
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 04 July 2018
Sec. Plant Breeding
Volume 9 - 2018 | https://doi.org/10.3389/fpls.2018.00957
This article is part of the Research TopicOrgan Modification for Edible Parts of Horticultural CropsView all 9 articles
The phytohormone auxin is involved in many aspects of plant growth and developmental processes. The tomato Aux/IAA transcription factor SlIAA9/ENTIRE/E plays an important role in leaf morphogenesis and fruit development, and the E gene encodes a protein from the Aux/IAA family of auxin response repressors. Both SlIAA9-RNAi transgenic and entire (e) mutant plants reduce the leaf complexity in tomato, but the underlying mechanism is not yet completely resolved. Auxin signaling is known to regulate target genes expression via Aux/IAA and ARFs (auxin response factors) transcriptional regulators. ARFs mediate a wide range of developmental processes. Through an Y2H (yeast two-hybrid) assay coupled with expression profiling of the SlARF genes family, we identified a group of ARFs: SlARF6A, SlARF8A, SlARF8B, and SlARF24. Pull-down and BiFC (Bimolecular Fluorescence Complementation) results demonstrated that these SlARFs interact with SlIAA9 in vitro and in vivo, and the e mutation altered the expression patterns of multiple SlARFs. The simple leaves of the e mutant were partially converted to wild-type compound leaves by VIGS (virus-induced gene silencing) of these four SlARFs. Furthermore, IAA content in these samples was significantly increased compared to the e mutant. In addition, SlARF6A and SlARF24 bound to the SlPIN1 promoter and act as transcriptional activators to regulate genes expression involved in leaflet initiation. It may also suggest that SlARFs regulate leaf morphology through direct binding to auxin-responsive genes in the absence of SlIAA9, providing an insight for the role of SlARFs in leaf shape development.
We firstly found that SlARF6A, SlARF8A, SlARF8B, and SlARF24 could regulate tomato leaf development in a redundant manner; Furthermore, SlARF6A and SlARF24 bound to the SlPIN1 promoter to regulate genes expression involved in leaflet initiation.
Leaves are one of the main organs of flowering plants, and exhibit a tremendous diversity in shape and size. The shape of leaves varies enormously within the same species and individual plants, and can be ascribed to ranging from simple to compound. Variation is one of the most conspicuous aspects of plant diversity in leaf shape. This diversity is often achieved by the adjustment of leaf blade dissection to form lobes or leaflets (Ben-Gera et al., 2012). Simple leaves comprise of a single continuous blade, whereas compound leaves are composed of multiple discontinuous blade units termed as leaflets (Koenig et al., 2009). Leaves are formed at the flanks of the shoot apical meristem (SAM). Following the initiation of new shoot morphogenesis, leaves establish the basic framework for shape and size. Subsequently, organogenesis of lateral appendages occurs through differentiation and expansion of leaf tissue (Shwartz et al., 2016). Furthermore, the wild type leaves consist of primary, secondary, and intercalary leaflets with lobed margins in tomato (Berger et al., 2009).
After the formation and differentiation of the leaf primordia in the SAM, the development of the leaf primordium occurs (Merelo et al., 2016). Previous studies have discovered that two mechanisms are involved in the development of the leaf primordia. The first occurs through mutual repression between KNOX proteins and ARP (AS1/RS2/PHAN) MYB-domain proteins (Waites et al., 1998; Tsiantis et al., 1999; Byrne et al., 2000; Ori et al., 2000). The second mechanism demonstrates that the formation of leaf delimitation is controlled by PINOID (PID) and auxin efflux carrier PIN-FORMED1 (PIN1) which mediates local auxin accumulation (Furutani et al., 2004). In leaf development, AS1 represses the expression of KNOX gene BP (BREVIPEDICELLUS), while the function of the local auxin maxima alongside AS1 remains partly dependent on BP regulation. The SHOOT MERISTEMLESS (STM) gene is needed for SAM formation and maintenance, which prevents AS1 gene expression in the meristem (Byrne et al., 2000). In addition, auxin activities and KNOX proteins might form a feedback loop to facilitate leaf meristem delimitation (Zgurski et al., 2005; Hay et al., 2006).
Studies have indicated that leaf development is coordinated by a cross-talk between different hormones (Shwartz et al., 2016) with auxin playing a crucial role. The precise distribution and location of auxin signaling regulates proper leaf development in a specific spatiotemporal developmental context (Bilsborough et al., 2011). The auxin maxima is concentrated on the leaflet initiation area in the development of tomato leaves, resulting in lamina growth patterns (Ben-Gera et al., 2016). Auxin acts as an inducer of organogenesis. There are postulated inhibitory fields around existing primordia which are thought to result from low concentrations of auxin (Reinhardt et al., 2003; de Reuille et al., 2006; Jonsson et al., 2006). Endogenous auxin levels and localization are altered in developing leaves leads to leaf simplification phenotypes (Shwartz et al., 2016). SlIAA9 is an auxin regulator belonging to the Aux/IAA transcription factors gene family. Not only do SlIAA9-RNAi plants display simple leaves and parthenocarpy instead of compound leaves and seeded fruit characteristic typically seen from wild type (AC), but also the silenced plants have auxin-related growth alterations (Wang et al., 2005). The adjustment of Aux/IAA and SlARF genes and the downregulation of MADS box genes mediate fruiting, and early fruit development is regulated by the regulatory and metabolic events both in the absence and presence of pollination/fertilization (Wang et al., 2009). Meanwhile, tomato e mutant is reported as a single-base deletion in the coding region of the SlIAA9 gene and exhibits single based lamina with primary leaves partially fused (Zhang et al., 2007), E mRNA is discovered throughout the leaf margin (Koenig et al., 2009). Thus, SlIAA9 plays a role in limiting lamina growth between developing leaflets by locally inhibiting auxin responses. GOB (GOBLET) encodes a NAC-domain transcription factor and its expression is intact in the simplified leaves of entire (e) mutants in tomato. Leaves of single gob or e mutants formed only primary leaflets, and downregulation of both GOB and E (SlIAA9) contributed to the complete abolishment of leaflet initiation. This indicates those auxin response and leaflet morphogenesis are modulated by GOB and E via partly redundant pathways (Blein et al., 2008; Ben-Gera et al., 2012). The tomato clau (clausa) mutant exhibits elaborate compound leaves. CLAU might negatively regulate the expression of GOB, and GOB expression is up-regulated in the compound leaf mutant lyr (lyrate). However, the enhancement of the clau phenotype by lyr indicates that clau and lyr affect GOB and leaf development in different pathways (Bar et al., 2015). Higher expression of LA (LANCEOLATE) during the early stages of leaf development result in a simpler leaf shape, likely regulated in part by gibberellic acid (GA) levels (Yanai et al., 2011). The expression of TKn1 in the leaf primordium is needed for compound structure formation (Hareven et al., 1996). miR164 negatively regulates GOB-like genes, and leaf-specific overexpression of miR164 induces a loss of secondary leaflet initiation and smooth leaflet margins (Berger et al., 2009). The miR160 targets a group of ARFs which antagonize lamina growth and auxin response in conjunction with E plants. Leaflet separation is assured by different type of auxin signal antagonists (Ben-Gera et al., 2016). Auxin, E, GOB, LYR, and mir160-targeted ARFs collaborate to specify leaflet initiation and promote leaflet separation (Kimura et al., 2008). However, the underlying molecular mechanism of how functional redundancies among SlARF proteins regulate leaf shape development in tomato remains an open question.
Aux/IAA protein can repress ARF transcription factors via protein to protein interaction (Tiwari et al., 2001), and the degradation of Aux/IAA proteins can relieve ARF proteins for auxin-responsive gene transcription (Tan et al., 2007). SlIAA9 protein mediates leaf morphogenesis by participating in auxin signal transduction (Wang et al., 2005; Goetz et al., 2007). Furthermore, ARFs represent essential factors in the transduction of auxin signaling, and multiple ARFs were previously shown to interact with IAA9 (Korasick et al., 2014; Piya et al., 2014). In this work, we showed that tomato plants with a silenced SlIAA9 complex change from simple to complex leaf morphology, providing an insight for the significant role of functional redundancies among SlARF proteins in leaf morphogenesis. This indicates that SlARFs may mediate phenotypic plasticity in foliar organogenesis, and that further studies on SlARFs may reveal insights into the evolution of plant leaves.
Tomato plants (Solanum lycopersicum cv. Ailsa Craig) and e mutants in the Ailsa Craig background were grown under standard greenhouse conditions (14 h day/10 h night cycle, 25/20°C day/night temperature, 60–75% relative humidity). The e mutation plants prepared for VIGS assay were kept in a growth chamber (16 h day/8 h night cycle, 20–22°C, 50% relative humidity).
Total RNA from all samples was isolated using the TRIzol reagent (Invitrogen, United States). The RNA was treated with DNase I at 37°C for 30 min to remove residual genomic DNA. Using the HiScript II 1st cDNA Synthesis Kit (Vazyme, China) to synthesis the first-strand cDNA according to the manuscript’s protocol. The cDNA concentrations were normalized according to actin expression levels for qRT-PCR analysis. qRT-PCRs were performed using the power SYBR Premix Ex Taq kit and the TaKaRa two-step method (TaKaRa, Japan). PCR products were quantified using the Roche Light Cycler 480 Real-Time PCR Detection System and the SYBR Green I Master Kit (Roche, Switzerland). The PCR program was as follows: 95°C for 45 s; 40 cycles of 95°C for 10 s, 58°C for 25 s, and 72°C for 20 s. For all qRT-PCR experiments, at least three biological replicates were performed, and each reaction was run in triplicate.
