
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 01 April 2022
Sec. Plant Pathogen Interactions
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.849043
This article is part of the Research Topic Necrotrophic Fungal Plant Pathogens, Volume II View all 7 articles
 Peipei Li†
Peipei Li† Yifan Xu†
Yifan Xu† Ketao Wang*†
Ketao Wang*† Wenlei Guo
Wenlei Guo Yujie Gu
Yujie Gu Shiheng Lyu
Shiheng Lyu Jianqin Huang
Jianqin Huang Haiping Lin
Haiping Lin Chunying Huang
Chunying Huang Zhen Xu
Zhen Xu Yan Li*
Yan Li*
Hickory (Carya cathayensis) is a critical tree species of the genus Carya from the Juglandaceae family that contains nutrient-rich nuts. Due to large-scale soil degradation, the pests and diseases of hickory are becoming more and more serious. Thaumatin-like proteins (TLPs) are vital proteins involved in the complex defense process of plant pathogens. In this study, 40 CcTLP genes were identified genome-widely and phylogenetically grouped into three subfamilies. The sequence of CcTLPs had a conservative pattern, such as eight stable disulfide bonds, REDDD, and G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C structure. In total, 57 cis-elements related to stress-responsive, light-responsive, phytohormone-responsive, and plant-responsive were discovered. Under salicylate (SA), methyl jasmonate (MeJA), and ethephon (ETH) treatments, the expressions of CcTLP28, CcTLP29, CcTLP30, CcTLP31, CcTLP32, CcTLP33, CcTLP37, CcTLP38, and CcTLP39 had different patterns. This is an indication that most of the TLP genes were upregulated by SA and downregulated by MeJA. Notably, seven TLP genes were significantly upregulated under the Botryosphaeria dothidea inoculation, especially CcTLP31, with an over 20-fold change. Nine genes were shown by subcellular localization analysis to be located at the plasma membrane and cytoplasm. The knowledge of the disease-resistant function of the CcTLP family in hickory is promoted by these results. A foundation reference for the molecular breeding of this plant in the future is provided by our findings.
Plants are constantly attacked by various pathogens (e.g., bacteria, fungi, and viruses) in nature. In the long-term evolutionary process, plants and pathogens interact, adapt, and co-evolve. Plants have gradually established a series of complex defense mechanisms that coordinate well against the infection of pathogenic bacteria. Systemic-acquired resistance (SAR) is an essential defense mechanism induced by pathogenetic invasion (van Loon et al., 2006). Pathogenesis-related proteins (PRs) are induced in pathological or related situations intensely involved in the SAR process. So far, PRs have been identified and divided into 17 categories (PR1 to PR17) (van Loon et al., 2006; Dodds and Rathjen, 2010; Hamamouch et al., 2011). The PR-5 family, named as thaumatin-like proteins (TLPs), was first discovered in the fruit of Thaumatococcus daniellii, a shrub plant growing in the West African rainforest (Wel and Loeve, 1972). Afterward, TLP genes were explored in nematodes (Kitajima and Sato, 1999), insects (Brandazza et al., 2004), fungi (Grenier et al., 1999; Sakamoto et al., 2006), and multiple plants, such as Arabidopsis thaliana, Oryza sativa, Populus trichocarpa, Manihot esculenta, and Cucumis melo (Reimmann and Dudler, 1993; Abad et al., 1996; Midoro-Horiuti et al., 2000; van Loon et al., 2006; Liu et al., 2010b,2020; Petre et al., 2011; Irigoyen et al., 2020). A typical TLP protein sequence contains a highly conserved motif: G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C (Jami et al., 2007; Tachi et al., 2009), a REDDD structure, and 5–8 disulfide linkages composed of 10 or 16 cysteine residues. These structures are thought to be associated with an antifungal function (Hu and Reddy, 1997). With the presence of these disulfide linkages, correct folding under unsuitable circumstances, such as extreme heat, low pH, and protease degradation, is allowed (Fierens et al., 2009). The N-terminal signal peptides of TLPs consist of 15–30 amino acids, with which the transportation from the ribosome to the extracellular fluid through the endoplasmic reticulum is ensured (Anžlovar and Dermastia, 2003). It was shown that the 3D structures of TLPs contained three domains, and domain I was located between domain II and III. There is a cleft structure between domains I and II, which is usually acidic and associated with pathogenic resistance (Rajam et al., 2007; Ghosh and Chakrabarti, 2008).
Members of the TLP family have been reported in recent studies to participate in multiple biological processes under biotic or abiotic stress (Fierens et al., 2009; Petre et al., 2011). For example, it has been found in several studies that TLPs increase the β-1,3-glucanase activity or are performed as xylanase inhibitors to destroy fungal cell walls (Jami et al., 2007; Martin et al., 2007). The resistance to rice sheath blight Rhizocotonia solani and sheath rot Rocladittm oryzae, is improved by the overexpression of TLPs in elite indica rice cultivars (Kalpana et al., 2006). Transgenic cassava with an overexpressing rice TLP gene significantly delayed anthracnose disease and enhanced fungal tolerance compared with wild types (Odeny Ojola et al., 2018). Moreover, an overexpression of the grape TLP gene in A. thaliana resulted in its more robust resistance to powdery mildew and Pseudomonas syringae pv. tomato DC3000 compared with the wild type (Yan et al., 2017). In addition, it has been found that BanTLPs purified from banana extracts can serve as a defense against Penicillium expansum through the disturbance of the plasma membrane and cell wall disorganizing (Jiao et al., 2018). Recently, it has been shown that GbTLPs in Gossypium barbadense were significantly upregulated after Verticillium dahlia infection, suggesting a role for TLP in disease defense (Zhang et al., 2021). Furthermore, the tolerance of tobacco to salt, oxidative, and drought stress was enhanced by AdTLPs expression (Barthakur et al., 2001; Singh et al., 2013). Notably, it has been reported that SA and JA/ETH are involved in plant immunity. Resistance against biotrophic and hemi-biotrophic microbes is mediated by SA signaling, while JA and ethylene signaling participate in resistance against necrotrophs in plants (Gimenez-Ibanez et al., 2017).
Hickory (Carya cathayensis) is a commercially cultivated nut tree (Zhang and Xu, 2011), mainly distributed in China (Zhang and Xu, 2011). Its nut has a high nutritional value for human health, containing several nutrients, such as unsaturated fatty acids, flavonoids, dietary fibers, minerals, vitamins, and others (Huang et al., 2021, 2022; Li et al., 2022). With global climate change and soil over-utilization, soil degradation, biotic, and abiotic stresses are becoming more serious, resulting in the recent outbreak of a canker disease caused by Botryosphaeria dothidea (Zhang and Xu, 2011). This disease causes damage or death to hickory trees, bringing considerable losses in nut production and restricting its expanding cultivation. It has been revealed that B. dothidea causes infection symptoms on the trunk and branches, leading to tissue necrosis and disease spots around the infection sites, and disrupting the transportation of water and nutrients in the plants (Slippers et al., 2007). Therefore, we must explore the resistance genes in C. cathayensis against B. dothidea, which will contribute to the management of this disease.
In this study, we conducted the bioinformatics analyses, real-time fluorescence quantitative polymerase chain reaction (RT-qPCR), and Agrobacterium-mediated transient expression in tobacco for the genome-wide identification and analyzing the TLP family members in hickory (C. cathayensis). The objectives of this study were: (1) The identification, genomic location analysis, and protein characterization of the CcTLP genes, (2) to perform the multiple-alignment and phylogenetic analysis of the CcTLPs genes, (3) to analyze the gene structure, motif distribution, and cis-acting element of the CcTLPs genes, (4) to perform a differential expression profiling of the TLP genes under salicylate (SA), methyl jasmonate (MeJA), and ethephon (ETH) treatments, and infection by B. dothidea, and (5) to perform a subcellular localization assay of the TLP genes. With these studies, the disease resistance of the CcTLP family members in hickory will be better understood, and guidance for its molecular breeding improvement in the future will be provided.
The whole genomic sequences of pecan (Carya illinoinensis), walnut (Juglans regia), and hickory (C. cathyensis) are obtained from the Portal of Juglandaceae (Guo et al., 2020). Genomic data of ginkgo (Ginkgo biloba), amborella (Amborella trichopoda), waterlily (Nymphaea coloratar), soybean (Glycine max), grape (Vitis vinifera), rice (Oryza sativa), and Phalaenopsis (Phalaenopsis aphrodite) were obtained from the National Center for Biotechnology Information (NCBI) genome database. The protein sequences of A. thaliana were derived from TAIR1.
A TLP keyword search of the NCBI for genes was conducted to find the coding sequence (CDS) of TLP, which were downloaded in FASTA format. The obtained TLP seed sequences were then searched against the previously downloaded whole-genome protein sequences using the BLASTX program (Camacho et al., 2009) (NCBI-BLAST 2.9.0 software, E-value of 10–10) to look for similar homologous protein sequences of the candidate TLP genes in the 10 species. The seed files of TLP domains (PF00314) were downloaded from the Pfam database.2 Candidate TLP protein sequences were further screened using the Hidden Markov Model algorithm of the HMMER software (Finn et al., 2011) and finally renamed as CcTLP1–40.
A portal, ExPASy,3 was used to identify and analyze the physicochemical properties of protein sequences (Gasteiger, 2003). Additionally, we predicted the location and transmembrane (TM) domains using Cell PLoc 2.0 (Chou and Shen, 2008) and TMHMM 2.0.4 The motif features of TLP sequences and signal peptides were analyzed with the MEME Suite and SignalP 4.0 (Petersen et al., 2011). Genome structure visualization was accomplished using the Gene Structure Display Server 2.0 online website (Hu et al., 2015) with a gff file. Promoter sequences were extracted using a pipeline of SAMtools and bedtools (Danecek et al., 2021). Then, the cis-acting elements were predicted using PlantCARE (Lescot, 2002).
MCSCAN software (Wang et al., 2012) was used to run the Pairwise Synteny Search program, with the whole-genome CDS of hickory and the gff file as input. The obtained anchor pairwise results were filtered with a 30-gene threshold for small fragment gene blocks. The identified TLP genes were searched in the final gene blocks.
