- 1Laboratory of Metabolic and Developmental Sciences, School of Life Sciences and Biotechnology, The Joint International Research, Shanghai Jiao Tong University, Shanghai, China
- 2Zhiyuan College, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Biology, Tokyo Gakugei University, Koganei, Japan
- 4Shanghai Collaborative Innovation Center of Agri-Seeds/Joint Center for Single Cell Biology, Shanghai Jiao Tong University, Shanghai, China
The vacuole is an important organelle with multiple functions in plants, and the tonoplast that wraps the vacuole also plays essential roles in intracellular trafficking and ion homeostasis. Previous studies found that tonoplast proton pumps regulate embryo development and morphogenesis through their effects on vacuole biogenesis and distribution, as well as polar auxin transport and concomitant auxin gradient. However, the precise roles of the tonoplast proton pumps in gametophyte development remain unclear. Here we demonstrated that the lack of two types of tonoplast proton pumps or the absence of V-ATPase alone leads to abnormal development and nuclear localization of female gametophyte (FG), and slowed endosperm nuclei division after fertilization of the central cell. We further revealed that V-ATPase regulates auxin levels in ovules through coordinating the content and localization of PIN-FORMED 1 (PIN1) protein, hence influencing nuclear spacing between centra cell and egg cell, and subsequent endosperm development. Collectively, our findings revealed a crucial role of V-ATPase in auxin-mediated FG development in Arabidopsis and expanded our understanding of the functions of tonoplast proton pumps in seed plants reproductive development.
Introduction
The vacuole, as an organelle with a single membrane, forms compartments and is a sophisticated endomembrane system unique to plant cells (Marty, 1999). As an important barrier for material exchange into and out of the vacuole, the vacuolar membrane, also referred to as tonoplast, harbors a rich variety of transporters, among which the tonoplast proton pumps are the most fundamental proteins. There are two distinct proton pumps, namely the vacuolar H+-pyrophosphatase (V-PPase, EC 3.6.1.1) and the vacuolar H+-ATPase (V-ATPase, EC 3.6.4.10) (Hedrich et al., 1989), that have been reported in the literature, both exhibit completely different structural and functional properties. The V-PPase and V-ATPase catalyze the hydrolysis of Mg2+-PPi and Mg2+-ATP complex, respectively, and extract energy for pumping H+ ions into the vacuolar lumen, thus maintaining a proton gradient (ΔpH) across the tonoplast (Maeshima, 2000; Martinoia et al., 2007).
Whereas V-PPase has a relatively simple structure and is encoded by AVP1/FUGU5 in Arabidopsis thaliana (Arabidopsis hereafter) (Ferjani et al., 2011; Kriegel et al., 2015; Jiang et al., 2020), the V-ATPase is a versatile protein complex that broadly occurs in all cell types (Marshansky and Futai, 2008). It exhibits a unique differential targeting on endomembrane compartments, which is mediated by different isoforms of its subunit a (Lupanga et al., 2020). In Arabidopsis, V-ATPase complex containing VHA-a2 or VHA-a3 is localized at the tonoplast (Krebs et al., 2010; Jiang et al., 2020). According to previous studies, fugu5-1, a point mutation line of AVP1, lacks V-PPase activity and exhibits basically the same growth conditions as wild type (Kriegel et al., 2015; Jiang et al., 2020); vha2 (vha-a2 vha-a3), T-DNA insertion line of VHA-a2 and VHA-a3, lacks V-ATPase activity at the tonoplast and exhibits overall impaired growth phenotypes (Krebs et al., 2010); and fap3, the triple mutant obtained by crossing fugu5-1 with vha2, lacks both V-PPase and V-ATPase functions, and displays a hindered vegetative growth and an aberrant cotyledon boundary during embryogenesis due to blocked PIN-FORMED (PIN)1-mediated auxin transport (Kriegel et al., 2015; Jiang et al., 2020). Tonoplast proton pumps regulate embryo development through PIN1-mediated auxin transportation. However, tracking back along the reproductive developmental process, gametophyte development of plants lacking both tonoplast proton pumps remained poorly understood.
During the reproductive growth of angiosperms, male gametophytes are always produced in great redundancy, while female gametophytes (FGs) are produced in less abundance due to evolutionary specialization. Thus, FG development is indispensable for the generational alternation in higher plants. In most angiosperms with polygonum-type FGs, megaspore mother cell (MMC) undergoes meiosis to produce four megaspores and one of them becomes the functional megaspore, which gives rise to an FG. The development process of FGs can be divided into 8 stages from FG1 to FG8 (Christensen et al., 1997). At FG6 stage, with the fusion of polar nuclei, a fixed pattern of seven cells is formed: an egg cell and two synergid cells at the micropylar end, a central cell in the central area, and three antipodal cells at the chalazal end (Yang et al., 2010). The development of FGs determines the portion of fertile embryos and the number of seeds produced. Besides, FGs also influence many aspects of plant reproduction, including male-female crosstalk and maternal effects on seed development, highlighting the significance of untangling its developmental regulatory network.
Plant hormones, such as brassinosteroids (BRs), gibberellin (GA), cytokinin (CK), and auxin have been shown to play essential roles in FGs development (Pérez-España et al., 2011; Yuan et al., 2016; Gomez et al., 2020). BR, CK, and GA generally contribute indirectly to FG development through their effects on specific proteins that manipulate auxin levels among embryo sacs and change the FG fate (Pagnussat et al., 2009; Luca et al., 2013; Panoli et al., 2015). Auxin, due to its ubiquitous distribution and long-range mobility, has a crucial direct impact on FG development; as a signal molecule, auxin synthesis, transport, and dynamic concentration gradient are deeply involved in the whole process of female reproductive growth (Serbes et al., 2019). It is reported that auxin also acts as a positional cue for cell fate determination, and that the accurate positioning of FG nuclei is important for their identity (Sun et al., 2021). The build-up of auxin gradient relies on polar auxin transportation (PAT) through various organelles and the endomembrane transport system. Previously, we showed that tonoplast proton pumps regulate the intracellular trafficking of PIN1 protein, hence influencing auxin homeostasis during embryonic development in Arabidopsis (Jiang et al., 2020). Whether tonoplast proton pumps functions in FGs development, however, remains to be explored.
In this work, to fill knowledge gap about the precise function of tonoplast proton pumps in Arabidopsis FGs development, we conducted a multiscale phenotypic analysis of mutants lacking both V-PPase and V-ATPase activity, and found that these mutants exhibit an abnormal nuclear-spacing between FG nucleus and delayed endosperm division. We are also proposing a working model linking tonoplast proton pumps and PAT in FGs developmental process. Our results provide novel insights into roles of tonoplast proton pumps in the reproductive developmental process.
Materials and methods
Plant material and growth conditions
All materials used in this study were generated in Arabidopsis ecotype Columbia 0 (Col-0) background. Seeds were soaked with sterile water for 1 min, surface sterilized with a 3% NaClO solution for 5 min, then rinsed 5 times in sterile distilled water. Sterile seeds were grown on agar plates containing 1/2 Murashige and Skoog (MS) medium [2.215 g L–1, 0.75% agar (w/v), 1.5% sucrose (w/v), pH = 5.75]. Plates were incubated in the dark at 4°C for 2 days and cultured in a growth chamber with a photoperiod of 16 h/8 h, light/dark cycling, and a temperature of 22 ± 1°C. After 7 days, the plants on the dishes were transferred to a mixture of soil:vermiculite:perlite (10:10:1). In this paper, to make the mutant vha2 grow better during vegetative stages, both vha2 and its contemporaneous control plants were grown under 24-h long-day light.
Identification of the T-DNA insertion lines and construction of transgenic plants
For mutants of tonoplast proton pumps used in this study, vha2 [crossing vha-a2 (SALK_142642) with vha-a3 (SALK_029786) from Salk Institute for Biological Studies] and fap3 [obtained by crossing fugu5-1 with vha2] were both described in detail in our previous work, as well as ProPIN1:PIN1-YFP and R2D2 marker line (Jiang et al., 2020; Yu et al., 2020). For quantification of fluorescent signals of R2D2, we used the DII-Venus relative to mDII-ntdTomato for the semi-quantitative analysis of auxin level in plants, which is represented by DII/mDII (Benková et al., 2003; Liao et al., 2015). The construction of ProES1:H2B-GFP were based on the previously published literature (Pagnussat et al., 2007). To generate the ProVHA-a3:VHA-a3-GFP, the promoter and genomic DNA sequences were amplified by PCR using genomic DNA of Col as templates, and then cloned into the pHB vectors (Biovector NTCC Inc.) digested by EcoRI and HindIII, respectively. The sequences of oligonucleotide primer sets used are listed in Supplementary Table 1.
RNA extraction and qRT-PCR assays
The materials used for total RNA extraction in this study were inflorescence apices of Arabidopsis, which were harvested with tweezers and quick-frozen in liquid nitrogen. Subsequent RNA extraction and reverse transcription were performed using the RNAprep Pure Plant Kit (TIANGEN) and ReverAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) following the manufacturers’ protocols. qRT-PCR assays were conducted for three technical replicates of three biological replicates, and the results are shown as the mean ± standard deviations. The details were carried out as described previously (Zhang et al., 2016). The sequences of oligonucleotide primer sets used are listed in Supplementary Table 1.
In vitro treatment assays
To analyze the development status of FG in different stages upon 1-naphthylacetic acid (NAA; Sigma-Aldrich 317918) and picloram (Sigma-Aldrich 1918021), vigorously growing inflorescence apices were used as experimental materials. Floral buds larger than stage 10 were removed and the remaining inflorescences apices were immersed in 0.01% (v/v) Silwet L-77 solutions containing 1 μM NAA, or 1 μM picloram. After 24 h, wash the inflorescences apices with distilled water to remove residual agent. The materials were collected after 2 days.
