
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 25 May 2022
Sec. Crop and Product Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.884572
This article is part of the Research Topic Insights in Crop and Product Physiology: 2021 View all 10 articles
Postharvest deterioration can result in qualitative and quantitative changes in the marketability of horticultural commodities, as well as considerable economic loss to the industry. Low temperature and controlled atmosphere conditions (low O2 and elevated CO2) are extensively employed to prolong the postharvest life of these commodities. Nevertheless, they may suffer from chilling injury and other physiological disorders, as well as excessive water loss and bacterial/fungal decay. Research on the postharvest physiological, biochemical, and molecular responses of horticultural commodities indicates that low temperature/controlled atmosphere storage is associated with the promotion of γ-aminobutyrate (GABA) pathway activity, with or without the accumulation of GABA, delaying senescence, preserving quality and ameliorating chilling injury. Regardless of whether apple fruits are stored under low temperature/controlled atmosphere conditions or room temperature, elevated endogenous GABA or exogenous GABA maintains their quality by stimulating the activity of the GABA shunt (glutamate GABA succinic semialdehyde succinate) and the synthesis of malate, and delaying fruit ripening. This outcome is associated with changes in the genetic and biochemical regulation of key GABA pathway reactions. Flux estimates suggest that the GABA pool is derived primarily from glutamate, rather than polyamines, and that succinic semialdehyde is converted mainly to succinate, rather than γ-hydroxybutyrate. Exogenous GABA is a promising strategy for promoting the level of endogenous GABA and the activity of the GABA shunt in both intact and fresh-cut commodities, which increases carbon flux through respiratory pathways, restores or partially restores redox and energy levels, and improves postharvest marketability. The precise mechanisms whereby GABA interacts with other signaling molecules such as Ca2+, H2O2, polyamines, salicylic acid, nitric oxide and melatonin, or with phytohormones such as ethylene, abscisic acid and auxin remain unknown. The occurrence of the aluminum-activated malate transporter and the glutamate/aspartate/GABA exchanger in the tonoplast, respectively, offers prospects for reducing transpirational water in cut flowers and immature green fruit, and for altering the development, flavor and biotic resistance of apple fruits.
Fruits, vegetables and nuts are a crucial part of a healthy diet, which can help reduce risk factors for non-communicable diseases. Increasingly, consumers are concerned with the nutritional quality of these commodities (Kyriacou and Rouphael, 2018; Ziv and Fallik, 2021). Postharvest deterioration can result in qualitative and quantitative changes in their marketability, as well as incredible economic losses to the horticultural industry. Low temperature (LT) and controlled atmosphere (CA) conditions (low O2 and elevated CO2) are extensively employed to prolong the postharvest life of horticultural crops. However, horticultural crops may suffer from chilling injury and other physiological disorders, as well as excessive water loss and fungal decay (e.g., Lum et al., 2016b; Tarkowski et al., 2020; Ziv and Fallik, 2021).
The exogenous application of biostimulants, including naturally occurring plant metabolites and hormones such as polyamines (PA), salicylate, jasmonate, melatonin and γ-aminobutyrate (GABA), is being studied to improve plant tolerance/resistance to abiotic and biotic stresses under both open and closed environmental conditions (Bor and Turkan, 2019; Podlešákova et al., 2019; Akula and Mukherjee, 2020; Godoy et al., 2021; Shelp et al., 2021). The metabolism, transport, and signaling role(s) of GABA in plants were recently reviewed (Shelp et al., 2021; Xu et al., 2021b; Suhel et al., 2022). Stress-induced promotion of GABA pathways in vegetative plants, and the physiological, biochemical and molecular responses associated with enhancing stress tolerance via genetic manipulation of GABA metabolism and GABA receptors or the use of exogenous GABA were described (Shelp et al., 2021). Of particular interest is the demonstration that drought-induced GABA accumulation in the guard cell functions as an abscisic acid-independent mechanism for reducing stomatal reopening and transpirational water loss, thereby improving drought tolerance (Bown and Shelp, 2016; Mekonnen et al., 2016; Shelp et al., 2021; Xu et al., 2021b). GABA binds to aluminum-activated malate transporters (ALMT9/12 signaling pathway) and negatively regulates malate and/or Cl– transport (Xu et al., 2021b).
This review focuses on postharvest horticultural commodities, with emphasis on botanical fruits, though some discussion of root, leaf and ornamental crops, as well as walnuts and mushrooms, is also included. First, we describe how LT and CA storage conditions improve marketability and promote GABA metabolism. Second, we discuss the genetic and biochemical control of GABA metabolism and signaling in apple fruits, and the use of exogenous GABA to preserve the postharvest quality of stored and fresh-cut horticultural commodities (i.e., delaying senescence, and enhancing resistance to chilling, browning, disease and physiological disorders) by promoting GABA shunt activity, energy generation, and antioxidant and secondary pathways. Third, we discuss prospects for enhancing postharvest drought tolerance, pathogen resistance, and flavor using exogenous GABA. Finally, we briefly comment on the safety and commercial production of GABA.
During the postharvest storage of horticultural commodities, temperature and/or atmospheric conditions are adjusted so that ethylene production and respiratory rates are reduced, and ripening/senescence is delayed, resulting in the preservation of nutritional and sensory quality (Table 1). LT storage of mulberry leaves in air preserves color, while enhancing GABA accumulation (Li E. et al., 2018), as is often found in the vegetative organs of many plant species (Shelp et al., 2021). This result might be attributed to the elevated activity of glutamate (Glu) decarboxylase (GAD) and limited activity of the catabolic enzyme GABA transaminase (GABA-T) (Figure 1). In contrast, the LT storage of zucchini fruit promotes GABA catabolism, without causing its accumulation (Palma et al., 2014). The loss of GABA and the increase in GABA-T activity is more substantive in a chilling-tolerant cultivar than a chilling-sensitive cultivar, suggesting that GABA catabolism replenishes the tricarboxylic acid cycle (TCAC) to generate reducing equivalents and energy that could alleviate oxidative damage (Shelp et al., 2021). The authors have interpreted the increase in diamine oxidase (DAO) activity and putrescine (Put) accumulation as support for the involvement of Put catabolism in GABA production and the alleviation of chilling injury (Figure 1). Conditioning at 15°C prior to LT storage improves the tolerance in the chilling-sensitive zucchini cultivar by decreasing the GABA level and increasing the ATP level and activities of enzymatic antioxidants (peroxidase, catalase) (Carvajal et al., 2015). Improved chilling tolerance in peaches by hot water treatment prior to LT storage is associated with membrane stability (as indicated by less electrolyte leakage and lower malondialdehyde accumulation), and the maintenance of high levels of amino acid (including GABA and proline), polyamines (PAs) and radical scavenging capacity (phenols) (Wang L. et al., 2021).
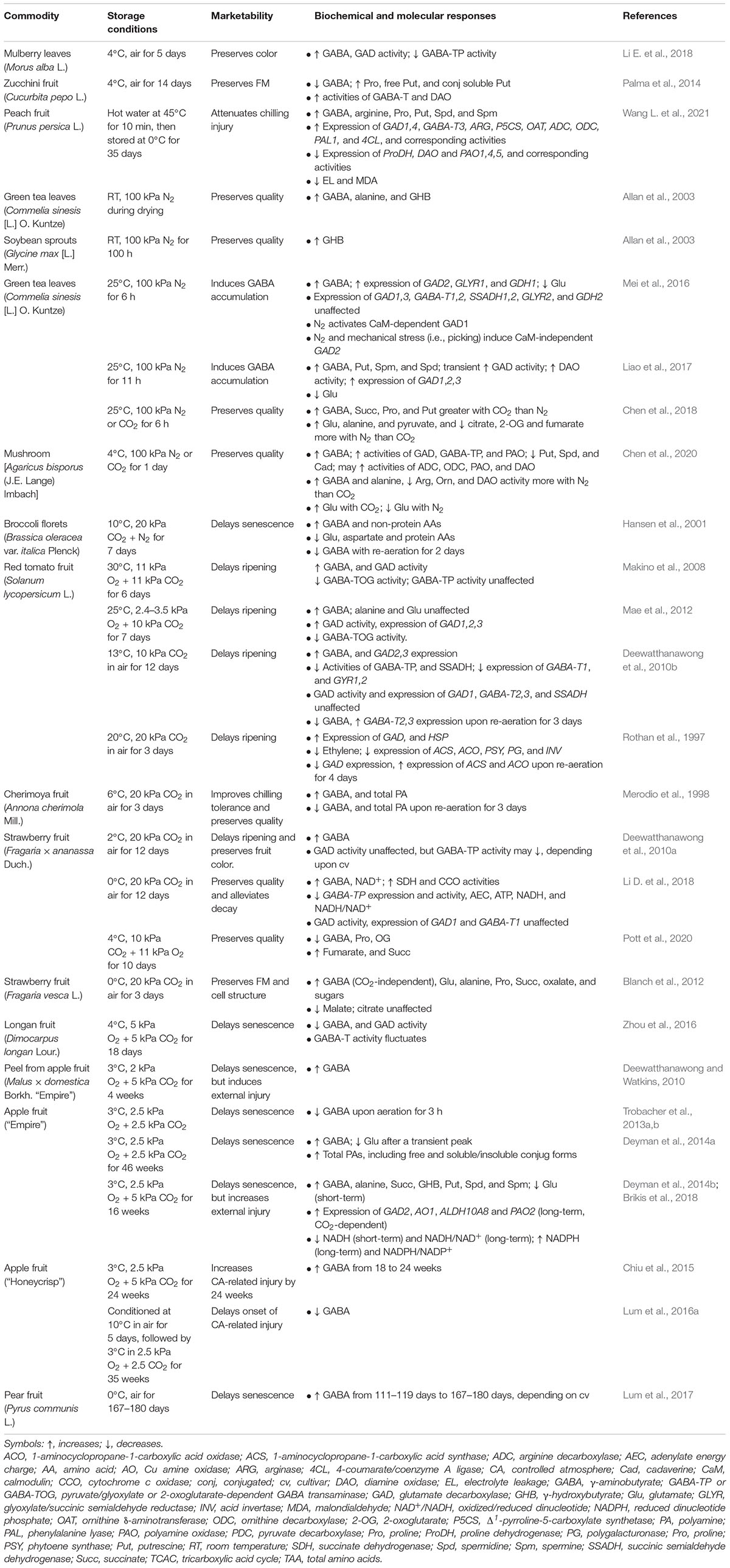
Table 1. Postharvest storage conditions improve the marketability of horticultural commodities and promote GABA metabolism.
