
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 19 December 2018
Sec. Plant Abiotic Stress
Volume 9 - 2018 | https://doi.org/10.3389/fpls.2018.01892
Aim: Heavy metal pollution is serious in China, and abscisic acid (ABA) is an important stress hormone. How it regulates plant tolerance to cadmium remains unclear, so we aimed to explore the molecular mechanism responsible for enhanced cadmium resistance in Arabidopsis wild-type and mutant plants and Brassica napus seedlings.
Methods: Arabidopsis/B. napus were cultured hydroponically for 28/15 days and then treated with 20/10 μM Cd/Cd+ABA (5 μM) for 3/4 days. Chlorophyll degradation rate, SPAD values, proline, MDA, ABA, , and Cd concentrations were measured in root vacuoles and protoplasts; root to shoot and Cd concentration ratios were determined and NRT1.5-, NRT1.8-, BnNRT1.5-, and BnNRT1.8-related gene expression was studied.
Results: Cytoplasmic ABA levels in root cells of bglu10 and bglu18 Arabidopsis mutants were significantly lower than those in the wild-type, apparently making the latter more resistant to Cd. long-distance transporter NRT1.5 responded to ABA signaling by downregulating its own expression, while NRT1.8 did not respond. Concomitantly, proton pump activity in wild-type plants was higher than in the bglu10 and bglu18 mutants; thus, more and Cd accumulated in the vacuoles of wild-type root cells. ABA application inhibited Cd absorption by B. napus. BnNRT1.5 responded to exogenous ABA signal by downregulating its own expression, while the lack of response by BnNRT1.8 resulted in increased amount of accumulating in the roots to participate in the anti-cadmium reaction.
Conclusion: NRT1.5 responds to the ABA signal to inhibit its own expression, whereas unresponsiveness of NRT1.8 causes accumulation of in the roots; thus, enhancing Cd resistance. In Arabidopsis, because of proton pump action, more and Cd accumulate in the vacuoles of Arabidopsis root cells, thereby reducing damage by Cd toxicity. However, in B. napus, the addition of exogenous ABA inhibited Cd absorption. Our data provide a sound basis to the theoretical molecular mechanism involved in hormone signaling during response of plants to heavy metal stress.
Nitrogen (N) is an essential macronutrient that plays a key role in plant growth and development, and in crop yield (Hirel et al., 2007; Wang et al., 2012; Krapp et al., 2014; Ruffel et al., 2014; Vidal et al., 2014). Nitrates () are some of the most abundant N sources in natural and agricultural systems (von Wiren et al., 2000). Absorption, transport, sensing, and responses to have been extensively studied (Krapp, 2015; O’Brien et al., 2016). In addition to its role as a nutrient acts as a signaling molecule that regulates gene expression and many processes, including plant growth, root system architecture (Krouk et al., 2010; Alvarez et al., 2012), leaf development (Rahayu et al., 2005), seed dormancy (Alboresi et al., 2005), and flowering (Marin et al., 2011). During growth and development plants inescapably experience various forms of unfavorable environmental conditions. Under such circumstances, plays a key role in the processes whereby plants try to prevent any potential damage. NRT1.5 and NRT1.8 have been identified as two essential long-distance transporters (Lin et al., 2008; Li et al., 2010). Arabidopsis NRT1.5 is expressed mainly in root pericycle cells and functions in the loading of into the xylem. On the other hand, Arabidopsis NRT1.8 is expressed predominantly in xylem parenchyma cells within the vascular bundles, where it functions to remove from the xylem vessels. AtNRT1.5 works together with AtNRT1.8 to fine-tune long-distance transport from roots to shoots (Lin et al., 2008; Li et al., 2010). Studies showed that NRT1.8 was strongly upregulated by Cd stress in roots, while the nrt1.8-1 mutant showed a nitrate-dependent Cd2+-sensitive phenotype. This finding suggests that NRT1.8 regulated distribution may play an important role in Cd2+ tolerance in plants (Li et al., 2010). NRT1.5 functions to mediate reallocation to roots, stress-responsive gene expression and metabolism; consequently salt, drought, and Cd2+ tolerance are affected by NRT1.5; further, the mRNA level of NRT1.5 is reportedly downregulated by salt, drought, and Cd treatments; thus, lending support to the hypothesis that reallocation to roots might be a common response to stress, coordinately regulated by the NRT1.8 and NRT1.5 (Chen et al., 2012).
The plant hormone abscisic acid (ABA) regulates plant growth, seed dormancy, leaf senescence, and plant responses to abiotic forms of stress (Fujii and Zhu, 2009; Cutler et al., 2010; Gonzalez-Guzman et al., 2012; Munemasa et al., 2015; Zhao et al., 2016). Consistently, endogenous ABA level is well-known to increase under stress (Lee et al., 2006; Wang et al., 2011; Ondzighi-Assoume et al., 2016; Takahashi et al., 2018); further, it is regulated by a dynamic balance among biosynthesis, degradation, transport, conjugation, and deconjugation reactions (Finkelstein, 2013). Among conjugates, ABA glucose ester (ABA-GE) is the predominant form. ABA-GE is located in the vacuoles, in xylem sap, and probably in the cell wall (Dietz et al., 2000). BGLU10, a member of the β-glucosidase family in Arabidopsis, is localized in vacuoles, where it hydrolyzes ABA-GE to produce active ABA; this protein plays a key role in drought tolerance (Wang et al., 2011). Similarly, β-GLUCOSIDASE1 (BGLU18) has been shown to function in the endoplasmic reticulum (ER) to release ABA from ABA-GE in response to salt stress (Lee et al., 2006). Thus, the release of ABA from ABA-GE pools is an important mechanism for regulating ABA levels both locally and within the plant as a whole in response to stress.
Studies have shown that Cd stress triggers ethylene (ET) and jasmonic acid (JA) signaling, which converged at EIN3/EIN3-Like1 (EIL1) to modulate the expression of ethylene response factors and hence to upregulate NRT1.8. In contrast, ET and JA signaling mediated the downregulation of NRT1.5 via EIN3/EIL1, and other unknown component(s). These processes enhanced stress tolerance and decreased plant growth (Zhang G.B. et al., 2014). Similarly, ABA acts as a stress response hormone; therefore, we asked, what is the relationship between ABA and NRT1.5 and NRT1.8 in the face of stress? We used Arabidopsis ABA mutants (bglu10 and bglu18) and wild-type (Col-0) for experimental studies under Cd stress.
The available data indicate that the vacuole is involved in ion homeostasis of the cytosol by storing products of primary and secondary metabolism, and by osmoregulation, thus contributing to plant defense responses under biotic and abiotic stress. In addition, the vacuole is known to be significantly related to N use efficiency (NUE) (Andreev, 2001; Han et al., 2016; Kim et al., 2017; Liu et al., 2018; Takeda et al., 2018). Vacuolar compartmentalization of toxic or excess essential heavy metals mainly relies on tonoplast energization and the associated establishment of a proton motive-force due to the H+ translocating activities of V-ATPase and V-PPase and various tonoplast-localized transporters (Sharma et al., 2016). The exposure of barley seedlings to Cd led to substantially elevated transcript levels of V-ATPase subunits VHA-c and VHA-E, with the magnitude of increase being greater in the case of the latter (Finkemeier et al., 2003; Sharma et al., 2004). In a proteomic analysis of barley leaf tonoplasts, an isoform of V-PPase was observed to be upregulated by twofold during the Cd treatment (Schneider et al., 2009; Khoudi et al., 2012). As these observations indicate that V-ATPase and V-PPase seem to play an important role in the ability of plants to resist Cd, therefore, we measured V-ATPase and V-PPase activities in the Arabidopsis wild-type and in the mutants used here as experimental materials.
In both, Brassica napus and Arabidopsis, NRT1.5 responded to the ABA signal by downregulating its expression under Cd stress, whereas NRT1.8 did not respond, thus resulting in nitrate accumulation in the root to enhance its ability to resist Cd. As for Arabidopsis, the wild-type showed higher proton pump activities (V-PPase and V-ATPase), which led to less Cd being transported to the shoot, thus reducing damage caused by Cd toxicity. However, in B. napus, the addition of exogenous ABA directly inhibited Cd absorption by plants and enhanced their resistance to Cd toxicity.
First, we examined the Cd phenotype by using Arabidopsis wild-type and ABA mutants (Figure 1A). There were no phenotypic differences between the two under control conditions. However, when plants were cultivated for 4 weeks under control conditions and then exposed for 3 days to 20 μM Cd, Col-0 showed more resistance to Cd, while bglu10 and bglu18 mutants displayed more sensitivity to Cd (Figure 1A).
