- 1Department of Agricultural Biotechnology, National Institute of Agricultural Sciences, Rural Development Administration, Jeonju, South Korea
- 2Department of Biotechnology, Alagappa University, Karaikudi, Tamil Nadu, India
- 3Department of Botany, St. Xavier’s College, Palayamkottai, Tamil Nadu, India
- 4Independent Researcher, Madurai, Tamil Nadu, India
- 5Department of Biotechnology, School of Life Sciences, Pondicherry University, Puducherry, India
Secondary metabolites are incontestably key specialized molecules with proven health-promoting effects on human beings. Naturally synthesized secondary metabolites are considered an important source of pharmaceuticals, food additives, cosmetics, flavors, etc., Therefore, enhancing the biosynthesis of these relevant metabolites by maintaining natural authenticity is getting more attention. The application of exogenous jasmonates (JAs) is well recognized for its ability to trigger plant growth and development. JAs have a large spectrum of action that covers seed germination, hypocotyl growth regulation, root elongation, petal expansion, and apical hook growth. This hormone is considered as one of the key regulators of the plant’s growth and development when the plant is under biotic or abiotic stress. The JAs regulate signal transduction through cross-talking with other genes in plants and thereby deploy an appropriate metabolism in the normal or stressed conditions. It has also been found to be an effective chemical elicitor for the synthesis of naturally occurring secondary metabolites. This review discusses the significance of JAs in the growth and development of plants and the successful outcomes of jasmonate-driven elicitation of secondary metabolites including flavonoids, anthraquinones, anthocyanin, xanthonoid, and more from various plant species. However, as the enhancement of these metabolites is essentially measured via in vitro cell culture or foliar spray, the large-scale production is significantly limited. Recent advancements in the plant cell culture technology lay the possibilities for the large-scale manufacturing of plant-derived secondary metabolites. With the insights about the genetic background of the metabolite biosynthetic pathway, synthetic biology also appears to be a potential avenue for accelerating their production. This review, therefore, also discussed the potential manoeuvres that can be deployed to synthesis plant secondary metabolites at the large-scale using plant cell, tissue, and organ cultures.
Introduction
Plants produce a variety of low molecular weight organic compounds, which are classified as primary or secondary metabolites. It requires primary metabolites for growth and development, whereas secondary metabolites serve as defence molecules that protect plants from adverse conditions (Verpoorte et al., 2000; Afrin et al., 2015). Secondary metabolites are produced by plants in their roots, stems, leaves, and other aerial regions with therapeutic beneficial, and have been utilized to treat many diseases since time immemorial (Pan et al., 2014; Raomai et al., 2015). The majority of secondary metabolites are obtained from wild plants, and that consequences their over-exploitation to eventual extinction. Chemical synthesis generates massive quantities of secondary metabolites, minimizing the need of their extraction from plants. However, the structure of many secondary metabolites is either unknown, extremely complicated, or their metabolic pathways are unknown. Consumers always prefer naturally derived products rather than chemically derived counterparts (Namdeo, 2007). However, the synthesis of secondary metabolites in a plant is relatively low and highly depending on the physiological and developmental stages of that plant (Thakur et al., 2013). To resolve this issue, a variety of in vitro techniques have widely been used to stimulate the production of bioactive secondary metabolites. Under natural conditions, secondary metabolites can be exogenously induced by plant hormone substances (Zhao et al., 2005; Ji et al., 2019). Accordingly, many biotechnological approaches have been introduced and explored for enhancing the production of secondary metabolites from medicinal plants. Among a few of the techniques used are cell line screening, elicitation, precursor feeding, hairy root culture, biotransformation, and others (Namdeo, 2007; Datta et al., 2011). Although many plant species have successfully been propagated in cell cultures, not all of them produce adequate secondary metabolites. When comparing with other techniques, treating undifferentiated cells by elicitors, such as methyl jasmonates (MJ), salicylic acid (SA), and chitosan might often accelerate the synthesis of secondary metabolites in most plants (Khanam et al., 2022).
Jasmonates is the collective term for jasmonic acid and its derivatives. It was originally isolated from the essential oil of Jasminum grandiflorum (Demole et al., 1962). JAs are cyclopentanone molecules generated from α-linolenic acid. It is the major precursor of several chemicals in this category, such as MJ (Ho et al., 2020). The biosynthesis of JAs has hitherto been explored in several monocotyledonous and dicotyledonous plants by having Arabidopsis thaliana and Solanum lycopersicum as model plants (Ruan et al., 2019). It was discovered with two phases of synthesis, viz., the formation of an intermediate, oxophytodienoic acid (OPDA), and biosynthesis of JAs and their derivatives (Figure 1). Jasmonate synthesis commences when the Phospholipase A1 (PLA1) mediated release of α-linolenic acid (α-LeA) from the sn1-position of plastid membranal galactolipids and which endows the intermediate product, OPDA through sequential steps mediated by several enzymes of the plastid (Wasternack and Song, 2017). In an initial step, the released α-LeA is oxygenated to 13S-hydroperoxy-octadecatrienoic acid (13-HPOT) by an enzyme, 13-lipoxygenase (13-LOX). Hitherto, four known 13-LOXs (LOX2, LOX3, LOX4, and LOX6) have been elucidated with tissue-specific roles including the synthesis of jasmonate in plants and are thereby predominantly involved in wound healing (Caldelari et al., 2011; Wasternack and Hause, 2013). Subsequently, Allene oxide synthase (AOS) catalyzes the conversion of 13-HPOT into an intermediate, 13-HPOT-allene oxide and further cyclization reaction in the hydrocarbon chain of HPOT by Allene oxide cyclase (AOC) ends up in the final intermediate of phase-I, OPDA. Allene oxide intermediates can also be converted into α-and β-ketol via spontaneous hydrolysis (Lu et al., 2014). The second phase of jasmonate synthesis is carried out in peroxisomes. For jasmonate synthesis, plant cells need to traffic plastid-synthesized OPDA into peroxisomes. OPDA is required to be exported from plastids and imported into peroxisomes. Though the influx mechanisms of OPDA to peroxisomes are well understood, the efflux of the protein through double-membraned plastids has long been a mystery. In a recent finding, Guan et al. (2019) discovered a protein called JASSY with the activity of OPDA effluxion. JASSY, a plastid outer membranal protein, has been implicated in OPDA effluxion out of the plastid in several mechanistic studies (Simm et al., 2013). However, none of the studies described the specificity of OPDA towards JASSY (Wasternack and Hause, 2019). The exported OPDA can be influxed into peroxisomes from the cytosol by either ATP-dependent Binding Cassette protein, COMATOSE (CTS) or ion-trapping mechanisms (Theodoulou et al., 2005). The knockout mutant of either of these two transporters perturbs jasmonate synthesis but not OPDA formation (Guan et al., 2019), indicating their auspicious role in OPDA trafficking. In peroxisomes, OPDA is first reduced by OPDA reductase (OPR3) to 3-oxo-2-[Z]-(pentenyl)-cyclopentane-1-octanoic acid (OPC-8: 0). Subsequently, carboxyl-CoA is ligated by OPC-8: 0 CoA ligase 1 (OPCL1) at the carbonyl end of OPC-8:0, which produces OPC-8:0-CoA. OPC-8:0-CoA further undergoes β-oxidation thrice, which shortens its hydrocarbon chain at the carbonyl end and gives rise to the final product of Jasmonic acid (Sanders et al., 2000; Andersson et al., 2006; Lu et al., 2014). Several derivatives are then formed from jasmonic acid, among which MJ and jasmonoyl-L-isoleucine (JA-Ile) exhibit profound effects on plant physiology. MJ and JA-Ile are formed through the catalyzed reaction of enzymes, Jasmonic acid carboxyl methyltransferase (JMT) and Jasmonate resistance-1 (JAR1), respectively. These derivative reactions are deployed in the cytoplasm of plant cells (Ruan et al., 2019).
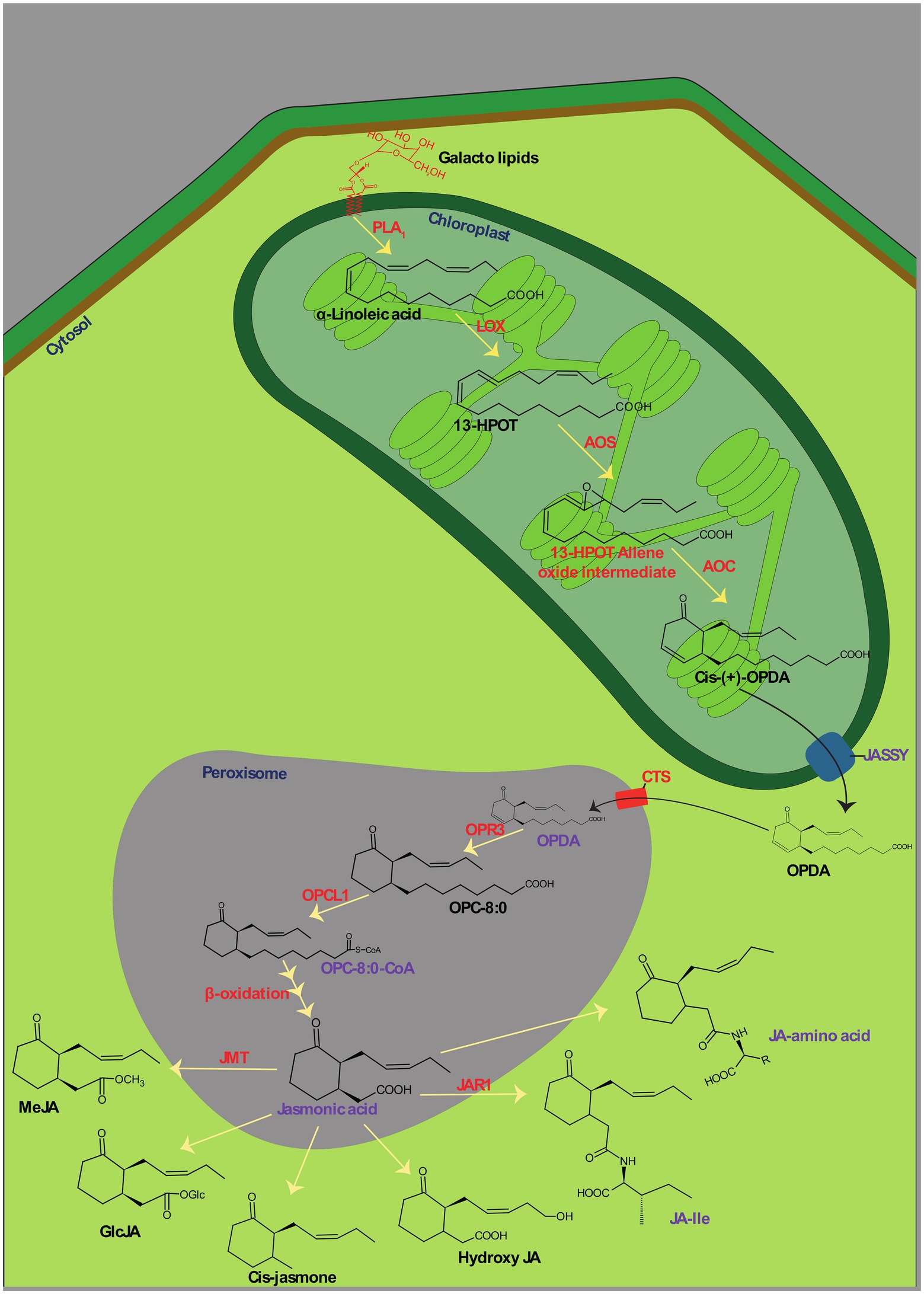
Figure 1. Biosynthesis of jasmonic acid (JA) and its direct derivatives. PLA1, Phospholipase A1; LOX, 13-lipoxygenase; HPOT, 13S-hydroperoxyoctadecatrienoic acid; AOS, Allene oxide synthase; AOC, Allene oxide cyclase; OPDA, Oxophytodienoic acid; OPR3, OPDA reductase; OPC-8:0, 3-oxo-2(29-[Z]-pentenyl)cyclopentane-1-octanoic acid; OPCL1, OPC-8:0 CoA ligase 1; JMT, Jasmonic acid carboxyl methyltransferase; JAR1, Jasmonate resistance-1; MeJA, Methyl jasmonate; GlcJA, Jasmonic acid Glycosyl ester; JA-Ile, Jasmonoyl-L-isoleucine.
Jasmonates can coordinate a lot of cellular activities, including plant growth and development and regulation of plant responses to biotic and abiotic stresses (Afrin et al., 2015). They are also involved in floral development, fruit ripening, tendril coiling, potato tuberization, trichome formation, and arbuscular micorrhizal fungi association with plants (Browse, 2005; Balbi and Devoto, 2008; Reinbothe et al., 2009; Yoshida et al., 2009). From gymnosperms to angiosperms, they act as unique and conserved elicitors for the production of secondary metabolites (Zhao et al., 2005; Pauwels et al., 2009). The elicitation process induces the crosstalk between JAs and their receptors in the plasma membrane. It also triggers a cascade of defence responses in the cells, including the production of reactive oxygen and nitrogen species (ROS and RNS) and the induction of oxidative stress-protective enzymes (Giri and Zaheer, 2016). This results in the synthesis and accumulation of signaling molecules such as JA, SA, nitric oxide (NO), and ethylene (ET), as well as the regulation of secondary metabolite biosynthesis gene expression (Zhao et al., 2005; Baenas et al., 2014; Rahimi et al., 2015). Several excellent reviews on JAs role in plant development, immunity, and abiotic stress tolerance have recently been published (Yan and Xie, 2015; Sharma and Laxmi, 2016; Wasternack and Strnad, 2016; Huang et al., 2017; Nguyen et al., 2022). In this review, we provide updated information on the mechanisms of action of JAs in plant growth and development and further elaborate on their role in the elicitation of secondary metabolites in important medicinal plants (Figure 2).
