- 1Plant Science and Natural Products, Institute of Biology, Leiden University, Leiden, Netherlands
- 2Business Unit Horticulture, Wageningen University and Research Center, Bleiswijk, Netherlands
Western flower thrips (WFT) Frankliniella occidentalis (Pergande) is a key agricultural pest of cultivated tomatoes. Induced host plant resistance by activating jasmonic acid (JA) signaling pathway constitutes a promising method for WFT control. The phytotoxin coronatine (COR), produced by Pseudomonas syringae pv. tomato DC3000 (Pst), mimics the plant hormone JA-Isoleucine and can promote resistance against herbivorous arthropods. Here we determined the effect of Pst and COR on tomato resistance against WFT, induction of JA and salicylic acid (SA) associated defenses, and plant chemistry. Additionally, we investigated the presence of other components in Pst-derived and filtered culture medium, and their interactive effect with COR on tomato resistance to WFT. Our results showed that infiltration of COR or Pst reduced WFT feeding damage in tomato plants. COR and Pst induced the expression of JA-associated gene and protein marker. COR also induced expression of a SA-related responsive gene, although at much less magnitude. Activation of JA defenses in COR and Pst infiltrated plants did not affect density of type VI leaf trichomes, which are defenses reported to be induced by JA. An untargeted metabolomic analysis showed that both treatments induced strong changes in infiltrated leaves, but leaf responses to COR or Pst slightly differed. Application of the Pst-derived and filtered culture medium, containing COR but not viable Pst, also increased tomato resistance against WFT confirming that the induction of tomato defenses does not require a living Pst population to be present in the plant. Infiltration of tomato plants with low concentrations of COR in diluted Pst-derived and filtered culture medium reduced WFT feeding damage in a greater magnitude than infiltration with an equivalent amount of pure COR indicating that other elicitors are present in the medium. This was confirmed by the fact that the medium from a COR-mutant of Pst also strongly reduced silver damage. In conclusion, our results indicate that induction of JA defenses by COR, Pst infection, the medium of Pst and the medium of a Pst COR- mutant increased resistance against WFT. This was not mediated by the reinforcement of leaf trichome densities, but rather the induction of chemical defenses.
Introduction
The western flower thrips (WFT), Frankliniella occidentalis [Pergande], is one of the most serious greenhouse and field insect pests of vegetable and ornamental crops worldwide. It is a highly polyphagous insect that can feed on more than 200 wild and cultivated host species (Lewis, 1997) by piercing and sucking epidermal/mesophyll plant cells, which results in damaged areas of a silvery appearance (silver damage). WFT cause direct damage by feeding on leaves, flowers, and fruits, or indirect damage through the transmission of plant viruses (de Jager et al., 1995a,b), being the main vector of tospoviruses, such as tomato spotted wilt virus (Maris et al., 2003). Current control of WFT mainly depends on the use of pesticides and biological control. Use of pesticides leads to residue problems on marketable crops, human health risks, toxicity to non-target beneficial organisms, and environmental contamination (Bielza, 2008; Demirozer et al., 2012; Gao et al., 2012; Mouden et al., 2017). Therefore, multiple and complementary tactics are necessary in the framework of integrated pest management (IPM) programs.
Enhancement of constitutive and/or inducible host plant defenses against WFT has recently been discussed as a promising alternative for thrips control (Steenbergen et al., 2018). Plants defend themselves against herbivores by employing a plethora of physical and chemical arsenals, including trichomes, defensive enzymes, and secondary metabolites that can be present in the plant before attack or induced after detecting the presence of the attacker. Induced plant defenses against herbivory are mainly controlled by the phytohormones jasmonic acid (JA), salicylic acid (SA), and ethylene (ET), and fine-tuned by other phytohormones such as abscisic acid, auxins, cytokinins, and gibberellins (Pieterse et al., 2012). Activation of JA-associated defenses has been reported to confer plant resistance against pierce-sucking arthropods such as spider mites and thrips (Li et al., 2002; Ament et al., 2004). In particular, WFT have been reported to be susceptible to JA-associated induced defenses in diverse plant species such as Arabidopsis, Chinese cabbage (Brassica rapa), cotton (Aphis gossypii), and tomato (Solanum lycopersicum) (Omer et al., 2001; Abe et al., 2008; Abe et al., 2009; Escobar-Bravo et al., 2017).
In tomato (S. lycopersicum), induction of JA-related defenses has been associated to increased levels of defensive type-VI glandular trichomes and their derived exudates, proteins such as proteinase inhibitors and polyphenol oxidases (PPO), and secondary metabolites (Boughton et al., 2005; Degenhardt et al., 2010; Escobar-Bravo et al., 2017). Type-VI trichomes are important physical and chemical defense barriers, and their absence increases tomato susceptibility against herbivory (Kang et al., 2010a,b). Overexpression of certain proteinase inhibitors has been reported to increase plant resistance against WFT (Annadana et al., 2002; Outchkourov et al., 2004), and enhanced PPO activities can confer enhanced resistance against beet armyworm (Spodoptera exigua), cotton bollworm (Helicoverpa armigera) (Bhonwong et al., 2009), and cutworm (Spodoptera litura) (Mahanil et al., 2008). Accordingly, application of natural or synthetic elicitors activating these JA-associated defenses can increase tomato resistance against various insects, including WFT (Thaler, 1999; Thaler et al., 2002; Escobar-Bravo et al., 2017).
The phytotoxin coronatine (COR) produced by several pathovars of Pseudomonas syringae acts as a virulence factor in P. syringae pv. tomato (Pst), allowing this pathogen to successfully develop high populations in the plant (Zhao et al., 2001; Uppalapati and Bender, 2005; Zheng et al., 2012). COR is a polyketide formed by the coupling of coronafacic acid (CFA) and coronamic acid (CMA) through an amide bond (Bender et al., 1993). Its structure mimics a bioactive JA conjugate (JA-Isoleucine), thus having the ability to stimulate JA-associated defense responses (reviewed by Geng et al., 2014), but also affecting ET and auxin signaling pathways (Uppalapati et al., 2005). Both JA and COR can induce chlorosis, ET emission, inhibition of root elongation, volatile production, biosynthesis of stress-associated compounds and anti-herbivore proteins (Uppalapati et al., 2005). Consequently, infiltration with COR-producing P. syringae or infiltration of pure COR in Arabidopsis enhanced plant resistance against arthropod herbivores, such as the caterpillar Trichoplusia ni (Cui et al., 2005). In tomato, P. syringae infection (López-Gresa et al., 2011) or COR application (Uppalapati et al., 2005) also induces metabolomic changes in the plant. All these studies suggest that Pst may potentially be used to increase resistance against WFT in tomato. Yet, the effects of Pst and COR infiltration on tomato defenses against herbivory may differ. Activation of defense signaling pathways in Pst-infected plants is not only mediated by the phytotoxin COR, but also by an array of virulence factors such as exopolysaccharides effectors secreted by the type III secretion system, and cell-wall-degrading enzymes (Zhao et al., 2003; He et al., 2004). Thus, research on the possible use of other Pst-derived defense elicitors for their practical application in agricultural systems is crucial, as Pst is a plant pathogen.
Here, we first investigated the effect of COR or Pst DC3000 infiltration on tomato defenses against WFT. In particular, we determined their effect on WFT-associated feeding damage, as well as variations in type-VI leaf trichome densities, leaf metabolome, expression of defense-associated genes, and tomato growth. In a further attempt to test the possible role of other Pst DC3000 associated defense elicitors in tomato-WFT interaction, we also studied the effect of dilution series of the Pst-derived filtered medium and a COR-deficient Pst strain on tomato resistance against WFT and activation of JA signaling pathway.
Materials and Methods
Plants, Insect, and Bacterial Strains
The tomato cultivar “Moneymaker” (S. lycopersicum) was used in all experiments. Tomato seeds were germinated on filter paper soaked with MilliQ water and incubated at 20°C. Five days later, germinated seeds were planted in plastic pots (11 cm × 11 cm × 12 cm) filled with potting soil and maintained in a climate room at 20°C, 70% RH, 113.6 μmol photons m-2⋅s-1 of photosynthetically active radiation (PAR) and L16:D8 photoperiod.
Western flower thrips, F. occidentalis (Pergande), were maintained on chrysanthemum flowers (cultivar Euro Sunny) in a climate room at 23°C, 60% RH, and L12:D12 photoperiod.
Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) NCPPB4369 was obtained from the National Collection of Plant Pathogenic Bacteria (NCPPB, London, United Kingdom). P. syringae pv. tomato DB29 (Pst DB29, a cmaA cfaA double mutant of Pst DC3000) (Brooks et al., 2004) was kindly provided by Prof. Barbara Kunkel from Washington University in St. Louis. Both Pst DC3000 and Pst DB29 were stored in 30% glycerol at -80°C for long-term preservation.
Experimental Design
To determine the effect of COR and Pst DC3000 on tomato defenses against WFT (Experiment 1), 4-week-old tomato plants were infiltrated with: (1) 5 μM COR solution, (2) a 108 cfu ml-1 of Pst DC3000 suspension, or (3) a mock solution of sterilized MilliQ water. For this, four leaflets (two top leaflets of leaf 2 and 3 from the bottom) were pressure-infiltrated with 400 μl (100 μl for each leaflet) of one of the treatments on their abaxial leaf sides using a 1-ml needleless syringe. Seven days after infiltration, plants were sampled for type VI trichome density, metabolomics, gene expression, and polyphenol oxidase (PPO) activity analysis, or used for non-choice whole plant thrips bioassays.
With the aim to explore whether COR and other defense elicitors present in Pst DC3000-derived medium (without viable bacteria) increases tomato resistance against WFT, three additional experiments were conducted. First, to test if the COR present in Pst DC3000-derived medium can enhance tomato resistance against WFT (Experiment 2), tomato plants at four leaf-stage were infiltrated with 100 μl of (1) mock solution (MilliQ water), (2) blank medium (described in Generation of Pst-derived and Blank Medium below), (3) blank medium supplemented with 0.68 μM COR (blank medium + COR), (4) 108 cfu ml-1 of Pst DC3000 suspension (Pst DC3000), or (5) Pst DC3000-derived medium (Pst DC3000 medium, without viable bacteria) containing 0.68 μM COR. The COR in the Pst DC3000-derived medium was produced by the bacteria during 6-day cultivation, and the concentration was measured before the start of the experiment. Second, to test the existence of interactions of COR with other defense elicitors present in Pst DC3000-derived medium on tomato resistance against WFT (Experiment 3), 4-week-old tomato plants were infiltrated with 0.2×, 0.4×, 0.6×, 0.8×, and 1.0× concentrations of (1) blank medium, (2) 0.68 μM COR diluted with blank medium, or (3) Pst DC3000-derived medium containing 0.68 μM COR. Third, to confirm the effect of other defense elicitors, present in Pst DC3000-derived medium, on tomato resistance against WFT (Experiment 4), 4-week-old tomato plants were infiltrated with (1) blank medium, (2) 0.14 μM COR diluted with blank medium, (3) culture medium derived from Pst DB29, a COR- mutant bacteria of Pst DC3000, diluted five fold with blank medium, or (4) five fold diluted Pst DB29-derived medium containing 0.14 μM COR. In these three experiments, four leaflets (two top leaflets of each of leaves 2 and 3 from the bottom) from one tomato plant were pressure-infiltrated on their abaxial leaf sides with about 400 μl in total of corresponding treatments as described above. Seven days after infiltration, parts of the plants were sampled for PPO activity measurements and the other part was used for non-choice whole plant thrips bioassays.
