
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 26 September 2018
Sec. Functional Plant Ecology
Volume 9 - 2018 | https://doi.org/10.3389/fpls.2018.01357
This article is part of the Research Topic New Perspectives on the Biology of Nectaries and Nectars View all 18 articles
 Paul A. Egan1*
Paul A. Egan1* Lynn S. Adler2
Lynn S. Adler2 Rebecca E. Irwin3
Rebecca E. Irwin3 Iain W. Farrell4
Iain W. Farrell4 Evan C. Palmer-Young2†
Evan C. Palmer-Young2† Philip C. Stevenson4,5
Philip C. Stevenson4,5Crop domestication can lead to weakened expression of plant defences, with repercussions for herbivore and pathogen susceptibility. However, little is known about how domestication alters traits that mediate other important ecological interactions in crops, such as pollination. Secondary metabolites, which underpin many defence responses in plants, also occur widely in nectar and pollen and influence plant-pollinator interactions. Thus, domestication may also affect secondary compounds in floral rewards, with potential consequences for pollinators. To test this hypothesis, we chemically analysed nectar and pollen from wild and cultivated plants of highbush blueberry (Vaccinium corymbosum L.), before conducting an artificial diet bioassay to examine pollinator-pathogen interactions. Our results indicated that domestication has significantly altered the chemical composition of V. corymbosum nectar and pollen, and reduced pollen chemical diversity in cultivated plants. Of 20 plant metabolites identified in floral rewards, 13 differed significantly between wild and cultivated plants, with a majority showing positive associations with wild compared to cultivated plants. These included the amino acid phenylalanine (4.5 times higher in wild nectar, 11 times higher in wild pollen), a known bee phagostimulant and essential nutrient; and the antimicrobial caffeic acid ester 4-O-caffeoylshikimic acid (two times higher in wild nectar). We assessed the possible biological relevance of variation in caffeic acid esters in bioassays, using the commercially available 3-O-caffeoylquinic acid. This compound reduced Bombus impatiens infection by a prominent gut pathogen (Crithidia) at concentrations that occurred in wild but not cultivated plants, suggesting that domestication may influence floral traits with consequences for bee health. Appreciable levels of genetic variation and heritability were found for most floral reward chemical traits, indicating good potential for selective breeding. Our study provides the first assessment of plant domestication effects on floral reward chemistry and its potential repercussions for pollinator health. Given the central importance of pollinators for agriculture, we discuss the need to extend such investigations to pollinator-dependent crops more generally and elaborate on future research directions to ascertain wider trends, consequences for pollinators, mechanisms, and breeding solutions.
Humans have domesticated and selectively bred crops for millennia (Smith, 2001). While domestication has delivered many agronomic benefits, including enhanced yield and other desirable traits (Evans, 1996; Bai and Lindhout, 2007; Dempewolf et al., 2017), negative or unintended consequences of this process have included reduced genetic diversity (Van de Wouw et al., 2010; Meyer et al., 2012), and the impaired function or disappearance of potentially useful traits (Rosenthal and Dirzo, 1997; Li et al., 2017) such as physical or chemical defences against herbivory and disease (Wink, 1988; Jones, 1998; Gols et al., 2008; Sujana et al., 2012). Plant defence traits can impose considerable resource allocation costs, and can thereby trade-off with fitness or yield (Kempel et al., 2011). Hence, where selection is consistently imposed over many generations to maximise crop yield, agronomic gains may accrue at the risk of compromising other traits from which resources are diverted (Rosenthal and Dirzo, 1997; Brown and Rant, 2013). It is only relatively recently, however, that the extent of domestication impacts on crop ecological function has come to be fully appreciated (Meyer et al., 2012; Chen et al., 2015; Whitehead et al., 2017). This topic has garnered increased attention due to growing interest in the agronomic potential of traits traditionally neglected in crops, such as herbivore-induced plant volatiles (HIPVs) and extrafloral nectaries involved in the attraction of pest natural enemies (Rasmann et al., 2005; Rodriguez-Saona et al., 2009; Chen et al., 2015; Heil, 2015; Li et al., 2017).
In contrast to impacts on crop interactions with herbivores and pathogens, very little is known about the effect of domestication on interactions with pollinators and the traits which mediate these interactions. What little is known on this topic to date has mostly come from the study of ornamental plants, where widespread loss of floral scent is a reported consequence of breeding (Vainstein et al., 2001; Pichersky and Dudareva, 2007). This lack of information on domestication effects on pollinator-relevant traits, including floral reward chemistry, is surprising, given the importance of insect pollination for agriculture. About 75% of the world’s staple food crops rely to some extent on the ecosystem service of pollination (Klein et al., 2007), which contributes an estimated $351 billion (USD) annually to global food production (Lautenbach et al., 2012). Pollinators also have the potential to contribute to human nutritional health and need for micronutrients (Chaplin-Kramer et al., 2014; Ellis et al., 2015), and improve global food security (Bailes et al., 2015).
Although historically studied separately, plant-herbivore and plant-pollinator interactions are interconnected (Strauss and Armbruster, 1997; Adler et al., 2001; Irwin et al., 2003; Strauss and Irwin, 2004; Kessler and Halitschke, 2009; Lucas-Barbosa, 2016; Muola et al., 2017; Nepi et al., 2018). For example, a broad range of defence-related secondary metabolites (including alkaloids, phenolics, and cyanogenic glycosides) are expressed in floral rewards, including nectar and pollen (Baker, 1977; Rhoades and Bergdahl, 1981; Adler, 2000; Adler and Irwin, 2005; Heil, 2011; Arnold et al., 2014; Irwin et al., 2014; Stevenson et al., 2017). These compounds range widely in concentration – from trace amounts, to concentrations similar to other plant tissues (Stevenson et al., 2017, and references therein) – and in ecological function. The ecological effects of secondary compounds in floral rewards include warding off nectar robbers and microbial growth (Irwin et al., 2004; González-Teuber and Heil, 2009; Barlow et al., 2017), enhancing pollinator memory (Wright et al., 2013), and reducing pollinator infection by pathogens and parasites (Baracchi et al., 2015; Richardson et al., 2015; Erler and Moritz, 2016; Palmer-Young et al., 2016). Evidence now suggests that secondary metabolites occur ubiquitously in floral rewards, and commonly show phenotypic integration across herbivore- and pollinator-consumed materials in wild and cultivated plants (Adler et al., 2006, 2012; Kessler and Halitschke, 2009; Manson et al., 2012; Cook et al., 2013; Richardson et al., 2016; Palmer-Young et al., in press). It is therefore of great interest to assess whether domestication effects on plant defence are also mirrored for floral reward chemistry, with potential ramifications for ecological function.
We used blueberry as a crop system to examine the effect of domestication on floral reward chemistry and its potential consequence for pollinators. We included in this study wild and cultivated plants of Vaccinium corymbosum L. (highbush blueberry; Ericaceae), including cultivated hybrid and introgressed crosses with V. angustifolium Ait. (lowbush blueberry). The relatively recent and well-documented history of domestication of Vaccinium species (including cranberry, lingonberry, and bilberry) has rendered the genus a useful model for the study of domestication effects (Rodriguez-Saona et al., 2011; Song and Hancock, 2011; Rivera et al., 2015, 2016; Hernandez-Cumplido et al., 2018). The history of domestication of highbush and lowbush blueberry dates back to 1908 and 1909, respectively, when the first wild plants were collected from Greenfield, New Hampshire, United States, for cultivation and selective breeding (Gough, 1993). This initial highbush collection, together with two additional wild selections from New Jersey (Ehlenfeldt, 2009), formed some of the first early cultivars of V. corymbosum (‘Brooks,’ ‘Sooy,’ ‘Rubel’). Together with their crosses (‘Katherine,’ ‘Pioneer’), these first selections continue to make the largest genetic contributions to modern highbush cultivars (Ehlenfeldt, 1994; Lobos and Hancock, 2015), which form the basis of the global blueberry industry.
We examined nectar and pollen chemistry in eight cultivars or interspecific crosses of V. corymbosum, and as a basis for comparison, sampled from three wild populations within ca. 40 km of where the original founding cultivar ‘Brooks’ was collected. To examine potential domestication effects on pollinator health via changes in the pathogens of pollinators, we conducted an artificial diet bioassay with the common eastern bumble bee, Bombus impatiens Cresson. Bees were infected with the pathogen Crithidia, before manipulating dietary levels of a caffeic acid ester at concentrations representative of wild and cultivated plant nectar to compare their effect on infection. Crithidia species are Trypanosomatid gut pathogens that infect Bombus species via faecal-oral contamination (Durrer and Schmid-Hempel, 1994). Infection by Crithidia can reduce learning (Gegear et al., 2005, 2006), colony growth rates (Shykoff and Schmid-Hempel, 1991) and queen founding success (Brown et al., 2003), and is associated with reduced reproduction in wild bumble bee colonies (Goulson et al., 2018). In Massachusetts, United States Crithidia can infect up to 80% of Bombus (Gillespie, 2010).
Specifically, we tested the following hypotheses: (1) Domestication has altered chemical composition and reduced chemical diversity of nectar and pollen; (2) Nectar and pollen chemical traits show genetic variation and heritability, but cultivars are phenotypically less variable than wild populations, consistent with their clonal propagation; (3) Nectar and pollen chemical concentration and composition are genetically correlated, which could constrain targeted breeding for floral reward chemistry; and (4) Altered expression of nectar antimicrobial compounds, such as caffeic acid esters, can influence pollinator-pathogen interactions.
Eight cultivars were selected for inclusion in this study, based on suitable replication across sampled farms: ‘Patriot’ (P), ‘Reka’ (R), ‘Liberty’ (L), ‘Bonus’ (B), ‘Friendship’ (F), ‘Northland’ (N), ‘BlueCrop’ (BC), and ‘Spartan’ (S). The genetic origin of the majority of these cultivars is pure V. corymbosum (cultivars B, L, N, R, and S), whereas cultivars BC, F, P are interspecific hybrids with minor parentage from V. angustifolium (Supplementary Table 1). A range of ploidy levels are known to exist within the genus Vaccinium. However, commercially grown highbush cultivars, including those with minor percentages of V. angustifolium, are reported to be tetraploids (Rowland and Hammerschlag, 2005). The cultivars included in this study, and domesticated highbush plants generally, are typically propagated by vegetative means (softwood or hardwood cuttings) (Gough, 1993), meaning that all sampled plant individuals of the same cultivar were genetic clones.
