
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 04 August 2022
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.954478
This article is part of the Research Topic Adaptation Mechanisms of Grass and Forage Plants to Stressful Environments View all 42 articles
 Carl A. Frisk1,2*†
Carl A. Frisk1,2*† Georgianna Xistris-Songpanya1
Georgianna Xistris-Songpanya1 Matthieu Osborne1
Matthieu Osborne1 Yastika Biswas1
Yastika Biswas1 Rainer Melzer1,2‡
Rainer Melzer1,2‡ Jon M. Yearsley1,2*‡
Jon M. Yearsley1,2*‡Identifying how various components of climate change will influence ecosystems and vegetation subsistence will be fundamental to mitigate negative effects. Climate change-induced waterlogging is understudied in comparison to temperature and CO2. Grasslands are especially vulnerable through the connection with global food security, with perennial ryegrass dominating many flood-prone pasturelands in North-western Europe. We investigated the effect of long-term waterlogging on phenotypic responses of perennial ryegrass using four common varieties (one diploid and three tetraploid) grown in atmospherically controlled growth chambers during two months of peak growth. The climate treatments compare ambient climatological conditions in North-western Europe to the RCP8.5 climate change scenario in 2050 (+2°C and 550 ppm CO2). At the end of each month multiple phenotypic plant measurements were made, the plants were harvested and then allowed to grow back. Using image analysis and principal component analysis (PCA) methodologies, we assessed how multiple predictors (phenotypic, environmental, genotypic, and temporal) influenced overall plant performance, productivity and phenotypic responses. Long-term waterlogging was found to reduce leaf-color intensity, with younger plants having purple hues indicative of anthocyanins. Plant performance and yield was lower in waterlogged plants, with tetraploid varieties coping better than the diploid one. The climate change treatment was found to reduce color intensities further. Flooding was found to reduce plant productivity via reductions in color pigments and root proliferation. These effects will have negative consequences for global food security brought on by increased frequency of extreme weather events and flooding. Our imaging analysis approach to estimate effects of waterlogging can be incorporated into plant health diagnostics tools via remote sensing and drone-technology.
Predicting plant responses caused by climate change is a fundamental challenge that will increasingly impact coming generations (Parmesan and Hanley, 2015). Identifying how the various components of climate change are likely to affect plant responses will inform options of optimal mitigation strategies to minimize any negative effects (Canadell and Raupach, 2008; Pareek et al., 2020). While this is a complex issue affecting all ecosystems, it is especially important for grassland ecosystems due to the unavoidable connection with global food security through agriculture, crop lands and pasture lands (Tester and Langridge, 2010; Kipling et al., 2016a; Lecerf et al., 2019; Raza et al., 2019). There is a growing concern that climate change will result in ecological transformation of grasslands (Kipling et al., 2016b; Wang et al., 2018) which will alter plant phenology (Jentsch et al., 2009; Munson and Long, 2017), biodiversity (Bellocchi and Picon-Cochard, 2021), and productivity (Goliński et al., 2018; Qi et al., 2018).
Grasslands are defined as ecosystems dominated by the Poaceae (grass) taxonomic family, with the ecology varying widely depending on species composition, edaphic factors, topography, management, and climate. Previous studies have suggested that elevated CO2 levels are likely to increase photosynthetic capability in grasses, increasing Net Primary Production (NPP) and thereby total yield (Ergon et al., 2018; Yiotis et al., 2021). Meanwhile, higher temperatures are likely to extend the length of the growth season, providing longer time for sustained growth (Höglind et al., 2013; Pembleton et al., 2020). While increased ambient temperatures and elevated CO2 act long-term and are the main components of many climate models (e.g., Thornley and Cannell, 1997; Huntingford et al., 2013), altered precipitation regimes are equally important, and predicted to act both short- and long-term (Dore, 2005; Cullen et al., 2009; Brown et al., 2019). Previous grass research from altered precipitation regimes has focused on the effects of prolonged droughts, reduced water-availability and increased desertification (Farfan-Vignolo and Asard, 2012; Cullen et al., 2014; Bothe et al., 2018; Buttler et al., 2019; Yates et al., 2019), however many areas might see the opposite effect, leading to increased flooding (Kiely, 1999; Rosenzweig et al., 2002).
Climate change-induced alterations to precipitation regimes are expected to contribute to increased frequency of extreme weather events, such as severe storms and extreme flooding (Easterling et al., 2000; Semmler and Jacob, 2004). This is partly due to warmer temperatures increasing evaporation, and warmer air being able to hold more moisture, increasing the total amount of water vapor in atmospheric circulation (Hu et al., 2000; O’Gorman and Muller, 2010). Additionally, predicted changes to the precipitation seasonality are likely to change the frequency and distribution of rain events, potentially enhancing the drought-flooding dichotomy (Feng et al., 2013; Kumar, 2013).
Perennial ryegrass (Lolium perenne) is a common cool season pasture grass grown extensively throughout its native Eurasian-range and cultivated worldwide due to its high nutritional quality and palatability for livestock (Hunt and Easton, 1989; Hannaway et al., 1999; Smit et al., 2005; Minneé et al., 2019; Tubritt et al., 2020). Many genetically different varieties of perennial ryegrass are bred and cultivated to match the climatological conditions of specific target regions, which in turn causes differences in plant health and yield depending on local environmental suitability (Grogan and Gilliland, 2011; Helgadóttir et al., 2018). Varieties suitable in current conditions under regular precipitation regimes might prove unsuitable under periods of increased flooding (Mustroph, 2018). Flooding can cause long-term waterlogging, affecting overall plant health and total yield (McFarlane et al., 2003; Striker, 2012) and grassland ecosystem function (Fay et al., 2008). It could also impact plant morphology due to phenotypic plasticity (Münzbergová et al., 2017; Mizutani and Kanaoka, 2018). Previous studies have shown that plants can survive the unfavorable conditions but alters their phenotype in the process as a response, with some varieties of the same species being differentially susceptible to the stressor (Song, 2009; Tong et al., 2021; Stasnik et al., 2022). Decrease in growth due to an altered phenotypic response can have devastating impacts on global food security due to the bottom-up reliance of agricultural system productivity from grassland areas (Hazell and Wood, 2008; Baldos and Hertel, 2014). In addition, economic consequences would be especially severe for countries like Ireland and the United Kingdom with large land areas consisting of ryegrass dominated pasture lands and many rivers currently prone and predicted in the future to flood (Blöschl et al., 2019).
Here, we quantify the effects from waterlogging on perennial ryegrass performance and plant health in the light of climate change. We hypothesized that waterlogging would decrease perennial ryegrass performance and lower plant yield, and that the climate change treated plants would perform better under waterlogging than the ambiently treated plants. We further explored whether waterlogging and climate change impacted the phenotypic plasticity of the plants. We investigated this using atmospherically controlled growth chambers and multiple commercial high-producing perennial ryegrass varieties with varying genetic backgrounds in an image analysis framework.
This study used four atmospherically controlled CONVIRON BDW40 walk-in growth chambers located in the PÈAC (Programme for Experimental Atmospheres and Climate) facility in Rosemount Environmental Research Station belonging to the University College Dublin in Dublin, Ireland. This facility has been used in previous studies to investigate plant responses to elevated CO2 (Batke et al., 2018; Yiotis et al., 2021) and atmospheric paleoclimatic reconstruction (Evans-Fitz. Gerald et al., 2016; Porter et al., 2017; Yiotis et al., 2017). Two chambers were chosen to represent typical North-western European climatological conditions (CO2-levels at 415 ppm and ambient temperature conditions) while two chambers were chosen to represent the predicted 2050 combined climate change climatological conditions according to the RCP8.5 scenario [elevated CO2-levels (550 ppm) and a 2°C increase in temperature (IPCC, 2014)]. The growth chamber climatological baselines (ambient conditions) were constructed from the last thirty years of meteorological data (1989–2018) collated from the meteorological station located at Cork Airport and publicly accessible via the Irish Meteorological Services (Met Éireann). The entire experiment simulated conditions from May to September but only climatological conditions replicating two months of optimal pasture growth (June and July) (McHugh et al., 2020) were used to investigate perennial ryegrass responses to waterlogging (Table 1).
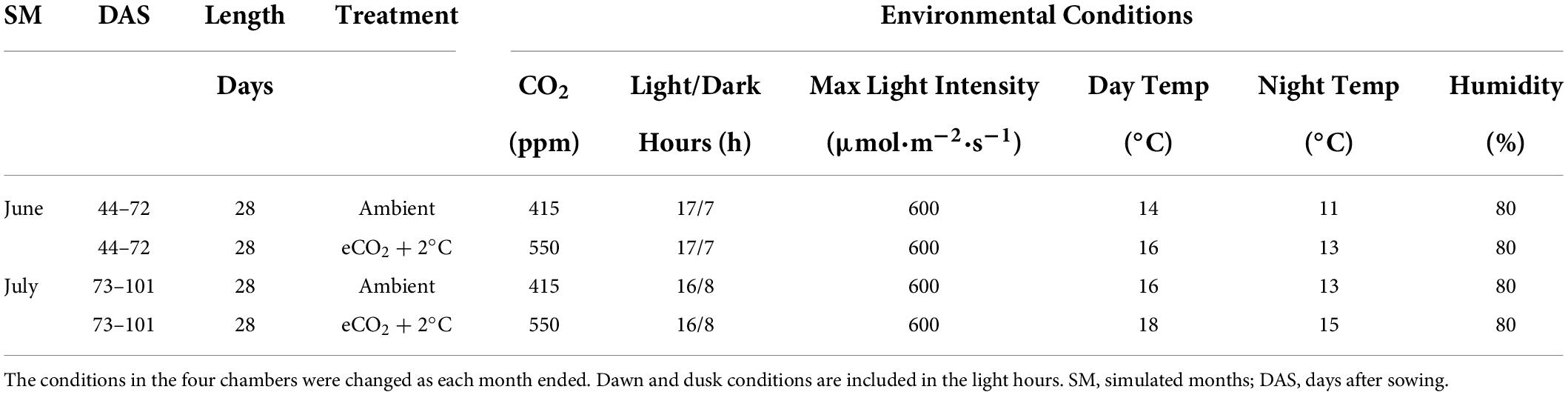
Table 1. Experimental Growth Chamber setup for the two ambient and the two climate chambers simulating the months June and July in North-western Europe.
Four common internationally grown varieties of perennial ryegrass were used for the experiment: Aberchoice, Abergain, Carraig, and Dunluce (European Commision, 2019). The varieties vary in heading date and ploidy (Table 2), which allowed for an intra-species comparison to identify whether genetic factors might contribute to the response to waterlogging and climate change. At the simulated start of May each chamber was populated with 80 PVC cores (50 cm × 16 cm (ϕ)) filled with John Innes No2 compost (320 cores in total for the four chambers), with the John Innes No2 (Westland) being a loam-mixture compost with peat, horticultural grit, and added nutrients. Each core was sealed inside a plastic bag to allow half of the cores to be waterlogged further on. The 50 cm tall cores allowed for the simulation of largely natural grassland root depth (Wedderburn et al., 2010; Cougnon et al., 2017). Each core was sown with ten seeds from one of the four varieties (DAS 0, Days after sowing) and allowed to germinate. At DAS 43, the most centrally germinated seedling was kept, and the other germinated seeds discarded. The seedlings were then cut to a height of 5 cm to simulate equal growth between all replicates. Waterlogging was initiated in 40 of the 80 cores per chamber on DAS 48, with the additional watering being slowly initiated over three days to reach a stable water level 2 cm above the soil. Non-waterlogged cores continued to be watered normally, once to twice weekly to keep soil moisture around 25%. Waterlogging was actively enforced for one month and then allowed to dissipate naturally. Waterlogging experiments in grasses tend to last around 15 days (e.g., Liu and Jiang, 2015; de la Cruz Jiménez et al., 2017; Ploschuk et al., 2017), with few experiments lasting up to 30 days (McFarlane et al., 2003). The longer duration was implemented due to the increased chance (and consequences) of climate changed-induced extreme flooding events (Dore, 2005; Trenberth, 2011). Chamber treatment, waterlogging, and variety placement were stratified equally among the chambers and then randomized within chambers. The stratification allowed for equal numbers of each variety in each chamber, with half of the 80 cores per chamber being waterlogged for equal comparison for all factors.
