- 1Laboratory of Plant Molecular Genetics and Breeding, Southwest University of Science and Technology, Mianyang, China
- 2Engineering Research Center for Biomass Resource Utilization and Modification of Sichuan Province, Mianyang, China
The Wx gene encodes a granule-bound starch synthase (GBSS) and plays a key role in determining rice eating and cooking qualities (ECQs). SSIII-2 (SSIIIa), a member of the soluble starch synthases, is responsible for the synthesis of long chains of amylopectin. To investigate the effects of Wx and its interaction with SSIII-2 on grain ECQs, a population from a hybrid combination was established as a research material. The genotypes of SSIII-2 and the single nucleotide polymorphisms (SNPs) on intron1, exon6, and exon10 of Wx, and the physicochemical indicators and rapid visco analyzer (RVA) profile characteristics were analyzed. The results revealed various effects of SSIII-2 on rice quality under different backgrounds of Wx alleles. There was no obvious difference between different SSIII-2 alleles under the same background of Wxa, whereas there was a significant diversity under the same background of Wxb. Wxa had a dominant epistasis to SSIII-2 because the effect of SSIII-2 was masked by the massive synthesis of GBSS under Wxa. The apparent amylose content (AAC) was mainly controlled by the In1G/T SNP, and rice gel consistency (GC) was regulated by the Ex10C/T SNP. The combined effects of three SNPs had a significant influence on all ECQs and RVA profile parameters, except for gelatinization temperature. In1T-Ex6A-Ex10C and In1T-Ex6A-Ex10T were classified as being low AAC type. TT-AA-CC and TT-AA-TT had a low AAC and a soft GC. The combined effects of different SNPs of Wx are very important for rice quality breeding.
Introduction
Rice yield has increased significantly over the past decade due to the introduction of semi-dwarfness and the exploitation of heterosis. However, with the constantly increasing demands from society, rice quality cannot meet the requirements of consumers. Despite many studies that have attempted to improve rice quality (Aluko et al., 2004; Zhou et al., 2010; Nelson et al., 2011), the genetic mechanism that controls quality remains ambiguous, and only a few quality-related genes have been identified (Li et al., 2011, 2014; Wang et al., 2012).
Rice quality is determined by starch, which is composed of two polysaccharides: amylose and amylopectin. High amylose content (AC) varieties have firm and separate grains once cooked, whereas low AC cultivars are tender, glossy, and cohesive (Jobling, 2004). The percentage of amylose content in total starch is measured as the apparent amylose content (AAC) using a spectrophotometer, because it contains both of amylose and long-chain amylopectin content. According to the standard Chinese rice resources evaluation, AAC can be classified as waxy (0–2%), very low (2–10%), low (10–20%), intermediate (20–25%), and high (25–33%). In addition, the most important indices of grain quality are eating and cooking qualities (ECQs) that are determined by three physical and chemical indices: AAC, rice gel consistency (GC), and gelatinization temperature (GT). A rice starch rapid visco analyzer (RVA) is widely used to evaluate rice grain quality because it provides a rapid and accurate determination of ECQs for a small amount of sample (Bao and Xia, 1999).
The Wx gene, encoding a granule-bound starch synthase (GBSS), was reported to be the main controller of AAC (Nakamura, 2002; Septiningsih et al., 2003; Li et al., 2010). Moreover, Wx also plays a key role in determining the physicochemical properties, such as GC, GT, and most RVA parameters (Tan et al., 1999; Fan et al., 2005; He et al., 2006; Wang et al., 2007). The Waxy gene is located on chromosome 6 and consists of 13 exons and 12 introns. Various single nucleotide polymorphisms (SNPs) of Wx were found, including a G to T (G-T) SNP of the first intron, A to G (A-G) SNP of the fourth exon, A to C (A-C) SNP of the sixth exon, and C to T (C-T) SNP of the tenth exon (Sano, 1984; Mikami et al., 2008). At least four alleles of Wx (Wxa, Wxb, Wxop, and Wxin) have been identified by these nucleotide polymorphisms (Wanchana et al., 2003). The wx mutant allele was caused by a 23-bp insertion in its second exon, which led to a premature expression of Wx protein. Mutations of Wx are associated with the ECQs of rice. For example, the G-T single nucleotide variation of the intron 1 has a strong influence on the normal expression of Wx. All rice varieties with a G express GBSS mRNA at normal levels. In contrast, the transcript level of GBSS in rice varieties with a T was lower (Wang et al., 1995). Ayres et al. (1997) found that this difference in the expression of GBSS was caused by a single-base change (G-T) in the putative 5′ leader intron splice site that interfered with mRNA processing. The allele Wxin appeared frequently in accessions of aromatic and tropical japonica, which exhibits an intermediate AAC, and is determined by a mutation in exon 6 at position +1,083. This mutation altered a Tyr to Ser in the active site of the enzyme and reduced its specific activity. The results of Tran et al. (2011) showed that the Ex10C/T SNP of Wx mainly affected GC. Rice with a C at exon 10 had soft, viscous gels once cooked; however, a sample with a T had short, firm gels.
The synthesis of amylopectin is more complex than that of amylose. The enzymes involved in amylopectin synthesis including soluble starch synthase (SSS), starch branching enzyme (SBE), and starch debranching enzyme (DBE) (Ball and Morell, 2003). SSIII is one of the soluble starch synthases and the SSIII-1 (SSIIIb) gene is mainly expressed in leaves, whereas SSIII-2 (SSIIIa) is preferentially expressed in endosperm and is responsible for the synthesis of long-chain amylopectin (Lloyd et al., 1999). Previous studies have found that the long-chain amylopectin content, with a polymerization degree of more than 30, was reduced by about 60% after silencing SSIII-2, and it also had some influence on starch GT (Fujita et al., 2007).
Five putative alleles at the Wx loci have been identified and indicated to be responsible for the variation of ECQs. However, the combined genetic effects of different Wx alleles on grain ECQs remain unclear. Previous studies have not identified the effects of the interaction of Wx and the SSIII-2 gene on rice ECQs. To breed rice cultivars with superior and novel grain qualities to meet the demands of consumers, it is necessary to clarify the correlation between the different Wx allele polymorphism and the grain qualities of cultivars, and analyze the interaction of Wx and SSIII-2 on rice quality. In this study, a F4 population was derived from a combination of CG133R, a restorer with a high AAC, and glutinous rice (javanica 22) as research materials. The effect of different haplotypes of Wx and their interaction with SSIII-2 on ECQs were investigated. This results of this study will be useful for the molecular breeding of rice quality.