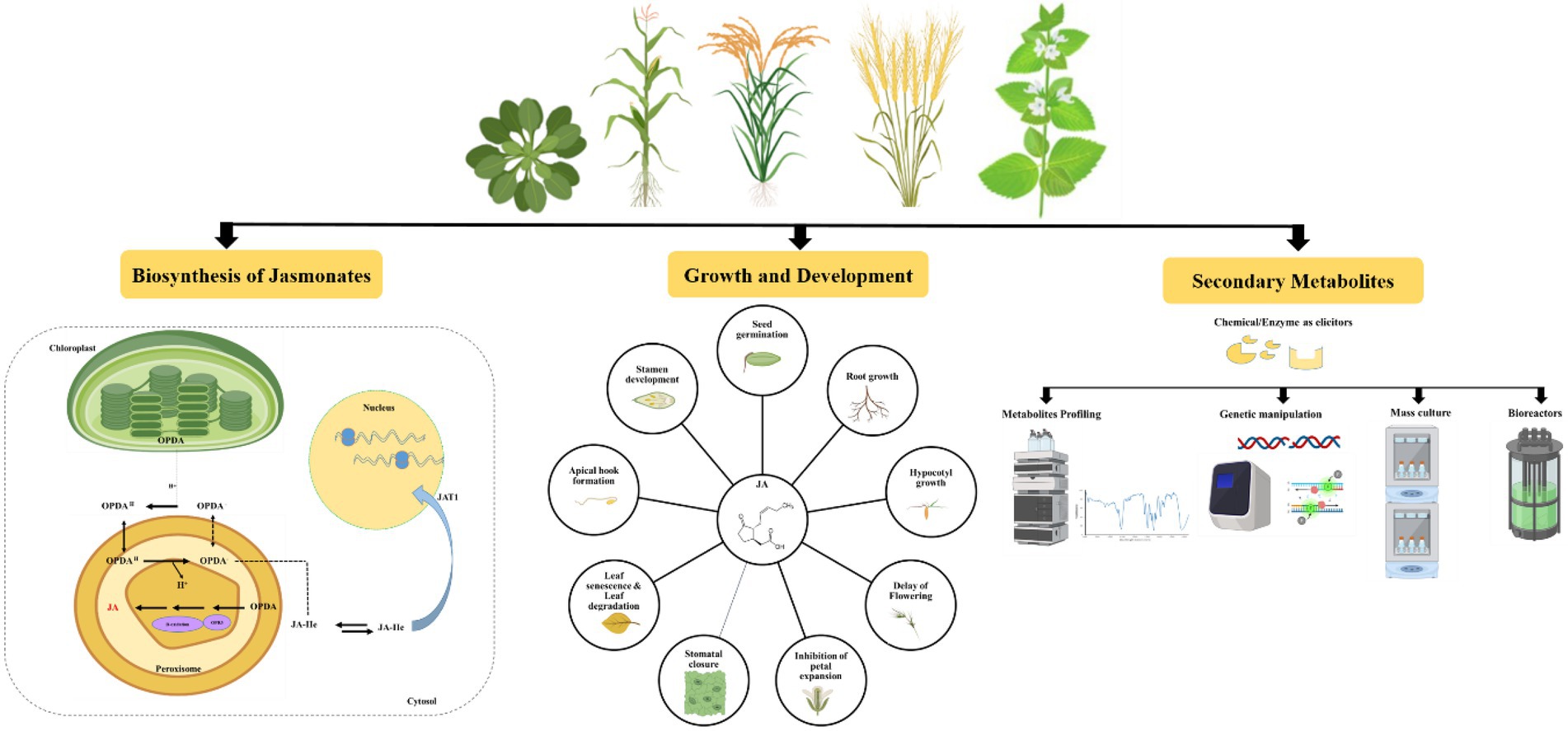
Figure 2. Schematic representation of jasmonates biosynthesis, role in growth and development and elicitation of secondary metabolites.
Regulation of plant growth and development
Jasmonates are lipid-derived hormones that act synergistically with endogenous hormones in response to environmental signals to regulate plant growth, development and defence (Figure 3). In the last decade, research has been carried out to examine the role of JA in plant maturation and development (Ghorbel et al., 2021). According to previous studies, JAs are involved in a variety of plant expanding processes, including root primary growth, leaf senescence, and reproductive development (Wasternack, 2007; Kim et al., 2015).
Effects on seed germination
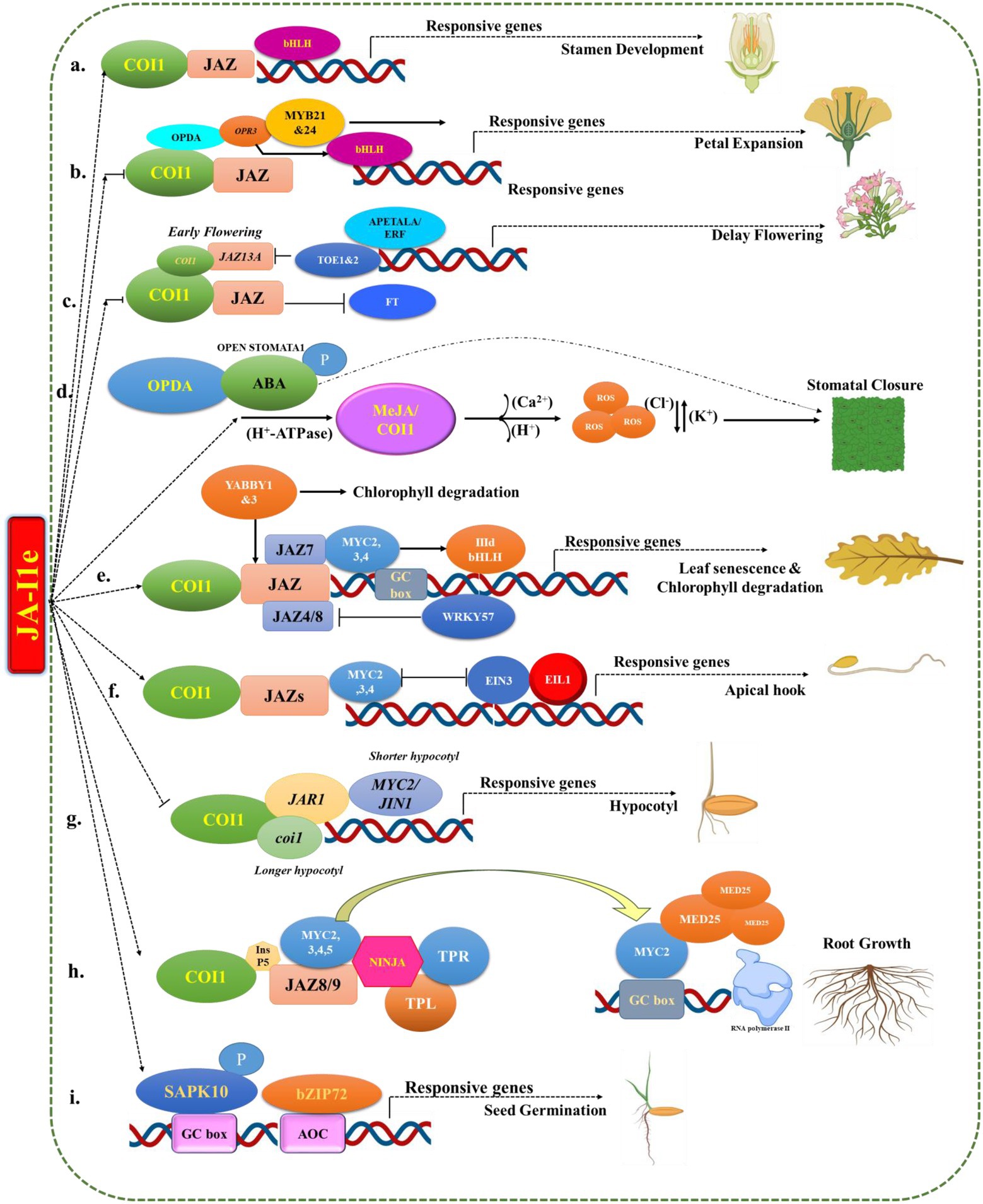
Phytohormones such as ABA (Abscisic acid), IAA (Indoleacetic acid), and JA have been shown to promote seed germination (Xiao et al., 2018). Seed germination is suppressed by both ABA and JA, but their interactions during this process are unknown (Tang et al., 2020). In Arabidopsis, JA inhibition occurs without the involvement of the COI1 co-receptor (Dave et al., 2011). Cold-stimulated seeds germination resulted in an increase in endogenous JA after overexpression of JA biosynthesis-related genes in Triticum aestivum plants, so JA promotes cold-stimulated germination (Xu et al., 2015b; Avramova, 2017). A novel SAPK10-bZIP72-AOC’ rice pathway has recently been found, in which ABA stimulates the production of JA, which synergistically suppresses rice seed germination (Wang et al., 2020). Auto-phosphorylation activates SAPK10 (Serine/threonine-protein kinase), which stabilizes bZIP72 transcription factor (TF; Basic leucine zipper) and improves its binding to the G-box cis-element of the AOC (Assimilable organic carbon) promoter, boosting AOC transcription in the presence of high JA concentrations. Interestingly, ABA sensitivity was reduced after inhibiting JA biosynthesis (Wang et al., 2020).
Inhibition of root growth
Plants with mutations in the JA-Ile (Jasmonoyl-isoleucine) COI1 (Coronatine-insensitive 1) co-receptor are resistant to the inhibition of primary root maturation caused by JAs (Yan et al., 2009). In addition, inositol pentakisphosphate (InsP5) enhances COI1–JAZ9 (Jasmonate Zim Domain) interactions, which reduces the influence of JAs on root elongation and maturation (Mosblech et al., 2011). Coronatine-O-methyloxime is a competitive JA antagonist that suppresses coronatine inhibitory effect on primitive root growth by blocking COI1–JAZs interactions (Monte et al., 2014). The ERF (Ethylene-responsive element-binding factor) associated amphiphilic repression (EAR) domain is absent from the majority of JAZ proteins (13 members) in Arabidopsis. To crush JA responses, they should interact with TPL (TOPLESS) and TPL-related proteins (TPRs) via the EAR of NINJA proteins. JAZ8 and JAZ13, two non-canonical JAZ proteins, do not require NINJA (novel interactor of JAZ) proteins to interact with TPLs/TPRs, instead of using their single EAR domain directly (Thireault et al., 2015; Chini et al., 2016). Overexpression of NINJA proteins or modified JAZ proteins (containing a deletion or mutation in the Jas domain) decreased the inhibitory effect of JA on primary root development, which was surprising. However, the NINJA/TPL or combination mutations in JAZ7, JAZ8, JAZ10, and JAZ13 increased this inhibitory effect (Thireault et al., 2015; Thatcher et al., 2016). In Arabidopsis, bHLH (basic helix–loop–helix) transcription factors (MYC2 and its homologs MYC3/4/5) interact with JAZ proteins (Qi et al., 2015). At the primary root apex, MYC2/3/4 gives way to JAs, which inhibit primary root growth (Gasperini et al., 2015). MYC2 also lowers the activity of root meristematic cells and suppresses primary root growth by inhibiting the expression of PLETHORA genes (PLT1 and PLT2; Chen et al., 2011). MYC2 also interacts with the MED25 (MEDIATOR25) subunit to inhibit RNA polymerase II, reducing its inhibitory action (Chen et al., 2011). MYC3–MED25 interactions have been demonstrated to be disrupted by JAZ9 (Gimenez-Ibanez et al., 2014). Inhibition of MYC2 ubiquitination and phosphorylation by PLANT U-BOX PROTEIN10 decreases JA-mediated root maturation inhibition in the presence of mitogen-activated protein kinase 3/6 (Chico et al., 2014; Sethi et al., 2014).
Inhibition of hypocotyl growth
Jasmonate suppresses hypocotyl elongation in Arabidopsis under a variety of light stress conditions, including far-red and blue wavelengths via COI1 (Chen et al., 2013). JA-deficient mutant JAR1 (Jasmonic acid-resistant 1) plants cultivated under far-red light were found to have extended hypocotyls (Robson et al., 2010). Furthermore, either grown in the dark or under fared lights (Chen et al., 2013) or red light or a low R/FR light ratio, coi1 mutants exhibit longer hypocotyls than WT plants (Robson et al., 2010; Chen et al., 2013). The MYC2/JIN1 (Jasmonate-insensitive mutant 1) mutant has a shorter hypocotyl when grown under far-red light and a low R/FR light ratio (Robson et al., 2010), but it has a longer hypocotyl when grown under blue light (Yadav et al., 2005). In rice, JA inhibits the maturity of coleoptiles and the growth of the plant. JA prevents the maturity of ear shoots in Zea mays (Yan et al., 2012; Yang et al., 2012).
Delay of flowering
Jasmonate inhibits the transition from vegetative to reproductive maturity in Arabidopsis. Flowering is inhibited by the interaction of COI1 and JAZ. Indeed, both coi1 mutant and JAZ13A transgenic plants showed early flowering. Furthermore, plant flowering is controlled by TARGET OF EAT TFs (TOE1 and TOE2), which are APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) domain TFs. In fact, their association with JAZ proteins limits blooming by inactivating FLOWERING LOCUS T transcription. Overexpression of TOE1 and/or TOE2 on the other hand, inhibits the coi1 early-blooming phenotype (Zhai et al., 2015).
Inhibition of petal expansion
Jasmonate suppresses petal growth in Arabidopsis via the COI1 pathway. In fact, when compared to wild type, coi1 mutants have bigger petals at anthesis. The aos and opr3 genes of Arabidopsis JA-deficient mutants showed a similar pattern (Brioudes et al., 2009; Reeves et al., 2012). MYB21 (Myeloblastosis 21) and MYB24 proteins are repressed by JA during the formation of sexual organs, resulting in petal growth restriction, indicating that MYB21 and MYB24 are crucial for petal expansion (Reeves et al., 2012). MYB21 expression was shown to be higher in the petals of aos and coi1 plants, resulting in continuous petal expansion and hence grand petals (Reeves et al., 2012). Furthermore, opr3 plants have a higher level of bHLH TF BIGPETALp expression. This transcription factor regulates post-mitotic cell growth, resulting in expanded petals and cells (Brioudes et al., 2009).
Regulation of stomatal closure
In plants, stomata aid in the regulation of gas exchange, water loss and the control of plant resistance to phytopathogens. JA is also involved in stomatal closure. In reality, Methyl-JA/COI1 activates an H+-ATPase in the plasma membrane. The depolarization of the membrane causes an influx of Ca2+ and an efflux of H+ (Yin et al., 2016). Methyl-JA/COI1 also causes the generation of reactive oxygen species (ROS), the stimulation of Cl− channels (resulting in Cl− efflux), and the stimulation of K+ efflux (through K+ channels), resulting in the loss of turgor guard cells and stomatal closure (Yan et al., 2015). Plants close their stomata in response to drought and salt stress. The activation of OPEN STOMATA1 protein kinase by JA and ABA pathways has been shown to modulate stomatal closure in Arabidopsis (Yin et al., 2016). Plants also generate OPDA rather than jasmonate in drought situations. In these circumstances, OPDA is more effective than JA. In Arabidopsis, OPDA interacts with ABA to improve stomatal closure (Savchenko et al., 2014).
Induction of leaf senescence and chlorophyll degradation
In Arabidopsis, JA promotes leaf senescence. COI1 is required for this impact to occur (Qi et al., 2015). JAZ7 also found to inhibit leaf senescence in dark-grown plants (Yu et al., 2016). Many members of the NAC TF family (such as NAC019, NAC055, and NAC072) promote chlorophyll degradation by acting downstream of MYC2/3/4 (Melotto et al., 2006). Subgroup IIId bHLH TFs inhibit leaf senescence by binding competitively to their target promoters and inhibiting MYC2/3/4 activity (Qi et al., 2015). In the presence of JA, WRKY57 interacts physically with JAZ4/8 and acts as a negative regulator of leaf senescence (Jiang et al., 2014). The proteins YABBY1 and YABBY3 interact with JAZs, promoting chlorophyll degradation (Boter et al., 2015).
