- 1Center for Agricultural Water Research in China, China Agricultural University, Beijing, China
- 2Department of Plant and Environmental Sciences, Faculty of Science, University of Copenhagen, Taastrup, Denmark
- 3Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
Stomatal conductance (gs) and water use efficiency (WUE) of tomato leaves exposed to different irrigation regimes and at ambient CO2 (a[CO2], 400 ppm) and elevated CO2 (e[CO2], 800 ppm) environments were simulated using the “Ball-Berry” model (BB-model). Data obtained from a preliminary experiment (Exp. I) was used for model parameterization, where measurements of leaf gas exchange of potted tomatoes were done during progressive soil drying for 5 days. The measured photosynthetic rate (Pn) was used as an input for the model. Considering the effect of soil water deficits on gs, an equation modifying the slope (m) based on the mean soil water potential (Ψs) in the whole root zone was introduced. Compared to the original BB-model, the modified model showed greater predictability for both gs and WUE of tomato leaves at each [CO2] growth environment. The models were further validated with data obtained from an independent experiment (Exp. II) where plants were subjected to three irrigation regimes: full irrigation (FI), deficit irrigation (DI), and alternative partial root-zone irrigation (PRI) for 40 days at both a[CO2] and e[CO2] environment. The simulation results indicated that gs was independently acclimated to e[CO2] from Pn. The modified BB-model performed better in estimating gs and WUE, especially for PRI strategy at both [CO2] environments. A greater WUE could be seen in plants grown under e[CO2] associated with PRI regime. Conclusively, the modified BB-model was capable of predicting gs and WUE of tomato leaves in various irrigation regimes at both a[CO2] and e[CO2] environments. This study could provide valuable information for better predicting plant WUE adapted to the future water-limited and CO2 enriched environment.
Introduction
Plant stomata aperture play a predominant role in modulating the diffusion of CO2 and H2O vapor between leaf and atmosphere (Buckley and Mott, 2013), and optimizing photosynthetic and transpiration rates, hereby the water use efficiency (WUE) at leaf scale (Liu et al., 2009). It is well-established that both reduced irrigation regimes, especially alternate partial root-zone irrigation (PRI) and elevated atmospheric CO2 environment (e[CO2]) could induce partial stomatal closure and synergistically enhance WUE (Pazzagli et al., 2016). Therefore, a better understanding of how to model stomatal conductance for water vapor (gs) is essential for the accurate prediction of leaf transpiration and improvement of plant WUE in response to the future water limited and CO2 enriched environments.
A number of approaches has been tested for modeling gs under well-watered conditions (Gutschick and Simonneau, 2002). Among those, the Ball-Berry model (BB-model) describing the linear coupling relation of gs to photosynthetic rate (Pn), relative humidity (hs), and CO2 concentration (Cs) on the leaf surface (Ball et al., 1987) has been broadly adopted and utilized from leaf to plant scale due to its apparent accuracy and simplicity (Miner et al., 2017). However, the Pn-gs relationship of BB-model would be changed under water stress (Damour et al., 2010), hence modified model is needed for simulating gs, for instance, by incorporating empirical functions coupling with abscisic acid (ABA), leaf water potential or soil water potential (Sala and Tenhunen, 1996; Gutschick and Simonneau, 2002; Bauerle et al., 2004; Damour et al., 2010).
For drought-prone areas, more efficient irrigation techniques need to be developed and implemented in order to achieve optimal crop yield and quality (Du et al., 2015). PRI strategy has been demonstrated to save considerable amount of irrigation water without significantly reducing yield as compared to full irrigation (FI) (Kang and Zhang, 2004; Wei et al., 2016). It is well-known that reduced plant water consumption under PRI is resulted from a decreased gs, which is primarily regulated by the root-to-shoot ABA signaling triggered in the roots exposed to drying soil (Davies et al., 2002; Liu et al., 2006). A modified BB-model based on the temporal and spatial change of soil water potential (Ψs) in the soil columns has been reported to be capable of predicting gs and WUE of PRI treated potato leaves, indicating an enhancement of WUE for PRI in relation to FI plants (Liu et al., 2009).
Plants grown at e[CO2] generally possess an increased Pn but decreased gs, resulting in a greater WUE as compared to those growth at a[CO2] (Pazzagli et al., 2016). An better understanding of the coordination between gs and Pn in response to e[CO2] is crucial for simulating the WUE at leaf level. The magnitude of e[CO2] effect on gs is modulated substantially together with other environmental variables, if gs is independently acclimated to e[CO2] from Pn, this would alter the sensitivity of gs to [CO2], Pn, and/or hs, and thereby requiring re-parameterization of the BB-model for plants grown under different CO2 environment (Ainsworth and Rogers, 2007). As lower gs and higher WUE are anticipated under both e[CO2] and reduced irrigation strategies, and a synergic interaction of those two factors would further decrease gs and enhance WUE (da Silva et al., 2017). However, this will complicate the influence of e[CO2] associated with PRI regime on the prediction of gs and WUE using the BB-model.
To date, the change of gs and WUE of tomato leaves in response to PRI strategy at e[CO2] have not been depicted by any model. Therefore, the objective of this study was to examine whether gs is independently acclimated to e[CO2] and if the BB-model is capable of predicting leaf gs and WUE of tomato leaves exposed to different irrigation regimes in combination with two CO2 growth conditions. Such modeled gs might provide an effective way for estimating transpiration rate at canopy scale and optimizing WUE of tomato plant in the future drier and CO2 enriched environment.
Materials and Methods
The BB-Model and Its Modification
The BB-model (Ball et al., 1987) describes the relationship between leaf stomatal conductance (gs) and photosynthetic rate (Pn), relative humidity (hs) and CO2 concentration (Cs) on the leaf surface:
where g0 is the residual stomatal conductance if Pn is zero, m is the slope of relation between gs and Pnhs/Cs (the Ball-index), also called the stomatal sensitivity factor. Under well-watered condition, the m is a constant and this model is a simple linear correlation between gs and Ball-index. However, under soil water deficits, m could be varied largely, and the relationship between gs and Pnhs/Cs becomes curvilinear (Sala and Tenhunen, 1996). Accounting for the effect of soil water deficits on leaf gs, numerous approaches have been used to adjust the m. The modified m could be based on an exponential function related to the ABA concentration in the xylem sap (Gutschick and Simonneau, 2002). Our earlier studies found that plant ABA could be empirically expressed as a linear function of the mean soil water potential (Ψs) in the root zone (Liu et al., 2008). Hereby, in this study, the xylem sap ABA was replaced by Ψs:
where mi is the initial slope of the BB-model without soil water deficits, β is a constant.
In Equation (1), Cs was calculated as:
where Ca is the atmospheric CO2 concentration (i.e., 400 or 800 ppm in this study); gb is the boundary layer conductance and shown to be 9.29 mol m−2 s−1 in the leaf chamber according to the manufacture's directions. The hs is computed as the ratio of two partial pressures of water vapor at the leaf surface and in the leaf internal space of stomata, es/ei. The es was obtained by Equation (4) as shown by Gutschick and Simonneau (2002):
By rearranging Equation (4), hs was calculated as:
where ea is the partial pressure of water vapor in the air and is obtained during gas exchange measurement. ei could be computed by Equation (5) from the leaf temperature (T, °C).
