
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 15 July 2021
Sec. Plant Abiotic Stress
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.657960
This article is part of the Research TopicSalt Tolerance: Molecular and Physiological Mechanisms and Breeding ApplicationsView all 27 articles
KARRIKINS INSENSITIVE2 (KAI2) is the receptor gene for karrikins, recently found to be involved in seed germination, hypocotyl development, and the alleviation of salinity and osmotic stresses. Nevertheless, whether KAI2 could regulate cold tolerance remains elusive. In the present study, we identified that Arabidopsis mutants of KAI2 had a high mortality rate, while overexpression of, a bioenergy plant, Sapium sebiferum KAI2 (SsKAI2) significantly recovered the plants after cold stress. The results showed that the SsKAI2 overexpression lines (OEs) had significantly increased levels of proline, total soluble sugars, and total soluble protein. Meanwhile, SsKAI2 OEs had a much higher expression of cold-stress-acclimation-relate genes, such as Cold Shock Proteins and C-REPEAT BINDING FACTORS under cold stress. Moreover, the results showed that SsKAI2 OEs were hypersensitive to abscisic acid (ABA), and ABA signaling genes were w significantly affected in SsKAI2 OEs under cold stress, suggesting a potential interaction between SsKAI2 and ABA downstream signaling. In SsKAI2 OEs, the electrolyte leakage, hydrogen peroxide, and malondialdehyde contents were reduced under cold stress in Arabidopsis. SsKAI2 OEs enhanced the anti-oxidants like ascorbate peroxidase, catalase, peroxidase, superoxide dismutase, and total glutathione level under cold stress. Conclusively, these results provide novel insights into the understanding of karrikins role in the regulation of cold stress adaptation.
Plants are the sessile organisms which often exposed to a broad range of adverse environmental conditions. Among a large number of adverse conditions, cold (chilling and freezing) stress significantly limit crop growth and agricultural productivity. Under cold conditions, plants activate their cold resistance mechanism called cold acclimation (Thomashow, 1999; Stockinger et al., 2001; Shi et al., 2014b). Cold acclimation enhances the endogenous as well as inducible components accumulation. The endogenous components, which promote cold tolerance, have extensively been studied and mainly refer to metabolites with anti-oxidant activity (Winkel-Shirley, 2002), with hormonal responses (Eremina et al., 2016) or osmoprotective functions to limit ice nucleation and to overcome the freeze-induced dehydration inside the plant cells (Janská et al., 2010). Furthermore, other regulatory molecules such as polyamines, reactive oxygen species, nitric oxide have also been described to be involved in cold tolerance (Cuevas et al., 2008; Zhao et al., 2009; Puyaubert and Baudouin, 2014; van Buer et al., 2016).
Karrikins, a group of chemical compounds, are present in burnt or charred plant material and its smoke. Karrikins are also produced by the pyrolysis of cellulose and simple sugars (Flematti et al., 2011). To date, natural origin within the plant has not discovered. Karrikins are potent promoters of seed germination of various plants (Flematti et al., 2004). Karrikins promote photomorphogenesis in seedling and negatively regulate the hypocotyl elongation (Nelson et al., 2010). Karrikins inhibited the hypocotyl length under red light, and the length of Arabidopsis thaliana seedlings hypocotyl treated with one micromolar KAR2 was almost half of the hypocotyl of untreated Arabidopsis thaliana seedlings (Nelson et al., 2010; Waters and Smith, 2013). It has also been reported that karrikins might regulate cotyledon expansion and chlorophyll accumulation in the seedlings of Brassica tournefourtii and Lactuca sativa (Nelson et al., 2010).
Recently, karrikins role against abiotic stresses has also been discovered. For example, it has been found that karrikins might play an essential role in the chilling response of tea plants (Zhao et al., 2012). In tomato, seed primed in butenolide (a karrikin) produced significantly more vigorous seedlings than the water-primed seeds. Vigor indices of seedlings produced by butenolide-primed seeds were significantly higher under different abiotic stresses conditions (salinity, temperature, or osmoticum) compared to control or water-primed seeds (Neeru and Van, 2007). In a bioenergy plant, Sapium sebiferum, KAR1 has been reported to alleviate osmotic and salinity stresses by regulating redox homeostasis (Shah et al., 2020). KARRIKINS INSENSITIVE2 (KAI2), which encodes an α/β-fold hydrolase, is a receptor gene for karrikins (Scaffidi et al., 2012; Li et al., 2013). Hydrophobic pocket in KAI2 has a conserved catalytic triad (Ser–His–Asp) (Kagiyama et al., 2013) and, KAI2 also has a hydrolyzes pocket (Hamiaux et al., 2012), which may bind to the karrikins (Boyer et al., 2012; Hamiaux et al., 2012). KAI2 was reported to be involved in the stomatal closure, regulation of cuticle formation, membrane integrity, and anthocyanin biosynthesis, which contributes to plant alleviation to the osmotic stress (Li et al., 2017). Recently, it has been reported that the karrikins-KAI2 signaling system provided stress tolerance by inhibiting germination in Arabidopsis under unfavorable conditions (Wang et al., 2018).
In this study, the homologous gene of SsKAI2 was identified in an ornamental and bio-energetic woody perennial plant Sapium sebiferum and characterized in Arabidopsis thaliana under cold stress. After finding the SsKAI2 alleviation of the cold stress tolerance in Arabidopsis, we conducted several experiments to find out the possible mechanism in the SsKAI2 improved Arabidopsis under cold stress.
Full sequance of SsKAI2 was found by local blasting amino acids sequance of Arabidopsis KAI2 in Blast-2.2.31. S. sebiferum flower-bud transcriptome (Yang et al., 2015) (Accession: SRX656554)1 was used to built a local blast library. Bio-informatics analysis of SsKAI2 is given in Supplementary Figure 1. The full cDNA sequence of all genes with the translated amino acid sequence is given in Supplementary Data Sheet 1. The full-length open reading frame (ORF) of the SsKAI2 gene was found by the NCBI ORF finding tool. Neighbor-Joining method was used to built the evolutionary history (Saitou and Nei, 1987). The bootstrap consensus tree built from 500 replicates (Felsenstein, 1985) is representing the taxa evolutionary history. Branches matching to partitions reproduced in <50% bootstrap replicates were distorted. The evolutionary distances were calculated by using the p-distance method (Nei and Kumar, 2000) and were represented in the number of amino acid differences per site. The analysis involved 30 amino acid sequences. All positions containing missing data or gaps were excluded. In the final dataset, there were 249 positions. Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016).
Gene-specific primers were designed by Primer Premier 5 to amplify the full-length ORF of SsKAI2 (Supplementary Table 1). The ORF of the SsKAI2 gene was sequenced from Sangon Biotech (Shanghai) Co., Ltd. Cloned gene sequence double digested at Sal1 from start and Sma1 from stop codon site. Full-length ORF of SsKAI2 was inserted into the expression vector pOCA30 under the control of the CaMV35S promoter, and the resulting 35S:SsKAI2 plasmid was transformed into the Agrobacteria EHA105 strain. The floral dip method was performed for the transformation of the recombinant expression vector in Arabidopsis. Atkai2 mutants, previously described in Waters et al. (2012), were gifted by Dr. Jiayang Li from the Chinese Academy of Sciences.
Sapium sebiferum seedlings were established by our previously developed method (Shah et al., 2018). The seed of the Arabidopsis Columbia-0 (Col-0) genotype was obtained from the Arabidopsis Biological Resources Center (Columbus, OH, United States). SsKAI2 was identified, cloned, transformed to Arabidopsis, and homozygous SsKAI2 OEs lines were selected for further experiments. Seeds of wild-type and SsKAI2 OEs were surfaces sterilized with 70% (v/v) ethanol for 2 min, then incubated in 10% (v/v) sodium hypochlorite (NaClO) for 10 min at room temperature, and washed thrice with double distilled water. The sterilized seeds were plated on ½ Murashige and Skoog (MS) medium supplemented with 1% (w/v) sucrose and 0.8% (w/v) agar and placed at 4 degrees Celsius (°C) for 2 days. Seeds were germinated in a growth room 16/8 h (day/night) photoperiod at 22°C. Seven-day-old Arabidopsis seedlings were transferred from ½ MS medium to the soil and grown in a chamber at 22°C, with 16/8 h (long-day conditions) photoperiods, approximately 120 μmol/m2/s radiation strength, and 75% humidity.
Cold resistant plants have developed a coping mechanism called cold acclimation (Thomashow, 1999). Cold acclimation mechanism includes the accumulation of soluble sugars (Guy and Huber, 1992), and proline (Verbruggen and Hermans, 2008), stimulation of antioxidants activity, and changes in the plant transcriptome and proteome (Thomashow, 1999; Zuther et al., 2019). Cold acclimation makes the plants ready in low temperatures to face the upcoming freezing temperatures. So, for phenotypical analysis under cold stress, 5-day-old Arabidopsis seedlings were cold acclimatized to 4°C for 12 h and then subjected to cold treatment at −20°C for an hour. The plants were again kept at 4°C for 12 h, the plants were then shifted to a plant growth room with a 16/8 h photoperiod at 22°C, approximately 120 μmol photons/m2/s, and 75% humidity. The recovery rate was measured 10 days after the cold-shock treatment. Photographs were taken by a Nikon D90 having Nikon DX AF-S NIKKOR 18-105 mm lens (Nikon Corporation, Tokyo, Japan).
Electrolyte leakage was determined by the previously reported method in the study of Nishiyama et al. (2011). In detail, after placing 15-day-old plants at 0–, – 4−, – 8−, – 12−, – 16−, and −20°C for an hour, five leaves of different plants of each genotype were collected, then plant samples were shifted to the 50 mL tubes containing 40 mL of double distilled water for 24 h. The electric conductivity (EC) of water was determined by the electric conductivity meter. The tubes having 40 ml of water were autoclaved for 20 min at 121°C, and the EC was measured again. The following equation calculated the percentage of electrolyte leakage.
For biochemical analysis, each line of every genotype was subjected to cold acclimation temperature (4°C). The samples were randomly taken from the leaves of five plants of each treatment after 0 (control at 22°C), 3−, 6−, and 12 h of cold treatment (4°C). Samples were immediately frozen in liquid nitrogen, the stored in to −80°C. The total proline, total soluble sugar (TSS), total soluble protein (TSP), hydrogen peroxide (H2O2), malondialdehyde (MDA), total glutathione (GSH), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) contents were determined by using a Proline assay kit, a plant soluble sugar content test kit, a total protein quantitative assay kit, an H2O2 assay kit, an MDA assay kit, a T-GSH assay kit, a POD assay kit, a SOD assay kit, an APX assay kit, and a CAT assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, as previously described by Ni et al. (2018).
Epidermal peels from mature leaves removed with forceps and were incubated in MES/KCl (2-(N-morpholino) ethanesulfonic acid/potassium chloride) buffer supplemented with 0, 10, 30, and 50 μM ABA for 2 h. Stomata were visualized under an epifluorescent microscope using 100× lenses (Eisele et al., 2016). Stomatal aperture was measured by analyzing pictures in ImageJ 1.52a.
