- Department of Plant and Soil Sciences, University of Kentucky, Lexington, KY, United States
Legumes are able to form a symbiotic relationship with nitrogen-fixing soil bacteria called rhizobia. The result of this symbiosis is to form nodules on the plant root, within which the bacteria can convert atmospheric nitrogen into ammonia that can be used by the plant. Establishment of a successful symbiosis requires the two symbiotic partners to be compatible with each other throughout the process of symbiotic development. However, incompatibility frequently occurs, such that a bacterial strain is unable to nodulate a particular host plant or forms nodules that are incapable of fixing nitrogen. Genetic and molecular mechanisms that regulate symbiotic specificity are diverse, involving a wide range of host and bacterial genes/signals with various modes of action. In this review, we will provide an update on our current knowledge of how the recognition specificity has evolved in the context of symbiosis signaling and plant immunity.
Introduction
The legume-rhizobial symbiosis starts with a signal exchange between the host plant and its microsymbiont (Oldroyd, 2013). Recognition of compatible bacteria by the host induces cortical cell divisions to form root nodule primordia, and simultaneously initiates an infection process to deliver the bacteria into the nodule cells. Infection of most legumes involves the development of plant-made infection threads that initiate in the root hair. The infection threads harboring dividing bacteria grow through the epidermal cell layer into the nodule cells, where the bacteria are released and internalized in an endocytosis-like process. In nodule cells, individual bacteria are enclosed by a membrane of plant origin, forming an organelle-like structure called the symbiosome, within which the bacteria further differentiate into nitrogen-fixing bacteroids (Jones et al., 2007; Oldroyd et al., 2011).
Symbiotic nodule development involves synchronous differentiation of both nodule and bacterial cells. Legume nodules can be grouped into two major types: indeterminate (e.g., pea, clovers, and Medicago) and determinate (e.g., soybeans, common bean, and Lotus) (Nap and Bisseling, 1990; Hirsch, 1992). Indeterminate nodules originate from cell divisions in the inner cortex and possess a persistent apical meristem. Consequently, indeterminate nodules are cylindrical in shape, with a developmental gradient from the apex to the base of the nodule, which can be divided into different nodule zones (Nap and Bisseling, 1990). In contrast, determinate nodules result from cell divisions in the middle/outer cortex of the root, lack a persistent meristem, and are spherical in shape. Cell divisions of a determinate nodule cease at early developmental stages and the mature nodule develops through cell enlargement; as such, the infected cells develop more or less synchronously to the nitrogen-fixing stage. In both nodule types, the symbiotic nodule cells undergo genome endoreduplication, leading to polyploidization and cell enlargement. Parallel to the nodule cell development is the differentiation of the nitrogen-fixing bacteroids. Depending on the host, but independent of the nodule type, such bacterial differentiation can be terminal or reversible. Terminal differentiation is featured by genome endoreduplication, cell elongation, increased membrane permeability, and loss of reproductive ability, while in reversible differentiation the bacteroids retain cell size and DNA content similar to free-living bacteria (Kereszt et al., 2011; Oldroyd et al., 2011; Haag et al., 2013). Compared to free-living bacteria, the bacteroids display dramatic changes in transcriptome, cell surface structure and metabolic activities so that they become better adapted to the intracellular environment and dedicated to nitrogen fixation (Mergaert et al., 2006; Prell and Poole, 2006; Haag et al., 2013).
Both legumes and rhizobial bacteria are phylogenetically diverse. No single rhizobial strains can form symbiosis with all legumes, and vice versa. Specificity occurs at both species and genotypic levels (Broughton et al., 2000; Perret et al., 2000; Wang et al., 2012). This can take place at early stages of the interaction so that the same bacterial strains can infect and nodulate one host plant but not another (Yang et al., 2010; Wang et al., 2012; Tang et al., 2016; Fan et al., 2017). Incompatibility also frequently happens at later stages of nodule development such that nitrogen-fixing efficiency differs significantly between different plant-bacteria combinations (Wang et al., 2012, 2017, 2018; Yang et al., 2017). Symbiotic specificity results from the changing of signals from both host and bacterial sides; as such, various recognition mechanisms have evolved during the process of co-adaptation. Knowledge of the genetic and molecular basis of symbiotic specificity is important for developing tools for genetic manipulation of the host or bacteria in order to enhance nitrogen fixation efficiency. In this review, we will discuss our current understanding of the evolution of specificity in the root nodule symbiosis.
Specificity Mediated by Flavonoids and the Flavonoid-NodD Recognition
Under nitrogen-limiting conditions, legume roots secrete a cocktail of flavonoid compounds into the rhizosphere, and they serve to activate the expression of a group of bacterial nodulation (nod) genes, leading to the synthesis of the Nod factor, a lipochitooligosaccharidic signal that is essential for initiating symbiotic development in most legumes (Oldroyd et al., 2011). Induction of nod gene expression is mediated by the flavonoid-activated NodD proteins, which are LysR-type transcription regulators (Long, 1996). NodDs activate nod gene expression through binding to the conserved DNA motifs (nod boxes) upstream of the nod operons (Rostas et al., 1986; Fisher et al., 1988).
NodD proteins from different rhizobia are adapted to recognizing different flavonoids secreted by different legumes, and this recognition specificity defines an early checkpoint of the symbiosis (Peck et al., 2006). Despite the absence of direct evidence for physical interaction between the two molecules, flavonoids have been shown to be able to stimulate the binding of NodD to nod gene promoters in Sinorhizobium meliloti (Peck et al., 2006). It is well documented that inter-strain exchange of nodD genes can alter the response of the recipient strain to a different set of flavonoid inducers and hence the host range (Horváth et al., 1987; Perret et al., 2000). For example, the transfer of nodD1 from the broad host range symbiont Rhizobium sp. NGR234 to the restricted host range strain Rhizobium leguminosarum biovar trifolii ANU843 enabled the recipient strain to nodulate the non-legume Parasponia, because the wide-host-range NodD1 protein is capable of recognizing a broader spectrum of flavonoid inducers (Bender et al., 1988).
