
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 23 December 2021
Sec. Plant Development and EvoDevo
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.764074
REVEILLE (RVE) genes generally act as core circadian oscillators to regulate multiple developmental events and stress responses in plants. It is of importance to document their roles in crops for utilizing them to improve agronomic traits. Soybean is one of the most important crops worldwide. However, the knowledge regarding the functional roles of RVEs is extremely limited in soybean. In this study, the soybean gene GmMYB133 was shown to be homologous to the RVE8 clade genes of Arabidopsis. GmMYB133 displayed a non-rhythmical but salt-inducible expression pattern. Like AtRVE8, overexpression of GmMYB133 in Arabidopsis led to developmental defects such as short hypocotyl and late flowering. Seven light-responsive or auxin-associated genes including AtPIF4 were transcriptionally depressed by GmMYB133, suggesting that GmMYB133 might negatively regulate plant growth. Noticeably, the overexpression of GmMYB133 in Arabidopsis promoted seed germination and plant growth under salt stress, and the contents of chlorophylls and malondialdehyde (MDA) were also enhanced and decreased, respectively. Consistently, the expressions of four positive regulators responsive to salt tolerance were remarkably elevated by GmMYB133 overexpression, indicating that GmMYB133 might confer salt stress tolerance. Further observation showed that GmMYB133 overexpression perturbed the clock rhythm of AtPRR5, and yeast one-hybrid assay indicated that GmMYB133 could bind to the AtPRR5 promoter. Moreover, the retrieved ChIP-Seq data showed that AtPRR5 could directly target five clients including AtPIF4. Thus, a regulatory module GmMYB133-PRR5-PIF4 was proposed to regulate plant growth and salt stress tolerance. These findings laid a foundation to further address the functional roles of GmMYB133 and its regulatory mechanisms in soybean.
CIRCADIAN CLOCK ASSOCIATED 1 (CCA1)-like proteins are a subgroup of MYB-related family, which generally contain the consensus sequence SHAQK(Y/F)F within a single MYB repeat (Du et al., 2013). To date, numerous CCA1-like MYB transcription factors have been identified in different plant species. The best-known roles of CCA1-like MYB genes are their participation in circadian rhythm regulation, therefore enabling plants to make proper responses in the day/night time or under adverse environmental conditions (Carre and Kim, 2002; Nguyen and Lee, 2016). Basically, the morning expressed CCA1-like MYBs, CCA1 and its homolog LATE ELONGATED HYPOCOTYL 1 (LHY1), serve as central oscillators to control circadian rhythms directly through repressing the afternoon expressed PSEUDO-RESPONSE REGULATOR (PRR) genes. Sequentially, the midday-expressed CCA1-like MYBs, REVEILLE (RVE) genes such as RVE4, RVE6, and RVE8, can activate the expressions of the oscillator genes including PRRs and the evening-expressed genes (Nohales, 2021). Recently, it has been proposed that the balance between the activating and repressing MYB-like factors is more important in the regulation of the circadian clock than the presence or absence of any specific factor (Carre and Kim, 2002).
A substantial number of studies indicated that CCA1-like genes might be involved in a wide array of developmental processes including hypocotyl elongation, leaf senescence, flowering, seed germination, hormone signaling pathway, biosynthesis of anthocyanin and isoflavonoid, diurnal carbon allocation and growth, histone modifications, and iron homeostasis. For example, LHY1 and CCA1 regulate photoperiodic flowering in Arabidopsis (Muroya et al., 2021); RVE4/6/8 in Arabidopsis repress plant growth by controlling the cell size (Gray et al., 2017); RVE1/2 are able to regulate seed germination and dormancy (Yang et al., 2020); a few CCA1-like genes such as MYBD and RVE8/LCL5 are positively involved in the regulation of anthocyanin biosynthesis (Nguyen et al., 2015; Perez-Garcia et al., 2015); MYBH participates in the mediation of hypocotyl elongation and leaf senescence (Kwon et al., 2013; Huang et al., 2015). Moreover, increasing evidence revealed that CCA1-like genes perform important functions in stress responses. For instance, CCA1 enables to control the homeostasis of reactive oxygen species and oxidative stress response in Arabidopsis (Lai et al., 2012); MYBS3 in rice is required for cold tolerance (Su et al., 2010); overexpression of SgRVE6 confers physiological responses to cold stress in tobacco (Chen S. et al., 2020). More importantly, some regulatory mechanisms mediated by CCA1-like genes have been revealed in different plant species. For example, RVE4/8 are able to govern plant thermotolerance through regulating the expression of ETHYLENE RESPONSIVE FACTOR53 (ERF53) and ERF54 (Li et al., 2019a); RVE4/8 can be transferred from the cytoplasm to the nucleus, therefore, directly activating DREB1 expression to confer cold tolerance (Kidokoro et al., 2021); CCA1/LHY1-mediated outputs from circadian clock contribute to plant cold tolerance via affecting the CBF cold-response pathway (Dong et al., 2011). Evidently, CCA1-like MYBs play important roles in diverse biological processes. However, the understanding of their functional roles is limited to a few plant species such as Arabidopsis and rice. Therefore, more efforts are needed to understand the functional roles of CCA1-like genes in diverse plant species.
Noticeably, several reports revealed that different members of the CCA1-like subfamily might perform different, even opposing, regulatory functions. For example, three members of the RVE8 clade genes (RVE4, RVE6, and RVE8) in Arabidopsis promote clock pace in a partially redundant manner, while the remaining members, RVE3 and RVE5, play only minor roles in the regulation of clock function (Gray et al., 2017); two CCA1-like genes in Arabidopsis, MYBS1 and MYBS2, show converse roles in regulating glucose and abscisic acid (ABA) signaling during seed germination and early seedling development (Chen et al., 2017); CCA1 represses TOC1 expression by promoting histone deacetylation, whereas RVE8/LCL5 might facilitate TOC1 expression via pronouncing histone acetylation at the TOC1 promoter region (Farinas and Mas, 2011). More interestingly, recent studies indicated that CCA1-like counterparts in different plant species sometimes show distinct or opposite functional roles. For example, two LHY1 genes (GmLHY1a and GmLHY1b) in soybean (Glycine max) enable to decrease drought tolerance through suppressing ABA responses (Wang et al., 2021). However, in Arabidopsis, LHY1 not only inhibits the expression of the rate-limiting enzyme gene (9-CIS-EPOXYCAROTENOID DIOXYGENASE) of ABA biosynthesis but also activates ABA-responsive genes required for drought tolerance (Adams et al., 2018). Thus, it is of significance to document the roles of individual CCA1-like family members in different plant species.
Soybean is well-recognized for the rich source of seed protein and edible oil as well as health-promoting compounds. Previously, we identified 54 soybean CCA1-like genes by data mining against the soybean genome database (Bian et al., 2017a). However, only few soybean CCA1-like genes have been functionally studied such as GmLHYs, GmLCLs, GmMYB138a, GmMYB176, and GmMYB177, which are associated with the regulation of plant height and internode, isoflavonoid accumulation, ABA perception, and signaling pathway, or in response to stresses (Liao et al., 2008; Li et al., 2012; Bian et al., 2017a; Wang et al., 2021; Yuan et al., 2021). Recently, GmMYB133 was reported to be involved in the regulation of isoflavonoid accumulation (Bian et al., 2018). In this study, additional functional roles of GmMYB133 were addressed in Arabidopsis. Overexpression of GmMYB133 in Arabidopsis led to decreased hypocotyl length of seedlings, short leaf petiole, and late flowering as well as enhancement of salt tolerance during seed germination and plant growth. Consistently, nine auxin-associated genes, two light-responsive genes, and four salt tolerance-related genes were transcriptionally altered by GmMYB133 overexpression. Yeast one-hybrid assay indicated that GmMYB133 can bind to the promoter region of AtPRR5, and the rhythmic expression of AtPRR5 was extremely disrupted by GmMYB133 overexpression. Moreover, the retrieved ChIP-Seq data indicated that AtPRR5 can directly target its client genes such as AtCCA1, AtLHY1, AtPIF4, AtBBX24, and AtIAA19. These findings provided solid information to address the functional roles of GmMYB133 and its regulatory mechanism in soybean, especially hypocotyl elongation and salt tolerance.
The protein sequences of GmMYB133 and other CCA1-like transcription factors in soybean and Arabidopsis were downloaded from Phytozome1 and TAIR2. Multiple sequence alignment was performed by MEGA X and DNAMAN. The protein homology analysis was calculated using the online tool MUSCLE of EMBL-EBI3, and the heat map of the identity was generated using the TBtools software (Chen C. et al., 2020). The phylogenetic tree of GmMYB133 and other CCA1-like transcription factors was constructed by the maximum likelihood method with 1,000 bootstrap replications using MEGA X (Kumar et al., 2018). The number on the line indicates the branch length.
The Arabidopsis Col-0 and soybean (cultivar “Jilin 32”) were used in this study. Generally, Arabidopsis plants were grown in growth chambers under long days (16 h light/8 h dark) at 20°C with 70–80% relative humidity. To generate transgenic lines overexpressing GmMYB133, the full-length cDNA of GmMYB133 was amplified and cloned into the Gateway vector pEarleyGate101 through recombination. Transgenic Arabidopsis was generated using the floral dip method (Tsuda et al., 2012). Positive transgenic lines were screened on murashige and skoog (MS) with 10 μg/ml of glufosinate, followed by real-time PCR (RT-PCR) for confirmation with gene-specific primers. Primer information is listed in Supplementary Table 1.
To generate β-glucuronidase (GUS) reporter lines, the 1,605 bp fragment of ATG upstream of GmMYB133 was cloned and constructed into the Gateway vector pMDC162 to obtain pGmMYB133:GUS, and the floral dip method (Tsuda et al., 2012) was used to generate transgenic Arabidopsis. Positive transgenic lines were screened on MS with 50 μg/ml of hygromycin, followed by RT-PCR for confirmation. Primer information is listed in Supplementary Table 1.
To conduct histochemical GUS assay, the transgenic samples with pGmMYB133:GUS (7-day-old seedling, root, rosette leaf, cauline leaf, inflorescences, pod wall, and seeds) were collected and immersed in the GUS staining solution as previously described (Bian et al., 2017b). After vacuuming for 15 min, the samples were incubated in staining solution at 37°C overnight and then destained with 75% ethanol. To investigate the expression of GmMYB133 under drought and salt, 7-day-old transgenic Arabidopsis seedlings with pGmMYB133:GUS were separately transferred to MS solid medium with 150 mM NaCl or 300 mM mannitol, and samples were collected for histochemical GUS staining and GUS activity assay at 3 days after stress treatment, respectively.
Fluorometric GUS assay was performed according to the method described by Jefferson et al. (1987). In brief, Arabidopsis seedlings were homogenized in 1 ml extraction buffer and centrifuged at 12,000 rpm for 10 min at 4°C. Notably, 100 μl supernatant was used to perform the assay of GUS activity in 400 μl reaction buffer with 1 mM 4-methylumbelliferyl-β-D-glucuronide. Fluorescence was measured using a fluorescence spectrophotometer at an excitation/emission wavelength of 365/455 nm. The protein concentration in the extracts was determined according to the method of bicinchoninic acid (BCA) assay (Smith et al., 1985). GUS activity was counted as picomoles of 4-methylumbelliferone per minute per milligram of protein.
For the analysis of circadian rhythm, vernalized Arabidopsis seeds of untransformed (UT) control and T3 transgenic plants overexpressing GmMYB133 as well as soybean seeds were sown and grown under 12 h light/12 h dark at 22°C for 10 days and then transferred into the condition of constant light. Samples of soybean and Arabidopsis were collected every 4 h from 0 to 72 h, respectively, and then frozen in liquid nitrogen and stored at −80°C for RNA extraction.
