- 1Shandong Provincial Key Laboratory of Plant Stress, College of Life Sciences, Shandong Normal University, Jinan, China
- 2State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, China
- 3Biotechnology Research Center, Shandong Academy of Agricultural Sciences, Jinan, China
Cyanobacteria have evolved various strategies to sense and adapt to biotic and abiotic stresses including active movement. Motility in cyanobacteria utilizing the type IV pili (TFP) is useful to cope with changing environmental conditions. The model cyanobacterium Synechocystis sp. PCC 6803 (hereafter named Synechocystis) exhibits motility via TFP called thick pili, and uses it to seek out favorable light/nutrition or escape from unfavorable conditions. Recently, a number of studies on Synechocystis thick pili have been undertaken. Molecular approaches support the role of the pilin in motility, cell adhesion, metal utilization, and natural competence in Synechocystis. This review summarizes the most recent studies on the function of thick pili as well as their formation and regulation in this cyanobacterium.
Introduction
Cyanobacteria are the only prokaryotes capable of performing oxygenic photosynthesis and still significantly contribute to primary production on a global scale. They are adaptive in a wide range of ecological habitats. Certain species of cyanobacteria were observed to deal with changeable environment via type IV pili (TFP). For example, in the model cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis), phototactic motility driven by TFP allows such cyanobacterium to respond to fluctuations in the intensity and spectral quality of light (Yoshihara and Ikeuchi, 2004).
The cells of Synechocystis are covered by two distinct types of pili as extracellular appendages. One morphotype is specified as thick pili with an external diameter of 5 nm and a length of more than 2 μm, and another morphotype is defined by thin pili with a diameter of 3–4 nm and a length of less than 1 μm (Bhaya et al., 2000; Yoshihara et al., 2001). Thin pili distribute along the entire cell surface and align in bundles (Yoshihara et al., 2001). So far, genes involved in the formation of thin pili and their roles are unknown. In contrast, Synechocystis thick pili have been well dissected.
Thick pili of Synechocystis, which belong to TFP, have much in common with that of heterotrophs (Wilde and Mullineaux, 2015). Synechocystis cells use thick pili for extension, adhesion to the substrate and retraction to pull the cell across the surface. Multiple motility-related genes have been identified for the function of pili in this model organism (Yoshihara and Ikeuchi, 2004; Schuergers and Wilde, 2015). Besides motility, TFP have been shown to be involved in a range of cellular processes, such as natural transformation (NT) (Bhaya et al., 2000; Yoshihara et al., 2001, 2002), biofilm formation (Chandra et al., 2017; Allen et al., 2019), and metal acquisition (Lamb et al., 2014; Lamb and Hohmann-Marriott, 2017). The biogenesis of thick pili is regulated at multiple levels and has also been studied in Synechocystis (Kizawa et al., 2016; Gonçalves et al., 2018; Hu et al., 2018). This review summarizes recent advances on thick pili, including their function, biogenesis and regulation in this cyanobacterium.
Pilus Apparatuses and Their Encoding Genes
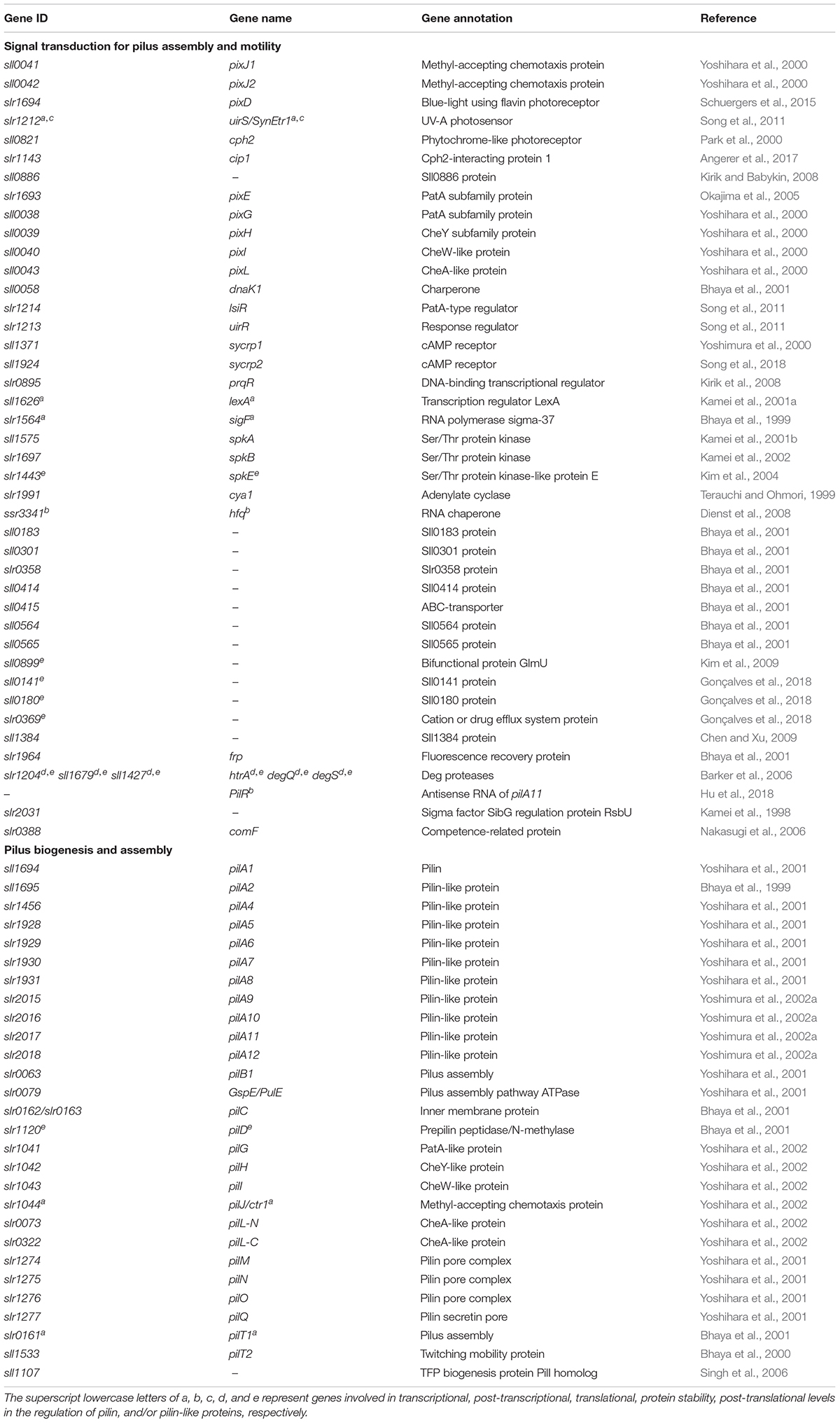
Thick pilus apparatus is built up with PilA1 and other components for biogenesis and assembly. PilA1 constitutes the major pilin subunit and anchors on the inner membrane, extends across the periplasm space, and/or the outer membrane (Wendt and Pakrasi, 2019). PilA2–PilA8 seem to be dispensable for pilus biogenesis and motility (Bhaya et al., 2000; Yoshihara et al., 2001). Whereas the specific role of these pili-like proteins is unclear. Furthermore, PilD contributes to excising the N-terminal signal peptide and methylation of PilA1 (Linhartová et al., 2014). Assembly of the pilus complex requires two ATPases, PilB, and PilT. The extension motor PilB energizes assembly of thick pili, whereas PilT is required for pilus depolymerization. PilB and PilT are located at the pilus base, while PilC is embedded in the inner membrane (Schuergers and Wilde, 2015). The RNA chaperone Hfq localizes to the pilus base via interaction with PilB1 (Schuergers et al., 2014). PilQ functions as the pore for pilus secretion across the outer membrane. PilMNO proteins are thought to connect the PilQ secretin pore with inner membrane proteins (Schuergers and Wilde, 2015; Figure 1). Besides, there might be other unknown components to be characterized in the pilus apparatus.
