
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 29 April 2022
Sec. Crop and Product Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.874338
The involvement of PpCBF6 in phytosulfokine α (PSKα)-ameliorated chilling injury (CI) by suppressing the expression of lipoxygenase 5 (LOX5) in peach fruit was revealed. The peaches were immersed in distilled water and PSKα solution. PSKα application inhibited the progression of CI index and weight loss, and the reduction of firmness and total soluble solids content in peaches. The endogenous PSKα accumulation and gene expression of PSK receptor 1 (PSKR1) and PSKR2 were up regulated by PSKα application. The superoxide anion (O2–) production rate, hydrogen peroxide (H2O2) production and reactive oxygen species (ROS) content decreased by PSKα application. Furthermore, PSKα application reduced the gene expression of 12 PpLOXs and LOX activity. The gene expression of 6 PpCBFs was enhanced by PSKα application. Importantly, after PSKα application, among 12 PpLOXs, the decrease in gene expression of PpLOX5 was the lowest, and among 6 PpCBFs, the increase in gene expression of PpCBF6 was the highest. Further results suggested that PpCBF6 bound to the C-repeat/dehydration responsive element (CRT/DRE) motif in PpLOX5 promoter, and repressed its transcription. Thus, PpCBF6 was involved in the PSKα-retarded CI by inhibiting the expression of PpLOX5 in peaches.
Refrigeration is commonly employed to maintain fruit quality and to extend storage period (Belay et al., 2020). However, peaches are susceptible to chilling injury (CI) when subjected to cold stress (Nilo et al., 2010). The main CI symptom in peaches is internal browning (Chen et al., 2019), which causes certain economic losses. Thus, it is vital to hunt for potent approaches to relieve CI in peaches.
Phytosulfokine α (PSKα), a plant sulfonated pentapeptide growth regulator, has been verified to motivate many biochemical activities (Aghdam and Luo, 2021a). PSKα perception by leucine-rich repeat PSK receptor (PSKR) kinase in plasma membrane is vital for triggering a series of physiological reactions (Aghdam et al., 2021b). Accordingly, PSKα application promoted energy status and scavenged reactive oxygen species (ROS) overproduction, and thus retarded senescence in broccoli florets throughout cold storage (Aghdam and Luo, 2021b). PSKα treatment boosted cyclic guanosine monophosphate accumulation and ameliorated membrane damage, therefore delaying petals senescence and extending vase life of cut rose flowers (Aghdam et al., 2021a). PSKα treatment retarded senescence in strawberries by inducing the phenylpropanoid pathway throughout cold storage (Aghdam et al., 2021b). However, whether PSKα treatment could relieve CI in peaches remains to be explored.
Cold storage would result in the elevation of lipoxygenase (LOX) level in postharvest fruit (Sheng et al., 2016). LOX catalyzes the hydroperoxidation of unsaturated fatty acids to produce hydroperoxide and a lot of ROS, leading to cell membrane damage and browning. Therefore, to inhibit CI in cold sensitive fruit, it is critical to apply potent measures to reduce LOX level. Accordingly, PSKα treatment could retard the increase in gene expression and activity of LOX in broccoli florets (Aghdam and Flores, 2021c), indicating that PSKα application is an effective technology to relieve cell membrane damage in postharvest vegetable. However, the effects of PSKα treatment on LOX level in peaches remain to be revealed.
Besides, the motivation of transcriptional factors (TFs) is an important defense response to cold stress in postharvest fruit (Peng et al., 2020; Jiao, 2021). C-repeat binding factors (CBFs) are the most widely investigated cold resistance TFs (Xiao et al., 2010; Liang et al., 2013). Transcription level of CBF1 in tomatoes was positively correlated with chilling tolerance, and negatively correlated with CI severity, suggesting that CBFs can effectively reflect chilling tolerance in fruit (Arae et al., 2017). Moreover, CBFs could be activated by lots of exogenous applications in plants. Methyl jasmonate treatment promoted the gene expression of CBF6, and thus enhanced the chilling tolerance in peaches (Cao et al., 2021). NO treatment enhanced the tolerance to cold stress by up regulating CBF1 expression in kiwifruit (Jiao, 2021). However, the modulation of CBFs by PSKα treatment remains to be explored.
Moreover, CBFs modulate the expression of downstream genes by binding to the C-repeat/dehydration responsive element (CRT/DRE) motif (CCGAC) in their promoters (Cao et al., 2021). Accordingly, CBF3 bound to the CRT/DRE motif in the promoter of ureidoglycolate amidohydrolase, and enhanced its expression in rice (Li et al., 2015). CBF6 suppressed the expression of vacuolar invertase by interacting with the CRT/DRE-binding site in its promoter, and thus retarded CI in peaches (Cao et al., 2021). However, the regulation of LOXs by CBFs in peaches remains to be investigated.
The aims of this research were to explore the effects of PSKα application on the decrease in CI severity and the induction of PpLOX5 and PpCBF6, and the modulation of PpLOX5 promoter by PpCBF6 in peaches.
Peaches (Prunus persica Batsch cv. ‘Yuhua No. 3’) were obtained at 80% ripeness from the orchard in Nanjing, China. The chosen uniform peaches absence of visual defects were divided into two groups each of three biological replicates at random. For each biological replicate of each group, 400 fruit were used.
(1) Control (CK): The peaches were immersed in distilled water.
(2) PSKα: The peaches were immersed in 300 nM PSKα.
The PSKα concentration was determined according to my preliminary experiments (Supplementary Figure 1). The aforementioned fruit were immersed for 10 min and air dried for 40 min thereafter. The peaches were stored at 4 ± 1°C for 35 days under 80–90% relative humidity afterward. For each biological replicate, 30 peaches were used to determine the CI degree, weight loss, firmness, and total soluble solids content every 7 days. For each biological replicate, 20 peaches were used to detect the physiological indicators (O2– production rate, H2O2 production, ROS content, LOX activity, and gene expression of PpLOXs and PpCBFs) every 7 days, which were preserved at −80°C. For CI severity calculation, peaches were removed to 20°C for 3 days after each time point. Other indicators were evaluated immediately after each time point.
For CI index calculation, the severity of internal browning in each fruit was recorded: 0 = none, 1 ≤ 5%, 2 = 6–25%, 3 = 26–50%, 4 ≥ 50%. CI index = Σ (CI severity × number of peaches at the CI severity)/(5 × total number of peaches in the replicate).
For weight loss assay, the peaches were weighed before and after each time point.
Firmness was determined using the firmness analyzer (FT327, Effegi, Alfonsine, Italy) with a probe of a 7.5 mm penetration depth.
For total soluble solids content determination, 5.0 g peach samples were ground, and centrifuged at 9,000 g for 15 min. The collected supernatant was assayed using WYT-4 hand-held refractometer (Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China) afterward.
Five gram of peach samples were incubated with 8 M urea for 60 min at 4°C as described by Song et al. (2017). After pH was adjusted to 2.0–3.0 using 1 M HCl, the homogenate was incubated for 15 min at 4°C, followed by a centrifugation at 12,000 g for 30 min at 4°C. The endogenous PSKα accumulation was detected using the protocol of Aghdam et al. (2021b).
For O2– production rate detection, 5.0 g peach samples were homogenized in 50 mM phosphate buffer (pH 7.8), followed by a centrifugation at 11,000 g for 20 min at 4°C thxereafter. The O2– production rate was measured following the protocol of Wang et al. (2020). The data were expressed as mmol/kg on a fresh weight (FW) basis.
For H2O2 production measurement, 5.0 g peach samples were homogenized using cold acetone, followed by a centrifugation at 11,000 g for 20 min at 4°C thereafter. The H2O2 production was assayed using the protocol of Yang et al. (2016). The data were expressed as μmol/kg on a FW basis.
The ROS content was assayed using fluorescence spectrophotometer (Cary Eclipse, VARIAN, United States) using the protocol of Jing et al. (2016). The maximum excitation and emission wavelengths were 485 and 530 nm, respectively. The slit width was 5 nm. The data were expressed as a.u./mg on a FW basis.
The total RNA was extracted according to the protocol of MiniBEST Plant RNA Extraction Kit (Takara Bio Inc., China). After purified, the total RNA was used for cDNA library construction. The clean reads were mapped to peach genome. The read numbers were transformed to FPKM (Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced) value for gene expression quantification. The differentially expressed genes was analyzed using edgeR with following criteria: False discovery rate (FDR) < 0.05 and |log2Fold change| ≥ 1. Three biological replicates were included for each assay.
Five gram of peach samples were extracted in 0.1 M phosphate buffer (pH 6.8) containing 1% (w/v) polyvinylpyrrolidone, and centrifuged at 10,000g for 15 min at 4°C thereafter. LOX activity was determined using the protocol of Yao et al. (2019). The data were expressed as U/g on a FW basis.