Pst Cultivation and Suspension Preparation
Pst DC3000 and Pst DB29 were cultured in a King’s B medium plate (King et al., 1954) supplemented with 100 μg ml-1 rifampicin and grown for 2 days at 28°C prior to use (Katagiri et al., 2002). The activated P. syringae pv. tomato (Pst) was then transferred to King’s B liquid medium supplemented with 100 μg ml-1 rifampicin in a shaking incubator (200 rpm) at 28°C for 8 to 12 h (Katagiri et al., 2002).
To prepare Pst DC3000 suspension, the obtained Pst DC3000 King’s B liquid culture was centrifuged at 4,000 rpm for 10 min at 4°C. The supernatant was discarded, and the bacteria pellet was re-suspended in sterilized MilliQ water. The bacteria suspension was diluted with sterilized MilliQ water to reach a concentration of 108 colony-forming units (cfu) ml-1, estimated by an optical density at 600 nm of 0.5, which was used for the experiments.
Generation of Pst-Derived and Blank Medium
Pst-DC3000- and Pst-DB29-derived medium were obtained following the protocol of Palmer and Bender (1993) with some modifications. Briefly, 100 μl of the Pst King’s B liquid culture obtained as described above was added to 20 ml Hoitink and Sinden medium optimized for COR production (also known as HSC) (nutrients per liter with a pH = 6.8: 1.0 g NH4Cl, 0.2 g MgSO4⋅7H2O, 4.1 g KH2PO4, 3.6 g K2HPO4⋅3H2O, 0.3 g KNO3, 20 μM FeCl3, 20 g glucose) supplemented with 100 μg ml-1 rifampicin and grown in a shaking incubator (200 rpm) at 20°C for 6 days. The Pst culture (20 ml) was centrifuged at 4,000 rpm for 30 min at 4°C. The supernatants were filtered using 0.22 μm regenerated cellulose (RC) filters (Sartorius AG, Göttingen, Germany) to remove Pst from the medium. The absence of active bacteria in the Pst-derived medium was confirmed by spraying the filtered Pst-derived medium on plates of King’s B medium and culturing the plates at room temperature. No colonies were detected at 3 days after the initial culture. Blank medium used as a control in Experiment 2, 3, and 4 was generated by incubating fresh HSC medium supplemented with 100 μg ml-1 rifampicin in a shaking incubator (200 rpm) at 20°C for 6 days. Thereafter, the HSC culture was centrifuged and the resulting supernatant was filtered using 0.22 μm RC filters. Presence of bacteria in the blank medium was also checked as described above for Pst-derived medium.
Measurements of Coronatine Concentration in Pst DC3000-Derived Medium
Concentration of COR in the Pst DC3000-derived medium was determined by HPLC as described by Palmer and Bender (1993) with slight modifications. In short, 20 ml of Pst DC3000-derived filtered medium was adjusted to pH = 9 and extracted twice with 20 ml of ethyl acetate. The aqueous phase was adjusted to pH = 2 and then extracted three times with 20 ml ethyl acetate. The ethyl acetate phase was dried in 45°C water bath in 50 ml tubes. The COR was recovered by re-dissolving twice with 250 μM 20% acetonitrile. Three samples were analyzed on a reverse-phase C-8 column (150 mm × 4.6 mm, 5 μm particle size, Agilent Zorbax Eclipse XDB) at 208 nm. The mobile phases, A and B, were MilliQ water and HPLC-grade acetonitrile, respectively. The flow rate was kept constant at 1 ml/min. The gradient elution was as follows: 0.00 min at 80% A, 5.00 min at 35% A, 7.00 min/10% A, 8.00 min/10% A, 8.10 min/80% A, 10.00 min/80% A. The injection volume was 50 μl, and the column temperature was 25°C. Calibration curves for quantification of COR were constructed by using dilution series of commercially available COR (Sigma-Aldrich, St. Louis, MO, United States).
Growth of Pst DC3000 in Infiltrated Tomato Leaves
Bacteria growth in the leaflets of Pst DC3000-infiltrated plants was confirmed at 7 days after infiltration. For this, one of the Pst DC3000-infiltrated leaflets was surface sterilized by placing it in a 70% ethanol solution for 1 min, blotted briefly on paper towels and rinsed in sterile distilled water for 1 min. Thereafter, a leaf disk (1.5 cm diameter) was punched and placed in a 3-ml microfuge tube with 100 μl sterile distilled water. The samples were ground and subsequently vortexed for 10 s; 10 μl of the leaf disk solution was plated on King’s B medium supplemented with 100 μg ml-1 rifampicin and incubated at room temperature. Number of cfu was recorded at 3 days after incubation.
Non-choice Whole Plant Thrips Bioassay
A non-choice whole plant thrips bioassay was used to test tomato resistance against WFT (Leiss et al., 2009b). For this, plants were placed inside individual WFT-proof cages consisting of transparent plastic cylinders (50 cm height, 20 cm diameter), closed at the top with displaceable lids made of nylon gauze of 120 μm mesh size. Ten adult WFT (eight females and two males) were released into each cage. Plants were maintained in a climate room with 113.6 μmol photons m-2⋅s-1 of PAR, 16L:8D of photoperiod, 25°C and 70% RH for 7 days. WFT feeding damage (hereafter referred as “silver damage”) was evaluated in all the leaves of the plant 7 days after infestation, and expressed as the damaged area in millimeter square. Evaluation of WFT-associated leaf damage in the whole plant has been proved to correlate well with resistance-associated parameters such as number of larvae, adult survival, adult abundance, and preference (De Kogel et al., 1997; Jiang et al., 2005; Badenes-Pérez and López-Pérez, 2018), and it has been used in multitude of studies determining host plant resistance to WFT (e.g., Leiss et al., 2009a,b, 2013; Mirnezhad et al., 2010; Thoen et al., 2016; Escobar-Bravo et al., 2017; Badenes-Pérez and López-Pérez, 2018; Escobar-Bravo et al., in press1). Silver damage symptoms caused by infestation with 10 adult WFT were very subtle at 7 days after infestation, and it did not result in significant loss of leaf tissues (see Supplementary Figure S1). Thus, WFT development and feeding was not limited by the available plant material in the host plant.
Measurement of PPO Activity
Polyphenol oxidase (PPO) activity was measured in one of the infiltrated leaflets belonging to the second leaf from the bottom using the protocol described in Stout et al. (1998). In short, 0.150 g of frozen and ground plant material was homogenized in a 2 ml tube with 1.25 ml ice-cold 0.1 M pH 7.0 potassium phosphate buffer containing 7% polyvinyl polypyrolidine and 0.4 ml of 10% Triton X-100. The extracts were vortexed for 2 min and centrifuged at 11,000 × g for 10 min at 4°C. Five microliters of the enzyme extract were added to 1 ml of 2.92 mM chlorogenic acid solution in pH 8.0 potassium phosphate buffer. The optical density (OD) at 470 nm was recorded in a spectrophotometer (UV-1800, Shimadzu) every 10 s for 1 min. PPO activity was expressed as changes in OD values per min per gram of fresh weight.
Gene Expression Analysis by RT-qPCR
Expression of the JA- and SA-associated marker genes, the wound-inducible proteinase inhibitor II (WIPI-II, also known as PI-II) and the pathogenesis-related protein 6 (PR-P6, also known as PR-1b) (Alba et al., 2015), respectively, were determined in mock-, COR-, and Pst DC3000-treated plants at 7 days after infiltration. The two infiltrated leaflets of leaf 3 from the bottom were flash frozen, homogenized, and stored at -80°C until analysis. Around 100 mg of the leaf material was used for RNA isolation. Total RNA was extracted using a phenol/LiCl method (Verdonk et al., 2003) followed by DNase (Ambion) treatment. Single strand cDNA was synthesized from 4 μg total RNA in a 20-μl reaction using a M-MuLV Reverse Transcriptase (Fermentas) according to manufacturer’s recommendations. The quantity of targeted synthesized cDNAs was analyzed with real-time quantitative reverse transcription-PCR (qRT-PCR) in CFX96TM Optics Module (BIO-RAD) using iQTM SYBR Green Supermix (BIO-RAD). The PCR protocol was set up in 20 μl reactions containing 0.25 μM of each primer and 1 μl of cDNA. The PCR program was as follows: 50°C for 5 min, 95°C for 2 min, 40 cycles of 95°C for 15 s, 60°C for 1 min, followed by a melting curve analysis. Four biological replicates (i.e., individual plants) for each treatment were used for qRT-PCR analysis and two technical replicates were analyzed per treatment. Actin was used as internal standard for the normalization of expression levels for both targeted genes. The normalized expression (NE) of both genes was calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001). To illustrate the levels of gene expressions in plot, NE values were scaled to the treatment with the lowest average NE, which was set to 1. The gene specific qRT-PCR primers are shown in Supplementary Method S1.
Trichome Density Measurement
Type-VI glandular trichome density was determined on non-infiltrated leaflets of mock-, COR-, and Pst DC3000-treated plants at 7 days after infiltration. For this, the second terminal leaflet of the third leaf from the bottom was used. Two pictures were taken in the middle section of the leaflet, at both sides of the midrib, in the adaxial and abaxial leaf sides by using a Leica stereomicroscope (MZ16, Leica Microsystems, Wetzlar, Germany). Each picture corresponded to an area of 12 mm2. Trichome number was counted on the pictures using the software 64-bit Fiji ImageJ2. The average of these two measurements was calculated for each leaflet and expressed as number of type-VI trichomes per centimeter square.
Nuclear Magnetic Resonance (NMR) Analysis
NMR metabolomic analysis was performed on mock-, COR-, or Pst DC3000-infiltrated leaflets at 7 days after infiltration. For this, plant material was freeze-dried and ground using a tissue lyser (Qiagen, Hilden, Germany). Twenty milligrams of fine powder were extracted with 1.5 ml of 80% methanol-d4 in KH2PO4 buffer (90 mM, pH = 6.0) containing 0.02% (w/v) trimethyl silyl-3-propionic acid sodium salt-d4 (TMSP). Plant extracts were vortexed for 1 min, ultra-sonicated for 15 min, and centrifuged at 13,000 rpm for 15 min at room temperature. Eight hundred microliters of the supernatant was transferred to the NMR tubes for analysis.
The 1H NMR spectra were acquired using a 600-MHz Bruker AV-600 spectrometer equipped with cryo-probe operating at a proton NMR frequency of 600 MHz at 25°C, as described in López-Gresa et al. (2012). Deuterated methanol served as internal lock. Each 1H NMR spectrum consisted of 128 scans requiring 10 min acquisition time with a digital resolution of 0.25 Hz/point, a pulse angle of 30° (10.8 μs), and a recycle delay of 1.5 s per scan. A pre-saturation sequence was used to suppress the residual water signal with low power selective irradiation at the H2O frequency during the recycle delay. Spectra were Fourier transformed with a 0.3-HZ line broadening and zero-filled to 32 K points. Phase and baseline correction of the resulting spectra were done manually, followed by a calibration to TMSP at 0.00 ppm using Topspin (version 2.1, Bruker). 1H NMR spectra were then converted and saved as ASCII files using AMIX (v. 3.7, Bruker Biospin). Spectral intensities were scaled to the intensity of the internal standard TMSP and reduced to integrated regions, referred to as buckets, of equal width (0.04 ppm) corresponding to the region of δ 10.0–0.2. The regions in the range of δ 4.92–4.70 and δ 3.33–3.28, corresponding to water and methanol, respectively, were removed prior to statistical analyses.