Ten-day-old soybean seedlings were subjected to the treatments of cold, drought, and salt stresses as previously described (Li et al., 2015): For cold stress, the ground-above tissues were harvested at 0, 6, 12, 24, 48, and 72 h after seedlings were transferred to 4°C; for drought stress, the ground-above tissues were collected at 0, 2, 4, 6, 8, and 10 days of water stress; for salinity stress, the ground-above tissues were harvested at 0, 6, 12, 24, 48, and 72 h after 200 mM NaCl solution was applied to seedlings. Then, the collected samples were frozen in liquid nitrogen and stored at −80°C for RNA extraction. Each treatment had at least six pots of seedlings, and three biological replicates were performed for each treatment.
Arabidopsis seeds from GmMYB133-overexpressing transgenic lines and UT control were sterilized and planted on MS solid medium, kept at 4°C for 2 days, and grown in a growth chamber under 16 h light/8 h dark at 22°C. At least 30 9-day-old seedlings were used for hypocotyl length assay, and three biological replicates were performed. Statistical significance of the data was analyzed using independent-samples t-test. Error bars indicate SE and p-value < 0.01 (**).
Germination assay of Arabidopsis seeds was conducted as previously described (Li et al., 2018). In brief, Arabidopsis seeds from GmMYB133-overexpressing transgenic lines and UT control were sterilized and sown on MS solid medium with or without 150 mM NaCl, kept at 4°C for 2 days, and then cultured under a photoperiod of 16/8 h (light/dark) at 22°C. The germination rates were monitored for 10 days. Data were analyzed using Graphpad Prism 8 software4.
To investigate the response of adult plants to salt stress, 3-week-old transgenic Arabidopsis plants and UT control were supplied with or without 200 mM NaCl, respectively. After salt stress was applied to Arabidopsis plants for 9 days, samples were collected for the extraction and determination of MDA and total chlorophylls. Additionally, 7-day-old transgenic Arabidopsis plants and UT control were subjected to cold stress (−9°C) for 1 h according to the procedure described by Jiang et al. (2020), and phenotypic difference and MDA content were investigated.
Extraction and determination of MDA were conducted according to the method described by Wu et al. (2021). In brief, 1 g of leaves from GmMYB133-overexpressing transgenic lines and UT control were homogenized in 10% trichloroacetic acid (TCA) and centrifuged for supernatant, which subsequently was mixed with 0.6% thiobarbituric acid in 10% TCA. The mixture was boiled for 15 min and placed on ice immediately, followed by a centrifuge at 10,000 g for 20 min. The absorbance of supernatants was spectrophotometrically measured at 450, 532, and 600 nm, and the following formula was used to count the MDA content of each supernatant: [6.459 × (A532 − A600) − 0.569 × A450]/fresh weight. Chlorophylls were extracted from leaves using acetone. Total chlorophyll content was spectrophotometrically measured as previously described (Li X. Y. et al., 2020).
Three biological replicates were performed with three-technique replicates for the extraction and determination of MDA and chlorophylls. Statistical significance of the data was analyzed using independent-samples t-test. Error bars indicate SE and p-value < 0.01 (**).
Total RNAs of Arabidopsis and soybean were extracted using OminiPlant RNA Kit (CWBIO, China) and RNAprep Pure Plant Plus Kit (TIANGEN, China), respectively. The first-strand cDNA was generated using StarScript II First-strand cDNA Synthesis Mix With gDNA Remover Kit (GenStar, China). Quantitative real-time PCR (qRT-PCR) was performed using the Bio-Rad CFX Connect Real-Time PCR Detection System with the reagent of 2 × RealStar Green Fast Mixture (GenStar, China). AtACTIN8 and GmUBIQUITIN-3 (GmSUBI3) were used as the internal reference for Arabidopsis and soybean, respectively. The data were analyzed using Bio-Rad CFX Manager. Three biological replicates with three techniques were conducted for each sample. Primer information is listed in Supplementary Table 1. Statistical significance of the data was analyzed using independent-samples t-test. Error bars indicate SE and p-value < 0.05 (*) or < 0.01 (**).
The evening element was predicted using the online program PlantPAN 3.0 with TFmatrixID_0029 and TFmatrixID_0030.5
To perform Yeast One-Hybrid (Y1H) assay, the full-length cDNAs of two genes (GmMYB133 and AtPRR5) and the promoter fragments of eleven genes (AtPRR5, AtPIF4, AtBBX24, AtIAA19, AtIAA29, AtSAUR21, AtSAUR26, AtCAX3, AtEARLI1, AtMPK3, and AtAZI1) were amplified using gene-specific primers (Supplementary Table 1). The full-length cDNAs of GmMYB133 and AtPRR5 were constructed into the vector of pB42AD as baits (pB42AD-GmMYB133 and pB42AD-AtPRR5), while promoter fragments of the above eleven genes were cloned into the vector pLacZi as preys [pLacZi-pAtPRR5-1 and pLacZi-pAtPRR5-2, pLacZi-pAtPIF4(EE), pLacZi-pAtPIF4(G-box), pLacZi-pAtBBX24, pLacZi-pAtIAA19, pLacZi-pAtIAA29, pLacZi-pAtSAUR21, pLacZi-pAtSAUR26, pLacZi-pAtCAX3, pLacZi-pAtEARLI1, pLacZi-pAtMPK3, and pLacZi-pAtAZI1]. Different plasmid combinations were separately co-transfected into yeast competent cell EGY48, including pB42AD-GmMYB133/pLacZi-pAtPRR5-1, pB42AD-GmMYB133/pLacZi-pAtPRR5-2, pB42AD-GmMYB133/pLacZi-pAtPIF4(EE), pB42AD-GmMYB133/pLacZi-pAtPIF4(G-box), pB42AD-GmMYB133/pLacZi-pAtBBX24, pB42AD-GmMYB133/pLacZi-pAtIAA19, pB42AD-GmMYB133/pLacZi-pAtIAA29, pB42AD-GmMYB133/pLacZi-pAtSAUR21, pB42AD-GmMYB133/pLacZi-pAtSAUR26, pB42AD-GmMYB133/pLacZi-pAtCAX3, pB42AD-GmMYB133/pLacZi-pAtEARLI1, pB42AD-GmMYB133/pLacZi-pAtMPK3, pB42AD-GmMYB133/pLacZi-pAtAZI1, sixteen negative controls (pB42AD/pLacZi, pB42AD-GmMYB133/pLacZi, pB42AD-AtPRR5/pLacZi, pB42AD/pLacZi-pAtPRR5-1, pB42AD/pLacZi-pAtPRR5-2, pB42AD/pLacZi-pAtPIF4-EE, pB42AD/pLacZi-pAtPIF4-G-Box, pB42AD/pLacZi-pAtBBX24, pB42AD/pLacZi-pAtIAA19, pB42AD/pLacZi-pAtIAA29, pB42AD/pLacZi-pAtSAUR21, pB42AD/pLacZi-pAtSAUR26, pB42AD/pLacZi-pAtCAX3, pB42AD/pLacZi-pAtEARLI1, pB42AD/pLacZi-pAtMPK3, and pB42AD/pLacZi-pAtAZI1), and one positive control (pB42AD-AtRVE8/placZi-pAtPRR5).
The ChIP-Seq data of AtPRR5 (Nakamichi et al., 2012) were searched and obtained against Arabidopsis thaliana using AtPRR5 as an assayed protein with the Encyclopedia of Plant Genome (ENPG) database.6 Enrichment peaks for the binding of AtPRR5 to the genomic regions of AtLHY1, AtCCA1, AtPIF4, AtIAA19, and AtBBX24 were retrieved from the ChIP-Seq data of AtPRR5 and visualized through the program ENPG using the default parameters.
Previously, GmMYB133 was revealed as a CCA1-like player in the regulation of isoflavonoid accumulation (Bian et al., 2018). To provide additional cues of its functional roles, we searched its homologs in soybean and Arabidopsis against the Phytozome and TAIR databases using the amino-acid sequence of GmMYB133 as a query, and sixteen proteins were obtained accordingly, e.g., GmRVE5/6/8/8a and AtRVE3/4/5/6/8. Multiple sequence alignment indicated that GmMYB133 and its homologs harbor a conserved MYB domain and the consensus sequence SHAQ(Y/F)F (Supplementary Figure 1). Phylogenetic analysis showed that all the MYB proteins were clustered into three groups, and GmMYB133 was distributed at the same clade with GmRVE5 (Supplementary Figure 1). In soybean, the protein identities between GmMYB133 and its homologs ranged from 46.44 to 90.94%, and GmMYB133 shared the maximum protein identity with GmRVE5 (Figure 1 and Supplementary Figure 1), which has not been functionally characterized. In Arabidopsis, GmMYB133 was closely related to the RVE8 clade proteins (AtRVE3, AtRVE4, AtRVE5, AtRVE6, and AtRVE8) known as the players to control hypocotyl elongation, flowering, leaf petiole growth, or respond to abiotic stresses (Perez-Garcia et al., 2015; Gray et al., 2017; Li et al., 2019a). The identities between GmMYB133 and the AtRVE8 clade genes were up to 48.44%–56.86% at the protein level with AtRVE5 showing maximum identity (Figure 1).
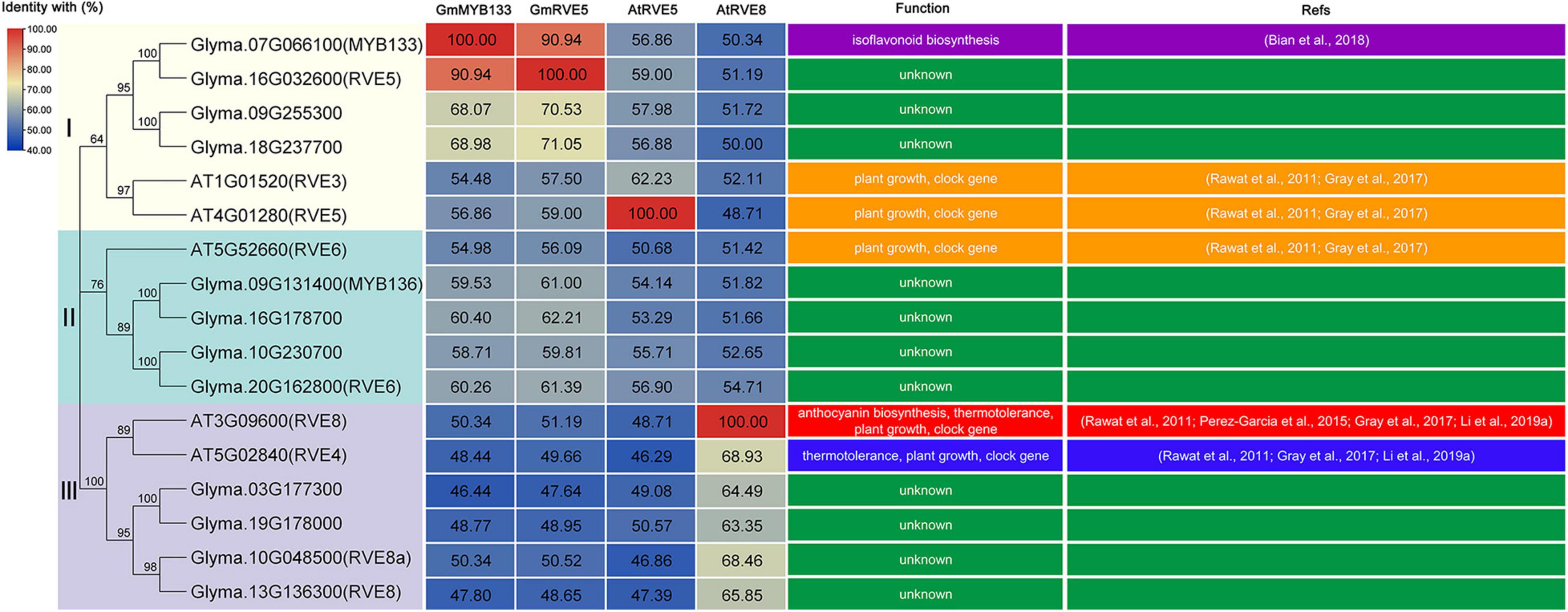
Figure 1. Sequence analysis of GmMYB133 and its homologous proteins in soybean and Arabidopsis. Phylogenetic relationship (left panel) and protein identities (middle panel) of GmMYB133 with its homologs as well as their functional roles (right panel), which were experimentally validated in previous studies. These proteins were clustered into three groups (I, II, and III); the numbers in the middle panel represent the percentage identity between proteins; the color scale and the numbers indicate the identities of GmMYB133, GmRVE5, AtRVE5, and AtRVE8 with their homologs; and low identity is indicated by blue color and high identity is indicated by red color.