Total RNA in peach samples was acquired following the protocol of E.Z.N.A.™ Plant RNA Kit (Omega, United States). The first-strand cDNA was synthesized using the protocol of Jiao (2021) thereafter. The primers in quantitative real-time polymerase chain reaction (qRT-PCR) tests for PpPSKR1, PpPSKR2, 12 PpLOXs, 6 PpCBFs, and β-actin were designed (Table 1). The gene expression was assayed according to the protocol of Jiao (2021). Three replicates were included for each assay.
A yeast one-hybrid (Y1H) assay was performed using the protocol of the Clontech ® Matchmaker ® one-hybrid system. The three tandem copies of the CRT/DRE-binding site (CCGAC) (-405 to -401 bp) and adjacent nucleotides in the PpLOX5 promoter (Supplementary Text 1) were ligated into pAbAi vector. The PpLOX5-AbAi and p53-AbAi were introduced into the Y1H Gold strain thereafter. Positive yeast cells were transformed with the pGADT7-AD, which contained the coding sequences (CDS) of PpCBF6. The basal activity of the promoter was detected on the SD medium lacking Ura with Aureobasidin A (AbA). Whether PpCBF6 could bind to PpLOX5 promoter was judged by the growth status of co-transformants on SD/-Leu medium in the presence of AbA.
The sequences of PpLOX5 promoter were inserted into the pGreen II 0800-LUC vector. The CDS of PpCBF6 were inserted into the pGreen II 62-SK vector. The plasmids were introduced into Agrobacterium tumefaciens strain GV3101, and transiently expressed in tobacco thereafter. After 3 days, LUC and REN were determined using the dual luciferase reporter (DLR) assay system (Promega). Nine independent replicates were conducted for each combination. The data were expressed as the relative LUC/REN ratio.
Statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL, United States). Each data was analyzed with one-way analysis of variance. The significant differences at p < 0.05 were determined using Duncan test.
The CI disorder in peaches was initially found at 14 days. The CI index and weight loss continuously increased following control and PSKα treatments throughout storage. PSKα treatment markedly delayed the increase in CI index and weight loss. Following 35 days of storage, PSKα treatment caused the decrease in CI index and weight loss by 35 and 23% (Figures 1A,B).
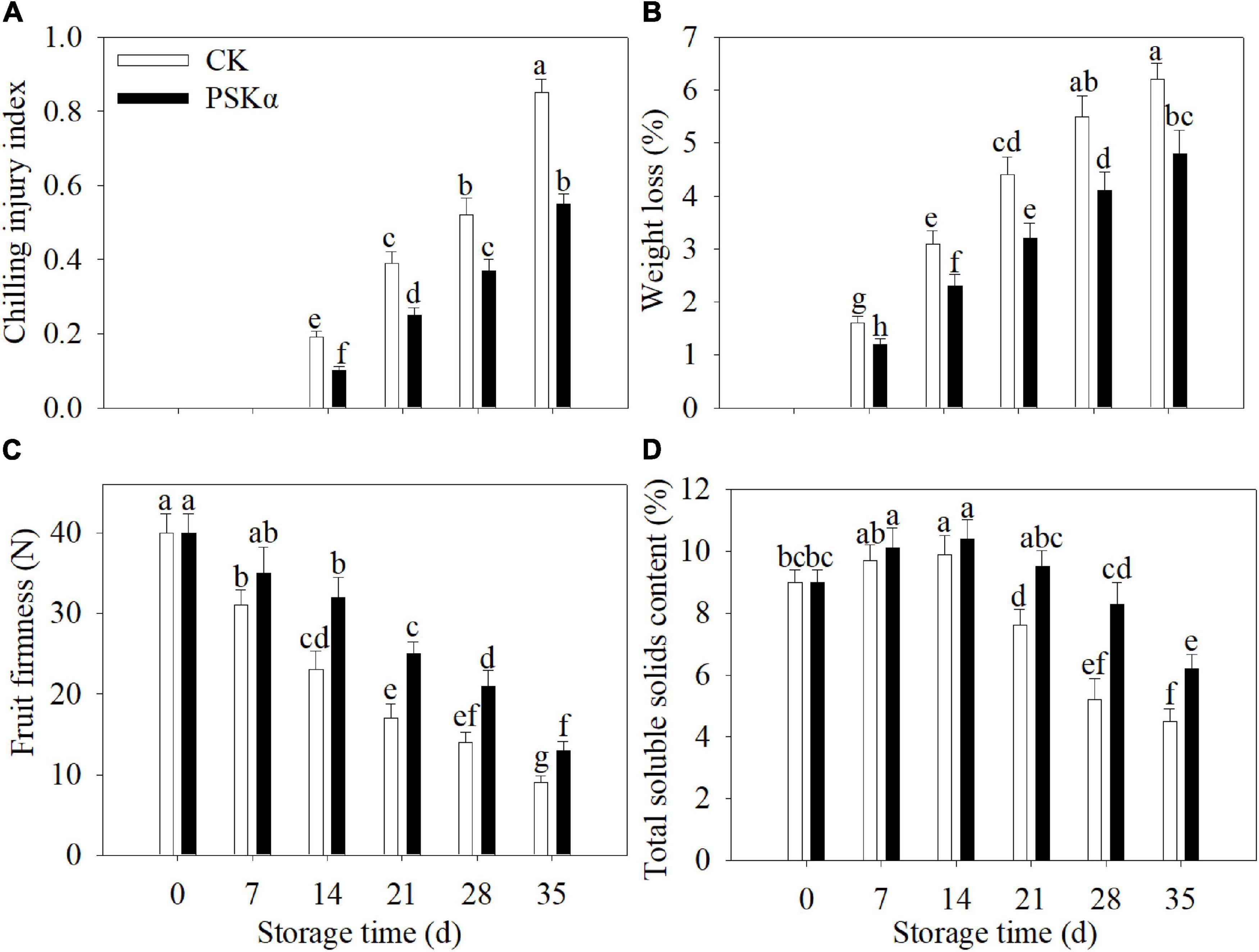
Figure 1. PSKα application reduced the CI degree (A) and weight loss (B) and maintained the firmness (C) and total soluble solids content (D) in peaches. The CI degree (A) was analyzed after peaches were removed to 20°C for 3 days after each time point. The weight loss (B), firmness (C), and total soluble solids content (D) were detected immediately after each time point. For each biological replicate, after each time point, 30 peaches were used to determine the CI degree (A) and weight loss (B) and maintained the firmness (C) and total soluble solids content (D). Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
The firmness continuously decreased in peaches following control and PSKα treatments throughout storage. PSKα treatment markedly delayed the decrease in firmness. Following 35 days of storage, PSKα treatment boosted the firmness by 44% (Figure 1C).
The total soluble solids content increased firstly and decreased thereafter in peaches following control and PSKα treatments throughout storage. PSKα treatment markedly retarded the decrease in total soluble solids content. Following 35 days of storage, PSKα treatment caused the elevation of total soluble solids content by 38% (Figure 1D).
The endogenous PSKα accumulation and gene expression of PSKR1 and PSKR2 increased firstly and decreased thereafter following control and PSKα applications throughout storage in peaches. PSKα application promoted the endogenous PSKα accumulation and gene expression of PSKR1 and PSKR2. Following 35 d of storage, the endogenous PSKα accumulation and gene expression of PSKR1 and PSKR2 in PSKα-immersed peaches was 2.5, 2.4, and 1.6 times of the control (Figure 2).
