- Shandong Province Key Laboratory for Agricultural Microbiology, Laboratory of Plant Virology, Department of Plant Pathology, College of Plant Protection, Shandong Agricultural University, Tai’an, China
Sugarcane mosaic virus (SCMV; genus Potyvirus) induces maize dwarf mosaic disease that has caused serious yield losses of maize in China. Cross-protection is one of the efficient strategies to fight against severe virus strains. Although many mild strains have been identified, the spontaneous mutation is one of the challenging problems affecting their application in cross-protection. In this study, we found that the substitution of cysteine (C) at positions 57 or 60 in the zinc finger-like motif of HC-Pro with alanine (A; C57A or C60A) significantly reduced its RNA silencing suppression activity and SCMV virulence. To reduce the risk of mild strains mutating to virulent ones by reverse or complementary mutations, we obtained attenuated SCMV mutants with double-mutations in the zinc finger-like and FRNK motifs of HC-Pro and evaluated their potential application in cross-protection. The results showed that the maize plants infected with FKNK/C60A double-mutant showed symptomless until 95 days post-inoculation and FKNK/C60A cross-protected plants displayed high resistance to severe SCMV strain. This study provides theoretical and material bases for the control of SCMV through cross-protection.
Introduction
Maize dwarf mosaic (MDM) is one of the most serious viral diseases, which threatens the safety of maize production in all maize-growing regions of China (Gao et al., 2011; Wu et al., 2012; Xie et al., 2016). Sugarcane mosaic virus (SCMV) belonging to the genus Potyvirus is the prevalent virus inducing MDM in China (Jiang and Zhou, 2002; Yan et al., 2016; Chen H. et al., 2017). However, little is known about effective measures to prevent SCMV.
Cross-protection is an environmentally safe method to control plant viral diseases (Pechinger et al., 2019) and has been used to control multiple plant viruses in the laboratory or field (Rast, 1972; Krstic et al., 1995; Cong et al., 2019; Cheng et al., 2020). Mild viruses can protect plants from subsequent infection of the same or closely related severe strains (Zhang and Qu, 2016; Zhang et al., 2018). However, screening mild mutants for cross-protection is a time-consuming task by traditional treating with nitrous acid, ultraviolet irradiation, or heat (Yeh and Cheng, 1989; Yang et al., 2002). Reverse genetics provides a faster and more effective way for screening mild mutants (Kung et al., 2014; Huang et al., 2019; Tuo et al., 2020). The stability of the mild mutants is one of the most important factor affecting the application of cross-protection (Ziebell and MacDiarmid, 2017). The reverse or complementary mutations increase the risk of mild strains becoming virulent ones (Sherwood, 1987; Loebenstein and Carr, 2006; Xu et al., 2020). Compared with that of viral mutants with a single mutation, the possibility of reversion to virulent ones for mutants carrying multiple mutations was lower (Maassab and DeBorde, 1985; Tosh et al., 2008; Liu et al., 2017). Therefore, mild strains with double or more mutations are preferred in cross-protection (Lin et al., 2007; Cong et al., 2019; Tuo et al., 2020). In addition, cross-protection only works well between closely related viruses (Matthews, 1949; Pechinger et al., 2019), and the local dominant strains should be used to screen mild mutants for cross-protection.
Potyviruses encode two polyproteins which are cleaved into 11 mature proteins by three virus-encoded proteinases (Chung et al., 2008; Olspert et al., 2015). Multifunctional helper component-proteinase (HC-Pro) of potyvirus is involved in RNA silencing suppression, aphid transmission, viral movement and virulence (Pirone and Blanc, 1996; Sáenz et al., 2002; Valli et al., 2014; Ivanov et al., 2016). Previous studies have revealed the role of HC-Pro FRNK motif in potyvirus virulence (Shiboleth et al., 2007; Gao et al., 2012; Kung et al., 2014; Xu et al., 2020). The N-terminal domain of potyviral HC-Pro contains a highly conserved cysteine (C)-rich region that belongs to the zinc finger-like motif (Robaglia et al., 1989). Mutations in the conserved C residues within the zinc finger-like motif have a strong debilitating effect on the self-interaction activity of PVY HC-Pro (Urcuqui-Inchima et al., 1999). The mutation of C at position 310 in HC-Pro zinc finger-like motif to serine (S) has profound effects on the virulence of tobacco vein mottling virus (TVMV) (Atreya and Pirone, 1993). So far, there has been no report on the role of the conserved C residues in the zinc finger-like motif of SCMV HC-Pro in its RNA silencing suppression (RSS) activity and the virulence of SCMV that mainly infects monocot crops.
In this study, we found that the zinc finger-like motif of HC-Pro was involved in its RSS activity and SCMV virulence. Our previous study has shown that the attenuated SCMV mutant with single-mutation in HC-Pro FRNK motif could protect maize plants from severe strain, while a spontaneous mutation restored its virulence (Xu et al., 2020). To reduce this risk, we obtained attenuated SCMV mutants with double-mutations in HC-Pro zinc finger-like and FRNK motifs, and evaluated their potential in cross-protection. This study provided theoretical and practical bases for the control of SCMV via cross-protection.
Materials and Methods
Plant Growth and Virus Inoculation
Plants of maize (Zea mays) inbred line B73, Nicotiana benthamiana, and GFP-expressing N. benthamiana (16C) were cultivated in a growth chamber with 16 h light (24°C) and 8 h dark (22°C) cycles.
The pSCMV-based constructs were inoculated onto leaves of the three-leaf staged maize plants as described previously (Xu et al., 2020). Crude extracts from the maize leaves infected with SCMV carrying gfp reporter gene (SCMV-GFP) or SCMV mutants were ground in 20 mM phosphate-buffered saline (pH 7.2) and inoculated onto maize leaves via mechanical rub. These experiments were repeated thrice independently.
Plasmid Construction
The infectious clone based on SCMV-BJ isolate (pSCMV) was kindly provided by Professor Yule Liu from Tsinghua University, China. Site-directed mutagenesis was performed as described previously (Liu and Naismith, 2008). The primers used for mutation were listed in Supplementary Table 1. For transient expression in 16C N. benthamiana leaves, the full-length coding sequence of SCMV HC-Pro or its mutants were ligated into pBin121 vector between BamHI and SacI restriction sites.
RNA Silencing Suppression Assay
Plasmids pBin-GUS and the plasmids expressing wild-type SCMV HC-Pro (pBin-HC) or its mutants were transformed into Agrobacterium cells, respectively. The transformed Agrobacterium cultures were grown overnight in the Luria-Bertani culture medium containing 50 μg/mL kanamycin and 50 μg/mL rifampicin followed by 3 h of incubation in an induction buffer [10 mM MgCl2, 150 μM acetosyringone and 10 mM 2-(N-Morpholino) ethane sulfonic acid (MES)] at room temperature. Agrobacterium cultures (OD600 = 0.3) were individually mixed with Agrobacterium cells harboring plasmid pBin-GFP in a ratio of 1:1 before infiltration into 16C N. benthamiana leaves. Green fluorescence was photographed using a Canon 800D camera under UV light. The experiments were repeated thrice independently.
RNA Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from maize and N. benthamiana 16C leaf tissues, and the first-strand cDNA for reverse transcription polymerase chain reaction (RT-PCR) was synthesized as described previously (Xu et al., 2020). The Quantitative Real-Time RT-PCR (qRT-PCR) was performed using ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) on a PCR machine (LC96, Roche, Basel, Switzerland). The house-keeping genes including maize ZmUbi gene (GenBank accession: XM_008647047) and N. benthamiana actin gene (GenBank accession: AY179605) were used as internal controls for qRT-PCR (Gao et al., 2012; Chen H. et al., 2017) (Supplementary Table 1). Each qRT-PCR was performed with three biological replicates and three technical replicates.
Enzyme-Linked Immunosorbent Assay
The maize upper non-inoculated leaves were extracted with coating buffer (15 mM Na2CO3 and 35 mM NaHCO3, pH 9.6), added to the microplate wells, and incubated at 37°C for 4 h. The rabbit polyclonal antibody against SCMV CP was used as the primary antibody. Alkaline phosphatase-conjugated goat anti-rabbit IgG (1:50000, v/v) was used as the secondary antibody. Furthermore, the absorbance value at 405 nm was measured using a Multi-function Microplate Reader (BioTek Synergy™ Mx, Winooski, VT, United States). The ELISA was performed with three biological replicates and repeated thrice independently.
Western Blotting
Western blotting was performed as described previously (Sun and Suzuki, 2008). The primary antibodies against SCMV CP, SCMV HC-Pro and GFP were prepared in the Laboratory of Plant Virology, Shandong Agricultural University (Wang et al., 2014; Xu et al., 2018, 2019). The horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) was used as the secondary antibody (Sigma-Aldrich, St. Louis, MO, United States). Quantification of SCMV CP and GFP accumulation levels were estimated using ImageJ software (Wyrsch et al., 2015). The samples from three biological replicates were detected separately.
