- 1Henan Province Engineering Research Center for Forest Biomass Value-added Products, College of Forestry, Henan Agricultural University, Zhengzhou, China
- 2Department of Biology, East Carolina University, Greenville, NC, United States
Edible plant oil (EPO) is an indispensable nutritional resource for human health. Various cultivars of oil-bearing plants are grown worldwide, and the chemical compositions of different plant oils are diverse. The extremely complex components in oils lead to diverse standards for evaluating the quality and safety of different EPOs. The environment poses great challenges to the EPO safety and quality during the entire industrial chain, including plant cultivation, harvesting, oil processing, and storage. Environmental risk factors include heavy metal or pesticide residue pollution, insect or harmful microbial infestation, and rancidity. Here, the diverse components in oil and various oil-producing processes are discussed, including plant species, oil yield, and composition complexity, environmental factors that degrade oil quality. Additionally, we propose a whole-industrial-chain monitoring system instead of current single-link-monitoring approach by monitoring and tracking the quality and safety of EPOs during the entire process of plant cultivation, raw materials harvest, oil process, and EPOs storage. This will provide guidance for monitoring the quality and safety of EPOs, which were challenged by the deteriorating environment.
Graphical Abstract Graphical Abstract The quality and safety of edible plant oil is very important. In the entire production and industrial chain, including cultivation, harvesting, processing, and storage, it is necessary to layers of checks and set evaluation indicators to ensure the quality and safety of edible plant oils, which were challenged by the deteriorating environment.
Introduction
Edible plant oil (EPO) is obtained from the seeds, pulps, fruits, and plumules of certain plants. As one of the three major energy resources for human life activities, EPO is majorly used in cooking, but also used in a small amount for cosmetics, health supplement capsules, and other purposes. According to the information provided by the U.S. Department of agriculture (USDA), the market of EPO was close to 203 million tons in 2019 (https://www.qianzhan.com/analyst/detail/220/200713-f26ac6c2.html). When used in cooking, oils can change the sensory properties of food such as color, fragrance, and taste in the cooking process, and they also provide diversified flavor and enhance the sense of satiety. As an indispensable part of human dietary nutrition, the safety of cooking oils is paramount to human health. With the increasing of EPO consumption and the aggravation of environmental pollution in recent years, the quality and safety of EPO has gained more attention, and this poses huge challenge to the EPO industry. A strict monitoring system is required throughout the cultivation of oil plants, the processing, transportation, and storage of EPO.
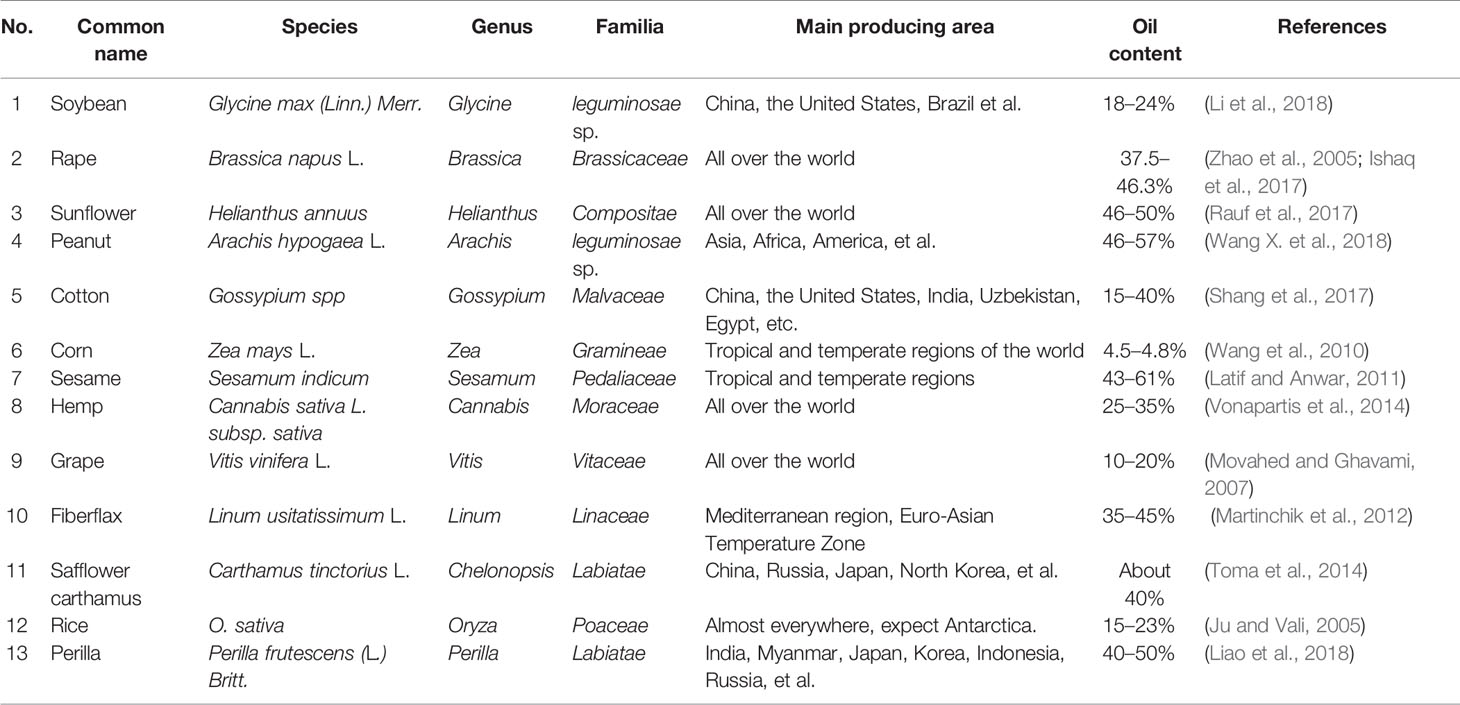
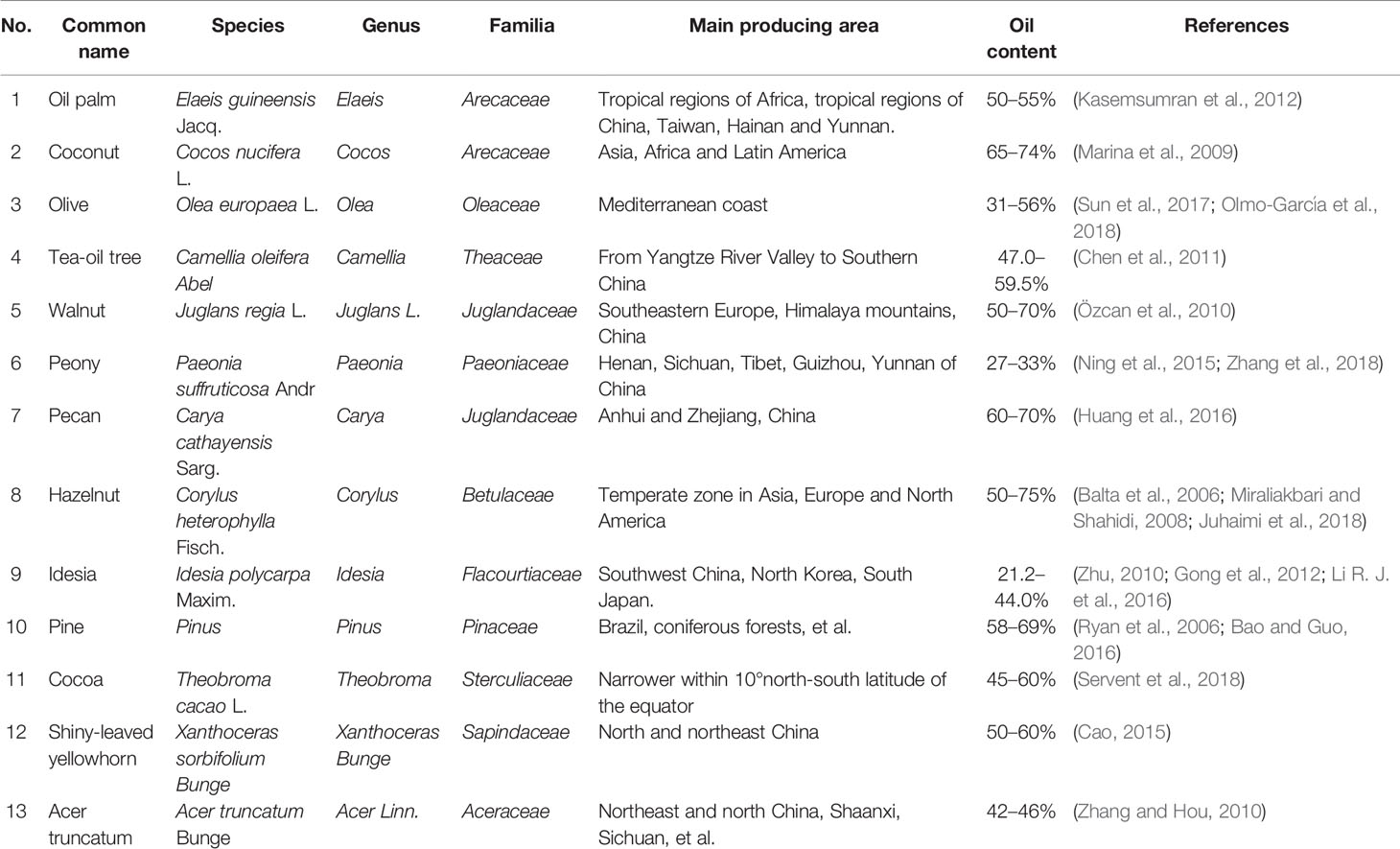
Plant can be used to produce edible oil from their seeds, germs, and/or fruits. In the early human history, sesame (Sesamum indicum) oil and olive (Olea europaea) oil were commonly used as EPOs. With the development of agriculture, processing and inspection technologies, more and more plants have been developed for EPO production. Many herbaceous plants produce a high percentage of EPO. However, the oil content, composition, and biological activity of different plant species or plant parts greatly vary (Table 1). Although sesame has the highest oil content among the herbal oil crops, sesame oil is not a commonly used edible oil because of its low global production and inefficient processing technology (Yi et al., 2017). Nowadays, soybean (Glycine max) oil and rapeseed (Brassica napus) oil are globally produced in the largest quantities. Additionally, many woody plants are also used for EPO production (Table 2). Oil-seed camellia, oil palm, olive, and coconut (Cocos nucifera) are the four well-known woody edible oil plants in the world, as they possess a high oil content. Among bulk herbaceous edible oils, the unsaturated fatty acids (UFAs) are the highest, approaching 80%, in peanut oil and rapeseed oil. While among EPOs from woody plants, olive oil and oil-seed camellia (Camellia oleifera) oil exceed 80% of UFAs, and camellia oil reaches 90%. Therefore, the EPO quality of most woody plants is better than that of herbaceous plants. With the advantage of no use of cultivated land, the development and utilization of woody oil will play an important role in global grain and edible oil security.
Here, we review the distribution of edible oil-bearing plants in the world and the complicated chemical composition of EPO, including their importance for our health. This review will also focus on the environmental risks during the process of EPO, from the cultivation of oil-bearing plants and the harvest of raw materials to the production and storage of EPO. The industrial chain monitoring system for the quality and safety of EPO is also proposed.
Global Production of EPO
The yield of oil-bearing plants is the guarantee of the source of EPO. As the growing of global population, the demand for EPO is also on the rise and many oil-bearing plants are being grown on a large scale, widely using agricultural machinery and high-tech methods, such as cell engineering, genome editing, and tissue culture. As more attraction to human health, people are preferred to consume more healthy oils with higher UFAs, such as olive oil, walnut oil, and corn oil (Weinstock et al., 2006; Geng et al., 2018; Wang et al., 2011; Lin et al., 2018). In China, camellia oil and olive oil have maintained a high growth rate, significantly higher than the overall growth rate of the domestic oil industry.
Among the common EPOs, palm oil has the highest annual yield, showing an increased trend in recent years. Soybean oil is with the second highest annual yield, followed by rapeseed oil (https://www.qianzhan.com/analyst/detail/220/200713-f26ac6c2.html). Overall, the global production of major EPO is increasing annually (Table 3). Palm oil is mainly produced in southeastern Asia, including Malaysia and Indonesia (Figure 1A) (Esteki et al., 2018). In 2016, palm oil from Indonesia and Malaysia was accounted for approximately 85% of global palm oil production, including palm kernel oil. The main producers of soybean oil are China, the United States, Argentina, and Brazil, among which China’s soybean oil production ranks first in the world (Figure 1B). The European Union, China, and Canada are the largest producers of rapeseed oil. In 2016, the European Union led the world in rapeseed oil production, accounting for 35% of the total, followed by China in which accounted for approximately 23% of the total yield (Figure 1C). Olive oil is mainly produced by the Mediterranean coastal countries, including Spain, Italy, Greece, and Turkey. These countries are account for 90% of the world’s total olive oil production, of which Spanish olive oil production ranks the first in the world. In southeast Asian countries, Philippines is rich in coconut oil. China and India are major producers of peanut oil, and Ukraine is the world’s largest producer of sunflower seed oil (Figure 1D).
