- Key Laboratory of Traditional Chinese Medicine Resources and Chemistry of Hubei Province, Hubei University of Chinese Medicine, Wuhan, China
Artemisia argyi Levl. et Van is an important Asteraceae species with a high medicinal value. There are abundant A. argyi germplasm resources in Asia, especially in China, but the evolutionary relationships of these varieties and the systematic localization of A. argyi in the family Asteraceae are still unclear. In this study, the chloroplast (cp) genomes of 72 A. argyi varieties were systematically analyzed. The 72 varieties originated from 47 regions in China at different longitudes, latitudes and altitudes, and included both wild and cultivated varieties. The A. argyi cp genome was found to be ∼151 kb in size and to contain 114 genes, including 82 protein-coding, 28 tRNA, and 4 rRNA genes. The number of short sequence repeats (SSRs) in A. argyi cp genomes ranged from 35 to 42, and most of them were mononucleotide A/T repeats. A total of 196 polymorphic sites were detected in the cp genomes of the 72 varieties. Phylogenetic analysis demonstrated that the genetic relationship between A. argyi varieties had a weak relationship with their geographical distribution. Furthermore, inverted repeat (IR) boundaries of 10 Artemisia species were found to be significantly different. A sequence divergence analysis of Asteraceae cp genomes showed that the variable regions were mostly located in single-copy (SC) regions and that the coding regions were more conserved than the non-coding regions. A phylogenetic tree was constructed using 43 protein-coding genes common to 67 Asteraceae species. The resulting tree was consistent with the traditional classification system; Artemisia species were clustered into one group, and A. argyi was shown to be closely related to Artemisia lactiflora and Artemisia montana. In summary, this study systematically analyzed the cp genome characteristics of A. argyi and compared cp genomes of Asteraceae species. The results provide valuable information for the definitive identification of A. argyi varieties and for the understanding of the evolutionary relationships between Asteraceae species.
Introduction
Artemisia argyi Levl. et Van is a perennial herb belonging to the Artemisia genus in the family Asteraceae. Artemisia argyi contains an abundance of active ingredients, such as flavonoids, phenolic acids, and other volatile components, endowing A. argyi with a wide range of pharmacological activities (Mei et al., 2016). For example, 1, 8-cineole and borneol have strong antibacterial and anti-inflammatory activities (Mulyaningsih et al., 2010; Martins et al., 2017; Bansod et al., 2021). Eupatilin is commonly used to treat gastritis and peptic ulcers (Lee et al., 2017; Kim et al., 2018). Moxa wool from A. argyi leaves is the main combustion material in moxibustion therapy (Cardini and Weixin, 1998). With the development of the health industry, A. argyi is widely used in medicine, healthcare, food, and cleaning products (Liu et al., 2018; Cui et al., 2021); therefore, the market demand for A. argyi is growing rapidly.
Artemisia argyi is widely distributed in eastern Asia, including China, Japan, South Korea, and Mongolia (Lee et al., 2009). Artemisia argyi is rich in germplasm resources, with abundant genetic variations occurring in different ecotypes. Our previous studies demonstrate significant differences in agronomic traits, moxa content, and active ingredients among different A. argyi varieties (Chen C. J. et al., 2021). In China, “Qichun Qi AI” (growing in Qichun County, Hubei Province), “Tangyin Bei AI” (Tangyin County, Henan Province), “Ningbo Hai AI” (Ningbo City, Zhejiang Province), and “Anguo Qi AI” (Anguo City, Hebei Province) are the four representative varieties with superior quality. The abundant germplasm resources of A. argyi also bring great challenges in planting, and varietal complexity often occurs in cultivation, seriously affecting the yield and quality of A. argyi.
The chloroplast (cp) is an organelle in plants and algae (Du et al., 2021; Li et al., 2021) that plays a significant role in plant cell function, including in photosynthesis, carbon fixation, and stress response. The cp is a semiautonomous organelle with a relatively independent genome containing its own genetic system for replication, transcription, and translation (Favier et al., 2021). The cp genome is a double-stranded circular DNA molecule with a conserved quadripartite structure, including a large single-copy (LSC) region, a small single-copy (SSC) region, and two copies of inverted repeat (IR) regions (Song et al., 2017; Gao et al., 2018; Wang M. et al., 2021). The LSC and SSC regions are usually separated by two IR regions.
The comparative genomic studies have demonstrated that most of the angiosperm cp genomes are highly conserved in gene order and content (Chen J. et al., 2021), and generally range in length from 120 to 160 kb (Wang Y. et al., 2021). Angiosperm cp genomes usually encode 110–130 genes (Feng et al., 2020). These genes are divided into three functional categories, namely, photosynthesis-related, transcription/translation-related, and biosynthesis-related genes. Some significant genomic changes have occurred throughout the process of evolution, comprising large-scale genome rearrangements, the contraction and expansion of IR regions, and the loss of specific introns. With the rapid development of sequencing technology, many cp genomes have been reported since the assembly of the Nicotiana tabacum cp genome (Shinozaki et al., 1986). Furthermore, cp genomes are extensively used in the fields of species identification, phylogeny, and genetic diversity analysis due to their relatively small size, maternal inheritance, conserved structure, and low nucleotide substitution rate (Qiao et al., 2016).
Although the cp genome of A. argyi has been reported previously (Kang et al., 2016), the structural characteristics of the A. argyi cp genome and the evolutionary relationships between A. argyi varieties remain unclear. In this study, 72 wild and cultivated A. argyi varieties were collected from 15 domestic provinces and 1 municipality in China. We sequenced and assembled the cp genomes of these 72 varieties and here report 51 different A. argyi cp genomes. Furthermore, we systematically analyzed cp genome characteristics of A. argyi, including GC content, gene number and order, repeat sequences, and simple sequence repeats (SSRs). The evolutionary relationships between A. argyi and other Asteraceae plants were also explored based on their cp genomes. These results provide important information for the identification of A. argyi varieties and the evolution of Asteraceae plants.
Materials and Methods
Plant Materials
In this study, 72 A. argyi germplasm resources from 47 regions in China were collected and planted in the A. argyi Germplasm Resource Garden at Hubei University of Chinese Medicine (Hubei Province, China; 114.264E, 30.451N). Detailed sampling information is shown in Supplementary Table 1. Maps were drawn using Lantuhui v2.8.0.1 Fresh leaves were collected from each variety and immediately frozen in liquid nitrogen prior to DNA extraction for sequencing. We also downloaded 67 cp genomes of plants in the family Asteraceae from NCBI,2 including the published cp genome of A. argyi (Supplementary Table 6).
DNA Sample Preparation and Chloroplast Genome Sequencing
Total genomic DNA was extracted from each sample using a modified cetyltrimethyl ammonium bromide (CTAB) method (Doyle et al., 1987). Agarose gels (1%) and a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, United States) were used to evaluate the quality and quantity of the extracted DNA. Genomic DNA samples were fragmented to a size of ∼350 bp by sonication. Index codes were added to each sample and the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, United States) was used for the preparation of DNA libraries. Subsequently, DNA fragments were end polished, A-tailed, ligated, and amplified by PCR. PCR products were then purified with the AMPure XP system (Beckman Coulter, Brea, CA, United States) and the DNA concentration was measured using the Qubit 3.0 Fluorometer. Libraries that passed quality inspection were sequenced by Beijing Nuohe Zhiyuan Technology (Beijing) Co., Ltd.3 using the Illumina HiSeq Xten-PE150 platform (Illumina, San Diego, CA, United States). The original fluorescence image files were transformed into short reads (raw data). The program fastp (v0.19.7) was used for clean reads by removing the joints and low-quality sequences (Phred quality < 5), and clean reads were recorded in FASTQ format. The raw data of 72 cp genome sequencing have been submitted to NCBI under the accession number PRJNA821831 and the 72 assembled cp genomes have been submitted to the China National Gene Bank Database (https://db.cngb.org/) under accession number CNP0002996.
Assembly and Annotation of Chloroplast Genomes
We assembled the cp genomes of A. argyi varieties using NOVOPlasty v4.24 (Dierckxsens et al., 2017) and selected the rbcL gene of A. argyi from NCBI (NC030785.1) as a seed sequence; the default settings were used for all other parameters. The Plastid Genome Annotator (PGA) was used to annotate the cp genomes, using Artemisia annua Linn (MF623173.1) as the reference. The annotation results were verified by Geneious 8.0.25 (Kearse et al., 2012). The program tRNAscan-SE6 (Lowe and Chan, 2016) was used to determine the boundaries of all tRNA genes. A circular map of the A. argyi cp genome was drawn using Chloroplot.7 Four junctions between IR and SC regions were verified using high-fidelity PCR amplification and Sanger sequencing. The primers are listed in Supplementary Table 4.
Feature Analysis of the Chloroplast Genome
Relative synonymous codon usage (RSCU) was analyzed using MEGA7. REPuter8 was used to detect the size and location of repeat sequences with a minimal length of 19 bp (Kurtz and Schleiermacher, 1999) and an identity of 90%. MISA9 was used to identify SSRs (Thiel et al., 2003). Molecular markers for SSR identification are listed in Supplementary Table 2 (Curci et al., 2016). To reveal highly variable regions in cp genomes for the identification of A. argyi varieties, we used MAFFT V7.471 (Kazutaka Katoh, Japan) for the alignment (Katoh and Standley, 2013); DnaSP v6 software was then used for a sliding window analysis (Librado and Rozas, 2009) with the window length set to 600 bp and a step size of 200 bp.
Comparative Analysis of Chloroplast Genomes
To compare the IR boundaries, cp genomes of nine Artemisia species downloaded from NCBI were compared with the A. argyi cp genome using IRscope10 (Amiryousefi et al., 2018). To determine the differences in the cp genomes of 11 Asteraceae species, A. argyi was used as the reference, and complete cp genome sequence annotations in BED format were converted and uploaded to the online analysis program mVISTA11 (Kawabe et al., 2018).
Phylogenetic Analysis
The complete cp genomes of 72 A. argyi varieties were aligned and uploaded to RAxML v8.2.12 (Stamatakis, 2014) to construct a phylogenetic tree using the maximum likelihood (ML) method, and A. annua (MF623173.1) was used as the outgroup. For the phylogenetic analysis of Asteraceae species, cp genomes of 67 Asteraceae species were selected, and Platycodon grandiflorus (Jacq.) A. DC. (MZ 202358.1) and Codonopsis minima Nakai. (NC036311.1) were used as the outgroup. The cp genomes of 67 selected Asteraceae species were downloaded from NCBI, manually annotated, and aligned using MAFFT v7.471 software. Phylogenetic analyses were performed using the coding DNA sequences (CDSs), LSCs, SSCs, IRs, introns, protein-coding genes common to all of the selected species, and the full-length cp genomes. RAxML v8.2.12 was used to construct the phylogenetic tree with 1,000 bootstrap replicates to estimate node support, and the best tree based on bootstrap scores was used for further analysis.