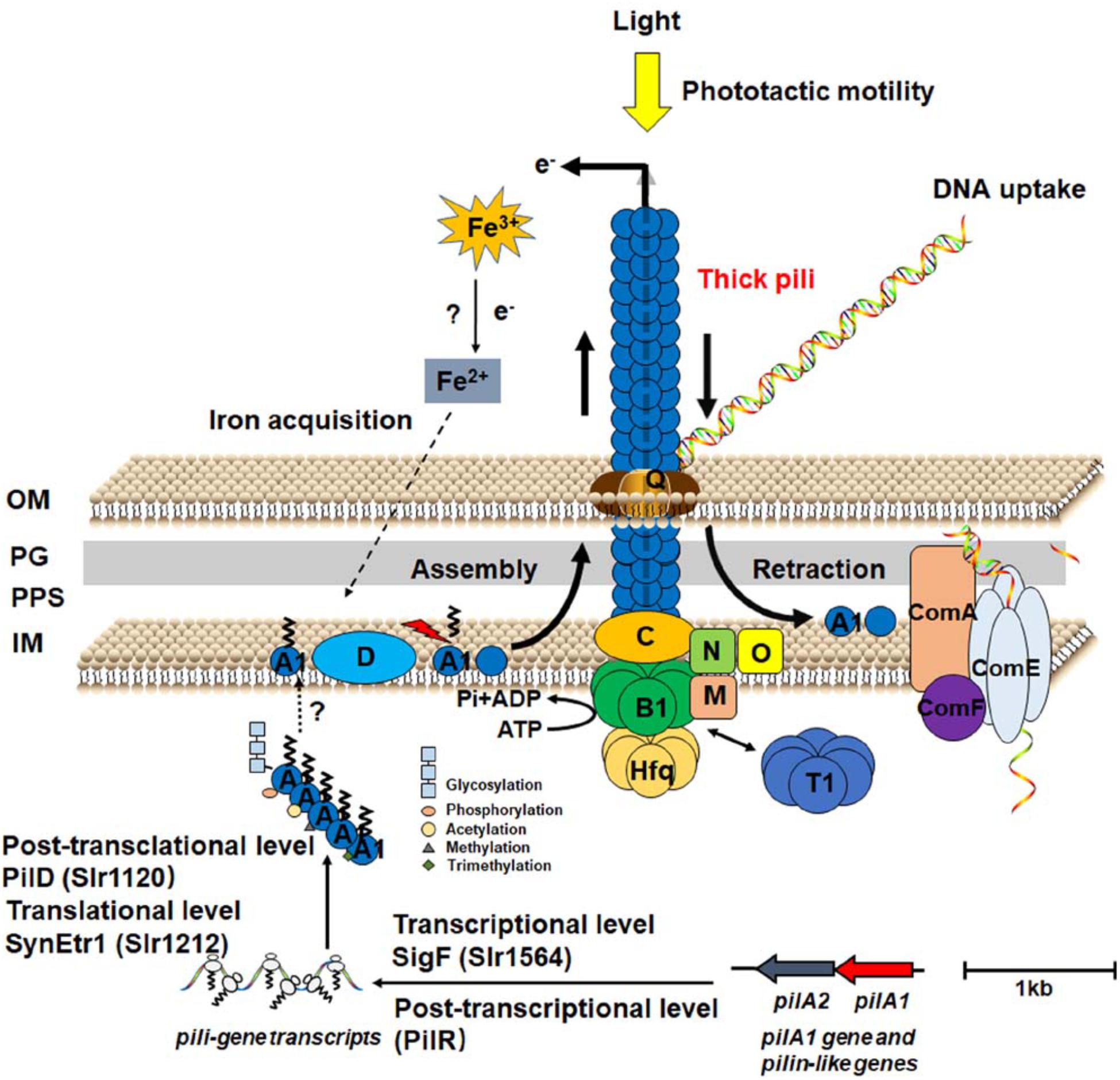
Figure 1. Schematic illustration of the assembly, retraction, function and regulation of thick pili apparatus in Synechocystis. A1, B1, C, D, O, M, N, Q, and T1 are short for Pil-related proteins. OM, outer membrane; PG, peptidoglycan; PPS, periplasmic space; IM, inner membrane; SynEtr1, ethylene receptor; SigF, sigma factor; PilR, antisense RNA of PilA11; Hfq, RNA chaperone; ComA/E/F, competence protein.
Motility and Phototaxis
Motility, including both gliding and twitching motility, is a frequently observed and beneficial feature among cyanobacteria. The cells of Synechocystis sense and shift their location via twitching motility in response to environmental stimuli. TFP are extended by PilB, then adhere to the substrate. Next, pilus retraction is induced by PilT, and cells are pulled across the surface (Burrows, 2012). Thick pilus retraction drags Synechocystis cells in a jerky motion across moist surfaces (Burriesci and Bhaya, 2008; Wilde and Mullineaux, 2015). The direction of movement correlates with localization of PilB patches at the inner membrane (Schuergers et al., 2015). The identification of key genes involved in the motility of Synechocystis began from the observation of some spontaneous mutants with different motility (Tajima et al., 2011; Kanesaki et al., 2012; Trautmann et al., 2012; Morris et al., 2014; Ding et al., 2015). There are two distinct major groups of Synechocystis substrains: the motile PCC and non-motile GT-lineages (Morris et al., 2017). Genes critical for motility and pilus biosynthesis may have mutations among strains, for instance a frameshift mutation in pilC (Bhaya et al., 2000), insertion in spkA, deletion upstream and within slr2031, and/or deletion of a single nucleotide within slr0322 (Ding et al., 2015). Widespread genomic variations may contribute to the discovery of Synechocystis strain-specific mutations related to motility.
