- Departamento de Química Orgánica, Ciudad Universitaria, Facultad de Ciencias Exactas y Naturales, Consejo Nacional de Investigaciones Científicas y Técnicas, Centro de Investigaciones en Hidratos de Carbono (CIHIDECAR/CONICET), Universidad de Buenos Aires, Buenos Aires, Argentina
In the current review, compositional data on fucoidans extracted from more than hundred different species were surveyed through the available literature. The analysis of crude extracts, purified extracts or carefully isolated fractions is included in tabular form, discriminating the seaweed source by its taxonomical order (and sometimes the family). This survey was able to encounter some similarities between the different species, as well as some differences. Fractions which were obtained through anion-exchange chromatography or cationic detergent precipitation showed the best separation patterns: the fractions with low charge correspond mostly to highly heterogeneous fucoidans, containing (besides fucose) other monosaccharides like xylose, galactose, mannose, rhamnose, and glucuronic acid, and contain low-sulfate/high uronic acid proportions, whereas those with higher total charge usually contain mainly fucose, accompanied with variable proportions of galactose, are highly sulfated and show almost no uronic acids. The latter fractions are usually the most biologically active. Fractions containing intermediate proportions of both polysaccharides appear at middle ionic strengths. This pattern is common for all the orders of brown seaweeds, and most differences appear from the seaweed source (habitat, season), and from the diverse extraction, purification, and analytitcal methods. The Dictyotales appear to be the most atypical order, as usually large proportions of mannose and uronic acids appear, and thus they obscure the differences between the fractions with different charge. Within the family Alariaceae (order Laminariales), the presence of sulfated galactofucans with high galactose content (almost equal to that of fucose) is especially noteworthy.
Introduction: Aim of the Review
Fucoidans are sulfated polysaccharides present in the cell walls of the Phaeophyceae (brown seaweeds) composed usually by fucose (Fuc) as the main monosaccharide, but accompanied by very variable amounts of other monosaccharides like galactose (Gal), xylose (Xyl), mannose (Man), rhamnose (Rha), and/or glucuronic acid (GlcA). The scientific literature on different aspects of fucoidans is steadily growing, mostly due to the diverse biological activities found for samples from many different species of seaweeds. This bioactivity (antiviral, anticoagulant, antitumoral, antioxidant, among others) has been reviewed extensively (Cosenza et al., 2017; Senthilkumar et al., 2017; Wang et al., 2019). Many studies attempted to explore the structural details of fucoidans, but it was very difficult to find a common trait in the different fucoidans so far analyzed (Bilan and Usov, 2008; Kopplin et al., 2018). This marks a big difference with red seaweed sulfated galactans, showing an unchallenged disaccharidic repeating structure modified by the position of sulfation, the series of the α-galactose units and its possible presence as a 3,6-anhydro ether (Usov, 2011). For these galactans, it has been found that the taxonomic order (or sometimes the family) to which the seaweed yielding the galactan belongs has a strong influence on the characteristics of these galactans, i.e., chemotaxonomy appears to be in effect (Miller, 1997; Stortz and Cerezo, 2000). For instance, within the brown seaweeds, it has been postulated that the fucoidans from the Laminariales tend to have just α-3-linked Fuc units, whereas those of the Fucales show more proportions of a α-(1,3)-α-(1,4) alternating structure (Deniaud-Bouët et al., 2014), as a chemotaxonomical trait related to structure. A previous review by Ale et al. (2011) has tried to establish some relationship with taxonomy, with the focus set on extraction methods, qualitative compositional data, and structural features. In this review, compositional data on fucoidans originated in different taxonomic groups of the Phaeophyceae will be presented. Two hypotheses are put into consideration: (a) that there is a relationship between some of these compositional features and the taxonomic classification, and (b) that various other factors produce the differences in composition.
Taxonomy of the Phaeophyceae
The taxonomy of brown algae (Heterokonta, Ochrophyta, Phaeophyceae) had many controversies throughout the history (Silberfeld et al., 2014). Order delineation in the Phaeophyceae has traditionally been based on the type of life cycle, reproductive aspects, mode of growth, and filamentous vs. parenchymatous construction of the thallus (Rousseau and de Reviers, 1999a, b). However, with the advent of molecular systematics, new insights were brought, thoroughly reshaping the evolutionary concepts of brown algae. Rousseau and de Reviers (1999b) and de Reviers et al. (2007) have provided a detailed evolution of classificatory concepts within the Phaeophyceae. Several changes in the classification at the ordinal level have been set between the Oltmanns (1922), comprising 8 orders to the present times classification, encompassing 18 orders (Silberfeld et al., 2014; Figure 1). Major changes were produced after the DNA sequencing of brown seaweeds started in 1993 (Draisma et al., 2003; de Reviers et al., 2007). Different molecular markers can be used, but phylogenetic studies of Phaeophyceae have mostly utilized the rDNA sequences, which include four subunits (18S, 5.8S, 26S, and 5S), containing regions which are highly conserved as well as others highly variable. Most information arose from studies on the 18S subunit of rDNA, although those studies had limited results for more recent Phaeophycean lineages (Tan and Druehl, 1996). In this way, Rousseau et al. (2001) utilized the 26S sequence, which altogether with a larger taxonomic sampling, solved some of the earlier divergences. Thus, a phylogenetic tree was constructed (Draisma et al., 2001, 2003). It has been concluded that morphological characters, many times useful to understand the ecology of brown seaweeds, have no value at all for phylogeny. Different degrees of organization, diffuse or apical growth, or life stages have appeared and disappeared repeatedly in the history of the different taxonomic groups.
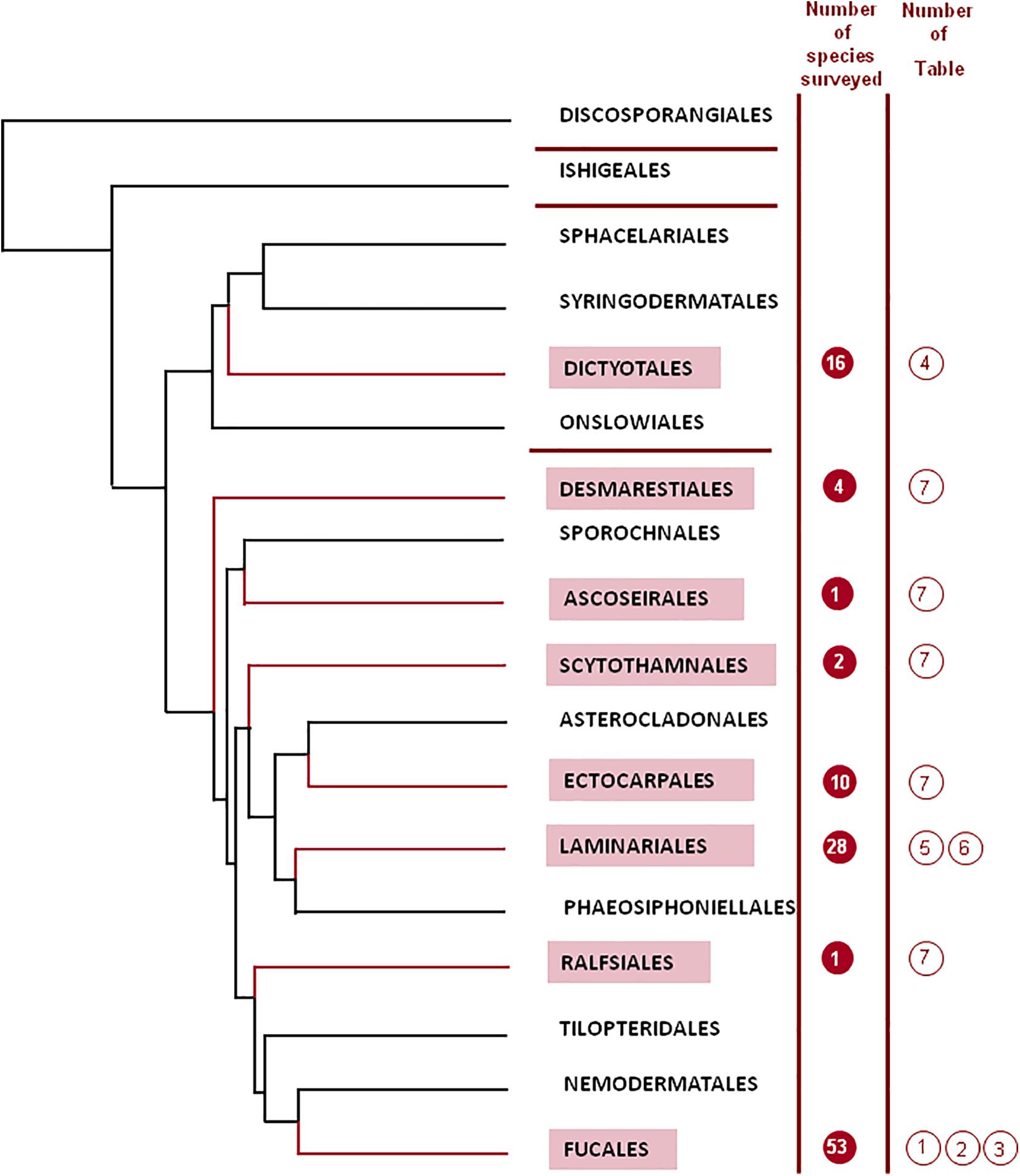
Figure 1. Phylogenetic tree for the different orders of the Phaeophyceae (adapted from Silberfeld et al., 2014; reproduced with kind permission from the authors). One diverging branch from the order Scytothamnales containing the family Bachelotiaceae has been removed from the figure for the sake of simplicity.
Silberfeld et al. (2014) have introduced a thorough phylogenetic analysis based on a dataset generated previously (Silberfeld et al., 2011), including seven markers, for a total of 6804 nucleotides, determined for 91 Phaeophycean taxa, including minor orders for which there were very few studies. In this way, the shape of phylogenetic trees changed sharply the previous knowledge (Silberfeld et al., 2011; Charrier et al., 2012). Figure 1 depicts the outcome of the tree for the 18 orders determined by Silberfeld et al. (2014), grouped in four subclasses (Discosporangiophycidae and Ishigeophycidae, including one order each, Dictyotophycidae, including four orders, and Fucophycidae, including the remaining 12 orders).
Polysaccharides From the Phaeophyceae: The Fucoidans
Most macroalgae exhibit polysaccharides as their most abundant constituents. Taking into account their function, they can be classified into two main groups: storage and structural polysaccharides. The formers are polymers such as starch/glycogen or laminaran considered as food reserve materials, whereas the latters are structural elements of the cell walls, intercellular tissues and mucilaginous matrix. Sulfated polysaccharides are a group of anionic structural polysaccharides, useful for the seaweed in the marine environment to avoid desiccation. Their gross composition is characteristic of each algal group (galactans in red seaweeds, fucoidans in brown seaweeds, rhamnoglucuronans, and arabinogalactans in green seaweeds, van den Hoek et al., 1996), whereas more or less subtle differences appear often depending on the order, family, genus and species, as well as sometimes on the season, geographic location, or reproductive stage (Mackie and Preston, 1974). Other roles of the polysaccharides might include participations in cell-cell communication (Deniaud-Bouët et al., 2014), and in cell division processes (Skriptsova, 2015).
In macroalgae, the cell walls comprise a fibrillar skeleton immersed in an amorphous matrix. In the case of the Phaeophyceae, the fibrillar skeleton is mainly made up of cellulose [a linear β-(1→4)-glucan], and the surrounding matrix is composed predominantly by alginic acid or its salts, together with a system of sulfated polysaccharides (the fucoidans; Mackie and Preston, 1974). In this way, the cell wall is composed of two different layers: the inner layer consisting of a skeleton of microfibrils providing rigidity to the cell wall, and the outermost layer, which is usually observed as a poorly crystalline matrix in which the set of microfibrils is embedded. There is also evidence that the matrix does not penetrate the fibers, but remains attached to this layer through hydrogen bonds (Davis et al., 2003). It has been suggested that fucoidans might play a key role in cell wall architecture, cross-linking cellulose and alginates (Kloareg et al., 1986). Besides this function, as occurs with other sulfated polysaccharides, the fucoidans help to protect the plant from desiccation. When the fronds are in contact with sea water the sulfate hemiester groups are strongly associated with magnesium ions, which are highly hydrated and thus retain water in the fronds (Percival, 1979). In a more modern model for the Fucales (Deniaud-Bouët et al., 2014, 2017; Torode et al., 2016), it has been proposed that two networks are assembled in the cell wall; the first one contains the fucoidans interlocking a cellulose (or other β-glucans) network, and the second one contains alginate crosslinked by polyphenols. The rigidity is controlled by the alginate structure and its calcium cross-linking capabilities, whereas the fucoidans participate mostly in adaptation to the osmotic stress.
More than one century ago, Kylin has isolated for the first time (from different seaweed species of the genera Fucus, Laminaria, and Ascophyllum) a group of sulfated polysaccharides with a high Fuc content and called them “fucoidin” (Kylin, 1913). Originally the name fucoidin (later changed to the more systematic fucoidan) was coined for the polysaccharides from those species, but this term was rapidly extended to any fucose-rich polysaccharides, including not only those becoming from brown seaweeds, but also to those present in echinoderms (Olatunji, 2020). As noted above, fucoidans are sulfated polysaccharides present mainly in the intercellular tissue of mucilaginous matrix of the cell walls of brown algae (Deniaud-Bouët et al., 2017).
Fucoidans comprise a family of diverse molecules containing, in addition to Fuc, varying proportions of Gal, Man, Xyl and GlcA (Figure 2). Acetate esters have also been found, especially in modern studies (see below). In the early studies extensive purification was carried out in an effort to isolate a “fucan” containing only Fuc residues, assuming that the remaining monosaccharides were originated in other, contaminating polysaccharides. Nevertheless, even in the allegedly pure samples, small proportions of Gal, Xyl, and/or uronic acid persisted (Percival, 1979). Later, only in a few species a pure fucan was isolated after purification (see below). Thus, most of the samples so far isolated are heterofucans (Deniaud-Bouët et al., 2014).
