- 1State Key Laboratory of Crop Stress Biology for Arid Areas, Shaanxi Key Laboratory of Apple, College of Horticulture, Northwest A&F University, Yangling, China
- 2College of Forestry, Northwest A&F University, Yangling, China
Melatonin has been reported to play roles in regulating carbohydrate levels and plant growth. However, little is known about the exact mechanism by which melatonin regulates sugar levels and growth in plants. In this study, it was found that high levels of melatonin inhibited the growth of wild-type (WT) apple plants and induced significant accumulations of fructose, glucose, and sucrose in apple leaves, while MdFRK2 expression was significantly downregulated. MdFRK2 promoter transiently expressed in tobacco leaves further supported that the expression of MdFRK2 could be inhibited by exogenous melatonin. After applying exogenous melatonin, the suppression of MdFRK2 expression was significantly rescued in transgenic apples overexpressing MdFRK2 via the 35S promoter. Fructose, glucose, and sucrose concentrations increased less as compared to WT apple plants. Wild-type plants showed a stunted phenotype 21 days after melatonin treatment, while MdFRK2-overexpressing plants exhibited slightly inhibited growth, indicating that the downregulated MdFRK2 expression in response to melatonin was involved in melatonin-mediated growth inhibition. Taken together, these results demonstrate the involvement of MdFRK2 in melatonin-induced sugar accumulation and growth inhibition. Our findings shed light on the roles played by MdFRK2 in connecting melatonin action and plant growth.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), a small, highly conserved molecule involved in the process of biological evolution, is widely present in organisms including bacteria, unicellular eukaryotes, macroalgae, fungi, higher plants, invertebrates, and vertebrates (Tan et al., 2010). Although melatonin is known mainly as an animal hormone and neurotransmitter, its role in plants is currently being extensively investigated (Erland et al., 2015). Melatonin acts as a plant growth and development regulator as well as a biostimulant or perhaps even as a DAMP when some cells are leaking as a consequence of (a) biotic stresses (Hardeland et al., 2012; Versluys et al., 2017). It plays a role in the plant photoperiod response, scavenging of reactive oxygen species and protection of plants against bacterial pathogens by activating expression of defense-related genes (Park et al., 2013; Wang et al., 2013, 2015; Liang et al., 2015; Qian et al., 2015; Mandal et al., 2018). As a result, exogenous melatonin is widely used to improve resistance against biotic/abiotic stress or to regulate plant growth. Although one melatonin receptor has been identified in Arabidopsis thaliana (Wei et al., 2018), the understanding of the functional mechanisms of melatonin action in plants is very limited.
Exogenous melatonin significantly affects carbohydrate/sugar concentrations in maize (Zea mays) (Zhao et al., 2015a), apple (Malus) (Wang et al., 2015), Arabidopsis (Zhao et al., 2015b), and Prunus avium × Prunus cerasus (Sarropoulou et al., 2012). Carbohydrate fuels all the processes of plant metabolic pathways and satisfies the extra requirements for ATP, NADPH, and other metabolites. Soluble sugar molecules such as sucrose, glucose (Rolland et al., 2006) and fructose (Cho and Yoo, 2011) act as signaling molecules that regulate the expression of both various metabolism- and defense-related genes and metabolic processes that control plant growth and development (Rolland et al., 2006; Janse van Rensburg et al., 2019). The novel ‘sweet immunity’ concept predicts that sweet, endogenous saccharides such as sucrose, raffnose family oligosaccharides, fructans, and galactinol may act as signaling molecules that are activated by exposure to stress and hence initiate signal amplification and lead to more rapid and robust activation of defense, immunity and stress tolerance (Bolouri Moghaddam and Van den Ende, 2012, 2013; Versluys et al., 2017). As a signaling molecule, hexose accumulation in leaves represents the onset of senescence and inhibits growth, as this accumulation tends to repress photosynthesis (Cho and Yoo, 2011). As reported (Zhao et al., 2015a), high doses of melatonin stopped seedling growth and caused leaf senescence by inducing excessive accumulations of sucrose, hexose, and starch in the leaves of maize seedlings.
In plant cells, sugar concentrations are highly regulated by sugar metabolism, which involves the breakdown of sucrose by invertase and sucrose synthase (SUSY), the phosphorylation of the resulting hexoses and the interconversion between hexose phosphates and UDP-glucose, and the synthesis of sucrose via SPS and SPP (Pego and Smeekens, 2000; Ruan, 2014; Li et al., 2018). In maize seedlings, sucrose synthesis and hydrolysis increased in response to exogenous melatonin application, as reflected by the elevated gene expression and enzymatic activities of SPS and acid invertase (Zhao et al., 2015a). However, the expression of FRK2, a gene encoding fructokinase (FRK), which phosphorylates fructose to fructose-6-phosphate (F6P), was downregulated in both the source and sink parts of the maize leaves, especially under high concentrations of melatonin applications (Zhao et al., 2015a).
Fructokinase is the gateway to fructose metabolism, and FRK2 orthologs are the major fructose-phosphorylating high-affinity enzymes in tomato (Kanayama et al., 1997, 1998), rice (Oryza sativa) (Jiang et al., 2003), maize (Zhang et al., 2003), and potato (Solanum tuberosum) (Renz and Stitt, 1993). Suppression of the FRK2 orthologs resulted in phenotypes of plants (increased sugar concentrations and inhibited growth) that were similar to those of plants treated with high concentrations of melatonin. For example, antisense suppression of the FRK2 ortholog (StFRK1) in potato resulted in increased levels of sugars (fructose, glucose, sucrose) and reduced aerial growth (Davies et al., 2005). In tomato, RNA interference (RNAi)-LeFRK2 plants exhibited diminished stem growth and wilted leaves—phenotypes that are related to a reduction in the area of active xylem in the stem (German et al., 2003). RNAi-FRK2 aspen plants also presented elevated concentrations of fructose, glucose, and sucrose and decreased cell wall fiber thickness due to the decreased carbon flux to cell wall polysaccharide precursors (Roach et al., 2012). Additionally, our results showed that overexpressing MdFRK2 in apple decreased the concentrations of fructose, glucose, and sucrose while both SPS and neutral invertase expression and enzyme activity decreased (Yang et al., 2018). Overall, there seems to be an intimate link between FRK2 expression and melatonin application. It can be speculated that FRK2 is involved in melatonin-mediated variations in sugar metabolism and stunted growth.
To test this hypothesis, we investigated the influence of exogenous melatonin on sugar concentrations and the expression of genes related to sugar metabolism in mature leaves of apple. The MdFRK2 promoter coupled to a β-glucuronidase (GUS) reporter gene was transiently expressed in tobacco leaves to further confirm the influence of melatonin on MdFRK2 expression. Last, transgenic apple lines overexpressing MdFRK2 were used to further investigate the role of MdFRK2 in regulating sugar concentrations and plant growth under exogenous melatonin application. In this study, our results show that melatonin regulates sugar accumulation and plant growth by inhibiting FRK2 expression, providing new insights into the roles played by melatonin in regulating sugar concentrations in plants.
Materials and Methods
Plant Materials and Treatments
Wild-type Malus domestica cv. ‘Royal Gala’ tissue-cultured plants were initially grown on an MS medium supplemented with 0.2 mg L−1 IAA and 0.3 mg L−1 6-BA for 4 weeks. They were then transferred to a rooting medium (MS + 0.5 mg IBA and 0.5 mg IAA) for about 60 days. After rooting, the tissue-cultured plants were transplanted to small black plastic pots with good drainage (12 cm × 12 cm) that were filled with soil and sand, and were transferred to a culture room maintained at 23°C and a 14-h photoperiod supplemented with fluorescent light (60 μM m−2 s−1). One month after acclimating to controlled conditions, plants of similar growth and size were selected for exogenous melatonin treatments. The control was, supplied with thirty milliliters water every 3 days. Treatments received thirty milliliters melatonin solution at concentrations of 0, 100, 500, 1,000, and 5,000 μM as described before (Wang et al., 2013), applied to the soil in each pot every 3 days for 14 days. Afterward, plant heights were measured, and the fourth to seventh mature leaves from the base of the stem (fully mature leaves) were harvested and stored at −80°C.
We obtained MdFRK2-OE transgenic apple lines (L1, L4, and L9) with the cauliflower mosaic virus 35S promoter (CaMV 35S) promoter (Supplementary Figure S1). Compared to untransformed WT lines, MdFRK2 expression increased by 9.2-, 13.0-, and 13.2-fold in lines L1, L4, and L9, respectively. To test whether MdFRK2 is involved in melatonin-mediated variations in sugar metabolism and growth inhibition, MdFRK2-OE tissue-cultured plants (L1, L4, and L9) were selected to receive exogenous melatonin treatments. The growth and culture conditions of the transgenic plants are the same as those of WT described above. In a previous study, 1,000 μM of melatonin initially had an inhibitory effect on the growth of WT plants, so we selected 1,000 μM as the most appropriate concentration for the treatment of the MdFRK2-OE lines. Healthy and uniform plants of WT and transgenic lines (L1, L4, and L9) were selected to receive melatonin application. Regarding the WT and transgenic lines (L1, L4, and L9), control group was irrigated with standard water, and another group was irrigated with 1,000 μM of melatonin dissolved in the irrigation water. The treatment method was similar to that mentioned above. We recorded the day on which the melatonin treatment was started as the first day, and the plant height was measured every 7 days.