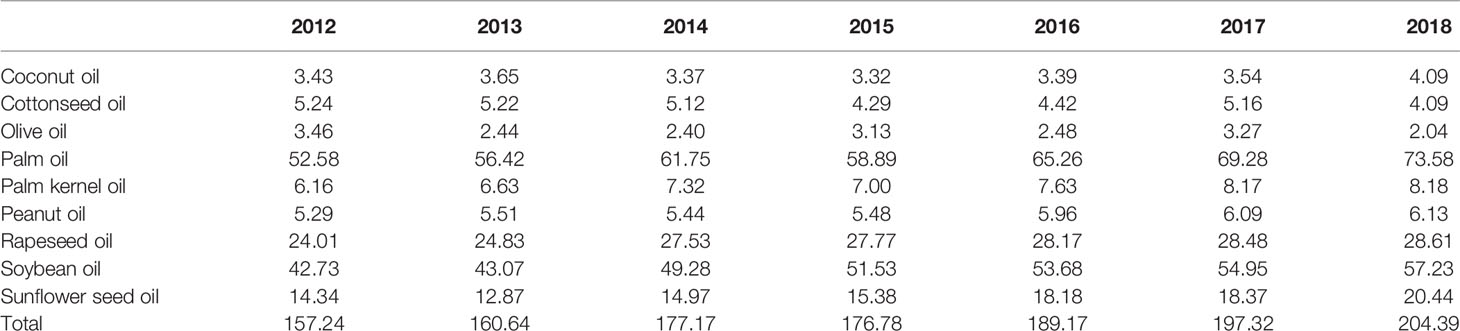
Table 3 Global yield of common EPOs during 2012 to 2018 from U.S. Department of Agriculture (USDA) (millions tons).
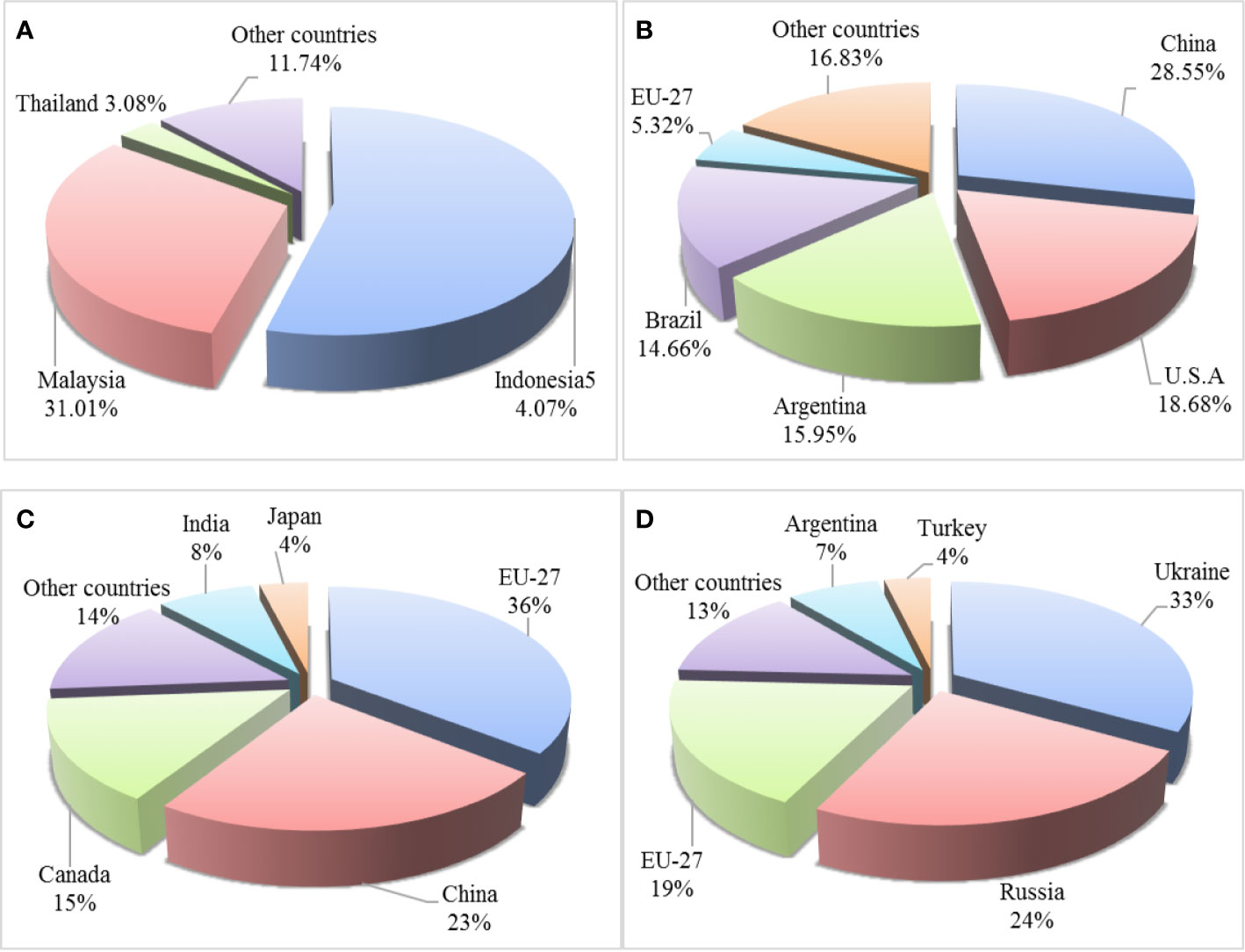
Figure 1 Major countries producing palm oil (A), soybean oil (B), rapeseed oil (C), and sunflower seed oil (D) in 2016.
Complexity of the Chemical Composition of EPOs
EPOs contain complex chemical components, and are generally rich in fatty acids, microelements and active compounds, and flavor substances (Kim et al., 2010; Puch et al., 2010; Ascensión et al., 2014; Wang et al., 2019). These components together constitute the unique physicochemical properties of EPOs. EPOs are also rich in the fat-soluble vitamins A, D, E, and K, among which vitamin E has antioxidant properties and can devour the free radicals that lead to aging and carcinogenesis.
Fatty Acid Composition in EPOs
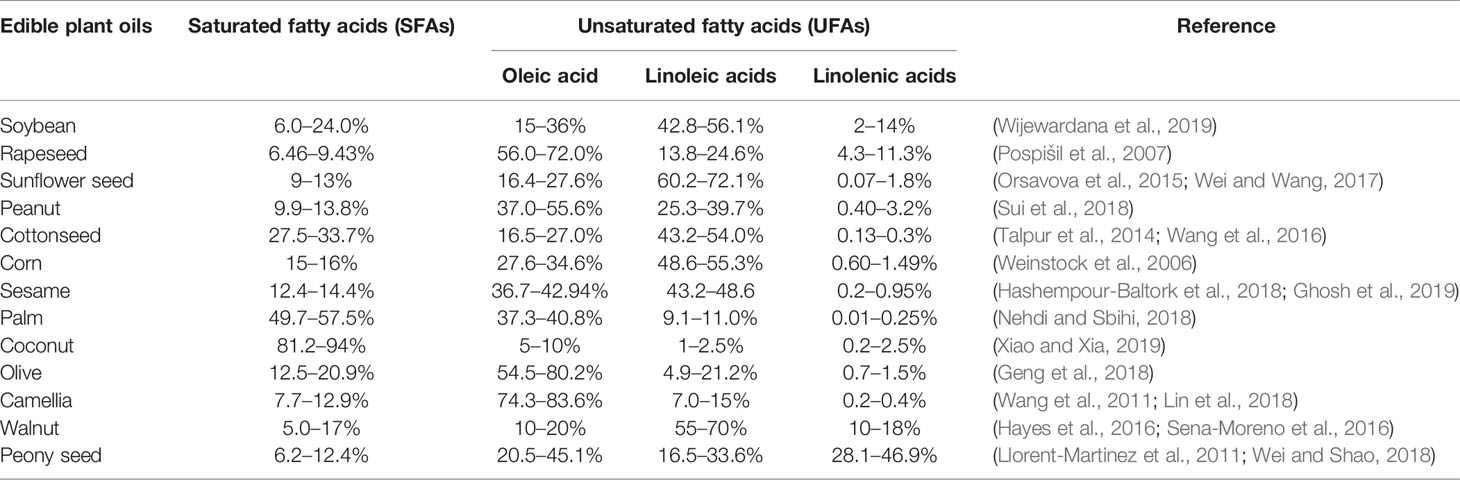
Fatty acids are the major composition of oils. A fatty acid is an organic substance consisting of a long aliphatic hydrocarbon chain that contains one carboxyl group at one end. Fatty acids are divided into saturated fatty acids (SFAs) and UFAs (Esteki et al., 2018). The human body can synthesize the required SFAs and UFAs with only one double bond. Fatty acids containing two or more double bonds must be obtained from the diet, and therefore, the latter are called essential fatty acids, among which linolenic acid and linoleic acid are the most important.
UFAs play important roles in the human body, such as maintaining the relative fluidity of cell membranes to ensure the normal physiological function of cells, esterifying cholesterol, and reducing cholesterol and triglyceride in the blood (Assmann et al., 2018). They are the precursors of prostaglandin synthesis, reducing blood viscosity, increasing blood microcirculation and the activity of brain cells, and enhancing memory and thought processes. There are 16 or 18 carbon atoms in the most abundant fatty acids, which are oleic acid, linoleic acid, linolenic acid, and SFA. However, different oils have different fatty acid compositions (Table 4).
Trace Components in EPOs
Many kinds of trace components have been detected in EPOs and their contents vary in different EPOs (Figure 2). With the development of detection technology, more and more trace components in EPOs will be characterized.
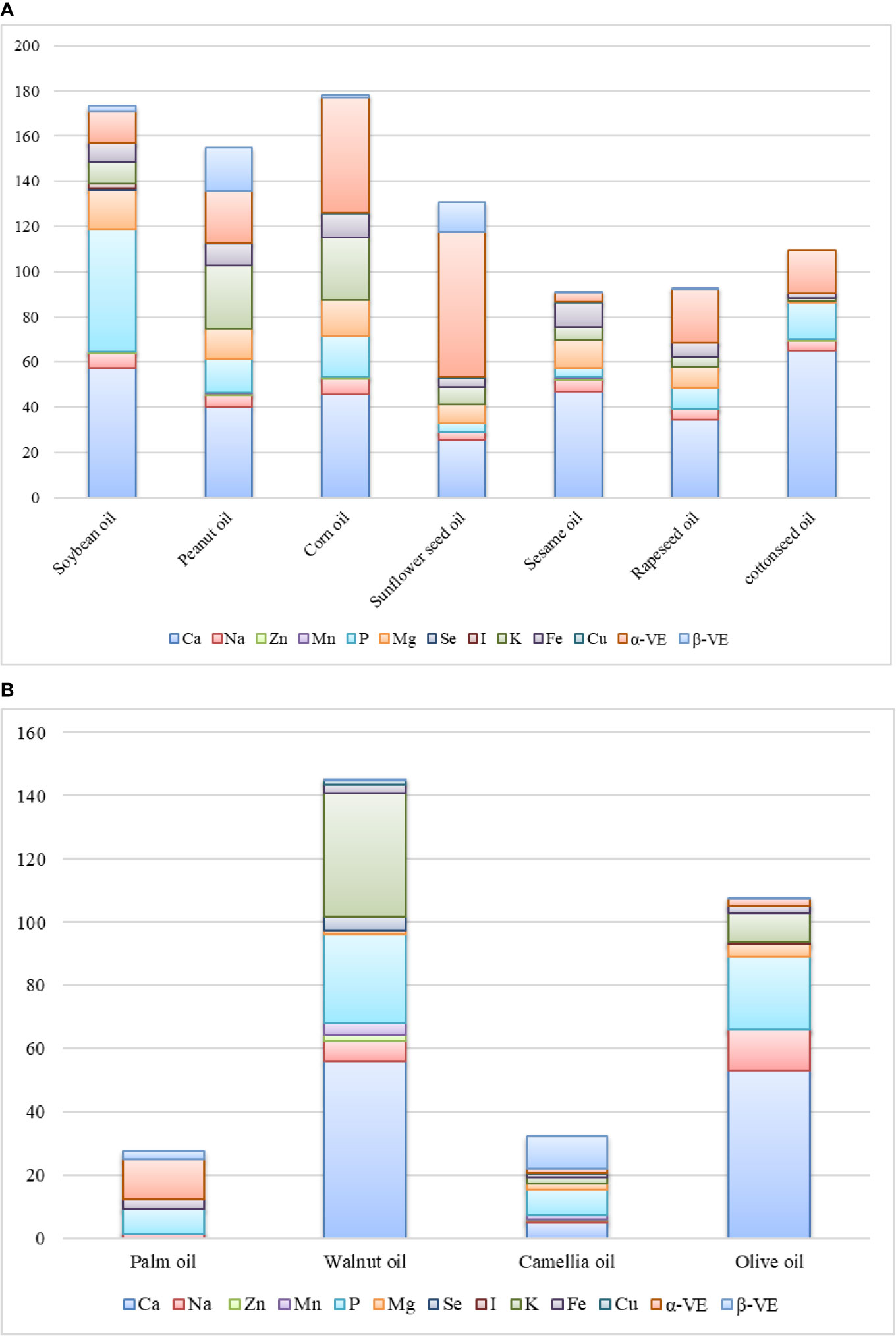
Figure 2 Microactive compositions in (A) herbal edible plant oils (mg/100 g) and (B) woody edible plant oils (mg/100g).
