
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 22 June 2021
Sec. Plant Breeding
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.670284
This article is part of the Research TopicBiological and Genetic Basis of Agronomical and Seed Quality Traits in LegumesView all 30 articles
Manteca yellow dry beans (Phaseolus vulgaris L.) have many quality traits that appeal to consumers, including fast cooking times, creamy texture, and sweet, buttery flavor. They are native to Chile and consumed in regions of South America and Africa but are largely unfamiliar to United States consumers. While cooking time, flavor, and texture have not been prioritized in United States dry bean breeding programs, genetic variability exists such that these traits could be addressed through breeding. In this study, a recombinant inbred line (RIL) population was developed from a cross between Ervilha (Manteca) and PI527538 (Njano), yellow dry beans with contrasting cooking time and sensory attributes. The population and parents were grown for 2 years in Michigan and evaluated for cooking time and sensory attribute intensities, including total flavor, beany, vegetative, earthy, starchy, sweet, bitter, seed-coat perception, and cotyledon texture. Cooking time ranged 19–34 min and exhibited high broad-sense heritability (0.68). Sensory attribute intensities also exhibited variation among RILs, although broad-sense heritability was low, with beany and total flavor exhibiting the highest (0.33 and 0.27). A linkage map of 870 single nucleotide polymorphisms markers across 11 chromosomes was developed for quantitative trait loci (QTL) mapping, which revealed QTL for water uptake (3), cooking time (6), sensory attribute intensities (28), color (13), seed-coat postharvest non-darkening (1), seed weight (5), and seed yield (2) identified from data across 2 years. Co-localization was identified for starchy, sweet, and seed-coat perception on Pv01; for total flavor, beany, earthy, starchy, sweet, bitter, seed-coat perception, cotyledon texture, and color on Pv03; water uptake and color on Pv04; total flavor, vegetative, sweet, and cotyledon texture on Pv07; cooking time, starchy, sweet, and color on Pv08; and water uptake, cooking time, total flavor, beany, starchy, bitter, seed-coat perception, cotyledon texture, color, and seed-coat postharvest non-darkening on Pv10. The QTL identified in this work, in particular CT8.2 and CT10.2, can be used to develop molecular markers to improve seed quality traits in future dry bean varieties. Considering yellow dry beans already excel in quality and convenience, they might be an ideal market class to signal a new focus on consumer-valued traits in the United States.
Dry beans (Phaseolus vulgaris L.) are widely regarded as a nutritious and affordable food (Akibode and Maredia, 2011). The species encompasses many different market classes grown and consumed around the world with many regional preferences (Siddiq and Uebersax, 2012). There is variability not just for seed size, color, and shape, but also end-use quality attributes, including cooking time, mineral concentration and bioavailability, color, flavor, and texture (Katuuramu et al., 2018; Bassett et al., 2020b). Some market classes may be of particular interest to modern consumers looking to incorporate beans into their diets for their nutritional benefits and also looking for convenience not typically associated with dry beans considering their often long cooking times (Sloan, 2015).
The Manteca yellow bean market class has multiple quality traits of value to consumers (Leakey, 2000; Wiesinger et al., 2016, 2018). Manteca are pale yellow with a gray hilum. They are Andean beans native to Chile (Leakey, 1992) and currently consumed in South America and Africa (Wiesinger et al., 2018). Manteca are appreciated for their sweet, buttery flavor (Leakey, 2000) as well as fast cooking time and high iron bioavailability (Wiesinger et al., 2016, 2018). United States consumers are largely unfamiliar with this yellow market class. Its novel color has the potential to set the market class apart from familiar market classes and signal the presence of quality attributes if introduced more broadly.
Current dietary guidelines recommend cup (∼56 g) of pulse per day, but less than 50% of the population meets that recommendation (Britten et al., 2012). There is an opportunity to increase utilization of dry beans by addressing consumer preferences for convenience and flavor as well as developing bean products to reach new consumers (IPSOS, 2010; Karlsen et al., 2016; Hooper et al., 2019; Winham et al., 2019). While United States dry bean breeders have always prioritized quality traits, they primarily have focused on seed size, shape, color, and canning quality and production-related traits with minor if any consideration for cooking time and flavor (Kelly and Cichy, 2012). As a result, genetic variability exists for cooking time, flavor, and texture in modern cultivars as well as the breeding lines used for their development (Bassett et al., 2020b). There is an opportunity to address these consumer-valued traits through breeding to increase dry bean consumption, and Manteca beans are an ideal target for this effort, as they already excel in these traits and provide novelty to those unfamiliar with them.
Cooking time has been reported to be controlled by few genes and have moderate to high heritability, with narrow sense heritability values estimated between 0.74 and 0.90 (Elia et al., 1997; Jacinto-Hernandez et al., 2003). Genotypic cooking time patterns are stable across environments (Cichy et al., 2019; Katuuramu et al., 2020). Following screening of 206 accessions of the Andean Diversity Panel (ADP), several significant single nucleotide polymorphisms (SNPs) associated with cooking time were identified on Pv02, Pv03, and Pv06 (Cichy et al., 2015b). A more recent screening of 430 accessions of the ADP revealed additional significant SNPs on Pv03, Pv04, Pv06, Pv07, Pv08, and Pv11 (Bassett et al., 2020b). In addition, a recent quantitative trait loci (QTL) mapping study using a recombinant inbred line (RIL) population developed from two ADP accessions revealed QTL for cooking time on Pv01, Pv02, Pv03, Pv05, Pv06, Pv10, and Pv11 (Berry et al., 2020). With further study, marker-assisted selection may be a feasible method for breeding faster cooking beans, which could reduce the need to phenotype for cooking time and allow greater incorporation of the fast-cooking trait in breeding programs.
Flavor is a major influence on consumer food choices (Glanz et al., 1998), but evaluating flavor and texture is time consuming and requires trained panelists. As it stands, little is understood about consumer preference in regard to flavor and texture in dry beans apart from a general preference for beans that are sweet and soft and for bean products without a beany “off” flavor (Kinsella, 1979; Bott and Chambers, 2006; Mkanda et al., 2007; Hooper et al., 2019). A few studies have identified genetic variability for sensory attributes, including flavor and texture acceptability, seed-coat perception, seed-coat roughness, cotyledon mealiness, and beany flavor intensity (Koehler et al., 1987; Rivera et al., 2013). A recent study identified genetic variability in the Andean Diversity Panel (ADP) for total, beany, vegetative, earthy, starchy, bitter, and sweet flavor intensities as well as seed-coat perception and cotyledon texture (Bassett et al., 2020b). Using a genome-wide association approach, significant SNPs were identified for many of these traits. As for cooking time, the potential for marker-assisted selection could reduce the need for extensive phenotyping and allow breeders to incorporate flavor and texture into their breeding programs more easily. With a greater understanding of consumer preference for flavor and texture, new varieties could be developed that appeal to consumers and are suitable for use as ingredients in products.
In this study, a yellow dry bean RIL population developed from two ADP accessions with contrasting cooking time and sensory characteristics was screened for cooking time and sensory attribute intensities to elucidate their genetic control and aid in the development of molecular markers for these traits.
A RIL population of 242 F5:F7-F8 lines was developed from a cross between two yellow bean genotypes of the Andean gene pool: Ervilha (ADP0512) and PI527538 (ADP0468) (Figure 1; Bassett and Cichy, 2020). The RILs were developed by advancing F2 seed via single seed descent to the F5 generation and then bulking seeds from individual plants to form RILs.
Ervilha is a pale yellow Manteca seed type with a gray hilum with a seed weight of 52.8 (g per 100 seeds) in this study (Supplementary Table 1). Ervilha was originally collected at a marketplace in Angola in 2010 (Cichy et al., 2015a). PI527538 is a yellow-green Njano seed type with hints of purple and a black hilum with a seed weight of 48.0 (g per 100 seeds) in this study (Supplementary Table 1). PI527538 was originally collected in Burundi in 1985 (Cichy et al., 2015a). Both genotypes are likely members of race Nueva Granada. These genotypes were selected to develop a RIL population after a screening of 206 lines of the Andean Diversity Panel (ADP) for cooking time, mineral concentration, and iron bioavailability (Cichy et al., 2015b; Katuuramu et al., 2018). Ervilha cooks about 10 min faster than PI527538 when soaked, and this relative difference in cooking time is stable across growing environments, although specific cooking times vary depending on the growing environment and storage conditions (Cichy et al., 2015b; Katuuramu et al., 2020).
The genotypes were grown at the Montcalm Research Farm in Lakeview, MI in 2016 and 2017. The soil type is Eutric Glossoboralfs (coarse-loamy and mixed) and Alfic Fragiorthods (coarse-loamy, mixed, and frigid). Two row plots 4.75 m long with 0.5 m spacing between rows were arranged in a randomized complete block design with two replications per genotype. In 2016, 100 seeds were planted per plot due to limited seed, and in 2017, 160 seeds were planted per plot. Standard agronomic practices were followed as described in the MSU SVREC 2017 Farm Research Report (Kelly et al., 2017). Plants were hand-pulled at maturity and threshed with a Hege 140 plot harvester (Wintersteiger, Salt Lake City, UT, United States). Following harvest, seeds were cleaned by hand to remove field debris, off types, and damaged seed. Seed weights (g per 100 seeds) and seed yield (kg per ha) were recorded for each field replicate.
For both years, images were collected for one field replicate of each genotype using a custom machine vision system as described in Mendoza et al. (2017). For each image, a 60 × 15 mm petri dish was filled with representative seeds cleaned of debris and damaged seeds. The EOS Rebel T3i software settings were consistent across each image as follows: lens aperture f = 5.6, shutter speed 1/125, white balanced, and ISO = 100. Following image collection, each image was cropped to center the petri dish and minimize background. To examine the relationship among color, cooking time, and sensory attributes, CIELAB values were obtained using a custom batch macro in ImageJ developed by Bornowski et al. (2020) that applies a gamma correction of 0.5, excludes background pixels outside the petri dish, and measures each slice of the LAB stack. CIELAB uses three values to describe color: L∗ for black (0) to white (100), a∗ for green (−) to red (+), and b∗ for blue (−) to yellow (+). These values were collected relative to the imaging conditions and reflect average color of seeds without calibration for the purpose of observing differences among lines rather than determining absolute color.
Variability in seed-coat postharvest darkening among genotypes was observed after the first year, so the potential presence of the non-darkening trait in this population was explored. Genotypes grown in 2017 were stored for approximately 2 years in opaque paper bags in a cool, dry barn prior to evaluation for seed-coat postharvest darkening in January 2020. Samples that appeared visibly darkened after this storage period were given a score of 1 and those that remained light were given a score of 0.
For each year, two field replicates of 30 seed per genotype were equilibrated to 10–14% moisture in a 4°C humidity chamber prior to evaluating for cooking time. Each 30 seed sample was soaked for 12 h in distilled water prior to cooking time evaluation using an automated Mattson cooker method (Wang and Daun, 2005). Genotypes were cooked in a random order to minimize seed aging effects. Seed weights after soaking were recorded for each sample to determine water uptake. Mattson cookers loaded with soaked seeds were placed into 4 L stainless steel beakers with 1.8 L of boiling distilled water on Cuisinart CB-30 Countertop Single Burners to cook. The Mattson cookers (Michigan State University Machine Shop, East Lansing, MI, United States) use twenty-five 65 g stainless steel rods with 2 mm diameter pins to pierce beans as they finish cooking in each well. As the pins drop, a custom software reports the cooking time associated with each pin. A low boil was maintained during cooking, and the 80% cooking times were recorded and regarded as the time required to fully cook each sample. Final cooked seed weights were recorded.
Ervilha, PI527538, and the RILs were evaluated in duplicate using a modified Quantitative Descriptive Analysis (QDA) approach (Stone et al., 1974), in which four panelists per session independently evaluated samples using a non-consensus approach to limit group bias. For the purposes of this study, the QDA approach was modified as described by Bassett et al. (2020b) to increase suitability for implementation in public breeding programs with limited resources. In brief, seeds from each field replicate were prepared for sensory evaluation in the same order that they were cooked for cooking time evaluation to minimize seed aging effects. Sensory evaluation sessions were held daily with four panelists per session until each genotype had been evaluated twice for each year. Twelve genotypes were evaluated at each session including Ervilha and PI527538 as controls. Each sample was evaluated using 5-point attribute intensity scales (low → high intensity) for total, beany, vegetative, earthy, starchy, bitter, and sweet flavor intensities as well as seed-coat perception and cotyledon texture. The scale for seed-coat perception ranged from imperceptible (1) to tough and lingering (5). For cotyledon texture, the scale ranged from mushy (1) to very gritty/firm (5) (Bassett et al., 2020b). This sensory evaluation protocol was approved by the Institutional Review Board of Michigan State University (IRB# x16-763e Category: Exempt 6).
Panelists were recruited from the USDA (East Lansing, MI, United States) and Michigan State University dry bean breeding programs due to their familiarity with dry beans and their availability for long-term sensory evaluation projects. Initially, seven panelists were trained using a diverse set of dry bean genotypes selected from the USDA and MSU dry bean programs with the intention of exposing panelists to a wide range of attribute intensities. This initial set included dark red kidney, Jacob’s cattle, white kidney, and yellow beans. A training set of genotypes exhibiting extreme attribute intensities identified in the ADP (Bassett et al., 2020b) was used to train eleven panelists for the second year. This training set was grown at the MSU Montcalm Research Center in Lakeview, MI alongside the RIL population.