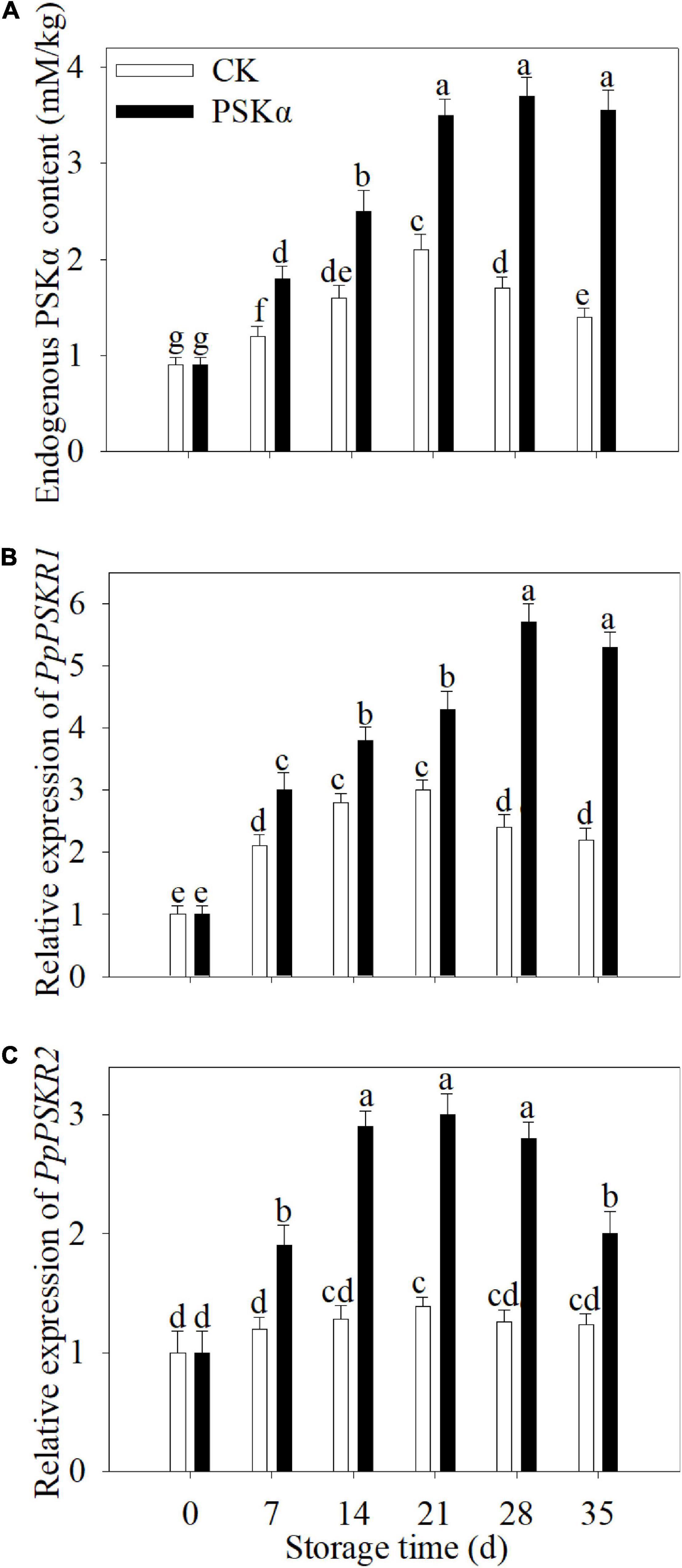
Figure 2. PSKα application elevated the endogenous PSKα accumulation (A) and gene expression of PSKR1 (B), and PSKR2 (C) in peaches. The endogenous PSKα accumulation (A) and gene expression of PSKR1 (B) and PSKR2 (C) were determined immediately after each time point. For each biological replicate, after each time point, 20 peaches were used to detect the endogenous PSKα accumulation (A) and gene expression of PSKR1 (B) and PSKR2 (C). Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
The O2– production rate, H2O2 production and ROS accumulation continuously increased following control and PSKα applications throughout storage in peaches. PSKα application suppressed the elevation of O2⋅– production rate, H2O2 production and ROS content. Following 35 days of storage, the O2⋅− production rate, H2O2 production and ROS accumulation in PSKα-immersed peaches were suppressed by 22, 36, and 24% (Figure 3).
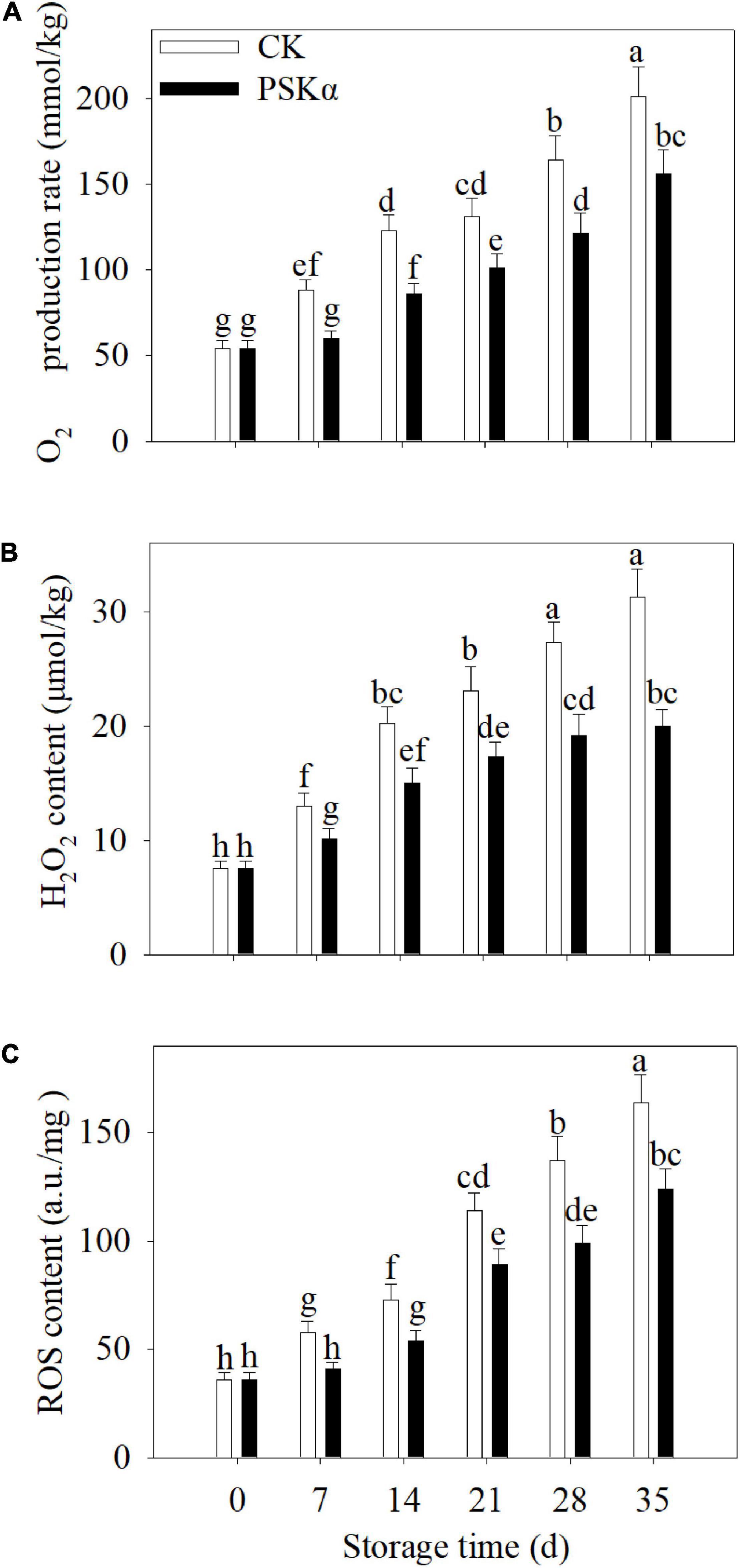
Figure 3. PSKα application retarded the elevation of the O2– production rate (A), H2O2 content (B) and ROS accumulation (C) in peaches. The O2– production rate (A), H2O2 content (B), and ROS accumulation (C) were assayed immediately after each time point. For each biological replicate, after each time point, 20 peaches were used to detect the O2– production rate (A), H2O2 content (B), and ROS accumulation (C). Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
Twelve PpLOXs were identified using transcriptome. Following PSKα application, the gene expression of PpLOX2-1 (LOC18773995), PpLOX2-1 (LOC18787524), PpLOX2-1 (LOC18787140), PpLOX3-1 (LOC18781374), and PpLOX3-1 (LOC18783171) showed no significant change. The gene expression of PpLOX6 (LOC18793349), PpLOX6 (LOC18766622), PpLOX5 (LOC18774987), PpLOX5 (LOC18773359), PpLOX5 (LOC18774870), PpLOX5 (LOC18774983), and PpLOX5 (LOC18775056) decreased after PSKα application (Table 2).
