
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 28 July 2022
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.917528
This article is part of the Research TopicAdaptation Mechanisms of Grass and Forage Plants to Stressful EnvironmentsView all 42 articles
Raising crops production via improving photosynthesis has always been focused. Recently excavating and increasing the photosynthetic capacity of non-leaf organs becomes an important approach to crops yield increase. Here we studied the photosynthetic characteristics of the flag leaf and the non-leaf organs including the sheath, the glume and the lemma under greenhouse. The relative water content (RWC), the stomatal characteristics, the photosynthetic pigment contents, the enzyme activities in C3 and C4 pathway and the malate content of the flag leaf and the non-leaf organs on 7, 14, 21, and 28 days after anthesis (denoted by 7DAA, 14DAA, 21DAA, and 28DAA) were determined under well-watered (CK) and water-stressed (D) treatments. Drought stress significantly reduced the RWC of the flag leaf and the non-leaf organs, while the variation of RWC in the glume and the lemma was lower than in the flag leaf. The chlorophyll a content, the chlorophyll b content, the total chlorophyll content and the xanthophyll content in the flag leaf were significantly decreased under D. However, drought stress significantly increased the photosynthetic pigment contents in the glume at the late stage (21DAA and 28DAA). In addition, the induced activities of PEPC, NADP-MDH, NADP-ME, NAD-ME, and PPDK in non-leaf organs under drought stress suggested that the C4 photosynthetic pathway in non-leaf organs compensated the limited C3 photosynthesis in the flag leaf. Non-leaf organs, in particular the glume, showed the crucial function in maintaining the stable photosynthetic performance of oat.
Oat is an important crop ranking around sixth in the cereal cultivated area (FAO, 2019), versatilely utilized as grains and forage. Oat grain contains high levels of β-glucan and dietary fiber components and is a source of food, pharmaceutical and industrial products (Zaheri and Bahraminejad, 2012; Marshall et al., 2013; Gorash et al., 2017). In China, oat is grown over a wider area, mainly in the north and southwest, with an annual harvested area of 0.7 million ha, yielding 8.5 million tons (Diao, 2017; Zhou et al., 2018). The regions cultivating oat in China is characterized by arid and semiarid climate especially in northwest (Li et al., 2019), where water deficit is one of the major constraints for the growth and production of oats (Stevens et al., 2004; Hakala et al., 2020). Drought caused oat grains yield loss of 32%–69% (Varga et al., 2013; Zhao et al., 2021), which was associated with a marked reduction in photosynthesis ability under drought stress (Marcińska et al., 2017). Over 90% of grain yield originated from photosynthetic production, and traditionally, the flag leaf acted as the main assimilation organ for the cereal crops (Evans et al., 1980). Nevertheless, other organs, such as the reproductive structures, were often considered to be the carbon sinks, while were shown to be photosynthetically active (Hu et al., 2014; Sanchez-Bragado et al., 2014; Wang et al., 2020). Recently ear photosynthesis has been focused on its prominent compensation, especially subjected to water deficit (Tambussi et al., 2005; Abebe et al., 2010; Hein et al., 2016; Lou et al., 2018). The reported ear photosynthetic contribution to grain filling ranged from 12% to 65% (Maydup et al., 2010; Sanchez-Bragado et al., 2014; Hu et al., 2019) and the proportion was enhanced with the decrease of water supply (Zhang et al., 2011).
Drought stress inhibited all stages of plant growth and development, especially during the reproductive stage. When subjected to water deficit, the leaves would wilt and senesce due to the water loss (Abebe et al., 2010). Nevertheless, the ear of cereal crops was more resilient, maintaining the stable moisture state under drought stress (Tambussi et al., 2005; Li et al., 2017). In comparison with the leaves, a higher RWC (relative water content) of the non-leaf organs was reported in wheat (Wardlaw, 2002; Tambussi et al., 2005), barley (S’anchez-D’ıaz et al., 2002) and cotton (Hu et al., 2014), which could be explained by the higher osmotic adjustment and the xeromorphic anatomy. The osmotic adjustment was substantially higher in non-leaf organs than in leaves (Tambussi et al., 2005; Hein et al., 2016). In addition, the unique anatomical characteristics, including the thicker wax layer, smaller intercellular spaces and thicker cells, contributed to the higher water use efficiency in the non-leaf organs than in leaves (Blum, 1985; Li et al., 2006). The better photosynthetic performance of non-leaf organs was associated with the ability to maintain a steadier water state under drought stress conditions.
Apart from the higher ability to adjust water state under drought stress, the higher photosynthetic efficiency pathways were considered to sustain grain-filling in the non-leaf organs of C3 plants. A large body of evidence indicated C4 pathway, C4-like pathway or C3-C4 intermediate pathway might conduct photosynthesis in non-leaf organs of wheat (Ziegler-Jöns, 1989; Rangan et al., 2016; Balaur et al., 2018), barley (Nutbeam and Duffus, 1976), cucumber (Sui et al., 2017), tobacco and celery (Hibberd and Quick, 2002). Baluar et al. (2018) observed that similar to maize leaf, the glume, the lemma and the awn had the Kranz anatomy with two types of chloroplasts in wheat. In addition, the isotope labeling experiment indicated that most of 14C was detected in malate after assimilating 14CO2 by the illuminated ear of wheat (Singal et al., 1986). Some important photosynthetic enzymes in the C4 pathway exhibited activities in non-leaf organs of C3 plants. The key enzyme, PEPC, was determined with higher activity in non-leaf organs than in leaves and the activity of PEPC increased under drought stress conditions (Hu et al., 2014; Jia et al., 2015; Wang et al., 2020). Previous studies also reported that photosynthetic enzymes involved in three classical C4 photosynthesis subtypes, NADP-ME, NAD-ME, and PEPCK, conducted carbon fixation in the non-leaf organs (Singal et al., 1986; Wei et al., 2003; Zhang, 2019). Besides the anatomical and zymologic evidences, the molecular evidence verified that a complete set of C4 specific genes including ppc, aat, mdh, me2, gpt, and ppdk were up-regulated in caryopsis and NAD-ME type C4 photosynthesis operated in developing wheat grains (Rangan et al., 2016). The C4 pathway originated later than the C3 pathway, while provided enhanced radiation-water-and nitrogen-use efficiency especially in sub-optimal environments (Paulus et al., 2013). Thus, investigating and utilizing the C4 photosynthetic ability in the non-leaf organs of C3 plants attracted the attention of plant physiologists and crop breeders.
Avena sativa cv. Junma seeds were sown in the plastic pots (height 25 cm, diameter 27 cm) in the greenhouse of the Academy of Animal Science and Veterinary Medicine of Qinghai Province in June 2020. Each pot was filled with the field soil (weight 5 kg, maximum field capacity 35.44%) from Huangzhong County in Qinghai Province and 24 pots were used in this study. The field soil was mixed with 0.3 g urea (containing 46% N) and 0.3 g diammonium phosphate (containing 18% N and 46% P2O5) per kg soil. Before sowing, 2 L water was added to each pot. When the height of seedlings was around 10 cm, six plants were reserved in each pot. All plants were well-watered (75% of maximum field capacity, CK) till 7 days after anthesis, when half of the pots were started to water with 45% of maximum field capacity, denoted by D. CK and D treatments were controlled till seed maturation by weighing and watering the pot by using an electronic scale at 6:00 p.m. each day. The flag leaf, the sheath, the glume, and the lemma in the plant were collected at 7, 14, 21, and 28 days after anthesis, denoted by 7DAA, 14DAA, 21DAA, and 28DAA, under CK and D treatments with three replicates. All samples were saved in the refrigerator with −80°C for measuring the physiological parameters.
The flag leaf, the sheath, the glume, and the lemma samples were collected, immediately weighed to obtain the fresh weight (w1) and soaked into the distilled water for 30 h. After being weighed again, thus obtaining the saturated weight (w2), the samples were dried in an oven until constant weight (w3). The relative water content (RWC) was calculated based on the following formula.
The adaxial and abaxial surfaces of the leaf, the interior and exterior surfaces of the sheath, the glume and the exterior surfaces of the lemma on 7DAA were wiped with wet paper. The clear nail polish was smeared on both sides of four organs from 10:00 a.m. to 11:30 a.m. After 20 min, the dried nail polish was peeled away and pressed against the glass slide. The samples were observed under a microscope (DM2000 LED, Leica, Germany) at 40× magnification and the sight area was 272.03 μm × 207.61 μm. From each sample, 10 random sights were selected to record cell numbers, stoma numbers and guard cell length. Stomatal frequency and stomatal index were calculated by the following formula.
The flag leaf, the sheath, the glume, and the lemma samples were snipped into small strips and transferred to the centrifuge tubes with 10 ml of extracting solution. The extracting solution was mixed with acetone and absolute ethyl alcohol by the volume rate of 1:1. Absorbancy of extracting solution at 470, 663, and 645 nm was measured by using the full wavelength microplate analyzer (Multiskan GO, Thermo Fisher, United States). The chlorophyll a content (chla), the chlorophyll b content (chlb), the total chlorophyll content (total chl) and the xanthophyll content (xan) were calculated by the following formula.
where, V is the volume of extracting solution, W is the weight of the sample.
