- 1French Associates Institute for Agriculture and Biotechnology of Drylands, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Midreshet Ben-Gurion, Israel
- 2Dead-Sea & Arava Science Center, Neve Zohar, Israel
The tropical seagrass species, Halophila stipulacea, originated from the Indian Ocean and the Red Sea, subsequently invading the Mediterranean and has recently established itself in the Caribbean Sea. Due to its invasive nature, there is growing interest in understanding this species’ capacity to adapt to new conditions. One approach to understanding the natural tolerance of a plant is to compare the tolerant species with a closely related non-tolerant species. We compared the physiological responses of H. stipulacea exposed to different salinities, with that of its nearest freshwater relative, Vallisneria americana. To achieve this goal, H. stipulacea and V. americana plants were grown in dedicated microcosms, and exposed to the following salt regimes: (i) H. stipulacea: control (40 PSU, practical salinity units), hyposalinity (25 PSU) and hypersalinity (60 PSU) for 3 weeks followed by a 4-week recovery phase (back to 40 PSU); (ii) V. americana: control (1 PSU), and hypersalinity (12 PSU) for 3 weeks, followed by a 4-week recovery phase (back to 1 PSU). In H. stipulacea, leaf number and chlorophyll content showed no significant differences between control plants and plants under hypo and hypersalinities, but a significant decrease in leaf area under hypersalinity was observed. In addition, compared with control plants, H. stipulacea plants exposed to hypo and hypersalinity were found to have reduced below-ground biomass and C/N ratios, suggesting changes in the allocation of resources in response to both stresses. There was no significant effect of hypo/hypersalinity on dark-adapted quantum yield of photosystem II (Fv/Fm) suggesting that H. stipulacea photochemistry is resilient to hypo/hypersalinity stress. In contrast to the seagrass, V. americana exposed to hypersalinity displayed significant decreases in above-ground biomass, shoot number, leaf number, blade length and Fv/Fm, followed by significant recoveries of all these parameters upon return of the plants to non-saline control conditions. These data suggest that H. stipulacea shows remarkable tolerance to both hypo and hypersalinity. Resilience to a relatively wide range of salinities may be one of the traits explaining the invasive nature of this species in the Mediterranean and Caribbean Seas.
Introduction
Seagrasses (order: Alismatales) are a unique group of marine flowering plants (angiosperms) that re-entered the oceans and secondarily colonized marine habitats. Phylogenetic analysis of the Alismatales has indicated that the secondary return of seagrasses to the sea occurred three–four times independently through parallel evolution from a common aquatic (freshwater) ancestor of terrestrial origin (Les et al., 1997; Orth et al., 2006; Wissler et al., 2011). In returning to the sea, some 60–90 million years ago (Les et al., 1997), seagrasses were faced with the physiological challenges related to growing in marine conditions (Olsen et al., 2016). These challenges entailed adaptive morphological and physiological traits common across all seagrass species, causing them to be considered as a single functional group (Les et al., 1997; Olsen et al., 2016). These adaptive traits include blade or subovate leaves, epidermal chloroplasts, loss of the stomata, internal gas transport that facilitates root proliferation in permanently flooded anoxic sediments (den Hartog, 1970; Hemminga and Duarte, 2000), submarine (hydrophilous) pollination (Björk et al., 2008) and tolerance to high salinity environments (Touchette, 2007).
Halophila stipulacea (Forsk.) Aschers. is a small, dioecious tropical seagrass that was originally described from the Red Sea (Forskal, 1775; Lipkin, 1975). It is widely known as a euryhaline species because of its wide range of salinity tolerance (den Hartog, 1970; Por, 1971). H. stipulacea was first reported in the Mediterranean Sea in 1894, only 25 years after the opening of the Suez Canal, making it a Lessepsian migrant (Lipkin, 1975). Since then it has become established along the coastlines of Egypt, Lebanon, Turkey, Albania, Greece, Italy, Libya, Cyprus and Tunisia (Lipkin, 1975; Gambi et al., 2009; Sghaier et al., 2011). Surprisingly, in 2002, this seagrass was reported in the Caribbean Sea (Ruiz and Ballantine, 2004), and in just over 10 years has spread to most eastern Caribbean island nations and recently to the South American continent (Vera et al., 2014; Willette et al., 2014; van Tussenbroek et al., 2016). Studies from the Caribbean have shown that H. stipulacea is physically displacing local Caribbean seagrass species (e.g., Syringodium filiforme) by monopolizing their space (Willette and Ambrose, 2012; Steiner and Willette, 2014). It has been suggested that the invasiveness of H. stipulacea could be attributed to its ability to acclimate to a wide range of physiological conditions including water temperatures, light intensities, nutrient levels and salinities (Por, 1971; reviewed by Gambi et al., 2009; Sharon et al., 2009, 2011).
Salinity is a major environmental component that can influence the growth, function, structure, and distribution of seagrasses (Montague and Ley, 1993; Salo et al., 2014). Most seagrass species are adapted to grow at salinities ranging from 20 to 40 Practical Salinity Units (PSU; 35 PSU having a concentration for the major ions of 540 mM Cl- 460 mM Na+, and 50 mM Mg+; Touchette, 2007). While these salt concentrations are much higher than those found in saline habitats where terrestrial plants exist, changing salinities can also influence the structure and function of seagrass communities (Montague and Ley, 1993), including the disappearance of seagrass meadows (Zieman et al., 1999; Rudnick et al., 2005).
In terms of their ability to withstand marine salinities, seagrasses are halophytes, i.e., they can thrive in salt concentrations that would kill 99% of other plant species (Flowers and Colmer, 2008). Increases or decreases in salinity have been shown to affect the photosynthetic capacity, growth, pigment content, biomass and C/N balance in seagrasses (Fernández-Torquemada and Sánchez-Lizaso, 2011; Sandoval-Gil et al., 2012; Garrote-Moreno et al., 2015; Piro et al., 2015a,b). Several studies have been conducted to study salt tolerance in both temperate and tropical seagrasses (Koch et al., 2007; reviewed by Touchette, 2007; Sandoval-Gil et al., 2014; Collier et al., 2014). Koch et al. (2007) tested the response of three tropical seagrass species to hypersalinity (induced slowly and also pulsed). Based on growth and photosynthetic parameters, they demonstrated that Thalassia testudinum and Halodule wrightii are able to tolerate high salinities of 60 and 65 PSU, respectively. Interestingly, Sandoval-Gil et al. (2014) reported that inter and intra-specific divergences play an important role in determining the threshold of salinity tolerance in the temperate seagrasses Cymodocea nodosa and Posidonia oceanica. This is indicated by the intra-specific physiological plasticity that was observed between different populations of Cymodocea nodosa and P. oceanica. While Collier et al. (2014) also noticed inter-species specific hyposalinity thresholds, they proposed a response analogous to a stress-induced morphometric response (SIMR) – the seagrasses show up to a 400% increase in shoot density at sub-lethal salinities. This SIMR precedes mortality in two of the three seagrass species chosen for the study (Collier et al., 2014).
The only known study investigating salinity tolerance in H. stipulacea has shown that the epidermal concentrations of Na+ and Cl- are lower than the surrounding seawater, indicating the existence of some ion exclusion mechanisms (Beer et al., 1980). They also demonstrated that carbon-fixing enzymes such as phosphoenolpyruvate carboxylase are able to function in the presence of salt in vitro, an important adaptive mechanism to salinity. However, very few studies have measured responses of tropical seagrasses to both hyper and hyposalinities (Collier et al., 2014), and none have compared responses of seagrasses to differing salinities with that of their freshwater relatives. Comparing close halophytic and non-halophytic terrestrial relatives has proven a very fruitful approach to understanding halophytism in the Brassicaceae (Inan et al., 2004; Amtmann, 2009; Orsini et al., 2010; Oh et al., 2012; Bartels and Dinakar, 2013). These comparative studies have revealed a plethora of salt adaptation mechanisms including anatomical structures, tight control of entry and compartmentation of Na+ uptake, salt-resilient photochemistry, constitutive up- and down-regulation of stress tolerance genes and metabolites, and sub-functionalization and neo-functionalization of duplicated genes (Inan et al., 2004; Volkov et al., 2004; Kant et al., 2006; Stepien and Johnson, 2009; Dassanayake et al., 2011; Kazachkova et al., 2013; Oh et al., 2014). Similarly, the recent comparison of the Zostera marina genome with a freshwater relative (the duckweed, Spirodela polyrhiza) has revealed genetic changes related to adaptation to a marine environment (Olsen et al., 2016).
The order of Alismatales includes eleven families of aquatic freshwater species and four families that are fully marine species (i.e., seagrasses; Les et al., 1997; Wissler et al., 2011). The phylogenetically closest freshwater relative of H. stipulacea, the tropical seagrass species which is the focus of our study, is Vallisneria americana (Les et al., 1997). Both species belong to the Hydrocharitaceae family, and both the Halophila and Vallisneria genera are classified within the same subfamily (Hydrilloideae Luerss.; reviewed by Les et al., 2006). In fact, phylogenetic studies of the Hydrocharitaceae that compared both morphological characteristics and a suite of molecular markers (chloroplast rbcL, matK, trnK intron sequences, ribosomal ITS region sequences; Les et al., 2006), showed that H. stipulacea is more closely related to V. americana than to many other seagrass species, and the two species share some metabolic features such as production, accumulation, and use of carbohydrates (Kraemer et al., 1999). Although V. americana is considered a freshwater (aquatic) angiosperm (Kraemer et al., 1999), several studies suggest that V. americana can tolerate up to 20 PSU (Doering et al., 1999; Boustany et al., 2010). However, the salinity tolerance limits of V. americana varies between populations (Doering et al., 1999; Frazer et al., 2006; Boustany et al., 2010; Lauer et al., 2011).
Applying the comparative approach used to understanding salt tolerance mechanisms in terrestrial halophytes, the aim of this study was to lay the foundations for detailed molecular investigations into H. stipulacea halophytism by comparing the physiological responses to changes in salinity in the tropical seagrass H. stipulacea, with that of its closest freshwater relative, V. americana. To the best of our knowledge, this is the first comparison of physiological and growth responses of a seagrass species with its freshwater relative.
Materials and Methods
Plant Collection and Experimental Design
Intact H. stipulacea plants were collected from meadows growing at 6–8 m depth (Supplementary Figure S1) in the northern Gulf of Aqaba (North beach site; 29.546150° N 34.964819° E; Mejia et al., 2016) by SCUBA-diving. In order to reduce variability, only shoots less than 1 year-old were collected (i.e., shoots with less than 12 leaf scars on the vertical rhizome; Pérez and Romero, 1992). Plants were brought into our seagrass dedicated microcosm with controlled temperature, salinity and light (Figure 1A) and planted in 15 aquaria (40 cm width × 33 cm height, ∼45 L of seawater in each aquarium), placed in temperature-controlled water baths at 25°C and layered with 20 L of natural sediment (10 cm high; Figures 1A,B). Two months prior to collecting plants, sediment was collected from a location near the plant collection site, sieved (∼3 mm pore) to exclude macro-invertebrates and stones, autoclaved (to exclude potential microbial contaminations) and placed into aquaria. In each aquarium, 13 shoots (with their corresponding rhizomes and roots) were planted in 10 cm of sediment and the aquarium was filled with artificial seawater (Red Sea Salt, Israel) at a control salinity level of 40 PSU (the year-round average salinity of Eilat’s water1). Lighting was provided via T5 fluorescent lamps (Osram Lumilux HO 865/54W cool daylight with the color temperature of 6500 degrees Kelvin2). Photosynthetically active radiation (PAR) values and duration (10–12 h of light, ∼100–120 μmol photons s-1 m-2) at the bottom of the aquaria were set to mimic midday light at the site during November 2016 at 6–8 m depth. Independent powerheads were installed to obtain proper water circulation in each aquarium. Salinity, pH, and temperature were measured in each aquarium daily using a digital salinity/conductivity/temperature meter (WTW 340i, WTW, Germany). Conditions (25°C, 40 PSU) in the microcosms were maintained for 21 days to allow plant acclimation before starting experiments.

FIGURE 1. (A) The seagrass (Halophila stipulacea) dedicated microcosm fully controlled for water temperature, light, and salinity (the image is being published with the consent of the depicted individual; Photographed by Yoram Zvieli). (B) H. stipulacea growing in some of the aquaria (photographed by Gidon Winters). (C) The Vallisneria americana dedicated microcosm with aquaria and sediment type identical to those used in the seagrass setup (photographed by Gidon Winters).