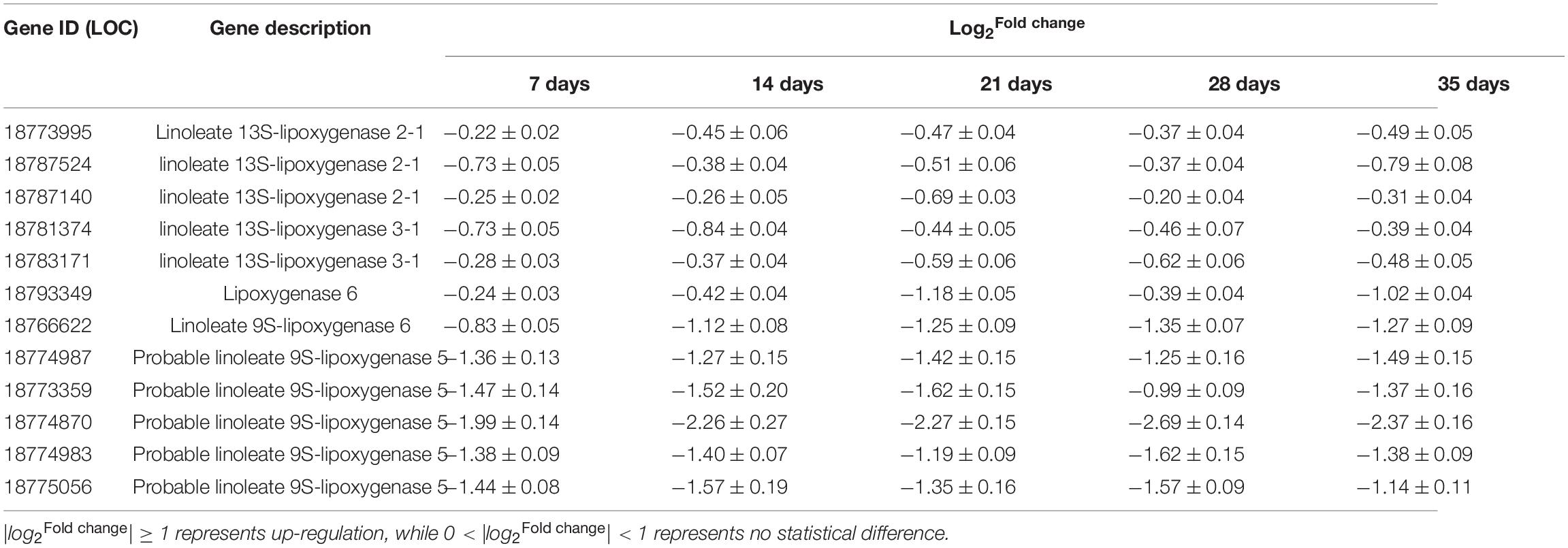
Table 2. The identification and quantification of PpLOXs in postharvest peaches after PSKα treatment using transcriptome.
To verify the results of transcriptome, qRT-PCR tests were carried out. Following 35 days of storage, there were no statistical differences in the gene expression of PpLOX2-1 (LOC18773995), PpLOX2-1 (LOC18787140), and PpLOX3-1 (LOC18781374) between CK and PSKα treatments. Following 35 days of storage, the gene expression of PpLOX2-1 (LOC18787524), PpLOX3-1 (LOC18783171), PpLOX6 (LOC18793349), PpLOX6 (LOC18766622), PpLOX5 (LOC18774987), PpLOX5 (LOC18773359), PpLOX5 (LOC18774870), PpLOX5 (LOC18774983), and PpLOX5 (LOC18775056) in PSKα-immersed peaches decreased by 16, 17, 19, 21,18, 18, 29, 17, and 15% (Figure 4).
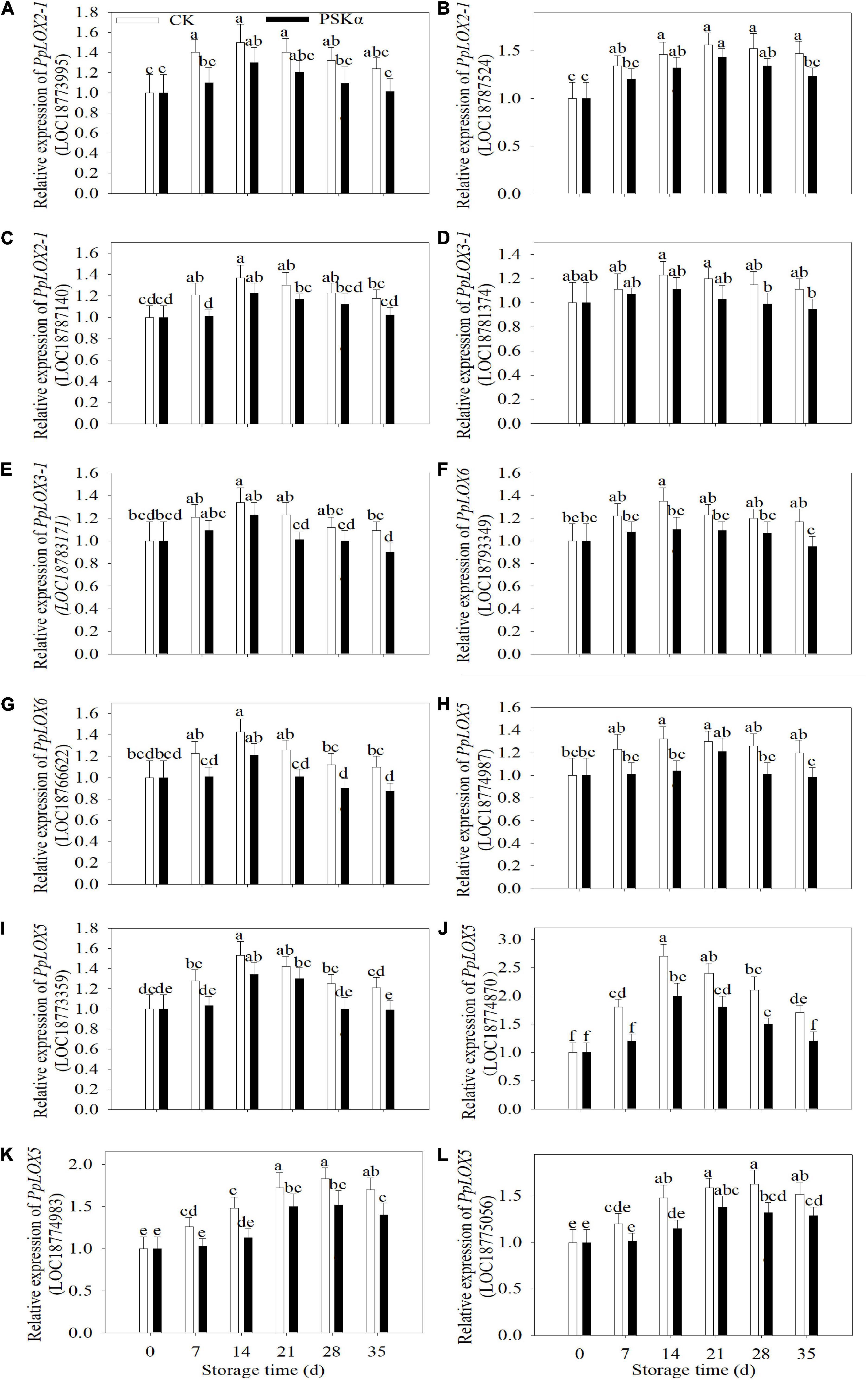
Figure 4. Effects of PSKα application on the gene expression of PpLOX2-1 (LOC18773995) (A), PpLOX2-1 (LOC18787524) (B), PpLOX2-1 (LOC18787140) (C), PpLOX3-1 (LOC18781374) (D), PpLOX3-1 (LOC18783171) (E), PpLOX6 (LOC18793349) (F), PpLOX6 (LOC18766622) (G), PpLOX5 (LOC18774987) (H), PpLOX5 (LOC18773359) (I), PpLOX5 (LOC18774870) (J), PpLOX5 (LOC18774983) (K), and PpLOX5 (LOC18775056) (L) in peaches. The gene expression of PpLOXs were determined immediately after each time point. For each biological replicate, after each time point, 20 peaches were used to detect the gene expression of PpLOXs. Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
The LOX activity increased firstly and decreased thereafter following control and PSKα applications throughout storage in peaches. PSKα treatment suppressed the LOX activity. The LOX activity after PSKα treatment was at the summit on 21 days, which decreased by 27%. Following 35 days of storage, the LOX activity in PSKα-immersed peaches decreased by 35% (Figure 5).
