- 1College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou, China
- 2Zhejiang Provincial Key Laboratory for Genetic Improvement and Quality Control of Medicinal Plants, Hangzhou Normal University, Hangzhou, China
- 3College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, China
The stems of Dendrobium officinale have been used as a rare and valuable Chinese tonic medicine, known as “Tiepi Fengdou”, since the Qing dynasty. Because of the increased market demand and continued exploitation of this plant, the reserves of wild D. officinale resources have been depleted, and D. officinale products on the market are being increasingly adulterated. Such changes have strongly affected the sustainable utilization of this valuable medicinal plant resource and the development of related industries. In this study, a species-specific DNA marker was developed for the rapid and accurate authentication of D. officinale. In total, 36 start codon-targeted (SCoT) polymorphism primers were screened in 36 definite Dendrobium species, and a distinct species-specific DNA amplicon (SCoT13-215) for D. officinale was obtained. After the sequence was cloned and sequenced, a sequence-characterized amplified region marker was developed (named SHF/SHR) and validated through PCR amplification of all 38 Dendrobium samples. The marker’s specificity for D. officinale was confirmed through the consistent amplification of a clear 197-bp band. This SCAR marker can be used to rapidly, effectively, and reliably identify D. officinale among various Dendrobium species and may play an important role in ensuring the quality of medicinal preparations and protecting the germplasm of this important medicinal species.
Introduction
Dendrobium officinale Kimura et Migo is an important orchid plant endemic to China that has been identified separately from other Dendrobium species in the Pharmacopeia of the People’s Republic of China (2010 Edition). This plant is primarily distributed in Yunnan, Guangxi, Zhejiang, Anhui, Fujian, and Sichuan Provinces of China (Tsi et al., 1999; Chinese Pharmacopoeia Commission, 2015; Teixeira da Silva et al., 2016). The stems of D. officinale form a rare and valuable Chinese herb that is highly valued for its promising medicinal functions (Ding et al., 2003; Feng et al., 2017; Lu et al., 2018). The D. officinale-derived herb is known as “Tiepi Fengdou”, and the international medical plant community refers to this herb as a “medicinal giant panda”. D. officinale contains abundant polysaccharides, dendrobium alkaloids, flavonoids, and other bioactive substances, which are beneficial to human stomach health, exhibiting such activities as clearing heat and toxic material, enhancing immunity, reducing blood sugar concentration, and delaying aging, and this plant is recognized as a high-end health care product (Wei et al., 2016; Tang et al., 2017; Li et al., 2019). D. officinale has high medicinal value and is a valuable plant resource for this traditional Chinese medicine. Therefore, this plant has high research value and broad development and utilization prospects.
However, due to the increasing market demand, the wild germplasm resources of D. officinale have been over-harvested which, together with the destruction of the natural habitat, has caused wild D. officinale resources to be critically endangered (Teixeira da Silva et al., 2016). The scarcity of D. officinale has resulted in medicinal D. officinale materials being mixed and adulterated with other materials, particularly other Dendrobium species, in clinical practice (Feng et al., 2015b; Zhu et al., 2018). Because of the similarities in morphological characteristics among Dendrobium species, identification is difficult and their use is confusing, with “synonym” and “homonym” phenomena being commonly observed (Jin and Huang, 2015). More seriously, some illegal elements have been offered as legitimate resources, which leads to low-quality material. These problems affect the protection of D. officinale resources and the development of the D. officinale industry (Tang et al., 2017; Cheng et al., 2019). Thus, to better protect and clinically utilize D. officinale resources, it is very important to identify a rapid and accurate method to identify D. officinale from its adulterants.
Initially, morphological (Wahba et al., 2014; Moudi and Go, 2017), microscopic (Zhao et al., 2017, 2018), or chemical analyses (Luo et al., 2013; Ye et al., 2017) were utilized for herb identification; however, in the majority of cases, these analyses are inadequate to correctly identify plant species (Teixeira da Silva et al., 2016; Kumar et al., 2018). Moreover, these morphological, microscopic, and chemical indicators are easily affected by environmental factors. Compared with the above-mentioned traditional plant authentication methods, DNA molecular markers can be used to detect organisms, tissues, organs, and even cells at different developmental stages (Feng et al., 2015a; Teixeira da Silva et al., 2016). The number of DNA molecular markers covering the whole genome is large, and they present high polymorphic and genetic stability; their use is not limited by environmental factors or gene expression levels. These markers have been widely used in molecular identification, phylogenetic evolution, and genetic diversity analyses of plant species (Teixeira da Silva et al., 2016; Lu et al., 2018). To date, numerous DNA marker techniques, including random amplified polymorphic DNA (RAPD) (Ding et al., 2009; Khosravi et al., 2009; Xue et al., 2010), amplified fragment length polymorphism (AFLP) (Li et al., 2008; Wahba et al., 2014), inter simple sequence repeat (ISSR) (Shen et al., 2006; Wang et al., 2009), simple sequence repeat (SSR) (Lu et al., 2012, 2013) and sequence-related amplified polymorphism (SRAP) (Ding et al., 2008), have been utilized for genetic correlation, mapping and diversity studies of Dendrobium species. Sequence-characterized amplified regions (SCARs) are monologs codominant markers that are screened using PCRs with a pair of specific oligonucleotide primers designed from a specific nucleotide sequence generated by RAPD, AFLP, ISSR and inter-retrotransposon amplified polymorphism (IRAP) techniques (Marieschi et al., 2016; Kumar et al., 2018). Compared with conventional molecular markers, such as RAPDs, AFLPs, ISSRs, and SSRs, SCAR markers are less sensitive to reaction conditions and are more reproducible because of their high levels of specificity. The use of SCAR markers is highly convenient and provides accurate fingerprint identification at the inter- and/or intraspecific level (Marieschi et al., 2016; Kumar et al., 2018; Yang et al., 2019). The natural distribution of D. officinale is often complex (Jin and Huang, 2015), the SCAR markers can play a primary role in distinguishing with other Dendrobium germplasm, which will continue help conservation an utilization of D. officinale.
Start codon-targeted (SCoT) markers, developed based on the translation start codon, are PCR-based gene-targeted markers (Collard and Mackill, 2009). SCoT markers use a single primer that amplifies the genomic region based on the conserved region surrounding the translation initiation codon ATG (Mulpuri et al., 2013; Feng et al., 2015a). SCoT markers can be used as powerful tools in the characterization of germplasm collections; their applications may include screening for genetic diversity (Igwe et al., 2017), identifying species (Hao et al., 2018), and performing phylogenetic studies (Jalilian et al., 2018). In this study, we report the suitability of polymorphic SCoT markers in developing a species-specific SCAR marker. Species-specific SCAR markers have been applied to identify many different important medicinal herbs from their close relatives or adulterants (Devaiah and Venkatasubramanian, 2008; Jiang et al., 2018). Different medicinal herbs act in different medicinal pathways; therefore, it is highly important to establish a stable and effective identification method for quality control, which necessitates developing a rapid and reliable method for the identification of D. officinale.
