
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 01 December 2021
Sec. Plant Metabolism and Chemodiversity
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.791124
This article is part of the Research TopicThe Back-and-Forth between Metabolism and Photosystem IView all 7 articles
Photosynthesis is the process that harnesses, converts and stores light energy in the form of chemical energy in bonds of organic compounds. Oxygenic photosynthetic organisms (i.e., plants, algae and cyanobacteria) employ an efficient apparatus to split water and transport electrons to high-energy electron acceptors. The photosynthetic system must be finely balanced between energy harvesting and energy utilisation, in order to limit generation of dangerous compounds that can damage the integrity of cells. Insight into how the photosynthetic components are protected, regulated, damaged, and repaired during changing environmental conditions is crucial for improving photosynthetic efficiency in crop species. Photosystem I (PSI) is an integral component of the photosynthetic system located at the juncture between energy-harnessing and energy consumption through metabolism. Although the main site of photoinhibition is the photosystem II (PSII), PSI is also known to be inactivated by photosynthetic energy imbalance, with slower reactivation compared to PSII; however, several outstanding questions remain about the mechanisms of damage and repair, and about the impact of PSI photoinhibition on signalling and metabolism. In this review, we address the knowns and unknowns about PSI activity, inhibition, protection, and repair in plants. We also discuss the role of PSI in retrograde signalling pathways and highlight putative signals triggered by the functional status of the PSI pool.
Photosynthesis, the primary source of oxygen and organic compounds, is vital for life on Earth. Photosynthetic activity in plants is intrinsically associated with productivity and yield (Raines, 2011) through allocation of assimilated carbon and biomass accumulation. Therefore, efficient photosynthesis is essential to the problem of boosting crop growth and productivity that is required to match increasing food and fuel demands by the growing global population (Fischer and Edmeades, 2010; Ray et al., 2012; Long et al., 2015; Simkin et al., 2017). Accumulating evidence supports an increase in photosynthetic capacity as a viable route to increase the yield of crop plants (Long et al., 2015; Kromdijk et al., 2016; von Caemmerer and Furbank, 2016; Simkin et al., 2017; Salesse-Smith et al., 2018).
Although the study of photosynthesis is a pillar of the plant sciences, many questions remain concerning its regulation, and how photosynthetic activity influences other processes within the cell and throughout the organism. Unlike decades of extensive research on the damage and repair of photosystem II (PSII) (e.g., Aro et al., 1993; Zavafer and Mancilla, 2021), outstanding questions relating to damage and repair of PSI, the other light-harnessing reaction centre protein complex of the thylakoid membrane, have been less well-studied. Beside PSI protection and inactivation, the impact of PSI inactivation on chloroplast metabolism and retrograde signalling have remained poorly understood. However, absorbance measurements of P700, the special chlorophylls at the PSI reaction centre, are now commonly used to assess PSI quantum yield and electron transport reactions involving PSI (Klughammer and Schreiber, 2008, 2016; Schreiber and Klughammer, 2016), which has improved the understanding of factors regulating PSI activity and/or inactivation.
Here we review the current knowns and unknowns about PSI activity, inhibition, protection, and repair in plants. We also discuss the role of PSI in retrograde signalling pathways and highlight putative signals triggered by the functional status of the PSI pool. Considering the importance of understanding PSI metabolism and regulation, new directions for PSI research are suggested.
In general, photosynthesis converts light energy into chemical energy, which is stored as carbohydrate molecules synthesised from carbon dioxide (CO2) and water. In plants, photosynthesis is often separated into two distinct processes; photochemistry and CO2 assimilation/fixation, although these steps are inter-related. During photochemistry, chlorophyll and other photosynthetic pigments absorb light energy that is used to extract electrons from water in the lumen and transport them through the thylakoid membrane to reduce the oxidised form of nicotinamide adenine dinucleotide phosphate (NAD+), producing its reduced form (NADPH) in the stroma. This process also generates a proton gradient across the thylakoid membrane that produces the energy carrier molecule adenosine triphosphate (ATP). During CO2 assimilation, ATP and NADPH generated from the photochemical phase are used to reduce CO2 molecules to produce carbohydrates and their derivative products. These processes are shown in Figure 1 and its animated version in the Supplementary Material (Supplementary Video 1).
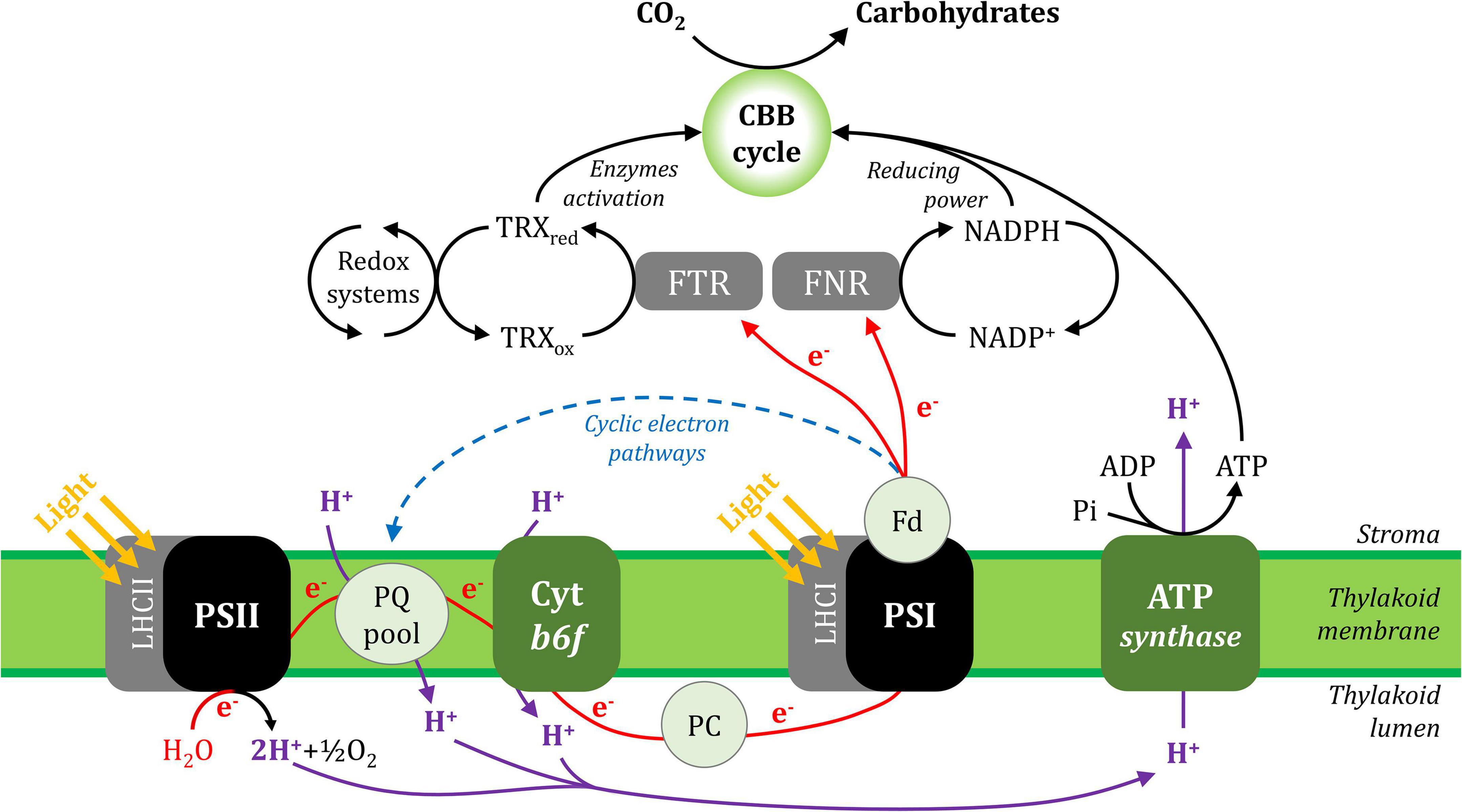
Figure 1. A simplified scheme of the photosynthetic electron transport chain in the thylakoid membrane and its interaction with CO2 assimilation in the Calvin-Benson-Bassham cycle. Linear electron (e–) transport is shown with red lines and cyclic electron transport is represented with a blue dashed line. The proton (H+) fluxes are represented in purple lines. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CBB cycle, Calvin-Benson-Bassham cycle; Cyt b6f, cytochrome b6f; Fd, ferredoxin; FNR, ferredoxin:NADP+ oxidoreductase; FTR, ferredoxin:thioredoxin reductase; LHCI, light-harvesting complex I; LHCII, light-harvesting complex II; NADP+, oxidised nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PC, plastocyanin; Pi, inorganic phosphate; PQ, plastoquinone; PSI, photosystem I; PSII, photosystem II; TRXox, oxidised thioredoxin; TRXred, reduced thioredoxin.