Confocal microscopy
Confocal laser-scanning microscopy (CLSM) observation of male and FG was strictly performed as described previously (Christensen et al., 1997), but with the modification that we used the Upright Laser Confocal Microscope (Nikon & Nikon Ni-E A1 HD25). For the collection of male and FGs, we took the vigorously growing buds from outside to inside of inflorescence apices. For the analysis of flowers containing GFP constructs, we first carefully peeled the pistils from the flowers in different periods and fixed them in 4% paraformaldehyde for 30 min and then immersed in Clearsee agent according to Clearsee method (Kurihara et al., 2015). Cell walls were stained with 0.02% Fluorescent Brightener 28 (FB28) in Clearsee agent for over 3 h, as described in our previous study (Zu et al., 2022). Before observation, materials were soaked in Clearsee reagent to wash away the cell wall stain for more than half an hour. For the ovule wrapped inside the pistils, we tapped the corresponding position of the coverslip with the tip of the tweezers to exposed the ovule. For CLSM, GFP was excited with an argon laser at a wavelength of 488 nm, and emission was detected between 500 and 530 nm. FB28 was excited with an argon laser at a wavelength of 395 nm, and emission was detected between 450 and 480 nm. Measurements of nuclear spacing and fluorescence intensity were obtained using the Image J software.
Male gametophyte observation assays
Male gametophyte analyses, including Alexander staining, scanning electron microscopy (SEM), in vitro culture of pollen grains, DAPI staining of pollen grains and aniline blue staining of pollen tube were performed as described previously (Johnson-Brousseau and McCormick, 2004; Li et al., 2013).
Quantification and statistical analysis
Data analysis and statistical graphs were carried out using GraphPad Prism 8 and Microsoft Office Excel software. For comparison between two groups, Two-tailed Student’s t-test was used, and for more than two groups, Duncan’s test was used. The specific statistical methods for each assay are described in figure legends. Quantification of the phenotypes were performed using the ImageJ software.1
Accession numbers
Arabidopsis genes mentioned in this article are as follows: AVP1/FUGU5, AT1G15690; VHA-a2, AT2G21410; VHA-a3, AT4G39080; PIN1, AT1G73590; PIN3, AT1G70940; PIN4, AT2G01420; ARF1, AT1G59750; ARF2, AT5G62000; ARF3, AT2G33860; ARF4, AT5G60450; ARF5, AT1G19850; YUC2, AT4G13260; YUC5, AT5G43890; YUC8, AT4G28720; YUC9, AT1G04180.
Results
Vacuolar H+-ATPase deficiency results in ovule and seed abortion
To explore the role of the tonoplast proton pumps in gametophyte development of Arabidopsis, we focused on the reproductive development of fap3 mutant lacking both V-PPase and V-ATPase activities. Most of mature flowers at stage 13 (72%, 112/156) obviously had morphological abnormalities (Supplementary Figure 1A) and 97% (118/121) ovules of fap3 were aborted before fertilization (Supplementary Figure 1B). Further examinations with Alexander staining, CLSM, and SEM on pollen grains revealed that 72% (64/89) pollen grains of fap3 are not fertile due to defective pollen development (Supplementary Figures 1C–E), indicating that loss-of-function of both V-PPase and V-ATPase activities severely affects male gametophyte development as well. Based on CLSM observation and statistics, only 2% (3/121) FGs of fap3 could develop normally, and the rest prematurely aborted (Supplementary Figure 1F), confirming the roles of both V-PPase and V-ATPase in FG development. Collectively, the above results indicated that the lack of two tonoplast proton pumps can have extremely serious consequences for the entire process of plant reproduction. Therefore, fap3 is unlikely an ideal material for studying the role of tonoplast proton pumps in plant FG development. Since the overall plant growth, male and female transmission of fugu5-1 are comparable to those of the wild type Col-0 (Jiang et al., 2020; Supplementary Table 1), we next focused our study on V-ATPase mutant vha2.
As mentioned above, while VHA-a1 mediates TGN/EE targeting of V-ATPase, VHA-a2 and VHA-a3 mediate V-ATPase’s tonoplastic localization (Krebs et al., 2010; Kriegel et al., 2015; Luo et al., 2015; Lupanga et al., 2020). Therefore, vha2 was used to investigate its specific role in plant gametophyte development. Considering that an extended illumination time can improve vegetative growth of vha2 (Krebs et al., 2010), vha2 mutants were grown under continuous light conditions, which greatly improved inflorescence growth to a level almost indistinguishable from Col-0 (Supplementary Figure 2) and eased our analyses. Interestingly, most flowers of vha2 displayed normal morphologies, as only 17% (19/112) of them were abnormal (Figure 1A). Correspondingly, most siliques elongated normally and bore seeds, and only 14% (21/150) of them were obviously shorter (Figure 1B and Supplementary Figure 2). Consistently, vha2 abnormal siliques had a higher ovule abortion rate and developed only few seeds (Figures 1B,C). As the stigma was morphologically defective (Supplementary Figure 3A), when abnormal vha2 pistils were hand-pollinated with the Col-0 pollen, ovules could develop till the FG7 stage and remained unfertilized as examined at 48 h after pollination (HAP) (Supplementary Figure 3B). Hence, it appears that the premature abortion prior to fertilization could be derived from abnormal growth of the pollen tube on the defective stigma (Supplementary Figure 3C). Based on the above observations, morphologically normal vha2 flowers were mainly used in our following experiments.
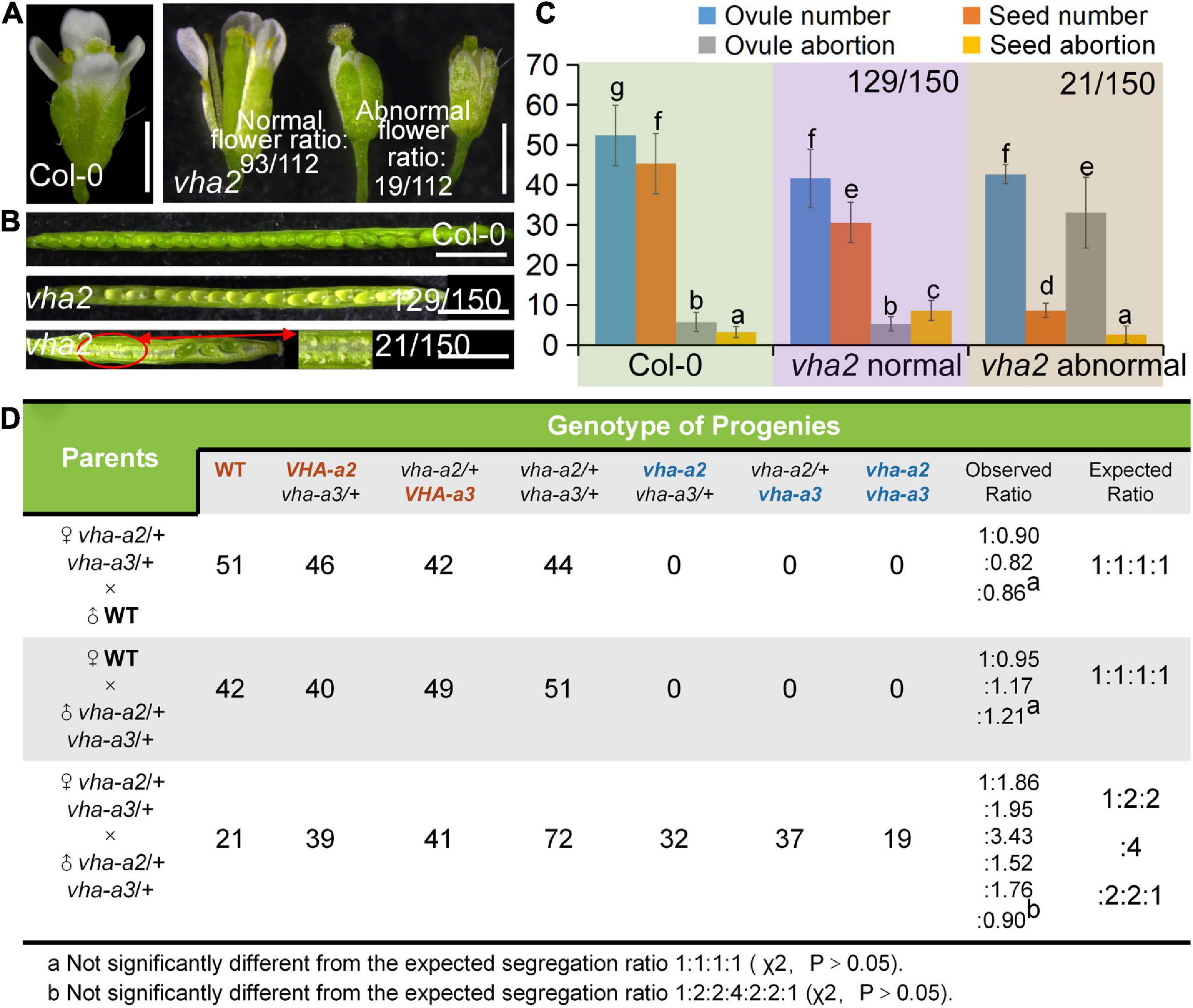
Figure 1. Lack of V-ATPase results in abnormal embryonic development. (A) Flower development at stage-13 in Col-0 and vha2. Statistics on the ratio of normal and abnormal microspores in vha2 are shown in each image. Bar = 1 mm. (B) Seed development in Col-0 and vha2. Two types of siliques of vha2 correspond to those in (A). (C) Statistics analysis of ovule number, seed number, ovule abortion, and seed abortion in Col-0 and vha2. Bars represent the mean ± SD of three biological replicates (n = 30). Lowercase letters indicate statistically significant differences between different stages (P< 0.05). Theoretically, ovule number = seed number + ovule abortion + seed abortion. (D) Both male and female transmission of vha-a2, and vha-a3 are comparable to VHA-a2 and VHA-a3 of WT. Genotypes consistent with wild type are marked in red font, homozygous mutant genotypes are marked in blue font, and heterozygous mutant genotypes are marked in black font.