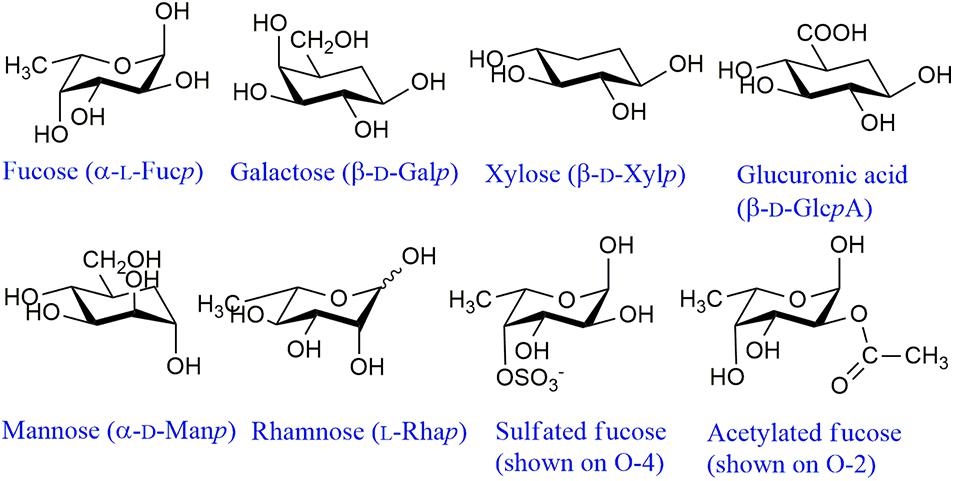
Figure 2. Main structural monosaccharidic units of fucoidans. These monosaccharides can appear as terminal non-reducing units or linked through any of the free hydroxyl groups. Usually Fuc and GlcA appear linked through O-3 or O-4, Xyl through O-4, Gal through O-3 or O-6 and Man through O-2 (Sakai et al., 2003; Bilan et al., 2010, 2017, 2018). The structural features of Rha are unknown. For representative structures of fucoidans (see Deniaud-Bouët et al., 2017).
Fucoidans From Different Species of Phaeophyceae
In this section, the main chemical characteristics of fucoidans extracted from different species of brown seaweeds reported so far to the best of our knowledge (with compositional data provided) will be described in tabular form. They will be shown separately for each of the different orders (Figure 1). When numerous species of an order were studied, separations in families or genera are also displayed. It is worth noting that depending on the way that the analyses were expressed in the original papers, the uronic acids in the following tables were indicated as a percentage of the total sample (in most cases) or as part of the molar ratio of all the monosaccharides. Thus, these molar ratios might or might not include the uronic acid components. The main monosaccharidic units appearing in fucoidans are shown in Figure 2. When the authors have isolated a large number of fractions, only those more abundant or representative are listed in the tables. The reported presence of acetyl groups is indicated qualitatively with the “Ac” acronym. It should be noted that the geographic location and season of harvest of the seaweed can also have significant effects on the composition of the extracted fucoidans (e.g., Zvyagintseva et al., 2003). The extraction and fractionation procedures are schematically displayed, neglecting defatting and depigmenting steps, as well as usual procedures like dialysis or single alcohol precipitations. The methods used for monosaccharide and sulfate quantitation are also shown.
Fucales
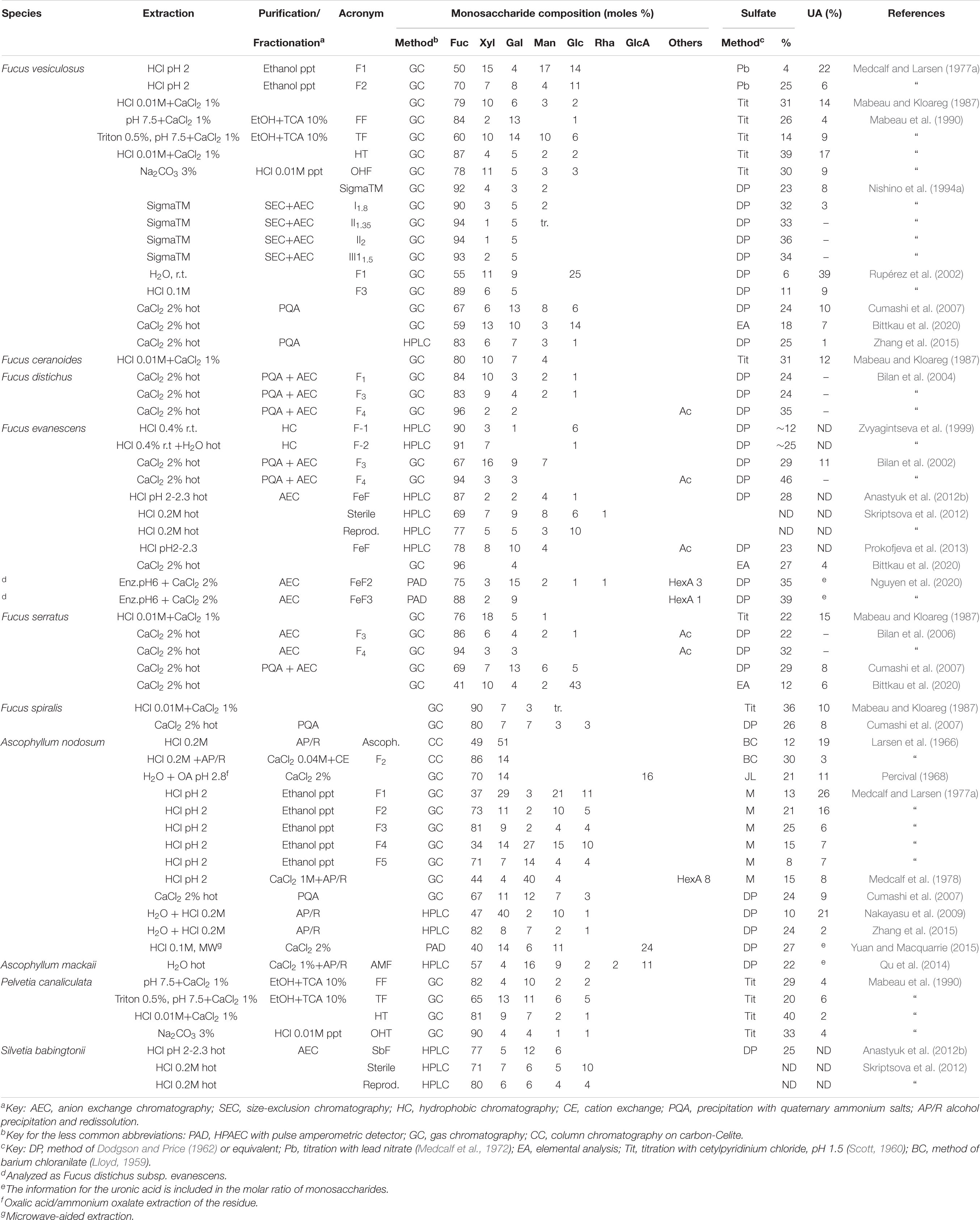
As expected, samples of fucoidans from this is order were the most studied. Samples from five different families of the Fucales have been studied. Two species from the Fucaceae, i.e., Fucus vesiculosus and Ascophyllum nodosum appear in the earlier studies by Kylin (1913). The polysaccharides from these species were studied extensively by different research groups (see below). However, the family with more species studied was the Sargassaceae. Considering only the genus Sargassum, studies on the fucoidans from 26 different species were found in the current survey.
The extraction of fucoidans from Fucus vesiculosus was originated in the early Kylin studies, when Fuc was characterized after hydrolysis as phenyl-L-fucosazone; pentoses in the hydrolyzate were also reported (Kylin, 1913). Different products from this species were extensively studied (Table 1). Originally, the presence of Xyl was ascribed to a contaminating xylan that accompanied the fucoidan (Percival and McDowell, 1967). As a matter of fact, they reported the isolation of a xylan, although uronic acid residues were found in the xylan fraction and, furthermore, the authors were not able to separate any fraction composed just by Fuc residues. The studies by Nishino et al. (1994a) on a commercial sample from this seaweed were highly comprehensive: they were able to separate 13 different fractions and analyze them thoroughly, showing structures ranging from typical fucans (containing mainly Fuc and sulfate, and free of uronic acids) to heteropolysaccharides with low sulfate content and high content of uronic acids. In a minor fraction, they were able to find an appreciable amount of glucosamine (11.5%). In an interesting study using microwave extraction of this seaweed, Rodríguez-Jasso et al. (2011) showed that depending on the pressure and extraction time, fucoidans with different ratios Fuc/Gal were obtained (ranging from 100% Fuc to a 1:1 ratio), plus variable proportions of Xyl and sulfation degrees. Another species from the same genus that has been studied is Fucus evanescens. Zvyagintseva et al. (1999) separated the polysaccharides using a chromatography system on a hydrophobic resin. It is interesting to note that in a subsequent work Zvyagintseva et al. (2003) analyzed specimens of three different seaweeds (F. evanescens, Laminaria cichorioides, and Saccharina japonica) collected at different places, at various stages of development and at different seasons, and found some notable differences, particularly for the F. evanescens equivalent fractions obtained in different geographic locations (ratio Fuc/sulfate between 1 and 2.1; Fuc proportion from 56 to 80%; molecular masses from 14–40 to 150–500 kDa).
It should be mentioned that the high proportions of Glc found in some unpurified extracts are probably becoming from laminaran. This has occurred, for instance, in the sample of Fucus serratus isolated by Bittkau et al. (2020), as lower proportions of this monosaccharide have been found in other studies (Table 1). The studies of Bilan et al. (2002, 2004, 2006) on different Fucus species, carried out with careful separations involving anion exchange chromatography have shown in all cases that at high ionic strengths, they were able to isolate, with good yields, a fucan sulfate almost devoid of other monosaccharides (Fuc ≥ 94%, Table 1, fraction F4).
Ascophyllum nodosum is the other characteristic species from the family Fucaceae which has been thoroughly studied since the early studies of Kylin (1913), followed by further reports indicating the presence of a sulfated polysaccharide with a Fuc/Gal ratio of 8:1 (Percival and McDowell, 1967). The name ascophyllan was coined (to distinguish from the fucoidan characteristic of Fucus vesiculosus) for the isolated polysaccharide, composed of Fuc, Xyl, and sulfate groups, along with uronic acids. Medcalf and Larsen (1977a, b) determined a complex mixture of polysaccharides in this seaweed, and concluded that the fucan constituted the backbone of the molecule, whereas the ascophyllan-like components were attached as branches. Besides, they also determined that the uronic acid present was not glucuronic acid, as indicated in previous reports, but mannuronic and guluronic acid, i.e., the components of alginic acid, suggesting that contamination with this polysaccharide was difficult to avoid. For the fucoidans of this seaweed, an attempt was made to compare the results of the various researchers (Table 1), taking into account that most extractions were carried out in acid medium. However, the original Fuc/Xyl ratio close to 1 found by Larsen et al. (1966) was only reproduced by Nakayasu et al. (2009). Medcalf and Larsen (1977a) found a series of highly heterogeneous fractions, whereas 1 year later, using the same seaweed sample, Medcalf et al. (1978) found a polysaccharide with a Fuc/Gal ratio close to 1. The proportion of uronic acids in purified samples varied between 2 and 21%, whereas the content of sulfate varied between 8 and 24%. In summary, no common pattern between the determinations carried out by different researchers was observed.
Within the Fucaceae, it is clear that polysaccharides from the genus Fucus tend to be fucose-rich (more than 70% of the monosaccharides), although reports diverge, and important proportions of other monosaccharides appear in some cases (Table 1). On the other hand, in the genus Ascophyllum, important proportions of Xyl and uronic acid-containing fractions appear, although some purification steps allowed to obtained fucans equivalent to those of Fucus, suggesting that mixtures of different kinds of polymers appear in all the samples that have been surveyed in this study, and they might change their proportions in the different species, and using different extraction and purification methods.
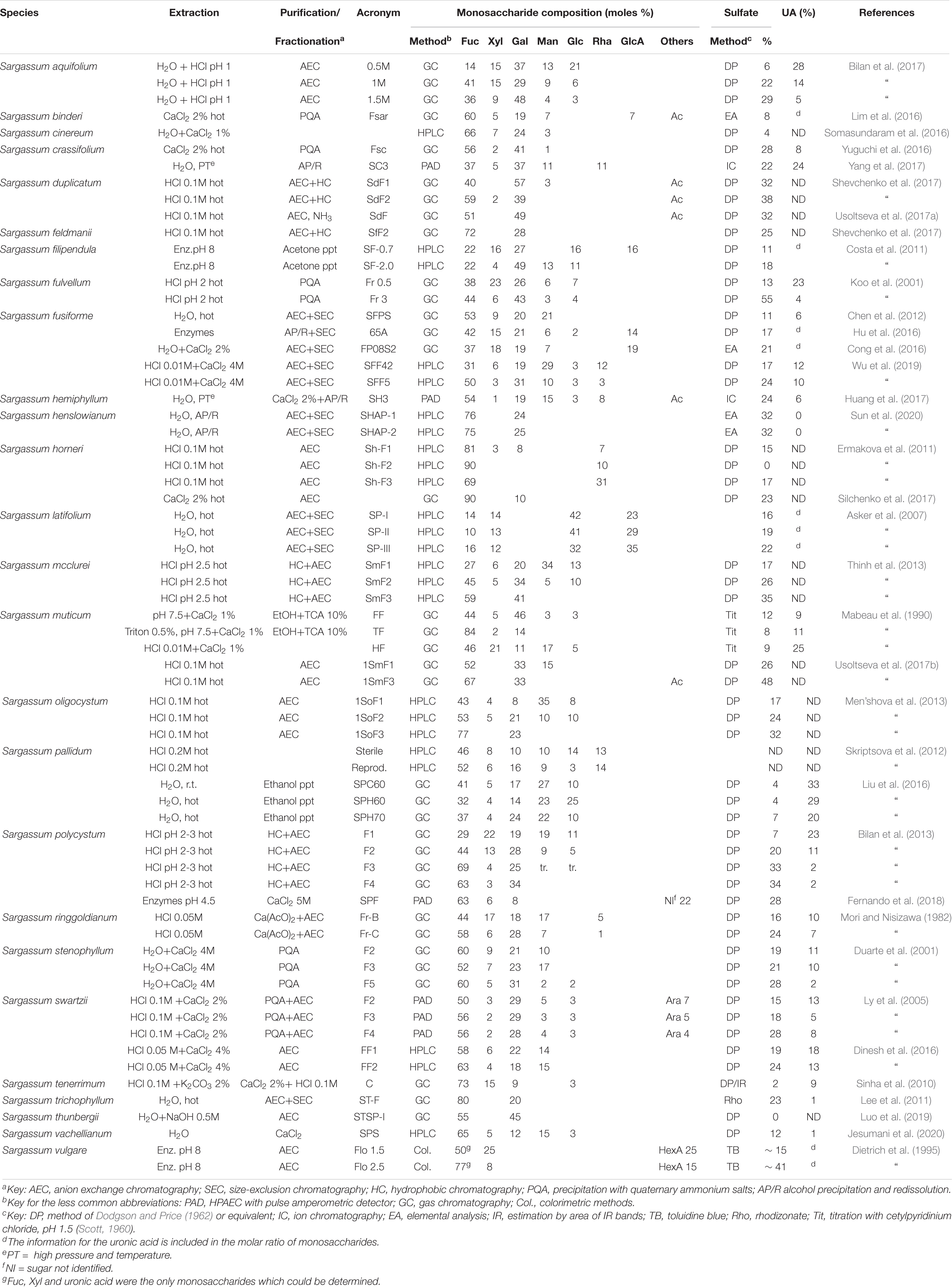
The family Sargassaceae comprises much more species than the Fucaceae (512 against 18, Guiry and Guiry, 2020). This family has the largest number of species studied from the point of view of its polysaccharides. The fucoidans from at least 26 different species of the genus Sargassum alone were analyzed. Table 2 shows the results for the different fucoidans isolated from this genus. For S. horneri, Ermakova et al. (2011) postulated the presence of Rha in substantial amounts within the polysaccharides (Table 2). However, their NMR spectra did not show the presence of this sugar, and in a further work by the same group (Silchenko et al., 2017) the fucoidans were purified without any trace of Rha. In S. latifolium, Asker et al. (2007) isolated three fractions where Glc and GlcA are the major components and Fuc is a minor one, not responding to the classical fucoidan composition. Other atypical polysaccharides were reported in S. pallidum (Liu et al., 2016) carrying high-mannose fucoidans, rich in uronic acids and scarcely sulfated, and in S. thunbergii (Luo et al., 2019), where a fucoidan completely devoid of sulfate groups was reported (Table 2).