The evidence for the importance of flavonoids in determining host range primarily comes from bacterial genetics, and the plant genes involved are less studied. Since legume roots secrete a complex mixture of flavonoid compounds, it is difficult to pinpoint which flavonoids play a more critical role, and when and where they are produced. Recent studies in soybeans and Medicago truncatula have highlighted key flavonoids required for rhizobial infection (reviewed in Liu and Murray, 2016). These so called “infection flavonoids” are strong inducers of nod genes, secreted by roots, highly accumulated at the infection sites, and show increased biosynthesis in response to infection by compatible rhizobia. Although luteolin was the first flavonoid identified that can induce nod gene expression across a wide range of rhizobial strains, it is not legume-specific, mainly produced in germinating seeds, and has not been detected in root exudates or nodules. In contrast, methoxychalcone has been shown to be one of the strong host infection signals from Medicago and closely related legumes that form indeterminate nodules, while genistein and daidzein are crucial signals from soybeans that form determinate nodules. Part of the flavonoid compounds may also function as phytoalexins, acting to reinforce symbiosis specificity (Liu and Murray, 2016). For example, Bradyrhizobium japonicum and Mesorhizobium loti, but not the Medicago symbiont S. meliloti, are susceptible to the isoflavonoid medicarpin produced by Medicago spp. (Pankhurst and Biggs, 1980; Breakspear et al., 2014), and the soybean symbionts B. japonicum and S. fredii are resistant to glyceollin when exposed to genistein and daidzein (Parniske et al., 1991).
Specificity Mediated by Nod-Factor Perception
Nod factors produced by rhizobia are an essential signaling component for symbiosis development in most legumes. Nod factors are lipochito-oligosaccharides, consisting of four or five 1,4-linked N-acetyl-glucosamine residues that carry a fatty acyl chain of varying length attached to the C-2 position of the non-reducing end and various species-specific chemical decorations at both the reducing and non-reducing ends (Dénarié et al., 1996). The common nodABC genes contribute to the synthesis of the chitin backbone, while other strain-specific nod genes act to modify the backbone by changing the size and saturation of the acyl chain, or adding to the terminal sugar units with acetyl, methyl, carbamoyl, sulfuryl or glycosyl groups. Structural variations in Nod factors are a key determinant of host range, because these Nod factors have to be recognized by the host in order to initiate infection and nodulation (Perret et al., 2000; D’Haeze and Holsters, 2002).
Nod factors are perceived by Nod-factor receptors (e.g., NFR1 and NFR5 in Lotus japonicus), which are LysM-domain-containing receptor kinases (Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003). Direct binding of Nod factors to the extracellular LysM domains of the receptor complex leads to activation of the downstream nodulation signaling pathways (Broghammer et al., 2012). Specificity in Nod-factor binding is widely thought to be critical for recognition between the prospective symbiotic partners. This hypothesis has been strongly supported by genetic evidence even though such binding specificity has not been demonstrated. The best examples are from the pea-R. leguminosarum symbiosis where bacterial nod gene mutants that lead to changed Nod factor composition or structure exhibited genotype-specific nodulation (Firmin et al., 1993; Bloemberg et al., 1995). This alteration of host range corresponds to allelic variations at the Sym2/Sym37/PsK1 locus, an orthologous region of NFR1 that contains a cluster of LysM receptor kinases (Zhukov et al., 2008; Li et al., 2011). In this case, allelic variation coupled with gene duplication and diversification contribute to alterations in symbiotic compatibility.
Nod factor recognition presumably plays a more critical role in determining host range at species level, which has been best illustrated on the bacterial side. However, natural polymorphisms in Nod-factor receptors that are responsible for nodulation specificity between different legumes have not been well studied at the genetic level, simply because the plants cannot be interbred. Nevertheless, transferring NFR1 and NFR5 of L. japonicus into M. truncatula enables nodulation of the transformants by the L. japonicus symbiont Mesorhizobium loti (Radutoiu et al., 2007).
Specificity Mediated by Perception of Rhizobial Exopolysaccharides
In addition to Nod factors, rhizobial surface polysaccharides such as exopolysaccharides (EPS), lipopolysaccharides (LPS), and capsular polysaccharides (KPS) are also thought to be important for establishing symbiotic relationships (Fraysse et al., 2003; Becker et al., 2005; Jones et al., 2007; Gibson et al., 2008). These surface components are proposed to be able to suppress plant defense, but their active roles in promoting bacterial infection and nodulation remain elusive and are dependent on the specific interactions studied.
Exopolysaccharides have been shown to be required for rhizobial infection in multiple symbiotic interactions. This has been best illustrated in the Sinorhizobium-Medicago symbiosis, in which succinoglycan, a major EPS produced by S. meliloti, is required for the initiation and elongation of infection threads, and increased succinoglycan production enhances nodulation capacity (Leigh et al., 1985; Reinhold et al., 1994; Cheng and Walker, 1998; Jones, 2012). However, the symbiotic role of EPS is very complicated in the Mesorhizobium-Lotus interaction (Kelly et al., 2013). For instance, a subset of EPS mutants of M. loti R7A displayed severe nodulation deficiencies on L. japonicus and L. corniculatus, whereas other mutants formed effective nodules (Kelly et al., 2013). In particular, R7A mutants deficient in production of an acidic octasaccharide EPS were able to normally nodulate L. japonicus, while exoU mutants producing a truncated pentasaccharide EPS failed to invade the host. It was proposed that full-length EPS serves as a signal to compatible hosts to modulate plant defense responses and allow bacterial infection, and R7A mutants that make no EPS could avoid or suppress the plant surveillance system and therefore retain the ability to form nodules. In contrast, strains that produce modified or truncated EPS trigger plant defense responses resulting in block of infection (Kelly et al., 2013).
