- 1Research Center on Ecological Sciences, Jiangxi Agricultural University, Nanchang, China
- 2Department of Agronomy, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 3Department of Agronomy, Sub-Campus Depalpur, Okara, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 4Biology Department, Collage of Science, Jouf University, Sakaka, Saudi Arabia
- 5Department of Botany and Plant Physiology, Faculty of Agrobiology, Food, and Natural Resources, Czech University of Life Sciences Prague, Prague, Czechia
- 6Department of Plant Physiology, Slovak University of Agriculture, Nitra, Slovakia
- 7Department of Agriculture, Guru Nanak Dev University, Amritsar, India
- 8Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt
- 9Department of Field Crops, Faculty of Agriculture, Siirt University, Siirt, Turkey
- 10Department of Biology, Al-Jumum University College, Umm Al-Qura University, Makkah, Saudi Arabia
Global warming in this century increases incidences of various abiotic stresses restricting plant growth and productivity and posing a severe threat to global food production and security. The plant produces different osmolytes and hormones to combat the harmful effects of these abiotic stresses. Melatonin (MT) is a plant hormone that possesses excellent properties to improve plant performance under different abiotic stresses. It is associated with improved physiological and molecular processes linked with seed germination, growth and development, photosynthesis, carbon fixation, and plant defence against other abiotic stresses. In parallel, MT also increased the accumulation of multiple osmolytes, sugars and endogenous hormones (auxin, gibberellic acid, and cytokinins) to mediate resistance to stress. Stress condition in plants often produces reactive oxygen species. MT has excellent antioxidant properties and substantially scavenges reactive oxygen species by increasing the activity of enzymatic and non-enzymatic antioxidants under stress conditions. Moreover, the upregulation of stress-responsive and antioxidant enzyme genes makes it an excellent stress-inducing molecule. However, MT produced in plants is not sufficient to induce stress tolerance. Therefore, the development of transgenic plants with improved MT biosynthesis could be a promising approach to enhancing stress tolerance. This review, therefore, focuses on the possible role of MT in the induction of various abiotic stresses in plants. We further discussed MT biosynthesis and the critical role of MT as a potential antioxidant for improving abiotic stress tolerance. In addition, we also addressed MT biosynthesis and shed light on future research directions. Therefore, this review would help readers learn more about MT in a changing environment and provide new suggestions on how this knowledge could be used to develop stress tolerance.
Introduction
Plants are sessile organisms that face a variety of environmental stress (drought, salinity, heat, cold stress, heavy metals stress, and nutrient deficiency) (Rasheed et al., 2021a,b), which have devastating impacts on their performance in terms of growth and productivity (Sharma et al., 2019). These abiotic stresses disrupt plant physiological and metabolic functioning development processes (Jeandroz and Lamotte, 2017) and induce the production of reactive oxygen species (ROS), lipid peroxidation and accumulation of various osmolytes, and significant yield losses (Arif et al., 2016; Singh et al., 2017; Batool et al., 2022a,b; Imran et al., 2022). The intensity of these abiotic stresses is steadily increasing due to rapid climate change, and appropriate measures need to be taken to address these stresses (Beebe et al., 2011; Jeandroz and Lamotte, 2017; Ali et al., 2019). Therefore, plants have developed diverse mechanisms to counter these abiotic stresses (Rasheed et al., 2020a,b). Such tools include plant growth regulators, different osmolytes synthesis, and accumulation to protect against stress-induced damages for maintaining cellular homoeostasis and optimum plant growth (Yancey, 2005; Burg and Ferraris, 2008; Beebe et al., 2011; Liang et al., 2013; Singh et al., 2017).
Melatonin (MT) is one such molecule considered a vital plant growth regulator under stress conditions. It is a pineal molecule discovered in bovine pineal glands (Lerner et al., 1958; Reiter, 1991). MT received its name in 1957 when it was reported to play a role in the skin lightening of frogs and involves in controlling circadian rhythms in diverse vertebrates (Lerner et al., 1958; Tan et al., 2018). The maximum MT levels during the night indicate its importance in nocturnal signalling (Reiter, 1991). In plants, the MT presence was discovered in various monocot and dicot families (Reiter et al., 2001; Nawaz et al., 2016). Its presence in diverse plant parts (root, stem, leaves, fruit, flower, and seeds) in apple, banana, cucumber, onion, rice, and tomato, indicates its importance in plant growth and development across the plant kingdom (Nawaz et al., 2016; Wei et al., 2018).
The MT role in response to different stresses has been comprehensively studied (Debnath et al., 2019). MT plays an important role in seed germination, biomass productivity, photosynthesis, fruit maturation, membrane integrity, osmoregulation, leaf senescence and plants responses to abiotic stresses (Lee et al., 2014; Shi et al., 2015b). MT-mediated gene expression regulation protects plants against stress conditions, for example, the activation of antioxidant machinery of plants (Debnath et al., 2019); thus, it is considered an essential bio-stimulant to improve crop production in adverse conditions. MT triggered an antioxidant defence system under stress conditions, favouring ROS scavenging and acting as a stress protecting molecule (Khan et al., 2020a). This property of MT makes it a promising molecule that can be used for exogenous application under stress conditions. In this review, we have explored the physiological and biochemical role of MT under diverse abiotic stresses. We also discussed the possible mechanism of MT under different stresses. Moreover, we have also shed light on engineered MT biosynthesis, its crosstalk with other hormones, and future research to provide a complete picture of MT-mediated abiotic stress tolerance.
Biosynthesis of Melatonin in Plants
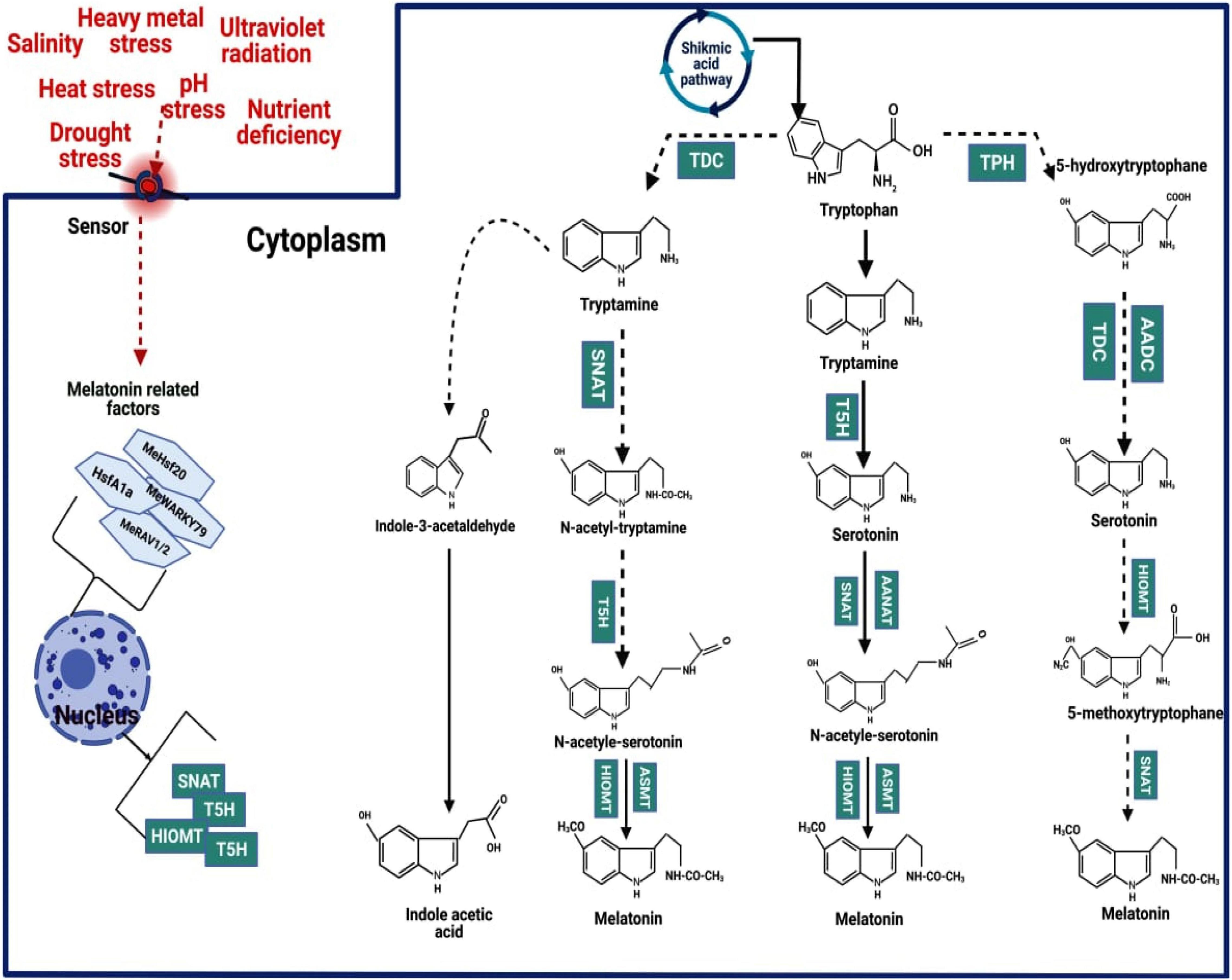
In the MT biosynthesis pathway, the tryptophan (TTP) precursor, which is also a precursor of indole-3-acetic acid (IAA), comes from the shikimic acid pathway (Posmyk and Janas, 2009; Arnao and Hernández-Ruiz, 2014; Nawaz et al., 2016; Zhao et al., 2019). The TTP is converted in MT by four enzymatic reactions catalysed by four diverse enzymes (Figure 1). The enzyme tryptophan decarboxylase (TDC) firstly changed TTP into tryptamine. After that, the enzyme tryptamine 5-hydroxylase (T5H) converts tryptamine into serotonin. These two steps are crucial for the synthesis of serotonin in plants. Nevertheless, in some plants, a different pathway operates in which tryptophan is converted by tryptophan 5-hydroxylase (TPH) to 5-hydroxytryptophan, which is then catalysed by tryptophan decarboxylase or aromatic L-amino acid decarboxylase (TDC/AADC) to serotonin (Zuo et al., 2014). Subsequently, arylalkylamine N-acetyltransferase (AANAT) or N-acetyltransferase (SNAT) converts serotonin into N-acetyl-serotonin. Moreover, SNAT can also convert tryptamine into N-acetyl-tryptamine; however, T5H cannot convert N-acetyl-tryptamine into N-acetyl-serotonin. In the last step, N-acetyl-serotonin methyltransferase (ASMT) or hydroxyindole-O-methyltransferase (HIOMT) catalysed the N-acetyl-serotonin into MT. HIOMT can also convert serotonin into 5-methoxytryptamine, converted into MT by SNAT (Zhang et al., 2014; Tan et al., 2016).
Generally, MT and its intermediate accretion in different sub-cellular sites depend on the order of enzymes reaction involved in MT biosynthesis. For instance, the accumulation of serotonin occurs in the endoplasmic reticulum when TTP is converted into T5H, while serotonin accumulates in the cytoplasm in the TDC enzyme. Likewise, the conversation of serotonin into N-acetyl-serotonin occurs in the chloroplast, where serotonin conversion into 5-methoxytryptamine by ASMT accumulation occurs in the cytoplasm. Finally, MT synthesis occurs in the chloroplast (Miller et al., 2010). The order of enzymes reaction in MT biosynthesis alters the subcellular sites of intermediates and MT formation (Back et al., 2016). For instance, the first and second enzymatic reactions result in the formation of serotonin in the cell endoplasmic reticulum (ER), while the third and fourth enzymatic reaction leads to the formation of serotonin in the cell cytoplasm (Back et al., 2016). The synthesis of MT in the chloroplast occurs when the final step enzyme is SNAT whereas ASMT/COMT is involved in the terminal reaction that occurs in the cytoplasm. Nonetheless, depending on the sites of biosynthesis, both MT and serotonin levels are strongly affected by the capability of anabolic and catabolic flow (Back et al., 2016). TTP and serotonin are significantly accumulated in senesced leaves, while tryptamine and N-acetylserotonin are not significantly increased (Back et al., 2016). Thus, these events can be explained by the quick conversion of tryptamine to serotonin by T5H and serotonin conversion N-acetylserotonin by SNAT (Kang et al., 2009a,2010).
Moreover, a significant accumulation of serotonin is not attained when enzymes competing for serotonin as a substrate are present at the same sub-cellular site. For instance, serotonin is quickly metabolised into phenylpropanoid amides (feruloyl serotonin) by serotonin N-hydroxycinnamoyl transferase, which is expressed in the cell cytoplasm (Kang et al., 2009b). Moreover, MT can also be quickly metabolised into 2-hydroxymelatonin (2-OHMel) and cyclic 3-hydroxymelatonin (3-OHMel) by MT-2-hydroxylase (M2H) and melatonin 3-hydroxylase (M3H), respectively, when MT is present in plant chloroplast and cytoplasm, respectively (Byeon and Back, 2015; Lee et al., 2016). In-plant chloroplast MT provides a significant defence to plants against oxidative stresses. The plant chloroplast and mitochondria are significant sites of MT biosynthesis, and it does not preclude the possibility that some MT is not also formed in cell cytosol (Tan and Reiter, 2019). The diverse pathways, along with different sub-cellular sites for MT production, play an important role in the steady-state level of MT and in the induction of MT synthesis in responses to various stresses to cope with adverse impacts (Back et al., 2016).
Melatonin: The Stress Protectant
Melatonin is an excellent antioxidant molecule with the appreciable potential to scavenge ROS and improve stress tolerance (Figure 2). Its exogenous application improves various physiological and biochemical processes and plants’ responses to diverse abiotic stress conditions. It improves chlorophyll contents, photosynthetic efficiency, protein accumulations, and RuBisCO activities and triggers the antioxidant defence system, inducing stress tolerance (Figure 2). MT also stimulates different signalling pathways in response to stress conditions. Here we briefly described the prominent roles of MT mediated tolerance in plants against various abiotic stresses.