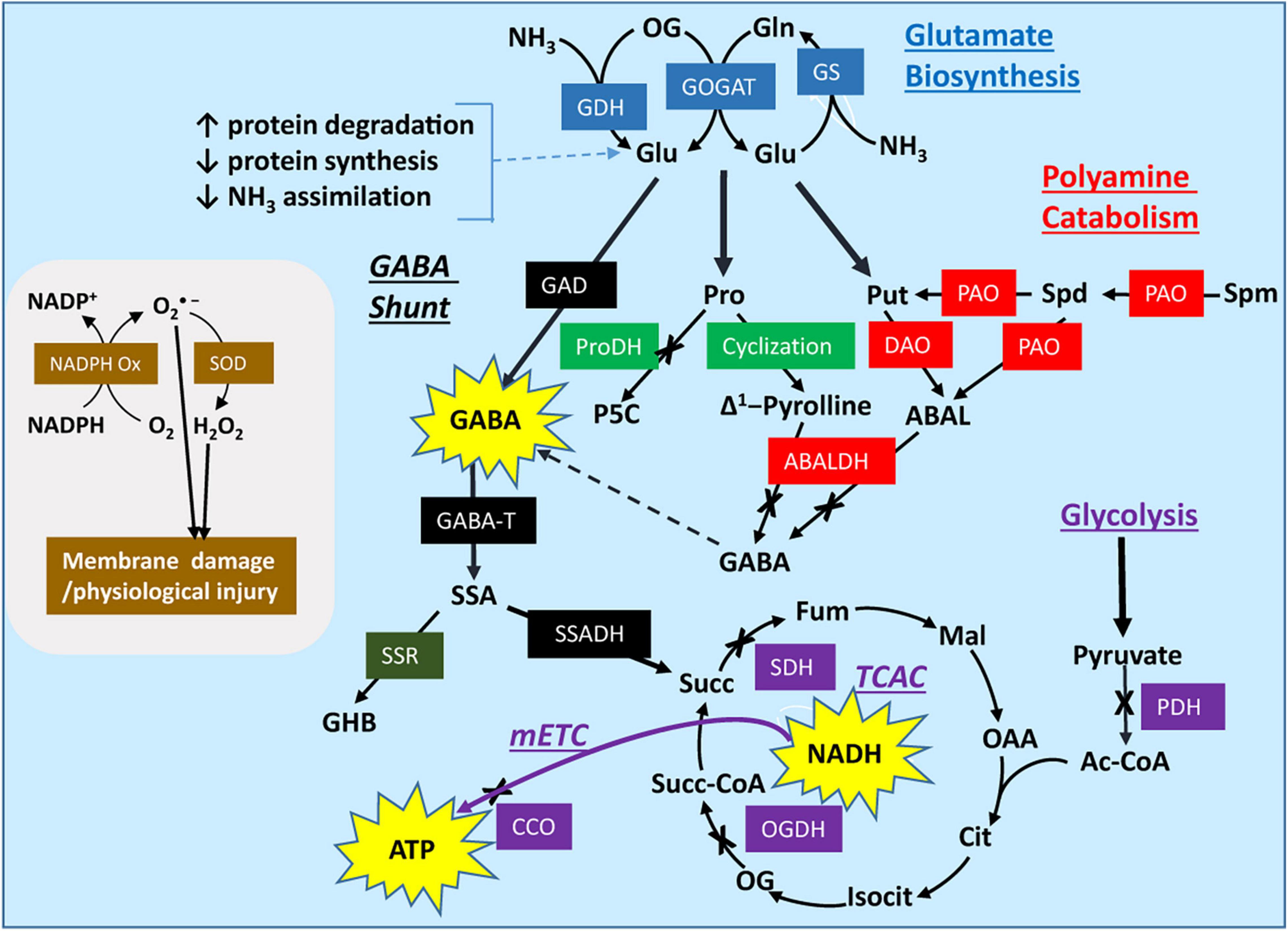
Figure 1. Modeling the postharvest impact of low temperature and controlled atmosphere conditions on activities of the GABA shunt, polyamine catabolism, proline catabolism, respiratory processes, and oxidant systems in horticultural commodities. Low temperature, low O2 and elevated CO2 can limit the activities of pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, succinate dehydrogenase, and cytochrome c oxidase, leading to less NADH, FADH and ATP generation and more protein turnover. This is accompanied by a shift in redox balance. The elevated NADPH/NADP+ ratio stimulates H2O2 production via NADPH oxidase and superoxide dismutase, and stimulates the expression/activities of non-enzymatic and enzymatic antioxidants (not shown). Under these conditions, the availability of Glu and the synthesis of polyamines, proline and GABA increase. Polyamines often accumulate, but evidence suggests that only about 3% of the stress-induced GABA is derived from putrescine or spermidine catabolism, which may be explained, at least in part, by O2 and NAD+ limitation of DAO, PAO, and ABALDH activities. Proline also accumulates, in part due to the decline in proline dehydrogenase activity, but there is no direct evidence for the conversion of proline into GABA via ABALDH (because 4-aminobutanal and △1-pyrroline are in rapid non-enzymatic equilibrium, their oxidation is often considered to be catalyzed by ABALDH). The limiting activities are to some extent overcome by H+ stimulation or Ca2+/calmodulin activation of glutamate decarboxylase, which increases the biosynthesis of GABA and the carbon flux through succinic semialdehyde to succinate via GABA transaminase and succinic semialdehyde dehydrogenase, respectively. Only a minor portion of the NADPH is recycled via the diversion of succinic semialdehyde into γ-hydroxybutyrate. Consequently, stress-derived succinate stimulates the production of NADH and ATP via the non-cyclic tricarboxylic acid cycle and the mitochondrial electron transport chain. A representative oxidant system is shown on the left; it involves NADPH oxidase and superoxide dismutase, and contributes to membrane damage and physiological injury [Please refer to Shelp et al. (2021) for more detailed graphical representations]. Symbols: ↑, increase; ↓, decrease; colored rectangles, enzymes; X, biochemical reaction potentially inhibited by stress; thick arrows, multiple biochemical steps; moderately thick arrows, the GABA shunt. ABAL, 4-aminobutanal; ABALDH, 4-aminobutanal dehydrogenase; Ac-CoA, acetyl-CoA; Cit, citrate; CCO, cytochrome oxidase; DAO, diamine oxidase; Fum, fumarate; GABA; γ-aminobutyrate; GABA-T, pyruvate/glyoxylate-dependent GABA transaminase; GAD, glutamate decarboxylase; GDH, glutamate dehydrogenase; GHB, γ-hydroxybutyrate; Glu, glutamate; GOGAT, glutamate synthase; GS, glutamine synthetase; Isocit, isocitrate; MAL, malate; mETC, mitochondrial electron transport chain; NADPH Ox, NADPH oxidase; OG, 2-oxoglutarate; OGDH, 2-oxoglutarate dehydrogenase; PAO, polyamine oxidase; Pro, proline; PDH, pyruvate dehydrogenase; ProDH, proline dehydrogenase; Put, putrescine; SDH, succinate dehydrogenase; SOD, superoxide dismutase; Spd, spermidine; Spm, spermine; SSADH, succinic semialdehyde dehydrogenase; SSR, succinic semialdehyde reductase; Succ, succinate; Succ-CoA; succinyl-CoA; TCAC, tricarboxylic acid cycle; See Table 4 legend for the remaining abbreviations.
Anoxia preserves the quality of drying green tea leaves and of soybean sprouts stored at room temperature (RT) and promotes GABA accumulation and the diversion of succinic semialdehyde (SSA) from succinate to γ-hydroxybutyrate (GHB) (Allan et al., 2003; Table 1 and Figure 1). Several mechanisms could account for the accumulation of GABA: calmodulin (CaM) activation of CsGAD1; elevated expression of CsGAD2; oxidation of Put/proline; and, feedback inhibition of GABA transaminase (CsGABA-T) activity (Mei et al., 2016; Liao et al., 2017; Shelp et al., 2021; Table 1). Complete inhibition of DAO activity by aminoguanidine (4–11 h of treatment) suggests that about 25% of the GABA is derived from the PA degradation pathways (Liao et al., 2017; Figure 1). However, this interpretation can be challenged. Based upon the increasing accumulation of Put with aminoguanidine over the same time period, we estimate that Put degradation would account for only 3% of the anoxia-induced rate of GABA accumulation. Notably, the spermidine (Spd) pool also decreases at an estimated rate of approximately 3% of the rate for GABA accumulation, suggesting that the terminal oxidation of Spd can substitute for the terminal oxidation of Put (Shelp et al., 2012b). This re-assessment of the published data is consistent with our recent interpretation of the ΔGABA/ΔPut stoichiometry published for wheat roots treated simultaneously with salinity and aminoguanidine (Shelp et al., 2021). While increasing DAO activity seems contrary to our interpretation of the metabolite data, it could reflect an “anticipation response” to the return to normoxia, as proposed for alanine transaminase and glutamate dehydrogenase (Limami et al., 2008).
The quality of green tea leaves at RT and of broccoli florets at LT is preserved under anaerobic conditions imposed by either anoxia or elevated CO2 (Hansen et al., 2001; Chen et al., 2018; Table 1). However, the accumulation of GABA and succinate, and the depletion of Glu is more rapid with CO2 than with N2, whereas the accumulation of alanine is faster with N2. There is greater Put and NADH accumulation, and less NADPH, citrate, 2-oxoglutarate (OG) and fumarate accumulation with CO2 than air. Thus, the GABA shunt is more active with CO2, but the inhibition of the TCAC and mitochondrial electron transport chain (mETC) occurs more quickly with N2 (Chen et al., 2018). While the storage of mushrooms with 100% CO2 at LT also stimulates the production of Glu-derived GABA, storage with N2 stimulates the production of both Glu-and PA-derived GABA (Chen et al., 2020), perhaps due in part to protein degradation. Notably, AbGAD, unlike most plant GADs, does not possess a CaM-binding domain, and therefore its activity is likely to be stimulated by cytosolic acidification only.
Elevated CO2 in air at LT improves chilling tolerance in cherimoya fruit (Merodio et al., 1998), and delays ripening/senescence in tomato and strawberry fruits (Deewatthanawong et al., 2010a,b; Blanch et al., 2012; Li D. et al., 2018; Table 1). These positive outcomes are typically accompanied by the accumulation of GABA and occasionally PAs, as well as limited flux of GABA-carbon through the GABA shunt into the TCAC and the mETC. Elevated CO2, in combination with low O2, delays the ripening/senescence of tomato fruit stored at RT (Makino et al., 2008; Mae et al., 2012), and longan (Zhou et al., 2016), strawberry (Pott et al., 2020) and “Empire” apple (Deewatthanawong and Watkins, 2010; Trobacher et al., 2013a; Deyman et al., 2014a,b; Brikis et al., 2018) fruits stored at LT. These findings have been attributed to the elevated generation of GABA from Glu, rather than PAs, and enhanced flux of GABA-carbon through the GABA shunt into a non-cyclic TCAC for generation of ATP (Shelp et al., 2012b; Brikis et al., 2018). Wang C. et al. (2014) previously suggested that Glu-derived GABA accumulation in melon roots can alleviate hypoxia damage by accelerating PA biosynthesis and conversion, as well as preventing PA degradation.
