
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 21 January 2020
Sec. Plant Abiotic Stress
Volume 10 - 2019 | https://doi.org/10.3389/fpls.2019.01698
This article is part of the Research TopicUnifying Insights into the Desiccation Tolerance Mechanisms of Resurrection Plants and SeedsView all 9 articles
Plant cell walls define the shape of the cells and provide mechanical support. They function as osmoregulators by controlling the transport of molecules between cells and provide transport pathways within the plant. These diverse functions require a well-defined and flexible organization of cell wall components, i.e., water, polysaccharides, proteins, and other diverse substances. Cell walls of desiccation tolerant resurrection plants withstand extreme mechanical stress during complete dehydration and rehydration. Adaptation to the changing water status of the plant plays a crucial role during this process. This review summarizes the compositional and structural variations, signal transduction and changes of gene expression which occur in cell walls of resurrection plants during dehydration and rehydration.
Plants as sessile organisms cope with environmental challenges by adopting a wide spectrum of strategies (Bartels and Salamini, 2001). Drought, a pervasive stress, causes water deficit (Bray, 1997) and may even lead to desiccation, a condition where only the bound water is left in the plant cells (Ramanjulu and Bartels, 2002; Zhang and Bartels, 2018). Although seeds of higher plants withstand desiccation (Bewley, 1979), vegetative tissues of most plants do not tolerate a water content which is below 60–30% (Challabathula and Bartels, 2013; Zhang and Bartels, 2018). However, some bryophytes, ferns, and a few angiosperms can survive in an extremely arid environment (Alpert, 2000). The desiccation tolerant plants, termed resurrection plants, can equilibrate their vegetative tissues with nearly 0% relative humidity (Gaff, 1971), stay in a dehydrated, quiescent stage for months, and resurrect once water is available again. Water loss leads to plasmolysis and subsequently causes mechanical stress (Moore et al., 2006; Plancot et al., 2019). Desiccation tolerant tissues can avoid or resist detrimental effects of this stress through increased vacuolation and/or cell wall folding (Figure 1) (Webb and Arnott, 1982; Moore et al., 2006; Farrant et al., 2007) which requires structural flexibility as well as physiological and molecular responses in the cell wall. In this review, we will focus on the changes in polysaccharide composition, cell wall signaling, and transcriptional changes, which are linked to reversible cell wall folding in resurrection plants (Figure 1).

Figure 1 Surface images of Craterostigma plantagineum leaves taken with a scanning electron microscope. Micrographs were taken from untreated (RWC = 100%), desiccated (RWC = 2%) and rehydrated (RWC = 90%) leaves.
The cell walls encapsulate plant cells and provide mechanical strength. They define the morphology, and are implicated in plant growth and responses to environmental stresses (Pilling and Höfte, 2003; Hamann, 2014). Important building blocks of cell walls are cellulose, callose, pectin, and hemicelluloses. Pectin is the most abundant component and accounts for up to 50% (w/w) of the cell wall in Arabidopsis thaliana (Zablackis et al., 1995). Cellulose and callose are linear homopolysaccharides and are composed of β-(1,4)- and β-(1,3)-linked glucose residues, respectively. Cellulose microfibrils are interconnected by hemicelluloses and pectin and form rigid structures which build up the mechanical scaffold of the cell wall (Nishiyama, 2009; Wang et al., 2012). Pectin is a heterogenous matrix of homogalacturonan, rhamnogalacturonan-I, and rhamnogalacturonan-II. Homogalacturonan is typically most abundant and accounts for about 65% of pectin. Rhamnogalacturonan-I accounts for 20–35% and rhamnogalacturonan-II is a minor component (Mohnen, 2008). α-(1,4)-Linked D-galacturonic acid is a building block of homogalacturonan, where it is arranged in linear chains. Galacturonic acid is also the building block of rhamnogalacturonan-II and, together with rhamnose, the backbone of rhamnogalacturonan-I. Rhamnogalacturonan-I and rhamnogalacturonan-II are more complex than homogalacturonan, because galacturonic acid and rhamnose are substituted by other sugar residues. The biosynthesis of pectin has been reviewed recently (Harholt et al., 2010; Lampugnani et al., 2018) and will not be described further. Xyloglucan and xylan are the most abundant hemicelluloses in dicot cell walls and crosslink cellulose fibrils (Park and Cosgrove, 2015; Simmons et al., 2016). Xyloglucan has a β-(1,4)-linked glucose backbone with side chains which contain xylose, galactose (possibly acetylated), fucose, and arabinose. Xylan is made of β-(1,4)-linked xylose residues with side chains of α-arabinofuranose and α-galacturonic acid. Modifications such as transglucosylation, acetylation, or methylesterification and cross-linking of the different cell wall components play a major role in modifying the mechanical properties of plant cell walls (O’Neill et al., 2001; Ryden et al., 2003; Caffall and Mohnen, 2009; Caffall et al., 2009; Park and Cosgrove, 2015). Analyzing the behavior of the polysaccharide matrix in response to stress is essential to understand the flexibility of cell walls. Upon desiccation, the vacuole shrinks, and the cell contents are drawn inwards, which results in more tension between the plasmalemma and the cell wall (Levitt and Levitt, 1987). Callose synthesis is induced in response to different stresses and it functions as a local cell wall stabilizer (Nielsen et al., 2012; De Storme and Geelen, 2014). Upon desiccation most resurrection plants undergo extensive folding of the cell wall, a process which is quickly reversed during rehydration (Phillips et al., 2008; Jung et al., 2019). Controlled cell wall folding prevents tearing of the plasmalemma from the cell wall, which is essential to maintain cell integrity (Thomson and Platt, 1997; Farrant and Sherwin, 1998; Vicré et al., 1999; Farrant, 2000; Vicré et al., 2004b). The degree of folding depends on the leaf morphology and the leaf area e.g. the leaves of the desiccation tolerant grass Oropetium thomaeum (VanBuren et al., 2017) are narrow and the degree of folding is less than in Craterostigma plantagineum. Cells of desiccated leaves show the most extensive folding after dehydration compared to cells of roots or stems.
In resurrection plants, changes in homogalacturonan, rhamnogalacturonan-I, rhamnogalacturonan-II, and hemicelluloses were investigated in leaves of C. plantagineum, C. wilmsii, and Lindernia brevidens during dehydration and rehydration to understand cell wall plasticity (Vicré et al., 1999; Jung et al., 2019). Higher levels of de-methylesterified homogalacturonan were found upon desiccation which was reversed after rehydration. Homogalacturonan is synthesized in the methylesterified form and subsequently de-methylesterified in the cell wall, which suggests de novo synthesis of homogalacturonan during rehydration (Zhang and Staehelin, 1992; Staehelin and Moore, 1995; Sterling et al., 2001). A high proportion of de-methylesterified homogalacturonan upon desiccation in combination with calcium (Vicré et al., 1999) leads to the formation of the so-called “egg-box” structures (Figure 2) (Grant et al., 1973; Jarvis, 1984; Moore et al., 1986; Lloyd, 1991) which are proposed to strengthen the cell wall (Vicré et al., 1999; Jung et al., 2019). Highly de-methylesterified homogalacturonan provides additional binding sites for pectin binding proteins which might be important to sense the cell wall hydration status (Giarola et al., 2016; Jung et al., 2019). A role of homogalacturonan in desiccation tolerance is supported by a report that correlates accumulation of homogalacturonan with desiccation resistance in the green algae Zygnema sp. (Herburger et al., 2019). Changes in rhamnogalacturonan-I, rhamnogalacturonan-II, and the hemicelluloses may reinforce the cell wall upon desiccation in resurrection plants. In C. wilmsii and C. plantagineum the xyloglucan levels increased upon desiccation (Vicré et al., 1999; Jung et al., 2019). More xyloglucan points to an increase of interconnected cellulose fibrils and thus enhances cell wall rigidity (Moore et al., 1986; Fry, 1989; Park and Cosgrove, 2015). Xylan, another cellulose-linking cell wall component, is also increased upon desiccation but motile and flexible cell wall components like β-1,4-galactan and α-1,5-arabinan do not change (Jung et al., 2019). In C. plantagineum, dehydration leads to changes in rhamnogalacturonan-II (Jung et al., 2019). In the studied resurrection plants, the changes in the pectin composition lead to a more rigid cell wall upon dehydration (Vicré et al., 1999; Jung et al., 2019). Crosslinking of homogalacturonan via Ca2+ and rhamnogalacturonan-II via borate strengthens the cell wall (Kobayashi et al., 1996).