Materials and Methods
Plant Material and DNA Isolation
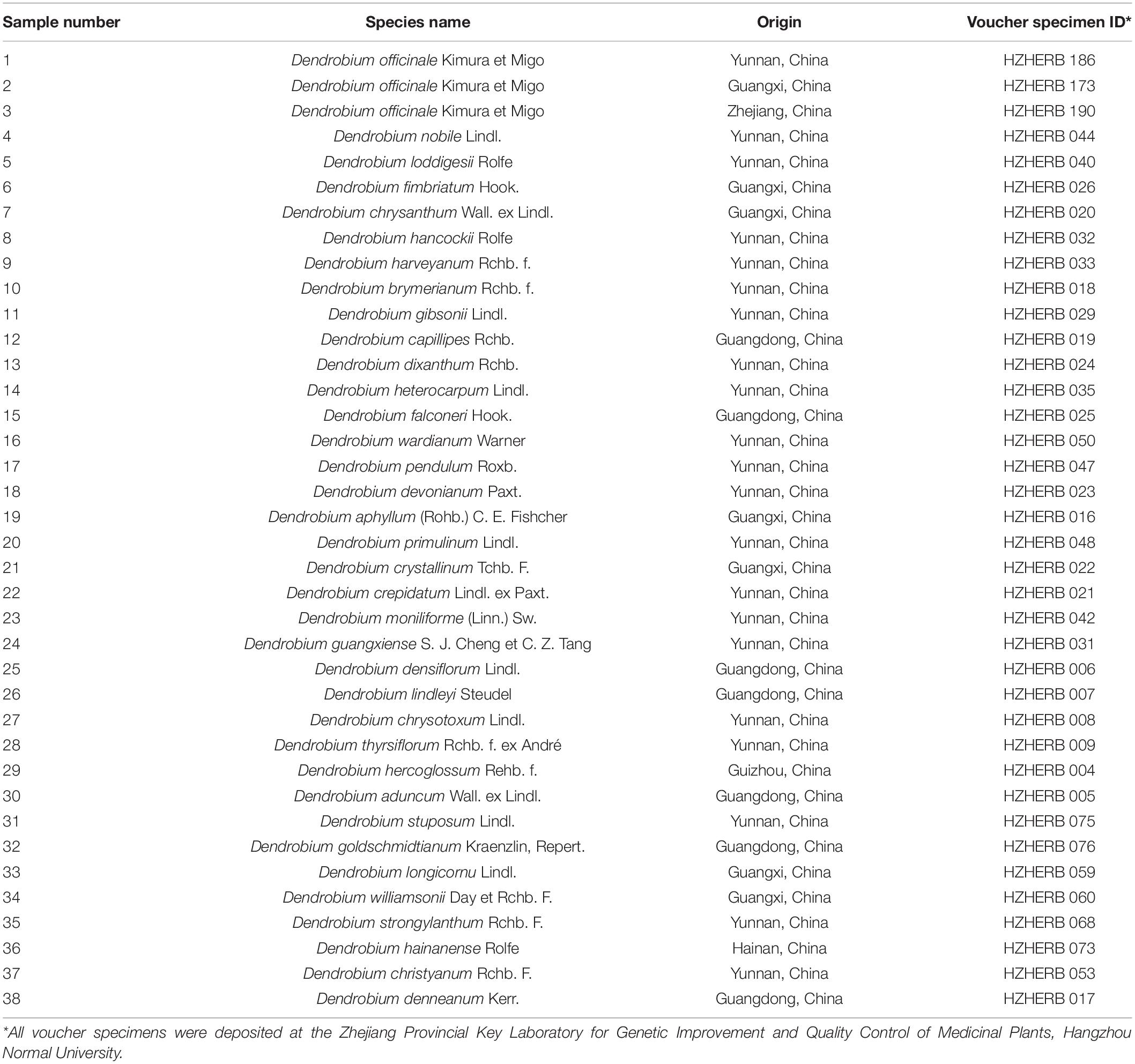
A total of 36 definite Dendrobium species collected from their primary distribution areas in China were used to screen for specific markers by mixing fresh leaves from 3 to 4 individuals (Table 1). Furthermore, 15 D. officinale populations were selected for validating the SCAR marker by using fresh leaves from at least 10 individuals (Table 2). These species were identified and authenticated by HW after flowering. All voucher specimens of the collected accessions were deposited at the Zhejiang Provincial Key Laboratory for Genetic Improvement and Quality Control of Medicinal Plants, Hangzhou Normal University. The experimental research on Dendrobium species was in keeping with the guidelines of Hangzhou Normal University. Total genomic DNA was extracted from the fresh leaves of the collected samples as described in a previous study (Feng et al., 2013). The integrity and quality of DNA were determined using 0.8% agarose gel electrophoresis, and the genomic DNA concentration was measured using a UV spectrometer. Stock DNA was diluted to a working solution of 50 ng/μL.
PCR Amplification With SCoT Primers
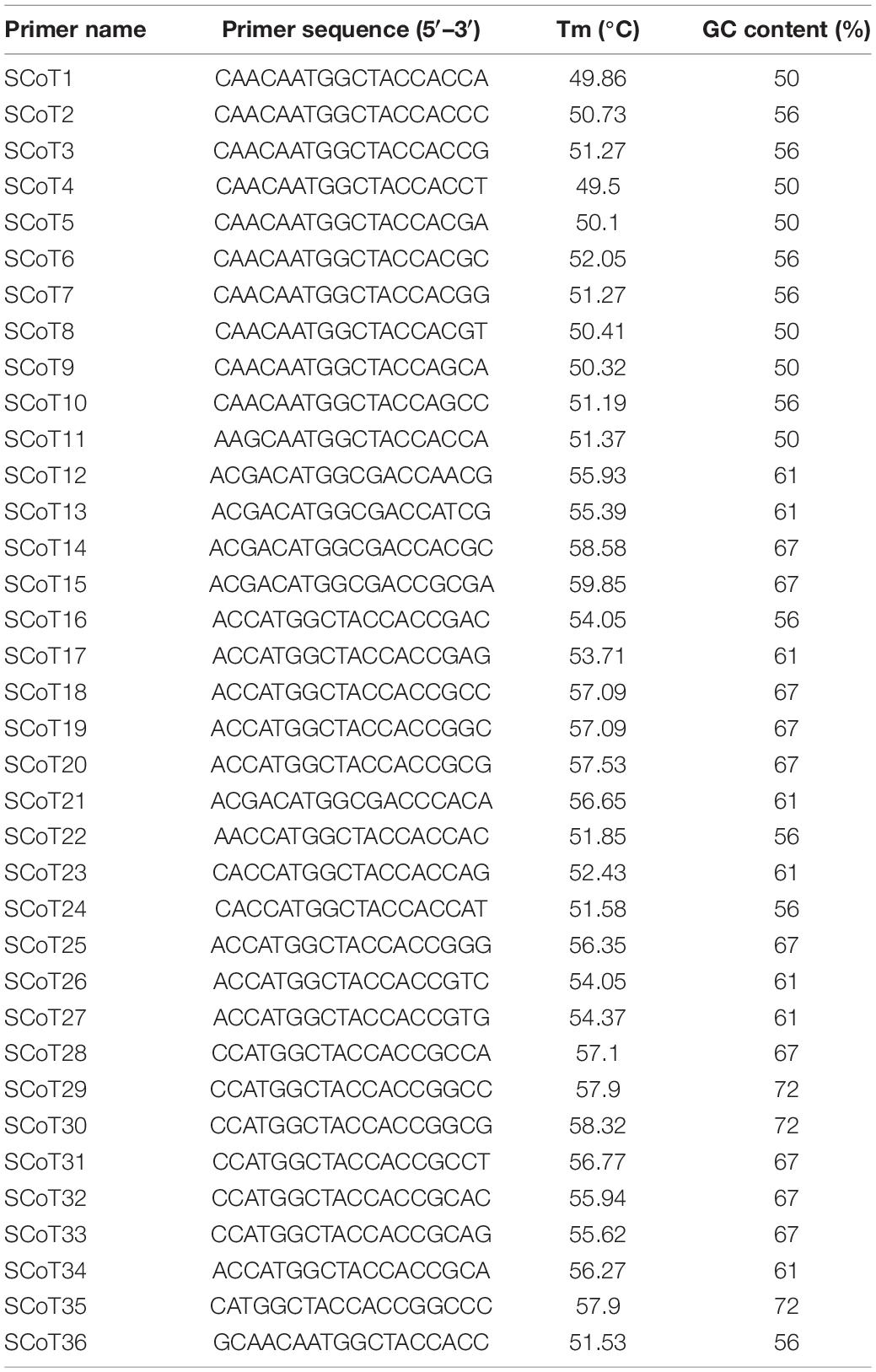
For the initial screening, a total of 36 SCoT primers (Table 3) were synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd., Shanghai, China, based on the study by Collard and Mackill (2009). PCR analysis was performed in a total volume of 20 μL containing 2 μL 1 × PCR buffer [100 mM (NH4)2SO4, 100 mM KCl, 1% Triton X-100, pH 8.8], 2 μL Mg2+ (25 mM), 0.8 μL dNTPs (10 mM), 1 μL primer (10 μM), 0.5 μL Taq DNA polymerase (2 U/μL) (TaKaRa Bio., Kyoto, Japan) and 50 ng genomic DNA template. Amplification reactions were performed in a thermal cycler (MJ Research PTC-100, Waltham, MA, United States) using the following parameters: 5 min at 94°C; 35 cycles of 1 min at 94°C, 1 min at 50–60°C (depending on the annealing temperature of each primer) and 2 min at 72°C; and a final extension at 72°C for 10 min. After purification with an EZ-10 spin column PCR product purification kit (Sangon Biotech, Shanghai, China), the PCR products were electrophoresed on a 1.5% agarose gel alongside Trans2K DNA markers (TransGen Biotech Co., Ltd., Beijing, China), followed by staining with GelStain (TransGen Biotech Co., Ltd.) and photography was performed using a Molecular Imager® Gel DocTM XR + System with Image LabTM Software (Bio-Rad, Philadelphia, PA, United States).