Statistical Analysis
All statistical analyses were performed using the SPSS software package (version 23; SPSS Inc., Chicago, IL, United States). Effect of mock-solution, COR, Pst DC3000, or Pst DC3000-derived medium infiltration on silver damage symptoms, type VI trichome density, PPO activity, and normalized expression of WIPI-II and PR-P6 (Experiments 1 and 2) were analyzed by one-way ANOVA, followed by Fisher’s least significant difference (LSD) post hoc test. Residuals of the data were first tested for normality and homogeneity of variance. Data on silver damage and WIPI-II and PR-P6 expression obtained from Experiment 1 and PPO activity determined in Experiment 2 were Log transformed prior to analysis to meet ANOVA assumptions. Effect of treatments (blank medium, blank medium + COR, and Pst DC3000-derived medium), concentration (0.2, 0.4, 0.6, 0.8, and 1.0×), and the interaction between these two factors (Experiment 3) on silver damage symptoms, and PPO activity was determined by generalized linear models (GLM) using linear distribution and identity link functions, followed by LSD post hoc test. Data on silver damage were Log-transformed prior to analysis. Effect of COR, Pst DB29-derived medium, and their interaction (Experiment 4) on silver damage and PPO activity was analyzed by GLM using linear distribution and identity link functions, followed by LSD post hoc test. Data on silver damage were Log-transformed prior to analysis. Patterns of chemical shifts detected by NMR in leaf extracts of mock-, COR-, or Pst DC3000-treated plants were subjected to multivariate analysis using the SIMCA-P 15 software package (Umetrics, Umeå, Sweden). Supervised partial least squares discriminant analysis (PLS-DA) was applied to determine the variation in X variables (chemical shifts) modeled by the Y explanatory variable corresponding to mock, COR or Pst DC3000 treatments. R2X and R2Y is the cumulative variation explained by the PLS-DA model in variable X and Y, respectively. Q2 is the cumulative predicted variation in Y, according to cross-validation. The final model was the one with minimum number of latent variables showing the highest value of Q2. The chemical shifts with a variable importance in projection (VIP) > 1 were selected as the important X variables, some of which were identified and tested using a nonparametric analysis followed by non-parametric Kruskal–Wallis test. Detailed statistical results are shown in Supplementary Table S1.
Results
Infiltration of COR or Pst DC3000 Increases Tomato Resistance Against WFT
Infiltration of tomato plants with COR or Pst DC3000 reduced silver damage by 47% and 37%, respectively, compared to the mock-treated plants (ANOVA: P < 0.05, Figure 1). Overall, this reduction was evident in both infiltrated and non-infiltrated leaves of COR- and Pst DC3000-treated plants (ANOVA: P < 0.05, Supplementary Figure S2).
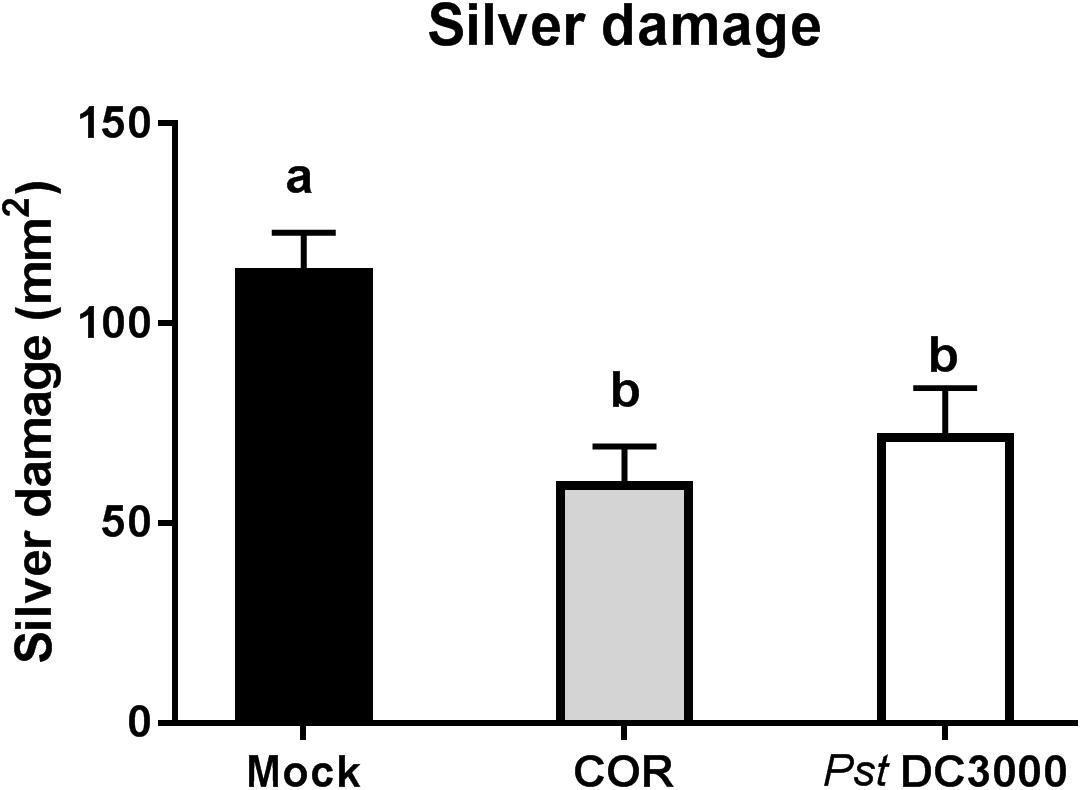
FIGURE 1. Effect of COR and Pst DC3000 on tomato resistance against WFT. Silver damage symptoms (mean ± SEM, n = 15) in tomato plants infiltrated with mock solution (mock), coronatine (COR), or Pseudomonas syringae pv. tomato DC3000 (Pst DC3000). Plants were infested with western flower thrips (WFT) at 7 days after the initial treatment and evaluated 7 days after WFT infestation. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
COR and Pst DC3000 Induced JA-Signaling, and COR Induced Both JA and SA
To further determine the mechanism of COR and Pst DC3000-mediated induction of tomato defenses against WFT, expression of JA- and SA-responsive genes, as well as the activity of the JA-associated defensive protein PPO, were analyzed at 7 days after infiltration. Both COR and Pst DC3000 infiltration strongly induced PPO activity in infiltrated tomato leaves (ANOVA: P < 0.05, Figure 2A). Similarly, the expression of WIPI-II, a JA marker gene, was about 900 and 1,300 times higher in COR- and Pst DC3000-infiltrated plants, respectively, than in mock-treated leaves of control plants (ANOVA: P < 0.05, Figure 2B). Interestingly, for PR-P6, a SA marker gene, a 28-times higher expression was observed in COR-treated plants, but not in mock and Pst DC3000-infiltrated plants (ANOVA: P < 0.05, Figure 2C).
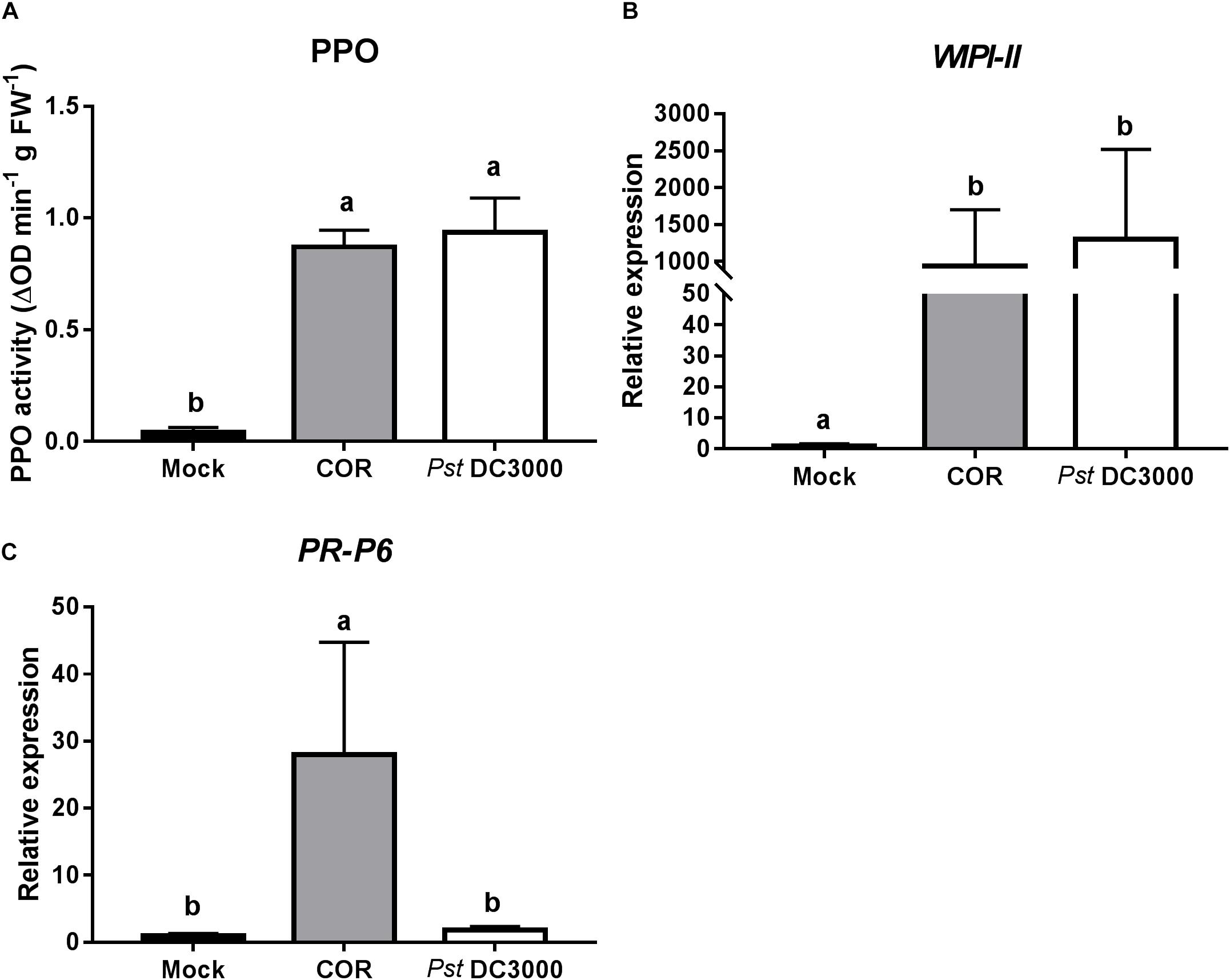
FIGURE 2. Effect of COR and Pst DC3000 on jasmonic acid- and salicylic acid-associated responses. (A) Polyphenol oxidase (PPO) activity (mean ± SEM, n = 5) and relative transcript levels of (B) the JA-responsive gene wound inducible proteinase inhibitor-II (WIPI-II) and (C) the SA-responsive gene pathogenesis related protein 6 (PR-P6) (mean ± SEM, n = 5) were measured in tomato plants at 7 days after infiltration with coronatine (COR), Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), or a mock solution (mock). The analysis was performed on infiltrated leaflets from the bottom second/third leaf. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
Infiltration of Tomato Plants With COR or Pst DC3000 Does Not Increase Type-VI Trichome Density
To determine whether COR and Pst DC3000 induce trichome-associated defenses against WFT, type-VI trichome density was determined on both adaxial and abaxial leaf sides of mock-, COR-, and Pst DC3000-treated plants. Surprisingly, type-VI trichome density in the adaxial leaf side was marginally decreased by COR or Pst DC3000 infiltration (ANOVA: P = 0.071, Figure 3A). However, type-VI trichome density on abaxial leaf sides was slightly reduced in COR-treated plants in comparison to Pst DC3000- and mock-treated plants (ANOVA: P < 0.05, Figure 3B).