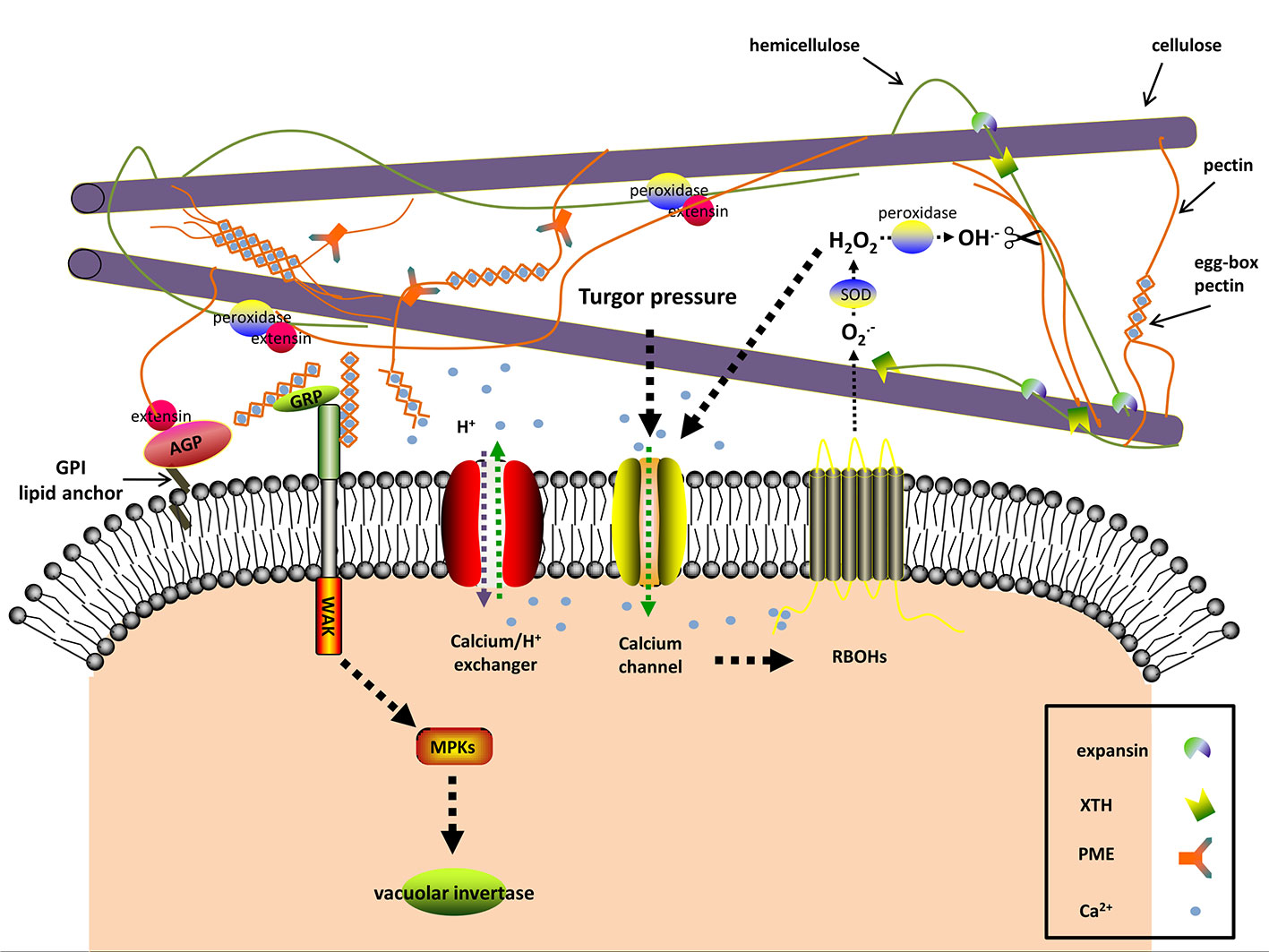
Figure 2 The predicted interactions among apoplastic proteins and signaling molecules in resurrection plants during dehydration. Dehydration induces turgor pressure changes, which are sensed by mechanosensitive (MS) calcium channels As a consequence [Ca2+]cyt levels rise. The plasma membrane-localized NADPH oxidases (respiratory burst oxidase homologs, RBOHs) are activated through binding [Ca2+]cyt and produce O2−, a substrate of the cell wall superoxide dismutase (SOD). The apoplastic H2O2 as the product of SOD also leads to Ca2+ influx. Cell wall peroxidases produce hydroxyl radicals (OH−) with apoplastic H2O2 as substrate. The reactive OH− is able to rupture glycosidic bonds and leads to cell wall loosening. In addition to OH−, expansin and xyloglucan endotransglucosylase/hydrolase (XTH) may contribute to loosening the cell wall by disrupting the interaction between hemicellulose and cellulose during the early stages of dehydration and rehydration. Cell wall peroxidases facilitate wall stiffness by reinforcing the cross-linking of extensin with cell wall polysaccharides. Excessive [Ca2+]cyt is toxic and thus [Ca2+]cyt is transported to the extracellular space by Ca2+ efflux systems (Ca2+ exchangers are shown). The alkalization in the apoplast affects the activity of many cell wall proteins, one of which is pectinmethylesterase (PME). PME exerts its demethylesterifying role on pectin in alkalized apoplast and generates negatively charged pectins, which form the egg-box pectin gelatin with apoplastic Ca2+ and increase wall stiffness. Wall-associated kinase (WAK) forms a complex with the glycine-rich protein (GRP) and can detect the egg-box pectin. This then in turn activates the vacuolar invertase activity. The classic (glycosylphosphatidyl inositol) GPI-anchored cell wall arabinogalactan proteins (AGPs) are involved in signal transduction between the intracellular and extracellular compartments and act as plasticizers in resurrection plants against desiccation. The signaling pathway and the interactions in the apoplast are hypothesized according to the available literatures and the current research on cell walls of resurrection plants.
The plant cell wall has a complex signaling system which monitors cell wall integrity by detecting chemical and physical modifications of the cell wall polymers and then transduces this information into the cell to trigger appropriate responses (Seifert and Blaukopf, 2010; Voxeur and Höfte, 2016). Signaling pathways resemble yeast signaling mechanisms and can be activated by various stimuli including drought stress (Hamann, 2014). Different molecules, including reactive oxygen species (ROS) and hormones, are integrated in cell wall-mediated signaling cascades (Miller et al., 2010; Choudhury et al., 2017; Novaković et al., 2018). An overview of the different physical and chemical signals and pathways is provided in the following paragraphs.
Turgor pressure is the result of the osmotic pressure in the symplast and the mechanical strength of the cell wall (Cleland, 1971; Lewicki, 1998; Ringli, 2010). Altered turgor pressure can be perceived as physical signal by cell wall sensors such as ion channels, leading to the flow of Ca2+ between intra- and extracellular spaces according to the plasma membrane tension (Seifert and Blaukopf, 2010; Hamann, 2014; Hamilton et al., 2015) (Figure 2). Dehydration lowers the turgor pressure, thus stopping cell expansion and growth (Tardieu et al., 2014). The resurrection plant Myrothamnus flabellifolia maintains cell turgor and copes with the mechanical stress by increasing cell wall elasticity with plasticizers, i.e., arabinose-containing polymers (Moore et al., 2013). These polymers might act as “mechanosensors” as well (Moore et al., 2006; Moore et al., 2008; Le Gall et al., 2015).
Turgor pressure changes, as a result of dehydration, are perceived as mechanical signals by a specialized plasma membrane localized-mechanosensory gauge, termed mechano-sensitive or stretch-activated ion channel (Figure 2) (Seifert and Blaukopf, 2010; Hamilton et al., 2015). The pore-forming mechano-sensitive ion channels control ion passage via sensing the membrane tension and thus trigger the downstream signaling. In this way the change of mechanical force is sensed at the membrane-wall interface and acts as cell wall integrity sensor (Seifert and Blaukopf, 2010; Wolf et al., 2012; Basu and Haswell, 2017). In plants, three mechanosensitive channel families have been characterized, namely MscS-like channels, two-pore domain K+ channels, and Mid1-complementing activity channels (Hamilton et al., 2015; Basu and Haswell, 2017). The non-selective mechanosensitive-like channels are classified into three groups, among which the plastid-localized group II MscS-like 2 and MscS-like 3 are correlated with plastid osmotic stress and abscisic acid (ABA) induction. Overexpression of plasma membrane-localized group III MscS-like 10 can result in H2O2-associated cell death (Veley et al., 2014; Hamilton et al., 2015). The Mid1-complementing activity channels (Ca2+-permeable mechanosensitive channels) are tightly associated with Ca2+ influx and involved in regulating Ca2+ homoeostasis (Hamilton et al., 2015). The transcriptome analysis of the angiosperm resurrection plant C. plantagineum and the desiccation-tolerant lichen Cladonia rangiferina revealed elevated expression of genes encoding pore calcium channels and other non-defined ion channels upon dehydration (Rodriguez et al., 2010; Junttila et al., 2013; Giarola et al., 2015).
Many studies identified breakdown products of pectin as signaling molecules (Bidhendi and Geitmann, 2016; Cosgrove, 2016; Voxeur and Höfte, 2016). Pectin-derived oligosaccharides (OGAs) were first discovered in plant pathogen studies and belong to a class of elicitors, leading to damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), which are related to wounding or diseases (De Lorenzo et al., 2018; Nürnberger and Kemmerling, 2018). The cell wall surveillance system is able to distinguish the degree of polymerization and conformation of OGAs and to trigger different responses, accordingly (Cabrera et al., 2008; Osorio et al., 2008; Cabrera et al., 2010). In A. thaliana de-methylesterified pectin stretches bind to calcium and form so-called “egg-box” structures which are recognized by cell wall-associated protein kinases (WAKs) (Decreux and Messiaen, 2005). Dehydration leads to a higher level of de-methylesterified pectin and an increase in the concentration of calcium in the cell wall of the resurrection species Craterostigma (Vicré et al., 1999; Vicré et al., 2004a; Jung et al., 2019), which is the basis for the “egg-box” formation and the pectin-WAK association. These results support a role for OGAs as signal molecules in resurrection plants during water deficit (Figure 2). The xyloglucan-derived OGAs also regulate cell wall expansion (Pilling and Höfte, 2003; Seifert and Blaukopf, 2010). Fry et al. (1990) observed the modulating effects of xyloglucan-derived OGAs in plant growth. Takeda et al. (2002) proposed the involvement of the xyloglucan metabolism in cell elongation. During dehydration the structure and distribution of xyloglucan are significantly altered in resurrection plants (Vicré et al., 1999; Vicré et al., 2004a; Vicré et al., 2004b). Therefore, it is tempting to speculate that xyloglucan is involved in defense responses under dehydration, when other intrinsic defense systems are shut-down.
