- 1Research and Innovation Center, AMOREPACIFIC, Yongin, South Korea
- 2Natural Products Research Institute, College of Pharmacy, Seoul National University, Seoul, South Korea
Coumestrol (CMS) derivatives are unique compounds, which function as phytoalexins; they are derived from soybean roots, following abiotic and biotic stresses. As a phytoalexin, CMS forms a defense system that enables plants to maintain their viability. However, it is still challenging to achieve the mass production of phytoalexins, which exhibit pharmacological values, via plant breeding. Here, the synthesis of CMS derivatives from the seedling, plant, and adventitious root (AR) of Glycine max were investigated under artificial light, as well as via a chemical elicitor treatment. In the presence of constant light, as well as under treatment with methyl jasmonate, the CMS monoglucoside (coumestrin; CMSN) and malonyl CMSN (M-CMSN) contents of the AR culture (4 weeks) increased drastically. The two CMS derivatives, CMSN and M-CMSN, were obtained as a mixture of isomers, which were identified via nuclear magnetic resonance analysis. These derivatives were also observed in a soybean plant that was grown on artificial soil (AS; 5 weeks) and a Petri dish (9 days) although in considerably lesser quantities than those observed in the AR culture. Compared with the two other media (AS and the Petri dish), the AR culture achieved the superior synthesis of CMSN and M-CMSN within a relatively short cultivation period (<1 month) in laboratory-scale (3 L) and pilot-scale (1,000 L) bioreactors. The isoflavone content of AR under the constant light conditions was three-fold that under dark conditions. Significant quantities of malonyl daidzin and malonyl genistin were produced in the root of AS and the seedling of Petri dish, respectively. Flavonol glycosides were not produced in the AR culture under the dark and light conditions, as well as in AS under the dark condition. However, significant contents of kaempferol glycosides were produced in the leaves of AS and seedling of Petri dish, following the light treatment. Thus, we proposed that the established soybean AR-cultivation approach represented a better method for biosynthesizing phytoalexins, such as the CMS derivatives, as plant-derived functional materials.
Introduction
Plants exert selective effects on bacteria in bulk soil to acquire various features for metabolic fitness (Ling et al., 2022). Important microbial interactions occur among thousands of commensals, pathogens, and symbionts and plant in the rhizosphere, and these result in numerous challenges to the plant (Ling et al., 2022). Fabaceae plants exhibit distinctive symbiotic assimilation in root nodules, thereby inducing the corresponding genes in the rhizobacterial community (Dakora, 2000). Many Fabaceae plants facilitate the synthesis of signature polyphenols, such as isoflavonoids, via well-known biological pathways (Sugiyama et al., 2017); isoflavone conjugates undergo different modifications when plants are subjected to different stresses (biotic and abiotic) (Ahmad et al., 2017). Under certain extrinsic stresses, such as biotic and abiotic stresses, phytoalexins are converted using existing isoflavonoids via induced metabolic pathways (Yoshikawa, 1978). Pterocarpans are representative inducible phytoalexins that are formed through the ring closure of isoflavonoids (Uchida et al., 2017). Among the pterocarpans available in soybean, glyceollins and their derivatives have attracted enormous attention because of their biological effects (Nwachukwu et al., 2013; Bamji and Corbitt, 2017), and their biosynthetic pathways have been elucidated (Sukumaran et al., 2018; Vadivel et al., 2019).
Moreover, compared with the large quantities of glyceollins produced in the seeds and leaves of soybean, low quantities of coumestan, the oxidation product of pterocarpans, have also been reported (Figure 1). Although coumestan derivatives were first identified in soybean roots 40 years ago (Le-Van, 1984), their studies only began a decade ago (Yuk et al., 2011a; Jeon et al., 2012; Yun et al., 2020, 2021; Cox et al., 2021; Mun et al., 2021). Considering that plant-derived coumestan exhibits a wide range of pharmacological activities (Tu et al., 2021), researchers have attempted to mass-produce coumestrol (CMS), a coumestan exhibiting phytoestrogenic activity, from soybean adventitious root (AR) (Lee et al., 2020). Although most plants produce conjugated forms of these phytochemicals (coumestan), the knowledge and applications of coumestans are only based on aglycones. Based on the biosynthetic pathway of CMS (Ha et al., 2019), we believed that conjugated or complex coumestan derivatives could be produced employing intrinsic enzymes. Further, we observed that the laboratory (lab)-scale mass production of another phytoalexin from a soybean AR culture could be achieved. Thus, here, we identified new CMS derivatives, which were biosynthesized from soybean AR, following abiotic stress. Furthermore, the soybean AR was cultivated on a pilot scale to achieve the mass production of CMS derivatives. Additionally, soybean plants and seedlings were cultivated in the short term in artificial soil (AS) and a Petri dish, respectively, to confirm the existence of the CMS derivatives in soybean.
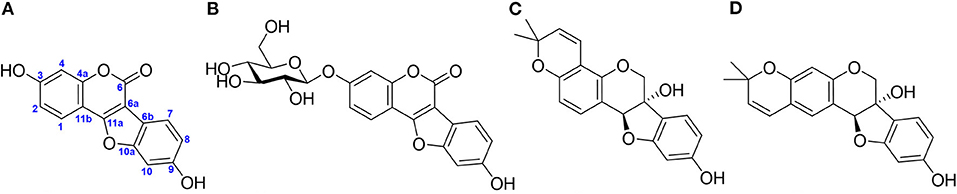
Figure 1. Representative phytoalexins in soybean reported in the literature. (A) Coumestrol (CMS), (B) CMS 3-O-glucoside, (C) glyceollin I, and (D) glyceollin II.
Materials and Methods
Chemicals and Reagents
Daidzein, daidzin, daidzin 6″-O-acetate, daidzin 6″-O-malonate, genistein, genistin, genistin 6″-O-acetate, genistin 6″-O-malonate, glycitein, glycitin, glycitin 6″-O-acetate, and glycitin 6″-O-malonate were purchased from FUJIFILM Wako Pure Chemical Corp (Osaka, Japan). CMS; dimethyl sulfoxide (DMSO); Gelrite™; indole-3-butyric acid (IBA); ascorbic acid; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; methyl jasmonate (MJ); and phosphate-buffered saline were purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA). Mass-grade formic acid (FA), acetonitrile, methanol, and water were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The other chemicals employed in this study were of American Chemical Society grade or higher. The Sinhwakong soybean variety (an elite cultivar) was supplied by the National Institute of Crop Science (Cheonju, Republic of Korea).
Soybean Cultivation Methods
AR Culture
The soybean AR culture was performed, following a previous method (Lee et al., 2020). Briefly, the ARs were propagated in a full-strength Murashige and Skoog medium (MSO) (Duchefa, Haarlem, The Netherlands) (Murashige and Skoog, 1962) containing 4 mg/L IBA and 30 g/L sucrose in 3 L bulb-type bubble bioreactors. The bioreactor culture was initiated via inoculation with wet ARs at a density of 4.0 g/L and an aeration volume of 0.1 air volume/culture volume per min (vvm) employing an air flow meter (RMA series; Dwyer Instruments, Michigan, USA). The ARs were maintained by subculturing in a fresh liquid medium every 3 weeks under dark conditions at 22 ± 1°C. To increase the phytochemical content, the ARs were cultured under fluorescent light in the bioreactor and with treatment employing a physical elicitor (Yun et al., 2021) for 4 weeks under the aforementioned conditions. Afterward, to increase the phytochemical productivity, the ARs were cultured in a half-strength MSO (2 L) containing 4 mg/L IBA and 30 g/L sucrose under bright light, as well as with treatment employing 50 μM MJ for 5 days before harvesting. The control samples were cultured under the same conditions but in the dark (Figure 2A). AR was mass-produced in a bulb-type bioreactor at an operating capacity of 1,000 L under the same conditions as those in the lab-scale experiment, except for the volume of the half-strength MSO (500 L) and the addition of a fluorescent lamp (Figure 2B). The bright-light conditions were as follows: 60 photosynthetic photon flux density (PPFD) (4,500 lx) and a fluorescent lamp (FHF32W/865; OSRAM GmbH, Munich, Germany). The bright light was supplied for 16 h, followed by 8 h of darkness in a 24 h cycle. After 4 weeks of culturing in the bioreactor, the liquid medium was removed, and the ARs were dried in a convection oven for 24 h at 60°C. The lab-scale and pilot-scale cultures were produced in three batches under the light and dark conditions, respectively.