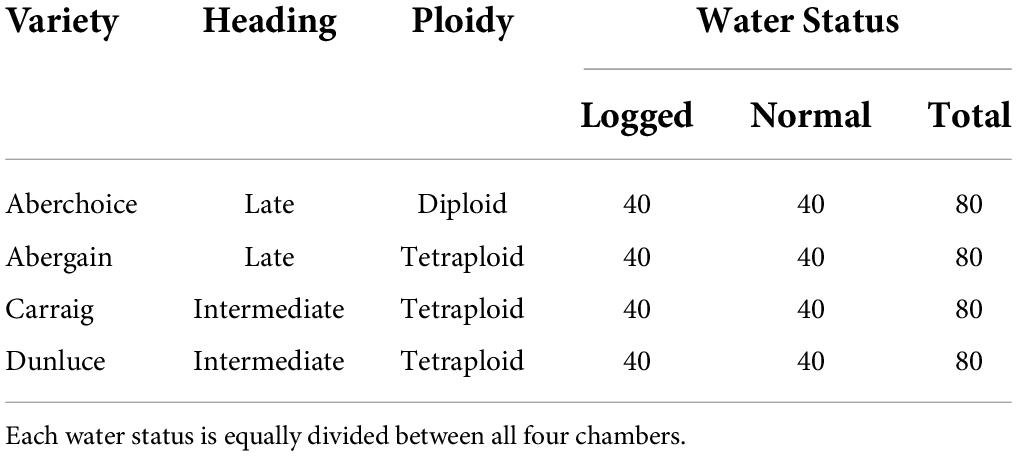
Table 2. Perennial ryegrass (Lolium perenne) varieties grown and water status for all chambers and variety replicates.
Multiple sets of phenotypic data were collected each month to identify the effects on plant performance from the waterlogging. Soil and Plant Analyzer Development (SPAD) readings were conducted using a SPAD-502 Plus Chlorophyll Meter (Konica Minolta) to sample leaf chlorophyll content (e.g., Bothe et al., 2018; Dong et al., 2019). Three representative leaves were sampled from each plant, with each leaf being sampled at three places and averaged for a reliable measurement. Soil moisture was measured at 10 cm depth using a HH2 Moisture Meter with a calibrated WET sensor type WET-2 attachment (Delta-T Devices). At the end of each month (DAS 72, June and 101, July) the maximum height of the plants were measured. After being measured, the plants were harvested by cutting the grasses using scissors at 5 cm above the soil-level, leaving 5 cm of basal grass tissue intact, simulating natural grazing. The plants were then allowed to recover, grow and then be harvested again the subsequent month. The harvested material from each plant was placed on a flat white background and photographed using a high-resolution LUMIX DC-G9 camera (Panasonic) with an accompanied ColorChecker Classic chart (X-rite). This enabled the harvested material to be processed using color-corrective image analysis techniques to identify differences in leaf color hues (e.g., Hu et al., 2013; Li et al., 2014; Zhang et al., 2014; de la Cruz Jiménez et al., 2017). The leaves were photographed immediately after harvest to prevent structural and color degradation (Løkke et al., 2013; Yamauchi and Watada, 2019). After the photographs all harvest material from each plant was oven dried at 65°C for one week and then weighed to measure dried biomass per plant.
The first stage of image analysis was to convert the color filter array (CFA) in each RAW image from the LUMIX DC-G9 camera into a color-corrected red-green-blue (RGB) image. This was done using a standardized pipeline with the following nine steps: (1) subtract a black value from all pixels in the CFA, (2) set negative pixel values to zero, (3) divide all pixels by the maximum pixel value, (4) correct the white balance by scaling red and blue pixels relative to green pixels in the CFA, (5) convert pixels to unsigned 16-bit integers, demosaiced the CFA into a true-color image using a gradient-corrected linear interpolation (Malvar et al., 2004), (6) transform the image from the camera’s color space to RGB, (7) identify the 24 colors on the X-Rite ColorChecker Classic chart within the image, (8) estimate an affine color-transformation matrix that minimizes the sum of squared deviations between the RGB colors in the image to the known colors of the 24 colored squares, (9) apply the color-transformation matrix to produce a color-correct RGB image. The camera’s image metadata was used to obtain values for black level, white-balance correction and camera color space to RGB conversion.
The second stage of the image analysis extracted RGB values from pixels that corresponded to ryegrass leaves. Ryegrass leaves were placed upon a flat, white, rectangular background which enabled the image to be cropped to the white background. The cropped image was then converted into an HSV color-space and an initial mask created with hue values in the range 0.0–0.4 and 0.875–1.0 (corresponding to yellow, green, and red hues), saturation in the range 0.2–1.0 and value in the range 0.0–0.9. Regions of the mask with connected components containing fewer than 100 pixels were removed before the mask was refined using 50 iterations of an active contours region growing algorithm and saturation values retained if they were in the range 0.25–1.0. The final mask was used to extract the position of pixels in the mask and their RGB values. All image processing was performed using MatLAB (version R2021a.) and its image processing toolbox (Mathworks, 2021).
The ryegrass leaf RGB values from the image analysis were further processed by calculating the median value for each hue from each image of the harvested ryegrass. Median values were used due to their robust statistical properties against outliers and skewed distributions (Chen, 1998; von Hippel, 2005). To dimensionally reduce the three hues into one variable the hues were analyzed using principal component analysis (PCA) from the R package vegan (Oksanen et al., 2020). Although variable standardization is normally recommended (Jollife and Cadima, 2016), the hues were analyzed without scaling and centering to preserve the relative values (Lever et al., 2017). We did not expect the removal of scaling to have a detrimental effect on model fitting due to the three bands having similar variances. The principal components (PC) were first tested for normality using the Shapiro-Wilks test (Shapiro and Wilk, 1965) and then analyzed using Kendall’s tau rank correlation (Kendall, 1938) and Wilcoxon’s signed rank test (Wilcoxon, 1945) to test if there were any correlations and differences in mean values between the harvest groups (DAS 72 and 101) and between each water status. Kendall’s tau was used due to the higher robustness and efficiency compared to the otherwise commonly used Spearman’s rho (Spearman, 1904; Croux and Dehon, 2010). The first principal component was further used as a response variable to build a linear model to identify relevant covariates responsible for the combined hues. A similar approach has previously been used by Golzarian and Frick (2011) to investigate early growth stages of grasses. Multiple predictor variables were used to build the linear regression model: phenotypic (dried biomass, maximum height and SPAD measurements as a proxy for chlorophyll content), environmental (soil moisture as a proxy for waterlogging and chamber treatment as proxy for ambient and climate change climatological conditions), genetic (variety as a proxy for ploidy and heading date) and temporal (progression of the season and recovery from the waterlogging). Although mixed-models are generally recommended in this type of hierarchical ecological design (Piepho et al., 2003) the estimation of random effects from a low number of levels is very similar to fitting fixed effects models, and can in some cases cause worse model fits due to zero variance estimates (Harrison et al., 2018). The model terms were subsequently analyzed using a Type II ANOVA (Langsrud, 2003; Smith and Cribbie, 2014). Model selection was then performed to analyze how removing variables in the full model would impact the AIC values and model understanding (Bozdogan, 1987; Aho et al., 2014). All statistical analyses were performed in the statistical software R (version 4.1.3.) (R Core Team, 2022).
To estimate the effects of long-term waterlogging on perennial ryegrass we grew 320 plants in fully atmospherically controlled growth chambers, simulating typical current climatic conditions in North-western Europe and conditions as predicted in 2050 according to RCP8.5 (550 ppm CO2 and a mean temperature increase of 2°C). Half of the plants were subjected to waterlogging 48 days after sowing (DAS) for one month. After waterlogging had begun, visible differences were observed between waterlogged and non-waterlogged plants at DAS 72 and continued to be visible at DAS 101 (Figure 1). The waterlogged plants by DAS 72 had stunted growth with dark brown leaf hues. The leaf morphology of the waterlogged plants also varied to the non-waterlogged plants, because leaf unfolding was disrupted, causing a concave and folded appearance of many leaves in waterlogged plants (Figure 2). By DAS 101 many plants had started to change in leaf color, with many leaves presenting light green shades. This contrasts with the non-waterlogged plants that had darker green shades and a lush appearance. Leaf morphology also differed at DAS 101 between waterlogged and non-waterlogged plants, where the leaves of the waterlogged plants remained concave and light green while the non-waterlogged plants had tall, wide and lush leaves. The median true colors of the harvests at DAS 72 (Figure 3) and DAS 101 (Figure 4) showed a substantial variation between plants of each water treatment and chamber condition, with darkening of leaves as the growth season progressed. No direct visual difference could be observed between the two climate treatments (ambient vs 2050 RCP8.5 scenario).

Figure 1. Perennial Ryegrass (Lolium perenne) whole plant appearance examples images for selected harvests and water status. (A) DAS 72 non-waterlogged. (B) DAS 72 waterlogged. (C) DAS 101 non-waterlogged. (D) DAS 101 waterlogged. Examples images are not color corrected.

Figure 2. Perennial Ryegrass (Lolium perenne) leaf morphology example images for selected harvests and water status. (A) DAS 72 non-waterlogged. (B) DAS 72 waterlogged. (C) DAS 72 waterlogged. (D) DAS 101 non-waterlogged. (E) DAS 101 waterlogged. Examples images are not color corrected.
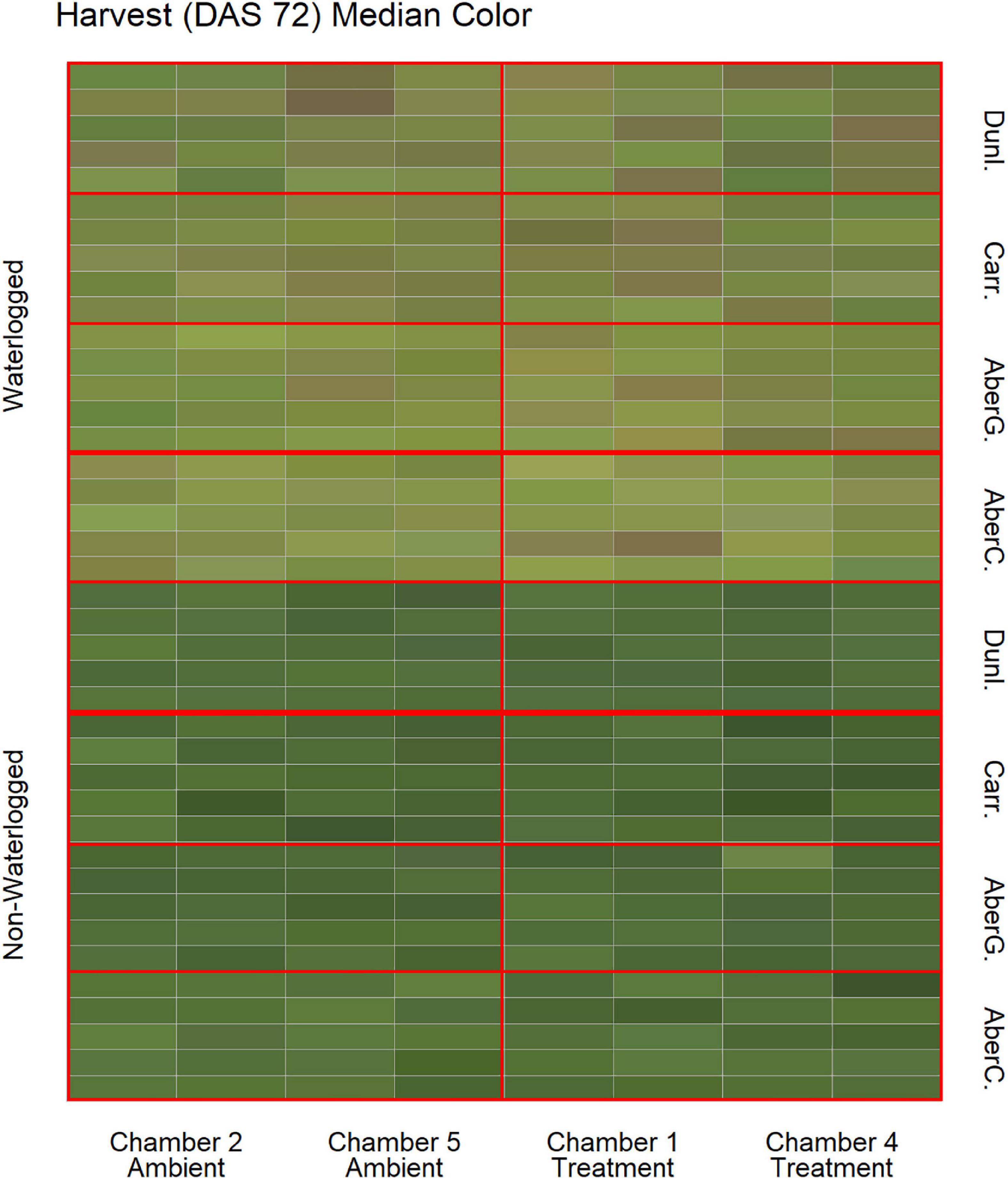
Figure 3. Median color (RGB) of the harvested material for all cores on DAS 72 as identified by the image analysis, sorted on water status, climate treatment and variety. Each cell represents the median true color of the harvested material of a plant. AberC, Aberchoice; AberG, Abergain; Carr, Carraig; Dunl, Dunluce.