It is necessary to notice that gs is used as an input variable for computing hs (Equation 5). The gs could be obtained by rearranging the BB-model as:
Equation (7) could then be elaborated as a quadratic equation of gs:
gs was solved as:
where A = (Cs/gb), B = (Cs-g0Cs/gb-mPn/gb) and C = −(g0Cs+mPnea/ei). By applying PROC NLIN (SAS 9.4 Ins. Inc.) of gs on the remaining variables, the parameters g0 and m were derived. Here, m could be replaced by Equation (2) to consider the effect of soil water deficits on gs.
After estimating gs by Equation (9), leaf transpiration rate (Tr) could be calculated as:
where Pa is the air pressure (1013 hPa). WUE of tomato leaves was then computed as:
The aim of this study was to examine the capability of the BB-model in predicting gs and WUE for tomato leaves at e[CO2] in combination with different irrigation regimes as this has not been done up to date; therefore, we have taken the observed Pn as an input for the BB-model rather than developing a coupled model for Pn. Moreover, we compared the performance of the original BB-model (without m modification) and the modified BB-model (with m modification by Equation 2) in simulating gs and WUE in order to evaluate the importance of soil water deficits on the model performance under different irrigation regimes and CO2 enriched environment.
Data
The data for this study are from two pot experiments conducted in a climate-controlled greenhouse at the experimental farm of the Faculty of Science, University of Copenhagen, Taastrup, Denmark. The experimental setups have been detailed elsewhere (Yan et al., 2017; Wei et al., 2018) and are only summarized here. In both experiments, the tomato seeds (Solanum lycopersicum L., cv. Elin) were sown on 26th Sept. 2016. Half of the plants were grown in a greenhouse cell with ambient CO2 concentration of 400 ppm (a[CO2]), and another half were grown in a cell with elevated CO2 concentration of 800 ppm (e[CO2]). In the first experiment (Exp. I), plants were grown in 1.5 L pots filled with peat substance. Since 31st Oct., plants were subjected to progressive soil drying by withholding irrigation from the pots for 5 days when the gs decreased to ca. 10% of that on 31st Oct. (i.e., when pot weight ca. 320 g). Gas exchange measurements (Pn and gs) were made at midday around 10:00 h with a portable photosynthetic system (LiCor-6400XT, LI-Cor, NE, USA). Measurements were performed on one leaf per plant at 20°C chamber temperature and 1200 μmol m−2 s−1 photon flux density, and at a [CO2] of 400 ppm for a[CO2] and 800 ppm for e[CO2] treatment, respectively. The mean volumetric soil water content in the pots was monitored by weighing the pots daily. The mean Ψs was then obtained based on the water retention curve for the peat substance (Figure 1). Other environmental variables such as ea and T were also obtained during gas exchange measurements. The data obtained from this experiment was used for parameterization.
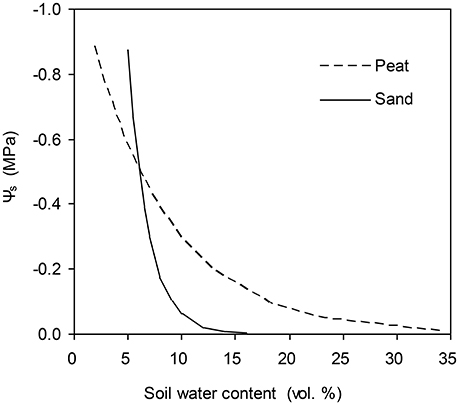
Figure 1. Water retention curves of the peat substrate used in experiment one and of the sandy soil used in experiment two.
The models were then validated by data obtained from another experiment (Exp. II). In this experiment, tomato plants were grown in pots with roots divided into two equal compartments. The pots (10 L) were filled with a sandy soil. The water retention curve of the sandy soil is shown in Figure 1. Three weeks after transplanting, plants were subjected to three irrigation regimes: (1) full irrigation (FI), in which both soil columns were irrigated daily to 18% (vol.); (2) alternative partial root-zone drying (PRI), in which only one soil column was watered daily to 70% irrigation amount of FI while the other was allowed to dry until the soil water content had decreased to ca. 6%; then the irrigation was shifted; (3) deficit irrigation (DI), in which the same amount of water for PRI was irrigated evenly to the two soil columns. The irrigation treatments lasted 40 days and each soil compartment of the PRI plants had experienced five drying/wetting cycles. The mean soil water content of each soil column was determined by TDR (Time Domain R ctometry; TRASE, Soil Moisture Equipment Corp., USA). The Pn and gs and other environmental variables were obtained in the same way as for experiment I.
Statistics
The performance of the original and the modified BB-model was compared by evaluating the coefficient of determination (r2), the mean absolute error (MAE) and the root mean square of error (RMSE) of the linear regressions between the measured and the observed values of gs and WUE. Analysis of covariance (ANCOVA) (SAS 9.4 Ins. Inc.) was performed to reveal the regression lines between vapor pressure deficit (VPD) in the atmosphere and leaf WUE.
Results
Model Parameterization
The data from experiment I was used for model parameterization. In brief, during the 5 days of soil drying Ψs decreased from −0.01 to −0.53 MPa and −0.01 to −0.61 MPa, gs decreased from 0.61 to 0.03 mol m−2 s−1 and 0.39 to 0.03 mol m−2 s−1, and Pn decreased from 15.3 to 1.54 mmol m−2 s−1 and 18.3 to 3.77 mmol m−2 s−1, for the plants grown at [CO2] concentration of 400 and 800 ppm, respectively.
To calculate the slope (m) of the BB-model, we first calculated hs by using the observed gs values. The Ball-index, viz. Pnhs/Cs was then computed. By plotting the observed gs against the Ball-index, an exponential, rather than a linear relationship between the two variables was found in both a[CO2] and e[CO2] (400 and 800 ppm) environment (Figure 2). Thus, m was not a constant indicating that gs is not linearly correlated with Pn and hs. Accordingly, the effects of soil water deficits on m (i.e., Equation 2) must be taken into account. This was done by incorporating Equation (2) into Equation (9). The simulation results show that the initial slope of the BB-model (i.e., mi) was 33.43 and 41.55 at a[CO2] and e[CO2], respectively; while the both of actual slope (m) decreased exponentially with declining Ψs (Figure 3; Table 1). Also, the mi was significantly greater for plants grown under e[CO2] than under a[CO2]. From Figures 4, 5 it can be seen that the modified BB-model significantly improved the gs and WUE simulations as compared with the original BB-model due to the higher r2, lower MAE and RMSE values in both a[CO2] and e[CO2] environment.
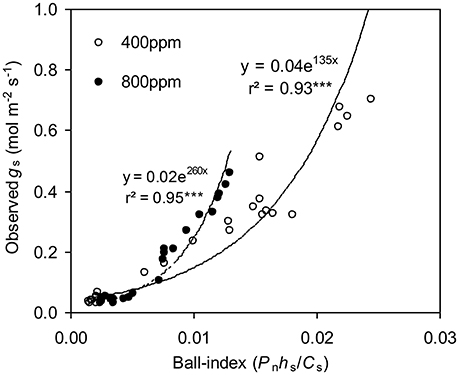
Figure 2. Relationship between the observed stomatal conductance (gs) and Ball-index (Pnhs/Cs) of a tomato leaf during progressive soil drying at [CO2] concentration of 400 and 800 ppm, respectively in experiment one.