Materials and Methods
Materials
In this study, a hybrid combination of CG133R (restorer, AAC ≈ 28%) and Javanica22 (a natural mutant from javanica rice variety Xiangdali, AAC ≈ 0.7%) was established. The alleles of the two parents differed in terms of AGPlar, AGPsma, Wx, and the SSIII-2 gene. In the third generation, 68 plants that differed in Wx and SSIII-2 loci were selected as research materials. In the fourth generation, a line of 120 plants was selected as the research material. These plants only had polymorphism on the Wx locus and came from the F3 generation. These plants were planted in an experimental agricultural field at the Southwest University of Science and Technology and were maintained regularly. At maturity, the seeds from each plant were harvested for the investigation of physical and chemical indicators and RVA profile characteristics.
Methods
DNA Extraction
About 100 mg rice leaf tissues were collected from the main stem of each individual plant at the tillering stage and were ground using a Fastprep Sample Rapid Crushing System (MP Biomedicals, Santa Ana, CA, USA) for DNA extraction as described previously (Murray and Thompson, 1980).
DNA Amplification and Restriction Digestion
PCR-AccI and WXE10, which were developed by Cai et al. (2002) and Tran et al. (2011), were used to detect the +1 base type of the first intron and the tenth exon C/T polymorphism of Wx, respectively. To genotype the SNP on exon 6 of Wx, primers of Exon6 were designed exactly as described by Dobo et al. (2010). SSIII-2 M1 was designed to detect A/G polymorphism according to Tian et al. (2010). All the primers were synthesized by Sangon Biotech (Shanghai, China).
The PCR consisted of a 15 μL reaction mixture with 2 μL of template DNA, 7.5 μL of 2 × Reaction Mix, 1.5 μL of 1 μmol L−1 primer, 0.2 μL of 2.5 U μL−1 Golden DNA Polymerase and 3.8 μL of ddH2O. The PCR reaction was performed using a BIO RAD DNA Engine Dyad Peltier Thermal Cycler (BIO RAD, Hercules, CA, USA).
The PCR-ACCI and WXE10 amplification products were digested with restriction endonuclease ACCI and ApaI, respectively, in a 15 μL reaction mixture containing 10 μL of amplification product, 1.5 μL of 10 × buffer, 0.5 μL of restriction endonuclease, and 3 μL of ddH2O. The digest reaction was performed at 37°C for 3 h.
The products of WXE10, EXon6, PCR-AccI, and SSIII-2 M1 were detected using 2.5% agarose gel in 0.5 × Tris-Borate EDTA (TBE) buffer. The gel was run at 150 V for 40 min and stained with GreenView. The size of the amplification products was estimated using a 100 bp DNA ladder.
Determination of Physical and Chemical Indexes of Starch
AAC was assayed by the colorimetric method with iodine-potassium iodide. GC was measured according to Chinese national standard GB/T 17891–1999. In the third generation, GT was assayed by the alkaline spreading value (ASV) method according to GB/T 17891–1999. In the fourth generation, GT was measured with differential scanning calorimetry (DSC) as described by Yan et al. (2010). Each sample was tested three times.
RVA Profile Characteristics Measurement
RVA profile characteristics were measured using a rapid RVA (Model No. RVA4500, NewPortSci. Co., Warriewood, Australia), according to the American Association of Cereal Chemists standard method AACC61-02. Rice starch viscosity characteristics included the following original parameters: peak viscosity (PKV), hot paste viscosity (HPV), and cool paste viscosity (CPV), and three secondary parameters: breakdown (BDV), setback (SBV), and consistency (CSV). In addition, pasting temperature (PaT) and pasting time (PeT) were also recorded. Each measurement was repeated twice.
Statistical Analysis
Data were classified according to the genotyping results. An analysis of variance (ANOVA) was performed using the Data Processing System version 9.50 (DPS, http://www.chinadps.net). Multiple comparisons of the Duncan method were performed using the Statistical Product and Service Solutions (SPSS) software version 19.
Results
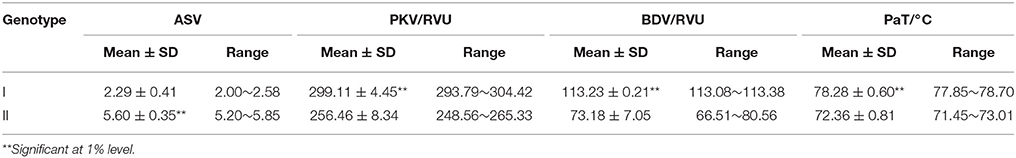
Effects of SSIII-2 Under Different Wx Background
There was no obvious difference in the tested characteristic values between different SSIII-2 alleles under the same background of Wxa (SNP of Wx loci with G) (data not shown). However, significant differences under the background of Wxb (SNP of Wx loci with T) were detected (Table 1). The results of a multiple comparison showed that the ASV, PKV, BDV, and PaT varied significantly between different polymorphisms of the SSIII-2 alleles. The ASV of SSIII-2-II type (same as CG133R) increased by 144.54%, and its PKV, BDV, and PaT decreased by 14.58, 36, and 7.23%, respectively, compared with the SSIII-2-I type (same as Javanica22). The effects of SSIII-2 alleles on rice quality varied under the different backgrounds of Wx alleles, which implied an interaction between Wx and SSIII-2.
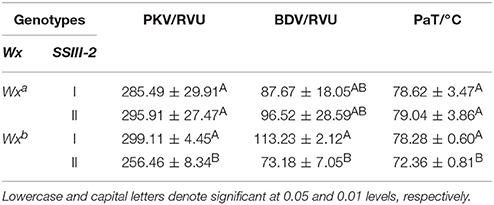
Interaction Effect of Wx and SSIII-2
The differences in AAC and ASV between the partial combinations of different Wx alleles and SSIII-2 (Table 2) were both extremely significant. The AAC of the genotype of Wxb-I and Wxb-II was significantly lower than that of Wxa-I and Wxa-II. However, there was no significant difference in the AAC between the genotypes of Wxa-I and Wxa-II. Moreover, the AAC of the Wxb-I genotype was similar to that of Wxb-II. The ASV of Wxb-I was significantly lower than that of Wxb-II, while there was no significant difference in the ASV of Wxa-I and Wxa-II.