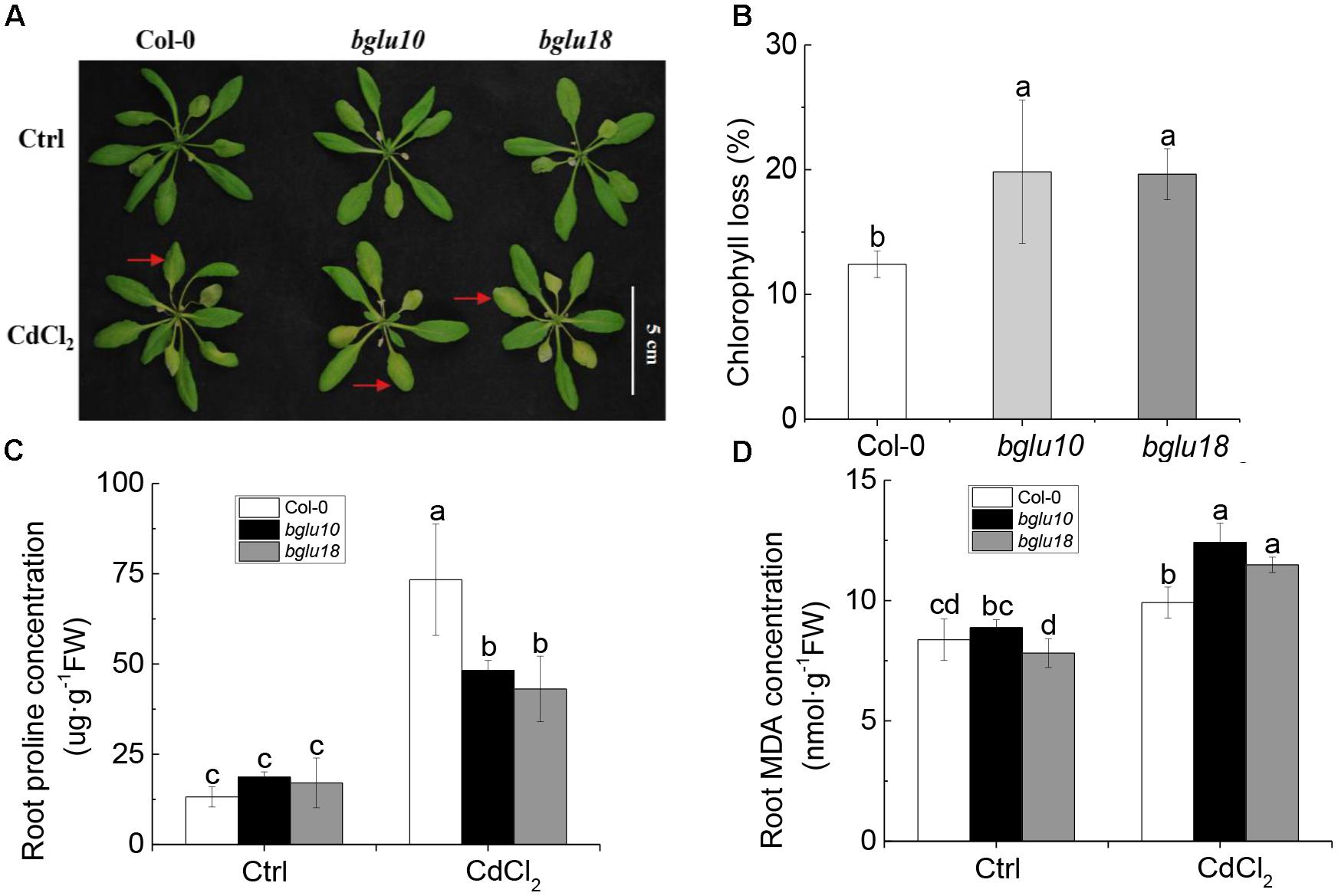
Figure 1. Arabidopsis abscisic acid (ABA) mutants (bglu10 and bglu18) are more sensitive than wild-type (Col-0) under 20 μM cadmium stress. (A) Photograph showing the higher tolerance of the wild-type (Col.0) as compared with ABA mutants (bglu10 and bglu18). (B) Chlorophyll loss in Cd2+ treated plants relative to control. (C) Effects of Cd2+ stress on proline in Col-0, bglu10, and bglu18 plants. (D) Effects of Cd2+ stress on malondialdehyde (MDA) in Col-0, bglu10, and bglu18 plants. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
Leaf chlorophyll is an important indicator of plant tolerance to Cd (DalCorso et al., 2008). We observed that after Cd stress, chlorophyll degradation rate in Col-0 was 12%, while the corresponding rates in bglu10 and bglu18 were both 20%, which was significantly higher than that of Col-0. This finding demonstrated that Col-0 was more tolerant to Cd than either of the ABA mutants (Figure 1B).
Proline and malondialdehyde (MDA) are also important indicators of stress tolerance. Proline was able to maintain the stability of the membrane structure and to eliminate reactive oxygen species. The accumulation of proline is positively correlated with plant stress tolerance. As for MDA, it is one of the most important products of membrane lipid peroxidation; it is cytotoxic, because it promotes cross-link polymerization of living macromolecules, such as proteins and nucleic acids. After Cd stress, proline concentration in roots of Col-0 was significantly higher than in roots of either ABA mutant. In contrast, root MDA was significantly lower in Col-0 than in ABA mutants (Figures 1C,D). Our data suggest that after Cd stress, Col-0 showed higher Cd tolerance when compared to either of the ABA mutants tested.
In view of the phenotypic differences shown in Figure 1, because the materials are ABA mutants, we determined the ABA distribution and content differences in root cells under Cd stress (Figures 2A,B). We found that, compared with bglu10 and bglu18, the ABA content in Col-0 root vacuoles accounted for 77.0% of protoplast ABA content, which is much lower than the ABA contents found in the ABA mutants, which were 91.9 and 88.5%, respectively (Figure 2A). Therefore, we conclude that the amount of ABA in the cytoplasm of Col-0 root cells was significantly higher than that in either the bglu10 or the bglu18 ABA mutant (Figure 2B).
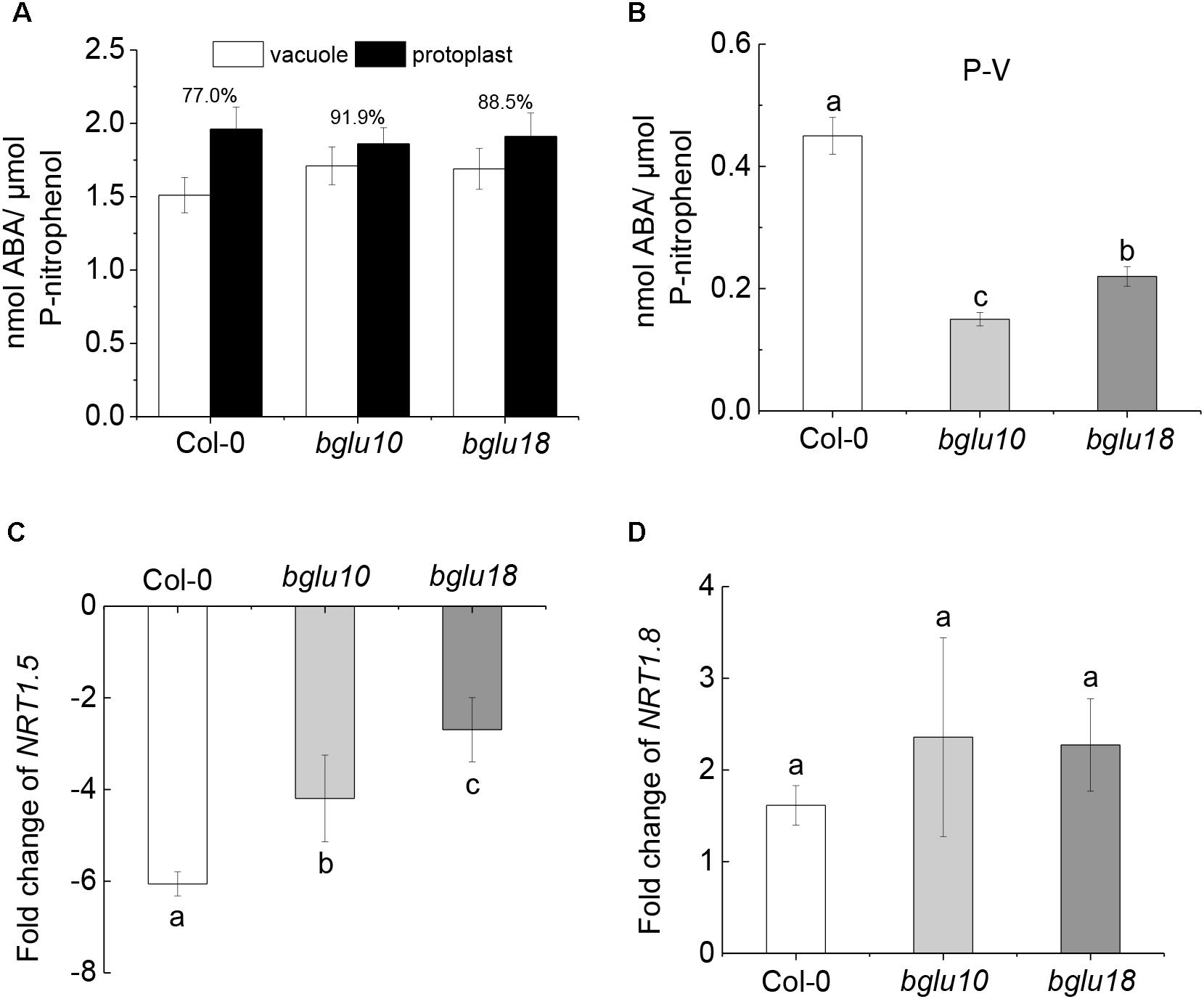
Figure 2. Distribution of ABA content in Arabidopsis wild-type (Col-0) and ABA mutants (bglu10 and bglu18) root cells under Cd stress and the effect of endogenous ABA on NRT1.5 and NRT1.8. (A) ABA distribution between the vacuole and protoplast; values above the bars represent the percentage of vacuolar ABA relative to the total ABA in the protoplasts. (B) Total ABA accumulated in cytoplasm (P-V) calculated as total ABA in the protoplast – total ABA in the vacuole. (C) The fold change of NRT1.5 down-regulation in roots calculated as the expression of NRT1.5 under normal treatment divided by the expression of NRT1.5 under Cd stress. (D) The fold change of NRT1.8 up-regulation in roots calculated as the expression of NRT1.8 under Cd stress divided by the expression of NRT1.8 under normal treatment. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
We took the Arabidopsis roots that were grown under control conditions for 4 weeks and then treated them with 200 μM Cd for 6 h. We then tested for the gene expression of NRT1.5 and NRT1.8. The expression of NRT1.5 was significantly inhibited after Cd treatment, regardless of the material. In contrast, the expression of NRT1.8 was significantly induced (Supplementary Figures 1a,b). However, fold change of NRT1.5 down-regulation and NRT1.8 up-regulation in the wild-type and the mutants was different after exposure to Cd stress. In this case, fold change of NRT1.5 down-regulation in Col-0 was significantly higher than fold change in bglu10 or bglu18. On the other hand, although the expression of NRT1.8 was induced, there was almost no difference in fold change of NRT1.8 up-regulation between the wild-type and the ABA mutants (Figures 2C,D). These results indicated that NRT1.5, but not NRT1.8, responded to ABA signaling.