Phototactic movement, also known as phototaxis, mediated by thick pili allows Synechocystis to move either toward or away from a light source according to light wavelength and intensity. Synechocystis harbors a variety of photoreceptors to perceive and respond to the direction, quantity, and quality of illumination (Choi et al., 1999; Ng et al., 2003). Photoreceptors responsible for phototaxis include blue/green light-absorbing proteins PixJ1 and Cph2, blue-light receptor PixD, and UV-A receptor UirS (also known as ethylene receptor, SynEtr1) (Yoshihara et al., 2000; Wilde et al., 2002; Okajima et al., 2005; Song et al., 2011; Sugimoto et al., 2017). Analysis of phototactic movement on low concentration of agar or agarose plates can be achieved at macroscopic and microscopic scales, which represents group behavior and single-cell motility, respectively (Jakob et al., 2017). Individual cells directly and accurately sense the position of light rather than respond to a spatiotemporal gradient in light intensity. During this process, Synechocystis cells act as spherical microlenses sensing and moving toward the light source (Schuergers et al., 2016). By fluorescent labeling of thick pili, quantitative analyses of cell tracking indicate asymmetric distribution of cells along the light axis for directional cell motility (Nakane and Nishizaka, 2017). However, the details of Synechocystis phototaxis need to be further explored.
Cell Adhesion
Synechocystis may transit from motile states to cell adhesive states, like flocculation and sessile biofilm. These processes include cell-cell and cell-substrate adhesion through cell surface fractions, such as TFP. TFP in Synechocystis have recently been revealed to be responsible for biofilm and floc formation (Allen et al., 2019; Conradi et al., 2019). Mutants lacking TFP were unable to aggregate, and loss of pilC was shown to significantly reduce biofilm formation and prevent flocculation as well. Except for PilC, the PilB1 and PilT1 proteins are required for flocculation, but PilA1 may be completely absent, indicating the active cycles of thick pilus extension and retraction is dispensable for flocculation. Besides, the minor pilins encoded by the pilA9-slr2019 operon have been implicated in cell-cell adhesion in flocculation and the processes switching between cell-cell adhesion and cell surface adhesion depend on cyclic AMP (cAMP) levels (Chandra et al., 2017). It was speculated that alteration of surface-attached behavior responsible by TFP might confer Synechocystis the resistance to adverse stimuli during the environmental adaptation.
Natural Competence
Natural transformation is a generally conserved mechanism of horizontal gene transfer among bacteria (Lorenz and Wackernagel, 1994). Natural competence in Synechocystis was reported in the late 1980s (Williams, 1988), making this alga as an important model cyanobacterial strain for molecular genetic studies. The process of NT in bacteria mainly includes DNA uptake, transport, processing, and recombination (Johnston et al., 2014). Furthermore, genomic analysis in cyanobacteria has cataloged the genes that are involved in NT post entry of the DNA into the periplasm in a similar manner (Cassier-Chauvat et al., 2016). Synechocystis TFP have been proven to be crucial for the uptake of extracellular DNA from the extracellular milieu (Yoshihara et al., 2001). Subsequently, PilQ is found to bind DNA for transport into the periplasm, one strand of the dsDNA is then degraded by an endonuclease, and the other strand is transported across the inner membrane via the competence gene products (Com) ComA (Slr0197), ComE (Sll1929), and ComF (Yoshihara et al., 2001; Nakasugi et al., 2006). After internalization, the ssDNA strand may be bound by recombination mediator proteins, which would recruit recombinases into a complex to promote homologous recombination (Salleh et al., 2019). Nevertheless, many details of these processes are still unclear in Synechocystis.
Recently, this conserved mechanism of horizontal gene transfer by TFP competence pili during NT has been revealed in Vibrio cholerae (Ellison et al., 2018). The surface pili directly binding to DNA via their tips and then internalizing DNA through retraction could be observed using fluorescent probes. It is likely that similar processes occur during DNA uptake in Synechocystis. Besides TFP, NT of Synechocystis is also affected by the physiological factors, including phase of cell growth, foreign DNA concentration and incubation time of cells and DNA (Zang et al., 2007). Genome analysis deciphered that pilus structural and assembly proteins and Com proteins are highly conserved across the known species of competent cyanobacteria species (Wendt and Pakrasi, 2019). For instance, the introduction of TFP assembly protein PilN from Synechococcus elongatus PCC 7942 into S. elongatus UTEX 2973 successfully recovered its natural transformability (Li et al., 2018). Whereas, mutants defective in comF were not transformable, confirming its role in natural transformation. The transcripts of comA in such mutants was not significantly affected, indicating an alternative pathway independent of ComA may exist in Synechocystis (Nakasugi et al., 2006). Therefore, to a large extent, the presence of these conserved proteins may explain why some cyanobacterial species are naturally competent whereas others are not.
Metal Utilization
Being considered as microbial nanowires, TFP may enable metal acquisition for microbial cells and facilitate electron donation to extracellular electron acceptors in some bacteria such as Shewanella oneidensis MR–1 (Gorby et al., 2006). Nevertheless, Pirbadian et al. (2014) found that S. oneidensis does not produce conductive pili. Recently, it was revealed that microbial nanowires in G. sulfurreducens that were earlier thought to be TFP were assembled by polymerized chains of the hexaheme cytochrome OmcS (Wang et al., 2019). Later, Lovley and Walker (2019) evaluate the available evidence on the in vivo expression of electrically conductive pili and OmcS filaments and support that both of these two proteins are required for electron transfer in G. sulfurreducens. Electrically conductive Synechocystis nanowires have also been investigated using scanning tunneling microscopy (Gorby et al., 2006) and subsequently conductive atomic force microscopy (Sure et al., 2015). Further evidences should be provided to determine whether nanowires in this strain are assembled by PilA1. In addition, Synechocystis ΔpilA1 mutants exhibit slower growth rates than wild type on oxidized iron minerals, indicating the role of PilA1 in electron transport to iron oxides. Physiological and spectroscopic data suggested the role of Synechocystis PilA1 in oxidization of iron minerals (Lamb et al., 2014), enhancement of manganese acquisition (Lamb and Hohmann-Marriott, 2017) and non-metallic element arsenic deposition (Sure et al., 2016).
Genes Involved in Pilin Regulation
Comparative genomic analysis clearly showed that genes encoding core components of TFP display high sequence similarity among divergent bacterial groups (Pelicic, 2008). The Synechocystis genome contains pilin and pilin-like genes that are organized mainly in polycistronic operons (Kaneko et al., 1996). Table 1 summarizes genes involved in motility, including regulatory pilin genes (Supplementary Figure S1A).
