
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 20 May 2021
Sec. Plant Breeding
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.637284
 Xiaojing Zhou1
Xiaojing Zhou1 Jianbin Guo1
Jianbin Guo1 Manish K. Pandey2
Manish K. Pandey2 Rajeev K. Varshney2
Rajeev K. Varshney2 Li Huang1
Li Huang1 Huaiyong Luo1
Huaiyong Luo1 Nian Liu1
Nian Liu1 Weigang Chen1
Weigang Chen1 Yong Lei1
Yong Lei1 Boshou Liao1
Boshou Liao1 Huifang Jiang1*
Huifang Jiang1*Peanut is an important legume crop worldwide. To uncover the genetic basis of yield features and assist breeding in the future, we conducted genome-wide association studies (GWAS) for six yield-related traits of the Chinese peanut mini-core collection. The seed (pod) size and weight of the population were investigated under four different environments, and these traits showed highly positive correlations in pairwise combinations. We sequenced the Chinese peanut mini-core collection using genotyping-by-sequencing approach and identified 105,814 high-quality single-nucleotide polymorphisms (SNPs). The population structure analysis showed essentially subspecies patterns in groups and obvious geographical distribution patterns in subgroups. A total of 79 significantly associated loci (P < 4.73 × 10–7) were detected for the six yield-related traits through GWAS. Of these, 31 associations were consistently detected in multiple environments, and 15 loci were commonly detected to be associated with multiple traits. Two major loci located on chromosomal pseudomolecules A06 and A02 showed pleiotropic effects on yield-related traits, explaining ∼20% phenotypic variations across environments. The two genomic regions were found 46 putative candidate genes based on gene annotation and expression profile. The diagnostic marker for the yield-related traits from non-synonymous SNP (Aradu-A06-107901527) was successfully validated, achieving a high correlation between nucleotide polymorphism and phenotypic variation. This study provided insights into the genetic basis of yield-related traits in peanut and verified one diagnostic marker to facilitate marker-assisted selection for developing high-yield peanut varieties.
Peanut (Arachis hypogaea L.) is an important oilseed crop and a key source of oil and protein. It is grown in more than 100 countries, with a world production of 45.95 m tons from an area of 28.5 m ha (FAOSTAT, 2018). It is expected that the current demand of peanut will increase at a much higher growth rate in the next decades (Foley et al., 2011; Tilman et al., 2011). This poses a big challenge to researchers in enhancing peanut productivity at a faster pace. Genomics-assisted breeding (GAB) has been successfully demonstrated to facilitate the process of improved varietal development in peanut (Pandey et al., 2020). Identification of stable major loci and closely associated markers for yield-related traits in peanut will provide applicable opportunity for GAB to accelerate the development of high-yield varieties.
Seed size, seed weight, pod size, and pod weight have direct impact on peanut yield. Some efforts have been made on the genetic basis of these yield-related traits. Based on temporary separated populations, such as F2, BC2F2:3, and F2:3, dozens of main effect quantitative trait loci (QTLs) were identified (Fonceka et al., 2012; Shirasawa et al., 2012; Huang et al., 2015; Chen W. et al., 2016). Based on recombinant inbred line (RIL) populations, some major effect QTLs were detected in multiple environments (Luo et al., 2018; Zhang et al., 2019; Zhou et al., 2019). Luo et al. (2018) identified two stable major QTLs located on chromosomal pseudomolecules A05 and A07 which simultaneously affected the traits of pod length (PL), pod width (PW), and hundred-pod weight (HPW). Zhou et al. (2019) reported stable major QTLs on B06 that were also pleiotropic for the above-mentioned three pod-related traits. Zhang et al. (2019) mapped a stable major QTL for hundred-seed weight (HSW) and seed length (SL) on A02 and a stable QTL for HSW and seed width (SW) on B06. Recently, Gangurde et al. (2020) identified the stable major QTLs for HPW and HSW on A05, A06, B05, and B06 using nested-association mapping populations. In addition, a few studies have reported associated loci for yield-related traits using the natural populations of peanut (Pandey et al., 2014; Zhao et al., 2017; Zhang et al., 2017; Wang J. et al., 2019). Zhao et al. (2017) identified a major SSR marker that was consistently detected to be associated with SL and HSW in different environments. Although some stable major QTLs for seed- and pod-related traits have been reported in different peanut germplasms, there is no information yet on diagnostic markers available in breeding.
As the important affected factors of yield, seed (pod) size and weight have been extensively studied in some crops, leading to better understanding of the genetic basis. A large number of QTLs were identified, and dozens of important genes were cloned. In rice, the genes that controlled grain-related traits, such as GS3, GW2, BG1, D61, GW5/qSW5, and qGL3/qGL3.1, simultaneously affected two or more traits of grain size or weight (Fan et al., 2006; Zuo and Li, 2014; Peng et al., 2015). In Brassica napus, the gene ARF18 affected both seed weight and silique length (Liu et al., 2015), and three major QTLs simultaneously controlled seed weight and silique length (Yang et al., 2012; Li et al., 2015). Compared with the research progress in other crops, in peanut it is still far from comprehensively understanding the genetic factors controlling these traits.
Up to now, most of the QTLs for yield-related traits in peanut were found using bi-parental populations through linkage mapping. The bi-parental population involves only two parents that allow a limited number of meiosis, leading to limited recombination events in the population (Korte and Farlow, 2013). Compared with linkage mapping, association analysis employs natural populations to discover genomic regions associated with target traits in a relatively high resolution and unbiased manner in broad-based and diverse accessions. With the availability of high-throughput sequencing technology and the development of bioinformatics and statistical methods, genome-wide association study (GWAS) using large-scale single-nucleotide polymorphisms (SNPs) has become an important alternative in dissecting the genetic basis of quantitative traits. In addition, the reference genome sequences of both wild diploid progenitors Arachis ipaensis and Arachis duranensis (Bertioli et al., 2016; Chen X. et al., 2016; Lu et al., 2018), as well as the assembled allotetraploid cultivated peanut A. hypogaea (Bertioli et al., 2019; Chen et al., 2019; Zhuang et al., 2019), are important resources for sequence-based trait mapping and genetics research.
In order to better understand the genetic basis of yield-related traits in peanut, we sequenced 250 accessions of the Chinese peanut mini-core collection by genotyping-by-sequencing (GBS) method and investigated the phenotypic data for six yield-related traits in four environments. By exploiting the genomic variation from the population and performing GWAS for seed (pod) size and weight in multi-environments, the significantly associated loci were identified, and stable major associations were found. Two associated genomic regions were investigated for candidate gene discovery and making validated diagnostic markers available for breeding.
A total of 250 accessions of the Chinese peanut mini-core collection were obtained from the China National Gene Bank, Oil Crops Research Institute, Chinese Academy of Agricultural Sciences, Wuhan, China. These materials were maintained by self-pollination for several generations. Detailed information of the accessions is listed in Supplementary Table 1.
For phenotyping, the population was planted in two consecutive years (2015 and 2016) at the experimental fields of Nanchong, Sichuan, China, and Wuhan, Hubei, China. The four experimental environments were designated as E1 (Nanchong in 2015), E2 (Wuhan in 2015), E3 (Nanchong in 2016), and E4 (Wuhan in 2016), respectively. A random block design with three replications was adopted for generating phenotyping data across four environments. Twelve plants per accession were planted in a 2.5-m-long single row, maintaining a 30-cm distance between rows. The traits of PL, PW, SL, SW, HPW, and HSW were measured after drying the harvested pods according to the described standard procedures (Jiang et al., 2006). The minimum, maximum, mean, standard deviation (SD), and coefficients of variation (CV) of phenotypic data for each trait in each environment were analyzed using the MEANS procedure of SAS (Schlotzhauer and Littell, 1997). The broad-sense heritability was calculated according to Hallauer and Miranda (1998) as h2 = σ2g/(σ2g + σ2ge/n + σ2e/nr), where σ2g was the genetic variance, σ2ge was the interaction variance of the genotype with environment, σ2e was the variance of residual error, n was the number of environments, and r was the number of replications. The multi-environment joint variances were obtained using the general linear model (GLM) procedure of SAS (Schlotzhauer and Littell, 1997). Correlation coefficients between traits across environments were calculated using the CORR procedure of SAS (Schlotzhauer and Littell, 1997).
The genomic DNA was extracted from a single plant of each accession using a modified cetyltrimethyl ammonium bromide method. For constructing GBS sequencing libraries, initially, 10 ug of genomic DNAs from each accession was incubated with MseI (New England Biolabs, Ipswich, MA, United States), T4 DNA ligase (NEB), ATP, and the Y-adapter containing a barcode, followed by digestion at 37°C and heating at 65°C to inactivate the enzymes. Then, two additional enzymes, HaeIII and NlaI (NEB), were simultaneously added into the same tube to further digest the fragments at 37°C. The digested fragments with ligations were purified using the Agencourt AMPure XP System, followed by PCR amplifications with purified samples and Phusion Master Mix (NEB) after adding a universal primer and an index primer to each sample. The PCR reactions were purified using Agencourt AMPure XP (Beckman) and electrophoresed on 2% (w/v) agarose gel, followed by fragment isolation of 340–410 bp (including indexes and adaptors) using a Gel Extraction Kit (Qiagen, Valencia, CA, United States). These fragments were then purified using the Agencourt AMPure XP System and were then diluted for sequencing on the Illumina HiSeq platform by Novogene-Beijing, China.