MicroRNA (miRNA) is a class of non-coding single-stranded RNA molecules with a length of approximately 22 nucleotides that participate in the post-transcriptional gene expression regulation in plants and animals. The network profile between miRNAs and CcTLPs was further analyzed to better understand the miRNA regulation network of hickory TLP genes. The psRNATarget online server (Dai et al., 2018) was used to identify the miRNAs interacting with the hickory TLP genes. Then, the complex networks integrating miRNAs and TLP genes were visualized using Cytoscape (Shannon et al., 2003).
Multiple sequence alignment of TLP genes was achieved using MAFFT software (Katoh, 2002) under local pair mode, and the maxiterate parameter was set to 1,000. Then, TrimAL software (Capella-Gutierrez et al., 2009) was utilized to remove the spurious sequences and poorly aligned regions. The final alignment result was removed with more than 20% gaps, a similarity score lower than 0.001, and minimum conservation of 60%. The phylogenetic tree was built using the maximum likelihood method using IQTREE software (Nguyen et al., 2015). The s parameter was used to estimate the optimal substitution model automatically during the calculation process, and the bootstrap value was set to 1,000. JTT + I + G4 and JTT + R7 were the best-fit substitution models for hickory and green plants trees sorted by Bayesian information criterion (BIC) scores. The generated tree file was transfigured with the Evolview3 website (Subramanian et al., 2019).
In this experiment, the 6-month hickory seedlings growing in the Zhejiang Agriculture and Forestry University greenhouse (50 m, N30°23′, E119°72′) under a 16-h light/8-h dark photoperiod and at 22–25°C temperature were sprayed with 100 μM of SA, MeJA, and ETH until the first drop of liquid on the leaf surface. After treatments for 0, 2, 6, 12, 24, 48 h, 3–6 leaves from each plant were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until use. This experiment was conducted with three independent biological replicates for each treatment.
The 2-year hickory seedlings were selected for B. dothidea inoculation in the Zhejiang Agriculture and Forestry University greenhouse (50 m, N30° 23′, E119° 72′). Before inoculation, B. dothidea was grown on potato dextrose agar (PDA) at 28°C in the dark. After 7 days, a 5-mm-diameter fungal agar plug was acquired using a punch and immediately placed on the surfaces of the wound site of the hickory stalk caused by the inoculating needle. In addition, a 5-mm-diameter empty PDA was used as control. The plug was removed after 2 days and the diameter of the disease areas was measured in six seedlings at 3, 4, 5, 6, 7, 9, 10, 11, 12, 14, and 16 days after inoculation (dpi) using rulers. The disease areas were collected at 0, 2, 7, and 16 dpi (Wang et al., 2020). All samples were immediately frozen in liquid nitrogen and then stored at −80°C until use. Each treatment contained three independent biological replicates.
Total RNA was extracted using a Quick RNA Isolation Kit (Huayueyang, China). cDNA was synthesized using the PrimeScript™ 1st strand cDNA Synthesis Kit (Takara, Japan) according to the manufacturer’s instructions. The gene-specific primers of TLPs and SA-synthesis and signaling-related genes were designed using the online software Primer 3.5 All primers used in this study are listed in Supplementary Table 1. The quantitative reverse transcription (qRT-PCR) was conducted using the SYBR Green Master Mix reagent (Applied Biosystems) and CFX 96 Real-Time system (Applied Biosystems), according to the manufacturer’s instructions. CcActin was used as an internal standard for normalization. The reaction procedure was 40 cycles with 95°C for 10 s and 55°C for 30 s. Formula 2–ΔΔCT was applied to calculate the relative expression. Each sample was conducted with three biological replicates and three technical replicates in the RT-qPCR experiment. The relative expression level of CcTLP genes was calculated by the standard curve and then normalized by the CcActin expression level. R-package pheatmap was utilized to plot the expression heatmap of CcTLPs with SA, MeJA, and ETH treatments for 0, 2, 6, 12, 24, and 48 h.
The full-length CDS of CcTLP28, CcTLP29, CcTLP30, CcTLP31, CcTLP32, CcTLP33, CcTLP37, CcTLP38, and CcTLP39 were amplified with PCR. The gene-specific primers of the TLPs were designed using the Snapgene software and are listed in Supplementary Table 2. The PCR products were cloned into the binary vector 35s:GFP (modified from pCAMBIA 1300). The resulting plasmids with the correct sequence were introduced into Agrobacterium tumefaciens strains GV3101 and were cultured on the luria broth (LB) solid medium counting 50 μg/ml gentamicin (Geta), 50 μg/ml rifampicin (Rif), and 50 μg/ml kanamycins (Kana) at 28°C in the dark for 2 days. Then, a single colony was obtained, transformed into a liquid LB medium, and cultured at 28°C. After another 2 days, the cultures of A. tumefaciens (OD600 = 0.5–0.6) were centrifuged at 5,000 rpm at room temperature for 10 min and re-suspended in MMA buffer (10 mM MES, 10 mM MgCl2, and 150 μM acetosyringone, pH = 5.6) to an OD600 of 1.0 and then incubated at room temperature in the darkness. After 2∼3 h, the suspension was injected into the 4-week-old tobacco (Nicotiana benthamiana) leaves. The plasma membrane marker (pm-rk) was used as a plasma membrane marker and was co-transformed with CcTLPs in tobacco (Nelson et al., 2007). After 2 days, green fluorescent protein (GFP) fluorescence was observed using laser confocal fluorescence microscopy (excitation: 488 nm; emission: 495–515 nm; LSM 800, Zeiss, Germany).
Statistical analyses were conducted using the one-way analysis of variance (ANOVA) procedure with SPSS (ANCOVA; SPSS26, SPSS Inc., Chicago, IL, United States). Significant differences among the groups were compared according to Duncan’s new multiple range test at p = 0.05.
After blasting and searching against the HMM seed model with whole-genome protein sequences, 40 TLP genes with typical thaumatin-like domains were found in C. cathayensis (Table 1). The number of TLP genes in 10 other green plants (G. biloba, A. trichopoda, N. coloratar, O. sativa, P. aphrodite, A. thaliana, G. max, V. vinifera, J. regia, and C. illinoinensis) are also listed in Table 1. Specifically, it has been shown that the amborella genome owns the least TLP genes. Compared with amborella, the number of TLP genes is almost two times in water lily belonging to the same Amborellales-Nymphaeales-Austrobaileyales (ANA) clade due to the Nymphaeaceae-specific whole-genome duplication (WGD) event (Zhang et al., 2020). Soybean and walnut genomes have the highest number of TLP genes, 62 and 66, respectively, almost three times than that of amborella, which may be attributed to that modern soybean was a diploid species from ancient tetraploid (Schmutz et al., 2010) and high quality of walnut genome (Marrano et al., 2020). From the information detected by the synteny analysis, four tandem duplications occurred in the distribution of TLP genes in hickory: CcTLP07 and CcTLP08 on the scaffold 18,053, CcTLP15, CcTLP16, and CcTLP17 on the scaffold 25,681, CcTLP21 and CcTLP22 on the scaffold 28,723, CcTLP29, CcTLP30, CcTLP31, CcTLP32, and CcTLP33 on the scaffold 54,619. Further synteny analysis with chromosome-level pecan genome showed these tandem duplicated TLP-located scaffolds originated from different chromosomes (Supplementary Figure 1). Five pairs of genes were revealed by gene synteny analysis to be duplicated from the WGD event, containing CcTLP15-CcTLP22, CcTLP16-CcTLP22, CcTLP17-CcTLP21, CcTLP18-CcTLP25, and CcTLP31-CcTLP38, which implied a potential influence of WGD event on CcTLP gene expansion.
Protein characteristics of hickory TLP gene family were further analyzed and presented in Table 2, such as Instability index (II), Aliphatic index (AI), Grand average of hydropathicity (GRAVY), and isoelectric point (pI). The II ranges from 26.65 (CcTLP29) to 73.31 (CcTLP08), and the AI values were between 52.1 (CcTLP35) and 83.67 (CcTLP27). According to GRAVY values, 35% (14 out of 40) of TLP genes code as hydrophobic, while others code as hydrophilic proteins. The pI values of 10 out of 40 CcTLP proteins reached values higher than seven, while the remaining ones reached values lower than seven. The vast majority of TLP genes were shown by sublocalization predication to be distributed in the cytoplasm. The exceptions were CcTLP14 and CcTLP18, located in the cell membrane/cytoplasm and vacuole, respectively. Finally, most of the genes contained transmembrane domains (Table 2). Notably, it was also found that some of these TLPs had N-signal peptides and transmembrane domains (TM) (Table 2).
The multi-sequence alignment was further performed and was shown in Figure 1. Most hickory TLP peptide sequences (35 out of 40) contain 16 cysteine residues and form eight stable disulfide bonds. However, CcTLP07, CcTLP09, and CcTLP27 lost five cysteines that only allowed them to encode type S (Small) TLPs with anti-fungal activity (Liu et al., 2010a). Moreover, it was observed that the CcTLP02 and CcTLP09 sequences were missing one and three amino acids, respectively. The REDDD structure from several genes has undergone varying degrees of mutation and deletion. For instance, CcTLP27 and CcTLP40 completely lost their REDDD structures, which may lead to an inability to maintain proper topologies (Liu et al., 2012). It was found that CcTLP24, CcTLP08, CcTLP29, CcTLP30, and CcTLP32 suffered 2–3 site mutations that may have affected their anti-microbial function. Notably, most of the hickory TLP sequences reversed the core domain sequence G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C. Still, CcTLP27 missed part of the sequence: G-X-[GF]-X-C-X-T.
