
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 13 December 2022
Sec. Plant Biotechnology
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1097622
This article is part of the Research Topic Omics technology in agriculture: molecular breeding for sustainable crop production View all 7 articles
 Quan Zhang1†
Quan Zhang1† Jianyin Xie1,2†
Jianyin Xie1,2† Xueqiang Wang1†
Xueqiang Wang1† Miaosong Liu1
Miaosong Liu1 Xiaoyang Zhu1
Xiaoyang Zhu1 Tao Yang1
Tao Yang1 Najeeb Ullah Khan1
Najeeb Ullah Khan1 Chen Sun1
Chen Sun1 Jinjie Li1
Jinjie Li1 Zhanying Zhang1
Zhanying Zhang1 Zichao Li1
Zichao Li1 Hongliang Zhang1,2*
Hongliang Zhang1,2*The grain number per panicle (GNP) is an important yield component. Identifying naturally favorable variations in GNP will benefit high-yield rice breeding. Here, we performed a genome-wide association study using a mini-core collection of 266 cultivated rice accessions with deep sequencing data and investigated the phenotype for three years. Three genes, i.e., TOTOU1 (TUT1), Grain height date 7 (Ghd7), and Days to heading 7/Grain height date 7.1/Pseudo-Response Regulator37 (DTH7/Ghd7.1/OsPRR37), which regulate GNP, were found in the quantitative trait loci (QTL) identified in this study. A stable QTL, qGNP1.3, which showed a strong correlation with variations in GNP, was repeatedly detected. After functional and transgenic phenotype analysis, we identified a novel gene, regulator of grain number 1a (RGN1a), which codes for protein kinase, controlling GNP in rice. The RGN1a mutation caused 37.2%, 27.8%, 51.2%, and 25.5% decreases in grain number, primary branch number per panicle, secondary branch number per panicle, and panicle length, respectively. Furthermore, breeding utilization analysis revealed that the additive effects of the dominant allelic variants of RGN1a and DTH7 played a significant role in increasing the grain number per panicle in japonica rice. Our findings enrich the gene pool and provide an effective strategy for the genetic improvement of grain numbers.
Grain yield in rice (Oryza sativa L.) comprises three components: grain number per panicle (GNP), grain weight, and effective panicle number. Increasing the number of grains per panicle is an effective strategy for improving rice yield in modern breeding. In recent years, many genes that affect panicle development have been identified. However, rice germplasm resources still contain many potentially excellent alleles for controlling the number of grains to be identified. Therefore, enriching the gene pool is important for yield improvement in cultivated rice.
Over the decades of rice research, a series of important grain-number genes have been cloned from germplasm resources. Grain number 1a (Gn1a) was identified in the near-isogenic lines of Habataki in the Koshihikari background, regulating grain numbers by influencing cytokinin accumulation in inflorescence meristems (Ashikari et al., 2005). IDEAL PLANT ARCHITECTURE 1/SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 14 (IPA1/OsSPL14) was detected in two pairs of rice combinations (Taichung Native 1 (TN1) and Shaoniejing (SNJ), Nipponbare (NIP) and ST-12), affecting the tiller number and grain number per panicle (Jiao et al., 2010; Miura et al., 2010). FRIZZY PANICLE (FZP)/SMALL GRAIN AND DENSE PANICLE 7 (SGDP7)/CONTROL OF SECONDARY BRANCH 1 (COS1) controls secondary branches per panicle, thus influencing grain number. The functional variation site located in the upstream regulatory region of FZP has been identified using map-based cloning (Bai et al., 2017; Huang et al., 2018). NUMBER OF GRAINS 1 (NOG1) was isolated from wild rice, and upregulation of NOG1 expression significantly increased the number of grains per panicle (Huo et al., 2017).
In addition to the previously mentioned genes, map-based cloning in mutants identified many genes associated with grain number. The mutation of LAX PANICLE1 (LAX1) resulted in the lateral spikelets being abolished (Komatsu et al., 2003). LAX PANICLE2 (LAX2)/GRAIN NUMPER PER-PANICLE 4 (GNP4) was also cloned from loss-of-function mutants and regulated reproductive branching (Tabuchi et al., 2011; Zhang et al., 2011a). DROUGHT AND SALT TOLERANCE (DST) in the gain-of-function mutant reg1 increased panicle branches and grain numbers (Li et al., 2013). O. sativa SHORT INTERNODES1 (OsSHI1) was isolated from the shi1 mutant (60Co-γ irradiation-induced in 93-11 background), modulating IPA1 transcriptional activity to influence plant architecture and grain number per panicle (Duan et al., 2019). Both GRAIN SIZE AND NUMBER1 (GSN1) and ERECTA1 (OsER1) act upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade while exhibiting the opposite grain number regulation pattern (Guo et al., 2018; Guo et al., 2020a). The REGULATOR OF GRAIN NUMBER1 (RGN1) was obtained from a rare rice germplasm with abnormal panicle branches, affecting grain number and panicle architecture (Li et al., 2022).
With the development of sequencing technology, genes controlling grain number have been discovered through the MutMAP approach or genome-wide association study (GWAS) relying on high-throughput sequencing data. LARGE1 and LARGE2 were isolated from the F2 population by MutMAP analysis. LARGE1 encodes the Mei2-like protein, and its overexpression lines positively regulate grain number per panicle and reduce grain size and weight (Lyu et al., 2020). Mutation of LARGE2, encoded by a HECT-domain E3 ubiquitin ligase, results in a large panicle and an increase in grain number (Huang et al., 2021). Given the complexity of the grain number per panicle, a few genes were mapped using GWAS. Only Gnd5, a novel GRAS transcription factor that positively regulates grain number per panicle, has been identified using GWAS of the japonica population (Cui et al., 2022).
In this study, we identified three genes, TOTOU1 (TUT1), Grain height date 7 (Ghd7), and Days to heading 7/Grain height date 7.1/Pseudo-Response Regulator37 (DTH7/Ghd7.1/OsPRR37), through a genome-wide association study, which have been reported to regulate grain number. Meanwhile, qGNP1.3, a stable signal segment in the whole genome Manhattan map detected multiple times over years of studying the phenotype, and regulator of grain number 1a (RGN1a) has been shown to participate in panicle development. Breeding utilization analysis showed that aggregating the favorable alleles of RGN1a and DTH7 further improved grain number in the japonica population.
A panel of 266 Oryza sativa accessions was used from the core collection (Zhang et al., 2011b). All accessions were planted in Sanya, Hainan Province (18°20′N), under normal cultivation conditions in 2010, 2012, and 2013. Each variety (30 plants) was grown in three rows of 10 plants per row. The main panicles of five plants per variety in the middle row were randomly selected to determine panicle phenotype statistics. The average GNP of five plants was used for the analysis. The three years of phenotypic data were marked as 2010_HN, 2012_HN, and 2013_HN.
The sequencing data of the 266 Oryza sativa accessions were obtained from the 3,000 Rice Genome Project (3K-RG) database (Wang et al., 2018), which had an average sequencing depth of 14x and generated > 10 million single nucleotide polymorphisms (SNPs) when compared with the Nipponbare reference genome.
A total of 4,625,141, 3,562,186, and 3,149,160 high-quality SNPs with missing rates ≤ 50% and minor allele frequencies ≥ 2% were first identified in the full, indica and japonica populations. Principal component (PC) and kinship analyses were conducted using GAPIT to verify the population structure (Tang et al., 2016). A total of 514,177 SNPs (missing rates ≤ 50% and minor allele frequencies ≥ 5%) were filtered using linkage disequilibrium (LD) pruning and used to construct the neighbor-joining tree in MEGA 7.0 (Kumar et al., 2016).