Dietrich et al. (1995) studied the polysaccharides from Sargassum vulgare, differentiating whole plants and floaters. The fucoidan fractions corresponded to sulfated xylofucans containing important proportions of uronic acids. The proportion of sulfate is clearly higher in floaters. The ratio Fuc/Xyl/HexA varied between 1:0.5:0.5 and 1:0.1:0.2. However, only Fuc, Xyl and uronic acid have been determined in this investigation, missing other sugars possibly present.
For Sargassum fusiforme, the presence of galacturonic acid was detected (Hu et al., 2014). However, it has been shown later that this monosaccharide was part of a contaminating polysaccharide which could be separated by careful fractionation (Cong et al., 2016; Hu et al., 2016).
For the remaining members of the Fucales, the data is shown in Table 3. Mian and Percival (1973) carried out studies on Bifurcaria bifurcata and Himanthalia lorea. The data is shown only partially in Table 3, as Gal could not be quantified. Fractionation by ion exchange chromatography showed fractions with high uronic acid/low sulfate content using lower ionic strengths, and high sulfate, high Fuc, low uronic acid content in the later elutions. This behavior was observed for many further studies, regardless of the taxonomy of the seaweed. In some cases, like for Nizamuddinia zanardinii, the authors have devoted a lot of work in order to search for different extraction methods (Alboofetileh et al., 2019a,b,c). In Table 3 we have included the analysis of one extraction method, as the characteristics of the polysaccharides appear to be quite similar.
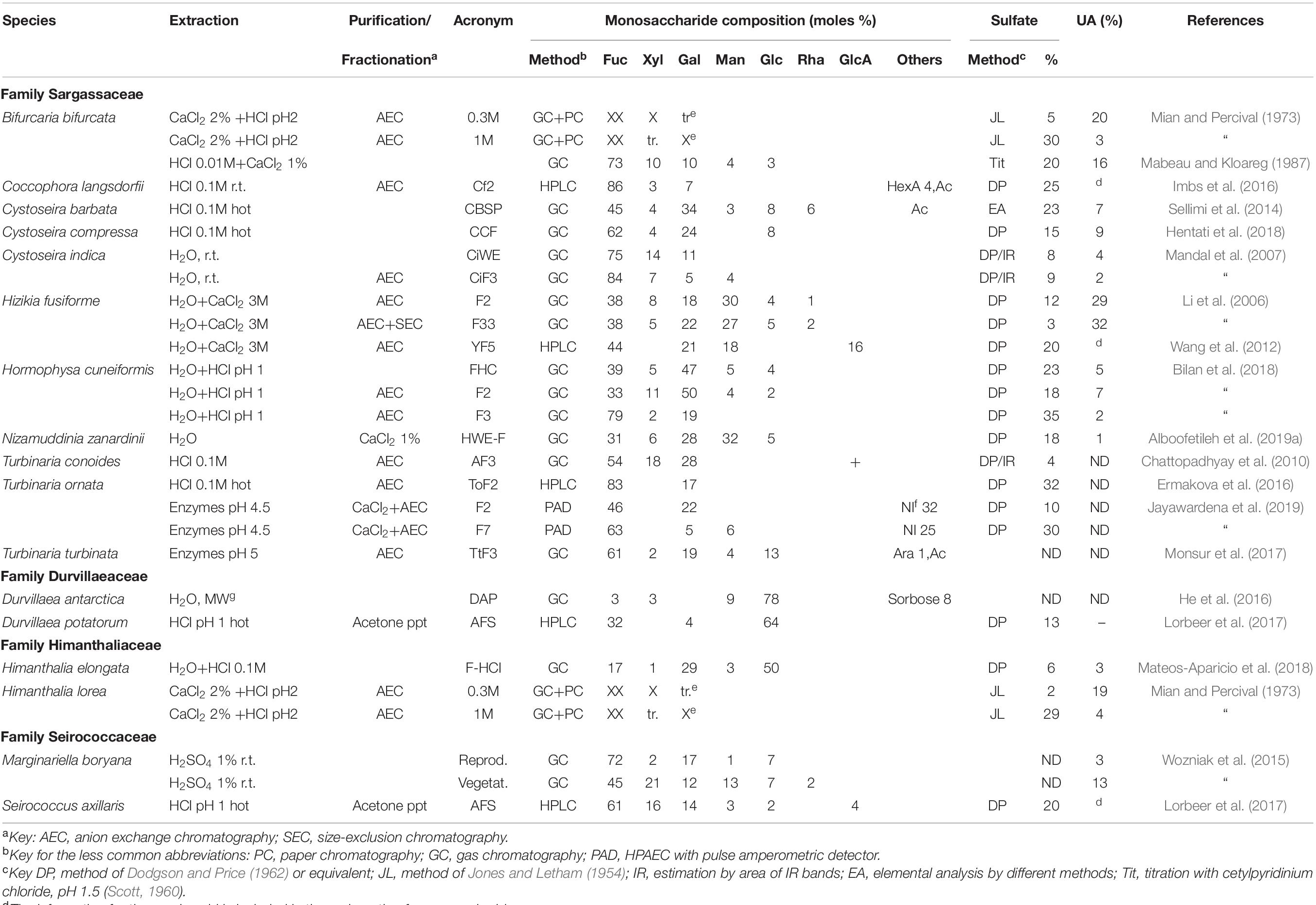
Table 3. Reported compositions of the fucoidans from the order Fucales not belonging to the family Fucaceae or to the genus Sargassum (Sargassaceae).
For Marginariella boryana, Wozniak et al. (2015) analyzed the polysaccharides extracted from vegetative structures (blades and vesicles) and receptacles (reproductive structures) separately. The proportions of Xyl, Man, and uronic acid increase significantly in the vegetative structures (Table 3). Within the family Durvillaeaceae two species were studies. Both in Durvillaea antarctica (He et al., 2016) and D. potatorum (Lorbeer et al., 2017), the proportion of Glc was so large that it obscured the analysis of the fucoidan constituents, even when purification procedures (successful with other seaweeds) to avoid contamination with laminaran were carried out (Lorbeer et al., 2017).
Most of the fucoidans analyzed from the Fucales were galactofucans, usually with small proportions of Xyl, with the exception of those of Ascophyllum nodosum (Table 1). Man and GlcA appeared in variable amounts.
Dictyotales
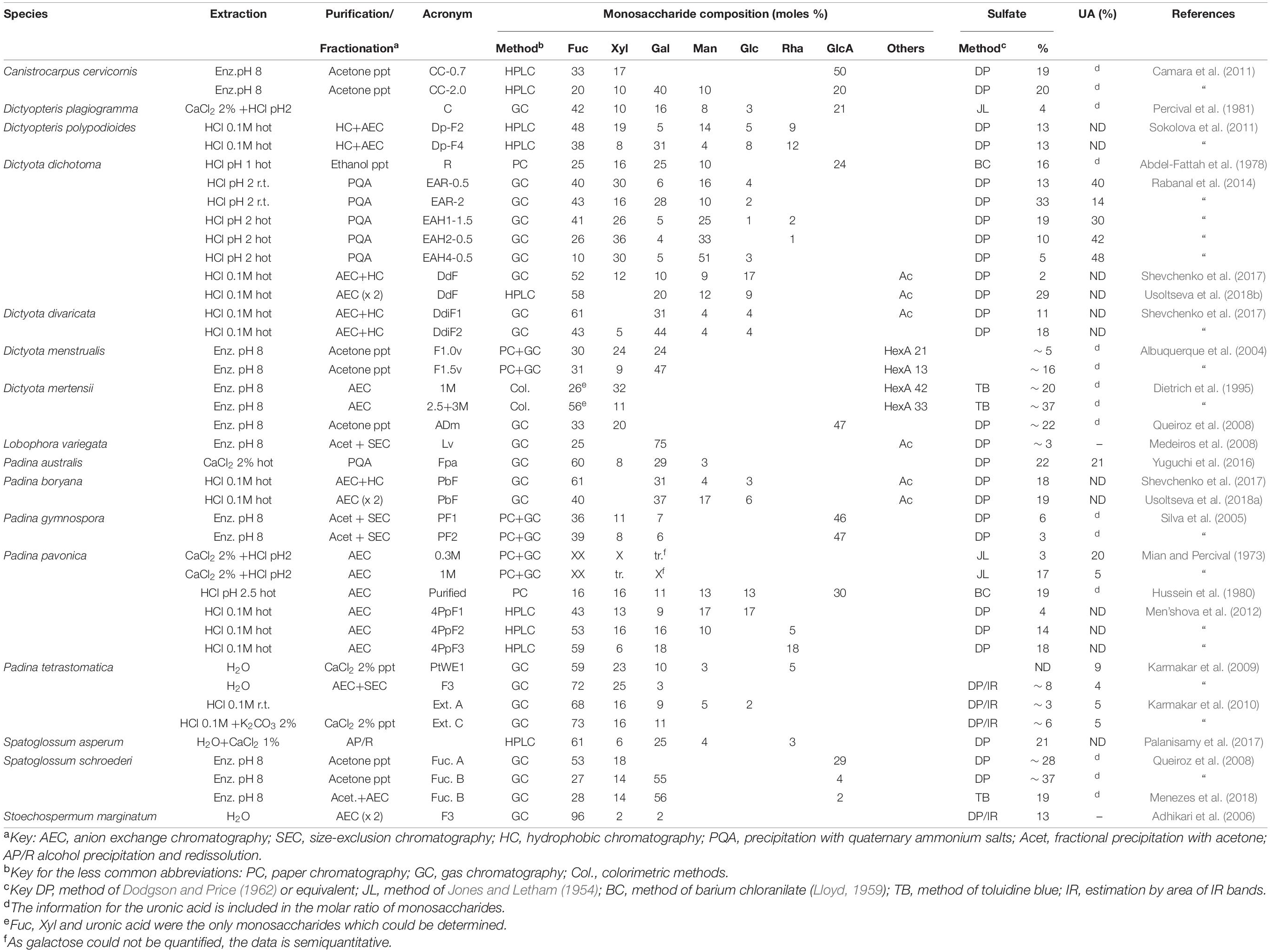
The data on the fucoidans from different species of the order Dictyotales is shown in Table 4. It should be mentioned that for Dictyota mertensii, the information is incomplete, as only Fuc, Xyl and uronic acid have been determined (Dietrich et al., 1995).
Padina pavonica was studied by Mian and Percival (1973), named then as P. pavonia. As occurred with the other seaweeds studied in that paper, the data on the table are incomplete, as Gal could not be quantified. Fraction 0.3M was rich in Fuc and Xyl, whereas fraction 1M was richer in Fuc, together with Gal. For this seaweed, Men’shova et al. (2012) carried out a seasonal study which showed that the proportion of Gal of the fucoidans increased markedly in all fractions when stepping down from spring to summer.
The fucoidans from the Dictyotales appear to be more heterogeneous than most of those of the Fucales. High proportions of Man and Rha appeared often (Table 4). However, an almost pure fucan sulfate was reported to be present in Stoechospermum marginatum (Adhikari et al., 2006) after careful purification.
Laminariales
Two species of Laminariales have been included in the early studies of Kylin (1913). They are Laminaria digitata and Saccharina lattisima (as Laminaria saccharina).
Many different species from the Laminariales have been studied thereafter, including species from four families (Agaraceae, Alariaceae, Laminariaceae, and Lessoniaceae). In order to keep up with the Silberfeld et al. (2014) taxonomy, we have included also a species from the Chorda genus (family Chordaceae) which has been recently proposed to be included in a new order, the Chordales (Starko et al., 2019). The data for the family Laminariaceae are shown in Table 5, whereas those of the remaining families appear in Table 6. It is worth noting that the species studied as Laminaria cichorioides and L. japonica are included in Table 5 as Saccharina cichorioides and S. japonica, respectively, in order to keep up with the newer taxonomy (Guiry and Guiry, 2020).