Calcium participates in multiple biological processes and has different functions in the cell wall. Besides a structural role in forming “egg-box” structures, calcium can move in and out of the cell and functions as second messenger (Parre and Geitmann, 2005; Bose et al., 2011; Kurusu et al., 2013). The majority of Ca2+ is localized in the apoplast and vacuole (Medvedev, 2005). In the apoplast, the excess of free Ca2+ is sequestered via the formation of “egg-box” structures (Voxeur and Höfte, 2016), which also serves as reservoir for cytosolic calcium ([Ca2+]cyt). Transient [Ca2+]cyt elevation is a ubiquitous signal when plant cells encounter abiotic or biotic stress (Bose et al., 2011), which can be induced by OGAs (Moscatiello et al., 2006), and ascorbate (Makavitskaya et al., 2018). [Ca2+]cyt elevation activates ROS production, and vice versa. ROS can also cause Ca2+ influx thus facilitating signal propagation (Seifert and Blaukopf, 2010; Kurusu et al., 2013) (Figure 2). Because Ca2+ reacts with proteins and other substances in the cytoplasm, high concentrations of [Ca2+]cyt are detrimental. Therefore it is necessary to maintain the basal [Ca2+]cyt levels with the help of cytosolic buffering systems and Ca2+ efflux systems (Ca2+-ATPases and Ca2+ exchangers) (Bose et al., 2011) (Figure 2). Repetitive Ca2+ influx and efflux give rise to cytosolic calcium oscillations, which vary in magnitude, frequency, and shape and are related to the severity and type of stress (Bose et al., 2011). Long term drought in soybean induced large Ca2+ efflux from mesophyll cells, accompanied by large K+ efflux and H+ influx, which may prime the ABA signal transduction in guard cells and finally lead to stomata closure (Mak et al., 2014). Similar to soybean, the apoplastic Ca2+ was also increased in the resurrection plant C. wilmsii upon dehydration, but with no significant change of K+ in the cell wall (Vicré et al., 2004a), which suggests that the resurrection plants may have a specific Ca2+ signaling mechanism. Mihailova et al. (2018) speculated that the accumulation of Ca2+ in the cell wall of C. wilmsii resulted from electrolyte leakage. This explanation may overlook the fact that neither the apoplastic K+ nor the phosphate significantly increased. The apoplastic Ca2+ in C. wilmsii was quantified using secondary ion mass spectrometry technology. However, the studies of Ca2+ signature require more real-time data and Ca2+ levels should be determined using microelectrode ion flux measurement and dynamic calcium imaging (Krebs et al., 2012).
The proton influx and efflux across the plasma membrane can lead to apoplastic alkalization or acidification, which dictates the activities of pH-dependent cell wall modifying enzymes and finally affects cell wall structures. Water deficit, similar to other stresses such as salinity or pathogen infection tends to decrease proton concentrations in the apoplast (Geilfus, 2017). Increased apoplastic pH inhibits expansin activity and activates pectin-methylesterases, which together with elevated [Ca2+]apo eventually strengthen the cell wall (Wolf et al., 2012) (Figure 2). The cell wall pH also varies spatially with a lower pH in the growing tip, thereby promoting cell wall loosening in apical tips (Moore et al., 2008; Mangano et al., 2018). Systemic apoplastic alkalinization is considered as a stress signal stimulating ABA accumulation in guard cells and stomatal closure during dehydration (Geilfus, 2017; Karuppanapandian et al., 2017). Therefore it is essential to consider the effect of pH on the activity of cell wall modifying enzymes in more detail.
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radical (OH−), superoxide anion (O2−), and nitric oxide (NO) are a group of reactive molecules with partially reduced or active forms of oxygen (Choi et al., 2017). Historically ROS were only considered to be toxic for cell metabolism, but now it is widely accepted that ROS also act as important transmitters for both intra- and intercellular signaling (Hamann et al., 2009; Choudhury et al., 2017; Mittler, 2017). The apoplastic ROS trigger multiple downstream responses which demands a precise signal perception and transduction from the apoplast to the nucleus (Wrzaczek et al., 2013; Kangasjärvi and Kangasjärvi, 2014). The ROS signaling in the extracellular compartment has not been well deciphered. The predicted ROS sensing and transduction involve plasma membrane receptor-like kinases (RLKs), ion channels, aquaporins, redox balancing substances, plasma membrane lipid oxidation, and modification of cysteine residues in relevant proteins (Dynowski et al., 2008; Spoel and Loake, 2011; Kangasjärvi and Kangasjärvi, 2014). In resurrection plants not many studies on apoplastic ROS signaling exist. Based on observations of Ramonda nathaliae Jovanović et al. (2011) proposed that controlled production of ROS is a vital part in sensing dehydration and inducing multiple responses.
In plants, a considerable amount of ROS is generated intracellularly due to photosynthesis, mitochondrial respiration, photorespiration, and other processes caused by diverse stresses (Kimura et al., 2017; Mittler, 2017). In the extracellular space, the plasma membrane-localized NADPH oxidases (respiratory burst oxidase homologs) and cell wall peroxidases are the main sources for ROS production (Suzuki et al., 2011; O’Brien et al., 2012; Choudhury et al., 2017; Kimura et al., 2017). The respiratory burst oxidase homologs are activated via the influx of apoplastic Ca2+, internal Ca2+ binding, and phosphorylation (Baxter et al., 2014) (Figure 2). Under stress respiratory burst oxidase homologs are not only ROS producers, but also transmit ROS waves from one cell to neighboring cells (Choi et al., 2017). The apoplastic H2O2 is derived from spontaneous chemical reactions or superoxide dismutase-mediated mutation of superoxide which is generated from respiratory burst oxidase homologs (Figure 2) (Baxter et al., 2014), xanthine dehydrogenase (Ma et al., 2016), and oxalate oxidase (Voothuluru and Sharp, 2013). The comparative genome analysis of the desiccation tolerant lycophyte Selaginella tamariscina and the desiccation-sensitive Selaginella moellendorffii showed that the number of ROS-producing genes such as respiratory burst oxidase homologues and oxalate oxidase genes are much lower in the genome of the desiccation tolerant S. tamariscina compared to S. moellendorffii (Xu et al., 2018). This indicates that S. tamariscina may produce less apoplastic ROS and thus alleviates stress to cell membranes during water deficit. Choudhury et al. (2017) suggested that the ROS detoxification mechanisms within the cell walls are less effective than intracellular mechanisms, because they rely on low levels of ascorbate and glutathione, CuZn-superoxide dismutases, or cell wall peroxidases. This causes the accumulation of extracellular ROS which facilitates rapid systemic auto-propagating ROS waves (Choi et al., 2017; Choudhury et al., 2017). Despite being known as ROS-scavengers peroxidases also produce hydroxyl radicals from H2O2 which are capable of cleaving cell wall polysaccharides (Figure 2) (Fry, 1998; Passardi et al., 2004). Peroxidases in cell walls interact with polysaccharides and extensins, and supply phenoxy radicals for cell wall lignification and suberization (Figure 2) (Passardi et al., 2004; Tenhaken, 2015). Hence, peroxidases have dual functions: they contribute to wall loosening by releasing hydroxyl radicals and they boost wall stiffness by solidifying the extensin cross-linkages and supporting cell wall lignification and suberization (Novaković et al., 2018). Reinforcing the cell wall is an effective way to increase mechanical strength and to counteract increasing osmotic stress in response to dehydration. However, cell wall loosening is necessary for cell growth. In some resurrection plants the activities of peroxidases are highly increased upon rehydration, but do not change during dehydration (Sherwin and Farrant, 1998; Rodriguez et al., 2010; Deeba et al., 2016; Yobi et al., 2017). The limited activity of peroxidases may facilitate cell wall loosening and help reversible cell wall folding. In other resurrection plants, such as X. viscosa, peroxidases are up-regulated during dehydration but down-regulated upon rehydration, which is a prerequisite for cell wall stiffness under drought (Sherwin and Farrant, 1998; Ingle et al., 2007). Low-level substrates or decreased activity of peroxidases tend to generate hydroxyl radicals which lead to cell wall loosening and on the contrary high amounts of peroxidases, substrates, and ROS facilitate cell wall stiffness (Tenhaken, 2015).
The decoding of environmental cues and detection of cell wall perturbation under dehydration require special sensing mechanisms. Components of these sensors are members of RLK sub-families. RLKs generally consist of an extracellular domain, presiding over the perception of signals, a transmembrane region, and an intracellular kinase domain which triggers the downstream intracellular signaling (Ringli, 2010; Tenhaken, 2015; Novaković et al., 2018). Cell wall RLKs have been demonstrated to exert pivotal roles in plant development, growth and responses under various stresses, among which the well-characterized Catharanthus roseus protein kinase1-like receptor kinases (CrRLKs) and cell wall-associated protein kinases (WAKs) are candidates for cell wall integrity sensors (Novaković et al., 2018). In A. thaliana, there are 17 members of CrRLK (Lindner et al., 2012). Their involvement in Ca2+ signaling and ROS production during pollen tube growth, root hair elongation, or stress responses have been confirmed particularly for THESEUS1, FERONIA, and ANXUR (Hématy et al., 2007; Cheung and Wu, 2011; Denness et al., 2011; Boisson-Dernier et al., 2013; Feng et al., 2018). The FERONIA triggered-signaling is additionally regulated by a group of small peptides, RALFs (rapid alkalinization factors), which bind to FERONIA and also regulate a H+-ATPase and thus adjust the extracellular pH which subsequently determines activities of cell wall-remodeling enzymes (Murphy and De Smet, 2014). In analogy to FERONIA, the C. plantagineum WAK1 (CpWAK1) is a binding partner for the cell wall protein CpGRP1 (C. plantagineum glycine-rich protein1) (Giarola et al., 2016), and the A. thaliana WAK1 showed binding to both the AtGRP-3 protein and OGAs (Figure 2) (Decreux and Messiaen, 2005). WAKs are connected with turgor pressure as it was demonstrated that Arabidopsis plants silenced for WAKs had impaired cell expansion and reduced expression and activity of the vacuolar invertase (Kohorn et al., 2006). CpGRP1 and CpWAK1 accumulate in opposite directions upon dehydration and rehydration with more CpGRP1 and less CpWAK1 in desiccated samples compared to hydrated or rehydrated samples (Giarola et al., 2016). It was recently demonstrated that also CpGRP1 interacts with pectin and that the interaction is dependent on the homogalacturonan methylesterification status of pectin (Jung et al., 2019). The CpGRP1 protein binds stronger to homogalacturonan isolated from desiccated leaves than to homogalacturonan from hydrated leaves, where the degree of methylesterification is lower than in hydrated leaves. The data imply that both CpWAK1 and CpGRP1, or the CpWAK1-CpGRP1 complex participate in sensing changes in the cell wall organization and might trigger cell wall remodeling processes during dehydration (Figure 2).