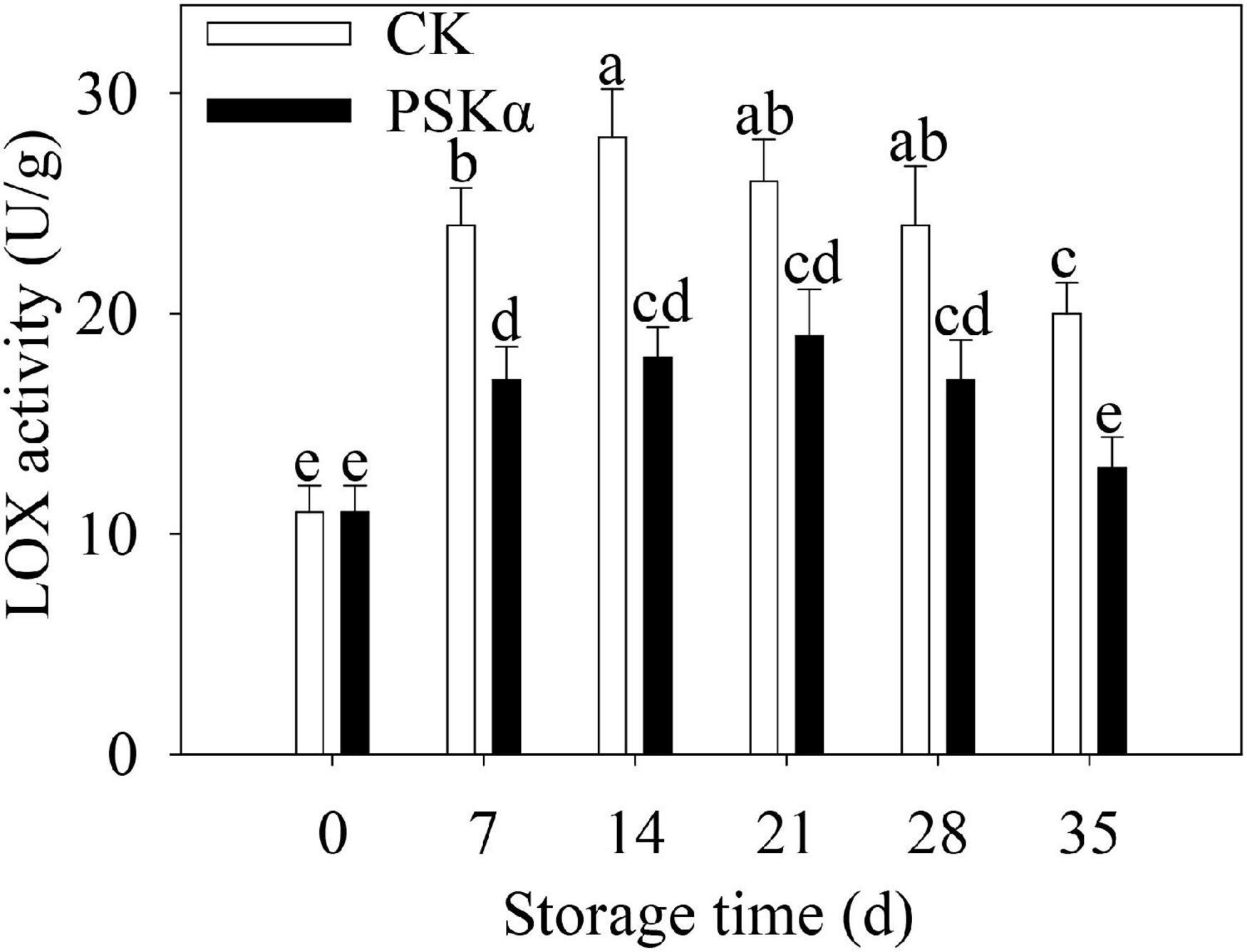
Figure 5. PSKα application suppressed the LOX activity in peaches. The LOX activity were determined immediately after each time point. For each biological replicate, after each time point, 20 peaches were used to detect the LOX activity. Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
Six PpCBFs were identified using transcriptome. During storage, PSKα application enhanced the gene expression of six PpCBFs (Table 3).
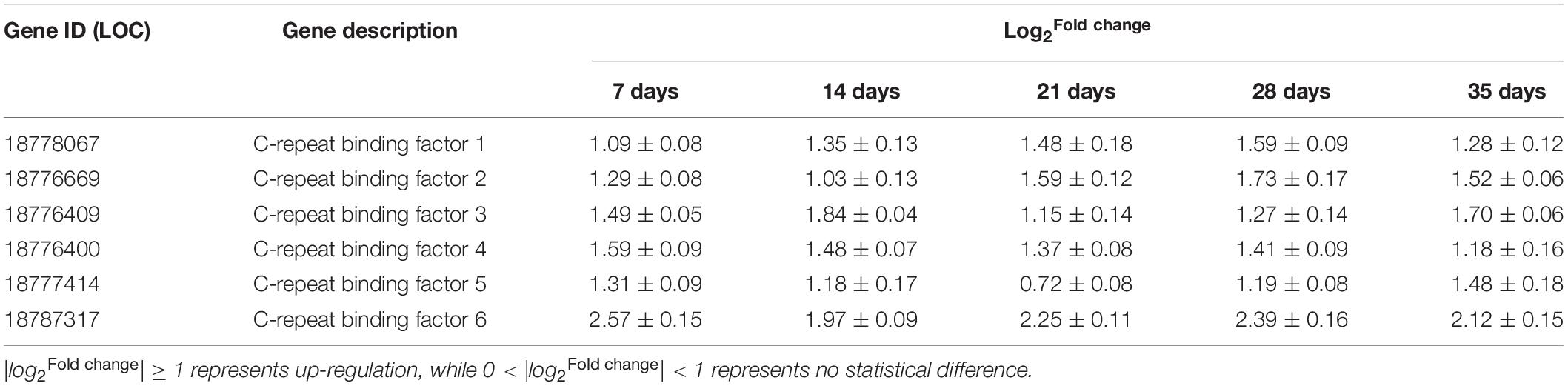
Table 3. The identification and quantification of PpCBFs in postharvest peaches after PSKα treatment using transcriptome.
To verify the results of transcriptome, qRT-PCR tests were carried out. Following 35 days of storage, the gene expression of PpCBF1 (LOC18778067), PpCBF2 (LOC18776669), PpCBF3 (LOC18776409), PpCBF4 (LOC18776400), PpCBF5 (LOC18777414) and PpCBF6 (LOC18787317) in PSKα-immersed peaches was 1.2, 1.4, 1.3, 1.2, 1.2, and 2.1 times of the control (Figure 6).
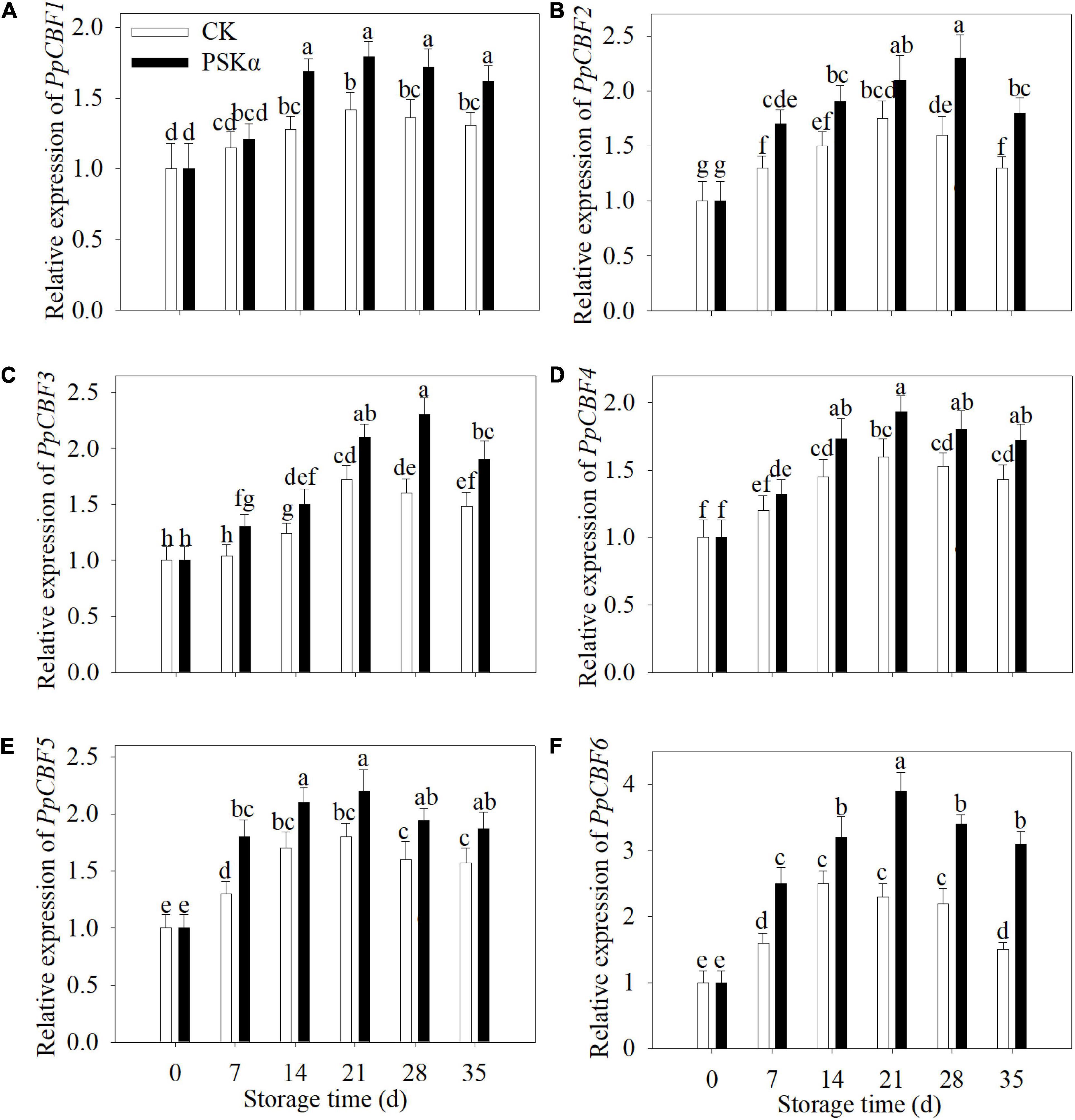
Figure 6. PSKα application enhanced the gene expression of PpCBF1 (A), PpCBF2 (B), PpCBF3 (C), PpCBF4 (D), PpCBF5 (E), and PpCBF6 (F) in peaches. The gene expression of PpCBFs was detected immediately after each time point. For each biological replicate, after each time point, 20 peaches were used to detect the gene expression of PpCBFs. Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
The promoter sequences of PpLOX5 were characterized, and a putative CRT/DRE-binding site (CCGAC) was identified (Supplementary Text 1). Then, Y1H assay was performed to explore the interaction between PpCBF6 and PpLOX5 promoter. the results in Y1H assay showed that yeast cells co-transformed with pGADT7-PpCBF6 and pAbAi-PpLOX5 promoter grew in the presence of 200 ng/ml AbA, indicating that PpCBF6 bound to the CRT/DRE motif in the PpLOX5 promoter (Figure 7).
