- National Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, China
Indole compounds with their unique properties of mimicking peptide structures and reversible binding to enzymes are of great exploitative value in the regulation of plant growth. They stimulate root and fruit formation and activate the plant’s immune system against biotic and abiotic factors harmful to the plant. Analysis of target recognition, receptor recognition, key activation sites and activation mechanisms of indoles in plant to enhance crop growth or disease resistance is a crucial step for further developing compounds as plant growth regulators and immune inducers. Therefore, this review focused on the mechanism of action of indoles in regulating plant growth and enhancing plant resistance to biotic and abiotic stresses.
Introduction
Synthesized or extracted artificially, plant growth regulators, also known as phytohormones, possess a physiological effect that is comparable to that of natural plant hormones. Within plants, they bind to hormone receptors in plant cells to form complexes that recognize hormone signals, which in turn trigger a series of physiological and biochemical reactions in the plant, ultimately leading to morphological changes in the plant (Nikonorova et al., 2021). Plant immune inducers act as a catalyst to activate the immune system, making it better defend against agricultural pests and diseases. Within plants, the induction of salicylic acid (SA) and jasmonic acid (JA) biosynthesis can be induced (Gozzo and Faoro, 2013), resulting in the hypersensitive reaction (HR) of the plant cell, which leads to its death to protect the plant from further colonization of pests and diseases (Chen et al., 2014).
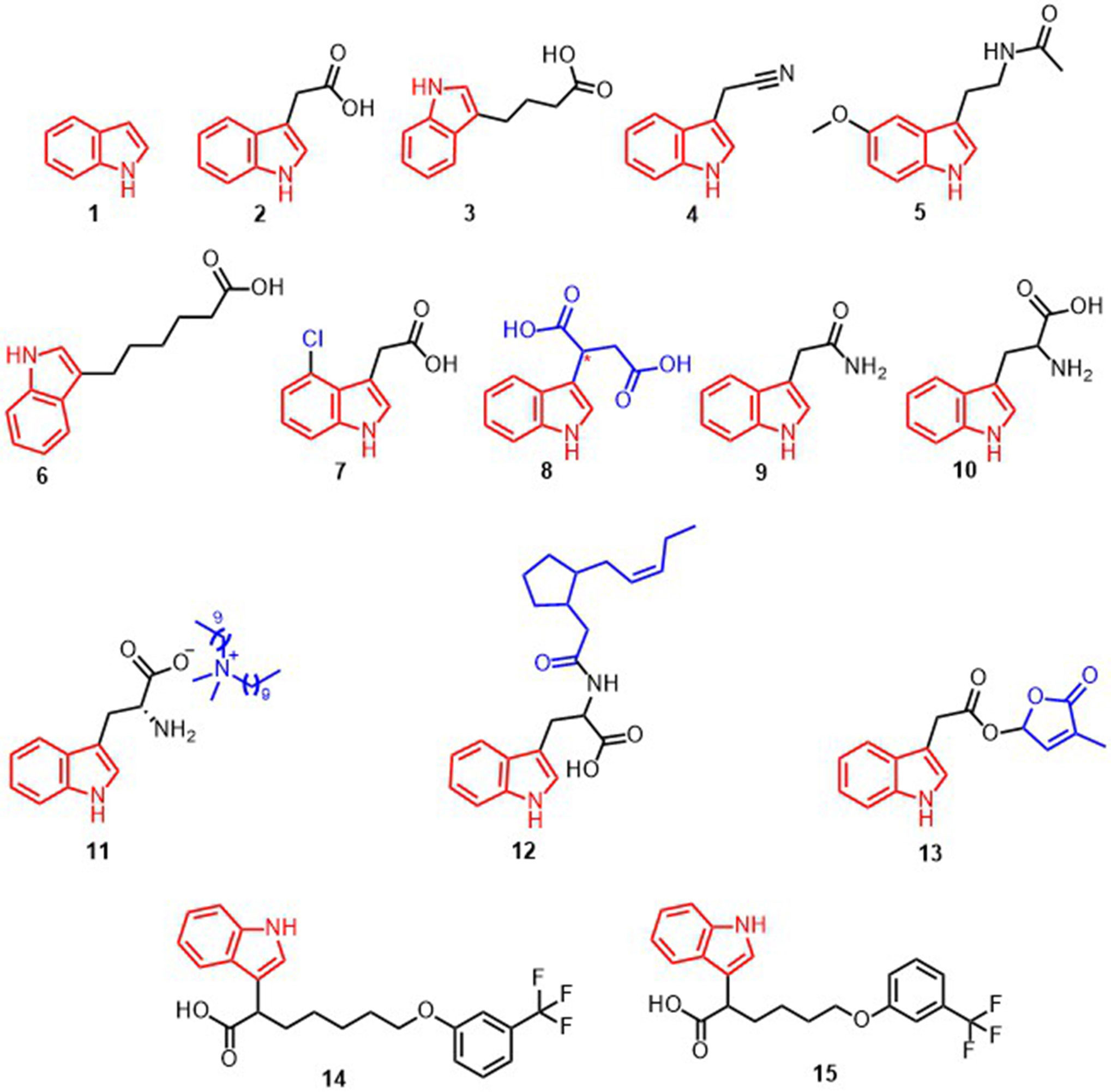
Indole 1 (Figure 1) is a significant structure in drug discovery, as it functions as a scaffold for various receptors (de Sá Alves et al., 2009; Zhang and Chen, 2014b). Indole-based compounds, such as indoleacetic acid (IAA) 2 (Figure 1) (Chen et al., 2020) and indole-3-butyric acid (IBA) 3 (Figure 1) (Li et al., 2018), are commonly used as plant growth regulators in agricultural settings. Indole-3-acetonitrile (IAN) 4 (Figure 1) has been documented to be an effective plant growth regulator, with its efficacy being ten-fold that of IAA. Additionally, it is converted to IAA with growth-regulating effects in plants (Osborne, 1952; Sun et al., 2018). The emergence of indole compounds has revealed a multitude of indole derivatives that can activate plant immunity. Studies conducted by Stahl et al. and Ye et al. have demonstrated that indole, a plant organic volatile, can augment plant immunity to herbivorous insects (Stahl et al., 2016; Ye et al., 2019). Studies have revealed that MT 5 (Figure 1) can increase plant resistance to pathogens by activating MAPK pathways, resulting in the expression of numerous plant protection genes (Lee and Back, 2016a). To further exploit compounds as plant growth regulators and plant immune inducers, identifying targets, recognizing receptors, determining key activation points, and understanding activation mechanisms are necessary (Kusajima, 2019). An analysis of indole compounds about plant growth regulators and plant immune inducers is rarely documented. Therefore, this review examines the mechanism of action of indole compounds with regard to their application in the regulation of plant growth and activation of plant immunity. Our goal is to furnish a reliable source of knowledge for academics in related fields.
Plant growth promoters
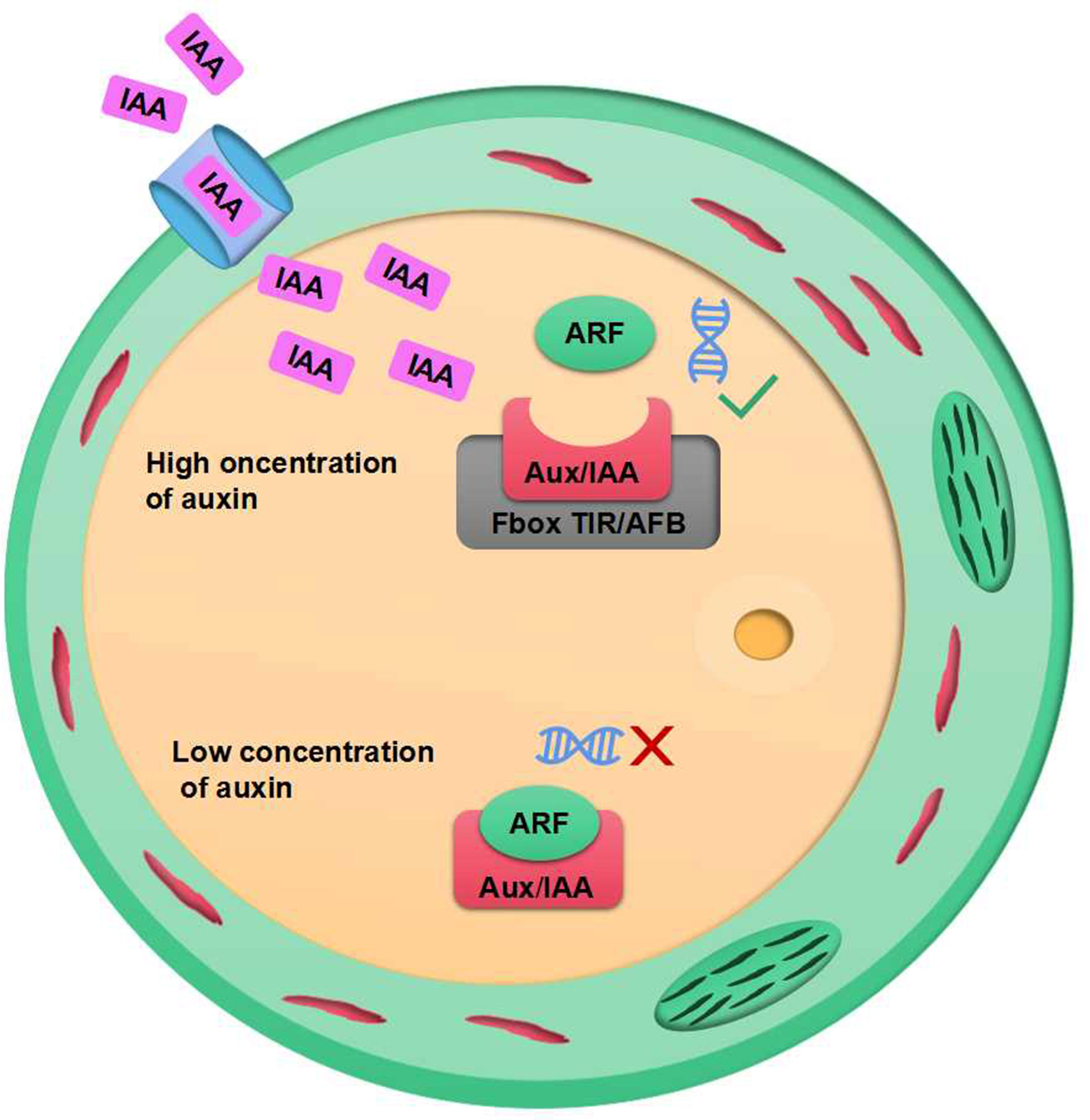
Plant growth promoters are a form of growth regulators that can encourage cell division, elongation, and the growth of vegetation, as well as the maturation of reproductive organs (Cai et al., 2020). The indole compounds with growth-regulating abilities are widespread. However, IAA is the most common and has a major impact on the growth and development of plants. IAA acts as a signal between rhizobium and plants. Experimental studies have demonstrated the application of Stenotrophomonas maltophilia Sg3, Proteus mirabilis BjB17, Providencia rettgeri AlDp5, Bacillus thuringiensis TNJbx.3.3 and Bacillus cereus GR12, which are capable of synthesizing IAA, increased the number of pods of edamame beans (Zhang et al., 2022). The secretion of root in Arabidopsis can trigger Falciphora oryza to produce IAA, thus promoting the development of the lateral root of Arabidopsis (Sun et al., 2020). Gomes et al. and Zhang et al. (Gomes and Scortecci, 2021; Zhang et al., 2022) conducted reviews which revealed that IAA can modulate the transcription and expression of numerous genes through the ubiquitination complex, which is downstream of the repressor and activator of gene transcription factors. When there is a high growth hormone level in the cell, the ubiquitination complex is triggered by transport inhibitor response (TIR) proteins that are part of the growth hormone signaling pathway. This leads to the breakdown of Aux/IAA repressor molecules, thus allowing transcription factors to activate gene transcription in response to the growth hormone. At low growth hormone levels, cells tend to favor Aux/IAA due to dimer-mediated gene transcription by transcriptional auxin response factor (ARF) (as seen in Figure 2).