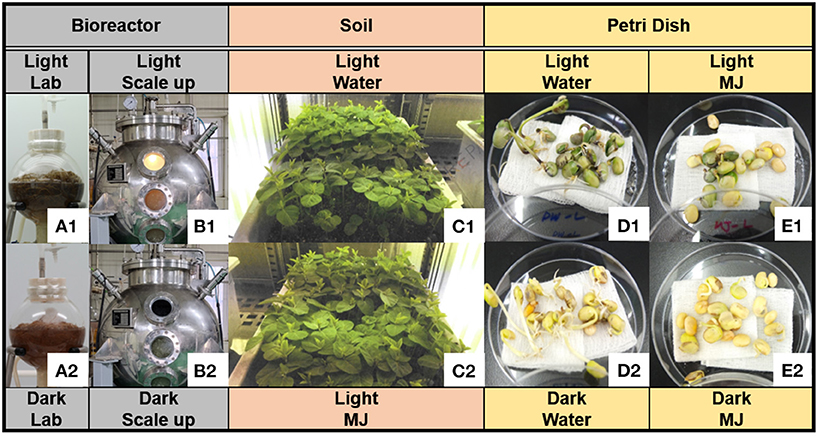
Figure 2. Three cultivation methods of soybean. (A) Lab-scale culture of soybean adventitious root (AR) [(A1) light condition; (A2) dark condition], (B) pilot-scale culture of soybean AR, (C) soybean plants cultured in artificial soil (AS) with water (C1) and methyl jasmonate (MJ) (C2) under light–dark cycle conditions, (D) seedlings in water under (D1) light and (D2) dark conditions, and (E) seedlings in MJ under (E1) light and (E2) dark conditions.
Plant Culture in AS
To compare the chemometric differences between the continuous bioreactor culture and soil cultivation, soybeans were cultured for 5 weeks in AS. The seeds were sterilized with a 2% sodium hypochlorite solution for 20 min. A hundred seeds were sown in a plastic pot (500 × 300 × 200 mm3; width × length × height) containing a horticultural substrate (Hanareum; Shinsung Mineral Co., Republic of Korea) and irrigated with tap water (3 L) every 3–4 days, following the overhead irrigation method. The physicochemical characteristics of the horticultural substrate were as follows: pH, 5–7; electrical conductivity, <1.2 dS/m; bulk density, <0.3 g/m3; coconut peat, 51.5%; pearlite, 15%; peat moss, 10%; vermiculite, 13%; zeolite, 10%; humic acid, 0.1%; and fertilizer, 0.4%. The light intensity was increased from 70 PPFD (5,200 lx) to a maximum of 200 PPFD (14,800 lx) depending on the growth phase, and the photoperiod was repeated under light (16 h) and dark (8 h) conditions in a 24 h cycle. The plant was cultivated in a growth chamber (GC-1000; JeioTech Co, Daejeon, Republic of Korea) for 5 weeks at a day/night temperature and humidity of 23 ± 2°C and 50 ± 5%, respectively (Figure 2C). To increase the phytochemical content of the roots, 50 μM MJ (2 L) in water was applied via the bottom irrigation method 5 days before harvesting. The plants, which were utilized as the controls, were supplied by regular tap water rather than MJ. After 5 weeks of cultivation, the grown plants were uprooted whole, and the accompanying soil particles were removed using running water. Thereafter, the roots were dried for 24 h at 60°C.
Germination of Seedlings in a Petri Dish
The soybeans were washed two times and soaked in pure water for 15 h. Thereafter, the soaked soybeans were sterilized with 70% (v/v) aqueous ethyl alcohol for 10 s and washed with pure water. Twenty prepared seeds were loaded onto a Petri dish (in triplicates) and covered with a four-layered gauze sheet. Pure water (10 mL) or 10 μM MJ was added to each Petri dish as a growth medium. A set of growth media was cultured without light, while the other set was exposed to light (10,000 lx). The Petri dishes were placed in a plant growth chamber (GC-1000; JeioTech Co) at a temperature of 20°C and relative humidity of 40%. Next, pure water (5 mL) was added to the Petri dish after three culture days (Figures 2D,E). The seedlings were collected after three, six, and nine culture days and freeze-dried via immersion in liquid nitrogen.
Purification and Identification of the CMS Derivatives
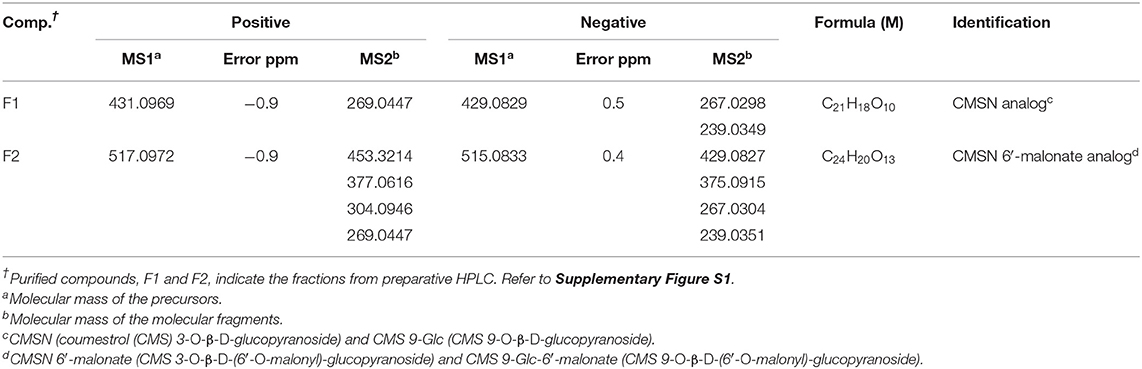
Powdery cultured soybean AR was dissolved in 60% (v/v) aqueous methanol (50 mg/mL) and sonicated for 20 min. The methanolic extracts were centrifuged for 10 min at 4,000 × g and 25°C, after which the supernatants were filtered through a 0.45 μm polyvinylidene fluoride (PVDF) syringe filter (Pall Corp., Port Washington, NY, USA). The filtered solutions were injected (200–1,000 μL per injection) into a preparative high-performance liquid chromatography (HPLC) column that was equipped with a 172-diode array detector, 321 binary solvent pump, and GX-271 liquid handler (Gilson Inc., Middleton, WI, USA). Two fractions (F1 and F2) were collected for a single peak under programmed elution conditions employing a preparative separation column (ZORBAX Eclipse XDB C18, 80 Å, 5 μm, 21.2 × 150 mm; Agilent Technologies, Santa Clara, CA, USA) at 30°C with a column heater (CO-2060; JASCO Corp., Tokyo, Japan). Each fraction in the repeated injections was combined, evaporated, and freeze-dried. The details of the preparative elution program and peak collection are presented in Supplementary Figure S1.