To generate the genotype data for GWAS, the method was essentially according to Jia et al. (2013). Only the unique sequences mapped to unique locations in the progenitor genome sequences of peanut were retained for SNP detection using the software of Burrows-Wheeler Aligner (BWA, version: 0.7.8) and Genome Analysis Toolkit (GATK, version 2.4). We used Bayesian algorithm to detect the polymorphism SNP site on a population scale and calculated genotype likelihoods from reads for each individual at each genomic location and the allele frequencies in the sample. Only the SNPs that had biallelic candidate SNP loci, had minor allele frequency (MAF) ≥ 0.05, and contained genotype calls of over 50 accessions were left for subsequent imputation. Genotype imputation was conducted for the remaining SNPs with missing data using the function infer sporadic missing genotype data of Beagle software, version 3.1.0 (Browning and Yu, 2009). Furthermore, the imputed genotypes with probability of less than 60% were removed from the dataset, and each marker was required to be present in ≥80% of population individuals. The imputation of the genotypes of 250 peanut accessions reduced the missing genotype calls from 35.02 to 3.04%. Four accessions were selected from the association population and sequenced for nearly 10 times as much data as before. The specificity of the genotype data before and after imputation was assessed using the sequencing data of the four accessions as quality control (SupplementaryTable 2). Finally, the remaining SNPs were annotated using the package Annovar (Wang et al., 2010).
The final set of high-quality SNPs for the 250 peanut accessions was used to assess the population structure (Q), phylogenetic trees, principal component analysis (PCA), and relative kinship (K). The number of groups (k) was determined using Structure 2.2 (Pritchard et al., 2000). Five independent simulations were performed for values of k ranging from 1 to 10. For each simulation, 10,000 iterations before a burn-in length of 10,000 Markov Chain Monte Carlo replications were conducted. The optimal k value was determined by the posterior probability [LnP(D)] (Pritchard et al., 2000) and delta k (Evanno et al., 2005). The Q values were calculated as the probability that the genomic variation of each individual came from each population. The boundary value of membership probabilities was set as 0.75 by comprehensively comparing the results of the population structure, phylogenetic trees, PCA, and K. The accessions with membership probabilities ≥0.75 were assigned to corresponding groups, and the accessions with membership probabilities <0.75 in any given group were assigned to a mixed group. Phylogenetic trees were inferred using pairwise genetic distances matrix data of all individuals, calculated by TreeBest v1.9.21. The bootstrap was set to 1,000 times to assess the branch reliability. PCA was performed using the package GCTA2. Tracy–Widom test was used to determine the significance level of the eigenvectors.
We divided several geographic regions in China into southern, northern, and central regions. The northern China group included the provinces of Shandong, Hebei, Henan, Liaoning, Beijing, Heilongjiang, Shanxi, and Shaanxi. The southern China group included the provinces of Guangdong, Guangxi, Fujian, Zhejiang, Guizhou, Yunnan, and Hainan. The central China group included the provinces of Hubei, Sichuan, Hunan, Anhui, Jiangxi, and Jiangsu.
The software GEMMA3 was used to perform the GWAS analysis. Firstly, the models of the GLM (Q), MLM (PCA + K), and MLM (Q + K) were compared, and quantile (QQ) plots were used to evaluate the most suitable model for association analysis. The MLM (PCA + K) model exhibited the best performance to eliminate false positives and was selected for GWAS. We defined the whole-genome significance cutoff as the Bonferroni test threshold. The threshold was set as −log10(0.05/105814) = 6.33. Those with P < 4.73 × 10–7 were defined as significant trait-associated SNPs. The Manhattan and QQ plots were drawn using R (Turner, 2018). To identify the putative candidate genes associated with each trait, the regions of 1.3 Mb upstream or downstream of associated SNPs were used to search genes according to the linkage disequilibrium (LD) decay of the population. The genes were defined as candidate associated genes within the genomic regions as described in Wei et al. (2015). The squared correlation coefficient (r2) was used to assess the LD using the Tassel 5 software (Bradbury et al., 2007). The significance of r2 was calculated based on Fisher’s exact test. The r2 was calculated between each pair of SNPs on the same chromosome (Weir, 1996). Decay of r2 value was analyzed across all chromosomes (Alhaddad et al., 2013). The value where r2 dropped to 50% of its maximum was set as the background level (Wei et al., 2015). The decay of LD with genetic distance was estimated by interval rather than marker pairs individually to reduce the influence of outliers as per Mather et al. (2007). We combined r2 values with P < 0.05 of all chromosomes into an interval series of 0–0.5, 0.5–1, 1–1.5, 1.5–2, 2–2.5, 2.5–3, 3–3.5, 3.5–4, 4–4.5, and 4.5–5 Mb based on marker distance. We estimated the averaged r2 for each interval and assumed the r2 value with 0-Mb marker distance to be 1 as previously described (Yan et al., 2009). The non-linear regression function was deployed to fit the trend of LD decay.
The diagnostic SNP marker was amplified by PCR and sequenced to determine the base variation. The sequences of the primer were from the peanut reference genome of Tifrunner4. Primers were designed using Primer 3 software5 based on the flanking sequences of the SNP variant and the specificity of the primers. The primer left sequence for PCR was GAGATAAATTTCTTTCATATTTTACG, and the primer right sequence for PCR was TTGTCCCCTGATCCAGCATA. PCR product was sequenced using the primer of GAGATAAATTTCTTTCATATTTTACG.
Six yield-related traits, including PL, PW, SL, SW, HPW, and HSW, of the Chinese peanut mini-core collection were measured in four environments (E1–E4). All the traits exhibited a large phenotypic variation (Table 1 and Supplementary Figure 2). The trait of HSW showed the highest CV (average: 28.97%), while the trait of SW showed the lowest CV (average: 10.46%). Among environments, the highest CV (30.41%) was observed for HSW in E1 (range: 24–117 g; mean: 63 g), while the lowest CV (10.55%) was found for SW in E4 (range: 0.65–1.2 cm; mean: 0.92 cm) (Table 1).
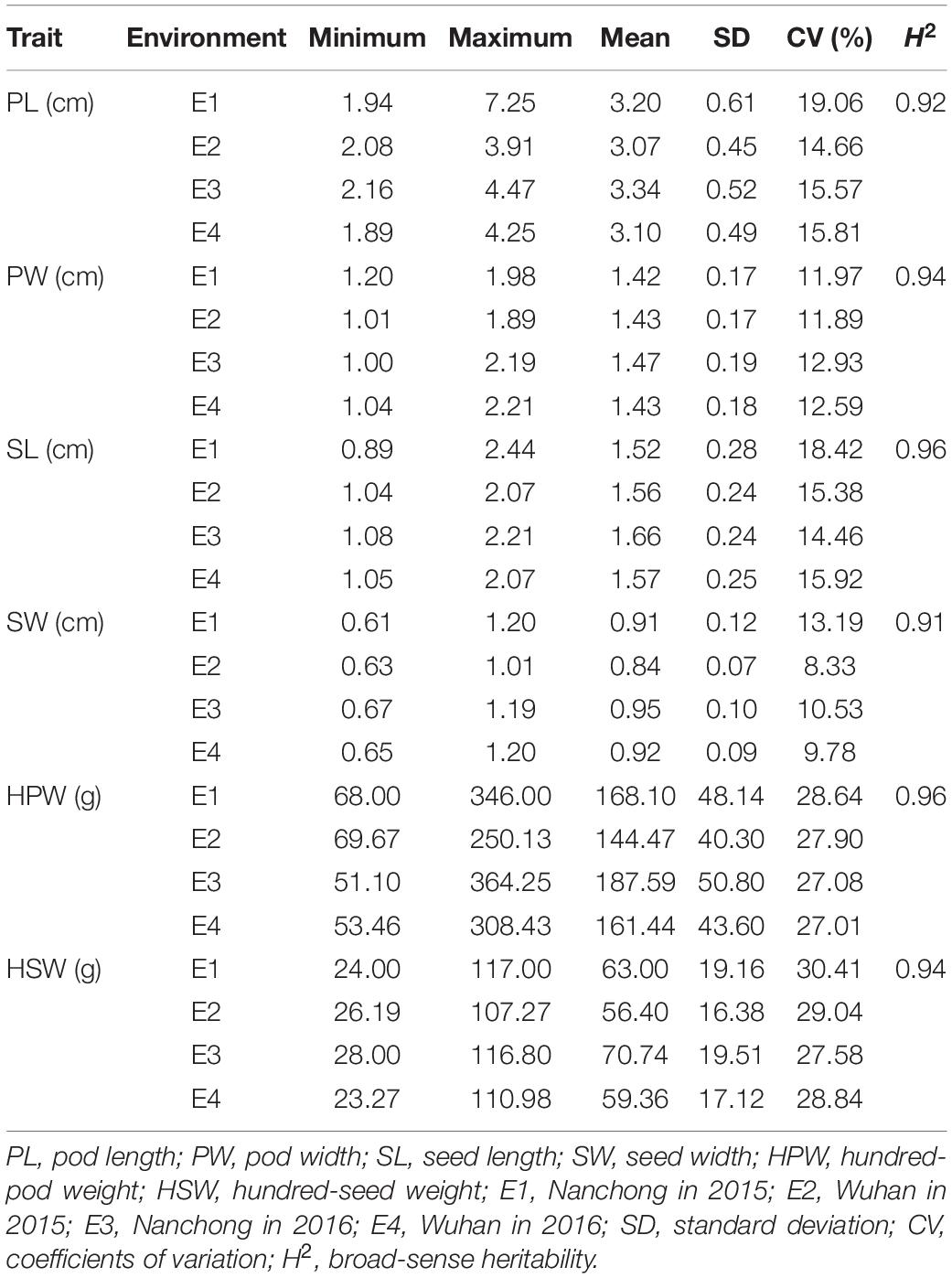
Table 1. Phenotypic performance of the six yield-related traits in the Chinese peanut mini-core collection under four environments.