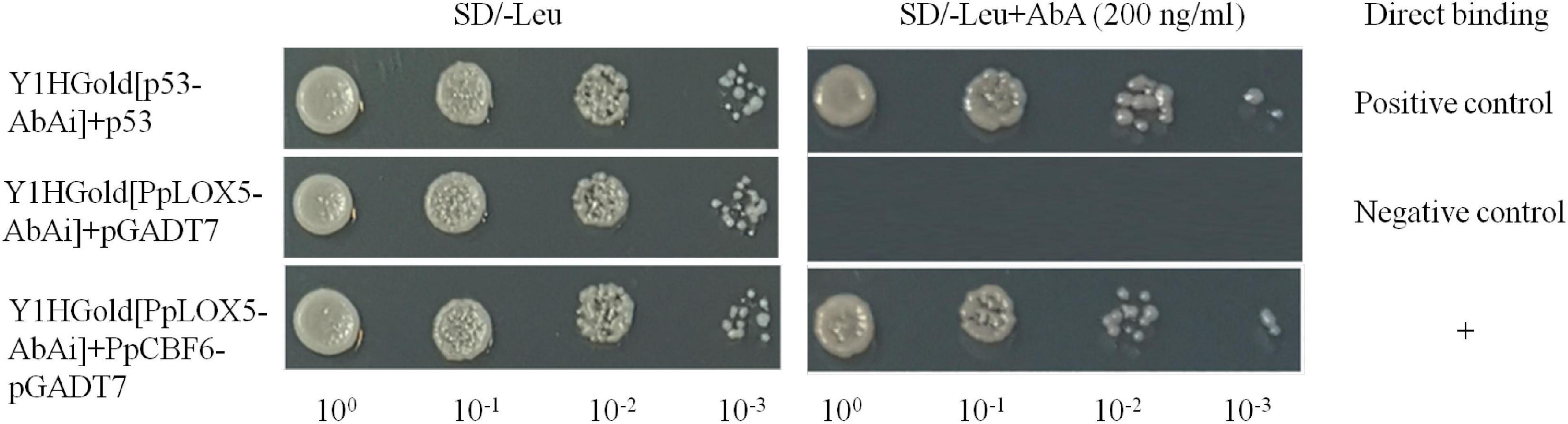
Figure 7. The interaction between PpCBF6 and PpLOX5 promoter. The direct binding of PpCBF6 protein to PpLOX5 promoter was tested on the basis of the ability of Y1HGold [PpLOX5-AbAi] + PpCBF6-pGADT7 to grow on SD/-Leu in the presence of 200 ng/ml AbA.
Furthermore, as indicated from the DLR assay, compared with the control that was cotransfected with the empty vector, the relative LUC/REN ratio decreased when the promoter-LUC reporter construct was cotransfected with PpCBF6, suggesting that PpCBF6 suppressed PpLOX5 expression. (Figure 8).
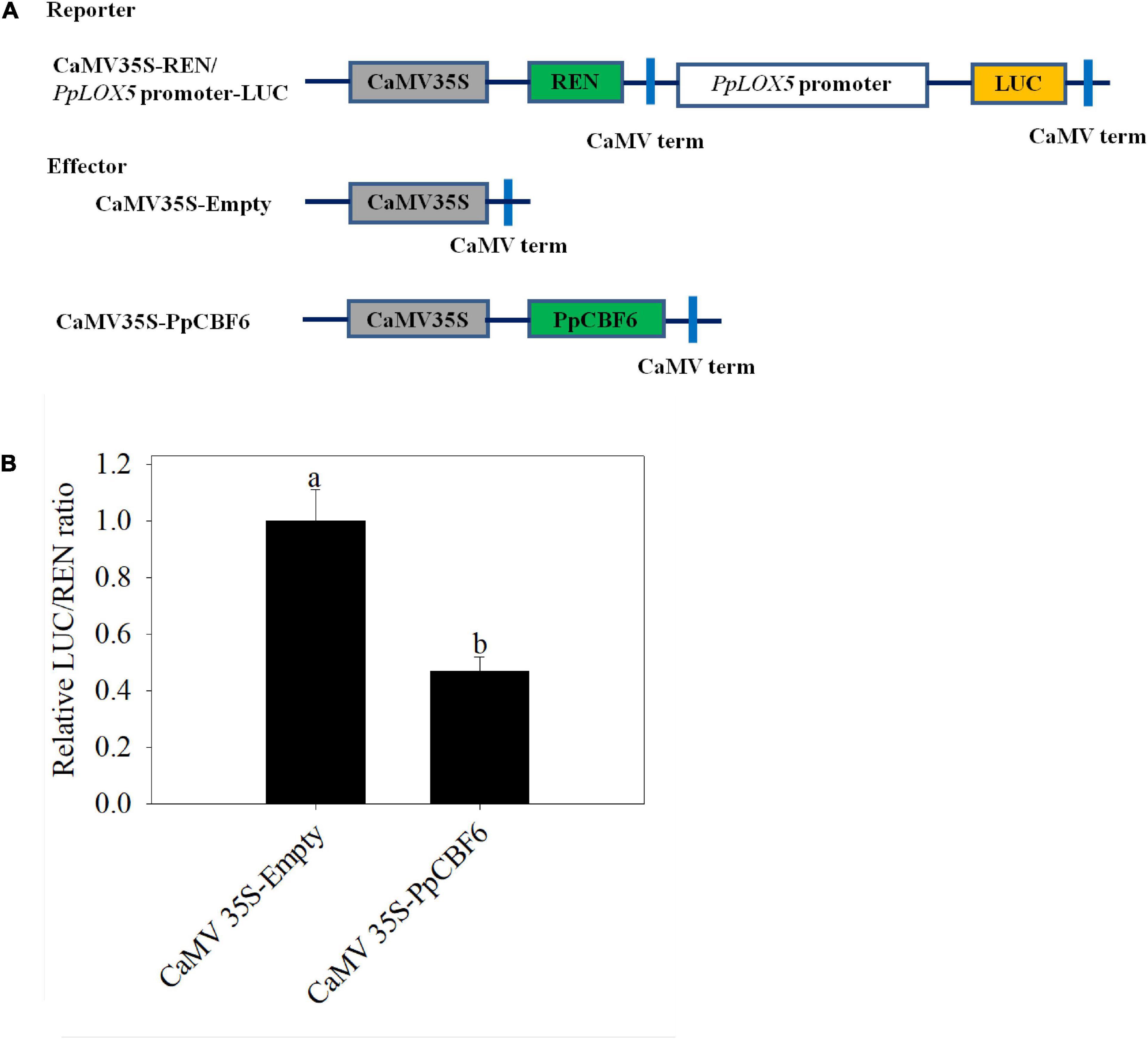
Figure 8. The regulation of PpLOX5 promoter by PpCBF6. (A) Schematic of the reporter and effecter constructs. (B) The inhibition of PpLOX5 promoter by PpCBF6 protein. Nine independent replicates were included for each combination. Transactivation was indicated by the LUC/REN ratio. Values represent the mean ± standard deviation. Values not with the same letter are significantly different at p < 0.05.
