
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Plant Sci., 19 September 2018
Sec. Plant Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fpls.2018.01375
This article is part of the Research TopicHydrogen Sulfide Metabolism and Signalling in PlantsView all 11 articles
Hydrogen sulfide (H2S), an endogenous gaseous molecule, is considered as a signaling agent, in parallel with other low molecular weight reactive substances, mainly hydrogen peroxide (H2O2) and nitric oxide (NO), in various plant systems. New studies are now revealing that the postharvest application of H2S, through H2S donors such as sodium hydrosulfide (NaSH) or sodium sulfide (Na2S), can inhibit fruit ripening and senescence programs in numerous fruits. We discuss here current knowledge on the impact of H2S in postharvest physiology of several climacteric and non-climacteric fruits such as banana, apple, pear, kiwifruit, strawberry, mulberry fruit, and grape. Although there is still a considerable lack of studies establishing the mechanisms by which H2S signaling is linked to fruit metabolism, we highlight several candidate mechanisms, including a putative cross-talk between H2S and ethylene, reactive oxygen and nitrogen species, oxidative/nitrosative stress signaling, sulfate metabolism, and post-translational modification of protein cysteine residues (S-sulfhydration) as being functional in this H2S postharvest action. Understanding H2S metabolism and signaling during postharvest storage and the interplay with other key player molecules would therefore provide new, improved strategies for better fruit postharvest storage. To achieve this understanding, postharvest fruit physiology research will need to focus increasingly on the spatial interaction between H2S and ethylene perception as well as on the interplay between S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation under several postharvest conditions.
The continuously increasing world population demands more effective food production strategies, better agriculture management systems, and less postharvest losses (Kader, 2005). Undertaken research by various international and national organizations led by FAO indicated that about one-third of all food produced on the planet and about a half of all fruit and vegetables are lost and not consumed (Porat et al., 2018). Postharvest attributes of horticultural products are associated with the use of synthetic chemicals; however, the application of chemicals as germicides raises several issues related to pathogen resistance and food safety (Deng et al., 2013). Therefore, research has set as a priority the establishment of consumer friendly postharvest treatments (Gong et al., 2018). Toward this goal, several low molecular weight compounds, such as hydrogen sulfide (H2S), nitric oxide (NO), hydrogen peroxide (H2O2), hydrogen gas (H2), carbon dioxide (CO2), and chlorine dioxide (ClO2) have been applied to perishable horticultural products (Gong et al., 2018). Herein, we focus upon the latest research data linked with the physiological aspect of H2S application in the fruit postharvest behavior along with future perspectives.
Hydrogen sulfide research began upon animal systems, but during the last decade, a plethora of scientific data originated from plants (Wang, 2002). A significant body of evidence suggest that at minor concentrations H2S can exert signaling properties during the acclimation of plants to abiotic stress, plant growth and development, and specific physiological processes through an interplay with hormones, reactive oxygen species (ROS), and other signaling compounds, like NO (Hancock et al., 2011; Christou et al., 2014; Hancock and Whiteman, 2014, 2016; Ziogas et al., 2015; Antoniou et al., 2016).
In plants, H2S is produced through sulfite reductase, which catalyzes the reduction of sulfite to sulfide, or through two cysteine-dependent reactions involving members of the O-acetylserine(thiol)lyase (OAS-TL) gene family. L-cysteine desulfhydrase (DES, EC 4.4.1.1) converts L-cysteine to H2S, ammonia, and pyruvate while β-cyanoalanine synthase produces H2S through the detoxification of cyanide at the expense of cysteine (Hatzfeld et al., 2000). Although the biochemical aspects of H2S are extended, it has been suggested that H2S exerts its biological activity mainly via the oxidative post-translational modification of cysteine residues (RSH) to persulfides (RSSH; Filipovic and Jovanović, 2017).
Fruit ripening is accompanied by various biochemical and physiological changes, which are orchestrated by multiple genetically programmed processes (Gong et al., 2018). Based on the physiological differences in respiratory pattern during ripening, fleshy fruits have been categorized as climacteric and non-climacteric. Fleshy fruits have long been categorized to climacteric or non-climacteric according to various biochemical differences of their respiratory pattern during ripening. A characteristic burst of ethylene production has been observed to climacteric fruits like banana, apple, and kiwifruit, while non-climacteric products, like strawberry and grape, withhold ethylene production at basal level (Cherian et al., 2014). Climacteric fruits such as banana, apple, and kiwi display a well-characterized peak in respiration with a concomitant burst of ethylene at the onset of ripening. In contrast, non-climacteric fruits, which include strawberry and grape, do not show a dramatic change in respiration, and ethylene production remains at a basal level (Cherian et al., 2014). During the last decade, research has focused on the role of low molecular compounds that manipulate metabolic pathways linked with freshness and extended postharvest life of horticultural produce (Gong et al., 2018). Although the potential mechanism is poorly understood (Fotopoulos et al., 2015), increasing evidence suggest that H2S significantly influences the postharvest life of fruits from perennial plants (Table 1).