For gradual ramping of salinities up from 40 to 60 PSU (5 PSU/day), artificial Red Sea salt mixtures (which also contained a complete set of micronutrients and appropriate salts but negligible nitrates or phosphates3) were dissolved in distilled water and added daily to the aquariums in parallel to replacing 2–4 liters of aquarium water until the desired final salt concentrations in the aquariums were reached (Figure 2A). Similarly, for the gradual ramping down from 40 to 25 PSU, aquarium water that was set at 40 PSU (acclimation period) was gradually replaced by adding small amounts (2–4 liters/day) of distilled water (0 PSU) until the desired final salt concentration (25 PSU) in the aquaria was reached. This ramping up/down process has been previously shown to be slow enough to prevent (or at least attenuate) osmotic shock (Kahn and Durako, 2006; Griffin and Durako, 2012). A set of control plants (n = 5 aquaria) were maintained at 40 PSU throughout the entire experiment (Figure 2A). In all three treatments, throughout the entire period of the experiment, 10% of the water (25, 40, and 60 PSU) was replaced each week. The plants were maintained under these conditions for 22 days, and harvested at various time points (Figure 2A). Salinities in the aquaria were then ramped either up (from the 25 PSU hyposalinity treatment) or down (from the 60 PSU hypersalinity treatment) to the control salinity (40 PSU) at the same rate and method as at the beginning of the experiment (5 PSU/day). After returning to control salinities of 40 PSU, the plants were allowed to recover at this salinity level over a period of another 21 days and then harvested for analysis. To avoid experimental bias, all tanks and treatments were completely randomized.
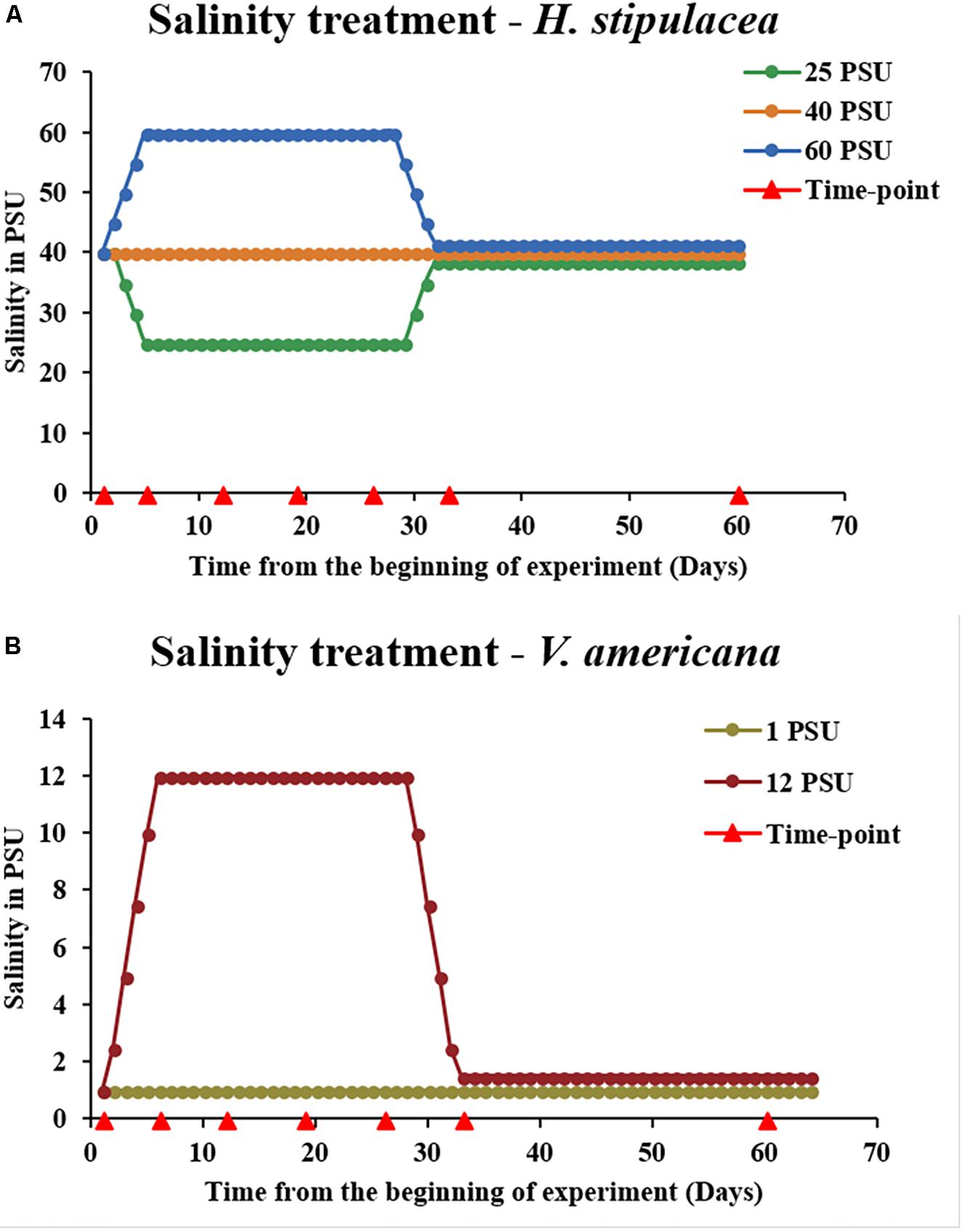
FIGURE 2. Experimental design for investigating the physiological response of H. stipulacea (A) to control, hyper and/or hyposalinity (40, 60, and 25 PSU, respectively) and its freshwater relative, V. americana (B) to control and hypersalinity (1 and 12 PSU, respectively). In both species, there were five tanks for each treatment (n = 5). Salinities were kept at these levels for 3 weeks followed by a recovery phase of another 4 weeks (salinities returned to control levels of 40 and 1 PSU, for H. stipulacea and V. americana, respectively). Physiological, fitness and photosynthetic parameters were performed throughout the experiment at various time-points (red arrowheads). Graphical depiction of salinity levels during the recovery phase in (A) (25 and 60 PSU) and (B) (12 PSU) were slightly modified for visual clarification by separation of the lines.
Throughout the entire experiment, plants were measured and sampled for a suite of fitness, physiological and molecular parameters. Measurements were taken at the following time points: day 0 – just before starting the salinity treatments; day 5 – the day on which the final salinity concentrations were reached; day 12, day 19, day 26 – at 1-week intervals (when plants were exposed to 1, 2, and 3 weeks, respectively, of high/low salinities); day 33 – 1 day after ramping up/down back to the control 40 PSU for recovery; day 60 – after 4 weeks of recovery at control salinity of 40 PSU (Figure 2A). Plant harvesting was always performed at the same time of day (11:30–12:30) in order to take into account any effects of the circadian clock. All growth and physiological measurements were carried out on the second youngest leaf pair, which exhibits the least variability within the shoots and is the most representative tissue (Durako and Kunzelman, 2002).
For the parallel V. americana experiments, plants were obtained from a commercial supplier in Israel4 in October 2016, after which they were grown in sediment, light, temperature and water circulation conditions similar to the H. stipulacea plants (Figure 1C).
Preliminary work (with other plants) tested the effects of different salinities on H. stipulacea (15, 25, 40, 60, and 65 PSU; Supplementary Figure S2). The fact that previous studies determining salinity thresholds in V. americana used different populations and varying durations of stress, frequency, and intensities (Boustany et al., 2015), made it difficult to compare across different salinity stress studies. Hence, preliminary experiments were conducted to test the highest tolerance threshold for V. americana (0, 10, 12, and 15 PSU; Supplementary Figure S3). The results of these experiments (Supplementary Figure S3) confirmed the work of Twilley and Barko (1990) showing the highest tolerance for V. americana to be around 12–13 PSU. Thus, for V. americana, we selected 12 PSU as the higher salinity concentration to stress the plants as the plants died at 15 PSU and there were no significant effects of hypersalinity at 10 PSU (Supplementary Figure S3). Control levels were chosen as 1 PSU (as opposed to 0 PSU), due to the fact that V. americana is mostly found growing in estuary water, where natural minimal salinities are larger than 0 PSU (Twilley and Barko, 1990; Kraemer et al., 1999).
Similarly, for H. stipulacea we selected 25 and 60 PSU since lower salinity (15 PSU) prevented any recovery of leaf number and Fv/Fm (Supplementary Figures S2A,C). The hypersalinity treatment of 60 PSU was chosen since our preliminary results showed that going up to 65 PSU seemed lethal to H. stipulacea (Supplementary Figure S2).
The idea of the comparison between H. stipulacea and V. americana was to compare plants exposed to salinities that yielded a response to salt but were not lethal. Hence, the final salinity conditions selected for the V. americana experiments were 1 PSU (control) and 12 PSU (hypersalinity). Starting from control levels, salinity was ramped up to 12 PSU (Figure 2B) at 2.5 PSU/day (by replacing freshwater with saline mixtures as described above for H. stipulacea) to avoid osmotic shock. Measurements were taken at the same time points as for H. stipulacea on the leaves from the second youngest shoot.
Growth and Physiological Measurements
Growth and biochemical parameters including leaf number, leaf area, dry weight of above/below-ground tissues, chlorophyll, and carotenoid content, and carbon/nitrogen ratios in above/below-ground tissues were measured over the course of the experiment as physiological indicators of stress. For leaf and shoot counts, three plants were selected randomly per aquarium, plants were marked, and the number of leaves and shoots were counted for the same three plants at every time-point; these three measurements were averaged into a single biological replicate, and this was performed for five tanks (n = 5) in each condition (control, hypo, and hypersalinity). Leaf area was measured by selecting the second youngest leaves from three random plants from each aquarium (at each time point these three measurements were averaged into a single biological replicate, n = 5 aquaria in each treatment), and digitally scanned (Cannon Lide 110 scanner). The leaf area was then measured using ImageJ (Abramoff et al., 2004; Mejia et al., 2016). Because the morphology of V. americana differs from that of H. stipulacea (Figures 1B,C), leaf counts were determined as for H. stipulacea except that we also measured the number of leaves per shoot (there are more leaves per shoot for V. americana than in H. stipulacea). Additionally, blade length was measured as indicated in Doering et al. (1999). The three measurements from the same aquarium were averaged into a single biological replicate, n = 5 aquaria in each treatment.
Biomass and C/N Ratios
For biomass and C/N ratios, three plants were randomly selected per aquarium at the end of the experiment (day 60 only, due to the need to sacrifice the entire plant for this measurement) and dried in the oven at 60°C for 24 h. The above- and below-ground tissues were then weighed to compare biomass across the three treatments. For determining C/N ratios, the dried tissues were later ground to powder in a TissueLyser (Retsch GmbH & Co. KG) and 6 mg of the tissue was weighed in a tin capsule and analyzed in a Flash 2000 Organic Elemental Analyzer (Thermo Scientific, USA). Calibration was performed using 2–3 mg of 2,5-Bis(5-tert-butyl-benzoxazol-2-yl)thiophene (BBOT) containing 6.51% N, 72.53% C, 6.09% H, and 7.44% S. Each sample was run for 660 s and was recalculated with a blank (empty capsule) and BBOT standard measurements.
Chlorophyll Florescence and Pigment Content
For determination of quantum efficiency of photosystem II (Fv/Fm; Genty et al., 1989), five plants were selected per aquarium and fluorescence was measured using a PAM (Pulse Amplitude Modulation) chlorophyll fluorometer (PAM-2500, Walz, Germany). The same five plants were measured over time (at every time point) for any change in quantum efficiency. Measurements from the same aquarium were averaged into a single biological replicate, with n = 5 aquaria in each treatment. Leaves were dark adapted for 10 s prior to the measurement using a dark adaption leaf clip (Winters et al., 2011). For measuring chlorophyll and carotenoids, 100 mg fresh tissue was harvested from three plants in each aquarium at each time point. Pigments were extracted in 100% methanol, kept overnight at 4°C in the dark, and measured according to Lichtenthaler (1987) in a microplate reader (EPOCH 2, Biotek Instruments, Inc., United States) according to Warren (2008). Concentrations were normalized to the fresh weight (FW) of the sampled tissues.
Statistical Analysis
For H. stipulacea, a one-way ANOVA with repeated measures (IBM SPSS version 19) with a Greenhouse-Geisser correction was performed to identify the effects of each salinity treatment on the physiological data such as leaf number, leaf area, shoot number, blade length, quantum yield, and chlorophyll content (treated as dependent variables) for measurements at different time-points. Post hoc mean comparisons with the Tukey–Kramer HSD test were performed to identify specific salinity or time points which exhibited significant differences. For measurements of biomass and C/N ratios that were taken only at the last time point (day 60), a one-way ANOVA (SPSS version 19) was performed. Since the V. americana experiment, included only control (1 PSU) and hypersalinity (12 PSU), pairwise comparisons were performed (control vs. hypersalinity) to find the specific time point which displayed significant differences. All the treatment effects were considered statistically significant at P < 0.05. Normality of data was tested using Shapiro–Wilk’s test and QQ plots. Homogeneity of variances was tested using the Levene’s test.
Results
Changes in Salinity Have No Effect on H. stipulacea’s Capability to Produce New Leaves While V. americana Exhibits Growth Reduction and Recovery
In order to examine the effect of hypo and hypersalinity on the growth of the tropical seagrass, H. stipulacea and its closest freshwater relative, V. americana, the seagrass was exposed to 40, 25, and 60 PSU (control, hyposalinity, and hypersalinity treatments, respectively) while V. americana was exposed to 1 and 12 PSU (control and hypersalinity treatments, respectively). Figure 3 shows images of H. stipulacea and V. americana plants at various time points (Figure 2) during the experiment.