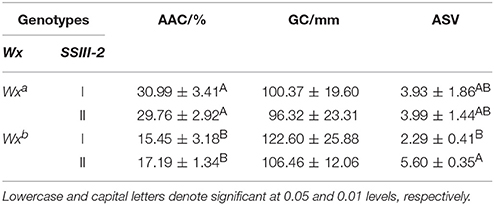
Table 2. Comparison of physicochemical indexes among different combinations of Wx and SSIII-2 alleles.
The RVA profile characteristics of rice for different combinations of the Wx and SSIII-2 alleles were analyzed by multiple comparisons (Table 3). We found significant differences in the PKV, BDV, and PaT among the combinations. The PKV and PaT of Wxb-II were significantly lower than the corresponding values of Wxb-I, Wxa-I, and Wxa-II. The BDV of Wxb-II was also significantly lower than that of Wxb-I, but there was no significant difference in the BDV of Wxa-I and Wxa-II.
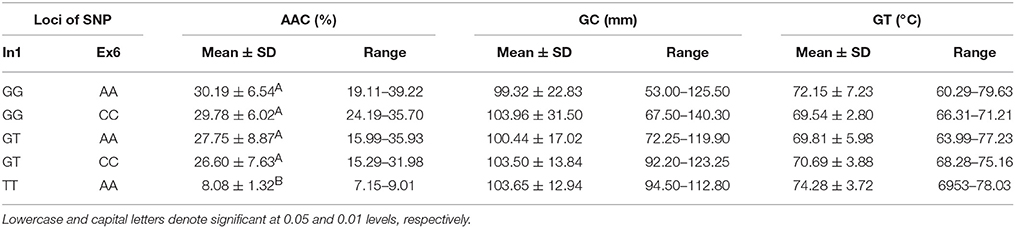
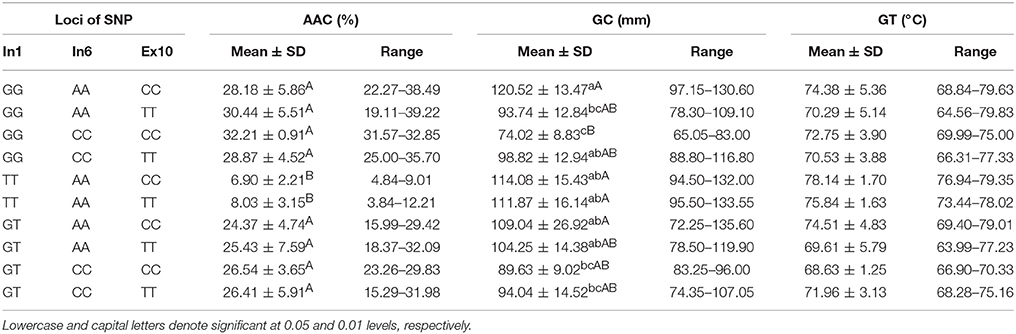
Combined Effects of Wx (In1G/T) and Wx (Ex6A/C)
As shown in Table 4, five haplotypes of Wx were identified by the different SNPs of Wx (In1G/T) and Wx (Ex6A/C): GG-AA, GG-CC, GT-AA, GT-CC, and TT-AA. There was no significant difference in their GC and GT. However, there was a significant difference in their AAC. The mean AAC was only 8.08% (range of 7.15–9.01%) in the rice line with haplotype TT-AA, whereas the mean AAC in the other four haplotypes was in the range of 26.60–30.19%.
All the RVA profile characteristics are provided in Figure 1. The HPV, CPV, SBV, SCV, and PaT of haplotype TT-AA were significantly different to those of the other haplotypes. However, the BDV of haplotype GG-CC was significantly less than that of the other haplotypes.
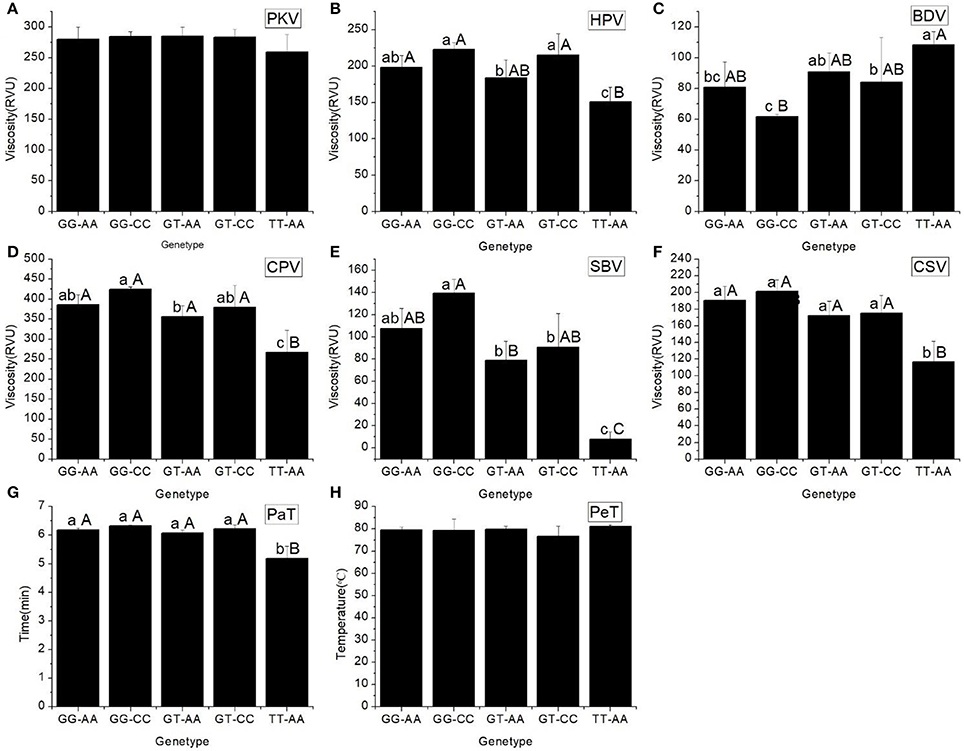
Figure 1. RVA profile characteristics for different haplotypes of In1G/T and Ex6A/C polymorphisms of Wx. (A) Peak viscosity (PKV). (B) Hot paste viscosity (HPV). (C) Cool paste viscosity (CPV). (D) Breakdown valve (BDV). (E) Setback valve (SBV). (F) Consistency valve (CSV). (G) Pasting temperature (PaT). (H) Pasting time (PeT). GG-AA, GG-CC, GT-AA, GT-CC and TT-AA represent different haplotypes of In1G/T and Ex6A/C polymorphisms of Wx. Lowercase and capital letters above column denote significant at 0.05 and 0.01 levels, respectively.