Phytosulfokine α application was verified to suppress the progression of CI degree and weight loss and the decrease in firmness and total soluble solids content (Figure 1), and to induce endogenous PSKα signaling (Figure 2), illustrating that PSKα treatment could be applied as an efficient approach to elevate tolerance to cold stress in peaches.
Phytosulfokine α treatment inhibited the gene expression of PpLOXs and LOX activity (Table 2 and Figures 4, 5). Low-temperature storage would cause the progression of the LOX level in postharvest fruit (Sheng et al., 2016). LOX catalyzes the hydroperoxidation of unsaturated fatty acids. Meanwhile, this process produces lots of ROS (Porta, 2002). ROS overproduction participates in the peroxidation of cell membrane lipid, resulting in cell membrane damage and cell necrosis. These physiological processes would consequently lead to browning, a typical symptom of CI. PSKα application was shown to be an effective approach to reduce O2– production rate, H2O2 content and ROS accumulation in peaches (Figure 3), illustrating that PSKα treatment maintained redox equilibrium in peaches. Therefore, the PSKα treatment-suppressed LOX level may facilitate to alleviate redox stress, therefore relieving CI in peaches. Additionally, the gene expression of PpCBFs was promoted by PSKα treatment (Table 3 and Figure 6). CBFs are the clearest cold signal transduction pathway in plants (Álvaro et al., 2019). Accordingly, CBF1 promoted the expression and activity of catalase, and down regulated H2O2 content, thereby ameliorating oxidative damage and boosting chilling tolerance in transgenic tomato (Hsieh et al., 2002). Also, overexpression of CBFc from Prunus mume in Arabidopsis promoted the activity of superoxide dismutase and peroxidase, thereby boosting cold resistance (Peng et al., 2016). Therefore, the PSKα application-boosted gene expression of PpCBFs (Table 3 and Figure 6) may function in chilling tolerance through avoiding ROS overproduction (Figure 3). In a word, this work proved that PSKα application regulated the ROS level by weakening the gene expression of PpLOX5 and LOX activity and boosting the gene expression of PpCBF6, and thus inhibited CI in peaches (Figures 1–6). which is consistent with a previous report suggesting that PSKα treatment enhanced the ROS scavenging capacity by elevating the expression of alternative oxidase and uncoupling protein in broccoli florets (Aghdam and Luo, 2021b). Thus, this work would provide new evidences to prove that PSKα application is an effective approach to maintain redox equilibrium in fruits and vegetables easy to suffer CI, and expand our horizon regarding the effects of PSKα on suppression of CI.
Moreover, the promotion of chilling tolerance following exogenous applications in postharvest fruit is mediated by endogenous signals (Jiao, 2021). Both of the results of transcriptome and qRT-PCR tests suggested that the down regulation of gene expression of PpLOX5 (LOC18774870) by PSKα treatment is the lowest (Table 2 and (Figures 4, 5), and the up regulation of gene expression of PpCBF6 (LOC18787317) by PSKα treatment is the highest (Table 3 and Figure 6). Thus, I investigated the involvement of PpCBF6 in the PSKα-suppressed PpLOX5 in peaches afterwards. As seen from the Y1H assay, PpCBF6 recognized the CRT/DRE motif in the promoter of PpLOX5 (Figure 7). What’s more, the negative regulation of PpLOX5 transcription by PpCBF6 was verified using the DLR assay (Figure 8). Accordingly, a previous study revealed that NF-YC transcription factor bound to LOX3 promoter in Arabidopsis thaliana (Breeze, 2014). Overexpression of EREBP1 (a APETALA2/ethylene responsive factor transcription factor) in rice elevated the expression of chloroplastic LOX (Jisha et al., 2015). This work would broaden our perceptions regarding the molecular mechanisms of the modulation of genes in membrane lipid metabolism by transcription factors. Based on the above results, it can be inferred that when the PSKα-immersed peaches were subjected to cold stress, PSKα perception in the plasma membrane by leucine-rich repeat PSKR1 and PSKR2 may be fundamental for the up regulation of gene expression of PpCBF6. Then, PpCBF6 bound to PpLOX5 promoter, and weakened its transcription. This suppression retarded ROS accumulation, thus relieving CI (Figure 9). Thus, a possible novel molecular mechanism underlying the PSKα treatment-relieved CI in peaches was elucidated in this work.
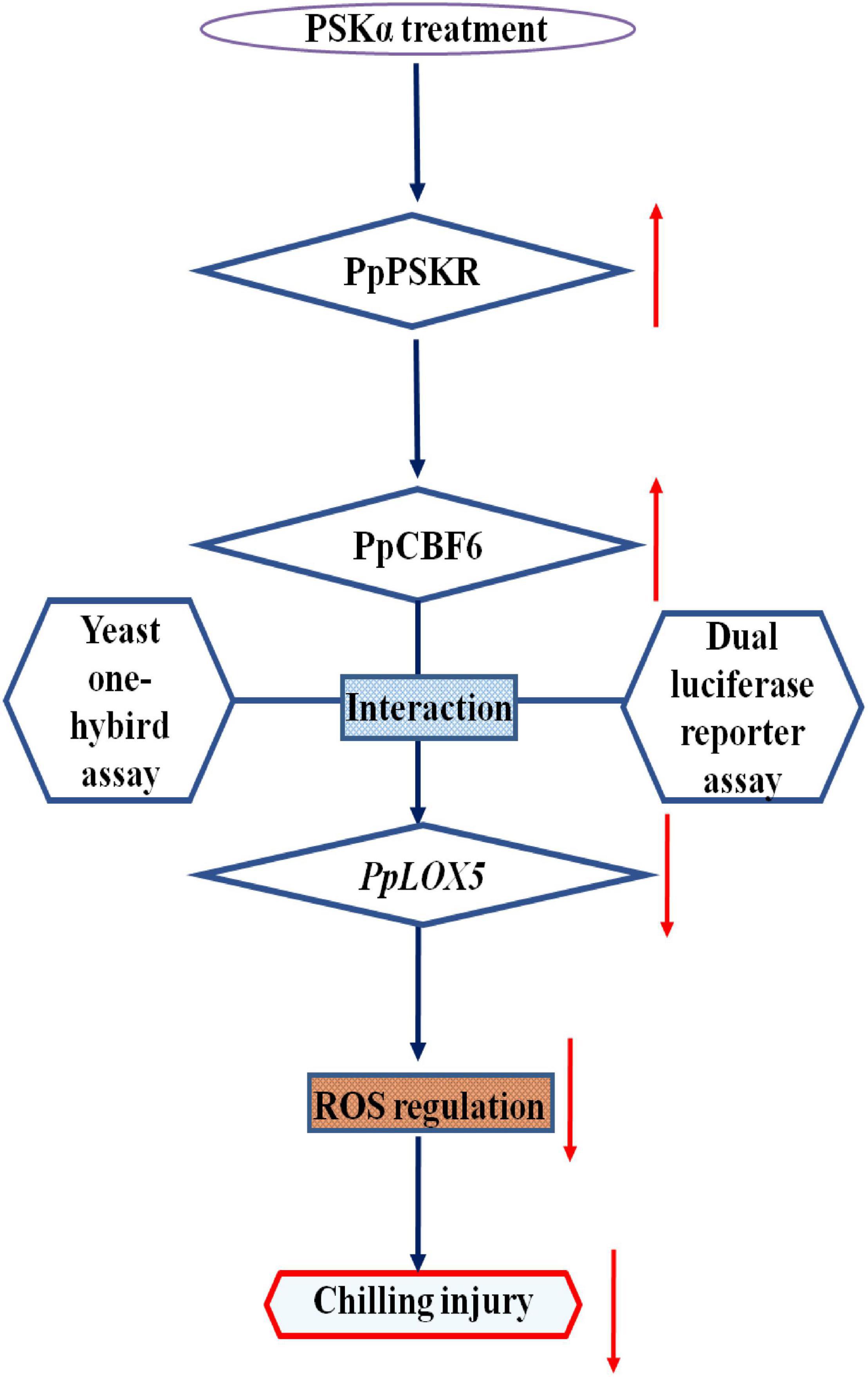
Figure 9. Proposed model of the involvement of PpCBF6 in the PSKα application-retarded CI by inhibiting PpLOX5 expression in peaches.