Cross-Protection Assay
Cross-protection experiments was conducted as described in our previous study with minor improvement (Xu et al., 2020). The time between the inoculation of mild strain and wild-type SCMV-GFP were set to 15 and 20 days, respectively. Western blotting was used to determine the accumulation levels of wild-type SCMV-GFP at 20 days after the challenge inoculation. Three independent experiments were carried out. Agrobacterium cells carrying empty vector pCB301-Rz were used as control.
Genetic Stability Assay
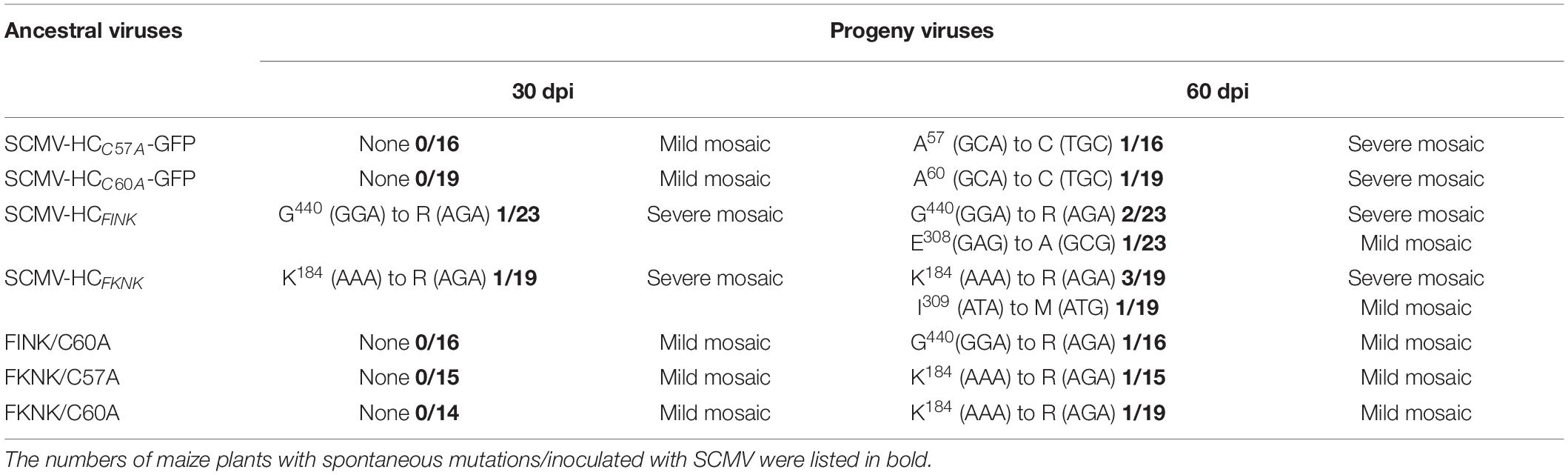
The genetic stability of SCMV mutants was tested through successive passages in maize plants. The crude extracts from upper non-inoculated maize leaves infected with SCMV mutants were used for inoculating healthy maize leaves. The mutants were successively transferred for four generations in maize plants at 15 day intervals. The HC-Pro coding sequences of the fourth generation SCMV progeny in maize plants were sequenced. In addition, the HC-Pro coding sequences from the SCMV progeny in maize plants infected with SCMV mutants were sequenced at 30 days post inoculation (dpi) and 60 dpi, respectively.
Results
Mutations on the Conserved C57 and C60 of Helper Component-Proteinase Reduced Its RNA Silencing Suppression Activity and Sugarcane Mosaic Virus Virulence
The N-terminus of HC-Pro contains a highly conserved cysteine (C)-rich region, which belongs to the zinc finger-like motif. By alignment of HC-Pro amino acid sequences of 12 potyviruses, we found that C at positions 57 and 60 (C57 and C60) in the zinc finger-like motif of SCMV HC-Pro were highly conserved (Figure 1). Furthermore, C57 was located in the KITC motif involved in aphid transmission (Figures 1, 2A). Then we investigated the role of C57 and C60 in HC-Pro RSS activity and SCMV virulence.
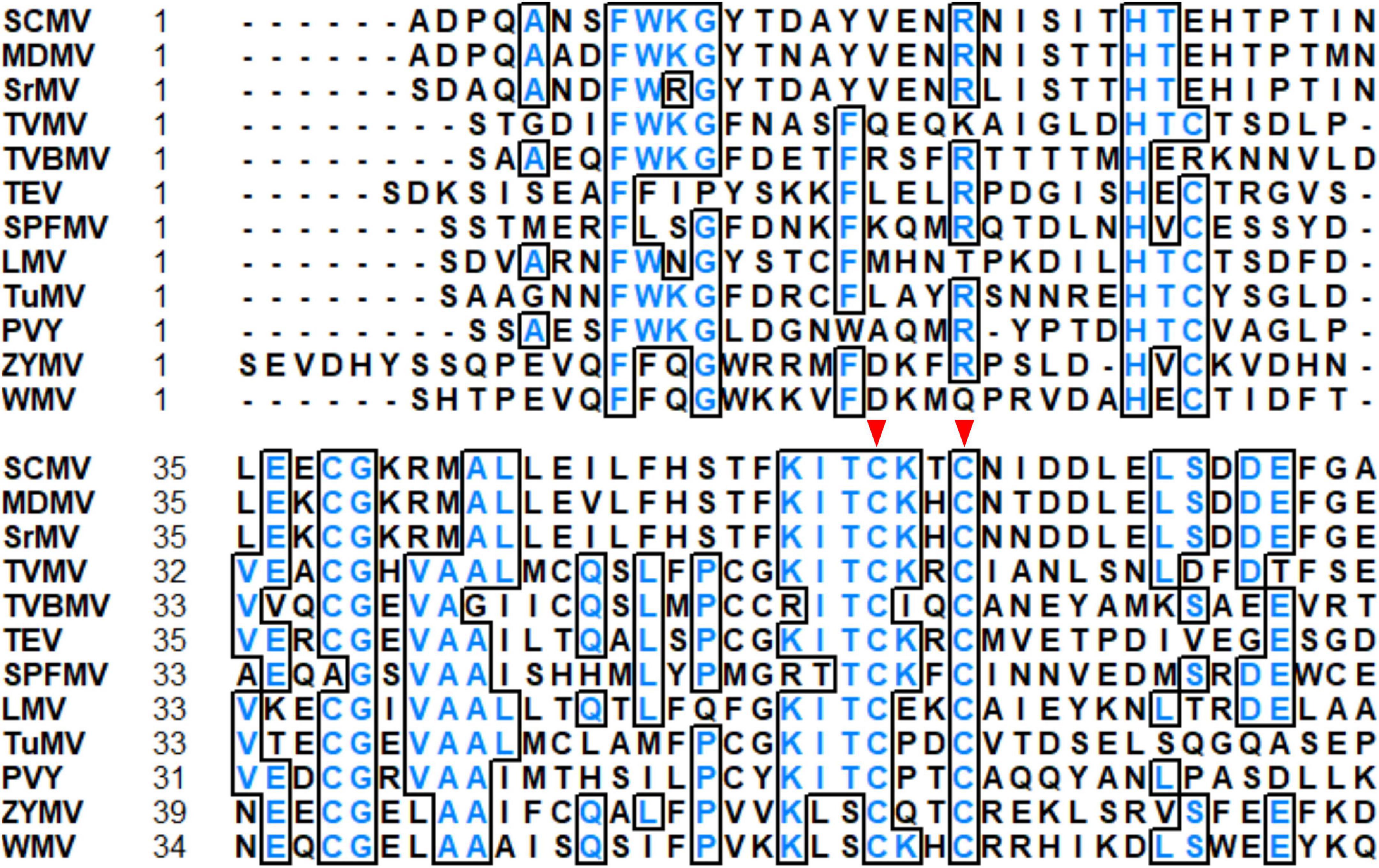
Figure 1. Alignment of partial HC-Pros sequences of 12 potyviruses. The highly conserved cysteine residues at positions 57 and 60 (C57 and C60) in SCMV HC-Pro were pointed by the red triangles. The analysis was performed with the sequences of SCMV (GenBank accession: AY042184), maize dwarf mosaic virus (MDMV, GenBank accession: AJ001691), sorghum mosaic virus (SrMV, GenBank accession: U57358), tobacco vein mottling virus (TVMV, GenBank accession: NP_734329), tobacco vein banding mosaic virus (TVBMV, GenBank accession: EF219408), tobacco etch virus (TEV, GenBank accession: DQ986288), sweet potato feathery mottle virus (SPFMV, GenBank accession: KU511268), lettuce mosaic virus (LMV, GenBank accession: AJ306288), turnip mosaic virus (TuMV, GenBank accession: AB093596), potato virus Y (PVY, GenBank accession: AB185833), zucchini yellow mosaic virus (ZYMV, GenBank accession: AY188994) and watermelon mosaic virus (WMV, GenBank accession: AB218280).