Hydroxyproline-rich proteins are composed of highly O-glycosylated proteoglycans and exist in two forms in plants, one of which is insoluble and localized in the apoplast (Deepak et al., 2010; Shivaraj et al., 2018). The arabinogalactan proteins (AGPs) and extensins are two members of the hydroxyproline-rich protein family. Both strengthen the cell wall through crosslinking with other cell wall components and participate in signal transduction (Pilling and Höfte, 2003; Deepak et al., 2010; Ringli, 2010; Seifert and Blaukopf, 2010). AGPs have effects on cell expansion, growth, and pattern formation. The consensus structure of AGPs comprises a large carbohydrate moiety of type II arabinogalactans (β-(1,3)-galactan backbone decorated with arabinose and other polysaccharides in side chains) O-linked to the hydroxyproline (Hyp) residues of the polypeptide backbone (repetitive AlaHyp, SerHyp, and ThrHyp peptides) with an N-terminal signal sequence for secretion and a C-terminal glycosylphosphatidylinositol (GPI) lipid anchor tethering AGPs to the plasma membrane (Figure 2) (Knoch et al., 2014; Lamport et al., 2014; Tan et al., 2018). AGPs form a diverse class of proteins due to variable compositions of the peptide backbone and the carbohydrate moieties. Moore et al. (2006; 2013) supported the notion that AGPs serve as “plasticizers” to maintain cell wall flexibility during desiccation in the resurrection species Mohria caffrorum, M. flabellifolia, C. plantagineum, the grass-like Xerophyta spp., and the grass Erograstis nindensis. OGAs released from AGPs may facilitate to maintain intracellular osmotic pressure during dehydration according to the analysis of AGP genes in rice (Ma and Zhao, 2010). However, the function of AGPs is probably not only restricted to the release of OGAs as signaling molecules, but AGPs may act as sensors (Ringli, 2010; Seifert and Blaukopf, 2010; Lamport et al., 2014; Novaković et al., 2018).
Extensins are characterized by the repetitive SerHyp4 and SerHyp2 motif and the Tyr-Lys-Tyr sequence with Ser residues decorated with single galactose and minor arabinogalactan moieties attached to hydroxyproline residues (Shivaraj et al., 2018; Tan et al., 2018). The self-assembling extensins usually function as positively charged scaffolds, interacting with de-methylesterified pectin via adsorption (Cannon et al., 2008; Valentin et al., 2010). Extensins facilitate the crosslinking of rhamnogalacturonan-II via borate and also contribute to ion-regulated cell wall integrity (Chormova and Fry, 2016; Tan et al., 2018). Besides a structural role, the proline-rich extensin-like receptor kinases have an effect on cell wall signal transduction. The T-DNA mutant perk4 (defective extensin-like receptor kinase) displayed decreased sensitivity to ABA and lower [Ca2+]cyt and Ca2+ channel currents upon ABA treatment (Bai et al., 2009). This supports a role of an extensin-like receptor kinase in ABA signaling and Ca2+ homeostasis.
The remodeling of the polysaccharide composition under dehydration or rehydration is catalyzed by different cell wall modifying proteins and enzymes. Understanding the activity and regulation of these proteins and enzymes is crucial to decipher the folding process. Cell wall modifying enzymes are a group of cell wall proteins regulating cell wall composition and rheology. Here, three members of cell wall modifying proteins and their functions in resurrection plants are described.
Expansins are hypothesized to prompt acid-induced growth and cell wall remodeling under abiotic stress by disrupting hydrogen-bonds between xyloglucan and cellulose microfibrils without lytic activity (Figure 2) (Cosgrove, 2015; Tenhaken, 2015). Plant expansins fall into two major families: α-expansins and β-expansins according to phylogenetic analyses (Cosgrove, 2015). The expression and activity of α-expansins were studied in C. plantagineum leaves (Jones and McQueen-Mason, 2004). Among the three expansin transcripts the CplExp1 transcript level was correlated with expansin activity which increased in the early stages of dehydration and rehydration corresponding to cell wall extensibility (Jones and McQueen-Mason, 2004). This suggests a role of expansin-induced wall extension in the early stages of dehydration and rehydration. However, dehydration tends to alkalize the apoplast which leads to the question how the activity of acid-activated expansins can be triggered upon dehydration (Geilfus, 2017).
Apart from expansins, the xyloglucan endotransglucosylases/hydrolases (XTHs) are other candidates for unzipping the hemicellulose (xyloglucan)-cellulose network via hydrolysis or transglucosylation to increase the cell wall extensibility (Figure 2) (Sasidharan et al., 2011; Tenhaken, 2015). In contrast to expansins, XTHs exhibit two activities: irreversible xyloglucan hydrolysis (XEH) and reversible xyloglucan endotransglucosylation (XET), suggesting roles for XTHs in cell wall loosening and re-assembling (Rose et al., 2002). Transgenic A. thaliana and tomato plants overexpressing a xyloglucan endotransglucosylase/hydrolase CaXTH3 from hot pepper showed enhanced tolerance to salt and dehydration stress (Cho et al., 2006; Choi et al., 2011). In the resurrection plant, Haberlea rhodopensis, one putative XTH gene HrhDR35 was up-regulated during early dehydration to desiccation and rehydration (Georgieva et al., 2012), which corresponds to expansin expression in C. plantagineum (Jones and McQueen-Mason, 2004). Based on these observations it is suggested that XTHs contribute to improve dehydration tolerance through increasing wall extensibility and cell wall reconstruction after stress relief in both desiccation tolerant and desiccation sensitive plants. However, XTHs may not always contribute to cell wall extensibility as was shown for cell walls of ripening tomato fruit (Saladié et al., 2006)
The degree of pectin methylesterification is an important factor for cell wall structure and has an effect on cellular growth and cell wall responses during dehydration (Wolf et al., 2009). Pectinmethylesterases de-methylesterify pectin and thus generate negatively charged pectin (Figure 2). This reaction is affected by the apoplastic pH and the degree of methylesterification of galacturonic acid (Micheli, 2001; Wolf et al., 2012). The released pectin transfers Ca2+ to promote the formation of egg-box pectin gelatin which enhances the mechanical stability (Wolf et al., 2012; Voxeur and Höfte, 2016). Under dehydration the cell wall texture is presumably modified by the egg-box gelatin through activating pectinmethylesterases and inhibiting expansins due to the increased pH of the apoplast (Figure 2) (Wolf et al., 2012). Upon dehydration de-methylesterified pectin increases in the cell wall of C. wilmsii, C. plantagineum, and L. brevidens which is probably due to pectinmethylesterase activities during dehydration (Vicré et al., 1999; Vicré et al., 2004a; Jung et al., 2019).
The above-described cell wall proteins are not sufficient to explain the cell wall behavior during dehydration/rehydration in resurrection plants. Analyses of genome sequences have identified several other cell wall proteins in resurrection plants (Giarola et al., 2015). Giarola et al. (2015; 2016) identified a cysteine-rich protein localized in the apoplast and down-regulated during dehydration but up-regulated during rehydration, while the expression level of the CpGRP1 protein was enhanced during desiccation, which is consistent with the dehydration-induced GRP1 from Boea hygrometrica (Wang et al., 2009). Also aquaporins and plasma membrane intrinsic proteins accumulated upon dehydration or in the presence of ABA in C. plantagineum, suggesting that water channels are associated with ABA signaling during dehydration (Mariaux et al., 1998).
In the past 10 years transcriptome-wide changes upon dehydration and rehydration have been reported for the dicot resurrection species C. plantagineum (Rodriguez et al., 2010), H. rhodopensis (Gechev et al., 2013), M. flabellifolia (Ma et al., 2015), and Boea hygrometrica (Xiao et al., 2015; Zhu et al., 2015), and for the monocot resurrection species Oropetium thomaeum (VanBuren et al., 2015) and Sporobolus stapfianus (Yobi et al., 2017). This information can be used to identify genes which are related to cell wall compartments. This will allow a comprehensive study of the molecular mechanisms which are activated to adapt the cell walls to the reducing cell volume caused by water loss in resurrection plants.