The compounds in the purified fractions were identified via quadrupole time-of-flight high-resolution electrospray ionization mass spectrometry (Q-TOF-HR ESI/MS). An UltiMate 3000 (Thermo Fisher Scientific), which was equipped with a CORTECS C18 column (90 Å, 1.6 μm, 2.1 mm × 100 mm; Waters Corp., Milford, MA, USA), was employed for the separation. The column temperature and flow rates were 40°C and 0.5 mL/min, respectively. The mobile phase comprised 0.1% (v/v) FA in water (Solvent A) and 0.1% (v/v) FA in acetonitrile (Solvent B). Further, the following linear gradient was applied: 86% A/14% B at 0 min, 86% A/14% B at 1.5 min, 74% A/26% B at 3 min, 74% A/26% B at 5 min, 20% A/80% B at 5.5 min, 86% A/14% B at 6 min, and 86% A/14% B at 7 min. The precursors and fragments were mass-detected using a Triple TOF 5600+ System (AB Sciex LLC, Framingham, MA, USA) under the following conditions: ionization mode, positive and negative; MS scan type, full scanning and information-dependent acquisition scanning; ionization source, ESI; MS scan range, 200–2,000 m/z; MS/MS scan range, 30–2,000 m/z; nebulizing gas pressure (ion source 1), 50 psi; heating gas pressure (ion source 2), 50 psi; curtain gas pressure, 25 psi; desolvation temperature, 500°C; ion spray voltage floating, 5.5 kV (positive) and 4.5 kV (negative); declustering potential (DP), 60 (positive) and −60 (negative); collision energy (CE), 10 (positive) and −10 (negative); CE, 35 ± 15 (positive) and −35 ± 15 (negative); and collision gas, He. The purity of the compounds was tentatively calculated via a spectral purity check employing an ACQUITY photodiode array detector (PDA) and the Empower 3 software (Waters Corp.) (Fabre et al., 1995; Singh et al., 2012). Each of the prepared compounds was dissolved in 500 μL of DMSO-d6, after which one-dimensional (1D) (1H and 13C) and two-dimensional (2D) [heteronuclear single-quantum coherence (HSQC) and heteronuclear multiple bond correlation (HMBC)] analyses were performed using a high-resolution nuclear magnetic resonance (NMR) spectrometer (600 MHz) (AVANCE 600; Bruker, Billerica, MA, USA). The structures of the compounds were determined using the Mnova 11 software (Mestrelab Research, Santiago de Compostela, A Coruña, Spain).
Determination of the Chemical Composition
The freeze-dried ARs, the plants from AS, and the soybean seedlings were pulverized using a Tubemill™ (IKA, Staufen, Germany) for 1 min at 25,000 rpm. The powdered samples (50 mg each) were mixed in 1.0 mL of 10% (v/v) DMSO in methanol, followed by 10 min of sonication. Thereafter, they were properly diluted using 10% (v/v) DMSO in methanol. The diluted samples were filtered through a 0.45 μm PVDF syringe filter (Pall Corp.).
The contents of the 12 isoflavones, CMS, and purified flavonol glycosides in the samples were analyzed using an ACQUITY UPLC system that was equipped with a binary pump, PDA detector, and mass detector (QDa™; Waters Corp.), as well as a CORTECS C18 column (90 Å, 1.6 μm, 2.1 × 100 mm; Waters Corp.). The flow rate, column temperature, and injection volume were 0.5 mL/min, 30°C, and 1 μL, respectively. Gradient elution was performed employing solvents A and B (the aforementioned linear gradient was applied).
The optimal conditions for mass detection, as well as the validated quantification method, have been described in the literature (Rha et al., 2021). The CMS monoglucoside (coumestrin; CMSN) and malonyl CMSN (M-CMSN) contents of the samples were analyzed employing the aforementioned instrument in different injection sets at a cone voltage of 5 V in the positive acquisition mode. The data were collected and processed using Empower 3 (Waters Corp.).
Statistical Analysis
Data are expressed as the mean ± standard error of the mean for a set of five plants and a set of three extracts. One-way analysis of variance was performed using comparisons of each pair by Student's t-test or comparisons for all pair by Tukey-Kramer's honestly significant difference test at an α = 0.05 level using JMP 16 (SAS Institute Inc., Cary, NC, USA).
Results
Biomass Obtained via the Three Cultivation Methods
Employing the 3 L and 1,000 L bioreactors, the yields of the dried ARs were ~4.0 and 3.0 g/L, respectively. The lengths of the shoots and roots of the AS-grown control and test (elicitor) plants were similar, in the ranges of 250–300 and 200–300 mm, respectively. The dry weights of the shoots and roots of the control culture were 5.21 ± 0.77 and 0.59 ± 0.11 g/10 plants, respectively, and those of the test culture were 4.97 ± 0.38 and 0.51 ± 0.04 g/10 plants, respectively. The dry weights of the shoots and roots of the control and elicitor cultures were not significantly different (p < 0.05). The initial dry weight of a soybean seedling was 2.75 ± 0.04 g/20 beans, while that of the 3-day seedling decreased to 2.06 ± 0.03 g/20 beans in the four germination groups. The dry weights reduced after germination for all the treatments and stagnated during the water and light treatments, while those for the other treatments decreased during the 9-day culture period (Supplementary Figure S2).
Newly Identified CMS Derivatives
AR cultivation availed substantial CMSN derivatives along with considerable root masses. Moreover, different chromatograms, which indicated the presence of the different molecules, were observed. Further, we observed two major CMSN derivatives, including their isomers. Here, we elucidated their structures.
Confirmation of the Structure of F1
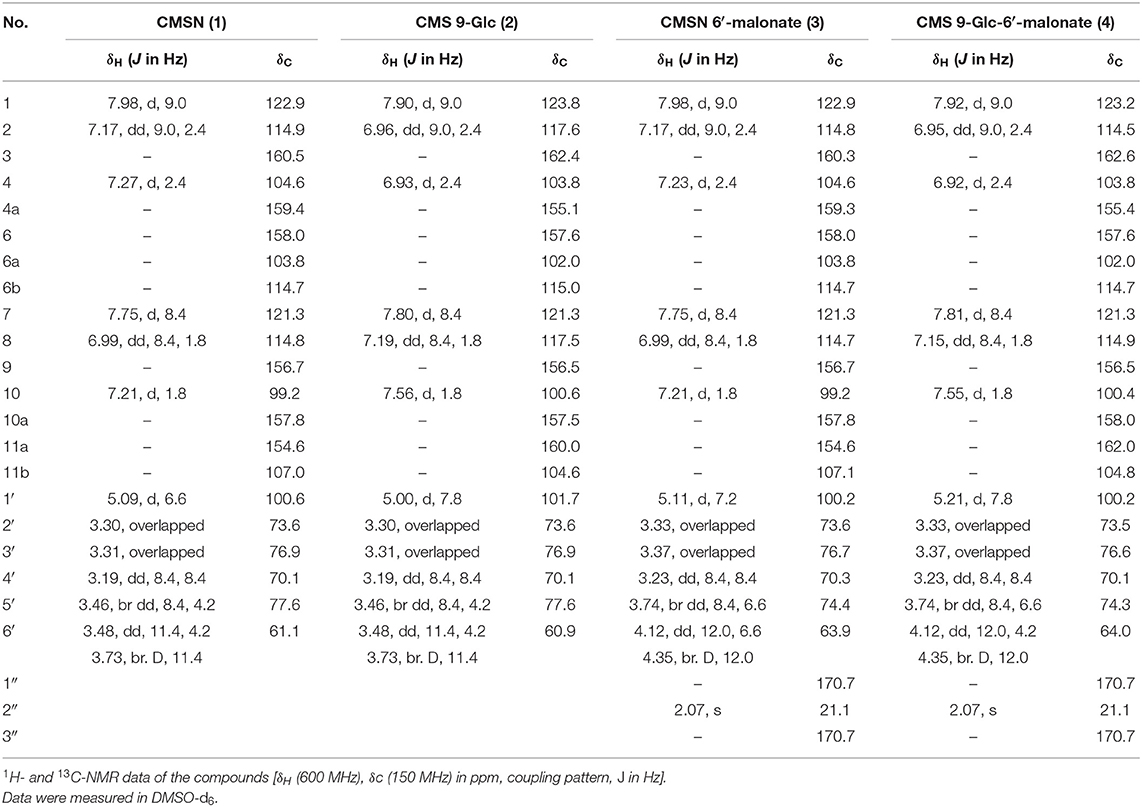
