challenging factors interaction conditions.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
PG, AF, VT, and LL conceived the ideas and designed the methodology. PG, AF, GR, and PB collected the data. PG, GR, and PB analyzed the data. PG, AF, PB, VT, and LL wrote the original draft. AF and LL critically revised the original draft. All the authors gave final approval for the publication.
This project was funded by the Emilia-Romagna region, Italy, in the framework of the Regional Development Program PSR 2014-2020, Measure 16.1.01–Focus Area 4B (project 5150325 – INBIOS: Sviluppo di un approccio INtegrato a base di BIOStimolanti per la sostenibilità delle produzioni agrarie).
We thank the “Romeo ed Enrica Invernizzi” Foundation (Milan, Italy) for the kind support to the metabolomic facility at the Università Cattolica del Sacro Cuore.
PB was employed by company oloBion-OMICS LIFE LAB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.956391/full#supplementary-material
Anas, M., Liao, F., Verma, K. K., Sarwar, M. A., Mahmood, A., Chen, Z. L., et al. (2020). Fate of nitrogen in agriculture and environment: agronomic, ecophysiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 53, 1–20. doi: 10.1186/s40659-020-00312-4
Adesemoye, A. O., Torbert, H. A., and Kloepper, J. W. (2008). Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 54, 876–886. doi: 10.1139/W08-081
Agnolucci, M., Avio, L., Pepe, A., Turrini, A., Cristani, C., Bonini, P., et al. (2019). Bacteria associated with a commercial mycorrhizal inoculum: Community composition and multifunctional activity as assessed by illumina sequencing and culture-dependent tools. Front. Plant Sci. 9:1956. doi: 10.3389/fpls.2018.01956
Agnolucci, M., Battini, F., Cristani, C., and Giovannetti, M. (2015). Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol. Fertil. Soils 51, 379–389. doi: 10.1007/s00374-014-0989-5
Ahmed, M., Rauf, M., Mukhtar, Z., and Saeed, N. A. (2017). Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 24, 26983–26987. doi: 10.1007/s11356-017-0589-7
Azizi, S., Tabari Kouchaksaraei, M., Hadian, J., Fallah Nosrat Abad, A. R., Modarres Sanavi, S. A. M., Ammer, C., et al. (2021). Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. For. Ecol. Manage. 497:119478. doi: 10.1016/j.foreco.2021.119478
Cheng, Y. T., Zhang, L., and He, S. Y. (2019). Plant-microbe interactions facing environmental challenge. Cell Host Microbe 26, 183–192. doi: 10.1016/j.chom.2019.07.009
Congreves, K. A., Otchere, O., Ferland, D., Farzadfar, S., Williams, S., and Arcand, M. M. (2021). Nitrogen use efficiency definitions of today and tomorrow. Front. Plant Sci. 12:637108. doi: 10.3389/fpls.2021.637108
Courty, P. E., Smith, P., Koegel, S., Redecker, D., and Wipf, D. (2015). Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. CRC. Crit. Rev. Plant Sci. 34, 4–16. doi: 10.1080/07352689.2014.897897
Dalla Costa, M., Rech, T. D., Primieri, S., Pigozzi, B. G., Werner, S. S., and Stürmer, S. L. (2021). Inoculation with isolates of arbuscular mycorrhizal fungi influences growth, nutrient use efficiency and gas exchange traits in micropropagated apple rootstock ‘Marubakaido.’ Plant Cell. Tissue Organ Cult. 145, 89–99. doi: 10.1007/s11240-020-01994-0
Dellero, Y. (2020). Manipulating amino acid metabolism to improve crop nitrogen use efficiency for a sustainable agriculture. Front. Plant Sci. 11:602548. doi: 10.3389/fpls.2020.602548
Di Benedetto, N. A., Corbo, M. R., Campaniello, D., Cataldi, M. P., Bevilacqua, A., Sinigaglia, M., et al. (2017). The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 3, 413–434. doi: 10.3934/microbiol.2017.3.413
Galloway, J. N., Winiwarter, W., Leip, A., Leach, A. M., Bleeker, A., and Erisman, J. W. (2014). Nitrogen footprints: Past, present and future. Environ. Res. Lett. 9:115003. doi: 10.1088/1748-9326/9/11/115003
Ganugi, P., Fiorini, A., Ardenti, F., Caffi, T., Bonini, P., Taskin, E., et al. (2022). Nitrogen use efficiency, rhizosphere bacterial community, and root metabolome reprogramming due to maize seed treatment with microbial biostimulants. Physiol. Plant. 174, 1–15. doi: 10.1111/ppl.13679
Garcia, K., Doidy, J., Zimmermann, S. D., Wipf, D., and Courty, P. E. (2016). Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci. 21, 937–950. doi: 10.1016/j.tplants.2016.07.010
Giovannini, L., Palla, M., Agnolucci, M., Avio, L., Sbrana, C., Turrini, A., et al. (2020). Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 10:106. doi: 10.3390/agronomy10010108
