- 1Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran
- 2Plant Virology Research Center, College of Agriculture, Shiraz University, Shiraz, Iran
- 3Institute for Sustainable Plant Protection, National Research Council of Italy, Turin, Italy
- 4Laboratory of Virology, Department of Plant Sciences, Wageningen University & Research, Wageningen, Netherlands
Tomato spotted wilt virus (TSWV) is a devastating plant pathogen, causing huge crop losses worldwide. Unfortunately, due to its wide host range and emergence of resistance breaking strains, its management is challenging. Up to now, resistance to TSWV infection based on RNA interference (RNAi) has been achieved only in transgenic plants expressing parts of the viral genome or artificial microRNAs targeting it. Exogenous application of double-stranded RNAs (dsRNAs) for inducing virus resistance in plants, namely RNAi-based vaccination, represents an attractive and promising alternative, already shown to be effective against different positive-sense RNA viruses and viroids. In the present study, the protection efficacy of exogenous application of dsRNAs targeting the nucleocapsid (N) or the movement protein (NSm) coding genes of the negative-sense RNA virus TSWV was evaluated in Nicotiana benthamiana as model plant and in tomato as economically important crop. Most of the plants treated with N-targeting dsRNAs, but not with NSm-targeting dsRNAs, remained asymptomatic until 40 (N. benthamiana) and 63 (tomato) dpi, while the remaining ones showed a significant delay in systemic symptoms appearance. The different efficacy of N- and NSm-targeting dsRNAs in protecting plants is discussed in the light of their processing, mobility and biological role. These results indicate that the RNAi-based vaccination is effective also against negative-sense RNA viruses but emphasize that the choice of the target viral sequence in designing RNAi-based vaccines is crucial for its success.
Introduction
Tomato spotted wilt virus (TSWV), genus Orthotospovirus, family Tospoviridae, belongs to the list of the ten most economically important viruses in the world (Scholthof et al., 2011) and is able to cause high yield losses in a variety of crops and ornamentals, in tropical and subtropical regions (Pappu et al., 2009; Mitter et al., 2013; Turina et al., 2016). It has a wide host range and is transmitted by thrips in a persistent manner (Whitfield and Rotenberg, 2015). The TSWV particles are spherical and surrounded by a host-derived membrane with a diameter of 80–120 nm. The TSWV genome is of negative/ambisense polarity and consists of three linear single-stranded RNAs (ssRNA) named large (L), medium (M), and small (S) according to their sizes (Turina et al., 2016). The L segment codes for the RNA-dependent RNA polymerase (RdRp) in negative sense, while M and S segments are ambisense in their genome organization; the M RNA encodes the glycoprotein precursor (GP) of the mature membrane glycoproteins GN and GC and the cell-to-cell movement protein (NSm) while the S RNA codes for the nucleocapsid protein (N), and the silencing suppressor protein (NSs).
Up to now, few resistance genes against TSWV have been identified and introgressed in commercial cultivars (Turina et al., 2016); however, the frequent appearance of resistance-breaking isolates, together with the difficulty of selecting and incorporating new resistance genes stress the needs of developing new strategies for protecting plants against TSWV infection.
RNA interference (RNAi) is an RNA-mediated regulatory mechanism, conserved in most eukaryotes, consisting in the sequence-specific degradation of target RNA guided by the complementary small RNAs (sRNAs; Meister and Tuschl, 2004). Beside its crucial activity in regulating growth and development, this mechanism plays a critical role in host defense against subcellular pathogens (Baulcombe, 2004; Padmanabhan et al., 2009; Agius et al., 2012). RNAi is triggered by double-stranded RNAs (dsRNA) or hairpin RNAs (hpRNA) that are processed by specific nucleases called DICER or DICER-LIKE (DCL) into sRNAs able to guide the cleavage of complementary single-stranded RNAs, such as messenger RNAs or viral genomic/antigenomic RNAs (Meister and Tuschl, 2004).
Virus infection is associated with the accumulation of viral small RNAs (vsRNAs), originated by the host plant RNAi machinery from viral dsRNAs or hpRNAs. VsRNAs are able to direct the degradation of complementary viral single-stranded RNAs through the plant RNAi pathways (Agius et al., 2012). As a consequence, viruses are both inducers and targets of RNAi, through a process regarded as a natural antiviral defense mechanism in plants (Guo et al., 2016). Since the discovery of such siRNA-mediated antiviral defense mechanism, a number of transgene- or virus-based silencing technologies have been developed in order to protect plants from pathogens (Dietzgen and Mitter, 2006; Guo et al., 2016). DsRNAs or hpRNAs transcribed from engineered inverted repeats were shown to be potent inducers of a gene silencing response when directed against transgenes or viral pathogens (Smith et al., 2000; Marjanac et al., 2009; Hameed et al., 2017). Different RNAi approaches, all based on plant transformation, were established to induce resistance against TSWV, including the expression of viral genomic sequences (Yazhisai et al., 2015), artificial microRNAs (miRNAs; Mitter et al., 2016), or synthetic trans-acting siRNAs (tasiRNAs; Carbonell et al., 2019). However, transgenic approaches are time-consuming, expensive and require efficient plant transformation protocols; moreover, they are prone to significant regulation and acceptance issues, particularly in the case of food crops.
A promising technique is the RNAi-based vaccination, that exploits the RNAi machinery of the plants to protect them from viral infection. Several studies have shown that dsRNAs homologous to viral sequences, when topically applied to plants, can induce RNAi-mediated defense, interfering with infection by plant positive RNA viruses and viroids in a sequence-specific manner [reviewed in Mitter et al. (2017b); Dubrovina and Kiselev (2019))]. Exogenous application of naked dsRNAs (Tenllado and Díaz-Ruíz, 2001; Tenllado et al., 2004; Petrov et al., 2015) or spraying of bacterially expressed dsRNAs (Tenllado et al., 2003; Gan et al., 2010) were demonstrated to protect plants up to 5 days post treatment. Moreover, loading dsRNAs onto clay nanosheets increased dsRNA stability and efficacy up to 20 days post spray (Mitter et al., 2017a). These approaches interfered with the infection of potyviruses, bromoviruses, tobamoviruses, and potexviruses (Tenllado and Díaz-Ruíz, 2001; Tenllado et al., 2004; Petrov et al., 2015; Mitter et al., 2017b).
In the present study, we tested the protective effect of exogenous application of dsRNAs against TSWV, a negative sense RNA virus, belonging to the Tospoviridae family, both in the model plant Nicotiana benthamiana and in tomato (Solanum lycopersicum L.), one of the most important horticultural crops worldwide. For this purpose, we selected two genomic regions, one covering the N gene, which encodes a structural protein, and another covering the NSm gene encoding a non-structural protein. The dsRNAs were synthesized in vitro and applied on plant leaves that were further inoculated with TSWV. Plants were monitored for local and systemic symptom development and for the presence of TSWV. Persistence and movement of the dsRNAs in plants were also studied.
Materials and Methods
Biological Material
Nicotiana benthamiana and tomato plants were maintained in a growth chamber at 24°C with a light/dark cycle of 16/8 h. Plants with 4–5 fully expanded leaves were used for the bioassays. Plants were inoculated with a TSWV pepper isolate [P105, PLAVIT collection, IPSP-CNR Torino, Italy, World Data Center for Microorganism (WDCM) no. 1057, http://www.wfcc.info/ccinfo/collection/col_by_country/i/39/].
In vitro dsRNA Synthesis
To produce dsRNA molecules, a two-step PCR approach followed by in vitro transcription was used (Voloudakis et al., 2015). In the case of N- and NSm-targeting dsRNAs, total RNA from TSWV-infected N. benthamiana plants was extracted using Trizol (Life Technologies, United States) according to manufacturer’s instructions. 1 μg of total RNA extracted from TSWV-infected N. benthamiana plants was used as template for cDNA synthesis, using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, United States), according to manufacturer’s instructions. Viral fragments were amplified with specific primers designed using Primer3 software1. The T7 RNA polymerase promoter/binding site sequence was added at the 5 ì end of both the forward and the reverse primers (Supplementary Table 1). PCR was carried out in a final volume of 50 μl, containing 10X reaction buffer, 1 μl cDNA template (diluted 1:5), 200 μM dNTPs, 0.2 μM of each primer, 1.5 mM MgCl2, and 2.0 units of Platinum Taq DNA polymerase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, United States). After an initial denaturation at 95°C for 10 min, the first 10 cycles were performed as follows: 95°C for 30 s, 40°C for 45 s, 72°C for 1 min, followed by 35 cycles each of 95°C for 30 s, 55°C for 45 s, and 72°C for 1 min. After a final extension at 72°C for 7 min, PCR fragments were loaded on 1% agarose gel and purified by the DNA Clean and Concentrator kit (Zymo Research, Irvine, United States). To in vitro synthesize sense and antisense viral derived ssRNAs, 1 μg of purified DNA template was used in 50 μl transcription reaction containing T7 reaction buffer, 500 μM rNTPs, 5 mM DTT, 50 units of T7 RNA polymerase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, United States), conducted at 37°C for 2 h. After removing DNA template by TURBO RNase-free DNAse (Ambion, Thermo Fisher Scientific, Waltham, MA, United States), dsRNAs were obtained by mixing the specific sense and antisense ssRNAs, then incubating at 95°C for 3 min, and at 37°C for 30 min. The formation of dsRNAs was confirmed by treatment with Mung Bean Nuclease (New England Biolabs, MA, United States).