Pn (net photosynthetic rate) and Gs (stomatal conductance) of the flag leaf, the sheath, the glume, and the lemma were determined by using a portable photosynthesizer (Li-6800, Li-Cor, United States) at 9:00 a.m.–11:30 a.m. We chose the gasket of air chamber with the smallest size of 2 cm2. The 3 × 3 light source provided independent control of red and blue light intensities.
The samples saved in the −80°C refrigerator were used to determine the activities of Rubisco, PEPC, PPDK, NAD-ME, NADP-ME, and NADP-MDH. A 0.1 g sample was ground using a mortar at 4°C and then the grinding media of 1 ml was added. Samples were centrifuged at 8,000 g for 10 min at 4°C. The supernatant was used for the activities assays. The grinding media and the reaction mixture solutions were assay kits from the company of Suzhou Comin Biotechnology Co., Ltd. The absorbancy was recorded at 20 s and 5 min 20 s from the starting, respectively, at the wavelength of 340 nm using the full wavelength microplate analyzer (Multiskan GO, Thermo Fisher, United States).
An 0.1 g of sample was immediately frozen in liquid N2, ground thoroughly and soaked in the centrifuge tube with 1 ml of distilled water. The tube was placed in the 4°C refrigerator overnight. Following centrifugation at 8,000 g for 10 min at 4°C, the supernatant was filtered with the needle-type filter. The malate content was measured using an HPLC system (High Performance Liquid Chromatography, L3000, RIGOL, China) containing a Rigol C18 reversed-phase column (250 nm × 4.6 nm, 5 μm). The mobile phase was 0.016 M NaH2PO4 (pH 4.0) with a flow rate of 0.8 ml/min. The column temperature and the sampling time was 25°C and 30 min, respectively. The injection volume was 10 μl. Samples were detected at 214 nm.
Total RNA was extracted from the samples using the Pure Plant Total RNA Extraction Kit (TSINGKE, Beijing) as described in the manufacturer’s instructions. The ratio of absorbancy at 260–280 nm was measured by Nanophotometer of Implen (Implen, Germany). RNA integrity was detected by agarose gel electrophoresis. All samples were stored at-80°C. All RNA samples served as templates for the cDNA synthesis by the Goldenstar RT6 cDNA Synthesis Mix(TSINGKE, Beijing). The reverse transcribed products was kept at −20°C.
Quantitative real-time PCR (qRT-PCR) was performed with Step One Plus real-time quantitative PCR instrument (Thermo Fisher, America) and using the SYBR Green I PCR master mix kit (TSINGKE, Beijing) according to the Cmanufacturer’s instructions. The relative amount of gene expression was calculated using the expression of actin as internal control gene. qRT-PCR primers were as following (Table 1). The relative quantity of gene expression was calculated using 2–ΔΔCT method (Livak and Schmittgen, 2001).
The Relative water content, the stomata characteristics, the photosynthetic pigment contents, the photosynthetic parameters, the photosynthetic enzyme activities and the malate content data were subjected to ANOVA. The statistical analysis was conducted with the R software package. Duncan’s multiple range test was used to compare mean differences among treatments at the 5% probability level.
Stomata were found on the adaxial and abaxial surface of the flag leaf, the interior and the exterior surface of sheath and the glume and the exterior surface of the lemma in oat (Figure 1). The adaxial surface of the flag leaf had a higher stomatal frequency, stomatal index and guard cell length than of the interior surface of three non-leaf organs (Table 2). Nevertheless, the lower stomatal frequency was found in the abaxial surface of the flag leaf than of the exterior surface of the sheath and the glume, and the exterior surface of the sheath had a higher stomatal index than of the flag leaf.
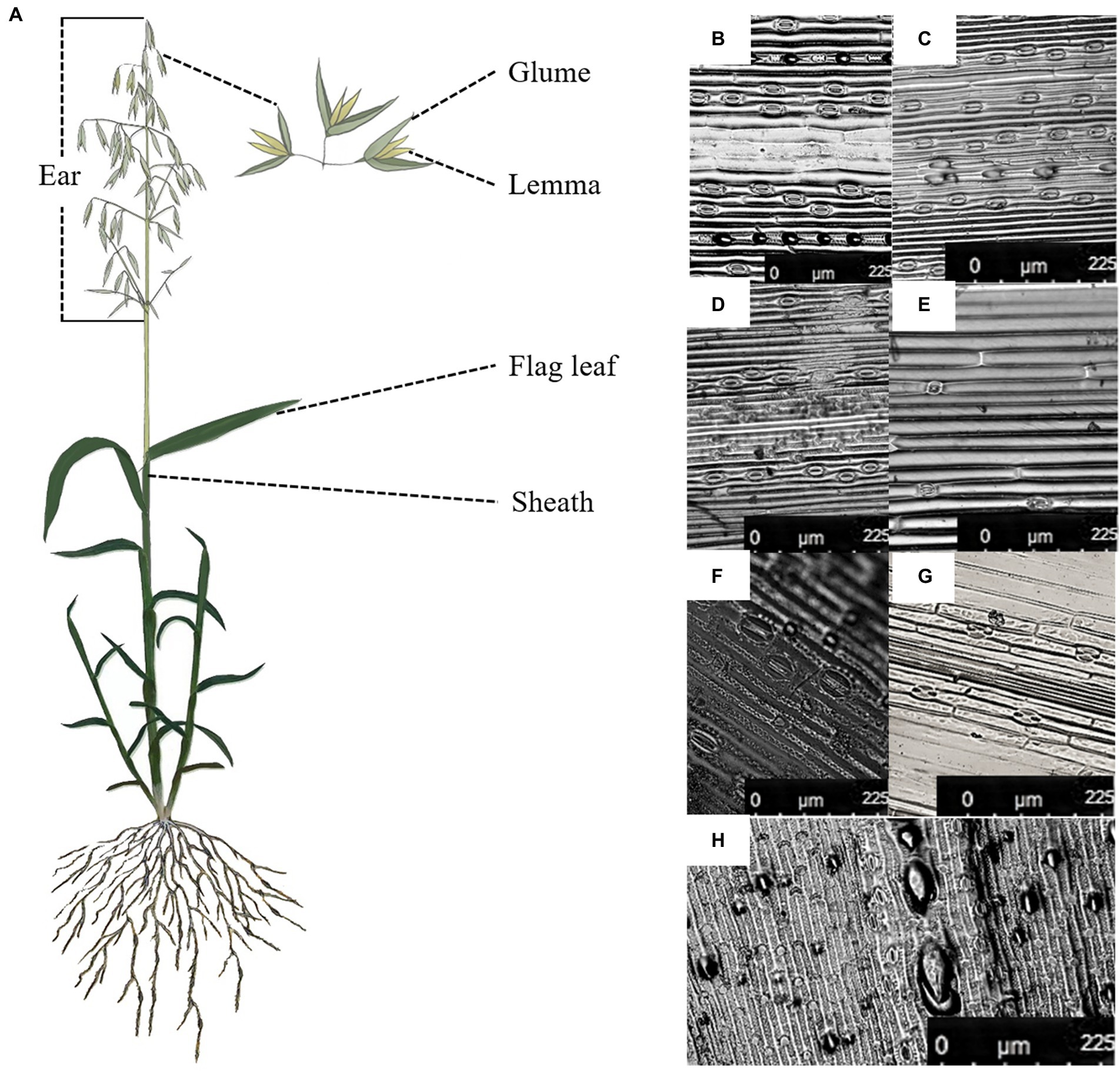
Figure 1. Stoma distribution in different green organs of oat on 7DAA. (A), The flag leaf and the non-leaf organs in the oat plant, (B), the adaxial surface of the flag leaf, (C), the abaxial surface of the flag leaf, (D), the exterior surface of the sheath, (E), the interior surface of the sheath, (F), the exterior surface of the glume, (G), the interior surface of the glume, (H), the exterior surface of the lemma.
On 21DAA and 28DAA, the flag leaf showed a significant decrease in the RWC under drought stress (Table 3). In addition, the RWC was significantly reduced under drought stress in the sheath on 14DAA, 21DAA, and 28DAA, in the glume on 21DAA and 28DAA and the lemma on 21DAA. Nevertheless, the variation of the RWC in the glume and the lemma was lower than in the flag leaf and the sheath.