Gundi, J. S., Santos, M. S., Oliveira, A. L. M., Nogueira, M. A., Hungria, M. (2018). Development of liquid inoculants for strains of Rhizobium tropici group using response surface methodology. Afr J Biotechnol 17, 411–421. doi: 10.5897/AJB2018.16389
Harrison, J., Brugière, N., Phillipson, B., Ferrario-Mery, S., Becker, T., Limami, A., et al. (2000). Manipulating the pathway of ammonia assimilation through genetic 399 engineering and breeding: Consequences to plant physiology and plant development. Plant Soil 221, 81–93. doi: 10.1023/A:1004715720043
Hestrin, R., Hammer, E. C., Mueller, C. W., and Lehmann, J. (2019). Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2:233. doi: 10.1038/s42003-019-0481-8
Hu, Y., and Chen, B. (2020). Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid, malic acid accumulation, and improvement of nitrogen assimilation in roots of water-stressed maize plants. Mycorrhiza 30, 329–339. doi: 10.1007/s00572-020-00952-0
Jan, R., Asaf, S., Numan, M., Lubna, and Kim, K. M. (2021). Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 11:968. doi: 10.3390/agronomy11050968
Khalid, M., Hassani, D., Bilal, M., Asad, F., and Huang, D. (2017). Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 58:35. doi: 10.1186/s40529-017-0189-3
Koegel, S., Mieulet, D., Baday, S., Chatagnier, O., Lehmann, M. F., Wiemken, A., et al. (2017). Phylogenetic, structural, and functional characterization of AMT3; an ammonium transporter induced by mycorrhization among model grasses. Mycorrhiza 27, 695–708. doi: 10.1007/s00572-017-0786-8
Kollah, B., Patra, A. K., and Mohanty, S. R. (2016). Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 23, 4358–4369. doi: 10.1007/s11356-015-5857-9
Kuila, D., and Ghosh, S. (2022). Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 3:100107. doi: 10.1016/j.crmicr.2022.100107
Kumar, V., Kumar, P., and Khan, A. (2020). Optimization of PGPR and silicon fertilization using response surface methodology for enhanced growth, yield and biochemical parameters of French bean (Phaseolus vulgaris L.) under saline stress. Biocatal. Agric. Biotechnol. 23:101463. doi: 10.1016/j.bcab.2019.101463
Lenth, R. V. (2009). Response-surface methods in R, using RSM. J. Stat. Softw. 32, 1–17. doi: 10.18637/jss.v032.i07
López-Bellido, R. J., and López-Bellido, L. (2001). Efficiency of nitrogen in wheat under Mediterranean conditions: Effect of tillage, crop rotation and N fertilization. F. Crop. Res. 71, 31–46. doi: 10.1016/S0378-4290(01)00146-0
Maris, S. C., Abalos, D., Capra, F., Moscatelli, G., Scaglia, F., Reyes, G. E. C., et al. (2021). Strong potential of slurry application timing and method to reduce N losses in a permanent grassland. Agricult. Ecosyst. Environ. 311:107329. doi: 10.1016/j.agee.2021.107329
Marulanda-Aguirre, A., Azcón, R., Ruiz-Lozano, J. M., and Aroca, R. (2008). Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus coinoculated: Physiologic and biochemical traits. J. Plant Growth Regul. 27, 10–18. doi: 10.1007/s00344-007-9024-5
Matsumura, A., Taniguchi, S., Yamawaki, K., Hattori, R., Tarui, A., Yano, K., et al. (2013). Nitrogen uptake from amino acids in maize through arbuscular mycorrhizal symbiosis. Am. J. Plant Sci. 4, 2290–2294. doi: 10.4236/ajps.2013.412283
Mazumdar, D., Saha, S., and Ghoshet, S. (2021). RSM based optimization of plant growth promoting rhizobacteria and nitrogen dosage for enhanced growth and yield of mustard (Brassica campestris L.). J. Plant Nutr. 44, 2228–2244. doi: 10.1080/01904167.2021.1889585
Moreau, D., Bardgett, R. D., Finlay, R. D., Jones, D. L., and Philippot, L. (2019). A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 33, 540–552. doi: 10.1111/1365-2435.13303
Mus, F., Crook, M. B., Garcia, K., Garcia Costas, A., Geddes, B. A., Kouri, E. D., et al. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82, 3698–3710. doi: 10.1128/AEM.01055-16
Naili, F., Neifar, M., Elhidri, D., Cherif, H., Bejaoui, B., and Aroua, M. (2018). Optimization of the effect of PGPR-based biofertlizer on wheat growth and yield. Biometric. Biostat. Int. J. 7, 226–232. doi: 10.15406/bbij.2018.07.00213
Neumann, G., Bott, S., Ohler, M. A., Mock, H. P., Lippmann, R., Grosch, R., et al. (2014). Root exudation and root development of lettuce (Lactuca sativa l. Cv. Tizian) as affected by different soils. Front. Microbiol. 5:2. doi: 10.3389/fmicb.2014.00002
Oldroyd, G. E. D., Murray, J. D., Poole, P. S., and Downie, J. A. (2011). The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45, 119–144. doi: 10.1146/annurev-genet-110410-132549