The molecular formula of F1, as determined by Q-TOF-HR ESI/MS [positive [M + H]+, m/z 431.0969 (calculated (calc.) for C21H19, 431.0973) and negative [M – H]−, m/z 429.0829 (calc. for C21H17, 429.0827)], was C21H18O10 (Table 1, Supplementary Figure S3). The 1H-NMR spectrum (600 MHz, DMSO-d6) revealed the presence of four 1,2,4-trisubstituted benzene-ring moieties and two hemiacetal signals; we confirmed via the integral of 1H-NMR that the ratio of major to minor components was 8:1 (Supplementary Figure S4). The major-component signals were detected at [δH 7.98 (1H, d, J = 9.0 Hz, H-1), δH 7.27 (1H, d, J = 2.4 Hz, H-4), and δH 7.17 (1H, dd, J = 9.0, 2.4 Hz, H-2)] and [δH 7.75 (1H, d, J = 8.4 Hz, H-7), δH 7.21 (1H, d, J = 1.8 Hz, H-10), and δH 6.99 (1H, dd, J = 8.4, 1.8 Hz, H-8)] corresponding to the two 1,2,4-trisubstituted benzene ring moieties and at δH 5.09 (1H, d, J = 6.6 Hz, H-1′) as a hemiacetal signal, as well as at δH 3.73 (1H, br d, J = 11.4 Hz, H-6′b) and δH 3.48 (1H, dd, J = 11.4, 4.2 Hz, H-6′a) corresponding to the oxymethylene signal and δH 3.46 (1H, br dd, J = 8.4, 4.2 Hz, H-5′), δH 3.31 (1H, overlapped, H-3′), δH 3.30 (1H, overlapped, H-2′), and δH 3.31 (1H, overlapped, H-3′) corresponding to four oxygenated methine signals, respectively (Table 2). The 1H-NMR spectrum revealed two 1,2,4-trisubstituted benzene ring moieties of minor components at [δH 7.90 (1H, d, J = 8.4 Hz, H-1), δH 7.21 (1H, d, J = 1.8 Hz, H-4), and δH 6.99 (1H, dd, J = 8.4, 1.8 Hz, H-2)] and [δH 7.75 (1H, d, J = 8.4 Hz, H-7), δH 7.21 (1H, d, J = 1.8 Hz, H-10), and δH 6.99 (1H, dd, J = 8.4, 1.8 Hz, H-8)], as well as a minor anomeric proton at δH 5.09 (1H, d, J = 6.6 Hz, H-1′). The 13C-NMR spectrum (150 MHz, DMSO-d6, δC) mainly exhibited 21 carbon signal components along with weak minor peaks, which confirmed that F1 comprised a phenolic compound and sugar (Supplementary Figure S5). Regarding the major component, a carbonyl carbon signal (δC 158.0), five oxygenated quaternary carbon signals (δC 160.5, 159.4, 157.8, 156.7, 154.6), three olefinic quaternary carbon signals (δC 114.7, 107.0, 103.8), and six olefinic methine carbon signals (δC 122.9, 121.3, 114.9, 114.8, 104.6, 99.2) were detected as the signals of an aglycone, which was identified as a CMS. The carbon signals of the minor component were detected as very weak signals at δC 162.4 (C-3), 160.0 (C-11a), 157.6 (C-5), 157.5 (C-10a), 155.1 (C-4a), 123.8 (C-1), 121.3 (C-7), 117.6 (C-2), 117.5 (C-8), 115.0 (C-6b), 104.6 (C-11b), 103.8 (C-4), 100.6 (C-10), 102.0 (C-6a), and 100.6 (C-10). The chemical shifts of two anomeric carbons (δC 101.6 and 101.7 corresponding to the major and minor components, respectively) overlapped with eight oxymethine (δC 77.6, 76.9, 73.6, 70.1) and two oxymethylene (δC 61.1) carbon signals (Table 2, Supplementary Figure S5). The HMQC spectrum enabled the identification of the proton and carbon signals of the corresponding major and minor protons, respectively (Supplementary Figure S6). The aforementioned 1H- and 13C-NMR, as well as HMQC data, indicated that F1 was a mixture (8:1) of a glycosylated CMS and its analog. CMS comprising two 1,2,4-trisubstituted benzene ring moieties and sugar was constructed by its correlation spectroscopy (COSY) and HMBC (Figure 3, Supplementary Figures S7, S8). The COSY spectrum revealed the presence of glucopyranose [δH 5.09 (H-1′) or δH 5.00 (H-1′), δH 3.30 (H-2′), δH 3.31 (H-3′), δH 3.19 (H-4′), δH 3.46 (H-5′), δH 3.48 (H-6′a), and δH 3.73 (H-6′b)]; the coupling constants (J = 6.6 Hz) of δH 5.09 (H-1′) and (J = 7.8 Hz) of δH 5.00 (H-1′) confirmed the presence of an anomeric hydroxy group exhibiting a β-configuration. Regarding the major component, the COSY of H-1 (δH 7.98) with H-2 (δH 7.17)/H-7 (δH 7.75) with H-8 (δH 6.99), as well as HMBC from H-1 to C-11b (δC 107.0); H-2 to C-3 (δC 160.5), C-4a (δC 159.4), and C-11a (δC 154.6); H-4 (δH 7.27) to C-2 (δC 114.9), C-11a, and C-11b; H-7 to C-6 (δC 158.0), C-6a (δC 103.8), and C-9 (δC 156.7); H-8 to C-6b (δC 114.7) and C-10 (δC 99.2); and H-10 (δH 7.21) to C-8 (δC 114.8), C-9 (δC 156.7), and C-10a (δC 157.8) confirmed that the CMS skeleton was a major component. HMBC of a methylene H-1′ (δH 5.09) with an oxygenated quaternary carbon C-3 (δC 160.5) corresponding to CMS 3-O-β-d-glucopyranose (CMSN, 1) indicated the major component (Figure 3, Supplementary Figure S8). In the gHMBC spectrum of the minor component (Supplementary Figure S9), the signal of the minor anomeric proton (δH 5.00, H-1′) exhibited the cross-peak with the signal of the oxygenated olefinic quaternary carbon (δC 156.5, C-9); the long-range correlations of H-10 (δH 7.56) with C-9 and C10a (δC 157.5) and of H-7 (δH 7.80) with C-10a confirmed that the O-β-d-glucopyranose was positioned at C-9 as a novel compound. A previous 1H-NMR report of CMS confirmed that H-1, H-2, H-4, H-7, H-8, and H-10 were detected at δH 7.85, 6.93, 6.91, 7.69, 6.95, and 7.17 in a DMSO-d6 solvent, respectively (Sheng et al., 2016). Three H-1, H-2, and H-4 CMCN (CMS 3-Glc, 1) signals were observed as major components at δH 7.98, 7.17, and 7.27, respectively, with down-shifted values corresponding to the glycosylation effect. Similarly, H-7, H-8, and H-10 signals were observed at δH 7.80, 7.19, and 7.56, respectively, and were downshifted because of the position of O-β-d-glucopyranose to C-9 of CMS 9-O-β-d-glucopyranose (2), another novel compound. Overall, F1 was identified as CMS 3-O-β-d-glucopyranose (CMSN, 1), a major component, and CMS 9-O-β-d-glucopyranose (CMS 9-Glc, 2).