The broad-sense heritability was 0.92, 0.94, 0.96, 0.91, 0.96, and 0.94 for PL, PW, SL, SW, HPW, and HSW, respectively (Table 1). The high heritability indicated that genetic factors played a predominant role in determining the variation for these traits. To pinpoint their relationships, correlation coefficients were calculated between the traits across environments (Table 2). Highly positive correlations were observed in all pairs of the six yield-related traits across environments (Table 2). The highest correlation coefficient was observed between HPW and HSW, which varied from 0.90 in E1 to 0.92 in E4, with an average of 0.91 (Table 2). The strong and stable relationship with each other across environments indicated that these traits might be affected by common genetic factors.
The GBS library was constructed for each accession, and massively parallel sequencing was performed to generate ∼311 Gb data and 589,167,078 tags. Polymorphic reads defined by GATK software and a total of 3,070,141 SNPs were initially identified among the 250 peanut accessions. After filtering, a total of 105,814 SNPs were retained. The chromosomal pseudomolecule A03 (6,737 SNPs) had the largest number of SNPs, followed by A04 (6,502 SNPs), while the smallest number of SNPs was detected on chromosomal pseudomolecule A08 (2,233 SNPs), followed by A07 (3,518 SNPs) (Figures 1A,B). Functional annotation detected ∼86% SNPs located in intergenic regions, while 14% SNPs were in genic regions. The largest number of SNPs in genic regions was in introns of annotated genes followed by untranslated regions and coding regions of annotated genes (Supplementary Table 3). A total of 2,085 SNPs were found in the coding regions, and 3,688 SNPs were in the gene upstream regions.
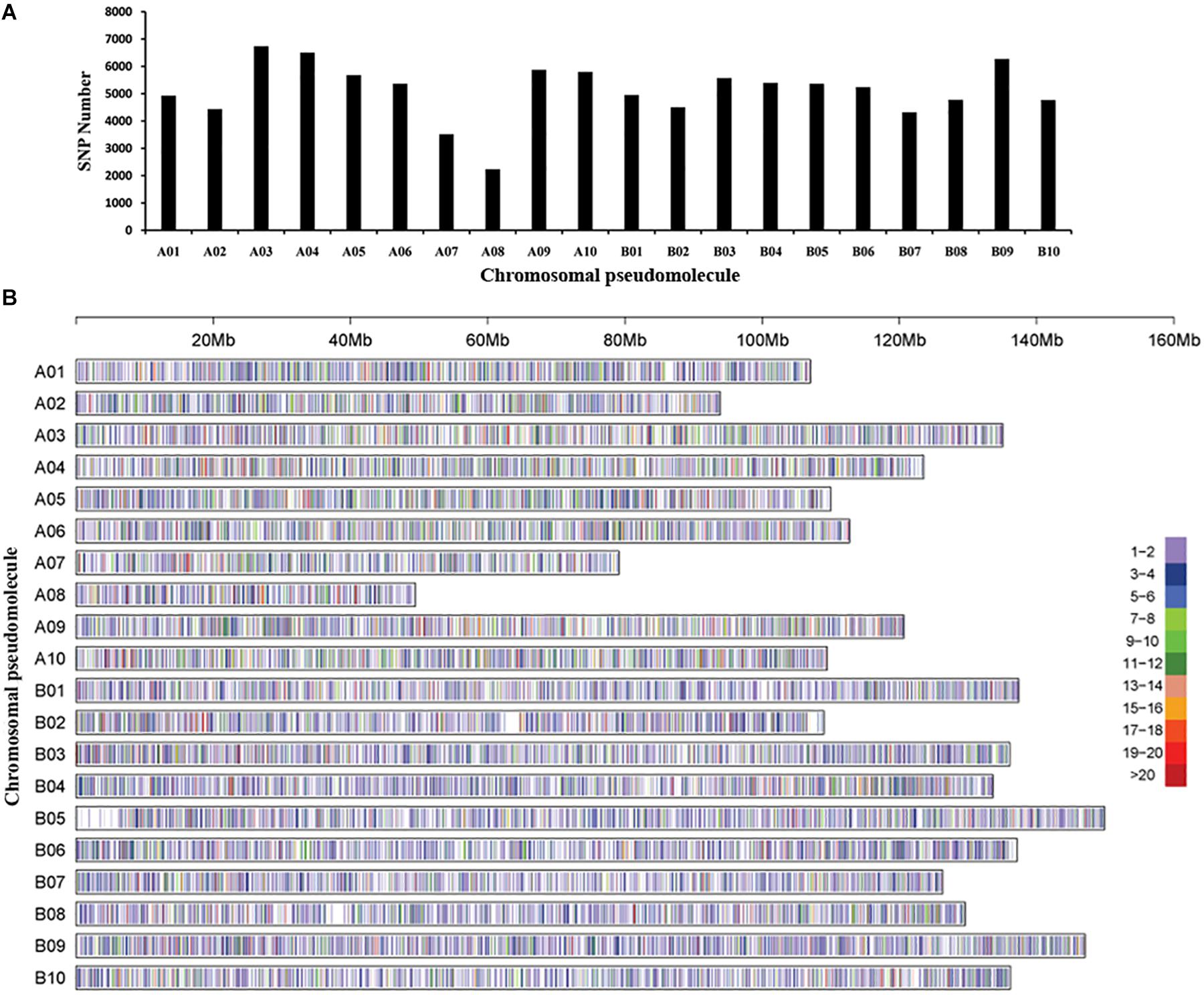
Figure 1. Single-nucleotide polymorphism (SNP) density and distribution across the genome of the peanut. (A) The distribution of the number of SNPs on each chromosomal pseudomolecule. (B) SNP density on each chromosomal pseudomolecule. The horizontal axis shows the length of the pseudomolecule; the number of SNPs per 20-kb window is shown as color index.
The LD was estimated using the r2 of SNP pairs. Decay of r2 value was analyzed across all chromosomes. Almost 25.7% r2 showed statistical significance (P < 0.05). The value where r2 dropped to half of its maximum was set as the background level. The extent of LD was found in the peanut panel at approximately 1.3 Mb, which is the point of 50% decay r2 value (r2 = 0.41; Supplementary Figure 3).
The population structure analysis was estimated based on the SNP genotype of the Chinese peanut mini-core collection. Bayesian clustering analysis detected significant changes in both delta k-value and LnP(D) value when k was increased from 1 to 2, suggesting two as the most likely number of inferred clusters. The first group, called G I, comprised 74 accessions, of which 64 accessions (86.49%) were subspecies of ssp. fastigiata. The second group, named G II, included 146 accessions, of which 95 accessions (65.07%) were subspecies of spp. hypogaea. In the mixed group, there were 15 accessions (50%) from ssp. fastigiata and 13 accessions (43.33%) from ssp. hypogaea (Figure 2A, Supplementary Table 1, and Supplementary Figure 4). In spite of the discrepancies, the population structure was obviously associated with the classification of botanical subspecies (Supplementary Table 1 and Supplementary Figure 4). Based on color system classification, the comparison of the phylogenetic tree and structure analysis (Figure 2B) showed that the majority accessions of a group in structure analysis also showed close phylogenetic relationships in the neighbor-joining tree analysis. The PCA plot of the top two principal components of this population based on structure result also indicated a clear separation (Figure 2C). In the association panel, a kinship coefficient less than 0.25 accounted for 95.23%, indicating that most of the accessions had a weak relationship (Supplementary Figure 5). The K matrix was visualized using a heat map, in which the groups were clearly separated (Figure 2D).
