
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 14 November 2022
Sec. Plant Systematics and Evolution
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1059379
This article is part of the Research Topic Rise to the Challenges in Plastome Phylogenomics View all 10 articles
Clematis is one of the largest genera of Ranunculaceae with many phylogenetic problems left to be resolved. Clematis species have considerable genome size of more than 7 Gbp, and there was no whole-genome reference sequence published in this genus. This raises difficulties in acquiring nuclear genome data for its phylogenetic analysis. Previous studies based on Sanger sequencing data, plastid genome data, and nrDNA sequences did not well resolve the phylogeny of Clematis. In this study, we used genome skimming and transcriptome data to assemble the plastid genome sequences, nuclear single nucleotide polymorphisms (SNPs) datasets, and single-copy nuclear orthologous genes (SCOGs) to reconstruct the phylogenetic backbone of Clematis, and test effectiveness of these genome partitioning methods. We also further analyzed the discordance among nuclear gene trees and between plastid and nuclear phylogenies. The results showed that the SCOGs datasets, assembled from transcriptome method, well resolved the phylogenetic backbone of Clematis. The nuclear SNPs datasets from genome skimming method can also produce similar results with the SCOGs data. In contrast to the plastid phylogeny, the phylogeny resolved by nuclear genome data is more robust and better corresponds to morphological characters. Our results suggested that rapid species radiation may have generated high level of incomplete lineage sorting, which was the major cause of nuclear gene discordance. Our simulation also showed that there may have been frequent interspecific hybridization events, which led to some of the cyto-nuclear discordances in Clematis. This study not only provides the first robust phylogenetic backbone of Clematis based on nuclear genome data, but also provides suggestions of genome partitioning strategies for the phylogenomic study of other plant taxa.
With the rapid development of molecular biotechnology, the cost of high-throughput sequencing continues to decrease. Using genomic data to reconstruct phylogeny and explore the origin and evolutionary history of plant taxa is growing rapidly (Zimmer and Wen, 2015; Wen et al., 2017; Marks et al., 2021; Kress et al., 2022). Compared to previous studies using the Sanger sequencing method, the application of genomic data has greatly improved the resolution of the phylogenetic trees (Valcárcel and Wen, 2019; Li et al., 2021; Khan et al., 2021). Genomic data can not only generate better resolved phylogenies of plant taxa, but can also alleviate the problem of stochastic error due to insufficient information from small datasets (Yu et al., 2018; Watson et al., 2020).
In recent years, the plastid genome (plastome) has been considered to be the most important source of data and widely applied for phylogenetic reconstruction of green plant phylogeny at almost all taxonomic levels (Li et al., 2019; Zhai et al., 2019; Zhang et al., 2020; Zhao et al., 2021). However, the uni-parental inherited plastid genome sometimes showed conflicting phylogenetic signals with the bi-parental inherited nuclear genome data (cyto-nuclear discordance) due to chloroplast capture, incomplete lineage sorting (ILS) (Rose et al., 2021), or other factors such as sampling error, stochastic error, paralogs, and so on (Zou and Ge, 2008). Comprehensive understanding of evolutionary process for a plant taxon requires both cytoplasmic and nuclear genome evidence and an in depth analysis of their phylogenetic discordance (Lee-Yaw et al., 2019).
Clematis L. is one of the largest genera in the family Ranunculaceae with about 300 wild species, most of which are diploid (Tamura, 1995; Wang and Bartholomew, 2001; Wang and Li, 2005). The taxonomy of Clematis has been considered to be difficult. Many classifications published in recent years held different views on many issues, including the delineation of the genus, infrageneric classification, and species delimitation (Tamura, 1995; Johnson, 1997; Grey-Wilson, 2000; Wang and Li, 2005). Previous molecular phylogenetic studies, based on the nuclear ribosomal DNA (nrDNA), the plastid fragments, and the complete plastome data, have solved many of those problems, such as genus delineation and the identification of the sister group of Clematis (Miikeda et al., 2006; Xie et al., 2011; Lehtonen et al., 2016; Jiang et al., 2017; He et al., 2021). However, all the previously published studies had limitations of not establishing a robust phylogenetic framework within Clematis, and its extensive cyto-nuclear discordance remains to be analyzed by inclusion of more nuclear genome data.
There are several reasons that may contribute to the difficulties in reconstructing a robust phylogeny of Clematis. Firstly, according to previous molecular studies, species radiation events may have happened during the late Neogene and the Quaternary (Xie et al., 2011; He et al., 2021). Small number of DNA sequences with insufficient informative loci often failed to resolve the relationships among recently radiated groups (Zhao et al., 2021). Secondly, interspecific hybridization may have happened or may be not uncommon in Clematis (Lyu et al., 2021), that may cause cyto-nuclear discordance during phylogenetic reconstruction. Thirdly, Clematis species have relatively large genome size (7.18 Gbp−16.43 Gbp, https://cvalues.science.kew.org/search) and there is no high-quality whole genome data available, which raise technical difficulties for genome-partitioning selection.
The genome-partitioning methods for phylogenomic study of plant taxa generally include reduced-representation Genome Sequencing (RRGS), genome skimming, transcriptome sequencing or RNA-seq, and target enrichment sequencing (Zimmer and Wen, 2015; Yu et al., 2018; McKain et al., 2018). Among them, genome skimming, which randomly captures a certain percentage of total genomic DNA (Dodsworth, 2015; Thode et al., 2020; Wikström et al., 2020), has been widely applied for phylogenetic studies (Wen et al., 2018; Su et al., 2021; He et al., 2021). One of the advantages of genome skimming method is that fresh, silica-gel dried, or even herbarium materials can be used for this method (Liu et al., 2019; Wang et al., 2020). Using genome skimming data, cytoplasmic genome and tandemly repeated nrDNA can be assembled for phylogenetic reconstruction (Yu et al., 2018; Fonseca and Lohmann, 2020). According to the recently developed method by Liu et al. (2021), genome skimming data with high sequencing depth (10 × or more) can be used for assembling single-copy nuclear genes for phylogenetic studies. Other studies have shown that genome skimming data with low sequencing depth (less than 1 ×) can be used to obtain single nucleotide polymorphisms (SNPs) from nuclear genome for phylogenetic reconstruction (Olofsson et al., 2019).
In contrast, transcriptome method has irreplaceable advantages for obtaining single-copy nuclear genes (One Thousand Plant Transcriptomes Initiative, 2019), and plant genome size is not the factor affecting sequencing depth because the transcribed gene content is small and very stable among seed plants (around 0.03 Gbp, Novák et al., 2020). However, the application of the transcriptome method is limited by plant material, which requires fresh plant tissue (or stored in RNA stabilization solution), or at least silica gel dried material (He et al., 2022). For a large genus like Clematis, a considerable proportion of species samples may be from herbarium specimens. It is difficult to obtain transcriptome data from all samples. In recent years, using targeted enrichment sequencing method to obtain nuclear gene has attracted much attention in phylogenetic studies (Vargas et al., 2019; Stull et al., 2020). This method can also use herbarium material for DNA extraction. However, comparing to RNA-seq method, the target enrichment method has much more complicated experimental process, relatively smaller amount of data, too much missing data, and low data reusability (McKain et al., 2018).
For Clematis, an accurate and well-supported phylogenetic backbone still remains to be reconstructed by nuclear genome data. Obtaining high-depth sequencing data (10 × means at least 70 Gbp for each sample in Clematis) to assemble nuclear genes is not economically viable for Clematis. In this study, using genome skimming (with low depth) and transcriptome data, we try to answer the following questions: to what extent nuclear genome data may improve the phylogenetic inference of Clematis? can genome skimming data with low sequencing depth provide more nuclear phylogenetic information? if the nuclear single nucleotide polymorphisms (SNPs) data from genome skimming method can be used for Clematis phylogenetic reconstruction? which one, incomplete lineage sorting or hybridization, may have caused the cyto-nuclear discordance of Clematis? This study will also shed light on the genome partitioning selection for phylogenomic analysis of other similar taxa with recent species radiation and considerable genome size.
Because the major purpose of this study is to check the robustness of the phylogenetic backbones inferred by different datasets, we chose a phylogenetically representative sampling scheme with only key species of Clematis in this study. A total of 32 species (about 1/10 of total species) were used for our phylogenomic analysis, covering all the subgenera of both Tamura (1995) and Wang and Li (2005). This sampling scheme also covers 11 sections (of the total 17) in the classification of Tamura (1995), and 9 sections (of the total 15) in the classification of Wang and Li (2005). Although we did not include several small sections (like sect. Archiclematis, sect. Pterocarpa, and sect. Angustifoliae), our sampling represented all the major lineages (clades) of Clematis included in previous studies (Miikeda et al., 2006; Xie et al., 2011; He et al., 2021). Furthermore, our previous studies showed that some sections, such as sect. Clematis and sect. Viorna (Reichb.) Prantl (sensu Wang and Li, 2005), may be polyphyletic. So, our samples also included problematic species of those sections (Supplementary Table S1).
The plant materials are mostly collected from the field, only with two samples from herbarium specimens. Among all the 32 sampled species, genome skimming data of 28 were newly generated for this study, and those of the other four species were retrieved from previous studies (Supplementary Table S1). Because specimen materials cannot yield RNA-seq data, transcriptomes of only 28 species were sequenced in this study. According to Jiang et al. (2017), Anemoclema glaucifolium (Franch.) W. T. Wang was chosen as an outgroup.
Transcriptome sequencing followed the method of He et al. (2022). Total RNAs were extracted at Biomarker Technologies Corporation (https://www.biomarker.com.cn) from silica gel dried leaves using TRIzon Reagent (TRIzon, CoWin Biosciences, Jiangsu, PR China). Then the RNAs were reversed into cDNA, and paired-end reads of 2 ×150 libraries were generated and sequenced on a NovaSeq 6000 platform (Illumina, San Diego, California, USA). About 6 Gbp of raw reads were obtained for each samples. The raw reads were then filtered and trimmed using fastp v.0.20 (Chen et al., 2018). The clean transcriptomes were de novo assembled using Trinity v.2.5.1 (Grabherr et al., 2011) with default parameters. All the transcriptome data were deposited in GenBank (Supplementary Table S2).