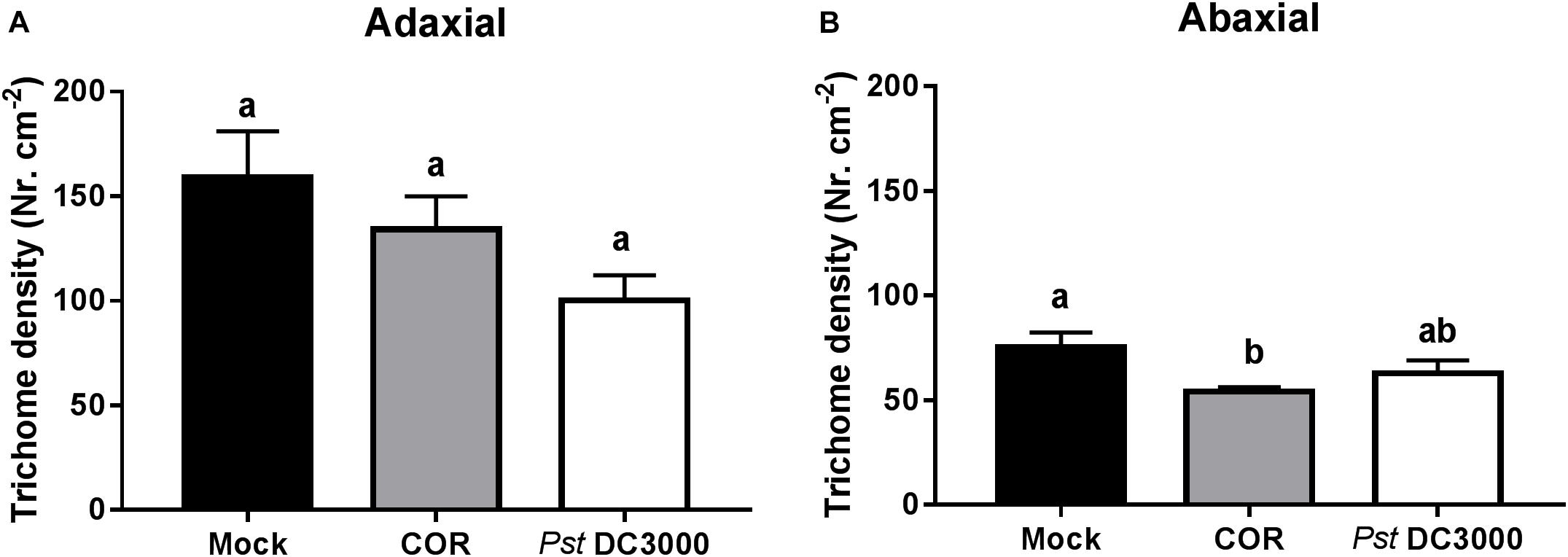
FIGURE 3. Effect of COR and Pst DC3000 on type VI trichome density. Type VI trichome density (mean ± SEM, n = 10) on (A) adaxial or (B) abaxial leaf side was determined in tomato plants infiltrated with coronatine (COR), Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), or a mock solution (mock) at 7 days after the initial treatments. The analysis was performed in leaflets collected from the third youngest leaf. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
COR and Pst DC3000 Induced Similar Metabolomic Changes in Infiltrated Tomato Leaves
A total of 244 signals were obtained from 1H NMR measurement of the mock-, COR-, and Pst DC3000-treated tomato plants. The multivariate PLS-DA analysis of the NMR signal profiles resulted in a model with five latent variables (LVs) that cumulatively explained 74.6% of the total metabolomic variation and 91.1% of the elicitor agent treatments, with a 40.7% total model predictability (model statistics: R2X = 0.746, R2Y = 0.911, and Q2 = 0.407) (Figure 4). The first LV explained 23.3% of the variance and separated mock – from both COR – and Pst DC3000-treated plants (Figure 4A). The second LV explained 15.8% and separated COR – from Pst DC3000-treated plants. The discriminated patterns among mock-, COR-, and Pst DC3000-treated plants were mainly explained by 80 signals with VIP scores higher than 1 (Figure 4B and Supplementary Figure S3). Among these 80 NMR signals, 22 were identified, which corresponded to 16 different compounds (Figure 4C), including isoleucine (δ 0.96), leucine (δ 1.00), valine (δ 1.04), alanine (δ 1.48), acetate (δ 1.92), glutamate (δ 2.04), malic acid (δ 2.48), aspartic acid (δ 2.64, 2.68, 2.80), citric acid (δ 2.72), gamma-aminobutyric acid (GABA, δ 3.00), ethanolamine (δ 3.12), sucrose (δ 5.40), chlorogenic acid (δ 6.40, 6.44, 6.88), rutin (δ 6.52, 7.00), fumaric acid (δ 6.56), and phenylalanine (δ 7.56). Both COR and Pst DC3000 treatments significantly increased leaf content of aspartic acid, ethanolamine and fumaric acid. However, increased GABA and rutin levels were only observed in Pst DC3000-treated plants (Figures 4D–H). For the other identified compounds, we did not find significant differences among treatments.
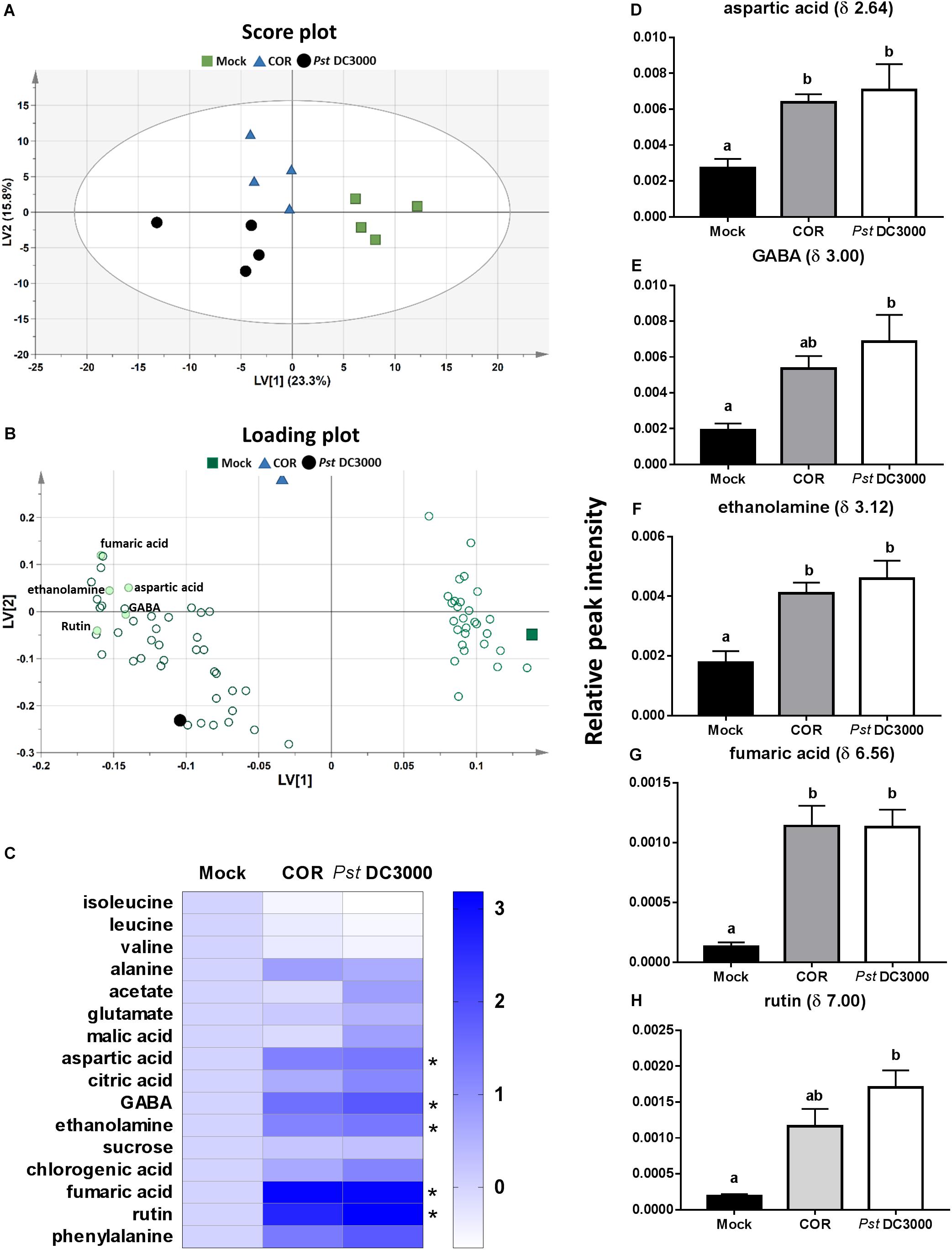
FIGURE 4. Metabolome responses of tomato plants to COR and Pst DC3000 infiltration. Leaf metabolites were analyzed on tomato leaves infiltrated with coronatine (COR), Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), or a mock solution (mock) by NMR at 7 days after the initial treatment. Partial least square-discriminant analysis (PLS-DA) was performed based on 1H-NMR spectra (n = 4 individual plants), and resulted in five latent variables (LVs) that cumulatively explained 74.6% of the total metabolomic variation and 91.1% of the treatment response, with a 40.7% total model predictability. (A) Score plot showing the first two LVs. The ellipse represents the Hotelling T2 with 95% confidence in score plot. (B) Loading plot showing important metabolites contributing most to the model (VIP score > 1). (C) Heatmap of the identified 16 compounds. Each of the three Heatmap columns represents the log2 fold change of relative peak intensity from one of the treatments Mock, COR, or Pst DC3000 in comparison to Mock. Thus, all log2 fold change of compounds in mock treatment was 0 (fold change = 1) as shown in the first column. (D–H) Relative peak intensities (mean ± SEM, n = 4) of five metabolites (aspartic acid, GABA, ethanolamine, fumaric acid, and rutin) identified in the 1H NMR spectra that significantly differed among treatments. Different letters indicate significant differences among treatments tested by Mann–Whitney U test, P < 0.05.