There are small quantities of trace elements in the human body that are necessary for human survival and health. Because the human body cannot automatically synthesize trace elements, they must be obtained from the diet, for example the EPOs (Llorent-Martinez et al., 2011).
The content of trace elements and active compounds in EPOs is very low, but their biological activities have some unique health functions that are very meaningful.
High quantities of phosphorus (P), magnesium (Mg), and potassium (K) are found in soybean oil (Figure 2A). Walnut oil also contains high amounts of P (Juranović Cindrić et al., 2018). P protects human tissue cells and enhances the role of cell membranes. P boosts the effectiveness of the vitamin B family. Phospholipids are formed when P combines with fat in the blood, and this compound plays a structural and metabolic role in the human cell membrane and plays a role in the body tissue structure (Taketani et al., 2015).
Olive oil contains the largest quantities of calcium (Ca) at 53 mg/100 g (Gouvinhas et al., 2015). The function of Ca is to maintain strong bones and healthy teeth, to maintain regular heart rhythms, to relieve symptoms of insomnia, to assist with the metabolism of Fe in the body, and to strengthen the nervous system, especially its stimulating communication function (Marsh et al., 2015).
EPOs also contain other trace elements, including Cu, Zn, and Mn, which play important roles in the development and function of the hair, skin, bone tissue, brain, liver, heart, and other internal organs (Haase and Rink, 2014; Marsh et al., 2015; Schofield, 2017). Most common EPOs contain Cu, except palm oil, but the content of Cu in different EPOs is significantly different. Soybean oil, walnut oil, and olive oil contain selenium (Se) and/or iodine (I), which are not found in other EPOs (Figure 2).
The content of alpha-tocopherol (α-VE) in sunflower seed oil and corn oil is 64.12 mg/100 g and 50.94 mg/100 g, respectively (Figure 2). α-VE is a very important vasodilator and anticoagulant, which can reduce wrinkles and the oxygen consumption of cells and further help to reduce leg cramps and hand and foot stiffness (Jiang, 2017).
The majority of oils also contain a small amount (0.1–1.0%) of saturated and unsaturated hydrocarbons. For example, squalene, molecular formula C30H50, is a colorless oily liquid with a pleasant odor; after oxygen absorption, it becomes viscous linseed oil. Squalene was found in some herbaceous and woody oils. Squalene has strong biological activity that robustly transports reactive oxygen species in the blood, enhances the body physiological functions, improves human immunity, and helps the cells resist ultraviolet rays. Squalene is widely used in various fields such as medicine, beauty, and cosmetics. International research has been carried out on the exploration of natural resources and chemical synthesis methods so that squalene can be produced without killing sharks (Kim and Karadeniz, 2012). It is found that the content of squalene in woody oil is higher than that in herbaceous oil.
Among all plant oils, corn oil contains the highest quantities of phytosterols, followed by rapeseed oil (Zou et al., 2018). Phytosterols consist of a variety of ingredients such as β-glutosterol, oil sterol, rapeseed sterol, and soya sterol. Phytosterols can inhibit the absorption of cholesterol, have significant preventive and therapeutic effects on cardiovascular diseases and cervical cancer, and also have strong anti-inflammatory effects (Wang F. C. et al., 2017). Therefore, eating EPO with higher content of phytosterols is a healthy choice.
Carotenoids have been found in soybean and rapeseeds (Syed and Shinwari, 2016). Both chlorophyll A and B are photosensitive substances and are the source of photooxidation of oils (Tena et al., 2018). However, under the condition of no light, chlorophyll A and chlorophyll B capture the free radicals produced during the initial stage of oil oxidation, which subsequently inhibits the automatic oxidation of oils. Particularly, chlorophyll A has a strong antioxidant effect, but different oxidation conditions such as substrate and temperature can affect chlorophyll antioxidant action, even with stronger antioxidant effects at low temperatures (Bhattacharya et al., 2017). This may be one of the reasons for the long storage time of soybean oil and rapeseed oil.
Polyphenols, also known as tannins, are found in herceous edible oils and are one of antioxidant components (Wang X. et al., 2017). It is a generic term for plant components containing multiple hydroxyphenols in their molecules. Polyphenols have strong antioxidant activity in oils and fats and display anti-cancer activity. Polyphenols are also used to ameliorate radiation damage (Rueda et al., 2016).
Flavonoids are a group of substances that contain alpha-phenyl phenylthiopyranone or beta-phenylthiopyranone. Flavonoids are beneficial to the body and act as antioxidants (Cassidy and Minihane, 2017). At present, flavonoids have been found in many EPOs, such as soybean oil, rapeseed oil, and corn oil (Li X. J. et al., 2016). The trace substance of herbaceous and woody oils is basically the same, but the content is different.
Flavor Substances in EPOs
EPOs are important raw materials in our daily consumption of food, as well as in food processing. They not only provide heat energy and essential fatty acids for human beings but also endow food with a pleasant flavor (Tekaya et al., 2018). With the improvement in living standards, in addition to containing the necessary nutrients in EPOs, the flavor is a major factor that is taken into account when choosing an EPO.
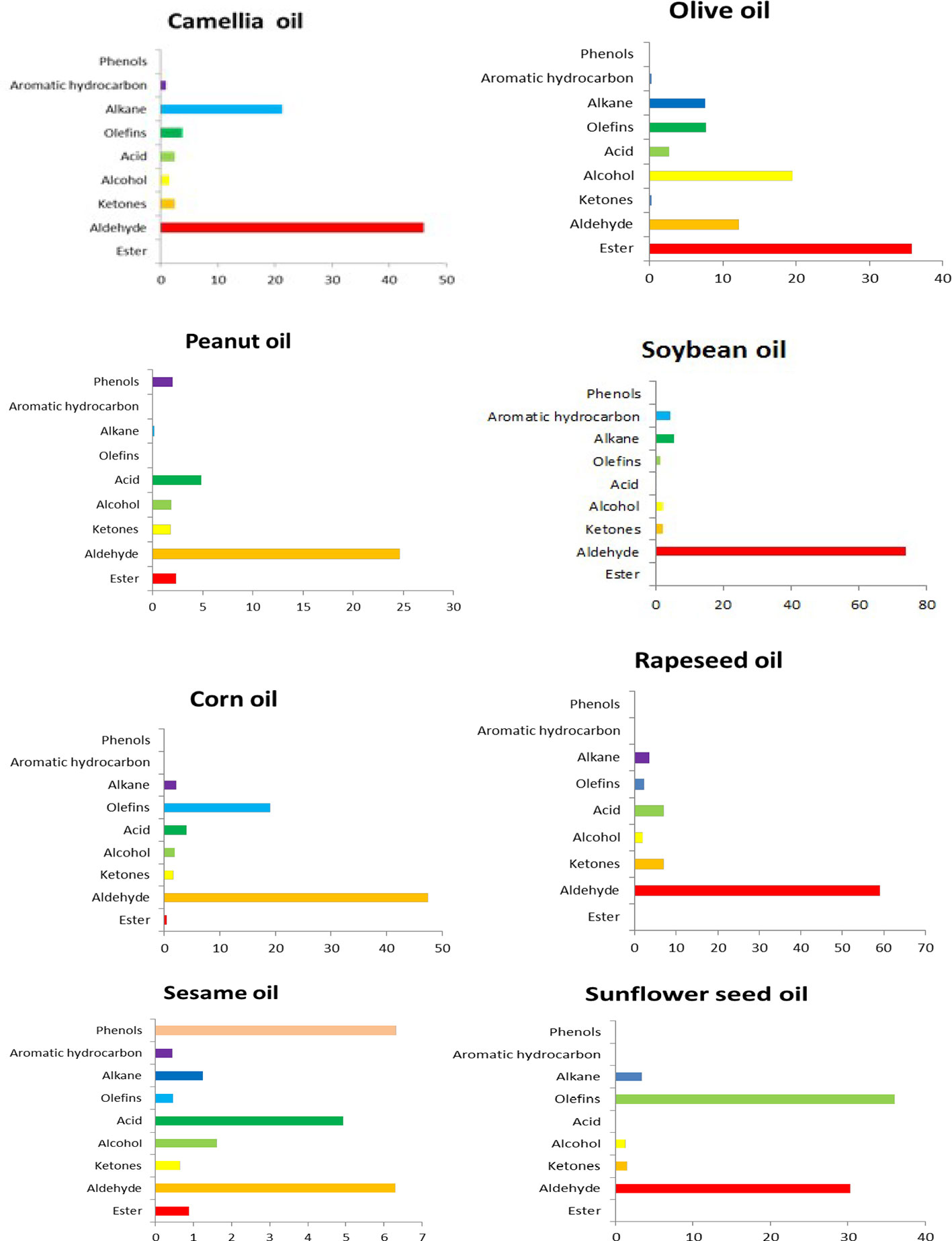
Flavor substances are one of the important indicators of the sensory quality of EPOs (Fujikawa et al., 2002). The unique flavor of different EPOs is not formed by one or several compounds but is formed by the synergy of various components (Zhang et al., 2012; Yang et al., 2015; Liu et al., 2017). Phenols, including tocopherols, polyphenols, phytosterols, and pigments, are important components of natural vegetable oils. Although the contents of these substances are relatively low, they are closely related to the quality of edible oil, which directly affects the functionality and oxidation stability of edible oil. It was found through comparison that the flavor substances in EPOs mainly include alcohols, aldehydes, ketones, alkanes, alkenes, and furans, and the variety and content of flavor components in one EPO is different from each other (Zhang et al., 2012). The volatile flavors of tea oil, olive oil, soybean oil, corn oil, peanut oil, sunflower oil, sesame oil, and rapeseed oil were compared using solid phase micro-extraction-mass spectrometry, and it was found that olive oil contained the largest amount of esters, and the other EPOs had high amounts of aldehyde (Hu et al., 2018).
As an important flavor substance in oils and fats, esters are easily decomposed or oxidized by heat to form aldehydes and other volatile short-chain secondary oxidation products. No olefin substances were detected in peanut oil. Acidic substances were not detected in sunflower oil or soybean oil. Phenolic substances were only found in peanut oil and sesame oil. All these results indicated that the type and content of flavor substances in different EPOs are not the same (Figure 3).
Hazardous Substances in EPOs
Due to improper cultivation, process, and storage, some hazardous substances are also detected in EPOs. These substances can be divided into two types: (i) biological hazards and (ii) harmful chemical substances (Ji et al., 2016).
For EPOs, biological hazards usually arise from the oil-bearing plants that are infected by microorganisms or infested by pests. During plant growing, raw materials harvesting and storing, microorganisms, parasites, and insects attack plants, destroy their epidermis, and leave harmful secretions in or on the surface of seeds or fruits, contaminating the raw materials of EPO process (Bhat and Reddy, 2016; Zhou et al., 2017).
The majority of harmful chemical components detected in EPOs come from pesticide residues, heavy metals, or plasticizers (Hu et al., 2016). It should also be noted that in the process of cooking with edible oils, high temperature will change the structure of the oil and produce harmful trans-fatty acids, which are harmful to human health (Ginter and Simko, 2016). Improper use of storage containers can also cause some harmful substances to enter the EPOs, such as some plasticizers and submicrometre plastics from plastic containers, which may pose a health threat if they enter the internal circulation of human body. Heavy metals such as lead in glass containers can also contaminate EPOs (Li et al., 2013; Sungur et al., 2015).