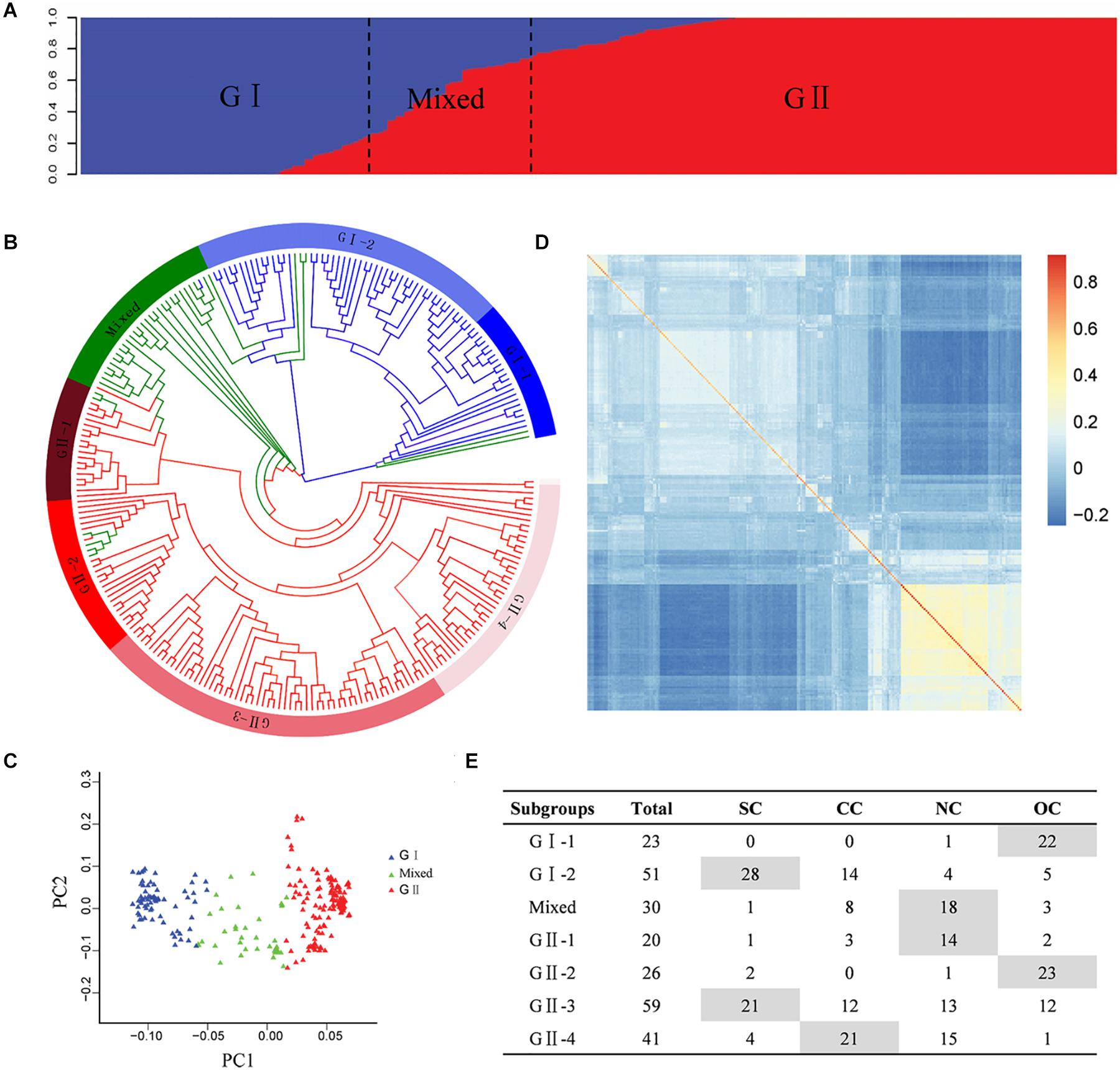
Figure 2. Genetic structures of the Chinese peanut mini-core collection. (A) Population structure. (B) Phylogenetic tree. The layer rings indicate the group and subgroup name. (C) Principal component analysis. (D) Heat map of pairwise relative kinship estimates. (E) The geographic origin of each accession in each cluster. SC, southern China; CC, central China; NC, northern China; OC, countries other than China. The information of botanical subspecies, origin geographic region, and group classification of each accession is provided in Supplementary Table 1.
According to phylogenetic relationships and stated location of origin, accessions in each group were further classified into several subgroups, which exhibited obviously geographic distribution patterns (Figures 2B,E and Supplementary Table 1). The G I group was classified into two subgroups, with G I-1 mainly containing ssp. fastigiata accessions from countries other than China and G I-2 mainly consisting of ssp. fastigiata accessions from southern China. The G II group was classified into four subgroups, with G II-1 to G II-4 mainly consisting of ssp. hypogaea accessions from northern China, countries other than China, southern China, and central China, respectively (Figures 2B,E). The mixed group had accessions mainly from north China.
A total of 10, 38, 20, and 57 significantly associated SNP loci (P < 4.73 × 10–7) were identified for the six yield-related traits under four environments of E1, E2, E3, and E4, respectively (Figure 3A and Supplementary Table 4). The larger number of association loci of a single trait in each environment was 15 for SL in E4, 14 for PL in E4, and 14 for HSW in E1, while no significant association could be detected for SW in E2 and SL in E1 and E3 (Supplementary Table 4). For each trait, a total of 15, 7, 15, 6, 20, and 16 non-redundant associations were identified for PL, PW, SL, SW, HPW, and HSW, respectively (Figure 3B and Supplementary Tables 4, 5). Most of the associations were distributed on chromosomal pseudomolecules A02, A06, B04, and B06, while a small number of associations were detected on A03, A04, A05, B07, B08, B09, and B10. The Manhattan plots and quantile–quantile plots of each trait under different environments are shown in Supplementary Figures 6–11.
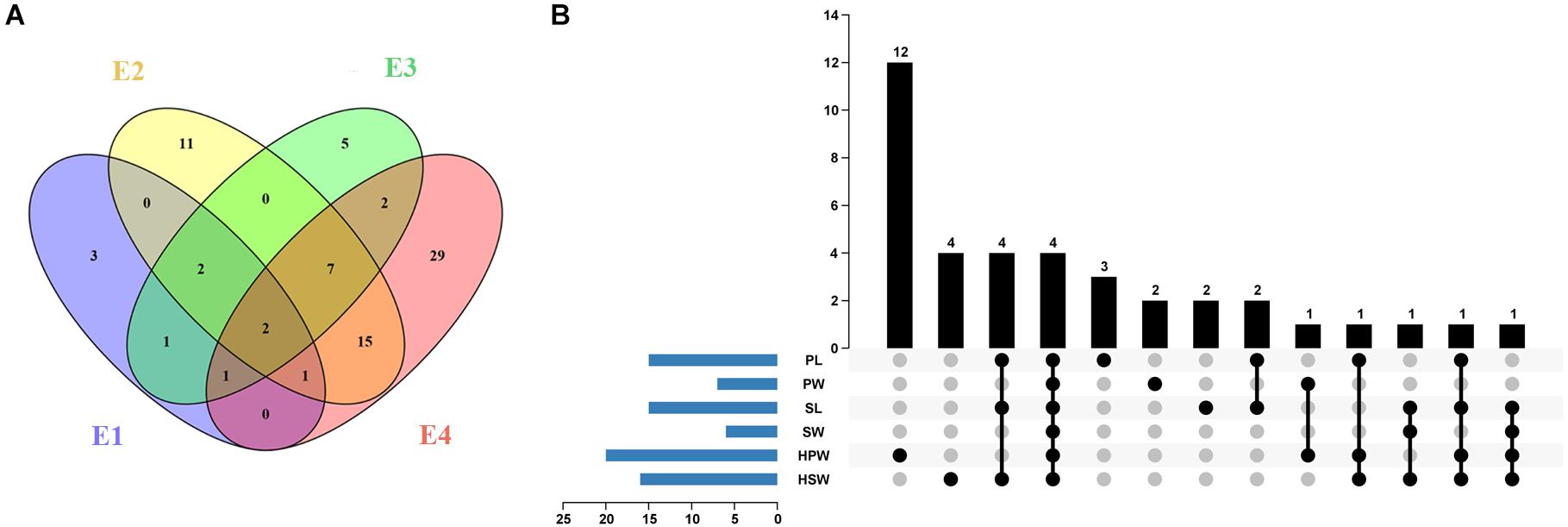
Figure 3. The profile of the associated loci for the six yield-related traits of the Chinese peanut mini-core collection under four different environments. (A) The Venn diagram of associated loci for six traits under four environments (E1–E4). (B) The statistical column diagram of the associations of the six yield-related traits and their interactive upset plot. E1, Nanchong in 2015; E2, Wuhan in 2015; E3, Nanchong in 2016; E4, Wuhan in 2016; PL, pod length; PW, pod width; SL, seed length; SW, seed width; HPW, hundred-pod weight; HSW, hundred-seed weight.
Among the 79 significantly associated loci identified for the six yield-related traits (Figure 3A and Supplementary Table 5), 31 were detected in at least two environments (Figure 3A and Supplementary Table 6). The associated marker A02-86439145 for PW and HSW was repeatedly detected across four environments, while 11 other such associated markers, such as B06-129049126 for PL and B04-14788349 for PW and HSW, were detected across three environments. A set of 15 markers was found to be associated with multiple traits (Figure 3B and Supplementary Table 7). Of these, four markers (A02-86439145, A06-108577126, A06-108613799, and B04-14788349) were found to be significantly associated with all six yield-related traits. Six markers (A06-108797911, B06-127760084, B06-128026082, B06-128423460, B06-129334953, and B06-129337040) were significantly associated with three of these traits. The high and positive phenotypic correlations among the yield-related traits and the common associated markers detected by GWAS suggested that it was possible to improve the multiple traits of seed (pod) size and weight through GAB.
The markers A06-108577126 and A02- 86439145 were repeatedly detected to be significantly associated with all six yield-related traits. The marker A06-108577126 was identified with ∼20% phenotypic variation explained (PVE) of PW in three environments and of HPW, HSW, PL, SL, and SW in two environments (Table 3). The highest phenotypic variance explained by this significant association was 36.27% for SL in E2. A similar phenomenon was observed with the marker A02-86439145 (Table 3). The results suggested that the candidate genomic regions on A06 and A02 might contain major and stable loci regulating the yield-related traits.