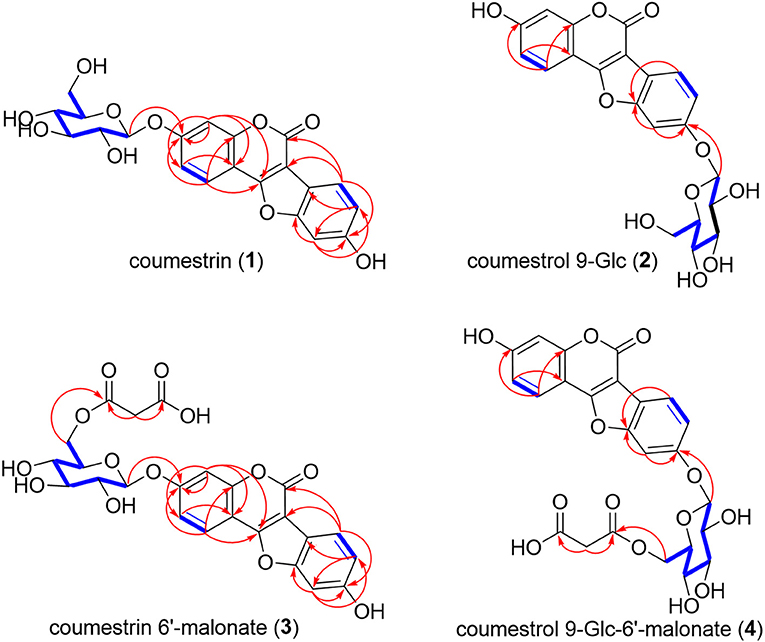
Figure 3. COSY and HMBC for the structural interpretations of coumestrin (CMSN), malonyl CMSN (M-CMSN), and the isomers. 1H–1H coupling and 1H–13C long-range correlations observed in the COSY and HMBC spectra of CMSN (1), CMS 9-Glc (2), CMSN 6′-malonate (3), and CMS 9-Glc-6′-malonate (4), respectively. The bold blue lines indicate the coupling correlations between the proton and proton signals in the COSY spectrum, and the red arrows show the J2 and J3 correlations between the proton and carbon signals in the HMBC spectrum.
Confirmation of the Structure of F2
The Q-TOF-HR ESI/MS data of the positive molecule [M + H]+ at m/z 517.0972 (calc. for C24H21, 517.0977), as well as the negative molecule [M – H]− at m/z 515.0833 (calc. for C24H19, 515.0831) confirmed that the molecular formula of F2 was C24H20O13 (Table 1, Supplementary Figure S3). The 1H-NMR (600 MHz, DMSO-d6) and 13C-NMR (150 MHz) spectra of F2 were almost similar to those of F1, except for the presence of one additional malonate and two extra carbonyl carbon (δC 170.7, δC 170.7) and extra methylene (δH 2.07; δC 21.1) signals (Table 2, Figure 2, and Supplementary Figures S9, S10). The mass spectrometry and 1D NMR analyses revealed that the structure of F2 corresponded to CMSN containing malonate. Moreover, we observed that the ratio of the major component to the minor one of each peak integral value of 1H-NMR was 8:1, corresponding to the ratio of CMSN (1) to coumestrol 9-Glc (2) in F1 (Supplementary Figure S9). The HMQC spectrum revealed the identities of the proton and carbon signals, as well as their corresponding protons (Table 2, Supplementary Figure S11). In the gHMBC spectrum, H-6′a (δH 4.12) and H-6′b (δH 4.35), which were downshifted via carbonylation, exhibited correlations with C-1″ (δC 170.7), and H-2″ (δH 2.07) exhibited correlations with C-1″ and C-3″ (δC 170.7) (Figure 3, Supplementary Figure S12), indicating that a malonate group was positioned at C-6′ of glucopyranose. HMBC and COSY indicated that the major and minor components of F2 were CMSN 6′-malonate (3) and CMS 9-O-β-d-(6′-O-malonyl)-glucopyranose [CMS 9-Glc-6′-malonate, (4)], respectively (Figure 3, Supplementary Figures S12, S13). Thus, 4 of F2 was identified as a novel compound.
Chemical Composition of the Derivatives Obtained via Cultivation
Based on the area under the curve of the maximum ultraviolet (UV) absorbance (220–460 nm) (Supplementary Figures S14, S15), the purities of F1 and F2, as obtained via peak collection employing preparative HPLC, were 70% and 64%, respectively. The purities of F1 and F2 were exploited to quantify the CMSN and M-CMSN contents of all the samples, respectively.
The CMS derivatives were present in the ARs, beans, leaves, roots, and seedlings of the soybean (Figure 4). A significant amount of M-CMSN (~400 μg/g) was produced in the ARs, while a four-fold-lower quantity of CMSN was produced under light (Figure 4A1). Under dark conditions for the AR culture, 0.75-fold-lower amounts of the CMS derivatives were obtained than the amounts under the light conditions. Notably, CMS was not detected in the ARs, although its traces were detected in the AR extract owing to the degradation of the CMS derivatives at the extraction stage (Figure 4A2). Similarly, M-CMSN was obtained in a 10-fold-higher amount (7.6 μg/g) in the roots than in the leaves (Figure 4B). Compared with the utilization of the control (water) in AS, the MJ treatment could not effectively increase the production of the CMS derivatives (Figure 4B2). Lower amounts of M-CMSN were detected in the untreated and soaked soybeans (Figure 4C). The contents of the CMS derivatives in the soybean seedlings increased gradually with the cultivation period (Figures 4D,E). The M-CMSN content of the soybean seedling was 8–14-fold higher than the CMSN content. Thus, darkness and water contributed majorly to the production of M-CMSN (Figures 4D,E). Compared with the results for AR, the CMS derivative contents of the plants and seedlings were substantially lower.
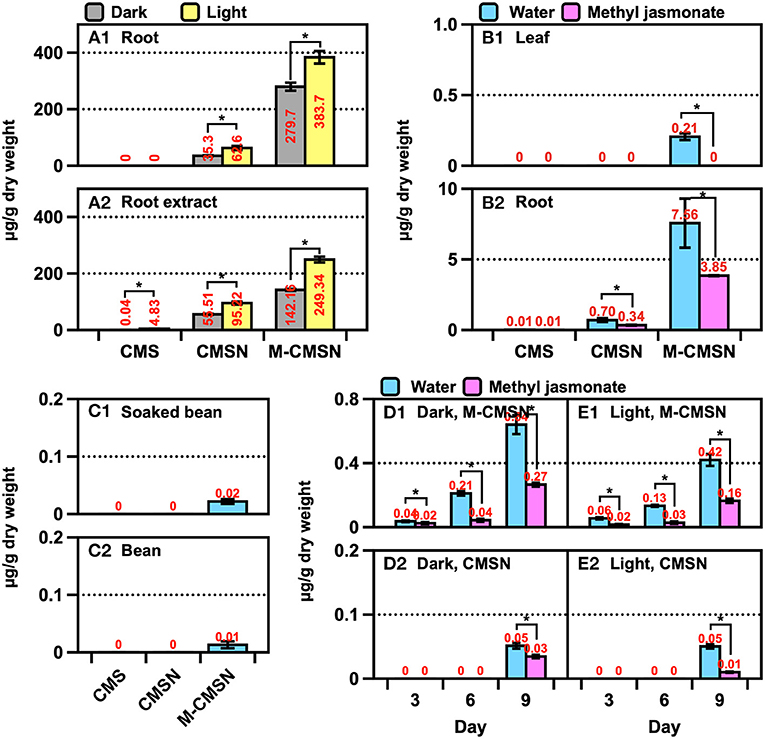
Figure 4. Changes in the CMSN and M-CMSN contents of (A) soybean AR, (B) soybean plants cultivated in artificial soil (AS), (C) untreated and soaked soybean, and (D,E) soybean seedlings. The data are expressed as the mean ± standard error of the mean. Asterisk “*” on the bar indicates significant differences of the comparisons for each pair by Student's t-test at an α = 0.05 level.
