
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 19 May 2021
Sec. Plant Abiotic Stress
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.672368
Climate change has created an environment where heat stress conditions are becoming more frequent as temperatures continue to raise in crop production areas around the world. This situation leads to decreased crop production due to plant sensitivity to heat stress. Reproductive success is critically dependent on plants’ ability to produce functional pollen grains, which are the most thermo-sensitive tissue. Flavonols are plant secondary metabolites known for their potent antioxidative activity, essential for male fertility in several species including tomato, and implicated in heat stress tolerance. Since flavonols are highly abundant in fruits of the tomato high pigment 2 (hp2) mutant, we tested the level of flavonols in pollen of this mutant, under the hypothesis that increased accumulation of flavonols would render pollen more tolerant to heat stress. Indeed, pollen from two alleles of the hp2 mutant was found to have flavonols levels increased by 18 and 280% compared with wild-type (WT) under moderate chronic heat stress (MCHS) conditions. This mutant produced on average 7.8-fold higher levels of viable pollen and displayed better germination competence under heat stress conditions. The percentage of fully seeded fruits and the number of seeds per fruit were maintained in the mutant under heat stress conditions while decreased in wild-type plants. Our results strongly suggest that increased concentrations of pollen flavonols enhance pollen thermotolerance and reproductive success under heat stress conditions. Thus, the high flavonols trait may help frame the model for improving crop resilience to heat stress.
Crop productivity is often dependent on efficient sexual reproduction, a complex process that is very sensitive to environmental stresses, in particular heat stress, leading to considerable yield losses. Sensitivity to heat stress during the reproductive phase of plant development was documented in various crop species, and it was shown to be manifested by morphological alterations of anthers, style elongation, bud abscission and reduced fruit number, weight, and seed set. The most heat stress sensitive processes include meiosis, pollen germination, pollen tube growth, pollen/pistil interactions, fertilization, formation of the endosperm, and embryo development (Rudich et al., 1977; Warrag and Hall, 1984; Monterroso and Wien, 1990; Abdul-Baki and Stommel, 1995; Peet et al., 1998; Erickson and Markhart, 2002; Firon et al., 2006). Severity of the damage is dependent on the nature of the heat stress experienced by the plants (i.e., intensity, duration, occurrence of heat spikes etc.), and whether or not thermotolerance was acquired. In tomato (Solanum lycopersicum), a prolonged stress of day temperatures exceeding 32°C with night temperature above 20°C caused reduced fruit set, fruit weight, total yield, and seed production (El Ahmadi and Stevens, 1979; Peet et al., 1998). Prolonged exposure to temperature of 36°C led to the development of abnormal anthers and pollen grains and style elongation (Rudich et al., 1977; Giorno et al., 2013). Predictions of the effect of temperature increment on major crops yield show that each degree-Celsius increase in global mean temperature would, on average, reduce yields by 3.1–7.4% (Zhao et al., 2017). Therefore, a comprehensive understanding of how plants respond to heat stress conditions to mitigate possible damage is fundamental for designing measures to sustain adequate productivity under increasingly warming climate.
Pollen development is considered the most heat-sensitive stage during plant development (Lohani et al., 2020), as it was shown to be more sensitive than both the sporophyte and female gametophyte (Peet et al., 1998; Young et al., 2004; Wang et al., 2019). Pollen viability was reported to be compromised by high temperatures in crop plants such as wheat (Begcy et al., 2018), rice (Jagadish et al., 2007), sorghum (Djanaguiraman et al., 2018), soybean (Djanaguiraman et al., 2013), and tomato (Firon et al., 2006). The occurrence of heat stress during male reproductive development, 8–13 days before anthesis, impairs pollen viability and function, leading to decreased fruit and seed set (Iwahori, 1966; Peet et al., 1998; Zinn et al., 2010; De Storme and Geelen, 2014; Muller and Rieu, 2016). Heat stress causes disruption of meiotic cell division, abnormal pollen morphology and size, and reduced grain number, viability, and germination capacity (Peet et al., 1998; Pressman et al., 2002; Firon et al., 2006; Prasad et al., 2006; Endo et al., 2009; Djanaguiraman et al., 2013; Giorno et al., 2013; Begcy et al., 2019). In tomato, optimal growth conditions are approximately 26–28°C during the day and 20–22°C during the night. Pollen heat stress related damage was observed after short episodes of high temperatures at 40°C, as well as after chronic exposure to milder heat stress of 32/28°C or 31/25°C day/night for several months (Iwahori, 1966; Firon et al., 2006).
Flavonols are plant secondary metabolites, among the most abundant groups of flavonoids, which are present in large quantities in various tissues of many plant species (Buer et al., 2010). Flavonols are important for stress defense and proper pollen function (Falcone Ferreyra et al., 2012), being essential for maintenance of pollen fertility. Mutants deficient in chalcone synthase (CHS), the first dedicated enzyme in the flavonol biosynthesis branch, cannot produce pollen tubes. The infertility phenotype of this mutant can be rescued by exogenous application of flavonols like quercetin or kaempferol (Mo et al., 1992; Ylstra et al., 1992; Falcone Ferreyra et al., 2012).
Pollen flavonols are synthesized in the tapetum, the innermost cell layer of the anther, and transported to microspores during pollen development, becoming a major component of the pollen coat (Hsieh and Huang, 2007; Fellenberg and Vogt, 2015). It has been suggested that flavonols might also contribute to pollen wall plasticity to facilitate rapid pollen tube growth (Derksen et al., 1999).
Flavonols are potent antioxidants that have been implicated in abiotic stress tolerance because they can inhibit the generation of free radicals and scavenge reactive oxygen species (ROS; Brown et al., 1998; Rice-Evans, 2001; Melidou et al., 2005; Agati et al., 2012; Brunetti et al., 2013; Martinez et al., 2016). In particular, several studies pointed at the involvement of flavonols in the response to heat stress. In tomato, flavonols content in leaves and pollen was increased in response to heat stress (Martinez et al., 2016; Paupière et al., 2017). In leaves, heat stress specific activation of the flavonol-biosynthesis pathway was observed, suggesting a specific role for flavonols in mitigating the oxidative stress developed under high temperatures (Martinez et al., 2016). Using a complementary approach, a non-targeted metabolomics study in pollen suggested that the most significant metabolic response to a 2 h heat stress was the 2-fold increase in total flavonoids, which include flavonols (Paupière et al., 2017). In addition, several studies correlated the reduction of flavonols with a reduction in heat stress tolerance. For example, a CHS-deficient mutant variety of Morning Glory (Ipomoea purpurea), which does not produce flavonols, shows reduced pollen fertility under high temperatures (Coberly and Rausher, 2003). In tomato, knockdown of Heat shock factor A2 (HsfA2) resulted in reduced pollen viability in response to heat stress and decreased chalcone isomerase (CHI) expression (Fragkostefanakis et al., 2016). The tomato anthocyanin reduced (are) mutant has reduced flavonol levels in pollen grains and tubes. This mutant produces fewer pollen grains and is impaired in pollen viability, germination, tube growth, and tube integrity, resulting in reduced seed set. Consistent with flavonols acting as ROS scavengers, are shows elevated levels of ROS in pollen tubes and inhibited tube growth that could be rescued by antioxidant treatment (Muhlemann et al., 2018). That study showed that flavonols support pollen development and tube growth under heat stress by reducing the abundance of ROS.
The pleiotropic high pigment 2 (hp2) natural mutant in tomato is characterized by enhanced light responsiveness resulting in increased pigmentation and increased plastid number and size (Mustilli et al., 1999; Levin et al., 2003; Kolotilin et al., 2007). Metabolomic studies revealed that the darker red color of ripe fruits of the hp2 mutant is caused by increased levels of secondary metabolites, mainly carotenoids and flavonoids. Total carotenoids in hp2 fruits were increased up to 3.2-fold (Kolotilin et al., 2007), and the flavonoids rutin and a quercetin derivative were found to be induced 2.9 and 4.4-fold, respectively (Bino et al., 2005).
The gene underlying hp2 is the tomato homolog of the Arabidopsis DEETIOLATED1 (DET1) gene (Solyc01g056340), initially identified as a negative regulator of photomorphogenesis (Pepper et al., 1994; Mustilli et al., 1999; Levin et al., 2003). Three hp2 alleles are known: hp2, hp2j, and hp2dg. In hp2, a nine base pairs deletion within a putative nucleolar localization signal of DET1 results in a three amino acids deletion. hp2j and hp2dg are the result of a conserved amino acid substitution within a protein-protein interaction domain (Mustilli et al., 1999; Jones et al., 2012). These alleles share similar phenotypes but also differences, as for example, the hp2dg allele has more intense pigmentation at the green fruit stage than hp2 (Konsler, 1973; van Tuinen et al., 1997) while hp2j has shorter hypocotyls and higher hypocotyl anthocyanin content than hp2 (Mustilli et al., 1999; Levin et al., 2003). While DET1 is expressed throughout the tomato fruit in almost all tissues and ripening stages tested,1 no major transcriptional changes were reported for DET1 in the hp2 mutants. Downregulation of DET1 specifically in tomato fruit resulted in a significant increase in carotenoids, flavonoids, and chlorophyll levels, along with increase in plastids number and area per cell (Davuluri et al., 2005; Enfissi et al., 2010), supporting earlier studies that define DET1 as regulator of secondary metabolites biosynthesis.
DEETIOLATED1 is a component of an E3 ligase complex involved in protein ubiquitination and degradation by the 26S proteasome (Bernhardt et al., 2006; Zhang et al., 2008). Among the proteins shown to be targets of the DET1 complex are positive regulators of photomorphogenesis such as ELONGATED HYPOCOTYL5 (HY5) in Arabidopsis (Osterlund et al., 2000) and upstream regulators of chloroplast biogenesis, flavonoid accumulation and carotenoid biosynthesis such as GOLDEN2-LIKE (GLK2), BBX20, and MBD5 in tomato (Powell et al., 2012; Nguyen et al., 2014; Li et al., 2016; Tang et al., 2016; Xiong et al., 2019). Interestingly, ubiquitin ligases, which contain DET1 as a complex unit, were suggested to play a role in abiotic stress responses as they mediate ABA signaling in Arabidopsis (Irigoyen et al., 2014; Seo et al., 2014). The involvement of DET1 in abiotic stress tolerance was further demonstrated by improved seeds germination of the det1 Arabidopsis mutant under salt and osmotic stress conditions (Fernando and Schroeder, 2016). However, the possible effect of the tomato det1 (hp2) mutant on abiotic stress response or stress tolerance was not evaluated prior to this study. Taking into account that the hp2 mutant has increased content of antioxidants capable of counteracting ROS produced under stress, we hypothesized that hp2 may be more tolerant to heat stress. Considering the high sensitivity of pollen to heat stress, such hypothesis requires that pollen of hp2 would also be enriched with antioxidants, not only leaves and fruits. This information was not available prior to our study. We therefore set to investigate pollen characteristics of hp2, i.e., pollen flavonols content and pollen viability, under conditions of heat stress, a major abiotic stress in tomato.