The analysis of transcriptome data showed that several cell wall-related genes which are involved in different processes such as the regulation of cell wall plasticity and cell wall dynamics, catabolic processes, and cell wall organization are differentially modulated upon dehydration thus suggesting the importance of cell wall remodeling during the acquisition of desiccation tolerance (Rodriguez et al., 2010; Gechev et al., 2013; Xiao et al., 2015; Zhu et al., 2015). One main obstacle to the interpretation of RNA expression data resides in the fact that genes encoding cell wall modifying enzymes belong to large gene families and often different enzyme isoforms in these families are differently regulated upon dehydration. Table 1 summarizes dehydration-induced changes in the expression of genes encoding cell wall modifying proteins and enzymes which were reported for resurrection species. A good example of enzyme isoforms which display an opposite expression upon dehydration is represented by xyloglucan endotransglucosylases (XTHs) in H. rhodopensis. Transcriptome data showed that several XTHs isoforms are down-regulated during dehydration (Gechev et al., 2013) (Table 1) but the presence of a dehydration-induced XTH was previously identified by cDNA-AFLP experiments (Georgieva et al., 2012). Genes encoding XTHs, expansins, pectinmethylesterases, and pectinacetylesterases are abundant in hydrated leaves of C. plantagineum and down-regulated upon dehydration (Rodriguez et al., 2010). The stage where the maximum transcript expression is registered can also be hardly used as indicator for protein activity as this can be affected by additional factors, e.g., the binding with specific inhibitors, changes in the apoplastic pH, the substrate accessibility, and/or the accumulation of ROS upon dehydration.
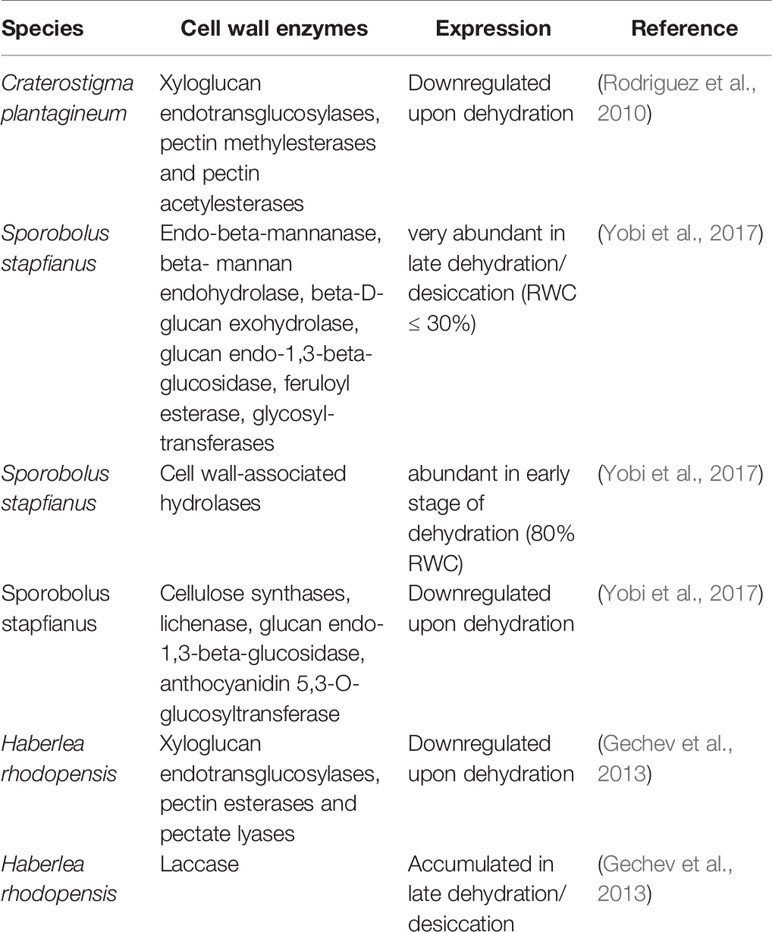
Table 1 Dehydration-induced expression changes of cell wall enzymes which were reported for resurrection species.
Our knowledge of structural changes in cell walls of dehydration sensitive species upon dehydration stress is limited and thus it is difficult to identify which mechanisms are specific for cell wall folding in resurrection species. Increased cell wall extensibility is observed upon dehydration in the resurrection species Craterostigma and it appears to be essential for cell wall folding and survival (Jones and McQueen-Mason, 2004). Conversely, sensitive plants subjected to drought stress tend to increase the stiffness of their cell walls (Lu and Neumann, 1998; Tenhaken, 2015). Expansins and XTHs have been proposed to be good candidates to increase cell wall extensibility in resurrection species. Additionally, other cell wall modifying proteins or enzymes, e.g., pectinmethylesterases are emerging as possible modulators of cell wall stiffness by acting on the methylesterification level of pectin. Finally, transcriptome data suggest the involvement of several classes of cell wall modifying enzymes and cell wall modifying enzyme inhibitors but the sole transcript data are far from providing a clear picture of how the different classes of cell wall proteins from these species are recruited and coordinated to achieve cell folding in resurrection species.
Cell wall remodeling is a pivotal drought tolerance mechanism for plants (Tenhaken, 2015), which includes two opposite effects: stiffening and loosening. Both effects contribute to the ability to overcome mechanical stress, while stiffening preferentially occurs in desiccation sensitive plants and loosening is essential for cell wall folding in resurrection plants. Maintaining the integrity of cell walls during dehydration and rehydration in resurrection plants involves many components ranging from changes in polysaccharide composition to differential RNA expression. The activation of pathways leading to more flexible components on the one hand and adding more stability to the cell wall on the other hand, suggests a tightly controlled folding process during dehydration which finally keeps the plasmalemma and the photosynthetic apparatus intact in resurrection plants.
PC, NJ, and VG wrote the manuscript. DB and VG supervised the work and corrected the manuscript.
PC acknowledges a fellowship from CSC and part of the work was supported by the DFG project Smartwall (BA 712-18-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
OGAs, Oligosaccharides; WAKs, Wall-associated protein kinases; ROS, Reactive oxygen species; RLKs, Receptor-like kinases; ABA, abscisic acid; GRP, Glycine-rich protein; AGPs, Arabinogalactan proteins; XTHs, Xyloglucan endotransglucosylases/hydrolases.
Alpert, P. (2000). The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecol. 151 (1), 5–17.doi: 10.1023/A:1026513800380
Bai, L., Zhang, G., Zhou, Y., Zhang, Z., Wang, W., Du, Y., et al. (2009). Plasma membrane-associated proline-rich extension-like receptor kinase 4, a novel regulator of Ca2+ signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J. 60 (2), 314–327. doi: 10.1111/j.1365-313X.2009.03956.x
Bartels, D., Salamini, F. (2001). Desiccation tolerance in the resurrection plant Craterostigma plantagineum. A contribution to the study of drought tolerance at the molecular level. Plant Physiol. 127 (4), 1346–1353. doi: 10.1104/pp.010765
Basu, D., Haswell, E. S. (2017). Plant mechanosensitive ion channels: an ocean of possibilities. Curr. Opin. Plant Biol. 40, 43–48. doi: 10.1016/j.pbi.2017.07.002
Baxter, A., Mittler, R., Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65 (5), 1229–1240. doi: 10.1093/jxb/ert375
Bewley, J. D. (1979). Physiological aspects of desiccation tolerance. Annu. Rev. Plant Phys. 30, 195–238. doi: 10.1146/annurev.pp.30.060179.001211
Bidhendi, A. J., Geitmann, A. (2016). Relating the mechanics of the primary plant cell wall to morphogenesis. J. Exp. Bot. 67 (2), 449–461. doi: 10.1093/jxb/erv535
Boisson-Dernier, A., Lituiev, D. S., Nestorova, A., Franck, C. M., Thirugnanarajah, S., Grossniklaus, U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PloS Biol. 11, e1001719. doi: 10.1371/journal.pbio.1001719
Bose, J., Pottosin, I., Shabala, S. S. S., Palmgren, M. G., Shabala, S. (2011). Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2, 85. doi: 10.3389/fpls.2011.00085
Bray, E. A. (1997). Plant responses to water deficit. Trends Plant Sci. 2, 48–54. doi: 10.1016/S1360-1385(97)82562-9
Cabrera, J. C., Boland, A., Messiaen, J., Cambier, P., Van Cutsem, P. (2008). Egg box conformation of oligogalacturonides: The time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology 18 (6), 473–482. doi: 10.1093/glycob/cwn027
Cabrera, J. C., Boland, A., Cambier, P., Frettinger, P., Van Cutsem, P. (2010). Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 20 (6), 775–786. doi: 10.1093/glycob/cwq034
Caffall, K. H., Mohnen, D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohyd. Res. 344, 1879–1900. doi: 10.1016/j.carres.2009.05.021
Caffall, K. H., Pattathil, S., Phillips, S. E., Hahn, M. G., Mohnen, D. (2009). Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol. Plant 2 (5), 1000–1014. doi: 10.1093/mp/ssp062
Cannon, M. C., Terneus, K., Hall, Q., Tan, L., Wang, Y., Wegenhart, B. L., et al. (2008). Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. U.S.A. 105 (6), 2226–2231. doi: 10.1073/pnas.0711980105
Challabathula, D., Bartels, D. (2013). Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci. 4, 482. doi: 10.3389/fpls.201300482
Cheung, A. Y., Wu, H. M. (2011). THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 14 (6), 632–641. doi: 10.1016/j.pbi.2011.09.001
Cho, S. K., Kim, J. E., Park, J.-A., Eom, T. J., Kim, W. T. (2006). Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 580 (13), 3136–3144. doi: 10.1016/j.febslet.2006.04.062
Choi, J. Y., Seo, Y. S., Kim, S. J., Kim, W. T., Shin, J. S. (2011). Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 30 (5), 867–877. doi: 10.1007/s00299-011-1032-z
Choi, W. G., Miller, G., Wallace, I., Harper, J., Mittler, R., Gilroy, S. (2017). Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 90 (4), 698–707. doi: 10.1111/tpj.13492
Chormova, D., Fry, S. C. (2016). Boron bridging of rhamnogalacturonan-II is promoted in vitro by cationic chaperones, including polyhistidine and wall glycoproteins. New Phytol. 209 (1), 241–251. doi: 10.1111/nph.13596
Choudhury, F. K., Rivero, R. M., Blumwald, E., Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J. 90 (5), 856–867. doi: 10.1111/tpj.13299
Cleland, R. (1971). Cell wall extension. Annu. Rev. Plant Physiol. 22 (1), 197–222. doi: 10.1146/annurev.pp.22.060171.001213
Cosgrove, D. J. (2015). Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 25, 162–172. doi: 10.1016/j.pbi.2015.05.014
Cosgrove, D. J. (2016). Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67 (2), 463–476. doi: 10.1093/jxb/erv511
De Lorenzo, G., Ferrari, S., Cervone, F., Okun, E. (2018). Extracellular DAMPs in plants and mammals: Immunity, tissue damage and repair. Trends Immunol. 39 (11), 937–950. doi: 10.1016/j.it.2018.09.006
De Storme, N., Geelen, D. (2014). Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Front. Plant Sci. 5, 138. doi: 10.3389/fpls.2014.00138
Decreux, A., Messiaen, J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46 (2), 268–278. doi: 10.1093/pcp/pci026
Deeba, F., Pandey, A. K., Pandey, V. (2016). Organ specific proteomic dissection of Selaginella bryopteris undergoing dehydration and rehydration. Front. Plant Sci. 7, 425. doi: 10.3389/fpls.2016.00425
Deepak, S., Shailasree, S., Kini, R. K., Muck, A., Mithöfer, A., Shetty, S. H. (2010). Hydroxyproline-rich glycoproteins and plant defence. J. Phytopathol. 158 (9), 585–593. doi: 10.1111/j.1439-0434.2010.01669.x
Denness, L., McKenna, J. F., Segonzac, C., Wormit, A., Madhou, P., Bennett, M., et al. (2011). Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species-and jasmonic acid-dependent process in. Arabidopsis. Plant Physiol. 156 (3), 1364–1374. doi: 10.1104/pp.111.175737
Dynowski, M., Schaaf, G., Loque, D., Moran, O., Ludewig, U. (2008). Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 414, 53–61. doi: 10.1042/Bj20080287
Farrant, J. M., Sherwin, H. W. (1998). “Mechanisms of desiccation tolerance in seeds and resurrection plants,” in Progress in seed science research (Geneva, New York: Communication Services of the New York State Agricultural Experimental Station), 109–120.