Candidate genes were selected in the genomic regions if they encode components of metabolic or signaling pathways known to be related to the corresponding phenotypes or based on the expression profile (for example, tissue-specific expression) of peanut (Clevenger et al., 2016). A total of 17 and 29 candidate genes for the yield traits were discovered in the genomic regions underlying the A06-108577126 and A02-86439145, respectively (Table 4). The AP2 transcription factor (Aradu.GG3LG) is the homologous gene of AINTEGUMENTA (ANT, AT4G37750) which has been reported to regulate seed size through regulating the growth of the embryo organ and the proliferation of cell number in Arabidopsis (Mizukami and Fischer, 2000). The EamA-like transporter family protein (Aradu.K0L5G) was found to be specifically expressed in seeds and pods according to expression profile using RNA sequencing data from 22 tissues of A. hypogaea L. (Clevenger et al., 2016). A set of 16 candidate genes was predicted to code for cytochrome P450s, which were reported to be involved in the regulation of cell proliferation in the embryo and endosperm (Zhang et al., 2012; Nagasawa et al., 2013). A set of 13 candidate genes was predicted to code for receptor kinases, which were reportedly involved in the CLAVATA–WUSCHEL (CLV-WUS) pathway that affected seed size by controlling the locule number (Somssich et al., 2016). Seven genes were predicted to code for transcription factors, which controlled fruit size by affecting the fertility, seed number, cell number, and cell size (De Folter et al., 2004; Swaminathan et al., 2008; Zhang et al., 2016). Two candidate genes were predicted to code for ubiquitin protein, which were reported to regulate fruit size by influencing cell proliferation (Wang Z. et al., 2016). Two genes were predicted to code for beta-fructofuranosidases, which were reported to affect seed size by affecting cell division and cell elongation (Jofuku et al., 2005). A single gene each encoded G-protein, cytokinin dehydrogenase, sugar transport protein, and UDP-glycosyltransferase protein, which was reported in regulating seed size (Hussain et al., 2020).
To validate the diagnostic marker for seed (pod) size and weight, we investigated the correlation between nucleotide polymorphism in the candidate genes and phenotypic variation in the association panel. There were 15 non-synonymous SNPs in seven candidate genes in the genomic region underlying the A06-108577126 and 23 non-synonymous SNPs in eight candidate genes in the genomic region underlying the A02-86439145. The allelic effect of each genotypic class for the associated target trait was estimated in different environments. Unfortunately, we did not find non-synonymous SNP to correlate with phenotypic variation in the genomic region underlying the A02-86439145, but in the genomic region underlying the A06-108577126, we found the non-synonymous SNP (G→T, Ala→Ser, located in Aradu.K0L5G) at A06-107901527 (Figure 4A) which showed that the GG genotype contained a significantly higher (P < 0.001) seed (pod)-related trait value than those having TT genotype in our panel under different environments (Figure 4B).
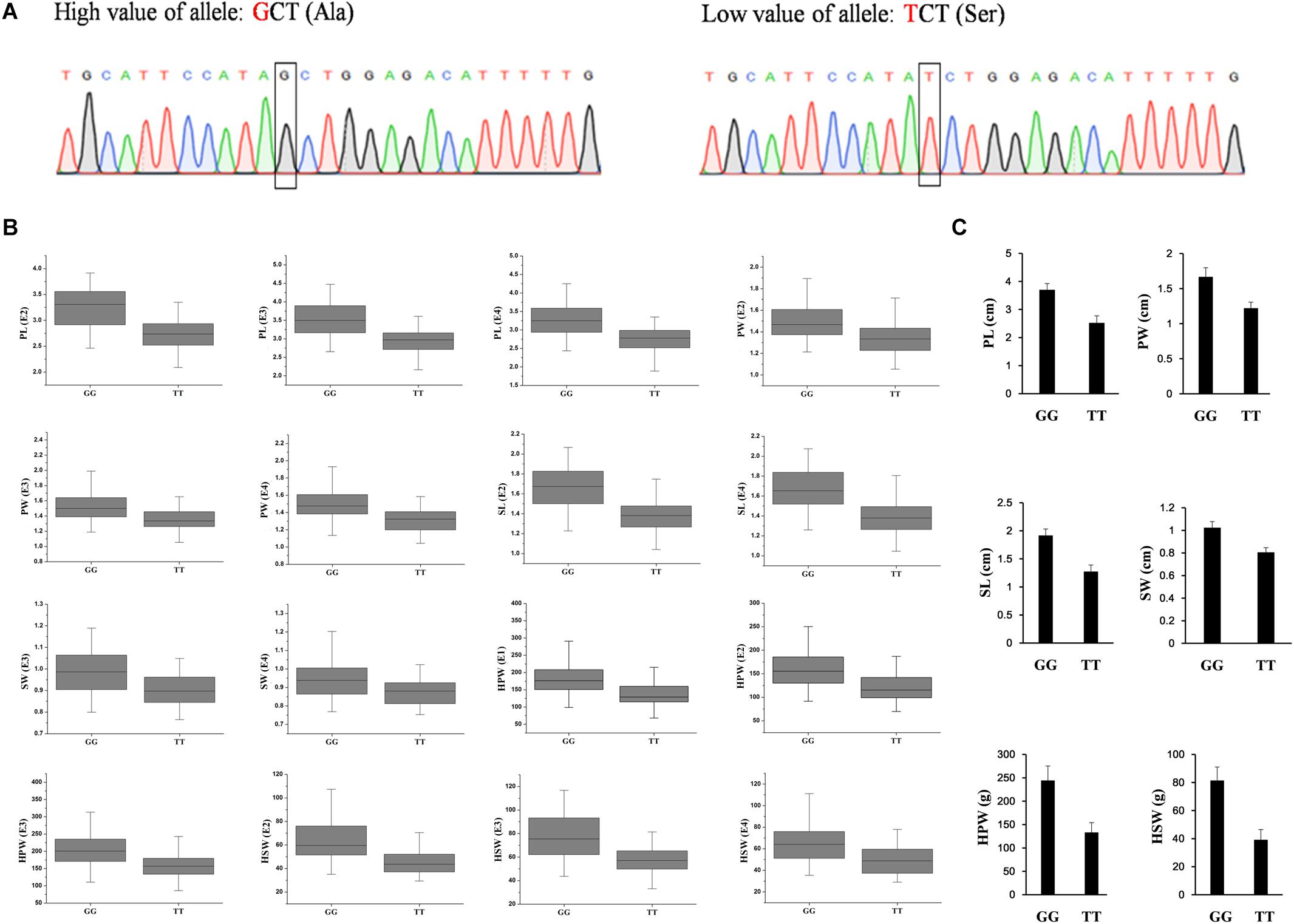
Figure 4. The validated diagnostic marker on A06 for the six yield-related traits. (A) The partial sequence diagrams included the diagnostic locus of the non-synonymous single-nucleotide polymorphism (G to T) after amplifying by polymerase chain reaction and sequencing. (B) The allelic effect at A06-107901527 for the significant associated target trait in different environments (P < 0.001) in the association panel. (C) The accessions with GG alleles show a significantly higher value of seed (pod) size and weight than those with TT allele (P < 0.001, Student’s t-test) in two extreme trait groups of peanuts. PL, pod length; PW, pod width; SL, seed length; SW, seed width; HPW, hundred-pod weight; HSW, hundred-seed weight; E1, Nanchong in 2015; E2, Wuhan in 2015; E3, Nanchong in 2016; E4, Wuhan in 2016.
In addition, two diverse extreme trait groups of 25 accessions with high trait values and 25 accessions with low trait values of seed (pod) size and weight were surveyed using SSR primers that amplified a fragment including the SNP. PCR product sequencing revealed a clear differentiation between the accessions with high and low values of seed (pod)-related traits, with the high value accessions having the GG genotype and the low value accessions having the TT genotype (Figure 4C). The results indicated that the SNP of Aradu-A06-107901527 could be used as a diagnostic marker for seed (pod) size and weight in peanut and would be useful for marker-assisted breeding of high-yield peanut varieties.
The Chinese peanut mini-core collection was selected from 6,390 peanut germplasm resources in our previous study (Jiang et al., 2008). Here we used an abundant diversity of the collection (Jiang et al., 2008, 2010, 2014) combined with GBS sequencing for genetic mapping to dissect the genetic basis of yield traits. The method has been shown to provide sufficient power to map major effect loci, even to the level of individual causal SNPs in some previous studies (Wang H. et al., 2016). Fortunately, the high-quality reference genomes for both peanut progenitors, A. duranensis (A genome) and A. ipaensis (B genome) (Bertioli et al., 2016; Chen X. et al., 2016; Lu et al., 2018), are available with annotations. Assemblies of the reference genome have also become available for cultivated peanut (Bertioli et al., 2019; Chen et al., 2019; Zhuang et al., 2019) and will increase the efficiency of such studies in the future. In this study, we have used progenitor genome sequence assemblies for calling SNPs in the Chinese peanut mini-core collection as reported in some recent publications (Chavarro et al., 2019; Gangurde et al., 2020; Huang et al., 2020; Yu et al., 2020). Finally, 105,814 high-quality genome-wide SNPs were retained in this study, which is, so far, the highest number of markers used for performing association analysis in peanut.
