
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 January 2023
Sec. Plant Abiotic Stress
Volume 13 - 2022 | https://doi.org/10.3389/fpls.2022.1053780
This article is part of the Research Topic Abiotic Stress Alleviation in Plants: Morpho-Physiological and Molecular Aspects View all 31 articles
Soil salinity severely inhibits leaf photosynthesis and limits agricultural production. Red to far-red light ratio (R/FR) affects leaf photosynthesis under salt stress, however, its regulation mechanism is still largely unknown. This study investigated the effects of different R/FR on plant growth, gas exchange parameters, photosynthetic electron transport, Calvin cycle and key gene expression under salt stress. Cucumber seedlings were exposed to four treatments including 0 mM NaCl and R/FR=7 (L7, control), 0 mM NaCl and R/FR=0.7 (L0.7), 80 mM NaCl and R/FR=7 (H7) and 80 mM NaCl and R/FR=0.7 (H0.7) for 9 days in an artificial climate chamber. The results showed that compared to L7 treatment, H7 treatment significantly reduced relative growth rate (RGR), CO2 assimilation rate (Pn), maximum photochemical efficiency PSII (Fv/Fm), most JIP-test parameters and total Rubisco activity, indicating that salt stress severely inhibited photosynthetic electron transport from PSII to PSI and blocked Calvin cycle in cucumber leaves. However, these suppressions were effectively alleviated by low R/FR addition (H0.7 treatment). Compared to H7 treatment, H0.7 treatment significantly increased RGR and Pn by 209.09% and 7.59%, respectively, enhanced Fv/Fm, maximum quantum yield for primary photochemistry (φPo), quantum yield for electron transport (φEo) and total Rubisco activity by 192.31%, 17.6%, 36.84% and 37.08%, respectively, and largely up-regulated expressions of most key genes involved in electron transport and Calvin cycle. In conclusion, low R/FR effectively alleviated the negative effects of salt stress on leaf photosynthesis by accelerating photosynthetic electron transport from PSII to PQ pool and promoting Calvin cycle in cucumber plants. It provides a novel environmentally friendly light-quality regulation technology for high efficiency salt-resistant vegetable production.
Soil salinity, a global environmental problem, occurs in approximately 7% of the world’s total land area and 20% of irrigated land (Shabala and Cuin, 2008). Salt stress not only negatively affects plant growth and production, but also induces a series of physiological and metabolic disorders, especially photosynthesis (Kalaji et al., 2011; Zhang J. et al., 2016). Salt stress-induced osmotic stress decreases water absorption in root, causes water loss in leaves, and thus plays negative roles in leaf photosynthesis. Salt stress also stimulates synthesis of reactive oxygen species (ROS), which seriously destructs photosynthetic organs and components, and inhibits leaf photosynthetic characteristics, such as CO2 assimilation rate, stomatal conductance, maximum photochemical efficiency PSII (Fv/Fm) (Ma et al., 2017; Niu et al., 2019; Gong et al., 2020). Therefore, it is necessary to improve salt tolerance by promoting leaf photosynthetic capacity.
Light quality participants in the regulation of growth, physiological and yield characteristics in vegetable crops. Red to far-red light ratio (R/FR), one of the important light environment factors, participants in seed germination, plant photomorphogenesis, physiological metabolism and gene expression (Demotes-Mainard et al., 2015; Holalu and Finlayson, 2017). R/FR is about 7 under light-emitting diode (LED) lamp, 1.14 under sunny condition and 0.09-0.7 under shade condition. Low R/FR induces shade avoidance syndrome (SAS) responses, such as increased internode, petiole, stem, leaf length and plant dry weight, apical dominance and early flowering (Franklin, 2008). Low R/FR also takes parts in several physiological changes, especially in leaf photosynthetic characteristics, it dramatically increases leaf net photosynthetic rate and effective photochemical quantum yield of PSII (ΦII), accelerates cyclic electron transport around PSI, but decreases leaf chlorophyll a/b in horticultural crops, such as tomato, soybean and lettuce (Zhen and Iersel, 2017; Kalaitzoglou et al., 2019; Yang et al., 2020).
R/FR not only influences plant growth and physiological metabolism, but also effectively alleviates injuries caused by abiotic stresses, including salt, cold and drought stresses and so on (Courbier and Pierik, 2019; Ahres et al., 2020; Gyugos et al., 2021). Under salt stress, low R/FR increased the stability of Phytochrome interaction factor (PIF) and upregulated brassinosteroid and auxin signaling, thus promoted hypocotyl growth in Arabidopsis (Hayes et al., 2019). Low R/FR also up-regulated SODCC.2, GPX1, APX2 and CAT1 gene expressions and increased antioxidant enzyme (e.g., SOD, POD and CAT) activities, thus enhanced salt resistance in tomato plants (Cao et al., 2018; Wang Y. et al., 2021). Cockburn et al. (1996) found that under salt stress, low R/FR enhanced PEP carboxylase activity, and caused accumulation of CAM isoform of PEP carboxylase isozyme and increased terpineol and soluble carbohydrate contents, finally improved salt tolerance in Mesembryanthemum crystallinum plant. However, these researches on salt tolerance of R/FR basically focus on plant growth and antioxidant capacity, little is known about how R/FR regulates leaf photosynthetic responses to salt stress. It is of great research significance to explore the regulation roles of R/FR on specific processes of photosynthetic electron transport chain and Calvin cycle simultaneously under salt stress.
Cucumber (Cucumis sativus L.) is an important worldwide economic vegetable crop, and its growth and production are severely limited by salt stress (Miao et al., 2020; Wang W. et al., 2021). Cucumber is sensitive to light quality, and its photomorphogenesis and photosynthetic characteristics are easily regulated by R/FR (Shibuya et al., 2015; Miao et al., 2019; Jeong et al., 2020). In the present study, we investigated the effect of R/FR on leaf gas exchange parameters, photosynthetic electron transfer capacity, Calvin cycle and key gene expression in salt-stressed cucumber plants. According to the regulation mechanism of R/FR on leaf photosynthesis under salt stress, this study will provide a more theoretical basic and new light quality control method to improve salt tolerance in cucumber production.
Cucumber (Cucumis sativus L.cv. ‘Jinchun 4’) seeds were germinated at 28°C, then sowed into a hydroponic tank in an artificial climate chamber. After the second true leaf has fully developed, all plants were exposed to four treatments, including 0 mM NaCl and R/FR=7 (L7, control), 0 mM NaCl and R/FR=0.7 (L0.7), 80 mM NaCl and R/FR=7 (H7) and 80 mM NaCl and R/FR=0.7 (H0.7). The hydroponic tank was filled with full strength Hoagland nutrient solution contained 0 mM or 80 mM NaCl, respectively. Red and far-red light were provided by LED lamps with maximum intensity at 660nm and 730nm, respectively. Red to far-red light ratio (R/FR) was calculated from photon irradiance for the bands 655-656 nm and 725-735nm according to Kotilainen et al. (2020). The light intensity and spectral distribution were determined by Avaspec-2048 fiber optic spectrometer (AVANTES, Netherlands) in the range 400-950nm with a spectral resolution of 1 nm (Figure 1). The day/night temperature, light intensity, photoperiod and relative humidity were 26°C/18°C, 250μmol·m2·s−1, 12h·d-1 and 60%-80%, respectively. The cucumber samples were harvested at day 9 after treatments and the experiments were repeated at least three times.
Four cucumber seedlings from each treatment were harvested on days 0 and 9 after treatments. Leaf area was measured using a LI-3000C portable leaf area meter (LI-COR, USA) and dry weight was fully dried at 80°C in an oven for 2 days. Relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), leaf dry weight ratio (LWR), and specific leaf area (SLA) were calculated according to Hunt (1978).
The samples from second leaves were harvested to measure photosynthetic pigment and carbohydrate contents. The photosynthetic pigments, soluble sugar and starch contents was measured using the method of Zhao X. et al. (2022), fructose and sucrose content was determined by resorcinol spectrophotometry according to Gao (2006). Four cucumber seedlings were selected for each treatment.