Results
Characteristics of the Artemisia argyi Chloroplast Genome
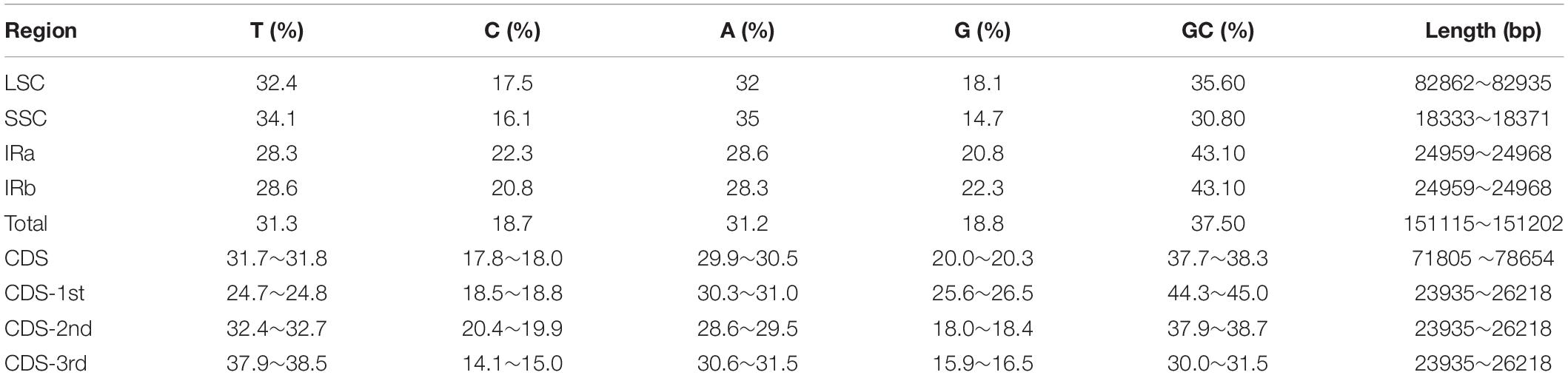
The locations at which the 72 A. argyi varieties were collected are shown in Figure 1. We obtained 32.93–44.18 Mb raw reads for each variety, providing 218- to 292-fold coverage of the A. argyi cp genome. Assembly and annotation yielded the complete cp genomes of A. argyi varieties. The results showed that the A. argyi cp genome was a circular double-stranded molecule ranging in size from 151,115 to 151,202 bp (Figure 2). The A. argyi cp genome was composed of the LSC (82,862–82,935 bp), the SSC (18,333–18,371 bp), IRa (24,959–24,968 bp), and IRb (24,959–24,968 bp). The average GC content was 35.6%. Specifically, the LSC, SSC, IRa, and IRb had 35.6, 30.8, 43.1, and 43.1% GC content, respectively. The CDS lengths ranged from 71,805 to 78,654 bp, and GC content in the CDSs ranged from 37.7 to 38.3% (Table 1). On the whole, the structural characteristics of cp genomes were identical between the 72 A. argyi varieties, indicating that cp genome structure was highly conserved in this species.
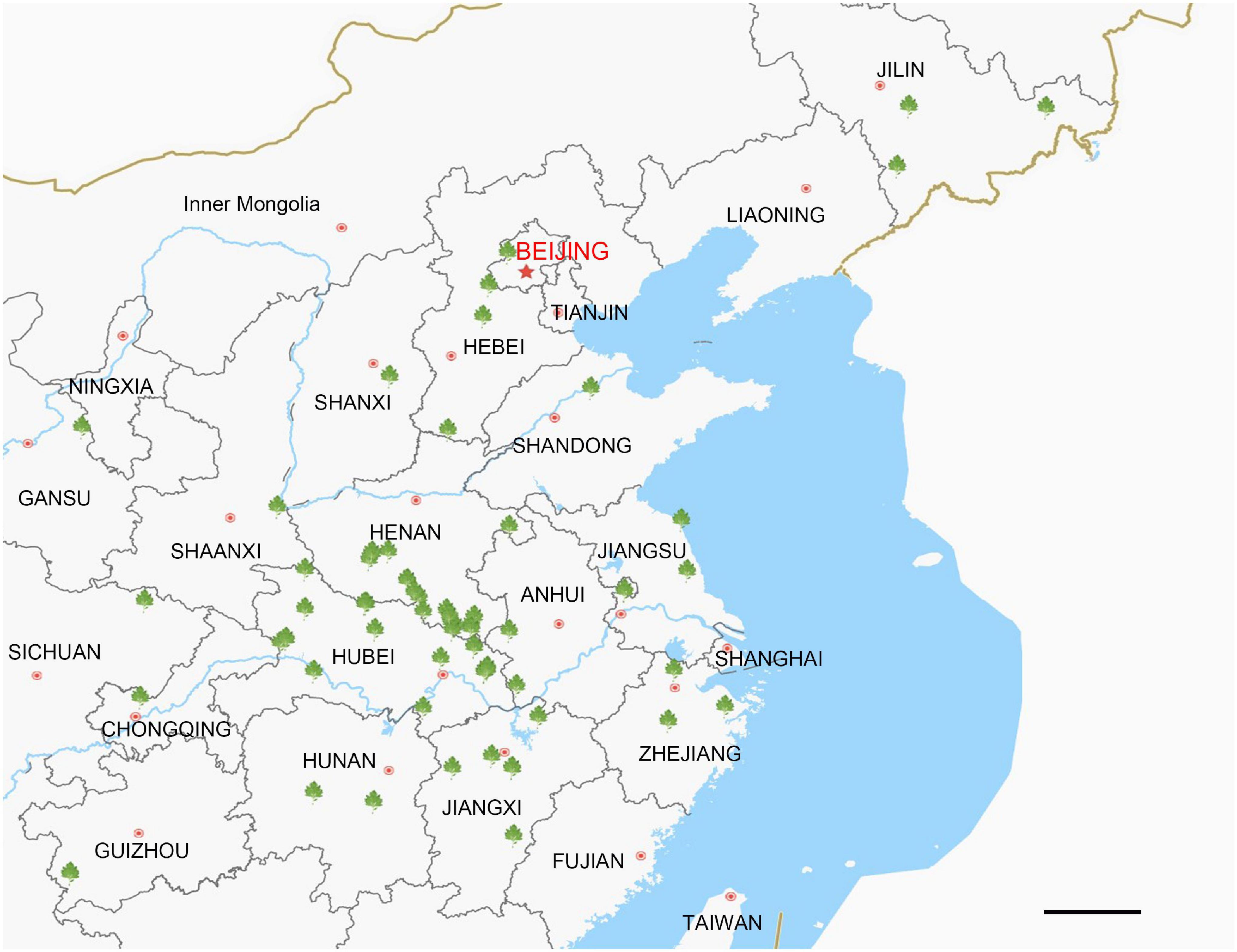
Figure 1. Collection locations for Artemisia argyi germplasm resources. The green symbols indicate collection points for Artemisia argyi varieties. There are 47 markers located in 15 domestic provinces in China. Scale bar = 20 km.
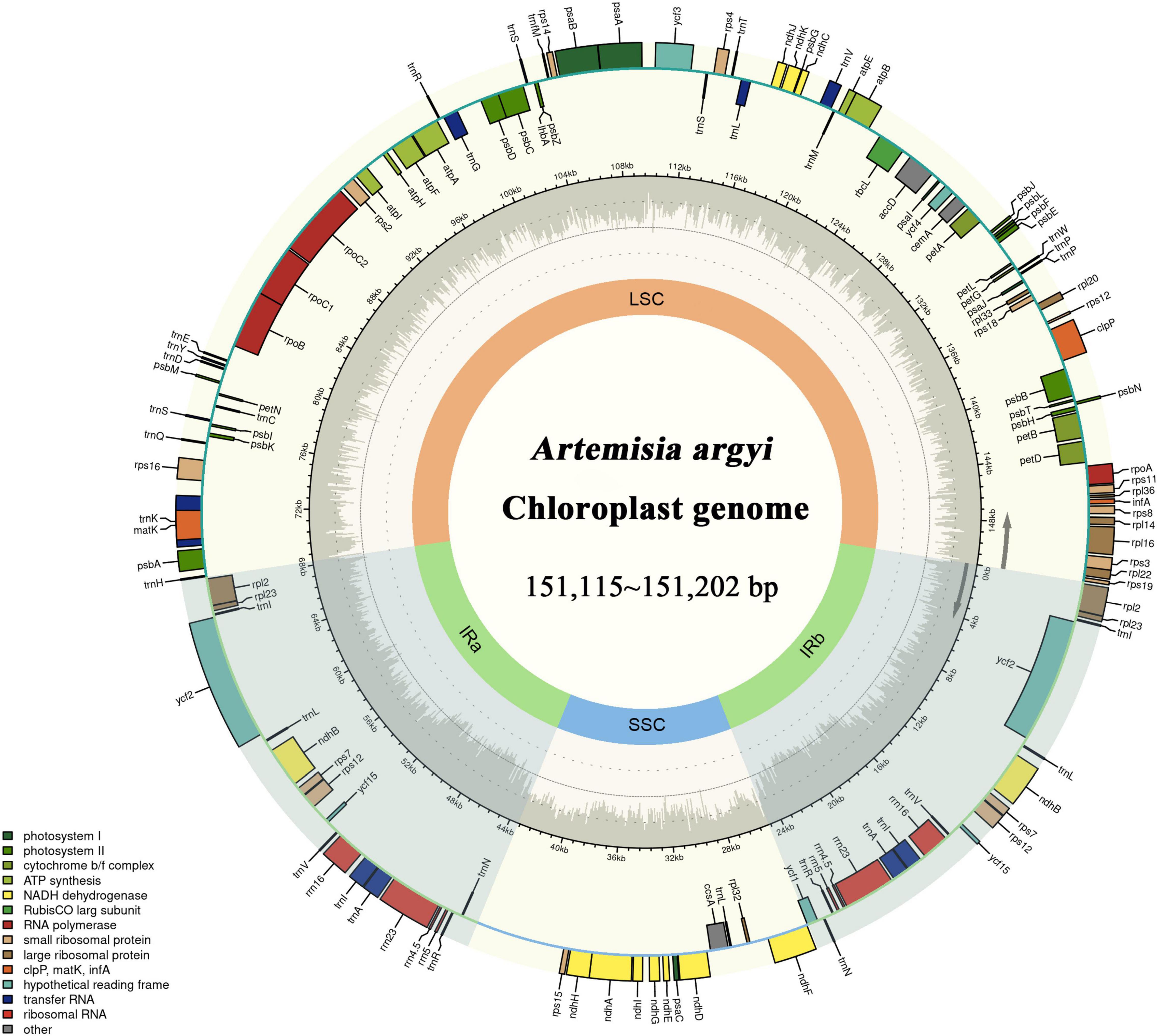
Figure 2. Gene map of the Artemisia argyi chloroplast (cp) genome. Genes drawn inside the circle are transcribed in the clockwise direction, and those on the outside are transcribed in the counterclockwise direction. The genes are color-coded based on their function. In the inner circle, darker gray corresponds to GC content and lighter gray corresponds to AT content of the Artemisia argyi cp genome. The SSC and LSC regions are separated by two darker areas representing inverted repeat (IR) regions (IRa and IRb).