Nectar and pollen from wild and cultivated highbush blueberry were sampled in western Massachusetts, United States, in May 2014. Wild samples were collected from three sites at least 5 km apart between May 15 to 26 [Harvard Forest (HF): 42°32′6″N, 72°11′19″W, Harvard Pond (HP): 42°29′54″N, 72°12′47″W, and Quabbin Reservoir (Q): 42°23′32″N, 72°24′11″W]. We sampled cultivars at several farms between May 19 and 28 from Cold Spring Orchard (42°15′6″N, 72°21′38″W; cultivars P, R, L, B, F, and N), Kenburn Orchard (42°36′43″N, 72°39′18″W; cultivar P), Nourse Farms (42°25′49″N, 72°35′18″W; cultivars BC and S) and Sobieski’s River Valley Farm (42°27′12″N, 72°35′43″W; cultivars BC and S). To ensure no large confounding effect of sampling across multiple farms, cultivar-by-farm interactions were tested but were not significant (data not shown) for nectar and pollen chemical composition and diversity, as quantified below. For analyses other than nectar and pollen chemical composition and diversity, we included only cultivars which had been sampled from a single farm (Cold Spring Orchard).
Ten wild plants were sampled per site, and 5 cultivated plants per cultivar per farm. To prevent pollinator access to nectar, flowers were bagged with mesh usually one day before collection for all plants except at Sobieski’s farm, where we obtained sufficient nectar without bagging; sometimes bags were left on plants for several days if weather was not conducive to collection (e.g., rain). Where a mixture of bagged and unbagged samples were collected for a cultivar (i.e., BC and S), we tested to affirm that bagging did not hold a significant effect on compound concentrations (data not shown). Nectar was typically collected from flowers using 2 μl glass microcapillary tubes inserted into the flower. Nectar was pooled across flowers within plants with a target of 20 μl per plant (range: 6.76–32.16 μl), and added to 80 μl ethanol in a 1.5 ml microcentrifuge tube. Anthers were collected using forceps, and thus our samples included the pollen sac and a small amount of filament in addition to granular pollen. We collected at least 5 mg per plant by pooling across flowers within plants. Nectar and pollen samples were placed on ice in a cooler in the field, frozen at -20°C, and then freeze-dried prior to analysis.
Freeze-dried nectar was redissolved in 50 μL MeOH. For extraction of pollen, dried samples were sonicated for 10 min in 1 ml MeOH, and incubated at room temperature for 24 h. Samples were then centrifuged at 13,000 rpm for 5 min, and supernatant transferred to glass vials for analysis. Sample analyses were performed by liquid chromatography electrospray ionisation mass spectroscopy (LC-ESIMS) and UV spectroscopy using a Micromass ZQ LC-MS (Waters, Elstree, Herts, United Kingdom). Aliquots of nectar were injected directly onto a Phenomenex (Macclesfield, Cheshire, United Kingdom) Luna C18(2) columns (150 × 3.0 mm i.d., 5 um particle size) and compounds eluted using MeOH (A), H2O (B) and formic Acid (C) with A = 0%, B = 90% at T = 0 min; A = 90%, B = 0% at T = 20 min and held for 10 min with C at 10% throughout the analyses. Column temperature was 30°C with flow rate = 0.5 ml min-1. High resolution MS spectra were used to provide additional data for compound identification and were recorded for a subset of samples using a Thermo LTQ-Orbitrap XL mass spectrometer (Waltham, MA, United States) with compound separation on an Accela LC system using similar elution parameters as described above. Except for identification of caffeoylquininc and caffeoylshikimic acids (see below), compounds were identified by comparison with mass spectra available via the NIST spectral database version 2.0 and with authentic standards available at Royal Botanic Gardens, Kew. Compounds were quantified using calibration curves with authentic standards based on UV absorbance or compound ionisation in the mass spectrometer. Where these were not available, compound quantities were determined using standard curves calculated from the UV absorbance of compounds with the same chromphore.
These methods also permitted the detection of two amino acids, phenylalanine and tryptophan. For convenience, we used the term ‘total amino acids’ to refer to the two amino acids only detected and quantified. Compound concentrations are for nectar expressed in μmol L-1 original volume, and for pollen in μmol kg-1 dry mass. We refer to 3-, 4-, and 5-O-caffeoylquinic acids (CQAs) collectively as chlorogenic acids, and specifically to 3-CQA as chlorogenic acid.
Our LC-MS analysis identified CQAs as principal secondary metabolites in nectar based on their mass spectral properties, which were similar to those reported previously for these compounds (Schütz et al., 2004). This identification was supported by comparison with authentic standards from our own in-house (Jodrell Laboratory) collection, and their accurate mass pseudomolecular ion of m/z 355.1038 which gave a protonated molecular formula [M+H]+ of C16H19O9. CQAs are esters of caffeic acid with a hydroxylated cyclohexanoic acid, quinic acid (Schütz et al., 2004). Our analysis also identified an additional caffeic acid ester in nectar of V. corymbosum which had a similar UV spectrum to the CQAs (indicative of a caffeoyl moiety). However, this ester recorded a pseudomolecular ion of m/z 337.0926 (calcd. 337.0918), corresponding to a protonated molecular formula [M+H]+ of C16H17O8, suggesting a dehydro derivative of CQA. Further comparison of the (-) MS2 data showed major fragment ions at m/z 291 and 179, indicative of caffeoylshikimic acids as reported in Phoenix dactylifera (Habib et al., 2014). Subsequent co-elution with an authentic laboratory standard and (-) MS2 showing similar fragments at 179, 135, 161, and 291 allowed assignment of the compound to 4-O-caffeoylshikimic acid (4-CSA).
As per our chemical analysis, 4-CSA largely differentiated wild and cultivated plants of V. corymbosum (see section “Results”), and thus warranted further biological evaluation. We tested chlorogenic acid (3-CQA) as a proxy for this compound, since no commercial source of 4-CSA was available, and different caffeic acid esters typically show equivalent biological activities (Guzman, 2014), likely owing to their common ortho-dihydroxy substitution (Stevenson et al., 1993). For example, in bioassays against various species of bacteria and yeast, 3-CQA, 5-CQA, and other caffeic acid esters frequently possessed identical minimum inhibitory concentration (MIC) values (Zhu et al., 2004; Xia et al., 2011; Guzman, 2014).
Bioassays were conducted with workers of Bombus impatiens parasitized with Crithidia. To test how 3-CQA affected Crithidia infection, we experimentally infected Bombus impatiens workers before feeding them 30% sucrose solution without or with 3-CQA (C16H18O9; molecular weight 354.31 g/mol; CAS number 327-97-9; Sigma-Aldrich, St. Louis, MO, United States) at two concentrations, low (2.8 μM [1 ppm] 3-CQA dissolved in 30% sucrose solution), and high (56.5 μM [20 ppm] dissolved in 30% sucrose solution). These represented low and high concentrations of 4-CSA found naturally in cultivated (4.44 μM ± 7.52 SD) and wild (22.03 μM ± 27.46 SD) plants, respectively (see section “Results”).
Thirty worker bees were tested per treatment (n = 90 bees total), evenly spread across three source colonies (Biobest Canada, Leamington, Canada; 10 bees per source colony per treatment). Bees were removed from their source colonies, randomly assigned to treatments, starved for 4–6 h, and then experimentally infected with Crithidia on the same day. Crithidia were isolated from wild B. impatiens collected in Massachusetts, United States (GPS coordinates: 42°24′25″N, 72°31′46″W), and maintained in B. impatiens colonies in the laboratory. Crithidia inoculum was prepared according to a standard protocol (Richardson et al., 2015). In brief, on the day of experimental inoculation, 10 workers were sacrificed from the infected colonies and their intestinal tracts ground individually in 300 μl of dH20. After vortexing the samples, we allowed them to settle at room temperature for 4 h. We then placed 10 μl of the clear solution on a haemocytometer and examined it for Crithidia. For samples that contained Crithidia, we transferred 50–150 μl of solution to a clean container and diluted to make an approx. 30% sucrose solution with 6000 Crithidia cells × 10 μl-1. Each bee was provisioned with 10 μl of inoculum containing 6000 Crithidia cells, which is within the range of concentrations of the pathogen that bees encounter (e.g., Schmid-Hempel and Schmid-Hempel, 1993; Otterstatter and Thomson, 2006). Immediately following inoculation, bees were housed individually in plastic containers. They were given one lump of honey bee collected pollen (Koppert Biological Systems, Howell, MI, United States) mixed with sugar water, and new sugar solution ad libitum of the appropriate treatment. Pollen and nectar were replaced daily for 7 days.
After 7 days, we sacrificed individuals and prepared intestinal tracts as when making Crithidia inoculum. We placed 10 μl of intestinal solution on a haemocytometer and counted the number of Crithidia cells in five subfields of the grid at 10x magnification, totalling 0.02 μl, with a dissecting microscope. We summed these counts and calculated the number of Crithidia cells per mL for each bee. We also removed the right forewing of each bee, mounted it on a microscope slide, and measured the radial cell length as an estimate of bee size (Harder, 1982).
Comparisons of chemical composition between wild and cultivated plant nectar and pollen were made on both an absolute and proportional basis, which describe two different aspects of composition. Hence, the quantitative concentration of compounds was used directly in the absolute composition analysis, whereas the relative concentration of compounds (their percent contribution on a molar basis relative to other compounds in the sample) was used in proportional composition analysis. For this, a proportional dataset was generated in which compound concentrations within a sample row were standardised by dividing each by the row total. Absolute composition was examined by plotting principle components analysis (PCA) ordinations using the ‘rda’ function in the R package ‘vegan’ (Oksanen et al., 2017). PCA provided good representation of the multivariate composition of nectar and pollen, in which the cumulative proportion of variance explained by the first two components was 0.84 and 0.65, respectively. For proportional analyses, unconstrained NMDS (Non-metric multidimensional scaling) ordinations were fitted and plotted based on a Bray-Curtis distance metric in ‘vegan.’
As a complement to ordinations, the hypothesis that wild and cultivated plants differed in nectar and pollen chemical composition was explicitly tested using permutational multivariate ANOVAs (function ‘adonis’ in vegan). For this, distance matrices were first generated for absolute composition based on Euclidean distance (to better preserve quantitative relationships), and for proportional composition using Bray–Curtis distance. Similar to univariate ANOVA, this technique relies on the assumption of multivariate homogeneity between groups (i.e., between wild and cultivated plants). According to homogeneity of multivariate dispersions tests (vegan function ‘betadisper’), this assumption was met for all variables except nectar proportional composition. As an alternative to permutational multivariate ANOVA, we therefore opted to compare nectar proportional composition based on axis scores extracted from NMDS ordination. NMDS axis 1 and 2 scores provided a reduced dimensionality representation of proportional composition, and were each fitted as a univariate response in generalised linear mixed models (GLMMs) using the R package ‘nlme’ (Pinheiro et al., 2017). The GLMMs were fitted with an explanatory variable of ‘origin’ (wild or cultivated), and a nested random effect of group ID (cultivar or population name). A variance structure was added to both models via the ‘weights’ function of nlme, to control for unequal variances between wild and cultivated plants. Models were otherwise validated by examining standardised residuals for normal distribution.