For yeast two-hybrid assay, the full-length coding sequence of each SlARF (Supplementary Table S1) and SlIAA9 (Solyc04g076850) were cloned from various tissues of Ailsa Craig. Recombinant plasmid pGBKT7-SlIAA9 and pGADT7-SlARF were constructed by inserting SlIAA9 and SlARF into pGBKT7 and pGADT7 vectors separately. The two plasmids were co-transformed into the yeast strain AH109 by small-scale yeast transformation method. The transformants grew on the SD/-Trp/-Leu drop-out medium. After colony formation, transformants were transferred to SD/-Leu/-Trp/-His/-Ade drop-out medium with 40 μg ml-1 X-gal.
The SlIAA9 ORF without the stop codon was constructed using the pUC-SPYNE/pSPYNE-35S vector to produce SlIAA9-YFPN fusions, and the SlARF ORFs without the stop codon were cloned into the pUC-SPYCE/pSPYCE-35S vector to generate SlARFs-YFPC fusion proteins. Protoplasts were extracted from 1-week-old Arabidopsis Col-0 suspension cell culture and the corresponding constructs were co-transformed into them. The transfected protoplasts were assayed for fluorescence after 12–18 h of expression. All primer sequences used in this analysis are listed in Supplementary Table S2.
The recombinant plasmids were transformed into BL21(DE3)pLysS chemically competent cells. SlIAA9-GST was purified with Glutathione Agarose (Thermo Fisher Scientific, United States) according to the company manual instruction. MBP, MBP-SlARF6A, MBP-SlARF8A, MBP-SlARF8B, and MBP-SlARF24 were purified as fusion proteins immobilized with amylose resin (New England Biolabs, United States) following standard protocols. Five micrograms of GST-SlIAA9 protein were pre-incubated with 10 μL pre-washed amylose resin in 150 μL incubation buffer (1 mM NaCl, 20 mM MgCl2, 0.2% Triton X-100, and 0.1 M HEPES at pH7.2) for 1 h at 4°C. The resin was collected by centrifugation and washed five times with washing buffer (20 mM Tris-HCl at pH 7.5, 300 mM NaCl, 0.1 mM EDTA, 0.5% Triton-X100). The pull-down proteins were detected by western blot with an α-GST antibody (Thermo Fisher Scientific, United States). All primer sequences used in this analysis are listed in Supplementary Table S2.
To generate the VIGS constructs, 208 bp, 157 bp, and 238 bp fragments of the gene SlARF6A, SlARF8A, and SlARF24 was amplified by sequence-specific primers (Supplementary Table S2), respectively. Since SlARF8A and SlARF8B are highly homologous, the fragment from SlARF8A was used to silence both SlARF8A and SlARF8B. All of the pTRV1, pTRV2, pTRV2-PDS, and pTRV2-host target genes were transformed into the Agrobacterium tumefactions strain GV3101 by electroporation. Cultures containing the pTRV1 and pTRV2 vectors were mixed in a 1:1 ratio, either individually or simultaneously (pTRV2-PDS, pTRV2, pTRV2-SlARF6A, pTRV2-SlARF8A, pTRV2-SlARF24, pTRV2-SlARF6A/8A, pTRV2-SlARF6A/24, pTRV2-SlARF8A/24, pTRV2-SlARF6A/8A/24). These were used to infect cotyledon of tomato e mutant plants before the emergence of true leaves. The infected plants were transferred to a growth chamber at 16 h day/8 h night cycle, 20–22°C and 50% RH. The phenotypes were analyzed 6–7 weeks after inoculation. The VIGS method was following the published protocol (Velasquez et al., 2009).
The sample leaves were frozen in liquid nitrogen. Three replicates were prepared for each leaf sample. The biomass for each replicate was 0.1 g. Subsequently, IAA extraction was performed by ESI-MS/MS following the published protocol (Liu et al., 2012).
For yeast one-hybrid assay, the –1479 bp fragment (upstream from the start codon) from the SlPIN1 (Solyc03g118740) promoter was amplified from Ailsa Craig genomic DNA and cloned into the pAbAi vector (Clontech). Recombinant plasmid pAbAi-SlPIN1 and pGADT7-SlARF were co-transformed into the yeast strain Y1HGold (Clontech) by small-scale yeast transformation method respectively. The transformants were plated on the SD/-Ura drop-out medium. Colonies were picked and diluted in sterile ddH2O to an OD600 of 0.5, and 3 μl of suspension was spotted on SD/-Ura/-Leu drop-out medium with or without AbA antibiotic at 30°C. Both pGAD-p53+p53-AbAi (positive control) and pGADT7+P1-AbAi (negative control) were included.
The full-length SlARFs ORF were amplified and cloned into the effector vector, pGreen II 62-SK. A –1479 bp fragment (upstream from the start codon) from the SlPIN1 promoter was amplified and cloned into the reporter vector, pGreen II 0800-LUC. Both the effector and reporter vector were respectively co-transformed into the Agrobacterium tumefactions strain GV3101 cells with the pSoup vector, then infiltrated into N. benthamiana young leaves and incubated 72 h in the dark. LUC and REN were analyzed using the dual luciferase assay reagents (Promega) with an Infinite M200 (Tecan). All primers used in this analysis are listed in Supplementary Table S2.
In order to dissect the mechanism of SlIAA9 regulating leaf shape development in tomato, a yeast two-hybrid (Y2H) screening was performed to identify the SlIAA9 interacting proteins from tomato cDNA Y2H library. After several screens, SlARF24 was screened out (Supplementary Table S3). To identify more SlARFs that may participate in this pathway, the full-length coding sequences of 15 SlARFs, including the candidate SlARF24 previously identified, were isolated and inserted into the yeast two hybrid vector pGADT7, including SlARF1, SlARF2B, SlARF3, SlARF4, SlARF5, SlARF6A, SlARF6B, SlARF8A, SlARF8B, SlARF9A, SlARF9B, SlARF10A, SlARF10B, and SlARF16A. The recombinant plasmid pGBKT7-SlIAA9 and pGADT7-SlARFs were co-transformed into the yeast, respectively. Specifically, we observed that yeast cells containing pGBKT7-SlIAA9 mated with pGADT7-SlARF6A, pGADT7-SlARF8A, pGADT7-SlARF8B, and pGADT7-SlARF24 respectively to grow under selection conditions (Figure 1A). This result suggests that SlIAA9 may interact with SlARF6A, SlARF8A, SlARF8B, and SlARF24 in yeast cells.
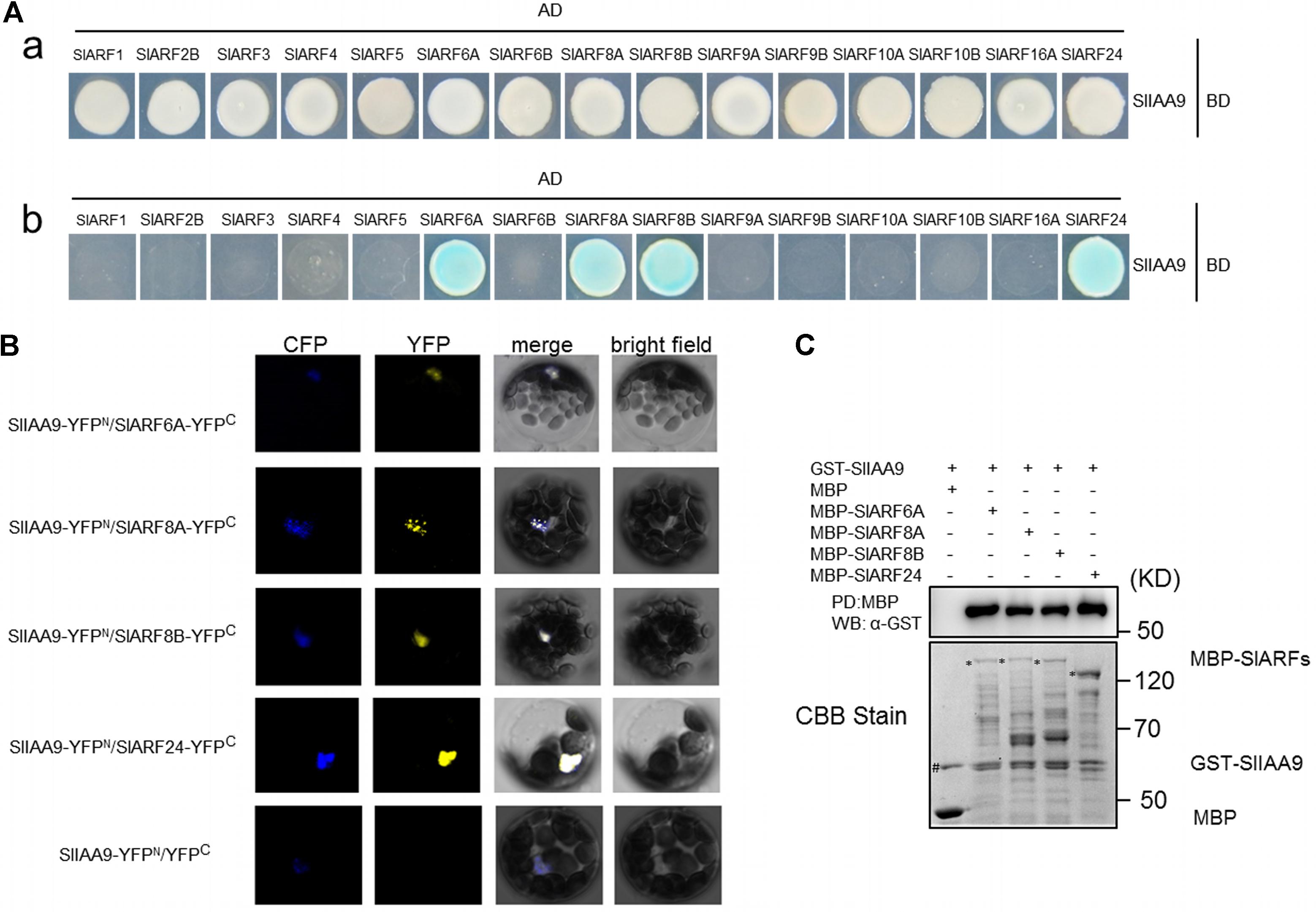
FIGURE 1. Interaction of SlIAA9 and the cloned SlARF proteins in vivo and in vitro. (A) The yeast cells grown on SD/-Leu/-Trp (a) and SD/-Ade/-His/-Leu/-Trp + 40 ug ml-1 X-gal (b). (B) SlIAA9 interacts with SlARF6A, SlARF8A, SlARF8B, and SlARF24 in Arabidopsis thaliana cell culture protoplasts. YFP fluorescence was detected when SlIAA9-YFPN was coexpressed with SlARF6A-YFPC, SlARF8A-YFPC, SlARF8B-YFPC, and SlARF24-YFPC, respectively. (C) GST-fused SlIAA9 proteins were incubated with MBP, MBP-SlARF6A, MBP-SlARF8A, MBP-SlARF8B or MBP-SlARF24 beads (PD:MBP). The incubated beads were washed and pelleted for immunoblot analysis with α-GST antibody. The protein inputs are indicated by Coomassie Brilliant Blue (CBB) staining.