GWAS was performed using a compressed mixed linear model (CMLM) with the first three PCs in GAPIT software (Tang et al., 2016). The conditional permutation test was executed as previously reported to define the suggestive thresholds (Zhao et al., 2018), and 196,787, 228,287, and 123,723 effective numbers of independent SNPs were first calculated and obtained by PLINK 1.9 (–indep-pairwise: 50 5 0.3, window size 50 SNPs, step size 5 SNPs, r2 ≥ 0.3) in the full, indica, and japonica populations, respectively (Purcell et al., 2007; Guo et al., 2020b). Then, the formula “-log10 (1/effective number of independent SNPs)”, as previously described, was used to set a significant threshold. Combining the above two methods, we set a significance threshold of P = 10-5 at a genome-wide level. Quantitative trait loci (QTL) detection using this method identified a region containing at least three clustered significant SNPs within a distance of < 170 kb from one another (Huang et al., 2010; Zhao et al., 2018). Based on genome structure annotation information from MSU-RGAP 7.0, non-synonymous SNPs were annotated using SnpEff (Cingolani et al., 2012). They were then separated from all SNPs identified in the 266 accessions using an in-house Perl script. LD heatmaps of target regions in the GWAS were constructed using “LD heatmap” in the R package (Shin et al., 2006).
Given that TUT1, Ghd7, and DTH7 all existed functional SNPs (-log(P) ≥ 3) in this study, haplotype analysis was based on the SNPs (P ≤ 10-3) in the 2 k gene promoter and exons. Grain number per panicle was evaluated after harvest. Haplotypes, which were used for statistical testing, contain four varieties at least.
Homologous protein sequences of RGN1a and RGN1b were downloaded from NCBI (National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/). Amino acid sequence alignments were assessed with DNAMAN version 6.0 software (Lynnon Corporation, San Ramon, CA, USA). A neighbor-joining tree with sequence homology to RGN1a and RGN1b was constructed in MEGA 7.0.
After the candidate gene analysis of grain number per panicle, we obtained the T-DNA insertion mutant from the POSTECH Biotech Center, Republic of Korea. The plants were sown at the Shangzhuang Experimental Farm of China Agricultural University in Beijing. We designed specific primers in the gene body (LP and RP) and T-DNA specific primers (RB) to identify the genotype of mutants.
Total RNA was extracted from young panicle. qRT-PCR was performed using TB Premix Ex Taq II with ROX Reference Dye II (Takara, #RR820A) on the Applied Biosystems 7500 Fast Real-Time PCR System. Relative gene expression level was analyzed using the comparative critical threshold (△△Ct) method (Livak and Schmittgen, 2001).
Primers used in the study are listed in Supplementary Table 1.
The genes used in this study can be found in the Rice Genome Annotation Project (http://rice.uga.edu/home_overview.shtml) with the following accession numbers: RGN1a (LOC_Os01g49580), RGN1b (LOC_Os01g49614), TUT1 (LOC_Os01g11040), Ghd7 (LOC_Os07g15770), and DTH7/Ghd7.1/OsPRR37 (LOC_Os07g49460).
We retrieved 4,625,141 high-quality SNPs (missing rates ≤ 50% and minor allele frequencies ≥ 2%) from the 3K-RG project as genotypes. Principal component and kinship analyses using 4,625,141 SNPs revealed an apparent population structure. The indica and japonica subpopulations were clearly separated (Figure 1A; Supplementary Figure 1). The neighbor-joining tree using 514,177 SNPs also showed distinct differentiation between the two subspecies (Figure 1B).

Figure 1 Population structure of 266 rice accessions. (A) Principal component analysis (PCA) of different subpopulations. (B) Neighbor-joining tree for all accessions; green lines represent indica, and red lines represent japonica rice. (C) The phenotype statistics of grain number per panicle in full, indica, and japonica populations over three years. The numbers above violins are mean phenotypic values, and different letters indicate significant differences at P < 0.05 according to one-way ANOVA. (D–F) The distribution of the grain number per panicle in full population among different years.
As the population structure analysis showed two O. sativa subspecies, abundant differences in GNP existed between the indica and japonica subspecies. Overall, indica rice had more grains per panicle than japonica rice did (Figure 1C). The variation of GNP in the indica variety ranged from 38.9 to 323.4 (2010), 55.8 to 359.7 (2012), and 47.5 to 399.1 (2013), and from 35.2 to 265.1 (2010), 46.7 to 253.7 (2012), and 53.2 to 298.5 (2013) in japonica. The phenotypic data were normally distributed and suitable for association analysis (Figures 1D–F; Supplementary Figure 2).
We used a rigorous compressed mixed linear model (CMLM) for GWAS to identify important QTLs for GNP. Using the phenotype of GNP in 2010, 210, 12, and 325 SNPs were identified by GWAS at -log(P) ≥ 5 in the full, indica, and japonica populations, respectively (Figure 2A; Supplementary Figure 3); 176, 121, and 10 significant SNPs (P ≤ 10-5) were identified by GWAS on GNP_2012 in the full, indica, and japonica populations, respectively (Figure 2B; Supplementary Figure 3); and 363, 14, and 7 significant SNPs were identified by GWAS on GNP_2013 in the full, indica, and japonica populations, respectively (Figure 2C; Supplementary Figure 3). A total of 126 and 29 significant SNPs were detected in the two and three GWAS populations, respectively. In total, 44 SNPs associated with GNP were repeatedly detected from the full population among different years; only 8 and 3 SNPs were detected repeatedly from the indica and japonica populations, respectively (Supplementary Figure 4). Given that the linkage disequilibrium decay value reported in rice is up to 167 kb (Huang et al., 2010), we defined a QTL as having at least three significant SNPs within distances ≤ 170 kb between adjacent ones (Zhao et al., 2018). Phenotypic data from three years were used to detect QTLs successively. In total, 17 QTLs were detected in 2010, including 9 associated regions in the full population, one in the indica population, and seven in the japonica population; 19 QTLs were detected in 2012, including 9, 8, and 2 associated regions in the full, indica, and japonica populations, respectively; and 20 QTLs were detected in 2013, including 19 associated regions in the full population, and 1 QTL was detected in the japonica population (Table 1; Supplementary Table 2).
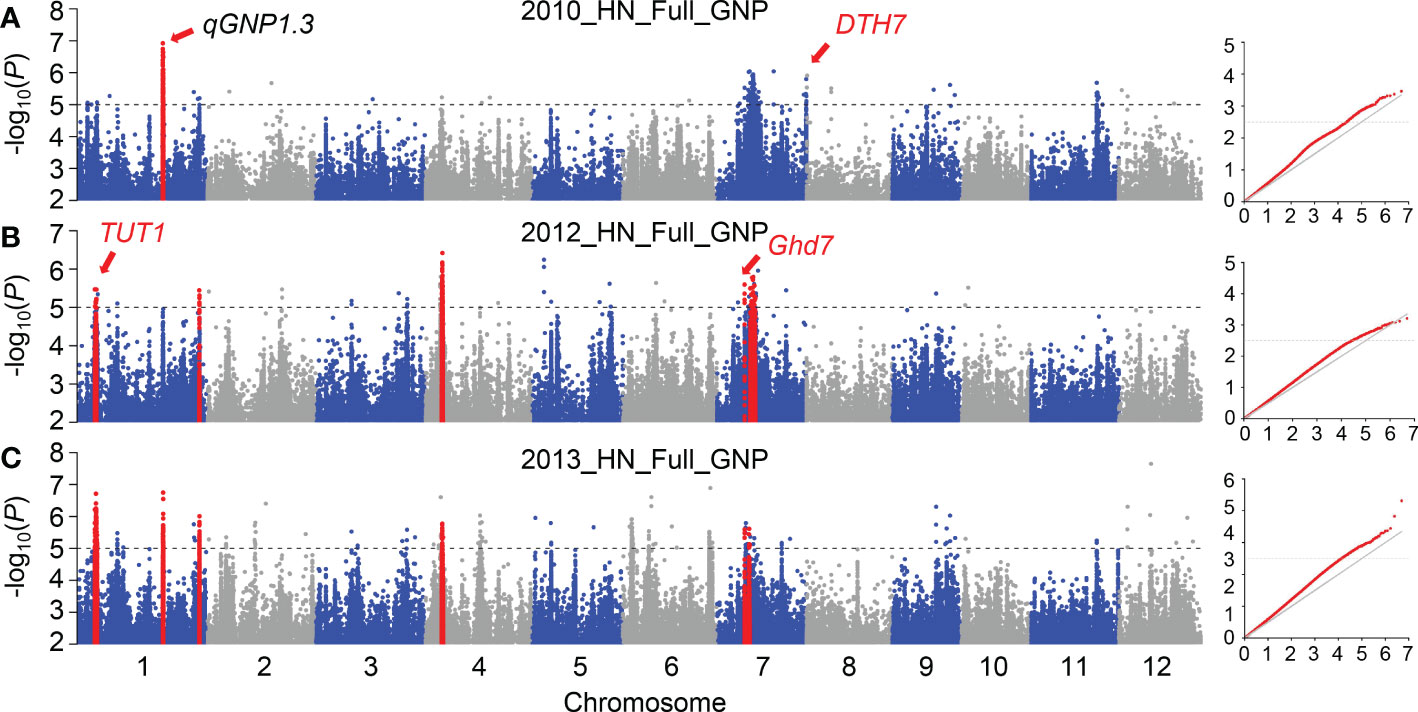
Figure 2 GWAS results of GNP in different years. Quantile–quantile plots and Manhattan plots for the GWAS in 2010 (A), 2012 (B), and 2013 (C) using CMLM. Red points in the Manhattan plot represent the QTLs that were identified at least two years. In quantile-quantile plots, red points show the CMLM model. In the Manhattan plots, the gene in red was previously cloned. A dotted horizontal line for each figure indicates the significance threshold (P = 10−5).