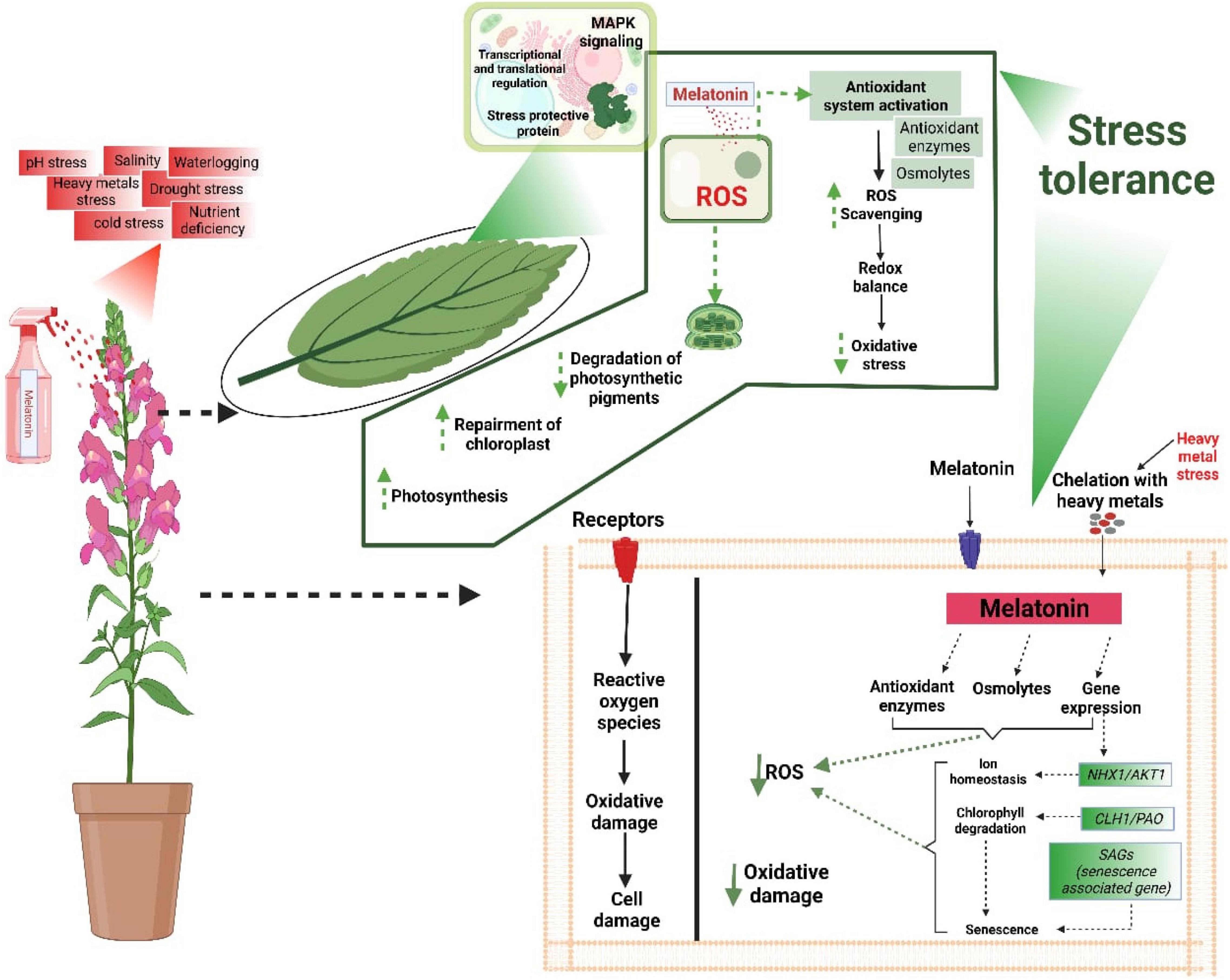
Figure 2. MT being an amphiphilic molecule free crosses cellular membranes and directly scavenges the ROS by increasing the anti-oxidant activities. MT also improves osmolytes accumulation, protects photosynthetic apparatus, maintains redox balance, and affects the signalling transduction and genes expression linked with different stresses to induce stress tolerance.
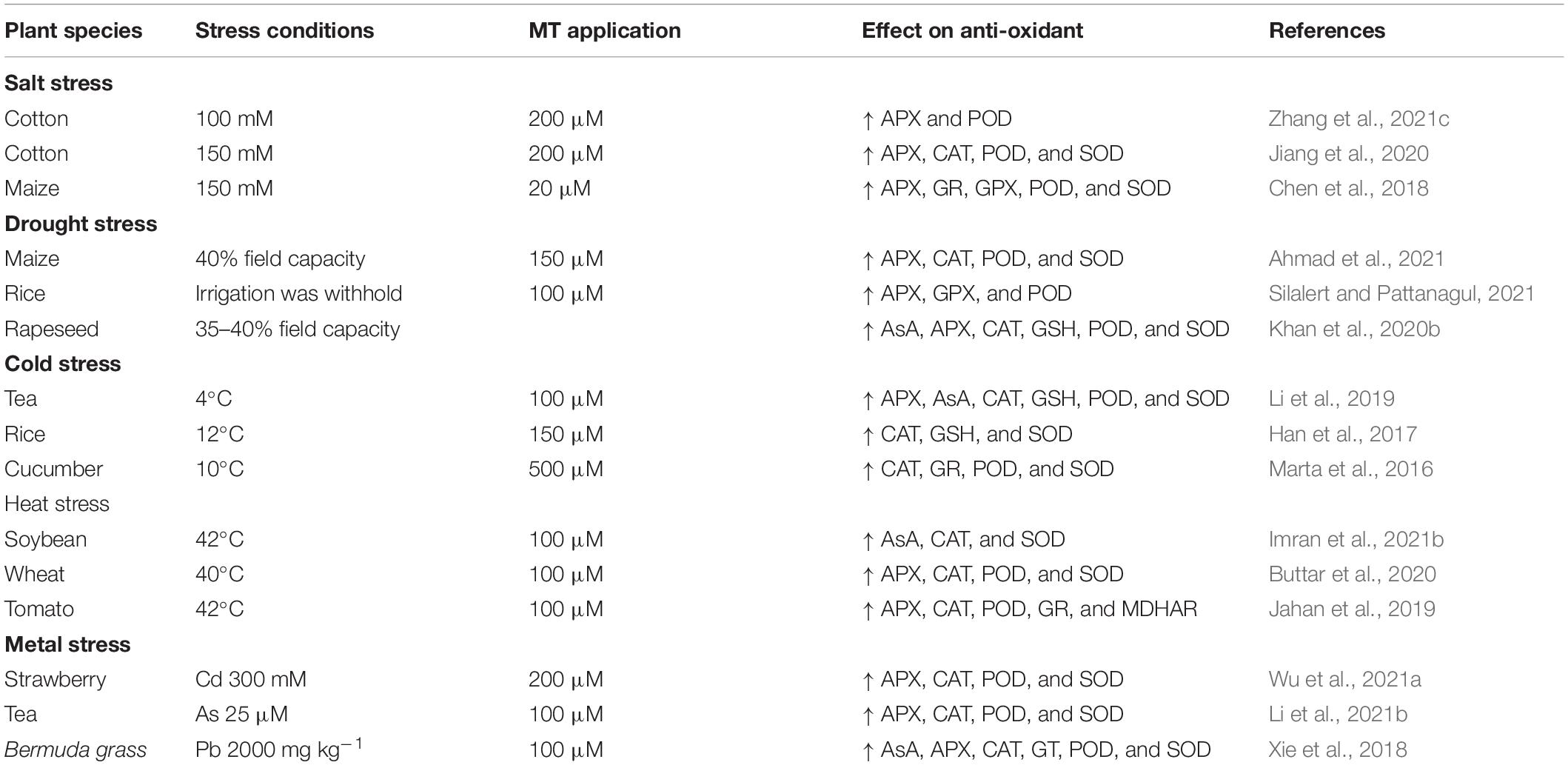
Melatonin Induces Salinity Tolerance in Plants
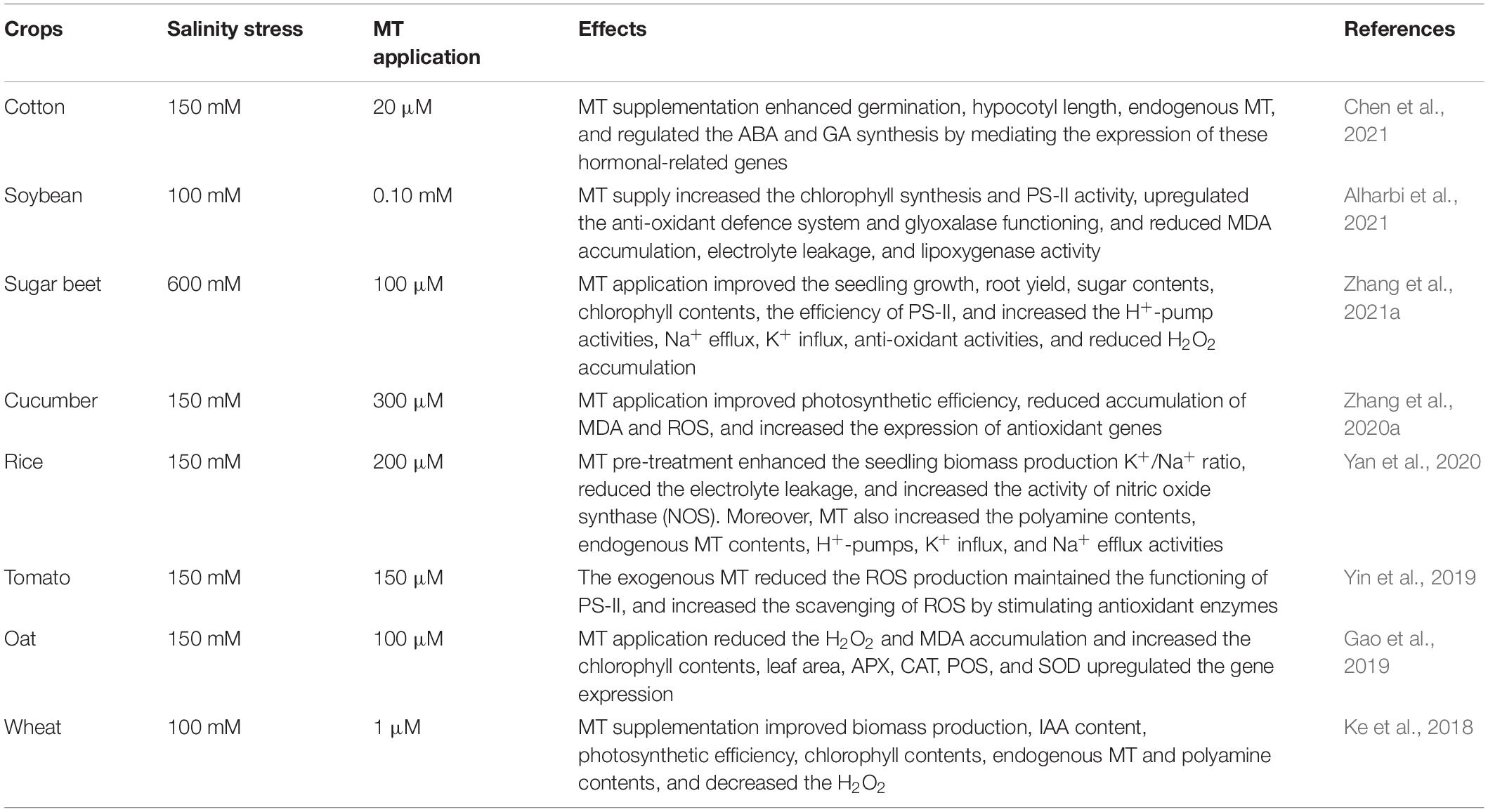
Salt stress significantly limits crop growth and development and threatens global food production. It mainly induces osmotic stress, ionic and nutritional imbalance, and ROS, resulting in a significant loss in plant growth (Abbasi et al., 2016; Dustgeer et al., 2021; Sultan et al., 2021; Seleiman et al., 2022). Globally, many plant growth regulators (PGR) reported improving salt tolerance to achieve agricultural sustainability (Bastam et al., 2013). Salt stress-induced a reduction in crop productivity by decreasing the photosynthetic efficiency (Meloni et al., 2003). Reduced photosynthetic efficiency can be caused by the closing of stomata and the negative effect of salinity on photosynthetic parameters (Meloni et al., 2003). However, MT application considerably improved the effectiveness of PS-II (Table 1) for photochemical and non-photochemical quenching, which favours increased photosynthetic efficacy under salt stress (Li et al., 2017).
MT application regulates the ROS, protecting the photosynthetic apparatus and improving the photosynthetic efficiency and subsequent growth under salt stress, as shown in maize (Chen et al., 2018). MT supply improves sugar accumulation, chlorophyll biosynthesis, and protection of PS-II under salt-stressed conditions (Zhang et al., 2021a). MT supplementation enhances gene expression of various antioxidant, photosynthesis and ROS scavenging enzymes, confining salt tolerance in Phaseolus vulgaris and rice (Yan et al., 2021).
Moreover, MT also substantially maintains the ionic balances to counter the salt stress. For example, MT application increased the K+ accumulation, decreased the Na+ accretion, and kept the higher K+/Na+ ratio to induce salinity tolerance in maize seedlings (Jiang et al., 2016). The improved ionic homoeostasis in plants is linked with the upregulation of the transcription of the different genes such as MdNHX1 and MdAKT1, which substantially confer the salt tolerance in MT treated Malus hupehensis seedlings (Li et al., 2012). Likewise, MT treatment also increased the expression of NHX1 and SOS2 in rapeseed seedlings which were associated with a lower Na+/K+ ratio (Zhao et al., 2018). Moreover, the interaction of Ca2+/CaM (Ca2+/Calmodulin) and MT is also considered to be involved in overcoming the harmful effects of salt stress. Ca and MT interaction induces long-distance signalling, bringing salt stress tolerance in Dracocephalum kotschyi (Vafadar et al., 2020).
Additionally, MT supplementation also caused a reduction in ROS production (Table 1; Wang et al., 2016a; Zheng et al., 2017) through enhanced activities of antioxidant enzymes (APX, CAT, GR, GPX, POD, and SOD) under salt stress (Jiang et al., 2016; Chen et al., 2018). It also increased the actions of the H+-pump, which subsequently promoted the K+ influx and Na+ efflux. It enhanced the activity of antioxidants (APX, CAT, POD, SOD, AsA, and GSH) and the accumulation of soluble sugars, proline, and glycine betaine, favouring the increase in salt tolerance (Zhang et al., 2021a). In conclusion, MT improves plant growth under salt stress by enhancing photosynthetic efficiency, K+ influx, and Na+ efflux, reducing ROS production, improving antioxidant activities, and accumulating compatible solutes. Therefore, exogenous application of MT can improve salt stress in crops.