IBA (Figure 1) is a type of auxin structurally similar to IAA, with two methylene groups to its side chain (Dong et al., 2018; Damodaran and Strader, 2019). The indole ring of IBA is too elongated to successfully bind to the TIR1-Aux/IAA pocket, which is a necessary component of the peroxidase enzymes IBR1, IBR3, IBR10, and ECH2 that are involved in the β-oxidation process leading to IAA production, resulting in a subsequent auxin-level signaling cascade (Fattorini et al., 2017; Aihebaier et al., 2019). It is yet to be determined whether IBA is an IAA-independent signaling molecule.
A new form of growth factor, indole-3-hexanoic acid (IHA) 6, has been identified (illustrated in Figure 1). Structurally, it is analogous to IAA and IBA. It is derived from a novel pyridine carboxylate. It is recognized directly or indirectly by TIR1, the protein responsible for receiving signals from IHA, thus exhibiting a reaction similar to IAA (Napier, 2014). Studies have indicated that IHA can regulate the secretion of growth hormones by converting to IBA, and can also inhibit the transformation of IBA to IAA. Additionally, IHA has been found to induce responses that are distinct from IBA, such as increased amounts of GH3.3 and ACS4 (Song et al., 2021). However, the signaling process of IHA requires further exploration and study.
4-Chloro-indole-3-acetic acid (4-Cl-IAA) 7 (Figure 1) is a variant of IAA, which is distinguished by the presence of a chlorine atom at the 4-position of the indole ring. It was initially isolated from immature pea seeds (Marumo et al., 1968). However, in peas, only 4-Cl-IAA was able to stimulate gibberellin biosynthesis, inhibit the expression levels of ethylene biosynthesis genes (PsACS4, PsACO2, and PsACO3) in the pericarp, and upregulate the expression levels of ethylene receptor and signaling-related genes (PsERS1, PsETR2, PsEBF1, and PsEBF2) in the pericarp thereby reducing ethylene signaling output for pericarp growth (Jayasinghege et al., 2017). Reports indicate that 4-Cl-IAA is a critical signaling molecule in the aging process of oat florets, yet its precise mode of action remains uncertain (Dziurka et al., 2019). Generally, the distance between the aromatic ring and the carboxyl-terminal of IAA, IBA, 4-Cl-IAA, and other structurally similar growth factors is optimally within 0.55 Å for the most preferred activity (Cao et al., 2019; Damodaran and Strader, 2019). Research has shown that the activity of certain compounds in regulating plant growth is affected by the spatial configuration of the compounds. For example, Indole-3-succinic acid (ISA) 8 (Figure 1) proved to be more efficient than IAA or IBA in stimulating the growth of certain seedlings. Through chromatographic and diastereomeric crystallographic splitting, Daniel and his team were able to isolate the enantiomers R-(-)-ISA and S-(+)-ISA of ISA. It was determined that the plant growth-promoting activity of R-(-)-ISA was more effective than that of S-(+)-ISA (Armstrong et al., 2002).
Indoleamine compounds are essential for the growth and development of plants and are involved in many significant biological processes. Such as stress response, growth and development, and reproduction. Indole-3-acetamide (IAM) 9 (Figure 1) is the precursor to the biosynthesis of IAA, which impacts plant growth through two pathways. Pathway 1 works towards the promotion of plant growth when IAM is converted to IAA by the specific hydrolase AMI1 (Pérez Alonso et al., 2020) (Figure 3). Pathway 2 is elucidated in depth through its inhibitory effects.
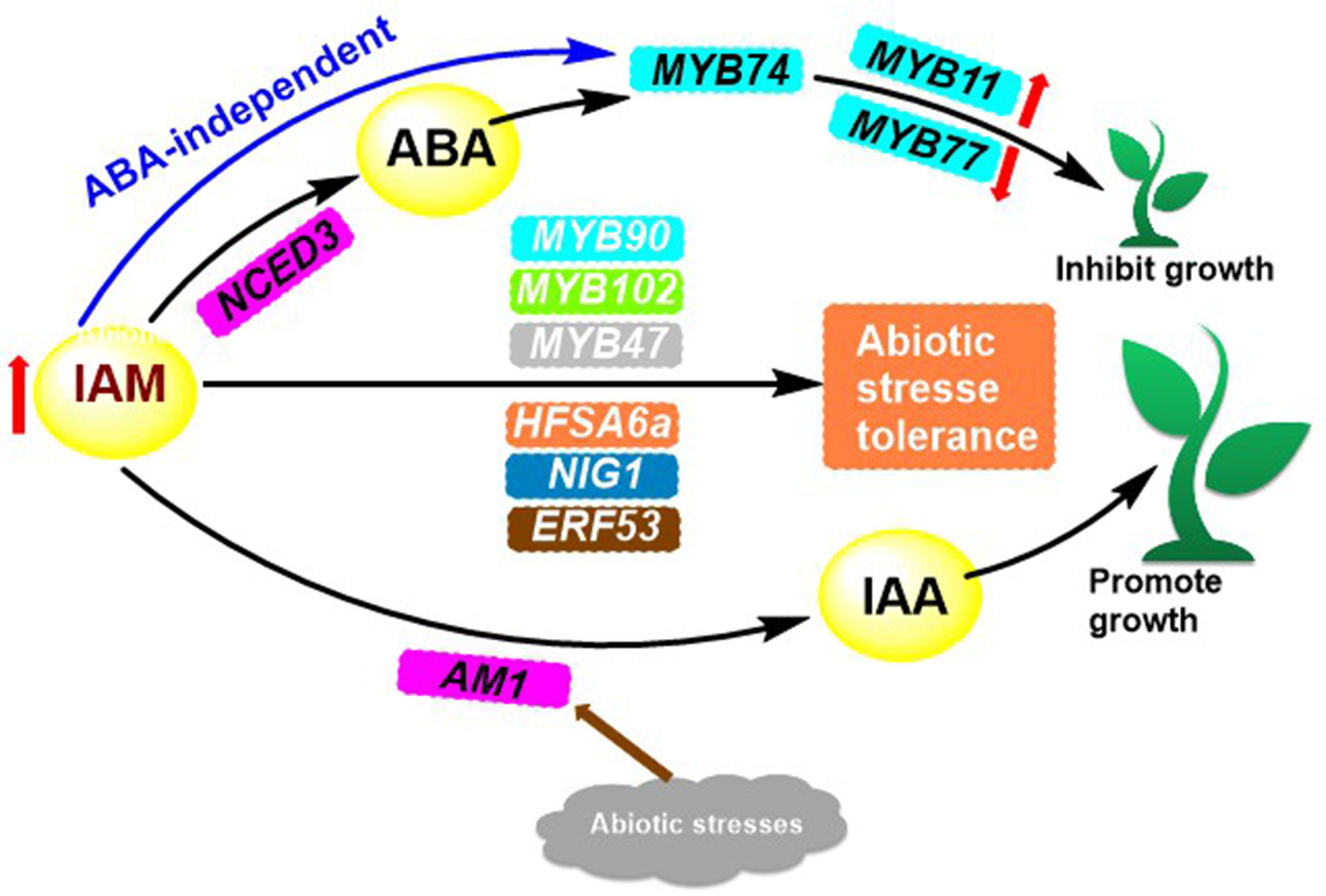
Figure 3 IAM accumulation-mediated transcriptional activation of MYB74 and its conversion to IAA regulates plant growth.