Banana (Musa acuminata) is a typical climacteric fruit, whose ripening initiation is characterized by a sudden increase of ethylene production. Ripe banana fruit suffers from extensive postharvest losses due to ethylene-associated texture softening, peel deterioration, and disease vulnerability (Liu et al., 1999). 1-methylcyclopropene (1-MCP) is a well-known blocker of membrane ethylene receptors, resulting to an extensive shelf life of fruits (Harris et al., 2000). However, the application of 1-MCP upon banana fruits is associated with the negative outcome of green color preservation or yellow color uneven allocation, resulting to limited commercial potential of 1-MCP in banana fruits, thus pinpointing the need to develop alternative mechanism that could manipulate the role of ethylene during ripening (Golding et al., 1998). Ge et al. (2017) showed that the coupled treatment of H2S with ethylene in banana fruits downregulated the expression profile of ethylene biosynthesis genes MaACS1, MaACS2, and MaACO1 and pectate lyase MaPL, while enhancing the expression profile of ethylene receptor genes MaETR, MaERS1, and MaERS2, compared with solo ethylene application. In addition, H2S treatment sustained fruit chlorophyll content, increased carotenoids, soluble proteins, and the overall antioxidant capacity (Ge et al., 2017). These results suggest that H2S delayed banana fruit ripening and senescence via an antagonizing effect with ethylene, through the alleviation of oxidative stress and inhibition of ethylene signaling (Ge et al., 2017).
Cold storage is widely used for the extent of postharvest life of many horticultural products (Wang, 1990). However, due to its tropical nature, banana fruit is prone to chilling injury (CI), which expressed as surface browning, pitting and inability to soften below 13°C (Jiang et al., 2004). Fumigation treatment with H2S depressed the development of CI in banana fruit during cold storage under various ripening stages (Luo et al., 2015). In this work, H2S also sustained peel firmness and reduced MDA content while phenylalanine ammonia lyase (PAL) activity and antioxidant capacity was increased with parallel increase in the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR). Finally, H2S fumigation increased proline content by promoting the activity of proline biosynthetic enzyme P5CS and suppressing the activity of proline dehydrogenase. A similar study by Li et al. (2016) indicated that banana fruits fumigated with H2S under chilling temperature conditions showed higher firmness and lower electrolyte leakage, MDA content, and ethylene production, again indicating a positive effect of H2S toward CI in banana fruit. Interestingly, H2S treatment enhanced the enzyme activity of H+-ATPase, Ca+2-ATPase, cytochrome C oxidase (CCO), and succinate dehydrogenase (SDH), highlighting a direct effect on energy metabolism and maintenance of energy charge (Li et al., 2016).
In recent years, there has been a rapid expansion in the sale of fresh-cut apples due to the advantages offered by ready-to-eat or ready-to-use fresh produce (Rico et al., 2007). However, exposure of cut fruit surfaces to atmospheric factors limits their potential for longer postharvest life when compared with intact fruits. The multifunctional signaling role of H2S in fresh-cut apples was established by Zheng et al. (2016). Treatment with H2S retarded postharvest spoilage of fresh-cut “Fuji” apples (Malus x pumila) via the modulation of the antioxidant metabolism and the regulation of senescence-related gene expression (Zheng et al., 2016). In detail, H2S treatment upon fresh-cut apples retained quality traits like ascorbic acid, flavonoids, total phenolics, reducing sugars, and soluble proteins. Molecular analysis revealed that the delayed postharvest senescence of apple fruits caused by H2S was linked with the suppression of genes involved in ethylene biosynthesis (MdACS1, MdACS3, MdACO1, and MdACO2) and signal transduction (MdETR1, MdERS1, MdERS2, MdERF3, MdERF4, and MdERF5; Zheng et al., 2016), thereby supporting the counteractive role of H2S in ethylene biosynthesis and signaling.
The positive effect of H2S treatment (applied as the H2S donor NaHS at 0.5–2.5 mM that could liberate about 0.05–0.5 ppm H2S gas into a closed container) on fresh-cut pear slices was also reported (Hu K.D. et al., 2014). Particularly, H2S fumigation on sliced pears (Pyrus pyrifolia cv. Dangshan) caused the maintenance of higher levels of reduced sugars and soluble proteins while reducing the accumulation of ROS and MDA in a dose-dependent manner. These findings further supported the role of H2S in antioxidant mechanism, since H2S fumigation upregulated the enzymatic activities of APX, CAT, and POD while reduced the enzymatic activities of polyphenol oxidase (PPO), lipoxygenase (LOX), and PAL. Interestingly, postharvest storage of pear slices was also prolonged by the inhibition of fungal pathogens (Aspergillus niger and Penicillium expansum) due to H2S fumigation (Hu K.D. et al., 2014), highlighting its importance as a potent postharvest fungicide agent.