Melatonin Induces Drought Tolerance in Plants
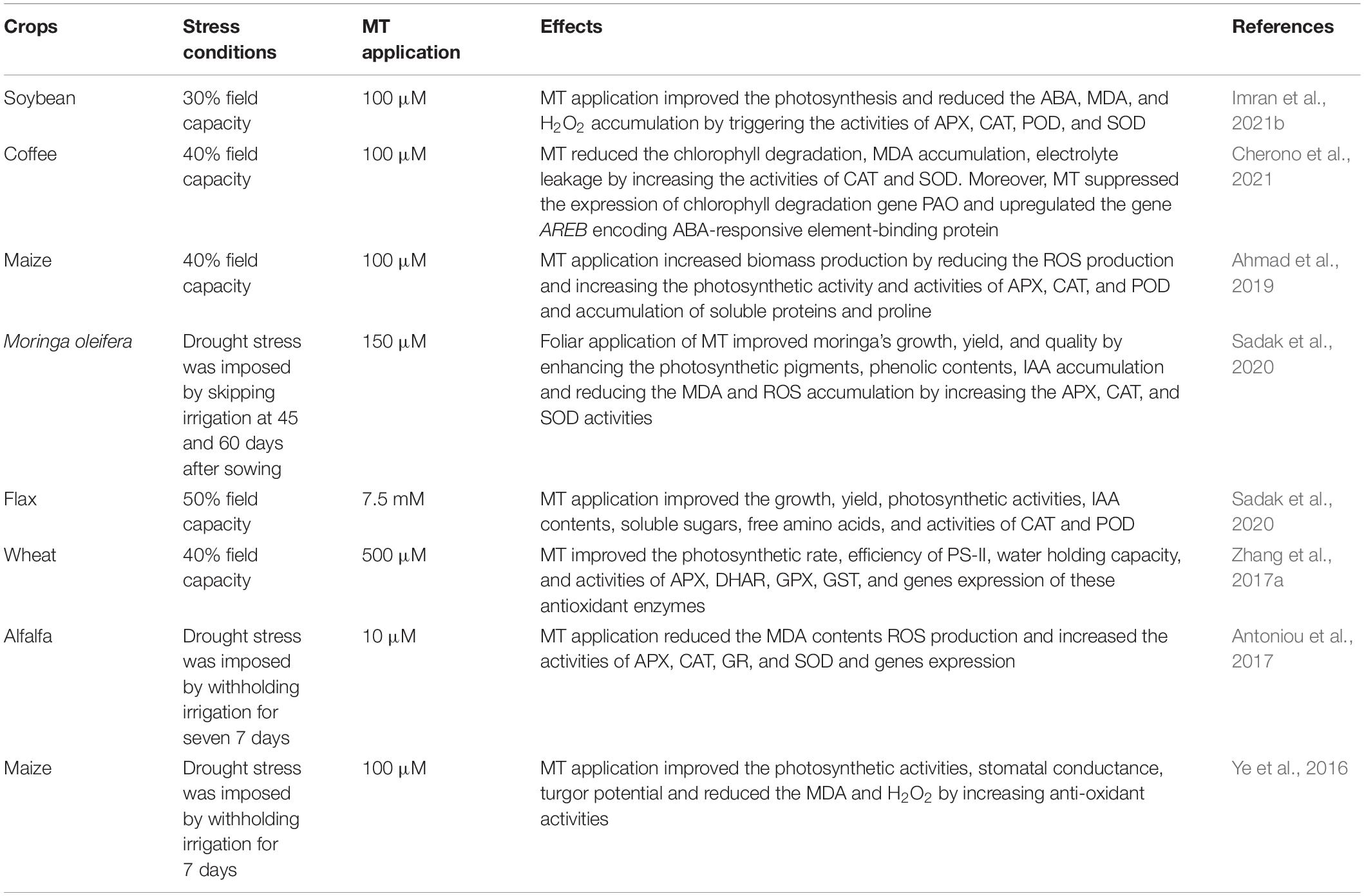
Drought is another significant abiotic stress that considerably limits crop growth and global food production (Hassan et al., 2017, 2020; Mehmood et al., 2021). The reduced water availability induces severe alterations in plant physiological processes, which consequently cause severe yield losses (Hassan et al., 2019, 2021). Melatonin is a potential PGR that confers tolerance in plants against different stress conditions, including drought stress (Meng et al., 2014; Kabiri et al., 2018). Melatonin regulates various physiological, biochemical, and molecular processes (Table 2), which improves the plant’s resistance to stand drought conditions (Campos et al., 2019). The regulation of photosynthetic processes and antioxidant defence system are the main processes controlled by MT under drought stress (Liang et al., 2019). Melatonin protects the photosynthetic apparatus from the effects of drought, which, therefore, improves the photosynthetic efficiency (Meng et al., 2014; Liang et al., 2018).
Melatonin also prevents chlorophyll degradation during drought and improves stomatal conductance and photosynthetic efficiency (Liang et al., 2018; Karaca and Cekic, 2019). Moreover, enhanced photosynthetic rate by MT supplementation is attributed to improved PS-II efficiency and better electron transport rates (Zhang et al., 2013; Liang et al., 2018). MT application also protects the chloroplast structure from oxidative stress damage resulting in a substantial increase in photosynthesis (Cui et al., 2017). MT supply also suppressed the expression of chlorophyll degradation genes [pheophorbide a oxygenase (PAO)], which improves the chlorophyll synthesis under stress conditions. MT also increases the expression of photosynthetic genes (RBCS2), thereby improving overall photosynthetic efficiency, assimilating production and crop growth under drought stress (Cherono et al., 2021).
Melatonin application as pre-treatments significantly improved seed germination, delayed senescence, and enhanced root growth under drought stress, resulting in improved plant development and final production (Wang et al., 2013; Zhang et al., 2013). Moreover, MT application also reduced the drought-induced impacts on growth by improving stomatal conductance, photosynthetic efficiency, leaf water status, reducing the electrolyte leakage and H2O2 accumulation, and increasing soluble sugars and proline accumulation (Liang et al., 2018; Ahmad et al., 2021). The MT mediated protection of the plants from damaging impacts of drought-induced oxidative stress is linked with increased ROS scavenging. The triggered ROS scavenging by MT is due to the stimulated antioxidant defence system under drought stress (Liu et al., 2015b; Cai et al., 2017; Gao et al., 2018; Campos et al., 2019). Water scarcity induced a significant increase in ABA accumulation in plants. Increased ABA level in plants increased oxidative stress linked with lipid peroxidation, electrolyte leakage, and chlorophyll degradation (Jiang et al., 2020). MT supplementation reduced the ABA accumulation under drought stress by downregulating the genes linked with ABA biosynthesis and upregulating the genes involved in ABA catabolism (Jiang et al., 2020). Additionally, in drought-stressed plants, MT appreciably increased the activities of antioxidants (APX, CAT, DHAR, GPX, GR, MDHAR, POD, and SOD) which declined the ROS production safeguarded the plants from drought-induced oxidative stress (Galano et al., 2013; Li et al., 2015; Kabiri et al., 2018; Campos et al., 2019). To summarise, MT improves photosynthetic efficiency, reduces drought-induced ROS production, ABA accumulation, and increases antioxidant activities and proline accumulation, which confer drought tolerance and can be used as a stress protectant under drought stress.
Melatonin Induces Cold Tolerance in Plants
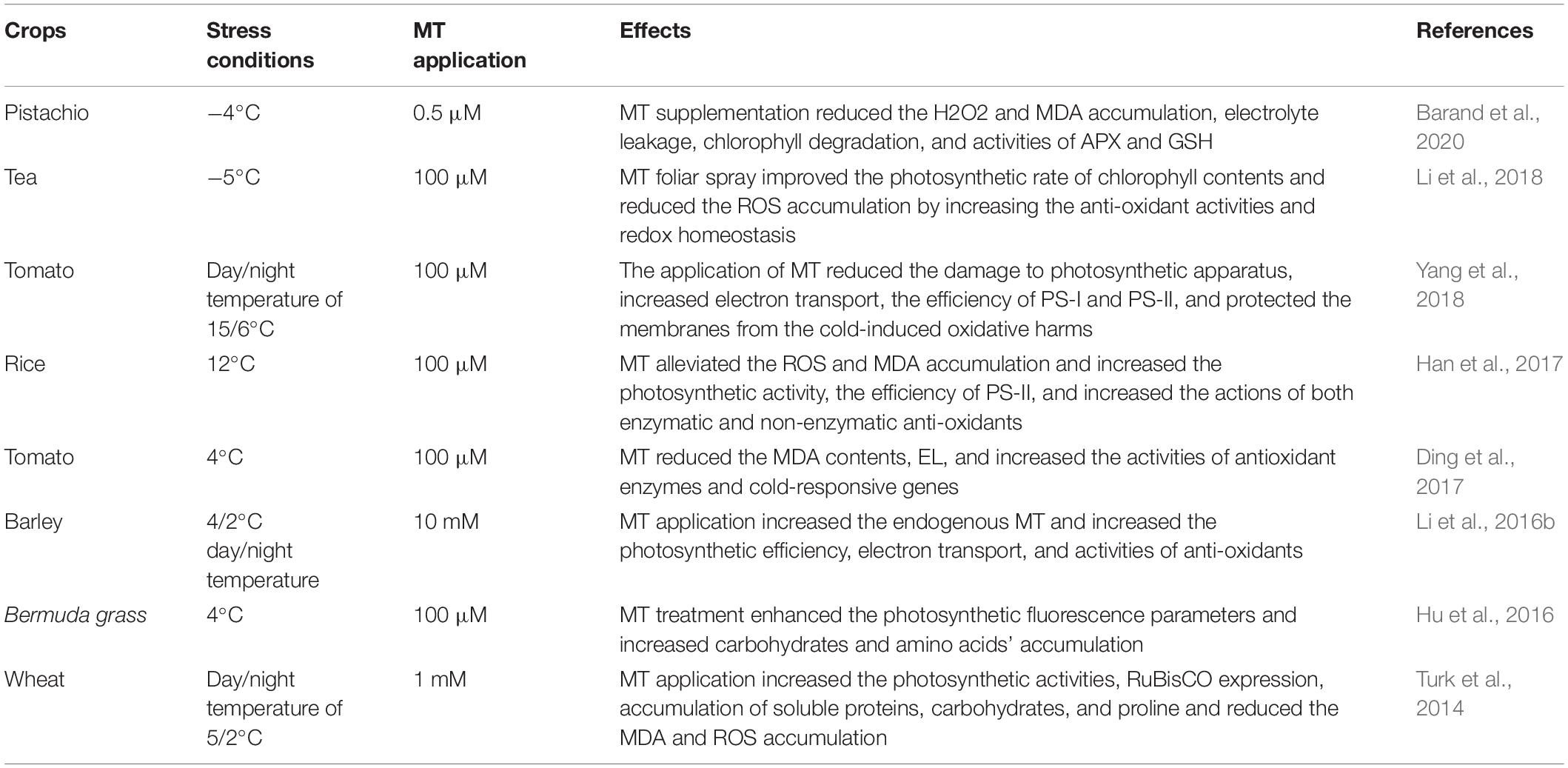
Cold stress also has devastating impacts on plants and considerably limits crop growth and production (Mishra et al., 2011). Cold stress induces substantial changes in plants’ physiological, molecular, and metabolic activity, altering the membrane permeability and antioxidant activity (Bajwa et al., 2014; Hu et al., 2016). Therefore, MT application improved the cold tolerance of Bermuda grass by increasing ROS scavenging (Table 3) through increased antioxidant activities (Shi et al., 2015a). Similarly, spraying the rice seedlings with different MT concentrations (0, 20, or 100 μM) significantly improved the rice growth by preventing ROS MDA accumulation and increasing the efficiency of PS-II (Liu et al., 2015a). The application of MT at lower concentrations (10 and 30 μm) appreciably improved root growth, shoot growth, and biomass production (Bajwa et al., 2014). The application of MT upregulated the cold-responsive genes (COR15a) and antioxidant genes (ZAT10 and ZAT12), which increases the cold tolerance (Bajwa et al., 2014). MT supply also reduced the cold-induced reduction in photosynthetic efficiency by increasing the antioxidant potential and redox homoeostasis, as shown in pea plants (Li et al., 2018). Foliar spray of MT (200 μM) helps in the alleviation of cold-induced growth suppression by improving stomatal conductance, photosynthetic efficiency, the quantum yield of PS-II, and reducing MDA accumulation by increasing CAT, POD, and SOD activities and increasing the expression of antioxidant genes including CmSOD, CmPOD, and CmCAT (Zhang et al., 2017d). The maize seedlings treated with MT (1 mM) under cold stress effectively mitigated the cold stress as shown by enhanced RWC, chlorophyll contents, activities of antioxidants, and lower MDA and H2O2 accumulation (Turk and Erdal, 2015). Moreover, MT application also induced a significant increase in uptake of nutrients like boron, calcium, copper, iron, potassium, phosphorus, sulphur, and zinc, which generated a considerable increase in maize growth under cold stress (Turk and Erdal, 2015). In conclusion, MT improved cold tolerance by improving photosynthetic activities, stomatal conductance, nutrient uptake, and reduced MDA and H2O2 through enhanced antioxidant activities and expression of antioxidant genes and has the potential to be used as a stress protectant under cold stress onset.