Linear electron flux begins with water-splitting at PSII and proceeds through sequential reduction and oxidation of cofactors within the thylakoid membrane (plastoquinone; PQ), the cytochrome b6f complex (cyt b6f), and the thylakoid lumen (plastocyanin; PC), before arriving at the donor side of PSI. Electron transport upstream of PSI will not be detailed here, but has been described in excellent reviews (Freeman and Guss, 2011; Borisova-Mubarakshina et al., 2019; Havaux, 2020; Malone et al., 2021; Sarewicz et al., 2021; Shevela et al., 2021). In the light, the PSI reaction centre receives excitation from both light-harvesting complex I (LHCI), which serves only PSI, and light-harvesting complex II (LHCII), which serves both PSI and PSII (Grieco et al., 2012, 2015; Wientjes et al., 2013; Rantala and Tikkanen, 2018). Excitation promotes PSI charge separation, whereby an electron is ejected from P700 via the monomeric form of chlorophyll a named A0 and phylloquinone A1 to the first iron-sulphur (FeS) cluster FX. Cofactors P700, A0, A1, and FX are bound to the PSI protein subunits PsaA and/or PsaB, which form the central protein heterodimer of PSI and bind the majority of the other subunits of the complex (Figure 2; Golbeck, 1992; Ben-Shem et al., 2003; Amunts et al., 2007; Amunts and Nelson, 2009; Qin et al., 2015; Kozuleva and Ivanov, 2016; Mazor et al., 2017). The electron hole formed by charge separation at P700 is filled from the PSI donor side by oxidation of reduced PC (detailed in Caspy et al., 2021). Electron flux through PSI terminates at the FA and FB clusters housed by the stromal PSI subcomplex PsaC, PsaD, and PsaE at the PSI acceptor side, where there also resides a docking site for oxidised ferredoxin (Fd). PsaC establishes close contact required for fast electron transfer between the respective FeS clusters of PSI and Fd, while PsaD and PsaE are responsible for guidance of Fd into the PSI binding pocket (Busch and Hippler, 2011; Marco et al., 2018; Caspy et al., 2020). Fd reduced by PSI primarily carries electrons to the Fd-NADP+-oxidoreductase (FNR) enzyme, which is responsible for producing reduced NADPH that powers the electron-consuming reactions of the chloroplast (reviewed in Hanke and Mulo, 2013). Fd also delivers electrons to the thioredoxin network of the chloroplast, which regulates the redox-dependent activity of CO2 assimilation enzymes of the Calvin-Benson-Bassham (CBB) cycle (Buchanan, 2016; Nikkanen et al., 2016). Under specific conditions, reduced Fd also injects electrons back into the PQ pool via cyclic electron transport (reviewed in Peltier et al., 2016).
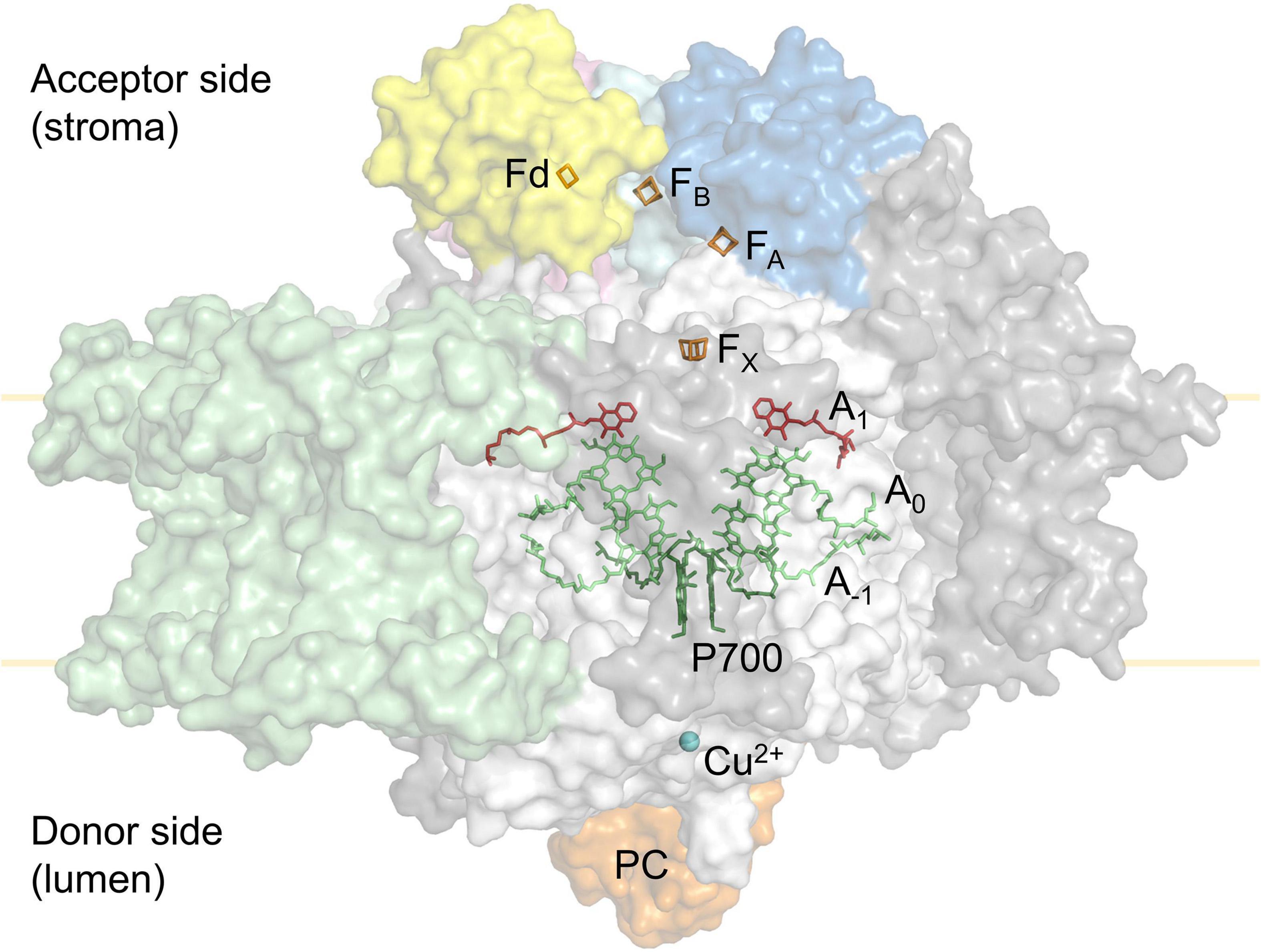
Figure 2. Simplified tertiary structure of the PSI:plastocyanin:ferredoxin complex (PDB accession 6YEZ; Caspy et al., 2020), showing protein subunits PsaA and PsaB (white), PsaC (cyan), PsaD (blue), PsaE (pink) and LHCI subunits (green). Other PSI subunits are coloured grey. Also shown are plastocyanin (orange) at the PSI donor side and ferredoxin (yellow) at the PSI acceptor side. Cofactors involved in electron transport are shown; Cu2+ (blue), P700 (dark green), A–1 and A0 chlorophylls (lime), A1 phylloquinone (red) in PSI, as well as the PSI 4Fe4S clusters FX, FA, FB (orange) and the ferredoxin (Fd) 2Fe2S cluster (orange).
ATP and NADPH molecules synthesised by photochemistry are used to reduce CO2 into sugar precursors through the CBB cycle, where ribulose-1,5-bisphosphate (RuBP) carboxylation is catalysed by RuBisCO and the resulting 3-phosphoglycerate is reduced to glyceraldehyde-3-phosphate (G3P) that is mostly used to regenerate the RuBP used in the CBB cycle. A portion of G3P also serves as a precursor for the synthesis of carbohydrates with myriad functions, including simple sugars (e.g., glucose and fructose), stored energy (e.g., starch), transported energy (e.g., sucrose), structural carbohydrates (e.g., cellulose), amino acids, fatty acids and many other compounds (Paul and Foyer, 2001; Kölling et al., 2015; Wingler, 2018). For each molecule of G3P, three molecules of CO2 are assimilated, while nine ATP and six NADPH are consumed during each round of the cycle (Benson et al., 1950; Raines, 2003). CO2 assimilation in the chloroplast is dependent on the entry and diffusion of CO2 from the atmosphere. Leaf pores known as stomates regulate CO2 uptake through changes in stomatal resistance and aperture, and are therefore a major limiting factor for CO2 assimilation and plant growth (Lawson and Blatt, 2014; Wang et al., 2014). Stomatal activity responds to changes in light and relative humidity, and is regulated by several coordinated and dynamic signalling mechanisms (Daloso et al., 2017; Devireddy et al., 2018). After entering through stomata, CO2 molecules concentrate in the intercellular air space and then pass across the cell wall, plasmalemma, cytosol, and chloroplast envelope before reaching the chloroplast stroma, where they are available to the CBB cycle (Evans and von Caemmerer, 1996; Evans et al., 2009; Tan et al., 2021).