Panelists were trained over multiple sessions using a consensus approach. Panelists then practiced using a non-consensus approach to improve their familiarity with the selected scales and their sensory evaluation skills. Panelist performance was assessed via ANOVA with FGenotype (p-value < 0.05) indicating ability to discriminate and Frep (p-value > 0.05) indicating consistency (Meilgaard et al., 1999; Armelim et al., 2006). Sensory evaluation commenced after successful training of each panelist. Following screening of the parents and RILs from both years, panel performance was assessed as during training.
Samples were prepared as described in Bassett et al. (2020b). Prior to each session, four seeds per panelist of each genotype scheduled for evaluation were soaked for 12 h in distilled water prior to cooking. Large tea bags filled with the soaked samples were boiled in distilled water for the cooking time determined by the Mattson cooker method, timed so they all finished cooking together. The cooked samples were poured into preheated (105°C) ceramic ramekins, covered with aluminum foil, and placed in a chafing dish to maintain temperature prior to evaluation. Samples were given a random letter code to mask their identity. Panelists were asked to refrain from wearing strong scents or eating during the hour before each session. Samples were served out of the ceramic ramekins with a plastic spoon onto paper plates. Lemon water was made available as a palate cleanser, and panelists were asked to drink water between samples.
PROC MIXED in SAS version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC, United States) was used to conduct analyses of variance (ANOVAs) for all traits. For seed weight, seed yield, water uptake, and cooking time, the fixed effects were genotype, year, and genotype by year with replicate as a random effect. For L∗, a∗, and b∗ color values, the fixed effects were genotype and year with no random effects. For the sensory attribute intensity traits, the fixed effects were genotype, year, and genotype by year with replicate, panelist (year), and session (year) as random effects. Least squares estimates for sensory traits were calculated via the LSMeans statement in PROC MIXED for visualization of trait distributions. Mean separation of parents was determined using pdiff in PROC MIXED. Density plots of traits were generated in R (R Core Team, 2017) using the sm package version 2.2–5.6 (Bowman and Azzalini, 2018).
To analyze both years combined while minimizing environmental effects, best linear unbiased predictors (BLUPs) were generated for each trait using the lme4 package (Bates et al., 2015) in R version 4.0.3 with genotype, year, genotype by year, and rep nested in year as random effects. For sensory traits, panelist nested in year and session nested in year were also included as random effects. For analysis within individual years, BLUPs were calculated for sensory traits with genotype, rep, panelist, and session included as random effects. The normality of trait distributions was assessed visually using Q–Q plots.
Broad sense heritability (H2) was calculated on a family mean basis for each trait using the following equation:
where is genotypic variance, is genotype year interaction variance, is environmental variance, y is number of years, and r is number of replications. For seed-coat postharvest non-darkening, the component was excluded from the equation as the trait was only assessed in 1 year. Variance components were calculated using PROC VARCOMP in SAS version 9.4 with method = restricted maximum likelihood method (reml) (Holland et al., 2003). Principal component analysis (PCA) among traits was conducted via singular value decomposition of the centered and scaled BLUPs from both years combined using the prcomp function in R.
Genomic DNA was extracted from young trifoliate leaf tissue from three plants each for the 242 RILs and the two parental lines (Ervilha and PI527538) using a Macherey-Nagel NucleoSpin Plant II kit. For each genotype, 10 μL aliquots of DNA at a minimum concentration of 50 ng/μL were loaded into 96-well plates with parental lines prepared in quintuplicate. The plates were genotyped at the USDA Beltsville Agricultural Research Center in Beltsville, MD (BARC) using the BARCBean12K BeadChip, which includes all SNPs from the BARCBean6K_3 (Song et al., 2015) and additional SNPs among a set of Andean accessions. Illumina’s GenomeStudio software was used to confirm variant calling. SNP positions were reported according to Phaseolus vulgaris v2.1 genome (DOE-JGI and USDA-NIFA1) positions. Raw SNPs (11,292 markers) were filtered to eliminate markers that were heterozygous or monomorphic (9,085 markers), duplicates (1,301 markers), or exhibiting extreme segregation distortion (p-value < 1e–10; 31 markers).
Linkage mapping was performed using MapDisto version 2.1.7 (Heffelfinger et al., 2017). Markers causing excessive map length and/or exhibiting aberrant segregation distortion patterns were excluded (five markers). A fixed-order genetic map of 439.53 cM was generated using the Kosambi function with the remaining 870 markers. Markers were grouped by chromosome with marker order reflecting physical positions. The Ripple function was used to confirm marker order.
Quantitative trait loci mapping was performed using QTL Cartographer version 2.5 (Wang et al., 2012). The composite interval mapping (CIM) procedure was performed with the parameters set to 10 cM window size and 1 cM walkspeed with forward and backward regression. BLUPs were used for all traits in QTL mapping for both years combined and for sensory traits for individual years, and means were used for analyses of all other traits for individual years. The LOD thresholds for each trait in each year and across years were determined using 1,000 permutations in scanone from rQTL with the extended Haley–Knott method (p-value < 0.05) (Broman et al., 2003; Feenstra et al., 2006). QTL regions were defined including all significant markers for each QTL peak. LOD information by position is available for each trait in the Supplementary Material. The constructed linkage maps with QTL overlaid were visualized using Mapchart 2.32 (Voorrips, 2002). Each QTL was named according to the guidelines for common bean QTL nomenclature (Miklas and Porch, 2010).
Genotype and year significantly affected water uptake, and cooking time (p-value < 0.05) and genotype by year significantly affected cooking time (p-value < 0.05) (Table 1 and Supplementary Table 1). The parental lines, Ervilha and PI527538 had water uptakes of 109.3 and 98.8% and cooking times of 21.0 and 29.7 min, respectively averaged across both years.
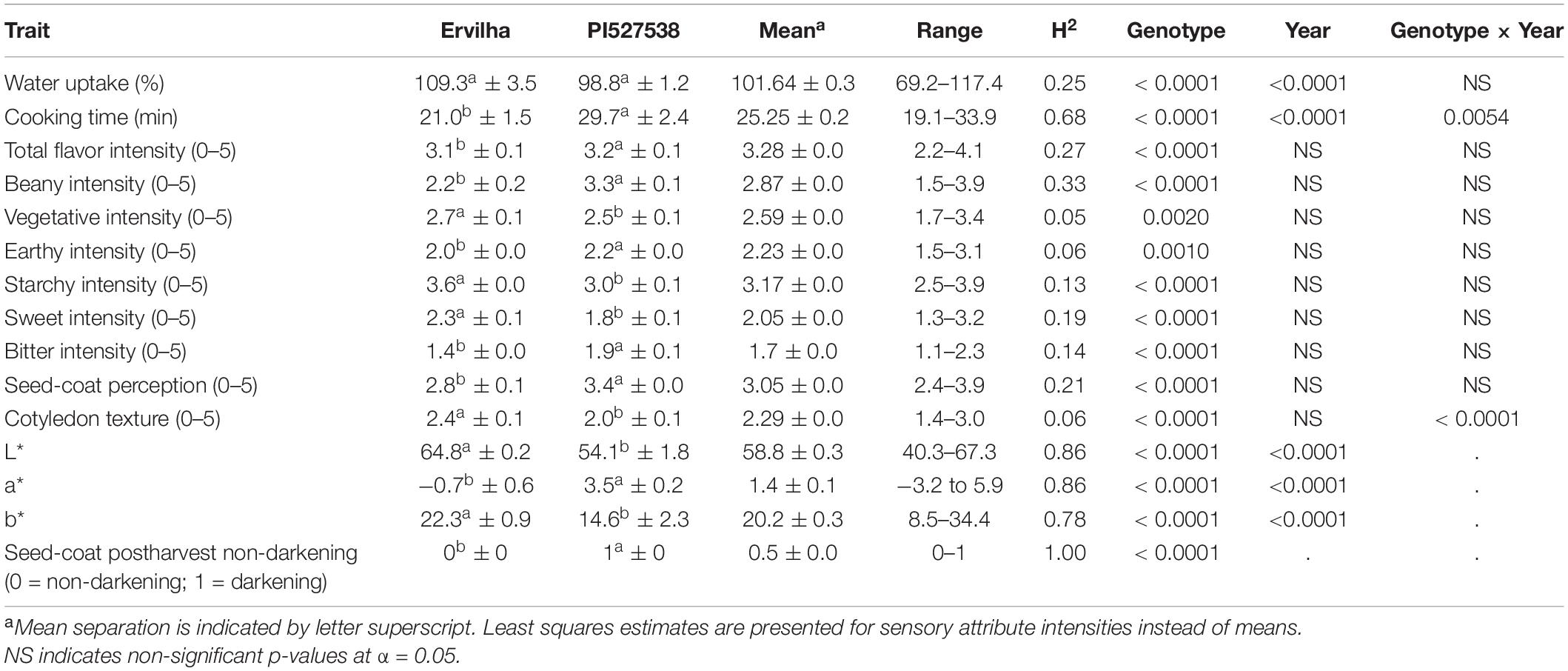
Table 1. Parental phenotypes, means with standard error, ranges, and broad-sense heritability (H2) estimates for the RILs for both years combined with ANOVA p-values for genotype, year, and genotype by year indicated.
Water uptake and cooking time for the RILs exhibited approximately normal distributions (Supplementary Table 2 and Figure 2). Averaged across both years, water uptake ranged 69.2–117.4%, and cooking time ranged 19.1–33.9 min (Table 1). Broad-sense heritability for cooking time was 0.68 and water uptake was 0.25 (Table 1).
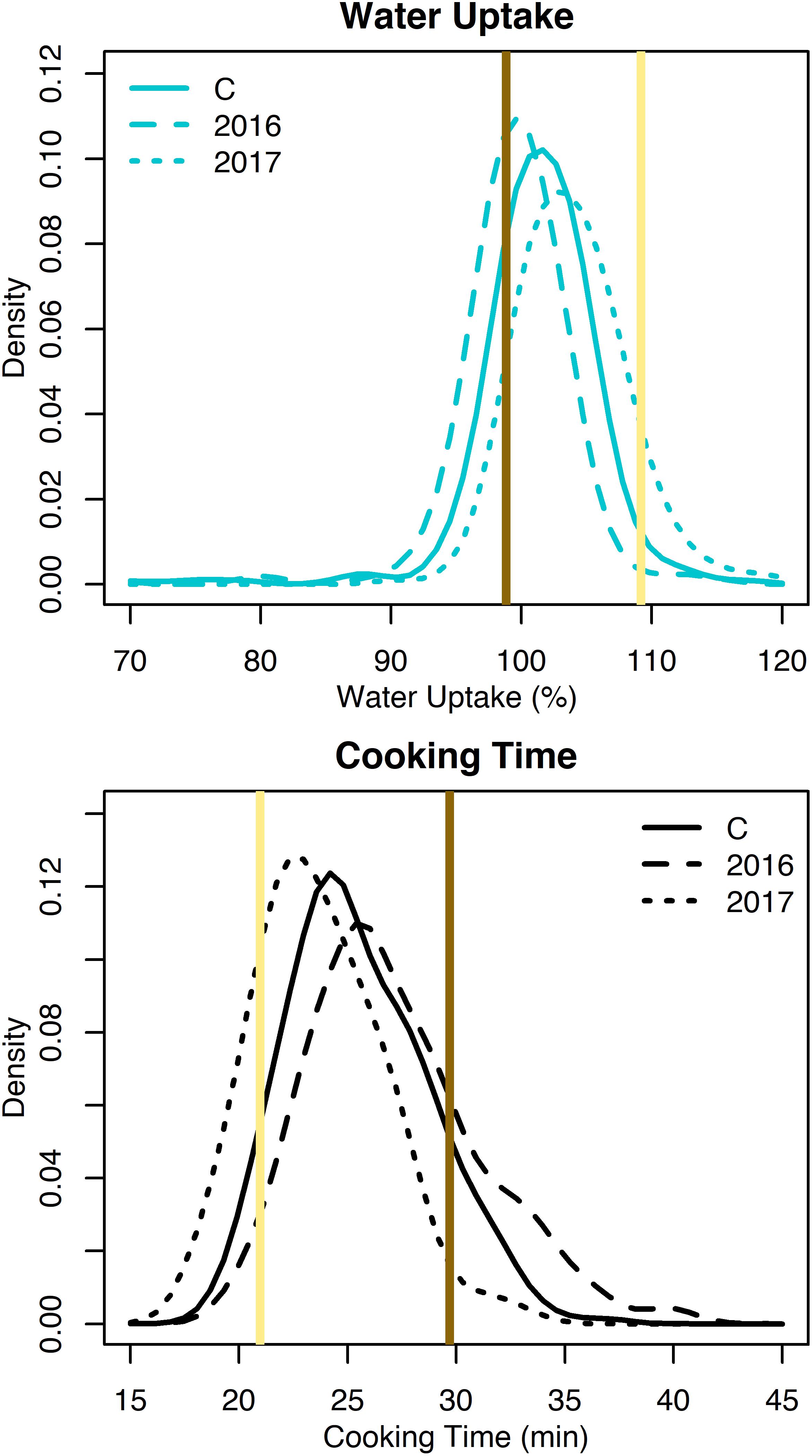
Figure 2. Density plots of water uptake and cooking time for the RILs from 2016, 2017, and both years combined (C). Means for Ervilha and PI527538 from both years combined are indicated in yellow and brown, respectively.