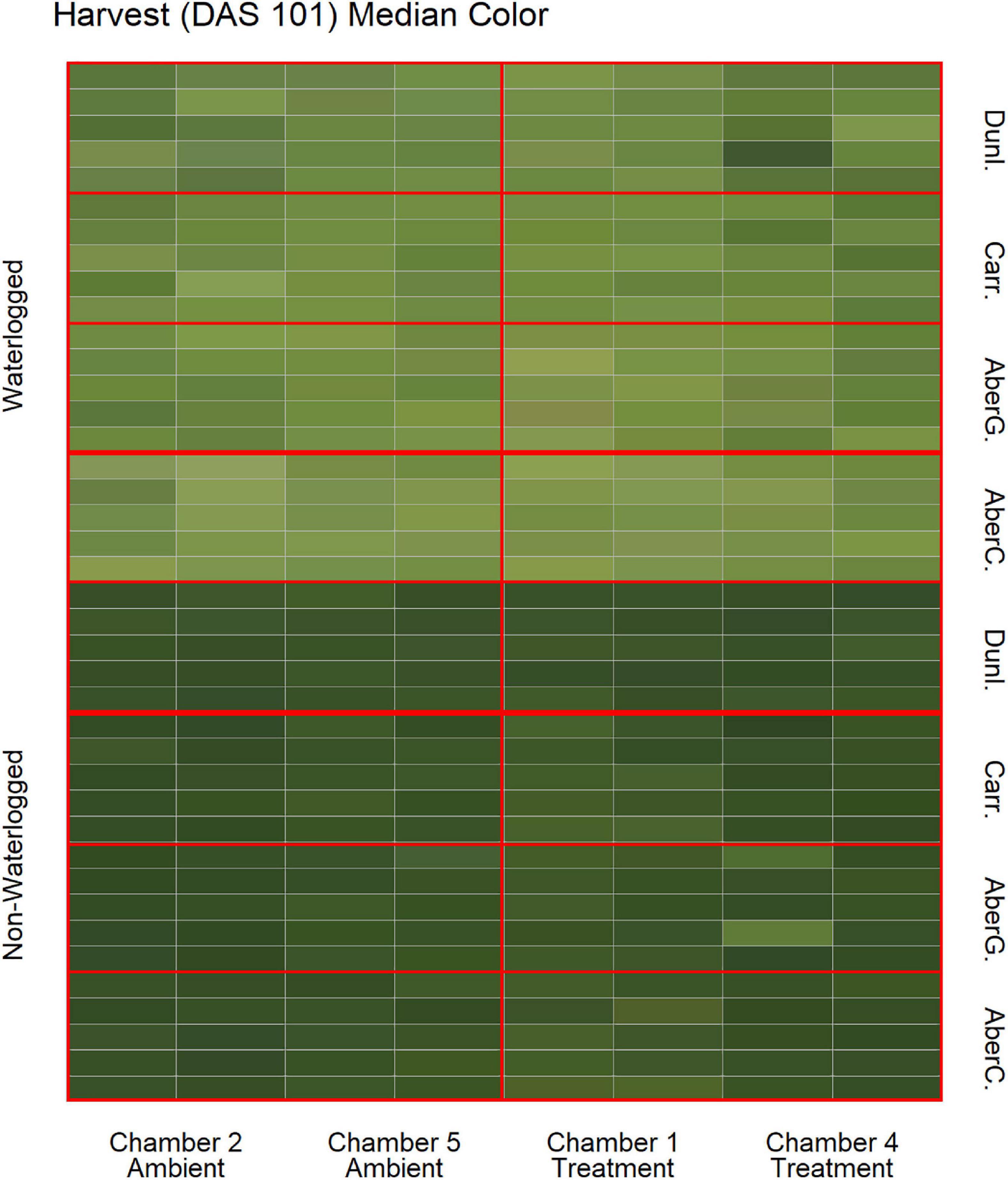
Figure 4. Median color (RGB) of the harvested material for all cores on DAS 101 as identified by the image analysis, sorted on water status, climate treatment and variety. Each cell represents the median true color of the harvested material of a plant. AberC, Aberchoice; AberG, Abergain; Carr, Carraig; Dunl, Dunluce.
To quantify the visible differences between the waterlogged and non-waterlogged plants, all plant leaves were harvested at DAS 72 and again at DAS 101, photographed and subjected to a color analysis. The isolated RGB hues from the color analysis were further modeled using a PCA-method. We hypothesized that there would be measurable color differences depending on the water status, with the waterlogging contributing to lighter colors. We also expected color differences between harvest dates, with darker colors as the growth season progressed. The PCA of the three RGB hues isolated from the harvested material from DAS 72 and 101 identified that the greatest variation (97.3%) could be explained by the first PC axis (PC1) (Figure 5). All three RGB hues showed a positive increase with the first axis (Supplementary Table 1), illustrating that the axis describes a light beige to dark brown hue divergence (Supplementary Figure 1). To simplify this main leaf describing characteristics we classified the axis as overall color intensity. The negative values of PC1 are therefore darker intensities, while the positive values are lighter intensities. The other two minor axes PC2 (1.8%) and PC3 (0.9%) describe pure color hue gradients, a green-purple and an orange-blue hue divergence respectively. Kendall’s tau and Wilcoxon’s signed rank test showed that the harvested material from the waterlogged plants collected on DAS 72 were not correlated in color to the non-waterlogged plants of the same harvest and were overall lighter (p < 0.001) and more purple (p < 0.001) (Table 3). The waterlogged plants on DAS 101 were positively correlated in color intensity with the non-waterlogged plants of the same harvest while being darker (p < 0.001) and greener (p < 0.001). The harvested material from the waterlogged plants were positively correlated in color intensity from DAS 72 to 101 with the plants becoming darker (p < 0.001) and greener (p < 0.001) as they started to recover from the waterlogging. The non-waterlogged plants were positively correlated in color intensity between the harvests periods and became overall darker (p < 0.001) as the growth season progressed. See Supplementary Material for the mean differences in PC axes values between the harvests and water status groupings (Supplementary Table 2).
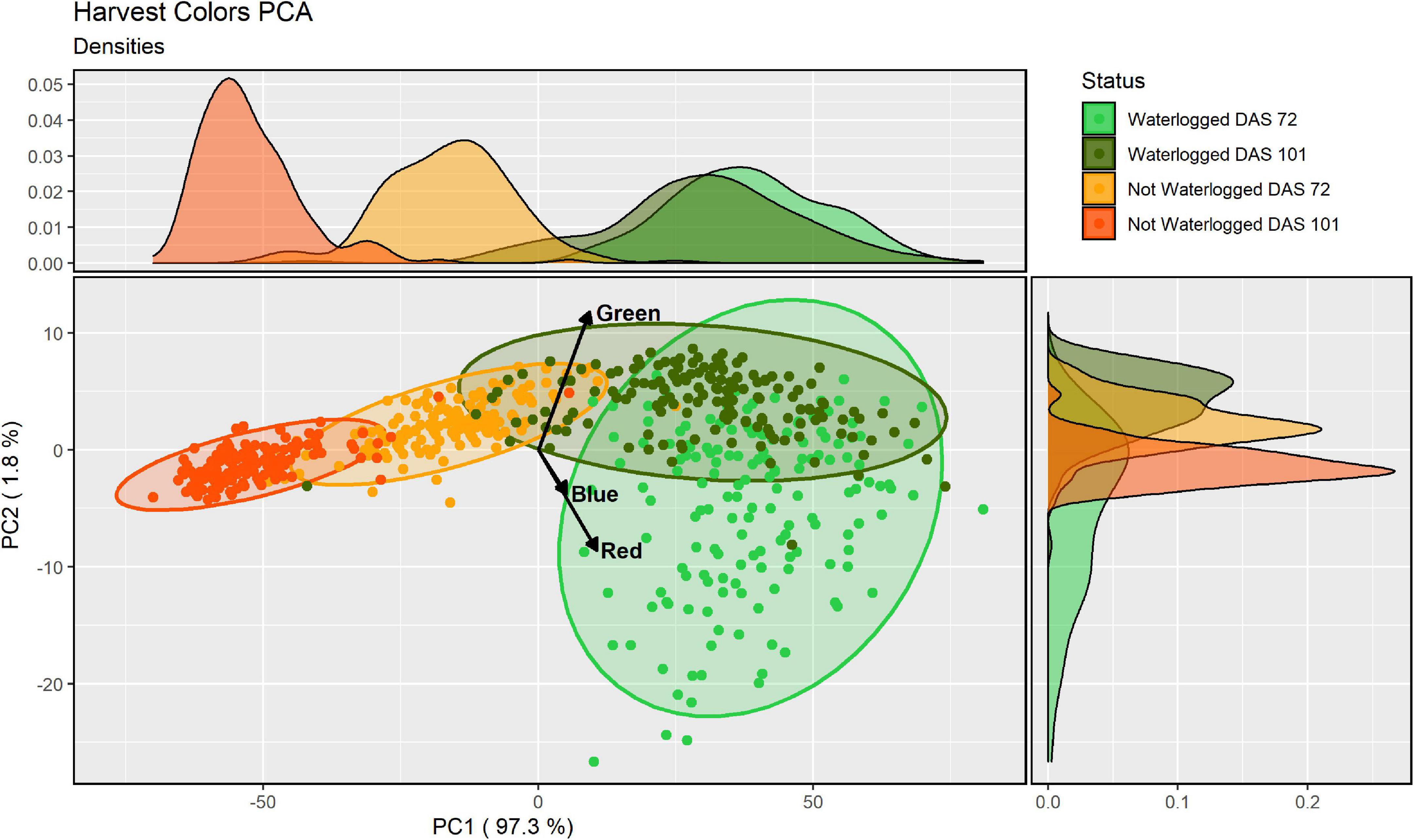
Figure 5. Principal component analysis (PCA)-analysis of the three RGB-hues of the harvested material for all cores, water status and harvest (DAS 72 and 101) as identified by the image analysis. Each ellipse represents a 95% confidence ellipse for each group. Side graphs shows the relative densities of each PCA axis and grouping, with the area under each curve representing the entire group distribution.
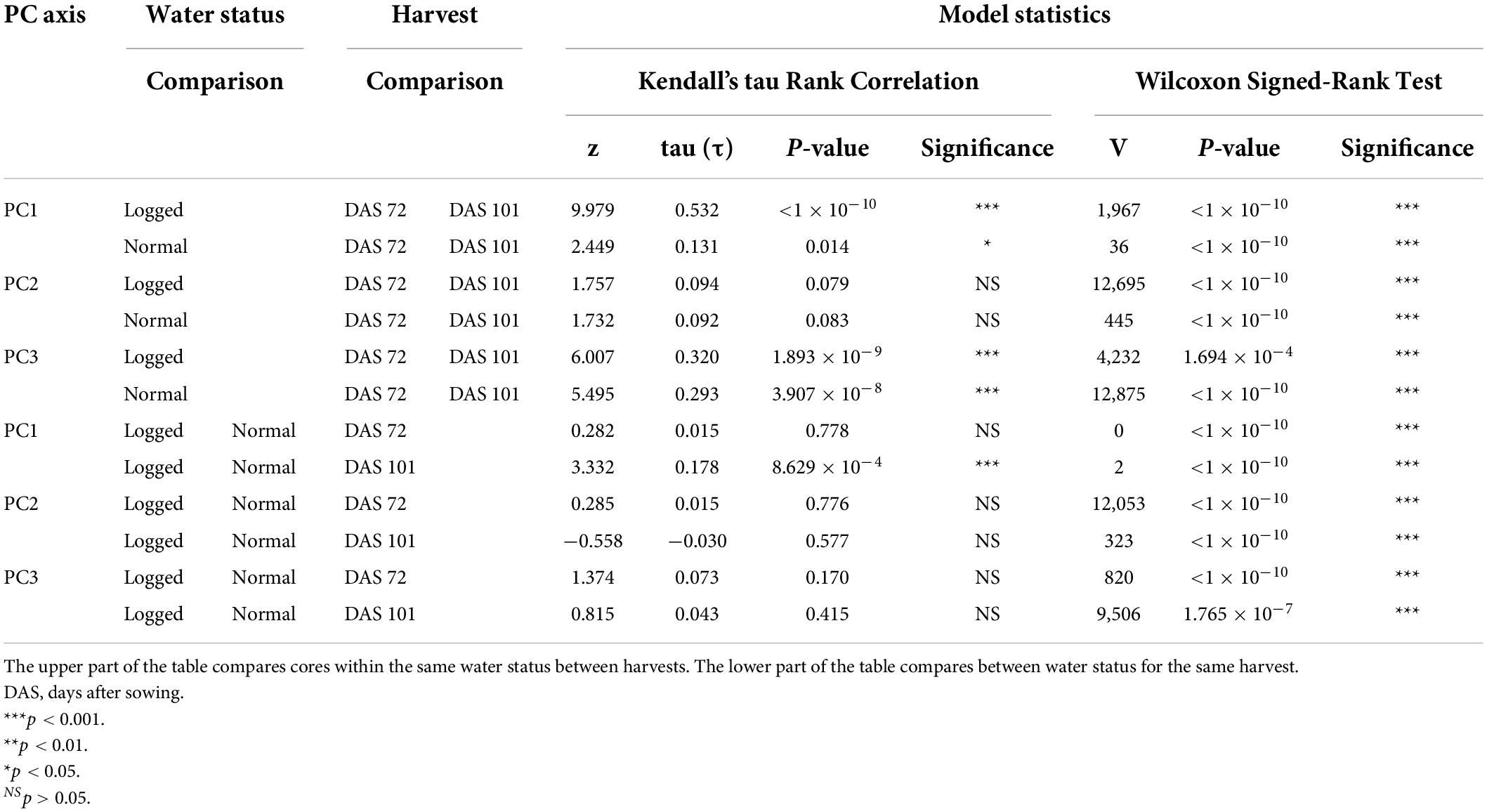
Table 3. Model statistics and significance levels for the comparison of PC axes values for the groupings harvested material (DAS 72 and 101) and water status of the cores in contrast to the other grouping.