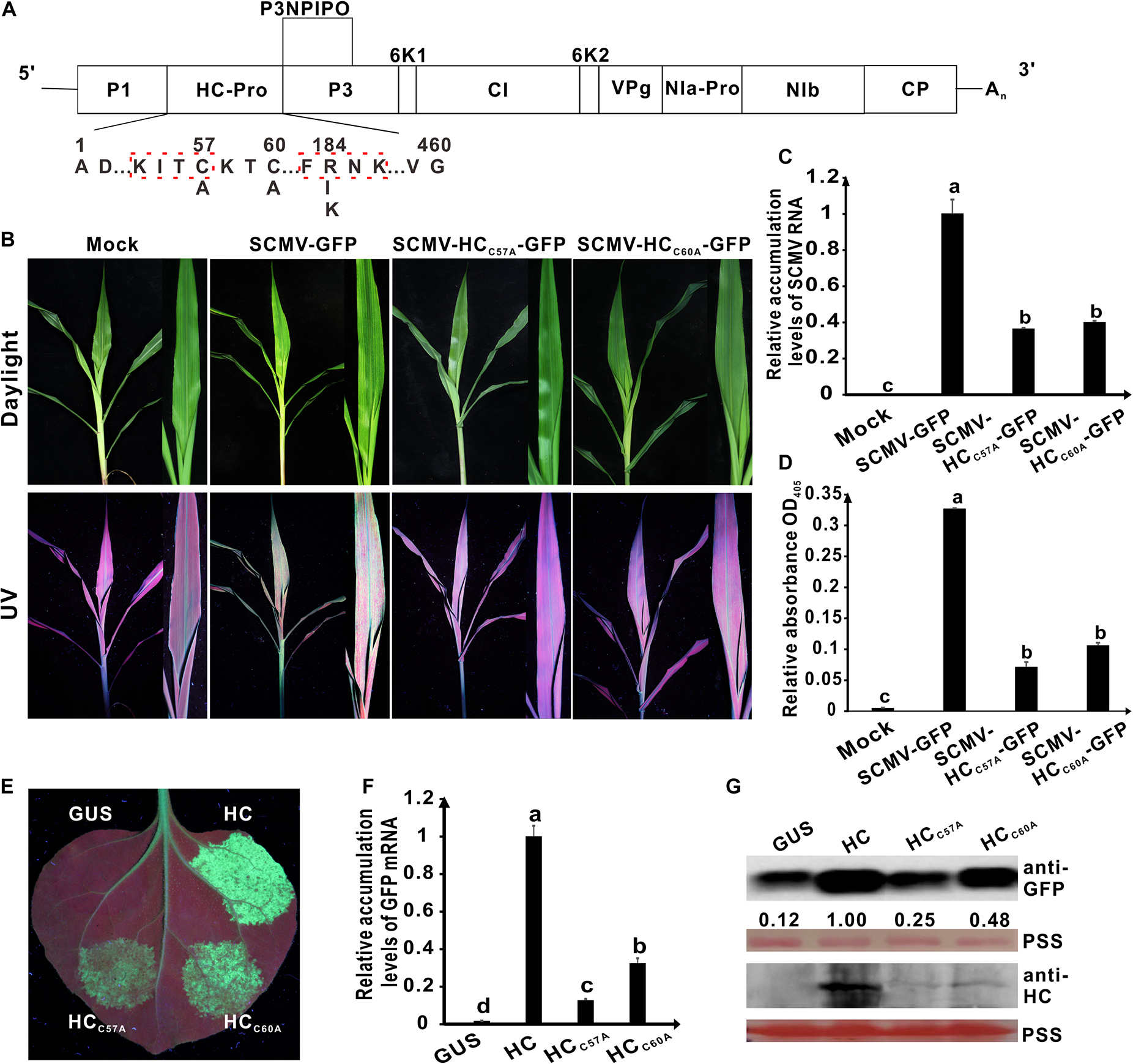
Figure 2. Effects of the mutations in the conserved C57 and C60 of wild type HC-Pro on its RNA silencing suppression activity and virulence of SCMV. (A) Genetic map of SCMV, showing the all mutations in HC-Pro. The numbers above the sequence indicate their position in SCMV HC-Pro and the letters below the sequence showed the substituted residues at that position. The highly conserved KITC and FRNK motifs in SCMV HC-Pro were marked by the red dotted boxs. (B) Symptoms of SCMV and two SCMV mutants in maize plants at 10 days post inoculation (dpi). The conserved C57 and C60 in wild type SCMV HC-Pro were mutated to A residues in HC-Pros of SCMV-HCC57A-GFP and SCMV-HCC60A-GFP, respectively. Mock, the maize plants inoculated with the empty vector pCB301-Rz. SCMV-GFP, the maize plants infected with wild type SCMV with gfp reporter gene. (C) The wild type and mutant SCMV RNA accumulation levels in the upper leaves of maize plants at 10 dpi. (D) ELISA analysis of the wild type and mutant SCMV accumulation levels in the upper leaves of maize plants at 10 dpi. (E) The wild type and mutants HC-Pro RSS activity in Agrobacterium co-infiltration assay. The N. benthamiana 16C leaves were infiltrated with a mixture of Agrobacterium cultures carrying pBin-GFP and either wild type or mutant HC-Pro and photographed under long-wavelength UV light at 3 days post agroinfiltration (dpai). The conserved C residues in wild type HC-Pro (HC) were mutated to A residues in HCC57A and HCC60A, respectively. The GUS was used as a negative control. (F) The GFP mRNA accumulation levels in agroinfiltrated 16C leaf patches. (G) Western blotting analysis of the accumulation levels of GFP and HC in agroinfiltrated leaf patches of 16C. The ponceau S staining (PSS) shows sample loadings. Band intensities were measured using the ImageJ software. Numbers indicate the accumulation levels of SCMV CP normalized to PSS staining. Error bars indicate the means ± standard deviation of three replicates. Statistical significance was determined by employing Tukey multiple range test for between-group comparisons. Different letters indicate significant differences (P < 0.05). The same below. The experiments were repeated thrice independently.
To explore the role of amino acid residues C57 and C60 in determining SCMV virulence, we carried out the site-directed mutagenesis using primers listed in Supplementary Table 1. The resulting plasmids pSCMV-HCC57A-GFP and pSCMV-HCC60A-GFP were agroinfiltrated to N. benthamiana leaves, respectively, and mechanically inoculated to maize plants 3 days later. Five plants were inoculated for each treatment. The corresponding amino acid of C57 or C60 was alanine (A) in HC-Pro derived from the progeny of SCMV mutants (Figure 2A). At 10 dpi, wild-type SCMV with the gfp reporter gene (SCMV-GFP) caused severe mosaic symptoms and induced strong GFP fluorescence under UV light in the upper non-inoculated leaves of maize. Compared with the wild-type SCMV-GFP, the symptoms caused by SCMV-HCC57A-GFP and SCMV-HCC60A-GFP were attenuated significantly under daylight and the fluorescence intensity of GFP markedly decreased under UV light in the maize upper leaves at 10 dpi (Figure 2B). Results of qRT-PCR showed that compared with the wild-type SCMV-GFP, SCMV RNA accumulation levels decreased by about 60% for SCMV-HCC57A-GFP and SCMV-HCC60A-GFP (Figure 2C). Enzyme-linked immunosorbent assay results showed that the accumulation levels of SCMV-HCC57A-GFP and SCMV-HCC60A-GFP were significantly (P < 0.05) lower than that of wild-type SCMV-GFP (Figure 2D). These results indicated that C57 and C60 of HC-Pro played an important role in determining SCMV virulence in maize plants.
To investigate the role of amino acid residues C57 and C60 in SCMV HC-Pro RSS activity, we cloned the HC-Pro coding sequence into pBin121 vector. The resulting plamids was named pBin-HC. The codons encoding C57 and C60 in pBin-HC were mutated to codon encoding Alanine (A) using primers listed in Supplementary Table 1, and the resulting plasmids were named pBin-HCC57A and pBin-HCC60A. They were transformed into Agrobacterium cells and then individually mixed with Agrobacterium harboring plasmid pBin-GFP in a ratio of 1:1. The mixtures were infiltrated into the fully expanded leaves of N. benthamiana 16C plants. The Agrobactium harboring plasmid pBin-GUS was used as a negative control. At 3 days post agroinfiltration (dpai), no GFP fluorescence was observed in the patches expressing GUS; GFP fluorescence was apparent in the patch expressing HC; however, GFP fluorescence in the patches expressing HCC57A or HCC60A was significantly reduced compared with wild type HC-Pro (Figure 2E). qRT-PCR showed that GFP mRNA accumulated only up to about 20%–30% of wild-type HC-Pro for HCC57A and HCC60A (Figure 2F). Western blotting results showed that GFP accumulation levels in 16C leaf patches expressing HC-Pro mutants HCC57A and HCC60A were significantly lower than that of wild-type HC-Pro (Figure 2G). Interestingly, the HC-Pro accumulation levels of mutants HCC57A and HCC60A were significantly lower than that of wild-type HC-Pro in 16C leaf patches (Figure 2F).