Although light energy is vital for photosynthetic electron transport, the same energy can damage the photosynthetic machinery when excitation/electron pressure in the photosystem exceeds the capacity of electron consumption by chloroplast sinks. As a result, excitation or electrons are transferred to O2, generating reactive oxygen species (ROS) that can oxidise proteins, lipids and metabolites, and can also generate signalling compounds (discussed below). These photo-oxidative conditions are usually triggered by changes in environmental conditions and can lead to a phenomenon known as “photoinhibition,” which is characterised as the inactivation of either or both photosystem(s) (Powles, 1984; Aro et al., 1993; Gururani et al., 2015). Photoinhibition negatively affects photosynthetic capacity and is thus deleterious for plant growth and crop yield (Takahashi and Murata, 2008; Kato et al., 2012; Simkin et al., 2017). Unlike PSII, which is frequently damaged in the light (Tyystjärvi and Aro, 1996), PSI is protected from photoinhibition by several mechanisms (see below). Nonetheless, PSI photoinhibition is induced when PSI is over-reduced, relative to the oxidised state of conventional stromal acceptors, whereupon O2 is utilised as an alternative electron acceptor. O2 reduction is thought to occur at the PSI acceptor side and/or at the phylloquinone A1 site, in each case producing the radical superoxide (O2•‒) that is disproportionated to hydrogen peroxide (H2O2) and O2 (Mehler, 1951; Asada et al., 1974; Takagi et al., 2016; Kozuleva et al., 2021). PSI photoinhibition is thought to be the result of the reaction between H2O2 and the FeS clusters, causing formation of hydroxyl radical (•OH) and inactivation of PSI electron transport (Sonoike et al., 1997; reviewed in Sonoike, 2011). Damage to protein subunits by O2•‒ and singlet oxygen (1O2) produced by excitation of O2 by triplet P700 (3P700) has also been associated with PSI inhibition (Takagi et al., 2016). Notably, the mechanism(s) of ROS production and PSI photoinactivation is not yet fully established.
PSI photoinhibition can be triggered by the combination of light and environmental stresses, such as low temperature, drought and salinity, all of which limit CO2 assimilation (Inoue et al., 1986; Terashima et al., 1994; Tjus et al., 1998; Munekage et al., 2008; Takahashi and Murata, 2008). PSI is also susceptible to photoinhibition when the PSI acceptor side capacity is overwhelmed by unregulated electron flow (Munekage et al., 2002; Suorsa et al., 2012; Tiwari et al., 2016; Kanazawa et al., 2017; Lima-Melo et al., 2019a,b) or by various regimes of artificial fluctuating light (Sejima et al., 2014; Kono and Terashima, 2016; Tikkanen and Grebe, 2018). A recent study showed that PSI photoinhibition is intensified in red and blue light, which preferentially excite PSII, when compared with white and green light (Oguchi et al., 2021). In other words, PSI is at risk of inhibition when chloroplast sink capacity is overwhelmed by photosynthetic electron transport activity.
Some studies have demonstrated the negative effects of PSI photoinhibition on CO2 fixation, and sugar and starch accumulation, which is attributed to decreased electron transport by a partly inactive PSI pool, and a subsequent decrease in reduced NADPH to power the CBB cycle (Zivcak et al., 2015; Gollan et al., 2017; Lima-Melo et al., 2019a,b). Time-resolved measurements of CO2 assimilation and photosynthetic electron transport during the onset and proceeding stages of PSI photoinhibition showed an initial rapid decrease in PSI oxidation and CBB activity, followed by slower rates of decline (Lima-Melo et al., 2019a). These results indicate that the level of PSI inactivation is proportional to the magnitude of energy imbalance between the donor and acceptor sides. Such imbalance decreases in the course of photoinhibition of PSI electron transport, which in turn results in a corresponding decline in the rate of PSI photoinhibition (Figure 3). The negative impact of PSI photoinhibition on CBB activity is particularly acute under low or “growth” light intensities, which are insufficient to fully energise the remaining active PSI centres in order to power stromal reactions (Gollan et al., 2017; Lima-Melo et al., 2019a,b). PSI photoinhibition is especially deleterious to plant fitness due to the fact that the restoration of PSI activity can take a period of days or longer, which is much slower than the mere minutes or hours taken to repair damaged PSII (Kudoh and Sonoike, 2002; Li et al., 2004; Huang et al., 2010; Lima-Melo et al., 2019b). This discrepancy can be explained by the dedicated and efficient PSII repair cycle (reviewed in Aro et al., 1993), while no such repair system for PSI has been identified. Replacement of damaged PSI reaction centre proteins or FeS clusters is widely thought to involve degradation and rebuilding of the entire PSI complex (Scheller and Haldrup, 2005). Nevertheless, PSI recovery appears to be more complex, revealed by employing different methods for evaluating the PSI activity. Decreased abundance of PSI subunit proteins, especially the core proteins PsaA and PsaB and their proteolytic fragments, has been used to demonstrate long-lasting PSI inhibition over several days (Kudoh and Sonoike, 2002; Zhang and Scheller, 2004; Lima-Melo et al., 2019b), while oxidation of P700 and FeS clusters by electron transport appears to recover slightly more quickly (Li et al., 2004; Zhang et al., 2011; Gollan et al., 2017; Lima-Melo et al., 2019b). Rates of CO2 assimilation also reflect PSI activity, which is required to provide both reduced NADPH and the proton motive force (PMF) that drives ATP production. Gas exchange measurements showed a more rapid recovery of CO2 assimilation after PSI photoinhibition compared to the recovery of PSI activity estimated with analysis of P700 absorbance (Lima-Melo et al., 2019b). Also, the measurement of CO2 assimilation revealed that higher light intensity further enhanced the activity of the partly-inactive PSI pool caused by PSI photoinhibition (Gollan et al., 2017; Lima-Melo et al., 2019a,b). These results indicate that electron consumption in the chloroplast may be partly independent from PSI activity and the P700 redox state, and that PSI activity can be enhanced by LHCII-derived excitation and/or activation of “reserve” PSI complexes (Lima-Melo et al., 2019b). Meanwhile, thermal dissipation of excitation energy from LHCII via oxidised P700+ in photoinhibited PSI centres (Tiwari et al., 2016; Shimakawa and Miyake, 2019) has prompted the suggestion that photoinhibited PSI does not require replacement at all (Li L. et al., 2018).
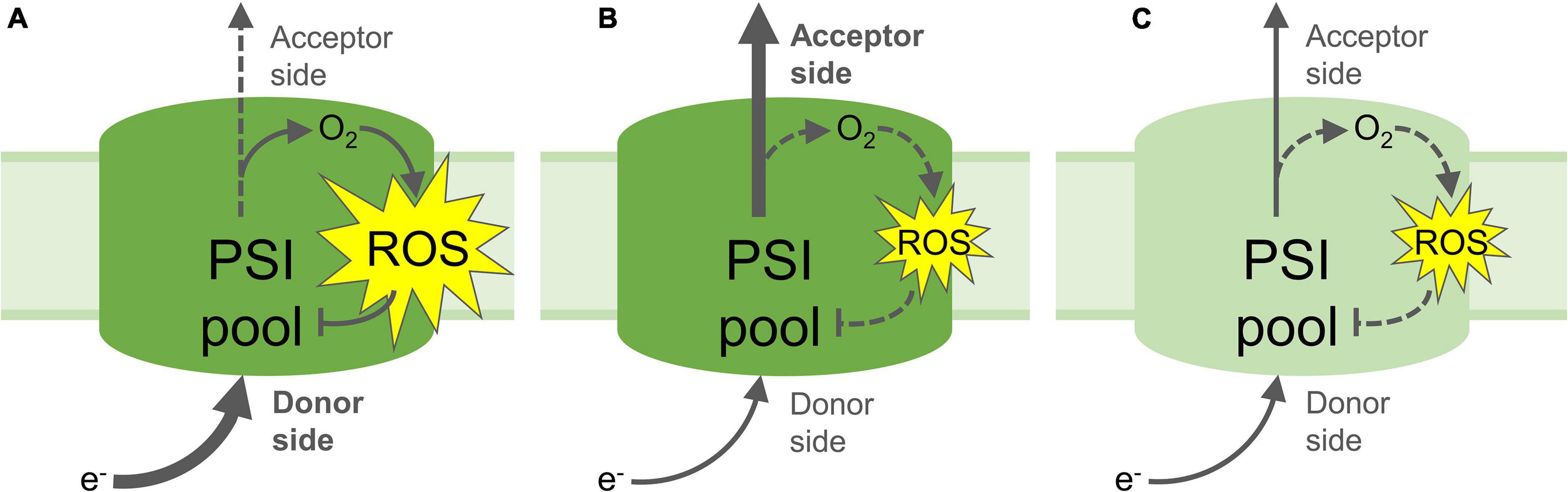
Figure 3. Proposed mechanisms of PSI photoinhibition and photoprotection. (A) Increased electron flow to the PSI donor side and/or decreased flow from the acceptor side lead to reduction of O2 and formation of reactive oxygen species (ROS), which result in inactivation of part of the PSI pool; (B) Non-photochemical quenching, photosynthetic control and/or PSII inactivation down-regulate the flow of electrons to the PSI donor side, limiting the formation of ROS and protecting PSI from photoinhibition. Upregulated electron acceptor capacity in the stroma through increased CO2 fixation or photorespiration can also increase flow of electrons from PSI, which may also prevent ROS formation and protect PSI; (C) Partial inactivation of the PSI pool by PSI photoinhibition down-regulates transport of electrons to the acceptor side, limiting further PSI photoinhibition and down-regulating electron-consuming reactions in the stroma, including ROS formation and signalling. Yellow stars represent ROS production; Arrows represent electron flow, heavy arrows represent high rate of electron flow, dashed arrows represent low rates of electron flow. Pale green PSI pool (C) denotes partial inactivation of the PSI pool through ROS-induced FeS cluster and/or protein damage.