Population structure is an important component in association mapping analysis, and it helps in reducing the detection of false positives among associated markers. The structure analysis classified the Chinese peanut mini-core collection into two groups and a mixed one. The peanut germplasm collections in previous studies could be divided into two to four groups, which were always associated with different botanical subspecies or botanical types (Wang et al., 2011; Jiang et al., 2014; Zhang et al., 2017; Zheng et al., 2018). In the present study, most of the accessions of two subspecies of ssp. fastigiata and ssp. hypogaea could be clearly divided into GI and GII, respectively, but it was hard to clearly distinguish the botanical types because many accessions in this population harbored features of mixed morphological types. The phenomenon may be due to the genetic exchange between varieties from different botanical types. Further analysis found that G I contained two subgroups of ssp. fastigiata accessions mainly from southern China and countries other than China, while G II contained four subgroups of ssp. hypogaea accessions mainly from northern China, southern China, central China, and countries other than China, suggesting that obvious geographical distribution patterns existed in the subgroups. A similar phenomenon was observed in many other crops, such as rice, soybean, and sorghum (Morris et al., 2013; Zhou et al., 2015; Wang H. et al., 2019). The result suggested that different geographic origins of accessions harbored different genetic characteristics, probably enabling peanut accessions to adapt to various ecological environments.
In this study, 79 significantly associated loci were identified for six yield-related traits (Figure 3B and Supplementary Table 5). The number of QTLs detected for seed (pod) size and weight was significantly higher than that identified in previous genetic mapping studies (Huang et al., 2015; Zhang et al., 2017; Zhao et al., 2017). Some of the associated loci identified in this study were consistent with QTLs obtained from segregating populations. The marker A02-86439145 was found to be associated with all six yield-related traits. The SNP A02-86439292 was another associated marker located 147 bp away from SNP A02-86439145 and explained 20.85% of the phenotypic variance for PL in E4. The genomic region underlying A02-86439145 and A02-86439292 was located within the QTL of q100SW2 for HSW in one environment (PVE: 24.69%) and qSL2 for SL in four environments (PVE ranged from 42.43 to 61.74%) in Huayu36 × 6-13 population (Zhang et al., 2019). Another significant associated marker B04-14788349 was detected to be associated with SW and HPW in two environments, PW and HSW in three environments, and PL and SL in a single environment. The genomic region underlying B04-14788349 was co-localized within the qSLB04 for SL, qSWB04.3 for SW, and qHSWB04.4 for HSW of the Zhonghua 5 × ICGV 86699 population (Zhou, 2018). Moreover, 18 associations were consistently detected in two environments, 11 associations were repeatedly detected in three environments, and two associations were detected across four environments (Figure 3A and Supplementary Table 6). The associations detected in different populations or environments achieved mutual corroboration, indicating that these associated loci were stable and reliable. On the other hand, most of the associated loci identified in this study were new, such as A06-108577126 for the six yield-related traits and B06-127760084 for HSW, PL, and SL. The results showed that GBS-based GWAS was effective in revealing the genetic basis of seed (pod) size and weight.
Two important stable major associations were identified on pseudomolecules A06 and A02, and the PVE of the associations for yield-related traits could reach about 20% under multi-environments (Table 3). Our analysis also suggested 1.3 Mb LD distance in the association panel, which was similar to the results of two previous studies by Zhao et al. (2017) and Liu et al. (2020). In the candidate genomic region underlying A06-108577126, the AP2 transcription factor (Aradu.GG3LG) was identified. Its homologous gene (ANT) had been well studied in Arabidopsis, and it controlled seed size through regulating the growth of the embryonic organ and the proliferation of cell number. However, information on the functional role of this gene in peanut is limited, making seed (pod) development a good concern in future research. In the same genomic region, the gene Aradu.K0L5G was found to be specifically expressed in seeds and pods. It was worth noting that the sequence polymorphism of the non-synonymous SNP located at A06-107901527 in Aradu.K0L5G was highly correlated with the phenotypic variation of the six yield-related traits. The SNP changed the base from G to T and resulted in the amino acid from Ala to Ser in Aradu.K0L5G. The homologous gene of Aradu.K0L5G in Arabidopsis (At4g32140) was reported to be a cell cycle-regulated gene, showing a significant fluctuation and a peak expression in the S phase (Menges et al., 2002). The two candidate genes represented excellently preferred candidates for follow-up analysis of causal polymorphisms and were ultimately confirmed by transformation. In the genomic regions underlying A06-108577126 and A02-86439145, no correlation between sequence variation and phenotypic variation was observed in the other 44 candidate genes for seed size and weight. Because the proteins encoded by these genes were reported to be involved in related pathways, these genes might be involved in regulating seed (pod) size and weight as well. We could not detect correlations between sequence variations and phenotypic variations in these genes, possibly because there were still not enough SNPs. Alternatively, some unknown genes or genomic components involved in trans-regulatory or epistatic interactions may be responsible for the indicated associated loci.
One of the main advantages of sequencing-based trait mapping approaches is the development of markers for target traits (Pandey et al., 2017). Diagnostic markers for some traits have been identified in peanut, such as rust resistance, late leaf spot resistance, and bacterial wilt resistance (Pandey et al., 2017; Luo et al., 2019). The diagnostic marker Aradu-A06-107901527 for seed (pod) size and weight was successfully validated in this study. Its sequence variation was correlated with phenotype variation and could be used to distinguish peanut accessions with corresponding high or low trait values. The allelic effect of the SNP was stable in multi-environments. This character allows breeders to avoid many difficulties in checking its stable performance in different places for higher field suitability. The diagnostic marker developed in this study could be applied to accelerate the marker-assisted breeding program of high-yield peanut varieties.
In conclusion, the results provided a large data set of genomic variation for diverse varieties and a comprehensive presentation of associated loci for six yield-related traits in peanut. Putative candidate genes underlying stable major associated loci were discovered, and the correlation was investigated between nucleotide polymorphism and phenotype variation. The diagnostic marker was successfully validated and could be deployed in GAB of high-yield peanut varieties.
The datasets presented in this study can be found in online repositories. The names of the repositories and accession numbers can be found below: PRJNA687812 and PRJNA695811.
XZ and HJ conceived and designed the study. XZ and JG analyzed the data. XZ, LH, HL, NL, WC, YL, and BL performed the field trials and phenotyping. XZ wrote the manuscript. MP and RV revised and improved the manuscript. All authors read and approved the final manuscript.
This research was supported by the National Natural Science Foundation of China (31871666), Special Fund for Basic Scientific Research of Central Public Welfare Research Institutes (1610172016002), National Program for Crop Germplasm Protection of China (2020NWB033), and National Crop Germplasm Resources Center (NCGRC2020-014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Pengfei Jin for his help in SNP calling. The authors are also grateful to Jiaojiao Zhao and Huaiwen Chen for their help in the experiments. Thanks to Kede Liu and Liyun Wan for their suggestions on the article. Thanks also to Bolun Yu, Zhihui Wang, and Xiaomeng Xue for their valuable discussion.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.637284/full#supplementary-material
Supplementary Figure 1 | The geographic distribution of the accessions used in this study. Each accession is displayed as a dot on the world map. The composition (top right) of botanical subspecies and the geographic origin composition (bottom right) of the material panel used are shown.
Supplementary Figure 2 | Frequency distribution of the phenotypic variation of the six traits in the Chinese peanut mini-core collection. The averaged phenotype value of four environments for each trait was used.
Supplementary Figure 3 | Linkage disequilibrium decay for the Chinese peanut mini-core collection.
Supplementary Figure 4 | The number of different botanical subspecies in each group.
Supplementary Figure 5 | Distribution of pairwise relative kinship estimates. The values of kinship less than 0 were ordered as 0.
Supplementary Figure 6 | Genome-wide association studies for pod length in four environments.
Supplementary Figure 7 | Genome-wide association studies for pod width in four environments.
Supplementary Figure 8 | Genome-wide association studies for seed length in four environments.
Supplementary Figure 9 | Genome-wide association studies for seed width in four environments.
Supplementary Figure 10 | Genome-wide association studies for hundred-pod weight in four environments.
Supplementary Figure 11 | Genome-wide association studies for hundred-seed weight in four environments.
Supplementary Table 1 | Summary of the Chinese peanut mini-core collection.
Supplementary Table 2 | Specificities and missing data proportions of the genotype dataset before and after the missing genotypes are inferred.
Supplementary Table 3 | Detailed list of the predicted effects of annotated single-nucleotide polymorphisms.
Supplementary Table 4 | For each of the six yield-related traits, the number of single-nucleotide polymorphisms (SNPs) and the number of repeatedly detected SNPs in different environments.
Supplementary Table 5 | Detailed information of the genome-wide significant association signals for the yield-related traits using the MLM (PCA + K) model.
Supplementary Table 6 | The repeated detected single-nucleotide polymorphism for each trait in different environments.
Supplementary Table 7 | The single-nucleotide polymorphism detected to be associated with multiple traits.
