
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Plant Sci., 17 February 2023
Sec. Plant Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1118271
This article is a correction to:
Phytochromes A and B Mediate Light Stabilization of BIN2 to Regulate Brassinosteroid Signaling and Photomorphogenesis in Arabidopsis
 Jiachen Zhao1†
Jiachen Zhao1† Guangqiong Yang1†
Guangqiong Yang1† Lu Jiang1
Lu Jiang1 Shilong Zhang1
Shilong Zhang1 Langxi Miao2
Langxi Miao2 Peng Xu2
Peng Xu2 Huiru Chen1
Huiru Chen1 Li Chen1
Li Chen1 Zhilei Mao1
Zhilei Mao1 Tongtong Guo1
Tongtong Guo1 Shuang Kou1
Shuang Kou1 Hong-Quan Yang1
Hong-Quan Yang1 Wenxiu Wang1*
Wenxiu Wang1*A Corrigendum on:
Phytochromes A and B mediate light stabilization of BIN2 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis.
By Zhao J, Yang G, Jiang L, Zhang S, Miao L, Xu P, Chen H, Chen L, Mao Z, Guo T, Kou S, Yang H-Q and Wang W (2022) 13:865019. doi: 10.3389/fpls.2022.865019
In the published article, there was an error in Figure 1 and Supplemental Figure 4. An antibody against a mammalian protein called BIN2 (Nouvsbio, NBP2-48690) to detect Arabidopsis BIN2 was used by mistake, which recognized Arabidopsis BIN2. The corrected Figure 1 and Supplemental Figure 4 and their captions appear below:
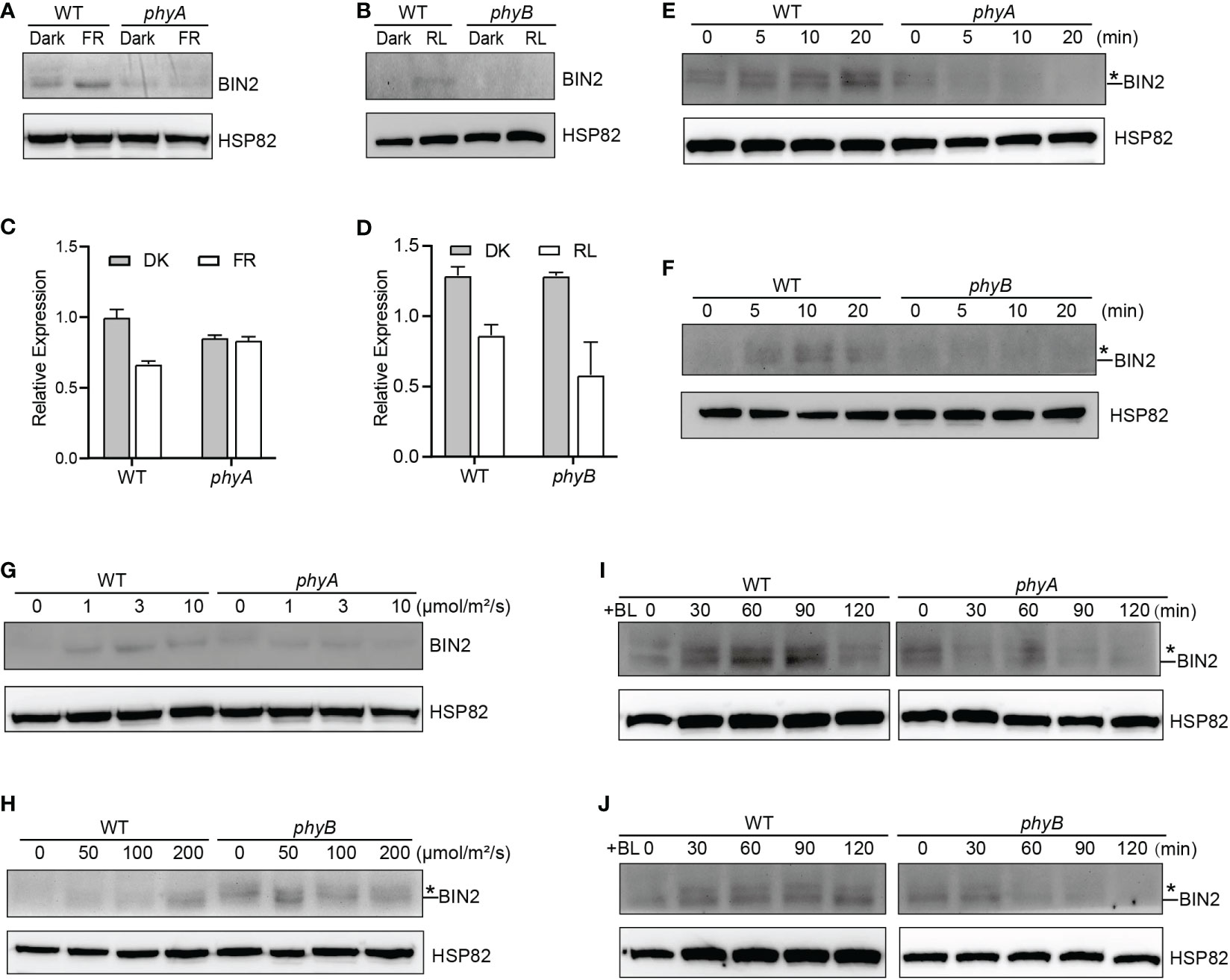
Figure 1 phyA and phyB Mediate Far-Red and Red lights Inhibition of BL-Induced Degradation of BIN2 Protein. (A, B) Western blotting assays showing phyA- and phyB-mediated far-red or red light inhibition of degradation of BIN2 protein. WT, phyA and phyB mutant seedlings were grown on MS plates in continuous darkness (DK) or far-red light (FR, 1 μmol/m2/s) (A) or red light (R, 50 μmol/m2/s) (B) for 5 d. (C, D) RT-qPCR assays showing the regulation of BIN2 expression by phyA or phyB in (A) and (B). Data correspond to the mean and standard deviation from three technical replicates. (E, F) Western blotting assays showing the effects of different exposure times of far-red or red light on the degradation of BIN2 protein. WT, phyA and phyB mutant seedlings were grown on MS plates supplemented with 2 µM BRZ in darkness for 7 d, and then exposed to far-red light (10 μmol/m2/s) (E) or red light (100 μmol/m2/s) (F) for the indicated lengths of time. (G, H) Western blot assays showing the effects of far-red or red light intensity on the degradation of BIN2 protein. WT and phyA mutant seedlings were grown on MS plates in far-red light at the fluence rates of 0, 1, 3, and 10 µmol/m2/s for 5 d, respectively (G); WT and phyB mutant seedlings were grown on MS plates supplemented with 2 µM BRZ in darkness for 7 d, and then exposed to the indicated light intensities of red light (H) for 30 min. (I, J) Western blot assays showing the effects of phyA or phyB on the BL-induced degradation of BIN2 protein. WT, phyA and phyB mutant seedlings were grown on MS plates supplemented with 2 μM BRZ in darkness for 7 d, and then treated with 1 μM BL, and then exposed to far-red (10 μmol/m2/s) (I) or red light (100 μmol/m2/s) (J) for the indicated lengths of time. Asterisks shown in (E), (F), (H), (I), and (J) denote the possible modified BIN2 protein upon BRZ treatment.