In this study, we compared the reproductive success of hp2 tomato mutants and wild-type (WT) isogenic lines under moderate chronic heat stress (MCHS) conditions (34/24°C day/night temperatures for 10–12 weeks during the plants’ reproductive stage), and measured pollen viability and flavonols level at the population scale using flow cytometry. We show that the hp2 mutants are more heat stress tolerant than their isogenic wild-type lines producing more seeds than the wild-types under MCHS, correlated with better pollen viability and higher pollen flavonols level. We also show that tomato cultivars known for being thermotolerant have increased flavonols content in pollen. Our findings indicate that the ability of pollen to accumulate high flavonols content can be employed as a marker predicting increased pollen thermotolerance and improved reproductive success under heat stress conditions. Moreover, our results may be translated into a biotechnology approach to enhance pollen flavonols levels in order to achieve heat stress tolerance in tomato and other crops.
In order to determine seed set rate, 5–10 ripe fruits from 8 to 10 plants per genotype were collected, weighed, and sectioned. Fruits with no or little amount of seeds (<10 seeds) were considered as “unseeded fruits,” while fully seeded fruits were considered as “seeded.” Fruit pictures were taken using Cannon PowerShot SX520HS camera. For each plant, the ratio of seeded fruits out of total was calculated. For seed count, seeds were extracted using sulfuric acid. Seeds were extracted with the placenta cells and jelly tissues attached, incubated for 3 h in a 2% sulfuric acid solution, then, transferred into net bags and rinsed with tap water. Clean seeds were left to air-dry, then spread over paper, and imaged using a smartphone camera (exposure time 0.25 s, Mi6, Xiaomi, China). Seed number was quantified automatically by image processing in ImageJ (version 1.50i) using the “Count Kernels in Directory” macro as described in (Severini et al., 2011) with several modification and adjustments (circularity 0.1–1.00, size ≥ 150).
To isolate pollen for in vitro germination, individual anther cones were detached from open flowers (3–4 anther cones per genotype/condition in three replicates), cut in transverse, and placed in 1.2 ml of pollen germination medium (2 mM boric acid, 2 mM calcium nitrate, 2 mM magnesium sulfate, and 1 mM potassium nitrate, supplemented with 10% w/v sucrose before use). Cut anther cones in solution were gently crushed and vortexed to release pollen into the medium, and then split into two 1.5 ml Eppendorf tubes each of 0.5 ml pollen suspension. One tube was then incubated at 34°C for 30 min (for in vitro heat stress), while the other tube was incubated at 22°C for 30 min (as control). Pollen (0.1 ml) from each condition was then transferred to a 96-well plate, covered, and incubated at 22°C for 1.5 h. Pollen was observed using an Olympus IX2 inverted microscope, and images were captured at X4 magnification using an Olympus XM10 camera. Scoring of germination was performed manually using the ImageJ (version 1.50i) Cell Counter plug-in (Schneider et al., 2012).
To isolate pollen, 3–4 anther cones per plant (three plants per genotype/condition), in 3–6 replicates, were detached from open flowers, cut in transverse, and placed in 5 ml of 10% sucrose pollen germination medium in a 15-ml centrifuge tube. Pollen was released from anther cones by gently crushing against the wall of the tube followed by vortexing for 30 s. Suspensions of pollen were subsequently filtrated through folded miracloth (Merck Millipore, Darmstadt, Germany) to remove tissue debris.
2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) and diphenylboric acid-2-aminoethyl ester (DPBA) were dissolved in DMSO. Pollen was allowed to hydrate in germination buffer for 15 min before H2DCFDA (Cayman chemical) or DPBA (2-aminoethyl diphenylborinate; Sigma) was added to a final concentration of 5 μM and 0.025%w/v, respectively. Pollen was incubated in stains for 30 min prior to flow cytometry analysis. A sample of unstained pollen was retained to calibrate the dichlorofluorescein (DCF) and DPBA fluorescence threshold. Mode of action of H2DCFDA: upon hydrolysis by cytoplasmic esterases, the DA moiety of H2DCFDA is cleaved-off, freeing the non-fluorescent H2DCF to become oxidized by ROS, forming green fluorescent DCF. Thus, only metabolically active cells are DCF-stained, and the mean fluorescent intensity (MFI) signal correlates with the rate of metabolic activity and level of ROS in the cell. Therefore, DCF staining is used to measure cell viability (Luria et al., 2019; Rutley and Miller, 2020).
Following staining, samples were analyzed in a BD LSRFortessa™ and the FITC laser-filter set (488 nm solid state laser, BP filter 530/30) compatible with both DCF and DPBA. Gating of singlet pollen grains was performed by first gating pollen from non-pollen events using Forward Scatter Area (FSC-A) and Side Scatter Area (SSC-A) parameters, and then applying a gate for double discrimination using Forward Scatter Height (FSC-H) and Forward Scatter Width (FSC-W). In the FITC-A channel, true fluorescence was gated from auto-fluorescence using the unstained sample as the auto-fluorescence threshold. Typically, > 3,000 singlet pollen grains were analyzed per sample. Data analyses were performed using FlowJo software.
Reference images of DCF and DPBA-stained pollen from Moneymaker (MM) and hp2 were acquired using Amnis® ImageStream®X Mk II (MilliporeSigma) imaging flow cytometer and INSPIRE (MilliporeSigma) software was used for data collection. Samples were acquired at 40X magnification and medium spend/medium resolution. The bright field images were acquired in Channel 1 and DCF or DPBA signal in Channel 2 (488 nm laser, BP filter 528/65). Image analyses were performed using the IDEAS® software.
Flow cytometry data analysis resulted in the percentage of pollen in each sample, according to the different pollen fractions: negative, high-DCF, and low-DCF. The sum of high-DCF and low-DCF pollen fractions provides the amount of positive (i.e., viable) pollen. This data were used directly to present % viability. Alternatively, the data were used to calculate the ratio between MCHS and control conditions using the formula:
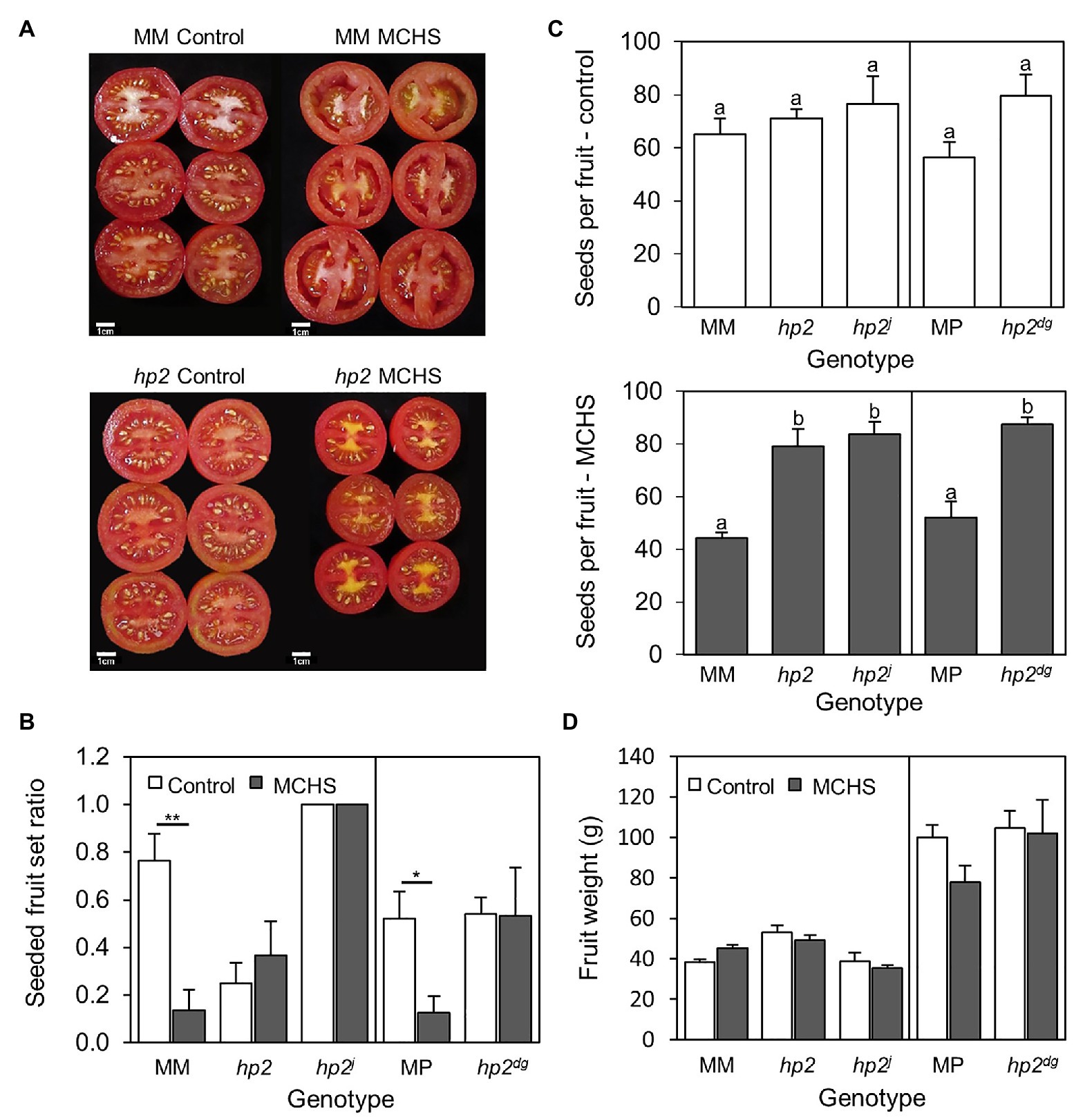
Figure 1. The hp2 mutant maintains normal seed production under MCHS conditions. (A) Visual differences in fruit seed set between hp2 and Moneymaker under control and MCHS conditions. (B) Rates of fruits full of seeds, under control and MCHS conditions. Fruits were scored as “full of seeds” when visually similar to fruits shown in (A) for control conditions. (C) Number of seeds per fruit under control (upper panel) and MCHS conditions (bottom panel). (D) Single fruit weight under control and MCHS conditions. About 5–10 ripe fruits (from 8 to 10 plants per genotype per condition) were analyzed in three replicates. Error bars represent SEM. *represents p < 0.05 and **represents p < 0.01. Different letters indicate statistically significant differences. MCHS, moderate chronic heat stress; MM, moneymaker; and MP, manapal.
Anther cones from three plants/replicates (3–4 per plant) per genotype/condition were detached from open flowers and flash frozen in liquid nitrogen. Total RNA was isolated from pairs of anther cones using RiboEx™ (GeneAll; Seoul, South Korea) according to the manufacturer’s protocol. RNA was treated with DNaseI (Thermo Fischer Scientific, California, United States) prior to cDNA synthesis. cDNA was synthesized from 0.5 μg of DNaseI-treated total RNA using qPCRBIO cDNA Synthesis Kit (PCR Biosystems, London, United Kingdom) following the manufacturer’s instructions. Relative expression of HSP17.6 (NM_001246984.3) was determined using quantitative real-time PCR (qRT-PCR) on an Applied Biosystems StepOnePlus Real-Time PCR System (Thermo Fischer Scientific, California, United States). Ubiquitin (Solyc07g064130) and EF1α (Solyc06g009970) were used as reference genes. Each 10 μl qRT-PCR reaction consisted of qPCRBIO SyGreen Blue Mix Hi-ROX (PCR Biosystems, London, United Kingdom), gene-specific primers, and 1:5 diluted cDNA template. Thermocycling conditions were performed according to manufacturer instructions. Data were analyzed using Applied Biosystems StepOne™ software v 2.3 (Thermo Fischer Scientific, California, United States). Primer sequences are listed in Supplementary Table S3.