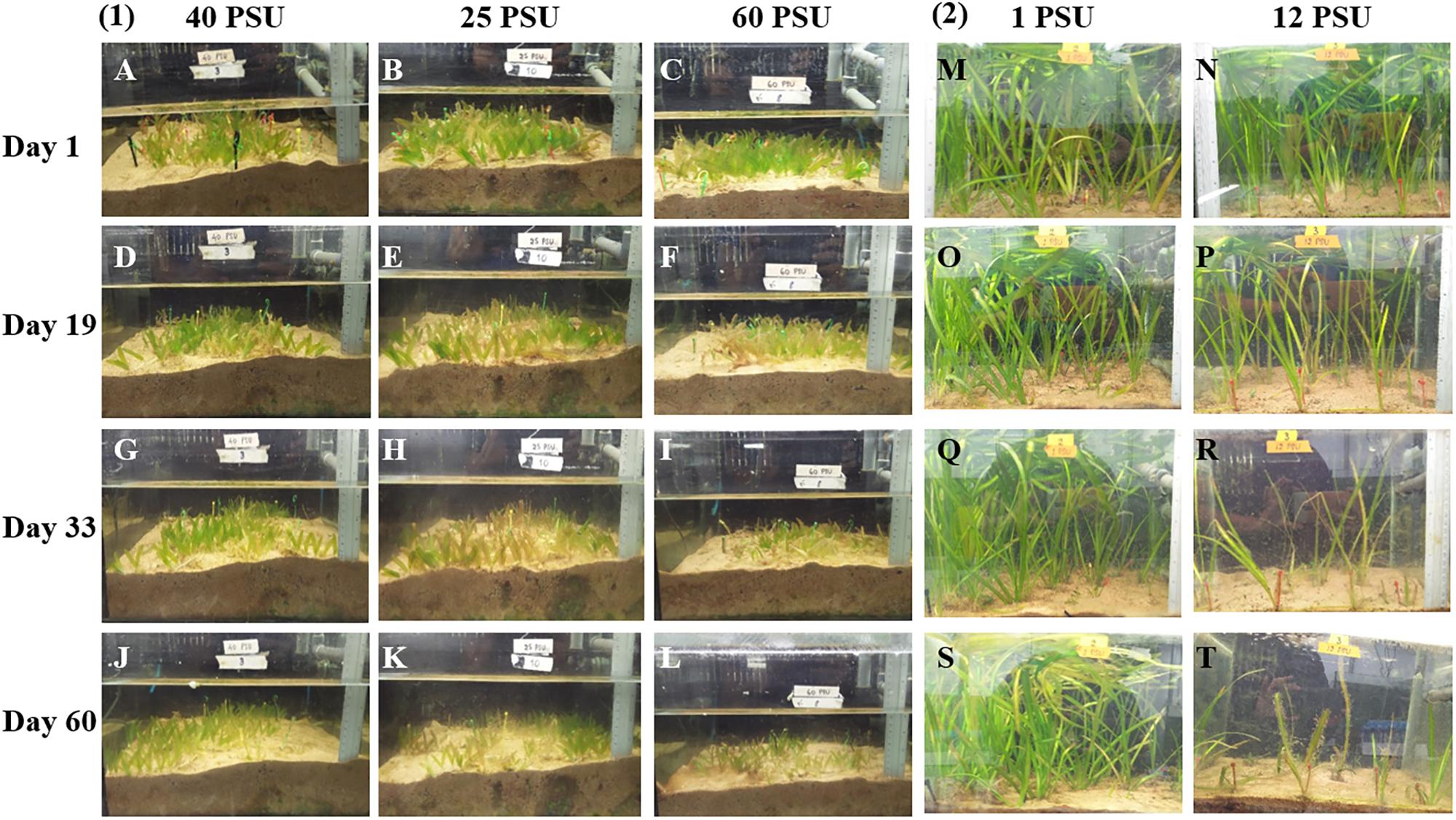
FIGURE 3. Photographs showing the differences in growth of H. stipulacea (A–L) and V. americana (M–T) plants after treatment with different salinities. Days 1–60 correspond to time points during the experiment (see Figure 2). (1) showing responses of H. stipulacea plants to control (40 PSU; A,D,G,J), hyposalinity (25 PSU; B,E,H,K) and hypersalinity (60 PSU; C,F,I,L). (2) showing responses of V. americana plants to control (1 PSU; M,O,Q,S) and hypersalinity (12 PSU; N,P,R,T).
Leaf Number
For H. stipulacea, a constant increase in leaf number was observed throughout the experiment in all three salinity treatments (Figure 4A; one-way ANOVA with repeated measures, P < 0.01; Table 1) although plants exposed to hyposalinity (25 PSU) showed a trend of lower number of leaves throughout the experiment, compared with plants in control and hypersalinity (40 and 60 PSU, respectively). While there was no significant interaction between the effect of the salinity treatment and time on the changes in leaf number in H. stipulacea (one-way ANOVA with repeated measures, P > 0.05; Table 1), more senescence was seen in leaves at hyposalinities at day 33 in comparison with control plants (Figures 3G,H). On the other hand, V. americana plants exposed to hypersalinity (12 PSU) showed a significant decrease (one-way ANOVA with repeated measures, P < 0.05; Table 2) in the number of leaves compared to control plants (1 PSU) (Figure 4B). Furthermore, although by the end of the salinity exposure (day 33), V. americana plants exposed to hypersalinity possessed half the number of leaves observed in control plants (Figure 4B), leaf number recovered to control levels by the end of the recovery period. Significant interaction between salinity over the time of the experiment was also seen (one-way ANOVA with repeated measures, P < 0.05; Table 2). V. americana exposed to hypersalinity suffered large losses in above-ground biomass (Figure 3R), and the effects of the salinity treatment were still visible even after 3 weeks into the recovery phase (Figure 3T), where plants had been returned to control salinity levels.
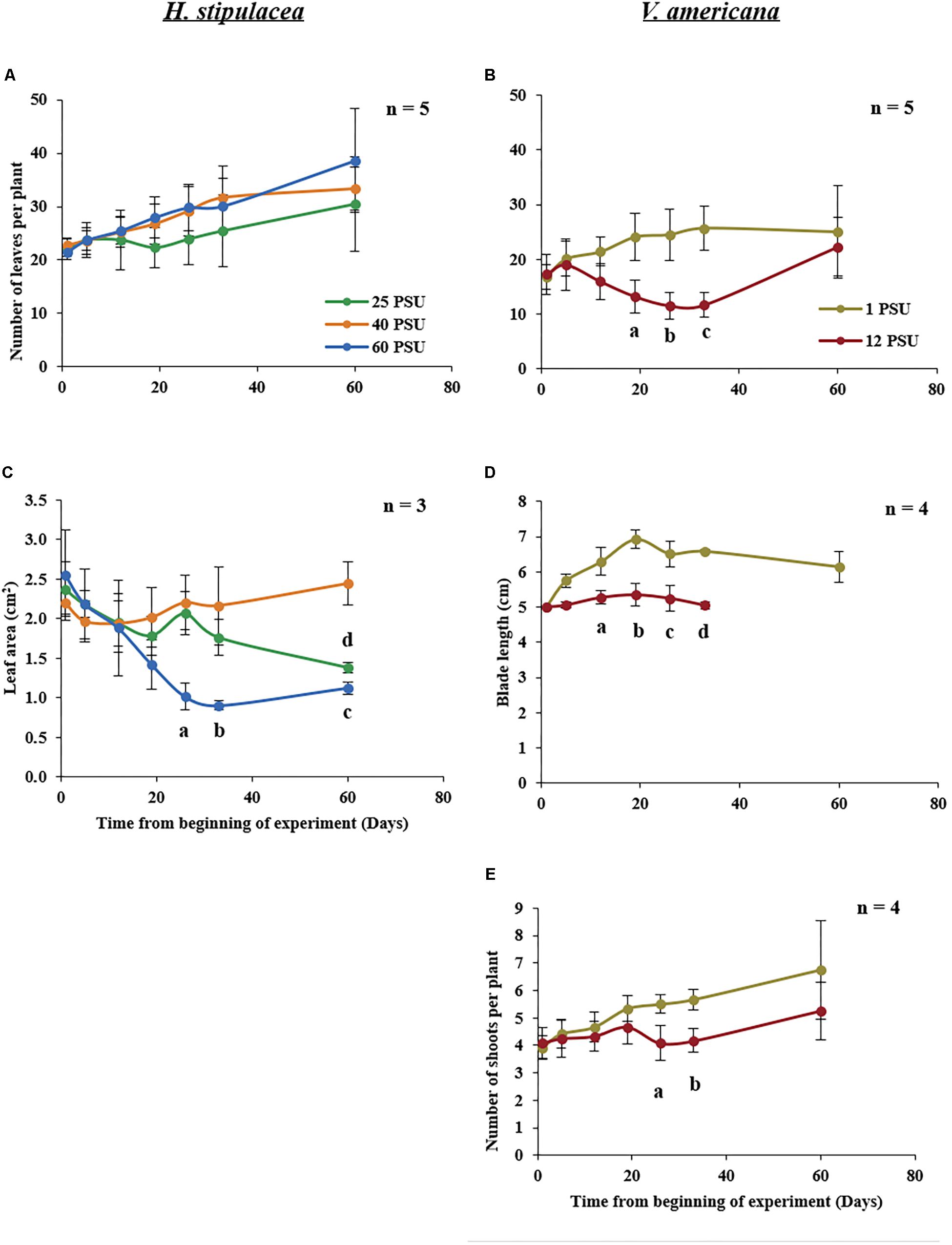
FIGURE 4. Growth measurements in H. stipulacea (Left; A,C) and V. americana (Right; B,D,E) throughout the experiment. H. stipulacea plants were exposed to control, hyper and hyposalinity (40, 60, and 25 PSU, respectively). V. americana plants were exposed to control and hypersalinity (1 and 12 PSU, respectively). Data represent mean ± SD in the number of leaves per plant (A,B), leaf area (cm2; C), blade length (cm, D) and shoot number per plant (E). Time-points with different letters are significantly different between treatments, P ≤ 0.05 as determined by Tukey–Kramer HSD for H. stipulacea and pairwise comparisons for V. americana.

TABLE 1. Results of one-way repeated measures ANOVA for the effects of the salinity treatment (25, 40, or 60 PSU) on different variables in H. stipulacea.
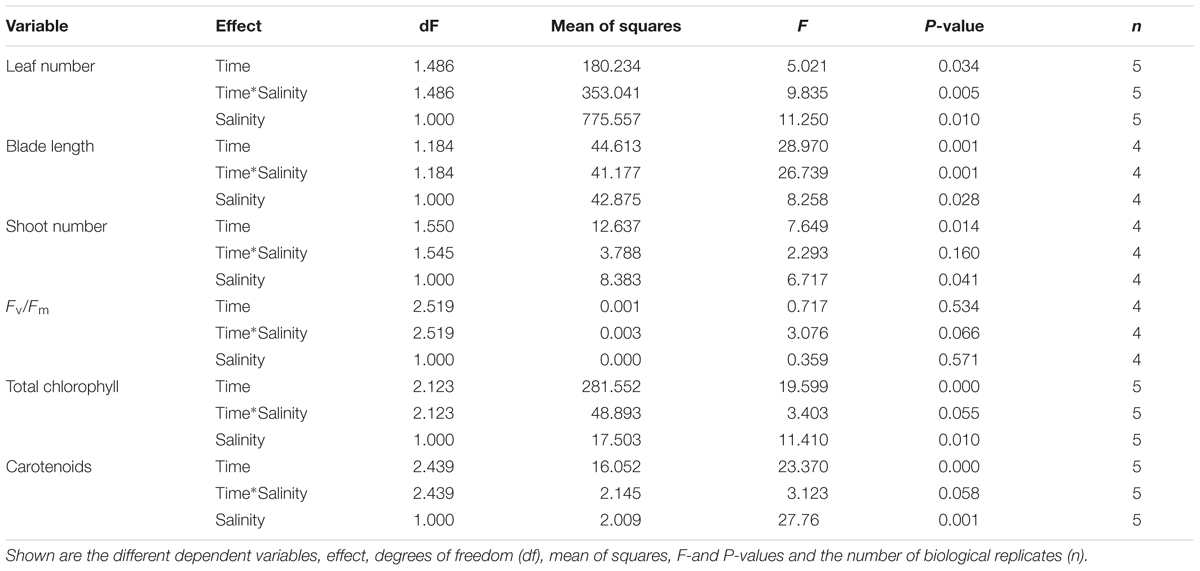
TABLE 2. Results of one-way repeated measures ANOVA for the effects of the salinity treatment (1 vs. 12 PSU) on different variables in V. americana.
Leaf Area and Blade Length
Exposure to hypersalinity resulted in a large decrease in leaf area (Figure 4C) in H. stipulacea (one-way ANOVA with repeated measures, P < 0.05; Table 1). A trend toward reduced leaf area was also observed in response to hyposalinity, but this decrease was smaller, slower and not statistically significant (one-way ANOVA with repeated measures followed by Tukey test, P > 0.05; Table 1). However, leaf area in plants exposed to hyposalinity continued to drop even after plants were returned to control salt levels until there was a significant leaf area reduction compared to control plants by day 60 (one-way ANOVA with repeated measures followed by Post hoc Tukey test, P < 0.05). On the other hand, leaf area in H. stipulacea plants exposed to hypersalinity stopped decreasing once plants were returned to control salt levels although leaf area did not recover to that of control plants at least over the period allowed for recovery in this experiment. The interaction between the salinity treatment and time was also significant (one-way ANOVA with repeated measures, P < 0.05; Table 1).