Selection, Cloning, and Sequencing of Species-Specific SCoT Fragments
Any SCoT band present in a particular species and absent in all the other species was considered a species-specific marker. To verify the reproducibility of the results, SCoT-PCR amplifications were performed at least twice, and only repeatable amplicons were selected. A unique band specific to D. officinale was excised and purified from an agarose gel using a SanPrep Column DNA Gel Extraction Kit (Shanghai Sangon Biological Engineering Technology and Service Co., Ltd., Shanghai, China). The purified product was ligated into a pMDTM 19-T vector (Takara Biomedical Technology Co., Ltd., Beijing, China) according to the manufacturer’s protocol and transformed into the ultracompetent Escherichia coli strain Trans5α using the heat shock method. The recombinant plasmids were isolated by red/white clone screening and sequenced bidirectionally using M13 universal primers at Shanghai Sunny Biotechnology Co., Ltd. (Shanghai, China).
Designing and Validating SCAR Primers
The obtained sequence was edited using the online tool VecScreen1 to remove vector sequences. The obtained sequence was identified by performing a BLASTN-based search2 of the nucleotide databases, and it was deposited in GenBank (GenBank accession number: MN746373). Forward and reverse oligonucleotide SCAR primers were designed using Primer Premier 5 (Lalitha, 2000) based on the obtained sequence information. The SCAR primer pair SHF/SHR was developed using the sequenced SCoT fragment (Table 4). The primer lengths ranged from 18 to 24 bp, and the optimum annealing temperature (Tm) value was adjusted to 65°C, with a range of 60 to 68°C. SCAR amplification was performed in 20 μL of reaction mixture containing 2 μL 1 × PCR buffer [100 mM (NH4)2SO4, 100 mM KCl, 1% Triton X-100, pH 8.8], 2 μL Mg2+ (25 mM), 0.8 μL dNTPs (10 mM), 1 μL of forward primer (10 μM), 1 μL of reverse primer (10 μM), 0.5 μL Taq DNA polymerase (2 U/μL) (TaKaRa Bio.), and 50 ng genomic DNA template. PCR analysis was performed in a thermal cycler (MJ Research PTC-100) using the following parameters: 5 min at 94°C; 35 cycles of 1 min at 94°C, 1 min at 65°C and 2 min at 72°C; and a final extension at 72°C for 10 min. The PCR products were run on 1.5% agarose gels and detected by GelStain (TransGen Biotech) staining. The specific amplified fragment was designated a species-specific SCAR marker.

Table 4. Characteristics of developed species-specific SCAR primer pair derived from cloned SCoT13-derived amplicon of Dendrobium officinale.
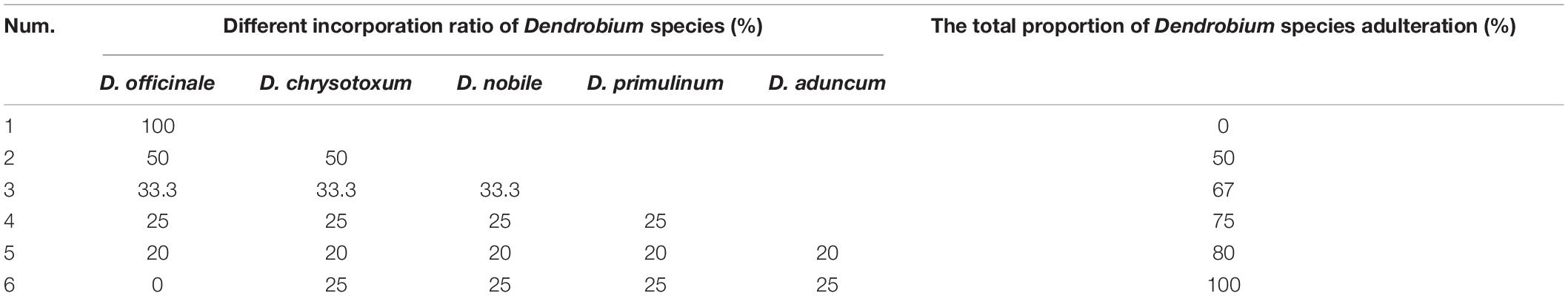
We also examined the sensitivity of the D. officinale genome DNA template concentration for SHF/SHR, and seven DNA template concentrations, specifically 0, 5, 10, 15, 20, 30, and 50 ng/μL, were set. To further verify the practicality of this marker, four Dendrobium samples of the D. chrysotoxum, D. nobile, D. primulinum, and D. aduncum species were utilized to simulate different adulterations of D. officinale (Table 5).
Results
Species-Specific SCoT Primer and Specific Loci Identification
A total of 38 samples from 36 Dendrobium species, including most of the Dendrobium species that are easily confused with D. officinale, were chosen to develop species-specific SCAR markers for D. officinale. After screening, 22 SCoT primers with clear and repeatable polymorphisms were selected, and they yielded 337 loci, of which 324 loci were polymorphic and represented 96% of the polymorphisms. Among the 324 polymorphic loci, only primer SCoT 13 yielded a sharp and consistent DNA band of 215 bp that was unique to D. officinale samples (Figure 1), which could provide a molecular tool for the identification of D. officinale.