One-way ANOVA test was used to detect significant differences between conditions and genotypes. Student’s t test was used for pair-wise comparisons either between conditions, per genotype, or between mutant and wild-type means, per condition. All statistical analyses were performed using JMP Version 3.2.2 (SAS Institute, Inc.).
One of the most prominent effects of heat stress on reproductive development is reduced seed set (Abdul-Baki, 1991; Sato et al., 2000; Xu et al., 2017). To test whether the hp2 mutant performs better than the wild-type under heat stress conditions, the three allelic lines, hp2, hp2j, and hp2dg, were grown in temperature-controlled greenhouses along with their corresponding isogenic wild-type lines; Moneymaker (MM; for hp2, hp2j) and Manapal (MP; for hp2dg). The plants grew under control temperatures of 26/20°C day/night in two greenhouses until the onset of flowering. From that stage throughout the rest of the experiment, the temperature in one of the greenhouses was set to reach at least 32°C during the day and at least 22°C during the night, creating conditions of MCHS, while the other greenhouse was maintained at control conditions (Supplementary Figure S1A). The impact of MCHS was evident, as flower damage, such as elongated style, abnormal anthers, and flower drop was prevalent in the stress greenhouse compared with the control greenhouse (Supplementary Figure S1B). Heat stress perception was confirmed at the molecular level by quantifying the expression level of Hsp17.6 gene (NM_001246984.3) as a heat stress reporter (Frank et al., 2009). Under MCHS conditions, Hsp17.6 was induced between 31 and 43-fold, depending on the genotype, relative to control conditions (Supplementary Figure S1C).
To evaluate reproductive performance of the hp2 mutant, we estimated the rate of fully seeded fruits (i.e., having a full seed set by visual inspection, Figure 1A), and counted the number of seeds per fruit under both MCHS and control conditions. Both wild-type lines, Moneymaker and Manapal, had a significant reduction of 5.4-fold and 4-fold in the proportion of seeded fruits per plant under MCHS conditions, respectively, whereas in all three hp2 genotypes, the proportion of seeded fruit was similar between control and MCHS conditions (Figure 1B). The number of seeds per fruit was very similar among all genotypes under control conditions, ranging from 56 to 80 seeds per fruit. Under MCHS conditions, the hp2 genotypes maintained a similar number of seeds (79–87 seeds per fruit) whereas a decrease to 44 and 52 seeds per fruit was observed in the Moneymaker and Manapal genotypes, respectively (Figure 1C). The apparent reduction in fruit size of hp2 under MCHS conditions compared with control conditions (Figure 1A) was addressed by measuring single fruit weight, but the effects were found to be non-significant (Figure 1D).
Since the seed set of all three hp2 mutants was not affected by heat stress conditions, we assumed that the hp2 pollen might be more tolerant than the wild-type pollen. Therefore, we evaluated pollen viability under normal and MCHS conditions in hp2 and wild-type lines using the H2DCFDA staining coupled with flow cytometry approach, as recently described (Luria et al., 2019; Rutley and Miller, 2020). DCF is the fluorescent molecule produced by ROS-mediated oxidation of H2DCFDA in viable cells and is an indication of ROS levels and metabolic activity (Muhlemann et al., 2018; Luria et al., 2019). Using this method, three pollen populations were detected: dead pollen, which do not produce a DCF signal (DCF-negative), and two populations of viable pollen, one having low metabolic activity (“low ROS”) and the other with high metabolic activity (“high ROS”; Figure 2A). Flow cytometry analysis revealed similar distribution of the three pollen populations between the wild-type and hp2 lines under control conditions with 71.1–79.3% viable pollen (low and high-ROS) across all genotypes. Under MCHS conditions, the percentage of viable pollen was dramatically reduced in all genotypes. However, the reduction was larger in the wild-type lines (0–0.3% high-ROS pollen fraction) compared with the hp2 lines (3–12% high-ROS pollen fraction). Similarly, the low-ROS viable pollen fraction was bigger in the hp2 lines (12.6–15.5%) compared with the wild-types (1.6–2.9%) under MCHS conditions. Overall, the viable pollen fraction (low and high-ROS) under MCHS conditions reached 1.6–3.2% of total pollen for the wild-types and 13.8–24.6% in hp2 which was significantly higher (p-value < 0.04; Figures 2B,C; Supplementary Figure S2).
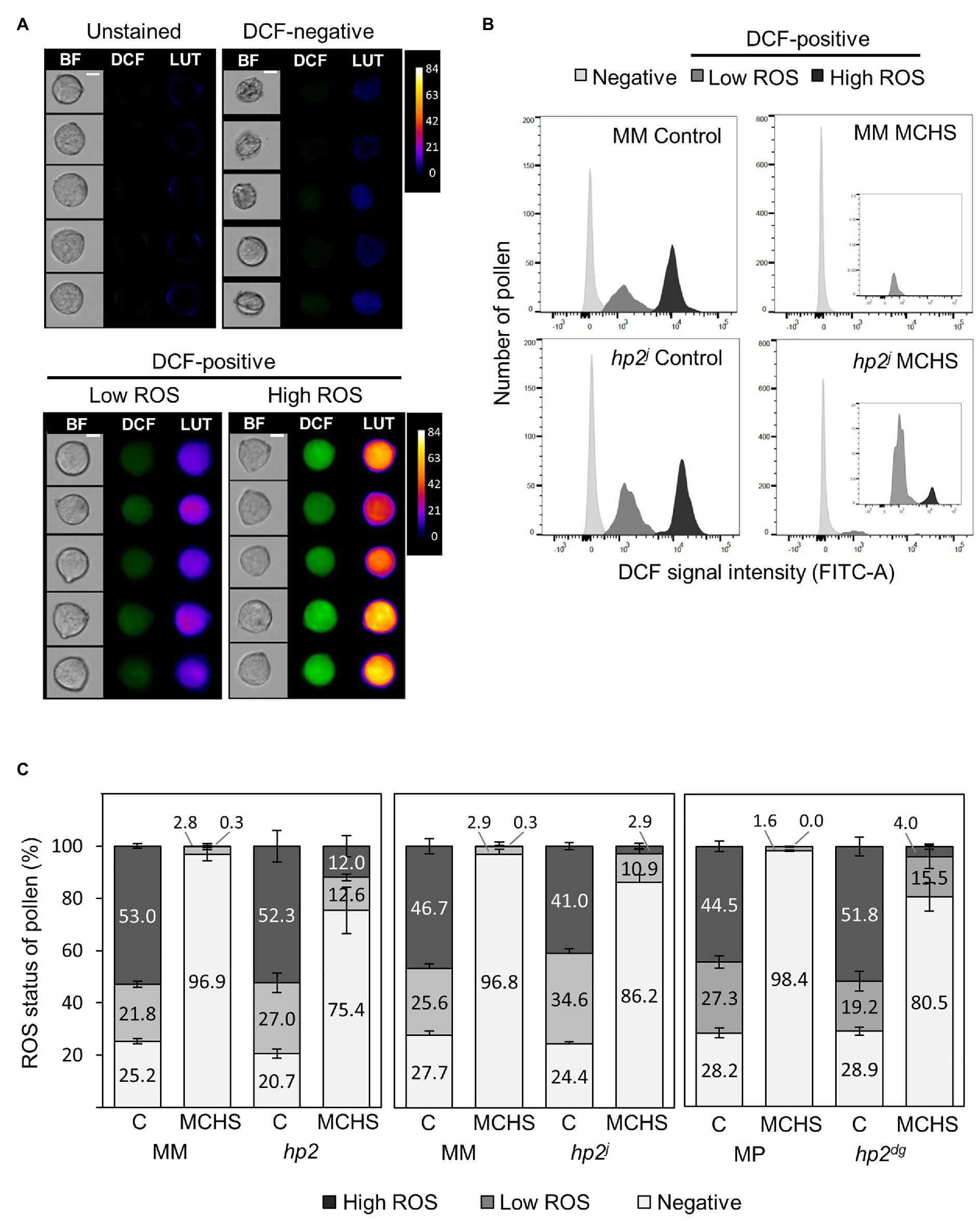
Figure 2. The hp2 mutant maintains higher pollen viability and metabolic activity under moderate chronic heat stress (MCHS) conditions. (A) Dichlorofluorescein (DCF) fluorescence of randomly selected pollen grains from unstained and 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA)-stained samples of Moneymaker wild type line under control conditions. Pseudo-color lookup table (LUT) images emphasize the scale of DCF fluorescent intensity. BF, bright field images. Scale bar = 10 μm. (B) Distribution of pollen by DCF-signal intensity. Inset boxes zoom into the DCF-positive fraction, which is smaller under MCHS conditions in all genotypes. (C) Distributions of reactive oxygen species (ROS) status (negative, low, or high) of pollen across genotypes and conditions. Numbers denote the percentage of each status fraction. Differences between hp and wild type lines in viable pollen (low, high, or both) under MCHS conditions were statistically significant (3–6 replicates, value of p < 0.04). Error bars represent SEM. MCHS, moderate chronic heat stress; C, control conditions; MM, moneymaker; and MP, manapal.
Temperature stress causes defects in developing pollen, often leading to deformed pollen and decreased grain size (Mercado et al., 1997; Porch and Jahn, 2001). In flow cytometry, the FSC-A value is proportional to cell size (Adan et al., 2017). The distribution of pollen by FSC-A values revealed two pollen subpopulations, of smaller-grain and larger-grain size (Figures 3A,B). Under normal conditions, the fraction of larger pollen was bigger in either mutant or wild-type lines. On the contrary, under MCHS conditions, the majority of pollen grains were of smaller size, demonstrating heat stress damage. Additionally, under MCHS conditions, the larger-pollen faction was bigger in the hp2 mutant compared with the related wild-type, implying better thermotolerance, in line with our results on pollen viability (Figures 3B,C; Supplementary Figures S3, S4). Indeed, positive correlation was found between ROS level of viable pollen and grain size across all samples, meaning that high-ROS pollen was overall larger than low-ROS pollen (Figure 3C; Supplementary Figure S3; Supplementary Table S2). The high-ROS larger pollen fraction did not differ between the mutant and wild-type lines under normal conditions, however, under MCHS conditions, both hp2 and hp2j alleles produced 4 and 7-fold higher proportion of larger high-ROS pollen compared with the Moneymaker background. In the Manapal genotype, the larger high-ROS pollen was completely obliterated by the stress, whereas in the hp2dg allele, 14.8% of larger high-ROS pollen survived (Figure 3D).