In V. americana, there was a significant difference over time in blade length between the control and stressed plants immediately after the plants were exposed to high salinity (one-way ANOVA with repeated measures, P < 0.05; Table 2 and Figure 4D). However, most of the leaves that were marked for blade length measurement were lost during the recovery phase and therefore no measurements could be taken for the last time point in the stressed V. americana plants. The increase in V. americana leaf number during the recovery phase was due to new leaf production (Figure 4B).
A significant decrease was also observed in shoot number (one-way ANOVA with repeated measures, P < 0.05; Table 2) in stressed V. americana plants in comparison to the plants at control salinity (Figure 4E). Pairwise comparisons showed significant differences at days 26 and 33 (one-way ANOVA with repeated measures followed by pairwise comparisons, P < 0.05). However, there was no significant interaction of salinity and time (one-way ANOVA with repeated measures, P > 0.05; Table 2). During recovery, V. americana plants produced new shoots and at day 60 there was no significant difference between control plants and plants that were exposed to hypersalinity (one-way ANOVA with repeated measures followed by pairwise comparisons; P > 0.05).
H. stipulacea Exhibits Stress-Resilient Photosynthetic Capacity
Measurements of chlorophyll fluorescence parameters can provide information about photochemistry that is driving photosynthesis (Maxwell and Johnson, 2000). In H. stipulacea, Fv/Fm, which evaluates the maximum efficiency of photosystem II (PSII; Maxwell and Johnson, 2000), showed no significant interaction between salinity and time (one-way ANOVA with repeated measures, P > 0.05; Table 1) throughout the experiment (Figure 5A). There were also no significant differences in total leaf chlorophyll content (Figure 5C), carotenoid levels (Figure 5E) or chlorophyll a and chlorophyll b (data not shown) between plants under control and salinity treatments over the time of the experiment (one-way ANOVA with repeated measures, P > 0.05; Table 1). However, in V. americana, Fv/Fm exhibited a strong trend (one-way ANOVA with repeated measures, P = 0.0534; Table 2) for differences between treatments over time. However, this lack of significant difference between control and salt-stressed plants lasted only until day 33 by which time Fv/Fm was significantly reduced (one-way ANOVA with repeated measures followed by Post hoc Tukey test, P < 0.05) in plants exposed to hypersalinity (Figure 5B). After return to control salinity, the stressed plants were able to recover and there was no significant difference between the control and stressed plants at day 60. While total chlorophyll and carotenoid contents for V. americana plants also displayed no significant difference between treatments over time (one-way ANOVA with repeated measures, P > 0.05; Table 2), there were strong trends that indicated some differential effect (Table 2) between control and salt-stressed V. americana plants (Figure 5D). This is suggested by differences in chlorophyll and carotenoid contents between the treatments shown toward the end of the experiment, after the return of salt-stressed plants to control salt conditions, where both total chlorophyll (Figure 5D) and carotenoid (Figure 5F) levels increased significantly above that of control plants (one-way ANOVA, followed by Pairwise comparisons, P < 0.05; Figure 5F).
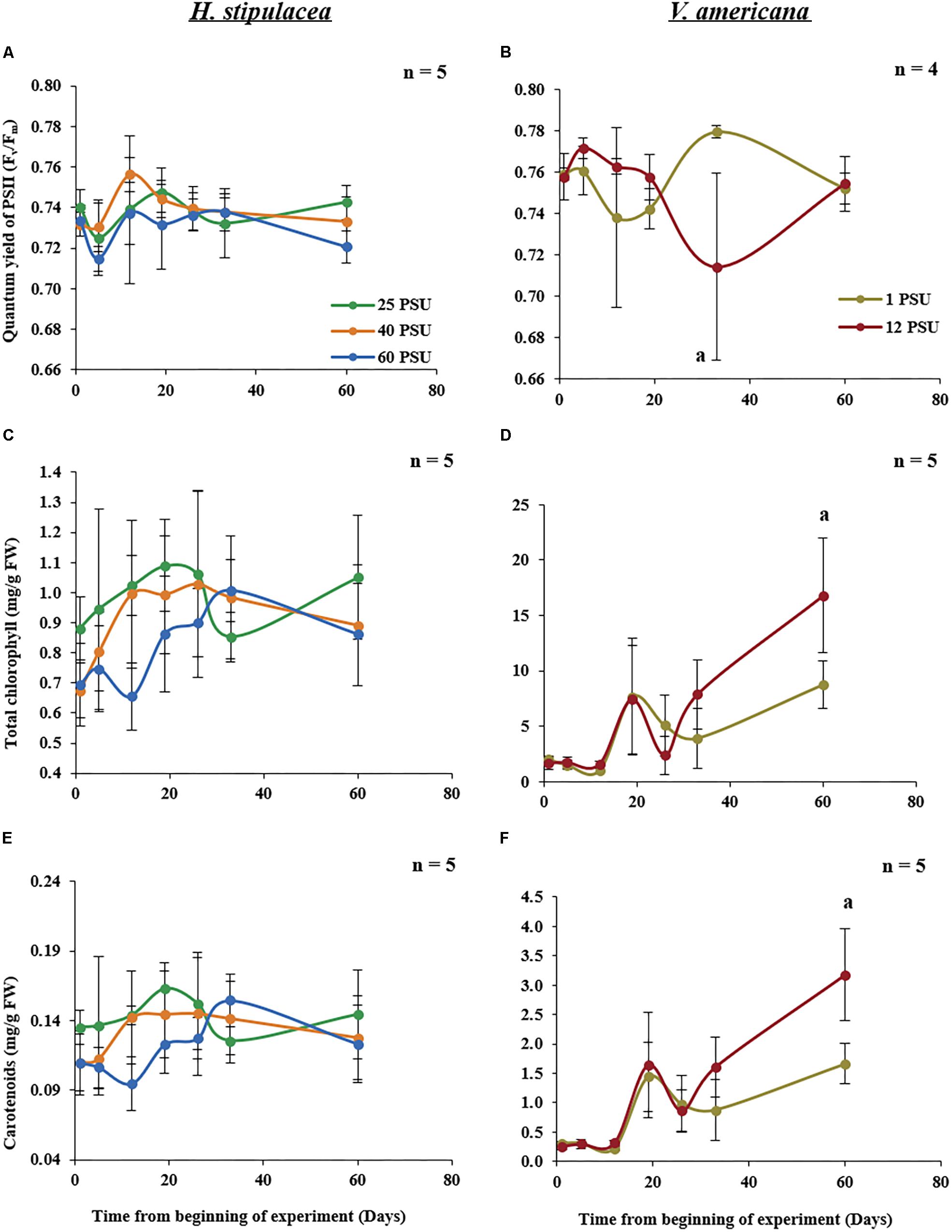
FIGURE 5. Photo-physiological parameters measured in H. stipulacea (Left; A,C,E) and V. americana (Right B,D,F) throughout the experiment. Data represent mean ± SD in dark-adapted quantum yield of PSII (Fv/Fm; A,B), total chlorophyll (chlorophyll a+b; C,D) and carotenoid (E,F) concentrations. Time-points with different letters are significantly different between treatments, P ≤ 0.05 as determined by Tukey–Kramer HSD for H. stipulacea and pairwise comparisons for V. americana.
Changes in Salinity Cause a Greater Effect on the Biomass and C/N Ratios of H. stipulacea Than V. americana
Biomass and C/N ratios of H. stipulacea and V. americana were determined from plants harvested at day 60 (27 days into the recovery period). A significant decrease (one-way ANOVA, P < 0.05; Table 3) in the biomass of both below- and above-ground tissues from H. stipulacea plants previously exposed to hypo or hypersalinity was observed compared to plants that were maintained during the entire experimental period in control salinities (Figure 6A). This finding indicates a long-lasting impact of the salinity treatment on H. stipulacea plants. A long-term effect of salinity on V. americana biomass was also observed but only in above-ground tissues (Figure 6B; one-way ANOVA, P < 0.05; Table 4). There was no significant difference (one-way ANOVA, P > 0.05; Table 4) in biomass of below-ground V. americana tissues between control and salt-treated plants.
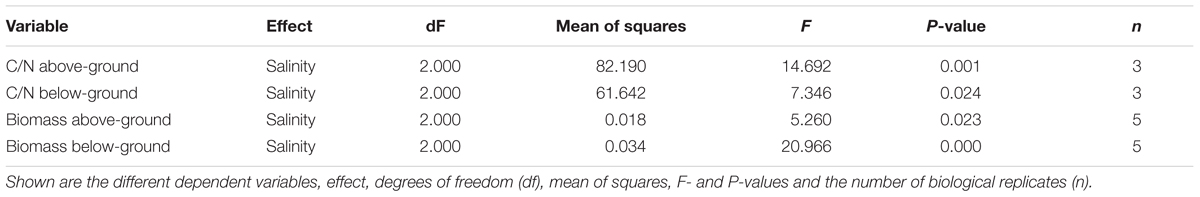
TABLE 3. Results of one-way ANOVA for the effects of the salinity treatment (25, 40, or 60 PSU) on different variables in H. stipulacea at day 60.
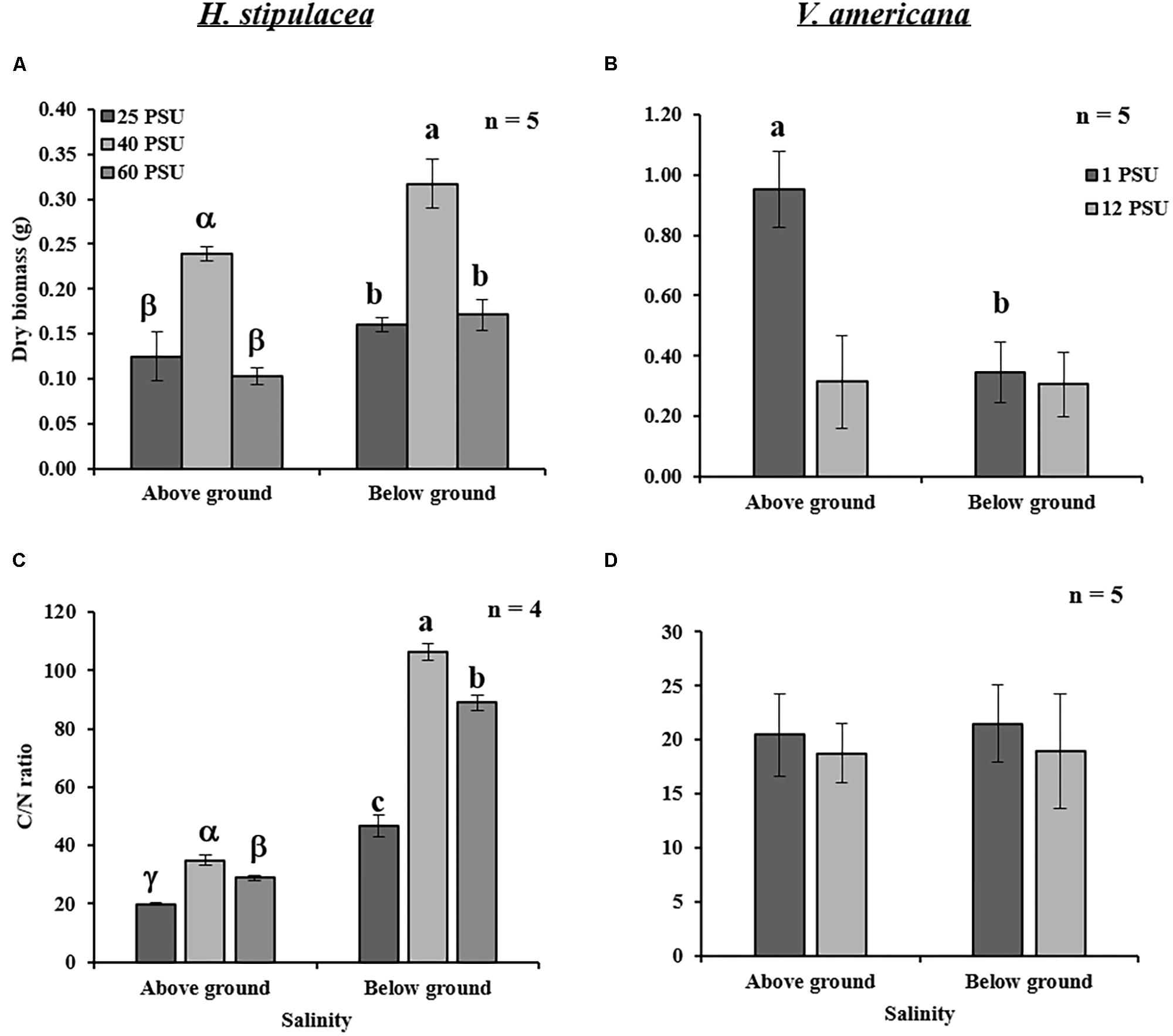
FIGURE 6. Effects of salinity treatments (see legend of Figure 5 for details) on the above- and below-ground dry biomass and C/N ratios in H. stipulacea (Left; A,C) and V. americana (Right; B,D) measured the end of the experiment (day 60 only). Data represent mean ± SD. Bars with different letters are significantly different between treatments, P ≤ 0.05 as determined by Tukey–Kramer HSD for H. stipulacea and pairwise comparisons for V. americana.