Subsequently, the interactions between SlIAA9 and SlARF6A, SlARF8A, SlARF8B, and SlARF24 were examined using BiFC in Arabidopsis mesophyll protoplasts. We transformed the Arabidopsis Col-0 protoplasts with SlIAA9-YFPN/SlARF6A-YFPC, SlIAA9-YFPN/SlARF8A-YFPC, SlIAA9-YFPN/SlARF8B-YFPC, SlIAA9-YFPN/SlARF24-YFPC, and SlIAA9-YFPN/YFPC. Strong YFP fluorescence signal was detected throughout the nucleus when SlIAA9-YFPN was co-expressed with SlARF6A-YFPC, SlARF8A-YFPC, SlARF8B-YFPC, and SlARF24-YFPC, whereas no fluorescence was detected in the control cells (Figure 1B). These results indicate that SlIAA9 might interact with multiple SlARF proteins in vivo.
In order to further confirm that the SlIAA9 protein could directly interact with SlARF6A, SlARF8A, SlARF8B, and SlARF24 proteins, a pull-down assay was performed. In this experiment, SlARF6A, SlARF8A, SlARF8B, or SlARF24 fused to maltose binding protein (MBP) immobilized on amylose-agarose beads were used as bait against GST-SlIAA9 fusion proteins. As shown in Figure 1C, GST-SlIAA9 could be pulled down by MBP-SlARF6A, MBP-SlARF8A, MBP-SlARF8B, as well as MBP-SlARF24, but not by MBP alone, demonstrating that SlARF6A, SlARF8A, SlARF8B, and SlARF24 proteins physically interact with SlIAA9 in vitro.
A previous study showed that the basal gene expression of SlIAA9 was high in roots, leaves, flowers, and fruits (Wang et al., 2005). For comparative purposes, qRT-PCR was performed to investigate the expression levels of SlIAA9 in wild type root, stem, leaf, flower, and fruit tissue (Supplementary Figure S1). These results indicated that SlIAA9 expressed in all tissues, but exhibits lower expression at the mature green (MG) stage in fruit tissue.
We analyzed the expression levels of the SlARF genes family in wild type (compound leaves) and e mutant (simple leaves) leaves. The expression levels of SlARF1, SlARF2A, SlARF2B, SlARF5, and SlARF18 in leaves of the e mutant were induced 2.14-, 5.79-, 5.90-, 3.19-, and 2.73-fold more than that of those in wild type leaves. While the SlARF3, SlARF6A, SlARF6B, SlARF7B, SlARF10A, SlARF10B, SlARF16A, SlARF16B, SlARF19, and SlARF24 showed decreased expression corresponding to 0.05-, 0.48-, 0.45-, 0.47-, 0.15-, 0.15-, 0.44-, 0.01-, 0.05-, and 0.48-fold, respectively. Other SlARFs showed no significant difference (Figure 2). These results establish that multiple SlARFs are regulated in the absence of SlIAA9 in the auxin signaling model. This is particularly indicated for the SlARF16B gene, which might have a unique and significant function in this pathway. In addition, the expression levels of SlARF6A, SlARF8A, SlARF8B, and SlARF24 displayed varying degrees of attenuation in e mutant plants compared to wild type plants, supporting the hypothesis that the SlARF6A, SlARF8A, SlARF8B, and SlARF24 interaction with SlIAA9 has compromised biological function in e mutant plants.
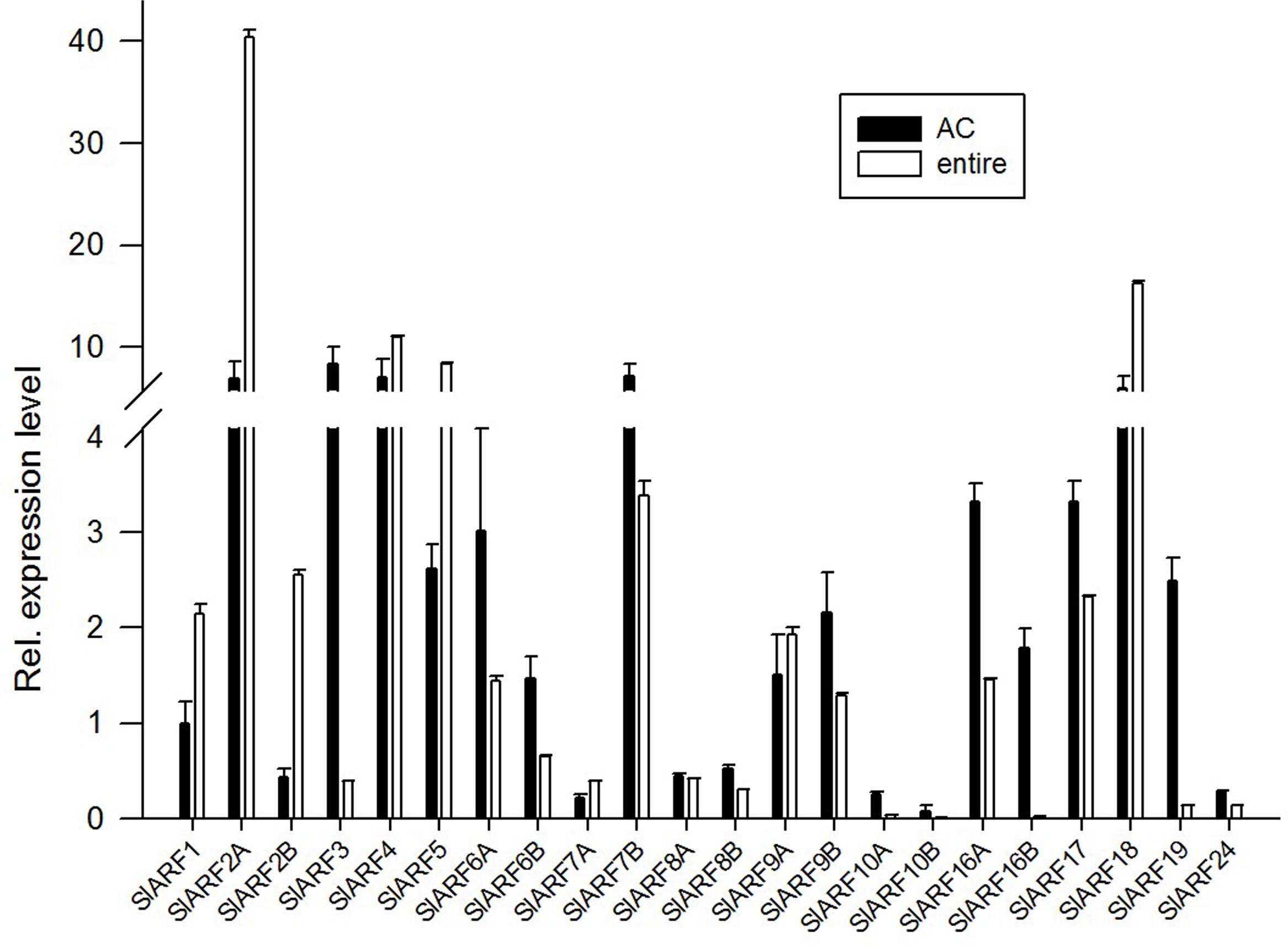
FIGURE 2. The expression patterns of Multiple SlARFs were altered by e mutation. The expression of SlARF genes family in wild type (compound leaves material) and e mutant (simple leaves material) leaves. The relative expression was referred using the level of SlARF1 in the wild type control plant as calibrator. Standard errors (SE) are shown (n = 3).