In the published article there was an error in the Materials and Methods, BIN2 Protein Degradation Assay in Arabidopsis, paragraph 1 and 2. Following the correction to Figure 1 and Supplemental Figure 4, the Materials and Methods needs to be updated accordingly.
The corrected paragraph appears below:
“For the assays of the influence of phyA and phyB on BIN2 protein degradation in far-red and red lights, WT, phyA and phyB seedlings were grown in darkness, far-red or red light (1 µmol/m2/s or 50 µmol/m2/s) for 5 d. For the assays of the influence of far-red or red light exposure time on BIN2 protein degradation, WT and phyA or phyB seedlings were grown on MS plates supplemented with 2 µM BRZ (Sigma-Aldrich, USA) in darkness for 7 d, and then exposed to far-red (10 µmol/m2/s) or red light (100 µmol/m2/s) for 0, 5, 10, and 20 min, respectively. For the assay of the effects of far-red light intensity on the degradation of BIN2 protein, WT and phyA seedlings were grown in far-red light at the fluence rates of 0, 1, 3, and 10 µmol/m2/s for 5 d, respectively. For the assay of the effects of red light intensity on the degradation of BIN2 protein, WT and phyB seedlings were grown on MS plates supplemented with 2 µM BRZ in darkness for 7 d, and then exposed to red light at the fluence rates of 0, 50, 100, and 200 µmol/m2/s for 30 min, respectively. For the assays of the effects of phyA or phyB on brassinolide (BL)-induced degradation of BIN2, WT and phyA or phyB seedlings were grown on MS plates supplemented with 2 µM BRZ in darkness for 7 d, and then transferred into liquid MS medium containing 1 µM eBL (Sigma-Aldrich, USA) and exposed to far-red (10 µmol/m2/s) or red light (100 µmol/m2/s) for 30, 60, 90, and 120 min, respectively.”
“Lysis buffer containing 1 mM Pefabloc, cocktail and 50 µM MG132 was used to extract total protein, and Bradford assay (Bio-Rad, United States) was used to determine the total protein concentration. The supernatant of total protein was mixed with 5 × SDS loading buffer and boiled for 5 min, and subjected to Western blot analysis with an antibody against Arabidopsis BIN2 (Jiang et al., 2019).”
In the published article there was an error in the Results, phyA and phyB Are Involved in Mediating Far-Red and Red Lights Inhibition of Brassinolide-Induced Degradation of BIN2 Protein, Respectively, paragraphs 1 and 2. Following the correction to Figure 1 and Supplemental Figure 4, the Results needs to be updated accordingly.