To understand how phenotypic, environmental, genetic and temporal factors contribute to the quantified colors of the harvested leaf material a linear regression model was created and analyzed. The first PC axis (PC1), identified as color intensity, was modeled using three phenotypic variables, two environmental variables, one genetic variable and one temporal variable. The model was analyzed using Type II ANOVA and AIC to see the model performance after subsequently removing each variable individually. We hypothesized that the effects from the predictor variables would be physiologically connected and cause differences in color intensity. We expected that higher soil moisture would result in lighter colors and cause negative physiological effects on growth by lowering biomass and maximum height. We also expected that the climate change conditions would enhance growth through increased temperature and CO2, causing darker colors, along with darker colors as the growth season progressed, with variations between varieties caused by inherent genetic differences.
Phenotypically, harvested material from plants with lighter color intensities had significantly lower dried biomass (F1,630 = 232.44, p < 0.001), significantly lower maximum height (F1,630 = 57.92, p < 0.001), and significantly lower SPAD values (F1,630 = 250.01, p < 0.001) (Table 4 and Supplementary Figures 2–4). For the water status, harvested material from cores with lighter color intensities had significantly higher soil moisture (F1,630 = 174.67, p < 0.001) (Supplementary Figure 5). The harvested material from plants in chambers in predicted 2050 climate change conditions had significantly lighter intensities than harvested material from plants in ambient conditions (F1,630 = 19.20, p < 0.001) (Supplementary Figure 6).
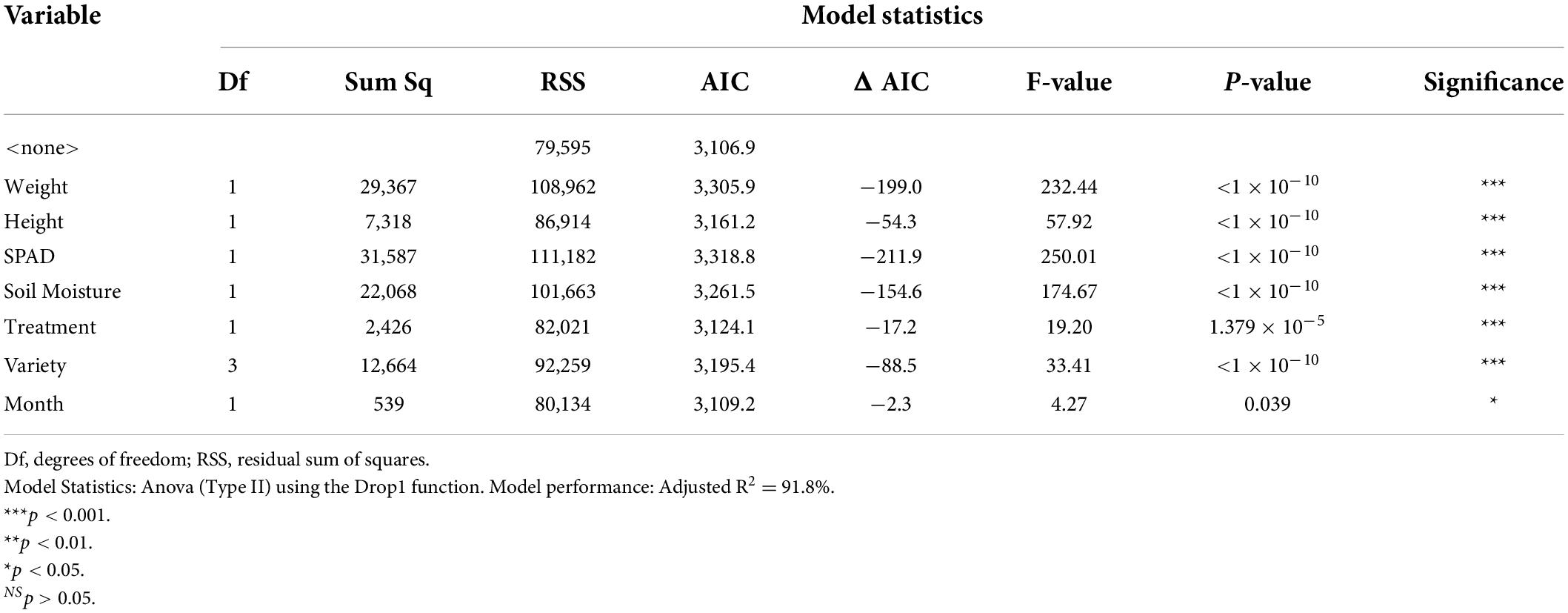
Table 4. Model statistics and significance levels for the linear model in regards to the first PCA axis (Color Intensity) isolated from the color analysis of the harvested perennial ryegrass material.
Genetically, there were significant differences in color intensity between the varieties (F3,630 = 33.41, p < 0.001). Aberchoice, the only diploid variety, had the lightest color intensities, while the three tetraploid varieties, Abergain, Dunluce, and Carraig had darker intensities in that order, with Carraig having the darkest leaf color intensities (Supplementary Figure 7). The harvested material became darker overall as the season progressed (F1,630 = 4.27, p = 0.039), with the leaves in DAS 101 being significantly darker than DAS 72. The AIC revealed that the variables had a differential importance to the model performance. SPAD values (ΔAIC = −211.9), dried biomass (ΔAIC = −199.0), and soil moisture (ΔAIC = −154.7) were the most influential variables able to predict color intensity, while progression of the season (ΔAIC = −2.3), climate treatment (ΔAIC = −17.2) and maximum height (ΔAIC = −54.3) were the least influential. Overall, the linear regression model had a very high accuracy in predicting leaf color intensity, with an adjusted R2 of 91.8%. See Supplementary Material for the linear model estimates (Supplementary Table 3).
Our aim was to investigate the phenotypic effect of long-term waterlogging on perennial ryegrass. We found that long-term waterlogging reduced perennial ryegrass productivity and changed the plant phenotype. Waterlogged plants were lighter in color than controls, with younger waterlogged plants expressing purple hues, suggestive of anthocyanins. We also found that waterlogged plants had significantly lower dried biomass and maximum height, demonstrating that waterlogging reduced overall plant performance and yield. Our study has experimentally determined the influence of waterlogging and climate change upon plant performance, with the effects of waterlogging largely overshadowing the effects of increased temperature and elevated CO2. The imaging methods could be developed as remote sensed diagnostics tools in combination with drone-technology to determine the influence of waterlogging on plant health in field environments.
Our results identified substantial variation in leaf coloration in perennial ryegrass caused by long-term waterlogging. This variation was mainly in color intensity, with waterlogging resulting in lighter color intensities. Previous studies have shown that waterlogging can reduce concentrations of plant pigments, mainly chlorophyll, resulting in lighter colors and reduced photosynthetic capability (e.g., Close and Davidson, 2003; Smethurst and Shabala, 2003; Pang et al., 2004; Li et al., 2011; Ou et al., 2011; Simova-Stoilova et al., 2012; Zhang et al., 2015; Barickman et al., 2019; Cotrozzi et al., 2021). Cotrozzi et al. (2021) found that waterlogging damaged the photosynthetic ability of durum wheat but that it depended on the duration, and that longer waterlogging was more detrimental. Li et al. (2011) found that waterlogging previous to anthesis could prevent photosynthetic damage in subsequent waterlogging events after anthesis, enhancing overall tolerance. Pang et al. (2004) found that barley varieties respond differently from waterlogging damage to photosystem II, and that recovery is at least partly genetically determined. We also observed significant variation in hue, with waterlogging resulting in red/blue (purple) shades. This was especially pronounced for the waterlogged plants in DAS 72, with large variation being observed between the plants, showing that varieties but also individual plants respond divergently to the same stimuli. These purple shades are likely caused by an accumulation of secondary anthocyanin metabolites, with multiple compounds having been identified in grasses (Clifford and Harborne, 1967; Fossen et al., 2002; Petrella et al., 2016). This suggests that individual plants respond differently to waterlogging by accumulating anthocyanins of varying degree. This is in agreement with previous research that has suggested that plants accumulate anthocyanins as a response to environmental stressors (Chalker-Scott, 1999), for example water availability as during drought (e.g., Close and Beadle, 2003; Kovinich et al., 2015; Li et al., 2018; Cirillo et al., 2021) and waterlogging (e.g., Close and Davidson, 2003; Smethurst and Shabala, 2003; Hussain et al., 2022). One of the main suggested benefits of increased anthocyanin accumulation is protection against DNA damaging UV-B radiation that can reduce photosynthetic capability (Teramura and Sullivan, 1994; Rozema et al., 1997; Hoch et al., 2001; Steyn et al., 2002). Anthocyanin accumulation has also been found as a response to phosphorus deficiency (e.g., Ulrychová and Sosnová, 1970; Shaikh et al., 2008; Sarker and Karmoker, 2011). This is a potential physiological cause of the observed purple shades in our waterlogged plants for two reasons. Firstly, phosphorus has been found to become soluble during excess water availability and migrate down the soil column (Sanyal and De Datta, 1991; Sinaj et al., 2002) and secondly, altered plant nutrient uptake during periods of excess water abundance might make the soil nutrients (e.g., phosphorus) temporarily unavailable (Elzenga and van Veen, 2010). These effects are expected to be especially pronounced for younger plants (Close and Beadle, 2003), as we indicated by our initial results, due to the undeveloped root system occupying only the upper part of the soil column. Additional root growth and reduction in soil moisture would allow the plants to reach the migrated phosphorus and start to recover via normalized nutrient levels, as observed with the reduction in purple hues from the waterlogging in DAS 101. Our results strengthen the consensus of recent studies that phenotypic color variations can effectively be quantified through image analysis and analyzed to detect physiological effects from waterlogging (e.g., de la Cruz Jiménez et al., 2017; Ventura et al., 2020), although detailed soil nutrient analysis would be needed to confirm our findings in connection to phosphorus availability. Future research is needed to validate the soil phosphorus availability in waterlogged scenarios in relation to anthocyanin accumulation in plant tissue and to what extent undeveloped root systems are influenced by this.
Our results showed reductions in dried biomass and height in response to long-term waterlogging for all perennial ryegrass varieties. These reductions in plant growth likely stems from lower photosynthetic capability caused by a physiological reduction in plant pigments as identified from the SPAD values and color analysis, but also from hindrance to root development. Complex interactions between soil moisture and root proliferation patterns are likely one of the main drivers governing growth performance via soil nutrient absorption (Brugge, 1985; Setter and Belford, 1990; Xu et al., 2013; Medlyn et al., 2016; Cougnon et al., 2017). Previous studies have linked waterlogging to reductions in grass performance caused by altered root development (Malik et al., 2002; Ploschuk et al., 2017). This agrees with our findings, as we observed a clear reduction in root proliferation in the waterlogged cores (visual inspection, results not shown), with long-term waterlogging having previously been shown to reduce root mass in ryegrass (McFarlane et al., 2003). Another potential consequence is reduced root respiration caused by a reduction in soil oxygen levels, which has been shown to have multiple negative feedbacks on grass biomass accumulation and nutrients absorption (Trought and Drew, 1980; Dunbabin et al., 1997; Fukao et al., 2019).