The genetic mechanism by which SlIAA9 regulates leaf shape development in tomato was examined by silencing SlARFs in the e mutant background. Using TRV-mediated VIGS we carried out a functional characterization assay of the four candidate SlARF genes identified through the previous expressional analysis. SlARF8A and SlARF8B share 82% amino acid identity (Supplementary Figure S2), therefore both genes were silenced in one VIGS construct. There was no detectable change in leaf shape after individually silencing pTRV2-SlARF6A, pTRV2-SlARF8A, and pTRV2-SlARF24 (Figure 3A). Gene expression analysis showed that the mRNA levels of SlARF6A, SlARF8A, SlARF8B, and SlARF24 were reduced to approximately 43%, 48%, 49%, and 51% respectively compared to the empty vector control (Figure 3B). Functional redundancies among ARF proteins have been described in Cucumis sativus, A. thaliana, and S. lycopersicum (Okushima et al., 2005; Liu and Hu, 2013; Hao et al., 2015). In accordance with this redundancy, we inoculated pTRV2-SlARF6A, pTRV2-SlARF8A, and pTRV2-SlARF24 constructs into e mutant plants using double or triple co-cultures of Agrobacterium. Interestingly, 80%, 73%, and 77% of the e mutant plants infiltrated with Agrobacterium triple co-cultures expressing pTRV2-SlARF6A/8A/24 were partially converted to compound leaves in three replicate experiments (Table 1). Meanwhile, the mRNA levels of SlARF6A, SlARF8A, SlARF8B, and SlARF24 in the e mutant plants inoculated with the Agrobacterium co-cultures of pTRV2-SlARF6A/8A/24 were reduced to approximately 60%, 57%, 69%, and 64% compared with the empty vector control, respectively (Figure 3C). Furthermore, the gene expression of other members of the SlARF family were not significantly changed after silencing the four candidate SlARFs (Supplementary Figure S3). Moreover, the fifth leaves of silencing of candidate SlARF genes in tomato e mutation were observed, the results presented that e mutant plants inoculated with pTRV2-SlARF6A/8A/24 triple combination cultures were partially converted to wild-type compound leaves, which generating more leaflets (Figure 4A). The total leaflets on the mature first five leaves of the pTRV2-SlARF6A/8A/24 inoculated plants were significantly increased compared to the e mutant plants (Figure 4B). The double co-cultures pTRV2-SlARF6A/8A, pTRV2-SlARF6A/24, and pTRV2-SlARF8A/24 could not restore the development of compound leaves (Figure 4A and Table 1) despite that the expression analysis revealed the target genes were down-regulated (Figure 3B). These results illustrated that simultaneously silencing of these four genes could restore the compound leaf shape in e mutant plants, and suggested functional redundancies among SlARF proteins in regulating tomato leaf shape development.
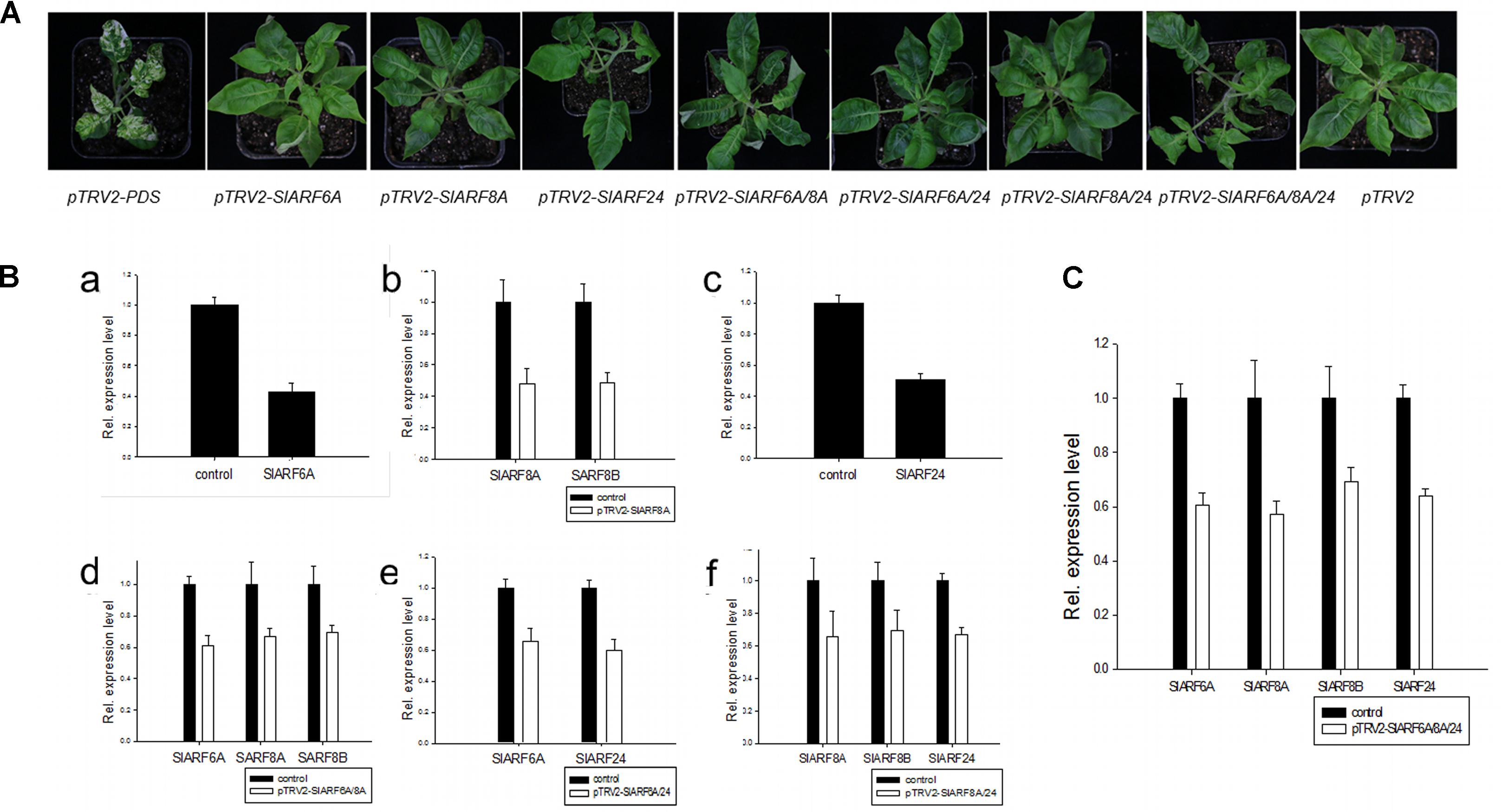
FIGURE 3. TRV-mediated virus-induced gene silencing (VIGS) of candidate SlARF genes in tomato e mutation leaves. SlARF8A and SlARF8B shared the same gene fragment in the VIGS vector. (A) Photographs of silencing tomato e mutation. (B) The candidate SlARF genes expression of injected with pTRV2-SlARF6A (a), pTRV2-SlARF8A (b), pTRV2-SlARF24 (c), pTRV2-SlARF6A/8A (d), pTRV2-SlARF6A/24 (e), pTRV2-SlARF8A/24 (f) cultures, respectively. (C) The SlARF genes expression of injected with pTRV2-SlARF6A/8A/24 culture. The expression of a specific SlARF was compared with the level in e mutant plant agroinfiltrated with empty vector (control sample). Standard errors (SE) are shown (n = 3).
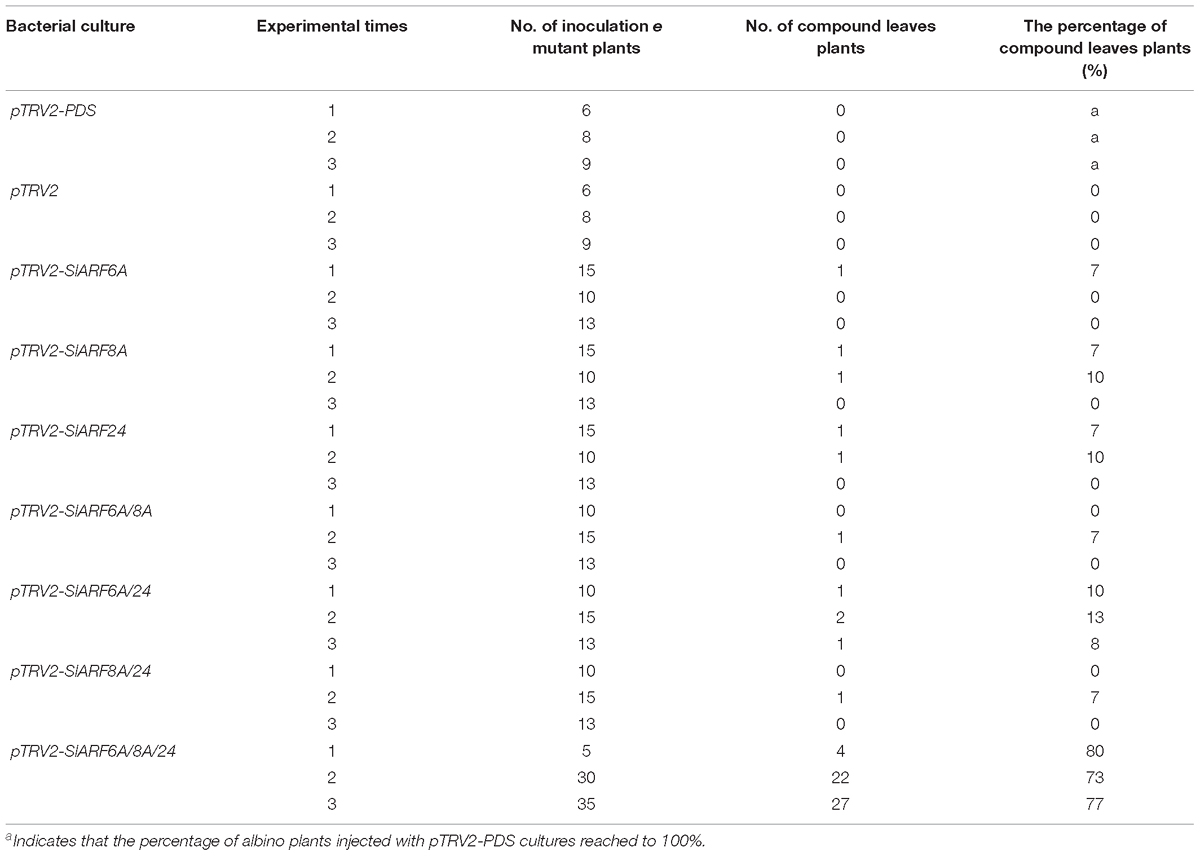
TABLE 1. Statistical information describing the TRV-mediated virus-induced gene silencing (VIGS) of genes in e mutant plants.