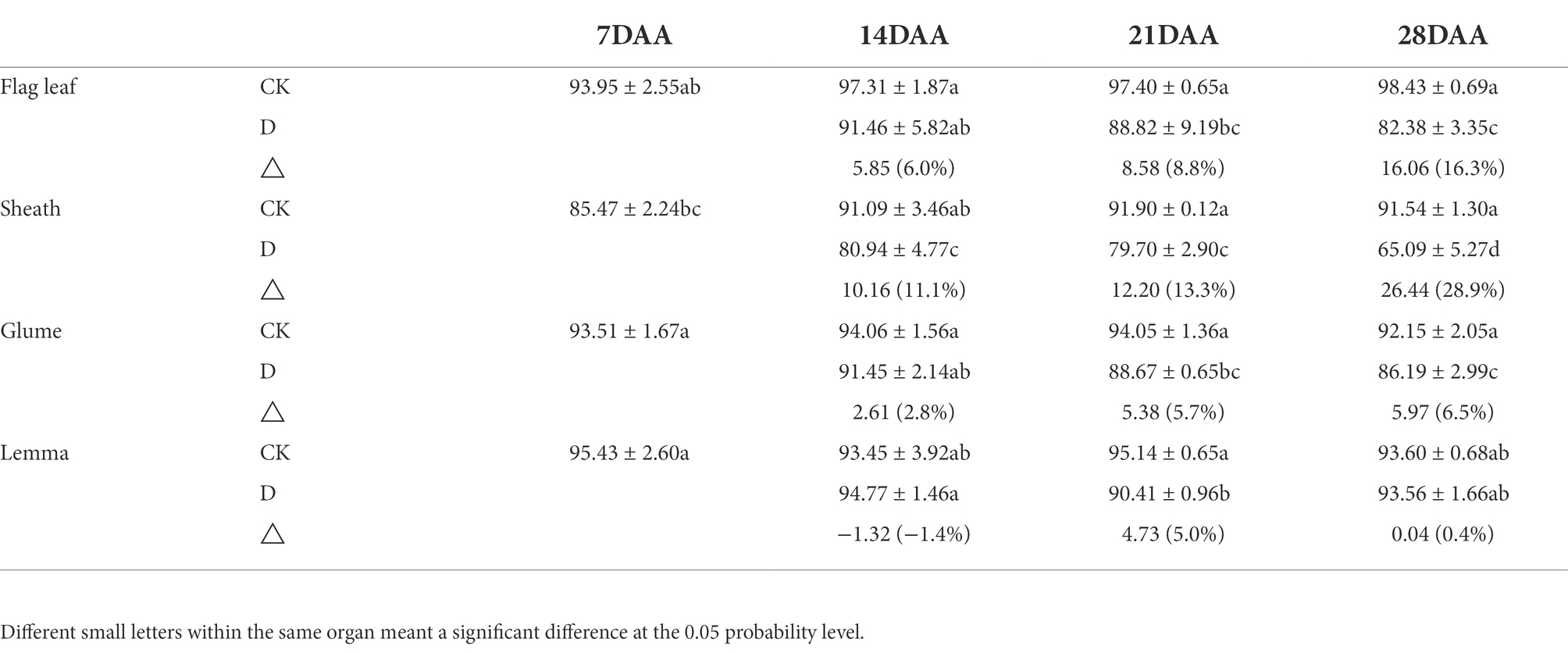
Table 3. The relative water content of the flag leaf, the sheath, the glume and the lemma under drought stress.
As the growth stage developed, the chla, the chlb, total chl and the xan gradually increased and maximized on 21DAA in the flag leaf under drought stress and CK (Figure 2). In the flag leaf drought stress significantly decreased the chla on 14DAA and 28DAA, the chlb on 14DAA, 21DAA, and 28DAA, total chl on 14DAA and 28DAA and the xan on 14DAA and 28DAA. As the growth stage developed, the chla, the chlb, total chl and the xan gradually increased and maximized on 14DAA under CK and maximized on 21DAA under drought stress in the sheath, the glume and the lemma. Drought stress led to the significant reductions of the chla, the chlb, total chl and the xan on 14DAA in the sheath. The chla on 28DAA, the chlb on 21DAA and 28DAA, total chl on 21DAA and 28DAA and the xan on 28DAA in the glume were increased significantly,while the markedly reduction of chlb was observed in the lemma on 28DAA under drought stress.
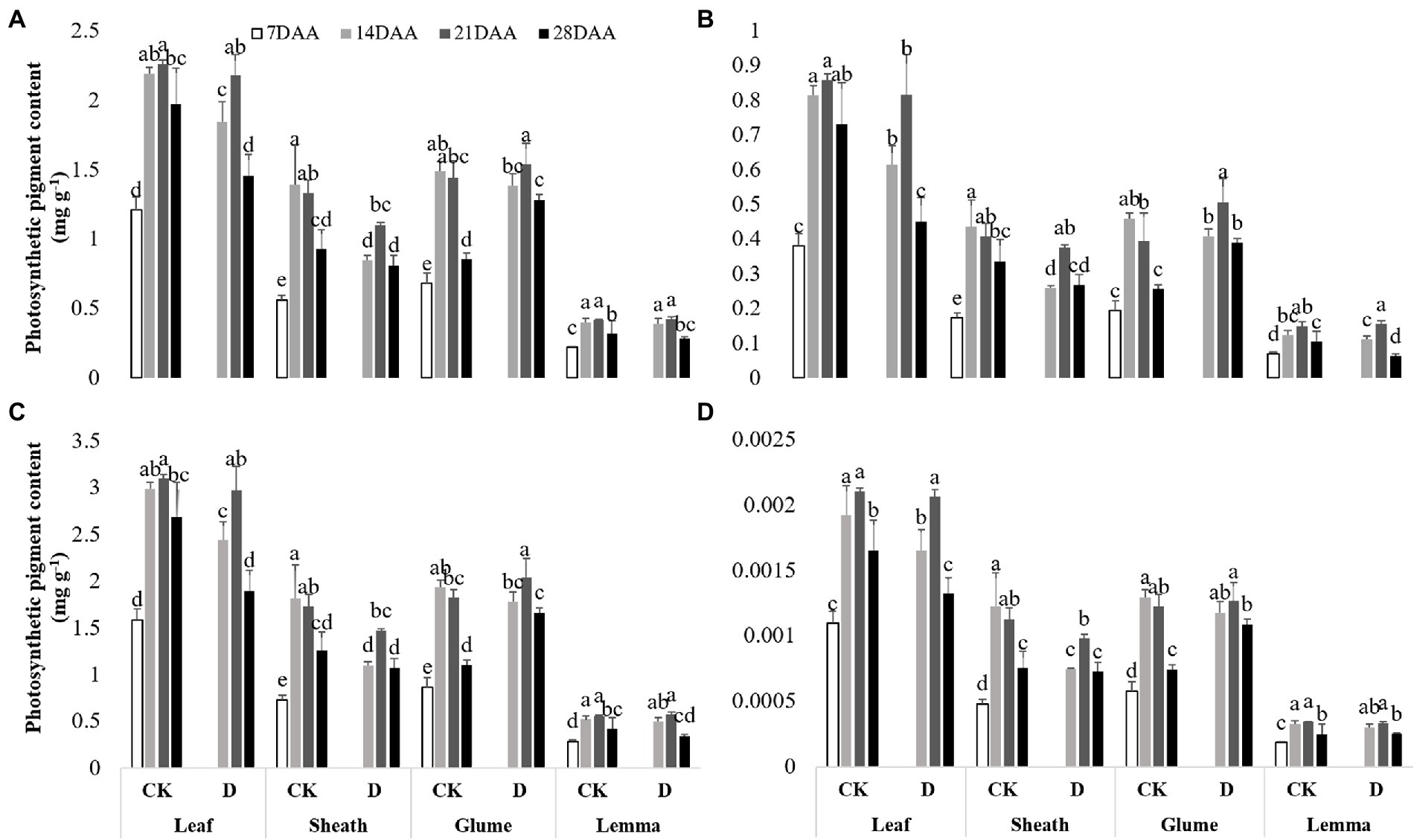
Figure 2. Photosynthetic pigment contents in the flag leaf, the sheath, the glume and the lemma under drought stress. (A), chlorophyll a content (chla), (B), chlorophyll b content (chlb), (C), total chlorophyll content (total chl), (D), xanthophyll content (xan). Different small letters within the same organ meant a significant difference at the 0.05 probability level.
As the stage developed, Pn was gradually increased and maximized on 14DAA in the flag leaf (Table 4). The significant decreases of Pn were found in the flag leaf on 14DAA, 21DAA, and 28DAA under water stress. Pn was increased significantly on 21DAA, but was decreased significantly on 28DAA in the sheath. In addition, drought stress led to a marked reduction of Pn in the glume on 14DAA, 21DAA, and 28DAA and in the lemma on 14DAA and 28DAA.