As a major biosynthetic precursor, tryptophan (Trp) 10 (Figure 1) can help enhance the metabolites of Clonostachys rosea, resulting in a stronger capability to support the growth of tomato roots (Han et al., 2022). A class of ionic liquids (ILs) 11 (Figure 1) that demonstrate good solubility was developed by incorporating ammonium cations into the structure of L-Trp. The utilization of Lettuce increases its biomass by a range of 12-20% and enhances the uptake of certain nutrients (Szymaniak et al., 2021). Jasmonoyl-L-Tryptophan (JA-Trp) 12 (Figure 1) is a class of compounds that has the ability to disrupt AUX1, thus resulting in a failure of IAA. However, endogenous JA-Trp plays a minor role in the regulation of plant growth (Staswick et al., 2017). Additionally, Trp can be converted to melatonin by L-Trp decarboxylase (PSID) and tryptophan-5-hydroxylase (CYP71P1) (Zhang et al., 2022). This conversion has been found to have an effect on plant growth, such as promoting root growth after germination (Park and Back, 2012), influencing flowering time and regulating plant sugar metabolism (Zhao et al., 2015; Lee et al., 2019). The extent to which melatonin influences root elongation is dependent on the availability of IAA. At low concentrations, its ability to increase the expression of genes related to IAA signal transduction (IAA19 and IAA24) and IAA biosynthesis (YUC1, YUC2, YUC3, YUC6, and TAR2) as well as some PIN proteins, has been demonstrated to facilitate lateral root development Auxin, coupled with its downstream signal nitric oxide, can activate the growth hormone signaling pathway (Wang et al., 2016; Wen et al., 2016), resulting in the production of adventitious roots in plants (Wen et al., 2016) (Figure 4). Zhang et al. reviewed (Zhang et al., 2022) that the first MT receptor in Arabidopsis was the candidate G protein-coupled receptor 2 (CAND2), a membrane protein that readily binds to MT (Wei et al., 2018). Research has demonstrated that the introduction of melatonin from an external source can induce the upregulation of the genes RPOTm and RPOTmp through the CAND2 receptor and its G protein alpha subunit (GP A1) (Bychkov et al., 2022). Arabidopsis Cand2/pmrt1, which is located at the plasma membrane, is known to interact with GPA1 and control stomatal movement by means of the NADPH oxidase-mediated reactive oxygen species (ROS) signaling pathway (Li et al., 2020). Recently, Zhao et al. reported that exogenous MT can promote the expression of PITDC and PICOMT1 and increase the content of endogenous MT. And the endogenous MT can promote the expression of lignin biosynthesis-related genes (PIPAL, PICCR, PICAD, PICOMT, and PIPOD) and increases lignin accumulation, improving the strength of Paeonia lactiflora Pall stems (Zhao et al., 2022).
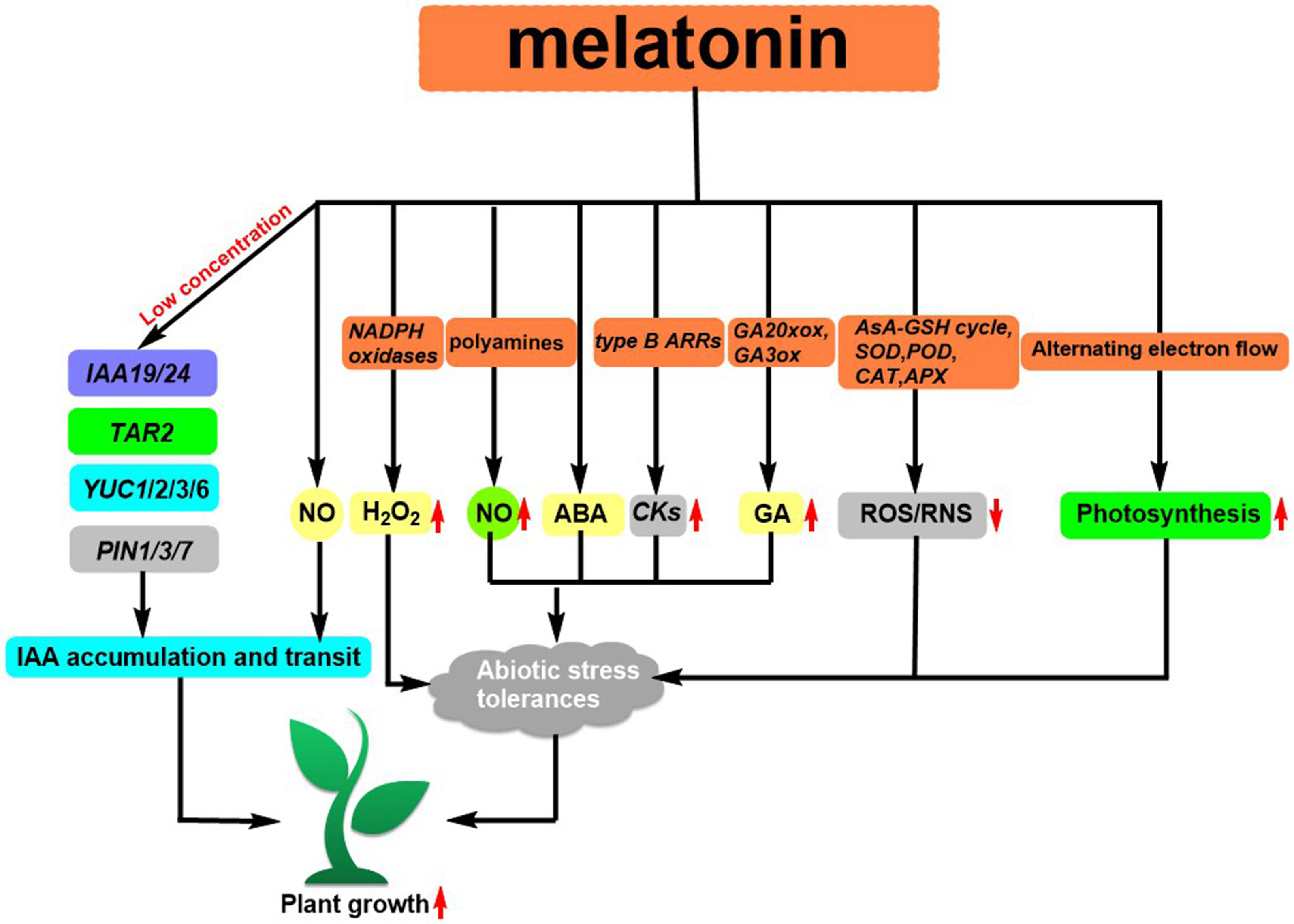
Figure 4 Melatonin regulates IAA biosynthesis and transport as well as regulates abiotic stress responses in plant.
Plant growth restrainers
Plant growth inhibitors are compounds, either man-made or natural, that impede the development of the entire plant or a particular part of the plant (Tuyen et al., 2018; Ellis et al., 2019).