Some pome fruit are particularly sensitive to LT, CA storage (e.g., “Honeycrisp” apples and “Cold Snap” pears) (Chiu et al., 2015; Lum et al., 2016a,2017; Table 1). In these cases, a dramatic increase in the GABA level coincides with CA-or senescence-related injury and is likely due to the disruption of cellular compartmentation and the release of acidic vacuolar contents to the cytosol (Bown and Shelp, 2006). Interestingly, conditioning of “Honeycrisp” apples at 10°C improves the resistance to CA-related injury, decreases the GABA level and increases the ratios of NAD(P)H/NAD(P) + (Lum et al., 2016a).
Overall, these studies indicate that LT, CA-mediated improvements in the postharvest marketability of horticultural products is generally associated with the promotion of GABA biosynthesis and GABA shunt activity, with or without the accumulation of GABA. The onset of CA-or senescence-related injury during prolonged storage may also be associated with the accumulation of GABA. Discrepancies in data from the various studies might be explained by: pretreatment and conditioning of plant materials prior to storage; the use of different cultivars and single time point determinations, rather than time courses; excessive handling or wounding of plant materials prior to metabolite extraction; and, the use of non-saturating levels of Glu and inhibitory levels of GABA and pyruvate, respectively, in in vitro assays of GAD and GABA-TP activities [for examples, compare Zhou et al. (2016), Li D. et al. (2018), and Chen et al. (2020) with Snedden et al. (1995), Van Cauwenberghe and Shelp (1999), Clark et al. (2009a), and Shelp et al. (2012a)]. Interpretation could vary somewhat because: (i) it is not possible to directly assess the Ca2+/CaM stimulation of GAD activity in situ; (ii) the existence of a 2-OG-dependent plant GABA-T is questionable [for examples, compare Makino et al. (2008) and Deewatthanawong et al. (2010b) with Clark et al. (2009b), Koike et al. (2013), Trobacher et al. (2013a), Shimajiri et al. (2013), and Shelp et al. (2021)]; (iii) the expression of a gene does not establish that the encoded protein is operational; (iv) the understanding of precursor/product relations and flux is often incomplete (e.g., pool sizes alone do not indicate flux; Put accumulation does not establish greater contribution than Glu to GABA generation) (Shelp et al., 2012a); and, (v) there is often a failure to consider the importance of multiple isoforms of the GABA pathway enzymes (Shelp et al., 2012c,2021).
Gene sequences for the key steps in GABA metabolism in apple fruit have been identified, allowing elucidation of the biochemical properties and subcellular location of multiple isoforms of the encoded proteins (Table 2 and Figure 1). Three cytosolic GADs are present, but unlike MdGAD3, MdGAD1,2 are Ca2+/CaM-dependent and more sensitive to pH (Trobacher et al., 2013b). There are also two mitochondrial pyruvate/glyoxylate-dependent GABA transaminases (MdGABA-Ts, designated as GABA-TP), two mitochondrial NAD+-dependent SSADHs (MdSSADH1,2 or MdALDH5F1,2), and two NADPH-dependent glyoxylate/succinic semialdehyde reductases (MdGLYR1,2 or MdSSR1,2) with different subcellular locations (Trobacher et al., 2013a; Brikis et al., 2017, 2018; Zarei et al., 2017). MdGLYR1 is cytosolic, whereas MdGLYR2 is both plastidial and mitochondrial. Two of the six apple fruit FAD-dependent polyamine oxidases (MdPAO2,4) are peroxisomal and likely catalyze the back-conversion of Spm and Spd to Spd and Put, respectively (Brikis et al., 2018). Three of the five MdCuAOs identified are peroxisomal (MdCuAO1,4-5), but only one of these, MdCuAO1, has been shown to exclusively utilize diamines (diaminopropane, Put and cadaverine) as substrates (Zarei et al., 2015a; Brikis et al., 2018). A candidate plastidial diamine oxidase activity has not yet been identified. Two NAD+-dependent 4-aminobutanal dehydrogenases (ABALDH) exist in apple fruit (MdALDH10A8,9 or MdAMADH1,2): one is peroxisomal and the other plastidial (Zarei et al., 2015b,2016; Brikis et al., 2018).
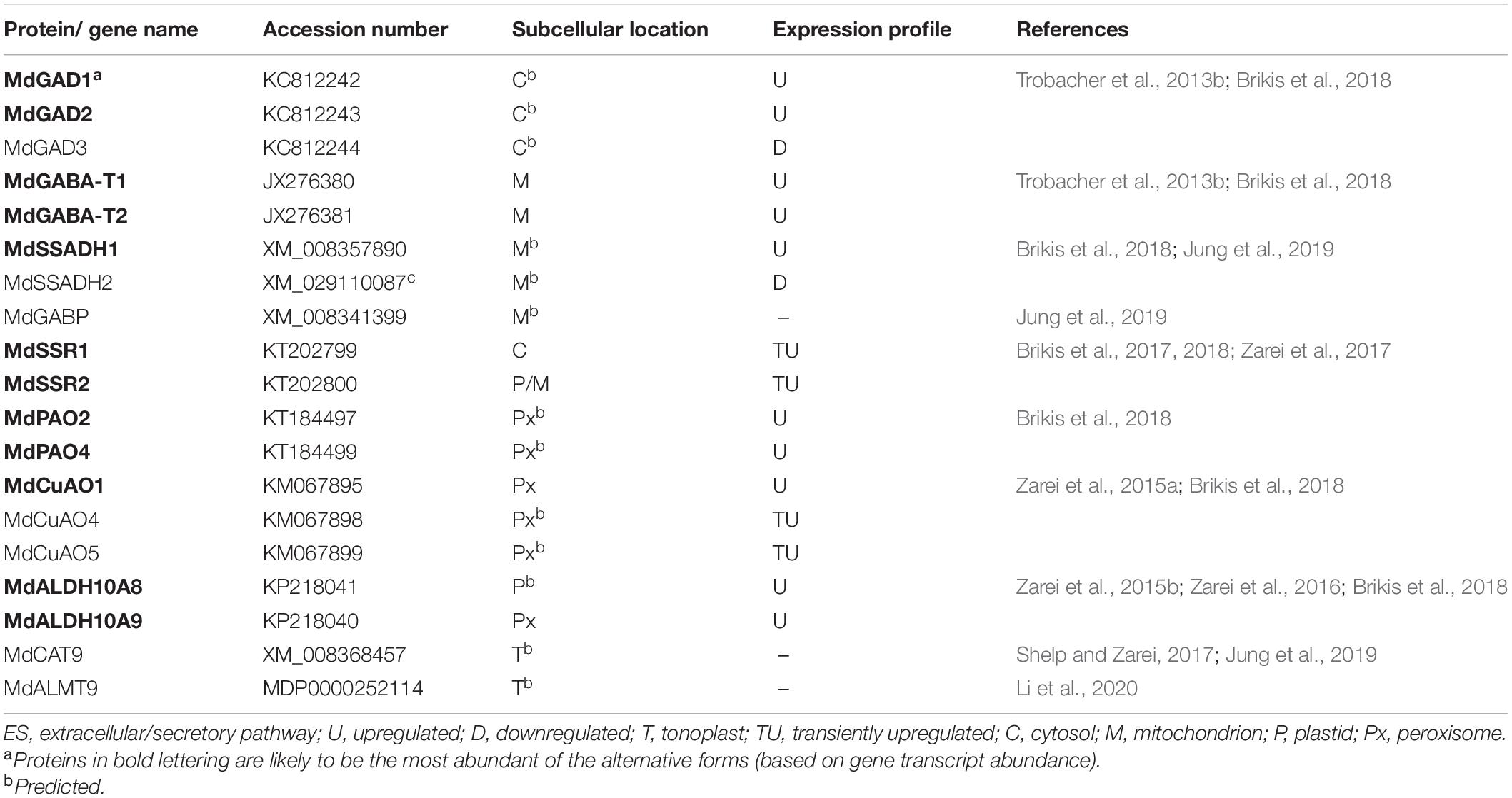
Table 2. Key proteins/genes of GABA metabolism and signaling in apple fruits subjected to low temperature, controlled atmosphere storage.
The temporal patterns of specific metabolites have been compared to the expression of genes encoding the most biochemically relevant proteins in intact “Empire” apple fruit stored under LT and low O2 with ambient or elevated CO2 (0°C, 2.5 kPa O2 and 0.03 or 5 kPa CO2) (Brikis et al., 2018; Table 2). Five kPa CO2 is known to elicit symptoms of external, but not internal, CO2-induced injury in this cultivar within 16 weeks (Deyman et al., 2014b). Under LT, low-O2 and ambient-CO2 storage, there is a transient increase in amino acid availability, including Glu, early in the storage period (2–4 weeks), probably a reflection of protein hydrolysis (Brikis et al., 2018). This is accompanied by a rapid peak in the expression of alanine transaminase (MdAla-T), a marker of hypoxia (Cukrov et al., 2016), as well as in the pool of alanine (2–4 weeks), which decline slowly to a steady basal level (from 8 to 16 weeks) (Brikis et al., 2018). A rapid accumulation of GABA is also transient (2–4 weeks), but the pool size is approximately 60% of that for alanine, suggesting that the alanine is derived from both Ala-T and GABA-TP reactions. Notably, MdGAD1 expression increases linearly up to 12 weeks and then remains steady, whereas MdGAD2 expression increases up to only 4 weeks and then decreases, and MdGAD3 expression decreases over the storage period. Succinate does not accumulate, but the burst of GABA is followed by a much smaller transient increase in GHB (Figure 1). Nevertheless, MdSSADH1 expression increases up to 8 weeks and then slowly declines, whereas MdSSADH2 expression decreases over the storage period. The expression of MdSSR1 is transiently increased, peaking at 4–8 weeks, and may be correlated with GHB. While Put, Spd and spermine (Spm) represent potential precursors for GABA, their levels are only 1–5% of that for GABA. Furthermore, the Put level declines only slightly with the increase in GABA, while Spd accumulates slightly and Spm dramatically declines. The expression of MdPAO2,4, MdCuAO1 and MdALDH10A8,9 rapidly increases, peaking after 8, 2 and 4–8 weeks, respectively, whereas the expression of MdCuAO4-5 is transiently increased, peaking at 4 weeks.