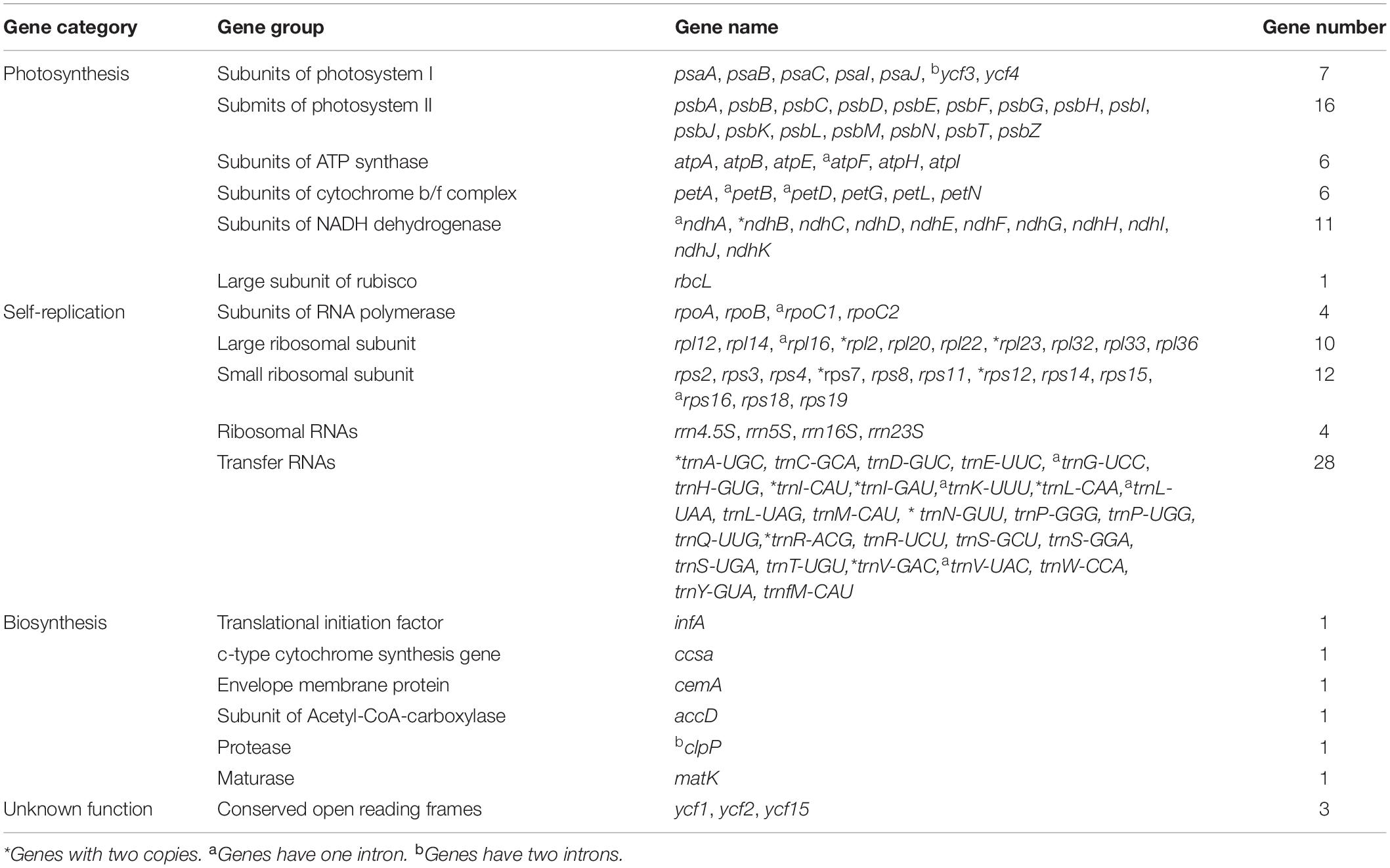
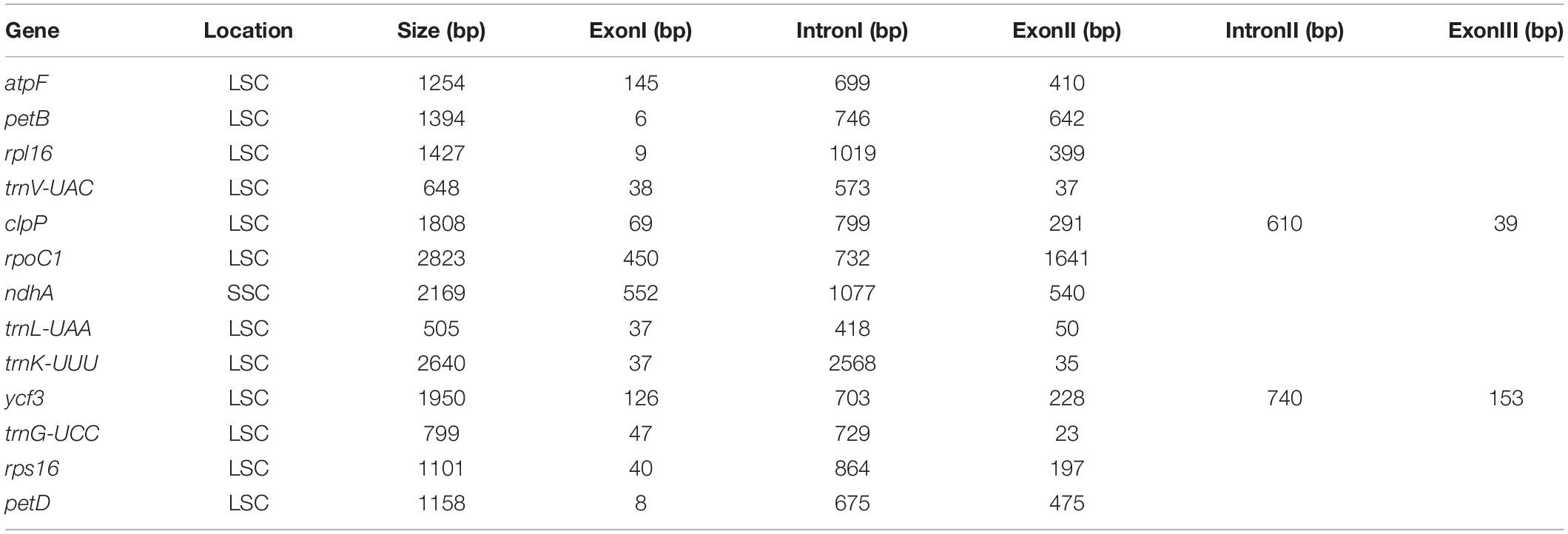
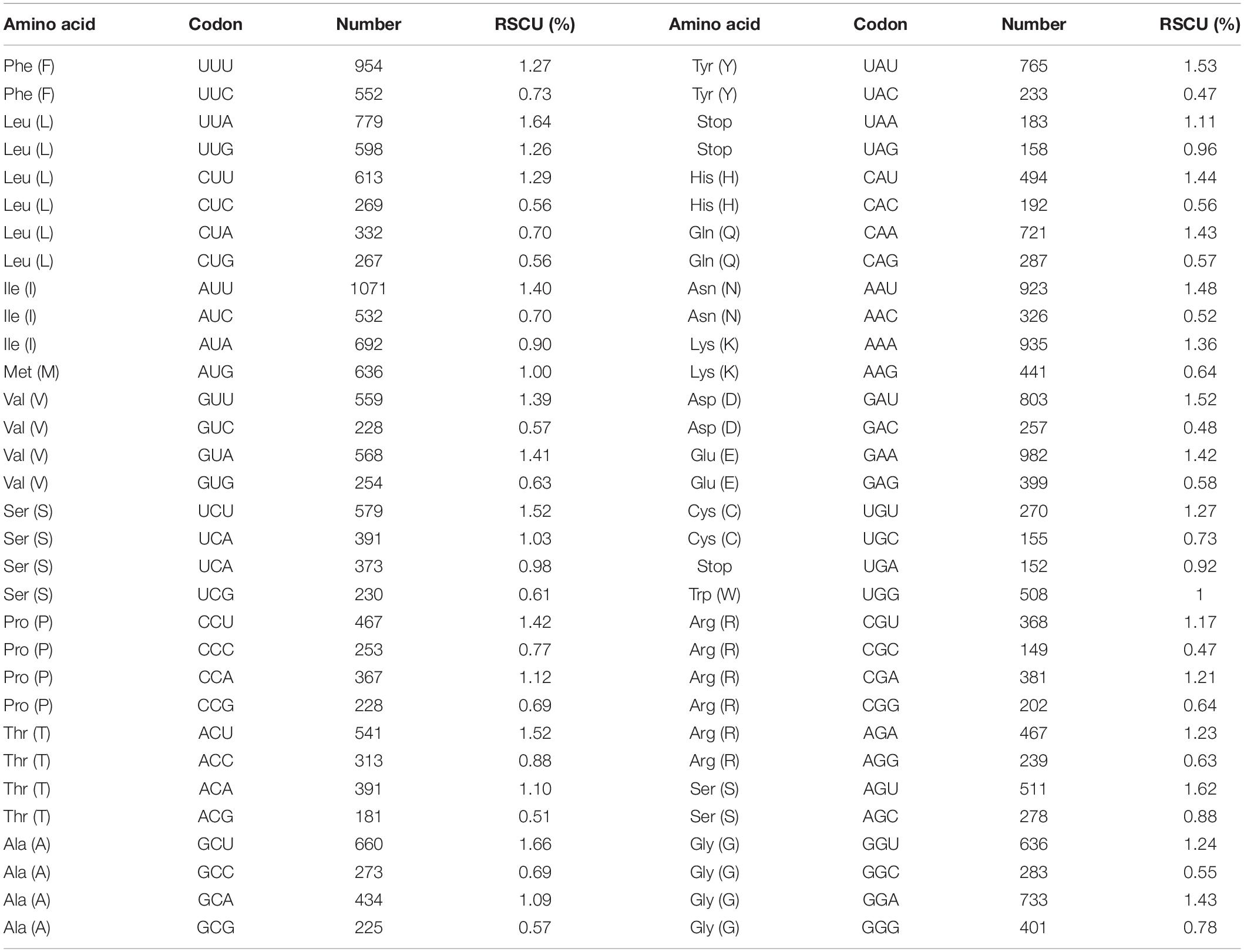
The A. argyi cp genome contained 114 genes: 82 protein-coding, 28 tRNA, and 4 rRNA genes. These genes were divided into three functional categories; there were 47 photosynthesis-related genes, 28 related to self-replication, and 6 genes related to biosynthesis (Table 2). In the IR regions, 18 genes were duplicated in total. Among these duplicated genes, seven were protein-coding (ndhB, rpl2, rpl23, rps7, RPS12, ycf2, and ycf15), seven were tRNA (tRNA-UGC, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and four were mRNA genes (rrn4.5s, rrn5s, rrn16s, and rrn23s). The only single-copy gene located in the IR region was ycf1. The results of gene structure analysis showed that there were 13 intron-containing genes in the A. argyi cp genome, 12 of which were located in the LSC and one of which was in the SSC (ndhA). Of those 13 genes, 11 contained one intron (atpF, petB, rpl16, trnV-UAC, rpoC1, ndhA, trnL-UAA, trnK-UUU, trnG-UCC, rps16, and petD) and two contained two introns (clpP and ycf3) (Table 3). We then performed RSCU analysis of the protein-coding genes. The results showed that there were 30 codons with RSCU > 1.00. Notably, there were 29 codons ending with A or U, indicating that the A. argyi cp genome preferentially contained synonymous codons ending with A or U (Table 4).
Repeat Sequence and Short Sequence Repeat Analysis of the Artemisia argyi Chloroplast Genome
To further analyze the characteristics of the A. argyi cp genome, four types of repetitive sequences were analyzed, namely, forward repeats, reverse repeats, palindromic repeats, and complementary repeats. The A. argyi varieties S07 (Anguo), S09 (Tangyin), S29 (Qichun), and S71 (Ninghai) were used as representative varieties, and each had 47 repeats in the cp genome. We first analyzed the length of these repeats which ranged from 19 to 48 bp. Most repeats were 20–29 bp, accounting for 80.3% of the total. The longest repeat was a palindrome sequence in the LSC that was 48 bp long. The location of these repeats was then investigated. The results showed that most forward and palindrome repeats and all reverse and complementary repeats were located in the LSC (Figure 3A).