EPOs Affected by Environmental Risks
Oil-bearing plants are affected by various external environmental factors, such as heavy metals, pests, and diseases, and pesticides and herbicides used during plant growth and development, which will directly affect the safety of EPOs. With the continuous development of society, there has been increasing concern over the quality and safety of edible oils. The requirements for the quality of oil-bearing plants are also increasing. The improvement of the quality of oil-bearing plants has become a necessary factor for modern edible oil production. Therefore, in order to fundamentally improve the quality of oil-bearing plants, it is necessary to comprehensively analyze the planting safety of oil-bearing plants (Figure 4A).
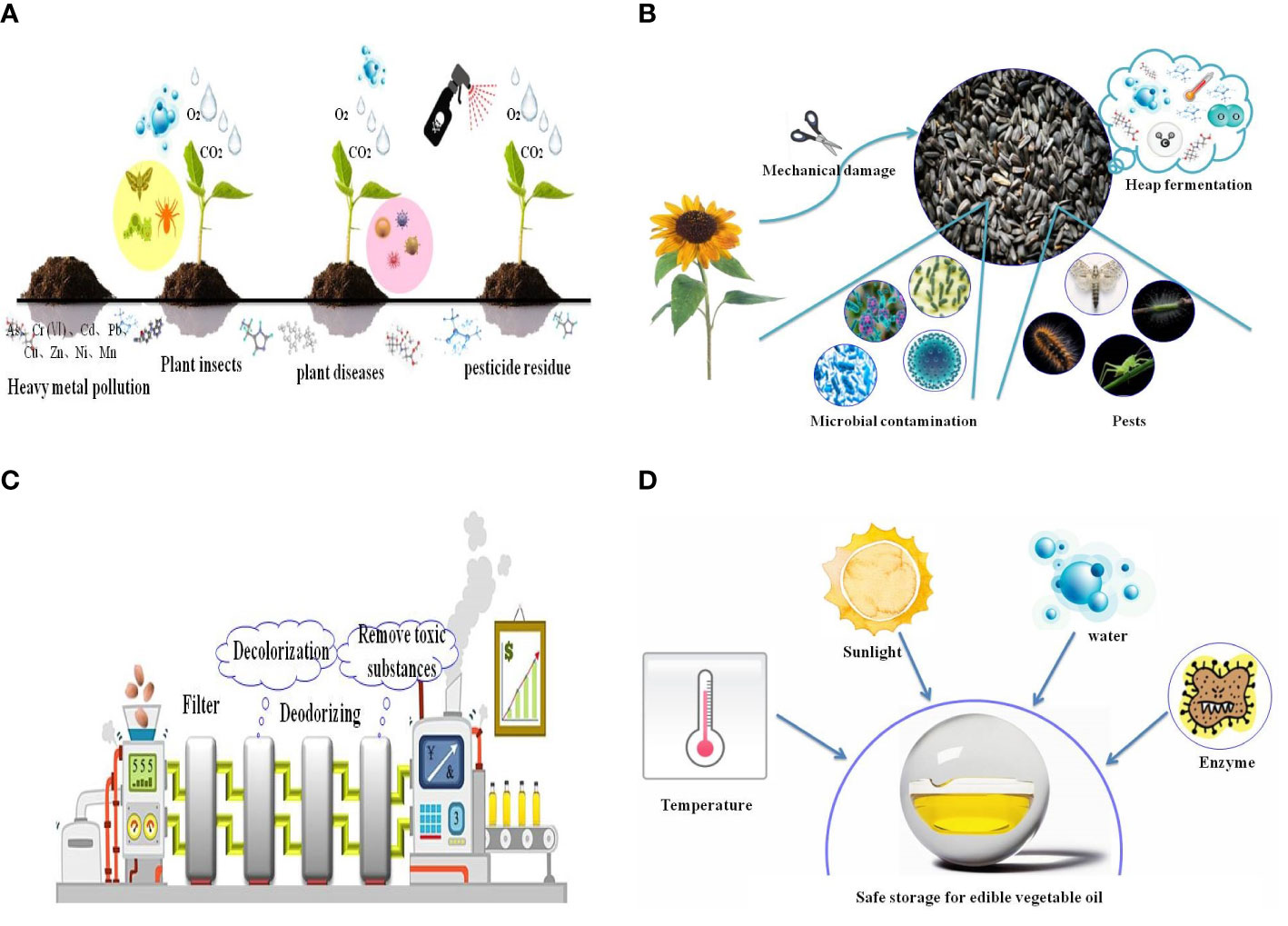
Figure 4 Environmental risks in (A) oil-bearing plant cultivation, (B) the harvesting and post-harvest process of EPOs, (C) during EPO processing, and (D) during EPO storage. The graph takes sunflower seeds as an example to show the risks that oil crops will encounter during harvesting and post-harvesting process.
Risks During Oil-Bearing Plant Cultivation
At present, oil-bearing plants are threatened by diseases, pests, air and soil pollution, pesticide residues, and other environmental factors (Wang Y. et al., 2018). The major pollution affecting oil quality and safety is heavy metals and pesticides. Many contaminants have been identified during the planting stage that will enter into the vegetable oil. Some fungi can infect oil plants and produced large amounts of toxins that are very harmful to our human body. Aspergillus flavus and A. parasiticus can infect peanuts causing a severe decline in yield, and what’s more, aflatoxin produced by them is serious carcinogen (Zhang et al., 2017).
Pesticides play an important role in controlling diseases and pests of oil plants, but at the same time, they also pollute the environment and oil plants. During oil processing, pesticides will migrate and eventually accumulate in edible oils. Pesticide residues in EPOs have become a major factor that adversely affects human health (Wei et al., 2008; Yao, 2016). Common pesticides are endosulfan, chlorpyrifos, cypermethrin, and HCH, classified as persistent organic pollutants because of their persistence, bioaccumulation, long-distance migration, and adverse effects on organisms (Frigo et al., 2002). Some studies suggest that the absorption and accumulation of organic pollutants by plants depends not only on the nature of the pollutants but also on the oil content of oil plants. Oil plants with higher oil content absorbed more lipophilic substances such as toxaphene (McLachlan, 1996; Wei et al., 2006).
Most oil-bearing plants become contaminated with heavy metals such as As, Cr (VI), Cd, Pb, Cu, Zn, Ni, Hg, and Mn during their growth stage, which will severely affect the safety of edible oil (Xue et al., 2019). When heavy metals sufficiently accumulate in cultivated soil, they can directly injure crop growth, resulting in reduced yield and quality (Sun et al., 2016). More seriously, heavy metal can be absorbed into the plant cells and contaminate the oils. In order to ensure the supply safety of oil-bearing raw materials, it is necessary to perform sufficient testing and treatment technology in order to reduce the safety hazards caused by edible oil raw materials in the planting stage.
In addition, with the extensive use of plastic products, micro-plastics have been detected in various ecological environments around the world, which has aroused widespread concern. It has been proved that microplastics could been absorbed by plants and affected their growth, which posed a potential risk to our food safety (Li et al., 2020; Sun et al., 2020). Microplastics may enter the human body through oil-bearing plants and pose a threat to human health, and this risk will be exacerbated due to the adsorption properties of microplastics to heavy metal ions (Mao et al., 2020; Yu et al., 2020).
Risks During Harvesting and Post-Harvest
After raw materials are harvested, they may not be immediately pressed into EPOs, so they often need to be stored for a period of time (Figure 4B). Some oil-bearing plants have a higher oil content, especially sunflower seeds, with an oil content of 45 to 60% and UFA content of more than 90%, which is prone to heat, mildew, oil rancidity, and deterioration during storage (Mangin et al., 2017). It is difficult to store sunflower seeds without spoilage, and advanced technology is required for preservation. At present, the methods for preserving and storing vegetable oil raw materials include physical preservation techniques such as cryopreservation, cold shock-ultraviolet and microwave irradiation, and chemical preservatives such as thiophanate, diazolid, and carbendazim. However, these chemical preservatives will also pollute oil raw materials.
Water and temperature play a dominant role in seed storage (Cheong et al., 2018). The moisture content of the seeds affects the preservation time and quality of EPO raw materials. Seeds should be fully dried before storage because water causes deterioration. Storage sites should be kept ventilated and dry, strictly disinfected, sterilized, and moisture-proof., and it is important to remove any impurities, rotten fruit, or mildew-contaminated seeds. The preservation methods for various EPO raw materials will be different, but the ultimate goal is to ensure that the raw materials do not deteriorate and prevent various mildew reactions. If the raw materials are rancid and deteriorated and contain molds, then they cannot be used in edible oil production. It has been reported that with the prolongation of storage time, the quality and yield of oil from the seeds was decreased (Bonte et al., 2017; Han et al., 2017).
Risks During Oil Processing
Pressed extraction of EPOs is the most traditional way to extract oil, and it is also the most widely used method currently (Yara-Varon et al., 2017). Compared with solvent extraction, pressed extraction has the advantages of simple operation, no solvent pollution, high quality of crushed oil, and retention of the unique flavor of EPOs (Figure 4C). Pressing extraction of edible oil can be divided into hot-pressed and cold-pressed (Pereira et al., 2014). Cold-pressed is a method of producing oil by low-temperature pressing through an oil mill. The oil plants are not heated, nor are they are stir-fried at low temperature before pressing. During extraction at the oil mill, the entire pressing process is performed at low temperatures, which ensures that the oil will contain the maximum amount of flavor substances (Fawzy, 2013; Yang et al., 2013; Sielicka et al., 2014). The hot-pressed method is the opposite, and the oil pressing process occurs at a continuous high temperature environment (Siger et al., 2017). At high temperatures, oil plants can increase oxidation stability, but hot-pressing also has some drawbacks. High temperature may lead to loss of many nutrients and flavor substances in EPOs (Soria and Villamiel, 2010; Teh and Birch, 2014; Koubaa et al., 2016). For example, high temperature caused vitamin E damage and loss. Research showed that the content of vitamin E in cold-pressed and hot-pressed sunflower seed oil was 45.7 mg/100 g and 13.2 mg/100 g, respectively. This indicates that vitamin E decomposed to a certain extent during the baking process, thus resulting in a decrease in the vitamin E content (Wei and Wang, 2017). Therefore, cold-pressed method becomes more popular.
Risks During Oil Storage
Improper storage conditions or long term of storage decreased the quality of EPOs (Figure 4D). Severe deterioration of EPOs will result in adverse effects on human health, and lose their edible value. Conditions that deteriorate EPOs are light, heat, oxygen in the air, water, and enzymes in oils, and the high UFA content of some EPOs requires additional storage measures to ensure safety (Jabeur et al., 2015). Consequently, storage conditions are very important in the storage process. If the standard of containers for EPOs is not strict, EPOs will be contaminated with plasticizers, heavy metal, synthetic antioxidants (Carvalho et al., 2019), or other toxic substances during storage.
The types of rancidity are oxidative rancidity, hydrolytic rancidity, and ketone rancidity (Haman et al., 2017). Although the processes of the rancidities are different, any rancidity will eventually lead to the production of a certain amount of alcohols, aldehydes, and ketones, which will cause serious harm to the consumer. Severe rancidity will be accompanied by a pungent unpleasant smell due to the aldehydes, ketones, and other oxides. The fatty acid composition of the oil directly affects the shelf life of EPOs (Buratti et al., 2018). SFAs must be acidified by enzymes or fungi, or conditions must be optimal for the existence of hydrogen peroxide, and therefore, oils with a higher content of UFAs are more likely to be acidified, which poses a challenge to store EPOs with high-quality.
The physical factors that affect the storage safety of EPOs are heat, water, oxygen, and other factors. The rate of the rancidity reaction increases one fold with an increase in storage temperature of 10°C, and therefore, the better choice is to maintain the process of oils production and storage at a low temperature. The effect of the moisture content in oils is very complex on the oxidation of oils, and the moisture content also affects the growth of microorganisms that can cause the spoilage of oils. Rancidity can be prevented by reducing the water content through refining and dehydration. Oxygen plays an important role in rancidity because the higher the oxygen content, the faster the occurrence of ketone rancidity and oxidation rancidity. Oxygen can be removed by adding nitrogen to filled bottles and vacuum packaging or adding antioxidants to reduce the oxygen content of edible oils. During the storage of edible oil, direct sunlight and metal contact should be avoided, because radiation can significantly increase the rate of free radical formation and increase the sensitivity of fatty acid oxidation; metal ions can catalyze the oxidation of oil and greatly increase the decomposition rate of hydrogen peroxide.
