- The State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
Verticillium dahliae is a wide-host-range fungal pathogen that causes soil-borne disease in hundreds of dicotyledonous hosts. In search of V. dahliae Vd991 cell death-inducing proteins, we identified a pectate lyase (VdPEL1) that exhibited pectin hydrolytic activity, which could induce strong cell death in several plants. Purified VdPEL1 triggered defense responses and conferred resistance to Botrytis cinerea and V. dahliae in tobacco and cotton plants. Our results demonstrated that the mutant VdPEL1rec lacking the enzymatic activity lacked functions to induce both cell death and plant resistance, implying that the enzymatic activity was necessary. In addition, VdPEL1 was strongly induced in V. dahliae infected Nicotiana benthamiana and cotton roots, and VdPEL1 deletion strains severely compromised the virulence of V. dahliae. Our data suggested that VdPEL1 contributed to V. dahliae virulence and induced plant defense responses. These findings provide a new insight for the function of pectate lyase in the host–pathogen interaction.
Introduction
Plants are exposed to a multitude of pathogens that cause massive yield and quality losses annually. To ward off invading microorganisms, plants have evolved elaborate systems to provide better immunity against pathogens. Plants utilize pattern recognition receptors (PRRs) to perceive pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and rapidly activate PAMP-triggered immunity (PTI). This recognition initiates a cascade of signaling events resulting in induction of a battery of immune responses, including Ca2+ influx, the burst of reactive oxygen species (ROS), the accumulation of defense hormones, the expression of defense-related genes and callose deposition (Boller and Felix, 2009; Couto and Zipfel, 2016). In turn, during the coevolution of hosts and microbes, pathogens also employ numerous effectors to interfere with PTI and establish successful infection, which is regarded as effector-triggered susceptibility (ETS) (Chisholm et al., 2006; Jones and Dangl, 2006; Saijo et al., 2017). As an adaption to ETS, the effectors are recognized by the resistance (R) proteins, and subsequently lead to robust immunity termed effector-triggered immunity (ETI) (Houterman et al., 2008; Stergiopoulos and de Wit, 2009). Generally, ETI is associated with stronger immune responses, such as the hypersensitive response (HR). Plant immune responses initiate from local tissues located at the site of the infection and subsequently extend to other non-infected tissues by long-distance intercellular communications, generating a systemic acquired resistance (SAR) that is effective against a broad spectrum of pathogens in the whole plant (Kulye et al., 2012).
The plant cell wall is the first barrier, which provides mechanical strength and rigidity to protect against pathogenic infection. Correspondingly, pathogens secrete numerous of cell wall-degrading enzymes (CWDEs) to permit them to invade plant tissue and supply themselves with nutrients (Kikot et al., 2009; Klöckner et al., 2016). On the one hand, CWDEs serve as virulence factor in pathogens and play an essential role in infection process. Previous studies suggested that the deletion of the Xylanase Xyn11A gene caused a marked effect on the ability of Botrytis cinerea to infect tomato leaves and grape berries (Brito et al., 2006). And an endoxylanase named xynB contributed to virulence in Xanthomonas oryzae pv. oryzae (Pandey and Sonti, 2010). Alternatively, plants can also sense CWDEs or self-molecules (released from plant cell wall polysaccharides) as inducers of immune responses (Misas-Villamil and van der Hoorn, 2008). Recently, it was widely reported that glycoside hydrolase 12 (GH12) proteins, VdEG1 and VdEG3 from Verticillium dahliae, XEG1 from Phytophthora sojae and BcXYG1, a secreted xyloglucanase from B. cinerea contributed to virulence and simultaneously triggered plant immunity as PAMPs (Gui Y.-J. et al., 2017; Zhu et al., 2017a,b). Oligogalacturonides (OGs) were regarded as DAMPs to activate the plant immune responses (De Lorenzo et al., 2011). The enzymatic activity of VdCUT11(a cutinase from V. dahliae) was required for activating immune responses in Nicotiana benthamiana, implying that the cutinase degradation products might induce the plant resistance (Gui Y. et al., 2017).
Pectin exists widely in the plant cell walls and cell lining and maintains wall integrity and cell–cell cohesion. Due to the complexity of this highly branched polysaccharide, the degradation of pectin requires a variety of enzymes, such as pectin lyases, pectate disaccharide-lyases, and pectate lyases. Among these, pectate lyases have received the most attention. Pectate lyases randomly cleave α-1,4-polygalacturonic acid via a β-elimination reaction. They can also macerate and disassemble the plant cell wall and tissues in a manner similar to that occurring in fungal diseases (Collmer, 1986). For instance, the deletion of the pectate lyase gene CcpelA and PelB in Colletotrichum coccodes, resulted in attenuated virulence on green tomato fruit and reduced susceptibility on avocado (Persea americana) fruit, respectively (Yakoby et al., 2001; Ben-Daniel et al., 2011). The deletion of the pectate lyase genes BcPG1 and BcPG2 also reduced virulence in B. cinerea (Have et al., 1998; Kars et al., 2005). However, pectic enzymes can also elicit plant defense responses through direct or indirect ways. For instance, a pectate lyase, from Erwinia carotovora bacteria, induced defense responses against Erwinia soft rot in potato plants (Wegener, 2002).T4BcPG1, an endopolygalacturonase from B. cinerea, activated grapevine defense reactions (Poinssot et al., 2003).
Verticillium dahliae is a wide-host-range pathogen that can infect a large number of dicotyledonous hosts, including ecologically important plants and many high-value crops worldwide such as cotton, tobacco, potato, and tomato (Fradin and Thomma, 2006; Klosterman et al., 2009; Inderbitzin et al., 2014). It infects plants primarily through the formation of hyphae, which can puncture the plant root surface and colonize in xylem vessels (Zhao et al., 2014). It was demonstrated that V. dahliae secrets a large amount of CWDEs, including 13 pectate lyases. However, the specific roles of the pectate lyases in V. dahliae remain largely unknown.
The main objectives of the current study were to: (1) isolate and investigate cell death-inducing proteins in V. dahliae Vd991; (2) determine whether VdPEL1 is secreted into the apoplast in order to induce cell death; (3) examine the relationship between the enzymatic activity and cell death-inducing activity; and (4) investigate the function of VdPEL1 in immune responses and virulence.
Materials and Methods
Fungal Cultures, Plants Grown
The V. dahliae strains, including Vd991 wild type strains, targeted deletion strains and complementary transformants, were maintained and cultured on PDA media or in liquid Czapek media for 7 days at 25°C. B. cinerea strain BC-98 and Agrobacterium tumefaciens AGL-1 were grown on PDA media at 25°C and LB (Kan and Rif) medium at 28°C, respectively. Cotton (Gossypium hirsutum cv. Junmian 1) and N. benthamiana were grown at 23 and 27°C, respectively, in a greenhouse with a day/night period of 14/10 h.
Separation, Purification, and Characterization of Proteins Secreted From V. dahliae
To produce large amounts of secreted proteins, Vd991 was grown in Czapek media at 25°C for 14 days, shaken at 150 rpm (Bailey, 1996), and then filtered through two layers of filter paper. The supernatants were collected and precipitated with 70% (NH)4SO4 overnight at 4°C. The precipitate was collected by centrifugation at 15,000 ×g for 10 min at 4°C, and then resuspended in 10 mM Tris-HCl (pH 7.5) and 1 mM EDTA (TE). Crude protein was further purified by ion-exchange chromatography and eluted with a linear sodium chloride gradient from 0.0 to 1.0 M in TE. Fractions corresponding to absorbance peaks were desalted and concentrated using a 10-kDa ultrafiltration device and tested their ability to induce cell death activity. The fraction with cell death activity was excised from the SDS-PAGE gel and identified using mass spectrometry (MS) analysis (Beijing Protein Innovation, Beijing, China) as described (Heese et al., 2007). The tandem MS + MS/MS data were automatically analyzed using the Mascot search engine (Matrix Science, London, United Kingdom).
Cloning, Expression, and Purification of Recombinant Protein
VdPEL1 fragment (amplified with primers VdPEL1F/VdPEL1R; Supplementary Table S2) without the predicted signal peptides and stop codons was inserted into the pPICZαA plasmid at the BamHI and EcoRI sites (Ma et al., 2015). The recombinant plasmid pPICZαA-VdPEL1 was linearized with PmeI and transformed into Pichia pastoris KM71H for expression. The transformed yeasts were grown and induced in BMGY (buffered glycerol-complex medium) and BMMY (buffered methanol-complex medium), respectively (Easy Select Pichia Expression Kit; Invitrogen). The supernatant was collected (3,000 ×g for 10 min at 4°C) and then purified using nickel affinity chromatography (Ma et al., 2015; Zhang et al., 2017). The purified VdPEL1 or VdPEL1rec were kept in protein buffer (20 mM Tris, pH 8.0) and further detected via SDS-PAGE and Western blotting. The concentration of the purified protein was measured using an Easy II Protein Quantitative Kit (BCA) and the protein was then stored at -80°C.
Site-Directed Mutagenesis
To examine the relationship between the enzymatic activity and cell death-inducing activity of VdPEL1, we constructed a VdPEL1rec mutant with loss of the enzymatic activity. According to multiple sequence alignment, we found that Asp-125 and Asp-147 residues might be the critical catalytic sites of VdPEL1. Two Asp residues of VdPEL1 were replaced by Ala residues using the Quick Change™ Site-Directed Mutagenesis Kit (Stratagene, United States) to cause the loss of pectate lyase activity of VdPEL1. Primers used in these constructions are listed in Supplementary Table S2. The VdPEL1rec mutant was confirmed by sequence alignment and the enzyme assays.
Enzyme Activity Assays
Hydrolase activity was measured using the 2-cyanoacetamide spectrophotometric method (Bach and Schollmeyer, 1992). The purified VdPEL1 or VdPEL1rec (500 ng) and substrate (2.5% polygalacturonic acid) were co-incubated at 30°C in a medium buffered with MES or Tris 10 mM at indicated pH (total volume: 350 μl). Fifteen minutes later, all samples were incubated for 10 min at 100°C to terminate the assays, and the reduced sugars released by VdPEL1 or VdPEL1rec were measured at 274 nm using a spectrophotometer. The reduced sugars were quantified using a standard calibration curve obtained with polygalacturonic acid. The experiment was repeated three times.
Immunoblot Analysis
To confirm whether VdPEL1 was secreted into the apoplast, transient expression in N. benthamiana was performed. Three sequences, encoding VdPEL1 protein with the putative signal peptide, VdPEL1 protein without the putative signal peptide and PR1 SP-VdPEL121-255 protein replaced the signal peptide from pathogenesis-related protein 1 (PR1), were amplified with primers VdPEL1-T-F/VdPEL1-T-R, VdPEL121-255-F/VdPEL121-255-R, and PR1 SP-VdPEL121-255-F/PR1 SP-VdPEL121-255-R, respectively (Supplementary Table S2). Three sequences were cloned into the pYBA1132 vector which contained a C-terminal GFP tag at the XbaI and BamHI sites (Yan et al., 2012). To confirm whether the enzymatic activity of VdPEL1 was related to the cell death-inducing activity, the sequences encoding the mature VdPEL1 protein and site-directed mutagenized VdPEL1rec with the putative signal peptide (amplified with primers VdPEL1F/VdPEL1R and VdPEL1rec F/VdPEL1rec R, Supplementary Table S2) were cloned into pYBA1132 vector at the XbaI and BamHI sites, and then transformed into the A. tumefaciens strain GV3101. Agroinfiltration assays were performed on N. benthamiana plants. To determine whether fusion proteins were expressed, plant total protein extractions, and immunoblots were assessed as previously described (Yu et al., 2012). All the proteins were analyzed via immunoblots using anti-GFP-tag primary monoclonal antibody. The blots were visualized using the Odyssey® LI-COR Imaging System. Rubisco was used to confirm the equal protein loading.