In conclusion, PSKα application reduced CI degree and weight loss, and maintained the firmness and total soluble solids content in peaches. The endogenous PSKα production and gene expression of PSKR1 and PSKR2 were enhanced by PSKα application. The elevation of O2– production rate, H2O2 production and ROS content was delayed by PSKα application. Moreover, PSKα application weakened the gene expression of PpLOX5 and LOX activity. The gene expression of PpCBF6 was promoted by PSKα application. Furthermore, PpCBF6 interacted with a PpLOX5 promoter fragment containing the CRT/DRE motif, and inhibited its expression. Thus, PpCBF6 mediated the PSKα-retarded CI by weakening the expression of PpLOX5 in peaches.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
CJ: conceptualization, formal analysis, investigation, methodology, data curation, writing, supervision, project administration, and funding acquisition.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.874338/full#supplementary-material
Aghdam, M., and Luo, Z. (2021a). Harnessing cGMP signaling pathways for improving fruits and vegetables marketability. Sci. Hortic. 291:110587. doi: 10.1016/j.scienta.2021.110587
Aghdam, M., and Luo, Z. (2021b). Exogenous application of phytosulfokine α (PSKα) delays senescence in broccoli florets during cold storage by ensuring intracellular ATP availability and avoiding intracellular ROS accumulation. Sci. Hortic. 276:109745. doi: 10.1016/j.scienta.2020.109745
Aghdam, M., and Flores, F. (2021c). Employing phytosulfokine α (PSKα) for delaying broccoli florets yellowing during cold storage. Food Chem. 355:129626. doi: 10.1016/j.foodchem.2021.129626
Aghdam, M., Sayyari, M., and Luo, Z. (2021b). Exogenous phytosulfokine α application delays senescence and promotes antioxidant nutrient accumulation in strawberry fruit during cold storage by triggering endogenous phytosulfokine α signaling. Postharvest Biol. Tec. 175:111473. doi: 10.1016/j.postharvbio.2021.111473
Aghdam, M., Ebrahimi, A., Sheikh-Assadic, M., and Naderi, R. (2021a). Endogenous phytosulfokine α (PSKα) signaling delays petals senescence and prolongs vase life of cut rose flowers (Rosa hybrida cv. Angelina). Sci. Hortic. 289:11044. doi: 10.1016/j.scienta.2021.110444
Álvaro, C., Carlos, P., Mari-Cruz, C., Fernanda, R., Julio, S., and José, L. (2019). Nitric oxide deficiency decreases CBF-dependent and CBF-independent induction of cold acclimation. J. Exp. Bot. 70, 3283–3296. doi: 10.1093/jxb/erz115
Arae, T., Isai, S., Sakai, A., Mineta, K., and Chiba, Y. (2017). Coordinated regulations of mRNA synthesis and decay during cold acclimation in Arabidopsis cells. Plant Cell Physiol. 58, 1090–1102. doi: 10.1093/pcp/pcx059
Belay, Z., Caleb, O., Vorster, A., Heerden, C., and Opara, U. (2020). Transcriptomic changes associated with husk scald incidence on pomegranate fruit peel during cold storage. Food Res. Int. 135:109285. doi: 10.1016/j.foodres.2020.109285
Breeze, E. (2014). Action of the AtNF-Y transcription factors in plant stress responses. Cent. Eur. J. Energ. Mat. 7, 131–144. doi: 10.1007/128_2010_47
Cao, K., Wei, Y., Chen, Y., Jiang, S., Chen, X., Wang, X., et al. (2021). PpCBF6 is a low-temperature-sensitive transcription factor that binds the PpVIN2 promoter in peach fruit and regulates sucrose metabolism and chilling injury. Postharvest Biol. Technol. 181:111681. doi: 10.1016/j.postharvbio.2021.111681
Chen, M., Guo, H., Chen, S., Li, T., Li, M., Rashid, A., et al. (2019). Methyl jasmonate promotes phospholipid remodeling and jasmonic acid signaling to alleviate chilling injury in peach fruit. J. Agr. Food Chem. 67, 9958–9966. doi: 10.1021/acs.jafc.9b03853
Hsieh, T., Lee, J., Yang, P., Chiu, L., Charng, Y., Wang, Y., et al. (2002). Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 135, 1086–1094. doi: 10.1104/pp.003442
Jiao, C. (2021). IP3 mediates NO-enhanced chilling tolerance in postharvest kiwifruit. Postharvest Biol. Tec. 176:111463. doi: 10.1016/j.postharvbio.2021.111463
Jing, G., Zhou, J., and Zhu, S. (2016). Effects of nitric oxide on mitochondrial oxidative defence in postharvest peach fruits. J. Sci. Food Agric. 96, 1997–2003. doi: 10.1002/jsfa.7310
Jisha, V., Dampanaboina, L., Vadassery, J., Mithöfer, A., Kappara, S., and Ramanan, R. (2015). Overexpression of an AP2/ERF type transcription factor OsEREBP1 confers biotic and abiotic stress tolerance in rice. PLoS One 10:e0127831. doi: 10.1371/journal.pone.0127831
Li, J., Qin, R., Li, H., Xu, R., Yang, Y., Ni, D., et al. (2015). Low-temperature-induced expression of rice ureidoglycolate amidohydrolase is mediated by a c-repeat/dehydration-responsive element that specifically interacts with rice c-repeat-binding factor 3. Front. Plant Sci. 6:1011.
Liang, L., Zhang, B., Yin, X., Xu, C., and Chen, K. (2013). Differential expression of the CBF gene family during postharvest cold storage and subsequent shelf-life of peach fruit. Plant Mol. Biol. Rep. 31, 1358–1367. doi: 10.1007/s11105-013-0600-5
Nilo, R., Saffie, C., Lilley, K., Baeza-Yates, R., Cambiazo, V., Campos-Vargas, R., et al. (2010). Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC Genomics 11:43. doi: 10.1186/1471-2164-11-43
Peng, T., Guo, C., Yang, J., Xu, M., Zuo, J., Bao, M., et al. (2016). Overexpression of a Mei (Prunus mume) CBF gene confers tolerance to freezing and oxidative stress in Arabidopsis. Plant Cell Tissue Organ Cult. 126, 373–385. doi: 10.1007/s11240-016-1004-7
Peng, Y., Wang, Y., Fei, J., and Sun, C. (2020). Isolation and expression analysis of two novel C-repeat binding factor (CBF) genes involved in plant growth and abiotic stress response in mangrove Kandelia obovata. Ecotoxicology 29, 718–725. doi: 10.1007/s10646-020-02219-y
Porta, H. (2002). Plant lipoxygenases. physiological and molecular features. Plant Physiol. 130, 15–21. doi: 10.1104/pp.010787
Sheng, L., Zhou, X., Liu, Z., Wang, J., Zhou, Q., Wang, L., et al. (2016). Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 117, 1–8. doi: 10.1016/j.postharvbio.2016.01.015
Song, H., Wang, X., Hu, W., Yang, X., Diao, E., Shen, T., et al. (2017). A cold induced phytosulfokine peptide is related to the improvement of loquat fruit chilling tolerance. Food Chem. 232, 434–442. doi: 10.1016/j.foodchem.2017.04.045
Wang, Y., Yang, Q., Jiang, H., Wang, B., Bi, Y., Li, Y., et al. (2020). Reactive oxygen species-mediated the accumulation of suberin polyphenolics and lignin at wound sites on muskmelons elicited by benzo (1, 2, 3)-thiadiazole-7-carbothioic acid S-methyl ester. Postharvest Biol. Technol. 170:111325. doi: 10.1016/j.postharvbio.2020.111325
Xiao, H., Siddiqua, M., Braybrook, S., and Nassuth, A. (2010). Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 29, 1410–1421. doi: 10.1111/j.1365-3040.2006.01524.x
Yang, Q., Wang, F., and Rao, J. (2016). Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in kiwifruit. PLoS One 11:e0162159. doi: 10.1371/journal.pone.0162159
Keywords: phytosulfokine α, chilling injury, lipoxygenase 5, PpCBF6, peaches
Citation: Jiao C (2022) PpCBF6 Is Involved in Phytosulfokine α-Retarded Chilling Injury by Suppressing the Expression of PpLOX5 in Peach Fruit. Front. Plant Sci. 13:874338. doi: 10.3389/fpls.2022.874338
Received: 14 February 2022; Accepted: 28 March 2022;
Published: 29 April 2022.
Edited by:
Vittorio Farina, University of Palermo, ItalyReviewed by:
Claudia Anabel Bustamante, Centro de Estudios Fotosintéticos y Bioquímicos (CEFOBI), ArgentinaCopyright © 2022 Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caifeng Jiao, MjkxNDU0MjkxQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.