‘Indicator compound analysis’ (or multilevel pattern analysis) was implemented using R package ‘indicspecies’ (Cáceres and Legendre, 2009) to further probe any potential differences in chemical composition between wild and cultivated plants. This analysis used a permutational approach to test for compound association (or differences in abundance) between different groups (De Cáceres, 2013), in our case wild and cultivated plants. Compound association values (i.e., point-biserial correlation coefficients) and their corresponding p-values (testing the null hypothesis that the correlation is zero) were generated using the ‘multipatt’ function, based on 999 permutations, and use of a corrected correlation coefficient (specified as ‘r.g’ in multipatt). This correlation coefficient is similar to Pearson correlation, and is likewise bounded between zero and one.
Chemical diversity was assessed both in terms of compound richness (the total number of compounds per sample), and through calculation of a chemical diversity index (H′C) per sample, following previous authors (Epps et al., 2007; Meier and Bowman, 2008). Calculation of this index used the same equation as for Shannon–Wiener diversity (computed using the vegan ‘diversity’ function). Hence, H′C took into account information on compound concentration as well as compound richness. We used linear mixed models (LMMs) to examine potential differences in H′C between wild and cultivated plants for nectar and pollen. LMMs were fitted using the nlme package, specifying H′C as a response, ‘origin’ (cultivated or wild) as a fixed effect, and group ID (cultivar or population name) as a nested random effect. We used ANOVAs to test for significant differences in H′C between cultivars, as well as between wild populations. All models were validated by checking residuals for equal variance and normal distribution.
We examined genetic variation, genetic correlation, and heritability (as described below) for the six cultivars sampled at Cold Spring Orchard for six traits summarising nectar and pollen chemistry: chemical diversity (H′C), chemical composition (i.e., NMDS axis 1 scores), total amino acids, total phenolic acids, total flavonols, and total flavan-3-ols. The latter were only included for pollen analyses, as these compounds were not identified in nectar. Chemical composition was quantified via NMDS, as described above. Since our estimates of genetic variation (VG) and covariance (CovG) were based on the use of clones, which therefore included both additive and other genetic effects, these are considered total genetic estimates (de Araújo and Coulman, 2004). We hence report estimates of total genetic variation, total genetic correlation, and broad-sense (or clonal) heritability (H2) – the genetic contribution to cultivar phenotypic variance. High values of H2 hence indicate that a focal trait is largely under genetic control, whereas low values mean that most phenotypic variance is due to environmental factors.
Genetic variation and heritability were analysed by fitting variance component (or random effects) models using the R packages ‘rptR’ (Stoffel et al., 2017), ‘lme4,’ and its extension ‘lmerTest’ (Kuznetsova et al., 2017). Each trait was fitted as a univariate response, and ‘cultivar’ as a random effect. We used the ‘rand’ function in lmerTest to assess the magnitude and significance of genetic variation, based on the chi-squared (χ2) statistic, and its corresponding p-value from a likelihood ratio test. From the same model, the ‘rpt’ function in rptR was used to provide estimates of H2 and its standard error. A bootstrapping procedure (n = 1000 iterations) was implemented within the function to test significance for H2.
Genetic correlations between traits were analysed using Bayesian multivariate mixed models, implemented in the package ‘MCMCglmm’ (Hadfield, 2010). For within-material correlations (e.g., between total amino acids and total flavonols), two models were fitted; one which included all nectar traits as a multivariate response, and a second including all pollen traits. For between-material correlations (e.g., between total amino acids across nectar and pollen), a combined model was fitted which included all nectar and pollen traits as a multivariate response. Use of multivariate models in this sense allowed genetic variance (VG) and covariance (CovG) components to be estimated for and between traits, respectively. Based on these components, genetic correlation (rG) was calculated for a given set of traits, A and B, as: rG = CovG(A,B)/sqrt[VG(A), VG(B)]. Following Houslay and Wilson (2017), we reported the posterior mean of rG estimates, and assessed the statistical support for rG through plotting 95% credible intervals for the posterior distribution. When this interval did not include zero, we considered genetic correlations to be statistically supported. All traits were fitted as a Gaussian response, aside from nectar total amino acids and total phenolic acids, for which an ‘exponential’ family was used. For each model, we employed uninformative parameter-expanded priors for the random effects, and allowed for different trait means, unstructured (co)variances, and trait-specific intercepts. The within-material and between-material models were run for 160,000/1,500,000 iterations, respectively, discarding the first 10,000 iterations as burn-in, and sampling every 75 iterations thereafter. Convergence was assessed by means of the Gelman-Rubin criterion, in which four chains were run to check that they converged to the same posterior distribution. As additional diagnostics, we examined trace and autocorrelation plots for consistent variation and non-autocorrelation between iterations, respectively, and checked that posterior estimates were robust to different starting priors.
As an alternative to direct comparisons of genetic variation in traits between wild and cultivated plants (which was not possible since we have no basis to partition genetic variation from phenotypic variation in wild plants), we compared the phenotypic variability of nectar and pollen composition. Phenotypic variability in chemical composition was measured as the Euclidean distance (or dispersion) of plant individuals from their respective cultivar/population multivariate centroid, following the similar use of this approach in community ecology (Anderson et al., 2006). Hence, to examine whether phenotypic variability differed between wild and cultivated plants, we extracted individual plant distances to their respective group centroids for both nectar and pollen (using the ‘betadisper’ function in vegan) and fitted these distances as a response variable in LMMs. All other model details then followed the same as for ‘chemical diversity’ above.
To test how 3-CQA affected Crithidia infection, we used a generalised linear mixed model in SAS version 9.4 with Crithidia cells per mL-1 (log x+1 transformed) as the response, nectar treatment (control, low or high 3-CQA) as a fixed effect, bee size as a covariate, and bee source colony as a random effect. Tukey’s HSD tests were used for post hoc pairwise comparisons. Only five bees total (out of 90 bees) died during the course of the experiment, with two deaths in the control treatment, one in the low 3-CQA treatment, and one in the high 3-CQA treatment. These bees were excluded from statistical analysis. Given that so few bees died during the experiment, and that deaths were spread across all treatments, no analysis of mortality was conducted.
Across nectar and pollen samples from wild and cultivated plants, we identified 20 compounds belonging to five chemical classes (12 flavonols, 3 caffeic acid esters, 2 amino acids, 2 flavan-3-ols, and 1 norisoprenoid; Supplementary Table 2). Nectar contained 9 compounds, pollen 18, and 7 were shared across the material types. Chemical composition of wild and cultivated plants of V. corymbosum was highly distinct for nectar (permutational MANOVA: F = 10.5, p < 0.001) and pollen (F = 15.0, p < 0.001; Figures 1A,B). Indicator compound analysis revealed six nectar compounds and nine pollen compounds which significantly differentiated these groups (Table 1). Of these compounds, a majority (4 out of 6 in nectar; 5 out of 9 in pollen) were more positively associated with wild plants, including the caffeic acid ester 4-O-caffeoylshikimic acid (4-CSA). In terms of proportional chemical composition, nectar of wild and cultivated plants was moderately distinct (LMM – NMDS axis 1: F = 0.05, p = 0.828; NMDS axis 2: F = 13.6, p = 0.005; Figure 1C), whereas large differences existed between groups for pollen (permutational MANOVA: F = 30.0, p < 0.001; Figure 1C).
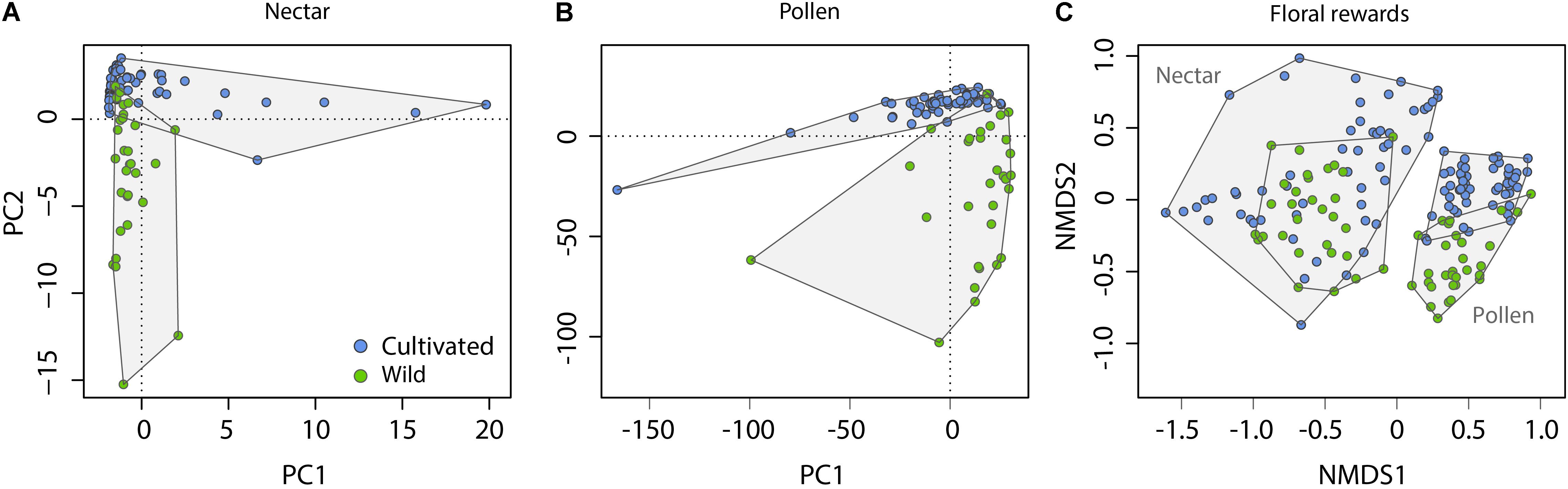
FIGURE 1. Differentiation of nectar and pollen chemical composition in relation to plant origin (Cultivated vs. Wild). Information on the quantitative concentration of compounds was used to construct PCA ordinations for absolute composition (A,B), whereas relative concentrations of compounds (their percent contribution relative to other compounds in the sample) was used to construct NMDS ordinations for proportional composition (C). Outlying data points in (A) are cultivar ‘Spartan’ (four rightmost points) and ‘Northland’; and in (B) are cultivar ‘Pioneer.’