Elicitor Activity and Systemic Resistance Assays
To detect the induction of cell-death, 300 nM purified VdPEL1 or VdPEL1rec, PEVC and BSA were injected into leaves of 4-week-old N. benthamiana plants and 2-week-old plants of cotton, tomato, and soybean with an injector. These plants were kept in a glasshouse with a day/night period of 14/10 h, and cell-death responses were investigated after 48 h of treatment with the recombinant proteins. The accumulation of ROS in the plant leaves was stained by using 3′3-diaminobenzidine (DAB) solution as described previously (Bindschedler et al., 2006). To visualize callose deposition, 4-week-old N. benthamiana leaves were treated with 300 nM recombinant proteins and stained with aniline blue at 24 h post-treatment, as described previously (Chen et al., 2012). To assay electrolyte leakage, 4-week-old N. benthamiana leaves were infiltrated with 300 nM purified VdPEL1 or VdPEL1rec and PEVC. The corresponding N. benthamiana leaves at different time points were harvested and submerged in sterile water at 4°C. Ion conductivity was measured using a conductivity meter.
The purified VdPEL1 or VdPEL1rec and PEVC were individually syringe-infiltrated into 4-week-old N. benthamiana leaves. A total of 5 μl of 2 × 106 conidia/ml B. cinerea or 1 × 106 conidia/ml V. dahliae were placed on the infiltrated area or inoculated by the root-dip method, respectively. The inoculated plants were placed in a greenhouse with a day/night period of 14/10 h. The lesion development of B. cinerea on the N. benthamiana leaves was evaluated at 2 days post-inoculation by determining the average lesion diameter. In addition, disease symptoms were observed at 12 days V. dahliae post-inoculation on N. benthamiana. All the experiments were performed three times.
Generation of VdPEL1 Deletion and Complementary Mutants
The wild-type VdPEL1 gene and 500 bp flanking sequences of the target gene were amplified from the V. dahliae Vd991 genomic DNA (gDNA). Two flanking sequences of the target gene and a hygromycin resistance cassette were constructed into a fusion fragment using a nested PCR reaction, which was subsequently introduced into the binary vector pGKO2-Gateway. To generated complementary transformants, the donor vector pCT-HN containing VdPEL1 gene was integrated into the mutant transformants using an Agrobacterium-mediated transformation method (Liu et al., 2013). All the mutants were identified using PCR with the corresponding primers.
RNA Extraction and qRT-PCR
To measure the expression of VdPEL1 gene during infection, 4-week-old N. benthamiana plants or 2-week-old cotton seedlings were inoculated with 1 × 106 V. dahliae conidia/ml or 5 × 106 conidia/ml by the root-dip method, respectively. We selected 10 indicated time points during different stages of post-inoculation to determine expression patterns of VdPEL1 using quantitative PCR (qPCR). All the samples were obtained at the time points indicated and stored at –80°C. Total RNA from V. dahliae infected plants was extracted with the E.Z.N.A.® Total Fungal RNA Kit I. To measure the expression of defense-related genes, the leaves of 4-week-old N. benthamiana plants were treated with 300 nM purified VdPEL1 or VdPEL1rec and PEVC. The leaves were obtained at the time points indicated, immediately frozen in liquid nitrogen, and stored at -80°C. A EasyPure Plant RNA Kit (TransGen Biotech) was used to extract the total plant RNA. The gDNA was digested by the DNase I (TransGen Biotech). And the gDNA remover was added when the cDNA was synthesized. To further investigate whether or not the gDNA was absolutely removed, the gene encoding the actin (GenBank: X63603.1) in tobacco and β-tubulin (VDAG_10074) in V. dahliae were detected by PCR, respectively.
qRT-PCR was performed using a TransStart Green qPCR SuperMix UDG according to the manufacturer’s instructions (TransGen Biotech). qRT-PCR was performed under the following conditions: an initial 95°C denaturation step for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The cotton 18S gene, N. benthamiana EF-1a and β-tubulin (VDAG_10074) of V. dahliae were used as endogenous plant controls and used to quantify fungal colonization, respectively. All primers are listed in Supplementary Table S2. The relative transcript levels among various samples were determined using the 2-ΔΔCT method with three independent determinations (Livak and Schmittgen, 2001).
Pathogenicity Assays
To test whether VdPEL1 functioned as a virulence factor of V. dahliae, the wild-type strain and derived mutants, including the VdPEL1 deletion and complementary mutants were used in this study. Four-week-old N. benthamiana plants or 2-week-old cotton seedlings were inoculated with 1 × 106 conidia/ml or 5 × 106 conidia/ml by the root-dip method, respectively (Zhou et al., 2013). After 21 days post-inoculation on cotton or 14 days post-inoculation on N. benthamiana, disease symptom and fungal biomass was determined as previously described (Santhanam et al., 2013). Discoloration in vascular tissues was observed by cutting root longitudinal sections at 3 weeks after inoculation. Real-time qPCR was performed using a qPCR SYBR premix Ex Taq II kit (TaKaRa, Kyoto, Japan). t-tests were performed to determine statistical significance at p-values <0.05 between two treatments groups.
Statistical Analysis
All the experiments and data presented here were performed at least three repeats. The data are presented as the mean and standard deviations. Statistical Analysis System (SAS) software was used to perform the statistical analysis via Student’s t-test.
Results
Identification, Purification, and Characterization of VdPEL1
To identify the defense response proteins from V. dahliae, the induction of cell death in N. benthamiana leaves was used as an index to fractionate proteins from culture filtrates via anion exchange chromatography. Due to different affinities for chromatographic column, fractionation of a 70% ammonium sulfate precipitate generated three primary peaks (Figure 1A). Fractions corresponding to peak 1 could induce cell death in N. benthamiana leaves (Figure 1B). Further fractionate and purify, SDS-PAGE analysis showed a single obvious band between 25 and 35 kDa (Figure 1C). To identify amino acid sequence of the peak 1 band, tryptic digestion was performed, and the peptides generated were analyzed by liquid chromatography-MS. Many peptides with high credibility were co-matched a protein encoded by the gene (Gene ID: 20707618) (Supplementary Figure S1). The bioinformatics analysis found that the gene encodes a pectate lyase in V. dahliae Vd991, and we designated this protein as VdPEL1. Transient expression of VdPEL1 in N. benthamiana leaves showed that the protein triggers cell death 5 days after infiltration (Figure 1D). Immunoblot analysis using a-GFP antibody detected the expression of VdPEL1 in the leaves (Figure 1E).
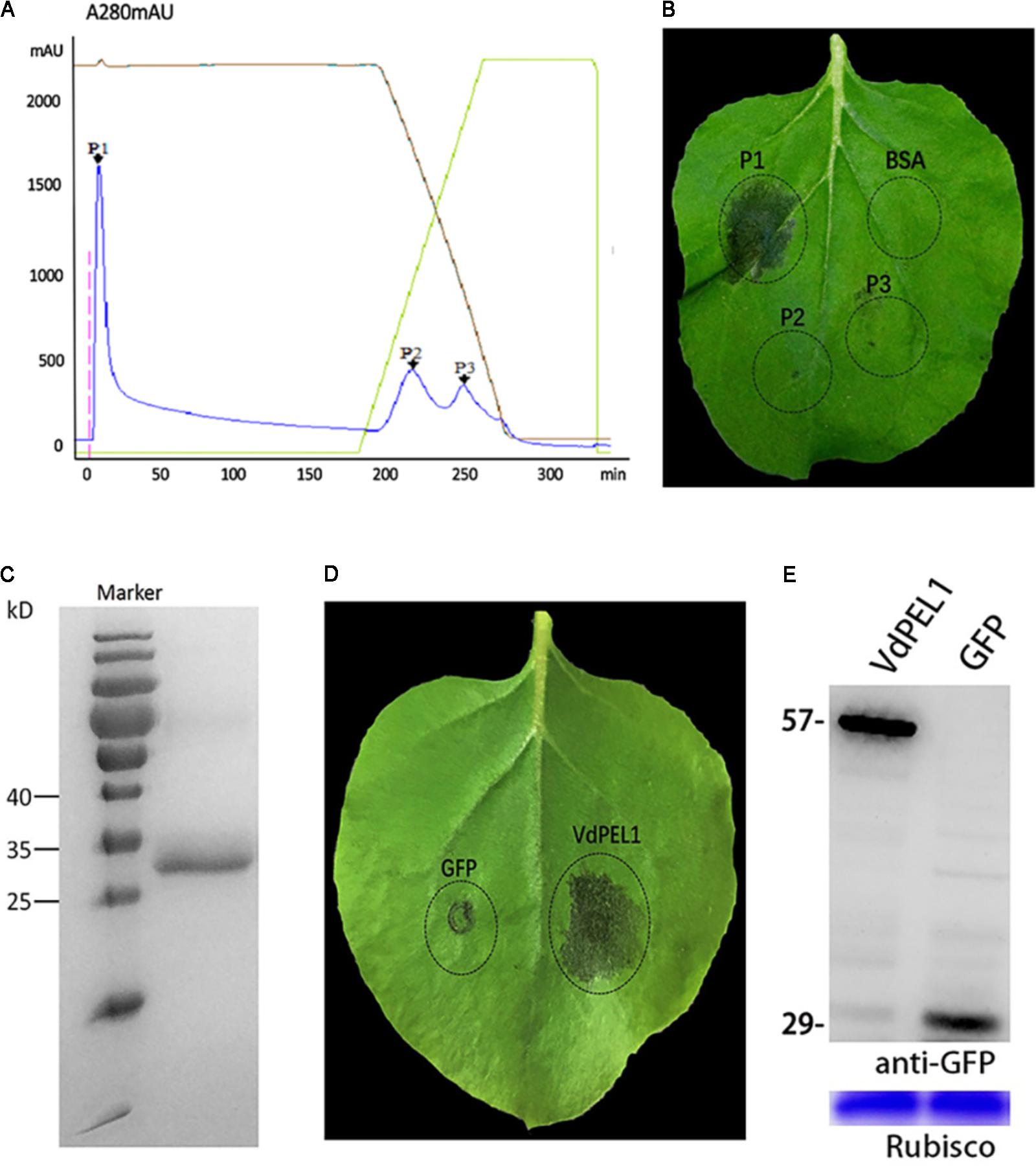
FIGURE 1. VdPEL1 purified from V. dahliae culture supernatants induces cell death in N. benthamiana. (A) Ion exchange chromatography of supernatant proteins, resulting in three peaks. (B) Two-month-old N. benthamiana leaves were infiltrated with fractions from the peaks or a BSA control. (C) SDS-PAGE analysis of peak A proteins showed a single band with a molecular mass between 25 and 35 kDa. (D) Transient expression of VdPEL1 in N. benthamiana leaves 5 days after inoculation with Agrobacterium strains. (E) Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing GFP and VdPEL1.