Tobacco plants (Nicotiana tabacum L., ‘Samsun NN’) were grown at 25°C, and the 16-h photoperiod was supplemented with lamps at 120 μM m−2 s−1. After 1 month, 35 plants were separated into seven groups (one group consisted of at least four plants as one biological replicate). Five groups were irrigated with different concentrations of exogenous melatonin (10, 100, 500, 1,000, or 5,000 μM) while another two groups were well-watered as controls.
Analysis of Soluble Sugars
Soluble sugars were obtained and derivatized according to the method of Yang et al. (2018). Each sample (0.1 g) was extracted in 1.4 ml of 75% methanol, and ribitol was added as an internal standard. After fractionation of non-polar metabolites in chloroform, 5 μL of the polar phase was transferred to 2.0 ml Eppendorf vials to measure the metabolites in each sample, such as sorbitol, sucrose, glucose, and fructose. The metabolites were dried under vacuum without heating and then derivatized with methoxyamine hydrochloride and N-methyl-N-trimethylsilyl-trifluoroacetamide sequentially. Afterward, the metabolites were analyzed with a Shimadzu GCMS-2010SE device (Shimadzu Corporation, Kyoto, Japan). The metabolites were identified by comparing their fragmentation patterns with those from a mass spectral library generated on our GC/MS system and those from an annotated quadrupole GC/MS spectral library downloaded from the Golm Metabolome Database1. The quantifications were based on standard curves generated for each metabolite and internal standard.
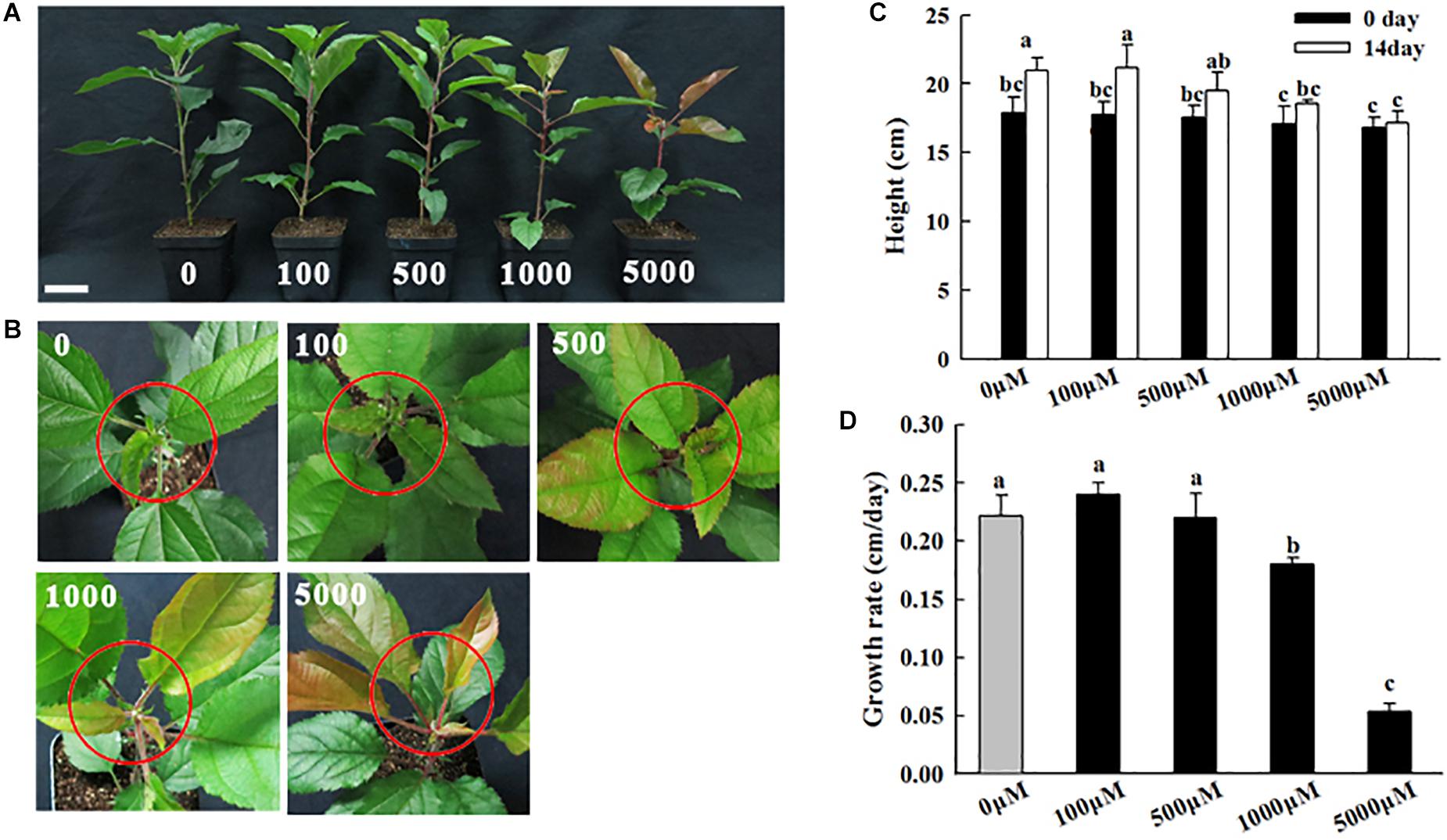
Figure 1. Phenotypes of apple plants under different concentrations of melatonin treatment (0, 100, 500, 1,000, and 5,000 μM). (A) Growth performance, (B) shoot tip phenotypes, (C) stem height, and (D) growth rate were recorded after 14 days of treatment. The plant height was considered the average of 6 plants per treatment. The red circles indicate the shoot tips. Values are means of four replicates SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test. Scale bars represent 5 cm.
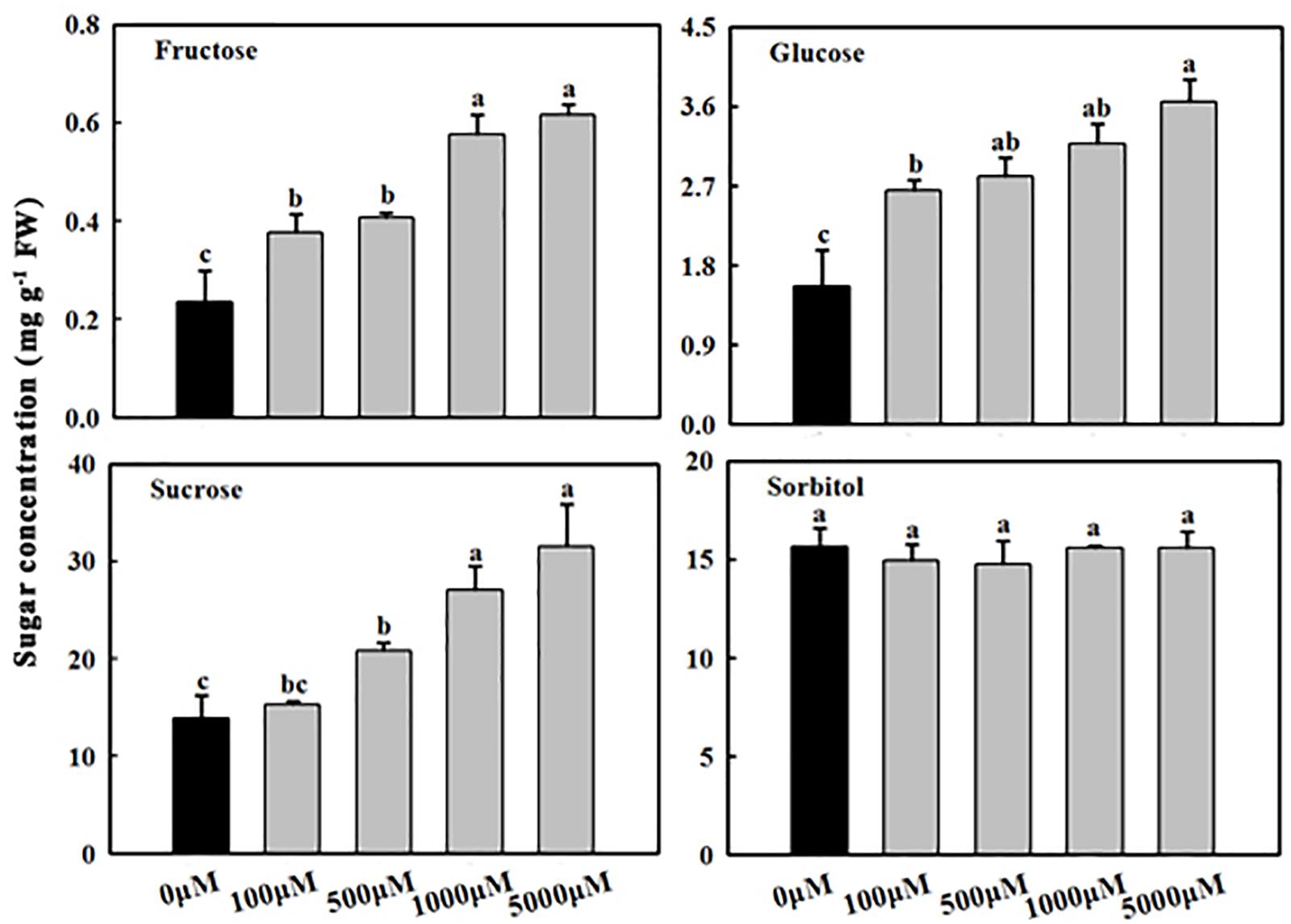
Figure 2. Effects of exogenous melatonin on fructose, glucose, sucrose, and sorbitol concentrations in apple leaves. Samples were obtained from fully mature leaves (the fourth to seventh leaves from the base of the stem) of WT plants after 14 days of melatonin applications (0, 100, 500, 1,000, and 5,000 μM). Values are means of four replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test.