As negative control, a dsRNA targeting the replication initiator protein (Rep) coding region of the geminivirus tomato yellow leaf curl Sardinia virus (TYLCSV) was used. In this case, DNA was extracted from TYLCSV-infected N. benthamiana plants using the TLES method described in Maffei et al. (2014). The Rep-targeting dsRNA was synthetized as described above, using specific primers reported in Supplementary Table 1.
Virus Inoculation and dsRNAs Exogenous Application
In order to ensure the uniformity of the TSWV inoculum in all the experiments, a TSWV inoculum stock was prepared from systemically infected N. benthamiana leaves. For this, symptomatic leaf tissue was cut in small slices, split into 500 mg aliquots and stored in liquid nitrogen until use. Inoculation was performed by homogenizing each aliquot in 50 ml of inoculation buffer (5 mM diethyldithiocarbamic acid, 1 mM ethylenediaminetetraacetic acid, and 5 mM sodium sulfite), and applying a 50 μl-dose to the upper side of two different leaves of each plant previously dusted with carborundum. When required, 10 μg of dsRNAs were added to the inoculum, just before inoculation.
Nicotiana benthamiana plants were used to evaluate the effect of dsRNAs on local lesions. Systemic infection induced by TSWV was evaluated in N. benthamiana and tomato plants, at different time points from the inoculation. Plants were inoculated with TSWV together with dsRNAs (N and NSm). The positive control group was inoculated with TSWV only and water. As negative controls, one group of plants received TSWV and the Rep-targeting dsRNAs originated from TYLCSV, while another one received only water. A total of thirteen N. benthamiana and seven tomato plants per treatment were analyzed in three and one different experiments, respectively. Symptoms development was monitored until 40 days post inoculation (dpi) in the case of N. benthamiana and 63 dpi in the case of tomato. Presence of viral RNA was checked by PCR in newly emerged leaves that did not receive the inoculum.
In planta dsRNA Movement
In order to evaluate the persistence/movement of dsRNAs in the plants that were not inoculated with TSWV, 10 μg of dsRNAs were mixed with 100 μl of sterile water and mechanically inoculated on two carborundum-dusted fully expanded leaves (50 μl per leaf) by gentle rubbing. Two leaf disks from each treated (local) and first expanded leaf from the apex (systemic) were collected at 1, 3, 6, and 9 dpi. Three plants were used for each time point. Just before sampling, leaves were washed with Triton X-100 (0.05%) and water to eliminate residual dsRNAs present on the leaf surface. Total RNA was extracted by Trizol reagent (Life Technologies, United States). cDNA synthesis was performed with random primers using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, United States), according to manufacturer’s instructions.
Detection of TSWV and dsRNAs by RT-PCR
Tomato spotted wilt virus infection was evaluated by RT-PCR with a primers pair (TSWV_L_fw/TSWV_L_rv, amplicon size 646 bp; Supplementary Table 1) designed on the L RNA, a genomic component unrelated to the N and NSm genes used to design the dsRNAs. In the dsRNAs mobility experiment, the presence of N- and NSm-targeting dsRNAs was estimated by PCR using primers TSWV_N_fw/TSWV_N_rv and TSWV_NSm_fw/TSWV_NSm_rv, respectively (Supplementary Table 1). PCR was performed in a final volume of 25 μl, containing 2.5 μl reaction buffer, 1 μl cDNA template, 200 μM each dNTPs, 0.4 μM each primer, 2 mM MgCl2, and 1 unit Platinum Taq DNA polymerase (Invitrogen). After an initial denaturation step for 4 min at 95°C, 30 cycles consisting each of 30 s at 95°C, 30 s at 58°C, and 30 s at 72°C were performed, followed by a final extension step for 7 min at 72°C.
Detection of dsRNAs by Quantitative RT- PCR
Quantitative RT-PCR (qRT-PCR) was carried out using iCycler iQTM Real-Time PCR Detection System (BioRad Laboratories, Hercules, CA, United States), with the following cycling parameters: 1 cycle at 95°C for 5 min, 45 cycles, each consisting of 15 s at 95°C and 1 min at 60°C. A melting curve was recorded at the end of each run to assess the specificity of amplification. All reactions were performed with three technical replicates. RT-PCR efficiency was calculated using standard curves constructed with serial dilutions of cDNA extracted from infected plants. Data acquisition and analysis were handled by the BioRad iCycler software (version 3.06070) that calculates Ct values and standard curves. qRT_TSWV_N_fw/qRT_TSWV_N_rv (Mason et al., 2002) and qRT_TSWV_NSm_fw/qRT_TSWV_NSm_rv primers pairs (Supplementary Table 1) were used to amplify exogenous dsRNAs, while the primer pair qRT_NbCOX_fw/qRT_NbCOX_rv (Supplementary Table 1) was used for the amplification of the N. benthamiana gene Niben101Scf02399 coding for the cytochrome c oxidase (NbCOX), used as reference gene (Nerva et al., 2017). The relative dsRNAs amount was estimated for each sample using the ΔCt method, where ΔCt is |CtdsRNA – Ctcox|.
Small RNA Analysis by High Throughput Sequencing (HTS)
Small RNA populations originating from N- and NSm-targeting dsRNAs were analyzed in samples collected for the dsRNA movement investigation at 1 dpi. Small RNA libraries preparation and sequencing were performed by Novogene (United Kingdom) Company Limited (United Kingdom). After adapter removal with fastp (Chen et al., 2018) and low-quality filtering and artifact removal with fastx-toolkit2, clean reads were mapped to the TSWV genome segment targeted by the applied exogenous dsRNA (S segment, Acc. Num. DQ376178.1; M segment, Acc. Num. KJ575621.1) using bowtie v1-3-0 (Langmead et al., 2009), with 0 mismatches. Mapping results were visualized using Misis (Seguin et al., 2014). Sequence data have been submitted to the Sequence Read Archive (SRA) with the BioProject ID PRJNA672300.
Results
Selection of Viral Genomic Sequences for dsRNA Production
In order to select the genomic regions of TSWV most suitable as target by dsRNAs, we first considered the vsRNAs profile of TSWV available in the literature (Mitter et al., 2013; Margaria et al., 2015), starting from the hypothesis that the genomic regions characterized by a high number of mapping vsRNAs could be more subjected to RNAi-mediated degradation. According to Mitter et al. (2013), most vsRNAs detected in TSWV-infected N. benthamiana plants mapped to the M segment, followed by the S segment, while the L RNA had the least number of mapping vsRNAs. Therefore, we focused our attention on the M and S segments. Among the two open reading frames (ORFs) of the M segment, GP and NSm, the GP ORF has the higher number of mapping vsRNAs (Mitter et al., 2013). However, we decided to select the NSm coding region as dsRNA target, since the movement protein has an important effect on viral systemic spread while the glycoproteins are not needed for in planta replication and intercellular spread of the virus; indeed, amiRNAs targeting the GP fragment did not show any antiviral protective effect (Carbonell et al., 2019). In the case of the S segment containing the N and NSs ORFs, we focused on the N coding region, since it codes for the nucleocapsid protein, a structural protein essential for virions formation. In addition, artificial miRNAs targeting the NSs ORF were not effective in protecting plants against TSWV infection (Mitter et al., 2016; Carbonell et al., 2019).
Since previous work showed that the exogenous dsRNAs must be longer than 300 bp to effectively interfere with virus infection (Tenllado and Díaz-Ruíz, 2001), we designed to produce dsRNAs longer than this threshold, i.e., 761 bp for the N-targeting dsRNA and 603 bp for the NSm-targeting dsRNA.
Finally, to better evaluate the dsRNAs protective effect, we decided to include a dsRNA homologous to a portion of the gene encoding the Rep pf TYLCSV as a negative control. The length of the Rep-targeting dsRNA (598 bp) was similar to the N- and NSm-targeting dsRNAs, but, due to the lack of homology with the TSWV genome, this dsRNA was not expected to show any protective effect against TSWV.
Production of dsRNAs
The method employed to produce dsRNAs, based on two sequential PCR reactions, coupled with in vitro transcription (Voloudakis et al., 2015) typically yielded 50–80 μg of dsRNAs, starting from 1 μg of DNA template. The double-stranded nature of the molecules obtained was confirmed by incubating the in vitro-transcribed RNAs before and after the annealing step with Mung Bean Nuclease, an enzyme that specifically degrades single-stranded nucleic acids (Figure 1).