Ortiz, N., Armada, E., Duque, E., Roldán, A., and Azcón, R. (2015). Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 174, 87–96. doi: 10.1016/j.jplph.2014.08.019
Pacheco, I., Ferreira, R., Correia, P., Carvalho, L., Dias, T., and Cruza, C. (2021). Microbial consortium increases maize productivity and reduces grain phosphorus concentration under field conditions. Saudi J. Biol. Sci. 28, 232–237. doi: 10.1016/j.sjbs.2020.09.053
Paul, K., Saha, C., Nag, M., Mandal, D., Naiya, H., Sen, D., et al. (2020). A tripartite interaction among the basidiomycete rhodotorula mucilaginosa, N2-fixing endobacteria, and rice improves plant nitrogen nutrition. Plant Cell 32, 486–507. doi: 10.1105/tpc.19.00385
Peng, S., Buresh, R. J., Huang, J., Yang, J., Zou, Y., Zhong, X., et al. (2006). Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. F. Crop. Res. 96, 37–47. doi: 10.1016/j.fcr.2005.05.004
Peng, Y., He, Y., Wu, Z., Lu, J., and Li, C. S. (2014). Screening and optimization of low-cost medium for Pseudomonas putida Rs-198 culture using RSM. Braz. J. Microbiol. 45, 1229–1237. doi: 10.1590/s1517-83822014000400013
Pereira, S. I. A., Abreu, D., Moreira, H., Vega, A., and Castro, P. M. L. (2020). Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 6:e05106. doi: 10.1016/j.heliyon.2020.e05106
Qu, Q., Zhang, Z., Peijnenburg, W. J. G. M., Liu, W., Lu, T., Hu, B., et al. (2020). Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 68, 5024–5038.
Ribaudo, C., Rondanini, D. P., Curá, J. A., and Fraschina, A. A. (2001). Response of Zea mays to the inoculation with Azospirillum on nitrogen metabolism under greenhouse conditions. Biol. Plant. 44, 631–634. doi: 10.1023/A:1013779712106
Rosenblueth, M., Ormeño-Orrillo, E., López-López, A., Rogel, M. A., Reyes-Hernández, B. J., Martínez-Romero, J. C., et al. (2018). Nitrogen fixation in cereals. Front. Microbiol. 9:1794. doi: 10.3389/fmicb.2018.01794
Salvador, G. L. O., Araújo, F. F., de Araujo Pereira, A. P., Bonifácio, A., and Ferreira Araujo, A. S. (2022). Rhizobacteria and arbuscular mycorrhizal fungus presented distinct and specific effects on soybean growth when inoculated with organic compost. Rhizosphere 22:100513. doi: 10.1016/j.rhisph.2022.100513
Santi, C., Bogusz, D., and Franche, C. (2013). Biological nitrogen fixation in non-legume plants. Ann. Bot. 111, 743–767. doi: 10.1093/aob/mct048
Tajini, F., Trabelsi, M., and Drevon, J. J. (2012). Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 19, 157–163. doi: 10.1016/j.sjbs.2011.11.003
The, S. V., Snyder, R., and Tegeder, M. (2021). Targeting nitrogen metabolism and transport processes to improve plant nitrogen use efficiency. Front. Plant Sci. 11:628366. doi: 10.3389/fpls.2020.628366
Udvardi, M., and Poole, P. S. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64, 781–805. doi: 10.1146/annurev-arplant-050312-120235
van Bueren, L. E. T., and Struik, P. C. (2017). Diverse concepts of breeding for nitrogen use efficiency. A review. Agron. Sustain. Dev. 37:50. doi: 10.1007/s13593-017-0457-3
van Dam, N. M., and Bouwmeester, H. J. (2016). Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 21, 256–265. doi: 10.1016/j.tplants.2016.01.008
Verzeaux, J., Hirel, B., Dubois, F., Lea, P. J., and Tétu, T. (2017). Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: Basic and agronomic aspects. Plant Sci. 264, 48–56. doi: 10.1016/j.plantsci.2017.08.004
Wickham, H., and Chang, W. (2007). ggplot2: An implementation of the grammar of graphics. GNU general public license. Available online at: https://cran.r-project.org/web/packages/ggplot2 (accessed May 03, 2022).
Keywords: biostimulants, Bacillus megaterium, Rhizoglomus irregulare, Funneliformis mosseae, nutrients uptake, plant growth promoting rhizobacteria
Citation: Ganugi P, Fiorini A, Rocchetti G, Bonini P, Tabaglio V and Lucini L (2022) A response surface methodology approach to improve nitrogen use efficiency in maize by an optimal mycorrhiza-to-Bacillus co-inoculation rate. Front. Plant Sci. 13:956391. doi: 10.3389/fpls.2022.956391
Received: 30 May 2022; Accepted: 11 July 2022;
Published: 11 August 2022.
Edited by:
Maurizio Ruzzi, University of Tuscia, ItalyReviewed by:
Philipp Franken, Friedrich Schiller University Jena, GermanyCopyright © 2022 Ganugi, Fiorini, Rocchetti, Bonini, Tabaglio and Lucini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Fiorini, YW5kcmVhLmZpb3JpbmlAdW5pY2F0dC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.