Inhibition of apical hook formation
The COI1-JAZs-MYC2/3/4 cascade suppresses the development of an apical hook in dark-grown plants (Song et al., 2014). In dark stress circumstances, JA activates the transcription factors of MYC2, MYC3, and MYC4, which physically interact with and decrease the transcriptional activity of EIN3/EIL1 (Ethylene-insensitive3/ETHYLENE-INSENSITIVE3-like 1). Apical hook curvature is prevented and the HOOKLESS1 gene, which controls apical hook formation, is downregulated (Song et al., 2014; Zhang et al., 2014). MYC2 also promotes the formation of EIN3 BINDING F-BOX PROTEIN1, which causes EIN3 degradation (Zhang et al., 2014).
Stamen development in Arabidopsis
Many male sterile Arabidopsis plants have been reported, among which JA-deficient mutants such as coi1, lox3 (Lipoxygenases), lox4, aos, opr3, fad3 (Acyl-lipid omega-3 desaturase), fad7, fad8, dad1 (Defective in anther dehiscence1), JAZ13A and JAZ10.4 mutations, as well as CYP94B3 (Jasmonoyl-isoleucine-12-hydroxylase) overexpression lines were discovered, all of which indicated impaired stamen development (Song et al., 2013). Exogenous administration of JA rescues stamen growth in plants deficient in JA production but not in JA signalling mutants, which is surprising (Jewell and Browse, 2016). Furthermore, in a coi1 background, re-expressing COI1 in a variety of tissues, such as the filament epidermis or anthers, can restore anther dehiscence, filament elongation, and pollen maturation (Jewell and Browse, 2016). The R2R3-MYB TFs MYB21, MYB24, and MYB57 interact directly with JAZs (Song et al., 2011). Delayed anther dehiscence, a non-viable pollen grain, and short filaments are all symptoms of the myb21 myb24 double mutant. Overexpression of MYB21 proteins in coi1-1 plants results in restored stamen formation (Song et al., 2011). MYB21 and MYB24 regulate the stamen formation by physically connecting to MYC2, MYC3, MYC4, and MYC5 (Qi et al., 2015). Overexpression of MYC5 and MYC3 enhances stamen maturation and productivity in plants (Qi et al., 2015).
Elicitation of secondary metabolites using jasmonates
Apart from their role in plant growth and development, JAs act as a major elicitor for the enhancement of secondary metabolites. Among the JAs, MJ has been extensively used as an elicitor to enhance a wide range of secondary metabolites for more than two decades. It has been reported to influence the production of phytochemicals in different in vitro culture systems, such as adventitious root culture, callus culture, multiple shoot culture, cell suspension culture, and hairy root culture (Nabi et al., 2021). MJ is the most frequently used elicitor, and it has been found to have a significant influence on secondary metabolite accumulation in plant cells and organs (Baenas et al., 2014; Giri and Zaheer, 2016). Exogenous application of MJ increases the concentration of phenols (Ahn et al., 2014), alkaloids (Zhou et al., 2015), terpenoids (Onrubia et al., 2013), coumarin (Dučaiová et al., 2016), anthocyanin (Ram et al., 2013) and polyamines (Cao et al., 2014), not only in plant cell cultures but also in whole plants (Ahn et al., 2014; Dučaiová et al., 2016; Ho et al., 2020). MJ was used as an effective elicitor in the root suspension of Ajuga bracteosa, leading to an increased phenolic and flavonoid content (Saeed et al., 2017). In addition, increased expression of genes and transcription factors related to secondary metabolite biosynthesis has also been reported. In recent years, elicitation of metabolites has been demonstrated in bioreactors as a prelude to large-scale commercial production of phytochemicals.
Enhanced accumulation of high valued metabolites
In cell suspension cultures
A dramatic increase in rosmarinic acid content in cultured cells of Lithospermum erythrorhizon was observed after their exposure to MJ (Mizukami et al., 1993). MJ elicitation resulted in the induction of xanthones in cell suspension cultures of Centaurium spp. (Beerhues and Berger, 1995). A marked increase in the alkannin pigment content in cells and medium of suspension cultures of Alkanna tinctoria after treatment with MJ was observed (Urbanek et al., 1996). The addition of 0.5 μM MJ provoked a twofold to threefold increase in anthocyanin production over that of the control in cell cultures of Vaccinium pahalae (Fang et al., 1999). Supplementation of 100 μM MJ and 25 g/L sucrose produced 24 mg/L cephalomaninein in the cell suspension cultures of Taxus chinensis (Lan et al., 2002). Synthetic JAs, such as pentafluoropropyl jasmonate, 2-hydroxyethyl jasmonate and 2-hydroxyethoxyethyl jasmonate were found to promote ginsenoside production in cell suspension cultures of Panax notoginseng (Wang et al., 2005). A sixfold increase of phenolic compounds, flavanols and flavonols after JA elicitation was observed in Hypericum perforatum cell suspension cultures (Gadzovska et al., 2007). Taxus cuspidata var. nana cell suspension culture was reported to have a fourfold increased accumulation of paclitaxel, an anticancer diterpenoid, upon elicitation with 21 mg/L JA (Tachinbana et al., 2007). A concentration of 100 mg/L of MJ induced peruvoside production in Thevetia peruviana cell suspension cultures (Zabala et al., 2010). Suspension cultures of Habanero pepper exposed to MJ have led to the accumulation of capsaicinoids and vanillin (Gutiérrez-Carbajal et al., 2010). Sequential application of MJ, SA, and yeast extract to Argemone mexicana cell cultures resulted in a ninefold increase in sanguinarine accumulation over unexposed control cultures (Trujillo-Villanueva et al., 2012).
Leaf-derived cell culture of Adhatoda vasica elicited with 20 μM MJ resulted in a 3.7-fold higher yield of vasicine in comparison with control cultures (Bhambhani et al., 2012). The combined treatment with cyclodextrins (50 mM) and MJ (100 μM) resulted in enhancement of ajmalicine and catharanthine productivity and increased gene transcript accumulation in Catharanthus roseus cell cultures (Almagro et al., 2014). The combined treatment of UV-C and MJ highly induced the total intracellular stilbene production to its maximum in cell suspension cultures of Vitis vinifera L. (Xu et al., 2015a). MJ yielded the maximum gymnemic acid content of 135.41 ± 0.43 mg g−1 dry cell weight, after 72 h elicitor application in Gynema sylvestre cell suspension cultures (Chodisetti et al., 2015). Treatment of Hypericum perforatum cell cultures with 100 μM/L MJ on day 15, resulted in 2.7 times more flavonoid production (Wang et al., 2015). Ali et al. (2015) observed enhanced accumulation of total phenolic content, total flavonoid content, and the highest radical scavenging activity in suspension cultures of Artemisia absinthium treated with 1.0 mg/L of MJ, JA, and GA, each.
A synergistic combination of MJ (0.1 mM) and 2-hydroxypropyl-β-cyclodextrin (20 mM) increased the production of intracellular anthraquinones in suspension cultures of Rubia tinctorum (Perassolo et al., 2016). The ginsenoside biosynthesis-related genes and ginsenoside accumulation were highly induced by 100 μM MJ in combination with 200 μM of sodium nitro prusside in adventitious root cultures of Panax ginseng (Rahimi et al., 2016). A combination of 0.1 mM MJ and 0.1 mM SA in the immobilized cells of Ginkgo biloba increased the production of bilobalide and ginkgolides A, B, and C than in the unelicited cultures (Sukito and Tachibana, 2016). In vitro cell suspension culture of Momordica dioica elicited with JA produced higher amounts of flavonols, hydroxycinnamic acids, and hydroxybenzoic acids (Chung et al., 2017). The addition of 150 μM MJ enriched the yield of essential oil in Coriandrum sativum embryogenic cultures (Ali et al., 2019).
In callus cultures
The addition of 100 μM MJ increased the paclitaxel (an anticancer alkaloid) content from 2.37 to 90 μg g−1 and cephalomannine content from 5.14 to 29.14 μg g−1 (dry weight) in callus cultures of Taxus × media var. Hatfieldii (Furmanowa et al., 1997). Elicitation of the calli by MJ induced a 38% increase in total polyphenol production in Eritrichium sericeum (Inyushkina et al., 2007). Ram et al. (2013) found that 5 μM MJ promoted anthocyanin production in rose callus cultures. The contents of six naphthoquinone compounds were increased in the MJ-treated callus tissues of Arnebia euchroma and in particular, the bioactive component acetylshikonin nearly doubled its content due to MJ elicitation (Hao et al., 2014). In callus cultures of Phyllanthus pulcher, 1 mM of MJ resulted in the highest yield for total flavonoid and phenolic contents and antioxidant activity (Danaee et al., 2015). A significant increase in antioxidant activity was observed in the calli of three Opuntia species in media with 50 μM JA (Camarena-Rangel et al., 2017). Callus cultures of Gardenia jasminoides elicited by 200 μM MJ showed the maximum content of total chlorogenic acid (a polyphenolic antioxidant) and its derivatives and displayed a much higher antioxidant capacity (Liu et al., 2018).
In adventitious root cultures
Indole-3-butyric acid with MJ at 100 μM synergistically stimulated both root growth and ginsenoside accumulation in Panax ginseng adventitious root cultures (Kim et al., 2007). The growth of adventitious roots, the contents of triptolide and alkaloids were increased 1.04, 1.64 and 2.12-folds, respectively, when MJ was at 50 μM in adventitious root cultures of Tripterygium wilfordii (Li et al., 2015). Andrographolide (an antiviral diterpenoid) content of 10.8-fold was obtained after the first week of elicitation with 25 μM JA in adventitious root cultures of Andrographis paniculata (Zaheer and Giri, 2017). The maximum accumulation of flavonoids was induced on the third day with the addition of H2O2 combined with MJ in root cultures of Stevia rebaudiana (Alvarado-Orea et al., 2020).
In whole plant cultures
Treatment of Glycyrrhiza glabra in vitro plantlets with 0.1–2 mM MJ enhanced the production of glycyrrhizin, a saponin, by 3.8 times (Shabani et al., 2009). After 4 weeks of treatment with 0.025 mg/L of TDZ coupled with 0.1 mM MJ, the production of anti-inflammatory triterpenoids (madecassoside and asiaticoside) from whole plant cultures of Centella asiatica was found to be increased by 2.40-and 2.44-folds, respectively (Yoo et al., 2011). Shoot cultures of Bacopa monnieri elicited with a combination of 25 μM MJ and SA resulted in a fivefold increased accumulation of Bacoside A, a memory-boosting triterpenoid saponin (Largia et al., 2015). An increased accumulation of anti-inflammatory alkaloids (pteropodine, isopteropodine, speciophylline, rumberine, hameline and palmirine) was reported in JA elicited Hamelia patens (Flores-Sanchez et al., 2016). The highest dioscorealide B (a phenolic compound) contentwas recorded in the 100 μM JA elicited in vitro shoots of Dioscorea membranacea (Jirakiattikul et al., 2020).
In hairy root cultures
Biondi et al. (2000) demonstrated an enhancement in the levels of methyl putrescine, a polyamine in normal and hairy root cultures of Hyoscyamus muticus by using JA and MJ. MJ up-regulated the biosynthesis of sesquiterpene lactones in hairy root cultures of Cichorium intybus after 72 h of exposure (Malarz et al., 2007). In hairy root culture of Taxus × media var. Hicksii, the supplementation of 100 μM of phenylalanine together with 100 μM of MJ resulted in the enhancement of paclitaxel production from 40.3 to 568.2 μg L−1 (Syklowska-Baranek et al., 2009). The highest rhinacanthin (an antiviral naphthoquinone) content was observed after treatment with 10 μM MJ which was about 1.7 fold higher than control hairy root cultures of Rhinacanthus nasutus (Cheruvathur and Thomas, 2014). The elicitation of hairy roots of Solanum trilobatum with 4 μM MJ for 2 weeks boosted the accumulation of the alkaloid solasodine by 6.5-fold more than untransformed roots. They also noticed a significant improvement in total phenolics, total flavonoids and radical scavenging activity of MJ elicited hairy roots (Shilpha et al., 2015). Isatis tinctoria hairy root cultures elicited with 179.54 μM MJ caused 11-fold increased flavonoid production (Gai et al., 2019). The highest quantity of triterpenoids (60.25 mg/g DW) was produced in hairy root cultures of Centella asiatica treated with 400 μM MJ (Baek et al., 2020). A sneak peek of recent reports pertaining to jasmonate elicitation has been given in Table 1.
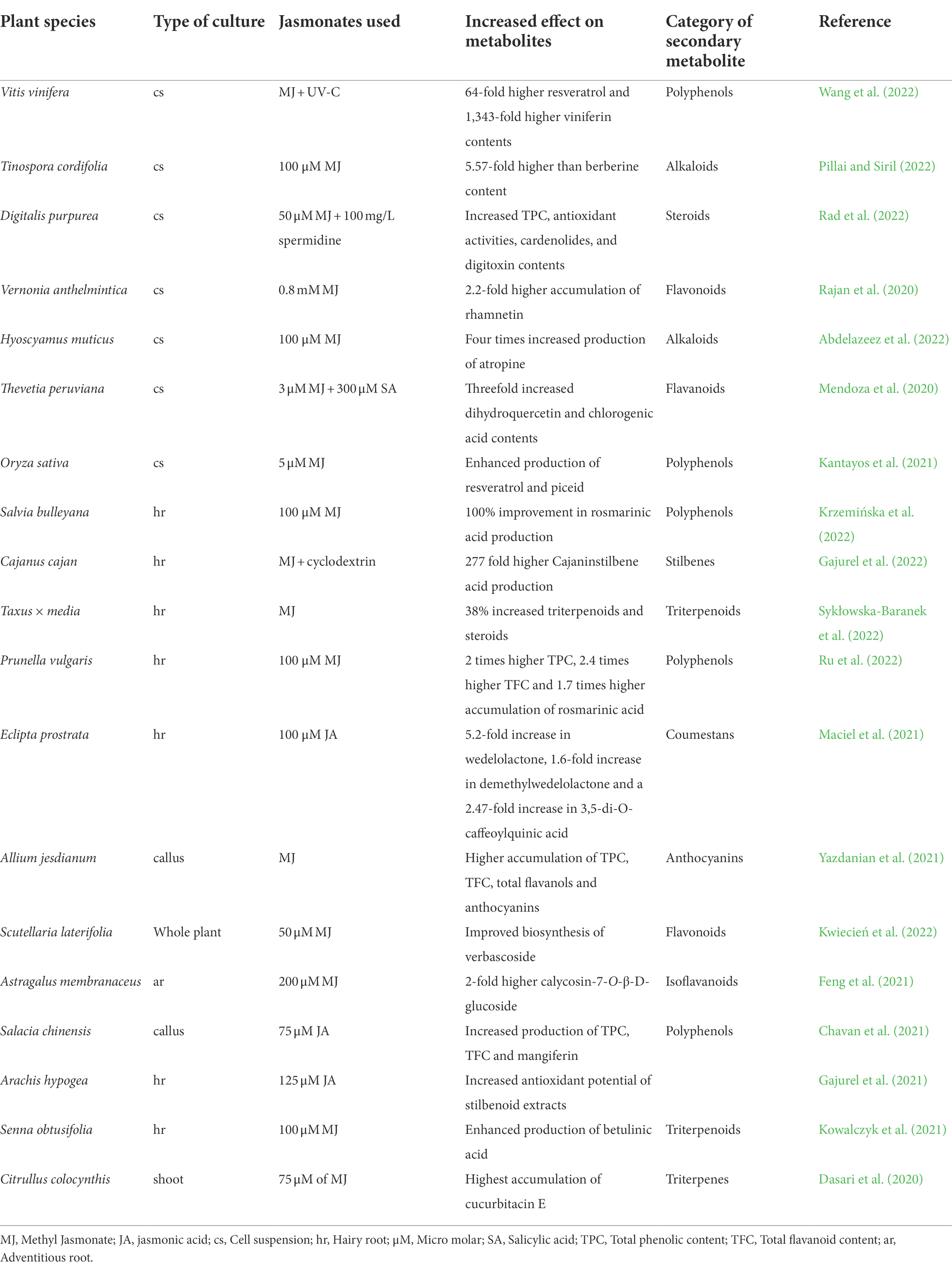
Table 1. Recent reports on the use of jasmonates for enhanced production of a variety of secondary metabolites through different in vitro culture systems.