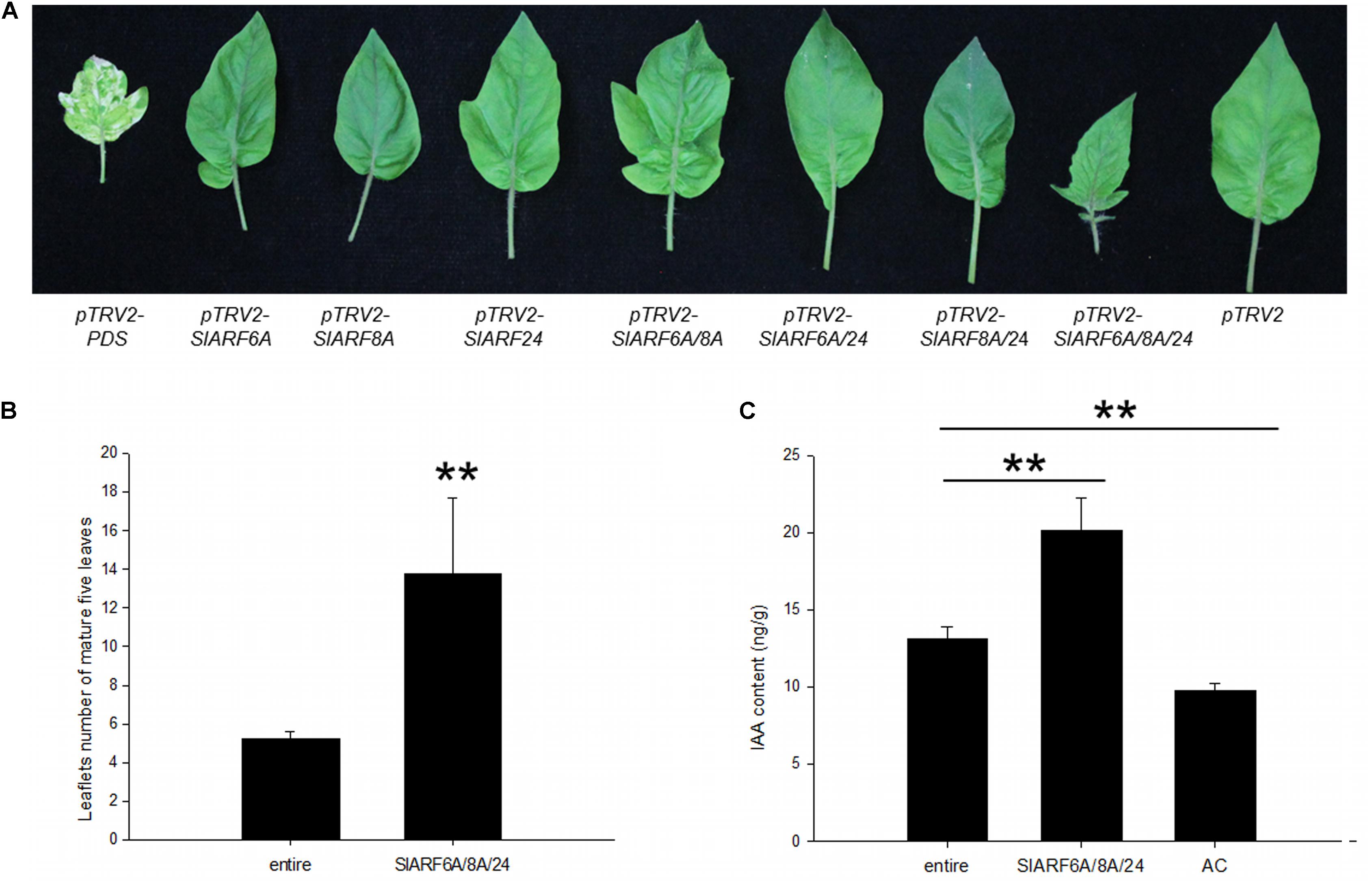
FIGURE 4. Leaves phenotypes and the free IAA contents of silencing of candidate SlARF genes in tomato e mutation. (A) The mature fifth leaves of the indicated phenotypes. (B) Quantification of the total leaflets number of the mature first five leaves (n= 5). (C) The IAA contents in e mutant and silencing of all the four SlARF genes materials. Standard errors (SE) are shown (n = 3). ∗P < 0.05, ∗∗P < 0.01.
To analyze whether these phenotypic changes are regulated by auxin, the free IAA levels were quantified using UFLC-ESI-MS/MS. IAA levels of young leaves that had all four SlARFs silenced reached 20.14 ng/g, which were significantly higher than the e mutant plants (13.14 ng/g). Interestingly, the concentration of IAA in e mutant was observed to have a higher basal level than the wild type control plants (9.74 ng/g) (Figure 4C). This result suggested that the development of compound leaves inoculated with pTRV2-SlARF6A/8A/24 were induced by auxin through promoting auxin response.
The leaf growth and development in tomato is likely driven by the leaf shape related genes (Hareven et al., 1996; David-Schwartz et al., 2009; Illing et al., 2009; Yanai et al., 2011; Pinon et al., 2013). Subsequently, the transcript levels of these leaf shape related genes in tomato were evaluated through qRT-PCR. The SlPIN1 and phan expression in pTRV2-SlARF6A/8AB/24 silenced plants was induced 1.8- and 2.5-fold compared to e mutant plants, respectively. In contrast, the expression level of LYR was reduced by 4.6-fold compared to e mutant plants (Figure 5A). Thus, after silencing the four SlARFs in e mutant, the expression of those genes were reverted back to wild type level. We hypothesize that SlARF proteins may regulate leaf shape development by regulating the expression of SlPIN1.
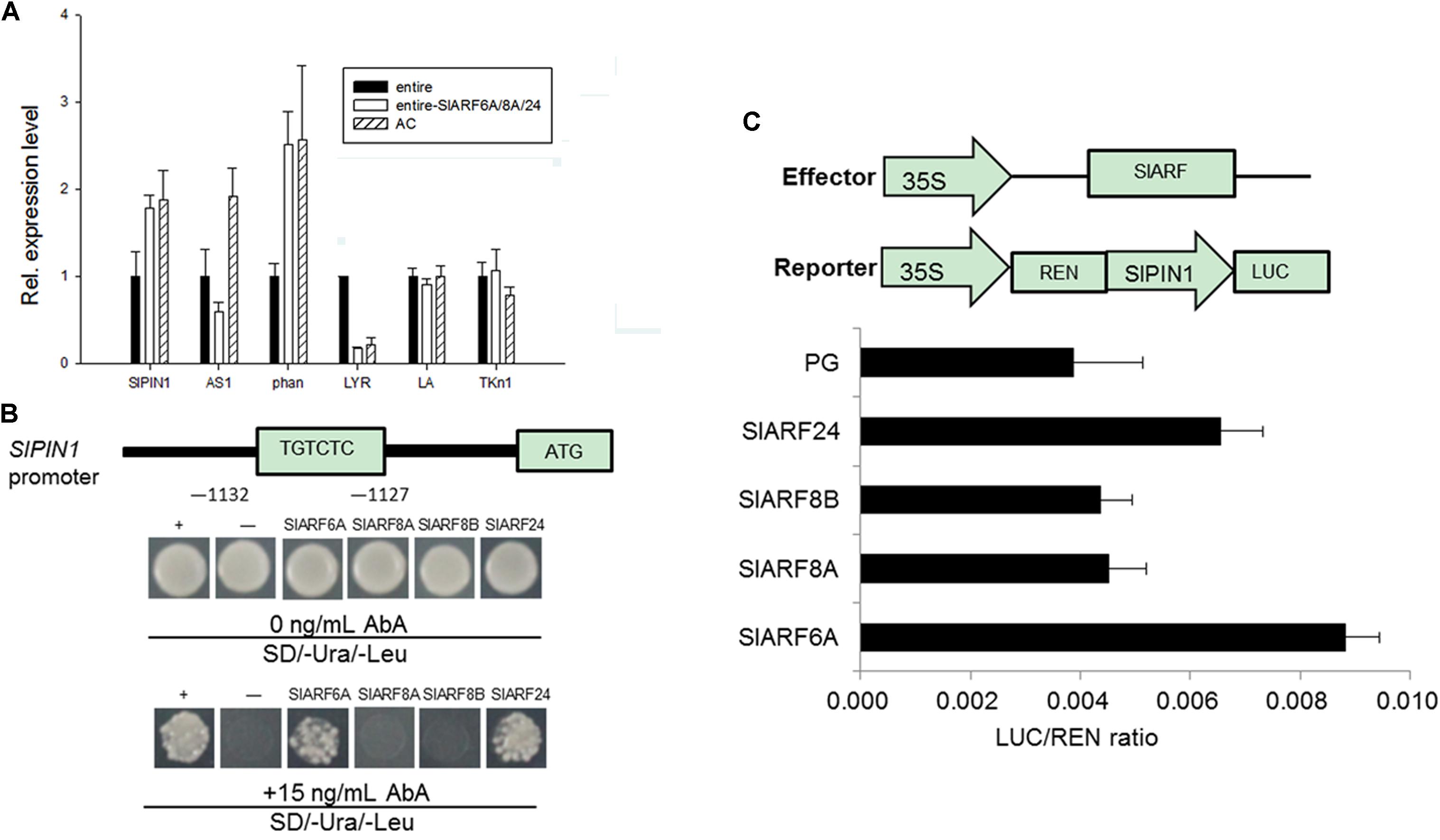
FIGURE 5. Quantitative real-time PCR analysis of leaf shape related genes and SlARF6A and SlARF24 bind to the SlPIN1 promoter. (A) The expression of SlPIN1, AS1, and phan in the leaves of e mutant, silencing of all the four SlARF genes materials and wild type plants. (B) Yeast-one hybrid (Y1H) assay of SlARF6A and SlARF24 binding to SlPIN1 promoter. The yeast cells grown on SD/-Ura/-Leu and SD/-Ura/-Leu + 15 ng ml-1 AbA. (C) Transcription activity assay in tobacco to examine the interaction between candidate SlARFs and SlPIN1 promoter. The schematic diagrams constructs used for the dual LUC assay; REN (Renilla luciferase), a control for activity normalization; PG, the empty vector of pGreen II 62-SK. Standard errors (SE) are shown (n = 3).