Genotype significantly affected all sensory attributes (p-value < 0.05) (Table 1). Year did not significantly affect any sensory attributes, and genotype by year only significantly affected cotyledon texture (p-value < 0.05). Rep effects were insignificant for all sensory attributes, which indicates panelists were consistent across reps, although significant panelist and session effects were observed (Supplementary Table 3). For the parents Ervilha and PI527538, respectively with least squares estimates averaged across both years, the total flavor intensities were 3.1 and 3.2; beany intensities were 2.2 and 3.3; vegetative intensities were 2.7 and 2.5; earthy intensities were 2.0 and 2.2; starchy intensities were 3.6 and 3.0; sweet intensities were 2.3 and 1.8; bitter intensities were 1.4 and 1.9; seed-coat perceptions were 2.8 and 3.4; and cotyledon textures were 2.4 and 2.0 (Table 1).
Least squares estimates for all sensory attribute intensities varied minimally across years and exhibited approximately normal distributions (Supplementary Table 2 and Figure 3). Across both years, least squares estimates ranged 2.2–4.1 for total flavor intensity, 1.5–3.9 for beany intensity, 1.7–3.4 for vegetative intensity, 1.5–3.1 for earthy intensity, 2.5–3.9 for starchy intensity, 1.3–3.2 for sweet intensity, 1.1–2.3 for bitter intensity, 2.4–3.9 for seed-coat perception, and 1.4–3.0 for cotyledon texture (Table 1). While panelists were able to differentiate among genotypes using 5-point scales, sensory attribute ranges did not exceed 2.4, suggesting panelists did not make full use of the scales. This could reflect the limited differences in sensory attribute intensities observed between the parents.
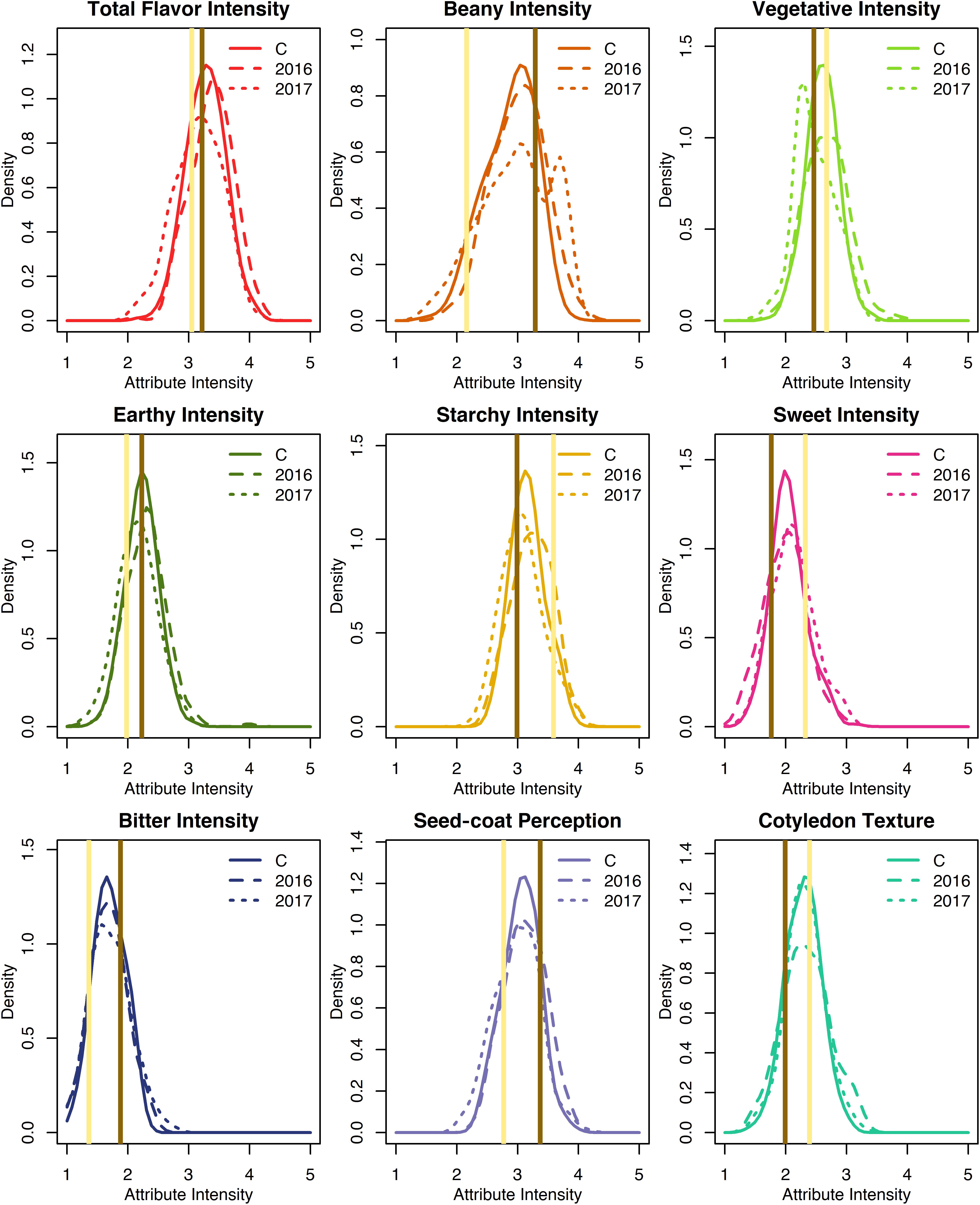
Figure 3. Density plots of least squares estimates of sensory attribute intensities for the RILs from 2016, 2017, and both years combined (C). Attribute intensities for Ervilha and PI527538 from both years combined are indicated in yellow and brown, respectively.
Broad-sense heritability for sensory attribute intensities were low, ranging from 0.05 to 0.33 (Table 1). Beany intensity and total flavor intensity exhibited the highest broad-sense heritability (0.33 and 0.27), while vegetative intensity, earthy intensity, and cotyledon texture exhibited the lowest (0.05, 0.06, and 0.06).
Genotype significantly affected L∗, a∗, b∗, and seed-coat postharvest non-darkening (p-value < 0.05) (Table 1). Year significantly affected L∗, a∗, and b∗ (p-value < 0.05). For the parents Ervilha and PI527538, respectively averaged across both years, L∗ values were 64.8 and 54.1; a∗ values were −0.7 and 3.5; b∗ values were 22.3 and 14.6; and seed-coat postharvest non-darkening values were 0 (non-darkening) and 1 (darkening).
The L∗, a∗, and b∗ for the RILs varied minimally across years and exhibited approximately normal distributions (Supplementary Table 2 and Figure 4). Averaged across both years, L∗ ranged from 40.3–67.3; a∗ ranged from −3.2 to 5.9; and b∗ ranged from 8.5 to 34.4 (Table 1). Seed-coat postharvest darkening was only determined for seeds from 1 year (2017), and progeny exhibiting both non-darkening and darkening were observed. Broad-sense heritability was high for L∗ (0.86), a∗ (0.86), b∗ (0.78), and seed-coat postharvest non-darkening (1.00).
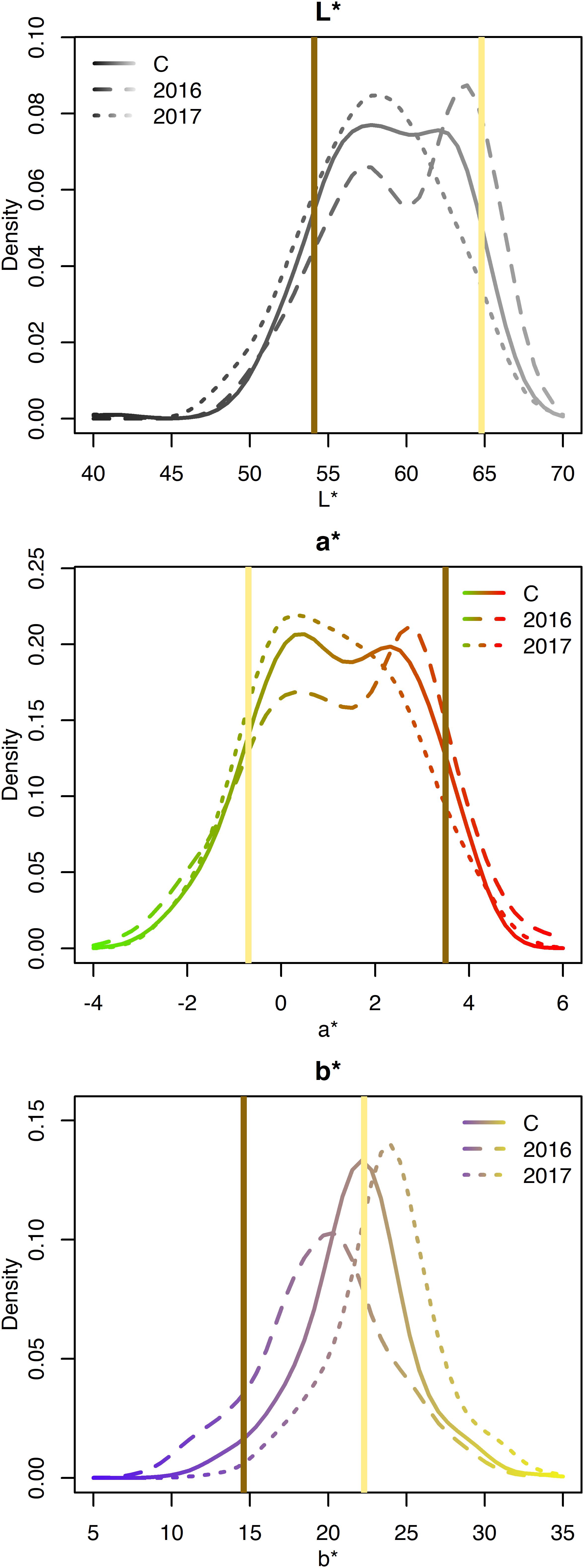
Figure 4. Density plots of CIELAB values for the RILs from 2016, 2017, and both years combined (C). Attribute intensities for Ervilha and PI527538 from both years combined are indicated in yellow and brown, respectively.
Genotype, year, and genotype by year significantly affected seed weight and seed yield (p-value < 0.05) (Supplementary Table 1). For the parents Ervilha and PI527538, respectively averaged across both years, the seed weights were 52.8 and 48.0 g per 100 seeds. Seed yield data for Ervilha is not available for 2016 (Supplementary Table 2), and fewer seeds were planted per plot in 2016, making averages across years misleading. In 2017, the seed yields for Ervilha and PI527538, respectively, were 1,731.4 and 2,384.4 kg/ha.
The seed weight for the RILs exhibited approximately normal distributions (Supplementary Table 2 and Supplementary Figure 1). Seed yield for the RILs varied substantially across years due to reduced seeds planted per plot in 2016 but exhibited approximately normal distributions. Averaged across both years, seed weight ranged 39.1–68.4 g per 100 seeds and seed yield ranged 751.0–3,283.9 kg per ha (Supplementary Table 2).
Broad-sense heritability for seed weight (0.89) was high and for seed yield was moderate (0.57) (Supplementary Table 1).
A PCA for the seed quality trait relationship was conducted and the first two principal components (PCs) explained approximately 52% of the variance (Figure 5). The first PC separates the genotypes approximately by beany, earthy, and bitter intensities as well as L∗, a∗, b∗, and seed-coat postharvest non-darkening and represents over a third of the variation (38.5%). The second PC separates the genotypes approximately by cooking time; total flavor, vegetative, starchy, and sweet intensities; and cotyledon texture and seed-coat perception. The second PC represents over an eighth of the variance (13.0%). The remaining PCs accounted for 11.1, 7.4, 6.0, 5.4, 4.2, 3.2, 2.9, 2.5, 2.2, 1.6, 1.1, and 0.9% of the variance, respectively (data not shown).