There is also variation in waterlogging tolerance between grass species (e.g., Rubio et al., 1995; Rubio and Lavado, 1999; Xiao and David, 2019) and wheat cultivars (e.g., Ghobadi et al., 2017; Cotrozzi et al., 2021) based on local adaptation and specific genotypes. Genotype specific tolerance to waterlogging has been found between perennial ryegrass varieties (Liu and Jiang, 2015; Byrne et al., 2017). This was expanded upon by Pearson et al. (2011) that found multiple quantitative trait loci (QTLs) that code for morphological traits influencing the tolerance to waterlogging in perennial ryegrass. This is one likely explanation for the differential performance to waterlogging amongst our varieties, with the tetraploid varieties generally tolerating waterlogging better than the diploid variety. However, our study only included one diploid variety, introducing a potential risk for bias in evaluating the contribution of ploidy to waterlogging resilience. We did observe substantial variation between the tetraploid varieties, suggesting that other aspects of the genotype could influence the response to the environmental stressors. It is possible that the difference in performance is due to genotypic root development, which has been observed previously between perennial ryegrass varieties (Bonos et al., 2004; Wedderburn et al., 2010; Deru et al., 2014). Although, there is no clear connection between ploidy and stress tolerance to waterlogging or other environmental stressors (Yu et al., 2012; Kemesyte et al., 2017; Tozer et al., 2017; Lee et al., 2019). Future research would benefit from including a wide range of varieties of varying genotypes grown under combinations of environmental stressors coupled with detailed genomics analyses. This could reveal the genetic basis of stress tolerance, and potential genetic trade-offs that occur between phenotypic traits.
We hypothesized that increases in temperature and CO2 (predicted 2050-levels) would enhance plant performance to climate change-induced waterlogging, but our results showed that perennial ryegrass responded with lighter leaf shades, suggesting reduced photosynthetic capability and reduced yields. This is in contrast to the theory that climate change will generally increase photosynthesis and productivity (Chen et al., 1996; Dusenge et al., 2019; Yiotis et al., 2021). Other studies have suggested that combined stressors brought by climate change could bring further negative effects than each stressor individually (Ahuja et al., 2010; Zandalinas et al., 2021). The developmental period of the grasses could be relevant here, with our study investigating the performance during the first few months including young plants. It is uncertain if these effects are specific to younger plants, or applicable to mature ones as well. Waterlogging has previously been shown to affect grain-crops differently depending on species and development, with wheat being able to sustain growth regardless of the period of waterlogging, while barley being disproportionately affected in later development (Ploschuk et al., 2020, 2021). Daepp et al. (2001) showed that the effects of elevated CO2 will depend on the developmental stage of the ryegrass, suggesting that some stages are more sensitive to than others. For grasses in general, elevated CO2 has been suggested to intensify the reproductive period from increases in NPP (Kurganskiy et al., 2021). Climate change will lead to multiple changes in the environment: increased temperature and CO2 may have positive effects by extending the growing season for certain species while simultaneously flooding or drought may have negative effects on overall plant growth (Richardson et al., 2013). To understand the effects of climate change we need to consider all environmental effects, their relative contribution to the overall impact and possible interactions to different developmental stages (Tubiello et al., 2007; Parmesan and Hanley, 2015; Gray and Brady, 2016; Zhou et al., 2020). In our case, the effect of waterlogging seems to largely overshadow the effects of increased temperature and elevated CO2.
To our knowledge this is the first study to investigate the combined effects of climate change (both temperature and CO2) and waterlogging experimentally in any plant. Previous studies have indicated that the interactive effects from elevated CO2 in isolation with waterlogging are inconclusive. Shimono et al. (2012) observed that soy bean (Glycine max) dry weight was significantly heavier during elevated CO2 (∼580–600 ppm), but did not see an overall interactive effect with waterlogging. Pérez-Jiménez et al. (2018) observed that the stress response of sweet cherry (Prunus avium) was significantly reduced after being waterlogged during elevated CO2 (800 ppm) compared to ambient levels, suggesting that elevated CO2 can reduce the waterlogging stress response. It is possible that the 200 ppm difference between these two studies is responsible for the difference in response, or that the study species inherently respond differently to the increase. One recent modeling-study has suggested that climate change will reduce waterlogging stress in barley (Liu et al., 2021), but not enough to compensate the reduction in yield (∼35% on average) due to high temperatures stress and that the development of resilient varieties to waterlogging will be required. However, increases in flooding due to climate change would cause more severe impacts than from current precipitation regimes (Mirza, 2011; Iglesias et al., 2012). The decrease in overall dried biomass for all varieties as a consequence of waterlogging predicted by our study has implications for global food security. A reduction in overall plant yield due to poor plant growth from flooding would potentially cause increases in fodder prices with follow-up consequences to all agricultural sectors and industries relying on fodder from pasture lands (Hazell and Wood, 2008; Kipling et al., 2016b; Manik et al., 2019). Early detection of waterlogging and identification of varieties resistant to waterlogging, will likely be important to employ mitigation strategies that could minimize reductions in plant health and production yield in pasture lands. Areas prone to current waterlogging are likely to experience increased frequency with climate change, with the utilization of appropriate drainage systems (natural or artificial) have previously been shown to be effective (Singh, 2017; Manik et al., 2019). The identification and breeding of plant lineages resilient to waterlogging damage is likely the most efficient (and cost-effective) approach of mitigating reductions in yield and performance and will be fundamental in a climate change future (Boru et al., 2001; Kole et al., 2015; Rivero et al., 2022).
We demonstrate that image analysis approaches can be used as diagnostic tools to investigate plant performance reductions caused by waterlogging. While most field monitoring would be time consuming and most satellite-based remote sensing products would be low resolution for this type of analysis other more navigable high-resolution options are available, for example drones (Bansod et al., 2017; Cracknell, 2018; Simic Milas et al., 2018). Drones have been shown to be a useful and cost-effective tool for agricultural surveying (Tripicchio et al., 2015; Puri et al., 2017; Kulbacki et al., 2018) and plant ecological investigation (Cruzan et al., 2016; Tay et al., 2018; Zellweger et al., 2019; Sun et al., 2021). The main benefits comes from the use of high-resolution multi- and hyperspectral cameras which capture a wide-range of light wavelengths used to infer plant physiological parameters (e.g., Li et al., 2020; Tao et al., 2020; Papp et al., 2021). Our results showed that differences in color intensity could be observed between perennial ryegrass varieties, suggesting that the imaging analysis method could be developed further to identify closely related varieties or perhaps different grass species using remote-sensed color distributions. Recent studies have also shown the usefulness of drones to monitor the effects of waterlogging on agricultural systems (e.g., Boiarskii et al., 2019; Den Besten et al., 2021; León-Rueda et al., 2021), illustrating that our imaging approaches could be adapted to work as diagnostic tools with drone-technology. Applications using integrated monitoring of plant health will become increasingly important as climate change-induced extreme weather events become more prevalent.
The data supporting the findings of this study is now publicly available in the general-purpose open-access repository Zenodo developed by the European OpenAIRE program and operated by CERN (doi: 10.5281/zenodo.6334191).
CF: design of the research, performance of the research, data analysis, collection or interpretation, and writing the manuscript. GX-S, MO, and YB: performance of the research and data analysis, collection, or interpretation. RM and JY: design of the research, data analysis, collection or interpretation, and writing the manuscript. All authors contributed to the article and approved the submitted version.
This project was funded under the EPA Research Programme 2014-2020 (Grant: 2018-CCRP-MS.52). The EPA Research Programme is a Government of Ireland initiative funded by the Department of the Environment, Climate and Communications. It is administered by the Environmental Protection Agency, which has the statutory function of coordinating and promoting environmental research.
Thanks goes out to Sónia Negrão for providing assistance with the equipment acquisition. GX-S, MO, and YB acknowledges funding from the Thomas Crawford Hayes Fund administered by the four NUI constituent Universities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.954478/full#supplementary-material
Aho, K., Derryberry, D., and Peterson, T. (2014). Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95, 631–636. doi: 10.1890/13-1452.1
Ahuja, I., de Vos, R. C. H., Bones, A. M., and Hall, R. D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15, 664–674. doi: 10.1016/j.tplants.2010.08.002
Baldos, U. L. C., and Hertel, T. W. (2014). Global food security in 2050: The role of agricultural productivity and climate change. Aust. J. Agric. Resour. Econ. 58, 554–570. doi: 10.1111/1467-8489.12048
Bansod, B., Singh, R., Thakur, R., and Singhal, G. (2017). A comparision between satellite based and drone based remote sensing technology to achieve sustainable development: a review. J. Agric. Environ. Int. Dev. 111, 383–407. doi: 10.12895/jaeid.20172.690
Barickman, T. C., Simpson, C. R., and Sams, C. E. (2019). Waterlogging Causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 160, 1–15. doi: 10.3390/plants8060160
Batke, S., Holohan, A., Hayden, R., Fricke, W., Porter, A. S., and Christiana, C. M. (2018). The pressure is on – Epiphyte water-relations altered under elevated CO2. Front. Plant Sci. 871:1758. doi: 10.3389/fpls.2018.01758
Bellocchi, G., and Picon-Cochard, C. (2021). Effects of climate change on grassland biodiversity and productivity. Agronomy 11:1047. doi: 10.3390/agronomy11061047
Blöschl, G., Hall, J., Viglione, A., Perdigão, R. A. P., Parajka, J., Merz, B., et al. (2019). Changing climate both increases and decreases European river floods. Nature 573, 108–111. doi: 10.1038/s41586-019-1495-6
Boiarskii, B., Hasegawa, H., Muratov, A., and Sudeykin, V. (2019). Application of UAV-derived digital elevation model in agricultural field to determine waterlogged soil areas in Amur region, Russia. Int. J. Eng. Adv. Technol. 8, 520–523.