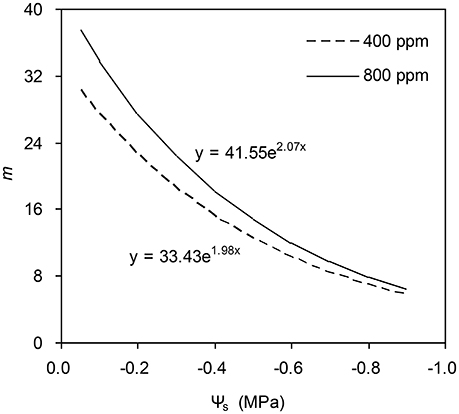
Figure 3. Simulation results of the effect of soil water deficits on the slope of the BB-model for a tomato leaf at [CO2] concentration of 400 and 800 ppm, respectively (details see Equation 2). Data is from experiment one (n = 25).
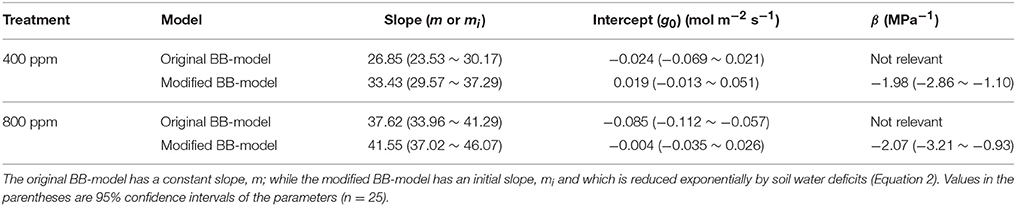
Table 1. Parameters of the original and modified BB-models at [CO2] concentration of 400 and 800 ppm obtained from the multi-regression (Equation 9) over the data of experiment one.
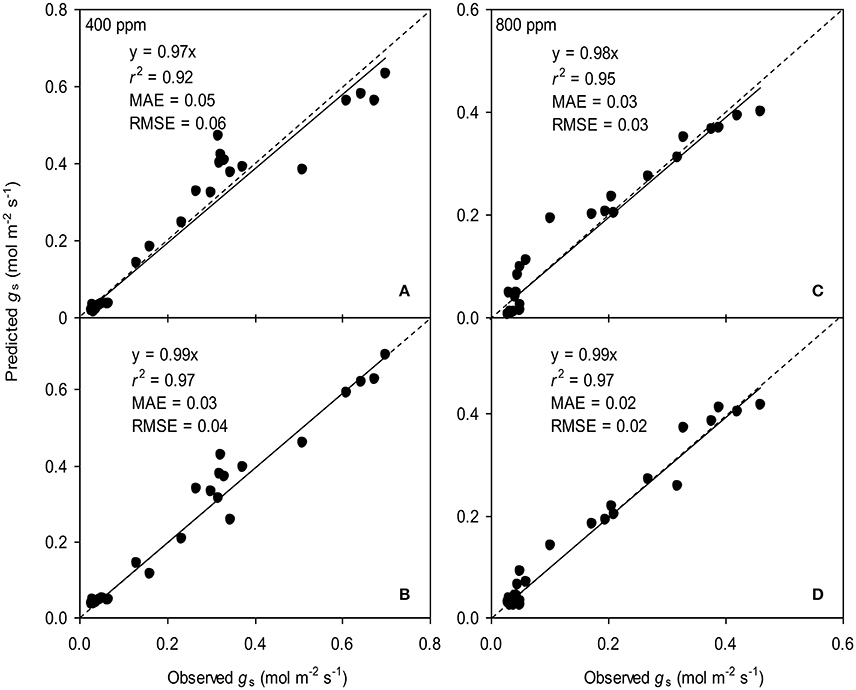
Figure 4. Observed and predicted stomatal conductance (gs) of tomato leaves predicted with the original (A,C) and the modified BB-model (B,D) at [CO2] concentration of 400 and 800 ppm, respectively. Data is from experiment one for model parameterization. The dashed lines indicate the 1:1 relationship.
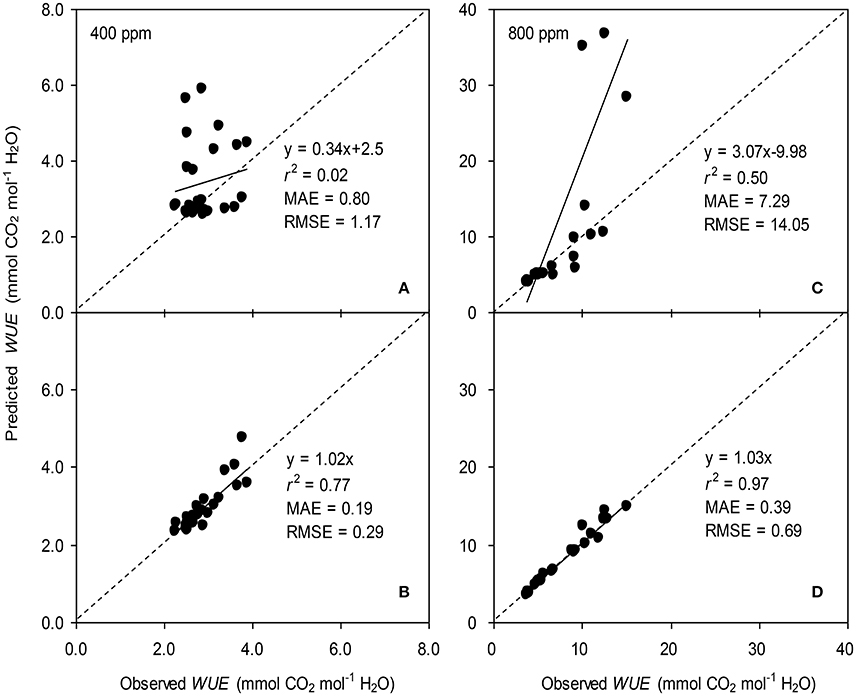
Figure 5. Observed and predicted water use efficiency (WUE) of tomato leaves predicted with the original (A,C) and the modified BB-model (B,D) at [CO2] concentration of 400 and 800 ppm, respectively. Data is from experiment one for model parameterization. The dashed lines indicate the 1:1 relationship.
Model Validation
The BB-models (with or without m modifications) were validated by the data obtained from the experiment II (Wei et al., unpublished). Shortly, for the FI plants Ψs was kept above −0.001 MPa; for the DI plants, Ψs ranged between −0.001 and −0.112 MPa; for the PRI plants, Ψs of the wet soil column was maintained above −0.001 MPa while that of the dry soil column ranged from −0.001 to −0.398 MPa during the treatment period. The model simulations indicated that both models were able to explain more than 71% of the variation in gs; for any FI, DI or PRI tomato plant, the modified BB-model was obviously superior to the original BB-model in predicting leaf gs owing to its equal or higher r2, lower MAE and RMSE values at both a[CO2] and e[CO2] (Figures 6, 7).