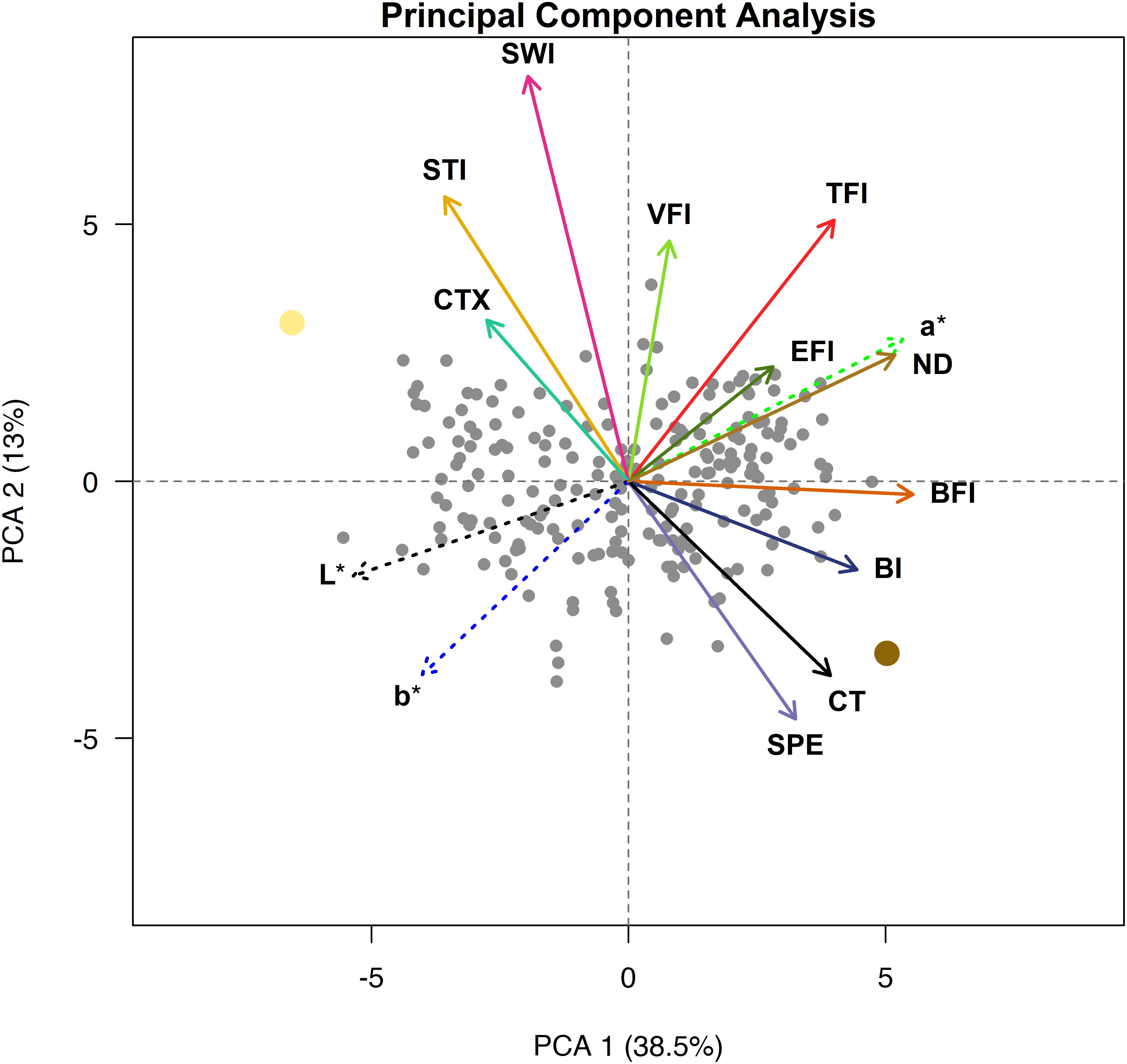
Figure 5. Principal component analysis biplot with loadings for cooking time (CT), total flavor intensity (TFI), beany intensity (BFI), vegetative intensity (VFI), earthy intensity (EFI), starchy intensity (STI), sweet intensity (SWI), bitter intensity (BI), seed-coat perception (SPE), cotyledon texture (CTX), L*, a*, and b*. Ervilha and PI527538 are indicated in yellow and brown, respectively.
The PCA biplot highlights distinct groupings of traits that tend to be observed together. Loadings that group together highlight strong positive relationships within each group, and groups of loadings opposite of each other highlight strong negative relationships between groups. Loadings for starchy intensity, sweet intensity, and cotyledon texture are positioned close to each other and opposite cooking time and seed-coat perception. Loadings for beany intensity and bitter intensity also group together and are somewhat opposite starchy intensity, sweet intensity, and cotyledon texture. The loadings for total flavor intensity earthy intensity, a∗, and seed-coat postharvest non-darkening group together, opposite of loadings for L∗ and b∗. The loading for vegetative intensity does not appear to group with or opposite of other loadings but lies in between loadings for total flavor intensity and sweet intensity. The genotypes are spread across the biplot, with Ervilha and PI527538 positioned opposite each other.
A linkage map was developed with 870 SNPs spread across eleven chromosomes for a total map length of 439.53 cM with a marker density of one SNP per 0.51 cM (Table 2). Significant QTL were identified using BLUPs from both years combined for water uptake, cooking time, total flavor intensity, beany intensity, vegetative intensity, earthy intensity, starchy intensity, sweet intensity, bitter intensity, seed-coat perception, cotyledon texture, L∗, a∗, b∗, seed-coat postharvest non-darkening, seed weight, and seed yield (Tables 3–5, Figures 6–8, and Supplementary Figure 2). Additional QTL were also identified in individual years for these traits (Supplementary Tables 4–7).
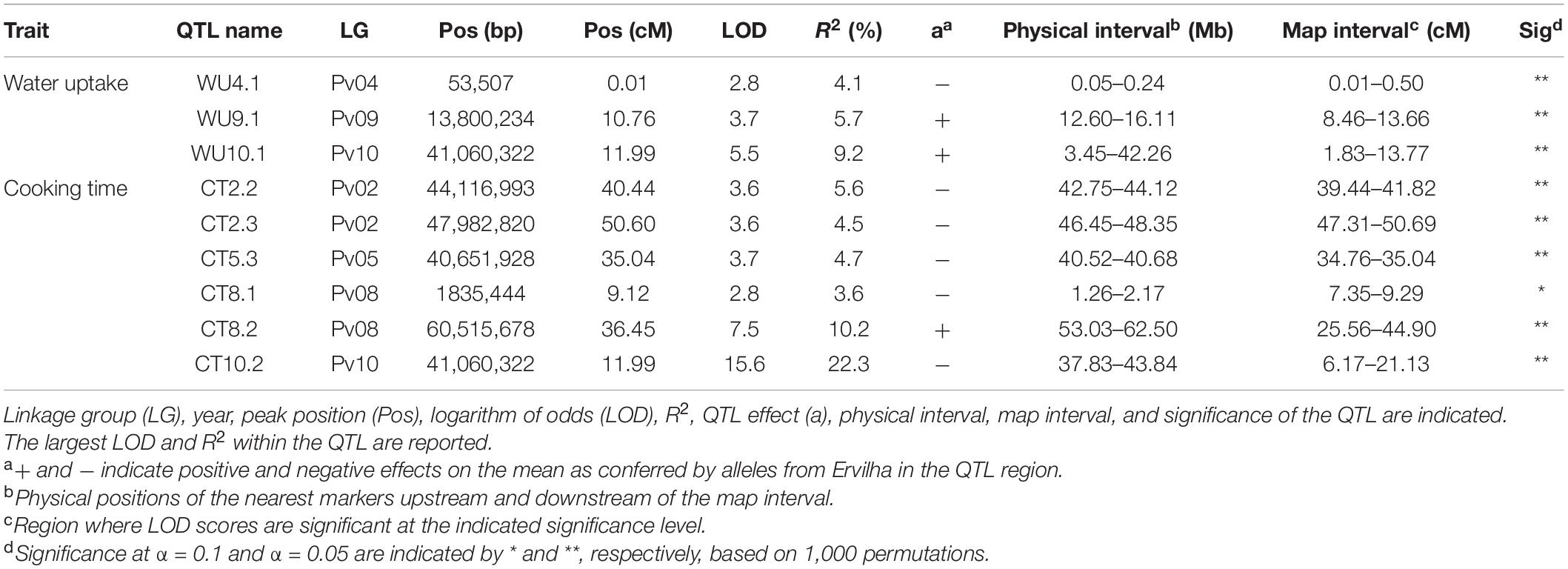
Table 3. Quantitative trait loci identified in the RIL population (N = 242) using BLUPs from samples grown in Entrican, MI in 2016 and 2017 for water uptake and cooking time.
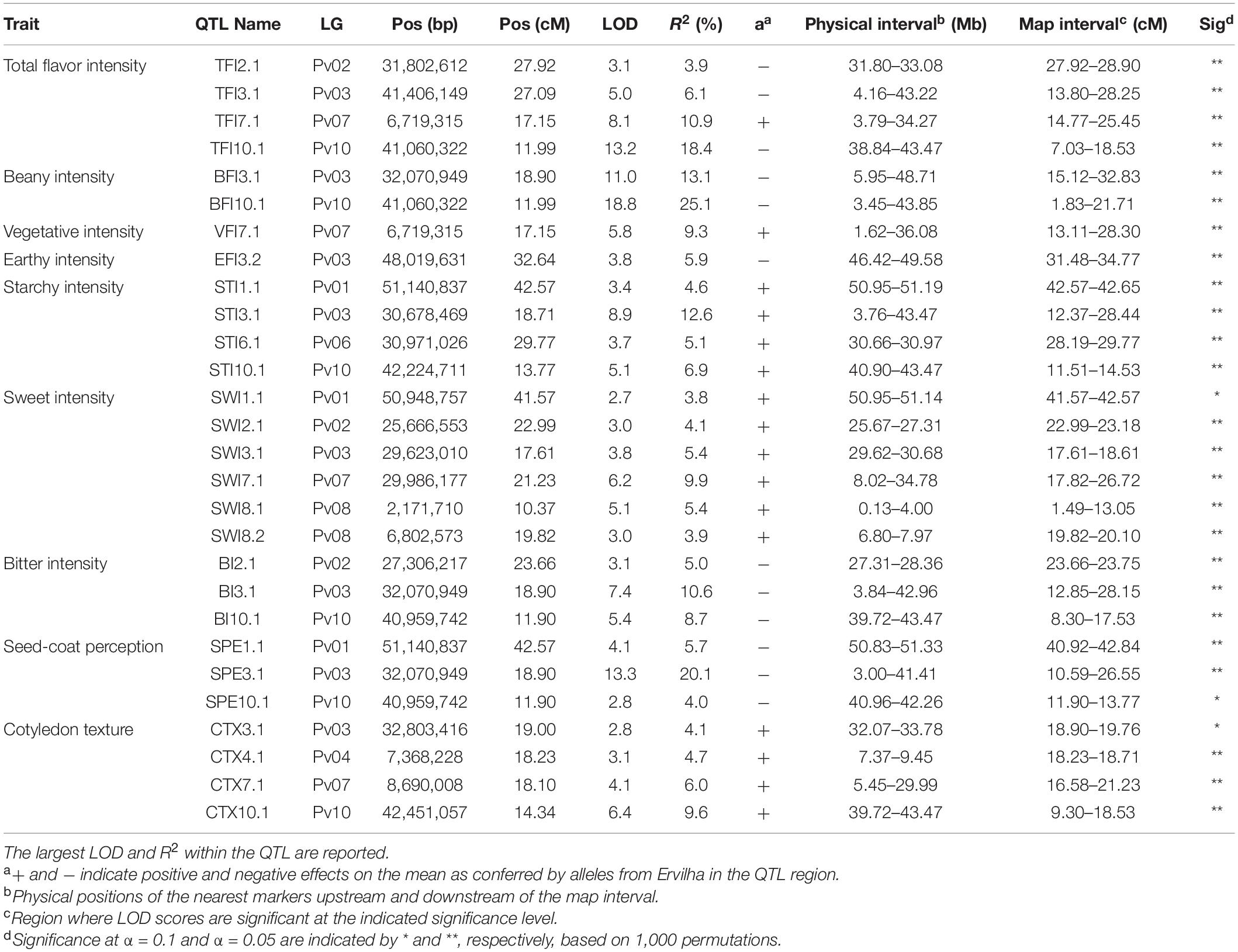
Table 4. Quantitative trait loci identified in the RIL population (N = 242) using BLUPs from samples grown in Entrican, MI in 2016 and 2017 for sensory attributes. Linkage group (LG), year, peak position (Pos), logarithm of odds (LOD), R2, QTL effect (a), physical interval, map interval, and significance of the QTL are indicated.