With LT, low-O2 and elevated-CO2 storage, a pronounced transient peak of GABA is accompanied by a strong transient peak of succinate, and smaller transient peaks of GHB, Put, Spd and Spm (Brikis et al., 2018). With prolonged storage, only GABA and GHB exhibit subsequent increases. These changes are accompanied by minor, yet significant, increases in the expression of MdGAD1, MdCuAO1 and MdALDH10A8,9. Thus, the GABA pattern might be interpreted as a CO2-induced shift from Glu/CaM-mediated stimulation/activation of GAD activity to H+-mediated stimulation of GAD activity (Trobacher et al., 2013b; Brikis et al., 2018). The patterns for succinate, GHB and Put might be explained by a combination of: elevated GABA production; differential effects of shifting redox balance on the activities of SSADH, TCAC, SSR, and ABALDH; and, limiting O2 availability for DAO activity in bulky apple fruit (Shelp et al., 2012b; Brikis et al., 2018). Based on changing pool sizes, we can estimate the maximum rates of GABA and succinate synthesis to be ∼50 nmol g–1 fresh mass (FM) wk–1, and the maximum rates of GHB synthesis and Put/Spd depletion to be ∼0.2 and ∼1.5 nmol g–1 FM wk–1, respectively. Thus, the terminal oxidation of PAs and the direct decarboxylation of Glu can account for approximately 3 and 97%, respectively, of GABA synthesis. Moreover, only 3% of the SSA is diverted from succinate to GHB production. Overall, this study suggests that both genetic and biochemical mechanisms are involved in the metabolism of GABA in apple fruit stored under LT, CA conditions.
Han et al. (2018) have monitored the expression of the GABA shunt enzymes and the levels of important metabolites in “Cripps Pink” apple fruit stored at RT in air for 70 days. The MdGADs exhibit different expression patterns, with MdGAD1 expression increasing gradually with time, MdGAD2 expression increasing until 30 days and then decreasing, and MdGAD3 expression decreasing. The expression of MdGABA-T1,2 and MdSSADH1 increases gradually from 0 to 30 days, peaking at the same time as the ethylene climacteric peak (30 days). Thus, the expression of MdGAD1, MdGAD2, MdGABA-T1,2, and MdSSADH1 in “Cripps Pink” apple fruit responds strongly under RT storage, essentially as in “Empire” apple fruit stored under LT, low O2 and ambient or elevated CO2 (Brikis et al., 2018). These findings, in conjunction with those of Brikis et al. (2018), lead us to conclude that the postharvest expression patterns for GABA shunt genes in apple fruits are more influenced by development, than by environment.
The temporal patterns for GABA (i.e., slow decrease of approximately 60% from 10 to 40 days, followed by a dramatic increase at 70 days, presumably due to fruit aging and cellular disintegration at the end of storage), succinate and malate (slow decrease of 40 and 20%, respectively, from 30 to 70 days) indicate that GABA does not accumulate under storage at RT, and that GABA is probably catabolized to succinate and malate (Han et al., 2018). The application of exogenous GABA increases the expression of MdGAD1, MdGAD2, MdGABA-T1,2 and MdSSADH1, restrains the decrease in malate and succinate levels, decreases respiration and ethylene production rates, and delays the ethylene production peak (Han et al., 2018; Table 3). Notably, the application of exogenous Ca2+ decreases the Glu level (before 30 days), increases the levels of GABA, succinate and malate (10–60 days) and expression of MdGAD1 (before 30 days), MdGAD2 (20–40 days), MdGABA-T1/2 (10–20 days) and MdSSADH (20–40 days), suppresses the respiration rate, and decreases the ethylene production peak (Han et al., 2021).
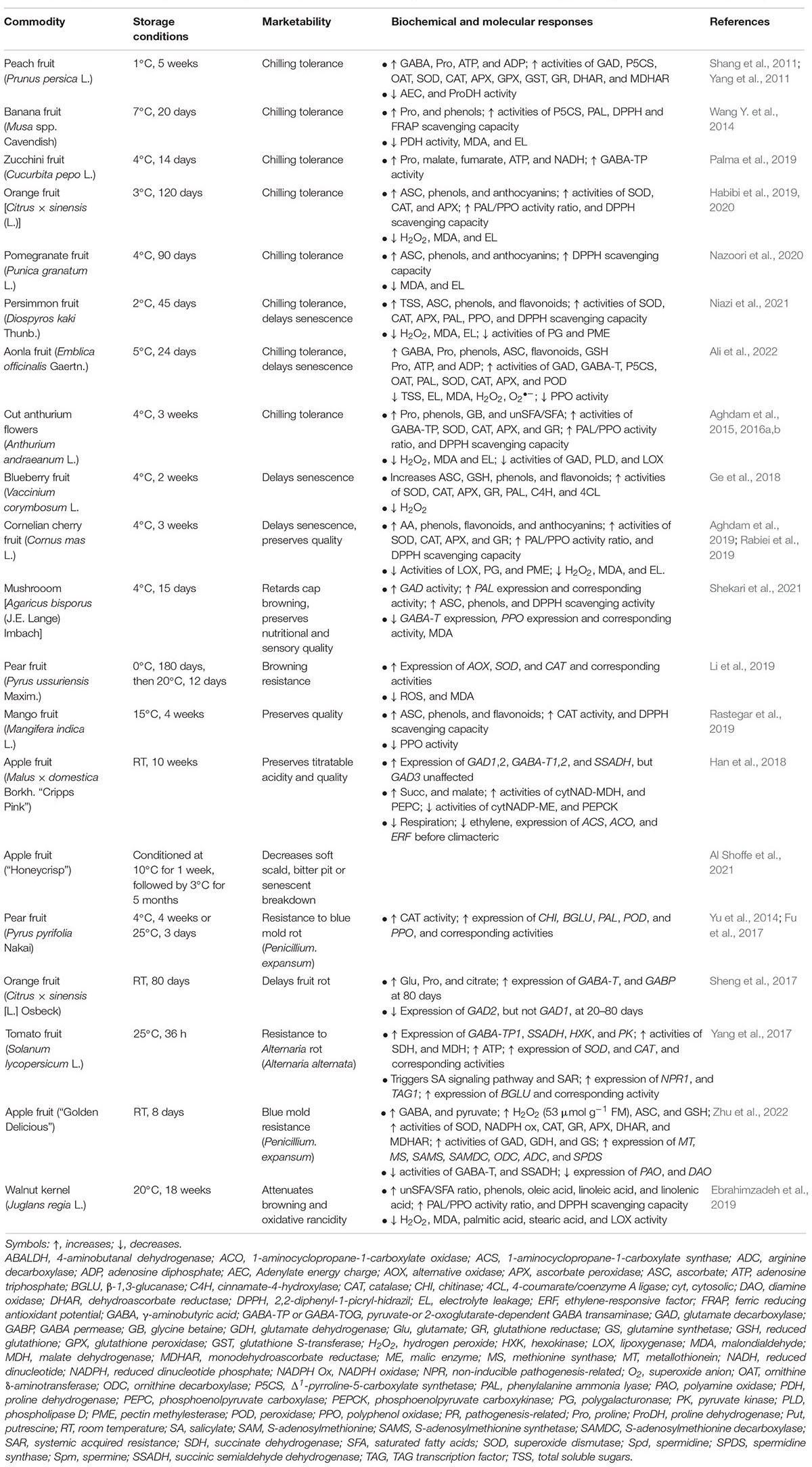
Table 3. The application of exogenous GABA improves the postharvest marketability of horticultural commodities by promoting GABA and antioxidant pathways.
Together, these studies suggest that elevated endogenous GABA or exogenous GABA maintains the quality of apple fruit by stimulating the activity of the GABA shunt and the synthesis of malate, and delaying fruit ripening. Notably, the inhibition of ethylene-mediated ripening by 1-methylcyclopropene increases the GABA level in “Empire” and “Honeycrisp” apples and in “AC Harrow Crisp” pears stored under LT, CA conditions (Deyman et al., 2014a; Lum et al., 2016a; Flaherty et al., 2018). While the interaction between ethylene and GABA biosynthesis requires further study, exogenous GABA seems to elicit similar responses as LT, CA conditions.
The attenuation of LT injury in peach, banana, orange, pomegranate, persimmon and aonla fruits, as well as cut anthurium flowers, by exogenous GABA is evident from the preservation of membrane fluidity and stability (decrease in electrolyte leakage), which is accompanied by decreases in reactive oxygen species (ROS; e.g., hydrogen peroxide and superoxide radical), greater antioxidant and radical-scavenging capacities, the maintenance of intracellular ATP and NADH, and the accumulation of potential osmolytes (i.e., soluble sugars, PAs and proline) (Shang et al., 2011; Yang et al., 2011; Wang Y. et al., 2014; Aghdam et al., 2015, 2016a,2016b; Habibi et al., 2019, 2020; Nazoori et al., 2020; Niazi et al., 2021; Ali et al., 2022; Table 3).
Similar mechanisms are involved in: the delay of senescence and preservation of quality in LT-stored blueberries, cherries and mushrooms, RT-stored apples, and conditioned LT-stored apples (Ge et al., 2018; Han et al., 2018; Aghdam et al., 2019; Rabiei et al., 2019; Al Shoffe et al., 2021; Shekari et al., 2021); browning resistance and the preservation of quality in LT-stored pear and mango (Li et al., 2019; Rastegar et al., 2019); resistance against fungal infection in LT-or RT-stored pear, orange, strawberry and tomato fruits (Yu et al., 2014; Fu et al., 2017; Sheng et al., 2017; Yang et al., 2017); and resistance against various pathogens and browning in RT-stored walnut kernels (Ebrahimzadeh et al., 2019; Table 3). Notably, pathogen resistance is promoted by salicylate signaling and disease resistance proteins, and maintaining the integrity of the cell wall barrier (Yu et al., 2014; Fu et al., 2017; Yang et al., 2017; Gao et al., 2018a; Zhao et al., 2021; Table 3), and the loss of apple fruit acidity is retarded by accumulating malate and suppressing ethylene biosynthesis (Han et al., 2018; Table 3).
Hou et al. (2022) have shown that the fresh-cut process does not affect the organoleptic quality of carrots stored under LT for hours, though it appears to enhance GABA biosynthesis from both Glu and PAs (Table 4). This result is consistent with the previously reported impact of wounding/mechanical damage on GABA accumulation (Shelp et al., 2012a). Notably, the resistance to browning and bacterial pathogens in fresh-cut pear, apple and potato during prolonged LT storage is improved by both CA and exogenous GABA via the mechanisms described above (Gao et al., 2018a,b; Wang D. et al., 2021; Zhao et al., 2021).