Nucleotide Sequence Analysis of VdPEL1
The open reading frame of VdPEL1 is 765 bp encoding a 255 amino acid protein. The first 20 N-terminal amino acids encode a signal peptide (Signal IP 4.1 server), and no transmembrane helices of VdPEL1 were found, indicating that the protein was likely to be secreted to the extracellular space. VdPEL1 has a conserved domain (39–229 amino acids) and belongs to the pectate lyase super family similar to pectate lyase A. BLAST results showed VdPEL1 homologues were present in a large number of pectate lyases of necrotrophic and hemibiotrophic plant pathogens. Phylogenetic analysis showed that all V. dahliae pectate lyase members were sorted into four distinct groups comprising five, two, one, and five pectate lyases each, respectively (Figure 2). The reference pectate lyases known to be involved in virulence and triggering defense responses in fungi and bacterial were also distributed into four branches. These results suggested that the function of pectate lyases members is significantly diverse in V. dahliae.
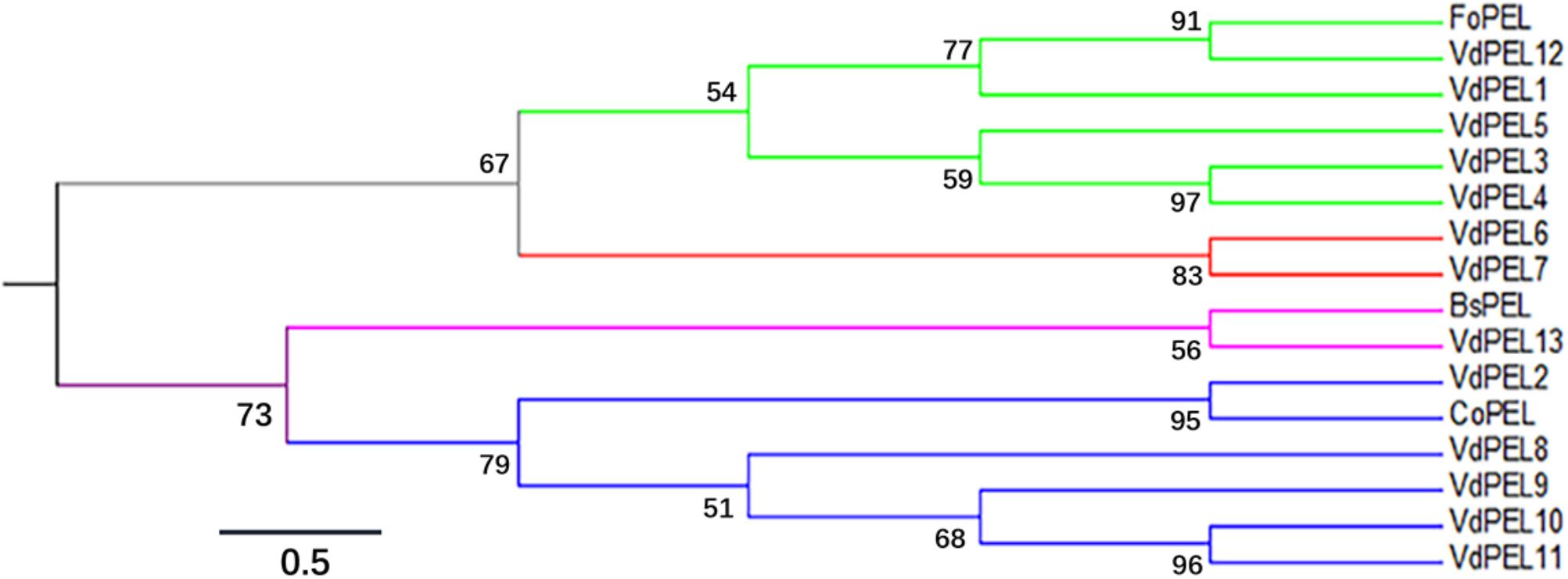
FIGURE 2. Phylogenetic relationships between the V. dahliae pectate lyase family members and pectate lyase from other fungi. The phylogeny was constructed by Mega 6.0 using maximum-likelihood (parameters: 1,000 bootstraps, Jones-Taylor-Thornton model). Branch colors indicated the four groups of pectate lyase family members in V. dahliae. The sequence data of all the proteins can be found in the GenBank/EMBL data libraries under the accession numbers: VdPEL1 (EGY15301.1), VdPEL2 (EGY22111.1), VdPEL3 (EGY18351.1), VdPEL4 (EGY13381.1), VdPEL5 (EGY19202.1), VdPEL6 (XP_009649735.1), VdPEL7 (EGY16604.1), VdPEL8 (EGY14058.1), VdPEL9 (EGY16402.1), VdPEL10 (EGY14238.1), VdPEL11 (XP_009651657.1), VdPEL12 (PAU54057.1), VdPEL13 (PAU53142.1), FoPEL (BAC74094), BsPEL (1EE6_A), and CoPEL (XP_369589).
VdPEL1 Induces Cell Death in Several Plants
To examine the necrosis-inducing activity of VdPEL1 in N. benthamiana, VdPEL1 was expressed in the P. pastoris using the pPICZaA vector (pPICZαA: VdPEL1), which could secrete proteins into the culture media. Purified VdPEL1, with a size of 29 kDa (Supplementary Figure S4), was infiltrated into the mesophyll of N. benthamiana leaves using a syringe with different concentrations from 100 nM to 1 μM. The necrosis area occurred and increased with increasing concentrations of VdPEL1 after infiltration for 2 days compared with bovine serum albumin (BSA) or PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same manner as VDPEL1), which had no HR response at 1 μM and even at 10 μM (Figure 3A). In parallel, the HSR203J gene and HIN1 genes, which are described for HR-marker genes in tobacco plants (Takahashi et al., 2004), were significantly induced expression in VdPEL1 treated leaves (Figure 3B), suggesting that VdPEL1 can trigger a severe HR. To examine the host specificity of VdPEL1, we infiltrated VdPEL1 (300 nM) into the leaves of various plant species. The results demonstrated that VdPEL1 induced localized cell death in tomato, soybean (Glycine max), and cotton (Gossypium hirsutum) plants (Figure 3C). Therefore, VdPEL1 had a necrosis-inducing activity in diverse plant species.
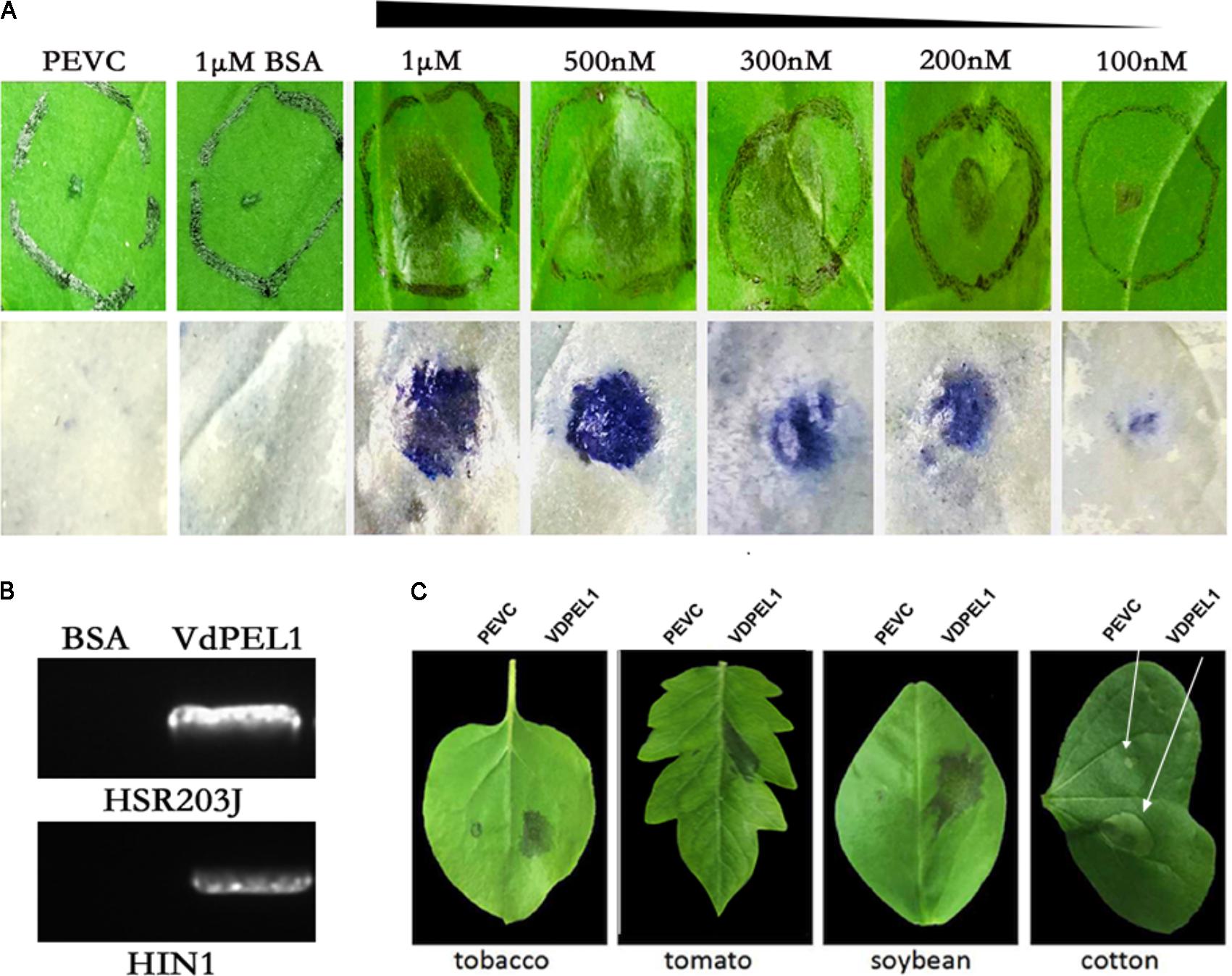
FIGURE 3. VdPEL1 induces cell death in several plants. (A) N. benthamiana leaves were infiltrated with purified VdPEL1 protein (100 nM to 1 μM), BSA (1 μM), and PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same manner as VdPEL1). Two days post-infiltration, N. benthamiana leaves were photographed and stained with trypan blue. (B) N. benthamiana leaves were infiltrated with purified 300 nM VdPEL1 and 300 nM BSA. The expression of the HSR203J gene and HIN1 gene were detected 12 h after infiltration of the recombinant VdPEL1. (C) Treatment of tobacco, tomato, soybean, and cotton leaves with purified 300 nM VdPEL1 and 300 nM BSA. Two days after post-infiltration, different plants leaves were photographed.