After 3 days of 20 μM Cd treatment, there was a significant difference in proton pump activity between the wild-type and the ABA mutants tested. V-ATPase (Figure 3A) and V-PPase (Figure 3B) activities were significantly higher in Col-0 than in bglu10 or bglu18. This suggests an increased ability of Col-0 plants to transport Cd2+ into the vacuole. The distribution of Cd2+ in vacuoles and protoplasts is shown in Figure 3C. As the ratio of vacuolar to protoplasmic Cd2+ is higher in Col-0 than that in bglu10 or bglu18, the Cd2+ remaining in the cytoplasm in Col-0 is significantly lower than in bglu10 or bglu18 (Figure 3D). At the same time, the proton pump activity also influenced the distribution of in the cells. Additionally, the ratio of vacuolar to protoplasmic in Col-0 was higher than in bglu10 or bglu18 (Figure 3E), therefore, remaining in the cytoplasm in Col-0 was significantly lower than in bglu10 or bglu18 (Figure 3F).
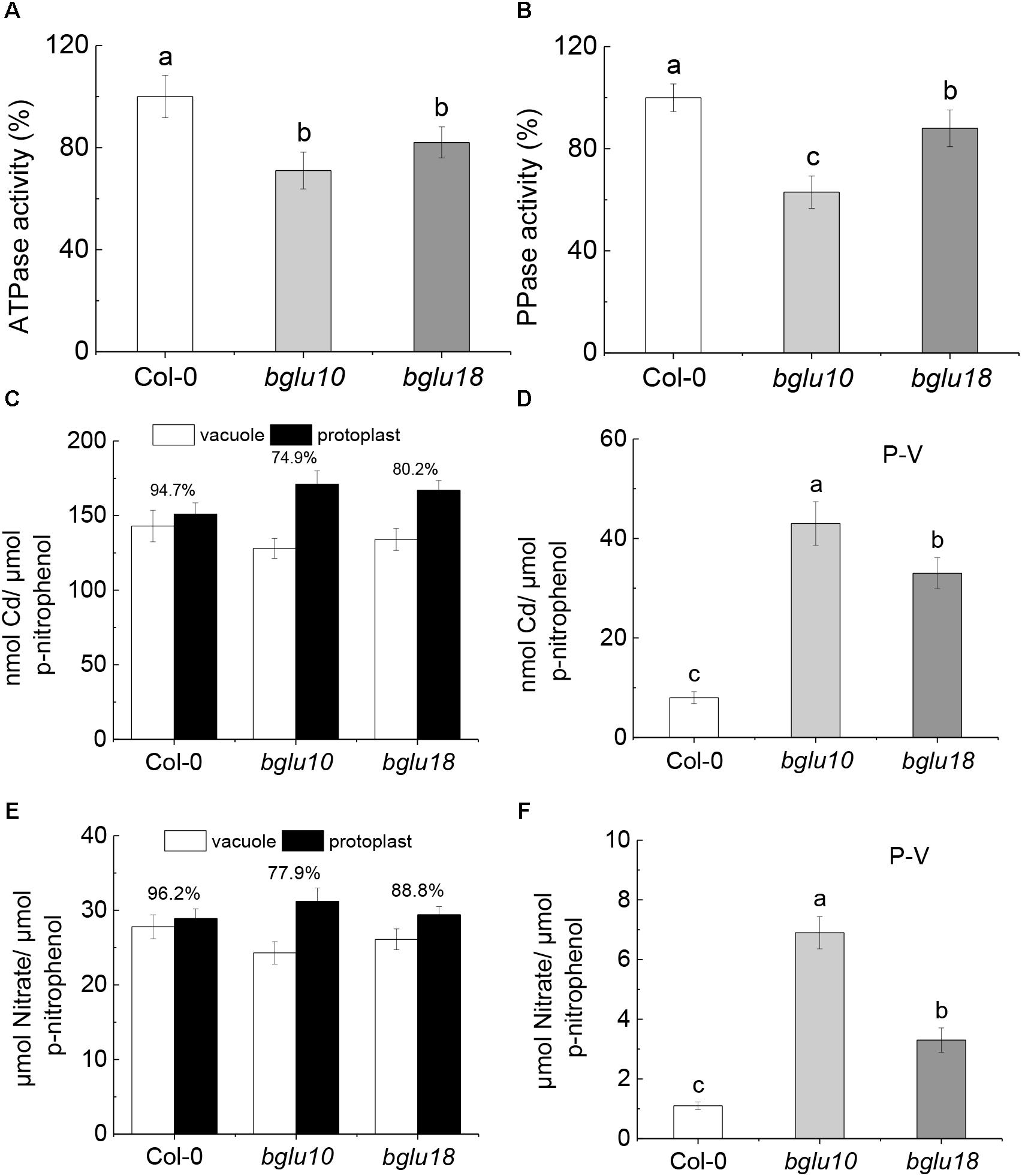
Figure 3. Vacuolar proton pump activity in Arabidopsis wild-type (Col-0) and ABA mutants (bglu10 and bglu18) root cells under Cd stress. (A) V-ATPase and (B) V-PPase activities in roots of Arabidopsis wild-type measured and ABA mutants under treatment with 20 μmol L-1 CdCl2. The proton pumps activity of the wild-type under Cd condition was set to 100%, and the specific activity of the root tonoplast proton pumps were expressed as percentage of that in the wild-type under Cd condition. (C) Cd2+ distribution between the vacuole and protoplast in roots; values above the bars represent the percentage of vacuolar Cd2+ relative to the total Cd2+ in protoplasts; (D) roots total Cd2+ accumulated in cytoplasm (P-V) calculated as total Cd2+ in protoplast – total Cd2+ in vacuole. (E) distribution between the vacuole and protoplast in roots; values above the bars represent the percentage of vacuolar relative to the total in protoplasts; (F) roots total accumulated in cytoplasm (P-V) calculated as total in protoplast – total in vacuole. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
Previous research demonstrated that stress decouples nitrate assimilation from photosynthesis through stress-initiated nitrate allocation to roots (SINAR), which is mediated by nitrate transporters NRT1.8 and NRT1.5, and functions to promote stress tolerance (Li et al., 2010; Chen et al., 2012). Here, we showed that ABA produced by Arabidopsis wild-type and ABA mutants differed in response to Cd stress. The cytoplasmic ABA levels in Col-0 plants were significantly higher than those in bglu10 or bglu18, which resulted in a much higher degree of inhibition of expression of NRT1.5 in the former, whereas the level of expression of NRT1.8 differed slightly between wild-type and mutants (Figure 2). The function of NRT1.5 is to load the xylem nitrate into the shoot, thus, after Cd stress, Col-0 had more nitrate in the root than bglu10 or bglu18 (Figure 4A). Concomitantly, due to the difference in the activity of the proton pump, the amount of nitrate remaining in the cytoplasm in Col-0 was reduced, as was the nitrate transported to the shoot (Figures 3E,F). The overall result of this was more nitrate left in the roots in Col-0, thereby reflecting wild-type plant resistance to Cd.