In vivo plants
Interestingly, a 40–70 fold increase in the level of furanocoumarins was observed in the leaves of Apium graveolens by the exposure of MJ vapours (Miksch and Boland, 1996). JA at 50 μM concentration for 4 days resulted in increased camptothecin (an alkaloid) production up to 11 times (Song and Byun, 1998). In two cultivars of Ocimum basilicum, foliar application of 0.5 mM MJ raised the percentages of linalool and 1,8-cineole (terpene alcohols) and increased their antioxidant activity (Talebi et al., 2018). The elicitation of Maritime and Monterey pine seedlings with 5 mM MJ resulted in increased total mono and sesquiterpenes, which led to increased resistance against pine weevil, Hylobius abietis (Lundborg et al., 2019). Foliar spray of JA (400 ppm) improved the accumulation of antidiabetic potential triterpenoids, withanolide A and withanolide B in Withania somnifera (Singh et al., 2020). Punica granatum elicited with 200 μM MJ displayed an increased accumulation of flavonols and phenols (Chang et al., 2021). Bean varieties such as Phaseolus vulgaris, Glycine max, and Vigna radiata demonstrated a higher accumulation of isoflavanoids on MJ treatment (Gómez et al., 2022).
Influence of jasmonates on gene transcripts
Elicitation treatment in general stimulates the production of secondary metabolites through the involvement of signal compounds. In the last decade, elicitation studies were blended with gene expression analysis through RT-PCR and qRT-PCR techniques to confirm the mechanism and mode of action of elicitation. MJ treatment resulted in a 50-fold induction of transcripts encoding the key triterpene biosynthetic enzyme β-amyrin synthase in Medicago truncatula cell suspension (Suzuki et al., 2005). Hairy root cultures of Panax ginseng elicited with MJ revealed the increased transcription of relevant responsive genes such as squalene synthase, squalene epoxidase, and dammarenediol synthase-II (Kim et al., 2009). In case of 65-day-old plantlets of licorice treated with MJ (0.1, 1 and 2 mM) exhibited increased expression of two key biosynthetic enzymes for terpenoid biosynthesis such as squalene synthase and beta-amyrin synthase (Shabani et al., 2010).
Methyl jasmonates application resulted in induction of pathogenesis-related genes in two cultivars of Gossypium hirsutum (Zambounis et al., 2012). MJ treatment increased the transcript levels of terpene biosynthesis genes such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase, 1-deoxy-D-xylulose-5-phosphate reductoisomerase, and hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase, as well as parthenolide biosynthetic genes such as germacrene A synthase, germacren in Tanacetum parthenium (Majdi et al., 2015). Treatment of Solanum trilobatum hairy roots with 4 μM MJ has upregulated the expression of hmgr (HMGCoA reductase) gene, the key regulator of solasodine biosynthetic pathway (Shilpha et al., 2015). MJ at 25 μM promoted the expression of PAL genes by sevenfold on day 16 of elicitor treatment in Ocimum tenuiflorum suspension cell cultures (Vyas and Mukhopadhyay, 2018). The maximum yields of alkaloids and the highest levels of the expression of biosynthetic genes such as strictosidine synthase, geissoschizine synthase, deacetylvindoline acetyltransferase, and peroxidase were observed under 100 μM MJ in combination with 100 μM of AgNO3 after 7 days in Catharanthus roseus in vitro propagated shoots (Paeizi et al., 2018). Up-regulation of critical genes involved in the rubber biosynthesis pathway was exhibited by MJ treated Hevea brasiliensis barks (Liu et al., 2018). Soybean cell cultures treated with MJ treatment had the most significant effect on the expression of isoflavonoid biosynthesis genes (Jeong et al., 2018).
The simultaneous elicitation of MJ and putrescine in Catharanthus roseus shoots resulted in the up-regulation of signaling and biosynthetic genes of alkaloids production (Khataee et al., 2019). The relative expression levels of phenylpropanoid pathway genes, such as PAL, C4H, 4CL, and HPPR in the tyrosine-derived pathways were increased in MJ elicited hairy root cultures of Mentha spicata in comparison to untreated controls (Yousefian et al., 2020). The selected genes in the tanshinone and phenolic acid biosynthetic pathways were up-regulated with MJ elicitation in hairy root cultures of Salvia przewalskii (Li et al., 2020). Transcriptome analysis of MJ treated Carthamus tinctorius demonstrated the up-regulation of flavonoid biosynthesis pathway genes (Chen et al., 2020). MJ elicited callus cultures of Capparis spinosa showed the highest rutin content and increased expression patterns of rutin biosynthesis genes (Kianersi et al., 2021). Hairy root cultures of Scutellaria bornmuelleri exhibited enhanced expression of two important genes involved in the flavonoid biosynthesis pathway (Gharari et al., 2020). Besides, Table 2 presents a glimpse of the most recent reports correlated with gene expression and jasmonate elicitation.
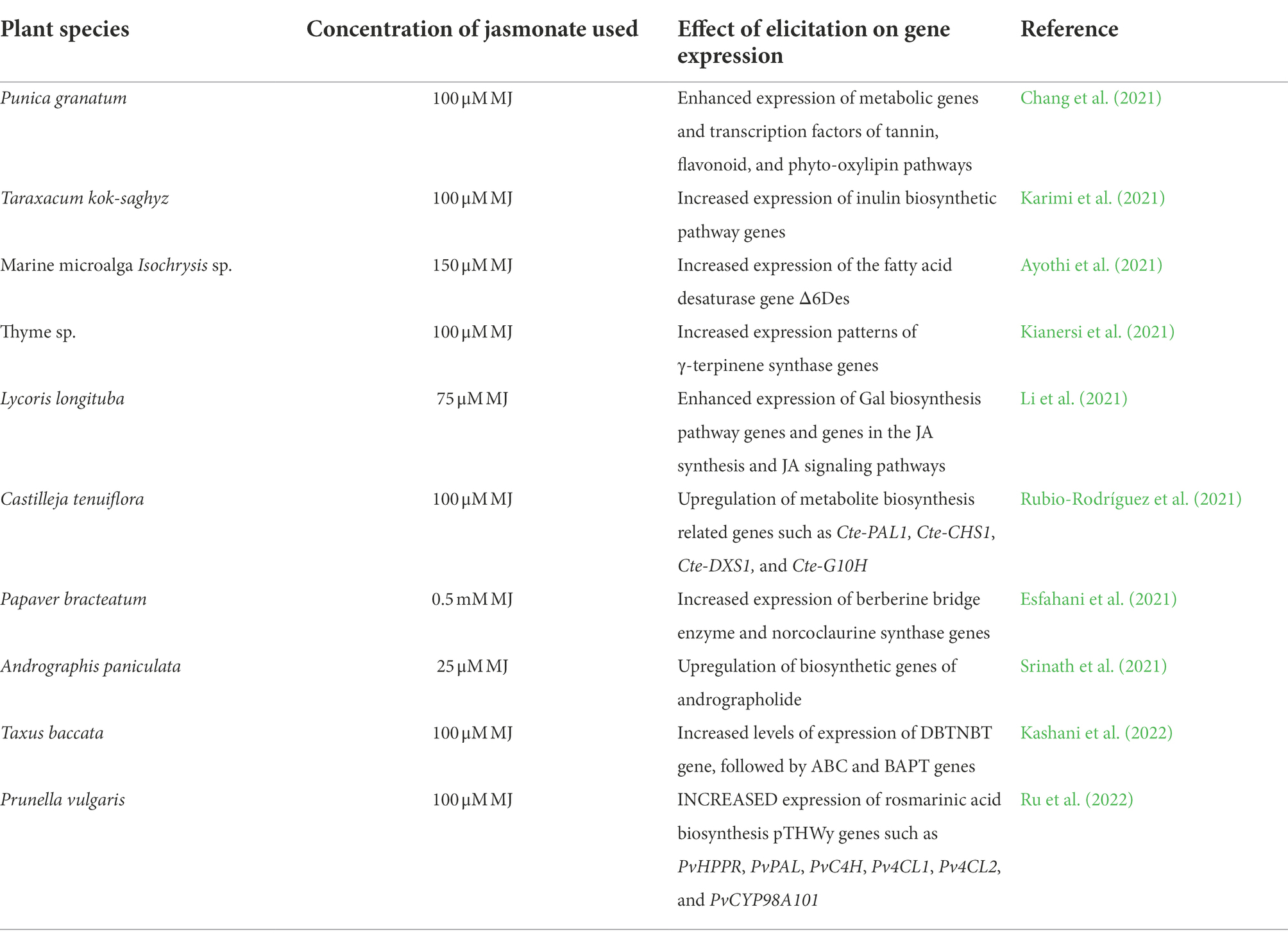
Table 2. Reports on influence of jasmonates on gene expression during elicitation of secondary metabolites.
Transcription factors are promising metabolic engineering targets due to their ability to regulate the transcription of multiple biosynthetic pathway genes. Jasmonate elicitation results in activation of TFs which regulate gene expression through specific binding to cis-acting elements in the promoters of target genes and so results in JAs-induced accumulation of secondary metabolites. These TFs belong to different families, including AP2/ERF, bHLH, MYB and WRKY TFs (De Geyter et al., 2012). De Geyter et al. (2012) and Zhou and Memelink (2016) provided in-depth reviews of various families of TFs and the effects of JAs on them. Some important studies published later are highlighted therein.
In Vitis vinifera, combined elicitation by MJ and cyclodextrins provoked the activation of additional regulatory pathways involving the upregulation of MYB15, NAC and WRKY transcription factors, protein kinases and calcium signal transducers, which in turn resulted in a greater trans-resveratrol production (Almagro et al., 2014). Induced by MJ treatment, the expression of a large number of genes involved in phenylpropanoid biosynthesis and many genes encoding transcription factors such as cytochrome P450s, glycosyltransferases, methyltransferases, and transporters in Salvia sclarea (Hao et al., 2015). MJ has been shown to activate the promoters of Ethylene Response Factor (ERF) family transcription factors such as ERF29, ERF210, and ERF199 at the nicotine-regulatory locus NICOTINE2 in Nicotiana tabacum. These transcription factors have been shown to be most effective in controlling nicotine biosynthetic pathway genes (Shoji and Hashimoto, 2015). Many genes encoding transcription factors belonging to the ERF, bHLH, MYB, and WRKY families also responded and were upregulated due to MJ elicitation in Gentiana macrophylla (Cao et al., 2016). Sharma et al. (2019) reported the inducible nature of transcription factor WsMYC2 in response to MJ elicitation in Withania somnifera. They have also witnessed the involvement of WsMYC2 in the biosynthesis of triterpenoid withanolides and in inducing phytosterol via key pathway genes. MJ application in pear calli significantly enhanced flavonoid accumulation and upregulated the expression of the flavonoid biosynthesis pathway structural genes (PcCHS, PcCHI, PcF3H, PcDFR, PcANS, PcANR2a, and PcLAR1). In addition to PcMYB10, which is a known positive regulator of anthocyanin biosynthesis in pear, several novel MYB candidates that may regulate flavonol and proanthocyanidin biosynthesis were revealed (Premathilake et al., 2020).
Elicitation of bioreactor-based cultures
Mass culturing of plant cells and tissues along with an enhanced accumulation of phytochemicals in bioreactors is the next step in large-scale, and commercial production of plant-based metabolites. In recent years, reports on bioreactor-based plant cultures have been blooming. Elicitation of bioreactor cultures further results in a large-scale production of plant secondary metabolites. Investigations on MJ elicitation in 5-L bioreactor suspension cultures of Panax ginseng demonstrated the highest ginsenoside accumulation at 200 μM (Thanh et al., 2005). A single 200 μM MJ treatment of Panax ginseng roots increased ginsenoside accumulation in airlift bioreactors (Ali et al., 2006). Mass cultivation of Silybum marianum hairy roots in a bioreactor resulted in the highest production of silymarin after MJ treatment (100 μM) for 3 weeks (Rahimi et al., 2012). In a pilot-scale bioreactor of 500 L capacity, elicitor-treated (100 μM MJ) adventitious roots of Echinacea angustifolia resulted in the maximum accumulation of total phenolics, total flavonoids, and total caffeic acid derivatives. Among the caffeic acid derivatives, the accumulation of echinacoside is approximately threefold more in MJ-treated adventitious roots than in non-MJ-treated roots cultured in bioreactors (Cui et al., 2013). MJ increased the scopolamine productivity by 146% in hairy root cultures of Hyoscyamus niger grown in a hybrid bubble column/spray bioreactor (Jaremicz et al., 2014). Surprisingly, adventitious root cultures of Tripterygium wilfordii grown in bubble column bioreactors showed 3.55-fold, 49.11-fold, and 10.40-fold increased accumulation of triptolide, wilforgine, and wilforine, respectively, upon MJ elicitation (Miao et al., 2014). In vitro shoots of Vitis flexuosa cultured in continuous immersion bioreactors recorded a greater quantity of total phenolics after MJ elicitation combined with manipulation of the NH4+ and NO3 – ratio (Park et al., 2015). The adventitious roots of Eleutherococcus koreanum in airlift bioreactors subjected to MJ treatment for 1 week showed the highest production of five target bioactive compounds (i.e.) eleutherosides B and E, chlorogenic acid, and total phenolics and flavonoids (Lee et al., 2015).