RNA Extraction and qRT-PCR Assays
The total RNA was extracted from each sample using an RNAprep plant kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. The RNA was then reverse-transcribed into cDNA with a PrimeScriptTM RT reagent kit and gDNA Eraser to avoid possible genomic DNA contamination (TaKaRa, Dalian, China). The primer sequences used for quantitative real-time polymerase chain reaction (qRT-PCR) assays are presented in Supplementary Table S1. Mdactin (CN938023) was chosen as an internal control for data normalization. qRT-PCR was performed on an ABI 7300 Real-Time PCR instrument (Thermo Fisher Scientific) using a SYBR Green Premix Ex Taq Kit (TaKaRa, Kyoto, Japan). The PCR conditions were as follows: predenaturing at 94°C for 5 min followed by 40 cycles of 95°C for 15 s and 58°C for 60 s. For each sample, total RNA was extracted from three biological replicates. The data were analyzed using the ddCT method.
Assays of Enzyme Activities
The enzymes in the mature leaves of WT and transgenic lines were extracted as described by Li et al. (2012), with some modifications. Briefly, a 0.5 g sample was homogenized in 2 ml of 200 mM Hepes-KOH (pH 8.0) buffer, containing 5 mM MgCl2, 2 mM EDTA, 2.5 mM dithiothreitol (DTT), 2 mM benzamidine, 2 mM ε-aminocaproic acid, 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 1% bovine serum albumin (BSA) (w/v), 2% glycerol (v/v), 0.05% Triton X-100 (v/v) and 2% polyvinylpyrrolidone (PVP) (w/v). The extract was then centrifuged at 16,000 g for 20 min at 4°C, after which 1 ml of the supernatant was immediately desalted in a Sephadex G25 PD-10 column (GE Healthcare, United Kingdom) and subsequently equilibrated with the above mentioned extraction buffer containing a concentration of 50 mM Hepes-KOH (pH 7.4) but without Triton X-100, DTT, BSA, and PVP.
We determined the activity of SPS as described by Li et al. (2016). The assay mixture (200 μl) contained 50 mM Hepes-KOH (pH 7.4), 4 mM MgCl2, 1 mM EDTA, 4 mM F6P, 3 mM UDP-glucose, 20 mM G6P and 100 μl of the sample. The reaction was carried out at 27°C for 30 min, then it was stopped by boiling in water for 3 min. The blank was run for each assay by adding denatured extracts. After centrifugation for 2 min at 12,000 g, 75 μl of the reaction mixture was used for UDP measurements in a spectrophotometric assay. The assay mixture (1.0 mL) contained 50 mM Hepes-KOH (pH 7.0), 0.8 mM phosphoenolpyruvate, 0.3 mM NADH, 5 mM MgCl2, 14 U of lactate dehydrogenase, and 4 U of pyruvate kinase (to start the reaction).
Neutral invertase was incubated at 37°C for 60 min in a 200 μl assay mixture that contained 100 mM phosphate-citrate buffer (pH 7.2), 100 mM sucrose, and 50 μl of the desalted extract or denatured extract (as a blank). The assay was stopped by boiling for 3 min before adding 0.75 M Tris-HCl buffer (pH 8.5). The blanks contained the same mixture, but the extract was boiled for 5 min before being mixed. The level of glucose produced from sucrose was determined by the enzyme-coupling method (Li et al., 2016).
Sorbitol dehydrogenase activity was assayed in a 1.0 ml reaction mixture that contained 1 mM NAD+, 300 mM sorbitol, and 0.2 ml of desalted extract in 100 mM Tris-HCl (pH 9.6), and NADH production was determined spectrophotometrically at 340 nm.
Hexokinase (HXK) and FRK activities were assayed via a continuous spectrophotometric assay as described by Li et al. (2016), with minor modifications. For HXK, the 0.5 ml assay mixture contained 50 mM Tris-HCl (pH 8.0), 2.5 mM ATP, 4 mM MgCl2, 1 U of G6P dehydrogenase, 0.33 mM NAD+, 1 mM glucose and 25 ml of desalted extract. For FRK, one unit of phosphoglucoisomerase was also added, and 0.4 mM fructose was used instead of glucose.
Promoter Cloning and GUS Activity Analysis
Based on the genome contig sequence of MDC019777.147, we designed primers to clone the promoter of MdFRK2 (Supplementary Table S1). As the promoter sequence, the upstream 1.8 kb region of MdFRK2 mRNA was cloned from the genomic DNA of ‘Royal Gala’ apple. To examine the transient gene expression in tobacco, the MdFRK2 promoter (PMdFRK2) was introduced into a pC0390GUS vector to generate PMdFRK2-GUS. The empty pC0390GUS vector served as a negative control. Agrobacterium-mediated transient transformation was conducted with samples of fully expanded tobacco leaves in accordance with a previously described protocol (Sparkes et al., 2006).
At 48 h after infiltration, the tissues were irrigated with 25 mL of different concentrations of exogenous melatonin (0, 10, 100, 500, 1,000, or 5,000 μM). After 12 h of irrigation, leaves were stained for 2 h using a GUS histochemical assay as previously described (Xu et al., 2011), while two leaves from each plant were harvested to analyze the GUS activity.
Statistical Analysis
The data were expressed as the means ± standard deviations (SDs) of three replicate samples per measurement. All statistical analyses were performed using IBM SPSS Statistics 21 software (SPSS, Inc., Chicago, IL, United States), and graphs were generated with Sigma Plot 12.5. The data were analyzed using one-way ANOVA and Duncan’s multiple range test, where differences were considered significant at P < 0.05.
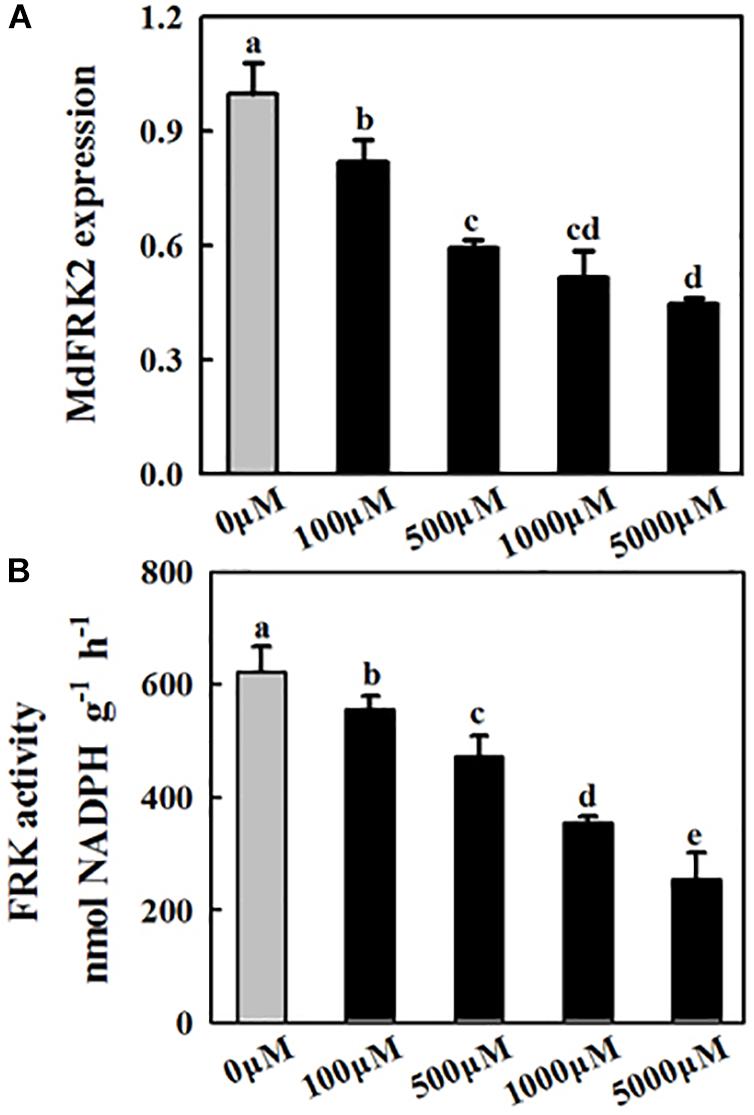
Figure 3. Effects of exogenous melatonin on MdFRK2 expression (A) and FRK activity (B) in apple leaves. Samples were obtained from fully mature leaves (the fourth to seventh leaves from the base of the stem) of WT plants after 14 days of melatonin applications (0, 100, 500, 1,000, and 5,000 μM). The total RNA was isolated, and qRT-PCR was performed with gene-specific primers. For the qRT-PCR assay, the MdActin gene served as an internal control. Values are means of four replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test.