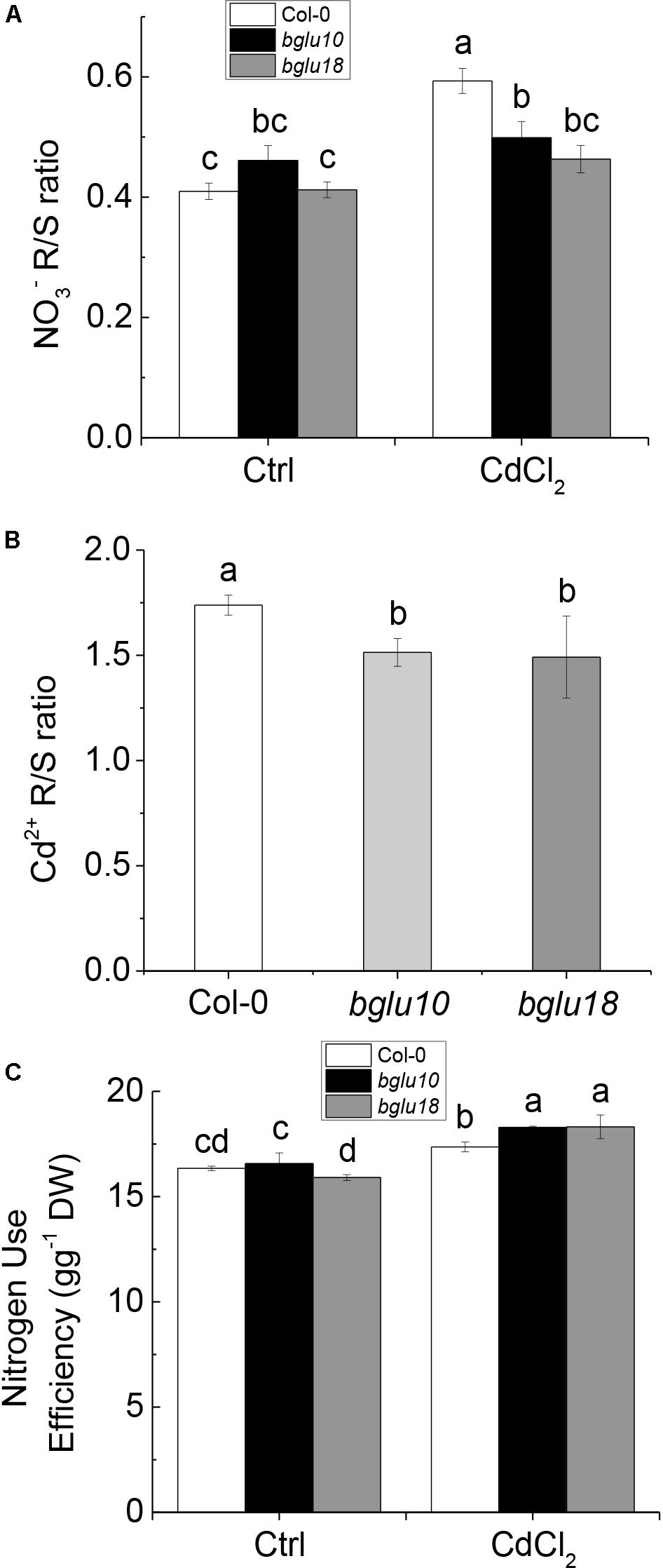
Figure 4. Nitrate and cadmium distribution in Arabidopsis wild-type (Col-0) and ABA mutants (bglu10 and bglu18) as well as NUE (nitrogen use efficiency). (A) [] ration between root and shoot under control and Cd treatment. (B) [Cd2+] ration between root and shoot. (C) NUE of wild-type and ABA mutants under normal treatment and Cd stress. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
On the other hand, due to the difference in the activity of the proton pump, the content of Cd in the cytoplasm of Col-0 was lower than in bglu10 or bglu18 (Figures 3C,D); thus, more Cd accumulated in the roots (Figure 4B), the net result of which was that Cd-induced damage was not as severe in Col-0 plants as in either of the ABA mutants.
In summary, the combined effects of nitrate and proton pump activity increased the resistance of Col-0 plants to Cd. Furthermore, the resistance of Col-0 to Cd was higher than that of bglu10 or bglu18, but the NUE was significantly lower in Col-0 plants than in either bglu10 or bglu18 (Figure 4C). In order to verify the anti-Cd mechanism in plants, we treated B. napus with exogenous ABA and arrived at the following results.
After treatment with exogenous ABA, the cotyledons of B. napus showed more severe yellowing than under Cd treatment alone due to the joint effects of both, ABA and Cd. ABA accelerated senescence of cotyledons, while Cd stress promoted cotyledon yellowing in. However, in this case the new leaves showed no trace of Cd poisoning, while the new leaves of B. napus under Cd-treatment alone showed obvious yellowing. Cd poisoning mainly affected new leaves; thus, the addition of exogenous ABA increased the anti-Cd ability of B. napus (Figures 5A,C). Further, after the addition of exogenous ABA, the proline concentration of B. napus was significantly higher than under Cd treatment alone (Figure 5B), whereas MDA concentration was significantly lower (Figure 5D). This confirmed that the addition of exogenous ABA enhanced Cd resistance of B. napus.
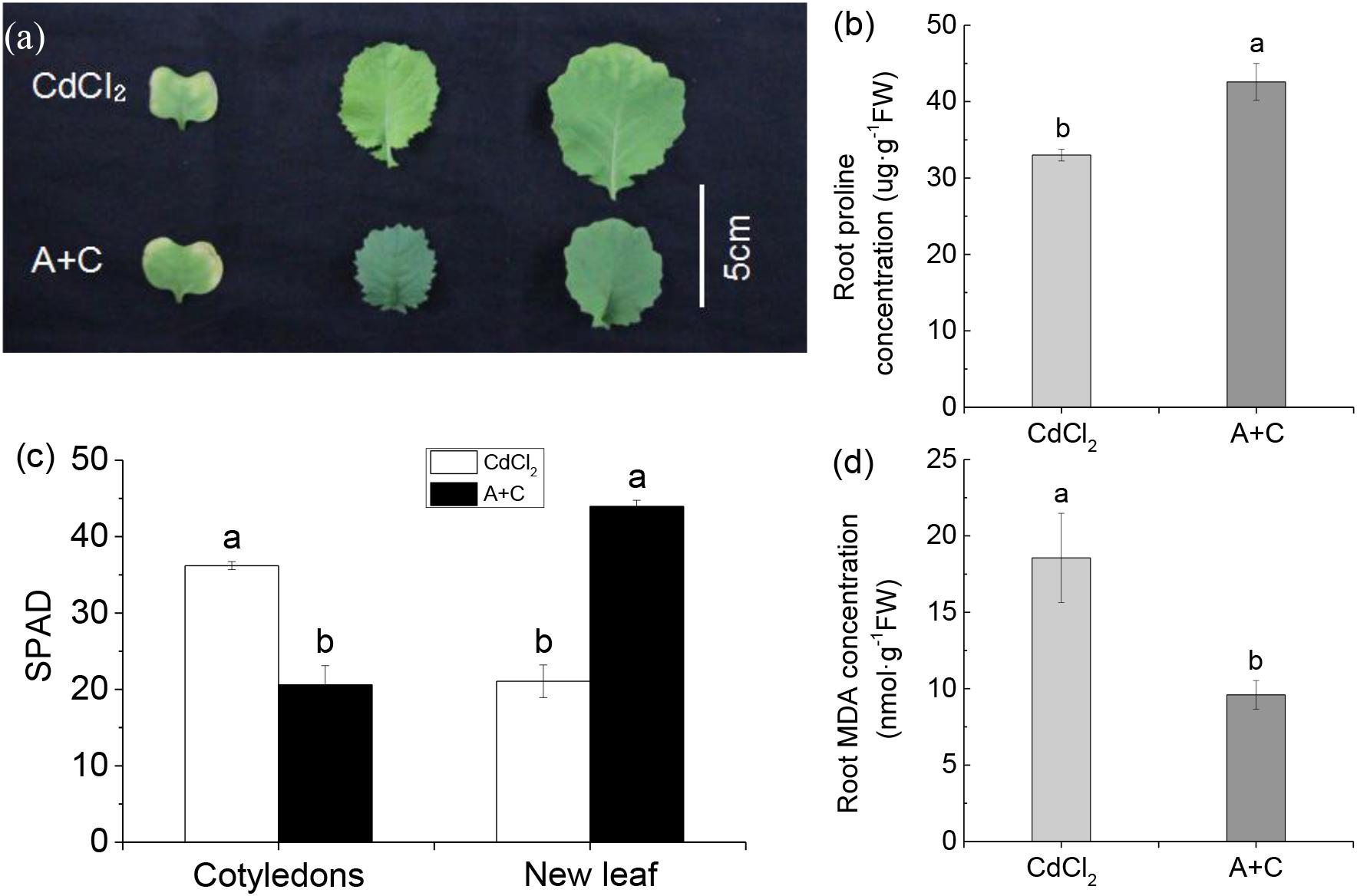
Figure 5. Under Cd stress, B. napus (814) showed stronger tolerance after adding exogenous ABA. (a) Photograph showing higher tolerance of the treatment with 5 μM exogenous ABA and 10 μM Cd (A+C) as compared with 10 μM Cd treatment. The leaves from left to right are cotyledons, the first new leaf and the second new leaf. (b) Root proline concentration in different treatments. (c) The SPAD of and new leaf. (d) Root MDA (Malonaldehyde) concentration in different treatments. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
After the addition of exogenous ABA, the expression level of BnNRT1.5 was significantly downregulated (sixfold), compared to Cd treatment alone (Figure 6A). However, there was no difference in the expression level of BnNRT1.8 (Figure 6B). Under CK (normal culture) and ABA treatments, we arrived at the same conclusion: BnNRT1.5 responded to ABA signal and the expression level was downregulated, while BnNRT1.8 did not respond. Further, content in the shoots and roots under Cd treatment alone was significantly higher than in the case of Cd treatment followed by ABA addition (A+C) (Figure 6C). However, in the (A+C) treatment, the concentration ratio between root and shoot was significantly higher than under the Cd treatment alone (Figure 6D). This indicated that the addition of exogenous ABA caused a greater proportion of to be distributed in the root of B. napus seedlings, thereby enhancing their resistance to Cd.