To date, several genes reported to regulate the expression of pilA1 at the transcriptional level have been studied ctr1 (slr1044), which encodes a putative methyl-accepting chemotaxis protein (MCP) (Chung et al., 2001); sigF (slr1564), which encodes an alternative sigma factor (Bhaya et al., 1999); pilT1 (slr0161), which is responsible for pilus depolymerization (Bhaya et al., 2000); lexA (sll1626), which is involved in cell motility (Kamei et al., 2001a). Of these, Ctr1 protein functions as a transducer, SigF and LexA may play roles as transcriptional factors that positively regulates the expression of pilA1 (Asayama and Imamura, 2008; Kizawa et al., 2016). While the pilA1 transcript influenced by PilT1 has not been revealed.
In addition, the RNA chaperone Hfq (Ssr3341) was predicted to play important roles in phototaxis in post-transcriptional regulation in Synechocystis (Schuergers et al., 2014). The antisense RNA PilR acts as a direct negative regulator of PilA11 and cell motility (Hu et al., 2018). Disruption in the second ethylene-binding domain of ethylene receptor, named SynEtr1ΔTM2 cells, showed a large increase in both transcriptional and translational levels of PilA1 (Lacey and Binder, 2016). The ΔDeg (slr1204/sll1679/sll1427) mutant appeared to be hyperpiliated with thick pili, indicating the regulation of PilA1 stability by Deg proteases (Barker et al., 2006).
Moreover, post-translational modifications (PTMs) have been characterized as one of the most important factors in Synechocystis pilus function over the past two decades. Various PTMs of pilin in Synechocystis, have also been proposed or verified to be important factors in phototactic movement (Sergeyenko and Los, 2000; Kim et al., 2004, 2009, 2011). For instance, Kamei et al. (2001b) found that six Serine/Threonine protein kinases participate in cell motility control, and phosphorylation participates in the phototactic movement process. Later, it was interesting to identify pilin phosphorylated at Serine 59 (Chen et al., 2015) and acetylated at lysine 58 (Mo et al., 2015), implying cross-talk between serine phosphorylation and lysine acetylation. PilD (Slr1120) has been predicted to be responsible for pre-pilin leader peptide cleavage as well as N-terminal methylation (Bhaya et al., 2001). PilD was also identified to play a role in PilA1 glycosylation (Linhartová et al., 2014). Pilin has been confirmed to be glycosylated in Neisseria (Chamot-Rooke et al., 2007) and Pseudomonas (Voisin et al., 2007), and several pilin-glycosylation genes have also been characterized in Synechocystis. Notably, Kim et al. (2011) discovered trimethylation at the C-terminal lysine and O-glycosylation within the pilus peptide in Synechocystis, indicating an indispensable role of PTMs for pilus assembly and pilus-mediated motility. sll0899 is involved in O-glycosylation between amino acids 67 and 75 in pilin and inactivation of sll0899 produces increased molecular mass of pilins (Kim et al., 2009). sll0141, sll0180, and slr0369 genes, which encoded putative inner membrane translocase components of TolC-mediated secretion, are also responsible for pilus glycosylation. Nevertheless, motility assays confirmed that Sll0141 and Slr0369 are not essential for motility (Gonçalves et al., 2018). The three Deg mutants with less fucose in cells were found to have impaired motility, implying impairment of PilA1 glycosylation (Cheregi et al., 2015). Additionally, other types of PTMs and functional associations with PilA1 may exist in Synechocystis, similar to other bacteria (Stimson et al., 1996; Naessan et al., 2008). Moreover, it will be more meaningful to dissect the specific role of such modification sites on the mature pili in this model organism.
Motility Signaling Pathway
Currently, increasing evidence suggest that Synechocystis motility is associated with several signaling pathways (Supplementary Figure S1B), for example the cAMP and/or cyclic-di-GMP-mediated pathway (Bhaya et al., 2006; Yoshimura et al., 2010; Savakis et al., 2012; Xie et al., 2018). Two cAMP receptor-like proteins, named as Sycrp2 and Sycrp1, are known to be involved in twitching motility (Song et al., 2018). Sycrp1 has binding affinity for cAMP and directly binds to the upstream region of slr1667, and positively regulates the expression of pilA9–pilA10–pilA11–slr2018 gene cluster (Yoshimura et al., 2002a). Sycrp2 does not bind cAMP (Yoshimura et al., 2000, 2002b) but may interact and work with Sycrp1 without functional redundancy (Song et al., 2018). Photoreceptor Cph2 with the GGDEF domain acts as a blue-light triggered c-di-GMP producer and thereby inhibits cell motility in blue light. Furthermore, Cph2 modulates motility by interacting with Cip1 (Slr1143, Cph2-interacting protein 1) under red light (Angerer et al., 2017). MCP–CheA–CheY systems include sll0038–sll0043, slr1041–slr1044 and slr0322, slr0073, sll1291–sll1296 three gene clusters (Chung et al., 2001; Yoshihara et al., 2002). Jakob et al. (2019) have confirmed the direct interaction of the PixD-PixE complex with PilB1, suggesting that blue-light dependent negative phototaxis is controlled by the PixD-PixE signal transduction system. Using a computer-assisted video microscope motion analysis system, researchers found that Ca2+ plays a significant role in regulating Synechocystis photo-orientation and motility (Moon et al., 2004).
Additionally, histidine kinases (Hik18, Hik36, and Hik43) were predicted or demonstrated to be involved in phototaxis (Xu and Wang, 2019). Hik18 regulates positive phototaxis by suppressing pilus biosynthesis and expression of regulatory genes through the interplay with positive phototaxis/motility two-component proteins (Shin et al., 2008). An ethylene-responsive signaling pathway affecting phototaxis was also characterized in Synechocystis (Kuchmina et al., 2017). Endogenous ethylene produced by heterologous expression of the Pseudomonas syringae ethylene-forming enzyme accelerates positive phototaxis. Ethylene mainly inactivates transcription from the csiR1/lsiR promoter. This promoter is under the control of UirS and its response regulator UirR. Synechocystis might use ethylene as an environmental signal in aquatic environments (Lacey and Binder, 2016). Further details of signal transduction regulating motility in Synechocystis remain to be explored.
Perspective
There is substantial investigation of biological function of the thick pili in the model cyanobacterium Synechocystis. For instance, emerging evidence suggest the signaling pathway of motility is mediated by thick pili, which actively sense and respond to several environmental conditions. Apart from the subunit of the thick pilus apparatus, some regulatory proteins and small RNAs involved in these processes are likely to directly or indirectly influence thick pilus biogenesis and function. To uncover these regulation of pilus genes or their coding products, we may resort to mutant library and high-throughput “-omics” to facilitate elucidation of pilus-involved signaling pathways. In addition, thick pili of Synechocystis may also be significantly used in large-scale applications for biofuel biotechnology owing to their characteristics of biofilm formation and adhesion, which will render cell immobilization, biomass harvesting, and product purification more convenient. Although there is a contentious debate over microorganisms which can produce conductive pili, the studies on the Synechocystis thick pili may also have practical applications in the sustainable composite materials, and further bioavailability of metals in the future.