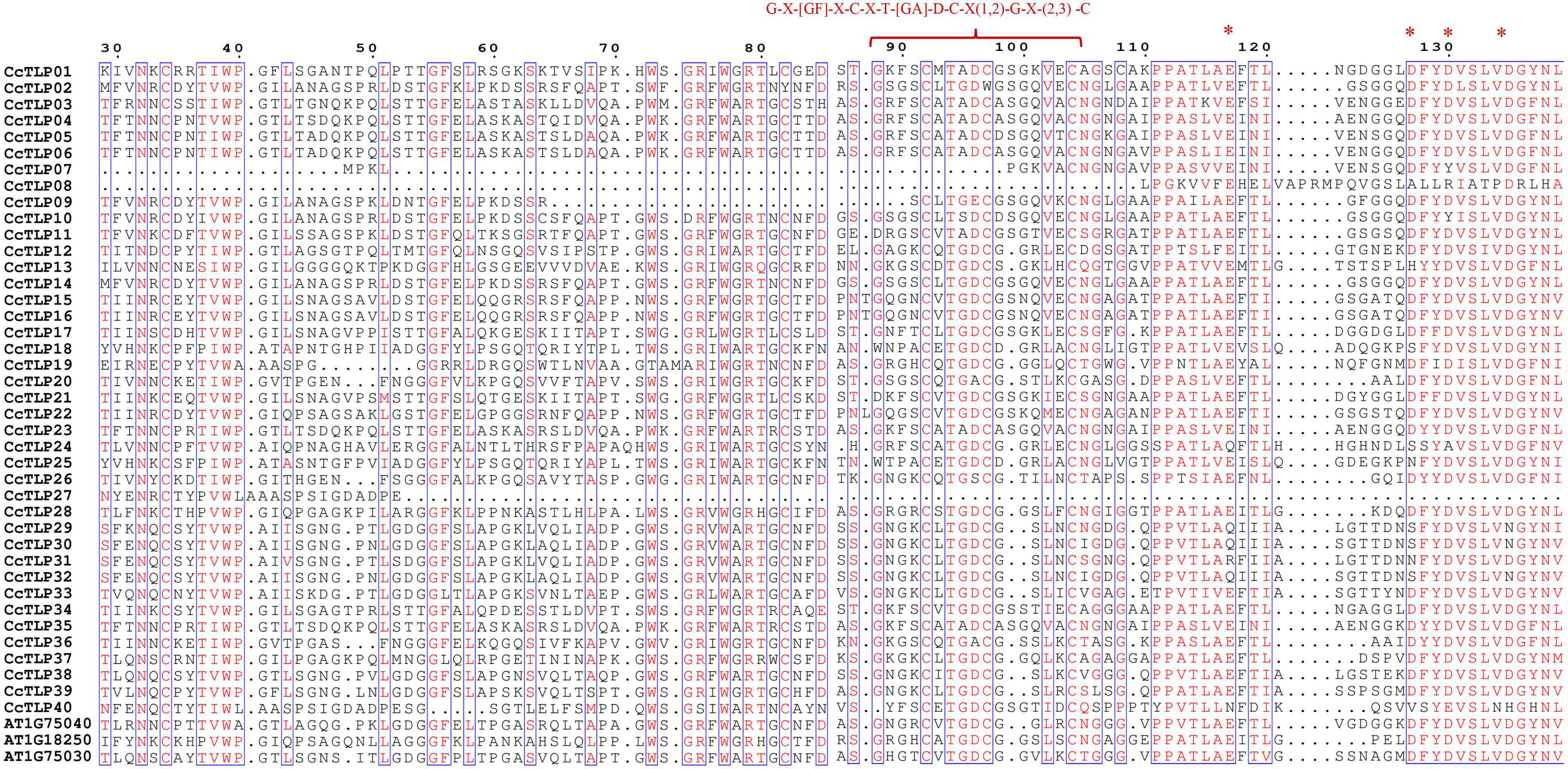
Figure 1. Multiple alignment sequence of 43 conserved thaumatin-like protein (TLP) regions. Bracket indicated the G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C region, asterisk signs represented the highly conversed REDDD structure.
A total of 424 TLP genes were identified in 11 green plants to analyze the evolutionary relationship of TLP genes among green plants. The phylogenetic tree was distinctly divided into five subfamilies (Figure 2A). The green clade had the largest number of TLP genes (115, including 18 hickory TLP genes). Notably, this clade contains 37 gingko TLP genes and basal amborella TLP genes. This finding is an indication that the clade represents a complete evolutionary history. Blue, red, and purple clades had 117 (27.6%), 70 (16.5%), and 42 (9.91%) TLP genes, respectively. The red clade had 33 Juglandaceae species, consisting of 47.1% of the 70 TLP genes. This finding is a suggestion that the Juglandaceae-specific TLP gene family expansion was within the red clade.
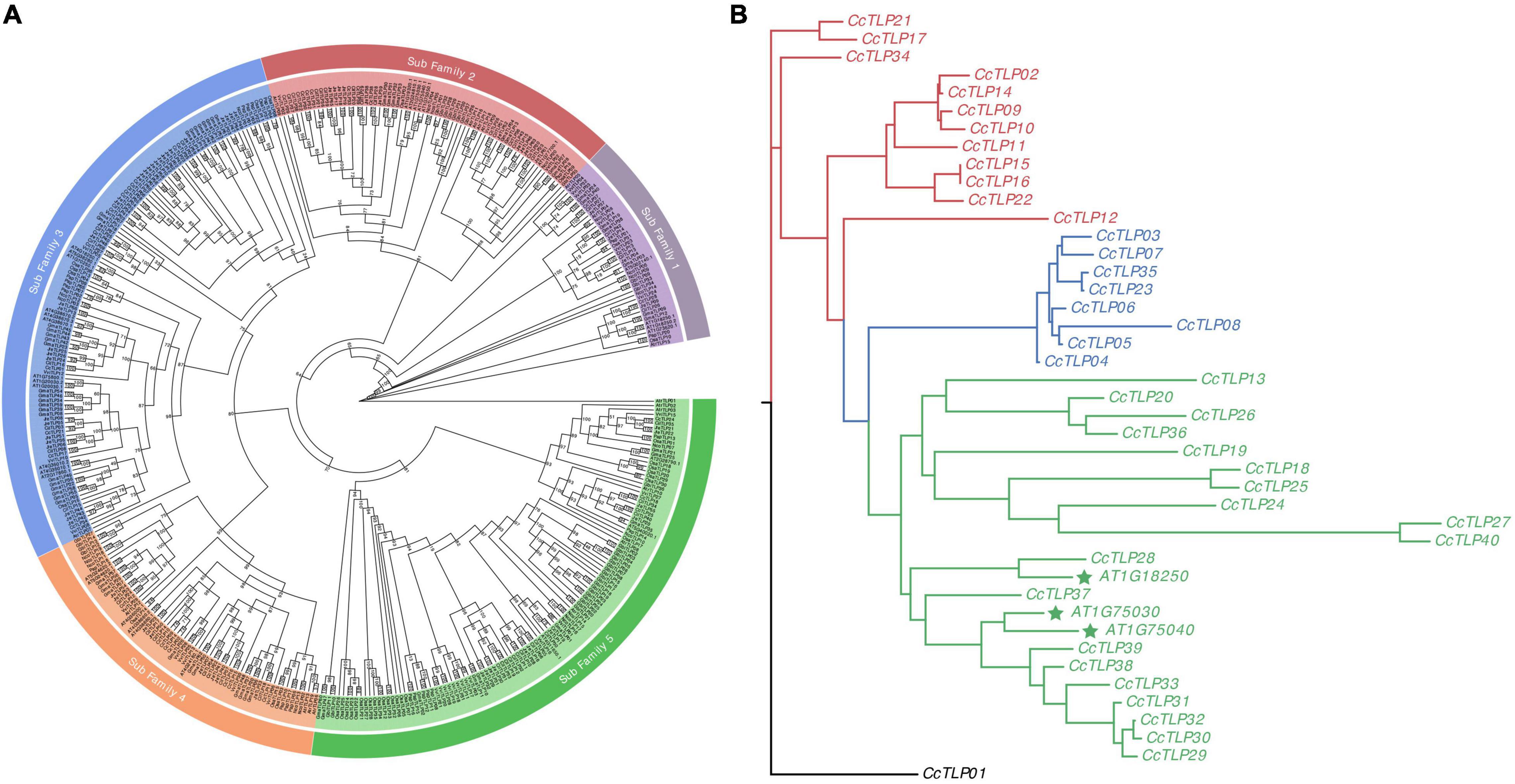
Figure 2. Hickory phylogenetic tree constructed with 424 plant TLP genes (A) and full-length peptides sequences of 40 and three TLPs from hickory and three Arabidopsis thaliana, respectively (B). The phylogenetic tree of 424 plant TLP genes was plotted with the maximum-likelihood estimation, JTT + R7 substitution model, and 1,000 bootstrap times. The phylogenetic tree was divided into five clades: Purple, dark red, blue, orange, and green. The 43 TLP peptides sequences were aligned using the MAFFT software. Then, the phylogenetic tree was inferred with IQTree with the parameters of the JTT + I + G4 substitution model and 1,000 times bootstrap. The maximum-likelihood tree could be divided into three groups, presented by red, green, and blue lines.
A maximum-likelihood tree was constructed using 43 full-length TLP sequences (40 from hickory and three from Arabidopsis thaliana) to explore the further phylogenetic relationship among TLP genes in hickory (Figure 2B). The maximum-likelihood tree can be divided into three groups: group I (red line), group II (blue line), and group III (green line). The richest group III contained 22 TLP genes, including 19 CcTLP genes and three Arabidopsis TLP genes, which have been significantly studied. Within group III, CcTLP27 and CcTLP40 owned the longest clades, 2.6292 and 2.5801, respectively, indicating that these two genes were highly diverged. This result was consistent with the loss of REDDD in these two genes. The gene count in groups I and II was 12 and 8, and gene branch lengths in these two groups were nearly the same apart from CcTLP08. These results are suggestions that the genetic variation within group I and group II was low, and TLP gene functions were potentially similar.
Differences in the type and arrangement order of exons and introns could impact gene function. The gene structure of CcTLPs was analyzed and is shown in Figure 3B. Six CcTLP genes (CcTLP02, CcTLP35, CcTLP24, CcTLP40, CcTLP19, and CcTLP31) maintained only one exon, with no intron element within the genes. In the remaining, most of the genes (20 out of 35) contained two exons and one intron elements, 14 out of 35 genes had three exons and two introns, and remarkably CcTLP28 comprised four exons. Peptide sequence regions that have a significant impact on protein function or structure should be more conservative, which are called motifs. According to the motif analysis results, five motif patterns were found within 40 CcTLP genes (Figure 3C). Most of the CcTLP genes (82.5%) maintained the complete five motif patterns, while CcTLP09 and CcTLP40 lost one motif pattern, CcTLP02, CcTLP07, and CcTLP19 lost two motifs, and CcTLP08 and CcTLP27 only maintained two unbroken motifs.