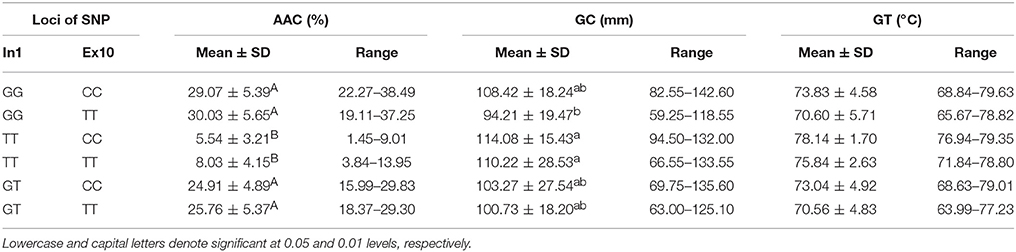
Combined Effects of Wx (In1G/T) and Wx (Ex10C/T)
The tested materials had six combinations of different SNPs of Wx (In1G/T) and Wx (Ex10C/T) (Table 5). The results of a multiple comparison showed that AAC and GC varied significantly among the different haplotypes. The haplotype TT-CC had the highest GC and the lowest AAC, and the haplotype GG-TT had the highest AAC and lowest GC.
Our results showed that the starch pasting properties of the different haplotypes of Wx (In1G/T SNP) and Wx (Ex10C/T SNP) altered significantly (Figure 2). Three basic parameters of the RVA profile (PKV, HPV, and CPV), two secondary parameters (SBV and CSV), and the PaT of haplotypes TT-CC and TT-TT dramatically decreased compared with the other haplotypes. The BDV of TT-TT was the highest among the different haplotypes. When compared with the other haplotypes, the PeT of haplotypes GG-CC and GG-TT were the highest and lowest, respectively.
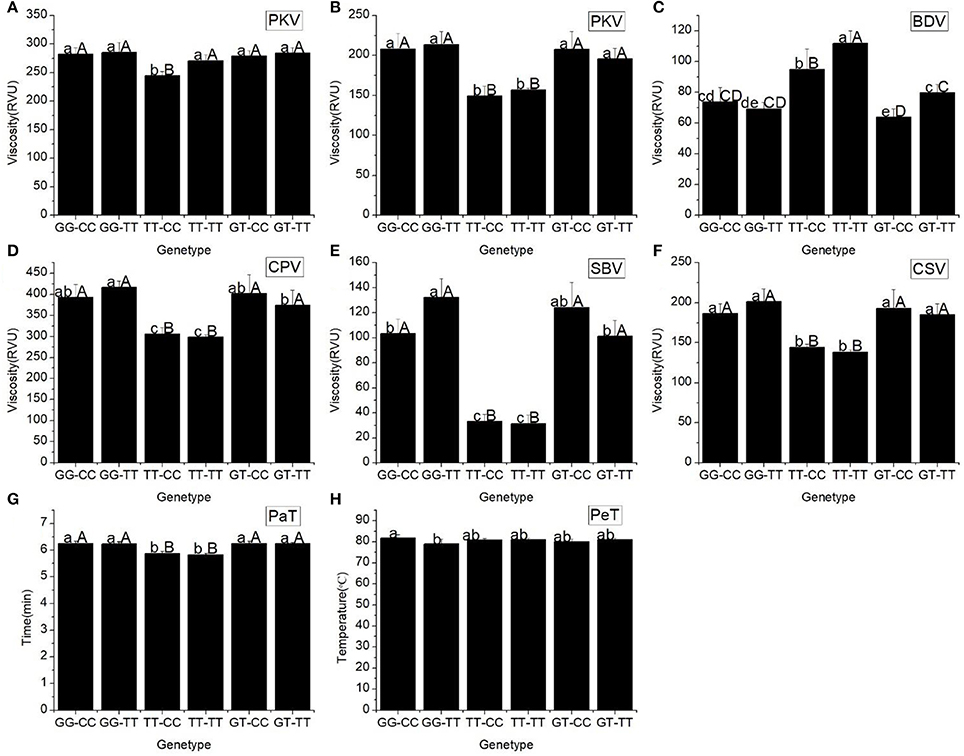
Figure 2. RVA profile characteristics for different haplotypes of In1G/T and Ex10C/T polymorphisms of Wx. (A) Peak viscosity (PKV). (B) Hot paste viscosity (HPV). (C) Cool paste viscosity (CPV). (D) Breakdown valve (BDV). (E) Setback valve (SBV). (F) Consistency valve (CSV). (G) Pasting temperature (PaT). (H) Pasting time (PeT). GG-CC, GG-TT, TT-CC, TT-CC, GT-CC and GT-TT represent different haplotypes of In1G/T and Ex10C/T polymorphisms of Wx. Lowercase and capital letters above column denote significant at 0.05 and 0.01 levels, respectively.