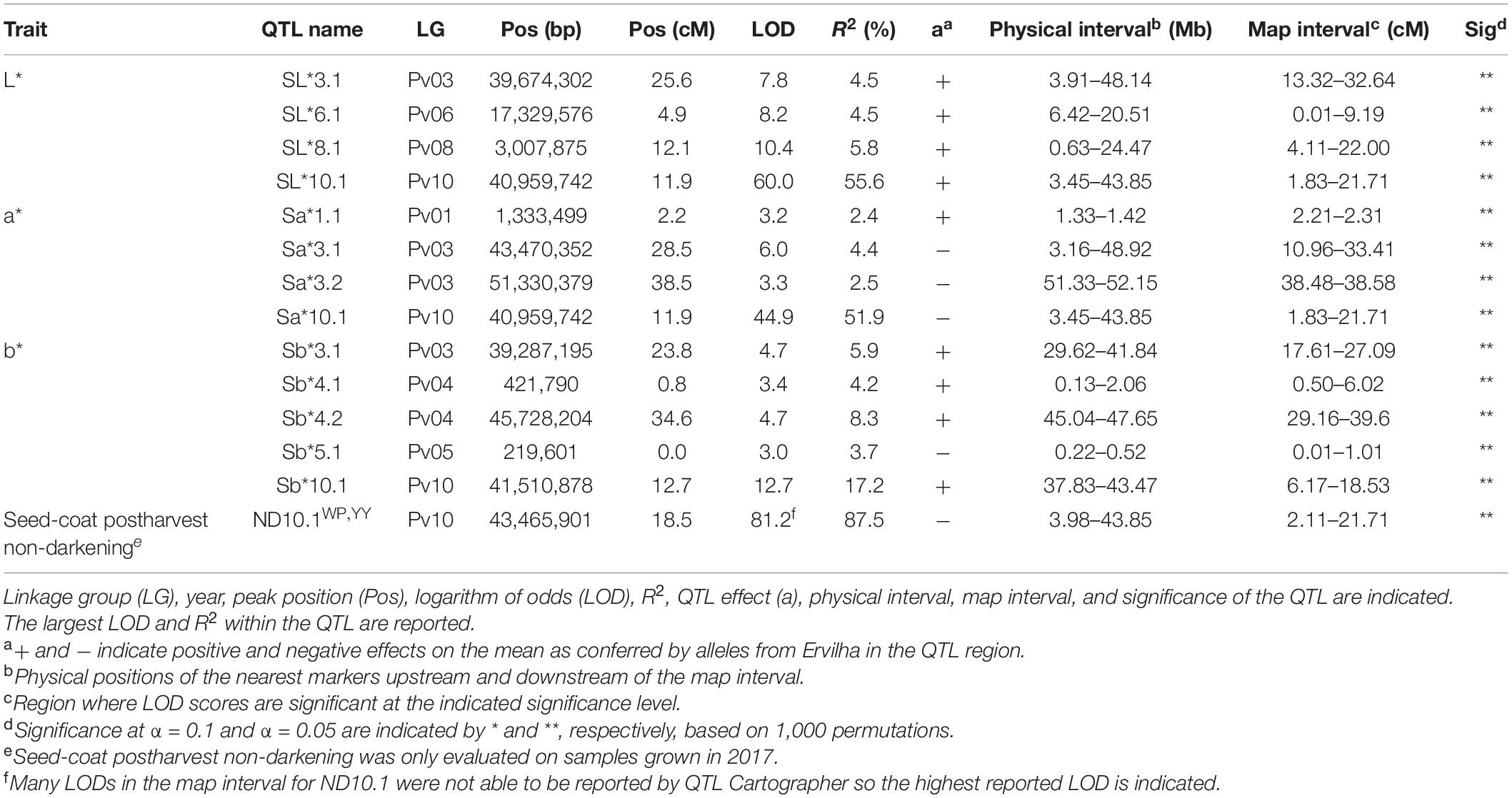
Table 5. Quantitative trait loci identified in the RIL population (N = 242) using BLUPs from samples grown in Entrican, MI in 2017 for color and seed-coat postharvest non-darkening.
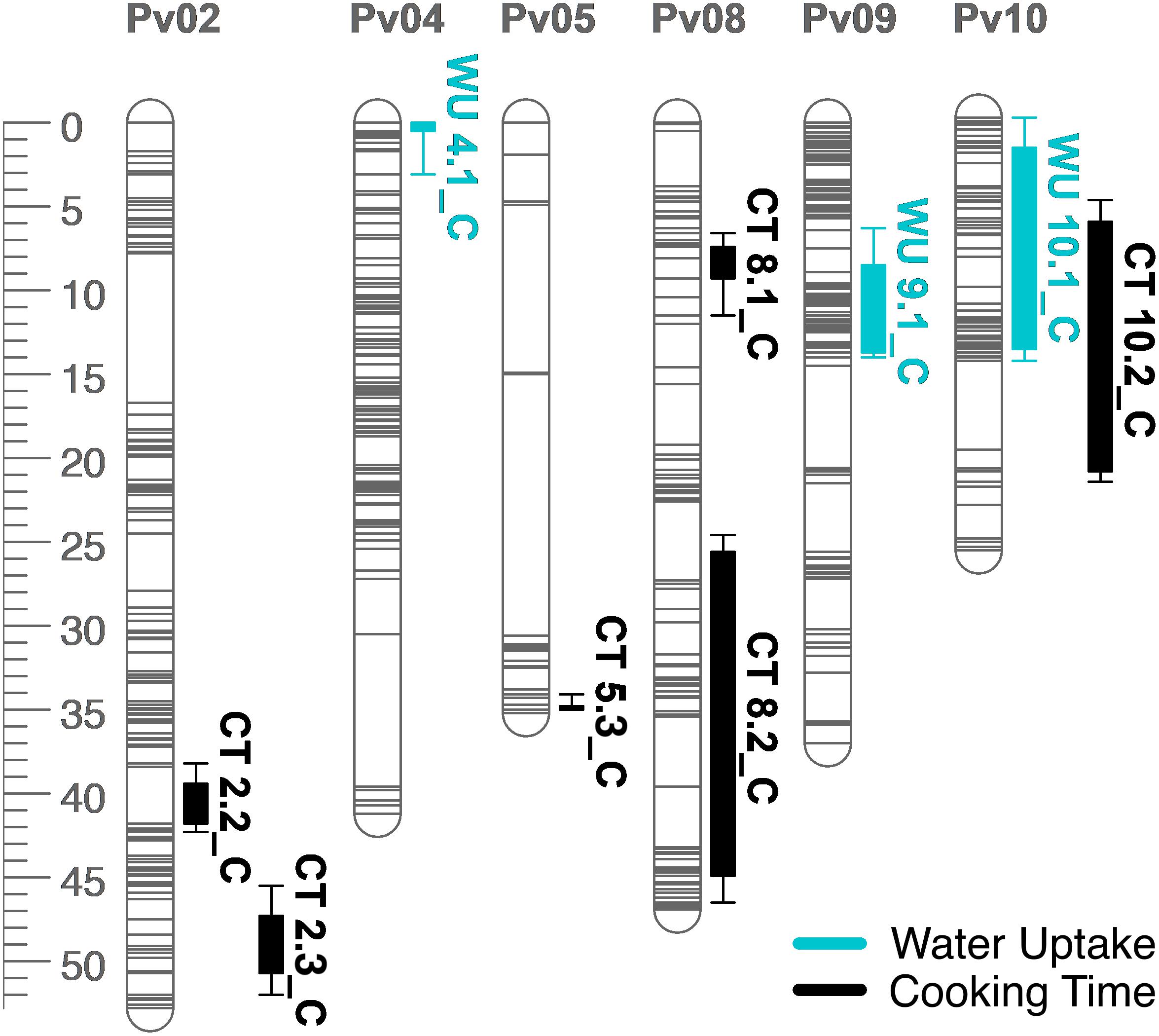
Figure 6. QTL map for water uptake (WU) and cooking time (CT) in the RIL population. Size is in cM. All QTL were detected in both years combined.
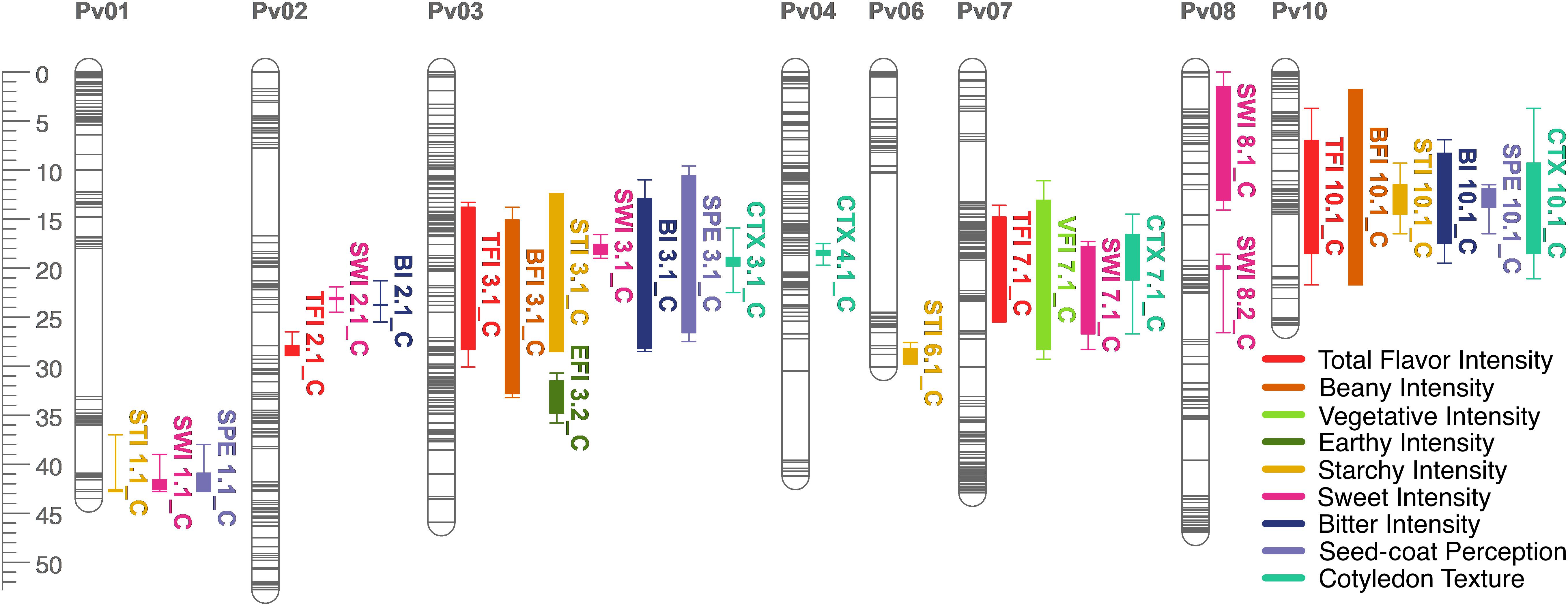
Figure 7. QTL map for total flavor intensity (TFI), beany intensity (BFI), vegetative intensity (VFI), earthy intensity (EFI), starchy intensity (STI), sweet intensity (SWI), bitter intensity (BI), seed-coat perception (SPE), and cotyledon texture (CTX) in the RIL population. Size is in cM. All QTL were detected in both years combined.
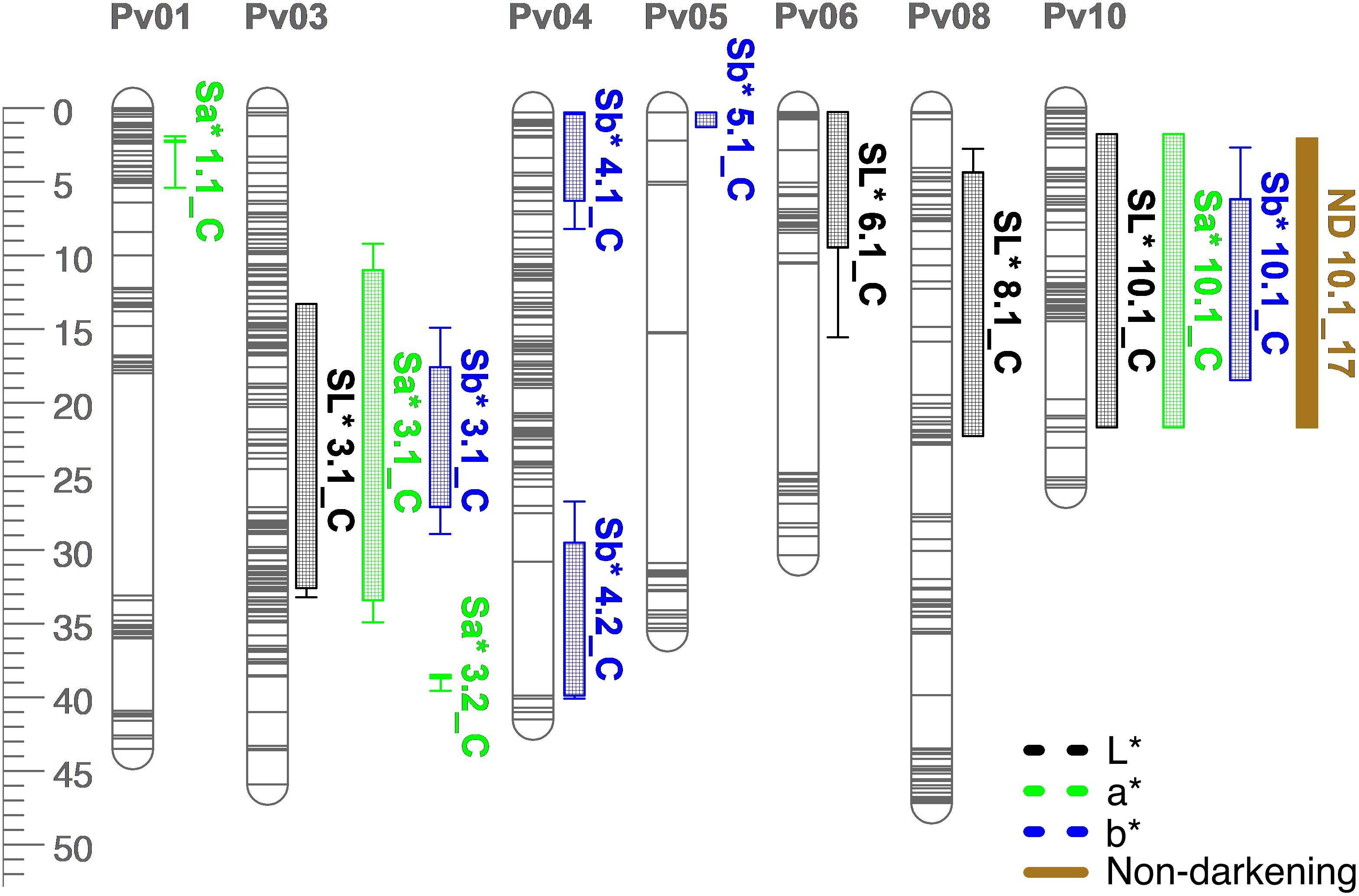
Figure 8. QTL map for L*, a*, b*, and seed-coat postharvest non-darkening (ND) in the RIL population. Size is in cM. All QTL were detected in both years combined.