EPS production is common in rhizobial bacteria, and the composition of EPS produced by different species varies widely (Skorupska et al., 2006). Several studies have suggested the involvement of the EPS structures in determining infective specificity (Hotter and Scott, 1991; Kannenberg et al., 1992; Parniske et al., 1994; Kelly et al., 2013). Recently, an EPS receptor (EPR3) has been identified in L. japonicus, which is a cell surface-localized protein containing three extracellular LysM domains and an intracellular kinase domain (Kawaharada et al., 2015). EPR3 binds rhizobial EPS in a structurally specific manner. Interestingly, Epr3 gene expression is contingent on Nod-factor signaling, suggesting that the bacterial entry to the host is controlled by two successive steps of receptor-mediated recognition of Nod factor and EPS signals (Kawaharada et al., 2015, 2017). The receptor-ligand interaction supports the notion that EPS recognition plays a role in regulation of symbiosis specificity. However, natural variation in host-range specificity that results from specific recognition between host receptors and strain-specific EPS has not been demonstrated in any legume-rhizobial interactions. It is noteworthy that acidic EPS of bacterial pathogens also promote infection to cause plant disease (Newman et al., 1994; Yu et al., 1999; Aslam et al., 2008; Beattie, 2011). Thus, rhizobial EPS might also be recognized by host immune receptors to induce defense responses that negatively regulate symbiosis development.
Specificity Mediated by Host Innate Immunity
Symbiotic and pathogenic bacteria often produce similar signaling molecules to facilitate their invasion of the host (Deakin and Broughton, 2009). These molecules include conserved microbe-associated molecular patterns (MAMPs) and secreted effectors (D’Haeze and Holsters, 2004; Fauvart and Michiels, 2008; Deakin and Broughton, 2009; Soto et al., 2009; Downie, 2010; Wang et al., 2012; Okazaki et al., 2013). The host has evolved recognition mechanisms to distinguish between, and respond differently to, pathogens and symbionts (Bozsoki et al., 2017; Zipfel and Oldroyd, 2017). However, this discrimination is not always successful; as a result, recognition specificity frequently occurs in both pathogenic and symbiotic interactions. In the legume-rhizobial interaction, effector- or MAMP-triggered plant immunity mediated by host receptors also plays an important role in regulating host range of rhizobia (Yang et al., 2010; Wang et al., 2012; Faruque et al., 2015; Kawaharada et al., 2015; Tang et al., 2016).
Several dominant genes have been cloned in soybeans (e.g., Rj2, Rfg1, and Rj4) that restrict nodulation by specific rhizobial strains (Yang et al., 2010; Tang et al., 2016; Fan et al., 2017). In these cases, restriction of nodulation is controlled in a similar manner as ‘gene-for-gene’ resistance against plant pathogens. Rj2 and Rfg1 are allelic genes that encode a typical TIR-NBS-LRR resistance protein conferring resistance to multiple B. japonicum and Sinorhizobium fredii strains (Yang et al., 2010; Fan et al., 2017). Rj4 encodes a thaumatin-like defense-related protein that restricts nodulation by specific strains of B. elkanii (Tang et al., 2016). The function of these genes is dependent on the bacterial type III secretion system and its secreted effectors (Krishnan et al., 2003; Okazaki et al., 2009; Yang et al., 2010; Tsukui et al., 2013; Tsurumaru et al., 2015; Tang et al., 2016; Yasuda et al., 2016). These studies indicate an important role of effector-triggered immunity in the regulation of nodulation specificity in soybeans. As discussed earlier, rhizobial Nod factors and surface polysaccharides could play a role in suppression of defense responses (Shaw and Long, 2003; D’Haeze and Holsters, 2004; Tellström et al., 2007; Jones et al., 2008; Liang et al., 2013; Cao et al., 2017), but these signaling events apparently are not strong enough to evade effector-trigged immunity in incompatible interactions.
Many rhizobial bacteria use the type III secretion system to deliver effectors into host cells to promote infection, and in certain situations, the delivered effector(s) are required for Nod-factor independent nodulation as demonstrated in the soybean-B. elkanii symbiosis (Deakin and Broughton, 2009; Okazaki et al., 2013, 2016). On the other hand, however, recognition of the effectors by host resistance genes triggers immune responses to restrict rhizobial infection. The nodulation resistance genes occur frequently in natural populations, raising a question why host evolve and maintain such seemingly unfavorable alleles. This could happen because of balancing selection, as the same alleles may also contribute to disease resistance against pathogens, considering that some rhizobial effectors are homologous to those secreted by bacterial pathogens (Dai et al., 2008; Kambara et al., 2009). Alternatively, legume may take advantage of R genes to exclude nodulation with less efficient nitrogen-fixing strains and selectively interact with strains with high nitrogen fixation efficiency, which is the case of the soybean Rj4 allele.
A single dominant locus, called NS1, was also identified in M. truncatula that restricts nodulation by S. meliloti strain Rm41 (Liu et al., 2014). Unlike R gene-controlled host specificity in soybeans, which depends on bacterial type III secretion system, Rm41 strain lacks genes encoding such a system. It will be interesting to know what host gene(s) control this specificity and what bacterial signals are involved.
Specificity in Nitrogen Fixation
Symbiotic specificity is not confined to the early recognition stages; incompatible host-strain combinations can lead to formation of nodules that are defective in nitrogen fixation (Fix-). For example, a screen of a core collection of Medicago accessions using multiple S. meliloti strains showed that ∼40% of the plant-strain combinations produced nodules but failed to fix nitrogen (Liu et al., 2014). The Fix- phenotype was not due to a lack of infection but caused by bacteroid degradation after differentiation (Yang et al., 2017; Wang et al., 2017, 2018).