Fifteen-day-old Sapium sebiferum seedlings were subjected to cold stress (4°C), salt stress (200mM NaCl), and osmotic stress (300mM mannitol). The samples were randomly taken from the leaves of five plants of each treatment after 0 h (control at 22°C), 3−, 6−, and 12 h of. Samples were immediately frozen in liquid nitrogen, and stored in to −80°C. S. sebiferum flower-bud transcriptome (Accession: SRX656554, see text footnote 2) (Yang et al., 2015) was used to build the local blast library in blast-2.2.31. The full sequences of all genes were searched by local blasting Arabidopsis amino-acids sequence. Full-length mRNA sequences of SsKAI2 is available in Supplementary Data Sheet 1. Primers for quantitative real-time PCR were designed in primer premier 6, and a list of all primers is available in Supplementary Table 1. Expression of cold-acclimation-related genes, ABA-responsive genes under cold stress were investigated in each line after 0 h (control at 22°C), 3−, 6−, and 12 h of cold treatment (4°C). The samples were randomly taken from the leaves of five plants of each treatment. Samples were immediately frozen in liquid nitrogen, the stored in to −80°C. RNA from already frozen and stored samples at −80°C was extracted by E.Z.N.A® plant RNA extraction kit (Omega Bio-tek, Inc., Norcross, GA, United States) using the standard protocol. 500 nanograms of RNA of each sample was reverse transcribed by cDNA Synthesis SuperMix (TransGen Biotech., Shanghai, China) according to the standard protocol. Each cDNA sample was diluted 25 times with double distilled water. The reaction for RT–qPCR was prepared according to the standard protocol of QuantiNova SYBR Green PCR Master Mix (QIAGEN, Pudong, Shanghai, China) then run in the Light Cycler®96 (Roche Diagnostics, Indiana, United States). Following the program was set in qPCR: preheating, 95°C for 10 min; amplification (45 cycles) at 95°C for 10 s, at 60°C for 20 s, and 72°C for 20 s; melting curve at 95°C for 2 min, and at 60°C for 30 s, then continuously increased to 95°C. The 2–ΔΔCt method was used to calculate the relative gene expression, as described by Livak and Schmittgen (2001).
The statistical analyses were done in R Studio 1.1.442. All data were presented in the form of mean ± standard deviation. One-way analysis of variance (ANOVA) was used to test the significant difference between the treatments. The significant difference between the means of different treatments was determined by using the Tukey test at P < 0.05.
The SsKAI2 homolog with 77.8% sequence similarity with Atkai2 was identified from the S. sebiferum transcriptome database (Supplementary Figure 1A). Then, the phylogenetic analysis of the KAI2 protein sequences was carried out from more than 30 plant species (Supplementary Data Sheet 1). The results showed that SsKAI2 had the highest sequence identity with perennial woody plants, such as Jatropha caucus and Populus euphratica, which also belong to the Euphorbiaceae family (Figure 1A). We investigated the time-course expression pattern of SsKAI2 in response to the abiotic stresses (osmotic, salt, and cold) in the 25-day-old S. sebiferum seedlings. The results showed that the expression of KAI2 in Sapium sebiferum was increased under cold stress (4°C), salinity (200 mM NaCl), and osmotic stress (300mM mannitol) as compared to control condition (Figures 1B–D). These results suggested that KAI2 is a stress-responsive gene, which might have a role in the acclimation of abiotic stresses.
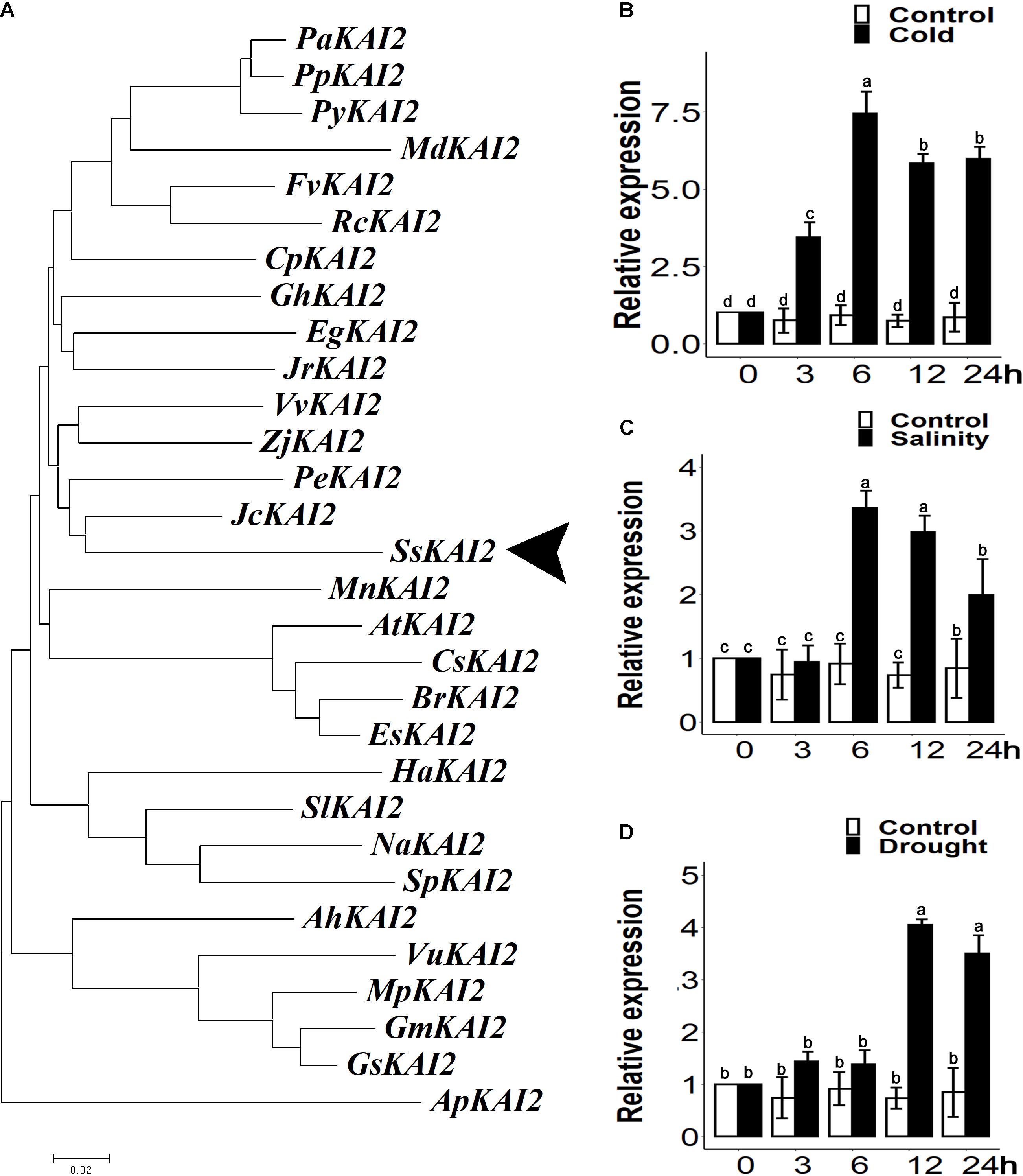
Figure 1. Phylogenetic analysis and relative expression of KAI2 in Sapium sebiferum under cold, salinity, and osmotic stresses. (A) Phylogenic analysis of SsKAI2 protein with its homologs from other species. (B) SsKAI2 relative expression under cold (4°C), salinity (200 mM NaCl) (C), and osmotic stress (300 mM mannitol) (D). Twenty-five-day-old seedlings were used to determine the SsKAI2 expression level under abiotic stresses. Leaf samples were collected after 3−, 6−, 12−, and 24 h of each treatment. Sapium sebiferum UBQ10 was used as a reference gene; control treatment at 0 h was considered as 1. qPCR was used to determine gene expression, one-way ANOVA was used to analyze all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). Bars with uncommon letters show significant difference at P < 0.05.
Cold stress is one of the unfavorable environmental factors that restrict plant growth and development and might cause mortality in the plant. Cold stress alters the structure of the cell membrane, which makes it leaky and results in the loss of ions that are essential for proper functioning in the cell (Uemura et al., 1995). The membrane injury in the plant exposed to cold temperatures is measured by the rises in electrical conductivity resulting from the leakage of the electrolyte from the plant tissues. Exogenous application of KAR1 showed 96 ± 3.3% survival rate in Arabidopsis under cold stress (Supplementary Figure 2). The results showed that SsKAI2 OEs, kai2 mutant, and wild-type Arabidopsis started wilting 2 h later cold-shock treatment. On the third day of post-cold-shock treatment, the plants started to regenerate new apical leaves. The recovery rate was recorded on the 10th day after treatment (Figure 2A). The results showed that two overexpression lines SsKAI2 OE1 and OE2 showed 90 ± 3% and 95 ± 2% recovery rate, respectively, while wild-type was 45 ± 10%, and kai2 was 30 ± 10% recovered after cold stress (Figure 2B). Further, we determined the electrolyte leakage of SsKAI2 OEs, kai2, and wild-type Arabidopsis after subjecting plants in 0−, – 4−, – 8−, – 12−, – 16−, and −20°C for an hour. The results showed that cold stress increased electrolyte leakage in SsKAI2 OEs, wild-type, and kai2 plants. Overall, the electrolyte leakage of SsKAI2 OEs was significantly lower than wild-type plants and kai2 plants (Figure 2C). These results are suggesting that KAI2 is involved in the regulation of cold stress alleviation.