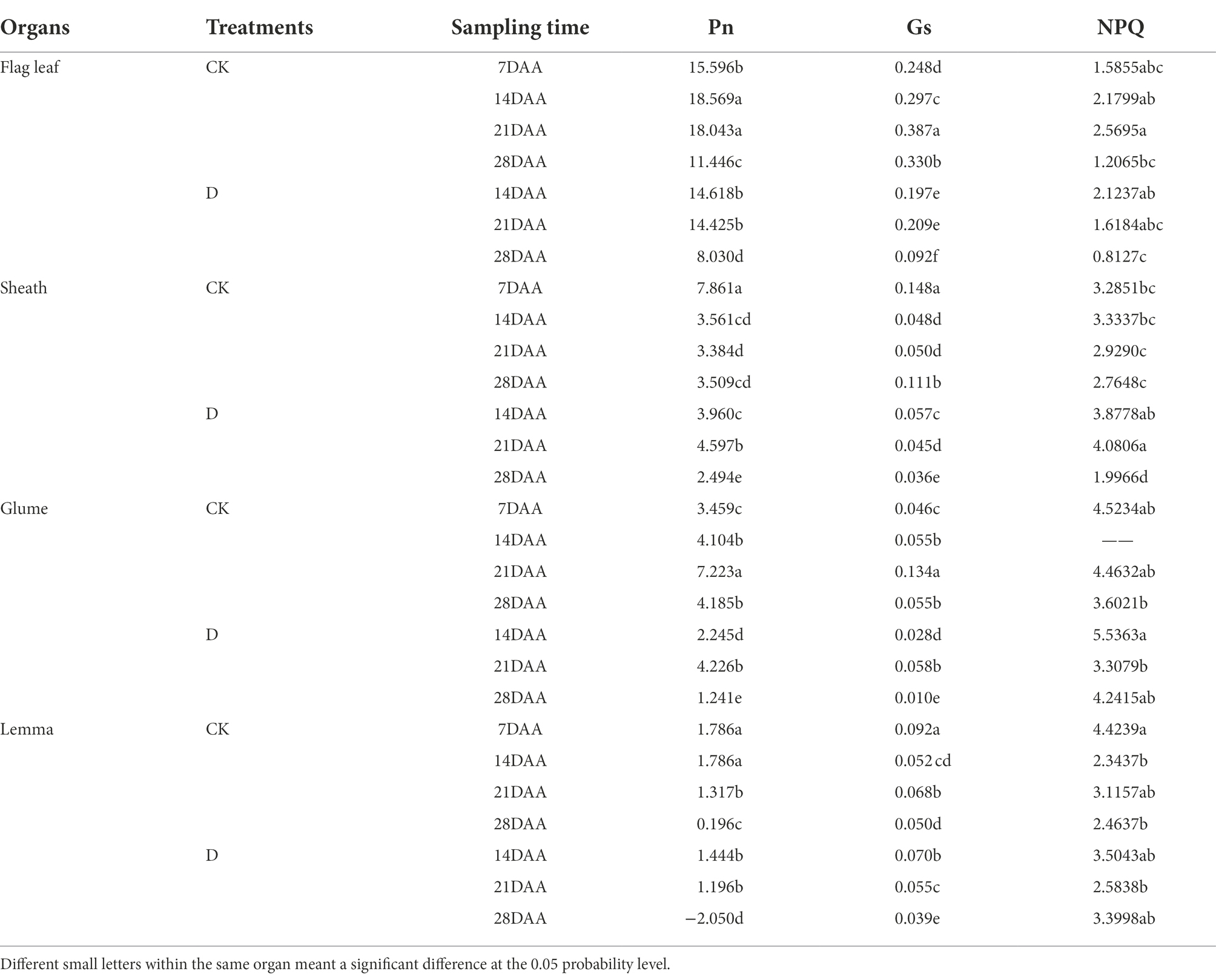
Table 4. Photosynthetic parameters in the flag leaf, the sheath, the glume and the lemma under drought stress.
Gs was reduced significantly on 14DAA, 21DAA, and 28DAA in the flag leaf. Drought stress significantly increased Gs in the sheath on 14DAA, but decreased on 21DAA, and 28DAA. Gs was decreased significantly in the glume on 14DAA, 21DAA, and 28DAA and in the lemma on 21DAA and 28DAA, but increased on 14DAA in the lemma.
As the stage developed, NPQ was first increased then decreased in the flag leaf under CK, while was decreased under drought stress. NPQ was significantly increased on 21DAA under drought stress, but was decreased on 28DAA in the sheath. Under CK and D treatments, NPQ was higher in sheath, glume and lemma than in flag leaf.
Drought stress significantly decreased the Rubisco activity in the flag leaf on 14DAA, 21DAA, and 28DAA (Figure 3A-I). In addition, Rubisco activity was significantly declined in the sheath and the glume on 28DAA (Figures 3A-II,III).
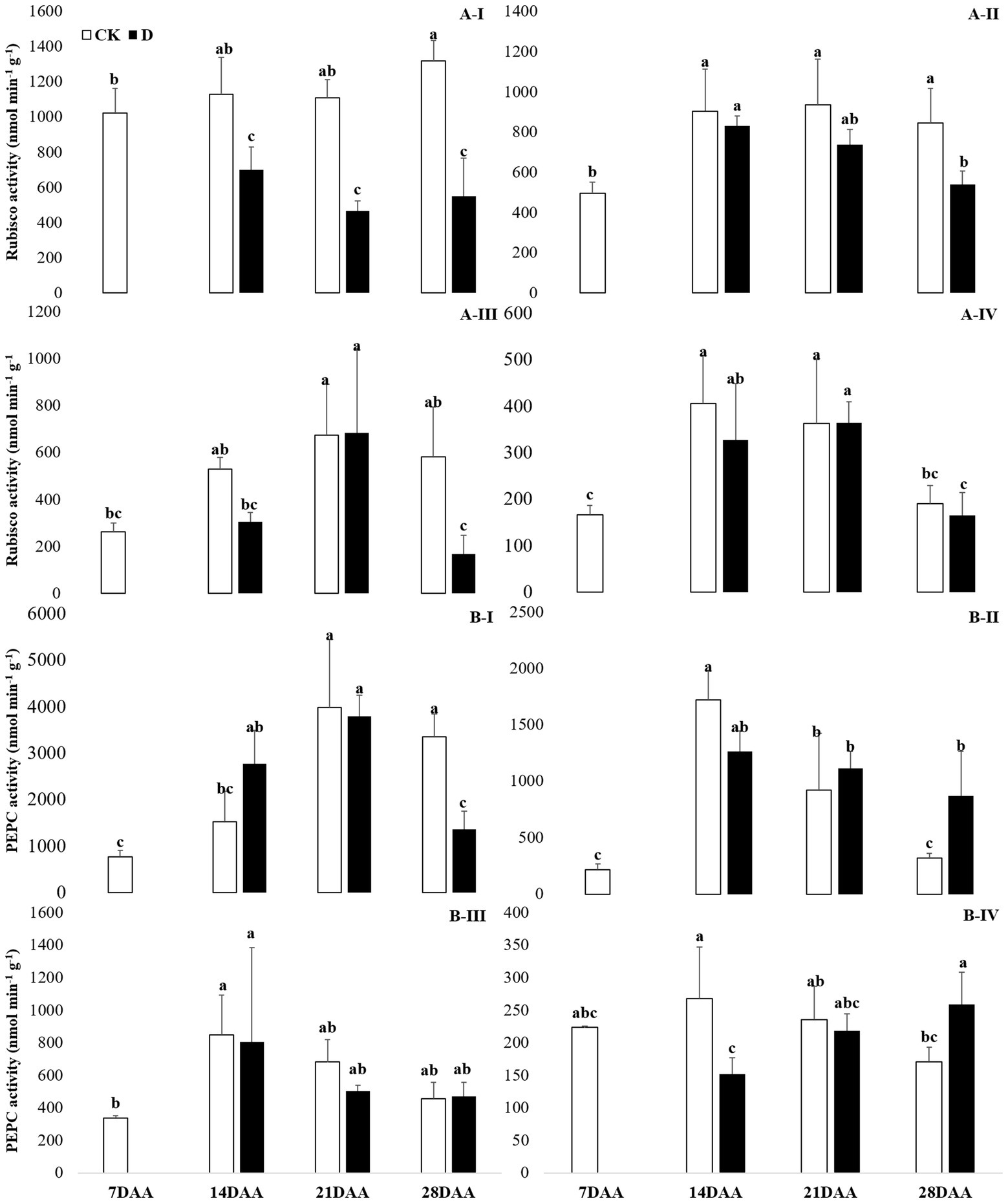
Figure 3. Rubisco (A) and PEPC (B) activity in the flag leaf (I), the sheath (II), the glume (III) and the lemma (IV) under drought stress. Different small letters within the same organ meant a significant difference at the 0.05 probability level.
A significant reduction of PEPC activity was found in the flag leaf on 28DAA under water stress (Figure 3B-I). Drought stress decreased PEPC activity in the lemma on 14DAA (Figure 3B-IV), but increased significantly in the sheath and the lemma on 28DAA (Figures 3B-II,III).
Under drought stress, NADP-MDH activity was increased significantly in the flag leaf on 14DAA, 21DAA, and 28DAA (Figure 4A-I). In addition, drought stress led to the markedly decreases of NADP-MDH activity in the sheath on 14DAA, 21DAA, and 28DAA (Figure 4A-II). NADP-MDH activity was significantly increased in the glume on 14DAA and in the lemma on 21DAA (Figure 4A-III), but decreased significantly in the lemma on 14DAA (Figure 4A-IV).