These results showed that the C57 and C60 residues in HC-Pro zinc finger-like motif played a critical role in SCMV virulence and HC-Pro RSS activity in plants.
Three Sugarcane Mosaic Virus Mutants With Double-Mutations in Helper Component-Proteinase Displayed Reduced Virulence
In our previous study, we found that arginine (R) at position 184 (R184) of the FRNK motif was also involved in SCMV virulence in maize plants (Xu et al., 2020). Double-mutant plasmids pSCMV-HCFINK/C57A, pSCMV-HCFINK/C60A, pSCMV-HCFKNK/C57A and pSCMV-HCFKNK/C60A were obtained according to the above described method using the primers listed in Supplementary Table 1. The progeny of four SCMV double-mutants as follows: C57 or C60 to A (C57A or C60A) and R184 to I or K (FINK or FKNK) of SCMV HC-Pro, respectively (Figure 2A). At 10 dpi, the upper leaves of maize plants inoculated with wild-type SCMV showed severe mosaic and yellowing symptoms; whereas the maize plants inoculated with four SCMV double-mutants presented as symptomless (Figure 3A). Results of qRT-PCR showed that SCMV RNA accumulated up to 20% of wild type SCMV for FINK/C60A, FKNK/C57A and FKNK/C60A mutants, while the accumulation levels of SCMV RNA in maize plants inoculated with FINK/C57A and pCB301 were similar and significantly lower (P < 0.5) than that of the other three SCMV double-mutants (Figure 3B). Western blotting results showed that FINK/C60A, FKNK/C57A and FKNK/C60A mutants accumulated to similar level, but significantly lower than that of wild type SCMV (Figure 3C). And in the upper leaves of maize plants inoculated with FINK/C57A and pCB301, SCMV CP could not be detected (Figure 3C). Thus, the double-mutant FINK/C60A, FKNK/C57A and FKNK/C60A, but not FINK/C57A, were capable of systemically infecting maize plants and were candidates for eliciting cross-protection.
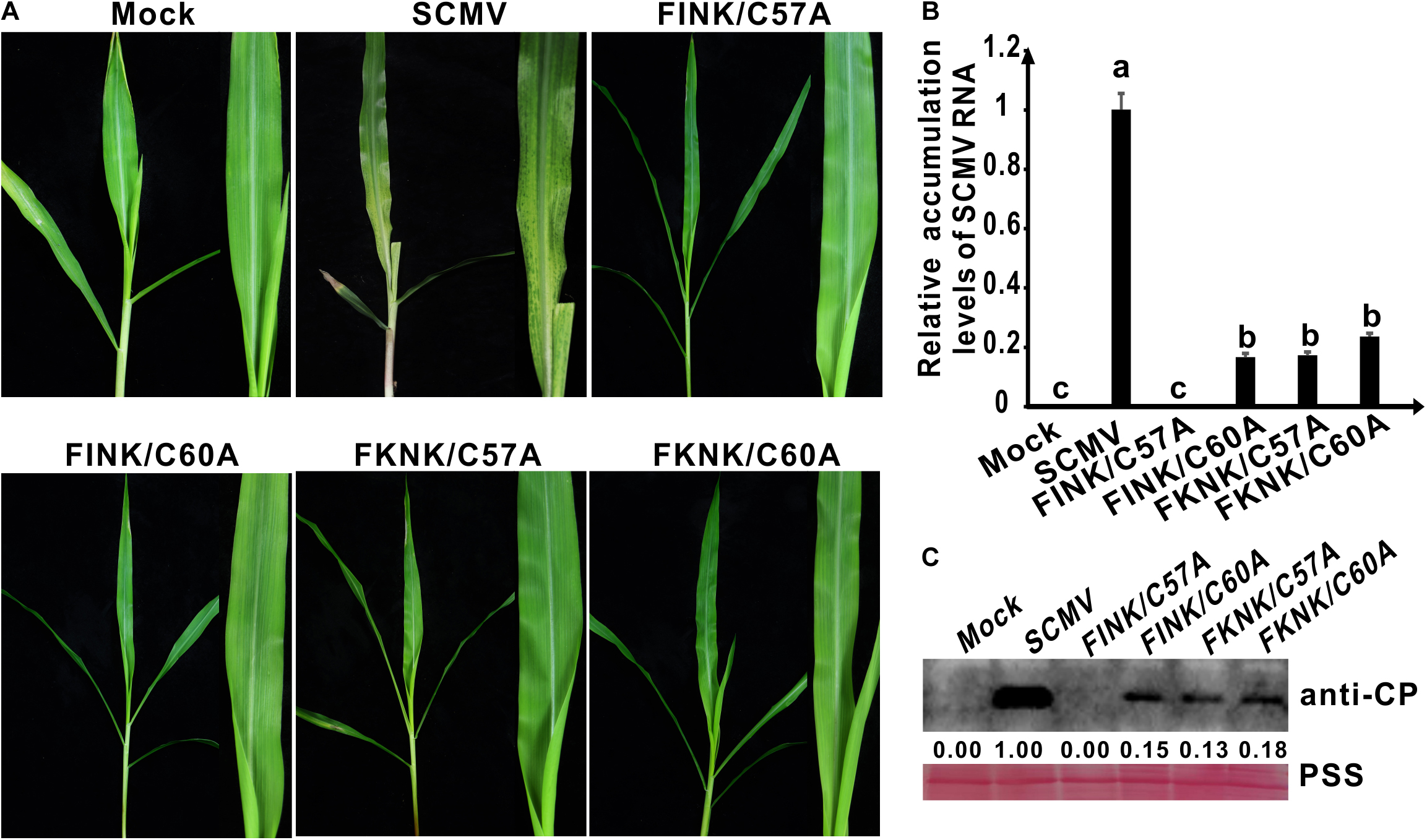
Figure 3. Symptoms and accumulation levels of SCMV double-mutants in maize plants. (A) Symptoms of wild-type SCMV and its double-mutants in maize plants at 10 dpi. Mock, the maize plants inoculated with the empty vector pCB301-Rz. SCMV, the maize plants infected with wild-type SCMV. FINK/C60A, the conserved C60 was mutated to A residues and R184 was mutated to I residues in SCMV HC-Pro. FKNK/C57A, the conserved C57 were mutated to A residues and R184 were mutated to K residues in SCMV HC-Pro. FKNK/C60A, the conserved C60 was mutated to A residues and R184 were mutated to K residues in SCMV HC-Pro. (B) The wild-type and mutant SCMV RNA accumulation levels in the upper leaves of maize plants at 10 dpi. (C) Western blotting analysis of the wild type and mutant SCMV accumulation levels in the upper leaves of maize plants at 10 dpi. The ponceau S staining (PSS) shows sample loadings. Band intensities were measured using the ImageJ software. Numbers indicated the accumulation levels of SCMV CP normalized to PSS staining. The experiments were repeated thrice independently. The statistical analyses as above.
Attenuated Double-Mutant FKNK/C60A Could Protect Maize Plants Against Severe Strain
To test the cross-protection efficacy of three SCMV double-mutants, the first fully expanded leaves of the maize plants primarily inoculated with FINK/C60A, FKNK/C57A, and FKNK/C60A, respectively, were mechanically inoculated with wild-type SCMV-GFP with an interval of 15 or 20 days. At 20 days post challenge inoculation, the non-protected maize plants showed clear mosaic symptom under daylight and strong GFP fluorescence under UV light. With an interval of 15 days, fifteen of the eighteen maize plants protected by FINK/C60A and eleven of the nineteen maize plants protected by FKNK/C57A showed mosaic symptom and strong GFP fluorescence, whereas only five of the seventeen maize plants protected by FKNK/C60A showed mild mosaic and weak GFP fluorescence. With an interval of 20 days, eight of the nineteen maize plants protected by FINK/C60A and five of the sixteen maize plants protected by FKNK/C57A showed mild mosaic and weak GFP fluorescence, and no maize plants protected by FKNK/C60A showed mosaic symptoms and GFP fluorescence (Figure 4A). Western blotting analysis showed that GFP was accumulated in control and the treatments of FINK/C60A or FKNK/C57A, but not in the treatment of FKNK/C60A with an interval of 20 days (Figure 4B). Cross-protection tests indicated that FKNK/C60A double-mutant conferred the best cross-protection efficiency and provided complete cross-protection to the infection of wild-type SCMV with an interval of 20 days.