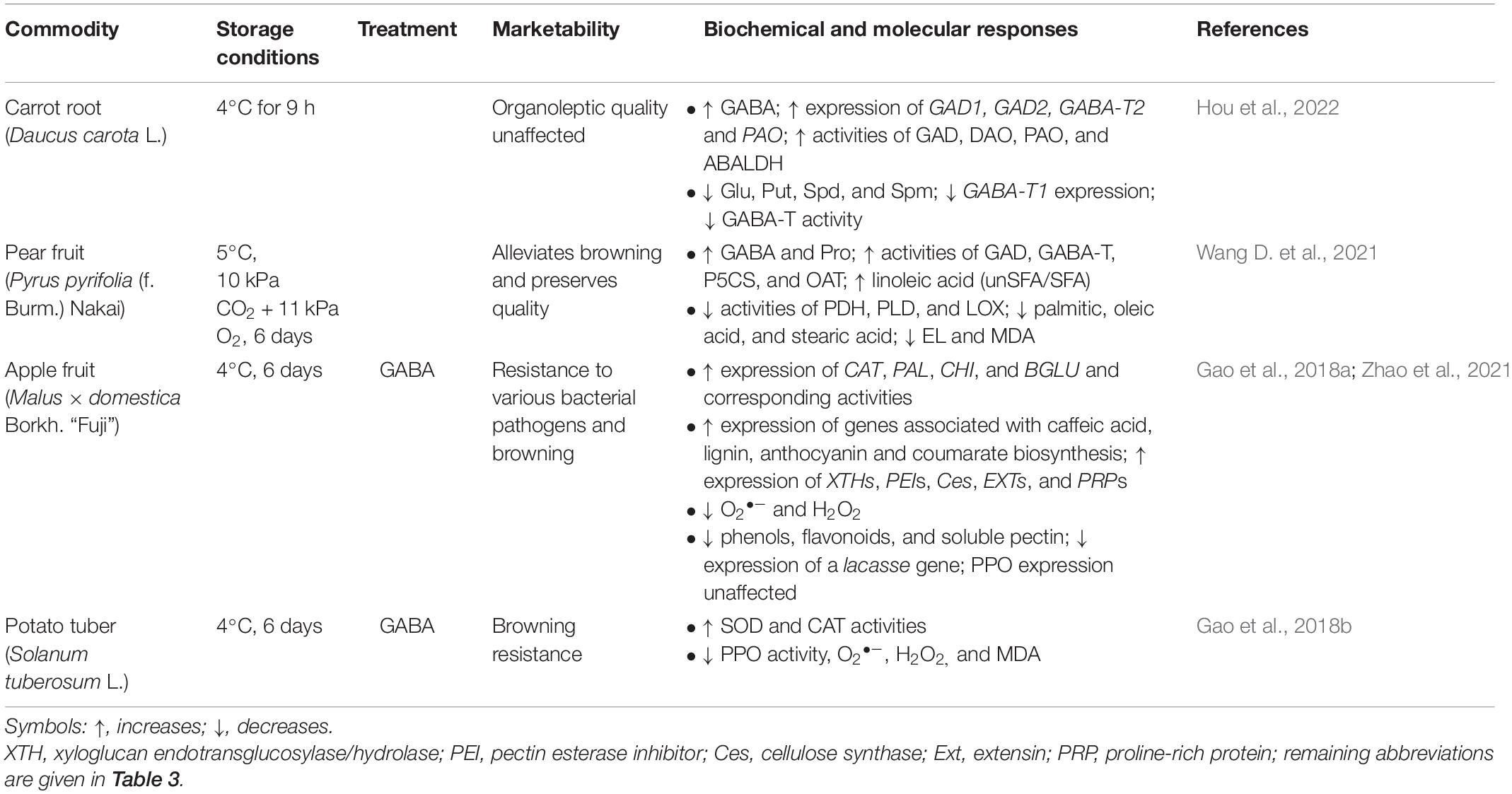
Table 4. The postharvest marketability of fresh-cut horticultural commodities is improved by low temperature, controlled atmosphere conditions or exogenous GABA.
In summary, the application of exogenous GABA to postharvest fruits, vegetables (including mushrooms), cut flowers, and walnuts delays senescence, attenuates chilling injury and fungal/bacterial-induced decay, and helps to preserve sensory and nutritional quality. GABA can promote activities of the GABA shunt, and the TCAC, antioxidant, secondary and phytohormone pathways, which in turn, reduce the stress-induced ROS level. However, the precise mechanisms whereby GABA interacts with other signaling molecules such as Ca2+, H2O2, PAs, salicylic acid, nitric oxide and melatonin, or with phytohormones such as ethylene, abscisic acid and auxin remain unknown (Bor and Turkan, 2019; Podlešákova et al., 2019; Seifikalhor et al., 2019; Suhel et al., 2022).
In cut flowers, excessive transpiration can result in a loss of turgor, premature wilting of flowers and leaves, and accelerate flower senescence. Water loss via the stomata can also result in a loss of FM and quality in leafy vegetables and immature green fruits. Therefore, it may be beneficial to manipulate endogenous GABA by applying exogenous GABA to restrict stomatal opening and prevent water loss (Xu et al., 2021a). Stomatal closure may also aid in preventing bacterial and fungal pathogens from entering leaves or fruits (Gahir et al., 2021). Thus, regulation of stomatal function may be a promising strategy for improving postharvest quality and safety of horticultural products (van Meeteren and Aliniaeifard, 2016).
Malate is the predominant organic acid in ripe apple fruit, and most of this is found in the vacuole. The transport of malate across the apple tonoplast is probably mediated by the apple ALMT9 (MdMa1) (Li et al., 2020; Table 2). Both the full-length protein, MdMa1, and its naturally occurring truncated protein, mdma1, localize to the tonoplast; when expressed in Xenopus laevis oocytes and Nicotiana benthamiana cells, MdMa1 mediates a malate-dependent inward-rectifying current, whereas the ma1-mediated transmembrane current is much weaker, indicating that ma1 has significantly lower malate transport activity than Ma1. RNA interference suppression of MdMa1 expression in “McIntosh” apple leaves, “Empire” apple fruit, and “Orin” apple calli significantly decreases the malate level. Notably, the most highly-related ortholog in Arabidopsis, ALMT9, transports mainly Cl– into the vacuole, but is subject to negative regulation by cytosolic GABA (Bown and Shelp, 2016; Xu et al., 2021a,b). Thus, the application of GABA on apple fruit during LT storage could reduce malate accumulation and the acidity of apple fruits. Bai et al. (2015) have suggested that a major network of genes, including MdALMT9, is associated with the developmental regulation of apple fruit acidity in “Golden Delicious,” but such a network has not been investigated during the ripening period (Ban and Xu, 2020). It could have implication for breeding apples or other fruits in order to preserve or enhance their flavor during postharvest storage.
Tomato SlCAT9 encodes a tonoplast Glu/Asp/GABA exchanger and its expression increases in tomato fruit during ripening (Snowden et al., 2015; Table 2). Such an exchanger might provide a mechanism for remobilizing GABA from the vacuole during cellular Glu uptake (Chung et al., 1992). On the other hand, ripening-specific overexpression of SlCAT9 increases the accumulation of GABA, Glu and Asp by approximately 20-, one- and sixfold, respectively (Snowden et al., 2015). Notably, greater Glu and Asp accumulation in the vacuole contribute to umami taste development in tomato fruit during ripening (Takayama and Ezura, 2015). Elevated GABA accumulation in the vacuole of immature fruit might deter insect pests and pathogens, whereas lower GABA accumulation in tomato fruit during ripening might be beneficial for attracting insects and animals for successful seed dispersal (Takayama and Ezura, 2015; Shelp et al., 2021). The properties of apple CAT9 have not yet been characterized (Table 2), but they could have implications for altering the development, flavor and biotic resistance of apple fruits.
Natural GABA is ubiquitous in plants and animals, and exogenous GABA is readily catabolized (Tuin and Shelp, 1994; Hijaz and Killiny, 2019; Oketch-Rabah et al., 2021). Nevertheless, the application of exogenous GABA to horticultural commodities during postharvest storage is likely to result in GABA accumulation. GABA is marketed worldwide as a dietary ingredient, food supplement and medicinal agent/drug. Available evidence suggests that GABA ingestion is not associated with adverse health events, probably due to the inability of GABA to cross the human blood–brain barrier (Boonstra et al., 2015; Oketch-Rabah et al., 2021). Also, GABA meets the statutory requirement of reasonable certainty of no harm to the environment (The United States Environmental Protection Agency, 2004).
Large scale commercial production of GABA would be necessary to support its use in postharvest storage of horticultural commodities. While chemical synthesis of GABA is feasible, this process requires expensive and hazardous reagents and generates unwanted by-products (Grewal, 2020; Oketch-Rabah et al., 2021). GABA can be formed from Glu using purified GAD and the coenzyme pyridoxal-5’-phosphate, but the purification of GAD is expensive and the enzyme tends to be unstable. The preferred manufacturing method for commercial production of GABA is fermentation by lactic acid bacteria because of their GRAS (Generally Recognized As Safe) status, high stress tolerance, and ability to release GABA into the extracellular matrix (Grewal, 2020; Jin et al., 2021; Laroute et al., 2021; Yogeswara et al., 2021).
Research on the postharvest physiological, biochemical, and molecular responses of horticultural commodities to LT and CA storage provides valuable information for conceiving new strategies to improve their marketability. These storage conditions are generally associated with the promotion of GABA pathway activity, with or without the accumulation of GABA, delaying senescence, preserving quality and ameliorating chilling injury. Induction and co-ordinated gene expression, together with the biochemical properties and subcellular location of the corresponding encoded proteins, suggest that MdGAD1,2, MdGABA-T1,2, MdSSADH1, MdCuAO1, and MdALDH10A8,9 are important determinants of GABA pathway activity in stored apple fruits, regardless of the storage condition. Notwithstanding, the targeted metabolite profiles suggest that protein hydrolysis, Ca2+/CaM activation or H+ stimulation of GAD activity, and changing redox balance are especially significant under LT, CA conditions. Furthermore, flux estimates suggest that the GABA pool is primarily derived from Glu, rather than PAs, and that SSA is converted mainly to succinate, rather than GHB.