Next, we examined the male and female transmission of vha-a2 and vha-a3 and found that transmission of the vha-a2 and vha-a3 mutant alleles is comparable to VHA-A2 and VHA-A3 in WT alleles (Figure 1D). For a more rigorous follow-up study, we need to demonstrate that VHA-a2 and VHA-a3 are expressed during gametophyte development. So we examined V-ATPase expression by constructing a transgenic line for VHA-a3, the predominant form of the a subunit on the tonoplast, which is fully redundant with VHA-a2. Fluorescence images of a ProVHA-a3: VHA-a3-GFP transgenic line confirmed the expression of V-ATPase during the whole process of Arabidopsis gametophyte development and early embryo development as well (Figures 2A–I).
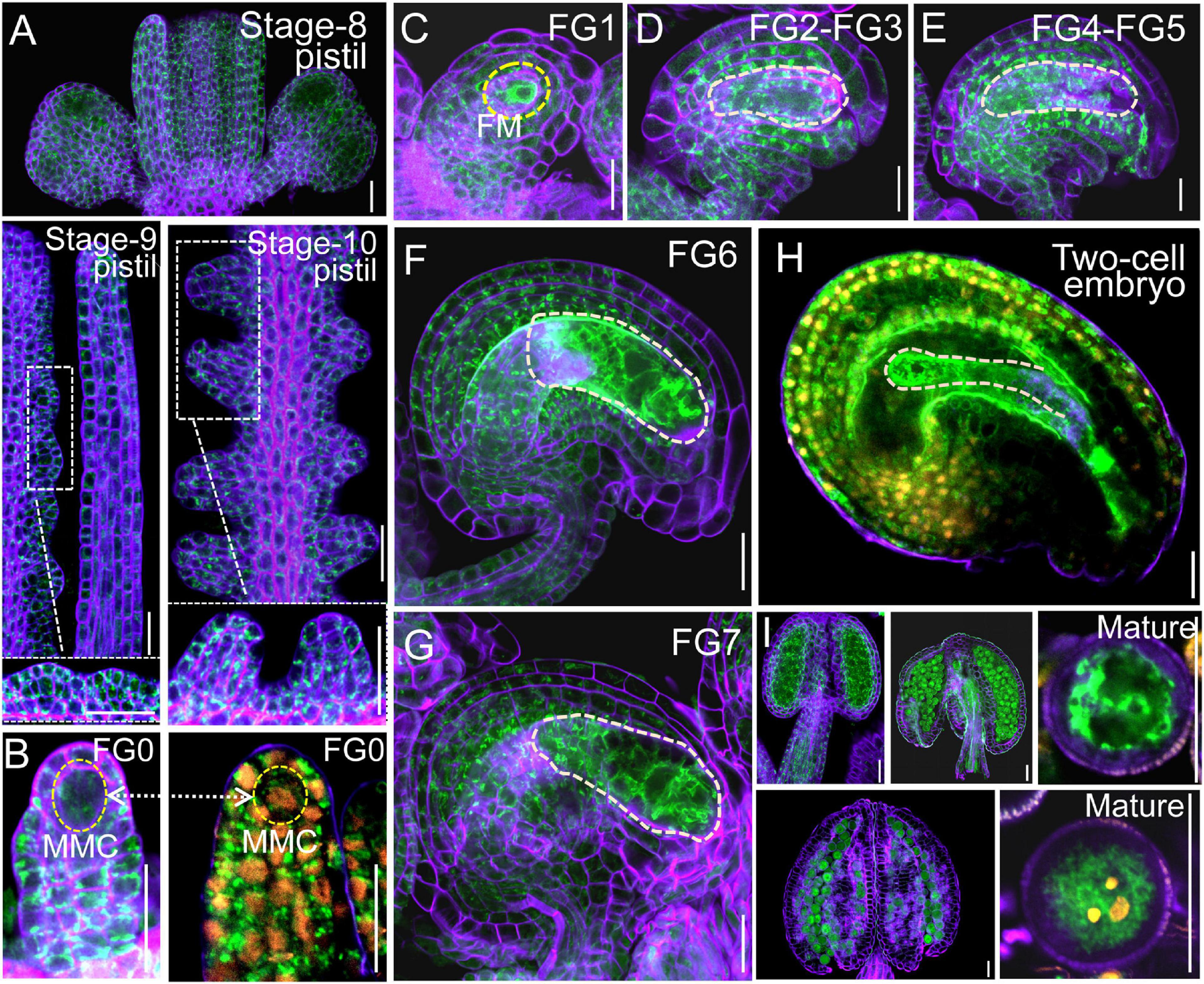
Figure 2. VHA-a3 is constitutively expressed in reproductive organs. Representative stage-8, stage-9, stage-10 pistil (A), ovules during FG0 stage (B), FG1 stage (C), FG2-FG3 stage (D), FG4-FG5 stage (E), FG6 stage (F), FG7 stage (G), two-cell embryo (H), anthers and mature pollen grain (I) from the Pro VHA-a3: VHA-a3-GFP plants. The nuclear staining with DAPI in FG0 stage (B) to demonstrate that MMC is present in this developmental stage. Yellow dotted circles in (D–G) indicate the embryo sac and in (H) indicate the two-cell embryo; Orange dots indicate the nucleus by DAPI staining of mature pollen grain. The purple part is the outline of pollen grains stained with FM4-64. Bars = 20 μm. MMC megaspore mother cell, FM functional megaspore.
Loss of vacuolar H+-ATPase activity affects pollen development
vha2 normal siliques wrapped normal ovules, but the ratio of seed abortion in vha2 normal siliques was higher than that of Col-0 (Figures 1B,C). To gain insights into the seed abortion in vha2 normal siliques, we further examined male and FG development in vha2. For male fertility, in vitro pollen germination combined with alexander staining, fluorescence microscopy and SEM were performed to examine the pollen viability. 77% (40/52) anthers from vha2 were stained purplish red by Alexander dye that was consistent with Col-0 (Figure 3A), while only 23% (12/52) anthers showed light blue pollen coloration (Figure 3A), indicating that most pollen grains were vigorous. Further CLSM implied that pollen grains with large vacuoles in infertile anthers are arrested in pollen mitosis I (PMI) stage (Figure 3B). SEM images of vha2 revealed that the majority of pollen grains (64%, 83/130) are oval-shaped similar to Col-0 (Figure 3C), and that the remaining portion of pollen grains are slightly wrinkled (Figure 3C). In vitro pollen germination demonstrated that there are no significant differences between vha2 and Col-0 regarding pollen tube length and width (Figures 3D,E). Therefore, the slightly wrinkled pollens had similar fertility to Col-0 normal ones. Furthermore, DAPI staining further demonstrated that vha2 mature pollen has a normal nuclear division (Figure 3F). The abovementioned results implied that even though a small portion of pollens are slightly wrinkled, the loss of V-ATPase function does not significantly affect pollen fertility.
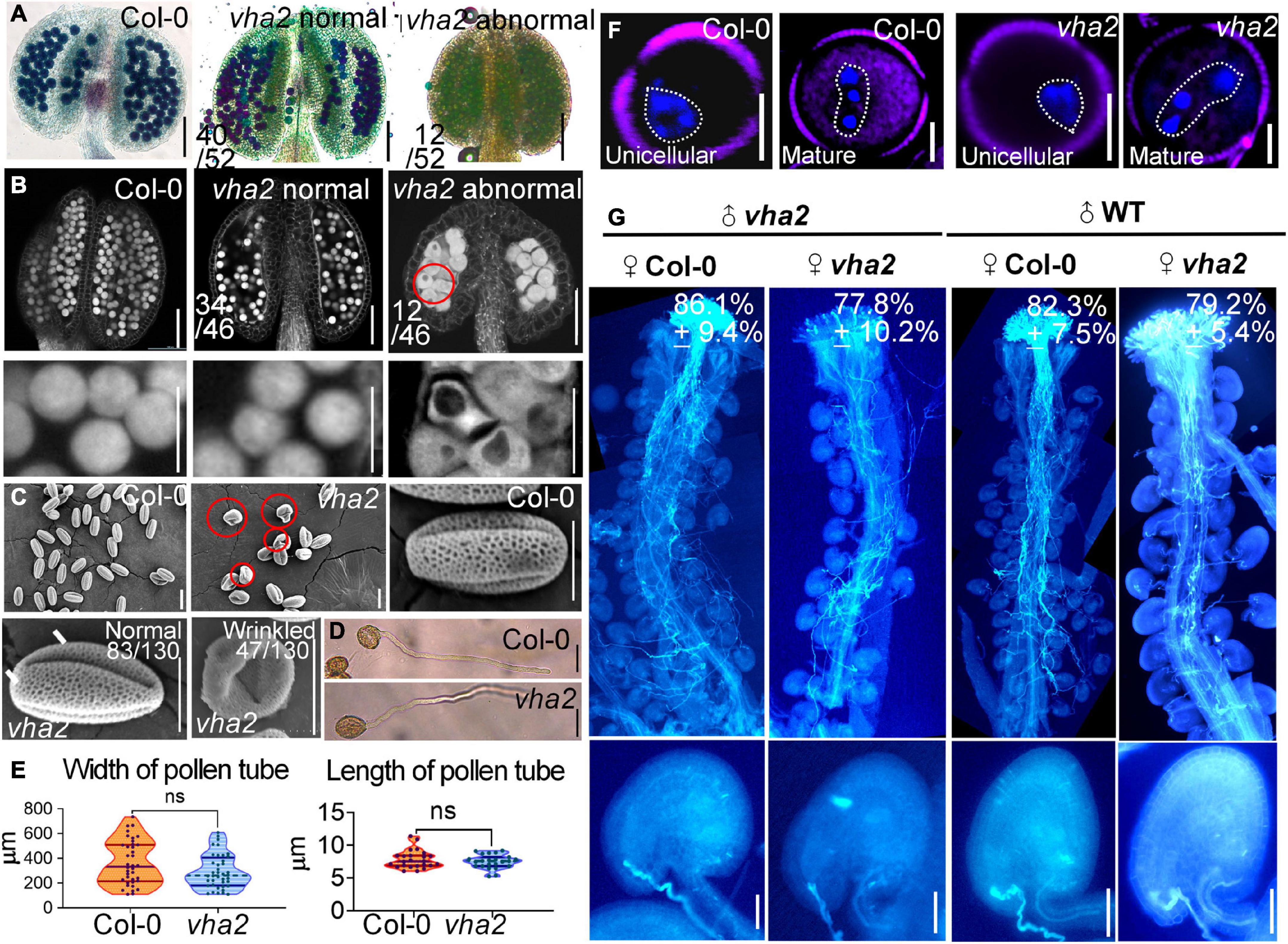
Figure 3. Lack of V-ATPase affects pollen development. (A) Alexander’s staining of pollen from Col-0 and vha2. The purplish red color indicates viable pollen. Bar = 100 μm. (B) Anthers visualized by CLSM. Col-0 and vha2 anthers at stage 12. Statistics on the ratio of normal and abnormal flowers in vha2 are shown in each image. Scale bars represent 100 μm (main image) and 10 μm (partial enlargement). Red circle indicates the pollen grain that should have matured was stagnant at PMI stage with big vacuole. (C) SEMs observation on pollen grains from Col-0 and vha2. The scale bar is marked in each image. Bars = 10 μm. (D) A tetrad of mature pollen of Col-0 and vha2 from in vitro pollen germination. Bars = 50 μm. (E) Quantitative analysis of pollen germination. Results shown are means ± standard errors (SE, n = 3). In total, 30 tetrads were examined for each genotype. (F) DAPI staining of unicellular and mature pollen grains from Col and vha2. The purple part is the outline of pollen grains stained with FM4-64. Bars = 5 μm. (G) Aniline blue staining of Col-0 and vha2 pistils at 24 h after pollination (HAP) with Col-0 and vha2 pollen. The whole pistil was overlaid by three overlapping images with Photoshop (Adobe). Numbers in (G) are quantification of targeted ovules out of total ovules. Results are means ± SD (n = 15). Bars = 20 μm.