Bonos, S. A., Rush, D., Hignight, K., and Meyer, W. A. (2004). Selection for deep root production in tall fescue and perennial ryegrass. Crop Sci. 44, 1770–1775. doi: 10.2135/cropsci2004.1770
Boru, G., Van Ginkel, M., Kronstad, W. E., and Boersma, L. (2001). Expression and inheritance of tolerance to waterlogging stress in wheat. Euphytica 117, 91–98. doi: 10.1023/A:1003929803920
Bothe, A., Westermeier, P., Wosnitza, A., Willner, E., Schum, A., Dehmer, K. J., et al. (2018). Drought tolerance in perennial ryegrass (Lolium perenne L.) as assessed by two contrasting phenotyping systems. J. Agron. Crop Sci. 204, 375–389. doi: 10.1111/jac.12269
Bozdogan, H. (1987). Model selection and akaike’s information criterion (AIC): the general theory and its analytical extensions. Psychometrika 52, 345–370. doi: 10.1007/BF02294361
Brown, J. N., Ash, A., MacLeod, N., and McIntosh, P. (2019). Diagnosing the weather and climate features that influence pasture growth in Northern Australia. Clim. Risk Manag. 24, 1–12. doi: 10.1016/j.crm.2019.01.003
Brugge, R. (1985). A mechanistic model of grass root growth and development dependent upon photosynthesis and nitrogen uptake. J. Theor. Biol. 116, 443–467. doi: 10.1016/S0022-5193(85)80281-2
Buttler, A., Mariotte, P., Meisser, M., Guillaume, T., Signarbieux, C., Vitra, A., et al. (2019). Drought-induced decline of productivity in the dominant grassland species Lolium perenne L. depends on soil type and prevailing climatic conditions. Soil Biol. Biochem. 132, 47–57. doi: 10.1016/j.soilbio.2019.01.026
Byrne, N., Gilliland, T. J., Mchugh, N., Delaby, L., Geoghegan, A., and O’donovan, M. (2017). Establishing phenotypic performance of grass varieties on Irish grassland farms. J. Agric. Sci. 155, 1633–1645. doi: 10.1017/S0021859617000740
Canadell, J. G., and Raupach, M. R. (2008). Managing forests for climate change mitigation. Science 320, 1456–1457. doi: 10.1126/science.1155458
Chalker-Scott, L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70, 1–9. doi: 10.1111/j.1751-1097.1999.tb01944.x
Chen, D. X., Hunt, H. W., and Morgan, J. A. (1996). Responses of a C3 and C4 perennial grass to CO2 enrichment and climate change: comparison between model predictions and experimental data. Ecol. Modell. 87, 11–27. doi: 10.1016/0304-3800(94)00199-5
Chen, Z. (1998). A note on bias robustness of the median. Stat. Probab. Lett. 38, 363–368. doi: 10.1016/S0167-7152(98)00049-2
Cirillo, V., D’Amelia, V., Esposito, M., Amitrano, C., Carillo, P., Carputo, D., et al. (2021). Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology (Basel). 10, 1–15. doi: 10.3390/biology10020139
Clifford, H. T., and Harborne, J. B. (1967). Anthocyanin composition of and distribution in the Poaceae (Gramineae). Proc. Linn. Soc. London 178, 125–127. doi: 10.1111/j.1095-8312.1967.tb00968.x
Close, D. C., and Beadle, C. L. (2003). The ecophysiology of foliar anthocyanin. Bot. Rev. 69, 149–161. doi: 10.1016/j.jplph.2020.153161
Close, D. C., and Davidson, N. J. (2003). Long-term waterlogging: Nutrient, gas exchange, photochemical and pigment characteristics of Eucalyptus nitens saplings. Russ. J. Plant Physiol. 50, 843–847. doi: 10.1023/B:RUPP.0000003284.25827.95
Cotrozzi, L., Lorenzini, G., Nali, C., Pisuttu, C., Pampana, S., and Pellegrini, E. (2021). Transient waterlogging events impair shoot and root physiology and reduce grain yield of durum wheat cultivars. Plants 10:2357. doi: 10.3390/plants10112357
Cougnon, M., De Swaef, T., Lootens, P., Baert, J., De Frenne, P., Shahidi, R., et al. (2017). In situ quantification of forage grass root biomass, distribution and diameter classes under two N fertilisation rates. Plant Soil 411, 409–422. doi: 10.1007/s11104-016-3034-7
Cracknell, A. P. (2018). The development of remote sensing in the last 40 years. Int. J. Remote Sens. 39, 8387–8427. doi: 10.1080/01431161.2018.1550919
Croux, C., and Dehon, C. (2010). Influence functions of the spearman and kendall correlation measures. Stat. Methods Appl. 19, 497–515. doi: 10.1007/s10260-010-0142-z
Cruzan, M. B., Weinstein, B. G., Grasty, M. R., Kohrn, B. F., Hendrickson, E. C., Arredondo, T. M., et al. (2016). Small unmanned aerial vehicles (Micro-Uavs, Drones) in plant ecology. Appl. Plant Sci. 4, 1–11. doi: 10.3732/apps.1600041
Cullen, B. R., Johnson, I. R., Eckard, R. J., Lodge, G. M., Walker, R. G., Rawnsley, R. P., et al. (2009). Climate change effects on pasture systems in south-eastern Australia. Crop Pasture Sci. 60, 933–942. doi: 10.1071/CP09019
Cullen, B. R., Rawnsley, R. P., Eckard, R. J., Christie, K. M., and Bell, M. J. (2014). Use of modelling to identify perennial ryegrass plant traits for future warmer and drier climates. Crop Pasture Sci. 65, 758–766. doi: 10.1071/CP13408
Daepp, M., Nösberger, J., and Lüscher, A. (2001). Nitrogen fertilization and developmental stage alter the response of Lolium perenne to elevated CO2. New Phytol. 150, 347–358. doi: 10.1046/j.1469-8137.2001.00109.x
de la Cruz Jiménez, J., Cardoso, J. A., Leiva, L. F., Gil, J., Forero, M. G., Worthington, M. L., et al. (2017). Non-destructive phenotyping to identify brachiaria hybrids tolerant to waterlogging stress under field conditions. Front. Plant Sci. 8:167. doi: 10.3389/fpls.2017.00167
Den Besten, N., Steele-Dunne, S., de Jeu, R., and van der Zaag, P. (2021). Towards monitoring waterlogging with remote sensing for sustainable irrigated agriculture. Remote Sens. 13, 1–20. doi: 10.3390/rs13152929
Deru, J., Schilder, H., van der Schoot, J. R., and van Eekeren, N. (2014). Genetic differences in root mass of Lolium perenne varieties under field conditions. Euphytica 199, 223–232. doi: 10.1007/s10681-014-1129-x
Dong, T., Shang, J., Chen, J. M., Liu, J., Quian, B., Ma, B., et al. (2019). Assessment of portable chlorophyll meters for measuring crop leaf chlorophyll concentrations. Remote Sens. 11:2706. doi: 10.3390/rs11222706
Dore, M. H. I. (2005). Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 31, 1167–1181. doi: 10.1016/j.envint.2005.03.004
Dunbabin, J. S., Hume, I. H., and Ireson, M. E. (1997). Effects of irrigation frequency and transient waterlogging on the production of a perennial ryegrass-white clover pasture. Aust. J. Exp. Agric. 37, 165–171. doi: 10.1071/EA96057
Dusenge, M. E., Duarte, A. G., and Way, D. A. (2019). Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 221, 32–49. doi: 10.1111/nph.15283
Easterling, D. R., Meehl, G. A., Parmesan, C., Changnon, S. A., Karl, T. R., and Mearns, L. O. (2000). Climate extremes: observations. Model Impact. Sci. 289, 2068–2074. doi: 10.1126/science.289.5487.2068
Elzenga, J. T. M., and van Veen, H. (2010). “Waterlogging and plant nutrient uptake,” in Waterlogging Signalling and Tolerance in Plants, eds S. Mancuso and S. Shabala (Berlin: Springer), 23–35. doi: 10.1007/978-3-642-10305-6_2
Ergon, A., Seddaiu, G., Korhonen, P., Virkajärvi, P., Bellocchi, G., Jørgensen, M., et al. (2018). How can forage production in nordic and mediterranean europe adapt to the challenges and opportunities arising from climate change? Eur. J. Agron. 92, 97–106. doi: 10.1016/j.eja.2017.09.016
European Commision (2019). Common catalogue of varieties of agricultural plant species (Document ID: C2019/013/01). Off. J. Eur. Union 37, 1–812.
Evans-Fitz.Gerald, C., Porter, A. S., Yiotis, C., Elliott-Kingston, C., and McElwain, J. C. (2016). Co-ordination in morphological leaf traits of early diverging angiosperms is maintained following exposure to experimental palaeo-atmospheric conditions of sub-ambient O2 and elevated CO2. Front. Plant Sci. 7:1368. doi: 10.3389/fpls.2016.01368
Farfan-Vignolo, E. R., and Asard, H. (2012). Effect of elevated CO2 and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 59, 55–62. doi: 10.1016/j.plaphy.2012.06.014
Fay, P. A., Kaufman, D. M., Nippert, J. B., Carlisle, J. D., and Harper, C. W. (2008). Changes in grassland ecosystem function due to extreme rainfall events: Implications for responses to climate change. Glob. Chang. Biol. 14, 1600–1608. doi: 10.1111/j.1365-2486.2008.01605.x
Feng, X., Porporato, A., and Rodriguez-Iturbe, I. (2013). Changes in rainfall seasonality in the tropics. Nat. Clim. Chang. 3, 811–815. doi: 10.1038/nclimate1907
Fossen, T., Slimestad, R., Øvstedal, D. O., and Andersen, ØM. (2002). Anthocyanins of grasses. biochem. Syst. Ecol. 30, 855–864. doi: 10.1016/S0305-1978(02)00028-5
Fukao, T., Barrera-Figueroa, B. E., Juntawong, P., and Peña-Castro, J. M. (2019). Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front. Plant Sci. 10:340. doi: 10.3389/fpls.2019.00340
Ghobadi, M. E., Ghobadi, M., and Zebarjadi, A. (2017). Effect of waterlogging at different growth stages on some morphological traits of wheat varieties. Int. J. Biometeorol. 61, 635–645. doi: 10.1007/s00484-016-1240-x
Goliński, P., Czerwiński, M., Jørgensen, M., Mølmann, J. A. B., Golińska, B., and Taff, G. (2018). Relationship between climate trends and grassland yield across contrasting European locations. Open Life Sci. 13, 589–598. doi: 10.1515/biol-2018-0070
Golzarian, M. R., and Frick, R. A. (2011). Classification of images of wheat, ryegrass and brome grass species at early growth stages using principal component analysis. Plant Methods 7:28. doi: 10.1186/1746-4811-7-28
Gray, S. B., and Brady, S. M. (2016). Plant developmental responses to climate change. Dev. Biol. 419, 64–77. doi: 10.1016/j.ydbio.2016.07.023
Grogan, D., and Gilliland, T. J. (2011). A review of perennial ryegrass variety evaluation in Ireland. Irish J. Agric. Food Res. 50, 65–81.
Hannaway, D., Fransen, S., Cropper, J., Teel, M., Chaney, M., Griggs, T., et al. (1999). Perennial Ryegrass (Lolium perenne L.). A Pacific Northwest Ext. Publ. Corvallis: Oregon State University, 1–20.
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 2018, 1–32. doi: 10.7717/peerj.4794
Hazell, P., and Wood, S. (2008). Drivers of change in global agriculture. Philos. Trans. R. Soc. B Biol. Sci. 363, 495–515. doi: 10.1098/rstb.2007.2166
Helgadóttir, A., Aavola, R., Isolahti, M., Marum, P., Persson, C., Aleliūnas, A., et al. (2018). Adaptability and phenotypic stability of Lolium perenne L. cultivars of diverse origin grown at the margin of the species distribution. J. Agron. Crop Sci. 204, 493–504. doi: 10.1111/jac.12273
Hoch, W. A., Zeldin, E. L., and McCown, B. H. (2001). Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol. 21, 1–8. doi: 10.1093/treephys/21.1.1
Höglind, M., Thorsen, S. M., and Semenov, M. A. (2013). Assessing uncertainties in impact of climate change on grass production in Northern Europe using ensembles of global climate models. Agric. For. Meteorol. 170, 103–113. doi: 10.1016/j.agrformet.2012.02.010
Hu, H., Oglesby, R. J., and Saltzman, B. (2000). The relationship between atmospheric water vapor and temperature in simulations of climate change. Geophys. Res. Lett. 27, 3513–3516. doi: 10.1029/2000GL011680
Hu, H., Zhang, J., Sun, X., and Zhang, X. (2013). Estimation of leaf chlorophyll content of rice using image color analysis. Can. J. Remote Sens. 39, 185–190. doi: 10.5589/m13-026
Hunt, W. F., and Easton, H. S. (1989). Fifty years of ryegrass research in New Zealand. Proc. N Z. Grassl. Assoc. 23, 1–23. doi: 10.33584/jnzg.1989.50.1876
Huntingford, C., Zelazowski, P., Galbraith, D., Mercado, L. M., Sitch, S., Fisher, R., et al. (2013). Simulated resilience of tropical rainforests to CO2 -induced climate change. Nat. Geosci. 6, 268–273. doi: 10.1038/ngeo1741
Hussain, S., Mehmood, U., Ashraf, U., and Naseer, M. A. (2022). “Combined salinity and waterlogging stress in plants: limitations and tolerance mechanisms,” in Climate Change and Crop Stress (INC), eds A. K. Shanker, C. Shanker, A. Anand, and M. Maheswari (Cambridge, MA: Academic Press), 95–112. doi: 10.1016/b978-0-12-816091-6.00017-1
Iglesias, A., Quiroga, S., Moneo, M., and Garrote, L. (2012). From climate change impacts to the development of adaptation strategies: challenges for agriculture in Europe. Clim. Change 112, 143–168. doi: 10.1007/s10584-011-0344-x
IPCC (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC.