The total genomic DNAs were extracted from silica-dried samples at Biomarker Technologies Corporation (https://www.biomarker.com.cn) using a genomic DNA extraction kit following manufacturer instructions (Tiangen Biotech Co. Ltd., Beijing, China). For the specimen samples, the total DNAs were obtained from the Herbarium of Institute of Botany, the Chinese Academy of Sciences (PE), and the extraction method was according to Li, 2013. Then, 2 ×150 bp paired-end libraries were constructed and sequenced using an illumina NovaSeq 6000 platform (Illumina, San Diego, California, USA). The newly sequenced samples yielded around 6 Gbp of raw data. In order to assemble the draft genome of Clematis, we extracted total DNA from a C. brevicaudata DC. sample and constructed a library for sequencing, finally obtaining raw data of about 200 Gbp. All the genome skimming data were deposited in GenBank (Supplementary Table S1).
We used genome skimming data to assemble the complete plastid genome sequence using GetOrganelle v.1.7.5 (Jin et al., 2020). Detailed assembling process followed He et al. (2021). The assembled plastome sequences were annotated using Plann v.1.1.2 (Huang and Cronk, 2015) and manually adjusted by Geneious Prime v.2020 (Kearse et al., 2012).
Nuclear single-copy orthologs (SCOGs) were obtained from transcriptome data followed the pipeline of He et al. (2022). We used CD-HIT v.4.6.2 (Fu et al., 2012) to remove redundant sequences and TransDecoder v.5.0 (https://github.com/TransDecoder/TransDecoder/releases) to predict protein-coding regions. The assembly completeness of each sample was assessed using BUSCO v.5.2.2 (Simao et al., 2015). Subsequently, we constructed transcriptome homology scans using Proteinortho v.6.0.10 (Lechner et al., 2011) in the Diamond mode (Buchfink et al., 2015), and then searched the resulting clusters to identify gene families using a Python script “get_seq_from_proteinortho.py” (https://github.com/HeJian151004/get_seq_from_proteinortho). We then deleted all the organelle genome sequences from the SCOGs using the script “del_chloro_mito_from_fasta.py” (https://github.com/HeJian151004/del_chloro_mito_from_fasta), and used Treeshrink v.1.3.9 (Mai and Mirarab, 2018) to delete sequences that may be incorrectly clustered (showing unexpectedly long branches in the gene tree). Finally, we selected two SCOGs datasets with alignment length at least 1,000 bp (SCOG1000) and 3,000 bp (SCOG3000) for phylogenetic analysis.
For the genome skimming data, we further mined the nuclear SNPs data for phylogenetic inference. In brief, we assembled a draft genome as a reference, and then mapped the genome skimming data of other species to this reference genome to obtain the SNPs dataset. We used two methods to obtain the SNPs data, the GATK and the Geneious pipelines. Detailed process of both pipelines are as follows (also shown in Figure 1).
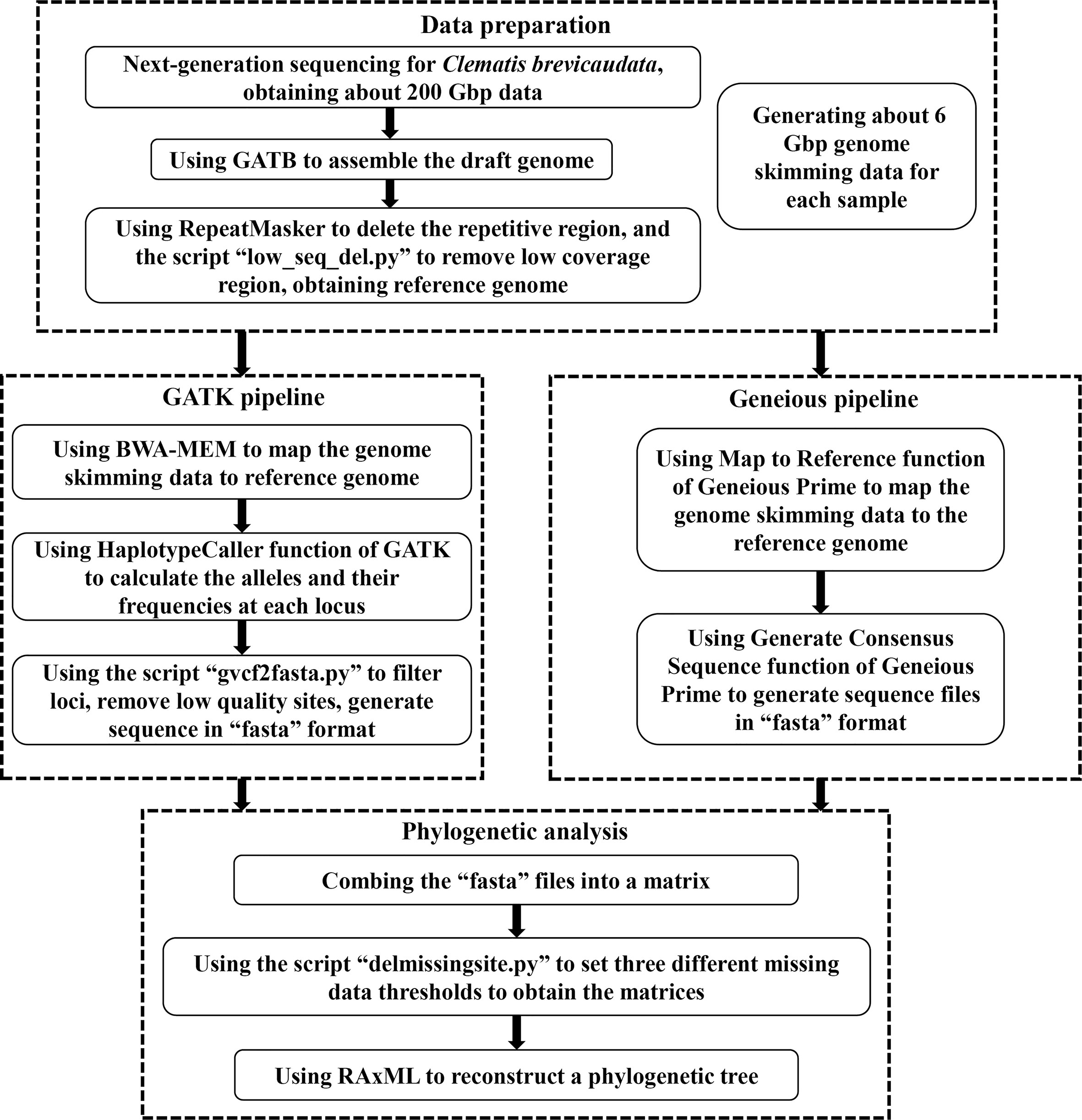
Figure 1 A flow chart of acquiring nuclear single nucleotide polymorphisms (SNPs) dataset from genome skimming data in this study.
First, we used the GATB-Minia (https://github.com/GATB/gatb-minia-pipeline) to assemble the draft genome (Drezen et al., 2014). We obtained a draft genome of 7.81Gbp, which is too large to be applied for downstream analysis. Therefore, we used the RepeatMasker v.4.0.9 (Chen, 2004) to exclude the repetitive regions in the draft genome. We further deleted the low coverage regions by the following processes: the genome skimming data of five distantly related Clematis species [C.leschenaultiana DC., C. repens Finet et Gagnep., C. songorica Bunge, C. tibetana Kuntze, C. viridis (W. T. Wang and M. C. Chang) W. T. Wang] were mapped to the draft genome by Map to Reference function of Geneious Prime v.2020 (Kearse et al., 2012). Then we used a script “low_seq_del.py” (https://github.com/Jhe1004/low_seq_del) to remove the regions that none of the five samples were matched. After removing the duplicate and low coverage regions, we finally obtained a reference genome of 616 Mbp.
The GATK pipeline used BWA-MEM v.0.7.1 (Li, 2013) to map each genome skimming data back to the reference genome to generate “bam” format files. Then the HaplotypeCaller function of GATK v.4.2.5 (Mckenna et al., 2010) was applied to calculate the alleles and their frequencies at each locus. Then, GATK output the result as the “vcf” format file. Then, we used script “gvcf2fasta.py” (https://github.com/Jhe1004/gvcf2fasta) to convert “vcf” file to the “fasta” sequence. We filtered and deleted the site that met any of the following three criteria: (1) coverage less than 4, (2) site quality score less than 20, and (3) heterozygous.
The Geneious pipeline applied the Map to Reference function of Geneious Prime v.2020 (Kearse et al., 2012) to map the genome skimming data of each sample to the reference genome using Custom Sensitivity option with Allow Gaps off. Then we used the Generate Consensus Sequence function (using Trim to Reference Sequence option, and Most Common Bases for heterozygous sites) to generate sequence file of each sample, and finally saved these sequences as “fasta” files. All the alignments of this study, including the complete plastid genome sequences, SCOGs, and nuclear SNPs datasets, are deposited on Zenodo with the identifier https://doi.org/10.5281/zenodo.7215665.
Plastid genome structure and gene arrangement in Clematis species were checked according to the method of Liu et al. (2018), and then multiple sequence alignments were done using MAFFT v.7.471 (Katoh and Standley, 2013), after removing one inverted repeat (IR) region (He et al., 2019; He et al., 2021). We used both maximum likelihood (ML) and Bayesian inference (BI) methods for phylogenetic reconstruction. ML trees were generated by RAxML v.8.2.12 (Stamatakis, 2014) under the GTR+G model with bootstrap percentages computed after 100 replicates. BI analysis was performed using MrBayes v.3.2.3 (Ronquist et al., 2012) and the best substitution model (TVM+I+G) was tested by the AIC in jModelTest v.2.1.10 (Darriba et al., 2012). Markov chain Monte Carlo (MCMC) chains run 2,000,000 generations, sampling every 100 generations. The first 25% of the trees were discarded as burn-in, and the remaining trees were used to generate the consensus tree.
For the two SCOGs datasets, we applied both concatenation- and coalescent-based methods for phylogenetic reconstruction. For the concatenation method, genes of all the datasets were concatenated. Then, we used RAxML v.8.2.12 (Stamatakis, 2014) to reconstruct phylogeny with the GTR+G model and 100 replicates of bootstrap. For the coalescent-based method, single-gene trees were reconstructed by RAxML with the parameters as above. All gene trees were then inputted in ASTRAL v.4.4.4 (Zhang et al., 2018) for species tree inference.
The nuclear SNPs matrices obtained by both GATK and Geneious pipelines had a high proportion of missing data at many loci. Therefore, we set three missing data (percentage of gaps per alignment column, Duvall et al., 2020) thresholds for each pipeline and obtained six matrices: GATK-0.4MS, GATK-0.5MS, GATK-0.6MS (40%, 50%, and 60% missing data); Geneious-0MS, Geneious-0.05MS, Geneious-0.1MS (0, 5%, and 10% missing data). We used SNP-sites v.2.5.1 (Page et al., 2016) to remove the invariant sites. Then, all matrices were analyzed using ML method implemented in RAxML v.8.2.12 with “ASC_GTRGAMMA” model (Stamatakis, 2014) and 100 bootstrap replicates.