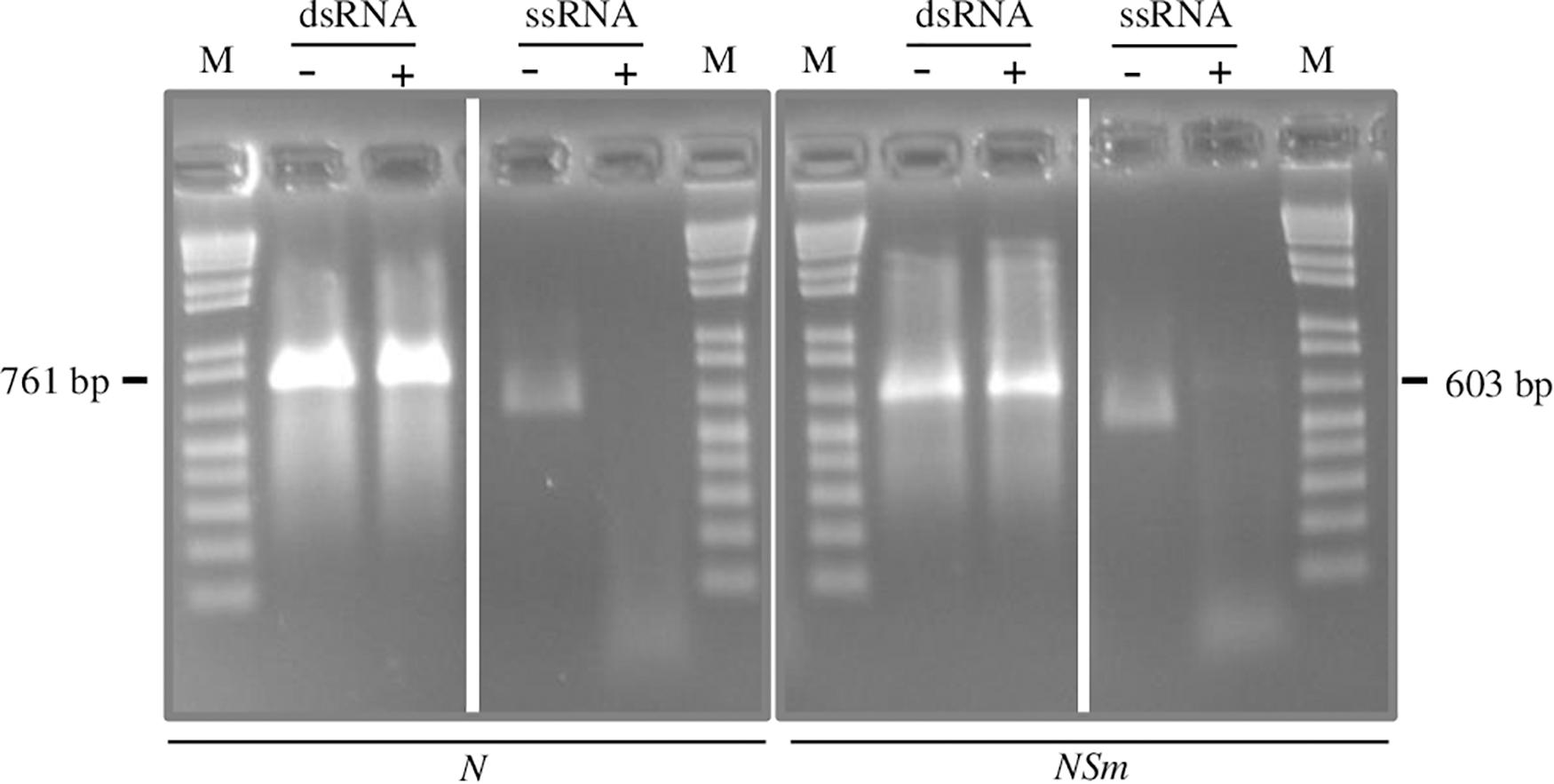
Figure 1. Confirmation of double-strand structure of N- and NSm-targeting dsRNAs by specific degradation with Mung Bean Nuclease. A single-stranded RNA transcript is used as control. +, Mung Bean Nuclease treatment; -, mock treatment; and M, 100 bp DNA Ladder (New England Biolabs, MA, United States). Nucleotide sequence length of synthetized fragments is shown on the sides.
Different Antiviral Efficacy of Exogenous Application of dsRNAs Targeting the N or NSm Genomic Regions of TSWV
At 7 dpi, typical chlorotic and necrotic local lesions occurred on leaves of N. benthamiana plants inoculated with TSWV alone, with no significant difference (p-value < 0.05) in their average number compared to plants inoculated with TSWV + NSm-targeting dsRNAs and with TSWV + Rep-targeting dsRNA. At the same time point, plants inoculated with TSWV + N-targeting dsRNAs showed drastic reduction in the number of local lesions (Figure 2), highlighting a protective effect of the N-targeting dsRNAs.
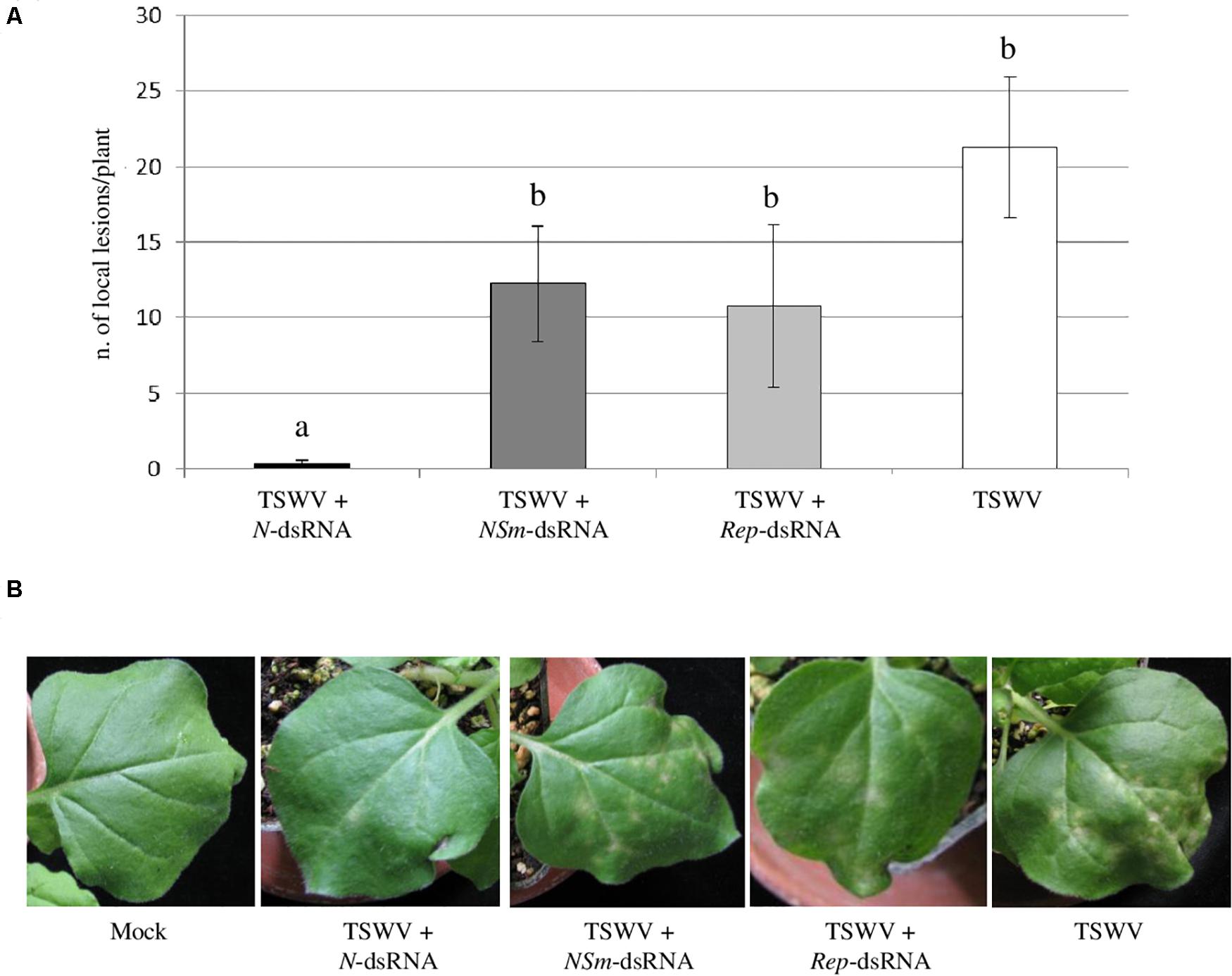
Figure 2. Effect of dsRNAs on production of TSWV-induced local lesions on N. benthamiana leaves. (A) Histogram reporting the number of local lesions per plant. Results are expressed as mean values of local lesions per plant counted at 7 dpi on 2 inoculated leaves for each plant, for a total of 13 plants per treatment, except for Rep-targeting dsRNAs treated plants for which 4 plants were considered. Different letters indicate significant differences at P < 0.05 (Kruskal–Wallis test followed by post hoc Wilcoxon test). (B) Local lesions at 7 dpi on N. benthamiana plants treated with N-, NSm- and Rep-targeting dsRNAs and inoculated with TSWV or inoculated with TSWV alone. Different letters indicate statistically significant differences.