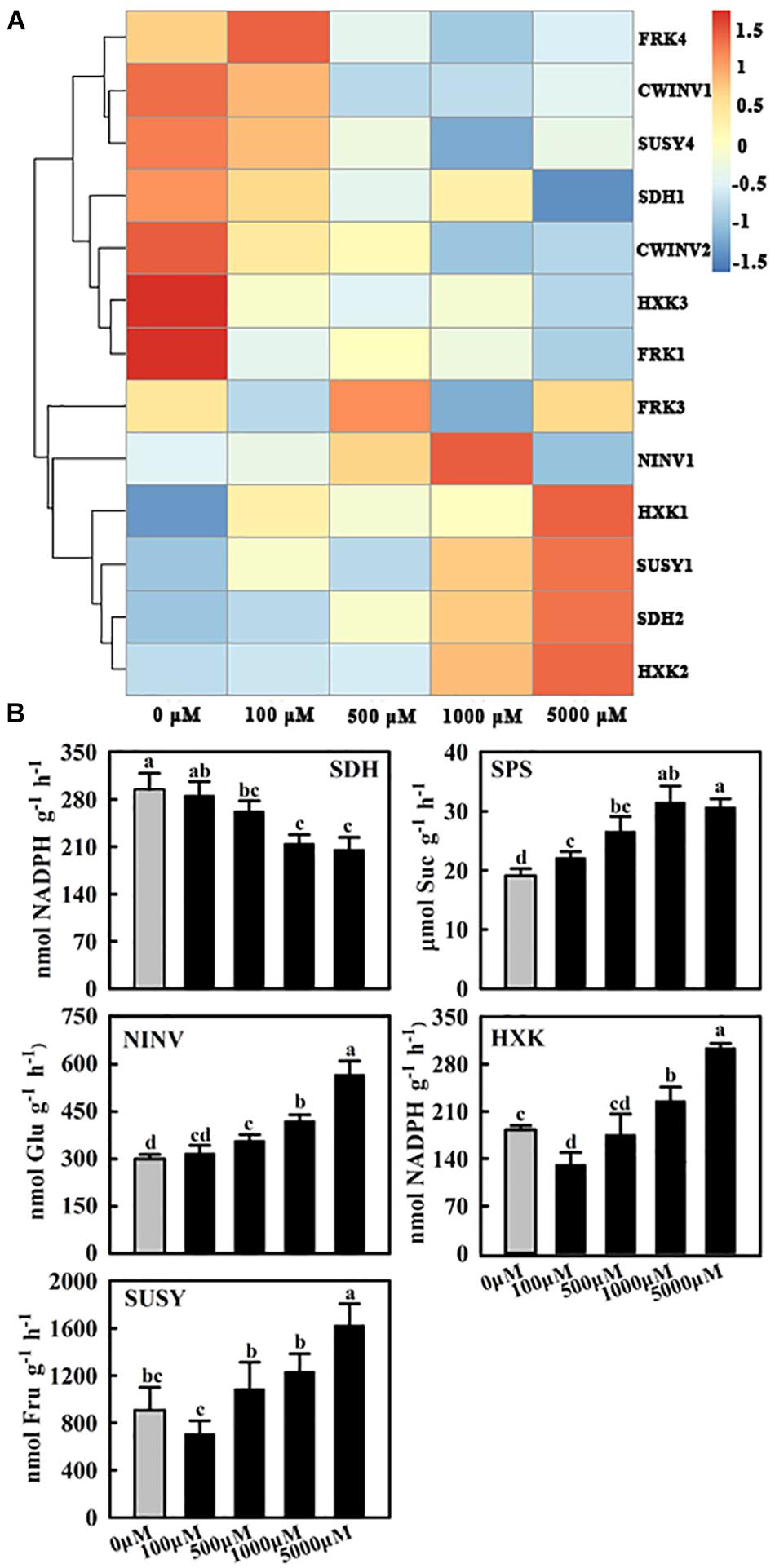
Figure 4. Effects of exogenous melatonin on the relative mRNA expression of genes (A) and activities of enzymes (B) involved in sugar metabolism. Samples were obtained from fully mature leaves (the fourth to seventh leaves from the base of the stem) of WT plants after 14 days of melatonin applications (0, 100, 500, 1,000, and 5,000 μM). The expression was normalized to that of MdActin mRNA. The data represent the means ± SD of three replicates. SPS, sucrose-phosphate synthase; NINV, neutral invertase; SUSY, sucrose synthase; SDH, sorbitol dehydrogenase; HXK, hexokinase. Values are means of four replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test.
Results
Apple Plants Growth Is Affected by Melatonin
To investigate the concentration-dependent effects of melatonin on the growth of apple plants via phenotypic analysis, 2-month-old apple plants grown in soil were irrigated with different concentrations of melatonin solution (0, 100, 500, 1,000, and 5,000 μM) for 14 days (see section “Materials and Methods”). Compared with the control treatment, the low-concentration melatonin treatments (0, 100, 500 μM) caused no significant phenotypic changes; however, the high-concentration melatonin treatments (1,000 and 5,000 μM) inhibited apple plants growth after 14 days of treatment, as reflected by the shoot height and growth rate. Compared to the control, the plant growth rates in response to the 5,000 μM melatonin treatment were reduced by 50.03% (Figures 1A,C). Additionally, when treated with high concentrations of melatonin (1,000 and 5,000 μM), the young leaves and shoot tips of plants clearly appeared red in color (Figure 1B). These observations implied that high concentrations of exogenous melatonin inhibited stem growth and induced leaf anthocyanin accumulations.
Sugar Metabolism in Mature Apple Leaves Is Affected by Melatonin
As reported previously (Wang et al., 2013; Zhao et al., 2015a), the concentrations of sucrose, glucose, and fructose in the melatonin treatment group were higher than those in the control (water) treatment group (Figure 2). The melatonin concentrations were positively correlated with the sugar concentrations (sucrose, glucose, and fructose) (Figure 2). Compared to the control, the concentrations of fructose, glucose, and sucrose in mature leaves treated with 1,000 μM of melatonin increased 2.47-, 2.02-, and 1.95-fold, respectively. However, sorbitol concentrations did not significantly differ between the treated groups and the control group.
The Expression of Genes and Enzymes Related to Sugar Metabolism Is Affected by Melatonin
To understand how melatonin regulates carbohydrates in apple mature leaves, the expression of key genes and the activities of enzymes involved in sugar metabolism were subsequently assessed. The transcript levels of MdFRK2 clearly decreased as the melatonin concentrations increased (Figure 3A), and FRK activity showed a similar trend (Figure 3B). NINV activity was significantly upregulated after melatonin treatment, although the expression of MdNINV1 was inhibited by melatonin (Figures 4A,B). The activity of SPS, the major enzyme participating in the sucrose biosynthesis pathway, was also upregulated after melatonin treatment, especially in response to the 1,000 and 5,000 μM treatments (Figure 4B). SDH, the key enzyme regulating sorbitol degradation, was downregulated after melatonin application. HXK activity raised gradually with the increase of melatonin concentration (Figure 4B).
Melatonin Inhibits the Activity of the MdFRK2 Promoter
To examine the hypothesis that the expression of MdFRK2 is inhibited by melatonin, a β-glucuronidase (GUS) reporter gene was fused downstream from the MdFRK2 promoter (MdFRK2P), and then the resulting MdFRK2P::GUS construct was introduced into tobacco by Agrobacterium-mediated transient transformation (Figure 5A). The transgenic plants were treated with different concentrations of melatonin (0, 10, 100, 500, 1,000, and 5,000 μM), after which histochemical staining was performed. As shown in Figure 5B, the transgenic plants treated with low concentrations of melatonin showed a strong blue precipitate, while high concentrations of melatonin showed a slight blue precipitate (Figure 5B). To accurately measure the level of GUS activity, quantitative GUS activity assays were performed. The highest activity occurred in the transgenic lines treated with 10 μM of melatonin (Figure 5C), but the MdFRK2 promoter activity decreased as exogenous melatonin concentrations in the transgenic lines increased. These results suggested that the MdFRK2 promoter activity could be inhibited by melatonin.