VdPEL1 Is Secreted Into the Apoplast in Order to Induce Cell Death in N. benthamiana
Bioinformatic analysis showed that VdPEL1 has a signal peptide with 20 amino acids and no transmembrane helices, implying that VdPEL1 was likely to be an extracellular protein. To test whether VdPEL1 was localized to the plant apoplast to induce cell death, we constructed three A. tumefaciens strains: VdPEL121-255 (deleted the N-terminal signal peptide), VdPEL1 (complete protein with the N-terminal signal peptide), and PR1 SP-VdPEL121-255 (replaced the signal peptide from PR1) (Figure 4A). All the strains were infiltrated into N. benthamiana leaves. As expected, VdPEL1 and PR1 SP-VdPEL121-255 were secreted from the plant cells to the apoplast and developed the typical necrosis, whereas expression of VdPEL121-255 (remaining inside the cell) and green fluorescent protein (GFP as a negative control) didn’t appear to cause a cell death response (Figure 4B). Western blot assays showed that the accumulation of all the examined proteins was similar in N. benthamiana 5 days post-infiltration (Figure 4C). These results indicated that VdPEL1 must be secreted into the apoplast to trigger cell death.
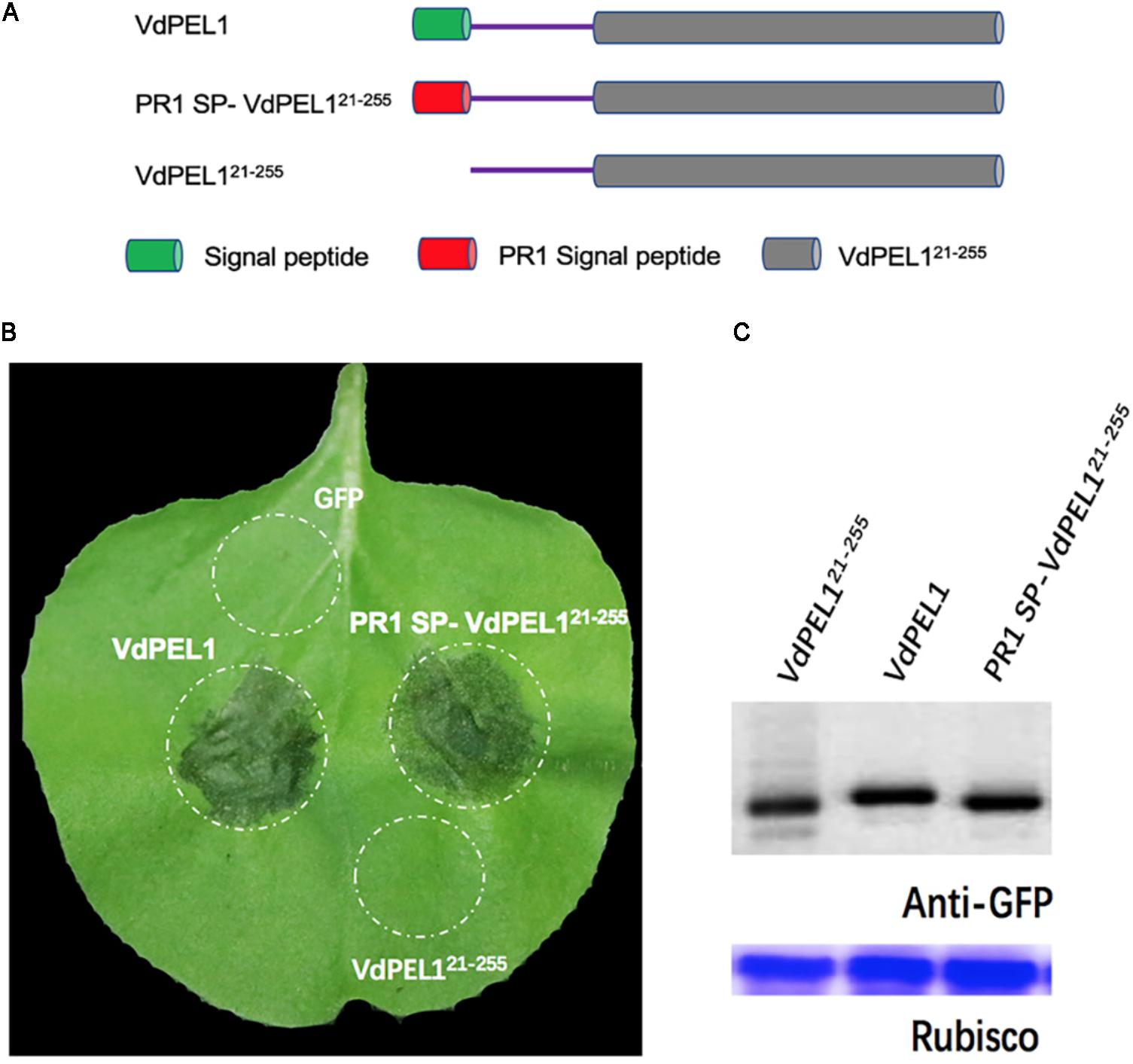
FIGURE 4. VdPEL1 is secreted into the apoplast in order to induce cell death. (A) Schematic presentation of the constructs examined. VdPEL1 (complete protein with the N-terminal signal peptide), VdPEL121-255 (deleted the N-terminal signal peptide), and PR1 SP-VdPEL121-255 (the native signal peptide replaced by the signal peptide from pathogenesis-related protein 1, PR1). (B) Cell death induction was detected in N. benthamiana leaves 5 days after infiltration with the various A. tumefaciens strains examined. (C) Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing the examined proteins from a pYBA1132 vector.
The Enzymatic Activity of VdPEL1 Is Required for Cell Death-Inducing Activity in N. benthamiana
Previously, pectate lyases from fungi were shown to degrade polygalacturonic acid via a β-elimination reaction (Kashyap et al., 2001). We assessed the hydrolase activity of VdPEL1 by determining the level of reducing sugar using polygalacturonic acid as substrates. We found that purified VdPEL1 had a hydrolase activity, which was affected by the temperature, Ca2+ concentration and pH (Supplementary Figure S2). Calcium (Ca2+) is known to be an essential co-factor for pectate lyases activity. The region around Ca2+ is believed to be the catalytic center site, especially aspartic acids, which are present in diverse members of the pectate lyase family (Heffron et al., 1995). As shown in Supplementary Figure S3, VdPEL1 contained two corresponding catalytic residues (D125 and D147) by sequence alignment. To examine the relationship between the enzymatic activity and cell death-inducing activity of VdPEL1, we replaced D125 and D147 with alanine (Ala) residues using site-directed mutagenesis and expressed the mutant proteins (VdPEL1rec) in P. pastoris (Figure 5A and Supplementary Figure S4). A hydrolase assay showed that pectate lyase activity of VdPEL1rec was abolished (Supplementary Table S1). In addition, purified VdPEL1 induced visible cell death in N. benthamiana, at a much higher level than the cell death symptoms after infiltration with VdPEL1rec (Figure 5B). We transiently expressed the wild type (VdPEL1) and mutant (VdPEL1rec) proteins in N. benthamiana. As expected, VdPEL1 triggered the cell death symptoms, while VdPEL1rec and GFP lacked the ability to produce necrosis at 5 days after agroinfiltration (Figure 5B). Two proteins in N. benthamiana were confirmed using immunoblot analysis (Figure 5C). These results suggested that the enzymatic activity of VdPEL1 is required for cell death-inducing activity in N. benthamiana.
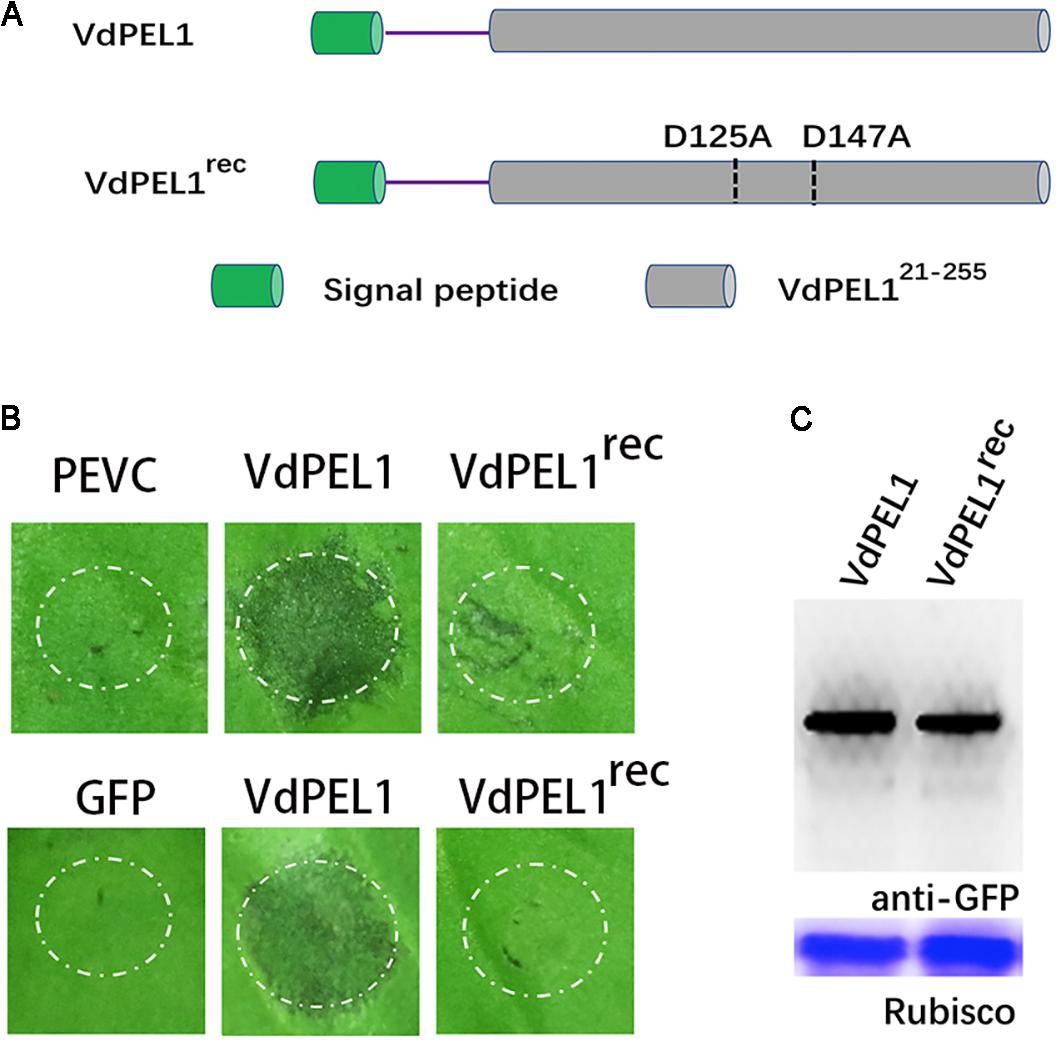
FIGURE 5. The enzymatic activity of VdPEL1 is required for cell death-inducing activity in N. benthamiana. (A) Schematic presentation of the examined constructs. VdPEL1 (the complete protein), VdPEL1rec (D125 and D147 replaced with alanine, Ala). (B) Upper pictures, cell death induction was detected in N. benthamiana leaves 2 days after infiltration with purified VdPEL1, VdPEL1rec, and PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same manner as VdPEL1). Lower pictures, cell death induction was detected in N. benthamiana leaves 5 days after infiltration with the examined various A. tumefaciens strains. (C) Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing the examined proteins from a pYBA1132 vector.