All the N. benthamiana plants inoculated with TSWV alone showed typical systemic symptoms of chlorosis, necrosis, stunting, and wilting at 11 dpi. At the same time point, similar symptoms were observed on 92% of plants treated with TSWV + NSm-targeting dsRNAs and 75% of plants treated with TSWV + Rep-targeting dsRNAs; conversely, only 15% of plants treated with TSWV + N-targeting dsRNAs appeared symptomatic. At later observation (25 dpi), all plants treated with TSWV + NSm-targeting dsRNAs or with TSWV + Rep-targeting dsRNAs showed the systemic TSWV symptoms. In the case of plants treated with TSWV + N-targeting dsRNAs, the rate of symptomatic plants reached 46% at 17 dpi and remained unchanged until the end of the experiment (40 dpi; Figure 3). The RT-PCR performed on a subset of N. benthamiana plants confirmed visual symptoms evaluation (not shown).
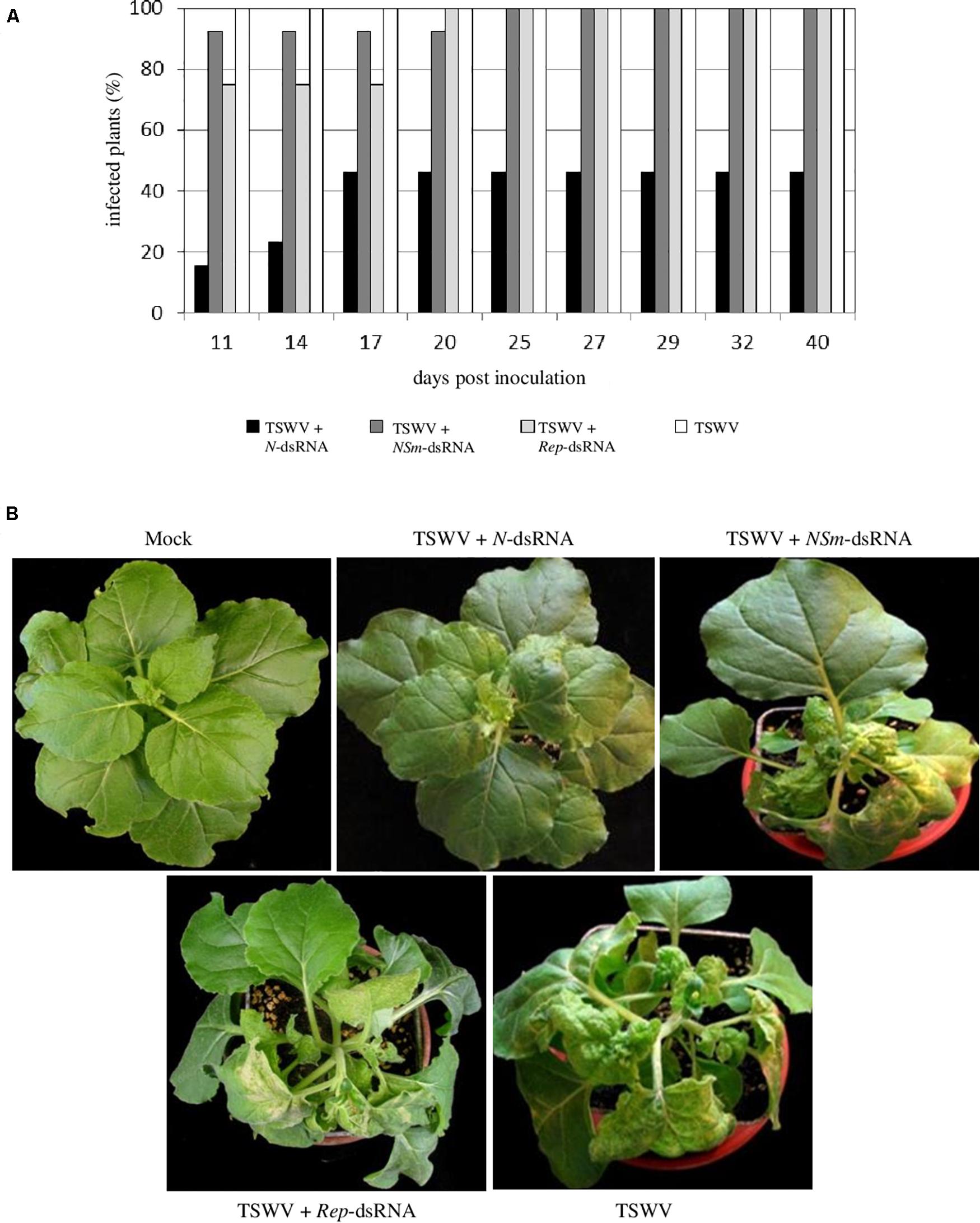
Figure 3. Efficacy of N-, NSm- and Rep-targeting dsRNA treatments against TSWV infection in N. benthamiana. (A) Percentage of TSWV-infected plants treated or not with dsRNAs. (B) Systemic symptoms at 14 dpi on N. benthamiana plants treated with N-, NSm- and REP-targeting dsRNAs and inoculated with TSWV or inoculated with TSWV alone.
In the case of tomato plants, most of the plants (71%) treated with TSWV + NSm-targeting dsRNAs and 100% of the plants inoculated only with TSWV were already infected at 21 dpi, but none of the plants inoculated with TSWV and treated with N-targeting dsRNAs became infected for the entire duration of the experiment, at 64 dpi (Figure 4A).
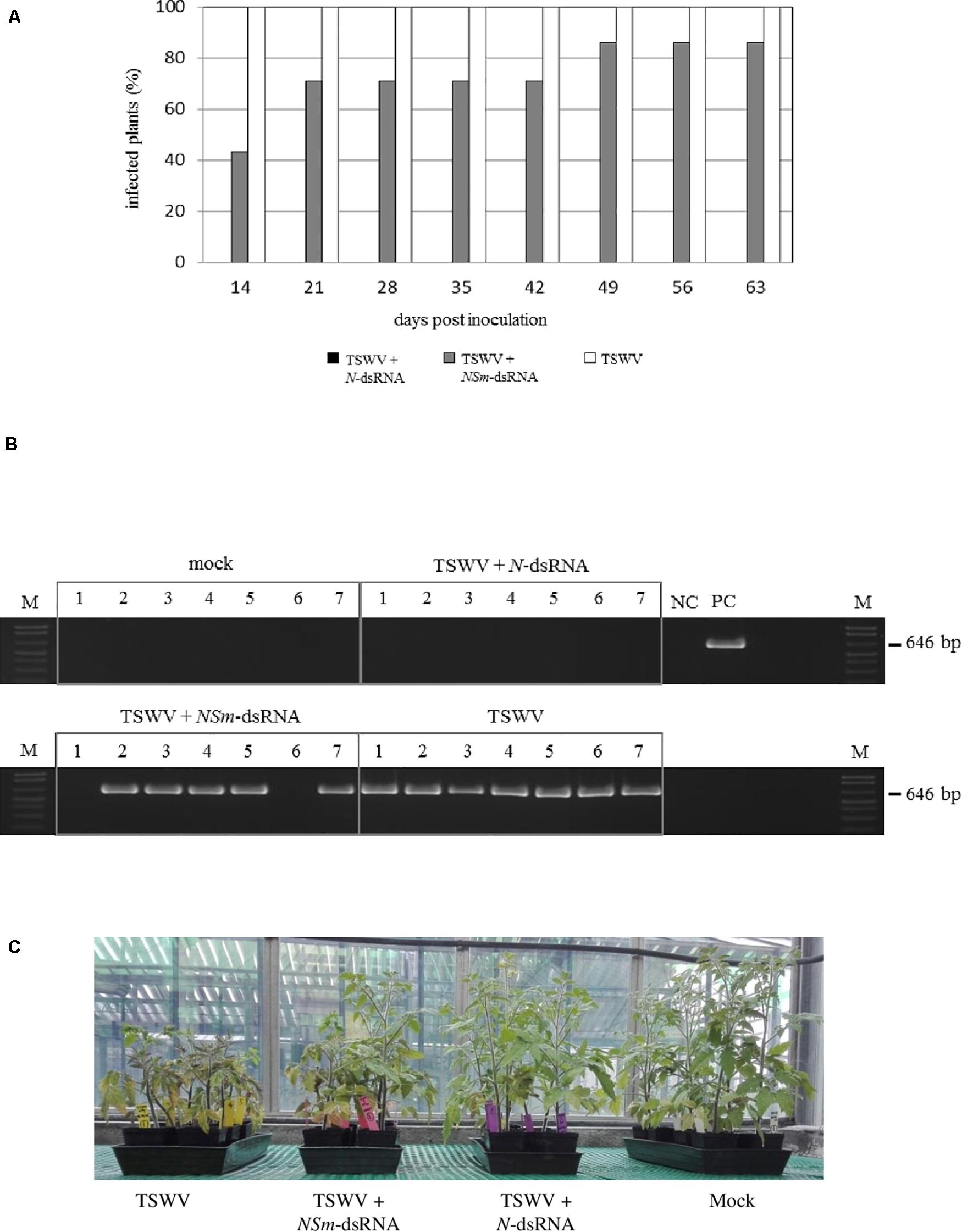
Figure 4. Efficacy of N- and NSm-targeting dsRNA treatments against TSWV infection in tomato; seven plants (1–7) were used for each thesis. (A) Percentage of TSWV infection in plants treated or not with dsRNAs at several time points. (B) RT-PCR for the evaluation of TSWV infection in tomato plants at 28 dpi; primers targeting TSWV L genomic segment were used. NC, negative control (Mock-inoculated plant); PC, positive control (TSWV-infected plant); and M, 100 bp DNA Ladder (New England Biolabs, MA, United States). Size of amplified fragment is shown on the side. (C) Tomato plants treated with N- and NSm-targeting dsRNAs and inoculated with TSWV or inoculated with TSWV alone, at 30 dpi.