In this study, we explored the discordance among nuclear gene trees, between plastid and nuclear gene trees, and analyzed the possible biological causes. We tried to exclude factors such as sampling errors, stochastic errors, and paralogs (Zou and Ge, 2008), and tested the role of incomplete lineage sorting (ILS) and hybridization on the discordance of gene trees.
First, we examined the conflict among nuclear gene trees (the SCOG1000 dataset). In order to reduce the influence of stochastic error, gene trees with average support values more than 60 were chosen for analysis. We used Phyparts v.0.0.1 (Smith et al., 2015) to compare each nuclear gene tree with the species tree, calculated the proportion of gene trees concordant with the species tree at each node, and displayed them with pie charts. Meanwhile, to further visualize single-gene tree conflicts, we built cloud tree plots using the python package Toytree v.2.0.5 (Eaton, 2020).
The causes of nuclear gene tree conflicts were explored using a multiple species coalescent (MSC) model implemented in a simulation analysis to investigate whether ILS could be used to explain the conflict among nuclear gene trees (Yang et al., 2020; Morales-Briones et al., 2021). If the coalescent model fit the empirical gene trees well, the simulated gene trees would be consistent with the empirical gene trees, and ILS can explain the tree discordance. We used the function “sim.coaltree.sp” in the R package Phybase v.1.5 (Liu and Yu, 2010) to simulate 10,000 gene trees under the MSC model (the input coalescent species tree was constructed using the SCOG1000 dataset). Finally, we calculated the distances between each empirical gene tree and the species tree using DendroPy v.4.5.2 (Sukumaran and Holder, 2010), then showed the distance distribution between simulated gene trees and species tree using a histogram plot.
We also analyzed the causes of cyto-nuclear discordance and carried out a coalescent simulation study (Rose et al., 2021). We used the “sim.coaltree.sp” function in the R package Phybase v.1.5 (Liu and Yu, 2010) to simulate 10,000 gene trees, and then used PhyParts v.0.0.1 (Smith et al., 2015) to compare these simulated gene trees to the plastome phylogeny. If the discordant nodes are supported by a certain proportion of simulated gene trees, then it is probable that the conflict was caused by incomplete lineage sorting.
The genome skimming data size of each sample ranged from 5.07 Gbp (C. songorica) to 6.20 Gbp (C. brevicaudata), and the Q20 was 96.0%−98.9%, Q30 was 89.4%−96.9% (Supplementary Table S1). The data size of transcriptomes ranged from 5.41 Gbp (C. viridis) to 6.76 Gbp (C. sibirica Miller), and the Q20 was 97.3%−98.5%, Q30 was 93.0%−95.6%. The number of de novo transcripts varied from 53,429 (C. tibetana) to 147,758 (C. macropetala Ledeb.), and 37,188−114,171 transcripts were kept after removing redundancy. The N50 length of the transcripts ranged from 712 bp to 1,547 bp, and completeness of the assemblies comparing to BUSCO ranged from 54.2% (C. reticulata Walter) to 75.9% (C. terniflora DC.) (Supplementary Table S2). The size of C. brevicaudata genome draft was 7.81 Gbp with the contig N50 being 2,579 bp, and the number of contigs longer than 500 bp was 4,293,110 in total size of 6.19 Gbp.
We acquired a total of 32 Clematis plastome sequences ranging from 159,284 bp (C. reticulata) to 159,847 bp (C. viridis). The number and arrangement of the plastid genes of all the Clematis species are identical, all contained a pair of datIRs (31,023−31,082 bp.) separated by a large single copy region (79,074−79,693 bp) and a small single copy region (17,978−18,229 bp). All plastomes encoded a set of 112 genes, including 79 protein-coding genes, 29 transfer RNAs and four ribosomal RNAs (Supplementary Table S3). After removing IRa and poor alignment region, we finally obtained a matrix with aligned length of 128,149 bp for phylogenetic analysis.
For the transcriptome data, we obtained 9,900 SCOGs by homologous clusters after removing 106 organelle genes. We further discarded 3,782 genes, which were shorter than 1,000 bp, in subsequent analyses. Finally, the SCOG1000 dataset contained 6,118 genes (4,393 genes with average support value over 60), and SCOG3000 dataset contained 699 genes.
The data amount of nuclear SNPs matrix obtained by GATK and Geneious pipelines are different. The lengths of the matrices obtained by GATK pipeline are 21,767bp (GATK-0.4MS), 48,933 bp (GATK-0.5MS), and 100,223 bp (GATK-0.6MS), whereas those of the Geneious pipeline are 99,179 bp (Geneious-0MS), 375,536 bp (Geneious-0.05MS), and 2,066,289 bp (Geneious-0.1MS), respectively. The proportion of missing data in sect. Naravelia Prantl and sect. Naraveliopsis Hand.-Mazz. were significantly higher than those in other species. Because only 2.59 Gbp genome skimming data were available online for C. fusca Turcz. (Supplementary Table S1), high percentage of missing data was also present in its nuclear SNPs sequence.
For the plastid genome data, except a few clades with relatively weak support values, the majority of branches received full support (Figure 2A). Sect. Naraveliopsis Hand.-Mazz., sect. Atragene (L.) DC., sect. Naravelia (DC.) Prantl, sect. Cheiropsis DC., sect. Meclatis (Spach) Baillon, and sect. Fruticella Tamura (sensu Tamura, 1995) were shown to be monophyletic. Whereas, some sections, such as sect. Campanella Tamura, sect. Clematis (sensu Tamura, 1995), and sect. Tubulosae Decne. were not supported in the plastid phylogeny, and species of these sections were nested together.
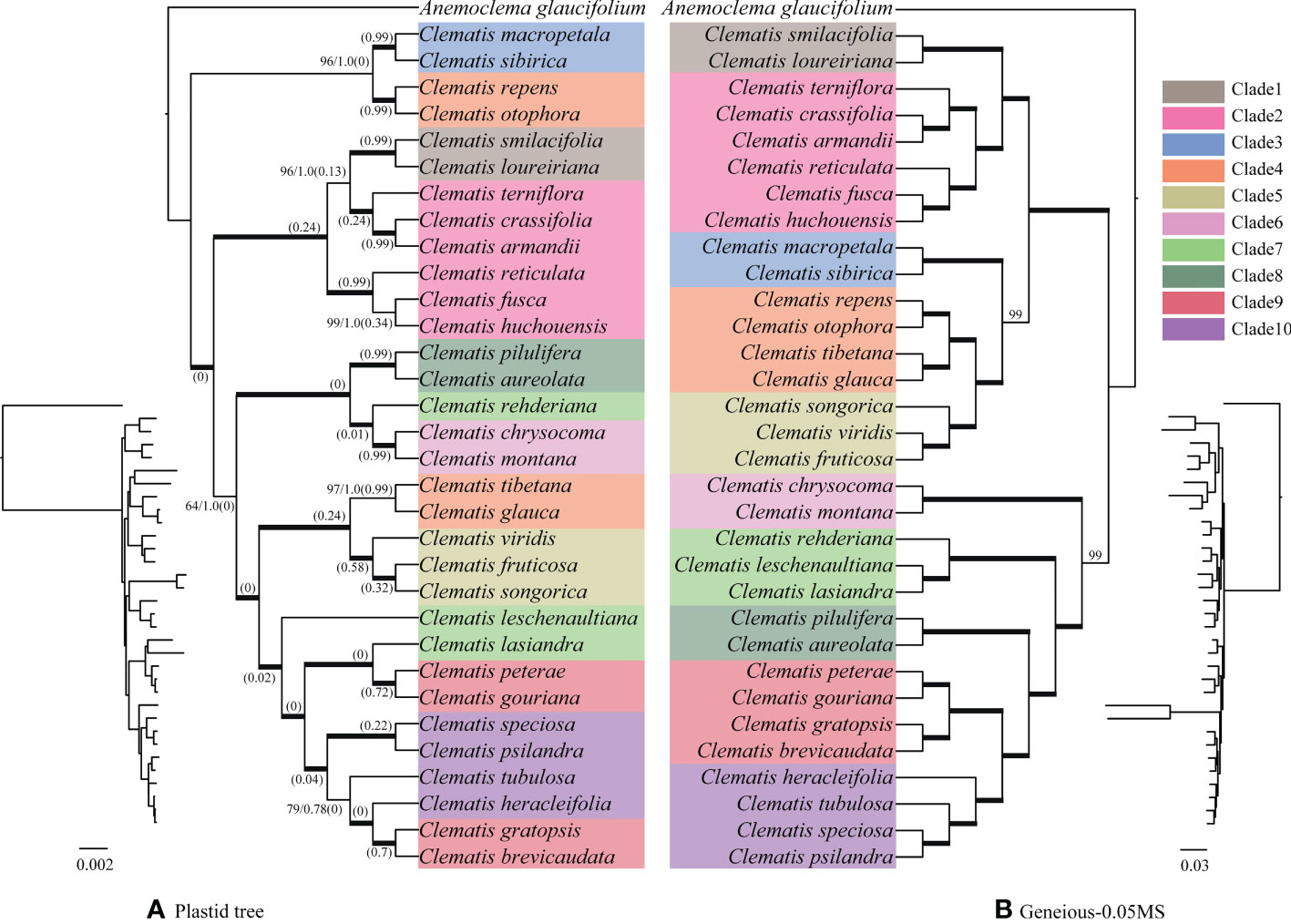
Figure 2 Bayesian phylogeny (A) of Clematis inferred from the plastid genome data and maximum likelihood phylogeny (B) inferred from nuclear SNPs of Geneious-0.05MS dataset. Cyto-nuclear conflicts are shown. In the plastid phylogeny (A), bold branches show that the clades are 100% supported by both posteriori probability and ML bootstrap values. Otherwise, these two statistical values were marked on the branches. Numbers in brackets show the contribution of incomplete lineage sorting (ILS) to the conflicts between the simulated and plastid gene trees based on the multispecies coalescent model. Ten major clades were marked on the nuclear SNPs tree (B) with different colors. Species in plastid tree were marked with the same color with those in the nuclear SNPs tree.