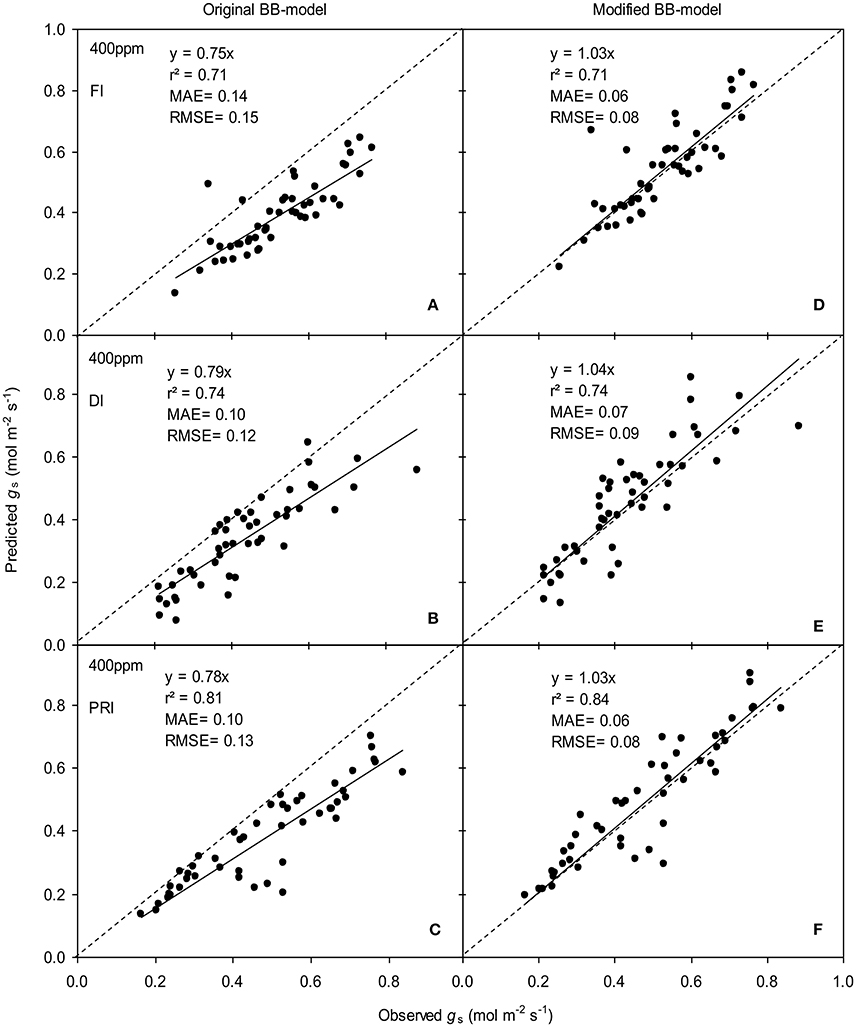
Figure 6. Observed and predicted stomatal conductance (gs) of experiment two data with the original (A–C) and the modified BB-model (D–F) of a tomato leaf under full irrigation (FI) (A,D) (n = 48), deficit irrigation (DI) (B,E) (n = 48) and alternative partial root-zone irrigation (PRI) (C,F) (n = 48), treatments at [CO2] concentration of 400 ppm. The predictability of the models was compared by the coefficient of determination (r2), the mean absolute error (MAE), and the root mean square of error (RMSE) of the linear regressions between the observed and predicted gs. The dashed lines indicate the 1:1 relationship.
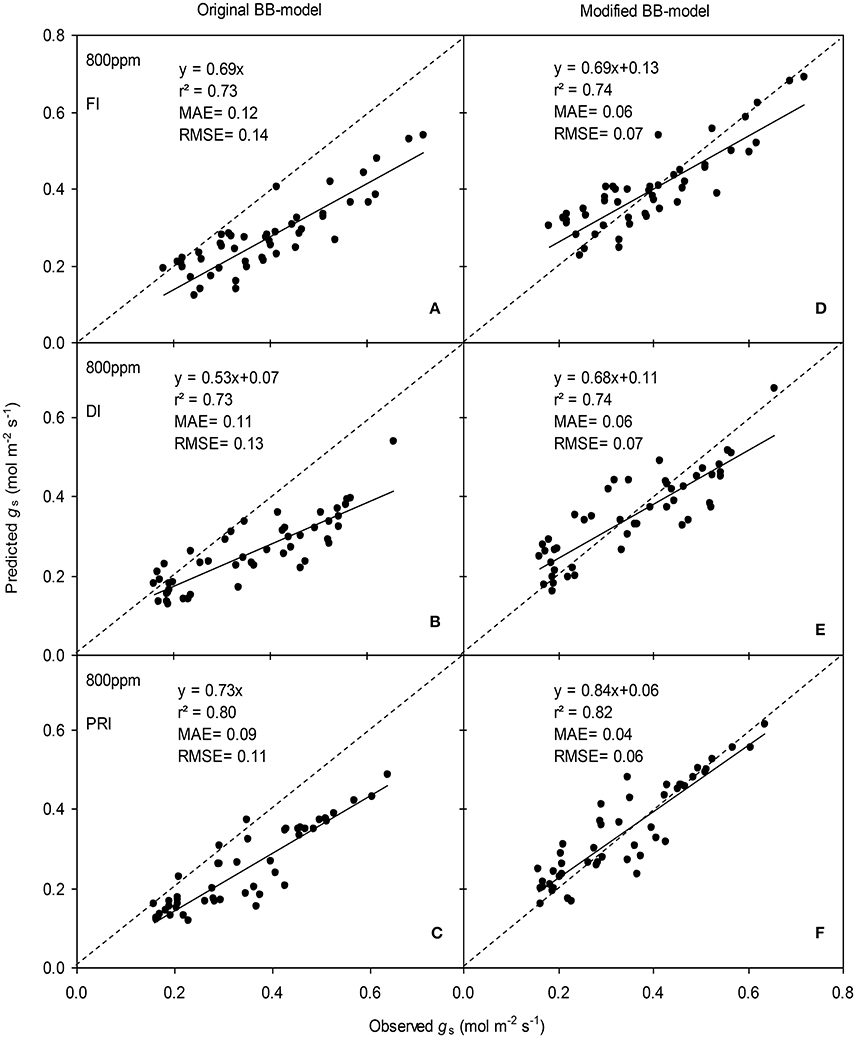
Figure 7. Observed and predicted stomatal conductance (gs) of experiment two data with the original (A–C) and the modified BB-model (D–F) of a tomato leaf under full irrigation (FI) (A,D) (n = 48), deficit irrigation (DI) (B,E) (n = 48) and alternative partial root-zone irrigation (PRI) (C,F) (n = 48), treatments at [CO2] concentration of 800 ppm. The predictability of the models was compared by the coefficient of determination (r2), the mean absolute error (MAE), and the root mean square of error (RMSE) of the linear regressions between the observed and predicted gs. The dashed lines indicate the 1:1 relationship.