In both species, we didn’t observe any difference in the reproductive stage; all the asymptomatic plants, including those treated with dsRNAs, exhibited a normal flowering, while no flowering was observed in symptomatic plants, due to the heavy symptomatology.
The virus was detected in young leaves of all the symptomatic plants while it was absent in all asymptomatic plants treated with TSWV + N-targeting dsRNAs (Figure 4B), confirming visual inspection (Figure 4C).
Taken together, these results show that exogenous application of dsRNAs homologous to the N gene can completely suppress or robustly contrast viral replication and protects plants from TSWV infection, both in N. benthamiana and tomato, by inhibiting or strongly delaying symptom development, while dsRNAs targeting the NSm gene have almost no protective effect.
Systemic Movement of the Applied dsRNAs
In order to investigate the persistence and the systemic movement of N and NSm-targeting dsRNAs applied on the leaves, in the absence of virus infection, we analyzed by RT-PCR the presence of both dsRNAs at 1, 3, 6, and 9 dpi in both treated (local persistence) or young untreated leaves (systemic movement) of N. benthamiana plants (Figure 5A). Both dsRNAs could be detected until 9 dpi in the treated leaves indicating that they persist there for several days after application. Moreover, we detected both N and NSm-targeting dsRNAs also in untreated leaves at all time points, although with a lower intensity compared to treated tissues.
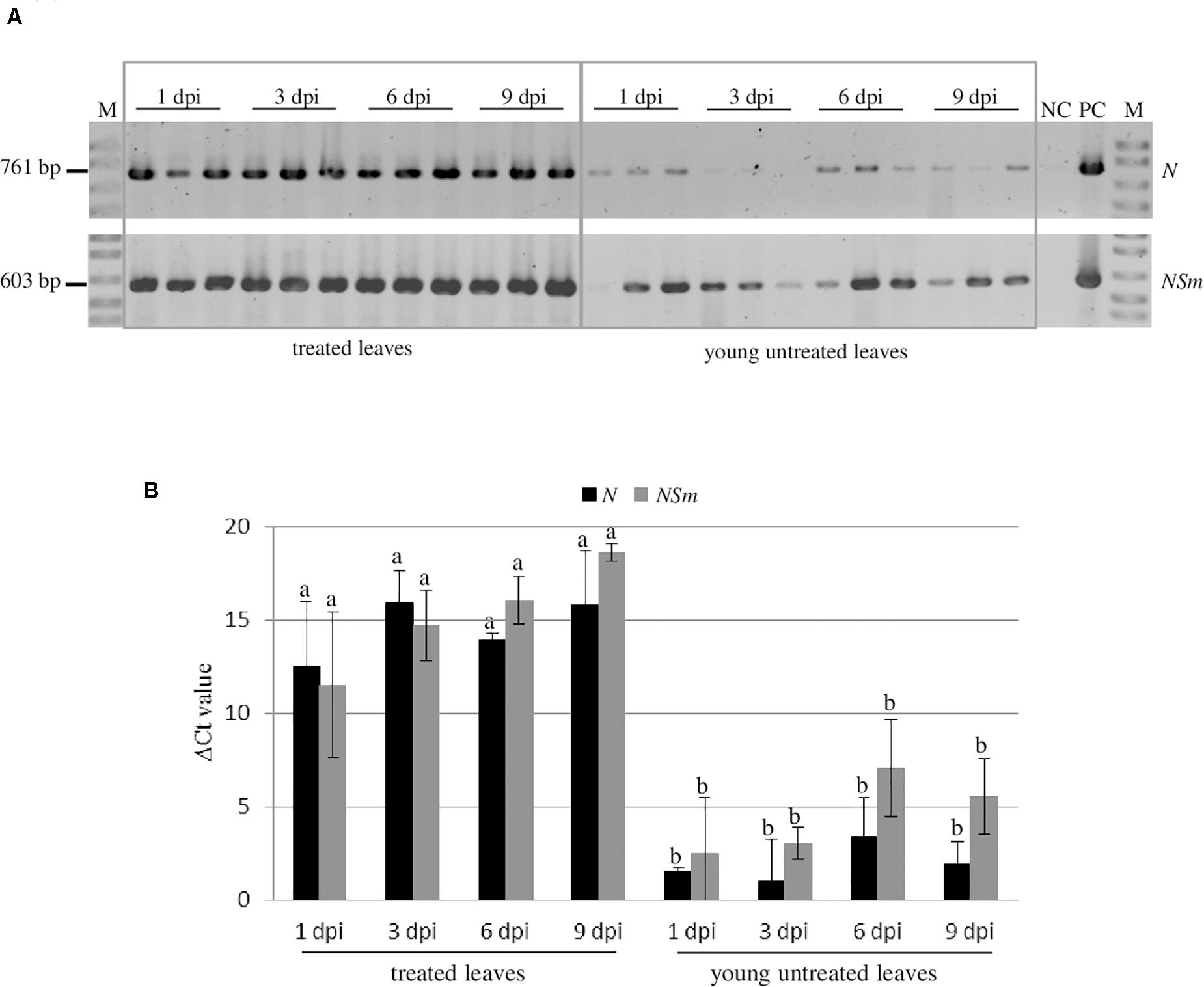
Figure 5. Persistence and systemic movement of N- and NSm-targeting dsRNAs. Three biological replicates were used. (A) Detection of the N- and NSm-targeting dsRNAs in treated leaves and untreated young leaves of N. benthamiana at different time points by end-point RT-PCR. NC, negative control (Mock-inoculated plant); PC, positive control (TSWV-infected plant); and M, 100 bp DNA Ladder (New England Biolabs, MA, United States). Sizes of amplified fragments are shown on the side. (B) Quantification of N- and NSm-targeting dsRNAs by qRT-PCR. The Ct values obtained for the dsRNAs were normalized with the Ct values obtained for the COX transcript used as reference. Vertical lines on each bar represent standard deviations. Different letters indicate statistically significant differences (p < 0.05, ANOVA). Different letters indicate statistically significant differences.
When the quantitative evaluation of the dsRNAs was performed by qRT-PCR, we observed that most of the applied N- or NSm-targeting dsRNAs remained in the treated leaves and only a limited amount of them was transported systemically to the young untreated leaves. Interestingly, the dsRNAs systemic movement, even if very limited, could be observed at 1 dpi and remained steadily low until 9 dpi (Figure 5B).
These results indicate that both dsRNAs considered are able to move systemically within the plant, but that the majority of them remains in treated leaves. No significant differences in persistence or systemic movement capacity were observed between the N- and NSm-targeting dsRNAs.
Analysis of the sRNAs Populations Originating From N- and NSm-Targeting dsRNAs
In order to evaluate if N- and NSm-targeting dsRNAs were processed by Dicer and were able to originate the silencing effector molecules (siRNAs), we sequenced the sRNAs populations of treated (local), and newly emerged non-treated (systemic) N. benthamiana leaves, 1 day post exogenous dsRNAs application. After adapter removal and quality filtering, 18 to 28 million of reads were obtained (Supplementary Tables 2, 3). Sequences with length ranging from 20 to 25 nt were selected for further analyses. In the case of leaves treated with dsRNAs (local), the percentage of reads mapping to the N- and NSm-targeting dsRNAs regions was 0.39 and 0.50, respectively (Figure 6); when newly emerged non-treated leaves were considered, only few reads mapped to the corresponding viral region, i.e., 53 reads for the N-targeting dsRNA and 89 reads for NSm-targeting dsRNA. These results indicate that both dsRNAs are effectively introduced into the plants and processed, thus originating siRNAs that can guide the cleavage of cognate sequences. However, in agreement with the very limited amount of dsRNAs that move systemically into the plant, only few dsRNA-derived sRNAs were found in the newly emerged non-treated leaves.