Host genetic control of nitrogen fixation specificity is very complicated in the Medicago-Sinorhizobium symbiosis, involving multiple linked loci with complex epistatic and allelic interactions. By using the residual heterozygous lines identified from a recombination inbred line population, Zhu and colleagues were able to clone two of the underlying genes, namely NFS1 and NFS2, that regulate strain-specific nitrogen fixation concerning the S. meliloti strains Rm41 and A145 (Wang et al., 2017, 2018; Yang et al., 2017). NFS1 and NFS2 both encode nodule-specific cysteine-rich (NCR) peptides (Mergaert et al., 2003). The NFS1 and NFS2 peptides function to provoke bacterial cell death and early nodule senescence in an allele-specific and rhizobial strain-specific manner, and their function is dependent on host genetic background. NCRs were previously shown to be positive regulators of symbiotic development, essential for terminal bacterial differentiation and for maintenance of bacterial survival in the nodule cells (Van de Velde et al., 2010; Wang et al., 2010; Horváth et al., 2015; Kim et al., 2015). The discovery of NFS1 and NFS2 revealed a negative role that NCRs play in regulation of symbiotic persistence, and showed that NCRs are host determinants of symbiotic specificity in M. truncatula and possibly also in closely related legumes that subject their symbiotic bacteria to terminal differentiation.
The genomes of M. truncatula and closely related galegoid legumes contain a large number of NCR-encoding genes that are expressed exclusively in the infected nodule cells (Montiel et al., 2017). These NCR genes, similar to bacterial type III effectors or MAMPs, can play both positive and negative roles in symbiotic development and both roles are associated with the antimicrobial property of the peptides. On one hand, the host uses this antimicrobial strategy for promoting terminal bacteroid differentiation to enhance nitrogen fixation efficiency (Oono and Denison, 2010; Oono et al., 2010; Van de Velde et al., 2010; Wang et al., 2010). On the other hand, some rhizobial strains cannot survive the antibacterial activity of certain peptide isoforms. The vulnerability of particular bacterial strains in response to a peptide is contingent on the genetic constitution of the bacteria as well as the genetic background of the host. It was proposed that this host-strain adaptation drives the coevolution of both symbiotic partners, leading to the rapid amplification and diversification of the NCR genes in galegoid legumes (Wang et al., 2017; Yang et al., 2017).
Host-range specificity in the ability to fix nitrogen has also been documented in legumes (e.g., soybeans) where the bacteria undergo reversible differentiation. In soybeans, this type of incompatibility was associated with the induction of phytoalexin accumulation and hypersensitive reaction in the nodule cells (Parniske et al., 1990). No NCR genes exist in the soybean genome, implying the involvement of novel genetic mechanisms that control this specificity. Work is in progress in our lab to identify the host genes that are involved.
Conclusion and Future Perspectives
Specificity in the legume-rhizobial symbiosis results from a suite of signal exchanges between the two symbiotic partners (summarized in Figure 1). Recent studies have just begun to reveal the underlying molecular mechanisms that regulate this specificity, and there are many challenging questions waiting to be answered. Effector-triggered immunity has been shown to be an important factor in determining host range of rhizobia in soybeans but the cognate effectors have not been clearly defined. In addition, what are the genes that control nodulation specificity in the Medicago-Sinorhizobium interaction where the bacterial partner lacks the type III secretion system? Cloning and characterization of the NS1 locus in M. truncatula (Liu et al., 2014) will provide novel insights into this question. We now know that NCR peptides regulate nitrogen fixation specificity in Medicago and possibly in other closely related legumes, but we lack mechanistic understanding of how these peptides work. Do the pro- and anti-symbiotic peptides interact with the same bacterial targets? How do the amino-acid substitutions affect the peptide structure and function? How is nitrogen fixation specificity regulated in the NCR-lacking legumes such as soybeans where bacteria undergo reversible differentiation? These are just a handful of outstanding questions that need to be addressed. Answering these questions will certainly enrich our knowledge of how specificity is controlled and allow us to use such knowledge to develop tools for genetic improvement of symbiotic nitrogen fixation in legumes.