To verify the reliability of our results, we first checked whether the reported genes were located in candidate QTLs. We found that TUT1 in qGNP1.1, Ghd7 in qGNP7.1, and DTH7 in qGNP7.8, were significantly associated with the grain number per panicle (Table 1; Figures 3A, D; Supplementary Figure 5A).
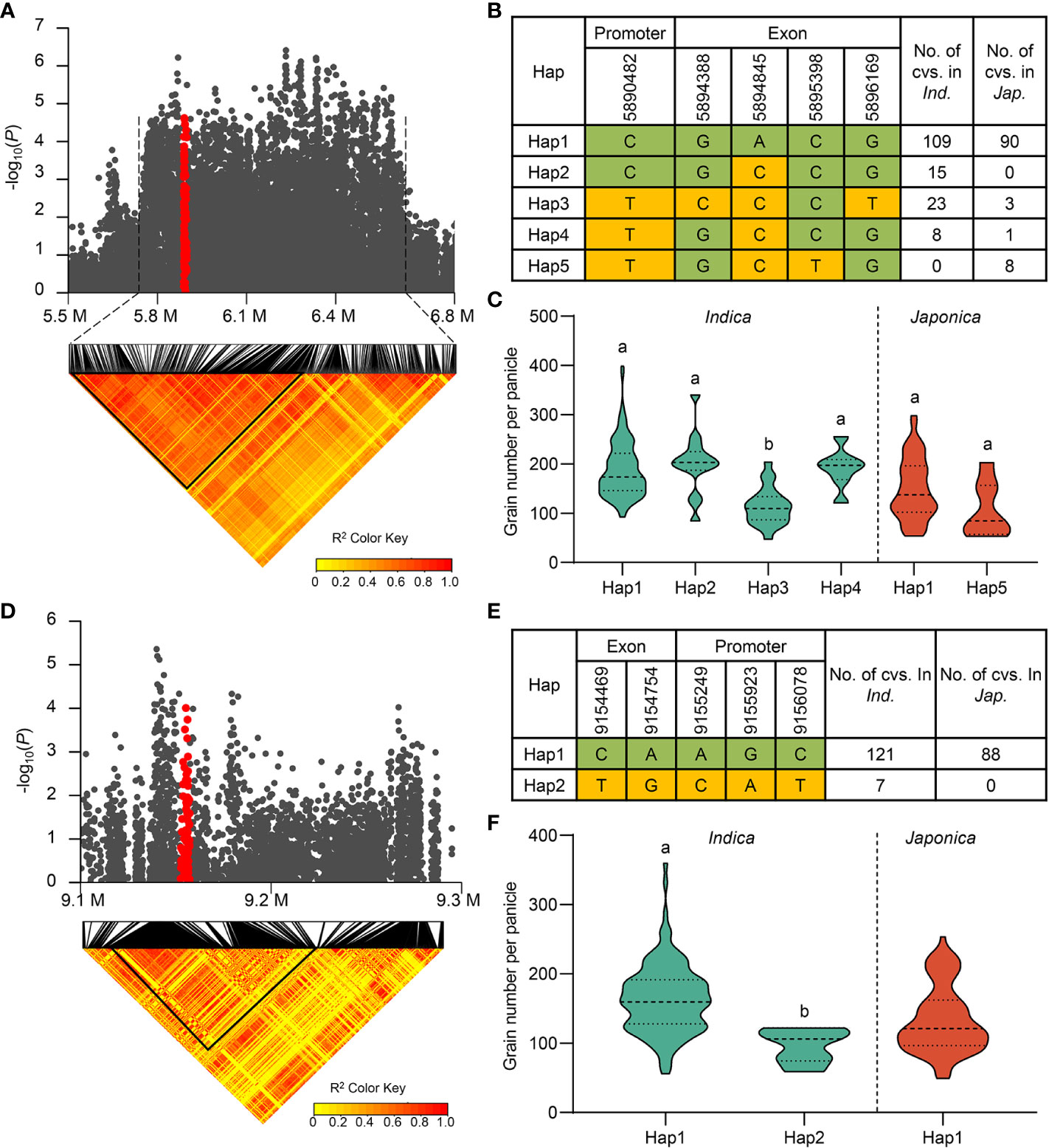
Figure 3 Exploration of TUT1 and Ghd7 for grain number per panicle. (A) Regional Manhattan plot (top) and pairwise LD analysis (bottom) of qGNP1.1 containing TUT1 for GNP. Red dots represent all SNPs within TUT1. (B) Different haplotypes of TUT1 in the indica and japonica subgroups. (C) Comparison of GNP traits among haplotypes of TUT1 in indica and japonica. (D) Regional Manhattan plot (top) and pairwise LD analysis (bottom) of qGNP7.1 containing Ghd7 for GNP. Red dots represent all the SNPs within Ghd7. (E) Ghd7 haplotypes in the indica and japonica subgroups. (F) Comparison of GNP trait among haplotypes of Ghd7 in indica and japonica. The yellow and green in (B, D), respectively, represent major and minor alleles. In (C, F), the green violins represent indica, and the red violins represent japonica rice, and different letters indicate significant differences (P < 0.05) detected by one-way ANOVA.
Mutant tut1 negatively regulates panicle development in rice and decreases the spikelet number per panicle (Bai et al., 2015). The TUT1 haplotype in qGNP1.1 was analyzed using SNPs (P ≤ 10-3) in exons (non-synonymous SNPs) and the 2 k promoter. There were a total of five haplotypes in the full population (Figure 3B). The average GNP values of three favorable haplotypes (TUT1Hap1, TUT1Hap2, and TUT1Hap4) were 65.1%, 76.1%, and 68.3% higher than that of the inferior haplotype TUT1Hap3 in the indica population, respectively. However, there was no difference in the japonica subpopulation (Figure 3C).
Ghd7 encodes a CCT protein, a core factor that regulates heading date and plant height and increases the panicle branch in rice (Xue et al., 2008; Weng et al., 2014). In this study, we found that Ghd7 is located in qGNP7.1. There were three significant SNPs in the promoter region of Ghd7 and two significant non-synonymous SNPs in the exon. Using the five SNPs, we identified two Ghd7 haplotypes in 216 rice accessions. Ghd7Hap2 only existed in the indica population, and Ghd7Hap1 exhibited a better grain-number phenotype than Ghd7Hap2 in the indica population (Figures 3D-F).
DTH7/Ghd7.1/OsPRR37 encodes a pseudo-response regulator, a major genetic locus affecting the heading date and grain number per panicle (Liu et al., 2013; Koo et al., 2013; Yan et al., 2013; Gao et al., 2014). Based on three non-synonymous SNPs and four promoter SNPs, we detected five haplotypes of DTH7 in qGNP7.8. DTH7 Hap4 showed higher grain numbers in both indica and japonica subpopulations, whereas, as inferior haplotypes, DTH7Hap2 and DTH7Hap5 had fewer grains in the indica and japonica subpopulations (Supplementary Figures 5B, C).