The original model showed poor predictability for WUE (0.21 < r2 < 0.64) of tomato leaves (Figures 8, 9). The modified BB-model performed better in predicting leaf WUE than did the original BB-model for any FI, DI or PRI tomato plant owing to its greater r2 (0.46 < r2 < 0.75), lower MAE and RMSE values in both a[CO2] and e[CO2] conditions. Figure 10 shows there was a negative relationship between VPD and WUE. The correlation between predicted VPD and WUE was not different among the FI, DI, and PRI treatments under the same BB-model and [CO2] environment (Table 2).
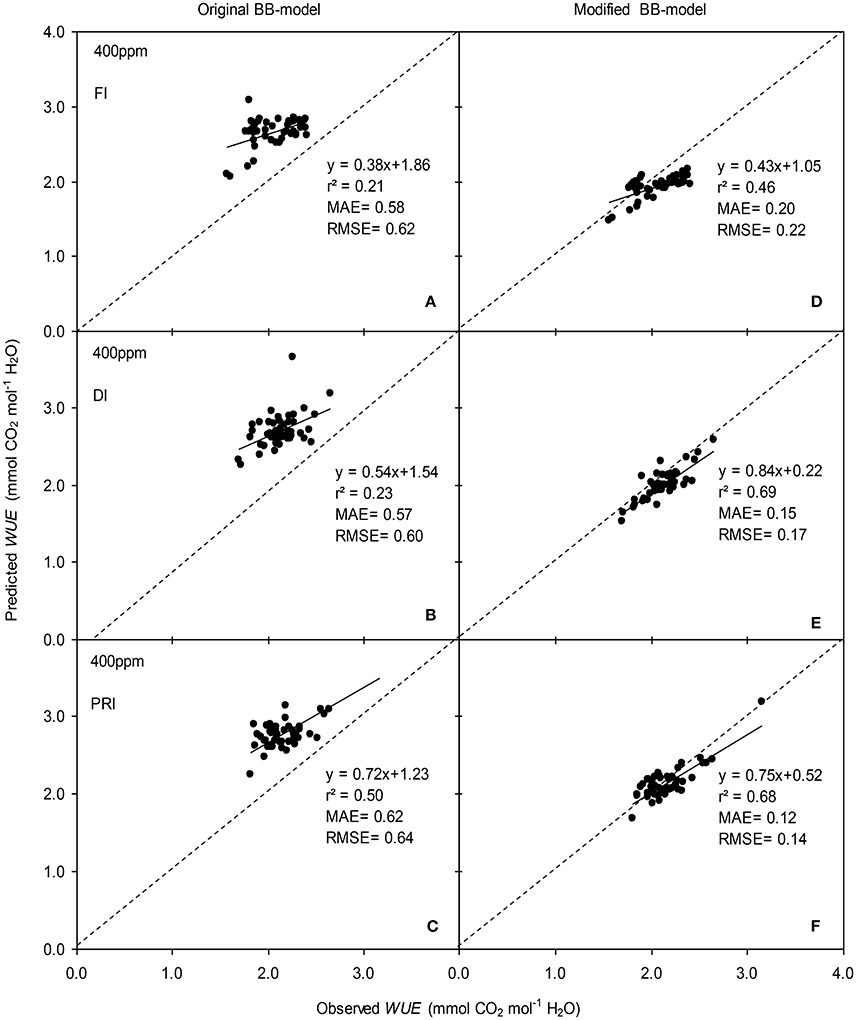
Figure 8. Observed and predicted water use efficiency (WUE) of experiment two data with the original (A–C) and the modified BB-model (D–F) of a tomato leaf under full irrigation (FI) (A,D) (n = 48), deficit irrigation (DI) (B,E) (n = 48) and alternative partial root-zone irrigation (PRI) (C,F) (n = 48), treatments at [CO2] concentration of 400 ppm. The predictability of the models was compared by the coefficient of determination (r2), the mean absolute error (MAE), and the root mean square of error (RMSE) of the linear regressions between the observed and predicted gs. The dashed lines indicate the 1:1 relationship.
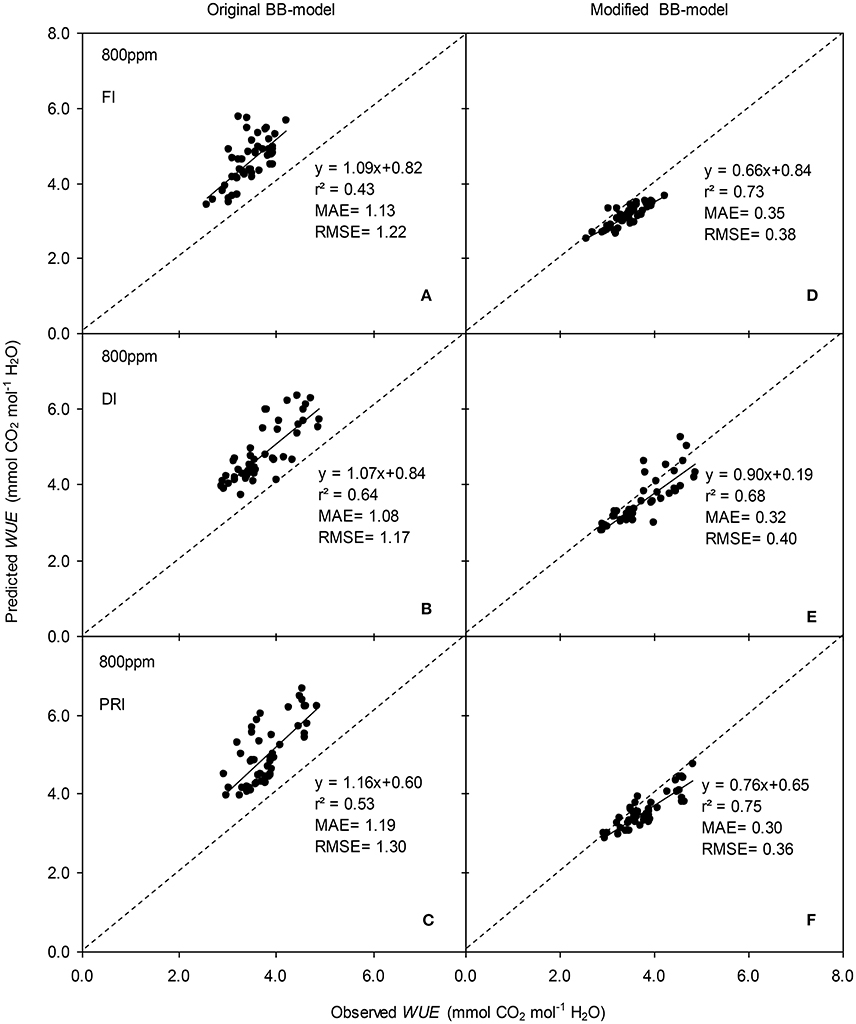
Figure 9. Observed and predicted water use efficiency (WUE) of experiment two data with the original (A–C) and the modified BB-model (D–F) of a tomato leaf under full irrigation (FI) (A,D) (n = 48), deficit irrigation (DI) (B,E) (n = 48) and alternative partial root-zone irrigation (PRI) (C,F) (n = 48), treatments at [CO2] concentration of 800 ppm. The predictability of the models was compared by the coefficient of determination (r2), the mean absolute error (MAE), and the root mean square of error (RMSE) of the linear regressions between the observed and predicted gs. The dashed lines indicate the 1:1 relationship.