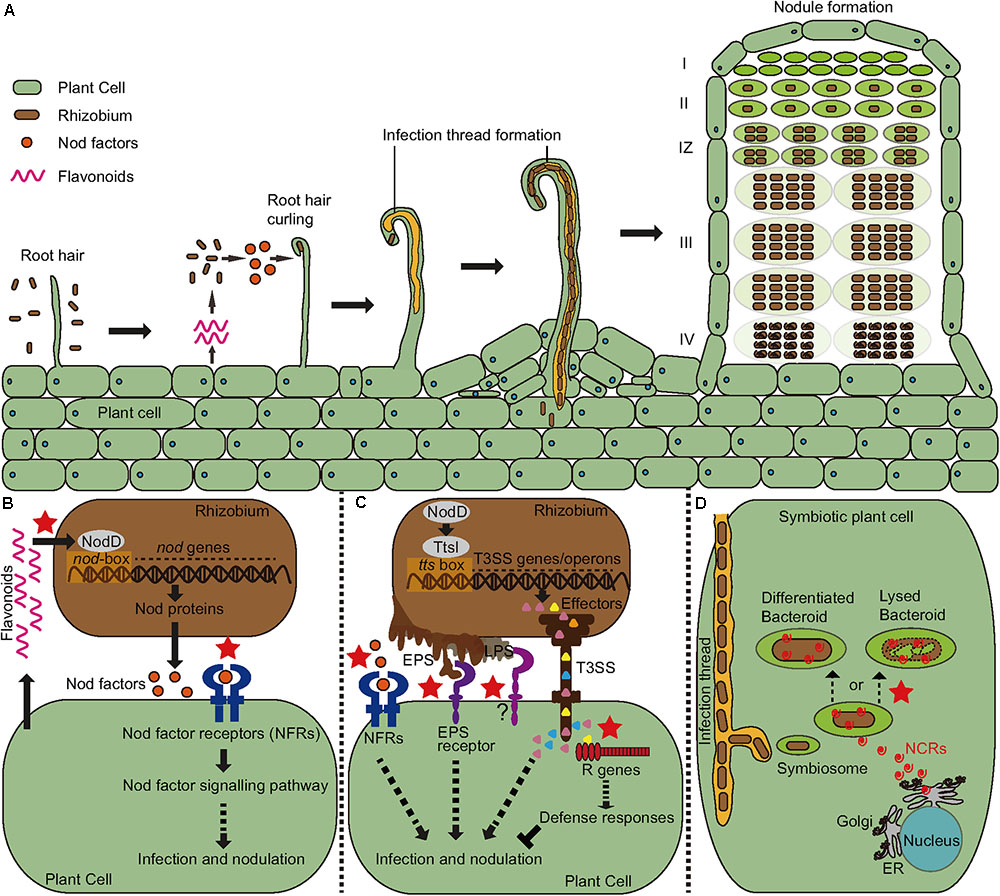
FIGURE 1. Symbiosis signaling and plant immunity involved in recognition specificity in the legume-rhizobial interactions (indicated by the red stars). (A) The process of infection and nodule development. A mature indeterminate nodule contains a meristem zone (I), an infection zone (II), an interzone (IZ), a nitrogen fixing zone (III), and a senescent zone (IV). (B) The host secretes flavonoids to induce the expression of bacterial nodulation (nod) gene through the activation of NodD proteins. The enzymes encoded by the nod genes lead to the synthesis of Nod factors (NF) that are recognized by host Nod factor receptors (NFRs). Recognition specificity occurs both between Flavonoids and NodDs and between NF and NFRs. (C) In addition to NF signaling, bacteria also produce extracellular polysaccharides (EPS) and type III effectors to facilitate their infection in compatible interactions, but these molecules may also induce immune responses causing resistance to infection in incompatible interactions. (D) Certain legumes such as Medicago encode antimicrobial nodule-specific cysteine-rich (NCR) peptides to drive their bacterial partners to terminal differentiation that is required for nitrogen fixation. However, some rhizobial strains cannot survive the antibacterial activity of certain peptide isoforms, leading to formation of nodules defective in nitrogen fixation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by United States Department of Agriculture/National Institute of Food and Agriculture, Agriculture and Food Research Initiative Grant 2014-67013-21573, Kentucky Science and Engineering Foundation Grant 2615-RDE-015, and the Kentucky Soybean Promotion Board.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aslam, S. N., Newman, M. A., Erbs, G., Morrissey, K. L., Chinchilla, D., Boller, T., et al. (2008). Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 18, 1078–1083. doi: 10.1016/j.cub.2008.06.061
Beattie, G. A. (2011). Water relations in the interaction of foliar bacterial pathogens with plants. Annu. Rev. Phytopathol. 49, 533–555. doi: 10.1146/annurev-phyto-073009-114436
Becker, A., Fraysse, N., and Sharypova, L. (2005). Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant Microbe Interact. 18, 899–905. doi: 10.1094/MPMI-18-0899
Bender, G. L., Nayudu, M., Le Strange, K. K., and Rolfe, B. G. (1988). The nodD1 gene from Rhizobium strain NGR234 is a key determinant in the extension of host range to the nonlegume Parasponia. Mol. Plant Microbe Interact. 1, 259–266. doi: 10.1094/MPMI-1-259
Bloemberg, G. V., Kamst, E., Harteveld, M., Drift, K. M., Haverkamp, J., Thomas-Oates, J. E., et al. (1995). A central domain of Rhizobium NodE protein mediates host specificity by determining the hydrophobicity of fatty acyl moieties of nodulation factors. Mol. Microbiol. 16, 1123–1136. doi: 10.1111/j.1365-2958.1995.tb02337.x
Bozsoki, Z., Cheng, J., Feng, F., Gysel, K., Vinther, M., Andersen, K. R., et al. (2017). Receptor-mediated chitin perception in legume roots is functionally separable from Nod factor perception. Proc. Natl. Acad. Sci. U.S.A. 114, 8118–8127. doi: 10.1073/pnas.1706795114
Breakspear, A., Liu, C., Roy, S., Stacey, N., Rogers, C., Trick, M., et al. (2014). The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 26, 4680–4701. doi: 10.1105/tpc.114.133496
Broghammer, A., Krusell, L., Blaise, M., Sauer, J., Sullivan, J. T., Maolanon, N., et al. (2012). Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc. Natl. Acad. Sci. U.S.A. 109, 13859–13864. doi: 10.1073/pnas.1205171109
Broughton, W. J., Jabbouri, S., and Perret, X. (2000). Keys to symbiotic harmony. J. Bacteriol. 182, 5641–5652. doi: 10.1128/JB.182.20.5641-5652.2000
Cao, Y., Halane, M. K., Gassmann, W., and Stacey, G. (2017). The role of plant innate immunity in the legume-rhizobium symbiosis. Annu. Rev. Plant Biol. 68, 535–561. doi: 10.1146/annurev-arplant-042916-041030
Cheng, H. P., and Walker, G. C. (1998). Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180, 5183–5191.