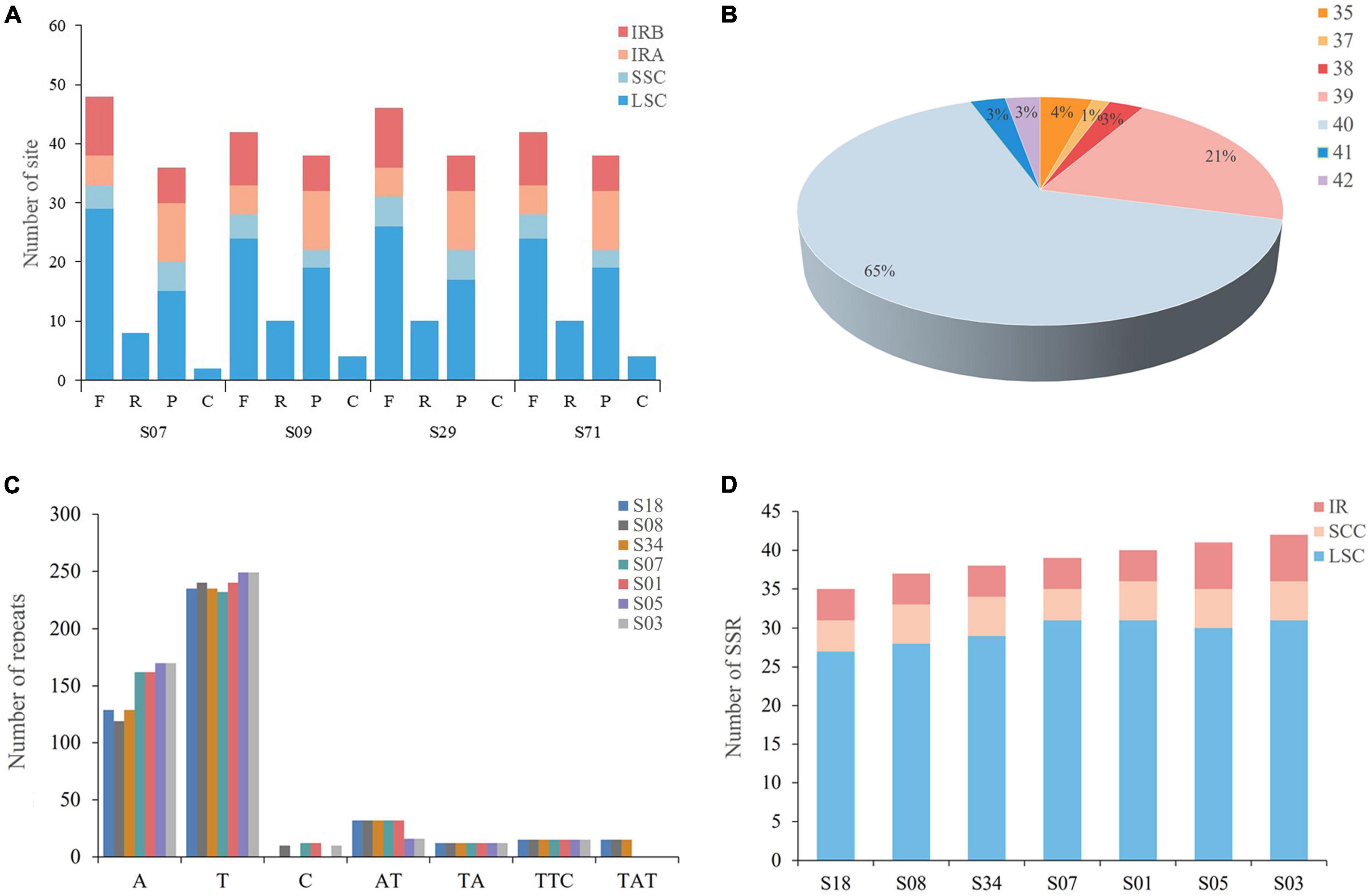
Figure 3. Types and distributions of repeat sequences and short sequence repeats (SSRs) in Artemisia argyi chloroplast (cp) genomes. (A) Number and position of repeat sequences in four Artemisia argyi cp genomes (S07, S09, S29, and S71). (B) Proportion of SSRs in 72 Artemisia argyi cp genomes. (C) Distribution of repeats classified by type. (D) The number of SSR loci in different cp genome regions.
We then determined the distribution of SSRs in the cp genomes of 72 A. argyi varieties. A total of 2,849 SSRs were identified. The number of SSRs in each cp genome ranged from 35 to 42, and 65.3% of A. argyi varieties had 40 SSRs in the cp genome (Figure 3B). To explore the characteristics of SSRs, seven varieties with different numbers of SSRs were selected for further analysis of SSR types and distributions: S18 (35 SSRs), S08 (37 SSRs), S34 (38 SSRs), S07 (39 SSRs), S01 (40 SSRs), S05 (41 SSRs), and S03 (42 SSRs). The percentages of mononucleotide, dinucleotide, and trinucleotide SSRs were 86.7, 8.6, and 4.7%, respectively (Figure 3C). With respect to distribution, a great majority of SSRs (73.2–79.5%) were located in the LSC. The IR regions had four to six SSRs; the SSC had four to five SSCs, and all trinucleotide repeats were located in the SSC. It is worth mentioning that 85.3% of the SSRs were mononucleotide A/T repeats, indicating an A/T nucleotide bias in A. argyi cp SSRs (Figure 3D). In addition, we developed several molecular markers based on SSR analysis of cp genomes to identify these A. argyi varieties, and Sanger sequencing of PCR amplification demonstrated that these SSRs were usable (Supplementary Figure 1 and Supplementary Table 2).
Comparative Analysis of Chloroplast Genomes in 72 Artemisia argyi Varieties
To detect the nucleotide diversity in 72 A. argyi cp genomes, the level of sequence variability was calculated using DnaSP v6.0. A total of 150,753 sites (excluding sites with gaps/missing data) were investigated, including 196 polymorphic sites in total (Figure 4). Among these polymorphic sites, there were 155 singleton variable sites and 41 parsimony-informative sites. The nucleotide diversity (Pi) values ranged from 0 to 0.00233 with an average of 0.00007, indicating that A. argyi cp genomes had high sequence similarity. In general, there were more variable sites in non-coding regions than in coding regions, and the variations in SC regions had greater divergence than variations in IR regions. The Pi values of 11 protein-coding genes (trnK-UUU, petL, rpl20, psaI, ycf4, psbA, rpl32, ndhF, ccsA, trnL-UAG, and rpl32) were higher than 0.0004; trnK-UUU, petL, and rpl20 had the highest Pi values. Moreover, all the 11 genes were located in SC regions, explaining why SC regions had greater variation than IR regions. And these highly variable regions could be used to identify A. argyi varieties. Using the four representative A. argyi varieties (S07, S09, S29, and S71) as an example, 30 polymorphic sites were detected in the cp genomes, including 24 single-nucleotide polymorphisms (SNPs) and six small insertions and deletions (INDELs). Combined with the analysis of repeat sequences, we selected two repeats and one SNP for distinguishing these four A. argyi varieties (Figure 5). Based on these variant sites, three specific molecular markers were developed and validated through high-fidelity PCR and Sanger sequencing, and the results indicated that these markers were fit for use (Supplementary Figure 2 and Supplementary Table 3).
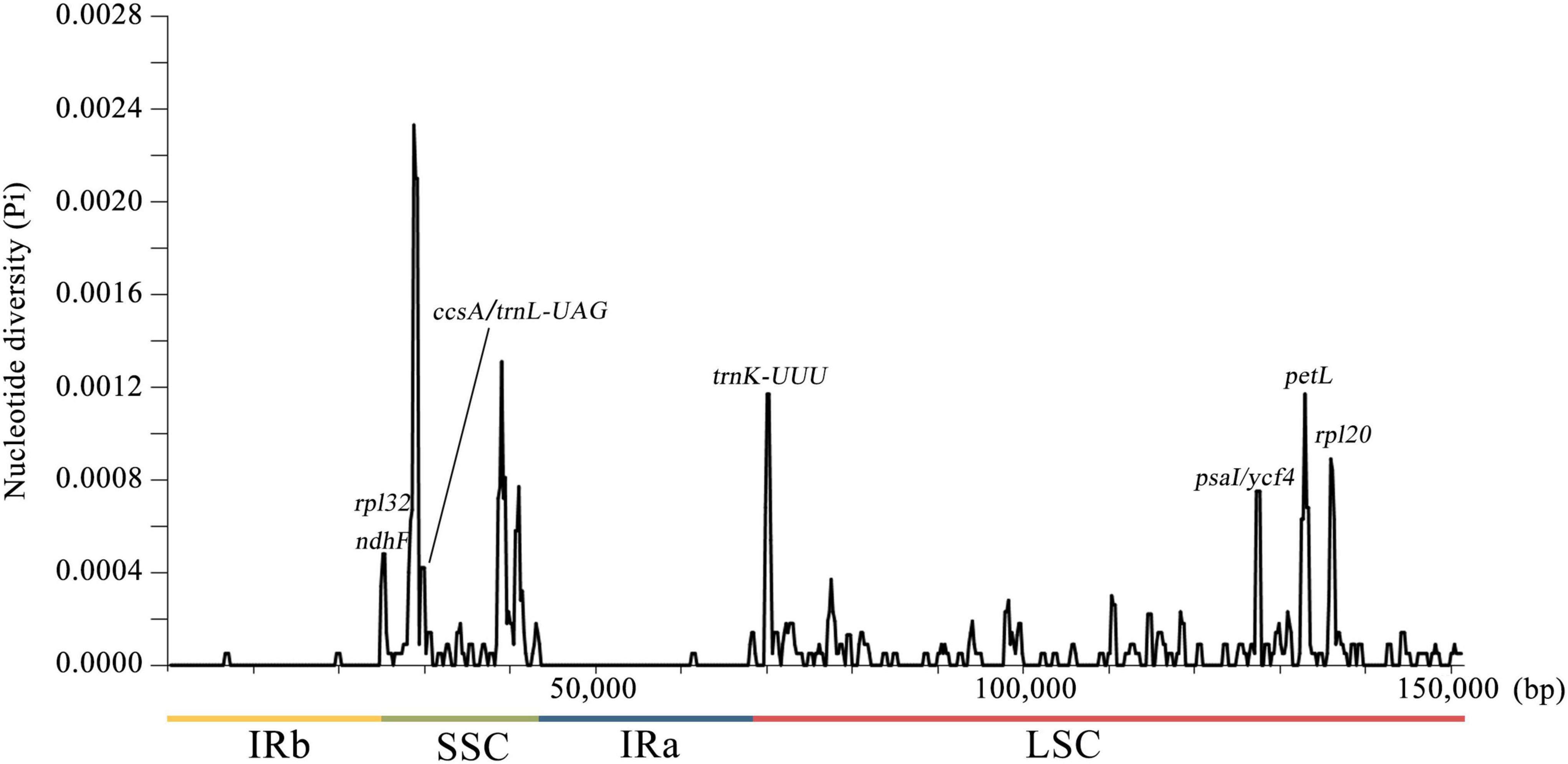
Figure 4. Sliding window analysis of 72 complete chloroplast (cp) genomes of Artemisia argyi. The x-axis represents the midpoint of the window and the y-axis represents the nucleotide diversity (Pi) of each window. The window length is 600 bp with a 200-bp step size.