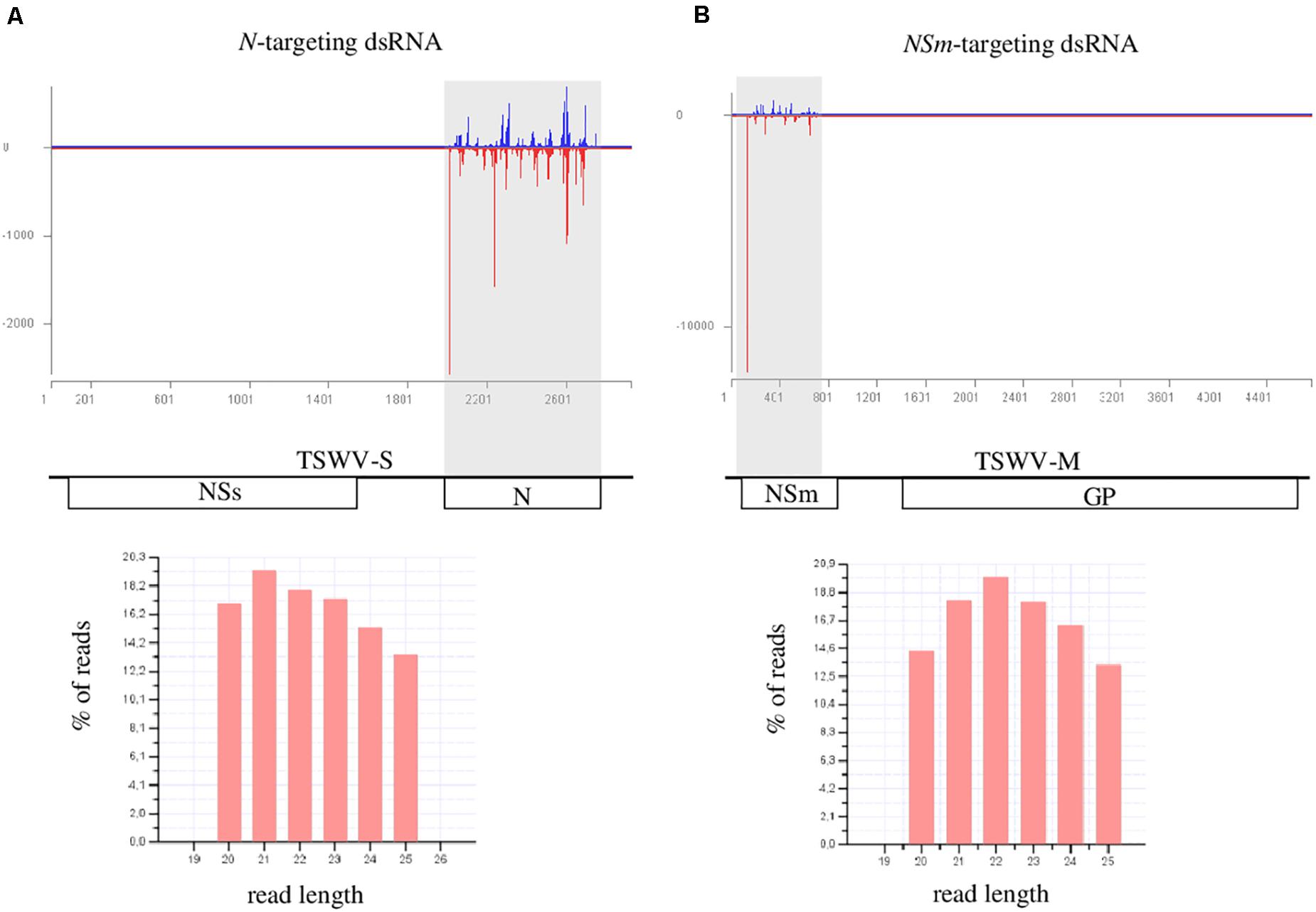
Figure 6. HTS analysis of small RNAs in dsRNAs-treated N. benthamiana leaves, 1 day post treatment. (A) upper panel: distribution of TSWV-targeted small RNAs along the S segment of TSWV genome (TSWV-S; Genbank acc. N. DQ376178.1); viral sense and antisense small RNAs are reported in blue and red, respectively; N (nucleoprotein); and NSs (non-structural protein) genes are represented along the viral RNA S segment; lower panel: length distribution of the small RNAs reads mapping to the TSWV S segment. (B) upper panel: distribution of TSWV-targeted small RNAs along the M segment of TSWV genome (TSWV-M; Genbank acc. N. KJ575621.1); NSm (non-structural protein); and GP (glycoproteins precursor) genes are represented along the viral RNA M segment; lower panel: length distribution of the small RNAs reads mapping to the TSWV M segment.
The size distribution of sRNAs originating from the dsRNAs was basically uniform; in both cases, even if the amount of 21- and 22-nt reads was slightly higher than the number of other reads, no well-defined peaks related to a particular read size was observed (Figure 6). This may suggest that the inoculated dsRNA was not processed into sRNAs and the protective effect was possibly dsRNA- and not RNAi-mediated.
Discussion
Previous work on engineering RNAi-mediated transgenic resistance indicated that constructs targeting the N and NSm genes of TSWV successfully protected plants against viral infection (Mackenzie and Ellis, 1992; Vaira et al., 1995; Prins et al., 1996; Herrero et al., 2000). Subsequent work showed that N gene segments as short as 110-nt were sufficient to efficiently induce RNA silencing, and hence virus resistance (Jan et al., 2000). Recently, the use of chimeric transgenes derived from different orthotospoviruses further extended the usefulness of this approach generating a broad-spectrum resistance against this virus group at the genus level (Bucher et al., 2006; Peng et al., 2014). However, even if the use of transgenic plants has been established as a powerful tool for plant protection, concerns about potential negative effects on human health and environment, together with limited acceptance by consumers dictated the development of alternative non-transgenic strategies exploiting RNAi, such as exogenous application of dsRNAs. In the present study, we tested the efficacy of the RNAi-based vaccination against the economically important virus TSWV, by applying exogenous synthetic dsRNAs on plant leaves.
We established that the RNAi-based vaccination approach is effective also against negative-strand RNA viruses, both in the model plant N. benthamiana and in the economically important tomato crop. However, we found that, in our conditions, the dsRNAs targeting the N gene are able to protect the plant, while those targeting the NSm gene are not. It is interesting to note that a slight reduction in the percentage of infected plants among those treated with Rep- and NSm-targeting dsRNAs was observed in respect to plants inoculated only with the virus. Such reduction is possibly related to the ability of exogenous dsRNAs to activate a sequence-unrelated pattern-induced immunity response (Niehl et al., 2016).
Overall, the different behavior of the N- and NSm-targeting dsRNAs requires some considerations.
First, we can exclude a possible effect of the dsRNA length, since the two dsRNAs are similar in size and include almost the entire coding region. Indeed, previous work on other plant viruses showed that dsRNAs with a size range from 600 to 900 nt can be very effective (Tenllado and Díaz-Ruíz, 2001).
Second, the RNAi-based vaccination has been demonstrated to be dose-dependent (Tenllado and Díaz-Ruíz, 2001; Dubrovina and Kiselev, 2019). Actually, in our experiments, the dose of dsRNAs applied to leaves (10 μg/plant) was in line with those currently used by other authors (Dubrovina and Kiselev, 2019). Furthermore, even when we increased the amount of the NSm-targeting dsRNAs up to 30 μg/plant, no protection against the virus was obtained (data not shown), demonstrating the intrinsic inefficacy of targeting this region.
The accessibility of dsRNAs to DCLs, frequently estimated by the abundance of originating vsRNAs, is another important aspect to consider regarding the effectiveness of RNAi-based vaccination. In our case, we can exclude that the viral sequences targeted by dsRNAs induce different response in terms of vsRNA abundance, since the amount of vsRNAs mapping to the N and NSm coding regions was similar (Mitter et al., 2013; Margaria et al., 2015; Ramesh et al., 2017). Indeed, the analysis of sRNAs populations in dsRNAs-treated plants confirmed that both N- and NSm-targeting dsRNAs were successfully recognized and processed by the endogenous RNAi machinery.
The ability of dsRNAs to move systemically in the plant can also be relevant for their efficacy. Actually, the systemic transport of exogenously applied dsRNAs is not well understood. Konakalla et al. (2016) observed that dsRNAs can move systemically in tobacco plants already 1 day following leaf application (Konakalla et al., 2016), a result also confirmed in tomato plants (Gogoi et al., 2017). Moreover, rice and maize roots soaked in a dsRNAs-containing solution can absorb dsRNAs (Li et al., 2015). On the other hand, other groups reported that exogenous dsRNAs remain mostly in treated leaves, at least in squash and watermelon plants (Kaldis et al., 2018), or in tomato (San Miguel and Scott, 2016). Such contradictory reports could be explained by the existence of an active dsRNA long-distance movement process mediated by proteins binding specific RNA-motifs (Kehr and Kragler, 2018). Our results showed that both N- and NSm-targeting dsRNAs behave similarly: a small quantity of dsRNAs can move systemically and reside in young untreated leaves already 1 day after their application on the older treated leaves, but the majority of them persist in the treated leaves. According to this, dsRNAs-derived sRNAs were mainly recovered in the leaves treated with exogenous dsRNAs and only few of them were found in newly emerged non-treated leaves. These results may suggest that, as well as dsRNAs, sRNAs also remain mostly in the treated leaves and only a small fraction is subjected to systemic transport. However, we cannot exclude the possibility that the detected sRNAs were produced in the systemic leaves after the processing of the dsRNAs coming from inoculated tissues. Taken together these data allow to rule out the possibility that the lack of protection from TSWV infection of the NSm-targeting dsRNA is connected with its movement ability or to the mobility of derived sRNAs.