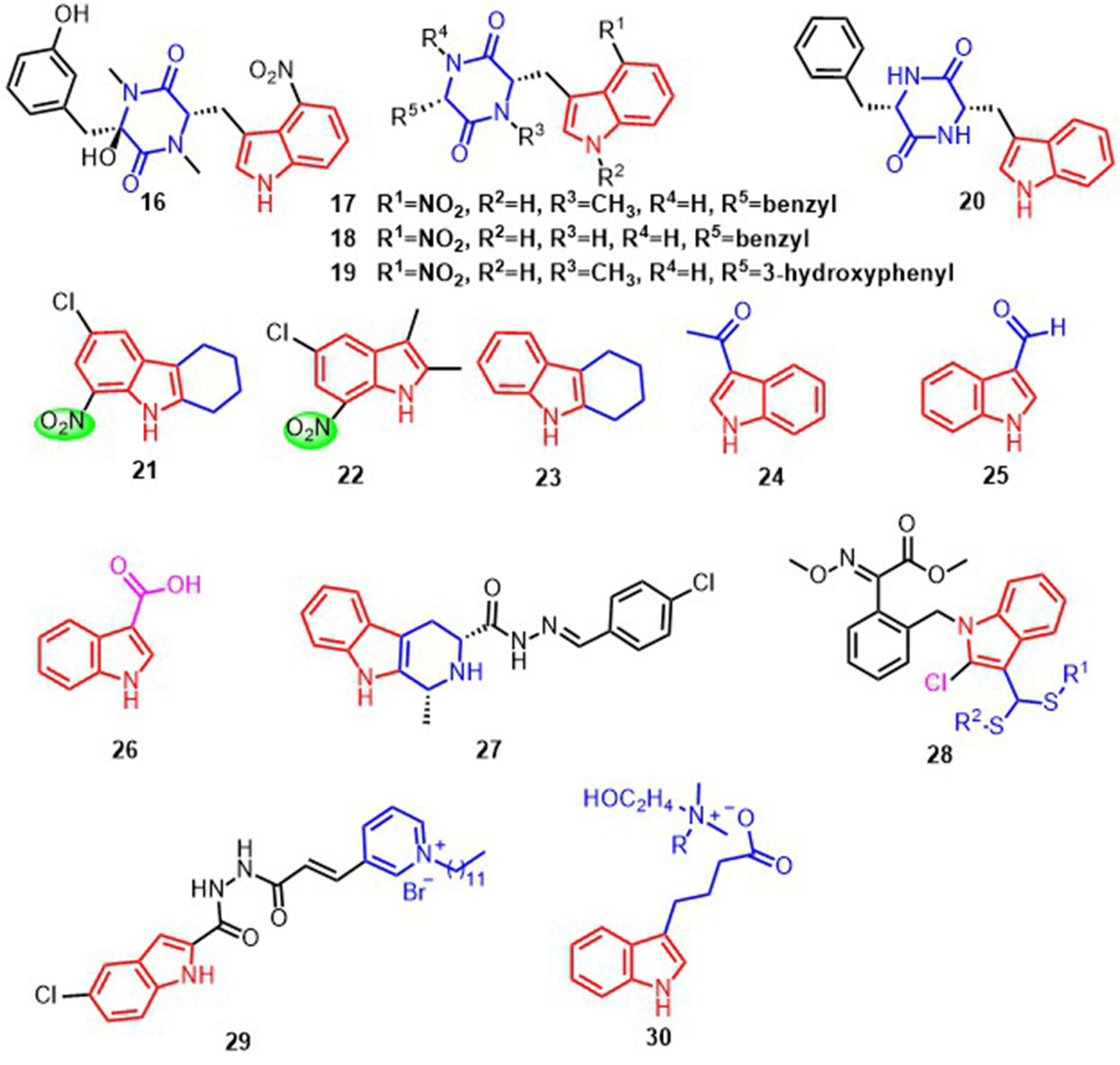
An investigation into the biological activity of chemicals associated with root-parasitic plants revealed that IAA had a potent inhibitory effect on the seed germination of certain root-parasitic plants. Subsequent introduction of the 3-methylfuran-2(5H)-one structure into the carboxylic acid portion of IAA resulted in the formation of compound 13 (Figure 1), which was found to have dual activity, both inducing seed germination and suppressing the growth of embryonic roots after germination (Kuruma et al., 2021). The IAA analogs also showed significant inhibition of root growth in Brassica napus. In particular, compounds 14 and 15 inhibited up to 96% and 95% of B. napus roots at 100 mg/L, respectively, and persisted with 92% and 93% inhibition when the concentration was decreased to 10 mg/L (Wang et al., 2022). The conformational analysis demonstrated that the number of substituents on the benzene ring and electronic effects influenced the inhibitory action of B. napus roots. It was seen that CF3-substituted compounds were the most successful, and the presence of a long-chain alkyl group at the alpha position of the compounds increased their affinity for the TIR1 receptor. In addition, the benzene ring at the alkyl terminus facilitated the binding of the compounds to the TIR1 receptor (Hayashi, 2012; Wang et al., 2022). IAM (Figure 1), a precursor of IAA biosynthesis, has a bifurcated effect on plant growth. In pathway 1, it has a stimulatory effect, while in pathway 2, it has an inhibitory effect. This is caused by the increased levels of IAM in the plant, which leads to the expression of NCED3, a rate-limiting enzyme involved in the biosynthesis of abscisic acid (ABA). This, in turn, results in the overexpression of R2R3 MYB transcription factor genes MYB74 or direct induction of MYB74 overexpression, independent of ABA (Pérez Alonso et al., 2020; Ortiz García et al., 2022). Overexpression of MYB74 has been observed to have an effect on certain genes associated with the proliferation of hyphal tissue cells (e.g., MYB11, MYB77), as well as genes related to the formation of lateral roots in plants, which ultimately leads to a decrease in plant growth (Figure 3).
Thaxtomins are a type of indole derivative featuring a 4-Nitroindole and diketopiperazine structure (King and Calhoun, 2009). Thaxtomin A 16 and thaxtomin C 17, isolated from natural materials, the pre-emergence and post-emergence inhibitory activities against of B. campestris and A. retroflexus are more than 60%. And a study of such compounds by Zhang et al. found that compounds 16, 17, 18, and 19 (Figure 5), with R5 as benzyl, showed significant inhibitory activity (≥85%) against B. campestris and A. retroflexus (King and Calhoun, 2009; Zhang et al., 2015). The nitro group at R1 is also critical for the growth inhibition of B. campestris and A. retroflexus. For example, compound 20 (Figure 5) with the nitro removed exhibited only 10% pre-emergence inhibition activity against B. campestris and A. retroflexus. In addition, the benzyl portion on R5 and the hydroxyl group on the diketopiperazine structure affect the crop selection properties of such compounds. Protoporphyrinogen oxidase (PPO) may be a potential target for compounds 16, 17, 18, and 19, which indirectly affect chlorophyll synthesis and inhibit plant growth (Duke et al., 2019). Another class of compounds with 7-Nitroindole structure, 21 and 22 (Figure 5), can reduce the dry biomass of the weeds of Ipomoea grandifolia and Senna alata by 40% and 37%. This leads to a decrease in plant ATP synthesis and CO2 fixation, interfering plant development (de Souza et al., 2020). In addition, the introduction of methylene structure can improve the lipophilicity of these compounds and promote their entry into plant cells to exert inhibitory effects. For example, compound 23 (Figure 5) inhibited seed germination and root length of plants by 22% and 49.6%, respectively.
The introduction of the methyl ketone structure at the indole 3-position of compound 24 (Figure 5) produced a considerable inhibition of germination and shoot growth of the seeds of Amaranthus tricolor (Chotpatiwetchkul et al., 2022). At a concentration of 400-800 μM, the germination of seeds was completely inhibited. Compound 24 demonstrated inhibitory effects against hydroxyphenylpyruvate dioxygenase (HPPD), potentially interrupting the transformation of HPP to homogentisate and subsequently impeding the formation of tocopherols and plastoquinones. This disruption in the production of carotenoids may result in abnormal plant growth or death (Ndikuryayo et al., 2017; Chotpatiwetchkul et al., 2022). The conformational analysis showed that replacing the 7-position of the indole ring in compound 24 with C to N could enhance the inhibitory activity against HPPD (Chotpatiwetchkul et al., 2022).
Biological stress resistance
Biological stress is a general term for various biological factors that are unfavorable to plant survival and development. It is usually caused by infection and competition, such as diseases, pests, weed hazards, etc (Moustafa-Farag et al., 2019).
Indole can serve as signals for some chewing insect infestations or for necrotic pathogens to invade plants. Studies have found that the indole biosynthesis rate in maize and rice quickly increases when exposed to herbivorous insect attacks. Indole has been demonstrated to bolster plant immunity when faced with pathogenic threats by prompting the build-up of H2O2, which activates the MAPK cascade and phosphorylates protein-like transcription factors. This leads to the activation of defense genes (Jalmi and Sinha, 2015; Mittler and Blumwald, 2015; Perez and Brown, 2015; Shen et al., 2018; Ye et al., 2019), including JA and plant antitoxin biosynthesis genes, cure-associated proteins, and antioxidant enzymes (Gozzo and Faoro, 2013; Shen et al., 2018). In Camellia sinensis, indole is the expression of early defense genes involved in Ca2+ signal, MPK signal, and JA biosynthesis, and the production of secondary metabolites associated with JA and defense is initiated, thus increasing the resistance of Camellia sinensis to herbivores (Ye et al., 2021).
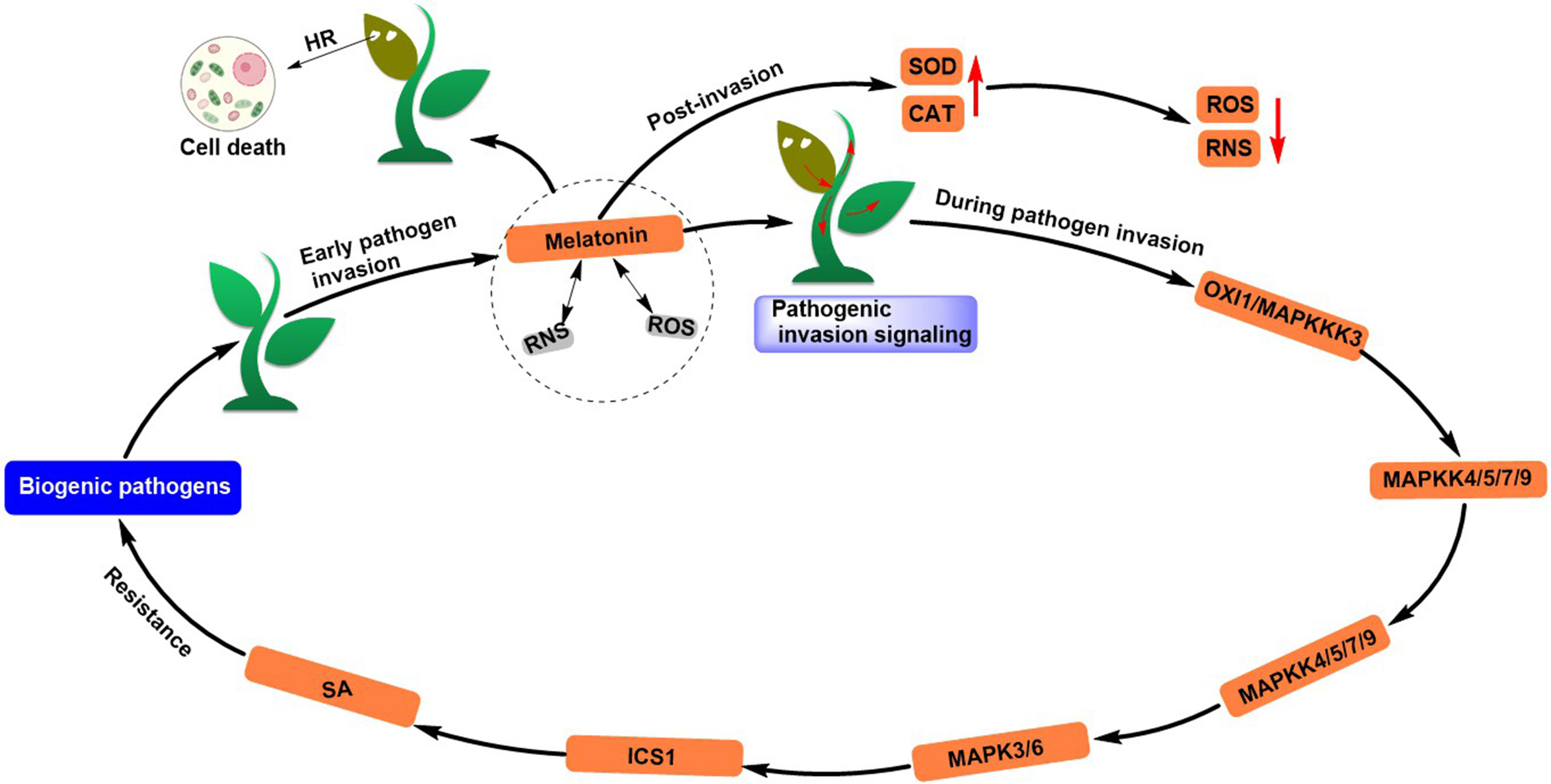
MT also plays a critical role in enhancing plant resistance to biotic stresses. Zhao et al. reviewed (Zhao et al., 2020) that MT, together with ROS and reactive nitrogen species (RNS), promotes cell death and prevents pathogen invasion by forming an integrated feedforward loop during the early stages of pathogen invasion (Gaupels et al., 2017; Arnao and Hernandez-Ruiz, 2018). In addition, the MT-ROS-RNS composition transmits pathogen invasion signals from the starting site to the entire plant and confers plant biological tolerance early in infection. During pathogen invasion, MT acts upstream of SA and accumulates it, and SA further mediates immune response dependent on MAPK signaling cascade. Moreover, MT may also improve plant immunity by altering cell wall composition and influencing crosstalk between auxin and JA signaling pathways. MT further removes excess ROS and RNS by activating gene expression of antioxidant enzymes (SOD, H2O2, etc.) and promotes redox homeostasis in plant systems (Reiter et al., 2009; Arnao and Hernández-Ruiz, 2019) (Figure 6). MT increased early in pathogen invasion and was restored to normal levels by expression of metabolic genes (IDO or 2-OGDD) (Tan et al., 2007; Lee et al., 2016b; Yu et al., 2018).