Kiwifruit is a typical climacteric fruit, and its ripening is closely associated with ethylene biosynthesis. Harvested kiwifruit undergoes a rapid increase of ethylene production after storage, leading to a limited postharvest life at room temperature (Minas et al., 2012; Minas et al., 2014; Tanou et al., 2015; Ainalidou et al., 2016). There is solid evidence that H2S delayed kiwifruit (Actinidia chinensis Planch. cv. Jinkui) postharvest ripening, expressed as titratable acidity (TA) and ethylene production. Treatment with H2S also increased the activity of SOD, POD, and CAT resulting in direct decrease of accumulated ROS, ultimately protecting kiwifruit cell membranes during postharvest storage (Zhu et al., 2014). The previously presented diverse positive effects of H2S on kiwifruit postharvest behavior were supported by another report pinpointing that fumigation of fresh-cut kiwifruit with H2S prolonged postharvest storage time, alleviated senescence, and prevented tissue softening (Gao et al., 2013). Furthermore, H2S treatment decreased MDA content, increased LOX activity, and reduced ROS production (Gao et al., 2013).
Strawberry (Fragaria × ananassa Duch.) is a non-climacteric fruit that is highly acceptable by consumers for its excellent sensory traits. However, strawberry fruit is highly prone to deterioration during harvest and storage as a result of its soft texture, while it is also susceptibility to fungal pathogens during postharvest period (Wills, 1996). A recent study revealed that H2S prolonged postharvest life of strawberry fruits (cv. Bao Jiao) according to the applied dosage (Hu et al., 2012). H2S fumigation on strawberry fruits retained higher content of reducing sugars, soluble proteins, free amino acids, and sustained flesh freshness and firmness. Additionally, H2S treatment kept respiration intensity and polygalacturonase (PG) activity at low levels. H2S modulated the antioxidant metabolism by increasing the enzymatic activity of CAT, POD, APX, and GR and lowering the activity of LOX and overall ROS levels, thus alleviating lipid peroxidation (Hu et al., 2012).
Zhang et al. (2014) reported a synergistic effect of H2S and NO toward the prolongation of postharvest life of strawberry fruits. Notably, the combination of H2S and NO in strawberry fruits suppressed fruit decay, inhibited the respiration rate, maintained crust color, and prolonged fruit firmness. The combination of H2S and NO also increased the enzymatic activity of chitinase (CHI), beta-1,3-glucanase (GNS) and decreased the activities of pectin methylestaerase (PME), PG, and endo-β-1,4-glucanase (EGase), consequently extending the shelf-life of strawberry fruits after harvest (Zhang et al., 2014), supporting the idea of a possible interplay between H2S and NO in postharvest life extension.
The production and consumption of mulberry fruit has witnessed a rapid increase during the past decade, due to recognized nutritional values and biological activities. However, fruits easily lose their postharvest commercial value due to rapid ripening rate. Hu H. et al. (2014) found that fumigation with H2S, released from 0.8 mm NaHS solution, enhanced the intercellular H2S content via the enhanced activity of D-cysteine desulfhydrase and L-cysteine desulfhydrase in mulberry fruits (Morus indica L. Dianmian-1). In addition, H2S delayed the decay of mulberry fruits, depressed respiration rate, and maintained quality characteristics (e.g., TA and ascorbic acid). Meanwhile, H2S fumigation exerted a protective role against senescence induced oxidative stress by terminating the propagation of lipid peroxidation and enhancing various antioxidant enzymes activity (Hu H. et al., 2014).
Grapes are subject to postharvest senescence during storage, in the syndromes of rachis browning, serious water loss, berry softening, off-flavor occurrence, as well as decay caused mainly by Botrytis cinerea, which reduces the commodity and consumption of grapes (Crisosto et al., 2001). The active role of H2S in postharvest senescence of grape berries (Vitis vinifera L × Vitis labrusca L, cv. Kyoho) was previously reported in the work of Ni et al. (2016). Exogenous application of H2S attenuated the rotting and threshing of grape berries. Prior to postharvest storage, fumigation of grape berries with H2S preserved in high levels several quality markers like firmness, soluble solids, TA, ascorbic acid, flavonoids, total phenolics, reducing sugars, and soluble proteins. As a result, the positive role of H2S in preserving chlorophyll and carotenoid content in both grape rachis and pulp was established. In the same work, H2S fumigation reduced the accumulation of ROS and MDA in grape pulp, while increased the activity of antioxidant enzymes and minimized LOX activity (Ni et al., 2016). Notably, the authors raised a question whether H2S might act as an antagonist to ethylene-induced fruit senescence.
Solid evidence indicated that H2S contributes to the maintenance of postharvest shelf life via pathogen inhibition (Fu et al., 2014; Hu K.D. et al., 2014). It has been reported that endogenous H2S plays a crucial role in plant defense when agricultural crops suffer from fungal infections (Bloem et al., 2012). In the work of Fu et al. (2014), H2S exerted a positive antifungal effect against postharvest pathogen, namely, A. niger and Penicillium italicum, when they were inoculated on apples, kiwifruit, pear, sweet oranges, and mandarin. In the same study, H2S inhibited spore germination, germ tube elongation, and mycelial growth, produce abnormal mycelial contractions, and stimulate antioxidative enzyme activity in fruits. Similar results were also reported by Hu K.D. et al. (2014) where fumigation of pear fruits with H2S resulted in direct inhibition of the pathogens A. niger and P. expansum. The positive effect of H2S fumigation toward the inhibition of postharvest fungal attacks highlights the commercial importance of H2S.