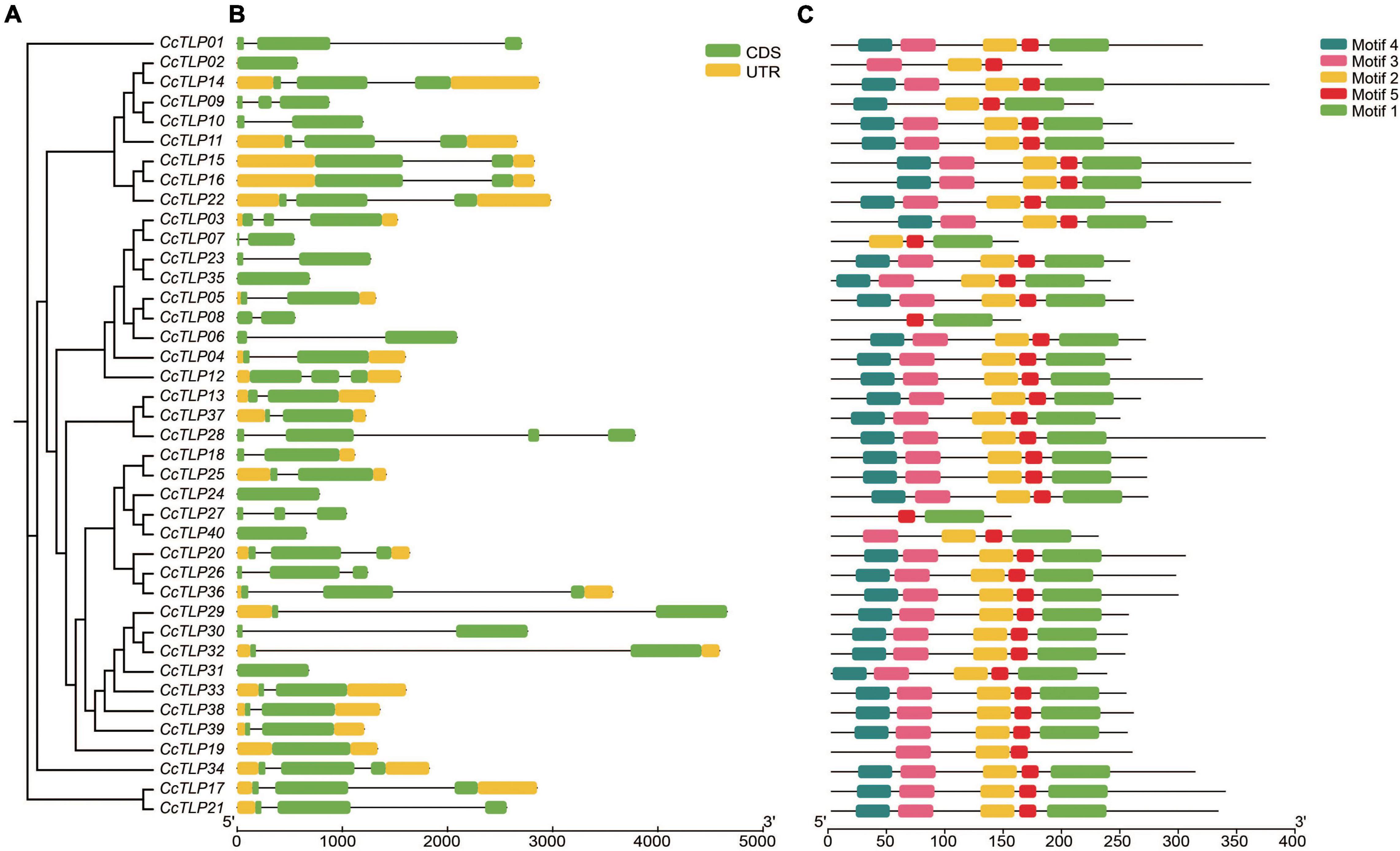
Figure 3. Rectangular phylogenetic tree, gene structure, and motif distribution of CcTLP genes. (A) The phylogenetic tree was inferred based on 40 hickory TLP peptides sequences. (B) Gene structure of CcTLP genes including 5′ and 3′-UTR (yellow bar), exon (green bar), and intron (black line). (C) Motif patterns in 40 CcTLP genes.
Cis-acting elements are important short regions in the promoter sequence recognized by specific transcriptional factors, thereby regulating the activity of the promoter and the targeted gene expression. A batch of cis-acting elements was found by predicting the 2 kb CcTLP promoter sequences, such as light-responsive elements, hormonal regulation elements, and stress-related elements. Then, 65 emphasis elements related to stress-responsive, light-responsive, phytohormone-responsive, and plant-responsive were selected for classifying and accounting (Figure 4). Remarkably, the CcTLP11 promoter sequence contained 16 ARE elements involved in the antioxidative response. The CcTLP09 promoter sequence covered 12 ABRE elements, and 10 MYC elements participated in the phytohormone regulation process. The CcTLP genes were revealed by these results to be potentially involved in various life activity regulations, such as the antipathogen process, abiotic stress, and plant development.
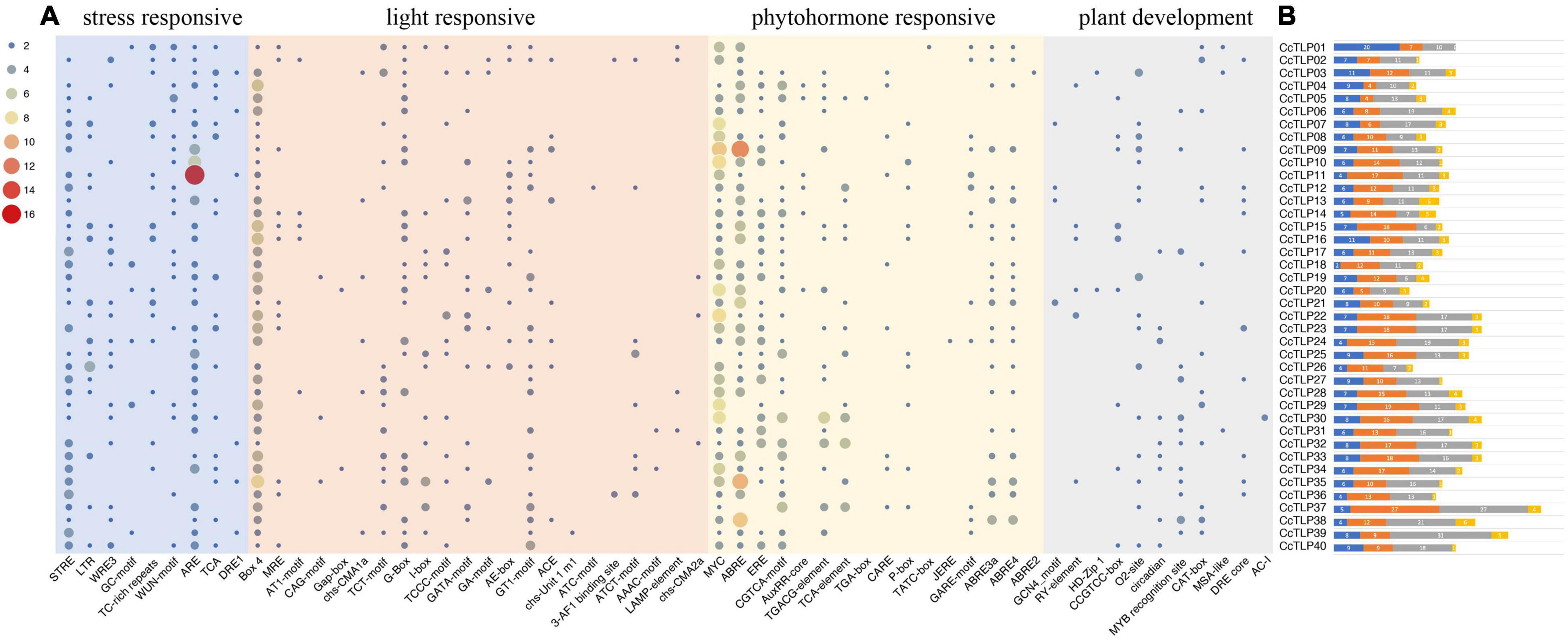
Figure 4. The predicted Cis-acting elements distribution pattern in 40 CcTLP gene promoter regions. (A) These cis-acting elements were classified into four groups: stress-responsive, light-responsive, phytohormone-responsive, and plant-responsive, as shown in the heatmap. (B) The total count of these four categories is displayed in the bar plot. Blue: stress-responsive. Orange: light-responsive. Gray: Phytohormone-responsive. Yellow: Plant-responsive.
In Figure 5, thirty-one miRNAs and 20 CcTLPs were predicted to be involved in the post-transcriptional process. Most of the CcTLPs (16 out of 29) were connected with one or two miRNAs. In contrast, CcTLP17, CcTLP26, CcTLP29, CcTLP34, and CcTLP38 were the most highly active genes to be influenced by four miRNAs. In addition, 24 out of 31 miRNAs targeted one or two TLP genes. Notably, miRNA4221 and miRNA5021 interacted with five TLP genes, suggesting that they had extensive roles in regulating the expression of TLP genes.