To investigate the relationship between the candidate SlARFs proteins and SlPIN1 promoter, a fragment of the SlPIN1 promoter 1,479 bp upstream from the start codon was used in an Y1H assay (Figure 5B). Y1H results demonstrated that this fragment could interact with SlARF6A and SlARF24 protein, confirming that SlARF6A and SlARF24 protein recognize the cis-element in the SlPIN1 promoter in yeast.
To determine whether SlARF6A and SlARF24 function as activator or repressor, we used a LUC (dual luciferase) assay to test how SlARF6A and SlARF24 interact with the SlPIN1 promoter. The same fragment of SlPIN1 used in the Y1H assay was introduced into the pGreen II 0800-LUC vector to generate the reporter construct (Figure 5C). The effector and reporter construct were transiently expressed in tobacco leaves and the relative LUC activity was determined. This result revealed that LUC activity was 2.29- and 1.69-fold higher in the presence of the SlARF6A and SlARF24 effector and reporter construct than in the negative control (Figure 5C), implying that both SlARF6A and SlARF24 may function as a transcriptional activator. This result revealed that the cis-element from the SlPIN1 promoter was bound by SlARF6A and SlARF24.
Over the past 10 years, SlIAA9 has been shown to be involved in fruit development, leaf morphogenesis, and fruit parthenocarpy in A. thaliana and S. lycopersicum (Wang et al., 2005, 2009; Goetz et al., 2007). In this study, we aimed to identify interacting partners of SlIAA9. The initial Y2H assay identified several candidates, including Aux/IAA proteins, ubiquitin related proteins, gibberellin beta-hydroxylase protein, MADs box interactor-like protein, and SlARF24 (Supplementary Table S3). More SlARFs (SlARF6A, SlARF8A, SlARF8B, and SlARF24) were further found to interact with SlIAA9 in vivo and in vitro (Figure 1), indicating that other SlARFs may play redundant roles with SlARF24 in regulating leaf development. SlIAA9 and miR160-targeted ARFs SlARF10A, SlARF10B, or SlARF17, appear to partially act in a functionally redundant manner, but remain necessary for local inhibition of lamina growth between initiating leaflets (Ben-Gera et al., 2016). However, the elucidation of the molecular mechanism on how these functional redundancies among SlARF proteins regulate tomato leaf shape has been hampered by complexity of the protein in planta. Understanding the mechanisms involved in leaf shape development in tomato can provide new insights into understanding these same mechanisms in other species such as A. thaliana, Glycine max, and C. hirsuta.
SlARFs may regulate leaf morphology through binding to the promoter of SlPIN1 or other auxin-responsive genes in the absence of SlIAA9 (Figure 5). SlARF8 and SlIAA9 proteins, together with another unknown protein, may form a regulatory complex to control fruiting and growth, offering a possible explanation for the role of SlIAA9 in parthenocarpy (Goetz et al., 2007). SlARF6 and SlARF8 also play conserved roles in regulating development and growth of flower and vegetable organs in dicots (Liu et al., 2014). The Osarf24-1 mutant presents reduced sensitivity to aberrant auxin signaling and auxin-deficient phenotypes (Sakamoto, 2013). Here, we firstly found that SlARF6A, SlARF8A, SlARF8B, and SlARF24 could regulate tomato leaf development in a redundant manner.
It has been illustrated that several SlARF genes might serve unique functions in tomato development. SlARF2A functions in the regulation of tomato fruit ripening as a recognized auxin signaling component (Breitel et al., 2016). Down-regulation of SlARF4 results in a dark-green fruit phenotype with increased chloroplasts densities (Jones et al., 2002). Furthermore, SlARF4 involves in the control of sugar metabolism during fruit development in tomato (Sagar et al., 2013). Both auxin and gibberellin responses are modulated by SlARF7 during fruit formation and development in tomato (De Jong et al., 2011). Compared with wild type fruits, the fruits of SlARF7-RNAi transgenic lines presented seedless, heart-shaped, and thick pericarp phenotypes in tomato (De Jong et al., 2009). Cell division is negatively regulated by SlARF9 during early fruit development in tomato (De Jong et al., 2015). Primexine formation is modulated by ARF17, which is crucial for pollen wall patterning, partially through regulation of CalS5 gene expression in Arabidopsis (Yang et al., 2013). A 165-bp deletion in ARF18 gene simultaneously affects silique length and seed weight in polyploid rapeseed (Liu et al., 2015).
Moreover, there are functional redundancies among ARF proteins in Cucumis sativus, A. thaliana, and S. lycopersicum (Okushima et al., 2005; Liu and Hu, 2013; Hao et al., 2015). A constitutive expression pattern was exhibited in almost all of the ARF genes in cucumber (Liu and Hu, 2013). In A. thaliana, arf7 arf19 double mutant presented an obvious auxin-related phenotype that were not detectable in the single mutant, suggesting that there are functional redundancies between ARF7 and ARF19 proteins (Okushima et al., 2005). Simultaneous silencing of SlARF2A and SlARF2B genes leaded to severe ripening inhibition, clarifying a functional redundancies between SlARF2A and SlARF2B proteins (Hao et al., 2015). Our VIGS results provided evidence that functional redundancies among SlARF proteins resulted in the change from a simple leaf to a complex one in tomato e mutant plants (Figure 3). Due to the far evolutionary relationship among candidate SlARFs, clarifying there are functional compensation among the candidate SlARFs. It should be noted that this study has only examined the function of SlARF6A, SlARF8A, SlARF8B, and SlARF24, because only 15 tomato SlARF genes were isolated from the full-length cDNA sequences out of the 22 SlARF genes family. It has been reported that SlARF17 and SlIAA9 do not interact in yeast (Ben-Gera et al., 2016). There are still another six SlARF genes that have not yet been characterized by us. Our result showed that the expression levels of SlARF16B were 0.01-fold less in leaves of the e mutant compared to those in wild type. A previous study describes that the Pto-ARF16 was affected by Pto-miR160a associated with tree growth and wood property traits in Populus tomentosa (Tian et al., 2016). In A. thaliana, ARF10 and ARF16 were targeted by miR160 to control the formation of root cap cell, and miR160-uncoupled production of ARF16 reflected pleiotropic effects (Wang et al., 2005). Thus, we hypothesize that SlARF16B is regulated in the absence of SlIAA9 in the auxin signaling model, which needs to further evaluated with additional experiments. Consequently, the e mutation likely alters the expression patterns of other SlARF genes through this mechanism. The hetero-dimerization between Aux/IAA and ARF proteins likely able to play unique cellular functions (Piya et al., 2014). However, how the SlIAA9-SlARFs complex functions during tomato leaf development is still not yet completely resolved.