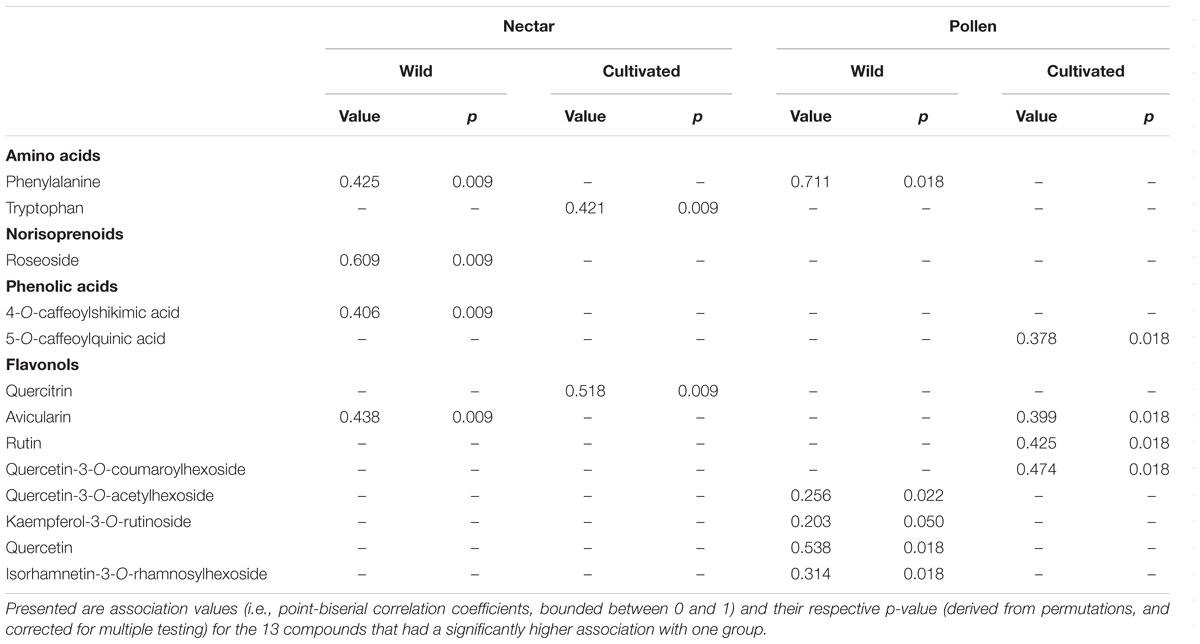
TABLE 1. ‘Indicator compound analysis’ of nectar and pollen chemical composition in wild and cultivated plants of Vaccinium corymbosum.
Wild plant nectar consistently lacked the flavonoid glycoside quercitrin, which was abundant in all cultivated plants examined (Supplementary Table 1). In contrast, the ester 4-CSA occurred in all wild plants sampled (n = 30), but in only 84% of cultivated plants (n = 55; Supplementary Table 2). As a whole, the chemical diversity index of wild (H′C = 1.59 ± 0.21 SE) and cultivated (H′C = 1.46 ± 0.13) plants did not differ significantly (LMM: t = 0.52, df = 9, p = 0.618; Figure 2A inset). Significant differences in nectar chemical diversity index were, however, seen between individual cultivars (ANOVA: F7,47 = 11.76, p < 0.001; Figure 2A), but not between wild populations (F2,27 = 0.71, p = 0.501; Figure 2A).
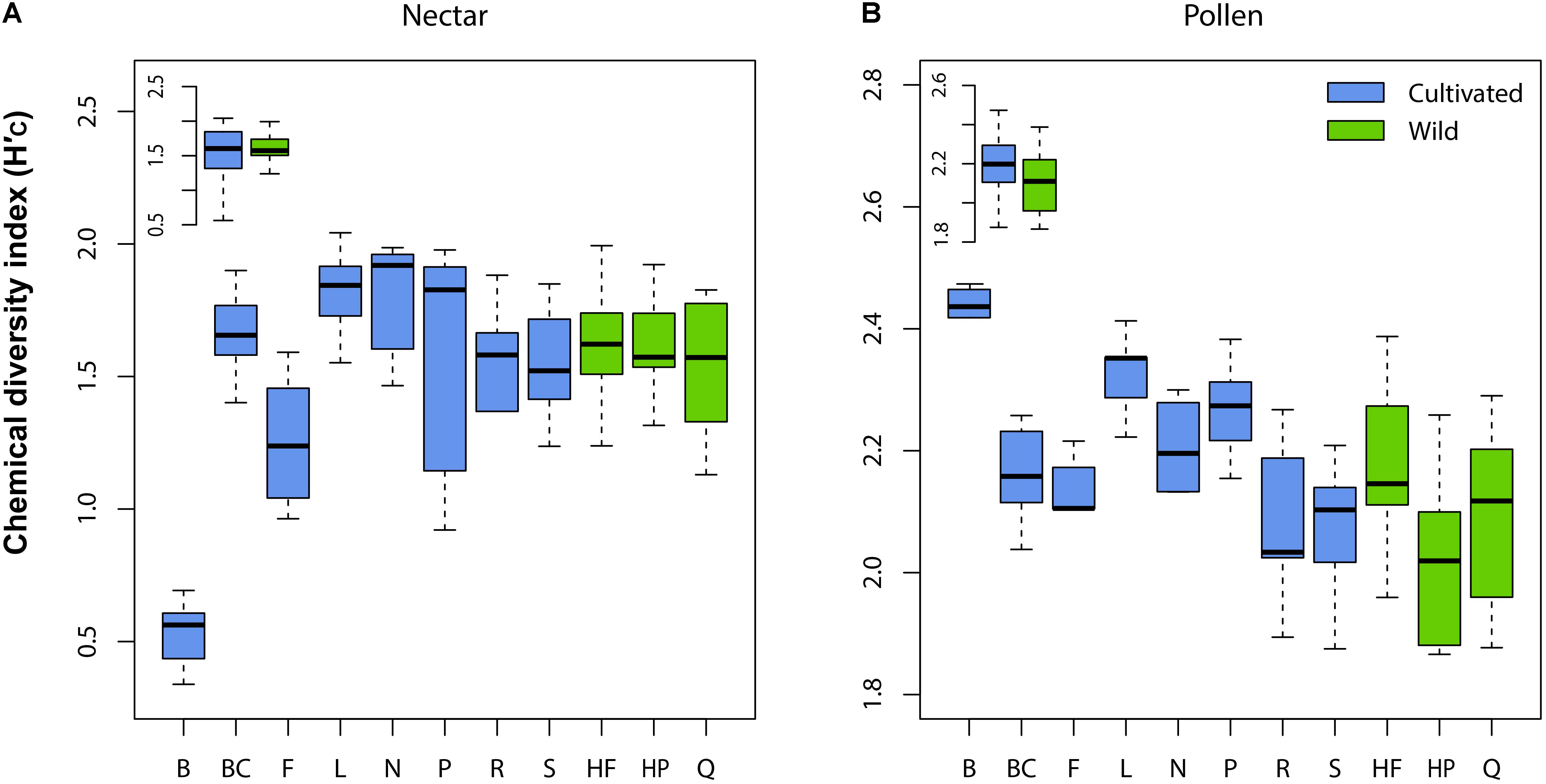
FIGURE 2. Variation in chemical diversity index (H′C) across cultivars and wild populations of Vaccinium corymbosum for nectar (A) and pollen (B). Significant differences between cultivars were observed for both material types, but not between wild populations. No differences in H′C existed overall between wild and cultivated plants (inset in A,B). Abbreviations for cultivars/populations are listed in Methods.
Pollen samples of several wild plants contained four flavonoid glycosides absent in cultivated plants (including glycosides of kaempferol, quercetin, and isorhamnetin). However, because these compounds occurred only sporadically (present in only 7-23% of cases; Supplementary Table 2), we found no significant difference in pollen chemical diversity index between wild (H′C = 2.10 ± 0.17 SE) and cultivated (H′C = 2.19 ± 0.22) plants (LMM: t = 1.32, df = 9, p = 0.220; Figure 2B inset). Similar to nectar, pollen chemical diversity index differed significantly across individual cultivars (ANOVA: F7,46 = 7.94, p < 0.001; Figure 2B), but not between wild populations (F2,27 = 2.75, p = 0.082).
For both nectar and pollen, we observed high levels of total genetic variation and broad-sense heritability among cultivars for most chemical traits (Table 2). Notable exceptions included total amino acids in pollen, and total phenolic acids and total flavonols in nectar, which exhibited little to no genetic variation. Weak to moderate genetic correlations were observed between nectar traits, in contrast to mostly strong genetic correlation in pollen traits (Table 2). Between-material genetic correlations (conducted for the same trait across nectar and pollen) were non-significant for all traits (Table 2). While direct comparison of genetic variation between wild and cultivated plants was not possible, differences in the phenotypic variability of chemical composition were significant for both nectar (LMM: t = 2.54, df = 9, p = 0.032; Figure 3A inset) and pollen (t = 2.59, df = 9, p = 0.029; Figure 3B inset). Cultivated plants exhibited 62.1 and 47.1% less phenotypic variation than wild plants for nectar and pollen, respectively. Thus, more variation could typically be found within single wild populations than in the majority of cultivars examined (Figures 3A,B).
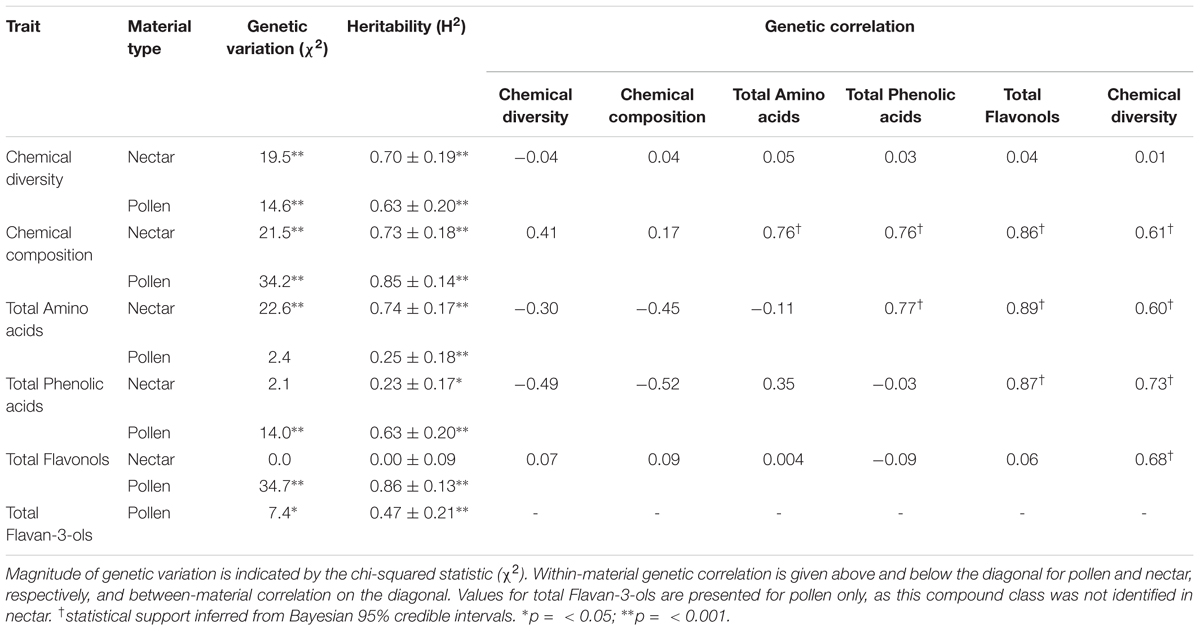
TABLE 2. Genetic variation, broad-sense heritability, and genetic correlation of nectar and pollen chemistry across six Vaccinium corymbosum cultivars (‘Bonus,’ ‘Friendship,’ ‘Liberty,’ ‘Northland,’ ‘Patriot,’ ‘Reka’ – n = 5 plants per cultivar, n = 4 for ‘Northland’).