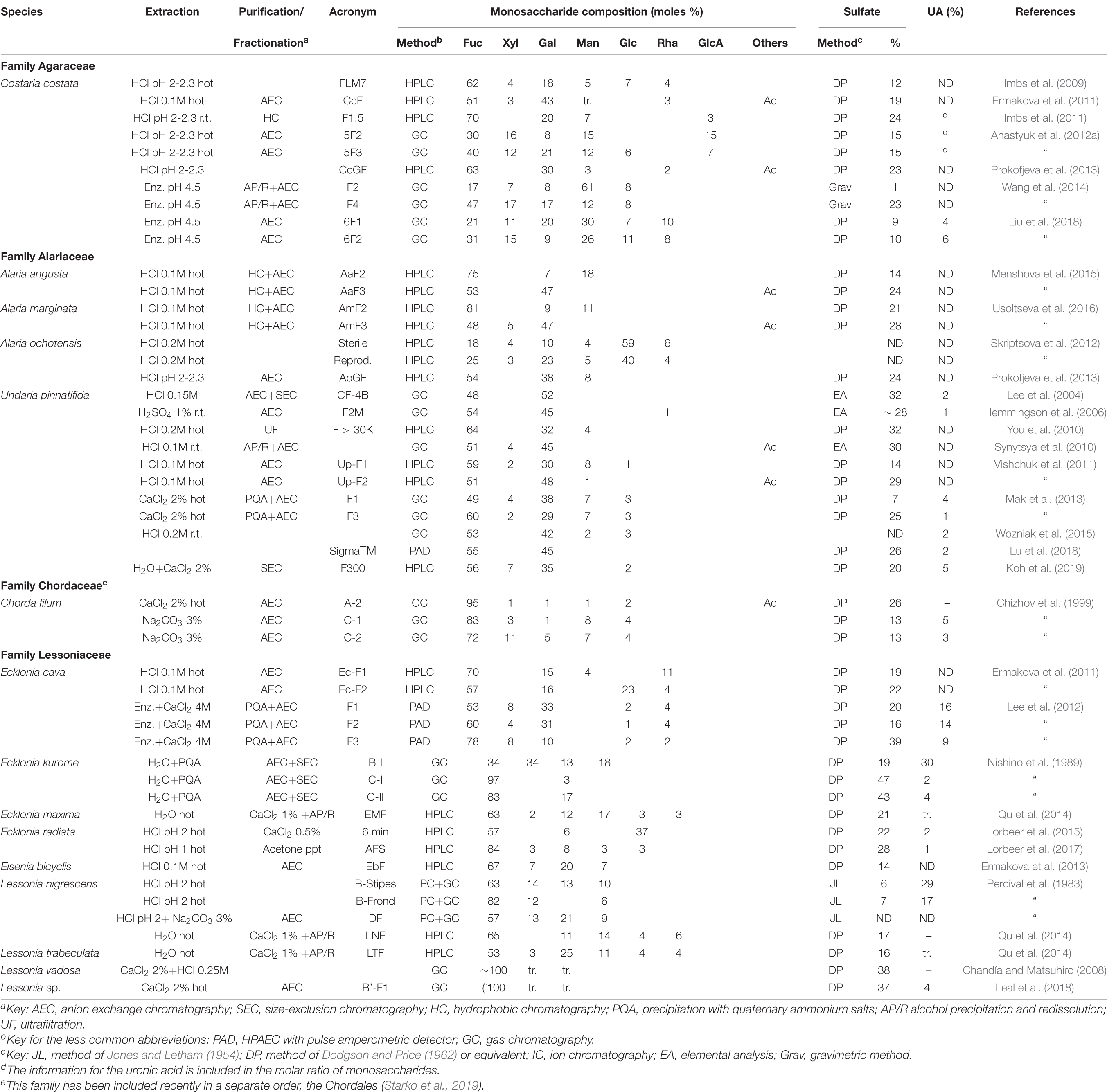
Table 6. Reported compositions of the fucoidans from the order Laminariales (families other than the Laminariaceae).
Many galactofucans have been found within the Laminariaceae family, usually with low proportions of Xyl or Man. However, several fractions containing almost pure fucans have been found in Laminaria angustata, L. hyperborea, Macrocystis pyrifera, Saccharina cichorioides, and S. japonica (Table 5). For L. angustata, Nishino et al. (1994b) have isolated a homogalactan sulfate, probably in the only case that an almost fucose-free product is found within the “fucoidan” fractions of brown seaweeds. The trend showing mixtures of polysaccharides separable by charge also occurs for the products from the Laminariales: usually heterogeneous polymers, containing high proportions of uronic acids, and low sulfation appear in the early-eluting fractions of anion exchange chromatography, whereas highly sulfated fucans or galactofucans appear in the late-eluting fractions.
Seasonal differences were also observed: for Costaria costata, Imbs et al. (2009) determined that the proportion of Fuc, Gal, Glc, and sulfate increased from spring to summer, whereas those of Man, Rha, and Xyl decreased. This trend is similar to that observed by Men’shova et al. (2012) for Padina pavonica (see above). In another study, carried out for Saccharina cichorioides (as Laminaria cichorioides), it has been shown that after the summer, and through fall, the proportion of Fuc decreases again, whereas that of Man increases clearly (Anastyuk et al., 2010).
On the basis of chemical degradations and NMR spectroscopy, Bilan et al. (2010) arrived to many structural features of the fucoidans from Saccharina lattisima. Ehrig and Alban (2015) have shown the large effect of the marine habitat and season on the characteristics of the isolated fucoidans of this seaweed. Samples picked up in the Baltic Sea showed more laminaran contamination and lower fucoidan yields, fucose, and sulfate content than those collected around the Faroe Islands (regardless of the season), although the uronic acid content was similar. Regarding the season effects, the proportion of sulfate was higher in fucoidans from seaweeds collected in September than in May. Anion-exchange chromatography separation showed that only from the September-collected seaweed it was possible to obtain high yields of a high-fucose fraction with the highest biological activity. However, in a further work from the same group (Bittkau et al., 2020), the authors have isolated such a fraction with high fucose and sulfate content from the same North Atlantic location, in July without the need of any purification, suggesting that the year of collection has a major effect on the composition of the isolated fucoidans.
A study carried out with an unidentified species of Alaria (Alaria sp., Vishchuk et al., 2012) was later ascertained as being A. ochotensis (Prokofjeva et al., 2013). In the Alaria species studied so far, it is noteworthy to mention the presence of fucogalactans with approximately equal proportions of Fuc and Gal (Table 6).
For Costaria costata, high proportions of Man have been encountered in the polymers, especially in the less charged fractions isolated in some studies (Wang et al., 2014). In any case, Man appears conspicuously in most of the studies carried out on fucoidans of any origin.
The polysaccharides from Undaria pinnatifida were studied by many research groups, probably due to the fact that this seaweed, native from northeastern Asia, is very invasive and now is widespread all around the world (Casas et al., 2004; Thornber et al., 2004). It is worth noting that most of the studies have shown the presence of a galactofucan with high proportions of Gal, sometimes leveling out with Fuc. The proportion of other sugars (Man, Xyl and uronic acids) is usually low, whereas the proportion of sulfate is considerable, but lower than those of other species (Table 6).
Other Orders
The analysis of the fucoidans of different species of the order Ectocarpales appears in Table 7. In this survey, only reports for ten different species (belonging to three families) of the order have been found. Highly sulfated galactofucans or homofucans coexist with polysaccharides containing significant proportions of Man, GlcA and/or Xyl.
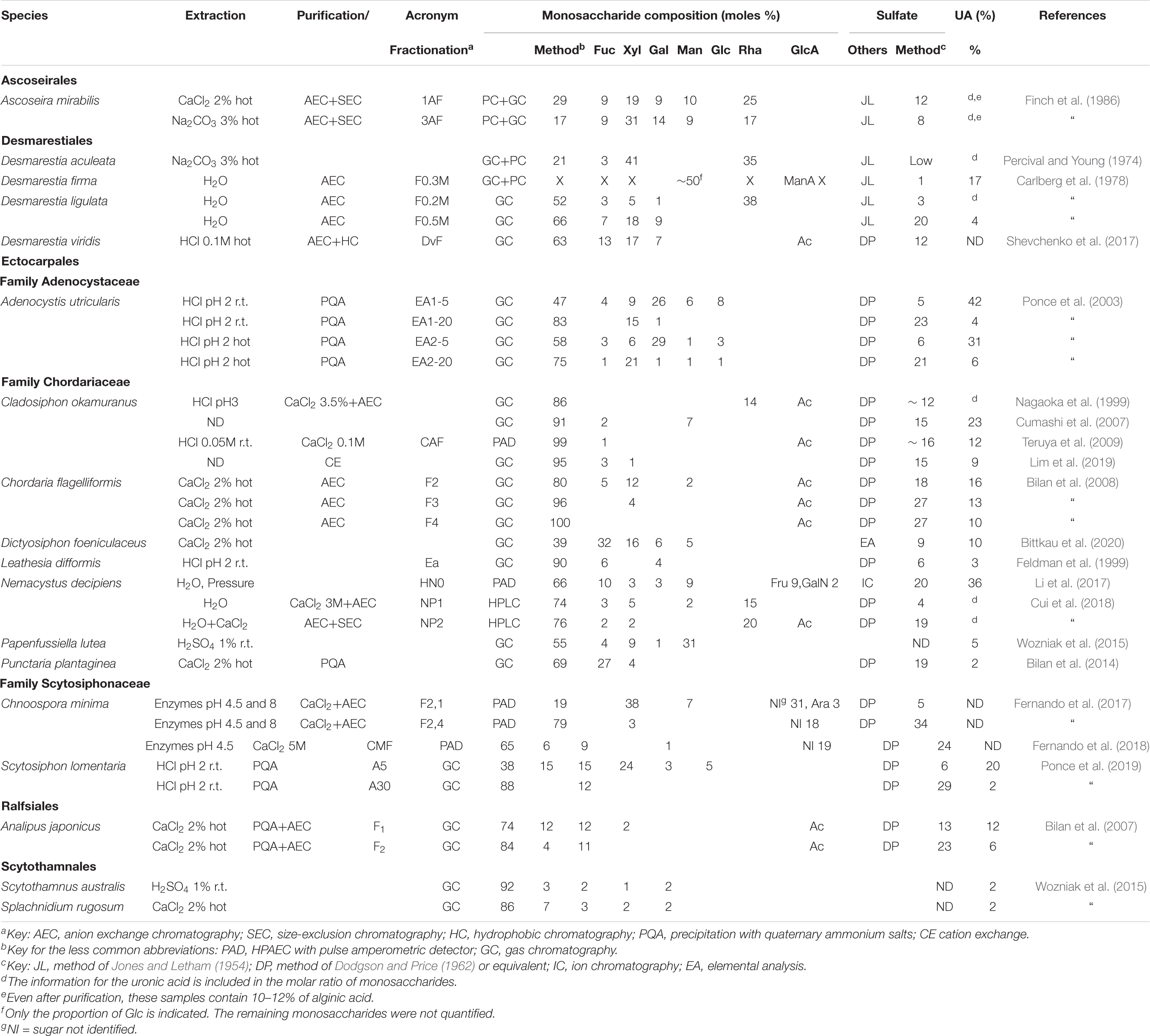
Table 7. Reported compositions of the fucoidans from the orders Ascoseirales, Desmarestiales, Ectocarpales, Ralfsiales, and Scytothamnales.
The analysis of the fucoidans from four species from the Desmarestiales is also shown in Table 7. It should be taken into account that these seaweeds contain free sulfuric acid in their vacuoles (Carlberg et al., 1978), making them very labile when taken out from the marine environment. This requires special techniques in order to obtain neutral extracts unaffected by the strong acid.
To the best of our knowledge, the fucoidans from only one species from the Ascoseirales and Ralfsiales, and two of the Scytothamnales have been studied (Table 7). The fucoidans from the three samples from the Ralfsiales and Scytothamnales appear to be particularly rich in Fuc and poor in uronic acids, whereas the Ascoseira sample was quite heterogeneous (Finch et al., 1986, Table 7).
Concluding Remarks
The current review has surveyed most of the compositional data on fucoidans extracted from different species, in many cases after purification; more than 100 species were screened through the literature. Besides the obvious purpose of providing a reliable source of compositional data gathered in a set of tables, this review attempted to foresee if there is any correlation of these compositional data with their taxonomy, or if other factors are more important than the taxonomic origin.
These general considerations can be deduced from the analysis of the compositional data:
1. Separation by charge is the most efficient method to obtain “pure” fucoidan fractions. Either using anion-exchange chromatography with increasing concentrations of salt as eluant, or by precipitating with cationic detergents and redissolving at increasing ionic strengths, two main type of polymers can be separated: (a) those appearing at low ionic strengths, usually highly heterogeneous in their monosaccharidic composition (containing Fuc, Xyl, Gal, Man, Rha, GlcA), with low-sulfate content, and high uronic acid content, and b) those appearing at high ionic strengths, containing mainly Fuc, accompanied with variable proportions of Gal, highly sulfated and containing little (or none) uronic acids. Fractions containing intermediate proportions of both polysaccharides appear at medium ionic strengths. Figure 3 depicts the composition of fractions belonging to each of the first groups from selected seaweeds, showing clearly the marked differences between both groups. This behavior is observed for samples from the orders Fucales, Laminariales, Ascoseirales, Desmarestiales, Ectocarpales, and Ralfsiales (Mian and Percival, 1973; Carlberg et al., 1978; Bilan et al., 2002, 2013, 2016, 2018; Ponce et al., 2003, 2019; Ozawa et al., 2006; Mak et al., 2013); however, for the Dictyotales, the trend is obscured due to the abundance of Man and/or uronic acids in the products separated at each ionic strength (Table 4). It has been postulated that the biological activity is concentrated on the galactofucan components (Ponce et al., 2003, 2019; Croci et al., 2011).
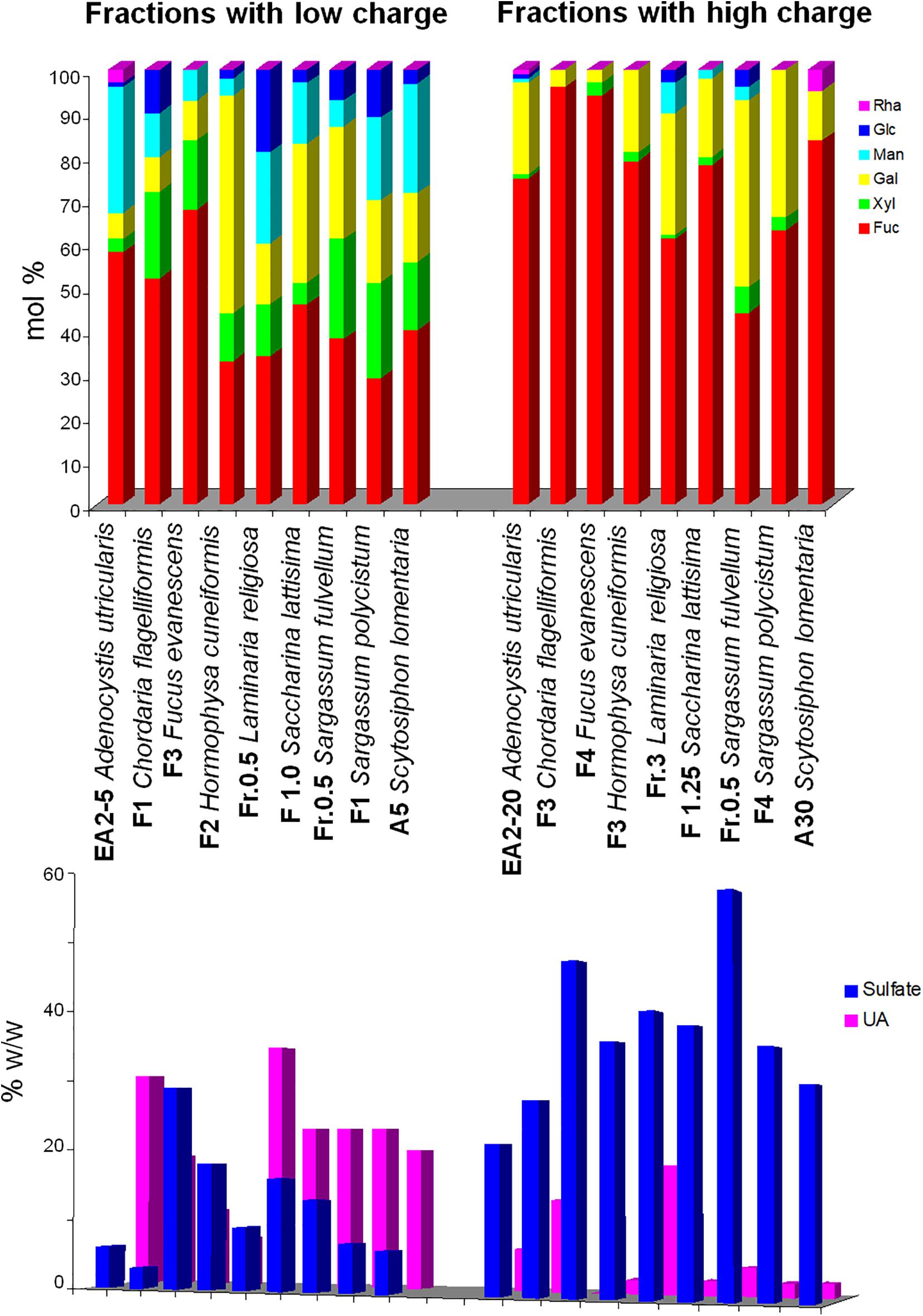
Figure 3. Difference in selected reported compositions of fucoidans submitted to charge-based separation methods. Fractions on the left side were eluted or redissolved at low ionic strengths, whereas those on the right side were eluted or redissolved at higher ionic strengths. Upper panel, neutral monosaccharide composition (mol/100 mols); lower panel, sulfate and uronic acid content. The data were reported by Koo et al. (2001), Bilan et al. (2002, 2008, 2010, 2013, 2018), and Ponce et al. (2003, 2019).