The safety of edible oil storage is also affected by chemical factors. Some edible oils are naturally pigmented, and the pigments easily form pigmented peroxide complexes, thus accelerating the degradation of oils (Wang H. C. et al., 2018). The addition of antioxidants to oils will increase the shelf life of edible oils. At present, the main synthetic antioxidants are BHA, BHT, PG, TBHQ, and THBP (Cini et al., 2014; Kim et al., 2016). Natural antioxidants mainly include vitamin C, vitamin E, carotenoids, and polyphenols such as flavonoids and tea polyphenols. In addition, rosemary extract as a natural antioxidant has been accepted by more and more people. Rosemary extract significantly improved the scavenging ability of free radicals in oil, delayed deterioration, and extended the shelf life of oils (Yang et al., 2016; Bañares et al., 2019).
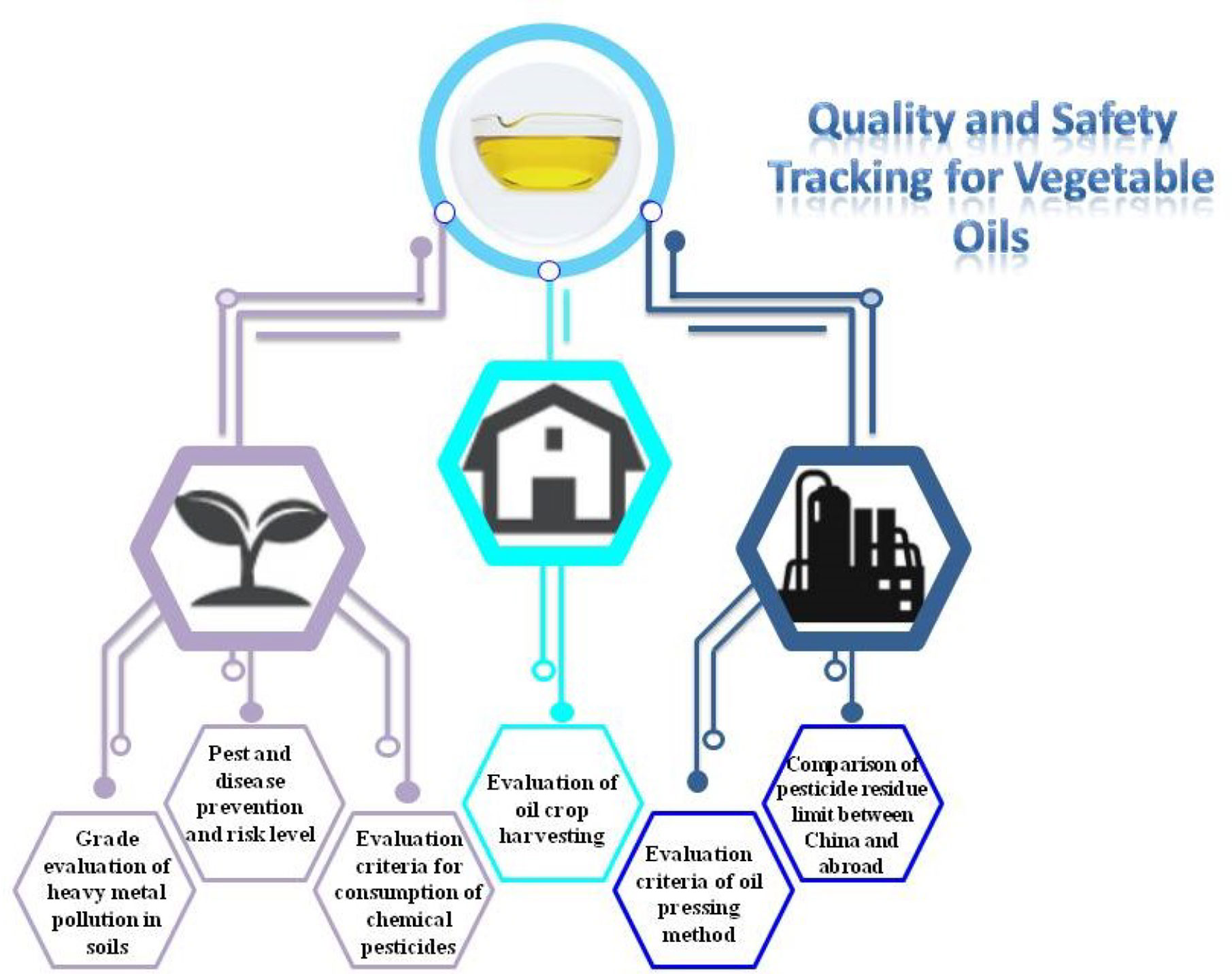
Industrial Chain Monitoring the Quality and Safety of EPOs
Edible oil industry is a complicate supplier chain, which is involved in plant planting, seed storage, transportation, production, processing, oil storage, and transportation. These links are interrelated, mutually restrictive, and interlinked. Security problems taking place in any link will affect the EPO quality. Therefore, in order to ensure the safety of edible oil, we must seize every link of the edible oil industry and take proper safety measures to monitor the entire process. A strict and reasonable evaluation system is the key to ensure the quality and safety of EPOs.
Monitoring During Cultivation Stage
During cultivation stage, oil-bearing plants mainly encounter three major elements: natural environment, pests and diseases, and exposure to chemical pesticides. Natural environment includes air, water, light and soil, while atmospheric pollution mainly includes inhalable particulate matter, SO2, and nitrogen oxides. Some studies have shown that SO2 can affect carbohydrate synthesis in plants, which causes plant cell membrane lipid peroxidation, permeability damage, ion exosmosis, and the increase of ethylene production in vivo before injury, leading to premature maturation and senescence of plants. This is a great damage to oil crops at seedling stage and eventually affects the yield of oil plants. Thus, it is important to monitor the air quality in the growing environment of oil plants.
Soil is the basis of providing nutrients for plant growth and development, and therefore, good soil conditions are required for growing oil-bearing plants. It is necessary to strengthen soil quality monitoring. Oil-bearing plants inevitably encounter diseases, insect pests, and weeds during their growth and development, which not only affect plant growth but also affect the quality of fruit and ultimately affect the quality of EPOs. In India, the fungus Sclerotinia sclerotiorum occurred on soybean and caused an approximately 40% reduction in soybean yield (Wrather et al., 2001). Pesticides are effective measures to control the effects of pests and weeds. The use of pesticides can increase yield and economic benefits, but it also affects the soil and fruit. Therefore, we should strictly control the soil conditions, the degree of pests and diseases, and the use of pesticides. Common control technologies of pest and disease are as follows (Li and Zhang, 2018):
1. Biological control and the use of pesticides/fungicides are effective preventive measures for eliminating hidden dangers over time.
2. Various cultivation modes such as rotation and interplanting can be adopted.
3. Using resistant varieties under good cultivation management can enhance plant resistance to stress.
4. New technologies and methods can be used to identify pests and diseases more rapidly.
To solve the key problems of pesticide residues, it is important to establish a good evaluation system (Li, 2002). It has been reported that the greater the amount of pesticides sprayed, the better the control of pests/diseases and the higher the yield (Gagic et al., 2017), but the more the amount of pesticide residues, the accumulation of pesticide residues to a certain amount will cause great harm to human and the environment. It is best to use environmentally friendly pesticides with excellent control, little impact on natural enemies, low toxicity, and no residues. The use of chemical pesticides should be strictly controlled (Cao et al., 2018). There are many types of pesticides, and the standards of pesticide usage vary in different countries. Therefore, it is necessary to establish a strict and uniform evaluation criterion for the usage of pesticides and pesticide residues.
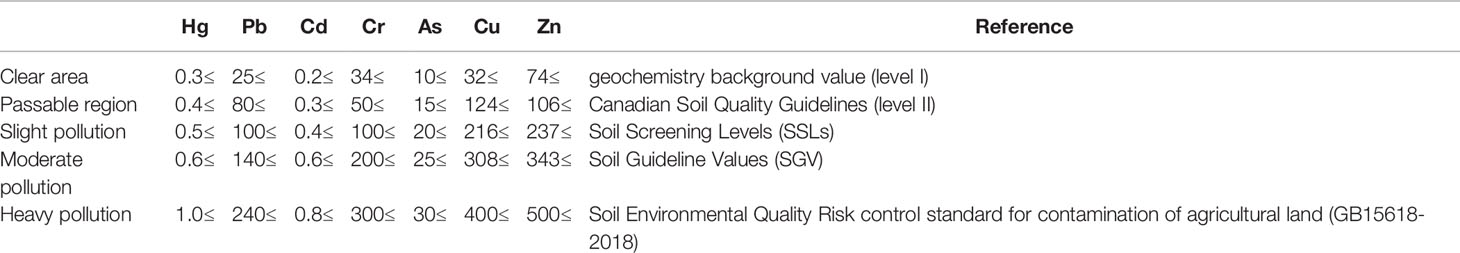
Heavy metals not only pollute the soil but also negatively affect food safety when plants are contaminated with the heavy metals from the soil (Fryzova et al., 2018). Therefore, it is necessary to control the amounts of heavy metals in the soil. It is indispensable to establish relevant soil heavy metal standards and limits (Table 5) and regularly monitor the content of heavy metals in the soil where oil plants are planted. Once the content of heavy metals in soil exceeds the standard, the cultivation of oil plants should be prohibited. If the soil is slightly polluted by heavy metals, the oil plants with weak adsorption capacity should be selected. Furthermore, it is still necessary to monitor the content of heavy metals in processing raw materials. The varieties with strong resistance should be selected before planting to reduce the amount of pesticide. Some genetically modified oil crops may have strong resistance and weak adsorption capacity of heavy metals. However, the safety of genetically modified organisms is still controversial. Thus, the supervision of genetically modified oil crops must be in place, and the products may be clearly marked.
Monitoring During Plant Harvesting Stage
Fruits and seeds are the most common parts of oil plants used for oil extraction. Due to the damage by animal encroachment or machine, a proper harvesting method should be selected during harvesting and a process of checking the integrity of the seeds or fruits is necessary after harvesting. Because damaged seeds and fruits are easier infected by microorganisms, reducing the quality of raw materials. For each kind of oil plants, the exact harvesting time should be firstly considered. Early harvesting may result in a lower oil content in seeds, while late harvesting may reduce yields as some mature seeds fall off from the plants (Matthäus et al., 2018). After harvesting, when the seeds and fruits are not immediately pressed, they need to be carefully stored in the most optimal environment. The main factors affecting the safe storage of oil are moisture, temperature, relative humidity, pests, microorganisms (Mmongoyo et al., 2017; Sobolev et al., 2019), and so on. These factors must be detected frequently during seeds and fruits storage. The evaluation of the harvest mainly includes a survey of the degree of damage to seeds and fruits during harvesting, and storage conditions should be strictly controlled.
Monitoring During Oil Processing Stage
EPOs can be obtained from the processing of plant seeds or fruits. At present, there are three main processing methods: hot pressing, cold pressing, and solvent extraction; each of the method has its own advantages and disadvantages. Compared with other two methods, cold pressing method maybe the better oil pressing method for obtaining high quality oil. In addition, there is also a method called supercritical CO2 extraction (Sookwong, and Mahatheeranont, 2017). No matter which oil pressing method, it is hard to obtain complete-pesticide-free oil, and trace amount of pesticide residues may be still detectable in extracted edible oils. To remove as more as possible the pesticide residue in oil, different counties have made different criterion for limiting the pesticides. Among them, the European Union is the organization with the strictest detection criteria. Therefore, the EU standards can be used to evaluate pesticide residues after processing.
Prospective
The quality and safety of edible oil are related to human health, attracting the attention of all human beings. Its evaluation depends on different testing data, testing depends on instruments to complete, and advanced scientific instruments are the material basis to promote more accurate testing. At present, compared with developed countries, developing countries’ technology and means of detecting pesticide residues and harmful substances in EPOs remain slightly inferior. Recently, a new method, the quick, easy, cheap, effective, rugged, and safe (QuEchERS) procedure using high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), has been applied in the detection of pesticide residues (Song et al., 2018). This method is simple, fast, effective, and has good purification effect (Deng et al., 2018). It is also necessary to develop rapid detection techniques for the detection of different hazardous substances in raw materials and EPOs. In the future, with the continuous development of science and technology, more advanced instruments can be used for detection and a multi-stage evaluation system will be established (Figure 5) to deal with the the deteriorating environment, which will help to improve the safety and quality of EPOs.
Of course, the establishment of a multi-stage evaluation system for EPOs requires the joint efforts of all countries in the world. People in different countries have different lifestyles and dietary habits, which is also influenced by religious beliefs and cultural differences. Furthermore, the detection standards of pesticide residues in edible oils in different countries and organizations are various, and subsequently, further communication and improvement is required. This will also be conducive to the global trade of EPOs. In each stage of the edible oil industry, strict monitoring of environmental conditions is required to provide a good and safe environment for the cultivation, harvesting and processing of oil crops, and ultimately to ensure the safety of edible oil production under environmentally friendly conditions.