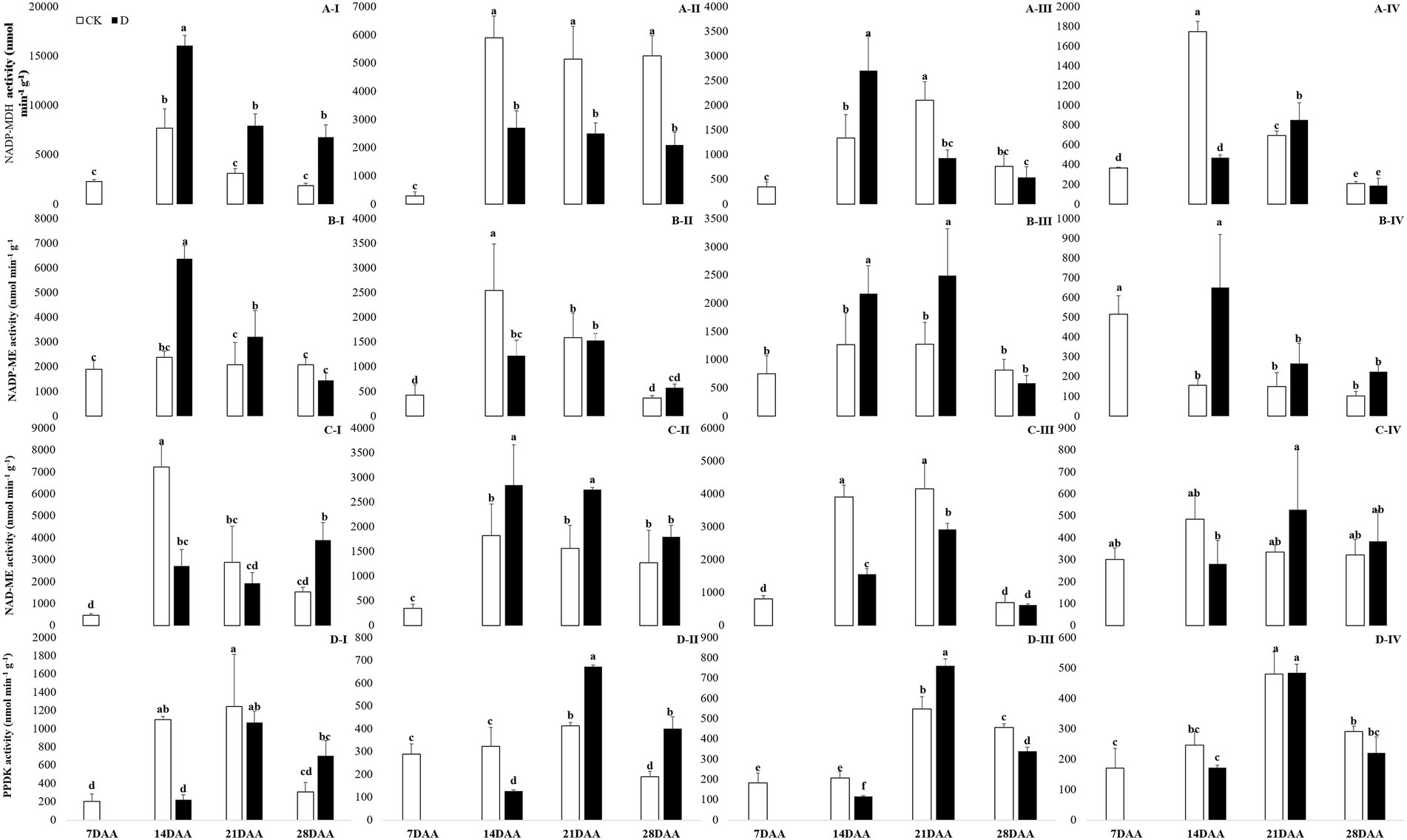
Figure 4. NADP-MDH (A), NADP-ME (B), NAD-ME (C) and PPDK (D) activity in the flag leaf (I), the sheath (II), the glume (III) and the lemma (IV) under drought stress. Different small letters within the same organ meant a significant difference at the 0.05 probability level.
NADP-ME activity was significantly increased in the flag leaf on 14DAA and 21DAA under water stress (Figure 4B-I). Drought stress significantly decreased NADP-ME activity in the sheath on 14 DAA (Figure 4B-II), but increased in the glume on 14DAA and 21DAA (Figure 4B-III) and in the lemma on 14DAA (Figure 4B-IV).
NAD-ME activity was significantly decreased in the flag leaf on 14DAA, but increased on 28DAA under drought stress (Figure 4C-I). Drought stress decreased significantly NAD-ME activity in the glume on 14DAA and 21DAA (Figure 4C-III), but increased significantly in the sheath on 14DAA and 21DAA (Figure 4C-II).
PPDK activity was significantly decreased in the flag leaf on 14DAA under water stress (Figure 4D-I). Drought stress significantly reduced PPDK activity in the sheath on 14DAA (Figure 4D-II) and in the glume on 14DAA and 28DAA (Figure 4D-III), but increased in the sheath and the glume on 21DAA (Figures 4D-II,III).
PEPC was significantly downregulated in the flag leaf on 14DAA and 28 DAA under drought stress and was significantly decreased as the stage developed (Figure 5A). In the glume, PEPC was significantly downregulated on 14DAA and in the lemma on 14DAA and 21DAA under drought stress (Figure 5A). MDH was significantly upregulated in the leaf at 14DAA and significantly down-regulated in the sheath on 21DAA under drought stress (Figure 5B). In addition, MDH was significantly upregulated in the glume and the lemma on 14DAA, 21DAA, and 28DAA under drought stress (Figure 5B). Under drought stress, the sheath, glume and lemma had the higher relative expression of PEPC and MDH than flag leaf (Figure 5).
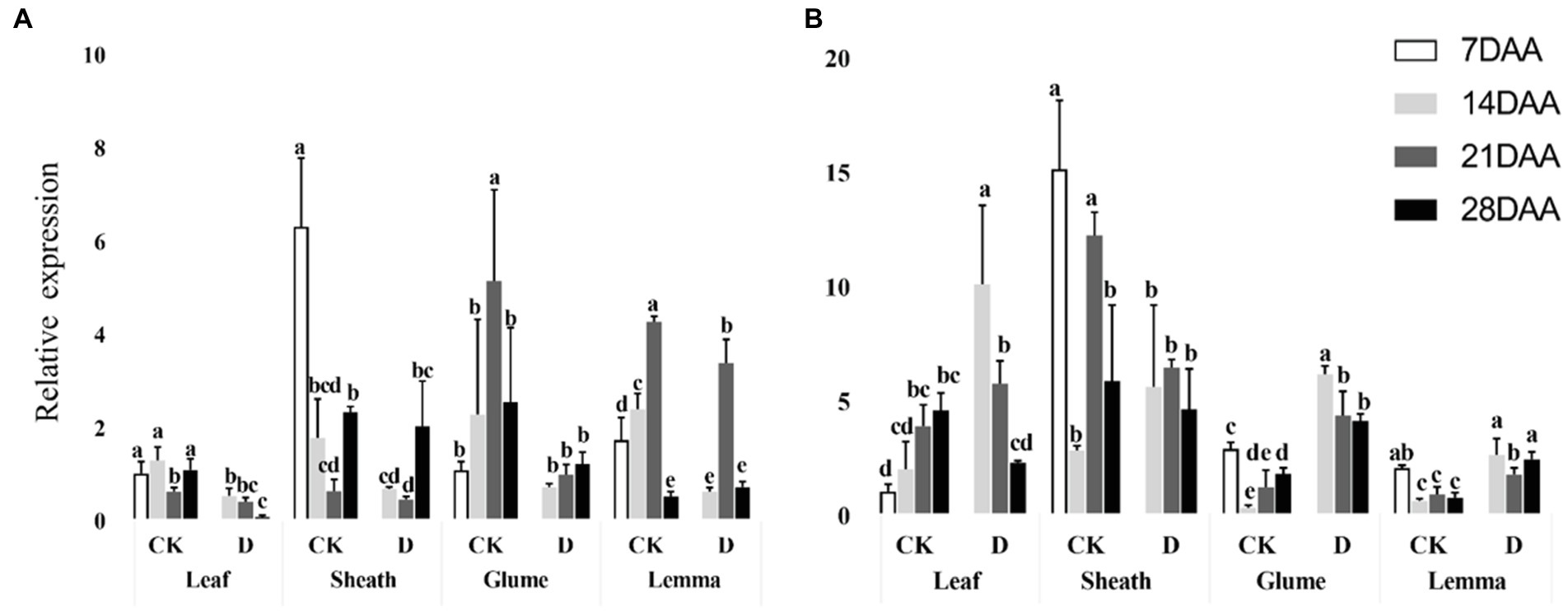
Figure 5. Relative expression of PEPC gene (A) and MDH gene (B) in different organs under control and drought stress. Different small letters within the same organ meant a significant difference at the 0.05 probability level.