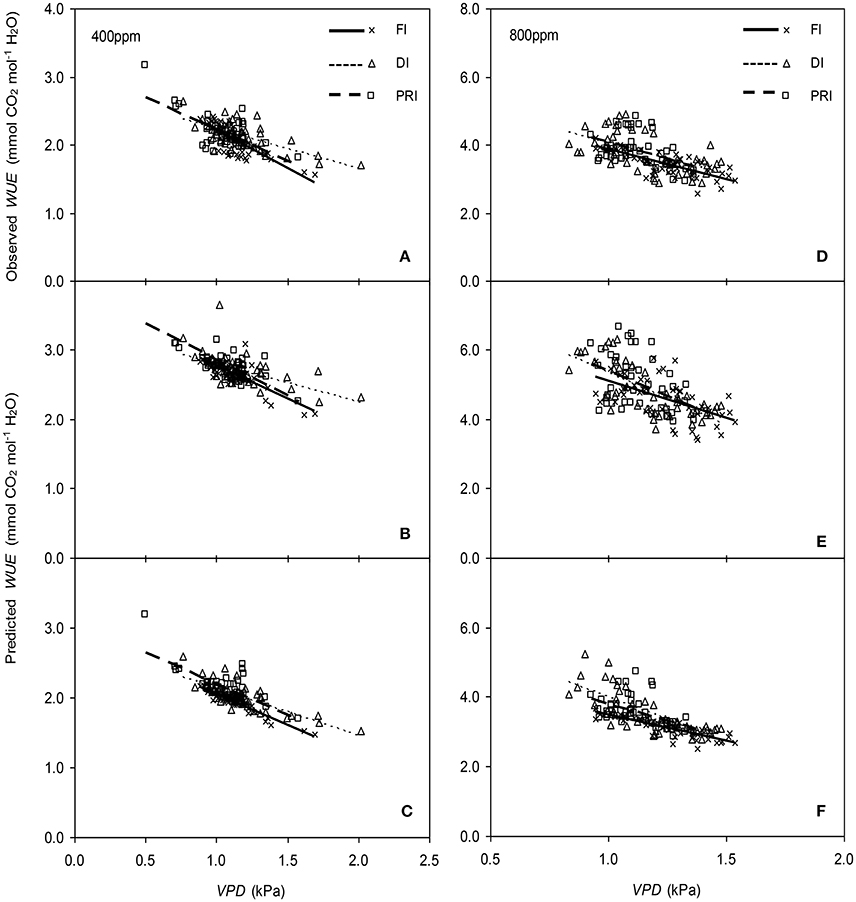
Figure 10. Relationships between the vapor pressure deficit (VPD) of the atmosphere and the measured photosynthetic water use efficiency (WUE) (A,D), and the simulated WUE by the original (B,E), and the modified BB-model (C,F) of tomato leaves under full irrigation (FI), deficit irrigation (DI) and alternative partial root-zone irrigation (PRI) regimes at [CO2] concentration of 400 and 800 ppm, respectively. The Comparison of regression lines under different irrigation treatments are seen in Table 2.
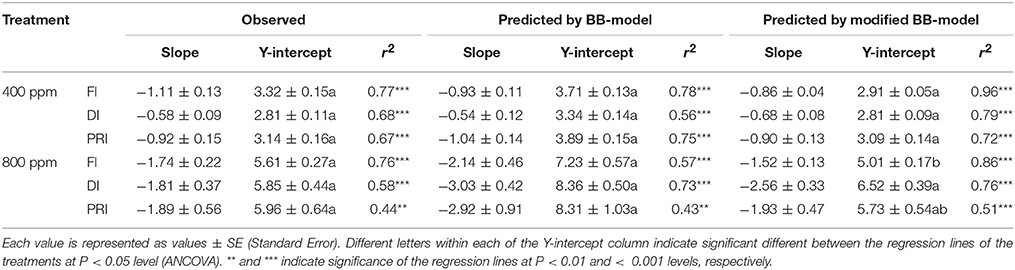
Table 2. Comparison of the regression lines between vapor pressure deficit of the air (VPD) and the predicted WUE under different irrigation treatments at [CO2] concentration of 400 and 800 ppm, respectively (Figure 10).
Discussion
In the two pot experiments, the simulation of the BB-model in predicting gs and WUE of tomato leaves grown in a[CO2] and e[CO2] environment under different irrigation regimes was performed. For both a[CO2] and e[CO2] plants, the original and modified BB-model could explain more than 71% of the observed variation in gs for all irrigation regimes; the modified BB-model was notably superior to the original model in predicting gs and WUE, although the two models showed relatively poor simulation in WUE of tomato leaves (r2 < 0.75) under different irrigation treatments.
It is well-known that partial stomatal closure leading to decrease in leaf gs during progressive soil drying was mainly induced by the increased root-to-shoot chemical signaling (ABA) in moderate soil moisture deficit (Liu et al., 2005). Moreover, changes in environmental conditions especially hs or VPD might mediate ABA action and influence leaf gas exchange (Wilkinson and Davies, 2002). A high hs or lowered VPD may decrease the delivery and concentration of ABA in the guard cells, resulting in minimal increase in gs and carbon assimilation without greater increase in transpiration (Speirs et al., 2013). In the current study, mostly the parameters ei and ea in the BB-model could well characterize the alteration in hs or VPD and modulation on ABA catabolism in relation to the growth environment of the plants. In addition, the modulation of CO2 concentration in combination with VPD and soil water deficits on the ABA signaling and its regulation on gs has not been well illustrated and needs further investigations (Yan et al., 2017).
A better understanding of whether there is physiological acclimation of gs to water stress and e[CO2] is crucial for describing plant responses using the BB-model (Miner et al., 2017). The influence of soil water stress on the slope (m) has been contradictory. Misson et al. (2004) measured 350% variation in m of ponderosa pine during the developing season; similarly, Héroult et al. (2013) found that two eucalyptus species had significant reductions in m under drought. However, Xu and Baldocchi (2003) showed that m remains relatively constant for blue oak even under severe water stress. Hence, disparate proposals have been suggested to account for the effect of water deficit on m of the BB-model (Buckley and Mott, 2013). To emphasize the significance of the root-to-shoot ABA signaling in regulating gs during mild soil drying, an ABA-based module was incorporated into the original BB-model to modify the m (Gutschick and Simonneau, 2002; Bauerle et al., 2004). The common point of those proposals for m modification has been that the initial slope of the BB-model, i.e., mi is scaled downward in response to progressive soil drying (Liu et al., 2009). In the present study, the modified m is related to soil water potential (Ψs) and it was found that such modification remarkably improved the predictability of model especially for the drought stressed tomato leaves grown at either a[CO2] and e[CO2] environment (Figures 4, 5). Besides, the m obtained from the model parameterization is higher (Table 1) than that observed mean value in C3 crops (Miner et al., 2017).