Dai, W. J., Zeng, Y., Xie, Z. P., and Staehelin, C. (2008). Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J. Bacteriol. 190, 5101–5110. doi: 10.1128/JB.00306-08
Deakin, W. J., and Broughton, W. J. (2009). Symbiotic use of pathogenic strategies: rhizobial protein secretion systems. Nat. Rev. Microbiol. 7, 312–321. doi: 10.1038/nrmicro2091
Dénarié, J., Debellé, F., and Promé, J. C. (1996). Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65, 503–535. doi: 10.1146/annurev.bi.65.070196.002443
D’Haeze, W., and Holsters, M. (2002). Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 12,79R–105R. doi: 10.1093/glycob/12.6.79R
D’Haeze, W., and Holsters, M. (2004). Surface polysaccharides enable bacteria to evade plant immunity. Trends Microbiol. 12, 555–561. doi: 10.1016/j.tim.2004.10.009
Downie, J. A. (2010). The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 34, 150–170. doi: 10.1111/j.1574-6976.2009.00205.x
Fan, Y., Liu, J., Lyu, S., Wang, Q., Yang, S., and Zhu, H. (2017). The soybean Rfg1 gene restricts nodulation by Sinorhizobium fredii USDA193. Front. Plant Sci. 8:1548. doi: 10.3389/fpls.2017.01548
Faruque, O. M., Miwa, H., Yasuda, M., Fujii, Y., Kaneko, T., Sato, S., et al. (2015). Identification of Bradyrhizobium elkanii genes involved in incompatibility with soybean plants carrying the Rj4 allele. Appl. Environ. Microbiol. 81, 6710–6717. doi: 10.1128/AEM.01942-15
Fauvart, M., and Michiels, J. (2008). Rhizobial secreted proteins as determinants of host specificity in the rhizobium–legume symbiosis. FEMS Microbiol. Lett. 28, 1–9. doi: 10.1111/j.1574-6968.2008.01254.x
Firmin, J. L., Wilson, K. E., Carlson, R. W., Davies, A. E., and Downie, J. A. (1993). Resistance to nodulation of cv. Afghanistan peas is overcome by nodX, which mediates an O-acetylation of the Rhizobium leguminosarum lipo-oligosaccharide nodulation factor. Mol. Microbiol. 10, 351–360. doi: 10.1111/j.1365-2958.1993.tb01961.x
Fisher, R. F., Egelhoff, T. T., Mulligan, J. T., and Long, S. R. (1988). Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 2, 282–293. doi: 10.1101/gad.2.3.282
Fraysse, N., Couderc, F., and Poinsot, V. (2003). Surface polysaccharide involvement in establishing the rhizobium–legume symbiosis. FEBS J. 270, 1365–1380. doi: 10.1046/j.1432-1033.2003.03492.x
Gibson, K. E., Kobayashi, H., and Walker, G. C. (2008). Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 42, 413–441. doi: 10.1146/annurev.genet.42.110807.091427
Haag, A. F., Arnold, M. F., Myka, K. K., Kerscher, B., Dall’Angelo, S., Zanda, M., et al. (2013). Molecular insights into bacteroid development during Rhizobium–legume symbiosis. FEMS Microbial. Rev. 37, 364–383. doi: 10.1111/1574-6976.12003
Hirsch, A. M. (1992). Developmental biology of legume nodulation. New Phytol. 122, 211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x
Horváth, B., Bachem, C. W., Schell, J., and Kondorosi, A. (1987). Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 6, 841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x
Horváth, B., Domonkos,Á., Kereszt, A., Szűcs, A., Ábrahám, E., Ayaydin, F., et al. (2015). Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc. Natl. Acad. Sci. U.S.A 112, 15232–15237. doi: 10.1073/pnas.1500777112
Hotter, G. S., and Scott, D. B. (1991). Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J. Bacteriol. 173, 851–859. doi: 10.1128/jb.173.2.851-859.1991
Jones, K. M. (2012). Increased production of the exopolysaccharide succinoglycan enhances Sinorhizobium meliloti 1021 symbiosis with the host plant Medicago truncatula. J. Bacteriol. 194, 4322–4331. doi: 10.1128/JB.00751-12
Jones, K. M., Kobayashi, H., Davies, B. W., Taga, M. E., and Walker, G. C. (2007). How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nat. Rev. Microbiol. 5, 619–633. doi: 10.1038/nrmicro1705
Jones, K. M., Sharopova, N., Lohar, D. P., Zhang, J. Q., VandenBosch, K. A., and Walker, G. C. (2008). Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc. Natl. Acad. Sci. U.S.A. 105, 704–709. doi: 10.1073/pnas.0709338105
Kambara, K., Ardissone, S., Kobayashi, H., Saad, M. M., Schumpp, O., Broughton, W. J., et al. (2009). Rhizobia utilize pathogen-like effector proteins during symbiosis. Mol. Microbiol. 71, 92–106. doi: 10.1111/j.1365-2958.2008.06507.x
Kannenberg, E. L., Rathbun, E. A., and Brewin, N. J. (1992). Molecular dissection of structure and function in the lipopolysaccharide of Rhizobium leguminosarum strain 3841 using monoclonal antibodies and genetic analysis. Mol. Microbiol. 6, 2477–2487. doi: 10.1111/j.1365-2958.1992.tb01424.x
Kawaharada, Y., Kelly, S., Nielsen, M. W., Hjuler, C. T., Gysel, K., Muszynski, A., et al. (2015). Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523, 308–312. doi: 10.1038/nature14611
Kawaharada, Y., Nielsen, M. W., Kelly, S., James, E. K., Andersen, K. R., Rasmussen, S. R., et al. (2017). Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat. Commun. 8:14534. doi: 10.1038/ncomms14534
Kelly, S. J., Muszyński, A., Kawaharada, Y., Hubber, A. M., Sullivan, J. T., Sandal, N., et al. (2013). Conditional requirement for exopolysaccharide in the Mesorhizobium–Lotus symbiosis. Mol. Plant Microbe Interact. 26, 319–329. doi: 10.1094/MPMI-09-12-0227-R
Kereszt, A., Mergaert, P., and Kondorosi, E. (2011). Bacteroid development in legume nodules: evolution of mutual benefit or of sacrificial victims? Mol. Plant Microbe Interact. 24, 1300–1309. doi: 10.1094/MPMI-06-11-0152
Kim, M., Chen, Y., Xi, J., Waters, C., Chen, R., and Wang, D. (2015). An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc. Natl. Acad. Sci. U.S.A. 112, 15238–15243. doi: 10.1073/pnas.1500123112