As stated in the previous section, the level of PSI inactivation is proportional to the magnitude of energy imbalance between the donor and acceptor sides, and accordingly distinct mechanisms for PSI photoinhibition avoidance are present at both sides (reviewed in Shimakawa and Miyake, 2018). Mechanisms for protection against PSI photoinhibition at the PSI donor side include inactivation of the PSII reaction centre, dissipation of absorbed light energy as heat and restriction of electron flow through cyt b6f (reviewed in Tikkanen and Aro, 2014). Each of these photoprotective strategies down-regulates the flow of electrons to the PSI donor side, reducing electron pressure on the PSI acceptor side and minimising O2 reduction. Over-supply of energy to PSII results in the generation of 1O2 in the PSII reaction centre, which damages the core D1 protein and suspends PSII activity while D1 is replaced (reviewed in Aro et al., 1993; Nixon et al., 2010). This light-induced inactivation of PSII not only relieves the excitation pressure on the remaining PSII complex, but also protects PSI from over-reduction (Tikkanen et al., 2014; Huang et al., 2016). Dissipation of excess excitation from LHCII, known as non-photochemical quenching (NPQ), involves protonation of the PsbS protein and activation of the xanthophyll cycle, both of which are triggered by acidification of the thylakoid lumen (reviewed in Jahns and Holzwarth, 2012; Ruban, 2016). Although NPQ is most often related to PSII photoprotection, NPQ also protects PSI, both directly through quenching part of the LHCII antenna pool functionally associated with PSI (Tikkanen and Grebe, 2018; Hepworth et al., 2021) and indirectly, through down-regulation of PSII activity and relief of electron pressure on the downstream photosynthetic electron transport chain (Han et al., 2010; Sonoike, 2011; Chaux et al., 2015). Aside from activating NPQ, acidification of the thylakoid lumen and subsequent formation of a pH gradient (ΔpH) across the thylakoid membrane also slows proton-coupled electron transport through the cyt b6f complex (reviewed in Tikhonov, 2014). This regulation mechanism, known as “photosynthetic control,” is arguably the most important form of protection against over-reduction of both donor and acceptor sides of PSI during sudden increases in light. Plants lacking functional proton gradient regulation 5 (PGR5) protein, which is essential for lumen protonation and thus induction of both NPQ and photosynthetic control, undergo severe PSI inhibition during increases in light intensity (Munekage et al., 2002; Suorsa et al., 2012; Kono et al., 2014; Tiwari et al., 2016; Gollan et al., 2017; Takagi and Miyake, 2018; Lima-Melo et al., 2019a,b). In the pgr5 mutant, high light-induced PSI photoinhibition is not caused by missing NPQ (Tikkanen et al., 2015; Gollan et al., 2017), demonstrating the importance of photosynthetic control specifically in rapid induction of PSI protection under changing environmental conditions (Rantala et al., 2020).
As PSI photoprotection relies on sufficient acceptor side capacity, improved rates of electron channelling toward strong stromal sinks can alleviate or guard against PSI photoinhibition (Padmasree et al., 2002; Alric and Johnson, 2017; Wada et al., 2018; Yamamoto and Shikanai, 2019). This has been clearly demonstrated through the study of flavodiiron (FLV) proteins, which oxidise electron carriers down-stream of PSI in cyanobacteria, algae, lower-order land plants and gymnosperms, but have been lost from angiosperms (Zhang et al., 2009; Gerotto et al., 2016; Ilík et al., 2017). The introduction of FLV proteins into angiosperm chloroplasts has clearly highlighted the value of stromal sink strength in protecting against PSI over-reduction, which appears to lie at least partly in enhanced electron transport and subsequent lumen protonation, triggering induction of NPQ and photosynthetic control (Wada et al., 2018; Yamamoto and Shikanai, 2019). A major natural electron sink in the chloroplast is the reduction of CO2 into sugars through CBB cycle activity. Indeed, a protective effect of elevated CO2 concentration against PSI photoinhibition during fluctuating light was recently observed, but was reported to be independent from mechanisms induced by thylakoid ΔpH (Tan et al., 2021). On the other hand, PSI oxidation by photorespiration, which is another major chloroplast electron sink that involves oxygenation rather than carboxylation of RuBP, decreases electron pressure at the PSI donor side by oxidising the electron transport chain (Huang et al., 2015; Osei-Bonsu et al., 2021) and by inducing lumen acidification and photoprotection (Furutani et al., 2020; Wada et al., 2020). Interestingly, weakening of chloroplast sinks in higher plants through down-regulation of CO2 fixation has been shown to induce photoprotection mechanisms that minimise PSI photoinhibition (Kohzuma et al., 2009; Joliot and Alric, 2013; Li Y.T. et al., 2018; Wada et al., 2019). Together, these results suggest existence of a regulatory link between CO2 assimilation and photosynthetic electron transport that protects PSI from over-reduction, although further research is required to determine how this feedback affects the donor and acceptor sides of PSI.
The prospective role of O2 as an electron sink for photoprotection through ROS production has been long speculated, and remains controversial (Ort and Baker, 2002; Miyake, 2010; Driever and Baker, 2011; Ivanov et al., 2012; Cai et al., 2017; Huang et al., 2019, 2021). Although considered not to be a major route for electron flow in leaves (Driever and Baker, 2011), O2 reduction by PSI through the so-called water-water cycle (WWC) has been shown to be a genuine mechanism of PSI oxidation (Asada, 2000; Ort and Baker, 2002; Miyake, 2010; Huang et al., 2019). The WWC relies on enzymatic dismutation of O2•‒ formed by PSI (Mehler, 1951), followed by detoxification of the resulting H2O2 in the chloroplast by ascorbate peroxidase (APX) using ascorbate as an electron donor. Ascorbate is regenerated through oxidation of glutathione (GSH), ultimately drawing on the reducing power of NADPH (Foyer and Halliwell, 1976; Foyer and Shigeoka, 2011). Protection from photoinhibition by the WWC has been described to occur through ROS scavenging and electron sink activities (Asada, 1999, 2000; Miyake, 2010), although it appears that the WWC plays only a minor role in PSI protection, depending on the plant species, sample type and the stress conditions studied (Driever and Baker, 2011; Huang et al., 2021). However, the proposed interaction between PSI and the chloroplast antioxidant network, wherein reducing power from PSI both produces ROS and drives ROS scavenging (in which the WWC has an important role), implicates PSI in chloroplast signalling, as discussed below.
As described above, PSI is a major site of O2 reduction and ROS formation in the chloroplast, which not only induces PSI photoinhibition, but also promotes ROS-dependent chloroplast retrograde signalling (reviewed in Gollan et al., 2015; Mullineaux et al., 2018). In particular, the relatively long-lived ROS H2O2 can move to the nucleus and instigate gene expression through modifying redox-sensitive transcription factors (Exposito-Rodriguez et al., 2017), but can also regulate transcription indirectly by reacting with protein thiol groups or changing the redox state of the antioxidant network (Chan et al., 2016; König et al., 2018; Noctor et al., 2018). Transcriptional reprogramming by H2O2 signalling is a vital component of both abiotic and biotic stress responses (Vanderauwera et al., 2005; Maruta et al., 2012; Sewelam et al., 2014; Dietz et al., 2016; Smirnoff and Arnaud, 2019; Gollan and Aro, 2020). Relatively low expression of many abiotic stress-responsive genes, which are classical markers for H2O2 signalling, was observed after PSI photoinhibition (Gollan et al., 2017), indicating a negative impact of PSI photoinhibition on H2O2 signalling. This was taken to indicate lower levels of O2•‒ and H2O2 in the chloroplast, suggesting that O2 reduction is decreased by down-regulated PSI electron transport in a similar way to the decline in CO2 assimilation, as discussed above. However, the abundance of O2•‒ and H2O2, along with the activity and expression of ROS-scavenging enzymes, was equivalent in control and PSI photoinhibited leaves after 1 hour of high light stress (Lima-Melo et al., 2019a). This finding indicates that chloroplast H2O2 deficiency takes place during earlier stages after PSI photoinhibition, or that deficient H2O2 signalling may have occurred indirectly, such as by decreased photorespiration and subsequently lower H2O2 production in the peroxisome (Vandenabeele et al., 2004; Sewelam et al., 2014).