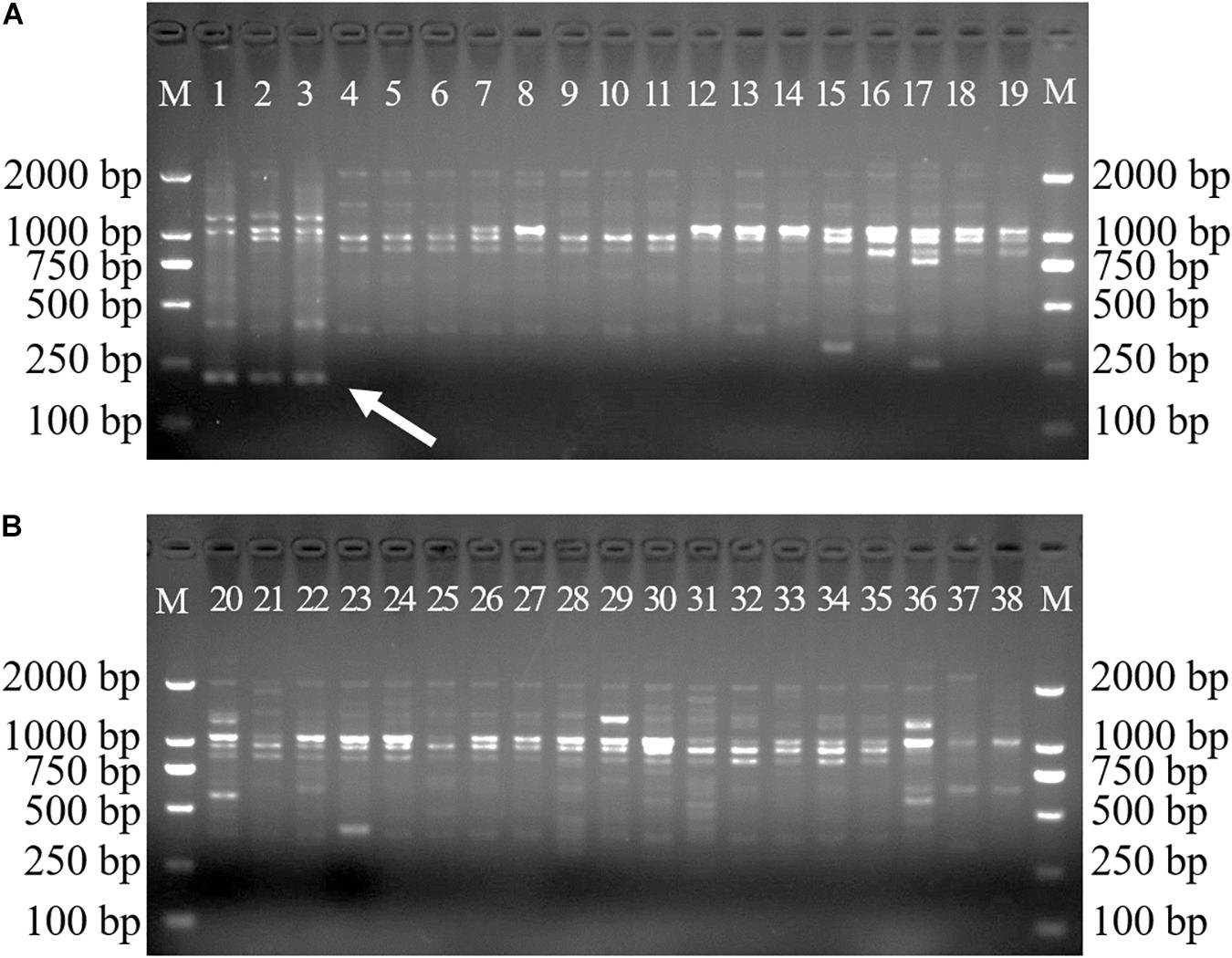
Figure 1. SCoT13 profiles of different Dendrobium species. Two 1.5% agarose gels were used for 38 Dendrobium species electrophoresis analysis (A,B). Lane 1∼38: Details of 38 Dendrobium species are provided in Table 1. Lane M: Trans2K DNA Marker with lengths (bp); Arrowheads represent specific amplified bands in all the D. officinale individuals.
DNA Sequence of the Species-Specific Fragment and Development of SCAR Marker
The species-specific band obtained in all three D. officinale samples, named SCoT13-215, was cloned, sequenced, and deposited in GenBank (GenBank accession number: MN746373). The nucleotide sequence was 48.84% A + T and 51.16% G + C, as shown in Figure 2. The BLAST results revealed that the sequence had no homology with other sequences in GenBank, and no repeats were observed within the sequence. A SCAR primer pair, named SHF/SHR, was developed using Primer 3 based on the sequence SCoT13-215 (Table 4 and Figure 2).
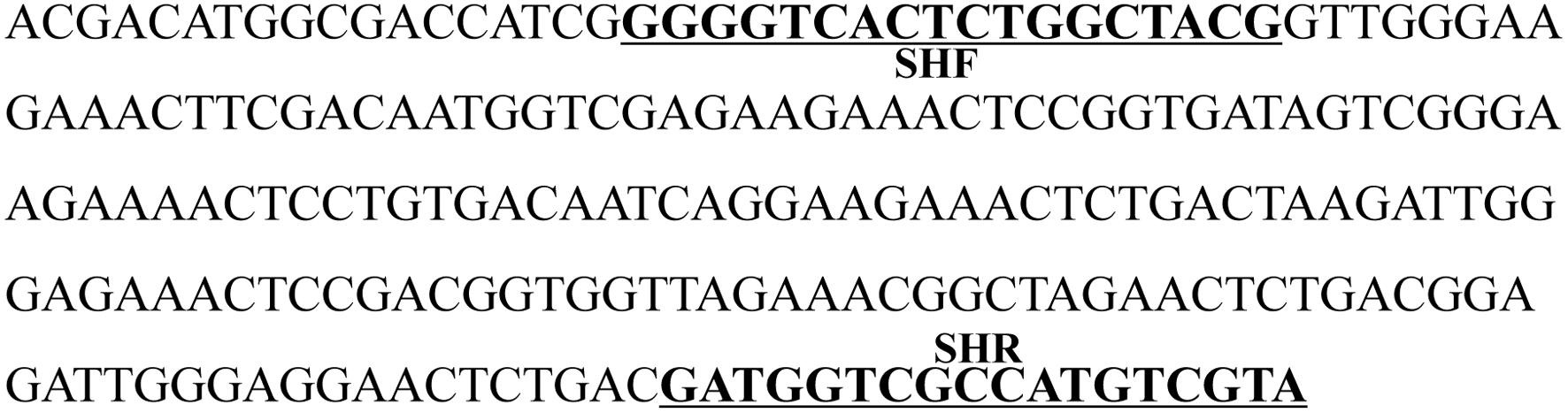
Figure 2. Nucleotide sequence of the SCoT marker specific to D. officinale. The sequence was named SCoT13-215, and has been deposited in GenBank (Accession number: MN746373), the underlined bold sequences represent the forward primer (SHF) and reverse primer (SHR).
SCAR-PCR Amplification of the Designed Species-Specific SCAR Primer
Gradient PCR was performed to determine the best annealing temperature of the SCAR primers within the range of 60 to 68°C, and the optimal annealing temperature was determined to be 65°C. The newly developed SCAR primer pair SHF/SHR was validated by amplifying the sample DNAs listed in Table 1 at the optimal annealing temperature of 65°C. All the samples belonging to D. officinale were amplified and produced a sharp unique band of 197 bp, but no amplicons were produced for the other Dendrobium species (Figure 3). To further verify the stability and specificity of this SCAR marker, a PCR analysis of 15 different D. officinale samples (Table 2) using the primer pair SHF/SHR was performed, and all samples of D. officinale samples produced the specific amplicon at 197 bp (Figure 4). Thus, the SCAR primer pair (SHF/SHR) developed in this study was suggested to be a species-specific marker of D. officinale.