Alhaddad, H., Khan, R., Grahn, R. A., Gandolfi, B., Mullikin, J. C., Cole, S. A., et al. (2013). Extent of linkage disequilibrium in the domestic cat, Felis silvestris catus, and its breeds. PLoS One 8:e53537. doi: 10.1371/journal.pone.0053537
Bertioli, D. J., Cannon, S. B., Froenicke, L., Huang, G., Farmer, A. D., Cannon, E. K., et al. (2016). The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48, 438–446.
Bertioli, D. J., Jenkins, J., Clevenger, J., Gao, D., Dudchenko, O., Seijo, G., et al. (2019). The genome sequence of peanut (Arachis hypogaea), a segmental allotetraploid. Nat. Genet. 51, 877–884.
Bradbury, P. J., Zhang, Z., Kroon, D., Casstevens, T. M., Ramdoss, Y., and Buckler, E. S. (2007). TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. doi: 10.1093/bioinformatics/btm308
Browning, B. L., and Yu, Z. (2009). Simultaneous genotype calling and haplotype phasing improves genotype accuracy and reduces false-positive associations for genome-wide association studies. Am. J. Hum. Genet. 85, 847–861. doi: 10.1016/j.ajhg.2009.11.004
Chavarro, C., Chu, Y., Holbrook, C. C., Isleib, T., Bertioli, D., Hovav, R., et al. (2019). Genetic analysis of seed and pod traits in a set of recombinant inbred lines (RILs) in peanut (Arachis hypogaea L.). bioRxiv [Preprint]. 738914.
Chen, W., Jiao, Y., Cheng, L., Huang, L., Liao, B., Tang, M., et al. (2016). Quantitative trait locus analysis for pod- and kernel-related traits in the cultivated peanut (Arachis hypogaea L.). BMC Genet. 17:25. doi: 10.1186/s12863-016-0337-x
Chen, X., Li, H., Pandey, M. K., Yang, Q., Wang, X., Garg, V., et al. (2016). Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proc. Natl. Acad. Sci. U.S.A. 113, 6785–6790. doi: 10.1073/pnas.1600899113
Chen, X., Lu, Q., Liu, H., Zhang, J., Hong, Y., Lan, H., et al. (2019). Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement. Mol. Plant 12, 920–934. doi: 10.1016/j.molp.2019.03.005
Clevenger, J., Chu, Y., Scheffler, B., and Ozias-Akins, P. (2016). A developmental transcriptome map for allotetraploid Arachis hypogaea. Front. Plant Sci. 7:1446. doi: 10.3389/fpls.2016.01446
De Folter, S., Busscher, J., Colombo, L., Losa, A., and Angenent, G. C. (2004). Transcript profiling of transcription factor genes during silique development in Arabidopsis. Plant Mol. Biol. 56, 351–366. doi: 10.1007/s11103-004-3473-z
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 14, 2611–2620. doi: 10.1111/j.1365-294x.2005.02553.x
Fan, C. C., Xing, Y. Z., Mao, H. L., Lu, T. T., Han, B., Xu, C. G., et al. (2006). GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. doi: 10.1007/s00122-006-0218-1
Foley, J. A., Ramankutty, N., Brauman, K. A., Cassidy, E. S., Gerber, J. S., Johnston, M., et al. (2011). Solutions for a cultivated planet. Nature 478, 337–342.
Fonceka, D., Tossim, H. A., Rivallan, R., Vignes, H., Faye, I., Ndoye, O., et al. (2012). Fostered and left behind alleles in peanut: interspecific QTL mapping reveals footprints of domestication and useful natural variation for breeding. BMC Plant Biol. 12:26. doi: 10.1186/1471-2229-12-26
Gangurde, S. S., Wang, H., Yaduru, S., Pandey, M. K., Fountain, J. C., Chu, Y., et al. (2020). Nested-association mapping (NAM)-based genetic dissection uncovers candidate genes for seed and pod weights in peanut (Arachis hypogaea). Plant Biotechnol. J. 18, 1457–1471. doi: 10.1111/pbi.13311
Hallauer, A. R., and Miranda, J. B. (1998). Quantitative Genetics in Maize Breeding, 2nd Edn. Ames, IA: Iowa State University Press.
Huang, L., He, H. Y., Chen, W. G., Ren, X. P., Chen, Y. N., Zhou, X. J., et al. (2015). Quantitative trait locus analysis of agronomic and quality-related traits in cultivated peanut (Arachis hypogaea L.). Theor. Appl. Genet. 128, 1103–1115. doi: 10.1007/s00122-015-2493-1
Huang, L., Liu, X., Pandey, M. K., Ren, X., Chen, H., Xue, X., et al. (2020). Genome-wide expression quantitative trait locus analysis in a recombinant inbred line population for trait dissection in peanut. Plant Biotechnol. J. 18, 779–790. doi: 10.1111/pbi.13246
Hussain, Q., Shi, J., Scheben, A., Zhan, J., Wang, X., Liu, G., et al. (2020). Genetic and signalling pathways of dry fruit size: targets for genome editing-based crop improvement. Plant Biotechnol. J. 18, 1124–1140. doi: 10.1111/pbi.13318
Jia, G., Huang, X., Zhi, H., Zhao, Y., Zhao, Q., Li, W., et al. (2013). A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 45, 957–961. doi: 10.1038/ng.2673
Jiang, H., Duan, N., and Ren, X. (2006). Descriptors and Data Standard for Peanut (Arachis spp.). Beijing: China Agriculture Press, 26–27.
Jiang, H., Huang, L., Ren, X., Chen, Y., Zhou, X., Xia, Y., et al. (2014). Diversity characterization and association analysis of agronomic traits in a Chinese peanut (Arachis hypogaea L.) mini-core collection. J. Integr. Plant Biol. 56, 159–169. doi: 10.1111/jipb.12132
Jiang, H., Ren, X., Huang, J., Liao, B., and Lei, Y. (2008). Establishment of peanut mini core collection in China and exploration of new resource with high oleat. Chin. J. Oil Crop Sci. 30, 294–299.
Jiang, H., Ren, X., Zhang, X., Huang, J., Wang, S., Lei, Y., et al. (2010). Genetic diversity of peanut mini-core collection detected by SSR markers. Chin. J. Oil Crop Sci. 32, 472–478.
Jofuku, K. D., Omidyar, P. K., Gee, Z., and Okamuro, J. K. (2005). Control of seed mass and seed yield by the floral homeotic gene APETA-LA2.Proc. Natl. Acad. Sci. U.S.A. 102, 3117–3122. doi: 10.1073/pnas.0409893102
Korte, A., and Farlow, A. (2013). The advantages and limitations of trait analysis with GWAS: a review. Plant Methods 9:29. doi: 10.1186/1746-4811-9-29
Li, J., Yu, C., Wu, H., Luo, Z., Ouyang, B., Cui, L., et al. (2015). Knockdown of a JmjC domain-containing gene JMJ524 confers altered gibberellin responses by transcriptional regulation of GRAS protein lacking the DELLA domain genes in tomato. J. Exp. Bot. 66, 1413–1426. doi: 10.1093/jxb/eru493
Liu, J., Hua, W., Hu, Z., Yang, H., Zhang, L., Li, R., et al. (2015). Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl. Acad. Sci. U.S.A. 112, 5123–5132.
Liu, N., Guo, J., Zhou, X., Wu, B., Huang, L., Luo, H., et al. (2020). High-resolution mapping of amajor and consensus quantitative trait locus for oil content to a ~0.8-Mb region on chromosome A08 in peanut (Arachis hypogaea L.). Theor. Appl. Genet. 133, 37–49. doi: 10.1007/s00122-019-03438-6
Lu, Q., Li, H., Hong, Y., Zhang, G., Wen, S., Li, X., et al. (2018). Genome sequencing and analysis of the peanut B-genome progenitor (Arachis ipaensis). Front. Plant Sci. 3:604. doi: 10.3389/fpls.2018.00604
Luo, H., Guo, J., Ren, X., Chen, W., Huang, L., Zhou, X., et al. (2018). Chromosomes A07 and A05 associated with stable and major QTLs for pod weight and size in cultivated peanut (Arachis hypogaea L.). Theor. Appl. Genet. 131, 267–282. doi: 10.1007/s00122-017-3000-7
Luo, H., Pandey, M. K., Khan, A. W., Wu, B., Guo, J., Ren, X., et al. (2019). Next-generation sequencing identified genomic region and diagnostic markers for resistance to bacterial wilt on chromosome B02 in peanut (Arachis hypogaea L.). Plant Biotechnol. J. 17, 2356–2369. doi: 10.1111/pbi.13153
Mather, K. A., Caicedo, A. L., Polato, N. R., Olsen, K. M., McCouch, S., and Purugganan, M. D. (2007). The extent of linkage disequilibrium in rice (Oryza sativa L.). Genetics 177, 2223–2232. doi: 10.1534/genetics.107.079616
Menges, M., Hennig, L., Gruissem, W., and Murray, J. A. (2002). Cell cycle-regulated gene expression in Arabidopsis. J. Biol. Chem. 277, 41987–42002.