Author Contributions
ZC designed and wrote the manuscript. XL revised the figures. YZ, XT, and BW made major revisions of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31600286), Open Funding Project of the State Key Laboratory of Biocatalysis and Enzyme Engineering (SKLBEE2018007), the China Postdoctoral Science Foundation (2017M610443), the Youth Foundation of Shandong Academy of Agricultural Sciences (2016YQN33), and the Development Plan for Youth Innovation Team of Shandong Provincial (2019KJE012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Christina Croney, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00241/full#supplementary-material
FIGURE S1 | Schematic illustration of genes involved in pilin regulation at different levels (A) and three potential signal pathways involved in motility in Synechocystis (B).
References
Allen, R., Rittmann, B. E., and Curtiss, R. (2019). Axenic biofilm formation and aggregation by Synechocystis sp. strain PCC 6803 are induced by changes in nutrient concentration and require cell surface structures. Appl. Environ. Microbiol. 85:e02192-18. doi: 10.1128/AEM.02192-18
Angerer, V., Schwenk, P., Wallner, T., Kaever, V., Hiltbrunner, A., and Wilde, A. (2017). The protein Slr1143 is an active diguanylate cyclase in Synechocystis sp. PCC 6803 and interacts with the photoreceptor Cph2. Microbiology 163, 920–930. doi: 10.1099/mic.0.000475
Asayama, M., and Imamura, S. (2008). Stringent promoter recognition and autoregulation by the group 3 sigma-factor SigF in the cyanobacterium Synechocystis sp. strain PCC 6803. Nucleic Acids Res. 36, 5297–5305. doi: 10.1093/nar/gkn453
Barker, M., de Vries, R., Nield, J., Komenda, J., and Nixon, P. J. (2006). The deg proteases protect Synechocystis sp. PCC 6803 during heat and light stresses but are not essential for removal of damaged D1 protein during the photosystem two repair cycle. J. Biol. Chem. 281, 30347–30355. doi: 10.1074/jbc.M601064200
Bhaya, D., Bianco, N. R., Bryant, D., and Grossman, A. (2000). Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Microbiol. 37, 941–951. doi: 10.1046/j.1365-2958.2000.02068.x
Bhaya, D., Nakasugi, K., Fazeli, F., and Burriesci, M. S. (2006). Phototaxis and impaired motility in adenylyl cyclase and cyclase receptor protein mutants of Synechocystis sp. strain PCC 6803. J. Bacteriol. 188, 7306–7310. doi: 10.1128/JB.00573-06
Bhaya, D., Takahashi, A., Shahi, P., and Grossman, A. R. (2001). Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 183, 6140–6143. doi: 10.1128/JB.183.20.6140-6143.2001
Bhaya, D., Watanabe, N., Ogawa, T., and Grossman, A. R. (1999). The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 96, 3188–3193. doi: 10.1073/pnas.96.6.3188
Burriesci, M., and Bhaya, D. (2008). Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC 6803. J. Photochem. Photobiol. B 91, 77–86. doi: 10.1016/j.jphotobiol.2008.01.012
Burrows, L. L. (2012). Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66, 493–520. doi: 10.1146/annurev-micro-092611-150055
Cassier-Chauvat, C., Veaudor, T., and Chauvat, F. (2016). Comparative genomics of DNA recombination and repair in cyanobacteria: biotechnological implications. Front. Microbiol. 7:1809. doi: 10.3389/fmicb.2016.01809
Chamot-Rooke, J., Rousseau, B., Lanternier, F., Mikaty, G., Mairey, E., Malosse, C., et al. (2007). Alternative Neisseria spp. type IV pilin glycosylation with a glyceramido acetamido trideoxyhexose residue. Proc. Natl. Acad. Sci. U.S.A. 104, 14783–14788. doi: 10.1073/pnas.0705335104
Chandra, A., Joubert, L. M., and Bhaya, D. (2017). Modulation of type IV pili phenotypic plasticity through a novel 7 chaperone-usher system in Synechocystis. BioRxiv [Preprint] doi: 10.1101/130278
Chen, Z., and Xu, X. D. (2009). DnaJ-like protein gene sll1384 is involved in phototaxis in Synechocystis sp. PCC 6803. Chin. Sci. Bull. 54, 4381–4386. doi: 10.1007/sll434-009-0674-5
Chen, Z., Zhan, J., Chen, Y., Yang, M. K., He, C. L., Ge, F., et al. (2015). Effects of β subunits of phycocyanins phosphorylation on state transition in the model cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 56, 1997–2013. doi: 10.1093/pcp/pcv118
Cheregi, O., Miranda, H., Gröbner, G., and Funk, C. (2015). Inactivation of the Deg protease family in the cyanobacterium Synechocystis sp. PCC 6803 has impact on the outer cell layers. J. Photochem. Photobiol. B 152, 383–394. doi: 10.1016/j.jphotobiol.2015.05.007
Choi, J. S., Chung, Y. H., Moon, Y. J., Kim, C., Watanabe, M., Song, P. S., et al. (1999). Photomovement of the gliding cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. 70, 95–102. doi: 10.1111/j.1751-1097.1999.tb01954.x
Chung, Y. H., Cho, M. S., Moon, Y. J., Choi, J. S., Yoo, Y. C., Park, Y. I., et al. (2001). ctr1, a gene involved in a signal transduction pathway of the gliding motility in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 492, 33–38. doi: 10.1016/S0014-5793(01)02227-X
Conradi, F. D., Zhou, R. Q., Oeser, S., Schuergers, N., Wilde, A., and Mullineaux, C. W. (2019). Factors controlling floc formation and structure in the cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 201:e00344-19. doi: 10.1128/JB.00344-19
Dienst, D., Duhring, U., Mollenkopf, H. J., Vogel, J., Golecki, J., Hess, W. R., et al. (2008). The cyanobacterial homologue of the RNA chaperone Hfq is essential for motility of Synechocystis sp. PCC 6803. Microbiology 154, 3134–3143. doi: 10.1099/mic.0.2008/020222-0
Ding, Q. L., Chen, G., Wang, Y. L., and Dong, W. (2015). Identification of specific variations in a non-motile strain of cyanobacterium Synechocystis sp. PCC 6803 originated from ATCC 27184 by whole genome resequencing. Int. J. Mol. Sci. 16, 24081–24093. doi: 10.3390/ijms161024081
Ellison, C. K., Dalia, T. N., Ceballos, A. V., Wang, J. C. Y., Biais, N., Brun, Y. V., et al. (2018). Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat. Microbiol. 3, 773–780. doi: 10.1038/s41564-018-0174-y
Gonçalves, C. F., Pacheco, C. C., Tamagnini, P., and Oliveira, P. (2018). Identification of inner membrane translocase components of TolC-mediated secretion in the cyanobacterium Synechocystis sp. PCC 6803. Environ. Microbiol. 20, 2354–2369. doi: 10.1111/1462-2920.14095
Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnalkova, A., et al. (2006). Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. U.S.A. 103, 11358–11363.