Previously, the expression pattern of GmMYB133 was investigated in root, stem, leaves, nodules, flowers, and differently developing embryos (Bian et al., 2018). To explore the spatial expression pattern of GmMYB133, its promoter region was cloned and fused with the GUS reporter gene. The transgenic Arabidopsis lines were generated for histochemical staining. As shown in Figures 2A–H, GUS signal was clearly detected in seedling, root, hypocotyl, rosette leaf, cauline leaf, stigma, anther, and pod wall. Further observation indicated that the GUS signal mainly appeared in the vasculature of cotyledons, leaves, and roots. Since GmMYB133 is homologous to the AtRVE8 clade genes, the rhythmic oscillation of GmMYB133 expression was examined under the constant light condition. The gene GmLHY (Glyma.16G017400) was set as a positive control. Consequently, although GmMYB133 showed oscillating expression to some extent, 24 h rhythmicity was not observed for its expression (Figure 2I). As expected, a typical rhythmic expression was observed for GmLHY (Figure 2J).
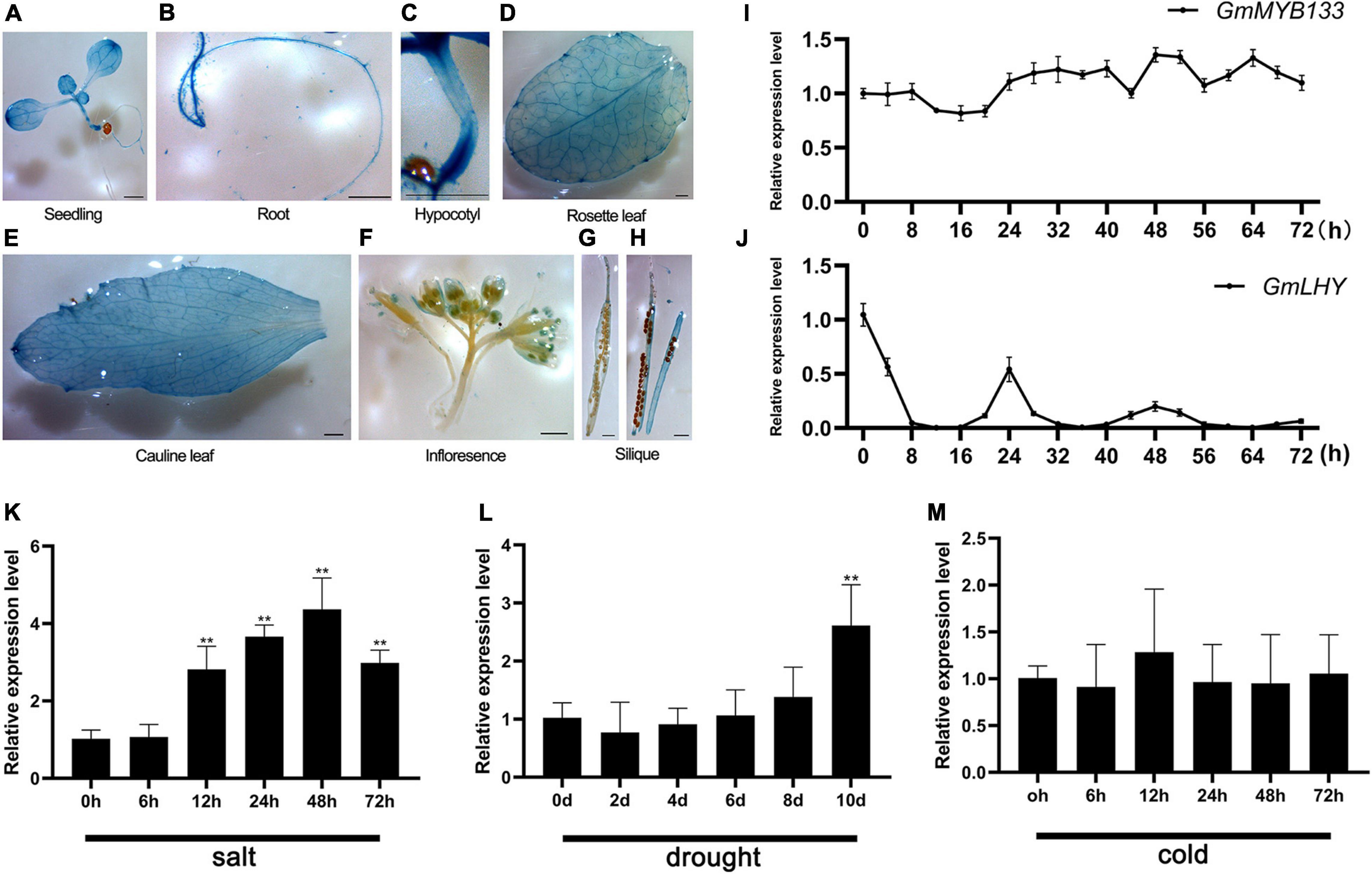
Figure 2. Spatial and diurnal expression patterns of GmMYB133 and its response to abiotic stresses. (A–H) Histochemical analysis of pGmMYB133:GUS activity in Arabidopsis. Transgenic plants were used for analysis including 7-day-old seedling, root, rosette leaf, cauline leaf, inflorescences, pod wall, and seeds. Scale bars indicate 1 mm. (I–J) Diurnal expression patterns of GmMYB133 and GmLHY (Glyma.16G017400) in soybean seedlings. Ten-day-old soybean seedlings were transferred into the condition of constant light, and the time course was set every 4 h from 0 h until 72 h. (K–M) Expression analysis of GmMYB133 in response to abiotic stresses. Ten-day-old soybean seedlings were exposed to salinity (K) and cold (M) stresses for 0, 6, 12, 24, 48, and 72 h and drought stress for 0, 2, 4, 6, 8, and 10 d (L). Error bars indicate SEs of three biological and three technical replicates. Values were normalized against the gene GmSUBI3. Significant differences are denoted by asterisks: **P < 0.01.
Several studies showed that CCA1-like MYBs play important roles in response to diverse stresses (Lai et al., 2012; Li et al., 2019a; Chen S. et al., 2020; Yuan et al., 2021). To investigate if GmMYB133 is involved in response to abiotic stresses, its expression pattern was determined under cold, drought, and salt stresses. When soybean seedlings were subjected to 200 mM NaCl stress, the transcript accumulation of GmMYB133 was gradually increased as stress prolongs and reached its maximum level at 48 h after salt stress with 5.69-fold changes as compared with control (Figure 2K). When soybean seedlings were exposed to drought stress, the expression of GmMYB133 remained a stable level until 10 days after stress, and its transcript accumulation was pronounced by 2.25-fold as compared with control (Figure 2L). However, GmMYB133 showed no significant change when cold stress was applied to soybean seedlings (Figure 2M). Furthermore, the above GUS reporter Arabidopsis was used to investigate the expression of GmMYB133 under drought and salt stress. As compared with the normal condition, a stronger GUS signal was observed in young leaves of seedlings under salt stress. No obvious difference was shown between normal condition and drought stress (Supplementary Figure 2A). Meanwhile, GUS activity was measured under drought and salt stress using the whole seedlings. As shown in Supplementary Figure 2B, no significant difference was observed for GUS activity between normal condition and salt stress as well as drought stress. Subsequently, the young leaves of the above seedlings were collected to investigate the expression of the GUS gene using the qRT-PCR approach. As compared with the normal condition, the expression of the GUS gene was significantly enhanced under both salt and drought stress. Especially under salt stress, a 149.5-fold increase was observed (Supplementary Figure 2C).
To investigate its functional roles, GmMYB133-overexpressing Arabidopsis lines were generated. Four transgenic lines (named as OX-16, OX-8, OX-14, and OX-15) were chosen as representatives for further study. As shown in Figure 3A, transgenic plants showed a compact structure with short-petiole leaf, especially OX-8, OX-14, OX-15, and qRT-PCR analysis indicated that GmMYB133 was, in fact, overexpressed in the four transgenic lines (Figure 3B). Consistent with the structural compactness of transgenic lines, a relatively low level of GmMYB133 transcripts was observed in the transgenic line OX16 and high level in OX-8, OX-14, and OX-15 (Figures 3A,B). Further observation indicated that the transgenic lines OX-8, OX-14, and OX-15 showed a clear delay of flowering under the long-day condition (Figure 3C). Noticeably, the hypocotyl lengths of OX-16, OX-8, OX-14, and OX-15 seedlings were decreased to 72.2%, 55.6%, 58.3%, 58.3% of UT control (Figures 3D,E), respectively, suggesting that overexpression of GmMYB133 might inhibit hypocotyl elongation in Arabidopsis.