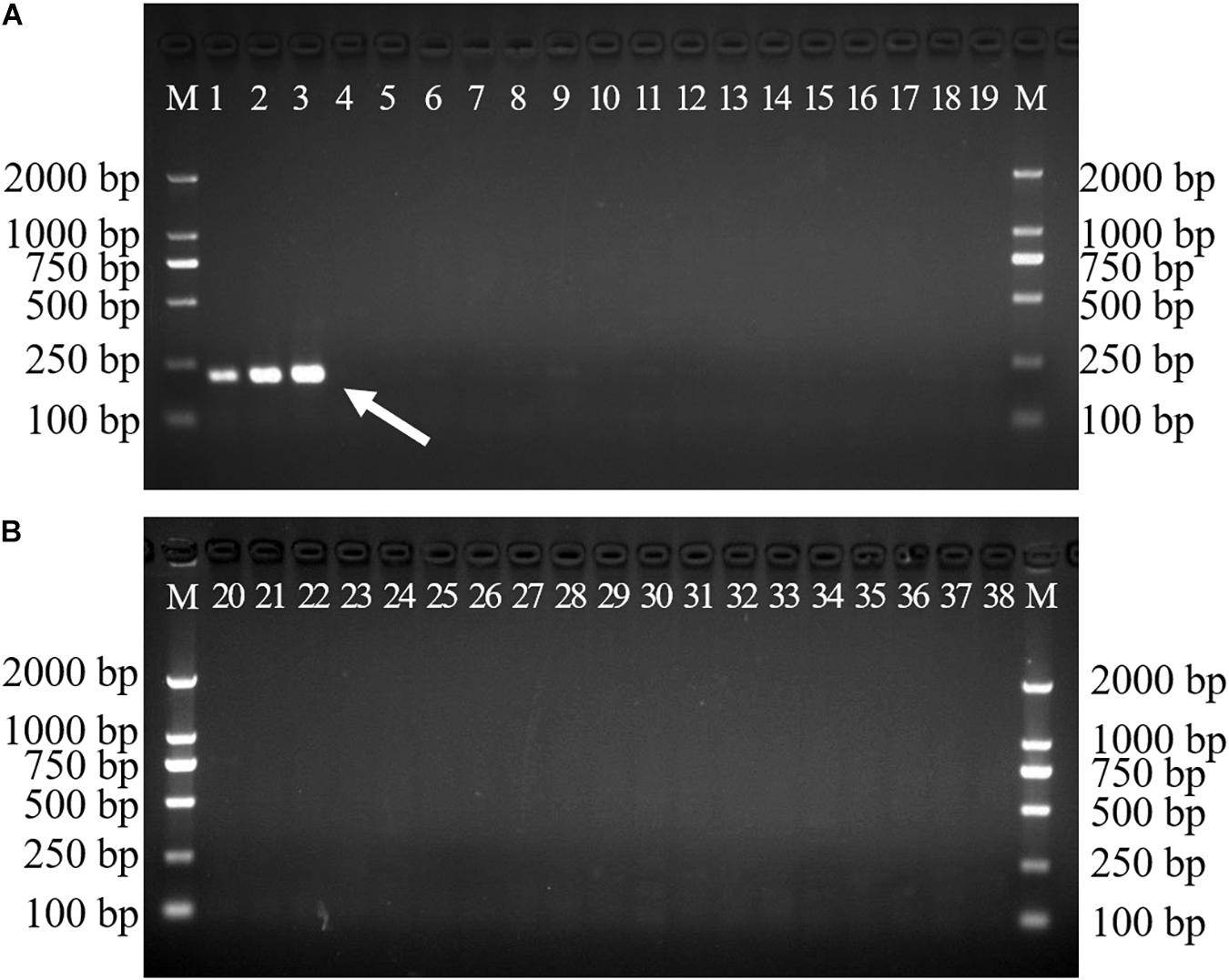
Figure 3. Amplification of the developed SCAR marker SHF/SHR in 38 different Dendrobium species. Two 1.5% agarose gels were used for 38 Dendrobium species electrophoresis analysis (A,B). Lane 1∼38: Details of 38 Dendrobium species are provided in Table 1. Lane M: Trans2K DNA Marker with lengths (bp); Arrowheads represent specific amplified bands in all the D. officinale individuals.
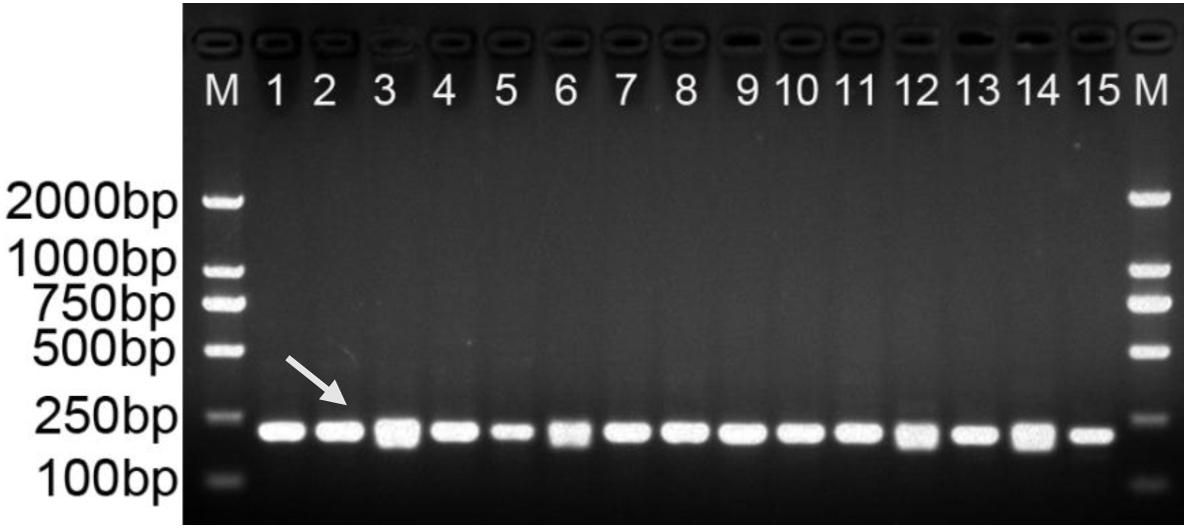
Figure 4. Amplification profiles of the primer pair SHF/SHR in 15 D. officinale individuals. Lane 1∼15: Details of 15 D. officinale individuals are provided in Table 2. Lane M: Trans2K DNA Marker with lengths (bp); Arrowheads represent specific amplified bands in all the D. officinale individuals.
Practicality of the Species-Specific SCAR Marker SHF/SHR
After diluting the D. officinale DNA template concentration to 5, 10, 15, 20, 30, and 50 ng/μL, our species-specific SCAR marker SHF/SHR produced a distinct, 197 bp band (Figure 5). This finding means that DNA concentrations as low as 5 ng are sufficient for D. officinale detection in practical applications. In addition, only the simulated samples containing D. officinale could detect the 197 bp band of the SCAR marker SHF/SHR, whereas this band was not detected in the adulterated samples of D. chrysotoxum, D. nobile, D. primulinum, and D. aduncum (Figure 6). Thus, this marker could provide a scientific tool for determining whether D. officinale is present in Chinese medicinal materials for clinical use.
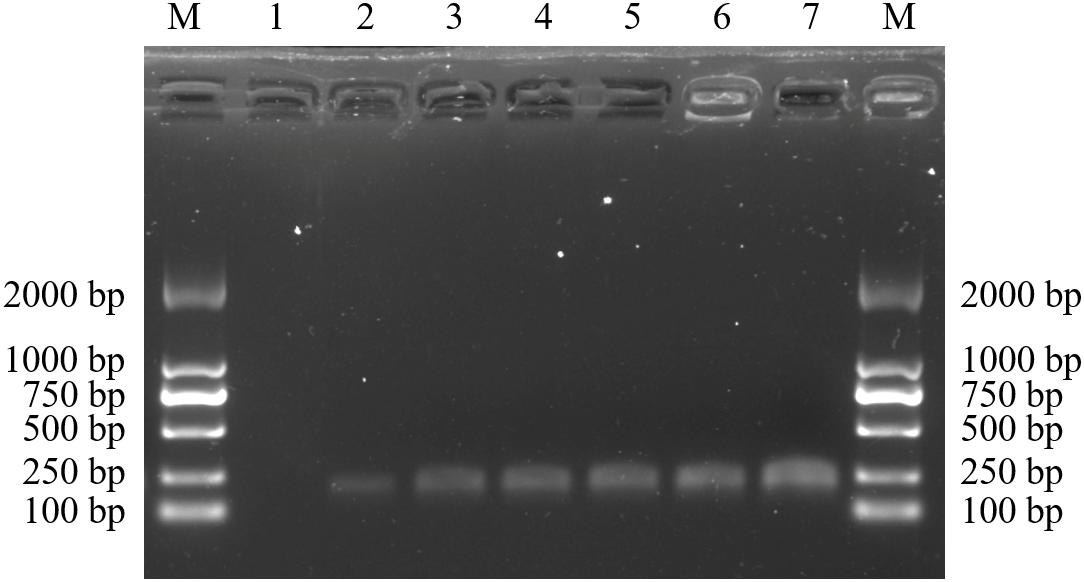
Figure 5. Amplification profiles of the primer pair SHF/SHR in different D. officinale genome DNA concentrations. Lane M: Trans2K DNA Marker with lengths (bp); Lane 1–7: 0, 5, 10, 15, 20, 30, and 50 ng/μL of D. officinale DNA template concentration.