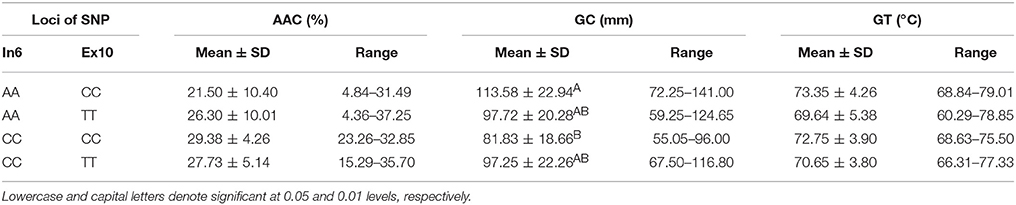
Combined Effects of Wx (Ex6A/C) and Wx (Ex10C/T)
Four haplotypes, AA-CC, AA-TT, CC-CC, and CC-TT, which originated from the combination of Wx (Ex6A/C) and Wx (Ex10C/T) (Table 6), differed from each other in terms of GC among the line we tested. Their GC was significantly different at the 0.01 level. The haplotype AA-CC had the longest gel length (113.58 ± 22.94 mm), whereas the haplotype CC-CC had the shortest gel length (81.83 ± 18.66 mm).
All RVA profile characteristics, except HPV, were significantly different (Figure 3) among the different Haplotypes. The HPV, CPV, SBV, CSV, PaT, and PeT of haplotypes CC-CC and CC-TT were significantly higher than the corresponding values for the other haplotypes. However, there was no difference in these RVA parameters between the haplotypes CC-CC and CC-TT, as well as between the haplotypes AA-CC and AA-TT.
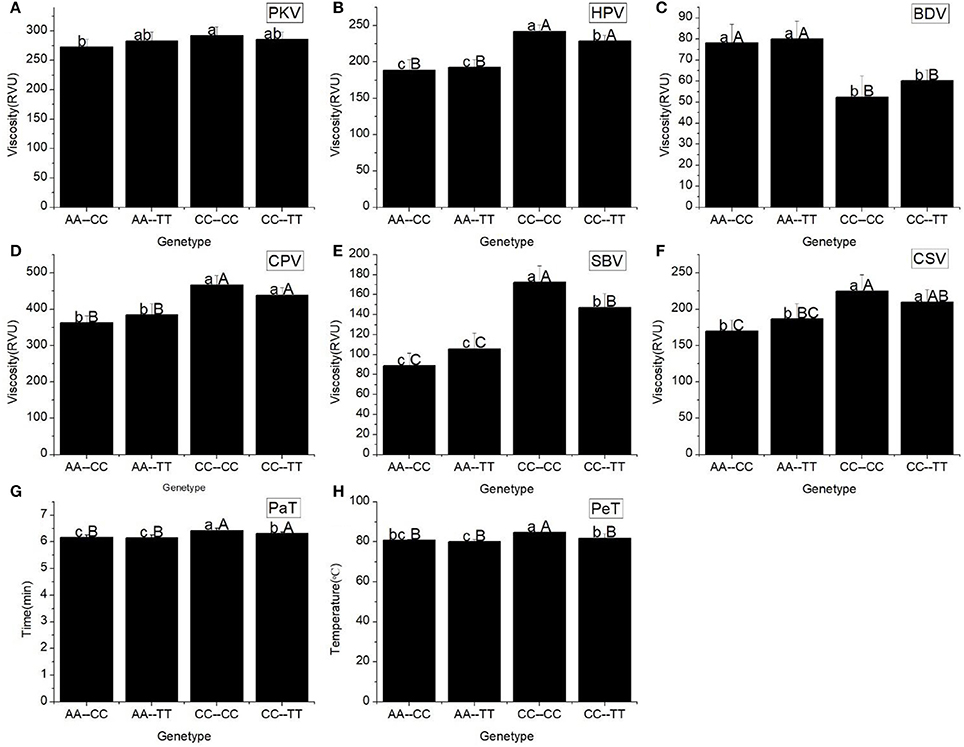
Figure 3. RVA profile characteristics for different haplotypes of Ex6A/C and Ex10C/T polymorphisms of Wx. (A) Peak viscosity (PKV). (B) Hot paste viscosity (HPV). (C) Cool paste viscosity (CPV). (D) Breakdown valve (BDV). (E) Setback valve (SBV). (F) Consistency valve (CSV). (G) Pasting temperature (PaT). (H) Pasting time (PeT). AA-CC, AA-TT, CC-CC and CC-TT represent different haplotypes of Ex6A/C and Ex10C/T polymorphisms of Wx. Lowercase and capital letters above column denote significant at 0.05 and 0.01 levels, respectively.
Combined Effects of Wx (In1G/T), Wx (Ex6A/C) and Wx (Ex10C/T)
According to the results of a multiple comparison (Table 7), ten haplotypes of Wx were identified by their different SNPs. The results indicated that a significant difference in AAC and GC existed among the different haplotypes. The TT-AA-CC and TT-AA-TT haplotypes had a low AAC (6.09 and 8.03%, respectively) and a soft GC (114.08 and 111.87 mm, respectively). The GC of haplotypes GG-AA-CC and GG-CC-CC were the hardest and softest, respectively.
RVA profile characteristics varied significantly among the different haplotypes (Figure 4). It should be noted that the maximum values of all RVA profile characteristics appeared in haplotype GG-CC-CC, except for BDV. The minimum values of two original parameters (PKV and HPV) and the secondary parameters (CPV and SBV) were found in haplotype TT-AA-CC. These results indicated that different combinations of the SNPs of Wx had a remarkable influence on the profile of RVA parameters.