Hu, J. L., Zhan, J., Chen, H., He, C., Cang, H., and Wang, Q. (2018). The small regulatory antisense RNA PilR affects pilus formation and cell motility by negatively regulating pilA11 in Synechocystis sp. PCC 6803. Front. Microbiol. 9:786. doi: 10.3389/fmicb.2018.00786
Jakob, A., Nakamura, H., Kobayashi, A., Sugimoto, Y., Wilde, A., and Masuda, S. (2019). The (PATAN)-CheY-like response regulator PixE interacts with the motor ATPase PilB1 to control negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. doi: 10.1093/pcp/pcz194 [Epub ahead of print].
Jakob, A., Schuergers, N., and Wilde, A. (2017). Phototaxis assays of Synechocystis sp. PCC 6803 at macroscopic and microscopic scales. BioProtoc. 7:e2328. doi: 10.21769/BioProtoc.2328
Johnston, C., Martin, B., Fichant, G., Polard, P., and Claverys, J. P. (2014). Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196. doi: 10.1038/nrmicro3199
Kamei, A., Hihara, Y., Geng, X. X., Kanehisa, M., and Ikeuchi, M. (2001a). Functional analysis of lexA-like gene, sll1626 in Synechocystis sp. PCC 6803 using DNA microarray. Photosynth. Res. 69, 273–274. doi: 10.1071/sa0403733
Kamei, A., Yuasa, T., Geng, X., and Ikeuchi, M. (2002). Biochemical examination of the potential eukaryotic-type protein kinase genes in the complete genome of the unicellular cyanobacterium Synechocystis sp. PCC 6803. DNA Res. 9, 71–78. doi: 10.1093/dnares/9.3.71
Kamei, A., Yuasa, T., Orikawa, K., Geng, X. X., and Ikeuchi, M. (2001b). A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 183, 1505–1510. doi: 10.1128/JB.183.5.1505-1510.2001
Kamei, A., Ogawa, T., and Ikeuchi, M. (1998). “Identification of a novel gene (slr2031) involved in high-light resistance in the cyanobacterium Synechocystis sp. PCC 6803,” in Photosynthesis: Mechanisms and Effects, ed. G. Garab (Dordrecht: Kluwer Academic Publishers), 2901–2905. doi: 10.1007/978-94-011-3953-3_680
Kaneko, T., Sato, S., Kotani, H., Tanaka, A., Asamizu, E., Nakamura, Y., et al. (1996). Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3, 109–136. doi: 10.1093/dnares/3.3.185
Kanesaki, Y., Shiwa, Y., Tajima, N., Suzuki, M., Watanabe, S., Sato, N., et al. (2012). Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 19, 67–79. doi: 10.1093/dnares/dsr042
Kim, Y. H., Kim, J. Y., Kim, S. Y., Lee, J. H., Lee, J. S., Chung, Y. H., et al. (2009). Alteration in the glycan pattern of pilin in a nonmotile mutant of Synechocystis sp. PCC 6803. Proteomics 9, 1075–1086. doi: 10.1002/pmic.200800372
Kim, Y. H., Park, K. H., Kim, S. Y., Ji, E. S., Kim, J. Y., Lee, S. K., et al. (2011). Identification of trimethylation at C-terminal lysine of pilin in the cyanobacterium Synechocystis PCC 6803. Biochem. Biophys. Res. Commun. 404, 587–592. doi: 10.1016/j.bbrc.2010.11.133
Kim, Y. H., Park, Y. M., Kim, S. J., Park, Y. I., Choi, J. S., and Chung, Y. H. (2004). The role of Slr1443 in pilus biogenesis in Synechocystis sp. PCC 6803: involvement in post-translational modification of pilins. Biochem. Biophys. Res. Commun. 315, 179–186. doi: 10.1016/j.bbrc.2004.01.036
Kirik, I. A., and Babykin, M. M. (2008). The sll0886 gene, controlling light-activated heterotrophic growth, is involved in regulation of phototaxis in cyanobacterium Synechocystis sp. PCC 6803. Russ. J. Genet. 44, 623–626. doi: 10.1134/s1022795408050177
Kirik, I. A., Nefedova, L. N., Fantin, Y. S., and Babykin, M. M. (2008). Inversion of phototaxis in cells of Synechocystis sp PCC 6803 determined by a mutation in the regulatory gene prqR. Russ. J. Genet. 44, 405–412. doi: 10.1134/S1022795408040042
Kizawa, A., Kawahara, A., Takimura, Y., Nishiyama, Y., and Hihara, Y. (2016). RNA-seq profiling reveals novel target genes of lexA in the cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 7:193. doi: 10.3389/fmicb.2016.00193
Kuchmina, E., Klähn, S., Jakob, A., Bigott, W., Enke, H., Dühring, U., et al. (2017). Ethylene production in Synechocystis sp. PCC 6803 promotes phototactic movement. Microbiology 163, 1937–1945. doi: 10.1099/mic.0.000564
Lacey, R. F., and Binder, B. M. (2016). Ethylene regulates the physiology of the cyanobacterium Synechocystis sp. PCC 6803 via an ethylene receptor. Plant Physiol. 171, 2798–2809. doi: 10.1104/pp.16.00602
Lamb, J. J., Hill, R. E., Eaton-Rye, J. J., and Hohmann-Marriott, M. F. (2014). Functional role of PilA in iron acquisition in the cyanobacterium Synechocystis sp. PCC 6803. PLoS One 9:e105761. doi: 10.1371/journal.pone.0105761
Lamb, J. J., and Hohmann-Marriott, M. F. (2017). Manganese acquisition is facilitated by PilA in the cyanobacterium Synechocystis sp. PCC 6803. PLoS One 12:e0184685. doi: 10.1371/journal.pone.0184685
Li, S. B., Sun, T., Xu, C. X., Chen, L., and Zhang, W. W. (2018). Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab. Eng. 48, 163–174. doi: 10.1016/j.ymben.2018.06.002
Linhartová, M., Bučinská, L., Halada, P., Ječmen, T., Šetlík, J., Komenda, J., et al. (2014). Accumulation of the Type IV prepilin triggers degradation of SecY and YidC and inhibits synthesis of Photosystem II proteins in the cyanobacterium Synechocystis PCC 6803. Mol. Microbiol. 93, 1207–1223. doi: 10.1111/mmi.12730
Lorenz, M. G., and Wackernagel, W. (1994). Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58, 563–602.