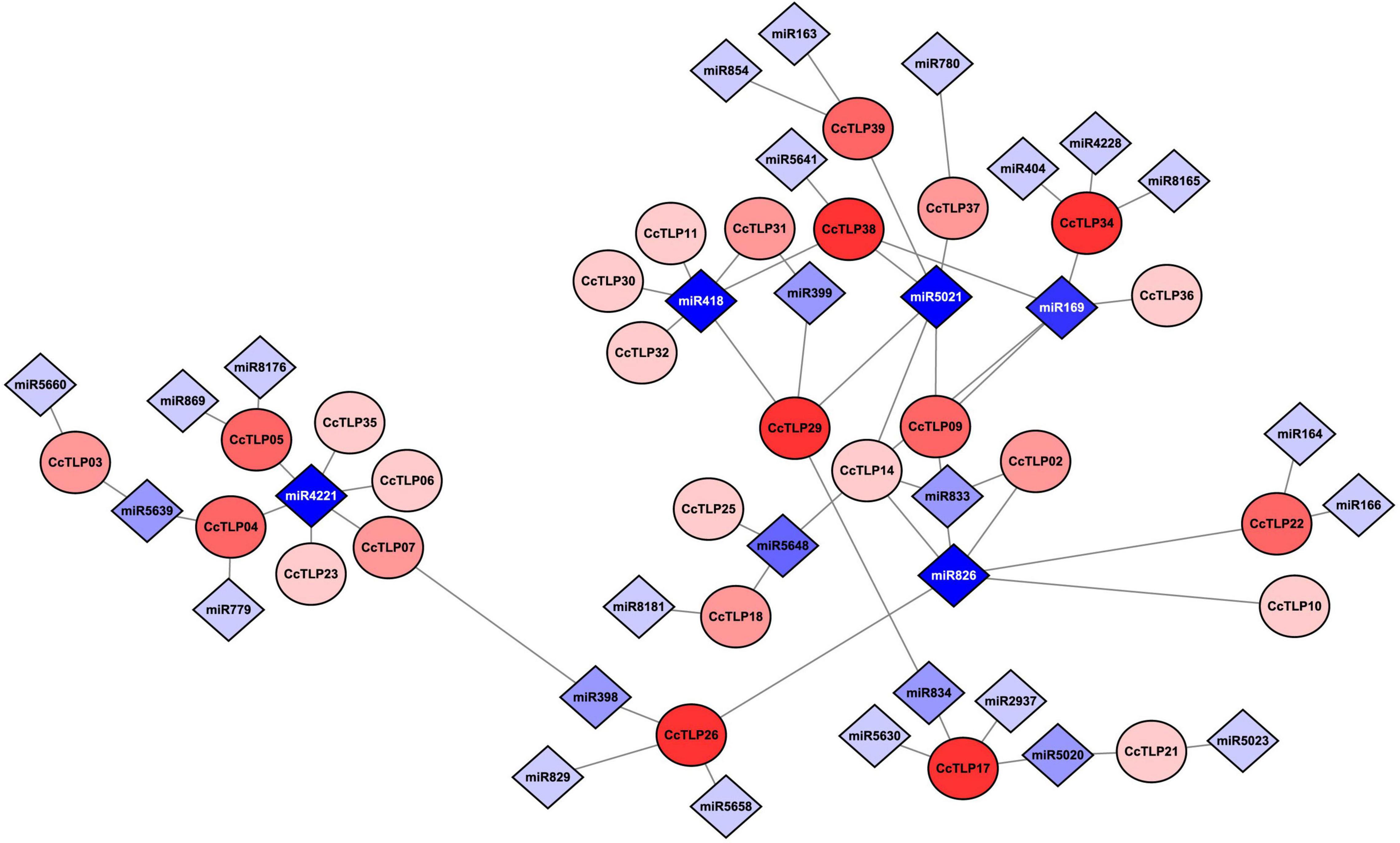
Figure 5. Interaction network diagram between 29 CcTLP genes and 31 micro RNAs (miRNAs). Circle elements represented CcTLP genes and rhombus elements represented miRNA interacting with CcTLP genes. The level of color depth indicated the co-regulation ability.
The SA and JA/ETH defense pathways are antagonistic, reflecting that enhanced resistance against biotrophs is often correlated with strengthened susceptibility to necrotrophs and vice versa. We selected nine CcTLP genes from the same clade with AT1G75040, AT1G75030, and AT1G18250 to quantify their expression levels in leaves after treatments with SA, MeJA, and ETH using qRT-PCR and to investigate the role of CcTLP genes, as demonstrated in Figure 6. It was shown that these most genes were all upregulated under SA treatment, especially CcTLP28, CcTLP29, CcTLP30, CcTLP33, and CcTLP38. However, CcTLP37, CcTLP38, and CcTLP39 were downregulated at all-time points under JA treatment. At the same time, CcTLP28 and CcTLP29 were significantly upregulated during all periods. Under ETH treatment, only two genes (CcTLP38 and CcTLP39) were found to be significantly upregulated. CcTLP29 and CcTLP30 had various changes in expression levels, being upregulated at some points and downregulated at others. These data are indications that CcTLP genes had different sensitivities to exogenous SA, JA, and ETH applications. It was suggested that the sequence of the CcTLP genes shared a high identity. Nonetheless, they had different expression patterns in response to the same hormone.
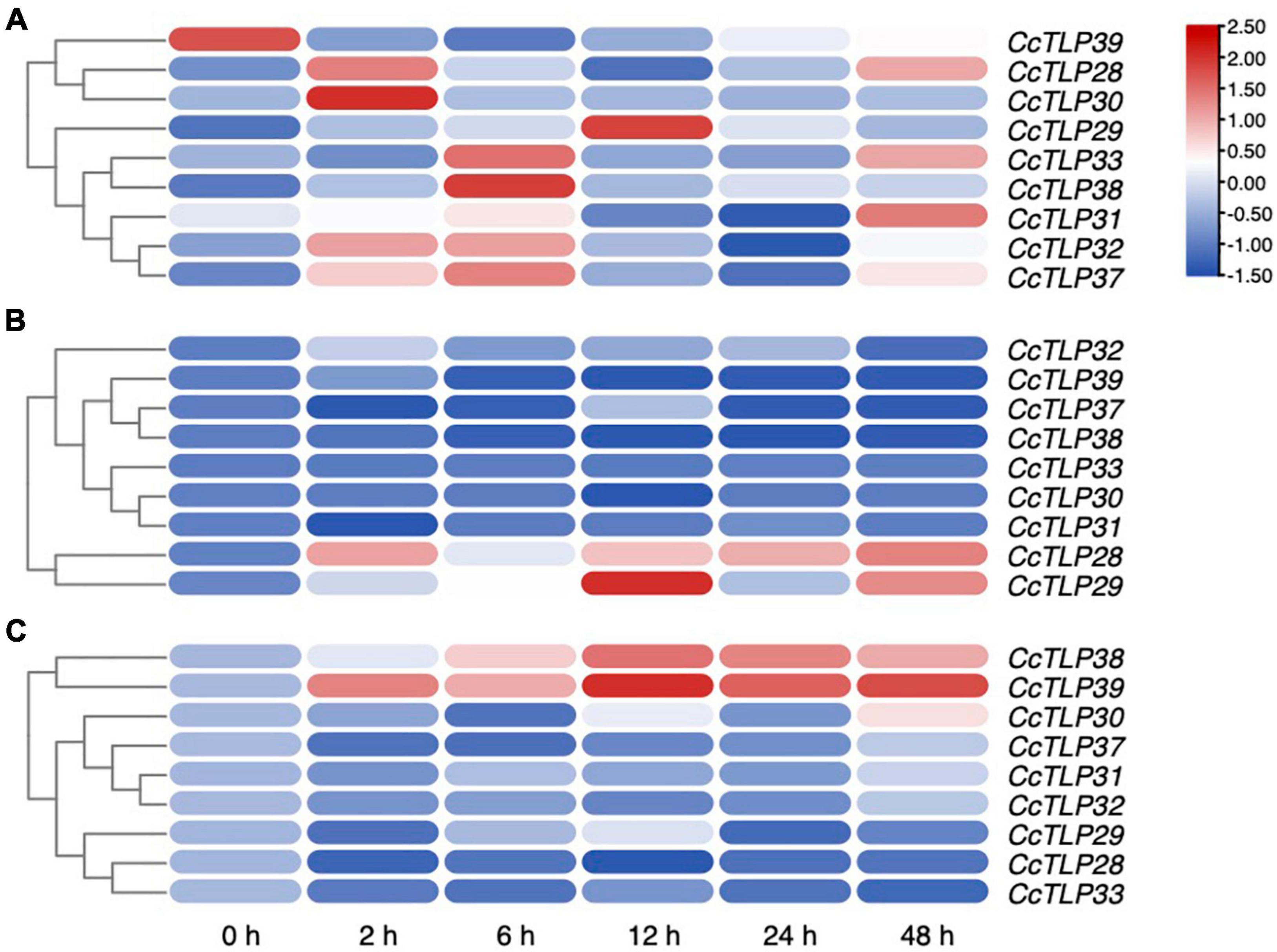
Figure 6. The expression patterns of nine CcTLP genes after plant hormones treatments. The relative expression of nine CcTLP genes after treatments with SA, MeJA, and ETH for 0, 2, 6,12, 24, and 48 h were calculated using quantitative real-time polymerase chain reaction (qRT-PCR) analysis. The expression level was represented by the mean relative expression value. The relative expression level of CcTLP genes was calculated by the standard curve and then normalized by the CcActin expression level. R-package pheatmap were utilized to plot the expression heatmap of CcTLPs after treatments with salicylate (SA), methyl jasmonate (MeJA), and ethephon (ETH) for 0, 2, 6, 12, 24, and 48 h. Color bar in the diagram showed the range of normalized signal intensities.
We further analyzed the changes in the previous nine CcTLP gene expressions to understand the function of TLP genes under infection conditions by B. dothidea. As shown in Figures 7A–C, B. dothidea caused dried and curly leaves, and the plant was led to death. When agar-containing mycelia was applied to the wound site, it became black, and the area enlarged was significantly different from control after 14 dpi. The diameters areas of the disease were broader after 3, 7, and 16 days of treatment (Figure 7D). Three CcTLP genes (CcTLP28, CcTLP29, and CcTLP30) were gradually upregulated with infection time. CcTLP31, CcTLP32, CcTLP33, and CcTLP38 could also be upregulated, and their expression levels were the highest after 2 dpi. Notably, CcTLP31 had an over 20-fold expression change. Based on these results, it is speculated that these genes were positively correlated with hickory resistance to B. dothidea (Figure 7E). In contrast, CcTLP37 and CcTLP39 were downregulated at the indicated time points compared with control (Figure 7E). This result is a suggestion that these CcTLP genes are negatively associated with hickory resistance to B. dothidea.