Gas exchange parameters were determined on the fully expanded second leaves at a similar position. Cucumber leaves exposed to different R/FR were measured under their own light quality conditions with a LI-6800 gas exchange analyzer and a standard transparent leaf chamber (LI-COR, USA). When leaves were clamped and reached steady-state condition in leaf chamber, gas exchange parameters such as CO2 assimilation rate (Pn), stomatal conductance (gsw), intercellular CO2 concentration (Ci) and transpiration rate (Tr) were measured. The light intensity was 250 μmol·m-2·s-1, leaf temperature was 26°C, relative humidity was 60%-70%, CO2 concentration was 400 μmol·mol-1 in leaf chamber. Four cucumber seedlings were selected for each treatment.
The CO2 response curve was measured with LI-6800 gas exchange analyzer using the same gas exchange parameters as described above. According to the procedure described by Long Bernacchi (2003), light intensity was 1500 μmol·m-2·s-1, CO2 concentration in leaf chamber was initially 400 μmol·mol-1 for 5 min, then followed by 400, 300, 250, 200, 150, 100, 50, 400, 600, 800, 1000, 1200, 1500 and 1800 μmol·mol-1. Three cucumber seedlings were selected for each treatment.
The maximum photochemical efficiency PSII (Fv/Fm) image was measured on the second leaves using a Maxi Imaging-pam fluorescence system (Walz, Germany). Details of procedure and measurement system were described in Perreault et al. (2010). After 30 min of dark adaptation, minimum fluorescence (Fo) was measured with a modulated light, then maximum fluorescence (Fm) was determined with a saturation pulse. Fv/Fm was calculated automatically using Fv/Fm =(Fm-Fo)/Fm.
The light induction transient of chlorophyll fluorescence (OJIP) curves were determined with a FluorPen FP 110 handheld chlorophyll fluorometer (Photon systems instruments, Czech Republic). After 30 min of dark adaptation, OJIP curve was induced by pulsed light of 3000 μmol·m-2·s-1. The relative variable fluorescence (Vt) was calculated as Vt=(Ft−Fo)/(Fp−Fo) according to Suzuki et al. (2011). Where, Vt and Ft represent relative variable fluorescence and fluorescence intensity at time t, respectively, Fo and Fp represent initial and maximum fluorescence intensity, respectively. JIP-test parameters were calculated according to Strasser et al. (2004). The formulas are as follows: Maximum quantum yield for primary photochemistry, φPo=TRo/ABS=[1-(Fo/Fm)], quantum yield for electron transport, φEo=ETo/ABS=[1-(Fo-Fm)](1-VJ), quantum yield for reduction of end electron acceptors at the PSI acceptor side, φRo=REo/ABS=[1-(Fo/Fm)](1-VJ), efficiency/probability that an electron moves further than QA-, ΨEo=ETo/TRo=1-VJ, efficiency/probability with which an electron from the intersystem electron carriers is transferred to reduce end electron acceptors at the PSI acceptor side, δRo=REo/ETo=(1-VI)/(1-VJ), QA reducing RCs per PSII antenna Chl, RC/ABS=φPo(VJ/Mo), performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors, PIABS=RC/ABS×[φPo/(1-φPo)]×[ΨEo/(1-ΨEo)], performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of PSI end acceptors, PItotal=PIABS·δRo/(1-δRo). Four cucumber seedlings were selected for each treatment.
The second leaves of four plants from each treatment were selected and snap frozen in liquid nitrogen, then stored at -80°C in a refrigerator. The initial and total Ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity and protein content were determined and calculated according to Zhang et al. (2013) and Wang et al. (2016).
Total RNA was extracted from the second leaves using TRIZOL reagent (Shanghai Blue Quarter Technology Development Co., Ltd., China) according to the method of Wang et al. (2009). Reverse transcription was performed with a CjamQTM Universal SYBR® qPCR Master Mix kit (Vazyme Biotech Co., Ltd, China) and real-time PCR (qPCR) was performed using a HiScript® III RT SuperMix kit (Vazyme Biotech Co., Ltd, China). The previously published primer sequences according to Miao et al. (2016) and Zhao H. et al. (2022) were used in this study (Table 1). Three cucumber seedlings were selected for each treatment.
All statistical analysis was carried out with SPSS 21.0 software (IBM Corporation, USA). The data were analyzed by one-way analysis of variance (ANOVA) and significant differences among means were assessed by Duncan’s test (P <0.05).
Generally, compared to L7 treatment, most plant parameters (e.g., Relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), specific leaf area (SLA) and total leaf area) were significantly increased by L0.7 treatment, while all plant parameters were significantly decreased by H7 and H0.7 treatments (Figure 2). Most plant parameters (e.g., RGR, NAR, LAR, LWR and total leaf area) were significantly higher for H0.7 treatment than for H7 treatment. These results indicated that salt stress seriously inhibited plant growth, however, the negative effects of salt stress on plant growth were effectively alleviated by low R/FR.
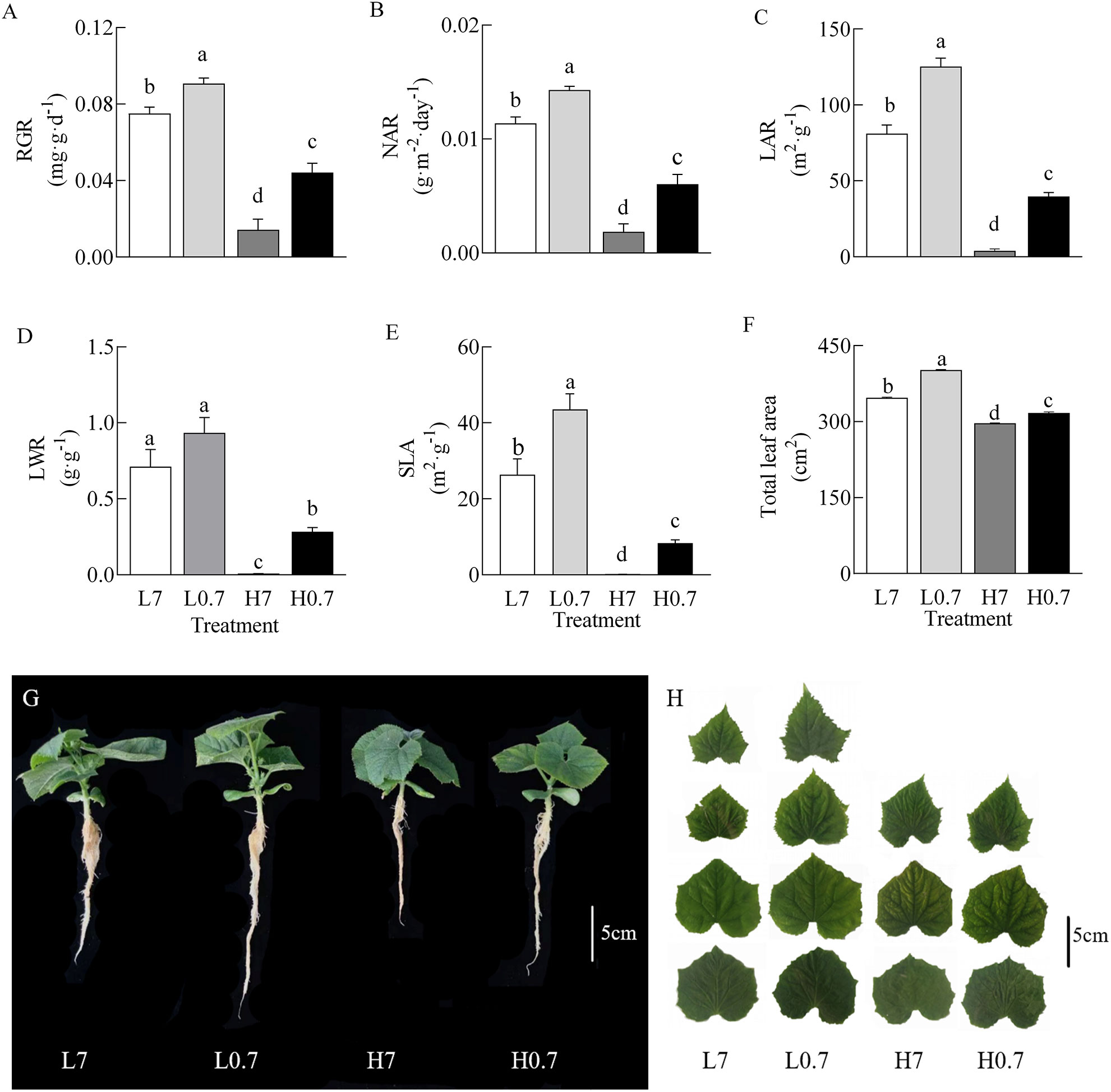
Figure 2 Effect of R/FR on growth parameters of cucumber seedlings under salt stress. (A), RGR, relative growth rate; (B), NAR, net assimilation rate; (C), LAR, leaf area ratio; (D), LWR, leaf dry weight ratio; (E), SLA, specific leaf area; (F), total leaf area; Cucumber seedlings (G) and total true leaves (H) on day 9 after treatment. L7, 0 mM NaCl and R/FR=7; L0.7, 0 mM NaCl and R/FR=0.7; H7, 80 mM NaCl and R/FR=7; H0.7, 80 mM NaCl and R/FR=0.7. Different letters indicate significant differences (P< 0.05; n= 4).