Several QTL were identified for water uptake and cooking time in both years combined. For water uptake, three QTL were identified: WU4.1, WU9.1, and WU10.1 (Table 3 and Figure 6). The total proportion of variance explained by the three QTL was 19.0%. For cooking time, six QTL were identified: CT2.2, CT2.3, CT5.3, CT8.1, CT8.2, and CT10.2 (Table 3 and Figure 6). The total proportion of variance explained by the six QTL was 50.9%. CT8.2 and CT10.2 were the most significant cooking time QTL identified. RILs with both fast-cooking alleles for these QTL cooked 5 min faster on average than RILs without fast-cooking alleles (Figure 9).
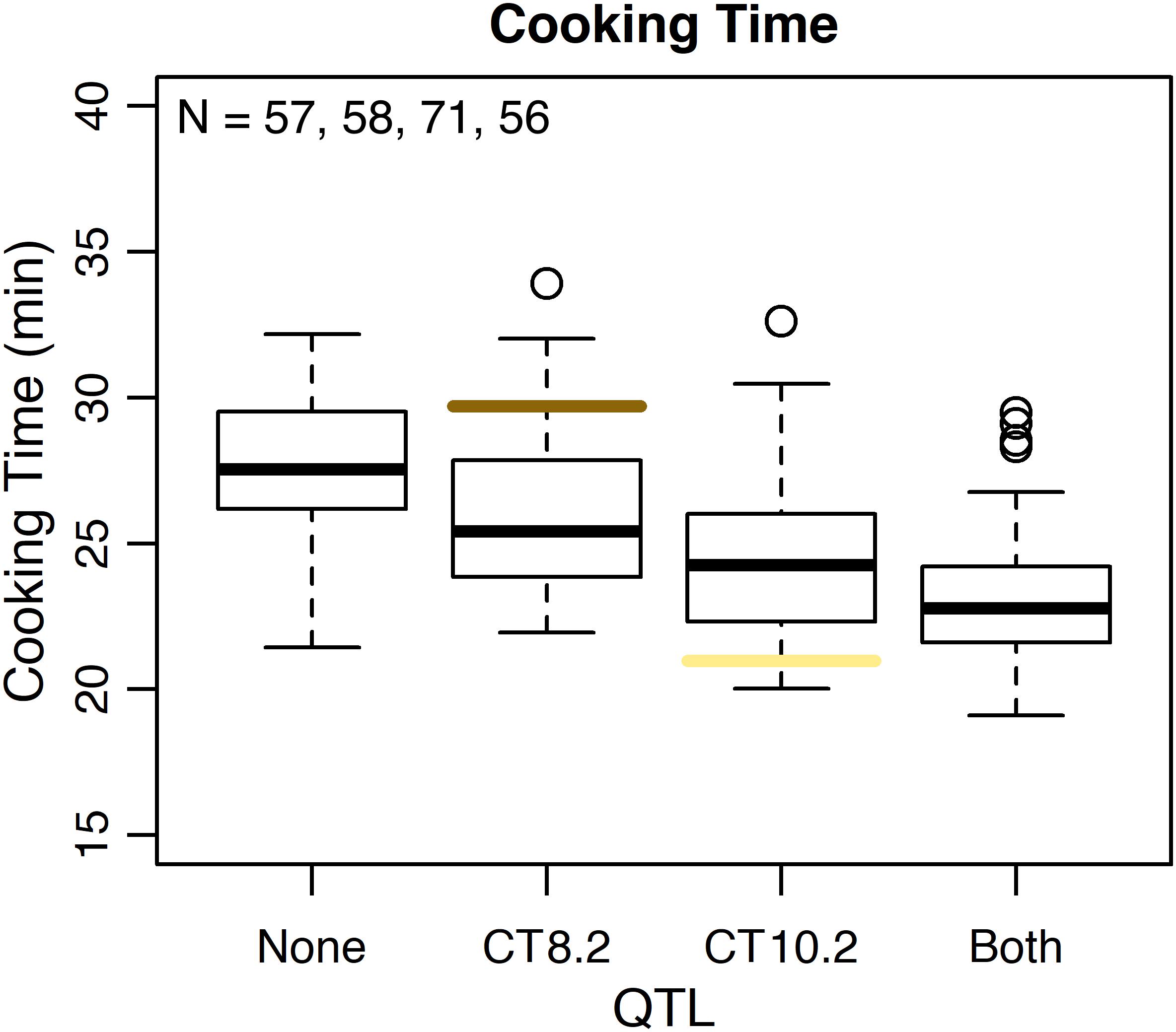
Figure 9. Phenotypic effect of CT8.2 and CT10.2 in the RIL population based on the peak SNP alleles and average cooking time across both years. Cooking times for RILs with no fast-cooking alleles (none), the fast-cooking CT8.2 allele (CT8.2), the fast-cooking CT10.2 allele (CT10.2), and both fast-cooking alleles (both) are displayed in boxplots. Means for Ervilha and PI527538 from both years combined are indicated in yellow and brown, respectively.
Many QTL were identified across all sensory characteristics in both years combined. For total flavor intensity, four QTL were identified: TFI2.1, TFI3.1, TFI7.1, and TFI10.1 (Table 4 and Figure 6). The proportion of variance explained by the four QTL was 39.3%. For beany intensity, two QTL were identified: BFI3.1 and BFI10.1 (Table 4 and Figure 7). The proportion of the variance explained by the two QTL was 38.2%. For vegetative intensity, one QTL was identified: VFI7.1 (Table 4 and Figure 7). The proportion of variance explained by VFI7.1 was 9.3%. For earthy intensity, one QTL was identified: EFI3.2 (Table 4 and Figure 7). The proportion of variance explained by EFI3.2 was 5.9%. For starchy intensity, four QTL were identified: STI1.1, STI3.1, STI6.1, and STI10.1 (Table 4 and Figure 7). The proportion of variance explained by the four QTL was 29.2%. For sweet intensity, six QTL were identified: SWI1.1, SWI2.1, SWI3.1, SWI7.1, SWI8.1, and SWI8.2 (Table 4 and Figure 7). The proportion of variance explained by the six QTL was 32.5%. For bitter intensity, three QTL were identified: BI2.1, BI3.1, and BI10.1 (Table 4 and Figure 7). The proportion of variance explained by the three QTL was 24.3%. For seed-coat perception, three QTL were identified: SPE1.1, SPE3.1, and SPE10.1 (Table 4 and Figure 7). The proportion of variance explained by the three QTL was 29.8%. For cotyledon texture, four QTL were identified: CTX3.1, CTX4.1, CTX7.1, and CTX10.1 (Table 4 and Figure 7). The proportion of variance explained by the four QTL was 24.4%.
Many QTL were identified for color traits across both years combined. For L∗, four QTL were identified: SL∗3.1, SL∗6.1, SL∗8.1, and SL∗10.1 (Table 5 and Figure 8). The total proportion of variance explained by four QTL was 70.4%. For a∗, four QTL were identified: Sa∗1.1, Sa∗3.1, Sa∗3.2, and Sa∗10.1 (Table 5 and Figure 8). The total proportion of variance explained by the four QTL was 61.2%. For b∗, five QTL were identified: Sb∗3.1, Sb∗4.1, Sb∗4.2, Sb∗5.1, and Sb∗10.1 (Table 5 and Figure 8). The total proportion of variance explained by the five QTL was 39.3%. For seed-coat postharvest non-darkening, one QTL was identified: ND.10.1 (Table 5 and Figure 8). Seed-coat postharvest non-darkening was only evaluated for 2017 seeds. The proportion of variance explained by ND10.1 was 87.5%, and Ervilha contributed an allele conferring a negative effect, reflecting its lack of darkening over time (Tables 1, 5).
While seed weight and seed yield were not central to this study, several QTL were identified for these traits as well. Additional information is available in the supplementary material (Supplementary Figure 2 and Supplementary Table 7).
Several QTL co-localized on Pv01, Pv03, Pv04, Pv07, Pv08, and Pv10. On Pv01, QTL for starchy intensity (STI1.1), sweet intensity (SWI1.1), and seed-coat perception (SPE1.1) co-localized. Alleles from Ervilha conferred positive effects for STI1.1 and SWI1.1 and a negative effect for SPE1.1. On Pv03, QTL for total flavor intensity (TFI3.1), beany intensity (BFI3.1), earthy intensity (EFI3.1 and EFI3.2), starchy intensity (STI3.1), sweet intensity (SWI3.1), bitter intensity (BI3.1), seed-coat perception (SPE3.1), cotyledon texture (CTX3.1), L∗ (SL∗3.1), a∗ (Sa∗3.1), and b∗ (Sb∗3.1) co-localized. Alleles from Ervilha conferred positive effects for STI3.1, SWI3.1, CTX3.1, SL∗3.1, and Sb∗3.1 and negative effects for TFI3.1, BFI3.1, EFI3.1, EFI3.2, BI3.1, SPE3.1, and Sa∗3.1. On Pv04, QTL for water uptake (WU4.1 and WU4.2) and b∗ (Sb∗4.1 and Sb∗4.2) co-localized. Alleles from Ervilha conferred positive effects for WU4.2, Sb∗4.1, and Sb∗4.2 and a negative effect for WU4.1. On Pv07, QTL for total flavor intensity (TFI7.1), vegetative intensity (VFI7.1), sweet intensity (SWI7.1), and cotyledon texture (CTX7.1 and CTX7.2) co-localized. Alleles from Ervilha conferred positive effects for TFI7.1, VFI7.1, SWI7.1, CTX7.1, and CTX7.2. On Pv08, QTL for cooking time (CT8.1), starchy intensity (STI8.1), sweet intensity (SWI8.1 and SWI8.2), and L∗ (SL∗8.1) co-localized. Alleles from Ervilha conferred positive effects for STI8.1, SWI8.1, SWI8.2, and SL∗8.1 and a negative effect for CT8.1. On Pv10, QTL for water uptake (WU10.1), cooking time (CT10.2), total flavor intensity (TFI10.1), beany intensity (BFI10.1), starchy intensity (STI10.1), bitter intensity (BI10.1), seed-coat perception (SPE10.1), cotyledon texture (CTX10.1), L∗ (SL∗10.1), a∗ (Sa∗10.1), b∗ (Sb∗10.1), and seed-coat postharvest non-darkening (ND10.1) co-localized. Alleles from Ervilha conferred positive effects for WU10.1, STI10.1, CTX10.1, SL∗10.1, and Sb∗10.1 and negative effects for CT10.2, TFI10.1, BFI10.1, BI10.1, SPE10.1, Sa∗10.1, and ND10.1.
The broad-sense heritability for cooking time was moderately high in this study, as was the case for previous reports looking at both broad-sense and narrow-sense heritability (Elia et al., 1997; Jacinto-Hernandez et al., 2003; Cichy et al., 2019; Bassett et al., 2020b). This supports the idea that marker-assisted selection for fast cooking time may be feasible with few molecular markers. Using marker-assisted selection as opposed to phenotyping could save breeding programs time and prevent the need to purchase specialized machinery specific for the evaluation of cooking time. It could also allow for early generation screening that would otherwise not be feasible due to limited seed and the large number of lines to be evaluated for cooking time.
Differences in sensory attribute intensities among genotypes were successfully detected, allowing the relationship among attributes in this population to be determined and for significant QTL to be identified for the evaluated sensory attributes. While significant panelist and session effects were identified (Supplementary Table 2), QDA does not rely on consensus among panelists, and these effects can be accounted for by using least squares estimates and BLUPs where appropriate. Although broad-sense heritability for sensory attributes tended to be low to very low, it is clear that genotype is important for flavor and texture. In the context of a breeding program, heritability can be improved by screening fewer lines with greater replication to better account for panelist and session effects while managing limited seed and personnel resources. As has been previously noted, panelists tend not to use the full range of the rating scales, which prevents detection of small differences between samples (Bassett et al., 2020b). In the case of this population, it is unlikely that this RIL population exhibited a full range of sensory attribute intensities, especially for traits with limited differences in the parents, so incomplete use of the scales likely reflects a lack of extreme differences among genotypes. However, increasing the size of the scales or using line scales that allow for continuous ratings may better reflect the diversity of attribute intensities exhibited in a population in future studies, which might return higher heritability for sensory traits. Year and genotype by year effects were not significant for sensory traits, apart from cotyledon texture, which had a significant genotype by year effect. This is encouraging because location of production and crop management practices have previously been identified as factors affecting sensory quality (Mkanda et al., 2007; Ferreira et al., 2012). This indicates that flavor and texture traits do not change across years in the same production environment, which is useful for meeting expectations of consistency for consumers and for product developers, who need consistent ingredients over time for their products to be successful.