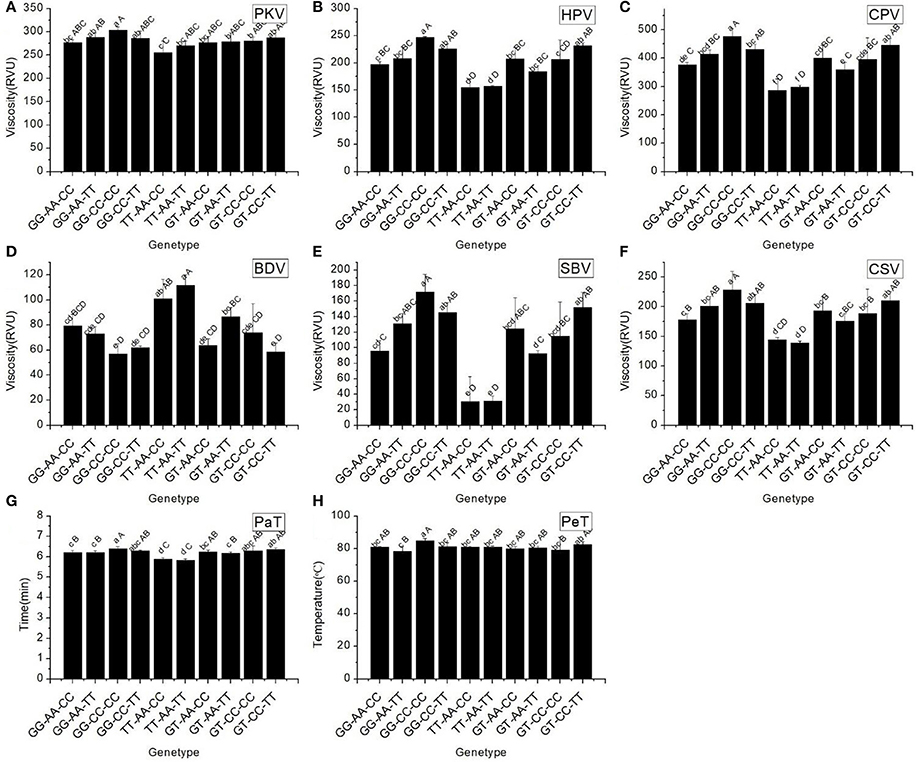
Figure 4. RVA profile characteristics for different haplotypes of In1G/T, Ex6A/C and Ex10C/T polymorphisms of Wx. (A) Peak viscosity (PKV). (B) Hot paste viscosity (HPV). (C) Cool paste viscosity (CPV). (D) Breakdown valve (BDV). (E) Setback valve (SBV). (F) Consistency valve (CSV). (G) Pasting temperature (PaT). (H) Pasting time (PeT). GG-AA-CC, GG-AA-TT, GG-CC-CC, GG-CC-TT, TT-AA-CC, TT-AA-TT, GT-AA-TT, GT-AA-TT, GT-CC-CC and GT-CC-TT represent different haplotypes of In1G/T, Ex6A/C and Ex10C/T polymorphisms of Wx. Lowercase and capital letters above column denote significant at 0.05 and 0.01 levels, respectively.
Discussion
As one of the most important objectives in rice breeding, ECQs has received increasing attention in recent years. However, due to the complexity in the quality inheritance, as well as the lack of effective measurements for breeding high quality rice, many high-yielding varieties are of poor quality. An understanding of the molecular mechanism involved in the formation of rice quality is essential for rice quality improvement. Generally, the amylopectin chain length and amylose molecular size have a synergistic effect on the quality of starch. Thus, a clarification of the effects of starch synthesis-related genes on grain quality is very important for quality breeding.
The results of this investigation indicated that SSIII-2 significantly affected the GT, PKV, BDV, and PaT of rice under the background of Wxb. The SSIII-2 gene contains 15 introns and 16 exons, and its third exon has repeated sequence polymorphism (Dian et al., 2005). Li et al. (2000) found that SSIII-2 was responsible for the synthesis of long-chain amylopectin in endosperm. Liu (2012) also found that SSIII-2 had a significant effect on rice GT by studying its near isogenic lines (NILs).
The biosynthesis of rice starch requires the association of multiple genes. For example, SSIII-2 not only participates in the synthesis of long-chain amylopectin, but also regulates the activity and expression level of SSS isozyme. Silencing SSIII-2 has been shown to result in an increase in SSI activity in rice (Fujita et al., 2007). Wu et al. (2006) found that SSI, SSII-3, SSIII-1, and SBE had no significant effect on rice quality under the background of Wxa, while there were obvious effects when Wxa was replaced by the recessive gene wx. Yan et al. (2006) found that the genetic effects of SBEI and SBEIII under different backgrounds (Wxa and Wxb) varied. The genetic effects of SBEI and SBEIII might be masked by Wx. Although SSIII-2 had no significant effect on rice quality under the background of Wxa, it had very significant effects on GT, PKV, BDV, and PaT under the background of Wxb. The expression level of Wxb genotype was significantly lower than that of Wxa genotype due to the first intron mutation of Wxa. This implies that the effect of SSIII-2 may be masked by the massive synthesis of GBSS under the Wxa genotype, which is a dominant controlling factor of rice quality and has a dominant epistasis effect on SSIII-2.
It is generally accepted that Wx is the major gene that controls rice AAC, and its three SNPs and one CT microsatellite repeat have been found in non-glutinous rice. Ayres et al. (1997) found that an SNP of In1G/T explained almost 80% of the variation in amylose content of their test materials. The genotype In1TT produced a low amylose content, while the AAC of genotype In1GG was significantly higher than that of genotype In1TT. Larkin and Park (2003) conducted a sequence analysis and found an A/C polymorphism in exon 6 and a C/T polymorphism in exon 10, which resulted in a non-conservative residue substitution. Plants with an A-C mutation in exon 6 led to an alteration of amino acid from Tyr to Ser and an intermediate AAC. The SNP Ex10TT was present only in rice with a high AAC. Tran et al. (2011) found that Ex10C/T could explain a significant component of gel consistency. In our study, a combination of In1G/T and Ex6A/C had a significant impact on AAC and no effect on GC. The combination of In1G/T and Ex10C/T had a significant effect on both AAC and GC. However, the combination of Ex6A/C and Ex10C/T had no effect on AAC but did have an effect on GC. This suggested that AAC was mainly controlled by In1G/T and rice GC was regulated by Ex10C/T.
The combined effect of different SNPs plays an important role in determining rice grain qualities. Our study showed that In1T-Ex6A-Ex10C (mean AAC = 6.90%) and In1T-Ex6A-Ex10T (mean AAC = 8.03%) were classified as the low AAC type, while the other haplotypes belonged to the high AAC type (>23%). The combined effects of three SNPs had a significant influence on all ECQs and RVA profile parameters except for GT. Dobo et al. (2010) identified three SNPs of Wx and measured its AAC in the US and European rice germplasm. Their results showed that a combination of these three SNPs accounted for 89.2% of the variation in the AAC of 85 US germplasm and 93.8% of the variation among 279 European rice varieties. The haplotypes In1T-Ex6A-Ex10C and In1T-Ex6C-Ex10C were found in low AAC varieties, and all varieties with intermediate levels of AAC had the In1G-Ex6C-Ex10C allele. High levels of AAC varieties had either the In1G-Ex6A-Ex10T or In1G-Ex6A-Ex10C allele. It has been reported that AAC and GC are mainly controlled by Wx, which is a minor gene affecting GT. SSIIa (SSII-3, ALK) was a dominant factor for controlling the GT (Gao et al., 2003; Umemoto and Aoki, 2005). In the present study, all combinations had no effect on GT. Thus, the same conclusion was reached, i.e., the different combination of the three SNPs of Wx had no significant influence on GT.