2. Acetate esters of the fucoidans are very common. As a matter of fact, this constituent has been found in almost every sample where it was searched. Determinations of acetyl groups are not very common, as they are only encountered through NMR spectra or specific colorimetric techniques. They are labile enough in mild alkaline or acid media as to get undetected when using some extraction procedures (Bernhard and Hammett, 1953; Wuts and Greene, 2006). Anyway, almost all of the seven tables report acetyl groups on some species. It is highly probable that searching in other species would have resulted in many more positive results.
3. In some cases, Man and Rha appear together, usually in fractions with lower sulfate contents. For Man, structural explanations have already been reported in terms of fucomannoglucuronans (Bilan et al., 2010), but for Rha no structural function has been found so far. Rha seems to appear in higher proportions within the order Dictyotales and the family Sargassaceae (Fucales).
4. The Dictyotales appear to be the most “atypical” order, as usually large proportions of Man and uronic acids appear. In one species which was highly fractionated, Man becomes the most important monosaccharide in the low-charged fractions, and it is still important in the fractions with more sulfate groups (Table 4; Rabanal et al., 2014). However, fractions with high proportions of monosaccharides different than Fuc were found in most of the taxa studied so far (see Tables).
5. The uronic acid content should be considered with due care. Sometimes it corresponds to GlcA actually comprising the fucoidan structure, but sometimes it corresponds to contamination with alginic acid (e.g., Finch et al., 1986; Lorbeer et al., 2017), a polysaccharide present in all of the brown seaweeds studied so far. By the same token, the Glc present in the samples should almost certainly correspond to contaminating laminarans (Lorbeer et al., 2017; Mateos-Aparicio et al., 2018). Only in a few cases, Glc has been shown to be part of the fucoidan structure (e.g., Duarte et al., 2001).
6. There are several factors to consider when comparing the compositional data of fucoidans from different seaweeds and research groups. The taxon is just one of them. Others like geographical location, year and season of harvest of the seaweed, extraction and purification methods, analytical methods, different parts or reproductive stages of the seaweeds are also of paramount importance in defining the final characteristics.
7. The geographic site of harvesting appears to be very important: Zvyagintseva et al. (2003) found marked differences between the fucoidans of Fucus evanescens collected in different spots of the southern Okhotsk Sea. Ehrig and Alban (2015) also found a significant difference between the composition and yields of fucoidans of Saccharina lattisima samples collected in the North Atlantic and in the Baltic Sea. This factor, together with the year of collection might explain the large differences in composition found for species studied by different groups (or at different times) even with similar extraction and purification procedures.
8. The season of harvesting has also influence over the composition of the fucoidans: a trend with increasing yields, and proportions of sulfate, Fuc, Gal and Glc (together with a decrease in the Man and Rha content) is observed as the collection month progressed from March to October, in the Northern Hemisphere (Imbs et al., 2009; Anastyuk et al., 2010; Men’shova et al., 2012; Ehrig and Alban, 2015).
9. The effect of the extraction conditions is more controversial: Ponce et al. (2003) and Wozniak et al. (2015) found very little differences when switching the extraction solvent from water to CaCl2 to diluted HCl. Alboofetileh et al. (2019b) found differences in yield and in sulfate content but a very similar monosaccharide composition using enzymes, ultrasound, or both combined. Rodríguez-Jasso et al. (2011) found a significant difference in composition and yields when changing the time and the pressure of a microwave-assisted water extraction. Nguyen et al. (2020) have shown a sharply different composition of the chemically and enzymatically-extracted crude products, being the latters richer in alginic acid and sulfate/Fuc ratios. After purification, the compositions might level off. However, the enzyme-aided extraction, also used by other groups (Dietrich et al., 1995; Albuquerque et al., 2004; Silva et al., 2005; Medeiros et al., 2008; Queiroz et al., 2008; Costa et al., 2011; Camara et al., 2011; Lee et al., 2012; Wang et al., 2014; Hu et al., 2016; Monsur et al., 2017; Fernando et al., 2017, 2018; Liu et al., 2018; Menezes et al., 2018; Song et al., 2018; Jayawardena et al., 2019; Alboofetileh et al., 2019a,b) appears to be an interesting prospect, considering cleaner chemical issues and the possibility of finding enhanced biological activities in comparison with chemically extracted products (Nguyen et al., 2020).
Some differences were found between the fucoidans isolated from reproductive and sterile tissue of five different seaweeds (Skriptsova et al., 2012, see Tables 1, 2, 5, 6). Usually the reproductive tissue is less heterogeneous, and carries more Fuc and less Glc than the sterile tissue. Regarding the extraction of fucoidans from different parts of the seaweeds, Percival et al. (1983) extracted separately the polysaccharides from fronds and stipes from Lessonia nigrescens, whereas Wozniak et al. (2015) compared the fucoidans isolated from reproductive structures and from vegetative structures in Marginariella boryana. The fucoidans from stipes and the vegetative structures, respectively, appear to be more heterogeneous (less Fuc and more uronic acids).
In order to obtain fucoidan samples devoid of contaminants, the best results were obtained by carrying out the extractions with dilute HCl or CaCl2, or using these agents after the extraction (for instance enzymatic) in order to precipitate the alginate in the first place, followed by a careful separation by charge (anion exchange chromatography eluting with increasing ionic strength, or precipitation with quaternary ammonium salts followed by redissolution with increasing ionic strengths). Further purification of each fraction by size-exclusion chromatography usually yield fucoidans devoid of alginic acid or laminaran contaminants.
The conclusion is that with so many variables determining the composition of the fucoidans, the subtle differences that might appear among the different higher taxa (order, family) surveyed in this review are overridden. Probably, comparisons carried out in the same labs with the same methods might help, or more profound structural studies might throw light on chemotaxonomical issues in the future.
Author Contributions
NP was involved in the conceptualization, formal analysis, investigation, writing, and visualization of this work. CS was involved in the conceptualization, formal analysis, writing, visualization, and funding of this work. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the University of Buenos Aires (20020170100255BA), National Research Council of Argentina-CONICET (PIP 298/14 and P-UE 22920160100068CO), and ANPCyT-Argentina (PICT 2017-1675).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Dr. María C. Rodríguez for her help on botanical/psychological issues, and to Dr. Marina Ciancia for her kind invitation to participate in this issue.
References
Abdel-Fattah, A. F., Hussein, M. M.-D., and Fouad, S. T. (1978). Carbohydrates of the brown seaweed Dictyota dichotoma. Phytochemistry 17, 741–743. doi: 10.1016/S0031-9422(00)94218-3
Adhikari, U., Mateu, C. G., Chattopadhyay, K., Pujol, C. A., Damonte, E. B., and Ray, B. (2006). Structure and antiviral activity o sulfated fucans from Stoechospermum marginatum. Phytochemistry 67, 2474–2482. doi: 10.1016/j.phytochem.2006.05.024
Alboofetileh, M., Rezaei, M., Tabarsa, M., Rittá, M., Donalisio, M., Mariatti, F., et al. (2019a). Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 124, 131–137. doi: 10.1016/j.ijbiomac.2018.11.201
Alboofetileh, M., Rezaei, M., Tabarsa, M., and You, S. (2019b). Bioactivities of Nizamuddinia zanardinii sulfated polysaccharides extracted by enzyme, ultrasound and enzyme-ultrasound methods. J. Food Sci. Technol. 56, 1212–1220. doi: 10.1007/s13197-019-03584-1
Alboofetileh, M., Rezaei, M., Tabarsa, M., You, S., Mariatti, F., and Cravotto, G. (2019c). Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 128, 244–253. doi: 10.1016/j.ijbiomac.2019.01.119
Albuquerque, I. R. L., Queiroz, K. C. S., Alves, L. G., Santos, E. A., Leite, E. L., and Rocha, H. A. O. (2004). Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 37, 167–171. doi: 10.1590/S0100-879X2004000200002
Ale, M. T., Mikkelsen, J. D., and Meyer, A. S. (2011). Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 9, 2106–2130. doi: 10.3390/md9102106
Anastyuk, S. D., Imbs, T. I., Semenova, M. L., Dmitrenok, P. S., and Zvyagintseva, T. N. (2012a). ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 90, 993–1002. doi: 10.1016/j.carbpol.2012.06.033
Anastyuk, S. D., Shevchenko, N. M., Dmitrenok, P. S., and Zvyagintseva, T. N. (2012b). Structural similarities of fucoidans from brown algae Silvetia babingtonii and Fucus evanescens, determined by tandem MALDI-TOF mass spectrometry. Carbohydr. Res. 358, 78–81. doi: 10.1016/j.carres.2012.06.015
Anastyuk, S. D., Shevchenko, N. M., Nazarenko, E. L., Imbs, T. I., Gorbach, V. I., Dmitrenok, P. S., et al. (2010). Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydr. Res. 345, 2206–2212. doi: 10.1016/j.carres.2010.07.043
Asker, M. M. S., Mohamed, S. F., Ali, F. M., and El-Sayed, O. H. (2007). Chemical structure and antiviral activity of water-soluble sulfated polysaccharides from Surgassum latifolium. J. Appl. Sci. Res. 3, 1178–1185.