We chose several leaf shape related genes in A. thaliana and S. lycopersicum to evaluate whether these genes could regulate the development of leaf shape. As a result, the expression levels of SlPIN1, phan, and LYR in leaves having SlARF6A, SlARF8A, SlARF8B, and SlARF24 simultaneously silenced through VIGS were restored to levels similar to those in wild-type plants (Figure 5A). The IAA levels in leaves having those same four SlARFs silenced were significantly increased compared to the control (Figure 4C). Auxin acts as a positional cue during leaf organogenesis, and auxin efflux carrier PIN1 is one of the main contributors to auxin localization (Reinhardt et al., 2003; Cheng et al., 2007). PIN1 localizes on the periphery of apical meristems directing auxin to convergence points, where auxin maxima is formed, subsequently auxin becomes directed subepidermally at the leaf initiation site to regulate leaf development (Heisler et al., 2005; Martinez et al., 2016). Genetic analyses have also demonstrated that PIN1 is required for leaflet initiation in compound leaves (Barkoulas et al., 2008). Accordingly, the cis-element of SlPIN1 was used for deep analysis. The cis-elements from SlPIN1 promoter was recognized and bound specifically by SlARF6A and SlARF24 in yeast and plants (Figure 5). Here, we propose that SlARF6A and SlARF24 may regulate leaf growth and development through direct binding to the SlPIN1 promoter. However, the effects of enhanced SlPIN1 transcription still needs to be further evaluated. This enhanced transcription may result to increased expression of SlPIN1 protein, changed SlPIN1 protein modification, or shift the location of SlPIN1.
Our data provide an insight to suggest that SlARF proteins work with SlIAA9 in a functionally redundant manner to dictate leaf shape. We propose that SlIAA9 interacts with multiple SlARF proteins to promote the formation of a regulatory complex which can directly block leaflet initiation genes. We also assume that this complex may act indirectly by preventing SlARFs from functioning as transcription activators. In the absence of SlIAA9, SlARFs may regulate leaf growth and development through direct binding to the promoter of SlPIN1 or unknown X genes induced by auxin (Figure 6). Future studies will be directed to dissect the relationship between SlIAA9 and SlARF6A, SlARF8A, SlARF8B, and SlARF24. We also intend to clone other SlARF genes to ascertain whether SlIAA9 has additional interactors with unique biological functions.
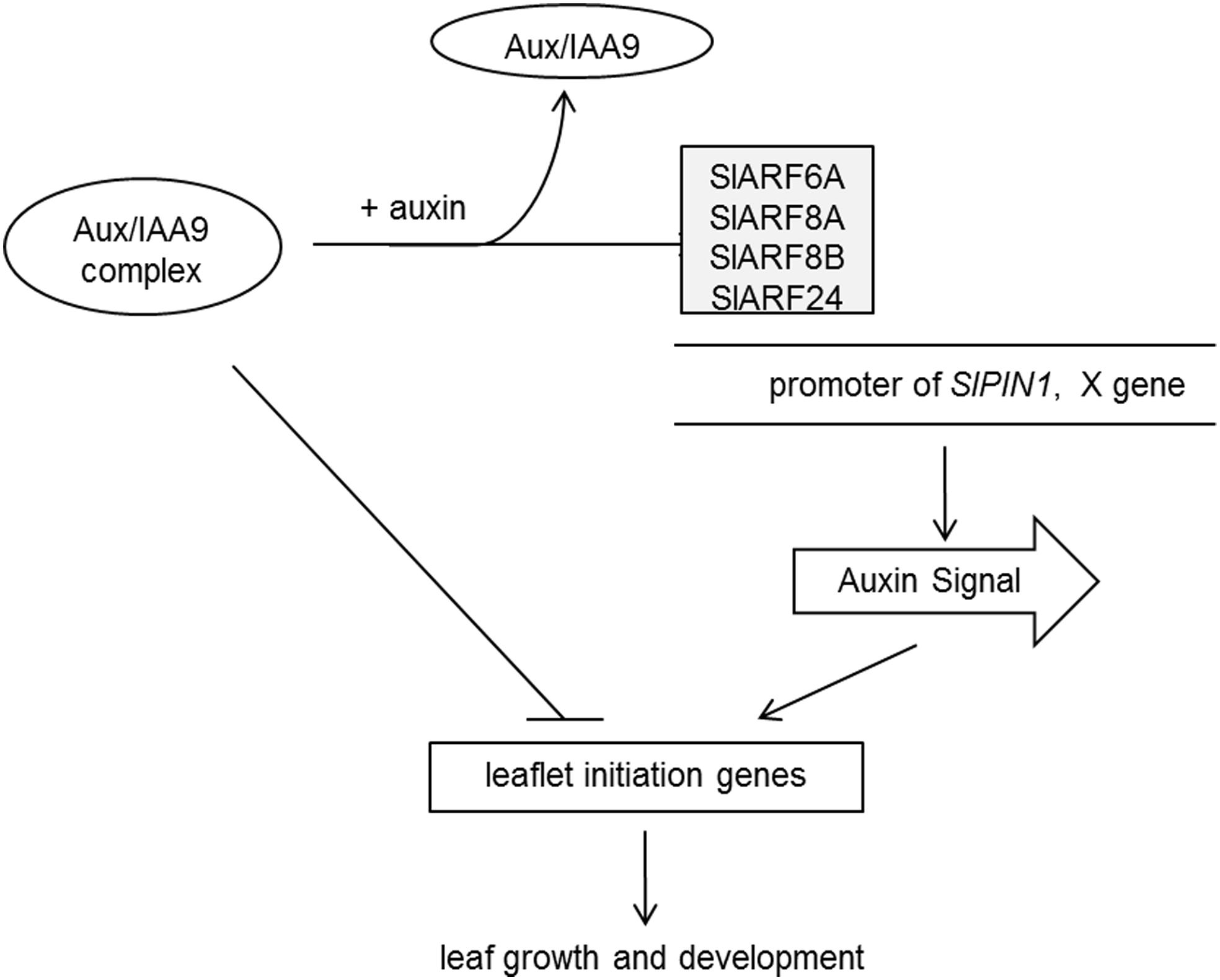
FIGURE 6. A proposed model of SlIAA9 complex in the control of tomato leaf forms. SlIAA9 and SlARF6A, SlARF8A, SlARF8B, and SlARF24 proteins, form a regulatory complex, which can directly block leaflet initiation genes or act indirectly by preventing SlARFs from functioning as transcriptional activators. In the absence of SlIAA9, SlARFs may regulate leaf growth and development through direct binding to the promoter of SlPIN1 or other auxin-responsive genes, which promoted auxin response.
In conclusion, this study posits a proposed molecular mechanism of SlIAA9 complex in the control of tomato leaf forms (Figure 6). Our results firstly demonstrate that SlARF6A, SlARF8A, SlARF8B, and SlARF24 directly interact with SlIAA9, and the simple leaves of the e mutant are partially converted to wild-type compound leaves by silencing of all the four SlARFs. Meanwhile, SlARF6A and SlARF24 bind to the SlPIN1 promoter to regulate genes expression involved in leaflet initiation. Further studies are still needed to explore the underlying mechanism of SlARF proteins in modulating tomato leaf shape.
LW designed and performed the experiments, data analysis, and drafted this manuscript. JZ supervised the experiments and revised the manuscript. JZ and ZT designed all the experiments.
This work was supported by grants from the National Natural Science Foundation of China (31471888 and 31772317) and the Fundamental Research Funds for the Central Universities (Program No. 2662015PY224).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Kevin L. Cox for critically revising of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00957/full#supplementary-material
ARF, auxin response factor; IAA, indole-3-acetic acid; LUC, firefly luciferase; qRT-PCR, quantitative real-time PCR; REN, renilla luciferase; VIGS, virus-induced gene silencing; YFP, yellow fluorescent protein.