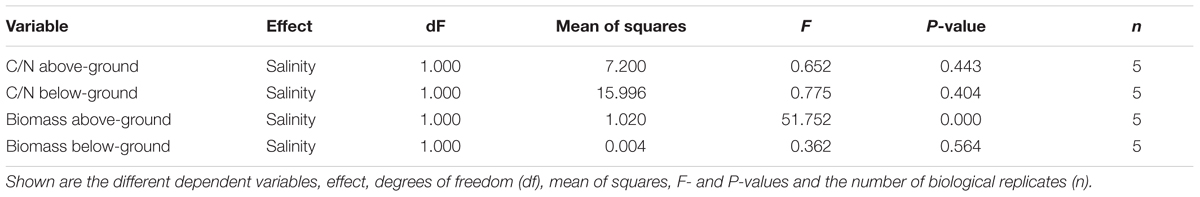
TABLE 4. Results of one-way ANOVA for the effects of the salinity treatment (1 vs. 12 PSU) on different variables in V. americana at day 60.
C/N ratios of H. stipulacea above- and below-ground tissues exposed to both hypo and hypersalinity exhibited a significant reduction at the end of the experiment (one-way ANOVA, P < 0.05; Table 3) compared to control plants (Figure 6C). However, no differences (one-way ANOVA, P < 0.05; Table 4) in V. americana C/N ratios between control and salt-treated plants were observed in either above- or below-ground tissues (Figure 6D).
Discussion
In terms of their ability to withstand marine salinities, seagrasses are defined as halophytes (Zhu, 2001; Touchette, 2007; Munns and Tester, 2008). This is probably even more so for the tropical H. stipulacea since it is dominant in the northern Red Sea, where salinity is rarely lower than 40–41 PSU (Katz et al., 2015). While it is obvious that H. stipulacea and V. americana are very different plants that exist in very different habitats and in particular, different salt environments, they are genetically very close (Les et al., 1997, 2006). Both these species are capable of extensive clonal growth (Lovett-Doust and Laporte, 1991), and both have a relatively wide, although not overlapping, salt range. Here, we are not comparing salt tolerance per se between the two species but comparing the responses of each plant to changes in salinity. In this context, it is important to note that the transcriptome and metabolome of the halophytic Arabidopsis relative, Eutrema salsugineum exhibit a much lower global response to salt stress than salt-sensitive Arabidopsis (Gong et al., 2005; Lugan et al., 2010; Kazachkova et al., 2013). However, at higher salinity levels than is required for Arabidopsis to respond, stress-mediated induction of the E. salsugineum transcriptome does occur suggesting that the stress-sensitive and stress-tolerant species possess different sensitivities to salt (Gong et al., 2005; Amtmann, 2009). A similar situation could occur between V. americana and H. stipulacea as the seagrass only exhibits an effect of salt on growth at a far higher salt level than its freshwater relative (Figures 3, 4).
Most seagrasses are thought to be sensitive to hypersalinity (Ogata and Matsui, 1965; Biebl and McRoy, 1971; Zieman, 1975; Adams and Bate, 1994; Kamermans et al., 1999; Van Katwijk et al., 1999). While there are a few studies on the effect of temperature and light on H. stipulacea (Angel et al., 1995; Schwarz and Hellblom, 2002; Sharon et al., 2009, 2011), this is, to the best of our knowledge, the first paper exploring the salinity range and effects of salinity on the physiology and growth of this euryhaline seagrass species.
Leaf Size Modulation – An Important Mechanism to Cope With Salinity in H. stipulacea
Among other traits such as ion homeostasis, photosynthesis, yield components and senescence, plant growth is an important trait associated with salt stress (Negrão et al., 2017). Plants reduce their growth rate immediately after the onset of stress and to begin to conserve and distribute their resources as needed (Skirycz and Inzé, 2010). Several reports on terrestrial plants suggest a reduction in leaf area as a general response to salinity stress (Marcelis and Van Hooijdonk, 1999; Wang and Nii, 2000; Maggio et al., 2007). Our results support this general response to stress – H. stipulacea suffered reductions in leaf area when exposed to hyper or hyposalinity (Figure 4C), with hyposalinity also causing a trend toward reduction in leaf number (Figure 4A). Previous studies showed that H. stipulacea modified leaf size in response to varying temperature (Procaccini et al., 1999), light levels (Schwarz and Hellblom, 2002; Mejia et al., 2016; Rotini et al., 2017) and hydrodynamics (Procaccini et al., 1999; Mejia et al., 2016). Hence, leaf size modulation may be an important mechanism in H. stipulacea to cope not only with salinity but with other abiotic stresses as well. It might be worth noting that under hyposalinity, leaf area was not very different from control plants until after day 33. This could be considered as a late response or damage, at least in comparison to H. stipulacea’s response to hypersalinity.
In contrast, V. americana showed a significant reduction in the number of leaves, number of shoots/plant and blade length (Figures 4B,D,E). In the case of leaf and shoot number, V. americana seemed to only show an effect of hypersaline stress 3 weeks after stress induction. These results are in agreement with other studies suggesting a similar effect of salinity on blade length, shoot number and leaf number in this freshwater species (Bourn, 1932, 1934; Haller et al., 1974; Frazer et al., 2006). Boustany et al. (2010) showed hardly any negative effects on V. americana plants of 3 weeks exposure to 18 PSU but nearly complete mortality after 10 weeks of exposure which also complements other reports including Davis and Brinson (1976), Staver (1986), and French and Moore (2003). In our study, during the recovery period, there was an increase in leaf and shoot number comparable to the levels of the plants grown in control conditions. This recovery was similar to the recovery observed by Doering et al. (2001), that summarized recovery of V. americana plants in two stages: (i) the allocation of energy to the production of new blades on individual shoots; (ii) the production of new shoots with blades. Though the recovery period used by Doering et al. (2001) was over 2 months (compared with our 1-month recovery period), our results demonstrating increases in shoot and leaf number, confirm similar recovery patterns. It is interesting to note that V. americana plants responded immediately to hypersalinity by slowing down the growth of existing leaves (Figure 4D). There were no measurements for the last time point after recovery because the leaves marked for measurements of blade length were lost (Figure 3T). This could be due to V. americana accumulating salt in older leaves and then shedding them, which is a known plant response to salinity (Munns and Tester, 2008), and it will thus be important to perform detailed measurement of ion concentrations in tissues of both V. americana and H. stipulacea.
H. stipulacea Photochemical Characteristics Are Resilient to Hypo and Hypersalinity
Quantum efficiency of photosystem II (Fv/Fm) has been used an as an indicator of stress in seagrasses (Biber et al., 2005; Massa et al., 2009; Winters et al., 2011; Dooley et al., 2013) including salinity stress (Ralph, 1998; Fernández-Torquemada and Sánchez-Lizaso, 2005; Koch et al., 2007). Studies on other seagrasses indicate that Fv/Fm is negatively affected by exposure to salinity stress over time (Ralph, 1998; Kahn and Durako, 2006; Pagès et al., 2010). H. stipulacea was reported to have ‘plastic’ photosynthetic capabilities at varying irradiances, and even showing an alteration in its PSI:PSII ratio (Sharon et al., 2009, 2011). Previous studies on H. stipulacea have also shown that enzymes involved in photosynthesis, RuBP and PEPcase, and located in the epidermal tissue, were active even under higher salinity conditions (Beer et al., 1980). H. stipulacea also displayed lower epidermal concentrations of Na+ and Cl- ions when compared to the exterior and other tissues (Beer et al., 1980). This might be one of the mechanisms by which H. stipulacea maintains photosynthetic capacity throughout the experiment in both hypo and hypersalinity conditions (Figure 5A). It is well-known that to maintain photosynthetic efficiency, plants change the concentrations of their photosynthetic pigments in response to changes in water quality and light regimes (Campbell et al., 2003; Ralph et al., 2007). Indeed, there were changes in number and ultrastructure of chloroplasts in H. stipulacea under high light conditions (Beer et al., 1980). In contrast to other seagrasses (Kahn and Durako, 2006; Pagès et al., 2010), our results show that there was no significant decrease or increase in the dark-adapted quantum yield (Fv/Fm) and chlorophyll content (Figures 5A,C) in response to salinity stress, indicating a rather stable photosynthetic capacity. In contrast, V. americana showed a reduction in Fv/Fm values during the experiment, but this took time and was evident only after day 19, which might be an indication of accumulative damage to the PSII reaction centers (Maxwell and Johnson, 2000). However, in terms of Fv/Fm, the V. americana plants demonstrated full recovery when returned to control salinity conditions as seen in the measurements at day 60 (Figure 5B). It is worth mentioning that although Fv/Fm measurements are widely used and are reliable diagnostic indicators of photophysiological stress (Murchie and Lawson, 2013) and photoinhibition (Winters et al., 2003), non-photochemical quenching, and effective quantum yield measurements (Ralph, 1998) will give us a more complete picture of the photosynthetic properties of H. stipulacea and V. americana. In V. americana plants, our results also show an increase in total chlorophyll and carotenoid content under hypersalinity (Figures 5D,F). This is in contrast to other studies which showed a reduction in chlorophyll content at higher salinities suggesting effects on the photochemical efficiency of the plants (Doering et al., 1999; French and Moore, 2003; Boustany et al., 2015). The increase in chlorophyll and carotenoid content observed in V. americana may be due to an increase in the number of chloroplasts as seen in other studies (Aldesuquy and Gaber, 1993; Misra et al., 1997). However, further studies are required to confirm these observations.
H. stipulacea Might Mobilize Stored Reserves From Below-Ground Tissues to Support Production of New Leaves
For H. stipulacea, measurements made after 27 days of recovery (day 60), showed significant hyper and hyposalinity-mediated reduction in above- and below-ground biomass (Figure 6A). Loss of above-ground biomass may be seen as a response to salinity by H. stipulacea in an attempt to survive by producing new leaves but with reduced leaf area. Exposure to both hypo and hypersalinity treatments caused a long-term change in the biomass of the rhizomes, which are also associated with storage of carbohydrates (e.g., sucrose; Gu et al., 2012). Indeed, stress was shown to reduce underground biomass in the temperate seagrass Z. marina. In Z. marina, light limitation suppressed production of new roots, led to a reduction of sucrose reserves, and caused a decrease in growth rate with increased sucrose synthase activity in leaf tissues toward the end of the stress (light limitation) period (Alcoverro et al., 1999). Sucrose synthase is a key enzyme involved in degradation of sucrose and increased activity of the enzyme indicated that sucrose was being used for the synthesis of more effective and compatible solutes (Touchette, 2007). It was previously shown that light or carbon limitation mobilizes stored reserves to support shoot or leaf proliferation at the expense of below-ground growth (Zimmerman and Alberte, 1996; Clabby and Osborne, 1997; Zimmerman et al., 1997). This ability, if present in H. stipulacea, needs to be confirmed through further studies including accumulation/depletion of specific amino acids or osmolytes in response to changing salinity.
The long-term loss of underground carbon storage was also evident by the C/N ratios measured at the end of the recovery period (Figures 6C,D). H. stipulacea plants that were exposed to high or low salinities showed significant decreases in the above- and below-ground C/N ratios even at day 60, 27 days after they were returned to control salinity levels (Figure 6C). There is a strong correlation between the C/N ratio of seagrasses with N concentration within the plant (Duarte, 1992). H. stipulacea showed higher C/N ratios in roots and rhizomes relative to leaves in plants at depths shallower than 24 m mainly due to lower nitrogen concentrations (Schwarz and Hellblom, 2002). In agreement with these results, our results showed that below-ground tissues exhibited a lower N concentration than the above-ground tissues under control and stressed conditions (Figure 6C). The reduction in C/N ratio observed under hypo and hypersalinities was due to an increase in N content (Supplementary Table S1) in comparison to control plants. These results are in line with known studies showing an increase in tissue nitrogen content and certain amino acids at hypersaline conditions (Pulich, 1986; Twilley and Barko,1990).
While studies have shown a significant decrease in both below- and above-ground biomass in V. americana as a response to increased salinities (Frazer et al., 2006; Boustany et al., 2015), only a few studies have shown that the roots might be more tolerant to increased salinity than shoots (Doering et al., 2001; Boustany et al., 2010). Boustany et al. (2010) reported that when V. americana plants were exposed to hypersalinity (8 PSU), there was an increase in the root:shoot ratio. Furthermore, at 18 PSU the plants lost all the above-ground biomass but 20% of the roots were able to regrow when the plants were returned to control conditions (Boustany et al., 2010). Doering et al. (2001) showed an 80% reduction in above-ground biomass in V. americana plants exposed to 18 PSU salinity, which recovered after 115 days of growth back in control salinity conditions. In the study presented here, exposure to hypersalinity of 12 PSU caused a 60% reduction in the above-ground V. americana biomass, even after recovery (Figure 6B). Although the duration of our experiment was not as long as the other studies, the effects of salinity on the shoots showed similar results, confirming that roots were more tolerant to hypersalinity than shoots.