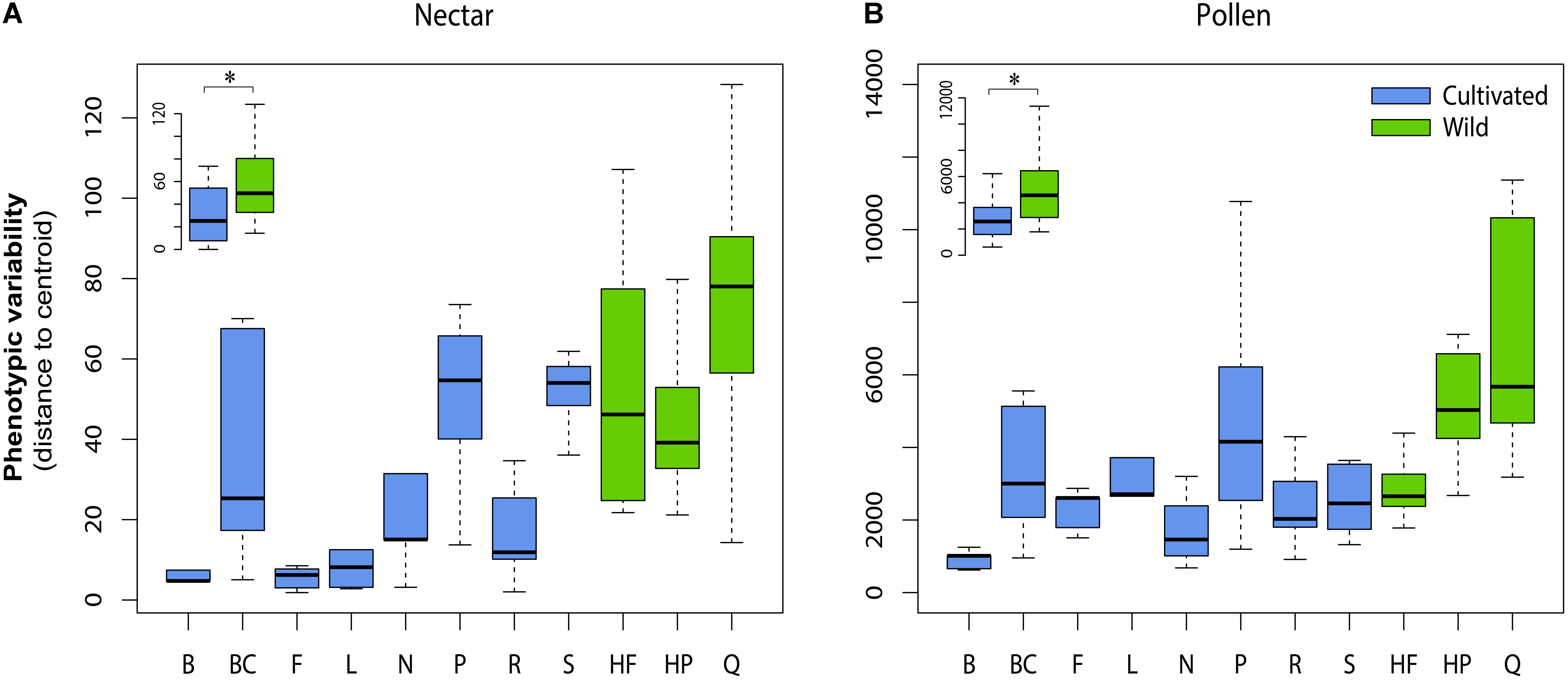
FIGURE 3. Phenotypic variability in the chemical composition of cultivars and wild populations of Vaccinium corymbosum for nectar (A) and pollen (B). Phenotypic variability was measured as the Euclidian distance (or dispersion) of plant individuals from their respective cultivar or population multivariate centroid. Significant differences existed overall between wild and cultivated plants for nectar and pollen (inset in A,B). Cultivar/population abbreviations are listed in Methods. ∗p = < 0.05.
Following dietary consumption of 3-CQA by bumble bees parasitised with the gut pathogen Crithidia, higher concentrations of 3-CQA significantly reduced infection (F2,79 = 4.15, p = 0.020). Pathogen load was on average reduced by 27% in the high 3-CQA treatment, as compared to a sucrose control or low 3-CQA (Figure 4). The covariate of bee size was not related to infection (F1,80 = 0.03, p = 0.856).
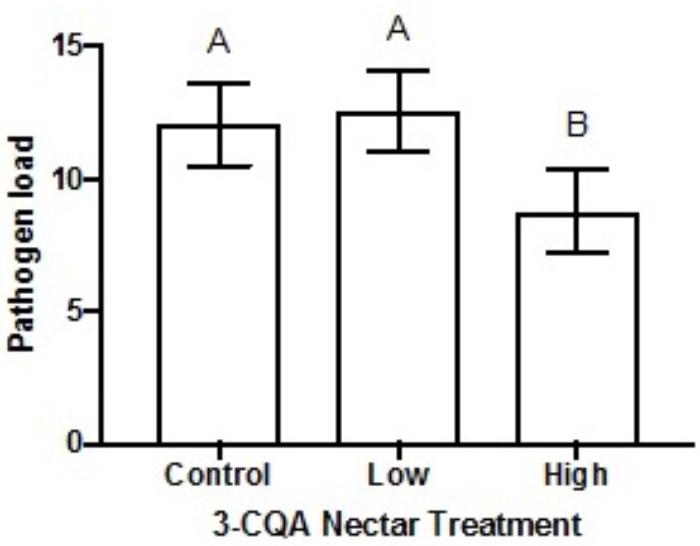
FIGURE 4. Mean pathogen load of Crithidia (log-transformed Crithidia per mL + 1) in Bombus impatiens workers ( ± SEM) given different dietary concentrations of 3-O-caffeoylquinic acid (3-CQA). The control treatment contained 30% sucrose solution. The low treatment corresponded to 2.8 μM ( = 1 ppm) 3-CQA dissolved in 30% sucrose solution, and the high treatment to 56.5 μM ( = 20 ppm) dissolved in 30% sucrose solution. Different upper-case letters above bars indicate statistically significant differences at p < 0.05.
Our study provides the first evidence that crop domestication can significantly alter floral reward chemistry, and that such alteration can potentially affect pollinator health via changes in pathogens. While several aspects of nectar and pollen chemistry differed markedly between cultivated and wild plants, this pattern did not hold for all chemical traits, indicating that some traits appeared robust to the domestication process. Of the four hypotheses initially posed, we found at least partial evidence to support each, as discussed in the following sections.
As hypothesised, wild and cultivated plants of V. corymbosum were differentiated in terms of nectar and pollen chemical composition. However, only pollen had reduced chemical diversity due to domestication; cultivated pollen lacked four flavonoid glycosides which were found in wild pollen. Of the 13 nectar and pollen compounds identified as underlying this differentiation, a majority were positively associated with (or more abundant in) wild plants. However, some examples of the opposite pattern (i.e., greater association with cultivated plants) were also observed for most compound classes (amino acids, phenolic acids, and flavonols), indicating that these differences did not follow a fixed biosynthetic pattern. Flavan-3-ols (catechin and epicatechin, identified only in pollen) did not specifically associate with either group. Of the compounds that differentiated wild or cultivated plants, most did so only for a single material (nectar or pollen). Only two compounds did so for both; phenylalanine showed a consistent pattern across nectar and pollen (positively associated with wild plants for both), whereas the flavonol avicularin showed opposite patterns in nectar and pollen (positive associations with wild plant nectar and cultivated plant pollen).
These results indicate that although domestication effects on the chemical composition and diversity of floral rewards were clear, it is difficult to make a priori predictions about which compounds (or compound classes) are affected and in which reward. This difficulty is surprising given the excellent knowledge of the domestication process for highbush blueberry (Hancock and Siefker, 1982; Gough, 1993; Ehlenfeldt, 1994, 2009), in which cultivar provenances, and the characteristics selected for by breeders, are unambiguously known. Selection has typically targeted fruit quality traits such as size, colour, firmness, flavour, and in more recent times, antioxidant content (Kalt et al., 2001). Among various antioxidants, blueberries are a rich source of chlorogenic acid (3-CQA) and anthocyanins, and these compounds correlate with each other in fruit across a wide range of blueberry cultivars (Yousef et al., 2016). Given ongoing selection for rich fruit colour – and by extension 3-CQA – it is reasonable to expect that other caffeic acid esters should increase in floral rewards with domestication. While our results indicated this is the case for 5-CQA in pollen, domestication instead appears to have reduced expression of 4-CSA in nectar. This finding also suggests that fruit chemistry is probably not an accurate proxy for floral reward chemistry. As a whole, given its general unpredictability, we suggest that floral reward chemistry deserves to be monitored in its own right as part of the crop improvement process for blueberry, and possibly entomophilous crops more generally.
The majority of nectar and pollen traits examined in cultivars exhibited significant genetic variation and broad-sense heritability, as predicted. The existence of genetic variation in traits is an important prerequisite for selective breeding. In principle, its occurrence offers breeders the potential to fine-tune floral reward chemistry, should such a need be identified. However, genetic correlations (or pleiotropy) may otherwise complicate this process (Luby and Shaw, 2009), and mean that selection cannot be precisely targeted to a trait without causing correlated responses in others, regardless of whether these are desired or not. However, based on two lines of evidence, our findings suggested that different genetic architectures underlie most nectar and pollen chemical traits examined. First, large differences in genetic variation existed for some of the same chemical traits in nectar and pollen; and, second, a majority of within- and between-material genetic correlations were weak or insignificant. Both findings suggest differential genetic regulation of these particular traits, and that pleiotropy does not impose a major constraint. Several pollen chemical traits were exceptions, however, in which large within-material genetic correlations indicate that selection for one trait cannot be achieved independently of others.
Other agronomic metrics of concern to plant breeders include trait and cultivar stability. Here, partitioning phenotypic variance into its total genetic and environmental components allowed us to calculate broad-sense heritability (H2). H2 also serves as a relative measure of trait stability; low values indicate that most phenotypic variance is the result of environmental plasticity. Somewhat contrary to the prevailing view of nectar traits as particularly plastic (Mitchell, 2004; Parachnowitsch et al., 2018), nectar H2 values were mostly high, and generally equivalent to those of pollen. However, total flavonols in nectar were especially unstable (i.e., subject to large environmental variation), as opposed to possessing high stability in pollen. ‘Bonus’ and ‘Friendship’ were the most stable cultivars, based on low variability in their multivariate chemical composition of nectar and pollen. These cultivars hence represent good candidates for cultivation, should stability in floral reward chemistry be an important criterion. Interestingly, the effect of interspecific breeding (to produce hybrid or introgressed cultivars) on stability was not consistent. ‘Friendship’ (a natural wild-collected hybrid between highbush and lowbush blueberry) was one of the most compositionally stable cultivars, whereas ‘BlueCrop’ and ‘Patriot’ (which consist of a 6.4 and 28% genetic contribution from lowbush blueberry, respectively (Lobos and Hancock, 2015) – Supplementary Table 1) were the least stable.