Malate content was significantly decreased in the flag leaf and the sheath on 14DAA under drought stress, but increased significantly in the flag leaf, the sheath and the glume on 28DAA (Figure 6). Drought stress significantly increased malate content in the lemma on 14DAA, but decreased significantly on 28DAA.
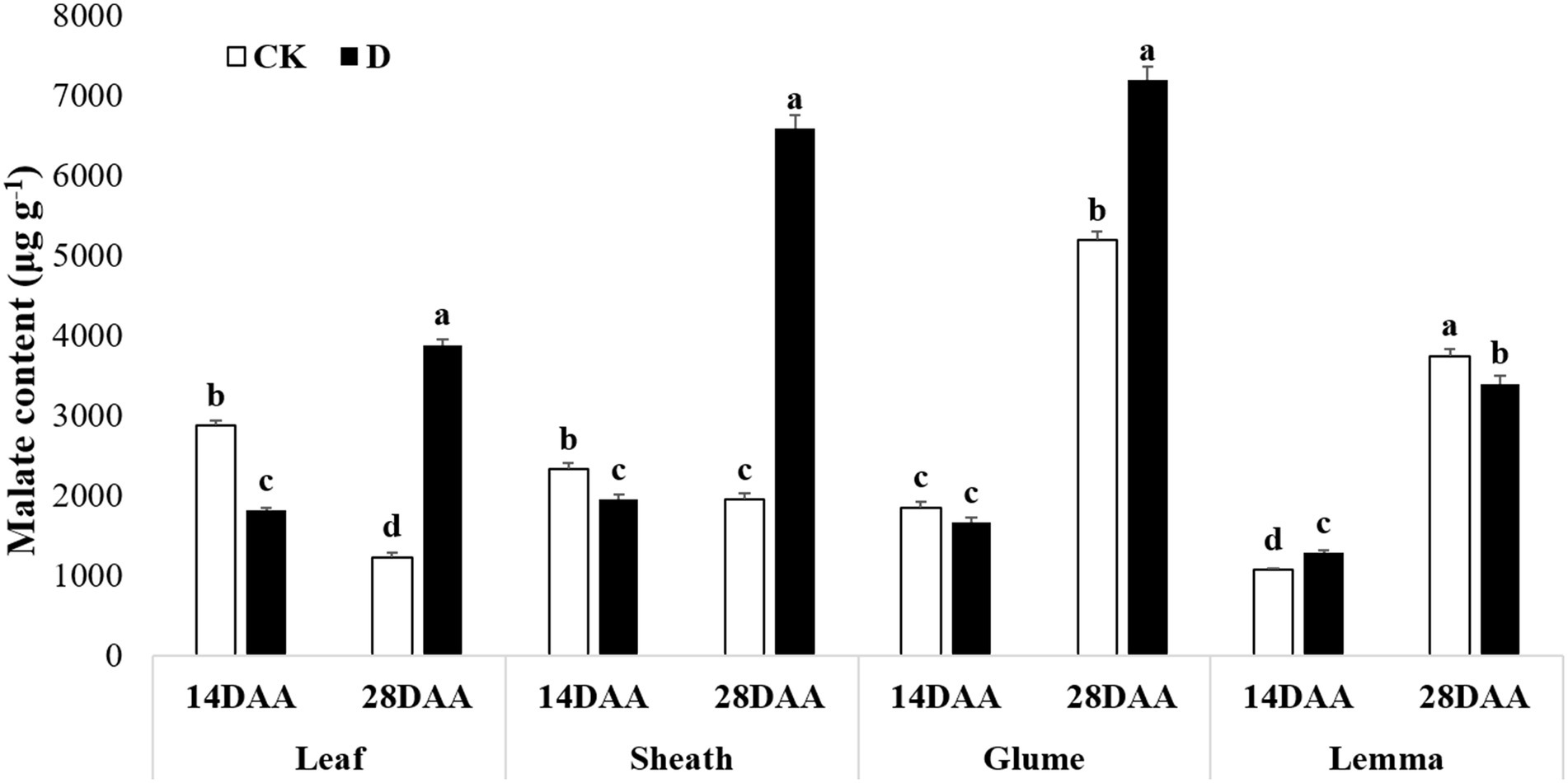
Figure 6. Malate content in the flag leaf, the sheath, the glume and the lemma under drought stress. Different small letters within the same organ meant a significant difference at the 0.05 probability level.
The greater tolerance of non-leaf organs to drought stress has been previously reported in some studies (Xu and Ishii, 1990; Wardlaw, 2002; Tambussi et al., 2005; Jia et al., 2015; Hein et al., 2016). In this study, the glume and the lemma in oat maintained the higher RWC under drought stress with the lower variation compared to the flag leaf (Table 3). The differences in the capacity to maintain high RWC between leaves and non-leaf organs could be due to the variance in sclerophyllous characteristics (Araus et al., 1986) and the capacity of osmotic adjustment (Hein et al., 2016). Compared to leaves, smaller and denser cells, lower intercellular spaces and thicker cellular walls were observed in non-leaf organs including the glume, the lemma and the awn in cereals, which contributed to reducing the damage resulted from drought stress (Li et al., 2006; Tambussi et al., 2007). In addition, drought stress could induce the accumulation of osmotic substances including the proline and the raffinose and increase the osmotic potential in ear organs (Tambussi et al., 2005; Li et al., 2006; Abebe et al., 2010; Jia et al., 2015). The non-leaf organs could ensure better physiological performance by maintaining a buffer function under drought conditions, performing better drought adaptation.
In addition to the differences of RWC, the non-leaf organs and the flag leaf had different changes in the content of photosynthetic pigments and photosynthetic parameters during the drought treatment. Stable photosynthetic performance implies a strong tolerance to drought stress. Chlorophyll content was lower in non-leaf organs than in leaves, which was found in wheat (Lu and Lu, 2004; Lou et al., 2018), cotton (Zhang et al., 2016) and soybean (Andrews and Svec, 1975). However, the greater photosynthetic activity could be measured per chlorophyll in non-leaf organs than in leaves (Andrews and Svec, 1975; Tambussi et al., 2007). In this study, the drought stress could significantly reduce the chla, chlb, total chl, xan and Pn in the flag leaf at three sampling times, while the photosynthetic pigments content in the glume were increased significantly at the late growth stage (21DAA and 28DAA; Figure 2; Table 4). Similarly, the chlorophyll content in non-leaf organs in cotton and wheat was influenced less by the drought condition, compared with leaves (Zhang et al., 2016; Lou et al., 2018). The regulation effect of the xanthophyll cycle to thermal dissipation was verified in rice and oilseed rape (Zhu et al., 2011). In this study, we found that the higher xan was measured in the glume on 28DAA under drought stress (Figure 2D), which reflected the xanthophyll cycle participated in dissipating excess energy in the glume of oat as well under drought conditions. Among the organs evaluated in this study, drought stress significantly reduced the Pn of the flag leaf, the glume and the lemma, not for the sheath, and even the Pn of the lemma on 28DAA was negative (Table 4). Under the drought condition, the green organs would close the stoma to reduce water evaporation, which attenuated dark reaction (Reddy et al., 2004). In addition, persistent drought stress might cause the degradation of photosynthetic pigments, the damage to membrane system and the reduction of synthetase activity (Ladjal et al., 2000; Jaleel et al., 2009). Previous studies reported that the reduction of Pn in bracts was significantly less than in leaves in cotton under drought stress (Wullschleger et al., 1990; Hu et al., 2014; Zhang et al., 2016). The photosynthesis was less sensitive to water deficit in the non-leaf organs than in leaves, showing a larger contribution to the seed yield (Martinez et al., 2003; Jia et al., 2015). NPQ value represents the ability to protect plants from photodamage by dissipating excess light energy under stress conditions. PSII (Photosynthetic system II) in plants was damaged initially during photosynthesis when confronted stress (Xiao et al., 2019). The higher NPQ value was determined in the glume of wheat than in leaves (Kong et al., 2015), which was consistent to our findings that the non-leaf organs had the higher NPQ value. Under drought stress, as the growth stage developed, the NPQ value in the flag leaf decreased, while the non-leaf maintained stable (Table 4). This suggested higher photosynthetic resistance of the non-leaf organs to the water deficit compared with leaves.