Surprisingly, C/N ratio measurements in V. americana did not show any significant change between the control and treated plants (Supplementary Table S2). This might have been due to recovery of the plants, as shown by the recovery of leaf number during the period from days 33 to 60 (Figure 4B). Similarly, Boustany et al. (2015) observed that recovery from salinity begins as soon as control salinity conditions are restored. Therefore, the possibility that C/N ratio in V. americana was affected by hypersalinity cannot be ruled out.
Although the emphasis in this study was on physiology, the results shown here also have important ecological implications. While H. stipulacea is native to the Red Sea and the Indian Ocean, it is invasive in the Mediterranean (Gambi et al., 2009) and the Caribbean (Steiner and Willette, 2014). With the increases in brine discharges in the Mediterranean, Red Sea and Persian Gulf (Bashitialshaaer et al., 2011) and with the Mediterranean Sea undergoing a process of ‘tropicalization’ (Bianchi and Morri, 2003; Borghini et al., 2014), it is becoming warmer and saltier (changes of 0.12°C ± 0.07 year-1 and 0.008 ± 0.006 year-1 in water temperatures and salinities, respectively in the Eastern Mediterranean; Ozer et al., 2017). This process will occur even faster after the recent doubling of the capacity of the Suez Canal (“Double Trouble”; Galil et al., 2015, 2017). Thus, the potential threat to local Mediterranean biodiversity posed by H. stipulacea is considered serious. Indeed, H. stipulacea has been included in the “100 Worst Invasive Alien Species in the Mediterranean” (Streftaris and Zenetos, 2006). This concern is even more warranted considering the alarming studies from the Caribbean showing that H. stipulacea is actually displacing local seagrass species (Steiner and Willette, 2014), and the studies from the Mediterranean showing vast declines in the local slow-growing P. oceanica (Marba and Duarte, 2010; Jordà et al., 2012).
Similar to the tolerance to increased salinities, the tolerance to reduced salinities might provide H. stipulacea an advantage in situations of terrestrial freshwater run-offs when local salinity levels temporarily drop (e.g., Katz et al., 2015; Winters et al., 2017).
The current study demonstrates that H. stipulacea is quite capable of tolerating, at least in the short-term (3–4 weeks), both increased (60 PSU) and decreased salinities (25 PSU). While we have no information about other seagrass species from this region, if indeed H. stipulacea has a wider salinity tolerance than other local species, it might have an advantage in the coming future compared with other seagrass species that might tolerate a much narrower range of salinity changes. We therefore hypothesize that tolerance to a wide range of salinities (both hypo and hypersalinities) could provide H. stipulacea with an advantage compared with other seagrass species, and might explain some of its opportunistic and invasive character. We might be seeing much more H. stipulacea in the impending future.
Conclusions and Perspectives
How do these closely related species – H. stipulacea and V. americana inhabit environments with such different salinities? Answering this question would help us not only in understanding the ability of H. stipulacea to survive in new environments (Dittami et al., 2017) and predict extension of this invasive species to other seas but also aid in elucidating salt tolerance mechanisms in seagrasses and plants in general.
Our results demonstrate that H. stipulacea has a remarkable tolerance to hyper and hyposalinity, and it is likely that H. stipulacea possesses salinity tolerance mechanisms that are absent in its close freshwater relative. The most visible differences in salt tolerance between the two species are leaf size modulation and the ability to produce new shoots probably at the expense of the below-ground tissue in H. stipulacea. Clearly, H. stipulacea is able to maintain its photosynthetic capability under both hyper and hyposalinity. This resilience to changing salinity may also be an important trait explaining the invasive nature of this species in the Caribbean Sea. Even though H. stipulacea does not exhibit much response to changing salinities in terms of growth and photosynthesis, the effects of hyper and hyposalinity in this seagrass cannot be dismissed considering the salt-mediated effects on biomass and C/N ratios. Contrasting responses to hypersalinity between H. stipulacea and V. americana are most evident just before and after the recovery phase; during this shift from hypersalinity back to control salinity levels, V. americana displays recovery of all growth and photochemical measurements to pre-stress values. The roots/below-ground tissues of V. americana are more tolerant to changing salinities in comparison with H. stipulacea. On-going work has moved on to molecular profiling in both these closely related species and holds great potential in separating molecular traits associated with adaption to an aquatic lifestyle (found in both H. stipulacea and V. americana) and those specifically associated with adaptation to high levels of salinity associated with the marine environment (found only in H. stipulacea). With recent studies on the microbiome of H. stipulacea (Mejia et al., 2016; Rotini et al., 2017) it is increasingly believed that plant–microbe interactions play an important role in plant adaptation to new environments. It would be interesting to study how much of H. stipulacea’s tolerance to salinity may be attributed to its associated microbes. More studies including accumulation of osmolytes and compartmentalization of Na+ ions in both these plant species would reveal interesting details regarding plant tolerance mechanisms. One way of comprehending ecological traits such as salinity is to combine phenotypic and physiological assessments with transcriptomic and their equivalent metabolic pathways (Exadactylos, 2015). With the emergence of molecular profiling and omics techniques in seagrass biology (Procaccini et al., 2007; Davey et al., 2016; Lee et al., 2016; Olsen et al., 2016), recent studies have focused on the effects of light, increased water temperature, salinity, high CO2 levels at the transcriptomic level in seagrasses. These studies are revealing new insights into mechanisms adapted by seagrasses to survive under various abiotic stresses (Franssen et al., 2011; Kong et al., 2014; Piro et al., 2015a,b; Salo et al., 2015; Marín-Guirao et al., 2017; Ruocco et al., 2017). Comparisons of the transcriptome and the metabolome of H. stipulacea and V. americana might reveal more about salinity tolerance mechanisms present in H. stipulacea.
Author Contributions
GW, SB, and MAO conceived and designed the experiments. GW performed the sampling of plants and contributed to the preparation of the samples. MAO performed the physiological measurements. MAO, GW, and SB wrote the manuscript.
Funding
We thank the ICA in Israel for supporting the seagrass microcosm facility. We also thank the Israeli ministry for Science and Technology for their financial support of the Dead-Sea & Arava Science Center (ADSSC). Our appreciation to the Goldinger Trust Jewish Fund for the Future for financial support of the project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Vu Hoang Nguyen for his help in performing the preliminary experiments for V. americana. We would also like to thank Nguyen Thi Chi, Thao Mai Thi Hieu, Valentine Mugirasoni, Sarah Wanjiru Gachie, and Hung Manh Nguyen for their help in sample collection during the experiments. Special thanks to Maria Dolores Camalle for help with statistical analysis. We also thank Liron Summerfield for her help in the CHNS elemental analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00950/full#supplementary-material
Footnotes
- ^http://www.iui-eilat.ac.il/Research/NMPmeteodata.aspx
- ^https://www.osram.com
- ^https://www.redseafish.com/red-sea-salts/red-sea-salt/
- ^http://www.ofra-aqua.co.il
References
Abramoff, M. D., Magalhaes, P. J., and Ram, S. J. (2004). Image processing with ImageJ. Biophoton. Int. 11, 36–42.
Adams, J. B., and Bate, G. C. (1994). The tolerance to desiccation of the submerged macrophytes Ruppia cirrhosa (Petagna) Grande and Zostera capensis Setchell. J. Exp. Mar. Biol. Ecol. 183, 53–62. doi: 10.1016/0022-0981(94)90156-2
Alcoverro, T., Zimmerman, R. C., Kohrs, D. G., and Alberte, R. S. (1999). Resource allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 87, 121–131. doi: 10.3354/meps187121
Aldesuquy, H. S., and Gaber, A. M. (1993). Effect of growth regulators on Vicia faba plants irrigated by sea water Leaf area, pigment content and photosynthetic activity. Biol. Plant. 35, 519–527. doi: 10.1007/BF02928026
Amtmann, A. (2009). Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Mol. Plant 2, 3–12. doi: 10.1093/mp/ssn094
Angel, D. L., Eden, N., and Susel, L. (1995). “The influence of environmental variables on Halophila stipulacea growth,” in Improving the Knowledge Base in Modern Aquaculture, eds H. Rosenthal, B. Moav, and H. Gordin (Ghent: European Aquaculture Society Special Publication), 103–128.
Bartels, D., and Dinakar, C. (2013). Balancing salinity stress responses in halophytes and non-halophytes: a comparison between Thellungiella and Arabidopsis thaliana. Funct. Plant Biol. 40, 819–831. doi: 10.1071/FP12299
Bashitialshaaer, R. A., Persson, K. M., and Aljaradin, M. (2011). Estimated future salinity in the Arabian Gulf, the Mediterranean Sea and the Red Sea consequences of brine discharge from desalination. Int. J. Acad. Res. 3, 133–140.
Beer, S., Shomer-Ilan, A., and Waisel, Y. (1980). Carbon metabolism in seagrasses: II. Patterns of photosynthetic CO2 incorporation. J. Exp. Bot. 31, 1019–1026. doi: 10.1007/s11120-017-0452-1
Bianchi, C. N., and Morri, C. (2003). Global sea warming and “tropicalization” of the Mediterranean Sea: biogeographic and ecological aspects. Biogeographia 24, 319–327. doi: 10.21426/B6110129
Biber, P. D., Paerl, H. W., Gallegos, C. L., Kenworthy, W. J., and Fonseca, M. S. (2005). “Evaluating indicators of seagrass stress to light,” in Estuarine Indicators, ed. S. A. Bortone (Boca Raton, FL: CRC Press), 193–209.
Biebl, R., and McRoy, C. P. (1971). Plasmatic resistance and rate of respiration and photosynthesis of Zostera marina at different salinities and temperatures. Mar. Biol. 8, 48–56. doi: 10.1007/BF00349344
Björk, M., Short, F., Mcleod, E., and Beer, S. (2008). Managing Seagrasses for Resilience to Climate Change IUCN Resilience Science Group Working Paper Series – No. 3. Gland: IUCN, 56.
Borghini, M., Bryden, H., Schroeder, K., Sparnocchia, S., and Vetrano, A. (2014). The Mediterranean is becoming saltier. Ocean Sci. 10, 693–700. doi: 10.5194/os-10-693-2014
Bourn, W. S. (1932). Ecological and physiological studies on certain aquatic angiosperms. Contrib. Boyce Thompson Inst. 4, 425–496. doi: 10.1016/j.aquatox.2016.06.006
Bourn, W. S. (1934). Sea-water tolerance of Vallisneria spiralis L. and Potamogeton foliosus Raf. Contrib. Boyce Thompson Inst. 6, 303–308.
Boustany, R., Michot, T., and Moss, R. (2010). Effects of salinity and light on biomass and growth of Vallisneria americana from Lower St. Johns River, FL, USA. Wetl. Ecol. Manag. 18, 203–217. doi: 10.1007/s11273-009-9160-8
Boustany, R. G., Michot, T. C., and Moss, R. F. (2015). Effect of nutrients and salinity pulses on biomass and growth of Vallisneria americana in lower St Johns River, FL, USA. R. Soc. Open Sci. 2:140053. doi: 10.1098/rsos.140053
Campbell, S. J., Rasheed, M. A., and Thomas, R. (2003). Monitoring of Seagrass Meadows in Cairns Harbour and Trinity Inlet: December 2002. DPI Information Series QI03059. Cairns, QLD: Department of Primary Industries, 20.
Clabby, G., and Osborne, B. A. (1997). Irradiance and nitrate-dependent variation in growth and biomass allocation of Mycelis muralis. An analysis of its significance for a functional categorization of ‘sun and ‘shade’plants. New Phytol. 135, 539–545. doi: 10.1046/j.1469-8137.1997.00677.x
Collier, C. J., Villacorta-Rath, C., van Dijk, K.-J., Takahashi, M., and Waycott, M. (2014). Seagrass proliferation precedes mortality during hyposalinity events: a stress-induced morphometric response. PLoS One 9:e94014. doi: 10.1371/journal.pone.0094014
Dassanayake, M., Oh, D. H., Haas, J. S., Hernandez, A., Hong, H., Ali, S., et al. (2011). The genome of the extremophile crucifer Thellungiella parvula. Nat. Genet. 43, 913–918. doi: 10.1038/ng.889
Davey, P. A., Pernice, M., Sablok, G., Larkum, A., Lee, H. T., Golicz, A., et al. (2016). The emergence of molecular profiling and omics techniques in seagrass biology; furthering our understanding of seagrasses. Funct. Integr. Genomics 16, 465–480. doi: 10.1007/s10142-016-0501-4
Davis, G. J., and Brinson, M. M. (1976). Submerged Macrophytes of the Pamlico River Estuary, North Carolina. Raleigh, NC: University of North Carolina.