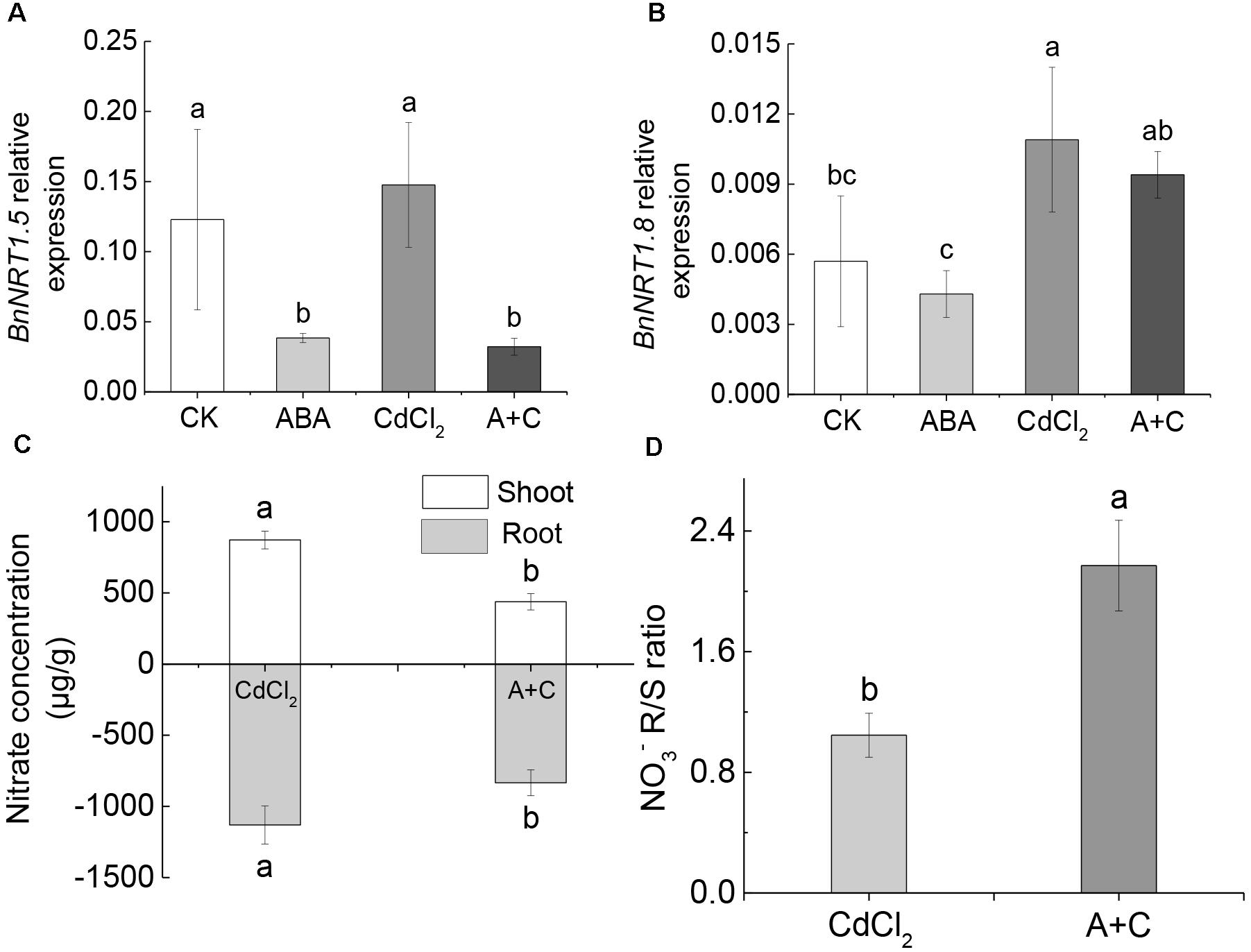
Figure 6. Exogenous ABA affects the distribution of under Cd conditions. (A) Gene expression of BnNRT1.5 in different treatments (CK, ABA, CdCl2, A+C). (B) Gene expression of BnNRT1.8. (C) The concentration of in the shoot and root. (D) [] ration between root and shoot. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
A number of studies have reported that the addition of exogenous ABA inhibited Cd absorption and increased Cd resistance in Arabidopsis and rice (Hsu and Kao, 2003; Uraguchi et al., 2009; Fan et al., 2014). Similarly, here we observed that after the addition of exogenous ABA, the absorption of Cd was also inhibited in B. napus, and that shoots and roots of B. napus were significantly lower in Cd content than under Cd treatment alone (Figures 7A,B).
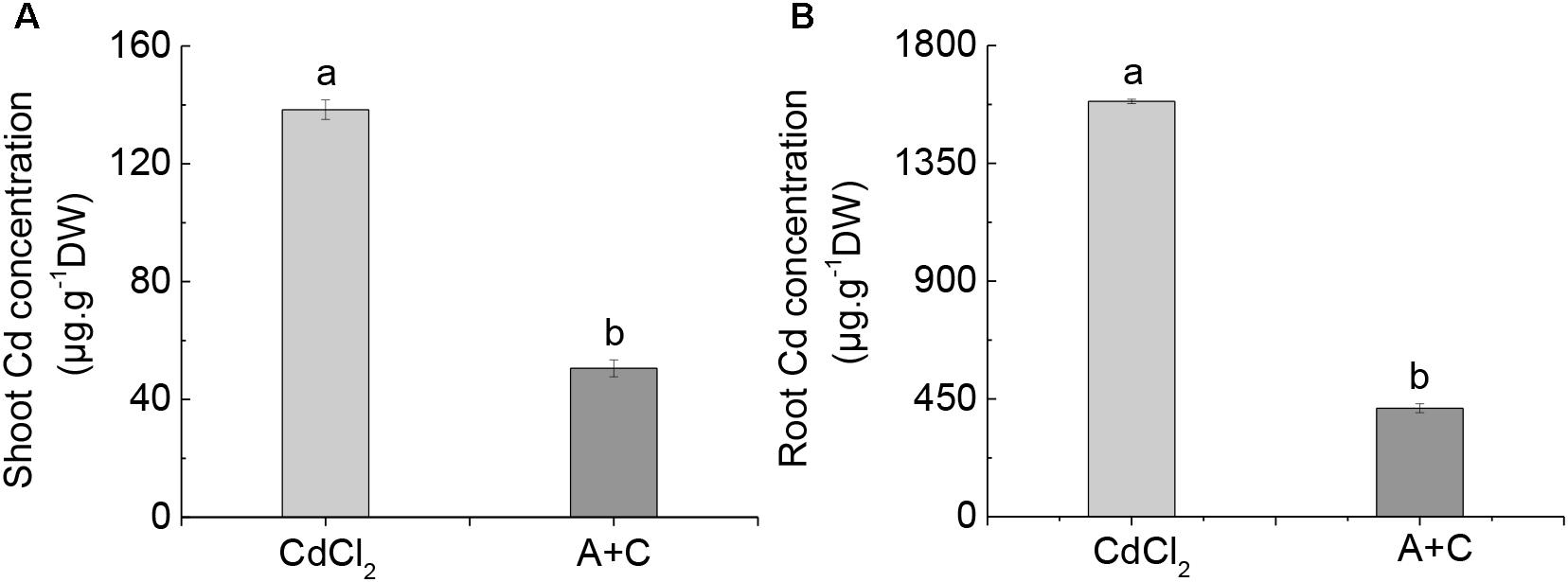
Figure 7. Effect of exogenous ABA on Cd uptake. Cd concentration in the shoot (A) and root (B) under Cd and (A+C) treatment. Data represent means ± SE (n = 4). Bars with the same letter indicate no significant difference at P < 0.05 level by the method of LSD.
Based on experimental data, we demonstrated that under Cd stress, NRT1.5 showed a response to ABA signaling, whereas NRT1.8 showed no response, thereby resulting in nitrate accumulation in the root. Concomitantly, because of the vacuolar action of the proton pump, and Cd were more distributed in the vacuoles of root cells. The and Cd R/S ratio values showed that more and Cd accumulated in the roots (Figures 4A,B). These two pathways together enhanced Cd resistance in Arabidopsis thaliana.