Figure 5. Plant morphology of S07, S09, S29, and S71 (A) and three polymorphic sites in chloroplast (cp) genomes of these four varieties (B).
Phylogenetic Analysis of 72 Artemisia argyi Varieties Based on Chloroplast Genomes
To investigate the genetic relationships between the 72 A. argyi varieties, a phylogenetic tree was constructed based on cp genomes using A. annua as the outgroup (Figure 6 and Supplementary Figure 4). The results showed that the varieties were divided into nine branches, with the largest branch containing 44 varieties. Because the selected germplasm resources contained both cultivated and wild varieties from wide geographical and altitudinal ranges (from 25.08 to 42.95°N, 104.9 to 129.52°E, and 2 to 1,592 m), we wondered whether the evolutionary relationships between varieties were associated with their collection locations. However, the phylogenetic analysis demonstrated that germplasm resources collected from similar longitudes or latitudes did not cluster together. Moreover, there was no obvious clustering between the cultivated and wild varieties, which may be due to the short cultivation history of A. argyi. Interestingly, there were 12 varieties outside the two largest branches of the phylogenetic tree, eight of which were collected at an altitude of above 500 m; this indicated that evolution of the cp genome likely occurred in the adaptive process of A. argyi varieties to grow at different altitudes. However, it is worth mentioning that most bootstrap values of the evolutionary tree were less than 70 due to high conservation of A. argyi cp genomes, indicating that the groups in this evolutionary tree were not much supported, and therefore other kinds of molecular markers would be necessary to assess phylogenetic relationships within the species A. argyi.
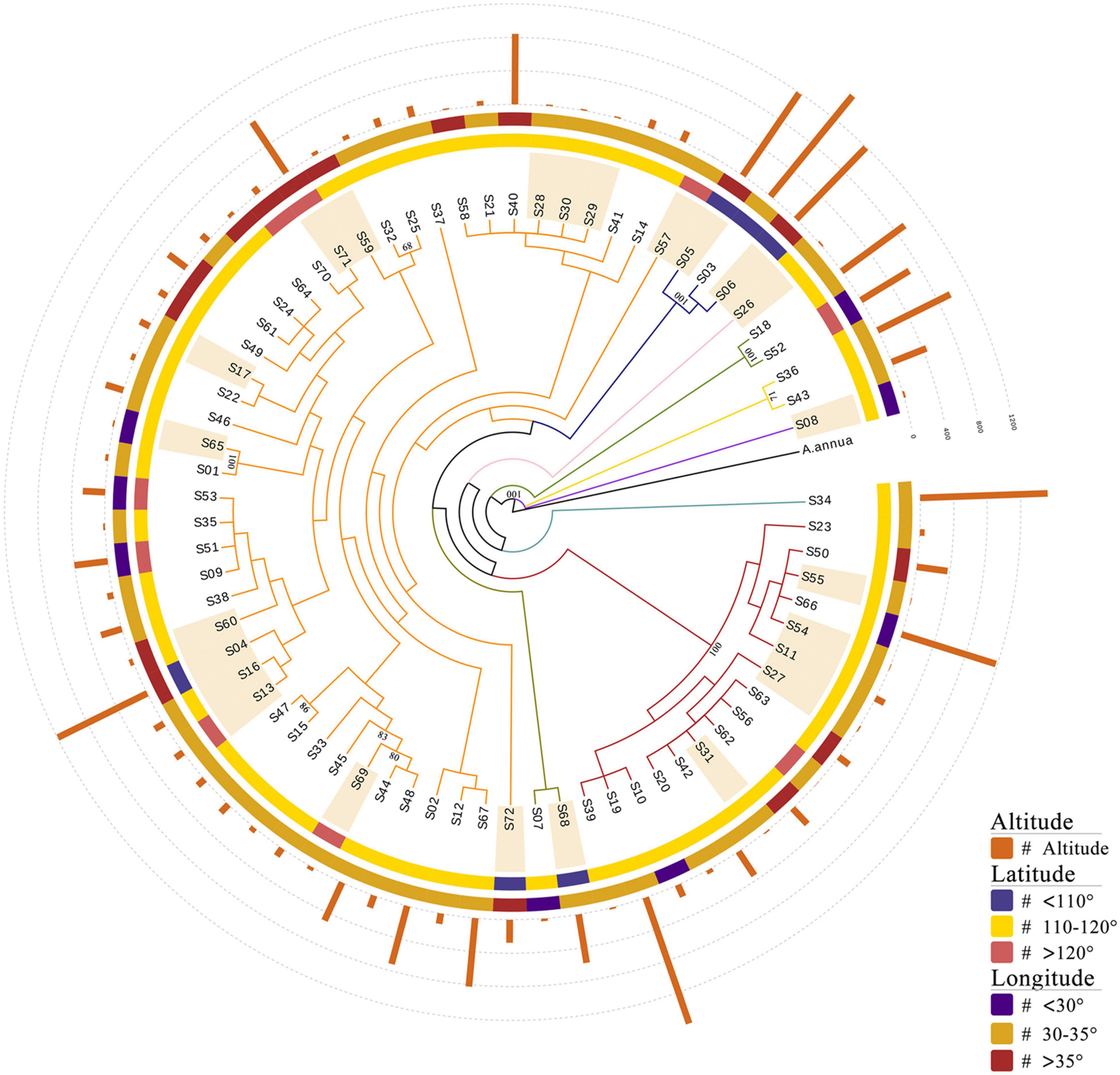
Figure 6. Phylogenetic analyses of 72 Artemisia argyi varieties. The tree is constructed using the ML method based on full-length chloroplast (cp) genomes. A. annua is used as the outgroup. Branches are color-coded. Sample numbers marked with light orange shadow are wild germplasm resources. Bootstrap values above 70 are marked in the figure.
Comparison of Boundary Regions in Artemisia Species
The boundary regions based on high throughput sequencing of A. argyi cp genomes were first validated by PCR and Sanger sequencing (Supplementary Figure 3 and Supplementary Table 4). To investigate species-specific gene loci, we compared IR/LSC and IR/SSC junction regions of the cp genomes in nine Artemisia species (Artemisia absinthium Linn., Artemisia annua, Artemisia frigida Willd., Artemisia giraldii Pamp., Artemisia lactiflora Wall ex DC., Artemisia maritima Linn., Artemisia montana Nakai., Artemisia ordosica Krasch and Artemisia scoparia Waldst. et Kit.) with A. argyi (S29). The results revealed that the length of IR regions ranged from 24,403 to 24,972 bp, and cp genomes in these species showed significant difference in IR/LSC and IR/SSC junction. In total, there were 10 genes around the boundaries: rpl22, rps19, rpl2, ycf1, ndhF, trmN, rpl2, trnH, psbA, and trnR. The characteristics of four boundaries, namely, IRa-SSC (JSA), IRa-LSC (JLA), IRb-LSC (JLB), and IRb-SSC (JSB), were then systematically analyzed in these species. The JLB boundary was located in rps19 in most of the Artemisia cp genomes (60%). The ycf1 gene was critical for the JSB and JSA boundaries. In four species, the JSB boundary was located in ycf1. In three species, the JSB boundary was only 1 bp away from ycf1. Furthermore, there were seven species in which the JSA boundary was in ycf1. For all the 10 species, the JLA boundary was between two genes; among these 10 species, the JLA boundaries were between rpl2 and trnH for five species, rpl2 and rps19 for two, and rps19 and trnH for three (Figure 7). These results demonstrated that the IR/LSC and IR/SSC junction regions varied between Artemisia species, likely due to an expansion or contraction of the IR region.
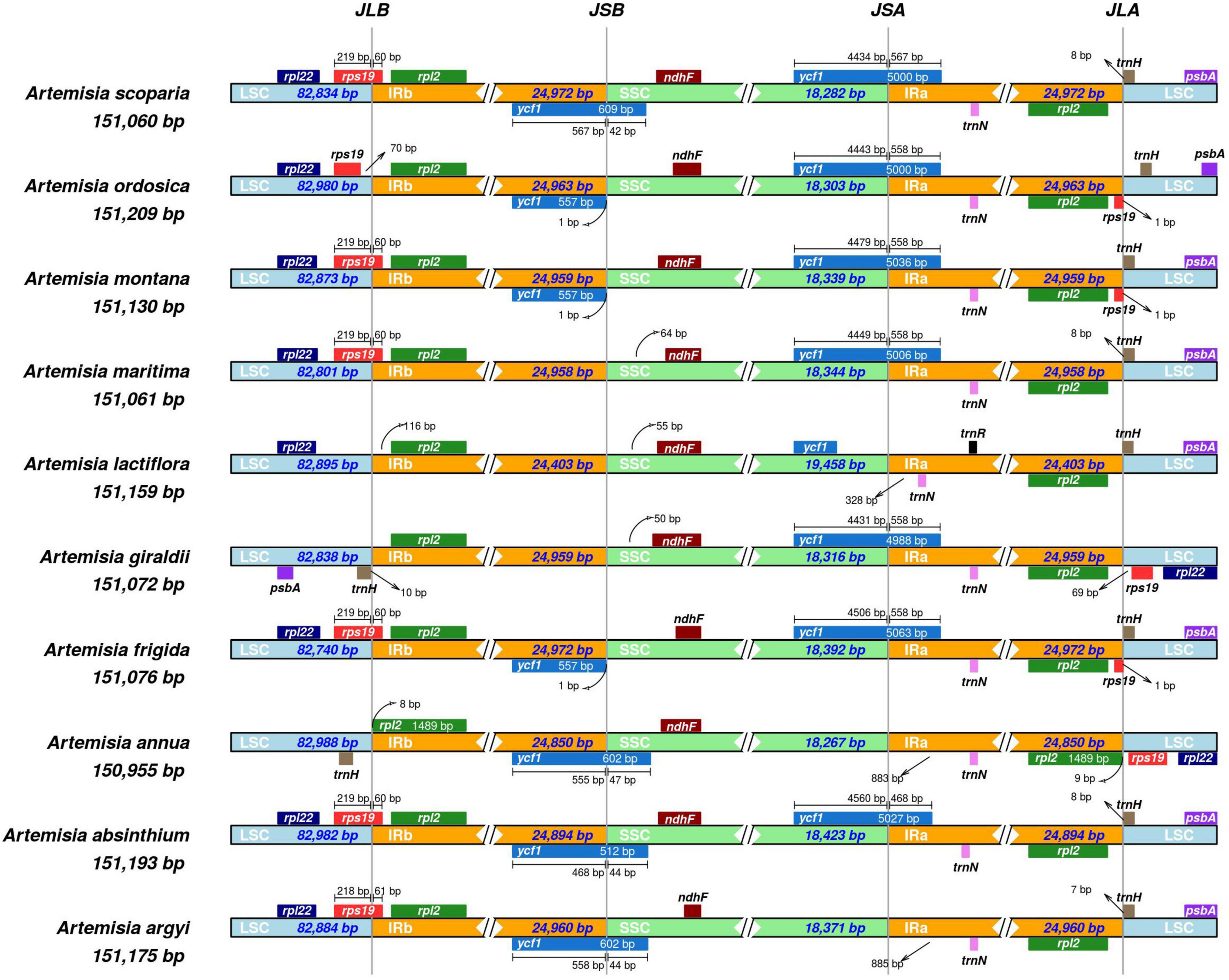
Figure 7. Comparison of the large single-copy (LSC), small single-copy (SSC), and inverted repeat (IR) regional boundaries of ten Artemisia species chloroplast (cp) genomes. JSA denotes the junction between IRa and SSC; JLA denotes the junction between IRa and LSC; JLB denotes the junction between IRb and LSC; JSB denotes the junction between IRb and SSC.