Treatment with 50 μM of MJ stimulated galanthamine and lycorine biosynthesis in Leucojum aestivum and L. aestivum bioreactor cultures (Ptak et al., 2017). Enhanced production of thapsigargin was achieved by growing in vitro shoots of Thapsia garganica in temporary immersion bioreactors using reduced nutrient supply in combination with MJ elicitation treatments (López et al., 2018). Cultivation of MJ elicited Ocimum basilicum suspension culture in bioreactors improved the cumulative productivities of betulinic acid, ursolic acid, and oleanolic acid (Pandey et al., 2019). The contents of total flavonoids and flavonoid monomers, including quercetin, kaempferide, epicatechin gallate, quercetin-3-O-glucose, and kaempferol-3-rutinoside, were significantly improved by MJ treatment in Orostachys cartilaginous cell cultures in balloon type bioreactors (Hao et al., 2020). Pueraria candollei var. mirifica cell suspension cultures in an airlift bioreactor exhibited enhanced production of deoxymiroestrol by MJ elicitation (Udomsin et al., 2020). In vitro seedlings of Dendrobium nobile grown in a temporary immersion bioreactor system were elicited with 10 μM MJ and resulted in a fivefold increased production of alkaloids (Zhang et al., 2022).
Conclusion and future perspectives
Secondary metabolite biosynthesis impulse under the action of phytohormones in general and JAs, in particular, is crucial for plant growth, development, and defense in a stressed environment. So far, tremendous efforts have led to the elucidation of some of the key genes involved in the physiological mechanisms deployed by the plant to grow and survive. The JAs-elicited plant secondary metabolism machinery involved several transcription factors, including AP2/ERFs (Verpoorte et al., 2000), WRKYs (Wang et al., 2007), bHLHs (Zhang et al., 2011), and R2R3-MYBs (Shan et al., 2009). For example, the interaction network with synthesis enzymes has been under elucidation in the plant model A. thaliana and some medicinal plant systems including Catharanthus roseus, Nicotina tabacum and Artemisia annua (De Geyter et al., 2012).
A better understanding of the accumulation of plant metabolites is garnering more attention due to the increased interest in naturally produced secondary metabolites in human health, plant protection, and nutraceutical enriched foods. To extend the application field, we suggested the inclusion of medicinal orphan crops that also represent a secondary metabolite gold mine that deserves to be investigated. Among those orphan crops, the transcriptional comprehension of certain secondary metabolite synthesis has been initiated (Kang et al., 2020; Hu et al., 2021; Wang et al., 2021a,b) paving the way for not only effective JA triggered health-oriented metabolic engineering but also engineered crop protection against adverse biotic and abiotic stresses. Under the ongoing environmental change status, JAs’ mediated metabolite elicitation machinery needs further investigation in diverse plant systems. Moreover, the synthetic biology approach offers a novel path to improve the production ratio of secondary metabolites and deserves more attention from scientists.
Author contributions
S-IS and SP conceived the review. S-IS, KR, ML, and SB wrote the manuscript. S-IS, YZ, JS, and MR made a critical revision of the review. SP, KR, ML, ST, and YZ performed the literature search. S-IS, ST, and SB prepared figures and tables. All authors contributed to the article and approved the submitted version.
Funding
This study was carried out with the support of Research Program for Agricultural Science and Technology Development (project no. PJ01672604), Rural Development Administration and 2022 Post-Doctoral Fellowship Program (project no. PJ01494301 and PJ01672604; SP and ST), and National Institute of Agricultural Sciences, Rural Development Administration, Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelazeez, W. M. A., Anatolievna, K. Y., Zavdetovna, K. L., Damirovna, A. G., Abou El-Dis, G. R., and Arnoldovna, T. O. (2022). Correction to: enhanced productivity of atropine in cell suspension culture of Hyoscyamus muticus L. Vitr. Cell. Dev. Biol. Plant. 1–13. doi: 10.1007/s11627-022-10273-w
Afrin, S., Huang, J.-J., and Luo, Z.-Y. (2015). JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 60, 1062–1072. doi: 10.1007/s11434-015-0813-0
Ahn, S. Y., Kim, S. A., Cho, K. S., and Yun, H. K. (2014). Expression of genes related to flavonoid and stilbene synthesis as affected by signaling chemicals and Botrytis cinerea in grapevines. Biol. Plant. 58, 758–767. doi: 10.1007/s10535-014-0437-2
Ali, M., Abbasi, B. H., and Ali, G. S. (2015). Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 120, 1099–1106. doi: 10.1007/s11240-014-0666-2
Ali, M., Mujib, A., Gulzar, B., and Zafar, N. (2019). Essential oil yield estimation by gas chromatography–mass spectrometry (GC–MS) after methyl jasmonate (MeJA) elicitation in in vitro cultivated tissues of Coriandrum sativum L. 3 Biotech 9, 1–16. doi: 10.1007/s13205-019-1936-9
Ali, M. B., Yu, K.-W., Hahn, E.-J., and Paek, K.-Y. (2006). Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 25, 613–620. doi: 10.1007/s00299-005-0065-6
Almagro, L., Gutierrez, J., Pedreño, M. A., and Sottomayor, M. (2014). Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult. 119, 543–551. doi: 10.1007/s11240-014-0554-9
Alvarado-Orea, I. V., Paniagua-Vega, D., Capataz-Tafur, J., Torres-López, A., Vera-Reyes, I., García-López, E., et al. (2020). Photoperiod and elicitors increase steviol glycosides, phenolics, and flavonoid contents in root cultures of Stevia rebaudiana. Vitr. Cell. Dev. Biol. 56, 298–306. doi: 10.1007/s11627-019-10041-3
Andersson, M. X., Hamberg, M., Kourtchenko, O., Brunnstrom, Å., McPhail, K. L., Gerwick, W. H., et al. (2006). Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana: formation of a novel oxo-phytodienoic acid-containing galactolipid, arabidopside E*. J. Biol. Chem. 281, 31528–31537. doi: 10.1016/S0021-9258(19)84066-8
Avramova, Z. (2017). The jasmonic acid-signalling and abscisic acid-signalling pathways cross talk during one, but not repeated, dehydration stress: a non-specific ‘panicky’ or a meaningful response? Plant Cell Environ. 40, 1704–1710. doi: 10.1111/pce.12967
Ayothi, P., Muthu, A., and Shanmugam, K. (2021). Iron and methyl jasmonate increase high-value PUFA production by elevating the expression of desaturase genes in marine microalga Isochrysis sp. J. Appl. Microbiol. 132, 2042–2053.
Baek, S., Ho, T.-T., Lee, H., Jung, G., Kim, Y. E., Jeong, C.-S., et al. (2020). Enhanced biosynthesis of triterpenoids in Centella asiatica hairy root culture by precursor feeding and elicitation. Plant Biotechnol. Rep. 14, 45–53. doi: 10.1007/s11816-019-00573-w
Baenas, N., García-Viguera, C., and Moreno, D. A. (2014). Elicitation: a tool for enriching the bioactive composition of foods. Molecules 19, 13541–13563. doi: 10.3390/molecules190913541
Balbi, V., and Devoto, A. (2008). Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 177, 301–318. doi: 10.1111/j.1469-8137.2007.02292.x
Beerhues, L., and Berger, U. (1995). Differential accumulation of xanthones in methyl-jasmonate-and yeast-extract-treated cell cultures of Centaurium erythraea and Centaurium littorale. Planta 197, 608–612. doi: 10.1007/BF00191567
Bhambhani, S., Karwasara, V. S., Dixit, V. K., and Banerjee, S. (2012). Enhanced production of vasicine in Adhatoda vasica (L.) Nees. Cell culture by elicitation. Acta Physiol. Plant. 34, 1571–1578. doi: 10.1007/s11738-011-0921-7
Biondi, S., Fornale, S., Oksman-Caldentey, K.-M., Eeva, M., Agostani, S., and Bagni, N. (2000). Jasmonates induce over-accumulation of methylputrescine and conjugated polyamines in Hyoscyamus muticus L. root cultures. Plant Cell Rep. 19, 691–697. doi: 10.1007/s002999900178
Boter, M., Golz, J. F., Giménez-Ibañez, S., Fernandez-Barbero, G., Franco-Zorrilla, J. M., and Solano, R. (2015). Filamentous flower is a direct target of JAZ3 and modulates responses to Jasmonate. Plant Cell 27, 3160–3174. doi: 10.1105/tpc.15.00220
Brioudes, F., Joly, C., Szécsi, J., Varaud, E., Leroux, J., Bellvert, F., et al. (2009). Jasmonate controls late development stages of petal growth in Arabidopsis thaliana. Plant J. 60, 1070–1080. doi: 10.1111/j.1365-313X.2009.04023.x
Browse, J. (2005). Jasmonate: an oxylipin signal with many roles in plants. Vitamins & Hormones 72, 431–456. doi: 10.1016/S0083-6729(05)72012-4
Caldelari, D., Wang, G., Farmer, E. E., and Dong, X. (2011). Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol. Biol. 75, 25–33. doi: 10.1007/s11103-010-9701-9
Camarena-Rangel, N. G., Barba-De la Rosa, A. P., Herrera-Corredor, J. A., and del Socorro Santos-Diaz, M. (2017). Enhanced production of metabolites by elicitation in Opuntia ficus-indica, Opuntia megacantha, and Opuntia streptacantha callus. Plant Cell Tissue Organ Cult. 129, 289–298. doi: 10.1007/s11240-017-1177-8
Cao, S., Cai, Y., Yang, Z., Joyce, D. C., and Zheng, Y. (2014). Effect of MeJA treatment on polyamine, energy status and anthracnose rot of loquat fruit. Food Chem. 145, 86–89. doi: 10.1016/j.foodchem.2013.08.019
Cao, X., Guo, X., Yang, X., Wang, H., Hua, W., He, Y., et al. (2016). Transcriptional responses and Gentiopicroside biosynthesis in methyl Jasmonate-treated Gentiana macrophylla seedlings. PLoS One 11:e0166493. doi: 10.1371/journal.pone.0166493
Chang, L., Wu, S., and Tian, L. (2021). Methyl jasmonate elicits distinctive hydrolyzable tannin, flavonoid, and phyto-oxylipin responses in pomegranate (Punica granatum L.) leaves. Planta 254, 1–19. doi: 10.1007/s00425-021-03735-9
Chavan, J. J., Kshirsagar, P. R., Jadhav, S. G., Nalavade, V. M., Gurme, S. T., and Pai, S. R. (2021). Elicitor-mediated enhancement of biomass, polyphenols, mangiferin production and antioxidant activities in callus cultures of Salacia chinensis L. 3 Biotech 11, 1–11. doi: 10.1007/s13205-021-02836-2
Chen, J., Sonobe, K., Ogawa, N., Masuda, S., Nagatani, A., Kobayashi, Y., et al. (2013). Inhibition of arabidopsis hypocotyl elongation by jasmonates is enhanced under red light in phytochrome B dependent manner. J. Plant Res. 126, 161–168. doi: 10.1007/s10265-012-0509-3
Chen, Q., Sun, J., Zhai, Q., Zhou, W., Qi, L., Xu, L., et al. (2011). The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during Jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 23, 3335–3352. doi: 10.1105/tpc.111.089870
Chen, J., Wang, J., Wang, R., Xian, B., Ren, C., Liu, Q., et al. (2020). Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 20, 1–12. doi: 10.1186/s12870-020-02554-6
Cheruvathur, M. K., and Thomas, T. D. (2014). Effect of plant growth regulators and elicitors on rhinacanthin accumulation in hairy root cultures of Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue Organ Cult. 118, 169–177. doi: 10.1007/s11240-014-0473-9
Chico, J.-M., Fernández-Barbero, G., Chini, A., Fernández-Calvo, P., Díez-Díaz, M., and Solano, R. (2014). Repression of Jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. Plant Cell 26, 1967–1980. doi: 10.1105/tpc.114.125047
Chini, A., Gimenez-Ibanez, S., Goossens, A., and Solano, R. (2016). Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 33, 147–156. doi: 10.1016/j.pbi.2016.07.005
Chodisetti, B., Rao, K., Gandi, S., and Giri, A. (2015). Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules—methyl jasmonate and salicylic acid. Vitr. Cell. Dev. Biol. 51, 88–92. doi: 10.1007/s11627-014-9655-8
Chung, I.-M., Rekha, K., Rajakumar, G., and Thiruvengadam, M. (2017). Jasmonic and salicylic acids enhanced phytochemical production and biological activities in cell suspension cultures of spine gourd (Momordica dioica Roxb). Acta Biol. Hung. 68, 88–100. doi: 10.1556/018.68.2017.1.8
Cui, H.-Y., Baque, M. A., Lee, E.-J., and Paek, K.-Y. (2013). Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol. Rep. 7, 297–308. doi: 10.1007/s11816-012-0263-y
Danaee, M., Farzinebrahimi, R., Kadir, M. A., Sinniah, U. R., Mohamad, R., and Taha, R. M. (2015). Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Brazilian J. Bot. 38, 265–272. doi: 10.1007/s40415-015-0140-3
Dasari, R., Gopu, C., Vankudoth, S., Dharavath, S., and Taduri, S. (2020). Enhancement of production of pharmaceutically important anti-cancerous compound; cucurbitacin E via elicitation and precursor feeding of in vitro culture of Citrullus colocynthis (L.) Schard. Vegetos 33, 323–334. doi: 10.1007/s42535-020-00110-z
Datta, A. K., Das, A., Bhattacharya, A., Mukherjee, S., and Ghosh, B. K. (2011). An overview on Withania somnifera (L.) Dunal _ The _ Indian ginseng@_. In.