The compounds indole-3-formaldehyde 25 and indole-3-carboxylic acid 26 (Figure 5) extracted from Purpureocillium lilaci-num had better immune activation for some plants infected by the tobacco mosaic virus (TMV). The application of 25 and 26 can increase the level of transcription of Nonexpresser of PR1 (NPR1), pathogenesis-related 1 (PR1), pathogenesis-related 2 (PR2), pathogenesis-related 5 (PR5) and phenylalanine ammonia-lyase(PAL), 25 and 26 can also upregulate the activity of defensive enzymes such as catalase (CAT) and peroxidase (POD) to reduce peroxide damage to membranes. In addition, 25 also improves (PAL) activity and transcription levels of isochorismate (ICS) and avrPphB susceptible 3 (PBS3) to facilitate SA accumulation. But 26 only mediates SA accumulation through the PAL pathway, triggering systemic acquired resistance in plants (SAR) (Sun et al., 2022). A class of compound 27 (Figure 5) reported by Wang et al. was also able to induce SA and PR2 expression and improve plant resistance to TMV by activating reactive oxygen species and antioxidant levels (Wang and Song, 2020).
In addition to the above indole compounds that enhance plant resistance to viruses via the SA pathway, Wei et al. reported that compound 28 (Figure 5) with a disulfide structure can promote photosynthesis by enhancing chlorophyll content, and also can enhance plant resistance to TMV, cucumber mosaic virus (CMV) and potato Y virus (PVY) by enhancing the activities of defense enzymes such as SOD, POD, PAL and CAT. Futhermore, compound 28 was able to increase malate dehydrogenase (MDH) activity and act with MDH signaling pathway (Figure 7) (Wei et al., 2019).
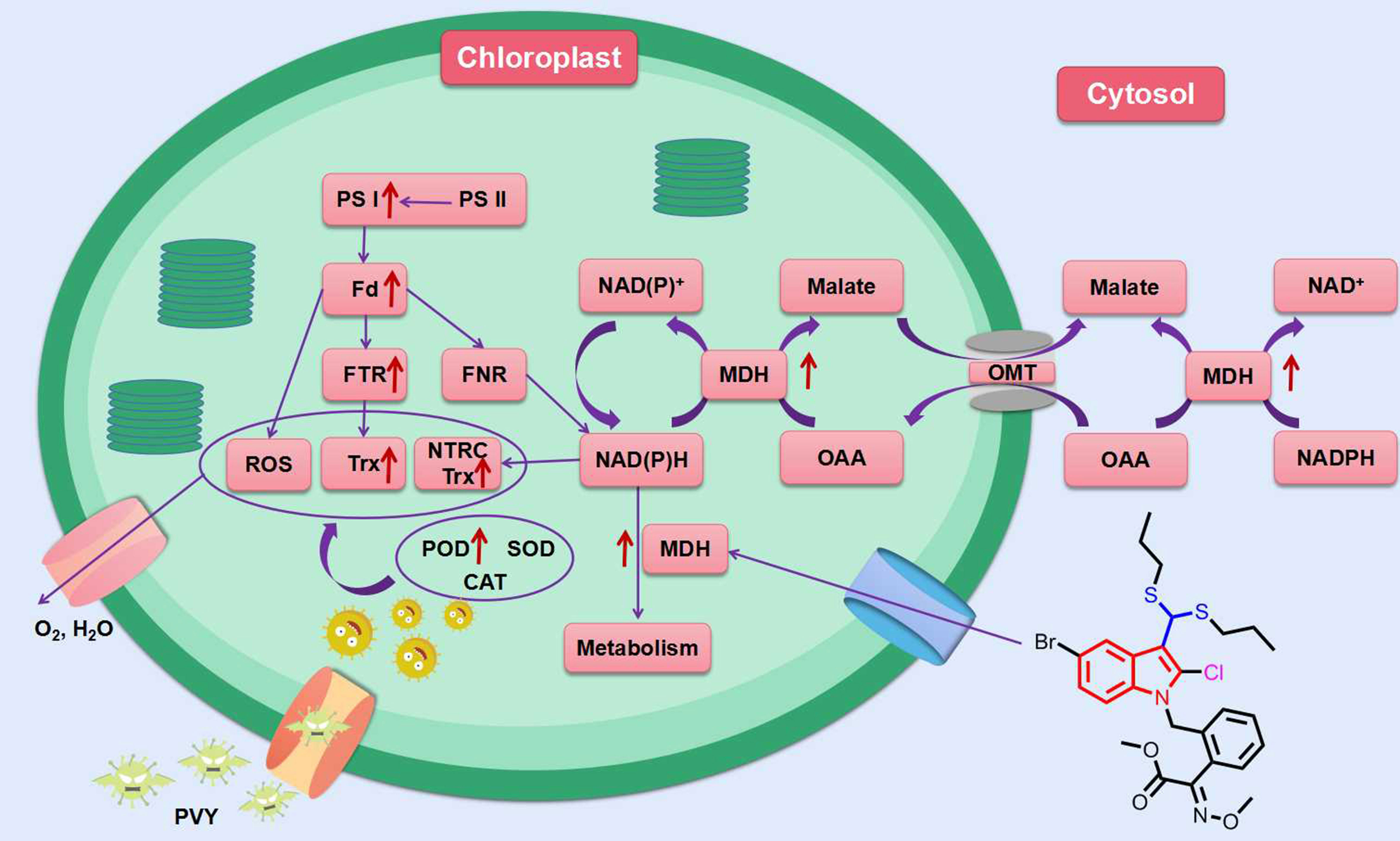
Figure 7 MDH signaling pathway in tobacco response to compound 28. Red arrows indicate that the protein is upregulated in this pathway. (Fd, ferredoxin; FNR, ferredoxin-NADP reductase; FTR, ferredoxin-thioredoxin reductase; MDH, malate dehydrogenase; NTRC, chloroplast NADPH-thioredoxin reductase; OAA, oxaloacetate; OMT, malate/OAA translocators; PS I, photosystem I; PS II, photosystem I; ROS, reactive oxygen species; Trx, thioredoxin). (Wei et al., 2019).