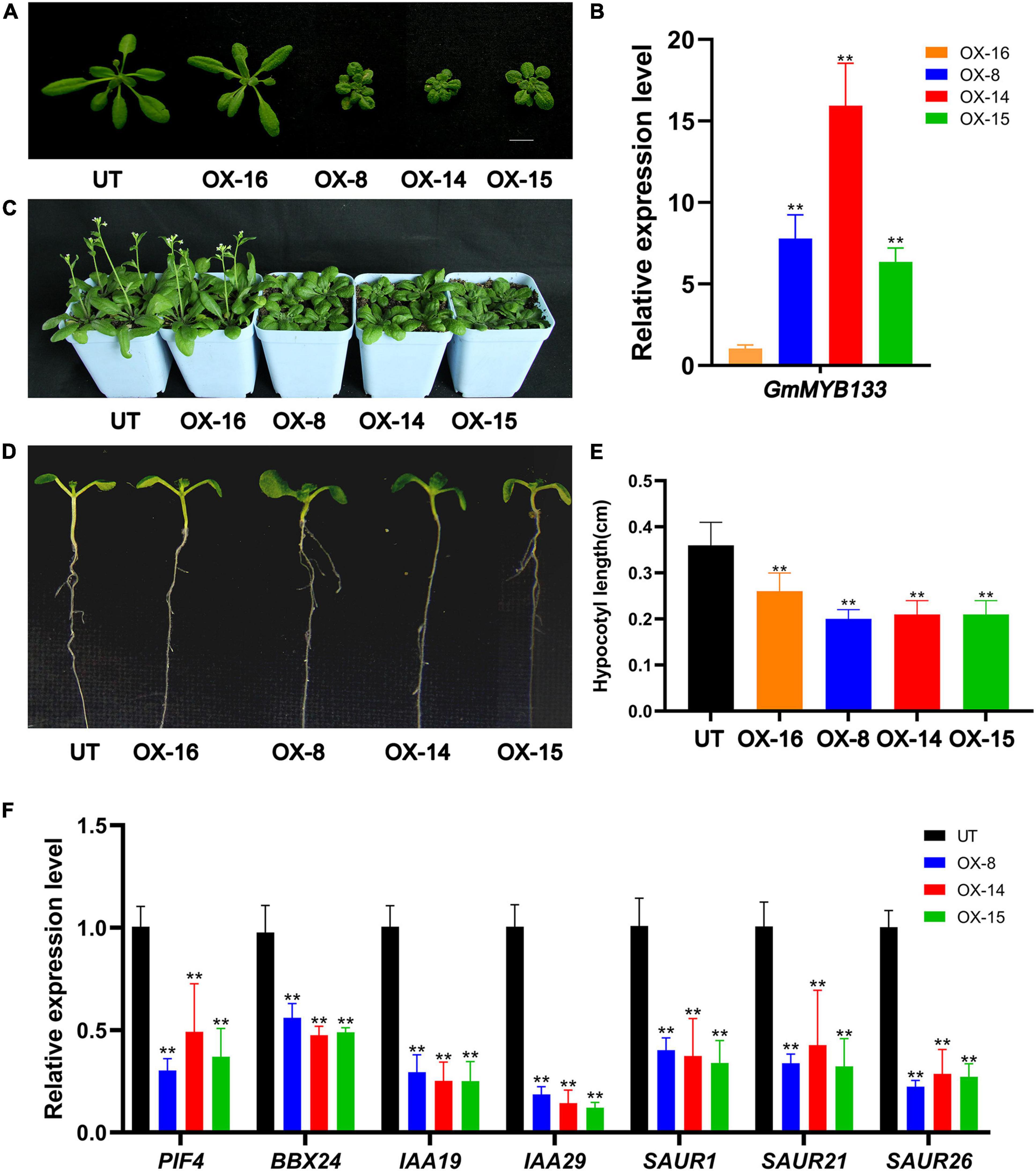
Figure 3. GmMYB133 negatively affects hypocotyl elongation in Arabidopsis. (A) Photograph of untransformed (UT) plant and four transgenic Arabidopsis lines overexpressing GmMYB133 (OX-16, OX-8, OX-14, and OX-15). (B) The expression of GmMYB133 in UT and four transgenic Arabidopsis lines. The expression level in the transgenic line OX-16 was set as 1. (C) Photograph of 36-day-old plants of UT and four transgenic Arabidopsis lines. (D,E) Photograph of hypocotyl elongation (D) and hypocotyl length assay (E) of UT and four transgenic lines. Nine-day-old seedlings were used for the hypocotyl length assay. (F) The expression patterns of light-responsive genes and auxin-associated genes in UT and three transgenic Arabidopsis lines (e.g., AtPIF4, AtBBX24, AtIAA19, AtIAA29, AtSAUR1, AtSAUR21, and AtSAUR26). Total RNAs for (B,F) were exacted from 14-day-old transgenic seedlings and UT. Values were normalized against the gene AtACTIN8, and the expression level in UT was set as 1. Error bars in (B,E,F) indicate SE of three biological and technical replicates, and significant differences are denoted by asterisks: **p < 0.01.
Numerous studies indicated that endogenous auxin and exogenous light signal antagonistically regulate hypocotyl growth (Xi et al., 2021). To provide some clues about how GmMYB133 can be involved in the regulation of hypocotyl elongation, seven genes were chosen to conduct expression analysis using the qRT-PCR approach, including two light-responsive genes (AtPIF4 and AtBBX24) and five auxin-associated genes such as AtIAA19, AtIAA29, AtSAUR1, AtSAUR21, and AtSAUR26. Molecular and/or genetic study has shown that AtPIF4, AtBBX24, AtIAA19, AtIAA29, and AtSAUR21 are positive regulators for hypocotyl elongation (Jiang et al., 2012; Spartz et al., 2012; Zhou et al., 2013; Shimizu et al., 2016; Li et al., 2021). As shown in Figure 3F, all the seven genes (AtPIF4, AtBBX24, AtIAA19, AtIAA29, AtSAUR1, AtSAUR21, and AtSAUR26) were transcriptionally suppressed by GmMYB133 overexpression. For example, the expressions of AtPIF4, AtIAA19, and AtIAA29 in three GmMYB133-overexpressing lines were decreased to 30.1%–49.1%, 25.0%–29.5%, and 12.1%–18.6% of UT control, respectively (Figure 3F). These observations suggested that GmMYB133 might act as a negative regulator in the regulation of hypocotyl elongation through governing the expressions of light-responsive and auxin-associated genes.
Since GmMYB133 showed salt-stress-inducible expression in soybean, its effect on plant tolerance to salt stress was investigated using the transgenic Arabidopsis with GmMYB133 overexpression. First, seeds of three transgenic lines and UT control were sown on solid MS medium supplemented with or without 150 mM NaCl, and then germination rates were dynamically surveyed from 1 to 10 days after vernalization treatment. As shown in Figures 4A,B, a similar germination tendency was observed between transgenic seeds and UT control under normal conditions, and germination rates reached nearly 100% at 4 days after vernalization treatment. However, in the presence of 150 mM NaCl, seed germination was obviously enhanced by GmMYB133 overexpression. As shown in Figures 4A,C, the germination rates for three transgenic lines were up to 68.0%–80.3%, whereas UT control only showed a 43.0% germination rate. Furthermore, phenotypic and physiological analyses of transgenic plants and UT control were performed in the presence of 200 mM NaCl. Consequently, the GmMYB133-overexpressing lines were insensitive to salt stress as compared with UT control. As indicated in Figures 4D,E, the leaves of three transgenic lines were greener than the UT control, and the content of total chlorophylls was significantly increased by GmMYB133 overexpression with 1.7–2.4-fold changes. Consistently, the MDA contents in the three transgenic lines were decreased to 9.3%–14.6% of UT control (Figure 4F). These results suggested that GmMYB133 might confer plant tolerance to salt stress in Arabidopsis. Additionally, transgenic plants and UT control were subjected to cold stress (−9°C) for 1 h, and phenotypic difference and MDA content were investigated accordingly. As shown in Supplementary Figure 3, no significant difference was observed in the aspects of phenotype and MDA content.
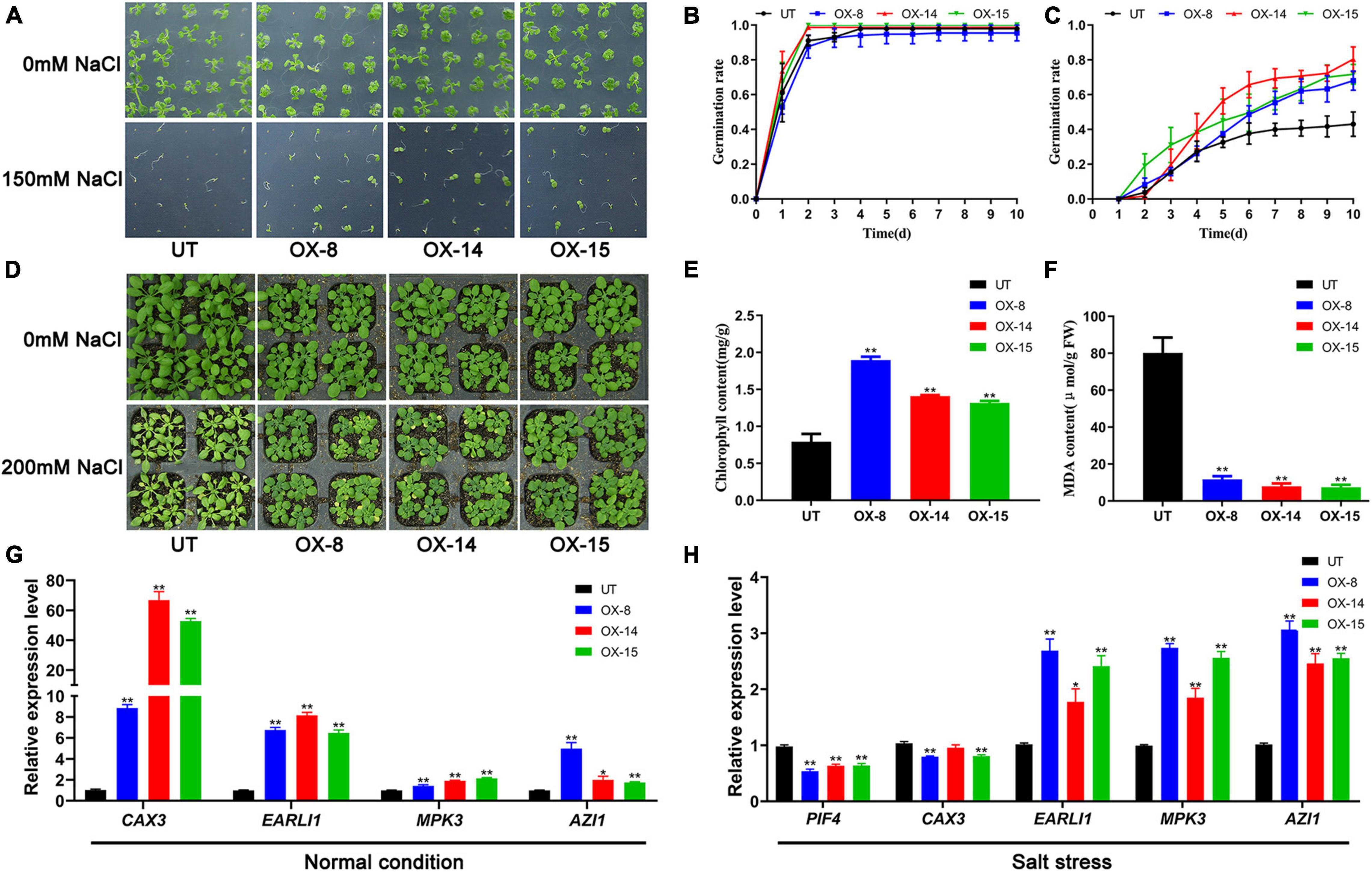
Figure 4. GmMYB133 confers plant tolerance to salt stress in Arabidopsis. (A) Photographs of seed germination for UT plant and three transgenic lines overexpressing GmMYB133 under normal condition and salt stress (150 mM NaCl), which were taken 10 days after culturing under the condition of 16/8 h (light/dark) at 22°C. (B,C) Germination assay of UT and three transgenic lines under normal condition (B) and salt stress (C). The seeds were sowed and cultured on MS solid medium supplied with or without 150 mM NaCl, and the germination rates were monitored for 10 days. (D) Photograph of 30-day-old plants for UT and three transgenic lines under normal condition and salt stress. The 21-day-old plants were exposed to 200 mM NaCl and normal condition for 9 days. (E,F) Quantitative analysis of total chlorophylls (E) and MDA (F) in UT and three transgenic lines under normal condition and salt stress. (G,H) The expression patterns of salt tolerance-associated genes in UT and three transgenic Arabidopsis lines under normal condition (G) and salt stress (H). The genes include AtCAX3, AtEARLI1, AtMPK3, AtAZI1, and AtPIF4. Total RNAs were exacted from 14-day-old transgenic seedlings and UT. Values were normalized against the gene AtACTIN8, and the expression level in UT was set as 1. Error bars in panels (B,C,E–H) indicate SE of three biological and technical replicates, and significant differences are denoted by asterisks: *p < 0.05, **p < 0.01.