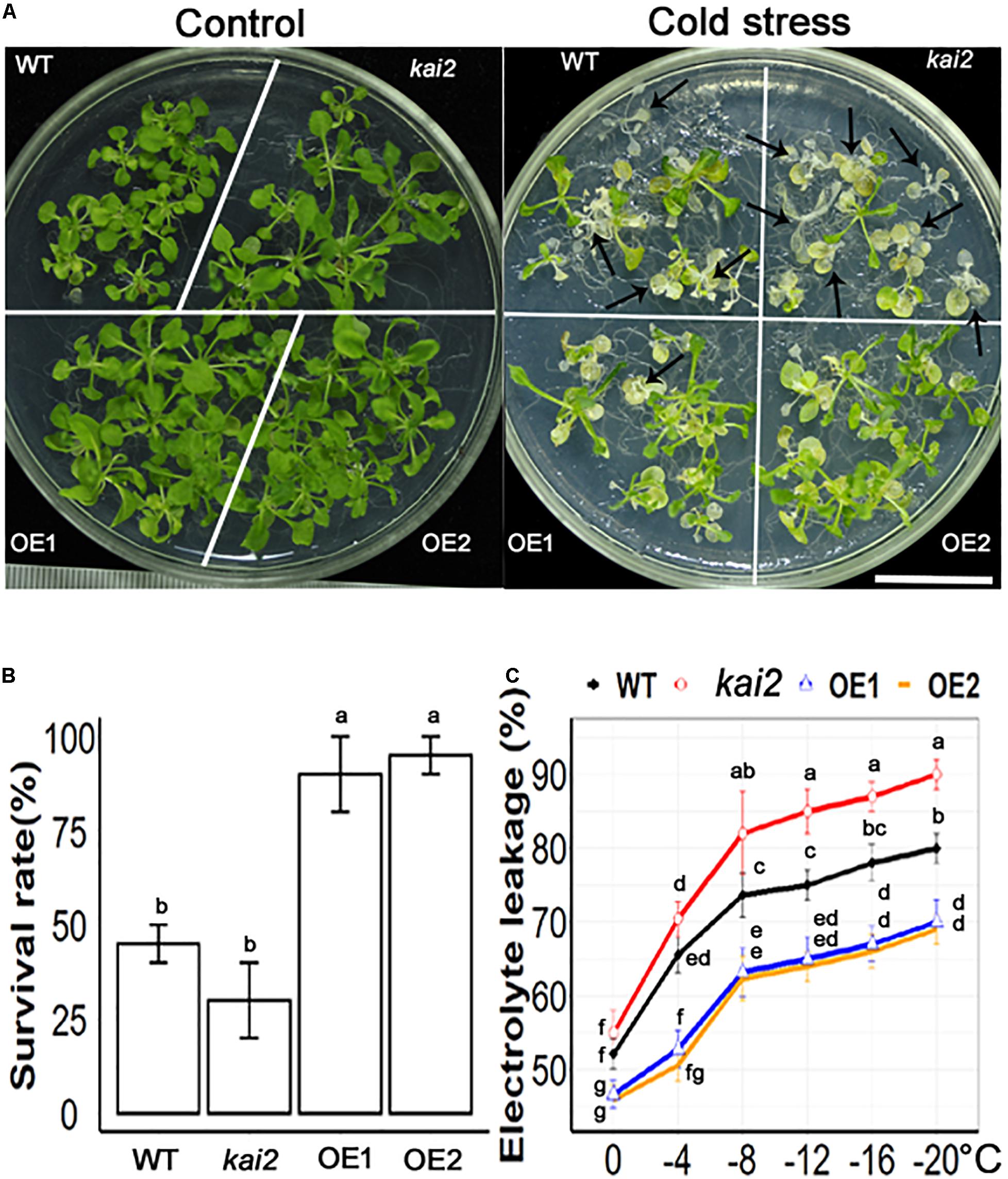
Figure 2. SsKAI2 alleviated cold stress in Arabidopsis. (A) Phenotypes of wild-type (WT), Atkai2, and SsKAI2 (OE1 and OE2) Arabidopsis at room temperature and under cold stress (−20°C). Pictures were taken after ten days after cold stress, white bar = 2cm. (B) Statistical presentation of survival rate after cold stress. (C) Electrolyte leakage was measured by subjecting 15-day-old plants at 0−, – 4−, – 8−, – 12−, – 16−, and −20°C for an hour. One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 5 in survival rate measurement and n = 3 in electrolyte leakage test). Bars or points with uncommon letters showing significant difference at P < 0.05.
Under abiotic stresses, the accumulation of the total soluble sugars is one of the primary acclimation symptoms. Then sugars modulate the expression of both abiotic and biotic stress-related genes in plants (Barau et al., 2015; Tarkowski and van den Ende, 2015). In this study, total soluble proteins (TSP), the total soluble sugars (TSS), and proline content in the leaves of different Arabidopsis lines were determined under cold stress. The results showed that the levels of TSS, TSP, and proline were all significantly increased in both SsKAI2 OEs in comparison with wild-type and kai2 mutant (Figures 3A,B). The results suggested that KAI2-regulated immediate induction of endogenous metabolites might play an important role in conferring the cold stress acclimation in Arabidopsis.
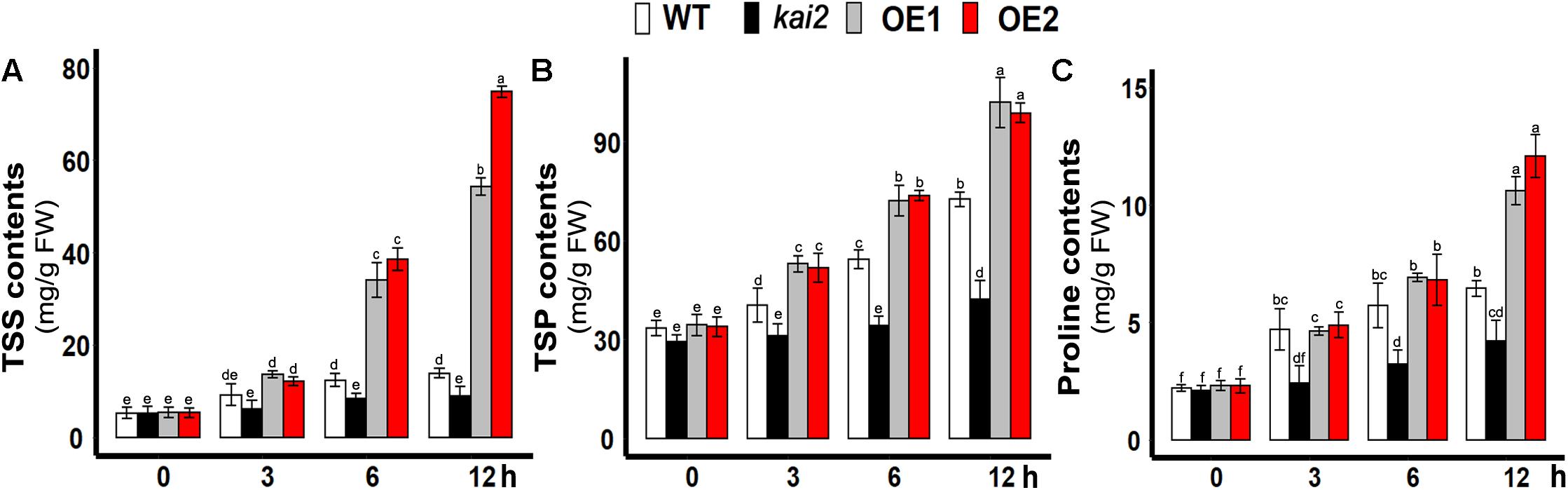
Figure 3. Overexpression of SsKAI2 promoted TSS and TSP under cold stress. (A) Total soluble sugars. (B) Total soluble protein contents. (C) Proline contents. Twenty-five-day-old plants were subjected to cold treatment. The samples were taken randomly from the leaves of three plants of each independent [(wild-type (WT), Atkai2, KAI2 over-expressed line 1 (OE1) and line 2 (OE2)] line after 3−, 6−, and 12 h under cold treatment (4°C). One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). An “h” at the X-axis of each graph represents time in hours under cold stress. Bars with uncommon letters showing significant difference at P < 0.05.
Like other abiotic stresses, cold stress can also increase the production of ROS in plants that can cause cellular oxidative damage when over-accumulated in cells (Karuppanapandian and Manoharan, 2008; Mafakheri et al., 2010). H2O2 is considered as a relatively long-lived molecule and moderately reactive, which can disseminate short distances away from its production site. H2O2 causes inactivation of enzymes by oxidizing their thiol groups. H2O2 enables it to diffuse the damage and also act as a messenger in the stress signaling response and thus can travel freely across membranes (Møller et al., 2007). ROS can cause oxidation of membrane lipids and degrade the cell membrane while MDA has been reported as an end product of lipid peroxidation, which is why MDA and H2O2 levels are markers of determining necrosis and cell damage in living organisms (MaBgorzata and Andrzej, 2016). In order to know whether the involvement of H2O2 in cold accumulation, we measured H2O2 content in SsKAI2 OEs, Atkai2, and WT under cold stress. The results showed that cold stress induced a significant increase of H2O2 content in the WT, while in the SsKAI2 OEs, the H2O2 content was not significantly increased in response to the cold stress (Figure 4A). Meanwhile, the results also revealed that the kai2 mutant had a higher increase of H2O2 level in comparison with WT after 6 h by cold treatment. An end product of lipid peroxidation, MDA, is a biochemical marker for the measurement of cell epidermal layer degradation. MDA level was increased in kai2 and wild-type Arabidopsis under cold stress, but on the other hand, SsKAI2 OEs had a decreased level of MDA contents with time under cold stress (Figure 4B). These results demonstrated that the stress-induced accumulation of H2O2 is strictly regulated by KAI2, which further led to enhanced stress tolerance in Arabidopsis.
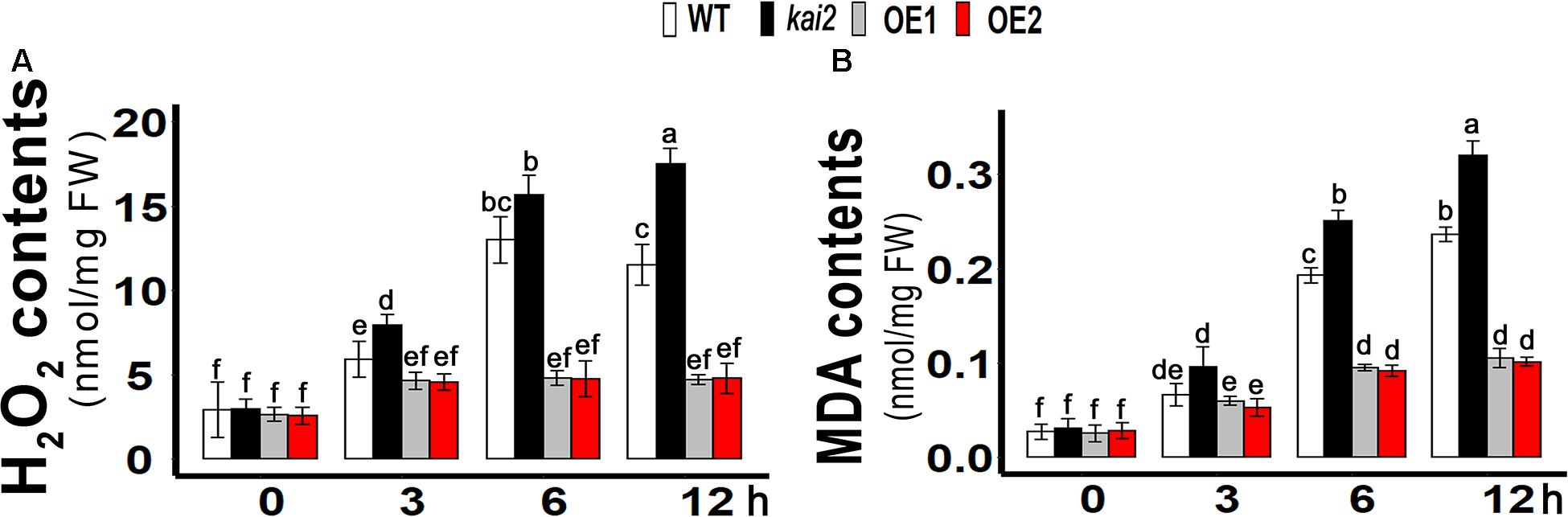
Figure 4. Overexpression of KAI2 in Arabidopsis reduced H2O2 and MDA levels under cold stress. (A) H2O2 contents. (B) MDA contents. The samples were taken randomly from the leaves of three plants of each independent (WT, Atkai2, OE1, and OE2) line after 0−, 3−, 6−, and 12 h under cold treatment (4°C). One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). Bars with uncommon letters showing significant difference at P < 0.05. An “h” at the X-axis of each graph represents time in hours under cold stress.
Ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) are the key enzymatic anti-oxidants which prevent the cell necrosis by scavenging ROS and alleviate oxidative stress. We further investigated the enzymatic anti-oxidants level of different Arabidopsis lines under cold stress. In this study, we demonstrated that the SOD activity was significantly higher in SsKAI2 OEs after six and 12 h of cold stress, but it was much lower in kai2 mutant in comparison with wild-type and SsKAI2 OEs during each time point of cold stress (Figure 5A). Nevertheless, under different time points of cold stress, the activity of other anti-oxidant enzymes, such as POD, CAT, and APX, was increased dramatically in SsKAI2 OEs (Figures 5B–D). Glutathione is a non-enzymatic anti-oxidant in the plant, which protects cellular damage from ROS under environmental stresses (Edwards et al., 2000). The results showed that SsKAI2 OEs could produce higher concentrations of T-GSH as compared to kai2 mutant and wild-type plants under cold stress (Figure 5E). These results suggested that KAI2 conferred cold stress via activating enzymatic and non-enzymatic anti-oxidant systems in Arabidopsis.