Krishnan, H. B., Lorio, J., Kim, W. S., Jiang, G., Kim, K. Y., DeBoer, M., et al. (2003). Extracellular proteins involved in soybean cultivar-specific nodulation are associated with pilus-like surface appendages and exported by a type III protein secretion system in Sinorhizobium fredii USDA257. Mol. Plant Microbe Interact. 16, 617–625. doi: 10.1094/MPMI.2003.16.7.617
Leigh, J. A., Signer, E. R., and Walker, G. C. (1985). Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. U.S.A. 82, 6231–6235. doi: 10.1073/pnas.82.18.6231
Li, R., Knox, M. R., Edwards, A., Hogg, B., Ellis, T. N., Wei, G., et al. (2011). Natural variation in host-specific nodulation of pea is associated with a haplotype of the SYM37 LysM-type receptor-like kinase. Mol. Plant-Microbe Interact. 24, 1396–1403. doi: 10.1094/MPMI-01-11-0004
Liang, Y., Cao, Y., Tanaka, K., Thibivilliers, S., Wan, J., Choi, J., et al. (2013). Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science 341, 1384–1387. doi: 10.1126/science.1242736
Limpens, E., Franken, C., Smit, P., Willemse, J., Bisseling, T., and Geurts, R. (2003). LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302, 630–633. doi: 10.1126/science.1090074
Liu, C. W., and Murray, J. D. (2016). The role of flavonoids in nodulation host-range specificity: an update. Plants 5:E33. doi: 10.3390/plants5030033
Liu, J., Yang, S., Zheng, Q., and Zhu, H. (2014). Identification of a dominant gene in Medicago truncatula that restricts nodulation by Sinorhizobium meliloti strain Rm41. BMC Plant Biol. 14:167. doi: 10.1186/1471-2229-14-167
Long, S. R. (1996). Rhizobium symbiosis: nod factors in perspective. Plant Cell 8, 1885–1898. doi: 10.1105/tpc.8.10.1885
Madsen, E. B., Madsen, L. H., Radutoiu, S., Olbryt, M., Rakwalska, M., Szczyglowski, K., et al. (2003). A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 425, 637–640. doi: 10.1038/nature02045
Mergaert, P., Nikovics, K., Kelemen, Z., Maunoury, N., Vaubert, D., Kondorosi, A., et al. (2003). A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 132, 161–173. doi: 10.1104/pp.102.018192
Mergaert, P., Uchiumi, T., Alunni, B., Evanno, G., Cheron, A., Catrice, O., et al. (2006). Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium–legume symbiosis. Proc. Natl. Acad. Sci. U.S.A. 103, 5230–5235. doi: 10.1073/pnas.0600912103
Montiel, J., Downie, J. A., Farkas, A., Bihari, P., Herczeg, R., Bálint, B., et al. (2017). Morphotype of bacteroids in different legumes correlates with the number and type of symbiotic NCR peptides. Proc. Natl. Acad. Sci. U.S.A. 114, 5041–5046. doi: 10.1073/pnas.1704217114
Nap, J. P., and Bisseling, T. (1990). Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science 250, 948–954. doi: 10.1126/science.250.4983.948
Newman, M. A., Conrads-Strauch, J., Scofield, G., Daniels, M. J., and Dow, J. M. (1994). Defense-related gene induction in Brassica campestris in response to defined mutants of Xanthomonas campestris with altered pathogenicity. Mol. Plant Microbe Interact. 7, 553–563. doi: 10.1094/MPMI-7-0553
Okazaki, S., Kaneko, T., Sato, S., and Saeki, K. (2013). Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. U.S.A. 110, 17131–17136. doi: 10.1073/pnas.1302360110
Okazaki, S., Tittabutr, P., Teulet, A., Thouin, J., Fardoux, J., Chaintreuil, C., et al. (2016). Rhizobium–legume symbiosis in the absence of Nod factors: two possible scenarios with or without the T3SS. ISME J. 10, 64–74. doi: 10.1038/ismej.2015.103
Okazaki, S., Zehner, S., Hempel, J., Lang, K., and Göttfert, M. (2009). Genetic organization and functional analysis of the type III secretion system of Bradyrhizobium elkanii. FEMS Microbiol. Lett. 295, 88–95. doi: 10.1111/j.1574-6968.2009.01593.x
Oldroyd, G. E. (2013). Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11, 252–264. doi: 10.1038/nrmicro2990
Oldroyd, G. E., Murray, J. D., Poole, P. S., and Downie, J. A. (2011). The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45, 119–144. doi: 10.1146/annurev-genet-110410-132549
Oono, R., and Denison, R. F. (2010). Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteroids. Plant Physiol. 154, 1541–1548. doi: 10.1104/pp.110.163436
Oono, R., Schmitt, I., Sprent, J. I., and Denison, R. F. (2010). Multiple evolutionary origins of legume traits leading to extreme rhizobial differentiation. New Phytol. 187, 508–520. doi: 10.1111/j.1469-8137.2010.03261.x
Pankhurst, C. E., and Biggs, D. R. (1980). Sensitivity of Rhizobium to selected isoflavonoids. Can. J. Microbiol 26, 542–545. doi: 10.1139/m80-092
Parniske, M., Ahlborn, B., and Werner, D. (1991). Isoflavonoid-inducible resistance to the phytoalexin glyceollin in soybean rhizobia. J. Bacteriol. 173, 3432–3439. doi: 10.1128/jb.173.11.3432-3439.1991
Parniske, M., Schmidt, P., Kosch, K., and Müller, P. (1994). Plant defense responses of host plants with determinate nodules induced by EPS-defective exob mutants of Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 7, 631–638. doi: 10.1094/MPMI-7-0631
Parniske, M., Zimmermann, C., Cregan, P. B., and Werner, D. (1990). Hypersensitive reaction of nodule cells in the Glycine sp./Bradyrhizobium japonicum-symbiosis occurs at the genotype-specific level. Plant Biol. 103, 143–148. doi: 10.1111/j.1438-8677.1990.tb00140.x
Peck, M. C., Fisher, R. F., and Long, S. R. (2006). Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. J. Bacteriol. 188, 5417–5427. doi: 10.1128/JB.00376-06
Perret, X., Staehelin, C., and Broughton, W. J. (2000). Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64, 180–201. doi: 10.1128/MMBR.64.1.180-201.2000