Author Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This project was partially supported by a project of Henan Provincial Science Research (192102110174), the key project of Henan Provincial Science Research, China (202102310635), the Training Program for Young Backbone University Teachers in of Henan Province (2019GGJS049) and the talent project of Henan Agriculture University, China.
References
Ascensión, R., Isabel, S., Manuel, O., Rafael, G., Luis, L., Carmen, C.-V. (2014). Characterization of Fatty Acid Profile of Argan Oil and Other Edible Vegetable Oils by Gas Chromatography and Discriminant Analysis. J. Chem. 2014, 1–8. doi: 10.1155/2014/843908
Assmann, K. E., Adjibade, M., Hercberg, S., Galan, P., Kesse-Guyot, E. (2018). Unsaturated Fatty Acid Intakes During Midlife Are Positively Associated with Later Cognitive Function in Older Adults with Modulating Effects of Antioxidant Supplementation. J. Nutr. 148, 1938–1945. doi: 10.1093/jn/nxy206
Balta, M. F., Yarılgaç, T., Aşkın, M. A., Küçük, M., Balta, F., Özrenk, K. (2006). Determination of fatty acid compositions, oil contents and some quality traits of hazelnut genetic resources grown in eastern Anatolia of Turkey. J. Food Composition Anal. 19, 681–686. doi: 10.1016/j.jfca.2005.10.007
Bañares, C., Martin, D., Reglero, G., Torres, C. F. (2019). Protective effect of hydroxytyrosol and rosemary extract in a comparative study of the oxidative stability of Echium oil. Food Chem. 290, 316–323. doi: 10.1016/j.foodchem.2019.03
Bao, Y., Guo, Y. (2016). Optimization of Ultrasonic-Assisted Aqueous Enzymatic Extraction of Pine Nut Oil and Its Oxidative Stability. Food Sci. 27, 60–68. doi: 10.7506/spkx1002-6630-201622009
Bhat, R., Reddy, K. R. N. (2016). Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 215, 425–437. doi: 10.1016/j.foodchem.2016.07.161
Bhattacharya, A., Biswas, P., Kar, P., Roychoudhury, P., Basu, S., Ganguly, S., et al. (2017). Nitric oxide sensing by chlorophyll a. Anal. Chim. Acta 985, 101–113. doi: 10.1016/j.aca.2017.07.026
Bonte, A., Schweiger, R., Pons, C., Wagner, C., Bruhl, L., Matthaus, B., et al. (2017). Metabolic Changes during Storage of Brassica napus Seeds under Moist Conditions and the Consequences for the Sensory Quality of the Resulting Virgin Oil. J. Agric. Food Chem. 65, 11073–11084. doi: 10.1021/acs.jafc.7b04149
Buratti, S., Malegori, C., Benedetti, S., Oliveri, P., Giovanelli, G. (2018). E-nose, e-tongue and e-eye for edible olive oil characterization and shelf life assessment: A powerful data fusion approach. Talanta 182, 131–141. doi: 10.1016/j.talanta.2018.01.096
Cao, J., Zhu, J., Wang, J., Hang, S., Wen, Y., He, J., et al. (2018). Effects of Diyaling application methods on rape aphids control and on yield, pesticide residue of rapeseed. Chin. J. Oil Crop Sci. 40, 556–570. doi: 10.7505/j.issn.1007-9084.2018.04.014
Cao, Y. (2015). Research on quality characteristics of different groups of xanthoceras sorbifolia. Food Sci. Technol. 40, 40–44. doi: 10.13684/j.cnki.spkj.2015.01.009
Carvalho, A. G., Silva, K. A., Silva, L. O., Costa, A. M., Akil, E., Coelho, M. A., et al. (2019). Jussara berry (Euterpe edulis M.) oil-in-water emulsions are highly stable: the role of natural antioxidants in the fruit oil. J Sci Food Agric. 99, 90–99. doi: 10.1002/jsfa.9147
Cassidy, A., Minihane, A. M. (2017). The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 105, 10–22. doi: 10.3945/ajcn.116.136051
Chen, Y. J., Wang, L. L., Chen, X. P., Ying, L., II (2011). Oil Content and Fatty Acid Composition of Camellia oleifera Seed in Guangxi. Hum. Hered. 71, 161. doi: 1002-6630(2011)08-0172-05
Cheong, A. M., Tan, C. P., Nyam, K. L. (2018). Stability of Bioactive Compounds and Antioxidant Activities of Kenaf Seed Oil-in-Water Nanoemulsions under Different Storage Temperatures. J. Food Sci. 83, 2457–2465. doi: 10.1111/1750-3841.14332
Cini, J. R. D. M., Silva, H. C. D., Coppo, R. L., Angilelli, K. G., Maia, E. C. R., Borsato, D., et al. (2014). Oxidation kinetics of biodiesel from soybean mixed with synthetic antioxidants BHA, BHT and TBHQ: Determination of activation energy. Fuel Process. Technol. 127, 111–116. doi: 10.1016/j.fuproc.2014.05.033
Deng, X., Zhou, Y., Zheng, W., Bai, L., Zhou, X. (2018). Dissipation Dynamic and Final Residues of Oxadiargyl in Paddy Fields Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry Coupled with Modified QuEChERS Method. Int. J. Environ. Res. Public Health 15, 1680. doi: 10.3390/ijerph15081680
Esteki, M., Ahmadi, P., Vander Heyden, Y., Simal-Gandara, J. (2018). Fatty Acids-Based Quality Index to Differentiate Worldwide Commercial Pistachio Cultivars. Molecules 24, 58. doi: 10.3390/molecules24010058
Fawzy, M. (2013). Healthy blends of high linoleic sunflower oil with selected cold pressed;oils: Functionality, stability and antioxidative characteristics. Ind. Crops Products. 43, 65–72. doi: 10.1016/j.indcrop.2012.07.013
Frigo, D. E., Burow, M. E., Mitchell, K. A., Tung-Chin, C., Mclachlan, J. A. (2002). DDT and its metabolites alter gene expression in human uterine cell lines through estrogen receptor-independent mechanisms. Environ. Health Perspect. 110, 1239–1245. doi: 10.1289/ehp.021101239
Fryzova, R., Pohanka, M., Martinkova, P., Cihlarova, H., Brtnicky, M., Hladky, J., et al. (2018). Oxidative Stress and Heavy Metals in Plants. Rev. Environ. Contam. Toxicol. 245, 129–156. doi: 10.1007/398_2017_7
Fujikawa, H., Ibe, A., Wauke, T., Morozumi, S., Mori, H. (2002). Flavor production from edible oils and their constituents by Penicillium corylophilum. Shokuhin Eiseigaku Zasshi. 43, 160–164. doi: 10.3358/shokueishi.43.160
Gagic, V., Kleijn, D., Báldi, A., Boros, G., Jørgensen, H. B., Elek, Z., et al. (2017). Combined effects of agrochemicals and ecosystem services on crop yield across Europe. Ecol. Lett. 20, 1427–1436. doi: 10.1111/ele.12850
Geng, S. X., Yang, C. S., Ning, D. L., Li, Y. J., Chen, H. Y. (2018). Analysis of the oil content and its fatty acid composition of fruits for introduced olive cultivars in Yunnan Province. J. Southwest Forest. Univ. (Nat. Sci.) 38, 193–199. doi: 10.11929/j.issn.2095-1914.2018.04.030
Ghosh, M., Upadhyay, R., Mahato, D. K., Mishra, H. N. (2019). Kinetics of lipid oxidation in omega fatty acids rich blends of sunflower and sesame oils using Rancimat. Food Chem. 272, 471–477. doi: 10.1016/j.foodchem.2018.08.072
Ginter, E., Simko, V. (2016). New data on harmful effects of trans-fatty acids. Bratisl Lek Listy. 117, 251–253. doi: 10.4149/bll_2016_048
Gong, B. C., Da-Wei, L., II, Jiang, X. B., Kai-Yun, W. U., Bai, J. J., Peng, J. L. (2012). Variation Analysis of Fruit Oil Content of Idesia polycarpa Maxim. from Different Populations. Acta Botanica Boreali-Occidentalia Sinica 32, 1680–1685. doi: 1000-4025(2012)08-1680-06
Gouvinhas, I., Machado, N., Cunha, M., Pereira, M., Matos, C., Gomes, S., et al. (2015). Trace Element Content of Monovarietal and Commercial Portuguese Olive Oils. J. Oleo Sci. 64, 1083–1093. doi: 10.5650/jos.ess15101
Haase, H., Rink, L. (2014). Multiple impacts of zinc on immune function. Metallomics 6, 1175–1180. doi: 10.1039/c3mt00353a
Haman, N., Romano, A., Asaduzzaman, M., Ferrentino, G., Biasioli, F., Scampicchio, M. (2017). A microcalorimetry study on the oxidation of linoleic acid and the control of rancidity. Talanta 164, 407–412. doi: 10.1016/j.talanta.2016.12.012
Han, Y., Mo, R., Yuan, X., Zhong, D., Tang, F., Ye, C., et al. (2017). Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere 180, 42–47. doi: 10.1016/j.chemosphere.2017.03.138
Hashempour-Baltork, F., Torbati, M., Azadmard-Damirchi, S., Peter Savage, G. (2018). Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 8, 107–113. doi: 10.15171/apb.2018.013
Hayes, D., Angove, M. J., Tucci, J., Dennis, C. (2016). Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 56, 1231–1241. doi: 10.1080/10408398.2012.760516
Hu, A. P., Liu, Y. L., Shi, L. K. (2016). Widespread occurrence of phthalic acid esters in raw oilseeds in China used for edible vegetable oil production. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 33, 1421–1427. doi: 10.1080/19440049.2016.1222631
Hu, H., Liu, H., Shi, A., Liu, L., Fauconnier, M. L., Wang, Q. (2018). The Effect of Microwave Pretreatment on Micronutrient Contents, Oxidative Stability and Flavor Quality of Peanut Oil. Molecules 24, 62. doi: 10.3390/molecules2401006224
Huang, J., Zhang, T., Zhang, Q., Chen, M., Wang, Z., Zheng, B., et al. (2016). The mechanism of high contents of oil and oleic acid revealed by transcriptomic and lipidomic analysis during embryogenesis in Carya cathayensis Sarg. BMC Genomics 17, 113. doi: 10.1186/s12864-016-2434-7
Ishaq, M., Razi, R., Khan, S. A. (2017). Exploring genotypic variations for improved oil content and healthy fatty acids composition in rapeseed (Brassica napus L.). J. Sci. Food Agric. 97, 1924–1930. doi: 10.1002/jsfa.7997
Jabeur, H., Zribi, A., Abdelhedi, R., Bouaziz, M. (2015). Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 169, 289–296. doi: 10.1016/j.foodchem.2014.07.118
Ji, N., Diao, E., Li, X., Zhang, Z., Dong, H. (2016). Detoxification and safety evaluation of aflatoxin B1 in peanut oil using alkali refining. J. Sci. Food Agric. 96, 4009–4014. doi: 10.1002/jsfa.7592
Jiang, Q. (2017). Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 8, 850–867. doi: 10.3945/an.117.016329
Ju, Y. H., Vali, S. R. (2005). Rice bran oil as a potential resource for biodiesel: a review. J. Sci. Ind. Res. 64, 866–882. doi: 10.1088/0960-1317/15/11/R01
Juhaimi, F. A., Uslu, N., Özcan, M. M. (2018). The effect of preultrasonic process on oil content and fatty acid composition of hazelnut, peanut and black cumin seeds. J. Food Process. Preservation. 42, e13335. doi: 10.1111/jfpp.13335
Juranović Cindrić, I., Zeiner, M., Hlebec, D. (2018). Mineral Composition of Elements in Walnuts and Walnut Oils. Int. J. Environ. Res. Public Health 15, 2674. doi: 10.3390/ijerph15122674
Kasemsumran, S., Thanapase, W., Punsuvon, V., Ozaki, Y. (2012). A feasibility study on nondestructive determination of oil content in palm fruits by visible-near infrared spectroscopy. J. Near Infrared Spectroscopy. 20, 687–694. doi: 10.1255/jnirs.1025
Kim, S. K., Karadeniz, F. (2012). Biological importance and applications of squalene and squalane. Adv. Food. Nutr. Res. 65, 223–233. doi: 10.1016/B978-0-12-416003-3.00014-7
Kim, J., Kim, D. N., Lee, S. H., Yoo, S. H., Lee, S. (2010). Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 118, 398–402. doi: 10.1016/j.foodchem.2009.05.011
Kim, J. M., Choi, S. H., Shin, G. H., Lee, J. H., Kang, S. R., Lee, K. Y., et al. (2016). Method validation and measurement uncertainty for the simultaneous determination of synthetic phenolic antioxidants in edible oils commonly consumed in Korea. Food Chem. 213, 19–25. doi: 10.1016/j.foodchem.2016.06.053
Koubaa, M., Mhemdi, H., Barba, F. J., Roohinejad, S., Greiner, R., Vorobiev, E. (2016). Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: An overview. Food Res. Int. 85, 59–66. doi: 10.1016/j.foodres.2016.04.007
Latif, S., Anwar, F. (2011). Aqueous enzymatic sesame oil and protein extraction. Food Chem. 125, 679–684. doi: 10.1016/j.foodchem.2010.09.064
Li, Q., Zhang, X. (2018). Effect of Diseases and Insect Pests on Soybean Yield in the Top Five Soybean Producing Countries: A Review. J. Agric. 2018 (8), 23–27.