The experimental evidence presented in this review suggested that H2S might influence fruit postharvest responses; however, it remains unclear how H2S could implement its anti-ripening effect. Recent evidence suggests that there is a putative interplay between H2S and ethylene (Ge et al., 2017). These authors demonstrated, as mentioned above, that H2S alleviated banana fruit ripening and expressed an antagonistic effect toward ethylene. In support, H2S fumigation at low concentration suppressed the expression of genes associated with ethylene biosynthesis under low ethylene environment in broccoli florets and apple slices (Li et al., 2014; Zheng et al., 2016), indicating that H2S function could be linked to ethylene perception. It has been recently proposed that H2S may bind to the copper ion of the same ethylene receptor as 1-MCP (Al Ubeed et al., 2018). However, the allosteric effect on the protein receptor would differ from coordination of H2S and 1-MCP (Pirrung et al., 2008), since several ethylene receptors are present in agricultural produce to which H2S may bind (Lacey and Binder, 2014). Even though H2S has an equal size to ethylene, it needs to be deprotonated so as to bind tightly to the receptor binding domain (Al Ubeed et al., 2018). Of relevance to postharvest physiology, the ability of H2S to bind to protein receptors was justified by Ge et al. (2017) where the parallel fumigation of H2S with ethylene increased the expression of ethylene receptor genes (MaETR, MaERS1, and MaERS2).
Aside from ethylene perception and signaling, evidence suggests that H2S can also function in autophagy. Analysis of the Arabidopsis des1 mutant impaired in the cytosolic production of H2S from cysteine (Cys) led to the conclusion that H2S acts as an inhibitor of autophagy (Álvarez et al., 2012). Accordingly, postharvest fruit senescence associated with cell organelle-specific autophagy process could also be associated with H2S, as already evidenced in animals (Wang et al., 2012). H2S-mediated signaling in autophagy might be based on the reversible post-translational modification of the enzymes involved in the ubiquitylation process or of other proteins involved in the initiation and completion of the autophagosome (Batoko et al., 2017).
As discussed elsewhere (Hancock and Whiteman, 2016), it is likely that H2S will interact with ROS and NO through sulfate metabolism; for example, the presence of H2S can lead to an increase in reduced glutathione (GSH), thus affecting redox state homeostasis. This mechanism can have profound effects on the gene expression and the activity of proteins (as discussed further below). Another possibility is that H2S signaling may be mediated by S-nitrosothiol (SNO) metabolism and signaling of cells as well as enzymatic (e.g., nitric oxide synthase, nitrate reductase, xanthine oxidase)-dependent NO production. Furthermore, the nucleophilic properties of this molecule and its capacity to react with oxygen, H2O2, or peroxynitrite (ONOO-) suggest that it acts by reducing cellular oxidative stress (Nagy, 2015), which is commonly observed during fruit ripening (Molassiotis et al., 2013). This is also justified by the fact that postharvest treatments of H2S can counteract oxidative damage and stimulate the antioxidant enzymes activity in several fruits (Hu et al., 2012; Ni et al., 2016).
Among the many kinds of amino acid residues susceptible to oxidative stress, sulfur-containing amino acids, like methionine (Met) and cysteine (Cys), are the most sensitive (Møller and Sweetlove, 2010). Meanwhile, thiol groups in Cys residues are the main protein targets of S-nitrosylation (i.e., the covalent bonding of an NO moiety to Cys thiol side chain, to form SNO and the resulting S-nitrosoprotein; Lounifi et al., 2013). Recent studies also suggest that the key H2S signaling action is achieved by the modification of Cys residues to form a persulfide group (also known as persulfidation; Aroca et al., 2015). Thus, ROS, NO, and H2S competitively target Cys residues to exert their biological action, and therefore it is likely that a tight link between oxidation, nitrosylation, and persulfidation exists, that in turn may control oxidative- and nitrosative-based climacteric fruit ripening events. More importantly, if these thiols are being modified by H2S, then they are no longer accessible to be modified by ROS and NO, so the capacity for such signaling may well be severely altered in the presence of H2S (Hancock and Whiteman, 2016). This hypothesis is supported by results in citrus cells showing that conformational changes induced in specific proteins by S-nitrosylation could lock the structure of these proteins in a state under which they are no more sensitive to irreversible carbonylation induced by ROS (Tanou et al., 2009). Current evidence also suggests that protein S-sulfhydration adheres closely to the generally acknowledged paradigm for S-nitrosylation. Indeed, many of the protein sites reported to undergo endogenous S-nitrosylation have also been found to undergo S-sulfydration (Lu et al., 2013). These observations clearly indicate that it is essential to better understand the interplay between S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation in fruit biology.