VdPEL1 Triggers Defense Responses and Systemic Resistance in N. benthamiana and Cotton
Plant immune system recognizes many cell death inducing proteins and activates host PTI, brings a series of typical characteristics such as accumulation of ROS, leakage of ion electrolytes, expression of defense genes, and callose deposition (Brutus et al., 2010; Zhang et al., 2014). To probe whether VdPEL1 induced immunity response, we first detected the accumulation of ROS. We observed intense staining in tobacco leaves treated with VdPEL1 (300 nM), whereas no DAB signal was detected in VdPEL1rec and PEVC treated leaves (Figure 6A). Meanwhile, VdPEL1 also induced electrolyte leakage and displayed an increase in conductivity over time, while VdPEL1rec or PEVC exhibited barely any change at the same concentration (Figure 6B). In addition, we examined the transcriptional induction of defense-responsive genes PR-1a and PR-5, which are involved in the SA-dependent defense pathway, PAL (phenylalanine ammonia lyase), NPR1 (the nonexpressor of PR1), and COI1 (CORONATINE INSENSITIVE 1), which is JA-responsive. As expected, the transcript levels of these defense genes were significantly up-regulated in N. benthamiana 24 h after treatment with VdPEL1 (Figure 6C). And VdPEL1rec induced a slight up-regulation of defense genes (NPR1 and PR-5) expression. Callose deposition was detected by aniline blue staining 24 h after VdPEL1, VdPEL1rec, PEVC, or flg22 treatment. N. benthamiana leaves inoculated with VdPEL1 or flg22 exhibited strong callose deposition compared with those inoculated with VdPEL1rec or PEVC, all of which exhibited low or undetectable levels of callose deposition (Figure 6D).
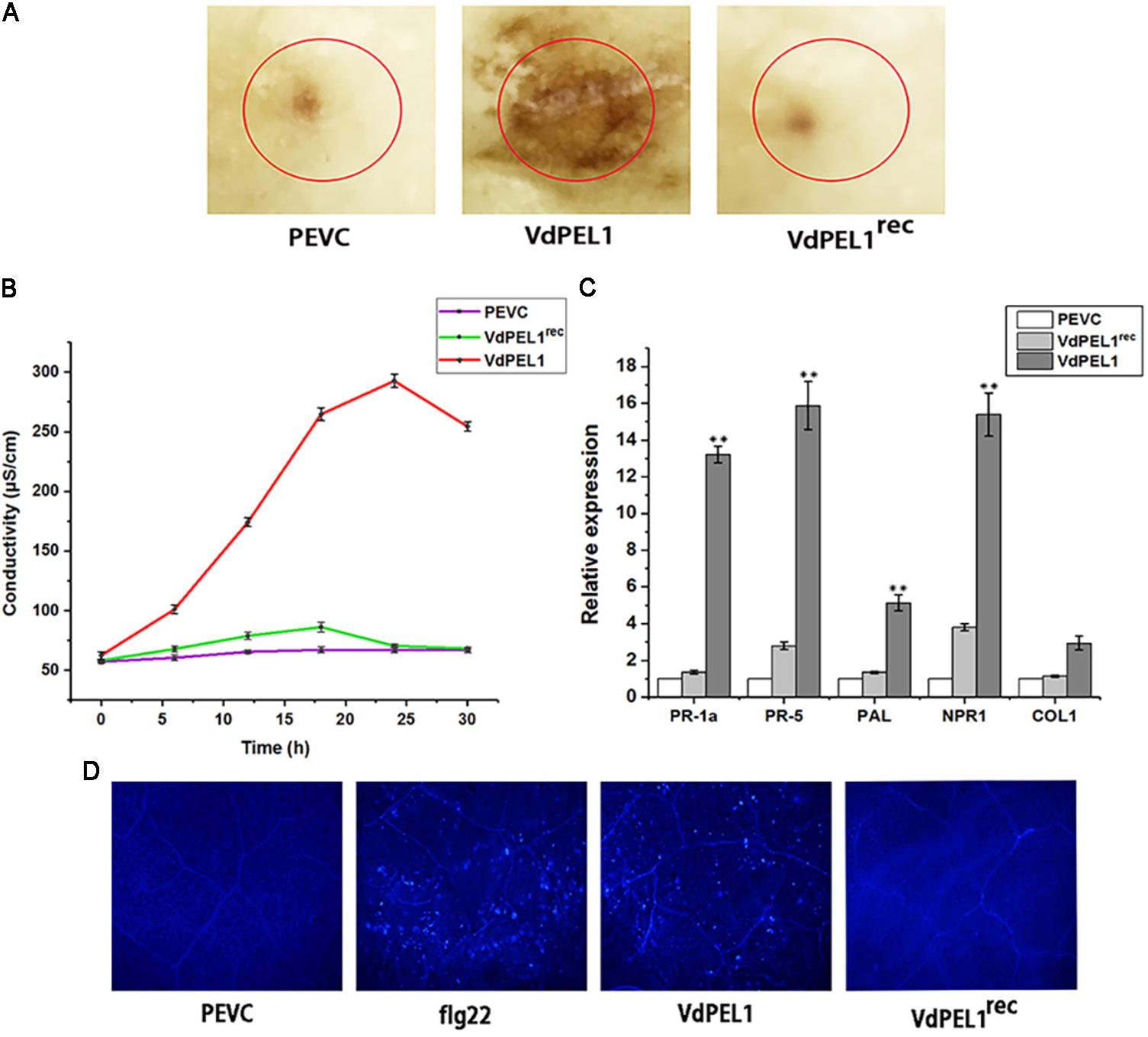
FIGURE 6. VdPEL1 induces defense responses in N. benthamiana. (A) ROS accumulation was detected in N. benthamiana leaves 12 h after infiltration of 300 nM purified VdPEL1, VdPEL1rec, and PEVC. The treated leaves were stained with DAB. (B) N. benthamiana leaves were infiltrated with 300 nM purified VdPEL1, VdPEL1rec, and PEVC. The conductivity was measured at the indicated time points. Error bars represent standard errors. (C) The transcripts of five defense response genes were measured in N. benthamiana leaves 24 h after infiltration of 300 nM purified VdPEL1, VdPEL1rec, and PEVC. qRT-PCR was performed. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between VdPEL1 and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value < 0.01. (D) Callose deposition in N. benthamiana leaves was detected 2 days after infiltration of 300 nM flg22, purified VdPEL1, VdPEL1rec, and PEVC; the treated leaves were stained with aniline blue.
Furthermore, to explore whether VdPEL1 conferred plants disease resistance, N. benthamiana leaves were pretreated with 300 nM recombinant protein VdPEL1, VdPEL1rec or PEVC, and 24 h later, they were inoculated with B. cinerea spore suspension. Leaves pretreatment with VdPEL1 restricted the development of B. cinerea infection, but leaves pretreatment with VdPEL1rec or PEVC displayed obvious lesion (Figure 7A). In addition, a histogram showed determination of B. cinerea lesion diameter (Figure 7B). In parallel, tobacco and cotton plants pretreatment with VdPEL1 had more resistance to V. dahliae, and significantly fewer verticillium wilt symptoms and fungal biomass compared with the plants treated with VdPEL1rec or PEVC controls (Figures 7C–F). These results collectively suggested that VdPEL1 had the capacity to trigger plant defense responses and confer disease resistance in N. benthamiana and cotton.
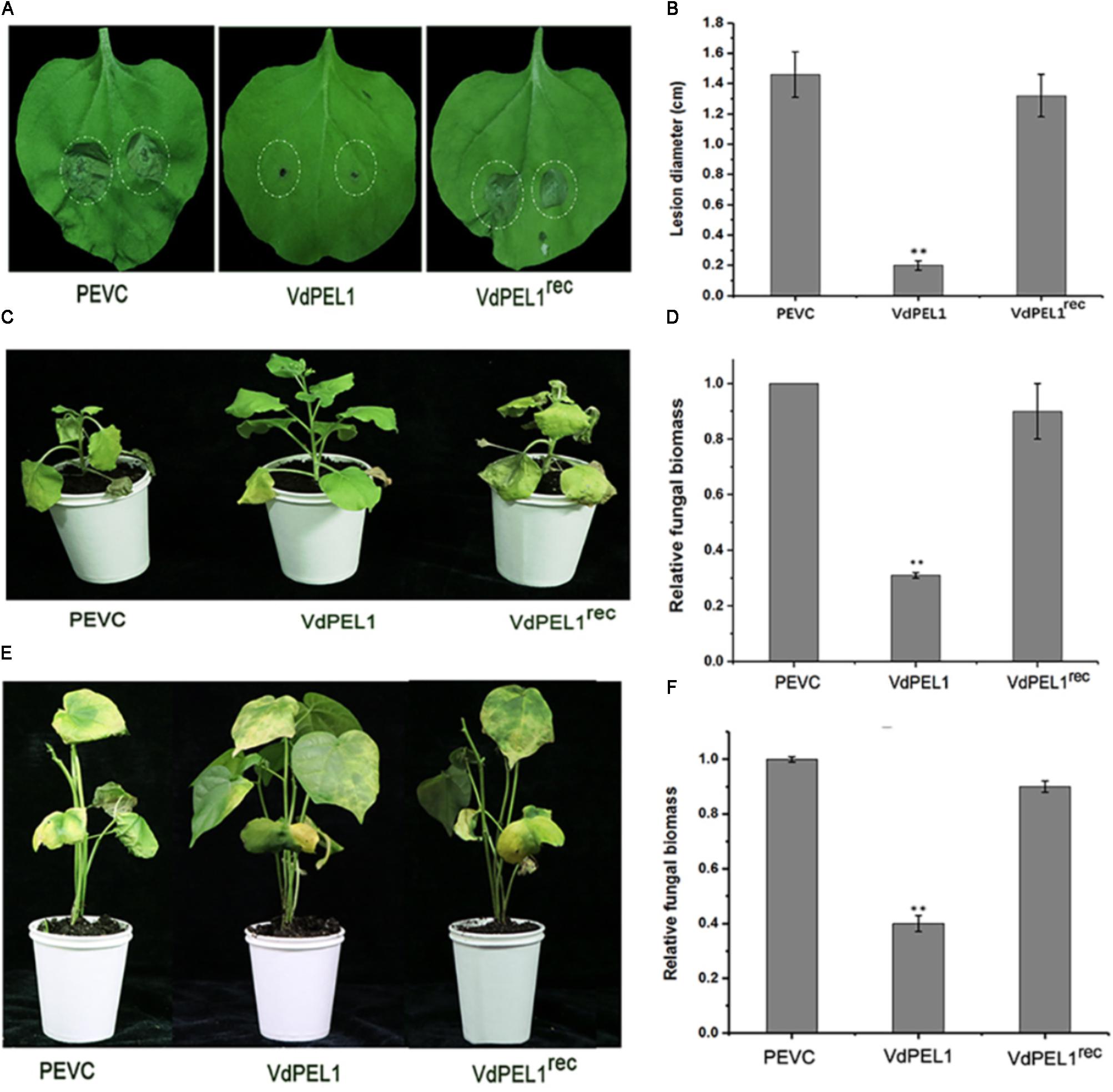
FIGURE 7. VdPEL1 confers disease resistance in N. benthamiana and cotton plants. (A) N. benthamiana leaves were pre-treated with 300 nM purified VdPEL1, VdPEL1rec, and PEVC. The treated leaves were inoculated with 5 μl of 2 × 106 conidia/ml Botrytis cinerea. Lesions symptoms were observed and photographed at 2 days post-inoculation. (B) Lesion diameter of B. cinerea on N. benthamiana leaves was measured after 2 days post-inoculation. The average lesion diameter on six leaves from six plants each was determined. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between VdPEL1 and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value < 0.01. (C) N. benthamiana leaves were pre-treated with 300 nM purified VdPEL1, VdPEL1rec, and PEVC and inoculated 24 h later with 1 × 106 conidia/ml V. dahliae. The phenotypes were observed and photographed at 12 days post-inoculation. (D) The fungal biomasses on the N. benthamiana plants were determined using qRT-PCR. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between VdPEL1 and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01. (E) Cotton leaves were pre-treated with 300 nM purified VdPEL1, VdPEL1rec, and PEVC and inoculated 24 h later with 1 × 106 conidia/ml V. dahliae. The phenotypes were observed and photographed at 21 days post-inoculation. (F) The fungal biomasses on cotton plants were determined using qRT-PCR. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between VdPEL1 and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01.