Abscisic acid is known as a stress hormone that takes part in the integration of signals. ABA induces different signaling pathways to help plants resist stress. ABA induces accumulation of protectants such as small hydrophilic proteins, sugars, and proline, or activates detoxifying mechanisms that confer stress tolerance by regulating redox balance or modifying ion transport to re-establish homeostasis (Ingram and Bartels, 1996; Pompeu et al., 2017). ABA can also affect stress-induced transcription factors or some of their target-gene expression can increase stress tolerance (Peleg et al., 2011; Qin et al., 2011; Sanghera et al., 2011). We found that Col-0 was significantly more resistant to the heavy metal than either bglu10 or bglu18 (Figure 1). Because of the Cd stress, the levels of active ABA produced by the wild-type and the ABA mutants were different, resulting in a phenotypic difference (Figure 2). NRT1.5 and NRT1.8 act as long-distance transporters of , and they respond to stress signals and act synergistically to allow more nitrate to accumulate in the root to enhance the level of plant resistance to stress (Lin et al., 2008; Li et al., 2010). The effects of NRT1.5 and NRT1.8 under adverse conditions are mediated by ethylene and JA (Zhang G.B. et al., 2014). In this study, we demonstrated that under Cd stress, NRT1.5 responded to the ABA signal and the expression level was downregulated, while NRT1.8 did not respond (Figure 2), which in turn caused more to accumulate in the roots (Figure 4A), thus, the Col-0 anti-cadmium ability is improved. The same conclusion was derived from experiments with B. napus (Figures 5, 6).
Further, V-ATPase and V-PPase play a vital role in the defense mechanisms to counter potential damage by heavy metal stress (Sharma et al., 2016). In this study, we found that Col-0, which is more resistant to Cd, showed higher V-ATPase and V-PPase activities (Figures 3A,B), which gave Col-0 a greater ability to sequester Cd in the vacuole, while a small amount of Cd remained in the cytoplasm (Figures 3C,D), caused more Cd to accumulate in the root (Figure 4B) and overall reduced the toxic effect of Cd on plants. Because there is a difference in the concentration of Cd in B. napus from the beginning (Figure 7), the degree of Cd toxicity in B. napus is different, endogenous ABA and exogenous ABA may differ in the way each counters Cd stress. Namely, the effect of endogenous ABA on the activity of the proton pump may cause accumulation of Cd in the root, while exogenous ABA seems to act by inhibiting Cd absorption to alleviate Cd toxicity. Therefore, we are not concerned about the proton pump activity in B. napus.
Vacuolar affects plant NUE (Han et al., 2016). At the same time, we found that Col-0, which showed a higher proton pump activity, accumulated more in the vacuoles of the roots and less in the cytoplasm, which resulted in less being transported up to the shoot (Figures 3E,F). Although the ability of the plant to resist Cd was enhanced, NUE was reduced (Figure 4C). This summarizes the roles of and V-ATPase and V-PPase in the improvement of Arabidopsis tolerance to Cd. This indicated a certain link between plant tolerance to stress and NUE. Indeed, generally high stress resistance would be associated with reduced NUE (Huang et al., 2018). However, it is unclear how enhanced resistance and NUE cooperate.
A possible mechanism for the NRT1.5 response to ABA signaling to trigger the accumulation of nitrate in the root and synergize with proton pump to enhance Arabidopsis resistance to Cd is schematized in Figure 8. According to this model, Cd stress induces ABA, which in turn inhibits the expression of NRT1.5, but has no effect on NRT1.8, thus causing more nitrate to be distributed in the roots; then it reduces NUE and improves Cd tolerance. Concomitantly, Cd stress enhanced the activity of the cell proton pumps in the roots, thereby causing more Cd and nitrate to be stored in the vacuole and to accumulate in the roots. More nitrate is allocated to the roots, while less Cd remains in the cytoplasm. Overall, these two processes enhance the resistance of A. thaliana to Cd. On the other hand, BnNRT1.5 also responded to the ABA signal and downregulated its own expression, whereas BnNRT1.8 showed no response. In addition, exogenous ABA hindered Cd absorption by seedlings, and then synergized with BnNRT1.5 to enhance Cd resistance in B. napus.
Arabidopsis thaliana wild-type Columbia-0 (Col-0) was used as the control for ABA conjugate hydrolysis mutants (bglu10 and bglu18). The functions of BGLU10 and BGLU18 have been confirmed in the reports of Wang and Lee. BGLU10, a member of the β-glucosidase family in Arabidopsis, is localized in vacuoles, where it hydrolyzes ABA-GE to produce active ABA; additionally, BGLU18 is localized in the ER, also hydrolyzing ABA-GE to produce active ABA (Lee et al., 2006; Wang et al., 2011). Mutants bglu10 and bglu18 used are BGLU10 and BGLU18 gene-deletion mutants, respectively. These were a gift from Zhang Jianhua, from the Chinese University of Hong Kong. B. napus (814) was provided by the Hunan Branch of Improvement Center of National Oil Crops, Hunan, China.
Arabidopsis plants were grown in a nutrient solution in plastic pots as described in Gong et al. (2003) and Han et al. (2016). The solution was changed every 3 days, with pH adjusted to 5.8 and 0.5 g L-1 MES (2- (4-Morpholino) ethanesulfonic acid) was added. Pots were arranged in a completely randomized design with six biological replications. The nutrient solution consisted of 1.25 mM KNO3, 0.625 mM KH2PO4, 0.5 mM MgSO4, 0.5 mM Ca (NO3)2⋅4H2O, 0.025 mM Fe-EDTA, 0.25 ml L-1 micronutrients (stock solution concentrations were the following: 70 mM H3BO3, 14 mM MnCl2, 1 mM ZnSO4, 0.5 mM CuSO4, and 0.2 mM NaMoO4).
Soaked B. napus seeds were sown onto gauze fixed to an enamel pan, and soaked with deionized water. After 6-days, seedlings were transplanted into 2-L black plastic pots containing nutrient solution. The experiment was laid in a completely randomized block design with six replicates. The nutrient solution consisted of 5.0 mM KNO3, 1.0 mM KH2PO4, 2.0 mM MgSO4⋅7H2O, 5.0 mM Ca(NO3)2⋅4H2O, 0.05 mM Fe-EDTA, 9 μM MnCl2⋅4H2O, 0.8 μM ZnSO4⋅7H2O, 0.3 μM CuSO4⋅5H2O, 0.1 μM NaMoO4⋅2H2O, and 50 μM H3BO3 (Zhang D. et al., 2014). The experiments were conducted at Hunan Agricultural University in a phytotron set at 70% relative humidity, 16 h/8 h light/dark cycle (A. thaliana) or 14 h/10 h light/dark cycle (B. napus), at constant temperature (22°C). The nutrient solution for Arabidopsis plants was changed every 3-days and, after 4 weeks of cultivation, they were treated for 3-days with 20 μM Cd. The nutrient solution for B. napus plants was changed every 5-days and, after 10-days of cultivation, they were treated for 4-days with either 10 μM Cd or 10 μM Cd added with 5 μM ABA. B. napus and Arabidopsis were analyzed separately.
Leaves (approximately 0.15 g) of A. thaliana were sampled and extracted in 10 ml 1:1 absolute ethanol: acetone for 24 h. Absorbance was then measured at 663, 645, and 652 nm to determine chlorophyll concentration. Chlorophyll loss (a) was calculated as the chlorophyll concentration under the control conditions (b) minus chlorophyll concentration under Cd stress, and (c) divided by concentration under control conditions, i.e., a = (b-c)/c∗100. MDA and proline were measured in root tissues. For MDA, 0.5 g of root tissue was ground in 5 ml 5% TCA, then centrifuged at 925 × g for 10 min. The supernatant was collected and used for determination of MDA concentration using the modified thiobarbituric acid–malondialdehyde (TBA–MDA) assay (Song et al., 2014). Proline was assayed according to the method described in Bates et al. (1973) and Sharma and Dubey (2005). Briefly, root tissues (0.5 g) were sampled and ground in 5 ml of 3% sulfosalicylic acid, then centrifuged at 22000 × g for 5 min. The supernatant was collected and used for determination of proline concentration by reaction with acidic ninhydrin (Chen et al., 2012).
Root tissues of A. thaliana (0.5 g) were collected to isolate intact protoplasts and vacuoles as described in Robert et al. (2007), with minor modifications as outlined in Huang et al. (2012) and Han et al. (2016). Purified protoplasts and vacuoles were subsampled and used to determine and Cd2+ concentrations (Vögeli-Lange and Wagner, 1990) and for enzyme activity assays (Ma et al., 2005). concentration in protoplasts and vacuoles were measured by a continuous flow auto-analyzer (Auto Analyzer 3, Bran and Luebbe, Norderstedt, Germany) as described previously (Han et al., 2016). The activities of acid phosphatase (ACP) and cytochrome oxidase (COX) were determined using plant ACP colorimetry and COX assay kits (GenMedSci, Inc., Shanghai, China) following the instructions by the manufacturer. ACP activity specific to vacuoles was determined and used to normalize accumulation. We measured in the protoplast outside the vacuole, which includes the cytosol and organelles, e.g., mitochondria and Golgi Apparatus (Robert et al., 2007). As most in the protoplast outside the vacuole is located in the cytosol (Krebs et al., 2010), we refer to distribution between vacuoles and cytosol rather than vacuole versus protoplast; Cd2+ concentrations in protoplasts and vacuoles were measured by inductively coupled plasma-mass spectrometry (ICP-MS, ELAN DRC-e, PerkinElmer, Shelton, United States) as described in Huang et al. (2012), with the corresponding modification.