Although we could not directly assess the genetic merit of the wild populations sampled, these likely represent good sources of genetically diverse germplasm. The cultivated genepool of V. corymbosum has not suffered genetic erosion to the same extent as more distantly domesticated crops, although a narrowing in diversity has nonetheless occurred in recent times (Boches et al., 2006). As discussed, we found little to no genetic variation for a minority of nectar and pollen traits examined, which limits their breeding potential. Given this situation, collection of wild germplasm with the aim to enhance genetic variation in floral reward chemistry could be a desirable and feasible prospect.
There was evidence to support our hypothesis that domestication effects on antimicrobial compounds in nectar (i.e., 4-CSA in V. corymbosum) could impact pollinator health via changes in pathogens. We found concentration-dependent effects of a nectar secondary compound on the gut pathogen Crithidia. Low levels of 3-CQA (as a chemically equivalent proxy for 4-CSA at its typical levels in cultivated plants) had no effects on pathogen counts, while high concentrations (within the natural range for 4-CSA in wild plants) significantly reduced Crithidia relative to low and control solutions. Our results extend prior research documenting the in vivo medicinal value of some secondary metabolites against Crithidia (Manson et al., 2010; Richardson et al., 2015). These effects may reflect the effects of these compounds on the bee immune system rather than direct toxicity to parasite cells, given that up to 2500 ppm 3-CQA had no direct effects on Crithidia growth in cell culture (Palmer-Young et al., 2016). Moreover, our results are consistent with other studies showing concentration-dependent effects of plant secondary metabolites on insects and their pathogens (Hunter and Schultz, 1993; Cory and Hoover, 2006; Mao et al., 2013; Palmer-Young et al., 2017). Future work should address whether high dietary consumption of 3-QCA or 4-CSA would benefit bee health via reduced pathogen load in natural settings, and whether there are sublethal costs of consuming caffeic acid esters over short and long time-periods. Nonetheless, utilising wild germplasm to breed new cultivars with enhanced levels or profiles of nectar antimicrobials may hold potential.
Beyond effects on pathogens, other facets of pollinator physiology, ecology, and behaviour are also likely influenced by domestication effects on floral reward chemistry. We observed significantly higher associations of the amino acid phenylalanine with wild plant nectar and pollen. The average concentration of this amino acid in wild plant nectar was nearly 4.5 times that of cultivated plants, and nearly 11 times that of cultivated plants for pollen (Supplementary Table 2). Phenylalanine is one of several essential amino acids in bee diets (de Groot et al., 1953). However, arguably its most important function is its strong phagostimulatory quality to bees (Inouye and Waller, 1984; Petanidou et al., 2006), believed to enhance nectar taste (Gardener and Gillman, 2002), and influence bee foraging preferences and behaviour at the community scale (Petanidou, 2007). Reduced phenylalanine levels in the nectar and pollen of cultivated V. corymbosum suggest large potential ramifications for pollinator attraction and crop pollination success. For instance, several highbush cultivars are relatively unattractive to bees (Filmer and Marucci, 1964; Huber, 2016). The reasons for this are unknown, but result in the need for ‘saturation pollination’ with honey bees (Gough, 1993). A diminished phagostimulatory quality of floral rewards provides one possible explanation for these observations. This hypothesis warrants further investigation.
The potential repercussions of crop domestication for pollinators, mediated through floral reward chemistry, have hitherto remained unexamined for animal-pollinated crops. Our findings on highbush blueberry suggest this topic warrants much wider attention for crops more generally. We therefore suggest future research in four complementary areas.
Establishing the overall scale and specific trends in domestication effects on floral reward chemistry across different types of crop plant would be of great or considerable interest, from both a fundamental and applied perspective. Is alteration of floral reward chemistry a typical outcome of domestication? Does the magnitude of effect depend on the identity of the crop organ(s) artificially selected, as is the case for effects on plant resistance (Whitehead et al., 2017)?
While the differential attractiveness of certain crop cultivars to pollinators is a well-known phenomenon (Henning et al., 1992; Klatt et al., 2013; Huber, 2016), to what extent could this be related to altered floral reward chemistry, potentially acting in concert with other floral traits? Conversely, could domestication have served to improve floral rewards, such as by reducing toxic or deterrent compound levels [analogous to the response of nectar toxins to plant invasion (Egan et al., 2016; Tiedeken et al., 2016)], and/or boosting levels of attractive or beneficial ones (e.g., pertaining to nutrition, disease resistance, behaviour, phagostimulation)? Are acute impacts of altered floral reward chemistry readily detectable for pollinators, or are negative effects more likely to be chronic, or only apparent in a context-specific manner such as due to disease (de Roode et al., 2013)?
Elucidation of the physiological and genetic mechanisms through which domestication can alter trait function would permit a deeper understanding of this process in crops. For instance, are some compound classes more likely to be affected than others? Does floral reward chemistry usually show independent genetic regulation, or genetic constraints, which may render it less or more robust to alteration than other plant tissues?
As has been recognised for other nectar-related traits (Prasifka et al., 2018), tailoring nectar and pollen chemistry for enhanced attraction and functional benefits for pollinators could represent a promising future direction for crop improvement and selective breeding. What are the practical implications for breeding? Can such benefits be unambiguously demonstrated in field settings? What role could wild germplasm play in helping to restore floral rewards which show diminished chemical function, as for other mutualism-related chemical traits (Stenberg et al., 2015)?
As a whole, progress on the above areas would offer great insight into crop domestication effects on floral reward chemistry, and the implications and potential breeding solutions for pollinators and crop pollination.
All datasets and R code used in this study will be made publicly available on Figshare.
LA, RI, PS, and PE conceived and designed the research. IF and PS performed the chemical analyses. PE analysed the data. PE wrote the manuscript with input and contributions from all authors.
This research was funded by the National Science Foundation (NSF: nsf.gov) (NSF DEB-1258096 to LA and PS, and NSF DEB-1256817/1638866 to RI), by the United States Department of Agriculture (USDA: usda.gov) [Cooperative State Research, Education, and Extension Service (CSREES) National Research Initiative (NRI) Arthropod and Nematode Biology and Management Program of the Grant USDA-AFRI 2013-02536 to LA, RI and PS; and Agricultural and Food Research Initiative (AFRI) Food, Agriculture, Natural Resources and Human Sciences Education and Literacy Initiative (ELI) Predoctoral Fellowship Award Number: 2016-67011-24698 to ECPY]. Additional financial support to PS from the Peter Sowerby Foundation is acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Biobest for donating bumble bee colonies; N. Milano for coordinating field collections of nectar and pollen; P. Anderson, K. Brevik, A. Hogeboom, J. Giacomini, and K. Staple for assistance with field collection, SJ Giacomini and J. Giacomini for laboratory assistance with the bumble bee infection experiment, and A. Brankin for chemical analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01357/full#supplementary-material
Adler, L. S. (2000). The ecological significance of toxic nectar. Oikos 91, 409–420. doi: 10.1371/journal.pone.0154381
Adler, L. S., and Irwin, R. E. (2005). Ecological costs and benefits of defenses in nectar. Ecology 86, 2968–2978. doi: 10.1890/05-0118
Adler, L. S., Karban, R., and Strauss, S. Y. (2001). Direct and indirect effects of alkaloids on plant fitness via herbivory and pollination. Ecology 82, 2032–2044. doi: 10.1086/303374
Adler, L. S., Seifert, M. G., Wink, M., and Morse, G. E. (2012). Reliance on pollinators predicts defensive chemistry across tobacco species. Ecol. Lett. 15, 1140–1148. doi: 10.1111/j.1461-0248.2012.01838.x
Adler, L. S., Wink, M., Distl, M., and Lentz, A. J. (2006). Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 9, 960–967. doi: 10.1111/j.1461-0248.2006.00944.x
Anderson, M. J., Ellingsen, K. E., and McArdle, B. H. (2006). Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 9, 683–693. doi: 10.1111/j.1461-0248.2006.00926.x
Arnold, S. E., Idrovo, M. E. P., Arias, L. J. L., Belmain, S. R., and Stevenson, P. C. (2014). Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. J. Chem. Ecol. 40, 878–881. doi: 10.1007/s10886-014-0467-4
Bai, Y., and Lindhout, P. (2007). Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann. Bot. 100, 1085–1094. doi: 10.1093/aob/mcm150
Bailes, E. J., Ollerton, J., Pattrick, J. G., and Glover, B. J. (2015). How can an understanding of plant–pollinator interactionscontribute to global food security? Curr. Opin. Plant Biol. 26, 72–79. doi: 10.1016/j.pbi.2015.06.002
Baker, H. G. (1977). Non-sugar chemical constituents of nectar. Apidologie 8, 349–356. doi: 10.1051/apido:19770405
Baracchi, D., Brown, M. J. F., and Chittka, L. (2015). Behavioural evidence for self-medication in bumblebees? F1000Res 4:73. doi: 10.12688/f1000research.6262.3
Barlow, S. E., Wright, G. A., Ma, C., Barberis, M., Farrell, I. W., Marr, E. C., et al. (2017). Distasteful nectar deters floral robbery. Curr. Biol. 27, 2552.e3–2558.e3. doi: 10.1016/j.cub.2017.07.012
Boches, P., Bassil, N. V., and Rowland, L. (2006). Genetic diversity in the highbush blueberry evaluated with microsatellite markers. J. Am. Soc. Horticult. Sci. 131, 674–686.