Bernhard, S. A., and Hammett, L. P. (1953). Specific effects in acid catalysis by ion-exchange resins. II. Hydrolysis of esters in water solution. J. Amer. Chem. Soc. 75, 5834–5835. doi: 10.1021/ja01119a017
Bilan, M. I., Grachev, A. A., Shashkov, A. S., Kelly, M., Sanderson, C. J., Nifantiev, N. E., et al. (2010). Further studies on the composition ans structure of a fucoidan preparation from the brown alga Saccharisima latissima. Carbohydr. Res. 345, 2038–2047. doi: 10.1016/j.carres.2010.07.009
Bilan, M. I., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2006). Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 341, 238–245. doi: 10.1016/j.carres.2005.11.009
Bilan, M. I., Grachev, A. A., Shashkov, A. S., Thuy, T. T. T., Van, T. T. T., Ly, B. M., et al. (2013). Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 377, 48–57. doi: 10.1016/j.carres.2013.05.016
Bilan, M. I., Grachev, A. A., Ustuzhanina, N. E., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2002). Structure of a fucoidan from the brown seawed Fucus evanescens C.Ag. Carbohydr. Res. 337, 719–730. doi: 10.1016/S0008-6215(02)00053-8
Bilan, M. I., Grachev, A. A., Ustuzhanina, N. E., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2004). A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 339, 511–517. doi: 10.1016/j.carres.2003.10.028
Bilan, M. I., Klochkova, N. G., Ustyuzhanina, N. E., Chizhov, A. O., Shashkov, A. S., Nifantiev, N. E., et al. (2016). Polysaccharides of algae 68. Sulfated polysaccharides from the Kamchatka brown alga Laminaria bongardiana. Russ. Chem. Bull. Int. Ed. 65, 2729–2736. doi: 10.1007/s11172-016-1643-1
Bilan, M. I., Shashkov, A. S., and Usov, A. I. (2014). Structure of a sulfated xylofucan from the brown alga Punctaria plantaginea. Carbohydr. Res. 393, 1–8. doi: 10.1016/j.carres.2014.04.022
Bilan, M. I., and Usov, A. I. (2008). Structural analysis of fucoidans. Nat. Prod. Comm. 3, 1639–1648. doi: 10.1177/1934578X0800301011
Bilan, M. I., Ustyuzhanina, N. E., Shashkov, A. S., Thanh, T. T. T., Bui, M. L., Tran, T. T. V., et al. (2017). Sulfated polysaccharides of the Vietnamese brown alga Sargassum aquifolium (Fucales, Sargassaceae). Carbohydr. Res. 449, 23–31. doi: 10.1016/j.carres.2017.06.016
Bilan, M. I., Ustyuzhanina, N. E., Shashkov, A. S., Thanh, T. T. T., Bui, M. L., Tran, T. T. V., et al. (2018). A sulfated galactofucan from the brown alga Hormophysa cuneiformis (Fucales, Sargassaceae). Carbohydr. Res. 469, 48–54. doi: 10.1016/j.carres.2018.09.001
Bilan, M. I., Vinogradova, E. V., Tsvetkova, E. A., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., et al. (2008). A sulfated glucuronofucan containing both fucofuranose and fucopyranose residues from the brown alga Chordaria flagelliformis. Carbohydr. Res. 343, 2605–2612. doi: 10.1016/j.carres.2008.06.001
Bilan, M. I., Zakharova, A. N., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2007). Polysaccharides of alga: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem. 33, 38–46. doi: 10.1134/S1068162007010049
Bittkau, K. S., Neupane, S., and Alban, S. (2020). Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 45:101759. doi: 10.1016/j.algal2019.101759
Camara, R. B. G., Costa, L. S., Fidelis, G. P., Nobre, L. D. T. B., Dantas-Santos, N., Cordeiro, L. S., et al. (2011). Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 9, 124–138. doi: 10.3390/md9010124
Carlberg, G. E., Percival, E., and Rahman, M. A. (1978). Carbohydrates of the seaweeds, Desmarestia ligulata and D. firma. Phytochemistry 17, 1289–1292. doi: 10.1016/S0031-9422(00)94576-X
Casas, G., Scrosati, R., and Piriz, M. L. (2004). The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol. Invasion. 6, 411–416. doi: 10.1023/B:BINV.0000041555.29305.41
Chandía, N. P., and Matsuhiro, B. (2008). Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 42, 235–240. doi: 10.1016/j.ijbiomac.2007.10.023
Charrier, B., Le Bail, A., and de Reviers, B. (2012). Plant Proteus: brown algal morphological plasticity and underlying developmental mechanisms. Trends Plant Sci. 17, 468–477. doi: 10.1016/j.tplants.2012.03.003
Chattopadhyay, N., Ghosh, T., Sinha, S., Chattopadhyay, K., Karmakar, P., and Ray, B. (2010). Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chem. 118, 823–829. doi: 10.1016/j.foodchem.2009.05.069
Chen, X., Nie, W., Fan, S., Zhang, J., Wang, Y., Lu, J., et al. (2012). A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophophosphamide-treated mice. Carbohydr. Polym. 90, 1114–1119. doi: 10.1016/j.carbpol.2012.06.052
Chizhov, A. O., Dell, A., Morris, H. R., Haslam, S. M., McDowell, R. A., Shashkov, A. S., et al. (1999). A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 320, 108–119. doi: 10.1016/S0008-6215(99)00148-2
Cong, Q., Chen, H., Liao, W., Xiao, F., Wang, P., Qin, Y., et al. (2016). Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 136, 899–907. doi: 10.1016/j.carbpol.2015.09.087
Cosenza, V. A., Navarro, D. A., Ponce, N. M. A., and Stortz, C. A. (2017). “Seaweed polysaccharides: structure and applications,” in Industrial Applications of Renewable Biomass Products. Past, Present, and Future, eds S. N. Goyanes and N. B. D’Accorso (Cham: Springer Int.), 75–116. doi: 10.1007/978-3-319-61288-1_3
Costa, L. S., Fidelis, G. P., Telles, C. B. S., Dantas-Santos, N., Camara, R. B. G., Cordeiro, S. L., et al. (2011). Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 9, 952–966. doi: 10.3390/md9060952
Croci, D. O., Cumashi, A., Ushakova, N. A., Preobrazhenskaya, M. E., Piccoli, A., Totani, L., et al. (2011). Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS One 6:e17283. doi: 10.1371/journal.pone.0017283
Cui, K., Tai, W., Shan, X., Hao, J., Li, G., and Yu, G. (2018). Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens. Int. J. Biol. Macromol. 120, 1817–1822. doi: 10.1016/j.ijbiomac.2018.09.079
Cumashi, A., Ushakova, N. A., Preobrazhenskaya, M. E., D’Incecco, A., Piccoli, A., Totani, L., et al. (2007). A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17, 541–552. doi: 10.1093/glycob/cwm014
Davis, T. A., Volesky, B., and Mucci, A. (2003). A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 37, 4311–4330. doi: 10.1016/S0043-1354(03)00293-8
de Reviers, B., Rousseau, F., and Draisma, S. G. A. (2007). “Classification of the Phaeophyceae from past to present and current challenges,” in Unraveling the Algae: the Past, Present and Future of Algal Systematic, eds J. Brodie and J. Lewis (Boca Raton, FL: CRC Press), 267–284. doi: 10.1201/9780849379901
Deniaud-Bouët, E., Hardouin, K., Potin, P., Kloareg, B., and Hervé, C. (2017). A review about brown algal cell walls and fucose-containing sulfated polysaccharides: cell wall context, biomedical properties, and key research challenges. Carbohydr. Polym. 175, 395–408. doi: 10.1016/j.carbpol.2017.07.082
Deniaud-Bouët, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B., and Hervé, C. (2014). Chemical and enzymatic fractionation of cell walls from fucales: insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 114, 1203–1216. doi: 10.1093/aob/mcu096
Dietrich, C. P., Farias, G. G. M., de Abreu, L. R. D., Leite, E. L., da Silva, L. F., and Nader, H. B. (1995). A new approach for the characterization of polysaccharides from algae: presence of four main acidic polysaccharides in three species of the class Phaeophyceae. Plant Sci. 108, 143–153. doi: 10.1016/0168-9452(95)04142-H
Dinesh, S., Menon, T., Hanna, L. E., Suresh, V., Sathuvan, M., and Manikannan, M. (2016). In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 82, 83–88. doi: 10.1016/j.ijbiomac.2015.09.078
Dodgson, K. S., and Price, R. C. (1962). A note on the determination of ester sulfate content of sulfated polysaccharides. Biochem. J. 84, 106–110. doi: 10.1042/bj0840106
Draisma, S. G. A., Peters, A. F., and Fletcher, R. L. (2003). “Evolution and taxonomy in the Phaeophyceae: effects of the molecular age on brown algal systematic,” in Out of the Past. Collected Reviews to Celebrate the Jubilee of the British Phycological Society, ed. T. A. Norton (Belfast: British Phycological Society), 87–102.
Draisma, S. G. A., Prud‘homme van Reine, W. F., Stam, W. T., and Olsen, J. L. (2001). A reassessment of phylogenetic relationships within the Phaeophyceae based on RUBISCO large subunit and ribosomal DNA sequences. J. Phycol. 37, 586–603. doi: 10.1046/j.1529-8817.2001.037004586.x
Duarte, M. E. R., Cardoso, M. A., Noseda, M. D., and Cerezo, A. S. (2001). Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 333, 281–293. doi: 10.1016/S0008-6215(01)00149-5
Ehrig, K., and Alban, S. (2015). Sulfated galactofucan from the brown alga Saccharina latissima – Variability of yield, structural composition, and bioactivity. Mar. Drugs 13, 76–101. doi: 10.3390/md13010076
Ermakova, S., Men´shova, R., Vishchuk, O., Kim, S.-M., Um, B.-H., Isakov, V., et al. (2013). Water-soluble polysaccharides from the brown alga Eisenia bicyclis: structural characteristics and antitumor activity. Algal Res. 2, 51–58. doi: 10.1016/j.algal.2012.10.002
Ermakova, S., Sokolova, R., Kim, S.-M., Um, B.-H., Isakov, V., and Zvyagintseva, T. (2011). Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 164, 841–850. doi: 10.1007/s12010-011-9178-2
Ermakova, S. P., Menshova, R. V., Anastyuk, S. D., Malyarenko (Vishchuk), O. S., Zakharenko, A. M., (Thinh), P. D., et al. (2016). Structure, chemical and enzymatic modification, and anticancer activity of polysaccharides from the brown alga Turbinaria ornata. J. Appl. Phycol. 28, 2495–2505. doi: 10.1007/s10811-015-0742-y
Feldman, S. C., Reynaldi, S., Stortz, C. A., Cerezo, A. S., and Damonte, E. B. (1999). Antiviral properties of fucoidans fractions from Leathesia difformis. Phytomedicine 6, 335–340. doi: 10.1016/S0944-7113(99)80055-5
Fernando, I. P. S., Sanjeewa, K. K. A., Samarakoon, K. W., Kim, H.-S., Gunasekara, U. K. D. S. S., Park, Y.-J., et al. (2018). The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 30, 3223–3232. doi: 10.1007/s10811-018-1415-4
Fernando, I. P. S., Sanjeewa, K. K. A., Samarakoon, K. W., Lee, W. W., Kim, H.-S., Kang, N., et al. (2017). A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 104, 1185–1193. doi: 10.1016/j.ijbiomac.2017.07.031
Finch, P., Percival, E., Slaiding, I. R., and Weigel, H. (1986). Carbohydrates of the antartic brown seaweed Ascoseira mirabilis. Phytochemistry 25, 443–448. doi: 10.1016/S0031-9422(00)85498-9
Guiry, M. D., and Guiry, G. M. (2020). AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online at: http://www.algaebase.org (accessed April 18, 2020).
Guo, H., Liu, F., Jia, G., Zhang, W., and Wu, F. (2013). Extraction optimization and analysis of monosaccharide composition of fucoidan from Saccharina japonica by capillary zone electrophoresis. J. Appl. Phycol. 25, 1903–1908. doi: 10.1007/s10811-013-0024-5
He, J., Xu, Y., Chen, H., and Sun, P. (2016). Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 17:1988. doi: 10.3390/ijms17121988
Hemmingson, J. A., Falshaw, R., Furneaux, R. H., and Thompson, K. (2006). Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta). J. Appl. Phycol. 18, 185–193. doi: 10.1007/s10811-006-9096-9
Hentati, F., Delattre, C., Ursu, A. V., Desbrières, J., Le Cerf, D., Gardarin, C., et al. (2018). Structural characterization and antioxidant activity of water-soluble polysaccharides from the tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 198, 589–600. doi: 10.1016/j.carbpol.2018.06.098
Hu, P., Li, Z., Chen, M., Sun, Z., Ling, Y., Jiang, J., et al. (2016). Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 139, 150–158. doi: 10.1016/j.carbpol.2015.12.019
Hu, P., Xue, R., Li, Z., Chen, M., Sun, Z., Jiang, J., et al. (2014). Structural investigation and immunological activity of a heteropolysaccharide from Sargassum fusiforme. Carbohydr. Res. 390, 28–32. doi: 10.1016/j.carres.2014.02.027
Huang, C.-Y., Kuo, C.-H., and Chen, P.-W. (2017). Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 23:E78. doi: 10.3390/molecules23010078
Hussein, M. M., Abdel-Aziz, A., and Salem, H. M. (1980). Sulphated heteropolysaccharides from Padina pavonia. Phytochemistry 19, 2131–2132. doi: 10.1016/S0031-9422(00)8220
Imbs, I., Ermakova, S. P., Malyarenko (Vishchuk), O. S., Isakov, V. V., and Zvyagintseva, (N.) (2016). Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 135, 162–168. doi: 10.1016/j.carbpol.2015.08.062
Imbs, T. I., Shevchenko, N. M., Semenova, T. L., Sukhoverkhov, S. V., and Zvyagintseva, T. N. (2011). Compositional heterogeneity of sulfated polysaccharides synthesized by the brown alga Costaria costata. Chem. Nat. Compd. 47, 96–97. doi: 10.1007/s10600-011-9839-y
Imbs, T. I., Shevchenko, N. M., Sukhoverkhov, S. V., Semenova, T. L., Skriptsova, A. V., and Zvyagintseva, T. N. (2009). Seasonal variations of the composition and structural characteristics of polysaccharides from the brown alga Costaria costata. Chem. Nat. Compd. 45, 786–791. doi: 10.1007/s10600-010-9507-7
Jayawardena, T. U., Fernando, I. P. S., Lee, W. W., Sanjeewa, K. K. A., Kim, H.-S., Lee, D.-S., et al. (2019). Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; inflamation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 131, 614–623. doi: 10.1016/j.ijbiomac.2019.03.105
Jesumani, V., Du, H., Pei, P., Aslam, M., and Huang, N. (2020). Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS One 15:e0227308. doi: 10.1371/journal.pone.0227308
Jones, A. S., and Letham, D. S. (1954). A submicro method for the estimation of sulphur. Chem. Ind. 662–663.
Karmakar, P., Ghosh, T., Sinha, S., Saha, S., Mandal, P., Ghosal, P. K., et al. (2009). Polysaccharides from the brown seaweed Padina tetrastromatica: characterization of a sulfated fucan. Carbohydr. Polym. 78, 416–421. doi: 10.1016/j.carbpol.2009.04.039
Karmakar, P., Pujol, C. A., Damonte, E. B., Ghosh, T., and Ray, B. (2010). Polysaccharides fom Padina tetrastromatica: features, chemical modification and antiviral activity. Carbohydr. Polym. 80, 513–520. doi: 10.1016/j.carbpol.2009.12.014
Kitamura, K., Matsuo, M., and Yasui, T. (1991). Fucoidan from brown seaweed Laminaria angustata var. Longissima. Agric. Biol. Chem. 55, 615–616. doi: 10.1271/bbb1961.55.615
Kloareg, B., Demarty, M., and Mabeau, S. (1986). Polyanionic characteristics of purified sulphated homofucans from brown algae. Int. J. Biol. Macromol. 8, 380–386. doi: 10.1016/0141-8130(86)90060-7
Koh, H. S. A., Lu, J., and Zhou, W. (2019). Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 212, 178–185. doi: 10.1016/j.carbpol.2019.02.040
Koo, J.-G., Choi, Y.-S., and Kwak, J.-K. (2001). Blood-anticoagulant activity of fucoidans from sporophylls of Undaria pinnatifida, Laminaria religiosa, Hizikia fusiforme and Sargassum fulvellum in Korea. J. Korean Fish. Soc. 34, 515–520.