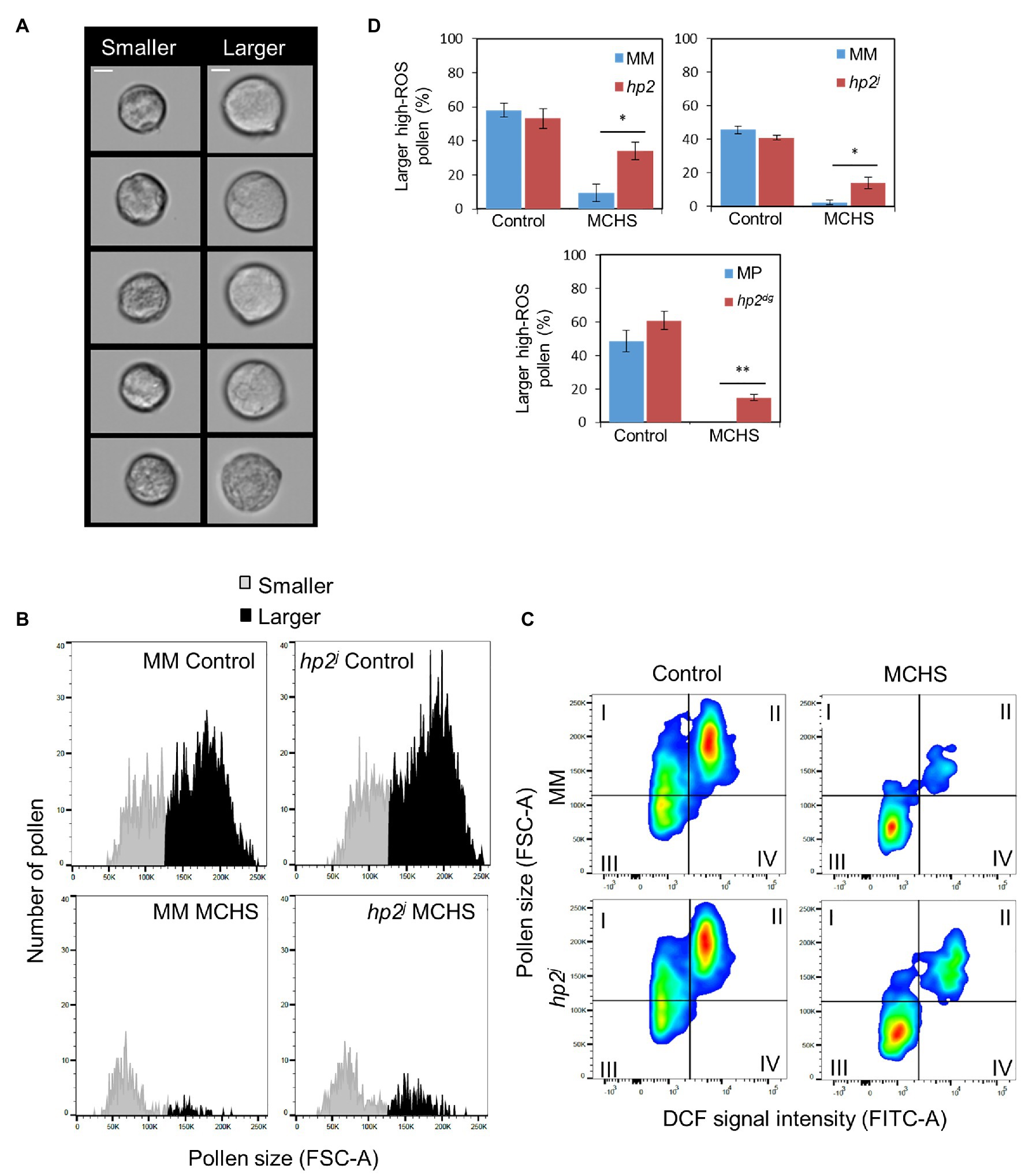
Figure 3. The hp2 mutant maintains a higher proportion of larger pollen under MCHS conditions. (A) Randomly selected pollen grains from smaller and larger subpopulations in a Moneymaker control sample. Scale bar = 10 μm. (B) Pollen grain size distribution by Forward Scatter Area (FSC-A) in an hp2j sample from control and MCHS conditions. (C) DCF-positive pollen populations plotted by grain size (FSC-A) vs. DCF signal intensity (FITC-A). The larger, high-ROS pollen faction is represented in quadrant II. (D) Rate of larger high-ROS pollen sub-population (quadrant II, section c) in all genotypes under control and MCHS conditions. Bars represent mean larger high-ROS pollen fraction of 3–6 replicates. Error bars represent standard error of the mean. *represents p < 0.05 and **represents p < 0.01. MCHS, moderate chronic heat stress; C, control conditions; MM, moneymaker; and MP, manapal.
To ask whether the improved viability of hp2 pollen under heat stress conditions could support thermotolerance during fertilization, we evaluated pollen germination in vitro under control and heat stress conditions. To this end, pollen was imbibed in germination medium and exposed to moderate heat stress conditions of 34°C for 30 min, and the proportion of germinated pollen grains was scored following 1.5 h of recovery at 22°C. We compared pollen of the MicroTom (MT) hp2dg introgression line (MT hp2dg; Sestari et al., 2014) with its MicroTom background line. Under control growth conditions (22°C), pollen germination rate was nearly 2-fold higher in hp2dg pollen compared with the wild-type MT line. Heat stress led to a 12.5 and a 5.9-fold reduction in pollen tube production rate in the MT and the hp2dg lines (corresponding to 2.5 and 9.9% germination), respectively (Figures 4A,B).
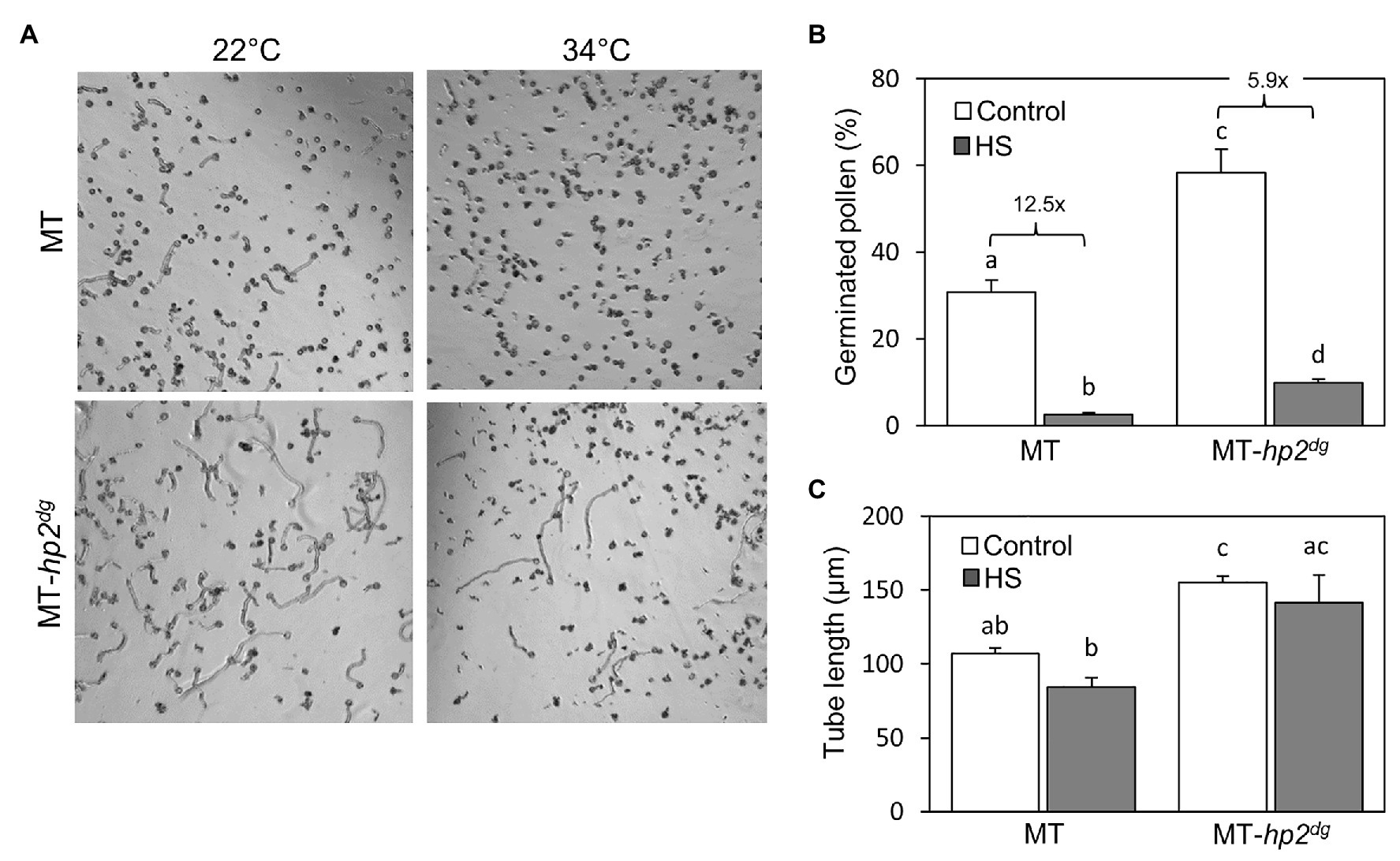
Figure 4. The hp2 mutant shows better pollen germination following in vitro heat stress treatment. (A) Germinated pollen of hp2dg and MicroTom (MT) background following an in vitro heat stress treatment of 34°C for 30 min, compared with germination without heat stress (22°C). (B) Percentage of germinated pollen for hp2dg and MicroTom background following an in vitro heat stress treatment, compared with control conditions. Numbers denote fold change between control and heat stress samples in each genotype. (C) Pollen tube length of hp2dg and MicroTom pollen following an in vitro heat stress treatment, compared with control conditions. Error bars represent SEM, derived from three replicates. Different letters indicate statistically significant differences. HS, heat stress; MT, MicroTom.
In addition, we measured the average length of pollen tubes and found that under both control and in vitro heat stress, hp2dg pollen had longer pollen tubes than wild-type indicating faster growth rate (Figure 4C). The heat stress treatment had a milder impact on tube length compared with pollen germination proportion, as the observed reductions in the MT and hp2dg lines were not statistically significant (Figure 4C). Taken together, these results suggest that the increased thermotolerance in the hp2dg line during tube production and growth contributed to the overall improved reproductive heat stress tolerance of the hp2 lines in vivo (Figure 1).
Considering the antioxidative property of flavonols and their established role in mitigating stress damage, the high level of flavonols in fruits of the hp2 mutant (Bino et al., 2005) prompted us to test the levels of flavonols in hp2 pollen. To this end, we stained mature pollen with the flavonol-specific stain DPBA (Sheahan and Rechnitz, 1992) and analyzed the fluorescence intensity using flow cytometry (Figure 5A). Signal intensity corresponds to levels of the major flavonols found in pollen, kaempferol, and quercetin. Under control conditions, the MFI of the DPBA signal was not different in the hp2 lines compared with the wild-type lines. However, the DPBA signal significantly increased in hp2 and hp2j lines under MCHS conditions, compared with only minor increase in the wild-type lines (Figure 5B). At the population level, we noticed that the distribution of pollen MFI values differed between wild-type and hp2 lines and between control and MCHS conditions (Figure 5C; Supplementary Figure S5). We quantified the fraction of pollen which hyper-accumulated flavonols (enhanced DPBA signal) and found that under MCHS conditions, the percentage of pollen which hyper-accumulated flavonols was higher in the hp2 lines compared with their corresponding wild-type lines (Figure 5D). In hp2, the percentage of enhanced DPBA pollen was 5.8% on average, which translate to 1.75-fold difference compared with the Moneymaker wild-type under the same conditions (3.3%). hp2j presented the strongest effect, reaching 35% of enhanced DPBA pollen under MCHS conditions, a 9.2-fold difference compared with the Moneymaker wild-type (3.8%). The weaker effect in hp2dg, a non-significant 1.4-fold higher in the mutant compared with the wild-type under MCHS conditions, may be attributed to the different genetic background compared with hp2 and hp2j (Figure 5D).