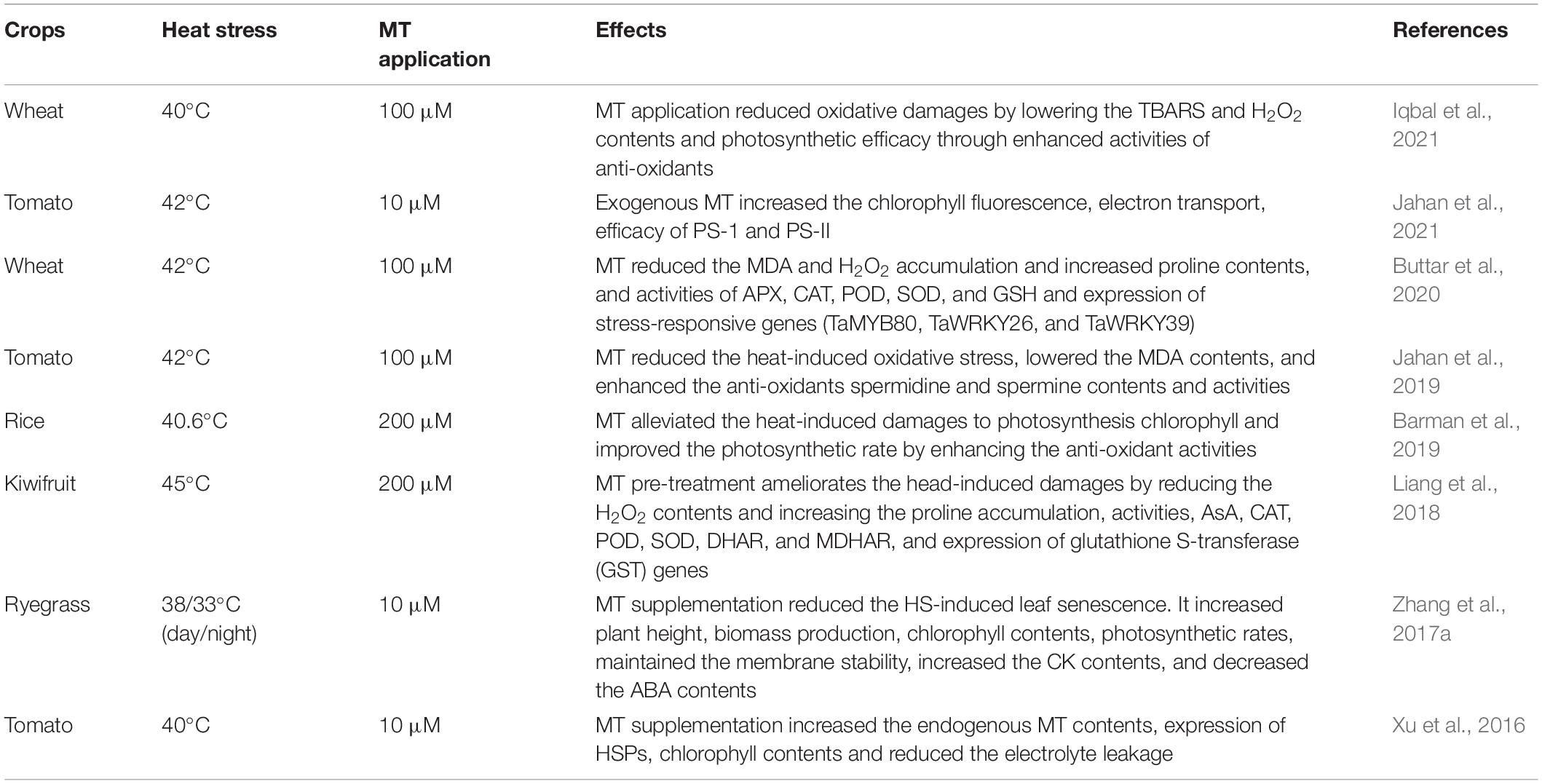
Melatonin Induces Heat Tolerance in Plants
Heat stress (HS) severely restricts plant growth, causes a severe reduction in crop yield, and is considered the most potent food security in this century (Hassan et al., 2021). Therefore, the use of plant growth regulators to protect plants against the adverse effects of this stress is imminent. MT application alleviated the negative impacts of HS (Table 4) and caused a significant increase in growth under HS in various crops (Table 5). MT supplementation maintains the photosynthesis under HS and favours a significant increase in growth (Ahammed et al., 2018). In kiwifruit, it was noticed that MT application effectively modulated the carbon fixation and improved the photosynthesis under HS by genes transcription (Liang et al., 2019). MT-treated seedlings showed increased tolerance to HS due to modulation of antioxidant activities, osmoregulatory system and methylglyoxal detoxification (Li et al., 2019).
Similarly, wheat MT supplementation suppressed the HS-induced damage by activating antioxidant machinery (Buttar et al., 2020). The supplementation of MT increases SOD activities APX, which counter the ROS and ensure the plants’ survival under HS conditions (Zhang et al., 2017b). Melatonin significantly attenuated HS-induced leaf senescence as indicated by reduced leaf yellowing and increased Fv/Fm ratio, reducing ROS production (Jahan et al., 2021). MT foliar spray also increased the plant growth regulators; for example, endogenous MT and GA contents in heat-stressed plants improved, significantly increasing HS tolerance (Jahan et al., 2021). MT application also reduced the ABA biosynthesis and gene expression, preventing the plants from ABA-induced oxidative damage (Jahan et al., 2021). A recent study indicated that MT supplementation in Lolium perenne induced substantial growth by reducing the ABA contents and increasing the endogenous MT and cytokinin contents (Zhang et al., 2017b). The MT application can reduce HS in tomato-induced protein misfolding, thus protecting the proteins from denaturation under HS (Xu et al., 2016). MT also increased the expression of heat shock proteins (HSPs) under HS conditions (Wang et al., 2015; Xu et al., 2016).
Calcium ions play an imperative role against HS tolerance in plants. MT application modulates Ca2+ influx through a non-selective Ca2+ permeable cation channel (Çelik and Naziroǧlu, 2012), stimulates Ca2+ transport across the cellular membranes, and ensures HS tolerance (Santofimia-Castaño et al., 2014). MT also increased the biosynthesis of total phenols and flavonoids, which conferred the HS tolerance (Meng et al., 2018). Therefore, in the light of the findings mentioned above, it is concluded that MT induced the HS by improving the photosynthetic efficiency, protecting the photosynthetic apparatus, reducing ROS and ABA accumulation, and increasing the Ca2+ influx antioxidant activities and expression of HSPs. It has enormous potential as a stress protectant used in the exogenous spray.
Melatonin Induces Ultraviolet Radiation Tolerance in Plants
Ultraviolet (UV) radiations are a severe threat to crop production, and their intensity is continuously increasing due to rapid ozone layer depletion. MT possesses an excellent potential to alleviate UV’s adverse impacts. It has been reported that MT application appreciably facilitated the UV-induced damages to DNA and UV radiations induced ROS in Nicotiana sylvestris and Malus hupehensis (Zhang et al., 2012; Ullah et al., 2019; Wei et al., 2019; Nazir et al., 2020). MT acts as a potent antioxidant to improve the UV resistance and regulates the expression of different UV signalling pathways, including the ubiquitin-degrading enzyme (COP1), transcription factors (HY5, HYH), and RUP1/2 (Yao et al., 2021). MT supply enhanced the expression of COP1, HY5, HYH, and RUP1/2 which play a significant role in UV-B signalling. Therefore, it regulates the plant antioxidant defence systems to protect them from the damaging impacts of UV-B stress (Yao et al., 2021).
In response to UV stress, endogenous MT accumulation in plant species (Alpine and Mediterranean species) provides UV tolerance (Simopoulos et al., 2005). Likewise, the roots of Glycyrrhiza uralensis exposed to UV-B showed a substantial increase in endogenous MT, reducing UV-induced damage to DNA (Zhang et al., 2012). MT application under UV radiation stress increased the endogenous MT and different phenolic compounds, including chlorogenic acid, phloridzin, and quercetin 3-galactoside, which confer UV tolerance (Wei et al., 2019). Though limited studies are conducted to determine the impact of MT against UV stress, more studies are direly needed to underpin the role of MT in mitigating the UV radiation stress in plants.
Melatonin Induces Waterlogging Tolerance in Plants
Waterlogging has been considered to affect crops’ survival, growth, and production in areas subjected to poor drainage and high rainfalls (Jackson and Colmer, 2005). Waterlogging affects plant growth and development, primarily creating anaerobic conditions and inducing ROS production. MT regulates plant growth and development under different stresses as an excellent antioxidant (Sun et al., 2021). For example, exogenous MT supplementation improved antioxidants activities and reduced the accumulation of MDA and H2O2 in tomato, pear, and alfalfa for water-logging tolerance (Zhang et al., 2019). Six alfalfa weeds grown under waterlogged conditions of 100 mM MT showed significant improvement in growth, physiological characteristics, photosynthetic efficiency, chlorophyll content, leaf polyamine content, and reduction in MDA and ROS accumulation due to increased antioxidant activity (Zhang et al., 2019). MT also maintains aerobic respiration protects the photosynthetic apparatus from oxidative damage and increases the expression of genes (MbT5H1, MbAANAT3, and MbASMT9) that subsequently improve tolerance to waterlogging stress (Zheng et al., 2017; Gu et al., 2021); for example, treated peach seedlings with MT (200 μM) and found improved chlorophyll concentration, stomatal movements, and reduced electrolyte leakage, lipid peroxidation, and MDA accumulation through increased POD and SOD activity under water deficit stress. MT supplementation enhanced the ADH activity and reserved the transition from aerobic to anaerobic respiration caused by waterlogging (Zheng et al., 2017).
Moreover, MT also controlled the anaerobic respiration enhancing the aerenchyma and suppressing the regulation of metabolic enzymes (Gu et al., 2021). MT improved the tolerance against waterlogging by reducing chlorosis and wilting (Zhang et al., 2013). Another study noted that foliar spray of (100 μ mol L–1) substantially enhances the efficiency of PS-II, photosynthetic rate and decreases the MDA accumulation through enhanced antioxidant activities in sorghum (Zhang et al., 2021b). The addition of MT improved waterlogging resistance by increasing the photosynthetic efficiency of photosynthetic pigments and reducing the accumulation of MDA and H2O2 through increased antioxidant activity. Thus it can be used as a stress protectant against waterlogging stress.
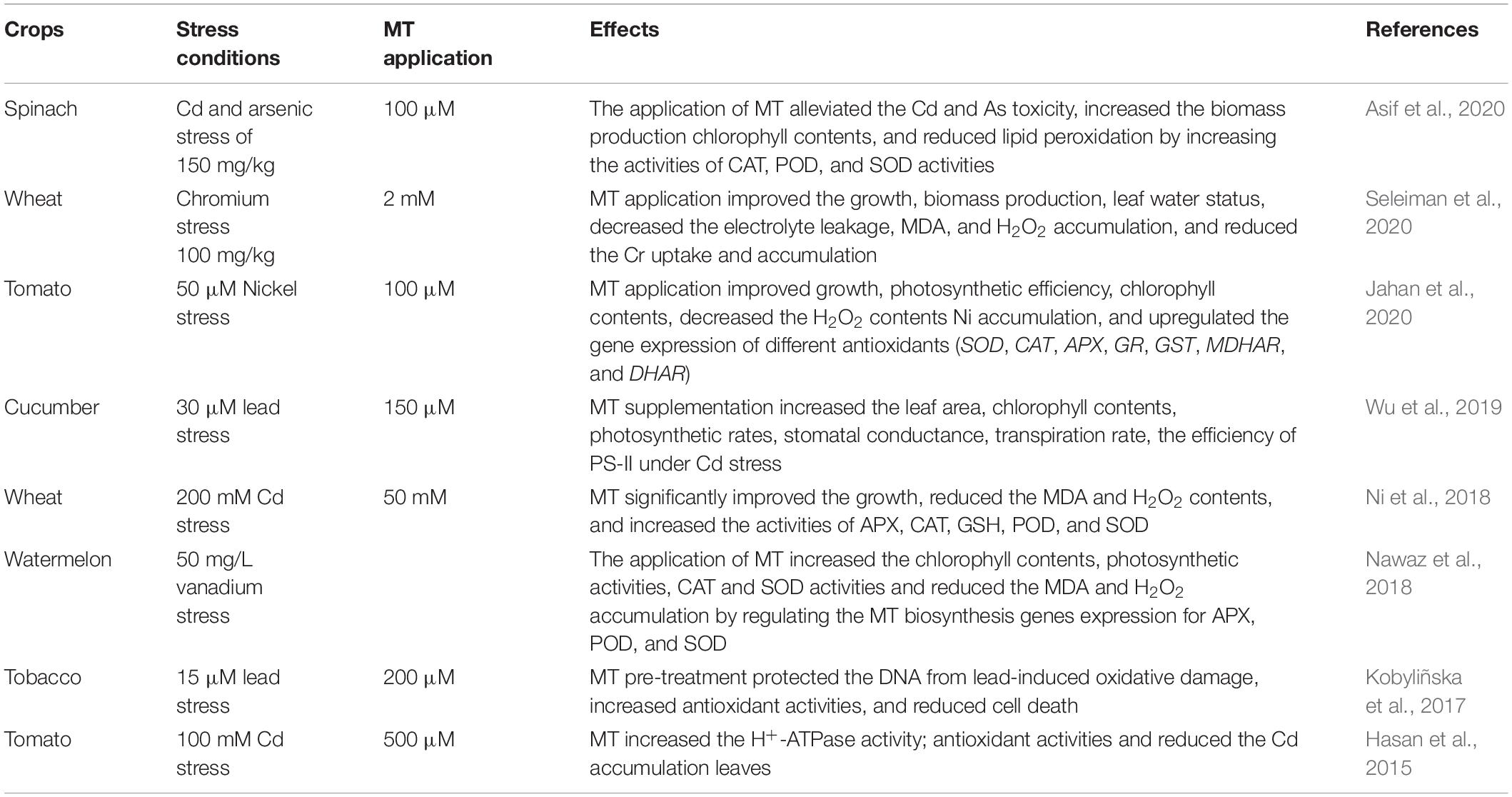
Melatonin Induces Heavy Metals Stress Tolerance in Plants
Heavy metals (HMs) are a severe threat to global food production. Their concentration in agricultural soil is rapidly increasing due to anthropogenic activities (Hassan et al., 2019; Chattha et al., 2021; Imran et al., 2021a; Rehman et al., 2022). The role of MT to regulate plants grown under different HMs is well explored (Hasan et al., 2015); nonetheless, MT-mediated growth regulation largely depends on MT application rate, heavy metal concentration, and plant species (Table 5). For instance, soybean grown under Al-stress (50 μM) showed a significant increase in growth and antioxidant activities with 1 μM MT compared to the 100 and 200 μM MT (Zhang et al., 2017b). Similarly, red cabbage plants grown under Cu stress showed a significant improvement in growth with 10 μM MT supplementation compared to 100 μM (Posmyk et al., 2008). Conversely, tomato plants grown under Cd stress (100 μM) showed a significant increase in plant growth with MT application of 100 μM as compared to lower rates (Hasan et al., 2015). MT application also reverses the lead-induced cell death and morphological deformation and membrane leakage in stressed plants compared to control (Li et al., 2016a; Kobyliñska et al., 2017).