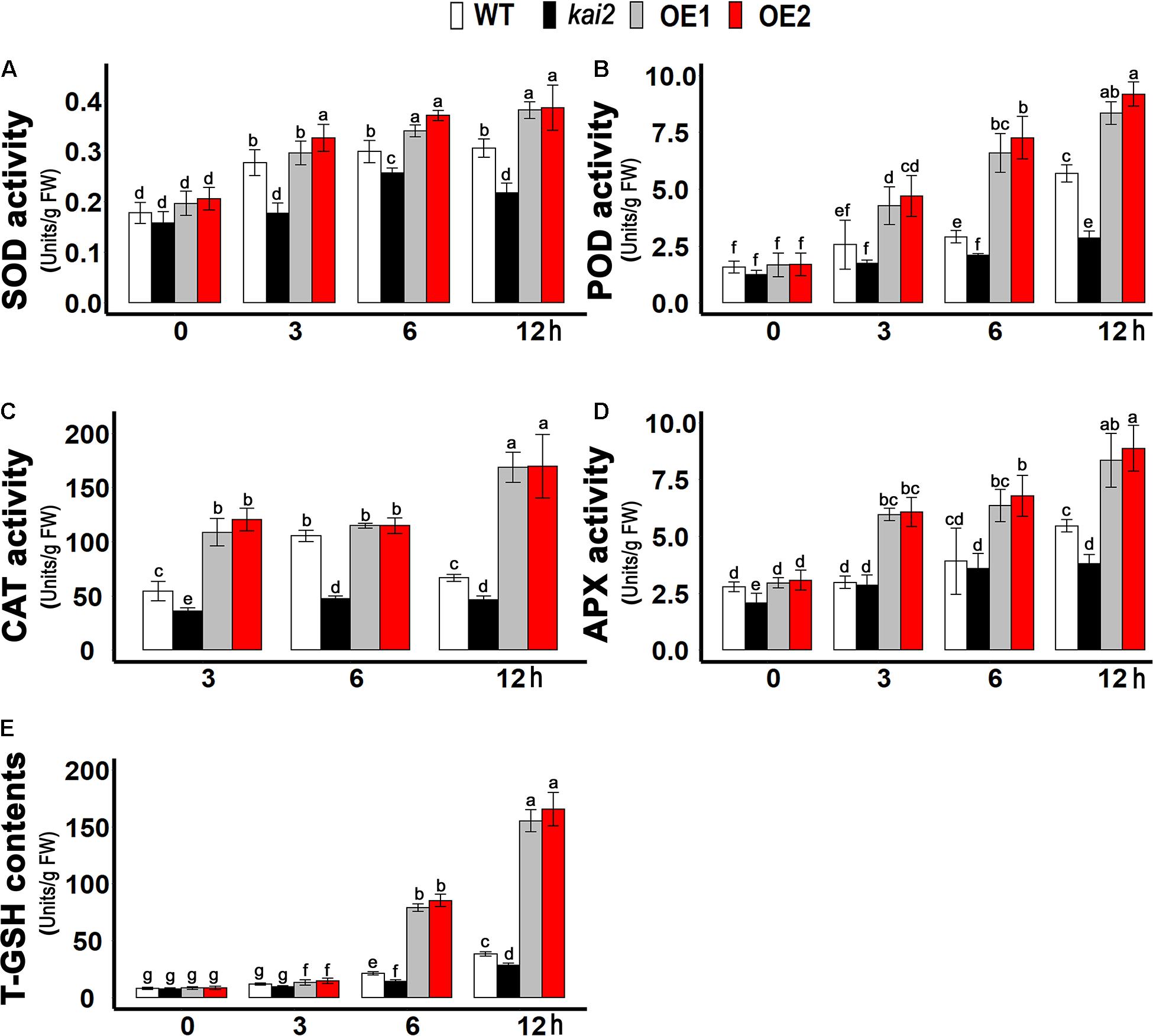
Figure 5. Anti-oxidants contents raised in SsKAI2 OEs under cold stress. (A) SOD. (B) POD. (C) CAT. (D) APX. (E) T-GSH. Twenty-five-day-old plants were subjected to cold treatment. The samples were randomly taken from the leaves of three plants of each genotype after 0−, 3−, 6−, and 12 h under cold treatment (4°C). One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). Bars with uncommon letters showing significant difference at P < 0.05.
During cold stress acclimation, cold-shock protein (CSP) genes, and C-repeat binding factors (CBFs) transcription factors are central regulators (Yamaguchishinozaki and Shinozaki, 1994; Chinnusamy et al., 2010). We found that the expression of all CSP genes was more significantly induced by cold treatment in the SsKAI2 OEs as compared to kai2 mutant and wild-type Arabidopsis (Figure 6A). Although the cold stress could significantly induce the expression of all CBF transcription factors in all the SsKAI2 OEs, the SsKAI2 OEs exhibited a much higher expression level than kai2 mutant and wild-type plants (Figure 6B). These results suggested that the KAI2 could potentially target the CSPs and CBFs in the regulation of cold acclimation in Arabidopsis.
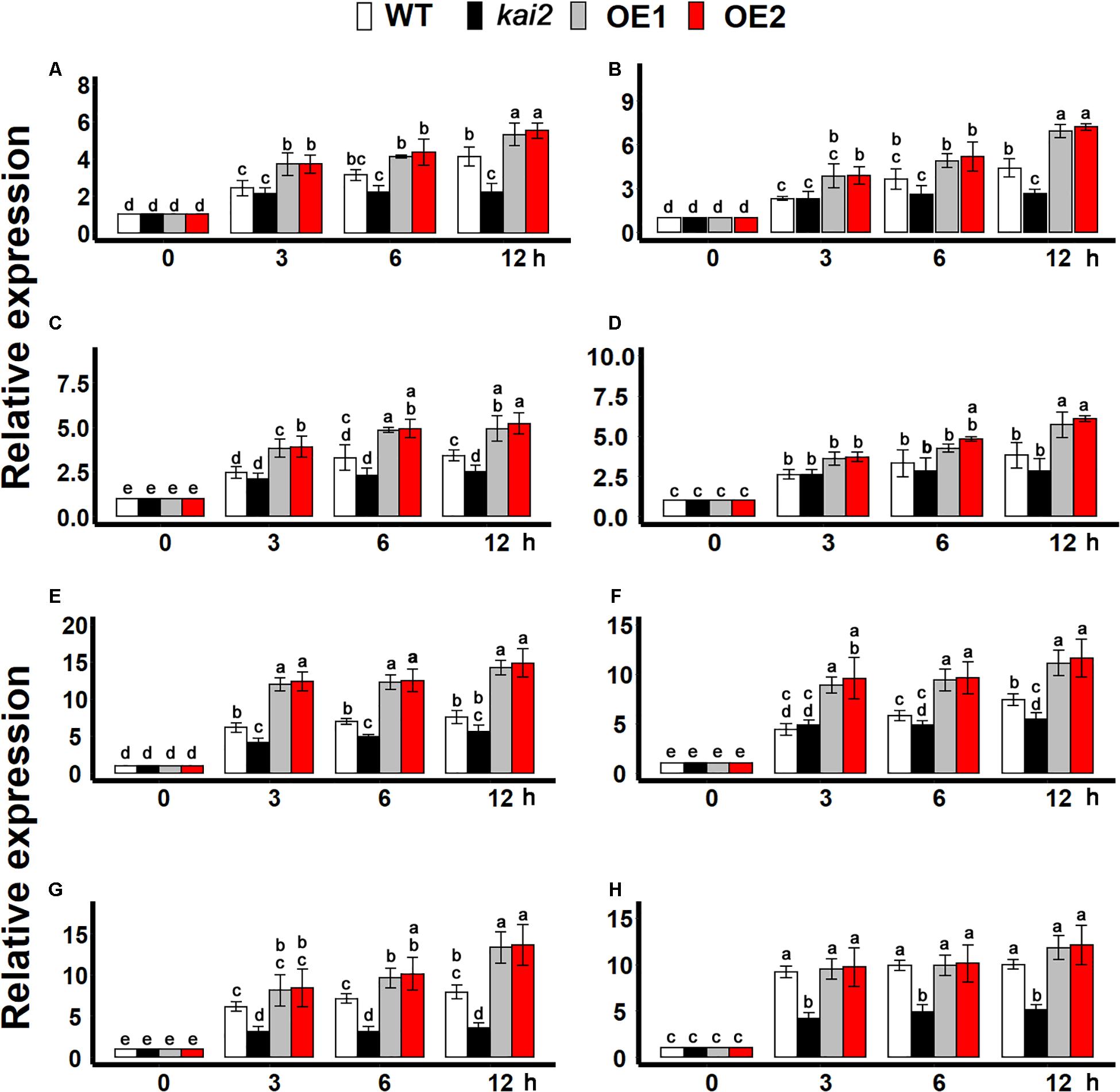
Figure 6. SsKAI2 OEs have enhanced cold-acclimation-related genes. (A) CSP1. (B) CSP2. (C) CSP3. (D) CSP4. (E) CBF1. (F) CBF2. (G) CBF3. (H) CBF4. Expression of cold-acclimation-related genes was investigated in three plants of each line after 0- (control at 22°C), 3−, 6−, and 12 h of cold treatment (4°C). The samples were randomly taken from the leaves of five plants of each treatment. Arabidopsis thaliana ACTIN 2 was taken as a reference gene, and control treatment at 0 h was considered as 1. One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). Bars with uncommon letters showing significant difference at P < 0.05. An “h” at the X-axis of each graph represents time in hours under cold stress.
Abscisic acid is the fundamental phytohormone that positively regulates the abiotic stress adaptation in various plants. To clarify whether karrikins could potentially interact with ABA in the regulation of cold acclimation, firstly we investigated the senstivity of SsKAI2 to ABA. Then we checked the expression level of ABA biosynthesis, ABA catabolism, and ABA signaling genes. The results showed that the seed germination in the SsKAI2 OEs was more likely to be inhibited in MS medium supplemented with ABA in comparison with wild-type, while Atkai2 seeds were less senstive to ABA as compare to wild-type (Figure 7A). The stomata started to close when SsKAI2 OEs leaves were incubated in ABA supplemented MES (2-(N-morpholino) ethane sulfonic acid) buffer (Figure 7B). Stomatal aperture decreased significantly in SsKAI2 OEs than wild-type and kai2 mutant when leaves were dipped in the medium containing 10 or 20 μM ABA. Stomata were completely closed when leaves were dipped in the solution containing 50 μM ABA (Figures 7C,D). These results demonstrated that overexpression of KAI2 could lead to hypersensitivity to ABA, suggesting a potential interaction between karrikins and ABA.