When Col-0 and vha2 pistils were pollinated with Col-0 and vha2 pollen separately, most ovules in Col-0 and vha2 pistils were targeted by the pollen tubes examined at 24 HAP (Figure 3G). This result indicated that vha2 has a comparable pollen tube guidance and reception to that of the Col-0, therefore, we focused our study on the development of FGs to further explore the mechanism behind seed abortion in normal vha2 siliques.
Vacuolar H+-ATPase deficiency leads to aberrant nuclear spacing during female gametophyte development and affects endosperm nuclei division rate
To see if there are defects in vha2 FGs, we examined vha2 ovules at different developmental stages using CLSM. There was no detectable morphological difference between WT and vha2 FGs until FG6 stage (Supplementary Figure 4 and Figure 4A). During the transition of late FG6 to early FG7, the distance between central cell and egg cell nuclei in more than half of vha2 embryo sacs was remarkably larger than that of Col-0 (Figures 4A–C). In Arabidopsis, late FG6-to-FG7 transition is an important step for embryo sac cell-fate decision and fertilization preparation. During this period, the pattern of eight nuclei and seven cells emerges, antipodal cells at chalaza end degenerates, and the distance between the central cell and egg cell nuclei is further shortened, preparing FGs for fertilization (Christensen et al., 1997; Sundaresan and Alandete-Saez, 2010).
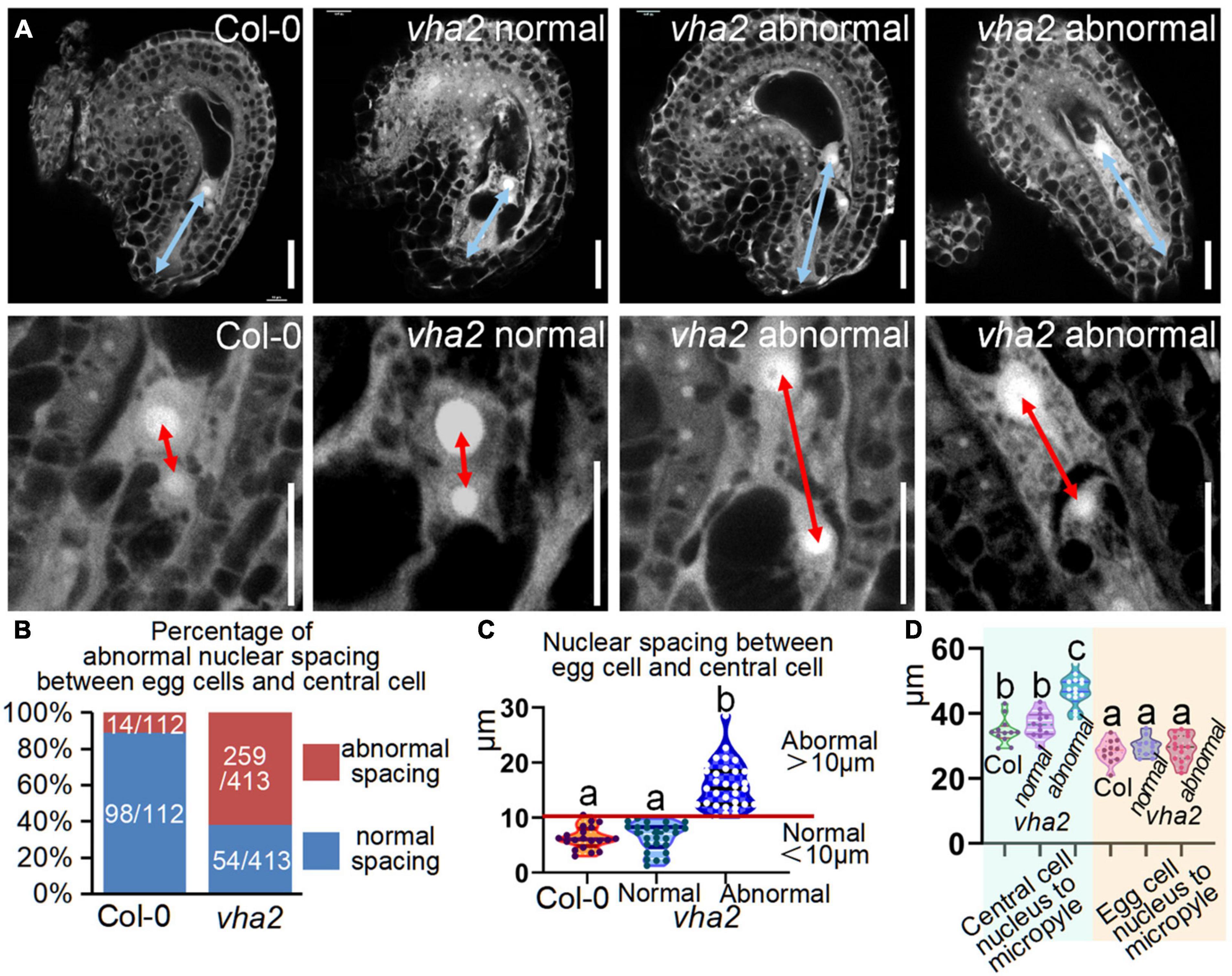
Figure 4. Lack of V-ATPase enlarges nuclear spacing of egg cells and central cells. (A) Embryo sac development of Col-0 and vha2 at late-FG6 stage visualized by fluorescence microscopy. The picture below is an enlarged view of the nuclear spacing between the egg cell and central cell in the above image. The blue double arrows represent the distance from the central cell nucleus to the micropylar end, and the red double arrows in enlarged views represent nuclear spacing between egg cell and central cell. Bars = 20 μm. (B) Statistics analysis of the proportion of abnormal nuclear spacing between egg cell and central cell in Col-0 and vha2. The number of ovules counted and the actual ratio is marked in the image. (C) Statistics analysis of nuclear spacing between egg cell and central cell in Col-0 and vha2. Below the red line is defined as normal spacing if the nuclear spacing between egg cell and central cell is less than 10 μm, and it was considered abnormal spacing if spacing exceeds 10 μm. Lowercase letters indicate statistically significant differences between different stages (P< 0.05). 100 ovules shown in the statistical graph come from 20 different inflorescence apices. (D) Statistics analysis of the distance from the central cell nucleus or egg cell nucleus to the micropylar end in Col and vha2. Lowercase letters indicate statistically significant differences between different stages (P< 0.05). 30 ovules shown in the statistical graph come from 3 different inflorescence apices.
Based on statistics of the distance in Col-0, we arbitrarily designated a distance less than 10 μm as normal spacing, and a distance larger than that as abnormal spacing, for convenient comparison and description. Under this criterion, the proportion of abnormal spacing in vha2 was more than a half, while that in the wild type was about 10% (Figures 4B,C). Nuclear spacing abnormity was clearly associated with the failed movement of the central cell to the proper position at the micropyle end (Figure 4D). This increased nuclear spacing phenotype was also observed in abnormal embryo sacs in fap3 (Supplementary Figure 5). Considering that vha2 exhibited higher premature seed abortion rate compared with Col-0 (Figure 1C) and that the fusion of sperm and diploid central cell during double fertilization is essential for subsequent embryo, endosperm, and even seedling development (Portereiko et al., 2006; Wu et al., 2012), it is reasonable to assume that the distance between the nuclei of central cell and the egg cell that is affected by V-ATPase, may influence the fertilization process as well as the following division of endosperm nuclei.
To formally test this hypothesis, we compared the endosperm development of vha2 to that of Col-0 at 18 HAP using CLSM. At 18 HAP, most egg cells and central cells were already fertilized, and endosperms consisted of four to eight nuclei in Col-0 (Figures 5A,C). In contrast, at the same stage, ∼80% egg cells and central cells remained unfertilized and ∼20% seeds had two endosperm nuclei in vha2. Until at 48 HAP, most vha2 endosperms contained four to eight nuclei (Figures 5B,C), while around 15% (23/158) nuclei of vha2 endosperms did not divide, which was in line with the slightly increased ratio of seed abortion in vha2 normal siliques (Figure 1C).