The results showed that Rubisco activity was markedly influenced by the drought stress in the flag leaf at three growth stages (Figure 3). These findings were in agreement with previous reports for wheat (Guliyev et al., 2008; Simova-Stoilova et al., 2020), rice (Gujjar et al., 2020), cotton (Carmo-Silva et al., 2012; Hu et al., 2014). Rubisco was regarded as the key enzyme of the Calvin cycle (Lawlor et al., 1989), the reduction of Rubisco activity and content resulted in the decline of photosynthetic ability (Hu et al., 2014). In addition to C3 pathway enzymes, some C4 pathway enzymes were activated in non-leaf organs in C3 plants and water deficit could induce the activities (Wei et al., 2003; Jia et al., 2015; Wang et al., 2020). In the present study, PEPC activity was significantly higher in the sheath and the lemma on 28DAA under drought treatment (Figure 3). The drought stress could significantly induce the activities of NADP-MDH, NADP-ME, NAD-ME, and PPDK in non-leaf organs (Figure 4). The previous studies reported that in non-leaf organs, the PEPC activity was significantly higher than in leaves and was induced under drought stress (Imaizumi et al., 1990; Jia et al., 2015; Zhang, 2019; Wang et al., 2020). The PEP in non-leaf organs was considered to have the possibility to recapture the respired CO2 in the dark reaction (Singal et al., 1986). Drought stress significantly improved the activities of NADP-MDH, NADP-ME, PPDK and induced the expression of NADP-MDH-7, NADP-ME-1, PPDK-1 in the glume of wheat, which was the explanation for the photosynthetic persistence and drought tolerance of non-leaf organs when confronted under water deficit (Jia et al., 2015; Zhang, 2019). In this study, the ear organs had the higher relative expression of PEPC and MDH than flag leaf under drought stress (Figure 5). Malate is the initial product of photosynthetic CO2 fixation in C4 plant leaves and ears in wheat as well (Singal et al., 1986). Photosynthetic enzymes in C4 cycle played important roles in the anaplerosis of intermediates, such as malate, and supplying carbon skeleton for the amino acids formation (Lea et al., 2001). Therefore, in accordance with the variation of enzyme activities in C4 cycle, malate content was significantly increased in the glume and the lemma (Zhang, 2019). The increase of malate content likely contributed to improve the osmotic adjustment and supply carbon skeleton. In this study, drought stress significantly increased the malate content in the lemma on 14DAA and in the sheath and the glume on 28DAA (Figure 6).
This study has evaluated the changes of the relative water content, the photosynthetic pigment contents, the photosynthetic parameters, the important enzyme activities in C3 and C4 pathway and the malate content in the flag leaf, the sheath, the glume and the lemma of oat under drought stress. These results suggest that C4 photosynthetic enzymes in non-leaf organs, especially in glume, play crucial functions in improving oat’ overall photosynthetic capacity, which contributes to maintaining the stable photosynthetic performance under drought stress conditions.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
HT and HW prepared the initial draft. QZ, WL, JZ, YC, and ZJ revised the manuscript. YS drew the Figure 1. HW designed the experiment. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (32001392), the China Agriculture Research System of MOF and MARA (CARS-34) and “the Fundamental Research Funds for the Central Universities,” Southwest Minzu University (2021PTJS30).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abebe, T., Melmaiee, K., Berg, V., and Wise, R. P. (2010). Drought response in the spikes of barley: gene expression in the lemma, palea, awn, and seed. Funct. Integr. Genomics 10, 191–205. doi: 10.1007/s10142-009-0149-4
Andrews, A. K., and Svec, L. V. (1975). Photosynthetic activity of soybean pods at different growth stages compared to leaves. Canadian J. Plant Sci. 55, 501–505. doi: 10.4141/cjps75-076
Araus, J. L., Alegre, L., Tapia, L., and Calafell, R. (1986). Relationship between leaf structure and gas exchange in wheat leaves at different insertion levels. J. Exp. Bot. 37, 1323–1333. doi: 10.1093/jxb/37.9.1323
Balaur, N., Badicean, D., Peterhaensel, C., Gardestrom, P., Mereniuc, L., and Vorontsov, V. (2018). The peculiarities of carbon metabolism in the ears of C3 cereals: The carbon metabolism and key genes expression in the photosyn-thetic active components of the ear of cereals. J. Tissue Cult. Bio. 1–14. doi: 10.29011/JTCB-102
Blum, A. (1985). Photosynthesis and transpiration in leaves and ears of wheat and barley varieties. J. Exp. Bot. 36, 432–440. doi: 10.1093/jxb/36.3.432
Carmo-Silva, A. E., Gore, M. A., Andrade-Sanchez, P., French, A. N., Hunsaker, D. J., and Salvucci, M. E. (2012). Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 83, 1–11. doi: 10.1016/j.envexpbot.2012.04.001
Diao, X. (2017). Production and genetic improvement of minor cereals in China. Crop J. 5, 103–114. doi: 10.1016/j.cj.2016.06.004
Evans, L. T., Wardlaw, I. F., and Fischer, R. A. (1980). “Wheat,” in Crop Physiology. ed. L. T. Evans (Cambridge: Cambridge University Press), 101–149.
FAO. (2019). Available at: http://faostat.fao.org (Accessed on July 9, 2021).
Gorash, A., Armonienė, R., Mitchell, F. J., and Danytė, V. (2017). Aspects in oat breeding: nutrition quality, nakedness and disease resistance, challenges and perspectives. Ann. Appl. Biol. 171, 281–302. doi: 10.1111/aab.12375
Gujjar, R. S., Banyen, P., Chuekong, W., Worakan, P., Roytrakul, S., and Supaibulwatana, K. A. (2020). A synthetic cytokinin improves photosynthesis in rice under drought stress by modulating the abundance of proteins related to stomatal conductance, chlorophyll contents, and rubisco activity. Plan. Theory 9, 1–21. doi: 10.3390/plants9091106
Guliyev, N., Bayramov, S., and Babayev, H. (2008). Effect of water deficit on RUBISCO and carbonic anhydrase activities in different wheat genotypes. Photosynthesis. Energy from the Sun. (Berlin: Springer), 1465–1468.
Hakala, K., Jauhiainen, L., Rajala, A. A., Jalli, M., Kujala, M., and Laine, A. (2020). Different responses to weather events may change the cultivation balance of spring barley and oats in the future. F. Crop Res. 259:107956. doi: 10.1016/j.fcr.2020.107956
Hein, J. A., Sherrard, M. E., Manfredi, K. P., and Abebe, T. (2016). The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 16, 248–212. doi: 10.1186/s12870-016-0922-1
Hibberd, J. M., and Quick, W. P. (2002). Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415, 451–454. doi: 10.1038/415451a
Hu, L., Zhang, Y., Xia, H., Fan, S., Song, J., Lv, X., et al. (2019). Photosynthetic characteristics of non-foliar organs in main C3 cereals. Physiol. Plant. 166, 226–239. doi: 10.1111/ppl.12838
Hu, Y. Y., Zhang, Y. L., Yi, X. P., Zhan, D. X., Luo, H. H., Soon, C. W., et al. (2014). The relative contribution of non-foliar organs of cotton to yield and related physiological characteristics under water deficit. J. Integr. Agric. 13, 975–989. doi: 10.1016/S2095-3119(13)60568-7
Imaizumi, N., Usuda, H., Nakamoto, H., and Ishihara, K. (1990). Changes in the rate of photosynthesis during grain filling and the enzymatic activities associated with the photosynthetic carbon metabolism in rice panicles. Plant Cell Physiol. 31, 835–844. doi: 10.1093/oxfordjournals.pcp.a077986
Jaleel, C. A., Manivannan, P., Wahid, A., Farooq, M., Somasundaram, R., and Panneerselvam, R. (2009). Drought stress in plants: a review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 7, 203–216. doi: 10.3763/ijas.2009.0459
Jia, S., Lv, J., Jiang, S., Liang, T., Liu, C., and Jing, Z. (2015). Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynthetica 53, 95–109. doi: 10.1007/s11099-015-0087-4
Kong, L., Sun, M., Xie, Y., Wang, F., and Zhao, Z. (2015). Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 6, 1–10. doi: 10.3389/fpls.2015.00358
Ladjal, M., Epron, D., and Ducrey, M. (2000). Effects of drought preconditioning on thermo tolerance of photosystem II and susceptibility of photosynthesis to heat stress in cedar seedlings. Tree Physiol. 20, 1235–1241. doi: 10.1093/treephys/20.18.1235