Brown, J., and Rant, J. (2013). Fitness costs and trade-offs of disease resistance and their consequences for breeding arable crops. Plant Pathol. 62, 83–95. doi: 10.1111/ppa.12163
Brown, M. J., Schmid-Hempel, R., and Schmid-Hempel, P. (2003). Strong context-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. J. Anim. Ecol. 72, 994–1002. doi: 10.1046/j.1365-2656.2003.00770.x
Cáceres, M. D., and Legendre, P. (2009). Associations between species and groups of sites: indices and statistical inference. Ecology 90, 3566–3574. doi: 10.1890/08-1823.1
Chaplin-Kramer, R., Dombeck, E., Gerber, J., Knuth, K. A., Mueller, N. D., Mueller, M., et al. (2014). Global malnutrition overlaps with pollinator-dependent micronutrient production. Proc. R. Soc. Lond. B Biol. Sci. 281:20141799. doi: 10.1098/rspb.2014.1799
Chen, Y. H., Gols, R., and Benrey, B. (2015). Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 60, 35–58. doi: 10.1146/annurev-ento-010814-020601
Cook, D., Manson, J. S., Gardner, D. R., Welch, K. D., and Irwin, R. E. (2013). Norditerpene alkaloid concentrations in tissues and floral rewards of larkspurs and impacts on pollinators. Biochem. Syst. Ecol. 48, 123–131. doi: 10.1016/j.bse.2012.11.015
Cory, J. S., and Hoover, K. (2006). Plant-mediated effects in insect–pathogen interactions. Trends Ecol. Evol. 21, 278–286. doi: 10.1016/j.tree.2006.02.005
de Araújo, M. R. A., and Coulman, B. (2004). Genetic variation and correlation of agronomic traits in meadow bromegrass (Bromus riparius Rehm) clones. Ciênc. Rural 34, 505–510. doi: 10.1590/S0103-84782004000200026
De Cáceres, M. (2013). How to Use the Indicspecies Package (ver. 1.7. 1). Lleida: Centre Tecnològic Forestal de Catalunya.
de Groot, A. P. (1953). Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecol. 3, 197–285.
de Roode, J. C., Lefèvre, T., and Hunter, M. D. (2013). Self-medication in animals. Science 340, 150–151. doi: 10.1126/science.1235824
Dempewolf, H., Baute, G., Anderson, J., Kilian, B., Smith, C., and Guarino, L. (2017). Past and future use of wild relatives in crop breeding. Crop Sci. 57, 1070–1082. doi: 10.2135/cropsci2016.10.0885
Durrer, S., and Schmid-Hempel, P. (1994). Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B Biol. Sci. 258, 299–302. doi: 10.1098/rspb.1994.0176
Egan, P. A., Stevenson, P. C., Tiedeken, E. J., Wright, G. A., Boylan, F., and Stout, J. C. (2016). Plant toxin levels in nectar vary spatially across native and introduced populations. J. Ecol. 104, 1106–1115. doi: 10.1111/1365-2745.12573
Ehlenfeldt, M. K. (1994). The genetic composition and tetrasomic inbreeding coefficients of highbush blueberry cultivars. HortScience 29, 1342–1345.
Ehlenfeldt, M. K. (2009). “Domestication of the highbush blueberry at Whitesbog, New Jersey, 1911–1916,” in Proceedings of the IX International Vaccinium Symposium 810, Oregon, OR, 147–152.
Ellis, A. M., Myers, S. S., and Ricketts, T. H. (2015). Do pollinators contribute to nutritional health? PLoS One 10:e114805. doi: 10.1371/journal.pone.0114805
Epps, K. Y., Comerford, N. B., Reeves, J. B. III, Cropper, W. P. Jr., and Araujo, Q. R. (2007). Chemical diversity–highlighting a species richness and ecosystem function disconnect. Oikos 116, 1831–1840. doi: 10.1111/j.0030-1299.2007.15853.x
Erler, S., and Moritz, R. F. (2016). Pharmacophagy and pharmacophory: mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera). Apidologie 47, 389–411. doi: 10.1007/s13592-015-0400-z
Filmer, R., and Marucci, P. (1964). “Honey bees and blueberry pollination,” in Proceedings 32nd Annals Blueberry Open House. New Jersey Agricultural Experiment Station, Orono, ME, 25–27.
Gardener, M. C., and Gillman, M. P. (2002). The taste of nectar–a neglected area of pollination ecology. Oikos 98, 552–557. doi: 10.1034/j.1600-0706.2002.980322.x
Gegear, R. J., Otterstatter, M. C., and Thomson, J. D. (2005). Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Anim. Behav. 70, 209–215. doi: 10.1016/j.anbehav.2004.09.025
Gegear, R. J., Otterstatter, M. C., and Thomson, J. D. (2006). Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. Lond. B Biol. Sci. 273, 1073–1078. doi: 10.1098/rspb.2005.3423
Gillespie, S. (2010). Factors affecting parasite prevalence among wild bumblebees. Ecol. Entomol. 35, 737–747. doi: 10.1111/j.1365-2311.2010.01234.x
Gols, R., Bukovinszky, T., van Dam, N. M., Dicke, M., Bullock, J. M., and Harvey, J. A. (2008). Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 34, 132–143. doi: 10.1007/s10886-008-9429-z
González-Teuber, M., and Heil, M. (2009). Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal. Behav. 4, 809–813. doi: 10.4161/psb.4.9.9393
Gough, R. E. (1993). The Highbush Blueberry and its Management. Boca Raton, FL: CRC Press. doi: 10.1201/9781482298000
Goulson, D., O’CONNOR, S., and Park, K. J. (2018). The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 43, 168–181. doi: 10.1111/een.12482
Guzman, J. D. (2014). Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 19, 19292–19349. doi: 10.3390/molecules191219292
Habib, H. M., Platat, C., Meudec, E., Cheynier, V., and Ibrahim, W. H. (2014). Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and quantification by using UPLC-DAD-ESI-MS. J. Sci. Food Agric. 94, 1084–1089. doi: 10.1002/jsfa.6387
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. doi: 10.18637/jss.v033.i02
Hancock, J., and Siefker, J. (1982). Levels of inbreeding in highbush blueberry cultivars. HortScience 17, 363–366. doi: 10.1007/BF00224252
Harder, L. D. (1982). Measurement and estimation of functional proboscis length in bumblebees (Hymenoptera: Apidae). Can. J. Zool. 60, 1073–1079. doi: 10.1139/z82-148
Heil, M. (2011). Nectar: generation, regulation and ecological functions. Trends Plant Sci. 16, 191–200. doi: 10.1016/j.tplants.2011.01.003
Heil, M. (2015). Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu. Rev. Entomol. 60, 213–232. doi: 10.1146/annurev-ento-010814-020753
Henning, J. A., Peng, Y.-S., Montague, M. A., and Teuber, L. R. (1992). Honey bee (Hymenoptera: Apidae) behavioral response to primary alfalfa (Rosales: Fabaceae) floral volatiles. J. Econ. Entomol. 85, 233–239. doi: 10.1093/jee/85.1.233
Hernandez-Cumplido, J., Giusti, M. M., Zhou, Y., Kyryczenko-Roth, V., Chen, Y. H., and Rodriguez-Saona, C. (2018). Testing the ‘plant domestication-reduced defense’ hypothesis in blueberries: the role of herbivore identity. Arthropod Plant Interact. 12, 483–493. doi: 10.1007/s11829-018-9605-1
Houslay, T. M., and Wilson, A. J. (2017). Avoiding the misuse of BLUP in behavioural ecology. Behav. Ecol. 28, 948–952. doi: 10.1093/beheco/arx023
Huber, G. (2016). An Investigation of Highbush Blueberry Floral Biology and Reproductive Success in British Columbia. Vancouver: University of British Columbia.
Hunter, M. D., and Schultz, J. C. (1993). Induced plant defenses breached? Phytochemical induction protects an herbivore from disease. Oecologia 94, 195–203. doi: 10.1007/BF00341317
Inouye, D. W., and Waller, G. D. (1984). Responses of honey bees (Apis mellifera) to amino acid solutions mimicking floral nectars. Ecology 65, 618–625. doi: 10.2307/1941424
Irwin, R. E., Adler, L. S., and Brody, A. K. (2004). The dual role of floral traits: pollinator attraction and plant defense. Ecology 85, 1503–1511. doi: 10.1890/03-0390
Irwin, R. E., Cook, D., Richardson, L. L., Manson, J. S., and Gardner, D. R. (2014). Secondary compounds in floral rewards of toxic rangeland plants: impacts on pollinators. J. Agric. Food Chem. 62, 7335–7344. doi: 10.1021/jf500521w
Irwin, R. E., Strauss, S. Y., Storz, S., Emerson, A., and Guibert, G. (2003). The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84, 1733–1743. doi: 10.1890/0012-9658(2003)084[1733:TROHIT]2.0.CO;2
Jones, D. A. (1998). Why are so many food plants cyanogenic? Phytochemistry 47, 155–162. doi: 10.1016/s0031-9422(97)00425-1
Kalt, W., Ryan, D. A., Duy, J. C., Prior, R. L., Ehlenfeldt, M. K., and Vander Kloet, S. (2001). Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium Section cyanococcus spp.). J. Agric. Food Chem. 49, 4761–4767. doi: 10.1021/jf010653e
Kempel, A., Schädler, M., Chrobock, T., Fischer, M., and van Kleunen, M. (2011). Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc. Natl. Acad. Sci. U.S.A. 108, 5685–5689. doi: 10.1073/pnas.1016508108
Kessler, A., and Halitschke, R. (2009). Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Funct. Ecol. 23, 901–912. doi: 10.1111/j.1365-2435.2009.01639.x
Klatt, B. K., Burmeister, C., Westphal, C., Tscharntke, T., and von Fragstein, M. (2013). Flower volatiles, crop varieties and bee responses. PLoS One 8:e72724. doi: 10.1371/journal.pone.0072724
Klein, A.-M., Vaissiere, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Lautenbach, S., Seppelt, R., Liebscher, J., and Dormann, C. F. (2012). Spatial and temporal trends of global pollination benefit. PLoS One 7:e35954. doi: 10.1371/journal.pone.0035954
Li, X., Garvey, M., Kaplan, I., Li, B., and Carrillo, J. (2017). Domestication of tomato has reduced the attraction of herbivore natural enemies to pest-damaged plants. Agric. For. Entomol. 20, 390–401. doi: 10.1111/afe.12271
Lobos, G. A., and Hancock, J. F. (2015). Breeding blueberries for a changing global environment: a review. Front. Plant Sci. 6:782. doi: 10.3389/fpls.2015.00782
Luby, J. J., and Shaw, D. V. (2009). Plant breeders’ perspectives on improving yield and quality traits in horticultural food crops. HortScience 44, 20–22.
Lucas-Barbosa, D. (2016). Integrating studies on plant–pollinator and plant–herbivore interactions. Trends Plant Sci. 21, 125–133. doi: 10.1016/j.tplants.2015.10.013
Manson, J. S., Otterstatter, M. C., and Thomson, J. D. (2010). Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162, 81–89. doi: 10.1007/s00442-009-1431-9
Manson, J. S., Rasmann, S., Halitschke, R., Thomson, J. D., and Agrawal, A. A. (2012). Cardenolides in nectar may be more than a consequence of allocation to other plant parts: a phylogenetic study of Asclepias. Funct. Ecol. 26, 1100–1110. doi: 10.1111/j.1365-2435.2012.02039.x
Mao, W., Schuler, M. A., and Berenbaum, M. R. (2013). Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. U.S.A. 110, 8842–8846. doi: 10.1073/pnas.1303884110
Meier, C. L., and Bowman, W. D. (2008). Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc. Natl. Acad. Sci. U.S.A. 105, 19780–19785. doi: 10.1073/pnas.0805600105
Meyer, R. S., DuVal, A. E., and Jensen, H. R. (2012). Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytol. 196, 29–48. doi: 10.1111/j.1469-8137.2012.04253.x
Mitchell, R. J. (2004). Heritability of nectar traits: why do we know so little? Ecology 85, 1527–1533. doi: 10.1890/03-0388
Muola, A., Weber, D., Malm, L. E., Egan, P. A., Glinwood, R., Parachnowitsch, A. L., et al. (2017). Direct and pollinator-mediated effects of herbivory on strawberry and the potential for improved resistance. Front. Plant Sci. 8:823. doi: 10.3389/fpls.2017.00823
Nepi, M., Grasso, D. A., and Mancuso, S. (2018). Nectar in plant–insect mutualistic relationships: from food reward to partner manipulation. Front. Plant Sci. 9:1063. doi: 10.3389/fpls.2018.01063
Oksanen, J., Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2017). “vegan: Community Ecology Package”. R package version 2.4-5 ed.).