Jentsch, A., Kreyling, J., Boettcher-Treschkow, J., and Beierkuhnlein, C. (2009). Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Glob. Chang. Biol. 15, 837–849. doi: 10.1111/j.1365-2486.2008.01690.x
Jollife, I. T., and Cadima, J. (2016). Principal component analysis: a review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 374, 20150202. doi: 10.1098/rsta.2015.0202
Kemesyte, V., Statkeviciute, G., and Brazauskas, G. (2017). Perennial ryegrass yield performance under abiotic stress. Crop Sci. 57, 1935–1940. doi: 10.2135/cropsci2016.10.0864
Kiely, G. (1999). Climate change in Ireland from precipitation and streamflow observations. Adv. Water Resour. 23, 141–151. doi: 10.1016/S0309-1708(99)00018-4
Kipling, R. P., Bannink, A., Bellocchi, G., Dalgaard, T., Fox, N. J., Hutchings, N. J., et al. (2016a). Modeling European ruminant production systems: facing the challenges of climate change. Agric. Syst. 147, 24–37. doi: 10.1016/j.agsy.2016.05.007
Kipling, R. P., Virkajärvi, P., Breitsameter, L., Curnel, Y., De Swaef, T., Gustavsson, A. M., et al. (2016b). Key challenges and priorities for modelling European grasslands under climate change. Sci. Total Environ. 566–567, 851–864. doi: 10.1016/j.scitotenv.2016.05.144
Kole, C., Muthamilarasan, M., Henry, R., Edwards, D., Sharma, R., Abberton, M., et al. (2015). Application of genomics-assisted breeding for generation of climate resilient crops: progress and prospects. Front. Plant Sci. 6:563. doi: 10.3389/fpls.2015.00563
Kovinich, N., Kayanja, G., Chanoca, A., Otegui, M. S., and Grotewold, E. (2015). Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 10:e1027850. doi: 10.1080/15592324.2015.1027850
Kulbacki, M., Segen, J., Knieć, W., Klempous, R., Kluwak, K., Nikodem, J., et al. (2018). “Survey of drones for agriculture automation from planting to harvest,” in Proceedings of the 2018 IEEE 22nd International Conference on Intelligent Engineering Systems (INES), (Las Palmas de Gran Canaria), 21–23. doi: 10.1109/INES.2018.8523943
Kumar, P. (2013). Hydrology: seasonal rain changes. Nat. Clim. Chang. 3, 783–784. doi: 10.1038/nclimate1996
Kurganskiy, A., Creer, S., Vere, N., De Griffith, G. W., Osborne, N. J., et al. (2021). Predicting the severity of the grass pollen season and the effect of climate change in Northwest Europe. Sci. Adv. 7:eabd7658. doi: 10.1126/sciadv.abd7658
Langsrud, Ø (2003). ANOVA for unbalanced data: use Type II instead. Stat. Comput. 13, 163–167. doi: 10.1023/A:1023260610025
Lecerf, R., Ceglar, A., López-Lozano, R., Van Der Velde, M., and Baruth, B. (2019). Assessing the information in crop model and meteorological indicators to forecast crop yield over Europe. Agric. Syst. 168, 191–202. doi: 10.1016/j.agsy.2018.03.002
Lee, M. A., Howard-Andrews, V., and Chester, M. (2019). Resistance of multiple diploid and tetraploid perennial ryegrass (Lolium perenne L.) varieties to three projected drought scenarios for the UK in 2080. Agronomy 9:159. doi: 10.3390/agronomy9030159
León-Rueda, W. A., León, C., Caro, S. G., and Ramírez-Gil, J. G. (2021). Identification of diseases and physiological disorders in potato via multispectral drone imagery using machine learning tools. Trop. Plant Pathol. 47, 152–167. doi: 10.1007/s40858-021-00460-2
Lever, J., Krzywinski, M., and Altman, N. (2017). Points of significance: principal component analysis. Nat. Methods 14, 641–642. doi: 10.1038/nmeth.4346
Li, B., Xu, X., Zhang, L., Han, J., Bian, C., Li, G., et al. (2020). Above-ground biomass estimation and yield prediction in potato by using UAV-based RGB and hyperspectral imaging. ISPRS J. Photogramm. Remote Sens. 162, 161–172. doi: 10.1016/j.isprsjprs.2020.02.013
Li, C., Jiang, D., Wollenweber, B., Li, Y., Dai, T., and Cao, W. (2011). Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 180, 672–678. doi: 10.1016/j.plantsci.2011.01.009
Li, L., Zhang, Q., and Huang, D. (2014). A review of imaging techniques for plant phenotyping. Sensors (Switzerland) 14, 20078–20111. doi: 10.3390/s141120078
Li, X., Lv, X., Wang, X., Wang, L., Zhang, M., and Ren, M. (2018). Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 69, 1208–1214. doi: 10.1071/CP18341
Liu, K., Harrison, M. T., Archontoulis, S. V., Huth, N., Yang, R., Liu, D. L., et al. (2021). Climate change shifts forward flowering and reduces crop waterlogging stress. Environ. Res. Lett. 16:9. doi: 10.1088/1748-9326/ac1b5a
Liu, M., and Jiang, Y. (2015). Genotypic variation in growth and metabolic responses of perennial ryegrass exposed to short-term waterlogging and submergence stress. Plant Physiol. Biochem. 95, 57–64. doi: 10.1016/j.plaphy.2015.07.008
Løkke, M. M., Seefeldt, H. F., Skov, T., and Edelenbos, M. (2013). Color and textural quality of packaged wild rocket measured by multispectral imaging. Postharvest Biol. Technol. 75, 86–95. doi: 10.1016/j.postharvbio.2012.06.018
Malik, A. I., Colmer, T. D., Lambers, H., Setter, T. L., and Schortemeyer, M. (2002). Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 153, 225–236. doi: 10.1046/j.0028-646X.2001.00318.x
Malvar, H. S., He, L. W., and Cutler, R. (2004). High-quality linear interpolation for demosaicing of Bayer-patterned color images. ICASSP, IEEE Int. Conf. Acoust. Speech Signal Proc. 3, 2–5. doi: 10.1109/icassp.2004.1326587
Manik, S. M. N., Pengilley, G., Dean, G., Field, B., Shabala, S., and Zhou, M. (2019). Soil and crop management practices to minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 10:140. doi: 10.3389/fpls.2019.00140
Mathworks (2021). MATLAB R2021a. The Language for Technical Computing. Version 9.10.0.1710957. Natick, MA: The MathWorks Inc.
McFarlane, N. M., Ciavarella, T. A., and Smith, K. F. (2003). The effects of waterlogging on growth, photosynthesis and biomass allocation in perennial ryegrass (Lolium perenne L.) genotypes with contrasting root development. J. Agric. Sci. 141, 241–248. doi: 10.1017/S0021859603003502
McHugh, O., Liu, J., Browne, F., Jordan, P., and McConnell, D. (2020). “Data-driven classifiers for predicting grass growth in northern ireland: a case study,” in Information Processing and Management of Uncertainty in Knowledge-Based Systems Communications in Computer and Information Science, 1237, (Cham: Springer International), 301–312. doi: 10.1007/978-3-030-50146-4_23
Medlyn, B. E., De Kauwe, M. G., and Duursma, R. A. (2016). New developments in the effort to model ecosystems under water stress. New Phytol. 212, 5–7. doi: 10.1111/nph.14082
Minneé, E. M. K., Kuhn-Sherlock, B., Pinxterhuis, I. J. B., and Chapman, D. F. (2019). Meta-analyses comparing the nutritional composition of perennial ryegrass (Lolium perenne) and plantain (Plantago lanceolata) pastures. J. N Z. Grasslands 81, 117–124. doi: 10.33584/jnzg.2019.81.402
Mirza, M. M. Q. (2011). Climate change, flooding in South Asia and implications. Reg. Environ. Chang. 11, 95–107. doi: 10.1007/s10113-010-0184-7
Mizutani, M., and Kanaoka, M. M. (2018). Environmental sensing and morphological plasticity in plants. Semin. Cell Dev. Biol. 83, 69–77. doi: 10.1016/j.semcdb.2017.10.029
Munson, S. M., and Long, A. L. (2017). Climate drives shifts in grass reproductive phenology across the western USA. New Phytol. 213, 1945–1955. doi: 10.1111/nph.14327
Münzbergová, Z., Hadincová, V., Skálová, H., and Vandvik, V. (2017). Genetic differentiation and plasticity interact along temperature and precipitation gradients to determine plant performance under climate change. J. Ecol. 105, 1358–1373. doi: 10.1111/1365-2745.12762
Mustroph, A. (2018). Improving flooding tolerance of crop plants. Agronomy 8, 1–25. doi: 10.3390/agronomy8090160
O’Gorman, P. A., and Muller, C. J. (2010). How closely do changes in surface and column water vapor follow clausius-clapeyron scaling in climate change simulations? Environ. Res. Lett. 5, 1–7. doi: 10.1088/1748-9326/5/2/025207
Oksanen, J., Guillaume Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). Vegan: Community Ecology Package. Available online at: https://cran.r-project.org/package=vegan.
Ou, L. J., Dai, X. Z., Zhang, Z. Q., and Zou, X. X. (2011). Responses of pepper to waterlogging stress. Photosynthetica 49, 339–345. doi: 10.1007/s11099-011-0043-x
Pang, J., Zhou, M., Mendham, N., and Shabala, S. (2004). Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust. J. Agric. Res. 55, 895–906. doi: 10.1071/AR03097
Papp, L., van Leeuwen, B., Szilassi, P., Tobak, Z., Szatmári, J., Árvai, M., et al. (2021). Monitoring invasive plant species using hyperspectral remote sensing data. Land 10:29. doi: 10.3390/land10010029
Pareek, A., Dhankher, O. P., and Foyer, C. H. (2020). Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 71, 451–456. doi: 10.1093/jxb/erz518
Parmesan, C., and Hanley, M. E. (2015). Plants and climate change: complexities and surprises. Ann. Bot. 116, 849–864. doi: 10.1093/aob/mcv169
Pearson, A., Cogan, N. O. I., Baillie, R. C., Hand, M. L., Bandaranayake, C. K., Erb, S., et al. (2011). Identification of QTLs for morphological traits influencing waterlogging tolerance in perennial ryegrass (Lolium perenne L.). Theor. Appl. Genet. 122, 609–622. doi: 10.1007/s00122-010-1473-8
Pembleton, K. G., Cullen, B. R., Rawnsley, R. P., and Ramilan, T. (2020). Climate change effects on pasture-based dairy systems in south-eastern Australia. Crop Pasture Sci. 72, 666–677. doi: 10.1071/CP20108
Pérez-Jiménez, M., Hernández-Munuera, M., Piñero, M. C., López-Ortega, G., and del Amor, F. M. (2018). Are commercial sweet cherry rootstocks adapted to climate change? Short-term waterlogging and CO2 effects on sweet cherry cv. ‘Burlat.’. Plant Cell Environ. 41, 908–918. doi: 10.1111/pce.12920
Petrella, D. P., Metzger, J. D., Blakeslee, J. J., Nangle, E. J., and Gardner, D. S. (2016). Anthocyanin production using rough bluegrass treated with high-intensity light. HortScience 51, 1111–1120. doi: 10.21273/HORTSCI10878-16
Piepho, H. P., Büchse, A., and Emrich, K. (2003). A Hitchhiker’s guide to mixed models for randomized experiments. J. Agron. Crop Sci. 189, 310–322. doi: 10.1046/j.1439-037X.2003.00049.x
Ploschuk, R. A., Grimoldi, A. A., Ploschuk, E. L., and Striker, G. G. (2017). Growth during recovery evidences the waterlogging tolerance of forage grasses. Crop Pasture Sci. 68, 574–582. doi: 10.1071/CP17137
Ploschuk, R. A., Miralles, D. J., Colmer, T. D., and Striker, G. G. (2020). Waterlogging differentially affects yield and its components in wheat, barley, rapeseed and field pea depending on the timing of occurrence. J. Agron. Crop Sci. 206, 363–375. doi: 10.1111/jac.12396
Ploschuk, R. A., Miralles, D. J., and Striker, G. G. (2021). Early- And late-waterlogging differentially affect the yield of wheat, barley, oilseed rape and field pea through changes in leaf area index, radiation interception and radiation use efficiency. J. Agron. Crop Sci. 207, 504–520. doi: 10.1111/jac.12486
Porter, A. S., Yiotis, C., Montañez, I. P., and McElwain, J. C. (2017). Evolutionary differences in Δ13C detected between spore and seed bearing plants following exposure to a range of atmospheric O2:CO2 ratios; implications for paleoatmosphere reconstruction. Geochim. Cosmochim. Acta 213, 517–533. doi: 10.1016/j.gca.2017.07.007
Puri, V., Nayyar, A., and Raja, L. (2017). Agriculture drones: a modern breakthrough in precision agriculture. J. Stat. Manag. Syst. 20, 507–518. doi: 10.1080/09720510.2017.1395171
Qi, A., Holland, R. A., Taylor, G., and Richter, G. M. (2018). Grassland futures in Great Britain – Productivity assessment and scenarios for land use change opportunities. Sci. Total Environ. 634, 1108–1118. doi: 10.1016/j.scitotenv.2018.03.395
Raza, A., Razzaq, A., Mehmood, S. S., Zou, X., Zhang, X., Lv, Y., et al. (2019). Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 8:34. doi: 10.3390/plants8020034
Richardson, A. D., Keenan, T. F., Migliavacca, M., Ryu, Y., Sonnentag, O., and Toomey, M. (2013). Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 169, 156–173. doi: 10.1016/j.agrformet.2012.09.012
Rivero, R. M., Mittler, R., Blumwald, E., and Zandalinas, S. I. (2022). Developing climate-resilient crops: improving plant tolerance to stress combination. Plant J. 109, 373–389. doi: 10.1111/tpj.15483
Rosenzweig, C., Tubiello, F. N., Goldberg, R., Mills, E., and Bloomfield, J. (2002). Increased crop damage in the US from excess precipitation under climate change. Glob. Environ. Chang. 12, 197–202. doi: 10.1016/S0959-3780(02)00008-0
Rozema, J., Van De Staaij, J., Björn, L. O., and Caldwell, M. (1997). UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol. Evol. 12, 22–28. doi: 10.1016/S0169-5347(96)10062-8
Rubio, G., Casasola, G., and Lavado, R. S. (1995). Adaptations and biomass production of two grasses in response to waterlogging and soil nutrient enrichment. Oecologia 102, 102–105. doi: 10.1007/BF00333316
Rubio, G., and Lavado, R. S. (1999). Acquisition and allocation of resources in two waterlogging-tolerant grasses. New Phytol. 143, 539–546. doi: 10.1046/j.1469-8137.1999.00482.x
Sanyal, S. K., and De Datta, S. K. (1991). “Chemistry of phosphorus transformations in soil,” in Advances in Soil Science. Advances in Soil Science, 16, ed. B. A. Stewart (New York, NY: Springer), 1–120. doi: 10.1007/978-1-4612-3144-8_1
Sarker, B. C., and Karmoker, J. L. (2011). Effects of phosphorus deficiency on accumulation of biochemical compounds in lentil (Lens culinaris Medik). Bangladesh J. Bot. 40, 23–27. doi: 10.3329/bjb.v40i1.7992
Semmler, T., and Jacob, D. (2004). Modeling extreme precipitation events - a climate change simulation for Europe. Glob. Planet. Change 44, 119–127. doi: 10.1016/j.gloplacha.2004.06.008
Setter, T., and Belford, B. (1990). Waterlogging: how it reduces plant growth and how plants can overcome its effects. J. Dep. Agric. West. Aust. Ser. 4, 51–55.