V-ATPase and V-PPase activities within microsomal membranes collected from the root tissues of A. thaliana were colorimetrically determined as Pi release after an incubation period of 40 min at 28°C (Zhu et al., 2001; Krebs et al., 2010; Han et al., 2015). Reactions were terminated by adding 40 mM citric acid. For the blank value, 10 μg of bovine serum albumin was used instead of tonoplast vesicles. The V-ATPase assay medium contained 25 mM Tris-MES (pH 7.0), 4 mM MgSO4⋅7H2O, 50 mM KCl, 1 mM NaN3, 0.1 mM Na2MoO4, 0.1% Brij 35, 500 μM NaVO4, and 2 mM Mg-ATP. Activity was expressed as the difference in Pi release measured in the absence and in the presence of 100 nM concanamycin A. V-PPase was assayed in a reaction medium containing 25 mM Tris-MES (pH 7.5), 2 mM MgSO4 × 7H2O, 0.1 mM Na2MoO4, 0.1% Brij 58, and 0.2 mM K4P2O7. V-PPase activity was calculated as the difference in Pi release measured in the absence and the presence of 50 mM KCl.
Nitrate was extracted from tissue samples (shoot: 1 g; root: 0.5 g) in deionized water for 30 min in a boiling water bath; next, 0.1 ml of the sample solution was taken, 0.4 ml of 5% salicylic acid-sulfuric acid solution was added, and mixed. After cooling, the mixture was cooled at room temperature for 20 min, and then 9.5 ml of an 8% sodium hydroxide solution was added. The sample was then allowed to cool to room temperature and spectrophotometrically determined for nitrate at 410 nm (Cataldo et al., 1975).
Whole, hydroponically grown seedlings of B. napus and A. thaliana were sampled, oven-dried to constant weight, first at 105°C for 30 min, followed by 70°C. N concentration was determined as described by Han et al. (2016) (N data is used to calculate NUE). For the Cd2+ assay, shoots and roots were sampled separately, dried, and weighed; Cd2+ concentration was then determined by ICP-MS, after digesting with 4:1 HNO3: HClO4 (Huang et al., 2012).
Endogenous ABA was extracted from the isolated vacuoles and protoplasts of each sample using 0.5 mL of homogenizing buffer (70% methanol, 0.1% formic acid); 2 ng ABA-d6 (Olchemim, Olomouc, Czechia) were added to the extracts as an internal standard (Balcke et al., 2012). The mixture was diluted twice using deionized water, and the ABA concentration of a 50-μL dilution of each sample was determined using the UPLC-TripleTOF 5600+ system (Sciex, Concord, ON, Canada).
Root samples were ground in liquid nitrogen. Total RNA was extracted with TRIzol (Ambion, United States). The first-strand cDNA was synthesized using the total RNA by PrimeScript reverse transcription (RT) reagent kit (TaKaRa, Shiga, Japan). The qRT-PCR assays for the detection of relative gene expression were performed using SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa, Shiga, Japan) with an Applied Biosystems StepOneTM Plus Real-time PCR System (Thermo Fisher Scientific, Waltham, MA, United States). The thermal cycles were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s, then 60°C for 30 s. Melt curve analysis to ensure the primer gene-specificity was conducted as follows: 95°C for 15 s, 60°C for 1 min, 60–95°C for 15 s (+0.3°C per cycle). The gene-specific primers for qRT-PCR assays are listed in Supplementary Table 1 (Bustin et al., 2009; Wang et al., 2014).
We used the SPSS software (IBM SPSS Statistic 19) for ANOVA and mean separation of main effects and interactions using LSD’s multiple range test at P < 0.05. Data are means and SE of three or six replicates from three independent experiments. Different letters associated with specific data (e.g., at the top of histogram bars in figures) indicate significant differences at P < 0.05.
TW and ZZ designed the experiments and all co-authors wrote the manuscript. TW performed most of the experiments. TW, YH, and ZZ analyzed the data.
This study was financially supported in part by the National Key R&D Program of China (2017YFD0200100 and 2017YFD0200103), the Hunan Provincial Recruitment Program of Foreign Experts, the National Oilseed Rape Production Technology System of China, “2011 Plan” supported by The Chinese Ministry of Education, and the Double First-Class Construction Project of Hunan Agricultural University (kxk201801005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Abdelbagi M. Ismail (IRRI) discussed the MS contents, and thank The Chinese University of Hong Kong supplied the plant materials mutant (bglu10 and bglu18).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01892/full#supplementary-material
Alboresi, A., Gestin, C., Leydecker, M. T., Bedu, M., Meyer, C., and Truong, H. N. (2005). Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 28, 500–512. doi: 10.1111/j.1365-3040.2005.01292.x
Alvarez, J. M., Vidal, E. A., and Gutiérrez, R. A. (2012). Integration of local and systemic signaling pathways for plant N responses. Curr. Opin. Plant Biol. 15, 185–191. doi: 10.1016/j.pbi.2012.03.009
Andreev, I. M. (2001). Functions of the vacuole in higher plant cells. Russ. J. Plant Physiol. 48, 672–680. doi: 10.1023/A:1016776523371
Balcke, G. U., Handrick, V., Bergau, N., Fichtner, M., Henning, A., Stellmach, H., et al. (2012). An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 8:47. doi: 10.1186/1746-4811-8-47
Bates, L., Waldren, R., and Teare, I. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1016/j.dental.2010.07.006
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi: 10.1373/clinchem.2008.112797
Cataldo, D. A., Maroon, M., Schrader, L. E., and Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6, 71–80. doi: 10.1080/00103627509366547
Chen, C. Z., Lv, X. F., Li, J. Y., Yi, H. Y., and Gong, J. M. (2012). Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 159, 1582–1590. doi: 10.1104/pp.112.199257
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R., and Abrams, S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679. doi: 10.1146/annurev-arplant-042809-112122
DalCorso, G., Farinati, S., Maistri, S., and Furini, A. (2008). How plants cope with cadmium: staking all on metabolism and gene expression. J. Integr. Plant Biol. 50, 1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x
Dietz, K. J., Sauter, A., Wichert, K., Messdaghi, D., and Hartung, W. (2000). Extracellular beta-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 51, 937–944. doi: 10.1093/jexbot/51.346.937
Fan, S. K., Fang, X. Z., Guan, M. Y., Ye, Y. Q., Lin, X. Y., Du, S. T., et al. (2014). Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front. Plant Sci. 5:721. doi: 10.3389/fpls.2014.00721
Finkelstein, R. (2013). Abscisic acid synthesis and response. Arabidopsis Book 11:e0166. doi: 10.1199/tab.0166
Finkemeier, I., Kluge, C., Metwally, M., Georgi, M., Grotjohann, N., and Dietz, K. J. (2003). Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare. Plant Cell Environ. 26, 821–833. doi: 10.1046/j.1365-3040.2003.01014.x
Fujii, H., and Zhu, J. K. (2009). Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. U.S.A. 106, 8380–8385. doi: 10.1073/pnas.0903144106
Gong, J. M., Lee, D. A., and Schroeder, J. I. (2003). Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100, 10118–10123. doi: 10.1073/pnas.1734072100
Gonzalez-Guzman, M., Pizzio, G. A., Antoni, R., Vera-Sirera, F., Merilo, E., Bassel, G. W., et al. (2012). Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24, 2483–2496. doi: 10.1105/tpc.112.098574
Han, Y. L., Liu, Q., Gu, J. D., Gong, J. M., Guan, C. Y., Lepo, J. E., et al. (2015). V-ATPase and V-PPase at the tonoplast affect NO3- content in Brassica napus by controlling distribution of NO3- between the cytoplasm and vacuole. J. Plant Growth Regul. 34, 22–34. doi: 10.1007/s00344-014-9439-8
Han, Y. L., Song, H. X., Liao, Q., Yu, Y., Jian, S. F., Lepo, J. E., et al. (2016). Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 170, 1684–1698. doi: 10.1104/pp.15.01377
Hirel, B., Le Gouis, J., Ney, B., and Gallais, A. (2007). The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58, 2369–2387. doi: 10.1093/jxb/erm097
Hsu, Y. T., and Kao, C. H. (2003). Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ. 26, 867–874. doi: 10.1046/j.1365-3040.2003.01018.x
Huang, J., Zhang, Y., Peng, J. S., Zhong, C., Yi, H. Y., Ow, D. W., et al. (2012). Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis. Plant Physiol. 158, 1779–1788. doi: 10.1104/pp.111.192872
Huang, X. Z., Hou, L. Y., Meng, J. J., You, H. W., Li, Z., Gong, Z. Z., et al. (2018). The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 11, 970–982. doi: 10.1016/j.molp.2018.05.001
Ingram, J., and Bartels, D. (1996). The molecular basis of dehydration tolerance in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 47, 377–403. doi: 10.1146/annurev.arplant.47.1.377