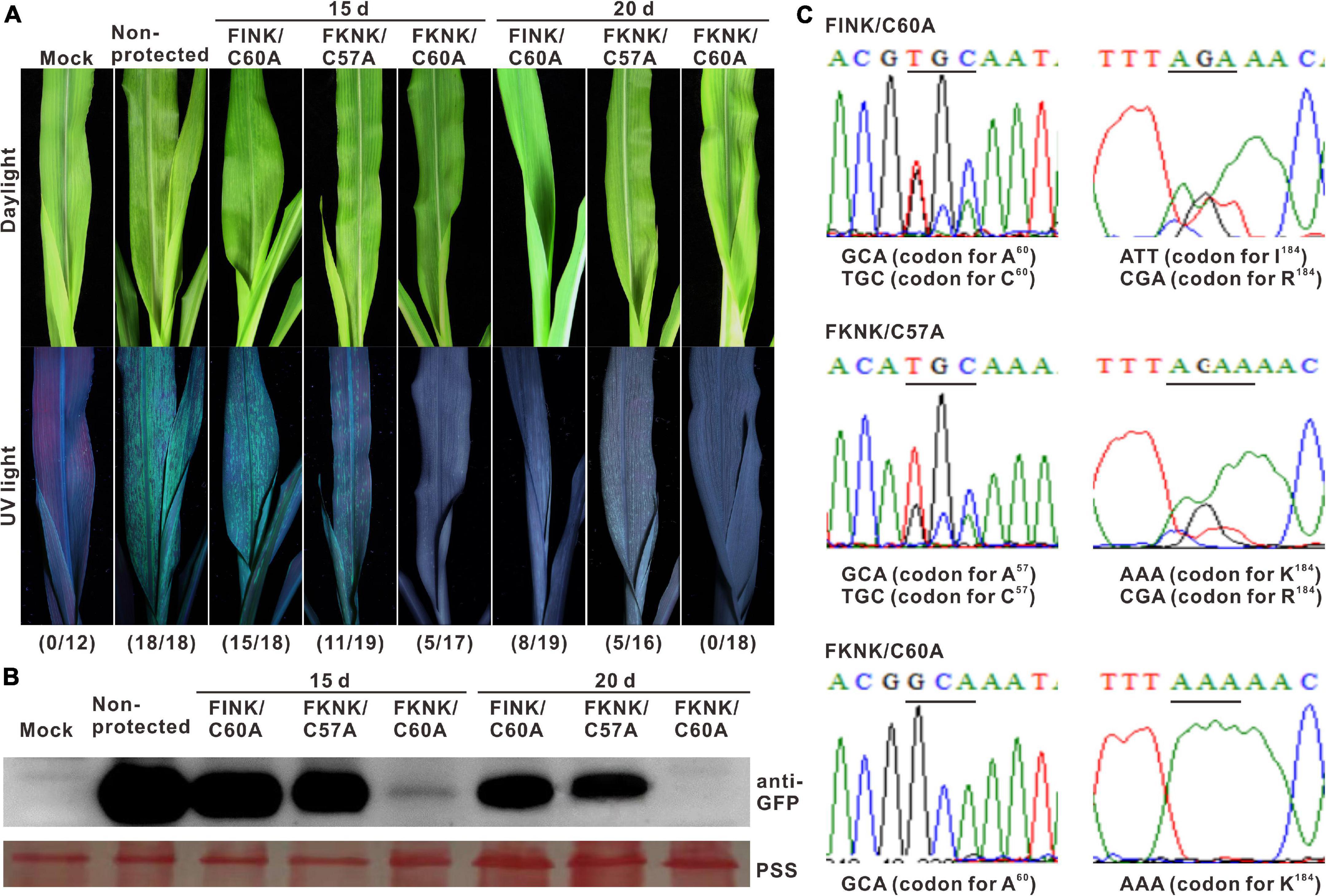
Figure 4. Cross protection efficacy of SCMV double-mutants against severe strain. (A) Symptoms of the maize plants challenged with SCMV-GFP at 20 days post-challenge inoculation with intervals of 15 or 20 dpi. Mock, the maize plants were inoculated with phosphate-buffered saline. Non-protected, the mazie plants were inoculated with pCB301-Rz vector. The numbers of symptomatic/inoculated maize plants were marked in brackets. (B) Western blotting analysis of the GFP accumulation levels in the maize plants challenged with wild-type SCMV-GFP at 20 days post-challenge inoculation. The ponceau S staining (PSS) shows sample loadings. (C) With the interval of 20 days, the sequencing result of HC-Pro RT-PCR products from the maize plants protected with FINK/C60A, FKNK/C57A or FKNK/C60A at 20 days post-challenge inoculation. The sites of point mutations in SCMV HC-Pro were underlined and the corresponding amino acid residues were indicated. The experiments were repeated thrice independently.
The HC-Pro encoding sequences of the SCMV progenies were sequenced at 20 days post-challenge inoculation. Sequencing results showed that, with the interval of 20 days, the codons of the amino acid at position 60 were GCA (codon for A60) and TGC (codon for C60), and the codons of the amino acid at position 184 were ATT (codon for I184) and CGA (codon for R184) in the treatment of FINK/C60A; the codons of the amino acid at position 57 were GCA (codon for A57) and TGC (codon for C57), and the codons of the amino acid at position 184 were AAA (codon for K184) and CGA (codon for R184) in the treatment of FKNK/C57A, indicating that mix infection of the wild type SCMV and mutant FINK/C60A or FKNK/C57A occurred in those maize plants. However, the codons of the amino acid at position 60 was GCA (codon for A60) and at position 184 was AAA (codon for K184) in the treatments of FKNK/C60A (Figure 4C), indicating the wild-type SCMV-GFP was completely excluded by FKNK/C60A mutant.
The Stability of FKNK/C60A Mutant in Maize Plants
The HC-Pro encoding sequence from the SCMV progeny in maize plants infected with FKNK/C60A was sequenced and aligned during four serial passages by mechanical inoculation in maize plants with an interval of 15 days. After successive transfer of 4 generations, neither of the maize plants infected with FKNK/C60A double-mutant showed severe mosaic symptoms. The sequencing results indicated no mutation in the HC-Pro coding sequence from the SCMV progenies in maize plants infected with FKNK/C60A mutant after four successive passages. Thus, FKNK/C60A double-mutant were stable genetically through successive passages in maize plants.
Then the HC-Pro coding sequences from the SCMV progeny in maize plants infected with SCMV mutants at 30 dpi and 60 dpi were sequenced and aligned. At 30 dpi, one of the twenty-three maize plants infected with SCMV-HCFINK showed severe mosaic symptoms. Sequence alignment showed that G440 codon (GGA) in HC-Pro was changed to R codon (AGA) (Table 1). One of the nineteen maize plants infected with SCMV-HCFKNK showed severe mosaic symptoms. Sequence alignment showed that AAA, the codon for K at position 184 (K184) of HC-ProFKNK, mutated to AGA (codon for R) (Table 1). At 60 dpi, one of the maize plants infected with SCMV-HCC60A-GFP or SCMV-HCC57A-GFP, respectively, showed severe mosaic symptoms. And GCA, the codons for A at position 57 or 60 (A57 or A60), were mutated to TGC, which is the codon for C (Table 1). One of the maize plants infected with SCMV-HCFINK showed mild mosaic symptoms, but GAG, the codons for glutamic acid (E) at position 308 (E308) of HC-ProFINK, mutated to GCG, which is the codons for A (Table 1). One of the maize plants infected with SCMV-HCFKNK showed mild mosaic symptoms, but ATA, the codon for I at position 309 (I309) of HC-ProFKNK, mutated to ATG, which is the codons for methionine (M) (Table 1). Although the spontaneous mutations of HC-Pro coding sequences from the SCMV progeny also occurred in maize plants infected with FINK/C60A, FKNK/C57A and FKNK/C60A double-mutants, all the maize plants still showed mild mosaic symptoms at 60 dpi (Table 1), indicating the attenuated symptoms caused by SCMV double-mutants were stable in maize plants at 60 dpi.
Discussion
In this study, our results showed that the mutation of C57 and C60 in the zinc finger-like motif of HC-Pro affected its RSS activity and SCMV virulence. The attenuated SCMV mutants with double-mutations in the zinc finger-like and FRNK motifs were obtained. The FKNK/C60A double-mutant only caused mild symptoms in maize plants until 95 dpi and could provide complete cross-protection to the infection of wild-type SCMV with an interval of 20 days, thus it was a promising mild strain for cross-protection.