The limited mobility of dsRNAs and derived sRNAs point out the possible limits in the large-scale use of the dsRNA-vaccination for crop protection against viruses and suggests that more effective application techniques such as the use of high-pressure spraying (Dalakouras et al., 2016) or the conjugation of dsRNAs with protein carriers (Numata et al., 2014), nanostructures (Mitter et al., 2017a), or abrasive substances could be developed.
Finally, the different antiviral efficacy of the N- and NSm-targeting dsRNAs could reside on the biological role of the N and NSm gene products. A successful infection by TSWV requires primarily the replication and transcription of the genetic viral elements to produce massive amounts of infectious ribonucleocapsid proteins (RNPs), the minimal infectious unit containing the three genomic RNAs tightly packed by the N protein and few copies of the viral RdRp. RNPs then associate with NSm and move intra- and inter-cellularly through a continuous endoplasmic reticulum network, supported by NSm-derived tubule structures that allow systemic spread (Zhu et al., 2019). Since the N protein is involved in RNPs aggregation immediately after RNA genome replication, it is likely that a certain amount of N coding RNA is required to synthesize enough N protein molecules to form new RNPs. Indeed, N gene is constitutively expressed since early time points of infection (Kormelink et al., 1994). Conversely, the NSm viral gene is only transiently expressed at the early stage of infection and its product is required for the RNPs to disseminate from the first initially infected cells. Therefore, one can argue that lower amount of NSm protein could be sufficient for this step. In this perspective, the degradation of the N coding RNA could have a crucial impact on viral replication due to the central role of its encoded protein; on the other hand, the NSm protein could play a secondary role and, even if targeted by vsRNAs, a small quantity of NSm-coding RNA could be sufficient to produce the required amount of movement protein. It is worth noting that, in a natural context, the inducers of antiviral RNAi and R gene-based host defense, namely dsRNAs and viral effector proteins, are deployed in the initial stages of the viral cycle (Zhu et al., 2019). The importance of the link between the biological role of N and NSm proteins and the ability of their encoding sequences to confer resistance is also supported by the observation that plants transformed with the N sequence were resistant at both plant and cellular level while plants transformed with the NSm sequence showed resistance at plant but not at cellular level, since the virus was still able to replicate in protoplasts of NSm-transgenic plants (Prins et al., 1996). However, NSm-transformed plants were resistant to TSWV infection probably because the NSm RNA was constitutively expressed in the whole plant at a level sufficient to contrast the systemic movement of the virus. Finally, it is worth mentioning that most of the successful reports on RNAi-based vaccination involved dsRNAs targeting capsid coding genes, except for one case where up to 66% of tobacco plants were protected from tobacco mosaic virus infection using dsRNAs targeting the movement protein (Sun et al., 2010).
To conclude, we demonstrated that the RNAi-based vaccination is effective also against membrane-encapsidated, multi-component, negative-sense RNA viruses and we added the Tospoviridae, a family including one of the most economically important plant viruses, to the list of viral families potentially targeted by this approach. Noteworthy, we showed that the choice of the viral region targeted by dsRNAs was crucial to induce resistance, highlighting that the viral cycle is a fundamental aspect to consider in the RNAi-based vaccination design.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material. Sequence data are available in the Sequence Read Archive (SRA) with the BioProject ID PRJNA672300.
Author Contributions
LM, ST, GA, AV, and EN conceived and designed the experiments. ST, MJ, and LM conducted the experiments. DM gave technical support in laboratory and greenhouse activities. SAAB supported ST activities. LM, ST, EN, GA, and AV wrote the manuscript. All authors read and approved the manuscript.
Funding
This work has been supported by Fondazione Cassa di Risparmio di Torino, project n. 2018.AI1517.U1797, by Italian Ministry of University and Research (MUR), GeMed Project decree n. R.0000750.28-05-2020, PRIMA2018-Section 2, and by a scientific cooperation agreement 2018–19 between CNR (Italy) and HCST/NCRD (Jordan).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Caterina Perrone and Elena Zocca for support in greenhouse plant management. We thank Dr. Richard Kormelink for critical reading.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.533338/full#supplementary-material
Footnotes
References
Agius, C., Eamens, A. L., Millar, A. A., Watson, J. M., and Wang, M. B. (2012). RNA silencing and antiviral defense in plants. Methods Mol. Biol. 894, 17–38. doi: 10.1007/978-1-61779-882-5_2
Bucher, E., Lohuis, D., van Poppel, P., Geerts-Dimitriadou, C., Goldbach, R., and Prins, M. (2006). Multiple virus resistance at a high frequency using a single transgene construct. J. Gen. Virol. 87(Pt 12), 3697–3701. doi: 10.1099/vir.0.82276-0
Carbonell, A., López, C., and Daròs, J. A. (2019). Fast-forward identification of highly effective artificial small RNAs against different tomato spotted wilt virus isolates. Mol. Plant Microbe Interact. 32, 142–156. doi: 10.1094/mpmi-05-18-0117-ta
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890.
Dalakouras, A., Wassenegger, M., McMillan, J. N., Cardoza, V., Maegele, I., Dadami, E., et al. (2016). Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 07:1327. doi: 10.3389/fpls.2016.01327
Dietzgen, R. G., and Mitter, N. (2006). Transgenic gene silencing strategies for virus control. Austral. Plant Pathol. 35, 605–618. doi: 10.1071/ap06064
Dubrovina, A. S., and Kiselev, K. V. (2019). Exogenous RNAs for gene regulation and plant resistance. Int. J. Mol. Sci. 20:21.
Gan, D. F., Zhang, J. A., Jiang, H. B., Jiang, T., Zhu, S. W., and Cheng, B. J. (2010). Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 29, 1261–1268. doi: 10.1007/s00299-010-0911-z
Gogoi, A., Sarmah, N., Kaldis, A., Perdikis, D., and Voloudakis, A. (2017). Plant insects and mites uptake double-stranded RNA upon its exogenous application on tomato leaves. Planta 246, 1233–1241. doi: 10.1007/s00425-017-2776-7
Guo, Q., Liu, Q., Smith, N. A., Liang, G. L., and Wang, M. B. (2016). RNA silencing in plants: mechanisms, technologies and applications in horticultural crops. Curr. Genom. 17, 476–489. doi: 10.2174/1389202917666160520103117
Hameed, A., Tahir, M., Asad, S., Bilal, R., Eck, J., Jander, G., et al. (2017). RNAi-mediated simultaneous resistance against three RNA viruses in potato. Mol. Biotechnol. 59, 73–83. doi: 10.1007/s12033-017-9995-9
Herrero, S., Culbreath, A. K., Csinos, A. S., Pappu, H. R., Rufty, R. C., and Daub, M. E. (2000). Nucleocapsid gene-mediated transgenic resistance provides protection against Tomato spotted wilt virus epidemics in the field. Phytopathology 90, 139–147. doi: 10.1094/phyto.2000.90.2.139
Jan, F. J., Fagoaga, C., Pang, S. Z., and Gonsalves, D. (2000). A minimum length of N gene sequence in transgenic plants is required for RNA-mediated tospovirus resistance. J. Gen. Virol. 81, 235–242. doi: 10.1099/0022-1317-81-1-235
Kaldis, A., Berbati, M., Melita, O., Reppa, C., Holeva, M., Otten, P., et al. (2018). Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol. Plant Pathol. 19, 883–895. doi: 10.1111/mpp.12572
Kehr, J., and Kragler, F. (2018). Long distance RNA movement. New Phytol. 218, 29–40. doi: 10.1111/nph.15025
Konakalla, N. C., Kaldis, A., Berbati, M., Masarapu, H., and Voloudakis, A. E. (2016). Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 244, 961–969. doi: 10.1007/s00425-016-2567-6
Kormelink, R., Storms, M., Van Lent, J., Peters, D., and Goldbach, R. (1994). Expression and subcellular location of the NSM protein of tomato spotted wilt virus (TSWV), a putative viral movement protein. Virology 200, 56–65. doi: 10.1006/viro.1994.1162
Langmead, B., Trapnell, C., Pop, M., and Salzberg, S. L. (2009). Ultrafast, and memory. (-)efficient alignment of short DNA sequences to the human genome. Genome Biol. 10:R25.