As shown above, three cloned genes regulating grain number were detected in our GWAS results for the full population. Given that qGNP1.1 containing TUT1 and qGNP7.1 containing Ghd7 were both identified in at least two years, we focused on QTLs that were detected multiple times in the full population (Figure 2; Table 1). qGNP1.3 showed the strongest signal in GWAS (Figure 2) and has been identified as having a major effect on grain number and secondary branches using linkage mapping (Deshmukh et al., 2010; Zhu et al., 2013). Candidate genes in a 120 kb region were screened, and five genes with non-synonymous SNPs (-log(P) ≥ 3) were identified (Supplementary Figure 6). Genes regulating grain number per panicle must be expressed during the young panicle development period. We compared the expression levels of the five genes mentioned using public expression data (Sato et al., 2011; Li et al., 2016), and found that only LOC_Os01g49580, LOC_Os01g49614, and LOC_Os01g49680 were expressed in the young panicle (Supplementary Figure 6). qGNP1.3 has been identified by comparing single-segment substitution lines (Nipponbare/NIP introgression segments in Guangluai 4 background) with the recurrent parent Guangluai 4 (Zhu et al., 2013). The significant non-synonymous SNPs of LOC_Os01g49580 and LOC_Os01g49614 exhibited polymorphism differences between NIP and Guangluai 4, in addition to LOC_Os01g49680. Protein functional analysis revealed that LOC_Os01g49580 and LOC_Os01g49614 were both annotated as protein kinase domain-containing proteins, and LOC_Os01g49680 encodes the DNA repair helicase XPB2 (Supplementary Figure 6). Several genes that regulate panicle development in rice encode protein kinases. For example, mitogen-activated protein kinase GSN1, OsMKKK10, OsMKK4, OsMPK6, and the receptor-like protein kinase OsER1 control spikelet number via the same pathway (Guo et al., 2018; Guo et al., 2020a), and glycogen synthase kinase 2/GSK2 and glycogen synthase kinase 3/GSK3 participate in phosphorylating or dephosphorylating processes to modulate grain size in rice (Gao et al., 2019; Lyu et al., 2020; Liu et al., 2021). These results prompted us to select LOC_Os01g49580 and LOC_Os01g49614 as candidate genes, named RGN1a and RGN1b, respectively (Figure 4A). Haplotype analysis of RGN1a or RGN1b showed they both had significant genetic variation in GNP in japonica subgroups (Figures 4B–E).
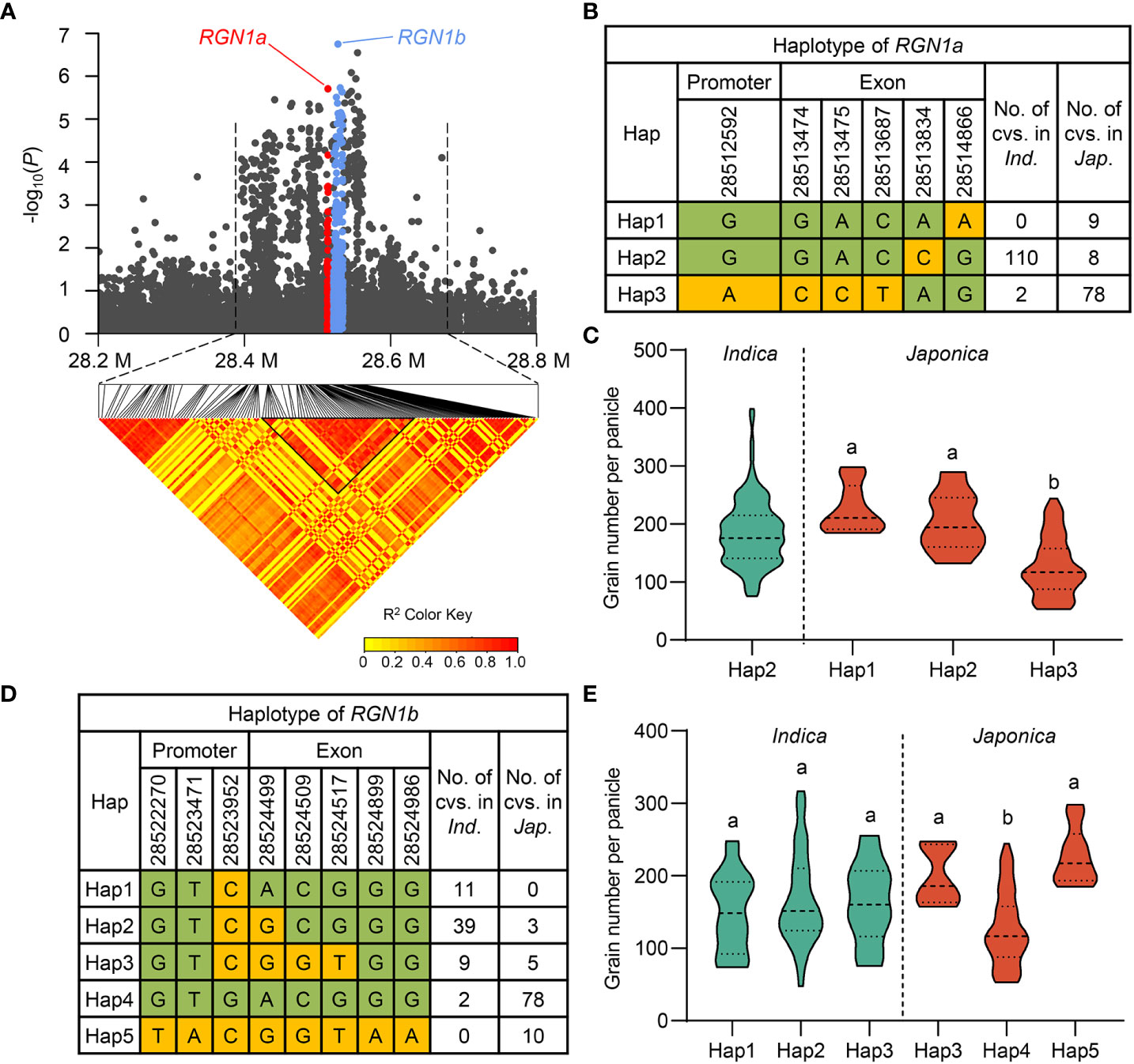
Figure 4 Exploration of RGN1a and RGN1b for grain number per panicle on chromosome 1. (A) Regional Manhattan plot (top) and pairwise LD analysis (bottom) of qGNP1.3 for GNP on chromosome 1. Red and cornflower blue dots represent all SNPs within RGN1a and RGN1b, respectively. (B, D) Different haplotypes of RGN1a or RGN1b in the indica and japonica subgroups. (C, E) A comparison of GNP traits among haplotypes of RGN1a and RGN1b in the indica and japonica subgroup. In (C, E), the green violins represent indica, the red violins represent japonica rice, and different letters indicate significant differences (P < 0.05) detected by one-way ANOVA.
Since RGN1a and RGN1b both belong to the protein kinase family, we first conducted amino acid sequence alignments between them. Both had a wall-associated receptor kinase N-terminal domain and a catalytic domain of the serine/threonine kinases and shared 95.43% similarity with the C-terminal structure (RGN1a, aa 562-913; RGN1b, aa 316-666) (Supplementary Figure 7). Further protein sequence alignments of RGN1a, RGN1b, and their homologs revealed that they are highly conserved in monocots (Supplementary Figure 8). This indicates that these two genes may perform similar functions in rice.