In the molecular arms race, potyvirus has evolved an effective RNA silencing suppressor (HC-Pro) to counteract the RNA silencing mechanism in plants (Anandalakshmi et al., 1998; Ivanov et al., 2016; Valli et al., 2018). Helper component-proteinase is a major virulence determinant of potyviruses and an important candidate for screening attenuated mutants (Seo et al., 2011; Huang et al., 2019; Bao et al., 2020; Cheng et al., 2020). Single or multiple amino acid mutations in the conserved motifs of HC-Pro affected potyviral virulence (Desbiez et al., 2010; Kung et al., 2014; Tuo et al., 2020). The N-terminal domain of multifunctional potyviral HC-Pro contains a zinc finger-like motif and a KITC motif (Govier and Kassanis, 1974; Atreya et al., 1992; Atreya and Pirone, 1993; Valli et al., 2014, 2018). The zinc finger-like motif in HC-Pro plays a crucial role in its self-interaction and viral virulence. The single amino acid changes C25 and C53 to glutamic (G) within the zinc finger-like motif is critical for the self-interaction of PVY HC-Pro (Urcuqui-Inchima et al., 1999). The substitution of threonine (T) at position 27 with I in HC-Pro zinc finger-like motif reduced the virulence of clover yellow vein virus (Yambao et al., 2008). The mutation of C at position 310 in the zinc finger-like motif of TVMV HC-Pro corresponding to C57 of SCMV HC-Pro to serine (S) has profound effects on the TVMV virulence, while the importance of C at position 313 of TVMV corresponding to C60 of SCMV HC-Pro remains unknown (Atreya and Pirone, 1993). This study found that the mutations on C57 and C60 in the zinc finger-like motif of HC-Pro reduced its RSS activity and SCMV virulence (Figure 2). Consistent with previous studies, our results also showed that viral symptom is correlated with the RSS activity of HC-Pro (Gal-On, 2000; Shiboleth et al., 2007; Chen L. et al., 2017). Interestingly, we noticed that the accumulation levels of HCC57A and HCC60A mutants were also reduced significantly (P < 0.05) compared to wild type HC-Pro in 16C leaf patches (Figure 2F), indicating HC-Pro mutants were more unstable than wild type HC-Pro in plants, which might be due to plant autophagy (Nakahara et al., 2012). Therefore, the attenuated virulence and RSS activity might be related to the decrease of HC-Pro accumulation. The KITC motif in HC-Pro plays a key role in aphid transmission (Atreya and Pirone, 1993; Blanc et al., 1998). Tobacco etch virus HC-Pro with the substitution of K for E in the KITC motif failed to interact with a ribosomal protein S2 in aphid heads and lost the aphid transmission activity (Fernandez-Calvino et al., 2010). The alanine (A) substitution at C16, C47 and C49 residues in the zinc finger-like motif of wheat streak mosaic virus HC-Pro abolished vector transmission, indicating that the conserved C residues in HC-Pro zinc finger-like motif might also be involved in aphid transmission (Young et al., 2007).
Cross-protection is an efficient method to control plant viruses. However, it has been applied only for a few crops in the field, such as Citrus sinensis, Manihot esculenta, Theobroma cacao, Solanum lycopersicum, Cucurbita pepo, Cucurbita melo, Cucumis sativus, and Glycine max (Kosaka and Fukunishi, 1993; Montasser et al., 1998; Owor et al., 2004; Zanutto et al., 2013; Ameyaw et al., 2016; Aranda and Freitas-Astúa, 2017; Agüero et al., 2018). The stability of mild strain is an important factor limiting the successful application of cross-protection (Ziebell and MacDiarmid, 2017). The spontaneous mutations increase the risk that attenuated mutants may become virulent strains (Loebenstein and Carr, 2006; Ambrós et al., 2018). Single mutation can alter the mild strain of pepino mosaic virus from mild pathotype to necrotic one in tomato and D. inoxia (Hasiów-Jaroszewska et al., 2011). Potyviral polymerases lack the mechanism of proofreading and repair resulting in rapid genetic changes (Roossinck, 1997). Potyvirus HC-Pro is also under continuous mutation (Torres-Barceló et al., 2008, 2009; Ambrós et al., 2018). A spontaneous mutation in HC-Pro of attenuated SCMV mutant could restore HC-Pro RSS activity and SCMV virulence (Xu et al., 2020). The attenuated mutants with two or more mutations might reduce such risk (Cong et al., 2019). The ZYMV GAC triple-mutant was stable after nine months in Chenopodium quinoa plants (Lin et al., 2007). The papaya plants infected with the double mutant of papaya leaf distortion mosaic virus showed symptomless leaves at 60 dpi (Tuo et al., 2020). The maize plants infected with FKNK/C60A double-mutant showed mild symptoms even at 95 dpi, and FKNK/C60A mutant should be a stable mild strain for cross-protection against severe strain. Mutations in RNA viruses occur continuously and randomly, and there are mutational hot-spots (Sanjuán and Domingo-Calap, 2016; Domingo, 2020). The 3‘ non-translated region containing numerous mutations from all cucumber mosaic virus populations is a mutational hot-spot (Schneider and Roossinck, 2001). In this study, we found that the high frequency of spontaneous mutations was observed in the central and C-terminal regions of HC-Pro (101–460 residues) from all the populations of SCMV mutants in maize plants (Table 1). Reverse mutations were not observed in HC-Pro zinc finger-like motif from the progeny of SCMV double-mutants in maize plants. Furthermore, their SCMV RNA accumulated up to about 50% of SCMV-HCC57A-GFP and SCMV-HCC60A-GFP mutants for SCMV double-mutants in the maize plants (Figures 2C, 3B). These results indicated that lower copy numbers of the double-mutants might impair the error-prone replication in HC-Pro zinc finger-like motif.
In summary, our results reveal C57 and C60 in the zinc finger-like motif of HC-Pro are involved in its RSS activity and virulence of SCMV in plants. The study also reports a promising attenuated SCMV double-mutant for cross-protection. These results provide a theoretical guide for the management of SCMV by cross-protection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
X-JX, Y-PT, and X-DL designed the experiments and wrote the manuscript. X-JX, S-YJ, QZ, and Z-YY performed the experiments. X-JX, CG, Y-PT, and X-DL analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funds from Shandong Modern Agricultural Technology and Industry System (SDAIT-02-10) and “Taishan Scholar” Construction Project (TS201712023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.788963/full#supplementary-material
References
Agüero, J., Gómez-Aix, C., Sempere, R. N., García-Villalba, J., García-Núñez, J., Hernando, Y., et al. (2018). Stable and broad spectrum cross-protection against Pepino mosaic virus attained by mixed infection. Front. Plant Sci. 9:1810. doi: 10.3389/fpls.2018.01810
Ambrós, S., de la Iglesia, F., Rosario, S. M., Butkoviæ, A., Elena, S. F., and Abergel, C. (2018). Engineered functional redundancy relaxes selective constraints upon endogenous genes in viral RNA genomes. Genome Biol. Evol. 10, 1823–1836. doi: 10.1093/gbe/evy141
Ameyaw, G., Domfeh, O., Dzahini-Obiatey, H., Ollennu, L., and Owusu, G. (2016). Appraisal of cocoa swollen shoot virus (CSSV) mild isolates for cross protection of cocoa against severe strains in Ghana. Plant Dis. 100, 810–815. doi: 10.1094/PDIS-09-15-0974-RE