Lovley, D. R., and Walker, D. J. F. (2019). Geobacter protein nanowires. Front. Microbiol. 10:2078. doi: 10.3389/fmicb.2019.02078
Mo, R., Yang, M., Chen, Z., Cheng, Z., Yi, X., Li, C., et al. (2015). Acetylome analysis reveals the involvement of lysine acetylation in photosynthesis and carbon metabolism in the model cyanobacterium Synechocystis sp. PCC 6803. J. Proteome Res. 14, 1275–1286. doi: 10.1021/pr501275a
Moon, Y. J., Park, Y. M., Chung, Y. H., and Choi, J. S. (2004). Calcium is involved in photomovement of cyanobacterium Synechocystis sp PCC 6803. Photochem. Photobiol. 79, 114–119. doi: 10.1111/j.1751-1097.2004.tb09865.x
Morris, J. N., Crawford, T. S., Jeffs, A., Stockwell, P. A., Eaton-Rye, J. J., and Summerfield, T. C. (2014). Whole genome re-sequencing of two ‘wild-type’ strains of the model cyanobacterium Synechocystis sp. PCC 6803. N. Z. J. Bot. 52, 36–47. doi: 10.1080/0028825X.2013.846267
Morris, J. N., Eaton-Rye, J. J., and Summerfield, T. C. (2017). Phenotypic variation in wild-type substrains of the model cyanobacterium Synechocystis sp. PCC 6803. N. Z. J. Bot. 55, 25–35. doi: 10.1080/0028825X.2016.1231124
Naessan, C. L., Egge-Jacobsen, W., Heiniger, R. W., Wolfgang, M. C., Aas, F. E., Røhr, A., et al. (2008). Genetic and functional analyses of PptA, a phospho-form transferase targeting type IV pili in Neisseria gonorrhoeae. J. Bacteriol. 190, 387–400. doi: 10.1128/JB.00765-07
Nakane, D., and Nishizaka, T. (2017). Asymmetric distribution of type IV pili triggered by directional light in unicellular cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 114, 6593–6598. doi: 10.1073/pnas.1702395114
Nakasugi, K., Svenson, C. J., and Neilan, B. A. (2006). The competence gene, comF, from Synechocystis sp. strain PCC 6803 is involved in natural transformation, phototactic motility and piliation. Microbiology 152, 3623–3631. doi: 10.1099/mic.0.29189-0
Ng, W. O., Grossman, A. R., and Bhaya, D. (2003). Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J. Bacteriol. 185, 1599–1607. doi: 10.1128/jb.185.5.1599-1607.2003
Okajima, K., Yoshihara, S., Fukushima, Y., Geng, X., Katayama, M., Higashi, S., et al. (2005). Biochemical and functional characterization of BLUF-type flavin-binding proteins of two species of cyanobacteria. J. Biochem. 137, 741–750. doi: 10.1093/jb/mvi089
Park, C. M., Kim, J. I., Yang, S. S., Kang, J. G., Kang, J. H., Shim, J. Y., et al. (2000). A second photochromic bacteriophytochrome from Synechocystis sp PCC 6803: spectral analysis and down-regulation by light. Biochemistry 39, 10840–10847. doi: 10.1021/bi992831r
Pelicic, V. (2008). Type IV pili: E pluribus unum? Mol. Microbiol. 68, 827–837. doi: 10.1111/j.1365-2958.2008.06197.x
Pirbadian, S., Barchinger, S. E., Leung, K. M., Byun, H. S., Jangir, Y., Bouhenni, R. A., et al. (2014). Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. U.S.A. 111, 12883–12888. doi: 10.1073/pnas.1410551111
Salleh, M. Z., Karuppiah, V., Snee, M., Thistlethwaite, A., Levy, C. W., Knight, D., et al. (2019). Structure and properties of a natural competence-associated pilin suggest a unique pilus tip-associated DNA receptor. mBio 10:e00614-19. doi: 10.1128/mBio.00614-19
Savakis, P., De Causmaecker, S., Angerer, V., Ruppert, U., Anders, K., Essen, L. O., et al. (2012). Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 85, 239–251. doi: 10.1111/j.1365-2958.2012.08106.x
Schuergers, N., Lenn, T., Kampmann, R., Meissner, M. V., Esteves, T., Temerinac-Ott, M., et al. (2016). Cyanobacteria use micro-optics to sense light direction. eLife 5:e12620. doi: 10.7554/eLife.12620
Schuergers, N., Nurnberg, D. J., Wallner, T., Mullineaux, C. W., and Wilde, A. (2015). PilB localization correlates with the direction of twitching motility in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 161, 960–966. doi: 10.1099/mic.0.000064
Schuergers, N., Ruppert, U., Watanabe, S., Nürnberg, D. J., Lochnit, G., Dienst, D., et al. (2014). Binding of the RNA chaperone Hfq to the type IV pilus base is crucial for its function in Synechocystis sp. PCC 6803. Mol. Microbiol. 92, 840–852. doi: 10.1111/mmi.12595
Schuergers, N., and Wilde, A. (2015). Appendages of the cyanobacterial cell. Life 5, 700–715. doi: 10.3390/life5010700
Sergeyenko, T. V., and Los, D. A. (2000). Identification of secreted proteins of the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol. Lett. 193, 213–216. doi: 10.1111/j.1574-6968.2000.tb09426.x
Shin, B. J., Oh, J., Kang, S., Chung, Y. H., Park, Y. M., Kim, Y. H., et al. (2008). Cyanobacterial hybrid kinase Sll0043 regulates phototaxis by suppressing pilin and twitching motility protein. J. Microbiol. 46, 300–308. doi: 10.1007/s12275-007-0212-6
Singh, A. K., Summerfield, T. C., Li, H., and Sherman, L. A. (2006). The heat shock response in the cyanobacterium Synechocystis sp strain PCC 6803 and regulation of gene expression by HrcA and SigB. Arch. Microbiol. 186, 273–286. doi: 10.1007/s00203-006-0138-0
Song, J. Y., Cho, H. S., Cho, J. I., Jeon, J. S., Lagarias, J. C., and Park, Y. I. (2011). Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 108, 10780–10785. doi: 10.1073/pnas.1104242108
Song, W. Y., Zang, S. S., Li, Z. K., Dai, G. Z., Liu, K., Chen, M., et al. (2018). Sycrp2 is essential for twitching motility in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 200:e00436-18. doi: 10.1128/JB.00436-18
Stimson, E., Virji, M., Barker, S., Panico, M., Blench, I., Saunders, J., et al. (1996). Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem. J. 316, 29–33. doi: 10.1042/bj3160029