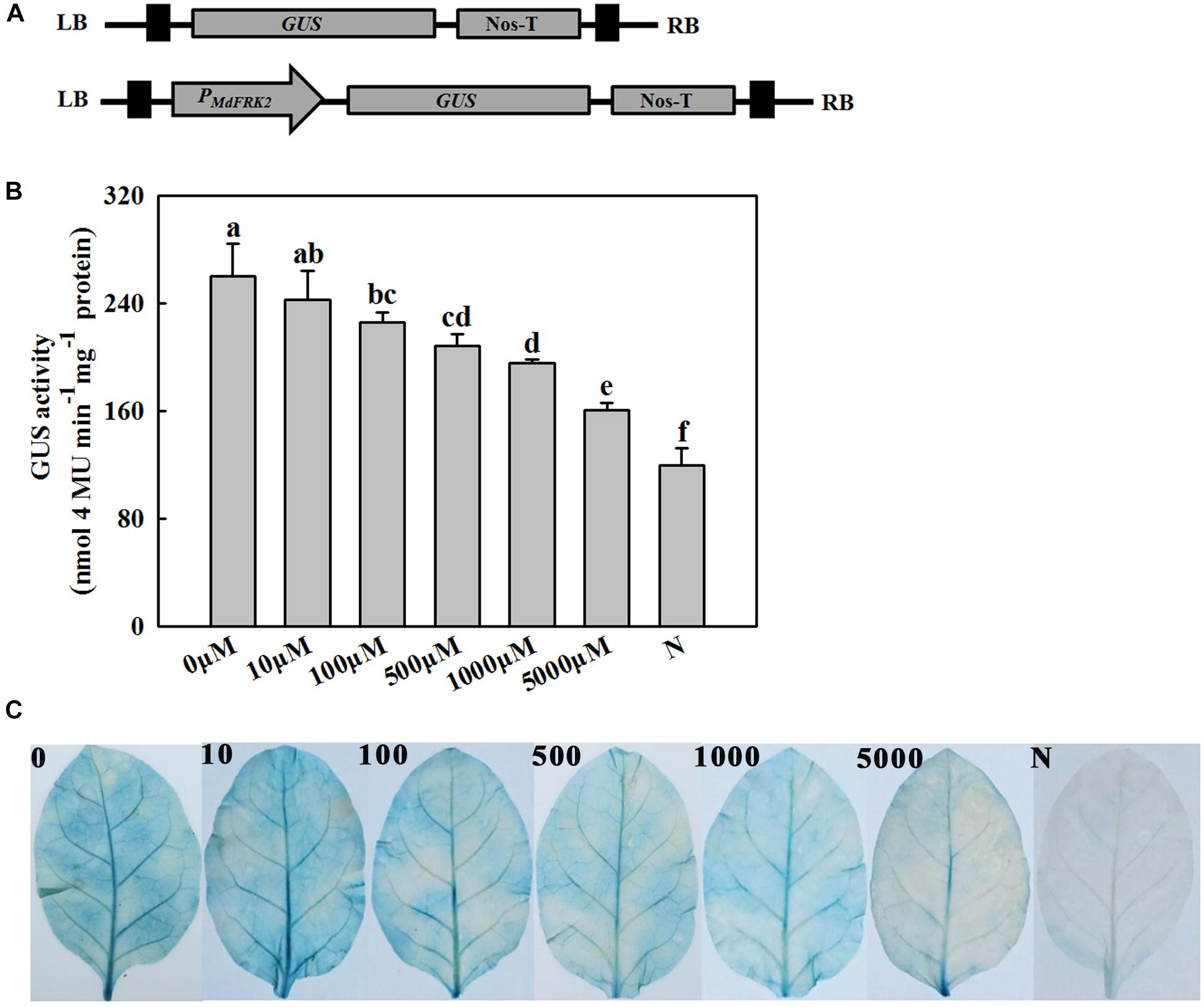
Figure 5. Analysis of the activity of the MdFRK2 promoter under melatonin treatment. Tobacco that transiently expressed the MdFRK2 promoter was irrigated with different concentrations of melatonin (0, 100, 500, 1,000, and 5,000 μM). (A) Schematic diagram of the vector construct. The MdFRK2 promoter (PMdFRK2) was cloned into pC0390GUS and fused with a GUS reporter. P0-GUS (empty pC0390GUS vector) served as a negative control. GUS, β-glucuronidase; LB, left border; NOS-T, Nos terminator; RB, right border. (B) Activity of the MdFRK2 promoter inhibited by melatonin. The vector PMdFRK2-GUS was transiently expressed in tobacco leaves by the Agrobacterium-mediated method, and the tobacco was then irrigated with different concentrations of melatonin (0, 10, 100, 500, 1,000, and 5,000 μM). The GUS activity in leaf extracts was measured using a microplate spectrophotometer. Values are means of three replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test. (C) Leaf phenotypes in response to different concentrations of melatonin treatments.
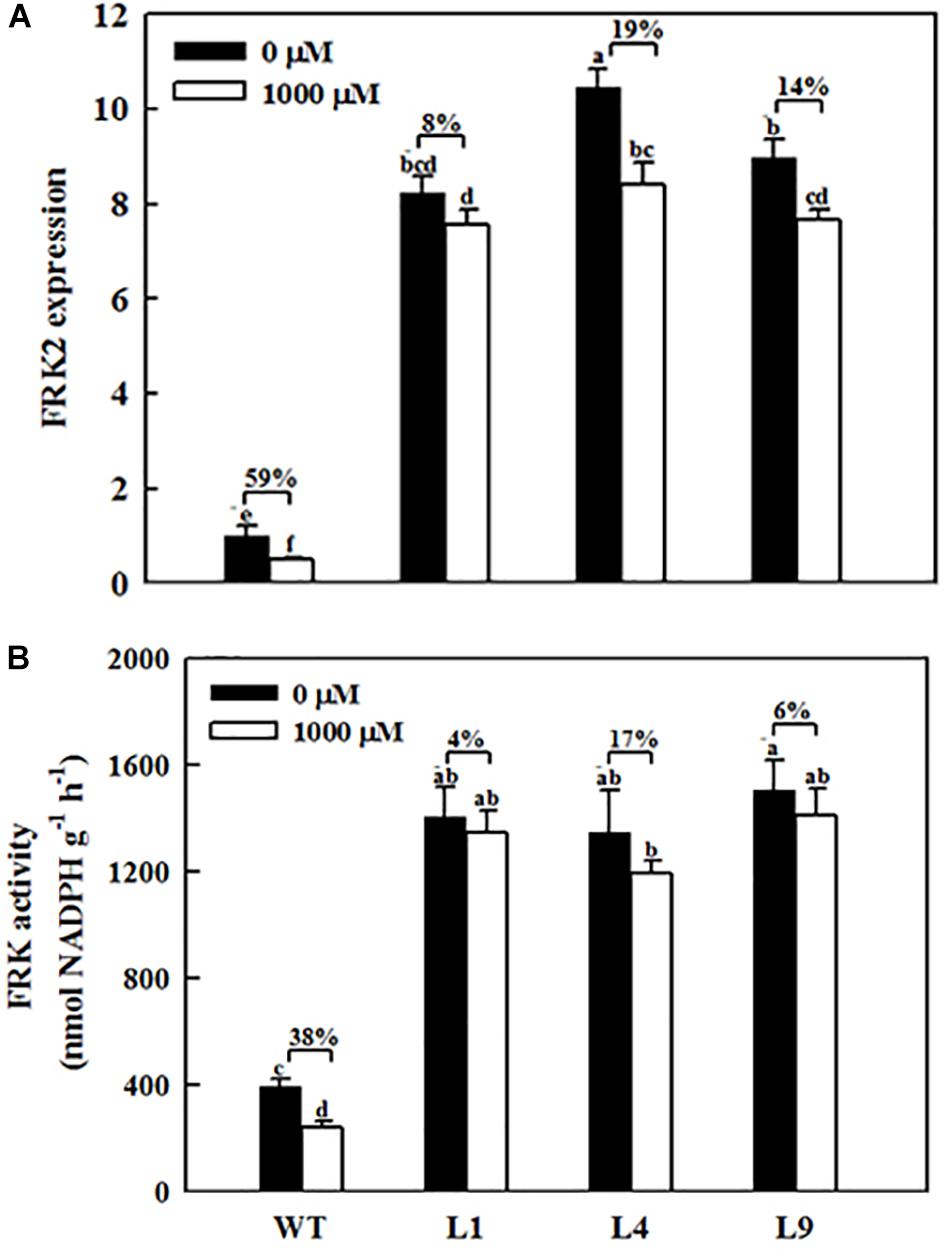
Figure 6. Effects of exogenous melatonin on MdFRK2 expression (A) and FRK activity (B) in mature leaves of WT and transgenic apple plants overexpressing MdFRK2. To confirm the link between MdFRK2 and melatonin, WT and MdFRK2-OE lines (L1, L4, and L9) were treated with 0 μM of melatonin (control) or 1,000 μM of melatonin for 14 days. Samples were obtained from fully mature leaves (the fourth to seventh leaves from the base of the stem) after 14 days of melatonin treatment. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test.
MdFRK2 Overexpression Counteracts the Hexose Accumulation Caused by Melatonin
MdFRK2 was expected to be involved in melatonin-mediated sugar metabolism in apple plants. To further determine the role of MdFRK2 in regulating sugar concentrations under melatonin treatment, we overexpressed MdFRK2 in apple plants with the 35S promoter. Compared with those in untransformed WT lines, MdFRK2 mRNA levels and FRK enzyme activity in the leaves of three transgenic apple lines, lines L1, L4, and L9, significantly increased (Supplementary Figure S1, Yang et al., 2018). 14 days after applying 1,000 μM of exogenous melatonin, we measured the MdFRK2 expression and sugar concentrations of these transgenic and WT apple plants (Figures 6, 7). As expected, compared with those in WT plants treated with control solutions, the transcript levels of MdFRK2 in WT plants treated with 1,000 μM of exogenous melatonin were reduced to 51%. However, compared with those in the three transgenic lines treated with water, MdFRK2 expression treated with 1,000 μM of exogenous melatonin decreased by 8% for L1, 19% for L4, and 14% for L9. Similarly, enzyme analysis exhibited evident decrease in the FRK activity in melatonin-treated WT plants as compared to the WT controls (Figure 6). Exogenous melatonin application resulted in significantly high concentrations of fructose, sucrose, and glucose in the WT lines (Figure 7). However, in the mature leaves of the MdFRK2-overexpression (OE) lines (L1, L4, and L9), exogenous melatonin treatment led to a slight increase in fructose, glucose, and sucrose concentrations (Figure 7).
MdFRK2 Overexpression Counteracts the Growth-Inhibitory Effect of Melatonin
There were no obvious alterations in growth performance between WT and MdFRK2-OE (L1, L4, and L9) lines (Yang et al., 2018). In the presence of exogenous melatonin, the transgenic lines (L1, L4, and L9) did not show marked growth inhibition nor did their shoot tips became red after 45 days of 1,000 μM of exogenous melatonin treatment (Figures 8B–D). However, the treated WT plants appeared significantly stunted at approximately 21 days after melatonin treatment (Figure 8A).