VdPEL1 Contributes to the Pathogenicity of V. dahliae
Pectate lyases are generally involved in the pathogenicity of fungi (Collmer, 1986). To determine the possible contribution of VdPEL1 to V. dahliae virulence, we assessed the expression patterns of VdPEL1 during different stages of post-inoculation. VdPEL1 was strongly expressed in tobacco and cotton at 1.5–3 days after inoculation, and then sharply declined from 3 days onward (Supplementary Figure S5). Next, we generated two independent VdPEL1 deletion lines (ΔVdPEL1-1 and ΔVdPEL1-2) and the complementary transformants (EC-1 and EC-2) by reintroducing the VdPEL1 gene (Supplementary Figure S6). All of the strains examined showed normal development, and there was no influence on radial growth and colony morphology (Supplementary Figure S7). To investigate the contribution of VdPEL1 to V. dahliae virulence, the wild type V. dahliae and VdPEL1 mutant strains were inoculated onto N. benthamiana plants and cotton plants. Interestingly, VdPEL1 deletion strains displayed significantly reduced virulence on tobacco plants compared with the wild-type V. dahliae. EC-1 and EC-2 recovered the high virulence phenotypes (Figure 8A). Similar results were observed in cotton plants 21 days after inoculation with all transformants. ΔVdPEL1-1 and ΔVdPEL1-2 decreased disease susceptibility, whereas EC-1 and EC-2 and wild strains caused more symptoms of necrosis, wilting, and vascular discoloration (Figure 8C). These results indicated that VdPEL1 played a positive role in V. dahliae virulence. This conclusion was further supported by the observation that the fungal biomass of VdPEL1 deletion lines was significantly lower than the biomass of the wild-type and complementary transformants in inoculated N. benthamiana and cotton plants (Figures 8B,D). These results confirmed that VdPEL1 contributed to virulence to V. dahliae.
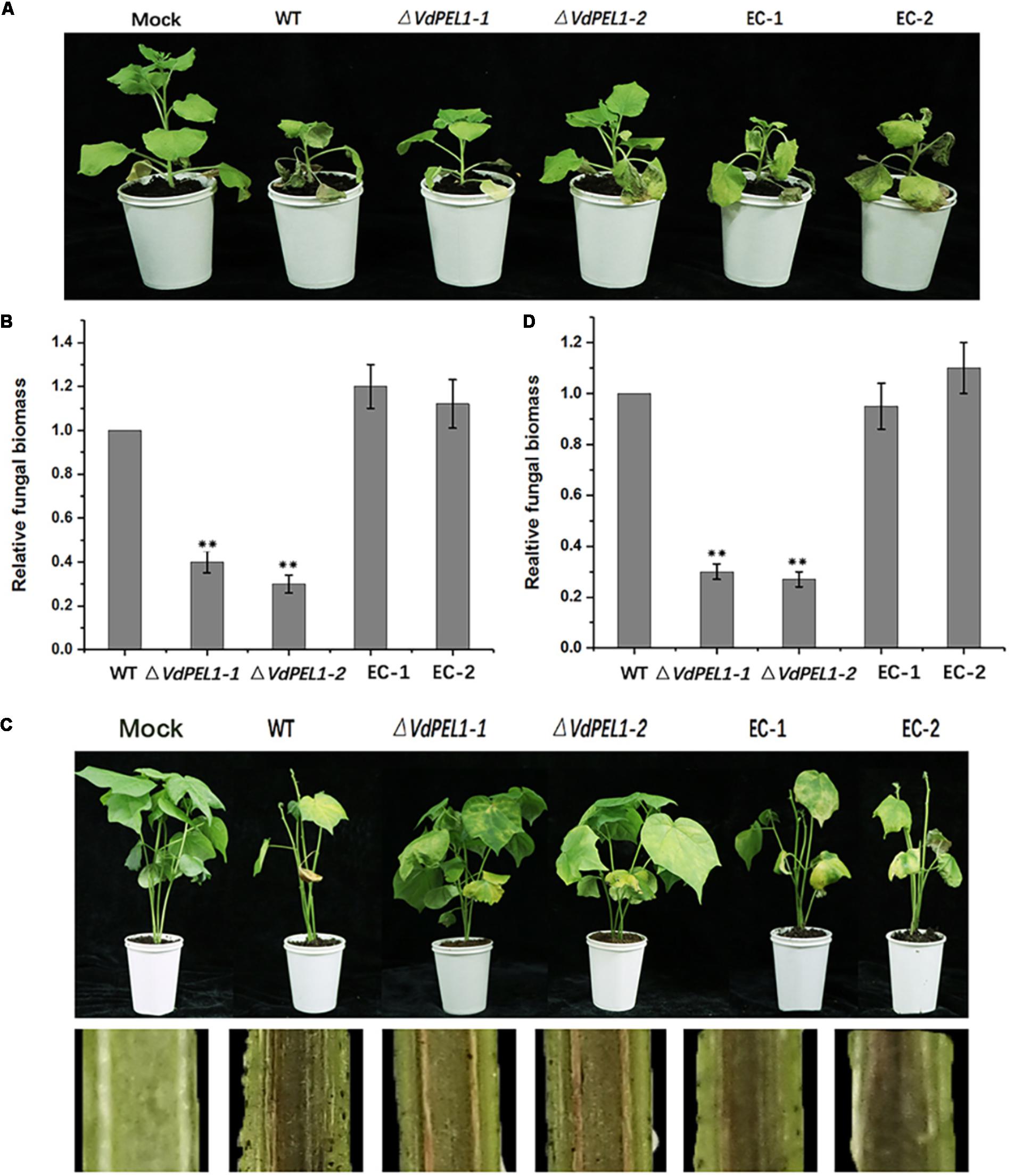
FIGURE 8. Detection of the virulence function of VdPEL1 during N. benthamiana and cotton pathogenesis. (A) Phenotypes of the inoculated N. benthamiana plants. Four-week-old seedlings of N. benthamiana were inoculated with sterile water (Mock), wild-type Verticillium dahliae (WT), VdPEL1 gene deletion strains (ΔVdPEL1-1 and ΔVdPEL1-2), and the complementary transformants (EC-1 and EC-2). The virulence phenotypes were photographed 12 days post-inoculation. (B) The fungal biomass quantification of WT, ΔVdPEL1-1 and ΔVdPEL1-2, and EC-1 and EC-2 inoculated on N. benthamiana was determined using qPCR. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between mutants and WT stain. Asterisks “∗∗” indicate statistically significant differences at a p-value < 0.01. (C) Phenotypes of inoculated cotton plants. Two-week-old seedlings of cotton were inoculated with sterile water (Mock), wild-type Verticillium dahliae (WT), VdPEL1 gene deletion strains (ΔVdPEL1-1 and ΔVdPEL1-2), and the complementary transformants (EC-1 and EC-2). The disease symptoms 21 days after inoculation are shown at the top, and the discoloration of the inoculation shoot longitudinal sections is shown at the bottom. (D) The fungal biomass quantification of WT, ΔVdPEL1-1 and ΔVdPEL1-2, and EC-1 and EC-2 inoculated on cotton plants were determined using qPCR. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between mutants and WT stain. Asterisks “∗∗” indicate statistically significant differences at a p-value < 0.01.
Discussion
The plant exocyst has recently emerged as an important battleground in plant–pathogen interactions (Žárský et al., 2013; Fujisaki et al., 2015). The plant cell wall serves as a natural barrier to limit the invasion of pathogens. To penetrate and colonize plants, phytopathogenic fungi produce a diverse group of plant CWDEs, which are involved in the generation of plant diseases and pathogenesis (King et al., 2011; Glass et al., 2013; Kubicek et al., 2014). Among the CWDEs, the pectate lyases are examined more closely because of their crucial roles in degrading plant pectin, which exists widely in plant cell walls and cell linings to maintain cell wall integrity. In this study, we identified a secreted pectate lyase VdPEL1 from V. dahliae culture supernatant, which has the ability to trigger immunity plant responses and contributes to V. dahliae virulence. In addition, our study also found that the enzymatic activity of VdPEL1 was necessary for induced cell death and PTI responses. Our data provide a new avenue to advance the understanding of host–pathogen interactions.
Verticillium dahliae, a soil-borne hemibiotrophic pathogen, attacks the plant roots and spreads to the leaves through the xylem vessels resulting in verticillium wilt of cotton and diseases in over 400 different plant species (Larsen et al., 2007; Vallad and Subbarao, 2008). Recent reports demonstrated that V. dahliae secreted a large amount of pectate lyases to catalyze the degradation of the pectin and facilitate penetration during its infection processes (Bartnicki-Garcia, 1968; Durrands and Cooper, 1988a,b; Pietro et al., 2009; Tzima et al., 2010). Although pectate lyases are particularly abundant and evolutionary preserved, phylogenetic analysis showed that the pectate lyases of V. dahliae are divided into four groups. According to the difference of virulence and defense responses in fungi and bacteria, pectate lyases were also distributed into four branches (Figure 2). We hypothesized that the pectate lyases play diverse functions in pathogenesis and confer the ability of V. dahliae to cause disease on such a broad host range.
The HR, a form of plant cell death in the tissues surrounding the lesion, is regarded as a plant defense response to block pathogen infection (Glazebrook, 2005; Jones and Dangl, 2006; Yamada et al., 2016). The ability to recognize a few nanograms of purified VdPEL1 resulting in rapid leaf tissue necrosis was observed in soybean, tomato, cotton, and N. benthamiana (Figure 3C). In addition, the range of plant species responding to VdPEL1 may be larger than we detected. We confirmed that VdPEL1 triggered defense responses, including the accumulation of ROS, leakage of ion electrolytes, deposition of callose, and expression of defense genes (Figure 6).
As we know, due to the diversity of the host and the inability of fungicides to affect the pathogen once in the plant vascular system, verticillium wilt diseases are difficult to control. The most sustainable manner to control these diseases is the use of resistant cultivars. Thus, it is relevant to identify the new PAMPs or DAMPs, which can provide materials for disease-resistant breeding. VdPEL1 could induce plant immunity and has the potential to be used in plant breeding and as a biological pesticide.