Two transcriptome-based datasets (SCOG1000 and SCOG3000) yielded highly congruent phylogenies in the coalescent-based and concatenated analyses (Figure 3 and Supplementary Figure S1). All nodes in the SCOG1000 dataset using coalescent method obtained 100% support values. Whereas, in the SCOG3000 dataset, sect. Fruticella was not 100% supported, and the position of C. songorica was different in the coalescent and concatenated analyses (Supplementary Figures S1, S2). For the SCOG1000 dataset, except sect. Campanella, sect. Viorna (sensu Wang and Li, 2005) and sect. Clematis (sensu Wang and Li, 2005), which was shown to be polyphyletic, other sections were supported (Figure 3). Sect. Clematis (sensu Tamura, 1995) and sect. Tubulosae were both supported and tested to be sister groups
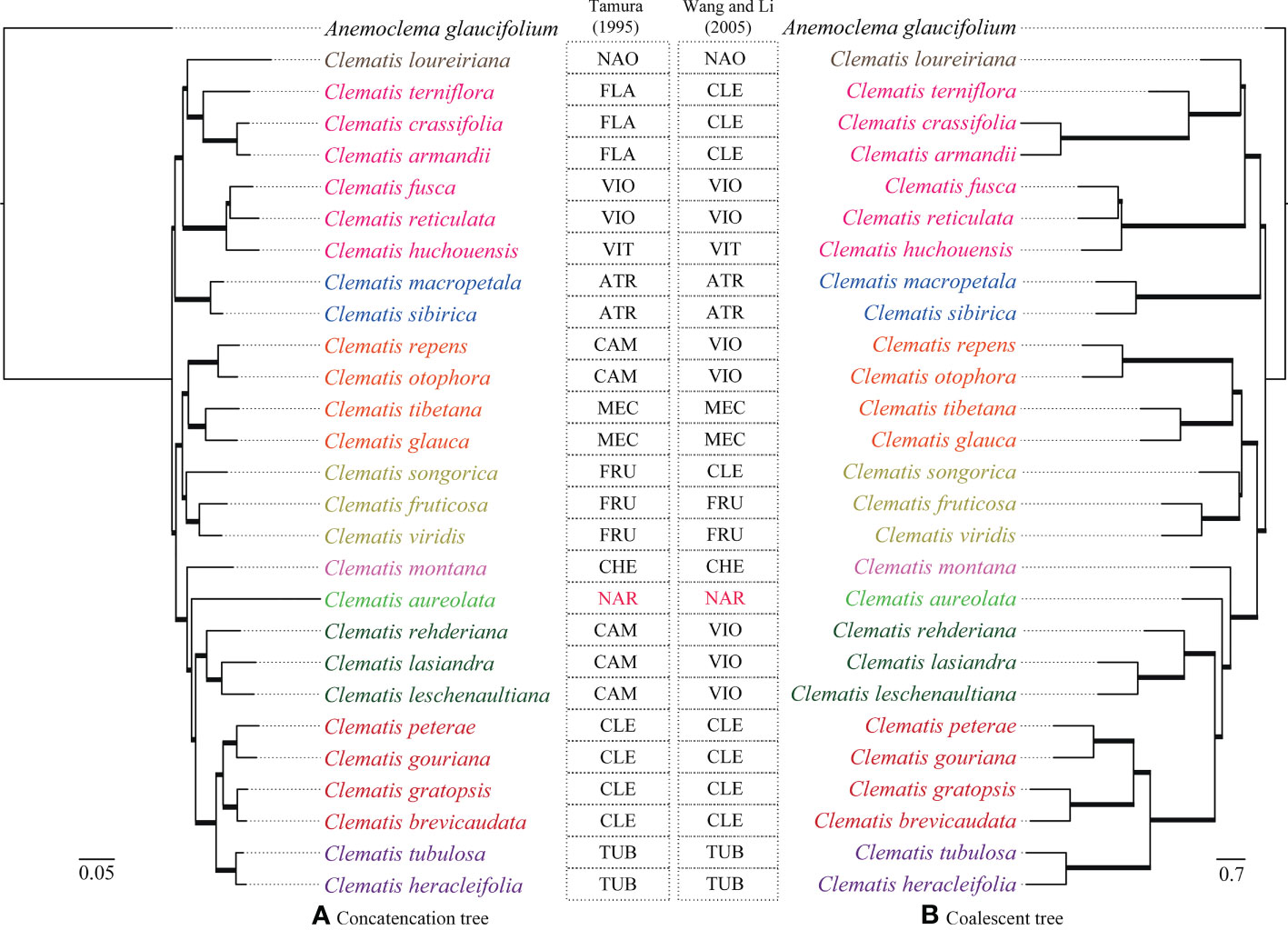
Figure 3 Phylogenetic trees inferred from SCOG1000 dataset by the concatenated (left) and the coalescence-based (right) methods. All clades of both trees are 100% supported and all the branches are in bold. Section abbreviations are: sect. Atragene (ATR), sect. Naraveliopsis (NAO), sect. Clematis (CLE), sect. Flammula (FLA), sect. Viorna (VIO), sect. Viticella (VIT), sect. Campanella (CAM), sect. Meclatis (MEC), sect. Fruticella (FRU), sect. Cheiropsis (CHE), sect. Tubulosae (TUB), Naravelia (NAR).
The nuclear SNPs phylogenies based on the GATK pipeline were slightly different in basal branches which were insufficiently supported (Supplementary Figures S3). Among them, GATK-0.4MS dataset yielded a phylogeny which was more consistent with the trees inferred from the Geneious pipeline (Supplementary Figure 4). The resolved clades were also largely consistent with the SCOG1000 species tree (Figure 3). However, although sect. Clematis (sensu Tamura, 1995) and sect. Tubulosae showed close relationship in the three GATK datasets, the former section was shown to be paraphyletic to the latter (Supplementary Figure S3).
The phylogenies inferred from the three datasets of Geneious pipeline were basically similar, but differed in support values (Figure 4 and Supplementary Figure S4). The Geneious-0.05MS dataset produced the most robust phylogeny, which was almost the same with the SCOG1000 species tree. Their major difference was the position of sect. Naraveliopsis (Figures 3, 4). Both SCOG1000 and the Geneious-0.05MS datasets had some well-supported incongruence with the plastid tree (Figure 2; Supplementary Figure S2). Because the nuclear SNPs dataset contains more samples than the SCOG1000 dataset, we discuss the phylogenetic relationships of Clematis mainly based on Geneious-0.05MS dataset (Figure 4). In this phylogenetic tree, ten major clades were resolved, and one section (sect. Campanella) in Tamura (1995) and two sections (sect. Clematis and sect. Viorna) in Wang and Li (2005) were shown to be polyphyletic. All the other sectional classification of the two systems were supported.
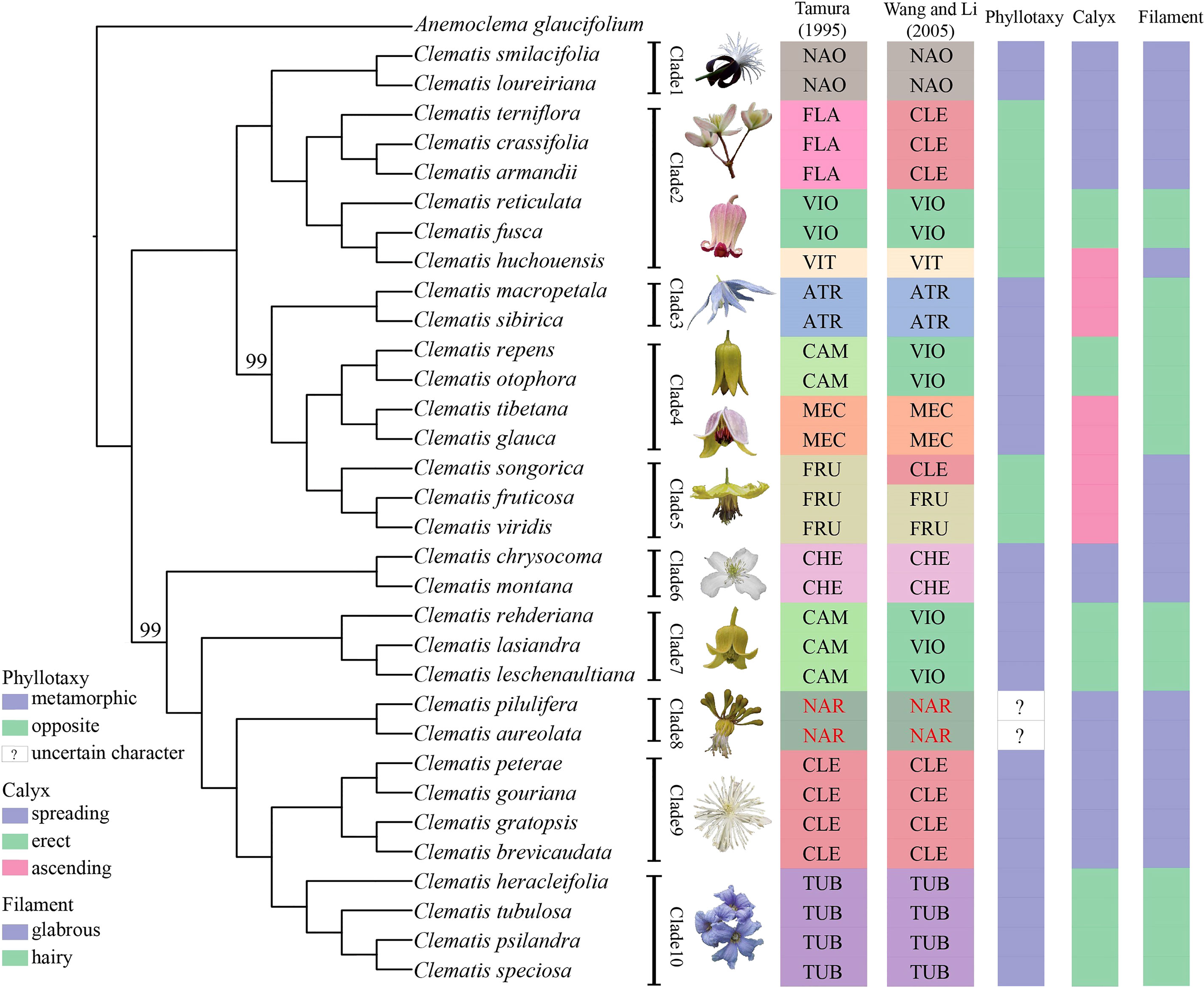
Figure 4 A maximum likelihood tree inferred form a nuclear SNPs dataset (Geneious-0.05MS). Two bootstrap values, which are less than 100, are marked above the branch, and all the other branches are fully supported. Section abbreviations follow Figure 3, and three important morphological characters are marked at right side of the tree.