Prell, J., and Poole, P. (2006). Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14, 161–168. doi: 10.1016/j.tim.2006.02.005
Radutoiu, S., Madsen, L. H., Madsen, E. B., Felle, H. H., Umehara, Y., Grønlund, M., et al. (2003). Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425, 585–592. doi: 10.1038/nature02039
Radutoiu, S., Madsen, L. H., Madsen, E. B., Jurkiewicz, A., Fukai, E., Quistgaard, E. M., et al. (2007). LysM domains mediate lipochitin–oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J. 26, 3923–3935. doi: 10.1038/sj.emboj.7601826
Reinhold, B. B., Chan, S. Y., Reuber, T. L., Marra, A., Walker, G. C., and Reinhold, V. N. (1994). Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J. Bacteriol. 176, 1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994
Rostas, K., Kondorosi, E., Horvath, B., Simoncsits, A., and Kondorosi, A. (1986). Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc. Natl. Acad. Sci. U.S.A. 83, 1757–1761. doi: 10.1073/pnas.83.6.1757
Shaw, S. L., and Long, S. R. (2003). Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132, 2196–2204. doi: 10.1104/pp.103.021113
Skorupska, A., Janczarek, M., Marczak, M., Mazur, A., and Król, J. (2006). Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb. Cell Fact. 5:7. doi: 10.1186/1475-2859-5-7
Soto, M. J., Domínguez-Ferreras, A., Pérez-Mendoza, D., Sanjuán, J., and Olivares, J. (2009). Mutualism versus pathogenesis: the give-and-take in plant–bacteria interactions. Cell. Microbiol. 11, 381–388. doi: 10.1111/j.1462-5822.2009.01282.x
Tang, F., Yang, S., Liu, J., and Zhu, H. (2016). Rj4, a gene controlling nodulation specificity in soybeans, encodes a thaumatin-like protein, but not the one previously reported. Plant Physiol. 170, 26–32. doi: 10.1104/pp.15.01661
Tellström, V., Usadel, B., Thimm, O., Stitt, M., Küster, H., and Niehaus, K. (2007). The lipopolysaccharide of Sinorhizobium meliloti suppresses defense-associated gene expression in cell cultures of the host plant Medicago truncatula. Plant Physiol. 143, 825–837. doi: 10.1104/pp.106.090985
Tsukui, T., Eda, S., Kaneko, T., Sato, S., Okazaki, S., Kakizaki-Chiba, K., et al. (2013). The type III secretion system of Bradyrhizobium japonicum USDA122 mediates symbiotic incompatibility with Rj2 soybean plants. Appl. Environ. Microbiol. 79, 1048–1051. doi: 10.1128/AEM.03297-12
Tsurumaru, H., Hashimoto, S., Okizaki, K., Kanesaki, Y., Yoshikawa, H., and Yamakawa, T. (2015). A putative type III secretion system effector encoded by the MA20_12780 gene in Bradyrhizobium japonicum Is-34 causes incompatibility with Rj4 genotype soybeans. Appl. Environ. Microbiol. 81, 5812–5819. doi: 10.1128/AEM.00823-15
Van de Velde, W., Zehirov, G., Szatmari, A., Debreczeny, M., Ishihara, H., Kevei, Z., et al. (2010). Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327, 1122–1126. doi: 10.1126/science.1184057
Wang, D., Griffitts, J., Starker, C., Fedorova, E., Limpens, E., Ivanov, S., et al. (2010). A nodule specific protein secretory pathway required for nitrogen-fixing symbiosis. Science 327, 1126–1129. doi: 10.1126/science.1184096
Wang, D., Yang, S., Tang, F., and Zhu, H. (2012). Symbiosis specificity in the legume–rhizobial mutualism. Cell Microbiol. 14, 334–342. doi: 10.1111/j.1462-5822.2011.01736
Wang, Q., Liu, J., Li, H., Yang, S., Körmöczi, P., Kereszt, A., et al. (2018). Nodule-specific cysteine-rich peptides negatively regulate nitrogen-fixing symbiosis in a strain-specific manner in Medicago truncatula. Mol. Plant Microbe Interact. 31, 240–248. doi: 10.1094/MPMI-08-17-0207-R
Wang, Q., Yang, S., Liu, J., Terecskei, K., Ábrahám, E., Gombár, A., et al. (2017). Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula. Proc. Natl. Acad. Sci. U.S.A. 114, 6854–6859. doi: 10.1073/pnas.1700715114
Yang, S., Tang, F., Gao, M., Krishnan, H. B., and Zhu, H. (2010). R gene-controlled host specificity in the legume–rhizobia symbiosis. Proc. Natl. Acad. Sci. U.S.A. 107, 18735–18740. doi: 10.1073/pnas.1011957107
Yang, S., Wang, Q., Fedorova, E., Liu, J., Qin, Q., Zheng, Q., et al. (2017). Microsymbiont discrimination mediated by a host-secreted peptide in Medicago truncatula. Proc. Natl. Acad. Sci. U.S.A. 114, 6848–6853. doi: 10.1073/pnas.1700460114
Yasuda, M., Miwa, H., Masuda, S., Takebayashi, Y., Sakakibara, H., and Okazaki, S. (2016). Effector-triggered immunity determines host genotype-specific incompatibility in legume–rhizobium symbiosis. Plant Cell Physiol. 57, 1791–1800. doi: 10.1093/pcp/pcw104
Yu, J., Peñaloza-Vázquez, A., Chakrabarty, A. M., and Bender, C. L. (1999). Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol 33, 712–720. doi: 10.1046/j.1365-2958.1999.01516.x
Zhukov, V., Radutoiu, S., Madsen, L. H., Rychagova, T., Ovchinnikova, E., Borisov, A., et al. (2008). The pea Sym37 receptor kinase gene controls infection-thread initiation and nodule development. Mol. Plant Microbe Interact. 21, 1600–1608. doi: 10.1094/MPMI-21-12-1600
Keywords: legume, nodulation, nitrogen fixation, rhizobial symbiosis, host specificity
Citation: Wang Q, Liu J and Zhu H (2018) Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 9:313. doi: 10.3389/fpls.2018.00313
Received: 27 November 2017; Accepted: 23 February 2018;
Published: 09 March 2018.
Edited by:
Jeanne Marie Harris, University of Vermont, United StatesReviewed by:
Arijit Mukherjee, University of Central Arkansas, United StatesDong Wang, University of Massachusetts Amherst, United States
Copyright © 2018 Wang, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Zhu, aHpodTRAdWt5LmVkdQ==
†These authors have contributed equally to this work.
 Qi Wang
Qi Wang Jinge Liu
Jinge Liu Hongyan Zhu
Hongyan Zhu