Kopplin, G., Rokstad, A. M., Mélida, H., Bulone, V., Skjåk-Bræk, G., and Aachmann, F. L. (2018). Structural characterization of fucoidan from Laminaria hyperborea: assessment of coagulation and inflammatory properties and their structure-function relationship. ACS Appl. Bio Mater. 1, 1880–1892. doi: 10.1021/acsabm.8b00436
Larsen, B., Haug, A., and Painter, T. J. (1966). Sulphated polysaccharides in brown algae-I. Isolation and preliminary characterization of three sulphated polysaccharides from Ascophyllum nodosum (L.) Le Jol. Acta Chem. Scand. 20, 219–230. doi: 10.3891/acta.chem.scand.20-0219
Leal, D., Mansilla, A., Matsuhiro, B., Moncada-Basualto, M., Lapier, M., Maya, J. D., et al. (2018). Chemical structure and biological properties of sulfated fucan from the sequential extraction of subAntartic Lessonia sp. (Phaeophyceae). Carbohydr. Polym. 199, 304–313. doi: 10.1016/j.carbpol.2018.07.012
Lee, J.-B., Hayashi, K., Hashimoto, M., Nakano, T., and Hayashi, T. (2004). Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 52, 1091–1094. doi: 10.1248/cpb.52.1091
Lee, J.-B., Takeshita, A., Hayashi, K., and Hayashi, T. (2011). Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 86, 995–999. doi: 10.1016/j.carbpol.2011.05.059
Lee, S.-H., Ko, C.-I., Ahn, G., You, S., Kim, J.-S., Heu, M. S., et al. (2012). Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 89, 599–606. doi: 10.1016/j.carbpol.2012.03.056
Li, B., Wei, X.-J., Sun, J.-L., and Xu, S.-Y. (2006). Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed. Hizikia fusiforme. Carbohydr. Res. 341, 1135–1146. doi: 10.1016/j.carres.2006.03.035
Li, G.-Y., Luo, Z.-C., Yuan, F., and Yu, X.-B. (2017). Combined process of high-pressure homogenization and hydrothermal extraction for the extraction of fucoidan with good antioxidant properties from Nemacystus decipients. Food Bioprod. Process. 106, 35–42. doi: 10.1016/j.fbp.2017.08.002
Lim, S. J., Aida, W. M. W., Maskat, M. Y., Latip, J., Badri, K. H., Hassan, O., et al. (2016). Characterisation of fucoidan extracted from Malaysian Sargassum binderi. Food Chem. 209, 267–273. doi: 10.1016/j.foodchem.2016.04.058
Lim, S. J., Aida, W. M. W., Schiehser, S., Rosenau, T., and Böhmdorfer, S. (2019). Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 272, 222–226. doi: 10.1016/j.foodchem.2018.08.034
Liu, N., Wu, X., Fu, X., Duan, D., Xu, J., and Gao, X. (2018). Characterization of polysaccharides extracted from a cultivated brown alga Costaria costata during the harvest period. J. Ocean. Univ. China 17, 1209–1217. doi: 10.1007/s11802-018-3621-8
Liu, X., Liu, B., Wei, X.-L., Sun, Z.-L., and Wang, C.-Y. (2016). Extraction, fractionation, and chemical characterisation of fucoidans from the brown seaweed Sargassum pallidum. Czech J. Food Sci. 34, 406–413. doi: 10.17221/322/2015-CJFS
Lloyd, A. G. (1959). Studies on sulphatases. 24. The use of barium chloranilate in the determination of the enzymically liberated sulphate. Biochem. J. 72, 133–136. doi: 10.1042/bj0720133
Lorbeer, A. J., Charoensiddhi, S., Lahnstein, J., Lars, C., Franco, C. M. M., Bulone, V., et al. (2017). Sequential extraction an characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 29, 1515–1526. doi: 10.1007/s10811-016-0990-5
Lorbeer, A. J., Lahnstein, J., Fincher, G. B., Su, P., and Zhang, W. (2015). Kinetics of conventional and microwave-assisted fucoidan extractions from the brown alga, Ecklonia radiata. J. Appl. Phycol. 27, 2079–2087. doi: 10.1007/s10811-014-0446-8
Lu, J., Shi, K. K., Chen, S., Wang, J., Hassouna, A., et al. (2018). Fucoidan extracted from the New Zealand Undaria pinnatifida-physicochemical comparison against five other fucoidans: unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 16:461. doi: 10.3390/md16120461
Luo, D., Wang, Z., and Nie, K. (2019). Structural characterization of a novel polysaccharide from Sargassum thunbergii and its antioxidant and anti-inflammation effects. PLoS One 14:e0223198. doi: 10.1371/journal.pone.0223198
Ly, B. M., Buu, N. Q., Nhut, N. D., Thinh, P. D., and Van, T. T. T. (2005). Studies on fucoidan and its production from vietnamese brown seaweeds. AJSTD 22, 371–380. doi: 10.29037/ajstd.173
Mabeau, S., and Kloareg, B. (1987). Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 38, 1573–1580. doi: 10.1093/jxb/38.9.1573
Mabeau, S., Kloareg, B., and Joseleau, J.-P. (1990). Fractionation and analysis of fucans from brown algae. Phytochemistry. 29, 2441–2445. doi: 10.1016/0031-9422(90)85163-A
Mackie, W., and Preston, R. D. (1974). “Cell wall and intercellular region polysaccharides,” in Algal Physiology and Biochemistry, ed. W. D. P. Stewart (Oxford: Blackwell Scientific Publications), 58–64.
Mak, W., Hamid, N., Liu, T., Lu, J., and White, W. L. (2013). Fucoidan from New Zealand Undaria pinnatifida: monthly variations and determination of antioxidant activities. Carbohydr. Polym. 95, 606–614. doi: 10.1016/j.carbpol.2013.02.047
Mandal, P., Mateu, C. G., Chattopadhyay, K., Pujol, C. A., Damonte, E. B., and Ray, B. (2007). Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 18, 153–162. doi: 10.1177/095632020701800305
Mateos-Aparicio, I., Martera, G., Goñi, I., Villanueva-Suárez, M.-J., and Redondo-Cuenca, A. (2018). Chemical structure and molecular weight influence the in vitro fermentability of polysaccharide extracts from the edible seaweeds Himanthalia elongata and Gigartina pistillata. Food Hydrocoll. 83, 348–354. doi: 10.1016/j.foodhyd.2018.05.016
Medcalf, D. G., and Larsen, B. (1977a). Fucose-containing polysaccharides in the brown alga Ascophyllum nodosum and Fucus vesiculosus. Carbohydr. Res. 59, 531–537. doi: 10.1016/S0008-6215(00)83190-0
Medcalf, D. G., and Larsen, B. (1977b). Structural studies on ascophyllan and the fucose-containing complexes from the brown alga Ascophyllum nodosum. Carbohydr. Res. 59, 539–546. doi: 10.1016/S0008-6215(00)83191-2
Medcalf, D. G., Root, C. F., Craney, C. L., Mukhopadhyhay, D., Miller, C. J., and Hopewell, W. D. (1972). Chemical characterization of mucilaginous polysaccharides from Ulvaceae species native to the Puget Sound. Proc. Int. Seaweed Symp 7, 541–547.
Medcalf, D. G., Schneider, T. L., and Barnett, R. W. (1978). Structural features of a novel glucuronogalactofucan from Ascophyllum nodosum. Carbohydr. Res. 66, 167–171. doi: 10.1016/S0008-6215(00)83249-8
Medeiros, V. P., Queiroz, K. C. S., Cardoso, M. L., Monteiro, G. R. G., Oliveira, F. W., Chavante, S. F., et al. (2008). Sulfated galactofucan from Lobophora variegata: anticoagulant and anti-inflammatory properties. Biochemistry 73, 1018–1024. doi: 10.1134/S0006297908090095
Menezes, M. M., Nobre, L. T. D. B., Rossi, G. R., Almeida-Lima, J., Melo-Silveira, R. F., Franco, C. R. C., et al. (2018). A low-molecular-weight galactofucan from the seaweed, Spatoglossum schröederi, binds fibronectin and inhibits capillary-like tube formation in vitro. Int. J. Biol. Macromol. 111, 1067–1075. doi: 10.1016/j.ijbiomac.2018.01.119
Menshova, R. V., Anastyuk, S. D., Ermakova, S. P., Shevchenko, M. N., Isakov, V. I., and Zvyagintseva, T. N. (2015). Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 132, 118–125. doi: 10.1016/j.carbpol.2015.06.020
Men’shova, R. V., Ermakova, S. P., Rachidi, S. M., Al-Hajje, A. H., Zvyagintseva, T. N., and Kanaan, H. M. (2012). Seasonal variations of the composition, structural features, and antitumor properties of polysaccharides from Padina pavonica (Lebanon) as a function of composition. Chem. Nat. Compd. 47, 870–875. doi: 10.1007/s10600-012-0091-x
Men’shova, R. V., Lepeshkin, F. D., Ermakova, S. P., Pokrovskii, O. I., and Zvyagintseva, T. N. (2013). Effect of pretreatment conditions of brown algae by supercritical fluids on yield and structural characteristics of fucoidans. Chem. Nat. Compd. 48, 923–926. doi: 10.1007/s10600-013-0429-z
Mian, A. J., and Percival, E. (1973). Carbohydrates of the brown seaweeds Himanthalia lorea, Bifurcaria bifurcata, and Padina pavonia. Part I. Extraction and fractionation. Carbohydr. Res. 26, 133–146. doi: 10.1016/S0008-6215(00)85030-2
Miller, I. J. (1997). The chemotaxonomic significance of the water-soluble red algal polysaccharides. Recent Res. Dev. Phytochem. 1, 531–565.
Monsur, H. A., Jaswir, I., Simsek, S., Amid, A., and Alam, Z. (2017). Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata). Int. J. Food Prop. 20, 1457–1469. doi: 10.1080/10942912.2016.1211144
Mori, H., and Nisizawa, K. (1982). Sugars constituents of sulfated polysaccharides from the fronds of Sargassum ringgoldianum. Bull. Jpn. Soc. Sci. Fish. 48, 981–986. doi: 10.2331/suisan.48.981
Nagaoka, M., Shibata, H., Kimura-Takagi, I., Hashimoto, S., Kimura, K., Makino, T., et al. (1999). Structural study of fucoidan from Cladosiphon Okamuranus TOKIDA. Glycoconj. J. 16, 19–26. doi: 10.1023/A:1006945618657
Nakayasu, S., Soegima, R., Yamaguchi, K., and Oda, T. (2009). Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 73, 961–964. doi: 10.1271/bbb.80845
Nguyen, T. T., Mikkelsen, M. D., Tran, V. H. N., Trang, V. T. D., Rhein-Knudsen, N., Holck, J., et al. (2020). Enzyme-assisted fucoidan extraction from brown macroalgae Fucus districhus subsp. evanescens and Saccharina lattisima. Mar. Drugs 18:296. doi: 10.3390/md18060296
Nishino, T., Nishioka, C., Ura, H., and Nagumo, T. (1994a). Isolation and partial characterization of a novel aminosugar-containing fucan sulphate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 255, 213–224. doi: 10.1016/S0008-6215(00)90980-7
Nishino, T., Takabe, Y., and Nagumo, T. (1994b). Isolation and partial characterization of a novel β-D-galactan sulfate from the brown seaweed Laminaria angustata var. longissima. Carbohydr. Polym. 23, 165–173. doi: 10.1016/0144-8617(94)90099-X
Nishino, T., Yokoyama, G., Dobashi, K., Fujihara, M., and Nagumo, T. (1989). Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 186, 119–129. doi: 10.1016/0008-6215(89)84010-8
Olatunji, O. (2020). “Fucoidan,” in Aquatic Biopolymers. Springer Series on Polymer and Composite Materials, ed. S. Kalia (Cham: Springer), 95–115. doi: 10.1007/978-3-030-34709-3_5
Oltmanns, F. (1922). Morphologie und Biologie der Algen. Phaeophyceae-Rhodophyceae, 2nd Edn, Vol. II. Jena: Gustav Fischer.
Ozawa, T., Yamamoto, J., Yamagishi, T., Yamazaki, N., and Nishizawa, M. (2006). Two fucoidans in the holdfast of cultivated Laminaria japonica. J. Nat. Med. 60, 236–239. doi: 10.1007/s11418-006-0046-2
Palanisamy, S., Vinosha, M., Marudhupandi, T., Rajasekar, P., and Prabhu, N. M. (2017). In vitro antioxidant and andibacterial activity of sulfated polysaccharides isolated from Spatoglossum asperum. Carbohydr. Polym. 170, 296–304. doi: 10.1016/j.carbpol.2017.04.085
Percival, E. (1968). Glucuronoxylofucan, a cell-wall component of Ascophyllum nodosum.Part. I. Carbohydr. Res. 7, 272–283. doi: 10.1016/S0008-6215(00)81200-8
Percival, E. (1979). The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br. Phycol. J. 14, 103–117. doi: 10.1080/00071617900650121
Percival, E., and McDowell, R. H. (1967). Chemistry and Enzymology of Marine Algal Polysaccharides. (New York, NY: Academic Press), 157–174.