Sugimoto, Y., Nakamura, H., Ren, S., Hori, K., and Masuda, S. (2017). Genetics of the blue light-dependent signal cascade that controls phototaxis in the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 58, 458–465. doi: 10.1093/pcp/pcw218
Sure, S., Ackland, M. L., Gaur, A., Gupta, P., Adholeya, A., and Kochar, M. (2016). Probing Synechocystis-arsenic interactions through extracellular nanowires. Front. Microbiol. 7:1134. doi: 10.3389/fmicb.2016.01134
Sure, S., Torriero, A. A. J., Gaur, A., Li, L. H., Chen, Y., Tripathi, C., et al. (2015). Inquisition of Microcystis aeruginosa and Synechocystis nanowires: characterization and modelling. Antonie Van Leeuwenhoek 108, 1213–1225. doi: 10.1007/s10482-015-0576-2
Tajima, N., Sato, S., Maruyama, F., Kaneko, T., Sasaki, N. V., Kurokawa, K., et al. (2011). Genomic structure of the cyanobacterium Synechocystis sp. PCC 6803 strain GT-S. DNA Res. 18, 393–399. doi: 10.1093/dnares/dsr026
Terauchi, K., and Ohmori, M. (1999). An adenylate cyclase, cya1, regulates cell motility in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 40, 248–251. doi: 10.1093/oxfordjournals.pcp.a029534
Trautmann, D., Voss, B., Wilde, A., Al-Babili, S., and Hess, W. R. (2012). Microevolution in cyanobacteria: re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 19, 435–448. doi: 10.1093/dnares/dss024
Voisin, S., Kus, J. V., Houliston, S., St-Michael, F., Watson, D., Cvitkovitch, D. G., et al. (2007). Glycosylation of Pseudomonas aeruginosa strain Pa5196 type IV pilins with mycobacterium-like alpha-1,5-linked d-Araf oligosaccharides. J. Bacteriol. 189, 151–159. doi: 10.1128/JB.01224-06
Wang, F. B., Gu, Y. Q., O’Brien, J. P., Yi, S. M., Yalcin, S. E., Srikanth, V., et al. (2019). Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell 177, 361–369. doi: 10.1016/j.cell.03.029
Wendt, K. E., and Pakrasi, H. B. (2019). Genomics approaches to deciphering natural transformation in cyanobacteria. Front. Microbiol. 10:1259. doi: 10.3389/fmicb.2019.01259
Wilde, A., Fiedler, B., and Börner, T. (2002). The cyanobacterial phytochrome Cph2 inhibits phototaxis towards blue light. Mol. Microbiol. 44, 981–988. doi: 10.1046/j.1365-2958.2002.02923.x
Wilde, A., and Mullineaux, C. W. (2015). Motility in cyanobacteria: polysaccharide tracks and Type IV pilus motors. Mol. Microbiol. 98, 998–1001. doi: 10.1111/mmi.13242
Williams, J. G. K. (1988). Construction of specific mutations in photosystem-II photosynthetic reaction center by genetic-engineering methods in Synechocystis-6803. Methods Enzymol. 167, 766–778. doi: 10.1016/0076-6879(88)67088-1
Xie, C. S., Wu, X. G., Zhao, J., Xu, X., Hu, J., and Cao, Y. (2018). Shotgun secretome analysis of Synechocystis sp. PCC 6803 response to phosphate limitation. Curr. Proteomics 15, 320–328. doi: 10.2174/1570164615666180419160122
Xu, W., and Wang, Y. C. (2019). Sequences, domain architectures, and biological functions of the serine/threonine and histidine kinases in Synechocystis sp. PCC 6803. Appl. Biochem. Biotechnol. 188, 1022–1065. doi: 10.1007/s12010-019-02971-w
Yoshihara, S., Geng, X., and Ikeuchi, M. (2002). pilG gene cluster and split pilL genes involved in pilus biogenesis, motility and genetic transformation in the cyanobacterium Synechocystis sp PCC 6803. Plant Cell Physiol. 43, 513–521. doi: 10.1093/pcp/pcf061
Yoshihara, S., Geng, X. X., Okamoto, S., Yura, K., Murata, T., Go, M., et al. (2001). Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp PCC 6803. Plant Cell Physiol. 42, 63–73. doi: 10.1093/pcp/pce007
Yoshihara, S., and Ikeuchi, M. (2004). Phototactic motility in the unicellular cyanobacterium Synechocystis sp PCC 6803. Photochem. Photobiol. Sci. 3, 512–518. doi: 10.1039/b402320j
Yoshihara, S., Suzuki, F., Fujita, H., Geng, X. X., and Ikeuchi, M. (2000). Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 41, 1299–1304. doi: 10.1093/pcp/pce010
Yoshimura, H., Hisabori, T., Yanagisawa, S., and Ohmori, M. (2000). Identification and characterization of a novel cAMP receptor protein in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 275, 6241–6245. doi: 10.1074/jbc.275.9.6241
Yoshimura, H., Kaneko, Y., Ehira, S., Yoshihara, S., Ikeuchi, M., and Ohmori, M. (2010). CccS and CccP are involved in construction of cell surface components in the cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Cell Physiol. 51, 1163–1172. doi: 10.1093/pcp/pcq081
Yoshimura, H., Yanagisawa, S., Kanhisa, M., and Ohmori, M. (2002a). Screening for the target gene of cyanobacterial cAMP receptor protein SYCRP1. Mol. Microbiol. 43, 843–853. doi: 10.1046/j.1365-2958.2002.02790.x
Yoshimura, H., Yoshihara, S., Okamoto, S., Ikeuchi, M., and Ohimori, M. (2002b). A cAMP receptor protein, SYCRP1, is responsible for the cell motility of Synechocystis sp. PCC 6803. Plant Cell Physiol. 43, 460–463. doi: 10.1093/pcp/pcf050
Keywords: thick pili, motility, phototaxis, DNA uptake, Synechocystis sp. PCC 6803
Citation: Chen Z, Li X, Tan X, Zhang Y and Wang B (2020) Recent Advances in Biological Functions of Thick Pili in the Cyanobacterium Synechocystis sp. PCC 6803. Front. Plant Sci. 11:241. doi: 10.3389/fpls.2020.00241
Received: 30 July 2019; Accepted: 17 February 2020;
Published: 10 March 2020.
Edited by:
Julian Eaton-Rye, University of Otago, New ZealandReviewed by:
David John Lea-Smith, University of East Anglia, United KingdomAran Incharoensakdi, Chulalongkorn University, Thailand
Copyright © 2020 Chen, Li, Tan, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuo Chen, Y2hlbnpodW80MzU3QDE2My5jb20=; Yan Zhang, eWFuemk4MjAxMDZAMTI2LmNvbQ==
 Zhuo Chen
Zhuo Chen Xitong Li1
Xitong Li1 Xiaoming Tan
Xiaoming Tan Yan Zhang
Yan Zhang