The corrected paragraphs appear below:
“The GSK3-like kinase BIN2 is the key negative regulator of BR signaling, and the regulation of BIN2 stability is important for BR signaling (Peng et al., 2008). Given our previous demonstrations that photoreceptors CRY1 and phyB inhibits auxin signaling by stabilizing the key auxin signaling repressors AUX/IAA proteins (Xu et al., 2018), and that CRY1 inhibits GA signaling by stabilizing the key GA signaling repressors DELLA proteins (Xu et al., 2021), we explored whether phyA and phyB might affect the stability of BIN2 to regulate BR signaling. To this end, we firstly performed immunoblot assays using an anti-BIN2 antibody to detect BIN2 protein level in WT, phyA and phyB mutant seedlings grown in continuous darkness or far-red or red light, respectively. The results showed that, in the WT background, much more BIN2 protein accumulated in far-red or red light than in the dark, whereas in the phyA or phyB mutant background, basically similar very low level of BIN2 was detected in the dark and far-red or red light (Figures 1A, B; Supplementary Figure 4). RT-qPCR analysis demonstrated that BIN2 expression was not increased, but decreased to a varied extent in the WT or phyA or phyB mutant seedlings exposed to far-red or red light (Figures 1C, D). These results indicated that far-red and red lights induce the accumulation of BIN2 protein and that phyA and phyB might be responsible for mediating this process in far-red and red lights, respectively. As the BR biosynthesis inhibitor BRZ promotes BIN2 protein content in the etiolated seedlings (Peng et al., 2008), we then analyzed the accumulation of BIN2 protein using the BRZ-treated WT, phyA and phyB mutant seedlings grown in darkness for 7 d, and then exposed to different lengths of time of far-red and red lights, respectively. We found that BIN2, accumulated faster in WT than in phyA and phyB mutant backgrounds within 30 min far-red and red lights irradiation, respectively (Figures 1E, F; Supplementary Figure 4). We further analyzed BIN2 levels in the etiolated WT, phyA and phyB mutant seedlings exposed to different fluence rates of far-red and red lights, respectively. As WT and phyB mutants have low BIN2 protein levels when growing under continuous dark or red light, we treated WT and phyB mutant seedlings with 2 µM BRZ in darkness for 7 d, and then exposed to different fluence rates of red light for 30 min; While WT and phyA seedlings were grown in far-red light at the fluence rates of 0, 1, 3, and 10 µmol/m2/s for 5 d, respectively. The results showed that, in the WT background, BIN2 protein level increased as the fluence rate of far-red or red light increased (Figures 1G, H; Supplementary Figure 4), but hardly increased in phyA or phyB mutant background (Figures 1G, H; Supplementary Figure 4). Taken together, these results demonstrate that phyA and phyB mediate far-red and red light-induced accumulation of BIN2 protein, respectively.”
“Given the previous demonstration that the exogenous application of an active BR, brassinolide (BL) induces BIN2 degradation (Peng et al., 2008), we further examined whether phyA and phyB would inhibit the BL-induced degradation of BIN2 protein under far-red and red lights. To minimize the potential endogenous BRs content difference in WT and phyA or phyB mutant with different treatments, we applied BRZ in the subsequent assays. We performed Western blotting assay using the BRZ treated WT, phyA and phyB mutant seedlings grown in darkness for 5 d, and then transferred to liquid medium containing 1 μM eBL exposed to different lengths of time of far-red and red lights. As shown in Figures 1I, J and Supplementary Figure 4, the effects of red and far-red lights were stronger than those of BL within two hours of BL treatment, making BIN2 protein slightly increase in WT background as the light exposure time increased. Moreover, BIN2 protein was degraded much faster in phyA and phyB mutant seedlings than in WT seedlings when exposed to far-red and red lights. These results indicate that phyA and phyB are able to inhibit the BL-induced degradation of BIN2 protein under far-red and red lights.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: Arabidopsis, phytochrome A (phyA), phytochrome B (phyB), brassinosteroid (BR), BRASSINOSTEROID-INSENSITIVE 2 (BIN2), BRI1-EMS SUPPRESSOR 1 (BES1), photomorphogenesis
Citation: Zhao J, Yang G, Jiang L, Zhang S, Miao L, Xu P, Chen H, Chen L, Mao Z, Guo T, Kou S, Yang H-Q and Wang W (2023) Corrigendum: Phytochromes A and B mediate light stabilization of BIN2 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis. Front. Plant Sci. 13:1118271. doi: 10.3389/fpls.2022.1118271
Received: 07 December 2022; Accepted: 19 December 2022;
Published: 17 February 2023.
Edited and Reviewed by:
Jigang Li, China Agricultural University, ChinaCopyright © 2023 Zhao, Yang, Jiang, Zhang, Miao, Xu, Chen, Chen, Mao, Guo, Kou, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiu Wang, d2FuZ3dlbnhpdTg1QHNobnUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.