There did not appear to be distinct groupings of genotypes based on cooking time and attribute intensity in the PCA biplot, indicating that there was a general mixing of these traits in the progeny (Figure 4). This suggests that extensive efforts at breaking linkages among traits are not needed to combine desired traits and achieve a target cooking time and sensory profile. Developing new yellow bean varieties with both fast cooking time and desirable flavor and texture would address two major factors influencing consumer purchasing decisions regarding dry beans and provide novelty for the many consumers unfamiliar with the yellow seed type (Leterme and Carmenza Muñoz, 2002; Eihusen and Albrecht, 2007; Winham et al., 2019).
Many QTL were identified in this study, with those for cooking time and sensory attribute intensities of particular interest. Two cooking time QTL (CT2.3 and CT8.2) cover physical ranges including ss715646000 (Pv02 48,676,223 bp), ss715646002 (Pv02 48,704,298 bp), S08_60104796 (Pv08 60,104,796 bp), and S08_62659170 (Pv08 62,659,170 bp), which are significant SNPs previously identified via genome-wide association in the ADP (Cichy et al., 2015b; Bassett et al., 2020b). Another recent study identified cooking time QTL on Pv02, Pv05, and Pv10, but the physical positions are not proximal to cooking time QTL identified in this study (Berry et al., 2020). All cooking time QTL identified in this study were detected in both years combined and have potential for use in marker-assisted selection. Because the LOD and R2 values for CT8.2 and CT10.2 are particularly high, these two cooking time QTL are the most compelling for marker development. The genetic control of sensory attributes is a new area of research in dry beans with limited study (Bassett et al., 2020b). For total flavor intensity, TFI2.1, TFI3.1, and TFI10.1 cover physical ranges including or in close proximity to S02_34288083 (Pv02 34,288,083 bp), S03_36213088 (Pv03 36,213,088 bp), S10_42515259 (Pv10 42,515,259 bp), and S10_42798266 (Pv10 42,798,266 bp), which were identified in association with total flavor intensity for the ADP (Bassett et al., 2020b). In addition, BFI10.1 and CTX3.1 cover physical ranges including or in close proximity to S10_42475118 (Pv10 42,475,118 bp) and S03_31659572 (Pv03 31,659,572 bp), which were also identified in the ADP in association with beany intensity and cotyledon texture, respectively (Bassett et al., 2020b). Otherwise, the QTL identified for sensory attributes in this study were novel. While most QTL identified for flavor and texture were consistent across years and many exhibited high LOD and R2 values, further validation would be beneficial before use in marker-assisted selection.
Three QTL were identified for water uptake and 13 QTL for CIELAB values. Some water uptake and CIELAB QTL were proximal to QTL and genetic markers identified in previous studies (Cichy et al., 2014; Mendoza et al., 2017; Erfatpour et al., 2018; Bassett et al., 2020b). WU4.2 and WU10.1 are near SNPs for water uptake identified in the ADP (Bassett et al., 2020b). SL∗10.1, Sa∗10.1, and Sb∗10.1 overlapped with the J-locus associated with postharvest non-darkening (Erfatpour et al., 2018). SL∗8.1, SL∗10.1, and Sa∗10.1 overlapped with QTL for L∗ and a∗ of canned black beans identified by Bornowski et al. (2020). Two seed coat lightness QTL fall within the ranges of known P. vulgaris color genes. The V color gene responsible to violet (blue to black) color falls within the SL∗6.1 interval and the Gy color gene (greenish yellow coat color) falls within the SL∗8.1 interval (Myers et al., 2019).
Seed-coat postharvest darkening was detected in PI527538 and half of the RILs. Seed-coat postharvest darkening describes the tendency of some genotypes to darken in color over time due to the presence of proanthocyanidin precursors in the seed coat (Beninger et al., 2005; Chen et al., 2015). This phenomenon has been most studied in pinto and cranberry beans but can be observed in other market classes. Lighter seed coats are perceived by consumers as indications of freshness or quality, so seeds exhibiting postharvest darkening have reduced market value (Nasar-Abbas et al., 2009; Erfatpour and Pauls, 2020). The J locus was previously identified on Pv10, and genotypes that are homozygous recessive at J do not exhibit postharvest darkening (Bassett, 2007; Elsadr et al., 2011; Erfatpour et al., 2018). The QTL identified for the non-darkening trait in this study overlaps with a previously identified QTL for non-darkening located between 40.16 and 40.30 Mb on Pv10 (Supplementary Table 5; Erfatpour et al., 2018). Flavan-3-ols, which include proanthocyandidins, have been previously associated with bitterness and astringency depending on their degree of polymerization (Robichaud and Noble, 1990; Peleg et al., 1999), so seed-coat postharvest darkening may alter flavor over time. The relationship between seed-coat postharvest darkening and flavor after beans have darkened was not examined in this study, but it remains practical to select against darkening when developing new varieties to ensure greater visual appeal to consumers, which would bypass flavor changes caused by darkening altogether. A SNP-based marker has been developed to allow marker-assisted selection for this trait (Erfatpour and Pauls, 2020).
As there is still much to be understood regarding flavor and texture in dry beans, other methods for assessing these sensory traits like GC-MS and texture measurements should be explored. Volatile concentrations and texture measurements have been used successfully as proxies for flavor and texture in studies looking at genetic control of sensory traits in other crops, and these measurements can be cheaper and easier to obtain than those generated by a descriptive panel (Zhang et al., 2015; Amyotte et al., 2017; Bauchet et al., 2017; Zhao et al., 2019). Apart from beany intensity (Vara-Ubol et al., 2004; Bott and Chambers, 2006), however, the contribution of volatiles to perceived flavors in dry beans is not well understood, and texture measurements have not been well explored outside of their use in the evaluation of firmness in canned samples (Kelly and Cichy, 2012). In addition, research assessing consumer preference for flavor and texture in dry beans is needed to define breeding targets for sensory attributes. Understanding which traits are most important for consumer preference and what the expectations are for different seed types will help breeders address flavor and texture with a focused, efficient approach.
Dry beans in the United States are sold as market classes rather than variety preserved. Variation exists within market classes for consumer-valued traits like cooking time, flavor, and texture so consumers are not able to make informed purchasing decisions taking these traits into account (Cichy et al., 2015b; Bassett et al., 2020b; Berry et al., 2020). In addition, the canning industry cannot receive the benefits of reduced energy costs and higher efficiency associated with fast-cooking genotypes if slow-cooking genotypes are present in the same cans (Bassett et al., 2020a). Because yellow beans are largely unfamiliar to United States consumers, there is an opportunity to develop new yellow bean varieties that prioritize these traits so that the yellow color can serve as a marker for convenience and culinary quality to consumers and the canning industry can produce quality canned products with yellow beans while benefitting from shorter processing times. Consumers are already seeking out unique flavors, textures, seed patterns, and colors from heirloom beans (Bullard, 2016), but heirlooms are not suited to modern farming practices, which makes them more expensive and less widely available than more familiar market classes. Yellow beans, the Manteca market class in particular, could serve this consumer interest while addressing grower needs.
This work adds to the currently limited pool of resources available for dry bean breeders to target fast cooking time, flavor, and texture in their breeding programs. The QTL identified in this work, in particular CT8.2 and CT10.2, can be used to develop molecular markers for the incorporation of fast cooking time into new bean varieties to benefit both consumers and the canning industry. For sensory attributes, many QTL for attribute intensities including total flavor, beany, earthy, starchy, sweet, bitter, seed-coat perception, and cotyledon texture were consistent across years and show potential for use in marker-assisted selection following identification of breeding targets informed by consumer preference. Consumers are seeking pulse products with improved culinary characteristics and unique appearance. Yellow dry beans like those used in this study are unfamiliar to United States consumers, but they tend to be fast cooking with desirable sensory attributes. With the recent increased interest in plant-based proteins, now is an opportune time to address consumer preference in dry beans to remain competitive with other pulses, and yellow beans might be an ideal vehicle to a fast-cooking, flavorful, and flourishing future of dry beans.
The phenotypic data, genotypic data, linkage map, and QTL results relevant for this study are provided as Supplementary Materials.
The studies involving human participants were reviewed and approved by the Institutional Review Board at Michigan State University (IRB# x16-763e Category: Exempt 6). The patients/participants provided their written informed consent to participate in this study.
Informed consent was obtained from all panelists.
AB contributed to methodology design, collected, analyzed, and visualized the data, and wrote the manuscript. DK developed the RIL population, collected leaf tissue for DNA extraction, and edited the manuscript. QS genotyped the RIL population and edited the manuscript. KC conceptualized the study, acquired the funding, designed field trials, and contributed to writing and editing the manuscript. All authors contributed to the article and approved the submitted version.
This material was based upon work that was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2017-67013-26212. This research was also supported by funding from the U.S. Department of Agriculture, Agriculture Research Services Projects 5050-21430-01000D (KC). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank members of the USDA-ARS and MSU dry bean breeding programs for their contributions as panelists. Additional thanks go to Hannah Cooperider, Hannah Peplinski, Anna Akariza, Gasana Elyvine, and Queen Iribagiza for their assistance with cooking samples and managing seeds and to Angel Torres for assistance with DNA extraction and field work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.670284/full#supplementary-material
Akibode, S., and Maredia, M. (2011). Global and regional trends in production, trade and consumption of food legume crops. Dep. Agric. Food Resour. Econ. Michigan. State Univ. 87:89.
Amyotte, B., Bowen, A. J., Banks, T., Rajcan, I., and Somers, D. J. (2017). Mapping the sensory perception of apple using descriptive sensory evaluation in a genome wide association study. PLoS One 12:e0171710. doi: 10.1371/journal.pone.0171710
Armelim, J. M., Canniatti-Brazaca, S. G., Spoto, M. H. F., Arthur, V., and Piedade, S. M. S. (2006). Quantitative descriptive analysis of common bean (Phaseolus vulgaris L.) under gamma radiation. J. Food Sci. 71, 8–12. doi: 10.1111/j.1365-2621.2006.tb12410.x
Bassett, A., and Cichy, K. (2020). QTL analysis of a yellow Phaseolus vulgaris recombinant inbred line population for a fast-cooking, flavorful, and flourishing future of dry beans. Annu. Rep. Bean Improv. Coop. 63, 95–96.
Bassett, A., Dolan, K. D., and Cichy, K. (2020a). Reduced retort processing time improves canning quality of fast-cooking dry beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 100, 3995–4004. doi: 10.1002/jsfa.10444
Bassett, A. N., Kamfwa, K., Ambachew, D., and Cichy, K. A. (2020b). Genetic variability and genome-wide association analysis of flavor and texture in cooked beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 134, 959–978. doi: 10.1007/s00122-020-03745-3
Bassett, M. J. (2007). “Genetics of seed coat color and pattern in common bean,” in Plant Breeding Reviews, ed. J. Janick (Hoboken, NJ: John Wiley & Sons, Inc), 239–315. doi: 10.1002/9780470168028.ch8
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bauchet, G., Grenier, S., Samson, N., Segura, V., Kende, A., Beekwilder, J., et al. (2017). Identification of major loci and genomic regions controlling acid and volatile content in tomato fruit: implications for flavor improvement. New Phytol. 215, 624–641. doi: 10.1111/nph.14615
Beninger, C. W., Gu, L., Prior, R. L., Junk, D. C., Vandenberg, A., and Bett, K. E. (2005). Changes in polyphenols of the seed coat during the after-darkening process in pinto beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 53, 7777–7782. doi: 10.1021/jf050051l
Berry, M., Izquierdo, P., Jeffery, H., Shaw, S., Nchimbi-Msolla, S., and Cichy, K. A. (2020). QTL analysis of cooking time and quality traits in dry bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 133, 2291–2305. doi: 10.1007/s00122-020-03598-w
Bornowski, N., Song, Q., and Kelly, J. D. (2020). QTL mapping of post-processing color retention in two black bean populations. Theor. Appl. Genet. 133, 3085–3100. doi: 10.1007/s00122-020-03656-3
Bott, L., and Chambers, E. (2006). Sensory characteristics of combinations of chemicals potentially associated with beany aroma in foods. J. Sens. Stud. 21, 308–321. doi: 10.1111/j.1745-459X.2006.00067.x
Bowman, A. W., and Azzalini, A. (2018). R Package “SM”: Nonparametric Smoothing Methods. Available online at: http://www.stats.gla.ac.uk/~adrian/sm doi: 10.1111/j.1745-459x.2006.00067.x
Britten, P., Cleveland, L. E., Koegel, K. L., Kuczynski, K. J., and Nickols-Richardson, S. M. (2012). Updated US Department of Agriculture Food Patterns meet goals of the 2010 Dietary Guidelines. J. Acad. Nutr. Diet. 112, 1648–1655. doi: 10.1016/j.jand.2012.05.021
Broman, K. W., Wu, H., Sen, Ś, and Churchill, G. A. (2003). R/qtl: QTL mapping in experimental crosses. Bioinformatics 19, 889–890. doi: 10.1093/bioinformatics/btg112
Bullard, G. (2016). Heirloom Beans Are a Thing, But Do They Really Taste Better than Bulk Beans? The Plate. Washington, DC: National Geographic. Available online at: https://www.nationalgeographic.com/culture/article/heirloom-beans-are-a-thing-but-do-they-really-taste-better-than-bulk-beans
Chen, P. X., Tang, Y., Marcone, M. F., Pauls, P. K., Zhang, B., Liu, R., et al. (2015). Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 185, 298–308. doi: 10.1016/j.foodchem.2015.03.100
Cichy, K. A., Fernandez, A., Kilian, A., Kelly, J. D., Galeano, C. H., Shaw, S., et al. (2014). QTL analysis of canning quality and color retention in black beans (Phaseolus vulgaris L.). Mol. Breed. 33, 139–154. doi: 10.1007/s11032-013-9940-y
Cichy, K. A., Porch, T. G., Beaver, J. S., Cregan, P., Fourie, D., Glahn, R. P., et al. (2015a). A Phaseolus vulgaris diversity panel for Andean bean improvement. Crop Sci. 55, 2149–2160. doi: 10.2135/cropsci2014.09.0653
Cichy, K. A., Wiesinger, J. A., Berry, M., Nchimbi-Msolla, S., Fourie, D., Porch, T. G., et al. (2019). The role of genotype and production environment in determining the cooking time of dry beans (Phaseolus vulgaris L.). Legum. Sci. 1, 1–15. doi: 10.1002/leg3.13
Cichy, K. A., Wiesinger, J. A., and Mendoza, F. A. (2015b). Genetic diversity and genome-wide association analysis of cooking time in dry bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 128, 1555–1567. doi: 10.1007/s00122-015-2531-z
Eihusen, J., and Albrecht, J. A. (2007). Dry bean intake of women ages 19-45. Rural. Rev. Undergrad. Res. Agric. Life Sci. 2:3.