A previous study indicated that RVA profile parameters were closely related to AAC and GC (Umemoto et al., 2002) as well as Wx genes. Zhang et al. (2012) found a significant effect on starch paste viscosity using four NILs with different Wx alleles as research materials. Our results clearly demonstrated that the RVA profile parameters of the different combinations of In1G/T and Ex6A/C were significantly different, except for PKV and PeT. This result shows that combined effects of different SNPs of Wx are important for rice quality breeding. There is a need to further explore the interaction of different genes that regulate ECQs to enhance the efficiency of quality breeding.
Author Contributions
BY and SX performed the genetic studies. LX and HY carried out the measure of physicochemical indicators and RVA profile characteristics. XX designed the overall project. BY analyzed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Construction Project of Professional Innovation Team for Key Scientific Research Platform of Southwest University of Science and Technology (Grant No.14tdgc07); The Key Program of Education Bureau of Sichuan Province, China (Grant No.17ZA0412).
References
Aluko, G., Martinez, C., Tohme, J., Castano, C., Bergman, C., and Oard, J. H. (2004). QTL mapping of grain quality traits from the interspecifc cross Oryza sativa × O. glaberrima. Theor. Appl. Genet. 109, 630–639. doi: 10.1007/s00122-004-1668-y
Ayres, N. M., McClung, A. M., Larkin, P. D., Bligh, H. F. J., Jones, C. A., and Park, W. D. (1997). Microsatellites and a single-nucleotide polymorphism differentiate apparent amylose classes in an extended pedigree of US rice germplasm. Theor. Appl. Genet. 94, 773–781. doi: 10.1007/s001220050477
Ball, S. G., and Morell, M. K. (2003). From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 54, 207–233. doi: 10.1146/annurev.arplant.54.031902.134927
Bao, J. S., and Xia, Y., W. (1999). Genetic control of paste viscosity characteristics in indica rice (Oryza sativa L.). Theor. Appl. Genet. 98, 1120–1124.
Cai, X. L., Liu, Q. Q., Tang, S. Z., Gu, M. H., and Wang, Z. Y. (2002). Development of a molecular marker for screening the rice cultivars with intermediate amylose content in Oryza sativa subsp. indica. J. Plant Physiol. Mol. Biol. 28, 137–144. doi: 10.3321/j.issn:1671-3877.2002.02.011
Dian, W., Jiang, H., and Wu, P. (2005). Evolution and expression analysis of starch synthase III and IV in rice. J. Exp. Bot. 56, 623–632. doi: 10.1093/jxb/eri065
Dobo, M., Ayres, N., Walker, G., and Park, W. D. (2010). Polymorphism in the GBSS gene affects amylose content in US and European rice germplasm. J. Cereal Sci. 52, 450–456. doi: 10.1016/j.jcs.2010.07.010
Fan, C. C., Yu, X. Q., Xing, Y. Z., Xu, C. G., Luo, L. J., and Zhang, Q. F. (2005). The main effects, epistatic effects and environmental interactions of QTLs on the cooking and eating quality of rice in a doubled-haploid line population. Theor. Appl. Genet. 110, 1445–1452. doi: 10.1007/s00122-005-1975-y
Fujita, N., Yoshida, M., Kondo, T., Saito, K., Utsumi, Y., Tokunaga, T., et al. (2007). Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 144, 2009–2023. doi: 10.1104/pp.107.102533
Gao, Z. Y., Zeng, D. L., Cui, X., Zhou, Y. H., Yan, M., Huang, D., et al. (2003). Map-based cloning of the ALK gene, which controls the GT of rice. Sci. China C Life Sci. 46, 661–668. doi: 10.1360/03yc0099
He, Y., Han, Y. P., Jiang, L., Xu, C. W., Lu, J. F., and Xu, M. L. (2006). Functional analysis of starch-synthesis genes in determining rice eating and cooking qualities. Mol. Breed. 18, 277–290. doi: 10.1007/s11032-006-5505-7
Jobling, S. (2004). Improving starch for food and industrial applications. Curr. Opin. Plant Biol. 7, 210–218. doi: 10.1016/j.pbi.2003.12.001
Larkin, P. D., and Park, W. D. (2003). Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.). Mol. Breed. 12, 335–339. doi: 10.1023/B:MOLB.0000006797.51786.92
Li, Q. F., Sun, S. S. M., Yuan, D. Y., Yu, H. X., Gu, M. H., and Liu, Q. Q. (2010). Validation of can did ate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol. Biol. Rep. 28, 49–57. doi: 10.1007/s11105-009-0124-1
Li, Y., Fan, C., Xing, Y., Jiang, Y., Luo, L., Sun, L., et al. (2011). Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269. doi: 10.1038/ng.977
Li, Y., Fan, C., Xing, Y., Yun, P., Luo, L., Peng, B., et al. (2014). Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 46, 398–404. doi: 10.1038/ng.2923
Li, Z. Y., Mouille, G., Kosar-Hashemi, B., Rahman, S., Clarke, B., Gale, K. R., et al. (2000). The structure and expression of the wheat starch synthase III gene. Motifs in the expressed gene define the lineage of the starch synthase III gene family. Plant Physiol. 123, 613–624. doi: 10.1104/pp.123.2.613
Liu, F., C. (2012). Investigation of the Effects of Starch Synthesis Related Genes on Rice (Oryza sativa L.) Qualities using near Isogenic Lines. Master's thesis, Yangzhou University.