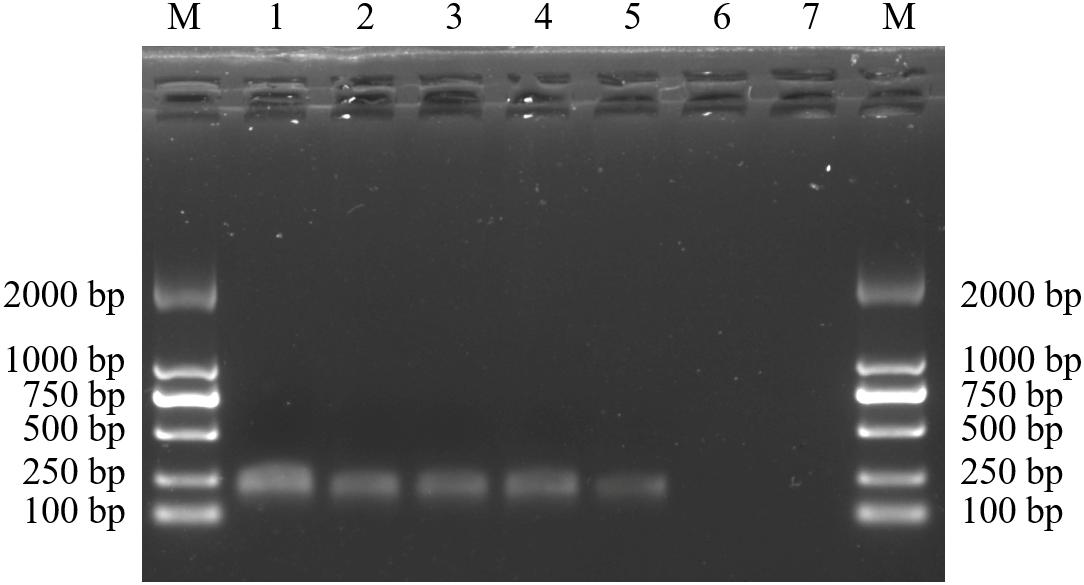
Figure 6. Amplification profiles of the primer pair SHF/SHR in different genome DNA composition of Dendrobium species. Lane M: Trans2K DNA Marker with lengths (bp); Lane 1–7: different proportions of Dendrobium species adulteration provided in Table 5.
Discussion
Species identification is necessary and important for product quality control, especially when the wrong species is deliberately provided. The stems of D. officinale are a rare traditional Chinese medicine, but in recent years, problems in the D. officinale market have become serious. Because of the numerous associated business entities, disordered competition, and similarity between the genuine D. officinale and its adulterants, it is highly common for other inferior Dendrobium species to enter the market as legitimate D. officinale, which has seriously affected the quality of D. officinale products on the market. Therefore, the authentication and traceability of D. officinale products are very important because of the increasing demand and the need to maintain the consumer quality of the products.
At present, many different DNA marker types, such as RAPD, ISSR, AFLP, and SSR, are available for the identification of medicinal plants, and they are also used in other fields, including taxonomy, physiology, and embryology (Teixeira da Silva et al., 2016). The identification of DNA molecular markers is particularly important when morphological identification is difficult, especially when samples are incomplete or damaged or the plants have become desiccated (Feng et al., 2018). Compared with the above-mentioned types of molecular markers, SCAR markers exhibit the advantages of high reliability and high detection sensitivity. In general, SCAR markers can be transformed from traditional DNA molecular markers, such as RAPDs (Zhang et al., 2015), AFLPs (Julio et al., 2006), SRAPs (Ji et al., 2014), and ISSRs (Albani et al., 2004). To increase the specificity of the amplification products, the sequence size of the PCR products amplified by SCAR primers is generally shorter than the original specific SCoT fragment. In recent years, an increasing number of SCAR markers have been designed for the identification of various medicinal plants, such as Akebiae Caulis (Moon et al., 2015), Ganoderma lucidum (Khan et al., 2016), Saraca asoca (Vinay, 2016), Physalis species (Feng et al., 2018), and Panax ginseng (Jiang et al., 2018).
Start codon-targeted markers are very popular because of their outstanding stability and polymorphic nature. SCoT-based SCAR markers have been used in the identification of many plant species or varieties, such as areca nut cultivars (Rajesh et al., 2016), Taxus media (Hao et al., 2018), and Physalis species (Feng et al., 2018). In our earlier study, we evaluated the genetic relationships of 36 Dendrobium species based on SCoT markers, and the findings indicated that a high degree of genetic diversity exists among Chinese Dendrobium species (Feng et al., 2015a). In this study, a specific 215 bp DNA sequence (SCoT13-215) of D. officinale obtained by the SCoT13 primer could be used for the identification of D. officinale by being transforming into a SCAR marker, and the specific band size amplified by the corresponding SCAR primer pair SHF/SHR was 197 bp, which was 18 bp shorter than the original specific SCoT sequence fragment.
The genus Dendrobium includes many species with different medicinal values, and D. officinale has the greatest value and accounts for a significant share in the global Chinese herbal medicine industry. The high value of D. officinale has resulted in the substitution of other Dendrobium species in place of D. officinale. To ensure the safe clinical use of D. officinale products, authentic raw materials are necessary. Thus, it is very important to develop a rapid and accurate method to identify D. officinale from its adulterants. The SCAR marker SHF/SHR developed in this study was able to amplify specific DNA fragments of a certain length in D. officinale samples, while no DNA bands were amplified in non-target Dendrobium species, which suggests that the SCAR marker can be used to distinguish D. officinale from other similar Dendrobium species. In addition, a minimum DNA template concentration of only 5 ng is sufficient for detection, which indicates that this method is highly sensitive.
Conclusion
A specific DNA sequence, SCoT13-215, was obtained for D. officinalis, and the SCAR primer pair SHF/SHR was developed for species identification of D. officinalis in this study. The SCAR marker was proven to be a potential tool for the rapid, effective, and reliable determination of D. officinale, which may be highly useful for ensuring the quality of medicinal preparations and protecting this valuable medicinal species’ germplasm.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
JL and SF conceived and designed the experiments, participated in the analysis, and drafted the manuscript. KZ, YC, and YG performed the experiments. JJ performed the statistical analysis. WC and HW collected the plant samples. All authors read and approved the final manuscript.
Funding
This work was funded by the Zhejiang Provincial Natural Science Foundation of China (LY20H280012); the Hangzhou Scientific and Technological Program of China (20191203B02); the National Natural Science Foundation of China (81503185); the key project at the central government level for the ability establishment of sustainable use for valuable Chinese medicine resources (2060302); the Zhejiang Provincial Key Research and Development Project Grants (2018C02030); and the college students’ science and technology innovation project of Zhejiang (2020R427072).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Albani, M. C., Battey, N. H., and Wilkinson, M. J. (2004). The development of ISSR-derived SCAR markers around the SEASONAL FLOWERING LOCUS (SFL) in Fragaria vesca. Theor. Appl. Genet. 109, 571–579. doi: 10.1007/s00122-004-1654-4
Cheng, J., Dang, P. P., Zhao, Z., Yuan, L. C., Zhou, Z. H., Wolf, D., et al. (2019). An assessment of the Chinese medicinal Dendrobium industry: supply, demand and sustainability. J. Ethnopharmacol. 229, 81–88. doi: 10.1016/j.jep.2018.09.001
Collard, B. C. Y., and Mackill, D. J. (2009). Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 27, 86–93. doi: 10.1007/s11105-008-0060-5
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the People’s Republic of China, 2015 Edn, Vol. part I. Beijing: China medical science and technology press, 282.