Comparative Analysis of Chloroplast Genomes in Asteraceae Species With mVISTA
The whole sequence identity of 10 Campanulales cp genomes including 8 Asteraceae and 2 Campanulaceae Juss. species was then plotted by mVISTA, using the A. argyi cp genome as a reference (Figure 8). Species belonging to different families had cp genomes with similar length, structure, and gene distribution, indicating that cp genomes were highly conserved in these species. The non-coding sequences were more variable than the coding sequences, and SC regions had higher levels of divergence than IR regions. Notably, intron-containing genes had higher levels of variability. In general, the species related to A. argyi based on classical taxonomy also had higher cp genome similarity. In addition, two species in the family Campanulaceae [P. grandiflorus (Jacq.) A. DC and C. minima (L.) A. Br. et Aschers.] had a larger number of variable regions, demonstrating a clear distinction from the family Asteraceae. The coding regions of 12 genes (ycf2, trnI-GAU, trnA-UGC, ycf1, ccsA, ndhD, ndhA, trnK-UUU, rpoB, rpoC1, rpoC2, and trnG-UCC) were relatively variable in non-Anthemidinae O. Hoffm. species in Asteraceae, and these genes may therefore be useful to identify Anthemideae Cass species. By comparison, the coding regions of four genes (trnA-UGC, rrn23, trnK-UUU, and rpoB) were relatively variable in non-Artemisia species of Anthemideae Cass, and could thus be used for identifying Artemisia species. Taken together, the results of the comparative cp genomic analysis provide valuable information for Asteraceae species identification at the molecular level.
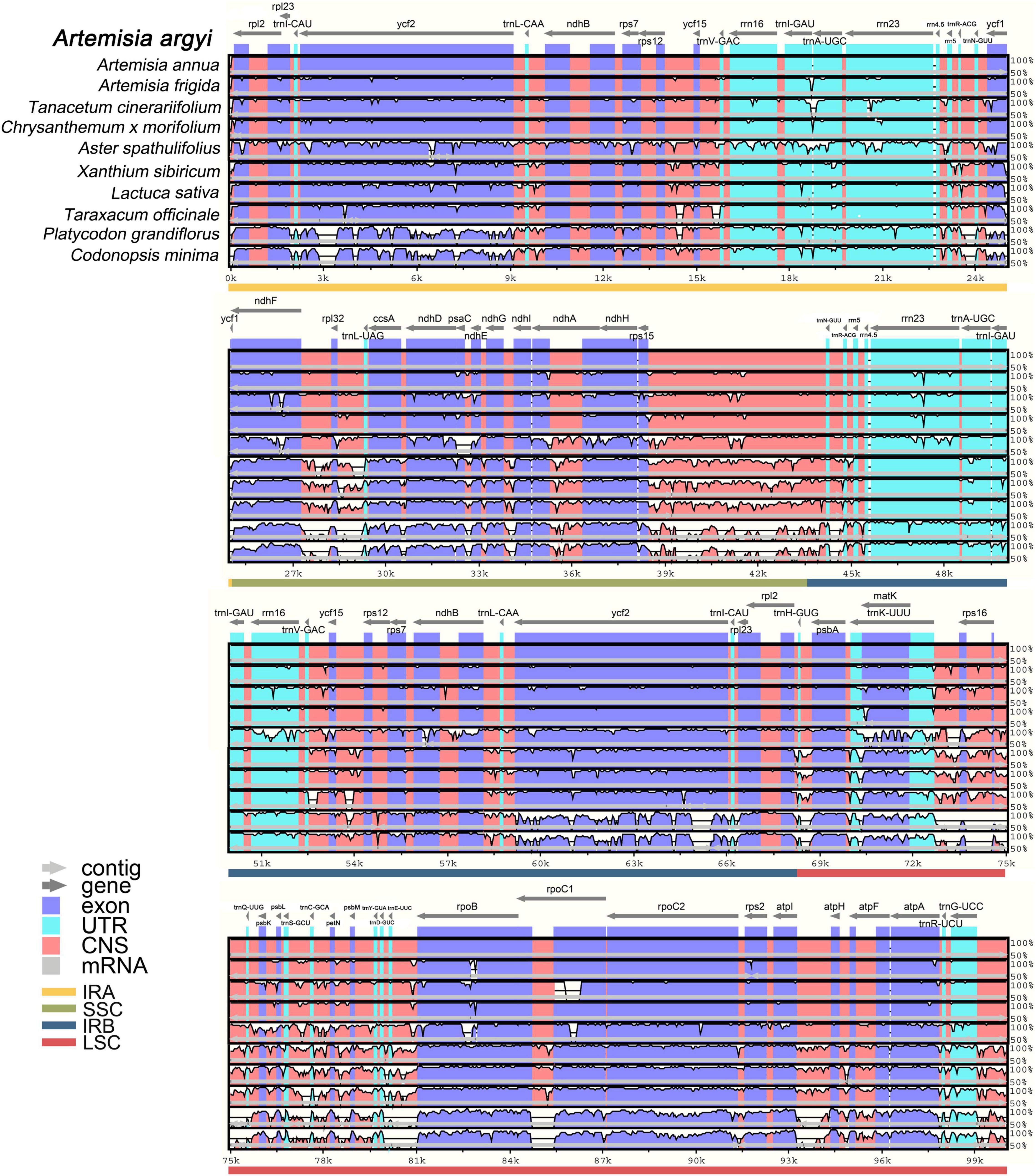
Figure 8. Full-length chloroplast (cp) genome alignment of Campanulales species with mVISTA. Using the cp genome of Artemisia argyi as a reference, the x-axis indicates the coordinates in cp genomes and the y-axis indicates the average percent identity, ranging from 50 to 100%. Arrows indicate gene orientation; cp genome regions are color-coded as exons, untranslated regions, conserved non-coding sequences, and mRNA.
Phylogenetic Analysis of 67 Asteraceae Species
To explore the evolutionary relationship of Asteraceae species, cp genomes of A. argyi and 66 other Asteraceae species were selected for phylogenetic analysis. Two Campanulaceae species (C. minima and P. grandiflorus) were used as the outgroup to detect whether Asteraceae and non-Asteraceae species can be divided into two groups based on their cp genomes. Phylogenetic trees were constructed using the ML method and seven regions of the genome: the complete cp genome, the CDS, the SSC, the LSC, the IR region, introns, and 43 protein-coding genes shared by the 67 species. The results showed that Lactuceae Cass could not be distinguished from Carduoideae Kitam based on the cp genome. Moreover, there were variations in the branches between the trees (Figure 9 and Supplementary Figure 5); the phylogenetic trees based on the IR, introns, and the 43 protein-coding genes were more consistent with the traditional classification system than those based on the full cp genome, the CDS, the SSC, or the LSC. The tree constructed from 43 common protein-coding genes had the highest reliability (89.6% of bootstrap values > 75), and the 10 Artemisia species included in this analysis were clustered in one branch, demonstrating a close genetic relationship. Among these 10 Artemisia species, A. argyi, A. latiflora, and A. montana formed a single group, suggesting a particularly close evolutionary relationship among the three species. Furthermore, four Chrysanthemum species and one Opisthopappus C. Shih species formed a larger branch with the 10 Artemisia species, suggesting that those Chrysanthemum and Opisthopappus species were closely related to Artemisia (Figure 9). In comparison, the other phylogenetic trees slightly differed from the traditional classification system (Supplementary Figure 5). This indicated that it was more reasonable to investigate the evolutionary relationship of Asteraceae species based on the shared protein-coding genes in their cp genomes.
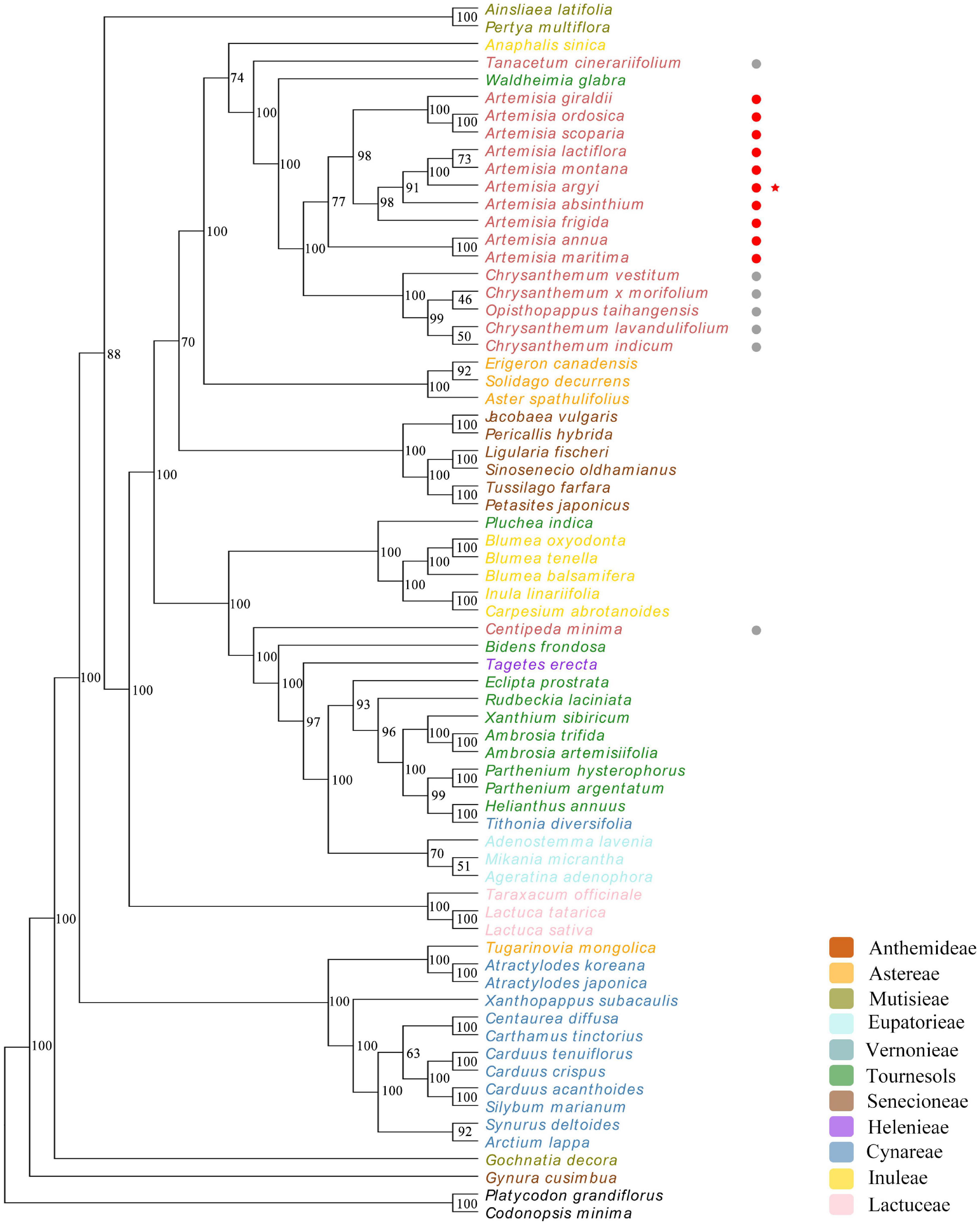
Figure 9. Phylogenetic tree constructed using 43 protein-coding genes common to 67 different Asteraceae species. Codonopsis minima and Platycodon grandiflorus from the family Campanulaceae are used as outgroup. The numbers above the branches represent the ML bootstrap values. Different tribes are color-coded. Non-Artemisia species of Anthemideae Cass are followed by gray circles, Artemisia species are followed by red circles, and Artemisia argyi is indicated with a red star.