Dave, A., Hernández, M. L., He, Z., Andriotis, V. M. E., Vaistij, F. E., Larson, T. R., et al. (2011). 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23, 583–599. doi: 10.1105/tpc.110.081489
De Geyter, N., Gholami, A., Goormachtig, S., and Goossens, A. (2012). Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 17, 349–359. doi: 10.1016/j.tplants.2012.03.001
Demole, E., Lederer, E., and Mercier, D. (1962). Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant caractéristique de l’essence de jasmin. Helv. Chim. Acta 45, 675–685. doi: 10.1002/hlca.19620450233
Dučaiová, Z., Sajko, M., Mihaličová, S., and Repčák, M. (2016). Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 79, 81–94. doi: 10.1007/s10725-015-0114-2
Esfahani, S. T., Karimzadeh, G., Naghavi, M. R., and Vrieling, K. (2021). Altered gene expression and root thebaine production in polyploidized and methyl jasmonate-elicited Papaver bracteatum Lindl. Plant Physiol. Biochem. 158, 334–341. doi: 10.1016/j.plaphy.2020.11.021
Fang, Y., Smith, M. A. L., and Pépin, M.-F. (1999). Effects of exogenous methyl jasmonate in elicited anthocyanin-producing cell cultures of ohelo (Vaccinium phalae). Vitr. Cell. Dev. Biol. 35, 106–113. doi: 10.1007/s11627-999-0019-8
Feng, Y., Zhao, Y., Ha, Y., Li, J., Su, Z., Quan, X., et al. (2021). Drought stress-induced methyl jasmonate accumulation promotes calycosin-7-O-β-d-glucoside production in Astragalus membranaceus adventitious roots. Plant Cell Tiss. Organ. Cult. 147, 561–568. doi: 10.1007/s11240-021-02147-7
Flores-Sanchez, I. J., Paniagua-Vega, D., Vera-Reyes, I., Cerda-Garcia-Rojas, C. M., and Ramos-Valdivia, A. C. (2016). Alkaloid biosynthesis and metabolic profiling responses to jasmonic acid elicitation in Hamelia patens plants by NMR-based metabolomics. Metabolomics 12:66. doi: 10.1007/s11306-016-0999-4
Furmanowa, M., Glowniak, K., Syklowska-Baranek, K., Zgórka, G., and Józefczyk, A. (1997). Effect of picloram and methyl jasmonate on growth and taxane accumulation in callus culture of Taxus× media var. Hatfieldii. Plant Cell. Tissue Organ Cult. 49, 75–79. doi: 10.1023/A:1005858329430
Gadzovska, S., Maury, S., Delaunay, A., Spasenoski, M., Joseph, C., and Hagege, D. (2007). Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ Cult. 89, 1–13. doi: 10.1007/s11240-007-9203-x
Gai, Q.-Y., Jiao, J., Wang, X., Zang, Y.-P., Niu, L.-L., and Fu, Y.-J. (2019). Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult. 137, 77–86. doi: 10.1007/s11240-018-01553-8
Gajurel, G., Hasan, R., and Medina-Bolivar, F. (2021). Antioxidant assessment of prenylated stilbenoid-rich extracts from elicited hairy root cultures of three cultivars of peanut (Arachis hypogaea). Molecules 26:6778. doi: 10.3390/molecules26226778
Gajurel, G., Nopo-Olazabal, L., Hendrix, E., and Medina-Bolivar, F. (2022). Production and secretion of Cajaninstilbene acid in hairy root cultures of pigeon pea (Cajanus cajan) co-treated with multiple elicitors. Plan. Theory 11:834. doi: 10.3390/plants11060834
Gasperini, D., Chételat, A., Acosta, I. F., Goossens, J., Pauwels, L., Goossens, A., et al. (2015). Multilayered Organization of Jasmonate Signalling in the regulation of root growth. PLoS Genet. 11:e1005300. doi: 10.1371/journal.pgen.1005300
Gharari, Z., Bagheri, K., Danafar, H., and Sharafi, A. (2020). Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol. Biochem. 148, 35–44. doi: 10.1016/j.plaphy.2020.01.002
Ghorbel, M., Brini, F., Sharma, A., and Landi, M. (2021). Role of jasmonic acid in plants: the molecular point of view. Plant Cell Rep. 40, 1471–1494. doi: 10.1007/s00299-021-02687-4
Gimenez-Ibanez, S., Boter, M., Fernández-Barbero, G., Chini, A., Rathjen, J. P., and Solano, R. (2014). The bacterial effector HopX1 targets JAZ transcriptional repressors to activate Jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 12:e1001792. doi: 10.1371/journal.pbio.1001792
Giri, C. C., and Zaheer, M. (2016). Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 126, 1–18. doi: 10.1007/s11240-016-0985-6
Gómez, K., Quenguan, F., Aristizabal, D., Escobar, G., Quiñones, W., García-Beltrán, O., et al. (2022). Elicitation of isoflavonoids in Colombian edible legume plants with jasmonates and structurally related compounds. Heliyon 8:e08979. doi: 10.1016/j.heliyon.2022.e08979
Guan, L. L., Denkert, N., Eisa, A., Lehmann, M., Sjuts, I., Weiberg, A., et al. (2019). JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proc. Natl. Acad. Sci. 116, 10568–10575. doi: 10.1073/pnas.1900482116
Gutiérrez-Carbajal, M. G., Monforte-González, M., de Miranda-Ham, M. L., Godoy-Hernández, G., and Vázquez-Flota, F. (2010). Induction of capsaicinoid synthesis in Capsicum chinense cell cultures by salicylic acid or methyl jasmonate. Biol. Plant. 54, 430–434. doi: 10.1007/s10535-010-0078-z
Hao, Y.-J., Cui, X.-H., Li, J.-R., An, X.-L., Sun, H.-D., Piao, X.-C., et al. (2020). Cell bioreactor culture of Orostachys cartilaginous A. Bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis. Acta Physiol. Plant. 42, 1–10. doi: 10.1007/s11738-019-3008-5
Hao, H., Lei, C., Dong, Q., Shen, Y., Chi, J., Ye, H., et al. (2014). Effects of exogenous methyl jasmonate on the biosynthesis of shikonin derivatives in callus tissues of Arnebia euchroma. Appl. Biochem. Biotechnol. 173, 2198–2210. doi: 10.1007/s12010-014-1025-9
Hao, X., Shi, M., Cui, L., Xu, C., Zhang, Y., and Kai, G. (2015). Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 62, 24–31. doi: 10.1002/bab.1236
Ho, T.-T., Murthy, H. N., and Park, S.-Y. (2020). Methyl Jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 21:716. doi: 10.3390/ijms21030716
Hu, Y., Ma, D., Ning, S., Ye, Q., Zhao, X., Ding, Q., et al. (2021). High-quality genome of the medicinal plant Strobilanthes cusia provides insights into the biosynthesis of Indole alkaloids. Front. Plant Sci. 12:742420. doi: 10.3389/fpls.2021.742420
Huang, H., Liu, B., Liu, L., and Song, S. (2017). Jasmonate action in plant growth and development. J. Exp. Bot. 68, 1349–1359. doi: 10.1093/jxb/erw495
Inyushkina, Y. V., Bulgakov, V. P., Veselova, M. V., Bryukhanov, V. M., Zverev, Y. F., Lampatov, V. V., et al. (2007). High Rabdosiin and Rosmarinic acid production in Eritrichium sericeum Callus cultures and the effect of the Calli on Masugi-nephritis in rats. Biosci. Biotechnol. Biochem. 71, 1286–1293. doi: 10.1271/bbb.60684
Jaremicz, Z., Luczkiewicz, M., Kokotkiewicz, A., Krolicka, A., and Sowinski, P. (2014). Production of tropane alkaloids in Hyoscyamus niger (black henbane) hairy roots grown in bubble-column and spray bioreactors. Biotechnol. Lett. 36, 843–853. doi: 10.1007/s10529-013-1426-9
Jeong, Y. J., An, C. H., Park, S.-C., Pyun, J. W., Lee, J., Kim, S. W., et al. (2018). Methyl jasmonate increases isoflavone production in soybean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J. Agric. Food Chem. 66, 4099–4105. doi: 10.1021/acs.jafc.8b00350
Jewell, J. B., and Browse, J. (2016). Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis. Plant J. 85, 634–647. doi: 10.1111/tpj.13131
Ji, J., Feng, Q., Sun, H., Zhang, X., Li, X., Li, J., et al. (2019). Response of bioactive metabolite and biosynthesis related genes to methyl jasmonate elicitation in Codonopsis pilosula. Molecules 24:533. doi: 10.3390/molecules24030533
Jiang, Y., Liang, G., Yang, S., and Yu, D. (2014). Arabidopsis WRKY57 functions as a node of convergence for Jasmonic acid– and Auxin-mediated signaling in Jasmonic acid–induced leaf senescence. Plant Cell 26, 230–245. doi: 10.1105/tpc.113.117838
Jirakiattikul, Y., Rithichai, P., Boonyeun, T., Ruangnoo, S., and Itharat, A. (2020). Improvement of dioscorealide B production by elicitation in shoot cultures of Dioscorea membranacea Pierre ex Prain & Burkill. Physiol. Mol. Biol. Plants 26, 585–591. doi: 10.1007/s12298-020-00762-w
Kang, S.-H., Pandey, R. P., Lee, C.-M., Sim, J.-S., Jeong, J.-T., Choi, B.-S., et al. (2020). Genome-enabled discovery of anthraquinone biosynthesis in Senna tora. Nat. Commun. 11:5875. doi: 10.1038/s41467-020-19681-1
Kantayos, V., Kim, J.-S., and Baek, S.-H. (2021). Alteration of resveratrol-dependent glycosyltransferase activity by elicitation in DJ-526 rice. GM Crops Food 12, 242–250. doi: 10.1080/21645698.2020.1859314
Karimi, A. A., Naghavi, M. R., Peyghambari, S. A., and Rasoulnia, A. (2021). Inulin content and expression of related genes in different tissues and cell suspension culture of Taraxacum kok-saghyz. Vitr. Cell. Dev. Biol. 57, 1009–1017. doi: 10.1007/s11627-021-10180-6
Kashani, K., Sabet, M. S., Jalali Javaran, M., and Moieni, A. (2022). Bottleneck removal of paclitaxel biosynthetic pathway by overexpression of DBTNBT gene under methyl-β-cyclodextrin and coronatine elicitation in Taxus baccata L. Plant Cell Tissue Organ Cult. 149, 485–495. doi: 10.1007/s11240-022-02279-4
Khanam, M. N., Anis, M., Javed, S. B., Mottaghipisheh, J., and Csupor, D. (2022). Adventitious root culture—an alternative strategy for secondary metabolite production: a review. Agron. 12:1178. doi: 10.3390/agronomy12051178
Khataee, E., Karimi, F., and Razavi, K. (2019). Alkaloids production and antioxidant properties in Catharanthus roseus (L.) G. Don. Shoots and study of alkaloid biosynthesis-related gene expression levels in response to methyl jasmonate and putrescine treatments as eco-friendly elicitors. Biol. Futur. 70, 38–46. doi: 10.1556/019.70.2019.05
Kianersi, F., Pour-Aboughadareh, A., Majdi, M., and Poczai, P. (2021). Effect of methyl Jasmonate on Thymol, Carvacrol, phytochemical accumulation, and expression of key genes involved in Thymol/Carvacrol biosynthetic pathway in Some Iranian thyme species. Int. J. Mol. Sci. 22:11124. doi: 10.3390/ijms222011124
Kim, O. T., Bang, K. H., Kim, Y. C., Hyun, D. Y., Kim, M. Y., and Cha, S. W. (2009). Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. 98, 25–33. doi: 10.1007/s11240-009-9535-9
Kim, J., Chang, C., and Tucker, M. L. (2015). To grow old: regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 6:20. doi: 10.3389/fpls.2015.00020
Kim, Y.-S., Yeung, E. C., Hahn, E.-J., and Paek, K.-Y. (2007). Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng CA Meyer. Biotechnol. Lett. 29, 1789–1792. doi: 10.1007/s10529-007-9442-2
Kowalczyk, T., Sitarek, P., Merecz-Sadowska, A., Szyposzyńska, M., Spławska, A., Gorniak, L., et al. (2021). Methyl Jasmonate effect on Betulinic acid content and biological properties of extract from Senna obtusifolia transgenic hairy roots. Molecules 26:6208. doi: 10.3390/molecules26206208
Krzemińska, M., Owczarek, A., Gonciarz, W., Chmiela, M., Olszewska, M. A., and Grzegorczyk-Karolak, I. (2022). The antioxidant, cytotoxic and antimicrobial potential of phenolic acids-enriched extract of elicited hairy roots of Salvia bulleyana. Molecules 27:992. doi: 10.3390/molecules27030992
Kwiecień, I., Miceli, N., D’Arrigo, M., Marino, A., and Ekiert, H. (2022). Antioxidant potential and enhancement of bioactive metabolite production in in vitro cultures of Scutellaria lateriflora L. by biotechnological methods. Molecules 27:1140. doi: 10.3390/molecules27031140
Lan, W. Z., Yu, L. J., Cheng, P., and Li, M. Y. (2002). Improvement of cephalomanine production in Taxus chinensis cells by a combination of sucrose and methyl jasmonate. Biotechnol. Lett. 24, 1253–1255. doi: 10.1023/A:1016269627346
Largia, M. J. V., Pothiraj, G., Shilpha, J., and Ramesh, M. (2015). Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.). Plant Cell Tissue Organ Cult. 122, 9–20. doi: 10.1007/s11240-015-0745-z
Lee, E. J., Park, S. Y., and Paek, K. Y. (2015). Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tissue Organ Cult. 120, 1–10. doi: 10.1007/s11240-014-0567-4
Li, J., Li, B., Luo, L., Cao, F., Yang, B., Gao, J., et al. (2020). Increased phenolic acid and tanshinone production and transcriptional responses of biosynthetic genes in hairy root cultures of Salvia przewalskii maxim. Treated with methyl jasmonate and salicylic acid. Mol. Biol. Rep. 47, 8565–8578. doi: 10.1007/s11033-020-05899-1
Li, Q., Xu, J., Zheng, Y., Zhang, Y., and Cai, Y. (2021). Transcriptomic and Metabolomic analyses reveals that exogenous methyl Jasmonate regulates Galanthamine biosynthesis in Lycoris longituba seedlings. Front. Plant Sci. 12:713795. doi: 10.3389/fpls.2021.713795
Li, Y., Zhao, L., Cui, L., Lei, J., and Zhang, X. (2015). Effects of elicitors on growth of adventitious roots and contents of secondary metabolites in Tripterygium wilfordii Hook. f. Sheng wu gong Cheng xue bao = Chinese J. Biotechnol. 31, 734–743.