Compared to L7 treatment, chlorophyll a, chlorophyll b, soluble sugar and starch contents were significantly increased by L0.7 treatment, however, all photosynthetic pigment and carbohydrate contents were significantly decreased by H7 treatment, chlorophyll a, soluble sugar, fructose and sucrose contents were significantly reduced by H0.7 treatments (Table 2). The chlorophyll a and all carbohydrate (e.g., soluble sugar, fructose, sucrose and starch) contents were significantly higher for H0.7 treatment than for H7 treatment. This indicated that the negative effects of salt stress on photosynthetic pigment and carbohydrate were dramatically reduced by low R/FR.
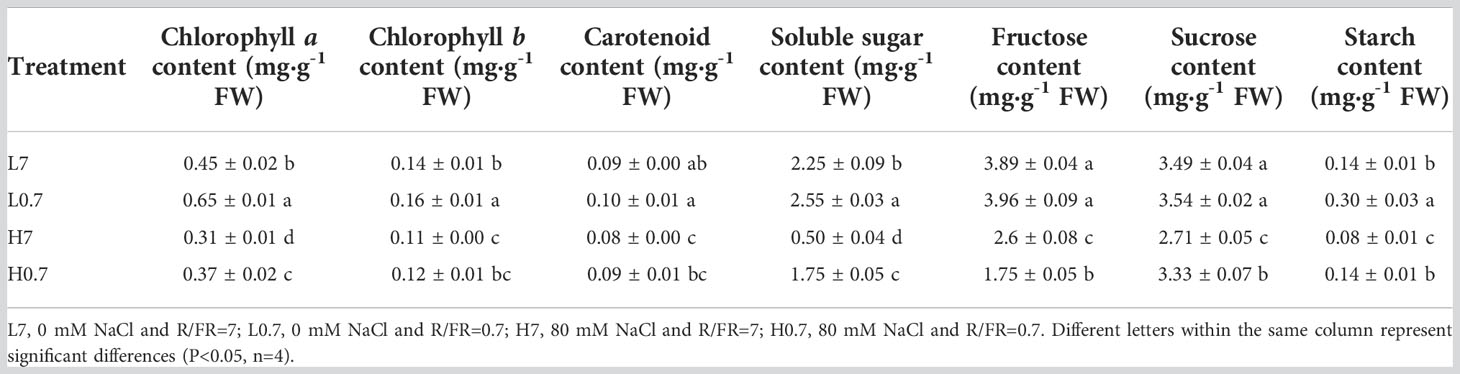
Table 2 Effect of R/FR ratio on photosynthetic pigment and carbohydrate contents in cucumber leaves under salt stress.
Compared to L7 treatment, Pn and gsw were significantly increased by L0.7 treatment, Ci was significantly enhanced by H0.7 treatment, while most gas exchange parameters (e.g., Pn, Ci and Tr) were significantly reduced by H7 treatment (Figure 3A–D). Compared to H7 treatment, Pn and Tr were significantly increased by H0.7 treatment.
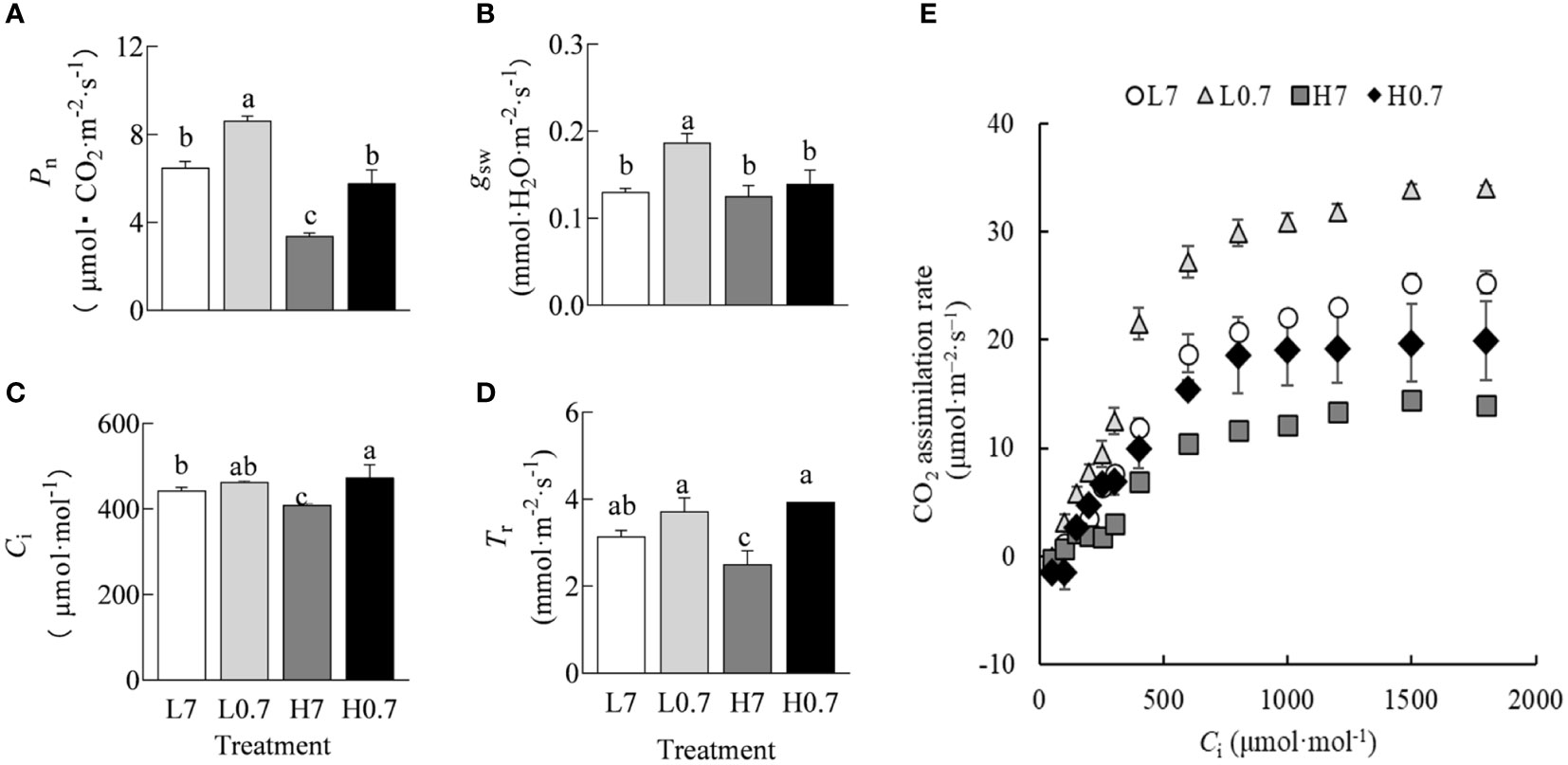
Figure 3 Effect of R/FR on gas exchange parameters in cucumber leaves under salt stress. (A), Pn, CO2 assimilation rate; (B), gsw, stomatal conductance; (C), Ci, intercellular CO2 concentration; (D), Tr, transpiration rate; (E), CO2 response curve. All data in (A–E) were reported as the arithmetic mean ± standard error (n=4 and n=3, respectively).
For all treatments, CO2 assimilation rate increased quickly with increasing Ci and reached maximum values when Ci was above 1000 μmol·mol-1 (Figure 3E). It was obvious that L0.7 treatment had the highest CO2 assimilation rate, followed by L7, H0.7 and H7 treatments.