Dittami, S. M., Heesch, S., Olsen, J. L., and Collén, J. (2017). Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J. Phycol. 53, 731–745. doi: 10.1111/jpy.12547
Doering, P., Chamberlain, R., Donohue, K., and Steinman, A. (1999). Effect of salinity on the growth of Vallisneria americana Michx. from the Caloosahahatchee estuary, Florida. Florida Sci. 62, 89–105. doi: 10.1098/rsos.140053
Doering, P. H., Chamberlain, R. H., and McMunigal, J. M. (2001). Effects of simulated saltwater intrusions on the growth and survival of wild celery, Vallisneria americana, from the Caloosahatchee Estuary (South Florida). Estuar. Coasts 24, 894–903. doi: 10.2307/1353180
Dooley, F. D., Wyllie-Echeverria, S., Roth, M. B., and Ward, P. D. (2013). Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquat. Bot. 105, 7–10. doi: 10.1016/j.aquabot.2012.10.007
Duarte, C. M. (1992). Nutrient concentration of aquatic plants: patterns across species. Limnol. Oceanogr. 37, 882–889. doi: 10.1016/j.scitotenv.2018.06.135
Durako, M., and Kunzelman, J. (2002). Photosynthetic characteristics of Thalassia testudinum measured in situ by pulse-amplitude modulated (PAM) measured: methodological and scale-based considerations. Aquat. Bot. 73, 173–185. doi: 10.1016/S0304-3770(02)00020-7
Exadactylos, A. (2015). “Molecular approach of seagrasses response related to tolerance acquisition to abiotic stress,” in Molecular Approaches to Genetic Diversity, eds M. Çalişkan, G. C. Oz, I. H. Kavakli, and B. Ozcan (Rijeka: InTech). doi: 10.5772/59425
Fernández-Torquemada, Y., and Sánchez-Lizaso, J. L. (2005). Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. J. Exp. Mar. Biol. Ecol. 320, 57–63. doi: 10.1016/j.jembe.2004.12.019
Fernández-Torquemada, Y., and Sánchez-Lizaso, J. L. (2011). Responses of two Mediterranean seagrasses to experimental changes in salinity. Hydrobiologia 669:21. doi: 10.1007/s10750-011-0644-1
Flowers, T. J., and Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytol. 179, 945–963. doi: 10.1111/j.1469-8137.2008.02531.x
Forskal, P. (1775). Flora Aegyptiaco-Arabica: Sive Descriptiones Plantarum, Quas per Aegyptum Inferiorem et Arabiam Felicem Detexit. Hanniae: Ex Officina Mölleri.
Franssen, S. U., Gu, J., Bergmann, N., Winters, G., Klostermeier, U. C., Rosenstiel, P., et al. (2011). Transcriptomic resilience to global warming in the seagrass Zostera marina, a marine foundation species. Proc. Natl. Acad. Sci. U.S.A. 108, 19276–19281. doi: 10.1073/pnas.1107680108
Frazer, T. K., Notestein, S. K., Jacoby, C. A., Littles, C. J., Keller, S. R., and Swett, R. A. (2006). Effects of storm-induced salinity changes on submersed aquatic vegetation in Kings Bay, Florida. Estuar. Coasts 29, 943–953. doi: 10.1007/BF02798655
French, G. T., and Moore, K. A. (2003). Interactive effects of light and salinity stress on the growth, reproduction, and photosynthetic capabilities of Vallisneria americana (wild celery). Estuar. Coasts 26, 1255–1268. doi: 10.1007/BF02803628
Galil, B., Marchini, A., Occhipinti-Ambrogi, A., and Ojaveer, H. (2017). The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manage. Biol. Invasions 8, 141–152. doi: 10.3391/mbi.2017.8.2.02
Galil, B. S., Boero, F., Campbell, M. L., Carlton, J. T., Cook, E., Fraschetti, S., et al. (2015). ‘Double trouble’: the expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol. Invasions 17, 973–976. doi: 10.1111/j.1749-4877.2012.00307.x
Gambi, M., Barbieri, F., and Bianchi, C. (2009). New record of the alien seagrass Halophila stipulacea (Hydrocharitaceae) in the western Mediterranean: a further clue to changing Mediterranean Sea biogeography. Mar. Biodivers. Rec. 2:e84. doi: 10.1017/S175526720900058X
Garrote-Moreno, A., Sandoval-Gil, J. M., Ruiz, J. M., Marín-Guirao, L., Bernardeau-Esteller, J., Muñoz, R. J., et al. (2015). Plant water relations and ion homeostasis of Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) in response to hypersaline stress. Mar. Biol. 162, 55–68. doi: 10.1007/s00227-014-2565-9
Genty, B., Briantais, J. M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Gong, Q., Li, P., Ma, S., Indu Rupassara, S., and Bohnert, H. J. (2005). Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 44, 826–839. doi: 10.1111/j.1365-313X.2005.02587.x
Griffin, N. E., and Durako, M. J. (2012). The effect of pulsed versus gradual salinity reduction on the physiology and survival of Halophila johnsonii Eiseman. Mar. Biol. 159, 1439–1447. doi: 10.1007/s00227-012-1923-8
Gu, J., Weber, K., Klemp, E., Winters, G., Franssen, S. U., Wienpahl, I., et al. (2012). Identifying core features of adaptive metabolic mechanisms for chronic heat stress attenuation contributing to systems robustness. Integr. Biol. 4, 480–493. doi: 10.1039/c2ib00109h
Haller, W. T., Sutton, D. L., and Barlowe, W. C. (1974). Effects of salinity on growth of several aquatic macrophytes. Ecology 55, 891–894. doi: 10.2307/1934427
Hemminga, M. A., and Duarte, C. M. (2000). Seagrass Ecology. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511525551
Inan, G., Zhang, Q., Li, P., Wang, Z., Cao, Z., Zhang, H., et al. (2004). Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 135, 1718–1737. doi: 10.1104/pp.104.041723
Jordà, G., Marbà, N., and Duarte, C. M. (2012). Mediterranean seagrass vulnerable to regional climate warming. Nat. Clim. Change 2, 821–824. doi: 10.1038/nclimate1533
Kahn, A., and Durako, M. (2006). Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. J. Exp. Mar. Biol. Ecol. 335, 1–12. doi: 10.1016/j.jembe.2006.02.011
Kamermans, P., Hemminga, M. A., and de Jong, D. J. (1999). Significance of salinity and silicon levels for growth of a formerly estuarine eelgrass (Zostera marina) population (Lake Grevelingen, The Netherlands). Mar. Biol. 133, 527–539. doi: 10.1007/s002270050493
Kant, S., Kant, P., Raveh, E., and Barak, S. (2006). Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant Cell Environ. 29, 1220–1234. doi: 10.1111/j.1365-3040.2006.01502.x
Katz, T., Ginat, H., Eyal, G., Steiner, Z., Braun, Y., Shalev, S., et al. (2015). Desert flash floods form hyperpycnal flows in the coral-rich Gulf of Aqaba, Red Sea. Earth Planet. Sci. Lett. 417, 87–98. doi: 10.1016/j.epsl.2015.02.025
Kazachkova, Y., Batushansky, A., Cisneros, A., Tel-Zur, N., Fait, A., and Barak, S. (2013). Growth platform-dependent and -independent phenotypic and metabolic responses of Arabidopsis and its halophytic relative, Eutrema salsugineum, to salt stress. Plant Physiol. 162, 1583–1598. doi: 10.1104/pp.113.217844
Koch, M., Schopmeyer, S., Kyhn-Hansen, C., Madden, C., and Peters, J. (2007). Tropical seagrass species tolerance to hypersalinity stress. Aquat. Bot. 86, 14–24. doi: 10.1016/j.aquabot.2006.08.003
Kong, F., Li, H., Sun, P., Zhou, Y., and Mao, Y. (2014). De novo assembly and characterization of the transcriptome of seagrass Zostera marina using Illumina paired-end sequencing. PLoS One 9:e112245. doi: 10.1371/journal.pone.0112245
Kraemer, G. P., Chamberlain, R. H., Doering, P. H., Steinman, A. D., and Hanisak, M. D. (1999). Physiological responses of transplants of the freshwater angiosperm Vallisneria americana along a salinity gradient in the Caloosahatchee Estuary (Southwestern Florida). Estuar. Coasts 22, 138–148. doi: 10.2307/1352934
Lauer, N., Yeager, M., Kahn, A., Dobberfuhl, D., and Ross, C. (2011). The effects of short term salinity exposure on the sublethal stress response of Vallisneria americana Michx. (Hydrocharitaceae). Aquat. Bot. 95, 207–213. doi: 10.1016/j.aquabot.2011.06.002
Lee, H., Golicz, A. A., Bayer, P., Jiao, Y., Tang, H., Paterson, A. H., et al. (2016). The genome of a southern hemisphere seagrass species (Zostera muelleri). Plant Physiol. 172, 272–283. doi: 10.1104/pp.16.00868
Les, D. H., Cleland, M. A., and Waycott, M. (1997). Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily. Syst. Bot. 22, 443–463. doi: 10.2307/2419820
Les, D. H., Moody, M. L., and Soros, C. L. (2006). A reappraisal of phylogenetic relationships in the monocotyledon family Hydrocharitaceae (Alismatidae). Aliso 22, 211–230. doi: 10.5642/aliso.20062201.18
Lichtenthaler, H. K. (1987). Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 131, 101–110. doi: 10.1016/S0176-1617(87)80271-7
Lipkin, Y. (1975). Halophila stipulacea, a review of a successful immigration. Aquat. Bot. 1, 203–215. doi: 10.1016/0304-3770(75)90023-6
Lovett-Doust, J., and Laporte, G. (1991). Population sex ratios, population mixtures and fecundity in a clonal dioecious macrophyte, Vallisneria americana. J. Ecol. 79, 477–489. doi: 10.2307/2260727
Lugan, R., Niogret, M. F., Leport, L., Guégan, J. P., Larher, F. R., Savouré, A., et al. (2010). Metabolome and water homeostasis analysis of Thellungiella salsuginea suggests that dehydration tolerance is a key response to osmotic stress in this halophyte. Plant J. 64, 215–229. doi: 10.1111/j.1365-313X.2010.04323.x
Maggio, A., Raimondi, G., Martino, A., and De Pascale, S. (2007). Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 59, 276–282. doi: 10.1016/j.envexpbot.2006.02.002
Marba, N., and Duarte, C. M. (2010). Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Change Biol. 16, 2366–2375. doi: 10.1111/j.1365-2486.2009.02130.x
Marcelis, L. F. M., and Van Hooijdonk, J. (1999). Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.). Plant Soil 215, 57–64. doi: 10.1023/A:1004742713538
Marín-Guirao, L., Entrambasaguas, L., Dattolo, E., Ruiz, J. M., and Procaccini, G. (2017). Molecular mechanisms behind the physiological resistance to intense transient warming in an iconic marine plant. Front. Plant Sci. 8:1142. doi: 10.3389/fpls.2017.01142
Massa, S. I., Arnaud-Haond, S., Pearson, G. A., and Serrao, E. A. (2009). Temperature tolerance and survival of intertidal populations of the seagrass Zostera noltii (Hornemann) in Southern Europe (Ria Formosa, Portugal). Hydrobiologia 619, 195–201. doi: 10.1007/s10750-008-9609-4
Maxwell, K., and Johnson, G. N. (2000). Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668. doi: 10.1093/jexbot/51.345.659
Mejia, A. Y., Rotini, A., Lacasella, F., Bookman, R., Thaller, M. C., Shem-Tov, R., et al. (2016). Assessing the ecological status of seagrasses using morphology, biochemical descriptors and microbial community analyses. A study in Halophila stipulacea (Forsk.) Aschers meadows in the northern Red Sea. Ecol. Indic. 60, 1150–1163. doi: 10.1016/j.ecolind.2015.09.014
Misra, A. N., Sahu, S. M., Misra, M., Singh, P., Meera, I., Das, N., et al. (1997). Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 39, 257–262. doi: 10.1023/A:1000357323205
Montague, C., and Ley, J. (1993). A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in Northeastern Florida Bay. Estuaries 16, 703–717. doi: 10.2307/1352429
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Murchie, E. H., and Lawson, T. (2013). Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 64, 3983–3998. doi: 10.1093/jxb/ert208
Negrão, S., Schmöckel, S. M., and Tester, M. (2017). Evaluating physiological responses of plants to salinity stress. Ann. Bot. 119, 1–11. doi: 10.1093/aob/mcw191
Ogata, E., and Matsui, T. (1965). Photosynthesis in several marine plants of Japan as affected by salinity, drying and pH, with attention to their growth habitats. Bot. Mar. 8, 199–217. doi: 10.1515/botm.1965.8.2-4.199
Oh, D. H., Dassanayake, M., Bohnert, H. J., and Cheeseman, J. M. (2012). Life at the extreme: lessons from the genome. Genome Biol. 13:241. doi: 10.1186/gb-2012-13-3-241