Previous studies showed that many fungal CWDEs, including xyloglucanases, glucanases, and cellulases, can trigger cell-death responses independent of their enzymatic activity (Ma et al., 2014, 2015; Gui Y.-J. et al., 2017; Zhu et al., 2017a). Endopolygalacturonase 1 (EG1) has two biological activities (enzymatic activity and elicitor activity) that are independent of each other in B. cinerea (Poinssot et al., 2003). However, an extracellular cutinase isolated from V. dahliae triggered plant defense responses required the enzymatic activity in N. benthamiana (Gui Y. et al., 2017). To test whether the enzymatic activity of VdPEL1 was required to trigger the plant immune responses, the site-directed mutagenesis of two residues in a conserved motif of VdPEL1 resulted in the loss of enzymatic activity. In contrast to VdPEL1, we surprisingly found that VdPEL1rec resulted in the loss of the cell death-inducing activity and the function of a series of plant defense responses and systemic resistance (Figures 6, 7). These results indicated that the enzymatic activity of VdPEL1 was necessary to trigger defense responses in N. benthamiana and cotton plants.
The pectate lyases have been implicated in pathogenicity and virulence in several plant pathogens. For example, in C. coccodes, the pectate lyase gene CcpelA contributes virulence on tomato, and in C. gleosporoides, deletion of the pectate lyase gene PelB resulted in a substantial loss of virulence on avocado (Persea americana) fruit (Yakoby et al., 2001; Ben-Daniel et al., 2011). Not all fungal pectate lyases have been conclusively shown to be involved in pathogenicity and virulence. For example, PelA (a pectate lyase gene form Fusarium graminearum) knock-out strains did not show attenuated virulence during the infection of wheat coleoptiles (Blum, 2017). The expression of VdPEL1 was most significantly up-regulated during the infection stage (1.5–3 days) before dropping sharply to the initial level (Supplementary Figure S5). The very strong and early expression of VdPEL1 may help the pathogen to extract nutrition, in addition to the more obvious role of physically facilitating invasion of the host tissue. Meanwhile, we observed that targeted VdPEL1 deletion resulted in significantly compromised virulence of Vd991 on tobacco and cotton plants (Figure 8).
Unlike effectors, which interferes with the plant defense response leading to ETS, VdPEL1 appeared to be a major virulence factor due to its enzymatic function, similar to VdCUT11 (Gui Y.-J. et al., 2017). It may indicate that VdPEL1 executes the pectate lyase activity to degrade pectin in the roots, which results in the invasion of the pathogens and the release of plant cell wall fragments (DAMPs). We speculated that the immunity triggered by VdPEL1 was likely to be mediated by the degradation of plant cell wall polymers that release pectin hydrolysis products (DAMPs), which in turn, trigger defense responses in plants.
The plant cell wall may be damaged by abiotic assaults or biotic assaults, resulting in the tissue or cellular damage, which is perceived as danger signals that function as DAMPs (Bianchi, 2006; Choi and Klessig, 2016). Generally, DAMPs appear in the apoplast and induce innate immune responses. For instance, cutin monomers and plant elicitor peptides (Peps), which are produced by pathogens depolymerization, can act as DAMPs (Chassot and Métraux, 2005; Huffaker et al., 2006; Yamaguchi et al., 2006). Similarly, OGs, fragments of the pectic polysaccharide homogalacturonan, can be released by pathogen-encoded hydrolytic enzymes to induce innate immune responses, including MAPK activation, callose deposition, ROS production and defense gene up-regulation (Decreux and Messiaen, 2005; Denoux et al., 2008).
Successful pathogens deliver effectors to surmount the host PTI response and establish infection (Jones and Dangl, 2006; Gimenez-Ibanez et al., 2009). For example, a RXLR effector suppressed XEG1-triggered immunity in oomycetes (Ma et al., 2015). In V. dahliae, carbohydrate-binding modules (CBMs) act as effectors, suppressing the GH12 protein and VdCUT11-triggered immunity and thus, facilitating host colonization (Gui Y. et al., 2017; Gui Y.-J. et al., 2017). Whether effectors mediate the suppression of VdPEL1 merits further investigation.
Author Contributions
YD and DQ designed the experiments. YY performed most of the experiments and wrote the paper. XY and BL participated in some part of the study.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 31701782 and 31371984).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to X. F. Dai from the Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, for the generous gift of the vectors for gene knockout and complementation. we also thank L. Yao from Beijing Academy of Agriculture and Forestry for providing the pYBA1132 plasmid and pPICZαA plasmid.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01271/full#supplementary-material
FIGURE S1. The MS/MS sequencing information. Proteins corresponding to Peak A were digested, and the peptides generated were analyzed using mass spectrometry. The detected peptides were covered with yellow color and matched against a protein from V. dahliae.
FIGURE S2. The analysis of the hydrolase activity of VdPEL1 with different temperatures, Ca2+ concentrations or pH. The reduced sugars were quantified using a standard calibration curve obtained with polygalacturonic acid. All the experiments were replicated three times. Standard errors from three biological replicates are shown.
FIGURE S3. Sequence alignment of the V. dahliae pectate lyase family proteins. Sequence alignment of all the V. dahliae pectate lyase family proteins and known cutinases from other fungi. The accession numbers of known pectate lyases from other fungi and bacteria are: 1EE6 (a pectate lyase from Bacillus sp. strain Ksm-P15), Pel A (pectate lyase A from Erwinia chrysanthemi). Two red triangles indicated possible catalytic residues of VdPEL1 (D125 and D147).
FIGURE S4. SDS-PAGE of VdPEL1 and VdPEL1rec recombinant proteins. VdPEL1 is the native protein; VdPEL1rec is a site-directed mutagenized protein, in which D125 and D147 were substituted with Ala. Two recombinant proteins were stained with Coomassie blue.
FIGURE S5. VdPEL1 expression analysis during infection of N. benthamiana and cotton roots. (A) The expression analysis of VdPEL1 in cotton roots. (B) The expression analysis of VdPEL1 in tobacco roots. The control (C) was mixed with non-inoculated conidia and cotton or tobacco root tissue. The housekeeping gene β-tubulin (VDAG_10074) was used as an endogenous control. Error bars represent standard errors.
FIGURE S6. Schematic view of the targeted deletion of VdPEL1 in V. dahliae. Two flanking sequences of the target gene and hygromycin resistance cassette were constructed into a fusion fragment. The fusion amplicon was integrated into the pGKO2-gateway vector using a homologous recombination method. The vectors were transferred into the Agrobacterium tumefaciens AGL-1 strain for fungal transformation with the wild-type (WT) strain Vd991. Two test primers were used to identify the fusion amplicon and positive targeted gene-deletion stains using PCR, respectively.
FIGURE S7. The targeted deletion of VdPEL1 does not affect radial growth and colony morphology. (A) The radial growth and colony morphology of the wild-type V. dahliae (WT), two VdPEL1 deletion strains (ΔVdPEL1-1 and ΔVdPEL1-2), and two ectopic transformants (EC-1 and EC-2) were determined after 11 days of incubation on PDA media at 25°C. (B) Colony diameters were determined at the time points indicated. Values shown are the average of three colony diameters. Error bars represent standard deviations.
TABLE S1. Hydrolysis activity test.
TABLE S2. Primers used in this study.
References
Bach, E., and Schollmeyer, E. (1992). An ultraviolet-spectrophotometric method with 2-cyanoacetamide for the determination of the enzymatic degradation of reducing polysaccharides. Anal. Biochem. 203, 335–339.
Bailey, B. A. (1996). Purification of a protein from culture filtrates of Fusarium oxysporum that Induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 85:1250. doi: 10.1094/Phyto-85-1250
Bartnicki-Garcia, S. (1968). Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu. Rev. Microbiol. 22, 87–108. doi: 10.1146/annurev.mi.22.100168.000511
Ben-Daniel, B.-H., Bar-Zvi, D., and Tsror Lahkim, L. (2011). Pectate lyase affects pathogenicity in natural isolates of Colletotrichum coccodes and in pelA gene-disrupted and gene-overexpressing mutant lines. Mol. Plant Pathol. 13, 187–197. doi: 10.1111/j.1364-3703.2011.00740.x
Bianchi, M. E. (2006). DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5. doi: 10.1189/jlb.0306164
Bindschedler, L. V., Dewdney, J., Blee, K. A., Stone, J. M., Asai, T., Plotnikov, J., et al. (2006). Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 47, 851–863. doi: 10.1111/j.1365-313X.2006.02837.x
Blum, A. (2017). Regulation and Cell Biology of Secondary Metabolite Production in Fusarium graminearum and Fusarium pseudograminearum. Ph.D. thesis, St Lucia: School of Agriculture and Food Sciences.
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Brito, N., Espino, J. J., and González, C. (2006). The endo-beta-1,4-xylanase xyn11A is required for virulence in Botrytis cinerea. MPMI 19, 25–32. doi: 10.1094/MPMI-19-0025
Brutus, A., Sicilia, F., Macone, A., Cervone, F., and De Lorenzo, G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. U.S.A. 107, 9452–9457. doi: 10.1073/pnas.1000675107
Chassot, C., and Métraux, J.-P. (2005). The cuticle as source of signals for plant defense. Plant Biosyst. 139, 28–31. doi: 10.1080/11263500500056344
Chen, M., Zeng, H., Qiu, D., Guo, L., Yang, X., Shi, H., et al. (2012). Purification and characterization of a novel hypersensitive response-inducing elicitor from Magnaporthe oryzae that triggers defense response in rice. PLoS One 7:e37654. doi: 10.1371/journal.pone.0037654
Chisholm, S. T., Coaker, G., Day, B., and Staskawicz, B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. doi: 10.1016/j.cell.2006.02.008
Choi, H. W., and Klessig, D. F. (2016). DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 16:232. doi: 10.1186/s12870-016-0921-2
Collmer, A. (1986). The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24, 383–409. doi: 10.1146/annurev.phyto.24.1.383
Couto, D., and Zipfel, C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. doi: 10.1038/nri.2016.77
De Lorenzo, G., Brutus, A., Savatin, D. V., Sicilia, F., and Cervone, F. (2011). Engineering plant resistance by constructing chimeric receptors that recognize damage-associated molecular patterns (DAMPs). FEBS Lett. 585, 1521–1528. doi: 10.1016/j.febslet.2011.04.043
Decreux, A., and Messiaen, J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46, 268–278. doi: 10.1093/pcp/pci026
Denoux, C., Galletti, R., Mammarella, N., Gopalan, S., Werck, D., De Lorenzo, G., et al. (2008). Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 1, 423–445. doi: 10.1093/mp/ssn019
Durrands, P. K., and Cooper, R. M. (1988a). Selection and characterization of pectinase-deficient mutants of the vascular wilt pathogen Verticillium albo-atrum. Physiol. Mol. Plant Pathol. 32, 343–362. doi: 10.1016/S0885-5765(88)80029-8
Durrands, P. K., and Cooper, R. M. (1988b). The role of pectinases in vascular wilt disease as determined by defined mutants of Verticillium albo-atrum. Physiol. Mol. Plant Pathol. 32, 363–371. doi: 10.1016/S0885-5765(88)80030-4
Fradin, E. F., and Thomma, B. P. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 7, 71–86. doi: 10.1111/j.1364-3703.2006.00323.x
Fujisaki, K., Abe, Y., Ito, A., Saitoh, H., Yoshida, K., Kanzaki, H., et al. (2015). Rice Exo70 interacts with a fungal effector,AVR-Pii, and is required for AVR-Pii-triggered immunity. Plant J. 83, 875–887. doi: 10.1111/tpj.12934
Gimenez-Ibanez, S., Hann, D. R., Ntoukakis, V., Petutschnig, E., Lipka, V., and Rathjen, J. P. (2009). AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 19, 423–429. doi: 10.1016/j.cub.2009.01.054
Glass, N. L., Schmoll, M., Cate, J. H. D., and Coradetti, S. (2013). Plant cell wall deconstruction by ascomycete fungi. Annu. Rev. Microbiol. 67, 477–498. doi: 10.1146/annurev-micro-092611-150044
Glazebrook, J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. doi: 10.1146/annurev.phyto.43.040204.135923
Gui, Y., Zhang, W., Zhang, D., Zhou, L., Short, D. P. G., Wang, J., et al. (2017). A Verticillium dahliae extracellular cutinase modulates plant immune. Responses. Mol. Plant Microbe Interact. 31, 260–273. doi: 10.1094/MPMI-06-17-0136-R
Gui, Y.-J., Chen, J.-Y., Zhang, D.-D., Li, N.-Y., Li, T.-G., Zhang, W.-Q., et al. (2017). Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environ. Microbiol. 19, 1914–1932. doi: 10.1111/1462-2920.13695
Have, A. T., Mulder, W., Visser, J., and van Kan, J. A. L. (1998). The Endopolygalacturonase gene Bcpg1Is required for full virulence of Botrytis cinerea. MPMI 11, 1009–1016. doi: 10.1094/MPMI.1998.11.10.1009
Heese, A., Hann, D. R., Gimenez-Ibanez, S., Jones, A. M. E., He, K., Li, J., et al. (2007). The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. U.S.A. 104, 12217–12222.