Melatonin restricts the HM translocation and increases genes expression of MT, thus increasing the endogenous concentration to combat the HM stress (Hasan et al., 2015). Moreover, MT directly scavenges the ROS by improving the antioxidant activities, conferring stress tolerance (Moustafa-Farag et al., 2020). For instance, MT spray enhanced the tolerance against ZnO by increasing the ATPase, RuBisCO, and antioxidant activities in wheat (Zuo et al., 2017). Similarly, MT enhanced the plant tolerance to HMs by modulating the antioxidant enzyme activities (Zhang et al., 2017b). Interestingly, HM induced the upregulation of MT biosynthetic enzymes genes from E. pisciphila tryptophan decarboxylase (EpTDC1 and EpSNAT1) and enhanced the MT biosynthesis improving the tolerance against the HMs in E. coli and A. thaliana (Yu et al., 2021). Strawberry seedlings grown under Cd showed a significant reduction in growth, biomass production, chlorophyll contents and activities of antioxidant enzymes. However, MT application (200 μmol) showed a substantial increase in growth biomass production through enhanced actions of APX, CAT, POD, and SOD, and reduced MDA accumulation (Wu et al., 2021a). MT application also improved the expression of MtPT4 and AM colonisation in Medicago truncatula plants, which improved the overall antioxidant activities and resultantly increased the growth under HM stress (Zhang et al., 2020b). In conclusion, MT alleviated the HMs induced deleterious effects by improving the photosynthetic activity, antioxidant activities, reduced HM uptake and MDA and ROS accumulation. It can be used as a potential stress protectant for managing HMs stress.
Melatonin Induce Elevated Ozone Tolerance in Plants
Ozone (O3) is a highly oxidising pollutant, and increasing O3 concentration severely affects plant growth as well as development (Serengil et al., 2011). MT plays an imperative role in plats responses to diverse abiotic stresses; nonetheless, its mechanism in alleviating the O3 is poorly understood. Mt crosstalk with various plant growth regulators helps stress alleviation; for example, grape leaves grown under O3 were treated with MT modulated ethylene biosynthesis and signalling. O3 induced a significant increase in genes expression linked with ethylene biosynthesis, while MT supplementation significantly inhibited the ethylene genes expression (Liu et al., 2021). Further MT application also improved the photosynthetic performance and antioxidant activities under O3. The over-expression of MT synthesis gene VvASMT1 (acetylserotonin methyltransferase 1) also alleviated the O3 stress and reduced the ethylene biosynthesis (Liu et al., 2021). The effect of diverse MT concentrations (0, 0.1, 0.5, 2.5, and 12.5 μM) was studied on apple plants grown under O3 stress. The exposure of apple plants to O3 induced a significant increase in MDA accumulation. However, MT application reduced the MDA accumulation by increasing the antioxidant activities (CAT, POD, and SOD). Further, MT also improved the accumulation of soluble proteins and non-enzymatic antioxidant activities and conferred the O3 tolerance (Qiu et al., 2019). Therefore, MT induced the O3 tolerance by favouring the antioxidant activities and reducing the MDA and ethylene accumulation. However, a wide range of studies is direly needed to underpin the mechanism linked with MT-induced O3 stress in plants.
Melatonin Induces Nutrient Deficiency Tolerance in Plants
The extensive agriculture practices continuously increase the nutrient deficiency problem, and it is considered to aggravate in the coming time. MT possesses an excellent potential to reduce the effects of nutrient deficiency. For instance, MT supplementation significantly increases the iron (Fe) concentration in roots and shoots and alleviates Fe deficiency (Zhou et al., 2016). In another study, MT supplementation enhanced the tolerance of wheat plants to potassium stress (K). MT upregulated the K transporter 1 (TaHAK1) gene expression, improved K absorption, and, therefore, alleviated K deficiency (Li et al., 2021a,b).
Similarly, MT supply reduced ROS production in sulphur (S) deprived plants and mitigated the S-induced deficiency by protecting the macromolecules and ultra-structures (Hasan et al., 2018). MT also promoted the S uptake and assimilation by regulating the genes expression involved in S metabolism and transportation (Hasan et al., 2018). Another investigation indicated the possible mechanism of MT application mediated improvement in growth and physiological parameters by a reduction in the electrolyte leakage, ROS production, and lipid peroxidation through increasing the activities and transcription of antioxidant enzyme genes and improved accumulation of phenols and flavonoids under Fe stress (Ahammed et al., 2020). Here, MT also increased the leaf Fe contents and increased the transcription levels of FRO2 and IRT1, which improved the Fe uptake under Fe deficient conditions (Ahammed et al., 2020). However, other element availability after MT application needs to be investigated. Therefore, nutrient availability can be improved by applying MT through various mechanisms and could be used as a stress protectant under nutrient deficiency.
Melatonin Induces Soil pH Stress Tolerance in Plants
Soil pH plays a critical role in plants growth, and any fluctuation in soil pH induces stress conditions for plants. MT could help plants withstand the soil fluctuations; for example, MT application improved the growth and yield of tomatoes under alkaline and acid pH stress (Liu et al., 2015a). Soil pH fluctuations can increase the endogenous MT and are reported to be increased by 12 times under pH stress in untreated plants (Arnao and Hernández-Ruiz, 2013). MT supplementation (0.1 and 1 μM) in soybean mitigated the Al-induced toxicity in acid soils through an enhanced accumulation of osmolytes and antioxidant activities (Zhang et al., 2017b). MT induced pH stress tolerance by activating MT receptors (MTNR1A and MTNR1B) and improving antioxidant defence (Arnao and Hernández-Ruiz, 2006).
Besides this, MT under alkaline stress also increased the accumulation of polyamines which conferred stress tolerance (Gong et al., 2017). MT also reduced oxidative stress, and membrane leakage in alkaline stressed conditions by scavenging the ROS (Hardeland, 2013; Gong et al., 2017). The increase in antioxidant activities preserves chloroplast grana, prevents chlorophyll degradation, and improves photosynthesis under alkaline stress (Debnath et al., 2018). MT’s protective role under sodic alkaline stress is also linked with NO signalling. Under alkaline stress, MT triggers NO accumulation by downregulation of expression of S-nitrosoglutathione reductase (Corpas and Barroso, 2015; Kaur et al., 2015; Wen et al., 2016). These findings suggested that NO is a downstream signal in plants’ tolerance to alkaline stress (Liu et al., 2015b). Similarly, MT application significantly improved the expression of acetyltransferase NSI-like genes and lowered the production of H2O2 under acidic soils (Moustafa-Farag et al., 2020). Little research is done on the ameliorative effect of exogenous MT in the context of plants grown under pH stress. Nonetheless, more studies are required to explore the mechanistic pathways of MT in inducing pH stress tolerance.
Mechanism of Melatonin Induced Stress Tolerance
Melatonin Mediated Upgrading of the Antioxidant Defence System Under Stress Conditions
Plants have different physiological and biochemical adaptations to cope with various abiotic stresses. ROS are produced in plants under other stress conditions (Hassan et al., 2019), which induce oxidative stress and cause damage to macromolecules and biological structures (Imran et al., 2021b; Iqbal et al., 2021). Thus, plants activate antioxidant defence systems to counter the deleterious impacts of abiotic stresses (Iqbal et al., 2021). MT is an excellent molecule that improves plant growth by triggering the antioxidant enzymes under stressed conditions (Table 6).
Drought and salt stress-induced ROS production was regulated by different plant growth regulators. These ROS act as plants’ internal defence systems to trigger the scavenging of ROS and reduce oxidative stress by increasing the activities of antioxidant enzymes (Liang et al., 2019).
Melatonin is a multi-functional antioxidant, and it substantially scavenges the ROS and improves stress tolerance (Arnao and Hernández-Ruiz, 2014). MT stimulates the enzymatic antioxidative defence system and protects against stress conditions (Ye et al., 2016). It also promotes the ABA degradation enzymes and scavenges the ROS by increasing the activities of APX, CAT, DHAR, GPX, GR, and SOD (Kabiri et al., 2018; Campos et al., 2019; Li et al., 2021a). Under salt stress, MT application significantly increased the photosynthetic rate and reduced oxidative stress, as discussed earlier (Zhang et al., 2017c). The MT-induced protection under salt stress is linked with improved light absorption, electron transport, the efficiency of PS-II, and reduction in oxidative stress induced by increase in activities of antioxidants (AsA, CAT, GSH, POD, and SOD) in melon crop (Zhang et al., 2017c).
Similarly, the application of MT under HS significantly increased proline accumulation. It reduced the MDA and H2O2 accumulation through the improved activity of CAT, POD, and SOD and the expression of genes linked with these enzymes (Jahan et al., 2019; Buttar et al., 2020). Similarly, MT application substantially improved the working of APX, CAT, POD, SOD, and GSH and, therefore, decreased ROS accumulation under HM and HS stress (Byeon et al., 2015; Hasan et al., 2015). MT also reduces the excessive ROS production induced by HM in rice, wheat, and watermelon by activating the SOD (Lee and Back, 2017; Nawaz et al., 2018). Similarly, other authors also reported that MT significantly improved the activities of APX, CAT, POD, SOD, and other antioxidant activities under waterlogging, cold, and ozone stress (Zhang et al., 2017c; Qiu et al., 2019; Gu et al., 2021). Thus, all these findings endorsed that MT supplementation effectively up-graded the antioxidant defence system to alleviate the effects of different abiotic stresses.
Interaction and Crosstalk of Melatonin With Other Hormones
Hormones play a critical role in plant growth and MT is widely involved in the metabolism of a range of hormones, including IAA, ABA, gibberellic acid (GA), cytokinin (CK), and ethylene (Arnao and Hernández-Ruiz, 2018). MT has similar chemical properties to IAA, and both these two hormones use tryptophan in their biosynthesis pathways as substrate (Wang et al., 2016b). MT acts as a growth regulator and it shows IAA-like activities (Pelagio-Flores et al., 2012). MT improves root development and vegetative growth in different crops, including Arabidopsis, barley, maize, rice, and tomato (Arnao and Hernández-Ruiz, 2018). MT regulates the formation of a root by IAA independent pathway in Arabidopsis (Pelagio-Flores et al., 2012).
Conversely, crosstalk between IAA and MT was also reported; for instance, an increase in endogenous IAA was reported in Brassica with external application of MT (Chen et al., 2009; Arnao and Hernández-Ruiz, 2018). Further application of IAA significantly improved the endogenous MT (Wang et al., 2016a). MT mediates mediate the biosynthesis of ABA, and it regulates the ABA metabolism, thus reducing the ABA accumulation under stress conditions. For instance, in apples, MT downregulated the MdNCED3, an essential ABA biosynthesis gene, consequently decreasing the ABA accumulation (Li et al., 2015). Likewise, MT downregulated the ABA under HS in perennial ryegrass and reduced the ABA contents (Zhang et al., 2017b). Similarly, MT also downregulated ABA signalling and improves stress tolerance (Fu et al., 2017). Interestingly MT also increased the expression of cold-responsive genes and reduced the ABA accumulation, therefore considerably increasing the cold tolerance (Fu et al., 2017).
Exogenous MT also ameliorated the impacts of salinity stress by regulating ABA biosynthesis and catabolism. In salty conditions, MT reduced the transcript levels of ABA synthesis-related genes (CsNCED1 and CsNCED2), which resulted in a reduction in ABA accumulation under stress conditions. Moreover, MT application increased the expression of genes (GA20ox and GA3ox) involved in GA, enhancing the GA accumulation under stress conditions (Zhang et al., 2014). In another study Zhang et al. (2017b) noted that MT induced CK activation and inhibition of ABA biosynthesis significantly inhibited the leaf senescence in ryegrass plants grown under HS. All this evidence suggests that MT can be a potential signalling molecule that triggers signalling transduction and improves plant growth and development under stress conditions.
Success Stories: Engineered Melatonin Biosynthesis to Enhance Abiotic Stress Tolerance
Melatonin is a natural hormone in plants and protects them against stress conditions. Thus, increasing the endogenous MT is crucial to combat the effects of abiotic stresses (Table 7). The transgenic strategy is an effective strategy to improve the endogenous MT level. Nonetheless, over-expression of MT responsive genes under various abiotic stresses is studied in few crops. Many studies reported that MT levels significantly increased under stress conditions (Xing et al., 2021; Qari et al., 2022).
Enzymes like AANAT and HIOMT are essential for the biosynthesis of MT, and the over-expression of these enzymes in tomatoes under drought stress increased the endogenous MT level (Wang et al., 2014). Higher MT levels improve the plant’s growth and tolerance to change, and resistance to drought and pesticides (Campos et al., 2019; Yan et al., 2019). For instance, in Arabidopsis, higher expression of FIT1, FRO2, and IRT1 genes after MT application restored the Fe deficiency (Zhou et al., 2016). In another study, the over-expression ASMT gene increased the endogenous MT level and provided cellular protection by increasing the expression of HSPs and triggering the HS tolerance (Xu et al., 2016). Moreover, in tomato over-expression of the HsfA1a gene, the COMT1 transcription factor was upregulated, which increased the MT biosynthesis and resistance against the Cd stress (Choi et al., 2017). Likewise, in rice crops, overexpression of chloroplast caffeic acid O-methyltransferase (COMT) increased the MT contents and improved the seedling growth under stress conditions (Choi et al., 2017).
Over-expression of MT biosynthesis pathway genes such as tryptophan decarboxylase-interacting protein 2 (MeTDC2), N-acetylserotonin O-methyltransferase-interacting protein 2 (MeASMT2), and N-acetylserotonin O-methyltransferase 3 (MeASMT3) significantly increased endogenous MT and improved stress tolerance (Wei et al., 2018). Ma et al. (2017) used the bacterium Pseudomonas fluorescens RG11 strain to increase the endogenous MT in grapes, which increased the salt tolerance in grapes and reduced the cellular damage by decreasing the ROS production (Ma et al., 2017). Moreover, a bacterial strain (Bacillus amyloliquefaciens) from grapevine roots significantly increased the endogenous MT production and facilitated the adverse impacts of drought by H2O2 scavenging (Jiao et al., 2016). Thus, all these findings suggested that a transgenic increase in endogenous MT could be a promising approach to improving stress tolerance.