Discussion
Chloroplast genome provides useful information for the study of evolution. Although the A. argyi cp genome has previously been reported (Kang et al., 2016), there is a lack of systematic research and evolutionary analysis of A. argyi varieties. In this study, cp genomes of 72 A. argyi varieties from 47 regions in China were determined. The length of A. argyi cp genomes ranged from 151,115 to 151,202 bp, placing them as medium-sized cp genomes compared to other Asteraceae species. GC content is generally considered an important indicator of species affinity (Chen J. et al., 2021). In A. argyi cp genomes, the GC content was significantly higher in IR regions than that in SC regions, which was mainly due to the abundance of rRNA genes in IR regions. The cp genome of A. argyi contained 114 genes, similar to the cp genome of A. annua (Shen et al., 2017). The A. argyi cp genome gained psbG and rpl12 but lost trnT-GGU in comparison to the cp genome of A. annua. Moreover, 13 intron-containing genes were detected in the A. argyi cp genome, all of which were located in the LSC. Among them, trnK-UUU had the largest intron, which contained matK (Wang Y. et al., 2021); matK is a well-known gene that is widely used for molecular identification and analysis of genetic relationships in medicinal plants (Hilu and Liang, 1997; Ramesh et al., 2022). Many intron-containing genes have important physiological functions; clpP is relevant to proteolysis (Shikanai et al., 2001) and petB encodes the B6 protein, the core component of the cytochrome b6f complex (Allen et al., 2011). Introns in these genes may be useful in the physiological function of A. argyi. Repeat sequence and SSR analyses have been widely used in studies of plant genetics, polymorphisms, and evolution. In the A. argyi cp genome, repeat sequences and SSRs were primarily located in the LSC region. Four A. argyi varieties from each of the four famous producing areas were used as representatives: S07, S09, S29, and S71. In these representative varieties, the types of repetitive sequences identified were mainly forward and palindromic repeats. The cp genomes of most A. argyi varieties contained ∼40 SSRs including mononucleotide, dinucleotide, and trinucleotide repeats. Most of the SSRs identified were mononucleotide A/T repeats, consistent with the cp genomes in most angiosperms (Hu et al., 2016).
Polymorphic sites and evolutionary relationships were here investigated in 72 A. argyi cp genomes. Overall, 196 polymorphic sites were detected. IR regions were found to be more conserved than SC regions. These polymorphic sites provide valuable information for the identification of A. argyi varieties at the molecular level. Previous studies indicated that A. argyi germplasm resources are rich in genetic diversity (Chen C. J. et al., 2021). Based on intraspecific evolutionary analysis of A. argyi, there was no obvious clustering of A. argyi varieties from similar longitudes, latitudes, or cultivation histories. However, germplasm resources collected from higher altitudes were more likely to cluster away from other germplasm resources based on their cp genomes. This may be the result of the following two factors: First, A. argyi has a short history of artificial domestication, so the cultivation and domestication have not yet had a remarkable impact on the conserved cp genomes. Second, the results suggest that altitude may have a greater impact on A. argyi evolution than longitude and latitude.
The morphological characteristics of Artemisia species are similar. By comparing cp genome sequences, the genetic differences between species can be clearly observed at the molecular level. Taken together, the cp genomes of ten selected Artemisia species were relatively conserved in the LSC/IR boundaries and more variable in the SSC/IR boundaries. Ycf1 and rps19 are considered pseudogenes that do not encode proteins (Vanin, 1985). There are a total of eight genes around the boundaries of these four regions in the A. argyi cp genome. Rps19 spans the LSC/IRb boundary and ycf1 spans the IRb/SSC boundary; this was also seen in A. scoparia and A. absinthium. However, rps19 was not detected in Aster spathulifolius, which is similar to some Asteraceae species including Centaurea diffusa Lam., Chrysanthemum indicum L., Chrysanthemum × morifolium Ramat. Jacobaea vulgaris Gaertn. and Lactuca sativa Linn. in the family Asteraceae (Liang et al., 2018). In addition to A. lactiflora, other Artemisia species contain these two pseudogenes, which have been duplicated in A. frigida, A. montana, and A. ordosica. Taken together, the copy numbers and positions of ycf1 and rps19 in Artemisia species are quite different, consistent with Arecaceae Bercht. and J. Presl (de Santana Lopes et al., 2018) and herbaceous bamboos (Wang et al., 2018). In contrast, rpl2, ndhF, and trnN are relatively conserved in the IR boundary of Artemisia. The mVISTA analysis of 11 Asteraceae cp genomes showed that variable regions were primarily located in SC regions and that the coding region was more conserved than the non-coding region, which is consistent with the results of the polymorphism analysis in the 72 A. argyi cp genomes. Relevant research indicates that this phenomenon is observed in most higher plants, such as the families Styracaceae DC. and Spreng. (Cai et al., 2021) and Rosaceae Juss. (Xue et al., 2019). The higher conservation observed in the IR region may be due to copy correction caused by gene transformation between the two IR regions (Zhang et al., 2016). In previous studies, several most informative coding regions (ycf1, rps16, ccsA, rbcL, ndhA, matK, clpP, and accD) were usually used for developing specific barcodes in the Asteraceae family, which is different from the identification of A. argyi varieties (Curci et al., 2015).
Asteraceae contains a wide variety of species that are extensively geographically distributed, which makes classification extremely complex at both the morphological and molecular levels. The cp genome is widely used to study the evolutionary relationships between species due to its high conservation and slow evolution rate. To explore the phylogenetic relationships between Asteraceae species and to clarify the phylogenetic position of A. argyi, 7 phylogenetic trees were constructed using different regions of the cp genomes of A. argyi and 66 other Asteraceae species. The resulting seven phylogenetic trees were roughly similar to one another and to the traditional taxonomic classification. The tree built using the 43 protein-coding genes shared by the 67 species was closest to the traditional classification system, a finding that is consistent with related studies in other species (Shen et al., 2017; Xue et al., 2019). We therefore conducted our main analysis using the tree constructed with the 43 common protein-coding genes. The results showed that A. argyi is closely related to other Artemisia species, and that Artemisia species are closely related to other Anthemideae Cass species. Anthemideae Cass species have a closer relationship with some Astereae Cass species and a slightly more distant relationship with some Senecioneae Cass species. It is worth mentioning that the evolutionary relationships of the 67 Asteraceae species based on cp genomes were not completely consistent with traditional taxonomic classifications. We speculate that this may be for three reasons: (1) those species underwent genetic variation in the process of evolution; (2) the evolutionary speed of different species is diverse, and for some rapidly evolving species, the phylogenetic relationships of these species may not be accurately reflected based on the relatively conserved cp genome (Moore et al., 2010); and (3) cp DNA is parthenogenetic, so the cp genomes can only reflect the evolutionary processes of maternal or paternal lines (Feng et al., 2020).
Conclusion
In this study, we sequenced the cp genomes of 72 A. argyi varieties from 47 regions in China, and the characteristics of A. argyi cp genomes were systematically analyzed. A comparative analysis of repeat sequences and SSRs in A. argyi cp genomes provided abundant variation sites for developing molecular markers of variety identification. Despite the high conservation of A. argyi cp genomes, 196 polymorphic sites are still detected. And the difference between the IR boundaries of ten Artemisia species provides useful information for identifying different Artemisia species. Moreover, the phylogenetic relationship of 67 Asteraceae species shows that A. argyi is closely related to A. lactiflora and A. Montana. Based on the relationship between Anthemideae Cass and other tribes, it appeared that A. Cass species had a closer relationship with some Astereae Cass species, and a slightly more distant relationship with some Senecioneae Cass species. These results provide key novel insights for A. argyi variety identification and understanding of the evolutionary history of the family Asteraceae.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The raw data of 72 cp genome sequencing have been deposited to NCBI under the accession number PRJNA821831 and the assembled 72 cp genomes were deposited in the CNGB Sequence Archive (CNSA) of the China National Gene Bank Database (https://db.cngb.org/) under the accession number CNP0002996.
Author Contributions
DLi and TZ designed the research. CC, TZ, YM, DLu, JL, ZW, and ML performed experiments. CC and TZ analyzed the data and wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the Young Qihuang Scholars Project of the State Administration of Traditional Chinese Medicine; the National Key Research and Development Program (2017YFC1700704); the Young and Middle-aged talents project of the Hubei Provincial Department of Education (Q20212001); and the Natural Science Foundation of Hubei Province (2021CFB225 and 2020CFB138); and Key project at central government level: The ability establishment of sustainable use for valuable Chinese Medicine Resources (2060302).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.906725/full#supplementary-material
Footnotes
- ^ https://www.ldmap.net/index.html
- ^ https://www.ncbi.nlm.nih.gov/
- ^ http://www.biomarker.com.cn/
- ^ https://github.com/ndierckx/NOVOPlasty
- ^ http://www.geneious.com
- ^ http://lowelab.ucsc.edu/tRNAscan-SE/
- ^ https://irscope.shinyapps.io/Chloroplot/
- ^ http://bibiserv.techfak.uni-bielefeld.de/reputer/
- ^ https://webblast.ipk-gatersleben.de/misa/
- ^ https://irscope.shinyapps.io/irapp/
- ^ http://genome.lbl.gov/vista/mvista/submit.shtml
References
Allen, J. F., de Paula, W. B., Puthiyaveetil, S., and Nield, J. (2011). A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci. 16, 645–655. doi: 10.1016/j.tplants.2011.10.004
Amiryousefi, A., Hyvonen, J., and Poczai, P. (2018). IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics 34, 3030–3031. doi: 10.1093/bioinformatics/bty220
Bansod, S., Chilvery, S., Saifi, M. A., Das, T. J., Tag, H., and Godugu, C. (2021). Borneol protects against cerulein-induced oxidative stress and inflammation in acute pancreatitis mice model. Environ. Toxicol. 36, 530–539. doi: 10.1002/tox.23058
Cai, X. L., Landis, J. B., Wang, H. X., Wang, J. H., Zhu, Z. X., and Wang, H. F. (2021). Plastome structure and phylogenetic relationships of Styracaceae (Ericales). BMC. Ecol. Evol. 21:103. doi: 10.1186/s12862-021-01827-4
Cardini, F., and Weixin, H. (1998). Moxibustion for correction of breech presentation: a randomized controlled trial. JAMA. 280, 1580–1584. doi: 10.1001/jama.280.18.1580
Chen, C. J., Luo, D. D., Miao, Y. H., Guo, L. P., and Liu, D. H. (2021). Diversity of Artemisia argyi germplasm resources based on agronomic and leaf phenotypic traits. Zhongguo Zhong Yao Za Zhi 46, 2773–2782. doi: 10.19540/j.cnki.cjcmm.20210125.101
Chen, J., Zang, Y., Shang, S., Liang, S., Zhu, M., Wang, Y., et al. (2021). Comparative chloroplast genomes of Zosteraceae species provide adaptive evolution insights into seagrass. Front. Plant Sci. 12:741152. doi: 10.3389/fpls.2021.741152
Cui, Y., Gao, X., Wang, J., Shang, Z., Zhang, Z., Zhou, Z., et al. (2021). Full-length transcriptome analysis reveals candidate genes involved in terpenoid biosynthesis in Artemisia argyi. Front. Genet. 12:659962. doi: 10.3389/fgene.2021.659962
Curci, P. L., Paola, D. D., Danzi, D., Vendramin, G. G., and Sonnante, G. (2015). Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS One 10:e0120589. doi: 10.1371/journal.pone.0120589
Curci, P. L., Paola, D. D., and Sonnante, G. (2016). Development of chloroplast genomic resources for Cynara. Mol. Ecol. Resour. 16, 562–573. doi: 10.1111/1755-0998.12457
de Santana Lopes, A., Gomes Pacheco, T., Nimz, T., do Nascimento Vieira, L., Guerra, M. P., Nodari, R. O., et al. (2018). The complete plastome of macaw palm [Acrocomia aculeata (Jacq.) Lodd. ex Mart.] and extensive molecular analyses of the evolution of plastid genes in Arecaceae. Planta 247, 1011–1030. doi: 10.1007/s00425-018-2841-x
Dierckxsens, N., Mardulyn, P., and Smits, G. (2017). NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45:e18. doi: 10.1093/nar/gkw955
Doyle, J. J., Doyle, J. L., Doyle, J. A., and Doyle, F. J. (1987). A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 19, 11–13.