To provide some hints about how GmMYB133 can respond to salt stress, four positive regulators for salt tolerance were chosen to conduct expression analysis using the qRT-PCR approach, including AtCAX3, AtEARLI1, AtMPK3, and AtAZI1. Consequently, the expressions of the four genes were significantly enhanced by GmMYB133 overexpression to a different extent. Especially, the two genes AtCAX3 and AtEARLI1 were transcriptionally elevated by 8.9–66.8- and 6.2–8.2-fold changes, respectively. Previous studies indicated that AtPIF4 is not only involved in the regulation of light-associated development but also acts as a negative regulator in response to salt stress. Furthermore, the expressions of the above four positive regulators and AtPIF4 were investigated in the transgenic lines with GmMYB133 overexpression and UT control under salt stress (150 mM NaCl). Consistently, AtPIF4 was transcriptionally decreased by GmMYB133 overexpression under salt stress, while the expressions of AtEARLI1, AtMPK3, and AtAZI1 were remarkably increased in the transgenic lines. However, no change or a slight decrease was observed for AtCAX3 between the transgenic lines and UT control under salt stress. Further observation indicated that the expression of AtCAX3 was remarkably increased in UT control under salt stress (Figure 4H and Supplementary Figure 4), which is consistent with the previous report that AtCAX3 expression was strongly induced by salt stress (Shigaki and Hirschi, 2000). These results implied that GmMYB133 might confer plant tolerance to salt stress through regulating the expressions of salt tolerance-related genes such as AtEARLI1, AtMPK3, and AtAZI1 in Arabidopsis.
It has been known that hypocotyl elongation and salt tolerance are controlled by the circadian clock (Rawat et al., 2011; Nakamichi et al., 2016; Gray et al., 2017). To explore if GmMYB133 affects the circadian clock, the rhythmic expressions of three core oscillator genes (AtCCA1, AtLHY1, and AtPRR5) were investigated in GmMYB133-overexpressing Arabidopsis lines and UT control under continuous light using the qRT-PCR approach. Consequently, all the examined oscillator genes sustained approximately 24-h circadian rhythm in UT control. However, overexpression of GmMYB133 obviously altered the rhythmic expressions of AtCCA1, AtLHY1, and AtPRR5. As shown in Figures 5A,B, AtCCA1 and AtLHY1 showed a 4-h rhythmic phase shift and reduced oscillation amplitude in transgenic lines as compared with type. Noticeably, the rhythmic expression of AtPRR5 was extremely disrupted by GmMYB133 overexpression. As indicated in Figure 5C, the oscillation amplitude of AtPRR5 was remarkably reduced by GmMYB133 overexpression, and a relatively stable expression level was observed for AtPRR5 in GmMYB133-overexpressing Arabidopsis lines. On the whole, the trough level of AtPRR5 was elevated by GmMYB133 overexpression. These results indicated that GmMYB133 might serve as a regulator of core circadian oscillators, especially AtPRR5, to control diverse biological processes.
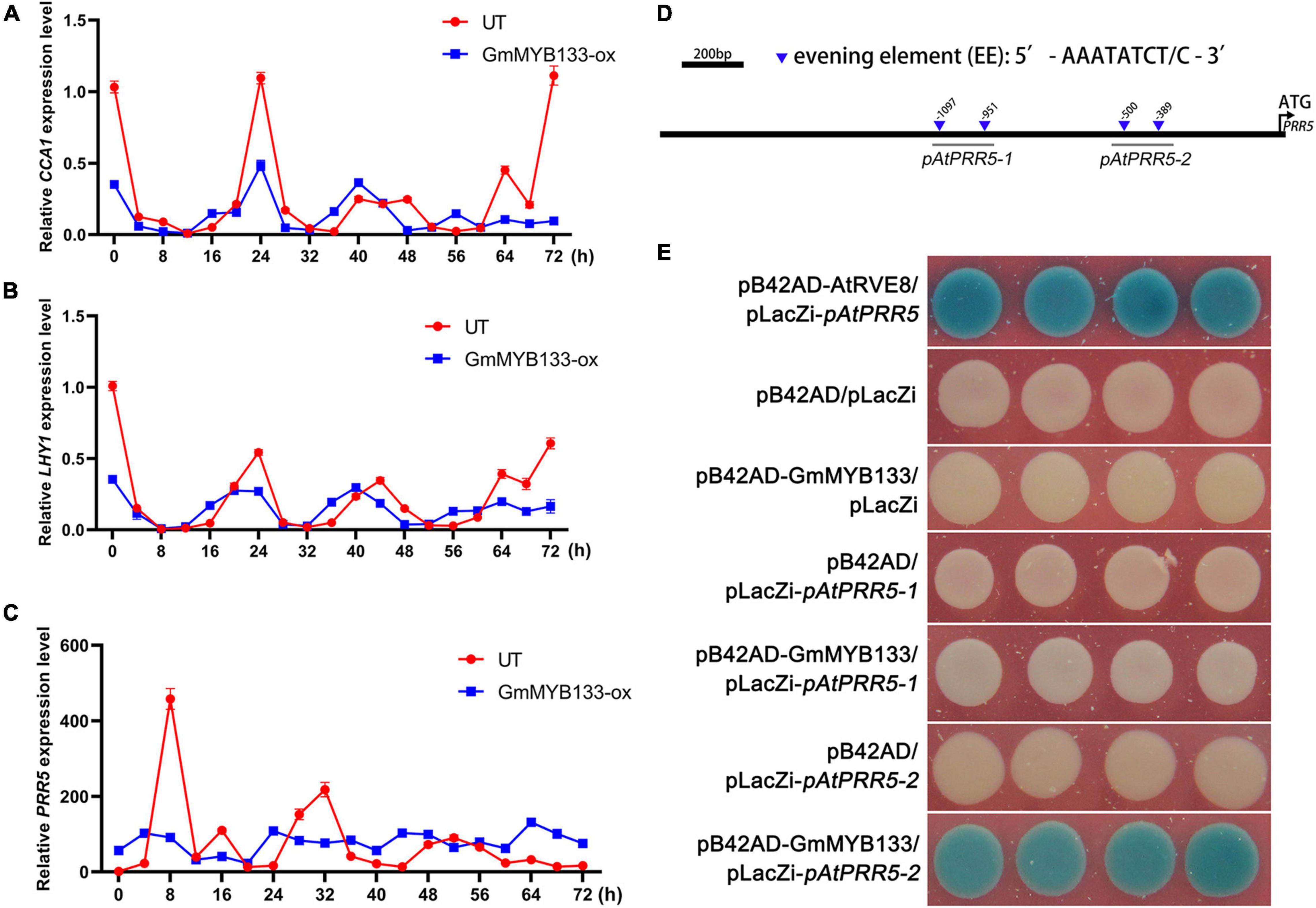
Figure 5. GmMYB133 might affect hypocotyl elongation and salt tolerance directly through regulating the expression of AtPRR5 in Arabidopsis. (A–C) Diurnal expression patterns of three core oscillator genes AtCCA1 (A), AtLHY1 (B), and AtPRR5 (C) in UT plant and the mix of three transgenic lines overexpressing GmMYB133 (OX-8, OX-14, and OX-15). Ten-day-old Arabidopsis seedlings were transferred into the condition of constant light, and the time course was set every 4 h from 0 h until 72 h. Total RNAs were exacted from transgenic seedlings and UT. Values were normalized against the gene AtACTIN8, and the expression level in UT at 0 h was set as 1. Error bars in panel (A–C) indicate the SE of three biological and technical replicates. (D) The schematic diagram of GmMYB133 promoter. The downward triangles indicate the evening element AAATATCT/C, and the number shows the start site of each element. Black lines labeled with pAtPRR5-1 or pAtPRR5-2 represent the examined regions for the Y1H assay. (E) Interaction assay between GmMYB133 and AtPRR5 promoter (pAtPRR5-1 and pAtPRR5-2) using the Y1H approach. Four plasmid combinations were set as negative controls (pB42AD/pLacZi, pB42AD-GmMYB133/pLacZi, pB42AD/pLacZi-pAtPRR5-1, and pB42AD/pLacZi-pAtPRR5-2) and one combination (pB42AD-AtRVE8/placZi-AtPRR5) as positive control.
Since the expression level and endogenous rhythm of AtPRR5 were significantly altered by GmMYB133 overexpression in Arabidopsis, it is worth exploring if GmMYB133 acts as a transcription factor to directly regulate the expression of AtPRR5. First, two CCA1-like protein binding sites (AAATATCT and AAATATCC) were observed in the promoter region of the gene AtPRR5 (Figure 5D). Thus, Y1H assay was performed using GmMYB133 as bait and two fragments of AtPRR5 promoter (named as pAtPRR5-1 and pAtPRR5-2) as preys. Consequently, like the positive control (pB42AD-AtRVE8/placZi-pAtPRR5), blue colonies were shown when GmMYB133 served as bait and the two fragments of AtPRR5 promoter (pAtPRR5-1 and pAtPRR5-2) as preys. As shown in Figure 5E, the colonies harboring the plasmid combination of GmMYB133 and pAtPRR5-2 showed strong blue color, while a very weak blue color was observed for the colonies with the plasmid combination of GmMYB133 and pAtPRR5-1. No blue color appeared in the four negative controls (pB42AD/pLacZi, pB42AD-GmMYB133/pLacZi, pB42AD/pLacZi-pAtPRR5-1, and pB42AD/pLacZi-pAtPRR5-2) (Figure 5E). These results indicated that physical interaction could occur between GmMYB133 protein and AtPRR5 promoter.
GmMYB133 can affect the expressions of AtPIF4, AtBBX24, AtIAA19, AtIAA29, AtSAUR21, AtSAUR26, AtCAX3, AtEARLI1, AtMPK3, and AtAZI1. To explore whether GmMYB133 acts as a transcription factor to directly target these genes, we first predicted the binding site of CCA1-like MYBs, the evening element, in their promoter regions using the online program PlantPAN 3.0. As shown in Supplementary Figure 5A, all the ten genes harbored 1–4 evening element(s) in their promoter regions. Furthermore, the Y1H assay was performed using GmMYB133 as bait and the promoter fragments with evening elements as prey. Consequently, no blue colony was observed when GmMYB133 served as bait and the promoter fragments of the above ten genes as prey, while blue colonies appeared for the positive control (Supplementary Figure 5B).
To further address GmMYB133-mediated mechanisms that control hypocotyl elongation and response to salt stress in Arabidopsis, we retrieved the ChIP-Seq data of AtPRR5 (Nakamichi et al., 2012) to obtain its target clients through the online program ENPG (see text footnote 6). Consequently, the peaks of the AtPRR5 binding site were observed for two rhythm-associated genes (AtCCA1 and AtLHY1), two light-responsive genes (AtPIF4 and AtBBX24), and AtIAA19 (Figures 6A–E). Further observation indicated that the peaks were mainly distributed in the promoter and/or UTR regions. Furthermore, AtPIF4 was chosen as a representative to confirm the binding of AtPRR5 using Y1H assay. AtPRR5 usually prefers to bind to the element G-box (CACGTG), and it was observed that AtPIF4 harbored one G-box element in its promoter region (Figure 6F). Y1H assay indicated that blue colonies appeared when AtPRR5 served as bait and the fragment of AtPIF4 promoter as prey, while no blue colony was observed for the negative control (Figure 6G). These results suggested that GmMYB133 might affect hypocotyl elongation and salt tolerance directly through regulating the expression of AtPRR5, which subsequently alters the transcriptional accumulations of its direct targets such as AtPIF4, AtBBX24, and AtIAA19.