Discussion
MdFRK2 Is Involved in Melatonin-Induced Sugar Accumulation in Mature Leaves
Melatonin, acting as a plant growth and development regulator as well as a biostimulant, has shown great potential in plant physiology (Arnao and Hernándezruiz, 2015). For example, melatonin can counteract abiotic stress (Hardeland et al., 2012; Park et al., 2013; Liang et al., 2015; Wang et al., 2015). Exogenous melatonin-induced sugar accumulations (including sucrose, glucose, and fructose) have been reported in different plant species (Sarropoulou et al., 2012; Wang et al., 2015; Zhao et al., 2015a,b). Additionally, melatonin applications have also been shown to inhibit FRK2 expression in maize plants (Zhao et al., 2015a). In accordance with the results of previous studies, we observed that, compared with applications of control solutions, applications of exogenous melatonin inhibited the expression of MdFRK2 (Figure 3) and caused higher concentrations of sucrose, fructose, and glucose in cells (Figure 2).
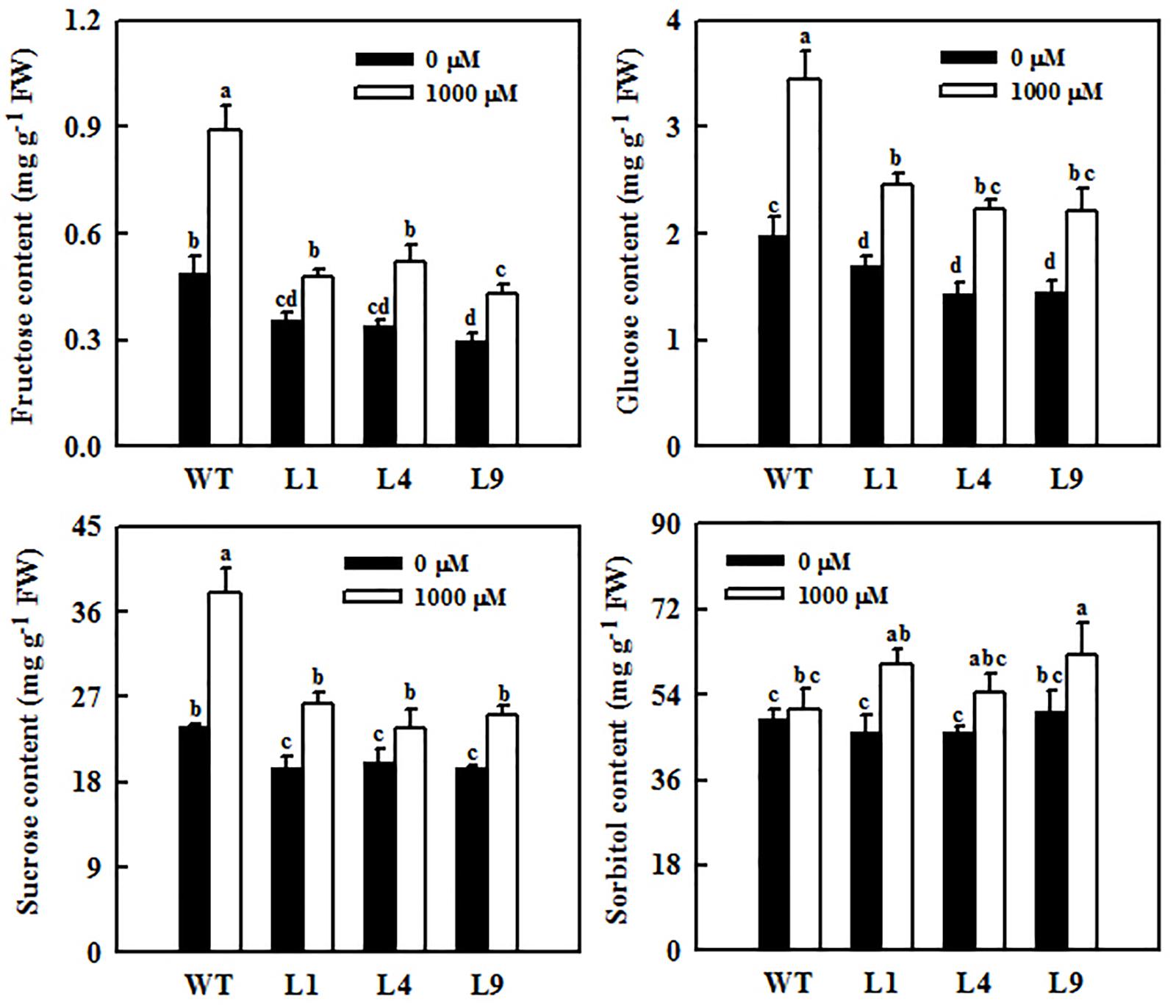
Figure 7. Changes in sugar concentrations in mature leaves of WT and transgenic apple plants overexpressing MdFRK2. Both WT and the transgenic plants were treated with 0 μM of melatonin (control) or 1,000 μM of melatonin for 14 days. Values are means of four replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test.
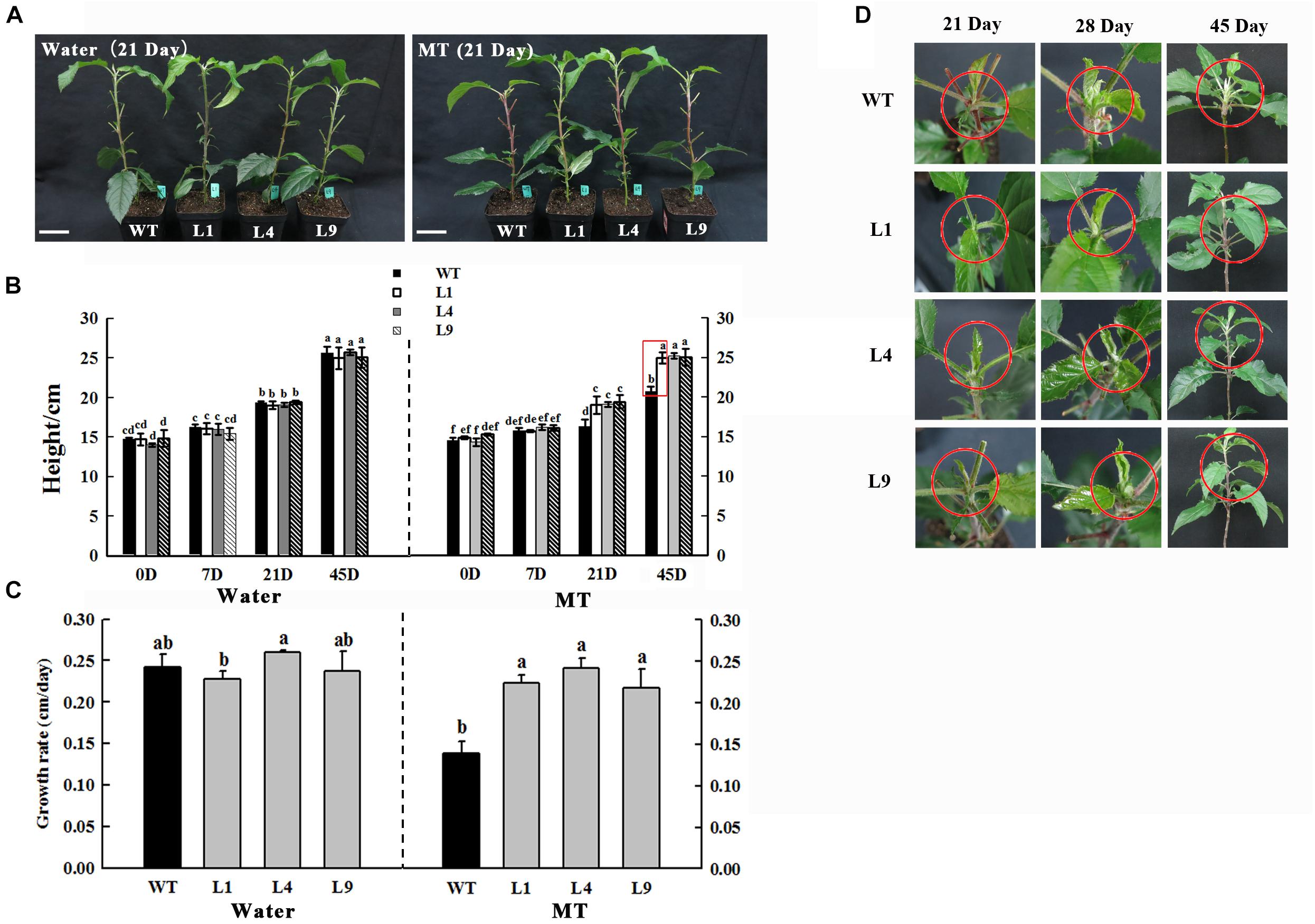
Figure 8. Phenotypes of WT and MdFRK2-OE (L1, L4, and L9) apple lines under 1,000 μM melatonin treatment. (A) Growth performance after 21 days of control (left) or melatonin treatment (right). (B) Stem height at 0, 7, 21, 45 days after melatonin treatment. (C) Growth rate and (D) shoot tip phenotypes at 21, 28, 45 days after melatonin treatment. We recorded the day on which the melatonin treatment started as the first day. The plant height was considered the average of six plants per treatment. The red circles indicate the shoot tips. Values are means of four replicates ± SD. Different letters above the column indicate significant difference at P < 0.05 by Duncan’s test. Scale bars represent 5 cm.