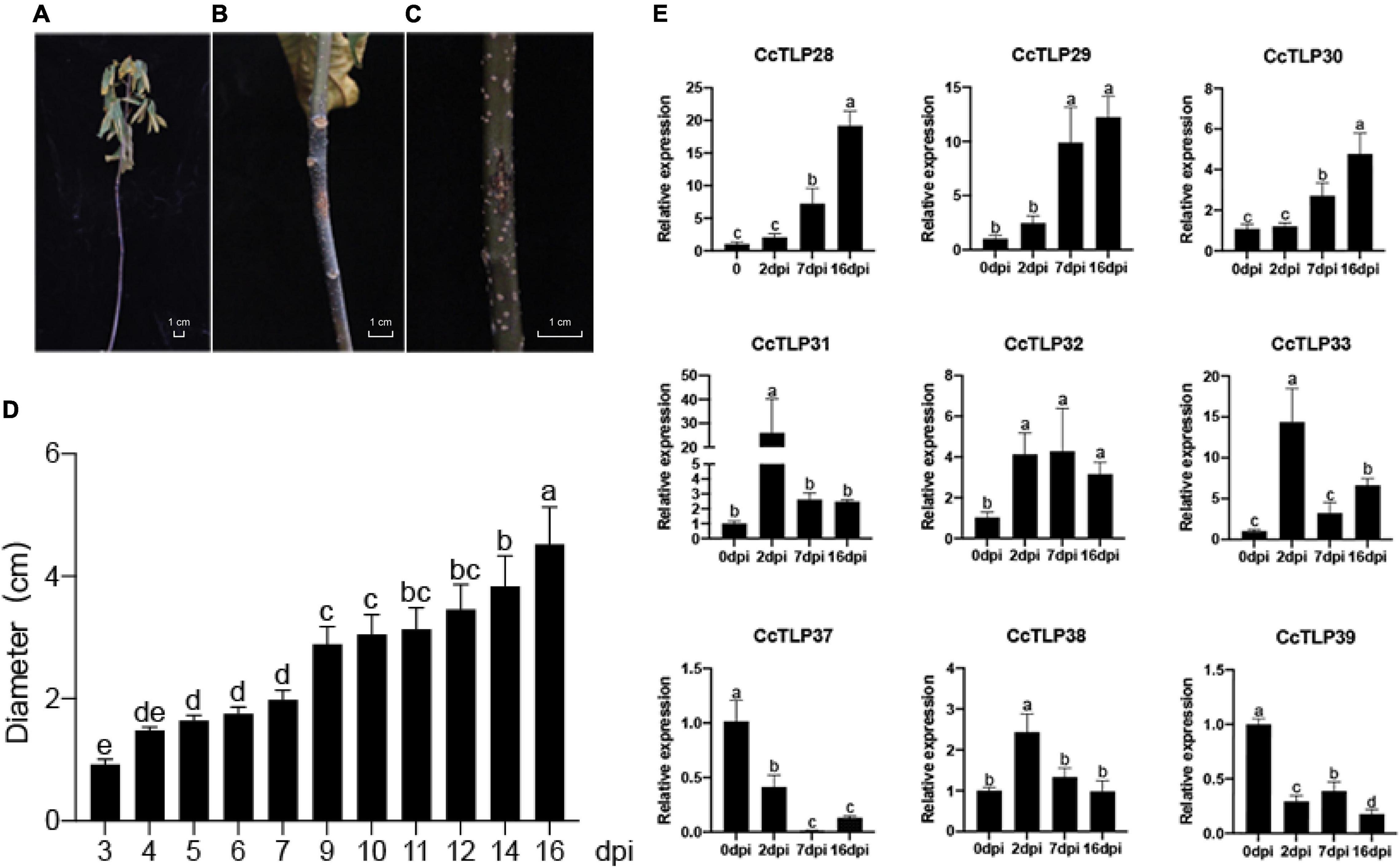
Figure 7. The phenotype and expression analysis of CcTLP genes in response to Botryosphaeria dothidea infection. (A) Plant phenotype after 14 days after inoculation (dpi). The leaves were dried and curly. (B,C) The disease areas of wound sites were applied to agar containing B. dothidea mycelia and empty 0.5-cm-diameter agar, respectively, after 14 dpi. (D) Analysis of the diameter length of disease areas at 3, 4, 5, 6, 7, 9, 10, 11, 12, 14, and 16 dpi. This is represented by the mean ± SE. Error bars were obtained from three technical repeats. (E) An expression analysis of CcTLP family genes under B. dothidea infection. Samples were collected at 0, 2, 7, and 16 days after treatment. Each bar is the mean ± SE from three technical repeats. Different letters in the error bar showed significant differences between the sample groups as determined using ANOVA (Duncan’s test, p < 0.05).
It has been reported that TLP proteins have antifungal functions. Subcellular localization analysis was further conducted to explore where they function. The GFP protein alone was present in the plasma membrane, cytoplasm, and nuclei, while the fluorescent signals of most CcTLPs-GFP were all found in the cytoplasm and plasma membrane (overlapped perfectly with the red fluorescence of the plasma membrane marker), as shown in Figure 8.
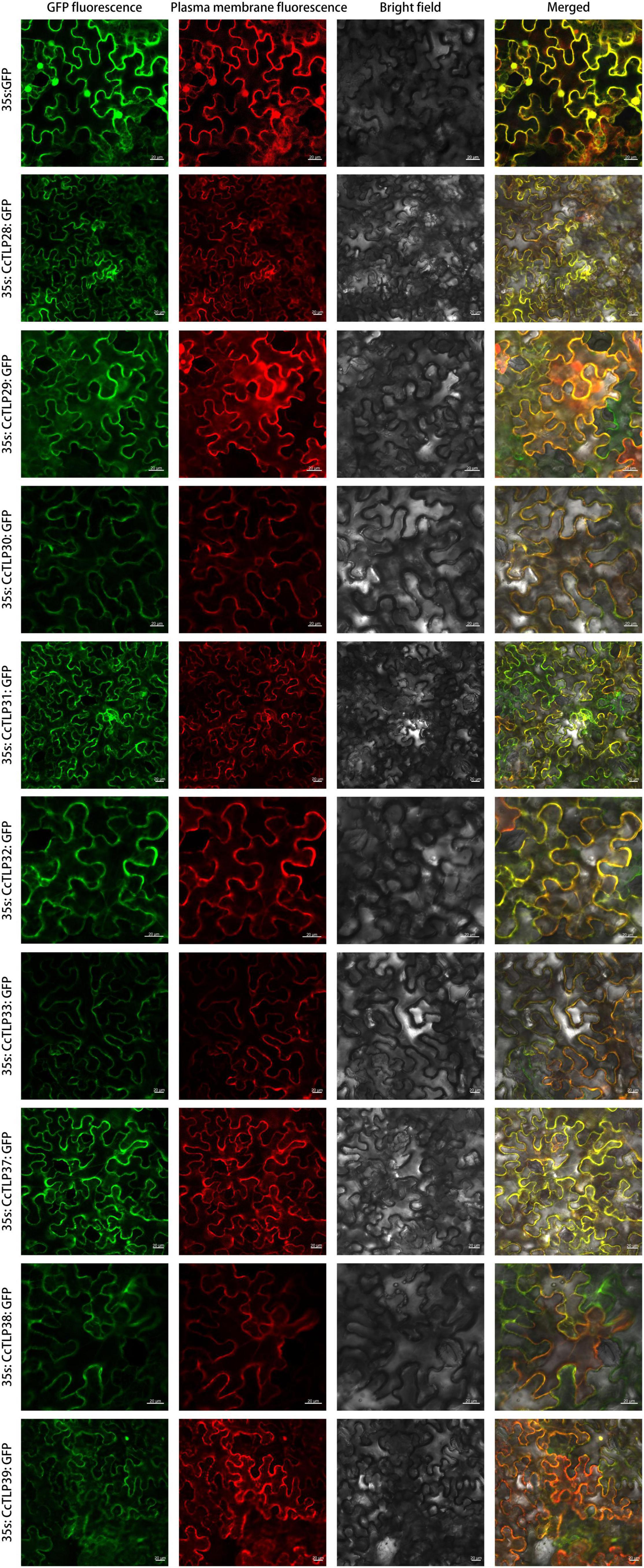
Figure 8. The subcellular localization analysis of the CcTLP28, CcTLP29, CcTLP30, CcTLP31, CcTLP32, CcTLP34, CcTLP37, CcTLP38, and CcTLP39 in Nicotiana. benthamiana. The transient expression of the 35S::CcTLP-EGFP fusing construct and the 35S::EGFP construct in N. benthamiana. Green fluorescent protein (GFP) fluorescence was observed 3 dpi using a confocal microscope. Scale bars: 20 μm.
Hickory is a critical economic tree species from the Juglandaceae family. Hickory nuts have several superior nutritional qualities, such as high quantities of mono-unsaturated fatty acids, thiamine, and dietary fibers, which are beneficial for human health (Miraliakbari and Shahidi, 2008; Bolling et al., 2011). Although these benefits may promote the cultivation and commercial exploitation of hickory, its planting area is still restricted to limited areas, such as Anhui, Hangzhou, Guizhou, and Hunan provinces in China. This stress situation primarily results from its weak resistance to the environment and biotic stress (Yang et al., 2015; Grauke et al., 2016). Therefore, it is vital to understand the genetic basis of hickory and exhume resistant gene resources to abiotic and pathogen threats.
The TLP family members have essential functions in plant development and response to adversity stresses (Singh et al., 2013). In this study, 40 TLP family member genes were identified from the hickory genome using a pipeline of bioinformatics approaches, with more members than amborella (19), water lily (33), Arabidopsis (32), rice (37), ginkgo (37), P. aphrodite (22), grape (33), but fewer than soybean (62), walnut (66), and pecan (43). The widely available TLP genes have a vital role in plants. It has been shown in previous studies that plant TLP gene families are formed by the evolutionary amplification of 10 common ancestral genes before the divergence of monocotyledons and dicots. The diversity of TLP genes in terrestrial plants is significantly better than that in animals and fungi (Liu et al., 2010a). During the long way of the evolution of the TLP gene family, WGD is thought to be an essential driver of TLP gene expansion and a significant source of functional evolution. In our research, we found that four tandem duplicate events appeared in the TLP family, accounting for 30%. It was shown by gene synteny analysis that five pairs of genes emerged from the WGD event. The expansion of the CcTLP family in hickory could jointly be explained by these results (Figure 2).