In addition to H2O2 signalling, PSI photoinhibition was also found to suppress production of the oxylipin hormone 12-oxo-phytodienoic acid (OPDA) and down-regulate expression of oxylipin-responsive genes, as well as decreasing the level of lipid peroxidation, during high light stress (Gollan et al., 2017; Lima-Melo et al., 2019a). Oxylipins are products of lipid oxidation, which can occur either enzymatically by lipoxygenase (LOX), or non-enzymatically by ROS, especially 1O2 and •OH (reviewed in Triantaphylidès et al., 2008; Farmer and Mueller, 2013; Wasternack and Feussner, 2018; Yalcinkaya et al., 2019). Decreased abundance of LOX and OPDA, and lower levels of lipid peroxidation (Gollan et al., 2017; Lima-Melo et al., 2019a), suggest that the enzymatic, rather than ROS-induced, signalling pathway is negatively affected by PSI photoinhibition, although both branches of oxylipin synthesis and signalling pathways appear to be closely interactive (Ramel et al., 2012; Gollan and Aro, 2020). OPDA regulates transcription for biotic and abiotic stress responses, as well as providing a precursor for jasmonic acid (JA), which regulates many stress-responsive and developmental processes (Wasternack and Hause, 2013; Raza et al., 2021). The observed effects of PSI photoinhibition on ROS and oxylipin signalling pathways highlight the importance of PSI activity in transcription regulation, although more work is required in this area to understand the contribution of PSI activity to hormone metabolism and chloroplast signalling.
PSI electron transport activity directly reduces the stromal electron carrier Fd, leading to both formation of NADPH reducing equivalents, which ultimately support biosynthesis of carbohydrates, and reduction of the thioredoxin network involved in redox regulation of stromal proteins. Although PSI is extremely well protected by regulation of electron flow to the donor side, PSI photoinhibition is induced when insufficient capacity of stromal acceptors leads to ROS formation, causing damage to FeS clusters and/or PSI core proteins; however, ROS formed by PSI over-reduction is an important component of chloroplast signalling and may also have an impact on the redox state of the cellular antioxidant network. The decrease in PSI activity caused by photoinhibition not only down-regulates carbohydrate metabolism, but also negatively affects transcriptional reprogramming through both ROS and metabolic (enzymatic) pathways. This suggests that photoinhibition of PSI during periods of sink weakness may be a mechanism to limit stromal metabolism and ROS formation, preventing excessive reduction of O2 and redox-sensitive stromal proteins. Because PSI photoinhibition is mainly avoided by several protective mechanisms, the impact of PSI inactivation on chloroplast metabolism and retrograde signalling seems to be particularly important under specific conditions, such as periods of fluctuating light intensity or low temperature stress. PSI photoinhibition is clearly an expensive option for protection of stromal over-reduction, given the impact on primary metabolism and long recovery time, although prevention of unregulated electron flow to the acceptor side is apparently worth the cost.
YL-M and PG devised the work. YL-M, MK, E-MA, and PG wrote the manuscript. All authors contributed to the article and approved the submitted version.
YL-M is supported by PNPD/CAPES (Bolsista CAPES/BRASIL, Proc. 88882.463229/2019-01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MK, E-MA, and PG are grateful for support from the Jane and Aatos Erkko Foundation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.791124/full#supplementary-material
Supplementary Video 1 | A simplified scheme of the photosynthetic electron transport chain in the thylakoid membrane.
Alric, J., and Johnson, X. (2017). Alternative electron transport pathways in photosynthesis: a confluence of regulation. Curr. Opin. Plant Biol. 37, 78–86. doi: 10.1016/j.pbi.2017.03.014
Amunts, A., Drory, O., and Nelson, N. (2007). The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447, 58–63. doi: 10.1038/nature05687
Amunts, A., and Nelson, N. (2009). Plant photosystem I design in the light of evolution. Structure 17, 637–650. doi: 10.1016/j.str.2009.03.006
Aro, E.-M., Virgin, I., and Andersson, B. (1993). Photoinhibition of photosystem II. inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–134. doi: 10.1016/0005-2728(93)90134-90132
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
Asada, K. (2000). The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. B Biol. Sci. 355, 1419–1431. doi: 10.1098/rstb.2000.0703
Asada, K., Kiso, K., and Yoshikawa, K. (1974). Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J. Biol. Chem. 249, 2175–2181.
Ben-Shem, A., Frolow, F., and Nelson, N. (2003). Crystal structure of plant photosystem I. Nature 426, 630–635. doi: 10.1038/nature02200
Benson, A. A., Bassham, J. A., Calvin, M., Goodale, T. C., Haas, V. A., and Stepka, W. (1950). The path of carbon in photosynthesis. V. Paper chromatography and radioautography of the products. J. Am. Chem. Soc. 72, 1710–1718. doi: 10.1021/ja01160a080
Borisova-Mubarakshina, M. M., Vetoshkina, D. V., and Ivanov, B. N. (2019). Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant. 166, 181–198. doi: 10.1111/ppl.12936
Buchanan, B. B. (2016). The path to thioredoxin and redox regulation beyond chloroplasts. Annu. Rev. Plant Biol. 67, 1–24. doi: 10.1146/annurev-arplant-043015-111949
Busch, A., and Hippler, M. (2011). The structure and function of eukaryotic photosystem I. Biochim. Biophys. Acta 1807, 864–877. doi: 10.1016/j.bbabio.2010.09.009
Cai, Y.-F., Yang, Q.-Y., Li, S.-F., Wang, J.-H., and Huang, W. (2017). The water-water cycle is a major electron sink in Camellia species when CO2 assimilation is restricted. J. Photochem. Photobiol. B Biol. 168, 59–66. doi: 10.1016/j.jphotobiol.2017.01.024
Caspy, I., Borovikova-Sheinker, A., Klaiman, D., Shkolnisky, Y., and Nelson, N. (2020). The structure of a triple complex of plant photosystem I with ferredoxin and plastocyanin. Nat. Plants 6, 1300–1305. doi: 10.1038/s41477-020-00779-779
Caspy, I., Fadeeva, M., Kuhlgert, S., Borovikova-Sheinker, A., Klaiman, D., Masrati, G., et al. (2021). Structure of plant photosystem I-plastocyanin complex reveals strong hydrophobic interactions. Biochem. J. 478, 2371–2384. doi: 10.1042/bcj20210267
Chan, K. X., Phua, S. Y., Crisp, P., McQuinn, R., and Pogson, B. J. (2016). Learning the languages of the chloroplast: retrograde signaling and beyond. Annu. Rev. Plant Biol. 67, 25–53. doi: 10.1146/annurev-arplant-043015-111854
Chaux, F., Peltier, G., and Johnson, X. (2015). A security network in PSI photoprotection: regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front. Plant Sci. 6:875. doi: 10.3389/fpls.2015.00875
Daloso, D. M., Medeiros, D. B., dos Anjos, L., Yoshida, T., Araújo, W. L., and Fernie, A. R. (2017). Metabolism within the specialized guard cells of plants. New Phytol. 216, 1018–1033. doi: 10.1111/nph.14823
Devireddy, A. R., Zandalinas, S. I., Gómez-Cadenas, A., Blumwald, E., and Mittler, R. (2018). Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci. Signal. 11:eaam9514. doi: 10.1126/scisignal.aam9514
Dietz, K.-J., Turkan, I., and Krieger-Liszkay, A. (2016). Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 171, 1541–1550. doi: 10.1104/pp.16.00375
Driever, S. M., and Baker, N. R. (2011). The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant, Cell Environ. 34, 837–846. doi: 10.1111/j.1365-3040.2011.02288.x
Evans, J. R., Kaldenhoff, R., Genty, B., and Terashima, I. (2009). Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 60, 2235–2248. doi: 10.1093/jxb/erp117
Evans, J. R., and von Caemmerer, S. (1996). Carbon dioxide diffusion inside leaves. Plant Physiol. 110, 339–346. doi: 10.1104/pp.110.2.339
Exposito-Rodriguez, M., Laissue, P. P., Yvon-Durocher, G., Smirnoff, N., and Mullineaux, P. M. (2017). Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 8:49. doi: 10.1038/s41467-017-00074-w
Farmer, E. E., and Mueller, M. J. (2013). ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 64, 429–450. doi: 10.1146/annurev-arplant-050312-120132
Fischer, R. A. T., and Edmeades, G. O. (2010). Breeding and cereal yield progress. Crop Sci. 50, S85–S98. doi: 10.2135/cropsci2009.10.0564
Foyer, C. H., and Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25.