Exogenous melatonin inhibited the expression of MdFRK2, which was confirmed by our experiments. First, MdFRK2 transcription was inhibited by different concentrations of melatonin: the higher the melatonin concentration was, the lower the expression of MdFRK2 and the activity of FRK (Figure 3). Second, in the presence of exogenous melatonin, GUS activity in transgenic tobacco that contained the MdFRK2 promoter-driven reporter gene decreased as the melatonin concentration increased (Figure 5). Third, compared with those in the FRK2-OE lines (L1, L4, and L9), which overexpressed the MdFRK2 gene under the control of the 35S promoter, MdFRK2 expression and FRK activity in the WT lines was clearly inhibited in response to 1,000 μM of melatonin (Figure 6). Melatonin consistently increased fructose accumulations more in the WT lines than in the FRK2-OE lines (L1, L4, and L9) (Figure 7). However, expression of MdFRK2 was also inhibited in the OE lines, suggesting that melatonin inhibit the own MdFRK2 promoter. There should exist a yet to be determined transcription factor that is responsible for melatonin-mediated MdFRK2 expression. Although one melatonin receptor has been identified in Arabidopsis thaliana (Wei et al., 2018), its signaling pathway is unknown in plant cells. The identification of the involved transcription factor will be helpful to understand the mechanism of melatonin in regulating sugar metabolism.
Fructokinase is crucial for fructose metabolism and negatively regulates the accumulation of fructose in plants (Pego and Smeekens, 2000). Expression patterns and functional analysis of FRK isoforms in tomato, rice, maize, and Populus suggest that FRK2 orthologs are the major FRK-encoding genes and the dominant fructose-phosphorylating enzymes in sink tissues (Kanayama et al., 1997, 1998; Pego and Smeekens, 2000; Jiang et al., 2003). MdFRK2, the gene that codes for the main fructose-phosphorylating enzyme in apple plants (Li et al., 2018), controls FRK activity and fructose concentrations in mature apple leaves (Yang et al., 2018). In this study, MdFRK2 expression was inhibited by exogenous melatonin applications at both low and high concentrations (Figures 3, 5), leading to a decrease in FRK activity and an increase in fructose levels (Figures 2, 3). These results are in agreement with those of a previous report with respect to decreases in FRK2 expression (Zhao et al., 2015a) and increases in fructose levels upon short-term exposure to a range of melatonin concentrations (10–100 μM) in maize seedlings (Zhao et al., 2015a). Taken together, these observations indicate a general role for melatonin in limiting FRK2 expression and increasing fructose levels.
Sucrose metabolism, including its synthesis and degradation, is induced by exogenous melatonin at the gene expression and enzyme activity levels. These phenomena are reflected by the increased activities of SPS and invertase, especially under high concentrations of melatonin (100 μM) (Zhao et al., 2015a). Our results further confirm previous findings, as the SPS and NINV activity significantly increased (Figure 4). Additionally, high concentrations of melatonin were found to counteract sucrose phloem loading, as indicated by the reduced expression of the tie-dyed2 (Tdy2) and Sxd1 genes (Zhao et al., 2015a). As the closest homolog of MdFRK2, LeFRK2 has been demonstrated to be required for phloem development and sugar transport (German et al., 2003). In tomato, suppression of LeFRK2 reduced the length and width of sieve elements and resulted in their low levels of callose deposition, which restricted the downward movement of sucrose and greatly elevated soluble sugar levels in tomato source leaves (German et al., 2003). Therefore, it is possible that exogenous melatonin inhibited the expression of MdFRK2 and then caused phloem interruption, thus leading to an abundance of sucrose in the leaves (Figure 2). The significantly increased sucrose levels may stimulate sweet immunity responses through sucrose sensing and signaling (Bolouri Moghaddam and Van den Ende, 2013).
Sugars fulfill many essential functions in plant cells (Pego and Smeekens, 2000). Plants have a system that controls sugar homeostasis to help avoid the negative effects associated with fluctuations in sugar concentrations. Excess fructose in the cytosol is harmful to plant growth and development. On the one hand, high concentrations of fructose can lead to stress reactions such as senescence. On the other hand, the inhibition of fructose phosphorylation can limit carbon flux to glycolysis and other pathways via F6P. Under high concentrations of fructose, apple plants have at least two alternative approaches to limit the production of fructose in cells: one involves sorbitol conversion to fructose via SDH catalysis (Yamada et al., 1998), and the other involves sucrose cleavage by SUSY or invertase (Pego and Smeekens, 2000; Ruan, 2014). Our results indicated that the decreased fructose concentrations in the leaves after low melatonin applications were partly limited by decreased sorbitol dehydrogenization, reflected by decreased SDH expression and activity (Figure 4). Correspondingly, the NINV activity increased (Figure 4), causing an increase in glucose levels. Interestingly, we observed opposite changes of SDH, SUSY, and NINV activities after melatonin treatment (Figure 4) compared to those in MdFRK2 overexpressing lines (Yang et al., 2018). These results indicated that carbohydrate metabolism changes in response to melatonin were mediated by MdFRK2.
MdFRK2 Is Involved in Growth Inhibition Mediated by Exogenous Melatonin
Compared to the control solutions and low-concentration melatonin treatments, shoot growth in response to high-concentration melatonin applications was significantly inhibited (Figures 1, 8). The observed growth inhibition effect was similar to that in previous reports on growth inhibition of maize seedlings in response to high concentrations of exogenous melatonin treatments (Zhao et al., 2015a). However, in the present study, it is interesting that the negative effect of growth was significantly alleviated in the MdFRK2-OE lines in response to the 1,000 μM melatonin treatment, as reflected by the similar shoot height between the MdFRK2-OE and WT lines (Figure 8A). Moreover, expression of MdFRK2 and activity of FRK were suppressed slightly less in the treated MdFRK2-OE plants than in WT plants after 14 days of melatonin treatment (Figure 6). These phenomena indicate that MdFRK2-OE counteracts the effects of the high concentration melatonin-mediated growth inhibition of MdFRK2-OE apple plants, suggesting that the reduced expression of MdFRK2 is an important cause of the stunted phenotype of WT plants (Figure 8).
FRK2 plays important physiological roles in regulating plant growth and development. For example, RNAi-MdFRK2 tomato plants displayed inhibited stem and root growth and reduced numbers of seeds per fruit (Odanaka et al., 2002). The young leaves of FRK2-antisense tomato plants showed inhibited growth and wilting because of the suppression of a particular area of active xylem (German et al., 2003). Antisense suppression of the FRK2 ortholog in potato (StFRK1) inhibited aerial growth and resulted in decreased tuber yields, possibly due to reduced phloem transport (Cho and Yoo, 2011). Reduced FRK2 activity led to reduced length and width of sieve elements, thus leading to inhibited stem water conductivity and restricted downward movement of sucrose (Damari-Weissler et al., 2009). As MdFRK2 expression is extremely high in actively growing sink tissues such as shoot tips in apple plants (Li et al., 2012), modulated expression of MdFRK2 in shoot tips might negatively affect stem development, potentially causing growth inhibition after melatonin treatment.
Previous studies showed that hexokinase1 (AtHXK1) overexpression inhibits growth (Jang et al., 1997; Dai et al., 1999), and the stunted growth occurs only when AtHXK1 expressed in photosynthetic tissues (Dai et al., 1999). Therefore, the expression of MdHXK1 was monitored in our study. We found that the MdHXK1 transcripts were upregulated in mature leaves, especially under high concentration of melatonin application (Figure 4). Although our results cannot provide the exact mechanism by which this occurs, these data allow us to speculate that the increased HXK1 expression may be also involved in the inhibitory effect (Figure 1B).
It has been demonstrated that suppression of the FRK2 orthologs resulted in increased sugar concentrations (German et al., 2003; Davies et al., 2005; Roach et al., 2012). Our data showed MdFRK2 represents a link in the cross-talk between increased sugar concentrations and the melatonin application. Current results also support that exogenous melatonin may mimic DAMPs, counteracting both abiotic and biotic stresses through elevated leaf sweetening (Versluys et al., 2017). Spraying exogenous melatonin on leafy vegetables may be very promising in terms of agronomical applications. Especially melatonin/fructan mixtures hold great promise, as natural and sustainable alternatives for toxic agrochemicals, to be used on leafy vegetables (Versluys et al., 2017). Further investigations of the role of melatonin in leafy vegetables will be done to support or refute these theories.
Author Contributions
JY and CZ performed the majority of experiments, analyzed, and discussed data. ZW, SS, RZ, YZ, and BM performed essential experiments. ML supervised work. JY wrote draft manuscript. All authors contributed to final manuscript.
Funding
This work was supported by the Program for the National Key Research and Development Program (Grant No. 2018YFD1000200), the National Natural Science Foundation of China (Grant No. 31872043), and the earmarked fund for the China Agriculture Research System (Grant No. CARS-28).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mr. Xuanchang Fu and Xiaowei Ma for maintaining the plants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00150/full#supplementary-material
Abbreviations
CaMV, cauliflower mosaic virus; DAMPs, damage-associated molecular patterns; F6P, fructose-6-phosphate; FRK, fructokinase; GUS, β-glucuronidase; HXK, hexokinase; MS, Murashige and Skoog medium; NINV, neutral invertase; SDH, sorbitol dehydrogenase; SPS, sucrose-phosphate synthase; SUSY, sucrose synthase.