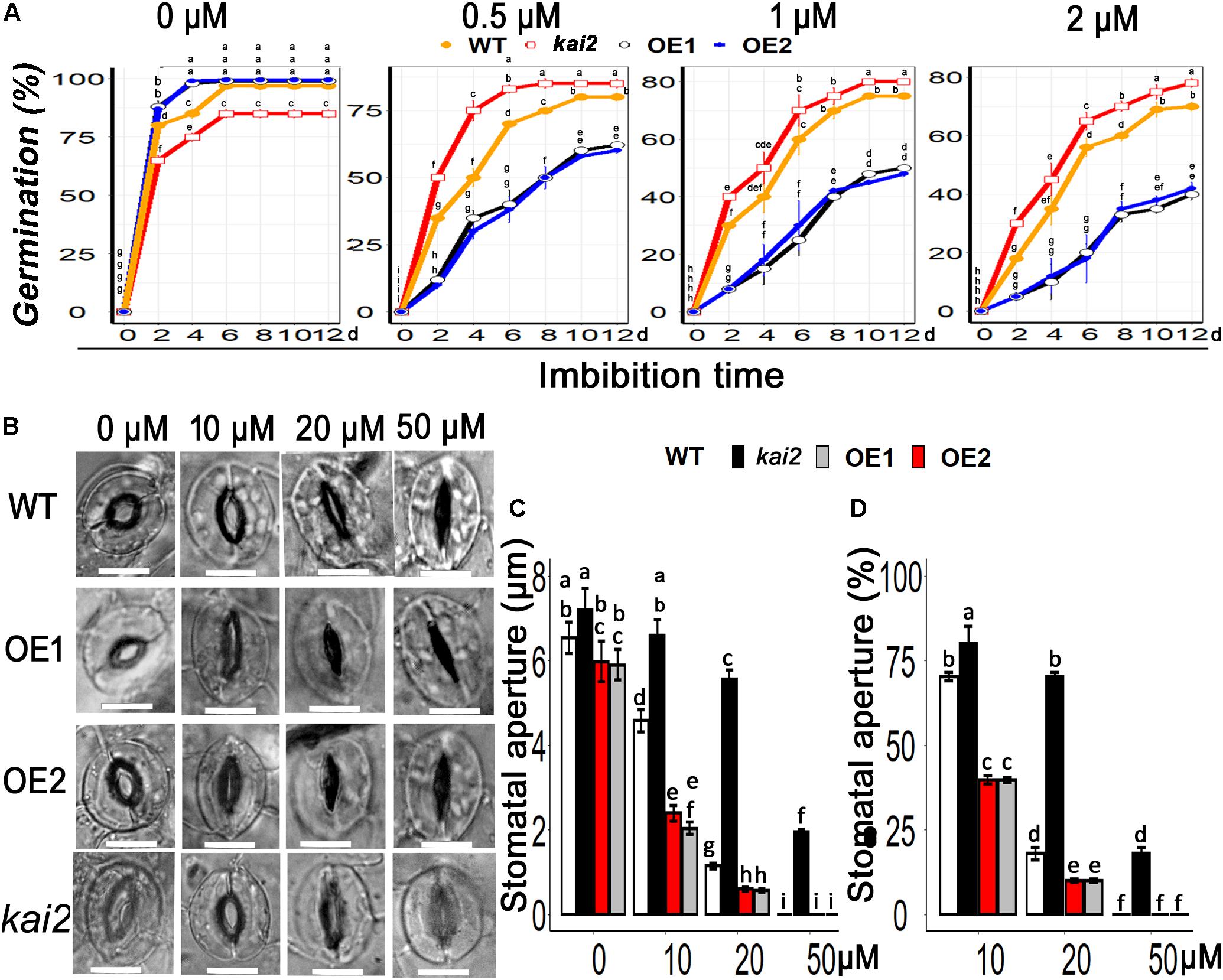
Figure 7. SsKAI2 OEs were hypersensitive to exogenous ABA. (A) An effect of ABA on seed germination in KAI2 overexpression line1 (OE1), line2 (OE2), WT (Col-0), and Atkai2 Arabidopsis. (B) KAI2 plants stomata showed hypersensitivity to ABA. 25-day-old plant leaves were selected for this treatment; white bar = 50 μM. (C) Stomatal aperture under different concentrations of ABA. (D) Stomatal aperture percentage closed after ABA treatment. Data shown are the mean ± SD of 15 replicates. One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 15). Bars or points with uncommon letters showing significant difference at P < 0.05.
Furthermore, to clarify the association of karrikins regulated cold acclimation to ABA, we determined the expression level of cold-responsive ABA biosynthesis genes such as NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) and ABSCISIC ALDEHYDE OXIDASE 3 (AAO3)(Qin and Zeevaart, 1999; Seo et al., 2004; Urano et al., 2009), ABA catabolic genes CYP707A family (Okamoto et al., 2006; Umezawa et al., 2010), and ABA signaling genes such as ABI3,ABI5, ABF1, MYB96, MYB3R2, and SIZ1 (Choi et al., 2000; Lee et al., 2005; Seo et al., 2009; Yuan et al., 2012; Guo et al., 2013; Liu et al., 2013; Dekkers et al., 2016; Skubacz et al., 2016). The results showed that the expression of NCED3 and AAO3 was not likely to be induced by cold treatments in the SsKAI2 OEs (Figures 8A,B). Under cold stress, the expression of CYP707A1, CYP707A2, CYP707A3, MYB96, and SnRK2.3 had no significant differences in all genotypes (Figures 8C,D,E,J,L). Under cold stress, the expression level of cold responsive ABA signaling genes such as SIZ1, and SnRK2.3 was significantly increased in SsKAI2 OEs. Meanwhile the expression of the key ABA signaling genes ABI3, ABI5, MYB3R2, ABF1 was also significantly increased in SsKAI2 OEs as compared to WT (Figures 8F–I,K). These results suggested that KAI2 potentially affected the ABA downstream signaling, which could contribute to the enhanced cold tolerance in the SsKAI2 OEs.
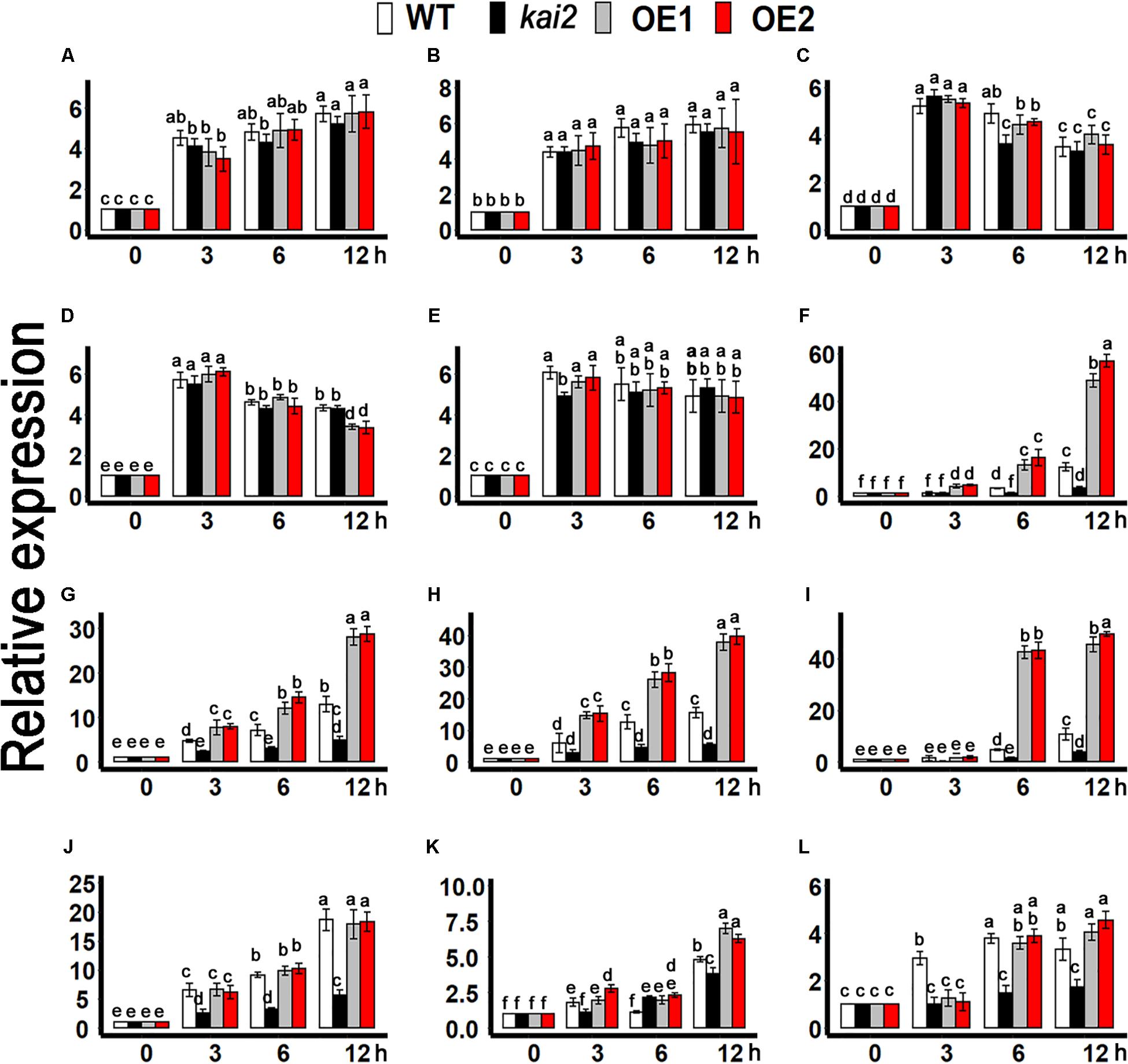
Figure 8. ABA-related genes expression in SsKAI2 OEs under cold stress. (A) NCED3. (B) AAO3. (C) CYP707A1. (D) CYP707A2. (E) CYP707A3. (F) ABI3. (G) ABI5. (H) ABF1. (I) MYB3R2. (J) MYB96. (K) SIZ1. (L) SnRK2.3. Twenty-five-day-old plants of SsKAI2 overexpression line1 (OE1), overexpression line1 (OE2), Atkai2, and WT (Col-0) Arabidopsis were subjected to cold stress (4°C), and samples were taken on given time points. The samples were randomly taken from the aerial of five plants of each treatment. Arabidopsis thaliana ACTIN2 was taken as a reference gene, control treatment at 0 h was considered as 1. The data shown in the figure are the mean ± SD of three replicates. One-way ANOVA was used to analyzed all data, and HSD Tukey’s test was used to perform multiple comparisons at P < 0.05 significant level (n = 3). Bars with uncommon letters showing significant difference at P < 0.05.