Li, H. C., Guan, R. B., Guo, H. M., and Miao, X. X. (2015). New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 38, 2277–2285. doi: 10.1111/pce.12546
Mackenzie, D. J., and Ellis, P. J. (1992). Resistance to tomato spotted wilt virus-infection in transgenic tobacco expressing the viral nucleocapsid gene. Mol. Plant Microbe Interact. 5, 34–40. doi: 10.1094/mpmi-5-034
Maffei, G., Miozzi, L., Fiorilli, V., Novero, M., Lanfranco, L., and Accotto, G. P. (2014). The arbuscular mycorrhizal symbiosis attenuates symptom severity and reduces virus concentration in tomato infected by Tomato yellow leaf curl Sardinia virus (TYLCSV). Mycorrhiza 24, 179–186. doi: 10.1007/s00572-013-0527-6
Margaria, P., Miozzi, L., Rosa, C., Axtell, M. J., Pappu, H. R., and Turina, M. (2015). Small RNA profiles of wild-type and silencing suppressor-deficient tomato spotted wilt virus infected Nicotiana benthamiana. Virus Res. 208, 30–38. doi: 10.1016/j.virusres.2015.05.021
Marjanac, G., Karimi, M., Naudts, M., Beeckman, T., Depicker, A., and De Buck, S. (2009). Gene silencing induced by hairpin or inverted repeated sense transgenes varies among promoters and cell types. New Phytol. 184, 851–864. doi: 10.1111/j.1469-8137.2009.03011.x
Mason, G., Provero, P., Vaira, A. M., and Accotto, G. P. (2002). Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnol. 2:20. doi: 10.1186/1472-6750-2-20
Meister, G., and Tuschl, T. (2004). Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349. doi: 10.1038/nature02873
Mitter, N., Koundal, V., Williams, S., and Pappu, H. (2013). Differential expression of tomato spotted wilt virus-derived viral small RNAs in infected commercial and experimental host plants. PLoS One 8:e76276. doi: 10.1371/journal.pone.0076276
Mitter, N., Worrall, E. A., Robinson, K. E., Li, P., Jain, R. G., Taochy, C., et al. (2017a). Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3:16207.
Mitter, N., Worrall, E., Robinson, K., Xu, Z., and Carroll, B. (2017b). Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 26, 49–55. doi: 10.1016/j.coviro.2017.07.009
Mitter, N., Zhai, Y., Bai, A. X., Chua, K., Eid, S., Constantin, M., et al. (2016). Evaluation and identification of candidate genes for artificial microRNA-mediated resistance to tomato spotted wilt virus. Virus Res. 211, 151–158. doi: 10.1016/j.virusres.2015.10.003
Nerva, L., Varese, G. C., Falk, B. W., and Turina, M. (2017). Mycoviruses of an endophytic fungus can replicate in plant cells: evolutionary implications. Sci. Rep. 05:1908.
Niehl, A., Wyrsch, I., Boller, T., and Heinlein, M. (2016). Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol. 211, 1008–1019. doi: 10.1111/nph.13944
Numata, K., Ohtani, M., Yoshizumi, T., Demura, T., and Kodama, Y. (2014). Local gene silencing in plants via synthetic dsRNA, and carrier peptide. Plant Biotechnol. J. 12, 1027–1034. doi: 10.1111/pbi.12208
Padmanabhan, C., Zhang, X. M., and Jin, H. L. (2009). Host small RNAs are big contributors to plant innate immunity. Curr. Opin. Plant Biol. 12, 465–472. doi: 10.1016/j.pbi.2009.06.005
Pappu, H. R., Jones, R. A. C., and Jain, R. K. (2009). Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Res. 141, 219–236. doi: 10.1016/j.virusres.2009.01.009
Peng, J. C., Chen, T. C., Raja, J. A. J., Yang, C. F., Chien, W. C., Lin, C. H., et al. (2014). Broad-spectrum transgenic resistance against distinct tospovirus species at the genus level. PLoS One 9:e0096073. doi: 10.1371/journal.pone.0096073
Petrov, N., Stoyanova, M., Andonova, R., and Teneva, A. (2015). Induction of resistance to potato virus Y strain NTN in potato plants through RNAi. Biotechnol. Biotechnol. Equ. 29, 21–26. doi: 10.1080/13102818.2014.984968
Prins, M., Resende, R. D., Anker, C., vanSchepen, A., deHaan, P., and Goldbach, R. (1996). Engineered RNA-mediated resistance to tomato spotted wilt virus is sequence specific. Mol. Plant Microbe Interact. 9, 416–418. doi: 10.1094/mpmi-9-0416
Ramesh, S. V., Williams, S., Kappagantu, M., Mitter, N., and Pappu, H. R. (2017). Transcriptome-wide identification of host genes targeted by tomato spotted wilt virus-derived small interfering RNAs. Virus Res. 238, 13–23. doi: 10.1016/j.virusres.2017.05.014
San Miguel, K., and Scott, J. G. (2016). The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 72, 801–809. doi: 10.1002/ps.4056
Scholthof, K. B. G., Adkins, S., Czosnek, H., Palukaitis, P., Jacquot, E., Hohn, T., et al. (2011). Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 12, 938–954. doi: 10.1111/j.1364-3703.2011.00752.x
Seguin, J., Otten, P., Baerlocher, L., Farinelli, L., and Pooggin, M. M. (2014). MISIS : a bioinformatics tool to view and analyze maps of small RNAs derived from viruses and genomic loci generating multiple small RNAs. J. Virol. Methods 195, 120–122. doi: 10.1016/j.jviromet.2013.10.013
Smith, N., Singh, S., Wang, M., Stoutjesdijk, P., Green, A., and Waterhouse, P. (2000). Gene expression - Total silencing by intron-spliced hairpin RNAs. Nature 407, 319–320. doi: 10.1038/35030305
Sun, Z. N., Yin, G. H., Song, Y. Z., An, H. L., Zhu, C. X., and Wen, F. J. (2010). Bacterially expressed double-stranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus y genome have different ability to protect tobacco from viral infection. Appl. Biochem. Biotechnol. 162, 1901–1914. doi: 10.1007/s12010-010-8968-2
Tenllado, F., and Díaz-Ruíz, J. R. (2001). Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 75, 12288–12297. doi: 10.1128/jvi.75.24.12288-12297.2001
Tenllado, F., Llave, C., and Diaz-Ruiz, J. R. (2004). RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res. 102, 85–96. doi: 10.1016/j.virusres.2004.01.019
Tenllado, F., Martínez-García, B., Vargas, M., and Díaz-Ruíz, J. R. (2003). Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 3:3. doi: 10.1186/1472-6750-3-3
Turina, M., Kormelink, R., and Resende, R. O. (2016). Resistance to tospoviruses in vegetable crops: epidemiological and molecular aspects. Annu. Rev. Phytopathol. 54, 347–371. doi: 10.1146/annurev-phyto-080615-095843
Vaira, A. M., Semeria, L., Crespi, S., Lisa, V., Allavena, A., and Accotto, G. P. (1995). Resistance to tospoviruses in Nicotiana benthamiana transformed with the N gene of tomato spotted wilt virus: correlation between transgene expression and protection in primary transformants. Mol. Plant Microbe Interact. 8, 66–73. doi: 10.1094/mpmi-8-0066
Voloudakis, A. E., Holeva, M. C., Sarin, L. P., Bamford, D. H., Vargas, M., Poranen, M. M., et al. (2015). “Efficient double-stranded RNA production methods for utilization in plant virus control,” in Plant Virology Protocols: New Approaches to Detect Viruses and Host Responses, 3rd Edn, eds I. Uyeda and C. Masuta (Totowa: Humana Press Inc), 255–274. doi: 10.1007/978-1-4939-1743-3_19
Whitfield, A. E., and Rotenberg, D. (2015). Disruption of insect transmission of plant viruses. Curr. Opin. Insect Sci. 8, 79–87. doi: 10.1016/j.cois.2015.01.009
Yazhisai, U., Rajagopalan, P. A., Raja, J. A. J., Chen, T. C., and Yeh, S. D. (2015). Untranslatable tospoviral NSs fragment coupled with L conserved region enhances transgenic resistance against the homologous virus and a serologically unrelated tospovirus. Trans. Res. 24, 635–649. doi: 10.1007/s11248-015-9865-9
Keywords: RNAi-based vaccination, double-stranded rnas, orthotospovirus, ambisense RNA, nucleocapsid protein, cell-to-cell movement protein
Citation: Tabein S, Jansen M, Noris E, Vaira AM, Marian D, Behjatnia SAA, Accotto GP and Miozzi L (2020) The Induction of an Effective dsRNA-Mediated Resistance Against Tomato Spotted Wilt Virus by Exogenous Application of Double-Stranded RNA Largely Depends on the Selection of the Viral RNA Target Region. Front. Plant Sci. 11:533338. doi: 10.3389/fpls.2020.533338
Received: 07 February 2020; Accepted: 09 November 2020;
Published: 26 November 2020.
Edited by:
Bruno Mezzetti, Marche Polytechnic University, ItalyReviewed by:
Tiziana Pandolfini, University of Verona, ItalyAlberto Carbonell, Polytechnic University of Valencia, Spain
Gabi Krczal, RLP AgroScience, Germany
Copyright © 2020 Tabein, Jansen, Noris, Vaira, Marian, Behjatnia, Accotto and Miozzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Miozzi, bGF1cmEubWlvenppQGlwc3AuY25yLml0
 Saeid Tabein
Saeid Tabein Marco Jansen
Marco Jansen Emanuela Noris
Emanuela Noris Anna Maria Vaira
Anna Maria Vaira Daniele Marian
Daniele Marian S. Ali Akbar Behjatnia2
S. Ali Akbar Behjatnia2 Gian Paolo Accotto
Gian Paolo Accotto Laura Miozzi
Laura Miozzi