Bar, M., Ben-Herzel, O., Kohay, H., Shtein, I., and Ori, N. (2015). CLAUSA restricts tomato leaf morphogenesis and GOBLET expression. Plant J. 83, 888–902. doi: 10.1111/tpj.12936
Barkoulas, M., Hay, A., Kougioumoutzi, E., and Tsiantis, M. (2008). A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40, 1136–1141. doi: 10.1038/ng.189
Ben-Gera, H., Dafna, A., Alvarez, J. P., Bar, M., Mauerer, M., and Ori, N. (2016). Auxin-mediated lamina growth in tomato leaves is restricted by two parallel mechanisms. Plant J. 86, 443–457. doi: 10.1111/tpj.13188
Ben-Gera, H., Shwartz, I., Shao, M. R., Shani, E., Estelle, M., and Ori, N. (2012). ENTIRE and GOBLET promote leaflet development in tomato by modulating auxin response. Plant J. 70, 903–915. doi: 10.1111/j.1365-313X.2012.04939.x
Berger, Y., Harpaz-Saad, S., Brand, A., Melnik, H., Sirding, N., Alvarez, J. P., et al. (2009). The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136, 823–832. doi: 10.1242/dev.031625
Bilsborough, G. D., Runions, A., Barkoulas, M., Jenkins, H. W., Hasson, A., Galinha, C., et al. (2011). Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. U.S.A. 108, 3424–3429. doi: 10.1073/pnas.1015162108
Blein, T., Pulido, A., Vialette-Guiraud, A., Nikovics, K., Morin, H., Hay, A., et al. (2008). A conserved molecular framework for compound leaf development. Science 322, 1835–1839. doi: 10.1126/science.1166168
Breitel, D. A., Chappell-Maor, L., Meir, S., Panizel, I., Puig, C. P., Hao, Y., et al. (2016). AUXIN RESPONSE FACTOR 2 intersects hormonal signals in the regulation of tomato fruit ripening. PLoS Genet. 12:e1005903. doi: 10.1371/journal.pgen.1005903
Byrne, M. E., Barley, R., Curtis, M., Arroyo, J. M., Dunham, M., Hudson, A., et al. (2000). Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408, 967–971. doi: 10.1038/35050091
Cheng, Y., Dai, X., and Zhao, Y. (2007). Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19, 2430–2439. doi: 10.1105/tpc.107.053009
David-Schwartz, R., Koenig, D., and Sinha, N. R. (2009). LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. Plant Cell 21, 3093–3104. doi: 10.1105/tpc.109.069948
De Jong, M., Wolters-Arts, M., Feron, R., Mariani, C., and Vriezen, W. H. (2009). The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 57, 160–170. doi: 10.1111/j.1365-313X.2008.03671.x
De Jong, M., Wolters-Arts, M., Garcia-Martinez, J. L., Mariani, C., and Vriezen, W. H. (2011). The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 62, 617–626. doi: 10.1093/jxb/erq293
De Jong, M., Wolters-Arts, M., Schimmel, B. C., Stultiens, C. L., De Groot, P. F., Powers, S. J., et al. (2015). Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J. Exp. Bot. 66, 3405–3416. doi: 10.1093/jxb/erv152
de Reuille, P. B., Bohn-Courseau, I., Ljung, K., Morin, H., Carraro, N., Godin, C., et al. (2006). Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 1627–1632. doi: 10.1073/pnas.0510130103
Furutani, M., Vernoux, T., Traas, J., Kato, T., Tasaka, M., and Aida, M. (2004). PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131, 5021–5030. doi: 10.1242/dev.01388
Goetz, M., Hooper, L. C., Johnson, S. D., Rodrigues, J. C., Vivian-Smith, A., and Koltunow, A. M. (2007). Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 145, 351–366. doi: 10.1104/pp.107.104174
Hao, Y., Hu, G., Breitel, D., Liu, M., Mila, I., Frasse, P., et al. (2015). Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet. 11:e1005649. doi: 10.1371/journal.pgen.1005649
Hareven, D., Gutfinger, T., Parnis, A., Eshed, Y., and Lifschitz, E. (1996). The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84, 735–744. doi: 10.1016/S0092-8674(00)81051-X
Hay, A., Barkoulas, M., and Tsiantis, M. (2006). ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133, 3955–3961. doi: 10.1242/dev.02545
Heisler, M. G., Ohno, C., Das, P., Sieber, P., Reddy, G. V., Long, J. A., et al. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899–1911. doi: 10.1016/j.cub.2005.09.052
Illing, N., Klak, C., Johnson, C., Brito, D., Negrao, N., Baine, F., et al. (2009). Duplication of the asymmetric leaves1/rough sheath 2/phantastica (ARP) gene precedes the explosive radiation of the Ruschioideae. Dev. Genes Evol. 219, 331–338. doi: 10.1007/s00427-009-0293-9
Jones, B., Frasse, P., Olmos, E., Zegzouti, H., Li, Z. G., Latche, A., et al. (2002). Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 32, 603–613. doi: 10.1046/j.1365-313X.2002.01450.x
Jonsson, H., Heisler, M. G., Shapiro, B. E., Meyerowitz, E. M., and Mjolsness, E. (2006). An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. U.S.A. 103, 1633–1638. doi: 10.1073/pnas.0509839103
Kimura, S., Koenig, D., Kang, J., Yoong, F. Y., and Sinha, N. (2008). Natural variation in leaf morphology results from mutation of a novel KNOX gene. Curr. Biol. 18, 672–677. doi: 10.1016/j.cub.2008.04.008
Koenig, D., Bayer, E., Kang, J., Kuhlemeier, C., and Sinha, N. (2009). Auxin patterns Solanum lycopersicum leaf morphogenesis. Development 136, 2997–3006. doi: 10.1242/dev.033811
Korasick, D. A., Westfall, C. S., Lee, S. G., Nanao, M. H., Dumas, R., Hagen, G., et al. (2014). Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc. Natl. Acad. Sci. U.S.A. 111, 5427–5432. doi: 10.1073/pnas.1400074111
Liu, H., Li, X., Xiao, J., and Wang, S. (2012). A convenient method for simultaneous quantification of multiple phytohormones and metabolites: application in study of rice-bacterium interaction. Plant Methods 8:2. doi: 10.1186/1746-4811-8-2
Liu, J., Hua, W., Hu, Z., Yang, H., Zhang, L., Li, R., et al. (2015). Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl. Acad. Sci. U.S.A. 112, E5123–E5132. doi: 10.1073/pnas.1502160112
Liu, N., Wu, S., Van Houten, J., Wang, Y., Ding, B., Fei, Z., et al. (2014). Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. J. Exp. Bot. 65, 2507–2520. doi: 10.1093/jxb/eru141
Liu, S. Q., and Hu, L. F. (2013). Genome-wide analysis of the auxin response factor gene family in cucumber. Genet. Mol. Res. 12, 4317–4331. doi: 10.4238/2013.April.2.1
Martinez, C. C., Koenig, D., Chitwood, D. H., and Sinha, N. R. (2016). A sister of PIN1 gene in tomato (Solanum lycopersicum) defines leaf and flower organ initiation patterns by maintaining epidermal auxin flux. Dev. Biol. 419, 85–98. doi: 10.1016/j.ydbio.2016.08.011
Merelo, P., Paredes, E. B., Heisler, M. G., and Wenkel, S. (2016). The shady side of leaf development: the role of the REVOLUTA/KANADI1 module in leaf patterning and auxin-mediated growth promotion. Curr. Opin. Plant Biol. 35, 111–116. doi: 10.1016/j.pbi.2016.11.016
Okushima, Y., Overvoorde, P. J., Arima, K., Alonso, J. M., Chan, A., Chang, C., et al. (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463. doi: 10.1105/tpc.104.028316
Ori, N., Eshed, Y., Chuck, G., Bowman, J. L., and Hake, S. (2000). Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127, 5523–5532.
Pinon, V., Prasad, K., Grigg, S. P., Sanchez-Perez, G. F., and Scheres, B. (2013). Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 110, 1107–1112. doi: 10.1073/pnas.1213497110
Piya, S., Shrestha, S. K., Binder, B., Stewart, C. N. Jr., and Hewezi, T. (2014). Protein-protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front. Plant Sci. 5:744. doi: 10.3389/fpls.2014.00744
Reinhardt, D., Pesce, E. R., Stieger, P., Mandel, T., Baltensperger, K., Bennett, M., et al. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260. doi: 10.1038/nature02081
Sagar, M., Chervin, C., Mila, I., Hao, Y., Roustan, J. P., Benichou, M., et al. (2013). SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 161, 1362–1374. doi: 10.1104/pp.113.213843
Sakamoto, T. (2013). Characterization of a Tos17 insertion mutant of rice auxin signal transcription factor gene, OsARF24. Am. J. Plant Sci. 4, 84–91. doi: 10.4236/ajps.2013.41013
Shwartz, I., Levy, M., Ori, N., and Bar, M. (2016). Hormones in tomato leaf development. Dev. Biol. 419, 132–142. doi: 10.1016/j.ydbio.2016.06.023
Tan, X., Calderon-Villalobos, L. I., Sharon, M., Zheng, C., Robinson, C. V., Estelle, M., et al. (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645. doi: 10.1038/nature05731
Tian, J., Chen, J., Li, B., and Zhang, D. (2016). Association genetics in Populus reveals the interactions between Pto-miR160a and its target Pto-ARF16. Mol. Genet. Genomics 291, 1069–1082. doi: 10.1007/s00438-015-1165-9
Tiwari, S. B., Wang, X. J., Hagen, G., and Guilfoyle, T. J. (2001). AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13, 2809–2822. doi: 10.1105/tpc.010289
Tsiantis, M., Schneeberger, R., Golz, J. F., Freeling, M., and Langdale, J. A. (1999). The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 284, 154–156. doi: 10.1126/science.284.5411.154
Velasquez, A. C., Chakravarthy, S., and Martin, G. B. (2009). Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J. Vis. Exp. 28:1292. doi: 10.3791/1292
Waites, R., Selvadurai, H. R., Oliver, I. R., and Hudson, A. (1998). The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93, 779–789. doi: 10.1016/S0092-8674(00)81439-7
Wang, H., Jones, B., Li, Z., Frasse, P., Delalande, C., Regad, F., et al. (2005). The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17, 2676–2692. doi: 10.1105/tpc.105.033415
Wang, H., Schauer, N., Usadel, B., Frasse, P., Zouine, M., Hernould, M., et al. (2009). Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21, 1428–1452. doi: 10.1105/tpc.108.060830
Yanai, O., Shani, E., Russ, D., and Ori, N. (2011). Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 68, 571–582. doi: 10.1111/j.1365-313X.2011.04716.x
Yang, J., Tian, L., Sun, M. X., Huang, X. Y., Zhu, J., Guan, Y. F., et al. (2013). AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol. 162, 720–731. doi: 10.1104/pp.113.214940
Zgurski, J. M., Sharma, R., Bolokoski, D. A., and Schultz, E. A. (2005). Asymmetric auxin response precedes asymmetric growth and differentiation of asymmetric leaf1 and asymmetric leaf2 Arabidopsis leaves. Plant Cell 17, 77–91. doi: 10.1105/tpc.104.026898
Keywords: auxin, development, leaf shape, SlARFs, SlIAA9, tomato
Citation: Wu L, Tian Z and Zhang J (2018) Functional Dissection of Auxin Response Factors in Regulating Tomato Leaf Shape Development. Front. Plant Sci. 9:957. doi: 10.3389/fpls.2018.00957
Received: 24 November 2017; Accepted: 14 June 2018;
Published: 04 July 2018.
Edited by:
Han Xiao, Shanghai Institutes for Biological Sciences (CAS), ChinaCopyright © 2018 Wu, Tian and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhong Zhang, emhhbmdqdW5obmdAbWFpbC5oemF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.