In addition to having an influence on these specific redox-based protein modifications, it is likely that many H2S-driven signaling components and mechanisms involved in fruit metabolism have yet to be unraveled. For example, H2S may exert its mode of action via the modulation of energy metabolism related with the TCA cycle, glycolysis, electron transport chain, sustaining high levels of ATP which delay fruit senescence (Fotopoulos et al., 2010; Li et al., 2017). In this regard, it has been recently proposed that H2S can have two effects on mitochondrial electron transport chain activity. It can feed electrons into the pathway, with a concomitant increase in ATP production, or, alternately, it can inhibit complex IV, thus inhibiting ATP production (Hancock and Whiteman, 2016). Critically, it is significant to note that, the signal transduction pathway activated by exogenously applied H2S donors-produced H2S in various fruit systems experience postharvest handling may differ from the pathway induced by endogenously H2S generation, as previously suggested for the extracellular and intracellular NO signaling in stressed plant cells (Molassiotis et al., 2010).
During the last decade, the manipulation of fruit postharvest loses is becoming a hot issue, and multiple lines of evidence discussed above propose an important role for H2S in postharvest fruit biology (Figure 1). In this sense, the exact role of H2S in fruit metabolism needs to be further characterized using high-throughput systems biology techniques such as transcriptomic, epigenomic, proteomic, and metabolomic approaches, while the synergistic effect of H2S with other molecules, such as ethylene and NO, should also be addressed. It is particularly significant to reveal how H2S could influence the ethylene perception mechanism and to characterize the functional significance of ethylene biosynthesis and response genes following H2S application through in silico studies at the genome-wide scale. Considering the importance of protein S-sulfhydration in various cellular responses, the regulatory system of post-translational modification of protein cysteine residues in fruit senescence must be elucidated. Although nitrosative and especially oxidative stress responses during fruit senescence are well studied, information regarding the interaction of H2S with nitro-oxidative postharvest conditions is scarce. Such understanding will lead to the establishment new technologies and strategies to preserve postharvest fruit quality and extent their postharvest life.
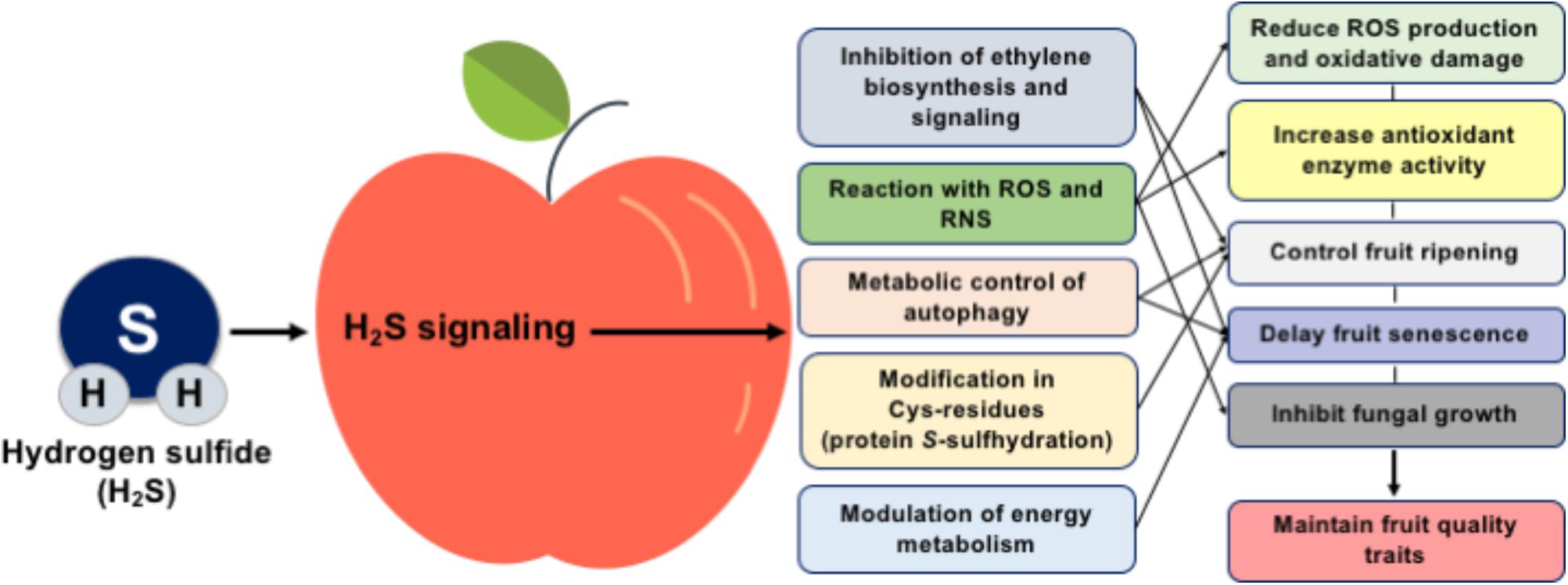
FIGURE 1. Schematic presentation of the mechanisms and potential benefits of H2S in postharvest fruit biology.