Members of the same branch were revealed by gene structure and conserved motif analyses to have similar gene structures. Most had the same number of conserved motifs and distribution patterns, implying some conserved function among CcTLP members (Figure 3). For instance, most of the CcTLPs contained specific receptor binding sites for antifungal activity: G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C and REDDD structure (Figure 1). A cis-acting element in the promoter interacts with specific transcription factors to form a transcription initiation complex that initiates gene-specific expression (Figure 4). CcTLPs contain many hormonal regulation elements, such as TGACG-motif involved in MeJA responsiveness and a TCA-element involved in SA responsiveness. Phytohormones, especially SA, JA, and ETH, function as key signaling molecules in the plant defense response under pathogen attack (Reymond and Farmer, 1998; Pieterse and van Loon, 1999; Thomma et al., 2001). It was shown by the results that the expression of CcTLP family genes could be influenced by SA, JA, and ETH. Especially under SA treatment, CcTPL28, CcTPL30, and CcTPL32 reached the highest expression after 2 h (Figure 6). The VqTLP29 transgenic lines have been revealed in previous studies to have improved resistance to powdery mildew and Pst DC3000 (Yan et al., 2017). In addition, the TLP transgenic poplars had an enhanced resistance against spot diseases (Sun et al., 2020). We accessed the expression of the CcTLP genes in hickory seedlings infected with B. dothidea to verify their roles in signaling pathways related to pathogen-induced stress. Similarly, it was found that the infection could significantly regulate seven CcTLP genes, except for CcTLP37 and CcTLP39 (Figure 7). Moreover, the expression of CcTLP31, CcTLP33, and CcTLP38 peaked under the B. dothidea infection after 2 days. Additionally, the expression of SA synthetic-related genes phenylalanine ammonia lyase (PAL) and the non-expressor of pathogenesis-related genes 1 (NPR1) were observed to be increased, indicating the possible increasement of SA content after infection (Supplementary Figure 3). Consequently, we could hypothesize how CcTLP genes participated in disease resistance. Under B. dothidea infection, there is an increase of SA endogenous levels. Then, transcription factors combine on the SA responsive elements, causing the upregulation of many TLP genes against pathogen attacks.
For the localization of TLP proteins, AdTLP and TaPR5 were identified as extracellular proteins (Wang et al., 2010; Singh et al., 2013). Other TLPs, RlemTLP, and CsTLP1, being predicted as extracellular, were found to be located in both the periphery of the plasma membrane and cytoplasm and also had an antifungal function (Garcia-Casado et al., 2000; Kim et al., 2009). The localization analysis of nine recombinant CcTLPs showed cytoplasm location (Figure 8), consistent with previous studies and location predictions (Table 2). Notably, it was observed that the fluorescent signals of most CcTLPs-GFP overlapped perfectly with the red fluorescence of the marker, suggesting that they were also present in the plasma membrane, which was different from the prediction. Therefore, the antifungal function of CcTLPs in hickory was inferred by us because of these results. Some of these TLPs contained N-signal peptides and transmembrane domains, which guided mature protein outward to participate in the defense response (Li et al., 2020). Therefore, it is likely that CcTLPs also have an antifungal function and are related to some cytoplasmic organs in cells.
Thaumatin-like proteins are indispensable parts of the plant immune system that accumulate rapidly to a high content under biotic stress. In this study, 40 hickory TLP genes were identified and classified by phylogenetic relationship, gene structure, and motif distribution. The significant structure of eight stable disulfide bonds, REDDD, G-X-[GF]-X-C-X-T-[GA]-D-C-X(1,2)-G-X-(2,3)-C were discovered and signed. Fifty-seven cis-elements within 40 CcTLP genes related with stress, light, phytohormone, and plant responses were discovered. Among these genes, nine CcTLP genes distributed in the same cluster as AtTLP can be regulated by plant hormones, especially salicylic acid (SA). It was shown by qRT-PCR that the expression of seven CcTLP genes was significantly induced among the nine genes under B. dothidea inoculation. The expression of CcTLP38, CcTLP32, CcTLP33, CcTLP30, and CcTLP31 was upregulated considerably with the treatments with SA and B. dothidea infection. The resistance against biotrophic and hemi-biotrophic microbes is mediated by SA-signaling. Therefore, when fungi attack plants, the endogenous level of SA may increase, causing the upregulation of the TLP gene secreted to the extracellular to achieve an antifungal effect. Using an assay of N. benthamiana, the CcTLP protein was indicated by the leave cell to be located in the plasma membrane and cytoplasm. The disease-resistant function in CcTLP family was explored in the results, providing helpful information for aiding the Chinese hickory molecular breeding improvement process.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
PL, YL, and JH conceived and designed this study. WG and PL analyzed the data. PL, YX, YG, and SL performed the experiments. PL and WG wrote the manuscript. YL, JH, and KW edited and reviewed the writing. HL and YG offered resources. All authors have read and approved this manuscript.
This research was financially supported by the Zhejiang Key Research and Development Project (2021C02037), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (2022C02009), the Key Research and Development Project of Zhejiang Province, China (2020C02005), the Zhejiang Agriculture New Variety Breeding Major Science and Technology Special (2021C02066-12), and the Zhejiang University Student Science and Technology Innovation Activity Plan (New Seedling talent Plan subsidy project, 2021R412004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.849043/full#supplementary-material
Abad, L. R., D’Urzo, M. P., Liu, D., Narasimhan, M. L., Reuveni, M., Zhu, J. K., et al. (1996). Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 118, 11–23. doi: 10.1016/0168-9452(96)04420-2
Anžlovar, S., and Dermastia, M. (2003). The comparative analysis of osmotins and osmotin-like PR-5 proteins. Plant Biol. 5, 116–124. doi: 10.1055/s-2003-40723
Barthakur, S., Babu, V., and Bansal, K. C. (2001). Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J. Plant Biochem. Biotechnol. 10, 31–37. doi: 10.1007/BF03263103
Bolling, B. W., Chen, C.-Y. O., McKay, D. L., and Blumberg, J. B. (2011). Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 24, 244–275. doi: 10.1017/S095442241100014X
Brandazza, A., Angeli, S., Tegoni, M., Cambillau, C., and Pelosi, P. (2004). Plant stress proteins of the thaumatin-like family discovered in animals. FEBS Lett. 572, 3–7. doi: 10.1016/j.febslet.2004.07.003
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al. (2009). BLAST+: architecture and applications. BMC Bioinform. 10:421. doi: 10.1186/1471-2105-10-421
Capella-Gutierrez, S., Silla-Martinez, J. M., and Gabaldon, T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. doi: 10.1093/bioinformatics/btp348
Chou, K.-C., and Shen, H.-B. (2008). Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 3, 153–162. doi: 10.1038/nprot.2007.494
Dai, X., Zhuang, Z., and Zhao, P. X. (2018). psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic Acids Res. 46, W49–W54. doi: 10.1093/nar/gky316
Danecek, P., Bonfield, J. K., Liddle, J., Marshall, J., Ohan, V., Pollard, M. O., et al. (2021). Twelve years of SAMtools and BCFtools. Gigascience 10:giab008. doi: 10.1093/gigascience/giab008
Dodds, P. N., and Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Fierens, E., Gebruers, K., Voet, A. R. D., De Maeyer, M., Courtin, C. M., and Delcour, J. A. (2009). Biochemical and structural characterization of TLXI, the Triticum aestivum L. thaumatin-like xylanase inhibitor. J. Enzyme Inhib. Med. Chem. 24, 646–654. doi: 10.1080/14756360802321831
Finn, R. D., Clements, J., and Eddy, S. R. (2011). HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, W29–W37. doi: 10.1093/nar/gkr367
Garcia-Casado, G., Collada, C., Allona, I., Soto, A., Casado, R., Rodriguez-Cerezo, E., et al. (2000). Characterization of an apoplastic basic thaumatin-like protein from recalcitrant chestnut seeds. Physiol. Plant. 110, 172–180. doi: 10.1034/j.1399-3054.2000.110205.x
Gasteiger, E. (2003). ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788. doi: 10.1093/nar/gkg563
Ghosh, R., and Chakrabarti, C. (2008). Crystal structure analysis of NP24-I: a thaumatin-like protein. Planta 228, 883–890. doi: 10.1007/s00425-008-0790-5
Gimenez-Ibanez, S., Boter, M., Ortigosa, A., García-Casado, G., Chini, A., Lewsey, M. G., et al. (2017). JAZ2 controls stomata dynamics during bacterial invasion. New Phytol. 213, 1378–1392. doi: 10.1111/nph.14354
Grauke, L. J., Wood, B. W., and Harris, M. K. (2016). Crop vulnerability: carya. HortScience 51, 653–663. doi: 10.21273/HORTSCI.51.6.653
Grenier, J., Potvin, C., Trudel, J., and Asselin, A. (1999). Some thaumatin-like proteins hydrolyse polymeric beta-1,3-glucans. Plant J. 19, 473–480. doi: 10.1046/j.1365-313X.1999.00551.x
Guo, W., Chen, J., Li, J., Huang, J., Wang, Z., and Lim, K. J. (2020). Portal of Juglandaceae: a comprehensive platform for Juglandaceae study. Hortic. Res. 7:35. doi: 10.1038/s41438-020-0256-x
Hamamouch, N., Li, C., Seo, P. J., Park, C.-M., and Davis, E. L. (2011). Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol. 12, 355–364. doi: 10.1111/j.1364-3703.2010.00675.x
Hu, B., Jin, J., Guo, A.-Y., Zhang, H., Luo, J., and Gao, G. (2015). GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297. doi: 10.1093/bioinformatics/btu817
Hu, X., and Reddy, A. S. N. (1997). Cloning and expression of a PR5-like protein from Arabidopsis: inhibition of fungal growth by bacterially expressed protein. Plant Mol. Biol. 34, 949–959. doi: 10.1023/A:1005893119263
Huang, C., Li, Y., Wang, K., Xi, J., Xu, Y., Hong, J., et al. (2021). Integrated transcriptome and proteome analysis of developing embryo reveals the mechanisms underlying the high levels of oil accumulation in Carya cathayensis Sarg. Tree Physiol. tab112. doi: 10.1093/treephys/tpab112