KARRIKINS INSENSITIVE2 (KAI2) is a receptor gene for karrikins, which encodes α/β-fold hydrolase, a hydrophobic pocket which may bind to the karrikins (Boyer et al., 2012; Hamiaux et al., 2012; Scaffidi et al., 2012; Li et al., 2013). KAI2 has been reported to be involved in the regulation of seed germination, hypocotyl development, and photomorphogenesis. Previously, KAI2 was reported to be involved in the stomatal closure, regulation of cuticle formation, membrane integrity, and anthocyanin biosynthesis, which contributes to plant alleviation of drought stress (Li et al., 2017). It has been reported that the karrikins-KAI2 signaling system provided stress tolerance by inhibiting germination in Arabidopsis under unfavorable conditions (Wang et al., 2018). A few studies have reported the involvement of KAI2 in the mitigation of environmental stresses such as osmotic and salinity in Arabidopsis, but there was not report regarding the role of karrikins-KAI2 in the regulation of cold stress. Cold resistant plants have induced level of TSS, TSP, and Proline contents (Hellmann, 2012; Keunen et al., 2013; Tarkowski and van den Ende, 2015), which are interlinked with the ROS homeostasis. Higher level of CSPs and CBFs gene expression is one of the fundamental characters of cold resistant plants (Fowler and Thomashow, 2002; Sasaki et al., 2007). Cold tolerance plants have induced expression level of ABA-responsive genes, which lead to stomata closure, and maintain the ROS balance (Thomashow, 1999; Wasilewska et al., 2008; Chinnusamy et al., 2010; Usadel et al., 2010; Hong et al., 2012; Shi et al., 2012; Jurczyk et al., 2019). In this study, we revealed that SsKAI2 OEs have higher levels of TSS, TSPs and Proline contents, and induced the expression level of CSPs and CBFs. SsKAI2 OEs were hypersensitive to ABA, and have induced the expression level of ABA-responsive genes, which are important characteristics of a cold resistant plant, and necessary for ROS homeostasis. In this study, we firstly reported that role of KAI2 in cold stress resistance in Arabidopsis, and revealed the biochemical and physiological mechanisms of KAI2 in the regulation of cold acclimation.
Among a large number of unfavorable conditions, cold (chilling and freezing) stress significantly limits the plant growth and development, and causes losses of the agricultural productivity. Cold resistant plants have developed a defensive system called cold acclimation (Thomashow, 1999; Stockinger et al., 2001; Shi et al., 2014b). Cold acclimation is a highly complicated process that includes an array of physiological, biochemical, and molecular modifications (Chinnusamy et al., 2010; Nakashima et al., 2014; van Buer et al., 2016). We found that overexpression of SsKAI2 in Arabidopsis recovered after cold stress. SsKAI2 overexpression lines (OEs) had significantly increased levels of proline, total soluble sugars, and total soluble protein. Under cold stress, cold-resistant plants produce an excessive level of soluble sugars, which directly interacted with the phosphate in the lipid headgroups of the cell membrane and decreased the membrane permeability (Strauss and Hauser, 1986). Soluble sugars accumulated in the apoplast of cold-stressed plants also suggested having a role in the protection of the plasma membrane (Valluru et al., 2008). At the same time, cold-resistant plant cells produce the proline, which helps the synthesis of specific proteins necessary for plasma membrane protection (Song et al., 2011; Tian et al., 2011; Melnikov et al., 2016). Furthermore, stress-resistant plants accumulate TSS and TSP to prevent the membrane damage and produce proline, which also plays a unique role in the synthesis of new proteins and may have a role in stress alleviation (Tarkowski and van den Ende, 2015; Li et al., 2018; Ni et al., 2018; Sadiq et al., 2018). In non-resistant plants, cold stress disrupts the cell membrane and cause leakage of electrolytes from the cytosol. Electrolytes leakage could cause the death of the plant (Demidchik et al., 2014). Our results are suggesting that SsKAI2 accumulated a significant amount of soluble sugars and proteins, which may strengthen the plasma membrane and protected SsKAI2 OEs from more electrolyte leakage under cold stresses.
Overproduction of ROS in plants under various abiotic stresses, including cold stress, causes oxidative cellular damage (Karuppanapandian and Manoharan, 2008; Mafakheri et al., 2010; van Buer et al., 2016). Among ROS, H2O2 is a relatively long-lived molecule and moderately reactive, disseminating short distances away from its production site. H2O2 enables it to diffuse the damage, act as a messenger in the stress signaling response, and travel freely across membranes (Møller et al., 2007). H2O2 can cause oxidation of membrane lipids and degrade the cell membrane, while MDA has been reported as an end product of lipid peroxidation, which is why MDA and H2O2 levels are markers of determining necrosis and cell damage in living organisms (MaBgorzata and Andrzej, 2016). In this study, we found that SsKAI2 OEs produced significantly lower amount of H2O2, and MDA level than WT under cold stress (Figures 3A,B). SsKAI2 had lower percentage of EL than WT under freezing temperature (Figure 2C), these results are consistent with previous report, demonstrating that a cold-sensitive S. lycopersicum genotype under cold stress produced significantly higher MDA and H2O2 content compared with controls. Han et al. (2017) found an increased level of MDA and EL contents in rice seedlings under cold stress. Similarly, Xue et al. (2019) reported that WT plants accumulate higher levels of H2O2 compared with transgenic Ammopiptanthus mongolicus under cold stress. These results are suggesting that SsKAI2 provided shield to cold stress via reducing H2O2 level, decreasing MDA content, and protecting plant cells from electrolyte leakage.
Various anti-oxidative defense systems scavenge ROS under steady-state conditions (Navrot et al., 2007). In anti-oxidative defense systems, ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) are the key enzymatic anti-oxidants that prevent cell necrosis by scavenging ROS and alleviate oxidative stress (Sairam et al., 2005; Fabio et al., 2007; Songbi and Bruria, 2013; Luis et al., 2018). When we investigated the activity of different enzymatic anti-oxidants such as APX, SOD, POD, and CAT in different Arabidopsis lines under cold stress, we found that the SOD activity was significantly higher in SsKAI2 OEs after six and 12 h of cold stress, but it was much lower in Atkai2 mutant than wild-type at each time point after cold stress (Figure 5A). Results here are agreement with a previous report, describing that cucumber seedling showed an induction in SOD activity under the cold stress (Zhao et al., 2016). Under different time points of cold stress, the activity of other anti-oxidant enzymes, such as POD, CAT, and APX, was increased dramatically in SsKAI2 OEs as compared to WT (Figures 5B–D). Previous studies showed an increased CAT activity in Cynodon dactylon, Capsella bursa pastoris, and Citrus reticulata, under cold stress (Shi et al., 2014a; Wani et al., 2018; Mohammadrezakhani et al., 2019). A higher activity of APX was detected in cold tolerant Jatropha macrocarpa, whereas reduction in APX activity was observed in cold sensitive Jatropha macrocarpa (Spano et al., 2017). Glutathione is a non-enzymatic anti-oxidant in the plant, which protects cellular damage from ROS under environmental stresses (Edwards et al., 2000). Cheng et al. (2016) observed the significantly higher GSH level in treated Citrullus lanatus compared with control samples under cold stress 24 h after treatment. Similarly, Wang Q. J. et al. (2016) demonstrated the increased GSH levels in transgenic apple seedlings as compared with WT under low temperature stress. We found that SsKAI2 OEs produced higher concentrations of T-GSH, while Atkai2 produced a significantly lower T-GSH contents than the WT plant under cold stress (Figure 5E). These results suggested that KAI2 conferred cold stress via activating enzymatic and non-enzymatic anti-oxidant systems in Arabidopsis.
During the process of cold acclimation, COLD SHOCK PROTEINS (CSPs) and C-REPEAT BINDING FACTORS (CBFs) were highly expressed in the cold-resistant plants. In the model plants Arabidopsis and poplar, the expression level of four CSP genes is differentially regulated in response to cold cues (Karlson and Imai, 2003; Benedict et al., 2006; Sasaki et al., 2007; Nakaminami et al., 2009). Overexpression of AtCBFs in the other plant species, or overexpression of CBFs from other species in Arabidopsis alleviated the freezing tolerance (Benedict et al., 2006; Tondelli et al., 2011). Previous studies revealed that the exogenous application with karrikins in Arabidopsis up-regulated the expression level of COLD SHOCK PROTEIN 2 (Baldrianová et al., 2015), which might be a reason for induction in cold resistance in Arabidopsis by the exogenous application of KAR1 (Supplementary Figure 2). It has also been reported that cold shock proteins were up-regulated by the transcription factors C-REPEAT BINDING FACTORS (CBFs) in response to cold stress (Fowler and Thomashow, 2002; Gilmour et al., 2004). In this study, SsKAI2 OEs has the highest level of CSPs and CBFs genes expression, while kai2 mutant exhibited the lowest expression level when compared to wild-type plants under cold stress (Figure 6). These results are consist with the previous studies showing that the cold resistant plants had a higher expression level of CSPs and CBFs (Fowler and Thomashow, 2002; Sasaki et al., 2007), suggesting that KAI2 might have a relationship with CSPs and CBFs in the regulation of cold acclimation in Arabidopsis.
Cold stress, same as other abiotic stresses, also cause water imbalance in plants and increase the abscisic acid (ABA) biosynthesis which could trigger the stomatal closure. Hence, stomatal closure is an adaptive strategy to drought, and cold (Shi and Yang, 2014; Jurczyk et al., 2019). In this study, SsKAI2 OEs were hypersensitive to ABA, and exogenous application of ABA, severely repressed seed germination, and caused induction in stomatal closure whereas Atkai2 mutants were not that sensitive to ABA as compared to WT (Figure 7), which are in agreement with the results of Li et al. (2017). However, it has also been suggested that ABA played a role in cold acclimation via triggering the expression of a set of stress responsive genes (Shi and Yang, 2014). ABA causes several changes in plant molecular, developmental, and physiological progressions resulting in plant adaptation to environmental stresses (Ton et al., 2009). Our results are consistent with Lee and Luan (2012), depicted that abiotic stresses stimulate ABA production which further triggers the expression of stomatal closure and stress-related genes. We found that SsKAI2 OEs promoted the expression level of ABA responsive genes, such as ABI3, ABI5, MYB96, MYB3R2, and ABF1 under cold stress (Figure 8), which is an agreement with the results of Li et al. (2017) suggesting that KAI2 might regulate the abiotic stress tolerance could be ABA-dependent. The relationship of ABA with redox homeostasis is well documented in different studies (Wang H. et al., 2016; Postiglione and Muday, 2020; Wenjing et al., 2020). Altogether, our results are suggesting that SsKAI2 enhanced response to ABA and induced expression level of ABA-responsive genes might be a pathway leading to redox homeostasis under cold stress.
Conclusively, in this study, the karrikins receptor gene KAI2 from the perennial woody plant Sapium sebiferum was the first time isolated and characterized under cold stress. The results of this report represented a novel function of KAI2 in the regulation of cold stress resistance in Arabidopsis by maintaining the redox homeostasis, increasing the ABA sensitivity, and inducing the expression of CSPs or CBFs genes. This study is providing foundations for researchers to explore abiotic stresses regulation functions of KAI2 in different plant species. Our discovery provides a foundation for the production of cold resistant plants. This study is beneficial for improving agronomic, horticultural, and forest plant research.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
FS, JN, and LW designed the experiments. JN, FS, YY, HH, and RW carried out the experiments. JN, FS, and YY analyzed the data and took photographs. FS wrote the manuscript. All authors read and approved the final manuscript.
This work was funded by the Anhui Natural Science Foundation (1908085QC141 and 2008085QC154), the Grant of the President Foundation of Hefei Institutes of Physical Science of Chinese Academy of Sciences (YZJJZX202013), the key program of 13th 5-year plan, CASHIPS (No. KP-2019-21), and the Supplementary Program of Science and Technology Service Program of the Chinese Academy of Sciences of Fujian Province (2019T3031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.657960/full#supplementary-material
Supplementary Figure 1 | Bioinformatics analysis of KAI2.
Supplementary Figure 2 | Exogenous application of KAR1 alleviated cold tolerance in Arabidopsis.
Supplementary Table 1 | List of primers.