Pst DC3000-Derived Medium Enhances Tomato Resistance Against WFT
To confirm that Pst DC3000-derived compounds are responsible for the induced resistance against WFT in tomato, we infiltrated plants with the Pst DC3000-derived medium (containing COR) but no viable bacteria, and compared the effect on tomato resistance against WFT with those triggered by the infiltration with water mock, blank medium control, blank medium + COR, and Pst DC3000. Silver damage symptoms were significantly reduced in tomato plants infiltrated with COR, Pst DC3000-, or Pst DC3000-derived medium compared to water mock or blank medium-treated plants (ANOVA: P < 0.05, Figure 5A). This reduction in silver damage was stronger in infiltrated leaves, when compared to systemic leaves (i.e., non-infiltrated leaves) (Supplementary Figure S4). In addition, PPO activity was induced in blank medium + COR-, Pst DC3000-, and Pst DC3000-derived medium-treated tomato plants compared to water mock and blank medium controls (ANOVA: P < 0.05, Figure 5B). This confirms the role of COR on the induction of tomato defenses against WFT, and that no bacterial infection is required to elicit WFT resistance.
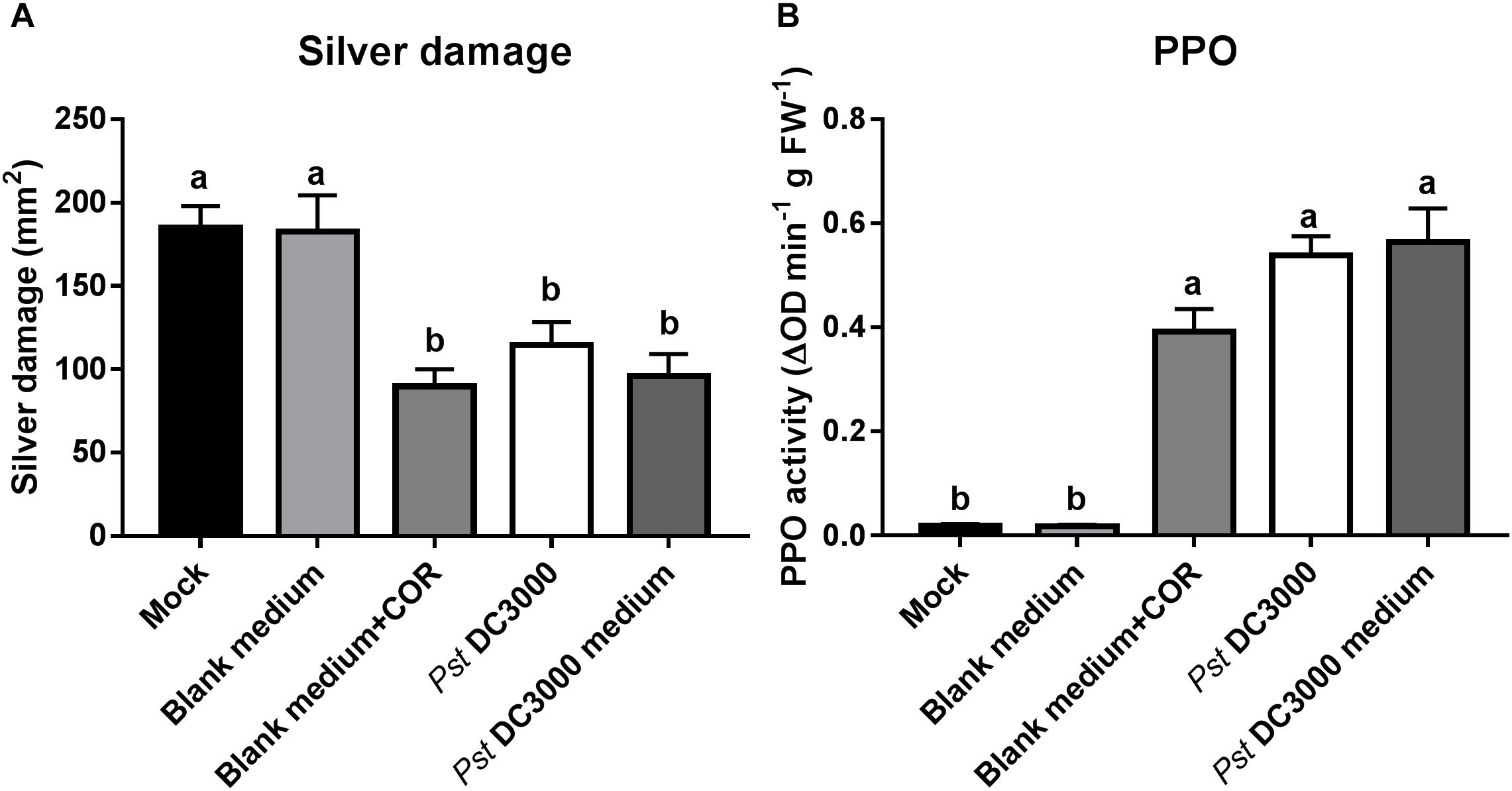
FIGURE 5. Effect of Pst DC3000-derived medium on tomato resistance against WFT and JA-associated responses. (A) Silver damage symptoms (mean ± SEM, n = 10) in tomato plants infiltrated with mock solution (mock), blank medium, 0.68 μM coronatine (COR) dissolved in blank medium (blank medium + COR), Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) suspension, or Pst DC3000-derived medium (containing 0.68 μM of COR). Plants were infested with western flower thrips (WFT) at 7 days after the initial treatment and evaluated 7 days after WFT infestation. (B) Polyphenol oxidase (PPO) activity (mean ± SEM, n = 5) was measured in the second leaf from the bottom of tomato plants pressure infiltrated with the above described treatments at 7 days after the initial treatment. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
Existence of Other Defense Elicitors Besides COR Existed in Pst DC3000-Derived Medium
In the previous experiment, plants infiltrated with COR or Pst DC3000-derived medium (containing 0.68 μM COR as in the COR treatments) showed a similar reduction in silver damage symptoms. Yet, the effect of other defense elicitors present in Pst DC3000-derived medium might have been masked by the high concentration of COR in the medium. Thus, we further assessed the effect of serial dilutions of blank medium, blank medium + COR, and Pst DC3000-derived medium on tomato resistance against WFT and PPO induction (Figure 6A). Pst DC3000-derived medium- and blank + COR-treated plants showed a significant reduction in silver damage symptoms (GML: P < 0.05 for treatment; P = 0.078 for dilution; P = 0.383 for the interaction). Notably, a stronger reduction in silver damage symptoms was observed in tomato plants treated with 0.2× concentration of Pst DC3000-derived medium when compared to 0.2× blank medium and 0.2× blank medium + COR. As these differences were only found at 0.2× concentration, this might explain why the interaction factor between treatment and dilution was not statistically significant. These results suggest that there might be other plant defense elicitors in Pst DC3000-derived medium that, maybe in combination with COR, trigger stronger plant defense responses against WFT than COR alone (i.e., in blank medium + COR treatment). Indeed, at 0.2× concentration, induction of the PPO activity was significantly higher in Pst DC3000-derived medium-treated plants than in those infiltrated with blank medium + COR (GLM: P < 0.05 for interaction) (Figure 6B and Supplementary Table S1). No significant differences in PPO activity between Pst DC3000-derived medium- and blank + COR-treated plants were observed at 0.6, 0.8, and 1.0× concentration.
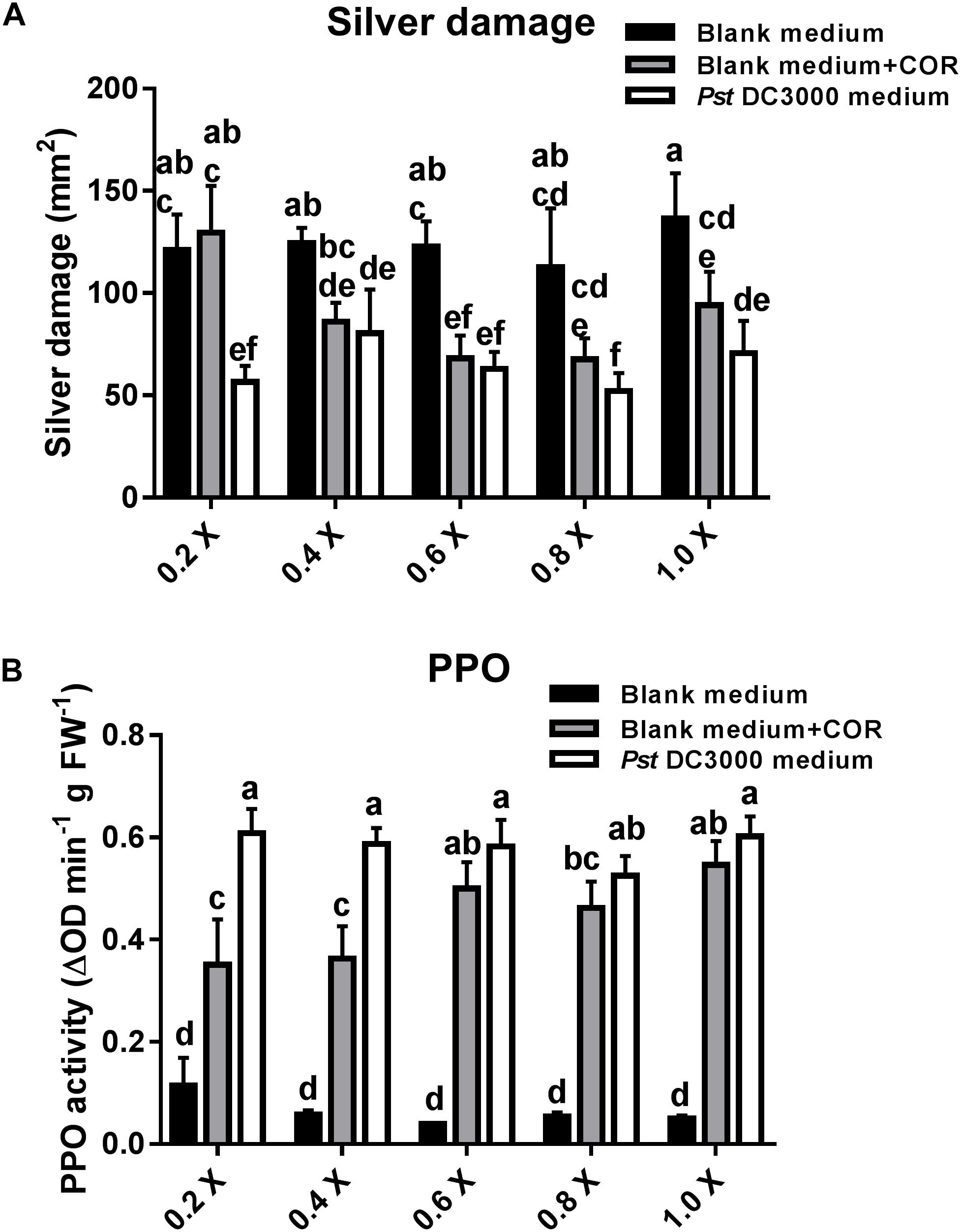
FIGURE 6. Effect of different concentrations of COR in Pst DC3000-derived medium on WFT resistance and JA-associated responses. (A) Silver damage symptoms (mean ± SEM, n = 7) in tomato plants infiltrated with 0.2, 0.4, 0.6, 0.8, or 1.0× concentrations of (1) blank medium, (2) blank medium + coronatine (COR) (0.64 μM), or (3) Pseudomonas syringae pv. tomato DC3000 (Pst DC3000)-derived medium (no viable bacteria, containing 0.64 μM of COR). Plants were infested with western flower thrips (WFT) at 7 days after the initial treatment and evaluated 7 days after WFT infestation. (B) Polyphenol oxidase (PPO) activity (mean ± SEM, n = 5) was measured in the second leaf from the bottom of plants infiltrated with the above described treatments at 7 days after the initial treatment. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
Confirmation of the Existence of Other Defense Elicitors in Pst DC3000-Derived Medium
Our previous results showed that while the effect of COR on tomato defenses against WFT was concentration-dependent, the effect of the Pst DC3000-derived medium was not, thus pointing out to the existence of other defense elicitors in Pst DC3000-derived medium. To further investigate this, we tested the effect of medium obtained from a COR defective mutant of Pst DC3000 (Pst DB29), blank medium, or both treatments supplemented with a low concentration of COR (0.14 μM) on WFT resistance and PPO activity (Figure 7). Silver damage symptoms did not significantly differ between plants infiltrated with blank medium and blank medium + COR (GLM: P = 0.994, for COR treatment) (Figure 7A), thus confirming our previous results. Yet, a small reduction in silver damage was observed in the infiltrated leaves of blank medium + COR (Supplementary Figure S5). Infiltration of plants with Pst DB29-derived medium without COR, however, significantly reduced silver damage symptoms when compared to blank medium and blank medium + COR treatments (GLM: P < 0.05 for the Pst DB29-derived medium; P < 0.05 for the interaction). This reduction was significant in both infiltrated and non-infiltrated leaves (Supplementary Figure S5). PPO activity was significantly induced by COR and Pst DB29-derived medium (GLM: P < 0.05 for COR treatment; P < 0.05 for the Pst DB29-derived medium). Furthermore, Pst DB29 + COR-treated plants showed a slight higher PPO induction when compared to Pst DB29-derived medium and blank medium + COR treatments (GLM: P = 0.104 for their interaction) (Figure 7B).