The cucumber leaves in L7 and L0.7 treatments showed a homogeneous Fv/Fm distribution centred around an Fv/Fm of 0.80, while leaf in H7 treatment had a heterogeneous distribution with a high Fv/Fm around the veins and an extremely low level of Fv/Fm (0.26) between the veins, Fv/Fm distribution in H0.7 treatment was relative homogeneous around an Fv/Fm of 0.77 (Figure 4A and Table S1).
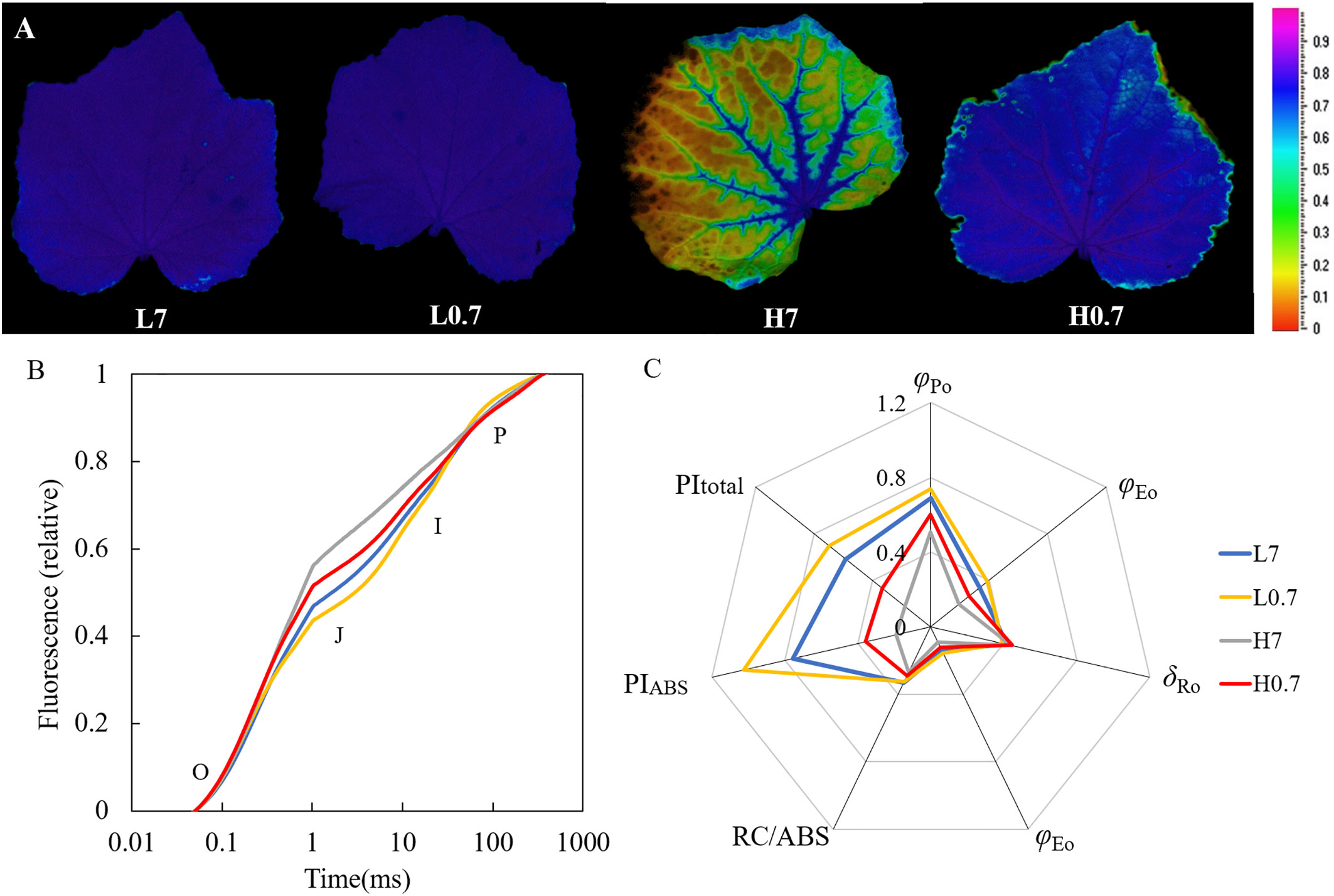
Figure 4 Effect of R/FR on chlorophyll fluorescence parameters in cucumber leaves under salt stress. (A), maximal photochemical quantum yield of PSII (Fv/Fm) images of cucumber leaves; (B), Chlorophyll a fluorescence transient (OJIP) curve; (C), JIP-test parameters calculated according to OJIP curve. Different letters indicate significant differences (P< 0.05; n= 4).
The typical polyphasic OJIP rise was found in L7 treatment (Figure 4B). The J (VJ, at 2ms) step was significantly higher for L0.7 treatment than for L7 treatment and was significantly higher for H0.7 treatment than for H7 treatment (Table S1). Compared to L7 treatment, φEo and PIABS were significantly increased by L0.7 treatment, however, most JIP-test parameters (e.g., φPo, φEo, φRo, RC/ABS, PIABS and PItotal) were reduced by H7 treatment, and φPo, φEo and PIABS were significantly decreased by H0.7 treatment (Figure 4C and Table S1). Compared with H7 treatment, φPo and φEo were significantly increased by H0.7 treatment.
Compared with L7 treatment, total Rubisco activity was statistically increased by L0.7 treatment, while total Rubisco activity was significantly reduced by H7 treatments (Figure 5). Total Rubisco activity was much higher for H0.7 treatment than for H7 treatment.
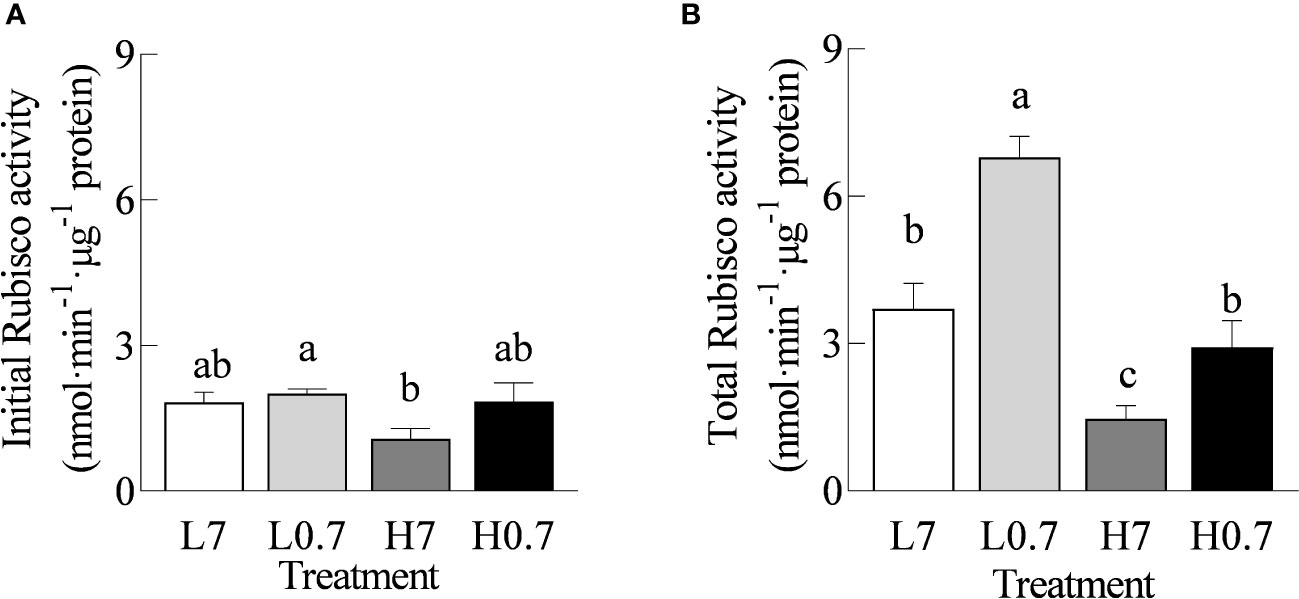
Figure 5 Effect of R/FR on Rubisco activity in cucumber leaves under salt stress. (A), Initial Rubisco activity; (B), Total Rubisco activity. Different letters indicate significant differences (P< 0.05; n= 4).