Conclusion and Future Perspectives
Melatonin has excellent properties for improving tolerance to abiotic stress. Melatonin alters different biochemical, molecular and physiological processes to induce stress tolerance in plants. MT protected the photosynthetic apparatus from oxidative damage caused by stress and increased the efficiency of photosynthesis. In addition, melatonin also stimulates cell signalling that controls diverse physiological and molecular aspects to confer stress tolerance in plants. Application of MT under various stresses reduced ROS production by activating antioxidant enzymes, accumulating compatible solutes, and increasing the expression of stress-responsive genes. However, many questions need to be answered by conducting a wide range of studies.
Future studies need to study the anatomical changes in leaves and roots of MT plants under different stresses. Similarly, researchers need to investigate the effect of MT application on fruit set, pollen viability, and abscission. The precise role of MT in signalling pathways under different stresses needs to be investigated. Other studies have reported the interaction of MT with different osmolytes and hormones. However, further studies are required to support the exchanges and interactions of MT with other osmolytes and hormones in individuals and combinations of various stresses. In addition, investigating the role of MT under different stresses would also unravel the potential of protecting spray in other crops. Recent improvements in plant genomics, transcriptomic, proteomic, and metabolomic will also to better understand hormone networks and their interaction and crosstalk under different stresses.
Regulation of gene expression and interactions with different hormones is also a crucial factor in MT that significantly increases stress tolerance. However, endogenous MT is not sufficient to cope with challenging conditions. Under such conditions, exogenous MT is resorted to increase endogenous MT to maintain average growth under stressful conditions. However, the cellular signalling pathways induced by MT require more profound studies in different crops under signal and combination of various stresses. ROS are mainly produced in plant chloroplast and mitochondria. Because MT functions as a signalling molecule, it would be interesting to study inter-organelle MT signalling under different stresses. In addition, the molecular mechanism of MT to increase the expression of antioxidant and stress-responsive genes should be investigated in more detail. Engineering MT signalling will open new perspectives on current knowledge to understand MT-induced stress tolerance. The effects of MT under nutrient deficiency, UV irradiation, and ozone stress are not fully explored. Therefore, a deeper understanding of MT under nutrient deficiency, UV, ozone, and pH stress needs further exploration. More intensive transcriptomic and proteomic studies would reveal how MT are affected by nutrient deficiency, UV radiation, ozone, and pH stress. Finally, the patterns of MT application in plant responses to individual and combined stresses under field conditions should also be investigated.
Author Contributions
MUH and GH conceived the idea. MUH prepared the original draft of manuscript. AM, MIA, RM, MA, HA, GH, MS, MB, SP, AES, and SHQ reviewed and edited the final version. GH supervised the study and provided funding. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Key Research and Development Program of China (2016YFD0300208), National Natural Science Foundation of China (41661070), and Key Disciplines (construction) of Ecology in the 13th Five-Year Plan of Jiangxi Agricultural University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to National Key Research and Development Program of China (2016YFD0300208), National Natural Science Foundation of China (41661070), and Key Disciplines (construction) of Ecology in the 13th Five-Year Plan of Jiangxi Agricultural University.
References
Abbasi, H., Jamil, M., Haq, A., Ali, S., Ahmad, R., Malik, Z., et al. (2016). Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: a review. Zemdirbyste Agricul. 103, 229–238.
Ahammed, G. J., Wu, M., Wang, Y., Yan, Y., Mao, Q., Ren, J., et al. (2020). Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 265:109205. doi: 10.1016/j.scienta.2020.109205
Ahammed, G. J., Xu, W., Liu, A., and Chen, S. (2018). COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front. Plant Sci. 9:998. doi: 10.3389/fpls.2018.00998
Ahmad, S., Kamran, M., Ding, R., Meng, X., Wang, H., Ahmad, I., et al. (2019). Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 7:e7793. doi: 10.7717/peerj.7793
Ahmad, S., Muhammad, I., Wang, G. Y., Zeeshan, M., Yang, L., Ali, I., et al. (2021). Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 21:368. doi: 10.1186/s12870-021-03160-w
Alharbi, B. M., Elhakem, A. H., Alnusairi, G. S., Soliman, M. H., Hakeem, K. R., Hasan, M. M., et al. (2021). Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 67, 208–220. doi: 10.17221/659/2020-pse
Ali, S., Eum, H.-I., Cho, J., Dan, L., Khan, F., Dairaku, K., et al. (2019). Assessment of climate extremes in future projections downscaled by multiple statistical downscaling methods over Pakistan. Atmosph. Res. 222, 114–133.
Antoniou, C., Chatzimichail, G., Xenofontos, R., Pavlou, J. J., Panagiotou, E., Christou, A., et al. (2017). Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 62:e12401. doi: 10.1111/jpi.12401
Arif, N., Yadav, V., Singh, S., Kushwaha, B. K., Singh, S., Tripathi, D. K., et al. (2016). “Assessment of antioxidant potential of plants in response to heavy metals,” in Plant Esponses to Xenobiotics, eds A. Singh, S. M. Prasad, and R. P. Singh (Berlin: Springer), 97–125. doi: 10.1007/978-981-10-2860-1_5
Arnao, M. B., and Hernández-Ruiz, J. (2006). The physiological function of melatonin in plants. Plant Signal. Behav. 1, 89–95. doi: 10.4161/psb.1.3.2640
Arnao, M. B., and Hernández-Ruiz, J. (2014). Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19, 789–797. doi: 10.1016/j.tplants.2014.07.006
Arnao, M. B., and Hernández-Ruiz, J. (2018). Melatonin and its relationship to plant hormones. Ann. Bot. 121, 195–207. doi: 10.1093/aob/mcx114
Arnao, M. B., and Hernández-Ruiz, J. (2013). Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 55, 149–155. doi: 10.1111/jpi.12055
Asif, M., Pervez, A., Irshad, U., Mehmood, Q., and Ahmad, R. (2020). Melatonin and plant growth-promoting rhizobacteria alleviate the cadmium and arsenic stresses and increase the growth of Spinacia oleracea L. Plant Soil Environ. 66, 234–241. doi: 10.17221/135/2020-pse
Back, K., Tan, D. X., and Reiter, R. J. (2016). Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 61, 426–437. doi: 10.1111/jpi.12364
Bajwa, V. S., Shukla, M. R., Sherif, S. M., Murch, S. J., and Saxena, P. K. (2014). Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 56, 238–245. doi: 10.1111/jpi.12115
Barand, A., Nasibi, F., Manouchehri Kalantari, K., and Moradi, M. (2020). The effects of foliar application of melatonin on some physiological and biochemical characteristics and expression of fatty acid desaturase gene in pistachio seedlings (Pistacia vera L.) under freezing stress. J. Plant Interact. 15, 257–265. doi: 10.1080/17429145.2020.1781271
Barman, D., Ghimire, O., Chinnusamy, V., Kumar, R., and Arora, A. (2019). Amelioration of heat stress during reproductive stage in rice by melatonin. Ind. J. Agric. Sci. 89, 1151–1156.
Bastam, N., Baninasab, B., and Ghobadi, C. (2013). Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Reg. 69, 275–284.
Batool, M., El-Badri, A. M., Hassan, M. U., Haiyun, Y., Chunyun, W., Zhenkun, Y., et al. (2022a). Drought stress in Brassica napus: effects, tolerance mechanisms, and management strategies. J. Plant Growth Reg. 1–25.
Batool, M., El-Badri, A. M., Wang, Z., Mohamed, I. A., Yang, H., Ai, X., et al. (2022b). Rapeseed morpho-physio-biochemical responses to drought stress induced by PEG-6000. Agronomy 12:579.
Beebe, S., Ramirez, J., Jarvis, A., Rao, I. M., Mosquera, G., Bueno, J. M., et al. (2011). “Genetic improvement of common beans and the challenges of climate change,” in Crop Adaptation to Climate Change, eds S. S. Yadav, R. Redden, J. L. Hatfield, H. Lotze-Campen, and A. Hall (Oxford, GB: John Wiley & Sons, Inc), 356–369. doi: 10.1002/9780470960929.ch25
Burg, M. B., and Ferraris, J. D. (2008). Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 283, 7309–7313. doi: 10.1074/jbc.R700042200
Buttar, Z. A., Wu, S. N., Arnao, M. B., Wang, C., Ullah, I., and Wang, C. (2020). Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 9:809. doi: 10.3390/plants9070809
Byeon, Y., and Back, K. (2015). Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 58, 343–351. doi: 10.1111/jpi.12220
Byeon, Y., Lee, H. Y., Hwang, O. J., Lee, H. J., Lee, K., and Back, K. (2015). Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 58, 470–478. doi: 10.1111/jpi.12232
Cai, S. Y., Zhang, Y., Xu, Y. P., Qi, Z. Y., Li, M. Q., Ahammed, G. J., et al. (2017). HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 62:e12387. doi: 10.1111/jpi.12387
Campos, C. N., Ávila, R. G., De Souza, K. R. D., Azevedo, L. M., and Alves, J. D. (2019). Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 211, 37–47.
Çelik, Ö, and Naziroǧlu, M. (2012). Melatonin modulates apoptosis and TRPM2 channels in transfected cells activated by oxidative stress. Physiol. Behav. 107, 458–465. doi: 10.1016/j.physbeh.2012.09.013
Chattha, M. U., Arif, W., Khan, I., Soufan, W., Bilal Chattha, M., Hassan, M. U., et al. (2021). mitigation of cadmium induced oxidative stress by using organic amendments to improve the growth and yield of mash beans [Vigna mungo (L.)]. Agronomy 11:2152.
Chen, L., Lu, B., Liu, L., Duan, W., Jiang, D., Li, J., et al. (2021). Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 162, 506–516. doi: 10.1016/j.plaphy.2021.03.029
Chen, Q., Qi, W.-B., Reiter, R. J., Wei, W., and Wang, B.-M. (2009). Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 166, 324–328. doi: 10.1016/j.jplph.2008.06.002
Chen, Y. E., Mao, J. J., Sun, L. Q., Huang, B., Ding, C. B., Gu, Y., et al. (2018). Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 164, 349–363. doi: 10.1111/ppl.12737
Cherono, S., Ntini, C., Wassie, M., Mollah, M. D., Belal, M. A., Ogutu, C., et al. (2021). Exogenous application of melatonin improves drought tolerance in coffee by regulating photosynthetic efficiency and oxidative damage. J. Am. Soc. Hortic. Sci. 146, 24–32. doi: 10.21273/jashs04964-20
Choi, G. H., Lee, H. Y., and Back, K. (2017). Chloroplast overexpression of rice caffeic acid O-methyltransferase increases melatonin production in chloroplasts via the 5-methoxytryptamine pathway in transgenic rice plants. J. Pineal Res. 63:e12412. doi: 10.1111/jpi.12412
Corpas, F. J., and Barroso, J. B. (2015). Functions of nitric oxide (NO) in roots during development and under adverse stress conditions. Plants 4, 240–252. doi: 10.3390/plants4020240
Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C., and Xi, Y. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 118, 138–149. doi: 10.1016/j.plaphy.2017.06.014
Debnath, B., Hussain, M., Irshad, M., Mitra, S., Li, M., Liu, S., et al. (2018). Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 23:388. doi: 10.3390/molecules23020388
Debnath, B., Islam, W., Li, M., Sun, Y., Lu, X., Mitra, S., et al. (2019). Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 20:1040. doi: 10.3390/ijms20051040
Ding, F., Liu, B., and Zhang, S. (2017). Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 219, 264–271.
Dustgeer, Z., Seleiman, M. F., Imran, K., Chattha, M., Alhammad, B. A., Jalal, R. S., et al. (2021). Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj Napoca 49, 12248–12248. doi: 10.15835/nbha49112248
Fu, J., Wu, Y., Miao, Y., Xu, Y., Zhao, E., Wang, J., et al. (2017). Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci. Rep. 7, 1–11. doi: 10.1038/srep39865
Galano, A., Tan, D. X., and Reiter, R. J. (2013). On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 54, 245–257. doi: 10.1111/jpi.12010
Gao, W., Feng, Z., Bai, Q., He, J., and Wang, Y. (2019). Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 20:1176. doi: 10.3390/ijms20051176
Gao, W., Zhang, Y., Feng, Z., Bai, Q., He, J., and Wang, Y. (2018). Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 23:1580. doi: 10.3390/molecules23071580
Gong, X., Shi, S., Dou, F., Song, Y., and Ma, F. (2017). Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by regulating the biosynthesis of polyamines. Molecules 22:1542.
Gu, Q., Chen, Z., Yu, X., Cui, W., Pan, J., Zhao, G., et al. (2017). Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 261, 28–37. doi: 10.1016/j.plantsci.2017.05.001
Gu, X., Xue, L., Lu, L., Xiao, J., Song, G., Xie, M., et al. (2021). Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Reg. 40, 2178–2190. doi: 10.1007/s00344-020-10263-5
Han, Q.-H., Huang, B., Ding, C.-B., Zhang, Z.-W., Chen, Y.-E., Hu, C., et al. (2017). Effects of melatonin on antioxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 8:785. doi: 10.3389/fpls.2017.00785
Hardeland, R. (2013). Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 55, 325–356. doi: 10.1111/jpi.12090
Hasan, M., Ahammed, G. J., Yin, L., Shi, K., Xia, X., Zhou, Y., et al. (2015). Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 6:601. doi: 10.3389/fpls.2015.00601
Hasan, M., Liu, C.-X., Pan, Y.-T., Ahammed, G. J., Qi, Z.-Y., and Zhou, J. (2018). Melatonin alleviates low-sulfur stress by promoting sulfur homeostasis in tomato plants. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-28561-0
Hassan, M. U., Aamer, M., Chattha, M. U., Ullah, M. A., Sulaman, S., Nawaz, M., et al. (2017). The role of potassium in plants under drought stress: mini review. J. Basic Appl. Sci. 13, 268–271. doi: 10.6000/1927-5129.2017.13.44
Hassan, M. U., Aamer, M., Umer Chattha, M., Haiying, T., Shahzad, B., Barbanti, L., et al. (2020). The critical role of zinc in plants facing the drought stress. Agriculture 10:396.