Oh, D. H., Hong, H., Lee, S. Y., Yun, D. J., Bohnert, H. J., and Dassanayake, M. (2014). Genome structures and transcriptomes signify niche adaptation for the multiple-ion-tolerant extremophyte Schrenkiella parvula. Plant Physiol. 164, 2123–2138. doi: 10.1104/pp.113.233551
Olsen, J. L., Rouzé, P., Verhelst, B., Lin, Y. C., Bayer, T., Collen, J., et al. (2016). The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530, 331–335. doi: 10.1038/nature16548
Orsini, F., D’urzo, M. P., Inan, G., Serra, S., Oh, D. H., Mickelbart, M. V., et al. (2010). A comparative study of salt tolerance parameters in 11 wild relatives of Arabidopsis thaliana. J. Exp. Bot. 61, 3787–3798. doi: 10.1093/jxb/erq188
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Ozer, T., Gertman, I., Kress, N., Silverman, J., and Herut, B. (2017). Interannual thermohaline (1979–2014) and nutrient (2002–2014) dynamics in the Levantine surface and intermediate water masses, SE Mediterranean Sea. Glob. Planet. Change 151, 60–67. doi: 10.1016/j.gloplacha.2016.04.001
Pagès, J. F., Pérez, M., and Romero, J. (2010). Sensitivity of the seagrass Cymodocea nodosa to hypersaline conditions: a microcosm approach. J. Exp. Mar. Biol. Ecol. 386, 34–38. doi: 10.1016/j.jembe.2010.02.017
Pérez, M., and Romero, J. (1992). Photosynthetic response to light and temperature of the seagrass Cymodocea nodosa and the prediction of its seasonality. Aquat. Bot. 43, 51–62. doi: 10.1016/0304-3770(92)90013-9
Piro, A., Marín-Guirao, L., Serra, I. A., Spadafora, A., Sandoval-Gil, J. M., Bernardeau-Esteller, J., et al. (2015a). The modulation of leaf metabolism plays a role in salt tolerance of Cymodocea nodosa exposed to hypersaline stress in mesocosms. Front. Plant Sci. 6:464. doi: 10.3389/fpls.2015.00464
Piro, A., Serra, I. A., Spadafora, A., Cardilio, M., Bianco, L., Perrotta, G., et al. (2015b). Purification of intact chloroplasts from marine plant Posidonia oceanica suitable for organelle proteomics. Proteomics 15, 4159–4174. doi: 10.1002/pmic.201500246
Por, F. D. (1971). One hundred years of Suez Canal—A century of Lessepsian migration: retrospect and viewpoints. Syst. Biol. 20, 138–159. doi: 10.2307/2412054
Procaccini, G., Mazzella, L., Alberte, R. S., and Les, D. H. (1999). Chloroplast tRNA Leu (UAA) intron sequences provide phylogenetic resolution of seagrass relationships. Aquat. Bot. 62, 269–283. doi: 10.1016/S0304-3770(98)00099-0
Procaccini, G., Olsen, J. L., and Reusch, T. B. (2007). Contribution of genetics and genomics to seagrass biology and conservation. J. Exp. Mar. Biol. Ecol. 350, 234–259. doi: 10.1016/j.jembe.2007.05.035
Pulich, W. M. (1986). Variations in leaf soluble amino acids and ammonium content in subtropical seagrasses related to salinity stress. Plant Physiol. 80, 283–286. doi: 10.1104/pp.80.1.283
Ralph, P. J. (1998). Photosynthetic responses of Halophila ovalis (R. Br.) Hook. f. to osmotic stress. J. Exp. Mar. Biol. Ecol. 227, 203–220. doi: 10.1016/S0022-0981(97)00269-4
Ralph, P. J., Durako, M. J., Enriquez, S., Collier, C. J., and Doblin, M. A. (2007). Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 350, 176–193. doi: 10.1016/j.jembe.2007.06.017
Rotini, A., Mejia, A. Y., Costa, R., Migliore, L., and Winters, G. (2017). Ecophysiological plasticity and bacteriome shift in the seagrass Halophila stipulacea along a depth gradient in the Northern Red Sea. Front. Plant Sci. 7:2015. doi: 10.3389/fpls.2016.02015
Rudnick, D., Ortner, P., Browder, J., and Davis, S. (2005). A conceptual ecological model of Florida Bay. Wetlands 25, 870–883. doi: 10.1672/0277-5212(2005)025[0870:ACEMOF]2.0.CO;2
Ruiz, H., and Ballantine, D. L. (2004). Occurrence of the seagrass Halophila stipulacea in the tropical West Atlantic. Bull. Mar. Sci. 75, 131–135.
Ruocco, M., Musacchia, F., Olivé, I., Costa, M. M., Barrote, I., Santos, R., et al. (2017). Genome-wide transcriptional reprogramming in the seagrass Cymodocea nodosa under experimental ocean acidification. Mol. Ecol. 26, 4241–4259. doi: 10.1111/mec.14204
Salo, T., Pedersen, M. F., and Boström, C. (2014). Population specific salinity tolerance in eelgrass (Zostera marina). J. Exp. Mar. Biol. Ecol. 461, 425–429. doi: 10.1016/j.jembe.2014.09.010
Salo, T., Reusch, T. B., and Boström, C. (2015). Genotype-specific responses to light stress in eelgrass Zostera marina, a marine foundation plant. Mar. Ecol. Prog. Ser. 519, 129–140. doi: 10.3354/meps11083
Sandoval-Gil, J. M., Marín-Guirao, L., and Ruiz, J. M. (2012). The effect of salinity increase on the photosynthesis, growth and survival of the Mediterranean seagrass Cymodocea nodosa. Estuar. Coast. Shelf Sci. 115, 260–271. doi: 10.1016/j.ecss.2012.09.008
Sandoval-Gil, J. M., Ruiz, J. M., Marín-Guirao, L., Bernardeau-Esteller, J., and Sánchez-Lizaso, J. L. (2014). Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Mar. Environ. Res. 95, 39–61. doi: 10.1016/j.marenvres.2013.12.011
Schwarz, A. M., and Hellblom, F. (2002). The photosynthetic light response of Halophila stipulacea growing along a depth gradient in the Gulf of Aqaba, the Red Sea. Aquat. Bot. 74, 263–272. doi: 10.1016/S0304-3770(02)00080-3
Sghaier, Y. R., Zakhama-Sraieb, R. I. B., and Charfi-Cheikhrouha, F. (2011). Occurrence of the seagrass Halophila stipulacea (Hydrocharitaceae) in the southern Mediterranean Sea. Bot. Mar. 54, 575–582. doi: 10.1515/BOT.2011.061
Sharon, Y., Levitan, O., Spungin, D., Berman-Frank, I., and Beer, S. (2011). Photoacclimation of the seagrass Halophila stipulacea to the dim irradiance at its 48-meter depth limit. Limnol. Oceanogr. 56, 357–362. doi: 10.4319/lo.2011.56.1.0357
Sharon, Y., Silva, J., Santos, R., Runcie, J. W., Chernihovsky, M., and Beer, S. (2009). Photosynthetic responses of Halophila stipulacea to a light gradient. II. Acclimations following transplantation. Aquat. Biol. 7, 153–157. doi: 10.3354/ab00148
Skirycz, A., and Inzé, D. (2010). More from less: plant growth under limited water. Curr. Opin. Biotechnol. 21, 197–203. doi: 10.1016/j.copbio.2010.03.002
Staver, L. W. (1986). Competitive Interactions of Submersed Aquatic Vegetation Under Varying Nutrient and Salinity Conditions. Doctoral dissertation, University of Maryland, College Park, MD.
Steiner, S. C. C., and Willette, D. A. (2014). Halophila stipulacea (Hydrochoritaceae, Angiospermae) is changing the seagrass landscape in the Commonwealth of Dominica, Lesser Antilles. Caribbean Nat. 22, 1–19.
Stepien, P., and Johnson, G. N. (2009). Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 149, 1154–1165. doi: 10.1104/pp.108.132407
Streftaris, N., and Zenetos, A. (2006). Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 7, 87–118. doi: 10.12681/mms.180
Touchette, B. (2007). Seagrass–salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. J. Exp. Mar. Biol. 350, 194–215. doi: 10.1016/j.jembe.2007.05.037
Twilley, R. R., and Barko, J. W. (1990). The growth of submersed macrophytes under experimental salinity and light conditions. Estuar. Coasts 13, 311–321. doi: 10.2307/1351922
Van Katwijk, M. M., Schmitz, G. H. W., Gasseling, A. P., and Van Avesaath, P. H. (1999). Effects of salinity and nutrient load and their interaction on Zostera marina. Mar. Ecol. Prog. Ser. 190, 155–165. doi: 10.3354/meps190155
van Tussenbroek, B. I., van Katwijk, M. M., Bouma, T. J., van der Heide, T., Govers, L. L., and Leuven, R. S. E. W. (2016). Non-native seagrass Halophila stipulacea forms dense mats under eutrophic conditions in the Caribbean. J. Sea Res. 115, 1–5. doi: 10.1016/j.seares.2016.05.005
Vera, B., Collado-Vides, L., and van Tussenbroek, B. I. (2014). Halophila stipulacea (Hydrocharitaceae): a recent introduction to the continental waters of Venezuela. Caribbean J. Sci. 48, 66–70. doi: 10.18475/cjos.v48i1.a11
Volkov, V., Wang, B., Dominy, P. J., Fricke, W., and Amtmann, A. (2004). Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ. 27, 1–14. doi: 10.1046/j.0016-8025.2003.01116.x
Wang, Y., and Nii, N. (2000). Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hort. Sci. Biotechnol. 75, 623–627. doi: 10.1080/14620316.2000.11511297
Warren, C. R. (2008). Rapid measurement of chlorophylls with a microplate reader. J. Plant Nutr. 31, 1321–1332. doi: 10.1080/01904160802135092
Willette, D. A., and Ambrose, R. F. (2012). Effects of the invasive seagrass Halophila stipulacea on the native seagrass, Syringodium filiforme, and associated fish and epibiota communities in the Eastern Caribbean. Aquat. Bot. 103, 74–82. doi: 10.1016/j.aquabot.2012.06.007
Willette, D. A., Chalifour, J., Debrot, A. O. D., Engel, M. S., Miller, J., Oxenford, H. A., et al. (2014). Continued expansion of the trans-Atlantic invasive marine angiosperm Halophila stipulacea in the Eastern Caribbean. Aquat. Bot. 112, 98–102. doi: 10.1016/j.aquabot.2013.10.001
Winters, G., Edelist, D., Shem-Tov, R., Beer, S., and Rilov, G. (2017). A low cost field-survey method for mapping seagrasses and their potential threats: an example from the northern Gulf of Aqaba, Red Sea. Aquat. Conserv. 27, 324–339. doi: 10.1002/aqc.2688
Winters, G., Loya, Y., Röttgers, R., and Beer, S. (2003). Photoinhibition in shallow-water colonies of the coral Stylophora pistillata as measured in situ. Limnol. Oceanogr. 48, 1388–1393. doi: 10.4319/lo.2003.48.4.1388
Winters, G., Nelle, P., Fricke, B., Rauch, G., and Reusch, T. B. H. (2011). Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Mar. Ecol. Prog. Ser. 435, 83–95. doi: 10.3354/meps09213
Wissler, L., Codoner, F., Gu, J., Reusch, T. B. H., Olsen, J. L., Procaccini, G., et al. (2011). Back to the sea twice: identifying candidate plant genes for molecular evolution to marine life. BMC Evol. Biol. 11:8. doi: 10.1186/1471-2148-11-8
Zhu, J. (2001). Plant salt tolerance. Trends Plant Sci. 6, 66–71. doi: 10.1016/S1360-1385(00)01838-0
Zieman, J., Fourqurean, J., and Frankovich, T. (1999). Seagrass die-off in Florida Bay: long-term trends in abundance and growth of turtle grass, Thalassia testudinum. Estuaries 22, 460–470. doi: 10.2307/1353211
Zieman, J. C. (1975). Seasonal variation of turtle grass, Thalassia testudinum König, with reference to temperature and salinity effects. Aquat. Bot. 1, 107–123. doi: 10.1016/0304-3770(75)90016-9
Zimmerman, R. C., and Alberte, R. S. (1996). Effect of light/dark transition on carbon translocation in eelgrass Zostera marina seedlings. Mar. Ecol. Prog. Ser. 36, 305–309. doi: 10.3354/meps136305
Keywords: seagrass, Halophila stipulacea, salinity, Vallisneria americana, seagrass physiology, aquatic plants physiology, photochemistry, invasive seagrass
Citation: Oscar MA, Barak S and Winters G (2018) The Tropical Invasive Seagrass, Halophila stipulacea, Has a Superior Ability to Tolerate Dynamic Changes in Salinity Levels Compared to Its Freshwater Relative, Vallisneria americana. Front. Plant Sci. 9:950. doi: 10.3389/fpls.2018.00950
Received: 07 November 2017; Accepted: 12 June 2018;
Published: 04 July 2018.
Edited by:
Peter Ian Macreadie, Deakin University, AustraliaReviewed by:
Jayakumar Bose, The University of Adelaide, AustraliaMathieu Pernice, University of Technology Sydney, Australia
Copyright © 2018 Oscar, Barak and Winters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gidon Winters, d2ludGVyc2dAYWRzc2Mub3Jn
 Michelle A. Oscar
Michelle A. Oscar Simon Barak
Simon Barak Gidon Winters
Gidon Winters