Anandalakshmi, R., Pruss, G. J., Ge, X., Marathe, R., Mallory, A. C., Smith, T. H., et al. (1998). A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. U. S. A. 95, 13079–13084. doi: 10.1073/pnas.95.22.13079
Aranda, M., and Freitas-Astúa, J. (2017). Ecology and diversity of plant viruses, and epidemiology of plant virus-induced diseases. Ann. Appl. Biol. 171, 1–4. doi: 10.1111/aab.12361
Atreya, C., Atreya, P., Thornbury, D., and Pirone, T. (1992). Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology 191, 106–111. doi: 10.1016/0042-6822(92)90171-K
Atreya, C. D., and Pirone, T. P. (1993). Mutational analysis of the helper component-proteinase gene of a potyvirus: effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proc. Natl. Acad. Sci. U. S. A. 90, 11919–11923. doi: 10.1073/pnas.90.24.11919
Bao, W., Yan, T., Deng, X., and Wuriyanghan, H. (2020). Synthesis of full-length cDNA infectious clones of soybean mosaic virus and functional identification of a key amino acid in the silencing suppressor Hc-Pro. Viruses 12:886. doi: 10.3390/v12080886
Blanc, S., Ammar, E., Garcia-Lampasona, S., Dolja, V., Llave, C., Baker, J., et al. (1998). Mutations in the potyvirus helper component protein: effects on interactions with virions and aphid stylets. J. Gen. Virol. 79, 3119–3122. doi: 10.1099/0022-1317-79-12-3119
Chen, H., Cao, Y., Li, Y., Xia, Z., Xie, J., Carr, J. P., et al. (2017). Identification of differentially regulated maize proteins conditioning Sugarcane mosaic virus systemic infection. New Phytol. 215, 1156–1172. doi: 10.1111/nph.14645
Chen, L., Yan, Z., Xia, Z., Cheng, Y., Jiao, Z., Sun, B., et al. (2017). A violaxanthin deepoxidase interacts with a viral suppressor of RNA silencing to inhibit virus amplification. Plant Physiol. 175, 1774–1794. doi: 10.1104/pp.17.00638
Cheng, D., Tian, Y., Geng, C., Guo, Y., Jia, M., and Li, X. (2020). Development and application of a full-length infectious clone of potato virus Y isolate belonging to SYR-I strain. Virus Res. 276:197827. doi: 10.1016/j.virusres.2019.197827
Chung, B. Y. W., Miller, W. A., Atkins, J. F., and Firth, A. E. (2008). An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. U. S. A. 105, 5897–5902. doi: 10.1073/pnas.0800468105
Cong, Q., Wang, Y., Liu, J., Lan, Y., Guo, Z., Yang, J., et al. (2019). Evaluation of Potato virus X mild mutants for cross protection against severe infection in China. Virol. J. 16:36. doi: 10.1186/s12985-019-1143-7
Desbiez, C., Girard, M., and Lecoq, H. (2010). A novel natural mutation in HC-Pro responsible for mild symptomatology of Zucchini yellow mosaic virus (ZYMV, Potyvirus) in cucurbits. Arch. Virol. 155, 397–401. doi: 10.1007/s00705-009-0569-4
Domingo, E. (2020). Molecular basis of genetic variation of viruses: error-prone replication. Virus Populations 2, 35–71. doi: 10.1016/B978-0-12-816331-3.00002-7
Fernandez-Calvino, L., Goytia, E., Lopez-Abella, D., Giner, A., Urizarna, M., Vilaplana, L., et al. (2010). The helper-component protease transmission factor of tobacco etch potyvirus binds specifically to an aphid ribosomal protein homologous to the laminin receptor precursor. J. Gen. Virol. 91, 2862–2873. doi: 10.1099/vir.0.022335-0
Gal-On, A. (2000). A point mutation in the FRNK motif of the potyvirus helper component-protease gene alters symptom expression in cucurbits and elicits protection against the severe homologous virus. Phytopathology 90, 467–473. doi: 10.1094/PHYTO.2000.90.5.467
Gao, B., Cui, X. W., Li, X. D., Zhang, C. Q., and Miao, H. Q. (2011). Complete genomic sequence analysis of a highly virulent isolate revealed a novel strain of Sugarcane mosaic virus. Virus Genes 43, 390–397. doi: 10.1007/s11262-011-0644-2
Gao, R., Tian, Y., Wang, J., Yin, X., Li, X., and Valkonen, J. P. (2012). Construction of an infectious cDNA clone and gene expression vector of Tobacco vein banding mosaic virus (genus Potyvirus). Virus Res. 169, 276–281. doi: 10.1016/j.virusres.2012.07.010
Govier, D. A., and Kassanis, B. (1974). A virus-induced component of plant sap needed when aphids acquire potato virus Y from purified preparations. Virology 61, 420–426. doi: 10.1016/0042-6822(74)90278-5
Hasiów-Jaroszewska, B., Borodynko, N., Jackowiak, P., Figlerowicz, M., and Pospieszny, H. (2011). Single mutation converts mild pathotype of the Pepino mosaic virus into necrotic one. Virus Res. 159, 57–61. doi: 10.1016/j.virusres.2011.04.008
Huang, X. D., Fang, L., Gu, Q. S., Tian, Y. P., Geng, C., and Li, X. D. (2019). Cross protection against the watermelon strain of Papaya ringspot virus through modification of viral RNA silencing suppressor. Virus Res. 265, 166–171. doi: 10.1016/j.virusres.2019.03.016
Ivanov, K. I., Eskelin, K., Bašiæ, M., De, S., Lõhmus, A., Varjosalo, M., et al. (2016). Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J. 85, 30–45. doi: 10.1111/tpj.13088
Jiang, J., and Zhou, X. (2002). Maize dwarf mosaic disease in different regions of China is caused by Sugarcane mosaic virus. Arch. Virol. 147, 2437–2443. doi: 10.1007/s00705-002-0890-7
Kosaka, Y., and Fukunishi, T. (1993). Attenuated isolates of soybean mosaic virus derived at a low temperature. Plant Dis. 77, 882–886. doi: 10.1094/PD-77-0882
Krstic, B., Ford, R. E., Shukla, D. D., and Tosic, M. (1995). Cross-protection studies between strains of sugarcane mosaic, maize dwarf mosaic, Johnsongrass mosaic, and sorghum mosaic potyviruses. Plant Dis. 79, 135–138. doi: 10.1094/PD-79-0135
Kung, Y., Lin, P., Yeh, S., Hong, S., Chua, N., Liu, L., et al. (2014). Genetic analyses of the FRNK motif function of Turnip mosaic virus uncover multiple and potentially interactive pathways of cross-protection. Mol. Plant Microbe Interact. 27, 944–955. doi: 10.1094/mpmi-04-14-0116-r
Lin, S. S., Wu, H. W., Jan, F. J., Hou, R. F., and Yeh, S. D. (2007). Modifications of the helper component-protease of Zucchini yellow mosaic virus for generation of attenuated mutants for cross protection against severe infection. Phytopathology 97, 287–296. doi: 10.1094/PHYTO-97-3-0287
Liu, H., and Naismith, J. H. (2008). An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 8:91. doi: 10.1186/1472-6750-8-91
Liu, L., Peng, B., Zhang, Z., Wu, Y., Miras, M., Aranda, M. A., et al. (2017). Exploring different mutations at a single amino acid position of Cucumber green mottle mosaic virus replicase to attain stable symptom attenuation. Phytopathology 107, 1080–1086. doi: 10.1094/PHYTO-03-17-0107-R
Loebenstein, G., and Carr, J. P. (2006). Natural Resistance Mechanisms Of Plants To Viruses. the Netherlands: Springer.
Maassab, H. F., and DeBorde, D. C. (1985). Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine 3, 355–369. doi: 10.1016/0264-410X(85)90124-0
Matthews, R. (1949). Studies on potato virus X II. Criteria of relationships between strains. Ann. Appl. Biol. 36, 460–474. doi: 10.1111/j.1744-7348.1949.tb06942.x
Montasser, M., Tousignant, M., and Kaper, J. (1998). Viral satellite RNAs for the prevention of cucumber mosaic virus (CMV) disease in field-grown pepper and melon plants. Plant Dis. 82, 1298–1303.