Khoudi, H., Maatar, Y., Gouiaa, S., and Masmoudi, K. (2012). Transgenic tobacco plants expressing ectopically wheat H+-pyrophosphatase (H+-PPase) gene TaVP1 show enhanced accumulation and tolerance to cadmium. J. Plant Physiol. 169, 98–103. doi: 10.1016/j.jplph.2011.07.016
Kim, J. H., Chen, C., Yun, H. R., Lee, Y. S., Yi, Y. B., Kim, T. Y., et al. (2017). Disorder of trafficking system of plasma membrane and vacuole antiporter proteins causes hypersensitive response to salinity stress in Arabidopsis Thaliana. J. Plant Biol. 60, 380–386. doi: 10.1007/s12374-017-0042-y
Krapp, A. (2015). Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr. Opin. Plant Biol. 25, 115–122. doi: 10.1016/j.pbi.2015.05.010
Krapp, A., David, L. C., Chardin, C., Girin, T., Marmagne, A., Leprince, A. S., et al. (2014). Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 65, 789–798. doi: 10.1093/jxb/eru001
Krebs, M., Beyhl, D., Görlich, E., Al-Rasheid, K. A., Marten, I., Stierhof, Y. D., et al. (2010). Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. U.S.A. 107, 3251–3256. doi: 10.1073/pnas.0913035107
Krouk, G., Crawford, N. M., Coruzzi, G. M., and Tsay, Y.-F. (2010). Nitrate signaling: adaptation to fluctuating environments. Curr. Opin. Plant Biol. 13, 265–272. doi: 10.1016/j.pbi.2009.12.003
Lee, K. H., Piao, H. L., Kim, H. Y., Choi, S. M., Jiang, F., Hartung, W., et al. (2006). Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126, 1109–1120. doi: 10.1016/j.cell.2006.07.034
Li, J. Y., Fu, Y. L., Pike, S. M., Bao, J., Tian, W., Zhang, Y., et al. (2010). The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22, 1633–1646. doi: 10.1105/tpc.110.075242
Lin, S. H., Kuo, H. F., Canivenc, G., Lin, C. S., Lepetit, M., Hsu, P. K., et al. (2008). Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20, 2514–2528. doi: 10.1105/tpc.108.060244
Liu, W., Jiang, Y. Y., Sun, J., Geng, S. Z., Pan, Z. M., Prinz, R. A., et al. (2018). Activation of TGF-β-activated kinase 1 (TAK1) restricts Salmonella Typhimurium growth by inducing AMPK activation and autophagy. Cell Death Dis. 9:570. doi: 10.1038/s41419-018-0612-z
Ma, J. F., Ueno, D., Zhao, F. J., and McGrath, S. P. (2005). Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220, 731–736. doi: 10.1007/s00425-004-1392-5
Marin, I. C., Loef, I., Bartetzko, L., Searle, I., Coupland, G., Stitt, M., et al. (2011). Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 233, 539–552. doi: 10.1007/s00425-010-1316-5
Munemasa, S., Hauser, F., Park, J., Waadt, R., Brandt, B., and Schroeder, J. I. (2015). Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 28, 154–162. doi: 10.1016/j.pbi.2015.10.010
O’Brien, J. A., Vega, A., Bouguyon, E., Krouk, G., Gojon, A., Coruzzi, G., et al. (2016). Nitrate transport, sensing, and responses in plants. Mol. Plant 9, 837–856. doi: 10.1016/j.molp.2016.05.004
Ondzighi-Assoume, C. A., Chakraborty, S., and Harris, J. M. (2016). Environmental nitrate stimulates abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. Plant Cell 28, 729–745. doi: 10.1105/tpc.15.00946
Peleg, Z., Apse, M. P., and Blumwald, E. (2011). “Engineering salinity and water stress tolerance in crop plants: getting closer to the feld,” in Plant Responses to Drought and Salinity Stress: Developments in a Post-Genomic Era, Vol. 57, ed. I. Turkan (London: Academic Press), 405–443. doi: 10.1016/B978-0-12-387692-8.00012-6
Pompeu, G. B., Vilhena, M. B., Gratão, P. L., Carvalho, R. F., Rossi, M. L., Martinelli, A. P., et al. (2017). Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 254, 771–783. doi: 10.1007/s00709-016-0989-4
Qin, F., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2011). Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 52, 1569–1582. doi: 10.1093/pcp/pcr106
Rahayu, Y. S., Walch-Liu, P., Neumann, G., Romheld, V., von Wiren, N., and Bangerth, F. (2005). Root-derived cytokinins as long-distance signals for NO3–induced stimulation of leaf growth. J. Exp. Bot. 56, 1143–1152. doi: 10.1093/jxb/eri107
Robert, S., Zouhar, J., Carter, C., and Raikhel, N. (2007). Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat. Protoc. 2, 259–262. doi: 10.1038/nprot.2007.26
Ruffel, S., Gojon, A., and Lejay, L. (2014). Signal interactions in the regulation of root nitrate uptake. J. Exp. Bot. 65, 5509–5517. doi: 10.1093/jxb/eru321
Sanghera, G. S., Wani, S. H., Hussain, W., and Singh, N. B. (2011). Engineering cold stress tolerance in crop plants. Curr. Genomics 12, 30–43. doi: 10.2174/138920211794520178
Schneider, T., Schellenberg, M., Meyer, S., Keller, F., Gehrig, P., Riedel, K., et al. (2009). Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics 9, 2668–2677. doi: 10.1002/pmic.200800806
Sharma, P., and Dubey, R. S. (2005). Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J. Plant Physiol. 162, 854–864. doi: 10.1016/j.jplph.2004.09.011
Sharma, S. S., Dietz, K. J., and Mimura, T. (2016). Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 39, 1112–1126. doi: 10.1111/pce.12706
Sharma, S. S., Kaul, S., Metwally, A., Goyal, K. C., Finkemeier, I., and Dietz, K. J. (2004). Cadmium toxicity to barley (Hordeum vulgare) as affected by varying Fe nutritional status. Plant Sci. 166, 1287–1295. doi: 10.1016/j.plantsci.2004.01.006
Song, W. Y., Yamaki, T., Yamaji, N., Ko, D., Jung, K. H., Fujii-Kashino, M., et al. (2014). A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. U.S.A. 111, 15699–15704. doi: 10.1073/pnas.1414968111
Takahashi, F., Suzuki, T., Osakabe, Y., Betsuyaku, S., Kondo, Y., Dohmae, N., et al. (2018). A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238. doi: 10.1038/s41586-018-0009-2
Takeda, E., Jin, N., Itakura, E., Kira, S., Kamada, Y., Weisman, L. S., et al. (2018). Vacuole-mediated selective regulation of TORC1-Sch9 signaling following oxidative stress. Mol. Biol. Cell 29, 510–522. doi: 10.1091/mbc.E17-09-0553
Uraguchi, S., Mori, S., Kuramata, M., Kawasaki, A., Arao, T., and Ishikawa, S. (2009). Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 60, 2677–2688. doi: 10.1093/jxb/erp119
Vidal, E. A., Moyano, T. C., Canales, J., and Gutierrez, R. A. (2014). Nitrogen control of developmental phase transitions in Arabidopsis thaliana. J. Exp. Bot. 65, 5611–5618. doi: 10.1093/jxb/eru326
Vögeli-Lange, R., and Wagner, G. J. (1990). Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves : implication of a transport function for cadmium-binding peptides. Plant Physiol. 92, 1086–1093. doi: 10.1104/pp.92.4.1086
von Wiren, N., Gazzarrini, S., Gojon, A., and Frommer, W. B. (2000). The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 3, 254–261. doi: 10.1016/S1369-5266(00)00073-X
Wang, P. T., Liu, H., Hua, H. J., Wang, L., and Song, C. P. (2011). A vacuole localized β-glucosidase contributes to drought tolerance in Arabidopsis. Chin. Sci. Bull. 56, 3538–3546. doi: 10.1007/s11434-011-4802-7
Wang, Y. Y., Hsu, P. K., and Tsay, Y. F. (2012). Uptake, allocation and signaling of nitrate. Trends Plant Sci. 17, 458–467. doi: 10.1016/j.tplants.2012.04.006
Wang, Z., Chen, Y., Fang, H., Shi, H., Chen, K., Zhang, Z., et al. (2014). Selection of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in Brassica napus under various stress conditions. Mol. Genet. Genomics 289, 1023–1035. doi: 10.1007/s00438-014-0853-1
Zhang, D., Hua, Y., Wang, X., Zhao, H., Shi, L., and Xu, F. (2014). A high-density genetic map identifies a novel major QTL for boron efficiency in oilseed rape (Brassica napus L.). PLoS One 9:e112089. doi: 10.1371/journal.pone.0112089
Zhang, G. B., Yi, H. Y., and Gong, J. M. (2014). The Arabidopsis ethylene/jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell 26, 3984–3998. doi: 10.1105/tpc.114.129296
Zhao, Y., Chan, Z., Gao, J., Xing, L., Cao, M., Yu, C., et al. (2016). ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. U.S.A. 113, 1949–1954. doi: 10.1073/pnas.1522840113
Keywords: ABA signaling, NRT1.5, NRT1.8, , Cd stress, proton pump activity
Citation: Wang T, Hua Y, Chen M, Zhang J, Guan C and Zhang Z (2018) Mechanism Enhancing Arabidopsis Resistance to Cadmium: The Role of NRT1.5 and Proton Pump. Front. Plant Sci. 9:1892. doi: 10.3389/fpls.2018.01892
Received: 29 August 2018; Accepted: 06 December 2018;
Published: 19 December 2018.
Edited by:
Sergey Shabala, University of Tasmania, AustraliaReviewed by:
Honghong Wu, University of California, Riverside, United StatesCopyright © 2018 Wang, Hua, Chen, Zhang, Guan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Zhang, emh6aDE0NjhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.