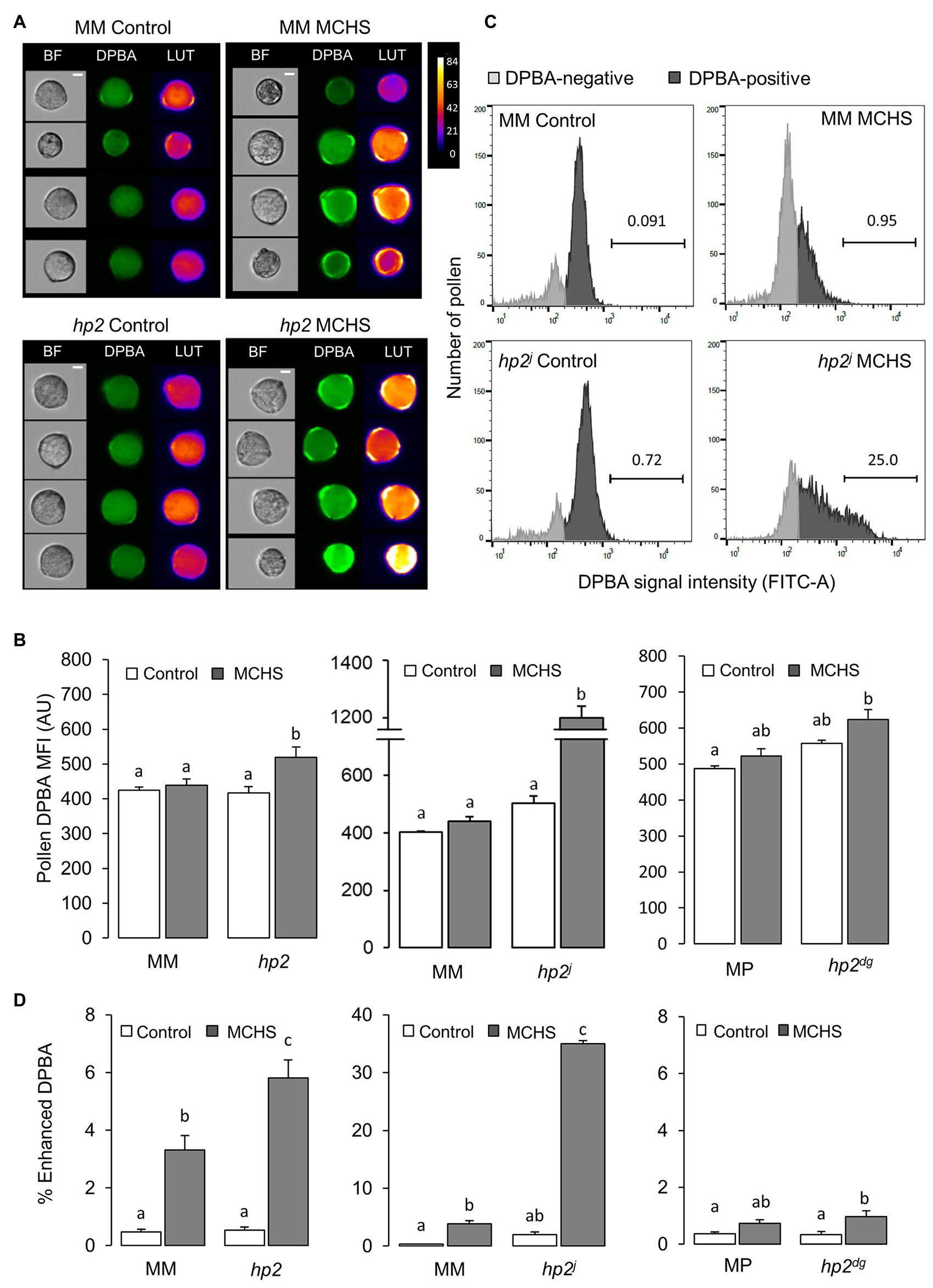
Figure 5. The hp2 mutant accumulates higher levels of flavonols in pollen in response to MCHS. (A) Randomly selected pollen grains from DPBA-stained samples of hp2 and Moneymaker under control and MCHS conditions. Pseudo-color LUT images indicate the scale of DCF fluorescent intensity. BF, bright field images. Scale bar = 10 μm. (B) Pollen flavonol levels under control and MCHS conditions by DPBA stain mean fluorescence signal intensity (MFI) presented in arbitrary units. (C) Pollen distribution by DPBA signal intensity in hp2j samples from control and MCHS conditions. Horizontal bars mark the enhanced-DPBA pollen subpopulation and the above number denotes the percentage of this subpopulation out of total DPBA-positive pollen. (D) Percentage of enhanced DPBA (i.e., enhanced flavonols) subpopulation under control and MCHS conditions. Error bars represent SEM, derived from 3 to 6 replicates. Different letters indicate statistically significant differences. MCHS, moderate chronic heat stress; MM, moneymaker; MP, manapal; and AU, arbitrary units.
Altogether, we show that hp2 accumulates higher levels of flavonols in pollen, and that its content increases in response to MCHS conditions. We therefore hypothesize that flavonols accumulation contribute to the observed pollen thermotolerance of hp2. These results suggest that flavonols take part in the response to heat stress more profoundly in the hp2 mutant.
To support the hypothesis that increased levels of flavonols are contributing to pollen thermotolerance, we compared the level of flavonols in pollen of two known heat stress tolerant cultivars, Saladette and CLN1621L (obtained from AVRDC, the world vegetable center), with two heat stress sensitive cultivars, M82 and Moneymaker.
The four cultivars (Saladette, CLN1621, M82, and Moneymaker) were grown in a non-controlled greenhouse during the summer hence reaching high-temperature conditions of 45/23°C day/night during flowering and fruit set. As a result, style elongation, a characteristic heat-stress phenotype, was abundant in flowers of M82 and Monemaker. In the heat stress tolerant cultivars CLN1621 and Saladette, most of the flowers appeared normal and undamaged (Figure 6A). The stress response was further validated by testing the expression of the heat stress molecular marker gene Hsp17.6. As expected, this gene was induced under heat stress relative to normal conditions, with the tolerant cultivars Saladette and CLN1621 showing no or non-significant induction, respectively (Figure 6B). In line with the style elongation phenotype abundance and Hsp17.6 induction, pollen viability tests further confirmed the heat stress tolerance of CLN1621 and Saladette. Under MCHS conditions, pollen viability rate was reduced to 3% of control in M82 and Moneymaker cultivars, while for CLN1621 and Saladette, pollen viability rates reduced to 43 and 22%, respectively compared with normal conditions (Figure 6C). Analyzing DPBA-stained pollen from these plants indicated that the tolerant cultivars accumulate about twice the amount of flavonols compared with the sensitive cultivars (Figure 6D).
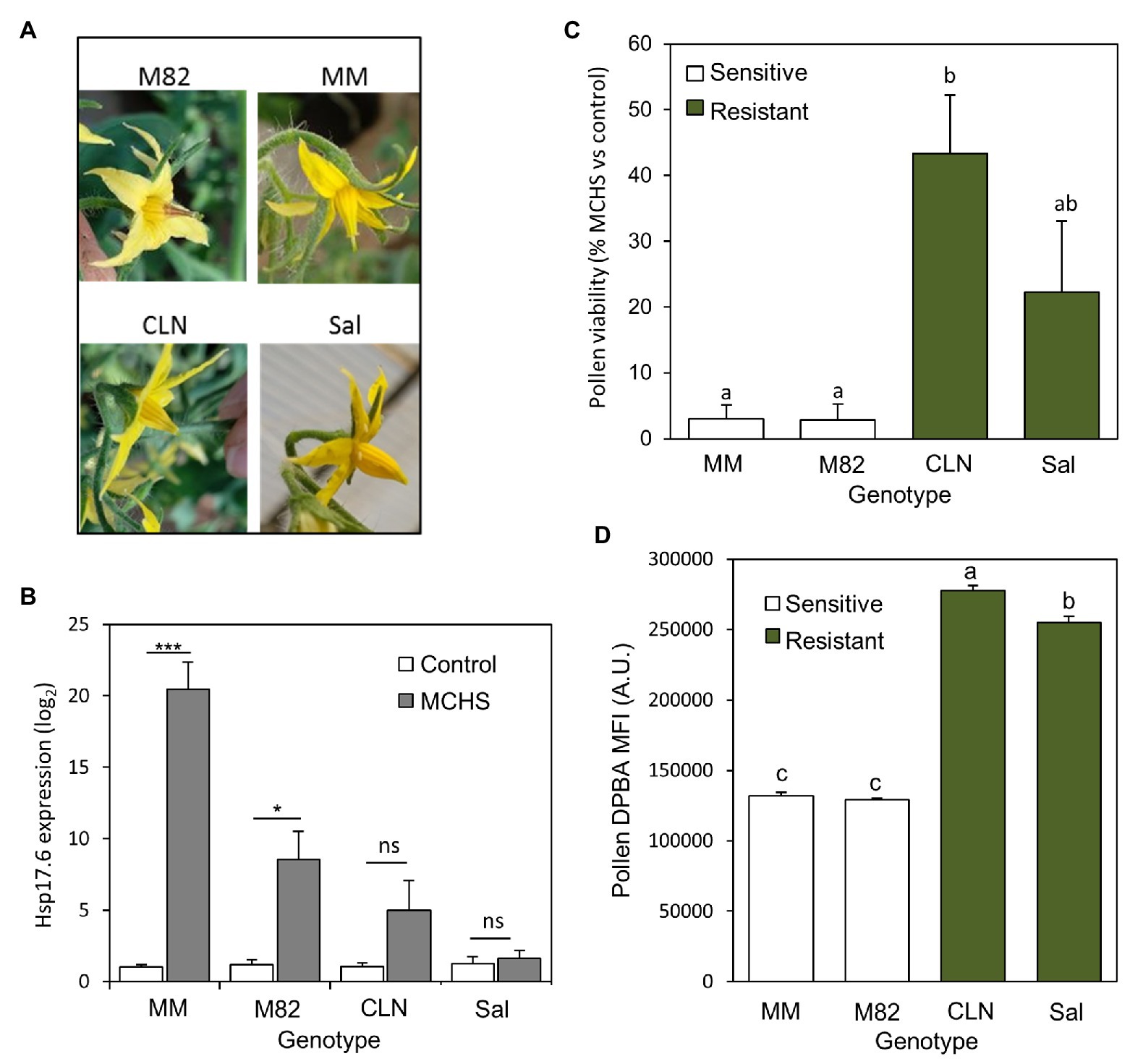
Figure 6. Flavonols levels are higher in pollen of heat stress tolerant tomato cultivars. (A) Typical flower appearance in response to heat stress in each of the four cultivars. (B) Expression level of the heat stress molecular marker gene Hsp17.6 in leaves of the four genotypes, under control and MCHS conditions. (C) Pollen viability rates of the four genotypes under MCHS conditions, relative to control conditions. Pollen viability rates were 96–97% in all four genotypes, under control conditions, therefore used as a reference to present the stress effect. (D) Pollen flavonols in the four genotypes, measured by diphenylboric acid-2-aminoethyl ester (DPBA) mean fluorescence signal intensity (MFI), in arbitrary units. Error bars represent SEM. *represents p < 0.05 and ***represents p < 0.001. Different letters indicate statistically significant differences. MCHS, moderate chronic heat stress; MM, moneymaker; CLN, CLN1621; Sal, saladette; and AU, arbitrary units.