Foyer, C. H., and Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. doi: 10.1104/pp.110.166181
Freeman, H. C., and Guss, J. M. (2011). Plastocyanin. Encycl. Inorg. Bioinorg. Chem. 1–18. doi: 10.1002/9781119951438.eibc0611
Furutani, R., Makino, A., Suzuki, Y., Wada, S., Shimakawa, G., and Miyake, C. (2020). Intrinsic fluctuations in transpiration induce photorespiration to oxidize P700 in photosystem I. Plants 12:1791. doi: 10.3390/plants9121761
Gerotto, C., Alboresi, A., Meneghesso, A., Jokel, M., Suorsa, M., Aro, E.-M., et al. (2016). Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc. Natl. Acad. Sci. U S A. 113, 12322–12327. doi: 10.1073/pnas.1606685113
Golbeck, J. H. (1992). Structure and function of photosystem I. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 293–324. doi: 10.1146/annurev.pp.43.060192.001453
Gollan, P. J., and Aro, E.-M. (2020). Photosynthetic signalling during high light stress and recovery: targets and dynamics. Philos. Trans. R. Soc. B Biol. Sci. 375:20190406. doi: 10.1098/rstb.2019.0406
Gollan, P. J., Lima-Melo, Y., Tiwari, A., Tikkanen, M., and Aro, E.-M. (2017). Interaction between photosynthetic electron transport and chloroplast sinks triggers protection and signalling important for plant productivity. Philos. Trans. R. Soc. B Biol. Sci. 372:20160390. doi: 10.1098/rstb.2016.0390
Gollan, P. J., Tikkanen, M., and Aro, E.-M. (2015). Photosynthetic light reactions: integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 27, 180–191. doi: 10.1016/j.pbi.2015.07.006
Grieco, M., Suorsa, M., Jajoo, A., Tikkanen, M., and Aro, E.-M. (2015). Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery - including both photosystems II and I. Biochim. Biophys. Acta 1847, 607–619. doi: 10.1016/j.bbabio.2015.03.004
Grieco, M., Tikkanen, M., Paakkarinen, V., Kangasjärvi, S., and Aro, E.-M. (2012). Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol. 160, 1896–1910. doi: 10.1104/pp.112.206466
Gururani, M. A., Venkatesh, J., and Tran, L.-S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8, 1304–1320. doi: 10.1016/j.molp.2015.05.005
Han, H., Gao, S., Li, B., Dong, X.-C., Feng, H.-L., and Meng, Q.-W. (2010). Overexpression of violaxanthin de-epoxidase gene alleviates photoinhibition of PSII and PSI in tomato during high light and chilling stress. J. Plant Physiol. 167, 176–183. doi: 10.1016/j.jplph.2009.08.009
Hanke, G., and Mulo, P. (2013). Plant type ferredoxins and ferredoxin-dependent metabolism. Plant, Cell Environ. 36, 1071–1084. doi: 10.1111/pce.12046
Havaux, M. (2020). Plastoquinone in and beyond photosynthesis. Trends Plant Sci. 25, 1252–1265. doi: 10.1016/j.tplants.2020.06.011
Hepworth, C., Wood, W. H. J., Emrich-Mills, T. Z., Proctor, M. S., Casson, S., and Johnson, M. P. (2021). Dynamic thylakoid stacking and state transitions work synergistically to avoid acceptor-side limitation of photosystem I. Nat. Plants 7, 87–98. doi: 10.1038/s41477-020-00828-823
Huang, W., Hu, H., and Zhang, S.-B. (2015). Photorespiration plays an important role in the regulation of photosynthetic electron flow under fluctuating light in tobacco plants grown under full sunlight. Front. Plant Sci. 6:621. doi: 10.3389/fpls.2015.00621
Huang, W., Sun, H., Tan, S.-L., and Zhang, S.-B. (2021). The water-water cycle is not a major alternative sink in fluctuating light at chilling temperature. Plant Sci. 305:110828. doi: 10.1016/j.plantsci.2021.110828
Huang, W., Yang, Y.-J., Hu, H., and Zhang, S.-B. (2016). Moderate photoinhibition of photosystem II protects photosystem I from photodamage at chilling stress in tobacco leaves. Front. Plant Sci. 7:182. doi: 10.3389/fpls.2016.00182
Huang, W., Yang, Y.-J., and Zhang, S.-B. (2019). The role of water-water cycle in regulating the redox state of photosystem I under fluctuating light. BBA - Bioenerg. 1860, 383–390. doi: 10.1016/j.bbabio.2019.03.007
Huang, W., Zhang, S.-B., and Cao, K.-F. (2010). The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth. Res. 103, 175–182. doi: 10.1007/s11120-010-9539-9537
Ilík, P., Pavloviè, A., Kouøil, R., Alboresi, A., Morosinotto, T., Allahverdiyeva, Y., et al. (2017). Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 214, 967–972. doi: 10.1111/nph.14536
Inoue, K., Sakurai, H., and Hiyama, T. (1986). Photoinactivation sites of photosystem I in isolated chloroplasts. Plant Cell Physiol. 27, 961–968. doi: 10.1093/oxfordjournals.pcp.a077213
Ivanov, A. G., Rosso, D., Savitch, L. V., Stachula, P., Rosembert, M., Oquist, G., et al. (2012). Implications of alternative electron sinks in increased resistance of PSII and PSI photochemistry to high light stress in cold-acclimated Arabidopsis thaliana. Photosynth. Res. 113, 191–206. doi: 10.1007/s11120-012-9769-y
Jahns, P., and Holzwarth, A. R. (2012). The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 1817, 182–193. doi: 10.1016/j.bbabio.2011.04.012
Joliot, P., and Alric, J. (2013). Inhibition of CO2 fixation by iodoacetamide stimulates cyclic electron flow and non-photochemical quenching upon far-red illumination. Photosynth. Res. 115, 55–63. doi: 10.1007/s11120-013-9826-9821
Kanazawa, A., Ostendorf, E., Kohzuma, K., Hoh, D., Strand, D. D., Sato-Cruz, M., et al. (2017). Chloroplast ATP synthase modulation of the thylakoid proton motive force: implications for photosystem I and photosystem II photoprotection. Front. Plant Sci. 8:719. doi: 10.3389/fpls.2017.00719
Kato, Y., Sun, X., Zhang, L., and Sakamoto, W. (2012). Cooperative D1 degradation in the photosystem II repair mediated by chloroplastic proteases in Arabidopsis. Plant Physiol. 159, 1428–1439. doi: 10.1104/pp.112.199042
Klughammer, C., and Schreiber, U. (2008). Saturation Pulse method for assessment of energy conversion in PS I. PAM Appl. Notes 1, 11–14.