VZ initiated the project, collected and analyzed the data, and wrote the manuscript with input from all authors. AM provided feedback and reviewed the article. VF reviewed the article. GT devised the project, the main concepts ideas, and proof outline and reviewed the article. VZ, AM, VF, and GT conceived the idea, designed the structure of the text, and wrote the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors apologize if some references related to the main theme of the current review could not be cited due to space constraints.
Ainalidou, A., Tanou, G., Belghazi, M., Samiotaki, M., Diamantidis, G., Molassiotis, A., et al. (2016). Integrated analysis of metabolites and proteins reveal aspects of the tissue-specific function of synthetic cytokinin in kiwifruit development and ripening. J. Proteomics 143, 318–333. doi: 10.1016/j.jprot.2016.02.013
Al Ubeed, H. M. S., Wills, R. B. H., Bowyer, M. C., and Golding, J. B. (2018). Comparison of hydrogen sulphide with 1-methylcyclopropene (1-MCP) to inhibit senescence of the leafy vegetable, pak choy. Postharvest Biol. Technol. 137, 129–133. doi: 10.1016/j.postharvbio.2017.11.020
Álvarez, C., García, I., Moreno, I., Pérez-Pérez, M. E., Crespo, J. L., Romero, L. C., et al. (2012). Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 24, 4621–4634. doi: 10.1105/tpc.112.105403
Antoniou, C., Savvides, A., Christou, A., and Fotopoulos, V. (2016). Unravelling chemical priming machinery in plants: the role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 33, 101–107. doi: 10.1016/j.pbi.2016.06.020
Aroca,Á., Serna, A., Gotor, C., and Romero, L. C. (2015). S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol. 168, 334–342. doi: 10.1104/pp.15.00009
Batoko, H., Dagdas, Y., Baluska, F., and Sirko, A. (2017). Understanding and exploiting autophagy signaling in plants. Essays Biochem. 61, 675–685. doi: 10.1042/EBC20170034
Bloem, E., Haneklaus, S., Kesselmeier, J., and Schnug, E. (2012). Sulfur fertilization and fungal infections affect the exchange of H2S and COS from Agricultural Crops. J. Agric. Food Chem. 60, 7588–7596. doi: 10.1021/jf301912h
Cherian, S., Figueroa, C. R., and Nair, H. (2014). ‘Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 65, 4705–4722. doi: 10.1093/jxb/eru280
Christou, A., Filippou, P., Manganaris, G. A., and Fotopoulos, V. (2014). Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 14:42. doi: 10.1186/1471-2229-14-42
Crisosto, C. H., Smilanick, J. L., and Dokoozlian, N. (2001). Table grapes suffer water loss, stem browning during cooling delays. Calif. Agric. 55, 39–42. doi: 10.3733/ca.v055n01p39
Deng, J., Li, W., Peng, G. L., and Hao, X. H. (2013). Study on the potential of antifungal activity of essential oils against fungal pathogens of fruits and vegetables. J. Chem. Pharm. Res. 5, 443–446.
Filipovic, M. R., and Jovanović, V. M. (2017). More than just an intermediate: hydrogen sulfide signalling in plants. J. Exp. Bot. 68, 4733–4736. doi: 10.1093/jxb/erx352
Fotopoulos, V., Christou, A., Antoniou, C., and Manganaris, G. A. (2015). Hydrogen sulfide: a versatile tool for the regulation of growth and defence responses in horticultural crops. J. Hortic. Sci. Biotechnol. 90, 227–234. doi: 10.1080/146203116.2015.11513176
Fotopoulos, V., Ziogas, V., Tanou, G., and Molassiotis, A. (2010). “Involvement of AsA/DHA and GSH/GSSG ratios in gene and protein expression and in the activation of defence mechanisms under abiotic stress conditions,” in Ascorbate-Glutathione Pathway and Stress Tolerance in Plants, ed. M. T. Chan (Dordrecht: Springer), 265–302.
Fu, L. H., Hu, K. D., Hu, L. Y., Li, Y. H., Hu, L. B., Yan, H., et al. (2014). An antifungal role of hydrogen sulfide on the postharvest pathogens aspergillus niger and penicillium italicum. PLoS One 9:e104206. doi: 10.1371/journal.pone.0104206
Gao, S. P., Hu, K. D., Hu, L. Y., Li, Y. H., Han, Y., Wang, H. L., et al. (2013). Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. Hortscience 48, 1385–1392.