Our findings indicate that the hp2 tomato mutant is more tolerant than wild-type plants to conditions of MCHS. Focusing on the male gametophyte for its known hyper-heat stress sensitivity, we found that under MCHS conditions, the hp2 lines maintained a bigger fraction of viable pollen, with increased metabolic activity (Figure 2). The active pollen fraction of the hp2 lines also kept its normal size under MCHS conditions, whereas the wild-type lines displayed a sharp decrease in the proportion of the functional, larger high-ROS pollen (Figure 3). Although ROS is generally regarded as a stress signal, in our experimental system, which involves a long term, chronic stress, the DCF-ROS signal is an indication of metabolic activity, rather than acute stress response. For this reason, pollen from control conditions always show higher DCF signal corresponding to higher ROS levels, compared with pollen developed under MCHS conditions.
By application of a short-term heat stress, we were able to identify better pollen germination in hp2dg following the stress (Figure 4). These data show better pollen thermotolerance of hp2, compared with wild-type. The hp2 lines maintained normal seeds production under MCHS, while the wild-type lines presented a sharp decrease in seeds set. We suggest that the improved pollen viability and normal seed set rate of hp2 under MCHS conditions are, at least in part, due to the capability of hp2 to accumulate higher levels of flavonols under heat stress conditions. Our finding that known thermotolerant tomato lines such as Saladette and CLN1621 have higher levels of pollen flavonols under normal conditions supports the assumption that flavonols are responsible for the improved thermotolerance of hp2 pollen.
Several studies pointed at the involvement of flavonols in the response to heat stress in plants. In tomato, flavonols content in leaves and pollen was increased about 2-fold in response to heat stress (Martinez et al., 2016; Paupière et al., 2017). Reduced levels of flavonols were functionally linked with a reduction in heat stress tolerance, as demonstrated by the heat stress sensitivity of the tomato are mutant, which has lower levels of flavonols in pollen. In this mutant, pollen viability is strongly reduced when plants are grown under heat stress conditions (Muhlemann et al., 2018). The hp2 mutant in tomato represents the opposite case, where flavonols content is elevated, offerings a unique opportunity for testing the effect of flavonols induction on pollen performance under heat stress conditions. Here, we show for the first time that the hp2 alleles increase pollen flavonols, adding to previous data showing increased flavonols in fruits of hp2 (Bino et al., 2005). Interestingly, flavonols levels increased in both the wild-type and hp2 pollen, although more dramatically in the latter (up to 2.44-fold induction for total pollen and 17.5-fold induction for enhanced-DPBA pollen in hp2j), under MCHS conditions (Supplementary Table S1), placing flavonols as a component of pollen heat stress response. These findings are in agreement with the function of flavonols as antioxidants and with previous findings showing their ability to protect against various environmental stresses, such as heat, cold, and drought (Di Ferdinando et al., 2012; Nakabayashi et al., 2014; Zandalinas et al., 2017; Muhlemann et al., 2018; Zhao et al., 2019). Thus, this antioxidative activity, may account, at least in part, for the improved ability of hp2 to maintain pollen viability and seed setting under MCHS conditions (Figures 1, 2). Nonetheless, taking into account the pleiotropic nature of hp2, it is reasonable that other metabolites, in addition to flavonols, may be involved in the improved thermotolerance of pollen. To test this hypothesis for secondary metabolites, for example, various pigmentation mutants of tomato could be utilized (Chattopadhyay et al., 2021). The importance of primary metabolites such as starch and soluble sugars for pollen thermotolerance was demonstrated by Firon et al. (2006); however, no alterations in soluble sugars were detected in fruits of the hp2 mutant (Bino et al., 2005). Thus, the question of whether primary metabolites are contributing to pollen thermotolerance of hp2 is yet to be addressed. Additionally, as was shown for starch levels in pollen, the specific stage of pollen development is also important in determining reproductive success under heat stress conditions (Firon et al., 2006). Therefore, flavonols levels in earlier stages of pollen development under heat stress conditions should be investigated.
Flavonols are highly abundant in the tapetum tissue of the anther and are transported as flavonol glycosides to the sporopollenin layer of the macrogametophyte during pollen development (Hsieh and Huang, 2007; Fellenberg and Vogt, 2015). Recent study in Arabidopsis identified FST1, a member of the nitrate/peptide transporter family that is expressed specifically in the tapetum and required for the accumulation and transport of pollen-specific flavonols to the pollen surface in Arabidopsis. In a mutant lacking this transporter, flavonols transport to the pollen is reduced, and pollen viability is hindered (Grunewald et al., 2020). Putative orthologous genes were identified in several plant species, including tomato. Our analysis of mature pollen flavonols do not distinguish flavonols originated from the pollen grain itself (i.e., the sporopollenin layer surrounding the pollen grain), and flavonols that may come from the tapetum tissue breakdown (or yet another tissue) during pollen maturation. Therefore, the induced level of flavonols we detected in pollen from hp2 may be the result of either enhanced biosynthesis in the tapetum, enhanced transport to the sporopollenin, or both. The issues of spatial localization of flavonols biosynthesis and transport in hp2 are yet to be investigated.
The cultivar Saladette was reported as heat-tolerant in 1977 (Rudich et al., 1977), and has been used as a heat-tolerant reference in numerous studies since. It was shown to cope better with high temperatures in terms of assimilate partitioning and metabolism, improved pollen viability, and higher fruit set under heat stress conditions (Rudich et al., 1977; Dinar et al., 1983; Dinar and Rudich, 1985; Abdul-Baki, 1991; Firon et al., 2006).
CLN1621L was found to be heat stress tolerant in two independent screens for tolerant tomato cultivars (Comlekcioglu and Kemal Soylu, 2010; Balyan et al., 2020). Under field conditions, when day/night temperatures were on average 37/27°C, CLN1621L produced a higher rate of seeded fruits and lower rate of aborted flowers compared with other cultivars tested (Comlekcioglu and Kemal Soylu, 2010). Comparing different seasons, CLN1621L performed better in the hot season (day/night temperatures of 35/28°C) in terms of fruit set rate and number of pollen grains per flower (Sangu et al., 2015). Despite many publications characterizing the physiological response to heat stress in Saladette and CLN1621L, no data were available for pollen secondary metabolites. Our findings that both thermotolerant cultivars have about twice as much flavonols in their pollen compared with thermosensitive cultivars (Figure 6) provides perhaps the first mechanistic explanation, at least in part, for their increased reproductive success under high temperatures, and an independent support to our hypothesis. However, it will be interesting to conduct a controlled heat stress experiment similarly to the hp2 system, in order to compare pollen flavonols levels between control and MCHS conditions.
The previously characterized phenotypes of hp2 (e.g., increased accumulation of fruits carotenoids, flavonoids, and vitamins) are caused by malfunction of the tomato DEETIOLATED1 gene (LEDET1, Solyc01g056340). DET1 acts as a component of an E3 ubiquitin ligase complex targeting proteins for degradation by the 26S proteasome (Bernhardt et al., 2006; Zhang et al., 2008). Thus, DET1 is affecting the abundance of different genes in various biosynthetic pathways, such as GLK2, BBX20, and MBD5, which act as upstream regulators of chloroplast biogenesis, flavonoid accumulation, and carotenoid biosynthesis in tomato (Powell et al., 2012; Nguyen et al., 2014; Li et al., 2016; Tang et al., 2016; Xiong et al., 2019). This dependency may explain the mode of action of the hp2 mutant, suggesting that when DET1 function is impaired, the 26S proteasome-mediated degradation of positive regulators of plastids biogenesis and carotenoids biosynthesis is hindered, resulting in increased number of plastids and enhanced pigmentation. This work is the first evaluation, to our knowledge, of the effect of hp2 secondary metabolites on pollen viability and performance. We show that the flavonols over-accumulation effect of hp2 in fruit is reproduced in pollen (Figure 5). As DET1 is the gene underlying hp2 mutant phenotypes, it is yet to be defined how DET1 function facilitates accumulation of flavonols in pollen under heat stress conditions. Based on current knowledge, we hypothesize that the function of the 26S proteasome in the tapetum is hindered when temperatures arise, leading to decreased degradation of positive regulators of flavonoids biosynthesis resulting in flavonols accumulation. The availability of three different allelic hp2 mutants (i.e., hp2, hp2j, and hp2dg), provides an intriguing opportunity to investigate allele-dependent effects. Indeed, some variation between the alleles was observed in our set of experiments. As the effect of hp2 and hp2j on pollen flavonols was evident both in terms of total pollen (Figure 5B) as well as enhanced-DPBA pollen (Figure 5D), the effect of hp2dg was observed only when looking at the enhanced-DPBA pollen, and the increase was smaller compared with hp2 and hp2j (Figure 5D). However in terms of seeds production, the magnitude of effect was the same for all three alleles (Figure 1C). This discrepancy may be explained by the different genetic background of the allelic lines (Moneymaker for hp2, hp2j and Manapal for hp2dg), or, may be the result of a specific effect of the hp2dg allele, hinting at a complex effect of DET1 function on pollen viability under heat stress conditions.
The marginal increase in pollen flavonols that we found under MCHS conditions in the wild-type lines Moneymaker and Manapal (Figure 5B) may suggest an additional pathway in which the response to heat stress could activate flavonols biosynthesis independently of the proteasome degradation machinery which involves DET1. To shed light on this issue, the function of the 26S proteasome under control and MCHS conditions should be tested.
The beneficial effect of hp2 in stress tolerance may extend beyond heat stress due to the fact that flavonols play an important role in protecting against oxidative stress which accompanies many other environmental stresses, such as cold, drought, and nutrient starvation (Nakabayashi et al., 2014; Zandalinas et al., 2017; Zhao et al., 2019; Song et al., 2020). Interestingly, a recent publication describes the tomato Micro-Tom high pigment 1, a closely related mutant to hp2, as chilling stress tolerant in terms of photosynthetic activity, activities of antioxidant enzymes, and accumulation of protecting osmolytes (Shahzad et al., 2020). It would be of interest to test whether pollen characteristics, such as viability, germination capacity, and flavonol levels are playing a role also in hp1 under chilling and heat stress conditions.
Finally, the enhanced accumulation of human-health promoting secondary metabolites drove the introgression of hp2 mutations into commercial tomato cultivars, facilitating the production of lycopene-rich fresh market tomatoes as well as processing cultivars for cost efficient lycopene extraction for the food and cosmetics industries (Levin et al., 2006; Ilahy et al., 2018). The identification of hp2 as a novel potential source for crop thermotolerance may increase its value as a biotechnology tool in plant breeding schemes. For this purpose, detailed characterization of yield traits, such as fruit number, total weight, and brix under control, MCHS, and commercial growth conditions should be performed using the original hp2 mutant lines (as in this study), as well as commercial cultivars that carry either of the hp2 alleles in various backgrounds (Ilahy et al., 2018).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
NR and ML-L designed and performed the experiments, analyzed the data, and wrote the manuscript. GoM and FW performed the experiments and analyzed the data. JH and GaM wrote the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by institutional grants to ML-L and grants to GaM and JH from BARD IS‐ 4652-13, GaM from BSF-2016605, and JH from NSF IOS 1656774.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.672368/full#supplementary-material
MCHS, Moderate chronic heat stress; WT, Wild-type; hp., high pigment; MM, Moneymaker; MP, Manapal; MT, MicroTom; CHS, Chalcone synthase; CHI, Chalcone isomerase; ROS, Reactive oxygen species; H2DCFDA, 2',7'-dichlorodihydrofluorescein diacetate; DCF, Dichlorofluorescein; DPBA, Diphenylboric acid-2-aminoethyl ester; MFI, Mean fluorescent intensity.