In general, compared to L7 treatment, expression levels of all key genes (e.g., psbA, psbB, psaA, psaB, rbcL, rbcS, Rca, TPI, FBPase, SBPase, Rupe and PRK) involved in photosynthetic electron transport and Calvin cycle were significantly up-regulated by L0.7 treatment, however, all gene expressions were obviously down-regulated by H7 treatment, only Rca gene expression was down-regulated by H0.7 treatments when compared to L7 treatment (Figure 6). The transcription levels of all genes except for rbcL and TPI were much higher in H0.7 treatment than H7 treatment.
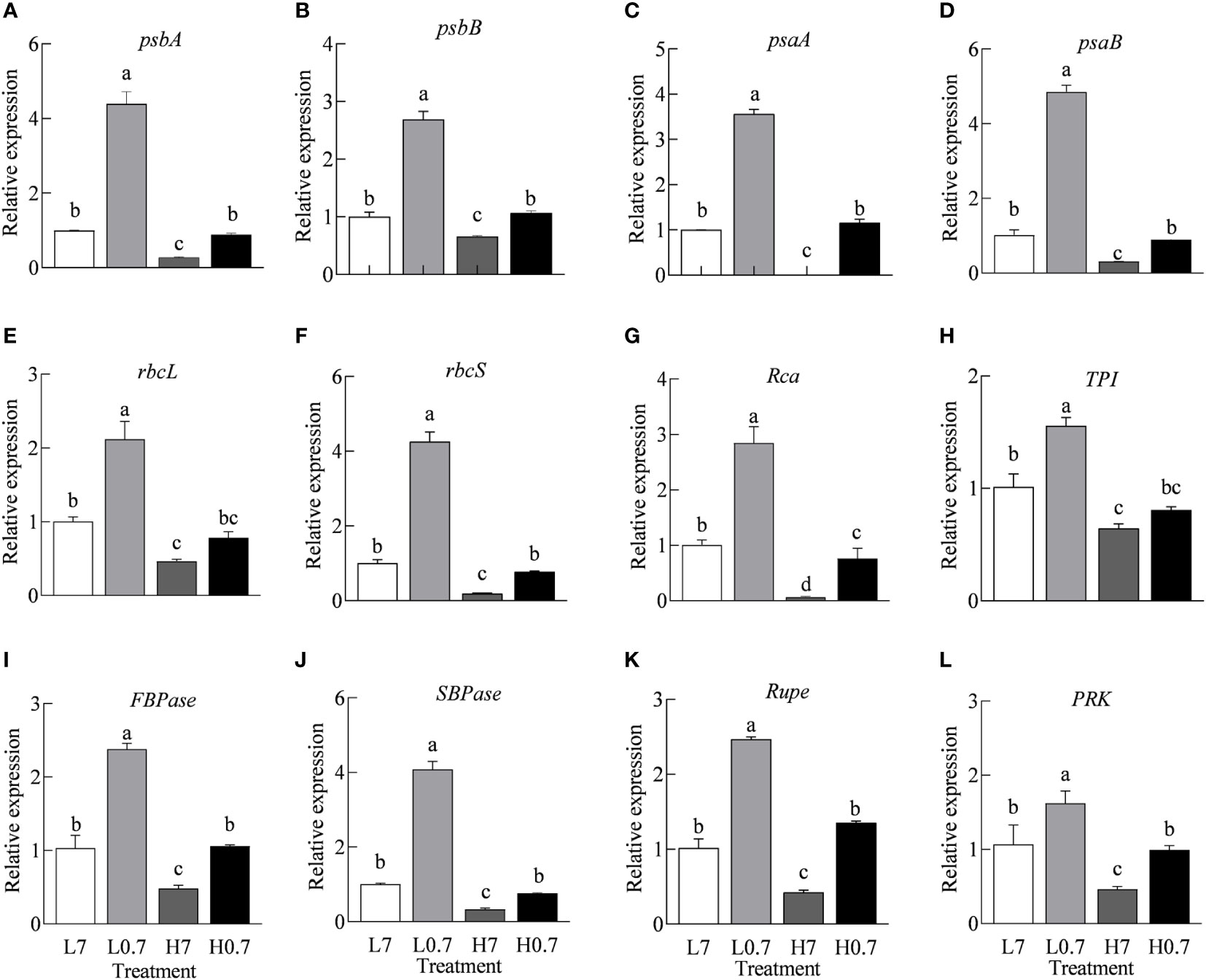
Figure 6 Effect of R/FR on relative expression levels of key genes involved in electron transport and Calvin cycle in cucumber leaves under salt stress. The relative expression levels of psbA (A), psbB (B), psaA (C) and psaB (D) genes involved in electron transport and relative expression levels of rbcL (E), rbcS (F), Rca (G), TPI (H), FBPase (I), SBPase (J), Rupe (K) and PRK (L) genes involved in Calvin cycle rbcL, ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit; rbcS, ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit; Rca, ribulose-1,5-bisphosphate carboxylase/oxygenase activase; TPI, propanosyl phosphate isomerase; FBPase, fructose-1,6-bisphosphatase; SBPase, scenic heptulose-1,7-bisphosphatase; Rupe, ribulose-5-phosphate epimerase; PRK, ribulose-5-phosphate kinase. Different letters indicate significant differences (P< 0.05; n= 3).
Compared to L7 treatment, chlorophyll a and b contents, most JIP-test parameters, expressions of genes (e.g., psbA, psbB, psaA and psaB) encoding Photosystem II and I (PSII and PSI) reaction center proteins were significantly reduced by H7 treatment, indicating that salt stress delayed reduction of primary electron acceptor quinone molecule in PSII (QA), and electron transfer from QA— to PQ pool until end acceptors (e.g., Fd, NADP) at PSI electron acceptor side, thus reduced photosynthetic electron transfer capacity (Figure 7); Meanwhile, expression levels of all key genes (e.g., rbcL, rbcS, Rca, TPI, FBPase, SBPase, Rupe and PRK) involved in Calvin cycle were statistically down-regulated, and Rubisco activity, sucrose and starch contents were significantly decreased by H7 treatments. These indicated that salt stress inhibited leaf photosynthesis mainly through disturbing photosynthetic electron transport and Calvin cycle.