Du, Z., Lu, K., Zhang, K., He, Y., Wang, H., Chai, G., et al. (2021). The chloroplast genome of Amygdalus L. (Rosaceae) reveals the phylogenetic relationship and divergence time. BMC Genomics 22:645. doi: 10.1186/s12864-021-07968-6
Favier, A., Gans, P., Boeri Erba, E., Signor, L., Muthukumar, S. S., Pfannschmidt, T., et al. (2021). The plastid-encoded RNA polymerase-associated protein PAP9 is a superoxide dismutase with unusual structural features. Front. Plant Sci. 12:668897. doi: 10.3389/fpls.2021.668897
Feng, S., Zheng, K., Jiao, K., Cai, Y., Chen, C., Mao, Y., et al. (2020). Complete chloroplast genomes of four Physalis species (Solanaceae): lights into genome structure, comparative analysis, and phylogenetic relationships. BMC Plant Biol. 20:242. doi: 10.1186/s12870-020-02429-w
Gao, X., Zhang, X., Meng, H., Li, J., Zhang, D., and Liu, C. (2018). Comparative chloroplast genomes of Paris Sect. Marmorata: insights into repeat regions and evolutionary implications. BMC Genomics 19:878. doi: 10.1186/s12864-018-5281-x
Hilu, K., and Liang, H. (1997). The matK gene: sequence variation and application in plant systematics. Am. J. Bot. 84, 830.
Hu, Y., Woeste, K. E., and Zhao, P. (2016). Completion of the chloroplast genomes of five Chinese Juglans and their contribution to chloroplast phylogeny. Front. Plant Sci. 7:1955. doi: 10.3389/fpls.2016.01955
Kang, S. H., Kim, K., Lee, J. H., Ahn, B. O., Won, S. Y., Sohn, S. H., et al. (2016). The complete chloroplast genome sequence of medicinal plant, Artemisia argyi. Mitochondrial DNA B Resour. 1, 257–258. doi: 10.1080/23802359.2016.1159926
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kawabe, A., Nukii, H., and Furihata, H. Y. (2018). Exploring the history of chloroplast capture in Arabis using whole chloroplast genome sequencing. Int. J. Mol. Sci. 19:602. doi: 10.3390/ijms19020602
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kim, Y. J., Kim, S., Jung, Y., Jung, E., Kwon, H. J., and Kim, J. (2018). Eupatilin rescues ciliary transition zone defects to ameliorate ciliopathy-related phenotypes. J. Clin. Invest. 128, 3642–3648. doi: 10.1172/JCI99232
Kurtz, S., and Schleiermacher, C. (1999). REPuter: fast computation of maximal repeats in complete genomes. Bioinformatics 15, 426–427. doi: 10.1093/bioinformatics/15.5.426
Lee, J. H., Lee, J. W., Sung, J. S., Bang, K. H., and Moon, S. G. (2009). Molecular authentication of 21 Korean Artemisia species (Compositae) by polymerase chain reaction-restriction fragment length polymorphism based on trnL-F region of chloroplast DNA. Biol. Pharm. Bull. 32, 1912–1916. doi: 10.1248/bpb.32.1912
Lee, J. H., Lee, Y. J., Lee, J. Y., and Park, Y. M. (2017). Topical application of Eupatilin Ameliorates atopic dermatitis-like skin lesions in NC/Nga mice. Ann. Dermatol. 29, 61–68. doi: 10.5021/ad.2017.29.1.61
Li, C., Cai, C., Tao, Y., Sun, Z., Jiang, M., Chen, L., et al. (2021). Variation and evolution of the whole chloroplast genomes of Fragaria spp. (Rosaceae). Front. Plant Sci. 12:754209. doi: 10.3389/fpls.2021.754209
Liang, F., Wen, X., Gao, H., and Ying, Z. (2018). Analysis of chloroplast genomes features of Asteraceae species. Genomics Appl. Biol. 37, 5437–5447. doi: 10.13417/j.gab.037.005437
Librado, P., and Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. doi: 10.1093/bioinformatics/btp187
Liu, M., Zhu, J., Wu, S., Wang, C., Guo, X., Wu, J., et al. (2018). De novo assembly and analysis of the Artemisia argyi transcriptome and identification of genes involved in terpenoid biosynthesis. Sci. Rep. 8:5824. doi: 10.1038/s41598-018-24201-9
Lowe, T. M., and Chan, P. P. (2016). tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44, 54–57. doi: 10.1093/nar/gkw413
Martins, A., Rodrigues, L. B., Cesario, F., de Oliveira, M. R. C., Tintino, C. D. M., Castro, F. F. E., et al. (2017). Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol). Biomed. Pharmacother. 96, 384–395. doi: 10.1016/j.biopha.2017.10.005
Mei, Q. X., Chen, X. L., Xiang, L., Liu, Y., Su, Y. Y., Gao, Y. Q., et al. (2016). DNA barcode for identifying folium Artemisiae Argyi from counterfeits. Biol. Pharm. Bull. 39, 1531–1537. doi: 10.1248/bpb.b16-00336
Moore, M. J., Soltis, P. S., Bell, C. D., Burleigh, J. G., and Soltis, D. E. (2010). Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc. Natl. Acad. Sci. U.S.A. 107, 4623–4628. doi: 10.1073/pnas.0907801107
Mulyaningsih, S., Sporer, F., Zimmermann, S., Reichling, J., and Wink, M. (2010). Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 17, 1061–1066. doi: 10.1016/j.phymed.2010.06.018
Qiao, J. W., Cai, M. X., Yan, G. X., Wang, N., Li, F., Chen, B. Y., et al. (2016). High-throughput multiplex cpDNA resequencing clarifies the genetic diversity and genetic relationships among Brassica napus, Brassica rapa and Brassica oleracea. Plant Biotechnol. J. 14, 409–418. doi: 10.1111/pbi.12395
Ramesh, G. A., Mathew, D., John, K. J., and Ravisankar, V. (2022). Chloroplast gene matK holds the barcodes for identification of Momordica (Cucurbitaceae) species from Indian subcontinent. Hortic. Plant J. 8, 89–98. doi: 10.1016/j.hpj.2021.04.001
Shen, X., Wu, M., Liao, B., Liu, Z., Bai, R., Xiao, S., et al. (2017). Complete chloroplast fenome sequence and phylogenetic analysis of the medicinal plant Artemisia annua. Molecules 22, 1330. doi: 10.3390/molecules22081330
Shikanai, T., Shimizu, K., Ueda, K., Nishimura, Y., Kuroiwa, T., and Hashimoto, T. (2001). The chloroplast clpP gene, encoding a proteolytic subunit of ATP-dependent protease, is indispensable for chloroplast development in tobacco. Plant Cell Physiol. 42, 264–273. doi: 10.1093/pcp/pce031
Shinozaki, K., Ohme, M., Tanaka, M., Wakasugi, T., Hayashida, N., Matsubayashi, T., et al. (1986). The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 5, 2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x
Song, Y., Chen, Y., Lv, J., Xu, J., Zhu, S., Li, M., et al. (2017). Development of chloroplast genomic resources for Oryza species discrimination. Front. Plant Sci. 8:1854. doi: 10.3389/fpls.2017.01854
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Thiel, T., Michalek, W., Varshney, R. K., and Graner, A. (2003). Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 106, 411–422. doi: 10.1007/s00122-002-1031-0
Vanin, E. F. (1985). Processed pseudogenes: characteristics and evolution. Annu. Rev. Genet. 19, 253–272. doi: 10.1146/annurev.ge.19.120185.001345
Wang, M., Wang, X., Sun, J., Wang, Y., Ge, Y., Dong, W., et al. (2021). Phylogenomic and evolutionary dynamics of inverted repeats across Angelica plastomes. BMC Plant Biol. 21:26. doi: 10.1186/s12870-020-02801-w
Wang, W., Chen, S., and Zhang, X. (2018). Whole-genome comparison reveals divergent IR borders and mutation hotspots in chloroplast genomes of Herbaceous Bamboos (Bambusoideae: Olyreae). Molecules 23:1537. doi: 10.3390/molecules23071537
Wang, Y., Wang, S., Liu, Y., Yuan, Q., Sun, J., and Guo, L. (2021). Chloroplast genome variation and phylogenetic relationships of Atractylodes species. BMC Genomics 22:103. doi: 10.1186/s12864-021-07394-8
Xue, S., Shi, T., Luo, W., Ni, X., Iqbal, S., Ni, Z., et al. (2019). Comparative analysis of the complete chloroplast genome among Prunus mume, P. armeniaca, and P. salicina. Hortic. Res. 6:89. doi: 10.1038/s41438-019-0171-1
Keywords: Artemisia argyi, Asteraceae, chloroplast genome, phylogenetic analysis, variety
Citation: Chen C, Miao Y, Luo D, Li J, Wang Z, Luo M, Zhao T and Liu D (2022) Sequence Characteristics and Phylogenetic Analysis of the Artemisia argyi Chloroplast Genome. Front. Plant Sci. 13:906725. doi: 10.3389/fpls.2022.906725
Received: 29 March 2022; Accepted: 06 May 2022;
Published: 20 June 2022.
Edited by:
Eleni Tani, Agricultural University of Athens, GreeceReviewed by:
Yun-peng Du, Beijing Academy of Agricultural and Forestry Sciences, ChinaHarald Schneider, Xishuangbanna Tropical Botanical Garden (CAS), China
Gabriella Sonnante, National Research Council (CNR), Italy
Copyright © 2022 Chen, Miao, Luo, Li, Wang, Luo, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Zhao, ZGF0aW5nc2RhdUAxMjYuY29t; Dahui Liu, bGl1ZGFodWlAaGJ0Y20uZWR1LmNu
 Changjie Chen
Changjie Chen Yuhuan Miao
Yuhuan Miao Dahui Liu
Dahui Liu