Exogenous GABA is a promising strategy for promoting the level of endogenous GABA and the activity of the GABA shunt, which results in increased carbon flux through respiratory pathways, leading to elevated levels of NADH, NADPH and ATP (Aghdam et al., 2018, 2020; Shelp et al., 2021). Adequate ATP and NADPH are essential for: (i) fortifying the activity of ROS avoidance and scavenging systems; (ii) promoting the accumulation of endogenous proline and PAs; (iii) promoting the activity of secondary pathways, which results in the generation of salicylate for promoting the expression and activity of PR proteins, as well as phenols, flavonoids, and anthocyanins for scavenging radicals; (iv) limiting the activity of phospholipase D and lipoxygenase, resulting in increased membrane stability and fluidity; and (v) enhancing NADPH oxidase activity for triggering H2O2 accumulation. As a result, chilling injury and fungal/bacterial decay are deterred during postharvest storage, delaying senescence, preserving nutritional quality, and improving the postharvest marketability of horticultural crops. The occurrence of the tonoplastic ALMT presents the opportunity to restrict transpirational water loss by applying exogenous GABA to negatively regulate malate influx into the vacuole and light-induced stomatal opening in cut flowers and immature green fruit. Also, both the ALMT transporter and tonoplast CAT exchanger present the opportunity to manipulate fruit flavor. Available evidence suggests that exogenous GABA does not adversely affect human or environment health, though further optimization of microbial fermentation is probably necessary to ensure an adequate commercial supply of GABA for use as a biostimulant in the postharvest storage of horticultural commodities.
MA conceived and wrote the original manuscript, prepared original figures, and reviewed the revised manuscript. EF conducted the bioinformatics analysis, and reviewed the original and revised manuscripts. BS conceived and administered the project, and revised the original manuscript and figures. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Imam Khomeini International University (MA) (12006) and the Natural Sciences and Engineering Research Council of Canada (BS) (400-367). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghdam, M. S., Jannatizadeh, A., Luo, Z., and Paliyath, G. (2018). Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 76, 67–81. doi: 10.1016/j.tifs.2018.04.003
Aghdam, M. S., Kakavand, F., Rabiei, V., Zaare-Nahandi, F., and Razavi, F. (2019). γ-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation. Sci. Hortic. 246, 812–817. doi: 10.1016/j.scienta.2018.11.064
Aghdam, M. S., Naderi, R., Jannatizadeh, A., Babalar, M., Sarcheshmeh, M. A., and Faradonbe, M. Z. (2016a). Impact of exogenous GABA treatments on endogenous GABA metabolism in anthurium cut flowers in response to postharvest chilling temperature. Plant Physiol. Biochem. 106, 11–15. doi: 10.1016/j.plaphy.2016.04.045
Aghdam, M. S., Naderi, R., Jannatizadeh, A., Sarcheshmeh, M. A. A., and Babalar, M. (2016b). Enhancement of postharvest chilling tolerance of anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Sci. Hortic. 198, 52–60. doi: 10.1016/j.scienta.2015.11.019
Aghdam, M. S., Naderi, R., Sarcheshmeh, M. A. A., and Babalar, M. (2015). Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharv. Biol. Technol. 110, 70–76. doi: 10.1016/j.postharvbio.2015.06.020
Aghdam, M. S., Palma, J. M., and Corpas, F. J. (2020). NADPH as a quality footprinting in horticultural crops marketability. Tr. Food Sci. Technol. 103, 152–161. doi: 10.1016/j.tifs.2020.07.002
Akula, R., and Mukherjee, S. (2020). New insights on neurotransmitters signaling mechanisms in plants. Plant Signal. Behav. 15:1737450. doi: 10.1080/15592324.2020.1737450
Al Shoffe, Y., Nock, J. F., Zhang, Y., and Watkins, C. B. (2021). Pre- and post-harvest γ-aminobutyric acid application in relation to fruit quality and physiological disorder development in ‘Honeycrisp’ apples. Sci. Hortic. 289:110431. doi: 10.1016/j.scienta.2021.110431
Ali, S., Anjum, M. A., Nawaz, A., Ejaz, S., Anwar, R., Khaliq, G., et al. (2022). Postharvest γ-aminobutyric acid application mitigates chilling injury of aonla (Emblica officinalis Gaertn.) fruit during low temperature storage. Postharv. Biol. Technol. 185:111803. doi: 10.1016/j.postharvbio.2021.111803
Allan, W. L., Peiris, C., Bown, A. W., and Shelp, B. J. (2003). Gammahydroxybutyrate accumulates in green tea and soybean sprouts in response to oxygen deficiency. Can. J. Plant Sci. 83, 951–953. doi: 10.4141/p03-085
Bai, Y., Dougherty, L., Cheng, L., and Xu, K. (2015). A co-expression gene network associated with developmental regulation of apple fruit acidity. Mol. Genet. Genom. 290, 1247–1263. doi: 10.1007/s00438-014-0986-2
Ban, S., and Xu, K. (2020). Identification of two QTLs associated with high fruit acidity in apple using pooled genome sequencing analysis. Hortic. Res. 7:171. doi: 10.1038/s41438-020-00393-y
Blanch, M., Sanchez-Ballesta, M. T., Escribano, M. I., and Merodio, C. (2012). Water distribution and ionic balance in response to high CO2 treatments in strawberries (Fragaria vesca L. cv. Mara de Bois). Postharv. Biol. Technol. 73, 63–71. doi: 10.1016/j.postharvbio.2012.06.003
Boonstra, E., de Kleijn, R., Colzato, L. S., Alkemade, A., Forstmann, B. U., and Nieuwenhuis, S. (2015). Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front. Psychol. 6:1520. doi: 10.3389/fpsyg.2015.01520
Bor, M., and Turkan, I. (2019). Is there a room for GABA in ROS and RNS signalling? Environ. Exp. Bot. 161, 67–73. doi: 10.1016/j.envexpbot.2019.02.015
Bown, A. W., and Shelp, B. J. (2006). Gamma-aminobutyrate: defense against invertebrate pests. Trends Plant Sci. 11, 424–427. doi: 10.1016/j.tplants.2006.07.002
Bown, A. W., and Shelp, B. J. (2016). Plant GABA: not just a metabolite. Trends Plant Sci. 21, 811–813. doi: 10.1016/j.tplants.2016.08.001
Brikis, C. J., Zarei, A., Chiu, G. Z., Deyman, K. L., Liu, J., Trobacher, C. P., et al. (2018). Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic. Res. 5:61. doi: 10.1038/s41438-018-0069-3
Brikis, C. J., Zarei, A., Trobacher, C. P., DeEll, J. R., Akama, K., Mullen, R. T., et al. (2017). Ancient plant glyoxylate/succinic semialdehyde reductases: GLYR1s are cytosolic, whereas GLYR2s are localized to both mitochondria and plastids. Front. Plant Sci. 8:601. doi: 10.3389/fpls.2017.006
Carvajal, F., Palma, F., Jamilena, M., and Garrido, D. (2015). Preconditioning treatment induces chilling tolerance in zucchini fruit improving different physiological mechanisms against cold injury. Ann. Appl. Biol. 166, 340–354. doi: 10.1111/aab.12189
Chen, Q., Li, M.-S., Ding, W., Tao, M.-M., Li, M.-R., Qi, Q., et al. (2020). Effects of high N2/CO2 in package treatment on polyamine-derived 4-aminobutyrate (GABA) biosynthesis in cold-stored white mushrooms (Agaricus bisporus). Postharv. Biol. Technol. 162:111093. doi: 10.1016/j.postharvbio.2019.111093
Chen, Q., Zhang, Y., Tao, M., Li, M., Wu, Y., Qi, Q., et al. (2018). Comparative metabolic responses and adaptive strategies of tea leaves (Camellia sinensis) to N2 and CO2 anaerobic treatment by a nontargeted metabolomics approach. J. Agric. Food Chem. 66, 9565–9572. doi: 10.1021/acs.jafc.8b03067
Chiu, G. Z., Shelp, B. J., Bowley, S. R., DeEll, J. R., and Bozzo, G. G. (2015). Controlled atmosphere-related injury in ‘Honeycrisp’apples is associated with γ-aminobutyrate accumulation. Can. J. Plant Sci. 95, 879–886. doi: 10.4141/CJPS-2015-061
Chung, I., Bown, A. W., and Shelp, B. J. (1992). The production and efflux of 4-aminobutyrate in isolated mesophyll cells. Plant Physiol. 99, 659–664. doi: 10.1104/pp.99.2.659
Clark, S. M., Di Leo, R., Dhanoa, P. K., Van Cauwenberghe, O. R., Mullen, R. T., and Shelp, B. J. (2009a). Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 60, 1743–1757. doi: 10.1093/jxb/erp044
Clark, S. M., Di Leo, R., Van Cauwenberghe, O. R., Mullen, R. T., and Shelp, B. J. (2009b). Subcellular localization and expression of multiple tomato γ-aminobutyrate transaminases that utilize both pyruvate and glyoxylate. J. Exp. Bot. 60, 3255–3267. doi: 10.1093/jxb/erp161
Cukrov, D., Zermiani, M., Brizzolara, S., Cestaro, A., Licausi, F., Luchinat, C., et al. (2016). Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front. Plant Sci. 7:146. doi: 10.3389/fpls.2016.00146
Deewatthanawong, R., and Watkins, C. B. (2010). Accumulation of γ-aminobutyric acid in apple, strawberry and tomato fruit in response to postharvest treatments. Acta Hortic. 877, 947–952. doi: 10.17660/actahortic.2010.877.127
Deewatthanawong, R., Nock, J. F., and Watkins, C. B. (2010a). γ-Aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharv. Biol. Technol. 57, 92–96. doi: 10.1016/j.postharvbio.2010.03.003
Deewatthanawong, R., Rowell, P., and Watkins, C. B. (2010b). γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharv. Biol. Technol. 57, 97–105. doi: 10.1016/j.postharvbio.2010.03.007
Deyman, K. L., Brikis, C. J., Bozzo, G. G., and Shelp, B. J. (2014a). Impact of 1-methylcyclopropene and controlled atmosphere storage on polyamine and 4-aminobutyrate levels in “Empire” apple fruit. Front. Plant Sci. 5:144. doi: 10.3389/fpls.2014.00144
Deyman, K. L., Chiu, G., Liu, J., Brikis, C. J., Trobacher, C. P., DeEll, J. R., et al. (2014b). Effects of elevated CO2 and 1-methylcyclopropene on storage-related disorders of Ontario-grown ‘Empire’ apples. Can. J. Plant Sci. 94, 857–865. doi: 10.4141/CJPS-2014-040