Using SCOG1000 (and average bootstrap value more than 60) dataset, high levels of gene tree discordances were detected mainly at deep nodes (Figure 5). Coalescent simulation analysis (Figure 6) showed similar pattern between empirical and simulated distance distributions, indicating that ILS alone can explain most of the gene tree conflicts. However, the contradiction between some nodes of the plastid and the nuclear species trees cannot be explained by ILS (Figure 2A). For example, species of Clade 9 (sect. Clematis sensu Tamura, 1995) and Clade 10 (sect. Tubulosae) (Figure 2B) were clustered together in the plastome phylogeny (Figure 2A), and Phyparts result showed no simulated gene trees were concordant to the empirical plastome tree. Moreover, some species of sect. Campanella (such as C. rehderiana) also showed different positions in the simulated gene trees and the plastome tree, suggesting that ILS can be excluded for explaining its cyto-nuclear discordance, and hybridization and introgression might be the main cause.
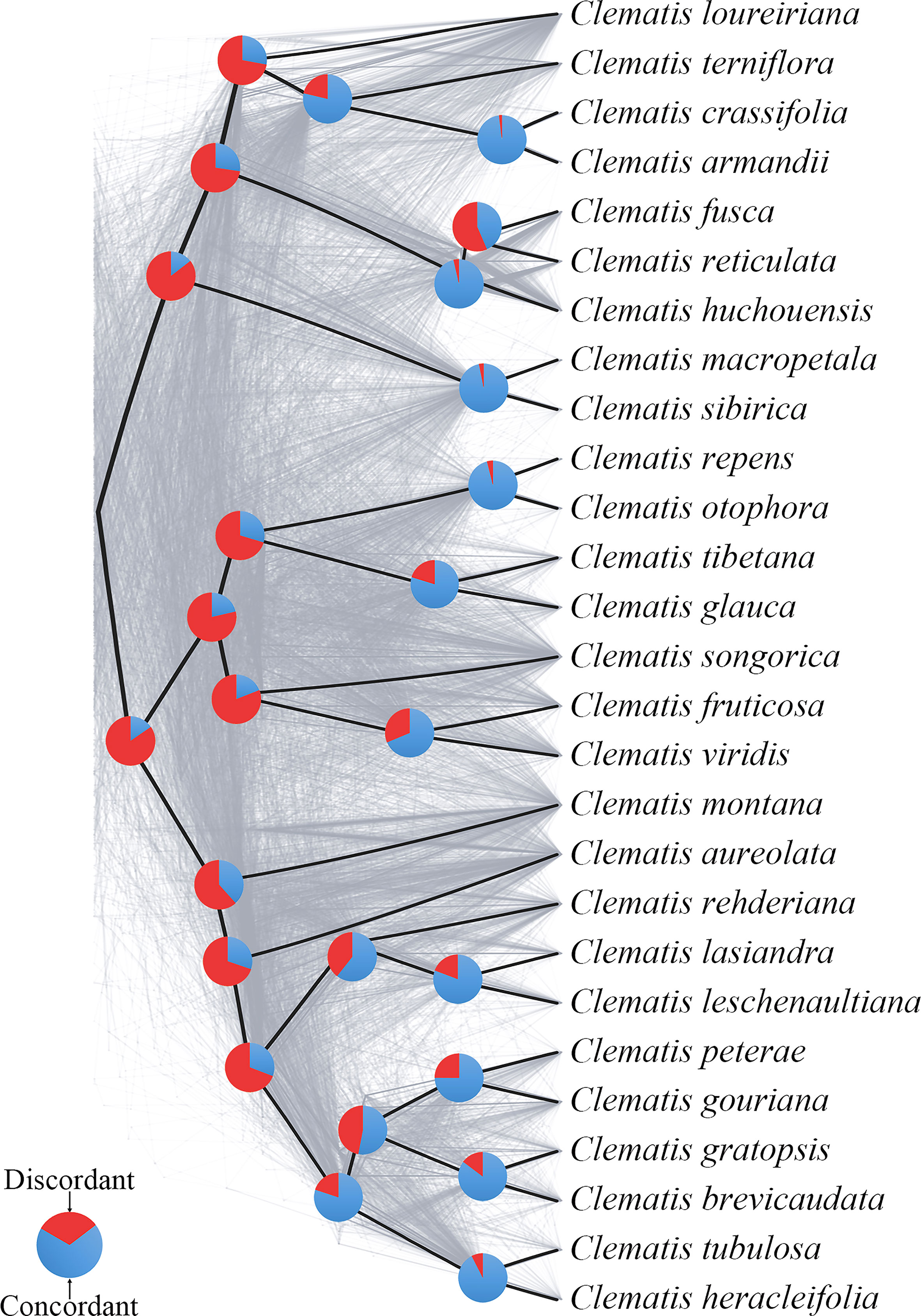
Figure 5 A cloud tree showing discordance among nuclear genes. The ASTRAL species tree (based on trees from SCOG1000 dataset with average bootstrap value more than 60) is in heavy black lines. All the branches are fully supported. The gray-colored trees (cloud tree) were sampled from 695 SCOGs (without missing taxa). Pie charts show the proportions of concordant and discordant topologies of gene trees comparing to the species tree.
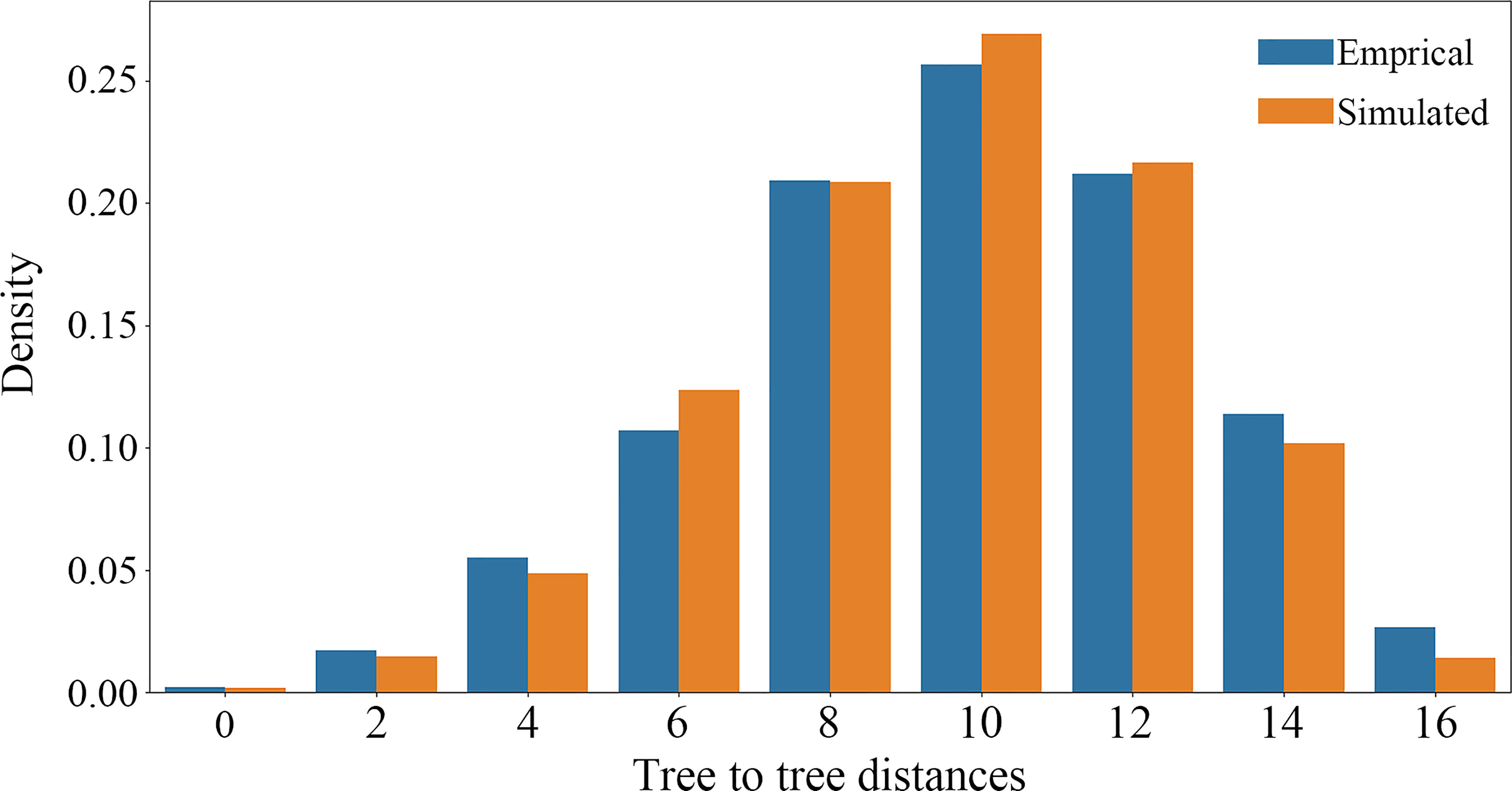
Figure 6 Coalescent simulations of tree-to-tree distance distributions between the ASTRAL species tree and the 4393 empirical (orange boxes) gene trees (based on trees from SCOG1000 dataset with average bootstrap value more than 60) and those from the 10,000 simulation trees (blue boxes).
Seed plants encompass a high level of diversity of genome size varying by more than 2,000-fold (Novák et al., 2020). Larger genomes generally contain more proportion of repeat sequences, transposable elements, and other non-transcribed low-copy sequences, while the amount of expressed genes are rather stable with about 0.03 Gbp (Kersey, 2019). For this reason, we do not need to consider the plant genome size when choosing transcriptome method for phylogenetic studies. However, when choosing genome skimming data, the size of plant genome becomes a vital issue that should be considered.
Clematis species have large genome size, which makes high-depth sequencing (10 × or more) unaffordable, and low-depth genome skimming data (less than 1 ×) of Clematis have been only used for assembling the plastome sequences or tandemly repeat nrDNA regions which have high copy numbers in the genome (He et al., 2021). The plastome phylogeny of Clematis (He et al., 2021) have better resolved the relationships within the genus than those of the Sanger sequencing data (e.g., Miikeda et al., 2006; Xie et al., 2011; Lehtonen et al., 2016). However, there were still some major clades with weak support and some clades are unexplainable taxonomically. The nrDNA sequences also failed to generate a robust tree due to insufficient phylogenetic information (He et al., 2021).