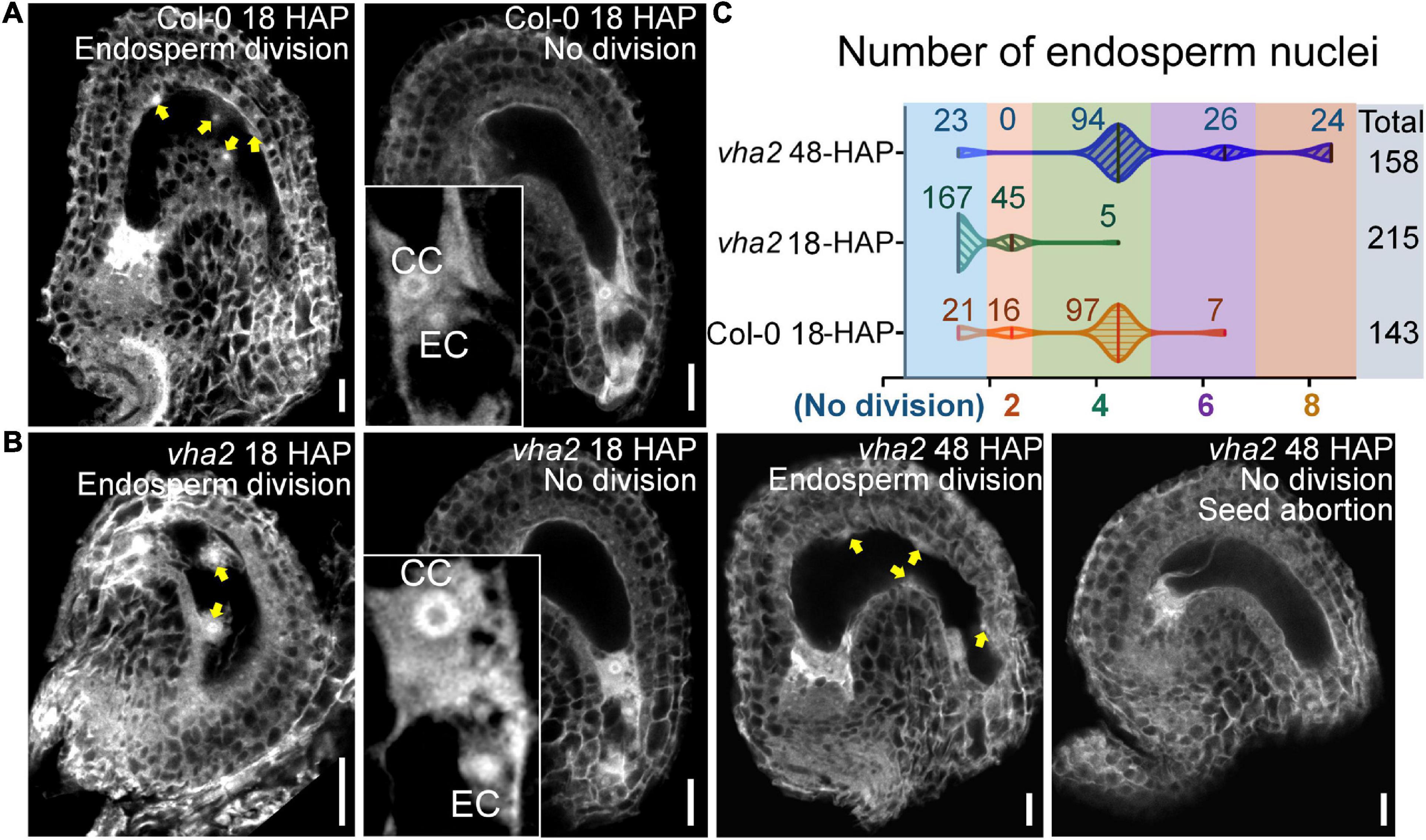
Figure 5. Lack of V-ATPase affects the division rate of endosperm nuclei. (A) Endosperm nuclei of Col-0 seeds at 18 h after pollination (HAP). (B) Endosperm nuclei of vha2 seeds at 18 HAP and 48 HAP. Yellow arrows in (A,B) indicate the endosperm nuclei. EC egg cell, CC central cell, graphs represent typical phenotype of all samples. Bars = 20 μm. (C) Quantitative analysis of endosperm nuclei number in Col-0 and vha2. Seeds shown in the statistical graph come from 5 different inflorescence apices.
The above results indicated that V-ATPase deficiency leads to the increased nuclear spacing between the central and the egg cells in mature FGs, thus slowing down subsequent division of endosperm nuclei. These findings also demonstrated that nuclear spacing in mature FGs is essential for subsequent fertilization and seed development. Given that auxin acts as a positional cue for individual cell specification during FG6-to-FG7 stages (Sun et al., 2021), and that V-ATPase is a modulator of the polar transport and distribution of auxin in Arabidopsis embryos and seedlings (Jiang et al., 2020), we speculated that V-ATPase may affect Arabidopsis FG development via auxin-mediated functions.
Vacuolar H+-ATPase regulates nuclear spacing between the egg and the central cells through PIN-FORMED 1-mediated auxin transport during female gametophyte development
At the late stages of FG development, auxin concentration in the embryo sac is relatively low and auxin transport in the sporophyte region surrounding the embryo sac mainly relies on the PIN1 protein (Luca et al., 2013; Panoli et al., 2015). Since our previous studies found that tonoplast proton pumps activities affect auxin gradient by altering the expression level and polar localization of PIN1 during embryonic development (Jiang et al., 2020), we first quantified the expression level of PIN1 gene as well as other genes associated with auxin biosynthesis and response in FGs, using qRT-PCR. We found that the expression level of PIN gene family members was generally lower in vha2 inflorescence (Figure 6A), especially that of PIN1, so was the expression of genes associated with auxin biosynthesis and signaling (Supplementary Figure 6). This result indicated that the auxin concentrations in vha2 inflorescence is generally low. We then detected fluorescent signals of PIN1-YFP in Col-0 and vha2 ovules and found that, PIN1-YFP signal in vha2 FG4 embryo sacs was indeed significantly weaker than that in Col-0 (Figures 6B,C). This reduced PIN1-YFP signal in vha2 FG4 embryo sacs indicated that the transportation of auxin in ovules is impeded, and that the inferior auxin content in the vha2 ovules might be a signal to affect the movement of the central cell to the proper position and increase the distance between the central cell and the egg cell.
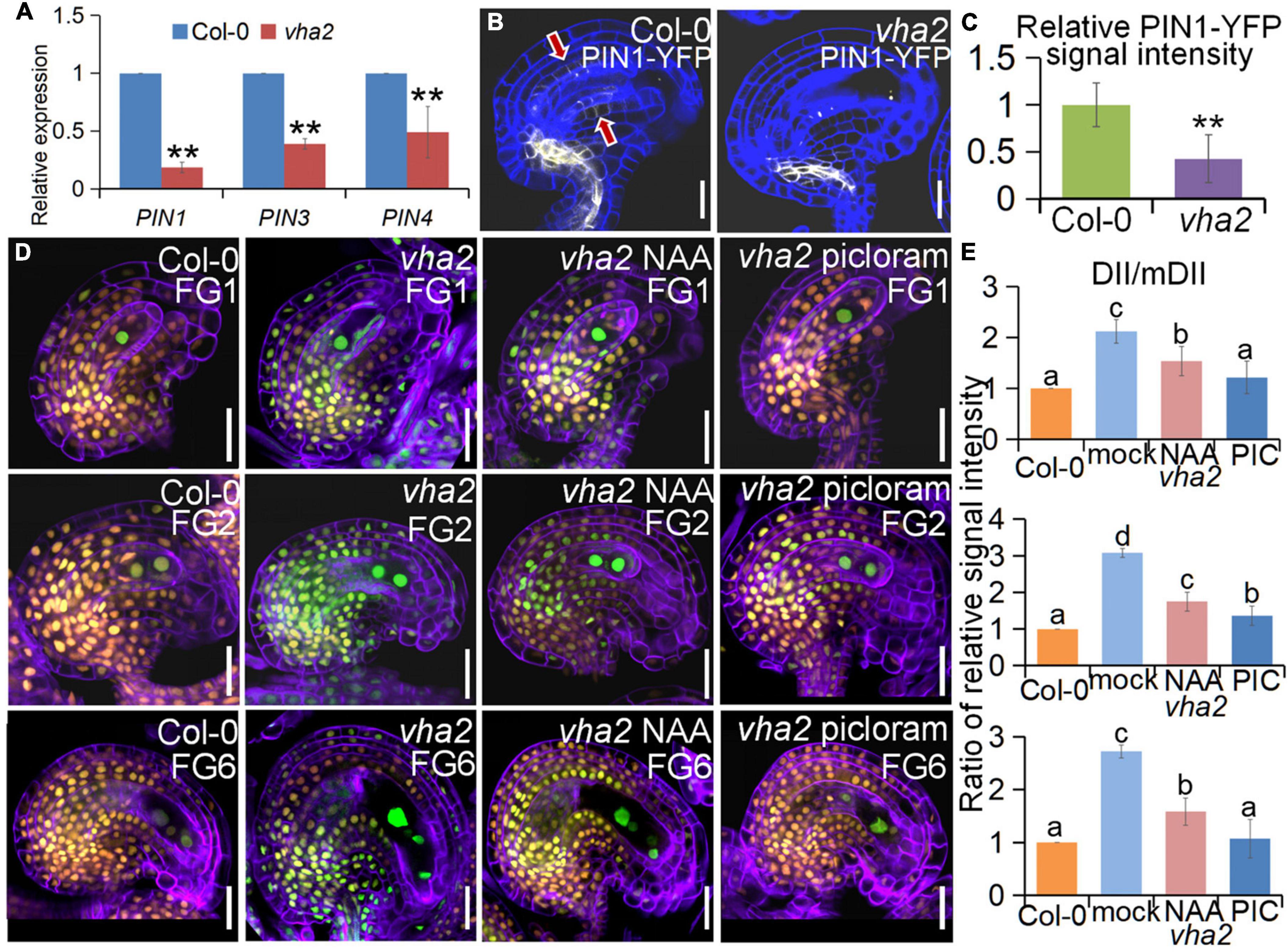
Figure 6. Lack of V-ATPase affects polar auxin transport and auxin distribution. (A) Relative transcript levels of PIN1, PIN3, and PIN4 in inflorescence apices of Col-0 and vha2. Bars correspond to arithmetic means ± SE of three technical replicates of three biological replicates. Asterisks indicate significant difference (Studert’s tw0-tailed t-test: **P < 0.01). (B) PIN1-YFP express ion in Col-0 and vha2 ovules. Bars = 20 μm. Graphs represent typical phenotype of all samples. (C) Quantitative analysis of PIN1-YFP fluorescence level in ovules. Bars correspond to relative YFP signal intensity. Relative fluorescence signal data were extracted from normalized mean gray levels in the above lines. Values correspond to arithmetic means ± SE of three biological replicates (n = 20). Asterisks indicate significant difference (Student’s two-tailed t-test: **P < 0.01). (D) R2D2 expression in Col-0 and vha2 ovules under picloram and NAA treatment during FG development. (orange for mDII-tdTomato and green for DII-Venus). Cell walls were stained with FB28 (purple). Bars = 20 μm. Graphs represent the typical phenotype of all samples. (E) Quantification of fluorescent signals of R2D2 (DII/mDII). Relative fluorescence signal data were extracted from normalized mean gray levels in the above lines. Values correspond to arithmetic means ± SE of three biological replicates (n = 20). Lowercase letters indicate statistically significant differences between different stages (P < 0.05). 10 ovules shown in the statistical graph come from 3 different inflorescence apices.