In soybean and wheat, the m significantly decreased when grown at e[CO2] (Bunce, 2004; Tausz-Posch et al., 2013). However, no change of m was noticed in five tree species (Medlyn et al., 2001) and soybean grown in a long-term free-air CO2 enrichment (FACE) experiment (Leakey et al., 2006), suggesting that acclimation of gs is mostly along with the photosynthetic acclimation, resulting in an unchanged m in e[CO2] condition (Ainsworth and Rogers, 2007; Gimeno et al., 2016). In contrast to those, here the m in e[CO2] was significantly greater than that in a[CO2] environment (Table 1), implying gs was independently acclimated to e[CO2] from Pn. A greater m indicates a higher sensitivity of stomata to the plant growth environment (such as radiation, humidity, soil water availability, and CO2 concentration). In this study, the intercept of the modified BB-model, i.e., g0 for tomato leaves was relatively lower than that obtained in an earlier potato study by Liu et al. (2009), and was not statistically significant from zero. A decreased g0 indicates a low stomatal conductance in the dark. It has been reported that at e[CO2] the simulated g0 could be lower, greater or no change as compared with that at a[CO2] (Medlyn et al., 2001; Bunce, 2004; Leakey et al., 2006). The BB-model not only requires environmental variables, also need the plant photosynthesis. Earlier studies have adopted the Farquhar's photosynthesis model (Farquhar et al., 1980) as an integrated module in the BB-model for simulating leaf Pn (Gutschick and Simonneau, 2002; Bauerle et al., 2004; Misson et al., 2004). However, such model is largely apt to a model of the correlation between Pn and gs resulted from the interdependence of those two factors, and it is useless for interpreting the causality (Liu et al., 2009). Numerous reports have confirmed that, applying the observed Pn as an input variable, the BB-model could performed successfully in predicting gs over different species (Misson et al., 2004; Liu et al., 2009). In addition, accompanied with decreased gs, leaf Pn would maintained or increased under PRI or e[CO2] (Pazzagli et al., 2016), which could complicate the simulation of Pn for plants grown under PRI strategy combined with e[CO2] environment. Thus, in this study, the measured leaf Pn was used to improve the model's performance in predicting gs across a wide range of soil water status.
Figures 6, 7 illustrated that the modified BB-model performed better than the original BB-model in predicting leaf gs of the three irrigation treatments at each atmospheric [CO2] concentration, especially for the PRI tomato plant with highest high r2, lowest MAE and RMSE values. It is evident that a decreased gs of leaf grown at e[CO2] would affect plant water use and further contribute to the varied Ψs (Kaminski et al., 2014). Likewise, the soil water heterogeneity induced by the PRI treatment could markedly altered the root-to-shoot ABA signaling involved in regulating gs, and the physiological responses, including leaf gs become more sensitive to the reduction of Ψs in the PRI than that in DI plant (Davies et al., 2002; Liu et al., 2008). Besides, previous study has revealed that xylem ABA concentration of PRI-treated potatoes is determined by Ψs and not by Ψs-dry (Liu et al., 2008), consistent with the results that the model performed much better when using mean Ψs in the whole root zone than using Ψs-dry to account for the effect of soil drying on gs (Liu et al., 2009). Therefore, a modification of m using Ψs incorporating into the BB-model is desired and necessary for achieving a better prediction of PRI tomato gs in the e[CO2] environment.
In the present study, similar to that for gs simulation, the modified BB-model also showed a better predictability for tomato WUE at leaf scale of all irrigation regimes in both [CO2] concentration as compared to the original model, particularly for PRI-treated plant, although the improvement of the prediction was less significant (Figures 8, 9). This could be ascribed to the accumulated errors in several variables for calculating WUE from the modeled gs. Nonetheless, regardless of the Ψs effect, the original and modified BB-model normally could not distinguish the influence of irrigation regimes on WUE of tomato leaves in either [CO2] condition (Figure 10; Table 2), indicating a robust relationship between WUE and VPD among different soil moisture condition and atmospheric [CO2] concentration. It is well-known that PRI combined with e[CO2] plants could lead to an increase of Pn, and a decrease of stomatal aperture, hence synergistically enhancing leaf WUE (Pazzagli et al., 2016). Thus, a greater WUE is expected for the PRI plant grown in e[CO2] environment as simulated by the modified gs and BB-model.
Collectively, tomato gs was independent acclimation to e[CO2] environment from Pn. Introducing Ψs of whole root zone into the BB-model could improve the model predictability of the model on gs and WUE of tomato leaves under combinations of different irrigation regimes and CO2 environments. This information is useful for better predicting WUE of tomato plant in a future drier and CO2 enriched environment.
Author Contributions
ZW: Done the experiment and finished the first manuscript; TD: The corresponding author, the work was supervised by him; XL: Help to do the experiment; LF: Help to do the experiment; FL: The corresponding author, the work was supervised by him.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (51725904, 51439006, 51621061), and the National Key Research Program (2016YFC0400207). ZW appreciates the Chinese Scholarship Council (CSC) for supporting his study at the Faculty of Science, University of Copenhagen, Denmark. Technical assistance by Rene Hvidberg Petersen and Lene Korsholm Jørgensen is gratefully acknowledged.
References
Ainsworth, E. A., and Rogers, A. (2007). The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270. doi: 10.1111/j.1365-3040.2007.01641.x
Ball, J. T., Woodrow, I. E., and Berry, J. A. (1987). “A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions,” in Progress in Photosynthesis Research, Vol. 4, Proceedings of the 7th International Congress on Photosynthesis, ed J. Biggins (Dordrecht: Martins Nijhoff), 221–224.