Li, L., Sun, Q. J., Xin, S. G., Yu, L., Jiang, Z. L. (2013). Detection of Phthalate Esters from Plastic Packaging Materials into Edible Oil by Gas Chromatography-Mass. Appl. Mechanics Mat. 395–396, 355–358. doi: 10.4028/www.scientific.net/AMM.395-396.355
Li, R. J., Gao, X., Li, L. M., Liu, X. L., Wang, Z. Y., Lu, S. Y. (2016). De novo Assembly and Characterization of the Fruit Transcriptome of Idesia polycarpa Reveals Candidate Genes for Lipid Biosynthesis. Front. Plant Sci. 7, 801. doi: 10.3389/fpls.2016.00801
Li, X. J., Shen, Y. B., Wu, G. C., Qi, X. G., Zhang, H., Wang, L., et al. (2016). Determination of Key Active Components in Different Edible Oils Affecting Lipid Accumulation and Reactive Oxygen Species Production in HepG2 Cells. J. Agric. Food Chem. 66, 11943–11956. doi: 10.1021/acs.jafc.8b04563
Li, Y., Yu, Z., Jin, J., Zhang, Q., Wang, G., Liu, C., et al. (2018). Impact of Elevated CO2 on Seed Quality of Soybean at the Fresh Edible and Mature Stages. Front. Plant Sci. 9, 1413. doi: 10.3389/fpls.2018.01413
Li, L. Z., Luo, Y. M., Li, R. J., Zhou, Q., Peijnenburg, W. J. G. M., Yin, N., et al. (2020). Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. doi: 10.1038/s41893-020-0567-9
Li, P. (2002). Review on Quality and Safety Standard of Oilseeds and Products in China. Rev. China Agric. Sci. Technol. 4, 20–23, 24. doi: 1008-0864(2002) 05-0020-05
Liao, B., Hao, Y., Lu, J., Bai, H., Guan, L., Zhang, T. (2018). Transcriptomic analysis of Perilla frutescens seed to insight into the biosynthesis and metabolic of unsaturated fatty acids. BMC Genomics 19, 213. doi: 10.1186/s12864-018-4595-z
Lin, P., Wang, K., Zhou, C., Xie, Y., Yao, X., Yin, H. (2018). Seed Transcriptomics Analysis in Camellia oleifera Uncovers Genes Associated with Oil Content and Fatty Acid Composition. Int. J. Mol. Sci. 19, 118. doi: 10.3390/ijms19010118
Liu, Y., Yang, Y., Hui, H. U., Liu, H., Shi, A., Liu, L., et al. (2017). Advance in flavor compounds analysis and flavor-enhancing technology of peanut oil. China Oils Fats 42, 30–34. doi: 1003-7969(2017)03-0030-05
Llorent-Martinez, E. J., Ortega-Barrales, P., Fernandez-de Cordova, M. L., Dominguez-Vidal, A., Ruiz-Medina, A. (2011). Investigation by ICP-MS of trace element levels in vegetable edible oils produced in Spain. Food Chem. 127, 1257–1262. doi: 10.1016/j.foodchem.2011.01.064
Mangin, B., Bonnafous, F., Blanchet, N., Boniface, M. C., Bret-Mestries, E., Carrere, S., et al. (2017). Genomic Prediction of Sunflower Hybrids Oil Content. Front. Plant Sci. 8, 1633d. doi: 10.3389/fpls.2017.01633d
Mao, R. F., Lang, M. F., Yu, X. Q., Wu, R. R., Yang, X. M., Guo, X. T. (2020). Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard Mater. doi: 10.1016/j.jhazmat.2020.122515
Marina, A. M., Che Man, Y. B., Nazimah, S. A. H., Amin, I. (2009). Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chemists’ Soc. 86, 301–307. doi: 10.1007/s11746-009-1351-1
Marsh, J. M., Davis, M. G., Flagler, M. J., Sun, Y., Chaudhary, T., Mamak, M., et al. (2015). Advanced hair damage model from ultra-violet radiation in the presence of copper. Int. J. Cosmet. Sci. 37, 532–541. doi: 10.1111/ics.12231
Martinchik, A. N., Baturin, A. K., Zubtsov, V. V., Viu, M. (2012). [Nutritional value and functional properties of flaxseed]. Vopr Pitan. 81, 4–10.
Matthäus, B., Özcan, M. M., Juhaimi, F. A., Adiamo, O. Q., Alsawmahi, O. N., Ghafoor, K., et al. (2018). Effect of the Harvest Time on Oil Yield, Fatty Acid, Tocopherol and Sterol Contents of Developing Almond and Walnut Kernels. J. Oleo Sci. 67, 39–45. doi: 10.5650/jos.ess17162
McLachlan, M. S. (1996). Bioaccumulation of Hydrophobic Chemicals in Agricultural Food Chains. Environ. Sci. Technol. 30, 252–259. doi: 10.1021/es9502738
Miraliakbari, H., Shahidi, F. (2008). Oxidative stability of tree nut oils. J. Agric. Food Chem. 56, 4751–4759. doi: 10.1021/jf8000982
Mmongoyo, J. A., Wu, F., Linz, J. E., Nair, M. G., Mugula, J. K., Tempelman, R. J., et al. (2017). Aflatoxin levels in sunflower seeds and cakes collected from micro- and small-scale sunflower oil processors in Tanzania. PloS One 12 (4), e0175801. doi: 10.1371/journal.pone.0175801
Movahed, S., Ghavami, M. (2007). Comparative and identification of fatty acid composition of Iranian and importing grape seed oil. Pajouhesh Sazandegi.
Nehdi, I. A., Sbihi, H. M. (2018). Chemical Composition of Date Palm (Phoenix dactylifera L.) Seed Oil from Six Saudi Arabian Cultivars. Food Chem. 83, 624–630. doi: 10.1111/1750-3841
Ning, C., Jiang, Y., Meng, J., Zhou, C., Tao, J. (2015). Herbaceous peony seed oil: A rich source of unsaturated fatty acids and γ-tocopherol. Eur. J. Lipid Sci. Technol. 117, 532–542. doi: 10.1002/ejlt.201400212
Olmo-García, L., Kessler, N., Neuweger, H., Wendt, K., Olmo-Peinado, J. M., Fernández-Gutiérrez, A., et al. (2018). Unravelling the distribution of secondary metabolites in olea europaea l.: exhaustive characterization of eight olive-tree derived matrices by complementary platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 23, 2419. doi: 10.3390/molecules23102419
Orsavova, J., Misurcova, L., Ambrozova, J. V., Vicha, R., Mlcek, J. (2015). Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 16, 12871–12890. doi: 10.3390/ijms160612871
Özcan, M. M., İMan, C., Arslan, D. (2010). Physicochemical properties, fatty acid and mineral content of some walnuts (Juglans regia L.) types. Agric. Sci. 1, 62–67. doi: 10.4236/as.2010.12009
Pereira, J. S. F., Pereira, L. S. F., Mello, P. A., Guimarães, R. C. L., Guarnieri, R. A., Fonseca, T. C. O., et al. (2014). Microwave-induced combustion of crude oil for further rare earth elements determination by USN–ICP-MS. Anal. Chim. Acta 844, 8–14. doi: 10.1016/j.aca.2014.07.043
Pospišil, M., Škevin, D., Mustapic´, Z., Nakic´, S. N., Butorac, J., Matijevic´, D., et al. (2007). Fatty acid composition in oil of recent rapeseed hybrids and 00-cultivars. Agriculturae Conspectus Sci. 72, 187–193.
Puch, F., Samsonvilleger, S., Guyonnet, D., Blachon, J. L., Rawlings, A. V., Lassel, T. (2010). Consumption of functional fermented milk containing borage oil, green tea and vitamin E enhances skin barrier function. Exp. Dermatol. 17, 668–674. doi: 10.1111/j.1600-0625.2007.00688.x
Rauf, S., Jamil, N., Tariq, S. A., Khan, M., Kausar, M., Kaya, Y. (2017). Progress in modification of sunflower oil to expand its industrial value. J. Sci. Food Agric. 97, 1997–2006. doi: 10.1002/jsfa.8214
Rueda, A., Samaniego-Sanchez, C., Olalla, M., Gimenez, R., Cabrera-Vique, C., Seiquer, I., et al. (2016). Combination of Analytical and Chemometric Methods as a Useful Tool for the Characterization of Extra Virgin Argan Oil and Other Edible Virgin Oils. Role of Polyphenols and Tocopherols. J. AOAC Int. 99, 489–494. doi: 10.5740/jaoacint.15-0121
Ryan, E., Galvin, K., O’Connor, T. P., Maguire, A. R., O’Brien, N. M. (2006). Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. Int. J. Food Sci. Nutr. 57, 219–228. doi: 10.1080/09637480600768077
Schofield, K. (2017). The Metal Neurotoxins: An Important Role in Current Human Neural Epidemics? Int. J. Environ. Res. Public Health 14, 1511. doi: 10.3390/ijerph14121511
Sena-Moreno, E., Pardo, J. E., Pardo-Giménez, A., Gómez, R., Alvarez-Ortí, M. (2016). Differences in Oils from Nuts Extracted by Means of Two Pressure Systems. Int. J. Food Properties. 19, 2750–2760. doi: 10.1080/10942912.2016.1144068
Servent, A., Boulanger, R., Davrieux, F., Pinot, M.-N., Tardan, E., Forestier-Chiron, N., et al. (2018). Assessment of cocoa (Theobroma cacao L.) butter content and composition throughout fermentations. Food Res. Int. 107, 675–682. doi: 10.1016/j.foodres.2018.02.070
Shang, X., Cheng, C., Ding, J., Guo, W. (2017). Identification of candidate genes from the SAD gene family in cotton for determination of cottonseed oil composition. Mol. Genet. Genomics 292, 173–186. doi: 10.1007/s00438-016-1265-1
Sielicka, M., Małecka, M., Purłan, M. (2014). Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 116, 388–394. doi: 10.1002/ejlt.201300356
Siger, A., Józefiak, M., Górnaś, P. (2017). Cold-pressed and hot-pressed rapeseed oil: The effects of roasting and seed moisture on the antioxi- dant activity, canolol, and tocopherol level. Acta Sci. Pol. Technol. Aliment. 16, 69–81. doi: 10.17306/J.AFS.2017.0458
Sobolev, V., Walk, T., Arias, R., Massa, A., Lamb, M. (2019). Inhibition of Aflatoxin Formation in Aspergillus Species by Peanut (Arachis hypogaea) Seed Stilbenoids in the Course of Peanut-Fungus Interaction. J. Agric. Food Chem. 67, 6212–6221. doi: 10.1021/acs.jafc.9b01969
Song, S., Zhu, K., Han, L., Sapozhnikova, Y., Zhang, Z., Yao, W. (2018). Residue Analysis of 60 Pesticides in Red Swamp Crayfish Using QuEChERS with High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 66, 5031–5038. doi: 10.1021/acs.jafc.7b05339
Sookwong, P., Mahatheeranont, S. (2017). Supercritical CO 2 Extraction of Rice Bran Oil -the Technology, Manufacture, and Applications. J. Oleo Sci. 66, 557–564. doi: 10.5650/jos.ess17019
Soria, A. C., Villamiel, M. (2010). Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 21, 323–331. doi: 10.1016/j.tifs.2010.04.003
Sui, N., Wang, Y., Liu, S. S., Yang, Z., Wang, F., Wan, S. B. (2018). Transcriptomic and Physiological Evidence for the Relationship between Unsaturated Fatty Acid and Salt Stress in Peanut. Front. Plant Sci. 9, 7. doi: 10.3389/fpls.2018.00007
Sun, J., Zhou, H. Y., Yue, M. W., Rao, Y. L., Yan, T. X., Yan, X. W. (2016). Effects of Heavy Metal Ions Stress on Seed Germination of Sesamum indicum and Its Seedling Growth. Subtropical Plant Sci. 45, 21–26. doi: 10.3969/j.issn.1009-7791.2016.01.005
Sun, R., Ye, R., Gao, L., Zhang, L., Wang, R., Mao, T., et al. (2017). Characterization and Ectopic Expression of CoWRI1, an AP2/EREBP Domain-Containing Transcription Factor from Coconut (Cocos nucifera L.) Endosperm, Changes the Seeds Oil Content in Transgenic Arabidopsis thaliana and Rice (Oryza sativa L.). Front. Plant Sci. 8, 63. doi: 10.3389/fpls.2017.00063
Sun, X. D., Yuan, X. Z., Jia, Y. B., Feng, L. J., Zhu, F. P., Dong, S. S., et al. (2020). Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. doi: 10.1038/s41565-020-0707-4
Sungur, S., Okur, R., Turgut, F. H., Ustun, I., Gokce, C. (2015). Migrated phthalate levels into edible oils. Food Addit. Contam. Part B Surveill. 8, 190–194. doi: 10.1080/19393210.2015.1041065
Syed, K., Shinwari, Z. K. (2016). Allelopathic effect of methanolic extracts of genetically modified and non-genetically modified canola on soybean. Toxicol. Ind. Health 32, 564–575. doi: 10.1177/0748233713501366
Taketani, Y., Imi, Y., Abuduli, M. (2015). [Bone and Nutrition. A novel function of phosphorus]. Clin. Calcium 25, 1015–1021.