Although plastome data have been successfully used for phylogenetic reconstruction of plant taxa at almost all taxonomic levels, studies have shown that the plastome data alone may sometimes not sufficiently resolve the phylogeny of closely related species due to frequent hybridization and introgression in plants (Liu et al., 2022). Therefore, care should be taken when using plastome data alone to resolve species relationships of plant taxa. This is also the case with Clematis. Evidences from horticulture (Yuan et al., 2010), molecular phylogenetic studies (Lyu et al., 2021), and the present study showed that there is widespread hybridization among Clematis species or even between sections. In this study, transcriptome data were successfully assembled with thousands of SCOGs which robustly resolved the phylogenetic framework of Clematis. The SCOG1000 dataset not only fully resolved Clematis phylogeny but also provided a tree that corresponded well to morphological groups. The RNA-seq method is easy, fast, efficient for acquiring highly reusable nuclear genome data, independent of plant genome size (Cheon et al., 2020), and maybe the best choice for phylogenetic study of Clematis so far. The major problem with transcriptome method is that it cannot be successfully applied for herbarium materials. If we want to include more herbarium samples, data partitioning method should be reconsidered.
Previous studies have used genome skimming data to obtain nuclear SNPs by mapping reads to the reference genome (Olofsson et al., 2019; Zhang et al., 2019). This study presented a further exploration of this method in Clematis. The phylogenies from the nuclear SNPs data by the two pipelines in this study were better resolved than the previous published nrDNA tree (He et al., 2021). Two different pipelines generated different amounts of data, and Geneious pipeline produced larger datasets than GATK pipeline. In the same way, Geneious pipeline generated more robust phylogeny which was almost the same with that reconstructed by SCOGs. Meanwhile, two herbarium samples (C. psilandra and C. speciosa) and four genome skimming data (C. fusca, C. macropetala, C. pilulifera, and C. reticulata) from other studies with lower sequencing depth clustered in the correct positions on the nuclear SNPs tree (Figure 4). Therefore, this method (especially the Geneious pipeline) is reliable and may play an important role in future phylogenetic study of Clematis with comprehensive sampling.
The problems of this method, however, also need to be mentioned. Because the sequencing depth is low, SNP genotyping and allele frequency estimation may be biased by those genome skimming data. So, the SNPs datasets may not be applied for population genetic analysis, such as STRUCTURE (Pritchard et al., 2000). Furthermore, this data may also not work well for analysis of reticulate evolution (such as HyDe, Blischak et al., 2018) and whole genome duplication detection (WGD, Yang et al., 2019).
Although previous phylogenetic studies used more samples (Xie et al., 2011; Lehtonen et al., 2016), insufficient resolution by small number of DNA regions has hindered our understanding of the evolution of Clematis. The plastome data took us a step forward in resolving the phylogeny of the genus (He et al., 2021). Plastome phylogeny, inferred by He et al. (2021), resolved six major clades in Clematis. Except a clade comprising only species of sect. Naravelia, all the other five clades contained three or more sections. Despite the smaller sample size of this study, all the six corresponding clades were also resolved by our plastome phylogenetic analysis. These clades (except sect. Naravelia clade) were difficult to be defined by morphology.
In this study, using nuclear SNPs and SCOG data, we reconstructed the first well resolved phylogenetic backbone of Clematis. Most of the morphologically defined sections were supported. Trees inferred from the nuclear genome data (Figures 2–4) were better corresponding to morphological characters than plastome phylogeny. The Geneious-0.05MS dataset resolved ten major clades in Clematis (Figures 2, 4). Clade 1 represents subtropical sect. Naraveliopsis which has conspicuous connective projections on the anthers. Clade 2 comprises species of sect. Flammula DC., sect. Viticella DC. and sect. Viorna (sensu Tamura, 1995). The synapomorphy may be their type II seedlings (or opposite seedling leaves, Essig, 1991). Clade 3 represents sect. Atragene, which has petal-like staminodes in the flowers. Clade 4, including sect. Meclatis and species of sect. Campanella with yellow flowers and hairy filaments and anthers, is characterized by its yellow and thick sepals. Clade 5 represents sect. Fruticella with erect shrubby stem. Clade 6 represents sect. Cheiropsis, which is characterized by its flowers arising from old or hornotinous branches. Clade 7 contains some species of sect. Campanella. Their shared characteristics are the type I seedling (or alternate seedling leaves, Essig, 1991), erect sepals, hairy stamen filaments and glabrous anthers. Clade 8 is sect. Naravelia which was recognized as a distinct genus by Tamura (1995) and Wang and Li (2005). Plants of this section possess leaf tendrils and spoon-shaped petals. Clade 9 represents the narrowly defined sect. Clematis (sensu Tamura, 1995), which is characterized by the type I seedling, small white flowers, spreading sepals, and glabrous stamens. Clade 10 represents sect. Tubulosae, which is characterized by the type I seedling, ternate leaves, erect herbaceous stem, erect sepals and hairy stamens. It should be pointed out that the range of our sample was relatively narrow, and future studies with more comprehensive sampling are needed to further elucidate the phylogenetic and taxonomic problems in Clematis.
Using nuclear genome data, we also gained new knowledge and insights into some taxonomic issues for Clematis in this study. Previous studies have suggested that sect. Campanella may be a polyphyletic group (He et al., 2021). Our nuclear genome phylogeny confirmed that C. repens and C. otophora (in sect. Campanella) are more closely related to sect. Meclatis (clade 4, Figure 4) rather than to other sect. Campanella species. Both C. repens and C. otophora have yellow flowers with thick sepals which are more similar to those of the sect. Meclatis. Two morphologically well diverged sections, sect. Clematis (sensu Tamura, 1995) and sect. Tubulosae, have shown to be very closely related or even cannot be clearly separated by Sanger sequencing data (Xie et al., 2011; Lehtonen et al., 2016; Yan et al., 2016). They were also nested together in our plastome tree (Figure 2), but were clearly separated by our SCOG1000 data (Figure 3) and Geneious-0.05MS data (clade 9 and clade 10, Figures 2–4). The simulation results showed that this cyto-nuclear discordance may be caused by hybridization events between the two sections (Figure 2A). Hybridization events between these two morphologically diverged sections have been also confirmed by other reports, horticultural evidence, and phylogenomic analysis (Makino, 1907; Yuan et al., 2010; Lyu et al., 2021).
Similar to other studies, our results showed that all the important morphological characters emphasized by taxonomists, such as phyllotaxy, calyx, and filament hairs (Tamura, 1995), may have evolved multiple times, and it is difficult to make subgeneric classification by using a few key characters. Specifically, we emphasis that seedling morphology (phyllotaxy as in this study), highlighted by Tamura (1995), should be based on observations but not speculation. Majority number of Clematis species have no real observation data of seedling morphology. Seedling status of many sections (such as sect. Naraveliopsis and sect. Fruticella) proposed by Tamura (1995) are likely to be wrong (Cheng et al., 2016). Based on our observation, seedling morphology of sect. Fruticella (not published) should be type I (metamorphic, Essig, 1991) and similar to that of sect. Meclatis (rather than type II proposed by Tamura, 1995). So, before using seedling morphology for taxonomic treatment, this character needs to be studied through comprehensive observation.
Our findings also shed light on the evolutionary history of Clematis. Studies have shown that Clematis may have experienced recent species radiation during the late Neogene and the Quaternary (Xie et al., 2011; He et al., 2021). Recent species radiation may lead to severe lineage sorting when the ancestral population was large (Pamilo and Nei, 1988), and this fits well with our simulation results (Figures 5, 6). Our results demonstrated that there are extensive gene tree conflicts at early diverged nodes, which can be explained by ILS. Meanwhile, our analysis of cyto-nuclear discordance (Figure 2) suggested that there may also have been widespread interspecific hybridization events in Clematis, which contributed to high level of incongruence between plastid and nuclear phylogenies. From our analysis, both ILS and interspecific hybridization in Clematis made its classification and phylogenetic analysis very difficult, especially using small number of DNA regions or plastome data alone.
There are several other genera in Ranunculaceae that are similar to Clematis, such as Anemone L., Aconitum L., and Delphinium L. These genera have not only large genome size (https://cvalues.science.kew.org/search) but also have hundreds of wild species (Tamura, 1995). In addition, they all have no high-quality whole genome reference available and few phylogenomic studies with comprehensive sampling. Resolving the phylogenetic framework of those taxa is highly possible to encounter the same conditions with Clematis: ineffectiveness of Sanger sequencing data and difficulty in genome partitioning selection. Furthermore, studies have shown that the plastid genome (or regions) data alone did not work well for the phylogenetic reconstruction of those taxa (Hoot et al., 2012; Jiang et al., 2017; Hong et al., 2017; Xiang et al., 2017). Our results suggested that transcriptome method may be the first choice for solving the problem, and if the samples are not suitable for RNA extraction, Geneious pipeline presented in this study (using low-depth genome skimming data) can be tried. Although this study did not test target enrichment data, this method is also recommended if the complicated experimental procedures are acceptable to the researchers.
Genome size may be an important factor in genome partitioning selection. If the genome size of concerning taxon is small (less than 1 Gbp), genome skimming method can easily obtain high sequencing depth at an acceptable cost, and is a good choice to solve phylogenetic problems. We have tried to obtain and successfully assembled SCOGs (not published) from 6 Gbp of genome skimming data from Epilobium L. (Onagraceae) samples using the method of Liu et al. (2021). The genome size of Epilobium species is about 0.2 Gbp, and our data was up to 30 × in sequencing depth. In this case, genome skimming method is better than transcriptome and target enrichment method. Using this data, we can acquire the plastome and nuclear SCOGs data from both transcribed region and non-transcribed (intron, spacer, repetitive regions, and so on) regions, and conduct a variety of downstream analysis, such as phylogenetic reconstruction, molecular dating, hybridization analysis, and WGD detection.
The data presented in the study are deposited in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/), accession number PRJNA838588 and PRJNA776151.
JX, RL, and JH, analyzed the data and prepared the draft. ML, JH, JJ, and LX conducted the sample gathering. JH, JC, and LX designed the study. JX, RL, and LX wrote and revised the manuscript. All the authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant numbers 32270223, 31670207).
We thank Ma Xin-Tang and Ban Qin, working in the Herbarium of Institute of Botany, the Chinese Academy of Sciences (PE), for kindly providing Clematis specimen samples. We are grateful to Dr. Xu Chao from Institute of Botany, the Chinese Academy of Sciences for extracting high quality DNAs from specimen samples for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1059379/full#supplementary-material
Supplementary Table S1 | Sample information of genome skimming data in this study.
Supplementary Table S2 | Sample information of transcriptome data in this study.
Supplementary Table S3 | Plastid genome features of the Clematis species in this study.