Nakahara, K. S., Masuta, C., Yamada, S., Shimura, H., Kashihara, Y., Wada, T. S., et al. (2012). Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc. Natl. Acad. Sci. U. S. A. 109, 10113–10118. doi: 10.1073/pnas.1201628109
Olspert, A., Chung, B. Y. W., Atkins, J. F., Carr, J. P., and Firth, A. E. (2015). Transcriptional slippage in the positive-sense RNA virus family Potyviridae. EMBO Rep. 16, 995–1004. doi: 10.15252/embr.201540509
Owor, B., Legg, J., Okao-Okuja, G., Obonyo, R., Kyamanywa, S., and Ogenga-Latigo, M. (2004). Field studies of cross protection with cassava mosaic geminiviruses in Uganda. J. Phytopathol. 152, 243–249. doi: 10.1094/PDIS.1998.82.12.1298
Pechinger, K., Chooi, K. M., MacDiarmid, R. M., Harper, S. J., and Ziebell, H. (2019). A new era for mild strain cross-protection. Viruses 11:670. doi: 10.3390/v11070670
Pirone, T. P., and Blanc, S. (1996). Helper-dependent vector transmission of plant viruses. Annu. Rev. Phytopathol. 34, 227–247. doi: 10.1146/annurev.phyto.34.1.227
Rast, A. T. B. (1972). M II-16, an artificial symptomless mutant of tobacco mosaic virus for seedling inoculation of tomato crops. Neth. J. Plant Pathol. 78, 110–112. doi: 10.1007/bf01980475
Robaglia, C., Durand-Tardif, M., Tronchet, M., Boudazin, G., Astier-Manifacier, S., and Casse-Delbart, F. (1989). Nucleotide sequence of potato virus Y (N strain) genomic RNA. J. Gen. Virol. 70, 935–947. doi: 10.1099/0022-1317-70-4-935
Roossinck, M. J. (1997). Mechanisms of plant virus evolution. Annu. Rev. Phytopathol. 35, 191–209. doi: 10.1146/annurev.phyto.35.1.191
Sáenz, P., Salvador, B., Simón-Mateo, C., Kasschau, K. D., Carrington, J. C., and García, J. A. (2002). Host-specific involvement of the HC protein in the long-distance movement of potyviruses. J. Virol. 76, 1922–1931. doi: 10.1128/JVI.76.4.1922-1931.2002
Sanjuán, R., and Domingo-Calap, P. (2016). Mechanisms of viral mutation. Cell. Mol. Life Sci. 73, 4433–4448. doi: 10.1007/s00018-016-2299-6
Schneider, W. L., and Roossinck, M. J. (2001). Genetic diversity in RNA virus quasispecies is controlled by host-virus interactions. J. Virol. 75, 6566–6571. doi: 10.1128/JVI.75.14.6566-6571.2001
Seo, J. K., Sohn, S. H., and Kim, K. H. (2011). A single amino acid change in HC-Pro of soybean mosaic virus alters symptom expression in a soybean cultivar carrying Rsv1 and Rsv3. Arch. Virol. 156, 135–141. doi: 10.1007/s00705-010-0829-3
Sherwood, J. L. (1987). “Mechanisms of cross-protection between plant virus strains,” in Plant Resistance To Viruses, eds D. Harnett Evered S. (Chichester: Wiley), 136–150. doi: 10.1002/9780470513569.ch10
Shiboleth, Y. M., Haronsky, E., Leibman, D., Arazi, T., Wassenegger, M., Whitham, S. A., et al. (2007). The conserved FRNK box in HC-Pro, a plant viral suppressor of gene silencing, is required for small RNA binding and mediates symptom development. J. Virol. 81, 13135–13148. doi: 10.1128/jvi.01031-07
Sun, L., and Suzuki, N. (2008). Intragenic rearrangements of a mycoreovirus induced by the multifunctional protein p29 encoded by the prototypic hypovirus CHV1-EP713. RNA 14, 2557–2571. doi: 10.1261/rna.1125408
Torres-Barceló, C., Daròs, J.-A., and Elena, S. F. (2009). Compensatory molecular evolution of HC-Pro, an RNA-silencing suppressor from a plant RNA virus. Mol. Biol. Evol. 27, 543–551. doi: 10.1093/molbev/msp272
Torres-Barceló, C., Martín, S., Daròs, J.-A., and Elena, S. F. (2008). From hypo-to hypersuppression: effect of amino acid substitutions on the RNA-silencing suppressor activity of the Tobacco etch potyvirus HC-Pro. Genetics 180, 1039–1049. doi: 10.1534/genetics.108.091363
Tosh, P. K., Boyce, T. G., and Poland, G. A. (2008). Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin. Proc. 83, 77–84. doi: 10.4065/83.1.77
Tuo, D., Zhou, P., Zhao, G., Yan, P., Tan, D., Li, X., et al. (2020). A double mutation in the conserved motifs of the helper component protease of papaya leaf distortion mosaic virus for the generation of a cross-protective attenuated strain. Phytopathology 110, 187–193. doi: 10.1094/PHYTO-09-19-0328-R
Urcuqui-Inchima, S., Walter, J., Drugeon, G., German-Retana, S., Haenni, A.-L., Candresse, T., et al. (1999). Potyvirus helper component-proteinase self-interaction in the yeast two-hybrid system and delineation of the interaction domain involved. Virology 258, 95–99. doi: 10.1006/viro.1999.9725
Valli, A., Gallo, A., Calvo, M., de Jesus Perez, J., and Garcia, J. A. (2014). A novel role of the potyviral helper component proteinase contributes to enhance the yield of viral particles. J. Virol. 88, 9808–9818. doi: 10.1128/JVI.01010-14
Valli, A. A., Gallo, A., Rodamilans, B., López-Moya, J. J., and García, J. A. (2018). The HCPro from the Potyviridae family: an enviable multitasking Helper Component that every virus would like to have. Mol. Plant Pathol. 19, 744–763. doi: 10.1111/mpp.12553
Wang, Y., Cong, Q., Lan, Y., Geng, C., Li, X., Liang, Y., et al. (2014). Development of new potato virus X-based vectors for gene over-expression and gene silencing assay. Virus Res. 191, 62–69. doi: 10.1016/j.virusres.2014.07.018
Wu, L., Zu, X., Wang, S., and Chen, Y. (2012). Sugarcane mosaic virus-Long history but still a threat to industry. Crop Prot. 42, 74–78. doi: 10.1016/j.cropro.2012.07.005
Wyrsch, I., Domínguez-Ferreras, A., Geldner, N., and Boller, T. (2015). Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol. 206, 774–784. doi: 10.1111/nph.13280
Xie, X., Chen, W., Fu, Q., Zhang, P., An, T., Cui, A., et al. (2016). Molecular variability and distribution of Sugarcane mosaic virus in Shanxi, China. PLoS One 11:e0151549. doi: 10.1371/journal.pone.0151549
Xu, X., Li, H., Cheng, D., Liu, L., and Li, X. (2020). A spontaneous complementary mutation restores the rna silencing suppression activity of hc-pro and the virulence of sugarcane mosaic virus. Front. Plant Sci. 11:1279. doi: 10.3389/fpls.2020.01279
Xu, X., Yu, W., Yang, G., Han, S., He, M., Yang, X., et al. (2019). Prokaryotic expression and antiserum preparation of helper component-proteinase of Sugarcane mosaic virus. Shandong Agric. Sci. 51, 87–91. doi: 10.14083/j.issn.1001-4942.2019.03.018
Xu, X., Zhang, J., Xu, D., Yin, F., Tian, Y., and Li, X. (2018). Prokaryotic expression and antiserum preparation of Sugarcane mosaic virus coat protein. Shandong Agric. Sci. 50, 106–109. doi: 10.14083/j.issn.1001-4942.2018.08.022
Yambao, M. L., Yagihashi, H., Sekiguchi, H., Sekiguchi, T., Sasaki, T., Sato, M., et al. (2008). Point mutations in helper component protease of clover yellow vein virus are associated with the attenuation of RNA-silencing suppression activity and symptom expression in broad bean. Arch. Virol. 153, 105–115. doi: 10.1007/s00705-007-1073-3
Yan, Z., Cheng, D., Liu, J., Tian, Y., Zhang, S., and Li, X. (2016). First report of Sugarcane mosaic virus group IV Isolates from the corn production fields in China. Plant Dis. 100:1508. doi: 10.1094/PDIS-11-15-1373-PDN
Yang, G., Qiu, B. S., Liu, X. G., Li, Y., and Wang, X. F. (2002). Nonsense mutations of replicase and movement protein genes contribute to the attenuation of an avirulent tomato mosaic virus. Virus Res. 87, 119–128. doi: 10.1016/S0168-1702(02)00025-4
Yeh, S. D., and Cheng, Y. H. (1989). Use of resistant Cucumis metuliferus for selection of nitrous-acid induced attenuated strains of papaya ringspot virus. Phytopathology 79, 1033–1039. doi: 10.1094/Phyto-79-1257
Young, B., Hein, G. L., French, R., and Stenger, D. C. (2007). Substitution of conserved cysteine residues in wheat streak mosaic virus HC-Pro abolishes virus transmission by the wheat curl mite. Arch. Virol. 152, 2107–2111. doi: 10.1007/s00705-007-1034-x
Zanutto, C. A., Corazza, M. J., de Carvalho Nunes, W. M., and Müller, G. W. (2013). Evaluation of the protective capacity of new mild Citrus tristeza virus (CTV) isolates selected for a preimmunization program. Sci. Agric. 70, 116–124. doi: 10.1590/S0103-90162013000200009
Zhang, X., and Qu, F. (2016). “Cross protection of plant viruses: recent developments and mechanistic implications,” in Current Research Topics in Plant Virology, eds A. Wang and X. Zhou (Cham: Springer International Publishing), 241–250. doi: 10.1007/978-3-319-32919-2_10
Zhang, X. F., Zhang, S., Guo, Q., Sun, R., Wei, T., and Qu, F. (2018). A new mechanistic model for viral cross protection and superinfection exclusion. Front. Plant Sci. 9:40. doi: 10.3389/fpls.2018.00040
Keywords: cross-protection, helper component-proteinase, RNA silencing suppression, spontaneous mutation, virulence, sugarcane mosaic virus
Citation: Xu X-J, Zhu Q, Jiang S-Y, Yan Z-Y, Geng C, Tian Y-P and Li X-D (2021) Development and Evaluation of Stable Sugarcane Mosaic Virus Mild Mutants for Cross-Protection Against Infection by Severe Strain. Front. Plant Sci. 12:788963. doi: 10.3389/fpls.2021.788963
Received: 04 October 2021; Accepted: 01 December 2021;
Published: 17 December 2021.
Edited by:
Wen-Ming Wang, Sichuan Agricultural University, ChinaReviewed by:
Baoshan Chen, Guangxi University, ChinaRasappa Viswanathan, Indian Council of Agricultural Research (ICAR), India
Copyright © 2021 Xu, Zhu, Jiang, Yan, Geng, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Dong Li, eGRvbmdsaUBzZGF1LmVkdS5jbg==; Yan-Ping Tian, eWFucGluZy50aWFuQHNkYXUuZWR1LmNu
 Xiao-Jie Xu
Xiao-Jie Xu Qing Zhu
Qing Zhu Zhi-Yong Yan
Zhi-Yong Yan Yan-Ping Tian
Yan-Ping Tian Xiang-Dong Li
Xiang-Dong Li