Percival, E., Rahman, M. D. A., and Weigel, H. (1981). Chemistry of the polysaccharides of the brown seaweed Dictyopteris plagiogramma. Phytochemistry 20, 1579–1582. doi: 10.1016/S0031-9422(00)98535-2
Percival, E., and Young, M. (1974). Carbohydrates of the brown seaweeds: part III. Desmarestia aculeata. Carbohydr. Res. 32, 195–201. doi: 10.1016/s0008-6215(00)82097-2
Percival, E. E., Venegas Jara, M. F., and Weigel, H. (1983). Carbohydrates of the brown seaweed Lessonia nigrescens. Phytochemistry 22, 1429–1432. doi: 10.1016/S0031-9422(00)84029-7
Ponce, N. M. A., Flores, M. L., Pujol, C. A., Becerra, M. B., Navarro, D. A., Córdoba, O., et al. (2019). Fucoidans from the phaeophyta Scytosiphon lomentaria: chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 478, 18–24. doi: 10.1016/j.carres.2019.04.004
Ponce, N. M. A., Pujol, C. A., Damonte, E. B., Flores, M. L., and Stortz, C. A. (2003). Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr. Res. 338, 153–165. doi: 10.1016/S0008-6215(02)00403-2
Prokofjeva, M. M., Imbs, T. I., Shevchenko, N. M., Spirin, P. V., Horn, S., Fehse, B., et al. (2013). Fucoidans and potential inhibitors of HIV-1. Mar. Drugs 11, 3000–3014. doi: 10.3390/md11083000
Qu, G., Liu, X., Wang, D., Yuan, Y., and Han, L. (2014). Isolation and characterization of fucoidans from five brown algae and evaluation of their antioxidant activity. J. Ocean. Univ. China 13, 851–856. doi: 10.1007/s11802-014-2260-y
Queiroz, K. C. S., Medeiros, V. P., Queiroz, L. S., Abreu, L. R. D., Rocha, H. A. O., Ferreira, C. V., et al. (2008). Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 62, 303–307. doi: 10.1016/j.biopha.2008.03.006
Rabanal, M., Ponce, N. M. A., Navarro, D. A., Gómez, R. M., and Stortz, C. A. (2014). The system of fucoidans from the brown seaweed Dictyota dichotoma: chemical analysis and antiviral activity. Carbohydr. Polym. 101, 804–811. doi: 10.1016/j.carbpol.2013.10.019
Rioux, L.-E., Turgeon, S. L., and Beaulieu, M. (2007). Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 69, 530–537. doi: 10.1016/j.carbpol.2007.01.009
Rodríguez-Jasso, R. M., Mussatto, S. I., Pastrana, L., Aguilar, C. N., and Teixeira, J. A. (2011). Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 86, 1137–1144. doi: 10.1016/j.carbpol.2011.06.006
Rousseau, F., Burrowes, R., Peters, A. F., Kuhlenkamp, R., and de Reviers, B. (2001). A comprehensive phylogeny of the Phaeophyceae based on nrDNA sequences resolves the earliest divergences. C. R. Acad. Sci. Paris 324, 305–319. doi: 10.1016/S0764-4469(01)01306-3
Rousseau, F., and de Reviers, B. (1999a). Phylogenetic relationships within the Fucales (Phaeophyceae) based on combined partial SSU + LSU rDNA sequence data. Eur. J. Phycol. 34, 53–64. doi: 10.1080/09670269910001736082
Rousseau, F., and de Reviers, B. (1999b). Circumscription of the order Ectocarpales (Phaeophyceae): bibliographical synthesis and molecular evidence. Cryptogamie Algol. 20, 5–18. doi: 10.1016/S0181-1568(99)80002-6
Rupérez, P., Ahrazem, O., and Leal, J. A. (2002). Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 50, 840–845. doi: 10.1021/jf010908o
Sakai, T., Kimura, H., and Kato, I. (2002). A marine strain of Flavobacteriaceae utilizes brown seaweed fucoidan. Mar. Biotechnol. 4, 399–405. doi: 10.1007/s10126-002-0032-y
Sakai, T., Kimura, H., Kojima, K., Shimanaka, K., Ikai, K., and Kato, I. (2003). Marine bacterial sulfated fucoglucuronomannan (SFGM) lyase digests brown algal SFGM into trisaccharides. Mar. Biotechnol. 5, 70–78. doi: 10.1007/s10126-002-0056-3
Saravana, P. S., Cho, Y.-J., Park, Y.-B., Woo, H.-C., and Chun, B. S. (2016). Structural, antioxidante, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 153, 518–525. doi: 10.1016/j.carbpol.2016.08.014
Schweiger, R. G. (1962). Methanolysis of fucoidan. II. The presence of sugars other than L-fucose. J. Org. Chem 27, 4270–4272. doi: 10.1021/jo01059a034
Scott, J. E. (1960). Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem. Anal. 8, 145–197. doi: 10.1002/9780470110249.ch4
Sellimi, S., Kadri, N., Barragan-Montero, V., Laouer, H., Hajji, M., and Nasri, M. (2014). Fucans from a Tunisian brown seaweed Cystoseira barbata: structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 66, 281–288. doi: 10.1016/j.ijbiomac.2014.02.041
Senthilkumar, K., Ramajayam, G., Venkatesan, J., Kim, S.-K., and Ahn, B.-C. (2017). “Biomedical applications of fucoidans, seaweed polysaccharides,” in Seaweed Polysaccharides – Isolation, Biological, and Biomedical Applications, eds J. Venkatesan, S. Anil, and S.-K. Kim (Amsterdam: Elsevier), 269–281. doi: 10.1016/B978-0-12-809816-5.00014-1
Shevchenko, N. M., Anastyuk, S. D., Menshova, R. V., Vishchuk, O. S., Isakov, V. I., Zadorozhny, P. A., et al. (2015). Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr. Polym. 121, 207–216. doi: 10.1016/j.carbpol.2014.12.042
Shevchenko, N. M., Usol´tseva (Men’shova), R. V., Ishina, I. A., Thinh, P. D., Ly, B. M., and Ermakova, S. P. (2017). Structural characteristic and in vitro antitumor activity of water-soluble polysaccharides from brown algae of the Russian far east and Vietnam. Chem. Nat. Compd. 53, 1–5. doi: 10.1007/s10600-017-1897-3
Silberfeld, T., Racault, M.-F. L. P., Fletcher, R. L., Couloux, A., Rousseau, F., and de Reviers, B. (2011). Systematics and evolutionary history of pyrenoid-bearing taxa in bown algae (Phaeophyceae). Eur. J. Phycol. 46, 361–377. doi: 10.1080/09670262.2011.628698
Silberfeld, T., Rousseau, F., and de Reviers, B. (2014). An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogam. Algol. 35, 117–156. doi: 10.7872/crya.v35.iss2.2014.117
Silchenko, A. S., Rasin, A. B., Kusaykin, M. I., Kalinovsky, A. I., Miansong, Z., Changheng, L., et al. (2017). Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargasum horneri. Carbohydr. Polym. 175, 654–660. doi: 10.1016/j.carbpol.2017.08.043
Silva, T. M. A., Alves, L. G., Queiroz, K. C. S., Santos, M. G. L., Marques, C. T., Chavante, S. F., et al. (2005). Partial characterization and anticoagulant activity of a heterofucan from the brown seawed Padina gymnospora. Braz. J. Med. Biol. Res. 38, 523–533. doi: 10.1590/S0100-879X2005000400005
Sinha, S., Astani, A., Ghosh, T., Schnitzler, P., and Ray, B. (2010). Polysaccharides from Sargassum tenerrimum: structural features, chemical modification and anti-viral activity. Phytochemistry 71, 235–242. doi: 10.1016/j.phytochem.2009.10.014
Skriptsova, A. V. (2015). Fucoidans from brown algae: biosynthesis, localization, and physiological role in the thallus. Russ. J. Mar. Biol. 41, 145–156. doi: 10.1134/S1063074015030098
Skriptsova, A. V., Shevchenko, N. M., Tarbeeva, D. V., and Zvyagintseva, T. N. (2012). Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 14, 304–311. doi: 10.1007/s10126-011-9413-4
Sokolova, R. V., Ermakova, S. P., Awada, S. M., Zvyagintseva, T. N., and Kanaan, H. M. (2011). Composition, structural characteristics and antitumor properties, of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Compd. 47, 329–334. doi: 10.1007/s10600-011-9925-1
Somasundaram, N., Shanmugam, S., Subramanian, B., and Jaganathan, R. (2016). Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15 S. Int. J. Biol. Macromol. 91, 1215–1223. doi: 10.1016/j.ijbiomac.2016.06.084
Song, Y., Wang, Q., Wang, Q., He, Y., Ren, D., Liu, S., et al. (2018). Structural characterization and antitumor effects of fucoidans from brown algae Kjellmaniella crassifolia farmed in northern China. Int. J. Biol. Macromol. 119, 125–133. doi: 10.1016/j.ijbiomac.2018.07.126
Starko, S., Soto Gomez, M., Darby, H., Demes, K. W., Kawai, H., Yotsukura, N., et al. (2019). A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol. Phylogenet. Evol. 136, 138–150. doi: 10.1016/j.ympev.2019.04.012
Stortz, C. A., and Cerezo, A. S. (2000). Novel findings in carrageenans, agaroids and “hybrid” red seaweed galactans. Curr. Top. Phytochem. 4, 121–134.
Sun, Q. L., Li, Y., Ni, L.-Q., Li, Y.-X., Cui, Y.-S., Jiang, S.-L., et al. (2020). Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 229:115487. doi: 10.1016/j.carbpol.2019.115487
Synytsya, A., Kim, W.-J., Kim, S.-M., Pohl, R., Synytsya, A., Kvasnièka, F., et al. (2010). Structure and antitumour activity of fucoidan isolated from sporophyllof Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 81, 41–48. doi: 10.1016/j.carbpol.2010.01.052
Tako, M., Takeda, S., Teruya, T., and Tamaki, Y. (2010). Chemical characterization of fucoidans from Laminaria angustata var. longissima. Nippon Shokuhin Kagaku Kogaku Kaishi 57, 495–502. (in Japanese) doi: 10.3136/nskkk.57.495
Tan, I. H., and Druehl, L. D. (1996). A ribosomal DNA phylogeny supports the close evolutionary relationships among the Sporochnales, Desmarestiales, and Laminariales (Phaeophyceae). J. Phycol. 32, 112–118. doi: 10.1111/j.0022-3646.1996.00112.x
Teruya, T., Tatemoto, H., Konishi, T., and Tako, M. (2009). Structural characteristics and in vitro macrophage of acetyl fucoidan from Cladosiphon okamuranus. Glycoconj. J. 26, 1019–1028. doi: 10.1007/s10719-008-9221-x
Thinh, P. D., Menshova, R. V., Ermakova, S. P., Anastyuk, S. D., Ly, B. M., and Zvyagintseva, T. N. (2013). Structural characteristics and anticancer activity of fucoidan from the brown alga Sargasum mcclurei. Mar. Drugs 11, 1456–1476. doi: 10.3390/md11051456
Thornber, C. S., Kinlan, B. P., Graham, M. H., and Stachowicz, J. J. (2004). Population ecology of the invasive kelp Undaria pinnatifida in California: environmental and biological controls on demography. Mar. Ecol. Prog. Ser. 268, 69–80. doi: 10.3354/meps268069
Torode, T. A., Siméon, A., Marcus, S. E., Jam, M., Le-Moigne, M. A., Duffieux, D., et al. (2016). Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. J. Exp. Bot. 67, 6089–6100. doi: 10.1093/jxb/erw369
Usoltseva (Menshova), R. V., Anastyuk, S. D., Shevchenko, M. N., Zvyagintseva, T. N., and Ermakova, S. P. (2016). The comparison of structure and anticancer activity(in)vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 153, 258–265. doi: 10.1016/j.carbpol.2016.07.103
Usoltseva, R. V., Anastyuk, S. D., Ishina, I. A., Isakov, V. V., Zvyagintseva, T. N., Thinh, P. D., et al. (2018a). Structural characteristics and anticancer activity in vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 184, 260–268. doi: 10.1016/j.carbpol.2017.12.071
Usoltseva, R. V., Anastyuk, S. D., Shevchenko, N. M., Surits, V. V., Silchenko, A. S., Isakov, V. V., et al. (2017a). Polysaccharides from brown algae Sargassum duplicatum: the structure and anticancer activity in vitro. Carbohydr. Polym. 175, 547–556. doi: 10.1016/j.carbpol.2017.08044
Usoltseva, R. V., Shevchenko, N. M., Malyarenko, O. S., Anastyuk, S. D., Kasprik, A. E., Zvyagintseva, N. V., et al. (2019). Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 221, 157–165. doi: 10.1016/j.carbpol.2019.05.079
Usoltseva, R. V., Shevchenko, N. M., Malyarenko, O. S., Ishina, I. A., Ivannikova, S. I., and Ermakova, S. P. (2018b). Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 180, 21–28. doi: 10.1016/j.carbpol.2017.10.006
Usoltseva, R. V., Zhao, P., Kusaikin, M. I., Jia, A., Yuan, W., Zhang, M., et al. (2017b). Structural characteristics and antitumor activity of fucoidans from the brown alga Sargasum muticum. Chem. Nat. Compd. 53, 219–223. doi: 10.1007/s10600-017-1956-9
Usov, A. I. (2011). Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 65, 115–217. doi: 10.1016/B978-0-12-385520-6.00004-2
van den Hoek, C., Mann, D., and Jahns, H. M. (1996). Algae: An Introduction To Phycology. Cambridge: Cambridge University Press.
Vishchuk, O. S., Ermakova, S. P., and Zvyagintseva, T. N. (2011). Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 346, 2769–2776. doi: 10.1016/j.carres.2011.09.034
Vishchuk, O. S., Ermakova, S. P., and Zvyagintseva, T. N. (2013). The effect of sulfated (1(3)-α-L-fucan from the brown alga Saccharina cichorioides Miyabe on resveratrol-induced apoptosis in colon carcinoma cells. Mar. Drugs 11, 194–212. doi: 10.3390/md11010194
Vishchuk, O. S., Tarbeeva, D. V., Ermakova, S. P., and Zvyagintseva, T. N. (2012). Structural characteristics and biological activity of fucoidans from the brown algae Alaria sp. and Saccharina japonica of different reproductive status. Chem. Biodiv. 9, 817–828. doi: 10.1002/cbdv.201100266
Wang, P., Zhao, X., Lv, Y., Liu, Y., Lang, Y., Wu, J., et al. (2012). Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr. Polym. 90, 602–607. doi: 10.1016/j.carbpol.2012.05.084
Wang, Q., Song, Y., He, Y., Ren, D., Kow, F., Qiao, Z., et al. (2014). Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydr. Polym. 107, 247–254. doi: 10.1016/j.carbpol.2014.02.071
Wang, Y., Xing, M., Cao, Q., Ji, A., Liang, H., and Song, S. (2019). Biological activities of fucoidan and the factors mediating Its therapeutic effects: a review of recent studies. Mar. Drugs 17:183. doi: 10.3390/md17030183
Wozniak, M., Bell, T., Dénes, A., Falshaw, R., and Itzhaki, R. (2015). Anti-HSV-1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 74, 530–540. doi: 10.1016/j.ijbiomac.2015.01.003
Wu, S., Zhang, X., Liu, J., Song, J., Yu, P., Chen, P., et al. (2019). Physicochemical characterization of Sargassum fusiforme fucoidan fractions and their antagonistic effect against P-selectin-mediated cell adhesion. Int. J. Biol. Macromol. 133, 656–662. doi: 10.1016/j.ijbiomac.2019.03.218
Wuts, P. G. M., and Greene, T. W. (2006). Greene’s Protective Groups in Organic Synthesis: Chapter 2, 4th Edn. Hoboken, NJ: John Wiley, doi: 10.1002/9780470053485.ch2
Yang, W.-N., Chen, P.-W., and Huang, C.-Y. (2017). Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 15:183. doi: 10.3390/md15060183
You, S., Yang, C., Lee, H., and Lee, B.-Y. (2010). Molecular characteristics of partially hydrolyzed fucoidans from sporophyll of Undaria pinnatifida and their in vitro anticancer activity. Food Chem. 119, 554–559. doi: 10.1016/j.foodchem.2009.06.054
Yuan, Y., and Macquarrie, D. J. (2015). Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars, and biochar from Ascophyllum nodosum through a biorefinery concept. Biores. Technol. 198, 819–827. doi: 10.1016/j.biortech.2015.09.090
Yuguchi, Y., Tran, V. T. T., Bui, L. M., Takebe, S., Suzuki, S., Nakajima, N., et al. (2016). Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 147, 69–78. doi: 10.1016/j.carbpol.2016.03.101
Zhang, W., Oda, T., Yu, Q., and Jin, J.-O. (2015). Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three others fucoidans. Mar. Drugs 13, 1084–1104. doi: 10.3390/md13031084
Zvyagintseva, T. N., Shevchenko, N. M., Chizhov, A. O., Krupnova, T. N., Sundukova, E. V., and Isakov, V. V. (2003). Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 294, 1–13. doi: 10.1016/S0022-0981(03)00244-2
Keywords: fucoidans, brown seaweeds, phaeophyceae, taxonomy, phylogeny
Citation: Ponce NMA and Stortz CA (2020) A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 11:556312. doi: 10.3389/fpls.2020.556312
Received: 27 April 2020; Accepted: 02 November 2020;
Published: 25 November 2020.
Edited by:
Cécile Hervé, Laboratoire de Biologie Intégrative des Modèles Marins, Station Biologique de Roscoff, FranceReviewed by:
Anne S. Meyer, Technical University of Denmark, DenmarkJasna Miroljub Nikolic, UMR 8227 Laboratoire de Biologie Intégrative des Modèles Marins, France
Copyright © 2020 Ponce and Stortz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nora M. A. Ponce, YXBvbmNlQHFvLmZjZW4udWJhLmFy; Carlos A. Stortz, c3RvcnR6QHFvLmZjZW4udWJhLmFy
 Nora M. A. Ponce
Nora M. A. Ponce Carlos A. Stortz
Carlos A. Stortz