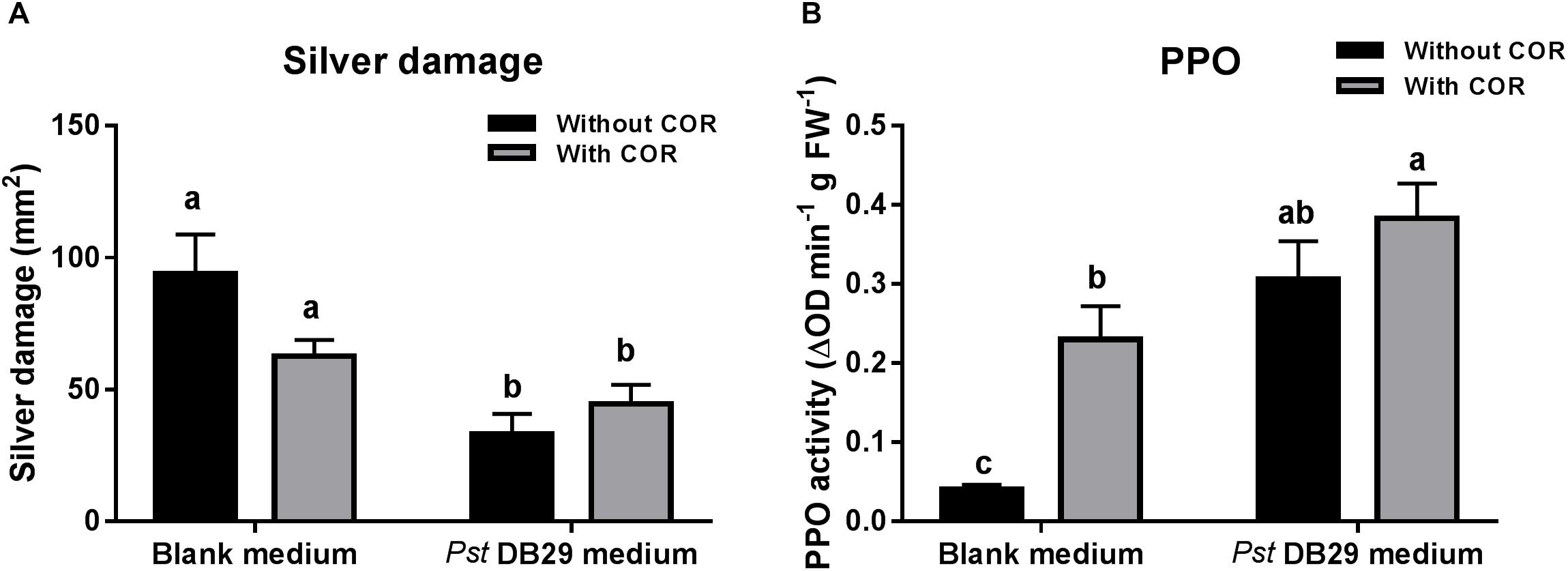
FIGURE 7. Effect of COR and Pst DB29 medium on WFT resistance and JA-associated responses. (A) Silver damage symptoms (mean ± SEM, n = 10) determined in tomato plants infiltrated with blank medium, 0.14 μM coronatine (COR) in blank medium, Pseudomonas syringae pv. tomato DB29 (Pst DB29)-derived medium diluted five fold with blank medium or 0.14 μM COR in Pst DB29-derived medium diluted five fold with blank medium. Plants were infested with western flower thrips (WFT) at 7 days after the initial treatment and evaluated 7 days after WFT infestation. (B) Polyphenol oxidase (PPO) activity (mean ± SEM, n = 5) was measured in the second leaf from the bottom of tomato plants infiltrated with the above described treatments at 7 days after the initial treatment. Different letters indicate significant differences among treatments tested by Fisher’s LSD test at P < 0.05.
Discussion
Activation of defense-associated signaling pathways by using natural or synthetic defense elicitors has shown to increase plant resistance against different arthropod herbivores, and it might be regarded as a valuable strategy for pest control in agriculture (Thaler, 1999) in combination with other IPM techniques, such as biological control. Here, we have shown that infiltration with COR, Pst DC3000, or Pst-derived medium increased tomato resistance against WFT through the induction of enzymatic and chemical defenses.
Our results first showed that infiltration of tomato plants with the COR-producing bacteria Pst DC3000 or COR alone significantly reduced WFT-associated damage in non-choice whole plant bioassays (Figure 1). This is in line with previous reports. Cui et al. (2005) described that the increased susceptibility to the caterpillar Trichoplusia ni in Arabidopsis plants infiltrated with virulent strains of P. syringae ES4326 was counteracted by COR, and that COR alone increased Arabidopsis resistance to this caterpillar. In tomato, Stout et al. (1999) described that infiltration with P. syringae pv. tomato significantly reduced Helicoverpa Zea larvae growth. Here we report on the effects of both Pst DC3000 and COR infiltration on tomato resistance against WFT. Furthermore, we show that not only COR, but that presence of other defense elicitors in Pst-derived medium can increase tomato resistance to WFT.
The enhancement of plant defenses against arthropod herbivores by infiltration of COR-producing Pst or COR itself has been explained by the strong induction of the JA-associated defense signaling pathway and suppression of the SA defense signaling (Cui et al., 2005). Analysis of the effect of COR and Pst DC3000 infiltration on the activation of JA and SA signaling pathways showed that both treatments strongly induced the expression of the JA-associated gene marker WIPI-II, which encodes for a proteinase inhibitor II protein (PI-II), and increased activity of the JA-related defensive enzyme PPO at 7 days after the infiltration. This agrees with previous results described by Stout et al. (1999), who found that Pst infiltration increased PI-II and PPO activities in infiltrated tomato plants. Pst DC3000 is reported to activate JA signaling in tomato (Zhao et al., 2003; Uppalapati et al., 2005) and Arabidopsis (He et al., 2004), which is proposed to be explained by the action of Pst DC3000-derived COR and type III effectors (He et al., 2004). Our results showed that application of COR also induced the expression of the SA-associated gene marker PR-P6, a pathogen defense-related gene (PR) (Figure 2C). Yet, the magnitude of the induction of PR-P6 was approximately 30 times lower than that of WIPI-II in COR-treated plants. Both COR treatment and infection with Pst DC3000 lead to slight increases of SA levels in Arabidopsis (Uppalapati et al., 2005) and tomato (Zhao et al., 2003). In tomato, this induction has been described to be stronger in COR deficient Pst, and thus it was suggested to be highly suppressed by the activation of JA signaling in COR-producing Pst (Zhao et al., 2003). The lack of induction of PR-P6 in Pst DC3000-infected tomato plants (Figure 2B) might be explained by our sampling time for gene expression analysis. Hence, induction of PRs has been generally observed 24 h after Pst infiltration (Zhao et al., 2003; Uppalapati et al., 2008; López-Gresa et al., 2011). Overall, the strong activation of JA-associated defenses by Pst DC3000 and COR infiltration might explain the increased tomato resistance against WFT. Previous studies have shown that induction of JA defenses can reduce WFT-associated damage in tomato and other plant species (Li et al., 2002; Abe et al., 2009; Escobar-Bravo et al., 2017). Activation of JA defense signaling is often associated with reduced plant growth (Guo et al., 2018). Interestingly, our results showed that neither COR or Pst DC3000 infiltration significantly affected plant dry biomass or height of tomato plants (Supplementary Figure S6). Additionally, we did not detect any Pst DC3000 colonies in systemic leaves of Pst DC3000-infiltrated plants, but only in the local leaves (Supplementary Figure S7), confirming that even localized Pst DC3000 infections have a great impact on tomato defenses against other biotic stressors.
Activation of JA signaling pathway through exogenous application of jasmonates, such as the volatile form of JA methyl jasmonate (MeJA) (Boughton et al., 2005; Maes and Goossens, 2010; Tian et al., 2012; Escobar-Bravo et al., 2017) is reported to increase type VI glandular trichome density in tomato leaves. We thus hypothesized that infiltration with Pst DC3000 or COR might induce these tomato defenses as well. Our results, however, showed that none of these treatments increased type VI trichome densities in newly formed leaves at 7 days after infiltration. This might be explained by differences in the magnitude of the induction of JA defenses when plants are treated with exogenous application of COR or Pst DC3000 infiltration, but also by the activation of different defense signaling pathways. Hence, although COR and MeJA application shared similar activities on tomato plants, some sets of genes are differently regulated by these two compounds (Uppalapati et al., 2005; Tsai et al., 2011). Both COR and Pst DC3000 are reported to induce JA, ET, and auxin signaling pathways (O’donnell et al., 2003; Cohn and Martin, 2005; Uppalapati et al., 2005), and COR slightly induced SA signaling as well. Whether the induction of these signaling pathways explains the lack of induction of trichomes in COR and Pst DC3000 infiltrated plants would require further research. Alternatively, COR-mediated activation of SA signaling might have attenuated JA-mediated induction of trichomes (Traw and Bergelson, 2003), as both signaling are known to interact via antagonistic crosstalk (Pieterse et al., 2012). Together, these results suggest that COR- and Pst-DC3000-mediated induction of tomato resistance against WFT is not explained by increased type-VI trichome densities.