To validate the function of qGNP1.3 in regulating grain number, we first obtained a T-DNA insertion mutant rgn1a in the Dongjin/DJ background. The T-DNA mutant rgn1a was accurately identified by electrophoresis and sequencing, showing that the T-DNA element was inserted in the first exon of RGN1a (Figures 5A, B). The grain number per panicle, primary branch, secondary branch per panicle, and panicle length of rgn1a significantly decreased by 37.2%, 27.8%, 51.2%, and 25.5%, respectively, compared to the wild type (Figures 5C–H). Comparison of GNP of individuals acquired by selfing from heterozygous T-DNA insertion mutant containing RGN1a, RGN1a/rgn1a, and rgn1a alleles, indicating that T-DNA insertion in RGN1a was co-segregated with the phenotype of grain number (Supplementary Figure 9). We also tested the expression levels of RGN1a in germplasm materials containing Hap2 and Hap3, and found no significant differences (Supplementary Figure 10). These results indicate that the natural functional variations of RGN1a are located in the coding region.
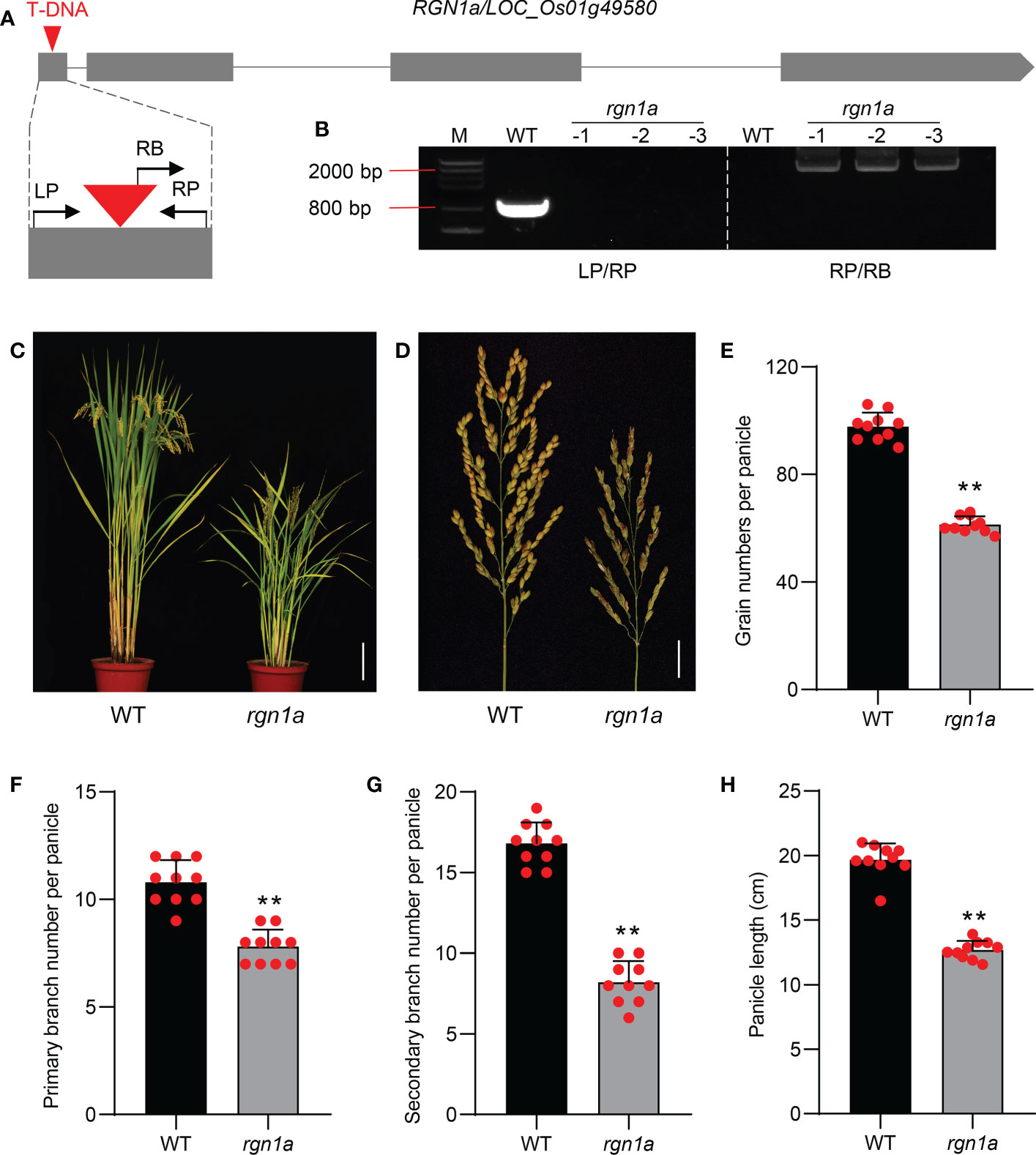
Figure 5 Identification and characterization of RGN1a controlling panicle development. (A) Schematic diagram of RGN1a gene with T-DNA insertion. Gray boxes and lines represent exons and introns, respectively. The insertion sites were in the first exon of RGN1a. LP and RP are the primers in the gene body, and RB is the primer in the T-DNA element. (B) PCR-analysis of WT and three rgn1a mutants using LP/RP and RB/RP. (C) Comparison of plant architecture of the wild type and rgn1a. Scale bar = 15 cm. (D) Comparison of panicle architecture of wild type and rgn1a. Scale bar = 2 cm. (E–H) Comparison of grain number per panicle, primary branch number per panicle, secondary branch number per panicle, and panicle length between wild and rgn1a mutant. P-values were determined using two-tailed Student’s t-tests. **P < 0.01. The data are shown as mean ± SD (n = 10).
We detected three haplotypes at RGN1a, but it only had japonica-specific allele variations for grain number per panicle, and no haplotype differences were present in indica accessions (Figures 4B, C). The favorable haplotype of RGN1a (Hap1 and Hap2) in japonica accessions contained 17 varieties, whereas the inferior haplotype of RGN1a (Hap3) contained 78 japonica germplasm resources (Figure 4B). We analyzed the geographical distribution of these materials in Asia and found varieties with favorable alleles that were mainly distributed in southwest China (Figure 6A). This result indicates that the different genotypes of RGN1a show regional distribution specificity. Furthermore, using information from four japonica clusters (temperate japonica/GJ-tmp, subtropical japonica/GJ-sbtrp, tropical japonica/GJ-trp, and admix japonica/GJ-admix) in the 3K-RG accessions (Wang et al., 2018), we analyzed the ascription of different genotypes of RGN1a. The varieties containing inferior RGN1a were present in all four japonica subpopulations. However, the varieties containing favorable alleles of RGN1a mainly belonged to the subtropical and tropical japonica subgroups (Figure 6B). These results implied that favorable alleles of RGN1a were more suitable for the growing conditions in subtropical and tropical regions and had not been used in temperate regions.