Ge, Y., Hu, K. D., Wang, S. S., Hu, L. Y., Chen, X. Y., Li, Y. H., et al. (2017). Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS One 12:e0180113. doi: 10.1371/journal.pone.0180113
Golding, J. B., Shearer, D., Wyllie, S. G., and Mcglasson, W. B. (1998). Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87–98. doi: 10.1016/S0925-5214(98)00032-5
Gong, T., Li, C., Bian, B., Wu, Y., Dawuda, M. M., and Liao, W. (2018). Advances in application of small molecule compounds for extending the shelf life of perishable horticultural products: a review. Sci. Hortic. 230, 25–34. doi: 10.1016/j.scienta.2017.11.013
Hancock, J. T., Lisjak, M., Teklic, T., Wilson, I. D., and Whiteman, M. (2011). Hydrogen sulfide and signaling in plants. CAB Rev. 6, 1–7. doi: 10.1079/PAVSNNR20110012
Hancock, J. T., and Whiteman, M. (2014). Hydrogen sulfide and cell signalling: team player or referee? Plant Physiol. Biochem. 78, 37–42. doi: 10.1016/j.plaphy.2014.02.012
Hancock, J. T., and Whiteman, M. (2016). Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2365, 5–14. doi: 10.1111/nyas.12733
Harris, D. R., Seberry, J. A., Wills, R. B. H., and Spohr, L. J. (2000). Effect of fruit maturity on efficiency of 1-methylcyclopropene to delay the ripening of bananas. Postharvest Biol. 20, 303–308. doi: 10.1016/S0925-5214(00)00150-2
Hatzfeld, Y., Maruyama, A., Schmidt, A., Noji, M., Ishizawa, K., and Saito, K. (2000). β-Cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis. Plant Physiol. 123, 1163–1172. doi: 10.1104/pp.123.3.1163
Hu, H., Shen, W., and Li, P. (2014). Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. Int. J. Food Sci. Tecnol. 49, 399–409. doi: 10.1111/ijfs.12313
Hu, K. D., Wang, Q., Hu, L. Y., Gao, S. P., Wu, J., Li, Y. H., et al. (2014). Hydrogen sulfide prolongs postharvest storage of fresh-cut pears (Pyrus pyrifolia) by alleviation of oxidative damage and inhibition of fungal growth. PLoS One 9:e85524. doi: 10.1371/journal.pone.0085524
Hu, L. Y., Hu, S. L., Wu, J., Li, Y. H., Zheng, J. L., Wei, Z. J., et al. (2012). Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 60, 8684–8693. doi: 10.1021/jf300728h
Jiang, Y., Joyce, D. C., Jiang, W., and Lu, W. (2004). Effects of chilling temperatures on ethylene binding by Banana Fruit. Plant Growth Regul. 43, 109–115. doi: 10.1023/B:GROW.0000040112.19837.5f
Kader, A. A. (2005). Increasing food availiability by reducing postharvest losses of fresh produce. Acta Hortic. 682, 2169–2176. doi: 10.17660/ActaHortic.2005.682.296
Lacey, R. F., and Binder, B. M. (2014). How plants sense ethylene gas — The ethylene receptors. J. Inorg. Biochem. 133, 58–62. doi: 10.1016/j.jinorgbio.2014.01.006
Li, D., Li, L., Ge, Z., Limwachiranon, J., Ban, Z., Yang, D., et al. (2017). Effects of hydrogen sulfide on yellowing and energy metabolism in broccoli. Postharvest Biol. Technol. 129, 136–142. doi: 10.1016/j.postharvbio.2017.03.017
Li, D., Limwachiranon, J., Li, L., Du, R., and Luo, Z. (2016). Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 208, 272–278. doi: 10.1016/j.foodchem.2016.03.113
Li, S.-P., Hu, K. D., Hu, L. Y., Li, Y. H., Jiang, A. M., Xiao, F., et al. (2014). Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J. Agric. Food Chem. 62, 1119–1129. doi: 10.1021/jf4047122
Liu, X., Shiomi, S., Nakatsuka, A., Kubo, Y., Nakamura, R., and Inaba, A. (1999). Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol. 121, 1257–1265. doi: 10.1104/pp.121.4.1257
Lounifi, I., Arc, E., Molassiotis, A., Job, D., Rajjou, L., and Tanou, G. (2013). Interplay between protein carbonylation and nitrosylation in plants. Proteomics 13, 568–578. doi: 10.1002/pmic.201200304
Lu, C., Kavalier, A., Lukyanov, E., and Gross, S. S. (2013). S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: a common paradigm for gasotransmitter signaling by H2S and NO. Methods 62, 177–181. doi: 10.1016/j.ymeth.2013.05.020
Luo, Z., Li, D., Du, R., and Mou, W. (2015). Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 183, 144–151. doi: 10.1016/j.scienta.2014.12.021
Minas, I. S., Tanou, G., Belghazi, M., Job, D., Manganaris, G. A., Molassiotis, A., et al. (2012). Physiological and proteomic approaches to address the active role of ozone in kiwifruit post-harvest ripening. J. Exp. Bot. 63, 2449–2464. doi: 10.1093/jxb/err418
Minas, I. S., Vicente, A. R., Dhanapal, A. P., Manganaris, G. A., Goulas, V., Vasilakakis, M., et al. (2014). Ozone-induced kiwifruit ripening delay is mediated by ethylene biosynthesis inhibition and cell wall dismantling regulation. Plant Sci. 229, 76–85. doi: 10.1016/j.plantsci.2014.08.016
Molassiotis, A., Tanou, G., and Diamantidis, G. (2010). NO says more than ‘YES’ to salt tolerance: salt priming and systemic nitric oxide signaling in plants. Plant Signal. Behav. 5, 209–212. doi: 10.4161/psb.5.3.10738
Molassiotis, A., Tanou, G., Filippou, P., and Fotopoulos, V. (2013). Proteomics in the fruit tree science arena: new insights into fruit defense, development, and ripening. Proteomics 13, 1871–1884. doi: 10.1002/pmic.201200428
Møller, I. M., and Sweetlove, L. J. (2010). ROS signalling-specificity is required. Trends Plant Sci. 15, 370–374. doi: 10.1016/j.tplants.2010.04.008
Nagy, P. (2015). “Chapter one - Mechanistic chemical perspective of hydrogen sulfide signaling,” in Methods in Enzymology, eds E. Cadenas and L. Packer (Cambridge, MA: Academic Press), 3–29.