To further validate our results, we treated Col-0 and vha2 inflorescences in vitro with auxin analogs, picloram and NAA, respectively, and then examined the content of auxin using an auxin-level marker R2D2 (a semiquantitative and rapid auxin-input reporter with DII-Venus and mDII-tdTomato) after treatment, in which the absence of DII fluorescence marks auxin accumulation (Benková et al., 2003; Liao et al., 2015). Transport of picloram need the plasma membrane-bound carrier PIC30, rather than PIN family proteins that NAA transportations relies on. We found that the overall auxin level of vha2 ovules along development is lower than that of Col-0 (Figures 6D,E). Auxin levels in vha2 ovule were slightly and greatly restored by NAA or picloram treatment, respectively (Figures 6D,E). Notably, picloram treatment restored nuclear spacing in vha2 embryo sacs to levels indistinguishable from Col-0 (Figures 7A,B), while NAA treatment hardly rescued the abnormal nuclear spacing albeit that a slight increase in normal proportions was observed (Figures 7A,B). These results indicated that, in vha2, PIN1 alone is not enough to transport sufficient NAA into ovules, resulting in the enlarged nuclear spacing. On the contrary, transportation of picloram does not require the participation of PIN1, so picloram could reach the ovules and to restore the abnormal nuclear distance between the egg cell and central cell.
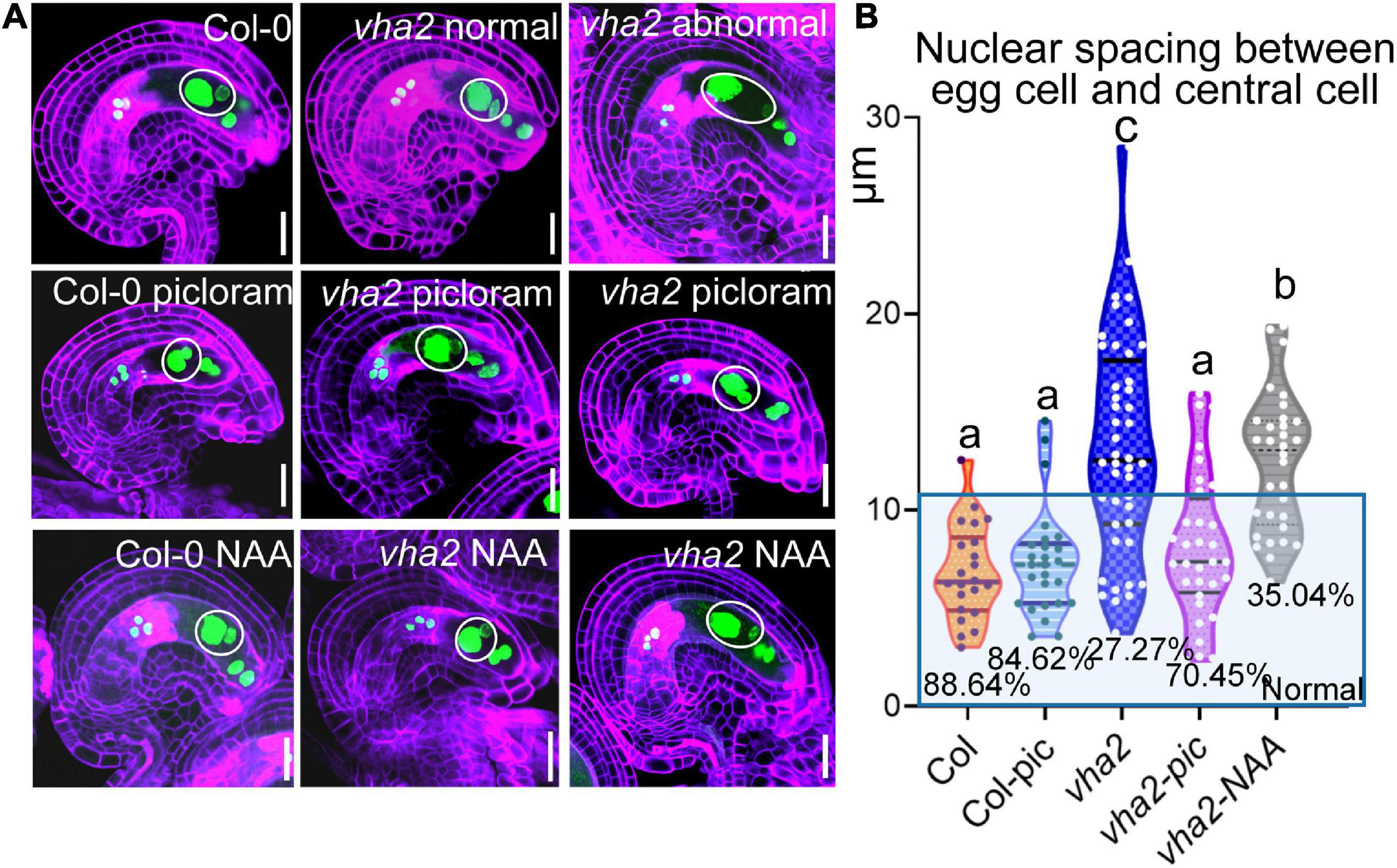
Figure 7. Picloram rescues the enlarged nuclear spacing of egg cells and central cells in vha2 through PIN-independent pathway. (A) Nuclear spacing of Col-0 and vha2 under picloram and NAA treatment at FG6 stage visualized using ProES1:H2B-GFP (green) by fluorescence microscopy. Cell walls were stained with FB28 (purple). Inflorescence apices grew for 2 days following 1 μM picloram or 1 μM NAA treatment for 24 h. In the white circles are nuclear of egg cells and central cells. Graphs represent the typical phenotype of all samples. Bars = 20 μm. (B) Statistics analysis of nuclear spacing between egg cell and central cell in Col and vha2. Dots in the blue box represent normal samples with nuclear spacing of egg cells and central cells less than 10 μm, and the proportions are shown in the chart. Lowercase letters indicate statistically significant differences between different stages (P < 0.05). 40 ovules shown in the statistical graph come from 10 different inflorescence apices.
In conclusion, V-ATPase regulates auxin levels in ovules through coordinating the content and localization of PIN-FORMED 1 (PIN1) protein, hence influencing nuclear spacing between centra cell and egg cell, and subsequent endosperm development (Figure 8).
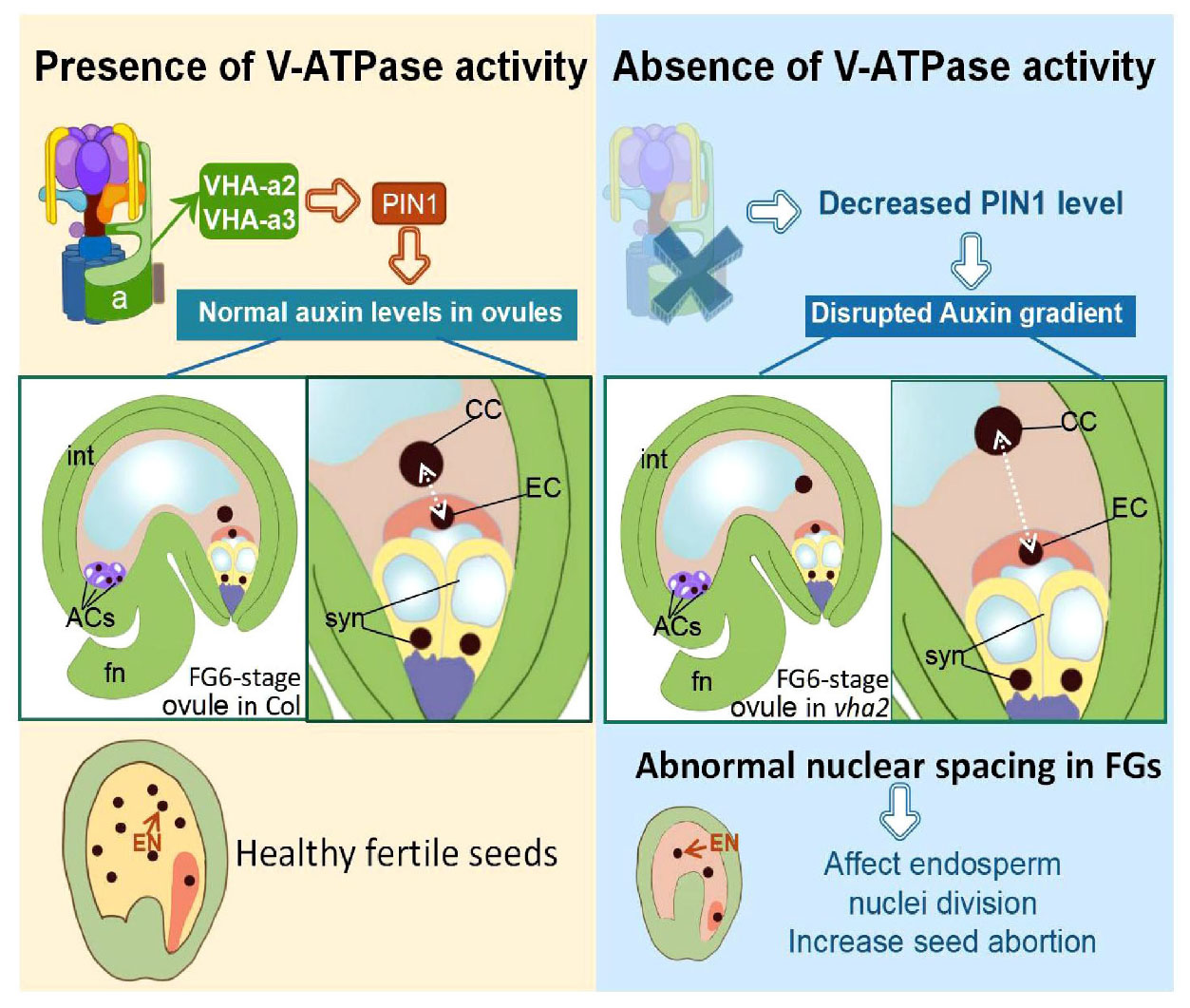
Figure 8. Schematic diagram of the role of V-ATPase on FGs and later endosperm development. Fn, funiculus; syn, synergid cell; EC, egg cell; CC, central cell; ACs, antipodal cells; int, integuments (inner and outer).