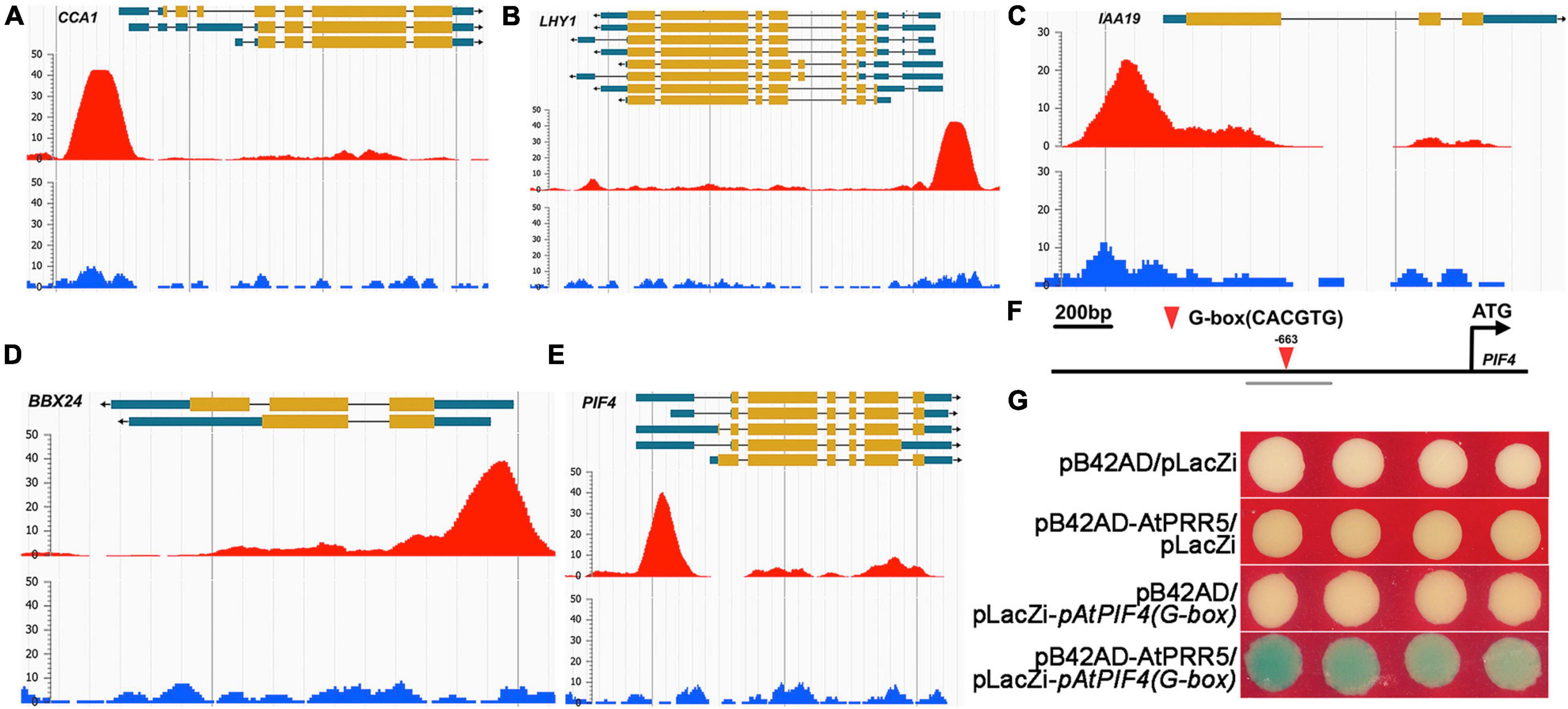
Figure 6. AtPRR5 might directly target circadian, light-responsive, and auxin-associated genes. (A–E) Enrichment peaks for the binding of AtPRR5 to the genomic regions of the circadian clock, light-responsive, and auxin-associated genes. The ChIP-Seq data of AtPRR5 were retrieved against Arabidopsis thaliana using AtPRR5 as assayed protein through the Encyclopedia of Plant Genome (ENPG) database (www.plantseq.org). Enrichment peaks for the binding of AtPRR5 to the genomic regions of AtCCA1, AtLHY1, AtPIF4, AtIAA19, and AtBBX24 were visualized through the program ENPG using the default parameters. The red peaks represent significant occupies of AtPRR5 at its target genes, and blue signals indicate background derived from input control. Gene structures are shown on the top of each panel. (F) The schematic diagram of the AtPIF4 promoter. The downward triangle indicates the G-box (CACGTG), and the number shows the start site of the G-box element. The gray line labeled with pAtPIF4(G-box) represents the examined region for the Y1H assay. (G) Interaction assay between AtPRR5 and AtPIF4 promoter using Y1H approach. Three plasmid combinations were set as negative controls [pB42AD/pLacZi, pB42AD-AtPRR5/pLacZi, and pB42AD/pLacZi-pAtPIF4(G-box)].
RVE genes are generally recognized as core circadian components to activate the expression of their downstream oscillator genes, therefore enabling plants to properly respond to the day/night time or adverse environmental stimuli (Gray et al., 2017; Chen S. et al., 2020; Li X. et al., 2020; Badhan et al., 2021). RVE genes have been documented to participate in the regulation of the first wave of heat shock-induced gene expression (Li et al., 2019a), cold tolerance (Chen S. et al., 2020), anthocyanin accumulation (Li X. et al., 2020), hypocotyl elongation (Rawat et al., 2011), growth of juvenile, and adult plants (Gray et al., 2017). However, the functional roles of RVE genes remained unclear in soybean. Previously, we reported that GmMYB133 affects isoflavonoid biosynthesis in soybean (Bian et al., 2018). In this study, we provided studies to support that GmMYB133 might act as an RVE transcription factor to regulate hypocotyl elongation and salt tolerance, which will enhance our understanding about the regulatory roles of RVE genes in soybean.
Sequence analysis showed that GmMYB133 is homologous to the RVE8 clade genes of Arabidopsis such as AtRVE3, AtRVE4, AtRVE5, AtRVE6, and AtRVE8 (Figure 1). In this study, we provided three aspects of studies to support that GmMYB133 acts as an RVE8 clade gene to negatively regulate plant growth. First, overexpression of GmMYB133 in Arabidopsis altered multiple circadian clock outputs such as decreased hypocotyl lengths of seedlings, short-petiole leaf, and late flowering (Figure 3). These observations are in agreement with the previous reports that AtRVE8-overexpressing Arabidopsis plants showed a late flowering phenotype (Rawat et al., 2011), while simultaneous mutation of RVE4, RVE6, and RVE8 in Arabidopsis facilitated the growth of hypocotyl and leaf petiole (Gray et al., 2017). It is not surprising since the RVE8 clade genes inhibit growth rate and adult cell size. Second, hypocotyl growth is regulated by a series of internal and external cues such as hormone and light (Reed et al., 2018; Kelly et al., 2021). In this study, five auxin-associated genes and two light-responsive genes AtPIF4 and AtBBX24 were, in fact, repressed by GmMYB133 overexpression in Arabidopsis (Figure 3F). These results are consistent with the previous reports that AtPIF4 was largely responsible for the effects of AtRVE4, AtRVE6, and AtRVE8 on hypocotyl growth (Gray et al., 2017), while mutation of AtBBX24 in Arabidopsis led to shorter hypocotyl (Jiang et al., 2012). Third, overexpression of GmMYB133 not only altered the expression intensity of three central oscillators such as AtCCA1, AtLHY1, and AtPRR5 in Arabidopsis but also caused a rhythmic phase shift. Thus, we assumed that GmMYB133 might regulate the photoperiodic plant growth (hypocotyl elongation, leaf petioles, and flowering) by affecting the expressions of the central oscillators such as AtCCA1, AtLHY1, and AtPRR5. Previous studies indicated that AtRVE4, AtRVE6, and AtRVE8 negatively affect hypocotyl elongation, while AtRVE3 and AtRVE5 do not contribute significantly to the effect (Gray et al., 2017). Also, AtRVE8 promotes the expression of AtPRR5 in Arabidopsis (Rawat et al., 2011). Although GmMYB133 showed maximum identity with AtRVE5 (56.86%) at the protein level in Arabidopsis (Figure 1), our results supported that GmMYB133 might perform functions similar to AtRVE4, AtRVE6, or AtRVE8, at least in the aspect of hypocotyl elongation. Noticeably, the rhythmic expressions of AtCCA1, AtLHY1, and AtPRR5 were perturbated by the overexpression of GmMYB133. However, no typical rhythmic expression was observed for GmMYB133 itself (Figure 2I). Previous report indicated that AtRVE3, AtRVE4, and AtRVE8, but not AtRVE5 and AtRVE6, showed rhythmic expression in Arabidopsis seedlings, and AtRVE4, AtRVE6, and AtRVE8 might perform partially redundant functions in speeding up the internal clock pace (Rawat et al., 2011). We speculated that GmMYB133 possibly works together with clock-associated factor (s) to control clock pace.
Recently, several studies indicated that RVE genes play important roles in plant response to abiotic stress including heat, cold, and drought. For example, AtRVE8 and its close homolog AtRVE4 enabled to affect plant thermotolerance by regulating the first wave of heat shock-induced gene expression in Arabidopsis (Li et al., 2019a); overexpression of SgRVE6 in tobacco conferred cold tolerance (Chen S. et al., 2020); drought tolerance of CRISPR-edited RVE7 protoplast was decreased in chickpea (Badhan et al., 2021). To our best knowledge, however, it remains unknown if RVE genes are involved in the regulation of salt stress tolerance. In this study, GmMYB133 showed a salt stress-inducible expression pattern (Figure 2K). Meanwhile, overexpression of GmMYB133 in Arabidopsis enhanced seed germination in salt stress (Figures 4A–C), and phenotypic and physiological analysis indicated that GmMYB133 could improve plant tolerance to salt stress in Arabidopsis (Figures 4D,E). Moreover, four salt tolerance-associated genes (AtCAX3, AtEARLI1, AtAZI1, and AtMPK3) were transcriptionally elevated by overexpression of GmMYB133 (Figure 4F). It was reported that AtCAX3 expression was strongly induced by salt stress, and cax3 mutant showed more sensitivity to salt stress (Zhao et al., 2008); EARLI1 in Arabidopsis greatly facilitated seed germination and seedling development in salt stress (Xu et al., 2011); AtAZI1 overexpression in Arabidopsis strongly conferred plant tolerance to high-salinity stress, and AtMPK3 acted as a positive regulator of AtAZI1 expression (Pitzschke et al., 2014). Thus, we speculated that GmMYB133 might be involved in the regulation of plant tolerance to salt stress through regulating the salt tolerance-associated genes in Arabidopsis. Noticeably, GmMYB133 can simultaneously coordinate the activation of the salt-tolerance genes and the inactivation of the light-responsive and auxin-associated genes (Figures 3F, 4G). It is reasonable since MYBs usually form a complex with themselves or other players to co-regulate diverse developmental processes or respond to various stresses.