Mizukami, Y., and Fischer, R. L. (2000). Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Sci. U.S.A. 97, 942–947. doi: 10.1073/pnas.97.2.942
Morris, G. P., Ramu, P., Deshpande, S. P., Hash, C. T., Shah, T., Upadhyaya, H. D., et al. (2013). Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. U.S.A. 110, 453–458. doi: 10.1073/pnas.1215985110
Nagasawa, N., Hibara, K. I., Heppard, E. P., Velden, K. A. V., Luck, S., Beatty, M., et al. (2013). GIANT EMBRYO encodes CYP78A13, required for proper size balance between embryo and endosperm in rice. Plant J. 75, 592–605. doi: 10.1111/tpj.12223
Pandey, M. K., Khan, A. W., Singh, V. K., Vishwakarma, M. K., Shasidhar, Y., Kumar, V., et al. (2017). QTL-seq approach identified genomic regions and diagnostic markers for rust and late leaf spot resistance in groundnut (Arachis hypogaea L.). Plant Biotechnol. J. 15, 927–941. doi: 10.1111/pbi.12686
Pandey, M. K., Pandey, A. K., Kumar, R., Nwosu, V., Guo, B., Wright, G., et al. (2020). Translational genomics for achieving higher genetic gains in groundnut. Theor. Appl. Genet. 133, 1679–1702. doi: 10.1007/s00122-020-03592-2
Pandey, M. K., Upadhyaya, H. D., Rathore, A., Vadez, V., Sheshshayee, M. S., Sriswathi, M., et al. (2014). Genome wide association studies for 50 agronomic traits in peanut using the ‘reference set’ comprising 300 genotypes from 48 countries of the semi-arid tropics of the world. PLoS One 9:105228. doi: 10.1371/journal.pone.0105228
Peng, Y., Hu, Y. Y., Mao, B. G., Xiang, H. T., Shao, Y., Pan, Y. L., et al. (2015). Genetic analysis for rice grain quality traits in the YVB stable variant line using RAD-seq. Mol. Genet. Genomics 291, 297–307. doi: 10.1007/s00438-015-1104-9
Pritchard, J. K., Stephens, M., and Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959.
Schlotzhauer, S. D., and Littell, R. C. (1997). SAS System for Elementary Statistical Analysis. Cary, NC: SAS Institute Inc.
Shirasawa, K., Koilkonda, P., Aoki, K., Hirakawa, H., Tabata, S., Watanabe, M., et al. (2012). In silico polymorphism analysis for the development of simple sequence repeat and transposon markers and construction of linkage map in cultivated peanut. BMC Plant Biol. 12:80. doi: 10.1186/1471-2229-12-80
Somssich, M., Je, B. I., Simon, R., and Jackson, D. (2016). CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143, 3238–3248. doi: 10.1242/dev.133645
Swaminathan, K., Peterson, K., and Jack, T. (2008). The plant B3 super family. Trends Plant Sci. 13, 647–655. doi: 10.1016/j.tplants.2008.09.006
Tilman, D., Balzer, C., Hill, J., and Befort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U.S.A. 108, 20260–20264.
Turner, S. D. (2018). Qqman: an R package for visualizing GWAS results using QQ and Manhattan plots. J. Open Source Softw. 3:731. doi: 10.21105/joss.00731
Wang, H., Xu, X., Vieira, F. G., Xiao, Y., Li, Z., Wang, J., et al. (2016). The power of inbreeding: NGS-based GWAS of rice reveals convergent evolution during rice domestication. Mol. Plant 9, 975–985. doi: 10.1016/j.molp.2016.04.018
Wang, H., Zhu, S. S., Dang, X. J., Liu, E. B., Hu, X. X., Eltahawy, M. S., et al. (2019). Favorable alleles mining for gelatinization temperature, gel consistency and amylose content in Oryza sativa by association mapping. BMC Genet. 20:34. doi: 10.1186/s12863-019-0735-y
Wang, J., Yan, C., Li, Y., Li, C., Zhao, X., Yuan, C., et al. (2019). GWAS discovery of candidate genes for yield-related traits in peanut and support from earlier QTL mapping studies. Genes 10:E803.
Wang, K., Li, M., and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38:e164. doi: 10.1093/nar/gkq603
Wang, M. L., Sukumaran, S., Barkley, N. A., Chen, Z., Chen, C. Y., Guo, B., et al. (2011). Population structure and marker-trait association analysis of the US peanut (Arachis hypogaea L.) mini-core collection. Theor. Appl. Genet. 123, 1307–1317. doi: 10.1007/s00122-011-1668-7
Wang, Z., Li, N., Jiang, S., Gonzalez, N., Huang, X., Wang, Y., et al. (2016). SCFSAP controls organ size by targeting PPD proteins for degradation in Arabidopsis thaliana. Nat. Commun. 7:11192.
Wei, X., Liu, K., Zhang, Y., Feng, Q., Wang, L., Zhao, Y., et al. (2015). Genetic discovery for oil production and quality in sesame. Nat. Commun. 6:8609.
Weir, B. S. (1996). Genetic Data Analysis 2: Methods for Discrete Population Genetic Data. Sunderland, MA: Sinauer Associates, 445.
Yan, J., Shah, T., Warburton, M. L., Buckler, E. S., McMullen, M. D., and Crouch, J. (2009). Genetic characterization and linkage disequilibrium estimation of a global maize collection using SNP markers. PLoS One 4:e8451. doi: 10.1371/journal.pone.0008451
Yang, P., Shu, C., Chen, L., Xu, J. S., Wu, J. S., and Liu, K. D. (2012). Identification of a major QTL for silique length and seed weight in oil seed rape (Brassica napus L.). Theor. Appl. Genet. 125, 285–296. doi: 10.1007/s00122-012-1833-7
Yu, B., Jiang, H., Pandey, M. K., Huang, L., Huai, D., Zhou, X., et al. (2020). Identification of two novel peanut genotypes resistant to aflatoxin production and their SNP markers associated with resistance. Toxins 12:156. doi: 10.3390/toxins12030156
Zhang, P., Allen, W. B., Nagasawa, N., Ching, A. S., Heppard, E. P., Li, H., et al. (2012). A transposable element insertion within ZmGE2 gene is associated with increase in embryo to endosperm ratio in maize. Theor. Appl. Genet. 125, 1463–1471. doi: 10.1007/s00122-012-1926-3
Zhang, S., Hu, X., Miao, H., Chu, Y., Cui, F., Yang, W., et al. (2019). QTL identification for seed weight and size based on a high-density SLAF-seq genetic map in peanut (Arachis hypogaea L.). BMC Plant Biol. 19:537. doi: 10.1186/s12870-019-2164-5
Zhang, X., Zhang, J., He, X., Wang, Y., Ma, X., and Yin, D. (2017). Genome-wide association study of major agronomic traits related to domestication in peanut. Front. Plant Sci. 8:1611. doi: 10.3389/fpls.2017.01611
Zhang, Y., Shen, Y. Y., Wu, X. M., and Wang, J. B. (2016). The basis of pod dehiscence: anatomical traits of the dehiscence zone and expression of eight pod shatter-related genes in four species of Brassicaceae. Biol. Plant. 60, 343–354. doi: 10.1007/s10535-016-0599-1
Zhao, J., Huang, L., Ren, X., Manish, K. P., Wu, B., Chen, Y., et al. (2017). Genetic variation and association mapping of seed-related traits in cultivated peanut (Arachis hypogaea L.) using single locus simple sequence repeat markers. Front. Plant Sci. 8:2105. doi: 10.3389/fpls.2017.02105
Zheng, Z., Sun, Z., Fang, Y., Qi, F., Liu, H., Miao, L., et al. (2018). Genetic diversity, population structure, and botanical variety of 320 global peanut accessions revealed through tunable genotyping-by-sequencing. Sci. Rep. 8:14500.
Zhou, X. (2018). “Quantitative trait locus analysis of seed size related traits using high density SNP genetic linkage map,” in Proceedings of the 8th Annual Conference of Oil Crops Committee of Chinese Crop Society, (Qingdao: Agriculture Press), 195.
Zhou, X., Lei, Y., Xia, Y., Qi, Y., Yan, L., Ren, X., et al. (2019). QTL mapping for traits of pod size and weight in cultivated peanut (Arachis hypogaea L.). Chin. J. Oil Crop Sci. 41, 869–877.
Zhou, Z., Jiang, Y., Wang, Z., Gou, Z., Lyu, J., Li, W., et al. (2015). Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 33, 408–414. doi: 10.1038/nbt.3096
Zhuang, W., Chen, H., Yang, M., Wang, J., Pandey, M. K., Zhang, C., et al. (2019). The Arachis hypogaea genome elucidates legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 51, 865–876.
Keywords: yield-related traits, mini-core collection, GWAS, diagnostic marker, peanut
Citation: Zhou X, Guo J, Pandey MK, Varshney RK, Huang L, Luo H, Liu N, Chen W, Lei Y, Liao B and Jiang H (2021) Dissection of the Genetic Basis of Yield-Related Traits in the Chinese Peanut Mini-Core Collection Through Genome-Wide Association Studies. Front. Plant Sci. 12:637284. doi: 10.3389/fpls.2021.637284
Received: 03 December 2020; Accepted: 24 March 2021;
Published: 20 May 2021.
Edited by:
Sergio J. Ochatt, INRA UMR 1347 Agroécologie, FranceReviewed by:
Mahendar Thudi, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), IndiaCopyright © 2021 Zhou, Guo, Pandey, Varshney, Huang, Luo, Liu, Chen, Lei, Liao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifang Jiang, cGVhbnV0bGFiQG9pbGNyb3BzLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.