Heffron, S., Henrissat, B., Yoder, M. D., Lietzke, S., and Jurnak, F. (1995). Structure-based multiple alignment of extracellular pectate lyase sequences. MPMI 8, 331–334.
Houterman, P. M., Cornelissen, B. J. C., and Rep, M. (2008). Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 4:e1000061. doi: 10.1371/journal.ppat.1000061
Huffaker, A., Pearce, G., and Ryan, C. A. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. U.S.A. 103, 10098–10103. doi: 10.1073/pnas.0603727103
Inderbitzin, P., Thomma, B. P. H. J., Klosterman, S. J., and Subbarao, K. V. (2014). “Verticillium alfalfae and V. dahliae, agents of Verticillium Wilt diseases,” in Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens, ed. C. Kole (Berlin: Springer), 65–97.
Jones, J. D. G., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kars, I., Krooshof, G. H., Wagemakers, L., Joosten, R., Benen, J. A. E., and van Kan, J. A. L. (2005). Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 43, 213–225. doi: 10.1111/j.1365-313X.2005.02436.x
Kashyap, D. R., Vohra, P. K., Chopra, S., and Tewari, R. (2001). Applications of pectinases in the commercial sector: a review. Bioresour. Technol. 77, 215–227.
Kikot, G. E., Hours, R. A., and Alconada, T. M. (2009). Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. J. Basic Microbiol. 49, 231–241. doi: 10.1002/jobm.200800231
King, B. C., Waxman, K. D., Nenni, N. V., Walker, L. P., Bergstrom, G. C., and Gibson, D. M. (2011). Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol. Biofuels 4:4. doi: 10.1186/1754-6834-4-4
Klöckner, A., Bühl, H., Viollier, P., and Henrichfreise, B. (2016). Deconstructing the chlamydial cell wall. Curr. Top. Microbiol. Immunol. 412, 1–33. doi: 10.1007/82_2016_34
Klosterman, S. J., Atallah, Z. K., Vallad, G. E., and Subbarao, K. V. (2009). Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 47, 39–62. doi: 10.1146/annurev-phyto-080508-081748
Kubicek, C. P., Starr, T. L., and Glass, N. L. (2014). Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 52, 427–451. doi: 10.1146/annurev-phyto-102313-045831
Kulye, M., Liu, H., Zhang, Y., Zeng, H., Yang, X., and Qiu, D. (2012). Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 35, 2104–2120. doi: 10.1111/j.1365-3040.2012.02539.x
Larsen, R. C., Vandemark, G. J., Hughes, T. J., and Grau, C. R. (2007). Development of a real-time polymerase chain reaction assay for quantifying Verticillium albo-atrumDNA in resistant and susceptible alfalfa. Phytopathology 97, 1519–1525. doi: 10.1094/PHYTO-97-11-1519
Liu, Z., Wu, Y., Yang, F., Zhang, Y., Chen, S., Xie, Q., et al. (2013). BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc. Natl. Acad. Sci. U.S.A. 110, 6205–6210. doi: 10.1073/pnas.1215543110
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, Y., Han, C., Chen, J., Li, H., He, K., Liu, A., et al. (2014). Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity. Mol. Plant Pathol. 16, 14–26. doi: 10.1111/mpp.12156
Ma, Z., Song, T., Zhu, L., Ye, W., Wang, Y., Shao, Y., et al. (2015). A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27, 2057–2072. doi: 10.1105/tpc.15.00390
Misas-Villamil, J. C., and van der Hoorn, R. A. (2008). Enzyme–inhibitor interactions at the plant–pathogen interface. Curr. Opin. Plant Biol. 11, 380–388. doi: 10.1016/j.pbi.2008.04.007
Pandey, A., and Sonti, R. V. (2010). Role of the feob protein and siderophore in promoting virulence of Xanthomonas oryzae pv. oryzae on rice. J. Bacteriol. 192, 3187–3203. doi: 10.1128/JB.01558-09
Pietro, A. D., Roncero, M. I. G., and RoldÁn, M. C. R. (2009). “From tools of survival to weapons of destruction: the role of cell wall-degrading enzymes in plant infection,” in The Mycota, ed. D. H. Howard (Berlin: Springer), 181–200.
Poinssot, B., Vandelle, E., Bentéjac, M., Adrian, M., Levis, C., Brygoo, Y., et al. (2003). The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. MPMI 16, 553–564. doi: 10.1094/MPMI.2003.16.6.553
Saijo, Y., Loo, E. P.-I., and Yasuda, S. (2017). Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 93, 592–613. doi: 10.1111/tpj.13808
Santhanam, P., van Esse, H. P., Albert, I., Faino, L., Nürnberger, T., and Thomma, B. P. H. J. (2013). Evidence for functional diversification within a fungal NEP1-Like protein family. MPMI 26, 278–286. doi: 10.1094/MPMI-09-12-0222-R
Stergiopoulos, I., and de Wit, P. J. G. M. (2009). Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. doi: 10.1146/annurev.phyto.112408.132637
Takahashi, Y., Berberich, T., Yamashita, K., Uehara, Y., Miyazaki, A., and Kusano, T. (2004). Identification of Tobacco HIN1 and two closely related genes as spermine-responsive genes and their differential expression during the tobacco mosaic virus-induced hypersensitive response and during leaf- and flower-senescence. Plant Mol. Biol. 54, 613–622. doi: 10.1023/B:PLAN.0000038276.95539.39
Tzima, A., Paplomatas, E. J., Rauyaree, P., and Kang, S. (2010). Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet. Biol. 47, 406–415. doi: 10.1016/j.fgb.2010.01.007
Vallad, G. E., and Subbarao, K. V. (2008). Colonization of resistant and susceptible Lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 98, 871–885. doi: 10.1094/PHYTO-98-8-0871
Wegener, C. B. (2002). Induction of defence responses against Erwinia soft rot by an endogenous pectate lyase in potatoes. Physiol. Mol. Plant Pathol. 60, 91–100. doi: 10.1006/pmpp.2002.0377
Yakoby, N., Beno-Moualem, D., Keen, N. T., Dinoor, A., Pines, O., and Prusky, D. (2001). Colletotrichum gloeosporioides pelBIs an important virulence factor in avocado fruit-fungus interaction. MPMI 14, 988–995. doi: 10.1094/MPMI.2001.14.8.988
Yamada, K., Yamashita-Yamada, M., Hirase, T., Fujiwara, T., Tsuda, K., Hiruma, K., et al. (2016). Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J. 35, 46–61. doi: 10.15252/embj.201591807
Yamaguchi, Y., Pearce, G., and Ryan, C. A. (2006). The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. U.S.A. 103, 10104–10109. doi: 10.1073/pnas.0603729103
Yan, X., Ye, Y., and Zeng, G. (2012). pYBA100: an ease-of-use binary vector with LoxP-FRT recombinase Site for plant transformation. Mol. Plant Breed. 10, 371–379.
Yu, X., Tang, J., Wang, Q., Ye, W., Tao, K., Duan, S., et al. (2012). The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 196, 247–260. doi: 10.1111/j.1469-8137.2012.04241.x
Žárskı, V., Kulich, I., Fendrych, M., and Peèenková, T. (2013). Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant Biol. 16, 726–733. doi: 10.1016/j.pbi.2013.10.013
Zhang, L., Kars, I., Essenstam, B., Liebrand, T. W. H., Wagemakers, L., Elberse, J., et al. (2014). Fungal endopolygalacturonases are recognized as microbe-associated molecular patterns by the Arabidopsis receptor-like protein responsiveness to botrytis polygalacturonases1. Plant Physiol. 164, 352–364. doi: 10.1104/pp.113.230698
Zhang, L., Ni, H., Du, X., Wang, S., Ma, X.-W., Nürnberger, T., et al. (2017). The Verticillium-specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytol. 215, 368–381. doi: 10.1111/nph.14537
Zhao, P., Zhao, Y.-L., Jin, Y., Zhang, T., and Guo, H.-S. (2014). Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 5, 94–98. doi: 10.1007/s13238-013-0009-9
Zhou, L., Zhao, J., Guo, W., and Zhang, T. (2013). Functional analysis of autophagy genes via agrobacterium-mediated transformation in the vascular wilt fungus Verticillium dahliae. J. Genet. Genomics 40, 421–431. doi: 10.1016/j.jgg.2013.04.006
Zhu, W., Ronen, M., Gur, Y., Minz-Dub, A., Masrati, G., Ben-Tal, N., et al. (2017a). BcXYG1, a secreted xyloglucanase from Botrytis cinerea, triggers both cell death and plant immune responses. Plant Physiol. 175, 438–456. doi: 10.1104/pp.17.00375
Keywords: Verticillium dahliae, pectate lyase, elicitor, plant immunity, virulence
Citation: Yang Y, Zhang Y, Li B, Yang X, Dong Y and Qiu D (2018) A Verticillium dahliae Pectate Lyase Induces Plant Immune Responses and Contributes to Virulence. Front. Plant Sci. 9:1271. doi: 10.3389/fpls.2018.01271
Received: 29 April 2018; Accepted: 14 August 2018;
Published: 13 September 2018.
Edited by:
Thomas Mitchell, The Ohio State University, United StatesReviewed by:
Raffaella Balestrini, Consiglio Nazionale delle Ricerche (CNR), ItalyPhilipp Franken, Leibniz-Institut für Gemüse- und Zierpflanzenbau (IGZ), Germany
Copyright © 2018 Yang, Zhang, Li, Yang, Dong and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijie Dong, bmtkb25neWlqaWVAMTYzLmNvbQ== Dewen Qiu, cWl1ZGV3ZW5AY2Fhcy5jbg==
 Yuankun Yang
Yuankun Yang Yi Zhang
Yi Zhang Beibei Li
Beibei Li Xiufen Yang
Xiufen Yang Yijie Dong
Yijie Dong Dewen Qiu
Dewen Qiu