Lloyd, J. R., Landschutze, V., and Kossmann, J. (1999). Simultaneous antisense inhibition of two starch synthase isoforms in potato tuber leads to accumulation of grossly modified amylopectin. Biochem. J. 338, 515–521. doi: 10.1042/bj3380515
Mikami, I., Uwatoko, N., Ikeda, Y., Yamaguchi, J., Hirano, H. Y., Suzuki, Y., et al. (2008). Allelic diversification at the wx locus in landraces of Asian rice. Theor. Appl. Genet. 116, 979–989. doi: 10.1007/s00122-008-0729-z
Murray, M. G., and Thompson, W. F. (1980). Rapid isolation of high molecular weight plant DNA. Nucl Acids Res. 8, 4321–4325. doi: 10.1093/nar/8.19.4321
Nakamura, Y. (2002). Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol. 43, 718–725. doi: 10.1093/pcp/pcf091
Nelson, J. C., McClung, A. M., Fjellstrom, R. G., Moldenhauer, K. A., Boza, E., Jodari, F., et al. (2011). Mapping QTL main and interaction influences on milling quality in elite US rice germplasm. Theor. Appl. Genet. 122, 291–309. doi: 10.1007/s00122-010-1445-z
Sano, Y. (1984). Differential regulation of waxy gene expression in rice endosperm. Theor. Appl. Genet. 64, 467–473. doi: 10.1007/BF00254822
Septiningsih, E. M., Prasetiyono, J., Lubis, E., Tai, T. H., Tjubaryat, T., Moeljopawiro, S., et al. (2003). Identification of quantitative trait loci for yield and yield components in an advanced backcross population derived from the Oryza sativa variety IR64 and the wild relative O. rufipogon. Theor. Appl. Genet. 107, 143–1441. doi: 10.1007/s00122-003-1373-2
Tan, Y. F., Li, J. X., Yu, S. B., Xing, Y. Z., Xu, C. G., and Zhang, Q. F. (1999). The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 99, 642–648. doi: 10.1007/s001220051279
Tian, Z. X., Yan, C. J., Qian, Q., Yan, S., Xie, H. L., Wang, F., et al. (2010). Development of gene-tagged molecular markers for starch synthesis-related genes in rice. Sci. Bull. 55, 3768–3777. doi: 10.1007/s11434-010-4124-1
Tran, N. A., Daygon, V. D., Resurreccion, A. P., Cuevas, R. P., Corpuz, H. M., and Fizgerald, M. A. (2011). A single nucleotide polymorphism in the Waxy gene explains a significant component of gel consistency. Theor. Appl. Genet. 123, 519–525. doi: 10.1007/s00122-011-1604-x
Umemoto, T., and Aoki, N. (2005). Single-nucleotide polymorphisms in rice starch synthase IIa that alter starch gelatinization and starch association of the enzyme. Funct. Plant Biol. 32, 763–768. doi: 10.1071/FP04214
Umemoto, T., Yano, M., Satoh, H., Shomura, A., and Nakamura, Y. (2002). Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 104, 1–8. doi: 10.1007/s001220200000
Wanchana, S., Toojinda, T., Tragoonrung, S., and Vanavichit, A. (2003). Duplicated coding sequence in the waxy allele of tropical glutinous rice (Oryzasativa, L.). Plant Sci. 165, 1193–1199. doi: 10.1016/S0168-9452(03)00326-1
Wang, L. Q., Liu, W. J., Xu, Y., He, Y. Q., Luo, L. J., Xing, Y. Z., et al. (2007). Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theor. Appl. Genet. 115, 463–476. doi: 10.1007/s00122-007-0580-7
Wang, S. K., Wu, K., Yuan, Q. B., Liu, X., Liu, Z., Lin, X., et al. (2012). Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954. doi: 10.1038/ng.2327
Wang, Z. Y., Zheng, F. Q., Shen, G. Z., Gao, J. P., Snustad, D. P., Li, M. G., et al. (1995). The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 7, 613–622. doi: 10.1046/j.1365-313X.1995.7040613.x
Wu, H. K., Liang, G. H., Gu, Y. J., Shan, L. L., Wang, F., Han, Y. P., et al. (2006). The effect of the starch-synthesizing genes on RVA profile characteristics in rice (O. sativa). Acta Agron Sin. 11, 1597–1603. doi: 10.3321/j.issn:0496-3490.2006.11.001
Yan, C. J., Fang, Y. W., Li, M., Peng, J. C., Liu, Q. Q., Tang, S. Z., et al. (2010). Genetic effect of PUL allelic variation on rice cooking and eating qualities. Acta Agron. Sin. 36, 728–735. doi: 10.1016/S1875-2780(09)60047-X
Yan, C. J., Tian, S., Zhang, Z. Q., Han, Y. P., Chen, F., Li, X., et al. (2006). The source of genes related to rice grain starch synthesis among cultivated varieties and its contribution to quality. Acta Agron. Sin. 6, 129–136. doi: 10.3321/j.issn:0578-1752.2006.05.001
Zhang, Z. J., Li, M., Fang, Y. W., Liu, F. C., Lu, Y., Meng, Q. C., et al. (2012). Diversification of the waxy gene is closely related to variations in rice eating and cooking quality. Plant Mol. Biol. Rep. 30, 462–469. doi: 10.1007/s11105-011-0362-x
Keywords: rice (Oryza stativa L.), Wx, SSIII-2, single nucleotide polymorphism, eating and cooking qualities, epistasis
Citation: Yang B, Xu S, Xu L, You H and Xiang X (2018) Effects of Wx and Its Interaction With SSIII-2 on Rice Eating and Cooking Qualities. Front. Plant Sci. 9:456. doi: 10.3389/fpls.2018.00456
Received: 27 November 2017; Accepted: 22 March 2018;
Published: 10 April 2018.
Edited by:
Junhua Peng, Center for Life Sci & Tech of China National Seed Group Co. Ltd., ChinaReviewed by:
Guangxiao Yang, Huazhong University of Science and Technology, ChinaLi Huang, Montana State University, United States
Copyright © 2018 Yang, Xu, Xu, You and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xunchao Xiang, eGlhbmd4dW5jaGFvQHN3dXN0LmVkdS5jbg==
 Bowen Yang
Bowen Yang Shunju Xu
Shunju Xu Liang Xu
Liang Xu Hui You
Hui You Xunchao Xiang
Xunchao Xiang