Ebrahimzadeh, A., Pirzad, F., Tahanian, H., and Aghdam, M. S. (2019). Influence of gum arabic enriched with GABA coating on oxidative damage of walnut kernels. Food Technol. Biotechnol. 57, 554–560. doi: 10.17113/ftb.57.04.19.6380
Flaherty, E. J., Lum, G. B., DeEll, J. R., Subedi, S., Shelp, B. J., and Bozzo, G. G. (2018). Metabolic alterations in postharvest pear fruit as influenced by 1-methylcyclopropene and controlled atmosphere storage. J. Agric. Food Chem. 66, 12989–12999. doi: 10.1021/acs.jafc.8b04912
Fu, D., Sun, Y., Yu, C., Zheng, X., Yu, T., and Lu, H. (2017). Comparison of the effects of three types of aminobutyric acids on the control of Penicillium expansum infection in pear fruit. J. Sci. Food Agric. 97, 1497–1501. doi: 10.1002/jsfa.7891
Gahir, S., Bharath, P., and Raghavendra, A. S. (2021). Stomatal closure sets in motion long-term strategies of plant defense against microbial pathogens. Front. Plant Sci. 12:761952. doi: 10.3389/fpls.2021.761952
Gao, H., Wu, S., Zeng, Q., Li, P., and Guan, W. (2018a). Effects of exogenous γ-aminobutyric acid treatment on browning and food-borne pathogens in fresh-cut apples. Postharv. Biol. Technol. 146, 1–8. doi: 10.1016/j.postharvbio.2018.08.007
Gao, H., Zeng, Q., Ren, Z., Li, P., and Xu, X. (2018b). Effect of exogenous gamma-aminobutyric acid treatment on the enzymatic browning of fresh-cut potato during storage. J. Food Sci. Technol. 55, 5035–5044. doi: 10.1007/s13197-018-3442
Ge, Y., Duan, B., Li, C., Tang, Q., Li, X., Wei, M., et al. (2018). γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 240, 303–309. doi: 10.1016/j.scienta.2018.06.044
Godoy, F., Olivos-Hernández, K., Stange, C., and Handford, M. (2021). Abiotic stress in crop species: improving tolerance by applying plant metabolites. Plants 10, 186. doi: 10.3390/plants10020186
Grewal, J. (2020). Gamma-aminobutyric acid (GABA): a versatile bioactive compound. Eur. J. Mol. Clin. Med. 7, 3068–3075. doi: 10.1007/s00726-020-02885-6
Habibi, F., Ramezanian, A., Guillén, F., Serrano, M., and Valero, D. (2020). Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 306:125634. doi: 10.1016/j.foodchem.2019.125634
Habibi, F., Ramezanian, A., Rahemi, M., Eshghi, S., Guillén, F., Serrano, M., et al. (2019). Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 99, 6408–6417. doi: 10.1002/jsfa.9920
Han, S., Liu, H., He, Y., Nan, Y., Qu, W., and Rao, J. (2021). Effects of calcium treatment on malate metabolism and γ-aminobutyric acid (GABA) pathway in postharvest apple fruit. Food Chem. 334, 127479. doi: 10.1016/j.foodchem.2020.127479
Han, S., Nan, Y., Qu, W., He, Y., Ban, Q., Lv, Y., et al. (2018). Exogenous γ-aminobutyric acid treatment that contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage. J. Agric. Food Chem. 66, 13473–13482. doi: 10.1021/acs.jafc.8b04674
Hansen, M. E., Sørensen, H., and Cantwell, M. (2001). Changes in acetaldehyde, ethanol and amino acid concentrations in broccoli florets during air and controlled atmosphere storage. Postharv. Biol. Technol. 22, 227–237. doi: 10.1016/s0925-5214(01)00093-x
Hijaz, F., and Killiny, N. (2019). Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal. Behav. 14:e1573096. doi: 10.1080/15592324.2019.1573096
Hou, Y., Ren, H., Wang, K., Cao, S., Zheng, Y., Wei, Y., et al. (2022). Influence of fresh-cut process on γ-aminobutyric acid (GABA) metabolism and sensory properties in carrot. J. Food Sci. Technol. 59, 552–561. doi: 10.1007/s13197-021-05039-y
Jin, Y. H., Hong, J. H., Lee, J.-H., Yoon, H., Pawluk, A. M., Yun, S. J., et al. (2021). Lactic acid fermented green tea with Levilactobacillus brevis capable of producing γ-aminobutyric acid. Fermentation 7:110. doi: 10.3390/fermentation7030110
Jung, S., Lee, T., Cheng, C.-H., Buble, K., Zheng, P., Yu, J., et al. (2019). 15 years of GDR: new data and functionality in the genome database for rosaceae. Nucleic Acids Res. 47, D1137–D1145. doi: 10.1093/nar/gky1000
Koike, S., Matsukura, C., Takayama, M., Asamizu, E., and Ezura, H. (2013). Suppression of γ-aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol. 54, 793–807. doi: 10.1093/pcp/pct035
Kyriacou, M. C., and Rouphael, Y. (2018). Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 234, 463–469. doi: 10.1016/j.scienta.2017.09.046
Laroute, V., Mazzoli, R., Loubière, P., Pessione, E., and Cocaign-Bousquet, M. (2021). Environmental conditions affecting GABA production in Lactococcus lactis NCDO 2118. Microorganisms 9:122. doi: 10.3390/microorganisms9010122
Li, C., Dougherty, L., Coluccio, A. E., Meng, D., El-Sharkawy, I., Borejsza-Wysocka, E., et al. (2020). Apple ALMT9 requires a conserved C-terminal domain. Plant Physiol. 182, 992–1006. doi: 10.1104/pp.19.01300
Li, D., Li, L., Xiao, G., Limwachiranon, J., Xu, Y., Lu, H., et al. (2018). Effects of elevated CO2 on energy metabolism and gamma-aminobutyric acid shunt pathway in postharvest strawberry fruit. Food Chem. 265, 281–289. doi: 10.1016/j.foodchem.2018.05.106
Li, E., Luo, X., Liao, S., Shen, W., Li, Q., Liu, F., et al. (2018). Accumulation of γ-aminobutyric acid during cold storage in mulberry leaves. Int. J. Food Sci. Technol. 53, 2664–2672. doi: 10.1111/ijfs.13875
Li, J., Zhou, X., Wei, B., Cheng, S., Zhou, Q., and Ji, S. (2019). GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chem. 297, 124903. doi: 10.1016/j.foodchem.2019.05.177
Liao, J., Wu, X., Xing, Z., Li, Q., Duan, Y., Fang, W., et al. (2017). γ-Aminobutyric acid (GABA) accumulation in tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 65, 3013–3018. doi: 10.1021/acs.jafc.7b00304
Limami, A. M., Glévarec, G., Ricoult, C., Cliquet, J.-B., and Planchet, E. (2008). Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J. Exp. Bot. 59, 2325–2335. doi: 10.1093/jxb/ern102
Lum, G. B., Shelp, B. J., DeEll, J. R., and Bozzo, G. G. (2016b). Oxidative metabolism is associated with physiological disorders in fruits stored under multiple environmental stresses. Plant Sci. 245, 143–152. doi: 10.1016/j.plantsci.2016.02.005
Lum, G. B., Brikis, C. J., Deyman, K. L., Subedi, S., DeEll, J. R., Shelp, B. J., et al. (2016a). Pre-storage conditioning ameliorates the negative impact of 1-methylcyclopropene on physiological injury and modifies the response of antioxidants and γ-aminobutyrate in ‘Honeycrisp’ apples exposed to controlled-atmosphere conditions. Postharv. Biol. Technol. 116, 115–128. doi: 10.1016/j.postharvbio.2016.01.013
Lum, G. B., DeEll, J. R., Hoover, G. J., Subedi, S., Shelp, B. J., and Bozzo, G. G. (2017). 1-Methylcylopropene and controlled atmosphere modulate oxidative stress metabolism and reduce senescence-related disorders in stored pear fruit. Postharv. Biol. Technol. 129, 52–63. doi: 10.1016/j.postharvbio.2017.03.008
Mae, N., Makino, Y., Oshita, S., Kawagoe, Y., Tanaka, A., Aoki, K., et al. (2012). Accumulation mechanism of γ-aminobutyric acid in tomatoes (Solanum lycopersicum L.) under low O2 with and without CO2. J. Agric. Food Chem. 60, 1013–1019. doi: 10.1021/jf2046812
Makino, Y., Soga, N., Oshita, S., Kawagoe, Y., and Tanaka, A. (2008). Stimulation of γ-aminobutyric acid production in vine-ripe tomato (Lycopersicon esculentum Mill.) fruits under modified atmospheres. J. Agric. Food Chem. 56, 7189–7193. doi: 10.1021/jf801516e
Mei, X., Chen, Y., Zhang, L., Fu, X., Wei, Q., Grierson, D., et al. (2016). Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 6:23685. doi: 10.1038/srep23685
Mekonnen, D. W., Flügge, U.-I., and Ludewig, F. (2016). Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 245, 25–34. doi: 10.1016/j.plantsci.2016.01.005
Merodio, C., Muñoz, M. T., Cura, B. D., Buitrago, D., Escribano, M., and Isabel, I. A. (1998). Effect of high CO2 level on the titres of γ-aminobutyric acid, total polyamines and some pathogenesis-related proteins in cherimoya fruit stored at low temperature. J. Exp. Bot. 49, 1339–1347. doi: 10.1093/jexbot/49.325.1339
Nazoori, F., ZamaniBahramabadi, E., Mirdehghan, S. H., and Rafie, A. (2020). Extending the shelf life of pomegranate (Punica granatum L.) by GABA coating application. J. Food Meas. Charact. 14, 2760–2772. doi: 10.1007/s11694-020-00521-1
Niazi, Z., Razavi, F., Khademi, O., and Aghdam, M. S. (2021). Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 285:110198. doi: 10.1016/j.scienta.2021.110198
Oketch-Rabah, H. A., Madden, E. F., Roe, A. L., and Betz, J. M. (2021). United States pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients 13:2742. doi: 10.3390/nu13082742
Palma, F., Carvajal, F., Jamilena, M., and Garrido, D. (2014). Contribution of polyamines and other related metabolites to the maintenance of zucchini fruit quality during cold storage. Plant Physiol. Biochem. 82, 161–171. doi: 10.1016/j.plaphy.2014.06.001
Palma, F., Carvajal, F., Jiménez-Muñoz, R., Pulido, A., Jamilena, M., and Garrido, D. (2019). Exogenous γ-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 136, 188–195. doi: 10.1016/j.plaphy.2019.01.023
Podlešákova, K., Ugena, L., Spichal, L., Dolezal, K., and De Diego, N. (2019). Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. Nat. Biotechnol. 48, 53–65. doi: 10.1016/j.nbt.2018.07.003
Pott, D. M., de Abreu e Limab, F., Soria, C., Willmitzer, L., Fernie, A. R., Nikoloski, Z., et al. (2020). Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 321:126747. doi: 10.1016/j.foodchem.2020.126747
Rabiei, V., Kakavand, F., Zaare-Nahandi, F., Razavi, F., and Aghdam, M. (2019). Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 243, 268–273. doi: 10.1016/j.scienta.2018.08.034