An untargeted metabolomic analysis of tomato leaves infiltrated with COR or Pst DC3000 revealed that both treatments induced similar but not the same metabolomic changes. Both COR and Pst DC3000 increased the leaf content of organic acids, phenolics, and amino acids (Figure 4). These results are in agreement with those reported by López-Gresa et al. (2010, 2011), where higher concentrations of amino acids, organic acids, rutin, and phenylpropanoids were detected in Pst-infected tomato plants. However, no comparison between the effects of COR and Pst infiltration on plant metabolome has been performed before. Interestingly, our results showed that the levels of the amino acid aspartic acid and the non-protein amino acid GABA, as well as the phenolic rutin, were slightly higher in Pst DC3000-infiltrated tomato leaves. Yet, these differences did not affect the levels of resistance of tomato plants against WFT, as both COR and Pst DC3000 significantly reduced silver damage symptoms in infiltrated plants (Figure 1). The increase in some of these compounds might have influenced tomato defenses against WFT. For instance, high concentrations of the flavonoid rutin (quercetin-3-O-β-rutinoside) have been reported to deter herbivore feeding (reviewed by Simmonds, 2001). On the other hand, increases in GABA levels are reported to occur in Pst DC3000-infected plants (Ward et al., 2010), but also in response to other biotic and abiotic stresses (Bouché et al., 2003). Although all the functions of GABA in plants have not been completely elucidated, it is induced upon herbivory or insects crawling on the leaf surface (Bown et al., 2002; Scholz et al., 2015), and it has a negative effect on arthropod’s performance when ingested by feeding in transgenic plants with elevated GABA levels or in GABA-enriched artificial diets (Ramputh and Bown, 1996; McLean et al., 2003; Scholz et al., 2015). Yet, whether its induction might affect tomato resistance against WFT would need further research.
Our results further showed that application of Pst DC3000-derived medium (without viable Pst bacteria and containing 0.68 μM of COR), COR (0.68 μM), or Pst DC3000, all increased tomato resistance against WFT (Figure 5A). Moreover, these treatments increased PPO activities in infiltrated leaves, indicating the activation of JA signaling (Figure 5B). Hence, infiltration of tomato plants with ca. seven times less COR than in our initial experiments (i.e., 5 μM, see Figure 1) resulted in a similar reduction in silver damage symptoms. This suggested that COR has a strong impact on tomato defenses even at low concentrations, and that we might have overlooked the possible effect of other defense elicitors present in Pst DC3000-derived medium. This prompted us to further investigate whether infiltration with much lower concentrations of COR alone or in Pst DC3000-derived medium had the same effects on tomato resistance against WFT. Notably, infiltration of tomato plants with a 0.2× concentration of Pst DC3000-derived medium (containing 0.14 μM of COR) resulted in a stronger reduction of silver damage symptoms than application of COR (0.14 μM) alone dissolved in blank medium. Moreover, induction of PPO activity was higher in plants infiltrated with a 0.2× concentration of Pst DC3000-derived medium than with a 0.2× concentration of COR or blank medium. Hence, this suggests that the presence of other defense elicitors in Pst DC3000-derived medium might increase tomato resistance against WFT, and that this induction is also probably explained by a stronger activation of JA signaling. Indeed, further assays using a COR deficient mutant of Pst DC3000, Pst DB29, showed that tomato plants infiltrated with Pst DB29-derived medium displayed lower silver damage symptoms after WFT infestation and induced PPO activities as well (Figures 7A,B). It should be noted that Pst DB29 is defective in the synthesis of COR precursors, CFA and CMA, both reported to induce some JA/wound associated plant responses in tomato, but at much less magnitude than COR (Uppalapati et al., 2005). Thus, activation of tomato defenses against WFT could not be explained by the presence of CFA and CMA in the Pst DB29-derived medium. We hypothesize that these responses might be explained by changes in the culture medium composition in terms of (1) primary or secondary metabolites modified in the medium by the Pst growth, or (2) presence of Pst-derived effectors. For instance, He et al. (2004) described that effectors secreted by the type III secretion system of Pst DC3000 can augment the JA-signaling pathway to promote virulence. Nevertheless, this requires further research.
In summary, our study shows that infiltration with COR and Pst DC3000 increases tomato resistance against WFT by activating JA-associated defenses, but not type-VI leaf trichome densities. Our results also show that Pst DC3000-derived medium contains other defense elicitors that can increase resistance against WFT in infiltrated tomato plants, thus providing a potential treatment for WFT control in agriculture systems.
Author Contributions
GC, RE-B, PK, and KL carried out the experimental design. GC conducted the infiltration, insect experiments, and chemical analyses. GC and RE-B performed the RT-qPCRs. GC, RE-B, PK, and KL performed the data analysis and interpretation. HK interpreted the NMR data. GC wrote the draft manuscript. All authors critically reviewed and approved the final version of the manuscript.
Funding
This work was supported by the Technology Foundation STW, project “Green Defense against Pests” (GAP) (Ref. 13553); we thank the companies involved in the GAP project: Rijk Zwaan, Düemmen Orange, Dekker Chrysanten, Deliflor Chrysanten, and Incotec for their financial support. GC was funded by the China Scholarship Council (CSC) of the Ministry of Education.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Erica Wilson for assistance with HPLC analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01417/full#supplementary-material
Footnotes
- ^ Escobar-Bravo, R., Ruijgrok, J., Kim, H. K., Grosser, K., Van Dam, N. M., Klinkhamer, P. G. L., et al. (in press). Light intensity-mediated induction of trichome-associated allelochemicals increases resistance against thrips in tomato. Plant Cell Physiol. doi: 10.1093/pcp/pcy166
- ^ http://fiji.sc/Fiji
References
Abe, H., Ohnishi, J., Narusaka, M., Seo, S., Narusaka, Y., Tsuda, S., et al. (2008). Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 49, 68–80. doi: 10.1093/pcp/pcm168
Abe, H., Shimoda, T., Ohnishi, J., Kugimiya, S., Narusaka, M., Seo, S., et al. (2009). Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol. 9:97. doi: 10.1186/1471-2229-9-97
Alba, J. M., Schimmel, B. C. J., Glas, J. J., Ataide, L. M. S., Pappas, M. L., Villarroel, C. A., et al. (2015). Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 205, 828–840. doi: 10.1111/nph.13075
Ament, K., Kant, M. R., Sabelis, M. W., Haring, M. A., and Schuurink, R. C. (2004). Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135, 1–13. doi: 10.1104/pp.103.038315
Annadana, S., Kuiper, G., Visser, P. B., de Kogel, W. D., Udayakumar, M., and Jongsma, M. A. (2002). Expression of potato multicystatin in florets of chrysanthemum and assessment of resistance to western flower thrips, Frankliniella occidentalis. Acta Hortic. 572, 121–129. doi: 10.17660/actahortic.2002.572.14
Badenes-Pérez, F. R., and López-Pérez, J. A. (2018). Resistance and susceptibility to powdery mildew, root-knot nematode, and western flower thrips in two types of winter cress (Brassicaceae). Crop Protoc. 110, 41–47. doi: 10.1016/j.cropro.2018.03.015
Bender, C. L., Liyanage, H., Palmer, D., Ullrich, M., Young, S., and Mitchell, R. (1993). Characterization of the genes controlling the biosynthesis of the polyketide phytotoxin coronatine including conjugation between coronafacic and coronamic acid. Gene 133, 31–38. doi: 10.1016/0378-1119(93)90221-N
Bhonwong, A., Stout, M. J., Attajarusit, J., and Tantasawat, P. (2009). Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J. Chem. Ecol. 35, 28–38. doi: 10.1007/s10886-008-9571-7
Bielza, P. (2008). Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 64, 1131–1138. doi: 10.1002/ps.1620
Bouché, N., Lacombe, B., and Fromm, H. (2003). GABA signaling: a conserved and ubiquitous mechanism. Trends Cell Biol. 13, 607–610. doi: 10.1016/j.tcb.2003.10.001
Boughton, A. J., Hoover, K., and Felton, G. W. (2005). Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 31, 2211–2216. doi: 10.1007/s10886-005-6228-7
Bown, A. W., Hall, D. E., and MacGregor, K. B. (2002). Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol. 129, 1430–1434. doi: 10.1104/pp.006114
Brooks, D. M., Hernández-Guzmán, G., Kloek, A. P., Alarcón-Chaidez, F., Sreedharan, A., Rangaswamy, V., et al. (2004). Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 17, 162–174. doi: 10.1094/MPMI.2004.17.2.162
Cohn, J. R., and Martin, G. B. (2005). Pseudomonas syringae pv. tomato type III effectors AvrPto and AvrPtoB promote ethylene-dependent cell death in tomato. Plant J. 44, 139–154. doi: 10.1111/j.1365-313X.2005.02516.x
Cui, J., Bahrami, A. K., Pringle, E. G., Hernandez-Guzman, G., Bender, C. L., Pierce, N. E., et al. (2005). Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. U.S.A. 102, 1791–1796. doi: 10.1073/pnas.0409450102
de Jager, K. M., Butôt, R. P. T., and Guldemond, A. (1995a). “Genetic variation in chrysanthemum for resistance to western flower thrips and Thrips tabaci,” in Thrips Biology and Management. NATO ASI Series (Series A: Life Sciences), eds B. L. Parker, M. Skinner, and T. Lewis (Boston MA: Springer), 403–406. doi: 10.1007/978-1-4899-1409-5_62
de Jager, C. M., Butôt, R. P. T., Klinkhamer, P. G. L., de Jong, T. J., Wolff, K., and van der Meijden, E. (1995b). Genetic variation in chrysanthemum for resistance to Frankliniella occidentalis. Entomol. Exp. Appl. 77, 277–287. doi: 10.1111/j.1570-7458.1995.tb02325.x
De Kogel, W. J., Van Der Hoek, M., and Mollema, C. (1997). Variation in performance of western flower thrips populations on susceptible and partially resistant cucumber. Entomol. Exp. Appl. 83, 73–80. doi: 10.1046/j.1570-7458.1997.00158.x
Degenhardt, D. C., Refi-Hind, S., Stratmann, J. W., and Lincoln, D. E. (2010). Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 71, 2024–2037. doi: 10.1016/j.phytochem.2010.09.010
Demirozer, O., Tyler-Julian, K., Funderburk, J., Leppla, N., and Reitz, S. (2012). Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Manag. Sci 68, 1537–1545. doi: 10.1002/ps.3389
Escobar-Bravo, R., Klinkhamer, P. G. L., and Leiss, K. A. (2017). Induction of jasmonic acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol. 58, 622–634. doi: 10.1093/pcp/pcx014
Gao, Y., Lei, Z., and Reitz, S. R. (2012). Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Manag. Sci. 68, 1111–1121. doi: 10.1002/ps.3305
Geng, X., Jin, L., Shimada, M., Kim, M. G., and Mackey, D. (2014). The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta 240, 1149–1165. doi: 10.1007/s00425-014-2151-x
Guo, Q., Major, I. T., and Howe, G. A. (2018). Resolution of growth–defense conflict: mechanistic insights from jasmonate signaling. Curr. Opin. Plant Biol. 44, 72–81. doi: 10.1016/j.pbi.2018.02.009
He, P., Chintamanani, S., Chen, Z., Zhu, L., Kunkel, B. N., Alfano, J. R., et al. (2004). Activation of a COI1-dependent pathway in Arabidopsis by Pseudomonas syringae type III effectors and coronatine. Plant J. 37, 589–602. doi: 10.1111/j.1365-313X.2003.01986.x
Jiang, R. F., Ma, D. Y., Zhao, F. J., and Mcgrath, S. P. (2005). Cadmium hyperaccumulation protects Thlaspi caerulescens from leaf feeding damage by thrips (Frankliniella occidentalis). New Phytol. 167, 805–814. doi: 10.1111/j.1469-8137.2005.01452.x
Kang, J. H., Liu, G., Shi, F., Jones, A. D., Beaudry, R. M., and Howe, G. A. (2010a). The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 154, 262–272. doi: 10.1104/pp.110.160192
Kang, J. H., Shi, F., Jones, A. D., Marks, M. D., and Howe, G. A. (2010b). Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 61, 1053–1064. doi: 10.1093/jxb/erp370
Katagiri, F., Thilmony, R., and He, S. Y. (2002). The Arabidopsis thaliana-Pseudomonas syringae interaction. Arabidopsis Book 1:e0039. doi: 10.1199/tab.0039
King, E. O., Ward, M. K., and Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44, 301–307.