Farrant, J. M., Brandt, W. B., Lindsey, G. G. (2007). An overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress 1 (1), 72–84. doi: 10.1002/9780470376881.ch3
Farrant, J. M. (2000). A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol. 151 (1), 29–39. doi: 10.1111/j.1469-8137.2010.03595.x
Feng, W., Kita, D., Peaucelle, A., Cartwright, H. N., Doan, V., Duan, Q., et al. (2018). The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ Signaling. Curr. Biol. 28 (5), 666–675. doi: 10.1016/j.cub.2018.01.023
Fry, S. C., McDougall, G. J., Lorences, E. P., Biggs, K. J., Smith, R. C. (1990). Oligosaccharins from xyloglucan and cellulose: modulators of the action of auxin and H+ on plant growth. Symp. Soc Exp. Biol. 44, 285–298.
Fry, S. C. (1989). The structure and functions of xyloglucan. J. Exp. Bot. 40 (210), 1–11. doi: 10.1093/Jxb/40.1.1
Fry, S. C. (1998). Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 332 (Pt 2), 507–515. doi: 10.1042/bj3320507
Gaff, D. F. (1971). Desiccation-tolerant flowering plants in southern Africa. Science 174 (4013), 1033–1034. doi: 10.1126/science.174.40131033
Gechev, T. S., Benina, M., Obata, T., Tohge, T., Sujeeth, N., Minkov, I., et al. (2013). Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell. Mol. Life Sci. 70 (4), 689–709. doi: 10.1007/s00018-012-1155-6
Geilfus, C. M. (2017). The pH of the apoplast: dynamic factor with functional impact under stress. Mol. Plant 10 (11), 1371–1386. doi: 10.1016/j.molp.2017.09.018
Georgieva, T., Christov, N. K., Djilianov, D. (2012). Identification of desiccation-regulated genes by cDNA-AFLP in Haberlea rhodopensis: a resurrection plant. Acta Physiol. Plant 34 (3), 1055–1066. doi: 10.1007/s11738-011-0902-x
Giarola, V., Krey, S., Frerichs, A., Bartels, D. (2015). Taxonomically restricted genes of Craterostigma plantagineum are modulated in their expression during dehydration and rehydration. Planta 241 (1), 193–208. doi: 10.1007/s00425-014-2175-2
Giarola, V., Krey, S., von den Driesch, B., Bartels, D. (2016). The Craterostigma plantagineum glycine-rich protein CpGRP1 interacts with a cell wall-associated protein kinase 1 (CpWAK1) and accumulates in leaf cell walls during dehydration. New Phytol. 210 (2), 535–550. doi: 10.1111/nph.13766
Grant, G. T., Morris, E. R., Rees, D. A., Smith, P. J. C., Thom, D. (1973). Biological Interactions between polysaccharides and divalent cations - egg-box model. FEBS Lett. 32 (1), 195–198. doi: 10.1016/0014-5793(73)80770-7
Hématy, K., Sado, P.-E., Van Tuinen, A., Rochange, S., Desnos, T., Balzergue, S., et al. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17 (11), 922–931. doi: 10.1016/j.cub.2007.05.018
Hamann, T., Bennett, M., Mansfield, J., Somerville, C. (2009). Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 57 (6), 1015–1026. doi: 10.1111/j.1365-313X.2008.03744.x
Hamann, T. (2014). The plant cell wall integrity maintenance mechanism—Concepts for organization and mode of action. Plant Cell Physiol. 56 (2), 215–223. doi: 10.1093/pcp/pcu164
Hamilton, E. S., Schlegel, A. M., Haswell, E. S. (2015). United in diversity: mechanosensitive ion channels in plants. Annu. Rev. Plant Biol. 66, 113–137. doi: 10.1146/annurev-arplant-043014-114700
Harholt, J., Suttangkakul, A., Scheller, H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153 (2), 384–395. doi: 10.1104/pp.110.156588
Herburger, K., Xin, A. Z., Holzinger, A. (2019). Homogalacturonan accumulation in cell walls of the green alga Zygnema sp. (Charophyta) increases desiccation resistance. Front. Plant Sci. 10, 540. doi: 10.3389/Fpls.2019.00540
Ingle, R. A., Schmidt, U. G., Farrant, J. M., Thomson, J. A., Mundree, S. G. (2007). Proteomic analysis of leaf proteins during dehydration of the resurrection plant Xerophyta viscosa. Plant Cell Environ. 30 (4), 435–446. doi: 10.1111/j.1365-3040.2006.01631.x
Jarvis, M. C. (1984). Structure and properties of pectin gels in plant-cell walls. Plant Cell Environ. 7 (3), 153–164. doi: 10.1111/1365-3040.Ep11614586
Jones, L., McQueen-Mason, S. (2004). A role for expansins in dehydration and rehydration of the resurrection plant Craterostigma plantagineum. FEBS Lett. 559 (1-3), 61–65. doi: 10.1016/S0014-5793(04)00023-7
Jovanović, Ž., Rakić, T., Stevanović, B., Radović, S. (2011). Characterization of oxidative and antioxidative events during dehydration and rehydration of resurrection plant Ramonda nathaliae. Plant Growth Regul. 64 (3), 231–240. doi: 10.1007/s10725-011-9563-4
Jung, N. U., Giarola, V., Chen, P., Paul Knox, J., Bartels, D. (2019). Craterostigma plantagineum cell wall composition is remodelled during desiccation and the glycine-rich protein CpGRP 1 interacts with pectins through clustered arginines. Plant J. 100, 661–676. doi: 10.1111/tpj.14479
Junttila, S., Laiho, A., Gyenesei, A., Rudd, S. (2013). Whole transcriptome characterization of the effects of dehydration and rehydration on Cladonia rangiferina, the grey reindeer lichen. BMC Genomics 14 (1), 870. doi: 10.1186/1471-2164-14-870
Kangasjärvi, S., Kangasjärvi, J. (2014). Towards understanding extracellular ROS sensory and signaling systems in plants. Adv. Bot. 2014, 538946. doi: 10.1155/2014/538946
Karuppanapandian, T., Geilfus, C. M., Muhling, K. H., Novak, O., Gloser, V. (2017). Early changes of the pH of the apoplast are different in leaves, stem and roots of Vicia faba L. under declining water availability. Plant Sci. 255, 51–58. doi: 10.1016/j.plantsci.2016.11.010
Kimura, S., Waszczak, C., Hunter, K., Wrzaczek, M. (2017). Bound by fate: The role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 29 (4), 638–654. doi: 10.1105/tpc.16.00947
Knoch, E., Dilokpimol, A., Geshi, N. (2014). Arabinogalactan proteins: focus on carbohydrate active enzymes. Front. Plant Sci. 5, 198. doi: 10.3389/Fpls.2014.00198
Kobayashi, M., Matoh, T., Azuma, J. (1996). Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 110 (3), 1017–1020. doi: 10.1104/pp.110.31017
Kohorn, B. D., Kobayashi, M., Johansen, S., Riese, J., Huang, L. F., Koch, K., et al. (2006). An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 46 (2), 307–316. doi: 10.1111/j.1365-313X.2006.02695.x
Krebs, M., Held, K., Binder, A., Hashimoto, K., Den Herder, G., Parniske, M., et al. (2012). FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 69 (1), 181–192. doi: 10.1111/j.1365-313X.2011.04780.x
Kurusu, T., Kuchitsu, K., Nakano, M., Nakayama, Y., Iida, H. (2013). Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 18 (4), 227–233. doi: 10.1016/j.tplants.2012.12.002
Lamport, D. T., Varnai, P., Seal, C. E. (2014). Back to the future with the AGP-Ca2+ flux capacitor. Ann. Bot. 114 (6), 1069–1085. doi: 10.1093/aob/mcu161
Lampugnani, E. R., Khan, G. A., Somssich, M., Persson, S. (2018). Building a plant cell wall at a glance. J. Cell Sci. 131 (2), jcs207373. doi: 10.1242/jcs.207373
Le Gall, H., Philippe, F., Domon, J. M., Gillet, F., Pelloux, J., Rayon, C. (2015). Cell wall metabolism in response to abiotic stress. Plants (Basel) 4 (1), 112–166. doi: 10.3390/plants4010112
Levitt, J., Levitt, J. (1987). Responses of plants to environmental stresses (Vol. 2) (New York: Academic Press).