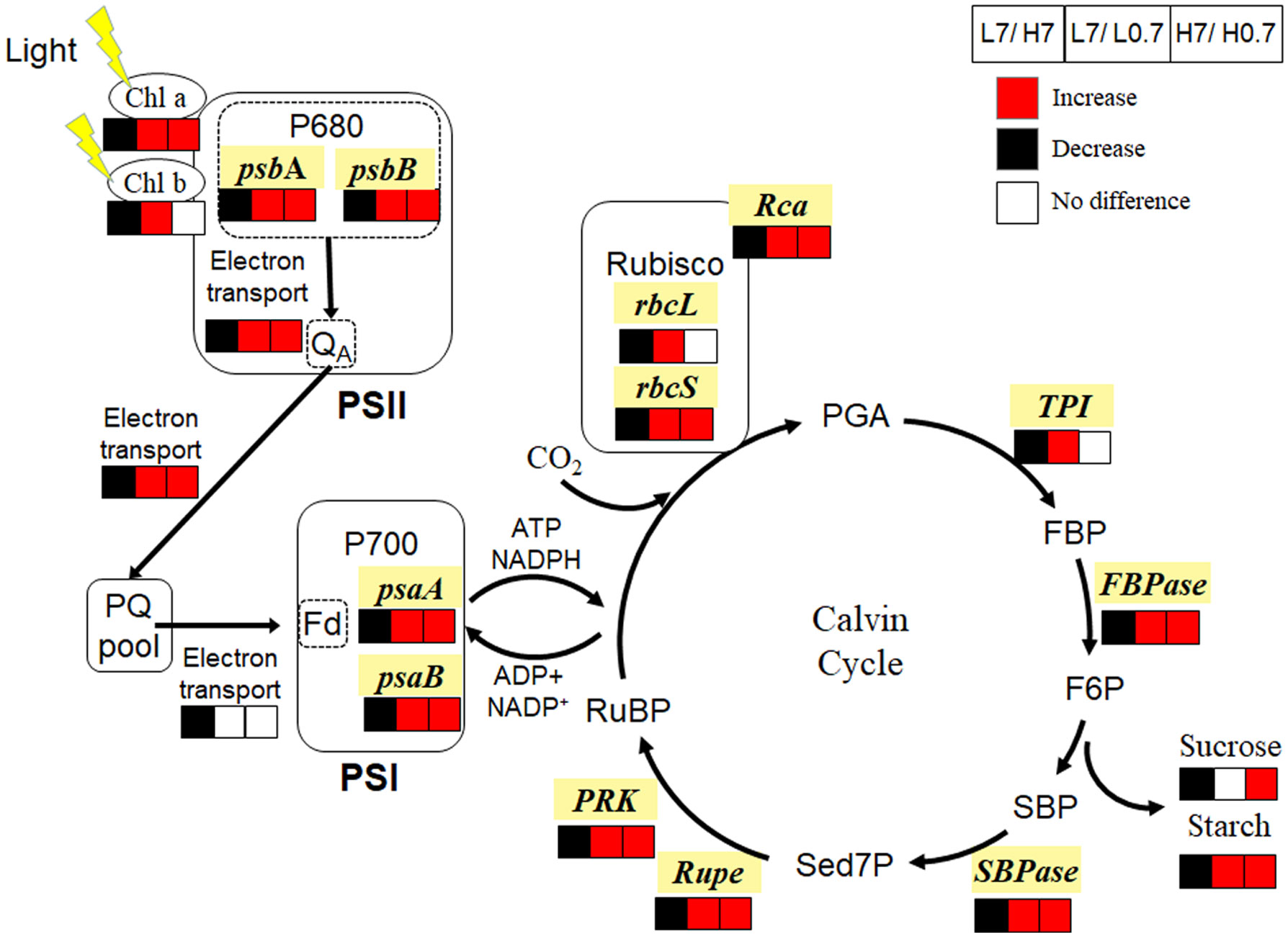
Figure 7 Effect of R/FR on photosynthetic electron transport and Calvin cycle in cucumber leaves under salt stress. Compared to L7 treatment, H7 treatment significantly decreased chlorophyll a, chlorophyll b, sucrose and starch contents, inhibited electron transport from PSII to PSI, and down-regulated expressions of genes (e.g., psbA, psbB, psaA, psaB, rbcL, rbcS, Rca, TPI, FBPase, SBPase, Rupe and PRK) involved in photosynthesis; While L0.7 treatment significantly increased chlorophyll a, chlorophyll b and starch contents, improved electron transport from PSII to PQ pool, and up-regulated expressions of genes (e.g., psbA, psbB, psaA, psaB, rbcL, rbcS, Rca, TPI, FBPase, SBPase, Rupe and PRK) involved in photosynthesis. Compared to H7 treatment, H0.7 treatment significantly enhanced chlorophyll a, sucrose and starch contents, promoted electron transport from PSII to PQ pool, and up-regulated expressions of genes (e.g., psbA, psbB, psaA, psaB, rbcS, Rca, FBPase, SBPase, Rupe and PRK) involved in photosynthesis. The red and black units represent enhancement and inhibition of photosynthesis apparatus or gene expression level, respectively, while white units represent no difference. Chl, chlorophyll; PSII, Photosystem II; P680, primary electron donor of PSII; QA, primary electron acceptor quinone molecule; PSI, Photosystem I; P700, primary electron Chl donor of PSI; PQ pool, plastoquinone pool; Rubisco, Ribulose-1,5-bisphosphate carboxylase-oxygenase; PGA, phosphoglycerate; FBP, fructose-1,6-bisphosphate; F6P, fructose-6-phosphate; SBP, Sedoheptulose-1, 7-bisphosphate; Sed7P, Sedoheptulose-7-bisphosphate; RuBP, ribulose-1,5-bisphosphate.
L0.7 treatment had the highest chlorophyll a and b contents, φEo, PIABS, psbA, psbB, psaA and psaB gene expressions in all treatments, suggesting that low R/FR accelerated electron transfer from QA— to PQ pool, thus enhanced photosynthetic electron transport capacity; In the meanwhile, expression levels of all key genes (e.g., rbcL, rbcS, Rca, TPI, FBPase, SBPase, Rupe and PRK) involved in Calvin cycle, and Rubisco activity were highest in L0.7 treatment, contributing to accelerated Calvin cycle and increased starch content in cucumber leaf. In all, low R/FR enhanced leaf photosynthesis mainly through promoting photosynthetic electron transport and Calvin cycle.
Compared to H7 treatment, chlorophyll a content, φPo, φEo, psbA, psbB, psaA and psaB gene expressions were significantly increased by H0.7 treatment, showing that low R/FR enhanced linear photosynthetic electron transport from PSII to PQ pool under salt stress; Simultaneously, expression levels of most genes (e.g., rbcS, Rca, FBPase, SBPase, Rupe and PRK) involved in Calvin cycle were up-regulated and Rubisco activity was enhanced by H0.7 treatment, resulting in increased sucrose and starch contents. In brief, low R/FR benefited leaf photosynthesis mainly through improving photosynthetic electron transport and Calvin cycle under salt stress.
Salt stress severely inhibits leaf photosynthesis and plant growth, and it has become a challenge in vegetable production. R/FR, an important light environmental factor, takes an active part in regulating salt tolerance in plants. Recently, great progress has been made in physiological characteristics and molecular mechanism of vegetable crops under salt stress, however, the regulation roles of R/FR on leaf photosynthetic characteristics under salt stress is still largely unclear (Cao et al., 2018; Wang Y. et al., 2021). In this study, we emphasized that low R/FR promoted photosynthetic electron transport chain and Calvin cycle, finally improved leaf photosynthesis in salt-stressed cucumber plant.
Growth parameters are common indicators of plant’s response to salt stress. Salt stress inhibits plant tissues and organs growth, delays plant growth and development, and reduces yield and quality in many plant species, such as tomato, lettuce, sorghum and so on (De Lacerda et al., 2005; Shao et al., 2015; Moncada et al., 2020; Wang W. et al., 2021). In the present study, both RGR and total leaf area were lower for H7 and H0.7 treatments than for L7 treatment, indicating that salt stress seriously inhibited cucumber plant growth (Figure 2). Low R/FR plays a vital role in regulating plant growth, it not only promotes cell wall extension which leads to increased leaf area, but also enhances leaf photosynthetic capacity, promotes dry matter accumulation and plant growth (Yang et al., 2020; Tan et al., 2022). In this study, H0.7 treatment had higher RGR and total leaf area than H7 treatment, suggesting that low R/FR alleviated the adverse effects of salt stress on plant growth.
Photosynthesis is a crucial process of absorbing light energy and synthesizing inorganic substance into organic compound in plant. Salt stress usually induces the decline in photosynthetic characteristics in horticultural crops, such as Ziziphus spina-christi (L.) Willd., Dianthus superbus L. and Coreopsis tinctoria Nutt. (Ma et al., 2017; Gorai et al., 2019; Jiang et al., 2021). In the current study, Pn, Ci and Tr were significantly lower for H7 treatment than for L7 treatment, indicating that salt stress decreased photosynthetic characteristics (Figure 3A–D). Salt stress inhibits water absorption, i.e., insufficient photosynthetic raw materials, leading to a reduction in leaf photosynthesis; On the other hand, salt stress-induced ROS (e.g., 1O2, O2–, H2O2 and HO) severely destroys chloroplast structure and metabolism through oxidation of membrane lipids and membrane proteins, thus adversely impacts photosynthetic capacity (Zhang M. et al., 2016). Furthermore, triose phosphate use (TPU) limitation is widely considered to be one of the main limiting factors of light-saturated photosynthetic rate (Long and Bernacchi, 2003). Interestingly, in CO2 response curve, CO2 assimilation rate plateaued or slightly declined at the higher CO2 concentrations in H7 treatment, suggesting that salt stress significantly decreased rate of triose phosphate use, i.e., CO2 utilization, leading to the reduction in sucrose and starch contents (Figure 3E and Table 2).
It is considered that low R/FR can largely increase superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activity, effectively alleviate oxidative damage on chloroplast induced by salt stress (Wang Y. et al., 2021). In this study, H0.7 treatment had higher chlorophyll a content, gas exchange parameters (e.g., Pn, Ci and Tr) and carbohydrate (e.g., sucrose and starch) contents than H7 treatment, indicating that low R/FR had a positive effect on leaf photosynthesis in salt-stressed cucumber plants. This can be further proved by low R/FR-induced improved photosynthetic electron transport and Calvin cycle characteristics below.