Liu, J.-P., Hu, J., Liu, Y.-H., Yang, C.-P., Zhuang, Y.-F., Guo, X.-L., et al. (2018). Transcriptome analysis of Hevea brasiliensis in response to exogenous methyl jasmonate provides novel insights into regulation of jasmonate-elicited rubber biosynthesis. Physiol. Mol. Biol. Plants 24, 349–358. doi: 10.1007/s12298-018-0529-0
López, C. Q., Corral, P., Lorrain-Lorrette, B., Martinez-Swatson, K., Michoux, F., and Simonsen, H. T. (2018). Use of a temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot cultures of Thapsia garganica. Plant Methods 14, 1–17. doi: 10.1186/s13007-018-0346-z
Lu, X., Zhang, F., Shen, Q., Jiang, W., Pan, Q., Lv, Z., et al. (2014). Overexpression of Allene oxide Cyclase improves the biosynthesis of Artemisinin in Artemisia annua L. PLoS One 9:e91741. doi: 10.1371/journal.pone.0091741
Lundborg, L., Sampedro, L., Borg-Karlson, A.-K., and Zas, R. (2019). Effects of methyl jasmonate on the concentration of volatile terpenes in tissues of maritime pine and Monterey pine and its relation to pine weevil feeding. Trees 33, 53–62. doi: 10.1007/s00468-018-1757-1
Maciel, G., Lopes, A. A., Cantrell, C. L., de Castro França, S., Bertoni, B. W., and Lourenço, M. V. (2021). Jasmonates promote enhanced production of bioactive caffeoylquinic acid derivative in Eclipta prostrata (L.) L. hairy roots. Plant Cell Tissue Organ Cult. 149, 363–369. doi: 10.1007/s11240-021-02201-4
Majdi, M., Abdollahi, M. R., and Maroufi, A. (2015). Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant Cell Rep. 34, 1909–1918. doi: 10.1007/s00299-015-1837-2
Malarz, J., Stojakowska, A., and Kisiel, W. (2007). Effect of methyl jasmonate and salicylic acid on sesquiterpene lactone accumulation in hairy roots of Cichorium intybus. Acta Physiol. Plant. 29, 127–132. doi: 10.1007/s11738-006-0016-z
Melotto, M., Underwood, W., Koczan, J., Nomura, K., and He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. doi: 10.1016/j.cell.2006.06.054
Mendoza, D., Arias, J. P., Cuaspud, O., Ruiz, O., and Arias, M. (2020). FT-NIR spectroscopy and RP-HPLC combined with multivariate analysis reveals differences in plant cell suspension cultures of Thevetia peruviana treated with salicylic acid and methyl jasmonate. Biotechnol. Reports 27:e00519. doi: 10.1016/j.btre.2020.e00519
Miao, G. P., Zhu, C. S., Yang, Y. Q., Feng, M. X., Ma, Z. Q., Feng, J. T., et al. (2014). Elicitation and in situ adsorption enhanced secondary metabolites production of Tripterygium wilfordii Hook. f. Adventitious root fragment liquid cultures in shake flask and a modified bubble column bioreactor. Bioprocess Biosyst. Eng. 37, 641–650. doi: 10.1007/s00449-013-1033-0
Miksch, M., and Boland, W. (1996). Airborne methyl jasmonate stimulates the biosynthesis of furanocoumarins in the leaves of celery plants (Apium graveolens). Experientia 52, 739–743. doi: 10.1007/BF01925585
Mizukami, H., Tabira, Y., and Ellis, B. E. (1993). Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 12, 706–709. doi: 10.1007/BF00233424
Monte, I., Hamberg, M., Chini, A., Gimenez-Ibanez, S., García-Casado, G., Porzel, A., et al. (2014). Rational design of a ligand-based antagonist of jasmonate perception. Nat. Chem. Biol. 10, 671–676. doi: 10.1038/nchembio.1575
Mosblech, A., Thurow, C., Gatz, C., Feussner, I., and Heilmann, I. (2011). Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 65, 949–957. doi: 10.1111/j.1365-313X.2011.04480.x
Nabi, N., Singh, S., and Saffeullah, P. (2021). Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. Vitr. Cell. Dev. Biol. Plant 57, 341–355. doi: 10.1007/s11627-020-10140-6
Namdeo, A. G. (2007). Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn. Rev. 1:69.
Nguyen, T. H., Goossens, A., and Lacchini, E. (2022). Jasmonate: a hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 67:102197. doi: 10.1016/j.pbi.2022.102197
Onrubia, M., Moyano, E., Bonfill, M., Cusidó, R. M., Goossens, A., and Palazón, J. (2013). Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J. Plant Physiol. 170, 211–219. doi: 10.1016/j.jplph.2012.09.004
Paeizi, M., Karimi, F., and Razavi, K. (2018). Changes in medicinal alkaloids production and expression of related regulatory and biosynthetic genes in response to silver nitrate combined with methyl jasmonate in Catharanthus roseus in vitro propagated shoots. Plant Physiol. Biochem. 132, 623–632. doi: 10.1016/j.plaphy.2018.10.015
Pan, W., Liu, K., Guan, Y., Tan, G. T., Hung, N.Van, Cuong, N. M., et al. (2014). Bioactive compounds from Vitex leptobotrys. J. Nat. Prod. 77, 663–667. doi: 10.1021/np400779v
Pandey, P., Singh, S., and Banerjee, S. (2019). Ocimum basilicum suspension culture as resource for bioactive triterpenoids: yield enrichment by elicitation and bioreactor cultivation. Plant Cell Tissue Organ Cult. 137, 65–75. doi: 10.1007/s11240-018-01552-9
Park, J.-A., Park, B.-J., Kim, A.-H., Park, S.-Y., and Paek, K.-Y. (2015). Airlift bioreactor system and nitrogen sources for biomass and antioxidant compound production from in vitro culture of Vitis flexuosa plantlets. Hortic. Environ. Biotechnol. 56, 358–365. doi: 10.1007/s13580-015-0006-4
Pauwels, L., Inzé, D., and Goossens, A. (2009). Jasmonate-inducible gene: what does it mean? Trends Plant Sci. 14, 87–91. doi: 10.1016/j.tplants.2008.11.005
Perassolo, M., Smith, M. E., Giulietti, A. M., and Talou, J. R. (2016). Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tissue Organ Cult. 124, 319–330. doi: 10.1007/s11240-015-0896-y
Pillai, S. K., and Siril, E. A. (2022). Exogenous elicitors enhanced Berberine production in the cell suspension cultures of Tinospora cordifolia (Willd.) Miers ex hook F. & Thoms. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 92, 209–218. doi: 10.1007/s40011-021-01310-6
Premathilake, A. T., Ni, J., Shen, J., Bai, S., and Teng, Y. (2020). Transcriptome analysis provides new insights into the transcriptional regulation of methyl jasmonate-induced flavonoid biosynthesis in pear calli. BMC Plant Biol. 20:388. doi: 10.1186/s12870-020-02606-x
Ptak, A., Morańska, E., Saliba, S., Zieliński, A., Simlat, M., and Laurain-Mattar, D. (2017). Elicitation of galanthamine and lycorine biosynthesis by Leucojum aestivum L. and L. aestivum ‘Gravety Giant’plants cultured in bioreactor RITA®. Plant Cell Tissue Organ Cult. 128, 335–345. doi: 10.1007/s11240-016-1113-3
Qi, T., Huang, H., Song, S., and Xie, D. (2015). Regulation of Jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 27, 1620–1633. doi: 10.1105/tpc.15.00116
Rad, M. M., Abdossi, V., Moradi, P., Rakhshandehroo, F., and Mehrafarin, A. (2022). Phytochemical changes of Digitalis purpurea L. in response to polyamines and methyl jasmonate application in callus culture. J. Plant Biochem. Biotechnol. 31, 310–319. doi: 10.1007/s13562-021-00678-w
Rahimi, S., Hasanloo, T., Najafi, F., and Khavari-Nejad, R. A. (2012). Methyl jasmonate influence on silymarin production and plant stress responses in Silybum marianum hairy root cultures in a bioreactor. Nat. Prod. Res. 26, 1662–1667. doi: 10.1080/14786419.2011.593518
Rahimi, S., Kim, Y.-J., Devi, B. S. R., Oh, J. Y., Kim, S.-Y., Kwon, W.-S., et al. (2016). Sodium nitroprusside enhances the elicitation power of methyl jasmonate for ginsenoside production in Panax ginseng roots. Res. Chem. Intermed. 42, 2937–2951. doi: 10.1007/s11164-015-2188-x
Rahimi, S., Kim, Y.-J., and Yang, D.-C. (2015). Production of ginseng saponins: elicitation strategy and signal transductions. Appl. Microbiol. Biotechnol. 99, 6987–6996. doi: 10.1007/s00253-015-6806-8
Rajan, M., Feba, K. S., Chandran, V., Shahena, S., and Mathew, L. (2020). Enhancement of rhamnetin production in Vernonia anthelmintica (L.) Willd. Cell suspension cultures by eliciting with methyl jasmonate and salicylic acid. Physiol. Mol. Biol. Plants 26, 1531–1539. doi: 10.1007/s12298-020-00829-8
Ram, M., Prasad, K. V., Singh, S. K., Hada, B. S., and Kumar, S. (2013). Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue Organ Cult. 113, 459–467. doi: 10.1007/s11240-013-0287-1
Raomai, S., Kumaria, S., Kehie, M., and Tandon, P. (2015). Plantlet regeneration of Paris polyphylla Sm. Via thin cell layer culture and enhancement of steroidal saponins in mini-rhizome cultures using elicitors. Plant Growth Regul. 75, 341–353. doi: 10.1007/s10725-014-9957-1
Reeves, P. H., Ellis, C. M., Ploense, S. E., Wu, M.-F., Yadav, V., Tholl, D., et al. (2012). A regulatory network for coordinated Flower maturation. PLoS Genet. 8:e1002506. doi: 10.1371/journal.pgen.1002506
Reinbothe, C., Springer, A., Samol, I., and Reinbothe, S. (2009). Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 276, 4666–4681. doi: 10.1111/j.1742-4658.2009.07193.x
Robson, F., Okamoto, H., Patrick, E., Harris, S.-R., Wasternack, C., Brearley, C., et al. (2010). Jasmonate and Phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22, 1143–1160. doi: 10.1105/tpc.109.067728
Ru, M., Li, Y., Guo, M., Chen, L., Tan, Y., Peng, L., et al. (2022). Increase in rosmarinic acid accumulation and transcriptional responses of synthetic genes in hairy root cultures of Prunella vulgaris induced by methyl jasmonate. Plant Cell Tissue Organ Cult. 149, 371–379. doi: 10.1007/s11240-022-02273-w
Ruan, J., Zhou, Y., Zhou, M., Yan, J., Khurshid, M., Weng, W., et al. (2019). Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 20:2479. doi: 10.3390/ijms20102479
Rubio-Rodríguez, E., Vera-Reyes, I., Sepúlveda-García, E. B., Ramos-Valdivia, A. C., and Trejo-Tapia, G. (2021). Secondary metabolite production and related biosynthetic genes expression in response to methyl jasmonate in Castilleja tenuiflora Benth. In vitro plants. Plant Cell Tissue Organ Cult. 144, 519–532. doi: 10.1007/s11240-020-01975-3
Saeed, S., Ali, H., Khan, T., Kayani, W., and Khan, M. A. (2017). Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa wall ex Benth., a high valued endangered medicinal plant. Physiol. Mol. Biol. Plants 23, 229–237. doi: 10.1007/s12298-016-0406-7
Sanders, P. M., Lee, P. Y., Biesgen, C., Boone, J. D., Beals, T. P., Weiler, E. W., et al. (2000). The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12, 1041–1061. doi: 10.1105/tpc.12.7.1041
Savchenko, T., Kolla, V. A., Wang, C.-Q., Nasafi, Z., Hicks, D. R., Phadungchob, B., et al. (2014). Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 164, 1151–1160. doi: 10.1104/pp.113.234310
Sethi, V., Raghuram, B., Sinha, A. K., and Chattopadhyay, S. (2014). A mitogen-activated protein kinase Cascade module, MKK3-MPK6 and MYC2, is involved in blue light-mediated seedling development in Arabidopsis. Plant Cell 26, 3343–3357. doi: 10.1105/tpc.114.128702
Shabani, L., Ehsanpour, A. A., Asghari, G., and Emami, J. (2009). Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ. J. Plant Physiol. 56, 621–626. doi: 10.1134/S1021443709050069
Shabani, L., Ehsanpour, A. A., and Esmaeili, A. (2010). Assessment of squalene synthase and beta-amyrin synthase gene expression in licorice roots treated with methyl jasmonate and salicylic acid using real-time qPCR. Russ. J. Plant Physiol. 57, 480–484. doi: 10.1134/S1021443710040047
Shan, X., Zhang, Y., Peng, W., Wang, Z., and Xie, D. (2009). Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 60, 3849–3860. doi: 10.1093/jxb/erp223
Sharma, M., and Laxmi, A. (2016). Jasmonates: emerging players in controlling temperature stress tolerance. Front. Plant Sci. 6:1129. doi: 10.3389/fpls.2015.01129
Sharma, A., Rather, G. A., Misra, P., Dhar, M. K., and Lattoo, S. K. (2019). Jasmonate responsive transcription factor WsMYC2 regulates the biosynthesis of triterpenoid withanolides and phytosterol via key pathway genes in Withania somnifera (L.) Dunal. Plant Mol. Biol. 100, 543–560. doi: 10.1007/s11103-019-00880-4
Shilpha, J., Satish, L., Kavikkuil, M., Joe Virgin Largia, M., and Ramesh, M. (2015). Methyl jasmonate elicits the solasodine production and anti-oxidant activity in hairy root cultures of Solanum trilobatum L. Ind. Crop. Prod. 71, 54–64. doi: 10.1016/j.indcrop.2015.03.083
Shoji, T., and Hashimoto, T. (2015). Stress-induced expression of NICOTINE2-locus genes and their homologs encoding ethylene response factor transcription factors in tobacco. Phytochemistry 113, 41–49. doi: 10.1016/j.phytochem.2014.05.017
Simm, S., Papasotiriou, D. G., Ibrahim, M., Leisegang, M. S., Müller, B., Schorge, T., et al. (2013). Defining the core proteome of the chloroplast envelope membranes. Front. Plant Sci. 4:11. doi: 10.3389/fpls.2013.00011
Singh, M., Poddar, N. K., Singh, D., and Agrawal, S. (2020). Foliar application of elicitors enhanced the yield of withanolide contents in Withania somnifera (L.) Dunal (Variety, Poshita). 3 Biotech 10, 1–8. doi: 10.1007/s13205-020-2153-2
Song, S. H., and Byun, S. Y. (1998). Elicitation of camptothecin production in cell cultures of Camptotheca acuminata. Biotechnol. Bioprocess Eng. 3, 91–95. doi: 10.1007/BF02932509
Song, S., Huang, H., Gao, H., Wang, J., Wu, D., Liu, X., et al. (2014). Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between Jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26, 263–279. doi: 10.1105/tpc.113.120394
Song, S., Qi, T., Huang, H., Ren, Q., Wu, D., Chang, C., et al. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23, 1000–1013. doi: 10.1105/tpc.111.083089
Song, S., Qi, T., Huang, H., and Xie, D. (2013). Regulation of stamen development by coordinated actions of Jasmonate, Auxin, and gibberellin in Arabidopsis. Mol. Plant 6, 1065–1073. doi: 10.1093/mp/sst054
Srinath, M., Shailaja, A., Bindu, B. B. V., and Giri, C. C. (2021). Molecular cloning and differential gene expression analysis of 1-deoxy-D-xylulose 5-phosphate synthase (DXS) in Andrographis paniculata (Burm f) Nees. Mol. Biotechnol. 63, 109–124. doi: 10.1007/s12033-020-00287-3
Sukito, A., and Tachibana, S. (2016). Effect of methyl jasmonate and salycilic acid synergism on enhancement of bilobalide and ginkgolide production by immobilized cell cultures of Ginkgo biloba. Bioresour. Bioprocess 3, 1–11. doi: 10.1186/s40643-016-0101-0
Suzuki, H., Reddy, M. S. S., Naoumkina, M., Aziz, N., May, G. D., Huhman, D. V., et al. (2005). Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta 220, 696–707. doi: 10.1007/s00425-004-1387-2
Sykłowska-Baranek, K., Kamińska, M., Pączkowski, C., Pietrosiuk, A., and Szakiel, A. (2022). Metabolic modifications in Terpenoid and steroid pathways triggered by methyl Jasmonate in Taxus × media hairy roots. Plants 11:1120. doi: 10.3390/plants11091120
Syklowska-Baranek, K., Pietrosiuk, A., Kokoszka, A., and Furmanowa, M. (2009). Enhancement of taxane production in hairy root culture of Taxus x media var. Hicksii. J. Plant Physiol. 166, 1950–1954. doi: 10.1016/j.jplph.2009.05.001
Tachinbana, S., Muranaka, T., and Itoh, K. (2007). Effect of elicitors and a biogenetic precursor on paclitaxel production in cell suspension cultures of Taxus cuspidata var. nana. Pak. J. Biol. Sci. 10, 2856–2861. doi: 10.3923/pjbs.2007.2856.2861
Talebi, M., Moghaddam, M., and Pirbalouti, A. G. (2018). Methyl jasmonate effects on volatile oil compounds and antioxidant activity of leaf extract of two basil cultivars under salinity stress. Acta Physiol. Plant. 40, 1–11. doi: 10.1007/s11738-018-2611-1
Tang, G., Ma, J., Hause, B., Nick, P., and Riemann, M. (2020). Jasmonate is required for the response to osmotic stress in rice. Environ. Exp. Bot. 175:104047. doi: 10.1016/j.envexpbot.2020.104047
Thakur, G. S., Sharma, R., Sanodiya, B. S., Baghel, R. K., Thakur, R., Singh, B. N., et al. (2013). In vitro induction of tuber formation for the synthesis of secondary metabolites in Chlorophytum borivilianum Sant. Et Fernand. African J. Biotechnol. 12, 2900–2907.