Footnotes
References
Arnao, M. B., and Hernándezruiz, J. (2015). Functions of melatonin in plants: a review. J. Pineal Res. 59, 133–150. doi: 10.1111/jpi.12253
Bolouri Moghaddam, M. R., and Van den Ende, W. (2012). Sugars and plant innate immunity. J. Exp. Bot. 63, 3989–3998. doi: 10.1093/jxb/ers129
Bolouri Moghaddam, M. R., and Van den Ende, W. (2013). Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 64, 1439–1449. doi: 10.1093/jxb/ert046
Cho, Y. H., and Yoo, S. D. (2011). Signaling role of fructose mediated by FINS1/FBP in Arabidopsisthaliana. PLoS Genet. 7:e1001263. doi: 10.1371/journal.pgen.1001263
Dai, N., Schaffer, A., Petreikov, M., Shahak, Y., Giller, Y., Ratner, K., et al. (1999). Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11, 1253–1266. doi: 10.1105/tpc.11.7.1253
Damari-Weissler, H., Rachamilevitch, S., Aloni, R., German, M. A., Cohen, S., Zwieniecki, M. A., et al. (2009). LeFRK2 is required for phloem and xylem differentiation and the transport of both sugar and water. Planta 230, 795–805. doi: 10.1007/s00425-009-0985-4
Davies, H. V., Shepherd, L. V., Burrell, M. M., Carrari, F., Urbanczyk-Wochniak, E., Leisse, A., et al. (2005). Modulation of fructokinase activity of potato (Solanum tuberosum) results in substantial shifts in tuber metabolism. Plant Cell Physiol. 46, 1103–1115. doi: 10.1093/pcp/pci123
Erland, L. A., Murch, S. J., Reiter, R. J., and Saxena, P. K. (2015). A new balancing act: themanyroles of melatonin and serotonin in plant growth and development. Plant Signal. Behav. 10:e1096469. doi: 10.1080/15592324.2015.106469
German, M. A., Dai, N., Matsevitz, T., Hanael, R., Petreikov, M., Bernstein, N., et al. (2003). Suppression of fructokinase encoded by LeFRK2 in tomato stem inhibits growth and causes wilting of young leaves. Plant J. 34, 837–846. doi: 10.1046/j.1365-313X.2003.01765.x
Hardeland, R., Madrid, J. A., Tan, D. X., and Reiter, R. J. (2012). Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 52, 139–166. doi: 10.1111/j.1600-079X.2011.00934.x
Jang, J. C., Patricia, L., and Sheen, Z. J. (1997). Hexokinase as a sugar sensor in higher plants. Plant Cell 9, 5–19. doi: 10.1105/tpc.9.1.5
Janse van Rensburg, H. C., Van den Ende, W., and Signorelli, S. (2019). Autophagy in plants: both a puppet and a puppet master of sugars. Front. Plant Sci. 10:14. doi: 10.3389/fpls.2019.00014
Jiang, H., Dian, W., Liu, F., and Wu, P. (2003). Isolation and characterization of two fructokinase cDNA clones from rice. Phytochemistry 62, 47–52. doi: 10.1016/S0031-9422(02)00428-4
Kanayama, Y., Dai, N., Granot, D., Petreikov, M., Schaffer, A., and Bennett, A. B. (1997). Divergent fructokinase genes are differentially expressed in tomato. Plant Physiol. 113, 1379–1384. doi: 10.1104/pp.113.4.1379
Kanayama, Y., Granot, D., Dai, N., Petreikov, M., Schaffer, A., Powell, A., et al. (1998). Tomato fructokinases exhibit differential expression and substrate regulation. Plant Physiol. 117, 85–90. doi: 10.1104/pp.117.1.85
Li, M., Feng, F., and Cheng, L. (2012). Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS One 7:e33055. doi: 10.1371/journal.pone.0033055
Li, M., Li, D., Feng, F., Zhang, S., Ma, F., and Cheng, L. (2016). Proteomic analysis reveals dynamic regulation of fruit development and sugar and acid accumulation in apple. J. Exp. Bot. 67, 5145–5157. doi: 10.1093/jxb/erw277
Li, M., Li, P., Ma, F., Dandekar, A. M., and Cheng, L. (2018). Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 5:60. doi: 10.1038/s41438-018-0064-8
Liang, C. Z., Zheng, G. Y., Li, W. Z., Wang, Y. Q., Hu, B., Wang, H. R., et al. (2015). Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 59, 91–101. doi: 10.1111/jpi.12243
Mandal, M. K., Suren, H., Ward, B., Borouierdi, A., and Kousik, K. (2018). Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 65:e12505. doi: 10.1111/jpi.12505
Odanaka, S., Bennett, A. B., and Kanayama, Y. (2002). Distinct physiological roles of fructokinase isozymes revealed by gene-specific suppression of Frk1 and Frk2 expression in tomato. Plant Physiol. 129, 1119–1126. doi: 10.1104/pp.000703
Park, S., Lee, D. E., Jang, H., Byeon, Y., Kim, Y. S., and Back, K. (2013). Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 54, 258–263. doi: 10.1111/j.1600-079X.2012.01029.x
Pego, J. V., and Smeekens, S. C. (2000). Plant fructokinases: a sweet family get-together. Trends Plant Sci. 5, 531–536. doi: 10.1016/S1360-1385(00)01783-0
Qian, Y., Tan, D. X., Reiter, R. J., and Shi, H. T. (2015). Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 5:15815. doi: 10.1038/srep15815
Renz, A., and Stitt, M. (1993). Substrate-specificity and product inhibition of different forms of fructokinases and hexokinases in developing potato-tubers. Planta 190, 166–175. doi: 10.1007/BF00196608
Roach, M., Gerber, L., Sandquist, D., Gorzsás, A., Hedenström, M., Kumar, M., et al. (2012). Fructokinase is required for carbon partitioning to cellulose in aspen wood. Plant J. 70, 967–977. doi: 10.1111/j.1365-313X.2012.04929.x
Rolland, F., Baena-gonzalez, E., and Sheen, J. (2006). Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 57, 675–709. doi: 10.1146/annurev.arplant.57.032905.105441
Ruan, Y. L. (2014). Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 65, 33–67. doi: 10.1146/annurev-arplant-050213-040251
Sarropoulou, V., Dimassi-theriou, K., Therios, I., and Koukourikou-petridou, M. (2012). Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 61, 162–168. doi: 10.1016/j.plaphy.2012.10.001
Sparkes, I. A., Runions, J., Kearns, A., and Hawes, C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025. doi: 10.1038/nprot.2006.286
Tan, D. X., Hardeland, R., Manchester, L. C., Paredes, S. D., Korkmaz, A., Sainz, R. M., et al. (2010). The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 85, 607–623. doi: 10.1111/j.1469-185X.2009.00118.x
Versluys, M., Tarkowski, Ł.P., and Van den Ende, W. (2017). Fructans as DAMPs or MAMPs: evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant Sci. 7:2061. doi: 10.3389/fpls.2016.02061
Wang, P., Sun, X., Chang, C., Feng, F., Liang, D., Cheng, L., et al. (2013). Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 55, 424–434. doi: 10.1111/jpi.12091
Wang, P., Sun, X., Xie, Y., Li, M., Zhang, S., Liang, D., et al. (2015). Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 57, 291–307. doi: 10.1111/jpi.12169
Wei, J., Li, D. X., Zhang, J. R., Shan, C., Rengel, Z., Song, Z. B., et al. (2018). Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 65:e12500. doi: 10.1111/jpi.12500
Xu, W., Yu, Y., Zhou, Q., Ding, J., Dai, L., Xie, X., et al. (2011). Expression pattern, genomic structure, and promoter analysis of the gene encoding stilbene synthase from Chinese wild Vitis pseudoreticulata. J. Exp. Bot. 62, 2745–2761. doi: 10.1093/jxb/erq447
Yamada, K., Oura, Y., Mori, H., and Yamaki, S. (1998). Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression. Plant Cell Physiol. 39, 1375–1379. doi: 10.1093/oxfordjournals.pcp.a029345
Yang, J. J., Zhu, L. C., Cui, W. F., Zhang, C., Li, D. X., Ma, B. Q., et al. (2018). Increasing activity of MdFRK2, a high affinity fructokinase, leads to upregulation of sorbitol metabolism and downregulation of sucrose metabolism in apple leaves. Hortic. Res. 5:71. doi: 10.1038/s41438-018-0099-x
Zhang, S., Nichols, S. E., and Dong, J. G. (2003). Cloning and characterization of two fructokinases from maize. Plant Sci. 165, 1051–1058. doi: 10.1016/S0168-9452(03)00298-X
Zhao, H., Su, T., Huo, L., Wei, H., Jiang, Y., Xu, L., et al. (2015a). Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case. J. Pineal Res. 59, 255–266. doi: 10.1111/jpi.12258
Keywords: apple, fructokinase, growth inhibition, MdFRK2, melatonin, sugar
Citation: Yang J, Zhang C, Wang Z, Sun S, Zhan R, Zhao Y, Ma B, Ma F and Li M (2019) Melatonin-Mediated Sugar Accumulation and Growth Inhibition in Apple Plants Involves Down-Regulation of Fructokinase 2 Expression and Activity. Front. Plant Sci. 10:150. doi: 10.3389/fpls.2019.00150
Received: 08 November 2018; Accepted: 28 January 2019;
Published: 19 February 2019.
Edited by:
Wim Van den Ende, KU Leuven, BelgiumReviewed by:
Mihir Kumar Mandal, Claflin University, United StatesOfer Stein, Agricultural Research Organization (ARO), Israel
Copyright © 2019 Yang, Zhang, Wang, Sun, Zhan, Zhao, Ma, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjun Li, bGltaW5nanVuQG53c3VhZi5lZHUuY24=
†These authors have contributed equally to this work
 Jingjing Yang1†
Jingjing Yang1† Chunxia Zhang
Chunxia Zhang Fengwang Ma
Fengwang Ma Mingjun Li
Mingjun Li