Supplementary Figure 1 | Phylogenetic trees constructed by concatenated and coalescence-based methods based on SCOG3000 dataset.
Supplementary Figure 2 | Bayesian phylogeny (A) of Clematis inferred from the plastid genome data and maximum likelihood phylogeny (B) inferred from of SCOG1000 data. Cyto-nuclear discordance is shown.
Supplementary Figure 3 | Maximum likelihood phylogenetic trees constructed from three nuclear SNPs data matrices obtained by GATK pipeline.
Supplementary Figure 4 | Maximum likelihood phylogenetic trees constructed from two nuclear SNPs data matrices obtained by Geneious pipeline.
Blischak, P. D., Chifman, J., Wolfe, A. D., Kubatko, L. S. (2018). HyDe: A Python package for genome-scale hybridization detection. Syst. Biol. 67, 821–829. doi: 10.1093/sysbio/syy023
Buchfink, B., Xie, C., Huson, D. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60. doi: 10.1038/nmeth.3176
Chen, N. S. (2004). Using repeat masker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinf. 5, 4–10. doi: 10.1002/0471250953.bi0410s05
Cheng, J., Yan, S. X., Liu, H. J., Lin, L. L., Li, J. Y., Liao, S., et al. (2016). Reconsidering the phyllotaxy significance of seedlings in Clematis. Phytotaxa 265, 131–138. doi: 10.11646/phytotaxa.265.2.4
Chen, S. F., Zhou, Y. Q., Chen, Y. R., Gu, J. (2018). Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Cheon, S., Zhang, J., Park, C., Teeling, E. (2020). Is phylotranscriptomics as reliable as phylogenomics? Mol. Biol. Evol. 37, 3672–3683. doi: 10.1093/molbev/msaa181
Darriba, D., Taboada, G., Doallo, R., Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772. doi: 10.1038/nmeth.2109
Dodsworth, S. (2015). Genome skimming for next-generation biodiversity analysis. Trends Plant Sci. 20, 525–527. doi: 10.1016/j.tplants.2015.06.012
Drezen, E., Rizk, G., Chikhi, R., Deltel, C., Lemaitre, C., Peterlongo, P., et al. (2014). GATB: genome assembly & analysis tool box. Bioinformatics 30, 2959–2961. doi: 10.1093/bioinformatics/btu406
Duvall, M. R., Burke, S. V., Clark, D. C. (2020). Plastome phylogenomics of poaceae: Alternate topologies depend on alignment gaps. Bot. J. Linn. Soc 192, 9–20. doi: 10.1093/botlinnean/boz060
Eaton, D. A. R. (2020). Toytree: A minimalist tree visualization and manipulation library for Python. Methods Ecol. Evol. 11, 187–191. doi: 10.1111/2041-210X.13313
Essig, F. B. (1991). Seedling morphology in Clematis (Ranunculaceae) and its taxonomic implications. SIDA 1991, 377–390.
Fonseca, L. H. M., Lohmann, L. G. (2020). Exploring the potential of nuclear and mitochondrial sequencing data generated through genome-skimming for plant phylogenetics: A case study from a clade of neotropical lianas. J. Syst. Evol. 58, 18–32. doi: 10.1111/jse.12533
Fu, L. M., Niu, B. F., Zhu, Z. W., Wu, S. T., Li, W. Z. (2012). CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152. doi: 10.1093/bioinformatics/bts565
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
He, J., Lyu, R. D., Luo, Y. K., Lin, L. L., Yao, M., Xiao, J. M., et al. (2021). An updated phylogenetic and biogeographic analysis based on genome skimming data reveals convergent evolution of shrubby habit in Clematis in the pliocene and pleistocene. Mol. Phylogenet. Evol. 164, 107259. doi: 10.1016/j.ympev.2021.107259
He, J., Lyu, R. D., Luo, Y. K., Xiao, J. M., Xie, L., Wen, J., et al. (2022). A phylotranscriptome study using silica gel-dried leaf tissues produces an undated robust phylogeny of ranunculaceae. Mol. Phylogenet. Evol. 174, 107545. doi: 10.1016/j.ympev.2022.107545
He, J., Yao, M., Lyu, R. D., Lin, L. L., Liu, H. J., Pei, L. Y., et al. (2019). Structural variation of the complete chloroplast genome and plastid phylogenomics of the genus Asteropyrum (Ranunculaceae). Sci. Rep. 9, 1–13. doi: 10.1038/s41598-019-51601-2
Hong, Y., Luo, Y., Gao, Q., Ren, C., Yuan, Q., Yang, Q. E. (2017). Phylogeny and reclassification of Aconitum subgenus Lycoctonum (Ranunculaceae). PLoS One 12, e0171038. doi: 10.1371/journal.pone.0171038
Hoot, S. B., Meyer, K. M., Manning, J. C. (2012). Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis on austral species. Syst. Bot. 37, 139–152. doi: 10.1600/036364412X616729
Huang, D. I., Cronk, Q. C. B. (2015). Plann: A command–line application for annotating plastome sequences. Appl. Plant Sci. 3, 1500026. doi: 10.3732/apps.1500026
Jiang, N., Zhou, Z., Yang, J. B., Zhang, S. D., Guan, K. Y., Tan, Y. H., et al. (2017). Phylogenetic reassessment of tribe anemoneae (Ranunculaceae): Non-monophyly of Anemone s. l. revealed by plastid datasets. PLoS One 12, e0174792. doi: 10.1371/journal.pone.0174792
Jin, J. J., Yu, W. B., Yang, J. B., Song, Y., dePamphilis, C. W., Yi, T. S., et al. (2020). GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21, 241. doi: 10.1186/s13059-020-02154-5
Katoh, K., Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kersey, P. J. (2019). Plant genome sequences: Past, present, future. Curr. Opin. Plant Biol. 48, 1–8. doi: 10.1016/j.pbi.2018.11.001
Khan, G., Nolzen, J., Schepker, H., Albach, D. C. (2021). Incongruent phylogenies and their implications for the study of diversification, taxonomy, and genome size evolution of Rhododendron. Am. J. Bot. 108, 1957–1981. doi: 10.1002/ajb2.1747
Kress, W. J., Soltis, D. E., Kersey, P. J., Soltis, P. S. (2022). Green plant genomes: What we know in an era of rapidly expanding opportunities. Proc. Natl. Acad. Sci. U.S.A. 119, e2115640118. doi: 10.1073/pnas.2115640118
Lechner, M., Findeiß, S., Steiner, L., Marz, M., Stadler, P. F., Prohaska, S. J. (2011). Proteinortho: detection of (co-) orthologs in large-scale analysis. BMC Bioinf. 12, 1–9. doi: 10.1186/1471-2105-12-124
Lee-Yaw, J. A., Grassa, C. J., Joly, S., Andrew, R. L., Rieseberg, L. H. (2019). An evaluation of alternative explanations for widespread cytonuclear discordance in annual sunflowers (Helianthus). New Phytol. 221, 515–526. doi: 10.1111/nph.15386
Lehtonen, S., Christenhusz, M. J. M., Falck, D. (2016). Sensitive phylogenetics of Clematis and its position in ranunculaceae. Bot. J. Linn. Soc 182, 825–867. doi: 10.1111/boj.12477
Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 1303, 3997. doi: 10.48550/arXiv.1303.3997
Li, H. T., Luo, Y., Gan, L., Ma, P. F., Gao, L. M., Yang, J. B., et al. (2021). Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biol. 19, 232. doi: 10.1186/s12915-021-01166-2
Liu, H. J., He, J., Ding, C. H., Lyu, R. D., Pei, L. Y., Cheng, J., et al. (2018). Comparative analysis of complete chloroplast genomes of Anemoclema, Anemone, Pulsatilla, and Hepatica revealing structural variations among genera in tribe anemoneae (Ranunculaceae). Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01097
Liu, B. B., Hong, D. Y., Zhou, S. L., Xu, C., Dong, W. P., Johnson, G., et al. (2019). Phylogenomic analyses of the Photinia complex support the recognition of a new genus Phippsiomeles and the resurrection of a redefined Stranvaesia in maleae (Rosaceae). J. Syst. Evol. 57, 678–694. doi: 10.1111/jse.12542
Liu, B. B., Ma, Z. Y., Ren, C., Hodel, R. G. J., Sun, M., Liu, X. Q., et al. (2021). Capturing single-copy nuclear genes, organellar genomes, and nuclear ribosomal DNA from deep genome skimming data for plant phylogenetics: A case study in vitaceae. J. Syst. Evol. 59, 1124–1138. doi: 10.1111/jse.12806
Liu, B. B., Ren, C., Kwak, M., Hodel, R. G. J., Xu, C., He, J., et al. (2022). Phylogenomic conflict analyses in the apple genus Malus s. l. reveal widespread hybridization and allopolyploidy driving diversification, with insights into the complex biogeographic history in the northern hemisphere. J. Integr. Plant Biol. 64, 1020–1043. doi: 10.1111/jipb.13246
Liu, L., Yu, L. (2010). Phybase: An r package for species tree analysis. Bioinformatics 26, 962–963. doi: 10.1093/bioinformatics/btq062
Li, J. L., Wang, S., Yu, J., Wang, L., Zhou, S. L. (2013). A modified CTAB protocol for plant DNA extraction. Chin. Bull. Bot. 48, 72–78. doi: 10.3724/SP.J.1259.2013.00072
Li, H. T., Yi, T. S., Gao, L. M., Ma, P. F., Zhang, T., Yang, J. B., et al. (2019). Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 5, 461–470. doi: 10.1038/s41477-019-0421-0
Lyu, R. D., He, J., Luo, Y. K., Lin, L. L., Yao, M., Cheng, J., et al. (2021). Natural hybrid origin of the controversial “species” Clematis × pinnata (Ranunculaceae) based on multidisciplinary evidence. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.745988
Mai, U., Mirarab, S. (2018). TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19, 272. doi: 10.1186/s12864-018-4620-2
Marks, R. A., Hotaling, S., Frandsen, P. B., VanBuren, R. (2021). Representation and participation across 20 years of plant genome sequencing. Nat. Plants 7, 1571–1578. doi: 10.1038/s41477-021-01031-8
McKain, M. R., Johnson, M. G., Uribe-Convers, S., Eaton, D., Yang, Y. (2018). Practical considerations for plant phylogenomics. Appl. Plant Sci. 6, e1038. doi: 10.1002/aps3.1038
Mckenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: A map reduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Miikeda, O., Kita, K., Handa, T., Yukawa, T. (2006). Phylogenetic relationships of Clematis (Ranunculaceae) based on chloroplast and nuclear DNA sequences. Bot. J. Linn. Soc 152, 153–168. doi: 10.1111/j.1095-8339.2006.00551.x
Morales-Briones, D. F., Kadereit, G., Tefarikis, D. T., Moore, M. J., Smith, S. A., Brockington, S. F., et al. (2021). Disentangling sources of gene tree discordance in phylogenomic data sets: Testing ancient hybridizations in amaranthaceae s. l. Syst. Biol. 70, 219–235. doi: 10.1093/sysbio/syaa066
Novák, P., Guignard, M. S., Neumann, P., Kelly, L. J., Mlinarec, J., Koblížková, A., et al. (2020). Repeat-sequence turnover shifts fundamentally in species with large genomes. Nat. Plants 6, 1325–1329. doi: 10.1038/s41477-020-00785-x
Olofsson, J. K., Cantera, I., Van de Paer, C., Hong-Wa, C., Zedane, L., Dunning, L. T., et al. (2019). Phylogenomics using low-depth whole genome sequencing: A case study with the olive tribe. Mol. Ecol. Resour. 19, 877–892. doi: 10.1111/1755-0998.13016
One Thousand Plant Transcriptomes Initiative (2019). One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679–685. doi: 10.1038/s41586-019-1693-2
Page, A. J., Taylor, B., Delaney, A. J., Soares, J., Seemann, T., Keane, J. A., et al. (2016). SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb. Genom. 2, e000056. doi: 10.1099/mgen.0.000056
Pamilo, P., Nei, M. (1988). Relationships between gene trees and species trees. Mol. Biol. Evol. 5, 568–583. doi: 10.1093/oxfordjournals.molbev.a040517
Pritchard, J. K., Stephens, M., Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959. doi: 10.1093/genetics/155.2.945
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Rose, J. P., Toledo, C. A., Lemmon, E. M., Lemmon, A. R., Sytsma, K. J. (2021). Out of sight, out of mind: widespread nuclear and plastid-nuclear discordance in the flowering plant genus Polemonium (Polemoniaceae) suggests widespread historical gene flow despite limited nuclear signal. Syst. Biol. 70, 162–180. doi: 10.1093/sysbio/syaa049
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V., Zdobnov, E. M. (2015). BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212. doi: 10.1093/bioinformatics/btv351
Smith, S. A., Moore, M. J., Brown, J. W., Yang, Y. (2015). Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evol. Biol. 15, 150. doi: 10.1186/s12862-015-0423-0
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stull, G. W., Soltis, P. S., Soltis, D. E., Gitzendanner, M. A., Smith, S. A. (2020). Nuclear phylogenomic analyses of asterids conflict with plastome trees and support novel relationships among major lineages. Am. J. Bot. 107, 790–805. doi: 10.1002/ajb2.1468
Su, C., Duan, L., Liu, P., Liu, J., Chang, Z., Wen, J. (2021). Chloroplast phylogenomics and character evolution of eastern Asian Astragalus (Leguminosae): Tackling the phylogenetic structure of the largest genus of flowering plants in Asia. Mol. Phylogenet. Evol. 156, 107025. doi: 10.1016/j.ympev.2020.107025
Sukumaran, J., Holder, M. T. (2010). DendroPy: A Python library for phylogenetic computing. Bioinformatics 26, 1569–1571. doi: 10.1093/bioinformatics/btq228
Tamura, M. (1995). “Clematis l,” in Die natürlichen pflanzenfamilien, vol. 17a . Ed. Hiepko, P. (Berlin: Duncker und Humbolt), 368–387.
Thode, V. A., Lohmann, L. G., Sanmartín, I. (2020). Evaluating character partitioning and molecular models in plastid phylogenomics at low taxonomic levels: A case study using Amphilophium (Bignonieae, bignoniaceae). J. Syst. Evol. 58, 1071–1089. doi: 10.1111/jse.12579
Valcárcel, V., Wen, J. (2019). Chloroplast phylogenomic data support Eocene amphi-pacific early radiation for the Asian palmate core araliaceae. J. Syst. Evol. 57, 547–560. doi: 10.1111/jse.12522
Vargas, O. M., Heuertz, M., Smith, S. A., Dick, C. W. (2019). Target sequence capture in the Brazil nut family (Lecythidaceae): Marker selection and in silico capture from genome skimming data. Mol. Phylogenet. Evol. 135, 98–104. doi: 10.1016/j.ympev.2019.02.020
Wang, W. T., Bartholomew, B. (2001). “Clematis l,” in Flora of China, vol. 6 . Eds. Wu, C. Y., Raven, P. (Beijing: Science Press, St. Louis, Missouri Botanical Garden Press), 333–386.
Wang, W. T., Li, L. Q. (2005). A new system of classification of the genus Clematis (Ranunculaceae). Acta Phytotax. Sin. 43, 431–488. doi: 10.1360/aps040130
Wang, Y. B., Liu, B. B., Nie, Z. L., Chen, H. F., Chen, F. J., Figlar, R. B., et al. (2020). Major clades and a revised classification of Magnolia and magnoliaceae based on whole plastid genome sequences via genome skimming. J. Syst. Evol. 58, 673–695. doi: 10.1111/jse.12588
Watson, L. E., Siniscalchi, C. M., Mandel, J. (2020). Phylogenomics of the hyperdiverse daisy tribes: Anthemideae, astereae, calenduleae, gnaphalieae, and senecioneae. J. Syst. Evol. 58, 841–852. doi: 10.1111/jse.12698
Wen, J., Harris, A. J., Ickert-Bond, S. M., Dikow, R., Wurdack, K., Zimmer, E. A. (2017). Developing integrative systematics in the informatics and genomic era, and calling for a global biodiversity cyberbank. J. Syst. Evol. 55, 308–321. doi: 10.1111/jse.12270
Wen, J., Harris, A. J., Kalburgi, Y., Zhang, N., Xu, Y., Zheng, W., et al. (2018). Chloroplast phylogenomics of the new world grape species (Vitis, vitaceae). J. Syst. Evol. 56, 297–308. doi: 10.1111/jse.12447
Wikström, N., Bremer, B., Rydin, C. (2020). Conflicting phylogenetic signals in genomic data of the coffee family (Rubiaceae). J. Syst. Evol. 58, 440–460. doi: 10.1111/jse.12566
Xiang, K. L., Aytaç, Z., Liu, Y., Espinosa, F., Jabbour, F., Byng, J. W., et al. (2017). Recircumscription of Delphinium subg. Delphinium (Ranunculaceae) and implications for its biogeography. Taxon 66, 554–566. doi: 10.12705/663.3
Xie, L., Wen, J., Li, L. Q. (2011). Phylogenetic analyses of Clematis (Ranunculaceae) based on sequences of nuclear ribosomal ITS and three plastid regions. Syst. Bot. 36, 907–921. doi: 10.1600/036364411X604921
Yang, Y., Li, Y., Chen, Q., Sun, Y., Lu, Z. (2019). WGDdetector: A pipeline for detecting whole genome duplication events using the genome or transcriptome annotations. BMC Bioinf. 20, 1–6. doi: 10.1186/s12859-019-2670-3
Yang, Y. Z., Sun, P. C., Lv, L. K., Wang, D. L., Ru, D. F., Li, Y., et al. (2020). Prickly waterlily and rigid hornwort genomes shed light on early angiosperm evolution. Nat. Plants 6, 215–222. doi: 10.1038/s41477-020-0594-6
Yan, S. X., Liu, H. J., Lin, L. L., Liao, S., Li, J. Y., Pei, L. Y., et al. (2016). Taxonomic status of Clematis acerifolia var. elobata, based on molecular evidence. Phytotaxa 268, 209–219. doi: 10.11646/phytotaxa.268.3.5
Yuan, T., Wang, L. Y., Roh, M. S. (2010). Confirmation of Clematis hybrids using molecular markers. Sci. Hortic. 125, 136–145. doi: 10.1016/j.scienta.2010.03.005
Yu, X. Q., Yang, D., Guo, C., Gao, L. M. (2018). Plant phylogenomics based on genome-partitioning strategies: Progress and prospects. Plant Divers. 40, 158–164. doi: 10.1016/j.pld.2018.06.005
Zhai, W., Duan, X., Zhang, R., Guo, C., Li, L., Xu, G., et al. (2019). Chloroplast genomic data provide new and robust insights into the phylogeny and evolution of the ranunculaceae. Mol. Phylogenet. Evol. 135, 12–21. doi: 10.1016/j.ympev.2019.02.024
Zhang, C., Rabiee, M., Sayyari, E., Mirarab, S. (2018). ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinf. 19, 15–30. doi: 10.1186/s12859-018-2129-y
Zhang, R., Wang, Y. H., Jin, J. J., Stull, G. W., Bruneau, A., Cardoso, D., et al. (2020). Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of leguminosae. Syst. Biol. 69, 613–622. doi: 10.1093/sysbio/syaa013
Zhang, B. W., Xu, L. L., Li, N., Yan, P. C., Jiang, X. H., Woeste, K. E., et al. (2019). Phylogenomics reveals an ancient hybrid origin of the Persian walnut. Mol. Biol. Evol. 36, 2451–2461. doi: 10.1093/molbev/msz112
Zhao, D. N., Ren, C. Q., Zhang, J. Q. (2021). Can plastome data resolve recent radiations? Rhodiola (Crassulaceae) as a case study. Bot. J. Linn. Soc 197, 513–526. doi: 10.1093/botlinnean/boab035
Zimmer, E. A., Wen, J. (2015). Using nuclear gene data for plant phylogenetics: Progress and prospects II. next-gen approaches. J. Syst. Evol. 53, 371–379. doi: 10.1111/jse.12174
Keywords: Clematis, cyto-nuclear discordance, genome partitioning, genome skimming, transcriptome, phylogenomics
Citation: Xiao J, Lyu R, He J, Li M, Ji J, Cheng J and Xie L (2022) Genome-partitioning strategy, plastid and nuclear phylogenomic discordance, and its evolutionary implications of Clematis (Ranunculaceae). Front. Plant Sci. 13:1059379. doi: 10.3389/fpls.2022.1059379
Received: 01 October 2022; Accepted: 24 October 2022;
Published: 14 November 2022.
Edited by:
Yu Song, University of Chinese Academy of Sciences, ChinaReviewed by:
Xin Yao, Xishuangbanna Tropical Botanical Garden (CAS), ChinaCopyright © 2022 Xiao, Lyu, He, Li, Ji, Cheng and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Cheng, Y2hlbmdqaW5AYmpmdS5lZHUuY24=; Lei Xie, eGllbGVpQGJqZnUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.