Devaiah, K. M., and Venkatasubramanian, P. (2008). Genetic characterization and authentication of Embelia ribes using RAPD-PCR and SCAR marker. Planta Med. 74, 194–196. doi: 10.1055/s-2008-1034279
Ding, G., Li, X., Ding, X., and Qian, L. (2009). Genetic diversity across natural populations of Dendrobium officinale, the endangered medicinal herb endemic to China, revealed by ISSR and RAPD markers. Russ. J. Genet. 45, 327–334. doi: 10.1134/S1022795409030119
Ding, G., Zhang, D. Z., Ding, X. Y., Zhou, Q., Zhang, W. C., and Li, X. X. (2008). Genetic variation and conservation of the endangered Chinese endemic herb Dendrobium officinale based on SRAP analysis. Plant Syst. Evol. 276, 149–156. doi: 10.1007/s00606-008-0068-1
Ding, X., Wang, Z., Zhou, K., Xu, L., Xu, H., and Wang, Y. (2003). Allele-specific primers for diagnostic PCR authentication of Dendrobium officinale. Planta Med. 69, 587–588. doi: 10.1055/s-2003-40651
Feng, S., He, R., Yang, S., Chen, Z., Jiang, M., Lu, J., et al. (2015a). Start codon targeted (SCoT) and target region amplification polymorphism (TRAP) for evaluating the genetic relationship of Dendrobium species. Gene 567, 182–188. doi: 10.1016/j.gene.2015.04.076
Feng, S., Jiang, Y., Wang, S., Jiang, M., Chen, Z., Ying, Q., et al. (2015b). Molecular identification of Dendrobium species (Orchidaceae) based on the DNA barcode ITS2 region and its application for phylogenetic study. Int. J. Mol. Sci. 16, 21975–21988. doi: 10.3390/ijms160921975
Feng, S., Jiao, K., Guo, H., Jiang, M., Hao, J., Wang, H., et al. (2017). Succinyl-proteome profiling of Dendrobium officinale, an important traditional Chinese orchid herb, revealed involvement of succinylation in the glycolysis pathway. BMC Genomics 18:598. doi: 10.1186/s12864-017-3978-x
Feng, S., Zhao, H., Lu, J., Liu, J., Shen, B., and Wang, H. (2013). Preliminary genetic linkage maps of Chinese herb Dendrobium nobile and D. moniliforme. J. Genet. 92, 205–212. doi: 10.1007/s12041-013-0246-y
Feng, S., Zhu, Y., Yu, C., Jiao, K., Jiang, M., Lu, J., et al. (2018). Development of species-specific SCAR markers, based on a SCoT analysis, to authenticate physalis (Solanaceae) species. Front. Genet. 9:192. doi: 10.3389/fgene.2018.00192
Hao, J., Jiao, K., Yu, C., Guo, H., Zhu, Y., Yang, X., et al. (2018). Development of SCoT-Based SCAR marker for rapid authentication of taxus media. Biochem. Genet. 56, 255-266. doi: 10.1007/s10528-018-9842-0
Igwe, D. O., Afiukwa, C. A., Ubi, B. E., Ogbu, K. I., Ojuederie, O. B., and Ude, G. N. (2017). Assessment of genetic diversity in Vigna unguiculata L. (Walp) accessions using inter-simple sequence repeat (ISSR) and start codon targeted (SCoT) polymorphic markers. BMC Genet. 18:98. doi: 10.1186/s12863-017-0567-6
Jalilian, H., Zarei, A., and Erfani-Moghadam, J. (2018). Phylogeny relationship among commercial and wild pear species based on morphological characteristics and SCoT molecular markers. Sci. Hortic. 235, 323–333. doi: 10.1016/j.scienta.2018.03.020
Ji, J. J., Huang, W., Yin, Y. X., Li, Z., and Gong, Z. H. (2014). Development of a SCAR marker for early identification of S-cytoplasm based on mitochondrial SRAP analysis in pepper (Capsicum annuum L.). Mol. Breed. 33, 679–690. doi: 10.1007/s11032-013-9984-z
Jiang, Q. T., Liu, L., Xiao, B. Y., Li, W. L., Luo, H. M., Nie, P., et al. (2018). Panax ginseng-specific sequence characterized amplified region (SCAR) marker for testing medicinal products. J. Cent. South Univ. 25, 1052–1062. doi: 10.1007/s11771-018-3805-9
Jin, X. H., and Huang, L. Q. (2015). Investigation of original materials of Chinese medicine “Shihu” and ”Tiepishihu”. Zhongguo Zhong Yao Za Zhi 40, 2475–2479.
Julio, E., Verrier, J. L., and Dorlhac De Borne, F. (2006). Development of SCAR markers linked to three disease resistances based on AFLP within Nicotiana tabacum L. Theor. Appl. Genet. 112, 335–346. doi: 10.1007/s00122-005-0132-y
Khan, M. A., Cheng, J. L., Mei, Z. Q., Wei, C. L., and Fu, J. J. (2016). Development of two novel specific SCAR markers by cloning improved RAPD fragments from the medicinal mushroom Ganoderma lucidium (Leysser: Fr) Karst. Genet. Mol. Res. 15:gmr.15038536. doi: 10.4238/gmr.15038536
Khosravi, A. R., Kadir, M. A., Kadzemin, S. B., Zaman, F. Q., and De Silva, A. E. (2009). RAPD analysis of colchicine induced variation of the Dendrobium Serdang beauty. Afr. J. Biotechnol. 8, 1455–1465.
Kumar, A., Rodrigues, V., Mishra, P., Baskaran, K., Shukla, A. K., Shasany, A. K., et al. (2018). ISSR-Derived species-specific SCAR marker for rapid and accurate authentication of Ocimum tenuiflorum L. Planta Med. 84, 117–122. doi: 10.1055/s-0043-116853
Lalitha, S. (2000). Primer premier 5. Biotech Softw. Intern. Rep. Comput. Softw. J. Scient 1, 270–272.