Hassan, M. U., Chattha, M. U., Khan, I., Chattha, M. B., Aamer, M., Nawaz, M., et al. (2019). Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities—a review. Environ. Sci. Pollut. Res. 26, 12673–12688. doi: 10.1007/s11356-019-04892-x
Hassan, M. U., Chattha, M. U., Khan, I., Chattha, M. B., Barbanti, L., Aamer, M., et al. (2021). Heat stress in cultivated plants: nature, impact, mechanisms, and mitigation strategies—a review. Plant Biosyst. 155, 211–234. doi: 10.1080/11263504.2020.1727987
Hu, Z., Fan, J., Xie, Y., Amombo, E., Liu, A., Gitau, M. M., et al. (2016). Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 100, 94–104. doi: 10.1016/j.plaphy.2016.01.008
Huang, Y.-H., Liu, S.-J., Yuan, S., Guan, C., Tian, D.-Y., Cui, X., et al. (2017). Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci. Rep. 7, 1–13. doi: 10.1038/s41598-017-12566-2
Imran, K., Zafar, H., Chattha, M. U., Mahmood, A., Maqbool, R., Athar, F., et al. (2022). Seed priming with different agents mitigate alkalinity induced oxidative damage and improves maize growth. Not. Bot. Horti Agrobot. Cluj Napoca 50, 12615–12615. doi: 10.15835/nbha50112615
Imran, K. H. A. N., Seleiman, M. F., Chattha, M. U., Jalal, R. S., Mahmood, F., Hassan, F. A., et al. (2021a). Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti Agrobot. Cluj Napoca 49, 12303–12303.
Imran, M., Aaqil Khan, M., Shahzad, R., Bilal, S., Khan, M., Yun, B.-W., et al. (2021b). Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 26:5116. doi: 10.3390/molecules26175116
Iqbal, N., Fatma, M., Gautam, H., Umar, S., Sofo, A., D’ippolito, I., et al. (2021). The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 10:1778. doi: 10.3390/plants10091778
Jackson, M., and Colmer, T. (2005). Response and adaptation by plants to flooding stress. Ann. Bot. 96, 501–505. doi: 10.1093/aob/mci205
Jahan, M. S., Guo, S., Baloch, A. R., Sun, J., Shu, S., Wang, Y., et al. (2020). Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ Saf. 197:110593. doi: 10.1016/j.ecoenv.2020.110593
Jahan, M. S., Guo, S., Sun, J., Shu, S., Wang, Y., Abou El-Yazied, A., et al. (2021). Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 167, 309–320. doi: 10.1016/j.plaphy.2021.08.002
Jahan, M. S., Shu, S., Wang, Y., Chen, Z., He, M., Tao, M., et al. (2019). Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 19:414. doi: 10.1186/s12870-019-1992-7
Jeandroz, S., and Lamotte, O. (2017). Plant responses to biotic and abiotic stresses: lessons from cell signaling. Front. Plant Sci. 8:1772. doi: 10.3389/fpls.2017.01772
Jiang, C., Cui, Q., Feng, K., Xu, D., Li, C., and Zheng, Q. (2016). Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 38, 1–9.
Jiang, D., Lu, B., Liu, L., Duan, W., Chen, L., Li, J., et al. (2020). Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. PeerJ 8:e10486. doi: 10.7717/peerj.10486
Jiao, J., Ma, Y., Chen, S., Liu, C., Song, Y., Qin, Y., et al. (2016). Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front. Plant Sci. 7:1387. doi: 10.3389/fpls.2016.01387
Kabiri, R., Hatami, A., Oloumi, H., Naghizadeh, M., Nasibi, F., and Tahmasebi, Z. (2018). Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 30, 155. doi: 10.2478/fhort-2018-0016
Kang, K., Kim, Y. S., Park, S., and Back, K. (2009a). Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant physiol. 150, 1380–1393. doi: 10.1104/pp.109.138552
Kang, K., Park, S., Kim, Y. S., Lee, S., and Back, K. (2009b). Biosynthesis and biotechnological production of serotonin derivatives. Appl. Microbiol. Biotech. 83, 27–34. doi: 10.1007/s00253-009-1956-1
Kang, K., Lee, K., Park, S., Kim, Y. S., and Back, K. (2010). Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J. Pineal Res. 49, 176–182. doi: 10.1111/j.1600-079X.2010.00783.x
Karaca, P., and Cekic, F. Ö (2019). Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 25, 601–609.
Kaur, H., Mukherjee, S., Baluska, F., and Bhatla, S. C. (2015). Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 10:e1049788. doi: 10.1080/15592324.2015.1049788
Ke, Q., Ye, J., Wang, B., Ren, J., Yin, L., Deng, X., et al. (2018). Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 9:914. doi: 10.3389/fpls.2018.00914
Khan, A., Numan, M., Khan, A. L., Lee, I.-J., Imran, M., Asaf, S., et al. (2020a). Melatonin: awakening the defense mechanisms during plant oxidative stress. Plants 9:407. doi: 10.3390/plants9040407
Khan, M. N., Khan, Z., Luo, T., Liu, J., Rizwan, M., Zhang, J., et al. (2020b). Seed priming with gibberellic acid and melatonin in rapeseed: consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crops Prod. 156:112850.
Kobyliñska, A., Reiter, R. J., and Posmyk, M. M. (2017). Melatonin protects cultured tobacco cells against lead-induced cell death via inhibition of cytochrome c translocation. Front. Plant Sci. 8:1560. doi: 10.3389/fpls.2017.01560
Lee, H. Y., Byeon, Y., and Back, K. (2014). Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 57, 262–268. doi: 10.1111/jpi.12165
Lee, K., and Back, K. (2017). Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J. Pineal Res. 62:e12392. doi: 10.1111/jpi.12392
Lee, K., Zawadzka, A., Czarnocki, Z., Reiter, R. J., and Back, K. (2016). Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 61, 470–478. doi: 10.1111/jpi.12361
Lerner, A. B., Case, J. D., Takahashi, Y., Lee, T. H., and Mori, W. (1958). Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 80, 2587–2587.
Li, C., Tan, D.-X., Liang, D., Chang, C., Jia, D., and Ma, F. (2015). Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 66, 669–680. doi: 10.1093/jxb/eru476
Li, C., Wang, P., Wei, Z., Liang, D., Liu, C., Yin, L., et al. (2012). The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 53, 298–306. doi: 10.1111/j.1600-079X.2012.00999.x
Li, G. Z., Liu, J., Chen, S. J., Wang, P. F., Liu, H. T., Dong, J., et al. (2021a). Melatonin promotes potassium deficiency tolerance by regulating HAK1 transporter and its upstream transcription factor NAC71 in wheat. J. Pineal Res. 70, e12727. doi: 10.1111/jpi.12727
Li, X., Ahammed, G. J., Zhang, X.-N., Zhang, L., Yan, P., Zhang, L.-P., et al. (2021b). Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 403:123922. doi: 10.1016/j.jhazmat.2020.123922
Li, H., Chang, J., Chen, H., Wang, Z., Gu, X., Wei, C., et al. (2017). Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 8:295. doi: 10.3389/fpls.2017.00295
Li, J., Yang, Y., Sun, K., Chen, Y., Chen, X., and Li, X. (2019). Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 24:1826. doi: 10.3390/molecules24091826
Li, X., Tan, D. X., Jiang, D., and Liu, F. (2016b). Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 61, 328–339. doi: 10.1111/jpi.12350
Li, M. Q., Hasan, M. K., Li, C. X., Ahammed, G. J., Xia, X. J., Shi, K., et al. (2016a). Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 61, 291–302. doi: 10.1111/jpi.12346
Li, X., Wei, J.-P., Scott, E. R., Liu, J.-W., Guo, S., Li, Y., et al. (2018). Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 23:165. doi: 10.3390/molecules23010165
Liang, D., Gao, F., Ni, Z., Lin, L., Deng, Q., Tang, Y., et al. (2018). Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules 23, 584. doi: 10.3390/molecules23030584
Liang, D., Ni, Z., Xia, H., Xie, Y., Lv, X., Wang, J., et al. (2019). Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Horticu. 246, 34–43.
Liang, X., Zhang, L., Natarajan, S. K., and Becker, D. F. (2013). Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011. doi: 10.1089/ars.2012.5074
Liu, C., Kang, H., Wang, Y., Yao, Y., Gao, Z., and Du, Y. (2021). Melatonin relieves ozone stress in grape leaves by inhibiting ethylene biosynthesis. Front. Plant Sci. 12:702874. doi: 10.3389/fpls.2021.702874
Liu, D.-D., Sun, X.-S., Liu, L., Shi, H.-D., Chen, S.-Y., and Zhao, D.-K. (2019). Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt stress. Molecules 24:1514. doi: 10.3390/molecules24081514
Liu, N., Gong, B., Jin, Z., Wang, X., Wei, M., Yang, F., et al. (2015b). Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J. Plant Physiol. 186, 68–77. doi: 10.1016/j.jplph.2015.07.012
Liu, J., Wang, W., Wang, L., and Sun, Y. (2015a). Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Reg. 77, 317–326. doi: 10.1007/s10725-015-0066-6
Ma, Y., Jiao, J., Fan, X., Sun, H., Zhang, Y., Jiang, J., et al. (2017). Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars. Front. Plant Sci. 7:2068. doi: 10.3389/fpls.2016.02068
Marta, B., Szafrañska, K., and Posmyk, M. M. (2016). Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 7:575. doi: 10.3389/fpls.2016.00575
Mehmood, M., Khan, I., Chattha, M. U., Hussain, S., Ahmad, N., Aslam, M. T., et al. (2021). Thiourea application protects maize from drought stress by regulating growth and physiological traits. Pak. J. Sci. 73, 355–363.
Meloni, D. A., Oliva, M. A., Martinez, C. A., and Cambraia, J. (2003). Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 49, 69–76. doi: 10.1186/s12870-020-02624-9
Meng, J. F., Xu, T. F., Wang, Z. Z., Fang, Y. L., Xi, Z. M., and Zhang, Z. W. (2014). The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 57, 200–212. doi: 10.1111/jpi.12159
Meng, J. F., Yu, Y., Shi, T.-C., Fu, Y.-S., Zhao, T., and Zhang, Z. W. (2018). Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 39, 35–42.
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
Mishra, A., Mishra, K. B., Höermiller, I. I., Heyer, A. G., and Nedbal, L. (2011). Chlorophyll fluorescence emission as a reporter on cold tolerance in Arabidopsis thaliana accessions. Plant Signal. 6, 301–310. doi: 10.4161/psb.6.2.15278
Moustafa-Farag, M., Mahmoud, A., Arnao, M. B., Sheteiwy, M. S., Dafea, M., Soltan, M., et al. (2020). Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 9:809. doi: 10.3390/antiox9090809
Nawaz, M. A., Huang, Y., Bie, Z., Ahmed, W., Reiter, R. J., Niu, M., et al. (2016). Melatonin: current status and future perspectives in plant science. Front. Plant Sci. 6:1230. doi: 10.3389/fpls.2015.01230
Nawaz, M. A., Jiao, Y., Chen, C., Shireen, F., Zheng, Z., Imtiaz, M., et al. (2018). Melatonin pre-treatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 220, 115–127. doi: 10.1016/j.jplph.2017.11.003
Nazir, M., Asad Ullah, M., Mumtaz, S., Siddiquah, A., Shah, M., Drouet, S., et al. (2020). Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 25:1072. doi: 10.3390/molecules25051072
Ni, J., Wang, Q., Shah, F. A., Liu, W., Wang, D., Huang, S., et al. (2018). Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23:799. doi: 10.3390/molecules23040799
Pelagio-Flores, R., Muñoz-Parra, E., Ortiz-Castro, R., and López-Bucio, J. (2012). Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 53, 279–288. doi: 10.1111/j.1600-079X.2012.00996.x
Posmyk, M. M., Kuran, H., Marciniak, K., and Janas, K. M. (2008). Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 45, 24–31. doi: 10.1111/j.1600-079X.2007.00552.x
Qari, S. H., Hassan, M. U., Chattha, M. U., Mahmood, A., Naqve, M., Nawaz, M., et al. (2022). Melatonin induced cold tolerance in plants: physiological and molecular responses. Front. Plant Sci. 13:843071. doi: 10.3389/fpls.2022.843071
Qiu, Y., An, K., Sun, J., Chen, X., Gong, X., Ma, L., et al. (2019). Investigating the effect of methyl jasmonate and melatonin on resistance of Malus crabapple ‘Hong Jiu’to ozone stress. Environ. Sci. Pollut. Res. 26, 27761–27768. doi: 10.1007/s11356-019-05946-w
Rasheed, A., Fahad, S., Aamer, M., Hassan, M., Tahir, M., and Wu, Z. (2020a). Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice. a review. Appl. Ecol. Environ. Res. 18, 4005–4023.
Rasheed, A., Fahad, S., Hassan, M., Tahir, M., Aamer, M., and Wu, Z. (2020b). A review on aluminum toxicity and quantitative trait loci maping in rice (Oryza sativa L). Appl. Ecol. Environ. Res. 18, 3951–3964.
Rasheed, A., Gill, R. A., Hassan, M. U., Mahmood, A., Qari, S., Zaman, Q. U., et al. (2021a). A critical review: recent advancements in the use of CRISPR/Cas9 technology to enhance crops and alleviate global food crises. Curr. Issues Mol. Biol. 43, 1950–1976. doi: 10.3390/cimb43030135
Rasheed, A., Hassan, M. U., Fahad, S., Aamer, M., Batool, M., Ilyas, M., et al. (2021b). “Heavy metals stress and plants defense responses,” in Sustainable Soil and Land Management and Climate Change, eds S. Fahad, O. Sonmez, S. Saud, D. Wang, C. Wu, M. Adnan, et al. (Boca Raton, FL: CRC Press), 57–82.
Rehman, S., Chattha, M. U., Khan, I., Mahmood, A., Hassan, M. U., Al-Huqail, A. A., et al. (2022). Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 11:822. doi: 10.3390/plants11060822
Reiter, R. J., Tan, D. X., Burkhardt, S., and Manchester, L. C. J. N. R. (2001). Melatonin in plants. Nutr. Rev. 59, 286–290.
Reiter, R. J. J. E. R. (1991). Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12, 151–180. doi: 10.1210/edrv-12-2-151
Sadak, M. S., Abdalla, A. M., Abd Elhamid, E. M., and Ezzo, M. (2020). Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Nat. Res. Cent. 44, 1–13.