Discussion
The life history of higher plants features an alternation between diploid sporophytes and haploid gametophytes. As model organism of angiosperms, Arabidopsis has been widely used to elucidate the underlying mechanisms of gametophyte developmental. In this study, we demonstrated that V-ATPase is essential for male and FG development, stigma patterning and early endosperm cell division. In this process, PIN1-mediated auxin transport is involved in the regulation of nuclear position downstream of the V-ATPase activity during FG development. These findings extend our understanding of the function of the tonoplast proton pump and auxin in the reproductive development of seed plants.
Vacuolar H+-ATPase has multiple regulatory functions in male and female gametophytes and seed development
In this study, the triple mutant fap3 lacking both tonoplast proton pumps was first used as the research material. Nevertheless, its severely impaired growth and uncompleted fertilizations in more than 90% gametophytes (Supplementary Figure 1) demonstrated that the lack of both tonoplast proton pumps severely affects the development of male and FGs, and that fap3 is not suited to study the functions of the two tonoplast proton pumps during gametophyte development at the same time. Therefore, we decided to study two tonoplast proton pumps separately. Since the growth status, together with male and female transmission rate of fugu5-1 mutant was comparable to that of Col-0, our work started to focus on V-ATPase instead of V-PPase.
After mutations of the a2 and a3 subunits that are responsible for tonoplast targeting of V-ATPase, male gametophytes in 26% (12/46) Arabidopsis anthers were arrested in PMI phase with large vacuoles (Figure 3). PMI is accompanied by vacuolar dynamics changes: a large central vacuole can be converted into several smaller vacuoles by convolution or fission (Yoko et al., 2003). It is well-known that endomembrane system proteins, including V-ATPases, are critical for the development of PMI (Whitley et al., 2009; Dettmer et al., 2010; Feng et al., 2017; Zhang et al., 2018). Our previous studies found that V-ATPase regulates the morphology and distribution of vacuoles during embryonic and seedling development, and that the loss of V-ATPase function in vha2 male gametophytes results in the failure of 26% (12/46) large central vacuoles to transform into small ones.
During flower development of vha2, ovules in 17% (19/112) abnormal flowers could develop up to the FG7 stage but could not complete fertilization, because the lack of an active V-ATPase severely affected stigma morphology. The growth of pollen tube was seriously hindered, as most pollen tubes did not grow to the micropylar end to fertilize the ovule (Supplementary Figure 3). Consequently, vha2 finally produced less than ten seeds. From FG development to double fertilization, the egg cell, as a female gamete, fuses with one sperm cell to give rise to the embryo. Meanwhile, the central cell, as the largest cell for nutrient storage, fuses with another sperm cell to form the endosperm. In vha2 normal flowers, loss of V-ATPase resulted in a mis-localization of the central cell, and the subsequent division speed of endosperm cell was affected as well (Figures 4, 5). This data indicates that the correct positioning of the nuclei of FG is essential for the double fertilization, and that V-ATPase plays a pivotal role in regulating the position of the nuclei.
The phytohormone auxin is involved in the regulation of vacuolar H+-ATPase in the coordinated development of plant sporophyte and gametophyte
During ovule development, the spatiotemporal distribution of auxin is essential for coordinated development of sporophyte and gametophyte (Pagnussat et al., 2007; Bencivenga et al., 2011). However, mechanisms underlying the spatial and temporal distribution of auxin remain ill-known. Auxin is a long-distance transported phytohormone, and the gradient around FG relies on the polar auxin transporter PIN1 (Luca et al., 2013; Panoli et al., 2015). The intracellular localization of PIN1 depends on the coordination of the endomembrane trafficking system (Gälweiler et al., 1998; Steinmann et al., 1999; Huang et al., 2010; Dhonukshe et al., 2015). In this study, we demonstrated that tonoplast V-ATPase plays a crucial role in the fine regulation of auxin and its function in FG development in Arabidopsis.
The spatial and temporal distribution of auxin in ovules is under the tight control of PIN1 (Luca et al., 2013; Panoli et al., 2015). Long-term exposure to NPA or BFA treatment disrupts FG development, a phenotype that has been reported to occur in pin1 mutants (Wang et al., 2021). In line with previous findings (Luca et al., 2013; Panoli et al., 2015), observation of the R2D2 auxin marker in Arabidopsis ovules confirmed that DII expression in FG is higher than that in the sporophyte (Figure 6), which implies that the auxin content in Arabidopsis FG is relatively low. Loss of V-ATPase decreased auxin content in ovules throughout the FG developmental stages (Figure 6), and this was mainly attributed to the abnormal localization of PIN1 (Figure 6). This result was also supported by the fact that picloram, but not NAA, was able to restore the position of the central cell nuclei (Figure 7).
Eukaryotic V-ATPase has gained a lot of unconventional cellular functions during evolution, such as the regulation of important intracellular proteins (Cross and Muller, 2004; Marshansky and Futai, 2008). In the current work, the epistatic regulation of V-ATPase exerted on PIN1-mediated auxin transport in ovules during FG development has been illustrated. Collectively, our findings revealed a crucial role of V-ATPase in auxin-related FG development in Arabidopsis and enhanced our understanding of the functions of tonoplast proton pumps in this vital process.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
W-HL designed the study, supervised the project, analyzed the data, organized the results, modified the manuscript, and acquired the funding. Y-TJ and J-XZ performed the experiment, analyzed the data, organized the results, and wrote the manuscript. R-HL and Y-CW helped performed the experiments and conducted association analysis. JS helped modify the manuscript. AF offered the materials and helped organized the results and the manuscript. All authors agreed to be accountable for the content of this manuscript, reviewed, and approved of the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32070342 and 32000222), the National Basic Research Program of China (grant no. 2014CB943404), Shanghai Jiao Tong University JiRLMDS Joint Research Fund (Project MDS-JF-2020-8), the Agri-X Interdisciplinary Fund of Shanghai Jiao Tong University (Agri-X20200204 and Agri-X2017006), the Bio-X Interdisciplinary Fund of Shanghai Jiao Tong University (20CX-04), the Scientific and Technological Innovation Funds of Shanghai Jiao Tong University (19×160020009), Grant-in-Aid for Scientific Research (B) (JP16H04803), and Grant-in-Aid for Scientific Research on Innovative Areas (JP25113002 and JP18H05487).
Acknowledgments
We thank Jian Xu (NUS Centre for BioImaging Sciences) for donating marker lines ProPIN1:PIN1-YFP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1006735/full#supplementary-material
Abbreviations
DAG, days after seed germination; HAP, Hours after pollination; V-PPase, H+-translocating pyrophosphatase; V-ATPase, vacuolar H+-ATPase; PIC, picloram; MMC, megaspore mother cell; FM, functional megaspore.