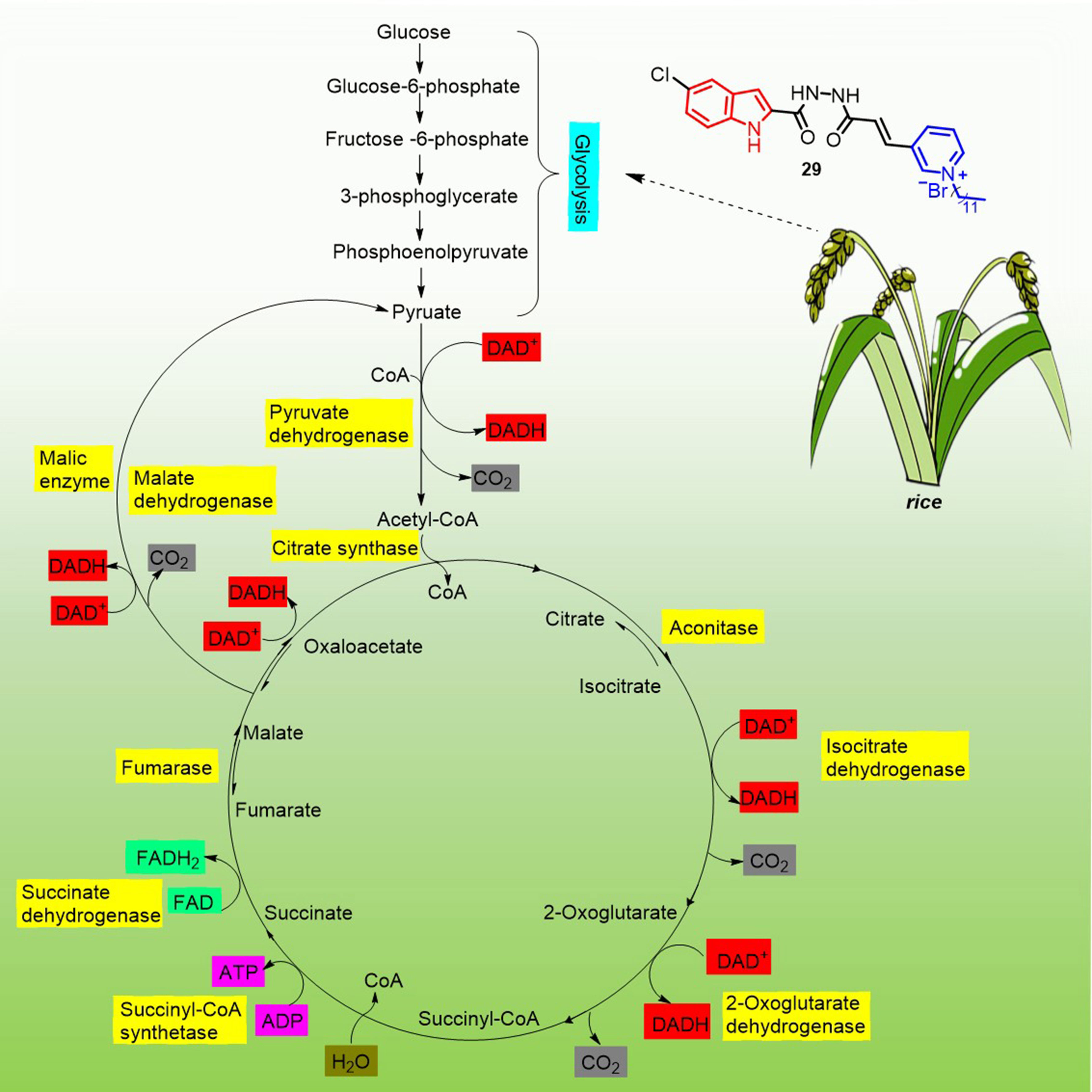
In 2022, Li et al. reported that indole derivatives 29 (Figure 5) containing pyridinium salts could regulate the conversion of glycolysis in rice to produce pyruvate, which was further decarboxylated to produce acetyl-CoA and subsequently entered the citric acid cycle where NAD+ was reduced to NADH. The NADH produced by this process was fed into the oxidative phosphorylation way (Li et al., 2022a). The result of the two closely linked ways improves plant resistance to Xanthomonas oryzae pv. oryzicola and X. oryzae pv. oryzae. by oxidizing nutrients and generating available chemical energy to give to the plant (Figure 8).
Abiotic stress resistance
Abiotic stress is the result of an abiotic factor on a plant in a given environment, which can disrupt its growth and development processes (Mittler, 2002).
IAA, one of the most abundant phytohormones in plants, not only promotes root growth but also enhances plant resistance to abiotic stresses. Studies have shown that the external application of IAA can significantly increase the activities of POD and SOD, as well as the contents of chlorophyll, carotenoid, and soluble protein in Cyphomandra betacea seedlings. Furthermore, it can reduce the Cd content in different organs and improve the resistance of plants to Cd (Li et al., 2022b). Salt stress in plants led to an overexpression of growth hormones, which manifested in increased root hair formation. This alteration augmented the capacity of plants to take up water during the drought (Germanà et al., 2015). IBA, was found to be effective in counteracting the inhibitory effects of Cd and mannitol on plant adventitious roots, and it was also successful in restoring the levels of soluble proteins that had been reduced due to Cd and mannitol (Li et al., 2018). Pernak et al. reported a class of ILs 30 composed of alkylated choline cations and IBA anions that exhibit excellent physical properties such as hydrophobicity and surface activity (Kaczmarek et al., 2020). Compound 30 were found to promote the uptake of essential material nutrients (P, K, Ca, Mg, Na, and Mn) by lettuce, while hindering the uptake of Fe, Zn, and Cu, resulting in a 20% increase in lettuce biomass production. However, the exact mechanism of action is yet to be determined. Additionally, IAM, as a precursor of IAA, can improve plant abiotic stress tolerance by enhancing the expression of abiotic stress-related genes, such as NIG1 and MYB47 (Kim and Kim, 2006; Ding et al., 2013).
Abiotic stress can produce a large amount of ROS and RNS in plants, resulting in oxidative damage to plant cells (Mittler, 2002). MT has been identified to possess antioxidant properties, which can stimulate the activity of antioxidant enzymes such as the Ascorbate-glutathione (AsA-GSH) cycle, SOD, POD, CAT, APX, and the expression of related genes. This helps to eliminate excess ROS and RNS, improving the resilience of plants to abiotic stresses (Shi et al., 2014; Li et al., 2016; Marta et al., 2016; Cui et al., 2017). MT can help to increase abiotic stress resistance through its downstream signals H2O2 and NO. For instance, under low-temperature stress, MT can inhibit sulfhydryl nitrosylation activity and promote NADPH oxidase activity to generate H2O2, protecting against low-temperature stress (Gong et al., 2017). In Fe-deficient plants, MT regulates the plant by modulating the polyamine-induced NO production (Zhou et al., 2016). Fu et al. revealed that MT has the potential to act as an antecedent to ABA, thereby regulating the plant’s response to low-temperature stress. Additionally, MT is known to assist plants in dealing with abiotic stresses (Arnao et al., 2018). Under heat stress, MT was able to up-regulate the expression of cytokinin (CKs) synthesis genes and their transcription factors type B ARRs (Zhang et al., 2017). Under salt stress, melatonin induced the expression of gibberellin (GA) synthesis genes GA20xox and GA3ox (Zhang et al., 2014a). In addition, MT resists the inhibitory effect of abiotic stress on plant photosynthesis by regulating photosynthetic carbon reduction, photorespiration, and O2-dependent alternate electron flow balance (Zhao et al., 2016; Chen et al., 2017; Li et al., 2017) (Figure 4).
Conclusion and perspectives
Investigating the role of indole compounds in the process of plant growth regulation, as well as their impact on plant resistance to both biological and abiotic stress, is the main focus of this review. The promotion of plant growth by indole analogs is closely related to IAA. For instance, IBA requires β-oxidation to form IAA, while IAM can be converted to IAA with the help of a specific hydrolase (AM1). Furthermore, melatonin is essential for enhancing IAA-related transduction genes, biosynthetic genes, and some PIN proteins, thus aiding in the development of plant roots. Indole compounds can boost plant resistance to various biotic stresses through direct or indirect action on SA, JA, and MDH pathways and increase the activity of associated defense response enzymes. Research has revealed that 26 and 27 can heighten plant defenses against TMV by augmenting the activity of defensive enzymes like CAT and POD and stimulating salicylic acid accumulation. Melatonin is the initial factor that triggers the increase of SA, which then activates the MAPK signaling cascade to regulate the immune response. In addition, melatonin can also resist the adverse effects of salt, drought, and cold on plants by promoting the activity of various antioxidant enzymes and the expression of related genes.
Numerous indole compounds have been observed to influence plant growth and stress tolerance. Yet, how these signals are detected by the plant and amplified for further regulation of development and stress resistance is largely unknown. Subsequent studies should focus on examining the effects of indole analog signals on biotic and abiotic stress signal receptors and how they may intensify the signal transduction process. Investigating the interplay between indole signals in plants and other phytohormones will be advantageous in comprehending the mechanism of indole compounds in regulating plant growth and resilience to stress.