Rastegar, S., Khankahdani, H. H., and Rahimzadeh, M. (2019). Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 14, 778–789. doi: 10.1007/s11694-019-00326-x
Rothan, C., Duret, S., Chevalier, C., and Raymond, P. (1997). Suppression of ripening-associated gene expression in tomato fruits subjected to a high CO2 concentration. Plant Physiol. 114, 255–263. doi: 10.1104/pp.114.1.255
Seifikalhor, M., Aliniaeifad, S., Hassani, B., Nikman, V., and Lastochkina, O. (2019). Diverse role of γ-aminobutyric acid in dynamic plant responses. Plant Cell Rep. 38, 847–867. doi: 10.1007/s00299-019-02396-z
Shang, H., Cao, S., Yang, Z., Cai, Y., and Zheng, Y. (2011). Effect of exogenous gamma-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 59, 1264–1268. doi: 10.1021/jf104424z
Shekari, A., Hassani, R. N., and Aghdam, M. S. (2021). Exogenous application of GABA retards cap browning in Agaricus bisporus and its possible mechanism. Postharv. Biol. Technol. 174:111434. doi: 10.1016/j.postharvbio.2020.111434
Shelp, B. J., Aghdam, M. S., and Flaherty, E. J. (2021). γ-Aminobutyrate (GABA) regulated plant defense: mechanisms and opportunities. Plants 10:1939. doi: 10.3390/plants10091939
Shelp, B. J., Bozzo, G. G., Trobacher, C. P., Zarei, A., Deyman, K. L., and Brikis, C. J. (2012b). Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 193–194, 130–135. doi: 10.1016/j.plantsci.2012.06.001
Shelp, B. J., Bozzo, G. G., Trobacher, C. P., Chiu, G., and Bajwa, V. S. (2012a). Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. I. Pathway structure. Botany 90, 651–668. doi: 10.1139/B2012-030
Shelp, B. J., Bozzo, G. G., Zarei, A., Simpson, J. P., Trobacher, C. P., and Allan, W. L. (2012c). Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. II. Integrated analysis. Botany 90, 781–793. doi: 10.1139/B2012-041
Shelp, B. J., and Zarei, A. (2017). Subcellular compartmentation of 4-aminobutyrate (GABA) metabolism in arabidopsis: An update. Plant Signal. Behav. 12:e1322244. doi: 10.1080/15592324.2017.1322244
Sheng, L., Shen, D., Luo, Y., Sun, X., Wang, J., Luo, T., et al. (2017). Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 216, 138–145. doi: 10.1016/j.foodchem.2016.08.024
Shimajiri, Y., Ozaki, K., Kainou, K., and Akama, K. (2013). Differential subcellular localization, enzymatic properties and expression patterns of γ-aminobutyric transaminases (GABA-T) in rice (Oryza sativa). J. Plant Physiol. 170, 196–201. doi: 10.1111/pbi.12050
Snedden, W. A., Arazi, T., Fromm, H., and Shelp, B. J. (1995). Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol. 108, 543–549. doi: 10.1104/pp.108.2.543
Snowden, C. J., Thomas, B., Baxter, C. J., Smith, J. A. C., and Sweetlove, L. J. (2015). A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 81, 651–660. doi: 10.1111/tpj.12766
Suhel, M., Husain, T., Pandey, A., Singh, S., Dubey, N. K., Prasad, S. M., et al. (2022). An appraisal of ancient molecule GABA in abiotic stress tolerance in plants, and its crosstalk with other signaling molecules. J. Plant Growth Regul. doi: 10.1007/s00344-022-10610-8
Takayama, M., and Ezura, H. (2015). How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 6:612. doi: 10.3389/fpls.2015.00612
Tarkowski, K. P., Signorelli, S., and Höfte, M. (2020). γ-Aminobutyric acid and related amino acids in plant immune responses: emerging mechanisms of action. Plant Cell Environ. 43, 1103–1116. doi: 10.1111/pce.13734
The United States Environmental Protection Agency (2004). L-Glutamic Acid and Gamma Aminobutyric Acid: Order Denying Objections to Issuance of Tolerance. 40 CFR Part 180, Vol. 69. Washington, DC: EPA.
Trobacher, C. P., Clark, S. M., Bozzo, G. G., Mullen, R. T., DeEll, J. R., and Shelp, B. J. (2013a). Catabolism of GABA in apple fruit: Subcellular localization and biochemical characterization of two γ-aminobutyrate transaminases. Postharv. Biol. Technol. 75, 106–113. doi: 10.1016/j.postharvbio.2012.08.005
Trobacher, C. P., Zarei, A., Liu, J., Clark, S. M., Bozzo, G. G., and Shelp, B. J. (2013b). Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 13:144. doi: 10.1186/1471-2229-13-144
Tuin, L. G., and Shelp, B. J. (1994). In situ [14C]glutamate metabolism by developing soybean cotyledons I. Metabolic routes. J. Plant Physiol. 143, 1–7. doi: 10.1016/s0176-1617(11)82089-4
Van Cauwenberghe, O. R., and Shelp, B. J. (1999). Biochemical characterization of partially purified GABA:pyruvate transaminase from Nicotiana tabacum. Phytochemistry 52, 575–581. doi: 10.1016/s0031-9422(99)00301-5
van Meeteren, U., and Aliniaeifard, S. (2016). “Stomata and postharvest physiology,” in Postharvest Ripening Physiology of Crops, ed. S. Pareek (Boca Raton, FL: CRC Press), 157–191.
Wang, C., Fan, L., Gao, H., Wu, X., Li, J., and Lv, G. (2014). Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia stressed melon roots. Plant Physiol. Biochem. 82, 17–26. doi: 10.1016/j.plaphy.2014.04.018
Wang, D., Li, D., Xu, Y., Li, L., Belwal, T., Zhang, X., et al. (2021). Elevated CO2 alleviates browning development by modulating metabolisms of membrane lipids, proline, and GABA in fresh-cut Asian pear fruit. Sci. Hortic. 281:109932. doi: 10.1016/j.scienta.2021.109932
Wang, L., Wang, Y., Hou, Y., Zhu, X., Zheng, Y., and Jin, P. (2021). Physiological and metabolomic analyses of hot water treatment on amino acids and phenolic metabolisms in peach cold tolerance. Postharv. Biol. Technol. 179:111593. doi: 10.1016/j.postharvbio.2021.111593
Wang, Y., Luo, Z., Huang, X., Yang, K., Gao, S., and Du, R. (2014). Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 168, 132–137. doi: 10.1016/j.scienta.2014.01.022
Xu, B., Sai, N., and Gilliham, M. (2021b). The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 187, 2005–2016. doi: 10.1093/plphys/kiab347
Xu, B., Long, Y., Feng, X., Zhu, X., Sai, N., Chirkova, L., et al. (2021a). GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilence. Nat. Commun. 12:1952. doi: 10.1038/s41467-021-21694-3
Yang, A., Cao, S., Yang, Z., Cai, Y., and Zheng, Y. (2011). γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 129, 1619–1622. doi: 10.1016/j.foodchem.2011.06.018
Yang, J., Sun, C., Zhang, Y., Fu, D., Zheng, X., and Yu, T. (2017). Induced resistance in tomato fruit by gamma-aminobutyric acid for the control of alternaria rot caused by Alternaria alternata. Food Chem. 221, 1014–1020. doi: 10.1016/j.foodchem.2016.11.061
Yogeswara, I. B. A., Kittibunchakul, S., Rahayu, E. S., Domig, K. J., Haltrich, D., and Nguyen, T. H. (2021). Microbial production and enzymatic biosynthesis of γ-aminobutyric acid (GABA) using Lactobacillus plantarum FNCC 260 isolated from Indonesian fermented foods. Processes 9:22. doi: 10.3390/pr9010022
Yu, C., Zeng, L., Sheng, K., Chen, F., Zhou, T., Zheng, X., et al. (2014). γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 159, 29–37. doi: 10.1016/j.foodchem.2014.03.011
Zarei, A., Brikis, C. J., Bajwa, V. S., Chiu, G. Z., Simpson, J. P., DeEll, J. R., et al. (2017). Plant glyoxylate/succinic semialdehyde reductases: comparative biochemical properties, function during chilling stress, and subcellular localization. Front. Plant Sci. 8:1399. doi: 10.3389/fpls.2017.01399
Zarei, A., Trobacher, C. P., Cooke, A. R., Meyers, A. J., Hall, J. C., and Shelp, B. J. (2015a). Apple fruit copper amine oxidase isoforms: peroxisomal MdAO1 prefers diamines as substrates, whereas extracellular MdAO2 exclusively utilizes monoamines. Plant Cell Physiol. 56, 137–147. doi: 10.1093/pcp/pcu155
Zarei, A., Trobacher, C. P., and Shelp, B. J. (2015b). NAD+-aminoaldehyde dehydrogenase candidates for 4-aminobutyrate (GABA) and β-alanine production during terminal oxidation of polyamines in apple fruit. FEBS Lett. 589, 2695–2700. doi: 10.1016/j.febslet.2015.08.005
Zarei, A., Trobacher, C. P., and Shelp, B. J. (2016). Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci. Rep. 6:35115. doi: 10.1038/srep35115
Zhao, P., Li, W., Zhen, C., Wang, K., Qin, Z., and Gao, H. (2021). Transcriptomic analysis of the effects of γ-aminobutyric acid treatment on browning and induced disease resistance in fresh-cut apples. Postharv. Biol. Technol. 181:111686. doi: 10.1016/j.postharvbio.2021.111686
Zhou, M., Ndeurumio, K. H., Zhao, L., and Hu, Z. (2016). Impact of precooling and controlled-atmosphere storage on γ-aminobutyric acid (GABA) accumulation in longan (Dimocarpus ongan Lour.) fruit. J. Agric. Food Chem. 64, 6443–6450. doi: 10.1021/acs.jafc.6b01738
Zhu, J., Li, C., Sun, L., Cheng, Y., Hou, J., Fan, Y., et al. (2022). Application of γ-aminobutyric acid induces disease resistance in apples through regulation of polyamine metabolism, GABA shunt and reactive oxygen species metabolism. Sci. Hortic. 291:110588. doi: 10.1016/j.scienta.2021.110588
Keywords: γ-aminobutyrate, biostimulants, horticultural commodities, marketability, postharvest stress
Citation: Aghdam MS, Flaherty EJ and Shelp BJ (2022) γ-Aminobutyrate Improves the Postharvest Marketability of Horticultural Commodities: Advances and Prospects. Front. Plant Sci. 13:884572. doi: 10.3389/fpls.2022.884572
Received: 26 February 2022; Accepted: 11 April 2022;
Published: 25 May 2022.
Edited by:
María Serrano, Miguel Hernández University of Elche, SpainReviewed by:
Milan Skalicky, Czech University of Life Sciences Prague, CzechiaCopyright © 2022 Aghdam, Flaherty and Shelp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barry J. Shelp, YnNoZWxwQHVvZ3VlbHBoLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.