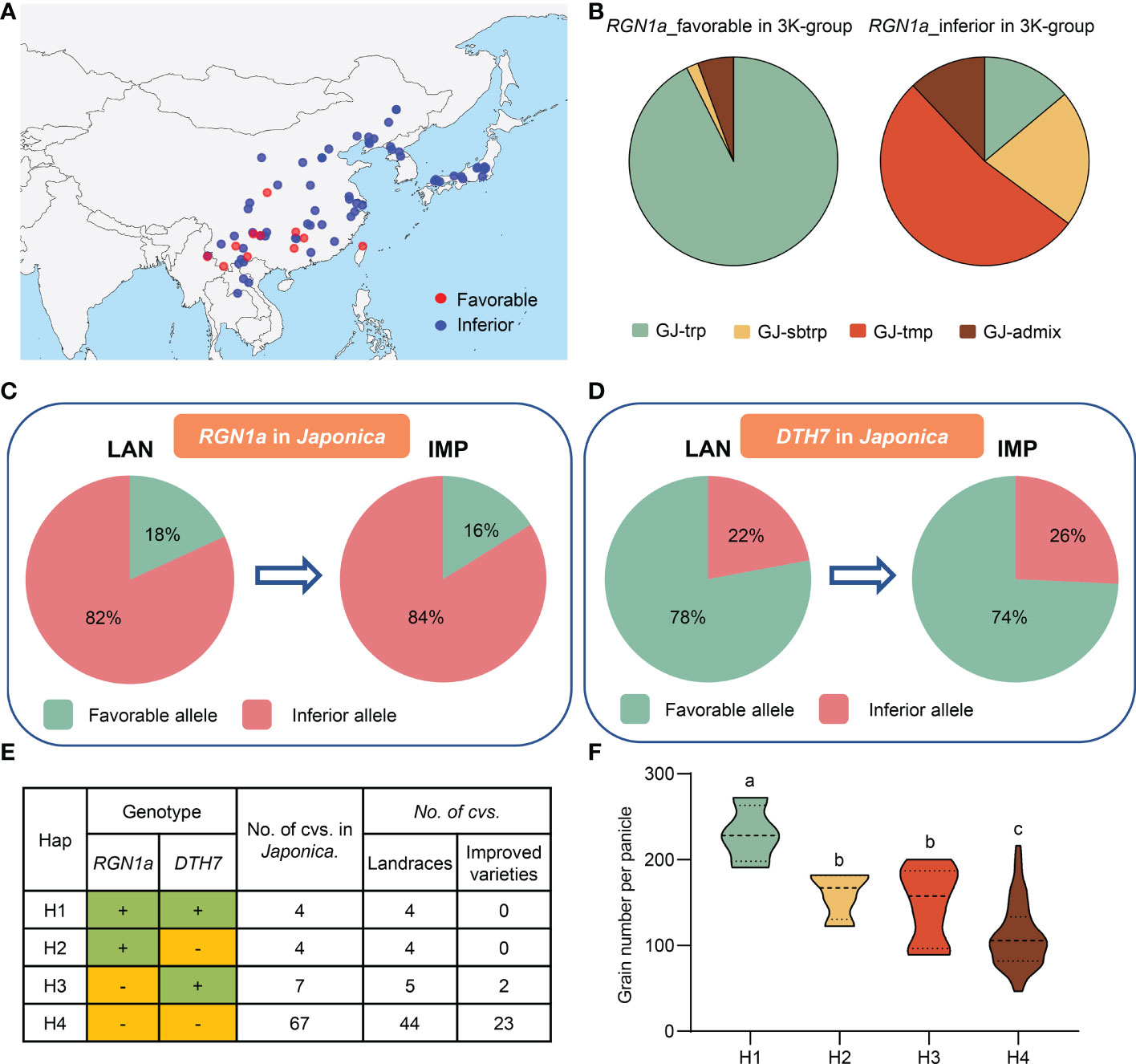
Figure 6 Breeding utilization of RGN1a and DTH7 in grain number per panicle. (A) The geographical distribution of RGN1a alleles among japonica varieties in Asia. (B) The frequency of RGN1a alleles among four japonica subgroups. (C) Allelic changes in RGN1a during japonica rice breeding. (D) Allelic changes in DTH7 during japonica rice breeding. (E) Combined haplotype analysis of RGN1a-DTH7 in the japonica subpopulation, ‘+’ and ‘–’ indicate favorable and inferior alleles. (F) Comparison of GNP trait among combined haplotypes of RGN1a and DTH7 in japonica subgroup. Different letters indicate significant differences (P < 0.05) detected by one-way ANOVA.
In this study, three cloned genes, TUT1, Ghd7, and DTH7, were significantly associated with GNP, and we identified a new gene, RGN1a, which also controlled panicle development. Both RGN1a and DTH7 showed considerable genetic variation in the japonica subpopulation. The favorable alleles of RGN1a and DTH7 had opposite proportions in landraces (18% and 84%, respectively) and improved varieties (16% and 74%, respectively). This indicated that DTH7 had been widely used to improve grain number, and the utilization of RGN1a remains undeveloped (Figures 6C, D; Supplementary Figure 11A). We also conducted a joint haplotype analysis of RGN1a and DTH7. A panel of 82 japonica accessions was divided into four haplotypes. H1 contained the favorable alleles RGN1a and DTH7 and showed the highest grain numbers. H2 with favorable RGN1a and H3 with favorable DTH7 exhibited similar phenotypes but less grain numbers than H1. H4, with inferior RGN1a and DTH7, had the lowest grain numbers (Figures 6E, F). This result confirmed that variety H1 containing favorable alleles of RGN1a and DTH7 could effectively increase the grain number per panicle. However, varieties of H1 are all present in landraces, indicating a broad prospect for the aggregation and utilization of RGN1a and DTH7 in improved species.
Grain yield is a vital research direction that needs continuous attention; however, as a complex quantitative trait, it is difficult to excavate eximious alleles in a natural population. GWAS is considered an effective method for gene mining. In recent years, many genes that control grain yield have been cloned into rice. OsSPL13, a transcription factor that positively regulates grain length, was identified in a GWAS performed on a japonica population containing 381 varieties (Si et al., 2016). GSE5, participating in regulating cell proliferation in spikelet hulls and controlling the grain size, was identified using a GWAS approach (Duan et al., 2017). Because the grain number per panicle is determined by panicle length, primary branch, and secondary branch, any changes in them will affect the GNP. To date, few GNP genes have been mapped through GWAS.
In this study, based on phenotypic and high-quality sequencing data, we performed GWAS in the full rice population and found a target gene, RGN1a, encoding a protein containing the catalytic domain of the serine/threonine kinases and wall-associated receptor kinase N-terminal domain. qGNP_J_1.3, containing RGN1a, was identified in the GWAS results of the japonica subgroup (Supplementary Table 2), indicating that the genetic variation in RGN1a was mainly concentrated in the japonica subgroup. A T-DNA insertion mutant of RGN1a in Dongjin (a japonica variety) background affected both panicle branch and grain numbers (Figures 5D–H), suggesting that RGN1a is a novel gene involved in the regulation of GNP. In addition, we also found RGN1a participates in regulating plant height and seed setting rate through phenotype investigation.
The cell wall-associated kinase (WAK) family plays an important role in cell expansion and disease resistance (Lally et al., 2001; Verica and He, 2002; Li et al., 2009; Zuo et al., 2015; Delteil et al., 2016) and is mainly composed of extracellular domains (wall-associated receptor kinase galacturonan-binding (GUS-WAK-bind) domain and epidermal growth factor (EGF)), cytoplasmic Ser/Thr kinase domain, and a transmembrane region. The conserved WAK proteins, maintaining approximately 80% similarity, are mainly expressed in the cytoplasmic kinase domain. In contrast, the sequences of five WAK genes in the extracellular domains of Arabidopsis shared only 40% to 64% identity (He et al., 1999; Zhang et al., 2005).
A total of 125 WAKs were identified from the rice genome of japonica Nipponbare and divided into five gene types: OsWAK-RLK, OsWAK-RLCK, OsWAK-RLP, OsWAK short gene, and OsWAK pseudogene (Zhang et al., 2005). Meanwhile, OsWAK-RLCK was defined as having only a cytoplasmic protein kinase domain with more than 40% identity to an OsWAK-RLK member. RNAi-mediated silencing of OsiWAK1 results in decreased plant height, pollen fertility, and flowers per panicle (Kanneganti and Gupta, 2011). Silencing DEFECT in RARLY EMBRYO SAC1 (OsDEES1) causes a functional defect in early embryo sac development and reduced pollen fertility (Wang et al., 2012). OsWAK11 influences grain size and leaf angle by regulating cell elongation rate (Yue et al., 2022). In our study, RGN1a lacked the extracellular domain of EGF compared to WAKs. While the T-DNA mutant rgn1a showed a phenotype similar to that of the OsiWAK1 or OsDEES1 RNAi lines, the grain number per panicle greatly decreased compared to the wild type (Figures 5C-H). Therefore, we believe that RGN1a can be classified as a novel OsWAK-RLCK protein, although further molecular experiments are needed to verify its kinase activity.
Based on candidate gene analyses in qGNP1.3, the amino acid sequence of RGN1b was found to be highly homologous to RGN1a (Supplementary Figure 7). Both genes have a GUS-WAK-binding domain and a cytoplasmic kinase domain and are distributed within a 23 kb region on chromosome 1. Interestingly, WAK genes and WAK-like genes in Arabidopsis usually lie in a tight cluster, such as WAK1-WAK5, WAKL1-WAKL8, and WAKL11-WAKL13, located in a region spanning less than 12 cM (He et al., 1999; Verica and He, 2002);. Gene structure and expression analyses of the rice WAK gene family revealed that localized gene duplication resulted in expanded rice (Zhang et al., 2005). Our results showed that RGN1a and RGN1b are closely distributed on chromosome 1, providing new evidence for this conclusion.