Lawlor, D. W., Kontturi, M., and Young, A. T. (1989). Photosynthesis by flag leaves of wheat in relation to protein, ribulose Bisphosphate carboxylase activity and nitrogen supply. J. Exp. Bot. 40, 43–52. doi: 10.1093/jxb/40.1.43
Lea, P. J., Chen, Z. H., Leegood, R. C., and Walker, R. P. (2001). Does phosphoenolpyruvate carboxykinase have a role in both amino acid and carbohydrate metabolism? Amino Acids. 20, 225–241. doi: 10.1007/s007260170041
Li, Y., Li, H., Li, Y., and Zhang, S. (2017). Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 5, 231–239. doi: 10.1016/j.cj.2017.01.001
Li, X., Wang, H., Li, H., Zhang, L., Teng, N., Lin, Q., et al. (2006). Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiol. Plant. 127, 701–709. doi: 10.1111/j.1399-3054.2006.00679.x
Li, X., You, Q., Ren, G., Wang, S., Zhang, Y., Yang, J., et al. (2019). Concurrent droughts and hot extremes in Northwest China from 1961 to 2017. Int. J. Climatol. 39, 2186–2196. doi: 10.1002/joc.5944
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lou, L., Li, X., Chen, J., Li, Y., Tang, Y., and Lv, J. (2018). Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS One 13, e0194625–e0194618. doi: 10.1371/journal.pone.0194625
Lu, Q., and Lu, C. (2004). Photosynthetic pigments composition and photosystem II photochemistry of wheat ears. Plant Physiol. Biochem. 42, 395–402. doi: 10.1016/j.plaphy.2004.02.008
Marcińska, I., Czyczyło-Mysza, I., Skrzypek, E., Grzesiak, M. T., Popielarska-Konieczna, M., Warchoł, M., et al. (2017). Application of photochemical parameters and several indices based on phenotypical traits to assess intraspecific variation of oat (Avena sativa L.) tolerance to drought. Acta Physiol. Plant. 39, 1–13. doi: 10.1007/s11738-017-2453-2
Marshall, A., Cowan, S., Edwards, S., Griffiths, I., Howarth, C., Langdon, T., et al. (2013). Crops that feed the world 9. Oats- a cereal crop for human and livestock feed with industrial applications. Food Secur. 5, 13–33. doi: 10.1007/s12571-012-0232-x
Martinez, D. E., Luquez, V. M., Bartoli, C. G., and Guiamét, J. J. (2003). Persistence of photosynthetic components and photochemical efficiency in ears of water-stressed wheat (Triticum aestivum). Physiol. Plant. 119, 519–525. doi: 10.1046/j.1399-3054.2003.00195.x
Maydup, M. L., Antonietta, M., Guiamet, J. J., Graciano, C., López, J. R., and Tambussi, E. A. (2010). The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). F. Crop. Res. 119, 48–58. doi: 10.1016/j.fcr.2010.06.014
Nutbeam, A. R., and Duffus, C. M. (1976). Evidence for C4 photosynthesis in barley pericarp tissue. Biochem. Biophys. Res. Commun. 70, 1198–1203. doi: 10.1016/0006-291X(76)91029-9
Paulus, J. K., Schlieper, D., and Groth, G. (2013). Greater efficiency of photosynthetic carbon fixation due to single amino-acid substitution. Nat. Commun. 4, 1518. doi: 10.1038/ncomms2504
Rangan, P., Furtado, A., and Henry, R. J. (2016). New evidence for grain specific C4 photosynthesis in wheat. Sci. Rep. 6, 1–12. doi: 10.1038/srep31721
Reddy, A. R., Chaitanya, K. V., and Vivekanandan, M. (2004). Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 161, 1189–1202. doi: 10.1016/j.jplph.2004.01.013
S’anchez-D’ıaz, M., Garc’ıa, J. L., Antol’ın, M. C., and Araus, J. L. (2002). Effects of soil drought and atmospheric humidity on yield, gas exchange, and stable carbon composition of barley. Photosynthetica 40, 415–421. doi: 10.1023/A:1022683210334
Sanchez-Bragado, R., Molero, G., Reynolds, M. P., and Araus, J. L. (2014). Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. J. Exp. Bot. 65, 5401–5413. doi: 10.1093/jxb/eru298
Simova-Stoilova, L., Kirova, E., and Pecheva, D. (2020). Drought stress response in winter wheat varieties – changes in leaf proteins and proteolytic activities. Acta Bot. Croat. 79, 121–130. doi: 10.37427/BOTCRO-2020-018
Singal, H. R., Sheoran, I. S., and Singh, R. (1986). In vitro enzyme activities and products of 14CO2 assimilation in flag leaf and ear parts of wheat (Triticum aestivum L.). Photosynthesis Res. 8, 113–122. doi: 10.1007/BF00035242
Stevens, E. J., Armstrong, K. W., Bezar, H. J., Griffin, W. B., and Hampton, J. G. (2004). “Fodder oats, an overview,” in Fodder oats, a world overview. eds. J. M. Suttie and S. G. Reynolds (Rome: Food and Agriculture Organization of the United Nations), 1–9.
Sui, X., Shan, N., Hu, L., Zhang, C., Yu, C., Ren, H., et al. (2017). The complex character of photosynthesis in cucumber fruit. J. Exp. Bot. 68, 1625–1637. doi: 10.1093/jxb/erx034
Tambussi, E. A., Bort, J., Guiamet, J. J., Nogués, S., and Araus, J. L. (2007). The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. CRC Crit. Rev. Plant Sci. 26, 1–16. doi: 10.1080/07352680601147901
Tambussi, E. A., Nogués, S., and Araus, J. L. (2005). Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221, 446–458. doi: 10.1007/s00425-004-1455-7
Varga, B., Varga-László, E., Bencze, S., Balla, K., and Veisz, O. (2013). Water use of winter cereals under well-watered and drought-stressed conditions. Plant Soil Environ. 59, 150–155. doi: 10.17221/658/2012-pse
Wang, H., Zhou, Q., and Mao, P. (2020). Ultrastructural and photosynthetic responses of pod walls in alfalfa to drought stress. Int. J. Mol. Sci. 21, 1–19. doi: 10.3390/ijms21124457
Wardlaw, I. F. (2002). Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Ann. Bot. 90, 469–476. doi: 10.1093/aob/mcf219
Wei, A. L., Wang, Z. M., Zhai, Z. X., and Gong, Y. S. (2003). Effect of soil drought on C4 photosynthetic enzyme activities of flag leaf and ear in wheat. Sci. Agric. Sin. 36, 508–512.
Wullschleger, S. D., Oosterhuis, D. M., and Rutherford, S. A. (1990). Importance of bracts in the carbon economy of cotton. Arkansas Farm Res. 39, 4.
Xiao, M., Li, Y., Wang, J., Hu, X., Wang, L., and Miao, Z. (2019). Study on the law of nitrogen transfer and conversion and use of fertilizer nitrogen in paddy fields under water-saving irrigation mode. Water 11, 1–13. doi: 10.3390/w11020218
Xu, H. L., and Ishii, R. (1990). Effects of water deficit on photosynthesis in wheat plants: V. Difference among plant parts in water relations. Japanese J. Crop Sci. 59, 384–389. doi: 10.1626/jcs.59.384
Zaheri, A., and Bahraminejad, S. (2012). Assessment of drought tolerance in oat (Avena sativa) genotypes. Ann. Biol. Res. 3, 2194–2201.
Zhang, X. (2019). Effects of Water Deficit on C3 and C4 Photosynthesis Enzymes and Primary Carbon Metabolism in Wheat Flag Leaves and Spikes. Yangling: Northwest Agriculture and Forestry University.
Zhang, C., Zhan, D. X., Luo, H. H., Zhang, Y. L., and Zhang, W. F. (2016). Photorespiration and photoinhibition in the bracts of cotton under water stress. Photosynthetica 54, 12–18. doi: 10.1007/s11099-015-0139-9
Zhang, Y., Zhang, Y., Wang, Z., and Wang, Z. (2011). Characteristics of canopy structure and contributions of non-leaf organs to yield in winter wheat under different irrigated conditions. F. Crop. Res. 123, 187–195. doi: 10.1016/j.fcr.2011.04.014
Zhao, B., Ma, B. L., Hu, Y., and Liu, J. (2021). Source–sink adjustment: a mechanistic understanding of the timing and severity of drought stress on photosynthesis and grain yields of two contrasting oat (Avena sativa L.) genotypes. J. Plant Growth Regul. 40, 263–276. doi: 10.1007/s00344-020-10093-5
Zhou, Q. P., Gou, X. L., Tian, L. H., Chen, Y., Gao, S., Bai, W., et al. (2018). Performances of early and late maturing oat varieties in cold regions. Chin. Sci. Bull. 63, 1722–1730. doi: 10.1360/N972018-00343
Zhu, S. Q., Chen, M. W., Ji, B. H., Jiao, D. M., and Liang, J. S. (2011). Roles of xanthophylls and exogenous ABA in protection against NaCl-induced photodamage in rice (Oryza sativa L) and cabbage (Brassica campestris). J. Exp. Bot. 62, 4617–4625. doi: 10.1093/jxb/err170
Keywords: non-leaf organ, C4 pathway, relative water content, oat, glume
Citation: Tian H, Zhou Q, Liu W, Zhang J, Chen Y, Jia Z, Shao Y and Wang H (2022) Responses of photosynthetic characteristics of oat flag leaf and spike to drought stress. Front. Plant Sci. 13:917528. doi: 10.3389/fpls.2022.917528
Received: 11 April 2022; Accepted: 12 July 2022;
Published: 28 July 2022.
Edited by:
Jin-Lin Zhang, Lanzhou University, ChinaReviewed by:
Ana María Mendez-Espinoza, Instituto de Investigaciones Agropecuarias (Chile), ChileCopyright © 2022 Tian, Zhou, Liu, Zhang, Chen, Jia, Shao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wang, enpiandoQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.