Ni, Z. J., Hu, K. D., Song, C. B., Ma, R. H., Li, Z. R., Zheng, J. L., et al. (2016). Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid. Med. Cell. Longev. 2016:4715651. doi: 10.1155/2016/4715651
Pirrung, M. C., Bleecker, A. B., Inoue, Y., Rodríguez, F. I., Sugawara, N., Wada, T., et al. (2008). Ethylene receptor antagonists: strained alkenes are necessary but not sufficient. Chem. Biol. 15, 313–321. doi: 10.1016/j.chembiol.2008.02.018
Porat, R., Lichter, A., Terry, L. A., Harker, R., and Buzby, J. (2018). Postharvest losses of fruits and vegetables during retail nad consumers’ homes: quantification, causes and means of prevention. Postharvest Biol. Tecnol. 139, 135–149. doi: 10.1016/j.postharvbio.2017.11.019
Rico, D., Martín-Diana, A. B., Barat, J. M., and Barry-Ryan, C. (2007). Extending and measuring the quality of fresh-cut fruit and vegetables: a review. Trends Food Sci. Technol. 18, 373–386. doi: 10.1016/j.tifs.2007.03.011
Tanou, G., Job, C., Rajjou, L., Arc, E., Belghazi, M., Diamantidis, G., et al. (2009). Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 60, 795–804. doi: 10.1111/j.1365-313X.2009.04000
Tanou, G., Minas, I. S., Karagiannis, E., Tsikou, D., Audebert, S., Papadopoulou, K. K., et al. (2015). The impact of sodium nitroprusside and ozone in kiwifruit ripening physiology: a combined gene and protein expression profiling approach. Ann. Bot. 116, 649–662. doi: 10.1093/aob/mcv107
Wang, D., Ma, Y., Li, Z. R., Kang, K., Sun, X., Pan, S., et al. (2012). The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy 8, 954–962. doi: 10.4161/auto.19927
Wang, R. (2002). Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16, 1792–1798. doi: 10.1096/fj.02-0211hyp
Wills, R. B. H. (1996). Enchancement of senescence in non-climacteric fruit and vegetables by low ethylene levels. Acta Hortic. 464, 159–162. doi: 10.17660/ActaHortic.1998.464.21
Zhang, C., Shi, J. Y., Zhu, L. P., Li, C. L., and Wang, Q. G. (2014). Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. Int. J. Agric. Biol. Eng. 7, 114–122.
Zheng, J. L., Hu, L. Y., Hu, K. D., Wu, J., Yang, F., and Zhang, H. (2016). Hydrogen sulfide alleviates senescence of fresh-cut apple by regulating antioxidant defense system and senescence-related gene expression. Hortscience 51, 152–158.
Zhu, L., Wang, W., Shi, J., Zhang, W., Shen, Y., Du, H., et al. (2014). Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. Int. J. Agric. Biol. Eng. 94, 2699–2704. doi: 10.1002/jsfa.6613
Keywords: ethylene, fruit ripening, hydrogen sulfide, postharvest biology, reactive nitrogen and oxygen species, S-sulfhydration
Citation: Ziogas V, Molassiotis A, Fotopoulos V and Tanou G (2018) Hydrogen Sulfide: A Potent Tool in Postharvest Fruit Biology and Possible Mechanism of Action. Front. Plant Sci. 9:1375. doi: 10.3389/fpls.2018.01375
Received: 15 July 2018; Accepted: 29 August 2018;
Published: 19 September 2018.
Edited by:
Luis C. Romero, Instituto de Bioquímica Vegetal y Fotosíntesis (IBVF), SpainReviewed by:
Tihana Teklić, Josip Juraj Strossmayer University of Osijek, CroatiaCopyright © 2018 Ziogas, Molassiotis, Fotopoulos and Tanou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgia Tanou, Z3Rhbm91QGFncm8uYXV0aC5ncg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.