Huang, C., Li, Y., Wang, K., Xi, J., Xu, Y., Si, X., et al. (2022). Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development. Food Chem. 370:130975. doi: 10.1016/j.foodchem.2021.130975
Irigoyen, M. L., Garceau, D. C., Bohorquez-Chaux, A., Lopez-Lavalle, L. A. B., Perez-Fons, L., Fraser, P. D., et al. (2020). Genome-wide analyses of cassava pathogenesis-related (pr) gene families reveal core transcriptome responses to whitefly infestation, salicylic acid and jasmonic acid. BMC Genomics 21:93. doi: 10.1186/s12864-019-6443-1
Jami, S. K., Swathi Anuradha, T., Guruprasad, L., and Kirti, P. B. (2007). Molecular, biochemical and structural characterization of osmotin-like protein from black nightshade (Solanum nigrum). J. Plant Physiol. 164, 238–252. doi: 10.1016/j.jplph.2006.01.006
Jiao, W., Li, X., Zhao, H., Cao, J., and Jiang, W. (2018). Antifungal activity of an abundant thaumatin-like protein from banana against penicillium expansum, and its possible mechanisms of action. Molecules 23:1442. doi: 10.3390/molecules23061442
Kalpana, K., Maruthasalam, S., Rajesh, T., Poovannan, K., Kumar, K. K., Kokiladevi, E., et al. (2006). Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci. 170, 203–215. doi: 10.1016/j.plantsci.2005.08.002
Katoh, K. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. doi: 10.1093/nar/gkf436
Kim, B.-G., Fukumoto, T., Tatano, S., Gomi, K., Ohtani, K., Tada, Y., et al. (2009). Molecular cloning and characterization of a thaumatin-like protein-encoding cDNA from rough lemon. Physiol. Mol. Plant Pathol. 74, 3–10. doi: 10.1016/j.pmpp.2009.07.001
Kitajima, S., and Sato, F. (1999). Plant pathogenesis-related proteins: molecular mechanisms of gene expression and protein function. J. Biochem. 125, 1–8. doi: 10.1093/oxfordjournals.jbchem.a022244
Lescot, M. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. doi: 10.1093/nar/30.1.325
Li, Y., Wang, J., Wang, K., Lyu, S., Ren, L., Huang, C., et al. (2022). Comparison analysis of widely-targeted metabolomics revealed the variation of potential astringent ingredients and their dynamic accumulation in the seed coats of both Carya cathayensis and Carya illinoinensis. Food Chem. 374:131688. doi: 10.1016/j.foodchem.2021.131688
Li, Z., Wang, X., Cui, Y., Qiao, K., Zhu, L., Fan, S., et al. (2020). Comprehensive genome-wide analysis of thaumatin-like gene family in four cotton species and functional identification of GhTLP19 involved in regulating tolerance to Verticillium dahlia and drought. Front. Plant Sci. 11:575015. doi: 10.3389/fpls.2020.575015
Liu, D., He, X., Li, W., Chen, C., and Ge, F. (2012). Molecular cloning of a thaumatin-like protein gene from Pyrus pyrifolia and overexpression of this gene in tobacco increased resistance to pathogenic fungi. Plant Cell. Tissue Organ. Cult. 111, 29–39. doi: 10.1007/s11240-012-0167-0
Liu, J.-J., Zamani, A., and Ekramoddoullah, A. K. M. (2010b). Expression profiling of a complex thaumatin-like protein family in western white pine. Planta 231, 637–651. doi: 10.1007/s00425-009-1068-2
Liu, J.-J., Sturrock, R., and Ekramoddoullah, A. K. M. (2010a). The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep. 29, 419–436. doi: 10.1007/s00299-010-0826-8
Liu, Y., Cui, J., Zhou, X., Luan, Y., and Luan, F. (2020). Genome-wide identification, characterization and expression analysis of the TLP gene family in melon (Cucumis melo L.). Genomics 112, 2499–2509. doi: 10.1016/j.ygeno.2020.02.001
Marrano, A., Britton, M., Zaini, P. A., Zimin, A. V., Workman, R. E., Puiu, D., et al. (2020). High-quality chromosome-scale assembly of the walnut (Juglans regia L.) reference genome. Gigascience 9, 1–16. doi: 10.1093/gigascience/giaa050
Martin, K., McDougall, B. M., McIlroy, S., Chen, J., and Seviour, R. J. (2007). Biochemistry and molecular biology of exocellular fungal β-(1,3)- and β-(1,6)-glucanases. FEMS Microbiol. Rev. 31, 168–192. doi: 10.1111/j.1574-6976.2006.00055.x
Midoro-Horiuti, T., Goldblum, R. M., Kurosky, A., Wood, T. G., and Brooks, E. G. (2000). Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. J. Immunol. 164, 2188–2192. doi: 10.4049/jimmunol.164.4.2188
Miraliakbari, H., and Shahidi, F. (2008). Antioxidant activity of minor components of tree nut oils. Food Chem. 111, 421–427. doi: 10.1016/j.foodchem.2008.04.008
Nelson, B. K., Cai, X., and Nebenführ, A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. doi: 10.1093/molbev/msu300
Odeny Ojola, P., Nyaboga, E. N., Njiru, P. N., and Orinda, G. (2018). Overexpression of rice thaumatin-like protein (Ostlp) gene in transgenic cassava results in enhanced tolerance to Colletotrichum gloeosporioides f. sp. manihotis. J. Genet. Eng. Biotechnol. 16, 125–131. doi: 10.1016/j.jgeb.2017.12.002
Petersen, T. N., Brunak, S., von Heijne, G., and Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786. doi: 10.1038/nmeth.1701
Petre, B., Major, I., Rouhier, N., and Duplessis, S. (2011). Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 11:33. doi: 10.1186/1471-2229-11-33
Pieterse, C. M., and van Loon, L. C. (1999). Salicylic acid-independent plant defence pathways. Trends Plant Sci. 4, 52–58. doi: 10.1016/S1360-1385(98)01364-8
Rajam, M. V., Chandola, N., Goud, P., Singh, D., Kashyap, V., Choudhary, M. L., et al. (2007). Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol. Plant. 51, 135–141. doi: 10.1007/s10535-007-0026-8
Reimmann, C., and Dudler, R. (1993). cDNA cloning and sequence analysis of a pathogen-induced thaumatin-like protein from rice (Oryza sativa). Plant Physiol. 101, 1113–1114. doi: 10.1104/pp.101.3.1113
Reymond, P., and Farmer, E. E. (1998). Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1, 404–411. doi: 10.1016/S1369-5266(98)80264-1
Sakamoto, Y., Watanabe, H., Nagai, M., Nakade, K., Takahashi, M., and Sato, T. (2006). Lentinula edode s tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol. 141, 793–801. doi: 10.1104/pp.106.076679
Schmutz, J., Cannon, S. B., Schlueter, J., Ma, J., Mitros, T., Nelson, W., et al. (2010). Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183. doi: 10.1038/nature08670
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Singh, N. K., Kumar, K. R. R., Kumar, D., Shukla, P., and Kirti, P. B. (2013). Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS One 8:e83963. doi: 10.1371/journal.pone.0083963
Slippers, B., Smit, W. A., Crous, P. W., Coutinho, T. A., Wingfield, B. D., and Wingfield, M. J. (2007). Taxonomy, phylogeny and identification of Botryosphaeriaceae associated with pome and stone fruit trees in South Africa and other regions of the world. Plant Pathol. 56, 128–139. doi: 10.1111/j.1365-3059.2006.01486.x
Subramanian, B., Gao, S., Lercher, M. J., Hu, S., and Chen, W.-H. (2019). Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 47, W270–W275. doi: 10.1093/nar/gkz357
Sun, W., Zhou, Y., Movahedi, A., Wei, H., and Zhuge, Q. (2020). Thaumatin-like protein(Pe-TLP)acts as a positive factor in transgenic poplars enhanced resistance to spots disease. Physiol. Mol. Plant Pathol. 112:101512. doi: 10.1016/j.pmpp.2020.101512
Tachi, H., Fukuda-Yamada, K., Kojima, T., Shiraiwa, M., and Takahara, H. (2009). Molecular characterization of a novel soybean gene encoding a neutral PR-5 protein induced by high-salt stress. Plant Physiol. Biochem. 47, 73–79. doi: 10.1016/j.plaphy.2008.09.012
Thomma, B. P., Penninckx, I. A., Cammue, B. P., and Broekaert, W. F. (2001). The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 13, 63–68. doi: 10.1016/S0952-7915(00)00183-7
van Loon, L. C., Rep, M., and Pieterse, C. M. J. (2006). Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. doi: 10.1146/annurev.phyto.44.070505.143425
Wang, J.-H., Gu, K.-D., Han, P.-L., Yu, J.-Q., Wang, C.-K., Zhang, Q.-Y., et al. (2020). Apple ethylene response factor MdERF11 confers resistance to fungal pathogen Botryosphaeria dothidea. Plant Sci. 291:110351. doi: 10.1016/j.plantsci.2019.110351
Wang, X., Tang, C., Deng, L., Cai, G., Liu, X., Liu, B., et al. (2010). Characterization of a pathogenesis-related thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus. Physiol. Plant. 139, 27–38. doi: 10.1111/j.1399-3054.2009.01338.x
Wang, Y., Tang, H., DeBarry, J. D., Tan, X., Li, J., Wang, X., et al. (2012). MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40:e49. doi: 10.1093/nar/gkr1293
Wel, H., and Loeve, K. (1972). Isolation and characterization of Thaumatin I and II, the sweet-tasting Proteins from Thaumatococcus daniellii benth. Eur. J. Biochem. 31, 221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x
Yan, X., Qiao, H., Zhang, X., Guo, C., Wang, M., Wang, Y., et al. (2017). Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 7:4269. doi: 10.1038/s41598-017-04105-w
Yang, J., Zhou, F., Xiong, L., Mao, S., Hu, Y., and Lu, B. (2015). Comparison of phenolic compounds, tocopherols, phytosterols and antioxidant potential in Zhejiang pecan [Carya cathayensis] at different stir-frying steps. LWT - Food Sci. Technol. 62, 541–548. doi: 10.1016/j.lwt.2014.09.049
Zhang, C. Q., and Xu, B. C. (2011). First report of canker on pecan (Carya cathayensis) caused by Botryosphaeria dothidea in China. Plant Dis. 95, 1319–1319. doi: 10.1094/PDIS-05-11-0457
Zhang, L., Chen, F., Zhang, X., Li, Z., Zhao, Y., Lohaus, R., et al. (2020). The water lily genome and the early evolution of flowering plants. Nature 577, 79–84. doi: 10.1038/s41586-019-1852-5
Keywords: TLP gene family, hickory, gene expressions, hormone, B. dothidea, subcellular localization
Citation: Li P, Xu Y, Wang K, Guo W, Gu Y, Lyu S, Huang J, Lin H, Huang C, Xu Z and Li Y (2022) Genome-Wide Identification of TLP Gene Family and Their Roles in Carya cathayensis Sarg in Response to Botryosphaeria dothidea. Front. Plant Sci. 13:849043. doi: 10.3389/fpls.2022.849043
Received: 11 January 2022; Accepted: 16 February 2022;
Published: 01 April 2022.
Edited by:
Paloma Melgarejo, Ministerio de Agricultura, Alimentación y Medio Ambiente, SpainReviewed by:
Jin Zhao, Agricultural University of Hebei, ChinaCopyright © 2022 Li, Xu, Wang, Guo, Gu, Lyu, Huang, Lin, Huang, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ketao Wang, d2FuZ2t0QHphZnUuZWR1LmNu; Yan Li, MjAxODAwNjFAemFmdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.