Although achievements of photosynthetic characteristics under salt stress have been made in various plant species, effects of R/FR on photosynthetic electron transport capacity under salt stress are still largely unclear (Kalaji et al., 2011; Giorio and Sellami, 2021). Salt stress easily causes oxidation of oxygen-evolving complex (OEC), reaction center protein D1 and antenna protein CP47 in PSII, disrupts their structures and inactivates their function, resulting less electrons (Gururani Mayank et al., 2015). Our study found that Fv/Fm, VJ and most JIP-test parameters (e.g., φPo, φEo, φRo, RC/ABS, PIABS and PItotal) were significantly lower for H7 treatment than for L7 treatment (Figure 4 and Table S1). These results indicated that salt stress suppressed the reduction of QA in PSII, and electron transfer from QA-, through PQ pool, to end acceptors (e.g., Fd, NADP) at PSI electron acceptor side, i.e., salt stress severely inhibited linear photosynthetic electron transport chain from PSII to PSI. In addition, salt stress largely down-regulated expression levels of psbA (encoding D1 protein in PSII), psbB (encoding CP47 protein in PSII), psaA and psaB (coding P700 core protein in PSI) genes, delayed the repair rate of PSII and decreased PSI activity, showing that salt stress played adverse roles in electron transport (Figure 6).
Studies have shown that R/FR could improve antioxidant enzyme activities, effectively relieve oxidative damage of salt stress, delay decomposition rate of proteins, eventually enhance leaf photosynthesis in plants (Cao et al., 2018). Our study clearly indicated that Fv/Fm, VJ in OJIP curve, φPo and φEo were significantly increased by H0.7 treatment when compared to H7 treatment, suggesting that low R/FR effectively increased electron transport from primary electron acceptor to PQ pool through QA. Furthermore, owing to up-regulated expression levels of psbA, psbB, psaA and psaB genes, the synthesis rate and efficiency of PSII and PSI were also improved by low R/FR (Figure 6). These results indicated that low R/FR improved the structure and function of PSII and PSI under salt stress, thus benefited photosynthetic electron transport under stress. Moreover, low R/FR also generated abundant ATP and NADPH for Calvin cycle by exhibiting strong electron transport capacity.
The Calvin cycle is an important component of carbon assimilation in photosynthesis. Rubisco is the key rate-limiting enzyme in Calvin cycle (Sharwood, 2017). The rbcL, rbcS, and Rca genes encode for large subunit, small subunit and activase of Rubisco, respectively. Previous studies found that salt stress can decrease Rubisco activity (Lin et al., 2018), which is consistent with the phenomenon observed in the current study. Under salt stress, total Rubisco activity, expression levels of rbcL, rbcS, and Rca genes and most key genes (TPI, FBPase, SBPase, Rupe and PRK) involved in Calvin cycle were remarkedly reduced (Figures 5, 6). The decline in expression and catalytic activity of Rubisco enzyme and other key enzymes involved in Calvin cycle severely restricts the ability of carbon assimilation (Sobhanian et al., 2010).
The previous studies showed that Low R/FR could enhance leaf photosynthesis by promoting carbon assimilation (Tan et al., 2022). Zhou et al. (2021) have suggested that low R/FR improved leaf photosynthetic capacity by up-regulating RBCS (encoding for small subunit of Rubisco) gene expression under calcium nitrate stress. In this study, low R/FR increased total Rubisco activity and up-regulated expression of most key genes (rbcS, Rca, FBPase, SBPase, Rupe and PRK) involved in Calvin cycle. These observations were consistent with the observations in tomato (Zhou et al., 2021) and spinach (Tan et al., 2022). These changes illustrating that low R/FR promoted carbon assimilation under salt stress through upregulating the expression levels and activity of Rubisco and other Calvin cycle enzymes.
Salt stress severely reduced leaf chlorophyll content, inhibited leaf photosynthesis and delayed plant growth. However, Low R/FR largely alleviated the adverse effects of salt stress on photosynthesis, it not only effectively improved linear photosynthetic electron transport from PSII to PQ pool, but also increased Rubisco activity and accelerated Calvin cycle, improved leaf photosynthesis and accelerated plant growth. In conclusion, low R/FR enhanced leaf photosynthesis by improving photosynthetic electron transport and Calvin cycle in salt-stressed cucumber plants. Therefore, usage of low red to far-red light ratio under salt stress condition could be advantageous for vegetable production.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
YM and XG performed experimental design, experimentation, data collection and analysis, manuscript preparation. BL contributed to project leadership, funding. WW performed experimental design and data analysis. LB revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
The research was financially supported by Shanxi Province Basic Research Program (20210302124242, 20210302123401), Shanxi Province Colleges and Universities’ Innovative Projects (2019L0382, 2019L0354), Shanxi Province Key R&D Plan (201703D211001-04-03), National Natural Science Foundation of China (31902029), High-level Foreign Expert Introduction Program in Ministry of Science and Technology (G2021004010L).
We thank Dr. Xiangzhen Wen for contributing to the experimental design. We thank Dr. Yaling Li for technical support throughout the experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1053780/full#supplementary-material
Ahres, M., Gierczik, K., Boldizsár, Á, Vítámvás, P., Galiba, G. (2020). Temperature and light-quality-dependent regulation of freezing tolerance in barley. Plants. 9 (1), 83. doi: 10.3390/plants9010083
Cao, K., Yu, J., Xu, D., Ai, K., Bao, E., Zou, Z. (2018). Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 18 (1), 92. doi: 10.1186/s12870-018-1310-9
Cockburn, W., Whitelam, G. C., Broad, A., Smith, J. (1996). The participation of phytochrome in the signal transduction pathway of salt stress responses in Mesembryanthemum crystallinum l. J. Exp. Bot. 47 (298), 647–653. doi: 10.1093/jxb/47.5.647
Courbier, S., Pierik, R. (2019). Canopy light quality modulates stress responses in plants. iScience 22, 441–452. doi: 10.1016/j.isci.2019.11.035
De Lacerda, C. F., Cambraia, J., Oliva, M. A., Ruiz, H. A. (2005). Changes in growth and in solute concentrations in sorghum leaves and roots during salt stress recovery. Environ. Exp. Bot. 54 (1), 69–76. doi: 10.1016/j.envexpbot.2004.06.004
Demotes-Mainard, S., Péron, T., Corot, A., Bertheloot, J., Gourrierec, J. L., Pelleschi-Travier, S., et al. (2015). Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 309, S0098847215000933. doi: 10.1016/j.envexpbot.2015.05.010
Franklin, K. A. (2008). Shade avoidance. New Phytol. 179 (4), 930–944. doi: 10.1111/j.1469-8137.2008.02507.x
Giorio, P., Sellami, M. H. (2021). Polyphasic OKJIP chlorophyll a fluorescence transient in a landrace and a commercial cultivar of sweet pepper (Capsicum annuum, l.) under long-term salt stress. Plants 10 (5), 887. doi: 10.3390/plants10050887
Gong, Z., Xiong, L., Shi, H., Yang, S., Zhu, J. K. (2020). Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 63 (5), 635–674. doi: 10.1007/s11427-020-1683-x
Gorai, M., Romdhane, R., Maraghni, M., Neffati, M. (2019). Relationship between leaf gas-exchange characteristics and the performance of Ziziphus spina-christi (L.) willd. seedlings subjected to salt stress. Photosynthetica 57 (3), 897–903. doi: 10.32615/ps.2019.093
Gururani Mayank, A., Venkatesh, J., Tran, L. S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8 (9), 1304–1320. doi: 10.1016/j.molp.2015.05.005
Gyugos, M., Ahres, M., Gulyás, Z., Szalai, G., Darkó, É, Mednyánszky, Z., et al. (2021). Light spectrum modifies the drought-induced changes of glutathione and free amino acid levels in wheat. Acta Physiol. Plant 43 (6), 90. doi: 10.1007/s11738-021-03253-x