Klughammer, C., and Schreiber, U. (2016). Deconvolution of ferredoxin, plastocyanin, and P700 transmittance changes in intact leaves with a new type of kinetic LED array spectrophotometer. Photosynth. Res. 128, 195–214. doi: 10.1007/s11120-016-0219-210
Kohzuma, K., Cruz, J. A., Akashi, K., Hoshiyasu, S., Munekage, Y. N., Yokota, A., et al. (2009). The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ. 32, 209–219. doi: 10.1111/j.1365-3040.2008.01912.x
Kölling, K., Thalmann, M., Müller, A., Jenny, C., and Zeeman, S. C. (2015). Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ. 38, 1965–1979. doi: 10.1111/pce.12512
König, K., Vaseghi, M. J., Dreyer, A., and Dietz, K. J. (2018). The significance of glutathione and ascorbate in modulating the retrograde high light response in Arabidopsis thaliana leaves. Physiol. Plant. 162, 262–273. doi: 10.1111/ppl.12644
Kono, M., Noguchi, K., and Terashima, I. (2014). Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol. 55, 990–1004. doi: 10.1093/pcp/pcu033
Kono, M., and Terashima, I. (2016). Elucidation of photoprotective mechanisms of PSI against fluctuating light photoinhibition. Plant Cell Physiol. 57, 1405–1414. doi: 10.1093/pcp/pcw103
Kozuleva, M., Petrova, A., Milrad, Y., Semenov, A., Ivanov, B., Redding, K. E., et al. (2021). Phylloquinone is the principal Mehler reaction site within photosystem I in high light. Plant Physiol. 186, 1848–1858. doi: 10.1093/plphys/kiab221
Kozuleva, M. A., and Ivanov, B. N. (2016). The mechanisms of oxygen reduction in the terminal reducing segment of the chloroplast photosynthetic electron transport chain. Plant Cell Physiol. 57, 1397–1404. doi: 10.1093/pcp/pcw035
Kromdijk, J., Głowacka, K., Leonelli, L., Gabilly, S. T., Iwai, M., Niyogi, K. K., et al. (2016). Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. doi: 10.1126/science.aai8878
Kudoh, H., and Sonoike, K. (2002). Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 215, 541–548. doi: 10.1007/s00425-002-0790-799
Lawson, T., and Blatt, M. R. (2014). Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. doi: 10.1104/pp.114.237107
Li, L., Aro, E.-M., and Millar, A. H. (2018). Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 23, 667–676. doi: 10.1016/j.tplants.2018.05.004
Li, Y.-T., Liang, Y., Li, Y.-N., Che, X.-K., Zhao, S.-J., Zhang, Z.-S., et al. (2018). Mechanisms by which bisphenol a affect the photosynthetic apparatus in cucumber (Cucumis sativus L.) leaves. Sci. Rep. 8:4253. doi: 10.1038/s41598-018-22486-22484
Li, X.-G., Wang, X.-M., Meng, Q.-W., and Zou, Q. (2004). Factors limiting photosynthetic recovery in sweet pepper leaves after short-term chilling stress under low irradiance. Photosynthetica 42, 257–262. doi: 10.1023/B:PHOT.0000040598.48732.af
Lima-Melo, Y., Alencar, V. T. C. B., Lobo, A. K. M., Sousa, R. H. V., Tikkanen, M., Aro, E. M., et al. (2019a). Photoinhibition of photosystem I provides oxidative protection during imbalanced photosynthetic electron transport in Arabidopsis thaliana. Front. Plant Sci. 10:916. doi: 10.3389/fpls.2019.00916
Lima-Melo, Y., Gollan, P. J., Tikkanen, M., Silveira, J. A. G., and Aro, E.-M. (2019b). Consequences of photosystem-I damage and repair on photosynthesis and carbon utilisation in Arabidopsis thaliana. Plant J. 97, 1061–1072. doi: 10.1111/tpj.14177
Long, S. P., Marshall-Colon, A., and Zhu, X.-G. (2015). Meeting the global food femand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. doi: 10.1016/j.cell.2015.03.019
Malone, L. A., Proctor, M. S., Hitchcock, A., Hunter, C. N., and Johnson, M. P. (2021). Cytochrome b6f - Orchestrator of photosynthetic electron transfer. BBA - Bioenerg. 1862:148380. doi: 10.1016/j.bbabio.2021.148380
Marco, P., Kozuleva, M., Eilenberg, H., Mazor, Y., Gimeson, P., Kanygin, A., et al. (2018). Binding of ferredoxin to algal photosystem I involves a single binding site and is composed of two thermodynamically distinct events. BBA - Bioenerg. 1859, 234–243. doi: 10.1016/j.bbabio.2018.01.001
Maruta, T., Noshi, M., Tanouchi, A., Tamoi, M., Yabuta, Y., Yoshimura, K., et al. (2012). H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 287, 11717–11729. doi: 10.1074/jbc.M111.292847
Mazor, Y., Borovikova, A., Caspy, I., and Nelson, N. (2017). Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3:17014. doi: 10.1038/nplants.2017.14
Mehler, A. H. (1951). Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 33, 65–77.
Miyake, C. (2010). Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol. 51, 1951–1963. doi: 10.1093/pcp/pcq173
Mullineaux, P. M., Exposito-Rodriguez, M., Laissue, P. P., and Smirnoff, N. (2018). ROS-dependent signalling pathways in plants and algae exposed to high light: comparisons with other eukaryotes. Free Radic. Biol. Med. 122, 52–64. doi: 10.1016/j.freeradbiomed.2018.01.033
Munekage, Y., Hojo, M., Meurer, J., Endo, T., Tasaka, M., and Shikanai, T. (2002). PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110, 361–371. doi: 10.1016/S0092-8674(02)00867-X
Munekage, Y. N., Genty, B., and Peltier, G. (2008). Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Physiol. 49, 1688–1698. doi: 10.1093/pcp/pcn140
Nikkanen, L., Toivola, J., and Rintamäki, E. (2016). Crosstalk between chloroplast thioredoxin systems in regulation of photosynthesis. Plant Cell Environ. 39, 1691–1705. doi: 10.1111/pce.12718
Nixon, P. J., Michoux, F., Yu, J., Boehm, M., and Komenda, J. (2010). Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 106, 1–16. doi: 10.1093/aob/mcq059
Noctor, G., Reichheld, J. P., and Foyer, C. H. (2018). ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 80, 3–12. doi: 10.1016/j.semcdb.2017.07.013
Oguchi, R., Terashima, I., and Chow, W. S. (2021). The effect of different spectral light quality on the photoinhibition of Photosystem I in intact leaves. Photosynth. Res. 149, 83–92. doi: 10.1007/s11120-020-00805-z
Ort, D. R., and Baker, N. R. (2002). A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 5, 193–198. doi: 10.1016/S1369-5266(02)00259-255
Osei-Bonsu, I., McClain, A. M., Walker, B. J., Sharkey, T. D., and Kramer, D. M. (2021). The roles of photorespiration and alternative electron acceptors in the responses of photosynthesis to elevated temperatures in cowpea. Plant Cell Environ. 44, 2290–2307. doi: 10.1111/pce.14026
Padmasree, K., Padmavathi, L., and Raghavendra, A. S. (2002). Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit. Rev. Biochem. Mol. Biol. 37, 71–119. doi: 10.1080/10409230290771465
Paul, M. J., and Foyer, C. H. (2001). Sink regulation of photosynthesis. J. Exp. Bot. 52, 1383–1400. doi: 10.1093/jexbot/52.360.1383
Peltier, G., Aro, E.-M., and Shikanai, T. (2016). NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu. Rev. Plant Biol. 67, 55–80. doi: 10.1146/annurev-arplant-043014-114752
Powles, S. B. (1984). Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35, 15–44. doi: 10.1146/annurev.pp.35.060184.000311
Qin, X., Suga, M., Kuang, T., and Shen, J.-R. (2015). Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995. doi: 10.1126/science.aab0214
Raines, C. A. (2003). The Calvin cycle revisited. Photosynth. Res. 75, 1–10. doi: 10.1023/A:1022421515027
Raines, C. A. (2011). Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 155, 36–42. doi: 10.1104/pp.110.168559
Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylidès, C., and Havaux, M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U S A. 109, 5535–5540. doi: 10.1073/pnas.1115982109
Rantala, S., Lempiäinen, T., Gerotto, C., Tiwari, A., Aro, E.-M., and Tikkanen, M. (2020). PGR5 and NDH-1 systems do not function as protective electron acceptors but mitigate the consequences of PSI inhibition. Biochim. Biophys. Acta - Bioenerg. 1861:148154. doi: 10.1016/j.bbabio.2020.148154
Rantala, S., and Tikkanen, M. (2018). Phosphorylation-induced lateral rearrangements of thylakoid protein complexes upon light acclimation. Plant Direct 2:e00039. doi: 10.1002/pld3.39
Ray, D. K., Ramankutty, N., Mueller, N. D., West, P. C., and Foley, J. A. (2012). Recent patterns of crop yield growth and stagnation. Nat. Commun. 3, 1293–1297. doi: 10.1038/ncomms2296
Raza, A., Charagh, S., Zahid, Z., Mubarik, M. S., Javed, R., Siddiqui, M. H., et al. (2021). Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 40, 1513–1541. doi: 10.1007/s00299-020-02614-z
Ruban, A. V. (2016). Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 170, 1903–1916. doi: 10.1104/pp.15.01935
Salesse-Smith, C. E., Sharwood, R. E., Busch, F. A., Kromdijk, J., Bardal, V., and Stern, D. B. (2018). Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 4, 802–810. doi: 10.1038/s41477-018-0252-254