Shaikh, N. P., Adjei, M. B., and Scholberg, J. M. (2008). Interactive effect of phosphorus and nitrogen on leaf anthocyanins, tissue nutrient concentrations, and dry-matter yield of Floralta limpograss during short day length. Commun. Soil Sci. Plant Anal. 39, 1006–1015. doi: 10.1080/00103620801925414
Shapiro, S. S., and Wilk, M. B. (1965). An Analysis of variance test for normality (Complete Samples). Biometrika 52, 591–611. doi: 10.2307/2333709
Shimono, H., Konno, T., Sakai, H., and Sameshima, R. (2012). Interactive effects of elevated atmospheric CO 2 and waterlogging on vegetative growth of soybean (Glycine max (L.) Merr.). Plant Prod. Sci. 15, 238–245. doi: 10.1626/pps.15.238
Simic Milas, A., Cracknell, A. P., and Warner, T. A. (2018). Drones–the third generation source of remote sensing data. Int. J. Remote Sens. 39, 7125–7137. doi: 10.1080/01431161.2018.1523832
Simova-Stoilova, L., Demirevska, K., Kingston-Smith, A., and Feller, U. (2012). Involvement of the leaf antioxidant system in the response to soil flooding in two Trifolium genotypes differing in their tolerance to waterlogging. Plant Sci. 183, 43–49. doi: 10.1016/j.plantsci.2011.11.006
Sinaj, S., Stamm, C., Toor, G. S., Condron, L. M., Hendry, T., Di, H. J., et al. (2002). Phosphorus exchangeability and leaching losses from two grassland soils. J. Environ. Qual. 31, 319–330. doi: 10.2134/jeq2002.0319
Singh, A. (2017). Waterlogging and salinity management for sustainable irrigated agriculture. ii: engineering measures and biodrainage. J. Irrig. Drain. Eng. 143:04017036. doi: 10.1061/(asce)ir.1943-4774.0001227
Smethurst, C. F., and Shabala, S. (2003). Screening methods for waterlogging tolerance in lucerne: Comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 30, 335–343. doi: 10.1071/FP02192
Smit, H. J., Tas, B. M., Taweel, H. Z., Tamminga, S., and Elgersma, A. (2005). Effects of perennial ryegrass (Lolium perenne L.) cultivars on herbage production, nutritional quality and herbage intake of grazing dairy cows. Grass Forage Sci. 60, 297–309. doi: 10.1111/j.1365-2494.2005.00480.x
Smith, C. E., and Cribbie, R. (2014). Factorial ANOVA with unbalanced data: a fresh look at the types of sums of squares. J. Data Sci. 12, 385–404. doi: 10.6339/jds.201407_12(3).0001
Song, J. (2009). Root morphology is related to the phenotypic variation in waterlogging tolerance of two populations of Suaeda salsa under salinity. Plant Soil 324, 231–240. doi: 10.1007/s11104-009-9949-5
Spearman, C. (1904). The proof and measurement of association between two things. Am. J. Psychol. 15, 72–101.
Stasnik, P., Großkinsky, D. K., and Jonak, C. (2022). Physiological and phenotypic characterization of diverse Camelina sativa lines in response to waterlogging. Plant Physiol. Biochem. 183, 120–127. doi: 10.1016/j.plaphy.2022.05.007
Steyn, W. J., Wand, S. J. E., Holcroft, D. M., and Jacobs, G. (2002). Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 155, 349–361. doi: 10.1046/j.1469-8137.2002.00482.x
Striker, G. G. (2012). “Flooding stress on plants: anatomical, morphological and physiological responses,” in Botany, ed. J. K. Mworia (Rijeka: InTechOpen), 3–28.
Sun, Z., Wang, X., Wang, Z., Yang, L., Xie, Y., and Huang, Y. (2021). UAVs as remote sensing platforms in plant ecology: Review of applications and challenges. J. Plant Ecol. 14, 1003–1023. doi: 10.1093/jpe/rtab089
Tao, H., Feng, H., Xu, L., Miao, M., Yang, G., Yang, X., et al. (2020). Estimation of the yield and plant height of winter wheat using UAV-based hyperspectral images. Sensors (Switzerland) 20, 1–19. doi: 10.3390/s20041231
Tay, J. Y. L., Erfmeier, A., and Kalwij, J. M. (2018). Reaching new heights: can drones replace current methods to study plant population dynamics? Plant Ecol. 219, 1139–1150. doi: 10.1007/s11258-018-0865-8
R Core Team (2022). A Language and Environment for Statistical Computing. Version 4.1.3. Vienna: R Foundation for Statistical Computing.
Teramura, A. H., and Sullivan, J. H. (1994). Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 39, 463–473. doi: 10.1007/BF00014599
Tester, M., and Langridge, P. (2010). Breeding technologies to increase crop production in a changing world. Science. 327, 818–822. doi: 10.1126/science.1183700
Thornley, J. H. M., and Cannell, M. G. R. (1997). Temperate grassland responses to climate change: an analysis using the hurley pasture model. Ann. Bot. 80, 205–221. doi: 10.1006/anbo.1997.0430
Tong, C., Hill, C. B., Zhou, G., Zhang, X. Q., Jia, Y., and Li, C. (2021). Opportunities for improving waterlogging tolerance in cereal crops—physiological traits and genetic mechanisms. Plants 10, 1–22. doi: 10.3390/plants10081560
Tozer, K. N., Carswell, K., Griffiths, W. M., Crush, J. R., Cameron, C. A., Chapman, D. F., et al. (2017). Growth responses of diploid and tetraploid perennial ryegrass (Lolium perenne) to soil-moisture deficit, defoliation and a root-feeding invertebrate. Crop Pasture Sci. 68, 632–642. doi: 10.1071/CP17154
Trenberth, K. E. (2011). Changes in precipitation with climate change. Clim. Res. 47, 123–138. doi: 10.3354/cr00953
Tripicchio, P., Satler, M., Dabisias, G., Ruffaldi, E., and Avizzano, C. A. (2015). “Towards smart farming and sustainable agriculture with drones,” in Proceedings of the 2015 International Conference on Intelligent Environments, 2015, (Prague), 140–143. doi: 10.1109/IE.2015.29
Trought, M. C. T., and Drew, M. C. (1980). The development of waterlogging damage in young wheat plants in anaerobic solution cultures. J. Exp. Bot. 31, 1573–1585. doi: 10.1093/jxb/31.6.1573
Tubiello, F. N., Soussana, J. F., and Howden, S. M. (2007). Crop and pasture response to climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19686–19690. doi: 10.1073/pnas.0701728104
Tubritt, T., Delaby, L., Gilliland, T. J., and O’Donovan, M. (2020). The relationship between the grazing efficiency and the production, morphology and nutritional traits of perennial ryegrass varieties. J. Agric. Sci. 158, 583–593. doi: 10.1017/S0021859620000982
Ulrychová, M., and Sosnová, V. (1970). Effect of phosphorus deficiency on anthocyanin content in tomato plants. Biol. Plant. 12, 231–235. doi: 10.1007/bf02920805
Ventura, I., Brunello, L., Iacopino, S., Valeri, M. C., Novi, G., Dornbusch, T., et al. (2020). Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Sci. Rep. 10, 16669. doi: 10.1038/s41598-020-73704-x
von Hippel, P. T. (2005). Mean, median, and skew: Correcting a textbook rule. J. Stat. Educ. 13, 1–13. doi: 10.1080/10691898.2005.11910556
Wang, D., Wang, L., Liu, J., Zhu, H., and Zhong, Z. (2018). Grassland ecology in China: Perspectives and challenges. Front. Agric. Sci. Eng. 5:24–43. doi: 10.15302/J-FASE-2018205
Wedderburn, M. E., Crush, J. R., Pengelly, W. J., and Walcroft, J. L. (2010). Root growth patterns of perennial ryegrasses under well-watered and drought conditions. N Z. J. Agric. Res. 53, 377–388. doi: 10.1080/00288233.2010.514927
Wilcoxon, F. (1945). Individual comparisons by ranking methods. Biometrics Bull. 1, 80–83. doi: 10.2307/3001968
Xiao, B., and David, D. (2019). Morphological and physiological responses of seashore paspalum and bermudagrass to waterlogging stress. J. Am. Soc. Hortic. Sci. 144, 305–313. doi: 10.21273/JASHS04737-19
Xu, C., Mcdowell, N. G., Sevanto, S., and Fisher, R. A. (2013). Our limited ability to predict vegetation dynamics under water stress. New Phytol. 200, 298–300. doi: 10.1111/nph.12450
Yamauchi, N., and Watada, A. E. (2019). Regulated chlorophyll degradation in spinach leaves during storage. J. Am. Soc. Hortic. Sci. 116, 58–62. doi: 10.21273/jashs.116.1.58
Yates, S., Jaškūnė, K., Liebisch, F., Nagelmüller, S., Kirchgessner, N., Kölliker, R., et al. (2019). Phenotyping a dynamic trait: leaf growth of perennial ryegrass under water limiting conditions. Front. Plant Sci. 10:344. doi: 10.3389/fpls.2019.00344
Yiotis, C., Evans-Fitzgerald, C., and McElwain, J. C. (2017). Differences in the photosynthetic plasticity of ferns and ginkgo grown in experimentally controlled low [o2]:[co2] atmospheres may explain their contrasting ecological fate across the triassic-jurassic mass extinction boundary. Ann. Bot. 119, 1385–1395. doi: 10.1093/aob/mcx018
Yiotis, C., Mcelwain, J. C., and Osborne, B. A. (2021). Enhancing the productivity of ryegrass at elevated CO2 is dependent on tillering and leaf area development rather than leaf-level photosynthesis. J. Exp. Bot. 72, 1962–1977. doi: 10.1093/jxb/eraa584
Yu, X., Luo, N., Yan, J., Tang, J., Liu, S., and Jiang, Y. (2012). Differential growth response and carbohydrate metabolism of global collection of perennial ryegrass accessions to submergence and recovery following de-submergence. J. Plant Physiol. 169, 1040–1049. doi: 10.1016/j.jplph.2012.03.001
Zandalinas, S. I., Sengupta, S., Fritschi, F. B., Azad, R. K., Nechushtai, R., and Mittler, R. (2021). The impact of multifactorial stress combination on plant growth and survival. New Phytol. 230, 1034–1048. doi: 10.1111/nph.17232
Zellweger, F., De Frenne, P., Lenoir, J., Rocchini, D., and Coomes, D. (2019). Advances in microclimate ecology arising from remote sensing. Trends Ecol. Evol. 34, 327–341. doi: 10.1016/j.tree.2018.12.012
Zhang, Y., Song, X., Yang, G., Li, Z., Lu, H., Kong, X., et al. (2015). Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field. Crop. Res. 179, 164–172. doi: 10.1016/j.fcr.2015.05.001
Zhang, Y., Tang, L., Liu, X., Liu, L., Cao, W., and Zhu, Y. (2014). Modeling dynamics of leaf color based on RGB value in rice. J. Integr. Agric. 13, 749–759. doi: 10.1016/S2095-3119(13)60391-3
Keywords: anthocyanins, chamber experiment, colors, flooding, grasslands, harvest, image analysis, Lolium perenne
Citation: Frisk CA, Xistris-Songpanya G, Osborne M, Biswas Y, Melzer R and Yearsley JM (2022) Phenotypic variation from waterlogging in multiple perennial ryegrass varieties under climate change conditions. Front. Plant Sci. 13:954478. doi: 10.3389/fpls.2022.954478
Received: 27 May 2022; Accepted: 11 July 2022;
Published: 04 August 2022.
Edited by:
Jin-Lin Zhang, Lanzhou University, ChinaReviewed by:
Julian Mario Peña-Castro, Universidad del Papaloapan, MexicoCopyright © 2022 Frisk, Xistris-Songpanya, Osborne, Biswas, Melzer and Yearsley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carl A. Frisk, Q2FybC5Gcmlza0BuaWJpby5ubw==; Jon M. Yearsley, Sm9uLlllYXJzbGV5QHVjZC5pZQ==
†Present address: Carl A. Frisk, Department of Urban Greening and Vegetation Ecology, Norwegian Institute of Bioeconomy Research, Ås, Norway
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.