Comparing the geographical distribution of rice germplasm in the favorable haplotype of RGN1a or RGN1b, these varieties are mostly located in the Yunnan, Guizhou, Hunan, Shanxi, and Guangxi provinces of China (Supplementary Figures 11A, B). RGN1a and RGN1b are adjacent to a ~23 kb block (Supplementary Figure 12), indicating that RGN1a and RGN1b were linked and received common selection in evolutionary history. Moreover, rgn1a reduced grain number, indicating that RGN1b may also regulate GNP. In the future, we also need to construct rgn1b and double RGN1a and RGN1b mutants to explore whether there is an additive effect on GNP regulation of these two genes.
We also discussed the breeding applications of RGN1a and RGN1b for variety improvement. The favorable alleles of RGN1a and RGN1b occupied a large proportion of indica and suggested that they had been widely used in the indica subgroup (Figures 4B–E). However, the favorable alleles of RGN1a and RGN1b in the japonica group were mostly found in landraces, especially in China (Supplementary Figures 11A, B). The few varieties containing favorable alleles of RGN1a or RGN1b located abroad were mainly improved varieties (Supplementary Figure 11). This result encourages us to use RGN1a and RGN1b in the genetic improvement of the grain number per panicle of japonica rice in China.
Here, we performed a GWAS for the grain number per panicle in the full rice population using a compressed mixed linear model. In our results, Ghd7, DTH7, and TUT1 were significantly associated with GNP. A novel region, qGNP1.3, contributes to the genetic variation of GNP in the japonica subgroup. The transgenic phenotype confirmed that RGN1a positively regulated the grain number per panicle. Favorable alleles of RGN1a have been used in the indica population, and the aggregation of RGN1a and DTH7 in the japonica population showed additive effects on GNP. There is no doubt that there will be a large increase in grain number per panicle when favorable RGN1a and other reported genes are utilized in improved japonica varieties.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
QZ and HZ designed the research. QZ performed most of experiments, and wrote the manuscript. QZ, JX, and XW performed data analysis. XW and XZ investigated the phenotype. ML and TY performed part of the experiments. CS, JL, and ZL provided technical assistance. NK assisted with revisions for the manuscript. ZZ and HZ provided funding support and supervised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (32172030, 31971922, 32072036), the project of the Administrative Bureau of Sanya Yazhou Bay Science and Technology City (SYND-2022-29), the Fundamental Research Funds for Central Universities of China Agricultural University (2022TC103).
We gratefully thank large scientific instrument sharing platform of College of Agronomy and Biotechnology for providing technical supports.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1097622/full#supplementary-material
Ashikari, M., Sakakibara, H., Lin, S., Yamamoto, T., Takashi, T., Nishimura, A., et al. (2005). Cytokinin oxidase regulates rice grain production. Science 309 (5735), 741–745. doi: 10.1126/science.1113373
Bai, X., Huang, Y., Hu, Y., Liu, H., Zhang, B., Smaczniak, C., et al. (2017). Duplication of an upstream silencer of FZP increases grain yield in rice. Nat. Plants 3 (11), 885–893. doi: 10.1038/s41477-017-0042-4
Bai, J., Zhu, X., Wang, Q., Zhang, J., Chen, H., Dong, G., et al. (2015). Rice TUTOU1 encodes a suppressor of cAMP receptor-like protein that is important for actin organization and panicle development. Plant Physiol. 169 (2), 1179–1191. doi: 10.1104/pp.15.00229
Cingolani, P., Platts, A., Wang, L., Coon, M., Nguyen, T., Wang, L., et al. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6 (2), 80–92. doi: 10.4161/fly.19695
Cui, D., Zhou, H., Ma, X., Lin, Z., Sun, L., Han, B., et al. (2022). Genomic insights on the contribution of introgressions from Xian/Indica to the genetic improvement of Geng/Japonica rice cultivars. Plant Commun. 3 (3), 100325. doi: 10.1016/j.xplc.2022.100325
Delteil, A., Gobbato, E., Cayrol, B., Estevan, J., Michel-Romiti, C., Dievart, A., et al. (2016). Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 16, 17. doi: 10.1186/s12870-016-0711-x
Deshmukh, R., Singh, A., Jain, N., Anand, S., Gacche, R., Singh, A., et al. (2010). Identification of candidate genes for grain number in rice (Oryza sativa l.). Funct. Integr. Genomics 10 (3), 339–347. doi: 10.1007/s10142-010-0167-2
Duan, E., Wang, Y., Li, X., Lin, Q., Zhang, T., Wang, Y., et al. (2019). OsSHI1 regulates plant architecture through modulating the transcriptional activity of IPA1 in rice. Plant Cell 31 (5), 1026–1042. doi: 10.1105/tpc.19.00023
Duan, P., Xu, J., Zeng, D., Zhang, B., Geng, M., Zhang, G., et al. (2017). Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol. Plant 10 (5), 685–694. doi: 10.1016/j.molp.2017.03.009
Gao, H., Jin, M., Zheng, X., Chen, J., Yuan, D., Xin, Y., et al. (2014). Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc. Natl. Acad. Sci. U. S. A. 111 (46), 16337–16342. doi: 10.1073/pnas.1418204111
Gao, X., Zhang, J., Zhang, X., Zhou, J., Jiang, Z., Huang, P., et al. (2019). Rice qGL3/OsPPKL1 functions with the GSK3/SHAGGY-like kinase OsGSK3 to modulate brassinosteroid signaling. Plant Cell 31 (5), 1077–1093. doi: 10.1105/tpc.18.00836
Guo, T., Chen, K., Dong, N., Shi, C., Ye, W., Gao, J., et al. (2018). GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell 30 (4), 871–888. doi: 10.1105/tpc.17.00959
Guo, T., Lu, Z., Shan, J., Ye, W., Dong, N., Lin, H. (2020a). ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice. Plant Cell 32 (9), 2763–2779. doi: 10.1105/tpc.20.00351
Guo, H., Zeng, Y., Li, J., Ma, X., Zhang, Z., Lou, Q., et al. (2020b). Differentiation, evolution and utilization of natural alleles for cold adaptability at the reproductive stage in rice. Plant Biotechnol. J. 18 (12), 2491–2503. doi: 10.1111/pbi.13424
He, Z., Cheeseman, I., He, D., Kohorn, B. (1999). A cluster of five cell wall-associated receptor kinase genes, Wak1-5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 39 (6), 1189–1196. doi: 10.1023/a:1006197318246
Huang, L., Hua, K., Xu, R., Zeng, D., Wang, R., Dong, G., et al. (2021). The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 33 (4), 1212–1228. doi: 10.1093/plcell/koab041
Huang, X., Wei, X., Sang, T., Zhao, Q., Feng, Q., Zhao, Y., et al. (2010). Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42 (11), 961–967. doi: 10.1038/ng.695
Huang, Y., Zhao, S., Fu, Y., Sun, H., Ma, X., Tan, L., et al. (2018). Variation in the regulatory region of FZP causes increases in secondary inflorescence branching and grain yield in rice domestication. Plant J. 96 (4), 716–733. doi: 10.1111/tpj.14062
Huo, X., Wu, S., Zhu, Z., Liu, F., Fu, Y., Cai, H., et al. (2017). NOG1 increases grain production in rice. Nat. Commun. 8 (1) 1497–1507. doi: 10.1038/s41467-017-01501-8
Jiao, Y., Wang, Y., Xue, D., Wang, J., Yan, M., Liu, G., et al. (2010). Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42 (6), 541–544. doi: 10.1038/ng.591