Otterstatter, M., and Thomson, J. (2006). Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133, 749–761. doi: 10.1017/S003118200600120X
Palmer-Young, E. C., Farrell, I., Adler, L. S., Milano, N. J., Egan, P. A., Junker, R. R., et al. (in press). Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monograph.
Palmer-Young, E. C., Sadd, B. M., Stevenson, P. C., Irwin, R. E., and Adler, L. S. (2016). Bumble bee parasite strains vary in resistance to phytochemicals. Sci. Rep. 6:37087. doi: 10.1038/srep37087
Palmer-Young, E. C., Tozkar, C. Ö, Schwarz, R. S., Chen, Y., Irwin, R. E., Adler, L. S., et al. (2017). Nectar and pollen phytochemicals stimulate honey bee (Hymenoptera: Apidae) immunity to viral infection. J. Econ. Entomol. 110, 1959–1972. doi: 10.1093/jee/tox193
Parachnowitsch, A. L., Manson, J. S., and Sletvold, N. (2018). Evolutionary ecology of nectar. Ann. Bot. doi: 10.1093/aob/mcy132 [Epub ahead of print].
Petanidou, T. (2007). “Ecological and evolutionary aspects of floral nectars in Mediterranean habitats,” in Nectaries and Nectar, eds S. W. Nicolson, M. Nepi, and E. Pacini (Berlin: Springer), 343–375.
Petanidou, T., Van Laere, A., Ellis, W. N., and Smets, E. (2006). What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 115, 155–169. doi: 10.1111/j.2006.0030-1299.14487.x
Pichersky, E., and Dudareva, N. (2007). Scent engineering: toward the goal of controlling how flowers smell. Trends Biotechnol. 25, 105–110. doi: 10.1016/j.tibtech.2007.01.002
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Heisterkamp, S., Van Willigen, B., et al. (2017). Package ‘nlme’. Linear and Nonlinear Mixed Effects Models, Version 3–1.
Prasifka, J. R. P., Mallinger, R. E., Portlas, Z. M., Hulke, B. S., Fugate, K. K., Paradis, T., et al. (2018). Using nectar-related traits to enhance crop-pollinator interactions. Front. Plant Sci. 9:812. doi: 10.3389/fpls.2018.00812
Rasmann, S., Köllner, T. G., Degenhardt, J., Hiltpold, I., Toepfer, S., Kuhlmann, U., et al. (2005). Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434, 732–737. doi: 10.1038/nature03451
Rhoades, D. F., and Bergdahl, J. C. (1981). Adaptive significance of toxic nectar. Am. Nat. 117, 798–803. doi: 10.1086/283765
Richardson, L. L., Adler, L. S., Leonard, A. S., Andicoechea, J., Regan, K. H., Anthony, W. E., et al. (2015). Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. Lond. B Biol. Sci. 282:20142471. doi: 10.1098/rspb.2014.2471
Richardson, L. L., Bowers, M. D., and Irwin, R. E. (2016). Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 97, 325–337. doi: 10.1890/15-0263.1
Rivera, M. J., Rodriguez-Saona, C., Egizi, A., Fonseca, D. M., Jennings, D. E., and Koppenhöfer, A. M. (2016). Cultivation and domestication of highbush blueberry (Vaccinium corymbosum) alters abundance, diversity and virulence of entomopathogenic nematodes. Agric. Ecosyst. Environ. 222, 148–155. doi: 10.1016/j.agee.2016.02.013
Rivera, M. J., Rodriguez-Saona, C., Jennings, D. E., and Koppenhöfer, A. M. (2015). Assessing the impact of cultivation and plant domestication of highbush blueberry (Vaccinium corymbosum) on soil properties and associated plant-parasitic nematode communities. Soil Biol. Biochem. 88, 25–28. doi: 10.1016/j.soilbio.2015.05.010
Rodriguez-Saona, C., Vorsa, N., Singh, A. P., Johnson-Cicalese, J., Szendrei, Z., Mescher, M. C., et al. (2011). Tracing the history of plant traits under domestication in cranberries: potential consequences on anti-herbivore defences. J. Exp. Bot. 62, 2633–2644. doi: 10.1093/jxb/erq466
Rodriguez-Saona, C. R., Rodriguez-Saona, L. E., and Frost, C. J. (2009). Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J. Chem. Ecol. 35, 163–175. doi: 10.1007/s10886-008-9579-z
Rosenthal, J. P., and Dirzo, R. (1997). Effects of life history, domestication and agronomic selection on plant defence against insects: evidence from maizes and wild relatives. Evol. Ecol. 11, 337–355. doi: 10.1023/A:1018420504439
Rowland, L., and Hammerschlag, F. (2005). Vaccinium spp. Blueberry. Biotechnology of Fruit and Nut Crops. Wallingford: CABI Publishing Cambridge, 222–246. doi: 10.1079/9780851996622.0222
Schmid-Hempel, P., and Schmid-Hempel, R. (1993). Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav. Ecol. Sociobiol. 33, 319–327. doi: 10.1007/BF00172930
Schütz, K., Kammerer, D., Carle, R., and Schieber, A. (2004). Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MS n. J. Agric. Food Chem. 52, 4090–4096. doi: 10.1021/jf049625x
Shykoff, J. A., and Schmid-Hempel, P. (1991). Parasites delay worker reproduction in bumblebees: consequences for eusociality. Behav. Ecol. 2, 242–248. doi: 10.1093/beheco/2.3.242
Smith, B. D. (2001). Documenting plant domestication: the consilience of biological and archaeological approaches. Proc. Natl. Acad. Sci. U.S.A. 98, 1324–1326. doi: 10.1073/pnas.98.4.1324
Song, G.-Q., and Hancock, J. F. (2011). “Vaccinium,” in Wild Crop Relatives: Genomic and Breeding Resources, ed. C. Kole (Berlin: Springer), 197–221. doi: 10.1007/978-3-642-16057-8_10
Stenberg, J. A., Heil, M., Åhman, I., and Björkman, C. (2015). Optimizing crops for biocontrol of pests and disease. Trends Plant Sci. 20, 698–712. doi: 10.1016/j.tplants.2015.08.007
Stevenson, P. C., Anderson, J. C., Blaney, W. M., and Simmonds, M. S. J. (1993). Developmental inhibition of Spodoptera litura (Fab.) larvae by a novel caffeoylquinic acid from the wild groundnut, Arachis paraguariensis (Chod et Hassl.). J. Chem. Ecol. 19, 2917–2933. doi: 10.1007/BF00980592
Stevenson, P. C., Nicolson, S. W., and Wright, G. A. (2017). Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Funct. Ecol. 31, 65–75. doi: 10.1111/1365-2435.12761
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Strauss, S. Y., and Armbruster, W. S. (1997). Linking herbivory and pollination—new perspectives on plant and animal ecology and evolution. Ecology 78, 1617–1618. doi: 10.2307/2266084
Strauss, S. Y., and Irwin, R. E. (2004). Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu. Rev. Ecol. Evol. Syst. 35, 435–466. doi: 10.1186/1471-2148-13-92
Sujana, G., Sharma, H. C., and Rao, D. M. (2012). Pod surface exudates of wild relatives of pigeonpea influence the feeding preference of the pod borer, Helicoverpa armigera. Arthropod Plant Interact. 6, 231–239. doi: 10.1007/s11829-011-9179-7
Tiedeken, E. J., Egan, P. A., Stevenson, P. C., Wright, G. A., Brown, M. J., Power, E. F., et al. (2016). Nectar chemistry modulates the impact of an invasive plant on native pollinators. Funct. Ecol. 30, 885–893. doi: 10.1111/1365-2435.12588
Vainstein, A., Lewinsohn, E., Pichersky, E., and Weiss, D. (2001). Floral fragrance. New inroads into an old commodity. Plant Physiol. 127, 1383–1389. doi: 10.1104/pp.010706
Van de Wouw, M., Kik, C., van Hintum, T., van Treuren, R., and Visser, B. (2010). Genetic erosion in crops: concept, research results and challenges. Plant Genet. Resour. 8, 1–15. doi: 10.1017/S1479262109990062
Whitehead, S. R., Turcotte, M. M., and Poveda, K. (2017). Domestication impacts on plant–herbivore interactions: a meta-analysis. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160034. doi: 10.1098/rstb.2016.0034
Wink, M. (1988). Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75, 225–233. doi: 10.1007/BF00303957
Wright, G. A., Baker, D. D., Palmer, M. J., Stabler, D., Mustard, J. A., Power, E. F., et al. (2013). Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 339, 1202–1204. doi: 10.1126/science.1228806
Xia, D., Wu, X., Shi, J., Yang, Q., and Zhang, Y. (2011). Phenolic compounds from the edible seeds extract of Chinese Mei (Prunus mume Sieb. et Zucc) and their antimicrobial activity. LWT Food Sci. Technol. 44, 347–349. doi: 10.1016/j.lwt.2010.05.017
Yousef, G. G., Brown, A. F., Guzman, I., Ballington, J. R., and Lila, M. A. (2016). Variations in chlorogenic acid levels in an expanded gene pool of blueberries. AIMS Agric. Food 1, 357–368. doi: 10.3934/agrfood.2016.3.357
Keywords: domestication, floral rewards, Vaccinium, crop evolution, pollinator-pathogen interactions, Bombus impatiens, pollinator health, phytochemicals
Citation: Egan PA, Adler LS, Irwin RE, Farrell IW, Palmer-Young EC and Stevenson PC (2018) Crop Domestication Alters Floral Reward Chemistry With Potential Consequences for Pollinator Health. Front. Plant Sci. 9:1357. doi: 10.3389/fpls.2018.01357
Received: 27 April 2018; Accepted: 28 August 2018;
Published: 26 September 2018.
Edited by:
Clay Carter, University of Minnesota Twin Cities, United StatesReviewed by:
Neelendra K. Joshi, University of Arkansas, United StatesCopyright © 2018 Egan, Adler, Irwin, Farrell, Palmer-Young and Stevenson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul A. Egan, cGF1bC5lZ2FuQHNsdS5zZQ==
†Present address: Evan C. Palmer-Young, Department of Entomology, University of California, Riverside, Riverside, CA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.