Santofimia-Castaño, P., Ruy, D. C., Fernandez-Bermejo, M., Salido, G. M., and Gonzalez, A. (2014). Pharmacological dose of melatonin reduces cytosolic calcium load in response to cholecystokinin in mouse pancreatic acinar cells. Mol. Cell. Biochem. 397, 75–86. doi: 10.1007/s11010-014-2174-4
Seleiman, M. F., Ali, S., Refay, Y., Rizwan, M., Alhammad, B. A., and El-Hendawy, S. E. (2020). Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 250:126239. doi: 10.1016/j.chemosphere.2020.126239
Seleiman, M. F., Aslam, M. T., Alhammad, B. A., Hassan, M. U., Maqbool, R., Chattha, M. U., et al. (2022). Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton Int. J. Exp. Bot. 91, 667–694. doi: 10.3389/fpls.2016.00957
Serengil, Y., Augustaitis, A., Bytnerowicz, A., Grulke, N., Kozovitz, A., Matyssek, R., et al. (2011). Adaptation of forest ecosystems to air pollution and climate change: a global assessment on research priorities. For. Biogeosci. 4:44. doi: 10.1016/j.envpol.2016.01.075
Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S., Bali, A. S., et al. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285. doi: 10.3390/biom9070285
Shi, H., Tan, D. X., Reiter, R. J., Ye, T., Yang, F., and Chan, Z. (2015b). Melatonin induces class A1 heat-shock factors (HSFA 1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 58, 335–342. doi: 10.1111/jpi.12219
Shi, H., Jiang, C., Ye, T., Tan, D.-X., Reiter, R. J., Zhang, H., et al. (2015a). Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694. doi: 10.1093/jxb/eru373
Silalert, P., and Pattanagul, W. (2021). Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti Agrobot. Cluj Napoca 49, 12417–12417. doi: 10.15835/nbha49312417
Simopoulos, A. P., Tan, D.-X., Manchester, L. C., and Reiter, R. J. (2005). Purslane: a plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 39, 331–332. doi: 10.1111/j.1600-079X.2005.00269.x
Singh, S., Tripathi, D. K., Singh, S., Sharma, S., Dubey, N. K., Chauhan, D. K., et al. (2017). Toxicity of aluminium on various levels of plant cells and organism: a review. Environ. Exp. Bot. 137, 177–193. doi: 10.1016/j.envexpbot.2017.01.005
Sultan, I., Khan, I., Chattha, M. U., Hassan, M. U., Barbanti, L., Calone, R., et al. (2021). Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 16:1810.
Sun, C., Liu, L., Wang, L., Li, B., Jin, C., and Lin, X. (2021). Melatonin: a master regulator of plant development and stress responses. J. Integrat. Plant Biol. 63, 126–145. doi: 10.1111/jipb.12993
Tan, D. X., Hardeland, R., Back, K., Manchester, L. C., Alatorre-Jimenez, M. A., and Reiter, R. J. (2016). On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J. Pineal Res. 61, 27–40. doi: 10.1111/jpi.12336
Tan, D. X., and Reiter, R. J. (2019). Mitochondria: the birth place, battle ground and site of melatonin metabolism in cells. Melatonin Res. 2, 44–66.
Tan, D. X., Xu, B., Zhou, X., and Reiter, R. J. (2018). Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 23:301. doi: 10.3390/molecules23020301
Turk, H., and Erdal, S. (2015). Melatonin alleviates cold-induced oxidative damage in maize seedlings by upregulating mineral elements and enhancing antioxidant activity. J. Plant Nutr. Soil Sci. 178, 433–439.
Turk, H., Erdal, S., Genisel, M., Atici, O., Demir, Y., and Yanmis, D. (2014). The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Reg. 74, 139–152.
Ullah, M. A., Tungmunnithum, D., Garros, L., Drouet, S., Hano, C., and Abbasi, B. H. (2019). Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum L. Int. J. Mol. Sci. 20:1787. doi: 10.3390/ijms20071787
Vafadar, F., Amooaghaie, R., Ehsanzadeh, P., Ghanati, F., and Sajedi, R. H. (2020). Crosstalk between melatonin and Ca2+/CaM evokes systemic salt tolerance in Dracocephalum kotschyi. J. Plant Physiol. 252:153237. doi: 10.1016/j.jplph.2020.153237
Wang, Q., An, B., Wei, Y., Reiter, R. J., Shi, H., Luo, H., et al. (2016b). Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front Plant Sci. 7:1882. doi: 10.3389/fpls.2016.01882
Wang, L., Liu, J., Wang, W., and Sun, Y. (2016a). Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54, 19–27.
Wang, L., Zhao, Y., Reiter, R. J., He, C., Liu, G., Lei, Q., et al. (2014). Changes in melatonin levels in transgenic ‘Micro-Tom’tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 56, 134–142. doi: 10.1111/jpi.12105
Wang, P., Sun, X., Li, C., Wei, Z., Liang, D., and Ma, F. (2013). Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 54, 292–302. doi: 10.1111/jpi.12017
Wang, P., Sun, X., Wang, N., Tan, D. X., and Ma, F. (2015). Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J. Pineal Res. 58, 479–489. doi: 10.1111/jpi.12233
Wei, Y., Liu, G., Chang, Y., Lin, D., Reiter, R. J., He, C., et al. (2018). Melatonin biosynthesis enzymes recruit WRKY transcription factors to regulate melatonin accumulation and transcriptional activity on W-box in cassava. J. Pineal Res. 65:e12487. doi: 10.1111/jpi.12487
Wei, Z., Li, C., Gao, T., Zhang, Z., Liang, B., Lv, Z., et al. (2019). Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 139, 630–641. doi: 10.1016/j.plaphy.2019.04.026
Wen, D., Gong, B., Sun, S., Liu, S., Wang, X., Wei, M., et al. (2016). Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 7:718. doi: 10.3389/fpls.2016.00718
Wu, S., Wang, Y., Zhang, J., Gong, X., Zhang, Z., Sun, J., et al. (2021a). Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 7, 13–22. doi: 10.1016/j.hpj.2020.06.002
Wu, Y., Fan, X., Zhang, Y., Jiang, J., Sun, L., Rahman, F. U., et al. (2021b). VvSNAT1 overexpression enhances melatonin production and salt tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 166, 485–494. doi: 10.1016/j.plaphy.2021.06.025
Wu, X., Li, H., Luo, H., Lin, D., and Zhou, X. (2019). “Effect of exogenous melatonin on photosynthetic characteristics in cucumber seedlings under cadmium stress: a rapid detection method for the cadmium resistance,” in Proceedings of the E3S Web of Conferences, (Les Ulis: EDP Sciences), 07023. doi: 10.1051/e3sconf/201913607023
Xie, C., Xiong, X., Huang, Z., Sun, L., Ma, J., Cai, S., et al. (2018). Exogenous melatonin improves lead tolerance of bermudagrass through modulation of the antioxidant defense system. Int. J. Phytoremed. 20, 1408–1417. doi: 10.1080/15226514.2018.1488813
Xing, X., Ding, Y., Jin, J., Song, A., Chen, S., Chen, F., et al. (2021). Physiological and transcripts analyses reveal the mechanism by which melatonin alleviates heat stress in chrysanthemum seedlings. Front. Plant Sci. 12:673236. doi: 10.3389/fpls.2021.673236
Xu, W., Cai, S. Y., Zhang, Y., Wang, Y., Ahammed, G. J., Xia, X. J., et al. (2016). Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 61, 457–469. doi: 10.1111/jpi.12359
Yan, F., Wei, H., Li, W., Liu, Z., Tang, S., Chen, L., et al. (2020). Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 206:111358. doi: 10.1016/j.ecoenv.2020.111358
Yan, F., Zhang, J., Li, W., Ding, Y., Zhong, Q., Xu, X., et al. (2021). Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 163, 367–375. doi: 10.1016/j.plaphy.2021.03.058
Yan, Y., Sun, S., Zhao, N., Yang, W., Shi, Q., and Gong, B. (2019). COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ. Pollut. 252, 51–61. doi: 10.1016/j.envpol.2019.05.052
Yancey, P. H. (2005). Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208, 2819–2830. doi: 10.1242/jeb.01730
Yang, W.-J., Du, Y.-T., Zhou, Y.-B., Chen, J., Xu, Z.-S., Ma, Y.-Z., et al. (2019). Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 20:652. doi: 10.3390/ijms20030652
Yang, X., Xu, H., Li, D., Gao, X., Li, T., and Wang, R. (2018). Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 56, 884–892. doi: 10.1007/s11099-017-0748-6
Yao, J. W., Ma, Z., Ma, Y. Q., Zhu, Y., Lei, M. Q., Hao, C. Y., et al. (2021). Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant Cell Environ. 44, 114–129. doi: 10.1111/pce.13879
Ye, J., Wang, S., Deng, X., Yin, L., Xiong, B., and Wang, X. (2016). Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 38, 1–13.
Yin, Z., Lu, J., Meng, S., Liu, Y., Mostafa, I., Qi, M., et al. (2019). Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 14, 453–463.
Yu, Y., Teng, Z., Mou, Z., Lv, Y., Li, T., Chen, S., et al. (2021). Melatonin confers heavy metal-induced tolerance by alleviating oxidative stress and reducing the heavy metal accumulation in Exophiala pisciphila, a dark septate endophyte (DSE). BMC Microbiol. 21:40. doi: 10.1186/s12866-021-02098-1
Zhang, H. J., Zhang, N., Yang, R. C., Wang, L., Sun, Q. Q., Li, D. B., et al. (2014). Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Rse. 57, 269–279. doi: 10.1111/jpi.12167
Zhang, J., Shi, Y., Zhang, X., Du, H., Xu, B., and Huang, B. (2017a). Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 138, 36–45.
Zhang, Y., Yang, S., and Chen, Y. J. B. P. (2017d). Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 61, 571–578.
Zhang, J., Zeng, B., Mao, Y., Kong, X., Wang, X., Yang, Y., et al. (2017b). Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct. Plant Biol. 44, 961–968. doi: 10.1071/FP17003
Zhang, Y., Yang, S., and Chen, Y. (2017c). Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 61, 571–578.
Zhang, L., Jia, J., Xu, Y., Wang, Y., Hao, J., and Li, T. J. I. V. C. (2012). Production of transgenic Nicotiana sylvestris plants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. In Vitro Cell Dev. Biol. Plant 48, 275–282. doi: 10.1007/s11627-011-9413-0
Zhang, N., Zhao, B., Zhang, H. J., Weeda, S., Yang, C., Yang, Z. C., et al. (2013). Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54, 15–23. doi: 10.1111/j.1600-079X.2012.01015.x
Zhang, P., Liu, L., Wang, X., Wang, Z., Zhang, H., Chen, J., et al. (2021a). Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L.). Plants 10:886. doi: 10.3390/plants10050886
Zhang, R., Yue, Z., Chen, X., Wang, Y., Zhou, Y., Xu, W., et al. (2021b). Foliar applications of urea and melatonin to alleviate waterlogging stress on photosynthesis and antioxidant metabolism in sorghum seedlings. Plant Growth Reg. 1–10.
Zhang, Y., Fan, Y., Rui, C., Zhang, H., Xu, N., Dai, M., et al. (2021c). Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front. Plant Sci. 12:1239. doi: 10.3389/fpls.2021.693690
Zhang, Q., Liu, X., Zhang, Z., Liu, N., Li, D., and Hu, L. (2019). Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 10:44. doi: 10.3389/fpls.2019.00044
Zhang, T., Shi, Z., Zhang, X., Zheng, S., Wang, J., and Mo, J. (2020a). Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 262:109070. doi: 10.13287/j.1001-9332.201706.005
Zhang, X., Zhang, H., Zhang, H., and Tang, M. (2020b). Exogenous melatonin application enhances Rhizophagus irregularis symbiosis and induces the antioxidant response of Medicago truncatula under lead stress. Front. Microbiol. 11:516. doi: 10.3389/fmicb.2020.00516
Zhao, D., Yu, Y., Shen, Y., Liu, Q., Zhao, Z., Sharma, R., et al. (2019). Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrin. 10, 249. doi: 10.3389/fendo.2019.00249
Zhao, G., Zhao, Y., Yu, X., Kiprotich, F., Han, H., Guan, R., et al. (2018). Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 19:1912. doi: 10.3390/ijms19071912
Zheng, X., Zhou, J., Tan, D.-X., Wang, N., Wang, L., Shan, D., et al. (2017). Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 8:483. doi: 10.3389/fpls.2017.00483
Zhou, C., Liu, Z., Zhu, L., Ma, Z., Wang, J., and Zhu, J. (2016). Exogenous melatonin improves plant iron deficiency tolerance via increased accumulation of polyamine-mediated nitric oxide. Int. J. Mol. Sci. 17:1777. doi: 10.3390/ijms17111777
Zhuang, W., Liu, T., Shu, X., Wang, H., Wang, Z., Wang, T., et al. (2020). Overexpression of MzASMT 1, a gene from Malus zumi mats, enhances salt tolerance in transgenic tobacco. Front. Plant Sci. 11:561903. doi: 10.3389/fpls.2020.561903
Zuo, B., Zheng, X., He, P., Wang, L., Lei, Q., Feng, C., et al. (2014). Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 57, 408–417. doi: 10.1111/jpi.12180
Keywords: abiotic stress, anti-oxidant defence, growth, genes regulation, melatonin, ROS, signalling crosstalk
Citation: Hassan MU, Mahmood A, Awan MI, Maqbool R, Aamer M, Alhaithloul HAS, Huang G, Skalicky M, Brestic M, Pandey S, El Sabagh A and Qari SH (2022) Melatonin-Induced Protection Against Plant Abiotic Stress: Mechanisms and Prospects. Front. Plant Sci. 13:902694. doi: 10.3389/fpls.2022.902694
Received: 23 March 2022; Accepted: 25 April 2022;
Published: 09 June 2022.
Edited by:
Daniel Kean Yuen Tan, The University of Sydney, AustraliaReviewed by:
Shalini Tiwari, Jawaharlal Nehru University, IndiaAndrzej Bajguz, University of Białystok, Poland
Copyright © 2022 Hassan, Mahmood, Awan, Maqbool, Aamer, Alhaithloul, Huang, Skalicky, Brestic, Pandey, El Sabagh and Qari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqin Huang, aGdxanhhdWhncUBqeGF1LmVkdS5jbg==; aGdxanhlc0BzaW5hLmNvbQ==
 Muhammad Umair Hassan
Muhammad Umair Hassan Athar Mahmood
Athar Mahmood Masood Iqbal Awan
Masood Iqbal Awan Rizwan Maqbool
Rizwan Maqbool Muhammad Aamer1,3
Muhammad Aamer1,3 Milan Skalicky
Milan Skalicky Marian Brestic
Marian Brestic Saurabh Pandey
Saurabh Pandey Ayman El Sabagh
Ayman El Sabagh Sameer H. Qari
Sameer H. Qari