PRR5 serves as the core oscillator gene to be involved in the regulation of a wide range of biological processes, including ABA biosynthesis and signaling, root cell proliferation, inhibitory expression of morning-phased clock genes, flowering, photoperiod-responsive growth, and abiotic stress tolerance such as cold, drought, and salt stresses (Nakamichi et al., 2007, 2012, 2016; Rawat et al., 2011; Li et al., 2019b; Yang et al., 2021). In this study, the clock rhythm of AtPRR5 was extremely perturbed by GmMYB133 overexpression, and the trough level of AtPRR5 was elevated by GmMYB133 overexpression (Figure 5C). Moreover, the Y1H assay indicated that GmMYB133 can bind to the promoter region of AtPRR5 (Figures 5D,E). These observations are in agreement with the previous study that AtRVE8 enabled to directly target AtPRR5 and disturbed its rhythmic expression (Rawat et al., 2011). Our results suggested that GmMYB133 and PRR5 possibly constitute a regulatory module to regulate plant growth and stress tolerance. Increasing evidence indicated that PIF4 serves as a molecular hub to positively affect hypocotyl elongation, flowering, and petiole growth by integrating the effects of light and hormones (Kunihiro et al., 2011; Galvao et al., 2019; Rosado et al., 2019; Li et al., 2021). Also, it was observed that the leaves of Arabidopsis pif4-1 mutant showed lower ion leakage under salt stress, whereas overexpression of AtPIF4 caused young seedlings more sensitive to salt stress (Sakuraba et al., 2017). In this study, GmMYB133 overexpression led to several phenotypes opposing to the ones of AtPIF4 mutation such as decreased hypocotyl length of seedlings, short-petiole leaf, and late flowering as well as improvement of plant tolerance to salt stress (Figures 3, 4), and, indeed, AtPIF4 were transcriptionally decreased in GmMYB133-overexpressing Arabidopsis under normal condition and salt stress (Figures 3F, 4H). Intriguingly, the retrieved ChIP-Seq data (Nakamichi et al., 2012) and our Y1H assay showed that AtPIF4 and AtIAA19 can be directly targeted by AtPRR5 (Figures 6A–E). Moreover, it was reported that AtPRR5 serves as transcriptional repressors of AtPIF4 to regulate photoperiodic growth (Takase et al., 2013; Zhu et al., 2016). Thus, we assumed that the regulatory module GmMYB133-AtPRR5 and the integrator AtPIF4 might constitute a triple hierarchy architecture to govern the expressions of their downstream genes, therefore, affecting hypocotyl elongation, leaf petiole growth, flowering, and salt stress tolerance in Arabidopsis.
GmMYB133 is homologous to the RVE8 clade genes of Arabidopsis. Like the RVE genes in Arabidopsis, GmMYB133 affected hypocotyl elongation, leaf petiole growth, and flowering in Arabidopsis. More importantly, GmMYB133 conferred plant tolerance to salt stress. Furthermore, a regulatory module GmMYB133-PRR5-PIF4 was proposed to regulate hypocotyl elongation, leaf petiole growth, flowering, and salt stress tolerance in Arabidopsis. These findings laid a foundation to further address the functional roles of GmMYB133 and its regulatory mechanisms in soybean.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
XL and SB designed the experiments. BS, WW, and SX performed the experiments. JC and RL performed the data analysis. XL, SB, and JC wrote the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant number 31872075, 2019–2022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Qingyu Wang, College of Plant Science of Jilin University, for providing soybean seeds (Jilin 32).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.764074/full#supplementary-material
Supplementary Figure 1 | Sequence analysis of GmMYB133 and its homologous proteins in soybean and Arabidopsis. (A) Sequence alignment of GmMYB133 protein with its homologs in other plant species. Amino-acid sequences were aligned by MEGA X and then imported into DNAMAN for shading. Protein homology ≥ 33% is shown as yellow, ≥ 50% as blue, ≥ 75% as pink, and 100% as black. The conserved MYB domain is marked with the black line, while the active site of the consensus sequence SHAQ(Y/F)F is labeled with the star. (B) Protein homology analysis of GmMYB133 with its homologs.
Supplementary Figure 2 | Histochemical analysis and GUS activity assay. (A) Histochemical staining and (B) fluorometric GUS assay of transgenic Arabidopsis with pGmMYB133:GUS under salt and drought stress. Seven-day-old transgenic Arabidopsis seedlings with pGmMYB133:GUS were subjected to salt (150 mM NaCl) or drought (300 mM mannitol) stress for 3 days. Scale bars indicate 1 mm. (C) The expression of GUS gene in young leaves of above transgenic Arabidopsis seedlings under salt and drought stress. Values were normalized against the gene AtACTIN8, and the expression level under the normal condition was set as 1. Error bars indicate SE of three biological and technical replicates, and significant differences are denoted by asterisks: **p < 0.01.
Supplementary Figure 3 | GmMYB133 might not confer plant tolerance to cold stress. (A) Photographs of 14-day-old seedlings of untransformed (UT) control and three transgenic lines overexpressing GmMYB133 under normal condition (left panel) and cold stress (right panel). Scale bars indicate 2 cm. (B) MDA contents of UT and transgenic lines under cold stress. Error bars in (B) indicate SE of three biological and technical replicates, and significant differences are denoted by asterisks: **p < 0.01.
Supplementary Figure 4 | Comparison of AtCAX3 expression under normal condition and salt stress. Statistical significance of the data was analyzed using one-way ANOVA with Duncan. Error bars refer to SE, and lowercase letters above all the bars indicate statistically significant differences.
Supplementary Figure 5 | GmMYB133 fails to bind to the promoters of light-responsive, auxin-related, and salt tolerance-associated genes. (A) The schematic diagrams of the promoters of the light-responsive, auxin-associated, and salt tolerance-related genes such as AtPIF4, AtBBX24, AtIAA19, AtIAA29, AtSAUR21, AtSAUR26, AtCAX3, AtEARLI1, AtMPK3, and AtAZI1. The downward triangles indicate the evening elements (EE), and the number shows the start site of each element. Gray lines represent the examined regions for Y1H assay. (B) Interaction assay between GmMYB133 and the promoters of the light-responsive, auxin-associated, and salt tolerance-related genes using the Y1H approach. Twelve plasmid combinations were set as negative controls [pB42AD/pLacZi, pB42AD-GmMYB133/pLacZi, pB42AD/pLacZi-pAtPIF4(EE), pB42AD/pLacZi-pAtBBX24, pB42AD/pLacZi- pAtIAA19, pB42AD/pLacZi-pAtIAA29, pB42AD/pLacZi-pAtSAUR21, pB42AD/pLacZi-pAtSAUR26, pB42AD/pLacZi-pAtCAX3, pB42AD/pLacZi-pAtEARLI1, pB42AD/pLacZi-pAtMPK3, and pB42AD/pLacZi-pAtAZI1] and one positive control (pB42AD-AtRVE8/placZi-pAtPRR5).
Adams, S., Grundy, J., Veflingstad, S. R., Dyer, N. P., Hannah, M. A., Ott, S., et al. (2018). Circadian control of abscisic acid biosynthesis and signalling pathways revealed by genome-wide analysis of LHY binding targets. New Phytol. 220, 893–907. doi: 10.1111/nph.15415
Badhan, S., Ball, A. S., and Mantri, N. (2021). First report of CRISPR/Cas9 mediated DNA-Free editing of 4CL and RVE7 genes in chickpea protoplasts. Int. J. Mol. Sci. 22:396. doi: 10.3390/ijms22010396
Bian, S., Jin, D., Li, R., Xie, X., Gao, G., Sun, W., et al. (2017a). Genome-wide analysis of CCA1-like proteins in soybean and functional characterization of GmMYB138a. Int. J. Mol. Sci. 18:2040. doi: 10.3390/ijms18102040
Bian, S., Li, X., Mainali, H., Chen, L., and Dhaubhadel, S. (2017b). Genome-wide analysis of DWD proteins in soybean (Glycine max): significance of Gm08DWD and GmMYB176 interaction in isoflavonoid biosynthesis. PLoS One 12:e0178947. doi: 10.1371/journal.pone.0178947
Bian, S., Li, R., Xia, S., Liu, Y., Jin, D., Xie, X., et al. (2018). Soybean CCA1-like MYB transcription factor GmMYB133 modulates isoflavonoid biosynthesis. Biochem. Biophys. Res. Commun. 507, 324–329. doi: 10.1016/j.bbrc.2018.11.033
Carre, I. A., and Kim, J. Y. (2002). MYB transcription factors in the Arabidopsis circadian clock. J. Exp. Bot. 53, 1551–1557. doi: 10.1093/jxb/erf027
Chen, C., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y., et al. (2020). TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi: 10.1016/j.molp.2020.06.009
Chen, S., Huang, H. A., Chen, J. H., Fu, C. C., Zhan, P. L., Ke, S. W., et al. (2020). SgRVE6, a LHY-CCA1-Like transcription factor from fine-stem stylo, upregulates NB-LRR gene expression and enhances cold tolerance in tobacco. Front. Plant Sci. 11:1276. doi: 10.3389/fpls.2020.01276
Chen, Y. S., Chao, Y. C., Tseng, T. W., Huang, C. K., Lo, P. C., and Lu, C. A. (2017). Two MYB-related transcription factors play opposite roles in sugar signaling in Arabidopsis. Plant Mol. Biol. 93, 299–311. doi: 10.1007/s11103-016-0562-8
Dong, M. A., Farre, E. M., and Thomashow, M. F. (2011). Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108, 7241–7246. doi: 10.1073/pnas.1103741108
Du, H., Wang, Y. B., Xie, Y., Liang, Z., Jiang, S. J., Zhang, S. S., et al. (2013). Genome-wide identification and evolutionary and expression analyses of MYB-related genes in land plants. DNA Res. 20, 437–448. doi: 10.1093/dnares/dst021
Farinas, B., and Mas, P. (2011). Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 66, 318–329. doi: 10.1111/j.1365-313X.2011.04484.x
Galvao, V. C., Fiorucci, A. S., Trevisan, M., Franco-Zorilla, J. M., Goyal, A., Schmid-Siegert, E., et al. (2019). PIF transcription factors link a neighbor threat cue to accelerated reproduction in Arabidopsis. Nat. Commun. 10:4005. doi: 10.1038/s41467-019-11882-7
Gray, J. A., Shalit-Kaneh, A., Chu, D. N., Hsu, P. Y., and Harmer, S. L. (2017). The REVEILLE clock genes inhibit growth of juvenile and adult plants by control of cell size. Plant Physiol. 173, 2308–2322. doi: 10.1104/pp.17.00109
Huang, C. K., Lo, P. C., Huang, L. F., Wu, S. J., Yeh, C. H., and Lu, C. A. (2015). A single-repeat MYB transcription repressor, MYBH, participates in regulation of leaf senescence in Arabidopsis. Plant Mol. Biol. 88, 269–286. doi: 10.1007/s11103-015-0321-2
Jefferson, R. A., Kavanagh, T. A., and Bevan, M. W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x
Jiang, B., Shi, Y., Peng, Y., Jia, Y., Yan, Y., Dong, X., et al. (2020). Cold-induced CBF-PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol. Plant 13, 894–906. doi: 10.1016/j.molp.2020.04.006
Jiang, L., Wang, Y., Li, Q. F., Bjorn, L. O., He, J. X., and Li, S. S. (2012). Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 22, 1046–1057. doi: 10.1038/cr.2012.34