Abdul-Baki, A. A. (1991). Tolerance of tomato cultivars and selected germplasm to heat stress. J. Am. Soc. Hort. Sci. 116, 1113–1116. doi: 10.21273/JASHS.116.6.1113
Abdul-Baki, A. A., and Stommel, J. R. (1995). Pollen viability and fruit set of tomato genotypes under optimum‐ and high-temperature regimes. HortScience 30, 115–117. doi: 10.21273/HORTSCI.30.1.115
Adan, A., Alizada, G., Kiraz, Y., Baran, Y., and Nalbant, A. (2017). Flow cytometry: basic principles and applications. Crit. Rev. Biotechnol. 37, 163–176. doi: 10.3109/07388551.2015.1128876
Agati, G., Azzarello, E., Pollastri, S., and Tattini, M. (2012). Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196, 67–76. doi: 10.1016/j.plantsci.2012.07.014
Balyan, S., Rao, S., Jha, S., Bansal, C., Das, J. R., and Mathur, S. (2020). Characterization of novel regulators for heat stress tolerance in tomato from Indian sub-continent. Plant Biotechnol. J. 18, 2118–2132. doi: 10.1111/pbi.13371
Begcy, K., Nosenko, T., Zhou, L. Z., Fragner, L., Weckwerth, W., and Dresselhaus, T. (2019). Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiol. 181, 683–700. doi: 10.1104/pp.19.00707
Begcy, K., Weigert, A., Egesa, A. O., and Dresselhaus, T. (2018). Compared to Australian cultivars, European summer wheat (Triticum aestivum) overreacts when moderate heat stress is applied at the pollen development stage. Agronomy 8:99. doi: 10.3390/agronomy8070099
Bernhardt, A., Lechner, E., Hano, P., Schade, V., Dieterle, M., Anders, M., et al. (2006). CUL4 associates with DDB1 and DET1 and its downregulation affects diverse aspects of development in Arabidopsis thaliana. Plant J. 47, 591–603. doi: 10.1111/j.1365-313X.2006.02810.x
Bino, R. J., De Vos, C. H. R., Lieberman, M., Hall, R. D., Bovy, A., Jonker, H. H., et al. (2005). The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome. New Phytol. 166, 427–438. doi: 10.1111/j.1469-8137.2005.01362.x
Brown, J. E., Khodr, H., Hider, R. C., and Rice-Evans, C. A. (1998). Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem. J. 330, 1173–1178.
Brunetti, C., Di Ferdinando, M., Fini, A., Pollastri, S., and Tattini, M. (2013). Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int. J. Mol. Sci. 14, 3540–3555. doi: 10.3390/ijms14023540
Buer, C. S., Imin, N., and Djordjevic, M. A. (2010). Flavonoids: new roles for old molecules. J. Integr. Plant Biol. 52, 98–111. doi: 10.1111/j.1744-7909.2010.00905.x
Chattopadhyay, T., Hazra, P., Akhtar, S., Maurya, D., Mukherjee, A., and Roy, S. (2021). Skin colour, carotenogenesis and chlorophyll degradation mutant alleles: genetic orchestration behind the fruit colour variation in tomato. Plant Cell Rep. doi: 10.1007/s00299-020-02650-9 [Epub ahead of print]
Coberly, L. C., and Rausher, M. D. (2003). Analysis of a chalcone synthase mutant in Ipomoea purpurea reveals a novel function for flavonoids: amelioration of heat stress. Mol. Ecol. 12, 1113–1124. doi: 10.1046/j.1365-294X.2003.01786.x
Comlekcioglu, N., and Kemal Soylu, M. (2010). Determination of high temperature tolerance via screening of flower and fruit formation in tomato. J. Agric. Sci. 20, 123–130.
Davuluri, G. R., van Tuinen, A., Fraser, P. D., Manfredonia, A., Newman, R., Burgess, D., et al. (2005). Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat. Biotechnol. 23, 890–895. doi: 10.1038/nbt1108
Derksen, J., van Wezel, R., Knuiman, B., Ylstra, B., and Van Tunen, A. J. (1999). Pollen tubes of flavonol-deficient Petunia show striking alterations in wall structure leading to tube disruption. Planta 207, 575–581. doi: 10.1007/s004250050520
De Storme, N., and Geelen, D. (2014). The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ. 37, 1–18. doi: 10.1111/pce.12142
Di Ferdinando, M., Brunetti, C., Fini, A., and Tattini, M. (2012). “Flavonoids as antioxidants in plants under abiotic stresses” in Abiotic Stress Responses in Plants. eds. P. Ahmad and M. Prasad (New York, NY: Springer), 159–179.
Dinar, M., and Rudich, J. (1985). Effect of heat stress on assimilate partitioning in tomato. Ann. Bot. 56, 239–248. doi: 10.1093/oxfordjournals.aob.a087008
Dinar, M., Rudich, J., and Zamski, E. (1983). Effects of heat stress on carbon transport from tomato leaves. Ann. Bot. 51, 97–103. doi: 10.1093/oxfordjournals.aob.a086454
Djanaguiraman, M., Perumal, R., Jagadish, S. V. K., Ciampitti, I. A., Welti, R., and Prasad, P. V. V. (2018). Sensitivity of sorghum pollen and pistil to high-temperature stress. Plant Cell Environ. 41, 1065–1082. doi: 10.1111/pce.13089
Djanaguiraman, M., Prasad, P. V. V., Boyle, D. L., and Schapaugh, W. T. (2013). Soybean pollen anatomy, viability and pod set under high temperature stress. J. Agron. Crop Sci. 199, 171–177. doi: 10.1111/jac.12005
El Ahmadi, A. B., and Stevens, M. A. (1979). Reproductive responses of heat-tolerant tomatoes to high temperature. Am. Soc. Hortic. Sci. J. 104, 686–691.
Endo, M., Tsuchiya, T., Hamada, K., Kawamura, S., Yano, K., Ohshima, M., et al. (2009). High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 50, 1911–1922. doi: 10.1093/pcp/pcp135
Enfissi, E. M. A., Barneche, F., Ahmed, I., Lichtlé, C., Gerrish, C., McQuinn, R. P., et al. (2010). Integrative transcript and metabolite analysis of nutritionally enhanced DE-ETIOLATED1 downregulated tomato fruit. Plant Cell 22, 1190–1215. doi: 10.1105/tpc.110.073866
Erickson, A. N., and Markhart, A. H. (2002). Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell Environ. 25, 123–130. doi: 10.1046/j.0016-8025.2001.00807.x
Falcone Ferreyra, M. L., Rius, S. P., and Casati, P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3:222. doi: 10.3389/fpls.2012.00222
Fellenberg, C., and Vogt, T. (2015). Evolutionarily conserved phenylpropanoid pattern on angiosperm pollen. Trends Plant Sci. 20, 212–218. doi: 10.1016/j.tplants.2015.01.011
Fernando, V. C. D., and Schroeder, D. F. (2016). Arabidopsis DDB1-CUL4 E3 ligase complexes in det1 salt/osmotic stress resistant germination. Plant Signal. Behav. 11:e1223004. doi: 10.1080/15592324.2016.1223004
Firon, N., Shaked, R., Peet, M. M., Pharr, D. M., Zamski, E., Rosenfeld, K., et al. (2006). Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Sci. Hortic. 109, 212–217. doi: 10.1016/j.scienta.2006.03.007
Fragkostefanakis, S., Mesihovic, A., Simm, S., Paupiere, M. J., Hu, Y., Paul, P., et al. (2016). HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 170, 2461–2477. doi: 10.1104/pp.15.01913
Frank, G., Pressman, E., Ophir, R., Althan, L., Shaked, R., Freedman, M., et al. (2009). Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 60, 3891–3908. doi: 10.1093/jxb/erp234
Giorno, F., Wolters-Arts, M., Mariani, C., and Rieu, I. (2013). Ensuring reproduction at high temperatures: the heat stress response during anther and pollen development. Plan. Theory 2, 489–506. doi: 10.3390/plants2030489
Grunewald, S., Marillonnet, S., Hause, G., Haferkamp, I., Ekkehard Neuhaus, H., Veß, A., et al. (2020). The tapetal major facilitator NPF2.8 is required for accumulation of flavonol glycosides on the pollen surface in Arabidopsis thaliana. Plant Cell 32, 1727–1748. doi: 10.1105/tpc.19.00801
Hsieh, K., and Huang, A. H. C. (2007). Tapetosomes in Brassica Tapetum accumulate endoplasmic reticulum–derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19, 582–596. doi: 10.1105/tpc.106.049049
Ilahy, R., Siddiqui, M. W., Tlili, I., Montefusco, A., Piro, G., Hdider, C., et al. (2018). When color really matters: horticultural performance and functional quality of high-lycopene tomatoes. CRC. Crit. Rev. Plant Sci. 37, 15–53. doi: 10.1080/07352689.2018.1465631
Irigoyen, M. L., Iniesto, E., Rodriguez, L., Puga, M. I., Yanagawa, Y., Pick, E., et al. (2014). Targeted degradation of abscisic acid receptors is mediated by the ubiquitin ligase substrate adaptor DDA1 in Arabidopsis. Plant Cell 26, 712–728. doi: 10.1105/tpc.113.122234
Iwahori, S. (1966). High temperature injuries in tomato. Engei Gakkai zasshi 35, 379–386. doi: 10.2503/jjshs.35.379
Jagadish, S. V. K., Craufurd, P. Q., and Wheeler, T. R. (2007). High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 58, 1627–1635. doi: 10.1093/jxb/erm003
Jones, M. O., Piron-Prunier, F., Marcel, F., Piednoir-Barbeau, E., Alsadon, A. A., Wahb-Allah, M. A., et al. (2012). Characterisation of alleles of tomato light signalling genes generated by TILLING. Phytochemistry 79, 78–86. doi: 10.1016/j.phytochem.2012.04.005
Kolotilin, I., Koltai, H., Tadmor, Y., Bar-Or, C., Reuveni, M., Meir, A., et al. (2007). Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol. 145, 389–401. doi: 10.1104/pp.107.102962
Levin, I., De Vos, R. C. H., Tadmor, Y., Bovy, A., Lieberman, M., Oren-Shamir, M., et al. (2006). High pigment tomato mutants—more than just lycopene (a review). Isr. J. Plant Sci. 54, 179–190. doi: 10.1560/IJPS_54_3_179
Levin, I., Frankel, P., Gilboa, N., Tanny, S., and Lalazar, A. (2003). The tomato dark green mutation is a novel allele of the tomato homolog of the DEETIOLATED1 gene. Theor. Appl. Genet. 106, 454–460. doi: 10.1007/s00122-002-1080-4
Li, Y., Deng, H., Miao, M., Li, H., Huang, S., Wang, S., et al. (2016). Tomato MBD5, a methyl CpG binding domain protein, physically interacting with UV-damaged DNA binding protein-1, functions in multiple processes. New Phytol. 210, 208–226. doi: 10.1111/nph.13745
Lohani, N., Singh, M. B., and Bhalla, P. L. (2020). High temperature susceptibility of sexual reproduction in crop plants. J. Exp. Bot. 71, 555–568. doi: 10.1093/jxb/erz426
Luria, G., Rutley, N., Lazar, I., Harper, J. F., and Miller, G. (2019). Direct analysis of pollen fitness by flow cytometry: implications for pollen response to stress. Plant J. 98, 942–952. doi: 10.1111/tpj.14286