The trend of the isoflavone content was similar to that of the CMS derivatives across the samples (Figures 5A,B). A significant amount of malonyl daidzin was produced from different isoflavones in AR, and over a two-fold higher amount was obtained under light (Figure 5A). Although the amounts of the daidzin and malonyl genistin accounted for the second-largest, acetylated isoflavones were undetected. The isoflavone composition of the plants was similar to that of ARs, while that of the leaves was 10-fold lower than that of the roots (Figure 5B). The chemical elicitor treatment afforded an approximately two-fold increase in the isoflavone content compared with that in the control (water). The kaempferol glycoside contents of the leaves obtained via the control or MJ treatments did not differ (Supplementary Figure S15). The contents of isoflavones (of which malonyl genistin accounted for the major component) of the soybean increased significantly (p < 0.05) by approximately 1.9-fold after soaking in water (Figure 6A1). The isoflavone content of the seedlings increased greatly across the growth days [the only difference in the malonyl isoflavone content was observed in the 9 days of darkness–water treatment (Figure 6B2)], and the isoflavone contents were not significantly different among the other treatments (Figures 6B,C). The kaempferol glycoside content of the seedlings increased significantly, following the illumination treatments (Supplementary Figure S15); moreover, the MJ treatment could hinder the production of kaempferol glycosides because it inhibited the growth of the seedlings.
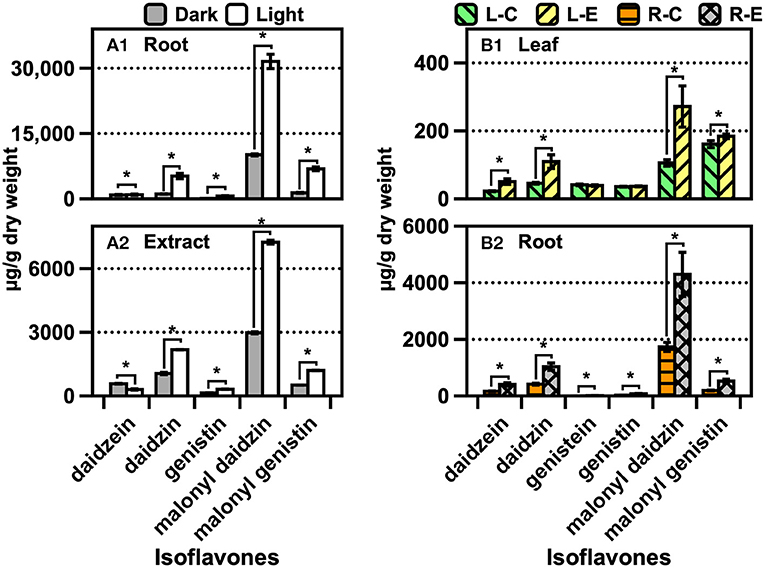
Figure 5. Contents of isoflavones and kaempferol glycosides of (A) soybean AR and (B) soybean plants cultivated in AS. The data are expressed as the mean ± standard error of the mean. Asterisk “*” on the bar indicates the significant differences of the comparisons for each pair by Student's t-test at an α = 0.05 level.
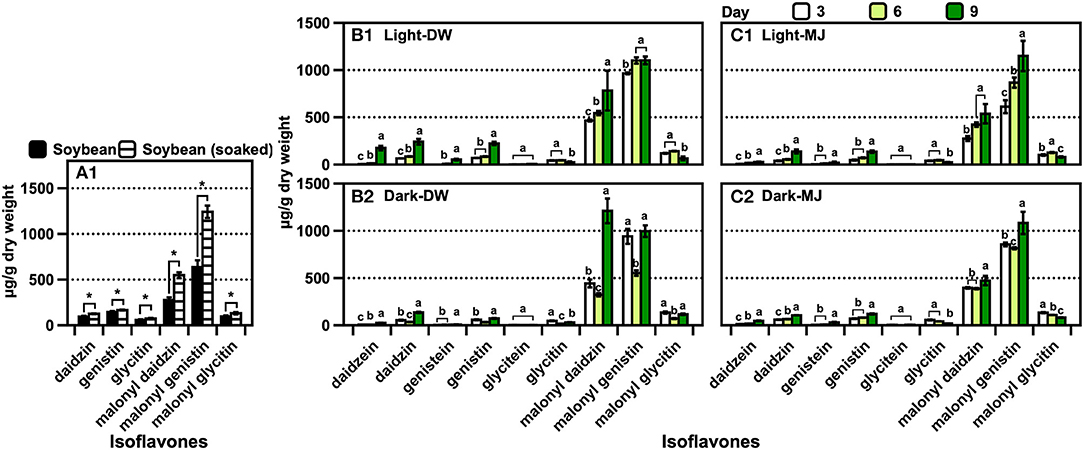
Figure 6. Isoflavone contents of (A) soybean before germination, (B) soybean seedlings in water, and (C) soybean seedlings in MJ solution. The data are expressed as the mean ± standard error of the mean. Asterisk “*” on the bar indicates the significant differences of the comparisons for each pair by Student's t-test at an α = 0.05 level. The lowercase letters on the bar in the same compounds indicate the significant differences of the comparisons for all the pairs by the Tukey–Kramer honestly significant difference test at an α = 0.05 level.
Discussion
Culture Methods
The difference between the yields obtained employing the lab-scale (4 g/L) and pilot-scale (3 g/L) bioreactors was mainly because of interferences, such as the low mass-transfer rate, hydraulic pressure, and shear stress of the large-scale reactor (Murthy et al., 2014), which reduced the yield. Thus far, no significant correlation has been calculated, thereby necessitating further optimization needs to scale up the productivity. The reproducible AR cultivation approach reported herein could represent a milestone in this research area and is more effective than the large-scale cultivation of plant cells, which is rarely adopted because of its many associating limitations (Lee et al., 2014). The dry weight yield of the root in the AS culture was ~0.2 g/L soil, which was lower than that of the root cultivated in the lab-scale bioreactor (4 g/L).
Newly Identified M-CMSN
The difference between the yields of the two bioreactors was mainly because of their light transparency. AR in the 3 L glass bioreactor was evenly exposed to the light source, and aeration facilitated the sufficient movement of the AR during cultivation (3 L bioreactor for 4 weeks; Supplementary Video S1). Conversely, AR in the 1,000 L stainless bioreactor was not fully exposed to the light source owing to the limitation of the light reflex and penetration (the light source was anchored on a fixed spot). Nevertheless, the periodic movement of AR in the bioreactor via aeration might have triggered the synthesis of phytoalexins, following light stress. It is well-known that the synthesis of CMSN can be facilitated by environmental factors and periodic changes in the plant growth cycle (Song et al., 2014; Mun et al., 2021). To date, only Romani et al. (2003) have detected M-CMSN in soybean roots via a tentative assignment by the UV spectra and mass fragmentation only. Additionally, Oshima et al. (2016) performed the structural analysis of M-CMSN in a soybean seed coat via NMR. Therefore, there are no definite evidence regarding the presence of M-CMSN, which contains a relatively unstable malonyl moiety, in soybean root. M-CMSN is produced from malonyl daidzin, which is abundant in soybean roots, via an intrinsic biological transformation against biotic and abiotic stresses. Employing the cultivation method, M-CMSN was detected in the roots and leaves of soybean; a small fraction of CMSN was also detected. However, CMS, an aglycone form of CMSN, was only detected in the root extract owing to its degradation during heating. Therefore, unlike the presence of CMS in a late-harvested soybean leaf, CMS might not be produced via the intrinsic metabolism of the root. To effectively produce M-CMSN, AR cultivation is preferred to the AS planting and Petri dish seedling methods. This indicates that field-grown roots might not be an alternative material for synthesizing these novel compounds. Furthermore, our results demonstrated the presence of CMS 9-glucoside and CMS 9-glucoside-6′-malonate, which could not be previously detected (Lee et al., 2020). The possible metabolic mechanisms of these compounds must be elucidated in subsequent studies.