Supplementary Data Sheet 1 | Proteins sequences and the list of genes used in the manuscript.
ABA, abscisic acid; APX, ascorbate peroxidase; CAT, catalase; CBFs, C-REPEAT BINDING FACTORS; CSPs, cold shock proteins; H2O2, hydrogen peroxide; KAI2, KARRIKINS INSENSITIVE2; KAR1, karrikin 1; KAR2, karrikin 2; KCl, potassium chloride; MDA, malondialdehyde; MES, 2-(N-morpholino) ethanesulfonic acid; POD, peroxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; TB, toluidine blue; T-GSH, total glutathione; TSP, total soluble protein; TSS, total soluble sugars.
Baldrianová, J., Èerný, M., Novák, J., Jedelský, P. L., Divíšková, E., and Brzobohatý, B. (2015). Arabidopsis proteome responses to the smoke-derived growth regulator karrikin. J. Proteom. 120, 7–20. doi: 10.1016/j.jprot.2015.02.011
Barau, J., Grandis, A., Teixeira, G. S., Zaparoli, G. H. A., Rio, M. C. S. D., Rincones, J., et al. (2015). Apoplastic and intracellular plant sugars regulate developmental transitions in witches’ broom disease of cacao. J. Exp. Bot. 66, 1325–1337. doi: 10.1093/jxb/eru485
Benedict, C., Skinner, J. S., Meng, R., Chang, Y., and Hurry, V. (2006). The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ. 29, 1259–1272. doi: 10.1111/j.1365-3040.2006.01505.x
Boyer, F.-D., De Saint Germain, A., Pillot, J.-P., Pouvreau, J.-B., Chen, V. X., Ramos, S., et al. (2012). Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: molecule design for shoot branching. Plant Physiol. 159, 1524–1544. doi: 10.1104/pp.112.195826
Cheng, F., Lu, J., Gao, M., Shi, K., Kong, Q., Huang, Y., et al. (2016). Redox signaling and CBF-responsive pathway are involved in salicylic acid-improved photosynthesis and growth under chilling stress in watermelon. Front. Plant Sci. 7:1519. doi: 10.3389/fpls.2016.01519
Chinnusamy, V., Zhu, J. K., and Sunkar, R. (2010). Gene regulation during cold stress acclimation in plants. Methods Mol. Biol. 639, 39–55. doi: 10.1007/978-1-60761-702-0_3
Choi, H. I., Hong, J. H. E., Ha, J. O., Kang, J. Y., and Kim, S. Y. (2000). ABFs, a Family of ABA-responsive element binding factors. J. Biol. Chem. 275, 1723–1730. doi: 10.1074/jbc.275.3.1723
Cuevas, J. C., López-Cobollo, R., Alcázar, R., Zarza, X., Koncz, C., Altabella, T., et al. (2008). Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol. 148, 1094–1105. doi: 10.1104/pp.108.122945
Dekkers, B. J., He, H., Hanson, J., Willems, L. A., Jamar, D. C., Cueff, G., et al. (2016). The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development. Plant J. 85, 451–465. doi: 10.1111/tpj.13118
Demidchik, V., Straltsova, D., Medvedev, S. S., Pozhvanov, G. A., Sokolik, A., and Yurin, V. (2014). Stress-induced electrolyte leakage: the role of K++-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 65, 1259–1270. doi: 10.1093/jxb/eru004
Edwards, R., Dixon, D. P., and Walbot, V. (2000). Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 5, 193–198. doi: 10.1016/S1360-1385(00)01601-0
Eisele, J. F., Florian, F., Bürgel, P. F., and Christina, C. (2016). A rapid and simple method for microscopy-based stomata analyses. PLoS One 11:e0164576. doi: 10.1371/journal.pone.0164576
Eremina, M., Rozhon, W., and Poppenberger, B. (2016). Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 73, 797–810. doi: 10.1007/s00018-015-2089-6
Fabio, R. C., Lima, J. P., Ferreira-Silva, S. L., Viegas, R. A., and Silveira, J. A. (2007). Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. Plant Physiol. 164, 591–600. doi: 10.1016/j.jplph.2006.03.004
Felsenstein, J. J. E. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x
Flematti, G. R., Ghisalberti, E. L., Dixon, K. W., and Trengove, R. D. (2004). A compound from smoke that promotes seed germination. Science 305, 977–977. doi: 10.1126/science.1099944
Flematti, G. R., Merritt, D. J., Piggott, M. J., Trengove, R. D., Smith, S. M., Dixon, K. W., et al. (2011). Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2, 2555–2559. doi: 10.1038/ncomms1356
Fowler, S., and Thomashow, M. F. (2002). Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14, 1675–1690. doi: 10.1105/tpc.003483
Gilmour, S. J., Fowler, S. G., and Thomashow, M. F. (2004). Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 54, 767–781. doi: 10.1023/B:PLAN.0000040902.06881.d4
Guo, L., Yang, H., Zhang, X., and Yang, S. (2013). Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 64, 1755–1767. doi: 10.1093/jxb/ert040
Guy, C. L., and Huber, H. (1992). Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol. 100:502. doi: 10.1104/pp.100.1.502
Hamiaux, C., Drummond, R. S., Janssen, B. J., Ledger, S. E., Cooney, J. M., Newcomb, R. D., et al. (2012). DAD2 is an αα/ββ hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22, 2032–2036. doi: 10.1016/j.cub.2012.08.007
Han, Q.-H., Huang, B., Ding, C.-B., Zhang, Z.-W., Chen, Y.-E., Hu, C., et al. (2017). Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 8:785. doi: 10.3389/fpls.2017.00785
Hellmann, H. (2012). Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 122, 357–368. doi: 10.1104/pp.122.2.357
Hong, Z., Lan, N., Liu, Y., Wang, Y., Zhang, A., Tan, M., et al. (2012). The C2H2-type zinc finger protein ZFP182 is involved in abscisic acid-induced antioxidant defense in rice. J. Integr. Plant Biol. 54, 500–510. doi: 10.1111/j.1744-7909.2012.01135.x
Janská, A., Maršík, P., Zelenková, S., and Ovesná, J. (2010). Cold stress and acclimation – what is important for metabolic adjustment? Plant Biol. 12, 395–405. doi: 10.1111/j.1438-8677.2009.00299.x
Jurczyk, B., Grzesiak, M., Pociecha, E., Wlazło, M., and Rapacz, M. (2019). Diverse stomatal behaviors mediating photosynthetic acclimation to low temperatures in Hordeum vulgare. Front. Plant Sci. 9:1963. doi: 10.3389/fpls.2018.01963
Kagiyama, M., Hirano, Y., Mori, T., Kim, S. Y., Kyozuka, J., Seto, Y., et al. (2013). Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18, 147–160. doi: 10.1111/gtc.12025
Karlson, D., and Imai, R. (2003). Conservation of the cold shock domain protein family in plants. Plant Physiol. 131, 12–15. doi: 10.1104/pp.014472
Karuppanapandian, T., and Manoharan, K. (2008). Uptake and translocation of tri-and hexa-valent chromium and their effects on black gram (Vigna mungo L. Hepper cv. Co4) roots. J. Plant Biol. 51, 192–201. doi: 10.1007/BF03030698
Keunen, E., Peshev, D., Vangronsveld, J., Ende, W., and Cuypers, A. (2013). Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ. 36, 1242–1255. doi: 10.1111/pce.12061
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lee, B. H., Henderson, D. A., and Zhua, J. K. (2005). The Arabidopsis cold-responsive transcriptome and its regulation by ICE1 W. Plant Cell 17, 3155–3175. doi: 10.1105/tpc.105.035568
Lee, S. C., and Luan, S. (2012). ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. doi: 10.1111/j.1365-3040.2011.02426.x
Li, H. Z., Zhou, X. E., Wu, Z.-S., Yi, W., Xu, Y., Li, S., et al. (2013). Crystal structures of two phytohormone signal-transducing αα/ββ hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 23:436. doi: 10.1038/cr.2013.19
Li, M., Xu, J., Wang, X., Fu, H., and Shi, L. (2018). Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 229, 132–141. doi: 10.1016/j.jplph.2018.07.009
Li, W., Nguyen, K. H., Chu, H. D., Ha, C. V., Watanabe, Y., Osakabe, Y., et al. (2017). The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genetics 13:e1007076. doi: 10.1371/journal.pgen.1007076
Liu, X., Zhang, H., Zhao, Y., Feng, Z., Li, Q., Yang, H.-Q., et al. (2013). Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 110, 15485–15490. doi: 10.1073/pnas.1304651110
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔΔΔ C T method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Luis, D. R. A., Corpas, F. J., López-Huertas, E., and Palma, J. M. (2018). “Plant superoxide dismutases: function under abiotic stress conditions,” in Antioxidants and Antioxidant Enzymes in Higher Plants, eds D. K. Gupta, J. M. Palma, and F. J. Corpas (Cham: Springer International Publishing), 1–26.
MaBgorzata, N., and Andrzej, G. (2016). The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016:3164734. doi: 10.1155/2016/3164734
Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Struik, P., and Sohrabi, Y. (2010). Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 4, 580–585.
Melnikov, S., Mailliot, J., Rigger, L., Neuner, S., Shin, B. S., Yusupova, G., et al. (2016). Molecular insights into protein synthesis with proline residues. EMBO Rep. 17:1776. doi: 10.15252/embr.201642943
Mohammadrezakhani, S., Hajilou, J., Rezanejad, F., and Zaare-Nahandi, F. (2019). Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J. Plant Interact. 14, 347–358. doi: 10.1080/17429145.2019.1629033
Møller, I. M., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
Nakaminami, K., Hill, K., Perry, S. E., Sentoku, N., Long, J. A., and Karlson, D. T. (2009). Arabidopsis cold shock domain proteins: relationships to floral and silique development. J. Exp. Bot. 60, 1047–1062. doi: 10.1093/jxb/ern351
Nakashima, K., Yamaguchishinozaki, K., and Shinozaki, K. (2014). The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 5:170. doi: 10.3389/fpls.2014.00170
Navrot, N., Rouhier, N., Gelhaye, E., and Jacquot, J. P. (2007). Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 129, 185–195. doi: 10.1111/j.1399-3054.2006.00777.x
Neeru, J., and Van, S. J. (2007). The potential of the smoke-derived compound 3-methyl-2H-furo[2,3-c]pyran-2-one as a priming agent for tomato seeds. Seed Sci. Res. 17, 175–181. doi: 10.1017/S0960258507785896
Nei, M., and Kumar, S. (2000). Molecular Evolution and Phylogenetics. Oxford: Oxford university press.