Hayes, S., Pantazopoulou, C. K., Van Gelderen, K., Reinen, E., Tween, A. L., Sharma, A., et al. (2019). Soil salinity limits plant shade avoidance. Curr. Biol. 29 (10), 1669–1676. doi: 10.1016/j.cub.2019.03.042
Holalu, S. V., Finlayson, S. A. (2017). The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J. Exp. Bot. 68 (5), 943–952. doi: 10.1093/jxb/erw479
Jeong, H. W., Lee, H. R., Kim, H. M., Kim, H. M., Hwang, H. S., Hwang, S. J. (2020). Using light quality for growth control of cucumber seedlings in closed-type plant production system. Plants 9 (5), 639. doi: 10.3390/plants9050639
Jiang, H., Li, Z., Jiang, X., Qin, Y. (2021). Effects of salt stress on photosynthetic fluorescence characteristics, antioxidant system, and osmoregulation of Coreopsis tinctoria nutt. HortScience 56 (9), 1066–1072. doi: 10.21273/HORTSCI15956-21
Kalaitzoglou, P., Van Ieperen, W., Harbinson, J., van der Meer, M., Martinakos, S., Weerheim, K., et al. (2019). Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00322
Kalaji, H. M., Bosa, K., Kościelniak, J., Żuk-Gołaszewska, K. (2011). Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 73, 64–72. doi: 10.1016/j.envexpbot.2010.10.009
Kotilainen, T., Aphalo, P. J., Brelsford, C. C., Böök, H., Devraj, S., Heikkilä, A., et al. (2020). Patterns in the spectral composition of sunlight and biologically meaningful spectral photon ratios as affected by atmospheric factors. Agric. For. Meteorol. 291, 108041. doi: 10.1016/j.agrformet.2020.108041
Lin, J., Li, J. P., Yuan, F., Yang, Z., Wang, B. S., Chen, M. (2018). Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia l. under salt stress. Photosynthetica 56 (4), 998–1009. doi: 10.1007/s11099-018-0824-6
Long, S., Bernacchi, C. (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? procedures and sources of error. J. Exp. Bot. 54 (392), 2393–2401. doi: 10.1093/jxb/erg262
Ma, X., Zheng, J., Zhang, X., Hu, Q., Qian, R. (2017). Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00600
Miao, Y., Chen, Q., Qu, M., Gao, L., Hou, L. (2019). Blue light alleviates ‘red light syndrome’by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. (Amsterdam) 257, 108680. doi: 10.1016/j.scienta.2019.108680
Miao, Y., Luo, X., Gao, X., Wang, W., Li, B., Hou, L. (2020). Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. (Amsterdam) 272, 109577. doi: 10.1016/j.scienta.2020.109577
Miao, Y., Wang, X., Gao, L., Chen, Q., Qu, M. (2016). Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agr. 15 (1), 87–100. doi: 10.1016/S2095-3119(15)61202-3
Moncada, A., Vetrano, F., Miceli, A. (2020). Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 10 (10), 1523. doi: 10.3390/agronomy10101523
Niu, M., Sun, S., Nawaz, M. A., Sun, J., Cao, H., Lu, J., et al. (2019). Grafting cucumber onto pumpkin induced early stomatal closure by increasing ABA sensitivity under salinity conditions. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01290
Perreault, F., Oukarroum, A., Pirastru, L., Sirois, L., Matias, W. G., Popovic, R. (2010). Evaluation of copper oxide nanoparticles toxicity using chlorophyll a fluorescence imaging in Lemna gibba. J. Bot. 2010 (4), 807–826. doi: 10.1155/2010/763142
Shabala, S., Cuin, T. A. (2008). Potassium transport and plant salt tolerance. Physiol. Plant 133 (4), 651–669. doi: 10.1111/j.1399-3054.2007.01008.x
Shao, Q. S., Shu, S., Du, J., Xing, W. W., Guo, S. R., Sun, J. (2015). Effects of NaCl stress on nitrogen metabolism of cucumber seedlings. Russ. J. Plant Physiol. 62 (5), 595–603. doi: 10.1134/S1021443715050155
Sharwood, R. E. (2017). Engineering chloroplasts to improve rubisco catalysis: P:rospects for translating improvements into food and fiber crops. New Phytol. 213 (2), 494–510. doi: 10.1111/nph.14351
Shibuya, T., Endo, R., Yuba, T., Kitaya, Y. (2015). The photosynthetic parameters of cucumber as affected by irradiances with different red:Far-red ratios. Biol. Plantarum 59 (1), 198–200. doi: 10.1007/s10535-014-0473-y
Sobhanian, H., Motamed, N., Jazii, F. R., Nakamura, T., Komatsu, S. (2010). Salt stress induced differential proteome and metabolome response in the shoots of Aeluropus lagopoides (Poaceae), a halophyte C4 plant. J. Proteome Res. 9 (6), 2882–2897. doi: 10.1021/pr900974k
Strasser, R., Tsimilli-Michael, M., Srivastava, A. (2004). Analysis of the chlorophyll a fluorescence transient (Berlin. Berlin: Springer Netherlands).
Suzuki, K., Ohmori, Y., Ratel, E. (2011). High root temperature blocks both linear and cyclic electron transport in the dark during chilling of the leaves of rice seedlings. Plant Cell Physiol. 52 (9), 1697–1707. doi: 10.1093/pcp/pcr104
Tan, T., Li, S., Fan, Y., Wang, Z., Ali Raza, M., Shafiq, I., et al. (2022). Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 10 (2), 300–309. doi: 10.1016/j.cj.2021.06.007
Wang, H., Gu, M., Cui, J. X., Shi, K., Zhou, Y. H., Yu, J. (2009). Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photoch. Photobio. B. 96 (1), 30–37. doi: 10.1016/j.jphotobiol.2009.03.010
Wang, W., Cai, L., Long, Z., Zhang, X., Zhao, F. (2021). Effects of non-uniform salt stress on growth, yield, and quality of tomato. Soil Sci. Plant Nutr. 67 (5), 545–556. doi: 10.1080/00380768.2021.1966834
Wang, X., Zhang, W., Miao, Y., Gao, L. (2016). Root-zone warming differently benefits mature and newly unfolded leaves of Cucumis sativus l. seedlings under sub-optimal temperature stress. PloS One 11 (5), e0155298. doi: 10.1371/journal.pone.0155298
Wang, Y., Bian, Z., Pan, T., Cao, K., Zou, Z. (2021). Improvement of tomato salt tolerance by the regulation of photosynthetic performance and antioxidant enzyme capacity under a low red to far-red light ratio. Plant Physiol. Biochem. 167, 806–815. doi: 10.1016/j.plaphy.2021.09.008
Yang, F., Liu, Q., Cheng, Y., Feng, L., Wu, X., Fan, Y., et al. (2020). Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 20 (1), 1–12. doi: 10.1186/s12870-020-02352-0
Zhang, J., Yang, D., Li, M., Shi, L. (2016). Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PloS One 11 (7), e0159622. doi: 10.1371/journal.pone.0159622
Zhang, L., Zhang, L., Sun, J., Zhang, Z., Ren, H., Sui, X. (2013). Rubisco gene expression and photosynthetic characteristics of cucumber seedlings in response to water deficit. Sci. Hortic. (Amsterdam) 161, 81–87. doi: 10.1016/j.scienta.2013.06.029
Zhang, M., Smith, J. A. C., Harberd, N. P., Jiang, C. (2016). The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 91 (6), 651–659. doi: 10.1007/s11103-016-0488-1
Zhao, H., Zhang, Z., Zhang, Y., Bai, L., Hu, X., Li, X., et al. (2022). Melatonin reduces photoinhibition in cucumber during chilling by regulating the Calvin-Benson cycle. Sci. Hortic. (Amsterdam) 299, 111007. doi: 10.1016/j.scienta.2022.111007
Zhao, X., Sui, X., Zhao, L., Gao, X., Wang, J., Wen, X., et al. (2022). Morphological and physiological response mechanism of lettuce (Lactuca sativa l.) to consecutive heat stress. Sci. Hortic. (Amsterdam) 301, 111112. doi: 10.1016/j.scienta.2022.111112
Zhen, S., Iersel, M. V. (2017). Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 209, 115–122. doi: 10.1016/j.jplph.2016.12.004
Keywords: red to far-red light ratio, salt stress, cucumber, photosynthetic electron transport, Calvin cycle
Citation: Miao Y, Gao X, Li B, Wang W and Bai L (2023) Low red to far-red light ratio promotes salt tolerance by improving leaf photosynthetic capacity in cucumber. Front. Plant Sci. 13:1053780. doi: 10.3389/fpls.2022.1053780
Received: 26 September 2022; Accepted: 30 November 2022;
Published: 06 January 2023.
Edited by:
Mohammad Golam Mostofa, Michigan State University, United StatesReviewed by:
Baizhao Ren, Shandong Agricultural University, ChinaCopyright © 2023 Miao, Gao, Li, Wang and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanxiu Miao, bWlhb3lhbnhpdUAxNjMuY29t
†These authors contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.