Polyphenol Metabolism of Soybean Roots
Polyphenols are biosynthesized in living plants to facilitate different activities, such as forming a defense system against exogenous matters, nutrient accumulation, and microorganism interaction in the rhizosphere of the plant (Sugiyama et al., 2017). Malonyl isoflavones, which are produced when symbiotic organisms in the rhizosphere secrete isoflavones in the form of aglycones or glycosides, account for most soybean polyphenols (Sugiyama et al., 2017). The metabolic responses to the synthesis of polyphenols in soybean plants can be altered by endogenous and exogenous factors, such as leaf maturation at the late-growth stage and defense pressure due to biological infections in the rhizosphere (Mun et al., 2021; Ling et al., 2022). Accordingly, other polyphenols can be generated from existing isoflavones via different endogenous enzymatic pathways (Ha et al., 2019). CMS and its derivatives have been converted from daidzein and its derivatives (daidzin and malonyl daidzin) via the internal cyclization of the molecule (Uchida et al., 2017). The corresponding enzymes were encoded employing transgenic plants, and the plant genes were expressed by heterologous microorganisms (Sohn et al., 2021). CMS occurrences have been largely elucidated, although the occurrences of CMSN have rarely been verified via mass spectrometry and NMR (Le-Van, 1984; Yuk et al., 2011b). Thus far, the presence of M-CMSN or its isomers has not been reported. The metabolic flux for generating M-CMSN in the ARs of soybean was higher than that in normal roots (Figure 4). We confirmed the presence of the isomer of M-CMSN via NMR analysis, and this represents a unique phenomenon in soybean ARs owing to the chemical induction for converting isoflavones into CMS derivatives. Many different polyphenols, including isoflavones, flavones, flavonols, and coumestan, as well as their corresponding derivatives, have been identified in soybean leaves (Song et al., 2014), while the less-diverse polyphenols have been detected in soybean roots. Similar to the composition of the isoflavones in soybean leaves, malonyl daidzin accounted for the highest proportion (~60%) in soybean roots. Many flavonols and glycosides are present in the leaves, but no flavonol was detected in the soybean ARs and the roots of the soybean planted in AS (data not shown). The isoflavone content of soybean leaves largely increased during the cultivation period of up to 120 days (Rha et al., 2021) probably because of the changes in the biosynthetic flux in mature soybean plants (Dhaubhadel et al., 2003). CMS was detected after 90 and 120 days in field-grown soybean leaves, and this is a well-known phenomenon that is due to the endogenous tolerance of a mature soybean plant to stress (Tripathi et al., 2016; Yun et al., 2016). However, no specific biosynthetic pathways have been revealed for generating M-CMSN and its isomers; thus, further studies are required to elucidate the unconventional occurrences of these compounds in soybean AR cultures.
Leguminous plants interact with different microorganisms by exchanging metabolites and signals to maintain their metabolic system. Alternatively, infectious biotic and abiotic stresses can be modulated in the rhizosphere, following the secretion of the aforementioned modified polyphenols, to facilitate the viability of the plant. The newly isolated compounds, which can influence biological and environmental factors, have been utilized in the pharmaceutical and healthcare industry. Therefore, our approach, AR cultivation, could represent a breakthrough for producing substantial amounts of M-CMSN with pharmaceutical and industrial applications. We attempted to elucidate the biological efficacy of M-CMSN, such as its proliferation effect on human follicle dermal papilla cells (data not shown). However, further concrete evidence would be reported in the future.
Conclusion
Soybean AR cultivation under abiotic stress induction generated two CMS derivatives, one of which is yet to be identified. AR cultivation can be an effective method for producing leguminous phytochemicals in a timely, cost-effective manner. Leguminous roots preserve their viabilities in distinctive rhizosphere environments via root cell–microorganism interactions. Accordingly, different approaches will further verify these findings for the production of significant phytochemicals under biotic or abiotic augmentation. Furthermore, understanding the changes in the metabolic flux in an environment would present substantial clues for a biotechnological approach to plant research in the future.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
EJL: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, visualization, and writing—review and editing. MCS: formal analysis, methodology, resources, and writing—review and editing. C-SR: conceptualization, data curation, formal analysis, software, supervision, validation, visualization, writing—original draft, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: EJL and C-SR declare employment with AMOREPACIFIC Corporation.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.923163/full#supplementary-material
Abbreviations
1D, one-dimensional; 2D, two-dimensional; AR, adventitious root; AS, artificial soil; CE, collision energy; CMS, coumestrol; CMSN, coumestrol monoglucoside/coumestrin; COSY, correlation spectroscopy; DMSO, dimethyl sulfoxide; DP, declustering potential; FA, formic acid; HMBC, heteronuclear multiple bond correlation; HPLC, high-performance liquid chromatography; HSQC, heteronuclear single-quantum coherence; IBA, indole-3-butyric acid; MJ, methyl jasmonate; MS, mass spectrometer; MSO, Murashige and Skoog medium; NMR, nuclear magnetic resonance; PDA, photodiode array; PPFD, photosynthetic photon flux density; PVDF, polyvinylidene fluoride; Q-TOF-HR ESI/MS, quadrupole time-of-flight high-resolution electrospray ionization mass spectrometry; UV, ultraviolet.
References
Ahmad, M. Z., Li, P., Wang, J., Rehman, N. U., and Zhao, J. (2017). Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Front. Plant Sci. 08:735. doi: 10.3389/fpls.2017.00735
Bamji, S. F., and Corbitt, C. (2017). Glyceollins: soybean phytoalexins that exhibit a wide range of health-promoting effects. J. Funct. Foods 34, 98–105. doi: 10.1016/j.jff.2017.04.020
Cox, L. D., Munholland, S., Mats, L., Zhu, H., Crosby, W. L., Lukens, L., et al. (2021). The induction of the isoflavone biosynthesis pathway is associated with resistance to common bacterial blight in Phaseolus vulgaris L. Metabolites 11, 433. doi: 10.3390/metabo11070433
Dakora, F. D. (2000). Commonality of root nodulation signals and nitrogen assimilation in tropical grain legumes belonging to the tribe Phaseoleae. Funct. Plant Biol. 27, 885–892. doi: 10.1071/pp00015
Dhaubhadel, S., McGarvey, B. D., Williams, R., and Gijzen, M. (2003). Isoflavonoid biosynthesis and accumulation in developing soybean seeds. Plant Mol. Biol. 53, 733–743. doi: 10.1023/B:PLAN.0000023666.30358.ae
Fabre, H., Le Bris, A., and Blanchin, M. D. (1995). Evaluation of different techniques for peak purity assessment on a diode-array detector in liquid chromatography. J. Chromatogr. A 697, 81–88. doi: 10.1016/0021-9673(94)00979-j
Ha, J., Kang, Y.-G., Lee, T., Kim, M., Yoon, M. Y., Lee, E., et al. (2019). Comprehensive RNA sequencing and co-expression network analysis to complete the biosynthetic pathway of coumestrol, a phytoestrogen. Sci. Rep. 9, 1934. doi: 10.1038/s41598-018-38219-6
Jeon, H. Y., Seo, D. B., Shin, H.-J., and Lee, S.-J. (2012). Effect of Aspergillus oryzae-challenged germination on soybean isoflavone content and antioxidant activity. J. Agric. Food Chem. 60, 2807–2814. doi: 10.1021/jf204708n
Lee, E.-J., Moh, S.-H., and Park, S.-Y. (2014). “Production of biomass and bioactive compounds in adventitious root cultures of Eleutherococcus koreanum Nakai,” in Production of Biomass and Bioactive Compounds Using Bioreactor Technology, eds. K.-Y. Paek, H. N. Murthy, and J.-J. Zhong (Dordrecht: Springer), 223–249.