Sarewicz, M., Pintscher, S., Pietras, R., Borek, A., Bujnowicz, Ł, Hanke, G., et al. (2021). Catalytic reactions and energy conservation in the cytochrome bc1 and b6f complexes of energy-transducing membranes. Chem. Rev. 121, 2020–2108. doi: 10.1021/acs.chemrev.0c00712
Scheller, H. V., and Haldrup, A. (2005). Photoinhibition of photosystem I. Planta 221, 5–8. doi: 10.1007/s00425-005-1507-1507
Schreiber, U., and Klughammer, C. (2016). Analysis of photosystem I donor and acceptor sides with a new type of online-deconvoluting kinetic LED-array spectrophotometer. Plant Cell Physiol. 57, 1454–1467. doi: 10.1093/pcp/pcw044
Sejima, T., Takagi, D., Fukayama, H., Makino, A., and Miyake, C. (2014). Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 55, 1184–1193. doi: 10.1093/pcp/pcu061
Sewelam, N., Jaspert, N., Van Der Kelen, K., Tognetti, V. B., Schmitz, J., Frerigmann, H., et al. (2014). Spatial H2O2 signaling specificity: H2O2 from chloroplasts and peroxisomes modulates the plant transcriptome differentially. Mol. Plant 7, 1191–1210. doi: 10.1093/mp/ssu070
Shevela, D., Kern, J. F., Govindjee, G., Whitmarsh, J., and Messinger, J. (2021). Photosystem II. eLS 2, 1–16. doi: 10.1002/9780470015902.a0029372
Shimakawa, G., and Miyake, C. (2018). Oxidation of P700 ensures robust photosynthesis. Front. Plant Sci. 9:1617. doi: 10.3389/fpls.2018.01617
Shimakawa, G., and Miyake, C. (2019). What quantity of photosystem I is optimum for safe photosynthesis? Plant Physiol. 179, 1479–1485. doi: 10.1104/pp.18.01493
Simkin, A. J., McAusland, L., Lawson, T., and Raines, C. A. (2017). Over-expression of the RieskeFeS protein increases electron transport rates and biomass yield. Plant Physiol. 175, 134–145. doi: 10.1104/pp.17.00622
Smirnoff, N., and Arnaud, D. (2019). Hydrogen peroxide metabolism and functions in plants. New Phytol. 221, 1197–1214. doi: 10.1111/nph.15488
Sonoike, K., Kamo, M., Hihara, Y., Hiyama, T., and Enami, I. (1997). The mechanism of degradation of PsaB protein, a reaction center subunit of photosystem I, upon photoinhibition. Plant Physiol. 53, 55–63. doi: 10.1023/A:1005852330671
Suorsa, M., Järvi, S., Grieco, M., Nurmi, M., Pietrzykowska, M., Rantala, M., et al. (2012). PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948. doi: 10.1105/tpc.112.097162
Takagi, D., and Miyake, C. (2018). PROTON GRADIENT REGULATION 5 supports linear electron flow to oxidize photosystem I. Physiol. Plant. 164, 337–348. doi: 10.1111/ppl.12723
Takagi, D., Takumi, S., Hashiguchi, M., Sejima, T., and Miyake, C. (2016). Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol. 171, 1626–1634. doi: 10.1104/pp.16.00246
Takahashi, S., and Murata, N. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13, 178–182. doi: 10.1016/j.tplants.2008.01.005
Tan, S.-L., Huang, X., Li, W.-Q., Zhang, S.-B., and Huang, W. (2021). Elevated CO2 concentration alters photosynthetic performances under fluctuating light in Arabidopsis thaliana. Cells 10:2329. doi: 10.3390/cells10092329
Terashima, I., Funayama, S., and Sonoike, K. (1994). The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193, 300–306. doi: 10.1007/BF00192544
Tikhonov, A. N. (2014). The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 81, 163–183. doi: 10.1016/j.plaphy.2013.12.011
Tikkanen, M., and Aro, E.-M. (2014). Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci. 19, 10–17. doi: 10.1016/j.tplants.2013.09.003
Tikkanen, M., and Grebe, S. (2018). Switching off photoprotection of photosystem I - a novel tool for gradual PSI photoinhibition. Physiol. Plant. 162, 156–161. doi: 10.1111/ppl.12618
Tikkanen, M., Mekala, N. R., and Aro, E.-M. (2014). Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim. Biophys. Acta 1837, 210–215. doi: 10.1016/j.bbabio.2013.10.001
Tikkanen, M., Rantala, S., and Aro, E.-M. (2015). Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 6:521. doi: 10.3389/fpls.2015.00521
Tiwari, A., Mamedov, F., Grieco, M., Suorsa, M., Jajoo, A., Styring, S., et al. (2016). Photodamage of iron-sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat. Plants 2:16035. doi: 10.1038/NPLANTS.2016.35
Tjus, S. E., Møller, B. L., and Scheller, H. V. (1998). Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol. 116, 755–764. doi: 10.1104/pp.116.2.755
Triantaphylidès, C., Krischke, M., Hoeberichts, F. A., Ksas, B., Gresser, G., Havaux, M., et al. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 148, 960–968. doi: 10.1104/pp.108.125690
Tyystjärvi, E., and Aro, E.-M. (1996). The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl. Acad. Sci. U S A. 93, 2213–2218. doi: 10.1073/pnas.93.5.2213
Vandenabeele, S., Vanderauwera, S., Vuylsteke, M., Rombauts, S., Langebartels, C., Seidlitz, H. K., et al. (2004). Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 39, 45–58. doi: 10.1111/j.1365-313X.2004.02105.x
Vanderauwera, S., Zimmermann, P., Rombauts, S., Vandenabeele, S., Langebartels, C., Gruissem, W., et al. (2005). Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 139, 806–821. doi: 10.1104/pp.105.065896
von Caemmerer, S., and Furbank, R. T. (2016). Strategies for improving C4 photosynthesis. Curr. Opin. Plant Biol. 31, 125–134. doi: 10.1016/j.pbi.2016.04.003
Wada, S., Suzuki, Y., and Miyake, C. (2020). Photorespiration enhances acidification of the thylakoid lumen, reduces the plastoquinone pool, and contributes to the oxidation of P700 at a lower partial pressure of CO2 in wheat leaves. Plants 9:319. doi: 10.3390/plants9030319
Wada, S., Takagi, D., Miyake, C., and Makino, A. (2019). Responses of the photosynthetic electron transport reactions stimulate the oxidation of the reaction center chlorophyll of photosystem I, P700, under drought and high temperatures in rice. Int. J. Mol. Sci. 20:2068. doi: 10.3390/ijms20092068
Wada, S., Yamamoto, H., Suzuki, Y., Yamori, W., Shikanai, T., and Makino, A. (2018). Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol. 176, 1509–1518. doi: 10.1104/pp.17.01335
Wang, Y., Noguchi, K., Ono, N., Inoue, S., Terashima, I., and Kinoshita, T. (2014). Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. U S A. 111, 533–538. doi: 10.1073/pnas.1305438111
Wasternack, C., and Feussner, I. (2018). The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol. 69, 363–386.
Wasternack, C., and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058. doi: 10.1093/aob/mct067
Wientjes, E., van Amerongen, H., and Croce, R. (2013). LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta 1827, 420–426. doi: 10.1016/j.bbabio.2012.12.009
Wingler, A. (2018). Transitioning to the next phase: the role of sugar signaling throughout the plant life cycle. Plant Physiol. 176, 1075–1084. doi: 10.1104/pp.17.01229
Yalcinkaya, T., Uzilday, B., Ozgur, R., Turkan, I., and Mano, J. (2019). Lipid peroxidation-derived reactive carbonyl species (RCS): their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 165, 139–149. doi: 10.1016/j.envexpbot.2019.06.004
Yamamoto, H., and Shikanai, T. (2019). PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 179, 588–600. doi: 10.1104/pp.18.01343
Zavafer, A., and Mancilla, C. (2021). Concepts of photochemical damage of photosystem II and the role of excessive excitation. J. Photochem. Photobiol. C Photochem. Rev. 47:100421. doi: 10.1016/j.jphotochemrev.2021.100421
Zhang, P., Allahverdiyeva, Y., Eisenhut, M., and Aro, E.-M. (2009). Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS One 4:e5331. doi: 10.1371/journal.pone.0005331
Zhang, S., and Scheller, H. V. (2004). Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol. 45, 1595–1602. doi: 10.1093/pcp/pch180
Zhang, Z., Jia, Y., Gao, H., Zhang, L., Li, H., and Meng, Q. (2011). Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta 234, 883–889. doi: 10.1007/s00425-011-1447-1443
Keywords: PSI, photoinhibition, P700, electron transport, ROS, metabolism, photoprotection, alternative electron flow
Citation: Lima-Melo Y, Kılıç M, Aro E-M and Gollan PJ (2021) Photosystem I Inhibition, Protection and Signalling: Knowns and Unknowns. Front. Plant Sci. 12:791124. doi: 10.3389/fpls.2021.791124
Received: 07 October 2021; Accepted: 11 November 2021;
Published: 01 December 2021.
Edited by:
Zhi-Yan (Rock) Du, University of Hawai‘i at Mânoa, United StatesReviewed by:
Anja Liszkay, UMR 9198 Institut de Biologie Intégrative de la Cellule (I2BC), FranceCopyright © 2021 Lima-Melo, Kılıç, Aro and Gollan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Gollan, cGV0ZXIuZ29sbGFuQHV0dS5maQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.