Talpur, M. Y., Kara, H., Sherazi, S. T., Ayyildiz, H. F., Topkafa, M., Arslan, F. N., et al. (2014). Application of multivariate chemometric techniques for simultaneous determination of five parameters of cottonseed oil by single bounce attenuated total reflectance Fourier transform infrared spectroscopy. Talanta 129, 473–480. doi: 10.1016/j.talanta.2014.04.002
Teh, S. S., Birch, E. J. (2014). Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrasonics Sonochem. 21, 346–353. doi: 10.1016/j.ultsonch.2013.08.002
Tekaya, M., Chehab, H., Flamini, G., Gharbi, I., Mahjoub, Z., Laamari, S., et al. (2018). Modification of pomological characteristics and flavour components of fruits and virgin olive oil following wastewater irrigation and soil tillage. J. Sci. Food Agric. 98, 2942–2952. doi: 10.1002/jsfa.8791
Tena, N., Aparicio, R., Garcia-Gonzalez, D. L. (2018). PhotooxidationEffect in Liquid Lipid Matrices: Answers from an Innovative FTIR Spectroscopy Strategy with “Mesh Cell” Incubation. J. Agric. Food Chem. 66, 3541–3549. doi: 10.1021/acs.jafc.7b05981
Toma, W., Guimarães, L. L., Brito, A. R. M. S., Santos, A. R., Cortez, F. S., Pusceddu, F. H., et al. (2014). Safflower oil: an integrated assessment of phytochemistry, antiulcerogenic activity, and rodent and environmental toxicity. Rev. Bras. Farmacognosia. 24, 538–544. doi: 10.1016/j.bjp.2014.09.004
Vonapartis, E., Aubin, M. P., Seguin, P., Mustafa, A. F., Charron, J. B. (2014). Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Composition Anal. 39, 8–12. doi: 10.1016/j.jfca.2014.11.004
Wang, H., Wang, T., Johnson, L. A. (2010). Effects of kernel breakage and fermentation on corn germ integrity and oil quality. J. Agric. Food Chem. 58, 10039–10044. doi: 10.1021/jf101564m
Wang, Y., Sun, D., Chen, H., Qian, L., Xu, P. (2011). Fatty acid composition and antioxidant activity of tea (Camellia sinensis L.) seed oil extracted by optimized supercritical carbon dioxide. Int. J. Mol. Sci. 12, 7708–7719. doi: 10.3390/ijms12117708
Wang, M. X., Zhou, D. Y., Ma, L., Xu, S. J., Wei, S. J. (2016). Analysis and Evaluation of Fatty Acid Composition in Cottonseed Oil. Food Sci. 36, 136–141. doi: 1002-6630(2016)22-0136-06
Wang, F. C., Acevedo, N., Marangoni, A. G. (2017). Encapsulation of phytosterols and phytosterol esters in liposomes made with soy phospholipids by high pressure homogenization. Food Funct. 8, 3964–3969. doi: 10.1039/c7fo00905d
Wang, X., Zeng, Q., Del Mar Contreras, M., Wang, L. (2017). Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 102, 184–194. doi: 10.1016/j.foodres.2017.09.089
Wang, H. C., Hou, Y. T., Hsieh, B. C. (2018). Direct Photometric Assay for Copper Chlorophyll Adulterants in Edible Oil by the Aid of an Ultraviolet-Photobleaching Pretreatment. J. Agric. Food Chem. 66, 8859–8863. doi: 10.1021/acs.jafc.8b02170
Wang, X., Xu, P., Yin, L., Ren, Y., Li, S., Shi, Y., et al. (2018). Genomic and Transcriptomic Analysis Identified Gene Clusters and Candidate Genes for Oil Content in Peanut (Arachis hypogaea L.). Plant Mol. Biol. Rep. 36, 518–529. doi: 10.1007/s11105-018-1088-9
Wang, Y., Jiao, A., Chen, H., Ma, X., Cui, D., Han, B., et al. (2018). Status and factors influencing on-farm conservation of Kam Sweet Rice (Oryza sativa L.) genetic resources in southeast Guizhou Province, China. J. Ethnobiol. Ethnomed. 14, 76. doi: 10.1186/s13002-018-0256-1
Wang, Y. L., Zhu, M. T., Mei, J., Luo, S. H., Leng, T., Chen, Y., et al. (2019). Comparison of Furans Formation and Volatile Aldehydes Profiles of Four Different Vegetable Oils During Thermal Oxidation. J. Food Sci. 84, 1966–1978. doi: 10.1111/1750-3841.14659
Wei, X., Shao, X. (2018). Rapid detection of adulterated peony seed oil by electronic nose. J. Food Sci. Technol. 55, 2152–2159. doi: 10.1007/s13197-018-3132-z
Wei, Z. W., Wang, J. G. (2017). Study on the quality and bioactive substances of hot and cold-pressed sunflower oil. Cereals Oils. 30, 28–30. doi: 1008-9578(2017)05-0028-03
Wei, F., Dong, Y., Qiong, A. N., Zhang, T. (2006). Uptake and accumulation of weathered DDT by oil plants. Ecol. Environ. 15, 1188–1191. doi: 10.16258/j.cnki.1674-5906.2006.06.013
Wei, F., Dong, Y. H., Qiong, A. N., Zhang, T. L., Liu, T. (2008). Residue of Organochlorine Pesticides in Seeds of Oil and Crop Plants. Soils 40, 647–652. doi: 10.13758/j.cnki.tr.2008.04.022
Weinstock, B. A., Janni, J., Hagen, L., Wright, S. (2006). Prediction of oil and oleic acid concentrations in individual corn (Zea mays L.) kernels using near-infrared reflectance hyperspectral imaging and multivariate analysis. Appl. spectrosc. 60, 9–16. doi: 10.1366/000370206775382631
Wijewardana, C., Reddy, K. R., Bellaloui, N. (2019). Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 278, 92–100. doi: 10.1016/j.foodchem.2018.11.035
Wrather, J. A., Anderson, T. R., Arsyad, D. M., Tan, Y., Ploper, L. D., Porta-Puglia, A., et al. (2001). Soybean disease loss estimates for the top ten soybean-producing counries in 1998. Can. J. Plant Pathology. 23, 115–121. doi: 10.1080/07060660109506918
Xiao, Y., Xia, W. (2019). Genetic control of fatty acid composition in coconut (Cocos nucifera), African oil palm (Elaeis guineensis), and date palm (Phoenix dactylifera). Planta 249, 333–350. doi: 10.1007/s00425-018-3003-x
Xue, P., Zhao, Q., Sun, H., Geng, L., Yang, Z., Liu, W. (2019). Characteristics of heavy metals in soils and grains of wheat and maize from farmland irrigated with sewage. Environ. Sci. Pollut. Res. Int. 26, 5554–5563. doi: 10.1007/s11356-018-3997-4
Yang, M., Zheng, C., Zhou, Q., Huang, F., Liu, C., Wang, H. (2013). Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Composition Anal. 29, 1–9. doi: 10.1016/j.jfca.2012.08.009
Yang, C., Liu, X., Wang, S., Chen, Z., Amp, S. (2015). Volatile Flavor Compounds in Vegetable Oils Using GC Chromatography-Mass Spectrometry Combined with Solid-Phase Microextract. J. Cere. Oils Ass. 30, 127–134. doi: 1003-0174(2015)10-0127-09
Yang, Y., Song, X. X., Sui, X. N., Qi, B. K., Wang, Z. J., Li, Y., et al. (2016). Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Products. 80, 141–147. doi: 10.1016/j.indcrop.2015.11.044
Yao, Z. J. (2016). Determination of 27 Pesticide Residues in Edible Oil by GPC-GC-MS/MS. Phys. Test. Chem. Anal. 52, 778–782. doi: 10.1197/lhjy-hx201607008
Yara-Varon, E., Li, Y., Balcells, M., Canela-Garayoa, R., Fabiano-Tixier, A. S., Chemat, F. (2017). Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 22, 1474. doi: 10.3390/molecules22091474
Yi, J. Y., Kim, H. J., Chung, M. S. (2017). Manufacture of low-benzo(a)pyrene sesame seed (Sesamum indicum L. oil using a self-designed apparatus. PloS One 12, e0173585. doi: 10.1371/journal.pone.0173585
Yu, F., Li, Y., Huang, G. Q., Yang, C. F., Chen, C., Zhou, T., et al. (2020). Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere. doi: 10.1016/j.chemosphere.2020.127650
Zhang, Y., Hou, X. L. (2010). Separation and purification of nervonic acid ethyl ester from Acer truncatum Bunge seed oil. China Oils And Fats 35, 28–31. doi: 1003-7969(2010)01-0028-04
Zhang, G., Weisheng, X. U., Liu, J., Liu, Y., Wang, X. (2012). Application of flavor finger-printing technology in identification of vegetable oil. China Oils Fats 37, 65–68. doi: 1003-7969(2012)11-0065-04
Zhang, C., Selvaraj, J. N., Yang, Q., Liu, Y. (2017). A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China. Toxins 9, 40. doi: 10.3390/toxins9010040
Zhang, Q., Yu, R., Sun, D., Rahman, M. M., Xie, L., Hu, J., et al. (2018). Comparative Transcriptome Analysis Reveals an Efficient Mechanism of alpha-Linolenic Acid in Tree Peony Seeds. Int. J. Mol. Sci. 20, 65. doi: 10.3390/ijms20010065
Zhao, J., Becker, H. C., Zhang, D., Zhang, Y., Ecke, W. (2005). Oil Content in a European × Chinese Rapeseed Population. Crop Sci. 45, 51–59. doi: 10.2135/cropsci2005.0051
Zhou, N. Z., Liu, P., Su, X. C., Liao, Y. H., Lei, N. S., Liang, Y. H., et al. (2017). Low-cost humic acid-bonded silica as an effective solid-phase extraction sorbent for convenient determination of aflatoxins in edible oils. Anal. Chim. Acta 970, 38–46. doi: 10.1016/j.aca.2017.02.029
Zhu, Z. (2010). Analysis of Oil Rate and Fatty Acids Content of Idesia palycarpa Fruits from Different Geographical Populations. Sci. Silvae Sinicae. 46, 176–180. doi: 10.3724/SP.J.1206.2010.00266
Keywords: plant oil, environmental factors, oil quality, edible safety, whole-industrial-chain monitoring
Citation: Zhou Y, Zhao W, Lai Y, Zhang B and Zhang D (2020) Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 11:1315. doi: 10.3389/fpls.2020.01315
Received: 29 May 2020; Accepted: 11 August 2020;
Published: 28 August 2020.
Edited by:
Stefania De Pascale, University of Naples Federico II, ItalyCopyright © 2020 Zhou, Zhao, Lai, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Lai, eGxhaXlvbmdAMTYzLmNvbQ==; Baohong Zhang, emhhbmdiQGVjdS5lZHU=; Dangquan Zhang, emhhbmdkYW5ncXVhbkAxNjMuY29t
†These authors have contributed equally to this work
 Ying Zhou1†
Ying Zhou1† Yong Lai
Yong Lai Baohong Zhang
Baohong Zhang Dangquan Zhang
Dangquan Zhang