Li, L., Yao, H., Li, X., Zhang, Q., Wu, X., Wong, T., et al. (2019). Destiny of Dendrobium officinale polysaccharide after oral administration: indigestible and nonabsorbing, ends in modulating gut microbiota. J. Agric. Food Chem. 67, 5968–5977. doi: 10.1021/acs.jafc.9b01489
Li, X., Ding, X., Chu, B., Zhou, Q., Ding, G., and Gu, S. (2008). Genetic diversity analysis and conservation of the endangered Chinese endemic herb Dendrobium officinale Kimura et Migo (Orchidaceae) based on AFLP. Genetica 133, 159–166. doi: 10.1007/s10709-007-9196-8
Lu, J., Liu, Y., Xu, J., Mei, Z., Shi, Y., Liu, P., et al. (2018). High-Density genetic map construction and stem total polysaccharide content-related QTL exploration for Chinese endemic Dendrobium (Orchidaceae). Front. Plant Sci. 9:398. doi: 10.3389/fpls.2018.00398
Lu, J. J., Kang, J. Y., Feng, S. G., Zhao, H. Y., Liu, J. J., and Wang, H. Z. (2013). Transferability of SSR markers derived from Dendrobium nobile expressed sequence tags (ESTs) and their utilization in Dendrobium phylogeny analysis. Sci. Hortic. 158, 8–15. doi: 10.1016/j.scienta.2013.04.011
Lu, J. J., Wang, S., Zhao, H. Y., Liu, J. J., and Wang, H. Z. (2012). Genetic linkage map of EST-SSR and SRAP markers in the endangered Chinese endemic herb Dendrobium (Orchidaceae). Genet. Mol. Res. 11, 4654–4667. doi: 10.4238/2012.December.21.1
Luo, C. P., He, T., and Chun, Z. (2013). Discrimination and chemical phylogenetic study of seven species of Dendrobium using infrared spectroscopy combined with cluster analysis. J. Mol. Struct. 1037, 40–48. doi: 10.1016/j.molstruc.2012.10.048
Marieschi, M., Torelli, A., Beghe, D., and Bruni, R. (2016). Authentication of Punica granatum L.: development of SCAR markers for the detection of 10 fruits potentially used in economically motivated adulteration. Food Chem. 202, 438–444. doi: 10.1016/j.foodchem.2016.02.011
Moon, B. C., Ji, Y., Lee, Y. M., Kang, Y. M., and Kim, H. K. (2015). Authentication of Akebia quinata D-ECNE. from its common adulterant medicinal plant species based on the RAPD-derived SCAR markers and multiplex-PCR. Genes Genom. 37, 23–32. doi: 10.1007/s13258-014-0225-6
Moudi, M., and Go, R. (2017). Morphological study of four sections of genus Dendrobium Sw. (Orchidaceae) in Peninsular Malaysia. Pakistan J. Bot. 49, 569–577.
Mulpuri, S., Muddanuru, T., and Francis, G. (2013). Start codon targeted (SCoT) polymorphism in toxic and non-toxic accessions of Jatropha curcas L. and development of a codominant SCAR marker. Plant Sci. 207, 117–127. doi: 10.1016/j.plantsci.2013.02.013
Rajesh, M. K., Sabana, A. A., Rachana, K. E., Rahman, S., Ananda, K. S., and Karun, A. (2016). Development of a SCoT-derived SCAR marker associated with tall-type palm trait in arecanut and its utilization in hybrid (dwarf x tall) authentication. Indian J. Genet. Plant Breed. 76, 119–122. doi: 10.5958/0975-6906.2016.00019.5
Shen, J., Ding, X., Liu, D., Ding, G., He, J., Li, X., et al. (2006). Intersimple sequence repeats (ISSR) molecular fingerprinting markers for authenticating populations of Dendrobium officinale Kimura et Migo. Biol. Pharm. Bull. 29, 420–422. doi: 10.1248/bpb.29.420
Tang, H., Zhao, T., Sheng, Y., Zheng, T., Fu, L., and Zhang, Y. (2017). Dendrobium officinale Kimura et Migo: a review on its ethnopharmacology, phytochemistry, pharmacology, and Industrialization. Evid. Based Complement. Alternat. Med. 2017:7436259. doi: 10.1155/2017/7436259
Teixeira da Silva, J. A., Jin, X. H., Dobránszki, J., Lu, J. J., Wang, H. Z., Zotz, G., et al. (2016). Advances in Dendrobium molecular research: applications in genetic variation, identification and breeding. Mol. Phylogenet. Evol. 95, 196–216. doi: 10.1016/j.ympev.2015.10.012
Tsi, Z. H., Chen, S. C., Luo, Y. B., and Zhu, G. H. (1999). Orchidaceae (3). Flora Republicae Popularis Sinicae. Beijing: Science Press.
Vinay, K. (2016). Rapid molecular authentication of Ashoka [Saraca asoca (Roxb.), de Wild], vulnerable medicinal plant species and its adulterant Polyalthia longifolia Benth by the development of SCAR markers and multiplex-PCR. Res. J. Biotechnol. 11, 59–68.
Wahba, L. E., Hazlina, N., Fadelah, A., and Ratnam, W. (2014). Genetic relatedness among Dendrobium (Orchidaceae) species and hybrids using morphological and AFLP markers. Hortscience 49, 524–530. doi: 10.21273/HORTSCI.49.5.524
Wang, H. Z., Feng, S. G., Lu, J. J., Shi, N. N., and Liu, J. J. (2009). Phylogenetic study and molecular identification of 31 Dendrobium species using inter-simple sequence repeat (ISSR) markers. Sci. Hortic. 122, 440–447. doi: 10.1016/j.scienta.2009.06.005
Wei, W., Feng, L., Bao, W. R., Ma, D. L., Leung, C. H., Nie, S. P., et al. (2016). Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 64, 881–889. doi: 10.1021/acs.jafc.5b05180
Xue, D. W., Feng, S. G., Zhao, H. Y., Jiang, H., Shen, B., Shi, N. N., et al. (2010). The linkage maps of Dendrobium species based on RAPD and SRAP markers. J. Genet. Genom. 37, 197–204. doi: 10.1016/S1673-8527(09)60038-2
Yang, X. D., Wu, Y. Y., Su, J. S., Ao, N., Guan, Z. Y., Jiang, J. F., et al. (2019). Genetic variation and development of a SCAR marker of anemone-type flower in chrysanthemum. Mol. Breed. 39:48. doi: 10.1007/s11032-019-0958-7
Ye, Z., Dai, J. R., Zhang, C. G., Lu, Y., Wu, L. L., Gong, A. G. W., et al. (2017). Chemical differentiation of Dendrobium officinale and Dendrobium devonianum by Using HPLC Fingerprints, HPLC-ESI-MS, and HPTLC Analyses. Evid. Based Complement. Alternat. Med. 2017:8647212. doi: 10.1155/2017/8647212
Zhang, C., Mei, Z., Cheng, J., He, Y., Khan, M. A., Luo, P., et al. (2015). Development of SCAR markers based on improved RAPD amplification fragments and molecular cloning for authentication of herbal medicines Angelica sinensis, Angelica acutiloba and Levisticum officinale. Nat. Prod. Commun. 10, 1743–1747. doi: 10.1177/1934578X1501001027
Zhao, Y., Han, B., Peng, H., Wang, X., Chu, S., Dai, J., et al. (2017). Identification of “Huoshan shihu” Fengdou: comparative authentication of the Daodi herb Dendrobium huoshanense and its related species by macroscopic and microscopic features. Microsc. Res. Tech. 80, 712–721. doi: 10.1002/jemt.22856
Zhao, Y., Zha, L., Han, B., and Peng, H. (2018). Compare the microscopic characteristics of stems of the 24 Dendrobium species utilized in the traditional Chinese medicine “Shihu”. Microsc. Res. Tech. 81, 1191–1202. doi: 10.1002/jemt.23117
Keywords: Dendrobium officinale, DNA markers, SCoT, SCAR marker, species identification
Citation: Zheng K, Cai Y, Chen W, Gao Y, Jin J, Wang H, Feng S and Lu J (2021) Development, Identification, and Application of a Germplasm Specific SCAR Marker for Dendrobium officinale Kimura et Migo. Front. Plant Sci. 12:669458. doi: 10.3389/fpls.2021.669458
Received: 18 February 2021; Accepted: 20 April 2021;
Published: 14 May 2021.
Edited by:
Jian Li Yang, Zhejiang University, ChinaReviewed by:
Xiaoyu Ding, Nanjing Normal University, ChinaMohamed A. M. Atia, Agricultural Research Center, Egypt
Altaf Ahmad, Aligarh Muslim University, India
Copyright © 2021 Zheng, Cai, Chen, Gao, Jin, Wang, Feng and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shangguo Feng, c2hhbmdndW8wMDdAMTI2LmNvbQ==; Jiangjie Lu, bHVqakBoem51LmVkdS5jbg==
 Kaixin Zheng1,2
Kaixin Zheng1,2 Huizhong Wang
Huizhong Wang Shangguo Feng
Shangguo Feng Jiangjie Lu
Jiangjie Lu