Lewicki, P. P. (1998). Effect of pre-drying treatment, drying and rehydration on plant tissue properties: a review. Int. J. Food Prop. 1 (1), 1–22. doi: 10.1080/10942919809524561
Lindner, H., Müller, L. M., Boisson-Dernier, A., Grossniklaus, U. (2012). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15 (6), 659–669. doi: 10.1016/j.pbi.2012.07.003
Lu, Z. J., Neumann, P. M. (1998). Water-stressed maize, barley and rice seedlings show species diversity in mechanisms of leaf growth inhibition. J. Exp. Bot. 49 (329), 1945–1952. doi: 10.1093/jexbot/49.3291945
Ma, H., Zhao, J. (2010). Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.). J. Exp. Bot. 61 (10), 2647–2668. doi: 10.1093/jxb/erq104
Ma, C., Wang, H., Macnish, A. J., Estrada-Melo, A. C., Lin, J., Chang, Y. H., et al. (2015). Transcriptomic analysis reveals numerous diverse protein kinases and transcription factors involved in desiccation tolerance in the resurrection plant Myrothamnus flabellifolia. Hortic. Res. 2, 15034. doi: 10.1038/Hortres.2015.34
Ma, X. F., Wang, W. M., Bittner, F., Schmidt, N., Berkey, R., Zhang, L. L., et al. (2016). Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell 28 (5), 1108–1126. doi: 10.1105/tpc.15.00880
Mak, M., Babla, M., Xu, S.-C., O’Carrigan, A., Liu, X.-H., Gong, Y.-M., et al. (2014). Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 98, 1–12. doi: 10.1016/j.envexpbot.2013.10.003
Makavitskaya, M., Svistunenko, D., Navaselsky, I., Hryvusevich, P., Mackievic, V., Rabadanova, C., et al. (2018). Novel roles of ascorbate in plants: induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 69 (14), 3477–3489. doi: 10.1093/jxb/ery056
Mangano, S., Pacheco, J. M., Marino-Buslje, C., Estevez, J. M. (2018). How does pH fit in with oscillating polar growth? Trends Plant Sci. 23 (6), 479–489. doi: 10.1016/j.tplants.2018.02.008
Mariaux, J. B., Bockel, C., Salamini, F., Bartels, D. (1998). Desiccation- and abscisic acid-responsive genes encoding major intrinsic proteins (MIPs) from the resurrection plant Craterostigma plantagineum. Plant Mol. Biol. 38 (6), 1089–1099. doi: 10.1023/a:1006013130681
Medvedev, S. S. (2005). Calcium signaling system in plants. Russ. J. Plant Physiol. 52 (2), 249–270. doi: 10.1007/s11183-005-0038-1
Micheli, F. (2001). Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6 (9), 414–419. doi: 10.1016/S1360-1385(01)02045-3
Mihailova, G., Kocheva, K., Goltsev, V., Kalaji, H. M., Georgieva, K. (2018). Application of a diffusion model to measure ion leakage of resurrection plant leaves undergoing desiccation. Plant Physiol. Bioch. 125, 185–192. doi: 10.1016/j.plaphy.2018.02.008
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33 (4), 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
Mittler, R. (2017). ROS are good. Trends Plant Sci. 22 (1), 11–19. doi: 10.1016/j.tplants.2016.08.002
Mohnen, D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277. doi: 10.1016/j.pbi.2008.03.006
Moore, P. J., Darvill, A. G., Albersheim, P., Staehelin, L. A. (1986). Immunogold localization of xyloglucan and rhamnogalacturonan-I in the cell-walls of suspension-cultured sycamore cells. Plant Physiol. 82 (3), 787–794. doi: 10.1104/Pp.82.3.787
Moore, J. P., Nguema-Ona, E., Chevalier, L., Lindsey, G. G., Brandt, W. F., Lerouge, P., et al. (2006). Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiol. 141 (2), 651–662. doi: 10.1104/pp.106.077701
Moore, J. P., Vicre-Gibouin, M., Farrant, J. M., Driouich, A. (2008). Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol. Plant 134 (2), 237–245. doi: 10.1111/j.1399-3054.2008.01134.x
Moore, J. P., Nguema-Ona, E. E., Vicre-Gibouin, M., Sorensen, I., Willats, W. G. T., Driouich, A., et al. (2013). Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237 (3), 739–754. doi: 10.1007/s00425-012-1785-9
Moscatiello, R., Mariani, P., Sanders, D., Maathuis, F. J. M. (2006). Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J. Exp. Bot. 57 (11), 2847–2865. doi: 10.1093/jxb/erl043
Murphy, E., De Smet, I. (2014). Understanding the RALF family: a tale of many species. Trends Plant Sci. 19 (10), 664–671. doi: 10.1016/j.tplants.2014.06.005
Nürnberger, T., Kemmerling, B. (2018). Pathogen-associated molecular patterns (PAMP) and PAMP-triggered immunity. Ann. Plant Revi. Online, 34, 16–47. doi: 10.1002/9781119312994.apr0362
Nielsen, M. E., Feechan, A., Böhlenius, H., Ueda, T., Thordal-Christensen, H. (2012). Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc. Natl. Acad. Sci. U.S.A. 109 (28), 11443–11448. doi: 10.1073/pnas.1117596109
Nishiyama, Y. (2009). Structure and properties of the cellulose microfibril. J. Wood Sci. 55 (4), 241–249. doi: 10.1007/s10086-009-1029-1
Novaković, L., Guo, T., Bacic, A., Sampathkumar, A., Johnson, K. (2018). Hitting the wall—sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 7 (4), 89. doi: 10.3390/plants7040089
O’Brien, J. A., Daudi, A., Finch, P., Butt, V. S., Whitelegge, J. P., Souda, P., et al. (2012). A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 158 (4), 2013–2027. doi: 10.1104/pp.111.190140
O’Neill, M. A., Eberhard, S., Albersheim, P., Darvill, A. G. (2001). Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294, 846–849. doi: 10.1126/science.1062319
Osorio, S., Castillejo, C., Quesada, M. A., Medina-Escobar, N., Brownsey, G. J., Suau, R., et al. (2008). Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J. 54 (1), 43–55. doi: 10.1111/j.1365-313X.2007.03398.x
Park, Y. B., Cosgrove, D. J. (2015). Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 56 (2), 180–194. doi: 10.1093/pcp/pcu204
Parre, E., Geitmann, A. (2005). Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220 (4), 582–592. doi: 10.1007/s00425-004-1368-5
Passardi, F., Penel, C., Dunand, C. (2004). Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 9 (11), 534–540. doi: 10.1016/j.tplants.2004.09.002
Phillips, J. R., Fischer, E., Baron, M., Van Den Dries, N., Facchinelli, F., Kutzer, M., et al. (2008). Lindernia brevidens: a novel desiccation-tolerant vascular plant, endemic to ancient tropical rainforests. Plant J. 54 (5), 938–948. doi: 10.1111/j.1365-313X.2008.03478.x
Pilling, E., Höfte, H. (2003). Feedback from the wall. Curr. Opin. Plant Biol. 6 (6), 611–616. doi: 10.1016/j.pbi.2003.09.004
Plancot, B., Gügi, B., Mollet, J. C., Loutelier-Bourhis, C., Govind, S. R., Lerouge, P., et al. (2019). Desiccation tolerance in plants: Structural characterization of the cell wall hemicellulosic polysaccharides in three Selaginella species. Carbohyd. Polym. 208, 180–190. doi: 10.1016/j.carbpol.2018.12.051
Ramanjulu, S., Bartels, D. (2002). Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 25 (2), 141–151. doi: 10.1046/j.0016-8025.2001.00764.x
Ringli, C. (2010). Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 153 (4), 1445–1452. doi: 10.1104/pp.110.154518
Rodriguez, M. C. S., Edsgärd, D., Hussain, S. S., Alquezar, D., Rasmussen, M., Gilbert, T., et al. (2010). Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum. Plant J. 63 (2), 212–228. doi: 10.1111/j.1365-313X.2010.04243.x
Rose, J. K., Braam, J., Fry, S. C., Nishitani, K. (2002). The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol. 43 (12), 1421–1435. doi: 10.1093/pcp/pcf171
Ryden, P., Sugimoto-Shirasu, K., Smith, A. C., Findlay, K., Reiter, W. D., McCann, M. C. (2003). Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 132 (2), 1033–1040. doi: 10.1104/pp.103.021873
Saladié, M., Rose, J. K., Cosgrove, D. J., Catalá, C. (2006). Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 47 (2), 282–295. doi: 10.1111/j.1365-313X.2006.02784.x
Sasidharan, R., Voesenek, L. A. C. J., Pierik, R. (2011). Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 30 (6), 548–562. doi: 10.1080/07352689.2011.615706