Author contributions
All authors have read and agreed to the published version of the manuscript. PS collected and analyzed the refences, wrote the draft of the manuscript. PS, YH and AL completed the Figures. XY and JW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The financial supports from NSFC (National Natural Science Foundation of China) (Nos. 32072445), the Program of Introducing Talents to Chinese Universities (D20023), Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY (2020)004], and the Specific Research Fund of The Innovation Platform for Academicians of Hainan Province (SQ2020PTZ0009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aihebaier, S., Muhammad, T., Wei, A., Mamat, A., Abuduaini, M., Pataer, P., et al. (2019). Membrane-protected molecularly imprinted polymer for the microextraction of indole-3-butyric acid in mung bean sprouts. ACS omega 4, 16789–16793. doi: 10.1016/j.tplants.2018.10.010
Armstrong, D. W., Liu, Y.-S., He, L., Ekborg-Ott, K. H., Barnes, C. L., Hammer, C. F., et al. (2002). Potent enantioselective auxin: indole-3-Succinic acid. J. Agric. Food Chem. 50, 473–476. doi: 10.1021/jf010991f
Arnao, M. B., Hernandez-Ruiz, J. (2018). Melatonin and its relationship to plant hormones. Ann. Bot. 121, 195–207. doi: 10.1093/aob/mcx114
Arnao, M. B., Hernández-Ruiz, J. (2019). Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 24, 38–48. doi: 10.1016/j.tplants.2018.10.010
Bychkov, I. A., Kudryakova, N. V., Shugaev, A. G., Kuznetsov, V. V., Kusnetsov, V. V. (2022). The melatonin receptor CAND2/PMTR1 is involved in the regulation of mitochondrial gene expression under photooxidative stress. Dokl. Biochem. Biophys. 502, 15–20. doi: 10.1134/s1607672922010021
Cai, L., Cai, L., Jia, H., Liu, C., Wang, D., Sun, X. (2020). Foliar exposure of Fe3O4 nanoparticles on nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 393, 122415. doi: 10.1016/j.jhazmat.2020.122415
Cao, X., Yang, H., Shang, C., Ma, S., Liu, L., Cheng, J. (2019). The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 20, 6343. doi: 10.3390/ijms20246343
Chen, Y., Dan, Z., Gao, F., Chen, P., Fan, F., Li, S. (2020). Rice GROWTH-REGULATING FACTOR7 modulates plant architecture through regulating GA and indole-3-Acetic acid metabolism. Plant Physiol. 184, 393–406. doi: 10.1104/pp.20.00302
Chen, L., Fan, J., Hu, Z., Huang, X., Amombo, E., Liu, A., et al. (2017). Melatonin is involved in regulation of bermudagrass growth and development and response to low k+ stress. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02038
Chen, M., Zhang, C., Zi, Q., Qiu, D., Liu, W., Zeng, H. (2014). A novel elicitor identified from magnaporthe oryzae triggers defense responses in tobacco and rice. Plant Cell Rep. 33, 1865–1879. doi: 10.1007/s00299-014-1663-y
Chotpatiwetchkul, W., Chotsaeng, N., Laosinwattana, C., Charoenying, P. (2022). Structure-activity relationship study of xanthoxyline and related small methyl ketone herbicides. ACS Omega 7, 29002–29012. doi: 10.1021/acsomega.2c02704
Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C., Xi, Y. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 118, 138–149. doi: 10.1016/j.plaphy.2017.06.014
Damodaran, S., Strader, L. C. (2019). Indole 3-butyric acid metabolism and transport in arabidopsis thaliana. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00851
de Sá Alves, F. R., Barreiro, E. J., Fraga, C. A. M. (2009). From nature to drug discovery: the indole scaffold as a 'privileged structure'. Mini-Rev. Med. Chem. 9, 782–793. doi: 10.2174/138955709788452649
de Souza, J. M., Fazolo, B. R., Ferreira Lacerda, J. W., Moura, M. D. S., Rodrigues Dos Santos, A. C., De Vasconcelos, L. G., et al. (2020). Rational design, synthesis and evaluation of indole nitrogen hybrids as photosystem II inhibitors. Photochem. Photobiol. 96, 1233–1242. doi: 10.1111/php.13295
Ding, Y., Liu, N., Virlouvet, L., Riethoven, J. J., Fromm, M., Avramova, Z. (2013). Four distinct types of dehydration stress memory genes in arabidopsis thaliana. BMC Plant Biol 13, 229. doi: 10.1186/1471-2229-13-229
Dong, H., Guo, M., Liang, Y., Fan, C., Ding, G., Zhang, W., et al. (2018). Preparation and characterization of indole-3-butyric acid nanospheres for improving its stability and utilization. Mater. Sci. Eng. C-Mater. Biol. Appl. 89, 175–181. doi: 10.1016/j.msec.2018.04.004
Duke, S. O., Lydon, J., Becerril, J. M., Sherman, T. D., Lehnen, L. P., Matsumoto, H. (2019). Protoporphyrinogen oxidase-inhibiting herbicides. Weed Sci. 39, 465–473. doi: 10.1017/s0043174500073239
Dziurka, K., Dziurka, M., Warchoł, M., Czyczyło-Mysza, I., Marcińska, I., Noga, A., et al. (2019). Endogenous phytohormone profile during oat (Avena sativa l.) haploid embryo development. In Vitro Cell. Dev. Biol.: Plant 55, 221–229. doi: 10.1007/s11627-019-09967-5
Ellis, G. D., Knowles, L. O., Knowles, N. R. (2019). Increasing the production efficiency of potato with plant growth retardants. Am. J. Potato Res. 97, 88–101. doi: 10.1007/s12230-019-09759-y
Fattorini, L., Veloccia, A., Della Rovere, F., D’angeli, S., Falasca, G., Altamura, M. (2017). Indole-3-butyric acid promotes adventitious rooting in arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 17, 1–14. doi: 10.1007/s12230-019-09759-y
Gaupels, F., Durner, J., Kogel, K. H. (2017). Production, amplification and systemic propagation of redox messengers in plants? The phloem can do it all. New Phytol 214, 554–560. doi: 10.1111/nph.14399
Germanà, M. A., Micheli, M., Chiancone, B., Bianco, C., Casales, F., Defez, R. (2015). Biotization of encapsulated vitro-derived propagules of carrizo citrange. Acta Hortic. 1065, 663–669. doi: 10.17660/ActaHortic.2015.1065.83
Gomes, G., Scortecci, K. (2021). Auxin and its role in plant development: structure, signalling, regulation and response mechanisms. Plant Biol. 23, 894–904. doi: 10.1111/plb.13303
Gong, B., Yan, Y., Wen, D., Shi, Q. (2017). Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in solanum lycopersicum. Physiol. Plant 160, 396–409. doi: 10.1111/ppl.12581
Gozzo, F., Faoro, F. (2013). Systemic acquired resistance (50 years after discovery): Moving from the Lab to the field. J. Agric. Food Chem. 61, 12473–12491. doi: 10.1021/jf404156x
Han, Z., Ghanizadeh, H., Zhang, H., Li, X., Li, T., Wang, Q., et al. (2022). Clonostachys rosea promotes root growth in tomato by secreting auxin produced through the tryptamine pathway. J. Fungi 8, 1166. doi: 10.3390/jof8111166
Hayashi, K. I. (2012). The interaction and integration of auxin signaling components. Plant Cell Physiol. 53, 965–975. doi: 10.1093/pcp/pcs035
Jalmi, S. K., Sinha, A. K. (2015). ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00769
Jayasinghege, C. P. A., Ozga, J. A., Waduthanthri, K. D., Reinecke, D. M. (2017). Regulation of ethylene-related gene expression by indole-3-acetic acid and 4-chloroindole-3-acetic acid in relation to pea fruit and seed development. J. Exp. Bot. 68, 4137–4151. doi: 10.1093/jxb/erx217
Kaczmarek, D. K., Kleiber, T., Wenping, L., Niemczak, M., Chrzanowski, Ł., Pernak, J. (2020). Transformation of indole-3-butyric acid into ionic liquids as a sustainable strategy leading to highly efficient plant growth stimulators. ACS Sustain. Chem. Eng. 8, 1591–1598. doi: 10.1021/acssuschemeng.9b06378
Kim, J., Kim, H. Y. (2006). Functional analysis of a calcium-binding transcription factor involved in plant salt stress signaling. FEBS Lett. 580, 5251–5256. doi: 10.1016/j.febslet.2006.08.050
King, R. R., Calhoun, L. A. (2009). The thaxtomin phytotoxins: Sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 70, 833–841. doi: 10.1016/j.phytochem.2009.04.013
Kuruma, M., Suzuki, T., Seto, Y. (2021). Tryptophan derivatives regulate the seed germination and radicle growth of a root parasitic plant, orobanche minor. Bioorg. Med. Chem. Lett. 43, 128085. doi: 10.1016/j.bmcl.2021.128085
Kusajima, M. (2019). Studies on the mechanism of agricultural chemicals focused on plant hormone signals. J. Pestic. Sci. 44, 270–274. doi: 10.1584/jpestics.j19-05
Lee, H. Y., Back, K. (2016a). Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal. Res. 60, 327–335. doi: 10.1111/jpi.12314
Lee, K., Zawadzka, A., Czarnocki, Z., Reiter, R. J., Back, K. (2016b). Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal. Res. 61, 470–478. doi: 10.1111/jpi.12361
Lee, H. Y., Lee, K., Back, K. (2019). Knockout of Arabidopsis Serotonin N-Acetyltransferase-2 Reduces Melatonin Levels and Delays Flowering. Biomolecules 9, 712. doi: 10.3390/biom9110712
Li, H., Chang, J., Chen, H., Wang, Z., Gu, X., Wei, C., et al. (2017). Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00295
Li, X., Tan, D.-X., Jiang, D., Liu, F. (2016). Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal. Res. 61, 328–339. doi: 10.1111/jpi.12350
Li, D., Wei, J., Peng, Z., Ma, W., Yang, Q., Song, Z., et al. (2020). Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in arabidopsis. J. Pineal. Res. 68, e12640. doi: 10.1111/jpi.12640
Li, H., Wu, S., Yang, X., He, H., Wu, Z., Song, B., et al. (2022a). Synthesis, antibacterial activity, and mechanisms of novel indole derivatives containing pyridinium moieties. J. Agric. Food Chem. 70, 12341–12354. doi: 10.1021/acs.jafc.2c04213
Li, S. W., Zeng, X. Y., Leng, Y., Feng, L., Kang, X. H. (2018). Indole-3-butyric acid mediates antioxidative defense systems to promote adventitious rooting in mung bean seedlings under cadmium and drought stresses. Ecotoxicol. Environ. Saf. 161, 332–341. doi: 10.1016/j.ecoenv.2018.06.003
Li, Z. Y., Zhu, J. P., Wang, Y. X., Lin, L. J., Liao, M. A., Wang, J., et al. (2022b). Effects of exogenous indole acetic acid on growth and cadmium accumulation of cyphomandra betacea seedlings. Int. J. Environ. Anal. Chem. 102, 771–779. doi: 10.1080/03067319.2020.1726336
Marta, B., Szafrańska, K., Posmyk, M. M. (2016). Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus l.) germinated under chilling stress. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00575
Marumo, S., Hattori, H., Abe, H., Munakata, K. (1968). Isolation of 4-chloroindolyl-3-acetic acid from immature seeds of pisum sativum. Nature 219, 959–960. doi: 10.1038/219959b0
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 161, 4–25. doi: 10.1016/s1360-1385(02)02312-9
Mittler, R., Blumwald, E. (2015). The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 27, 64–70. doi: 10.1105/tpc.114.133090
Moustafa-Farag, M., Almoneafy, A., Mahmoud, A., Elkelish, A., Arnao, M. B., Li, L., et al. (2019). Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10, 54. doi: 10.3390/biom10010054
Napier, R. M. (2014). “Auxin receptors and perception,” in Auxin and its role in plant development. Eds. Zažímalová, E., Petrášek, J., Benková., E. (Springer), pp101–pp116.