Footnotes
References
Bencivenga, S., Colombo, L., and Masiero, S. (2011). Cross talk between the sporophyte and the megagametophyte during ovule development. Sex. Plant Reprod. 24, 113–121. doi: 10.1007/s00497-011-0162-3
Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jürgens, G., et al. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. doi: 10.1016/S0092-8674(03)00924-3
Christensen, C. A., King, E. J., Jordan, J. R., and Drews, G. N. (1997). Megagametogenesis inArabidopsiswild type and the Gf mutant. Sex. Plant Reprod. 10, 49–64. doi: 10.1007/s004970050067
Cross, R. L., and Muller, V. (2004). The evolution of A-, F-, and V-type ATP synthases and ATPascs: Reversals in function and changes in the H+/ATP coupling ratio. Febs Lett. 576, 1–4. doi: 10.1016/j.febslet.2004.08.065
Dettmer, J., Schubert, D., Calvo-Weimar, O., Stierhof, Y. D., Schmidt, R., and Schumacher, K. (2010). Essential role of the V-ATPase in male gametophyte development. Plant J. Cell Mol. Biol. 41, 117–124. doi: 10.1111/j.1365-313X.2004.02282.x
Dhonukshe, P., Huang, F., Galvan-Ampudia, C. S., Mahonen, A. P., Kleine-Vehn, J., Xu, J., et al. (2015). Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 142, 2386–2387. doi: 10.1242/dev.127415
Feng, Q. N., Zhang, Y., and Li, S. (2017). Tonoplast targeting of VHA-a3 relies on a Rab5-mediated but Rab7-independent vacuolar trafficking route. J. Integr. Plant Biol. 59:4. doi: 10.1111/jipb.12526
Ferjani, A., Segami, S., Horiguchi, G., Muto, Y., Maeshima, M., and Tsukaya, H. (2011). Keep an eye on PPi: The vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23, 2895–2908. doi: 10.1105/tpc.111.085415
Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., et al. (1998). Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science 282, 2226–2230. doi: 10.1126/science.282.5397.2226
Gomez, M. D., Barro-Trastoy, D., Fuster-Almunia, C., Tornero, P., Alonso, J. M., and Perez-Amador, M. A. (2020). Gibberellin-mediated RGA-LIKE1 degradation regulates embryo sac development in Arabidopsis. J. Exp. Bot. 71, 7059–7072. doi: 10.1093/jxb/eraa395
Hedrich, R., Kurkdjian, A., Guern, J., and Flügge, U. I. (1989). Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 8, 2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x
Huang, F., Zago, M. K., Abas, L., Van Marion, A., Galvan-Ampudia, C. S., and Offringa, R. (2010). Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22, 1129–1142. doi: 10.1105/tpc.109.072678
Jiang, Y. T., Tang, R. J., Zhang, Y. J., Xue, H. W., Ferjani, A., Luan, S., et al. (2020). Two tonoplast proton pumps function in Arabidopsis embryo development. New Phytol. 225, 1606–1617. doi: 10.1111/nph.16231
Johnson-Brousseau, S. A., and McCormick, S. (2004). A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J. 39, 761–775. doi: 10.1111/j.1365-313X.2004.02147.x
Krebs, M., Beyhl, D., Gorlich, E., Al-Rasheid, K. A., Marten, I., Stierhof, Y. D., et al. (2010). Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. U.S.A. 107, 3251–3256. doi: 10.1073/pnas.0913035107
Kriegel, A., Andres, Z., Medzihradszky, A., Kruger, F., Scholl, S., and Delang, S. (2015). Job Sharing in the Endomembrane System: Vacuolar Acidification Requires the Combined Activity of V-ATPase and V-PPase. Plant Cell 27, 3383–3396. doi: 10.1105/tpc.15.00733
Kurihara, D., Mizuta, Y., Sato, Y., and Higashiyama, T. (2015). ClearSee: A rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168–4179. doi: 10.1242/dev.127613
Li, S., Ge, F. R., Xu, M., Zhao, X. Y., Huang, G. Q., Zhou, L. Z., et al. (2013). Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J. 74, 486–497. doi: 10.1111/tpj.12139
Liao, C. Y., Smet, W., Brunoud, G., Yoshida, S., Vernoux, T., and Weijers, D. (2015). Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12, 207–210. doi: 10.1038/nmeth.3279
Luca, C., Simona, M., Dola, S. R., Stefano, B., Irma, R. V., Anicet, D. F., et al. (2013). Maternal Control of PIN1 Is Required for Female Gametophyte Development in Arabidopsis. PLoS One 8:e66148. doi: 10.1371/journal.pone.0066148
Luo, Y., Scholl, S., Doering, A., Zhang, Y., Irani, N. G., Rubbo, S. D., et al. (2015). V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat. Plants 1, 15094–15094. doi: 10.1038/nplants.2015.94
Lupanga, U., Röhrich, R., Askani, J., Hilmer, S., Kiefer, C., Krebs, M., et al. (2020). The Arabidopsis V-ATPase is localized to the TGN/EE via a seed plant-specific motif. eLife 9:e60568. doi: 10.7554/eLife.60568
Maeshima, M. (2000). Vacuolar H+-pyrophosphatase. Biochim. Biophys. Acta Biomembranes 1465, 37–51. doi: 10.1016/S0005-2736(00)00130-9
Marshansky, V., and Futai, M. (2008). The V-type H+-ATPase in vesicular trafficking: Targeting, regulation and function. Curr. Opin Cell Biol. 20, 415–426. doi: 10.1016/j.ceb.2008.03.015
Martinoia, E., Maeshima, M., and Neuhaus, H. E. (2007). Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 58, 83–102. doi: 10.1093/jxb/erl183
Pagnussat, G. C., Alandete-Saez, M., Bowman, J. L., and Sundaresan, V. (2009). Auxin-Dependent Patterning and Gamete Specification in the Arabidopsis Female Gametophyte. Science 324, 1684–1689. doi: 10.1126/science.1167324
Pagnussat, G. C., Yu, H.-J., and Sundaresan, V. (2007). Cell-Fate Switch of Synergid to Egg Cell in Arabidopsis eostre Mutant Embryo Sacs Arises from Misexpression of the BEL1-Like Homeodomain Gene BLH1. Plant Cell 19, 3578–3592. doi: 10.1105/tpc.107.054890
Panoli, A., Martin, M. V., Alandete-Saez, M., Simon, M., and Sundaresan, V. (2015). Auxin Import nd Local Auxin Biosynthesis Are Required for Mitotic Divisions, Cell Expansion and Cell Specification during Female Gametophyte Development in Arabidopsis thaliana. PLoS One 10:e0126164. doi: 10.1371/journal.pone.0126164
Pérez-España, V. H., Sánchez-León, N., and Vielle-Calzada, J.-P. (2011). CYP85A1 is required for the initiation of female gametogenesis in Arabidopsis thaliana. Plant Signaling Behav. 6, 321–326. doi: 10.4161/psb.6.3.13206
Portereiko, M. F., Lloyd, A., Steffen, J. G., Punwani, J. A., Otsuga, D., and Drews, G. N. (2006). AGL80 Is Required for Central Cell and Endosperm Development in Arabidopsis. Plant Cell 18, 1862–1872. doi: 10.1105/tpc.106.040824
Serbes, I. E., Palovaara, J., and Groß-Hardt, R. (2019). “Chapter Fifteen - Development and function of the flowering plant female gametophyte,” in Current Topics in Developmental Biology, ed. U. GROSSNIKLAUS (Cambridge, MA: Academic Press), 401–434. doi: 10.1016/bs.ctdb.2018.11.016
Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C. L., Paris, S., et al. (1999). Coordinated Polar Localization of Auxin Efflux Carrier PIN1 by GNOM ARF GEF. Science 286, 316–318. doi: 10.1126/science.286.5438.316
Sun, Y., Wang, X., Pan, L., Xie, F., Dai, B., Sun, M., et al. (2021). Plant egg cell fate determination depends on its exact position in female gametophyte. Proc. Natl. Acad. Sci. U.S. A. 118:e2017488118. doi: 10.1073/pnas.2017488118
Sundaresan, V., and Alandete-Saez, M. (2010). Pattern formation in miniature: The female gametophyte of flowering plants. Development 137, 179–189. doi: 10.1242/dev.030346
Wang, J., Guo, X., Xiao, Q., Zhu, J., Cheung, A. Y., Yuan, L., et al. (2021). Auxin efflux controls orderly nucellar degeneration and expansion of the female gametophyte in Arabidopsis. New Phytol. 230, 2261–2274. doi: 10.1111/nph.17152
Zhang, W. T., Li, E., Guo, Y. K., Yu, S. X., Wan, Z. Y., Ma, T., et al. (2018). Arabidopsis VAC14 is critical for pollen development through mediating vacuolar organization. Plant Physiol. 177, 1529–1538. doi: 10.1104/pp.18.00495
Whitley, P., Hinz, S., and Doughty, J. (2009). Arabidopsis FAB1/PIKfyve proteins are essential for development of viable pollen. Plant Physiol. 151, 1812–1822. doi: 10.1104/pp.109.146159
Wu, J.-J., Peng, X.-B., Li, W.-W., He, R., Xin, H.-P., and Sun, M.-X. (2012). Mitochondrial GCD1 Dysfunction Reveals Reciprocal Cell-to-Cell Signaling during the Maturation of Arabidopsis Female Gametes. Dev. Cell 23, 1043–1058. doi: 10.1016/j.devcel.2012.09.011
Yang, W. C., Shi, D. Q., and Chen, Y. H. (2010). Female gametophyte development in flowering plants. Annu. Rev. Plant Biol. 61, 89–108. doi: 10.1146/annurev-arplant-042809-112203
Yoko, Y., Mikio, N., Ikuko, H. N., and Tetsuko, N. (2003). Behavior of Vacuoles during Microspore and Pollen Development in Arabidopsis thaliana. Plant Cell Physiol. 44, 1192–1201. doi: 10.1093/pcp/pcg147
Yu, S. X., Zhou, L. W., Hu, L. Q., Jiang, Y. T., Zhang, Y. J., Feng, S. L., et al. (2020). Asynchrony of ovule primordia initiation in Arabidopsis. Development 147:dev196618. doi: 10.1242/dev.196618
Yuan, L., Liu, Z., Song, X., Johnson, C., Yu, X., and Sundaresan, V. (2016). The CKI1 Histidine Kinase Specifies the Female Gametic Precursor of the Endosperm. Dev. Cell 37, 34–46. doi: 10.1016/j.devcel.2016.03.009
Zhang, Y., Zhang, Y. J., Yang, B. J., Yu, X. X., Wang, D., Zu, S. H., et al. (2016). Functional characterization of GmBZL2 (AtBZR1 like gene) reveals the conserved BR signaling regulation in Glycine max. Sci. Rep. 6:31134. doi: 10.1038/srep31134
Keywords: plant vacuole, V-ATPase, female gametophyte, egg cell, central cell, endosperm
Citation: Jiang Y-T, Zheng J-X, Li R-H, Wang Y-C, Shi J, Ferjani A and Lin W-H (2022) Tonoplast proton pumps regulate nuclear spacing of female gametophytes via mediating polar auxin transport in Arabidopsis. Front. Plant Sci. 13:1006735. doi: 10.3389/fpls.2022.1006735
Received: 29 July 2022; Accepted: 26 August 2022;
Published: 13 September 2022.
Edited by:
Binglian Zheng, Fudan University, ChinaReviewed by:
Duarte D. Figueiredo, Max Planck Institute of Molecular Plant Physiology, GermanyCaiji Gao, South China Normal University, China
Copyright © 2022 Jiang, Zheng, Li, Wang, Shi, Ferjani and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Hui Lin, d2hsaW5Ac2p0dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yu-Tong Jiang
Yu-Tong Jiang Ji-Xuan Zheng
Ji-Xuan Zheng Rong-Han Li
Rong-Han Li Yu-Chen Wang
Yu-Chen Wang Jianxin Shi
Jianxin Shi Ali Ferjani
Ali Ferjani Wen-Hui Lin
Wen-Hui Lin