Seifert, G. J., Blaukopf, C. (2010). Irritable Walls: The plant extracellular matrix and signaling. Plant Physiol. 153 (2), 467–478. doi: 10.1104/pp.110.153940
Sherwin, H. W., Farrant, J. M. (1998). Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul. 24 (3), 203–210. doi: 10.1023/A:1005801610891
Shivaraj, Y. N., Barbara, P., Gugi, B., Vicre-Gibo, M., Driouich, A., Govind, S. R., et al. (2018). Perspectives on structural, physiological, cellular, and molecular responses to desiccation in resurrection plants. Scientifica 2018, 18. doi: 10.1155/2018/9464592
Simmons, T. J., Mortimer, J. C., Bernardinelli, O. D., Pöppler, A. C., Brown, S. P., Deazevedo, E. R., et al. (2016). Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun. 7, 13902. doi: 10.1038/ncomms13902
Spoel, S. H., Loake, G. J. (2011). Redox-based protein modifications: the missing link in plant immune signalling. Curr. Opin. Plant Biol. 14, 358–364. doi: 10.1016/j.pbi.2011.03.007
Staehelin, L. A., Moore, I. (1995). The plant Golgi apparatus: structure, functional organization and trafficking mechanisms. Annu. Rev. Plant Phys. 46 (1), 261–288. doi: 10.1146/annurev.pp.46.060195.001401
Sterling, J. D., Quigley, H. F., Orellana, A., Mohnen, D. (2001). The catalytic site of the pectin biosynthetic enzyme α-1, 4-galacturonosyltransferase is located in the lumen of the Golgi. Plant Physiol. 127 (1), 360–371. doi: 10.1104/pp.127.1.360
Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M. A., Mittler, R. (2011). Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 14 (6), 691–699. doi: 10.1016/j.pbi.2011.07.014
Takeda, T., Furuta, Y., Awano, T., Mizuno, K., Mitsuishi, Y., Hayashi, T. (2002). Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc. Natl. Acad. Sci. U.S.A. 99 (13), 9055–9060. doi: 10.1073/pnas.132080299
Tan, L., Tees, D., Qian, J., Kareem, S., Kieliszewski, M. J. (2018). Intermolecular interactions between glycomodules of plant cell wall arabinogalactan-proteins and extensins. Cell Surf. 1, 25–33. doi: 10.1016/j.tcsw.2018.03.001
Tardieu, F., Parent, B., Caldeira, C. F., Welcker, C. (2014). Genetic and physiological controls of growth under water deficit. Plant Physiol. 164 (4), 1628–1635. doi: 10.1104/pp.113.233353
Tenhaken, R. (2015). Cell wall remodeling under abiotic stress. Front. Plant Sci. 5, 771. doi: 10.3389/Fpls.2014.00771
Thomson, W. W., Platt, K. A. (1997). Conservation of cell order in desiccated mesophyll of Selaginella lepidophylla ([Hook and Grev] Spring). Ann. Bot-London. 79, 439–447. doi: 10.1006/anbo.19960375
Valentin, R., Cerclier, C., Geneix, N., Aguie-Beghin, V., Gaillard, C., Ralet, M. C., et al. (2010). Elaboration of extensin-pectin thin film model of primary plant cell wall. Langmuir 26 (12), 9891–9898. doi: 10.1021/la100265d
VanBuren, R., Bryant, D., Edger, P. P., Tang, H. B., Burgess, D., Challabathula, D., et al. (2015). Single-molecule sequencing of the desiccation-tolerant grass Oropetium thomaeum. Nature 527 (7579), 508–U209. doi: 10.1038/nature15714
VanBuren, R., Wai, C. M., Zhang, Q., Song, X., Edger, P. P., Bryant, D., et al. (2017). Seed desiccation mechanisms co-opted for vegetative desiccation in the resurrection grass Oropetium thomaeum. Plant Cell Environ. 40 (10), 2292–2306. doi: 10.1111/pce.13027
Veley, K. M., Maksaev, G., Frick, E. M., January, E., Kloepper, S. C., Haswell, E. S. (2014). Arabidopsis MSL10 has a regulated cell death signaling activity that is separable from its mechanosensitive ion channel activity. Plant Cell 26 (7), 3115–3131. doi: 10.1105/tpc.114.128082
Vicré, M., Sherwin, H. W., Driouich, A., Jaffer, M. A., Farrant, J. M. (1999). Cell wall characteristics and structure of hydrated and dry leaves of the resurrection plant Craterostigma wilmsii, a microscopical study. J. Plant Physiol. 155 (6), 719–726. doi: 10.1016/S0176-1617(99)80088-1
Vicré, M., Farrant, J. M., Driouich, A. (2004a). Insights into the cellular mechanisms of desiccation tolerance among angiosperm resurrection plant species. Plant Cell Environ. 27 (11), 1329–1340. doi: 10.1111/j.1365-3040.2004.01212.x
Vicré, M., Lerouxel, O., Farrant, J., Lerouge, P., Driouich, A. (2004b). Composition and desiccation-induced alterations of the cell wall in the resurrection plant Craterostigma wilmsii. Physiol. Plant 120 (2), 229–239. doi: 10.1111/j.0031-9317.2004.0234.x
Voothuluru, P., Sharp, R. E. (2013). Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. J. Exp. Bot. 64 (5), 1223–1233. doi: 10.1093/jxb/ers277
Voxeur, A., Höfte, H. (2016). Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 26 (9), 950–960. doi: 10.1093/glycob/cww029
Wang, L., Shang, H., Liu, Y., Zheng, M., Wu, R., Phillips, J., et al. (2009). A role for a cell wall localized glycine-rich protein in dehydration and rehydration of the resurrection plant Boea hygrometrica. Plant Biol. 11 (6), 837–848. doi: 10.1111/j.1438-8677.2008.00187.x
Wang, T., Zabotina, O., Hong, M. (2012). Pectin-cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 51 (49), 9846–9856. doi: 10.1021/bi3015532
Webb, M. A., Arnott, H. J. (1982). Cell-wall conformation in dry seeds in relation to the preservation of structural integrity during desiccation. Am. J. Bot. 69, 1657–1668. doi: 10.1002/j.1537-2197.1982.tb13418.x
Wolf, S., Mouille, G., Pelloux, J. (2009). Homogalacturonan methyl-esterification and plant development. Mol. Plant 2 (5), 851–860. doi: 10.1093/mp/ssp066
Wolf, S., Hematy, K., Hofte, H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63, 381–407. doi: 10.1146/annurev-arplant-042811-105449
Wrzaczek, M., Brosché, M., Kangasjärvi, J. (2013). ROS signaling loops—production, perception, regulation. Curr. Opin. Plant Biol. 16 (5), 575–582. doi: 10.1016/j.pbi.2013.07.002
Xiao, L. H., Yang, G., Zhang, L. C., Yang, X. H., Zhao, S., Ji, Z. Z., et al. (2015). The resurrection genome of Boea hygrometrica: A blueprint for survival of dehydration. Proc. Natl. Acad. Sci. U.S.A. 112 (18), 5833–5837. doi: 10.1073/pnas.1505811112
Xu, Z., Xin, T., Bartels, D., Li, Y., Gu, W., Yao, H., et al. (2018). Genome analysis of the ancient tracheophyte Selaginella tamariscina reveals evolutionary features relevant to the acquisition of desiccation tolerance. Mol. Plant. 11 (7), 983–994. doi: 10.1016/j.molp.2018.05.00
Yobi, A., Schlauch, K. A., Tillett, R. L., Yim, W. C., Espinoza, C., Wone, B. W. M., et al. (2017). Sporobolus stapfianus: Insights into desiccation tolerance in the resurrection grasses from linking transcriptomics to metabolomics. BMC Plant Biol. 17 (1), 67. doi: 10.1186/s12870-017-1013-7
Zablackis, E., Huang, J., Muller, B., Darvill, A. G., Albersheim, P. (1995). Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107 (4), 1129–1138. doi: 10.1104/pp.107.41129
Zhang, Q., Bartels, D. (2018). Molecular responses to dehydration and desiccation in desiccation-tolerant angiosperm plants. J. Exp. Bot. 69 (13), 3211–3222. doi: 10.1093/jxb/erx489
Zhang, G. F., Staehelin, L. A. (1992). Functional compartmentation of the Golgi apparatus of plant cells: immunocytochemical analysis of high-pressure frozen-and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99 (3), 1070–1083. doi: 10.1104/pp.99.31070
Keywords: cell wall composition, cell wall signaling, transcriptomes, resurrection plants, dehydration, rehydration
Citation: Chen P, Jung NU, Giarola V and Bartels D (2020) The Dynamic Responses of Cell Walls in Resurrection Plants During Dehydration and Rehydration. Front. Plant Sci. 10:1698. doi: 10.3389/fpls.2019.01698
Received: 18 September 2019; Accepted: 02 December 2019;
Published: 21 January 2020.
Edited by:
John Moore, Stellenbosch University, South AfricaReviewed by:
Tsanko Savov Gechev, Plovdiv University “Paisii Hilendarski”, BulgariaCopyright © 2020 Chen, Jung, Giarola and Bartels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorothea Bartels, ZGJhcnRlbHNAdW5pLWJvbm4uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.