Kelly, G., Brandsma, D., Egbaria, A., Stein, O., Doron-Faigenboim, A., Lugassi, N., et al. (2021). Guard cells control hypocotyl elongation through HXK1, HY5, and PIF4. Commun. Biol. 4:14. doi: 10.1038/s42003-021-02283-y
Kidokoro, S., Hayashi, K., Haraguchi, H., Ishikawa, T., Soma, F., Konoura, I., et al. (2021). Posttranslational regulation of multiple clock-related transcription factors triggers cold-inducible gene expression in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 118:e2021048118. doi: 10.1073/pnas.2021048118
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Kunihiro, A., Yamashino, T., Nakamichi, N., Niwa, Y., Nakanishi, H., and Mizuno, T. (2011). PHYTOCHROME-INTERACTING FACTOR 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol. 52, 1315–1329. doi: 10.1093/pcp/pcr076
Kwon, Y., Kim, J. H., Nguyen, H. N., Jikumaru, Y., Kamiya, Y., Hong, S. W., et al. (2013). A novel Arabidopsis MYB-like transcription factor, MYBH, regulates hypocotyl elongation by enhancing auxin accumulation. J. Exp. Bot. 64, 3911–3922. doi: 10.1093/jxb/ert223
Lai, A. G., Doherty, C. J., Mueller-Roeber, B., Kay, S. A., Schippers, J. H., and Dijkwel, P. P. (2012). CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. U.S.A. 109, 17129–17134. doi: 10.1073/pnas.1209148109
Li, B., Gao, Z., Liu, X., Sun, D., and Tang, W. (2019a). Transcriptional profiling reveals a time-of-day-specific role of REVEILLE 4/8 in regulating the first wave of heat shock-induced gene expression in Arabidopsis. Plant Cell 31, 2353–2369. doi: 10.1105/tpc.19.00519
Li, B., Wang, Y., Zhang, Y., Tian, W., Chong, K., Jang, J. C., et al. (2019b). PRR5, 7 and 9 positively modulate TOR signaling-mediated root cell proliferation by repressing TANDEM ZINC FINGER 1 in Arabidopsis. Nucleic Acids Res. 47, 5001–5015. doi: 10.1093/nar/gkz191
Li, R., Jiang, X., Jin, D., Dhaubhadel, S., Bian, S., and Li, X. (2015). Identification of 14-3-3 family in common bean and their response to abiotic stress. PLoS One 10:e0143280. doi: 10.1371/journal.pone.0143280
Li, T., Li, B., Wang, L., Xie, Z., Wang, X., Zou, L., et al. (2021). Phytochrome-interacting factor 4 (PIF4) inhibits expression of SHORT HYPOCOTYL 2 (SHY2) to promote hypocotyl growth during shade avoidance in Arabidopsis. Biochem. Biophys. Res. Commun. 534, 857–863. doi: 10.1016/j.bbrc.2020.10.088
Li, X., Chen, L., and Dhaubhadel, S. (2012). 14-3-3 proteins regulate the intracellular localization of the transcriptional activator GmMYB176 and affect isoflavonoid synthesis in soybean. Plant J. 71, 239–250. doi: 10.1111/j.1365-313X.2012.04986.x
Li, X., Wu, T., Liu, H., Zhai, R., Wen, Y., Shi, Q., et al. (2020). REVEILLE transcription factors contribute to the nighttime accumulation of anthocyanins in ‘Red Zaosu’ (Pyrus bretschneideri Rehd.) pear fruit skin. Int. J. Mol. Sci. 21:1634. doi: 10.3390/ijms21051634
Li, X. Y., Gao, G. L., Li, Y. J., Sun, W. K., He, X. Y., Li, R. H., et al. (2018). Functional roles of two 14-3-3s in response to salt stress in common bean. Acta Physiol. Plant. 40:13. doi: 10.1007/s11738-018-2787-4
Li, X. Y., Hou, Y. M., Xie, X., Li, H. X., Li, X. D., Zhu, Y., et al. (2020). A blueberry MIR156a-SPL12 module coordinates the accumulation of chlorophylls and anthocyanins during fruit ripening. J. Exp. Bot. 71, 5976–5989. doi: 10.1093/jxb/eraa327
Liao, Y., Zou, H. F., Wang, H. W., Zhang, W. K., Ma, B., Zhang, J. S., et al. (2008). Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 18, 1047–1060. doi: 10.1038/cr.2008.280
Muroya, M., Oshima, H., Kobayashi, S., Miura, A., Miyamura, Y., Shiota, H., et al. (2021). Circadian clock in Arabidopsis thaliana determines flower opening time early in the morning and dominantly closes early in the afternoon. Plant Cell Physiol. 62, 883–893. doi: 10.1093/pcp/pcab048
Nakamichi, N., Kiba, T., Kamioka, M., Suzuki, T., Yamashino, T., Higashiyama, T., et al. (2012). Transcriptional repressor PRR5 directly regulates clock-output pathways. Proc. Natl. Acad. Sci. U.S.A. 109, 17123–17128. doi: 10.1073/pnas.1205156109
Nakamichi, N., Kita, M., Niinuma, K., Ito, S., Yamashino, T., Mizoguchi, T., et al. (2007). Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway. Plant Cell Physiol. 48, 822–832. doi: 10.1093/pcp/pcm056
Nakamichi, N., Takao, S., Kudo, T., Kiba, T., Wang, Y., Kinoshita, T., et al. (2016). Improvement of Arabidopsis biomass and cold, drought and salinity stress tolerance by modified circadian clock-associated PSEUDO-RESPONSE REGULATORs. Plant Cell Physiol. 57, 1085–1097. doi: 10.1093/pcp/pcw057
Nguyen, N. H., Jeong, C. Y., Kang, G. H., Yoo, S. D., Hong, S. W., and Lee, H. (2015). MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J. 84, 1192–1205. doi: 10.1111/tpj.13077
Nguyen, N. H., and Lee, H. (2016). MYB-related transcription factors function as regulators of the circadian clock and anthocyanin biosynthesis in Arabidopsis. Plant Signal. Behav. 11:e1139278. doi: 10.1080/15592324.2016.1139278
Nohales, M. A. (2021). Spatial organization and coordination of the plant circadian system. Genes 12:442. doi: 10.3390/genes12030442
Perez-Garcia, P., Ma, Y., Yanovsky, M. J., and Mas, P. (2015). Time-dependent sequestration of RVE8 by LNK proteins shapes the diurnal oscillation of anthocyanin biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 112, 5249–5253. doi: 10.1073/pnas.1420792112
Pitzschke, A., Datta, S., and Persak, H. (2014). Salt stress in Arabidopsis: lipid transfer protein AZI1 and its control by mitogen-activated protein kinase MPK3. Mol. Plant 7, 722–738. doi: 10.1093/mp/sst157
Rawat, R., Takahashi, N., Hsu, P. Y., Jones, M. A., Schwartz, J., Salemi, M. R., et al. (2011). REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 7:e1001350. doi: 10.1371/journal.pgen.1001350
Reed, J. W., Wu, M. F., Reeves, P. H., Hodgens, C., Yadav, V., Hayes, S., et al. (2018). Three auxin response factors promote hypocotyl elongation. Plant Physiol. 178, 864–875. doi: 10.1104/pp.18.00718
Rosado, D., Trench, B., Bianchetti, R., Zuccarelli, R., Rodrigues Alves, F. R., Purgatto, E., et al. (2019). Downregulation of PHYTOCHROME-INTERACTING FACTOR 4 influences plant development and fruit production. Plant Physiol. 181, 1360–1370. doi: 10.1104/pp.19.00833
Sakuraba, Y., Bulbul, S., Piao, W., Choi, G., and Paek, N. C. (2017). Arabidopsis EARLY FLOWERING3 increases salt tolerance by suppressing salt stress response pathways. Plant J. 92, 1106–1120. doi: 10.1111/tpj.13747
Shigaki, T., and Hirschi, K. (2000). Characterization of CAX-like genes in plants: implications for functional diversity. Gene 257, 291–298. doi: 10.1016/s0378-1119(00)00390-5
Shimizu, H., Torii, K., Araki, T., and Endo, M. (2016). Importance of epidermal clocks for regulation of hypocotyl elongation through PIF4 and IAA29. Plant Signal. Behav. 11:e1143999. doi: 10.1080/15592324.2016.1143999
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., et al. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. doi: 10.1016/0003-2697(85)90442-7
Spartz, A. K., Lee, S. H., Wenger, J. P., Gonzalez, N., Itoh, H., Inze, D., et al. (2012). The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 70, 978–990. doi: 10.1111/j.1365-313X.2012.04946.x
Su, C. F., Wang, Y. C., Hsieh, T. H., Lu, C. A., Tseng, T. H., and Yu, S. M. (2010). A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 153, 145–158. doi: 10.1104/pp.110.153015
Takase, M., Mizoguchi, T., Kozuka, T., and Tsukaya, H. (2013). The unique function of the Arabidopsis circadian clock gene PRR5 in the regulation of shade avoidance response. Plant Signal. Behav. 8:e23534. doi: 10.4161/psb.23534
Tsuda, K., Qi, Y., Nguyen, L. V., Bethke, G., Tsuda, Y., Glazebrook, J., et al. (2012). An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 69, 713–719. doi: 10.1111/j.1365-313X.2011.04819.x
Wang, K., Bu, T., Cheng, Q., Dong, L., Su, T., Chen, Z., et al. (2021). Two homologous LHY pairs negatively control soybean drought tolerance by repressing the abscisic acid responses. New Phytol. 229, 2660–2675. doi: 10.1111/nph.17019
Wu, J. Y., Yu, C. Y., Huang, L. L., and Gan, Y. B. (2021). A rice transcription factor, OsMADS57, positively regulates high salinity tolerance in transgenic Arabidopsis thaliana and Oryza sativa plants. Physiol. Plant. 173, 1120–1135. doi: 10.1111/ppl.13508
Xi, Y., Yang, Y., Yang, J., Zhang, X., Pan, Y., and Guo, H. (2021). IAA3-mediated repression of PIF proteins coordinates light and auxin signaling in Arabidopsis. PLoS Genet. 17:e1009384. doi: 10.1371/journal.pgen.1009384
Xu, D., Huang, X., Xu, Z. Q., and Schlappi, M. (2011). The HyPRP gene EARLI1 has an auxiliary role for germinability and early seedling development under low temperature and salt stress conditions in Arabidopsis thaliana. Planta 234, 565–577. doi: 10.1007/s00425-011-1425-9
Yang, L., Jiang, Z., Jing, Y., and Lin, R. (2020). PIF1 and RVE1 form a transcriptional feedback loop to control light-mediated seed germination in Arabidopsis. J. Integr. Plant Biol. 62, 1372–1384. doi: 10.1111/jipb.12938
Yang, M., Han, X., Yang, J., Jiang, Y., and Hu, Y. (2021). The Arabidopsis circadian clock protein PRR5 interacts with and stimulates ABI5 to modulate abscisic acid signaling during seed germination. Plant Cell 33, 3022–3041. doi: 10.1093/plcell/koab168
Yuan, L., Xie, G. Z., Zhang, S., Li, B., Wang, X., Li, Y., et al. (2021). GmLCLs negatively regulate ABA perception and signalling genes in soybean leaf dehydration response. Plant Cell Environ. 44, 412–424. doi: 10.1111/pce.13931
Zhao, J., Barkla, B. J., Marshall, J., Pittman, J. K., and Hirschi, K. D. (2008). The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227, 659–669. doi: 10.1007/s00425-007-0648-2
Zhou, X. Y., Song, L., and Xue, H. W. (2013). Brassinosteroids regulate the differential growth of Arabidopsis hypocotyls through auxin signaling components IAA19 and ARF7. Mol. Plant 6, 887–904. doi: 10.1093/mp/sss123
Keywords: GmMYB133, RVE gene, salt tolerance, soybean, hypocotyl elongation, PRR5
Citation: Shan B, Wang W, Cao J, Xia S, Li R, Bian S and Li X (2021) Soybean GmMYB133 Inhibits Hypocotyl Elongation and Confers Salt Tolerance in Arabidopsis. Front. Plant Sci. 12:764074. doi: 10.3389/fpls.2021.764074
Received: 25 August 2021; Accepted: 26 November 2021;
Published: 23 December 2021.
Edited by:
Chris Helliwell, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaReviewed by:
Kyoung Hee Nam, Sookmyung Women’s University, South KoreaCopyright © 2021 Shan, Wang, Cao, Xia, Li, Bian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuyan Li, eHV5YW5saUBqbHUuZWR1LmNu; Shaomin Bian, c2htYmlhbkBqbHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.