Kanneganti, V., Gupta, A. (2011). RNAi mediated silencing of a wall associated kinase, OsiWAK1 in Oryza sativa results in impaired root development and sterility due to anther indehiscence: Wall associated kinases from Oryza sativa. physiol. Mol. Biol. Plants 17 (1), 65–77. doi: 10.1007/s12298-011-0050-1
Komatsu, K., Maekawa, M., Ujiie, S., Satake, Y., Furutani, I., Okamoto, H., et al. (2003). LAX and SPA: major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. U. S. A. 100 (20), 11765–11770. doi: 10.1073/pnas.1932414100
Koo, B., Yoo, S., Park, J., Kwon, C., Lee, B., An, G., et al. (2013). Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol. Plant 6 (6), 1877–1888. doi: 10.1093/mp/sst088
Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33 (7), 1870–1874. doi: 10.1093/molbev/msw054
Lally, D., Ingmire, P., Tong, H., He, Z. (2001). Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 13 (6), 1317–1331. doi: 10.1105/tpc.13.6.1317
Li, D., Huang, Z., Song, S., Xin, Y., Mao, D., Lv, Q., et al. (2016). Integrated analysis of phenome, genome, and transcriptome of hybrid rice uncovered multiple heterosis-related loci for yield increase. Proc. Natl. Acad. Sci. U. S. A. 113 (41), E6026–E6035. doi: 10.1073/pnas.1610115113
Liu, T., Liu, H., Zhang, H., Xing, Y. (2013). Validation and characterization of Ghd7.1, a major quantitative trait locus with pleiotropic effects on spikelets per panicle, plant height, and heading date in rice (Oryza sativa l.). J. Integr. Plant Biol. 55 (10), 917–927. doi: 10.1111/jipb.12070
Liu, D., Yu, Z., Zhang, G., Yin, W., Li, L., Niu, M., et al. (2021). Diversification of plant agronomic traits by genome editing of brassinosteroid signaling family genes in rice. Plant Physiol. 187 (4), 2563–2576. doi: 10.1093/plphys/kiab394
Livak, K., Schmittgen, T. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Li, G., Xu, B., Zhang, Y., Xu, Y., Khan, N., Xie, J., et al. (2022). RGN1 controls grain number and shapes panicle architecture in rice. Plant Biotechnol. J. 20 (1), 158–167. doi: 10.1111/pbi.13702
Li, S., Zhao, B., Yuan, D., Duan, M., Qian, Q., Tang, L., et al. (2013). Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. U. S. A. 110 (8), 3167–3172. doi: 10.1073/pnas.1300359110
Li, H., Zhou, S., Zhao, W., Su, S., Peng, Y. (2009). A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol. Biol. 69 (3), 337–346. doi: 10.1007/s11103-008-9430-5
Lyu, J., Wang, D., Duan, P., Liu, Y., Huang, K., Zeng, D., et al. (2020). Control of grain size and weight by the GSK2-LARGE1/OML4 pathway in rice. Plant Cell 32 (6), 1905–1918. doi: 10.1105/tpc.19.00468
Miura, K., Ikeda, M., Matsubara, A., Song, X., Ito, M., Asano, K., et al. (2010). OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42 (6), 545–549. doi: 10.1038/ng.592
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81 (3), 559–575. doi: 10.1086/519795
Sato, Y., Antonio, B., Namiki, N., Takehisa, H., Minami, H., Kamatsuki, K., et al. (2011). RiceXPro: a platform for monitoring gene expression in japonica rice grown under natural field conditions. Nucleic Acids Res. 39, D1141–D1148. doi: 10.1093/nar/gkq1085
Shin, J., Blay, S., Mcneney, B., Graham, J. (2006). LDheatmap: An r function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J. Stat. Software 16 (c03). doi: 10.18637/jss.v016.c03
Si, L., Chen, J., Huang, X., Gong, H., Luo, J., Hou, Q., et al. (2016). OsSPL13 controls grain size in cultivated rice. Nat. Genet. 48 (4), 447–456. doi: 10.1038/ng.3518
Tabuchi, H., Zhang, Y., Hattori, S., Omae, M., Shimizu-Sato, S., Oikawa, T., et al. (2011). LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 23 (9), 3276–3287. doi: 10.1105/tpc.111.088765
Tang, Y., Liu, X., Wang, J., Li, M., Wang, Q., Tian, F., et al. (2016). GAPIT version 2: An enhanced integrated tool for genomic association and prediction. Plant Genome 9 (2). doi: 10.3835/plantgenome2015.11.0120
Verica, J. A., He, Z. (2002). The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiol. 129 (2), 455–459. doi: 10.1104/pp.011028
Wang, N., Huang, H., Ren, S., Li, J., Sun, Y., Sun, D., et al. (2012). The rice wall-associated receptor-like kinase gene OsDEES1 plays a role in female gametophyte development. Plant Physiol. 160 (2), 696–707. doi: 10.1104/pp.112.203943
Wang, W., Mauleon, R., Hu, Z., Chebotarov, D., Tai, S., Wu, Z., et al. (2018). Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557 (7703), 43–49. doi: 10.1038/s41586-018-0063-9
Weng, X., Wang, L., Wang, J., Hu, Y., Du, H., Xu, C., et al. (2014). Grain number, plant height, and heading date7 is a central regulator of growth, development, and stress response. Plant Physiol. 164 (2), 735–747. doi: 10.1104/pp.113.231308
Xue, W., Xing, Y., Weng, X., Zhao, Y., Tang, W., Wang, L., et al. (2008). Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 40 (6), 761–767. doi: 10.1038/ng.143
Yan, W., Liu, H., Zhou, X., Li, Q., Zhang, J., Lu, L., et al. (2013). Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res. 23 (7), 969–971. doi: 10.1038/cr.2013.43
Yue, Z., Liu, N., Deng, Z., Zhang, Y., Wu, Z., Zhao, J., et al. (2022). The receptor kinase OsWAK11 monitors cell wall pectin changes to fine-tune brassinosteroid signaling and regulate cell elongation in rice. Curr. Biol. 32 (11), 2454–2466. doi: 10.1016/j.cub.2022.04.028
Zhang, S., Chen, C., Li, L., Meng, L., Singh, J., Jiang, N., et al. (2005). Evolutionary expansion, gene structure, and expression of the rice wall-associated kinase gene family. Plant Physiol. 139 (3), 1107–1124. doi: 10.1104/pp.105.069005
Zhang, Z., Li, J., Yao, G., Zhang, H., Dou, H., Shi, H., et al. (2011a). Fine mapping and cloning of the grain number per-panicle gene (Gnp4) on chromosome 4 in rice (Oryza sativa l.). Agric. Sci. China 10 (12), 1825–1833. doi: 10.1016/S1671-2927(11)60182-X
Zhang, H., Zhang, D., Wang, M., Sun, J., Qi, Y., Li, J., et al. (2011b). A core collection and mini core collection of Oryza sativa l. @ in China. Theor. Appl. Genet. 122 (1), 49–61. doi: 10.1007/s00122-010-1421-7
Zhao, Y., Zhang, H., Xu, J., Jiang, C., Yin, Z., Xiong, H., et al. (2018). Loci and natural alleles underlying robust roots and adaptive domestication of upland ecotype rice in aerobic conditions. PloS Genet. 14 (8), e1007521. doi: 10.1371/journal.pgen.1007521
Zhu, J., Yang, M., Wang, Z., Wang, J., Zhou, Y., Yang, J., et al. (2013). Identification of grain number quantitative trait loci with single segment substituted lines in rice. Acta Agricult. Boreali-Sinica 28 (4), 23–30.
Keywords: GWAS, grain number per panicle, breeding, haplotype, rice
Citation: Zhang Q, Xie J, Wang X, Liu M, Zhu X, Yang T, Khan NU, Sun C, Li J, Zhang Z, Li Z and Zhang H (2022) Natural variation of RGN1a regulates grain number per panicle in japonica rice. Front. Plant Sci. 13:1097622. doi: 10.3389/fpls.2022.1097622
Received: 14 November 2022; Accepted: 28 November 2022;
Published: 13 December 2022.
Edited by:
Zhiwen Chen, Hainan Yazhou Bay Seed Laboratory, ChinaReviewed by:
Meng Jiang, Zhejiang University, ChinaCopyright © 2022 Zhang, Xie, Wang, Liu, Zhu, Yang, Khan, Sun, Li, Zhang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongliang Zhang, emhhbmdsQGNhdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.