Lee, E. J., Jiménez, Z., Seo, K.-H., Nam, G. B., Kang, Y.-G., Lee, T. R., et al. (2020). Mass production of coumestrol from soybean (Glycine max) adventitious roots through bioreactor: effect on collagen production. Plant Biotechnol. Rep. 14, 99–110. doi: 10.1007/s11816-019-00589-2
Le-Van, N. (1984). Coumestrin a coumestan derivative from soybean roots. Phytochemistry 23, 1204–1205. doi: 10.1016/s0031-9422(00)82649-7
Ling, N., Wang, T., and Kuzyakov, Y. (2022). Rhizosphere bacteriome structure and functions. Nat. Commun. 13, 836. doi: 10.1038/s41467-022-28448-9
Mun, B.-G., Kim, H.-H., Yuk, H. J., Hussain, A., Loake, G. J., and Yun, B.-W. (2021). A potential role of coumestrol in soybean leaf senescence and its interaction with phytohormones. Front. Plant Sci. 12:756308. doi: 10.3389/fpls.2021.756308
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Murthy, H. N., Lee, E.-J., and Paek, K.-Y. (2014). Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell, Tissue Organ Cult. 118, 1–16. doi: 10.1007/s11240-014-0467-7
Nwachukwu, I. D., Luciano, F. B., and Udenigwe, C. C. (2013). The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Res. Int. 54, 1208–1216. doi: 10.1016/j.foodres.2013.01.024
Oshima, A., Mine, W., Nakada, M., and Yanase, E. (2016). Analysis of isoflavones and coumestrol in soybean sprouts. Biosci. Biotechnol. Biochem. 80, 2077–2079. doi: 10.1080/09168451.2016.1196577
Rha, C.-S., Jang, E. K., Hong, Y. D., and Park, W. S. (2021). Supervised statistical learning prediction of soybean varieties and cultivation sites using rapid UPLC-MS separation, method validation, and targeted metabolomic analysis of 31 phenolic compounds in the leaves. Metabolites 11, 884. doi: 10.3390/metabo11120884
Romani, A., Vignolini, P., Galardi, C., Aroldi, C., Vazzana, C., and Heimler, D. (2003). Polyphenolic content in different plant parts of soy cultivars grown under natural conditions. J. Agric. Food Chem. 51, 5301–5306. doi: 10.1021/jf0212136
Sheng, J., Xu, T., Zhang, E., Zhang, X., Wei, W., and Zou, Y. (2016) Synthesis of coumestrol aureol. J. Nat. Prod. 79, 749–2753. doi: 10.1021/acs.jnatprod.6b00510
Singh, S., Singh, R., Banerjee, S., Negi, A. S., and Shanker, K. (2012). Determination of anti-tubercular agent in mango ginger (Curcuma amada Roxb.) by reverse phase HPLC-PDA-MS. Food Chem. 131, 375–379. doi: 10.1016/j.foodchem.2011.08.054
Sohn, S. I., Pandian, S., Oh, Y. J., Kang, H. J., Cho, W. S., and Cho, Y. S. (2021). Metabolic engineering of isoflavones: an updated overview. Front. Plant Sci. 12:670103. doi: 10.3389/fpls.2021.670103
Song, H.-H., Ryu, H. W., Lee, K. J., Jeong, I. Y., Kim, D. S., and Oh, S.-R. (2014). Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics 10, 833–841. doi: 10.1007/s11306-014-0640-3
Sugiyama, A., Yamazaki, Y., Hamamoto, S., Takase, H., and Yazaki, K. (2017). Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 58, 1594–1600. doi: 10.1093/pcp/pcx084
Sukumaran, A., McDowell, T., Chen, L., Renaud, J., and Dhaubhadel, S. (2018). Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 96, 966–981. doi: 10.1111/tpj.14083
Tripathi, P., Rabara, R. C., Reese, R. N., Miller, M. A., Rohila, J. S., Subramanian, S., et al. (2016). A toolbox of genes, proteins, metabolites and promoters for improving drought tolerance in soybean includes the metabolite coumestrol and stomatal development genes. BMC Genom. 17:102. doi: 10.1186/s12864-016-2420-0
Tu, Y., Yang, Y., Li, Y., and He, C. (2021). Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 169, 105615. doi: 10.1016/j.phrs.2021.105615
Uchida, K., Akashi, T., and Aoki, T. (2017). The missing link in leguminous pterocarpan biosynthesis is a dirigent domain-containing protein with isoflavanol dehydratase activity. Plant Cell Physiol. 58, 398–408. doi: 10.1093/pcp/pcw213
Vadivel, A. K. A., Renaud, J., Kagale, S., and Dhaubhadel, S. (2019). GmMYB176 regulates multiple steps in isoflavonoid biosynthesis in soybean. Front. Plant Sci. 10:562. doi: 10.3389/fpls.2019.00562
Yoshikawa, M. (1978). Diverse modes of action of biotic and abiotic phytoalexin elicitors. Nature 275, 546–547. doi: 10.1038/275546a0
Yuk, H., Lee, J., Curtis-Long, M. J., Lee, J., Kim, Y., Ryu, H., et al. (2011b). The most abundant polyphenol of soy leaves, coumestrol, displays potent α-glucosidase inhibitory activity. Food Chem. 126, 1057–1063. doi: 10.1016/j.foodchem.2010.11.125
Yuk, H. J., Curtis-Long, M. J., Ryu, H. W., Jang, K. C., Seo, W. D., Kim, J. Y., et al. (2011a). Pterocarpan profiles for soybean leaves at different growth stages and investigation of their glycosidase inhibitions. J. Agric. Food Chem. 59, 12683–12690. doi: 10.1021/jf203326c
Yun, D.-Y., Kang, Y.-G., Kim, M., Kim, D., Kim, E.-H., and Hong, Y.-S. (2020). Metabolomic understanding of pod removal effect in soybean plants and potential association with their health benefit. Food Res. Int. 138, 109797. doi: 10.1016/j.foodres.2020.109797
Yun, D.-Y., Kang, Y.-G., Lee, E. J., Kim, D., Kim, E.-H., and Hong, Y.-S. (2021). Metabolomics study for exploring metabolic perturbations in soybean adventitious roots by fluorescent light irradiation. Appl. Biol. Chem. 64, 26. doi: 10.1186/s13765-021-00598-2
Keywords: abiotic stress, artificial soil, coumestrol derivative, Glycine max, phytoalexin, plant cell culture, soybean seedling, specialized metabolism in legume crop
Citation: Lee EJ, Song MC and Rha C-S (2022) Mass Biosynthesis of Coumestrol Derivatives and Their Isomers via Soybean Adventitious Root Cultivation in Bioreactors. Front. Plant Sci. 13:923163. doi: 10.3389/fpls.2022.923163
Received: 19 April 2022; Accepted: 17 May 2022;
Published: 21 June 2022.
Edited by:
Mehran Dastmalchi, McGill University, CanadaReviewed by:
Koji Miyamoto, Teikyo University, JapanSailendra Singh, Migal-Galilee Research Institute, Israel
Copyright © 2022 Lee, Song and Rha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan-Su Rha, dGVhbWFuQGFtb3JlcGFjaWZpYy5jb20=
†These authors share first authorship
 Eun Jung Lee1†
Eun Jung Lee1† Myoung Chong Song
Myoung Chong Song Chan-Su Rha
Chan-Su Rha