Martinez, V., Mestre, T. C., Rubio, F., Girones-Vilaplana, A., Moreno, D. A., Mittler, R., et al. (2016). Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 7:838. doi: 10.3389/fpls.2016.00838
Melidou, M., Riganakos, K., and Galaris, D. (2005). Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radic. Biol. Med. 39, 1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009
Mercado, J. A., Mar Trigo, M., Reid, M. S., Valpuesta, V., and Quesada, M. A. (1997). Effects of low temperature on pepper pollen morphology and fertility: evidence of cold induced exine alterations. J. Hortic. Sci. 72, 317–326. doi: 10.1080/14620316.1997.11515518
Mo, Y., Nagel, C., and Taylor, L. P. (1992). Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. U. S. A. 89, 7213–7217. doi: 10.1073/pnas.89.15.7213
Monterroso, V. A., and Wien, H. C. (1990). Flower and pod abscission due to heat stress in beans. J. Am. Soc. Hortic. Sci. 115, 631–634. doi: 10.21273/JASHS.115.4.631
Muhlemann, J. K., Younts, T. L. B., and Muday, G. K. (2018). Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. U. S. A. 115, E11188–E11197. doi: 10.1073/pnas.1811492115
Muller, F., and Rieu, I. (2016). Acclimation to high temperature during pollen development. Plant Reprod. 29, 107–118. doi: 10.1007/s00497-016-0282-x
Mustilli, A. C., Fenzi, F., Ciliento, R., Alfano, F., and Bowler, C. (1999). Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 11, 145–157. doi: 10.1105/tpc.11.2.145
Nakabayashi, R., Yonekura-Sakakibara, K., Urano, K., Suzuki, M., Yamada, Y., Nishizawa, T., et al. (2014). Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 77, 367–379. doi: 10.1111/tpj.12388
Nguyen, C. V., Vrebalov, J. T., Gapper, N. E., Zheng, Y., Zhong, S., Fei, Z., et al. (2014). Tomato GOLDEN2-LIKE transcription factors reveal molecular gradients that function during fruit development and ripening. Plant Cell 26, 585–601. doi: 10.1105/tpc.113.118794
Osterlund, M., Hardtke, C., Wei, N., and Deng, X. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. doi: 10.1038/35013076
Paupière, M. J., Müller, F., Li, H., Rieu, I., Tikunov, Y. M., Visser, R. G. F., et al. (2017). Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 30, 81–94. doi: 10.1007/s00497-017-0301-6
Peet, M. M., Sato, S., and Gardner, R. G. (1998). Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant Cell Environ. 21, 225–231. doi: 10.1046/j.1365-3040.1998.00281.x
Pepper, A., Delaney, T., Washburnt, T., Poole, D., and Chory, J. (1994). DET1, a negative regulator of light-mediated development and gene expression in arabidopsis, encodes a novel nuclear-localized protein. Cell 78, 109–116. doi: 10.1016/0092-8674(94)90577-0
Porch, T. G., and Jahn, M. (2001). Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ. 24, 723–731. doi: 10.1046/j.1365-3040.2001.00716.x
Powell, A. L. T., Nguyen, C. V., Hill, T., Cheng, K. L. L., Figueroa-Balderas, R., Aktas, H., et al. (2012). Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336, 1711–1715. doi: 10.1126/science.1222218
Prasad, P., Boote, K., and Allen, L. J. (2006). Adverse high tempera‐ ture effects on pollen viability, seed-set, seed yield and harvest index of grain sorghum (Sorghum bicolor (L.) Moench) are more severe at elevated carbon dioxide. Agric. For. Meteorol. 139, 237–251. doi: 10.1016/j.agrformet.2006.07.003
Pressman, E., Peet, M. M., and Pharr, D. M. (2002). The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann. Bot. 90, 631–636. doi: 10.1093/aob/mcf240
Rice-Evans, C. (2001). Flavonoid antioxidants. Curr. Med. Chem. 8, 797–807. doi: 10.2174/0929867013373011
Rudich, J., Zamski, E., and Regev, Y. (1977). Genotypic variation for sensitivity to high temperature in the tomato: pollination and fruit set. Bot. Gaz. 138, 448–452. doi: 10.1086/336947
Rutley, N., and Miller, G. (2020). Large-scale analysis of pollen viability and oxidative level using h2DCFDA-staining coupled with flow cytometry. Methods Mol. Biol. 2160, 167–179. doi: 10.1007/978-1-0716-0672-8_11
Sangu, E., Tibazarwa, F. I., Nyomora, A., and Symonds, R. C. (2015). Expression of genes for the biosynthesis of compatible solutes during pollen development under heat stress in tomato (Solanum lycopersicum). J. Plant Physiol. 178, 10–16. doi: 10.1016/j.jplph.2015.02.002
Sato, S., Peet, M. M., and Thomas, J. F. (2000). Physiological factors limit fruit set of tomato (Lycopersicon esculentum mill.) under chronic, mild heat stress. Plant Cell Environ. 23, 719–726. doi: 10.1046/j.1365-3040.2000.00589.x
Schneider, C., Rasband, W., and Eliceiri, K. (2012). NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Seo, K. I., Lee, J. H., Nezames, C. D., Zhong, S., Song, E., Byun, M. O., et al. (2014). ABD1 is an Arabidopsis DCAF substrate receptor for CUL4-DDB1-based E3 ligases that acts as a negative regulator of abscisic acid signaling. Plant Cell 26, 695–711. doi: 10.1105/tpc.113.119974
Sestari, I., Zsögön, A., Rehder, G. G., Teixeira, L. D. L., Hassimotto, N. M. A., Purgatto, E., et al. (2014). Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv micro-tom) as a tool to produce nutrient-rich fruits. Sci. Hortic. 175, 111–120. doi: 10.1016/j.scienta.2014.06.010
Severini, A. D., Borras, L., and Cirilo, A. G. (2011). Counting maize kernels through digital image analysis. Crop Sci. 51, 2796–2800. doi: 10.2135/cropsci2011.03.0147
Shahzad, R., Ahmed, F., Wang, Z., Harlina, P. W., Nishawy, E., Ayaad, M., et al. (2020). Comparative analysis of two phytochrome mutants of tomato (micro-tom cv.) reveals specific physiological, biochemical, and molecular responses under chilling stress. J. Genet. Eng. Biotechnol. 18:77. doi: 10.1186/s43141-020-00091-1
Sheahan, J. J., and Rechnitz, G. A. (1992). Flavonoid-specific staining of Arabidopsis thaliana. BioTechniques 13, 880–883.
Song, Z., Luo, Y., Wang, W., Fan, N., Wang, D., Yang, C., et al. (2020). NtMYB12 positively regulates flavonol biosynthesis and enhances tolerance to low pi stress in Nicotiana tabacum. Front. Plant Sci. 10:1683. doi: 10.3389/fpls.2019.01683
Tang, X., Miao, M., Niu, X., Zhang, D., Cao, X., Jin, X., et al. (2016). Ubiquitin-conjugated degradation of golden 2-like transcription factor is mediated by CUL4-DDB1-based E3 ligase complex in tomato. New Phytol. 209, 1028–1039. doi: 10.1111/nph.13635
van Tuinen, A., Koornneef, M., Cordonnier-Pratt, M.-M., Pratt, L. H., Verkerk, R., and Zabel, P. (1997). The mapping of phytochrome genes and photomorphogenic mutants of tomato. Theor. Appl. Genet. 94, 115–122. doi: 10.1007/s001220050389
Wang, Y., Tao, H., Tian, B., Sheng, D., Xu, C., Zhou, H., et al. (2019). Flowering dynamics, pollen, and pistil contribution to grain yield in response to high temperature during maize flowering. Environ. Exp. Bot. 158, 80–88. doi: 10.1016/j.envexpbot.2018.11.007
Warrag, M. O. A., and Hall, A. E. (1984). Reproductive responses of cowpea (Vigna unguiculata (L.) Walp.) to heat stress. II. Responses to night air temperature. Field Crop Res. 8, 17–33. doi: 10.1016/0378-4290(84)90049-2
Xiong, C., Luo, D., Lin, A., Zhang, C., Shan, L., He, P., et al. (2019). A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 221, 279–294. doi: 10.1111/nph.15373
Xu, J., Wolters-Arts, M., Mariani, C., Huber, H., and Rieu, I. (2017). Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 213:156. doi: 10.1007/s10681-017-1949-6
Ylstra, B., Touraev, A., Moreno, R. M. B., Stöger, E., van Tunen, A. J., Vicente, O., et al. (1992). Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol. 100, 902–907. doi: 10.1104/pp.100.2.902
Young, L. W., Wilen, R. W., and Bonham-Smith, P. C. (2004). High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 55, 485–495. doi: 10.1093/jxb/erh038
Zandalinas, S. I., Sales, C., Beltrán, J., Gómez-Cadenas, A., and Arbona, V. (2017). Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 7:1954. doi: 10.3389/fpls.2016.01954
Zhang, Y., Feng, S., Chen, F., Chen, H., Wang, J., McCall, C., et al. (2008). Arabidopsis DDB1-CUL4 ASSOCIATED FACTOR1 forms a nuclear E3 ubiquitin ligase with DDB1 and CUL4 that is involved in multiple plant developmental processes. Am. Soc. Plant Biol. 20, 1437–1455. doi: 10.1105/tpc.108.058891
Zhao, M., Jin, J., Gao, T., Zhang, N., Jing, T., Wang, J., et al. (2019). Glucosyltransferase CsUGT78A14 regulates flavonols accumulation and reactive oxygen species scavenging in response to cold stress in Camellia sinensis. Front. Plant Sci. 10:1675. doi: 10.3389/fpls.2019.01675
Zhao, C., Liu, B., Piao, S., Wang, X., Lobell, D. B., Huang, Y., et al. (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. U. S. A. 114, 9326–9331. doi: 10.1073/pnas.1701762114
Keywords: crop productivity, thermotolerance, heat stress, tomato, pollen viability, flavonols, high pigment
Citation: Rutley N, Miller G, Wang F, Harper JF, Miller G and Lieberman-Lazarovich M (2021) Enhanced Reproductive Thermotolerance of the Tomato high pigment 2 Mutant Is Associated With Increased Accumulation of Flavonols in Pollen. Front. Plant Sci. 12:672368. doi: 10.3389/fpls.2021.672368
Received: 25 February 2021; Accepted: 07 April 2021;
Published: 19 May 2021.
Edited by:
Vicent Arbona, University of Jaume I, SpainReviewed by:
Sotirios Fragkostefanakis, Goethe University Frankfurt, GermanyCopyright © 2021 Rutley, Miller, Wang, Harper, Miller and Lieberman-Lazarovich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michal Lieberman-Lazarovich, bWljaGFsbEB2b2xjYW5pLmFncmkuZ292Lmls
†Present address: Fengde Wang, Institute of Vegetables and Flowers, Shandong Academy of Agricultural Sciences, Jinan, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.