Leiss, K. A., Choi, Y. H., Abdel-Farid, I. B., Verpoorte, R., and Klinkhamer, P. G. L. (2009a). NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J. Chem. Ecol. 35, 219–229. doi: 10.1007/s10886-008-9586-0
Leiss, K. A., Maltese, F., Choi, Y. H., Verpoorte, R., and Klinkhamer, P. G. L. (2009b). Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 150, 1567–1575. doi: 10.1104/pp.109.138131
Leiss, K. A., Cristofori, G., Van Steenis, R., Verpoorte, R., and Klinkhamer, P. G. L. (2013). An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 93, 63–70. doi: 10.1016/j.phytochem.2013.03.011
Li, C., Williams, M. M., Loh, Y. T., Lee, G. I., and Howe, G. A. (2002). Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 130, 494–503. doi: 10.1104/pp.005314
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
López-Gresa, M. P., Lisón, P., Kim, H. K., Choi, Y. H., Verpoorte, R., Rodrigo, I., et al. (2012). Metabolic fingerprinting of tomato mosaic virus infected Solanum lycopersicum. J. Plant Physiol. 169, 1586–1596. doi: 10.1016/j.jplph.2012.05.021
López-Gresa, M. P., Torres, C., Campos, L., Lisón, P., Rodrigo, I., Bellés, J. M., et al. (2011). Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ. Exp. Bot. 74, 216–228. doi: 10.1016/j.envexpbot.2011.06.003
López-Gresa, M. P., Maltese, F., Bellés, J. M., Conejero, V., Kim, H. K., Choi, Y. H., et al. (2010). Metabolic response of tomato leaves upon different plant–pathogen interactions. Phytochem. Anal 21, 89–94. doi: 10.1002/pca.1179
Maes, L., and Goossens, A. (2010). Hormone-mediated promotion of trichome initiation in plants is conserved but utilizes species and trichome-specific regulatory mechanisms. Plant Signal. Behav. 5, 205–207. doi: 10.4161/psb.5.2.11214
Mahanil, S., Attajarusit, J., Stout, M. J., and Thipyapong, P. (2008). Overexpression of tomato polyphenol oxidase increases resistance to common cutworm. Plant Sci. 174, 456–466. doi: 10.1016/j.plantsci.2008.01.006
Maris, P. C., Joosten, N. N., Peters, D., and Goldbach, R. W. (2003). Thrips resistance in pepper and its consequences for the acquisition and inoculation of Tomato spotted wilt virus by the western flower thrips. Phytopathology 93, 96–101. doi: 10.1094/PHYTO.2003.93.1.96
McLean, M. D., Yevtushenko, D. P., Deschene, A., Van Cauwenberghe, O. R., Makhmoudova, A., Potter, J. W., et al. (2003). Overexpression of glutamate decarboxylase in transgenic tobacco plants confers resistance to the northern root-knot nematode. Mol. Breed. 11, 277–285. doi: 10.1023/A:102348310
Mirnezhad, M., Romero-González, R. R., Leiss, K. A., Choi, Y. H., Verpoorte, R., and Klinkhamer, P. G. L. (2010). Metabolomic analysis of host plant resistance to thrips in wild and cultivated tomatoes. Phytochem. Anal. 21, 110–117. doi: 10.1002/pca.1182
Mouden, S., Sarmiento, K. F., Klinkhamer, P. G. L., and Leiss, K. A. (2017). Integrated pest management in western flower thrips: past, present and future. Pest Manag. Sci. 75, 813–822. doi: 10.1002/ps.4531
O’donnell, P. J., Schmelz, E. A., Moussatche, P., Lund, S. T., Jones, J. B., and Klee, H. J. (2003). Susceptible to intolerance–a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J. 33, 245–257. doi: 10.1046/j.1365-313X.2003.01619.x
Omer, A., Granett, J., Karban, R., and Villa, E. (2001). Chemically-induced resistance against multiple pests in cotton. Int. J. Pest Manag. 47, 49–54. doi: 10.1080/09670870150215595
Outchkourov, N. S., De Kogel, W. J., Wiegers, G. L., Abrahamson, M., and Jongsma, M. A. (2004). Engineered multidomain cysteine protease inhibitors yield resistance against western flower thrips (Frankliniella occidentalis) in greenhouse trials. Plant Biotechnol. J. 2, 449–458. doi: 10.1111/j.1467-7652.2004.00089.x
Palmer, D. A., and Bender, C. L. (1993). Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas syringae pv. glycinea. Appl. Environ. Microbiol. 59, 1619–1626.
Pieterse, C. M. J., Van Der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Ramputh, A. I., and Bown, A. W. (1996). Rapid γ-aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf-roller larvae. Plant Physiol. 111, 1349–1352. doi: 10.1104/pp.111.4.1349
Scholz, S. S., Reichelt, M., Mekonnen, D. W., Ludewig, F., and Mithöfer, A. (2015). Insect herbivory-elicited GABA accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response. Front. Plant Sci. 6:1128. doi: 10.3389/fpls.2015.01128
Simmonds, M. S. J. (2001). Importance of flavonoids in insect–plant interactions: feeding and oviposition. Phytochemistry 56, 245–252. doi: 10.1016/S0031-9422(00)00453-2
Steenbergen, M., Abd-el-Haliem, A., Bleeker, P., Dicke, M., Escobar-Bravo, R., Cheng, G., et al. (2018). Thrips advisor: exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 69, 1837–1848. doi: 10.1093/jxb/ery060
Stout, M. J., Brovont, R. A., and Duffey, S. S. (1998). Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J. Chem. Ecol. 24, 945–963. doi: 10.1023/A:1022350100718
Stout, M. J., Fidantsef, A. L., Duffey, S. S., and Bostock, R. M. (1999). Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol. Mol. Plant Pathol. 54, 115–130. doi: 10.1006/pmpp.1998.0193
Thaler, J. S. (1999). Induced resistance in agricultural crops: effects of jasmonic acid on herbivory and yield in tomato plants. Environ. Entomol. 28, 30–37. doi: 10.1093/ee/28.1.30
Thaler, J. S., Karban, R., Ullman, D. E., Boege, K., and Bostock, R. M. (2002). Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131, 227–235. doi: 10.1007/s00442-002-0885-9
Thoen, M. P. M., Kloth, K. J., Wiegers, G. L., Krips, O. E., Noldus, L. P., Dicke, M., et al. (2016). Automated video tracking of thrips behavior to assess host-plant resistance in multiple parallel two-choice setups. Plant Methods 12:1. doi: 10.1186/s13007-016-0102-1
Tian, D., Tooker, J., Peiffer, M., Chung, S. H., and Felton, G. W. (2012). Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236, 1053–1066. doi: 10.1007/s00425-012-1651-9
Traw, M. B., and Bergelson, J. (2003). Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 133, 1367–1375. doi: 10.1104/pp.103.027086
Tsai, C. H., Singh, P., Chen, C. W., Thomas, J., Weber, J., Mauch-Mani, B., et al. (2011). Priming for enhanced defence responses by specific inhibition of the Arabidopsis response to coronatine. Plant J 65, 469–479. doi: 10.1111/j.1365-313X.2010.04436.x
Uppalapati, S. R., Ayoubi, P., Weng, H., Palmer, D. A., Mitchell, R. E., Jones, W., et al. (2005). The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 42, 201–217. doi: 10.1111/j.1365-313X.2005.02366.x
Uppalapati, S. R., and Bender, C. L. (2005). Role of phytohormones and the phytotoxin coronatine in bacterial speck disease development in tomato. Phytopathology 95:S106.
Uppalapati, S. R., Ishiga, Y., Wangdi, T., Urbanczyk-Wochniak, E., Ishiga, T., Mysore, K. S., et al. (2008). Pathogenicity of Pseudomonas syringae pv. tomato on tomato seedlings: phenotypic and gene expression analyses of the virulence function of coronatine. Mol. Plant Microbe Interact. 21, 383–395. doi: 10.1094/MPMI-21-4-0383
Verdonk, J. C., De Vos, C. H. R., Verhoeven, H. A., Haring, M. A., Van Tunen, A. J., and Schuurink, R. C. (2003). Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62, 997–1008. doi: 10.1016/S0031-9422(02)00707-0
Ward, J. L., Forcat, S., Beckmann, M., Bennett, M., Miller, S. J., Baker, J. M., et al. (2010). The metabolic transition during disease following infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Plant J. 63, 443–457. doi: 10.1111/j.1365-313X.2010.04254.x
Zhao, Y., Thilmony, R., Bender, C. L., Schaller, A., He, S. Y., and Howe, G. A. (2003). Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 36, 485–499. doi: 10.1046/j.1365-313X.2003.01895.x
Zhao, Y. F., Jones, W. T., Sutherland, P., Palmer, D. A., Mitchell, R. E., Reynolds, P. H. S., et al. (2001). Detection of the phytotoxin coronatine by ELISA and localization in infected plant tissue. Physiol. Mol. Plant Pathol. 58, 247–258. doi: 10.1006/pmpp.2001.0334
Keywords: coronatine, Frankliniella occidentalis, induced plant defenses, jasmonic acid, Pseudomonas syringae, salicylic acid, Solanum lycopersicum, type VI glandular trichomes
Citation: Chen G, Escobar-Bravo R, Kim HK, Leiss KA and Klinkhamer PGL (2018) Induced Resistance Against Western Flower Thrips by the Pseudomonas syringae-Derived Defense Elicitors in Tomato. Front. Plant Sci. 9:1417. doi: 10.3389/fpls.2018.01417
Received: 31 May 2018; Accepted: 06 September 2018;
Published: 26 September 2018.
Edited by:
Brigitte Mauch-Mani, University of Neuchâtel, SwitzerlandReviewed by:
Richard Bostock, University of California, Davis, United StatesMathew G. Lewsey, La Trobe University, Australia
Copyright © 2018 Chen, Escobar-Bravo, Kim, Leiss and Klinkhamer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, Zy5jaGVuQGJpb2xvZ3kubGVpZGVudW5pdi5ubA==
 Gang Chen
Gang Chen Rocío Escobar-Bravo
Rocío Escobar-Bravo Hye Kyong Kim1
Hye Kyong Kim1 Kirsten A. Leiss
Kirsten A. Leiss