Elia, F. M., Hosfield, G. L., Kelly, J. D., and Uebersax, M. A. (1997). Genetic analysis and interrelationships between traits for cooking time, water absorption, and protein and tannin content of Andean dry beans. J. Am. Soc. Hortic. Sci. 122, 512–518. doi: 10.21273/JASHS.122.4.512
Elsadr, H. T., Wright, L. C., Pauls, K. P., and Bett, K. E. (2011). Characterization of seed coat post harvest darkening in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 123, 1467–1472. doi: 10.1007/s00122-011-1683-8
Erfatpour, M., Navabi, A., and Pauls, K. P. (2018). Mapping the non-darkening trait from ‘Wit-rood boontje’ in bean (Phaseolus vulgaris). Theor. Appl. Genet. 131, 1331–1343. doi: 10.1007/s00122-018-3081-y
Erfatpour, M., and Pauls, K. P. (2020). A R2R3-MYB gene-based marker for the non-darkening seed coat trait in pinto and cranberry beans (Phaseolus vulgaris L.) derived from ‘Wit-rood boontje.’. Theor. Appl. Genet. 133, 1977–1994. doi: 10.1007/s00122-020-03571-7
Feenstra, B., Skovgaard, I. M., and Broman, K. W. (2006). Mapping quantitative trait loci by an extension of the Haley-Knott regression method using estimating equations. Genetics 173, 2269–2282. doi: 10.1534/genetics.106.058537
Ferreira, J. J., del Castillo, R. R., Perez-Vega, E., Plans, M., Simó, J., and Casañas, F. (2012). Sensory changes related to breeding for plant architecture and resistance to viruses and anthracnose in bean market class Fabada (Phaseolus vulgaris L.). Euphytica 186, 687–696. doi: 10.1007/s10681-011-0540-9
Glanz, K., Basil, M., Maibach, E., Goldberg, J., and Snyder, D. (1998). Why Americans eat what they do: Taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J. Am. Diet. Assoc. 98, 1118–1126. doi: 10.1016/S0002-8223(98)00260-0
Heffelfinger, C., Fragoso, C. A., and Lorieux, M. (2017). Constructing linkage maps in the genomics era with MapDisto 2.0. Bioinformatics 33, 2224–2225. doi: 10.1093/bioinformatics/btx177
Holland, J. B., Nyquist, W. E., and Cervantes-Martínez, C. T. (2003). “Estimating and interpreting heritability for plant breeding: an update,” in Plant Breeding Reviews, ed. J. Janick (Hoboken, NJ: John Wiley & Sons, Inc), 9–112. doi: 10.1002/9780470650202.ch2
Hooper, S. D., Glahn, R. P., and Cichy, K. A. (2019). Single varietal dry bean (Phaseolus vulgaris L.) pastas: nutritional profile and consumer acceptability. Plant Foods Hum. Nutr. 74, 342–349. doi: 10.1007/s11130-019-00732-y
IPSOS (2010). Factors Influencing Pulse Consumption in Canada. Available online at: https://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/sis13117/$FILE/v4_factors_influencing_pulse_consumption_summary_feb24_2010.pdf
Jacinto-Hernandez, C., Azpiroz-Rivero, S., Acosta-Gallegos, J. A., Hernandez-Sanchez, H., and Bernal-Lugo, I. (2003). Genetic analysis and random amplified polymorphic DNA markers associated with cooking time in common bean. Crop Sci. 43:329. doi: 10.2135/cropsci2003.3290
Karlsen, M. C., Ellmore, G. S., and McKeown, N. (2016). Seeds-health benefits, barriers to incorporation, and strategies for practitioners in supporting consumption among consumers. Nutr. Today 51, 50–59. doi: 10.1097/NT.0000000000000135
Katuuramu, D. N., Hart, J. P., Porch, T. G., Grusak, M. A., Glahn, R. P., and Cichy, K. A. (2018). Genome-wide association analysis of nutritional composition-related traits and iron bioavailability in cooked dry beans (Phaseolus vulgaris L.). Mol. Breed. 38:44. doi: 10.1007/s11032-018-0798-x
Katuuramu, D. N., Luyima, G. B., Nkalubo, S. T., Wiesinger, J. A., Kelly, J. D., and Cichy, K. A. (2020). On-farm multi-location evaluation of genotype by environment interactions for seed yield and cooking time in common bean. Sci. Rep. 10, 3628. doi: 10.1038/s41598-020-60087-2
Kelly, J., Wright, E., and Bornowski, N. (2017). 2017 Dry Bean Yield Trials. Available online at: http://www.varietytrials.msu.edu/wp-content/uploads/2012/12/2014_Dry_Bean_Report.pdf (accessed April 2, 2015).
Kelly, J. D., and Cichy, K. A. (2012). “Dry bean breeding and production technologies,” in Dry Beans and Pulses Production, Processing and Nutrition, eds M. A. Uebersax and M. Siddiq (Hoboken, NJ: Wiley), 23–54. doi: 10.1002/9781118448298.ch2
Kinsella, J. E. (1979). Functional properties of soy proteins. J. Am. Oil Chem. Soc. 56, 242–258. doi: 10.1007/BF02671468
Koehler, H. H., Chang, C.-H., Scheier, G., and Burke, D. W. (1987). Nutrient composition, protein quality, and sensory properties of thirty-six cultivars of dry beans (Phaseolus vulgaris L.). J. Food Sci. 52, 1335–1340. doi: 10.1111/j.1365-2621.1987.tb14076.x
Leakey, C. L. A. (1992). Breeding on the C and J and B loci for modification of bean seedcoat flavonoids with the objective of improving food acceptibility. Annu. Rep. Bean Improv. Coop. 35, 13–17.
Leakey, C. L. A. (2000). Progress in developing tannin-free dry Phaseolus beans. Annu. Rep. Bean Improv. Coop. 43, 18–20.
Leterme, P., and Carmenza Muñoz, L. (2002). Factors influencing pulse consumption in Latin America. Br. J. Nutr. 88, 251–254. doi: 10.1079/bjn/2002714
Meilgaard, M. C., Civille, G. V., and Carr, B. T. (1999). Sensory Evaluation Techniques, 3rd Edn. Boca Raton, FL: CRC Press.
Mendoza, F. A., Kelly, J. D., and Cichy, K. A. (2017). Automated prediction of sensory scores for color and appearance in canned black beans (Phaseolus vulgaris L.) using machine vision. Int. J. Food Prop. 20, 83–99. doi: 10.1080/10942912.2015.1136939
Miklas, P. N., and Porch, T. (2010). Guidelines for common bean QTL nomenclature. Annu. Rep. Bean Improv. Coop. 53, 202–204.
Mkanda, A. V., Minnaar, A., and de Kock, H. L. (2007). Relating consumer preferences to sensory and physicochemical properties of dry beans (Phaseolus vulgaris). J. Sci. Food Agric. 87, 2868–2879. doi: 10.1002/jsfa.3046
Myers, J. R., Wallace, L. T., Mafi Moghaddam, S., Kleintop, A. E., Echeverria, D., Thompson, H. J., et al. (2019). Improving the health benefits of snap bean: genome-wide association studies of total phenolic content. Nutrients 11:2509. doi: 10.3390/nu11102509
Nasar-Abbas, S. M., Siddique, K. H. M., Plummer, J. A., White, P. F., Harris, D., Dods, K., et al. (2009). Faba bean (Vicia faba L.) seeds darken rapidly and phenolic content falls when stored at higher temperature, moisture and light intensity. LWT Food Sci. Technol. 42, 1703–1711. doi: 10.1016/j.lwt.2009.05.013
Peleg, H., Gacon, K., Schlich, P., and Noble, A. C. (1999). Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 79, 1123–1128. doi: 10.1002/(sici)1097-0010(199906)79:8<1123::aid-jsfa336>3.0.co;2-d
R Core Team (2017). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/
Rivera, A., Fenero, D., Almirall, A., Ferreira, J. J., Simó, J., Plans, M., et al. (2013). Variability in sensory attributes in common bean (Phaseolus vulgaris L.): a first survey in the Iberian secondary diversity center. Genet. Resour. Crop Evol. 60, 1885–1898. doi: 10.1007/s10722-013-9963-6
Robichaud, J. L., and Noble, A. C. (1990). Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 53, 343–353. doi: 10.1002/jsfa.2740530307
Siddiq, M., and Uebersax, M. A. (2012). “Dry beans and pulses production and consumption–an overview,” in Dry Beans and Pulses Production, Processing and Nutrition, 1st Edn, eds M. Siddiq and M. A. Uebersax (Hoboken, NJ: John Wiley & Sons, Inc), doi: 10.1002/9781118448298
Song, Q., Jia, G., Hyten, D. L., Jenkins, J., Hwang, E. Y., Schroeder, S. G., et al. (2015). SNP assay development for linkage map construction, anchoring whole-genome sequence, and other genetic and genomic applications in common bean. G3 Genes Genomes Genet. 5, 2285–2290. doi: 10.1534/g3.115.020594
Stone, H., Sidel, J., Oliver, S., Woolsey, A., and Singleton, R. C. (1974). Sensory evaluation by quantitative descriptive analysis. Food Technol. 28;51. doi: 10.1520/mnl1320170005
Vara-Ubol, S., Chambers, E., and Chambers, D. H. (2004). Sensory characteristics of chemical compounds potentially associated with beany aroma in foods. J. Sens. Stud. 19, 15–26. doi: 10.1111/j.1745-459X.2004.tb00133.x
Voorrips, R. E. (2002). MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 93, 77–78. doi: 10.1093/jhered/93.1.77
Wang, N., and Daun, J. K. (2005). Determination of cooking times of pulses using an automated Mattson cooker apparatus. J. Sci. Food Agric. 85, 1631–1635. doi: 10.1002/jsfa.2134
Wang, S., Basten, C. J., and Zeng, Z.-B. (2012). Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. Available online at: http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Wiesinger, J. A., Cichy, K. A., Glahn, R. P., Grusak, M. A., Brick, M. A., Thompson, H. J., et al. (2016). Demonstrating a nutritional advantage to the fast-cooking dry bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 64, 8592–8603. doi: 10.1021/acs.jafc.6b03100
Wiesinger, J. A., Cichy, K. A., Tako, E., and Glahn, R. P. (2018). The fast cooking and enhanced iron bioavailability properties of the Manteca yellow bean (Phaseolus vulgaris L.). Nutrients 10:1609. doi: 10.3390/nu10111609
Winham, D., Tisue, M., Palmer, S., Cichy, K., and Shelley, M. (2019). Dry bean preferences and attitudes among midwest hispanic and non-hispanic white women. Nutrients 11:178. doi: 10.3390/nu11010178
Zhang, J., Zhao, J., Xu, Y., Liang, J., Chang, P., Yan, F., et al. (2015). Genome-wide association mapping for tomato volatiles positively contributing to tomato flavor. Front. Plant Sci. 6:1042. doi: 10.3389/fpls.2015.01042
Keywords: sensory quality, taste, legume, fast cooking, Manteca
Citation: Bassett A, Katuuramu DN, Song Q and Cichy K (2021) QTL Mapping of Seed Quality Traits Including Cooking Time, Flavor, and Texture in a Yellow Dry Bean (Phaseolus vulgaris L.) Population. Front. Plant Sci. 12:670284. doi: 10.3389/fpls.2021.670284
Received: 20 February 2021; Accepted: 27 May 2021;
Published: 22 June 2021.
Edited by:
Petr Smýkal, Palacký University Olomouc, CzechiaReviewed by:
Ester Murube Torcida, Servicio Regional de Investigación y Desarrollo Agroalimentario (SERIDA), SpainCopyright © 2021 Bassett, Katuuramu, Song and Cichy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen Cichy, a2FyZW4uY2ljaHlAdXNkYS5nb3Y=
†ORCID: Amber Bassett, orcid.org/0000-0001-9013-8625; Karen Cichy, orcid.org/0000-0002-4311-0774
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.