Ndikuryayo, F., Moosavi, B., Yang, W. C., Yang, G. F. (2017). 4-hydroxyphenylpyruvate dioxygenase inhibitors: From chemical biology to agrochemicals. J. Agric. Food Chem. 65, 8523–8537. doi: 10.1021/acs.jafc.7b03851
Nikonorova, N., Murphy, E., Fonseca De Lima, C. F., Zhu, S., Van De Cotte, B., Vu, L. D., et al. (2021). The arabidopsis root tip (Phospho)Proteomes at growth-promoting versus growth-repressing conditions reveal novel root growth regulators. Cells 10, 7. doi: 10.3390/cells10071665
Ortiz García, P., Pérez Alonso, M. M., González Ortega Villaizán, A., SánchezParra, B., Ludwig Müller, J., Wilkinson, M. D., et al. (2022). The indole-3-Acetamide-Induced arabidopsis transcription factor MYB74 decreases plant growth and contributes to the control of osmotic stress responses. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.928386
Osborne, D. J. (1952). A synergistic interaction between 3-indolylacetonitrile and 3-indolylacetic acid. Nature 170, 210–211. doi: 10.1038/170210b0
Park, S., Back, K. (2012). Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal. Res. 53, 385–389. doi: 10.1111/j.1600-079x.2012.01008.x
Pérez Alonso, M. M., Ortiz García, P., Moya Cuevas, J., Lehmann, T., Sánchez Parra, B., Björk, R. G., et al. (2020). Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in arabidopsis. J. Exp. Bot. 72, 459–475. doi: 10.1093/jxb/eraa485
Perez, I. B., Brown, P. J. (2015). The role of ROS signaling in cross-tolerance: from model to crop. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00754
Reiter, R. J., Paredes, S. D., Manchester, L. C., Tan, D. X. (2009). Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 44, 175–200. doi: 10.1080/10409230903044914
Shen, Q., Liu, L., Wang, L., Wang, Q. (2018). Indole primes plant defense against necrotrophic fungal pathogen infection. PloS One 13, e0207607. doi: 10.1371/journal.pone.0207607
Shi, H., Jiang, C., Ye, T., Tan, D. X., Reiter, R. J., Zhang, H., et al. (2014). Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694. doi: 10.1093/jxb/eru373
Song, P., Xu, H., Zhang, J., Chen, H., Li, L., Qu, Y., et al. (2021). Functional analysis of indole 3-hexanoic acid as a novel auxin from arabidopsis thaliana. Planta 254, 69. doi: 10.1007/s00425-021-03719-9
Stahl, E., Bellwon, P., Huber, S., Schlaeppi, K., Bernsdorff, F., Vallat Michel, A., et al. (2016). Regulatory and functional aspects of indolic metabolism in plant systemic acquired resistance. Mol. Plant 9, 662–681. doi: 10.1016/j.molp.2016.01.005
Staswick, P., Rowe, M., Spalding, E. P., Splitt, B. L. (2017). Jasmonoyl-L-Tryptophan Disrupts IAA Activity through the AUX1 Auxin Permease. Front. Plant Sci. 8, 736. doi: 10.3389/fpls.2017.00736
Sun, X., Wang, N., Li, P., Jiang, Z., Liu, X., Wang, M., et al. (2020). Endophytic fungus falciphora oryzae promotes lateral root growth by producing indole derivatives after sensing plant signals. Plant Cell Environ. 43, 358–373. doi: 10.1111/pce.13667
Sun, Y., Wu, H., Zhou, W., Yuan, Z., Hao, J., Liu, X., et al. (2022). Effects of indole derivatives from purpureocillium lilacinum in controlling tobacco mosaic virus. pestic. Biochem. Physiol. 183, 105077. doi: 10.1016/j.pestbp.2022.105077
Sun, S. L., Yang, W. L., Fang, W. W., Zhao, Y. X., Guo, L., Dai, Y. J. (2018). The plant growth-promoting rhizobacterium variovorax boronicumulans CGMCC 4969 regulates the level of indole-3-Acetic acid synthesized from indole-3-Acetonitrile. Appl. Environ. Microbiol. 84, e00298–e00218. doi: 10.1128/aem.00298-18
Szymaniak, D., Kleiber, T., Wojcieszak, M., Materna, K., Pernak, J. (2021). Conversion of l-tryptophan derivatives into biologically active amino acid ionic liquids. ChemistrySelect 6, 5614–5621. doi: 10.1002/slct.202101084
Tan, D. X., Manchester, L. C., Di Mascio, P., Martinez, G. R., Prado, F. M., Reiter, R. J. (2007). Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phyt/oremediation. FASEB J. 21, 1724–1729. doi: 10.1096/fj.06-7745com
Tuyen, P., Xuan, T., Tu Anh, T., Mai Van, T., Ahmad, A., Elzaawely, A., et al. (2018). Weed suppressing potential and isolation of potent plant growth inhibitors from castanea crenata sieb. et zucc. Molecules 23, 345. doi: 10.3390/molecules23020345
Wang, Q., An, B., Wei, Y., Reiter, R. J., Shi, H., Luo, H., et al. (2016). Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in arabidopsis. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01882
Wang, X., Luo, M. J., Wang, Y. X., Han, W. Q., Miu, J. X., Luo, X. P., et al. (2022). Design, synthesis, and herbicidal activity of indole-3-carboxylic acid derivatives as potential transport inhibitor response 1 antagonists. Front. Chem. 10. doi: 10.3389/fchem.2022.975267
Wang, H., Song, H. (2020). Synthesis of four optical isomers of antiviral agent NK0209 and determination of their configurations and activities against a plant virus. J. Agric. Food Chem. 68, 2631–2638. doi: 10.1021/acs.jafc.9b07694
Wei, J., Li, D. X., Zhang, J. R., Shan, C., Rengel, Z., Song, Z. B., et al. (2018). Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in arabidopsis thaliana. J. Pineal. Res. 65, 2. doi: 10.1111/jpi.12500
Wei, C., Zhang, J., Shi, J., Gan, X., Hu, D., Song, B. (2019). Synthesis, antiviral activity, and induction of plant resistance of indole analogues bearing dithioacetal moiety. J. Agric. Food Chem. 67, 13882–13891. doi: 10.1021/acs.jafc.9b05357
Wen, D., Gong, B., Sun, S., Liu, S., Wang, X., Wei, M., et al. (2016). Promoting roles of melatonin in adventitious root development of solanum lycopersicum l. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00718
Ye, M., Glauser, G., Lou, Y., Erb, M., Hu, L. (2019). Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. Plant Cell. 31, 687–698. doi: 10.1105/tpc.18.00569
Ye, M., Liu, M., Erb, M., Glauser, G., Zhang, J., Li, X., et al. (2021). Indole primes defence signalling and increases herbivore resistance in tea plants. Plant Cell Environ. 44, 1165–1177. doi: 10.1111/pce.13897
Yu, Y., Lv, Y., Shi, Y. N., Li, T., Chen, Y. C., Zhao, D. K., et al. (2018). The role of phyto-melatonin and related metabolites in response to stress. Molecules 23, 1887. doi: 10.3390/molecules23081887
Zhang, M. Z., Chen, Q. (2014b). A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 89, 421–441. doi: 10.1016/j.ejmech.2014.10.065
Zhang, M., Gao, C., Xu, L., Niu, H., Liu, Q., Huang, Y., et al. (2022). Melatonin and indole-3-Acetic acid synergistically regulate plant growth and stress resistance. Cells 11, 3250. doi: 10.3390/cells11203250
Zhang, J., Shi, Y., Zhang, X., Du, H., Xu, B., Huang, B. (2017). Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass ( lolium perenne l.). Environ. Exp. Bot. 138, 36–45. doi: 10.1016/j.envexpbot.2017.02.012
Zhang, H., Wang, Q., Ning, X., Hang, H., Ma, J., Yang, X., et al. (2015). Synthesis and biological evaluations of a series of thaxtomin analogues. J. Agric. Food Chem. 63, 3734–3741. doi: 10.1021/jf506153t
Zhang, H. J., Zhang, N., Yang, R. C., Wang, L., Sun, Q. Q., Li, D. B., et al. (2014a). Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus l.). J. Pineal. Res. 57, 269–279. doi: 10.1111/jpi.12167
Zhao, D., Luan, Y., Shi, W., Tang, Y., Huang, X., Tao, J. (2022). Melatonin enhances stem strength by increasing the lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 73, 5974–5991. doi: 10.1093/jxb/erac165
Zhao, D., Wang, H., Chen, S., Yu, D., Reiter, R. J. (2020). Phytomelatonin: An emerging regulator of plant biotic stress resistance. Trends Plant Sci. 26, 70–82. doi: 10.1016/j.tplants.2020.08.009
Zhao, H. B., Su, T., Huo, L. Q., Wei, H. B., Jiang, Y., Xu, L. F., et al. (2015). Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case. J. Pineal Res. 59, 255–66. doi: 10.1111/jpi.12258
Zhao, H., Ye, L., Wang, Y., Zhou, X., Yang, J., Wang, J., et al. (2016). Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01814
Keywords: indole derivatives, plant growth regulators, plant immune inducers, biological stress, abiotic stresses, mechanisms of action
Citation: Sun P, Huang Y, Yang X, Liao A and Wu J (2023) The role of indole derivative in the growth of plants: A review. Front. Plant Sci. 13:1120613. doi: 10.3389/fpls.2022.1120613
Received: 10 December 2022; Accepted: 28 December 2022;
Published: 16 January 2023.
Edited by:
Yong Guo, Zhengzhou University, ChinaCopyright © 2023 Sun, Huang, Yang, Liao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, jwu6@gzu.edu.cn
 Ping Sun
Ping Sun