Nelson, D. C., Flematti, G. R., Riseborough, J. A., Ghisalberti, E. L., Dixon, K. W., and Smith, S. M. (2010). Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 107, 7095–7100. doi: 10.1073/pnas.0911635107
Ni, J., Wang, Q., Shah, F. A., Liu, W., Wang, D., Huang, S., et al. (2018). Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 23:799. doi: 10.3390/molecules23040799
Nishiyama, R., Watanabe, Y., Fujita, Y., Le, D. T., Kojima, M., Werner, T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23, 2169–2183. doi: 10.1105/tpc.111.087395
Okamoto, M., Kuwahara, A., Seo, M., Kushiro, T., Asami, T., Hirai, N., et al. (2006). CYP707A1 and CYP707A2, which encode abscisic acid 8’-Hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141:97. doi: 10.1104/pp.106.079475
Postiglione, A. E., and Muday, G. K. (2020). The Role of ROS homeostasis in ABA-induced guard cell signaling. Front. Plant Sci. 11:968. doi: 10.3389/fpls.2020.00968
Puyaubert, J., and Baudouin, E. (2014). New clues for a cold case: nitric oxide response to low temperature. Plant Cell Environ. 37, 2623–2630. doi: 10.1111/pce.12329
Qin, X., and Zeevaart, J. A. D. (1999). The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. U.S.A. 96, 15354–15361. doi: 10.1073/pnas.96.26.15354
Sadiq, M., Akram, N. A., and Ashraf, M. (2018). Impact of exogenously applied tocopherol on some key physio-biochemical and yield attributes in mungbean [Vigna radiata (L.) Wilczek] under limited irrigation regimes. Acta Physiol. Plant. 40:131. doi: 10.1007/s11738-018-2711-y
Sairam, R., Srivastava, G., Agarwal, S., and Meena, R. (2005). Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 49:85. doi: 10.1007/s10535-005-5091-2
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sasaki, K., Kim, M. H., and Imai, R. (2007). Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem. Biophys. Res. Commun. 364, 633–638. doi: 10.1016/j.bbrc.2007.10.059
Scaffidi, A., Waters, M. T., Bond, C. S., Dixon, K. W., Smith, S. M., Ghisalberti, E. L., et al. (2012). Exploring the molecular mechanism of karrikins and strigolactones. Bioorgan. Med. Chem. Lett. 22, 3743–3746. doi: 10.1016/j.bmcl.2012.04.016
Seo, M., Aoki, H., Koiwai, H., Kamiya, Y., Nambara, E., and Koshiba, T. (2004). Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol. 45, 1694–1703. doi: 10.1093/pcp/pch198
Seo, P. J., Xiang, F., Qiao, M., Park, J. Y., and Park, C. M. J. (2009). The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 151, 275–289. doi: 10.1104/pp.109.144220
Shah, F. A., Ni, J., Chen, J., Wang, Q., Liu, W., Chen, X., et al. (2018). Proanthocyanidins in seed coat tegmen and endospermic cap inhibit seed germination in Sapium sebiferum. PeerJ 6:e4690. doi: 10.7717/peerj.4690
Shah, F. A., Wei, X., Wang, Q., Liu, W., Wang, D., Yao, Y., et al. (2020). Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 11:216. doi: 10.3389/fpls.2020.00216
Shi, B., Lan, N., Zhang, A., Cao, J., Hong, Z., Qin, T., et al. (2012). OsDMI3 is a novel component of abscisic acid signaling in the induction of antioxidant defense in leaves of rice. Mol. Plant 5, 1359–1374. doi: 10.1093/mp/sss068
Shi, H., Ye, T., Zhong, B., Liu, X., and Chan, Z. (2014a). Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 56, 1064–1079. doi: 10.1111/jipb.12167
Shi, H., Ye, T., Zhong, B., Liu, X., Jin, R., and Chan, Z. (2014b). AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 203, 554–567. doi: 10.1111/nph.12812
Shi, Y., and Yang, S. (2014). “ABA regulation of the cold stress response in plants,” in Abscisic Acid: Metabolism, Transport and Signaling, ed. D. P. Zhang (Dordrecht: Springer), 337–363. doi: 10.1007/978-94-017-9424-4_17
Skubacz, A., Daszkowska-Golec, A., and Szarejko, I. (2016). The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 7:1884. doi: 10.3389/fpls.2016.01884
Song, S. Y., Chen, Y., Chen, J., Dai, X. Y., and Zhang, W. H. (2011). Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234, 331–345. doi: 10.1007/s00425-011-1403-2
Songbi, C., and Bruria, H. (2013). Effect of genotype and exogenous application of glycinebetaine on antioxidant enzyme activity in native gels of 7-day-old salt-stressed tomato (Solanum lycopersicum) seedlings. Sci. Hortic. 162, 106–116. doi: 10.1016/j.scienta.2013.07.001
Spano, C., Bottega, S., Ruffini Castiglione, M., and Pedranzani, H. E. (2017). Antioxidant response to cold stress in two oil plants of the genus Jatropha. Plant Soil Environ. 63, 271–276. doi: 10.17221/182/2017-PSE
Stockinger, E. J., Mao, Y., Regier, M. K., Triezenberg, S. J., and Thomashow, M. F. (2001). Transcriptional adaptor and histone acetyltransferase proteins in Arabidopsis and their interactions with CBF1, a transcriptional activatorinvolved in cold-regulated gene expression. Nucleic Acids Res. 29:1524. doi: 10.1093/nar/29.7.1524
Strauss, G., and Hauser, H. (1986). Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. U.S.A. 83, 2422–2426. doi: 10.1073/pnas.83.8.2422
Tarkowski, Ł. P., and van den Ende, W. (2015). Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front. Plant Sci. 6:203. doi: 10.3389/fpls.2015.00203
Thomashow, M. F. (1999). PLANT COLD ACCLIMATION: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599. doi: 10.1146/annurev.arplant.50.1.571
Tian, Y., Zhang, H., Pan, X., Chen, X., Zhang, Z., Lu, X., et al. (2011). Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgen. Res. 20, 857–866. doi: 10.1007/s11248-010-9463-9
Ton, J., Flors, V., and Mauch-Mani, B. (2009). The multifaceted role of ABA in disease resistance. Trends Plant Sci. 14, 310–317. doi: 10.1016/j.tplants.2009.03.006
Tondelli, A., Francia, E., Barabaschi, D., Pasquariello, M., and Pecchioni, N. (2011). Inside the CBF locus in Poaceae. Plant Sci. 180, 39–45. doi: 10.1016/j.plantsci.2010.08.012
Uemura, M., Joseph, R. A., and Steponkus, P. L. (1995). Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 109, 15–30. doi: 10.1104/pp.109.1.15
Umezawa, T., Okamoto, M., Kushiro, T., Nambara, E., Oono, Y., Seki, M., et al. (2010). CYP707A3, a major ABA 8’−hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 46, 171–182. doi: 10.1111/j.1365-313X.2006.02683.x
Urano, K., Maruyama, K., Ogata, Y., Morishita, Y., Takeda, M., Sakurai, N., et al. (2009). Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 57, 1065–1078. doi: 10.1111/j.1365-313X.2008.03748.x
Usadel, B., Bläsing, O. E., Gibon, Y., Poree, F., Höhne, M., Günter, M., et al. (2010). Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant Cell Environ. 31, 518–547. doi: 10.1111/j.1365-3040.2007.01763.x
Valluru, R., Lammens, W., Claupein, W., and Ende, W. V. D. (2008). Freezing tolerance by vesicle-mediated fructan transport. Trends Plant Sci. 13, 409–414. doi: 10.1016/j.tplants.2008.05.008
van Buer, J., Cvetkovic, J., and Baier, M. (2016). Cold regulation of plastid ascorbate peroxidases serves as a priming hub controlling ROS signaling in Arabidopsis thaliana. BMC Plant Biol. 16:163. doi: 10.1186/s12870-016-0856-7
Verbruggen, N., and Hermans, C. (2008). Proline accumulation in plants: a review. Amino Acids 35, 753–759. doi: 10.1007/s00726-008-0061-6
Wang, H., Yang, L., Li, Y., Hou, J., Huang, J., and Liang, W. (2016). Involvement of ABA-and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 107, 126–136. doi: 10.1016/j.plaphy.2016.05.040
Wang, L., Waters, M. T., and Smith, S. M. (2018). Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavourable to seedling establishment. New Phytol. 219, 605–618. doi: 10.1111/nph.15192
Wang, Q. J., Sun, H., Dong, Q. L., Sun, T. Y., Jin, Z. X., Hao, Y. J., et al. (2016). The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol. J. 14, 1986–1997. doi: 10.1111/pbi.12556
Wani, M. A., Jan, N., Qazi, H. A., Andrabi, K. I, and John, R. (2018). Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol. Plant. 40:167. doi: 10.1007/s11738-018-2747-z
Wasilewska, A., Vlad, F., Sirichandra, C., Redko, Y., Jammes, F., Valon, C., et al. (2008). An update on abscisic acid signaling in plants and more. Mol. Plant 1, 198–217. doi: 10.1093/mp/ssm022
Waters, M. T., Nelson, D. C., Scaffidi, A., Flematti, G. R., Sun, Y. K., Dixon, K. W., et al. (2012). Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139, 1285–1295. doi: 10.1242/dev.074567
Waters, M. T., and Smith, S. M. (2013). KAI2-and MAX2-mediated responses to karrikins and strigolactones are largely independent of HY5 in Arabidopsis seedlings. Mol. Plant 6, 63–75. doi: 10.1093/mp/sss127
Wenjing, W., Chen, Q., Singh, P. K., Huang, Y., and Pei, D. (2020). CRISPR/Cas9 edited HSFA6a and HSFA6b of Arabidopsis thaliana offers ABA and osmotic stress insensitivity by modulation of ROS homeostasis. Plant Signal. Behav. 15:1816321. doi: 10.1080/15592324.2020.1816321
Winkel-Shirley, B. (2002). Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 5, 218–223. doi: 10.1016/S1369-5266(02)00256-X
Xue, M., Guo, T., Ren, M., Wang, Z., Tang, K., Zhang, W., et al. (2019). Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. 143, 375–387. doi: 10.1016/j.plaphy.2019.07.019
Yamaguchishinozaki, K., and Shinozaki, K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6, 251–264. doi: 10.1105/tpc.6.2.251
Yang, M., Wu, Y., Jin, S., Hou, J., Mao, Y., Liu, W., et al. (2015). Flower bud transcriptome analysis of Sapium sebiferum (linn.) roxb. And primary investigation of drought induced flowering: pathway construction and g-quadruplex prediction based on transcriptome. PLoS One 10:e0118479. doi: 10.1371/journal.pone.0118479
Yuan, Z., Schumaker, K. S., and Guo, Y. (2012). Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 109, 12822–12827. doi: 10.1073/pnas.1202630109
Zhao, H., Ye, L., Wang, Y., Zhou, X., Yang, J., Wang, J., et al. (2016). Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 7:1814. doi: 10.3389/fpls.2016.01814
Zhao, M.-G., Chen, L., Zhang, L.-L., and Zhang, W.-H. (2009). Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 151, 755–767. doi: 10.1104/pp.109.140996
Zhao, Z., Tan, L., Dang, C., Zhang, H., Wu, Q., and An, L. (2012). Deep-sequencing transcriptome analysis of chilling tolerance mechanisms of a subnival alpine plant, Chorispora bungeana. BMC Plant Biol. 12:222. doi: 10.1186/1471-2229-12-222
Keywords: karrikins, KAI2, cold stress, redox homeostasis, abscisic acid
Citation: Shah FA, Ni J, Yao Y, Hu H, Wei R and Wu L (2021) Overexpression of Karrikins Receptor Gene Sapium sebiferum KAI2 Promotes the Cold Stress Tolerance via Regulating the Redox Homeostasis in Arabidopsis thaliana. Front. Plant Sci. 12:657960. doi: 10.3389/fpls.2021.657960
Received: 24 January 2021; Accepted: 07 April 2021;
Published: 15 July 2021.
Edited by:
Pasqualina Woodrow, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Chien Van Ha, Texas Tech University, United StatesCopyright © 2021 Shah, Ni, Yao, Hu, Wei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lifang Wu, bGZ3dUBpcHAuYWMuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.