Thanh, N. T., Murthy, H. N., Yu, K. W., Hahn, E. J., and Paek, K. Y. (2005). Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl. Microbiol. Biotechnol. 67, 197–201. doi: 10.1007/s00253-004-1759-3
Thatcher, L. F., Cevik, V., Grant, M., Zhai, B., Jones, J. D. G., Manners, J. M., et al. (2016). Characterization of a JAZ7 activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. J. Exp. Bot. 67, 2367–2386. doi: 10.1093/jxb/erw040
Theodoulou, F. L., Job, K., Slocombe, S. P., Footitt, S., Holdsworth, M., Baker, A., et al. (2005). Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 137, 835–840. doi: 10.1104/pp.105.059352
Thireault, C., Shyu, C., Yoshida, Y., St. Aubin, B., Campos, M. L., and Howe, G. A. (2015). Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J. 82, 669–679. doi: 10.1111/tpj.12841
Trujillo-Villanueva, K., Rubio-Piña, J., Monforte-González, M., Ramírez-Benítez, E., and Vázquez-Flota, F. (2012). The sequential exposure to jasmonate, salicylic acid and yeast extract promotes sanguinarine accumulation in Argemone mexicana cell cultures. Biotechnol. Lett. 34, 379–385. doi: 10.1007/s10529-011-0770-x
Udomsin, O., Yusakul, G., Kitisripanya, T., Juengwatanatrakul, T., and Putalun, W. (2020). The Deoxymiroestrol and Isoflavonoid production and their elicitation of cell suspension cultures of Pueraria candollei var. mirifica: from shake flask to bioreactor. Appl. Biochem. Biotechnol. 190, 57–72. doi: 10.1007/s12010-019-03094-y
Urbanek, H., Bergier, K., Saniewski, M., and Patykowski, J. (1996). Effect of jasmonates and exogenous polysaccharides on production of alkannin pigments in suspension cultures of Alkanna tinctoria. Plant Cell Rep. 15, 637–641. doi: 10.1007/BF00232468
Verpoorte, R., van der Heijden, R., and Memelink, J. (2000). Engineering the plant cell factory for secondary metabolite production. Transgenic Res. 9, 323–43; discussion 321. doi: 10.1023/A:1008966404981
Vyas, P., and Mukhopadhyay, K. (2018). Elicitation of Phenylpropanoids and expression analysis of PAL gene in suspension cell culture of Ocimum tenuiflorum L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 88, 1207–1217. doi: 10.1007/s40011-017-0858-8
Wang, H., Hao, J., Chen, X., Hao, Z., Wang, X., Lou, Y., et al. (2007). Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol. Biol. 65, 799–815. doi: 10.1007/s11103-007-9244-x
Wang, Y., Hou, Y., Qiu, J., Wang, H., Wang, S., Tang, L., et al. (2020). Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 228, 1336–1353. doi: 10.1111/nph.16774
Wang, X., Hu, H., Wu, Z., Fan, H., Wang, G., Chai, T., et al. (2021b). Tissue-specific transcriptome analyses reveal candidate genes for stilbene, flavonoid and anthraquinone biosynthesis in the medicinal plant Polygonum cuspidatum. BMC Genomics 22:353. doi: 10.1186/s12864-021-07658-3
Wang, R., Kumar, V., Sikron-Persi, N., Dynkin, I., Weiss, D., Perl, A., et al. (2022). Over 1000-fold synergistic boost in Viniferin levels by elicitation of Vitis vinifera cv. Gamay red cell cultures over accumulating phenylalanine. J. Agric. Food Chem. 70, 5049–5056. doi: 10.1021/acs.jafc.2c00107
Wang, J., Qian, J., Yao, L., and Lu, Y. (2015). Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2, 1–9. doi: 10.1186/s40643-014-0033-5
Wang, J., Xu, S., Mei, Y., Cai, S., Gu, Y., Sun, M., et al. (2021a). A high-quality genome assembly of Morinda officinalis, a famous native southern herb in the Lingnan region of southern China. Hortic. Res. 8:135. doi: 10.1038/s41438-021-00551-w
Wang, W., Zhao, Z.-J., Xu, Y., Qian, X., and Zhong, J.-J. (2005). Efficient elicitation of ginsenoside biosynthesis in cell cultures of Panax notoginseng by using self-chemically-synthesized jasmonates. Biotechnol. Bioprocess Eng. 10, 162–165. doi: 10.1007/BF02932587
Wasternack, C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100, 681–697. doi: 10.1093/aob/mcm079
Wasternack, C., and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann. Bot. 111, 1021–1058. doi: 10.1093/aob/mct067
Wasternack, C., and Hause, B. (2019). The missing link in jasmonic acid biosynthesis. Nat. Plants 5, 776–777. doi: 10.1038/s41477-019-0492-y
Wasternack, C., and Song, S. (2017). Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 68, 1303–1321. doi: 10.1093/jxb/erw443
Wasternack, C., and Strnad, M. (2016). Jasmonate signaling in plant stress responses and development – active and inactive compounds. New Biotechnol. 33, 604–613. doi: 10.1016/j.nbt.2015.11.001
Xiao, H.-M., Cai, W.-J., Ye, T.-T., Ding, J., and Feng, Y.-Q. (2018). Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal. Chim. Acta 1031, 119–127. doi: 10.1016/j.aca.2018.05.055
Xu, J., Wang, X., and Guo, W. (2015b). The cytochrome P450 superfamily: key players in plant development and defense. J. Integr. Agric. 14, 1673–1686. doi: 10.1016/S2095-3119(14)60980-1
Xu, A., Zhan, J.-C., and Huang, W.-D. (2015a). Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet sauvignon. Plant Cell Tissue Organ Cult. 122, 197–211. doi: 10.1007/s11240-015-0761-z
Yadav, V., Mallappa, C., Gangappa, S. N., Bhatia, S., and Chattopadhyay, S. (2005). A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 17, 1953–1966. doi: 10.1105/tpc.105.032060
Yan, Y., Christensen, S., Isakeit, T., Engelberth, J., Meeley, R., Hayward, A., et al. (2012). Disruption of OPR7 and OPR8 reveals the versatile functions of Jasmonic acid in maize development and defense. Plant Cell 24, 1420–1436. doi: 10.1105/tpc.111.094151
Yan, S., McLamore, E. S., Dong, S., Gao, H., Taguchi, M., Wang, N., et al. (2015). The role of plasma membrane H+-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J. 83, 638–649. doi: 10.1111/tpj.12915
Yan, C., and Xie, D. (2015). Jasmonate in plant defence: sentinel or double agent? Plant Biotechnol. J. 13, 1233–1240. doi: 10.1111/pbi.12417
Yan, J., Zhang, C., Gu, M., Bai, Z., Zhang, W., Qi, T., et al. (2009). The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21, 2220–2236. doi: 10.1105/tpc.109.065730
Yang, D.-L., Yao, J., Mei, C.-S., Tong, X.-H., Zeng, L.-J., Li, Q., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. U. S. A. 109, E1192–E1200. doi: 10.1073/pnas.1201616109
Yazdanian, E., Golkar, P., Vahabi, M. R., and Taghizadeh, M. (2021). Elicitation effects on Some secondary metabolites and antioxidant activity in callus cultures of Allium jesdianum Boiss. & Buhse.: methyl Jasmonate and Putrescine. Appl. Biochem. Biotechnol. 194, 601–619. doi: 10.1007/s12010-021-03643-4
Yin, Y., Adachi, Y., Nakamura, Y., Munemasa, S., Mori, I. C., and Murata, Y. (2016). Involvement of OST1 protein kinase and PYR/PYL/RCAR receptors in methyl Jasmonate-induced Stomatal closure in Arabidopsis guard cells. Plant Cell Physiol. 57, 1779–1790. doi: 10.1093/pcp/pcw102
Yoo, N. H., Kim, O. T., Kim, J. B., Kim, S. H., Kim, Y. C., Bang, K. H., et al. (2011). Enhancement of centelloside production from cultured plants of Centella asiatica by combination of thidiazuron and methyl jasmonate. Plant Biotechnol. Rep. 5, 283–287. doi: 10.1007/s11816-011-0173-4
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T., and Yamanaka, S. (2009). Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241. doi: 10.1016/j.stem.2009.08.001
Yousefian, S., Lohrasebi, T., Farhadpour, M., and Haghbeen, K. (2020). Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tissue Organ Cult. 142, 285–297. doi: 10.1007/s11240-020-01856-9
Yu, J., Zhang, Y., Di, C., Zhang, Q., Zhang, K., Wang, C., et al. (2016). JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis. J. Exp. Bot. 67, 751–762. doi: 10.1093/jxb/erv487
Zabala, M. A., Angarita, M., Restrepo, J. M., Caicedo, L. A., and Perea, M. (2010). Elicitation with methyl-jasmonate stimulates peruvoside production in cell suspension cultures of Thevetia peruviana. Vitr. Cell. Dev. Biol. 46, 233–238. doi: 10.1007/s11627-009-9249-z
Zaheer, M., and Giri, C. C. (2017). Enhanced diterpene lactone (andrographolide) production from elicited adventitious root cultures of Andrographis paniculata. Res. Chem. Intermed. 43, 2433–2444. doi: 10.1007/s11164-016-2771-9
Zambounis, A. G., Kalamaki, M. S., Tani, E. E., Paplomatas, E. J., and Tsaftaris, A. S. (2012). Expression analysis of defense-related genes in cotton (Gossypium hirsutum) after Fusarium oxysporum f. sp. vasinfectum infection and following chemical elicitation using a salicylic acid analog and methyl jasmonate. Plant Mol. Biol. Report. 30, 225–234. doi: 10.1007/s11105-011-0335-0
Zhai, Q., Zhang, X., Wu, F., Feng, H., Deng, L., Xu, L., et al. (2015). Transcriptional mechanism of Jasmonate receptor COI1-mediated delay of flowering time in Arabidopsis. Plant Cell 27, tpc.15.00619–tpc.15.02828. doi: 10.1105/tpc.15.00619
Zhang, B., Niu, Z., Li, C., Hou, Z., Xue, Q., Liu, W., et al. (2022). Improving large-scale biomass and total alkaloid production of Dendrobium nobile Lindl. Using a temporary immersion bioreactor system and MeJA elicitation. Plant Methods 18, 1–10. doi: 10.1186/s13007-022-00843-9
Zhang, Z., Xu, J., Sheng, Z., Sui, Y., and Palli, S. R. (2011). Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J. Biol. Chem. 286, 8437–8447. doi: 10.1074/jbc.M110.191684
Zhang, X., Zhu, Z., An, F., Hao, D., Li, P., Song, J., et al. (2014). Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 26, 1105–1117. doi: 10.1105/tpc.113.122002
Zhao, J., Davis, L. C., and Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 23, 283–333. doi: 10.1016/j.biotechadv.2005.01.003
Zhou, S. M., Chen, L. M., Liu, S. Q., Wang, X. F., and Sun, X. D. (2015). De novo assembly and annotation of the Chinese chive (Allium tuberosum Rottler ex Spr.) Transcriptome using the Illumina platform. PLoS One 10:e0133312. doi: 10.1371/journal.pone.0133312PONE-D-15-12465
Keywords: methyl jasmonate, jasmonic acid, plant growth, elicitation, secondary metabolites, medicinal plants
Citation: Sohn S-I, Pandian S, Rakkammal K, Largia MJV, Thamilarasan SK, Balaji S, Zoclanclounon YAB, Shilpha J and Ramesh M (2022) Jasmonates in plant growth and development and elicitation of secondary metabolites: An updated overview. Front. Plant Sci. 13:942789. doi: 10.3389/fpls.2022.942789
Edited by:
Shubhpriya Gupta, Palacký University, Olomouc, CzechiaReviewed by:
Adrian Garrido, University of Concepcion, ChileYue Yin, Ningxia Academy of Agriculture and Forestry Sciences, China
Javier Palazon, University of Barcelona, Spain
Copyright © 2022 Sohn, Pandian, Rakkammal, Largia, Thamilarasan, Balaji, Zoclanclounon, Shilpha and Ramesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo-In Sohn, c2lzb2huQGtvcmVhLmty
†These authors have contributed equally to this work
 Soo-In Sohn
Soo-In Sohn Subramani Pandian
Subramani Pandian Kasinathan Rakkammal
Kasinathan Rakkammal Muthiah Joe Virgin Largia3†
Muthiah Joe Virgin Largia3† Senthil Kumar Thamilarasan
Senthil Kumar Thamilarasan Sekaran Balaji
Sekaran Balaji Yedomon Ange Bovys Zoclanclounon
Yedomon Ange Bovys Zoclanclounon Manikandan Ramesh
Manikandan Ramesh