Bauerle, W. L., Toler, J. E., and Wang, G. G. (2004). Stomatal conductance of Acer rubrum ecotypes under varying soil and atmospheric water conditions: predicting stomatal responses with an abscisic acid-based model. Tree Physiol. 24, 805–811. doi: 10.1093/treephys/24.7.805
Buckley, T. N., and Mott, K. A. (2013). Modelling stomatal conductance in response to environmental factors. Plant Cell Environ. 36, 1691–1699. doi: 10.1111/pce.12140
Bunce, J. A. (2004). Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions. Oecologia 140, 1–10. doi: 10.1007/s00442-003-1401-6
Damour, G., Simonneau, T., Cochard, H., and Urban, L. (2010). An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 33, 1419–1438. doi: 10.1111/j.1365-3040.2010.02181.x
da Silva, J. R., Patterson, A. E., Rodrigues, W. P., Campostrini, E., and Griffin, K. L. (2017). Photosynthetic acclimation to elevated CO2 combined with partial rootzone drying results in improved water use efficiency, drought tolerance and leaf carbon balance of grapevines (Vitis labrusca). Environ. Exp. Bot. 134, 82–95. doi: 10.1016/j.envexpbot.2016.11.007
Davies, W. J., Wilkinson, S., and Loveys, B. (2002). Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol. 153, 449–460. doi: 10.1046/j.0028-646X.2001.00345.x
Du, T. S., Kang, S. Z., Zhang, J., and Davies, W. J. (2015). Deficit irrigation and sustainable water-resource strategies in agriculture for China's food security. J. Exp. Bot. 66, 2253–2269. doi: 10.1093/jxb/erv034
Farquhar, G. V., von Caemmerer, S. V., and Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. doi: 10.1007/BF00386231
Gimeno, T. E., Crous, K. Y., Cooke, J., O'Grady, A. P., Ósvaldsson, A., Medlyn, B. E., and Ellsworth, D. S. (2016). Conserved stomatal behaviour under elevated CO2 and varying water availability in a mature woodland. Funct. Ecol. 30, 700–709. doi: 10.1111/1365-2435.12532
Gutschick, V. P., and Simonneau, T. (2002). Modelling stomatal conductance of field grown sunf lower under varying soil water content and leaf environment: comparison of three models of stomatal response to leaf env ironment and coupling with an abscisic acid. based model of stomatal response to soil drying. Plant Cell Environ. 25, 1423–1434. doi: 10.1046/j.1365-3040.2002.00937.x
Héroult, A., Lin, Y. S., Bourne, A., Medlyn, B. E., and Ellsworth, D. S. (2013). Optimal stomatal conductance in relation to photosynthesis in climatically contrasting Eucalyptus species under drought. Plant Cell Environ. 36, 262–274. doi: 10.1111/j.1365-3040.2012.02570.x
Kaminski, K. P., Kørup, K., Nielsen, K. L., Liu, F. L., Topbjerg, H. B., Kirk, H. G., et al. (2014). Gas exchange, water use efficiency and yield responses of elite potato (Solanum tuberosum L.) cultivars to changes in atmospheric carbon dioxide concentration, temperature and relative humidity. Agric. For. Meteorol. 187, 36–45. doi: 10.1016/j.agrformet.2013.12.001
Kang, S. Z., and Zhang, J. H. (2004). Controlled alternate partial root zone irrigation: its physiological consequences and impact on water use efficiency. J. Exp. Bot. 55, 2437–2446. doi: 10.1093/jxb/erh249
Leakey, A. D., Bernacchi, C. J., Ort, D. R., and Long, S. P. (2006). Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ. 29, 1794–1800. doi: 10.1111/j.1365-3040.2006.01556.x
Liu, F. L., Andersen, M. N., and Jensen, C. R. (2009). Capability of the ‘Ball-Berry'model for predicting stomatal conductance and water use efficiency of potato leaves under different irrigation regimes. Sci. Hortic. 122, 346–354. doi: 10.1016/j.scienta.2009.05.026
Liu, F. L., Jensen, C. R., Shahanzari, A., Andersen, M. N., and Jacobsen, S. E. (2005). ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 168, 831–836. doi: 10.1016/j.plantsci.2004.10.016
Liu, F. L., Shahnazari, A., Andersen, M. N., Jacobsen, S. E., and Jensen, C. R. (2006). Physiological responses of potato (Solanum tuberosum L.) to partial root-zone drying: ABA signalling, leaf gas exchange, and water use efficiency. J. Exp. Bot. 57, 3727–3735. doi: 10.1093/jxb/erl131
Liu, F. L., Song, R., Zhang, X. Y., Shahnazari, A., Andersen, M. N., Plauborg, F., et al. (2008). Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environ. Exp. Bot. 63, 385–391. doi: 10.1016/j.envexpbot.2007.11.015
Medlyn, B. E., Barton, C. V. M., Broadmeadow, M. S. J., Ceulemans, R., De Angelis, P., Forstreuter, M., et al. (2001). Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 149, 247–264. doi: 10.1046/j.1469-8137.2001.00028.x
Miner, G. L., Bauerle, W. L., and Baldocchi, D. D. (2017). Estimating the sensitivity of stomatal conductance to photosynthesis: a review. Plant Cell Environ. 40, 1214–1238. doi: 10.1111/pce.12871
Misson, L., Panek, J. A., and Goldstein, A. H. (2004). A comparison of three approaches to modeling leaf gas exchange in annually drought-stressed ponderosa pine forests. Tree Physiol. 24, 529–541. doi: 10.1093/treephys/24.5.529
Pazzagli, P. T., Weiner, J., and Liu, F. L. (2016). Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manage. 169, 26–33. doi: 10.1016/j.agwat.2016.02.015
Sala, A., and Tenhunen, J. D. (1996). Simulations of canopy net photosynthesis and transpiration in Quercus ilex L. under the influence of seasonal drought. Agric For Meteorol. 78, 203–222.
Speirs, J., Binney, A., Collins, M., Edwards, E., and Loveys, B. (2013). Expression of ABA synthesis and metabolism genes under different irrigation strategies and atmospheric VPDs is associated with stomatal conductance in grapevine (Vitis vinifera L. cv Cabernet Sauvignon). J. Exp. Bot. 64, 1907–1916. doi: 10.1093/jxb/ert052
Tausz-Posch, S., Norton, R. M., Seneweera, S., Fitzgerald, G. J., and Tausz, M. (2013). Will intra-specific differences in transpiration efficiency in wheat be maintained in a high CO2 world? A FACE study. Physiol. Plant. 148, 232–245. doi: 10.1111/j.1399-3054.2012.01701.x
Wei, Z. H., Du, T. S., Li, X. N., Fang, L., and Liu, F. L. (2018). Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manage. 202, 174–182. doi: 10.1016/j.agwat.2018.02.027
Wei, Z. H., Du, T. S., Zhang, J., Xu, S. J., Cambre, P. J., and Davies, W. J. (2016). Carbon isotope discrimination shows a higher water use efficiency under alternate partial root-zone irrigation of field-grown tomato. Agric. Water Manage. 165, 33–43. doi: 10.1016/j.agwat.2015.11.009
Wilkinson, S., and Davies, W. J. (2002). ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ. 25, 195–210. doi: 10.1046/j.0016-8025.2001.00824.x
Xu, L., and Baldocchi, D. D. (2003). Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 23, 865–877. doi: 10.1093/treephys/23.13.865
Keywords: CO2, alternative partial root-zone irrigation, model simulation, stomatal conductance, water use efficiency, tomato
Citation: Wei Z, Du T, Li X, Fang L and Liu F (2018) Simulation of Stomatal Conductance and Water Use Efficiency of Tomato Leaves Exposed to Different Irrigation Regimes and Air CO2 Concentrations by a Modified “Ball-Berry” Model. Front. Plant Sci. 9:445. doi: 10.3389/fpls.2018.00445
Received: 14 December 2017; Accepted: 21 March 2018;
Published: 09 April 2018.
Edited by:
Hartmut Stützel, Leibniz University of Hanover, GermanyReviewed by:
Cecilia Brunetti, Istituto per la Valorizzazione del Legno e Delle Specie Arboree (CNR), ItalyClaudio Lovisolo, Università degli Studi di Torino, Italy
Copyright © 2018 Wei, Du, Li, Fang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taisheng Du, ZHV0YWlzaGVuZ0BjYXUuZWR1LmNu
Fulai Liu, ZmxAcGxlbi5rdS5kaw==
 Zhenhua Wei
Zhenhua Wei Taisheng Du
Taisheng Du Xiangnan Li
Xiangnan Li Liang Fang
Liang Fang Fulai Liu
Fulai Liu